Abstract
Mast cells store an impressive array of preformed compounds (mediators) in their secretory granules. When mast cells degranulate, these are released and have a profound impact on any condition in which mast cell degranulation occurs. The preformed mast cell mediators include well-known substances such as histamine, proteoglycans, proteases, and preformed cytokines, as well as several recently identified compounds. Mast cells have recently been implicated in a large number of novel pathological settings in addition to their well-established contribution to allergic reactions, and there is consequently a large current interest in the molecular mechanisms by which mast cells act in the context of a given condition. In many cases, preformed mast cell mediators have been shown to account for functions ascribed to mast cells, and these compounds are hence emerging as major players in numerous pathologies. In this review we summarize the current knowledge of preformed mast cell mediators.
Keywords: Mast cells, Mediators, Secretion, Granules, Inflammation
Introduction
Recent research has put the mast cell (MC) at the center stage of immunology. This development is in large part based on numerous studies demonstrating a crucial role for MCs in various types of disease. Most of these studies have utilized genetically MC-deficient mice, in which MC-deficiency is a result of defective c-kit signaling (reviewed in [1]). By using such experimental systems, it has been possible to demonstrate a detrimental role for MCs in, e.g., arthritis, experimental autoimmune encephalitis, bullous pemphigoid, atherosclerosis, cancer, and abdominal aortic aneurysm formation (reviewed in [1]). However, MCs have also been shown to possess a number of beneficial functions, most notably in the context of innate immunity towards bacteria and parasites (reviewed in [2]). In addition, recent research has revealed a role for MCs in the suppression of immune responses, including the promotion of allograft tolerance [3] and secretion of immunomodulatory IL-10 [4].
As a consequence of this development, many laboratories worldwide are currently studying the impact of MCs on many different immunological settings. Importantly, although it is established that MCs participate in various diseases, it is in many cases not clear exactly how they contribute, i.e., the molecular mechanisms. One major route by which MCs could affect a given condition is through effects mediated by the various preformed compounds (“mediators”) that are stored within the MC secretory granules, and are released upon MC degranulation. MC degranulation can be accomplished by a multitude of mechanisms, of which binding of multivalent antigen to IgE molecules bound to the high affinity IgE receptor, FcεRI, is the best characterized pathway [5–7]. In addition, MC degranulation can be triggered by various other mechanisms, including exposure to anaphylatoxins, stem cell factor, endothelin-1, and various neuropeptides [8]. However, it should be pointed out that MCs can be induced to secrete compounds without signs of ongoing degranulation [8]. It is also important to emphasize that stimuli which cause MC degranulation can induce de novo synthesis of numerous compounds, such as eicosanoids, cytokines, and chemokines [8, 9].
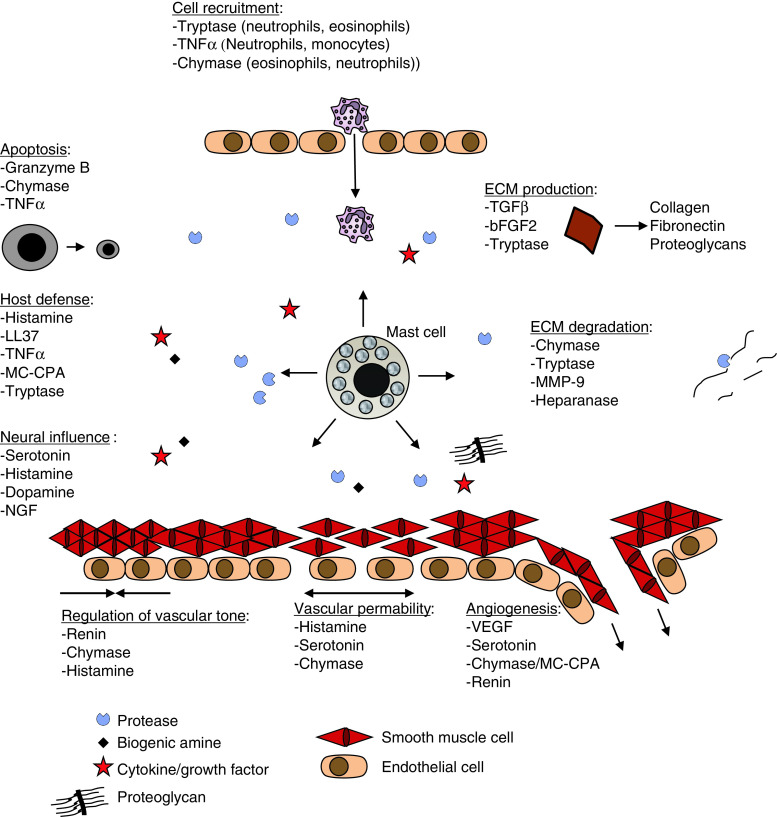
The preformed granule components include a number of biologically active substances (Table 1), and their release is likely to influence any pathological condition in which MC degranulation occurs (Fig. 1). In this review we focus on the preformed MC mediators and discuss their biological implications.
Table 1.
Preformed mediators stored within MC secretory granules
| Biogenic amines |
| Histamine |
| Serotonin |
| Dopamine |
| Polyaminesa |
| Lysosomal enzymes |
| β-Hexosaminidase |
| β-Glucuronidase |
| β-d-galactosidase |
| Arylsulfatase A |
| Cathepsin C |
| Cathepsin B |
| Cathepsin L |
| Cathepsin D |
| Cathepsin E |
| Proteoglycans |
| Serglycin (with heparin and/or CS GAG chains) |
| Proteases |
| Chymaseb |
| Tryptaseb |
| MC-CPA |
| Cathepsin G |
| Granzyme B |
| MMP-9 |
| Renin |
| Cytokines |
| TNFα |
| IL-4 |
| TGFβ |
| bFGF-2 |
| IL-15 |
| NGF |
| VEGF |
| Stem cell factor |
| Other |
| Heparanase |
| MBP |
| Peroxidase |
| LL-37/cathelicidine |
Fig. 1.
Examples of biological functions of secreted, preformed MC mediators
MC granule compounds
Biogenic amines
Out of all MC mediators, histamine is undoubtedly the most well known. It has been established since several decades that preformed histamine is a prominent component of MC granules and that histamine release accompanies MC degranulation [10]. Histamine is synthesized in one step, by decarboxylation of histidine, in a reaction catalyzed by histidine decarboxylase (HDC). Accordingly, HDC is expressed by MCs [11] and the HDC expression levels increase during the process of MC maturation [12]. Histamine possesses a multitude of biological activities, including induction of vasodilation, increased capillary permeability, bronchoconstriction, and bronchial smooth muscle contraction (Fig. 1). Recently, more insight into the in vivo function of histamine has been acquired through the genetic targeting of HDC [13]. By evaluating the HDC−/− strain in various animal models for disease, a role for histamine has been established in numerous pathological conditions, including allergic airway inflammation [14], systemic anaphylaxis [15], atherosclerosis [16], and experimental autoimmune encephalitis [17] (reviewed in [18]). However, it should be noted that HDC expression is not entirely restricted to MCs, and it is thus not certain that consequences of HDC deficiency result from histamine deficiency in MCs, as opposed to effects related to HDC expressed by cells other than MCs.
