Abstract
Mammalian oocytes grow and undergo meiosis within ovarian follicles. Fully grown oocytes are arrested at the first meiotic prophase by a mural granulosa origin “arrester” until a surge of luteinizing hormone (LH) from the pituitary at the mid-cycle stimulates the immature oocyte to resume meiosis. Recent evidence indicates that natriuretic peptide precursor type C (NPPC) produced by mural granulosa cells stimulates the generation of cyclic guanosine 3′,5′-monophosphate (cGMP) by cumulus cell natriuretic peptide receptor 2 (NPR2), which diffuses into oocyte via gap junctions and inhibits oocyte phosphodiesterase 3A (PDE3A) activity and cyclic adenosine 3′,5′-monophosphate (cAMP) hydrolysis and maintains meiotic arrest with a high intraoocyte cAMP level. This cAMP is generated through the activity of the Gs G-protein by the G-protein-coupled receptor, GPR3 and GPR12, and adenylyl cyclases (ADCY) endogenous to the oocyte. Further studies suggest that endocrine hormones, such as follicle-stimulating hormone (FSH), LH, 17β-estradiol (E2) and oocyte-derived paracrine factors (ODPFs), participate in oocyte meiosis possibly by the regulation of NPPC and/or NPR2. A detailed investigation of NPPC and NPR2 expression in follicle cells will elucidate the precise molecular mechanisms of gonadotropins, and control the arrest as well as resumption of meiosis.
Keywords: Meiosis, Cyclic nucleotides, Gonadotropin, Signal transduction
Introduction
In mammalian oocytes, meiosis is initiated during fetal life, and subsequently arrested for a prolonged period at the diplotene stage of the first meiotic prophase, morphologically identified by a characteristic nucleus commonly known as the germinal vesicle (GV) with a prominent nucleolus and is associated with partial condensation of the chromosomes [1]. Prophase arrest is maintained by inherent factors in the oocyte and correlates with low levels of activity by cell cycle regulatory proteins [2]. Once the growing oocyte reaches its full size and an antral space begins to form to divide the granulosa cells into two separate compartments, mural granulosa cells (MGCs) form the outer layers and the cumulus cells surround the oocyte and the oocyte acquires the ability to complete meiosis [3–5]. Nevertheless, fully grown mammalian oocytes are held in meiotic prophase arrest until the follicle responds to the preovulatory surge of luteinizing hormone (LH) from the pituitary gland during the estrous or menstrual cycle, shortly before ovulation [6–8]. Germinal vesicle breakdown (GVB) is the first change occurring, and is widely used as an endpoint for assessing the resumption of meiosis or oocyte maturation [9].
The progression of meiosis is subject to start and stop signals driven by both intrinsic and extrinsic factors. Because the entire follicle surrounding the oocyte must remain intact in order to preserve its normal function, the mechanisms that maintain meiotic arrest of the oocyte, as well as the mechanisms by which LH triggers resumption of meiosis, have therefore been technically challenging to study. When oocytes or cumulus–oocyte complexes (COCs) are removed from follicles and cultured, they spontaneously resume meiosis without hormonal stimulation [10]. This original observation, confirmed in numerous studies with oocytes of all mammalian species so far examined [4, 5, 11, 12], has led to general acceptance of the hypothesis that meiotic arrest in mammalian oocytes is maintained by a mural granulosa origin “arrester”. Studies have implicated one potential meiotic inhibitory molecule, a low-molecular-weight peptide known as oocyte maturation inhibitor (OMI) [13]. The action of OMI to maintain meiotic arrest is not species-specific, needs mediate of cumulus cells surrounding the oocyte [14], and can be reversed by LH [13], supporting the view that OMI has a physiological role in the control of oocyte maturation.
Recent studies with genetically altered mice, as well as directly inhibiting oocyte-specific proteins by microinjecting follicle-enclosed oocytes, have identified the components of the signaling network for the maintenance of meiotic arrest in fully grown mammalian oocytes. Oocyte cyclic adenosine 3′,5′-monophosphate (cAMP) is crucial for maintaining meiotic arrest and is generated by oocyte adenylyl cyclase (ADCY), which is controlled by the constitutive action of G-protein-coupled receptor, GPR3, and GPR12 via Gs protein [15, 16]. Cyclic guanosine 3′,5′-monophosphate (cGMP), produced by natriuretic peptide precursor type C (NPPC) and natriuretic peptide receptor 2 (NPR2, a guanylyl cyclase) in follicle cells, diffuses into the oocyte from companion cumulus cells via gap junctions and inhibits oocyte phosphodiesterase 3A (PDE3A) activity and cAMP hydrolysis and maintains meiotic arrest [17–20]. Further studies suggest that endocrine hormones, such as follicle-stimulating hormone (FSH), 17β-estradiol (E2), and oocyte-derived paracrine factors (ODPFs), may participate in oocyte meiotic arrest by stimulating the expression of NPPC and/or NPR2 [20]. Research investigating the regulation of NPPC and NPR2 in follicle cells may also pave the way for elucidating the mechanisms whereby LH stimulates mammalian oocyte maturation.
The endocrine control of meiotic arrest and resumption rests on a network of extracellular and intracellular molecular interactions. The purpose of this review is to highlight recent studies from several laboratories, including ours, elucidating how the oocyte maintains arrest and discussing potential mechanisms whereby LH acts to stimulate meiotic resumption in mammals. We hope that this review may provide a framework with which to understand how the initial signals activated by hormones tightly control the complex patterns of genes and protein expression that are required for meiosis.
Cyclic nucleotides involved in meiotic arrest
Meiotic arrest depends on a high level of cAMP within the oocyte. This cAMP is generated by the oocyte through the stimulation of the Gs G-protein by the G-protein-coupled receptor, GPR3 and GPR12, and adenylyl cyclases. Inhibition of oocyte cAMP-phosphodiesterase (PDE3A) activity is essential for sustaining elevated cAMP levels. More recently, cGMP, produced by follicle cells, diffuses through the gap junction network to the oocyte, and inhibits oocyte PDE3A activity and cAMP hydrolysis to maintain meiotic arrest [18, 19].
Cycle AMP
Intraoocyte cAMP is critical to the control of meiosis. It is well established that maintenance of meiotic arrest in meiotically competent oocytes depends on high levels of cAMP, whereas a drop in intraoocyte levels of this nucleotide is required for resumption of meiosis [1, 21, 22]. When released from their follicles, fully grown antral oocytes spontaneously undergo meiotic resumption in coincidence with a decrease in intraoocyte cAMP levels [23–25], which could be prevented by maintaining high levels of cAMP within oocytes [21, 26, 27]. High cAMP levels within the oocyte result in the phosphorylation of cyclin-dependent kinase 1 (CDK1) on Thr14 and Tyr15, rendering maturation promoting factor (MPF) inactive such that the oocyte maintain meiotic arrest [2, 12, 28, 29].
Cycle AMP synthesis
Cycle AMP could be produced either by the oocyte or by the follicle cells that surround it. It has been previously proposed that cAMP is produced by follicle cells and diffuses through gap junctions to the oocyte [12, 30–32]. However, the lack of specific inhibitors against gap junctions in the oocyte has complicated efforts to clarify their possible role in the maintenance of meiotic arrest.
