Abstract
Many hematological malignancies consist of tumor cells that are spontaneously recognized and killed by Vγ9Vδ2 T cells. These tumor cells generate high amounts of intracellular phosphorylated metabolites mimicking the natural ligands and display a wide range of stress-induced self-ligands that are recognized by Vγ9Vδ2 T cells via TCR-dependent and TCR-independent mechanisms. The intrinsic features of Vγ9Vδ2 T cells and that of tumor cells of hematological origin constitute an ideal combination from which to develop Vγ9Vδ2 T cell-based immune interventions. In this review, we will discuss the rationale, preclinical and clinical data in favor of this therapeutic strategy and the future perspectives of its development.
Keywords: Vγ9Vδ2 T cells, Lymphoma, MM, Immunotherapy, Zoledronic acid, Mevalonate pathway
Introduction
Hematological malignancies constitute the ideal setting for exploitation of the unique antitumor properties of Vγ9Vδ2 T cells. Lymphoproliferative disorders such as B cell lymphomas, chronic lymphocytic leukemia (CLL) and multiple myeloma (MM) consist of tumor cells that are spontaneously recognized and killed by Vγ9Vδ2 T cells. Many of these diseases are preceded by a premalignant phase [monoclonal B cell lymphocytosis (MBL) in the case of CLL and monoclonal gammopathy of undetermined significance (MGUS) in the case of MM] [1, 2] during which innate effector cells, such as Vγ9Vδ2 T cells, NK and NKT cells, play an important role in holding monoclonal B cells in check by exploiting their ability to recognize stress-induced self-ligands [3, 4].
Even when the monoclonal B cell population exceeds a critical threshold and the disease is classified as malignant, the immune system is still able to hold it in check and a significant tumor burden may be asymptomatic for a long time as in stage A CLL, indolent MM, early follicular lymphoma and many other settings [5]. Several clinical and laboratory evidences accredit innate effector cells such as NKT and Vγ9Vδ2 T cells with the ability to control tumor cells during the early phases of the disease [3, 4, 6]. Unfortunately, this control is antagonized by tumor cells via immune editing and other immune escape strategies, and tumor-specific immune surveillance gradually fades away. For instance, Vγ9Vδ2 T cells are normally represented and functionally active in early MM, but eventually become phenotypically and functionally abnormal as it progresses [6, 7].
The intrinsic susceptibility of hematopoietic tumor cells to immune cells and, in particular, of B cell tumors to Vγ9Vδ2 T cells, has only been partially deciphered. In general terms, malignant cells of both the myeloid and the B cell lineage may be very attractive to immune cells because they are professional antigen-presenting cells (APC) intrinsically committed to the recruitment of innate and adaptive immunity. More specifically related to Vγ9Vδ2 T cell immunosurveillance is the increased activity of the mevalonate (Mev) pathway detected in many hematopoietic tumor cells. This pathway generates intermediate metabolites like isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) that mimick the natural ligands of Vγ9Vδ2 T cells. Moreover, hematopoietic tumor cells often express a restricted set of endogenous stress determinants which are recognized by the NK-like activatory receptors expressed by Vγ9Vδ2 T cells (see also below) [8].
Lastly, hematological malignancies, and lymphoproliferative disorders in particular, are among those in which passive immunotherapy with monoclonal antibodies (mAbs) has more significantly improved the clinical outcome in association with conventional chemotherapy. Several in vitro and in vivo data indicate that Vγ9Vδ2 T cells are excellent candidates to further improve the efficacy of chemoimmunotherapy (CIT) because they are equipped with the appropriate lytic machinery and Fcγ receptors (FcγR) to exert antibody-dependent cell cytotoxicity (ADCC). Moreover, a recent report has shown that they potentiate the ADCC of NK cells against hematopoietic tumors [9].
Taken together, these data indicate that the intrinsic features of Vγ9Vδ2 T cells on the one hand, and that of tumor cells on the other, constitute an ideal combination from which to develop Vγ9Vδ2 T cell-based immune interventions in hematological malignancies. In this review, we will discuss the rationale, preclinical and clinical data in favor of this therapeutic strategy.
Vγ9Vδ2 T cells as privileged immune effectors against tumor cells in hematological malignancies
The peculiar capacity of Vγ9Vδ2 T cells to naturally recognize and kill tumor cells of hematological origin was initially reported by Fisch et al. [10–12]. They investigated the cytotoxic activity of more than 100 Vγ9Vδ2 T cell clones derived from healthy donors against an extensive panel of tumor cell lines, including the hematopoietic-derived K562, Molt-4, Daudi and Raji cell lines [10]. All Vγ9Vδ2 T cell clones were highly cytotoxic against the MHC class I negative Daudi cells, whereas they failed to lyse the MHC class I positive Raji cells. Notably, the ability to proliferate and kill Daudi cells was an intrinsic feature since, by contrast with conventional CD8+ cells, these clones did not require prior sensitization to perform their effector function [13]. Moreover, Vδ1 T cells did not proliferate to Daudi cells and IL-2 expanded Vδ1 T cell clones were unable to kill Daudi cells in vitro.
The peculiar capacity of Vγ9Vδ2 T cells to naturally recognize tumor cells of B cell origin was confirmed by showing that those from the peripheral blood of normal donors proliferated after stimulation with a large panel of B cell lymphoma cell lines, mostly derived from Burkitt lymphomas and expressing MHC class I molecules [12]. This proliferative response was lower than that observed against Daudi cells, leading to the conclusion that MHC class I molecules restrict the natural cytotoxic activity of Vγ9Vδ2 T cells against B cell lymphomas. However, the observations that a MHC class I expressing Daudi variant, transfected with the mouse β2-microglobulin, was also killed by Vγ9Vδ2 T cell clones, and that three MHC class I negative cell lines derived from solid tumors did not induce any expansion of Vγ9Vδ2 T cells led to reconsideration of the role of MHC class I molecules in the natural recognition of lymphoma B cells.
A key role of Vγ9Vδ2 T cells in the control of B cell malignancies was also illustrated by Street et al.’s demonstration that gene-targeted mice lacking β2-microglobulin and/or perforin have an high incidence of spontaneous disseminated B cell lymphomas [14]. By inoculating these tumors into a variety of gene-targeted, mutant or lymphocyte subset-depleted mice, they showed that NK cells and Vγ9Vδ2 T cells prevented the development of spontaneous B cell lymphomas through a perforin-dependent mechanism.
B cell lymphomas are not the only hematological tumors intrinsically able to activate Vγ9Vδ2 T cells. The myeloma cell line RPMI 8226 also induce the activation and proliferation of Vγ9Vδ2 T cell clones with the capacity to exert non-MHC restricted cytotoxic activity against an extended spectrum of tumor cell lines [15]. XG-7 is another myeloma cell line which is naturally recognized by Vγ9Vδ2 T cells. Zheng et al. showed that XG-7 cells selectively induce the proliferation of Vγ9Vδ2 T cells of healthy individuals. Under optimal conditions, bulk cultures contained more than 80% Vγ9Vδ2 T cells. XG-7 cells induced activated Vγ9Vδ2 T cells that exerted TCR-dependent cytotoxicity against several tumor cell lines other than the inducing one, including Molt-4, BJAB, EBV-transformed lymphoid cell lines (LCL), and two nasopharyngeal carcinoma cell lines [16].
These results indicate that B cell tumors are unique in their ability to activate Vγ9Vδ2 T cells. Once activated, however, Vγ9Vδ2 T cells also recognize and kill tumor cells unrelated to the inducing ones. Thus, B cell and solid tumors share common targets which are sufficient for recognition and killing by Vγ9Vδ2 T cells, but only the former are immunogenic enough to activate unprimed Vγ9Vδ2 T cells and trigger their effector functions. Indeed, most solid tumor cells are unable to activate Vγ9Vδ2 T cells unless they are manipulated to maximize Vγ9Vδ2 T cell immune reactivity. Targeting the Mev pathway to increase the production of endogenous IPP, or pulsing tumor cells with exogenous IPP or synthetic IPP analogs, has widely been used to achieve this goal (see also below) [17, 18]. IPP is very similar to the natural ligands recognized by Vγ9Vδ2 T cells, collectively termed phosphoantigens (pAgs), which are generated in the Mev and non-Mev pathways of bacteria and other pathogens during isoprenoid biosynthesis [19, 20]. Synthetic analogs, such as bromohydrin pyrophosphate (BrHPP), monoethyl pyrophosphate (EtPP), and 2-methyl-3-butenyl-1-pyrophosphate (2M3B1-PP) have been developed to mimick the natural pAgs recognized by Vγ9Vδ2 T cells and induce their activation in vitro and in vivo [21, 22]. Several reports indicate that antitumor capacity of Vγ9Vδ2 T cells is greatly enhanced by activation with synthetic pAgs (s-pAgs), tumor cells or APC, such as monocytes and dendritic cells (DCs), which have accumulated supra-physiological levels of endogenous IPP [6, 23]. The most effective antitumor activity is achieved when Vγ9Vδ2 T cells are activated with aminobisphosphonates (NBPs) or s-pAgs and challenged against tumor cells which have generated large amounts of intracellular IPP. Even if Vγ9Vδ2 T cells recognize pAgs generated in plant cells and microorganisms of various origin, the recognition of tumor cells that have accumulated intracellular IPP is species-specific, since NBP-pulsed tumor cell lines of murine origin fail to activate human Vγ9Vδ2 T cells, even if derived from B cells [24].
Relevance of the Mev pathway in hematological malignancies
The Mev pathway plays an important role in the survival and growth of mammalian cells by providing them with a variety of essential bioactive molecules. Mev is synthesized intracellularly from 3-hydroxy-3-methylglutaryl coenzyme A (HMGCoA) in a process catalyzed by HMG-CoA reductase (HMGR), the rate limiting enzyme in the pathway [25]. The Mev pathway converts mevalonate into both sterol isoprenoids, such as cholesterol, and nonsterol isoprenoids, such as farnesylpyrophosphate (FPP) or geranylgeranylpyrophosphate (GGPP). FPP and GGPP are generated by FPP synthase (FPPS), an enzyme acting downstream from HMGR in the Mev pathway. FPP and GGPP are hydrophobic molecules critical for the isoprenylation of a variety of proteins essential in cell growth and differentiation. This post-translational modification depends on the binding of FPP and GGPP to target proteins by farnesyltransferase (FT) or geranylgeranyltransferase (GGT). Upon isoprenylation, proteins are correctly anchored to cell membranes and acquire the ability to perform their normal function.
