Abstract
Parkinson’s disease (PD) is characterized by a progressive loss of dopaminergic neurons in the substantia nigra. The cause of neuronal death in PD is largely unknown, but several genetic loci, including PTEN-induced putative kinase 1 (PINK1), have been linked to early onset autosomal recessive forms of familial PD. PINK1 encodes a serine/threonine kinase, which phosphorylates several substrates and consequently leads to cell protection against apoptosis induced by various stresses. In addition, research has shown that inflammation largely contributes to the pathogenesis of PD, but the functional link between PINK1 and PD-linked neuroinflammation remains poorly understood. Therefore, in the present study, we investigated the functional role of PINK1 in interleukin (IL)-1β-mediated inflammatory signaling. We show that PINK1 specifically binds to TRAF6 and TAK1, and facilitates the autodimerization and autoubiquitination of TRAF6. PINK1 also enhances the association between TRAF6 and TAK1, phosphorylates TAK1, and stimulates polyubiquitination of TAK1. Furthermore, PINK1 leads to the potentiation of IL-1β-mediated NF-κB activity and cytokine production. These findings suggest that PINK1 positively regulates two key molecules, TRAF6 and TAK1, in the IL-1β-mediated signaling pathway, consequently up-regulating their downstream inflammatory events.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-012-1004-7) contains supplementary material, which is available to authorized users.
Keywords: IL-1β, Inflammation, Parkinson’s disease, PINK1, TAK1, TRAF6
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by progressive degeneration and death of dopaminergic neurons in the substantia nigra pars compacta [1]. Pathological hallmarks of PD include the presence of cytoplasmic inclusions known as Lewy bodies [2]. Although the etiology of PD remains poorly understood, several genetic loci have been implicated in the pathogenesis of familial forms of PD. Among them, the PTEN-induced putative kinase 1 (PINK1) gene encodes a serine/threonine kinase [3, 4] that phosphorylates substrates such as tumor necrosis factor (TNF) receptor-associated protein 1, thereby leading to cell protection against oxidative stress-induced apoptosis [5]. In cultured cells, overexpression of PINK1 protects cells against apoptotic stimuli [6, 7].
Inflammation is a complex biological response to harmful stimuli, such as pathogens, damaged cells, or irritants, and serves a protective role in the removal of these injurious stimuli. Inflammatory events in the neural system include microglial activation, astrogliosis, and lymphocytic infiltration. Many stimuli trigger activation of the transcription factor nuclear factor kappa B (NF-κB) both in astrocytes and microglia [8], resulting in the production of proinflammatory mediators including chemokines and cytokines [9]. Although some tissue inflammation is required for the repair of damaged tissue, an excessive inflammatory response, especially in the central nervous system, leads to significant cell death and consequent neural degeneration. Similarly, several lines of evidence indicate that inflammation is closely linked to the pathogenesis of PD. For example, autopsy of brain tissue from PD patients shows evidence of inflammation in the substantia nigra [10, 11].
Interleukin-1 (IL-1) is one of the principal cytokines responsible for inducing mediators that orchestrate various immune and inflammatory responses by activating NF-κB and activating protein 1 (AP-1) [12]. Interleukin-1 signaling is initiated by the ligand-induced formation of a receptor complex that consists of the IL-1 receptor (IL-1R) and the IL-1R accessory protein. Subsequently, the cytosolic adaptor protein myeloid differentiation marker (MyD88) is recruited to this complex [13]. MyD88, in turn, recruits IL-1R-associated kinase-1 (IRAK1) and IRAK4 by interacting with their death domains [14]. IRAK1 is presumably activated through phosphorylation by IRAK4 and interacts with TNF receptor-associated factor 6 (TRAF6) [15]. TRAF6 then moves from the membrane to the cytosol, where it associates with transforming growth factor-β (TGF-β)-activated kinase 1 (TAK1), a member of the mitogen-activated protein kinase (MAPK) kinase kinase (MAP3 K) family [16]. TRAF6 contains a RING domain and functions as an ubiquitin E3 ligase that conjugates Lys63-linked polyubiquitin chains to TRAF6 itself and to IRAK1. IRAK1 then facilitates the dimerization of TRAF6. In this active form, TAK1 is fully activated by polyubiquitinated TRAF6. This triggers the autophosphorylation of TAK1, which subsequently phosphorylates regulatory kinases in different downstream signaling pathways [17] and finally activates NF-κB to produce inflammatory cytokines [18, 19].
Despite intensive research, the functional relationship between PD-linked genes and the inflammatory response is poorly understood. Here, we examined the putative regulatory role of PINK1 in the IL-1β-mediated inflammatory signaling pathway. We demonstrate that PINK1 physically interacts with TRAF6 and TAK1 in cultured cells. PINK1 also promotes the autodimerization and autoubiquitination of TRAF6, and the polyubiquitination and phosphorylation of TAK1. As a result, PINK1 stimulates the transcriptional activity of NF-κB, resulting in further cytokine production upon IL-1β stimulation. These findings point to a new mechanism for regulating TRAF6 and TAK1 activation during inflammatory signaling events.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), fetal calf serum (FCS), and LipofectAMINE PLUS reagent were purchased from Invitrogen (Carlsbad, CA, USA). Protein A-Sepharose was obtained from GE Healthcare Biosciences (Piscataway, NJ, USA). Anti-PINK1 antibody was purchased from Abgent (San Diego, CA, USA), and anti-HA, anti-c-Myc, anti-TAK1, anti-TRAF6, anti-actin and anti-ubiquitin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Secondary goat anti-IgG and horseradish peroxidase-conjugated anti-rabbit and anti-mouse IgGs were purchased from Life Technologies (Grand Island, NY, USA), and anti-phospho-threonine IgG, anti-Flag antiserum, and recombinant human IL-1β were obtained from Sigma (St. Louis, MO, USA). Recombinant human IL-1 receptor antagonist (IL-1ra) was purchased from R&D Systems (Minneapolis, MN, USA) and enhanced chemiluminescence (ECL) reagent was purchased from Perkin-Elmer Life and Analytical Sciences (Waltham, MA, USA). Lys63-specific anti-ubiquitin antibody was obtained from Millipore (Temecula, CA, USA). All other chemicals used in the study were commercial products of analytical grade purchased from Sigma.
The mammalian construct encoding Myc-tagged wild-type hPINK1 (pBOS-3X-myc-hPINK1-WT) was a gift from J. Chung (Seoul National University, Seoul, Korea). Plasmid encoding Myc-tagged kinase-deficient hPINK1 mutant having two point mutations at K219A and D362A (pBOS-3X-myc-hPINK1-KD) was generated by the QuikChangeXL site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA), according to the manufacturer’s protocol. PCR was performed using the following primers (the mutated codon is underlined in each primer): K219A forward primer, 5′-CCCTTGGCCATCGCGATGATGTGGAAC-3′; K219A reverse primer, 5′-GTTCCACATCATCGCGATGGCCAAGGG-3′; D362A forward primer, 5′-ATCGCGCACAGAGCCCTGAAATCCGAC-3′; D362A reverse primer, 5′-GTCGGATTTCAGGGCTCTGTGCGCGAT-3′. Plasmids encoding Flag-TAK1 and Flag-TRAF6 were kindly provided by G. Takaesu (Keio University, Tokyo, Japan). Plasmid encoding GST-fused TAK1 was generated by subcloning TAK1 from pCMV-Flag-TAK1 into pGEX4T-1 vector using EcoRI and XhoI sites.
Cell culture and DNA transfection
PINK1-null (PINK1−/−) and control (PINK1+/+) mouse embryonic fibroblasts (MEFs) were provided by J. Shen (Harvard Medical School, Boston, USA), and 293 IL-1RI cells stably expressing type 1 IL-1 receptor were a kind gift from G. Takaesu (Keio University, Tokyo, Japan). Human embryonic kidney cells (HEK293) and PINK1−/− and +/+ MEFs were cultured in DMEM containing 10 % FBS, 100 Units/ml penicillin, and 100 μg/ml streptomycin at 37 °C in 5 % CO2. 293 IL-1RI cells were cultured in DMEM containing 10 % FCS, 100 Units/ml penicillin, and 100 μg/ml streptomycin at 37 °C in 5 % CO2. DNA transfection was performed using the LipofectAMINE PLUS reagent, according to the manufacturer’s protocol. The total amount of DNA transfected in each individual sample was adjusted using parental empty vector DNA.
DNA transfection and RNA interference
The siRNAs corresponding to human PINK1 against the following human PINK1 mRNA sequence, 5′-GAAAUCCGACAACAUCCUUUU-3′, and the negative control (NT-siRNA) with no known mammalian homology (siCONTROL Non-Targeting siRNA #1), were purchased from Bioneer (Seoul, Korea). siRNA transfection into 293 IL-1RI cells was performed using LipofectAMINE 2000 (Invitrogen), according to the manufacturer’s protocol.
