Abstract
Recently, additional subsets that extend the family of innate lymphocytes have been discovered. Among these newly identified innate lymphoid cells is a subset sharing phenotypic characteristics of natural killer cells and lymphoid tissue inducer cells. These cells co-express the transcription factor RORγt and activating NK cell receptors (NKR), but their lineage and functional qualities remain poorly defined. Here, we discuss recent proposals to place these NKR+RORγt+ innate lymphocytes on hematopoietic lineage maps. An overview of the transcriptional circuitry determining fate decisions of innate lymphocytes and a summary of current concepts concerning plasticity and stability of innate lymphocyte effector fates are provided. We will conclude by discussing the function of RORγt-expressing innate lymphocytes during inflammatory bowel diseases and in the immune response to tumors.
Keywords: Natural killer cells, Lymphoid tissue inducer cells, Natural helper cells, Innate lymphocytes, Innate lymphoid cells, Inflammatory bowel diseases, Tumor immunity, Plasticity
Introduction to innate lymphocyte lineages
Until very recently, natural killer (NK) cells were the lonely representative of innate lymphocytes on hematopoietic lineage maps. NK cells are the killer cells of the innate immune system and are an important innate source of IFN-γ. NK cells are required for immunity to various viral infections and limit tumor development [1–3]. Recently, NK cells have been joined by two distinct innate lymphocyte subsets, lymphoid tissue inducer (LTi) cells and natural helper (NH) cells [4] (also known as type 2 innate lymphocytes [5] or nuocytes [6]). Both of these innate lymphocyte subsets lack markers of mature hematopoietic lineages (Lin-negative) and still express cell surface molecules also found on lymphoid progenitors, such as the interleukin (IL)-7 receptor α chain (CD127) and the receptor tyrosine kinase Kit (CD117), and are therefore referred to as innate lymphoid cells (ILCs) [4, 7]. LTi cells were originally identified as lymphocytes that instruct prenatal organogenesis of lymph nodes and Peyer’s patches [7–9]. More recently, it has become clear that these cells are also present postnatally, and they have been implicated in the regeneration of lymphoid organs following viral infections [10] and may be involved in preserving T cell memory [11, 12]. Furthermore, it has been found that intestinal LTi cell populations produce cytokines, such as IL-17 and IL-22, involved in the regulation of epithelial homeostasis and in the defense against intestinal infections [13–20]. The latest addition to the family of innate lymphocytes are NH cells, which are an innate source of IL-5 and IL-13 [4] and have been shown to play a role during immunity to worm infections [5, 6, 21] and in the pathogenesis of infection-induced airway hyperreactivity [22]. ILC populations are well represented in the intestine (>20% of all lamina propria lymphocytes in the small intestine), whereas they are a minor subset in secondary lymphoid organs.
Intriguingly, the cytokine profiles of the various innate lymphocyte subsets strikingly resemble those of the major T helper (Th) cell effector populations (Fig. 1). These similarities extend to the transcriptional programs controlling effector functions and/or development of ILCs (Fig. 1). Similar to Th1 cells, the effector program of NK cells is partially controlled by the T-box transcription factor T-bet (Tbx21) [23]. RORγt instructs development and function of LTi cells and Th17 cells [9, 24–26]. The transcriptional program controlling NH cell function is unknown, but gene array data suggested expression of GATA-3 by NH cells [5], which is also required for Th2 effector fate decisions [27]. Collectively, these data suggest that the transcriptional programs, first shown to be required for T helper cell fate decisions, were already pre-formed in the evolutionary older innate immune system before adaptive immunity emerged.
Fig. 1.
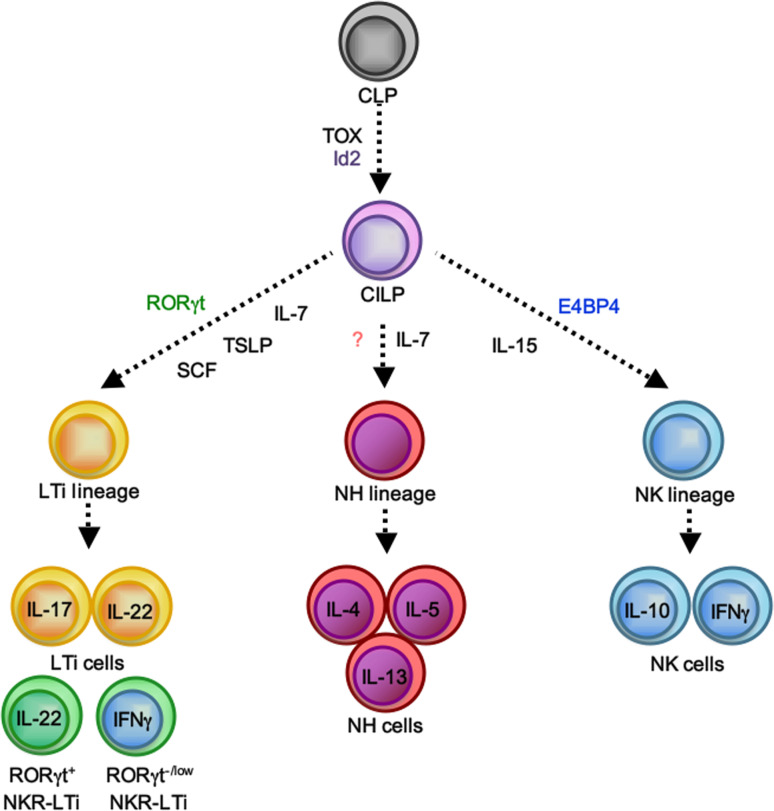
Innate lymphocyte lineages. All innate lymphocyte lineages can be generated from the common lymphoid progenitor (CLP) ([137], and A.M., A.D., unpublished data). Based on the finding that Id2 −/− and Tox −/− mice lack all innate lymphocyte lineages, a putative Id2 and/or Tox-dependent common ILC precursor (CILP, ILCP) has been proposed [28, 29]. After specification into the various lymphocyte lineages controlled by the expression of lineage-defining transcription factors and cytokines, various effector fates can be assumed
A meaningful and satisfying nomenclature for innate lymphocytes is still under development, and none of the circulating proposals has been generally embraced. Based on the above-mentioned similarities in transcriptional circuitry and effector functions between ILC and T helper cell subsets, it was proposed to adopt the nomenclature of the various T helper cell effector fates to classify ILC subsets (i.e., ILC1 for thymic NK cells, ILC2 for NH cells and ILC17 for LTi cells) [28]. While this nomenclature has the advantage of building on an accepted paradigm, there are also important differences between ILC and Th cells, and it remains unknown whether for example IL-17 production is the most important functional determinant of LTi lineage cells [29]. A nomenclature using the lineage or effector fate-determining transcription factor has been adopted by some to refer to the various LTi cell subsets as RORγt+ ILC [19, 30, 31]. While this seems to be meaningful, a lineage-defining transcription factor for NH cells is unknown. For the purpose of this review, we will refer to the various innate lymphocyte lineages by their historical names that were based on aspects of their functional programs (‘natural killer,’ ‘lymphoid tissue inducer,’ ‘natural helper’). It should be noted though that by using for example the term LTi cell, we are referring to an innate lymphocyte lineage rather than to a population of cells with homogenous functional qualities (Fig. 1).
In this review, we will focus on recent progress in understanding the relationship between innate lymphocyte subsets, in particular between LTi cells and NK cells. We will also put the spotlight on the transcriptional programs controlling cell fate decisions of innate lymphocytes, on new aspects of postnatal LTi cell function in the context of inflammatory bowel diseases and on their role in the defense against tumors.
Developmental relationship between innate lymphocyte subsets
Available evidence from gene knock-out mice supports the view that innate lymphocytes may share the developmental requirement for certain transcription factors. Mice lacking the transcription factor inhibitor of DNA binding 2 (Id2) and thymocyte selection-associated high mobility group box protein (TOX) show major defects in the development of NK cells and LTi cells [4, 32–34]. NH cells are also Id2-dependent, but the analysis of NH cells in TOX-deficient mice is still lacking [4, 33]. Collectively, the available data suggest a common developmental program of innate lymphocyte lineages [28, 29]. The fact that the three known innate lymphocyte lineages require Id2 for their development has led to the proposal of an Id2-dependent precursor to all innate lymphocyte lineages, which was dubbed common innate lymphoid progenitor (CILP) [29] or ILCP (Id2-expressing innate lymphoid cell precursor) [28]. Although the bone marrow-resident NK cell progenitor (NKp) (CD122+NK1.1−DX5− cells) already expresses Id2 [32, 35], Id2 is not required for NK lineage specification because the NKp normally develops in the absence of Id2 [32]. However, Id2 is required for the development of mature (CD122+NK1.1+DX5+) NK cells that are lacking in bone marrow and peripheral lymphoid organs of Id2-deficient mice [32]. Id2 represses E box proteins, and genetic deletion of E2A in Id2 −/− mice restores NK cell development and lymphorganogenesis [32, 36].
