Abstract
Naturally occurring antimicrobial peptides (AMPs) present several drawbacks that strongly limit their development into therapeutically valuable antibiotics. These include susceptibility to protease degradation and high costs of manufacture. To overcome these problems, researchers have tried to develop mimics or peptidomimetics endowed with better properties, while retaining the basic features of membrane-active natural AMPs such as cationic charge and amphipathic design. Protein epitope mimetics, multimeric (dendrimeric) peptides, oligoacyllysines, ceragenins, synthetic lipidated peptides, peptoids and other foldamers are some of the routes explored so far. The synthetic approach has led to compounds that have already entered clinical evaluation for the treatment of specific conditions, such as Staphylococcus (MRSA) infections. Should these trials be successful, an important proof-of-concept would be established, showing that synthetic oligomers rather than naturally occurring molecules could bring peptide-based antibiotics to clinical practice and the drug market for local and systemic treatment of medical conditions associated with multi-drug resistant pathogens.
Keywords: Antimicrobial peptides, Antimicrobial polymers, Synthetic approaches, Membrane-active, Dendrimeric peptides, Lipopeptides
Introduction
Since the identification, several decades ago, of cecropins in the pupae of Hyalophora cecropia moth, defensins in human neutrophils and magainins in skin secretions of the frog Xenopus laevis, our knowledge of antimicrobial peptides (AMPs) as crucial components of the innate immune system of multicellular organisms has expanded significantly. Several hundred peptides have been isolated from many sources, and detailed structure-activity relationship studies performed on many reference peptides belonging to different structural classes [1]. Notwithstanding these advances, however, translation of this science into antibiotics with new mechanisms of action that could be of much value to combat the growing health threat posed by resistant pathogenic microorganisms has not emerged as yet.
As drug candidates, AMPs present various disadvantages. First, because of their relatively large size [~20 amino acids], manufacturing antibacterial peptides can be costly and technically difficult. Second, many of these peptides are degraded quickly in the presence of proteases [2], are susceptible to pH changes or their activity is reduced in the presence of salts and divalent cations, such as those present in serum [3]. In addition to these built-in limitations, microbial resistance is also not a negligible factor. Although AMPs generally hit a cellular component that is believed per se to be not easy modifiable in its basic physico-chemical features by microbial targets, i.e., the lipid component of the plasma membrane, bacterial pathogens have traveled a long distance with AMPs throughout evolution, developing several mechanisms for conferring intrinsic or inducible resistance. These resistance mechanisms include structural modification of components of the bacterial outer membrane, such as lipopolysaccharide (LPS) or lipoteichoic acid (LTA), to reduce permeability by AMPs, AMPs-trapping molecules, efflux pumps to remove AMPs from the periplasmic space (see the review of Koprivnjak and Peschel within the MAR) and membrane-bound proteases that degrade peptides [4–6]. Although the use of peptides composed of d-amino acids is sometimes contemplated owing to their high resistance to proteolysis [7], this approach often significantly increases the cost of manufacturing with only limited pharmacokinetic advantages.
In order to overcome these limitations, focus has shifted from the production of these naturally occurring AMPs or their sequence analogs to developing mimics endowed with better properties such as multimeric peptides or peptidomimetics. The term peptidomimetic is used here in a broad sense, referring to any sequence designed to mimic a peptide structure and/or function, but whose backbone is not solely based on α-amino acids. Peptidomimetics can thus be based on semi-synthetic analogues or can be novel, fully synthetic macrocylic products, polymers that mimic a peptide primary structure through the use of amide bond isoesteres and/or modification of the native peptide backbone, including chain extension, or heteroatom incorporation [8]. Nonpeptidic compounds that are facially amphiphilic can also derive from a steroid scaffold or starting from a de novo design approach resulting in inexpensive synthetic oligomers. Classes of antimicrobial peptidomimetics will be briefly outlined here, with a focus on membrane-targeting compounds with therapeutic potential. Rather than being an encyclopedic account of synthetic routes to AMPs, the aim of this review is rather to highlight the most promising approaches, together with discussing the more conventional, typical examples that opened the field.
Mimicking antimicrobial peptides: selected approaches
Multimeric peptides
Peptide dendrimers—branched polymers with peptides attached centrally to a template or core matrix—have been developed for many years, for a number of different applications, such as protein mimetics, de novo design of artificial proteins, new biopolymers and biomaterials, and as immunogens and antigens [9]. In 1988, the multiple antigenic peptide (MAP) system was introduced as a novel approach to preparing peptide immunogens [10]. In this case, multiple peptide sequences can be added using standard solid-phase chemistry to an inner core of radially branched lysine residues. The main idea was to mimic a portion of a protein surface by bringing the potentially immunogenic peptide sequences together by assembling the peptide chains on a template as a peptide dendrimer, whereas in native proteins discontinuous bioactive peptide surfaces are held together in a particular conformation by the structural rigidity of the protein.
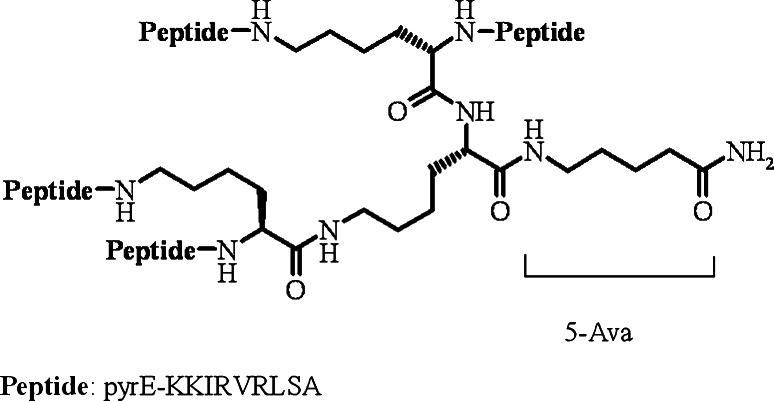
More recently, the MAP approach has been used for the development of peptide dendrimers with antimicrobial properties. Starting from a linear AMP sequence (QEKIRVRLSA) originally identified by selecting a random phage library against whole Escherichia coli cells [11], several cycles of rational modification and optimization led to the compound known as SB041 (Fig. 1). In this novel tetra-branched peptide, four identical peptides (pyrEKKIRVRLSA) have been linked to a lysine core, also carrying a lipophilic amino valeric acid chain aimed at enhancing the peptide’s membrane affinity, and a pyroglutamic acid at N-terminal end aimed at conferring more stability, avoiding the well-known cyclization process involving a Gln residue [12]. The peptide was found to be especially active against gram-negative strains, with a potency comparable (on molar basis) to that of the lipopeptides colistin and polymixin B; it bound E. coli and Pseudomonas aeruginosa LPS in vitro strongly, but this binding did not necessarily translate into LPS-neutralizing activity, as seen by checking the SB041 effects on LPS-induced activation of pattern recognition receptors (PRRs) in Raw-Blue cells, derived from RAW 264.7 macrophages [12]. Working on a very similar tetrabranched system, Luisa Bracci and colleagues from the University of Siena, Italy, have recently reported similar selectivity for gram-negative bacterial strains, coupled to LPS-neutralizing activity and even significant anti-endotoxin properties in sepsis animal models [13]. In the future, it would be interesting to check whether the discrepancies between these studies, such as for the reported neutralization of LPS by SB041 and related dendrimers, depend on the various assays of choice or not (e.g., Bracci and colleagues directly quantified TNF-α released in vitro from Raw 264.7 cells upon stimulation with a 50–200 smaller LPS amount than that used by Bruschi et al. [12]).
Fig. 1.