Immunoprecipitation and western blot analysis
Cells were rinsed twice with ice-cold phosphate-buffered saline (PBS), harvested in 1 % Nonidet P40 lysis buffer (50 mM Tris, pH 7.5, 1 % Nonidet P-40, 150 mM NaCl, 10 % glycerol, 1 mM Na3VO4, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mM EGTA, 1 mM EDTA, 10 mM NaF, and 0.2 mM phenylmethylsulfonyl fluoride), and briefly sonicated. Lysates were collected by centrifugation at 13,000 × g for 20 min at 4 °C. For immunoprecipitation, 1 μg of the appropriate antibody was incubated overnight at 4 °C with 0.5–1 mg of cell extracts prepared in cell lysis buffer. Thirty microliters of a 1:1 suspension of Protein A-Sepharose beads were added and incubated for 2 h at 4 °C with gentle rotation. Beads were pelleted by centrifugation at 10,000 × g for 30 s at 4 °C, and washed three times with 1 % Nonidet P40 lysis buffer. Immunocomplexes were dissociated by boiling in SDS-PAGE sample buffer, separated on an SDS-PAGE gel, and transferred to a nitrocellulose membrane. Membranes were then blocked in TBST buffer (20 mM Tris, pH 7.5, 137 mM NaCl, and 0.1 % Tween 20) containing 5 % nonfat dry milk for 1 h at room temperature, and incubated overnight at 4 °C in TBST buffer containing 3 % nonfat dry milk and the appropriate primary antibody. Membranes were washed three times in TBST and then incubated with the appropriate secondary IgG-coupled horseradish peroxidase antibody for 1 h at room temperature. The membranes were then washed three times with TBST, and signals were visualized with ECL reagent.
Immunocytochemistry
After DNA transfection, cells were washed twice with ice-cold PBS (pH 7.4), fixed with 3.7 % formaldehyde in PBS for 15 min, permeabilized with 0.2 % Triton X-100 for 20 min, blocked with 1 % bovine serum albumin for 30 min, and incubated overnight at 4 °C with the appropriate primary antibody. After washing with PBS, cells were incubated with FITC-, or TRITC-conjugated secondary antibody for 2 h. Where specified, the samples were stained with 4, 6-diamidino-2-phenylindole (DAPI) using the SlowFade Antifade kit (Invitrogen). Fixed cells were visualized using a LSM-510 META confocal microscope (Carl Zeiss, Göttingen, Germany).
In vitro PINK1 kinase assay
Two hundred and ninety three IL-1RI cells were transfected with Myc-tagged hPINK1-WT or hPINK1-KD mutant for 48 h. Cells were lysed in 1 % Nonidet P40 lysis buffer and lysates were immunoprecipitated with anti-Myc antibody overnight at 4 °C. The anti-Myc immunocomplexes were then incubated with 2 μg of bacterially expressed GST-TAK1, 6 mM ATP, and phosphatase inhibitors in the kinase reaction buffer (50 mM Tris–HCl, 10 mM MgCl2, 5 mM DTT) to a final volume 50 μl for 2 h at 30 °C, and the reaction products were analyzed by immunoblotting with anti-phospho-threonine antibody.
Luciferase reporter assay
HEK293, 293 IL-1RI, and PINK1+/+ and −/− MEF cells were grown in six-well plates at a density of 3 × 105 cells per well for 24 h. NF-κB-dependent firefly luciferase reporter and effector plasmids were co-transfected into the cells along with the Renilla luciferase plasmid. Forty-eight hours after transfection, cells were harvested in passive lysis buffer (Promega, Madison, WI, USA), and luciferase assays were performed using the Dual-Luciferase Reporter Assay System (Promega). The relative luciferase activity was calculated by dividing firefly luciferase activity by Renilla luciferase activity. Data represented three independent experiments performed in triplicate.
Enzyme-linked immunosorbent assay
After cells were cultured for the indicated times under the specified conditions, the amount of secreted IL-8 protein in the media was determined using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Biosource International, Camarillo, CA, USA), according to the manufacturer’s instruction.
Real-time PCR analysis for IL-8 mRNA expression
Total RNA was converted into cDNA using a random hexamer and viral reverse transcriptase (Promega). The cDNA was incubated with SYBR Green Real-time PCR master mix (Toyobo Co. Ltd., Osaka. Japan) that contained 10 pg/ml of the forward and reverse primers, and amplified using the Light-Cycler PCR system (Roche Applied Science, Indianapolis, IN, USA). Human specific primers were used to amplify IL-8 (forward, 5′-ATGACTTCCAAGCTGGCCGTGGCT-3′) and β-actin (5′-ACCAACTGGGACGACATGGAG-3′). These primers yielded a 297-bp product for IL-8 and a 349-bp PCR product for β-actin, which served as an internal control.
Statistical analysis
Statistical differences were determined using a one-way ANOVA with Tukey’s post test. All values were expressed as mean ± standard deviation (SD).
Results
PINK1 interacts with TRAF6 and TAK1 in mammalian cells
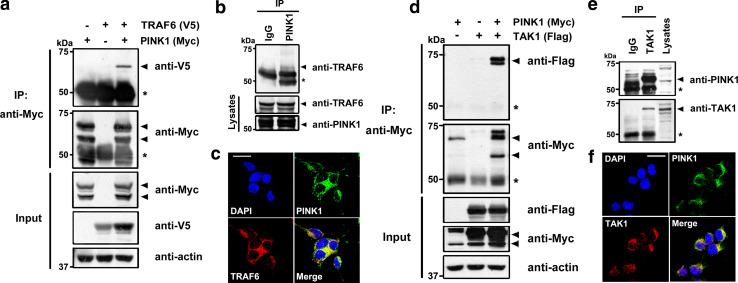
To examine the functional role of PINK1, we performed a yeast two-hybrid assay with full-length PINK1 as bait. After screening 5 × 106 human fetal brain cDNA library clones, a number of unknown as well as previously reported PINK1-binding partners were identified, including parkin [20], TRAF6, and TAK1 (data not shown). TRAF6 is an upstream adaptor molecule of the IL-1R complex, and functions as a ubiquitin E3 ligase responsible for downstream ubiquitination of TAK1 and activation in IL-1β-induced inflammatory signaling. In addition, TAK1, a member of the MAP3 K family and a downstream component of TRAF6 signaling, plays an essential role in the activation of JNK, p38, and IκB kinase (IKK) during IL-1β- and TNFα-mediated signaling [21]. We further explored the interactions of PINK1 with TRAF6 and TAK1, and their functional consequences, focusing on the regulation of the IL-1β-mediated inflammatory signaling pathway. First, we used a co-immunoprecipitation assay to test whether PINK1 binds to TRAF6 in mammalian cells. After HEK293 cells were transfected with Myc-tagged PINK1 and V5-tagged TRAF6, immunoprecipitation of cell lysates was performed using anti-Myc antibody, followed by immunoblot analysis with anti-V5 antibody. As shown in Fig. 1a, transiently transfected PINK1 selectively bound to TRAF6 in HEK293 cells. In addition, PINK1 appeared in two bands (Fig. 1a), consistent with the results of previous reports [3, 4]. To determine whether endogenous PINK1 binds to endogenous TRAF6, immunoprecipitation of HEK293 cell lysates was performed with anti-PINK1 IgG, followed by immunoblot analysis with anti-TRAF6 antibody. As shown Fig. 1b, endogenous PINK1 interacted with endogenous TRAF6, whereas no obvious interaction band was found in anti-immunocomplex samples prepared with preimmune IgG as a control (Fig. 1b). This result suggests that the specific interaction between PINK1 and TRAF6 is not an artifact of DNA transfection but a true interaction in the mammalian system. Moreover, immunocytochemical analysis revealed that endogenous PINK1 co-localizes with endogenous TRAF6, mainly in the cytoplasmic region of HEK293 cells (Fig. 1c).
Fig. 1.
PINK1 interacts with TRAF6 and TAK1 in mammalian HEK293 cells. a HEK293 cells were transfected with plasmids encoding Myc-tagged PINK1 and/or V5-tagged TRAF6 for 48 h. Immunoprecipitation (IP) of cell lysates was performed with anti-Myc antibody, and immunoblotted with anti-V5 antibody, as indicated. The proper expression of transiently transfected proteins was identified by immunoblotting with the indicated antibodies. An asterisk indicates IgG heavy chains. Equal loading of the samples was confirmed by immunoblotting with anti-actin antibody. b Where indicated, the immunoprecipitation of HEK293 cell lysates was performed with anti-PINK1 antibody, followed by immunoblotting with anti-TRAF6 antibodies. As a control, cell lysates were immunoprecipitated with preimmune IgG (IgG). The expression of PINK1 and TRAF6 in cell extracts was determined by immunoblotting with anti-PINK1 or anti-TRAF6 antibodies. c HEK293 cells were fixed, permeabilized, and labeled with anti-PINK1or anti-TRAF6 antibodies for 24 h. Cells were then stained with TRITC-conjugated and FITC-conjugated secondary antibodies and DAPI. Immunostained preparations were examined with a confocal microscope. Scale bar 20 μm. d Identification of specific binding between Myc-PINK1 and Flag-TAK1 in HEK293 cells. e Where indicated, immunoprecipitation of HEK293 cell lysates was performed with anti-TAK1 antibody, followed by immunoblot analysis with anti-PINK1 IgG. f Co-localization of PINK1 and TAK1 in HEK293 cells. Scale bar 20 μm
Next, we checked whether PINK1 binds to TAK1 in mammalian cells using the same co-immunoprecipitation/immunoblot assays. After HEK293 cells were transiently transfected with Myc-PINK1 and Flag-TAK1, anti-Myc immunoprecipitates from cell lysates were subjected to immunoblotting with anti-Flag antibodies. As shown Fig. 1d, transiently overexpressed PINK1 bound selectively to TAK1 in HEK293 cells. In a similar experiment, we assessed whether endogenous PINK1 interacts with endogenous TAK1 in mammalian cells. Immunoblot analysis of anti-TAK1 immunocomplexes with anti-PINK1 IgG revealed that endogenous PINK1 bound to endogenous TAK1 in HEK293 cells (Fig. 1e). This specific interaction was not detected when the immunoprecipitation was performed with non-specific IgG. Finally, immunocytochemical analysis showed that endogenous PINK1 co-localizes with endogenous TAK1 mainly in the cytoplasm of HEK293 cells (Fig. 1f).