Recent findings strongly suggest that the oocyte itself produces sufficient cAMP to maintain meiotic arrest by way of a constitutively active heterotrimeric G protein (Gs)-linked receptor, GPR3 or GPR12, which acts to stimulate at least one form of adenylate cyclase 3 [15, 16, 33–37]. If any of the components of this signaling pathway are eliminated in the follicle-enclosed oocyte, it could mature spontaneously [2]. However, GPR3 knockout mice are fertile, and only appear to fail in preventing meiosis resumption in aged mouse [37], indicating that additional pathway(s) for generation and maintenance of a sufficient cAMP level in young animals. GPR3 is the predominant receptor signaling meiotic arrest in mice, and GPR12 in rat [35]. In humans, RNA encoding GPR3, but not GPR12, is expressed in oocytes and meiotic arrest is maintained by a GPR3–Gs signaling pathway [38]. Follicle cells’ original cAMP diffusion through gap junctions is not sufficient by itself to maintain the meiotic arrest in the mouse oocyte [39]. Taken together, these data indicate that oocyte produces its own cAMP via G protein-linked receptor/Gs/ADCY pathway, which is essential for maintaining meiotic arrest.
Cycle AMP degradation
Intraoocyte steady-state levels of cAMP are synthesized by Gs/ADCY and degraded by phosphodiesterases (PDEs) [40]. However, the regulation of intraoocyte cAMP apparently occurs primarily by control of its degradation (by very active PDE), rather than by endogenous synthesis [41]. Maintenance of meiotic arrest is explained by constitutive cAMP signaling associated with undetectable cAMP–PDE activity [27, 42, 43]. PDE3A has been localized only to the oocyte [40, 41, 44–46], and the type 3 PDE inhibitor elevates intraoocyte cAMP and prevents the resumption of meiosis in many species including human [2, 47]. Furthermore, genetic ablation of PDE3A causes complete meiotic arrest even after prolonged incubation in vitro or after ovulation, and female sterility [42], demonstrating that PDE3A is the key molecule involved in the control of intraoocyte cAMP levels, thus regulating the arrest and resumption of meiosis [17]. The reason why Gs/ADCY activity is not sufficient to prevent spontaneous meiotic resumption may be that the increasing PDE3 activity during spontaneous maturation [17] is sufficient to decrease intraoocyte cAMP levels.
Cycle GMP
As early as 1980, it is found that cGMP levels are highest at dioestrus but lowest during oestrus in hamster ovary [48], suggesting a functional relationship between cGMP levels and meiotic arrest. More and more studies indicate that cGMP is involved in the regulation of oocyte maturation. Intraoocyte cGMP levels decrease during spontaneous meiotic resumption [23]. Injection of cGMP into the oocyte delays meiotic resumption, and inhibition of inosine monophosphate dehydrogenase (needed for cGMP production) causes meiotic resumption in follicle-enclosed oocytes [23, 49, 50]. Recently, it has been reported that inhibition of cGMP-specific PDE5 activity significantly and reversibly inhibits spontaneous maturation of mouse COCs [51]. Moreover, microinjection of a cGMP-specific PDE5 into oocytes causes meiotic maturation of wild-type oocytes, but this effect is absent in PDE3A-deficient oocytes [19]. The concentration of cGMP in GV-stage oocytes isolated from equine chorionic gonadotropin (eCG)-primed immature mice [19, 23] is sufficient to inhibit PDE3A activity via completion with cAMP in the hydrolysis process [52]. All these results indicate that an intraoocyte pool of cGMP is involved in the maintenance of meiotic arrest via regulation of PDE3A. Guanylyl cyclase agonists have inhibitory effects on spontaneous meiotic resumption in COCs, but not in isolated oocytes [24, 53], suggesting that the oocyte depends on the somatic cells for its supply of cGMP. Recent studies further show that cGMP passes through gap junctions from follicle cells into the oocyte, where it inhibits the hydrolysis of cAMP by PDE3A [18, 19]. This inhibition maintains a high level of cAMP in the oocyte and then blocks meiotic progression [18]. Thus, the production of cGMP in the somatic cells is essential for the maintenance of meiotic arrest. The precise mechanism for generation and maintenance of a sufficient cGMP in somatic cells needs further study.
Hormonal control of meiotic arrest
The synthesis of cGMP is accomplished by two distinct classes of guanylyl cyclases, soluble and particulate, activated by nitric oxide (NO) and natriuretic peptides, respectively [54]. Our recent study indicates that NPPC produced by follicular mural granulosa cells stimulates the generation of cGMP by cumulus cell NPR2, which is needed to inhibit oocyte PDE3A activity and thereby maintains meiotic arrest [20]. Further studies suggest that endocrine hormones, such as FSH, E2, and ODPFs, may participate in oocyte meiotic arrest by stimulating the expression of NPPC and/or NPR2 [20].
NPPC and its cognate receptor NPR2
The natriuretic peptide system forms a family of three structurally homologous but genetically distinct endogenous ligands, natriuretic peptide precursor A (NPPA; ANP), natriuretic peptide precursor B (NPPB; BNP), and NPPC (also known as CNP) [55]. NPPA and NPPB are cardiac hormones that are predominantly synthesized in atrial and ventricular cardiomyocytes, respectively, and play important roles in the regulation of cardiovascular homeostasis, primarily through guanylyl cyclase-coupled receptor NPR1 [56]. On the other hand, NPPC is expressed in a wide variety of central and peripheral tissues and acts locally as autocrine and paracrine regulator through NPR2 but little natriuretic activity [57]. The presence of NPR2 is reported in rat follicles, and the binding of its ligand NPPC varies during the estrous cycle [58].
More recently, it has been shown that Nppc mRNA was expressed by MGCs lining the inside of the follicle wall, and Npr2 mRNA was expressed predominantly by cumulus cells surrounding the oocyte [20]. NPPC increases cGMP and cAMP levels in oocytes and prevents spontaneous resumption of cumulus cell-enclosed, but not denuded, mouse oocytes in vitro. Moreover, meiotic arrest was not sustained in most Graafian follicles of Nppc or Npr2 mutant mice, and meiosis resumed precociously. All these results indicate that NPPC produced by follicular mural granulosa cells stimulates the generation of cGMP by cumulus cells NPR2, which diffuses into oocyte via gap junctions and inhibits PDE3A activity and cAMP hydrolysis and maintains meiotic arrest [19, 20, 59, 60]. Therefore, the granulosa cell ligand NPPC, binding to its receptor NPR2 in cumulus cells, prevents precocious meiotic maturation (Fig. 1), which is critical for maturation and ovulation synchrony and for normal female fertility.