Several epidemiological, preclinical and clinical data underscore the importance of the Mev pathway in hematological malignancies. Harwood et al. have shown that the activity of HMGR in freshly isolated leukocytes from patients with a variety of hematologic malignancies was significantly increased (up to 20-fold) when compared to that in leukocytes from normal subjects. The increased activity in patients with preleukemia was due to a rise in enzyme catalytic efficiency, whereas that in leukocytes from patients with overt leukemia or B cell lymphoma was due to a concomitant increase in both enzyme catalytic efficiency and enzyme protein concentration [26]. Shachaf et al. have confirmed the key role of the Mev pathway in B cell lymphoma by showing in a transgenic mouse model that inhibition of HMGR with atorvastatin prevents and reverses MYC-induced lymphomagenesis [27]. Lastly, epidemiological data from the European Case–Control Study EPILYMPH showed that statin use was associated with a reduced risk of lymphoma, with a similar reduction for all major histologic subtypes [28].
Since the Mev pathway generates intermediate metabolites that activate Vγ9Vδ2 T cells, one can speculate that tumor cells with a very active pathway are more inclined to signal to autologous Vγ9Vδ2 T cells compared to those with a limited activity. Several drugs are available to switch “on” or “off” the Mev pathway with opposite effects on Vγ9Vδ2 T cells. In general, drugs or strategies increasing intracellular IPP concentrations facilitate, whereas those that decrease them prevent, the activation of Vγ9Vδ2 T cells. For instance, silencing the IPP-consuming enzyme FPPS in Raji cells converted them into Vγ9Vδ2 T cell activators [29], whereas statins, which are irreversible HMGR inhibitors, abrogate IPP accumulation in tumor cells and abolish Vγ9Vδ2 T cell activation [6, 18, 30]. NBPs target FPPS downstream from HMGR and induce intracellular IPP accumulation. These drugs are extensively used to prevent and treat skeletal-related events in MM and other solid tumors. NBPs very avidly concentrate in the mineral bone where they are taken up by osteoclasts during bone resorption. Osteoclasts are then forced to apoptosis as a consequence of both defective protein prenylation and intracellular accumulation of triphosphoric acid 1-adenosin-50-yl ester 3-(3-methylbut-3-enyl) ester (ApppI). ApppI has recently been identified as a novel pro-apoptotic ATP analog, whose synthesis is forced in osteoclasts and other NBP-treated cells by the supra-physiological intracellular IPP concentrations provoked by ZA-induced FPPS inhibition [31]. Interestingly, ApppI is naturally produced by Daudi cells, the prototypic tumor cell targeted by Vγ9Vδ2 T cells. It has recently been proposed that ApppI acts as an inactive storage form of IPP because it binds to ecto-F1-ATP synthase, a mitochondrial ATP synthase ectopically expressed on the cell surface of tumor cells which activates Vγ9Vδ2 T cells in association with apolipoprotein A-1 [32, 33].
NBPs can be used to induce controlled IPP accumulation in APC and tumor cells to activate Vγ9Vδ2 T cells. We and others have shown that zoledronic acid (ZA), the most potent NBP currently available for clinical use, increases the immunosensitivity of myeloma and B cell lymphoma cells to Vγ9Vδ2 T cells. This strategy has also been applied to reconvert tumor cell lines and primary tumor cells not intrinsically susceptible to Vγ9Vδ2 T cell recognition and killing into sensitive targets [17, 18, 24]. Kato et al. showed that the majority of human solid tumor cell lines can be sensitized to Vγ9Vδ2 T cell-mediated cytotoxicity after pulsing with pamidronate, a second-generation NBP which also induces IPP accumulation by blocking FPPS [19]. Kunzmann et al. were the first to formulate the hypothesis [34], and Gober et al. the first to provide the proof-in-principle [18] that NBP activate Vγ9Vδ2 T cells by inducing an intracellular IPP accumulation in tumor cells as an upstream consequence of FPPS inhibition rather than by structural homologies with natural pAgs. The same mechanism has been reported by Roelofs et al., and by our group to explain the unique ability of monocytes and DC to induce the activation and proliferation of Vγ9Vδ2 T cells after short-term incubation with ZA [6, 35, 36].
Expression of NKG2D ligands and other TCR-independent target molecules in hematological malignancies
Besides a very active Mev pathway, tumor cells of hematological origin display a wide range of cell surface proteins that can be recognized by Vγ9Vδ2 T cells via TCR-independent mechanisms. Gomes et al. used gene expression profiling to screen a large panel of acute lymphoblastic leukemia and lymphoma cell lines, and primary hematopoietic tumor samples to identify markers of susceptibility versus resistance to Vγ9Vδ2 T cell-mediated cytotoxicity. They identified 10 genes encoding proteins whose cell surface expression was statistically different in Vγ9Vδ2 T cell-susceptible versus Vγ9Vδ2 T cell-resistant tumor cells. Three genes (ULBP1, TFR2 and IFITM1) were associated with increased susceptibility to Vγ9Vδ2 T cells, whereas seven genes (CLEC2D, NRP2, SELL, PKD2, KCNK12, ITGA6 and SLAMF1) were more expressed in resistant tumors [37].
ULBP1 is a member of the UL16-binding protein (ULBP) family of stress-inducible, nonclassic MHC proteins recognized by Vγ9Vδ2 T cells via the activating NK receptor member D of the lectin-like receptor family (NKG2D). The second group of NKG2D ligands are the stress-inducible MHC class I chain-related (MIC) protein A (MICA) and B (MICB) [38–40]. ULPB and MICA/B are both expressed on the cell surface of several hematological malignancies, such as B cell lymphomas, acute leukemias, CLL and MM [41–43]. Their expression and surface density strongly influence the susceptibility of tumor cells to innate effector cells such as NK cells and Vγ9Vδ2 T cells [44]. Lanca et al. have recently shown that the expression levels of ULBP1 in leukemia and lymphoma cells determines the tumor cell susceptibility to Vγ9Vδ2 T cell-mediated cytolysis, and that NKG2D blockade significantly inhibits lymphoma cell killing [45]. They draw the conclusion that Vγ9Vδ2 T cell-mediated surveillance of hematopoietic tumors is a two-step process where Vγ9Vδ2 T cells are activated through TCR stimulation via pAgs, but tumor cell recognition is mainly dependent on NKG2D.
Similar results have been reported by Girlanda et al., who propose a dual interaction between Vγ9Vδ2 T lymphocytes and myeloma cells involving both TCR triggering via pAgs and NKG2D-mediated signals. Vγ9Vδ2 T cells produced more IFN-γ and killed pamidronate-treated myeloma cells more efficiently if they expressed MICA on the cell surface. These authors also showed that MICA was expressed not only by myeloma cell lines and the majority of patients with active disease but also by MGUS individuals (six out of six), suggesting a role for MICA in the immune surveillance against MM (see also above) [4].
The expression of NKG2D ligands on tumor cells is not the only key factor promoting Vγ9Vδ2 T cell activation and tumor cell killing. Human Vγ9Vδ2 T cells frequently express both killer activatory (KARs), such as NKG2D, and killer inhibitory receptors (KIRs) that fine-tune their activation threshold [8]. The net balance between the expression of KARs and KIRs thus determines the ability of Vγ9Vδ2 T cells to recognize and kill tumor cells.
The intracellular cell adhesion molecule-1 (ICAM-1) is another cell surface molecule playing a key role in Vγ9Vδ2 T cell-mediated myeloma cell recognition and killing [46]. Uchida et al. have shown that the susceptibility of RPMI 8226 and U266 myeloma cell lines to the cytotoxic activity of Vγ9Vδ2 T cells requires both recognition of the Mev pathway metabolites, and expression of ICAM-1. The observation that the cytotoxic activity of Vγ9Vδ2 T cells against RPMI 8226 and U266 cells was not enhanced by ZA, and the video microscopic studies showing tight adhesion between Vγ9Vδ2 T cells and tumor cells led these authors to hypothesize that: (1) Mev metabolites in RPMI 8226 and U266 cells have already accumulated to maximal levels; and (2) adhesion molecules contribute to Vγ9Vδ2 T cell-mediated cytotoxicity. Indeed, treatment with anti-ICAM-1 blocking mAb inhibited the killing of RPMI 8226 and U266 cells, whereas blocking mAbs against other adhesion molecules (ICAM-3, LFA-3, VCAM-1, VLA-4) had no effect.
Altogether, these data indicate that tumor cells of hematological malignancies are very good candidates to activate Vγ9Vδ2 T cells because of their metabolic properties and equipment of cell surface proteins naturally recognized by Vγ9Vδ2 T cells (Fig. 1). This susceptibility can be further increased by manipulating the Mev pathway to generate an excess of IPP and, eventually, by modulating the expression of KARs and KIRs and their ligands on the cell surface of Vγ9Vδ2 T cells and tumor cells, respectively. Ranking of human tumor cells in function of their intrinsic or inducible susceptibility to Vγ9Vδ2 T cell recognition and killing can be postulated. At the top of the list are tumor cells of B cell origin, whereas at the bottom are cells from some solid tumors. So far, there are no data available that tumor cells other those of human origin display the same Vγ9Vδ2 T cell susceptibility (Fig. 2).
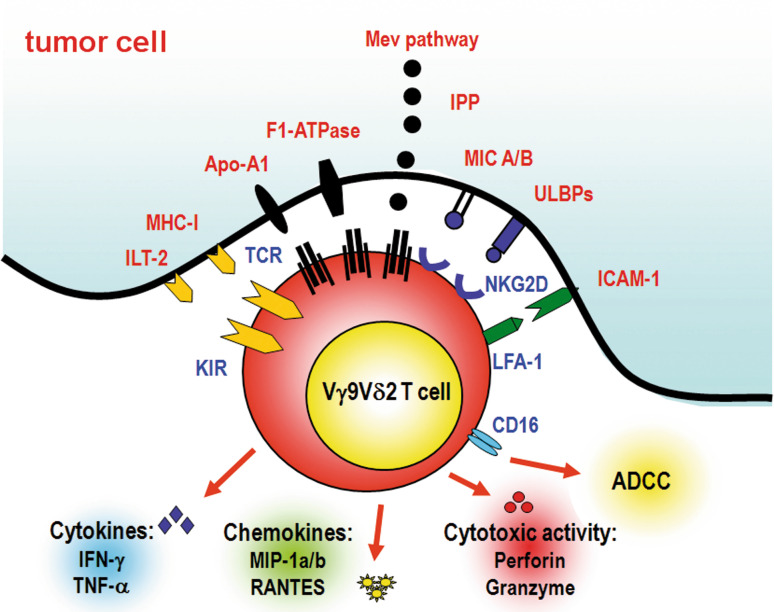
Fig. 1.
