Abstract
Alzheimer’s disease (AD) is by far the most commonly diagnosed dementia, and despite multiple efforts, there are still no effective drugs available for its treatment. One strategy that deserves to be pursued is to alter the expression and/or physiological action of endogenous proteins instead of administering exogenous factors. In this study, we intend to characterize the roles of the antioxidant, anti-inflammatory, and heavy-metal binding proteins, metallothionein-1 + 2 (MT1 + 2), in a mouse model of Alzheimer’s disease, Tg2576 mice. Contrary to expectations, MT1 + 2-deficiency rescued partially the human amyloid precursor protein-induced changes in mortality and body weight in a gender-dependent manner. On the other hand, amyloid plaque burden was decreased in the cortex and hippocampus in both sexes, while the amyloid cascade, neuroinflammation, and behavior were affected in the absence of MT1 + 2 in a complex manner. These results highlight that the control of the endogenous production and/or action of MT1 + 2 could represent a powerful therapeutic target in AD.
Keywords: Alzheimer’ disease, Tg2576, Metallothionein, Behavior, Amyloid plaques, Gliosis, Metals, Survival, Body weight
Introduction
Alzheimer’s disease (AD) is the most commonly diagnosed dementia, and is clinically defined by a progressive loss of cognitive functions and neuropathologically characterized by the presence of extracellular deposits of aggregated β-amyloid (Aβ) peptide (senile/amyloid plaques) and intracellular deposits of hyperphosphorylated tau protein (neurofibrillary tangles). This spectrum of pathology is usually accompanied by pronounced inflammation, oxidative stress, and neuronal death of the affected brain regions [10, 46].
Although inflammatory changes are observed throughout the AD brain, it is widely accepted that Aβ, particularly in aggregated or fibrillar forms, triggers pro-inflammatory reactions of microglia and astrocytes that are usually in close proximity to the senile plaques [37]. One of the effects derived from Aβ accumulation and Aβ-triggered inflammatory response that has been found in AD brains, and animal models of the disease, is the excessive generation of free radicals and subsequent increased levels of several lipid, protein and DNA oxidative stress markers, together with increased levels of antioxidant enzymes [35, 73]. Moreover, oxidative stress and Aβ are closely related to each other since Aβ induces oxidative stress in vitro and in vivo, and at the same time oxidative stress increases the production of Aβ [73]. Another important source of oxidative stress is transition metals such as Cu or Fe that together with Zn have been found to be altered in the AD brain where they may influence the synthesis and processing of APP and also induce Aβ aggregation and precipitation in a pH-dependent manner [13, 15].
Clioquinol (CQ) and PBT-2, which bind metals with weak reversible affinity and help in the restoration of physiologic metal levels in specific cellular compartments, are examples of novel metal-based therapies that have already reached clinical trial [11] and highlight the potential role of the natural antioxidant and metal-binding proteins metallothioneins (MTs) in modulating the progression of AD.
Metallothioneins are low-molecular-weight (6–7 kDa), cysteine-rich proteins with high metal content that are subdivided into four subfamilies (MT1–MT4) in mammals [75]. While MT1 and MT2 are present in most tissues, MT3 and MT4 are found primarily in the central nervous system (CNS) and the stratified squamous epithelia, respectively [30, 62, 67]. Although the primary biological role of MTs remains unknown, there is mounting evidence to suggest that MTs are essential proteins that confer a survival advantage in situations of biological stress or cellular damage [27, 56, 57, 80]. Indeed, from a physiological point of view, it is noteworthy that most, if not all, pro-oxidant and/or inflammatory conditions induce MT1 + 2 synthesis [3, 36, 69].
MT1 + 2 levels have been found to be consistently upregulated in several human neurodegenerative diseases including Alzheimer’s disease [57, 79]. Moreover, MT1 + 2 are also upregulated in different AD mouse models, including Tg2576 mice, which show a prominent up-regulation of these proteins in the vicinity of the amyloid plaques [21]. Recent in vitro studies in cultured rat cortical neurons show that MT2 reduces Aβ-induced changes in ionic homeostasis and the subsequent neurotoxicity by a metal swap between Zn7MT2 and Cu-Aβ [28]; such an attenuation of Aβ neurotoxicity has been confirmed independently [48], but a former study did not find any anti-Aβ effects of human MT2A on rat embryonic cortical neurons, in contrast to MT3 [45]. The reasons for such discrepancies with in vitro studies are unclear. In an eye model of Aβ42-induced toxicity in Drosophila, it has been demonstrated that zinc or copper ions exacerbate eye damage, while metallothioneins decrease it [43]. Moreover, preliminary studies from our laboratory, where we administered MT2 to old Tg2576 mice, indicate that MTs might modulate the amyloid pathology as well as other Aβ-related aspects of this AD model [57]. Apart from the expected actions related to their anti-inflammatory, anti-oxidant and metal-binding properties, MTs have been found to interact with transthyretin (TTR) [59], a homotetrameric protein that plays a critical role in modulating Aβ in vitro and in vivo by sequestering it and preventing its aggregation [71]. While the literature provides support for a potential involvement of the MT family in the pathogenesis of AD, there are many deficits in our understanding of how MTs may function. Therefore, in the present study we developed a double transgenic mouse line that develops AD-like pathology in addition to having a deficiency in MT1 and MT2, in order to assess the role of MTs in different aspects of AD pathology.
Materials and methods
Animals
The parental strains used in this study were mice deficient for both MT1 and MT2 (MT1 + 2KO) proteins [60] (The Jackson Laboratory, Bar Harbor, ME, USA) and the AD mouse model Tg2576, which expresses the human APP695 harboring the Swedish K670N/M671L mutations under the control of the hamster prion protein promoter [42] (Taconic Europe A/S; Ry, Denmark).
To produce the desired transgenic mice, a double crossing strategy was designed in order to obtain all the animals of the experiment in the same genetic background. In the first crossing, Tg2576 mice were crossed with MT1 + 2KO mice. From the resultant offspring (APP−/−/MT1 + 2+/− and APP+/−/MT1 + 2+/−), the APP+ were selected and backcrossed again with MT1 + 2KO mice to obtain the four genotypes that were used for the experiment: WT (APP−/−/MT1 + 2+/−), MT1 + 2KO (APP−/−/MT1 + 2−/−), APPWT (APP+/−/MT1 + 2+/−) and APPMT1 + 2KO (APP+/−/MT1 + 2−/−); the total number of mice per group engaged at weaning was 110, 80, 66, and 86, respectively (Fig. 1). These initial large groups were needed because significant mortality is caused by the APP transgene and analyses were to be carried out in young and old mice. Genotype was determined by PCR.
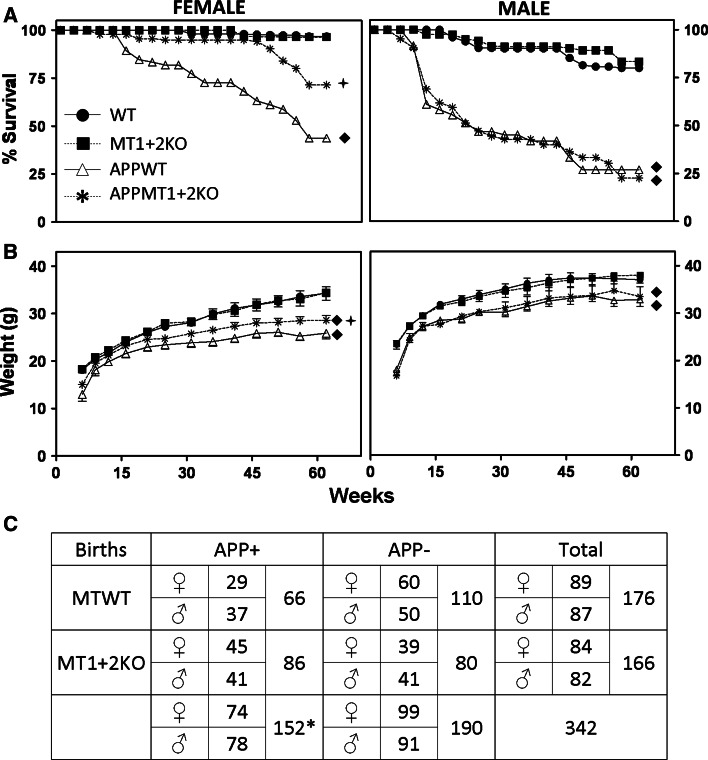
Fig. 1.
MT1 + 2-deficiency reduces APP-induced changes in mortality and body weight in females. Survival (a) and body weight (b) of 173 females (left) and 169 males (right) was followed up from weaning to killing. Mice carrying the hAPP transgene showed increased mortality rates and lower body weights than their APP− controls as revealed by Kaplan–Meier and generalized estimated equations (GEE) tests, respectively. This phenotype was reversed in part by MT1 + 2-deficiency in females. a Filled diamond, p ≤ 0.001 APP− versus APP+; plus, p ≤ 0.005 APPWT versus APPMT1 + 2KO. b Filled diamond, p ≤ 0.001 APP− versus APP+; plus, p ≤ 0.05 APPWT versusAPPMT1 + 2KO. c χ2 analysis of the offspring genotyped at weaning revealed a less than expected ratio for APP+ pups, particularly for APPWT mice (expected APP+ pups: 171 vs. 152 born; expected APPWT pups: 85.5 vs. 66 born; *χ2 = 4.22, p < 0.05 with 1 degree of freedom), again suggesting an hAPP-induced intrauterine/perinatal mortality that was partially prevented by the absence of MT1 + 2
After weaning at 3 weeks of age, the animals were housed with free access to food and water in a 12-h dark–light cycle under constant temperature. All mouse care and experimental procedures were approved by the Ethics Committee in Human and Animal experimentation from the Autonomous University of Barcelona. Body weight and mortality were monitored regularly from weaning until killing at approximately 14 months of age. Animals were killed by decapitation and the brain quickly removed on ice. The right hemisphere was dissected into cortex, hippocampus, cerebellum, and rest of the brain, frozen with liquid nitrogen, and stored at −80 °C. The left hemisphere was immersed in 4 % paraformaldehyde (PFA) and stored in 70 % ethanol at 4 °C. A small cohort of animals were killed at 5 months of age and the right hemispheres were dissected into prefrontal cortex and rest of the cortex and hippocampus, frozen with liquid nitrogen, and stored at −80 °C.