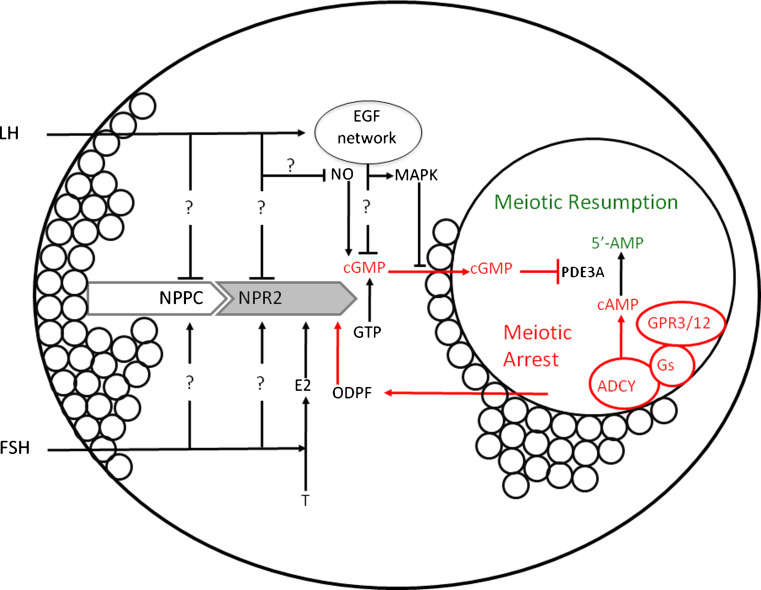
Fig. 1.
A proposed model for hormonal control of mammalian oocyte meiosis in pre-ovulatory follicle. NPPC produced by mural granulosa cells stimulates the generation of cGMP by cumulus cell NPR2, which diffuses into oocyte via gap junctions and inhibits oocyte PDE3A activity and cAMP hydrolysis and maintains meiotic arrest with a high intraoocyte cAMP level. This cAMP is generated through the activity of the Gs G-protein by GPR3/12 and ADCY endogenous to the oocyte. FSH, E2, and ODPFs may serve to maintain meiotic arrest by stimulating the expression of NPPC and/or NPR2 and the accumulation of cGMP, while the decrease of this cyclic nucleotide after LH treatment induces meiotic resumption by EGF network and/or by the down-regulation of NPPC and NPR2 expression. LH-induced peptides also activate MAPK and thus close the gap junctions throughout the somatic compartment, which reduces the flux of cGMP from the somatic cells to the oocyte. LH might also decrease nitric oxide level, and then decrease intraoocyte cGMP levels. FSH follicle-stimulating hormone, LH luteinizing hormone, EGF epidermal growth factor, MAPK mitogen-activated protein kinase, NO nitric oxide, T Testosterone, E2 17β-estradiol, ODPF oocyte-derived paracrine factor, NPPC natriuretic peptide precursor type C, NPR2 natriuretic peptide receptor 2, cAMP cyclic adenosine 3′,5′-monophosphate, cGMP cyclic guanosine 3′,5′-monophosphate, GPR3/12 G protein-coupled receptors 3 and 12, ADCY adenylyl cyclases, PDE3A phosphodiesterase 3A
It has been generally accepted that meiotic arrest in mammalian oocytes is maintained by the mural granulosa origin OMI. Porcine OMI has been partly characterized and purified, which is a low-molecular-weight peptide of approximately 2000 [61]. It is interesting that NPPC, a small peptide with 22 amino acid residues, has the character similar to OMI: low molecular weight peptide of 2197.6, predominant expression in MGCs, action via cumulus cells (with NPR2 receptor) to maintain meiotic arrest, and identical sequences among mouse, rat, pig and human [20, 62]. Whether OMI is NPPC remains to be determined. It is also possible that other factors (such as purine hypoxanthine present in the follicular fluid) redundantly participate in oocyte meiotic arrest, especially in the small antral follicles [27, 63]. A better understanding of the factors that maintain an oocyte in meiotic arrest may help in the development of strategies to improve culture conditions with particular regard to the quality of cytoplasmic maturation [64, 65].
FSH
The effect of Nppc and Npr2 mutant in fully grown follicles after eCG treatment, but not in follicles at earlier stages of growth indicates that the expression of these genes is controlled tightly by specific mechanisms. Thus, regulation of NPPC and NPR2 levels in granulosa cells of Graafian follicles is an integral aspect of the system that maintains oocyte meiotic arrest.
During follicular growth, some follicles at the early antral stage are ‘recruited’ to continue growing, which is dependent on the pituitary gonadotropin FSH [66, 67]. Although FSH could induce COCs maturation in vitro in a similar way as that of LH [28], it is essential for the steroidogenesis by stimulating aromatase enzyme activity (P450 aromatase), for the differentiation of the granulosa cells by inducing the expression of LH receptors and for the follicular antrum formation [68, 69]. Recently, FSH has also been suggested to involve in the regulation of NPPC and NPR2 expression. eCG, a glycoprotein hormone that possesses primarily FSH activity, can induce the expression of Nppc and Npr2 mRNA in the ovary [70]. However, FSH has no effect on the expression of Npr2 mRNA when cumulus cells are cultured in vitro [71]. It is possible that stimulation of Npr2 mRNA levels in cumulus cells by eCG in vivo is probably an indirect result of the FSH activity of eCG [72]. The increasing expression of NPPC and NPR2 in follicle cells, via cGMP, plays an important synchronizing role during follicle development by contributing to the maintenance of meiotic arrest until LH surge. Inappropriate decrease of NPPC and NPR2 in the growing follicles might disrupt normal follicular development [37].
E2
Although gonadotropins-induced increases in cAMP levels are associated with increased production of steroid hormones in follicle [73], the role of ovarian steroids on mammalian meiotic resumption is still far from clear. Testosterone and E2 have long been thought to play negative or dispensable roles during spontaneous meiotic resumption of oocytes [28]. Some studies suggest their involvement in promoting meiotic resumption of unprimed immature mouse COCs and denuded oocytes arrested in meiosis by isobutyl methylxanthine [74, 75], and of porcine COCs arrested in meiosis by hypoxanthine [76]. However, by using follicle-enclosed oocytes, other researchers question the meiosis-stimulating competence of these steroids in the mouse and rat [77, 78]. The different effects of testosterone and E2 on the meiotic resumption of mammalian oocyte may be due in part to the background of spontaneous oocyte maturation upon removal from the ovary, as well as methods of oocyte removal that pre-expose oocytes to sex steroids.
It is reported that injection of immature rats with the synthetic estrogen diethylstilbestrol (DES) results in increased Npr2 mRNA levels and NPPC binding in granulosa cells [70]. Moreover, E2 promotes and maintains expression of Npr2 mRNA by mouse cumulus cells, thereby augmenting their ability to produce cGMP in response to NPPC and maintenance of meiotic arrest by NPPC in vitro [71]. Testosterone has the same effect as E2 on the expression of Npr2 mRNA and meiotic arrest in vitro, which is likely due to aromatization of testosterone to estrogens, rather than direct androgen action because the non-aromatizable androgen dihydrotesterone is unable to stimulate the expression of Npr2 mRNA or to sustain responses to NPPC [71]. Thus, the physiological role of E2 may be involved in oocyte meiotic arrest through inducing the expression of NPR2 in cumulus cells, and the major role of testosterone in the ovary may possibly be to serve as estrogen precursors.
Oocyte-derived paracrine factors
Growth and development of the somatic and germ cell compartments of the ovarian follicle occur in a highly coordinated and mutually dependent manner. An increasing body of evidence indicates that MGCs have important endocrine functions, and oocyte-derived paracrine factors profoundly affects the differentiation of cumulus cells to control its own development [79]. Much of this recent interest has focused on oocyte-secreted transforming growth factor-β (TGF-β) superfamily members, in particular growth differentiation factor-9 (GDF-9), bone morphogenetic protein-15 (BMP-15; also called GDF-9B), and fibroblast growth factor 8B (FGF8B) [80].