It has also been known for a long time that serotonin is present in MC granules [19]. It was initially thought that serotonin was mainly present in MCs of rodents, and that human MCs lacked stored serotonin [20]. However, limited evidence early suggested the presence of serotonin also in human MCs [21] and this notion was further strengthened when Metcalfe et al. [22] demonstrated that human peripheral blood-derived MCs contain serotonin and that plasma serotonin levels were elevated in some patients with mastocytosis. Serotonin is synthesized in one step, by hydroxylation of tryptophan, in a reaction catalyzed by tryptophan hydroxylase (TPH). TPH is present in two isoforms, TPH1 and TPH2. Out of these, TPH1 appears to be the predominant isoform in MCs and its level of expression correlates positively with the degree of MC maturation [12]. Several functions of serotonin have been implied based on animal strains lacking TPH1 and TPH2, respectively ([23] and references therein). However, since serotonin expression is far from unique to MCs, a firm establishment of the function for MC serotonin has to await the conditional targeting of TPH1 in MCs.
Limited evidence suggests that dopamine may also be synthesized and stored in MCs and that MC activation results in depletion of cell-associated dopamine [24], the latter suggesting that dopamine is present within MC granules. However, mRNAs coding for the enzymes that catalyze the formation of dopamine from tyrosine, i.e., tyrosine hydroxylase and DOPA decarboxylase, have, to our knowledge, not been identified in MCs.
In a recent study, it was demonstrated that MC granules contain antizyme inhibitor 2 (AZIN2) [25]. Since AZIN2 is an activator of ornithine decarboxylase, a key enzyme in polyamine (putrescine, spermidine, spermine) synthesis, these findings suggest that polyamines may be present within MC granules. In support of this notion, depletion of polyamines was shown to inhibit the IgE-mediated release of serotonin from human MCs [25]. However, direct evidence for the presence of polyamines within MC granules has to date not been presented.
Lysosomal enzymes
MC secretory granules share many features with lysosomes, e.g., acidic pH and similar membrane components such as VAMP-8 [26, 27], and it has also been known for a long time that MC granules contain a number of components that are also present in lysosomes (Table 1). Hence, the distinction between lysosomes and secretory granules is not well defined and, consequently, secretory granules in many cells, including MCs, are often referred to as “secretory lysosomes” [5, 28]. Out of the lysosomal enzymes known to be present in MC granules, β-hexosaminidase is the most well known and, since β-hexosaminidase is ubiquitously present in MC granules of all subtypes and species, its release is frequently used as a means of quantifying the extent of MC degranulation. MC degranulation also leads to the release of a number of other saccharide-degrading enzymes, including β-glucuronidase, β-d-galactosidase, and arylsulfatase A [29, 30]. The biological function of these enzymes in the context of MCs is not clear. Most likely, they play a role in normal intracellular turnover processes but it cannot be excluded that they may have extracellular functions following MC degranulation.
It has also been known for some time that MC granules contain a number of lysosomal proteases. These include several cysteine cathepsins, e.g., cathepsin C, B, and L [31, 32], but also the aspartic acid proteases, cathepsin D [32] and cathepsin E [33]. Notably, IgE-mediated MC activation was shown to induce the release of cathepsin D, B, and L [32], thus indicating their presence in secretory granules.
The lysosomal enzymes all have a low pH optimum and, therefore, the traditional view has been that these enzymes are mainly active within the acidic environment of lysosomes/granules, and that they become rapidly inactivated after exposure to extracellular/cytosolic pH. However, recent research has revealed that several of the lysosomal cathepsins possess considerable enzymatic activity also after cellular release [34]. Hence, it cannot be excluded that lysosomal enzymes secreted as a consequence of MC degranulation can exert extracellular functions. However, extracellular biological functions of lysosomal enzymes secreted from MCs remain to be established.
Proteoglycans
It has been recognized for a long time that serglycin proteoglycan is a major constituent of MC granules. Serglycin, like all proteoglycans, contains a protein core to which heavily sulfated (and thereby negatively charged) glycosaminoglycan (GAG) chains are attached. Importantly, the nature of the GAG chains attached to the serglycin protein core varies to a large extent, depending on the cell type in which serglycin is expressed. In connective tissue-type MCs (CTMCs) of rodents, highly sulfated GAGs of heparin type are main constituents whereas in mucosal MCs (MMCs), highly sulfated (“oversulfated”) chondroitin sulfate (CS) is the predominant GAG component of serglycin [35, 36]. In contrast, serglycin in human MCs contains both heparin and CS, in ~2:1 ratio of heparin over CS [37, 38]. A distinguishing feature of MC serglycin, as opposed to serglycin produced by cell types other than MCs, is that the GAG chains have a remarkably high extent of sulfation, which enables them to engage in tight electrostatic interactions with the various basic compounds that co-exist in the granules (reviewed in [39]; see also under “Regulation of storage”). The reason behind the high extent of sulfation of MC serglycin is not fully understood, although recent data suggest that MC maturation correlates positively with the expression of a number of CS and heparin sulfotransferases [40].
Histologically, MCs are easily distinguished by their strong granular staining with various cationic dyes such as Toluidine Blue, Alcian Blue, Berberine sulfate, and May Grünwald/Giemsa. The staining with these dyes is most likely explained by their strong binding to serglycin present in MC granules, as indicated by the complete lack of metachromatic staining in serglycin−/− MCs [41]. The binding properties of these dyes can be employed to distinguish GAG subtypes and different MC subclasses, with Alcian Blue preferentially binding to MMCs, safranin preferentially binding to CTMCs, and Berberine sulfate staining predominantly for heparin rather than CS [42, 43].
Proteases
MCs granules constitute one of the major sites for stored proteases, with proteases accounting for more than 25% of the total MC protein [44]. Remarkably, the MC proteases are all stored as active enzymes, i.e., with their activation peptides removed before storage. The latter is in sharp contrast with most other proteases, e.g., the pancreatic digestive proteases, in which the activation peptides usually are cleaved off after secretion. The term “MC proteases” usually refers to a number of enzymes that are specifically expressed by MCs, including proteases of chymase, tryptase, and carboxypeptidase A (MC-CPA) type (Table 1). Notably, the specific MC protease expression profile differs extensively between MC subclasses and also between species (described in detail elsewhere [45, 46]). Recent studies involving the evaluation of various MC-protease knockout strains have revealed important roles for the MC proteases in numerous conditions in which MCs have previously been implicated, such as arthritis [47, 48], allergic airway inflammation [49], abdominal aortic aneurysm formation [50], and glomerulonephritis [51], as well as in the defense towards bacteria [52] and parasites [53]. Hence, MC proteases may in many cases account for the detrimental as well as beneficial effects ascribed to MCs.
In addition to expressing MC-specific proteases, MCs of different types have been shown to express other, non-MC-specific proteases. Early histological evidence suggested that human MCs contain cathepsin G [54], a serine protease also expressed by neutrophils, and mRNA encoding cathepsin G was identified in MC-containing skin from patients with urticaria pigmentosa [55]. It has also been demonstrated that human peripheral blood-derived MCs contain matrix metalloprotease 9 (MMP-9) [56] and that human cardiac MCs as well as HMC-1 cells (an MC-like cell line) contain renin [57]. Renin is responsible for the generation of angiotensin I from its precursor, angiotensinogen, whereas MC chymase is known to be one of the key enzymes involved in conversion of angiotensin I into the active component, i.e., angiotensin II [58]. Hence, MCs granules are equipped with all of the components necessary to generate angiotensin II from its precursors, suggesting a role for MCs in the modulation of angiotensin II-mediated events such as the regulation of blood pressure. Indeed, it has been demonstrated that MC-mediated mechanisms contribute to the angiotensin I-converting enzyme-independent regulation of arterial blood pressure [59].
More recently, it has been discovered that MCs express and store large amounts of granzyme B, a serine protease mainly implicated in apoptosis but also in extracellular matrix remodeling [60]. It was shown that MCs could induce apoptosis in target cells, through a mechanism involving granule-contained granzyme B. Hence, these data add a new dimension of MC function by introducing the possibility that MC degranulation may contribute to induction of apoptosis at inflammatory sites.