A recent report using an Id2 reporter mouse strain has found that while the common lymphoid progenitor (CLP) is Id2-negative, two populations of Lin-negative bone marrow-resident lymphoid precursor cells express Id2 [35]. Both Id2-positive subsets express CD127 and Sca1 and are negative for CD122 and CD135 (Flt3), but can be discriminated based on their expression of CD117 (Kit) into a Kit− and a Kitlow subset. Lin−Sca1+Kit− (LSK−) cells have been previously recognized, but their relationship to the major lymphocyte lineages remains unclear [37–39]. In vitro differentiation of these Id2+CD127+Sca1+ cells under conditions known to drive NK cell development revealed that these cells upregulate NK cell receptors (NKR), suggesting that they may constitute the earliest committed progenitor to the NK cell lineage, and they have been accordingly dubbed ‘pre-pro NK cells’ [35]. The transcriptional profiles of ‘pre-pro NK cells’ or their developmental potential in vivo were not addressed, and a previous report failed to generate any NK cell progeny after adoptive transfer of this population in vivo [38]. Future studies are needed to investigate the potential of Id2-expressing lymphoid progenitors into NH cells or LTi cells, subsets of which also express NK cell receptors [19, 28, 31].
Subsets of RORγt-expressing innate lymphocytes
RORγt+ ILC (LTi cells)
During prenatal lymphoid organogenesis, LTi cells develop in the fetal liver and home to the lymph node and Peyer’s patch anlagen where they instruct lymph organ development through close interaction with mesenchymal stroma involving TNF superfamily receptors [40–42]. Excellent recent reviews have comprehensively covered this topic, and we will focus on the role of LTi cells after birth [43–47].
A rather large pool of LTi lineage cells (ca. 10% of all lamina propria cells) is found in the lamina propria of the small intestine of adult mice, but such RORγt+ ILC are also present in other organs such as colon and liver, and in the spleen or lymph nodes where they represent a rather small lymphocyte subset [11, 16, 19, 48, 49]. Intestinal RORγt+ ILCs have an LTi function, as they are required for the postnatal formation of the multiple lymphoid follicles within the intestinal lamina propria [50–52]. After birth, LTi cells are scattered throughout the intestinal lamina propria. The initial event of lymphoid follicle formation is the clustering of LTi cells resulting in the development of 1,000–1,500 lymphoid clusters, referred to as cryptopatches (CP), that almost exclusively contain LTi cells and are surrounded by dendritic cells [50, 53, 54]. CPs develop around day 14 after birth in close vicinity to the small intestinal crypts, but it is unknown which positional cues LTi cells receive. The molecular events driving the formation of CPs are reminiscent of those identified for prenatal organogenesis of lymph nodes and involve crosstalk between LTi cells and mesenchymal organizer cells (reviewed in [54]). CP development is accompanied by an expansion of the RORγt+ ILC pool during the first 3–4 weeks after birth ([31]; A.D., manuscript submitted). Based on the finding that CP formation is not impeded in germ-free mice, it has been concluded that CP development is programmed and independent of environmental cues [53, 55].
Some (but not all) CPs further develop into intestinal lymphoid follicles (ILF) that contain B cells [52, 55]. Progression from CP to ILF is dependent on signals from the intestinal microbiota because germ-free mice lack ILF [53, 55–57]. Instruction of ILF formation requires epithelial sensing of commensal bacteria involving the pattern recognition receptor nucleotide-binding oligomerization domain-containing protein 1 (NOD1), which recognizes d-glutamyl-meso-diaminopimelic acid (iE-DAP) of gram-positive and some gram-negative bacteria [55, 58]. NOD1-dependent production of CCL20 contributes to the recruitment of CCR6-expressing B cells to CP [55]. Another report demonstrated an important role of the CXCR5/CXCL13 axis for the recruitment of B cells to ILF [59]. ILFs have been shown to contain B cells producing IgA specific for T cell-independent antigens, and ILFs have been identified as crucial structures supporting isotype switching of lamina propria B cells [51]. IgA-producing B cells are important to sequester commensal bacteria and to protect the intestinal barrier [60, 61].
In addition to being lymphocytes with LTi function, human and mouse RORγt+ ILCs constitutively secrete cytokines such as IL-22 and IL-17 [14, 16, 19]. Most importantly, these cytokines have been demonstrated to play an important role in the maintenance of intestinal epithelial homeostasis and in shaping the commensal microbiota by inducing epithelial expression of antimicrobial proteins [13, 15, 17, 49, 62–64]. The role of IL-22 produced by RORγt+ ILCs for epithelial homeostasis and antimicrobial effector functions will not be discussed here, as excellent recent reviews have extensively covered that topic [29, 65, 66].
The development of the prenatal and postnatal pool of LTi cells strictly depends on the transcription factor RORγt, and all LTi cells express this transcription factor [9, 19, 25]. Consequently, RORγt-deficient mice lack the development of secondary lymphoid organs (lymph nodes and Peyer’s patches) and of postnatally developing intestinal lymphoid follicles (i.e., CP and ILF) [9]. While initial studies have identified LTi cells in newborn mice as Lin−CD4+CD127+CD117+ lymphocytes [7], analysis of RORγt-reporter mice showed that the pool of RORγt+ ILCs in the intestine of newborn mice is composed of a CD4+ and a CD4− subset that are equally represented at birth [8, 31]. While the pool of CD4− RORγt+ ILCs expands in size during the first 3 weeks after birth, the population of CD4+ RORγt+ ILCs remains rather stable ([31] and A.D., manuscript submitted). The molecular signals driving the differential expansion of these distinct subsets are unknown.
Kit, IL-7 and TSLP are required for the maintenance of intestinal LTi cells
LTi lineage cells express high levels of the IL-7R, and IL-7 signaling has multifaceted roles for their development, function, recruitment and maintenance [43]. IL-7R signaling is a requirement for the development of Peyer’s patches, but not for the organogenesis of lymph nodes [8, 67–70]. Organogenesis of lymph nodes requires TRANCE (RANKL), which is dispensable for Peyer’s patch development [71, 72]. However, overexpression of IL-7 in mice lacking TRANCE signaling (Traf6 −/− mice) rescues lymph node development, demonstrating that LTi cells in lymph nodes are in principle IL-7 responsive [72]. The differential requirements for TRANCE or IL-7 are likely a consequence of the different availability of these factors in the various lymphoid tissue anlagen. In addition, IL-7 plays an important role in upregulating LTα1β2 expression by LTi cells residing in the mesenteric lymph nodes of newborn mice [73]. An important observation was that IL-7Rα-deficient (Il7ra −/−) and Il7 −/− mice have reduced numbers of CD3−α4β7−integrin+ LTi cells in mesenteric LN of newborn mice, whereas their numbers were normal in peripheral blood or spleen [73, 74]. Two non-exclusive models may explain this finding. The reduced expression of LTα1β2 results in decreased interactions with mesenchymal organizer cells leading to reduced production of chemokines and impaired recruitment of LTi cells [73]. In addition, IL-7 may also have a profound role on the survival and maintenance of the LTi cell pool [75, 76].
Consistent with the reduced numbers of LTi lineage cells in mesenteric and peripheral LNs of Il7ra −/− mice [73], the numbers of RORγt+ ILCs in the intestinal lamina propria of adult Il7ra −/− mice are also substantially reduced [19]. In contrast to LTi cells in LN of Il7 −/− mice [74, 75], intestinal LTi cell numbers were only mildly (2–3-fold) affected in mice lacking IL-7 [19, 77]. Similarly, deficiency in TSLP signaling, a cytokine also requiring the IL-7Rα chain for signaling [78], also resulted in mildly reduced numbers of intestinal LTi cells, whereas LTi cell numbers in peripheral lymph nodes were unaffected [19]. Our data suggest that IL-7 and TSLP are redundantly required for the maintenance of intestinal LTi cells of adult mice. The different requirements for the maintenance of LTi cells in lymph nodes and intestine likely reflect differential availability of these two cytokines. While in lymph nodes only IL-7 is available, in the intestine both IL-7 and TSLP are expressed.
LTi cells have been originally defined as Kit-expressing lymphoid cells [53]. Recent evidence has suggested an important role of the receptor tyrosine kinase Kit for the differentiation and maintenance of the intestinal LTi cell pool [79]. Newborn mice with impaired Kit signaling (Kit W/Wv mice) had significantly reduced numbers of intestinal LTi cells [79]. In vitro stimulation of fetal liver LTi cell progenitors with stem cell factor (SCF, the Kit ligand) alone did not lead to the generation of RORγt+ ILCs, but SCF acted synergistically with IL-7 [79]. A very similar, synergistic effect between Kit and IL-7 signaling was observed for the survival of LTi cells [79]. Thus, Kit signals are required for optimal IL-7 or TSLP-mediated differentiation and maintenance of the intestinal LTi cell pool.