Behavioral characterization
Mice were tested at 4–6 months of age and at 10 months of age to characterize their behavioral phenotype. Exploratory activity and anxiety were evaluated with the Hole Board and the Elevated Plus Maze tests as described elsewhere [4] and spatial memory and learning were assessed with the Morris Water Maze (MWM). Briefly, the MWM consists of a propylene round pool (120 cm diameter × 60 cm deep) virtually divided in four equal quadrants with a removable platform that can be adjusted at different heights (8-cm diameter). It contains water at 22 °C ± 1 made opaque by the addition of non-toxic liquid latex (Látex Compound Española S.A, Barcelona, Spain) and different cues placed in the pool and in the curtain that surrounds it. In the first phase, mice were challenged to escape from the pool by finding a hidden platform (submerged 1 cm below opaque water) using cues as spatial references. Animals were freely allowed to explore the pool for 60 s and after that, in case they failed to find the hidden platform, they were guided to it and required to remain on it for 15 s. Animals were trained for 8 non-consecutive days with four trials per day and the escape latency (time required to localize the hidden platform) in each trial was recorded. During this phase, several probe trial tests were carried out consisting of removing the platform and measuring the time the mice spent in the target quadrant (TQ) of the pool (the one with the hidden platform) looking for the platform. In the second phase, the reversal test, mice were challenged to re-learn a new platform location (3 days, four trials per day) and escape latency was measured in each trial. For the third phase (2 days, four trials per day) a big black cue was placed on top of the platform to make it visible above water level and escape latency was measured in each trial.
Protein extraction
Frozen prefrontal cortex samples from young (5 months old) mice and frozen cortex and hippocampus samples from old (14 months old) mice were processed using slightly different protein extraction protocols. Frozen prefrontal cortex samples from young mice were weighed with a precision scale and homogenized by sonication on ice (1:5 w/v) in PBS (pH 7.4) supplemented with protease (Roche; Basel, Switzerland) and phosphatase (Sigma-Aldrich; St. Louis, MO, USA) inhibitor cocktails. Frozen hippocampus samples were weighed with a precision scale and homogenized by sonication in 50 mM Tris–HCl (pH 7.6), 0.01 % NP-40, 150 mM NaCl, 2 mM EDTA, 3 % SDS, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 % deoxycholate and protease inhibitor cocktail (Sigma-Aldrich). Total homogenate samples were stored at −80 °C. Old mice cortical samples were also fractionated using the high-fidelity extraction procedure described by Lesné et al. [55] that separates proteins in known cellular compartments and allows quantifying and comparing four independent pools (fractions A–D) of transgene-derived Aβ species. Protein concentration from all homogenates and fractions were estimated with the BCA protein assay according to the manufacturer’s instructions (Pierce, Thermo Fisher Scientific Inc; Rockford, IL, USA).
Western blotting
Western blot was utilized to assess APP, Aβ, and other APP-derived proteolytic fragments such as CTF-β (6E10, Aβ1–16, 1:2,000, Signet, Dedham, MA, USA; WO2—Aβ5–8—1:50, in-house antibody), astrocytosis (anti-glial fibrillary acidic protein—GFAP—1:40,000, DakoCytomation, Denmark A/S) and microgliosis (anti-ionized calcium binding adaptor molecule 1—Iba1—1:3,000, Wako Pure Chemical industries, Osaka, Japan). Monoclonal anti-β-Actin (1:5000, Sigma) was used as loading control. Samples were run on NuPage NOVEX Invitrogen (Carlsbad, CA, USA) bis–Tris 4–12 % 20-well gels at 130–160 V for 1 h. Membranes were heated for 5 min in PBS, blocked in TBST (0.01 % Tween, 5 % skim milk powder), and incubated with the different antibodies (6E10 and Iba1: overnight at 4 °C; GFAP and Actin: 1 h room temperature, RT). Blots were rinsed in TBST and then incubated in secondary antibody 1 h RT (6E10, WO2 and Actin: polyclonal rabbit anti-mouse Ig/HRP 1:5,000, DakoCytomation; GFAP and Iba1: goat anti-mouse IgG biotin conjugate 1:10,000 and 1:5,000, respectively, Sigma) followed by further rinsing. Membranes were developed with ECL reagent (Amersham, GE Healthcare, Buckinghamshire, UK) and images were captured and quantified using the Bio-Rad Laboratories (Hercules, CA, USA) software QuantityOne ChemiDoc.
Enzyme-linked immunosorbent assay (ELISA)
Determination of Aβ1–40 and Aβ1–42 in prefrontal cortex total homogenates from young mice was done using a double-antibody capture ELISA DELFIA® (PerkinElmer, Waltham, MA, USA). Briefly, plates were coated with monoclonal antibody (mAb) G210 (for Aβ1–40) or mAb G211 (for Aβ1–42), blocked with 0.5 % (w/v) casein/PBS buffer (pH 7.4) and then biotinylated mAb WO2 was added to the wells. Bound antibody was detected with streptavidin-labeled Europium (Perkin Elmer) and analyzed with Wallac Victor 2 1420 Multilabel Plate Reader (PerkinElmer) with excitation wavelength at 340 nm and emission wavelength at 613 nm.
In 14-month-old mice, total homogenates determination of Aβ1–40 and Aβ1–42 was done using a Sandwich ELISA commercial kit (Invitrogen) according to the manufacturer’s instructions. Absorbance at 450 nm was measured with a Labsystems Multiskan Bichromatic (Helsinki, Finland) microtiter plate reader.
Inductively coupled plasma-mass spectrometry (ICP-MS)
Determination of Cu, Mn, Fe, and Zn content in the prefrontal cortex of young mice was carried out at The Mental Health Research Institute, as described by Cherny et al. [26].
Determination of old mice cortical metal content was done at the Servei d’Anàlisi Químic (SAQ) of the UAB. Briefly, samples were digested with HNO3 at 60 °C and further diluted in HNO3 1 %. ICP-MS was performed using an Agilent 7500ce spectrometer and samples were introduced using a glass nebulizer at a flow of 0.9 l/min.
Immunohistochemistry (IHC)
Fixed brains were paraffin-embedded and cut sagittally in 6–8-μm-thick sections for assessing the amyloid plaque load (primary antibody: 4G8—Aβ17–24—1:5,000, Signet; secondary antibody: anti-mouse IgG biotin conjugate 1:400, Sigma) and astrogliosis (primary antibody: anti-GFAP 1:900, DakoCytomation; secondary antibody: biotinylated anti-rabbit IgG 1:300, Vector Laboratories, Inc., Burlingame, CA, USA) surrounding the plaques in cortex and hippocampus of aged mice using standard procedures. For quantification, three non-consecutive sections per mouse were used. Two measures were taken in each region, the area occupied by specific signal (plaques or astrocytes) and the intensity (volume) of the specific signal were assessed and the mean values used for statistical analysis. Photographs of the cortex and hippocampus were taken with a Nikon Eclipse 400 (Nikon Corporation, Tokyo, Japan) microscope (4×), and the images were transformed to a gray scale and were analyzed using Scion image (Scion Corporation, Frederick, MD, USA) software.
RNase protection assay (RPA)
Cortex and hippocampus samples from young mice were used to extract total RNA and analyzed for different mRNA species by RPA using a host response multi-probe set that included the inflammation markers: ICAM, iNOS, A20, Mac-1, EB22/5, GFAP, and L32 (loading control) as previously described [58].
Statistical analysis
Data was analyzed using the Statistical Package for Social Sciences (SPSS) version 17.0. Males and females were analyzed separately and only exceptionally combined gender analysis was done. Survival was analyzed using the Kaplan–Meier survival test, using genotype as a factor with four levels (WT, MT1 + 2KO, APPWT, APPMT1 + 2KO). The rest of the data was analyzed using generalized linear model (GLZ) and generalized estimated equations (GEE) for repeated measures (i.e., body weight or acquisition in the MWM). In both cases, APP and MT were used as factors with two levels each: APP+ and APP− for APP and MTWT and MTKO for MT. In addition, in GEE analysis, “time” was used as a within-subject factor. In parameters such as amyloidosis, which is not present in APP− genotypes, only APP+ genotypes were analyzed, using MT as a grouping factor. Very occasionally, one animal per group was eliminated because its value was extreme according to SPSS criteria. Statistical significance was defined as p ≤ 0.05 and marginal significance as p ≤ 0.1.
Results
MT1 + 2 deficiency rescues APP-induced mortality and body weight loss in a gender-dependent manner
As expected, the expression of the human amyloid precursor protein (hAPP) transgene increased mortality, with the effects more conspicuous in males (≈75 %) than in females (≈40 %) (Fig. 1a). The absence of MT1 + 2 proteins showed a gender-specific effect in partially preventing the mortality in APP+ females but not in males (Fig. 1a). MT1 + 2-deficiency did not affect the survival of APP− mice (Fig. 1a). Interestingly, analysis of the Mendelian distribution of the mouse litters at weaning suggested the existence of perinatal mortality since APP+ mice were born at a less-than-expected ratio, with the APPWT pups the more affected group (Fig. 1c).
In agreement with the survival data, APP+ mice body weight was lower than that of APP− throughout the examined period, and MT1 + 2-deficiency partially reversed the phenotype in females (Fig. 1b).
