Abstract
Termites thrive on dead plant matters with the aid of microorganisms resident in their gut. The gut microbiota comprises protists (single-celled eukaryotes), bacteria, and archaea, most of which are unique to the termite gut ecosystem. Although this symbiosis has long been intriguing researchers of both basic and applied sciences, its detailed mechanism remains unclear due to the enormous complexity and the unculturability of the microbiota. In the effort to overcome the difficulty, recent advances in omics, such as metagenomics, metatranscriptomics, and metaproteomics have gradually unveiled the black box of this symbiotic system. Genomics targeting a single species of the unculturable microbial members has also provided a great progress in the understanding of the symbiotic interrelationships among the gut microorganisms. In this review, the symbiotic system organized by wood-feeding termites and their gut microorganisms is outlined, focusing on the recent achievement in omics studies of this multilayered symbiotic system.
Keywords: Gut bacteria, Insect, Lignocellulose, Nitrogen fixation, Whole genome amplification
Introduction
Termites (order Isoptera) are social insects phylogenetically close to cockroaches [1, 2]. They inhabit temperate to tropical regions and play a key role in the global carbon cycle as decomposers [3]. Most termites ingest exclusively dead plant matters (wood, leaves, grass, and humus), and some species are known as destructive pests of woody buildings. As exceptions, live plants of introduced species such as agricultural crops and plantation trees are severely damaged by termites in some regions [4].
Nearly 3,000 species of termites have been described (http://vsites.unb.br/ib/zoo/catalog.html) and they are conventionally classified into two large groups: lower and higher termites. The lower termites are phylogenetically basal and comprise six families, i.e., Mastotermitidae, Termopsidae, Hodotermitidae, Kalotermitidae, Serritermitidae, and Rhinotermitidae. All of these are wood-feeders, except the members of Hodotermitidae, which forage mainly on dead grasses. In general, the lower termites possess a simply formed gut (Fig. 1a, c).
Fig. 1.
Termites and their intestinal tract. a The lower termites Coptotermes formosanus. b The interface (wood and humus)-feeding higher termites Termes comis. c The gut of C. formosanus. d The gut of T. comis. F foregut, M midgut. Bars = 1 mm
The higher termites are phylogenetically apical and have evolved probably from the Rhinotermitidae [5, 6]. The higher termites comprise only the family Termitidae, which is divided into four subfamilies, i.e., Macrotermitinae, Apicotermitinae, Termitinae, and Nasutitermitinae. These termites include wood-feeders, litter-feeders, grass-feeders, soil (humus)-feeders, and lichen-feeders, and they occasionally dominate terrestrial ecosystems in tropical and subtropical regions. The higher termites possess a highly compartmentalized gut (Fig. 1b, d), including highly alkaline gut segment(s), except the fungus-growing macrotermitine termites, of which the gut is simply shaped and resembles those of lower termites.
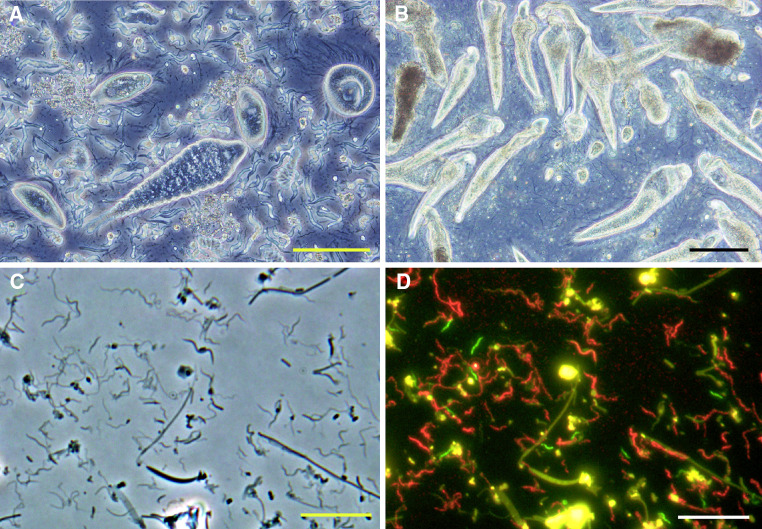
Although termites secrete their own digestive enzymes, including cellulases, from the salivary gland and/or midgut (reviewed in [7, 8]), the digestion of the recalcitrant foods largely depends on the activities of their gut microorganisms. Hence, to elucidate the mechanism of how the termites can survive on such recalcitrant and poor-quality foods, detailed investigation of the gut microbial ecosystem is essential. The gut microbiota (microbial community) comprises all the three domains of life, Eukaryotes (protists), Bacteria, and Archaea. In lower termites, an abundance of flagellated protists fill up the dilated portion, or paunch, of the hindgut (Fig. 2a, b), while most of the higher termites harbor only a small number of gut protists. Bacteria and archaea reside in the gut of both lower and higher termites. In general, termites harbor several hundreds or more bacterial species, most of which are found exclusively from the termite guts (reviewed in [9]). An obstacle in the study of these gut microorganisms is that the majority are as yet unculturable. Besides, the microbiota is too complex to manipulate experimentally. Therefore, conventional microbiological methods are less effective to clarify the detailed symbiotic mechanism in the termite gut ecosystem.
Fig. 2.
Microbiota in the hindgut of termites. a Phase-contrast image of the microbiota in the gut of the lower termite Reticulitermes speratus. b Phase-contrast image of the microbiota in the gut of the lower termite Coptotermes formosanus. c Phase-contrast image of the microbiota in the wood-feeding higher termite Nasutitermes takasagoensis. d FISH (fluorescent in situ hybridization) image of c. Texas-red signals: treponemes; 6FAM (green) signals: a novel group (FibS2) of the phylum Fibrobacteres [72]. Amorphous yellow colors were autofluorescence emitted from wood particles. Bars = 100 μm in a and b and 10 μm in c and d
In this review, I outline the termite gut ecosystem, focusing on the functional aspect of the symbiosis between wood-feeding termites and their gut microorganisms and also among the gut microorganisms. Particularly, recent studies using various omics techniques are summarized and future perspectives are discussed.
Lignocellulose digestion in the midgut
Hydrolysis with endogenous cellulases
Termites masticate the food materials with their mandibles and grind them into minute pieces using a sclerotized cuticular armature at the posterior terminus of the foregut (gizzard) [10, 11]. In the lower termite Coptotermes formosanus, the average size of the wood particles in the foregut and midgut is approximately 20 and 10 μm, respectively [12]. These wood particles are mixed with endo-β-1,4-glucanase (endoglucanase) and β-glucosidase, secreted from the salivary gland and/or midgut [7, 8]. Endoglucanases randomly cleave β-1,4 glycosidic bonds of amorphous sites of cellulose chains, and β-glucosidases hydrolyze cellobiose to glucose. In C. formosanus, the proportion of endoglucanase in the total midgut soluble proteins is extremely high, up to 6% [12]. With these two endogenous enzymes, termites most probably digest a part of the cellulosic materials on the surface of the wood particles and absorb glucose released in the midgut.
The expression of genes encoding these enzymes was verified by RT-PCR in the salivary gland and/or midgut of various termites [13–17] and also by transcriptome analysis of the salivary gland of the lower termite Hodotermopsis sjoestedti [18]. In the latter study, more than 10% of 851 expressed sequence tags (ESTs) were identified as transcripts for endoglucanases and a single clone was that for a β-glucosidase. The transcripts for these enzymes were also found in a transcriptome analysis of the gut tissue plus the salivary gland of the lower termite Reticulitermes flavipes [19]. Contribution of the microbiota to lignocellulose digestion in the foregut and midgut has never been found. The density of microbial cells are generally low in these gut regions [20, 21].
Glycoside hydrolases, including cellulases and hemicellulases, have been classified into more than 100 families based on the amino acid sequence similarities (http://www.cazy.org/) [22]. According to this classification, the endogenous endoglucanases of both lower and higher termites are members of the glycoside hydrolase family 9 (GHF9). To date, GHF9 endoglucanase genes have been found among diverse animals, including insects, a crayfish, an earthworm, sea squirts, clams, and snails [23]. In insects, endogenous GHF9 endoglucanase gene homologs have been found in the genomes or transcriptomes of various termites and cockroaches, a cricket, a louse, an aphid, a beetle, a honey bee, and a wasp [8]. Phylogenetic analyses suggested that these genes found in the metazoans share an ancient origin and that the genes have not been acquired by recent horizontal transfers from microorganisms [23, 24].
Presence of endogenous laccases
In the transcriptome analysis of the gut tissues plus salivary gland of R. flavipes, Tartar et al. [19] discovered transcripts for laccase homologs from the host tissues. RT-PCR and quantitative RT-PCR analysis showed that the gene expression of the laccase homologs was much higher in the salivary gland and foregut than other gut segments. Laccase is a phenoloxidase that is involved in lignin degradation in fungi [25] and cuticular melanization in insects [26]. Coy et al. [27] demonstrated that these laccases from R. flavipes are phylogenetically unique among those of prokaryotic and eukaryotic origins, and that recombinant enzymes expressed in a baculovirus-insect system exhibited strong activity towards several phenolic substrates, including lignin monomer sinapinic acid, while the enzymes displayed much lower or no activity against melanin precursors.