Using terminable fate labeling of fetal LTi cells, it has been noted that postnatal Kit expression by the progeny of fate-labeled fetal LTi cells is variable and may be used to distinguish various LTi cell subsets [31]. When fate labeling of LTi cells was terminated perinatally, only Kithigh cells were present in the first week after birth. However, from week 2 on, LTi cells with lower levels of Kit expression emerge. While the Kitlow subset does not express CD4, a CD4+ and a CD4− population of Kithigh cells exists. In that experimental system, all RORγt+ cells are labeled until fate labeling was stopped at day 3 after birth when all RORγt+ ILCs are Kithigh. In the most straightforward scenario, the appearance of CD4−Kitlow cells reflects downregulation of Kit by CD4−RORγt+ ILCs. The emergence of CD4−KitlowRORγt+ ILCs alternatively may be explained by selective proliferation of the small postnatal population of CD4−KitlowRORγt+ ILCs or recruitment of a distinct Kitlow subset to the intestinal lamina propria that could not migrate there before. A series of in vitro experiments supports the notion that Kitlow cells may be the progeny of a distinct subset of fetal liver RORγt+ cells. While Kithigh cells could be differentiated in vitro from RORγthigh α4β7−integrin+ fetal liver cells, Kitlow cells were the progeny of RORγtlowα4β7−integrin− fetal liver cells. It was not tested whether α4β7−integrin+ fetal liver cells were the progenitors of the α4β7−integrin subset. Although both the Kithigh and Kitlow LTi populations develop in an RORγt-dependent fashion, these data may suggest that Kitlow and Kithigh RORγt+ ILCs represent distinct populations and may have distinct RORγt-expressing fetal liver progenitors. Future studies analyzing the transcriptional program and effector profile of the CD4−Kitlow subset of RORγt+ ILCs are required to demonstrate whether this population represents an innate lymphocyte lineage independent of the LTi lineage.
NK cell receptor-expressing RORγt+ ILCs
Recent studies have identified a population of RORγt-expressing innate lymphocytes that co-expresses receptors commonly found on NK cells, such as NKp46 and NKG2D in mice or CD56, NKp46 and NKp44 in humans (NKR+RORγt+ ILC) [13–15, 17, 18, 63, 64]. Similar to NKR−RORγt+ ILCs, NKR+RORγt+ ILCs were shown to produce IL-22 and IL-17 at steady state in the spleen and intestine of mice, and in human fetal lymph nodes and tonsils. Excellent recent reviews have highlighted the role of these cells during infections and in the maintenance of intestinal homeostasis [29, 65, 80].
Because this peculiar lymphoid subset phenotypically resembles NK cells (NKR expression) and LTi cells (RORγt expression), it has been discussed whether NKR+RORγt+ ILCs are of the NK or LTi lineage. The various reports initially characterizing NKR+RORγt+ ILCs demonstrated that this subset shares a developmental program with LTi lineage cells because they are developmentally dependent on the transcription factor RORγt and LTi cells but not conventional NK cells require IL-7Rα signaling for differentiation and/or survival [13, 15, 19, 77]. In contrast, NKR+RORγt+ ILCs develop independently of IL-15, a growth factor crucial for NK cell development and survival [13, 15]. Mutations of the IL7R gene in humans cause severe combined immunodeficiency disease (SCID), and analysis of immune cells from these patients revealed a substantial reduction of T cells, whereas NK cells and B cells developed normally [81, 82]. Analysis of the human equivalent of NKR+RORγt+ ILCs (i.e., ‘NK-22 cells’) in patients suffering from SCID due to mutations in the IL7R gene revealed that human ‘NK-22 cells’ also require IL-7 signaling for their development or survival, whereas conventional NK cells are normally represented [19]. Mice genetically deficient for E4BP4 or NFIL3, a transcription factor selectively required for NK cell development, have normal numbers of intestinal NKR+RORγt+ ILCs ([83, 84] and H.M. Brady, personal communication). Collectively, these data demonstrate that the developmental program of human and mouse NKR+RORγt+ ILCs more closely resembles that of LTi lineage cells than those of the conventional NK lineage.
Initial evidence that LTi cells may differentiate into NKR-expressing cells came from clonal in vitro differentiation assays demonstrating the potential of fetal CD4+ LTi cells to upregulate NKRs and adopt functional characteristics of NK cells (cytotoxicity, IFN-γ production) [7]. Similar data have been provided for human LTi cells (Lin−CD127+CD117+ cells) that acquire the expression of NK cell receptors (CD56, NKp46) when cultured in vitro [14]. These data suggested that RORγt+ ILCs may be the progenitors of NK cells. However, analysis of a RORγt-fate map demonstrated that conventional NK cells at no time of their lineage development express RORγt, ruling out that RORγt-expressing LTi cells are the progenitors of conventional NK cells and demonstrating that NK cells and LTi cells are separate lymphocyte lineages [19, 77]. Recently, the origin of NKR+RORγt+ ILC cells has been rigorously addressed in vivo by using a combination of in vivo transfer of genetically tagged LTi cells and genetic lineage tracing of RORγt-expressing cells. Adoptive transfer of NKR−RORγt+ ILCs into lymphoid mice revealed, that these cells acquired the expression of NKR [19]. In contrast, NK cells did not upregulate RORγt in vivo or in vitro even if they were cultured under conditions known to induce RORγt expression by Th17 cells [19]. These data were confirmed by a study using terminable fate labeling of fetal LTi cells [31]. At the time point of termination of fate labeling (day 3 after birth), all RORγt+ ILCs were NKR-negative. However, 2 weeks later, NKR+RORγt+ cells emerged, consistent with the notion that NKR−RORγt+ ILCs upregulate the expression of NKR. Collectively, these data demonstrate that NKR+RORγt+ cells are the progeny of NKR−RORγt+ cells and belong to the LTi lineage of innate lymphocytes. Consequently, it has been proposed to refer to these cells as NKR-expressing LTi cells, NKR-LTi cells [19]. Importantly, these data also show that the pool of NKR+ innate lymphocytes is composed of at least two ILC lineages, NK cells and LTi-derived NKR-LTi cells [19]. While the population of LTi-derived NKR+ cells is small in spleen and LN (<15% of all NKR+ cells), it is rather substantial in the intestinal mucosa [19].
Interestingly, not all subsets of NKR−RORγt+ ILCs have the same propensity to upregulate NKRs. One report has found that the population of CD4−KitlowNKR−RORγt+ ILCs readily acquires NKRs, whereas the CD4+Kithigh and CD4−Kithigh subsets of NKR−RORγt+ ILCs do not [31]. However, others have reported that CD4+ LTi cells acquire NKR after in vitro culture in IL-2 [7] and that a sizeable population of CD4+KitlowNKR+RORγt+ ILCs exists in various organs [19]. It is possible that these at first sight contradictory data may be due to the different experimental strategies used.
Plasticity and stability of transcriptional programs within ILC lineages
It is now widely believed that NKR+RORγt+ ILCs are the direct progeny of NKR−RORγt+ LTi lineage cells [19, 31]. Using analysis of RORγt expression by LTi lineage cells (i.e., LTi cells and NKR-LTi cells) in the context of a RORγt-fate map, it became obvious that NKR-negative LTi cells stably express RORγt, but that a subset of NKR-LTi cells downregulates RORγt expression [19]. Interestingly, the extent of RORγt downregulation is dependent on the tissue microenvironment. While only very few ‘ex’-RORγt NKR-LTi cells (i.e., RORγt−/low NKR-LTi cells) were detected in the small intestine, the majority of NKR-LTi cells in colon, spleen and peripheral LN expressed only very low levels of RORγt [19]. Stable expression of RORγt by NKR+RORγt+ ILCs in the small intestine was confirmed in experiments using the terminable fate labeling strategy of fetal LTi cells described above [31]. Thus, RORγt+ ILCs undergo a three-step differentiation program: NKR−RORγt+ cells (LTi cells) → NKR+RORγt+ cells (RORγt+ NKR-LTi cells) → NKR+RORγt−/low cells (RORγt−/low NKR-LTi cells). The execution of the last step depends on the tissue context with some organs being permissive (spleen, lymph nodes, colon), whereas the small intestine is non-permissive for the downregulation of RORγt expression [19]. Collectively, these data demonstrate that expression of RORγt by LTi cells is not irreversibly fixed but may be destabilized under certain conditions, allowing for a certain degree of plasticity and potentially conferring functional flexibility to LTi lineage cells.
