Abstract
The series of seminal articles in this book clearly illustrate the multi-functional nature of γδ T cells. Some of the functions correlate with the tissue tropism of distinct γδ T cell subsets whereas others appear to result from oligoclonal selection. Here, we discuss the antigen-presenting cell (APC) function of the major subset of circulating γδ T cells, Vγ9/Vδ2 T cells, present in human blood. During tissue culture, Vγ9/Vδ2 T cells uniformly respond to a class of non-peptide antigens, so-called prenyl pyrophosphates, derived from stressed host cells or from microbes. It is this feature that distinguishes human (and primate) Vγ9/Vδ2 T cells from αβ and γδ T cells of all other species and that forms the basis for detailed studies of human Vγ9/Vδ2 T cells. One of the consequences of Vγ9/Vδ2 T cell activation is the rapid acquisition of APC characteristics (γδ T-APCs) reminiscent of mature dendritic cells (DCs). In the following discussion, we will discriminate between the potential use of γδ T-APCs as a cellular vaccine in immunotherapy and their role in anti-microbial immunity. Exploiting the APC function in γδ T-APCs represents a true novelty in current immunotherapy research and may lead to effective, anti-tumor immunity in cancer patients.
Keywords: Antigen presentation, γδ T cells, αβ T cells, Dendritic cells, Chemokines, Cytokines, Immunotherapy, Cancer
Discovery of human γδ T-APCs
γδ T cells expressing a Vγ9/Vδ2 T cell receptor are unique to humans and higher primates and differ fundamentally from all other ‘conventional’ and ‘unconventional’ T cells. Usually only comprising 1–5% of circulating T cells [1], they can expand considerably in many infections, at times to >50% of all circulating T cells within a few days [2]. Vγ9/Vδ2 T cells respond selectively in a non-MHC restricted manner to (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP), an intermediate of the microbial non-mevalonate pathway of isoprenoid biosynthesis utilized by most pathogenic bacteria. Of note, HMB-PP is not present in higher eukaryotes including humans [3, 4]. Vγ9/Vδ2 T cells also respond, albeit with a 10,000-fold lower potency in vitro, to isopentenyl pyrophosphate (IPP), the structurally related end product of both the mevalonate and non-mevalonate pathways with ubiquitous expression in all living prokaryotic and eukaryotic cells [2, 5].
Resting human blood Vγ9/Vδ2 T cells are characterized by an inflammatory migration program similar to cells of the innate immune system, such as monocytes and NK cells [6–9]. These migration properties include the expression of multiple receptors for inflammatory chemokines, such as CXCR3, CCR1, CCR2 and CCR5 [10, 11], which are a prerequisite for the recruitment of effector immune cells to sites of inflammation. These properties fit well with the proposed involvement of Vγ9/Vδ2 T cells in anti-microbial immunity [12]. Vγ9/Vδ2 T cells do not express CXCR1 and CXCR2, the two IL-8/CXCL8 receptors that are essential for the recruitment of neutrophils in the immediate early response to infection. And absence of CCR8 and CCR9, two chemokine receptors that are typically found on lymphocytes present in steady-state skin and intestine, respectively, agrees with their exclusion from healthy peripheral tissues. Stimulation with IPP induces dramatic changes in the migration properties, including the secretion of inflammatory chemokines that are responsible in part for the observed downmodulation of inflammatory chemokine receptors and the induction of CCR7, a chemokine receptor critically involved in the recruitment of immune cells to lymph nodes. CCR7, together with certain adhesion receptors such as CD62L, have been useful markers for distinguishing between cells participating in lymph node activities and those that do not. Best described are naïve and central-memory T (TCM) cells in blood as well as mature tissue DCs whose co-localization in the T cell area of lymph nodes is CCR7 dependent. Blood effector-memory T (TEM) cells lack CCR7 and, thus, are excluded from these sites under non-inflamed conditions [13]. Consequently, CCR7 expression in activated Vγ9/Vδ2 T cells suggests that they may be involved in lymph node activities. Indeed, γδ T cells are readily found in secondary lymphoid tissues [14, 15], both within B cell follicles and the T cell compartment [6]. The capacity to express the co-stimulatory molecules OX40 and CD70 [6] and the B cell attracting chemokine CXCL13 [16] upon activation points to a role in the control of humoral immunity. In support, B cells secrete large amounts of antibodies during co-culture with activated Vγ9/Vδ2 T cells [6, 17]. In this regard, activated Vγ9/Vδ2 T cells resemble the recently described subset of follicular B helper T (TFH) cells [18, 19].
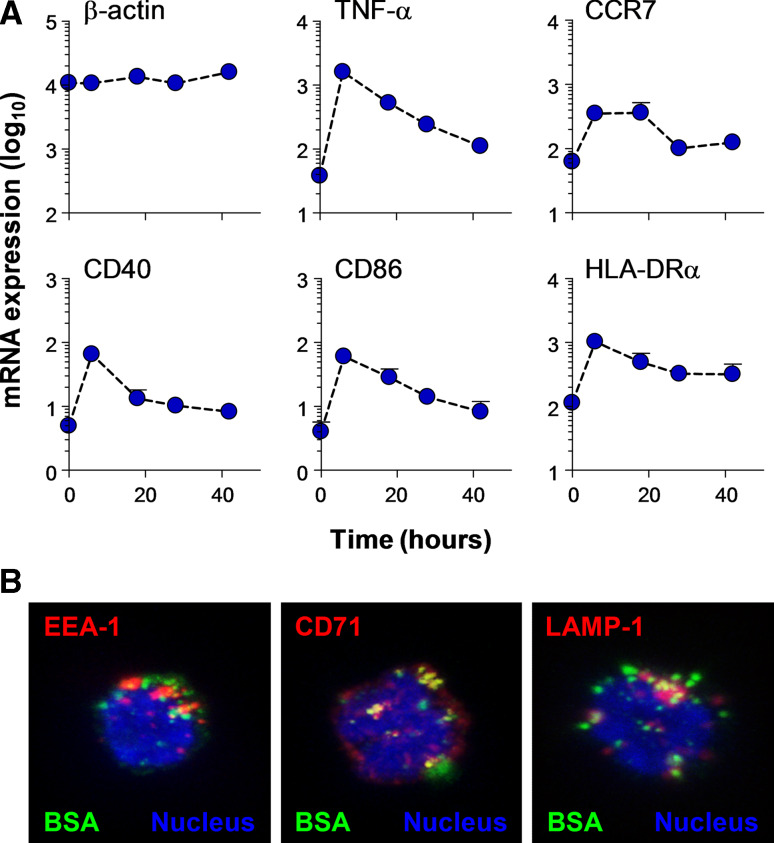
The presence of γ δ T cells in the T cell area of lymph nodes [6] also indicates a participation in αβ T cell responses. The following discussion emphasizes this view. Treatment of freshly isolated blood Vγ9/Vδ2 T cells with IPP or HMB-PP results in rapid upregulation or de novo expression of multiple markers that are typically associated with antigen-presenting cells (APCs), namely antigen-presenting molecules (MHC I and II), co-stimulatory molecules (CD80, CD83, CD86) and adhesion receptors (CD11a, CD18, CD54) [20] (Fig. 1a). These phenotypical features have been corroborated on the functional level by us [20–22] and others [23, 24]. Using human monocyte-derived DCs, freshly isolated monocytes and αβ T cells as positive and negative controls, we demonstrated that activated γδ T cells behave like APCs. The responses induced by antigen-presenting γδ T cells (γδ T-APCs) are both potent and professional [20]—‘potent’ with respect to the low numbers of γβ T-APCs required for inducing robust proliferation responses in memory αβ T cells and ‘professional’ in relation to their ability to turn naïve CD4+ and CD8+ αβ T cells into effector cells. In view of cellular immunotherapy, it may be important to emphasize that αβ T cell differentiation induced by γδ T-APCs led to T helper cell responses with a predominant pro-inflammatory cytokine (IFN-γ, TNF-α) profile. However, our understanding of the effects of γδ T-APC on T helper cell differentiation is at present still rudimentary. For instance, at low APC:responder cell ratios we noticed that some naïve αβ T cells differentiated into Th2-type (IL-4 producing) cells and Th0-type (IFN-γ plus IL-4 producing) cells [20]. Given the capacity of Vγ9/Vδ2 T cell responses to be polarized toward distinct effector cells [16], the cytokine milieu at the site of interactions between γδ T-APCs and CD4+ αβ T cells is expected to influence the outcome of the αβ T cell responses. The potential functional plasticity of γδ T-APCs in response to different culture conditions needs to be examined in more detail. Furthermore, initial data indicate that γδ T-APCs are “robust” APCs, as evidenced by the preservation of APC functions during prolonged tissue culture [22]. This is in clear contrast to monocyte-derived DCs that are known to become “exhausted” during prolonged tissue culture, as evidenced by losing their ability to induce T helper cell differentiation [25].
