Abstract
Non-coding RNAs (ncRNAs) are integral components of biological networks with fundamental roles in regulating gene expression. They can integrate sequence information from the DNA code, epigenetic regulation and functions of multimeric protein complexes to potentially determine the epigenetic status and transcriptional network in any given cell. Humans potentially contain more ncRNAs than any other species, especially in the brain, where they may well play a significant role in human development and cognitive ability. This review discusses their emerging role in Alzheimer’s disease (AD), a human pathological condition characterized by the progressive impairment of cognitive functions. We discuss the complexity of the ncRNA world and how this is reflected in the regulation of the amyloid precursor protein and Tau, two proteins with central functions in AD. By understanding this intricate regulatory network, there is hope for a better understanding of disease mechanisms and ultimately developing diagnostic and therapeutic tools.
Keywords: Non-coding RNAs, MicroRNAs, Alzheimer’s disease (AD), Amyloid-beta, APP (amyloid precursor protein), Tau, Frontotemporal dementia, FTLD, Gene regulatory networks
Introduction
Non-coding RNAs (ncRNAs) add another layer of complexity to the human brain: with 1011 neurons capable of sending rapid signals over great distances, and 1014 synapses as their specialized junctions, the brain is already an organ of inconceivable complexity [1]. For it to efficiently process and integrate the flow of information, the brain depends on the spatio-temporal control of gene expression that has to be orchestrated by an intricate regulatory network. Indeed, 80 % of the coding genome is expressed in the brain, exceeding gene expression in any other organ, and hence regulation of the transcriptome, epigenome, and proteome requires a highly complex network governing their coordinated outputs [2].
The advent of systems biology (and more recently deep-sequencing technologies) marks the culmination of a century of reductionism where signaling and developmental networks are no longer considered in terms of just a few canonical players. Instead the focus is now being placed on deciphering the networked interactions between many hierarchical levels of gene regulation that organize cells into a myriad of precisely sculpted and interacting organs and tissues. Systems-level analysis of these gene regulatory networks is currently focused on protein-coding genes, which make up a mere 2 % of the human transcriptional output. These ~20,000 protein-coding genes found in the human genome are remarkably similar in number, with largely orthologous functional overlaps, to those found in other organisms [3]. This includes the simple nematode, consisting of only 959 somatic cells of which 302 are neurons that make up the nervous system. This has created a conundrum of what could be responsible for higher organismal complexity and cognitive ability. Surprisingly, it is the extent of non-protein-coding DNA that increases with increasing developmental complexity and that may hold the key to understanding human development and higher brain functions [3]. The majority of human DNA bases are actually associated with at least one primary transcript and 98 % of these transcripts have little or no protein coding-ability and form a complex spectrum of ncRNAs [4]. Fortunately, RNA is no longer seen as an intermediate molecule bridging DNA and protein in line with the central dogma of gene expression but it is an integral component of biological networks with immense regulatory potential. RNA molecules not only encode sequence information enabling them to directly interact with both DNA and RNA, but they also possess remarkable structural plasticity. Highly structured RNAs provide docking sites for binding proteins thus bridging the gap between protein complexes and sequence information encoded in the genome more efficiently [5]. In addition, ncRNAs are not constrained by strict sequence conservation as required by the triplet code of an open reading frame and hence are freer to evolve. This allows ncRNAs to be more readily mutated, evolutionarily tested and incorporated into biological networks thus enhancing information flow and their potential biological capabilities. Indeed, ncRNAs show less sequence conservation than RNAs of protein-coding genes although their promoter regions are more conserved than their exons indicating that their regulation may be under more constraint. Interestingly, the most common diseases of the human brain are either not detected in other species, or else they manifest themselves in different ways, suggesting they could be due to disruptions in the non-coding portion of the genome which seems to have evolved to regulate human complexity. This hidden layer of RNA regulatory networks may therefore be central to developmental ontology, and its dysregulation could account for debilitating neurological disorders such as Alzheimer’s disease (AD), as discussed below.
Alzheimer’s disease
At present, there are an estimated 30 million people with dementia worldwide, with numbers expected to further increase to 80 million by 2040 [6]. Of all dementias, AD is the most frequent. It is a neurodegenerative disorder characterized by the loss of synapses and neurons that results in a progressive decline in memory and other cognitive functions, leading to dementia. Frontotemporal lobar degeneration (FTLD) is the second most common form of dementia presenting before the age of 65. It is more heterogeneous in nature than AD, being characterized by a broad spectrum of clinical symptoms including behavioral changes, language abnormalities, and motor impairment with memory functions often preserved until late in disease [7].
Histopathologically, the AD brain is characterized by deposition of both the amyloid-β (Aβ) peptide as amyloid plaques and of hyperphosphorylated forms of the microtubule-associated protein Tau as neurofibrillary tangles (NFTs). Aβ is a mainly 40 to 42-amino-acid-long polypeptide that is derived by proteolytic cleavage from the larger amyloid precursor protein (APP) [8]. In the AD brain, APP is first cleaved by the β-secretase BACE1 that generates the amino-terminus of Aβ and then by the γ-secretase complex that generates its carboxy-terminus. Additional products of APP processing are an amino-terminal fragment that is released by shedding, and AICD, the Aβ intracellular cytoplasmic domain [9]. α-Secretase cleaves APP within the Aβ domain, thereby precluding Aβ formation. Tau belongs to the family of microtubule-associated proteins. Its established function is in the axon where it binds to and stabilizes microtubules, but more recently the view has emerged that Tau has a scaffolding function, by interacting with a plethora of proteins in many cellular compartments [10]. Under pathological conditions Tau is subject to a vast range of post-translation modifications, which includes phosphorylation, nitration, glycation and truncation, to name a few. Tau phosphorylation is central to it (dys)function and negatively regulates Tau binding to microtubules. Tau is a phosphoprotein, with as many as 84 putative phosphorylation sites [11, 12]. In AD, Tau becomes increasingly phosphorylated (hyperphosphorylated) at both physiological and “pathological” sites causing it to detach from microtubules. Microtubules subsequently destabilize and axonal transport and other cellular functions become impaired. The intraneuronal distribution of Tau is altered in disease, with levels of Tau increasing in the somatodendritic compartment where it aggregates and eventually forms NFTs [13, 14]. Therefore the balance between Tau kinases and phosphatases is vital to fine-tune the phosphorylation state of Tau in order to regulate its biological activity.
The majority of AD cases are sporadic, with familial cases being estimated to account for less than 1 %. Autosomal dominant mutations have been identified in three genes; in APP itself as well as in presenilin 1 (PSEN1) and 2 (PSEN2) both of which encode a component of the γ-secretase complex [15]. Different from AD, the subset of FTLD with Tau pathology lacks an overt Aβ pathology. In AD, no mutations have been identified in the MAPT gene encoding Tau. These were found in familial cases of FTLD, where they are mainly located as a cluster in the exons that encode the microtubule-binding domain. A few mutations were also found in introns, affecting the splicing of what in humans encodes six major isoforms. As a slight distortion of the isoform ratio can cause neurodegeneration and dementia, the Tau proteome is obviously tightly regulated. Tau and Aβ ‘talk to each other’, synergistically impairing cellular functions such as those of mitochondria [16]. The human Tau and plaque pathology of AD has been faithfully reproduced in mutant Tau and APP transgenic mouse models [17]. These models have allowed many aspects of disease to be studied and have allowed predictions to be made concerning disease mechanisms that could be confirmed in human patients. Importantly, they are equally useful in dissecting the role of ncRNAs in human disease.
ncRNA species and biogenesis
ncRNAs can be divided into several categories, their widespread role however has remained contentious [18]. While well-known ncRNAs with “housekeeping” functions, such as ribosomal RNAs (rRNAs), transfer RNAs (tRNAs), small nuclear RNAs (snRNAs) and small nucleolar RNAs (snoRNAs), have long established roles in the spliceosomal and translational machinery, much focus is now being placed on the complex class of “regulatory” ncRNAs that predominantly affect the expression or function of protein-coding genes. These differ significantly in their biogenesis and mode of action, and can be broadly classified based on their size [19]. Small RNA species of less than 200 nucleotides include the microRNAs (miRNAs), endogenous small interfering RNAs (endo-siRNAs) and PIWI-interacting RNAs (piRNAs) [20]. While piRNAs and endo-siRNAs seem to function predominantly in the germ line, miRNAs have emerged as pivotal modulators of development and disease by regulating gene expression in both germ line and somatic tissue.
Discovered in C. elegans almost two decades ago, miRNAs now represent the most extensively studied class of ncRNAs [21]. Evolutionary conserved, these 19 to 24-nucleotide-long ncRNAs “fine-tune” gene expression by functioning as post-transcriptional gene silencers that control the translation of mRNA into protein. miRNA biogenesis is a tightly regulated multistep process starting with miRNA gene transcription, mediated mainly by RNA polymerase II, that produces a primary miRNA (pri-miRNA) transcript. Like mRNAs, these pri-miRNAs contain a 5′ cap and 3′ polyA tail and range in length from hundreds up to thousands of nucleotides [22, 23]. Subsequent processing by a series of nuclear enzymes including Drosha and DGCR8 cleaves the pri-miRNA transcript to generate a 70–100 nucleotide precursor miRNA (pre-miRNA) hairpin. Following nuclear export via Exportin 5, a second cleavage event takes place in the cytoplasm that is executed by the RNaseIII enzyme Dicer yielding mature double-stranded RNAs of ~22 nucleotides in length. One of the stands is then incorporated into the RNA-induced silencing complex (RISC), and binds imperfectly to the 3′ untranslated region (3′ UTR) of target mRNAs to induce its degradation or translational repression [24, 25]. Interestingly, some miRNAs have been shown to positively regulate gene expression, by enhancing mRNA translation and inducing gene expression via binding to the promoter of the target gene [26, 27]. Considering that there are 1,921 mature miRNAs currently known in the human genome and 1,157 in the mouse (miRBase.org v18) with each miRNA predicted to target several mRNAs, an intricate miRNA regulatory network is emerging which warrants intensive study [28].
