Abstract
Chs5p is a component of the exomer, a coat complex required to transport the chitin synthase Chs3p from the trans-Golgi network to the plasma membrane. The Chs5p N-terminal region exhibits fibronectin type III (FN3) and BRCT domains. FN3 domains are present in proteins that mediate adhesion processes, whereas BRCT domains are involved in DNA repair. Several fungi—including Schizosaccharomyces pombe, which has no detectable amounts of chitin—have proteins similar to Chs5p. Here we show that the FN3 and BRCT motifs in Chs5p behave as a module that is necessary and sufficient for Chs5p localization and for cargo delivery. The N-terminal regions of S. cerevisiae Chs5p and S. pombe Cfr1p are interchangeable in terms of Golgi localization, but not in terms of exomer assembly, showing that the conserved function of this module is protein retention in this organelle and that the interaction between the exomer components is organism-specific.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0596-z) contains supplementary material, which is available to authorized users.
Keywords: Yeast, Cell wall, Chitin, Golgi, Vesicular trafficking
Introduction
One of the least studied steps of secretion is protein trafficking between the trans-Golgi network (TGN) and the cell surface. The chitin synthase Chs3p, which produces chitin at the neck between mother and daughter cells and the lateral cell wall [1, 2], is a valuable model to investigate vesicular traffic. Chs3p is synthesized constitutively at the endoplasmic reticulum, its exit from this organelle being facilitated by the specific chaperone Chs7p [3, 4]. In the TGN, Chs3p is loaded in a subset of secretory vesicles coated by a protein complex termed the exomer ([5–7]; see below) and is delivered to the cell surface. Chs3p localizes predominantly to the mother-bud neck; there, Chs4p anchors the chitin synthase to the septin ring, activates it biochemically, and modulates its endocytic turnover [8–12]. After endocytosis Chs3p is not degraded in the vacuole; instead, it remains in a subpopulation of internal stores/endosomes termed chitosomes [13], from where it can be recycled to become reincorporated in the plasma membrane. Chs3p is one of the best-characterized cargoes that cycle between the plasma membrane and endosomes.
The term exomer refers to a S. cerevisie complex of proteins that form a vesicle coat required for the transport of certain proteins from the Golgi apparatus to the cell surface [7]. The exomer is composed of Chs5p, Chs6p, Bch1p, Bch2p, and Bud7p [5–7]. Chs5p acts as a scaffold to which Chs6p, Bch1p, Bch2p, and Bud7p (termed generically the ChAPs, from Chs5p-Arf1p-binding proteins) bind. All the exomer proteins localize in the TGN [5, 6]. The cargo specificity is provided by the ChAPs; deletion of CHS6 leads to a defect in chitin synthesis because of a defect in the transport of Chs3p to the plasma membrane [14, 15]; deletion of BUD7 leads to a defect in polarity [16], and deletion of BCH1 and BUD7 leads to a mating defect because of a missorting of Fus1p [17]. The role of BCH2 has not been established because in the analyses performed bch2Δ mutants showed no evident phenotype [5, 6]. Some ChAPs could have redundant roles for cargo selection since a bch1Δbud7Δ strain phenocopies a chs6Δ mutant in terms of its defect in chitin synthesis [5, 6]. According to its role as a scaffold for the binding of the ChAPs to the TGN vesicles, deletion of Chs5p results in disruption of the complex, and chs5Δ mutants show defects in chitin synthesis, the budding pattern, and mating [18, 19]. chs5Δ mutants show additional phenotypes, some of which might be due to their defect in the transport of Crh2p, a transglycosylase involved in cell wall synthesis [20].
The Chs5 N-terminal region contains two contiguous domains, FN3 and BRCT (see Fig. 1), which are only 1–5 residues apart (depending on the tool used to perform the motif search). FN3 domains are present in proteins involved in cell-surface binding processes, and their presence is rare in yeast [21]. They are protein regions of approximately 100 amino acids that exhibit a common tertiary structure but share little primary sequence similarity. They have seven β strands forming a sandwich of two antiparallel β sheets (one containing three strands termed A, B, and E, and the other containing four strands termed C′, C, F, and G [21, 22]). The alignment of FN3 motifs found in different proteins (SMART database [23]) allows the identification of a few conserved residues, which are present in S. cerevisiae Chs5p (Fig. 1) and in S. pombe Cfr1p (a protein with a structure similar to that of Chs5p [24]). Downstream from the FN3 domain there is a BRCT domain (Fig. 1). BRCTs are regions of 85–95 amino acids, characterized by their tertiary structure, that mediate protein-protein interactions and participate in DNA repair and in checkpoints [25, 26]. They comprise four β strands forming a β sheet surrounded by three α helices. The alignment of BRCT motifs in the SMART database leads to the identification of a few conserved residues that are present in most of these domains. Analysis of the three-dimensional structure predicts that some of the conserved residues cannot be altered without perturbing the functionality of the BRCT domains. In particular, a tryptophan located in helix α3, and a glycine located in a loop/turn connecting α1 and β2 are predicted to be important for the structure of the motif [25, 26]. Other residues relevant for the functionality of BRCT domains have been identified in analyses of cancer-associated mutations [25, 27].
Fig. 1.
Primary structure (upper line) and secondary structure (lower line E β strands; H α helices; C coil) corresponding to the Chs5p region comprising amino acids 76–255. The FN3 or BRCT domains are underlined. Amino acids forming β strands are depicted in red, and amino acids corresponding to α helices are in blue. The strands and helices have been named according to the nomenclature established for each domain. The amino acids missing in the Chs5ΔFN3i and Chs5ΔBRCTi proteins are double-underlined. Asterisks mark the conserved residues that were mutated in the Chs5W98C, Chs5PY143KL, Chs5W244H, and Chs5GA200TR proteins
In this work we analyzed the role of the FN3 and BRCT domains in the exomer component Chs5p. We found that both motifs are required for Chs5p localization to the Golgi, and that a truncated protein bearing only these domains is able to localize properly and to support Chs3p delivery to the cell surface. Mutation of some of the conserved residues in the FN3 and BRCT domains reduces Chs5p functionality. Finally, the region of Cfr1p containing the FN3 and BRCT domains localizes to the Golgi in both the fission and the budding yeast, showing that a conserved function of this module is to localize proteins to the Golgi. However, the S. pombe truncated protein is not able to support exomer assembly, and consequently Chs3p delivery to the cell surface, showing that the interaction between the exomer components is specific for each organism.
Materials and methods
Strains and growth conditions
The yeast strains used in this study are derivatives of the α-131-20, L-839 [18], and W303-1A strains. Cells were grown in batches at 32°C in Difco yeast nitrogen base without amino acids (YNB-aa) 6.7 g/l, containing 2% glucose and the required supplements, or in YEPD (1% yeast extract, 2% Bacto Peptone, 2% dextrose). For solid media, agar was added at 2%. Calcofluor (Blankophor, Bayer) resistance was tested by a plate assay on SD without uracil (SD-URA) medium buffered with 50 mM potassium biphthalate, pH 6.2, as described [18].