Tumor cell-induced activation of Vγ9Vδ2 T cells. The Mev pathway of tumor cells can generate supra-physiological amounts of phosphorylated Mev pathway metabolites, such as IPP, that mimick the natural p-Ags recognized by Vγ9Vδ2 T cells via their TCR. The Mev pathway is particularly active in tumor cells of hematopoietic origin. Other molecules that are recognized via TCR include ecto-F1-ATPase, a form of the mitochondrial ATP synthase ectopically expressed with ApoA-1 on the cell surface of tumor cells, including those of hematological origin. The ecto-F1-ATPase/Apo-1 complex is probably involved in the presentation of endogenous phosphorylated Mev metabolites, considering its capacity to bind ApppI. ApppI is an IPP-containing metabolite which is naturally produced in Daudi cells and formed in NBP-treated cells when intracellular IPP levels exceed a critical threshold as a consequence of FPPS inhibition. Tumor cells also display a wide range of cell surface proteins that are recognized via TCR-independent mechanisms, including a restricted set of endogenous stress determinants that are recognized via KARs, such as NKG2D, and KIRs. The expression of MHC class I molecules on tumor cells is generally sensed by Vγ9Vδ2 T cells as an inhibitory signal. The net balance between the expression of KARs and KIRs on Vγ9Vδ2 T cells, and the expression of their corresponding ligands on tumor cells, fine-tune the activation threshold and antitumor activity of Vγ9Vδ2 T cells. Another set of molecules that facilitate Vγ9Vδ2 T cell activation is represented by adhesion molecules such as LFA-1, CD6, CD2, and CD226. The interactions of these molecules with the corresponding ligands on tumor cells (ICAM-1, LFA-3, CD166 and others) help to stabilize the immunological synapse and deliver co-stimulatory signals. After productive interaction with tumor cells, fully activated Vγ9Vδ2 T cells proliferate, release cytokines (such as IFN-γ, TNF-α), chemokines (such as MIP-1α, MI-1β), and exert direct and indirect cytotoxic activity againt tumor cells, either alone or in association with other innate and adaptive immune effector cells (such as NK cells, CTL) and molecules (such as ADCC). Considering the immune adjuvant properties of Vγ9Vδ2 T cells, the immune performances of other effector cells are generally improved by their concurrent activation
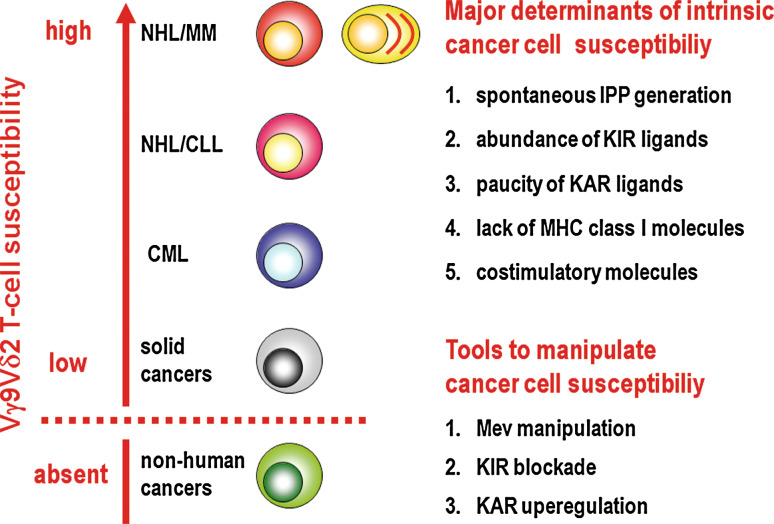
Fig. 2.
Ranking of human tumor cells in function of their intrinsic or inducible susceptibility to Vγ9Vδ2 T cell recognition and killing. At the top of the list are tumor cells of B cell origin, such as B cell lymphomas (NHL) and MM. These cells are the only ones immunogenic enough to fully activate unprimed Vγ9Vδ2 T cells. Once activated by these tumor cells, Vγ9Vδ2 T cells also recognize and kill tumor cells unrelated to the inducing ones. Data obtained with cell lines indicate that an hierarchical order also exist among B cell tumors. The activity rate of the Mev pathway and the reciprocal expression of cell surface ligands for KARs and KIRs of Vγ9Vδ2 T cells may determine their final immunogenicity and immune susceptibility. CML tumor cells display an intermediate susceptibility to Vγ9Vδ2 T cells. ZA-induced IPP accumulation is required to convert them into highly susceptible targets. Most solid tumor cells behave as CML cells, being unable to activate Vγ9Vδ2 T cells unless they are manipulated to maximize Vγ9Vδ2 T cell immune reactivity. However, there are some cell lines derived from solid cancer that never become susceptible to Vγ9Vδ2 T cells irrespective of any manipulation. So far, there are no data available that tumor cells other those of human origin are susceptible to Vγ9Vδ2 T cells, even if derived from B cells and treated with NBPs to increase their intracellular IPP content. This hierarchical rank of susceptibility is not irrevocable and it is possible to scale up the position of certain tumor cells in the list by manipulating the Mev pathway to generate an excess of IPP or modulating the expression of KIRs and KARs and their ligands on the cell surface of Vγ9Vδ2 T cells and tumor cells, respectively
Preclinical evidences of antitumor Vγ9Vδ2 T cell activity in hematological malignancies
As stated earlier, most of the studies about the susceptibility of hematopoietic tumor cells to Vγ9Vδ2 T cells have been conducted using Vγ9Vδ2 T cell clones or Vγ9Vδ2 T cells from healthy individuals. These studies have provided the proof-in-principle and dissected some of the mechanisms exploited by normal Vγ9Vδ2 T cells to kill tumor cells, but have devoted very little attention to a key issue, namely, whether Vγ9Vδ2 T cells from cancer patients are also able to perform the same way after a lengthy exposure to tumor cells and their immune editing activity.
Kunzmann et al. were among the first to explore the fitness of Vγ9Vδ2 T cells in patients with hematological malignancies and address the use of pamidronate to activate Vγ9Vδ2 T cells and generate antitumor activity in MM [34, 47]. In their initial in vitro study, they showed that NBPs (alendronate, ibandronate, and pamidronate) induce a dose-dependent activation and expansion of Vγ9Vδ2 T cells in primary peripheral blood mononuclear cell (PBMC) cultures of healthy donors at clinically relevant concentrations. They also showed that pamidronate stimulate bone marrow (BM) Vγ9Vδ2 T cells in 14 out of 24 tested patients, and that their activation was associated with a significant decrease in the number of autologous BM plasma cells. The failure to stimulate Vγ9Vδ2 T cells in all patients was interpreted as a consequence of a general dysfunction affecting T cell-mediated immunity.
Subsequent to these seminal papers, other groups have shown that MM Vγ9Vδ2 T cells can be induced to recognize and kill myeloma and lymphoma cells upon stimulation with NBPs or s-pAgs such as IPP and BrHPP [6, 7, 46, 48, 49]. We have shown that ZA induced the activation and proliferation of peripheral blood Vγ9Vδ2 T cells via autologous monocytes, which serve as endogenous IPP producers, and by enhancing the immunosensitivity of myeloma cells to Vγ9Vδ2 T cells. We also confirmed that the proliferative expansion of peripheral Vγ9Vδ2 T cells did not occur in all patients: approximately 50% of MM patients at diagnosis lack Vγ9Vδ2 T cell proliferation in response to monocyte-dependent ZA stimulation. These patients were defined as non-responders (NR), whereas those who showed total counts of Vγ9Vδ2 T cells after stimulation in the same range as normal donors were defined as responders (R). A very similar proportion of NR patients was reported by Wilhelm et al. who extended this analysis to other B cell lymphoid malignancies showing that approximately 20 and 80% of patients can be classified as NR in NHL and CLL, respectively [50, 79].
BrHPP has also been used to activate MM Vγ9Vδ2 T cells [7]. Burjanadze et al. compared the capacity of BrHPP and ZA to induce the proliferative expansion of Vγ9Vδ2 T cells and anti-myeloma cell cytotoxicity in MM patients at different time points throughout the disease. They showed that BrHPP triggered a 100-fold expansion of Vγ9Vδ2 T cells in almost 80% of newly diagnosed patients, whereas less vigorous expansions were observed when Vγ9Vδ2 T cells were obtained at the time of hematopoietic progenitor collection or in relapsing patients. An important finding of this study from the translational standpoint was that expanded Vγ9Vδ2 T cells efficiently killed myeloma cell lines and primary myeloma cells, but not normal CD34 cells.
Even if B cell malignancies are the most suitable candidates, other hematopoietic tumors are under investigation to exploit the intrinsic antitumor properties of Vγ9Vδ2 T cells. Kiladjian et al. have evaluated the phenotype, function, and expansion capacity of peripheral Vγ9Vδ2 T cells in patients with myelodysplastic syndromes (MDS) [51]. They found that Vγ9Vδ2 T cells proliferated in response to BrHPP and IL-2 in 60% of MDS patients irrespective of cytogenetic findings, WHO subtype or risk category. Seventy percent of NR MDS patients had a concomitant autoimmune disorder and a lower Vγ9Vδ2 T cell count in the peripheral blood. These findings led the authors to suggest that Vγ9Vδ2 T cells, like NK cells, are deeply involved in the immune surveillance defects occurring in MDS. Once expanded, however, Vγ9Vδ2 T cells display normal cytokine production and cytotoxic activity against MDS cell lines and primary tumor cells. The authors’ conclusion is that Vγ9Vδ2 T cells can be of particular value in the development of immunotherapy-based approaches in MDS, at least in the subset of R patients.