Another group of ncRNAs comprises that of the long non-coding RNAs (lncRNAs), a highly heterogenous group of transcripts that forms the bulk of the human non-coding transcriptome. They vary in size from approximately 200 to 100,000 bases, they may or may not be spliced, they are nuclear or cytoplasmic, polyadenylated or non-polyadenylated, and are usually transcribed by RNA polymerase II and/or III [29]. lncRNAs have been implicated in gene-regulatory roles, such as chromosome dosage compensation, cell cycle control, imprinting, epigenetic regulation, nuclear and cytoplasmic trafficking, splicing, translation, cell differentiation, and serving as organizational frameworks for subcellular structures or precursors for smaller ncRNAs [30]. Some studies suggest that they are at the heart of developmental regulation, determining the epigenetic status and transcriptional network in any given cell type. Natural antisense transcripts (NATs) are a class of lncRNA molecules that are abundant in the nervous system. They are transcribed from the opposite strand of either protein or non-protein coding genes. NATs function in a variety of ways, but have received much attention recently for their epigenetic regulatory functions, often exerted in cis, on their corresponding sense transcript. NATs often partially overlap with protein-coding genes and share therefore some sequence complementarity with the corresponding sense mRNA. By providing a scaffold for ubiquitously expressed histone-modifying complexes through specific secondary structures, NATs can effectively direct specific epigenetic modifications to target loci via sequence-specific interactions between the NAT and the DNA [31]. lncRNAs engaging in RNA–RNA, RNA–DNA, and RNA–protein interactions can thus regulate chromatin remodeling, transcription, mRNA processing, stability and localization, translation and protein stability, activity and secretion. This highly dynamic interplay between ncRNAs, the DNA sequence, epigenetic code, proteome, signaling molecules and environmental stimuli creates a gene regulatory network central to developmental ontology and vital for organ function.
ncRNAs in brain function and development
Not surprisingly, the nervous system has the broadest spectrum of ncRNA expression of all human tissues. Many are expressed in specific neuroanatomical regions, cell types or subcellular compartments suggesting specific functions within developmental programs and in brain homeostasis [32, 33]. miRNAs are predicted to exert post-transcriptional control to over 60 % of mammalian protein-coding genes and over 300 miRNAs are expressed in the mouse brain [34, 35]. Most of them show a highly specific expression pattern with regards to cell type and brain area, while others are more ubiquitous or are expressed in neuronal progenitor cells [36]. Dysregulation of miRNAs has been described in virtually all neurological diseases examined including Huntington’s disease [37, 38], Parkinson’s disease [39], schizophrenia [40], Down’s syndrome [41], and AD (see below). A role for miRNAs in memory functions is exemplified by the finding that fear-extinction learning in mice leads to increased expression of the brain-specific miR-128b, which disrupts the stability of several plasticity-related target genes and regulates the formation of fear-extinction memory [42]. The development and function of the normal brain is critically dependent on the integrity of miRNA pathways as evidenced by disease phenotypes resulting from conditional ablation of the miRNA machinery. For example, mutations in two genes implicated in FTLD, FUS (Fused in sarcoma) and TARDBP encoding TDP-43 (TAR DNA-binding protein 43), both components of the Drosha microprocessor complex, cause up to 50 % of familiar amyotrophic lateral sclerosis [43]. Fragile X syndrome can be attributed to mutations in the RISC complex component fragile X mental retardation protein I (FMRP) [44], while individuals with deletions at 22q11.2 may develop DGCR8 haplo-insufficiency and present with a high risk of developing schizophrenia [45]. Ablation of Dicer causes severely impaired brain development and synaptic plasticity, defects in dendritic arborization and axonal pathfinding, and eventually, neuronal cell death [46].
lncRNAs are emerging as potent regulators of the genome. They have been implicated in a wide variety of neuronal processes such as brain development and ageing, myelination of oligodendrocytes and lineage specification of GABAergic neurons, as well as signaling pathways and synaptic transmission [47, 48]. They also play a vital role in the development of the nervous system as shown in mice by knockout strategies. For example loss of the maternally expressed gene 3 (MEG3) results in increased microvessel density in the cortex [49]; loss of Evf2 reduces numbers of GABAergic interneurons in young mice and decreases synaptic inhibition in older mice [50]; and deletion of BC1 ncRNA alters the behavior and reduces survival under certain conditions [51]. In addition, NATs regulate brain functions such as neuronal specification and migration in the cortex, long-term memory formation, oligodendrocyte differentiation and stress responses [52]. In general lncRNAs are currently regarded to serve gene regulatory roles, functioning in both cis- and trans-acting pathways, to affect mRNA expression and protein function. However, unlike miRNAs, the function of lncRNAs is more elusive as they do not seem to operate via a common pathway and therefore no predictions can be made concerning their function based on their nucleotide sequence or secondary structure. Nevertheless, they are important players in brain molecular circuitry and are often dysregulated in disease.
Aberrant ncRNA expression in AD patients and animal models
Pathogenic processes represent a dynamic interplay between causative factors and the host tissue generating a transcriptomic response that involves both coding and non-coding genes. Profuse efforts have been made at attempting to define the molecular mechanisms underlying neurodegeneration. Transcription profiling has enabled us to attain a snapshot of gene activity in a tissue at a specific moment in time providing clues about the diverse mechanisms involved in the molecular pathogenesis of complex diseases like AD [53]. Various microarray platforms have been extensively used to identify altered genes and pathways during degeneration and to evaluate potential biomarkers and drug targets [54]. Microarray analysis of protein-coding genes has been applied across cellular and animal models of AD, patient biopsies and postmortem brain tissue, and most recently to patient derived cell lines [55, 56]. However, it was not until 2007 that the first study of differential miRNA profiles was published for human AD brain [57]. Since then, several groups have compared miRNA expression profiles of sporadic AD patients with age- and gender-matched normal controls (Table 1) [58–66]. This data has shown that indeed many miRNAs are dysregulated in various brain regions, different cell types and at several stages of disease progression. They have also provided insight into possible mechanisms of altered expression of key players in AD pathogenesis such as the genes encoding APP, BACE1 and Tau. The results of these studies however have been largely discordant showing either no or only very little overlap in miRNA changes [67]. One reason may be that most studies have used total brain homogenates consisting of a crude mixture of neurons, glia, blood vessels and meningeal cells. At postmortem, brain regions such as the hippocampus or entorhinal cortex, heavily affected in AD, show marked neurodegeneration and dramatic changes in cellular composition making a case–control comparison difficult. In other words, a putative down-regulation of neuronal transcripts may be due to changes in cellular composition rather than transcriptional repression of specific transcripts in the surviving neurons of an AD brain. In addition, stratification of patients into mechanistically relevant subgroups may be vital for analysis of results. For example, the correlation of miR-29a/b-1 deregulation with BACE1 expression is only seen in AD patients with increased BACE1 levels [60]. While this does not exclude a role for miRNAs in AD pathogenesis in general, the complex variables inherently associated with human studies poses limitations on data interpretation and clinical relevance. Follow-up studies using larger patient cohorts are needed to assess the biological and clinical relevance of the miRNA changes observed so far. In addition, detailed in situ hybridization studies will provide much needed insight into the cellular localization and subtypes of these dysregulated miRNAs.
Table 1.
microRNA dysregulation in Alzheimer’s disease (AD) patients and animal models obtained from profiling experiments
| Samples analyzed | De-regulated microRNAs | Trend | Target mRNAs identified | Ref |
|---|---|---|---|---|
| Human: hippocampus of 5 AD patients, 5 age-matched controls and 5 fetal brains | miR-9, miR-128 | Up | None | [57] |
| Human: hippocampus and superior temporal lobe of 23 AD patients and 23 controls | miR-146a | Up | CHF (miR-146a) | [58] |
| Human: temporal cortex of 6 AD, 6 MCI patients and 11 controls | miR-107 | Down | BACE1 | [59] |
| Human: anterior temporal cortex of 5 AD patients and 5 age-matched controls |
miR-9, -15a, -19b, -22, -26b, -29a/b-1, -93, -101, -106b, -181c, -210, -363, Let7i, |
Down | BACE1 (miR-29a/b-1, -15a, -9, -19b); APP (Let7i, miR-15a, -101, -106b) | [60] |
| miR-197, -320, -511 | Up | None | ||
| Human: hippocampus of 15 AD patients and 12 controls |
miR-9, -30c, -132, -146b, -210, -212, -425 |
Down | None | [61] |
|
miR-26a, -27a/b, -30e-5p, -34a, -92, -125b, -145, -200c, -381, -422a, -423 |
Up | None | ||
| Human: cerebellum of 15 AD patients and 12 controls | miR-9, -98, -132, -146b, -212, -425 | Down | None | |
|
miR-27a/b, -34a, -100, -125b, -381, -422a |
Up | None | ||
| Human: medial frontal gyrus of 15 AD patients and 12 controls |
miR-9, -26a, -132, -146b, -200c, -212, -425 |
Down | None | |
|
miR-27a/b, -29a/b, -30c/e-5p, -34a, -92, -100, -125b, -145, -148a, -381, -422a, -423 |
Up | None | ||
| Human: CSF from 10 AD patients and 10 controls |
Various—including miR-15b, -142-5p, -146b, -181a |
Down | None | |
| Human: white blood cells from 16 AD patients and 16 controls | Various—including miR-34a, -181b, -200a, Let-7f | Up | None | [62] |
| Human: anterior temporal cortex of 19 AD patients and 11 controls | miR-106b | Down | APP (miR-106b) | [63] |
| Human: temporal lobe cortex of 6 AD, 13 non-AD and 6 controls | miR-9, miR-125b, miR-146a | Up | None | [64] |
| Human: parietal lobe cortex of 5 AD patients and 5 controls |
Various—including miR-15a, -20b, -29b/c, -30e-5p, -95, -101, -148b, -181c, -368, -376a, -374, -582 |
Down | None | [65] |
|
Various—including miR-134, -188, -320, -572, -575, -601, -671, -765 |
Up | None | ||
| Human: frontal lobe of 7 AD patients and 4 controls | miR-29a | Down | NAV3 (miR-29a) | [66] |
| Human: temporal cerebral cortex–white matter vs. gray matter |
Various—including miR-9, -15a/b, -20a, -21, -30, -106a/b, -107, -29, -30, -34, -128, -181a/b, Let-7i |
Down | None | [135] |
| 30 miRNAs up-regulated | Up | None | ||
| Mouse: cerebral cortex from 3- and 6-month old APPSwe-PS1M146L and WT controls | Various—including miR-22, -29a/b/c, -101a/b, -106b, -125b, -181a | Down | None | [69] |
|
Various—including miR-28, -34a, -92a/b, -98, -346, Let-7b/c/d/e |
Up | BCL-2 (miR-34a) | ||
| Mouse: 4-month old Tg19959 | miR-103, -107 | Down | Cofilin (miR-103, -107) | [142] |
| Mouse: primary hippocampal neurons treated with Aβ42 | miR-9, -20b, -21, -148b, -181c, -361, -409, Let-7i | Down | SIRT1, TRIM2, BTBD3, TGFBI | [72, 132] |
miRNAs in bold indicate those repeatedly dysregulated in several studies. mRNAs identified to be regulated by miRNAs (shown in brackets) show either up-regulation (bold italic) or down-regulation (italic) in a subset of AD patients
CHF complement factor H; BACE1 beta-site APP-cleaving enzyme 1; APP amyloid precursor protein; NAV3 neuron navigator 3; SIRT1 sirtuin 1; TRIM2 tripartite motif-containing 2; BTBD3 BTB (POZ) domain containing 3; TGFBI transforming growth factor, beta-induced, 68 kDa
It remains hard to predict whether the miRNA changes observed in the above human AD studies are actually a cause or consequence of the disease process. Here, tissue culture and animal models have been useful to dissect the complexity inherently associated with human studies. While many AD mouse models have been effectively used for candidate miRNA approaches (reviewed in [68]), the first global miRNA study addressing amyloid pathology came from microarray experiments on the plaque-forming APPswe-PS1M146L mouse model evaluating miRNA changes both prior and during plaque deposition [69]. Thirty-seven miRNAs showed differential expression including several that had a similar deregulation in human AD patients. This included a down-regulation of miR-29a/b/c, miR-101a/b, miR-106b, miR-22 and miR-181a and an up-regulation of let-7 and miR-34a. Many miRNA changes observed in young mice prior to plaque deposition were maintained or accentuated as plaque morphology progressed. Functional studies also showed that increases in miR-34a may mediate apoptosis through modulation of the anti-apoptotic factor, Bcl2. Bcl2 is also targeted by miR-16 and -15a (mostly down-regulated in AD) and hence may represent a target affected by several deregulated miRNAs in AD [70]. To take these studies one step further, we have specifically addressed the contribution of Aβ itself, a known causative factor of AD [71], to the de-regulation of neuronal miRNA expression [72]. Exogenous application of Aβ42 to mouse primary hippocampal neurons evoked a rapid down-regulation of specific miRNAs including miR-9, -20b, -21, -148b, -181c, -361, -409, and let-7i. This response was paralleled in hippocampi of Aβ42-depositing APP23 mice at the onset of plaque formation with remarkable overlaps in human patients. While levels of some miRNAs were affected already prior to plaque development in APP23 mice, such as miR-409-3p and let-7i, others showed variable expression over time. Nevertheless, changes in miR-9, -15, -29, -34, -101, -106, -107, -146, and -181 seem to be a recurrent theme in both human and AD animal models warranting further investigation [73]. Interestingly, many of these miRNAs are implicated in modulating the expression and/or processing of key AD-related genes such as BACE1, APP, and MAPT as discussed below.