Fig. 1.
a Expression of APC markers by activated Vγ9/Vδ2 T cells, as analyzed by real-time PCR. Purified γδ T cells were co-cultured with EBV-transformed feeder cells in the presence of 100 nM HMB-PP and 100 U/ml IL-2 for up to 42 h. Quantitative RT-PCR was performed with RNA from MACS beads purified γδ T cells and specific primers corresponding to indicated proteins; β-actin and TNF-α served as internal controls. mRNA expression levels shown are mean ± SEM from up to four individual donors, relative to the house-keeping gene cyclophilin. b Uptake and sorting of soluble protein in γδ T-APCs. γδ T-APCs were cultured in the presence of 1 mg/ml FITC-BSA (green) for 1 h. After washing, cells were stained with antibodies to early endosomal markers EEA-1 or CD71 or lysosomal marker lamp-1 (red); nuclei are shown in blue. Confocal images of selected stacks of 15–20 z-planes are shown
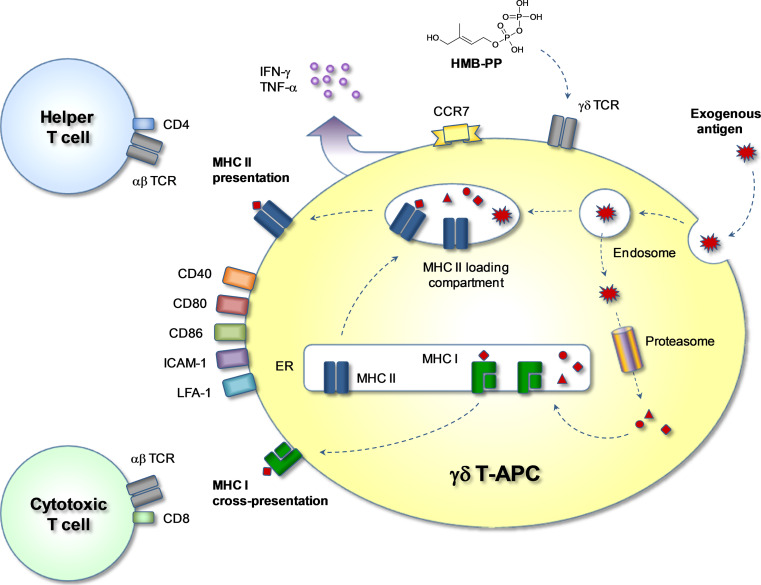
Of relevance to immunotherapy, human γδ T-APCs turned out to be cells with excellent antigen cross-presentation function [22], a process describing the uptake of exogenous antigen (such as microbial proteins or tumor antigens released into the microenvironment) and its routing to the MHC I pathway for induction of cytotoxic T cells [26, 27]. The classical MHC I pathway involves the cytosolic degradation of endogenous antigen, e.g., de novo synthesized protein of self or foreign (microbial) origin, leading to the cell surface localization of newly formed peptide-MHC I complexes. Those derived from endogenous metabolic proteins signify “healthy” cells whereas those derived from microbes or tumor proteins provide flags for recognition by cytotoxic T cells. By contrast, antigen cross-presentation occurs in specialized APCs and involves overcoming at least one cell membrane separating the internalized exogenous antigen from the peptide processing machinery in the cytosol. This feature enables APCs to induce cytotoxic effector T cells with specificity for tumor cells and infected tissue cells. Most APCs are poor antigen cross-presenting cells and the human DC subset specialized in this function is still a matter of debate [28]; however, it is certain that monocyte-derived DCs are not on the list of contestants. A number of experimental antigens were shown to be cross-presented by γδ T-APCs, including purified influenza matrix protein M1, inactivated influenza particles and extracts from influenza-infected cells [21, 22] (Fig. 1b). K. Gustafsson and colleagues recently demonstrated that Vγ9/Vδ2 T cells efficiently phagocytosed opsonized bacteria in a CD16-dependent manner [23]. In a follow-up study, this group has combined the tumor cell-killing activity of Vγ9/Vδ2 T cells with their APC properties by demonstrating PAX5 oncogene-specific CD8+ αβ T cell activation in response to Vγ9/Vδ2 T cells following the killing of PAX5-transduced Daudi cells and processing of tumor cell debris by Vγ9/Vδ2 T cells (Himoudi and Gustaffson, unpublished observations). The mechanism underlying antigen cross-presentation in γδ T-APCs involves the proteasome [21], indicating that the cytosolic (classical) MHC I pathway plays some part in this process. How exogenous antigen reaches the cytosol is not clear at present but may be facilitated by endocytic receptors that may include influenza hemagglutinin binding, sialic acid-containing cell surface proteins [21, 23]. An increase in “dwell-time” in APCs, i.e. a delay in lysosomal degradation, of endocytosed antigen was shown to favor antigen export to the cytosol and subsequent antigen cross-presentation [29]. In line with this notion, intracellular antigen degradation is considerably slower in γδ T-APCs as compared to monocyte-derived DCs, providing a rationale for their superior antigen cross-presentation capabilities [21, 22]. Finally, Landmeier and colleagues [24] reported the induction of EBV-specific CD8+ αβ T cell responses by EBV gene transduced γδ T cells, a process involving the classical pathway of antigen processing and peptide-MHC I presentation. Collectively, γδ T-APCs are expert APCs for activating both CD4+ and CD8+ αβ T cells whereas monocyte-derived DCs are excellent APCs for CD4+ αβ T cells but less so for CD8+ αβ T cells in response to exogenous antigens (Fig. 2). This, together with the secretion of predominantly pro-inflammatory cytokines, provides a strong rationale for the testing of tumor peptide-presenting γδ T-APCs in immunotherapy of cancer patients.
