Abstract
We summarize the clinical presentation and molecular basis of a unique group of congenital immunodeficiency disorders in which defects in immune tolerance mechanisms result in severe autoimmunity. Patients with severe, familial forms of multi-organ autoimmunity have been recognized and clinically described for more than 40 years (Clin Exp Immunol 1: 119–128, 1966; Clin Exp Immunol 2: 19–30, 1967). Some are characterized primarily by autoimmunity and others by autoimmunity combined with susceptibility to specific infectious organisms. The first mechanistic understanding of these disorders began to emerge approximately 10 years ago with the initial identification of causative genes. As a result, our understanding of how immune tolerance is established and maintained in humans has expanded dramatically. Data generated over the last 3–4 years including identification of additional gene defects and functional characterization of each identified gene product in human and animal models have added clarity. This, in turn, has improved our ability to diagnose and effectively treat these severe, life-threatening disorders. Inherited disorders characterized by immune dysregulation have dramatically expanded our understanding of immune tolerance mechanisms in humans. Recognition and diagnosis of these disorders in the clinic allows timely initiation of life-saving therapies that may prevent death or irreversible damage to vital organs.
Keywords: Autoimmunity, Immunodeficiency, Regulatory T cells
Introduction
The last decade has seen the identification of the molecular underpinnings of a unique class of congenital “immunodeficiency” disorders characterized more by susceptibility to autoimmunity than by susceptibility to infections. These discoveries, supported by studies in related animal models, have dramatically expanded our understanding of molecular and cellular mechanisms that contribute to the development and maintenance of immune tolerance. As manifested by the clinical symptoms and outcomes of patients with these “immune dysregulation” disorders, the inability to generate or maintain tolerance results in catastrophic consequences.
Virtually all components of the immune system participate in the development or maintenance of immune tolerance. In broad strokes however, immune tolerance mechanisms are generally divided into those that act centrally and those that act peripherally. Central immune tolerance mechanisms are those that are primarily mediated at the level of the thymus where the goal is to train-up a wide variety of T cells capable of responding to any foreign threat, while at the same time preventing any strongly autoreactive T cells from escaping the thymus into the periphery. This is accomplished through the mechanisms of positive selection and negative selection. Concomitantly, the thymus plays an essential role in the development of naturally arising FOXP3+ regulatory T cells (nTreg) from cells that have intermediate autoreactivity [3]. Peripheral immune tolerance mechanisms are those that are exerted at the level of the tissues and peripheral lymphoid organs [lymph nodes, gut-associated lymphoid tissue (GALT), etc.]. These are mediated by various cytokines and “regulatory” cells, chief among them being the CD4+CD25+ FOXP3+ regulatory T cells (Treg) that are generated in the thymus but exert their suppressive effect in the periphery. Interestingly, immune dysregulation disorders with molecular defects affecting central tolerance, peripheral tolerance, or a combination of the two have been described and will be discussed here.
APECED
Clinical features
Autoimmune polyendocrinopathy, candidiasis, and ectodermal dystrophy (APECED), also known as autoimmune polyglandular syndrome type 1 (APS1) (OMIM #240300), is a systemic disorder characterized most commonly by three basic clinical features: hypoparathyroidism, adrenal insufficiency, and mucocutaneous candidiasis. Typically, at least two of these need to be present to make a clinical diagnosis of APECED. Additional autoimmune manifestations are also common among patients with APECED including: gonadal dysfunction, enteropathy and pernicious anemia, autoimmune hepatitis, diabetes, alopecia areata, interstitial lung disease, and others [4, 5]. Among the most interesting findings of the past few years related to APECED has been the identification of a number of tissue-specific autoantigens to which pathogenic autoantibodies are generated. These include: NALP5, a protein expressed in parathyroid chief cells and associated with hypoparathyroidism [6]; CYPC17/CYPC21/CYPSCC, members of the cytochrome p450 family that are associated with adrenal insufficiency [7, 8], LPLUNC1 and KCNRG identified as autoantigens in APECED patients with interstitial lung disease [9, 10], and Intrinsic factor, present in gastric parietal cells and associated with development of pernicious anemia [8]. In addition, autoantibodies to type I interferons (IFN-α and ω) have been identified in virtually all APECED patients and have been proposed as an additional diagnostic criterion for the disease [11, 12]. Autoantibodies to IL-17 have also been identified and are thought to play a role in the unusual susceptibility to mucocutaneous candiasis in APECED [13]. The mechanism for this tremendous propensity to develop autoantibodies is not yet understood, but in a murine model of APECED, B cells were required for development of the typical autoimmune features of the disorder suggesting that autoantibodies play a critical role in the normal course of the disease [14].
Molecular basis
APECED is a disorder of dysfunctional central immune tolerance caused by defects in AIRE-1 (autoimmune regulator-1), a transcription factor expressed primarily in specialized thymic medullary epithelial cells (mTEC). AIRE-1 mediates expression of tissue-specific self-antigens (i.e. insulin, etc.) by mTECs. This serves as an important checkpoint in the thymus for negative selection of strongly autoreactive T cells and for generation of Tregs. The mechanism by which AIRE-1 mediates broad expression of tissue-specific proteins in mTECs is still not clear, but recent data suggest that it may regulate both transcription (by modulating chromatin accessibility and transcriptional activity) and RNA splicing and maturation [15, 16].