Direct and indirect regulation of APP by ncRNAs
Modulation of APP alternative splicing by miR-124
In human neurons, APP695 is the predominantly expressed isoform lacking both the Kunitz-type protease inhibitor (KPI) domain encoded by exon 7 and the OX2 domain encoded by exon 8. Longer isoforms encoding these domains are increased in AD brain and are associated with elevated Aβ production [74]. Similarly, they are increased in miRNA-deficient neurons in conditional Dicer knockout mice, suggesting that neuronal miRNAs are involved in regulating splicing of APP [75]. Subsequent loss and gain of function studies in neuronal cells showed that both miR-124 and PTBP1 can alter splicing of APP exons 7 and 8 but not 15. Of these miRNAs, miR-124 is abundantly expressed in neurons and has important functions in neuronal maintenance and splicing [76, 77]. In addition, its expression levels are reduced in a subset of AD patients [57, 75] indicating that the miR-124/PTBP pathway is important for the regulation of neuronal APP splicing.
Direct miRNA regulation of APP expression
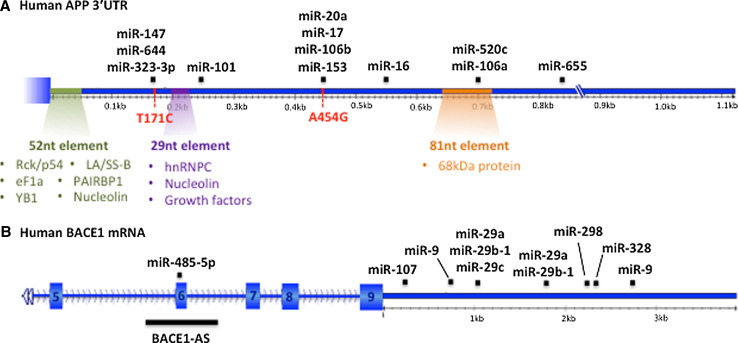
Elevated APP expression, as caused by APP promoter polymorphisms [78], gene duplications [79] or trisomy 21 [80] is associated with increased Aβ42 production and consequently, AD pathogenesis. Thus, understanding the mechanisms that regulate APP expression is important for elucidating the disease mechanisms behind AD. 5′ and 3′ untranslated regions (UTRs) of mRNAs are known to be critically involved in the control of translation or mRNA stability. The human APP 3′UTR is around 1,120 nucleotides in length and contains two polyadenylation sites which produce either a long or a short transcript. These differ by 258 nucleotides, with the longer transcript enhancing the translation of the APP mRNA [81]. APP has been an intensively studied miRNA target gene and several miRNAs have been identified in vitro to directly regulate the APP mRNA. The first studies came from C. elegans, where the worm APP homolog, APL-1, seems to be developmentally regulated by genes that are under control of the miRNA let-7 [82]. Subsequently, APP has developed an extensive repertoire of directly regulating miRNAs including members of the miR-20a family such as miRs-20a, -17 and -106b [63, 83], miRs-106a and 520c [84], miR-101 [85, 86], miR-16 [87], and miRs-147, -153, -323-3p, -644 and -655 [88, 89] (Fig. 1a). There are inconsistent reports for miR-106a, which was identified by Patel et al. to repress APP (position 693-717 on APP 3′UTR), but shown to have no effect by Hebert et al. [63, 84]. Conflicting data are also provided for miR-106b [63, 88], and miR-520c is normally not expressed in the brain and therefore may not be relevant for AD [63, 88]. Of the miRNAs regulating APP directly, miR-101 and -106b are down-regulated in human AD supporting a role for these in regulating APP expression and Aβ generation in disease [60, 65]. None of the reported miRNA-binding sites would be affected by alternative use of either of the two polyadenylation sites mentioned above, however, miRNA-binding sites do overlap with other factors (see below) known to affect APP expression through binding to cis-regulatory elements within the APP 3′UTR. Two of these regulatory elements are located proximal to the APP stop codon (Fig. 1a). A 52-nucleotide stabilizing element, located immediately downstream of the stop codon, is a binding motif for a total of six cytoplasmic proteins (nucleolin, Rck/p54, plasminogen activator inhibitor-RNA binding protein 1 (PAI/RBP1), Y-box binding protein 1 (YB1), autoantigen La/Sjögren syndrome antigen B (La/SS-B) and elongation factor 1α (EF1α)). Whether these are present in a single complex or bind the APP mRNA independently is not known [90]. Interestingly, Rck/p54 is part of the DEAD-box family of RNA helicases that modulates the secondary structure of mRNAs and whose overexpression increases both APP mRNA and protein levels [90]. Although its exact role in regulating APP mRNA stability is not known, it might alter the conformation of the APP 3′UTR modulating the interaction with other RNA-binding proteins and miRNAs. A 29-nucleotide destabilizing element, at position 197-225 after the APP stop codon, mediates the effect of several growth factors on APP expression, and binds both heterogeneous nuclear ribonucleoprotein (hnRNP) C and nucleolin [91, 92]. A more distal cis-regulatory sequence is an 81-nucleotide stabilizing element, which interacts with a 68-kDa protein of unknown function conferring TGFβ-mediated mRNA stability [93]. Interestingly, this 81-nucleotide element overlaps with a predicted miR-20 family-binding site, shown to be targeted by miR-106b/-520c (position 709-715). However, whether these systems are working in a synergistic or competitive fashion in brain remains to be determined.
Fig. 1.
a Regulation of APP expression, mediated through its 3′UTR, is influenced by several miRNA-binding sites, cis-acting regulatory elements and binding proteins, and single-nucleotide polymorphisms (SNPs). The human APP 3′UTR is ~1,120 nucleotides long. The alternative polyadenylation site leading to a shorter APP transcript is indicated by a broken line. The location of validated miRNA-binding sites are shown throughout the length of the shorter APP 3′UTR. SNPs that have been identified in AD and also overlap with miRNA-binding sites are indicated in red. The location of the three regulatory elements bound by various proteins which function to stabilize/destabilize APP mRNA is indicated. PAI/RBP1 plasminogen activator inhibitor-RNA binding protein 1, YB1 Y-box binding protein 1, La/SS-B autoantigen La/Sjögren syndrome antigen B, EF1α elongation factor 1a, hnRNPC heterogeneous nuclear ribonucleoprotein C. b ncRNA regulation of BACE1. BACE1-AS binds to and stabilizes BACE1 mRNA through complementarity with exon 6. The BACE1 mRNA is targeted by several miRNAs, predominantly through its 3′UTR but also via exon 6. The miR-29 family has two predicted binding sites, however, miR-29c only exerts repression through the first site and not the second. This has not been tested for the family members miR-29a and miR-29b-1
More recently, single-nucleotide polymorphisms (SNPs) located in the 3′UTR of APP that discriminate AD patients from healthy controls were shown to interfere with miRNA binding (Fig. 1a); [88]. Of these, SNP T171C specifically inhibited miR-147 binding (but not miR-644 or -323-3p), potentially leading to increased APP and Aβ production, while SNP A454G increased miR-20a binding (but not that of miR-153 or -17), thus theoretically enhancing the effect of miR-20a and reducing APP expression. These SNPs could therefore affect AD risk through modulation of APP expression via interfering with miRNA regulation. Indeed, disease-associated SNPs have been identified at miRNA loci potentially affecting several neurological diseases including multiple sclerosis and Parkinson’s disease [94].
In summary, the APP 3′UTR contributes significantly to the regulation of APP expression through several mechanisms including the repressive effects of several APP-binding miRNAs, alternative polyadenylation sites and various stabilizing/destabilizing cis-regulatory elements that interact with a host of RNA-binding complexes. The interplay between these regulatory mechanisms and how they are affected by AD-associated SNPs remains to be elucidated. As many peripheral organs and cell types express APP, determining the regulatory mechanisms that function in brain will be vital for determining the biological relevance to AD pathomechanisms.
ncRNA regulation of BACE1 indirectly affects APP processing and Aβ production
The β-secretase BACE1 is essential for proper cognitive, emotional and synaptic functions [95]. It is the rate-limiting enzyme in Aβ production. Importantly, increased protein, but not necessarily RNA levels, have been observed in postmortem AD brain, indicating possible disruptions of the post-transcriptional regulation of BACE1 under pathological conditions [60, 96]. Indeed, its mRNA expression is regulated by both miRNAs (miRs-9, -29a/b-1/c, -107, -298, -328 and -485-5p) and long ncRNAs such as BACE1-antisense (BACE1-AS) (Fig. 1b).
BACE1-AS is a ~2 kb conserved ncRNA transcribed from the opposite strand to BACE1 and is widely co-expressed with BACE1 in a variety of tissues and cell lines [97]. It contains 104 nucleotides that perfectly overlap with exon 6 of the BACE1 mRNA, allowing it to form an RNA duplex and stabilize the BACE1 mRNA in a concordant fashion. BACE1-AS is increased in response to cellular stress, including exposure to Aβ42, which subsequently increases BACE1 mRNA stability and protein levels, enhancing the amyloidogenic processing of APP and generating more Aβ peptides through a post-transcriptional feed-forward regulatory loop. BACE1-AS is also significantly up-regulated in AD brain and in the Tg19959 APP mouse model, potentially promoting the stability of the BACE1 sense transcript with direct implications for Aβ generation and AD pathogenesis.