Fig. 2.
Antigen processing and presentation in γδ T-APCs. Exogenous soluble or particulate antigens are taken up by endocytosis and are directed to MHC I or MHC II loading pathways. By default, endosomal antigen reaches the lysosomal compartment where proteolytic degradation takes place. By vesicular fusion, some of the degraded antigen reaches the MHC II loading compartment where peptides are loaded onto newly formed MHC II molecules. Peptide-MHC II complexes are then relocated to the cell surface for presentation to CD4+ αβ T cells. Alternatively, by a process called antigen cross-presentation, antigen reaches the cytosol where the proteasome produces antigen-derived peptides, which are then transported into the endoplasmic reticulum (ER) to the site of newly formed MHC I molecules. Peptide-MHC I molecules are finally displayed on the cell surface for presentation to CD8+ αβ T cells. Activation of γδ T cells by HMB-PP leads to upregulation of co-stimulatory molecules such as CD40, CD80 and CD86, adhesion molecules such as LFA-1 (CD11a/CD18) and ICAM-1 (CD54), and the lymphoid homing chemokine receptor CCR7. Activated cells release cytokines such as IFN-γ and TNF-α, thus creating a pro-inflammatory microenvironment favoring the generation of CD4+ Th1 cells and cytotoxic CD8+ T cells
In vivo relevance of γδ T-APCs
The in vivo relevance of the findings summarized above is an important issue and its investigation poses several fundamental problems. HMB-PP is the most active ligand for human Vγ9/Vδ2 T cells, exceeding IPP in potency by several orders of magnitude [4]. In fact, HMB-PP is so powerful that the amount released by neutrophils during phagocytosis of bacteria is sufficient to activate Vγ9/Vδ2 T cells during in vitro co-culture [30]. Our functional studies with HMB-PP fully support the well documented expansion of Vγ9/Vδ2 T cells observed in patients suffering from acute infections with HMB-PP+ bacteria [2, 3, 12]. For instance, Vγ9/Vδ2 T cells in M. tuberculosis patients may reach 35% of total blood T cells during the acute phase of infection. In addition, studies with acutely infected patients undergoing peritoneal dialysis demonstrated that peritoneal Vγ9/Vδ2 T cells were elevated in HMB-PP+ infections compared to HMB-PP− infections, suggesting increased recruitment and/or proliferation in response to HMB-PP released by invading bacteria [30, 31]. High γδ T cell levels do not persist and decline to normal levels during anti-microbial treatment [32]. This hyper-responsiveness of adult Vγ9/Vδ2 T cells to HMB-PP may be the result of a memory compartment established in newborn babies during exposure to environmental and/or pathogenic microbes [1, 33]. Collectively, the broad reactivity of human blood Vγ9/Vδ2 T cells to HMB-PP and related compounds resembles a microbial sensing pathway that is unique to humans and primates [12]. The role of such a novel pathway in anti-microbial immunity needs to be clarified. Mice and many other animals are also regularly exposed to HMB-PP+ microbes as well as to IPP from endogenous sources. Yet, for reasons that are not clear, such pre-selection of HMB-PP/IPP-reactive γδ T cells as seen in humans does not occur in these species. There is evidence for a human/primate-specific molecule on accessory cells that is needed for presentation of HMB-PP/IPP to Vγ9/Vδ2 T cells [34, 35]. Lack of such a presenting molecule may explain why homologues of Vγ9/Vδ2 T cells are missing in mice and other standard laboratory animals.
One of the major questions of relevance about γδ T-APCs concerns the site(s) where HMB-PP exposure and subsequent Vγ9/Vδ2 T cell activation and expansion occurs. The primary site of resting Vγ9/Vδ2 T cells is peripheral blood, which is also the site with high Vγ9/Vδ2 T cell numbers in patients infected with HMB-PP+ bacteria. However, and in agreement with αβ T cells and B cells, Vγ9/Vδ2 TCR triggering is unlikely to happen in the vascular compartment. Furthermore, most Vγ9/Vδ2 T cells lack CD4 and CD8 and, consequently, antigen recognition by their TCRs is not restricted by classical MHC molecules. The importance of CD4 and CD8 in the selection of thymocytes with TCR specificities for peptide-MHC complexes has recently been demonstrated [36]. This also means that Vγ9/Vδ2 T cells do not require contact with peptide-MHC presenting, professional APCs in lymph nodes where αβ T cell and B cell responses are initiated. Conversely, robust Vγ9/Vδ2 T cell proliferation depends on cell–cell contact with HMB-PP presenting cells and T cell growth factors (IL-2, IL-15, IL-21) [37–39], indicating the requirement for accessory cells (monocytes/DCs, αβ T cells). Adequate provision of growth factors may be met at sites of infection or, alternatively, in infection-draining lymph nodes. In the latter scenario, the cross-talk between γδ T-APCs and lymph node cells would be mutually beneficial in that microbe-specific αβ T cells become activated and vice versa activated accessory cells (that may include αβ T cells) provide the growth factors necessary for Vγ9/Vδ2 T cell expansion.
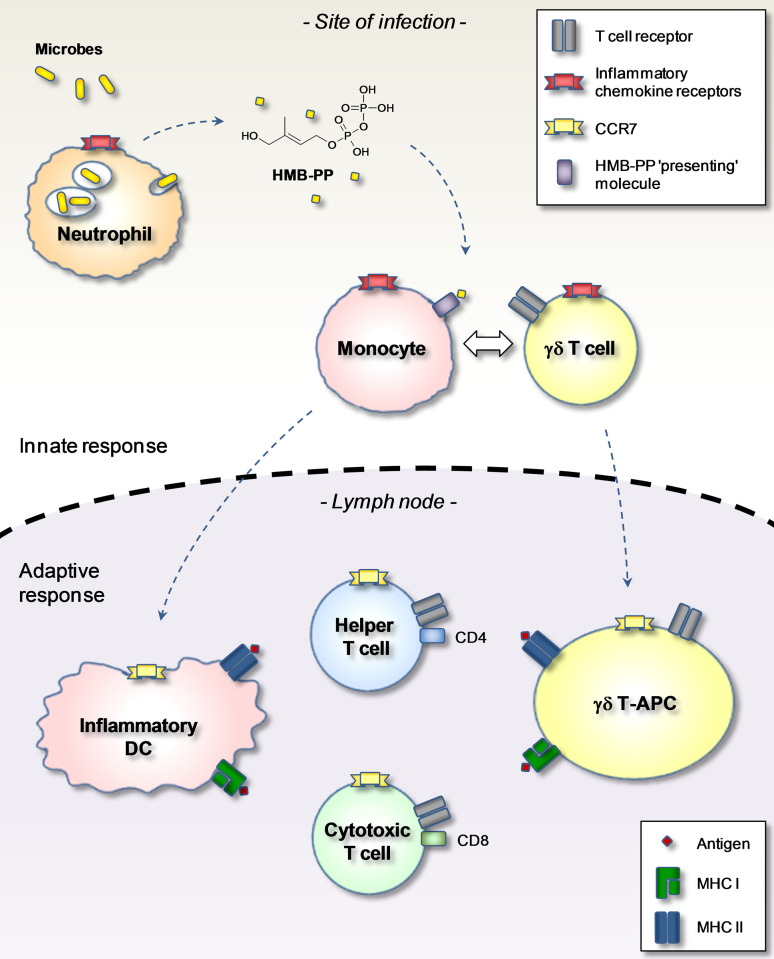
The following model (Fig. 3) incorporates the principal paradigm in chemokine research by linking changes in tissue homing properties with control of immune cell function [10, 11]. The majority of circulating Vγ9/Vδ2 T cells expresses receptors for inflammatory chemokines, including CXCR3, CCR1, CCR2 and CCR5, which enable their immediate (innate) recruitment to sites of inflammation. This process occurs independently from TCR triggering by microbe-derived HMB-PP. Once in the tissue, Vγ9/Vδ2 T cells become exposed to HMB-PP, either released by neutrophils during bacterial killing and presented by bystander monocytes [30] or presented on the surface of infected monocytes or dendritic cells [40, 41]. HMB-PP works best in the presence of “feeder” cells, including inflammatory monocytes or dendritic cells that may present HMB-PP to local Vγ9/Vδ2 T cells [12, 31]. Activated Vγ9/Vδ2 T cells then process bacterial antigens and become CCR7-expressing γδ T-APCs. γδ T cells may not proliferate at the site of infection but instead may do so after accessing the growth factor-rich environment in the draining lymph node. Thus, this model links γδ T cell expansion with induction of αβ T cell responses by antigen-presenting γδ T-APCs and, eventually, control of B cell responses. Finally, microbe-specific αβ T cells exit the lymph nodes and enter the site of infection in order to participate in the effector arm of anti-microbial immunity. Expanded γδ T cells may also leave the lymph nodes, accounting for the dramatic increase in blood Vγ9/Vδ2 T cells during acute infections. Whether lymph node-emigrant γδ T cells home to infected tissues is not clear, but initial studies in macaques support this possibility [42].