Together, these results suggest that PINK1 specifically binds to TRAF6 and TAK1 in mammalian cells.
Interactions between PINK1 and TRAF6/TAK1 increase upon IL-1β stimulation
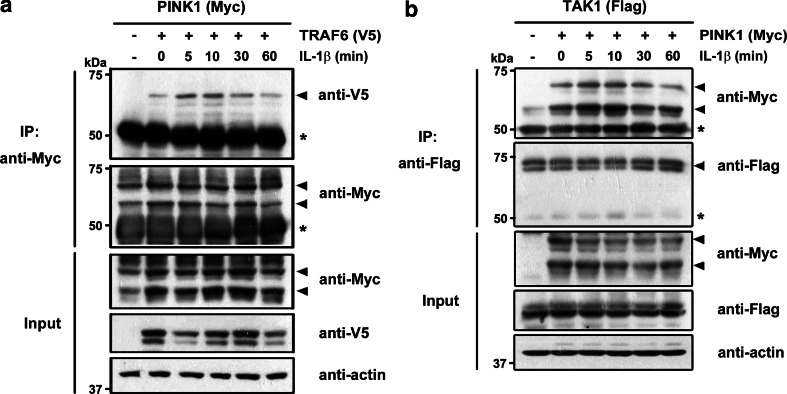
We next explored whether the interaction between PINK1 and TRAF6 changes with IL-1β stimulation. We used 293 IL-1RI cells stably expressing type I IL-1β receptor [22], which consequently show a much higher response to IL-1β stimulation. After co-transfection with Myc-PINK1 and V5-TRAF6, 293 IL-1RI cells were treated with IL-1β. Immunoblot analysis of anti-Myc immunocomplexes with anti-V5 antibodies revealed that stimulation with IL-1β induced the association of PINK1 with TRAF6 (Fig. 2a). In addition, the formation of this complex steadily increased for 30 min after IL-1β stimulation and disappeared thereafter (Fig. 2a), suggesting that PINK1 affects the function of TRAF6 through the transient formation of a PINK1-TRAF6 complex in IL-1-induced downstream signaling.
Fig. 2.
The association of PINK1 with TRAF6 and TAK1 is increased upon IL-1β stimulation. a, b 293 IL-1RI cells were co-transfected with Myc-PINK1 and either V5-TRAF6 (a) or Flag-TAK1 (b) for 42 h and treated with 10 ng/ml IL-1β for the indicated times. Cell lysates were subjected to immunoprecipitation (IP) with anti-Myc (a) or anti-Flag (b), followed by immunoblotting with anti-V5 (a) or anti-Myc IgGs (b), as indicated. An asterisk indicates IgG heavy chains and actin served as a loading control
We next investigated whether the association between PINK1 and TAK1 is affected by stimulation with IL-1β. After cells were transfected with Myc-PINK1 and Flag-TAK1 and stimulated with IL-1β in the same way, immunoblotting of anti-Myc immunocomplexes with anti-Flag antibody showed that PINK1 bound to TAK1 in the absence of the IL-1β stimulus in 293 IL-1RI cells (Fig. 2b). Moreover, addition of IL-1β caused an increase in this interaction, which reached a maximum at 10 min and decreased thereafter until 60 min post-stimulation (Fig. 2b).
Together, these results suggest that the binding of PINK1 to TRAF6 and TAK1 increases substantially upon stimulation with IL-1β.
Autodimerization of TRAF6 is up-regulated by overexpression of PINK1
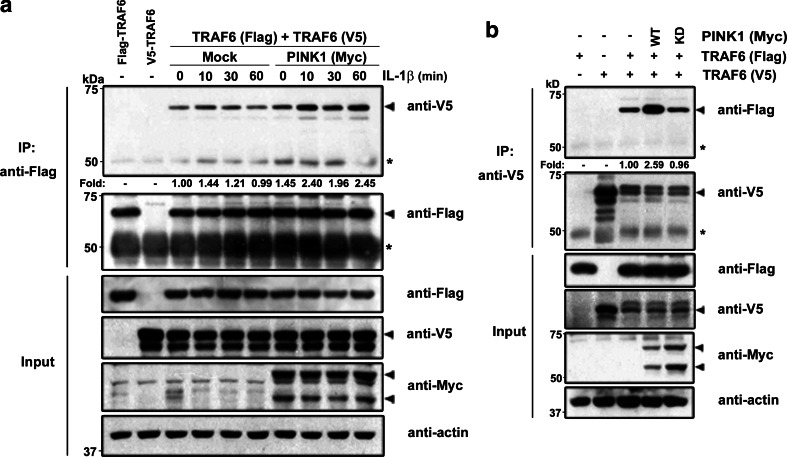
Autodimerization of TRAF6 is known to lead to Lys63-dependent auto-polyubiquitination following IL-1R engagement, suggesting that the ubiquitin ligase activity of TRAF6 is activated by dimerization and/or oligomerization [23, 24]. To test whether PINK1 also promotes autodimerization of TRAF6, 293 IL-1RI cells were transfected with FLAG-tagged and/or V5-tagged TRAF6 in the absence or presence of PINK1 and then stimulated with IL-1β. Immunoblot analysis of anti-FLAG immunocomplexes with anti-V5 antibodies revealed that TRAF6 autodimerizes weakly in the absence of PINK1 (Fig. 3a). However, overexpression of PINK1 led to the potentiation of TRAF6 autodimerization in 293 IL-1RI cells upon IL-1β treatment (Fig. 3a).
Fig. 3.
PINK1 increases TRAF6 autodimerization. a 293 IL-1RI cells were transfected with Flag-TRAF6, V5-TRAF6 s, or Myc-PINK1, either alone or in combination, for 42 h, and treated with 10 ng/ml IL-1β for the indicated times. Immunoprecipitation of cell lysates was performed with anti-FLAG antibody, and the immunocomplexes were immunoanalyzed with anti-V5 antibody. Equal loading of samples was confirmed with an anti-actin antibody. The relative binding affinities of samples were quantified, and are denoted below the upper panel. b 293 IL-1RI cells were co-transfected with Flag-TRAF6 and V5-TRAF6 alone or together with either Myc-tagged wild-type PINK1 (WT) or its kinase-deficient mutant (KD) for 42 h, and treated with 10 ng/ml IL-1β for 15 min. Cell extracts were immunoprecipitated with anti-V5 antibody, and the immunocomplexes were analyzed by immunoblotting with anti-Flag antibody. The asterisk indicates IgG heavy chains (a, b)
Next, we investigated whether dimerization of TRAF6 depends on the kinase activity of PINK1 with IL-1β treatment. Either Myc-tagged wild-type PINK1 or its dominant negative mutant was introduced into 293 IL-1RI cells along with FLAG- and V5-tagged TRAF6, and immunoprecipitation of cell lysates was performed with anti-V5-antibodies. Immunoblot analysis with anti-Flag antiserum revealed that TRAF6 autodimerization increased by 2.6-fold in the presence of wild-type PINK1 (Fig. 3b), but no such increase occurred in the presence of the dominant negative PINK1 mutant. These results suggest that PINK1 positively regulates TRAF6 autodimerization, which appears to be dependent upon the kinase activity of PINK1.
PINK1 stimulates autoubiquitination of TRAF6
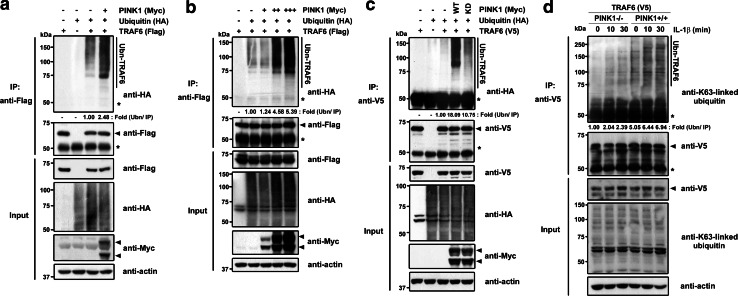
Considering the importance of TRAF6 ubiquitination in IL-1 signaling [23], we examined whether PINK1 can also provoke the polyubiquitination of TRAF6 after enhancing its autodimerization. We co-transfected HEK293 cells with V5-TRAF6 and HA-ubiquitin, with or without Myc-PINK1, and compared the ubiquitination patterns of TRAF6 in the absence or presence of PINK1. As shown in Fig. 4a, an in vivo ubiquitination assay of cell lysates revealed that the presence of PINK1 caused a significant increase in TRAF6 ubiquitination. The positive effect of PINK1 on TRAF6 polyubiquitination was shown to be dose-dependent (Fig. 4b). Moreover, wild-type PINK1 promoted TRAF6 ubiquitination, but the kinase-deficient PINK1 mutant did not (Fig. 4c). These results suggest that PINK1 stimulates the autoubiquitination of TRAF6.
Fig. 4.