Molecular and genetic manipulations
Plasmid KS+CHS5(SmaIATG NotISTOP), carrying a SmaI restriction site before the initial ATG and a NotI site before the stop codon, was used as a template to perform site-directed mutagenesis with a previously described method [28]. DNA sequencing was used to confirm the accuracy of the sequence in the alleles constructed. Then, the mutated DNA fragments were cloned into a modified pRS316 vector [29] lacking the NotI restriction site of the polylinker. To assess the level and localization of the mutated proteins, the HA epitope or the GFP was cloned as NotI DNA fragments into the NotI site at the C-terminal end of the protein. Deletions inside the FN3 and BRCT domains were produced as follows; ClaI sites were introduced between the codons coding for proline at amino acid position 124 and for asparagine at position 125, and between the codons for tyrosine at position 143 and for glutamic acid at position 144. Digestion with ClaI and plasmid religation produced the chs5 ΔFN3i allele. The chs5 ΔBRCTi allele was produced by digestion with the MluI enzyme and religation of a plasmid in which MluI restriction sites had been introduced between the codons for leucine at position 196 and for serine at position 197, and between the codons for asparagine at position 235 and for asparagine at position 236. To obtain the chs5 W98C mutant, the GCATGG sequence, coding for alanine at position 97 and tryptophan at position 98, was changed to GCATGC (which codes for alanine and cysteine and is a SphI restriction site). The chs5 PY143KL allele was produced by changing the CCATAC sequence, coding for proline and tyrosine at positions 142 and 143, respectively, by AAGCTT, coding for lysine and leucine and susceptible to digestion by HindIII. The chs5 W244H mutant was obtained by replacing the TGGGTG sequence (coding for tryptophan at position 244 and for valine at position 245) by CACGTG, which codes for histidine and valine and is susceptible to digestion by PmaCI. The chs5 GA200TR allele was produced by replacing the GGGGCG sequence, coding for glycine and alanine at positions 200 and 201, respectively, by ACGCGT, coding for threonine and arginine and susceptible to digestion by MluI. A truncated Chs5ΔCT protein, lacking the two C-terminal thirds of the protein, was produced by digestion with NotI and religation of the KS+CHS5(SmaIATG NotI1009 NotISTOP) plasmid, in which a NotI restriction site was inserted after the codon for the phenylalanine at position 274, which is 23 amino acids downstream from the BRCT domain. A Cfr1ΔCT truncated protein was constructed by digesting with NotI and religating a plasmid bearing a cfr1 + gene in which NotI sites had been introduced 60 bp downstream from the BRCT domain and before the stop codon. A plasmid expressing the Cfr1p FN3 and BRCT domains in S. cerevisiae was constructed by replacing the CHS5 ORF by a SmaI/NotI DNA fragment coding for the Cfr1p N-terminal end. This plasmid was used as a backbone to construct a plasmid expressing a Chs5:Cfr1 chimeric protein by cloning the CHS5 sequence coding for the C-terminal end of the protein as a NotI/NotI DNA fragment.
Protein techniques
Western blotting was performed as described [30]. To perform the electrophoresis, 4–12% gradient polyacrylamide gels and MES SDS buffer (NuPAGE, Invitrogen) were used. The α-HA (12CA5; Roche) and α-Tubulin (cloneB-5-1-2, SIGMA) antibodies were used at 1:5,000 dilutions.
Microscopy
Images were captured with a Leica DM RXA microscope equipped with a Photometrics Sensys CCD camera, using the Qfish 2.3 program, and processed using the Adobe 7.0 program. To analyze the localization of the sites of active chitin synthesis, cells were incubated in SD-URA medium buffered with 50 mM potassium biphthalate at pH 6.2 for 3 h in the presence of 50 μg/ml of Calcofluor.
Chitin measurement
The amount of chitin was determined in at least three independent experiments as described previously [31].
Brefeldin A treatment
Cells were treated with 25 μg/ml Brefeldin A (SIGMA B7651) for 15 min at 28ºC.
Results
The FN3 and BRCT domains are necessary and sufficient for Chs5p localization in the Golgi
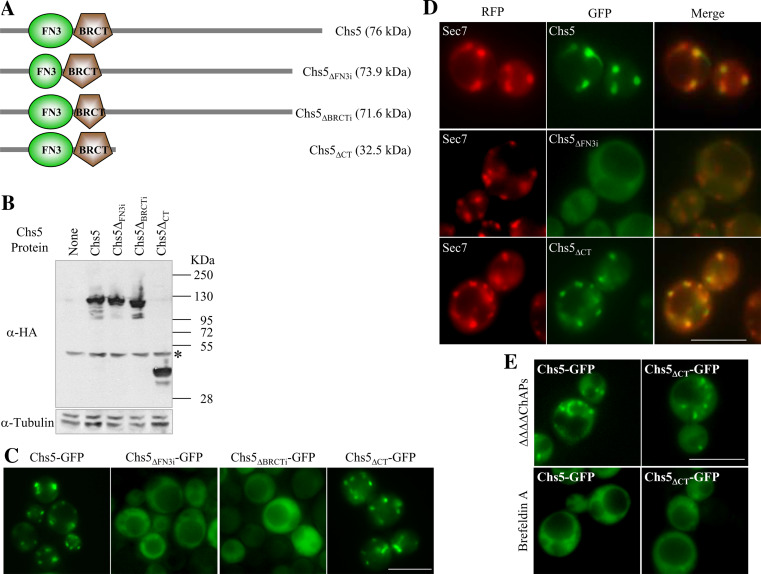
Different motif-searching programs detected the presence of FN3 and BRCT domains in the exomer component Chs5p (see Figs. 1, 2a). The presence of these domains in a Golgi protein involved in cell wall synthesis is intriguing, and we were prompted to uncover the function that each of the domains might play in the protein. Taking into account that tertiary structure is essential for the functionality of FN3 and BRCT domains, and that there is virtually no separation between these domains in Chs5p (Fig. 1), it was possible that eliminating one domain would interfere with the structure of the other. In order to overcome this problem, we produced mutant proteins in which 19 amino acids internal to the FN3 domain (Chs5ΔFN3i; “Materials and methods”; Fig. 2a) or 40 amino acids internal to the BRCT domain (Chs5ΔBRCTi; “Materials and methods”; Fig. 2a) were deleted. These deletions are 53 amino acids apart from each other. Additionally, we produced a truncated protein (Chs5ΔCT; “Materials and methods”; Fig. 2a) lacking the 397 C-terminal amino acids. Western blot analyses showed that all the truncated proteins were expressed in the cells (Fig. 2b). Observation of the GFP-fused control and truncated Chs5 proteins under a fluorescence microscope revealed that the Chs5ΔFN3i and the Chs5ΔBRCTi proteins were dispersed throughout the cytoplasm in 100% of the cells, while the localization of the Chs5ΔCT protein was similar to that of Chs5p (Fig. 2c). These results were obtained using the W303 and α-131-20 genetic backgrounds (Fig. 2 and results not shown). Sec7p is a Golgi-resident guanine nucleotide exchange factor for the Arf1 GTPase. Observation of an RFP-tagged Sec7 protein [32] in cells expressing GFP-fused Chs5 proteins showed that the internal deletions of the domains had an effect on Chs5p localization, but did not produce a general alteration in the TGN (central row panels in Fig. 2d and results not shown). Additionally, co-localization analyses confirmed that the presence of the FN3 and BRCT domains together was sufficient for protein