Cytokines
Through the pioneering work of Paul, Galli, Dorf and their and coworkers, it was realized that MCs are capable of generating cytokines [61–63]. Not only that, it was demonstrated that MCs can actually store preformed cytokines within secretory granules, as initially shown for tumor necrosis factor α (TNFα) [61]. Following the identification of the MC as a TNFα-producing cell, numerous functions for MC-derived TNFα have been demonstrated [64–67]. For example, it has been shown that MC TNFα is critical for inducing the lymph node hypertrophy that is associated with bacterial infection [68] and, interestingly, it was recently shown that the delivery of TNFα to the draining lymph nodes may be dependent on packaging of TNFα in heparin (serglycin)-containing MC-derived particles [66]. However, it should be pointed out that, in some experimental systems, the bulk of TNFα released by MCs may be derived from de novo synthesis rather than from preformed pools [69]. MCs have also been demonstrated to express and secrete a large number of additional cytokines, growth factors, and chemokines [8]. In many cases, these are most likely released as a consequence of de novo synthesis rather than being released from preformed pools. However, collective evidence suggests that a number of different cytokines/growth factors may actually be stored within the granules, including vascular endothelial growth factor (VEGF) [70, 71], IL-4 [72, 73], nerve growth factor (NGF) [74], IL-15 [75], basic fibroblast growth factor-2 (bFGF-2) [76], transforming growth factor-β (TGFβ) [77] and stem cell factor (SCF) [78] (Table 1). Notably, since MCs show a strong tendency of background immunohistochemical staining, due to unspecific binding of IgG to the secretory granule proteoglycans (serglycin), some caution should be taken when interpreting data showing positive staining for any compound in MC granule. Therefore, in order to firmly establish the presence of preformed compounds within MC granules, it is preferable that its presence is confirmed by other means. For example, rapid release (<30 min) after IgE cross-linking strongly supports the presence of a preformed compound within granules. Examples of the latter include the rapid release of TNFα, TGFβ, NGF, SCF, and IL-4 from various types of MCs after IgE-mediated degranulation. Another useful criterion that identifies a cytokine (or any compound) as a true MC product is if the corresponding mRNA can be identified. Further, lack of staining in MCs genetically targeted to lack the respective compound, as exemplified by the lack of positive IL-15 staining in IL-15−/− MCs [75], provides strong evidence for presence within granules.
Other granule components
Eosinophil major basic protein (MBP) has been identified in nasal and ileal human MCs and also in MCs from cutaneous mastocytosis specimens [79], although normal skin MCs do not stain for MBP [79, 80]. Early reports suggested that peroxidase may be present within MC granules [81, 82], but this finding has been questioned as most likely being the result of eosinophil contamination of the MC population studied [83]. Further, it has been shown that MC granules contain heparanase [84], an enzyme involved in degradation of heparin and its close relative, heparan sulfate. Heparanase exocytosed from MCs was shown to cleave heparan sulfate chains present in extracellular matrix, but it remains to be shown whether MC heparanase has a role in the intracellular turnover of the heparin chains present within MC granules. There is also limited evidence that MC granules contain preformed LL-37, an antimicrobial peptide belonging to the cathelicidin family, suggesting that MC degranulation could unleash direct anti-bacterial activity [85].
Regulation of storage
Several recent studies have pointed to a key role for serglycin in maintaining proper granule storage, with serglycin-deficient MCs displaying an almost complete inability to store a number of MC proteases as well as histamine and serotonin [12, 41, 86]. Importantly, the absence of serglycin does not affect the levels of mRNA coding for the respective proteases, suggesting effects at the level of storage rather than synthesis. The fate of the MC proteases in cells lacking serglycin is still not clear, although limited evidence suggests that they may become either degraded or secreted [87]. It is also noteworthy that serglycin does not have a universal role in promoting MC mediator storage, i.e., certain compounds rely strongly on serglycin for storage whereas others are stored independently of serglycin (reviewed in [39]). Although serglycin certainly has a key role in promoting storage of granular compounds, it should be emphasized that serglycin does not affect the actual granule biogenesis; when examining granules of bone marrow-derived MCs, approximately equal numbers of granules were found in WT and serglycin−/− cells and granules were of approximately equal size. However, granules from serglycin−/− cells lacked the dense core formation that is characteristic of WT counterparts, suggesting a key role for serglycin in promoting granular condensation [86]. An even more striking effect of serglycin-deficiency was observed in fully mature peritoneal MCs. Here, the WT cells contained granules entirely filled with highly electron dense material, whereas serglycin-deficient MCs instead contained only a few but dramatically enlarged vesicles virtually devoid of electron-dense content [88], the latter being reminiscent of the granule ultrastructure observed in MCs lacking N-deacetylase/N-sulfotransferase-2 [89]. Hence, the absence of serglycin appears to result in collapsed granule structure in mature MCs, although granule biogenesis occurs independently of serglycin.
Several observations point to additional complexity in terms of granule subtypes and subdivision. In human MCs, it has been known for a long time that the granules are ultrastructurally subdivided into regions containing scroll-like or crystalline structures, particles, or a mixture of such structural elements [90]. Although the mechanisms and implications for this subdivision have not been elucidated, an interesting observation is that tryptase and chymase are localized in regions of different ultrastructural appearance, with tryptase being predominantly contained within crystalline regions whereas chymase preferentially localizes to electron-dense regions of amorphous appearance [91]. In contrast, similar ultrastructural subdivision of rodent MC granules is not apparent (Fig. 2). On the other hand, recent evidence has suggested that different granules of individual murine MCs have distinct contents, with some granules containing serotonin and cathepsin D preferentially and with others instead containing histamine and TNFα [27]. Similar subdivision into preferentially serotonin- or histamine-containing granules is also supported by other studies [25, 92]. However, the mechanisms explaining this granule segregation remain to be clarified.
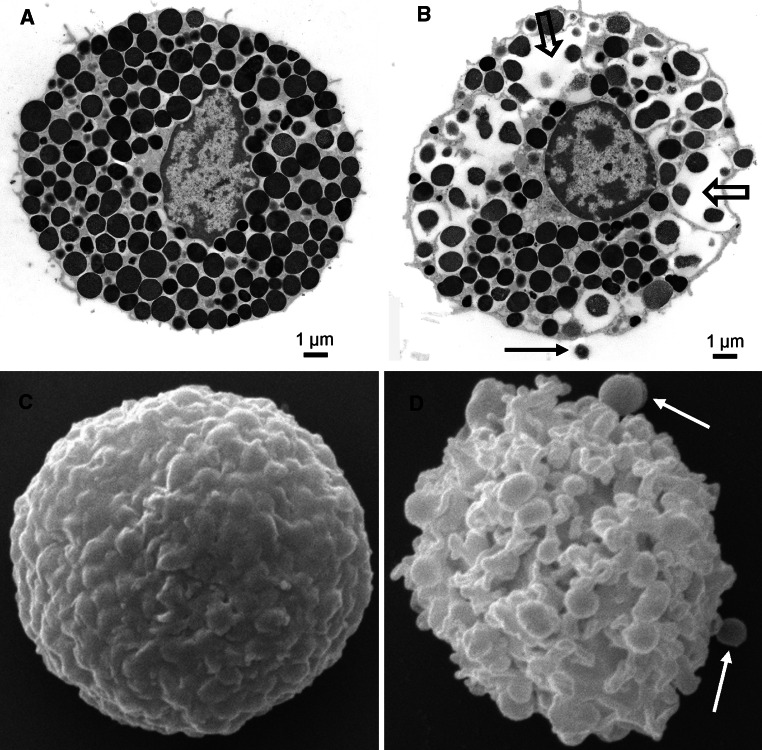
Fig. 2.