Fig. 3.
Generation of γδ T-APCs in acute microbial infection. γδ T cells at the site of infection become exposed to HMB-PP either released by neutrophils upon phagocytosis of invading pathogens and then presented on local monocytes/macrophages or presented by directly infected monocytes/macrophages (not shown). Monocytes provide crucial accessory signals for optimum activation of γδ T cells and receive survival and differentiation signals in return. Crosstalk between monocytes and γδ T cells induces both cell types to differentiate into lymph-node homing APCs. Following uptake and processing of microbial antigens, γδ T-APCs (and inflammatory DCs) relocate to the draining lymph nodes in order to initiate microbe-specific T helper and cytotoxic T cell responses
Besides recent progress, numerous questions about γδ T-APCs remain, which are difficult to address in humans. Obviously, the APC function of γδ T-APCs in anti-microbial immunity is linked with (1) their early (innate) recruitment to tissues infected with HMB-PP+ bacteria and (2) their subsequent relocation to draining lymph nodes. However, real-time imaging cannot be carried out in humans and, similarly, access to tissue material of patients with acute infections prior to treatment onset is close to impossible. Macaques have been used to study activation and expansion of γδ T cells in response to mycobacterial infections [43, 44]. These studies clearly demonstrated the massive and transient expansion of Vγ9/Vδ2 T cells mirrored by their accumulation in the blood of macaques during recall responses, replicating the findings seen in tuberculosis patients. However, γδ T cell relocation and their potential involvement in the control of M. tuberculosis-specific αβ T cell responses were not investigated. Co-administration of picostim, a synthetic γδ T cell activator with similar structure to HMB-PP, together with M. tuberculosis antigens by the i.v. route induced immediate cytokine responses by γδ T cells but did not modify the antigen-specific recall responses by cytotoxic αβ T lymphocytes [45]. Apparently, this treatment did not lead to γδ T-APC generation in vivo, which may be taken to suggest that blood does not provide the appropriate microenvironment for transforming γδ T cells into APCs. Of interest, γδ T cells with APC characteristics were recently discovered in mice [46]. Further studies in mice are limited by the lack of knowledge about γδ T cell-specific TCR ligands (in clear contrast to IPP and HMB-PP in humans) and by the fact that the expression of MHC class II molecules was highest in γδ T cells with lowest levels of cell surface TCRs (which might have obscured the discovery of murine γδ T-APCs in earlier studies). Mice carrying a transgene for γδ T cells with defined antigen-specificity will be invaluable for overcoming these obstacles. Cattle [47] and pigs [48, 49] were also reported to have γδ T cells with APC characteristics. Initially, αβ T cell responses in cattle were only seen with long-term cultured γδ T cell lines (not fresh γδ T cells), reminiscent of an early report with human αβ T cell clones [50]. More recent data revealed APC characteristics in primary bovine γδ T cells, including upregulation of MHC II during co-culture with DCs [51], and expression of CD40, CD80 and CD86 in response to mycobacterium Bacille Calmette-Guerin (Guzman and Hope, unpublished observations).
Collectively, in vitro-generated γδ T-APCs resemble monocyte-derived (inflammatory) DCs in their co-localization at sites of infections, their response to microbial stimuli (HMB-PP and toll-like receptor ligands, respectively) and their transition into potent APCs. Similar to monocytes, resting blood Vγ9/Vδ2 T cells have no APC functions, which exclude their involvement in the control of αβ T cell responses in the steady-state. We therefore suggest that γδ T-APCs are a product of acute responses, implying a role for these APCs at an early stage in our immune defence against infections (Fig. 3). The following outstanding questions about γδ T-APCs need to be addressed: (1) we do not know where in our body (blood, tissue, lymph nodes) γδ T-APCs are being generated; also, (2) we do not know whether the site of γδ T-APC generation is linked with the site of γδ T cell expansion as seen in the blood of patients with acute infections; and (3) we do not yet know to what extend γδ T-APCs contribute to infection control.