Unlike conventional miRNA regulation occurring via binding to sequences in the 3′UTR region, miR-485-5p was found to repress BACE1 by binding to its open reading frame in exon 6 [98]. Overexpression of miR-485-5p reduced BACE1 protein levels by 30 %, while knockdown of miR-485-5p increased BACE1 protein levels. However, co-expression of both miR-485-5p and BACE1-AS alleviated the effects of miR-485-5p on BACE1 protein reduction, indicating that the two ncRNAs compete for the same binding site on BACE1 mRNA (Fig. 1b). BACE1-AS may therefore enhance BACE1 mRNA stability by “masking” the binding site for miR-485-5p and preventing miRNA-induced translational repression of BACE1 mRNA. This interplay between miR-485-5p and BACE1-AS demonstrates an interface between two distinct groups of regulatory ncRNAs in the control of BACE1 gene expression.
More conventional miRNA repression through direct binding to the 3′UTR has also been shown for the BACE1 mRNA. Hebert et al. showed that BACE1 is repressed by miRs-29a, -29b-1 and -9 in vitro [60]. Significant down-regulation of the miR-29a/b-1 cluster was observed in the anterior temporal cortex in about 30 % of sporadic AD patients with high BACE1 protein levels and a similar correlation in expression was seen during brain development. In addition, decreasing miR-29a/b-1 levels led to an increase in Aβ production in cell culture and vice versa suggesting that the miR-29a/b-1 cluster is a potential major suppressor of BACE1 protein expression that may be implicated in AD pathogenesis. Zong et al. [99] added miR-29c to the list of ncRNAs shown to lower BACE1 protein levels in vitro. miR-29c exerts its repression through only one of its two predicted binding sites on the BACE1 3′UTR, and BACE1 protein was decreased in brain of transgenic mice showing a ~2-fold overexpression of miR-29c. Neuronal miR-107 is down-regulated at intermediate stages (Braak stage 3) of AD pathogenesis and its expression is negatively correlated to that of BACE1 [59]. A single miR-107 binding site mediating BACE1 repression in the HeLa cell system was validated, indicating that miR-107 may be involved in accelerating disease progression through control of BACE1. Finally, Boissonneault et al. [100] demonstrated that miR-298 and -328 bound specific sites in the BACE1 3′UTR, thereby exerting regulatory effects on BACE1 protein expression in cultured neuronal cells. BACE1 is thus a prime example of how mRNA expression is finely tuned by a particularly complex interplay between long and short ncRNA transcripts, and whose dysregulation can have profound effects on the downstream processing of APP and Aβ generation.
Other ncRNAs influencing Aβ generation
Alternative splicing not only affects APP metabolism directly but it is a central component of human brain complexity in general. Massone et al. [101] described a brain-expressed ncRNA termed 17A that is involved in this process. 17A is a 152-nucleotide long antisense transcript embedded within the G-protein-coupled receptor (GPR51) gene. It induces the synthesis of an alternatively spliced GPR51 isoform, which impairs GABAB signaling and enhances secretion of Aβ42. Synthesis of 17A is induced in response to inflammatory stimuli in a polymerase III-dependent manner and is up-regulated in the cerebral cortex of AD patients. Thus dysregulation of 17A in AD may contribute to plaque formation by affecting signaling pathways important in brain physiology and pathology.
Aβ formation is also influenced by membrane ceramides, major components of lipid rafts and increased in sporadic AD. They potentially contribute to pathology by facilitating the mislocalization of γ-secretase and BACE1 to lipid rafts thereby enhancing Aβ production [102]. Serine palmitoyltransferase (SPT) is the first rate-limiting enzyme in ceramide biosynthesis. SPT protein, but not its mRNA levels are up-regulated in sporadic AD brain suggesting post-transcriptional regulation. Indeed, loss of miRs-9, -29a/b-1, -137 and -181c, increases SPT and in turn, Aβ levels [103]. These miRNAs are all down-regulated in AD frontal cortex suggesting that they might influence Aβ generation through regulation of membrane lipid composition. Interestingly, miR-181c, -137 and -29a/b-1 are also developmentally regulated, with the highest expression in adult mice while SPT shows a negative correlation and decreases with age. Evidence also suggests that AD pathology may be more prevalent in females than in males [104] and indeed SPT protein levels are higher, with lower miRs-137, -181c, -29a/b-1 levels in females [103]. High fat diet increases plasma ceramide levels and aggravates the Aβ burden in animal models and indeed, miRs-137, -9 and -181c are down-regulated in mice fed a high fat diet. Together miRNA regulation of SPT provides a mechanism for the increased risk of AD-associated with age, gender and highly saturated fat diets.
Homeostasis of cholesterol can also have profound effects on Aβ metabolism and accumulation. APP, BACE1, and PSEN1 are all membrane proteins and their trafficking and proteolytic activities are affected by dysregulation of cholesterol that modulates membrane fluidity. Cholesterol is transported by the ATP-binding cassette transporter A1 (ABCA1) that mediates the efflux of cholesterol and phospholipids onto lipid-poor apolipoproteins (apo-A1 and apoE), to form high-density lipoprotein (HDL) particles [105]. In AD mouse models, deletion of ABCA1 enhances Aβ deposition, whereas overexpression dramatically reduces Aβ accumulation [106]. In addition, ABCA1 expression is elevated in human AD hippocampus where its levels are highly correlated with the severity of cognitive impairment [107]. miR-106b, which is down-regulated in AD, targets ABCA1 and indeed miR-106b-mediated ABCA1 repression resulted in increased Aβ levels by significantly reducing Aβ clearance [106]. Thus miR-106b can influence Aβ metabolism in several ways, either through direct regulation of APP itself, or via modulating APP trafficking, Aβ clearance as well as β- and γ-secretase activity through regulation of ABCA1.
Regulation of Tau metabolism by ncRNAs
In addition to Aβ, Tau is the other major player in AD pathogenesis. Its pathology can be exacerbated by disruptions in any of the highly regulated processes controlling Tau metabolism, including expression, subcellular localization, posttranslational modifications and clearance. Neurons are highly polarized cells containing both axonal and dendritic processes composed of differentiated microtubular networks. One main difference between axonal and dendritic microtubules is the asymmetric distribution of microtubule-associated proteins (MAP) that contribute to selectively shape the microtubule framework. In general, MAP2 is present in dendrites, whereas Tau is found in axons. This axonal localization of Tau is partly due to the prior transport of MAPT mRNA to the proximal segment of the neuronal compartment [108], a process requiring a 91-nucleotide sequence located in the MAPT 3′ untranslated region [109]. Nuclear factor 90 (NF90), a double-stranded RNA-binding protein, binds to this axonal targeting element and is required for the correct axonal localization of MAPT mRNA [110]. Interestingly, a family of small structured ncRNAs binds NF90. These brain-expressed ncRNAs are only found in humans and chimpanzees and while their physiological function remains to be established, their dysregulation may interfere with the dynamics of MAPT mRNA localization to the axon.
Unlike APP, MAPT mRNA is not known to be directly regulated by miRNAs making the link between Tau pathology and ncRNAs more indirect. Nevertheless, reducing global miRNA production by using a conditional knockout of Dicer in mouse brain induced changes in Tau metabolism including AD-like hyperphosphorylation and altered MAPT splicing [111, 112]. Indeed, loss of neuronal dicer mimicked several AD phenotypes including progressive neurodegeneration, enlarged ventricles, reduced brain size, neuroinflammation and impaired dendritic branching and spine length [113, 114]. In addition, Bilen et al. [115] have shown that reducing miRNA processing in dicer-1 knockout flies dramatically enhanced Tau-induced neurodegeneration. These studies together indicate that miRNA pathways affect Tau metabolism potentially contributing to several neuropathological features of AD.
The miR-132/PTBP2 pathway influences Tau exon 10 splicing
In the human brain, alternative splicing of MAPT exons 2, 3, and 10, produces six major Tau isoforms [116]. While exons 2 and 3 encode for inserts in the projection domain of Tau, exon 10 encodes a microtubule-binding repeat giving rise to either 3R or 4R isoforms. Humans have three 3R and three 4R isoforms, while mice only express 4R isoforms. Under normal physiological conditions the 4R/3R ratio is approximately 1, however, changes in this ratio can cause neurodegeneration and dementia [117, 118]. More than 35 mutations have already been identified in MAPT in patients with rare familial forms of FTLD, that cause aberrant splicing of MAPT exon 10 and hence, an imbalance in the 4R/3R ratio [119]. More recently, the splicing factor, SFPQ, was found to be relocalized from the nucleus to the cytoplasm in 4R human Tau mutant mice indicating additional roles for Tau other than in the axon [120]. Together, this indicates that proper regulation of MAPT splicing is important to maintain normal brain function. Smith et al. [112] have shown that several brain miRNAs, including miR-124, -9, -132 and -137, can regulate the 4R/3R ratio in neuronal cells. Interestingly, both miR-9 and miR-124 are down-regulated in AD and could therefore contribute to an altered Tau composition in disease. Using progressive supranuclear palsy (PSP), a major 4R tauopathy as a model, they showed that miR-132 is specifically down-regulated and that this correlated with increased protein levels of the neuronal splicing factor polypyrimidine tract-binding protein 2 (PTBP2). PTBP1 has been linked to MAPT exon 10 splicing in vitro [121], its expression levels are increased in adult brain of conditional dicer knockout mice with reduced miRNA expression [75], and both PTBP1 and 2 levels are changed in human disease showing alternative splicing patterns [122, 123]. Therefore, changes in the miR-132/PTBP2 pathway could influence abnormal splicing of MAPT exon 10 in brain and contribute to AD pathogenesis.
The miR-15/ERK1 pathway regulates Tau phosphorylation
Tau phosphorylation is regulated by a balanced interplay of kinases and phosphatases. Through the study of conditional knockout mice that are deficient for dicer in the forebrain, Hebert et al. [111] have shown that hyperphosphorylation of endogenous Tau at pathological sites coincided with an increase in mitogen-activated protein kinase 3 (MAPK3/ERK1). Furthermore they showed that the ERK1 3′UTR is directly regulated by the miR-15 family (including miR-15a, -16, -195 and -497) in mouse neuronal cells, and that miR-15a is specifically down-regulated in AD brains. Several other Tau kinases are also under miRNA-mediated regulation. GSK-3β has a critical role in Aβ production and NFT formation [124]. In smooth muscle, GSK-3β mRNA is directly repressed by miR-26a [125]. This miRNA is regulated by brain derived neurotrophic factor (BDNF), a neurotrophin with essential roles in neuronal development and plasticity [126]. Furthermore, miR-26a shows altered expression in AD [61]. However, whether this miR-26 pathway plays a role in regulating brain GSK-3β is yet to be determined.
miRNA regulation of Tau turnover
Degradation of Tau is partly mediated by the ubiquitin–proteasome system, which involves the E3 ubiquitin ligase CHIP (carboxyl terminus of the Hsc70-interacting protein) [127]. CHIP polyubiquitinates phosphorylated Tau at lysine residues and promotes its degradation. However, ubiquitination of lysines can be precluded by lysine acetylation, thus inhibiting proteasome-mediated degradation. Modulating lysine acetylation can therefore alter Tau levels, especially the hyperphosphorylated forms found in AD and other tauopathies. Tau is acetylated by the p300 acetyltransferase and deacetylated by SIRT1 [128], which is decreased in AD brain [129, 130]. SIRT1 directly interacts with and deacetylates Tau, and its deficiency in AD increases Tau acetylation and contributes to the accumulation of soluble forms of hyperphosphorylated Tau [128]. miR-9, -34 and -181c have all been shown to directly repress SIRT1 mRNA [131–133], and all show consistent deregulation in AD brain (Table 1). In addition, the co-chaperone BAG2 mediates a highly efficient Tau degradation pathway that preferentially degrades hyperphosphorylated, insoluble Tau [134]. This BAG2/Hsp70 complex operates in proximity to the microtubule where it captures and delivers Tau to the proteasome for ubiquitin-independent degradation. BAG2 is regulated by miR-128, which is increased in AD [57]. Hence disrupting levels of miRNAs impacting on Tau turnover may result in insufficient clearance and hence, accumulation of hyperphosphorylated Tau.