a, b Transmission electron micrographs showing an intact rat peritoneal MC (a) and a MC undergoing anaphylactic degranulation (b). The block arrows indicate regions where multiple granules have fused; the arrow depicts an exocytosed granule remnant. c, d Scanning electron micrographs showing an intact (c) and degranulated (d) rat peritoneal MC. Note the extensive membrane alterations in d. Arrows in d depict exocytosed granule remnants. Images are courtesy of Prof. Giuliano Zabucchi, Prof. Maria Rosa Soranzo, and Dr. Francesca Vita (Electron Microscopy Section of Centro Coordinamento e Sviluppo Progetti e Apparecchiature (CSPA), University of Trieste)
Sorting into MC granules
There is very limited knowledge of the mechanisms that lead to proper targeting of MC mediators into the secretory granules. For example, although it appears likely that the mannose-6-phosphate system has a role in sorting of typical lysosomal compounds and granzyme B into MC granules, there is so far no experimental evidence supporting such a notion. An attractive hypothesis would be that serglycin acts as an intracellular carrier and sorting vehicle for a number of granule compounds, i.e., those whose storage is serglycin-dependent. However, the evidence available so far suggests that serglycin-dependent mediators are correctly targeted to granules even in the absence of serglycin, but that serglycin rather has a role in promoting their retention within the granules [87].
Granule dynamics
The exact composition of the MC granules is a net result of a number of different processes interacting in a dynamic fashion. Undoubtedly, serglycin has a main function in promoting histamine storage but, interestingly, there also appears to exist an inverse relationship, as shown by the reduced proteoglycan (and protease) content of MCs lacking histamine [13]. Although the exact mechanism behind this finding is not clear, it appears likely that histamine, being positively charged, has a role in balancing the negative electric charge of serglycin. A reduction in histamine may thus lead to impaired proteoglycan storage, which, in turn, will lead to impaired protease storage. There is also evidence suggesting that MC mediators can show interdependence in terms of storage, in addition to their dependence on serglycin. One example of this is the strong interdependence of mouse mast cell protease 5 (mMCP-5) and MC-CPA, as shown by the complete lack of mMCP-5 protein in MC-CPA−/− MCs [93] and vice versa, i.e., a complete lack of MC-CPA protein in mMCP-5−/− MCs [94]. Notably, the interdependence of MC-CPA and mMCP-5 does not require that MC-CPA is enzymatically active, as indicated by the preserved mMCP-5 storage seen in mice in which the active site of MC-CPA is mutated [95]. As another example of interaction between different granule constituents, it has been shown that MCs lacking either cathepsin S or -C accumulate excessive amounts of MC-CPA and of mMCP-5, suggesting that MC-CPA and mMCP-5 levels in granules are controlled by proteolytic processing catalyzed by cathepsin S and -C [96]. Moreover, it has been shown that cathepsin C is essential for the processing of pro-chymases into active enzymes [97] and that cathepsin E located within granules has a role in the processing of pro-MC-CPA into mature enzyme [33]. Interestingly, the processing of MC protease precursors into active enzymes may in fact take place within the granules [98].
Another important example demonstrating potential interdependence of granule constituents is the findings of Zhao et al. [99] who demonstrated that MC proteases can degrade certain cytokines that were endogenously produced by MCs. Hence, MC proteases could have a role in down-regulating the magnitude of the effects caused by MC-produced cytokines. On a more controversial angle, it has been suggested that RNA and ribosomes may in fact be found associated with (and even within) MC secretory granules, implying an ongoing protein synthesis within this compartment [100].
The bulk of MC granule content is most likely the result of endogenous synthesis within the MC. However, there is substantial evidence suggesting that several compounds may be efficiently taken up by MCs and deposited in the granules. An early example of this phenomenon is the reported uptake of MBP by human skin MCs [79]. It has also been shown that MCs can take up peroxidase [83], dopamine [101], and histamine [102], and store these compounds within granules. We may thus envisage that tissue MCs may take up and act as reservoirs for various biologically active compounds, sequester these, and release them when needed. Further, we cannot exclude that some of the compounds that have been reported to be present in MC granules, especially when shown by immunohistological techniques only (without confirmation by mRNA analysis), may in fact be present in granules as a result of uptake from the surrounding milieu rather than having been synthesized by the MC itself.
Granule biogenesis
There is only limited knowledge of the mechanisms regulating MC granule biogenesis. Most of the general knowledge of secretory granule biogenesis comes from studies of neuroendocrine cells [103] and, considering that the secretory granules in MCs and neuroendocrine cells share many characteristics, it appears likely that mechanisms of granule biogenesis are similar in these cell types. In line with such a notion, it was recently shown that MC granule biogenesis was promoted by over-expression of secretogranin III, a protein that also has a key role in the formation of secretory granules in neuroendocrine cells [104].
There is also very little knowledge of the mechanisms that regulate the size of the MC secretory granules. However, it was recently proposed that the size of the MC secretory granules is tightly regulated by the fusion of Golgi-derived pro-granules of defined size (unit granule) either to each other or to pre-existing granules [105]. Moreover, there is evidence suggesting that synaptotagmin III has an important role in regulating the size of the MC secretory granules, although not being involved in the actual granule biogenesis [106].
Granule release
The signaling events involved in MC degranulation, in particular following stimulation through the IgE receptor, have been the subject of intense investigations and have been extensively reviewed in the past [1, 5–7]. MC activation through the IgE pathway typically leads to massive degranulation, referred to as anaphylactic degranulation (Fig. 2), in which a large portion of the stored granule compounds is released. Recent findings suggest that CD63 [107] and CD203c [108] are exposed on the surface of degranulated human MCs, but are only weakly expressed on non-activated cells, and thus can be used as selective markers for degranulated MCs. Anaphylactic degranulation has been shown to involve fusion of several granules into “degranulation channels”, followed by fusion of granule membranes with the plasma membrane and extrusion of membrane-free “granule remnants” into the surrounding milieu (reviewed in [109]) (Fig. 2). In addition to undergoing anaphylactic degranulation, it has been known for a long time that MCs can also release granule material in a slow fashion, a process termed “piecemeal degranulation” [90]. Typically, piecemeal degranulation leads to emptying of secretory granules without any visible plasma- or granule membrane alterations, and there is evidence that piecemeal degranulation involves vesicular transport of granule contents to the plasma membrane [90]. A feature that distinguishes MCs from many other granule-containing immune cells is that they, after undergoing massive degranulation, can rebuild their granular stores, i.e., re-granulate [110]. This process appears to be completed within 24 h [111].
Concluding remarks and future directions
MCs are currently emerging as highly versatile cells capable of influencing pathological conditions at multiple levels. Following this realization, it is also clear that strategies aimed at neutralizing various harmful effects of MCs may constitute attractive directions for developing new therapeutic regimens. As reviewed here, MCs contain a plethora of pathogenic compounds stored within their secretory granule, and many of these are potential (or already proven) targets that can be utilized for therapeutic intervention. Since many of the preformed MC mediators are not uniquely expressed by MCs, a major future challenge will be to establish their specific roles in the MC context, as opposed to functions of the same compound when expressed by other cell types. To this end, experiments in which MC-deficient mice are reconstituted with either WT MCs or MCs deficient in the expression of a gene of interest have in many cases been successful. However, considering that such reconstitution approaches often encounter problems due to uneven (or failure of) reconstitution of MCs to different organs/tissues [112], this approach has some limitations. We therefore anticipate that future research will also focus on conditional targeting of genes of interest in MCs, an approach that recently has been made possible through the generation of mouse strains expressing the Cre recombinase under control of MC-specific promoters [113, 114].