Lignin is a heterogeneous and amorphous phenylpropanoid polymer, constituting plant cell walls together with cellulose and hemicellulose. Lignin is extremely recalcitrant and protects cellulosic and hemicellulosic components. In a widely accepted view, termites and other wood-feeding insects are incapable of degrading a high-molecular-weight core of lignin [28, 29]. However, Geib et al. [30] first showed definitive evidence of a substantial degradation of lignin in a lower termite, Zootermopsis angusticollis. The authors observed significant levels of propyl side-chain oxidation, demethylation of ring methoxyl groups, and ring hydroxylation, which lead to depolymerization of lignin polymers. They implied that the observed pattern of lignin degradation by the termite is analogous to that by brown-rot fungi, which can remove cellulose and hemicellulose with minor modification to the lignin. Thus, termites may be able to, at least, loosen the lignin structures and efficiently extract cellulose and hemicellulose as energy and carbon sources.
The novel type of endogenous laccases expressed in the salivary gland, discovered in R. flavipes, might account for the lignin degradation in wood-feeding termites [19, 27], though further experimental evidence is needed to prove this hypothesis. At this point, the possibility that the endogenous laccases act for detoxification of phenolic compounds contained in ingested plant matters [31, 32] or for the sclerotization of the cuticular components in the foregut cannot be excluded. No convincing evidence of involvement of gut microorganisms in the degradation of lignin polymers has been obtained. For example, no genes related to lignin degradation were found in a metagenome analysis of the hindgut luminal microbiota of the wood-feeding higher termite Nasutitermes ephratae [33].
Lignocellulose digestion in the hindgut of lower termites
In general, the cellulose fibers in plant cell walls are composed of amorphous and crystalline sites. The latter cannot be broken down by endoglucanases; exoglucanases (cellobiohydrolases or exoglucohydrolases) are required for the reaction. In a classical view, exoglucanases act on the termini of cellulose chains to cleave cellobiosyl units and to loosen the crystalline structure of the cellulose fibers. This action produces additional amorphous sites on the cellulose fibers that endoglucanases can hydrolyze. Therefore, exoglucanases are indispensable for complete digestion of the cellulose fibers in plant cell walls.
In addition, hemicellulase is necessary for efficient digestion of cellulose, because the cellulose fibers are coated with hemicellulose. Hemicellulose is a collective term of noncellulosic polysaccharides such as xylan, mannan, and arabinose in plant cell walls. Hemicellulose constitutes about 15–35% of plant cell walls and itself can be an important energy and carbon source for termites. However, endogenous exoglucanases have never been found from termites, and endogenous hemicellulases appear to be absent or to play relatively minor roles in termites [34, 35]. Thus, these enzymes must be supplied by the symbiotic microbiota for an effective extraction of energy and carbon sources from ingested lignocellulosic materials.
Cellulolytic activity of cultured gut protists
A strong activity of hemicellulase and cellulase against crystalline cellulose has been detected in the hindgut homogenates of various lower termites [14, 35, 36]. In the hindgut of lower termites, the gut protists take up the partially digested wood particles or fragments of lignocellulosic materials into food vacuoles by phagocytosis. The wood particles inside the protist cells can be easily detected by autofluorescence emitted from the woody materials. However, the detailed fate of the wood particles remain unclear because the gut protists are resistant to cultivation. The termite gut protists comprise two distinct lineages of flagellates, belonging to either the phylum Parabasalia or the order Oxymonadida in the phylum Preaxostyla. They are unique to termites and the wood-feeding cockroaches Cryptocercus, and each termite species possesses a specific set of protist species. One termite species generally harbors several morphologically identifiable species of protists [37, 38]. A total number of the protist cells in a single gut can reach 104 to 105 [35, 39], and they account for more than a half of the total weight of the termite host [40].
An essential contribution of the hindgut microbiota to the lignocellulose digestion in lower termites has been demonstrated by eliminating gut protists [41, 42]. However, there have been only several examples of successful cultivation of protists from the termite gut [43]. Yamin [44, 45] succeeded in cultivation of the parabasalid protists Trichomitopsis termopsidis and Trichonympha sphaerica from the gut of the termite Z. angusticollis, under the condition that diminished coexisting bacteria by penicillin and streptomycin. Using these cultured protists, Yamin [45, 46] demonstrated that the protists are strictly anaerobic and ferment cellulose: n(C6H12O6) + n(2H2O) → n(2CH3COOH + 2CO2 + 4H2). This is consistent with a previous result obtained by Hungate who studied the physiology of a mixed population of the gut protists from Z. angusticollis [47, 48]. The acetate generated in the fermentation is considered to be the main energy and carbon source of the termite host [47–49]. The anaerobic nature of the protists is concordant with the fact that the central region of the paunch in the hindgut is anoxic [50].
Odelson and Breznak [51] treated this T. termopsidis culture with an antibiotic to eliminate methanogenic archaea, which were candidate contaminants in the culture. Then, they acquired its putatively axenic culture. They showed that the cultured T. termopsidis had an activity of endoglucanase, β-glucosidase, and probably exoglucanase because microcrystalline cellulose was degraded with the cell extracts of T. termopsidis. In addition, hydrolysis of xylan and starch was observed, indicating that the protist possesses hemicellulase and amylase [52]. However, no cultured strains of termite gut protists exist at present. Hence, recent studies of lignocellulose digestion by gut protists have been conducted with culture-independent approaches.
Presence of cellulase genes in gut protists
The presence of cellulase genes in the symbiotic gut protists has been verified in several termite species. Transcripts encoding putative endoglucanase belonging to GHF45 were obtained by RT-PCR from the protistan microbiota in the hindgut of the lower termite Reticulitermes speratus [53]. The origins of the genes were identified by in situ hybridization using probes specific to mRNA of the GHF45 endoglucanase genes; the cells of the parabasalid protists Trichonympha agilis and Teranympha mirabilis were detected. GHF45 endoglucanase genes were also obtained by PCR from isolated nuclei of the parabasalid protists Deltotrichonympha nana and Koruga bonita present in the hindgut of the lower termite Mastotermes darwiniensis [54]. Endoglucanase genes belonging to GHF7 were obtained by RT-PCR from the parabasalid protists Pseudotrichonympha grassii and Holomastigotoides mirabile present in the hindgut of the termite C. formosanus, and the functionality of one of the proteins was verified using a recombinant enzyme expressed in Escherichia coli [55]. In C. formosanus, an endoglucanase gene belonging to GHF5 was additionally found by screening a complementary DNA (cDNA) library constructed from the protistan gut microbiota for cellulase activity. The source of this gene was identified as the parabasalid protist Spirotrichonympha leidyi [56].
Transcripts encoding exoglucanase were obtained by RT-PCR from the cells of the protist P. grassii in the hindgut of C. formosanus [57]. The exoglucanase was identified as a member of GHF7 and phylogenetically related to those from fungi. A recombinant enzyme was expressed in E. coli and a cellulase activity against carboxymethylcellulose (CMC) was verified, though an exoglucanase activity against crystalline cellulase has never been successfully demonstrated using recombinant enzymes originating from any termite gut protists.
Metatranscriptome analysis of protistan gut microbiota
To comprehensively acquire genes involved in lignocellulose digestion by gut protists, metatranscriptome analyses were performed in several lower termite species. Todaka et al. [58] constructed a cDNA library from the entire protistan microbiota in the gut of the termite R. speratus. Since R. speratus harbors more than ten species of parabasalid and oximonad protists [59], the prepared library consisted of transcripts from heterogeneous strains of multiple protist species. Of 910 sequenced ESTs, nearly 10% were transcripts for enzymes involved in lignocellulose degradation. About half of them were homologs of GHF7 exoglucanase genes. The remaining comprised transcripts for endoglucanases (GHF5, 7, 45), xylanases (GHF8 and 11), GHF3 β-glucosidases, GHF43 arabinosidases, a GHF62 α-arabinofranosidase, and a GHF26 endo-1,4-β-mannanase. None of transcripts for enzymes involved in lignin degradation (i.e., lignin peroxidase, manganese peroxidase, and laccase) were found [58].
An abundance of transcripts for enzymes involved in lignocellulose degradation was consistent in metatranscriptome analyses of the protistan microbiota in the gut of the wood-feeding cockroach Cryptocercus punctulatus and lower termites belonging to different families: M. darwiniensis (family Mastotermitidae), H. sjoestedti (family Termopsidae), and Neotermes Koshunensis (family Kalotermitidae). In these termite and cockroach species, about 10% of approx. 1,000 sequenced ESTs for each were identified as transcripts for cellulolytic or hemicellulolytic enzymes [60], as in R. speratus (family Rhinotermitidae) [58]. The dominance of transcripts for GHF7 exoglucanases and the repertoires of GHFs were also consistent in these termite and cockroach species, though the frequency of the GHFs varied [60].
Tartar et al. [19] reported a metatranscriptome analysis of the microbiota in the hindgut of R. flavipes, which was performed simultaneously with the transcriptome analysis of the host gut tissues as mentioned above. Among 3,511 gene transcripts from the gut microbiota, various GHFs were identified, most of which are shared by the protistan gut microbiota of the above termite and cockroach species. The dominance of transcripts for GHF7 exoglucanases was also consistent. The authors suggested, based on the sequence similarities, that a substantial portion of the detected gene transcripts for cellulolytic and hemicellulolytic enzymes probably derived from prokaryotic gut symbionts. However, as the authors noted, the genes may be possessed by the gut protists because they might have been horizontally transferred from bacteria as strongly suggested in GHF5 endoglucanases of gut protists [56, 60].
The abundant transcripts for GHF7 exoglucanases and GHF10 or 11 xylanases in the protistan gut microbiota indicate that hemicellulose and crystalline cellulose are digested by the protistan gut microbiota. There has been no evidence that the endogenous endoglucanases and β-glucosidases secreted from the salivary gland and/or midgut of termites participate in the digestive process in the hindgut [14]. This dual digestive system for lignocellulose has realized the most efficient natural bioreactor which can degrade 65–99% of cellulose and hemicellulose contained in plant cell walls [28].