Our recent results show that microsurgical extirpation of oocytes from complexes (oocytectomy, OOX) significantly reduced expression of Npr2 mRNA in cumulus cells, which could be reversed by co-culture of cumulus cells with fully grown denuded oocytes [20]. Further study shows that each oocyte-derived paracrine growth factors GDF9, BMP15, and FGF8B slightly promotes expression of Npr2 mRNA by cumulus cells in vitro, and combinations of three proteins promote levels of Npr2 mRNA expression equivalent to those promoted by co-culture with cumulus cell-denuded oocytes [20]. We have not seen any precocious maturation in the ovaries from the aromatase inhibitor treated mice (data not shown), suggesting that ODPFs may be able to compensate for the expression of NPR2 when the estradiol signal is absent or reduced. Thus, oocytes themselves participate in the meiosis-arresting pathway not only by producing cAMP but also by promoting cumulus cell expression of NPR2 receptors to increase cGMP levels and then inhibit oocyte maturation. These results support the view that the signals originating from the oocytes play an essential role in orchestrating the growth and development of the follicle [80].
LH-induced meiotic resumption
In a normal reproductive cycle, the preovulatory surge of LH from the pituitary causes the resumption of meiosis in oocytes in Graafian follicles, as well as ovulation [1, 35, 81]. LH receptor activation stimulates Gs and activates adenylyl cyclase [82], and as a consequence, elevates cAMP levels [26, 83, 84] and PKA activity [85, 86] in the MGCs. Through a series of incompletely understood steps, LH causes a decrease in cAMP in the oocyte and then meiotic resumption [26, 87, 88].
LH regulates oocyte cAMP either by inhibition of GPR3 or GPR12/Gs/ADCY system, or stimulation of its cAMP phosphodiesterase. However, LH-induced signaling does not terminate GPR3-Gs-ADCY signaling [59], or stimulate of a Gi-mediated pathway in the oocyte [89]. It has been shown that LH increases PDE3A activity in mammalian oocytes [17] possibly by lowering cGMP, since cGMP inhibits PDE3A activity [22, 23]. Indeed, LH-stimulated decreases in cGMP occur in oocytes and whole follicles [19, 90]. Further research indicates that LH decreases intraoocyte cGMP levels by lowering cGMP levels in the somatic cells and by closing gap junctions between the somatic cells [18]. The resulting decrease in oocyte cGMP relieves the inhibition of PDE3A and causes a decrease in oocyte cAMP, which is sufficient to account for the stimulation of meiotic resumption [18]. Under the conditions of elevated intraoocyte cGMP levels, LH stimulation could not induce oocyte maturation [19]. Therefore, LH-induced cGMP decrease plays a major role in the resumption of meiosis. Other mechanisms, such as cGMP-independent stimulation of PDE3A [17, 91] and additional LH signaling pathways [92, 93], might function in parallel with cGMP regulation.
It is well known that LH induction of epidermal growth factor (EGF)-like growth factors in MGCs and EGF receptor (EGFR) transactivation in cumulus cells are essential for the regulation of a critical physiological process such as oocyte maturation and ovulation [28, 82, 94, 95]. The cGMP decrease in oocytes may be that the LH-induced peptides activate EGFR, which in turn activate mitogen-activated protein kinase (MAPK) [86]. MAPK phosphorylates and thus closes the gap junctions throughout the somatic compartment, which reduces the flux of cGMP from the somatic cells to the oocyte [18]. Although closure of these junctions is sufficient to induce oocyte maturation [88], the precise relationship between the loss of this coupling and the resumption of oocyte meiosis is inconsistent. Some studies in rodent oocytes suggest that gap junction disconnection precedes meiotic resumption [31, 96–98], while other studies in human, porcine, ovine, and murine oocytes suggest GVB precedes the decrease in gap junctions [87, 99]. It has also been shown that maturation can be induced even in the presence of open junctions [60]. Nevertheless, the EGFR kinase-dependent component of the cGMP decrease is required for LH-induced meiotic resumption [19, 100]. Anyway, the incomplete inhibition of the LH-induced cGMP decrease by the EGFR inhibitor AG1478 [19, 100], suggesting that EGFR activity is not required for most of the LH-induced cGMP decrease. These findings support the concept that two separate and partially redundant mechanisms contribute to the decrease in cGMP in response to LH.
The decrease of cGMP in the somatic cells could result from inhibition of a guanylyl cyclase or from stimulation of a cGMP phosphodiesterase. The increased activity of cGMP–PDEs is unable to be detected during LH stimulation [19, 101], and gonadotropins do not affect cGMP hydrolysis in cultured granulosa cells [102]. Furthermore, LH still causes a decrease in cGMP in the presence of cGMP special PDE5 inhibitors [19]. These results exclude the possibility that LH acts through activation of a cGMP–PDE. There is some evidence for regulation of the cyclase rather than the phosphodiesterase [101]. It is interesting that Nppc and Npr2 mRNA levels, and NPPC peptide levels are decreased in mouse follicle cells and human follicular fluid after treated by human chorionic gonadotropin (hCG), a pregnancy hormone that exhibits LH activity with a long serum half-life ([71, 103], and data not shown), which could decrease cGMP levels in the follicle cells. In contrast to the gonadotropin’s regulation of NPPC ligand, NPR2 receptor expressed in cumulus cells is unlikely to be regulated directly by the ovulatory LH stimulation, since few functional LH receptors are present in germ cells and cumulus cells [104]. Cycle GMP and cAMP in isolated mouse follicles decreases to the basal levels around 1 h [18, 100], and most GVB occurs between 2 and 3 h after hCG treatment ([105], and data not shown). However, the Nppc and Npr2 mRNA levels have not completely decreased to the basal levels during this period [71, 103]. It will be of interest to examine the regulation of NPPC release, and the phosphorylation sate of NPR2 that is critical for its hormone responsiveness [106]. Furthermore, LH might also increase PDE5 activity and decrease nitric oxide (NO) level, and then decrease intraoocyte cGMP levels [107].
It has been long hypothesized that the action of LH could either relieve an inhibitory, or maturation arresting, substance from the somatic cells, or, alternatively, provide a positive, maturation-promoting substance to override the follicular inhibition [1, 108]. Recent data is consistent with a model in which the inhibitory influence of NPPC/NPR2 on PDE3A activity is withdrawn upon exposure of the follicle to the preovulatory surge of LH (Fig. 1). It is also possible that additional modes of regulation of PDE3A (such as phosphorylation) contribute to the overall increase in PDE3A activity before meiotic maturation [109].