Primary sequence and structure of the dendrimeric (tetrameric) antimicrobial peptide SB041. 5-Ava 5-amino valeric acid, pyrE pyroglutamic acid. All amino acids have l-configuration. The peptide is amidated at the C-terminus (5-Ava)
Dendrimeric peptides usually display increased activity compared to their monomeric counterparts—a fact probably attributable to the higher local concentration of bioactive units for multimeric peptides—as well as greater stability to peptidases and proteases, possibly because of the steric hindrance of the branching core that would limit the cleavage rates of plasma peptidases, thus increasing the peptides’ pharmacokinetic properties [14].
Besides targeting bacterial pathogens, peptide-derivatized dendrimers have the potential for other anti-infective applications. For example, a very recent study has shown that the tetrabranched compound SB105 and its derivative SB105 A10 were able to inhibit replication of several strains of human cytomegalovirus (HCMV) in both primary fibroblasts and endothelial cells [15]. HCMV is the principal pathogen in bone marrow and solid-organ transplant recipients and among immunocompromised AIDS patients, these infections being a major cause of morbidity and mortality among these groups.
Apparently, in this case dendrimers exerted their inhibitory effect by blocking the initial attachment of virions to heparan sulfate on the cell surface, a novel mechanism that could make SB105 and SB105 A10 attractive candidates as members of an innovative class of antiviral drugs [15].
PEM molecules
The β-hairpin is an interesting scaffold for the development of new therapeutics. It is used by many natural proteins for molecular recognition, and it is suitable to mimetic design, for example, by transferring a loop sequence from the protein or peptide of interest onto a synthetic template that can act to stabilize hairpin backbone conformations [16].
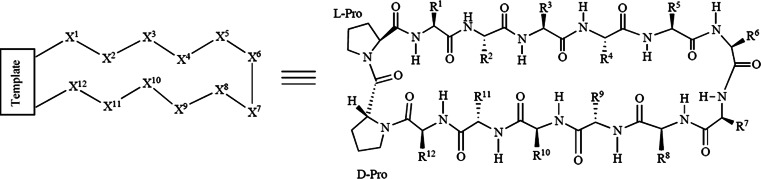
This kind of approach has been named Protein Epitope Mimetic (PEM) technology (Fig. 2), and it has been fruitfully applied to modify the sequence of membranolytic AMP protegrin I for the development of a new class of compounds. Iseganan, a protegrin derivative, has already undergone clinical trials. In 2004, IntraBiotics Pharmaceuticals, Inc. (now Ardea Biosciences, Inc.) ceased all development of Iseganan when two Phase III trials in oral mucositis failed, and a Phase II/III trial of the compound to prevent ventilator-associated pneumonia (VAP) was terminated after an independent data-monitoring committee found higher rates of VAP and mortality in the treatment arm [17].
Fig. 2.
Structure of a β-hairpin PEM molecule. PEMs typically comprise a peptide loop linked to a β-hairpin-stabilizing template (e.g., the d-Pro-l-Pro dipeptide shown here)
Researchers at the University of Zurich and Polyphor Ltd. (www.polyphor.com), by means of PEM technology, have developed epitope mimetics of protegrin I that specifically target P. aeruginosa via a mechanism of action that is distinct from the membrane-disrupting activity of the parent compound [18]. These mimetics contain loop sequences related to that of protegrin I, but linked to a d-proline–l-proline template, which contributes to the stabilizing β-hairpin conformations within the macrocycle [19, 20]. A hit-identification and lead-optimization campaign gave two lead compounds named POL7001 and POL7080 that were active in the nanomolar range against gram-negative Pseudomonas spp. In vitro studies evaluated the mimetics against more than 100 P. aeruginosa clinical isolates and found that the minimum inhibitory concentrations with antimicrobial activity against 90% of the isolates (MIC90) were 0.13 and 0.25 μg/ml for POL7001 and POL7080, respectively.
Different biochemical and genetic studies—including the characterization of spontaneous resistant mutants, selected at 5× MIC and with a frequency of ≤1 × 1010—showed that the peptidomimetics had a non-membrane-lytic mechanism of action and identified as target a homolog of the β-barrel protein LptD (Imp/OstA), which functions in outer-membrane biogenesis. LptD is an outer-membrane protein widely distributed in gram-negative bacteria that functions in the assembly of LPS in the outer leaflet of the outer membrane. If the function of LptD is impaired upon binding to the peptidomimetic, outer-membrane structure and biogenesis should become altered.
The in vivo efficacy of POL7001 and POL7080 was also evaluated in a mouse septicemia model using two P. aeruginosa strains. Both antibiotics demonstrated substantial activity against both strains with calculated median effective doses (ED50 values) in the range of 0.25–0.55 mg/kg. In contrast, median effective doses of the generic aminoglycoside antibiotic gentamicin were 3.1 and 2.9 mg/kg against the two strains, respectively [18].
This family of antibiotics may be useful in the treatment of nosocomial infections and chronic lung infections in patients with cystic fibrosis where P. aeruginosa is considered to grow as biofilms. Polyphor expected the lead mimetic, POL7080, to enter Phase I testing by the end of 2010.
Oligoacyllysines
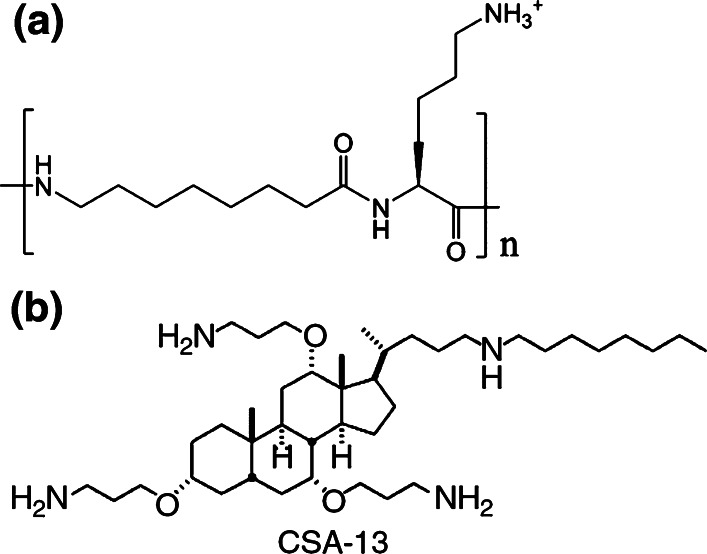
Oligoacyllysines (OAKs) are a novel group of antimicrobial copolymers, composed of tandem repeats of acyllysines, designed to mimic the primary structure and function of natural antimicrobial peptides (Fig. 3 a). This class of compounds is based on the assumption that acyl moieties might be able to substitute extensive sequences within the peptide backbone, thus enabling a gradual control of molecular hydrophobicity simply by changing the acyl chain length. While providing both hydrophobicity and positive charge features, these molecules are expected to limit the formation of defined secondary structures because of the optimal rotational freedom of the carbon atoms in an acyl chain [21].
Fig. 3.
a Acyl-lysyl oligomers (brackets define the building blocks); b chemical structure of ceragenin CSA-13
The first OAK investigated was found to exert antibacterial activity through membrane disruption, but recent findings indicate that drastically different killing rates (e.g., minutes vs. hours) of some of these OAKs can reflect a distinct mechanisms of action, such as interactions with intracellular targets and/or interference with intracellular processes [3, 22].