It was a surprising finding that transcriptional programs of innate lymphocytes show plasticity, challenging the view that the functional fate of innate lymphocytes is irreversibly fixed. Most of our knowledge concerning plasticity of lymphocyte effector fates comes from the T helper cell system [85, 86]. Plasticity is generally perceived to allow a certain degree of flexibility in effector and transcriptional programs. Such flexibility allows Th cells to adapt their effector profiles to various phases of an infection without the need for time-consuming de novo priming of pathogen-specific T cell responses [87]. Examples of plastic behavior have recently been documented for RORγt-expressing Th17 cells secreting IL-17, converting into T-bet-positive, IFN-γ-producing effector cells [88, 89]. Similar observations of abandoning a pre-programmed effector fate have been noted for other T helper cell lineages such as regulatory T cells [90, 91] and Th2 cells [92]. However, others have maintained that at least the fate of Foxp3-expressing T cells is stable [93].
An important question is, what does determine the relative stability of innate lymphocyte subsets? Three processes have been proposed to contribute to the relative stability of T helper cell subsets, which may also be applicable to innate lymphocytes, conditioning, circuitry and chromatin [85]. Conditioning means the sum of molecular cues that prime lineage decisions. These may vary for innate lymphocytes depending on the developmental stage (fetal vs. adult) or the priming conditions (different organs, microbiota, etc.), which may affect the stability of the resulting effector fate. Not much is currently known about the molecular signals determining the LTi lineage decision during fetal or adult life. Transcriptional circuits can further promote either stability or plasticity. For example, the transcriptional circuits driving Th1 or Th2 lineage decisions are self-reinforcing, leading to relative stability of the resulting effector fates, whereas the transcriptional circuitry of Treg and Th17 cells may allow for instabilities [85]. Heritable, epigenetic chromatin modifications are likely another important factor determining functional plasticity, and specifically ‘bivalent’ chromatin marks have been correlated with functional plasticity [86, 94–96]. While most of these parameters have not been analyzed at all for innate lymphocyte subsets, two questions regarding the plasticity within the LTi lineage have recently been addressed. (1) What are the factors that stabilize or reinforce RORγt expression by LTi lineage cells in the small intestine and which factors could be destabilizing RORγt in the colon, spleen or LN? (2) What is the functional profile of ‘ex’-RORγt+ ILCs?
Cytokines reinforcing or destabilizing RORγt expression by LTi lineage cells
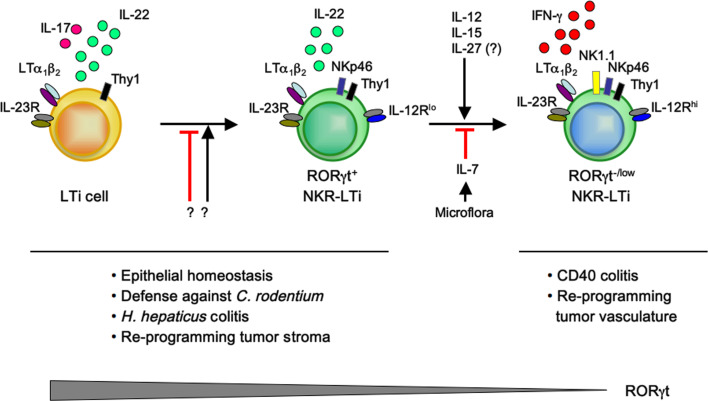
The molecular cues reinforcing RORγt expression are poorly defined. Recent data demonstrate that IL-7 is a factor stabilizing RORγt expression in RORγt+ NKR-LTi cells (Fig. 2). Transfer of RORγt+ NKR-LTi cells into mice lacking IL-7 led to downregulation of RORγt expression and accelerated generation of RORγt−/low NKR-LTi cells [19]. In contrast, transfer of RORγt+ NKR-LTi cells into mice overexpressing IL-7 maintained the cells in a RORγt-positive state. These data were further corroborated by blockade of IL-7 signaling in RORγt-fate map mice leading to accelerated transition of RORγt+ NKR-LTi cells into RORγt−/low NKR-LTi cells [19]. The induction and maintenance of RORγt in Th17 cells depend on IL-1, IL-6 and IL-23 [97]. However, these cytokines play a redundant role in the maintenance of RORγt+ NKR-LTi cells because RORγt+ NKR-LTi cells were normally represented in mice deficient for IL-1R, IL-6 or IL-23p19 [19].
Fig. 2.
Differentiation and plasticity of LTi lineage cells. LTi cells are RORγt-expressing innate lymphoid cells (CD127+CD117+) that produce IL-22 and IL-17, are responsive to IL-23 and express surface LTα1β2 [14, 16]. LTi cells can stably upregulate NKRs such as NKp46, NKG2D and NK1.1 [19, 31], but the molecular cues driving this differentiation step are unknown. Such RORγt+ NKR-LTi cells no longer produce IL-17, but are a substantial source of IL-22 [13–15, 17, 63]. RORγt+ NKR-LTi can downregulate RORγt expression and differentiate into RORγt−/low NKR-LTi cells that do not secrete IL-22 but present with an alternative, NK-like effector profile exemplified by the production of IFN-γ in response to stimulation with IL-12 or IL-23 [19]. Downregulation of RORγt is dependent on the organ microenvironment, and microbiota-induced IL-7 has been found to reinforce RORγt expression, whereas IL-12, IL-15 and potentially IL-27 may destabilize RORγt expression accelerating the generation of RORγt−/low NKR-LTi cells [19]. IL-22-producing LTi cells and RORγt+ NKR-LTi cells are involved in promoting intestinal homeostasis [15, 138] and are required for immunity against infections with attaching and effacing bacteria such as those with Citrobacter rodentium [13, 20, 63]. A subset of NKR−RORγt+ LTi cells producing IL-17 and IFN-γ may be involved in colitis onset following chronic inflammation with Helicobacter hepaticus [30]. IFN-γ-producing RORγt−/low NKR-LTi cells are the colitogenic cells when inappropriately triggered during innate CD40 colitis [19, 30, 115]
Previous data have indicated that in germ-free mice the fraction of RORγt+ NKR-LTi cells is smaller compared to conventional mice [13, 15]. These data could reflect reduced differentiation of RORγt+ NKR-LTi cells from their NKR-negative progenitors or accelerated conversion into RORγt−/low NKR-LTi cells. Interestingly, RORγt-fate map mice treated with antibiotics to reduce the commensal microflora revealed an increased fraction of RORγt−/low NKR-LTi cells in the small intestine [19]. Downregulation of RORγt expression by NKR-LTi cells in antibiotic-treated mice can be at least partially explained by the reduced expression of IL-7 in germ-free mice [19, 98]. Collectively, these data demonstrate that microbiota-induced expression of IL-7 is an important factor in stabilizing RORγt expression by NKR-LTi cells in the small intestine. An important issue is that stable expression of RORγt is found in the small intestine, which harbors much less bacteria than the colon where the majority of NKR-LTi cells downregulated RORγt expression. It is likely that destabilizing signals in the colon outcompete the reinforcing effects of IL-7 on RORγt expression.
Which factors are involved in destabilizing RORγt expression? Th17 cells and LTi lineage cells display very similar effector programs, and downregulation of RORγt has been demonstrated for Th17 cells in vitro and very recently in vivo [88, 89]. The IL-12 family of cytokines, in particular IL-12, IL-23 and IL-27, can destabilize RORγt expression in Th17 cells, leading to an effector fate conversion towards a Th1-like phenotype characterized by the production of IFN-γ. The appearance of such IFN-γ-producing ‘ex’-Th17 cells is closely correlated with various inflammatory syndromes such as colitis and experimental autoimmune encephalitis (EAE) [88, 89, 99, 100]. IL-12 is a cytokine known to activate NK cells via the IL-12 receptor complex, leading to the phosphorylation of signal-transducer and activator of transcription (STAT) 1 and STAT4 [101–103]. These transcription factors induce the expression of Tbx21 (T-bet) and the upregulation of Il12rb2 (encoding the IL-12-specific β2 chain of the IL-12R), allowing for the secretion of IFN-γ by T helper cells [104, 105]. Interestingly, mice lacking IL-12 or the IL-12Rβ2 chain showed an accumulation of RORγt+ NKR-LTi cells, suggesting that in the absence of IL-12 the progression to the RORγt− NKR-LTi fate is slowed (Fig. 2) [19]. IL-23 and IL-27 have been implicated in the destabilization of RORγt expression in Th17 cells [89, 99]. However, the LTi lineage populations are normally represented in mice lacking IL-23 [19]. Further experiments analyzing IL-23 deficiency in the context of a RORγt-fate map are required to fully investigate potential roles of IL-23 for LTi lineage plasticity and control of RORγt expression. The role of IL-27 for alternative effector fates of LTi cells has yet to be addressed.