Conclusions
NPPC produced by follicular mural granulosa cells stimulates the generation of cGMP by cumulus cell NPR2, which is essential to inhibit oocyte PDE3A activity and thereby maintain meiotic arrest in fully grown oocytes. Endocrine hormones participate in oocyte meiosis by the regulation of NPPC and/or NPR2. Although both FSH and LH may use the same signaling pathway in oocyte maturation in vitro, they have a different effect on the production of NPPC/NPR2 in the ovary: FSH/eCG stimulates, but LH/hCG decreases, their expression. It can be hypothesized that the increase of NPPC/NPR2 in follicle cells under FSH stimulation (during follicular growth) serves to prevent untimely oocyte maturation until their decrease after the LH surge, by which gonadotropins control oocyte maturation. A better understanding of these signaling networks during oocyte growth and maturation will provide new opportunities for the manipulation of follicular functions for contraception or the treatment of infertility.
Acknowledgments
This work was supported by National Basic Research Program of China (No. 2012CB944401, 2012CB944701).
Contributor Information
Meijia Zhang, Phone: +86-10-62732694, Email: zmeijia@cau.edu.cn.
Guoliang Xia, Phone: +86-10-62733456, FAX: +86-10-6273345, Email: glxiachina@sohu.com.
References
- 1.Eppig JJ, Vivieros MM, Marin-Bivens C, De La Fuente R. Regulation of mammalian oocyte maturation. In: Leung PCK, Adashi EY, editors. The ovary. Amsterdam: Elsevier Academic Press; 2004. pp. 113–129. [Google Scholar]
- 2.Solc P, Schultz RM, Motlik J. Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells. Mol Hum Reprod. 2010;16:654–664. doi: 10.1093/molehr/gaq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szybek K. In vitro maturation of oocytes from sexually immature mice. J Endocrinol. 1972;54:527–528. doi: 10.1677/joe.0.0540527. [DOI] [PubMed] [Google Scholar]
- 4.Erickson GF, Sorensen RA. In vitro maturation of mouse oocytes isolated from late, middle, and pre-antral Graafian follicles. J Exp Zool. 1974;190:123–127. doi: 10.1002/jez.1401900112. [DOI] [PubMed] [Google Scholar]
- 5.Sorensen RA, Wassarman PM. Relationship between growth and meiotic maturation of mouse oocyte. Dev Biol. 1976;50:531–536. doi: 10.1016/0012-1606(76)90172-X. [DOI] [PubMed] [Google Scholar]
- 6.Ducibella T. The cortical reaction and development of activation competence in mammalian oocytes. Hum Reprod Update. 1996;2:29–42. doi: 10.1093/humupd/2.1.29. [DOI] [PubMed] [Google Scholar]
- 7.Mehlmann LM, Mikoshiba K, Kline D. Redistribution and increase in cortical inositol 1,4,5-trisphosphate receptors after meiotic maturation of the mouse oocyte. Dev Biol. 1996;180:489–498. doi: 10.1006/dbio.1996.0322. [DOI] [PubMed] [Google Scholar]
- 8.Ducibella T. Biochemical and cellular insights into the temporal window of normal fertilization. Theriogenology. 1998;49:53–65. doi: 10.1016/S0093-691X(97)00402-0. [DOI] [PubMed] [Google Scholar]
- 9.Dekel N. Molecular control of meiosis. Trends Endocrin Met. 1995;6:165–169. doi: 10.1016/1043-2760(95)00079-W. [DOI] [PubMed] [Google Scholar]
- 10.Pincus G, Enzmann EV. The comparative behavior of mammalian eggs in vivo and in vitro: I. The activation of ovarian eggs. J Exp Med. 1935;62:665–675. doi: 10.1084/jem.62.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards RG. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature. 1965;208:349–351. doi: 10.1038/208349a0. [DOI] [PubMed] [Google Scholar]
- 12.Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130:791–799. doi: 10.1530/rep.1.00793. [DOI] [PubMed] [Google Scholar]
- 13.Tsafriri A, Pomerantz SH. Oocyte maturation inhibitor. Clin Endocrinol Meta. 1986;15:157–170. doi: 10.1016/S0300-595X(86)80047-0. [DOI] [PubMed] [Google Scholar]
- 14.Racowsky C, Baldwin KV. In vitro and in vivo studies reveal that hamster oocyte meiotic arrest is maintained only transiently by follicular-fluid, but persistently by membrana/cumulus granulosa-cell contact. Dev Biol. 1989;134:297–306. doi: 10.1016/0012-1606(89)90102-4. [DOI] [PubMed] [Google Scholar]
- 15.Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306:1947–1950. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- 16.Hinckley M, Vaccari S, Horner K, Chen R, Conti M. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol. 2005;287:249–261. doi: 10.1016/j.ydbio.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Richard FJ, Tsafriri A, Conti M. Role of phosphodiesterase type 3A in rat oocyte maturation. Biol Reprod. 2001;65:1444–1451. doi: 10.1095/biolreprod65.5.1444. [DOI] [PubMed] [Google Scholar]
- 18.Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136:1869–1878. doi: 10.1242/dev.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaccari S, Weeks JL, 2nd, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod. 2009;81:595–604. doi: 10.1095/biolreprod.109.077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330:366–369. doi: 10.1126/science.1193573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho WK, Stern S, Biggers JD. Inhibitory effect of dibutyryl camp on mouse oocyte maturation in vitro. J Exp Zool. 1974;187:383–386. doi: 10.1002/jez.1401870307. [DOI] [PubMed] [Google Scholar]
- 22.Hambleton R, Krall J, Tikishvili E, Honeggar M, Ahmad F, Manganiello VC, Movsesian MA. Isoforms of cyclic nucleotide phosphodiesterase PDE3 and their contribution to cAMP hydrolytic activity in subcellular fractions of human myocardium. J Biol Chem. 2005;280:39168–39174. doi: 10.1074/jbc.M506760200. [DOI] [PubMed] [Google Scholar]
- 23.Törnell J, Billig H, Hillensjo T. Resumption of rat oocyte meiosis is paralleled by a decrease in guanosine 3′, 5′-cyclic-monophosphate (cGMP) and is inhibited by microinjection of cGMP. Acta Physiol Scand. 1990;139:511–517. doi: 10.1111/j.1748-1716.1990.tb08953.x. [DOI] [PubMed] [Google Scholar]
- 24.Törnell J, Carlsson B, Billig H. Atrial natriuretic peptide inhibits spontaneous rat oocyte maturation. Endocrinology. 1990;126:1504–1508. doi: 10.1210/endo-126-3-1504. [DOI] [PubMed] [Google Scholar]
- 25.Grøndahl C, Breinholt J, Wahl P, Murray A, Hansen TH, Faerge I, Stidsen CE, Raun K, Hegele-Hartung C. Physiology of meiosis-activating sterol: endogenous formation and mode of action. Hum Reprod. 2003;18:122–129. doi: 10.1093/humrep/deg028. [DOI] [PubMed] [Google Scholar]