Diagnosis
A clinical diagnosis of APECED can be made in individuals with two of the three basic features of the disease (hypoparathyroidism, adrenal insufficiency, and mucocutaneous candidiasis). The presence of autoantibodies to type I interferons can also be suggestive, but a definitive diagnosis of APECED is typically made by sequencing of the AIRE1 gene. In almost all cases, the disease is inherited in an autosomal recessive fashion, although mutations in the SAND domain of AIRE-1 have been reported to generate a protein that acts in a dominant-negative fashion, causing an autosomal dominant form of the disease [17].
Treatment
The usual treatments for APECED focus on symptomatic therapy including hormone replacement for endocrinopathies and antifungals to treat mucocutaneous candidiasis (MCC). Immunosuppression has not been routinely utilized in APECED patients although there has been reported benefit of various combinations of cyclosporine, tacrolimus, methotrexate, azathioprine, and steroids for severe autoimmune renal, hepatic, and bowel disease [18, 19]. The development of pathogenic autoantibodies in many cases suggests that B cell depletion therapy (i.e. with anti-CD20 monoclonal antibody) may be useful and, in fact, murine studies support this idea. There is little information in the literature either in animal models or humans regarding the efficacy of bone marrow transplantation (BMT) for APECED. Because AIRE-1 deficiency primarily affects the function of a non-hematopoietically derived cell type, it is unclear whether BMT would provide significant clinical benefit since donor T cells would be subject to the same defects in thymic selection that led to disease in the first place.
IPEX syndrome
Clinical features and diagnosis
Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) (OMIM #304930) is an inherited syndrome of systemic autoimmunity that is characteristic of disorders caused by defective peripheral immune tolerance. The most common clinical features include enteropathy, dermatitis (most commonly eczematous), and endocrinopathy (type I diabetes or thyroiditis). Most patients also have other autoimmunity including hematologic disease (anemia, thrombocytopenia, neutropenia), hepatitis, nephritis, alopecia, and others. Significant failure to thrive, arising from the enteropathy and endocrine disease, is common. In some patients, the clinical course is fulminant, leading to death within the first months of life while in others, survival may extend into the second or third decades. Almost all patients, even those with “milder” disease, develop symptoms in infancy.
Similar to APECED, it has recently been recognized that patients with IPEX are prone to develop a diverse array of autoantibodies to a variety of autoantigen targets. Some of these are known to be pathogenic including antibodies that cause hepatitis and pemphigus nodularis [20–22].
Molecular basis
IPEX is caused by mutations in the FOXP3 gene, which encodes a DNA binding protein that is a member of the forkhead box transcription factor family. FOXP3 binds to and regulates a large number of target genes and is required for development of the suppressive function of CD4+ CD25+ FOXP3+ Tregs. The absence of functional FOXP3 leads to a deficiency of functional regulatory T cells and the consequent autoimmunity/immune dysregulation observed in IPEX.
Diagnosis
A diagnosis of IPEX is typically considered in patients with two or more of the characteristic autoimmune symptoms (i.e. enteropathy + diabetes, enteropathy + dermatitis, etc.). Interestingly, despite the significant immune dysregulation in IPEX, typical laboratory tests used to evaluate immune function are grossly normal. The one exception in most patients is a markedly elevated IgE level. Flow cytometry to measure the percentage or absolute number of FOXP3-expressing Tregs in the CD4+ T cell population is a useful initial screening test for IPEX, although some missense mutations may allow expression of some FOXP3 protein. Ultimately, sequencing of the FOXP3 gene is required to confirm a diagnosis of IPEX.
Treatment
Early recognition of IPEX syndrome is essential to optimize outcome. Patients typically require aggressive supportive care including elemental formula, parenteral nutrition, insulin, thyroid hormone, etc. Active disease is typically most effectively controlled in the short term by using T cell-directed immunosuppression with agents such as cyclosporine, tacrolimus, or rapamycin. In patients who have evidence of pathogenic autoantibodies, B cell depletion therapy with anti-CD20 antibodies has proven effective [21]. Since all patients with pathogenic FOXP3 mutations are expected to have a significantly shortened lifespan, bone marrow transplantation should be considered in most cases. To date, outcomes using reduced intensity conditioning regimens seem to be safer and at least as effective as fully myeloablative approaches. If patients can be identified and transplanted prior to the onset of diabetes or thyroid disease, BMT can lead to complete resolution of IPEX symptoms. If patients have diabetes or thyroid disease, these typically persist after transplant even though other IPEX symptoms are resolved.
CD25 deficiency
Clinical features and diagnosis
Deficiency of CD25 (OMIM #606367), the α-chain of the Interleukin-2 (IL-2) receptor, has been described in two unrelated male patients. In each case, the clinical features of disease had similarities with both IPEX and severe combined immune deficiency (SCID). Similar to IPEX, both patients developed severe, chronic diarrhea and villous atrophy in infancy (one at 6 weeks and the other at 8 months of age) [23, 24]. One also developed early onset insulin-dependent diabetes and eczema [23]. Subsequently, both patients developed autoantibodies, hepatosplenomegaly, lymphadenopathy, and lymphocytic infiltrates in various organs (gut, liver, etc.) indicative of ongoing immune dysregulation [23–25]. Unlike patients with FOXP3 mutations, serum IgE levels were either normal or only mildly elevated [23, 24].
In addition to autoimmune features, both CD25-deficient patients had infectious complications suggestive of a more extensive defect in cellular immunity. The most prominent of these was early onset, recurrent CMV pneumonitis in both patients. although persistent thrush, candidal esophagitis, chronic gastroenteritis, and EBV infection were also observed [23, 24]. Interestingly, an allogeneic skin graft was required in one patient and was not rejected despite no significant immune suppression being used [26].