While many miRNAs regulate various processes to fine-tune the balance of different Tau species, it is not known whether Tau itself and in what form may trigger miRNA deregulation and how this could contribute to AD pathogenesis. Interestingly, Wang et al. [135] have shown that alterations in miR-212 expression correlates with the density of NFTs and that this miRNA is also found to be down-regulated in AD patients. miR-454 also appears to change in correlation with the presence of a neurofibrillary pathology, and these changes are seen in both white and gray matter.
ncRNA regulation of synaptic plasticity, cytoskeletal structure, and inflammation
AD is a highly complex disease integrating cellular responses from various brain regions and many cell types. In addition to plaques and tangles as histopathological hallmarks, other pathological changes include inflammation, impaired axonal transport and reduced synaptic plasticity; they are all characteristics of AD and are all influenced by ncRNAs. Synaptic plasticity forms the basis of learning and memory, and plasticity failure may be a starting point for the neurodegenerative changes seen in AD [136]. Synapses are dynamic structures with the ability to reorganize the actin cytoskeleton and change morphology, and to alter numbers of neurotransmitter receptors and the amount of neurotransmitter released in response to neuronal activity. These changes generally involve the synthesis of new synaptic proteins directly in the participating compartments [137]. Recent research has revealed the existence of a complex network of synaptic ncRNAs, especially miRNAs, which function as translational repressors of pre-existing synaptic mRNAs. Several miRNAs have well-established functions in regulating synaptic plasticity including miRs-124, -125b, -132, -134, -138, and -219 [138]. In the hippocampus of AD patients, miR-132 is down-regulated [61], while miR-125b is consistently up-regulated in various brain regions including the hippocampus, cerebellum, medial frontal gyrus, and temporal lobe [61, 64]. This dysregulation could therefore potentially alter spine density and shape as well as synaptic events such as miniature excitatory postsynaptic currents (mEPSCs) in AD patients. In addition, brain cytoplasmic RNAs of 150 and 200 nucleotides, BC1 and BC200 respectively, are small somatodendritic, untranslated RNAs which also maintain long-term synaptic plasticity (LTP) by modulating local protein synthesis at the synapse. In the normal ageing brain, cortical BC200 levels decrease by 65 % after the age of 50 [139]. However, in brain areas affected by AD pathology, BC200 is not only significantly up-regulated but becomes mis-localized in the soma. Whether this mislocalization and over-expression of BC200 RNA is compensatory to, or causative of, synaptodendritic deterioration in AD neurons remains to be determined.
Aβ peptides and other forms of neurotoxic stress can also trigger aggregation of actin and the actin-severing protein cofilin into rod-like structures termed Hirano bodies, another prominent feature of the AD brain [140]. Any disruptions in cofilin metabolism can affect the structure, dynamics, and functions of the cytoskeleton. The rods not only destroy microtubule bundles but also interfere with neuronal transport, synaptic function and may even recruit phosphorylated Tau into neuropil threads [141]. Yao et al. [142] have shown that miRs-103 and -107 target the cofilin 3′UTR and repress its translation. In the plaque-forming Tg19959 AD mouse model they showed that decreased levels of brain miRs-103 and -107 correlate with increased cofilin protein levels and the presence of cofilin-actin rods. miR-107 expression is also reduced in human AD brain where it has been shown to target BACE1 [59]. Importantly, this highlights how disruption of one miRNA may contribute to both cytoskeletal pathology and increased Aβ production thus linking several aspects of AD pathology.
In AD, altered or excessive innate immune or inflammatory signaling is detrimental to brain function, contributing to the irreversible degeneration of brain cells. The Toll-like (TLR) and IL-1 (IL-1R) receptors play central roles in innate immune signaling pathways by functioning as extracellular sensors of pathogens and cytotoxic molecules such as Aβ. These TLR/IL-1 signaling pathways utilize interleukin-1 receptor-associated kinases (IRAKs) to initiate diverse downstream signaling processes that often lead to the induction of transcription factors such as NF-κB, AP-1, and IRFs [143]. miR-146a is emerging as a key regulator of the innate immune response and pro-inflammatory signaling through specific regulation of IRAK-1 [144]. miR-146a expression is also directly regulated by NF-κB forming a feedback loop in signaling regulation. In AD brain, there is significant up-regulation of miR-146a and a corresponding down-regulation of IRAK-1. Thus a dynamic interplay exists between pro-inflammatory miRNAs and transcription factors to mediate specific signaling pathways essential for brain homeostasis, neuroprotection, and repair.
Conclusions and future perspectives
It is clear that ncRNAs have an essential role not only under physiological conditions during development and organ function but also in neurodegeneration, a process that undermines the orchestrated functioning of the brain. We have illustrated that both long and short ncRNAs influence a wide variety of neuronal pathways implicated in the pathogenesis of AD, the most frequent form of neurodegenerative disease (Fig. 2). This highlights the importance of miRNAs as integral members of gene regulatory networks whereby dysregulation of a single miRNA may disrupt several pathways and have a great impact on cellular metabolism and disease progression. So far, most profiling studies on AD patients and animal models have used microarray technology, which is limited in the number of ncRNAs examined, with the exception of miRNA investigations. Accurate interrogation of ncRNA expression requires technologies that take an unbiased view of the transcriptional landscape. Deep-sequencing approaches provide a more comprehensive picture, which is essential if ncRNAs are ever to be incorporated into the molecular circuitry that defines a brain. RNA-seq is a relatively young technology with intensive bioinformatic demands, which so far has not been extensively used to investigate the genomics of neurodegeneration. Nevertheless, it looks set to deliver a window into the genome, revealing the transcriptional complexity of the human brain and potentially new players in the regulatory network governing AD pathogenesis. With a deeper understanding of the causes and consequences of these transcriptional changes, more reliable, affordable, efficient, and early diagnostic techniques together with more appropriate drug targets can be developed. While diagnostic techniques, such as imaging technologies, are developing rapidly, most techniques can only detect disease signals in more advanced disease stages, which is often too late for effective treatment. In contrast, molecular diagnostic strategies have the potential to detect the onset of disease pathogenesis thus allowing preventative or early treatment strategies to be employed. It is hoped that ncRNAs will develop into diagnostic and therapeutic tools that will help combat a disease for which at present no cure is available. The triplet nature of the genetic code, with defined codons read unidirectionally, has long been established for protein-coding genes. However, the language of non-coding genes is still largely elusive. A major challenge now lies in deciphering the functional elements in the primary sequence of non-coding genes, whether it be specific RNA sequences, structural motifs or regulatory elements, that determines their roles as RNA molecules. In addition, miRNA target genes need to be identified in order to establish a network of how miRNAs interact in coordinating their regulation of the proteome. Large scale genomics projects such as the Encyclopedia of DNA elements (ENCODE) and FANTOM (Functional Annotation of the Mammalian Genome) Consortium will provide a valuable resource on ncRNAs, divulging information on annotation, histone modifications, evolutionary conservation, cellular localization, interacting protein partners, and target genes [145].
Fig. 2.
ncRNAs affecting neuronal pathways implicated in AD pathogenesis. Schematic representation of a neuron showing APP processing and Aβ generation (enlarged). ncRNAs regulating neuronal processes affected in AD are indicated, showing those that are either up-regulated (red) or down-regulated (green) in AD patients together with relevant references in brackets. Arrows point to the associated targets shown to be regulated by the specific ncRNAs listed
As for AD, we are dealing with a slowly progressing chronic process that shows a high variability depending on age, gender, genotype and environmental factors encompassing anything from education to diet to exercise. Furthermore the degenerative process in the AD brain is not uniform, as some brain areas are affected earlier and/or more than others, making the task of deciphering the role of ncRNAs in this process even more challenging. Also it will be increasingly important to determine changes to ncRNAs at an early stage of disease in areas where AD is initiated, such as the entorhinal cortex. Furthermore, as AD is considered a synapse disorder, subcellular fractionations will be inevitable to determine changes in ncRNA expression and give insight into their function. As ncRNAs are ideal orchestrators of essential biological networks, integral to their (dys)function, they warrant further investigation to elucidate their contribution to the pathomechanisms of human disease, and to develop them as diagnostic and therapeutic tools.