Chronic myeloid leukemia (CML) is a myeloproliferative disease in which Vγ9Vδ2 T cells are under investigation as antitumor effector cells. The CML standard of care is currently represented by imatinib, a competitive inhibitor of the BCR-ABL tyrosine kinase. However, from 20 to 30% of CML patients display primary or develop secondary resistance to imatinib. One strategy to rescue these patients is to treat them with more potent tyrosine kinase inhibitors like dasatinib and nilotinib. An alternative strategy is the association with drugs that synergistically act with imatinib and other tyrosine kinase inhibitors. ZA was found to increase the antileukemia activity of imatinib both in vitro and in vivo and to inhibit the proliferation and induce the apoptosis of imatinib-resistant CML cells [23]. Since ZA also increase the susceptibility of tumor cells to Vγ9Vδ2 T cells (see above), D’Asaro et al. investigated the possibility of activating Vγ9Vδ2 T cells with ZA to generate immune-mediated antileukemia activity. Like patients with B cell malignancies and MDS patients, CML patients differ in the magnitude of their Vγ9Vδ2 T cell responses, and both R and NR patients were identified. Unlike patients with B cell malignancies, however, expanded Vγ9Vδ2 T cells failed to kill both CML cell lines and primary tumor cells if left untreated. Treatment of target cells with ZA was required to generate significant antitumor activity against autologous and allogeneic leukemia cells that could be detected in vitro and after adoptive transfer of activated Vγ9Vδ2 T cells and ZA-treated tumor cells into immunodeficient mice in vivo. Based on these results, the authors proposed intentional activation of Vγ9Vδ2 T cells with ZA and low doses of IL-2 as a novel strategy for CML immunotherapy, especially in patients resistant to imatinib or with suboptimal responses to other tyrosine kinase inhibitors.
The paucity of preclinical data about the therapeutic use of Vγ9Vδ2 T cells in hematological malignancies is also a consequence of the fact that mice lack the corresponding human Vγ9Vδ2 T cell counterpart [52] and therefore it is very difficult to exploit mouse models to investigate the antitumor activity of Vγ9Vδ2 T cells in vivo after activation with s-pAgs or NBPs [53].
To overcome the limitations imposed by the differences between mouse and human Vγ9Vδ2 T cell subsets, activated human Vγ9Vδ2 T cells and human cancer cell lines have adoptively been transferred into immunodeficient mice. A few studies have been performed in hematological malignancies. D’Asaro et al. showed that Vγ9Vδ2 T cells from healthy donors and CML patients, activated and expanded ex vivo with ZA and IL-2, exerted antitumor activity after adoptive transfer in Nod/SCID mice injected with the MM1 cells, a CML cell line which is resistant to any available tyrosine kinase inhibitor [23]. Malkovska et al. showed that the adoptive transfer of polyclonal human T cell populations containing a high percentage of Vγ9Vδ2 T cells or Vγ9Vδ2 T cell clones prolonged the survival of mice that received a lethal dose of Daudi cells [54]. Chen et al. extended these results by showing that when Vγ9Vδ2 T cells isolated from the tumor bed or the ascites of patients with colorectal and ovarian epithelial carcinoma are expanded ex vivo and adoptively transferred into Daudi cell-bearing BALB/c nude mice their survival rate is improved [55].
These studies have provided the proof-in-principle that Vγ9Vδ2 T cells activated with s-pAgs and NBPs can exert antitumor activity in vivo against human cancer cells of hematological and non-hematological origin, but it is important to consider that immunodeficient mice are not fully representative of the human setting because: (1) they lack almost all the immune components Vγ9Vδ2 T cells interact with; and (2) they do not recapitulate all the immune dysfunctions that tumor cells generate in immunocompetent hosts. To overcome these limitations, Gertner-Dardenne et al. have administered BrHPP and IL-2 in association with rituximab to cynomolgus macaques to show that the in vivo activation of Vγ9Vδ2 T cells improves the ability of rituximab to kill B cells in the peripheral blood and lymph nodes and provides the groundwork to improve the efficacy of cancer immunotherapy with therapeutic mAbs [56].
Therapeutic applications
Preclinical and clinical studies have paved the way for the development of two strategies for the treatment of patients with hematological malignancies: in vivo activation by administration of Vγ9Vδ2 T cells agonists or adoptive transfer of ex vivo activated Vγ9Vδ2 T cells (Fig. 3). The seven clinical studies that have specifically investigated the antitumor activity of Vγ9Vδ2 T cells are listed in Table 1. Since only two deal with hematological patients, we summarize the results as a whole and point out some analogies between hematological and solid cancer patients. As said above, in vivo stimulation of Vγ9Vδ2 T cells can be achieved with s-pAgs such as BrHPP and 2M3B1-PP or NBPs in association with IL-2. Other cytokines, such as IL-15, IL-17, and IL-21 can sustain the proliferation and immune fitness of Vγ9Vδ2 T cells, but are not yet available for clinical trials.
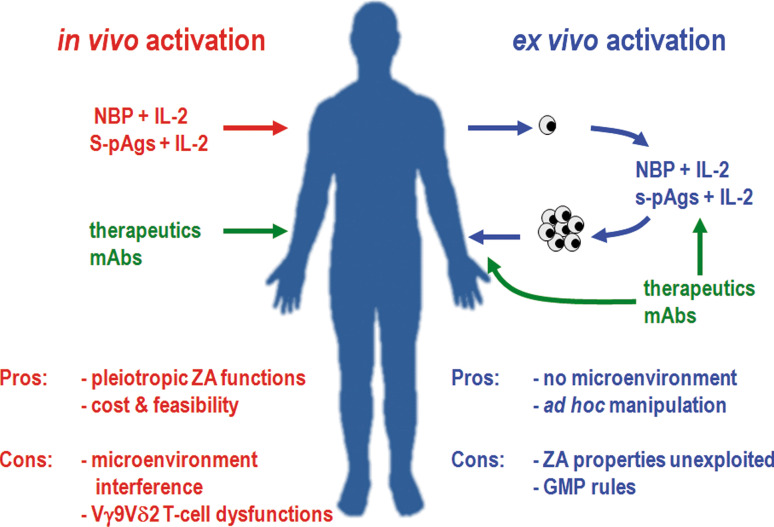
Fig. 3.
Therapeutic strategies for Vγ9Vδ2 T cell-based immune interventions. Two main strategies are under clinical investigation for the treatment of patients with hematological malignancies and other cancers: in vivo activation by administration of Vγ9Vδ2 T cells agonists (left) or adoptive transfer of ex vivo activated Vγ9Vδ2 T cells (right). In vivo stimulation of Vγ9Vδ2 T cells can be pursued with s-pAgs such as BrHPP and 2M3B1-PP or NBPs in association with IL-2. Other cytokines and growth factors can be useful, but they are not yet available for clinical studies. Ex vivo expansion of Vγ9Vδ2 T cells is induced with the same compounds. Each approach has its own pros and cons. Pros of in vivo stimulation are low cost, feasibility, and the possibility to exploit the pleiotropic direct and indirect antitumor activities of NBPs. Cons are the microenvironment interference and the immune dysfunctions eventually affecting Vγ9Vδ2 T cells in cancer patients. Pros of ex vivo activation are the possibility to generate large numbers of Vγ9Vδ2 T cells, and to improve their immune performances by appropriate stimulation in a controlled setting. On the other hand, adoptive therapies are expensive, time-consuming, and strictly regulated by GMP requirements. Vγ9Vδ2 T cell-based immunotherapy can be gainfully combined with therapeutic mAbs. mAbs can be administered in vivo or used ex vivo to load activated Vγ9Vδ2 T cells
Table 1.
List of clinical studies in which Vγ9Vδ2 T cells have intentionally been activated to generate antitumor activity
| Disease | Patients (n) | Vγ9Vδ2 activation | Activating agent(s) | Reference |
|---|---|---|---|---|
| NHL | 11 | In vivo | Pamidronate + IL-2 | Wilhelm et al. [50] |
| MM | 8 | In vivo | Pamidronate + IL-2 | |
| Breast cancer | 3 | In vivo | ZAa | Dieli et al. [63] |
| Prostate cancer | 6 | In vivo | ZA | |
| Prostate cancer | 9 | In vivo | ZA | Dieli et al. [64] |
| Prostate cancer | 9 | In vivo | ZA + IL-2 | |
| Breast cancer | 10 | in vivo | ZA + IL-2 | Meraviglia et al. [65] |
| Renal cell carcinoma | 7 | Ex vivo | 2M3B1-PPb + IL-2 | Kobayashi et al. [67] |
| Renal cell carcinoma | 10 | Ex vivo | BrHPPc + IL-2 | Bennouna et al. [68] |
| Multiple myeloma | 6 | Ex vivo | ZA + IL-2 | Abe et al. [69] |
aZoledronic acid
b2-methyl-3-butenyl-1-pyrophosphate
cBromohydrin pyrophosphate (IPH1101 Phosphostim®)
s-pAgs and NBPs cannot be considered equivalent. Both have pros and cons to be borne in mind when designing a clinical trial. One major difference is that the antitumor activity of s-pAgs is entirely dependent on their ability to activate Vγ9Vδ2 T cells, whereas NBPs have pleiotropic antitumor functions. Since FPPS is an ubiquitous enzyme and the Mev pathway is the only source of isoprenoids in mammalian cells, it is not surprising that ZA can affect the survival and function of many cell types other than tumor cells. Almost all these effects are due to inhibition of FPPS because they can be overcome by feeding cells with isoprenoid substrates to recover protein prenylation (reviewed in [57]). In vitro and in vivo studies have shown that ZA can target endothelial cells, macrophages, and fibroblasts in the tumor bed and convert the local microenvironment from a highly permissive partner to a tumor-hostile counterpart [53, 58–61]. It thus appears more logical to prefer NBPs in clinical trials designed to activate Vγ9Vδ2 T cells in vivo so that advantage can be taken of the pleiotropic direct and indirect antitumor activities of these compounds.
Despite these major premises and the widespread use of NBPs in clinical practice, very few trials have specifically investigated their antitumor activity. Even fewer are those which have sought to determine whether Vγ9Vδ2 T cells contribute to the antitumor effect induced by NBPs. For instance, Morgan et al. have recently reported the results of the MRC Myeloma IX Trial in which 1,960 MM patients were randomized to receive ZA or clodronate in association with, depending on age and other eligibility criteria, intensive or non-intensive chemotherapy. The ZA group displayed an increased progression-free survival and overall survival, but no data were reported about Vγ9Vδ2 T cells [62]. Credit is due to Kunzmann et al. for their provision of the first in vivo evidence that NBPs activate Vγ9Vδ2 T cells [47]. They monitored the proportion of peripheral blood Vγ9Vδ2 T cells in 10 patients after an initial course pamidronate alone to treat bone disease. Four patients (2 with MM) had an acute-phase reaction, coupled with a substantial increase in their percentage of peripheral blood Vγ9Vδ2 T cells.