localization at the Golgi (88% of RFP-Sec7 dots co-localized with Chs5-GFP dots, n = 268; 84% of RFP-Sec7 dots co-localized with Chs5ΔCT-GFP dots, n = 209). In order to determine whether the localization of the Chs5ΔCT-GFP protein in the Golgi was direct or mediated by its interaction with the ChAps, we analyzed its distribution in a ΔΔΔΔChAps strain, deleted for CHS6, BUD7, BCH1 and BCH2 [6]. We found that both Chs5p and Chs5ΔCTp exhibited a discrete localization as cytoplasmic dots in more than 90% of the cells, showing that the truncated protein localized to the Golgi even in the absence of the ChAps (Fig. 2e, upper panels). It is known that Arf1-dependent GTPase activity is required for Chs5p localization to the Golgi [6, 7]. In order to determine whether Chs5ΔCT also depended on this activity for its localization, we used Brefeldin A, an inhibitor of the GTP-exchange factors for Arf1p. Although the use of this drug has the caveat that the Golgi itself disperses with this treatment, Wang et al. [7] used it to show the dependence of the exomer assembly on Arf1p, and Trautwein et al. [6] used it to show that Chs5p and the ChAPs depend on Arf1p-GTP for Golgi localization. In more than 70% of the cells, both Chs5p and Chs5ΔCTp were dispersed throughout the cytoplasm (Fig. 2e, lower panels). The rest of the cells exhibited exaggerated Golgi structures (supplemental material, figure S1) that were not observed after 60 min of incubation in the presence of the drug. These results strongly suggested that the full-length and the C-terminal truncated Chs5 proteins had the same requirements for their localization, and confirmed that the presence of the FN3 and BRCT domains was sufficient for Chs5p localization in the Golgi.
Fig. 2.
The FN3 and BRCT domains are necessary and sufficient for localization of Chs5p to the Golgi. a Schematic representation of Chs5p and the indicated mutated proteins. The predicted molecular weight is given in kDa. b Western blots showing the level of the indicated HA-tagged Chs5 proteins (upper blot) compared to the level of tubulin (lower blot). kDa indicates the size of the proteins in the molecular weight standard. The asterisk marks an unspecific band recognized by the α-HA antibody. c Fluorescence micrographs of cells carrying the indicated Chs5 proteins fused to the GFP. d Fluorescence micrographs showing the localization of Sec7-RFP and the indicated GFP-fused Chs5 variants. e Localization of GFP-fused Chs5 and Chs5ΔCT proteins in a strain deleted for the four ChAp proteins (upper panels) or in cells treated with the Sec7 inhibitor Brefeldin A (lower panels). Bar 10 μm
The FN3 and BRCT domains are necessary and sufficient for chitin synthesis and Chs3p delivery to the cell surface
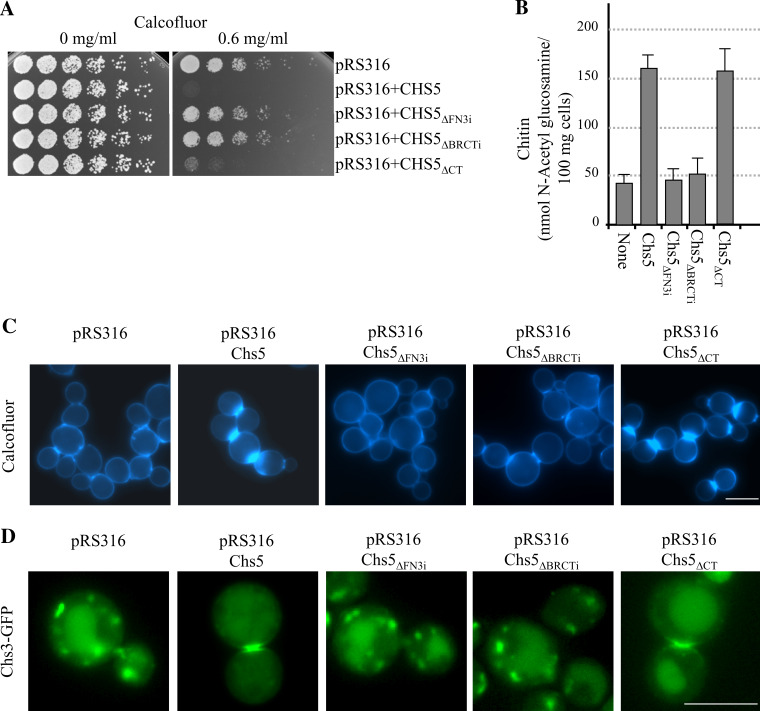
Wild-type budding yeast strains are sensitive to Calcofluor and exhibit defective growth in media supplemented with this chitin-binding dye [33]. However, strains with a defect in the Chs3p-dependent chitin synthesis pathway, such as chs3Δ, chs5Δ, and chs6Δ, are resistant to Calcofluor [14]. We tested the capacity of the mutant Chs5 proteins to complement the resistance to Calcofluor exhibited by the chs5Δ mutant [18]. Sensitivity to Calcofluor would show that the altered protein was functional, while resistance would show that it was not. We found that, in agreement with its altered localization, neither the Chs5ΔFN3i nor the Chs5ΔBRCTi proteins were able to complement the chs5Δ mutant (Fig. 3a). In contrast, the Chs5ΔCT protein was able to complement the resistance to Calcofluor of the chs5Δ strain (Fig. 3a). The same result was obtained in the W303, α-131-20 and L-839 genetic backgrounds, although in the W303 background higher Calcofluor concentrations (up to 1 mg/ml) were needed to observe the complementation of the chs5Δ mutant by the plasmid bearing the CHS5 gene (not shown). In order to obtain more accurate information about the functionality of the truncated proteins, we measured the amount of chitin in the same strains. We found that the level of chitin in cells bearing the Chs5ΔFN3i or the Chs5ΔBRCTi proteins was as low as that found in cells bearing an empty plasmid, while the level of the polymer in cells bearing the control Chs5 or the truncated Chs5ΔCT proteins was similar (Fig. 3b). In some mutants, chitin is abnormally distributed when the synthesis of this polymer is stimulated by incubating the cells with low amounts of Calcofluor [34]. We analyzed the distribution of the sites of active chitin synthesis by incubating the cells in the presence of a sublethal concentration of Calcofluor (50 μg/ml). As shown in Fig. 3c, the chs5Δ cells transformed with an empty plasmid or with plasmids expressing the Chs5ΔFN3i or the Chs5ΔBRCTi proteins only showed a weak staining around the cells. In contrast, chs5Δ cells expressing Chs5p or Chs5ΔCTp from centromeric plasmids exhibited strong fluorescence; in both cases this fluorescence was delimited to the neck between the mother and daughter cells. The same results were obtained in the W303, α-131-20, and L-839 genetic backgrounds (Fig. 3c and results not shown). These results showed that the Chs5ΔFN3i and the Chs5ΔBRCTi proteins were not able to promote chitin synthesis in response to the Calcofluor treatment, whereas Chs5ΔCT was able to do so. Since Chs5p is an exomer component, and since the exomer is involved in the transport of Chs3p to the membrane, we transformed the strains under study with a centromeric plasmid bearing a GFP-tagged Chs3 protein. We observed that Chs3p was retained in internal vesicles in 100% of the chs5Δ cells bearing the empty plasmid and the plasmids expressing the Chs5ΔFN3i and the Chs5ΔBRCTi proteins (n = 100–150; panels in Fig. 3d show a cell with the most representative localization for each strain). The distribution of Chs3-GFP in cells expressing Chs5p and Chs5ΔCTp was similar; in the case of cells bearing the pRS316Chs5 plasmid, Chs3p was observed at the mother-bud neck in 79% of the budded cells, in internal vesicles in 19% of the cells, and in both vesicles and the mother-bud neck in 2% of the cells (n = 126; Fig. 3d). In cells expressing the Chs5ΔCT truncated protein, Chs3p was observed at the mother-bud neck (75% of the cells, n = 108; Fig. 3d), in