By linking the octamer OAK derivative C12K–7α8 to a polystyrene bead, it has been possible to exploit one attribute—the binding affinity versus bacteria—and eliminate the other—the killing effect—in order to prepare a coating of polymeric surfaces for filtration/concentration of microorganisms from various liquid media and for the detection and/or depletion of bacteria [23]. These new resin-linked OAKs (ROAKs) were shown to be stable and to efficiently capture a variety of pathogens in different media upon brief incubation with ROAK beads or after continuous flow through a ROAK-packed column. The capture is likely to be mediated by nonspecific (physico-chemical) interactions with an external component(s) of the cell wall, such as LPS and LTA in gram-negative and gram-positive bacteria, respectively. A single ROAK bead is estimated to capture about 3,000 bacterial cells in culture medium, in contaminated saline or tap water, thus resulting in an efficient method for the detection of pathogens in large volumes of water (i.e., 10 CFU per 100 ml) [23]. Moreover, the ability of OAKs to remain active under a large spectrum of incubation conditions (such as different ionic strengths, pHs and temperatures) points to potential uses of OAKs in food safety as the antimicrobial properties were studied under incubation conditions relevant to food product preservation [23].
In a recent study, Amram Mor and colleagues challenged the OAK technology for its ability to generate effective and economically affordable antimicrobial compounds, and came up with a miniaturized OAK composed of only 3 lysyl residues and 2 acyls, named C12(ω7)K–β12, that preferentially targets gram-positive species by a bacteriostatic mode of action [24]. Since the excess hydrophobicity and the consequent self-assembly in aqueous media may result in enhanced hemolytic and cytotoxic effects along with decreased antimicrobial activity [25], this new OAK is endowed with an unsaturated N-terminal acyl moiety whose double bond is expected to interfere with self-assembly; more specifically, self-assembly is likely to be inhibited because of a dual effect imposed by the single double bond: reduced hydrophobicity and wobbly packing. In accordance with its design, C12(ω7)K-β12 demonstrated a non-aggregative state at biologically relevant concentrations, and a reduced hemolytic effect along with potent and selective antibacterial activity [24]. In terms of mechanism of action, the presented findings indicate that the observed bacteriostatic effect is linked to the shallow insertion of C12(ω7)K-β12 within plasma membrane anionic phospholipids, resulting in a slow perturbation of the membrane instead of a rapid damage. Moreover, data also demonstrated in vivo activity of C12(ω7)K-β12 in a peritonitis-sepsis model induced by gram-positive bacteria such as Staphylococcus aureus [24]. In a broader view, these results suggest that adding a N-terminal unsaturated acyl residue as anti-aggregation feature could be extended to become a strategy for improving the performance of lipopeptides in general.
Ceragenins
Another class of AMPs mimic has been developed by the synthesis of steroids with amine groups. This series of antimicrobial compounds, termed Ceragenins™, is based on derivatives of bile acids with covalently attached amines (Fig. 3b). This class of molecules has a net positive charge that is electrostatically attracted to negatively charged bacterial membranes and has a high binding activity for LPS and LTA, similar to AMPs. Ceragenins’ design can be considered as inspired by squalamine, a naturally occurring aminosterol with potent antimicrobial activity, isolated from shark liver [26].
The cationic steroid antimicrobial, CSA-13, is the most potent of the Ceragenin™ compounds tested to date and has been shown in vitro to effectively kill both gram-negative and gram-positive bacteria [27]. Although Ceragenins™ have been found to have weak hemolytic activity, they display broad-spectrum bactericidal activity [28], which gives them significant potential to be used as a therapy for several indications.
For example, the activity of CSA-13 against oral cariogenic and periodontopathic bacteria such as multiple isolates of Streptococcus mutans (anaerobic bacteria) and Porphyromonas spp. (obligate anaerobic bacteria) suggests that CSA-13 may be useful for the prevention and treatment of oral microbial diseases such as caries and periodontitis [29].
Recently, CSA-13 has also been demonstrated to have potential for treatment of Helicobacter pylori infections, including those caused by the clarithromycin- and/or metronidazole-resistant strains [30]. This study showed that CSA-13, contrary to linear peptides LL-37 and WLBU2, maintained strong bactericidal activity under harsh conditions such as the presence of mucin and after preincubation with pepsin at low pH. These conditions represent unique challenges related to H. pylori treatment, as these bacteria that inhabit the stomach are protected from the acidic environment by a thick mucus layer, and the effectiveness of many antimicrobial drugs is greatly diminished at acidic pH [31].
Eczema vaccinatum is a potentially fatal, disseminated viral skin infection that develops in individuals with atopic dermatitis after exposure to the vaccinia virus (VV). In another study, CSA-13 exhibited a potent antiviral activity by preferentially targeting and inactivating VV directly and by inducing AMPs with known antiviral activity against VV. In addition, topical administration of CSA-13 resulted in a significant reduction of the development of satellite lesions, making this synthetic agent a candidate for the treatment of disseminated viral skin infections [32].
Synthetic mimics of antimicrobial peptides
Although several approaches (e.g., d-aminoacids incorporation, cyclization etc.) can solve some relatively simple problems such as stability to blood proteases, more difficult issues may be represented by the expense of the materials, systemic toxicity and limited tissue distribution of modified AMPs.
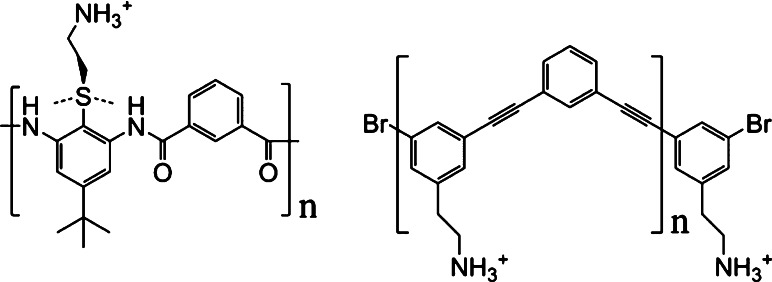
To overcome this hurdle, a synthetic approach was attempted, consisting of transferring the structural and biological properties of AMPs into the framework of inexpensive oligomers. An active program for the de novo design of synthetic mimics of antimicrobial peptides (SMAMPs) from inexpensive synthetic oligomers was carried out in several steps. First, a three-dimensional backbone was defined, using molecular dynamics and quantum force field calculations. Second, side groups were added computationally to maximize diversity and maintain drug-like properties. The best combinations of functional groups were then computationally selected to produce a cationic, amphiphilic structure resulting in two families of compounds: oligomers containing alternating 1,3-phenylene diamine units connected by a isophthalic acid and facially amphiphilic arylamide oligomers that utilized hydrogen bonding to produce conformationally stiff backbones (Fig. 4) [33, 34].
Fig. 4.
Representative SMAMP based on arylamide (a) and phenylene ethynylene oligomers (b)
Representative compounds were synthesized from these libraries, and their activity was optimized by increasing the rigidity of the backbone through hydrogen bonding and/or by introducing new substituents. This effort has led to the identification of a class of SMAMPs that possesses efficacy, safety and pharmaceutical qualities suitable for development as intravenous antibiotics [35].
The first compound in this series to undergo clinical evaluation was PMX-30063, currently being developed by Polymedix for the broad treatment of Staphylococcus (MRSA) infections. Phase I clinical results showed that it was possible to safely administer PMX-30063 without any serious side effects at single or multiple doses that exceeded those associated with full efficacy in animal models of infection. PMX-30063 has been shown to be effective in a variety of rat and mice models, including the thigh burden model, sepsis model and granuloma pouch model. Compared to vancomycin, PMX-30063 showed comparable to greater efficacy. The first application of PMX-30063 is planned to be an injectable formulation for broad use against Staphylococcus infections (pan-Staph—many forms of Staph, not simply MRSA). The clinical indications for this include skin and soft tissue structure infections, respiratory tract infections, urinary tract infections and complicated abdominal infections, including gynecological ones. Polymedix is also developing PMX-30063 and other related compounds for a larger panel of indications ranging from ocular infections and gastrointestinal infections, such as those caused by Clostridium difficile and Shigella, to fungal infections, tuberculosis and malaria, and also for the development of antimicrobial polymers for use in medical devices (source: company website, www.polymedix.com).