Use of γδ T-APCs in immunotherapy
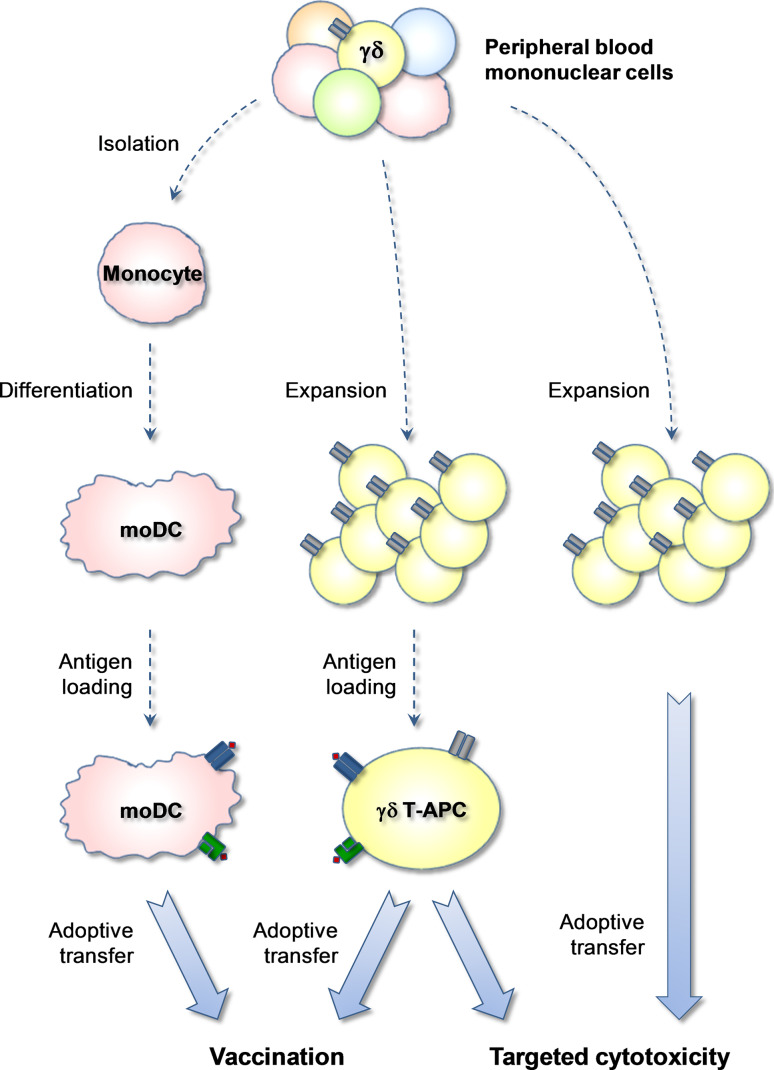
Since their discovery, DCs have become the prime focus in experimental immunotherapy of cancer patients [52]. The principal aim of this therapy is to overcome immune suppressive mechanisms and to establish effective T cell-mediated anti-tumor immunity. Much effort is being invested in the development of vaccine/adjuvant formulations that specifically target endogenous DCs. Alternatively, recent methods also include the ex vivo manipulation of DCs for subsequent infusion into cancer patients (Fig. 4). This latter strategy was substantially promoted by the identification of DC differentiation factors. Of note, the method for the in vitro generation of monocyte-derived DCs laid the foundation for numerous clinical trials [53]. Despite massive efforts worldwide, the use of DCs as cellular vaccines has not yet advanced beyond the experimental stage. PROVENGE, a blood cell-derived product developed by Dendreon (NJ, USA), is the only FDA-approved cellular vaccine today to be used specifically for the treatment of prostate cancer patients. It is not clear how or whether at all PROVENGE is related to monocyte-derived DCs.
Fig. 4.
In vitro generation of γδ T-APCs for use in immunotherapy. Treatment of PBMC with aminobisphosphonates or HMB-PP analogues and IL-2 leads to the expansion of Vγ9/Vδ2 T cells and subsequent re-stimulation in the presence of tumor antigens for 24 h generates tumor peptide-presenting γδ T-APCs for infusion into cancer patients. Presently, in vitro expanded Vγ9/Vδ2 T cells have already been adoptively transferred into cancer patients with moderate success (but no adverse effects). Treatment with γδ T-APCs differs from previous clinical trials with expanded human γδ T cells in their proposed APC function for induction of tumor-specific αβ T cell responses. However, similar to expanded γδ T cells, γδ T-APCs may also contribute directly to tumor cell killing. Previous cellular immunotherapy protocols have included monocyte-derived DCs (moDC) as tumor peptide-presenting APCs. Work with moDCs is somewhat problematic due to the limited numbers of moDCs that can be obtained during in vitro culture and their functional instability
We propose that human γδ T-APCs are an excellent choice of APCs for use in clinical research (Table 1). Probably, the most critical advantages of γδ T-APCs over monocyte-derived DCs are the relative ease of γδ T-APC generation and the practically unlimited number of γδ T-APCs that can be obtained during in vitro culture (Fig. 4). With PBMC from 50 ml of blood of healthy donors up to 109 γδ T cells routinely accumulate during 14 days of cell culture in the presence of IL-2 and either HMB-PP or zoledronate [30, 54]. Zoledronate, marketed by Novartis International (Basel, Switzerland) under the trade names Zometa, Reclast and Aclasta, is an aminobisphosphonate that is widely used in the treatment of patients with osteoporosis and metastatic bone disease [55, 56]. Relevant to the present discussion, zoledronate and other aminobisphosphonates are also potent activators of Vγ9/Vδ2 T cells [57, 58], which probably act by inducing enhanced levels of intracellular IPP [59]. Preliminary data show that expanded γδ T cells acquire APC characteristics in response to short-term stimulation much like primary γδ T cells prepared from fresh blood [20–22, 24]. We are now in the process of determining the functional quality of γδ T-APCs derived from expanded as compared to fresh blood γδ T cells.
Table 1.
Characteristics of γδ T cell- and monocyte-derived APCs
| gd T-APC | moDC | |
|---|---|---|
| Production | Easy and highly selective method >50-fold expansion during tissue culture (small blood samples from patients) | Blood monocytes do not proliferate (large leukapheresis samples) |
| Cell number | Large (routinely >108 cells/preparation) | Limited (approx. 107 cells/preparation) |
| Survival | Excellent survival during ex vivo preparation (may be frozen in large quantities for later use) | Limited survival (majority of monocytes die during ex vivo culture) |
| Purity | Uniform (effector-memory status) | Heterogeneous (immature–mature-exhausted) |
| APC function | Pro-inflammatory stable (no evidence for immunosuppression) | Variable, adjuvant-dependent (transient with short window of pro-inflammatory activity) |
An additional advantage over monocyte-derived DCs may lie in the functional uniformity of γδ T-APCs, as determined by the induction of primarily Th1-type responder cells. This is most likely due to the fact that the majority of activated Vγ9/Vδ2 T cells release large quantities of IFN-γ, which on the one hand induces T-bet, the master regulator of Th1 differentiation, and on the other hand suppresses Th2 and Th17 differentiation [60]. Further studies will show whether the pro-inflammatory nature of γδ T-APCs can be skewed either in response to polarising cytokines [16, 61, 62] or by engagement of Toll-like receptors and/or receptors for alternative DC maturation factors [63], towards APCs with Th2 cell-, Th17 cell- or Treg cell-inducing activities. Last but not least, we have shown that γδ T-APCs are substantially more efficient in antigen cross-presentation than monocyte-derived DCs [21, 22], a fact that is highly relevant for induction of tumor-specific CTL responses.