internal vesicles (22% of the cells), and in the neck and internal vesicles (3% of the cells). Since Chs5p is also required for the transport of Fus1p (a protein required for cell fusion during mating) and some unknown cargo required for polarity, we analyzed the capacity of the mutated Chs5 proteins to complement the mating and polarity defects of the chs5Δ mutant. We found that the ratios of trilobated to bilobated zygotes were 0.29 ± 0.02, 1.90 ± 0.1, 0.25 ± 0.02, 0.3 ± 0.03, and 1.95 ± 0.1 for the chs5Δ mutant transformed with the empty vector or the vector expressing the Chs5, Chs5ΔFN3i, Chs5ΔBRCTi, and Chs5ΔCT proteins, respectively. Additionally, Calcofluor staining allowed us to observe that the budding pattern was axial for the chs5Δ mutant transformed with the plasmid expressing the Chs5 and Chs5ΔCT proteins (supplemental material, figure S2). These results show that, at least for the assays performed and under the experimental conditions used, the Chs5ΔCT truncated protein was functional, whereas Chs5p with deletions in the FN3 or the BRCT domains was not.
Fig. 3.
The FN3 and BRCT domains are necessary and sufficient for the proper regulation of chitin synthesis. The Chs5 proteins used in this figure were untagged. a A total of 3 × 104 cells and serial 1:4 dilutions from a chs5Δ strain bearing the indicated plasmids were spotted onto buffered SD-URA plates supplemented with the indicated amounts of Calcofluor and incubated for 2 days at 32ºC. b Amount of chitin in a chs5Δ strain bearing centromeric plasmids producing the indicated Chs5 proteins. c Distribution of chitin in the same strains as in b incubated in the presence of 50 μg/ml of Calcofluor for 3 h. d Localization of a GFP-fused Chs3 protein in a chs5Δ strain bearing the indicated plasmids. Cells in the micrographs show the most representative Chs3p distribution for each strain. Bar 10 μm
Conserved residues in the FN3 and BRCT domains contribute to the localization/functionality of Chs5p
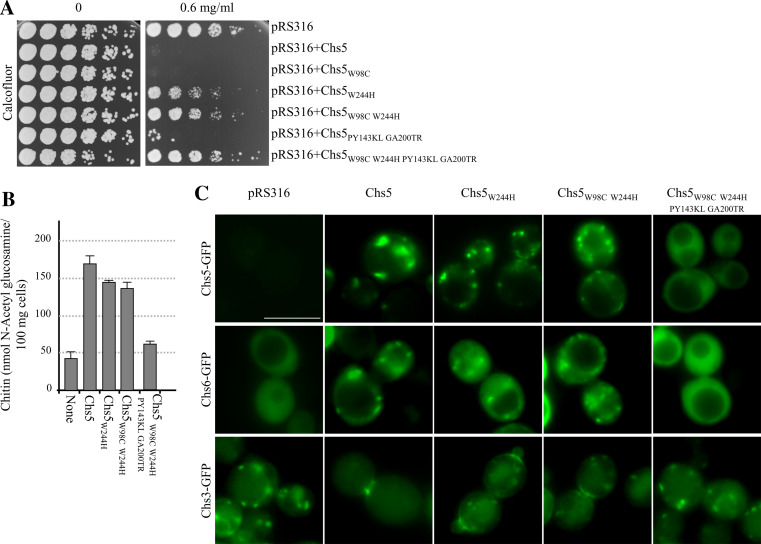
In order to analyze whether some of the conserved residues that are present in the FN3 and BRCT domains are relevant for the localization/function of Chs5p, we mutated the tryptophan at amino acid position 98 (in the FN3 domain; Chs5W98C protein) and/or the tryptophan at position 244 (in the BRCT domain; Chs5W244H and Chs5W98C W244H proteins), the proline and tyrosine at positions 142 and 143 (FN3 domain), and the glycine at position 200 and the alanine at position 201 (BRCT domain; Chs5PY143KL GA200TR protein), or all six amino acids (Chs5W98C W244H PY143KL GA200TR protein. See “Materials and methods” and Fig. 1). Initially, we analyzed the functionality of the mutated proteins by assessing their capacity to confer Calcofluor sensitivity to a chs5Δ mutant when expressed in centromeric plasmids. As shown in Fig. 4a, the degree of complementation conferred by the different mutated proteins was in the following order: the Chs5W98C protein was the most functional, followed by the Chs5PY143KL GA200TR protein, the Chs5W244H protein, the Chs5W98C W244H protein, and the Chs5W98C W244H PY143KL GA200TR protein, in whose presence the cells were almost as resistant to Calcofluor as the chs5Δ mutant transformed with an empty plasmid. Measurement of the chitin content confirmed that the level of resistance to Calcofluor was correlated with a decrease in the amount of this polymer in the cell wall of the strains under study (Fig. 4b and results not shown). Fluorescence microscopy analysis of cells bearing the mutated proteins fused to the GFP allowed us to correlate the degree of resistance to Calcofluor with a decrease in the signal observed in the Golgi and an increase in the diffuse fluorescence throughout the cytoplasm (Fig. 4b and results not shown). We next wished to evaluate the capacity of the mutated proteins to allow exomer assembly by observing the distribution of a GFP-fused Chs6 protein. We were unable to detect any specific signal when this fused protein was expressed from a centromeric plasmid in a WT strain (results not shown). When expressed from a multicopy plasmid, 30–50% of WT cells (depending on the culture conditions) exhibited a diffuse fluorescence, while 70–50% of the cells exhibited a discrete fluorescence in cytoplasmic dots (results not shown). When this protein was expressed in chs5Δ cells bearing centromeric plasmids that expressed different Chs5 proteins, the percentage of cells with discrete fluorescent dots was as follows: 0% in cells bearing the empty pRS316 vector and the Chs5W98C W244H PY143KL GA200TR protein (n = 150–200; see Fig. 4d); 59% in cells expressing Chs5p (n = 127), 48% in cells expressing the Chs5W244H protein (n = 143), and 44% in cells expressing the Chs5W98C W244H protein (n = 194). Finally, we evaluated the capacity of the Chs5 variants to deliver Chs3p to the cell surface by observing a GFP-fused Chs3 protein. When the chs5Δ cells bore the pRS316 empty vector or a plasmid expressing the Chs5W98C W244H PY143KL GA200TR mutant protein, 100% of the cells exhibited a discrete fluorescence in cytoplasmic dots (n = 150–200; see Fig. 4d); when the cells expressed the native Chs5 protein, Chs3p was observed at the mother-bud neck (71% of the cells), in internal vesicles (21% of the cells), and in both vesicles and the mother-bud neck (8% of the cells; n = 154, Fig. 4d). In chs5Δ mutants expressing the Chs5W244H mutant protein, Chs3p was observed at the mother-bud neck (23% of the cells), in internal vesicles (35% of the cells), and in the neck and internal vesicles (42% of the cells; n = 190). When the chs5Δ cells expressed the Chs5W98C W244H mutant protein, Chs3p was observed at the mother-bud neck (16% of the cells), in internal vesicles (41% of the cells), and in the neck and internal vesicles (43% of the cells; n = 165). These results showed that a decreased capacity of the Chs5 mutant proteins to localize in the Golgi was correlated with a low capacity to assemble the exomer, to deliver Chs3p to the cell surface, and, consequently, to support chitin synthesis. Additionally, these results confirmed that both domains participate in the localization of Chs5p in the TGN and that small alterations in these domains cause a decrease in Chs5p functionality.