Molecular basis
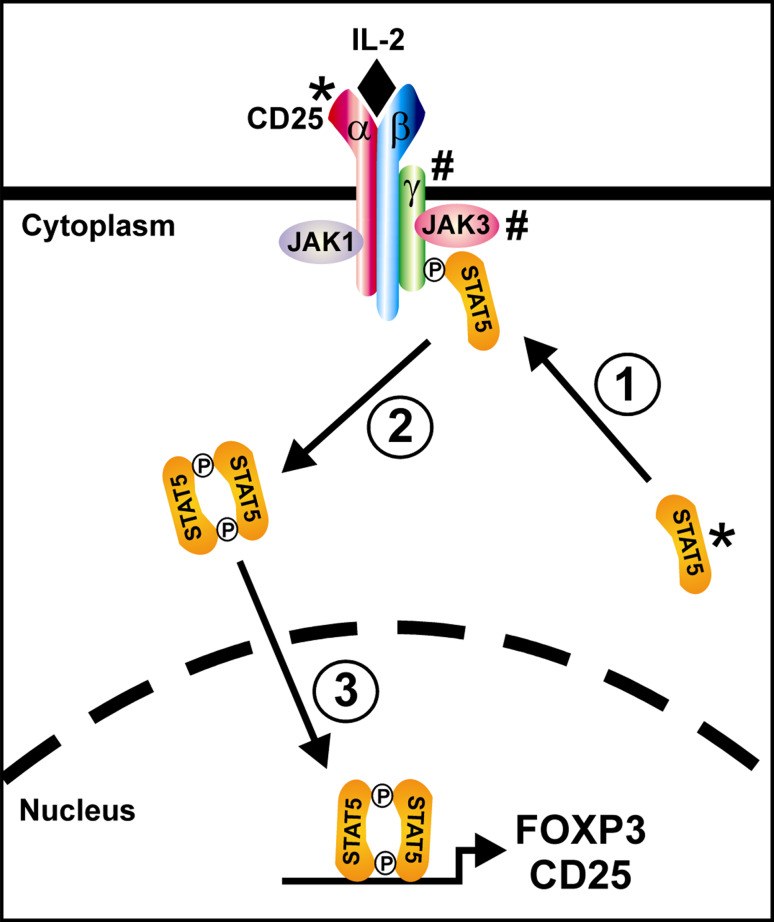
CD25 is the α-chain of the IL-2 receptor that, in concert with the β-chain (CD122) and common γ-chain (CD132), constitutes the complete IL-2 receptor (Fig. 1). Under normal circumstances, resting T cells express an IL-2 receptor consisting of only the β and γ chains. This minimal receptor binds IL-2 but only when it is present at high concentrations. Upon activation, T cells inducibly express the α-chain (CD25), which increases the affinity of the receptor for IL-2 by more than 100-fold (Torgerson, unpublished data). As opposed to effector T cells, regulatory T cells constitutively express CD25 so they are able to respond to low concentrations of IL-2 at all times. This ability to respond to low concentrations of IL-2 is critical for maintenance of FOXP3 expression in Tregs. In fact, in mice lacking either IL-2 or CD25, Tregs are generated normally in the thymus but there is a marked defect in the survival, maintenance, and competitive fitness of mature Tregs which appears to underlie the immune dysregulation observed in these models [27, 28]. Future efforts to assess Treg cells in CD25-deficient patients will hopefully help to determine whether a similar mechanism is at play in humans (Table 1).
Fig. 1.
The role of CD25 and STAT5 in IL-2 signaling. The high-affinity IL-2 receptor is composed of α (CD25), β (CD122), and γ (γc; CD132) protein subunits together with receptor-associated tyrosine kinases of the JAK protein kinase family (JAK1 and JAK3). Upon binding of IL-2, the receptor subunits are clustered and phosphorylated on cytoplasmic tyrosine residues by the JAK kinases. Via its SH2 domain, unphosphorylated STAT5 binds to the phosphotyrosine residues on the activated receptor thus bringing it into proximity with the JAK kinases (1). The STAT5 subunits are then phosphorylated on a specific tyrosine residue by the JAK kinases causing them to dimerize (2), translocate to the nucleus, and bind DNA thereby upregulating expression of CD25, stabilizing expression of FOXP3, and modulating expression of a number of other gene targets (3). Note that defects in CD25 and STAT5 (marked by *) cause an immune dysregulation phenotype whereas defects in γc and JAK3 (marked by #) cause a phenotype of severe combined immune deficiency (SCID)
Table 1.