MT1 + 2-deficiency partially reverses the behavioral phenotype of Tg2576 mice
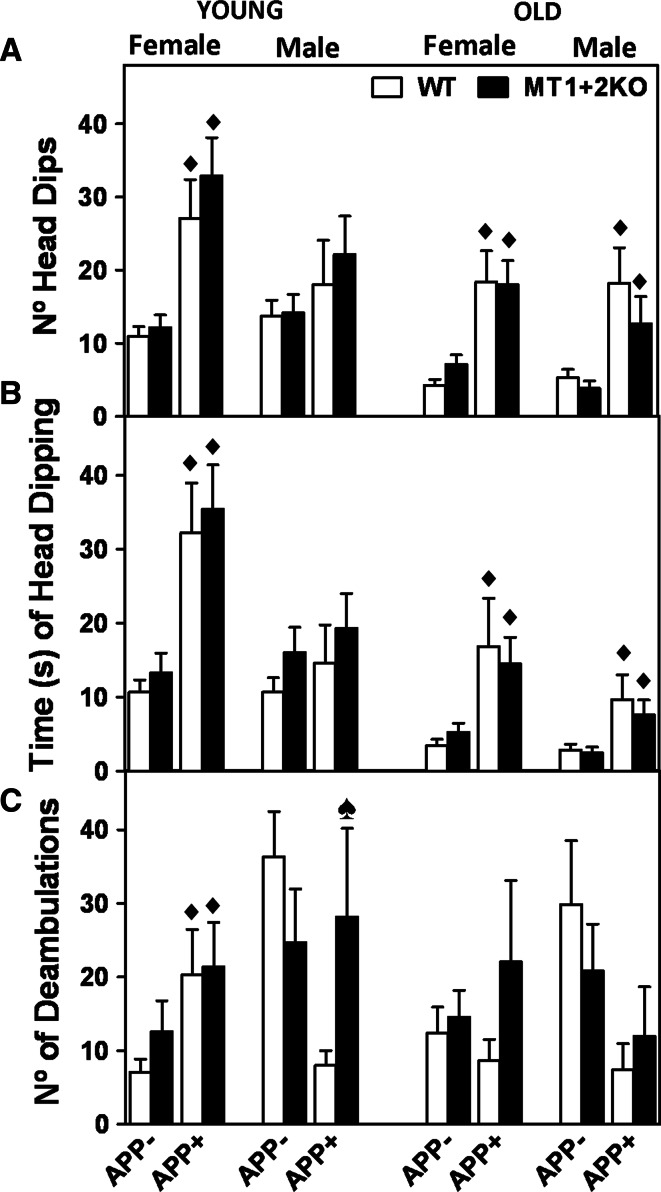
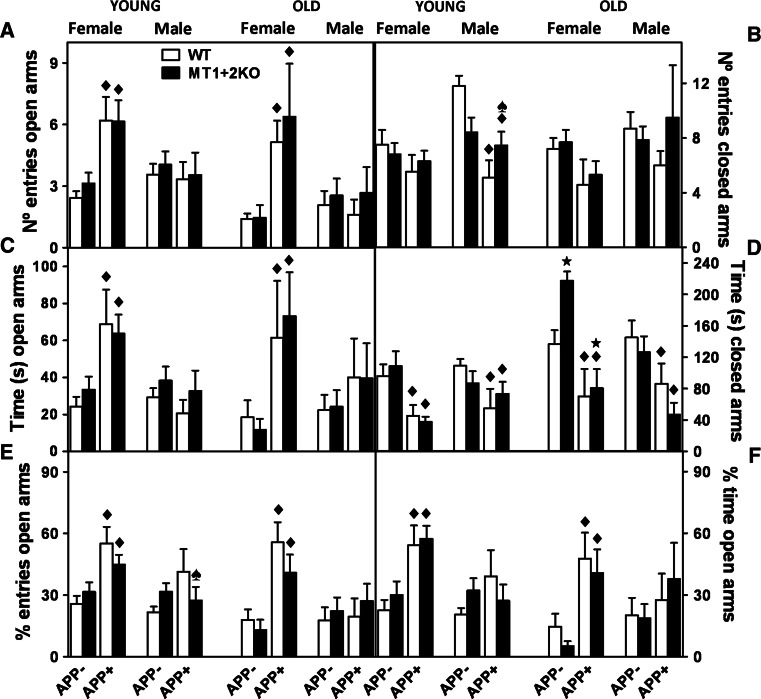
Deambulations, exploratory behavior, and anxiety were evaluated with the Hole-board (HB) and the Plus-Maze (PM) tests (Figs. 2, 3). Young APP+ males and old APP+ mice from both genders tended to be hypoactive in the HB, as evidenced by the decreased number of deambulations compared to APP− mice, while the opposite was observed in young females (Fig. 2c). Exploratory behavior (head dipping) in the HB was similarly increased in both genders of APP+ mice (Fig. 2a, b). Finally, APP+ mice were also significantly less anxious in the PM than their littermate controls, as evidenced by the increased time and number of entries in the open arms of the maze (Fig. 3a, c, e, f) and the decreased time spent in the closed ones (Fig. 3d). MT1 + 2-deficiency did not dramatically affect these behavioral phenotypes either in the presence or absence of the APP transgene (Figs. 2, 3). It did, however, significantly reverse the hypoactivity (Fig. 2c) as well as the diminished anxiety levels (Fig. 3b, e) of young APP+ males.
Fig. 2.
MT1 + 2-deficiency partially reverses hAPP-induced changes of behavior in the Hole-board test. a, b Exploratory behavior and c activity were evaluated with the Hole-board (HB) at 4 (young) and 10 (old) months of age. a The number of head dips and b time of head dipping were increased in both genders of APP+ mice, suggesting an increased exploratory behavior of APP+ mice compared to controls. c On the other hand, the number of deambulations tended to be decreased in APP+ genotypes, except for young APP+ females, hence suggesting hypoactivity. The absence of MT1 + 2 did not consistently affect exploratory behavior (a, b) but it did, however, significantly reverse the hypoactivity observed in young APP+ male mice (c). Data represents mean ± SEM (n = 9–28 and 5–20 for young and old mice, respectively), and they were analyzed using the generalized linear model (GLZ) with hAPP and MT1 + 2 deficiency as factors. Filled diamond, p ≤ 0.01 versus APP− mice; spade, p ≤ 0.04 significant interaction between both factors
Fig. 3.
MT1 + 2-deficiency partially reverses hAPP-induced changes of behavior in the Plus-Maze test. a The number of entries and c time spent in the open arms together with e the percentage of entries [(no. of entries open arms/no. entries open arms + no. of entries closed arms) × 100] and f the percentage of time spent in the open arms [(time spent open arms/time spent open arms + time spent closed arms) × 100] of the Plus-Maze (PM) test were used to asses anxiety levels. The number of entries in the closed arms (b) were used as an activity measure. As expected, and in line with HB results, APP+ animals from both sexes were less anxious (a, c, e, f) and tended to hypoactivity (b), and the absence of MT1 + 2 partly prevented these behaviors in young males (b, e). Data represents mean ± SEM (n = 9–28 and 5–20 for young and old mice, respectively), and they were analyzed using GLZ with hAPP and MT1 + 2 deficiency as factors. Filled diamond, p ≤ 0.03 versus APP− mice; star, p ≤ 0.04 versus MTWT mice; spade, p ≤ 0.025 significant interaction between both factors
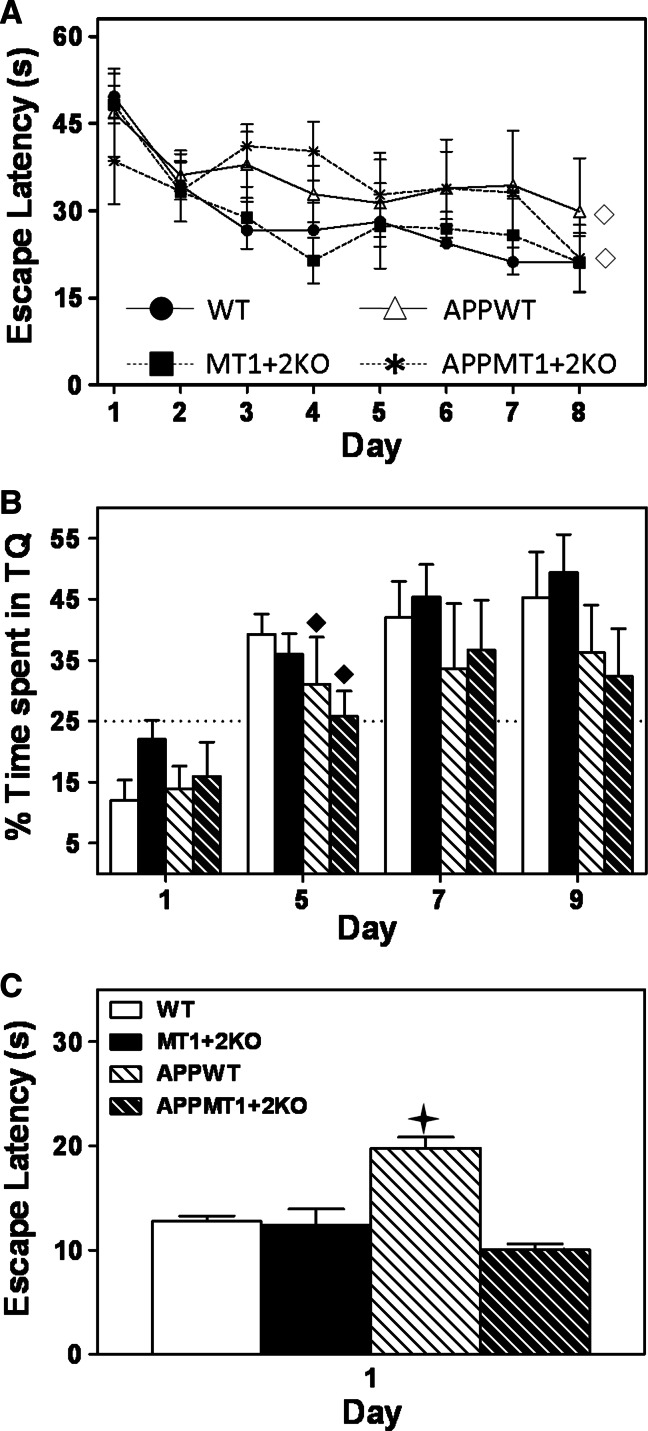
In the MWM test, when challenged to locate a submerged platform using external cues as reference, APP+ females showed impaired spatial memory and learning (Fig. 4a), and impaired retention during the probe trial tests (Fig. 4b). MT1 + 2-deficiency had no effect in any of these tests (Fig. 4a, b). When animals were challenged to learn a new location, there were no significant differences between genotypes in any of the variables measured (not shown). Finally, in the visible platform test (Fig. 4c), where mice have to switch to a stimulus-response strategy, all genotypes showed similar escape latencies except for APPWT females, which demonstrated increased escape latencies compared to their controls, perhaps reflecting an inability of this group to change the search strategy.
Fig. 4.