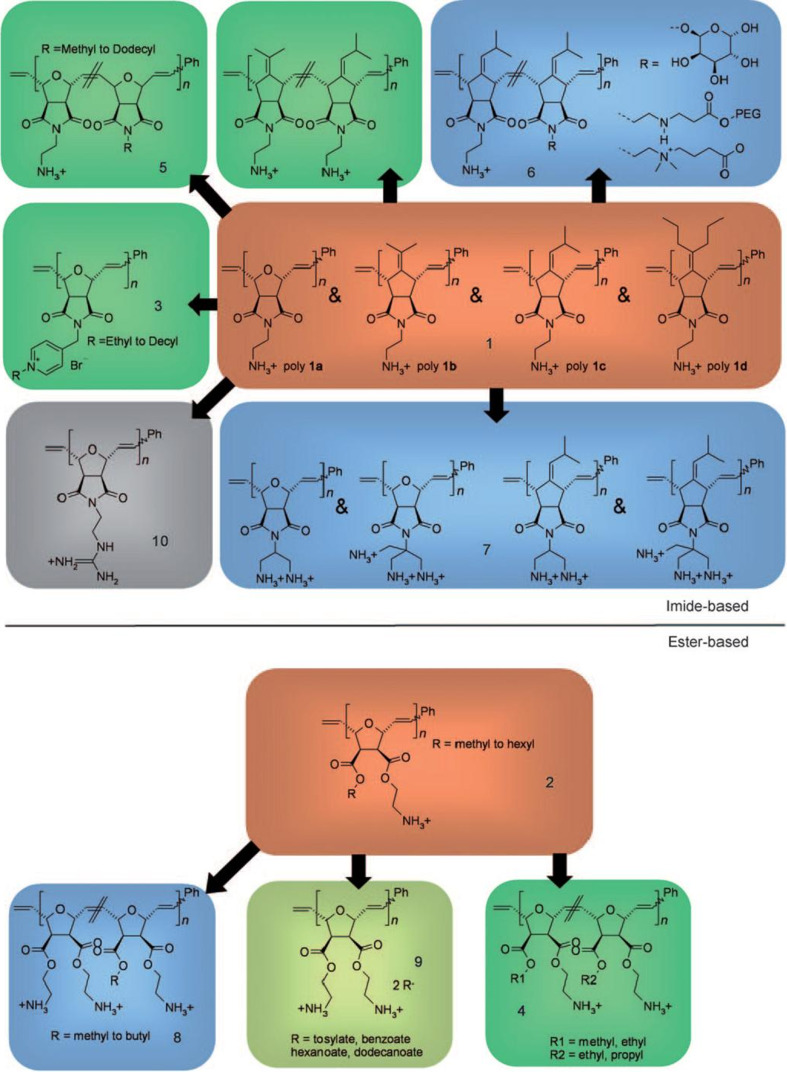
SMAMPs obtained by ring-opening metathesis polymerization (ROMP) have also received significant attention. In this case, poly-norbornene derivatives with facially amphiphilic repeat units have been prepared (Fig. 5), gradually varying the repeat unit and checking how different kinds of structural modifications, such as hydrophobicity, molecular weight and charge, impacted on the antibacterial and hemolytic activities of these polymers [36, 37]. “ROMP was chosen as a synthetic platform because it is a living polymerization technique, it yields molecules with low polydispersity over a wide range of molecular weights, and it is highly functional group tolerant,” wrote Gregory Tew and Karen Lienkamp in a recent review on the topic [37]. The membrane-disruptive properties of many ROMP-derived SMAMPs have been demonstrated, molecules whose biological activity ranged from inactive/non-hemolytic through active/non-hemolytic to active/toxic, depending on the modulation of structural parameters.
Fig. 5.
Library of ROMP-based SMAMP polymers. Two synthetic platform were devised, one based on norbornene-imide derivatives (a), the other on norbornene-ester derivatives (b). The parent series is marked in red and underwent hydrophobic modification (green), hydrophilic or charge-related variations (blue), or counterion exchange (light green). One SMAMP was modified with guanidinium groups (grey). Reproduced from [37], with permission
Last but not least, concerning SMAMPs, the Norwegian pharmaceutical company Lytix Biopharma AS has recently announced regulatory approval by Swedish authorities to commence Phase I/IIa clinical trials with Lytixar™ (LTX-109) for nasal decolonization of MRSA (www.lytixbiopharma.com). LTX-109 belongs to a group of extremely short (tripeptide) and stable synthetic antimicrobial peptidomimetics containing a modified tryptophan derivate as lipophilic bulk, which displayed a combination of high antibacterial activity against methicillin-resistant staphylococci and staphylococcal biofilms with low toxicity against human erythrocytes [38, 39].
Synthetic lipidated peptides
Anti-infective lipopeptides make up a large family of bacterial compounds that are primarily synthesized via non-ribosomal biosynthetic pathways and comprise a peptidyl portion conjugated to a fatty acid to form an acylated peptide. This class includes both cationic and anionic peptidic molecules with different spectra of activity, generally constrained by cyclization, such as polymyxins (polymyxin B and colistin), lipopeptaibols, echinocandin, laspartomycin, daptomycin (marketed under the trade name CUBICIN by Cubist Pharmaceuticals, www.cubist.com), and many others [8].
Several studies have revealed that mimicking of natural lipopeptide antibiotics by the attachment of an aliphatic chain to the N-terminus of native or designed short peptides can result in an enhancement of their antimicrobial activity [8, 40]. Therefore, the combination of an otherwise scarcely active peptidyl scaffold and a suitable N-terminal fatty acid chain represents a potentially winning approach to the development of potent antimicrobial agents, whereby spectra of activity may be modulated via modification of the N-terminal substituent [41].
The team led by Yechiel Shai recently reported a new family of ultra-short (4-mer) cationic lipopeptides active in vivo against fungi: C16-KAAK, C16-KLLK, C14-KLLK, C16-KKKK and C12-KLLK (amino acids in bold type are d-enantiomers) [41]. Mode-of-action studies supported a membranolytic or a detergent-like effect. The most efficient peptide C16-KAAK was tested further using an invasive pulmonary aspergillosis (IPA) animal model of infection and was found to significantly prolong the life of the treated animals with low toxicity effects and no damage to the treated lung tissues, offering a more efficient treatment toward the current standard antifungal therapy with amphotericin B [42].
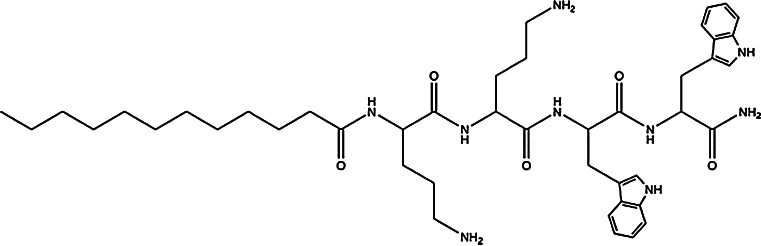
Another report described the solid phase synthesis, characterization, microbiological and toxicological evaluation of a library of ultra-short antimicrobial lipopeptides based on the Orn-Orn-Trp-Trp tetrapeptide motif conjugated with saturated fatty acids, which have inherent antimicrobial activity. The study indicates that the modification of the tetrapeptide amide H-Orn-Orn-Trp-Trp-NH2 with N-acyl substituents (Fig. 6) increased the antimicrobial potency resulting in a panel of compounds that exhibit excellent, broad-spectrum antimicrobial activity against a number of bacteria and fungi, including multidrug-resistant microorganisms in both planktonic and sessile (biofilm) cultures [43].
Fig. 6.