The direct contribution of prokaryotic gut microbiota to the hydrolysis of lignocellulose in lower termites is unclear. Although numerous bacterial strains that are capable of degrading cellulose, hemicellulose, and/or lignin monomers and dimers have been isolated from the gut of various lower termites [61], the evidence for their significant contribution has never been obtained thus far.
Lignocellulose digestion in the hindgut of wood-feeding higher termites
Alkaline gut segments and cellulase activity of bacteria
The higher termites generally lack the cellulolytic gut protists, and their gut microbiota comprises mostly bacteria and archaea. In most higher termites, after the partial digestion in the midgut, ingested food passes through a gut region called the mixed segment, which consists of both midgut (mesodermal) and hindgut (ectodermal) components (Fig. 1d) [11]. From this mixed segment, pH is elevated possibly by K+ secretion [20], and in the extreme anterior region of the hindgut, designated the P1 (proctodeal) section, pH reaches 10–12 [50, 62]. In wood-feeding higher termites, pH reaches 10 in P1 and steeply down to 7 in the following P3 section [50], while pH reaches 12 in P1 and still around 10 in the P3 section in soil-feeding termites [62]. Oxygen content is also steeply shifted in the P1 and P3 sections; anoxic in the central region of these two sections while aerobic in the midgut, P4, and P5 sections [50].
The function of the alkaline P1 section is still unclear, but there is agreement that it likely promotes the solubilization of lignocellulose and humus, which facilitates the degradation of those recalcitrant materials in the following gut sections [20]. In addition, the P1 section may play a significant role in the degradation of allochthonous microorganisms and the precipitation of tannins [62, 63]. In the proctodeal part of the mixed segment, bacteria densely colonize on its epithelium, though their role in the gut ecosystem is unknown. In the P1 section, bacterial cells exist only sparsely [64, 65].
It had long been believed that the degradation of lignocellulose in wood-feeding higher termites rely solely on their endogenous cellulases secreted in the midgut. Indeed, the endogenous cellulolytic activity in the midgut of the wood-feeding higher termite Nasutitermes walkeri theoretically meets the metabolic requirements of the termite, while almost no cellulase activity was detected in the hindgut [66]. Nevertheless, Tokuda and coworkers [36, 67] showed the first convincing evidence of the cellulolytic activity attributable to the bacterial microbiota in the hindgut of the wood-feeding higher termites Nasutitermes takasagoensis and N. walkeri. They succeeded in detecting the cellulase activity by using detergent to solubilize pellet extracts. The cellulase activity in the hindgut against crystalline cellulose is about a half of that in the midgut per individual termite [67].
Metagenome and metaproteome analysis of the hindgut luminal microbiota
To reveal the comprehensive functions of the bacterial gut microbiota, Warnecke et al. [33] performed metagenome and metaproteome analysis of the bacterial microbiota in the luminal fluid of the P3 section in the hindgut of the wood-feeding higher termite Nasutitermes ephratae. About 71 Mb of sequence data were generated and assembled into numerous contigs. Because the bacterial diversity in the hindgut of termites is enormously high, it was impossible to connect the sequence fragments into large contigs and very difficult to identify the taxonomic origin of the fragments. Therefore, only 9% of all contigs were classified beyond phylum level even with a composition-based phylogenetic classifier.
Nevertheless, the authors successfully identified bacterial groups which are putatively involved in the hydrolysis of lignocellulose: the genus Treponema in the phylum Spirochaetes and an uncultured lineage belonging to the phylum Fibrobacteres. The genus Treponema is the predominant bacterial group in wood-feeding lower and higher termites [68–72], and the cluster in the Fibrobacteres is dominant in wood-feeding higher termites and present as minorities in lower termites (Fig. 2c, d) [69, 72]. This is indicative that the bacterial microbiota in the gut of both lower and higher termites plays substantial roles in the hydrolysis of lignocellulose.
More than 700 glycoside hydrolase catalytic domains corresponding to 45 different GHFs were obtained in this metagenome analysis [33]. Among them, more than 100 gene modules were identified as the catalytic domains of GHF5 cellulases, GHF94 cellobiose/cellodextrin phosphorylases, GHF51 endoglucanase/arabinofuranosidases, and GHF8, 9, 44, 45, and 74 endoglucanases. About 100 gene modules corresponded to the catalytic domains of GHF10, 11, 26, and 43 xylanases. Catalytic modules corresponding to several potential pentosidases were also identified, including abundant GHF1 and 3 β-glucosidases. Various GHFs putatively involved in the hydrolysis of alpha carbohydrate and chitin were further identified.
The accompanying metaproteome analysis using a three-dimensional LC-MS/MS system verified at least GHF5 cellulases and GHF10 xylanases are expressed in the P3 luminal fluid. Functional genomic screens also confirmed the cellulase activities of GHF5 gene products against solubilized and crystalline cellulose [33]. Burnum et al. [73] further reported a comprehensive metaproteome analysis of the P3 luminal fluid of N. ephratae (or corniger). They obtained 886 proteins, 36 of which were identified as glycoside hydrolases classified into 15 different GHFs.
Fermentation and hydrogen production
Cellulose and hemicellulose are depolymerized into monomers in the hindgut of termites by protists and/or bacteria. The gut protists anaerobically ferment the monomeric carbohydrates and produce acetate, H2, and CO2, and the acetate is absorbed by the termite host as the main energy and carbon source. However, the molecular mechanism of the glycolysis and fermentation process remains unclear due to the difficulty in cultivation of the gut protists. The sequencing efforts in the metatranscriptome analyses of the protistan gut microbiota mentioned above were too small to depict detailed metabolic pathways including the glycolysis and fermentation process. Hence, only fragmental data are available at present about this topic.
Hydrogen production by gut protists
H2 is emitted by gut protists and bacteria as an end product of fermentation [47]. The partial pressure of H2 in the paunch of lower termites is very high; the values were 15–30 kPa in the lower termite Reticulitermes santonensis and 30–72 kPa in the lower termite Zootermopsis nevadensis [74], and 2–5 kPa in R. flavipes [75]. These values were measured using agarose-embedded guts with microelectrodes. However, since the hydrogen emission from the embedded guts was 30- to 50-fold higher than in live termites, actual values in vivo might be much lower [74]. This discrepancy was possibly caused by the damages to the H2-consuming bacteria and a limited concentration of O2 that was available to oxidize H2 compared to live termites [74].
Inoue et al. [76] identified two genes encoding iron-only hydrogenases (Fe-hydrogenase) during their ongoing metatranscriptome analysis of the protistan microbiota in the gut of C. formosanus. The source of the two Fe-hydrogenases was identified as the cells of the protist P. grassii by in situ hybridization analysis. It has been demonstrated that P. grassii possesses both GHF7 endo- and exoglucanase [55, 57] and is indispensable for the degradation of wood particles ingested by the termite host [39].
The two Fe-hydrogenases were expressed heterologously in E. coli, and one exhibited a strong H2 production activity and retain about a half of its activity even under a high H2 partial pressure comparable to that in the hindgut of termites [76]. The other Fe-hydrogenase was phylogenetically distinct and showed a weaker H2 production activity. These two recombinant enzymes showed different characteristics in optimum pH and K m values. When the cells of P. grassii were ruptured and fractionated by centrifugation, the hydrogen production was observed mainly in the fraction of hydrogenosomes, which are known as H2-producing organelles in certain anaerobic protists. On the other hand, a strong H2-uptake activity was observed in the fraction of intracellularly symbiotic bacteria, implying interspecies H2 transfer between the endosymbiotic bacteria and their protist host [76].
Hydrogen production by gut bacteria
Whereas the majority of termite gut bacteria are as yet unculturable, numerous bacterial strains have been isolated under aerobic or anaerobic conditions [61]. One of the anaerobic isolates, Treponema azotonutricum, ferments maltose to acetate, ethanol, CO2, and H2 [77]. In the metagenome analysis of the P3 luminal fluid of N. ephratae, gene modules coding for 159 diverse, putative Fe-hydrogenases were identified, the majority of which were binned to the genus Treponema by the composition-based classifier [33]. Thus, treponemes appear to be a dominant source for H2 in the hindgut of wood-feeding higher termites, while the gut protists are the predominant H2-producers in lower termites.
Acetogenesis and methanogenesis from H2 and CO2
In general, accumulated end products, especially H2, suppress fermentation process. Therefore, the generated H2 in the termite hindgut should be rapidly removed to keep its concentration low. Actually, the amount of emitted H2 from live termites is considerably smaller than the potential H2 production in the hindgut [74, 76]. It has been demonstrated with ample evidence that acetogenic and methanogenic prokaryotes are the major H2 sinks in termite guts.
Reductive acetogenesis by gut bacteria
Acetogenesis from H2 plus CO2 in the termite gut was discovered by Breznak and Switzer [78]. This is called reductive acetogenesis: 4H2 + 2CO2 → CH3COOH + 2H2O. The reductive acetogenesis occurs in the gut of various lower and higher termites [78], and it accounts for about one-quarter of all the acetates produced in the hindgut [74]. Several anaerobic bacteria showing the activity of the reductive acetogenesis have been isolated from termite guts [79, 80]. Among them, an axenic cultivation and its detailed physiological study of the homoacetogen Treponema primitia [77, 81, 82], isolated from Z. angusticollis, provided important information on the termite gut ecosystem, because treponemes dominate the bacterial microbiota in the gut of various wood-feeding lower and higher termites [68–72].