The following proposed scheme illustrates a simple method for the preparation and use of γδ T-APCs in the treatment of cancer patients. γδ T cells from fresh blood of cancer patients are expanded during in vitro culture in the presence of aminobisphosphonates and IL-2 [54]. Following the expansion, γδ T-APCs are loaded with tumor vaccines for a brief period (e.g., 24 h) and then processed for immediate infusion into cancer patients. Ideally, since γδ T-APCs are highly efficient in antigen cross-presentation, vaccines will include complex mixtures of tumor proteins or even extract from tumor biopsy material, which would circumvent the HLA haplotype restrictions commonly associated with defined tumor peptides. Due to safety concerns, however, initial tests will be conducted with well-described vaccine preparations. The proposed treatment procedure is repeated according to a prime-boost protocol. Initial phase I clinical trials will address safety issues and will help to define the optimal range of γδ T-APC dosage per infusion. We think that this novel treatment will not cause major adverse effects that would jeopardize the use of γδ T-APCs in the clinic. Several independent studies have shown that infusions of very large numbers (>109 cells/dose) of ex vivo expanded γδ T cells were well tolerated [64–67]. The same holds true for aminobisphosphonates and IL-2, two types of drugs currently used in the treatment of cancer patients and patients with osteoporosis [55, 56]. The observed mild/moderate side effects (acute-phase responses) seen in patients treated with zoledronate [55] may have been caused in part by cytokines released by zoledronate-responsive γδ T cells [57, 58, 68]. In fact, it is important to emphasize that human Vγ9/Vδ2 T cells (as opposed to other γδ T cell subsets) produce substantial quantities of cytokines upon activation, many of which (TNFα, IFNγ) are known to promote acute-phase responses [31]. The preparation of γδ T-APC with optimal APC qualities may require restimulation of expanded γδ T cells, a procedure that is expected to cause de novo cytokine production. Therefore, it will be essential to examine the level of residual cytokine production in the final γδ T-APC preparation, after washing and resuspension in infusion medium. Still, similar to current treatment with aminobisphosphonates or synthetic HMB-PP analogues, acute-phase responses resulting from treatment with γδ T-APCs may be reduced with co-administration of paracetamol/acetaminophen [55]. On a final note, success of the proposed immunotherapy protocol may also depend on the dosage of γδ T-APCs. Immunosuppression in cancer patients has been linked to impaired T cell responses, including proliferation of γδ T cells [69]. This situation may limit the number of ex vivo prepared γδ T-APCs that can be achieved from blood of cancer patients. Nevertheless, since treatment success will be evaluated in terms of parameters describing adaptive, tumor-specific immunity, APC quality may be more relevant than total numbers of infused cells. These concerns need to be tested in pilot clinical trials.
Concluding remarks
Considerable attention in immunotherapy research is currently focused on human Vγ9/Vδ2 T cells [70]. Solid in vitro data attest human Vγ9/Vδ2 T cells two types of qualities that promise to support the fight against cancer. Pro-inflammatory cytokines secreted by endogenous Vγ9/Vδ2 T cells stimulate innate anti-tumor immune mechanisms whereas cytotoxic activities in Vγ9/Vδ2 T cells are expected to be unleashed upon contact with tumor cells. Both of these defence mechanisms are targeted in cancer patients either by injection of aminobisphosphonates or synthetic HMB-PP analogues or by infusion of ex vivo expanded γδ T cells. Both functions are meant to act locally at the site of tumors, indicating that their effect is strictly coupled to proper homing mechanisms. However, the anti-inflammatory milieu in tumors may prevent this from happening. Our proposed treatment with γδ T-APCs does not target tumors directly and is therefore less affected by tumor-associated cytokines (Fig. 4). Instead, similar to DCs, infused γδ T-APCs target αβ T cells (and B cells) in secondary lymphoid tissues, such as spleen and lymph nodes, and consequently they need to enter these sites as opposed to tumor tissue. Subsequent engagement with αβ T cells is expected to result in the activation of tumor-specific αβ T cells of both denominations (T helper cells, CTLs). Treatment success probably requires the involvement of both CD4+ and CD8+ αβ T cells, emphasizing the importance in choosing the “right” vaccine, i.e., a complex vaccine bearing immunodominant epitopes able to form complexes with both MHC I and MHC II molecules. Of note, production of pro-inflammatory cytokines may be of added value to the proposed γδ T-APC treatment in overcoming T cell-mediated immune suppression and in the generation of anti-tumor effector T cells. Finally, direct tumor killing by γδ T-APCs may also occur once the change in the tumor milieu (e.g., production of inflammatory chemokines) allows their recruitment. It is important to emphasize that “classical” γδ T cell functions that are currently targeted in clinical trials are not expected to interfere with the proposed γδ T-APC therapy; quite the contrary, these functions may even be beneficial. Proof of principal studies in humans are urgently needed in order to translate the proposed new γδ T-APC therapy to the clinic.
Acknowledgments
Research has been supported by Grants from the Swiss National Science Foundation, European FP6 (MAIN-NoE, INNOCHEM), Welsh Office for Research and Development, Wellcome Trust, Cancer Research UK, Breast Cancer Campaign, Baxter Healthcare, and Cardiff University i3-IRG. M.E. is a RCUK Fellow in Translational Research in Experimental Medicine; B.M. is the recipient of a Royal Society Wolfson Research Merit Award.
Abbreviations
- APC
Antigen-presenting cells
- γδ T-APC
Antigen-presenting γδ T cells
- DC
Dendritic cells
- MHC
Major histocompatibility complex antigen
- TCR
T cell antigen receptor
- HMB-PP
(E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate
- IPP
Isopentenyl pyrophosphate
References
- 1.Caccamo N, Dieli F, Wesch D, Jomaa H, Eberl M. Sex-specific phenotypical and functional differences in peripheral human Vgamma9/Vdelta2 T cells. J Leukoc Biol. 2006;79:663–666. doi: 10.1189/jlb.1105640. [DOI] [PubMed] [Google Scholar]
- 2.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 3.Sicard H, Fournie JJ. Metabolic routes as targets for immunological discrimination of host and parasite. Infect Immun. 2000;68:4375–4377. doi: 10.1128/IAI.68.8.4375-4377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, Beck E, Wiesner J, Eberl M, Jomaa H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gammadelta T cells in Escherichia coli . FEBS Lett. 2001;509:317–322. doi: 10.1016/S0014-5793(01)03191-X. [DOI] [PubMed] [Google Scholar]
- 5.Eberl M, Hintz M, Reichenberg A, Kollas AK, Wiesner J, Jomaa H. Microbial isoprenoid biosynthesis and human gammadelta T cell activation. FEBS Lett. 2003;544:4–10. doi: 10.1016/S0014-5793(03)00483-6. [DOI] [PubMed] [Google Scholar]
- 6.Brandes M, Willimann K, Lang AB, Nam KH, Jin C, Brenner MB, Morita CT, Moser B. Flexible migration program regulates gamma delta T-cell involvement in humoral immunity. Blood. 2003;102:3693–3701. doi: 10.1182/blood-2003-04-1016. [DOI] [PubMed] [Google Scholar]