Structure of an antimicrobial synthetic lipopeptide with the sequence C12-Orn-Orn-Trp-Trp-NH2
Peptoids
Another simple but effective approach to mimicking cationic AMPs is the construction of peptoids. These are poly-N-substituted glycines in which side chains are attached to the backbone amide nitrogen rather than to the α-carbon [44]. Formally, these protease-resistant compounds are an example of the so-called ‘foldamers,’ “unnatural oligomers with the ability to display discrete folding properties, hence mimicking as well as complementing the behaviors known from biopolymers” [45].
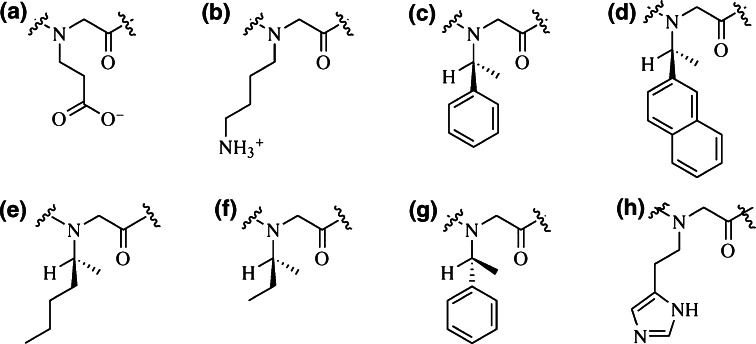
Selecting building blocks from an ad hoc constructed library of peptoid monomers tuned for hydrophobicity and side-chain charge (Fig. 7), Annelise Barron and her team have synthesized peptoid oligomers with helical structures and biomimetic sequences [46]. These peptoids, or ‘ampetoids,’ proved to have antimicrobial and hemolytic activities comparable to that of cationic AMPs, such as pexiganan, and extensive structure-activity characterization showed that in both AMP and ampetoids antimicrobial-hemolytic selectivity is modulated by the interplay among hydrophobicity, cationic charge and amphipathic design, which reinforces the idea that ampetoids function via a mechanism similar to that of AMPs. “Peptoids have greater potential than peptides to be used as pharmaceuticals and in biomaterials because of their improved stability, bioavailability, and highly tunable side-chain chemistry,” concluded the researchers [46].
Fig. 7.
N-Substituted glycine monomers used for the construction of helical peptoids (ampetoids) in the laboratory of Annelise Barron [46]. aN-(2-carboxyethyl) glycine; bN-(4-aminobutyl) glycine; c (S)-N-(1-phenylethyl) glycine; d (S)-N-(1-naphthylethyl) glycine; e (S)-N-(1-methylbutyl) glycine; f (S)-N-(sec-butyl) glycine; g (R)-N-(1-phenylethyl) glycine; hN-(methylimidazole) glycine. From [61]
More recently, Barron and colleagues used soft X-ray tomography to image changes in the subcellular organization of Candida albicans treated with two antifungal peptoids [47]. Peptoid treatment suppressed phenotypic switching of Candida—a process that leads to the formation of the pathogenic, invasive form that causes candidiasis—and resulted in striking changes in cell and organelle morphology [47]. In particular, with respect to control cells, stress response to peptoid treatment was visualized as clear differences in the nucleus and nucleolus, and in the number, size and location of lipidic bodies, with peptoid treatment causing the inclusion of lipidic bodies into the nucleus. Besides pointing to the potential usefulness of peptoids as antifungal agents, these results suggest that, similarly to AMPs, also peptoids may not only work as membrane disruptors, but may also have both intracellular and intranuclear effects.
Hybrid structures of α-amino acids and peptoids have also received wide attention for a number of possible applications, including as antimicrobial therapeutics [45]. In a recent advance along this trail, Trine Ryge and colleagues tested 20 de novo designed amphiphilic lysine-peptoid hybrids against a selection of clinically relevant gram-positive/-negative bacteria and fungi, including methicillin-resistant S. aureus and amphotericin-B-resistant C. albicans, finding good activity levels against most strains tested, with the most active compounds that displayed minimal inhibitory concentrations ranging from ≤1.6–6.25 μM [48].
Random-sequence copolymers
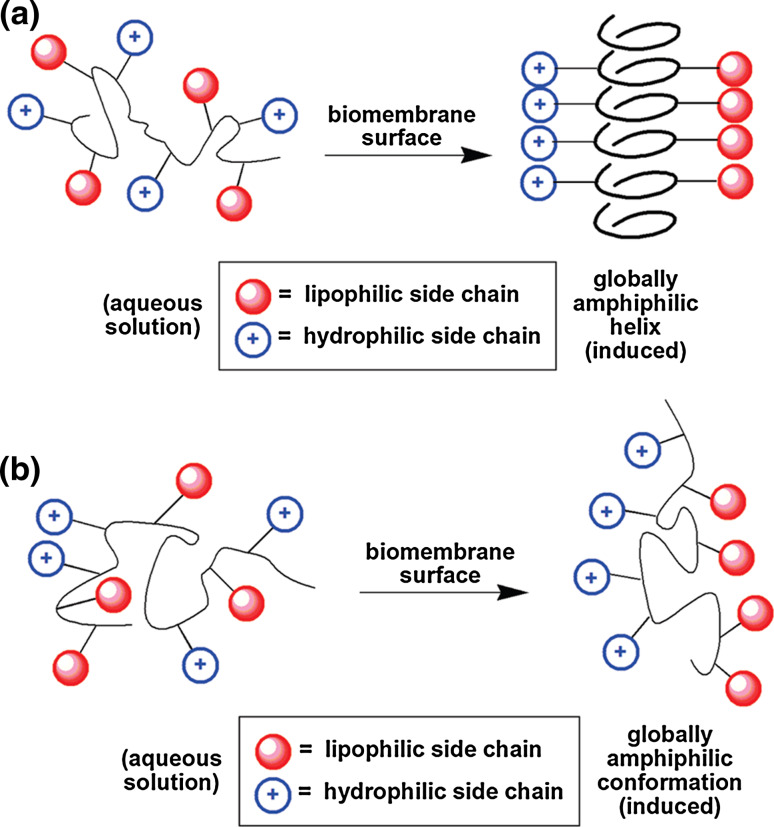
Moving from previous studies of the antibacterial properties of helix-forming foldamers, β-peptides and α/β-peptides [49, 50], Samuel Gellman later investigated those of flexible sequence-random polymers containing cationic and lipophilic subunits, such as amphiphilic nylon-3 copolymers generated via ring-opening polymerization of β-lactams, that act as functional mimics of host-defense peptides [51, 52]. The rationale behind this design lies in the hypothesis that rather than trying to reproduce the regular conformation of helical AMPs due to the segregation of cationic and lipophilic side chains in the folded state, with cationic groups arrayed along one side of the helix and lipophilic groups arrayed along the other, oligomers or polymers functionally-mimicking AMPs could be constructed that tend to adopt a globally amphiphilic but conformationally irregular structure at an interfacial environment (Fig. 8).
Fig. 8.
a Hypothesis explaining the activity of many host-defense peptides, involving the adoption of a globally amphiphilic helical conformation upon approach to a biomembrane surface; b alternative hypothesis, involving the adoption of a globally amphiphilic irregular conformation, which could explain the activity of α/β-peptides and random-sequence copolymers. Reproduced and modified from [54], with permission
Thus, amphiphilic random copolymers would avoid the necessity of synthesizing sequence-specific oligomers, with clear benefits on the associated costs and the possibility for up-scaling the synthesis process. Should this design be linked to a natural template, it might be recalled that Guangshun Wang and colleagues have shown that an analog of a fragment (17–32) of the human cathelicidin LL-37 containing several d-amino acids folds into a non-classical, non-α-helical 3D structure, a specific but irregular conformation that is globally amphiphilic in the presence of detergent micelles and retains antimicrobial activity [53].