Key features of genetic immune dysregulation syndromes
| Disorder | APECED | IPEX | CD25 deficiency | STAT5b deficiency | Itch deficiency | IL-10R or IL-10 deficiency |
|---|---|---|---|---|---|---|
| Gene product | AIRE-1 | FOXP3 | CD25 | STAT5b | ITCH/AIP4 | IL10R1, IL10R2, IL-10 |
| Number of patients reported | >150 | >70 |
3 patients (2 families) |
10 patients (8 families) |
10 patients (1 family) |
IL10R: 4 patients (3 families) |
|
IL-10: 2 patients (2 families) | ||||||
| Common clinical features (present in >50% of patients) | Hypoparathyroidism | Enteropathy | Enteropathy | Marked growth failure | Chronic lung disease/interstitial pneumonitis | Severe, early-onset, fistulating colitis |
| Adrenal insufficiency | Dermatitis (eczema) | Recurrent/persistent viral infections (CMV, EBV, etc.) | Chronic lung disease/interstitial pneumonitis | Hepatosplenomegaly | Follicular dermatitis | |
| Mucocutaneous candidiasis | Endocrinopathy | Hepatomegaly | Eczematous rash | Macrocephaly | ||
| Lymphadenopathy | Dysmorphic facies | |||||
| Additional clinical features | Gonadal insufficiency | Autoimmune hemolytic anemia, thrombocytopenia, or neutropenia | Eczema | Diarrhea/enteropathy | Hypothyroidism | Infections (pneumonia, otitis media, bronchitis, renal abscess, etc.), may be related to immune suppression |
| Diabetes mellitus | Autoimmune hepatitis | Persistent thrush, Candida esophagitis | Autoimmune thyroid disease | Hepatitis | ||
| Pernicious anemia | Renal insufficiency | Recurrent or severe viral infections | Enteropathy | |||
| Alopecia areata | Facies: prominent forehead and saddle nose | Insulin-dependent diabetes | ||||
| Vitiligo | ||||||
| Autoimmune hepatitis | ||||||
| Interstitial lung disease | ||||||
| Keratitis | ||||||
| Useful laboratory or diagnostic features | Hypocalcemia | Eosinophilia | CD25 expression on T cells absent or low (flow cytometry) | Normal plasma growth hormone | Itch mutation present | Tyrosine phosphorylation of STAT3 absent or decreased in cells stimulated with IL-10 (flow cytometry) |
| Hyperphosphatemia | Very elevated IgE | IgE typically normal | Very low plasma IGF-1 and IGFBP-3 | |||
| Low plasma cortisol | FOXP3 expression in CD4+ T cells typically low (flow cytometry) | Poor T cell proliferation | Elevated Prolactin | Immune workup typically normal | ||
| High plasma ACTH | FOXP3 mutation present | CD25 mutation present | Mild/moderate T cell lymphopenia | IL10 or IL10R mutation present | ||
| Antibodies to type I interferons (IFN-α or ω) typically present | Decreased NK cells | |||||
| STAT5B mutation present | ||||||
| AIRE-1 mutation present | ||||||
| Treatments | Symptomatic care | Symptomatic care | Symptomatic care | Growth hormone ineffective | Close pulmonary follow-up/PFT’s | Immune suppression largely ineffective |
| Antifungal therapy | T cell directed immune suppression (calcineurin inhibitors or rapamycin) | Immune suppression | IGF-1 therapy theoretically effective but untried | Immune suppression | Surgical management of fistulae and abscesses | |
| Possibly Rituximab for autoantibody mediated disease | IVIG | |||||
| Rituximab for autoantibody mediated disorders | Antimicrobials | |||||
| Immune suppression | ||||||
| Close pulmonary follow-up/PFT’s | ||||||
| Bone marrow transplant | Not routinely used | Effective (preferably early) | Effective | Probably effective for immunologic features but untried | Theoretically effective based on murine studies but untried | Effective |
Diagnosis
In both described cases, inheritance was autosomal recessive. One patient, the product of consanginuous parents, was homozygous for a 4 base pair deletion in the coding region of CD25 that causes a frameshift and early termination codon [24, 26]. The other patient had compound heterozygous mutations in the CD25 gene that led to a frameshift on one allele and a premature stop codon on the other [23]. In both cases, the mutations led to absence of CD25 expression on T cells. Flow cytometry is therefore a reasonable initial screening tool to evaluate patients suspected of having CD25 deficiency. Sequencing of the IL2RA (CD25) gene is, however, recommended to confirm the diagnosis in all cases.
Treatment
Both patients required significant supportive care to treat the symptoms of the disease. Because of the “SCID-like” features of this syndrome, one patient underwent a successful bone marrow transplant from a matched sibling donor and has done well [25, 26]. It is theoretically possible, however, that patients may respond to IL-2 therapy since the in vitro T cell proliferative defect could be overcome by treatment with exogenous high dose IL-2 or IL-15. Exogenous IL-2 may provide enough stimulation through the remaining “low-affinity” IL-2 receptor β-chain to allow Treg cells to survive and control autoreactive effector T cells.
STAT5b deficiency
Clinical features and diagnosis
Deficiency of the STAT5b transcription factor was first described in 2003 in patients with significant growth failure and autoimmunity [29]. Like other STAT transcription factors, STAT5b plays essential roles in cytokine and growth factor signaling, particularly in response to IL-2, IL-15, and growth hormone. As a result, deficiency of STAT5b (OMIM #245590) results in a combination of marked growth failure (heights −3.0 to −9.9 standard deviations below the mean for age) and immune deficiency/autoimmunity. Other than the growth phenotype, the most consistent clinical features are a chronic lymphocytic interstitial pneumonitits (LIP) described in 90% of patients and a rash (frequently eczematous) that is present in 80% of patients. The pneumonitis causes respiratory failure and death in some affected individuals. Autoantibodies were also prevalent (~50% of patients) [30].
Because STAT5b is essential for normal signaling from Interleukins-2 (IL-2) and 15 (IL-15), key growth factors for T cells and NK cells, respectively, patients typically have moderate T and NK cell lymphopenia and frequently suffer from recurrent viral infections with pathogens such as cytomegalovirus (CMV). Since signaling from IL-2 is essential for sustaining FOXP3 expression in natural Tregs, patients with STAT5b deficiency were found to have markedly decreased FOXP3 expression in CD4+ T cells and defective Treg function [30].