Acknowledgments
This work has been supported by the National Health & Medical Research Council (NHMRC), and the Australian Research Council (ARC).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513(5):532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 2.Ooi L, Wood IC. Regulation of gene expression in the nervous system. Biochem J. 2008;414(3):327–341. doi: 10.1042/BJ20080963. [DOI] [PubMed] [Google Scholar]
- 3.Mattick JS. The central role of RNA in human development and cognition. FEBS Lett. 2011;585(11):1600–1616. doi: 10.1016/j.febslet.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Amaral PP, Dinger ME, Mercer TR, Mattick JS. The eukaryotic genome as an RNA machine. Science. 2008;319(5871):1787–1789. doi: 10.1126/science.1155472. [DOI] [PubMed] [Google Scholar]
- 5.St Laurent G, 3rd, Wahlestedt C. Non-coding RNAs: couplers of analog and digital information in nervous system function? Trends Neurosci. 2007;30(12):612–621. doi: 10.1016/j.tins.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377(9770):1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 7.Liscic RM, Grinberg LT, Zidar J, Gitcho MA, Cairns NJ. ALS and FTLD: two faces of TDP-43 proteinopathy. Eur J Neurol. 2008;15(8):772–780. doi: 10.1111/j.1468-1331.2008.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gotz J. Tau and transgenic animal models. Brain Res Brain Res Rev. 2001;35(3):266–286. doi: 10.1016/s0165-0173(01)00055-8. [DOI] [PubMed] [Google Scholar]
- 9.Schonrock N, Matamales M, Ittner LM, Gotz J. MicroRNA networks surrounding APP and amyloid-beta metabolism—Implications for Alzheimer’s disease. Exp Neurol. 2012;235(2):447–454. doi: 10.1016/j.expneurol.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Brandt R, Leschik J. Functional interactions of tau and their relevance for Alzheimer’s disease. Curr Alzheimer Res. 2004;1(4):255–269. doi: 10.2174/1567205043332054. [DOI] [PubMed] [Google Scholar]
- 11.Hanger DP, Noble W. Functional implications of glycogen synthase kinase-3-mediated tau phosphorylation. Int J Alzheimers Dis. 2011;2011:352805. doi: 10.4061/2011/352805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen F, David D, Ferrari A, Gotz J. Posttranslational modifications of tau—role in human tauopathies and modeling in transgenic animals. Curr Drug Targets. 2004;5(6):503–515. doi: 10.2174/1389450043345236. [DOI] [PubMed] [Google Scholar]
- 13.Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wolfing H, Chieng BC, Christie MJ, Napier IA, Eckert A, Staufenbiel M, Hardeman E, Gotz J. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142(3):387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 14.Alonso AC, Grundke-Iqbal I, Iqbal K. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med. 1996;2(7):783–787. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- 15.Bertram L, Tanzi RE. Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9(10):768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- 16.David DC, Ittner LM, Gehrig P, Nergenau D, Shepherd C, Halliday G, Gotz J. Beta-amyloid treatment of two complementary P301L tau-expressing Alzheimer’s disease models reveals similar deregulated cellular processes. Proteomics. 2006;6(24):6566–6577. doi: 10.1002/pmic.200600634. [DOI] [PubMed] [Google Scholar]
- 17.Gotz J, Ittner LM. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat Rev Neurosci. 2008;9(7):532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- 18.Pauli A, Rinn JL, Schier AF. Non-coding RNAs as regulators of embryogenesis. Nat Rev Genet. 2011;12(2):136–149. doi: 10.1038/nrg2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattick JS, Amaral PP, Dinger ME, Mercer TR, Mehler MF. RNA regulation of epigenetic processes. Bioessays. 2009;31(1):51–59. doi: 10.1002/bies.080099. [DOI] [PubMed] [Google Scholar]
- 20.Suh N, Blelloch R. Small RNAs in early mammalian development: from gametes to gastrulation. Development. 2011;138(9):1653–1661. doi: 10.1242/dev.056234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 22.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23(20):4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10(12):1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 25.Eulalio A, Huntzinger E, Izaurralde E. Getting to the root of miRNA-mediated gene silencing. Cell. 2008;132(1):9–14. doi: 10.1016/j.cell.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 26.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318(5858):1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 27.Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci USA. 2008;105(5):1608–1613. doi: 10.1073/pnas.0707594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amaral PP, Clark MB, Gascoigne DK, Dinger ME, Mattick JS. lncRNAdb: a reference database for long noncoding RNAs. Nucleic Acids Res. 2011;39:146–151. doi: 10.1093/nar/gkq1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23(13):1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magistri M, Faghihi MA, St Laurent G, 3rd, Wahlestedt C. Regulation of chromatin structure by long non-coding RNAs: focus on natural antisense transcripts. Trends Genet. 2012 doi: 10.1016/j.tig.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao X, Yeo G, Muotri AR, Kuwabara T, Gage FH. Non-coding RNAs in the mammalian central nervous system. Annu Rev Neurosci. 2006;29:77–103. doi: 10.1146/annurev.neuro.29.051605.112839. [DOI] [PubMed] [Google Scholar]
- 33.Mercer TR, Dinger ME, Sunkin SM, Mehler MF, Mattick JS. Specific expression of long non-coding RNAs in the mouse brain. Proc Natl Acad Sci USA. 2008;105(2):716–721. doi: 10.1073/pnas.0706729105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, Lin C, Socci ND, Hermida L, Fulci V, Chiaretti S, Foa R, Schliwka J, Fuchs U, Novosel A, Muller RU, Schermer B, Bissels U, Inman J, Phan Q, Chien M, Weir DB, Choksi R, De Vita G, Frezzetti D, Trompeter HI, Hornung V, Teng G, Hartmann G, Palkovits M, Di Lauro R, Wernet P, Macino G, Rogler CE, Nagle JW, Ju J, Papavasiliou FN, Benzing T, Lichter P, Tam W, Brownstein MJ, Bosio A, Borkhardt A, Russo JJ, Sander C, Zavolan M, Tuschl T. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129(7):1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kosik KS, Krichevsky AM. The elegance of the microRNAs: a neuronal perspective. Neuron. 2005;47(6):779–782. doi: 10.1016/j.neuron.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Mehler MF, Mattick JS. Non-coding RNAs in the nervous system. J Physiol. 2006;575(Pt 2):333–341. doi: 10.1113/jphysiol.2006.113191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Packer AN, Xing Y, Harper SQ, Jones L, Davidson BL. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington’s disease. J Neurosci. 2008;28(53):14341–14346. doi: 10.1523/JNEUROSCI.2390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson R, Zuccato C, Belyaev ND, Guest DJ, Cattaneo E, Buckley NJ. A microRNA-based gene dysregulation pathway in Huntington’s disease. Neurobiol Dis. 2008;29(3):438–445. doi: 10.1016/j.nbd.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A. A microRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317(5842):1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perkins DO, Jeffries CD, Jarskog LF, Thomson JM, Woods K, Newman MA, Parker JS, Jin J, Hammond SM. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8(2):R27. doi: 10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuhn DE, Nuovo GJ, Martin MM, Malana GE, Pleister AP, Jiang J, Schmittgen TD, Terry AV, Jr, Gardiner K, Head E, Feldman DS, Elton TS. Human chromosome 21-derived miRNAs are overexpressed in down syndrome brains and hearts. Biochem Biophys Res Commun. 2008;370(3):473–477. doi: 10.1016/j.bbrc.2008.03.120. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Lin Q, Wei W, Coelho CM, Li X, Baker-Andresen D, Dudley K, Ratnu VS, Boskovic Z, Kobor MS, Sun YE, Bredy TW. The brain-specific microRNA miR-128b regulates the formation of fear-extinction memory. Nat Neurosci. 2011;14(9):1115–1117. doi: 10.1038/nn.2891. [DOI] [PubMed] [Google Scholar]
- 43.Ling SC, Albuquerque CP, Han JS, Lagier-Tourenne C, Tokunaga S, Zhou H, Cleveland DW. ALS-associated mutations in TDP-43 increase its stability and promote TDP-43 complexes with FUS/TLS. Proc Natl Acad Sci USA. 2010;107(30):13318–13323. doi: 10.1073/pnas.1008227107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, Tada T, Dolan BM, Sharp PA, Sheng M. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 2010;65(3):373–384. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, Wan X, Pavlidis P, Mills AA, Karayiorgou M, Gogos JA. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40(6):751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- 46.Tal TL, Tanguay RL. Non-coding RNAs-Novel targets in neurotoxicity. Neurotoxicology. 2012 doi: 10.1016/j.neuro.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, Cabili MN, Jaenisch R, Mikkelsen TS, Jacks T, Hacohen N, Bernstein BE, Kellis M, Regev A, Rinn JL, Lander ES. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458(7235):223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mercer TR, Qureshi IA, Gokhan S, Dinger ME, Li G, Mattick JS, Mehler MF. Long non-coding RNAs in neuronal-glial fate specification and oligodendrocyte lineage maturation. BMC Neurosci. 2010;11:14. doi: 10.1186/1471-2202-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon FE, Nutt CL, Cheunsuchon P, Nakayama Y, Provencher KA, Rice KA, Zhou Y, Zhang X, Klibanski A. Increased expression of angiogenic genes in the brains of mouse meg3-null embryos. Endocrinology. 2010;151(6):2443–2452. doi: 10.1210/en.2009-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bond AM, Vangompel MJ, Sametsky EA, Clark MF, Savage JC, Disterhoft JF, Kohtz JD. Balanced gene regulation by an embryonic brain ncRNA is critical for adult hippocampal GABA circuitry. Nat Neurosci. 2009;12(8):1020–1027. doi: 10.1038/nn.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewejohann L, Skryabin BV, Sachser N, Prehn C, Heiduschka P, Thanos S, Jordan U, Dell’Omo G, Vyssotski AL, Pleskacheva MG, Lipp HP, Tiedge H, Brosius J, Prior H. Role of a neuronal small non-messenger RNA: behavioural alterations in BC1 RNA-deleted mice. Behav Brain Res. 2004;154(1):273–289. doi: 10.1016/j.bbr.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 52.Salta E, De Strooper B. Non-coding RNAs with essential roles in neurodegenerative disorders. Lancet Neurol. 2012;11(2):189–200. doi: 10.1016/S1474-4422(11)70286-1. [DOI] [PubMed] [Google Scholar]
- 53.Hoerndli F, David DC, Gotz J. Functional genomics meets neurodegenerative disorders. Part II: application and data integration. Prog Neurobiol. 2005;76(3):169–188. doi: 10.1016/j.pneurobio.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Benetti F, Gustincich S, Legname G. Gene expression profiling and therapeutic interventions in neurodegenerative diseases: a comprehensive study on potentiality and limits. Expert Opin Drug Discov. 2012;7(3):245–259. doi: 10.1517/17460441.2012.659661. [DOI] [PubMed] [Google Scholar]