The reductive acetogenesis needs the Wood-Ljungdahl pathway for the synthesis of acetyl-CoA and acetate from H2 plus CO2. A key enzyme involved in this pathway is formyl tetrahydrofolate synthetase (FTHFS), and the FTHFS genes have been detected by PCR from the gut of various lower termites. The origins of the majority of these FTHFS genes were estimated to be treponemes [83–85]. In the metagenome analysis of the higher termite N. ephratae [33], 14–37 variants of all, except formate dehydrogenase, of the proteins related to the Wood-Ljungdahl pathway were obtained. Phylogenetic analysis of the FTHFS genes revealed that most of these derived from treponemes. Thus, the reductive acetogenesis in the gut of wood-feeding lower and higher termites is mainly attributable to treponemes.
Strains ZAS-1 and ZAS-2 of T. primitia were the first Treponema isolates from the termite gut [77, 81, 82]. They can grow on H2 plus CO2 as the sole energy and carbon source. They also grow on various mono- and disaccharides such as glucose, arabinose, xylose, maltose, and cellobiose, which are components of plant materials. Interestingly, strain ZAS-2 is capable of mixotrophic growth, i.e., the simultaneous utilization of organic substrates and H2 for energy. The mixotrophy of homoacetogens is a common trait, and they can generate more energy by mixotrophy than from H2 plus CO2 alone. This might be one reason why the H2-dependent reductive acetogenesis outcompetes the H2-dependent methanogenesis in the gut of wood-feeding termites. Otherwise, the outcompetition might be due to the difference in their specific localizations in the hindgut [21]. The H2 concentration is highest in the central region of the hindgut and outwardly lower in gradients [75]. Since treponemes are generally found in the gut luminal fluid or attached onto the cell surface of gut protists, they can utilize much more H2 than methanogens, the majority of which colonize the gut epithelium [74].
Methanogenesis by archaea
Methanogenic archaea are common in the hindgut of lower and higher termites, and they generate methane from H2 plus CO2: 4H2 + CO2 → CH4 + 2H2O. Thus, methanogens compete with acetogens for H2. In wood-feeding termites, homoacetogens generally outcompete methanogens in number and the activities; the rate of methane emission is only 10% of that of the reductive acetogenesis in the gut of lower termites [74]. Methanogens are localized on the epithelium of the hindgut [74] and/or inside the cells of certain protist species [86, 87]. Methanobrevibacters have been detected in many lower and higher termites [88–90], and three strains have been isolated from the lower termite R. flavipes: Methanobrevibacter cuticularis, Methanobrevibacter curvatus [91], and Methanobrevibacter filiformis [92]. The detailed ecology of methanogens in termite guts has recently been reviewed elsewhere [93–95].
Nitrogen metabolism
Wood-feeding termites can digest recalcitrant lignocellulosic materials with the aid of the gut microbiota. However, the termites must overcome another problem: nitrogen deficiency in the food dead wood. It has been suggested that the gut microbiota contribute to the solution of this problem in four ways [21]: (i) by removal of carbon in the form of CO2 or CH4 to decrease the C/N ratio of ingested food; (ii) by fixation of nitrogen from the atmosphere; (iii) by recycling nitrogen; (iv) by upgrading poor-quality nitrogenous compounds (e.g., NH3) to nutritionally valuable compounds such as vitamins and amino acids.
Nitrogen fixation
Nitrogen fixation is a prokaryotic activity requiring high energy: N2 + 8H+ + 8e− + 16ATP → 2NH3 + H2 + 16ADP + 16 Pi. Nitrogen fixation in termites was discovered in 1973 [96, 97]. Since then, the N2-fixing activity has been reported from various lower and higher termites and is particularly prominent in wood-feeding termites [98] (reviewed in [21]). Genes encoding nitrogenase (nifH) have been detected by PCR and RT-PCR from the gut of diverse termites. Phylogenetic analysis showed that the nifH genes from the termite guts constituted several monophyletic clusters, some of which are not closely related to those of known described species [98–100]. In the metagenome analysis of the hindgut microbiota of N. ephratae, 12 nifH homologues and 31–100 homologues of other nitrogenase components were identified. Although the nifH genes could not be assigned to a specific taxon in this metagenomic study, at least one variant of each of the other genes were binned to the genus Treponema or the phylum Fibrobacteres [33].
The involvement of treponemes in nitrogen fixation has been demonstrated by the successful isolation of Treponema azotonutricum strain ZAS-9 from the gut of Z. angusticollis [101]. T. azotonutricum grows by heterotrophy, fermenting mono- and disaccharides such as glucose, fructose, xylose, maltose, and cellobiose, which are components of plant materials [77]. Thus, the treponemes in termite guts are likely responsible for cellulolysis, fermentation, H2 production, H2-dependent acetogenesis, and nitrogen fixation.
Nitrogen fixation, recycling, and upgrade by bacterial endosymbionts of gut protists
One of the prominent features of the microbiota in lower termites is the cellular association of prokaryotes with the gut protists. Most protists in termite guts harbor prokaryotes intracellularly or on their cell surface. To date, bacteria belonging to the phyla Bacteroidetes [102–107], Spirochaetes [107–110], and Synergistetes [106] have been documented as ectosymbionts (Fig. 3a–c) and those belonging to the phyla Bacteroidetes [111, 112], Elusimicrobia [113–116] (formerly Termite Group 1 [71, 117]), Firmicutes [118], and Proteobacteria [119] as endosymbionts. Their diversity, localization, and host specificity have been reviewed in detail elsewhere [9, 94, 120, 121]; I here focus on their functions.
Fig. 3.
Cellular symbiosis between gut protists and bacteria. a Phase-contrast image of the anterior region of the parabasalid protist Trichonympha agilis from the termite Reticulitermes speratus. b FISH (fluorescent in situ hybridization) image of a. Hair-like treponeme cells were specifically detected with 6FAM-labeled probes (green). c FISH image of a. Bristle-like Bacteroidales cells were specifically detected with a Texas red-labeled probe. d Phase-contrast image of T. agilis. e FISH image of d. Rs-D17 cells were specifically detected with a Texas red-labeled probe. Other bacterial cells were detected with a 6FAM-labeled probe (green). f Transmission electron micrograph of Rs-D17. g Phase-contrast image of the parabasalid protist Pseudotrichonympha grassii from the termite Coptotermes formosanus. h FISH image of a P. grassii cell. CfPt1-2 cells were specifically detected with a 6FAM-labeled probe (green). Bars = 20 μm in a–e; 0.5 μm in e; 50 μm in g; 10 μm in h. Panels a–c, d–f, and g–h were originally published in [107], [122], and [123], respectively, and slightly modified
One intrinsic defeat in metagenome and metatranscriptome analysis is that these provide only poor information on the functions of and interrelationships among the individual members of the microbiota. In order to estimate the functions of an unculturable endosymbiotic bacterium of a termite gut protist, Hongoh et al. [122, 123] attempted to acquire the complete genome sequence of the endosymbiont. Two species of unculturable endosymbionts were targeted: phylotype Rs-D17 belonging to the candidate class “Endomicrobia” in the phylum Elusimicrobia (or Termite Group 1) [71] (Fig. 3d–f) and phylotype CfPt1-2 belonging to the order Bacteroidales in the phylum Bacteroidetes [111] (Fig. 3g, h).
Rs-D17 is a specific endosymbiont of the unculturable, parabasalid protist Trichonympha agilis present in the gut of R. speratus [114]. Because T. agilis appeared to comprise heterogeneous strains in a single gut, a single T. agilis cell was physically isolated using a micromanipulator. The T. agilis cell was ruptured with detergent, and prokaryotic cells that leaked out from its posterior part, where the density of Rs-D17 is high, were collected by micromanipulation. Several hundred cells of Rs-D17 were subjected to the isothermal whole genome amplification (WGA) using phi29 DNA polymerase. With this method, the whole Rs-D17 genomes were amplified more than 10 million-fold without cultivation of the bacterium [122].
As a result, a complete circular 1.1 Mb chromosome with almost no variation was successfully reconstructed. It contains 761 putative protein coding sequences (CDS) and additional 121 pseudogenes. The small genome size and abundant pseudogenes suggested that the genome has been experiencing a reductive evolution as in many known intracellular symbionts of insects. The pseudogenes derived from genes involved in functions such as DNA replication/repair, lipopolysaccharide biosynthesis, transport, and defense mechanisms. For instance, the gene encoding the chromosome replication initiation factor DnaA has been pseudogenized. In contrast, the genes required for biosynthesis of amino acids and cofactors are abundantly retained. Because the genes encoding glutamine synthetase GlnA and ammonium transporter AmtB have been pseudogenized, it is likely that Rs-D17 requires glutamine from the host cytoplasm and upgrades it to various compounds. The predicted pathways suggest that glucose-6-phosphate and hexuronates are the major energy and carbon sources for Rs-D17 [122].
The CfPt1-2 bacterium is a specific endosymbiont of the unculturable, parabasalid protist Pseudotrichonympha grassii [111, 112], which is indispensable gut symbiont for the wood digestion in C. formosanus as already mentioned above. Using the same method, a complete circular 1.1 Mb chromosome without variation was successfully reconstructed, which contains 758 CDSs and 21 pseudogenes [123]. The most striking feature of this bacterium, predicted from the genome sequence, was its ability to fix dinitrogen. The predicted pathways suggest that the fixed nitrogen, in the form of NH3, is assimilated initially by the action of glutamine synthetase and is then used for the biosynthesis of diverse amino acids and cofactors. This bacterium also possesses a gene encoding an ammonium transporter and a gene cluster encoding urease and a urea transporter. The ability to import and assimilate ammonium and urea implies that CfPt1-2 not only fixes atmospheric nitrogen but also recycles the putative nitrogen waste products of the host protist. CfPt1-2 possesses genes for importing and utilizing monosaccharides derived from lignocellulose, i.e., glucose, xylose, and hexuronates, which are likely to be the major energy and carbon sources [123]. The predicted function of CfPt1-2 is outlined in Fig. 4.