Fig. 4.
Conserved residues in the FN3 and BRCT domains are relevant for Chs5p localization and functionality. a A total of 3 × 104 cells and serial 1:4 dilutions from a chs5Δ strain bearing the indicated plasmids were spotted onto buffered SD-URA plates supplemented with the indicated amounts of Calcofluor and incubated for 2 days at 32ºC. The Chs5 proteins were untagged. b Amount of chitin in a chs5Δ strain bearing centromeric plasmids producing the indicated untagged Chs5 proteins. c Localization of the indicated Chs5 proteins fused to the GFP in chs5Δ cells (upper row panels) or localization of GFP-fused Chs6p (middle row panels) and GFP-fused Chs3p (lower row panels) in chs5Δ cells expressing the indicated untagged Chs5 proteins from centromeric plasmids. Cells in the micrographs show the most representative distribution of the GFP-fused proteins in each strain. Bar 10 μm
The Cfr1p FN3 and BRCT domains are sufficient to localize the protein to the Golgi in both Schizosaccharomyces pombe and Saccharomyces cerevisiae
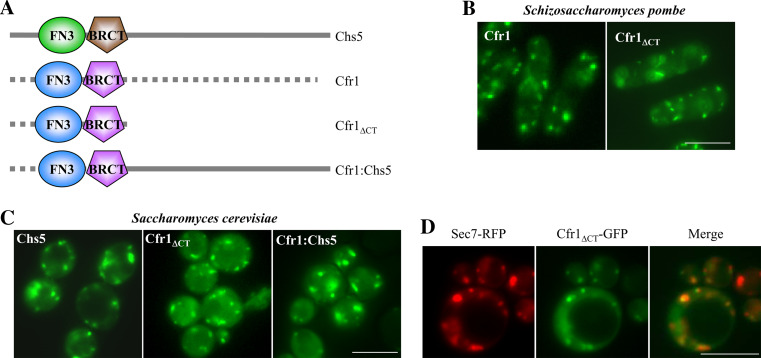
Cfr1p is a S. pombe protein required for mating that contains FN3 and BRCT domains in its N-terminal region [24]. In order to determine whether the FN3 and BRCT domains in the S. pombe Cfr1 protein were sufficient to localize it to the Golgi, as it occurs with the budding yeast Chs5p, we constructed a GFP-tagged Cfr1 protein lacking its C-terminal end (see “Materials and methods” and Fig. 5a). We found that in S. pombe the localization of the truncated and the full-length protein was similar (Fig. 5b). Next, we expressed in S. cerevisiae the GFP-fused Cfr1ΔCT truncated protein and a Cfr1:Chs5 chimeric protein in which the N-terminal region of Cfr1p was fused to the C-terminal region of Chs5p (see “Materials and methods” and Fig. 5a). When expressed in the budding yeast, most of the signal corresponding to both GFP-fused Cfr1ΔCT and Cfr1:Chs5 proteins localized as florescent dots in the cytoplasm, although some diffuse fluorescence was observed in the cytoplasm (Fig. 5c). Co-localization of the green dots with the TGN marker Sec7p fused to the RFP occurred in 88% of the dots (n = 173) in chs5Δ cells expressing the Chs5-GFP protein, in 81% of the dots (n = 139) in chs5Δ cells expressing the Cfr1ΔCT-GFP protein, and in 83% of the dots (n = 123) in chs5Δ cells expressing the Cfr1:Chs5-GFP protein (Fig. 5d), confirming that the proteins containing the Cfr1p FN3 and BRCT domains localized to the Golgi.
Fig. 5.