Regulated expression of RORγt within RORγt+ ILCs confers distinct functional fates
The functional consequences of the downregulation of RORγt expression in LTi lineage cells have recently been addressed [19]. Already the initial description of LTi cells indicated functional plasticity [7]. LTi cells that do not produce IFN-γ or display cell-mediated cytotoxicity [15] acquired such functional qualities after clonal culture in IL-2 [7]. Plasticity in the functional profiles of human LTi cells and NKR-LTi cells (‘NK-22’ cells) was also observed. NKR-LTi cells cultured with IL-7 alone maintained IL-22 production, which reinforces the concept that IL-7 stabilizes RORγt expression [106]. Addition of IL-2 to the NKR-LTi cell cultures led to reduced IL-22 expression and increased production of IFN-γ [106]. Interestingly, RORC expression was reduced when NKR-LTi cells were cultured in IL-2 plus IL-7 compared to those cultured in IL-1β plus IL-7 [106]. A large range of functional plasticity has been observed for human LTi cell clones established from various donors and maintained in culture with cytokines [107, 108]. Clones derived from Lin−CD117+CD127+ LTi-like cells or Lin−CD56+CD117+CD127+ LTi-like cells produced—in addition to IL-22 or IL-17—IFN-γ and TNF, but also IL-5, IL-13 and IL-2. Notably, the various functional fates were not accompanied by any changes in RORC mRNA expression.
The effector fates of NKR-LTi cells were further investigated in the context of a RORγt-fate map in mice. It became obvious that RORγt+ NKR-LTi cells produced IL-22 in response to IL-23 and IL-12. In contrast, RORγt−/low NKR-LTi cells produced IFN-γ in response to both IL-12 and IL-23 [19]. Interestingly, NKR-LTi cells that only recently downregulated RORγt expression could produce both IL-22 and IFN-γ. Analysis of transcriptional profiles of RORγt+ and RORγt−/low NKR-LTi cells revealed that RORγt expression correlated with the expression of Il23r, Il7ra and Il22, whereas downregulation of RORγt expression correlated with a transcriptional program reminiscent of conventional NK cells (increased expression of Il12rb2, Ifng and cytotoxic effector molecules such as granzyme B and perforin) [19]. These data may provide a molecular explanation for the observation that clonal culture of LTi cells in IL-2 turned them into potent IFN-γ-producing and cytotoxic ‘natural killer’ cells [7]. Although downregulation of RORγt expression correlates with a transcriptional program reminiscent of NK cells, it remains to be analyzed which underlying transcriptional circuitry drives these changes. Collectively, these data demonstrate that the effector fate of LTi lineage cells is not irreversibly fixed, but allows for alternative effector states that can be distinguished on the basis of RORγt expression.
Innate lymphoid cells and inflammatory bowel diseases
A series of recent data has implicated subsets of the LTi lineage of ILCs in the development of inflammatory bowel disease (IBD). Ulcerative colitis and Crohn’s disease are the main human forms of IBD, and represent severe inflammation of the intestinal tract that manifests with diarrhea, intestinal bleeding, leukocyte infiltration into the lamina propria and massive epithelial erosion [109–111]. The etiology of IBD is still incompletely understood, but is likely determined by genetic factors, inappropriate activation of immune cells and environmental factors. Genome-wide association studies have revealed that polymorphisms in the genes coding for IL-23 and the IL-23R are associated with the development of IBD [112]. Most of the research into IBD has focused on the role of T cell populations either in triggering IBD by the production of pro-inflammatory cytokines or by promoting disease through ineffective function of regulatory T cells. However, recent evidence suggests that IBD can be triggered in recombination activating gene 1 or 2-deficient (Rag1 −/− or Rag2 −/−) mice lacking all components of the adaptive immune system. Also these forms of innate colitis require IL-23, further stressing the importance of this cytokine for the development of inflammatory and autoimmune disorders [113].
Two mouse models of innate colitis have been described. Several weeks after Helicobacter (H.) hepaticus infection, Rag2 −/− mice on a 129SvEv background show signs of chronic intestinal inflammation that histopathologically displayed all the characteristics of human IBD [114]. Pathogenesis of Helicobacter-induced colitis is dependent on the production of IL-23 by myeloid cells and can be improved if IL-17 and/or IFN-γ is neutralized [30, 114]. The colitogenic IL-17 and IFN-γ-producing cell expresses RORγt, CCR6, CD25 and CD90 (Thy1), but not NKp46 [30]. This constellation of cell surface markers and the cytokine pattern are consistent with a cellular subset of the LTi lineage [115].
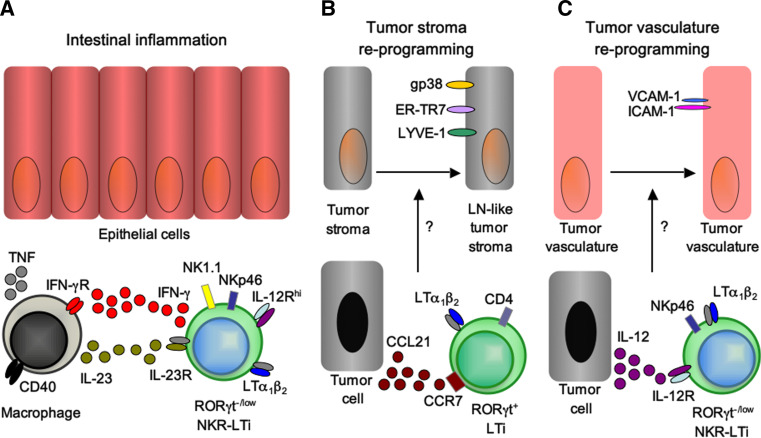
Another form of innate colitis can be induced in Rag-deficient mice by injecting an activating antibody specific for the costimulatory receptor CD40 expressed by myeloid cells [116]. CD40 has been involved in the pathogenesis of IBD by triggering IL-12 production and consequently disease-promoting IFN-γ responses [117]. In this model, colitis develops rather acutely (6–8 days after antibody injection) but yet histopathologically closely resembles human IBD [118]. Notably, Rag2 −/− animals deficient for IL-23p19 do not develop intestinal pathology upon injection with anti-CD40, implicating an essential role for IL-23 in mediating colitis [118]. In contrast to the Helicobacter model of innate colitis, CD40 colitis is independent of IL-17-secreting cells but IFN-γ production is a prerequisite [30, 118]. CD40 colitis requires the presence of innate lymphocytes because CD40 injection into Rag2 −/− Il2rg −/− mice that lack all lymphocytes do not develop colitis [19]. The role of innate lymphocytes for the onset of CD40 colitis is further supported by the observation that depletion of all innate lymphocytes by injection of Thy1 antibodies abrogates colitis [30]. Involvement of LTi lineage cells (i.e., RORγt+ ILCs) has been suggested by the finding that RORγt-deficient mice that lack all LTi lineage cells do not develop disease after injection of CD40 antibodies [30]. The question of which subset of RORγt+ ILCs promotes CD40 colitis has been recently addressed. RORγt− NKR-LTi cells but not RORγt+ NKR-LTi cells, LTi cells or NK cells isolated from RORγt-fate map mice produce IFN-γ upon stimulation with IL-23 [19], consistent with their effector profile discussed in detail above. To test the colitogenic potential of the various subsets of RORγt+ ILCs, an innate transfer colitis model has been developed. LTi lineage subsets are purified from RORγt-fate map mice and transferred into alymphoid Rag2 −/− Il2rg −/− mice [19]. Transfer of highly purified RORγt−/low NKR-LTi cells into lymphoid Rag2 −/− Il2rg −/− mice revealed that this LTi lineage subset is sufficient to trigger colitis after injection of CD40 antibodies. In contrast, conventional NK cells that can also produce IFN-γ do not allow for colitis induction, and selective depletion of conventional NK cells does not prevent colitis, demonstrating that NK cells are not required for the pathogenesis of CD40 colitis [19]. However, depletion of both conventional NK cells and RORγt−/low NKR-LTi cells abolished colitis, further supporting the view that RORγt−/low NKR-LTi cells are strictly required for disease onset [19]. The data from innate colitis models demonstrate that RORγt+ ILCs, if inappropriately stimulated, can cause inflammatory disorders in the absence of components of the adaptive immune system (Fig. 3a). Intriguingly, the involved ILC subsets show plastic alterations of their effector fate programs resembling the transition of Th17 cells into IFN-γ-producing ‘ex’-Th17 cells that have been correlated with various inflammatory and autoimmune disorders.
Fig. 3.