Fig. 4.
Schematic view of the multilayered symbiosis in the termite Coptotermes formosanus. The figure was originally published in [123] and modified here
The profile of the cluster of orthologous groups of proteins and the general features of the CfPt1-2 genome differ greatly from those of known Bacteroidales bacteria, but are strikingly similar to those of Rs-D17 [123]. The functional similarity of CfPt1-2 and Rs-D17 implies that the primary role of the endosymbionts of cellulolytic gut protists is efficient and stable biosynthesis of amino acids and cofactors, which are critically deficient in woody materials. The processes of nitrogen fixation and biosynthesis of amino acids and cofactors conducted by the endosymbionts are considered to be much more stable and efficient than those conducted by free-swimming gut bacteria. The endosymbionts can utilize ample carbon and energy sources without competition and their genomes have been reduced, streamlined, and specialized for the nitrogen metabolism. Actually, the genome size of CfPt1-2 is smaller than one-third of the N2-fixer T. azotonutricum [77]. The ability of CfPt1-2 to couple nitrogen fixation directly to cellulolysis probably facilitates highly efficient growth of the host cellulolytic protist, the termite, and the termite colony, without the limitation of nitrogen deficiency.
Conclusions
Termites have evolved a sophisticated, multilayered symbiotic system by harboring a complex gut microbiota. In this system, termites ingest woody materials and masticate and degrade them into fine particles by means of their mandibles, gizzard, and endogenous endoglucanase and β-glucosidase, which are secreted from the salivary gland and/or midgut. The discovery of these endogenous cellulases has raised a question: do termites really need the gut microbiota for their survival? Yes, they do. The necessity of the gut microbiota for the digestion of woody materials has been demonstrated in numerous studies. However, the complexity and formidable unculturability of the gut microbiota have hampered the clarification of the molecular mechanism of this symbiotic system. Recently, innovative technologies in omics sciences have been applied; metagenome analysis of the bacterial gut microbiota of a wood-feeding higher termite and metatranscriptome analyses of the protistan gut microbiota have revealed the presence of diverse glycoside hydrolase genes in both the bacterial and protistan microbiota. In the former analysis, bacterial genes required for fermentation, reductive acetogenesis, and nitrogen fixation were also identified. These functions have been recognized as essential bacterial activities in this symbiotic system, by the long-term efforts in cultivation of the fastidious microorganisms and in ecological, physiological, and biochemical studies of the whole insects and cultured gut symbionts. Furthermore, genomics targeting an unculturable, single bacterial species has succeeded by using isothermal whole genome amplification from only several hundred cells. The functional analysis of the complete genome sequences acquired from intracellular symbionts of gut protists revealed that the endosymbionts play crucial roles in the nitrogen metabolism, i.e., nitrogen fixation, recycling, and upgrade. Further investigations using both meta- and single-species-targeting genomics, transcriptomics, and proteomics will greatly promote the understanding of this highly evolved, complex symbiotic system.
References
- 1.Inward DJ, Vogler AP, Eggleton P. A comprehensive phylogenetic analysis of termites (Isoptera) illuminates key aspects of their evolutionary biology. Mol Phylogenet Evol. 2007;44:953–967. doi: 10.1016/j.ympev.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Lo N, Engel MS, Cameron S, Nalepa CA, Tokuda G, Grimaldi D, Kitade O, Krishna K, Klass KD, Maekawa K, Miura T, Thompson GJ. Save Isoptera: a comment on Inward et al. Biol Lett. 2007;3:562–565. doi: 10.1098/rsbl.2007.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugimoto A, Bignell DE, Macdonald J. Global impact of termites on the carbon cycle. In: Abe T, Bignell DE, Higashi M, editors. Termites: evolution, sociality, symbioses, ecology. Dordrecht: Kluwer Academic Publishers; 2000. [Google Scholar]
- 4.Vinson SB, editor. Economic impact and control of social insects. New York: Praeger; 1986. [Google Scholar]
- 5.Kambhampati S, Eggleton P. Taxonomy and phylogeny of termites. In: Abe T, Bignell DE, Higashi M, editors. Termites: evolution, sociality, symbiose, ecology. Dordrecht: Kluwer Academic Publishers; 2000. [Google Scholar]
- 6.Ohkuma M, Yuzawa H, Amornsak W, Sornnuwat Y, Takematsu Y, Yamada A, Vongkaluang C, Sarnthoy O, Kirtibutr N, Noparatnaraporn N, Kudo T, Inoue T. Molecular phylogeny of Asian termites (Isoptera) of the families Termitidae and Rhinotermitidae based on mitochondrial COII sequences. Mol Phylogenet Evol. 2004;31:701–710. doi: 10.1016/j.ympev.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe H, Tokuda G. Animal cellulases. Cell Mol Life Sci. 2001;58:1167–1178. doi: 10.1007/PL00000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe H, Tokuda G. Cellulolytic systems in insects. Annu Rev Entomol. 2010;55:609–632. doi: 10.1146/annurev-ento-112408-085319. [DOI] [PubMed] [Google Scholar]
- 9.Hongoh Y. Diversity and genomes of uncultured microbial symbionts in the termite gut. Biosci Biotechnol Biochem. 2010;74:1145–1151. doi: 10.1271/bbb.100094. [DOI] [PubMed] [Google Scholar]
- 10.Noirot C. The gut of termites (Isoptera). Comparative anatomy, systematics, phylogeny. I. Lower termites. Ann Soc Entomol Fr (NS) 1995;31:197–226. [Google Scholar]
- 11.Noirot C. The gut of termites (Isoptera). Comparative anatomy, systematics, phylogeny. II. Higher termites (Termitidae) Ann Soc Entomol Fr (NS) 2001;37:431–471. [Google Scholar]
- 12.Fujita A, Hojo M, Aoyagi T, Hayashi Y, Arakawa G, Tokuda G, Watanabe H. Details of the digestive system in the midgut of Coptotermes formosanus Shiraki. J Wood Sci. 2010;56:222–226. [Google Scholar]
- 13.Tokuda G, Lo N, Watanabe H, Slaytor M, Matsumoto T, Noda H. Metazoan cellulase genes from termites: intron/exon structures and sites of expression. Biochim Biophys Acta. 1999;1447:146–159. doi: 10.1016/s0167-4781(99)00169-4. [DOI] [PubMed] [Google Scholar]
- 14.Nakashima K, Watanabe H, Saitoh H, Tokuda G, Azuma JI. Dual cellulose-digesting system of the wood-feeding termite, Coptotermes formosanus Shiraki. Insect Biochem Mol Biol. 2002;32:777–784. doi: 10.1016/s0965-1748(01)00160-6. [DOI] [PubMed] [Google Scholar]
- 15.Tokuda G, Saito H, Watanabe H. A digestive beta-glucosidase from the salivary glands of the termite, Neotermes koshunensis (Shiraki): distribution, characterization and isolation of its precursor cDNA by 5′- and 3′-RACE amplifications with degenerate primers. Insect Biochem Mol Biol. 2002;32:1681–1689. doi: 10.1016/s0965-1748(02)00108-x. [DOI] [PubMed] [Google Scholar]
- 16.Tokuda G, Lo N, Watanabe H, Arakawa G, Matsumoto T, Noda H. Major alteration of the expression site of endogenous cellulases in members of an apical termite lineage. Mol Ecol. 2004;13:3219–3228. doi: 10.1111/j.1365-294X.2004.02276.x. [DOI] [PubMed] [Google Scholar]
- 17.Tokuda G, Miyagi M, Makiya H, Watanabe H, Arakawa G. Digestive beta-glucosidases from the wood-feeding higher termite, Nasutitermes takasagoensis: intestinal distribution, molecular characterization, and alteration in sites of expression. Insect Biochem Mol Biol. 2009;39:931–937. doi: 10.1016/j.ibmb.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Yuki M, Moriya S, Inoue T, Kudo T. Transcriptome analysis of the digestive organs of Hodotermopsis sjoestedti, a lower termite that hosts mutualistic microorganisms in its hindgut. Zool Sci. 2008;25:401–406. doi: 10.2108/zsj.25.401. [DOI] [PubMed] [Google Scholar]
- 19.Tartar A, Wheeler MM, Zhou X, Coy MR, Boucias DG, Scharf ME. Parallel metatranscriptome analyses of host and symbiont gene expression in the gut of the termite Reticulitermes flavipes . Biotechnol Biofuels. 2009;2:e25. doi: 10.1186/1754-6834-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bignell DE. Introduction to symbiosis. In: Abe T, Bignell DE, Higashi M, editors. Termites: evolution, sociality, symbioses, ecology. Dordrecht: Kluwer Academic Publishers; 2000. [Google Scholar]
- 21.Breznak JA. Ecology of prokaryotic microbes in the guts of wood- and litter-feeding termites. In: Abe T, Bignell DE, Higashi M, editors. Termites: evolution, sociality, symbioses, ecology. Dordrecht: Kluwer Academic Publishers; 2000. [Google Scholar]