Acknowledgments
The authors of this article receive support from The Swedish Research Council, Formas, King Gustaf V:s 80-year Anniversary Fund, Torsten and Ragnar Söderberg Foundation and The Swedish Cancer Foundation.
References
- 1.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–799. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 3.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, Strom TB, Zheng XX, Noelle RJ. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 4.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 5.Blank U, Rivera J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 2004;25:266–273. doi: 10.1016/j.it.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999;402:B24–B30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- 7.Rivera J, Fierro NA, Olivera A, Suzuki R. New insights on mast cell activation via the high affinity receptor for IgE. Adv Immunol. 2008;98:85–120. doi: 10.1016/S0065-2776(08)00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 9.Boyce JA. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol Rev. 2007;217:168–185. doi: 10.1111/j.1600-065X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 10.Riley JF. Histamine in tissue mast cells. Science. 1953;118:332. doi: 10.1126/science.118.3064.332. [DOI] [PubMed] [Google Scholar]
- 11.Rothschild AM, Schayer RW. Characterization of histidine decarboxylase from rat peritoneal fluid mast cells. Biochim Biophys Acta. 1959;34:392–398. doi: 10.1016/0006-3002(59)90291-4. [DOI] [PubMed] [Google Scholar]
- 12.Ringvall M, Rönnberg E, Wernersson S, Duelli A, Henningsson F, Åbrink M, Garcia-Faroldi G, Fajardo I, Pejler G. Serotonin and histamine storage in mast cell secretory granules is dependent on serglycin proteoglycan. J Allergy Clin Immunol. 2008;121:1020–1026. doi: 10.1016/j.jaci.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 13.Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, Tchougounova E, Hellman L, Gertsenstein M, Hirasawa N, Sakurai E, Buzas E, Kovacs P, Csaba G, Kittel A, Okada M, Hara M, Mar L, Numayama-Tsuruta K, Ishigaki-Suzuki S, Ohuchi K, Ichikawa A, Falus A, Watanabe T, Nagy A. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001;502:53–56. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- 14.Koarai A, Ichinose M, Ishigaki-Suzuki S, Yamagata S, Sugiura H, Sakurai E, Makabe-Kobayashi Y, Kuramasu A, Watanabe T, Shirato K, Hattori T, Ohtsu H. Disruption of l-histidine decarboxylase reduces airway eosinophilia but not hyperresponsiveness. Am J Respir Crit Care Med. 2003;167:758–763. doi: 10.1164/rccm.200206-619OC. [DOI] [PubMed] [Google Scholar]
- 15.Makabe-Kobayashi Y, Hori Y, Adachi T, Ishigaki-Suzuki S, Kikuchi Y, Kagaya Y, Shirato K, Nagy A, Ujike A, Takai T, Watanabe T, Ohtsu H. The control effect of histamine on body temperature and respiratory function in IgE-dependent systemic anaphylaxis. J Allergy Clin Immunol. 2002;110:298–303. doi: 10.1067/mai.2002.125977. [DOI] [PubMed] [Google Scholar]
- 16.Sasaguri Y, Wang KY, Tanimoto A, Tsutsui M, Ueno H, Murata Y, Kohno Y, Yamada S, Ohtsu H. Role of histamine produced by bone marrow-derived vascular cells in pathogenesis of atherosclerosis. Circ Res. 2005;96:974–981. doi: 10.1161/01.RES.0000166325.00383.ed. [DOI] [PubMed] [Google Scholar]
- 17.Musio S, Gallo B, Scabeni S, Lapilla M, Poliani PL, Matarese G, Ohtsu H, Galli SJ, Mantegazza R, Steinman L, Pedotti R. A key regulatory role for histamine in experimental autoimmune encephalomyelitis: disease exacerbation in histidine decarboxylase-deficient mice. J Immunol. 2006;176:17–26. doi: 10.4049/jimmunol.176.1.17. [DOI] [PubMed] [Google Scholar]
- 18.Ohtsu H. Progress in allergy signal research on mast cells: the role of histamine in immunological and cardiovascular disease and the transporting system of histamine in the cell. J Pharmacol Sci. 2008;106:347–353. doi: 10.1254/jphs.fm0070294. [DOI] [PubMed] [Google Scholar]
- 19.Benditt EP, Wong RL, Arase M, Roeper E. 5-Hydroxytryptamine in mast cells. Proc Soc Exp Biol Med. 1955;90:303–304. doi: 10.3181/00379727-90-22016. [DOI] [PubMed] [Google Scholar]
- 20.Sjoerdsma A, Waalkes TP, Weissbach H. Serotonin and histamine in mast cells. Science. 1957;125:1202–1203. doi: 10.1126/science.125.3259.1202. [DOI] [PubMed] [Google Scholar]
- 21.Morishima T. 5-Hydroxytryptamine (serotonin) and 5-hydroxytryptophan in mast cells of human mastocytosis. Tohoku J Exp Med. 1970;102:121–126. doi: 10.1620/tjem.102.121. [DOI] [PubMed] [Google Scholar]
- 22.Kushnir-Sukhov NM, Brown JM, Wu Y, Kirshenbaum A, Metcalfe DD. Human mast cells are capable of serotonin synthesis and release. J Allergy Clin Immunol. 2007;119:498–499. doi: 10.1016/j.jaci.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R, Boye P, Vilianovitch L, Sohr R, Tenner K, Hortnagl H, Bader M. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci USA. 2009;106:10332–10337. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freeman JG, Ryan JJ, Shelburne CP, Bailey DP, Bouton LA, Narasimhachari N, Domen J, Simeon N, Couderc F, Stewart JK. Catecholamines in murine bone marrow derived mast cells. J Neuroimmunol. 2001;119:231–238. doi: 10.1016/s0165-5728(01)00384-8. [DOI] [PubMed] [Google Scholar]
- 25.Kanerva K, Lappalainen J, Makitie LT, Virolainen S, Kovanen PT, Andersson LC. Expression of antizyme inhibitor 2 in mast cells and role of polyamines as selective regulators of serotonin secretion. PLoS One. 2009;4:e6858. doi: 10.1371/journal.pone.0006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiwari N, Wang CC, Brochetta C, Ke G, Vita F, Qi Z, Rivera J, Soranzo MR, Zabucchi G, Hong W, Blank U. VAMP-8 segregates mast cell-preformed mediator exocytosis from cytokine trafficking pathways. Blood. 2008;111:3665–3674. doi: 10.1182/blood-2007-07-103309. [DOI] [PubMed] [Google Scholar]
- 27.Puri N, Roche PA. Mast cells possess distinct secretory granule subsets whose exocytosis is regulated by different SNARE isoforms. Proc Natl Acad Sci USA. 2008;105:2580–2585. doi: 10.1073/pnas.0707854105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blott EJ, Griffiths GM. Secretory lysosomes. Nat Rev Mol Cell Biol. 2002;3:122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz LB, Austen KF. Enzymes of the mast cell granule. J Invest Dermatol. 1980;74:349–353. doi: 10.1111/1523-1747.ep12543620. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz LB, Lewis RA, Seldin D, Austen KF. Acid hydrolases and tryptase from secretory granules of dispersed human lung mast cells. J Immunol. 1981;126:1290–1294. [PubMed] [Google Scholar]
- 31.Wolters PJ, Laig-Webster M, Caughey GH. Dipeptidyl peptidase I cleaves matrix-associated proteins and is expressed mainly by mast cells in normal dog airways. Am J Respir Cell Mol Biol. 2000;22:183–190. doi: 10.1165/ajrcmb.22.2.3767. [DOI] [PubMed] [Google Scholar]