MT1 + 2-deficiency reverts APP-associated inability to switch from a spatial to a stimulus–response strategy. Spatial memory and learning of 6-month-old females were assessed in the Morris Water Maze (MWM). a In the hidden platform phase, APP+ females showed impaired learning and memory as evidenced by the increased escape latencies. b APP+ females also showed impaired retention as evidenced by the decreased percentage of time spent in the target quadrant (TQ) during the probe trial tests. The dotted line in this graph represents the % of time, that by chance and not because of a learning process, mice can spend in the TQ. No differences due to MT1 + 2 deficiency were observed in any of these parameters. c When tested in the visible platform test, which in contrast to the hidden platform phase is best solved using a stimulus–response strategy rather than a spatial one, APPWT females showed higher escape latencies than the rest of the genotypes including APPMT1 + 2KO females. Data represents mean ± SEM (n = 6–9), and they were analyzed using either repeated measures using the GEE in a or day-by-day analysis using the generalized linear model (GLZ) in b: open diamond, p ≤ 0.04, filled diamond, p ≤ 0.05 versus APP− mice. In the visible platform test (c), genotype was used as a factor using the GLZ: plus, p ≤ 0.001 versus APPMT1 + 2KO mice
MT1 + 2-deficiency reduces amyloid plaque load in cortex and hippocampus but has no effect on the associated astrocytosis
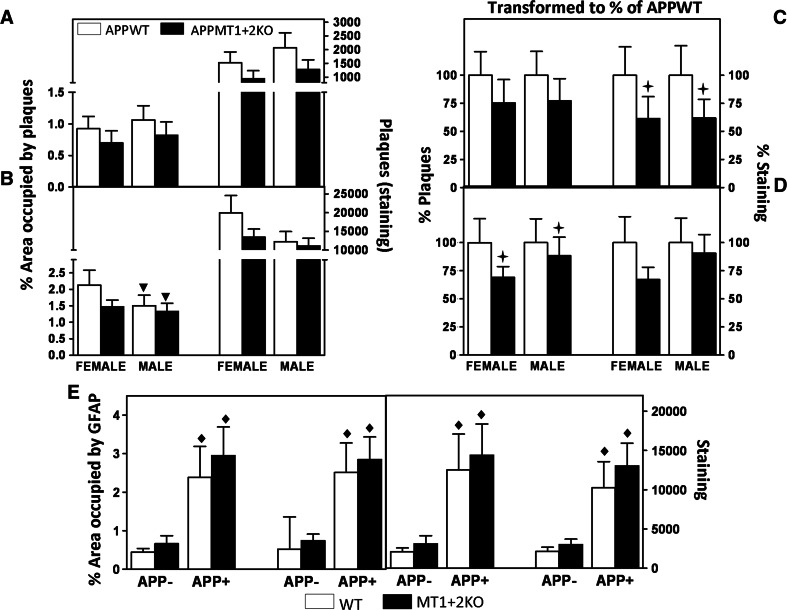
The amyloid plaque load of 14-month-old mice was evaluated by IHC. The results indicate that in the hippocampus, both sexes had similar amyloid load (Fig. 5a), while in the cortex, females tended to have a higher load than males (Fig. 5b); in both brain areas, MT1 + 2-deficiency clearly tended to decrease either the percentage of area occupied by plaques and/or the staining intensity (volume). Since the trends were similar, these values were transformed to percentage of the mean value of control mice (APPWT) (Fig. 5c, d) and a combined statistical analysis using MT, sex, and age as factors was carried out. With these normalized data, the results confirm that, especially in females (Fig. 5), MT1 + 2 deficiency significantly reduced the amyloid burden in both regions assessed, although marginal significance only was reached in some of the parameters measured (p = 0.062 and 0.065 for area occupied by plaques in the hippocampus and staining intensity in the cortex, respectively).
Fig. 5.
APPMT1 + 2KO mice present a lower amyloid plaque load in cortex and hippocampus than APPWT mice. The area occupied by plaques (left side) and the staining intensity (i.e., volume, right side) of Aβ immunostaining (IHC) of females and males were analyzed in hippocampus (a) and cortex (b). Females had a higher amyloid load than males in the cortex but not the hippocampus. In both sexes and brain areas, APPMT1 + 2KO mice tended to have a lower load than APPWT mice. In order to compare results from both sexes, the data was transformed to a percentage of the mean value of the control group (APPWT) (c, d). In the hippocampus (c), the absence of MT1 + 2 significantly decreased the amount of staining and the trend was similar for the percentage of area occupied by plaques (p = 0.062). In the cortex (d), MT1 + 2 deficiency significantly decreased the percentage of area occupied by plaques and the same trend could be observed in the amount of staining (p = 0.065). Evaluation of the associated astrocytosis by GFAP IHC in the cortex (e) revealed a clear hAPP-induced astrocytosis that was not affected by MT1 + 2 deficiency (area occupied by GFAP, left; staining intensity, right). Results are mean ± SEM (n = 7–10), and they were analyzed using GLZ with MT, sex, and age as factors. Inverse triangle, p < 0.05 versus females; plus, p ≤ 0.05 versus APPWT mice; filled diamond, p ≤ 0.001 versus APP− mice
Amyloid plaques typically induce gliosis, so we next assayed astrocytosis by GFAP IHC (Fig. 5e). In the hippocampus, which has a high baseline of GFAP immunostaining in control mice, no effects of the amyloid plaques were evident, while MT1 + 2-deficiency, if anything, tended to increase GFAP staining in males regardless of the presence of plaques (p = 0.075) (data not shown). In contrast, in the cortex a prominent, plaque-associated GFAP staining was demonstrated in both sexes, but no significant differences due to MT1 + 2 absence could be observed (Fig. 5e).
APP and its proteolytic fragments are increased in the hippocampus of females deficient for MT1 + 2
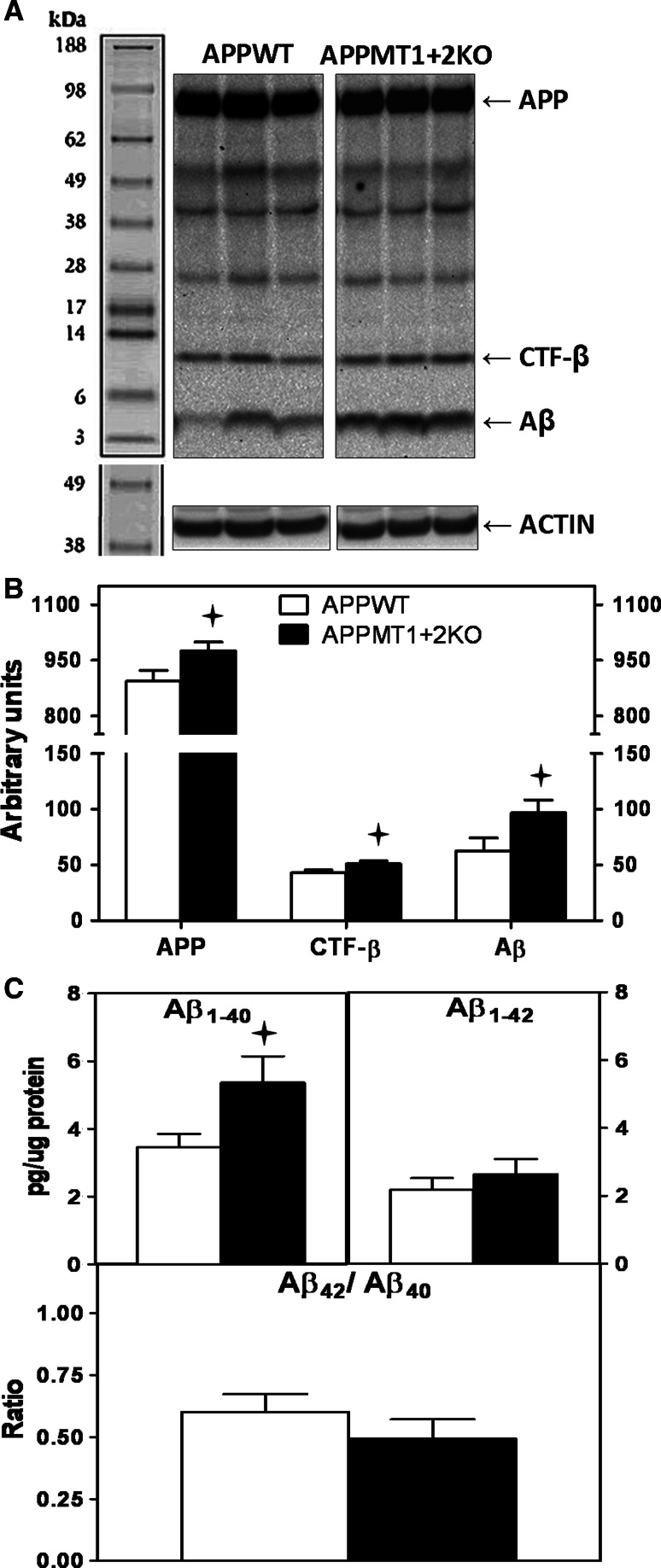
For further characterization of the amyloid content, total hippocampal homogenates were analyzed by western blot (WB) to assess the amount of APP and its proteolytic fragments (Fig. 6a). While no statistically significant differences between genotypes were found in males (not shown), the levels of APP, c-terminal fragment (CTF)-β and monomeric forms of Aβ were increased in APPMT1 + 2KO females compared to APPWT females (Fig. 6b). Further analysis by ELISA (only females were analyzed) revealed that Aβ1–40 was the primary form affected, showing a significant increase in mice deficient for MT1 + 2 (Fig. 6c).
Fig. 6.
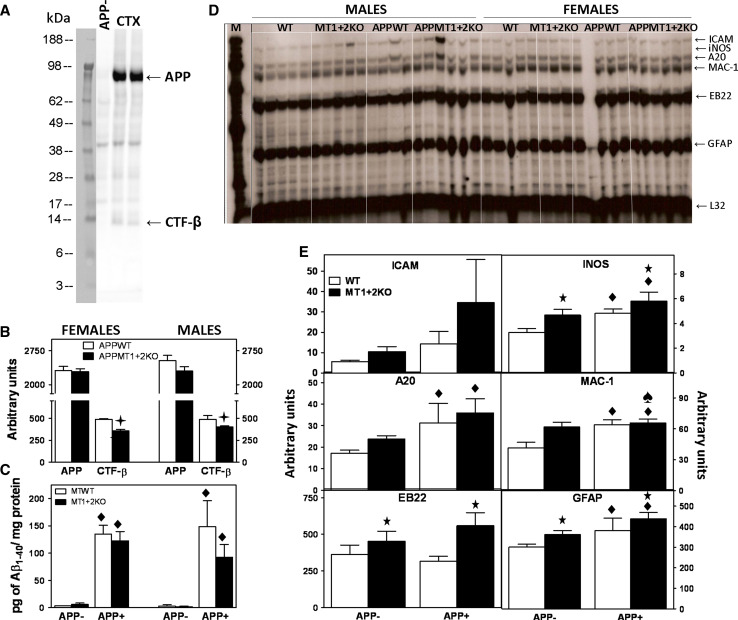
Increased APP, CTF-β and Aβ1–40 in the hippocampus of MT1 + 2 null females. Total hippocampal homogenates were assayed by WB and ELISA to further characterize the amyloid content. a Representative band pattern of the WB obtained with 6E10 antibody. Only the specific bands (only present in APP+ genotypes) were quantified, i.e., full-length APP (≈90 kDa), CTF-β (≈12 kDa) and Aβ (≈4 kDa). b MT1 + 2 deficiency significantly increased the levels of full-length APP and all its proteolytic fragments. c ELISA analysis evidenced that MT1 + 2 deficiency significantly increased Aβ1–40 levels but not Aβ1–42. Data represents mean ± SEM (n = 12–15). Plus, p at least ≤0.04 versus APPWT mice
APP and its proteolytic fragments are increased in the cortex of males deficient for MT1 + 2
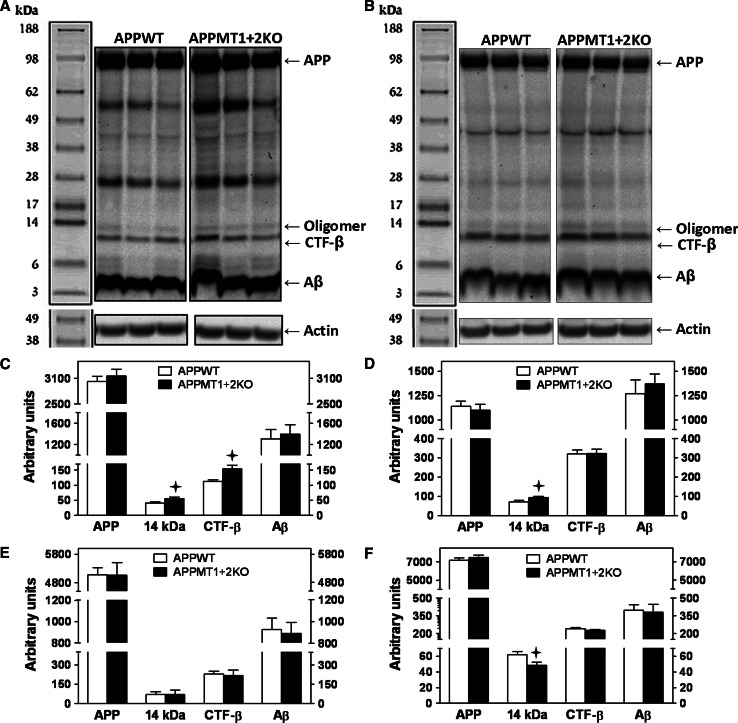
APP and its proteolytic fragments were studied by WB in total cortical homogenates as well as in the four independent cellular pools (fractions A–D) described by Lesné et al. [55]. In contrast to the hippocampus, in the cortex MT1 + 2-deficiency had a significant effect in males (Fig. 7a–d) rather than in females (Fig. 7e). However, a 14-kDa band, perhaps representing an oligomeric trimer of Aβ, was significantly decreased in the extracellular protein-enriched fraction of APPMT1 + 2KO females compared to controls (Fig. 7f).