- 22.Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucl Acids Res. 2009;37:D233–D238. doi: 10.1093/nar/gkn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davison A, Blaxter M. Ancient origin of glycosyl hydrolase family 9 cellulase genes. Mol Biol Evol. 2005;22:1273–1284. doi: 10.1093/molbev/msi107. [DOI] [PubMed] [Google Scholar]
- 24.Lo N, Watanabe H, Sugimura M. Evidence for the presence of a cellulase gene in the last common ancestor of bilaterian animals. Proc R Soc Lond B. 2003;270(Suppl 1):S69–S72. doi: 10.1098/rsbl.2003.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eggert C, Temp U, Dean JF, Eriksson KE. A fungal metabolite mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase. FEBS Lett. 1996;391:144–148. doi: 10.1016/0014-5793(96)00719-3. [DOI] [PubMed] [Google Scholar]
- 26.Arakane Y, Muthukrishnan S, Beeman RW, Kanost MR, Kramer KJ. Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proc Natl Acad Sci USA. 2005;102:11337–11342. doi: 10.1073/pnas.0504982102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coy MR, Salem TZ, Denton JS, Kovaleva ES, Liu Z, Barber DS, Campbell JH, Davis DC, Buchman GW, Boucias DG, Scharf ME. Phenol-oxidizing laccases from the termite gut. Insect Biochem Mol Biol. 2010;40:723–732. doi: 10.1016/j.ibmb.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Breznak JA, Brune A. Role of microorganisms in the digestion of lignocellulose by termites. Annu Rev Entomol. 1994;39:453–487. [Google Scholar]
- 29.Ohkuma M. Termite symbiotic systems: efficient bio-recycling of lignocellulose. Appl Microbiol Biotechnol. 2003;61:1–9. doi: 10.1007/s00253-002-1189-z. [DOI] [PubMed] [Google Scholar]
- 30.Geib SM, Filley TR, Hatcher PG, Hoover K, Carlson JE, Jimenez-Gasco MD, Nakagawa-Izumi A, Sleighter RL, Tien M. Lignin degradation in wood-feeding insects. Proc Natl Acad Sci USA. 2008;105:12932–12937. doi: 10.1073/pnas.0805257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johjima T, Inoue T, Ohkuma M, Noparatnaraporn N, Kudo T. Chemical analysis of food processing by the fungus-growing termite Macrotermes gilvus . Sociobiology. 2003;42:815–824. [Google Scholar]
- 32.Taprab Y, Johjima T, Maeda Y, Moriya S, Trakulnaleamsai S, Noparatnaraporn N, Ohkuma M, Kudo T. Symbiotic fungi produce laccases potentially involved in phenol degradation in fungus combs of fungus-growing termites in Thailand. Appl Environ Microbiol. 2005;71:7696–7704. doi: 10.1128/AEM.71.12.7696-7704.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warnecke F, Luginbühl P, Ivanova N, Ghassemian M, Richardson TH, Stege JT, Cayouette M, McHardy AC, Djordjevic G, Aboushadi N, Sorek R, Tringe SG, Podar M, Martin HG, Kunin V, Dalevi D, Madejska J, Kirton E, Platt D, Szeto E, Salamov A, Barry K, Mikhailova N, Kyrpides NC, Matson EG, Ottesen EA, Zhang X, Hernandez M, Murillo C, Acosta LG, Rigoutsos I, Tamayo G, Green BD, Chang C, Rubin EM, Mathur EJ, Robertson DE, Hugenholtz P, Leadbetter JR. Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature. 2007;450:560–565. doi: 10.1038/nature06269. [DOI] [PubMed] [Google Scholar]
- 34.Hungate RE. Studies on the nutrition of Zootermopsis II. The relative importance of termite and the protozoa in wood digestion. Ecology. 1938;19:1–25. [Google Scholar]
- 35.Inoue T, Murashima K, Azuma J-I, Sugimoto A, Slaytor M. Cellulose and xylan utilisation in the lower termite Reticulitermes speratus . J Insect Physiol. 1997;43:235–242. doi: 10.1016/s0022-1910(96)00097-2. [DOI] [PubMed] [Google Scholar]
- 36.Tokuda G, Lo N, Watanabe E. Marked variations in patterns of cellulase activity against crystalline- vs. carboxymethyl-cellulose in the digestive systems of diverse, wood-feeding termites. Physiol Entomol. 2005;30:372–380. [Google Scholar]
- 37.Yamin MA. Flagellates of the orders Trichomonadida Kirby, Oxymonadida Grassé, and Hypermastigida Grassi and Foà reported from lower termites (Isoptera families Mastotermitidae, Kalotermitidae, Hodotermitidae, Termopsidae, Rhinotermitidae, and Serritermitidae) and from the wood-feeding roach Cryptocercus (Dictyoptera: Cryptocercidae) Sociobiology. 1979;4:1–120. [Google Scholar]
- 38.Kitade O, Matsumoto T. Characteristics of the symbiotic flagellate composition within the termite family Rhinotermitidae (Isoptera) Symbiosis. 1998;25:271–278. [Google Scholar]
- 39.Yoshimura T. Contribution of the protozoan fauna to nutritional physiology of the lower termite, Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae) Wood Res. 1995;82:68–129. [Google Scholar]
- 40.Katzin LI, Kirby H. The relative weights of termites and their protozoa. J Parasitol. 1939;25:444–445. [Google Scholar]
- 41.Cleveland LR. Symbiosis between termites and their intestinal protozoa. Proc Natl Acad Sci USA. 1923;9:424–428. doi: 10.1073/pnas.9.12.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cleveland LR. The physiological and symbiotic relationships between the intestinal protozoa of termites and their host, with special reference to Reticulitermes flavipes Kollar. Biol Bull. 1924;46:178–227. [Google Scholar]
- 43.Trager W. The cultivation of a cellulose-digesting flagellate, Trichomonas termopsidis, and of certain other termite protozoa. Biol Bull. 1934;66:182–190. [Google Scholar]
- 44.Yamin MA. Axenic cultivation of the cellulolytic flagellate Trichomitopsis termopsidis (Cleveland) from the termite Zootermopsis . J Protozool. 1978;25:535–538. [Google Scholar]
- 45.Yamin MA. Cellulose metabolism by the flagellate Trichonympha from a termite is independent of endosymbiotic bacteria. Science. 1981;211:58–59. doi: 10.1126/science.211.4477.58. [DOI] [PubMed] [Google Scholar]
- 46.Yamin MA. Cellulose metabolism by the termite flagellate Trichomitopsis termopsidis . Appl Environ Microbiol. 1980;39:859–863. doi: 10.1128/aem.39.4.859-863.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hungate RE. Experiments on the nutrition of Zootermopsis. III. The anaerobic carbohydrate dissimilation by the intestinal protozoa. Ecology. 1939;20:230–245. [Google Scholar]
- 48.Hungate RE. Quantitative analyses of the cellulose fermentation by termite protozoa. Ann Entomol Soc Am. 1943;36:730–739. [Google Scholar]
- 49.Odelson DA, Breznak JA. Volatile fatty acid production by the hindgut microbiota of xylophagous termites. Appl Environ Microbiol. 1983;45:1602–1613. doi: 10.1128/aem.45.5.1602-1613.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brune A, Emerson D, Breznak JA. The termite gut microflora as an oxygen sink: microelectrode determination of oxygen and pH gradients in guts of lower and higher termites. Appl Environ Microbiol. 1995;61:2681–2687. doi: 10.1128/aem.61.7.2681-2687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Odelson DA, Breznak JA. Nutrition and growth characteristics of Trichomitopsis termopsidis, a cellulolytic protozoan from termites. Appl Environ Microbiol. 1985;49:614–621. doi: 10.1128/aem.49.3.614-621.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Odelson DA, Breznak JA. Cellulase and other polymer-hydrolyzing activities of Trichomitopsis termopsidis, a symbiotic protozoan from termites. Appl Environ Microbiol. 1985;49:622–626. doi: 10.1128/aem.49.3.622-626.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohtoko K, Ohkuma M, Moriya S, Inoue T, Usami R, Kudo T. Diverse genes of cellulase homologues of glycosyl hydrolase family 45 from the symbiotic protists in the hindgut of the termite Reticulitermes speratus . Extremophiles. 2000;4:343–349. doi: 10.1007/s007920070003. [DOI] [PubMed] [Google Scholar]
- 54.Li L, Fröhlich J, Pfeiffer P, König H. Termite gut symbiotic archaezoa are becoming living metabolic fossils. Eukaryot Cell. 2003;2:1091–1098. doi: 10.1128/EC.2.5.1091-1098.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe H, Nakashima K, Saito H, Slaytor M. New endo-beta-1, 4-glucanases from the parabasalian symbionts, Pseudotrichonympha grassii and Holomastigotoides mirabile of Coptotermes termites. Cell Mol Life Sci. 2002;59:1983–1992. doi: 10.1007/PL00012520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inoue T, Moriya S, Ohkuma M, Kudo T. Molecular cloning and characterization of a cellulase gene from a symbiotic protist of the lower termite, Coptotermes formosanus . Gene. 2005;349:67–75. doi: 10.1016/j.gene.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 57.Nakashima KI, Watanabe H, Azuma JI. Cellulase genes from the parabasalian symbiont Pseudotrichonympha grassii in the hindgut of the wood-feeding termite Coptotermes formosanus . Cell Mol Life Sci. 2002;59:1554–1560. doi: 10.1007/s00018-002-8528-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Todaka N, Moriya S, Saita K, Hondo T, Kiuchi I, Takasu H, Ohkuma M, Piero C, Hayashizaki Y, Kudo T. Environmental cDNA analysis of the genes involved in lignocellulose digestion in the symbiotic protist community of Reticulitermes speratus . FEMS Microbiol Ecol. 2007;59:592–599. doi: 10.1111/j.1574-6941.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- 59.Kitade O, Matsumoto T. Symbiotic protistan faunae of Reticulitermes (Isoptera: Rhinotermitidae) in the Japan Archipelago. Sociobiology. 1993;23:135–153. [Google Scholar]