PINK1 increases TRAF6 autoubiquitination. a HEK293 cells were transfected with HA-ubiquitin, Flag-TRAF6, or Myc-PINK1, either alone in combination, for 48 h, as indicated. Cell lysates were immunoprecipitated with anti-Flag antibody, followed by immunoblotting with an anti-HA antibody. The proper expression of transiently transfected proteins was confirmed by immunoblotting with anti-HA, anti-Flag, or anti-Myc antibodies. The asterisk indicates IgG heavy chains, and actin served as a loading control. b HEK293 cells were co-transfected with HA-ubiquitin or Flag-TRAF6 alone, and together with increasing amounts of Myc-PINK1 for 48 h. Immunoprecipitation of cell lysates was performed with an anti-Flag antibody, followed by immunoblot analysis with an anti-HA antibody. c HEK293 cells were transfected with either HA-ubiquitin or Flag-TRAF6, either alone or in combination with Myc-tagged wild-type PINK1 (WT) or its kinase-defective mutant (KD), for 48 h. Cell lysates were immunoprecipitated with anti-Flag antibody, and subjected to immunoblotting with anti-HA antibody. d Either PINK1−/− or +/+ MEFs were transfected with V5-TRAF6 for 42 h, and treated with 50 ng/ml IL-1β for the indicated times. Cell lysates were immunoprecipitated (IP) with anti-V5 antibody, and immunoblotted with anti-Lys63-specific ubiquitin antibody, as indicated
We then examined whether the PINK1-induced increase in Lys63-dependent autoubiquitination of TRAF6 actually occurs under physiological conditions by comparing the extent of IL-1β-stimulated ubiquitination of TRAF6 in PINK1−/− and PINK1+/+ MEFs. After PINK1−/− and +/+ MEFs were transiently transfected with V5-TRAF6, immunoprecipitation of cell lysates was performed with anti-V5 antibodies, followed by immunoblotting with anti-Lys63-specific ubiquitin antibodies. As shown in Fig. 4d, TRAF6 ubiquitination increased slightly after 10 min of IL-1β stimulation in PINK1−/− MEFs and decreased thereafter (Fig. 4d). By contrast, TRAF6 autoubiquitination increased significantly in PINK1 +/+ MEFs with IL-1β stimulus, and this increase was sustained for 30 min after IL-1β stimulation. Together, these results show that PINK1 promotes TRAF6 activity by facilitating the autodimerization and Lys63-dependent autoubiquitination of TRAF6 in PINK1−/− and PINK1 +/+ MEFs as well as in HEK293 and 293 IL-1RI cells.
PINK1 enhances formation of the TRAF6–TAK1 complex
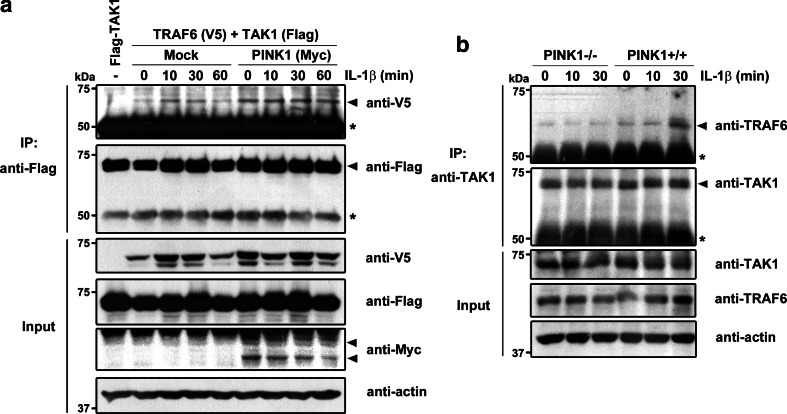
Lys63-linked polyubiquitination of IRAK1 and TRAF6 facilitates recruitment of the TAK1 and IKK complexes [24]. TAK1 then becomes a ubiquitination target of TRAF6 with help from the E2 enzyme Ubc13-Uev1A. In addition, IL-1β treatment induces recruitment of endogenous TAK1 to the TRAF6 complex, a crucial step for the activation of TAK1 catalytic activity, and subsequently stimulates NF-κB and MAPK activation. These previous findings led us to investigate whether PINK1 affects the formation of the TAK1–TRAF6 complex upon IL-1β stimulation. We transiently transfected 293 IL-1RI cells with V5-TRAF6 and Flag-TAK1, alone or together with Myc-PINK1, and treated them with IL-1β. Immunoblot analysis of anti-Flag immunocomplexes with anti-V5 antibodies revealed that the TRAF6-TAK1 complex forms in the absence of the IL-1β stimulus (Fig. 5a). We also found that, whereas IL-1β increases the extent of TRAF6–TAK1 interaction, PINK1 expression enhances the formation of this complex to much higher levels than those seen in the control cells, and maintains this complex more stably following an IL-1β stimulus (Fig. 5a). Furthermore, the association of TRAF6 with TAK1 peaked at 10 min in the absence of PINK1 and persisted 30 min after stimulation, whereas in the presence of PINK1, the complex was stably maintained for up to 60 min after IL-1β stimulation (Fig. 5a).
Fig. 5.
PINK1 enhances formation of the TRAF6-TAK1 complex. a 293 IL-1RI cells were transfected with FLAG-TAK1, V5-TRAF6, Myc-PINK1, either alone or in combination, for 42 h, and treated with 10 ng/ml of IL-1β for the indicated times. Cell lysates were immunoprecipitated with anti-FLAG antibody, and the immunocomplexes were analyzed by immunoblotting with anti-V5 antibodies. The relative levels of complex were quantified and are denoted below the upper panel. b Either PINK1−/− or +/+ MEFs were treated with 50 ng/ml IL-1β for the indicated times. Cell lysates were immunoprecipitated with anti-TAK1 antibody, and immunoblotted with anti-TRAF6 antibody. An asterisk indicates IgG heavy chains. Equal sample loading was confirmed by immunoblotting with anti-actin antibody
To understand whether PINK1 actually stimulates the binding of endogenous TRAF6 to endogenous TAK1, we compared the pattern of the formation and levels of the protein complex in response to IL-1β stimulation in PINK1−/− and PINK1 +/+ MEFs. The two MEF types were treated with 50 ng/ml IL-1β, and immunoprecipitation of cell lysates with anti-TAK1 antibodies was performed. Immunoblotting of the complex with anti-TRAF6 antibodies revealed that the association occurs even in the absence of IL-1β stimulation (Fig. 5b). In addition, endogenous TRAF6 bound more strongly to endogenous TAK1 at 10 min after IL-1β stimulation in PINK1 +/+ MEFs than in PINK1−/− MEFs (Fig. 5b). Together, these results suggest that PINK1 facilitates the interaction of TRAF6 and TAK1, which may consequently promote IL-1β-mediated downstream signaling.
PINK1 facilitates polyubiquitination of TAK1
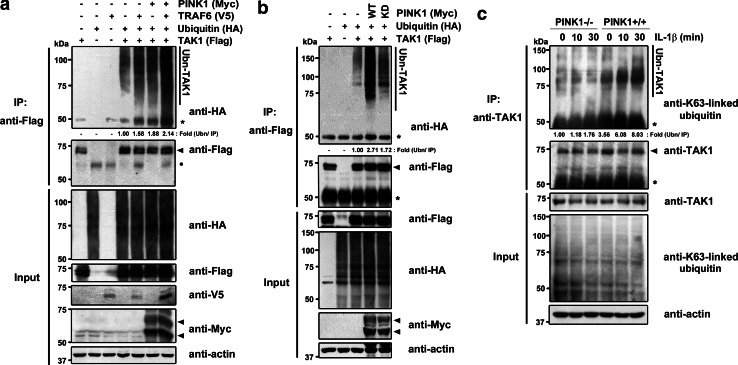
Several groups have recently shown that Lys63-linked polyubiquitination of TAK1 is required for proper TNFα, IL-1β, and TGF-β signaling [25, 26]. The polyubiquitination of TAK1 at Lys-34, catalyzed by TRAF6, activates TAK1 kinase activity. However, it remains unclear whether TAK1 is ubiquitinated in response to physiological stimuli that activate NF-κB, and if so, the functional role of TAK1 ubiquitination during cytokine-induced inflammatory signaling is unknown. To address this question, we first examined whether PINK1 facilitates TAK1 polyubiquitination. After HEK293 cells were transfected with Myc-PINK1, Flag-TAK1, V5-TRAF6, or HA-ubiquitin alone or in combination, cell lysates were immunoprecipitated with anti-Flag IgG, and the immunocomplexes were analyzed by immunoblotting with an antibody against HA tag. As reported previously, TRAF6 overexpression caused an increase in TAK1 polyubiquitination (Fig. 6a). In addition, PINK1 caused the up-regulation of TAK1 polyubiquitination. Moreover, the addition of both TRAF6 and PINK1 dramatically enhanced TAK1 polyubiquitination more than transfection with either of these molecules alone (Fig. 6a). This marked effect on TAK1 polyubiquitination was not observed with the kinase-defective PINK1 mutant (Fig. 6b).
Fig. 6.