The only clinical data from hematological patients about Vγ9Vδ2 T cells intentionally activated in vivo with pamidronate and IL-2 to induce antitumor activity are those reported by Wilhelm et al. in 11 patients with B cell lymphoma and 8 patients with MM [50]. One important aspect of this study was the selection of patients according to their R/NR status. In an initial series of unselected 10 patients, in fact, no activation or proliferation of Vγ9Vδ2 T cells was induced by even the highest IL-2 dose, and only 1 patient achieved stable disease. In second series, 9 selected R patients received a modified IL-2 treatment schedule. Vγ9Vδ2 T cell activation was observed in 5 patients (55%), and objective responses were achieved in 3 patients (33%). Only patients with significant in vivo proliferation of Vγ9Vδ2 T cells responded to the treatment, indicating that this functional screening test selected those most likely to benefit from the treatment.
The relationship between the clinical outcome and adequate numbers of circulating Vγ9Vδ2 T cells has also emerged from the clinical trials conducted in solid cancer [63–65]. Clinical responses were correlated with both the number of peripheral Vγ9Vδ2 T cells and their maturation towards an IFN-γ-producing effector phenotype that may induce more effective antitumor responses.
BrHPP and IL-2 have also been used to intentionally activate Vγ9Vδ2 T cells in vivo in association with IL-2 in patients with advanced solid tumors [66].
An alternative approach to exploit the antitumor properties of Vγ9Vδ2 T cells consist in their ex vivo activation and expansion followed by adoptive transfer to patients. Ex vivo expansion of Vγ9Vδ2 T cells can be obtained by stimulation with s-pAgs such as BrHPP and 2M3B1-PP or NBPs in association with IL-2. The first advantage of this approach is that ex vivo culture provides large numbers of Vγ9Vδ2 T cells which are then infused in vivo in a controlled setting. In addition, this approach potentially gives the possibility to improve the functional properties of infused Vγ9Vδ2 T cells, by stimulating them properly and relieving inhibitory signals mediated by Tregs or other cells. On the other hand, adoptive therapies are expensive, time-consuming, and strictly regulated by GMP requirements. A pilot study of adoptive immunotherapy in patients with advanced renal cell carcinoma using autologous Vγ9Vδ2 T cells activated ex vivo with 2M3B1-PP was well tolerated and induced antitumor effects [67]. Similarly, a clinical trial conducted in metastatic renal cell carcinoma showed that adoptive transfer of Vγ9Vδ2 T cells activated by BrHPP and IL-2 prolonged time to progression compared to placebo-receiving groups of historical controls [68]. In the hematological setting, Abe et al. have reported the results of a phase I trial in MM patients with adoptively transferred Vγ9Vδ2 T cells generated from autologous PBMC after a 14-day ex vivo culture with ZA and high doses IL-2 [69]. If the M protein level in the patient’s serum remained at baseline following four intravenous infusions, the patient underwent four more treatments at 4-week intervals. The percentages and total counts of Vγ9Vδ2 T cells, particularly those of effector memory cells, increased in the peripheral blood during treatment and remained high in comparison with baseline for 4 weeks after treatment. A similar increase of effector memory Vγ9Vδ2 T cells was also detected in the BM, suggesting a preferential recruitment and accumulation of these cells at the tumor site. M protein levels in the serum remained at baseline in 4 patients and increased in the other 2.
Future directions
The few clinical studies reported above have opened the way to the therapeutic application of Vγ9Vδ2 T cells in cancer. Even if the results have been encouraging, this form of immunotherapy is still in its infancy and in need of further developments. So far, the clinical outcome has been penalized by some aspects that are common requirements in trials exploring innovative approaches. For instance, patients with very advanced disease and severe immune dysfunctions have often been recruited, whereas the ideal setting to deliver immunotherapy is represented by patients with early-stage disease, low tumor burden, and a healthy immune system not yet impaired by repeated treatments and long-lasting tumor exposure. As said above, CLL and MM and other hematological malignancies are preceded by an indolent phase which can last for years. Immune interventions in the premalignant phase of these disease sound very reasonable from the immunological and ethical points of view, given the preserved immune functions and the lower incidence of side-effects of immunotherapy versus conventional chemotherapy.
Many hematological malignancies are very sensitive to chemotherapy, and achievement of a minimal residual disease (MRD) condition is another reasonable setting to deliver immune interventions aimed at preventing disease recurrence which is very likely initiated by subclones with clonogenic and chemoresistance properties. Matsui et al. identified a MM clonogenic cell population, termed side population (SP), which had very active intracellular drug detoxification and efflux capacities conferring resistance to cyclophosphamide, dexamethasone, lenadilomide and bortezomib in vitro [70]. SP cells resistant to conventional drugs such as fludarabine, bendamustin and rituximab have also been identified in CLL [71]. Interestingly, SP cells could be transiently eliminated from the peripheral blood of CLL patients who received autologous hCD40L/IL-2 gene-modified tumor cells as part of a tumor vaccine study [72]. A similar dissociation between sensitivity to chemotherapy on the one hand, and to immune effector mechanisms on the other, has also been reported in MM. Scheper et al. have shown that RPMI 8226 myeloma cells resistant to doxorubicin or mitoxantrone remain sensitive to in vitro killing by LAK or NK cells [73]. Shtil et al. have reported that mice immunized with MPC11 myeloma cells expressing GM-CSF and IL-12 generate cytotoxic T lymphocytes that kill parental and isogenic multidrug-resistant myeloma cells via perforin/granzyme B-mediated mechanisms [74]. The release of cytotoxic effector molecules, such as perforin and granzyme, is one of the weapons that Vγ9Vδ2 T cells employ to kill infected, activated or transformed cells and they can thus be reasonably presumed capable of recognizing and killing multidrug-resistant and clonogenic tumor cells, especially those manipulated to become more sensitive to Vγ9Vδ2 T cells [75, 76]. Indeed, Todaro et al. have shown that pretreatment of colon cancer stem cells with ZA potentiates their susceptibility to Vγ9Vδ2 T cell cytotoxicity [77]. This increased effector activity was dependent on IPP accumulation and TCR-mediated recognition of tumor cells. Vγ9Vδ2 T cells have recently been included among the immune effector cells playing a major role in the achievement of MRD negativity after non-myeloablative allogeneic transplantation in MM [78]. In seven of the nine patients who became MRD negative, the appearance of a new predominant Vγ9Vδ2 T cell clone was concomitant with the disappearance of the tumor cell clone.
Another apparent pitfall on the roadmap to successful Vγ9Vδ2 T cell-based immunotherapy is the NR status reported in a significant percentage of patients with hematological malignancies. The NR status does not automatically implies that Vγ9Vδ2 T cells are totally unable to produce cytokines and exert cytotoxic activity [6]. A dissociation between proliferation and activation of effector functions is a common feature of innate immunity, but NR patients are typically excluded from Vγ9Vδ2 T cell-based adoptive immunotherapy trials owing to the impossibility of increasing the number of cells and establishing a favorable effector-to-target cell ratio in vivo or ex vivo. Besides, the NR status may not just reflect a quantitative defect, but a more profound immune dysfunction provoked by the long-lasting exposure to tumor cells, such as unbalanced Vγ9Vδ2 T cell subset distribution or an excess of Treg over Vγ9Vδ2 T cells [6, 79]. Different strategies to recover Vγ9Vδ2 T cell proliferation in NR patients are currently under investigation and a quest for new and more potent Vγ9Vδ2 T cell activators is ongoing. Some groups have proposed to antagonize Treg cell activity to relieve the functional inhibition of Vγ9Vδ2 T cells and amplify adaptive immune response mediated by conventional CD8+ αβ T cells against tumor-associated antigens [79, 80]. We have recently shown that ZA-treated DCs from healthy donors are more effective than monocytes in activating Vγ9Vδ2 T cell since they are better IPP producers [36]. In the field of solid tumors, it has recently been shown that autologous DCs pretreated with ZA induce expansion of Vγ9Vδ2 T cells in NR patients [81]. In our laboratory, we are currently focusing on the superior ability of ZA-treated DC to stimulate Vγ9Vδ2 T cells in NR patients with hematological malignancies such as MM or CLL.
Another line of future research which appears very promising is the combination of old and new drugs with Vγ9Vδ2 T cell immunotherapy. As reviewed above, many of the mechanisms that regulate tumor cell recognition and killing by Vγ9Vδ2 T cells have been elucidated, and therefore it is now feasible to fine-tune these interactions to improve their antitumor activity. Besides increasing intracellular IPP concentrations in tumor cells by Mev pathway manipulation, other approaches, not mutually exclusive, can be set out to modulate the functional balance between KARs and KIRs on Vγ9Vδ2 T cells and their interactions with the corresponding ligands on tumor cells. For instance, certain cytotoxic drugs such as doxorubicin, etoposide, melphalan, bortezomib, and cisplatin increase the expression of NKG2D ligands and other stress-induced self molecules on dying tumor cells [82]. Other approaches are the inhibition of soluble MIC molecules, a mechanism exploited by tumor cells to escape NKG2D-mediated immune recognition [83], or functionally block KIRs to tip the balance in favor of KARs. IPH 2101 is a fully humanized anti-KIRs mAb currently under investigation in pre-treated high-risk MM [84]. Even if all these strategies have been developed to enhance the effector functions of NK cells, it is very possible that they can also be successfully applied to Vγ9Vδ2 T cells.
The challenge to gainfully combine immunotherapy with chemotherapy has been fuelled by the availability of new drugs that stimulate rather than impair host immunity. For instance, lenalidomide improves the effector function of Vγ9Vδ2 T cells against mantle cell lymphoma [85], while Cui et al. have shown that its association with ZA in MM patients reverses inhibition of Vγ9Vδ2 T cells by BM stromal cells [86]. Bortezomib is another drug potentially able to improve the antitumor performance of Vγ9Vδ2 T cells because it down-regulates MHC class I molecules and up-regulates NKG2D ligands expression on MM cells [82, 87]. Epigenetic drugs such as histone deacetylase inhibitors and DNA demethylating agents also up-regulate MICA/MICB expression on tumor cells and enhance their susceptibility to the cytotoxic activity of NKG2D expressing cells (reviewed in [88]).