- 60.Todaka N, Inoue T, Saita K, Ohkuma M, Nalepa CA, Lenz M, Kudo T, Moriya S. Phylogenetic analysis of cellulolytic enzyme genes from representative lineages of termites and a related cockroach. PLoS One. 2010;5:e8636. doi: 10.1371/journal.pone.0008636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wenzel M, Shönig M, Berchtold M, Kämpfer P, König H. Aerobic and facultatively anaerobic cellulolytic bacteria from the gut of the termite Zootermopsis angusticollis . J Appl Microbiol. 2002;92:32–40. doi: 10.1046/j.1365-2672.2002.01502.x. [DOI] [PubMed] [Google Scholar]
- 62.Brune A, Kuhl M. pH profiles of the extremely alkaline hindguts of soil-feeding termites (Isoptera: Termitidae) determined with microelectrodes. J Insect Physiol. 1996;42:1121–1127. [Google Scholar]
- 63.Bignell DE, Eggleton P. On the elevated intestinal pH of higher termites (Isoptera: Termitidae) Insect Soc. 1995;42:57–69. [Google Scholar]
- 64.Czolij RT, Slaytor M, O’Brien RW. Bacterial flora of the mixed segment and the hindgut of the higher termite Nasutitermes exitiosus Hill (Termitidae, Nasutitermitinae) Appl Environ Microbiol. 1985;49:1226–1236. [Google Scholar]
- 65.Tokuda G, Yamaoka I, Noda H. Localization of symbiotic clostridia in the mixed segment of the termite Nasutitermes takasagoensis (Shiraki) Appl Environ Microbiol. 2000;66:2199–2207. doi: 10.1128/aem.66.5.2199-2207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Slaytor M. Cellulose digestion in termites and cockroaches: what role do symbionts play? Comp Biochem Physiol B Biochem Mol Biol. 1992;103:775–784. [Google Scholar]
- 67.Tokuda G, Watanabe H. Hidden cellulases in termites: revision of an old hypothesis. Biol Lett. 2007;3:336–339. doi: 10.1098/rsbl.2007.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lilburn TG, Schmidt TM, Breznak JA. Phylogenetic diversity of termite gut spirochaetes. Environ Microbiol. 1999;1:331–345. doi: 10.1046/j.1462-2920.1999.00043.x. [DOI] [PubMed] [Google Scholar]
- 69.Hongoh Y, Deevong P, Inoue T, Moriya S, Trakulnaleamsai S, Ohkuma M, Vongkaluang C, Noparatnaraporn N, Kudo T. Intra- and interspecific comparisons of bacterial diversity and community structure support coevolution of gut microbiota and termite host. Appl Environ Microbiol. 2005;71:6590–6599. doi: 10.1128/AEM.71.11.6590-6599.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paster BJ, Dewhirst FE, Cooke SM, Fussing V, Poulsen LK, Breznak JA. Phylogeny of not-yet-cultured spirochetes from termite guts. Appl Environ Microbiol. 1996;62:347–352. doi: 10.1128/aem.62.2.347-352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hongoh Y, Ohkuma M, Kudo T. Molecular analysis of bacterial microbiota in the gut of the termite Reticulitermes speratus (Isoptera; Rhinotermitidae) FEMS Microbiol Ecol. 2003;44:231–242. doi: 10.1016/S0168-6496(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 72.Hongoh Y, Deevong P, Hattori S, Inoue T, Noda S, Noparatnaraporn N, Kudo T, Ohkuma M. Phylogenetic diversity, localization, and cell morphologies of members of the candidate phylum TG3 and a subphylum in the phylum Fibrobacteres, recently discovered bacterial groups dominant in termite guts. Appl Environ Microbiol. 2006;72:6780–6788. doi: 10.1128/AEM.00891-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burnum KE, Callister SJ, Nicora CD, Purvine SO, Hugenholtz P, Warnecke F, Scheffrahn RH, Smith RD, Lipton MS. Proteome insights into the symbiotic relationship between a captive colony of Nasutitermes corniger and its hindgut microbiome. ISME J. 2011;5:161–164. doi: 10.1038/ismej.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pester M, Brune A. Hydrogen is the central free intermediate during lignocellulose degradation by termite gut symbionts. ISME J. 2007;1:551–565. doi: 10.1038/ismej.2007.62. [DOI] [PubMed] [Google Scholar]
- 75.Ebert A, Brune A. Hydrogen concentration profiles at the oxic-anoxic interface: a microsensor study of the hindgut of the wood-feeding lower termite Reticulitermes flavipes (Kollar) Appl Environ Microbiol. 1997;63:4039–4046. doi: 10.1128/aem.63.10.4039-4046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Inoue J, Saita K, Kudo T, Ui S, Ohkuma M. Hydrogen production by termite gut protists: characterization of iron hydrogenases of parabasalian symbionts of the termite Coptotermes formosanus . Eukaryot Cell. 2007;6:1925–1932. doi: 10.1128/EC.00251-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Graber JR, Leadbetter JR, Breznak JA. Description of Treponema azotonutricium sp. nov. and Treponema primitia sp. nov., the first spirochetes isolated from termite guts. Appl Environ Microbiol. 2004;70:1315–1320. doi: 10.1128/AEM.70.3.1315-1320.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Breznak JA, Switzer JM. Acetate synthesis from H2 plus CO2 by termite gut microbes. Appl Environ Microbiol. 1986;52:623–630. doi: 10.1128/aem.52.4.623-630.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Breznak JA, Switzer JM, Seitz H-J. Sporomusa termitida sp. nov., an H2/CO2-utilizing acetogen isolated from termites. Arch Microbiol. 1988;150:282–288. [Google Scholar]
- 80.Kane MD, Breznak JA. Acetonema longum gen. nov. sp. nov., an H2/CO2 acetogenic bacterium from the termite, Pterotermes occidentis. Arch Microbiol. 1991;156:91–98. doi: 10.1007/BF00290979. [DOI] [PubMed] [Google Scholar]
- 81.Leadbetter JR, Schmidt TM, Graber JR, Breznak JA. Acetogenesis from H2 plus CO2 by spirochetes from termite guts. Science. 1999;283:686–689. doi: 10.1126/science.283.5402.686. [DOI] [PubMed] [Google Scholar]
- 82.Graber JR, Breznak JA. Physiology and nutrition of Treponema primitia, an H2/CO2-acetogenic spirochete from termite hindguts. Appl Environ Microbiol. 2004;70:1307–1314. doi: 10.1128/AEM.70.3.1307-1314.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salmassi TM, Leadbetter JR. Analysis of genes of tetrahydrofolate-dependent metabolism from cultivated spirochaetes and the gut community of the termite Zootermopsis angusticollis . Microbiology. 2003;149:2529–2537. doi: 10.1099/mic.0.26351-0. [DOI] [PubMed] [Google Scholar]
- 84.Ottesen EA, Hong JW, Quake SR, Leadbetter JR. Microfluidic digital PCR enables multigene analysis of individual environmental bacteria. Science. 2006;314:1464–1467. doi: 10.1126/science.1131370. [DOI] [PubMed] [Google Scholar]
- 85.Pester M, Brune A. Expression profiles of fhs (FTHFS) genes support the hypothesis that spirochaetes dominate reductive acetogenesis in the hindgut of lower termites. Environ Microbiol. 2006;8:1261–1270. doi: 10.1111/j.1462-2920.2006.01020.x. [DOI] [PubMed] [Google Scholar]
- 86.Lee MJ, Schreurs PJ, Messer AC, Zinder SH. Association of methanogenic bacteria with flagellated protozoa from a termite gut. Curr Microbiol. 1987;15:337–341. [Google Scholar]
- 87.Inoue J, Noda S, Hongoh Y, Ui S, Ohkuma M. Identification of endosymbiotic methanogen and ectosymbiotic spirochetes of gut protists of the termite Coptotermes formosanus . Microbes Environ. 2008;23:94–97. doi: 10.1264/jsme2.23.94. [DOI] [PubMed] [Google Scholar]
- 88.Tokura M, Ohkuma M, Kudo T. Molecular phylogeny of methanogens associated with flagellated protists in the gut and with the gut epithelium of termites. FEMS Microbiol Ecol. 2000;33:233–240. doi: 10.1111/j.1574-6941.2000.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 89.Brauman A, Dore J, Eggleton P, Bignell DE, Breznak JA, Kane MD. Molecular phylogenetic profiling of prokaryotic communities in guts of termites with different feeding habits. FEMS Microbiol Ecol. 2001;35:27–36. doi: 10.1111/j.1574-6941.2001.tb00785.x. [DOI] [PubMed] [Google Scholar]
- 90.Donovan SE, Purdy KJ, Kane MD, Eggleton P. Comparison of Euryarchaea strains in the guts and food-soil of the soil-feeding termite Cubitermes fungifaber across different soil types. Appl Environ Microbiol. 2004;70:3884–3892. doi: 10.1128/AEM.70.7.3884-3892.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leadbetter JR, Breznak JA. Physiological ecology of Methanobrevibacter cuticularis sp. nov. and Methanobrevibacter curvatus sp. nov., isolated from the hindgut of the termite Reticulitermes flavipes . Appl Environ Microbiol. 1996;62:3620–3631. doi: 10.1128/aem.62.10.3620-3631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leadbetter JR, Crosby LD, Breznak JA. Methanobrevibacter filiformis sp. nov., a filamentous methanogen from termite hindguts. Arch Microbiol. 1998;169:287–292. doi: 10.1007/s002030050574. [DOI] [PubMed] [Google Scholar]