LTi lineage subsets play important roles during inflammatory bowel disease and for tumor immunity. a RORγt−/low NKR-LTi cells induce innate CD40 colitis. Activation of lamina propria myeloid cells via CD40-ligation induces IL-23 secretion. IL-23 activates RORγt−/low NKR-LTi cells for the production of IFN-γ [19, 30, 115]. IFN-γ further activates myeloid cells resulting in secretion of large amounts of TNF that may mediate damage of intestinal epithelial cells. b LTi cells reprogram tumor stroma leading to tolerance against the tumor. Tumor cells secreting high levels of CCL21 attract RORγt+ LTi cells via CCR7 to the tumor. RORγt+ LTi cells reprogram the tumor stroma into a lymph node-like phenotype [125]. Lymph node-like tumor stroma dampens anti-tumor immunity by favoring the differentiation of regulatory T cells and by generating a tolerogenic microenvironment thereby preventing tumor rejection. c IL-12-stimulated NKR-LTi cells modifiy tumor vasculature leading to tumor clearance. IL-12 activated NKR-LTi cells reprogram the tumor vasculature to express VCAM-1 and ICAM-1, facilitating entry of effector cells into the tumor tissue resulting in efficient rejection of the tumor [130]
Intriguingly, available data from the analysis of lamina propria lymphocyte populations in IBD patients also implicate an NKR-expressing innate lymphocyte subset that produces IFN-γ after stimulation with IL-23 in the pathogenesis of Crohn’s disease [119]. Although the phenotype of the IFN-γ-producing subset is consistent with a conventional NK cell subset (NKp46+CD122+NKp44−CD127−), its ability to respond to IL-23 stimulation is unusual as the IL-23R may not be expressed by conventional NK cells in mice [19]. Thus, it is possible that these IFN-γ-producing cells are the human counterpart of RORγt−/low NKR-LTi cells. This is further supported by previous studies showing that Lin−CD127+CD117+ lamina propria cells differentiate into IFN-γ-producing NK-like cells that are increased in numbers in the lamina propria of patients suffering from Crohn’s disease [120]. Lin−CD127+CD117+ innate lymphocytes are RORγt+ and represent human LTi-like cells [14]. Collectively, these data argue that the IL-23-responsive NKp46+ ‘NK cells’ accumulating in patients with Crohn’s disease may in fact be derived from LTi cells and constitute the human equivalent of RORγt−/low NKR-LTi cells. These data demonstrate that RORγt+ ILCs, similarly to Th17 cells, can maintain and/or induce inflammatory disorders if inappropriately triggered and identify IL-23 as a central player in chronic inflammatory disorders mediated by innate and adaptive components of the immune system. In the future, genetic tools allowing for the specific interference with the various LTi lineage subsets will allow to better assess their respective contributions to intestinal pathologies and autoimmune diseases. Additionally, it will be necessary to investigate the exact composition and cellular sources of the cytokines produced during auto-aggressive diseases with a focus on cytokines that may destabilize or reinforce RORγt expression by LTi lineage cells.
Chronic inflammatory and autoimmune diseases are often accompanied by the neogenesis of inflammatory lymphoid follicles, and it has been speculated that LTi cells may support chronic inflammation by instructing the formation of such tertiary lymphoid follicles [54, 121]. Recently, it became clear however that formation of inflammation-induced lymphoid clusters in the intestine and in the bronchial system does not require LTi cells, and LTi-deficient mice developed even larger numbers of tertiary lymphoid follicles [122, 123]. Development of tertiary lymphoid clusters in the colon following repeated exposure to dextran sodium sulfate (DSS) involved B cells, and their formation could be blocked by the application of LTβR-Ig fusion proteins involving lymphotoxin or LIGHT signaling [124]. It has been suggested that the increased numbers of tertiary lymphoid clusters in LTi-deficient mice are necessary for sequestration of the commensal microflora [123].However, at the same time this compensatory increase of tertiary lymphoid clusters aggravates intestinal pathology following DSS-induced inflammation [123]. The data suggest that LTi cell deficiency can be compensated by increased B cell-instructed formation of inflammatory lymphoid clusters in an effort to maintain homeostasis, but this comes at the cost of aggravated pathology [123].
RORγt+ ILCs and the immune response to cancer
Recent data have implicated LTi lineage cells in the immune response to experimental melanoma. One report has found that B16 melanoma cells express the chemokine CCL21, leading to the recruitment of regulatory immune cells including Foxp3-expressing Treg, resulting in subdued tumor-specific T cell immunity [125]. Interference with CCL21 expression by tumors resulted in T cell-dependent rejection of the tumor, suggesting a potent immune evasion strategy [125]. Intriguingly, CCL21-expressing tumors recruit LTi cells in a CCR7-dependent manner to the tumor, and mice lacking all LTi cells (Rorc(γt)−/−) control the growth of CCL21-expressing melanoma [125]. LTi recruitment to the tumor is required for reprogramming of tumor stroma into lymphoid-like stroma reminiscent of the stroma formed by follicular reticular cells in the paracortex of lymph nodes [126, 127]. It is believed that lymphoid stroma is an important player in maintaining peripheral tolerance because it secures lymph node recruitment of Treg. In addition, lymphoid stroma can promote peripheral deletion of autoreactive T cells [128] and maintain homeostasis of naïve T cells [129]. Collectively, these data suggest that LTi cells are recruited to the vicinity of the tumor in a CCL21-CCR7-dependent manner and reprogram tumor stroma into lymphoid stroma required for the induction of tolerance to the tumor (Fig. 3b) [125].
Another important role of LTi lineage cells has been uncovered by a study using transplantable B16 melanoma cells engineered to continuously release IL-12. While control tumors grew continuously in mice, IL-12-expressing tumors were rejected [130]. Tumor rejection required innate lymphocytes as it occurred in Rag1 −/− mice but not in alymphoid Rag2 −/− Il2rg −/− mice. Transfer of innate lymphocyte populations into alymphoid mice revealed that NKR-LTi cells mediate tumor regression, but it is unclear whether RORγt+ or RORγt−/low NKR-LTi cells are required. Tumor rejection is independent of cytokines produced by LTi lineage cells (IL-17, IL-22) and does not require IFN-γ signaling or perforin. LTi cells upregulate ICAM-1 and VCAM-1 expression by mesenchymal stroma cells, a well-known hallmark of lymph node organogenesis [14, 131]. The hypothesis has been tested that NKR-LTi cells may instruct the expression of ICAM-1 and/or VCAM-1 by tumor vessels that are often devoid of these adhesion molecules [132–135]. Indeed, NKR-LTi cells facilitated increased expression of ICAM-1 and VCAM-1 by the tumor microvasculature allowing entry of immune cells into the tumor mass, an event that correlated with tumor rejection (Fig. 3c) [130]. Although IL-12 stimulation of NKR-LTi cells upregulates expression of LTα known to have anti-tumor properties [136], the rejection of IL-12-expressing melanoma cells does not require LTβR signaling. Both reports [125, 130] provide important data on additional functional qualities of RORγt+ ILCs reaching beyond the induction of lymphoid tissues in embryos and neonates or their production of cytokines. Intriguingly, they highlight the capacity of LTi lineage cells to modify and reprogram non-hematopoietic cells of mesenchymal origin.
Perspectives
The recent identification of new innate lymphocyte subsets has fundamentally changed our view of the innate immune system. To more precisely assign functions to these various ILC subsets will now require the generation of novel genetic tools to specifically manipulate the various ILC populations. Once these tools have become available, the role of individual ILC subsets for the onset of diseases widely believed to depend on cells of the adaptive immune system (e.g., tumor rejection, induction of intestinal inflammation, autoimmune diseases, allergic diseases) will need to be reevaluated [19, 22, 30, 130]. Another important avenue of future research will be to better define the transcriptional programs controlling cell fate decisions of innate lymphocytes. Such studies will also allow a reassessment of the issue of flexibility (plasticity) and stability of ILC effector programs including genome-wide assessments of activating, repressive and bivalent chromatin marks particularly at the relevant transcription factor and effector gene loci. It is an emerging picture that RORγt+ ILCs have the capacity to program non-hematopoietic cells (epithelial cells, mesenchymal stroma) to maintain organ homeostasis [10, 29], or to control tumor immunity by modifying tumor stroma or tumor vasculature [125, 130]. Future research will need to unravel the molecular programs induced by RORγt+ ILCs in mesenchymal stroma and how they are involved in controlling organ homeostasis and adaptive immunity.
Acknowledgments
We thank Elina Kiss for comments on the manuscript and the Diefenbach laboratory for discussions. The Diefenbach laboratory is supported by the Deutsche Forschungsgemeinschaft (Di764/3, SFB/CRC620, GRK1104, SGBM) and the BMBF (CCI).