Molecular basis
STAT5b is one of the seven members of the STAT family of transcription factors. These DNA binding proteins play key roles in cellular responses to a variety of cytokines and growth factors. STAT5b is particularly important for cellular responses to growth factor and to the cytokines IL-2 and IL-15. In transducing signals from IL-2, STAT5b plays a key role in sustaining FOXP3 protein expression in Tregs as described above (Fig. 1). Under normal circumstances, STAT5b is present in the cytoplasm as an inactive, non-phosphorylated dimer that is recruited to activated cytokine receptors where the STAT5b subunits are phosphorylated on critical tyrosine residues by receptor-associated tyrosine kinases. This activates the STAT5b dimer, causing it to localize to the nucleus where it binds DNA and regulates gene transcription of a variety of target genes including FOXP3. All STAT5b mutations identified to date are autosomal recessive and destroy the function of both STAT5b alleles in the cell [30].
Diagnosis
Because of the unique complex of marked growth failure, immune deficiency, and immune dysregulation, the physical examination and routine laboratory testing (lymphopenia, low NK cells, etc.) can strongly suggest a diagnosis of STAT5b deficiency. Endocrine testing typically shows normal plasma growth hormone levels, very low plasma IGF-1 and IGFBP-3, and elevated prolactin [30]. Definitive diagnosis is, however, made by sequencing of the STAT5B gene. In all cases described thus far, the disorder is inherited in an autosomal recessive fashion although there is a potential that autosomal dominant forms could be identified as in autosomal dominant hyper-IgE syndrome, where heterozygous mutant STAT3 proteins act in a dominant-negative manner to cause disease [31, 32].
Treatment
Most treatments for STAT5b deficiency have focused on symptomatic therapy and prophylaxis against infections. Immune suppression has met with mixed success although no therapies have been reported to be significantly beneficial for the severe lung disease that affects most patients [33]. To date, there have been no reports of bone marrow transplantation (BMT) for this disorder, although it is predicted that it would correct the significant immunodeficiency and immune dysregulation typically associated with STAT5b deficiency. While correction of these features may decrease morbidity and mortality, BMT would not be expected to improve the severe growth failure, a consideration that would need to be seriously weighed before attempting BMT.
Itch deficiency
Clinical features
Deficiency of the E3 ubiquitin ligase ITCH/AIP4 (OMIM # 613385) has recently been described in a single large Amish kindred with ten affected individuals [34]. The clinical features described in this cohort include a combination of dysmorphic facial features (triangular-shaped face, relative macrocephaly, hypertelorism, micrognathia), moderate to severe failure to thrive, developmental delay, and significant autoimmunity/immune dysregulation. The most consistent feature of immune dysregulation among this cohort was early-onset chronic lung disease, characterized primarily as an interstitial pneumonitis that was present in 90% of the patients, a feature not unlike that observed in patients with STAT5b deficiency (see above). In fact, three of the ten patients identified to date succumbed to chronic lung disease in childhood. In addition to pulmonary disease, 60% of the patients had evidence of at least one autoimmune disorder including thyroiditis, type I diabetes, chronic diarrhea/enteropathy, and hepatitis. Unfortunately, the full spectrum of functional immune deficits present in these patients remains to be carefully evaluated.
Molecular basis
Itch, an E3 ubiquitin ligase, catalyzes the transfer of ubiquitin to a number of important signaling proteins in the cell including phospholipase Cγ1 (PLCγ1), protein kinase C θ (PKCθ), Notch, and others [35, 36]. Addition of a single ubiquitin molecule to a target protein can alter its function and intracellular trafficking while addition of multiple ubiquitin molecules to a target protein can direct it to be degraded by the proteosome [37].
Mice lacking itch develop an immune dysregulation phenotype similar to that observed in humans including lymphoproliferation in the spleen, lymph nodes, and thymus, dermatitis, and chronic interstitial pulmonary inflammation that ultimately proves fatal. These mice demonstrate a skewing toward Th2 responses and chronically scratch their skin leading to their being named “itchy” mice [38]. The exact mechanism by which itch deficiency leads to autoimmunity is not fully understood, but in murine and in vitro models, itch appears to play an important role in development of T cell anergy by polyubiquitinating and downregulating PLCγ1 and PKCθ, two key signaling molecules that are induced by Ca+2/calcineurin signaling in activated T cells. Absence of itch therefore affects T cell anergy induction [39]. Itch was also recently shown to play a role in expression of Foxp3 and generation of regulatory T cells through modulation of the transcription factor TIEG1 [40]. Itch deficiency may therefore cause immune dysregulation by affecting both anergy induction in auto-reactive effector T cells and generation of Tregs.
Diagnosis
Until further information is available about additional clinical and laboratory features of itch deficiency, a definitive diagnosis can be made only by sequencing of the ITCH/AIP4 gene. Itch deficiency is inherited in an autosomal recessive manner. Thus far, all identified patients have the same insertion mutation (c.395_395insA) that leads to a frameshift and premature stop codon truncating the protein at only 139 amino acids (wild-type protein is 862 amino acids) [34].
Treatment
The therapies utilized for most of the patients reported to date have focused primarily on symptomatic treatment. Immunosuppression with corticosteroids, tacrolimus, rapamycin, and azathioprine was reported to be moderately effective for the hepatitis and enteropathy associated with the disorder although it is unclear whether the pulmonary disease was significantly responsive [34]. Interestingly, in mice, the itchy phenotype can be reproduced by transplantation of itchy-derived bone marrow into lethally-irradiated syngeneic hosts suggesting that the immune dysregulation observed in the disease is driven primarily by mutant lymphocytes and that bone marrow transplantation would likely resolve these aspects of the disorder [37].