Following this concept, polymers prepared from two types of β-lactams, bearing either cationic or lipophilic moieties (Fig. 9), displayed a profile of antibacterial activity and selectivity comparable to that of naturally occurring AMPs, with similar structure-activity relationships [54]. As for the mechanism by which ‘globally amphiphilic’ copolymers disrupt biological membranes, researchers suggested that a carpet-like mechanism, possibly involving the formation of variably sized toroidal pores, is more likely to explain the high prokaryote versus eukaryote selectivity manifested by some of their nylon-3 compounds [54], although no direct evidence was provided to support these claims.
Fig. 9.
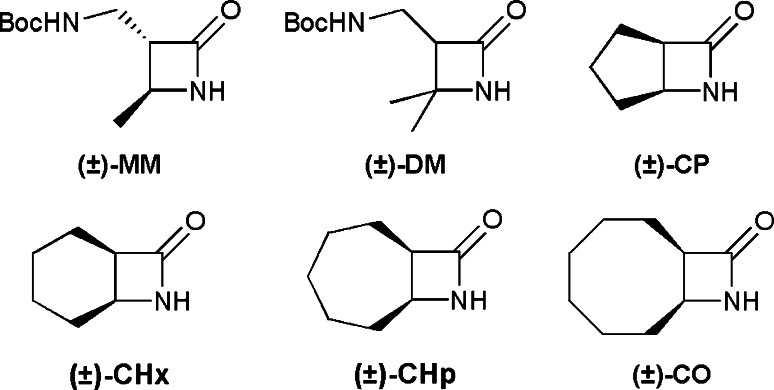
Monomers used for the synthesis of random-sequence nylon-3 copolymers in [54]. Polymers were prepared from two types of β-lactams, some that led ultimately to cationic subunits—MM (monomethyl) or DM (dimethyl)—and others that provided lipophilic subunits—CP (cyclopentyl), CHx (cyclohexyl), CHp (cycloheptyl), CO (cyclooctyl). All β-lactams were used as racemic mixtures. Reproduced from [54], with permission
Non-membrane directed AMP mimetics
Although AMPs are generally considered to kill their microbial targets through insertion and damage/permeabilization of the cytoplasmic membranes of target cells, multiple observations suggest that a number of defense peptides may also interact with intracellular targets such as DNA and RNA or protein synthesis/folding machinery, presumably interfering with their metabolic functions and thus leading to cell death [55–57]. Thus, it is not surprising that the quest for synthetic mimics of AMP might have generated compounds that do not act by perturbing the membrane structure. The PEM molecules POL7001 and POL7080 discussed above can be enrolled in this action-based class. Another example is the ROMP-based SMAMP poly guanidinium oxanorbornene (see Fig. 5). This compound displayed antibacterial activity against both gram-negative and gram-positive bacteria coupled to low hemolytic activity, but did not disrupt membranes in vesicle-dye leakage assays and fluorescence microscopy experiments [58]. On the basis of this experimental evidence, and since the guanidinium groups contained in this SMAMP is also found in polyarginine and other cell-penetrating peptides that are able to cross the membrane and bind anionic targets such as RNA and DNA, researchers believe intracellular targets may be responsible for the antimicrobial properties of polyguanidino-oxanorbornenes, which, they believe, could represent a new ‘proteomimetic activity’ [58]. Such a conclusion seems to be reinforced by the ability of similar polymers, with various chain lengths, to traverse membranes [59].
Conclusions
Over the last decade, there has been considerable interest in developing novel AMPs antibiotics, mainly because of their broad range of activity and the low tendency to induce antimicrobial resistance. However, despite extensive efforts devoted by biotech companies, it has proven difficult to accomplish this goal, mainly because of poor drug-like properties, such as limited bioavailability, an often unspecific mechanism of action and severe systemic toxicity [60].
More recently, a number of peptidomimetic approaches have identified potent and selective compounds that display broad antimicrobial activities in vitro and in vivo against resistant clinical isolates, coupled to chemical stability and limited toxicity towards mammalian cells.
The synthetic flexibility of the small peptidomimetic scaffolds allows fast structure modifications for rapid iterative lead-optimization campaigns. Moreover, up-scaling of the production of these molecules to the quantities required for preclinical and clinical development, it is likely (although not necessarily) swifter in terms of process development and less costly when compared to standard peptides. At this stage, it may be too soon to predict the potential of this approach—also because several issues, such as whether synthetic polymers can be utilized safely to modulate immune systems, await to be evaluated—but the results obtained to date suggest that many important opportunities lie ahead for the design of synthetic oligomers with AMP-like activity that could be beneficially utilized in various antimicrobial fields, including new antimicrobial materials or local and systemic treatment of medical conditions associated with multi-drug resistant pathogens.
Acknowledgments
Conflict of interest
One of the authors declares competing financial interests. Andrea Giuliani is an executive board member and minor shareholder of Spider Biotech S.r.l. (www.spiderbiotech.com), which is developing peptide-based anti-infectives.
Abbreviations
- AMP
Antimicrobial peptide
- MAP
Multiple antigenic peptide
- LPS
Lipopolysaccharide
- LTA
Lipoteichoic acid
- OAK
Oligoacyllysine
- PEM
Protein epitope mimetic
- ROMP
Ring-opening metathesis polymerization
- SMAMP
Synthetic mimic of antimicrobial peptides
- VV
Vaccinia virus
References
- 1.Giuliani A, Rinaldi AC. Antimicrobial peptides. Methods and protocols. Methods in molecular biology. New York: Humana Press; 2010. [Google Scholar]
- 2.van ‘t Hof W, Veerman EC, Helmerhorst EJ, Amerongen AV. Antimicrobial peptides: properties and applicability. Biol Chem. 2001;382:597–619. doi: 10.1515/BC.2001.072. [DOI] [PubMed] [Google Scholar]
- 3.Rotem S, Mor A. Antimicrobial peptide mimics for improved therapeutic properties. Biochim Biophys Acta. 2009;1788:1582–1592. doi: 10.1016/j.bbamem.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Tzeng Y-L, Ambrose KA, Zughaier S, Zhou X, Miller YK, Shafer WM, Stephens DS. Cationic antimicrobial peptide resistance in Neisseria meningitidis . J Bacteriol. 2005;187:5387–5396. doi: 10.1128/JB.187.15.5387-5396.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraus D, Peschel A. Molecular mechanisms of bacterial resistance to antimicrobial peptides. Curr Top Microbiol Immunol. 2006;306:231–250. doi: 10.1007/3-540-29916-5_9. [DOI] [PubMed] [Google Scholar]
- 6.Kraus D, Peschel A. Staphylococcus aureus evasion of innate antimicrobial defense. Future Microbiol. 2008;3:437–451. doi: 10.2217/17460913.3.4.437. [DOI] [PubMed] [Google Scholar]
- 7.Bessalle R, Kapitkovsky A, Gorea A, Shalit I, Fridkin M. All-d-magainin: chirality, antimicrobial activity and proteolytic resistance. FEBS Lett. 1990;274:151–155. doi: 10.1016/0014-5793(90)81351-N. [DOI] [PubMed] [Google Scholar]
- 8.Giuliani A, Pirri G, Bozzi A, Di Giulio A, Aschi M, Rinaldi AC. Antimicrobial peptides: natural templates for synthetic membrane-active compounds. Cell Mol Life Sci. 2008;65:2450–2460. doi: 10.1007/s00018-008-8188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tam JP, Spetzler JC (2001) Synthesis and application of peptide dendrimers as protein mimetics. Curr Protoc Protein Sci. Chapter 18:Unit 18.5 [DOI] [PubMed]