- 55.Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS, Carson CT, Laurent LC, Marsala M, Gage FH, Remes AM, Koo EH, Goldstein LS. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature. 2012;482(7384):216–220. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Courtney E, Kornfeld S, Janitz K, Janitz M. Transcriptome profiling in neurodegenerative disease. J Neurosci Methods. 2010;193(2):189–202. doi: 10.1016/j.jneumeth.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 57.Lukiw WJ. Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. NeuroReport. 2007;18(3):297–300. doi: 10.1097/WNR.0b013e3280148e8b. [DOI] [PubMed] [Google Scholar]
- 58.Lukiw WJ, Zhao Y, Cui JG. An NF-kappaB-sensitive micro RNA-146a-mediated inflammatory circuit in Alzheimer disease and in stressed human brain cells. J Biol Chem. 2008;283(46):31315–31322. doi: 10.1074/jbc.M805371200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang WX, Rajeev BW, Stromberg AJ, Ren N, Tang G, Huang Q, Rigoutsos I, Nelson PT. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J Neurosci. 2008;28(5):1213–1223. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A, De Strooper B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci USA. 2008;105(17):6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cogswell JP, Ward J, Taylor IA, Waters M, Shi Y, Cannon B, Kelnar K, Kemppainen J, Brown D, Chen C, Prinjha RK, Richardson JC, Saunders AM, Roses AD, Richards CA. Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J Alzheimers Dis. 2008;14(1):27–41. doi: 10.3233/jad-2008-14103. [DOI] [PubMed] [Google Scholar]
- 62.Schipper HM, Maes OC, Chertkow HM, Wang E. microRNA expression in Alzheimer blood mononuclear cells. Gene Regul Syst Bio. 2007;1:263–274. doi: 10.4137/grsb.s361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hebert SS, Horre K, Nicolai L, Bergmans B, Papadopoulou AS, Delacourte A, De Strooper B. microRNA regulation of Alzheimer’s Amyloid precursor protein expression. Neurobiol Dis. 2009;33(3):422–428. doi: 10.1016/j.nbd.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 64.Sethi P, Lukiw WJ. micro-RNA abundance and stability in human brain: specific alterations in Alzheimer’s disease temporal lobe neocortex. Neurosci Lett. 2009;459(2):100–104. doi: 10.1016/j.neulet.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 65.Nunez-Iglesias J, Liu CC, Morgan TE, Finch CE, Zhou XJ. Joint genome-wide profiling of miRNA and mRNA expression in Alzheimer’s disease cortex reveals altered miRNA regulation. PLoS ONE. 2010;5(2):e8898. doi: 10.1371/journal.pone.0008898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shioya M, Obayashi S, Tabunoki H, Arima K, Saito Y, Ishida T, Satoh J. Aberrant microRNA expression in the brains of neurodegenerative diseases: miR-29a decreased in Alzheimer disease brains targets neurone navigator 3. Neuropathol Appl Neurobiol. 2010;36(4):320–330. doi: 10.1111/j.1365-2990.2010.01076.x. [DOI] [PubMed] [Google Scholar]
- 67.Hebert SS, De Strooper B. Alterations of the microRNA network cause neurodegenerative disease. Trends Neurosci. 2009;32(4):199–206. doi: 10.1016/j.tins.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Delay C, Hebert SS. microRNAs and Alzheimer’s disease mouse models: current insights and future research avenues. Int J Alzheimers Dis. 2011;2011:894938. doi: 10.4061/2011/894938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X, Liu P, Zhu H, Xu Y, Ma C, Dai X, Huang L, Liu Y, Zhang L, Qin C. miR-34a, a microRNA up-regulated in a double transgenic mouse model of Alzheimer’s disease, inhibits bcl2 translation. Brain Res Bull. 2009;80(4–5):268–273. doi: 10.1016/j.brainresbull.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 70.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 2005;102(39):13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gotz J, Eckert A, Matamales M, Ittner LM, Liu X. Modes of Abeta toxicity in Alzheimer’s disease. Cell Mol Life Sci. 2011;68(20):3359–3375. doi: 10.1007/s00018-011-0750-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schonrock N, Ke YD, Humphreys D, Staufenbiel M, Ittner LM, Preiss T, Gotz J. Neuronal microRNA deregulation in response to Alzheimer’s disease amyloid-beta. PLoS ONE. 2010;5(6):e11070. doi: 10.1371/journal.pone.0011070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Delay C, Mandemakers W, Hebert SS. microRNAs in Alzheimer’s disease. Neurobiol Dis. 2012;46(2):285–290. doi: 10.1016/j.nbd.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 74.Donev R, Newall A, Thome J, Sheer D. A role for SC35 and hnRNPA1 in the determination of amyloid precursor protein isoforms. Mol Psychiatry. 2007;12(7):681–690. doi: 10.1038/sj.mp.4001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith P, Al Hashimi A, Girard J, Delay C, Hebert SS. In vivo regulation of amyloid precursor protein neuronal splicing by microRNAs. J Neurochem. 2011;116(2):240–247. doi: 10.1111/j.1471-4159.2010.07097.x. [DOI] [PubMed] [Google Scholar]
- 76.Papagiannakopoulos T, Kosik KS. microRNA-124: micromanager of neurogenesis. Cell Stem Cell. 2009;4(5):375–376. doi: 10.1016/j.stem.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 77.Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The microRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 2007;27(3):435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Theuns J, Brouwers N, Engelborghs S, Sleegers K, Bogaerts V, Corsmit E, De Pooter T, van Duijn CM, De Deyn PP, Van Broeckhoven C. Promoter mutations that increase amyloid precursor-protein expression are associated with Alzheimer disease. Am J Hum Genet. 2006;78(6):936–946. doi: 10.1086/504044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerriere A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, Dubas F, Frebourg T, Campion D. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet. 2006;38(1):24–26. doi: 10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- 80.Podlisny MB, Lee G, Selkoe DJ. Gene dosage of the amyloid beta precursor protein in Alzheimer’s disease. Science. 1987;238(4827):669–671. doi: 10.1126/science.2960019. [DOI] [PubMed] [Google Scholar]
- 81.de Sauvage F, Kruys V, Marinx O, Huez G, Octave JN. Alternative polyadenylation of the amyloid protein precursor mRNA regulates translation. EMBO J. 1992;11(8):3099–3103. doi: 10.1002/j.1460-2075.1992.tb05382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Niwa R, Zhou F, Li C, Slack FJ. The expression of the Alzheimer’s amyloid precursor protein-like gene is regulated by developmental timing microRNAs and their targets in Caenorhabditis elegans. Dev Biol. 2008;315(2):418–425. doi: 10.1016/j.ydbio.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fan X, Liu Y, Jiang J, Ma Z, Wu H, Liu T, Liu M, Li X, Tang H. miR-20a promotes proliferation and invasion by targeting APP in human ovarian cancer cells. Acta Biochim Biophys Sin (Shanghai) 2010;42(5):318–324. doi: 10.1093/abbs/gmq026. [DOI] [PubMed] [Google Scholar]
- 84.Patel N, Hoang D, Miller N, Ansaloni S, Huang Q, Rogers JT, Lee JC, Saunders AJ. MicroRNAs can regulate human APP levels. Mol Neurodegener. 2008;3:10. doi: 10.1186/1750-1326-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Long JM, Lahiri DK. microRNA-101 downregulates Alzheimer’s amyloid-beta precursor protein levels in human cell cultures and is differentially expressed. Biochem Biophys Res Commun. 2011;404(4):889–895. doi: 10.1016/j.bbrc.2010.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vilardo E, Barbato C, Ciotti M, Cogoni C, Ruberti F. microRNA-101 regulates amyloid precursor protein expression in hippocampal neurons. J Biol Chem. 2010;285(24):18344–18351. doi: 10.1074/jbc.M110.112664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu W, Liu C, Zhu J, Shu P, Yin B, Gong Y, Qiang B, Yuan J, Peng X. microRNA-16 targets amyloid precursor protein to potentially modulate Alzheimer’s-associated pathogenesis in SAMP8 mice. Neurobiol Aging. 2012;33(3):522–534. doi: 10.1016/j.neurobiolaging.2010.04.034. [DOI] [PubMed] [Google Scholar]
- 88.Delay C, Calon F, Mathews P, Hebert SS. Alzheimer-specific variants in the 3′UTR of amyloid precursor protein affect microRNA function. Mol Neurodegener. 2011;6:70. doi: 10.1186/1750-1326-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liang C, Zhu H, Xu Y, Huang L, Ma C, Deng W, Liu Y, Qin C. microRNA-153 negatively regulates the expression of amyloid precursor protein and amyloid precursor-like protein 2. Brain Res. 2012;1455:103–113. doi: 10.1016/j.brainres.2011.10.051. [DOI] [PubMed] [Google Scholar]
- 90.Broytman O, Westmark PR, Gurel Z, Malter JS. Rck/p54 interacts with APP mRNA as part of a multi-protein complex and enhances APP mRNA and protein expression in neuronal cell lines. Neurobiol Aging. 2009;30(12):1962–1974. doi: 10.1016/j.neurobiolaging.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rajagopalan LE, Malter JS. Growth factor-mediated stabilization of amyloid precursor protein mRNA is mediated by a conserved 29-nucleotide sequence in the 3′-untranslated region. J Neurochem. 2000;74(1):52–59. doi: 10.1046/j.1471-4159.2000.0740052.x. [DOI] [PubMed] [Google Scholar]
- 92.Zaidi SH, Malter JS. Nucleolin and heterogeneous nuclear ribonucleoprotein C proteins specifically interact with the 3′-untranslated region of amyloid protein precursor mRNA. J Biol Chem. 1995;270(29):17292–17298. doi: 10.1074/jbc.270.29.17292. [DOI] [PubMed] [Google Scholar]
- 93.Amara FM, Junaid A, Clough RR, Liang B. TGF-beta(1), regulation of Alzheimer amyloid precursor protein mRNA expression in a normal human astrocyte cell line: mRNA stabilization. Brain Res Mol Brain Res. 1999;71(1):42–49. doi: 10.1016/s0169-328x(99)00158-8. [DOI] [PubMed] [Google Scholar]
- 94.Glinsky GV. An SNP-guided microRNA map of fifteen common human disorders identifies a consensus disease phenocode aiming at principal components of the nuclear import pathway. Cell Cycle. 2008;7(16):2570–2583. doi: 10.4161/cc.7.16.6524. [DOI] [PubMed] [Google Scholar]
- 95.Laird FM, Cai H, Savonenko AV, Farah MH, He K, Melnikova T, Wen H, Chiang HC, Xu G, Koliatsos VE, Borchelt DR, Price DL, Lee HK, Wong PC. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci. 2005;25(50):11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang LB, Lindholm K, Yan R, Citron M, Xia W, Yang XL, Beach T, Sue L, Wong P, Price D, Li R, Shen Y. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003;9(1):3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- 97.Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, Finch CE, St Laurent G, 3rd, Kenny PJ, Wahlestedt C. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat Med. 2008;14(7):723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Faghihi MA, Zhang M, Huang J, Modarresi F, Van der Brug MP, Nalls MA, Cookson MR, St-Laurent G, 3rd, Wahlestedt C. Evidence for natural antisense transcript-mediated inhibition of microRNA function. Genome Biol. 2010;11(5):R56. doi: 10.1186/gb-2010-11-5-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zong Y, Wang H, Dong W, Quan X, Zhu H, Xu Y, Huang L, Ma C, Qin C. miR-29c regulates BACE1 protein expression. Brain Res. 2011;1395:108–115. doi: 10.1016/j.brainres.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 100.Boissonneault V, Plante I, Rivest S, Provost P. microRNA-298 and microRNA-328 regulate expression of mouse beta-amyloid precursor protein-converting enzyme 1. J Biol Chem. 2009;284(4):1971–1981. doi: 10.1074/jbc.M807530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Massone S, Vassallo I, Fiorino G, Castelnuovo M, Barbieri F, Borghi R, Tabaton M, Robello M, Gatta E, Russo C, Florio T, Dieci G, Cancedda R, Pagano A. 17A, a novel non-coding RNA, regulates GABA B alternative splicing and signaling in response to inflammatory stimuli and in Alzheimer disease. Neurobiol Dis. 2011;41(2):308–317. doi: 10.1016/j.nbd.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 102.Puglielli L, Ellis BC, Saunders AJ, Kovacs DM. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. J Biol Chem. 2003;278(22):19777–19783. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- 103.Geekiyanage H, Chan C. microRNA-137/181c regulates serine palmitoyltransferase and in turn amyloid beta, novel targets in sporadic Alzheimer’s disease. J Neurosci. 2011;31(41):14820–14830. doi: 10.1523/JNEUROSCI.3883-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu KP, Kuo MC, Tang KC, Chau AW, Ho IH, Kwok MP, Chan WC, Choi RH, Lam NC, Chu MM, Chu LW. Effects of age, education and gender in the Consortium to Establish a Registry for the Alzheimer’s Disease (CERAD)-Neuropsychological Assessment Battery for Cantonese-speaking Chinese elders. Int Psychogeriatr. 2011;23(10):1575–1581. doi: 10.1017/S1041610211001153. [DOI] [PubMed] [Google Scholar]