PINK1 facilitates TAK1 polyubiquitination. a Where specified, HEK293 cells were transfected with HA-ubiquitin, Flag-TAK1, V5-RAF6, or Myc-PINK1, either alone or in combination, for 48 h. Cell lysates were immunoprecipitated with anti-Flag antiserum, followed by immunoblotting with anti-HA antibody. The proper expression of transfected proteins in each cell lysate was confirmed by immunoblot analysis with the indicated antibodies. Asterisk and closed circle indicate IgG heavy chain and non-specific bands, respectively, and actin served as a loading control. b HEK293 cells were co-transfected with HA-ubiquitin plus Flag-TAK1, either alone or together with Myc-tagged wild-type PINK1 (WT) or its dominant-negative mutant (KD), for 48 h. Anti-Flag immunoprecipitates and cell lysates were analyzed by immunoblotting with anti-HA antibody. c PINK1−/− or +/+ MEFs were treated with 50 ng/ml IL-1β for the indicated times. Cell lysates were immunoprecipitated with anti-TAK1 antibody, and immunoblotted with anti-Lys63-specific ubiquitin antibody
We then addressed whether PINK1 can facilitate Lys63-linked polyubiquitin-conjugation to endogenous TAK1 in response to a physiological stimulus. When PINK1 +/+ MEFs were treated with IL-1β, there was an increase of Lys63-dependent polyubiquitination of TAK1, reaching its maximum at 30 min of post-stimulation. However, TAK1 polyubiquitination was severely reduced without a peak in PINK1−/− MEFs treated with IL-1β (Fig. 6c). These data suggest that PINK1 regulates TAK1 by enhancing its kinase activity in IL-1β signaling.
PINK1 potentiates TAK1 autophosphorylation
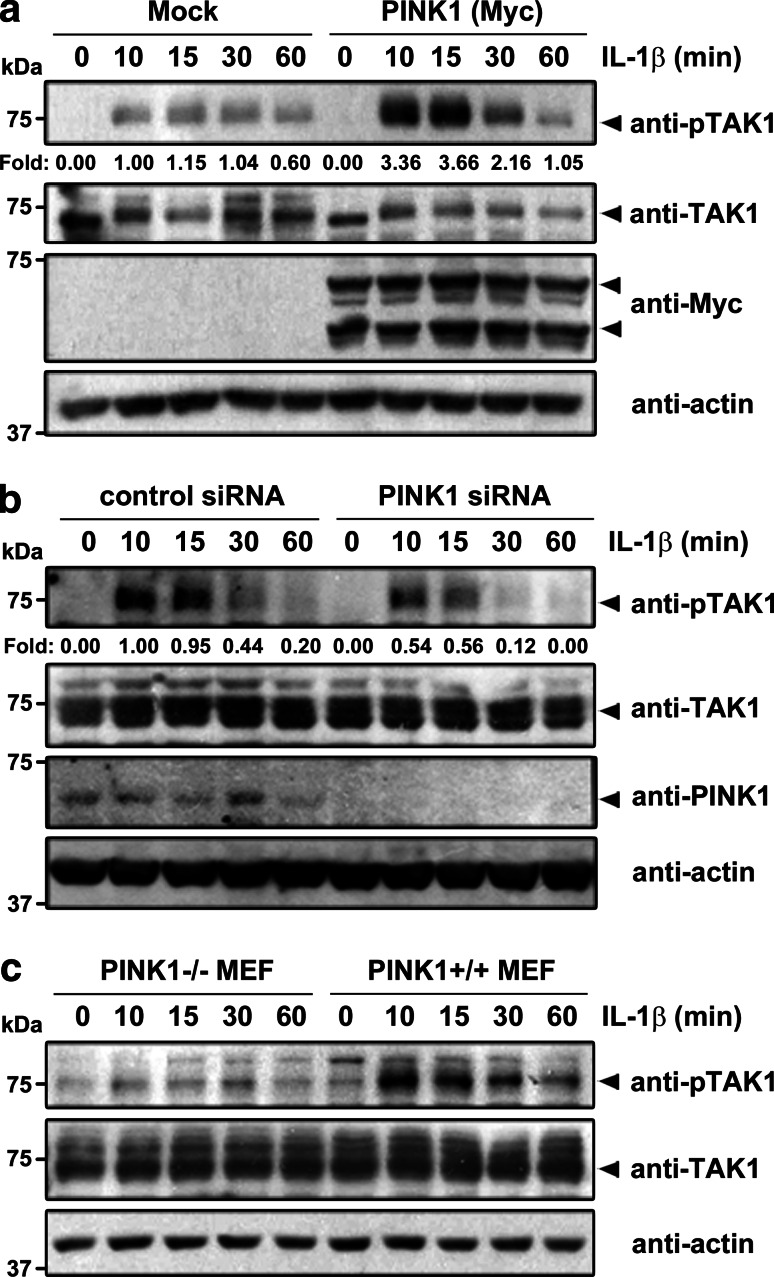
We next investigated the effect of PINK1 overexpression on basal and IL-1β-induced TAK1 activity. 293 IL-1RI cells were mock-transfected or transfected with Myc-tagged PINK1 and then treated with IL-1β. Consistent with previous reports that TAK1 activity is closely associated with the phosphorylation status at Thr187 [27, 28], immunoblot analysis with phospho-specific TAK1 (Thr187) antibodies revealed no significant activation of TAK1 in the absence of IL-1β. The addition of IL-1β led to a remarkable induction of TAK1 activity in 293 IL-1RI cells, which reached a maximum after 30 min of stimulation. Importantly, PINK1 overexpression enhanced baseline TAK1 activity and potentiated the extent of IL-1β-induced TAK1 activity by 3.6-fold (Fig. 7a). Next, we sought to determine the physiological significance of PINK1 in the regulation of TAK1 activity using PINK1-specific RNAi. In control cells, PINK1-siRNA efficiently reduced PINK1 protein levels (Fig. 7b). Following IL-1β stimulation, immunoblot analysis with phospho-TAK1 antiserum revealed increased and sustained TAK1 activity for up to 30 min in control cells treated with non-targeting siRNA (Fig. 7b). However, TAK1 activity was significantly diminished and only transiently maintained in PINK1-siRNA-treated cells (Fig. 7b). In addition to the knock-down effect of PINK1, we further validated, using PINK1−/− and +/+ MEFs, whether TAK1 phosphorylation is affected by endogenous PINK1. Immunoblot analysis of two cell lysates demonstrated that TAK1 phosphorylation was increased significantly in PINK1 +/+ MEFs compared to PINK1−/− MEFs (Fig. 7c).
Fig. 7.
PINK1 enhances TAK1 autophosphorylation. a 293 IL-1RI cells were either mock-transfected or transfected with Myc-PINK1 for 42 h and treated with 10 ng/ml IL-1β for the indicated times. Cell lysates were then immunoblotted with anti-TAK1 or anti-phospho-TAK1-T187 antibodies. Actin served as a loading control. The relative level of phopho-TAK1 was quantified and is denoted below the upper panel. b 293 IL-1RI cells were transfected with either scramble control siRNA, or siRNA against PINK1, for 42 h and then treated with 10 ng/ml IL-1β for the indicated times. c PINK1−/− or +/+ MEFs were treated with 50 ng/ml IL-1β for the indicated times. The levels of unphosphorylated TAK1 and TAK1 phosphorylated at Thr187 were determined by immunoblotting with anti-TAK1 or anti-phospho-TAK1 antibodies
In addition to TAK1, we determined whether PINK1 stimulates the phosphorylation of TRAF6 in response to IL-1β. After 293 IL-1RI cells were transfected with V5-TRAF6, Myc-hPINK1-WT or Myc-hPINK1-KD alone or in combination for 42 h followed by treatment with IL-1β for 15 min, cell lysates were immunoprecipitated with anti-V5 (Supple. S1). Immunoblotting of the complexes with anti-phospho-threonine antibody showed that there was no significant TRAF6 band corresponding to the size of its phosphorylated form (Supple. S1). These data suggest that PINK1 has no effect on TRAF6 phosphorylation.
Overall, these data suggest that PINK1 is critical for TAK1 activation via the up-regulation of TAK1 phosphorylation.
PINK1 directly phosphorylates TAK1 in vitro
We next investigated whether the kinase activity of PINK1 is required for the positive regulation of TRAF6 and TAK1. We firstly checked whether hPINK1-KD is still able to associate with TRAF6 or TAK1 under IL-1β treatment. After cells were transfected with Flag-TRAF6 alone or together with either Myc-hPINK1-WT or Myc-hPINK1-KD mutant, immunoblot analysis of anti-Flag immunocomplexes with anti-Myc antiserum revealed that, compared to wild-type PINK1, kinase-defective PINK1 mutant still binds to TRAF6 and TAK1 to a similar extent (Suppl. S2a and S2b).
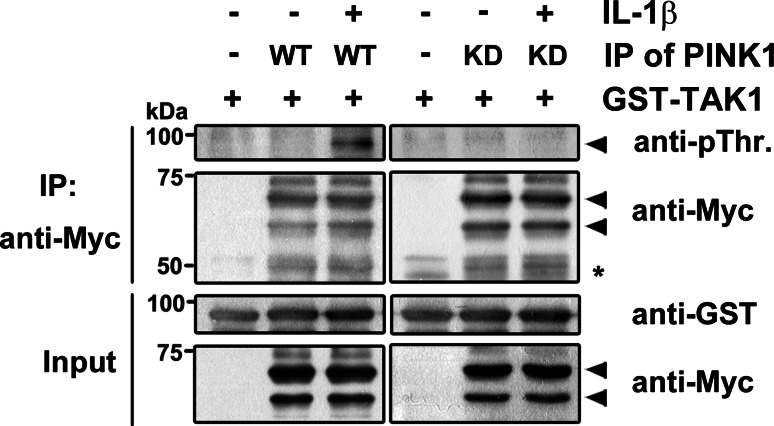
Based on the finding that PINK1 stimulates the phosphorylation of TAK1 (Fig 7a), but not TRAF6 (Suppl. 1), we further examined whether PINK1 directly phosphorylates TAK1 using in vitro kinase assay. After cells were transfected with Myc-hPINK1-WT or Myc-hPINK1-KD followed by IL-1β treatment, anti-Myc-PINK1 immunocomplexes were incubated with bacterially recombinant TAK1 fused with GST. Immunoblotting of reaction mixtures with anti-phospho-threonine antiserum revealed that there is a clear band corresponding to the size of phosphorylated GST-TAK1 in the presence of wild-type PINK1 (Fig. 8). As a control, this phospho-TAK1 band was completely abolished in the presence of kinase-defective PINK1 mutant (Fig. 8), suggesting that PINK1 directly phosphorylates TAK1. Taken together, these data suggest that PINK1 exert its positive effect on the regulation of TAK1 activity through the direct phosphorylation of TAK1.