References
- 1.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 3.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 5.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+ CD3-LTbeta+cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/S1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida H, Honda K, Shinkura R, Adachi S, Nishikawa S, Maki K, Ikuta K, Nishikawa SI. IL-7 receptor alpha+ CD3− cells in the embryonic intestine induces the organizing center of Peyer’s patches. Int Immunol. 1999;11:643–655. doi: 10.1093/intimm/11.5.643. [DOI] [PubMed] [Google Scholar]
- 9.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 10.Scandella E, Bolinger B, Lattmann E, Miller S, Favre S, Littman DR, Finke D, Luther SA, Junt T, Ludewig B. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9:667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- 11.Kim MY, Gaspal FM, Wiggett HE, McConnell FM, Gulbranson-Judge A, Raykundalia C, Walker LS, Goodall MD, Lane PJ. CD4(+)CD3(−) accessory cells costimulate primed CD4 T cells through OX40 and CD30 at sites where T cells collaborate with B cells. Immunity. 2003;18:643–654. doi: 10.1016/S1074-7613(03)00110-9. [DOI] [PubMed] [Google Scholar]
- 12.Lane PJ, Gaspal FM, Kim MY. Two sides of a cellular coin: CD4+CD3- cells regulate memory responses and lymph-node organization. Nat Rev Immunol. 2005;5:655–660. doi: 10.1038/nri1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC + CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 15.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. ROR gamma t and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O’Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, Hardwigsen J, Anguiano E, Banchereau J, Chaussabel D, et al. Influence of the transcription factor ROR gamma t on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 18.Hughes T, Becknell B, McClory S, Briercheck E, Freud AG, Zhang X, Mao H, Nuovo G, Yu J, Caligiuri MA. Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH 17 cytokine interleukin-22. Blood. 2009;113:4008–4010. doi: 10.1182/blood-2008-12-192443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, Flach M, Bengsch B, Thimme R, Holscher C, et al. Regulated expression of nuclear receptor ROR gamm at confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33:736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, Dekruyff RH, Umetsu DT. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14 iNKT cells. Immunity. 2004;20:477–494. doi: 10.1016/S1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 24.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor ROR gamma t directs the differentiation program of pro inflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 25.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 26.Kurebayashi S, Ueda E, Sakaue M, Patel DD, Medvedev A, Zhang F, Jetten AM. Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc Natl Acad Sci USA. 2000;97:10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/S0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 28.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 29.Sanos SL, Vonarbourg C, Mortha A, Diefenbach A. Control of epithelial cell function by interleukin-22-producing RORgamma t+ innate lymphoid cells. Immunology. 2011;132:453–465. doi: 10.1111/j.1365-2567.2011.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G. Lineage relationship analysis of RORgamma t+ innate lymphoid cells. Science. 2010;330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 32.Boos MD, Yokota Y, Eberl G, Kee BL. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J Exp Med. 2007;204:1119–1130. doi: 10.1084/jem.20061959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aliahmad P, de la Torre B, Kaye J. Shared dependence on the DNA-binding factor TOX for the development of lymphoid tissue-inducer cell and NK cell lineages. Nat Immunol. 2010;11:945–952. doi: 10.1038/ni.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 35.Carotta S, Pang SH, Nutt SL, Belz GT. Identification of the earliest NK-cell precursor in the mouse BM. Blood. 2011;117:5449–5452. doi: 10.1182/blood-2010-11-318956. [DOI] [PubMed] [Google Scholar]
- 36.de Pooter RF, Kee BL. E proteins and the regulation of early lymphocyte development. Immunol Rev. 2010;238:93–109. doi: 10.1111/j.1600-065X.2010.00957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Randall TD, Weissman IL. Characterization of a population of cells in the bone marrow that phenotypically mimics hematopoietic stem cells: resting stem cells or mystery population? Stem Cells. 1998;16:38–48. doi: 10.1002/stem.160038. [DOI] [PubMed] [Google Scholar]
- 38.Kumar R, Fossati V, Israel M, Snoeck HW. Lin−Sca1+ kit-bone marrow cells contain early lymphoid-committed precursors that are distinct from common lymphoid progenitors. J Immunol. 2008;181:7507–7513. doi: 10.4049/jimmunol.181.11.7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harman BC, Northrup DL, Allman D. Resolution of unique Sca-1high c-Kit- lymphoid-biased progenitors in adult bone marrow. J Immunol. 2008;181:7514–7524. doi: 10.4049/jimmunol.181.11.7514. [DOI] [PubMed] [Google Scholar]
- 40.De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 41.Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/S1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 42.Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, Luz A, Turetskaya RL, Tarakhovsky A, Rajewsky K, Nedospasov SA, Pfeffer K. Abnormal development of secondary lymphoid tissues in lymphotoxin beta-deficient mice. Proc Natl Acad Sci USA. 1997;94:9302–9307. doi: 10.1073/pnas.94.17.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Randall TD, Carragher DM, Rangel-Moreno J. Development of secondary lymphoid organs. Annu Rev Immunol. 2008;26:627–650. doi: 10.1146/annurev.immunol.26.021607.090257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7:344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 45.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 46.Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 47.Nishikawa SI, Hashi H, Honda K, Fraser S, Yoshida H. Inflammation, a prototype for organogenesis of the lymphopoietic/hematopoietic system. Curr Opin Immunol. 2000;12:342–345. doi: 10.1016/S0952-7915(00)00097-2. [DOI] [PubMed] [Google Scholar]
- 48.Kim MY, Rossi S, Withers D, McConnell F, Toellner KM, Gaspal F, Jenkinson E, Anderson G, Lane PJ. Heterogeneity of lymphoid tissue inducer cell populations present in embryonic and adult mouse lymphoid tissues. Immunology. 2008;124:166–174. doi: 10.1111/j.1365-2567.2007.02750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Maele L, Carnoy C, Cayet D, Songhet P, Dumoutier L, Ferrero I, Janot L, Erard F, Bertout J, Leger H, et al. TLR5 signaling stimulates the innate production of IL-17 and IL-22 by CD3(neg)CD127 + immune cells in spleen and mucosa. J Immunol. 2010;185:1177–1185. doi: 10.4049/jimmunol.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgamma t + cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 51.Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov II, Itoh K, Littman DR, Fagarasan S. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 52.Pabst O, Herbrand H, Worbs T, Friedrichsen M, Yan S, Hoffmann MW, Korner H, Bernhardt G, Pabst R, Forster R. Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur J Immunol. 2005;35:98–107. doi: 10.1002/eji.200425432. [DOI] [PubMed] [Google Scholar]
- 53.Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit + IL-7R + Thy1 + lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–1459. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eberl G. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nat Rev Immunol. 2005;5:413–420. doi: 10.1038/nri1600. [DOI] [PubMed] [Google Scholar]
- 55.Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 56.Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, Kaminogawa S, Takahashi-Iwanaga H, Iwanaga T, Kiyono H, et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- 57.Pabst O, Herbrand H, Friedrichsen M, Velaga S, Dorsch M, Berhardt G, Worbs T, Macpherson AJ, Forster R. Adaptation of solitary intestinal lymphoid tissue in response to microbiota and chemokine receptor CCR7 signaling. J Immunol. 2006;177:6824–6832. doi: 10.4049/jimmunol.177.10.6824. [DOI] [PubMed] [Google Scholar]
- 58.Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 59.Velaga S, Herbrand H, Friedrichsen M, Jiong T, Dorsch M, Hoffmann MW, Forster R, Pabst O. Chemokine receptor CXCR5 supports solitary intestinal lymphoid tissue formation, B cell homing, and induction of intestinal IgA responses. J Immunol. 2009;182:2610–2619. doi: 10.4049/jimmunol.0801141. [DOI] [PubMed] [Google Scholar]
- 60.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki K, Fagarasan S. How host-bacterial interactions lead to IgA synthesis in the gut. Trends Immunol. 2008;29:523–531. doi: 10.1016/j.it.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Malmberg KJ, Ljunggren HG. Spotlight on IL-22-producing NK cell receptor-expressing mucosal lymphocytes. Nat Immunol. 2009;10:11–12. doi: 10.1038/ni0109-11. [DOI] [PubMed] [Google Scholar]
- 63.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vivier E, Spits H, Cupedo T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat Rev Immunol. 2009;9:229–234. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- 66.Sonnenberg GF, Fouser LA, Artis D. Functional biology of the IL-22-IL-22R pathway in regulating immunity and inflammation at barrier surfaces. Adv Immunol. 2010;107:1–29. doi: 10.1016/B978-0-12-381300-8.00001-0. [DOI] [PubMed] [Google Scholar]
- 67.Adachi S, Yoshida H, Honda K, Maki K, Saijo K, Ikuta K, Saito T, Nishikawa SI. Essential role of IL-7 receptor alpha in the formation of Peyer’s patch anlage. Int Immunol. 1998;10:1–6. doi: 10.1093/intimm/10.1.1. [DOI] [PubMed] [Google Scholar]