- 105.Hirsch-Reinshagen V, Burgess BL, Wellington CL. Why lipids are important for Alzheimer disease? Mol Cell Biochem. 2009;326(1–2):121–129. doi: 10.1007/s11010-008-0012-2. [DOI] [PubMed] [Google Scholar]
- 106.Kim J, Yoon H, Ramirez CM, Lee SM, Hoe HS, Fernandez-Hernando C. miR-106b impairs cholesterol efflux and increases Abeta levels by repressing ABCA1 expression. Exp Neurol. 2012;235(2):476–483. doi: 10.1016/j.expneurol.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Akram A, Schmeidler J, Katsel P, Hof PR, Haroutunian V. Increased expression of cholesterol transporter ABCA1 is highly correlated with severity of dementia in AD hippocampus. Brain Res. 2010;1318:167–177. doi: 10.1016/j.brainres.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Litman P, Barg J, Rindzoonski L, Ginzburg I. Subcellular localization of tau mRNA in differentiating neuronal cell culture: implications for neuronal polarity. Neuron. 1993;10(4):627–638. doi: 10.1016/0896-6273(93)90165-n. [DOI] [PubMed] [Google Scholar]
- 109.Behar L, Marx R, Sadot E, Barg J, Ginzburg I. cis-acting signals and trans-acting proteins are involved in tau mRNA targeting into neurites of differentiating neuronal cells. Int J Dev Neurosci. 1995;13(2):113–127. doi: 10.1016/0736-5748(95)00001-w. [DOI] [PubMed] [Google Scholar]
- 110.Larcher JC, Gasmi L, Viranaicken W, Edde B, Bernard R, Ginzburg I, Denoulet P. Ilf3 and NF90 associate with the axonal targeting element of Tau mRNA. Faseb J. 2004;18(14):1761–1763. doi: 10.1096/fj.04-1763fje. [DOI] [PubMed] [Google Scholar]
- 111.Hebert SS, Papadopoulou AS, Smith P, Galas MC, Planel E, Silahtaroglu AN, Sergeant N, Buee L, De Strooper B. Genetic ablation of Dicer in adult forebrain neurons results in abnormal tau hyperphosphorylation and neurodegeneration. Hum Mol Genet. 2010;19(20):3959–3969. doi: 10.1093/hmg/ddq311. [DOI] [PubMed] [Google Scholar]
- 112.Smith PY, Delay C, Girard J, Papon MA, Planel E, Sergeant N, Buee L, Hebert SS. microRNA-132 loss is associated with tau exon 10 inclusion in progressive supranuclear palsy. Hum Mol Genet. 2011;20(20):4016–4024. doi: 10.1093/hmg/ddr330. [DOI] [PubMed] [Google Scholar]
- 113.Davis TH, Cuellar TL, Koch SM, Barker AJ, Harfe BD, McManus MT, Ullian EM. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci. 2008;28(17):4322–4330. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kawase-Koga Y, Low R, Otaegi G, Pollock A, Deng H, Eisenhaber F, Maurer-Stroh S, Sun T. RNAase-III enzyme Dicer maintains signaling pathways for differentiation and survival in mouse cortical neural stem cells. J Cell Sci. 2010;123(Pt 4):586–594. doi: 10.1242/jcs.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bilen J, Liu N, Burnett BG, Pittman RN, Bonini NM. microRNA pathways modulate polyglutamine-induced neurodegeneration. Mol Cell. 2006;24(1):157–163. doi: 10.1016/j.molcel.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 116.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 117.D’Souza I, Schellenberg GD. Regulation of tau isoform expression and dementia. Biochim Biophys Acta. 2005;1739(2–3):104–115. doi: 10.1016/j.bbadis.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 118.Caffrey TM, Joachim C, Paracchini S, Esiri MM, Wade-Martins R. Haplotype-specific expression of exon 10 at the human MAPT locus. Hum Mol Genet. 2006;15(24):3529–3537. doi: 10.1093/hmg/ddl429. [DOI] [PubMed] [Google Scholar]
- 119.Liu F, Gong CX. Tau exon 10 alternative splicing and tauopathies. Mol Neurodegener. 2008;3:8. doi: 10.1186/1750-1326-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ke Y, Dramiga J, Schutz U, Kril JJ, Ittner LM, Schroder H, Gotz J. Tau-mediated nuclear depletion and cytoplasmic accumulation of SFPQ in Alzheimer’s and Pick’s Disease. PLoS ONE. 2012;7(4):e35678. doi: 10.1371/journal.pone.0035678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang J, Gao QS, Wang Y, Lafyatis R, Stamm S, Andreadis A. Tau exon 10, whose missplicing causes frontotemporal dementia, is regulated by an intricate interplay of cis elements and trans factors. J Neurochem. 2004;88(5):1078–1090. doi: 10.1046/j.1471-4159.2003.02232.x. [DOI] [PubMed] [Google Scholar]
- 122.Tollervey JR, Wang Z, Hortobagyi T, Witten JT, Zarnack K, Kayikci M, Clark TA, Schweitzer AC, Rot G, Curk T, Zupan B, Rogelj B, Shaw CE, Ule J. Analysis of alternative splicing associated with aging and neurodegeneration in the human brain. Genome Res. 2011;21(10):1572–1582. doi: 10.1101/gr.122226.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sterne-Weiler T, Howard J, Mort M, Cooper DN, Sanford JR. Loss of exon identity is a common mechanism of human inherited disease. Genome Res. 2011;21(10):1563–1571. doi: 10.1101/gr.118638.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cai Z, Zhao Y, Zhao B (2012) Roles of Glycogen synthase kinase 3 in Alzheimer’s disease. Curr Alzheimer Res [Epub ahead of print] [DOI] [PubMed]
- 125.Mohamed JS, Lopez MA, Boriek AM. Mechanical stretch up-regulates microRNA-26a and induces human airway smooth muscle hypertrophy by suppressing glycogen synthase kinase-3beta. J Biol Chem. 2010;285(38):29336–29347. doi: 10.1074/jbc.M110.101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Caputo V, Sinibaldi L, Fiorentino A, Parisi C, Catalanotto C, Pasini A, Cogoni C, Pizzuti A. Brain derived neurotrophic factor (BDNF) expression is regulated by microRNAs miR-26a and miR-26b allele-specific binding. PLoS ONE. 2011;6(12):e28656. doi: 10.1371/journal.pone.0028656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, Grover A, De Lucia M, McGowan E, Lewis J, Prihar G, Kim J, Dillmann WH, Browne SE, Hall A, Voellmy R, Tsuboi Y, Dawson TM, Wolozin B, Hardy J, Hutton M. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet. 2004;13(7):703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- 128.Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, Meyers D, Cole PA, Ott M, Gan L. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67(6):953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gan L, Mucke L. Paths of convergence: sirtuins in aging and neurodegeneration. Neuron. 2008;58(1):10–14. doi: 10.1016/j.neuron.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Julien C, Tremblay C, Emond V, Lebbadi M, Salem N, Jr, Bennett DA, Calon F. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol. 2009;68(1):48–58. doi: 10.1097/NEN.0b013e3181922348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Saunders LR, Sharma AD, Tawney J, Nakagawa M, Okita K, Yamanaka S, Willenbring H, Verdin E. miRNAs regulate SIRT1 expression during mouse embryonic stem cell differentiation and in adult mouse tissues. Aging (Albany NY) 2010;2(7):415–431. doi: 10.18632/aging.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schonrock N, Humphreys DT, Preiss T, Gotz J. Target gene repression mediated by miRNAs miR-181c and miR-9 both of which are down-regulated by amyloid-beta. J Mol Neurosci. 2012;46(2):324–335. doi: 10.1007/s12031-011-9587-2. [DOI] [PubMed] [Google Scholar]
- 133.Zovoilis A, Agbemenyah HY, Agis-Balboa RC, Stilling RM, Edbauer D, Rao P, Farinelli L, Delalle I, Schmitt A, Falkai P, Bahari-Javan S, Burkhardt S, Sananbenesi F, Fischer A. microRNA-34c is a novel target to treat dementias. EMBO J. 2011;30(20):4299–4308. doi: 10.1038/emboj.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Carrettiero DC, Hernandez I, Neveu P, Papagiannakopoulos T, Kosik KS. The cochaperone BAG2 sweeps paired helical filament- insoluble tau from the microtubule. J Neurosci. 2009;29(7):2151–2161. doi: 10.1523/JNEUROSCI.4660-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang WX, Huang Q, Hu Y, Stromberg AJ, Nelson PT. Patterns of microRNA expression in normal and early Alzheimer’s disease human temporal cortex: white matter versus gray matter. Acta Neuropathol. 2011;121(2):193–205. doi: 10.1007/s00401-010-0756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 137.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127(1):49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 138.Siegel G, Saba R, Schratt G. microRNAs in neurons: manifold regulatory roles at the synapse. Curr Opin Genet Dev. 2011;21(4):491–497. doi: 10.1016/j.gde.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 139.Mus E, Hof PR, Tiedge H. Dendritic BC200 RNA in aging and in Alzheimer’s disease. Proc Natl Acad Sci USA. 2007;104(25):10679–10684. doi: 10.1073/pnas.0701532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Minamide LS, Striegl AM, Boyle JA, Meberg PJ, Bamburg JR. Neurodegenerative stimuli induce persistent ADF/cofilin-actin rods that disrupt distal neurite function. Nat Cell Biol. 2000;2(9):628–636. doi: 10.1038/35023579. [DOI] [PubMed] [Google Scholar]
- 141.Whiteman IT, Gervasio OL, Cullen KM, Guillemin GJ, Jeong EV, Witting PK, Antao ST, Minamide LS, Bamburg JR, Goldsbury C. Activated actin-depolymerizing factor/cofilin sequesters phosphorylated microtubule-associated protein during the assembly of Alzheimer-like neuritic cytoskeletal striations. J Neurosci. 2009;29(41):12994–13005. doi: 10.1523/JNEUROSCI.3531-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yao J, Hennessey T, Flynt A, Lai E, Beal MF, Lin MT. microRNA-related cofilin abnormality in Alzheimer’s disease. PLoS ONE. 2010;5(12):e15546. doi: 10.1371/journal.pone.0015546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li L. Regulation of innate immunity signaling and its connection with human diseases. Curr Drug Targets Inflamm Allergy. 2004;3(1):81–86. doi: 10.2174/1568010043483863. [DOI] [PubMed] [Google Scholar]
- 144.Cui JG, Li YY, Zhao Y, Bhattacharjee S, Lukiw WJ. Differential regulation of interleukin-1 receptor-associated kinase-1 (IRAK-1) and IRAK-2 by microRNA-146a and NF-kappaB in stressed human astroglial cells and in Alzheimer disease. J Biol Chem. 2010;285(50):38951–38960. doi: 10.1074/jbc.M110.178848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.The ENCODE Project Consortium A user’s guide to the encyclopedia of DNA elements (ENCODE) PLoS Biol. 2011;9(4):e1001046. doi: 10.1371/journal.pbio.1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]