Fig. 7.
Increased CTF-β and Aβ trimeric forms in the cortex of MT1 + 2 null males. a, b Representative WB band pattern obtained with 6E10 antibody in total cortical homogenates and fraction C (membrane-associated protein enriched fraction), respectively, of male mice. Apart from the specific bands mentioned in Fig. 6, a 14-kDa band, that by its size could represent a trimer of Aβ, was also quantified. MT1 + 2 deficiency in APP+ male mice significantly increased CTF-β and putative Aβ trimer levels in total cortical homogenates (c), and Aβ trimer levels in Fraction C (d). Analysis of female mice total cortical homogenates (e) showed no differences between APP+ genotypes, while in the extracellular protein enriched fraction (f) the 14-kDa band was significantly decreased by MT1 + 2 deficiency. Data represents mean ± SEM (n = 8–13). Plus, p at least ≤0.03 versus APPWT mice
In males, the absence of MT1 + 2 proteins significantly increased both CTF-β and the putative Aβ trimer in total cortical homogenates (Fig. 7a, c) and the latter also in the membrane-associated protein-enriched fraction (Fig. 7b, d), while no differences in full-length APP or Aβ could be detected in these pools and in any of the other assessed fractions (not shown).
APP-induced microgliosis but not astrocytosis is prevented by the absence of MT1 + 2
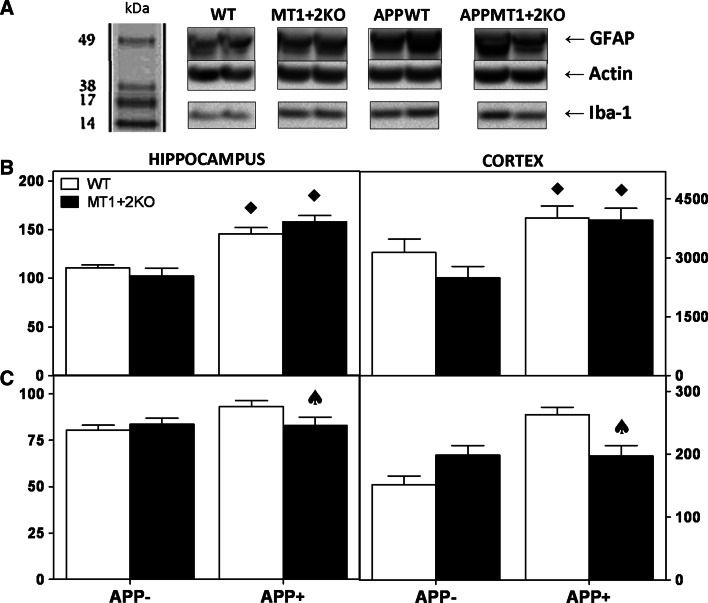
To further characterize the inflammatory response, hippocampal and cortical homogenates were analyzed by WB using anti-GFAP (astrocytes) and anti-iba1 (microglia) antibodies (Fig. 8a). In general, the presence of hAPP significantly increased both astrocytosis and microgliosis compared to APP− genotypes, an effect that was somewhat more prominent in males (Fig. 8) than in females (not shown). It is noteworthy that while the absence of MT1 + 2 had no effect on astrocytosis (Fig. 8a, b), it significantly prevented the APP-induced microgliosis (Fig. 8a, c).
Fig. 8.
APP-induced microgliosis is prevented by MT1 + 2 absence in males. Total cortex and hippocampus homogenates were assayed by WB. a Representative WB obtained with anti-GFAP and anti-Iba1 antibodies in cortex; anti-actin antibodies were used for loading controls and data normalization. b APP+ males showed significantly higher GFAP levels (reflecting astrocytosis) than APP− genotypes in both hippocampal (left) and cortical (right) homogenates. However, no effect due to MT1 + 2 absence was observed in GFAP levels in none of the regions assessed. c Similarly to astrocytosis, Iba1 levels were also increased by hAPP presence compared to controls (reflecting microgliosis), but in this case, MT1 + 2 deficiency significantly prevented this effect. Data represents mean ± SEM (n = 9) and was analyzed using GLZ with APP and MT deficiency as main factors. Filled diamond, p ≤ 0.001 versus APP−; spade, p ≤ 0.04 significant interaction between factors
Cortical metal content of old mice does not appear to be critically affected by the presence of APP or the absence of MT1 + 2
Total cortical homogenates were used to assess the manganese (Mn), copper (Cu), iron (Fe), and zinc (Zn) content by ICP-MS (Fig. 9). In general, female metal levels were higher than those of males in old mice (p < 0.05).
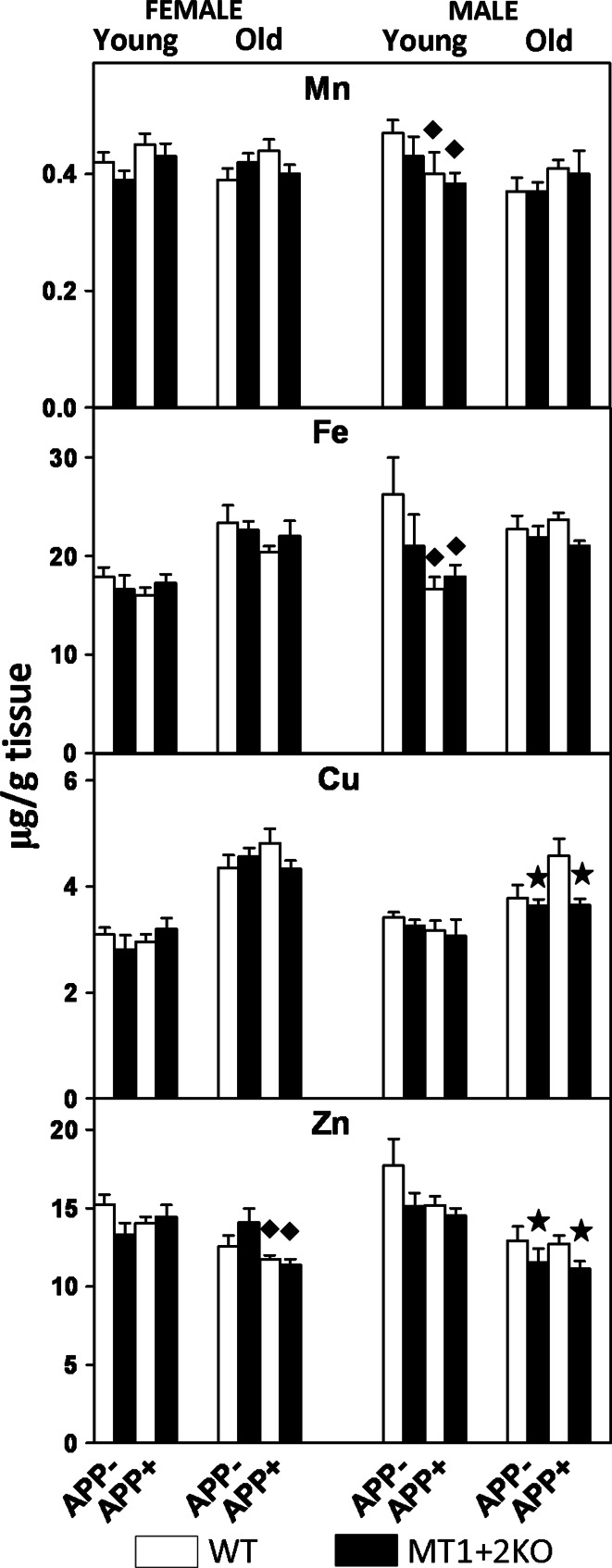
Fig. 9.
Total Mn, Fe, Cu, and Zn content are not critically affected by hAPP presence and MT1 + 2 absence. Prefrontal and total cortical homogenates from 5- and 14-month-old mice (young and old, respectively) were analyzed by ICP-MS. In general, metal content was higher in old females (left) than in males (right), and that despite statistically significant differences were observed, the effects of hAPP and MT1 + 2 deficiency were modest. Results are μg of metal/g of tissue (mean ± SEM; n = 5–8 and 9–12 for young and old mice, respectively), and they were analyzed using GLZ with APP and MT deficiency as main factors. Filled diamond, p < 0.05 versus APP− mice; star, p < 0.05 versus MTWT mice
Analysis of metal levels in females revealed that Zn levels were decreased in APP+ genotypes compared to controls while the other metals assessed were not affected by the presence of hAPP. The ablation of MT1 + 2 did not significantly affect metal content in this gender. In males, hAPP presence had no significant effect on any of the metals assessed although it clearly tended to increase Cu levels (p = 0.054). MT1 + 2 absence significantly decreased Cu and Zn levels and the same trend was observed in Fe levels (p = 0.078), with the effect more prominent in the APP+ mice.