The amino terminal end of Cfr1p directs protein localization to the Golgi in both Schizosaccharomyces pombe and Saccharomyces cerevisiae. a Schematic representation of S. cerevisiae Chs5p, S. pombe Cfr1p, a truncated Cfr1 protein missing the C-terminal end of the protein (Cfr1ΔCT), and a chimera bearing the N-terminal region of Cfr1p containing the FN3 and BRCT domains, and the C-terminal region of Chs5p (Cfr1:Chs5). b Localization of the full-length Cfr1 and the Cfr1ΔCT truncated proteins, fused to the GFP, in S. pombe. c Localization of the indicated proteins fused to the GFP in a chs5Δ S. cerevisiae strain. d Localization of Sec7-RFP and the C-terminal truncated Cfr1 protein in S. cerevisiae; the panel on the right shows a superimposition of the RFP and GFP images. Bar 10 μm
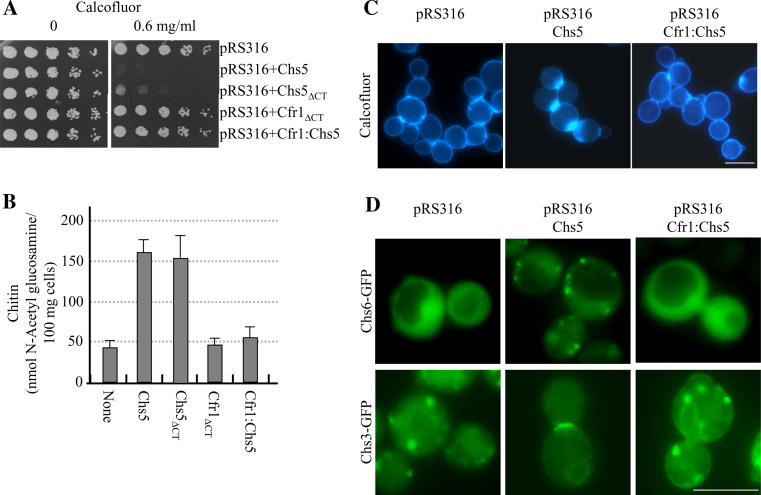
The Cfr1p FN3 and BRCT domains cannot regulate chitin synthesis in Saccharomyces cerevisiae
Upon finding that the FN3 and BRCT domains of Cfr1p were able to localize to the Golgi in S. cerevisiae, we wondered whether they were functional in the budding yeast; i.e., whether they were able to support exomer assembly and to regulate chitin synthesis. In order to address this question, we analyzed the capacity of the Cfr1ΔCT and Cfr1:Chs5 proteins to complement the defects of null chs5Δ mutants. We found that chs5Δ strains (in the W303 and the α-131-20 genetic backgrounds) bearing an empty vector or plasmids that expressed the Cfr1ΔCT or Cfr1:Chs5 proteins behaved similarly in terms of Calcofluor resistance (Fig. 6a) and chitin synthesis (Fig. 6b, c). These results showed that the N-terminal regions of Chs5p and Cfr1p are not interchangeable for chitin synthesis. In order to determine the reason for the inability of the Cfr1p N-terminal region to regulate this process, we analyzed the distribution of the exomer component Chs6p (fused to the GFP and expressed from a multicopy plasmid) and the chitin synthase Chs3p (fused to the GFP and expressed from a centromeric plasmid) in a chs5Δ strain transformed with an empty vector and the plasmids expressing the Chs5, Chs5ΔCT, Cfr1ΔCT, and Cfr1:Chs5 proteins. In the mutant strains bearing the empty plasmid, the fluorescence of the Chs6-GFP protein was dispersed throughout the cytoplasm and that of the GFP-Chs3 protein was observed in internal vesicles in 100% of the cells (n = 100–150; Fig. 6d). When the chs5Δ mutant cells expressed the full-length Chs5 protein, Chs6p was observed as discrete dots in the cytoplasm in 53% of the cells (n = 180), and Chs3p was observed at the mother-bud neck in 76% of the cells, in internal vesicles in 18% of the cells, and in both vesicles and the mother-bud neck in 6% of the cells (n = 174; Fig. 6d). When the chs5Δ mutant cells expressed the Chs5ΔCT protein, Chs6p was observed as discrete dots in the cytoplasm in 53% of the cells (n = 210), and Chs3p was observed at the mother-bud neck (75% of the cells), in internal vesicles (20% of the cells), and in the neck and internal vesicles (5% of the cells; n = 166). When the Cfr1ΔCT or the Cfr1:Chs5 proteins were expressed in the chs5Δ mutant, Chs6p was dispersed throughout the cytoplasm and, accordingly, Chs3p could not reach the cell surface in 100% of the cells (n = 150–180; Fig. 6d and results not shown). These results showed that the N-terminal region of Cfr1p was not able to promote chitin synthesis because it was not able to ensure Chs6p localization in the Golgi, and strongly suggested that the interaction between the scaffold and the other exomer components is organism-specific.
Fig. 6.
The amino terminal region of Cfr1p is not able to promote either chitin synthesis or Chs3p delivery. The Chs5 proteins used in this figure were untagged. a A total of 3 × 104 cells and serial 1:4 dilutions from a chs5Δ strain bearing the indicated plasmids were spotted onto buffered SD-URA plates supplemented with the indicated amounts of Calcofluor and incubated for 2 days at 32ºC. b Amount of chitin in a chs5Δ strain bearing centromeric plasmids producing the indicated Chs5 proteins. c Distribution of chitin in the same strains as in b, incubated in the presence of 50 μg/ml of Calcofluor for 3 h. d Localization of a GFP-fused Chs6 protein (upper panels) or Chs3 protein (lower panels) in strains bearing the indicated plasmids. Cells in the micrographs show the most representative distribution of the GFP-fused proteins in each strain. Bar 10 μm
Discussion
Vesicular transport is a major cellular activity that ensures protein trafficking between specific membrane-enclosed compartments. Protein transport from the ER to/from the cis- or intermediate-Golgi compartments has been subjected to detailed analysis ([35]; for a review see the FEBS letters special issue on the Golgi apparatus. Volume 583). Coat proteins participate in most of the vesicular traffic events in the cell and contribute to cargo-specificity. The TGN is viewed as a central station from which proteins are sorted and targeted to their final destination. One of the least well understood steps of secretion is the trafficking between the TGN and the plasma membrane. The exomer is a protein complex that forms a coat structure required for the transport of certain proteins from the TGN to the cell surface [5–7, 17–20, 36]. In this work we performed a structure-function analysis of Chs5p, a Golgi protein that is essential for the integrity of the complex and for the localization of the ChAps in the TGN [5–7]. Chs5p lacks all known sequences for retention at the Golgi apparatus, and the bioinformatic tools predict that it is a nuclear protein. It has been shown that E. coli-expressed Chs5p C-terminal end is able to interact with lipids in an overlay assay using phosphoinositide strips [7], suggesting that this part of the protein could mediate the recruitment of the protein to the Golgi membrane. Thus, it was expected that the N-terminal end of the protein, containing the FN3 and BRCT domains, would be responsible for the interaction with the ChAps and/or cargo proteins. In agreement with this notion, Chs5 amino acids 1–260 interact with Chs6p in a two-hybrid assay [7]. In order to uncover the function that those domains might play in the protein, we eliminated the central amino acids of each of them. We found that both domains had the same function in the protein, being required for Chs5p localization in the Golgi. This result was confirmed by mutating some of the conserved residues in the motifs. Substitution of tryptophan at position 244 (located on the α3 helix, at the center of a conserved hydrophobic pocket, and considered the hallmark of the BRCT domains) by a histidine had a significant impact on the functionality of the protein. Additional mutations in other conserved residues in the BRCT and FN3 domains reduced Chs5p functionality. These results confirmed that both domains are required for Chs5p localization in the Golgi and that the computer-predicted motifs are bona fide FN3 and BRCT domains. The amino-terminal end of the protein (amino acids 1–274) proved to be sufficient to promote protein localization to the TGN and to be functional in terms of exomer assembly and chitin synthesis. This is in agreement with the results of Wang et al. who found that amino acids 1–401 complemented the chitin synthesis defect of a chs5Δ strain [7]. The N-terminal region of the protein was able to localize to the TGN even in the absence of the ChAps, ruling out the possibility that these proteins might mediate the localization of the truncated Chs5 protein. Wang et al. [7] reported that the C-terminal end of Chs5p was able to interact with lipids in an overlay assay and suggested that this part of the protein facilitates the membrane recruitment of Chs5p. We have found that Chs5p is recruited to the Golgi even in the absence of its C-terminal end. Thus, either the C-terminal region of Chs5p does not interact with phosphoinositides in vivo or this interaction is not necessary for the localization of the protein to the Golgi. Also, it is possible that the lipid-Chs5p interaction plays some minor role in the functionality of the protein that cannot be detected under laboratory conditions. This does not mean that in nature, where environmental conditions are extreme, this part of the protein might have some relevance. It has been described that Chs5p runs more slowly than expected in polyacrylamide gels [36]; we found the same result for the Chs5ΔFN3i and the Chs5ΔBRCTi truncated proteins, but not for the Chs5ΔCT protein. Thus, the C-terminal region of the protein seems to undergo significant post-translational modifications. This region of the protein contains the 2 potential O-glycosylation sites, 5 out of the 6 potential N-glycosylation sites, the 2 potential SUMOylation sites, and 36 out of the 44 potential phosphorylation sites predicted by different programs (ExPASy Proteomics Server). However, the biological relevance of these modifications is difficult to establish, since this part of the protein seems to be dispensable for both localization and function.