Fig. 8.
PINK1 directly phosphorylates TAK1 in vitro. 293 IL-1RI cells were transfected with Myc-hPINK1-WT or Myc-hPINK1-KD for 42 h, and left untreated or stimulated with 10 ng/ml IL-1β for 15 min. For in vitro kinase assay, anti-Myc immunoprecipitates prepared from cell lysates were mixed with bacterially expressed GST-fused TAK1 and 6 mM of ATP, and the reaction products was then probed with anti-phospho-threonine antibody
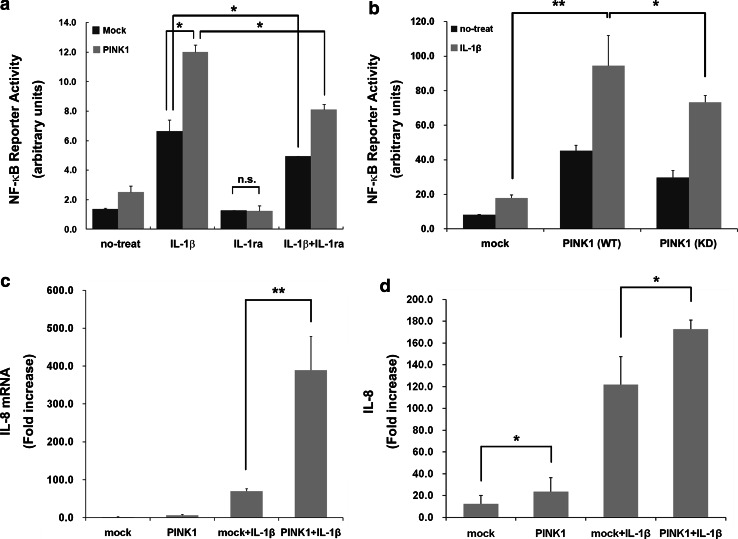
PINK1 potentiates NF-κB activation and IL-8 production in response to IL-1β
Based on the finding that TAK1 is required for TNFR1-, IL-1R-, and TLR-induced NF-κB activation [25, 26], we investigated the effect of PINK1 on NF-κB activity in the absence or presence of stimulation by IL-1β. Using an NF-κB luciferase reporter gene assay, we found that PINK1 dramatically enhanced the activity of NF-κB in 293 IL-1RI cells (Fig. 9a). In addition, wild-type PINK1 stimulated NF-κB in a dose-dependent manner (data not shown), irrespective of IL-1β stimulation (Fig. 9a). To further verify whether PINK1-induced up-regulation of NF-κB activity occurs through IL-1R-dependent signaling pathway, we tested the effect of interleukin-1 receptor antagonist (IL-1ra) on PINK1 action (Fig. 9a). After transfected with Myc-PINK1-WT or Myc-PINK1-KD, cells were then treated with interleukin-1 receptor antagonist (IL-1ra) alone or together with IL-1β. As shown in Fig. 9a, addition of IL-1ra abolished the stimulatory effect of PINK1 under IL-1β stimulation. Consistent with the previous finding, kinase-defective PINK1 mutant did not activate the NF-κB response to IL-1β (Fig. 9b). These results suggest that PINK1 positively regulates NF-κB activity, and this regulation is closely linked to the kinase activity of PINK1.
Fig. 9.
PINK1 potentiates production of IL-8 as well as NF-κB activation in response to IL-1β. a 293 IL-1RI cells were transfected with plasmids encoding NF-κB-responsive luciferase reporter and control Renilla luciferase reporter, either alone or together with Myc-hPINK1-WT, for 36 h, as indicated. Cells were then pretreated with 1 μg/ml of IL-1ra for 2 h, and left untreated or treated with 50 ng/ml IL-1β for 12 h. The relative luciferase activity was measured and normalized to Renilla activity. Error bars indicate ± SD in triplicate experiments. **p < 0.01 versus mock. b 293 IL-1RI cells were transfected with plasmids encoding NF-κB-responsive luciferase reporter vector and control Renilla luciferase reporter, either alone or together with Myc-tagged wild-type PINK1 (WT) or its dominant negative mutant (KD), for 36 h. Cells were left untreated or stimulated with 50 ng/ml IL-1β for 6 h. **p < 0.01 compared to IL-1β-treated mock sample. c 293 IL-1RI cells were either mock-transfected or transfected with Myc-PINK1 for 36 h, and treated with 50 ng/ml IL-1β for 12 h, as indicated. Subsequently, total RNA was extracted and transcribed to produce cDNA. Real-time PCR analysis of IL-8 mRNA was performed with IL-8 primers. **p < 0.01 compared to IL-1β-treated mock samples. d The amount of IL-8 protein in the media from (c) was measured by ELISA. **p < 0.01 compared to IL-1β-treated mock. The results are representative of three independent experiments
Lastly, we investigated whether the positive effect of PINK1 on IL-1R signaling events leads to the production and subsequent secretion of inflammatory cytokines, such as IL-8, in 293 IL-1RI cells, in the absence or presence of IL-1β stimuli. Consistent with the stimulatory effect of PINK1 on the upstream components of IL-1R signaling, PINK1 enhanced the levels of IL-8 mRNA as determined by real-time PCR (Fig. 9c) as well as the secretion of IL-8 protein as determined by ELISA (Fig. 9d). These results suggest that PINK1 acts as a positive regulator of IL-1β signaling, leading to the up-regulation of inflammatory cytokines in response to IL-1β stimulation.
Discussion
The loss of dopaminergic neurons is frequently observed in association with signs of inflammation such as activation of microglia, astrogliosis, and lymphocyte infiltration [10, 11]. Moreover, activated microglial cells, and proinflammatory mediators, including nitric oxide (NO), reactive oxygen species (ROS), IL-1β, TNF-α, IL-6, and chemokines have been observed in the substantia nigra of postmortem tissue from PD patients and in brain tissue from PD animal models. In vivo studies have also demonstrated that serum and cerebrospinal fluid of PD patients have increased levels of IL-1β, TNF-α, and IL-2, as well as increased levels of CD4 + and CD8 + T lymphocytes [29, 30], indicating peripheral activation of lymphocytes. These inflammatory events are thought to contribute directly to the pathogenesis of PD, based on the following reports. Administration of minocycline, which induces the paralysis of microglia, decreases the loss of dopaminergic neurons and improves the symptoms of PD in MPTP-treated animals [31]. In another model of PD, intracranial injection of lipopolysaccharide (LPS), a ligand of TLR4, was also shown to activate inflammation and subsequently induce the loss of dopaminergic neurons [32]. Those reports demonstrate the close relationship between brain inflammation and PD pathogenesis; however, the detailed mechanism and the signaling pathway(s) involved have not been clearly elucidated. Our present study provides evidence that PINK1, which is linked to familial PD, affects the IL-1β-initiated downstream signaling and inflammatory events. We show that PINK1 directly interacts with two members of the IL-1β-mediated downstream signaling pathway, TRAF6 and TAK1, and positively regulates their activation leading to enhanced IL-1β-mediated cytokine production.
Several PD-linked gene products are also known to associate in some manner with inflammatory signaling events. For example, parkin directly activates microglia and is neurotoxic to dopamine-producing neurons [33]. Once activated, microglial cells produce a wide variety of inflammatory mediators that promote an innate immune response, including inflammatory cytokines, chemokines, prostaglandins and leukotrienes, NO, ROS, and glutamate. In addition, α-synuclein may act as an initiator of inflammation in PD. Although α-synuclein-containing aggregates are formed intracellularly, we previously demonstrated that α-synuclein can be detected extracellularly [34]. Although the mechanisms responsible for the escape of α-synuclein from cells are not fully understood, extracellular α-synuclein activates microglia and induces production of proinflammatory mediators [35], providing a plausible link between α-synuclein pathology and initiation of a potentially pathogenic inflammatory response. Moreover, chronic administration of low-dose LPS triggers very similar neuroinflammatory and oxidative stress responses in the substantia nigra of parkin-deficient mice, which develop delayed and selective degeneration of DA neurons in the substantia nigra. Furthermore, basal and inflammatory cytokine-induced NF-κB activity is reduced in PINK1-deficient MEFs [36, 37]. The present work provides additional evidence of a direct interaction between PINK1 and TRAF6 and TAK1, and indicates that these interactions result in positive regulation of IL-1β-mediated downstream signaling. We also identified that PINK1 directly phosphorylates TAK1 (Fig. 8), but not TRAF6 (Suppl. 1). However, the interaction between PINK1 and TRAF6 is not dependent on the kinase activity of PINK1, and kinase-deficient PINK1 mutant still binds to TRAF6 (Suppl. 2a). Based on this finding and the previous observation that wild-type PINK1, but not its kinase-defective mutant, enhances the auto-dimerization or auto-ubiquitination of TRAF6 upon IL-1β treatment, we speculate that other unidentified protein(s) which selectively binds to wild-type PINK1, but not to kinase-dead PINK1 mutant, might contribute up-regulation of auto-dimerization or auto-ubiquitination of TRAF6. Or else, although kinase-deficient PINK1-KD mutant having double substitutions at K219A and D362A still binds to TRAF6 well, the conformational change of PINK1 after binding to TRAF6 might be required for the positive regulation of TRAF6 activity. Compared with wild-type PINK1, kinase-deficient PINK1-KD mutant could not carry out this process properly, which subsequently fails to stimulate TRAF6 activity.