- 32.Dragonetti A, Baldassarre M, Castino R, Demoz M, Luini A, Buccione R, Isidoro C. The lysosomal protease cathepsin D is efficiently sorted to and secreted from regulated secretory compartments in the rat basophilic/mast cell line RBL. J Cell Sci. 2000;113(Pt 18):3289–3298. doi: 10.1242/jcs.113.18.3289. [DOI] [PubMed] [Google Scholar]
- 33.Henningsson F, Yamamoto K, Saftig P, Reinheckel T, Peters C, Knight SD, Pejler G. A role for cathepsin E in the processing of mast-cell carboxypeptidase A. J Cell Sci. 2005;118:2035–2042. doi: 10.1242/jcs.02333. [DOI] [PubMed] [Google Scholar]
- 34.Turk V, Turk B, Turk D. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 2001;20:4629–4633. doi: 10.1093/emboj/20.17.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yurt RW, Leid RW, Jr, Austen KF. Native heparin from rat peritoneal mast cells. J Biol Chem. 1977;252:518–521. [PubMed] [Google Scholar]
- 36.Enerback L, Kolset SO, Kusche M, Hjerpe A, Lindahl U. Glycosaminoglycans in rat mucosal mast cells. Biochem J. 1985;227:661–668. doi: 10.1042/bj2270661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metcalfe DD, Lewis RA, Silbert JE, Rosenberg RD, Wasserman SI, Austen KF. Isolation and characterization of heparin from human lung. J Clin Invest. 1979;64:1537–1543. doi: 10.1172/JCI109613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson HL, Schulman ES, Metcalfe DD. Identification of chondroitin sulfate E in human lung mast cells. J Immunol. 1988;140:2708–2713. [PubMed] [Google Scholar]
- 39.Pejler G, Abrink M, Wernersson S. Serglycin proteoglycan: regulating the storage and activities of hematopoietic proteases. Biofactors. 2009;35:61–68. doi: 10.1002/biof.11. [DOI] [PubMed] [Google Scholar]
- 40.Duelli A, Rönnberg E, Waern I, Ringvall M, Kolset SO, Pejler G. Mast cell differentiation and activation is closely linked to expression of genes coding for the serglycin proteoglycan core protein and a distinct set of chondroitin sulfate and heparin sulfotransferases. J Immunol. 2009;183:7073–7083. doi: 10.4049/jimmunol.0900309. [DOI] [PubMed] [Google Scholar]
- 41.Åbrink M, Grujic M, Pejler G. Serglycin is essential for maturation of mast cell secretory granule. J Biol Chem. 2004;279:40897–40905. doi: 10.1074/jbc.M405856200. [DOI] [PubMed] [Google Scholar]
- 42.Enerback L. Berberine sulphate binding to mast cell polyanions: a cytofluorometric method for the quantitation of heparin. Histochemistry. 1974;42:301–313. doi: 10.1007/BF00492678. [DOI] [PubMed] [Google Scholar]
- 43.Tsai M, Takeishi T, Thompson H, Langley KE, Zsebo KM, Metcalfe DD, Geissler EN, Galli SJ. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc Natl Acad Sci USA. 1991;88:6382–6386. doi: 10.1073/pnas.88.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz LB, Irani AM, Roller K, Castells MC, Schechter NM. Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J Immunol. 1987;138:2611–2615. [PubMed] [Google Scholar]
- 45.Pejler G, Åbrink M, Ringvall M, Wernersson S. Mast cell proteases. Adv Immunol. 2007;95:167–255. doi: 10.1016/S0065-2776(07)95006-3. [DOI] [PubMed] [Google Scholar]
- 46.Pejler G, Ronnberg E, Waern I, Wernersson S. Mast cell proteases: multifaceted regulators of inflammatory disease. Blood. 2010;115:4981–4990. doi: 10.1182/blood-2010-01-257287. [DOI] [PubMed] [Google Scholar]
- 47.McNeil HP, Shin K, Campbell IK, Wicks IP, Adachi R, Lee DM, Stevens RL. The mouse mast cell-restricted tetramer-forming tryptases mouse mast cell protease 6 and mouse mast cell protease 7 are critical mediators in inflammatory arthritis. Arthritis Rheum. 2008;58:2338–2346. doi: 10.1002/art.23639. [DOI] [PubMed] [Google Scholar]
- 48.Shin K, Nigrovic PA, Crish J, Boilard E, McNeil HP, Larabee KS, Adachi R, Gurish MF, Gobezie R, Stevens RL, Lee DM. Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J Immunol. 2009;182:647–656. doi: 10.4049/jimmunol.182.1.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waern I, Jonasson S, Hjoberg J, Bucht A, Abrink M, Pejler G, Wernersson S. Mouse mast cell protease 4 is the major chymase in murine airways and has a protective role in allergic airway inflammation. J Immunol. 2009;183:6369–6376. doi: 10.4049/jimmunol.0900180. [DOI] [PubMed] [Google Scholar]
- 50.Sun J, Zhang J, Lindholt JS, Sukhova GK, Liu J, He A, Abrink M, Pejler G, Stevens RL, Thompson RW, Ennis TL, Gurish MF, Libby P, Shi GP. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation. 2009;120:973–982. doi: 10.1161/CIRCULATIONAHA.109.849679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scandiuzzi L, Beghdadi W, Daugas E, Åbrink M, Tiwari N, Brochetta C, Claver J, Arouche N, Zang X, Pretolani M, Monteiro RC, Pejler G, Blank U. Murine mast cell MCP4 chymase deteriorates renal function by contributing to inflammation and fibrosis in immune complex-mediated glomerulonephritis. J Immunol. 2010;185:624–633. doi: 10.4049/jimmunol.0902129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, Adachi R. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 53.Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, Lee DM. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J Immunol. 2008;180:4885–4891. doi: 10.4049/jimmunol.180.7.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schechter NM, Irani AM, Sprows JL, Abernethy J, Wintroub B, Schwartz LB. Identification of a cathepsin G-like proteinase in the MCTC type of human mast cell. J Immunol. 1990;145:2652–2661. [PubMed] [Google Scholar]
- 55.Schechter NM, Wang ZM, Blacher RW, Lessin SR, Lazarus GS, Rubin H. Determination of the primary structures of human skin chymase and cathepsin G from cutaneous mast cells of urticaria pigmentosa lesions. J Immunol. 1994;152:4062–4069. [PubMed] [Google Scholar]
- 56.Baram D, Vaday GG, Salamon P, Drucker I, Hershkoviz R, Mekori YA. Human mast cells release metalloproteinase-9 on contact with activated T cells: juxtacrine regulation by TNF-alpha. J Immunol. 2001;167:4008–4016. doi: 10.4049/jimmunol.167.7.4008. [DOI] [PubMed] [Google Scholar]
- 57.Silver RB, Reid AC, Mackins CJ, Askwith T, Schaefer U, Herzlinger D, Levi R. Mast cells: a unique source of renin. Proc Natl Acad Sci USA. 2004;101:13607–13612. doi: 10.1073/pnas.0403208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dell’Italia LJ, Husain A. Dissecting the role of chymase in angiotensin II formation and heart and blood vessel diseases. Curr Opin Cardiol. 2002;17:374–379. doi: 10.1097/00001573-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 59.Li M, Liu K, Michalicek J, Angus JA, Hunt JE, Dell’Italia LJ, Feneley MP, Graham RM, Husain A. Involvement of chymase-mediated angiotensin II generation in blood pressure regulation. J Clin Invest. 2004;114:112–120. doi: 10.1172/JCI20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pardo J, Wallich R, Ebnet K, Iden S, Zentgraf H, Martin P, Ekiciler A, Prins A, Mullbacher A, Huber M, Simon MM. Granzyme B is expressed in mouse mast cells in vivo and in vitro and causes delayed cell death independent of perforin. Cell Death Differ. 2007;14:1768–1779. doi: 10.1038/sj.cdd.4402183. [DOI] [PubMed] [Google Scholar]