- 26.Schultz RM, Montgomery RR, Belanoff JR. Regulation of mouse oocyte meiotic maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol. 1983;97:264–273. doi: 10.1016/0012-1606(83)90085-4. [DOI] [PubMed] [Google Scholar]
- 27.Eppig JJ, Ward-Bailey PF, Coleman DL. Hypoxanthine and adenosine in murine ovarian follicular fluid: concentrations and activity in maintaining oocyte meiotic arrest. Biol Reprod. 1985;33:1041–1049. doi: 10.1095/biolreprod33.5.1041. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Ouyang H, Xia G. The signal pathway of gonadotrophins-induced mammalian oocyte meiotic resumption. Mol Hum Reprod. 2009;15:399–409. doi: 10.1093/molehr/gap031. [DOI] [PubMed] [Google Scholar]
- 29.Tripathi A, Kumar KV, Chaube SK. Meiotic cell cycle arrest in mammalian oocytes. J Cell Physiol. 2010;223:592–600. doi: 10.1002/jcp.22108. [DOI] [PubMed] [Google Scholar]
- 30.Anderson E, Albertini DF. Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. J Cell Biol. 1976;71:680. doi: 10.1083/jcb.71.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bornslaeger EA, Schultz RM. Regulation of mouse oocyte maturation: effect of elevating cumulus cell cAMP on oocyte cAMP levels. Biol Reprod. 1985;33:698–704. doi: 10.1095/biolreprod33.3.698. [DOI] [PubMed] [Google Scholar]
- 32.Webb RJ, Marshall F, Swann K, Carroll J. Follicle-stimulating hormone induces a gap junction-dependent dynamic change in [cAMP] and protein kinase A in mammalian oocytes. Dev Biol. 2002;246:441–454. doi: 10.1006/dbio.2002.0630. [DOI] [PubMed] [Google Scholar]
- 33.Mehlmann LM, Jones TL, Jaffe LA. Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science. 2002;297:1343–1345. doi: 10.1126/science.1073978. [DOI] [PubMed] [Google Scholar]
- 34.Horner K, Livera G, Hinckley M, Trinh K, Storm D, Conti M. Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev Biol. 2003;258:385–396. doi: 10.1016/S0012-1606(03)00134-9. [DOI] [PubMed] [Google Scholar]
- 35.Mehlmann LM. Oocyte-specific expression of Gpr3 is required for the maintenance of meiotic arrest in mouse oocytes. Dev Biol. 2005;288:397–404. doi: 10.1016/j.ydbio.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freudzon L, Norris RP, Hand AR, Tanaka S, Saeki Y, Jones TL, Rasenick MM, Berlot CH, Mehlmann LM, Jaffe LA. Regulation of meiotic prophase arrest in mouse oocytes by GPR3, a constitutive activator of the Gs G protein. J Cell Biol. 2005;171:255–265. doi: 10.1083/jcb.200506194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ledent C, Demeestere I, Blum D, Petermans J, Hamalainen T, Smits G, Vassart G. Premature ovarian aging in mice deficient for Gpr3 . Proc Natl Acad Sci USA. 2005;102:8922–8926. doi: 10.1073/pnas.0503840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DiLuigi A, Weitzman VN, Pace MC, Siano LJ, Maier D, Mehlmann LM. Meiotic arrest in human oocytes is maintained by a Gs signaling pathway. Biol Reprod. 2008;78:667. doi: 10.1095/biolreprod.107.066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaccari S, Horner K, Mehlmann LM, Conti M. Generation of mouse oocytes defective in cAMP synthesis and degradation: endogenous cyclic AMP is essential for meiotic arrest. Dev Biol. 2008;316:124–134. doi: 10.1016/j.ydbio.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsafriri A, Chun SY, Zhang R, Hsueh AJW, Conti M. Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: studies using selective phosphodiesterase inhibitors. Dev Biol. 1996;178:393–402. doi: 10.1006/dbio.1996.0226. [DOI] [PubMed] [Google Scholar]
- 41.Thomas RE, Armstrong DT, Gilchrist RB. Differential effects of specific phosphodiesterase isoenzyme inhibitors on bovine oocyte meiotic maturation. Dev Biol. 2002;244:215–225. doi: 10.1006/dbio.2002.0609. [DOI] [PubMed] [Google Scholar]
- 42.Masciarelli S, Horner K, Liu C, Park SH, Hinckley M, Hockman S, Nedachi T, Jin C, Conti M, Manganiello V. Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest. 2004;114:196–205. doi: 10.1172/JCI21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Downs SM, Eppig JJ. The role of purines in the maintenance of meiotic arrest in mouse oocytes. Tokai J Exp Clin Med. 1986;11:463–469. [PubMed] [Google Scholar]
- 44.Reinhardt RR, Chin E, Zhou J, Taira M, Murata T, Manganiello VC, Bondy CA. Distinctive anatomical patterns of gene expression for cGMP-inhibited cyclic nucleotide phosphodiesterases. J Clin Invest. 1995;95:1528–1538. doi: 10.1172/JCI117825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiersma A, Hirsch B, Tsafriri A, Hanssen RG, Van de Kant M, Kloosterboer HJ, Conti M, Hsueh AJ. Phosphodiesterase 3 inhibitors suppress oocyte maturation and consequent pregnancy without affecting ovulation and cyclicity in rodents. J Clin Invest. 1998;102:532–537. doi: 10.1172/JCI2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayes MA, Sirard MA. Effect of type 3 and type 4 phosphodiesterase inhibitors on the maintenance of bovine oocytes in meiotic arrest. Biol Reprod. 2002;66:180–184. doi: 10.1095/biolreprod66.1.180. [DOI] [PubMed] [Google Scholar]
- 47.Sasseville M, Côté N, Guillemette C, Richard FJ. New insight into the role of phosphodiesterase 3A in porcine oocyte maturation. BMC Dev Biol. 2006;6:47. doi: 10.1186/1471-213X-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hubbard CJ. Ovarian cAMP and cGMP fluctuations in the hamster during the oestrous cycle. J Reprod Fertil. 1980;59:351–355. doi: 10.1530/jrf.0.0590351. [DOI] [PubMed] [Google Scholar]
- 49.Downs SM, Eppig JJ. Induction of mouse oocyte maturation in vivo by perturbants of purine metabolism. Biol Reprod. 1987;36:431–437. doi: 10.1095/biolreprod36.2.431. [DOI] [PubMed] [Google Scholar]
- 50.Eppig JJ. Maintenance of meiotic arrest and the induction of oocyte maturation in mouse oocyte-granulosa cell complexes developed in vitro from preantral follicles. Biol Reprod. 1991;45:824–830. doi: 10.1095/biolreprod45.6.824. [DOI] [PubMed] [Google Scholar]
- 51.Wang S, Ning G, Chen X, Yang J, Ouyang H, Zhang H, Tai P, Mu X, Zhou B, Zhang M, Xia G. PDE5 modulates oocyte spontaneous maturation via cGMP–cAMP but not cGMP–PKG signaling. Front Biosci. 2008;13:7087–7095. doi: 10.2741/3212. [DOI] [PubMed] [Google Scholar]
- 52.Zhang W, Colman RW. Conserved amino acids in metal-binding motifs of PDE3A are involved in substrate and inhibitor binding. Blood. 2000;95:3380–3386. [PubMed] [Google Scholar]