The concurrent administration of mAbs with activated Vγ9Vδ2 T cells is another strategy that can maximize the efficacy of chemoimmunotherapy in hematological malignancies. Vγ9Vδ2 T cells expanded in the presence of ZA and IL-2 express CD16, which raises the possibility of using them to boost ADCC. Tokuyama et al. have shown that Vγ9Vδ2 T cells used in combination with rituximab exert a greater cytotoxicity against CD20-positive cells in comparison with either agent alone and this effect is restricted to CD16+ Vγ9Vδ2 T cells [89]. Similar results have been reported using Vγ9Vδ2 T cells activated by BrHPP and IL-2 [56]. The combination of BrHPP and rituximab increased the ability of Vγ9Vδ2 T cells to bind to CD20+ cells and strongly enhanced their ADCC activity. Similar results were obtained with alemtuzumab, another mAb currently used in CLL. Altogether, these results indicate that activation of Vγ9Vδ2 T cells with NBP or s-pAgs can improve the efficacy of cancer immunotherapy by therapeutic mAbs. These results have paved the way to clinical trials in which rituximab, BrHPP and low doses IL-2 are given to patients with follicular B cell lymphoma. Final data on the response rate of a phase IIa trial in 45 patients relapsing after previous therapies, including at least one rituximab-containing regimen, were presented at the last EHA meeting [90]. The overall response rate was 45% (26% CR) suggesting a benefit for the combination of BrHPP and rituximab versus rituximab alone. Notably, responses were also observed in patients with unfavorable FcγR polymorphism. Vγ9Vδ2 T cells can also improve the ADCC mediated by other effector cells. For instance, Maniar et al. have shown that either adoptive transfer of in vitro expanded Vγ9Vδ2 T cells or systemic injection of Vγ9Vδ2 T cell-simulating agents such as ZA may improve clinical outcome through the enhancement of direct and ADCC tumor killing by NK cells [9]. The next step will be to investigate whether the novel anti-CD20 mAbs with an increased affinity for FcγR/CD16 can provide even better results in combination with activated Vγ9Vδ2 T cells, especially against tumor cells with low CD20 expression, such as CLL cells [91, 92].
Conclusions
Vγ9Vδ2 T cells are able to spontaneously recognize and kill tumor cells of hematological origin. B cell tumors are the most susceptible diseases, but effective antitumor activity can also be induced against many other tumor cell types by appropriate manipulation of target cells and activation of Vγ9Vδ2 T cells. Vγ9Vδ2 T cell-based immunotherapy is a promising approach to combine with conventional chemotherapy and therapeutic mAbs and improve the clinical outcome of patients with hematological malignancies.
Acknowledgments
This work was partially supported by Regione Piemonte (Ricerca Sanitaria, Ricerca Scientifica e Progetto Strategico ImmOnc), Fondazione Neoplasie Sangue Onlus (Torino, Italy), Associazione per lo Studio e la Cura delle Malattie del Sangue (Torino, Italy), and Novartis Farma.
Footnotes
B. Castella and C. Vitale contributed equally to this work.
References
- 1.Landgren O, Kyle RA. Multiple myeloma, chronic lymphocytic leukaemia and associated precursor diseases. Br J Haematol. 2007;139:717–723. doi: 10.1111/j.1365-2141.2007.06866.x. [DOI] [PubMed] [Google Scholar]
- 2.Shanafelt TD, Ghia P, Lanasa MC, Landgren O, Rawstron AC. Monoclonal B-cell lymphocytosis (MBL): biology, natural history and clinical management. Leukemia. 2010;24:512–520. doi: 10.1038/leu.2009.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhodapkar MV, Geller MD, Chang DH, Shimizu K, Fujii S, Dhodapkar KM, Krasovsky J. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197:1667–1676. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girlanda S, Fortis C, Belloni D, Ferrero E, Ticozzi P, Sciorati C, Tresoldi M, Vicari A, Spies T, Groh V, Caligaris-Cappio F, Ferrarini M. MICA expressed by multiple myeloma and monoclonal gammopathy of undetermined significance plasma cells costimulates pamidronate-activated gd lymphocytes. Cancer Res. 2005;65:7502–7508. doi: 10.1158/0008-5472.CAN-05-0731. [DOI] [PubMed] [Google Scholar]
- 5.Zenz T, Mertens D, Küppers R, Döhner H, Stilgenbauer S. From pathogenesis to treatment of chronic lymphocytic leukaemia. Nat Rev Cancer. 2010;10:37–50. doi: 10.1038/nrc2764. [DOI] [PubMed] [Google Scholar]
- 6.Mariani S, Muraro M, Pantaleoni F, Fiore F, Nuschak B, Peola S, Foglietta M, Palumbo A, Coscia M, Castella B, Bruno B, Bertieri R, Boano L, Boccadoro M, Massaia M. Effector gammadelta T cells and tumor cells as immune targets of zoledronic acid in multiple myeloma. Leukemia. 2005;19:664–670. doi: 10.1038/sj.leu.2403693. [DOI] [PubMed] [Google Scholar]
- 7.Burjanadzé M, Condomines M, Reme T, Quittet P, Latry P, Lugagne C, Romagne F, Morel Y, Rossi JF, Klein B, Lu ZY. In vitro expansion of gamma delta T cells with anti-myeloma cell activity by Phosphostim and IL-2 in patients with multiple myeloma. Br J Haematol. 2007;139:206–216. doi: 10.1111/j.1365-2141.2007.06754.x. [DOI] [PubMed] [Google Scholar]
- 8.Thedrez A, Sabourin C, Gertner J, Devilder MC, Allain-Maillet S, Fournié JJ, Scotet E, Bonneville M. Self/non-self discrimination by human gammadelta T cells: simple solutions for a complex issue? Immunol Rev. 2007;215:123–135. doi: 10.1111/j.1600-065X.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- 9.Maniar A, Zhang X, Lin W, Gastman BR, Pauza CD, Strome SE, Chapoval AI. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood. 2010;116:1726–1733. doi: 10.1182/blood-2009-07-234211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisch P, Malkovsky M, Braakman E, Sturm E, Bolhuis RL, Prieve A, Sosman JA, Lam VA, Sondel PM. γδ T cell clones and natural killer cell clones mediate distinct patterns of non-major histocompatibility complexrestricted cytolysis. J Exp Med. 1990;171:1567–1579. doi: 10.1084/jem.171.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisch P, Malkovsky M, Kovats S, Sturm E, Braakman E, Klein B, Voss SD, Morrissey LW, De Mars R, Welch WJ. Recognition by human Vγ9/Vδ2 T cells of a GroEL homolog on Daudi Burkitt’s lymphoma cells. Science. 1990;250:1269–1273. doi: 10.1126/science.1978758. [DOI] [PubMed] [Google Scholar]
- 12.Fisch P, Meuer E, Pende D, Rothenfuber S, Viale O, Kock S, Ferrone S, Fradelizi D, Klein G, Moretta L, Rammensee HG, Boon T, Coulie P, van der Bruggen P. Control of B cell lymphoma recognition via natural killer inhibitory receptors implies a role of human Vγ9/Vδ2 T cells in tumor immunità. Eur J Immunol. 1997;27:3368–3379. doi: 10.1002/eji.1830271236. [DOI] [PubMed] [Google Scholar]
- 13.Sturm E, Braakman E, Fisch P, Vreugdenhil RJ, Sondel P, Bolhuis RL. Human Vγ9-Vδ2 T cell receptor-γδ lymphocytes show specificity to Daudi Burkitt’s lymphoma cells. J Immunol. 1990;145:3202–3208. [PubMed] [Google Scholar]
- 14.Street SE, Hayakawa Y, Zhan Y, Lew AM, MacGregor D, Jamieson AM, Diefenbach A, Yagita H, Godfrey DI, Smyth MJ. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J Exp Med. 2004;199:879–884. doi: 10.1084/jem.20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selin LK, Stewart S, Shen C, Mao HQ, Wilkins JA. Reactivity of γδT cells induced by the tumour cell line RPMI 8226: functional heterogeneity of clonal populations and role of GroEL heat shock proteins. Scand J Immunol. 1992;36:107–117. doi: 10.1111/j.1365-3083.1992.tb02946.x. [DOI] [PubMed] [Google Scholar]
- 16.Zheng B, Lam C, Im S, Huang G, Luk W, Lau S, Yau KS, Wong CK, Yao K, Ng MH. Distinct tumour specificity and IL-7 requirements of CD56- and CD56+ subsets of human gd T Cells. Scand J Immunol. 2001;53:40–48. doi: 10.1046/j.1365-3083.2001.00827.x. [DOI] [PubMed] [Google Scholar]
- 17.Kato Y, Tanaka Y, Miyagawa F, Yamashita S, Minato N. Targeting of tumor cells for human γδ T cells by nonpeptide antigens. J Immunol. 2001;167:5092–5098. doi: 10.4049/jimmunol.167.9.5092. [DOI] [PubMed] [Google Scholar]
- 18.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor γδ cells recognized endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;19:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gd T cells. Nature. 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 20.Jomaa H, Feurle J, Luhs K, Kunzmann V, Tony HP, Herderich M, Wilhelm M. Vg9/Vd2 T cell activation induced by bacterial low molecular mass compounds depends on the 1-deoxy-d-xylulose 5-phosphate pathway of isoprenoid biosynthesis. FEMS Immunol Med Microbiol. 1999;25:371–378. doi: 10.1111/j.1574-695X.1999.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 21.Espinosa E, Belmant C, Pont F, Luciani B, Poupot R, Romagné F, Brailly H, Bonneville M, Fournié JJ. Chemical synthesis and biological activity of bromohydrin pyrophosphate, a potent stimulator of human gamma delta T cells. J Biol Chem. 2001;276:18337–18344. doi: 10.1074/jbc.M100495200. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka Y, Kobayashi H, Terasaki T, Toma H, Aruga A, Uchiyama T, Mizutani K, Mikami B, Morita CT, Minato N. Synthesis of pyrophosphate-containing compounds that stimulate Vgamma2Vdelta2 T cells: application to cancer immunotherapy. Med Chem. 2007;3:85–99. doi: 10.2174/157340607779317544. [DOI] [PubMed] [Google Scholar]
- 23.D’Asaro M, La Mendola C, Di Liberto D, Orlando V, Todaro M, Spina M, Guggino G, Meraviglia S, Caccamo N, Messina A, Salerno A, Di Raimondo F, Vigneri P, Stassi G, Fourniè JJ, Dieli F. Vgamma9Vdelta2 T lymphocytes efficiently recognize and kill zoledronate-sensitized, imatinib-sensitive, and imatinib-resistant chronic myelogenous leukemia cells. J Immunol. 2010;184:3260–3268. doi: 10.4049/jimmunol.0903454. [DOI] [PubMed] [Google Scholar]