We also demonstrate that PINK1 regulates NF-κB activity in inflammatory events involving cytokine release, suggesting that many diverse ‘loss-of-function’ mutations of PINK1 might contributes to the pathogenesis of PD.
While PINK1 contains a catalytic serine-threonine kinase domain and has autophosphorylation activity in vitro [38], little is known regarding its binding partners and/or putative substrates, largely due to technical difficulties in testing the kinase activity of PINK1 in vivo. Nonetheless, recombinant PINK1 directly phosphorylates TRAP1 in vitro, and PINK1 overexpression enhances H2O2-induced TRAP1 phosphorylation in PC12 cells [5]. In addition, TRAP1 depletion significantly enhances mitochondrial cytochrome c release and apoptosis in PC12 cells subjected to oxidative stress, indicating that TRAP1 is a substrate of PINK1 and acts as a key component mediating the cytoprotective action of PINK1. Other chaperones, Cdc37/Hsp90, have also been shown to interact with PINK1 [39]. Moreover, PINK1 interacts with HtrA2, a serine protease residing in the mitochondrial intermembrane space [40], and forms PINK1-HtrA2 complexes in mitochondria. Omi/HtrA2 activates pro-apoptotic proteins upon its release to the cytosol from damaged mitochondria. Although it is unclear whether HtrA2 is the direct target of PINK1 phosphorylation, interaction with PINK1 enhances HtrA2 phosphorylation in response to the activation of the p38 stress-signaling pathway. In addition to those roles of PINK1 and its various binding partners, the current work demonstrates that PINK1 directly binds to TRAF6 and TAK1, and consequently modulates IL-1β-mediated signaling and the downstream inflammatory response. However, it remains to be determined whether PINK1 directly phosphorylates TRAF6 and/or TAK1 as substrates.
Recently, PINK1 and parkin were shown to function in a common pathway to maintain the integrity of the mitochondrial network by preventing mitochondrial dysfunction and promoting degradation of defective mitochondria through autophagy. PINK1 directly phosphorylates parkin, resulting in a striking redistribution of parkin from the cytosol to mitochondria [41] and facilitating the selective clearance of depolarized mitochondria via autophagy [42]. In contrast to these findings, we recently demonstrated that PINK1 and parkin affect each other’s stability, solubility, and tendency to form cytoprotective aggresomes [20]. We also validated the functional relevance of this mutual interaction under pathological PD conditions [43]. These different patterns of mutual interaction observed between parkin and PINK1 could be explained by different cellular contexts [43]. Whereas PINK1 accumulation accompanied by enhanced parkin aggregate formation occurs under PD-mimicking conditions, the unique mitochondrial localization of these proteins and the resulting effects on mitochondrial dynamics may occur under normal cellular conditions [43]. Supporting this premise, the current work presents additional evidence that PINK1 contributes to the pathogenesis of PD by affecting the accompanying inflammatory response.
With regard to the subcellular location of PINK1, increasing evidence show both mitochondrial and cytosolic co-localization [3, 5, 7, 44]. These results demonstrate that full-length PINK1 translocates to the mitochondria depending on the mitochondria membrane potential, and its disruption causes the full-length PINK1 to accumulate in the mitochondria. On the other hand, cleaved forms of PINK1 are primarily localized to the cytosol. PINK1 is localized to mitochondrial membranes via an N-terminal targeting motif. In addition, overexpression of cytosolic PINK1 lacking the mitochondria localization signal, exhibits protective function against MPTP toxicity in mice and in cell culture. These reports all point to PINK1 having a dual localization and possibly two different functions, depending on the subcellular compartment [44, 45]. As for the functional roles of PINK1 in the cytoplasm, parkin, PINK1, and DJ-1 are shown to form a complex mainly within the cytosol, which then promotes ubiquitination and degradation of parkin substrates in neuroblastoma cells [46]. Furthermore, PINK1 exerts its cytoprotective function not only in mitochondria but also in the cytoplasm through activation of mTORC2 and Akt [47]. Besides, the current work provides an additional role of PINK1 within the cytoplasm to affect IL-1β-mediated downstream signaling.
Several binding partners of TRAF6 and TAK1 have been identified and are known to regulate their function or to be substrates. For example, TRAF6, a member of a family of RING domain ubiquitin ligases, binds to and catalyzes Lys-63-linked polyubiquitination to IKK [23]. TAK1 binds to adapter proteins TAK1-associated binding protein-1, -2, and -3 (TAB 1, 2, and 3) for its activation [48]. The requirement of TAB 1 for activation of TAK1 appears to depend on cellular stimuli, such as osmotic shock and not on cytokine-evoked activation of TAK1 [49]. The TAB 2/3 proteins were also identified by their association with TAK1 and shown to be important for TAK1 activation in response to IL-1β or TNF-α. In addition to these proteins, the present work demonstrates that PINK1 binds to TRAF6 and TAK1, and is thereby linked to IL-1β-mediated inflammatory signaling events.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank J. Chung and G. Takaesu for providing plasmids. We are also grateful to G. Takaesu for providing 293 IL-1RI cells and to J. Shen for PINK1−/− and +/+ MEFs. This study was supported by grants from the Brain Research Center of the 21st Century Frontier Research Program Technology (2009 K-001251 to K.C.C.) and from the Korea Science and Engineering Foundation (KOSEF) through the National Research Laboratory Program (2010-0018916 to K.C.C.) funded by the Ministry of Education, Science and Technology (MEST) of the Republic of Korea. This work was also partly supported by grants from KOSEF (2012-0000810 to K.C.C.) and from the Korea Health 21 R&D Project (A092004 and A111653 to K.C.C.), Ministry of Health, Welfare and Family Affairs.
Abbreviations
- DAPI
4, 6-Diamidino-2-phenylindole
- DMEM
Dulbecco’s modified Eagle’s medium
- ECL
Enhanced chemiluminescence
- FBS
Fetal bovine serum
- HEK293
Human embryonic kidney 293
- IKK
IκB kinase
- IL-1
Interleukin-1
- IL-1R
IL-1 receptor
- IRAK1
IL-1R-associated kinase-1
- LPS
Lipopolysaccharide
- MEFs
Mouse embryonic fibroblasts
- Myd88
Myeloid differentiation marker
- NF-κB
Nuclear factor-kappa B
- NO
Nitric oxide
- PD
Parkinson’s disease
- PINK1
PTEN-induced putative kinase 1
- ROS
Reactive oxygen species
- TAK1
TGF-β activated kinase-1
- TGF-β
Transforming growth factor-β
- TNF
Tumor necrosis factor
- TRAF6
TNF receptor-associated factor 6
- GST
Glutathion S-transferase
References
- 1.Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 2.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 3.Silvestri L, Caputo V, Bellacchio E, Atorino L, Dallapiccola B, Valente EM, Casari G. Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet. 2005;15:3477–3492. doi: 10.1093/hmg/ddi377. [DOI] [PubMed] [Google Scholar]
- 4.Beilina A, Van Der Brug M, Ahmad R, Kesavapany S, Miller DW, Petsko GA, Cookson MR. Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability. Proc Natl Acad Sci USA. 2005;19:5703–5708. doi: 10.1073/pnas.0500617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ. Hereditary early onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;21:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 7.Petit A, Kawarai T, Paitel E, Sanjo N, Maj M, Scheid M, Chen F, Gu Y, Hasegawa H, Salehi-Rad S, Wang L, Rogaeva E, Fraser P, Robinson B, St George-Hyslop P, Tandon A. Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J Biol Chem. 2005;7:34025–34032. doi: 10.1074/jbc.M505143200. [DOI] [PubMed] [Google Scholar]
- 8.Kaltschmidt B, Widera D, Kaltschmidt C. Signaling via NF-kappaB in the nervous system. Biochim Biophys Acta. 2005;30:287–299. doi: 10.1016/j.bbamcr.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 10.Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci Lett. 1996;211:13–16. doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- 11.Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from Parkinsonian patients. Neurosci Lett. 1994;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- 12.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/S1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 13.Janssens S, Burns K, Tschopp J, Beyaert R. Regulation of interleukin-1- and lipopolysaccharide-induced NF-kappaB activation by alternative splicing of MyD88. Curr Biol. 2002;19:467–471. doi: 10.1016/S0960-9822(02)00712-1. [DOI] [PubMed] [Google Scholar]