Changes in APP processing and inflammatory responses occur as early as 5 months of age in APP+ mice
A subset of male and female mice were killed at 5 months of age to assess the amyloid content by WB and ELISA, the inflammatory response by RNase protection assay (RPA) (Fig. 10), and the metal content by ICP-MS (Fig. 9).
Fig. 10.
Amyloidosis and inflammatory response in young mice. a Representative WB band pattern obtained with WO2 antibody in prefrontal cortex homogenates from 5-month-old mice. b WB analysis showed no differences between genotypes in the APP content but decreased levels of CTF-β in MT1 + 2 null mice from both genders, perhaps suggesting a decreased β-secretase activity. Although by WB the monomeric forms of Aβ could not be detected, ELISA analysis (c) revealed that at 5 months of age, Aβ1–40 but not Aβ1–42 levels were already increased in APP+ compared to APP− mice, although no differences due to the absence of MT1 + 2 could be observed at that age. d RNase protection assay (RPA) of prefrontal cortex mRNA hybridized with a set of host response genes. e Quantification of these hybrids in males showed that both hAPP presence and MT1 + 2 absence altered the mRNA levels at this early age, suggestive of an increased inflammatory response. Data represents mean ± SEM (n = 4–6) and was analyzed using GLZ. Plus, p < 0.05 versus APPWT mice; filled diamond, p ≤ 0.001 versus APP− mice; star, p < 0.05 versus MTWT mice; spade, p < 0.05 significant interaction between both factors
The levels of APP and its proteolytic fragments were assessed in the prefrontal cortex by WB (Fig. 10a), and while no differences in full-length APP were found due to the absence of MT1 + 2, CTF-β levels were decreased in both APPMT1 + 2KO males and females compared to controls (Fig. 10b). While monomeric Aβ was not detectable using WB, we also analyzed our samples using ELISA, where we demonstrated that only Aβ1–40 levels were increased in APP+ mice compared to APP− mice at this age (Fig. 10c). MT1 + 2 deficiency had no significant effect on Aβ levels.
A set of host response genes were assayed by RPA to evaluate the inflammatory response in the cortex (Fig. 10d). In female mice, there was no difference resulting from either APP presence or MT absence (not shown). In males, however, the response was affected by both factors (Fig. 10e). On the one hand, APP+ males had higher mRNA levels of iNOS, A20, MAC-1 and GFAP compared to APP− controls and on the other hand males deficient for MT1 + 2 proteins showed increased mRNA levels of iNOS, MAC-1, EB22, and GFAP compared to males heterozygous for MT1 + 2. It is noteworthy that the absence of MT1 + 2 prevented APPMT1 + 2KO males from the APP-induced increases in MAC-1 mRNA levels.
Metal analysis (Fig. 9) revealed that, in contrast to old mice, the levels of all metals examined (Mn, Fe, Cu, and Zn) were increased (p < 0.02) in the young male mice compared to the age-matched females. In females, neither hAPP presence nor MT1 + 2 absence had a significant effect on metal content, although Mn tended to be increased in APP+ genotypes (p = 0.051). Likewise, MT1 + 2 deficiency had no effect on metal content in males but hAPP presence significantly decreased Mn and Fe levels in APP+ genotypes. Analysis of data comparing young with old mice using GLZ with APP, MT, and age as factors revealed that, as expected, metal content was affected by age. However, the age effect was different depending on the metal, gender, and even genotype (age × APP interactions) of mice. In general terms, Fe and Cu levels increased with age, Zn decreased with age and Mn increased in females and decreased in males (Fig. 9).
Discussion
Probably the most striking result of this study is that contrary to expectations [31, 82, 83], MT1 + 2 isoforms appear to be detrimental factors, albeit only in females, regarding the survival of Tg2576 mice. APP-induced lethality is a phenomenon widely described in the literature in different AD models including Tg2576 mice, that may occur as adults but also before weaning [8, 19, 32, 44, 65, 70]. The APP mice show an average 10–20 % reduction in their body weight, consistent with their decreased life span and with no differences in food and water intake [74]. In line with these studies, when genotyped immediately after weaning, the APP+ genotypes, and more specifically the APPWT mice, were clearly underrepresented in our study, strongly suggesting that either intrauterine or early postnatal hAPP-induced mortality was indeed occurring, and that MT1 + 2 have a detrimental role when the hAPP transgene is expressed. This early negative effect of MT1 + 2 appears to be more prominent in female mice. The survival of the mice into adulthood followed the same trends, with a large mortality in the APP+ mice that was partially reversed by MT1 + 2-deficiency in females only. Mortality was much more severe in males, and it is feasible that in such a context MT1 + 2-deficiency cannot make a difference. In agreement with the survival data, MT1 + 2 deficiency partially protected against the APP-related lower body weights in females but not in males. In contrast, no effect of MT1 + 2-deficiency on survival or body weight was seen in APP− mice.
The mechanisms underlying mortality in APP mice are not well understood. Early experiments demonstrated that SOD1 could dramatically decrease the mortality of APP mice [19, 44]. SOD is a major antioxidant protein, and thus it was then proposed that reactive oxygen species could be a major mechanism of the hAPP-induced mortality. The prosurvival role of MTs was also attributed to their antioxidant effects [31, 82, 83], and indeed we also observe a tendency for increased mitochondrial damage in the brains of old MT1 + 2 KO mice (unpublished data), and thus the role seen in APP+ female mice was unexpected. Nevertheless, one study demonstrated that TNFα-induced mortality was dramatically reduced in MT-1 + 2KO mice [77], indicating that the roles of MTs on survival will depend on the context.
One obvious possibility to explain the phenotype of APPMT1 + 2KO versus APPWT mice is the alteration of the amyloid cascade, since amyloidosis and oxidative stress are closely related [7]. At 5 months of age, prior to amyloid deposition, WB analysis evidenced a reduction of CTF-β levels in the cortex of APPMT1 + 2KO mice in both genders without affecting APP and Aβ levels, suggesting that at this age MT-1 + 2 absence could be affecting the β-secretase pathway. At 14 months of age, when the amyloid pathology is firmly established in both hippocampus and cortex, a complex pattern of effects emerged, eventually with a region- and gender-dependent effect of MT1 + 2 deficiency on amyloidosis. Thus, in both the hippocampus and the cortex, MT1 + 2 deficiency decreased the amyloid load (as measured by IHC) in both male and female APP+ mice. Consistent with these data, we previously demonstrated that exogenous administration of Zn7MT2 tended to increase the amyloid plaque burden in the hippocampus of old Tg2576 mice [57]. Interestingly, when the precursor protein APP and its proteolytic fragments in the hippocampus were analyzed by WB, MT1 + 2 absence instead increased full-length APP, CTF-β, and Aβ levels in females only; ELISA analysis revealed that it was the Aβ1–40 form that was most affected. More APP protein means more substrate for α- and β-secretase proteolytic cleavage, thus leading to the observed increases in CTF-β and Aβ monomer. Yet, that does not fit well with the observed decrease of amyloid plaques in the hippocampus. This apparent contradiction might be reconciled considering the importance of the type of Aβ monomer that predominates. Although Aβ1–40 and to a lesser extent Aβ1–42 were increased in the APPMT1 + 2KO female mice, the Aβ1–42/Aβ1–40 ratio tendency was the opposite, and small alterations in this ratio dramatically affect aggregation kinetics since Aβ1–40 has been claimed to be anti-amyloidogenic [29, 47, 52]. Our results suggest that MT-1 + 2 absence could be shifting the balance of Aβ1–42/Aβ1–40 toward a less amyloidogenic composition and thus preventing plaque formation in hippocampus. Moreover, since Aβ1–40 has also been suggested to exert antioxidant effects [2, 5], the increased levels of Aβ1–40 of APPMT1 + 2KO females could explain at least in part their increased survival.
Paradoxically, no effects of MT1 + 2-deficiency were observed in the amyloid cascade (as measured by WB) in the hippocampus of APP+ male, while the opposite was seen in the cortex: clear signs of increased levels of proteolytic fragments of APP in males but not in females. Further analysis of different cortical compartments (extracellular, cytoplasmic, and membrane) did not clarify this complex pattern. Yet, a putative trimeric form of Aβ (14-kDa band) was detected in the cortical homogenates but not in hippocampus and it differed in APPMTKO mice compared to APPWT mice, increased in the membrane-enriched protein fraction of males and decreased in the extracellular-enriched protein fraction of females. Trimers have been proposed as the fundamental Aβ assembly unit in vivo [55] and the main toxic species of Aβ [78], and perhaps this could also be related to the lower mortality of the APPMTKO female mice. Incidentally, some of the beneficial effects of increased copper bioavailability (see below for further discussion) could be related to a specific decrease of Aβ trimers [24].
Another putative mechanism set in motion by MT1 + 2 deficiency is an altered inflammatory response in the brain [63]. At 5 months of age, the APP+ male mice already showed signs of neuroinflammation in the cortex, which was confirmed in old mice. MT-1 + 2 deficiency per se had a significant increasing effect, particularly in microglia/macrophages activation markers, at both ages. Remarkably, this upregulated microglial activation was not further increased by the concomitant expression of hAPP, in contrast to what happens in APPWT mice. It is therefore tempting to suggest that this heightened inflammatory response is somehow related to the lower amyloid plaque burden observed in APPMT1 + 2KO mice. It is difficult to clarify the nature of such a relationship, since one could consider that the reduction in plaques seen in APPMT1 + 2KO mice could be due to the increased microglia/macrophage activation and subsequent Aβ clearance, or alternatively microglia activation could be blunted because of a previous decrease in plaque formation. One way or another, it is noteworthy to comment that changes in microglia activation may be relevant for understanding the premature death as suggested by the increased mortality found in studies with impaired microglial recruitment [32].
There is compelling evidence relating AD pathology with metals such as Zn, Cu, and Fe, which have been reported to participate in Aβ aggregation [14] and in ROS production [7]. As expected, both aging and hAPP presence affected metal content, albeit changes were modest. Perhaps the only remarkable effect of MT1 + 2 deficiency is a decrease of Cu and Zn levels in aged APP+ male mice, which might have contributed to the lower amyloid plaques load of the APPMT1 + 2KO mice. Despite these decreased metal levels, it could be speculated that they would be more bioavailable considering the very high affinity of Zn and Cu for MTs [38]. Experiments with transgenic mice expressing hAPP show a beneficial effect of increased Cu bioavailability on life span, either by supplementing it with the diet [8, 70] or by using genetically modified mice [65]. Of notice, increased Cu bioavailability may be linked to decreased Aβ trimers [24]. Also, increased Zn bioavailability has been proven to be beneficial in other situations, e.g., after TNFα-induced lethal shock [77].