- 10.Tam JP. Synthetic peptide vaccine design: synthesis and properties of a high density multiple antigenic peptide system. Proc Natl Acad Sci USA. 1988;85:5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pini A, Giuliani A, Falciani C, Runci Y, Ricci C, Lelli B, Malossi M, Neri P, Rossolini GM, Bracci L. Antimicrobial activity of novel dendrimeric peptides obtained by phage display selection and rational modification. Antimicrob Agents Chemother. 2005;49:2665–2672. doi: 10.1128/AAC.49.7.2665-2672.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruschi M, Pirri G, Giuliani A, Nicoletto SF, Baster I, Scorciapino MA, Casu M, Rinaldi AC. Synthesis, characterization, antimicrobial activity and LPS-interaction properties of SB041, a novel dendrimeric peptide with antimicrobial properties. Peptides. 2010;31:1459–1467. doi: 10.1016/j.peptides.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Pini A, Falciani C, Mantengoli E, Bindi S, Brunetti J, Iozzi S, Rossolini GM, Bracci L. A novel tetrabranched antimicrobial peptide that neutralizes bacterial lipopolysaccharide and prevents septic shock in vivo. FASEB J. 2010;24:1015–1022. doi: 10.1096/fj.09-145474. [DOI] [PubMed] [Google Scholar]
- 14.Falciani C, Lozzi L, Pini A, Corti F, Fabbrini M, Bernini A, Lelli B, Niccolai N, Bracci L. Molecular basis of branched peptides resistance to enzyme proteolysis. Chem Biol Drug Des. 2007;69:216–221. doi: 10.1111/j.1747-0285.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- 15.Luganini A, Giuliani A, Pirri G, Pizzuto L, Landolfo S, Gribaudo G. Peptide-derivatized dendrimers inhibit human cytomegalovirus infection by blocking virus binding to cell surface heparan sulphate. Antiviral Res. 2010;85:532–540. doi: 10.1016/j.antiviral.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Obrecht D, Robinson JA, Bernardini F, Bisang C, DeMarco SJ, Moehle K, Gombert FO. Recent progress in the discovery of macrocyclic compounds as potential anti-infective therapeutics. Curr Med Chem. 2009;16:42–65. doi: 10.2174/092986709787002844. [DOI] [PubMed] [Google Scholar]
- 17.Lou K (2010) A new spin on protegrin. SciBX 3: doi:10.1038/scibx.2010.265
- 18.Srinivas N, Jetter P, Ueberbacher BJ, Werneburg M, Zerbe K, Steinmann J, Van der Meijden B, Bernardini F, Lederer A, Dias RL, Misson PE, Henze H, Zumbrunn J, Gombert FO, Obrecht D, Hunziker P, Schauer S, Ziegler U, Käch A, Eberl L, Riedel K, DeMarco SJ, Robinson JA. Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa . Science. 2010;327:1010–1013. doi: 10.1126/science.1182749. [DOI] [PubMed] [Google Scholar]
- 19.Jiang L, Moehle K, Dhanapal B, Obrecht D, Robinson JA. Combinatorial biomimetic chemistry: parallel synthesis of a small library of β-hairpin mimetics based on loop III from human platelet-derived growth factor. Helv Chim Acta. 2000;83:3097–3112. doi: 10.1002/1522-2675(20001220)83:12<3097::AID-HLCA3097>3.0.CO;2-1. [DOI] [Google Scholar]
- 20.Robinson JA, Demarco S, Gombert F, Moehle K, Obrecht D. The design, structures and therapeutic potential of protein epitope mimetics. Drug Discov Today. 2008;13:944–951. doi: 10.1016/j.drudis.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Radzishevsky IS, Rotem S, Bourdetsky D, Navon-Venezia S, Carmeli Y, Mor A. Improved antimicrobial peptides based on acyl-lysine oligomers. Nat Biotechnol. 2007;25:657–659. doi: 10.1038/nbt1309. [DOI] [PubMed] [Google Scholar]
- 22.Rotem S, Radzishevsky IS, Bourdetsky D, Navon-Venezia S, Carmeli Y, Mor A. Analogous oligo-acyl-lysines with distinct antibacterial mechanisms. FASEB J. 2008;22:2652–2661. doi: 10.1096/fj.07-105015. [DOI] [PubMed] [Google Scholar]
- 23.Rotem S, Raz N, Kashi Y, Mor A. Bacterial capture by peptide-mimetic oligoacyllysine surfaces. Appl Environ Microbiol. 2010;76:3301–3307. doi: 10.1128/AEM.00532-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarig H, Livne L, Held-Kuznetsov V, Zaknoon F, Ivankin A, Gidalevitz D, Mor A. A miniature mimic of host defense peptides with systemic antibacterial efficacy. FASEB J. 2010;24:1904–1913. doi: 10.1096/fj.09-149427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarig H, Rotem S, Ziserman L, Danino D, Mor A. Impact of self-assembly properties on antibacterial activity of short acyl-lysine oligomers. Antimicrob Agents Chemother. 2008;52:4308–4314. doi: 10.1128/AAC.00656-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore KS, Wehrli S, Roder H, Rogers M, Forrest JN, Jr, McCrimmon D, Zasloff M. Squalamine: an aminosterol antibiotic from the shark. Proc Natl Acad Sci USA. 1993;90:1354–1358. doi: 10.1073/pnas.90.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Epand RF, Savage PB, Epand RM. Bacterial lipid composition and the antimicrobial efficacy of cationic steroid compounds (Ceragenins) Biochim Biophys Acta. 2007;1768:2500–2509. doi: 10.1016/j.bbamem.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 28.Savage PB, Li C, Taotafa U, Ding B, Guan Q. Antibacterial properties of cationic steroid antibiotics. FEMS Microbiol Lett. 2002;217:1–7. doi: 10.1111/j.1574-6968.2002.tb11448.x. [DOI] [PubMed] [Google Scholar]
- 29.Isogai E, Isogai H, Takahashi K, Okumura K, Savage PB. Ceragenin CSA-13 exhibits antimicrobial activity against cariogenic and periodontopathic bacteria. Oral Microbiol Immunol. 2009;24:170–172. doi: 10.1111/j.1399-302X.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 30.Leszczyńska K, Namiot A, Fein DE, Wen Q, Namiot Z, Savage PB, Diamond S, Janmey PA, Bucki R. Bactericidal activities of the cationic steroid CSA-13 and the cathelicidin peptide LL-37 against Helicobacter pylori in simulated gastric juice. BMC Microbiol. 2009;9:187. doi: 10.1186/1471-2180-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5:321–331. doi: 10.1038/ncpgasthep1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howell MD, Streib JE, Kim BE, Lesley LJ, Dunlap AP, Geng D, Feng Y, Savage PB, Leung DY. Ceragenins: a class of antiviral compounds to treat orthopox infections. J Invest Dermatol. 2009;129:2668–2675. doi: 10.1038/jid.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu DH, Choi S, Chen B, Doerksen RJ, Clements DJ, Winkler JD, Klein ML, DeGrado WF. Nontoxic membrane-active antimicrobial arylamide oligomers. Angew Chem Int Ed. 2004;43:1158–1162. doi: 10.1002/anie.200352791. [DOI] [PubMed] [Google Scholar]