- 53.Bu S, Xie H, Tao Y, Wang J, Xia G. Nitric oxide influences the maturation of cumulus cell-enclosed mouse oocytes cultured in spontaneous maturation medium and hypoxanthine-supplemented medium through different signaling pathways. Mol Cell Endocrinol. 2004;223:85–93. doi: 10.1016/j.mce.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 54.Hanafy KA, Krumenacker JS, Murad F. NO, nitrotyrosine, and cyclic GMP in signal transduction. Med Sci Monit. 2001;7:801–819. [PubMed] [Google Scholar]
- 55.Rosenzweig A, Seidman CE. Atrial natriuretic factor and related peptide hormones. Annu Rev Biochem. 1991;60:229–255. doi: 10.1146/annurev.bi.60.070191.001305. [DOI] [PubMed] [Google Scholar]
- 56.Suga S, Nakao K, Hosoda K, Mukoyama M, Ogawa Y, Shirakami G, Arai H, Saito Y, Kambayashi Y, Inouye K, Imura H. Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology. 1992;130:229–239. doi: 10.1210/en.130.1.229. [DOI] [PubMed] [Google Scholar]
- 57.Koller KJ, Lowe DG, Bennett GL, Minamino N, Kangawa K, Matsuo H, Goeddel DV. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP) Science. 1991;252:120–123. doi: 10.1126/science.1672777. [DOI] [PubMed] [Google Scholar]
- 58.Jankowski M, Reis AM, MukaddamDaher S, Dam TV, Farookhi R, Gutkowska J. C-type natriuretic peptide and the guanylyl cyclase receptors in the rat ovary are modulated by the estrous cycle. Biol Reprod. 1997;56:59–66. doi: 10.1095/biolreprod56.1.59. [DOI] [PubMed] [Google Scholar]
- 59.Norris RP, Freudzon L, Freudzon M, Hand AR, Mehlmann LM, Jaffe LA. A G(s)-linked receptor maintains meiotic arrest in mouse oocytes, but luteinizing hormone does not cause meiotic resumption by terminating receptor-G(s) signaling. Dev Biol. 2007;310:240–249. doi: 10.1016/j.ydbio.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development. 2008;135:3229–3238. doi: 10.1242/dev.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsafriri A, Dekel N, Barami S. The role of oocyte maturation inhibitor in follicular regulation of oocyte maturation. J Reprod Fertil. 1982;64:541–551. doi: 10.1530/jrf.0.0640541. [DOI] [PubMed] [Google Scholar]
- 62.Ogawa Y, Itoh H, Yoshitake Y, Inoue M, Yoshimasa T, Serikawa T, Nakao K. Molecular cloning and chromosomal assignment of the mouse C-type natriuretic peptide (CNP) gene (Nppc): comparison with the human CNP gene (NPPC) Genomics. 1994;24:383–387. doi: 10.1006/geno.1994.1633. [DOI] [PubMed] [Google Scholar]
- 63.Downs SM, Coleman DL, Wardbailey PF, Eppig JJ. Hypoxanthine is the principal inhibitor of murine oocyte maturation in a low-molecular weight fraction of porcine follicular-fluid. Proc Natl Acad Sci USA. 1985;82:454–458. doi: 10.1073/pnas.82.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ponderato N, Crotti G, Turini P, Duchi R, Galli C, Lazzari G. Embryonic and foetal development of bovine oocytes treated with a combination of butyrolactone I and roscovitine in an enriched medium prior to IVM and IVF. Mol Reprod Dev. 2002;62:513–518. doi: 10.1002/mrd.10134. [DOI] [PubMed] [Google Scholar]
- 65.Coy P, Romar R, Payton RR, McCann L, Saxton AM, Edwards JL. Maintenance of meiotic arrest in bovine oocytes using the S-enantiomer of roscovitine: effects on maturation, fertilization and subsequent embryo development in vitro. Reproduction. 2005;129:19–26. doi: 10.1530/rep.1.00299. [DOI] [PubMed] [Google Scholar]
- 66.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17:121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 67.Zeleznik AJ. Dynamics of primate follicular growth: a physiological perspective. In: Leung PCK, Adashi EY, editors. The ovary. 2. Amsterdam: Elsevier Academic Press; 2004. pp. 45–53. [Google Scholar]
- 68.Terranova PF, Rice VM. Review: cytokine involvement in ovarian processes. Am J Reprod Immunol. 1997;37:50–63. doi: 10.1111/j.1600-0897.1997.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 69.Demeestere I, Centner J, Gervy C, Englert Y, Delbaere A. Impact of various endocrine and paracrine factors on in vitro culture of preantral follicles in rodents. Reproduction. 2005;130:147–156. doi: 10.1530/rep.1.00648. [DOI] [PubMed] [Google Scholar]
- 70.Noubani A, Farookhi R, Gutkowska J. B-type natriuretic peptide receptor expression and activity are hormonally regulated in rat ovarian cells. Endocrinology. 2000;141:551–559. doi: 10.1210/en.141.2.551. [DOI] [PubMed] [Google Scholar]
- 71.Zhang M, Su YQ, Sugiura K, Wigglesworth K, Xia G, Eppig JJ (2011) Estradiol promotes and maintains cumulus cell expression of natriuretic peptide receptor 2 (NPR2) and meiotic arrest in mouse oocytes in vitro. Endocrinology (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- 72.Licht P, Gallo AB, Aggarwal BB, Farmer SW, Castelino JB, Papkoff H. Biological and binding activities of equine pituitary gonadotrophins and pregnant mare serum gonadotrophin. J Endocrinol. 1979;83:311–322. doi: 10.1677/joe.0.0830311. [DOI] [PubMed] [Google Scholar]
- 73.LaPolt PS, Leung K, Ishimaru R, Tafoya MA, You-hsin Chen J. Roles of cyclic GMP in modulating ovarian functions. Reprod Biomed Online. 2003;6:15–23. doi: 10.1016/S1472-6483(10)62051-2. [DOI] [PubMed] [Google Scholar]
- 74.Gill A, Jamnongjit M, Hammes SR. Androgens promote maturation and signaling in mouse oocytes independent of transcription: a release of inhibition model for mammalian oocyte meiosis. Mol Endocrinol. 2004;18:97–104. doi: 10.1210/me.2003-0326. [DOI] [PubMed] [Google Scholar]
- 75.Jamnongjit M, Gill A, Hammes SR. Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc Natl Acad Sci USA. 2005;102:16257–16262. doi: 10.1073/pnas.0508521102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li M, Ai JS, Xu BZ, Xiong B, Yin S, Lin SL, Hou Y, Chen DY, Schatten H, Sun QY. Testosterone potentially triggers meiotic resumption by activation of intra-oocyte SRC and MAPK in porcine oocytes. Biol Reprod. 2008;79:897–905. doi: 10.1095/biolreprod.108.069245. [DOI] [PubMed] [Google Scholar]
- 77.Motola S, Popliker M, Tsafriri A. Are steroids obligatory mediators of luteinizing hormone/human chorionic gonadotropin-triggered resumption of meiosis in mammals? Endocrinology. 2007;148:4458–4465. doi: 10.1210/en.2007-0445. [DOI] [PubMed] [Google Scholar]
- 78.Tsafriri A, Motola S. Are steroids dispensable for meiotic resumption in mammals? Trends Endocrinol Metab. 2007;18:321–327. doi: 10.1016/j.tem.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 79.Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci. 2007;120:1330–1340. doi: 10.1242/jcs.000968. [DOI] [PubMed] [Google Scholar]