Although there are inconsistencies in the literature, a number of behavioral traits have been shown to be altered in AD mouse models including Tg2576 mice. Noteworthy, gender differences in several of these paradigms have been described in this model and also in wild-type animals [34, 50]. In general, APP+ genotypes tended to be hypoactive and less anxious compared to controls regardless of age and gender [66], which we also found in all cases except young female mice for deambulations. MT1 + 2 deficiency did not consistently affect the behavior of mice in the HB and PM tests, but nevertheless it tended to reverse the effect of hAPP on activity and on anxiety (significant in young males). Impaired cognition has also been widely described in the Tg2576 and other AD mouse models [25, 66] although not all authors have been able to demonstrate cognitive impairment [40]. In our experiments, APP+ females showed impaired spatial learning and memory and subsequent retention in the MWM. MT1 + 2 absence had no effect on these parameters but, interestingly, it reverted the inability of APP+ females to switch from a “spatial strategy”, appropriate for the hidden platform task, to the “stimulus–response strategy” needed for ensuing visible platform testing [49]. Taken together, this suggests that MT1 + 2 absence could be delaying or even preventing some of the hAPP-induced changes in behavior. Whether the underlying mechanisms could be related with those discussed above remains to be established. Importantly, it has been demonstrated that there is no obvious relationship between amyloid deposits and behavioral changes [41].
Age is the main risk factor for AD and one consequence of normal aging is depletion of sex steroids, which have been shown to significantly contribute to AD risk in both women and men [76]. In general, epidemiological evidence supports an increased prevalence and incidence of AD in women [1]. Moreover, AD pathology and AD-related cognitive decline have also been shown to be greater in females than in males [6, 72]. Despite several prospective studies have supported the concept that estrogen-based hormone therapy (HT) could effectively reduce the risk of AD and improve age-related deficits in cognition [20], findings from the Women’s Health Initiative Memory Study (WHIMS), the most exhaustive clinical evaluation of HT, failed to confirm these results, and moreover associated HT with increased AD risk [33]. Female and male brain demonstrate different vulnerabilities to CNS disorders, including AD [16], which might be due to sexual dimorphisms during development as well as to adult sex differences in hormones [76]. Both estrogens and androgens exert general neuroprotective actions relevant to several neurodegenerative conditions, some in a sex-specific manner, and regulate key processes implicated in AD pathogenesis [76]. For instance, immune and inflammatory responses are affected by sex steroids, which could be modulating the production of inflammatory and immunoregulatory cytokines such as IL-6 [51], a major regulator of MT1 + 2 [53, 64]. Interestingly, several AD mouse models, including the Tg2576 mice, reproduce these sexual differences, e.g., increased amyloid plaque load and overall higher Aβ40 in females compared to age-matched males [17]; worse cognitive performance in females than in males at determinate ages [23]; increased amyloid pathology and worse cognitive performance after ovariectomy/orchiectomy and improvement of these parameters when estrogens/androgens were administered [22, 68]. The mechanisms underlying this sexual disparity are still unclear, but it has been suggested that differences in the stress response [23] and differences in the accumulation of synaptic Zn with aging [54], could partly explain these divergences. In addition, it has been suggested that MTs had an intimate intracellular relationship with estrogenic activity [18, 81]. Moreover, testosterone and estrone have been reported to increase MT1 + 2 and Zn levels in brain and cerebellum [9] and MT1 + 2 levels in several organs including the brain, have been reported to be increased in females versus males suggesting sexual dimorphism in the regulation and distribution of this protein [12, 39, 61]. Taken all together, these results strongly suggest that sexual dimorphism occurs in both human and AD mouse models and that sexual steroids have a central role in modulating several aspects of AD pathology, and could explain the differences between genders observed in this study.
In summary, we have demonstrated that MT1 + 2 effectively alters the amyloid cascade, survival, body weight, and behavior in a complex manner in a mouse model of AD. Contrary to expectations, MT1 + 2 may in fact be detrimental to some extent. Considering their metal-binding capabilities, these results may provide some insight into the mechanisms underlying the effects of metal-based approaches to the treatment of AD, such as clioquinol and PBT2, which are thought to redistribute metals from extracellular collections and restore intracellular metal reserves where depleted [11].
Acknowledgments
The authors are grateful for grants from the Ministerio de Ciencia e Innovación y Cofinanciada por el Fondo Europeo de Desarrollo Regional (FEDER), SAF2002-01268, SAF2005-00671, SAF2008-00435, and SAF2011-23272 (J. Hidalgo). Y. Manso acknowledges her Ph.D. fellowship (AP2005-0588). P.A. Adlard is supported by the National Health and Medical Research Council of Australia, The Australian Research Council, The Alzheimer’s Association (USA), and the Joan and Peter Clemenger Trust.
Conflict of interest
A.I. Bush is a paid consultant and shareholder of Prana Biotechnology Ltd, and a paid consultant of Adenoa Inc, and a shareholder of Brighton Biotech Inc. P.A. Adlard is a paid consultant and shareholder of Prana Biotechnology Ltd.
References
- 1.Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, Dartigues JF, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A (1999) Gender differences in the incidence of AD and vascular dementia: the EURODEM Studies. EURODEM Incidence Research Group. Neurology 53:1992–1997 [DOI] [PubMed] [Google Scholar]
- 2.Andorn AC, Kalaria RN (2000) Factors affecting pro- and anti-oxidant properties of fragments of the b-protein precursor (bPP): implication for Alzheimer’s disease. J Alzheimers Dis 2:69–78 [DOI] [PubMed] [Google Scholar]
- 3.Andrews GK (2000) Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol 59:95–104 [DOI] [PubMed] [Google Scholar]
- 4.Armario A, Hernández J, Bluethmann H, Hidalgo J (1998) IL-6 deficiency leads to increased emotionality in mice: evidence in transgenic mice carrying a null mutation for IL-6. J Neuroimmunol 92:160–169 [DOI] [PubMed] [Google Scholar]
- 5.Atwood CS, Obrenovich ME, Liu T, Chan H, Perry G, Smith MA, Martins RN (2003) Amyloid-beta: a chameleon walking in two worlds: a review of the trophic and toxic properties of amyloid-beta. Brain Res Brain Res Rev. 43:1–16 [DOI] [PubMed] [Google Scholar]
- 6.Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA (2005) Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry 62:685–691 [DOI] [PubMed] [Google Scholar]
- 7.Barnham KJ, Masters CL, Bush AI (2004) Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 3:205–214 [DOI] [PubMed] [Google Scholar]
- 8.Bayer TA, Schäfer S, Simons A, Kemmling A, Kamer T, Tepest R, Eckert A, Schüssel K, Eikenberg O, Sturchler-Pierrat C, Abramowski D, Staufenbiel M, Multhaup G (2003) Dietary Cu stabilizes brain superoxide dismutase 1 activity and reduces amyloid Abeta production in APP23 transgenic mice. Proc Natl Acad Sci USA 100:14187–14192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beltramini M, Zambenedetti P, Wittkowski W, Zatta P (2004) Effects of steroid hormones on the Zn, Cu and MTI/II levels in the mouse brain. Brain Res 1013:134–141 [DOI] [PubMed] [Google Scholar]
- 10.Bertram L, Lill CM, Tanzi RE (2010) The genetics of Alzheimer disease: back to the future. Neuron 68:270–281 [DOI] [PubMed] [Google Scholar]
- 11.Biran Y, Masters CL, Barnham KJ, Bush AI, Adlard PA (2009) Pharmacotherapeutic targets in Alzheimer’s disease. J Cell Mol Med 13:61–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bremner I, Williams RB, Young BW (1981) The effects of age, sex, and zinc status on the accumulation of (copper, zinc)-metallothionein in rat kidneys. J Inorg Biochem 14:135–146 [DOI] [PubMed] [Google Scholar]
- 13.Bush AI (2003) The metallobiology of Alzheimer’s disease. Trends Neurosci 26:207–214 [DOI] [PubMed] [Google Scholar]
- 14.Bush AI, Moir RD, Rosenkrantz KM, Tanzi R (1995) Zinc and Alzheimer’s disease-response. Science 268:1921–1923 [DOI] [PubMed] [Google Scholar]
- 15.Bush AI, Pettingell WH, Multhaup G, d Paradis M, Vonsattel JP, Gusella JF, Beyreuther K, Masters CL, Tanzi RE (1994) Rapid induction of Alzheimer A beta amyloid formation by zinc. Science 265:1464–1467 [DOI] [PubMed] [Google Scholar]
- 16.Cahill L (2006) Why sex matters for neuroscience. Nat Rev Neurosci 7:477–484 [DOI] [PubMed] [Google Scholar]
- 17.Callahan MJ, Lipinski WJ, Bian F, Durham RA, Pack A, Walker LC (2001) Augmented senile plaque load in aged female beta-amyloid precursor protein-transgenic mice. Am J Pathol 158:1173–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cano-Gauci DF, Sarkar B (1996) Reversible zinc exchange between metallothionein and the estrogen receptor zinc finger. FEBS Lett 386:1–4 [DOI] [PubMed] [Google Scholar]
- 19.Carlson GA, Borchelt DR, Dake A, Turner S, Danielson V, Coffin JD, Eckman C, Meiners J, Nilsen SP, Younkin SG, Hsiao KK (1997) Genetic modification of the phenotypes produced by amyloid precursor protein overexpression in transgenic mice. Hum Mol Genet 6:1951–1959 [DOI] [PubMed] [Google Scholar]
- 20.Carlson MC, Zandi PP, Plassman BL, Tschanz JT, Welsh-Bohmer KA, Steffens DC, Bastian LA, Mehta KM, Breitner JC, Group CCS (2001) Hormone replacement therapy and reduced cognitive decline in older women: the Cache County Study. Neurology 57:2210–2216 [DOI] [PubMed] [Google Scholar]
- 21.Carrasco J, Adlard P, Cotman C, Quintana A, Penkowa M, Xu F, Van Nostrand WE, Hidalgo J (2006) Metallothionein-I and -III expression in animal models of Alzheimer disease. Neuroscience 143:911–922 [DOI] [PubMed] [Google Scholar]