- 7.Glatzel A, Wesch D, Schiemann F, Brandt E, Janssen O, Kabelitz D. Patterns of chemokine receptor expression on peripheral blood gamma delta T lymphocytes: strong expression of CCR5 is a selective feature of V delta 2/V gamma 9 gamma delta T cells. J Immunol. 2002;168:4920–4929. doi: 10.4049/jimmunol.168.10.4920. [DOI] [PubMed] [Google Scholar]
- 8.Cipriani B, Borsellino G, Poccia F, Placido R, Tramonti D, Bach S, Battistini L, Brosnan CF. Activation of C-C β-chemokines in human peripheral blood gammaδ T cells by isopentenyl pyrophosphate and regulation by cytokines. Blood. 2000;95:39–47. [PubMed] [Google Scholar]
- 9.Poggi A, Carosio R, Fenoglio D, Brenci S, Murdaca G, Setti M, Indiveri F, Scabini S, Ferrero E, Zocchi MR. Migration of V delta 1 and V delta 2 T cells in response to CXCR3 and CXCR4 ligands in healthy donors and HIV-1-infected patients: competition by HIV-1 Tat. Blood. 2004;103:2205–2213. doi: 10.1182/blood-2003-08-2928. [DOI] [PubMed] [Google Scholar]
- 10.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 11.Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25:75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Eberl M, Moser B. Monocytes and gammadelta T cells: close encounters in microbial infection. Trends Immunol. 2009;30:562–568. doi: 10.1016/j.it.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 14.Groh V, Porcelli S, Fabbi M, Lanier LL, Picker LJ, Anderson T, Warnke RA, Bhan AK, Strominger JL, Brenner MB. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989;169:1277–1294. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, Salerno A. Differentiation of effector/memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391–397. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vermijlen D, Ellis P, Langford C, Klein A, Engel R, Willimann K, Jomaa H, Hayday AC, Eberl M. Distinct cytokine-driven responses of activated blood gammadelta T cells: insights into unconventional T cell pleiotropy. J Immunol. 2007;178:4304–4314. doi: 10.4049/jimmunol.178.7.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caccamo N, Battistini L, Bonneville M, Poccia F, Fournie JJ, Meraviglia S, Borsellino G, Kroczek RA, La Mendola C, Scotet E, Dieli F, Salerno A. CXCR5 identifies a subset of Vgamma9 Vdelta2 T cells which secrete IL-4 and IL-10 and help B cells for antibody production. J Immunol. 2006;177:5290–5295. doi: 10.4049/jimmunol.177.8.5290. [DOI] [PubMed] [Google Scholar]
- 18.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 20.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T cells. Science. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 21.Meuter S, Eberl M, Moser B. Prolonged antigen survival and cytosolic export in cross-presenting human gammadelta T cells. Proc Natl Acad Sci USA. 2010;107:8730–8735. doi: 10.1073/pnas.1002769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandes M, Willimann K, Bioley G, Levy N, Eberl M, Luo M, Tampe R, Levy F, Romero P, Moser B. Cross-presenting human gammadelta T cells induce robust CD8+ alphabeta T cell responses. Proc Natl Acad Sci USA. 2009;106:2307–2312. doi: 10.1073/pnas.0810059106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y, Wu W, Wong WM, Ward E, Thrasher AJ, Goldblatt D, Osman M, Digard P, Canaday DH, Gustafsson K. Human gamma delta T cells: a lymphoid lineage cell capable of professional phagocytosis. J Immunol. 2009;183:5622–5629. doi: 10.4049/jimmunol.0901772. [DOI] [PubMed] [Google Scholar]
- 24.Landmeier S, Altvater B, Pscherer S, Juergens H, Varnholt L, Hansmeier A, Bollard CM, Moosmann A, Bisping G, Rossig C. Activated human gammadelta T cells as stimulators of specific CD8+ T-cell responses to subdominant Epstein Barr virus epitopes: potential for immunotherapy of cancer. J Immunother. 2009;32:310–321. doi: 10.1097/CJI.0b013e31819b7c30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 26.Cresswell P, Ackerman AL, Giodini A, Peaper DR, Wearsch PA. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol Rev. 2005;207:145–157. doi: 10.1111/j.0105-2896.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 27.Villadangos JA, Heath WR, Carbone FR. Outside looking in: the inner workings of the cross-presentation pathway within dendritic cells. Trends Immunol. 2007;28:45–47. doi: 10.1016/j.it.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Villadangos JA, Shortman K. Found in translation: the human equivalent of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1131–1134. doi: 10.1084/jem.20100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 30.Davey MS, Lin CY, Roberts GW, Heuston S, Brown AC, Chess JA, Toleman MA, Gahan CG, Hill C, Parish T, Williams JD, Davies SJ, Johnson DW, Topley N, Moser B, Eberl M. Human neutrophil clearance of bacterial pathogens triggers anti-microbial gammadelta T cell responses in early infection. PLoS Pathog. 2011;7:e1002040. doi: 10.1371/journal.ppat.1002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eberl M, Roberts GW, Meuter S, Williams JD, Topley N, Moser B. A Rapid crosstalk of human gammadelta T Cells and monocytes drives the acute inflammation in bacterial infections. PLoS Pathog. 2009;5:e1000308. doi: 10.1371/journal.ppat.1000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dieli F, Sireci G, Di Sano C, Champagne E, Fournie JJ, Salerno JI. Predominance of Vgamma9/Vdelta2 T lymphocytes in the cerebrospinal fluid of children with tuberculous meningitis: reversal after chemotherapy. Mol Med. 1999;5:301–312. [PMC free article] [PubMed] [Google Scholar]
- 33.Parker CM, Groh V, Band H, Porcelli SA, Morita C, Fabbi M, Glass D, Strominger JL, Brenner MB. Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J Exp Med. 1990;171:1597–1612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green AE, Lissina A, Hutchinson SL, Hewitt RE, Temple B, James D, Boulter JM, Price DA, Sewell AK. Recognition of nonpeptide antigens by human V gamma 9 V delta 2 T cells requires contact with cells of human origin. Clin Exp Immunol. 2004;136:472–482. doi: 10.1111/j.1365-2249.2004.02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarikonda G, Wang H, Puan KJ, Liu XH, Lee HK, Song Y, Distefano MD, Oldfield E, Prestwich GD, Morita CT. Photoaffinity antigens for human gammadelta T cells. J Immunol. 2008;181:7738–7750. doi: 10.4049/jimmunol.181.11.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Laethem F, Sarafova SD, Park JH, Tai X, Pobezinsky L, Guinter TI, Adoro S, Adams A, Sharrow SO, Feigenbaum L, Singer A. Deletion of CD4 and CD8 coreceptors permits generation of alphabetaT cells that recognize antigens independently of the MHC. Immunity. 2007;27:735–750. doi: 10.1016/j.immuni.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Pechhold K, Wesch D, Schondelmaier S, Kabelitz D. Primary activation of V gamma 9-expressing gamma delta T cells by Mycobacterium tuberculosis. Requirement for Th1-type CD4 T cell help and inhibition by IL-10. J Immunol. 1994;152:4984–4992. [PubMed] [Google Scholar]