IL-10 and IL-10 receptor deficiency
Clinical features
Deficiency of the IL-10 receptor subunits (IL10R1 and IL10R2) (OMIM #613148 and #612567) was first identified in 2009 in patients with severe, early-onset, fistulating colitis [41]. To date, only four patients with IL-10 receptor defects have been reported in the literature. In all cases, the colitis began before 1 year of age and was characterized by an aggressive course with recurrent abscesses and enterocutaneous fistulas. In addition to colitis, most patients also had a characteristic early-onset follicular rash. Some patients had infections including pneumonia, otitis media, bronchitis, and renal abscesses, but, in general, these were thought to be primarily associated with immune suppression since other immune parameters including serum immunoglobulins, lymphocyte subsets, and neutrophil oxidative burst were normal.
After identification of patients with IL-10 receptor deficiency, two additional patients with similar clinical features including early onset fistulating colitis and follicular rash were identified. Neither of these had identifiable mutations in the IL-10 receptor subunit genes and, furthermore, IL-10 signaling appeared intact. Additional investigation revealed a homozygous missense mutation (p.G113R) in Interleukin-10 itself [42] that was found to abrogate the functional activity of the IL-10 protein. Similar to the patients with IL-10 receptor deficiency, other measures of immune function were normal.
Molecular basis
Interleukin-10 is a pleiotropic cytokine that both suppresses and promotes immune responses. On the one hand, it has the ability to suppress production of inflammatory cytokines such as IFN-γ, TNF-α, GM-CSF, and IL-2 by macrophages and T cells [43, 44]. On the other hand, it enhances immune responses by promoting B cell survival, maturation, and immunoglobulin production. Like the patients with IL-10 or IL-10 receptor deficiency, mice lacking IL-10 develop spontaneous colitis [45]. IL-10 functions as a homodimer that binds to, and signals through, a tetrameric receptor composed of two IL10R1 chains and two IL10R2 chains. Activation of the receptor induces phosphorylation and activation of the transcription factors STAT1 and STAT3 although STAT3 is thought to be the dominant mediator for the anti-inflammatory effects of IL-10 [46].
Diagnosis
Patients with suspected IL-10 receptor deficiency can be rapidly identified by flow cytometry in which tyrosine phosphorylation of STAT3 in IL-10-stimulated peripheral blood mononuclear cells is measured using a phospho-STAT3 specific antibody. Absence of the IL10R1 or IL10R2 proteins can be directly detected by flow cytometry using specific antibodies but may not be as informative as the functional test of IL-10-stimulated STAT3 phosphorylation. The diagnosis can then be confirmed by sequencing of the IL10RA and IL10RB genes. In all cases, the mutations identified in the IL-10 receptor subunits were inherited in an autosomal recessive pattern. Both identified IL-10R1 mutations were missense mutations (p.T84I and p.G141R) that allow expression of a mutant receptor that is thought to be unable to bind IL-10 [41].
In contrast to IL-10 receptor deficiency, IL-10 deficiency cannot be diagnosed using either of the suggested flow cytometry tests. It is also unlikely to be detected by measuring IL-10 production by patient cells because both identified cases have the same homozygous point mutation in IL-10 (p.G113R) that allows expression of a non-functional protein. This would likely be measured as normal protein by most IL-10 quantification assays. Consequently, the most direct method to make the diagnosis is sequencing of the IL10 gene in patients with early onset (<1 year of age) colitis who have normal IL-10 signaling as judged by flow cytometry [42].
Treatment
In all cases of either IL-10 or IL-10R deficiency, immunosuppression with a wide variety of agents including corticosteroids, azathioprine, thalidomide, and TNF inhibitors was reported to be unsuccessful. Surgical intervention was required in most cases to manage abscesses and fistulae that formed. Theoretically, IL-10 supplementation may be of benefit in patients with IL-10 deficiency but this has not yet been tried. Ultimately, patients with both IL-10 and IL-10 receptor deficiency were successfully treated with bone marrow transplantation which led to resolution of symptoms in both forms of the disease [41, 42].
Conclusions
The identification of genetic defects in a variety of immune dysregulation syndromes is yielding important insights into basic mechanisms of autoimmunity in humans. There is hope that these new insights will provide potential targets for development of therapies to effectively manage other autoimmune disorders that affect larger segments of the population.