- 61.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 62.Plaut M, Pierce JH, Watson CJ, Hanley-Hyde J, Nordan RP, Paul WE. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989;339:64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- 63.Burd PR, Rogers HW, Gordon JR, Martin CA, Jayaraman S, Wilson SD, Dvorak AM, Galli SJ, Dorf ME. Interleukin 3-dependent and -independent mast cells stimulated with IgE and antigen express multiple cytokines. J Exp Med. 1989;170:245–257. doi: 10.1084/jem.170.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 65.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha [see comments] Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 66.Kunder CA, St John AL, Li G, Leong KW, Berwin B, Staats HF, Abraham SN. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J Exp Med. 2009;206:2455–2467. doi: 10.1084/jem.20090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piliponsky AM, Chen CC, Grimbaldeston MA, Burns-Guydish SM, Hardy J, Kalesnikoff J, Contag CH, Tsai M, Galli SJ. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am J Pathol. 2010;176:926–938. doi: 10.2353/ajpath.2010.090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, Abraham SN. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol. 2003;4:1199–1205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 69.Dastych J, Walczak-Drzewiecka A, Wyczolkowska J, Metcalfe DD. Murine mast cells exposed to mercuric chloride release granule-associated N-acetyl-beta-d-hexosaminidase and secrete IL-4 and TNF-alpha. J Allergy Clin Immunol. 1999;103:1108–1114. doi: 10.1016/s0091-6749(99)70186-7. [DOI] [PubMed] [Google Scholar]
- 70.Grutzkau A, Kruger-Krasagakes S, Baumeister H, Schwarz C, Kogel H, Welker P, Lippert U, Henz BM, Moller A. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF206. Mol Biol Cell. 1998;9:875–884. doi: 10.1091/mbc.9.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boesiger J, Tsai M, Maurer M, Yamaguchi M, Brown LF, Claffey KP, Dvorak HF, Galli SJ. Mast cells can secrete vascular permeability factor/vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of Fc epsilon receptor I expression. J Exp Med. 1998;188:1135–1145. doi: 10.1084/jem.188.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Horsmanheimo L, Harvima IT, Jarvikallio A, Harvima RJ, Naukkarinen A, Horsmanheimo M. Mast cells are one major source of interleukin-4 in atopic dermatitis. Br J Dermatol. 1994;131:348–353. doi: 10.1111/j.1365-2133.1994.tb08522.x. [DOI] [PubMed] [Google Scholar]
- 73.Gibbs BF, Arm JP, Gibson K, Lee TH, Pearce FL. Human lung mast cells release small amounts of interleukin-4 and tumour necrosis factor-alpha in response to stimulation by anti-IgE and stem cell factor. Eur J Pharmacol. 1997;327:73–78. doi: 10.1016/s0014-2999(97)89680-x. [DOI] [PubMed] [Google Scholar]
- 74.Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, Levi-Montalcini R. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci USA. 1994;91:3739–3743. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Orinska Z, Maurer M, Mirghomizadeh F, Bulanova E, Metz M, Nashkevich N, Schiemann F, Schulmistrat J, Budagian V, Giron-Michel J, Brandt E, Paus R, Bulfone-Paus S. IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nat Med. 2007;13:927–934. doi: 10.1038/nm1615. [DOI] [PubMed] [Google Scholar]
- 76.Qu Z, Huang X, Ahmadi P, Stenberg P, Liebler JM, Le AC, Planck SR, Rosenbaum JT. Synthesis of basic fibroblast growth factor by murine mast cells. Regulation by transforming growth factor beta, tumor necrosis factor alpha, and stem cell factor. Int Arch Allergy Immunol. 1998;115:47–54. doi: 10.1159/000023829. [DOI] [PubMed] [Google Scholar]
- 77.Lindstedt KA, Wang Y, Shiota N, Saarinen J, Hyytiainen M, Kokkonen JO, Keski-Oja J, Kovanen PT. Activation of paracrine TGF-beta1 signaling upon stimulation and degranulation of rat serosal mast cells: a novel function for chymase. Faseb J. 2001;15:1377–1388. doi: 10.1096/fj.00-0273com. [DOI] [PubMed] [Google Scholar]
- 78.Zhang S, Anderson DF, Bradding P, Coward WR, Baddeley SM, MacLeod JD, McGill JI, Church MK, Holgate ST, Roche WR. Human mast cells express stem cell factor. J Pathol. 1998;186:59–66. doi: 10.1002/(SICI)1096-9896(199809)186:1<59::AID-PATH140>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 79.Butterfield JH, Weiler D, Peterson EA, Gleich GJ, Leiferman KM. Sequestration of eosinophil major basic protein in human mast cells. Lab Invest. 1990;62:77–86. [PubMed] [Google Scholar]
- 80.Leiferman KM, Gleich GJ, Kephart GM, Haugen HS, Hisamatsu K, Proud D, Lichtenstein LM, Ackerman SJ. Differences between basophils and mast cells: failure to detect Charcot-Leyden crystal protein (lysophospholipase) and eosinophil granule major basic protein in human mast cells. J Immunol. 1986;136:852–855. [PubMed] [Google Scholar]
- 81.Christie KN, Stoward PJ. Endogenous peroxidase in mast cells localized with a semipermeable membrane technique. Histochem J. 1978;10:425–433. doi: 10.1007/BF01003006. [DOI] [PubMed] [Google Scholar]
- 82.Henderson WR, Kaliner M. Mast cell granule peroxidase: location, secretion, and SRS-A inactivation. J Immunol. 1979;122:1322–1328. [PubMed] [Google Scholar]
- 83.Rickard A, Lagunoff D. Eosinophil peroxidase accounts for most if not all of the peroxidase activity associated with isolated rat peritoneal mast cells. Int Arch Allergy Immunol. 1994;103:365–369. doi: 10.1159/000236655. [DOI] [PubMed] [Google Scholar]
- 84.Bashkin P, Razin E, Eldor A, Vlodavsky I. Degranulating mast cells secrete an endoglycosidase that degrades heparan sulfate in subendothelial extracellular matrix. Blood. 1990;75:2204–2212. [PubMed] [Google Scholar]
- 85.Di Nardo A, Yamasaki K, Dorschner RA, Lai Y, Gallo RL. Mast cell cathelicidin antimicrobial peptide prevents invasive group A Streptococcus infection of the skin. J Immunol. 2008;180:7565–7573. doi: 10.4049/jimmunol.180.11.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Braga T, Grujic M, Lukinius A, Hellman L, Abrink M, Pejler G. Serglycin proteoglycan is required for secretory granule integrity in mucosal mast cells. Biochem J. 2007;403:49–57. doi: 10.1042/BJ20061257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Henningsson F, Hergeth S, Cortelius R, Åbrink M, Pejler G. A role for serglycin proteoglycan in granular retention and processing of mast cell secretory granule components. Febs J. 2006;273:4901–4912. doi: 10.1111/j.1742-4658.2006.05489.x. [DOI] [PubMed] [Google Scholar]