- 38.Boullier S, Poquet Y, Debord T, Fournie JJ, Gougeon ML. Regulation by cytokines (IL-12, IL-15, IL-4 and IL-10) of the Vgamma9 Vdelta2 T cell response to mycobacterial phosphoantigens in responder and anergic HIV-infected persons. Eur J Immunol. 1999;29:90–99. doi: 10.1002/(SICI)1521-4141(199901)29:01<90::AID-IMMU90>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 39.Eberl M, Altincicek B, Kollas AK, Sanderbrand S, Bahr U, Reichenberg A, Beck E, Foster D, Wiesner J, Hintz M, Jomaa H. Accumulation of a potent gammadelta T-cell stimulator after deletion of the lytB gene in Escherichia coli . Immunology. 2002;106:200–211. doi: 10.1046/j.1365-2567.2002.01414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rojas RE, Torres M, Fournie JJ, Harding CV, Boom WH. Phosphoantigen presentation by macrophages to Mycobacterium tuberculosis–reactive Vgamma9 Vdelta2 + T cells: modulation by chloroquine. Infect Immun. 2002;70:4019–4027. doi: 10.1128/IAI.70.8.4019-4027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei H, Huang D, Lai X, Chen M, Zhong W, Wang R, Chen ZW. Definition of APC presentation of phosphoantigen (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate to Vgamma2 Vdelta 2 TCR. J Immunol. 2008;181:4798–4806. doi: 10.4049/jimmunol.181.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang D, Shen Y, Qiu L, Chen CY, Shen L, Estep J, Hunt R, Vasconcelos D, Du G, Aye P, Lackner AA, Larson M, Jacobs WR, Jr, Haynes BF, Letvin NL, Chen ZW. Immune distribution and localization of phosphoantigen-specific V{gamma}2 V{delta}2 T cells in lymphoid and non-lymphoid tissues in M tuberculosis infection. Infect Immun. 2008;76:426–436. doi: 10.1128/IAI.01008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, Kou Z, Wang Q, Jiang L, Estep J, Hunt R, Clagett M, Sehgal PK, Li Y, Zeng X, Morita CT, Brenner MB, Letvin NL, Chen ZW. Adaptive immune response of Vgamma2 Vdelta2 + T cells during mycobacterial infections. Science. 2002;295:2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cairo C, Hebbeler AM, Propp N, Bryant JL, Colizzi V, Pauza CD. Innate-like gammadelta T cell responses to mycobacterium Bacille Calmette-Guerin using the public V gamma 2 repertoire in Macaca fascicularis. Tuberculosis (Edinb) 2007;87:373–383. doi: 10.1016/j.tube.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cendron D, Ingoure S, Martino A, Casetti R, Horand F, Romagne F, Sicard H, Fournie JJ, Poccia F. A tuberculosis vaccine based on phosphoantigens and fusion proteins induces distinct gammadelta and alphabeta T cell responses in primates. Eur J Immunol. 2007;37:549–565. doi: 10.1002/eji.200636343. [DOI] [PubMed] [Google Scholar]
- 46.Cheng L, Cui Y, Shao H, Han G, Zhu L, Huang Y, O’Brien RL, Born WK, Kaplan HJ, Sun D. Mouse gammadelta T cells are capable of expressing MHC class II molecules, and of functioning as antigen-presenting cells. J Neuroimmunol. 2008;203:2–11. doi: 10.1016/j.jneuroim.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collins RA, Werling D, Duggan SE, Bland AP, Parsons KR, Howard CJ. Gammadelta T cells present antigen to CD4+ alphabeta T cells. J Leukoc Biol. 1998;63:707–714. doi: 10.1002/jlb.63.6.707. [DOI] [PubMed] [Google Scholar]
- 48.Takamatsu HH, Denyer MS, Wileman TE. A sub-population of circulating porcine gammadelta T cells can act as professional antigen presenting cells Vet. Immunol Immunopathol. 2002;87:223–224. doi: 10.1016/S0165-2427(02)00083-1. [DOI] [PubMed] [Google Scholar]
- 49.Takamatsu HH, Denyer MS, Stirling C, Cox S, Aggarwal N, Dash P, Wileman TE, Barnett PV. Porcine gammadelta T cells: possible roles on the innate and adaptive immune responses following virus infection. Vet Immunol Immunopathol. 2006;112:49–61. doi: 10.1016/j.vetimm.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Lanzavecchia A, Roosnek E, Gregory T, Berman P, Abrignani S. T cells can present antigens such as HIV gp120 targeted to their own surface molecules. Nature. 1988;334:530–532. doi: 10.1038/334530a0. [DOI] [PubMed] [Google Scholar]
- 51.Price SJ, Hope JC. Enhanced secretion of interferon-gamma by bovine gammadelta T cells induced by coculture with Mycobacterium bovis-infected dendritic cells: evidence for reciprocal activating signals. Immunology. 2009;126:201–208. doi: 10.1111/j.1365-2567.2008.02889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 53.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kondo M, Sakuta K, Noguchi A, Ariyoshi N, Sato K, Sato S, Sato K, Hosoi A, Nakajima J, Yoshida Y, Shiraishi K, Nakagawa K, Kakimi K. Zoledronate facilitates large-scale ex vivo expansion of functional gammadelta T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy. 2008;10:842–856. doi: 10.1080/14653240802419328. [DOI] [PubMed] [Google Scholar]
- 55.Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black M (2010) Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab (in press) [DOI] [PubMed]
- 56.Coleman R (2010) The use of bisphosphonates in cancer treatment. Ann N Y Acad Sci [DOI] [PubMed]
- 57.Kunzmann V, Bauer E, Wilhelm M. Gamma/delta T-cell stimulation by pamidronate. N Engl J Med. 1999;340:737–738. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- 58.Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F, Arcara C, Valerio MR, Meraviglia S, Di Sano C, Sireci G, Salerno A. Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003;102:2310–2311. doi: 10.1182/blood-2003-05-1655. [DOI] [PubMed] [Google Scholar]
- 59.Roelofs AJ, Jauhiainen M, Monkkonen H, Rogers MJ, Monkkonen J, Thompson K. Peripheral blood monocytes are responsible for gammadelta T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol. 2009;144:245–250. doi: 10.1111/j.1365-2141.2008.07435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wesch D, Glatzel A, Kabelitz D. Differentiation of resting human peripheral blood gamma delta T cells toward Th1- or Th2-phenotype. Cell Immunol. 2001;212:110–117. doi: 10.1006/cimm.2001.1850. [DOI] [PubMed] [Google Scholar]
- 62.Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells. J Immunol. 2010;184:7268–7280. doi: 10.4049/jimmunol.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pietschmann K, Beetz S, Welte S, Martens I, Gruen J, Oberg HH, Wesch D, Kabelitz D. Toll-like receptor expression and function in subsets of human gammadelta T lymphocytes. Scand J Immunol. 2009;70:245–255. doi: 10.1111/j.1365-3083.2009.02290.x. [DOI] [PubMed] [Google Scholar]
- 64.Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T, Minato N, Toma H. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother. 2007;56:469–476. doi: 10.1007/s00262-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kobayashi H, Tanaka Y, Shimmura H, Minato N, Tanabe K. Complete remission of lung metastasis following adoptive immunotherapy using activated autologous gammadelta T-cells in a patient with renal cell carcinoma. Anticancer Res. 2010;30:575–579. [PubMed] [Google Scholar]
- 66.Bennouna J, Bompas E, Neidhardt EM, Rolland F, Philip I, Galea C, Salot S, Saiagh S, Audrain M, Rimbert M, Lafaye-de Micheaux S, Tiollier J, Negrier S. Phase-I study of Innacell gammadelta, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1599–1609. doi: 10.1007/s00262-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakajima J, Murakawa T, Fukami T, Goto S, Kaneko T, Yoshida Y, Takamoto S, Kakimi K. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous gammadelta T cells. Eur J Cardiothorac Surg. 2010;37:1191–1197. doi: 10.1016/j.ejcts.2009.11.051. [DOI] [PubMed] [Google Scholar]
- 68.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–392. [PubMed] [Google Scholar]
- 69.Lamb LS, Jr, Lopez RD. Gammadelta T cells: a new frontier for immunotherapy? Biol Blood Marrow Transplant. 2005;11:161–168. doi: 10.1016/j.bbmt.2004.12.186. [DOI] [PubMed] [Google Scholar]
- 70.June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: tools, trials and tribulations. Nat Rev Immunol. 2009;9:704–716. doi: 10.1038/nri2635. [DOI] [PMC free article] [PubMed] [Google Scholar]