- 68.Honda K, Nakano H, Yoshida H, Nishikawa S, Rennert P, Ikuta K, Tamechika M, Yamaguchi K, Fukumoto T, Chiba T, et al. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer’s patch organogenesis. J Exp Med. 2001;193:621–630. doi: 10.1084/jem.193.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park SY, Saijo K, Takahashi T, Osawa M, Arase H, Hirayama N, Miyake K, Nakauchi H, Shirasawa T, Saito T. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity. 1995;3:771–782. doi: 10.1016/1074-7613(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 70.Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, Drago J, Noguchi M, Grinberg A, Bloom ET, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 71.Kim D, Mebius RE, MacMicking JD, Jung S, Cupedo T, Castellanos Y, Rho J, Wong BR, Josien R, Kim N, et al. Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J Exp Med. 2000;192:1467–1478. doi: 10.1084/jem.192.10.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshida H, Naito A, Inoue J, Satoh M, Santee-Cooper SM, Ware CF, Togawa A, Nishikawa S, Nishikawa S. Different cytokines induce surface lymphotoxin-alphabeta on IL-7 receptor-alpha cells that differentially engender lymph nodes and Peyer’s patches. Immunity. 2002;17:823–833. doi: 10.1016/S1074-7613(02)00479-X. [DOI] [PubMed] [Google Scholar]
- 73.Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J Exp Med. 2003;197:1191–1198. doi: 10.1084/jem.20021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chappaz S, Finke D. The IL-7 signaling pathway regulates lymph node development independent of peripheral lymphocytes. J Immunol. 2010;184:3562–3569. doi: 10.4049/jimmunol.0901647. [DOI] [PubMed] [Google Scholar]
- 75.Meier D, Bornmann C, Chappaz S, Schmutz S, Otten LA, Ceredig R, Acha-Orbea H, Finke D. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity. 2007;26:643–654. doi: 10.1016/j.immuni.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 76.Schmutz S, Bosco N, Chappaz S, Boyman O, Acha-Orbea H, Ceredig R, Rolink AG, Finke D. Cutting edge: IL-7 regulates the peripheral pool of adult RORgamma + lymphoid tissue inducer cells. J Immunol. 2009;183:2217–2221. doi: 10.4049/jimmunol.0802911. [DOI] [PubMed] [Google Scholar]
- 77.Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CA, Di Santo JP. IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J Exp Med. 2010;207:273–280. doi: 10.1084/jem.20092029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, Ziegler SF, Leonard WJ, Lodish HF. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 79.Chappaz S, Gartner C, Rodewald HR, Finke D. Kit ligand and Il7 differentially regulate Peyer’s patch and lymph node development. J Immunol. 2011;185:3514–3519. doi: 10.4049/jimmunol.1000665. [DOI] [PubMed] [Google Scholar]
- 80.Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 81.Lai SY, Molden J, Goldsmith MA. Shared gamma(c) subunit within the human interleukin-7 receptor complex. A molecular basis for the pathogenesis of X-linked severe combined immunodeficiency. J Clin Invest. 1997;99:169–177. doi: 10.1172/JCI119144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 83.Gascoyne DM, Long E, Veiga-Fernandes H, de Boer J, Williams O, Seddon B, Coles M, Kioussis D, Brady HJ. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118–1124. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- 84.Kamizono S, Duncan GS, Seidel MG, Morimoto A, Hamada K, Grosveld G, Akashi K, Lind EF, Haight JP, Ohashi PS, et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. J Exp Med. 2009;206:2977–2986. doi: 10.1084/jem.20092176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11:674–680. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 87.Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol. 2011;11:221–230. doi: 10.1038/nri2940. [DOI] [PubMed] [Google Scholar]
- 88.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 91.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hegazy AN, Peine M, Helmstetter C, Panse I, Frohlich A, Bergthaler A, Flatz L, Pinschewer DD, Radbruch A, Lohning M. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity. 2010;32:116–128. doi: 10.1016/j.immuni.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 93.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4 + T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 97.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 98.Shalapour S, Deiser K, Sercan O, Tuckermann J, Minnich K, Willimsky G, Blankenstein T, Hammerling GJ, Arnold B, Schuler T. Commensal microflora and interferon-gamma promote steady-state interleukin-7 production in vivo. Eur J Immunol. 2010;40:2391–2400. doi: 10.1002/eji.201040441. [DOI] [PubMed] [Google Scholar]
- 99.Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 100.Yang J, Yang M, Htut TM, Ouyang X, Hanidu A, Li X, Sellati R, Jiang H, Zhang S, Li H, et al. Epstein-Barr virus-induced gene 3 negatively regulates IL-17, IL-22 and RORgamma t. Eur J Immunol. 2008;38:1204–1214. doi: 10.1002/eji.200838145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, et al. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 102.Lee CK, Rao DT, Gertner R, Gimeno R, Frey AB, Levy DE. Distinct requirements for IFNs and STAT1 in NK cell function. J Immunol. 2000;165:3571–3577. doi: 10.4049/jimmunol.165.7.3571. [DOI] [PubMed] [Google Scholar]
- 103.Nguyen KB, Cousens LP, Doughty LA, Pien GC, Durbin JE, Biron CA. Interferon alpha/beta-mediated inhibition and promotion of interferon gamma: STAT1 resolves a paradox. Nat Immunol. 2000;1:70–76. doi: 10.1038/76940. [DOI] [PubMed] [Google Scholar]
- 104.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 105.Szabo SJ, Kim ST, Costa GL, Zhang XK, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/S0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 106.Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc Natl Acad Sci USA. 2010;107:10961–10966. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H. Human NKp44+ IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med. 2010;207:281–290. doi: 10.1084/jem.20091509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Crellin NK, Trifari S, Kaplan CD, Satoh-Takayama N, Di Santo JP, Spits H. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity. 2010;33:752–764. doi: 10.1016/j.immuni.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 109.Uhlig HH, Powrie F. Mouse models of intestinal inflammation as tools to understand the pathogenesis of inflammatory bowel disease. Eur J Immunol. 2009;39:2021–2026. doi: 10.1002/eji.200939602. [DOI] [PubMed] [Google Scholar]
- 110.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 111.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 114.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Diefenbach A, Vonarbourg C. Innate lymphocytes induce inflammatory bowel disease. Immunol Cell Biol. 2010;88:694–696. doi: 10.1038/icb.2010.82. [DOI] [PubMed] [Google Scholar]
- 116.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 117.Stuber E, Strober W, Neurath M. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996;183:693–698. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 119.Takayama T, Kamada N, Chinen H, Okamoto S, Kitazume MT, Chang J, Matuzaki Y, Suzuki S, Sugita A, Koganei K, et al. Imbalance of NKp44(+)NKp46(−) and NKp44(−)NKp46(+) natural killer cells in the intestinal mucosa of patients with Crohn’s disease. Gastroenterology. 2010;139:882–892. doi: 10.1053/j.gastro.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 120.Chinen H, Matsuoka K, Sato T, Kamada N, Okamoto S, Hisamatsu T, Kobayashi T, Hasegawa H, Sugita A, Kinjo F, et al. Lamina propria c-kit+ immune precursors reside in human adult intestine and differentiate into natural killer cells. Gastroenterology. 2007;133:559–573. doi: 10.1053/j.gastro.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 121.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 122.Halle S, Dujardin HC, Bakocevic N, Fleige H, Danzer H, Willenzon S, Suezer Y, Hammerling G, Garbi N, Sutter G, et al. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J Exp Med. 2009;206:2593–2601. doi: 10.1084/jem.20091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lochner M, Ohnmacht C, Presley L, Bruhns P, Si-Tahar M, Sawa S, Eberl G. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. J Exp Med. 2011;208:125–134. doi: 10.1084/jem.20100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sedy JR, Spear PG, Ware CF. Cross-regulation between herpesviruses and the TNF superfamily members. Nat Rev Immunol. 2008;8:861–873. doi: 10.1038/nri2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science. 2010;328:749–752. doi: 10.1126/science.1185837. [DOI] [PubMed] [Google Scholar]
- 126.Mueller SN, Ahmed R. Lymphoid stroma in the initiation and control of immune responses. Immunol Rev. 2008;224:284–294. doi: 10.1111/j.1600-065X.2008.00657.x. [DOI] [PubMed] [Google Scholar]
- 127.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 2009;9:618–629. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee JW, Epardaud M, Sun J, Becker JE, Cheng AC, Yonekura AR, Heath JK, Turley SJ. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 129.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 130.Eisenring M, vom Berg J, Kristiansen G, Saller E, Becher B. IL-12 initiates tumor rejection via lymphoid tissue-inducer cells bearing the natural cytotoxicity receptor NKp46. Nat Immunol. 2010;11:1030–1038. doi: 10.1038/ni.1947. [DOI] [PubMed] [Google Scholar]
- 131.Cupedo T, Jansen W, Kraal G, Mebius RE. Induction of secondary and tertiary lymphoid structures in the skin. Immunity. 2004;21:655–667. doi: 10.1016/j.immuni.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 132.Ryschich E, Schmidt J, Hammerling GJ, Klar E, Ganss R. Transformation of the microvascular system during multistage tumorigenesis. Int J Cancer. 2002;97:719–725. doi: 10.1002/ijc.10074. [DOI] [PubMed] [Google Scholar]
- 133.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 134.Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med. 2008;205:2125–2138. doi: 10.1084/jem.20080099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, Katsaros D, O’Brien-Jenkins A, Gimotty PA, Coukos G. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 136.Schrama D, thor Straten P, Fischer WH, McLellan AD, Brocker EB, Reisfeld RA, Becker JC. Targeting of lymphotoxin-alpha to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity. 2001;14:111–121. doi: 10.1016/S1074-7613(01)00094-2. [DOI] [PubMed] [Google Scholar]
- 137.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/S0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 138.Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Berard M, Kleinschek M, Cua D, Di Santo JP, Eberl G. RORgammat + innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011;12:320–326. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]