- 88.Zernichow L, Abrink M, Hallgren J, Grujic M, Pejler G, Kolset SO. Serglycin is the major secreted proteoglycan in macrophages and has a role in the regulation of macrophage tumor necrosis factor-alpha secretion in response to lipopolysaccharide. J Biol Chem. 2006;281:26792–26801. doi: 10.1074/jbc.M512889200. [DOI] [PubMed] [Google Scholar]
- 89.Forsberg E, Pejler G, Ringvall M, Lunderius C, Tomasini-Johansson B, Kusche-Gullberg M, Eriksson I, Ledin J, Hellman L, Kjellen L. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature. 1999;400:773–776. doi: 10.1038/23488. [DOI] [PubMed] [Google Scholar]
- 90.Dvorak AM. Piecemeal degranulation of basophils and mast cells is effected by vesicular transport of stored secretory granule contents. Chem Immunol Allergy. 2005;85:135–184. doi: 10.1159/000086516. [DOI] [PubMed] [Google Scholar]
- 91.Whitaker-Menezes D, Schechter NM, Murphy GF. Serine proteinases are regionally segregated within mast cell granules. Lab Investig. 1995;72:34–41. [PubMed] [Google Scholar]
- 92.Theoharides TC, Bondy PK, Tsakalos ND, Askenase PW. Differential release of serotonin and histamine from mast cells. Nature. 1982;297:229–231. doi: 10.1038/297229a0. [DOI] [PubMed] [Google Scholar]
- 93.Feyerabend TB, Hausser H, Tietz A, Blum C, Hellman L, Straus AH, Takahashi HK, Morgan ES, Dvorak AM, Fehling HJ, Rodewald HR. Loss of histochemical identity in mast cells lacking carboxypeptidase A. Mol Cell Biol. 2005;25:6199–6210. doi: 10.1128/MCB.25.14.6199-6210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stevens RL, Qui D, McNeil HP, Friend DS, Hunt JE, Austen KF, Zhang J. Transgenic mice that possess a disrupted mast cell protease 5 (mMCP-5) gene can not store carboxypeptidase A (mMC-CPA) protein in their granules. FASEB J. 1996;10:17772. [Google Scholar]
- 95.Schneider LA, Schlenner SM, Feyerabend TB, Wunderlin M, Rodewald HR. Molecular mechanism of mast cell-mediated innate defense against endothelin and snake venom sarafotoxin. J Exp Med. 2007;204:2629–2639. doi: 10.1084/jem.20071262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Henningsson F, Wolters P, Chapman HA, Caughey GH, Pejler G. Mast cell cathepsins C and S control levels of carboxypeptidase A and the chymase, mouse mast cell protease 5. Biol Chem. 2003;384:1527–1531. doi: 10.1515/BC.2003.169. [DOI] [PubMed] [Google Scholar]
- 97.Wolters PJ, Pham CT, Muilenburg DJ, Ley TJ, Caughey GH. Dipeptidyl peptidase I is essential for activation of mast cell chymases, but not tryptases, in mice. J Biol Chem. 2001;276:18551–18556. doi: 10.1074/jbc.M100223200. [DOI] [PubMed] [Google Scholar]
- 98.Rath-Wolfson L. An immunocytochemical approach to the demonstration of intracellular processing of mast cell carboxypeptidase. Appl Immunohistochem Mol Morphol. 2001;9:81–85. [PubMed] [Google Scholar]
- 99.Zhao W, Oskeritzian CA, Pozez AL, Schwartz LB. Cytokine production by skin-derived mast cells: endogenous proteases are responsible for degradation of cytokines. J Immunol. 2005;175:2635–2642. doi: 10.4049/jimmunol.175.4.2635. [DOI] [PubMed] [Google Scholar]
- 100.Dvorak AM, Morgan ES, Lichtenstein LM, Weller PF, Schleimer RP. RNA is closely associated with human mast cell secretory granules, suggesting a role(s) for granules in synthetic processes. J Histochem Cytochem. 2000;48:1–12. doi: 10.1177/002215540004800101. [DOI] [PubMed] [Google Scholar]
- 101.Rundquist I, Allenmark S, Enerback L. Uptake and turnover of dopamine in rat mast cells studied by cytofluorometry and high performance liquid chromatography. Histochem J. 1982;14:429–443. doi: 10.1007/BF01011855. [DOI] [PubMed] [Google Scholar]
- 102.Ohtsu H, Kuramasu A, Tanaka S, Terui T, Hirasawa N, Hara M, Makabe-Kobayashi Y, Yamada N, Yanai K, Sakurai E, Okada M, Ohuchi K, Ichikawa A, Nagy A, Watanabe T. Plasma extravasation induced by dietary supplemented histamine in histamine-free mice. Eur J Immunol. 2002;32:1698–1708. doi: 10.1002/1521-4141(200206)32:6<1698::AID-IMMU1698>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 103.Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J. 1998;332(Pt 3):593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Prasad P, Yanagihara AA, Small-Howard AL, Turner H, Stokes AJ. Secretogranin III directs secretory vesicle biogenesis in mast cells in a manner dependent upon interaction with chromogranin A. J Immunol. 2008;181:5024–5034. doi: 10.4049/jimmunol.181.7.5024. [DOI] [PubMed] [Google Scholar]
- 105.Hammel I, Lagunoff D, Galli SJ (2010) Regulation of secretory granule size by the precise generation and fusion of unit granules. J Cell Mol Med (in press) [DOI] [PMC free article] [PubMed]
- 106.Grimberg E, Peng Z, Hammel I, Sagi-Eisenberg R. Synaptotagmin III is a critical factor for the formation of the perinuclear endocytic recycling compartment and determination of secretory granules size. J Cell Sci. 2003;116:145–154. doi: 10.1242/jcs.00186. [DOI] [PubMed] [Google Scholar]
- 107.Schafer T, Starkl P, Allard C, Wolf RM, Schweighoffer T. A granular variant of CD63 is a regulator of repeated human mast cell degranulation. Allergy. 2010;65:1242–1255. doi: 10.1111/j.1398-9995.2010.02350.x. [DOI] [PubMed] [Google Scholar]
- 108.Hauswirth AW, Escribano L, Prados A, Nunez R, Mirkina I, Kneidinger M, Florian S, Sonneck K, Vales A, Schernthaner GH, Sanchez-Munoz L, Sperr WR, Buhring HJ, Orfao A, Valent P. CD203c is overexpressed on neoplastic mast cells in systemic mastocytosis and is upregulated upon IgE receptor cross-linking. Int J Immunopathol Pharmacol. 2008;21:797–806. doi: 10.1177/039463200802100404. [DOI] [PubMed] [Google Scholar]
- 109.Dvorak AM. Ultrastructural studies of human basophils and mast cells. J Histochem Cytochem. 2005;53:1043–1070. doi: 10.1369/jhc.5R6647.2005. [DOI] [PubMed] [Google Scholar]
- 110.Dvorak AM, Schleimer RP, Lichtenstein LM. Morphologic mast cell cycles. Cell Immunol. 1987;105:199–204. doi: 10.1016/0008-8749(87)90068-2. [DOI] [PubMed] [Google Scholar]
- 111.Xiang Z, Block M, Lofman C, Nilsson G. IgE-mediated mast cell degranulation and recovery monitored by time-lapse photography. J Allergy Clin Immunol. 2001;108:116–121. doi: 10.1067/mai.2001.116124. [DOI] [PubMed] [Google Scholar]
- 112.Tanzola MB, Robbie-Ryan M, Gutekunst CA, Brown MA. Mast cells exert effects outside the central nervous system to influence experimental allergic encephalomyelitis disease course. J Immunol. 2003;171:4385–4391. doi: 10.4049/jimmunol.171.8.4385. [DOI] [PubMed] [Google Scholar]
- 113.Musch W, Wege AK, Mannel DN, Hehlgans T. Generation and characterization of alpha-chymase-Cre transgenic mice. Genesis. 2008;46:163–166. doi: 10.1002/dvg.20378. [DOI] [PubMed] [Google Scholar]
- 114.Scholten J, Hartmann K, Gerbaulet A, Krieg T, Muller W, Testa G, Roers A. Mast cell-specific Cre/loxP-mediated recombination in vivo. Transgenic Res. 2008;17:307–315. doi: 10.1007/s11248-007-9153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]