- 14.Gan L, Li L. Regulations and roles of the interleukin-1 receptor associated kinases (IRAKs) in innate and adaptive immunity. Immunol Res. 2006;35:295–302. doi: 10.1385/IR:35:3:295. [DOI] [PubMed] [Google Scholar]
- 15.Inoue J, Gohda J, Akiyama T. Characteristics and biological functions of TRAF6. Adv Exp Med Biol. 2007;597:72–79. doi: 10.1007/978-0-387-70630-6_6. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is an ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;19:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 17.Martin MU, Wesche H. Summary and comparison of the signaling mechanisms of the Toll/interleukin-1 receptor family. Biochim Biophys Acta. 2002;11:265–280. doi: 10.1016/S0167-4889(02)00320-8. [DOI] [PubMed] [Google Scholar]
- 18.Huang Q, Yang J, Lin Y, Walker C, Cheng J, Liu ZG, Su B. Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat Immunol. 2004;5:98–103. doi: 10.1038/ni1014. [DOI] [PubMed] [Google Scholar]
- 19.Su J, Richter K, Zhang C, Gu Q, Li L. Differential regulation of interleukin-1 receptor associated kinase 1 (IRAK1) splice variants. Mol Immunol. 2007;44:900–905. doi: 10.1016/j.molimm.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Um JW, Stichel-Gunkel C, Lübbert H, Lee G, Chung KC. Molecular interaction between parkin and PINK1 in mammalian neuronal cells. Mol Cell Neurosci. 2009;40:421–432. doi: 10.1016/j.mcn.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Kishimoto K, Matsumoto K, Ninomiya-Tsuji J. TAK1 mitogen-activated protein kinase kinase kinase is activated by autophosphorylation within its activation loop. J Biol Chem. 2000;10:7359–7364. doi: 10.1074/jbc.275.10.7359. [DOI] [PubMed] [Google Scholar]
- 22.Cao Z, Henzel WJ, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science. 1996;23:1128–1131. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- 23.Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;13:351–361. doi: 10.1016/S0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 24.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;15:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 25.Fan Y, Yu Y, Shi Y, Sun W, Xie M, Ge N, Mao R, Chang A, Xu G, Schneider MD, Zhang H, Fu S, Qin J, Yang J. Lysine 63-linked polyubiquitination of TAK1 at lysine 158 is required for tumor necrosis factor alpha- and interleukin-1beta-induced IKK/NF-kappaB and JNK/AP-1 activation. J Biol Chem. 2010;19:5347–5360. doi: 10.1074/jbc.M109.076976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamazaki K, Gohda J, Kanayama A, Miyamoto Y, Sakurai H, Yamamoto M, Akira S, Hayashi H, Su B, Inoue J. Two mechanistically and temporally distinct NF-kappaB activation pathways in IL-1 signaling. Sci Signal. 2009;20:ra66. doi: 10.1126/scisignal.2000387. [DOI] [PubMed] [Google Scholar]
- 27.Kajino T, Ren H, Iemura S, Natsume T, Stefansson B, Brautigan DL, Matsumoto K, Ninomiya-Tsuji J. Protein phosphatase 6 down-regulates TAK1 kinase activation in the IL-1 signaling pathway. J Biol Chem. 2006;29:39891–39896. doi: 10.1074/jbc.M608155200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Y, Ge N, Xie M, Sun W, Burlingame S, Pass AK, Nuchtern JG, Zhang D, Fu S, Schneider MD, Fan J, Yang J. Phosphorylation of Thr-178 and Thr-184 in the TAK1 T-loop is required for interleukin (IL)-1-mediated optimal NF-kappaB and AP-1 activation as well as IL-6 gene expression. J Biol Chem. 2008;5:24497–24505. doi: 10.1074/jbc.M802825200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hisanaga K, Asagi M, Itoyama Y, Iwasaki Y. Increase in peripheral CD4 bright + CD8 dull + T cells in Parkinson disease. Arch Neurol. 2001;58:1580–1583. doi: 10.1001/archneur.58.10.1580. [DOI] [PubMed] [Google Scholar]
- 30.Bas J, Calopa M, Mestre M, Molleví DG, Cutillas B, Ambrosio S, Buendia E. Lymphocyte populations in Parkinson’s disease and in rat models of parkinsonism. J Neuroimmunol. 2001;113:146–152. doi: 10.1016/S0165-5728(00)00422-7. [DOI] [PubMed] [Google Scholar]
- 31.Wu Q, Combs C, Cannady SB, Geldmacher DS, Herrup K. Beta-amyloid activated microglia induce cell cycling and cell death in cultured cortical neurons. Neurobiol Aging. 2000;21:797–806. doi: 10.1016/S0197-4580(00)00219-0. [DOI] [PubMed] [Google Scholar]
- 32.Castano A, Herrera AJ, Cano J, Machado A. Lipopolysaccha-ride intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J Neurochem. 1998;70:1584–1592. doi: 10.1046/j.1471-4159.1998.70041584.x. [DOI] [PubMed] [Google Scholar]
- 33.Frank-Cannon TC, Tran T, Ruhn KA, Martinez TN, Hong J, Marvin M, Hartley M, Treviño I, O’Brien DE, Casey B, Goldberg MS, Tansey MG. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J Neurosci. 2008;28:10825–10834. doi: 10.1523/JNEUROSCI.3001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sung JY, Park SM, Lee CH, Um JW, Lee HJ, Kim J, Oh YJ, Lee ST, Paik SR, Chung KC. Proteolytic cleavage of extracellular secreted alpha-synuclein via matrix metalloproteinases. J Biol Chem. 2005;280:25216–25224. doi: 10.1074/jbc.M503341200. [DOI] [PubMed] [Google Scholar]
- 35.Park JY, Kim KS, Lee SB, Ryu JS, Chung KC, Choo YK, Jou I, Kim J, Park SM. On the mechanism of internalization of alpha-synuclein into microglia: roles of ganglioside GM1 and lipid raft. J Neurochem. 2009;110:400–411. doi: 10.1111/j.1471-4159.2009.06150.x. [DOI] [PubMed] [Google Scholar]
- 36.Verstrepen L, Bekaert T, Chau TL, Tavernier J, Chariot A, Beyaert R. TLR-4, IL-1R and TNF-R signaling to NF-kappaB: variations on a common theme. Cell Mol Life Sci. 2008;65:2964–2978. doi: 10.1007/s00018-008-8064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akundi RS, Huang Z, Eason J, Pandya JD, Zhi L, Cass WA, Sullivan PG, Büeler H. Increased mitochondrial calcium sensitivity and abnormal expression of innate immunity genes precede dopaminergic defects in Pink1-deficient mice. PLoS ONE. 2011;6:e16038. doi: 10.1371/journal.pone.0016038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sim CH, Lio DS, Mok SS, Masters CL, Hill AF, Culvenor JG, Cheng HC. C-terminal truncation and Parkinson’s disease-associated mutations down-regulate the protein serine/threonine kinase activity of PTEN-induced kinase-1. Hum Mol Genet. 2006;15:3251–3262. doi: 10.1093/hmg/ddl398. [DOI] [PubMed] [Google Scholar]
- 39.Weihofen A, Ostaszewski B, Minami Y, Selkoe DJ. Pink1 Parkinson mutations, the Cdc37/Hsp90 chaperones and Parkin all influence the maturation or subcellular distribution of Pink1. Hum Mol Genet. 2008;17:602–616. doi: 10.1093/hmg/ddm334. [DOI] [PubMed] [Google Scholar]
- 40.Strauss KM, Martins LM, Plun-Favreau H, Marx FP, Kautzmann S, Berg D, Gasser T, Wszolek Z, Müller T, Bornemann A, Wolburg H, Downward J, Riess O, Schulz JB, Krüger R. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson’s disease. Hum Mol Genet. 2005;14:2099–2111. doi: 10.1093/hmg/ddi215. [DOI] [PubMed] [Google Scholar]
- 41.Kim Y, Park J, Kim S, Song S, Kwon SK, Lee SH, Kitada T, Kim JM, Chung J. PINK1 controls mitochondrial localization of Parkin through direct phosphorylation. Biochem Biophys Res Commun. 2008;377:975–980. doi: 10.1016/j.bbrc.2008.10.104. [DOI] [PubMed] [Google Scholar]
- 42.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Um JW, Park HJ, Song J, Jeon I, Lee G, Lee PH, Chung KC. Formation of parkin aggregates and enhanced PINK1 accumulation during the pathogenesis of Parkinson’s disease. Biochem Biophys Res Commun. 2010;393:824–828. doi: 10.1016/j.bbrc.2010.02.090. [DOI] [PubMed] [Google Scholar]
- 44.Takatori S, Ito G, Iwatsubo T. Cytoplasmic localization and proteasomal degradation of N-terminally cleaved form of PINK1. Neurosci Lett. 2008;430:13–17. doi: 10.1016/j.neulet.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 45.Lin W, Kang UJ. Characterization of PINK1 processing, stability, and subcellular localization. J Neurochem. 2008;106:464–474. doi: 10.1111/j.1471-4159.2008.05398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiong H, Wang D, Chen L, Choo YS, Ma H, Tang C, Xia K, Jiang W, Ronai Z, Zhuang X, Zhang Z. Parkin, PINK1, and DJ-1 form a ubiquitin E3 ligase complex promoting unfolded protein degradation. J Clin Invest. 2009;119:650–660. doi: 10.1172/JCI37617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murata H, Sakaguchi M, Jin Y, Sakaguchi Y, Futami J, Yamada H, Kataoka K, Huh NH. A new cytosolic pathway from a Parkinson disease-associated kinase, BRPK/PINK1: activation of AKT via mTORC2. J Biol Chem. 2011;286:7182–7189. doi: 10.1074/jbc.M110.179390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;14:3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- 49.Inagaki M, Omori E, Kim JY, Komatsu Y, Scott G, Ray MK, Yamada G, Matsumoto K, Mishina Y, Ninomiya-Tsuji J. TAK1-binding protein 1, TAB 1, mediates osmotic stress-induced TAK1 activation but is dispensable for TAK1-mediated cytokine signaling. J Biol Chem. 2008;283:33080–33086. doi: 10.1074/jbc.M807574200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.