- 80.Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009;27:32–42. doi: 10.1055/s-0028-1108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol. 2002;64:69–92. doi: 10.1146/annurev.physiol.64.081501.131029. [DOI] [PubMed] [Google Scholar]
- 82.Hunzicker-Dunn M, Mayo K. Gonadotropin signaling in the ovary. In: Neill JD, editor. Knobil and Neill’s physiology of reproduction. 3. San Diego: Elsevier/Academic Press; 2006. pp. 547–592. [Google Scholar]
- 83.Hashimoto N, Kishimoto T, Nagahama Y. Induction and inhibition of meiotic maturation in follicle-enclosed mouse oocytes by forskolin. Dev Growth Differ. 1985;27:709–716. doi: 10.1111/j.1440-169X.1985.00709.x. [DOI] [PubMed] [Google Scholar]
- 84.Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27:1914–1924. doi: 10.1128/MCB.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hunzicker-Dunn M. Selective activation of rabbit ovarian protein kinase isozymes in rabbit ovarian follicles and corpora lutea. J Biol Chem. 1981;256:12185–12193. [PubMed] [Google Scholar]
- 86.Panigone S, Hsieh M, Fu M, Persani L, Conti M. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol. 2008;22:924–936. doi: 10.1210/me.2007-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eppig JJ, Downs SM. Chemical signals that regulate mammalian oocyte maturation. Biol Reprod. 1984;30:1–11. doi: 10.1095/biolreprod30.1.1. [DOI] [PubMed] [Google Scholar]
- 88.Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology. 2006;147:2280–2286. doi: 10.1210/en.2005-1011. [DOI] [PubMed] [Google Scholar]
- 89.Mehlmann LM, Kalinowski RR, Ross LF, Hewlett EL, Jaffe LA. Meiotic resumption in response to luteinizing hormone is independent of a Gi family G protein or calcium in the mouse oocyte. Dev Biol. 2006;299:345–355. doi: 10.1016/j.ydbio.2006.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hubbard CJ. Cyclic AMP changes in the component cells of Graafian follicles: possible influences on maturation in the follicle-enclosed oocytes of hamsters. Dev Biol. 1986;118:343–351. doi: 10.1016/0012-1606(86)90003-5. [DOI] [PubMed] [Google Scholar]
- 91.Han SJ, Vaccari S, Nedachi T, Andersen CA, Kovacina KS, Roth RA, Conti M. Protein kinase B/Akt phosphorylation of PDE3A and its role in mammalian oocyte maturation. EMBO J. 2006;25:5716–5725. doi: 10.1038/sj.emboj.7601431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eppig JJ, Downs SM. The effect of hypoxanthine on mouse oocyte growth and development in vitro: maintenance of meiotic arrest and gonadotropin-induced oocyte maturation. Dev Biol. 1987;119:313–321. doi: 10.1016/0012-1606(87)90037-6. [DOI] [PubMed] [Google Scholar]
- 93.Kawamura K, Ye Y, Liang CG, Kawamura N, Gelpke MS, Rauch R, Tanaka T, Hsueh AJW. Paracrine regulation of the resumption of oocyte meiosis by endothelin-1. Dev Biol. 2009;327:62–70. doi: 10.1016/j.ydbio.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 94.Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 95.Conti M, Hsieh M, Park JY, Su YQ. Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol. 2006;20:715–723. doi: 10.1210/me.2005-0185. [DOI] [PubMed] [Google Scholar]
- 96.Dekel N, Lawrence TS, Gilula NB, Beers WH. Modulation of cell-to-cell communication in the cumulus–oocyte complex and the regulation of oocyte maturation by LH. Dev Biol. 1981;86:356–362. doi: 10.1016/0012-1606(81)90193-7. [DOI] [PubMed] [Google Scholar]
- 97.Salustri A, Siracusa G. Metabolic coupling, cumulus expansion and meiotic resumption in mouse cumuli oophori cultured in vitro in the presence of FSH or dcAMP, or stimulated in vivo by hCG. J Reprod Fertil. 1983;68:335–341. doi: 10.1530/jrf.0.0680335. [DOI] [PubMed] [Google Scholar]
- 98.Sherizly I, Galiani D, Dekel N. Regulation of oocyte maturation: communication in the rat cumulus–oocyte complex. Hum Reprod. 1988;3:761–766. doi: 10.1093/oxfordjournals.humrep.a136780. [DOI] [PubMed] [Google Scholar]
- 99.Motlik J, Fulka J, Flechon JE. Changes in intercellular coupling between pig oocytes and cumulus cells during maturation in vivo and in vitro. J Reprod Fertil. 1986;76:31–37. doi: 10.1530/jrf.0.0760031. [DOI] [PubMed] [Google Scholar]
- 100.Norris RP, Freudzon M, Nikolaev VO, Jaffe LA. Epidermal growth factor receptor kinase activity is required for gap junction closure and for part of the decrease in ovarian follicle cGMP in response to LH. Reproduction. 2010;140:655–662. doi: 10.1530/REP-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patwardhan VV, Lanthier A. Cyclic GMP phosphodiesterase and guanylate cyclase activities in rabbit ovaries and the effect of in vivo stimulation with LH. J Endocrinol. 1984;101:305–310. doi: 10.1677/joe.0.1010305. [DOI] [PubMed] [Google Scholar]
- 102.Conti M, Kasson BG, Hsueh AJW. Hormonal regulation of 3′, 5′-adenosine monophosphate phosphodiesterases in cultured rat granulosa cells. Endocrinology. 1984;114:2361–2368. doi: 10.1210/endo-114-6-2361. [DOI] [PubMed] [Google Scholar]
- 103.Kawamura K, Cheng Y, Kawamura N, Takae S, Okada A, Kawagoe Y, Mulders S, Terada Y, Hsueh AJ 2011 Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes. Hum Reprod (Epub ahead of print) [DOI] [PubMed]
- 104.Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology. 1991;129:3200–3207. doi: 10.1210/endo-129-6-3200. [DOI] [PubMed] [Google Scholar]
- 105.Su YQ, Wigglesworth K, Pendola FL, O’Brien MJ, Eppig JJ. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology. 2002;143:2221–2232. doi: 10.1210/en.143.6.2221. [DOI] [PubMed] [Google Scholar]
- 106.Potter LR, Hunter T. Identification and characterization of the major phosphorylation sites of the B-type natriuretic peptide receptor. J Biol Chem. 1998;273:15533–15539. doi: 10.1074/jbc.273.25.15533. [DOI] [PubMed] [Google Scholar]
- 107.Pandey AN, Tripathi A, Premkumar KV, Shrivastav TG, Chaube SK. Reactive oxygen and nitrogen species during meiotic resumption from diplotene arrest in mammalian oocytes. J Cell Biochem. 2010;111:521–528. doi: 10.1002/jcb.22736. [DOI] [PubMed] [Google Scholar]
- 108.Conti M, Andersen CB, Richard F, Mehats C, Chun SY, Horner K, Jin C, Tsafriri A. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrin. 2002;187:153–159. doi: 10.1016/S0303-7207(01)00686-4. [DOI] [PubMed] [Google Scholar]
- 109.Andersen CB, Roth RA, Conti M. Protein kinase B/Akt induces resumption of meiosis in Xenopus oocytes. J Biol Chem. 1998;273:18705–18708. doi: 10.1074/jbc.273.30.18705. [DOI] [PubMed] [Google Scholar]