- 24.Kato Y, Tanaka Y, Tanaka H, Yamashita S, Minato N. Requirement of species-specific interactions for the activation of human gamma delta T cells by pamidronate. J Immunol. 2003;170:3608–3613. doi: 10.4049/jimmunol.170.7.3608. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 26.Harwood HJ, Jr, Alvarez IM, Noyes WD, Stacpoole PW. In vivo regulation of human leukocyte 3-hydroxy-3-methylglutaryl coenzyme A reductase: increased enzyme protein concentration and catalytic efficiency in human leukemia and lymphoma. J Lipid Res. 1991;32:1237–1252. [PubMed] [Google Scholar]
- 27.Shachaf CM, Perez OD, Youssef S, Fan AC, Elchuri S, Goldstein MJ, Shirer AE, Sharpe O, Chen J, Mitchell DJ, Chang M, Nolan GP, Steinman L, Felsher DW. Inhibition of HMGcoA reductase by atorvastatin prevents and reverses MYC-induced lymphomagenesis. Blood. 2007;110:2674–2684. doi: 10.1182/blood-2006-09-048033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fortuny J, de Sanjose S, Becker N, Maynadie’ M, Cocco PL, Staines A, Foretova L, Vornanen M, Brennan P, Nieters A, Alvaro T, Moffetta P. Statin use and risk of lymphoid neoplasms: results from the European Case–Control Study EPILYMPH. Cancer Epidemiol Biomarkers Prev. 2006;15:921–925. doi: 10.1158/1055-9965.EPI-05-0866. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Herold MJ, Kimmel B, Müller I, Rincon-Orozco B, Kunzmann V, Herrmann T. Reduced expression of the mevalonate pathway enzyme farnesyl pyrophosphate synthase unveils recognition of tumor cells by Vgamma9Vdelta2 T cells. J Immunol. 2009;182:8118–8124. doi: 10.4049/jimmunol.0900101. [DOI] [PubMed] [Google Scholar]
- 30.Thompson K, Rogers MJ. Statins prevent bisphosphonate-induced gd-T-cell proliferation and activation in vitro. J Bone Miner Res. 2004;19:278–288. doi: 10.1359/JBMR.0301230. [DOI] [PubMed] [Google Scholar]
- 31.Mönkkönen H, Auriola S, Lehenkari P, Kellinsalmi M, Hassinen IE, Vepsäläinen J, Mönkkönen J. A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Br J Pharmacol. 2006;147:437–445. doi: 10.1038/sj.bjp.0706628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scotet E, Martinez LO, Grant E, Barbaras R, Jenö P, Guiraud M, Monsarrat B, Saulquin X, Maillet S, Estève JP, Lopez F, Perret B, Collet X, Bonneville M, Champagne E. Tumor recognition following Vg9Vd2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Mookerjee-Basu J, Vantourout P, Martinez LO, Perret B, Collet X, Périgaud C, Peyrottes S, Champagne E. F1-adenosine triphosphatase displays properties characteristic of an antigen presentation molecule for Vgamma9Vdelta2 T cells. J Immunol. 2010;184:6920–6928. doi: 10.4049/jimmunol.0904024. [DOI] [PubMed] [Google Scholar]
- 34.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–392. [PubMed] [Google Scholar]
- 35.Roelofs AJ, Jauhiainen M, Mönkkönen H, Rogers MJ, Mönkkönen J, Thompson K. Peripheral blood monocytes are responsible for gammadelta T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol. 2009;144:245–250. doi: 10.1111/j.1365-2141.2008.07435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiore F, Castella B, Nuschak B, Bertieri R, Mariani S, Bruno B, Pantaleoni F, Foglietta M, Boccadoro M, Massaia M. Enhanced ability of dendritic cells to stimulate innate and adaptive immunity on short-term incubation with zoledronic acid. Blood. 2007;110:921–927. doi: 10.1182/blood-2006-09-044321. [DOI] [PubMed] [Google Scholar]
- 37.Gomes AQ, Correia DV, Grosso AR, Lança T, Ferreira C, Lacerda JF, Barata JT, Silva MG, Silva-Santos B. Identification of a panel of ten cell surface protein antigens associated with immunotargeting of leukemias and lymphomas by peripheral blood gammadelta T cells. Haematologica. 2010;95:1397–1404. doi: 10.3324/haematol.2009.020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, Spies T. Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc Natl Acad Sci USA. 1996;93:12445–12450. doi: 10.1073/pnas.93.22.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stephens HA. MICA and MICB genes: can the enigma of their polymorphism be resolved? Trends Immunol. 2001;22:378–385. doi: 10.1016/S1471-4906(01)01960-3. [DOI] [PubMed] [Google Scholar]
- 40.Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8ah T cells by NKG2D via engagement by MIC induced on virus infected cells. Nat Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 41.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gd T cells of MICA and MICB. Proc Natl Acad Sci USA. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salih HR, Antropius H, Gieseke F. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102:1389–1396. doi: 10.1182/blood-2003-01-0019. [DOI] [PubMed] [Google Scholar]
- 43.Carbone E, Neri P, Mesuraca M. HLA class I, NKG2D and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood. 2005;105:251–258. doi: 10.1182/blood-2004-04-1422. [DOI] [PubMed] [Google Scholar]
- 44.Kunzmann V, Wilhelm M. Anti-lymphoma effect of gammadelta T cells. Leuk Lymphoma. 2005;46:671–680. doi: 10.1080/10428190500051893. [DOI] [PubMed] [Google Scholar]
- 45.Lança T, Correia DV, Moita CF, Raquel H, Neves-Costa A, Ferreira C, Ramalho JS, Barata JT, Moita LF, Gomes AQ, Silva-Santos B. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to gamma}{delta T-cell cytotoxicity. Blood. 2010;115:2407–2411. doi: 10.1182/blood-2009-08-237123. [DOI] [PubMed] [Google Scholar]
- 46.Uchida R, Ashihara E, Sato K, Kimura S, Kuroda J, Takeuchi M, Kawata E, Taniguchi K, Okamoto M, Shimura K, Kiyono Y, Shimazaki C, Taniwaki M, Maekawa T. Gamma delta T cells kill myeloma cells by sensing mevalonate metabolites and ICAM-1 molecules on cell surface. Biochem Biophys Res Commun. 2007;354:613–618. doi: 10.1016/j.bbrc.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 47.Kunzmann V, Bauer E, Wilhelm M. Gamma/delta T-cell stimulation by pamidronate. N Engl J Med. 1999;340:737–738. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- 48.Saito A, Narita M, Yokoyama A, Watanabe N, Tochiki N, Satoh N, Takizawa J, Furukawa T, Toba K, Fuse I, Aizawa Y, Shinada S, Takahashi M. Enhancement of anti-tumor cytotoxicity of expanded gammadelta T cells by stimulation with monocyte-derived dendritic cells. J Clin Exp Hematop. 2007;47:61–72. doi: 10.3960/jslrt.47.61. [DOI] [PubMed] [Google Scholar]
- 49.Saitoh A, Narita M, Watanabe N, Tochiki N, Satoh N, Takizawa J, Furukawa T, Toba K, Aizawa Y, Shinada S, Takahashi M. Anti-tumor cytotoxicity of gammadelta T cells expanded from peripheral blood cells of patients with myeloma and lymphoma. Med Oncol. 2008;25:137–147. doi: 10.1007/s12032-007-9004-4. [DOI] [PubMed] [Google Scholar]
- 50.Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–206. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 51.Kiladjian JJ, Visentin G, Viey E, Chevret S, Eclache V, Stirnemann J, Bourhis JH, Chouaib S, Fenaux P, Caignard A. Activation of cytotoxic T-cell receptor gammadelta T lymphocytes in response to specific stimulation in myelodysplastic syndromes. Haematologica. 2008;93:381–389. doi: 10.3324/haematol.11812. [DOI] [PubMed] [Google Scholar]
- 52.Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 53.Coscia M, Quaglino E, Iezzi M, Curcio C, Pantaleoni F, Riganti C, Holen I, Mönkkönen H, Boccadoro M, Forni G, Musiani P, Bosia A, Cavallo F, Massaia M. Zoledronic acid repolarizes tumor-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J Cell Mol Med. 2010;14:2803–2815. doi: 10.1111/j.1582-4934.2009.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malkovska V, Cigel FK, Armstrong N, Storer BE, Hong R. Antilymphoma activity of human gamma delta T-cells in mice with severe combined immune deficiency. Cancer Res. 1992;52:5610–5616. [PubMed] [Google Scholar]
- 55.Chen J, Niu H, He W, Ba D. Antitumor activity of expanded human tumor-infiltrating gammadelta T lymphocytes. Int Arch Allergy Immunol. 2001;125:256–263. doi: 10.1159/000053824. [DOI] [PubMed] [Google Scholar]
- 56.Gertner-Dardenne J, Bonnafous C, Bezombes C, Capietto AH, Scaglione V, Ingoure S, Cendron D, Gross E, Lepage JF, Quillet-Mary A, Ysebaert L, Laurent G, Sicard H, Fournié JJ. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood. 2009;113:4875–4884. doi: 10.1182/blood-2008-08-172296. [DOI] [PubMed] [Google Scholar]
- 57.Roelofs AJ, Thompson K, Gordon S, Rogers MJ. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006;12:6222s–6230s. doi: 10.1158/1078-0432.CCR-06-0843. [DOI] [PubMed] [Google Scholar]
- 58.Scavelli C, Di Pietro G, Cirulli T, Coluccia M, Boccarelli A, Giannini T, Mangialardi G, Bertieri R, Coluccia AM, Ribatti D, Dammacco F, Vacca A. Zoledronic acid affects over-angiogenic phenotype of endothelial cells in patients with multiple myeloma. Mol Cancer Ther. 2007;6:3256–3262. doi: 10.1158/1535-7163.MCT-07-0311. [DOI] [PubMed] [Google Scholar]
- 59.Moschetta M, Di Pietro G, Ria R, Gnoni A, Mangialardi G, Guarini A, Ditonno P, Musto P, D’Auria F, Ricciardi MR, Dammacco F, Ribatti D, Vacca A. Bortezomib and zoledronic acid on angiogenic and vasculogenic activities of bone marrow macrophages in patients with multiple myeloma. Eur J Cancer. 2010;46:420–429. doi: 10.1016/j.ejca.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 60.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114:623–633. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007;67:11438–11446. doi: 10.1158/0008-5472.CAN-07-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morgan G, Davies FE, Gregory WM, Bell SE, Szubert AJ, Drayson MT, Ashcroft J, Owen RG, Cook G, Ross FM, Jackson GH, Russell NH, Child JA (2010) Optimising bone disease in myeloma; Zoledronic Acid plus Thalidomide combinations improves survival and bone endpoints: results of the MRC Myeloma IX Trial. In: ASH Annual Meeting, abstract 311