References
- 1.Blizzard RM, Chee D, Davis W. The incidence of parathyroid and other antibodies in the sera of patients with idiopathic hypoparathyroidism. Clin Exp Immunol. 1966;1:119–128. [PMC free article] [PubMed] [Google Scholar]
- 2.Blizzard RM, Chee D, Davis W. The incidence of adrenal and other antibodies in the sera of patients with idiopathic adrenal insufficiency (Addison’s disease) Clin Exp Immunol. 1967;2:19–30. [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson G, Lane PJ, Jenkinson EJ. Generating intrathymic microenvironments to establish T-cell tolerance. Nat Rev Immunol. 2007;7:954–963. doi: 10.1038/nri2187. [DOI] [PubMed] [Google Scholar]
- 4.Ahonen P, Myllarniemi S, Sipila I, Perheentupa J. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829–1836. doi: 10.1056/NEJM199006283222601. [DOI] [PubMed] [Google Scholar]
- 5.Husebye ES, Perheentupa J, Rautemaa R, Kampe O. Clinical manifestations and management of patients with autoimmune polyendocrine syndrome type I. J Intern Med. 2009;265:514–529. doi: 10.1111/j.1365-2796.2009.02090.x. [DOI] [PubMed] [Google Scholar]
- 6.Alimohammadi M, Bjorklund P, Hallgren A, Pontynen N, Szinnai G, Shikama N, Keller MP, Ekwall O, Kinkel SA, Husebye ES, Gustafsson J, Rorsman F, Peltonen L, Betterle C, Perheentupa J, Akerstrom G, Westin G, Scott HS, Hollander GA, Kampe O. Autoimmune polyendocrine syndrome type 1 and NALP5, a parathyroid autoantigen. N Engl J Med. 2008;358:1018–1028. doi: 10.1056/NEJMoa0706487. [DOI] [PubMed] [Google Scholar]
- 7.Betterle C, Dal Pra C, Mantero F, Zanchetta R. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr Rev. 2002;23:327–364. doi: 10.1210/er.23.3.327. [DOI] [PubMed] [Google Scholar]
- 8.Soderbergh A, Myhre AG, Ekwall O, Gebre-Medhin G, Hedstrand H, Landgren E, Miettinen A, Eskelin P, Halonen M, Tuomi T, Gustafsson J, Husebye ES, Perheentupa J, Gylling M, Manns MP, Rorsman F, Kampe O, Nilsson T. Prevalence and clinical associations of 10 defined autoantibodies in autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab. 2004;89:557–562. doi: 10.1210/jc.2003-030279. [DOI] [PubMed] [Google Scholar]
- 9.Alimohammadi M, Dubois N, Skoldberg F, Hallgren A, Tardivel I, Hedstrand H, Haavik J, Husebye ES, Gustafsson J, Rorsman F, Meloni A, Janson C, Vialettes B, Kajosaari M, Egner W, Sargur R, Ponten F, Amoura Z, Grimfeld A, De Luca F, Betterle C, Perheentupa J, Kampe O, Carel JC. Pulmonary autoimmunity as a feature of autoimmune polyendocrine syndrome type 1 and identification of KCNRG as a bronchial autoantigen. Proc Natl Acad Sci USA. 2009;106:4396–4401. doi: 10.1073/pnas.0809986106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shum AK, DeVoss J, Tan CL, Hou Y, Johannes K, O’Gorman CS, Jones KD, Sochett EB, Fong L, Anderson MS. Identification of an autoantigen demonstrates a link between interstitial lung disease and a defect in central tolerance. Sci Transl Med. 2009;1:9ra20. doi: 10.1126/scitranslmed.3000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin M. Anti-interferon auto-antibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 2006;3:e292. doi: 10.1371/journal.pmed.0030292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meager A, Visvalingam K, Peterson P, Moll K, Murumagi A, Krohn K, Eskelin P, Perheentupa J, Husebye E, Kadota Y, Willcox N. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 2006;3:e289. doi: 10.1371/journal.pmed.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachee-Chardin M, Toulon A, Bustamante J, Al-Muhsen S, Al-Owain M, Arkwright PD, Costigan C, McConnell V, Cant AJ, Abinun M, Polak M, Bougneres PF, Kumararatne D, Marodi L, Nahum A, Roifman C, Blanche S, Fischer A, Bodemer C, Abel L, Lilic D, Casanova JL. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavanescu I, Benoist C, Mathis D. B cells are required for Aire-deficient mice to develop multi-organ autoinflammation: a therapeutic approach for APECED patients. Proc Natl Acad Sci USA. 2008;105:13009–13014. doi: 10.1073/pnas.0806874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abramson J, Giraud M, Benoist C, Mathis D. Aire’s partners in the molecular control of immunological tolerance. Cell. 2010;140:123–135. doi: 10.1016/j.cell.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 16.Fierabracci A. Recent insights into the role and molecular mechanisms of the autoimmune regulator (AIRE) gene in autoimmunity. Autoimmun Rev. 2011;10:137–143. doi: 10.1016/j.autrev.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Cetani F, Barbesino G, Borsari S, Pardi E, Cianferotti L, Pinchera A, Marcocci C. A novel mutation of the autoimmune regulator gene in an Italian kindred with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, acting in a dominant fashion and strongly cosegregating with hypothyroid autoimmune thyroiditis. J Clin Endocrinol Metab. 2001;86:4747–4752. doi: 10.1210/jc.86.10.4747. [DOI] [PubMed] [Google Scholar]
- 18.Perheentupa J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab. 2006;91:2843–2850. doi: 10.1210/jc.2005-2611. [DOI] [PubMed] [Google Scholar]
- 19.Proust-Lemoine E, Saugier-Veber P, Lefranc D, Dubucquoi S, Ryndak A, Buob D, Lalau JD, Desailloud R, Weill J, Prin L, Lefebvre H, Wemeau JL. Autoimmune polyendocrine syndrome type 1 in north-western France: AIRE gene mutation specificities and severe forms needing immunosuppressive therapies. Horm Res Paediatr. 2010;74:275–284. doi: 10.1159/000297714. [DOI] [PubMed] [Google Scholar]