- 34.Scott RW, DeGrado WF, Tew GN. De novo designed synthetic mimics of antimicrobial peptides. Curr Opin Biotechnol. 2008;19:620–627. doi: 10.1016/j.copbio.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishitsuka Y, Arnt L, Majewski J, Frey S, Ratajczek M, Kjaer K, Tew GN, Lee KYC. Amphiphilic poly(phenyleneethynylene)s can mimic antimicrobial peptide membrane disordering effect by membrane insertion. J Am Chem Soc. 2006;128:13123–13129. doi: 10.1021/ja061186q. [DOI] [PubMed] [Google Scholar]
- 36.Lienkamp K, Madkour AE, Kumar KN, Nüsslein K, Tew GN. Antimicrobial polymers prepared by ring-opening metathesis polymerization: manipulating antimicrobial properties by organic counterion and charge density variation. Chemistry. 2009;15:11715–11722. doi: 10.1002/chem.200900606. [DOI] [PubMed] [Google Scholar]
- 37.Lienkamp K, Tew GN. Synthetic mimics of antimicrobial peptides–a versatile ring-opening metathesis polymerization based platform for the synthesis of selective antibacterial and cell-penetrating polymers. Chemistry. 2009;15:11784–11800. doi: 10.1002/chem.200900049. [DOI] [PubMed] [Google Scholar]
- 38.Haug BE, Stensen W, Kalaaij M, Rekdal Ø, Svendsen JS. Synthetic antimicrobial peptidomimetics with therapeutic potential. J Med Chem. 2008;51:4306–4314. doi: 10.1021/jm701600a. [DOI] [PubMed] [Google Scholar]
- 39.Flemming K, Klingenberg C, Cavanagh JP, Sletteng M, Stensen W, Svendsen JS, Flaegstad T. High in vitro antimicrobial activity of synthetic antimicrobial peptidomimetics against staphylococcal biofilms. J Antimicrob Chemother. 2009;63:136–145. doi: 10.1093/jac/dkn464. [DOI] [PubMed] [Google Scholar]
- 40.Shalev DE, Rotems S, Fish A, Mor A. Consequences of N-acylation on structure and membrane binding properties of dermaseptin derivative K4–S4-(1–13) J Biol Chem. 2006;281:9432–9438. doi: 10.1074/jbc.M513051200. [DOI] [PubMed] [Google Scholar]
- 41.Makovitzki A, Avrahami D, Shai Y. Ultrashort antibacterial and antifungal lipopeptides. Proc Natl Acad Sci USA. 2006;103:15997–16002. doi: 10.1073/pnas.0606129103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vallon-Eberhard A, Makovitzki A, Beauvais A, Latgé JP, Jung S, Shai Y. Efficient clearance of Aspergillus fumigatus in murine lungs by an ultrashort antimicrobial lipopeptide, palmitoyl-Lys-Ala-dAla-Lys. Antimicrob Agents Chemother. 2008;52:3118–3126. doi: 10.1128/AAC.00526-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laverty G, McLaughlin M, Shaw C, Gorman SP, Gilmore BF. Antimicrobial activity of short, synthetic cationic lipopeptides. Chem Biol Drug Des. 2010;75:563–569. doi: 10.1111/j.1747-0285.2010.00973.x. [DOI] [PubMed] [Google Scholar]
- 44.Zuckermann RN, Kerr JM, Kent SBH, Moos WH. Efficient method for the preparation of peptoids [oligo(N-substituted glycines)] by submonomer solid-phase synthesis. J Am Chem Soc. 1992;114:10646–10647. doi: 10.1021/ja00052a076. [DOI] [Google Scholar]
- 45.Olsen CA. Peptoid–peptide hybrid backbone architectures. Chem Bio Chem. 2010;11:152–160. doi: 10.1002/cbic.200900618. [DOI] [PubMed] [Google Scholar]
- 46.Chongsiriwatana NP, Patch JA, Czyzewski AM, Dohm MT, Ivankin A, Gidalevitz D, Zuckermann RN, Barron AE. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proc Natl Acad Sci USA. 2008;105:2794–2799. doi: 10.1073/pnas.0708254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uchida M, McDermott G, Wetzler M, Le Gros MA, Myllys M, Knoechel C, Barron AE, Larabell CA. Soft X-ray tomography of phenotypic switching and the cellular response to antifungal peptoids in Candida albicans . Proc Natl Acad Sci USA. 2009;106:19375–19380. doi: 10.1073/pnas.0906145106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryge TS, Frimodt-Møller N, Hansen PR. Antimicrobial activities of twenty lysine-peptoid hybrids against clinically relevant bacteria and fungi. Chemotherapy. 2008;54:152–156. doi: 10.1159/000119707. [DOI] [PubMed] [Google Scholar]
- 49.Schmitt MA, Weisblum B, Gellman SH. Unexpected relationships between structure and function in alpha, beta-peptides: antimicrobial foldamers with heterogeneous backbones. J Am Chem Soc. 2004;126:6848–6849. doi: 10.1021/ja048546z. [DOI] [PubMed] [Google Scholar]
- 50.Schmitt MA, Weisblum B, Gellman SH. Interplay among folding, sequence, and lipophilicity in the antibacterial and hemolytic activities of alpha/beta-peptides. J Am Chem Soc. 2007;129:417–428. doi: 10.1021/ja0666553. [DOI] [PubMed] [Google Scholar]
- 51.Mowery BP, Lee SE, Kissounko DA, Epand RF, Epand RM, Weisblum B, Stahl SS, Gellman SH. Mimicry of antimicrobial host-defense peptides by random copolymers. J Am Chem Soc. 2007;129:15474–15476. doi: 10.1021/ja077288d. [DOI] [PubMed] [Google Scholar]
- 52.Epand RF, Mowery BP, Lee SE, Stahl SS, Lehrer RI, Gellman SH, Epand RM. Dual mechanism of bacterial lethality for a cationic sequence-random copolymer that mimics host-defense antimicrobial peptides. J Mol Biol. 2008;379:38–50. doi: 10.1016/j.jmb.2008.03.047. [DOI] [PubMed] [Google Scholar]
- 53.Li X, Li Y, Han H, Miller DW, Wang G. Solution structures of human LL-37 fragments and NMR-based identification of a minimal membrane-targeting antimicrobial and anticancer region. J Am Chem Soc. 2006;128:5776–5785. doi: 10.1021/ja0584875. [DOI] [PubMed] [Google Scholar]
- 54.Mowery BP, Lindner AH, Weisblum B, Stahl SS, Gellman SH. Structure-activity relationships among random nylon-3 copolymers that mimic antibacterial host-defense peptides. J Am Chem Soc. 2009;131:9735–9745. doi: 10.1021/ja901613g. [DOI] [PubMed] [Google Scholar]
- 55.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 56.Hale JD, Hancock REW. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev Anti Infect Ther. 2007;5:951–959. doi: 10.1586/14787210.5.6.951. [DOI] [PubMed] [Google Scholar]
- 57.Cho JH, Kim SC. Non-membrane targets of antimicrobial peptides: novel therapeutic opportunities? In: Wang G, editor. Antimicrobial peptides: discovery, design and novel therapeutic strategies. England: Oxfordshire; 2010. pp. 128–140. [Google Scholar]
- 58.Gabriel GJ, Madkour AE, Dabkowski JM, Nelson CF, Nüsslein K, Tew GN. Synthetic mimic of antimicrobial peptide with nonmembrane-disrupting antibacterial properties. Biomacromolecules. 2008;9:2980–2983. doi: 10.1021/bm800855t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hennig A, Gabriel GJ, Tew GN, Matile S. Stimuli-responsive polyguanidino-oxanorbornene membrane transporters as multicomponent sensors in complex matrices. J Am Chem Soc. 2008;130:10338–10344. doi: 10.1021/ja802587j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giuliani A, Pirri G, Nicoletto SF. Antimicrobial peptides: an overview of a promising class of therapeutics. Centr Eur J Biol. 2007;2:1–33. doi: 10.2478/s11535-007-0010-5. [DOI] [Google Scholar]
- 61.Ross NT, Katt WP, Hamilton AD. Synthetic mimetics of protein secondary structure domains. Phil Trans R Soc A. 2010;368:989–1008. doi: 10.1098/rsta.2009.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]