Our results show that the FN3 and BRCT domains in Chs5p constitute a module that mediates protein localization to the Golgi, exomer assembly, and Chs3p delivery to the membrane. Both domains are required for this function since total deletion (results not shown) or small alterations in each domain abrogate the localization of the protein, exomer assembly, and chitin synthesis. This is a new function for the FN3 and BRCT domains that have been described to have roles in protein-protein interactions in the extracellular matrix and in DNA remodeling, respectively. One of the few exceptions is Ect2p, a guanine nucleotide exchange factor (GEF) for Rho A, in which the tandem BRCT domains are required for proper function of the protein during cytokinesis [37].
Proteins with consecutive FN3 and BRCT domains have only been found in fungi. With the exception of a hypothetical protein from the basidiomycete Filobasidiella neoformans, all other organisms bearing these proteins are ascomycetes. This suggests that that in these organisms these domains appeared as the consequence of a horizontal transmission of genetic information. The fact that in all the proteins bearing these domains the FN3 is always N-terminal with respect to the BRCT domain suggests that this organization is relevant for function; this is in agreement with the hypothesis that both motifs function as a single module, for which the three-dimensional structure provided by each domain is essential. With the exception of the S. cerevisiae Chs5 protein (involved in chitin synthesis and mating [18]) and the S. pombe Cfr1 protein (required for mating [24]), these fungal proteins have not been characterized. We found that the N-terminal region of Cfr1p was sufficient to localize the protein to the TGN in both S. pombe and in S. cerevisiae efficiently, showing that the conserved function of the module (formed by the FN3 and BRCT domains) is to localize proteins to this cellular organelle. However, although the pattern of localization of the Cfr1ΔCT or Cfr1:Chs5 proteins is similar to that of the Chs5W244H protein, the S. pombe domains are not able to promote exomer assembly or regulate chitin synthesis in S. cerevisiae, while the Chs5W244H mutant protein retains a high degree of functionality. These results are in agreement with our previous observation that Chs5p and Cfr1p can be expressed in S. pombe and S. cerevisiae, respectively, but they are not able to complement the phenotypes of the corresponding mutants ([24] and our unpublished results). We have found that the reason for the lack of complementation of the chitin synthesis defect in the S. cerevisiae chs5Δ mutant by Cfr1p is that the exomer component Chs6p is not able to localize in the Golgi and, therefore, Chs3p is not delivered to the cell surface. These results strongly suggest that the interaction between the scaffold and the other proteins in the exomer complex is specific to each organism. The N-terminal regions of Chs5p and Cfr1p are 46% identical and 63% similar. Probably the C-terminal region of the ChAps, which is responsible for their interaction with Chs5p [6], interacts with non-conserved residues in the Chs5/Cfr1 N-terminal region.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (TIFF 9,274 kb)
Supplementary material 2 (TIFF 3,236 kb)
Acknowledgments
We thank N. Skinner for language revision. We are indebted to B. Glick and to C. Roncero for the Sec7-RFP and the GFP-Chs3 plasmids, respectively, and to A. Spang for the strain deleted for the ChAPs. This work has been supported by grants BFU2007-61866 from the CICYT and GR231 from the Junta de Castilla y León, Spain. NdL was supported by a fellowship from the Spanish Ministry of Science. RMG and PBS were supported by postgraduate I3P fellowships from the Spanish council of research (CSIC).
Abbreviations
- TGN
Trans-Golgi network
- FN3
Fibronectin type III
- BRCT
Breast cancer susceptibility protein C-terminal domain
- ChAps
Chs5p-Arf1p-binding proteins
- GFP
Green fluorescent protein
- RFP
Red fluorescent protein
References
- 1.Shaw JA, Mol PC, Bowers B, Silverman SJ, Valdivieso MH, Durán A, Cabib E. The function of chitin synthases 2 and 3 in the Saccharomyces cerevisiae cell cycle. J Cell Biol. 1991;114:111–123. doi: 10.1083/jcb.114.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valdivieso MH, Mol PC, Shaw JA, Cabib E, Durán A. CAL1, a gene required for activity of chitin synthase 3 in Saccharomyces cerevisiae. J Cell Biol. 1991;114:101–109. doi: 10.1083/jcb.114.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trilla JA, Durán A, Roncero C. Chs7p, a new protein involved in the control of protein export from the endoplasmic reticulum that is specifically engaged in the regulation of chitin synthesis in Saccharomyces cerevisiae . J Cell Biol. 1999;145:1153–1163. doi: 10.1083/jcb.145.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kota J, Ljungdahl PO. Specialized membrane-localized chaperones prevent aggregation of polytopic proteins in the ER. J Cell Biol. 2005;168:79–88. doi: 10.1083/jcb.200408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchatjate S, Schekman R. Chs5/6 complex: a multiprotein complex that interacts with and conveys chitin synthase III from the trans-Golgi network to the cell surface. Mol Biol Cell. 2006;17:4157–4166. doi: 10.1091/mbc.E06-03-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trautwein M, Schindler C, Gauss R, Dengjel J, Hartmann E, Spang A. Arf1p, Chs5p and the ChAPs are required for export of specialized cargo from the Golgi. EMBO J. 2006;25:943–954. doi: 10.1038/sj.emboj.7601007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang CW, Hamamoto S, Orci L, Schekman R. Exomer: a coat complex for transport of select membrane proteins from the trans-Golgi network to the plasma membrane in yeast. J Cell Biol. 2006;174:973–983. doi: 10.1083/jcb.200605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeMarini DJ, Adams AE, Fares H, De Virgilio C, Valle G, Chuang JS, Pringle JR. A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J Cell Biol. 1997;139:75–93. doi: 10.1083/jcb.139.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trilla JA, Cos T, Durán A, Roncero C. Characterization of CHS4 (CAL2), a gene of Saccharomyces cerevisiae involved in chitin biosynthesis and allelic to SKT5 and CSD4 . Yeast. 1997;13:795–807. doi: 10.1002/(SICI)1097-0061(199707)13:9<795::AID-YEA139>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 10.Ono N, Yabe T, Sudoh M, Nakajima T, Yamada-Okabe T, Arisawa M, Yamada-Okabe H. The yeast Chs4p protein stimulates the trypsin-sensitive activity of chitin synthase 3 through an apparent protein-protein interaction. Microbiology. 2000;146:385–391. doi: 10.1099/00221287-146-2-385. [DOI] [PubMed] [Google Scholar]