- 20.Huter EN, Natarajan K, Torgerson TR, Glass DD, Shevach EM. Autoantibodies in Scurfy mice and IPEX patients recognize keratin 14. J Invest Dermatol. 2010;130:1391–1399. doi: 10.1038/jid.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGinness JL, Bivens MM, Greer KE, Patterson JW, Saulsbury FT. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) associated with pemphigoid nodularis: a case report and review of the literature. J Am Acad Dermatol. 2006;55:143–148. doi: 10.1016/j.jaad.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 22.Tsuda M, Torgerson TR, Selmi C, Gambineri E, Carneiro-Sampaio M, Mannurita SC, Leung PS, Norman GL, Gershwin ME. The spectrum of autoantibodies in IPEX syndrome is broad and includes anti-mitochondrial autoantibodies. J Autoimmun. 2010;35:265–268. doi: 10.1016/j.jaut.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Caudy AA, Reddy ST, Chatila T, Atkinson JP, Verbsky JW. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J Allergy Clin Immunol. 2007;119:482–487. doi: 10.1016/j.jaci.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Sharfe N, Dadi HK, Shahar M, Roifman CM. Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc Natl Acad Sci USA. 1997;94:3168–3171. doi: 10.1073/pnas.94.7.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aoki CA, Roifman CM, Lian ZX, Bowlus CL, Norman GL, Shoenfeld Y, Mackay IR, Gershwin ME. IL-2 receptor alpha deficiency and features of primary biliary cirrhosis. J Autoimmun. 2006;27:50–53. doi: 10.1016/j.jaut.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Roifman CM. Human IL-2 receptor alpha chain deficiency. Pediatr Res. 2000;48:6–11. doi: 10.1203/00006450-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 27.D’Cruz LM, Klein L. Development and function of agonist-induced CD25+ Foxp3+ regulatory T cells in the absence of interleukin-2 signaling. Nat Immunol. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 28.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin-2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 29.Kofoed EM, Hwa V, Little B, Woods KA, Buckway CK, Tsubaki J, Pratt KL, Bezrodnik L, Jasper H, Tepper A, Heinrich JJ, Rosenfeld RG. Growth hormone insenstivity associated with a STAT5b mutation. N Engl J Med. 2003;349:1139–1147. doi: 10.1056/NEJMoa022926. [DOI] [PubMed] [Google Scholar]
- 30.Nadeau K, Hwa V, Rosenfeld RG (2011) STAT5b deficiency: an unsuspected cause of growth failure, immunodeficiency, and severe pulmonary disease. J Pediatr (in press) [DOI] [PubMed]
- 31.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, Anderson VL, Darnell DN, Welch PA, Kuhns DB, Frucht DM, Malech HL, Gallin JI, Kobayashi SD, Whitney AR, Voyich JM, Musser JM, Woellner C, Schaffer AA, Puck JM, Grimbacher B. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 32.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, Metin A, Karasuyama H. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 33.Hwa V, Nadeau K, Wit JM, Rosenfeld RG. STAT5b deficiency: lessons from STAT5b gene mutations. Best Pract Res Clin Endocrinol Metab. 2011;25:61–75. doi: 10.1016/j.beem.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Lohr NJ, Molleston JP, Strauss KA, Torres-Martinez W, Sherman EA, Squires RH, Rider NL, Chikwava KR, Cummings OW, Morton DH, Puffenberger EG. Human itch E3 ubiquitin ligase deficiency causes syndromic multisystem autoimmune disease. Am J Hum Genet. 2010;86:447–453. doi: 10.1016/j.ajhg.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melino G, Gallagher E, Aqeilan RI, Knight R, Peschiaroli A, Rossi M, Scialpi F, Malatesta M, Zocchi L, Browne G, Ciechanover A, Bernassola F. Itch: a HECT-type E3 ligase regulating immunity, skin and cancer. Cell Death Differ. 2008;15:1103–1112. doi: 10.1038/cdd.2008.60. [DOI] [PubMed] [Google Scholar]
- 36.Qiu L, Joazeiro C, Fang N, Wang HY, Elly C, Altman Y, Fang D, Hunter T, Liu YC. Recognition and ubiquitination of notch by itch, a hect-type E3 ubiquitin ligase. J Biol Chem. 2000;275:35734–35737. doi: 10.1074/jbc.M007300200. [DOI] [PubMed] [Google Scholar]
- 37.Matesic LE, Copeland NG, Jenkins NA. Itchy mice: the identification of a new pathway for the development of autoimmunity. Curr Top Microbiol Immunol. 2008;321:185–200. doi: 10.1007/978-3-540-75203-5_9. [DOI] [PubMed] [Google Scholar]
- 38.Perry WL, Hustad CM, Swing DA, O’Sullivan TN, Jenkins NA, Copeland NG. The itchy locus encodes a novel ubiquitin protein ligase that is disrupted in a18H mice. Nat Genet. 1998;18:143–146. doi: 10.1038/ng0298-143. [DOI] [PubMed] [Google Scholar]
- 39.Venuprasad K. Cbl-b and itch: key regulators of peripheral T-cell tolerance. Cancer Res. 2010;70:3009–3012. doi: 10.1158/0008-5472.CAN-09-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venuprasad K, Huang H, Harada Y, Elly C, Subramaniam M, Spelsberg T, Su J, Liu YC. The E3 ubiquitin ligase Itch regulates expression of transcription factor Foxp3 and airway inflammation by enhancing the function of transcription factor TIEG1. Nat Immunol. 2008;9:245–253. doi: 10.1038/ni1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, Hatscher N, Pfeifer D, Sykora KW, Sauer M, Kreipe H, Lacher M, Nustede R, Woellner C, Baumann U, Salzer U, Koletzko S, Shah N, Segal AW, Sauerbrey A, Buderus S, Snapper SB, Grimbacher B, Klein C. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glocker EO, Frede N, Perro M, Sebire N, Elawad M, Shah N, Grimbacher B. Infant colitis—it’s in the genes. Lancet. 2010;376:1272. doi: 10.1016/S0140-6736(10)61008-2. [DOI] [PubMed] [Google Scholar]
- 43.Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interferon Cytokine Res. 1999;19:563–573. doi: 10.1089/107999099313695. [DOI] [PubMed] [Google Scholar]
- 44.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 45.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-P. [DOI] [PubMed] [Google Scholar]
- 46.Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol. 2004;172:567–576. doi: 10.4049/jimmunol.172.1.567. [DOI] [PubMed] [Google Scholar]