- 63.Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F, Arcara C, Valerio MR, Meraviglia S, Di Sano C, Sireci G, Salerno A. Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003;102:2310–2311. doi: 10.1182/blood-2003-05-1655. [DOI] [PubMed] [Google Scholar]
- 64.Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D’Asaro M, Gebbia N, Salerno A, Eberl M, Hayday AC. Targeting human gamma}delta T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meraviglia S, Eberl M, Vermijlen D, Todaro M, Buccheri S, Cicero G, La Mendola C, Guggino G, D’Asaro M, Orlando V, Scarpa F, Roberts A, Caccamo N, Stassi G, Dieli F, Hayday AC. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol. 2010;161:290–297. doi: 10.1111/j.1365-2249.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bennouna J, Medioni J, Rolland F, Misset JL, Campone M, Sicard H, Tiollier J, Romagne F, Douillard JY, Calvo F (2005) Phase I clinical trial of BromoHydrinPyroPhosphate, BrHPP (Phosphostim), a Vg9Vd2 T lymphocytes agonist in combination with low dose Interleukin-2 in patients with solid tumors. In: ASCO annual meeting
- 67.Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T, Minato N, Toma H. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother. 2007;56:469–476. doi: 10.1007/s00262-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bennouna J, Bompas E, Neidhardt EM, Rolland F, Philip I, Galéa C, Salot S, Saiagh S, Audrain M, Rimbert M, Lafaye-de Micheaux S, Tiollier J, Négrier S. Phase-I study of Innacell gammadelta, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1599–1609. doi: 10.1007/s00262-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abe Y, Muto M, Nieda M, Nakagawa Y, Nicol A, Kaneko T, Goto S, Yokokawa K, Suzuki K. Clinical and immunological evaluation of zoledronate-activated Vgamma9 gammadelta T-cell-based immunotherapy for patients with multiple myeloma. Exp Hematol. 2009;37:956–968. doi: 10.1016/j.exphem.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 70.Matsui W, Wang Q, Barber JP, Brennan S, Smith BD, Borrello I, McNiece I, Lin L, Ambinder RF, Peacock C, Watkins DN, Huff CA, Jones RJ. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008;68:190–197. doi: 10.1158/0008-5472.CAN-07-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gross E, L’Faqihi-Olive FE, Ysebaert L, Brassac M, Struski S, Kheirallah S, Fournié JJ, Laurent G, Quillet-Mary A. B-chronic lymphocytic leukemia chemoresistance involves innate and acquired leukemic side population cells. Leukemia. 2010;24:1885–1892. doi: 10.1038/leu.2010.176. [DOI] [PubMed] [Google Scholar]
- 72.Foster AE, Okur FV, Biagi E, Lu A, Dotti G, Yvon E, Savoldo B, Carrum G, Goodell MA, Heslop HE, Brenner MK. Selective elimination of a chemoresistant side population of B-CLL cells by cytotoxic T lymphocytes in subjects receiving an autologous hCD40L/IL-2 tumor vaccine. Leukemia. 2010;24:563–572. doi: 10.1038/leu.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scheper RJ, Dalton WS, Grogan TM, Schlosser A, Bellamy WT, Taylor CW, Scuderi P, Spier C. Altered expression of P-glycoprotein and cellular adhesion molecules on human multi-drug-resistant tumor cells does not affect their susceptibility to NK- and LAK-mediated cytotoxicity. Int J Cancer. 1991;48:562–567. doi: 10.1002/ijc.2910480414. [DOI] [PubMed] [Google Scholar]
- 74.Shtil AA, Turner JG, Durfee J, Dalton WS, Yu H. Cytokine-based tumor cell vaccine is equally effective against parental and isogenic multidrug-resistant myeloma cells: the role of cytotoxic T lymphocytes. Blood. 1999;93:1831–1837. [PubMed] [Google Scholar]
- 75.Dieli F, Troye-Blomberg M, Ivanyi J, Fournié JJ, Krensky AM, Bonneville M, Peyrat MA, Caccamo N, Sireci G, Salerno A. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vgamma9/Vdelta2 T lymphocytes. J Infect Dis. 2001;184:1082–1085. doi: 10.1086/323600. [DOI] [PubMed] [Google Scholar]
- 76.Qin G, Mao H, Zheng J, Sia SF, Liu Y, Chan PL, Lam KT, Peiris JS, Lau YL, Tu W. Phosphoantigen-expanded human gammadelta T cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian influenza viruses. J Infect Dis. 2009;200:858–865. doi: 10.1086/605413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Todaro M, D’Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, Orlando V, La Mendola C, Gulotta G, Salerno A, Dieli F, Stassi G. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol. 2009;182:7287–7296. doi: 10.4049/jimmunol.0804288. [DOI] [PubMed] [Google Scholar]
- 78.Galimberti S, Benedetti E, Morabito F, Petrini I, Battolla B, Papineschi F, Fazzi R, Ciabatti E, Martino M, Cuzzola M, Console G, Iacopino P, Petrini M. Different gamma/delta T clones sustain GVM and GVH effects in multiple myeloma patients after non-myeloablative transplantation. Leuk Res. 2006;30:529–535. doi: 10.1016/j.leukres.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Kunzmann V, Kimmel B, Herrmann T, Einsele H, Wilhelm M. Inhibition of phosphoantigen-mediated gammadelta T-cell proliferation by CD4+ CD25+ FoxP3+ regulatory T cells. Immunology. 2009;126:256–267. doi: 10.1111/j.1365-2567.2008.02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gong G, Shao L, Wang Y, Chen CY, Huang D, Yao S, Zhan X, Sicard H, Wang R, Chen ZW. Phosphoantigen-activated V gamma 2V delta 2 T cells antagonize IL-2-induced CD4 +CD25+ Foxp3+ T regulatory cells in mycobacterial infection. Blood. 2009;113:837–845. doi: 10.1182/blood-2008-06-162792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cabillic F, Toutirais O, Lavoué V, de La Pintière CT, Daniel P, Rioux-Leclerc N, Turlin B, Mönkkönen H, Mönkkönen J, Boudjema K, Catros V, Bouet-Toussaint F. Aminobisphosphonate-pretreated dendritic cells trigger successful Vgamma9Vdelta2 T cell amplification for immunotherapy in advanced cancer patients. Cancer Immunol Immunother. 2010;59:1611–1619. doi: 10.1007/s00262-010-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini A, Foà R, Santoni A. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood. 2009;113:3503–3511. doi: 10.1182/blood-2008-08-173914. [DOI] [PubMed] [Google Scholar]
- 83.Wang X, Lundgren AD, Singh P, Goodlett DR, Plymate SR, Wu JD. An six-amino acid motif in the alpha3 domain of MICA is the cancer therapeutic target to inhibit shedding. Biochem Biophys Res Commun. 2009;387:476–481. doi: 10.1016/j.bbrc.2009.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benson D, Bakan CE, Zhang S, Alghothani L, Liang J, Hofmeister C, Srivastava S, Smith MK, Greenfield CN, Andre P, Squiban P, Romagne F, Caligiuri MA, Farag S (2009) IPH2101, a novel anti-inhibitory KIR monoclonal antibody, and Lenalidomide combine to enhance the natural killer (NK) cell versus multiple myeloma (MM) effect. In: ASH annual meeting, abstract 3870
- 85.Gaidarova, Corral LG, Gleizer E, Young D, Brady H, Bennett B, Lopez-Girona A (2009) Lenalidomide enhances anti-tumor effect of γδ T cells against mantle cell lymphoma. In: ASH annual meeting, abstract 2616
- 86.Cui Q, Abe M, Miki H, Nakamura S, Watanabe K, Ikegame A, Hiasa M, Nakano A, Harada T, Fujii S, Kagawa K, Takeuchi K, Ozaki S, Matsumoto T (2010) Lenalidomide in combination with Zoledronic Acid restores the activation and anti-myeloma effects of γδT cells attenuated by the bone marrow microenvironment. In: ASH annual meeting, abstract 3124
- 87.Shi J, Tricot GJ, Garg TK, Malaviarachchi PA, Szmania SM, Kellum RE, Storrie B, Mulder A, Shaughnessy JD, Jr, Barlogie B, van Rhee F. Bortezomib down-regulates the cell-surface expression of HLA class I and enhances natural killer cell-mediated lysis of myeloma. Blood. 2008;111:1309–1317. doi: 10.1182/blood-2007-03-078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rey J, Veuillen C, Vey N, Bouabdallah R, Olive D. Natural killer and gammadelta T cells in haematological malignancies: enhancing the immune effectors. Trends Mol Med. 2009;15:275–284. doi: 10.1016/j.molmed.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 89.Tokuyama H, Hagi T, Mattarollo SR, Morley J, Wang Q, Fai-So H, Moriyasu F, Nieda M, Nicol AJ. Vgamma9Vdelta 2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs–rituximab and trastuzumab. Int J Cancer. 2008;122:2526–2534. doi: 10.1002/ijc.23365. [DOI] [PubMed] [Google Scholar]
- 90.Rossi JF, Solal-Celigny P, Soubeyran P, Delvail V, Ghesquieres H, Thieblemont C, Jourdan E, Kunzmann V, Feugier P, Casasnovas RO, Le Gouill S, van den Neste E, Lafaye De Micheaux S, Marzetto M, Beautier L, Squiban P, Sicard H, Laurent G (2010) A phase I/II study of IPH1101, gamma delta T cell agonist, in combination with Rituximab re-treatment, in patients with low grade follicular lymphoma. In: EHA annual meeting, abstract 1131
- 91.Bowles JA, Wang SY, Link BK, Allan B, Beuerlein G, Campbell MA, Marquis D, Ondek B, Wooldridge JE, Smith BJ, Breitmeyer JB, Weiner GJ. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood. 2006;108:2648–2654. doi: 10.1182/blood-2006-04-020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Romeuf C, Dutertre CA, Le Garff-Tavernier M, Fournier N, Gaucher C, Glacet A, Jorieux S, Bihoreau N, Behrens CK, Béliard R, Vieillard V, Cazin B, Bourel D, Prost JF, Teillaud JL, Merle-Béral H. Chronic lymphocytic leukaemia cells are efficiently killed by an anti-CD20 monoclonal antibody selected for improved engagement of FcgammaRIIIA/CD16. Br J Haematol. 2008;140:635–643. doi: 10.1111/j.1365-2141.2007.06974.x. [DOI] [PubMed] [Google Scholar]