- 11.Grabinska KA, Magnelli P, Robbins PW. Prenylation of Saccharomyces cerevisiae Chs4p affects chitin synthase III activity and chitin chain length. Eukaryot Cell. 2007;6:328–336. doi: 10.1128/EC.00203-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reyes A, Sanz M, Duran A, Roncero C. Chitin synthase III requires Chs4p-dependent translocation of Chs3p into the plasma membrane. J Cell Sci. 2007;120:1998–2009. doi: 10.1242/jcs.005124. [DOI] [PubMed] [Google Scholar]
- 13.Chuang JS, Schekman RW. Differential trafficking and timed localization of two chitin synthase proteins, Chs2p and Chs3p. J Cell Biol. 1996;135:597–610. doi: 10.1083/jcb.135.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roncero C, Valdivieso MH, Ribas JC, Durán A. Isolation and characterization of Saccharomyces cerevisiae mutants resistant to Calcofluor white. J Bacteriol. 1988;170:1950–1954. doi: 10.1128/jb.170.4.1950-1954.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziman M, Chuang JS, Tsung M, Hamamoto S, Schekman R. Chs6p-dependent anterograde transport of Chs3p from the chitosome to the plasma membrane in Saccharomyces cerevisiae . Mol Biol Cell. 1998;9:1565–1576. doi: 10.1091/mbc.9.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zahner JE, Harkins HA, Pringle JR. Genetic analysis of the bipolar pattern of bud site selection in the yeast Saccharomyces cerevisiae . Mol Cell Biol. 1996;16:1857–1870. doi: 10.1128/mcb.16.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barfield RM, Fromme JC, Schekman R. The exomer coat complex transports Fus1p to the plasma membrane via a novel plasma membrane sorting signal in yeast. Mol Biol Cell. 2009;20:4985–4996. doi: 10.1091/mbc.E09-04-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos B, Durán A, Valdivieso MH. CHS5, a gene involved in chitin synthesis and mating in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:2485–2496. doi: 10.1128/mcb.17.5.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos B, Snyder M. Specific protein targeting during cell differentiation: polarized localization of Fus1p during mating depends on Chs5p in Saccharomyces cerevisiae . Eukaryotic cell. 2003;2:821–825. doi: 10.1128/EC.2.4.821-825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Pena JM, Rodriguez C, Alvarez A, Nombela C, Arroyo J. Mechanisms for targeting of the Saccharomyces cerevisiae GPI-anchored cell wall protein Crh2p to polarised growth sites. J Cell Sci. 2002;115:2549–2558. doi: 10.1242/jcs.115.12.2549. [DOI] [PubMed] [Google Scholar]
- 21.Bateman A, Chothia C. Fibronectin type III domains in yeast detected by a hidden Markov model. Curr Biol. 1996;6:1544–1547. doi: 10.1016/S0960-9822(02)70765-3. [DOI] [PubMed] [Google Scholar]
- 22.Main AL, Harvey TS, Baron M, Boyd J, Campbell ID. The three-dimensional structure of the tenth type III module of fibronectin: an insight into RGD-mediated interactions. Cell. 1992;71:671–678. doi: 10.1016/0092-8674(92)90600-H. [DOI] [PubMed] [Google Scholar]
- 23.Schultz J, Milpetz F, Bork P, Ponting CP. SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci USA. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cartagena-Lirola H, Duran A, Valdivieso MH. The Schizosaccharomyces pombe cfr1 + gene participates in mating through a new pathway that is independent of fus1 + . Yeast. 2006;23:375–388. doi: 10.1002/yea.1361. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Morera S, Bates PA, Whitehead PC, Coffer AI, Hainbucher K, Nash RA, Sternberg MJ, Lindahl T, Freemont PS. Structure of an XRCC1 BRCT domain: a new protein-protein interaction module. EMBO J. 1998;17:6404–6411. doi: 10.1093/emboj/17.21.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huyton T, Bates PA, Zhang X, Sternberg MJ, Freemont PS. The BRCA1 C-terminal domain: structure and function. Mutat Res. 2000;460:319–332. doi: 10.1016/s0921-8777(00)00034-3. [DOI] [PubMed] [Google Scholar]
- 27.Williams RS, Chasman DI, Hau DD, Hui B, Lau AY, Glover JN. Detection of protein folding defects caused by BRCA1-BRCT truncation and missense mutations. J Biol Chem. 2003;278:53007–53016. doi: 10.1074/jbc.M310182200. [DOI] [PubMed] [Google Scholar]
- 28.Kunkel TA, Roberts JD, Zakour RA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-X. [DOI] [PubMed] [Google Scholar]
- 29.Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae . Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharifmoghadam MR, Valdivieso MH. The Schizosaccharomyces pombe Map4 adhesin is a glycoprotein that can be extracted from the cell wall with alkali but not with beta-glucanases and requires the C-terminal DIPSY domain for function. Mol Microbiol. 2008;69:1476–1490. doi: 10.1111/j.1365-2958.2008.06375.x. [DOI] [PubMed] [Google Scholar]
- 31.Bulawa CE, Slater M, Cabib E, Au-Young J, Sburlati A, Adair WL, Jr, Robbins PW. The S. cerevisiae structural gene for chitin synthase is not required for chitin synthesis in vivo. Cell. 1986;46:213–225. doi: 10.1016/0092-8674(86)90738-5. [DOI] [PubMed] [Google Scholar]
- 32.Losev E, Reinke CA, Jellen J, Strongin DE, Bevis BJ, Glick BS. Golgi maturation visualized in living yeast. Nature. 2006;441:1002–1006. doi: 10.1038/nature04717. [DOI] [PubMed] [Google Scholar]
- 33.Roncero C, Durán A. Effect of Calcofluor white and Congo red on fungal cell wall morphogenesis: in vivo activation of chitin polymerization. J Bacteriol. 1985;163:1180–1185. doi: 10.1128/jb.163.3.1180-1185.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanz M, Castrejon F, Duran A, Roncero C. Saccharomyces cerevisiae Bni4p directs the formation of the chitin ring and also participates in the correct assembly of the septum structure. Microbiology. 2004;150:3229–3241. doi: 10.1099/mic.0.27352-0. [DOI] [PubMed] [Google Scholar]
- 35.Lee MC, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- 36.Santos B, Snyder M. Targeting of chitin synthase 3 to polarized growth sites in yeast requires Chs5p and Myo2p. J Cell Biol. 1997;136:95–110. doi: 10.1083/jcb.136.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JE, Billadeau DD, Chen J. The tandem BRCT domains of Ect2 are required for both negative and positive regulation of Ect2 in cytokinesis. J Biol Chem. 2005;280:5733–5739. doi: 10.1074/jbc.M409298200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (TIFF 9,274 kb)
Supplementary material 2 (TIFF 3,236 kb)