- 93.Purdy KJ. The distribution and diversity of Euryarchaeota in termite guts. Adv Appl Microbiol. 2007;62:63–80. doi: 10.1016/S0065-2164(07)62003-6. [DOI] [PubMed] [Google Scholar]
- 94.Hongoh Y, Ohkuma M. Termite gut flagellates and their methanogenic and eubacterial symbionts. In: Hackstein JHP, editor. Microbiology monographs 19: (Endo)symbiotic Methanogenic Archaea. Berlin: Springer; 2011. [Google Scholar]
- 95.Brune A. Methanogens in the digestive tract of termites. In: Hackstein JHP, editor. Microbiology monographs 19: (Endo)symbiotic Methanogenic Archaea. Berlin: Springer; 2011. [Google Scholar]
- 96.Breznak JA, Brill WJ, Mertins JW, Coppel HC. Nitrogen fixation in termites. Nature. 1973;244:577–580. doi: 10.1038/244577a0. [DOI] [PubMed] [Google Scholar]
- 97.Benemann JR. Nitrogen fixation in termites. Science. 1973;181:164–165. doi: 10.1126/science.181.4095.164. [DOI] [PubMed] [Google Scholar]
- 98.Ohkuma M, Noda S, Kudo T. Phylogenetic diversity of nitrogen fixation genes in the symbiotic microbial community in the gut of diverse termites. Appl Environ Microbiol. 1999;65:4926–4934. doi: 10.1128/aem.65.11.4926-4934.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Noda S, Ohkuma M, Usami R, Horikoshi K, Kudo T. Culture-independent characterization of a gene responsible for nitrogen fixation in the symbiotic microbial community in the gut of the termite Neotermes koshunensis . Appl Environ Microbiol. 1999;65:4935–4942. doi: 10.1128/aem.65.11.4935-4942.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Noda S, Ohkuma M, Kudo T. Nitrogen fixation genes expressed in symbiotic microbial community in the gut of the termite Coptotermes formosanus . Microbes Environ. 2002;17:139–143. [Google Scholar]
- 101.Lilburn TG, Kim KS, Ostrom NE, Byzek KR, Leadbetter JR, Breznak JA. Nitrogen fixation by symbiotic and free-living spirochetes. Science. 2001;292:2495–2498. doi: 10.1126/science.1060281. [DOI] [PubMed] [Google Scholar]
- 102.Stingl U, Maass A, Radek R, Brune A. Symbionts of the gut flagellate Staurojoenina sp. from Neotermes cubanus represent a novel, termite-associated lineage of Bacteroidales: description of ‘Candidatus Vestibaculum illigatum’. Microbiology. 2004;150:2229–2235. doi: 10.1099/mic.0.27135-0. [DOI] [PubMed] [Google Scholar]
- 103.Noda S, Inoue T, Hongoh Y, Kawai M, Nalepa CA, Vongkaluang C, Kudo T, Ohkuma M. Identification and characterization of ectosymbionts of distinct lineages in Bacteroidales attached to flagellated protists in the gut of termites and a wood-feeding cockroach. Environ Microbiol. 2006;8:11–20. doi: 10.1111/j.1462-2920.2005.00860.x. [DOI] [PubMed] [Google Scholar]
- 104.Noda S, Hongoh Y, Sato T, Ohkuma M. Complex coevolutionary history of symbiotic Bacteroidales bacteria of various protists in the gut of termites. BMC Evol Biol. 2009;9:e158. doi: 10.1186/1471-2148-9-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Desai MS, Strassert JFH, Meuser K, Hertel H, Ikeda-Ohtsubo W, Radek R, Brune A. Strict cospeciation of devescovinid flagellates and Bacteroidales ectosymbionts in the gut of dry-wood termites (Kalotermitidae) Environ Microbiol. 2010;12:2120–2132. doi: 10.1111/j.1462-2920.2009.02080.x. [DOI] [PubMed] [Google Scholar]
- 106.Hongoh Y, Sato T, Dolan MF, Noda S, Ui S, Kudo T, Ohkuma M. The motility symbiont of the termite gut flagellate Caduceia versatilis is a member of the “Synergistes” group. Appl Environ Microbiol. 2007;73:6270–6276. doi: 10.1128/AEM.00750-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hongoh Y, Sato T, Noda S, Ui S, Kudo T, Ohkuma M. Candidatus Symbiothrix dinenymphae: bristle-like Bacteroidales ectosymbionts of termite gut protists. Environ Microbiol. 2007;9:2631–2635. doi: 10.1111/j.1462-2920.2007.01365.x. [DOI] [PubMed] [Google Scholar]
- 108.Cleveland LR, Grimstone AV. The fine structure of the flagellate Mixotricha paradoxa and its associated micro-organisms. Proc R Soc Lond B. 1964;159:668–686. [Google Scholar]
- 109.Iida T, Ohkuma M, Ohtoko K, Kudo T. Symbiotic spirochetes in the termite hindgut: phylogenetic identification of ectosymbiotic spirochetes of oxymonad protists. FEMS Microbiol Ecol. 2000;34:17–26. doi: 10.1111/j.1574-6941.2000.tb00750.x. [DOI] [PubMed] [Google Scholar]
- 110.Noda S, Ohkuma M, Yamada A, Hongoh Y, Kudo T. Phylogenetic position and in situ identification of ectosymbiotic spirochetes on protists in the termite gut. Appl Environ Microbiol. 2003;69:625–633. doi: 10.1128/AEM.69.1.625-633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Noda S, Iida T, Kitade O, Nakajima H, Kudo T, Ohkuma M. Endosymbiotic Bacteroidales bacteria of the flagellated protist Pseudotrichonympha grassii in the gut of the termite Coptotermes formosanus . Appl Environ Microbiol. 2005;71:8811–8817. doi: 10.1128/AEM.71.12.8811-8817.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Noda S, Kitade O, Inoue T, Kawai M, Kanuka M, Hiroshima K, Hongoh Y, Constantino R, Uys V, Zhong J-H, Kudo T, Ohkuma M. Cospeciation in the triplex symbiosis of termite gut protists (Pseudotrichonympha spp.), their hosts, and their bacterial endosymbionts. Mol Ecol. 2007;16:1257–1266. doi: 10.1111/j.1365-294X.2006.03219.x. [DOI] [PubMed] [Google Scholar]
- 113.Stingl U, Radek R, Yang H, Brune A. “Endomicrobia”: cytoplasmic symbionts of termite gut protozoa form a separate phylum of prokaryotes. Appl Environ Microbiol. 2005;71:1473–1479. doi: 10.1128/AEM.71.3.1473-1479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ohkuma M, Sato T, Noda S, Ui S, Kudo T, Hongoh Y. The candidate phylum ‘Termite Group 1’ of bacteria: phylogenetic diversity, distribution, and endosymbiont members of various gut flagellated protists. FEMS Microbiol Ecol. 2007;60:467–476. doi: 10.1111/j.1574-6941.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 115.Ikeda-Ohtsubo W, Desai M, Stingl U, Brune A. Phylogenetic diversity of ‘Endomicrobia’ and their specific affiliation with termite gut flagellates. Microbiology. 2007;153:3458–3465. doi: 10.1099/mic.0.2007/009217-0. [DOI] [PubMed] [Google Scholar]
- 116.Ikeda-Ohtsubo W, Brune A. Cospeciation of termite gut flagellates and their bacterial endosymbionts: Trichonympha species and ‘Candidatus Endomicrobium trichonymphae’. Mol Ecol. 2009;18:332–342. doi: 10.1111/j.1365-294X.2008.04029.x. [DOI] [PubMed] [Google Scholar]
- 117.Ohkuma M, Kudo T. Phylogenetic diversity of the intestinal bacterial community in the termite Reticulitermes speratus . Appl Environ Microbiol. 1996;62:461–468. doi: 10.1128/aem.62.2.461-468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fröhlich J, König H. Rapid isolation of single microbial cells from mixed natural and laboratory populations with the aid of a micromanipulator. Syst Appl Microbiol. 1999;22:249–257. doi: 10.1016/S0723-2020(99)80072-1. [DOI] [PubMed] [Google Scholar]
- 119.Sato T, Hongoh Y, Noda S, Hattori S, Ui S, Ohkuma M. Candidatus Desulfovibrio trichonymphae, a novel intracellular symbiont of the flagellate Trichonympha agilis in termite gut. Environ Microbiol. 2009;11:1007–1015. doi: 10.1111/j.1462-2920.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 120.Brune A, Stingl U. Prokaryotic symbionts of termite gut flagellates: phylogenetic and metabolic implications of a tripartite symbiosis. Prog Mol Subcell Biol. 2006;41:39–60. doi: 10.1007/3-540-28221-1_3. [DOI] [PubMed] [Google Scholar]
- 121.Ohkuma M. Symbioses of flagellates and prokaryotes in the gut of lower termites. Trends Microbiol. 2008;16:345–352. doi: 10.1016/j.tim.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 122.Hongoh Y, Sharma VK, Prakash T, Noda S, Taylor TD, Kudo T, Sakaki Y, Toyoda A, Hattori M, Ohkuma M. Complete genome of the uncultured Termite Group 1 bacteria in a single host protist cell. Proc Natl Acad Sci USA. 2008;105:5555–5560. doi: 10.1073/pnas.0801389105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hongoh Y, Sharma VK, Prakash T, Noda S, Toh H, Taylor TD, Kudo T, Sakaki Y, Toyoda A, Hattori M, Ohkuma M. Genome of an endosymbiont coupling N2 fixation to cellulolysis within protist cells in termite gut. Science. 2008;322:1108–1109. doi: 10.1126/science.1165578. [DOI] [PubMed] [Google Scholar]