- 22.Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ (2007) Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci 27:13357–13365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinton LK, Billings LM, Green KN, Caccamo A, Ngo J, Oddo S, McGaugh JL, LaFerla FM (2007) Age-dependent sexual dimorphism in cognition and stress response in the 3xTg-AD mice. Neurobiol Dis 28:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crouch PJ, Hung LW, Adlard PA, Cortes M, Lal V, Filiz G, Perez KA, Nurjono M, Caragounis A, Du T, Laughton K, Volitakis I, Bush AI, Li QX, Masters CL, Cappai R, Cherny RA, Donnelly PS, White AR, Barnham KJ (2009) Increasing Cu bioavailability inhibits Abeta oligomers and tau phosphorylation. Proc Natl Acad Sci USA 106:381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK (1999) Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci 2:271–276 [DOI] [PubMed] [Google Scholar]
- 26.Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI (2001) Treatment with a copper–zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron 30:665–676 [DOI] [PubMed] [Google Scholar]
- 27.Chiaverini N, De Ley M (2010) Protective effect of metallothionein on oxidative stress-induced DNA damage. Free Radic Res 44:605–613 [DOI] [PubMed] [Google Scholar]
- 28.Chung RS, Howells C, Eaton ED, Shabala L, Zovo K, Palumaa P, Sillard R, Woodhouse A, Bennett WR, Ray S, Vickers JC, West AK (2010) The native copper- and zinc-binding protein metallothionein blocks copper-mediated Abeta aggregation and toxicity in rat cortical neurons. PLoS One 5:e12030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng Y, Tarassishin L, Kallhoff V, Peethumnongsin E, Wu L, Li YM, Zheng H (2006) Deletion of presenilin 1 hydrophilic loop sequence leads to impaired gamma-secretase activity and exacerbated amyloid pathology. J Neurosci 26:3845–3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durnam DM, Palmiter RD (1981) Transcriptional regulation of the mouse metallothionein-I gene by heavy metals. J Biol Chem 256:5712–5716 [PubMed] [Google Scholar]
- 31.Egli D, Yepiskoposyan H, Selvaraj A, Balamurugan K, Rajaram R, Simons A, Multhaup G, Mettler S, Vardanyan A, Georgiev O, Schaffner W (2006) A family knockout of all four Drosophila metallothioneins reveals a central role in copper homeostasis and detoxification. Mol Cell Biol 26:2286–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD (2007) Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med 13:432–438 [DOI] [PubMed] [Google Scholar]
- 33.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J, Study WsHIM (2004) Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 291:2959–2968 [DOI] [PubMed] [Google Scholar]
- 34.File SE (2001) Factors controlling measures of anxiety and responses to novelty in the mouse. Behav Brain Res 125:151–157 [DOI] [PubMed] [Google Scholar]
- 35.Guglielmotto M, Giliberto L, Tamagno E, Tabaton M (2010) Oxidative stress mediates the pathogenic effect of different Alzheimer’s disease risk factors. Front Aging Neurosci 2:3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Günther V, Lindert U, Schaffner W (2012) The taste of heavy metals: gene regulation by MTF-1. Biochim Biophys Acta (in press) [DOI] [PubMed]
- 37.Heneka MT, O’Banion MK, Terwel D, Kummer MP (2010) Neuroinflammatory processes in Alzheimer’s disease. J. Neural Transm 117:919–947 [DOI] [PubMed] [Google Scholar]
- 38.Hidalgo J, Aschner M, Zatta P, Vašák M (2001) Roles of the metallothionein family of proteins in the central nervous system. Brain Res Bull 55:133–145 [DOI] [PubMed] [Google Scholar]
- 39.Hidalgo J, Giralt M, Garvey JS, Armario A (1987) Sex and restraint stress differences in rat metallothionein and Zn levels. Rev Esp Fisiol 43:427–431 [PubMed] [Google Scholar]
- 40.Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O’Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K (1998) Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med 4:97–100 [DOI] [PubMed] [Google Scholar]
- 41.Holcomb LA, Gordon MN, Jantzen P, Hsiao K, Duff K, Morgan D (1999) Behavioral changes in transgenic mice expressing both amyloid precursor protein and presenilin-1 mutations: lack of association with amyloid deposits. Behav Genet 29:177–185 [DOI] [PubMed] [Google Scholar]
- 42.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G (1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274:99–102 [DOI] [PubMed] [Google Scholar]
- 43.Hua H, Münter L, Harmeier A, Georgiev O, Multhaup G, Schaffner W (2011) Toxicity of Alzheimer’s disease-associated Aβ peptide is ameliorated in a Drosophila model by tight control of zinc and copper availability. Biol Chem 392:919–926 [DOI] [PubMed] [Google Scholar]
- 44.Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, Younkin S, Borchelt DR, Hsiao KK, Carlson GA (1999) SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci 2:157–161 [DOI] [PubMed] [Google Scholar]
- 45.Irie Y, Keung WM (2003) Anti-amyloid beta activity of metallothionein-III is different from its neuronal growth inhibitory activity: structure–activity studies. Brain Res 960:228–234 [DOI] [PubMed] [Google Scholar]
- 46.Ittner LM, Götz J (2011) Amyloid-β and tau—a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci 12:65–72 [DOI] [PubMed] [Google Scholar]
- 47.Kim J, Onstead L, Randle S, Price R, Smithson L, Zwizinski C, Dickson DW, Golde T, McGowan E (2007) Abeta40 inhibits amyloid deposition in vivo. J Neurosci 27:627–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim JH, Nam YP, Jeon SM, Han HS, Suk K (2012) Amyloid neurotoxicity is attenuated by metallothionein: dual mechanisms at work. J Neurochem 121:751–762 [DOI] [PubMed] [Google Scholar]
- 49.King DL, Arendash GW (2002) Behavioral characterization of the Tg2576 transgenic model of Alzheimer’s disease through 19 months. Physiol Behav 75:627–642 [DOI] [PubMed] [Google Scholar]
- 50.King DL, Arendash GW, Crawford F, Sterk T, Menendez J, Mullan MJ (1999) Progressive and gender-dependent cognitive impairment in the APP(SW) transgenic mouse model for Alzheimer’s disease. Behav Brain Res 103:145–162 [DOI] [PubMed] [Google Scholar]
- 51.Kovacs EJ, Messingham KA, Gregory MS (2002) Estrogen regulation of immune responses after injury. Mol Cell Endocrinol 193:129–135 [DOI] [PubMed] [Google Scholar]
- 52.Kuperstein I, Broersen K, Benilova I, Rozenski J, Jonckheere W, Debulpaep M, Vandersteen A, Segers-Nolten I, Van Der Werf K, Subramaniam V, Braeken D, Callewaert G, Bartic C, D’Hooge R, Martins IC, Rousseau F, Schymkowitz J, De Strooper B (2010) Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J 29:3408–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee DK, Carrasco J, Hidalgo J, Andrews GK (1999) Identification of a signal transducer and activator of transcription (STAT) binding site in the mouse metallothionein-I promoter involved in interleukin-6-induced gene expression. Biochem J 337:59–65 [PMC free article] [PubMed] [Google Scholar]
- 54.Lee JY, Cole TB, Palmiter RD, Suh SW, Koh JY (2002) Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc Natl Acad Sci USA 99:7705–7710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH (2006) A specific amyloid-beta protein assembly in the brain impairs memory. Nature 440:352–357 [DOI] [PubMed] [Google Scholar]
- 56.Lindeque JZ, Levanets O, Louw R, van der Westhuizen FH (2010) The involvement of metallothioneins in mitochondrial function and disease. Curr Protein Pept Sci 11:292–309 [DOI] [PubMed] [Google Scholar]
- 57.Manso Y, Adlard PA, Carrasco J, Vašák M, Hidalgo J (2011) Metallothionein and brain inflammation. J Biol Inorg Chem 16:1103–1113 [DOI] [PubMed] [Google Scholar]
- 58.Manso Y, Serra M, Comes G, Giralt M, Carrasco J, Cols N, Vasák M, González-Duarte P, Hidalgo J (2010) The comparison of mouse full metallothionein-1 versus alpha and beta domains and metallothionein-1-to-3 mutation following traumatic brain injury reveals different biological motifs. J Neurosci Res 88:1708–1718 [DOI] [PubMed] [Google Scholar]
- 59.Martinho A, Gonçalves I, Cardoso I, Almeida MR, Quintela T, Saraiva MJ, Santos CR (2010) Human metallothioneins 2 and 3 differentially affect amyloid-beta binding by transthyretin. FEBS J 277:3427–3436 [DOI] [PubMed] [Google Scholar]
- 60.Masters BA, Kelly EJ, Quaife CJ, Brinster RL, Palmiter RD (1994) Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci USA 91:584–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ono S, Koropatnick DJ, Cherian MG (1997) Regional brain distribution of metallothionein, zinc and copper in toxic milk mutant and transgenic mice. Toxicology 124:1–10 [DOI] [PubMed] [Google Scholar]
- 62.Palmiter RD, Findley SD, Whitmore TE, Durnam DM (1992) MT-III, a brain-specific member of the metallothionein gene family. Proc Natl Acad Sci USA 89:6333–6337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Penkowa M, Carrasco J, Giralt M, Moos T, Hidalgo J (1999) CNS wound healing is severely depressed in metallothionein I- and II-deficient mice. J Neurosci 19:2535–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Penkowa M, Moos T, Carrasco J, Hadberg H, Molinero A, Bluethmann H, Hidalgo J (1999) Strongly compromised inflammatory response to brain injury in interleukin-6-deficient mice. Glia 25:343–357 [PubMed] [Google Scholar]
- 65.Phinney AL, Drisaldi B, Schmidt SD, Lugowski S, Coronado V, Liang Y, Horne P, Yang J, Sekoulidis J, Coomaraswamy J, Chishti MA, Cox DW, Mathews PM, Nixon RA, Carlson GA, St George-Hyslop P, Westaway D (2003) In vivo reduction of amyloid-beta by a mutant copper transporter. Proc Natl Acad Sci USA 100:14193–14198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pugh PL, Richardson JC, Bate ST, Upton N, Sunter D (2007) Non-cognitive behaviours in an APP/PS1 transgenic model of Alzheimer’s disease. Behav Brain Res 178:18–28 [DOI] [PubMed] [Google Scholar]
- 67.Quaife CJ, Findley SD, Erickson JC, Froelick GJ, Kelly EJ, Zambrowicz BP, Palmiter RD (1994) Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry 33:7250–7259 [DOI] [PubMed] [Google Scholar]
- 68.Rosario ER, Carroll J, Pike CJ (2010) Testosterone regulation of Alzheimer-like neuropathology in male 3xTg-AD mice involves both estrogen and androgen pathways. Brain Res 1359:281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sato M, Bremner I (1993) Oxygen free radicals and metallothionein. Free Rad Biol Med 14:325–337 [DOI] [PubMed] [Google Scholar]
- 70.Schäfer S, Pajonk FG, Multhaup G, Bayer TA (2007) Copper and clioquinol treatment in young APP transgenic and wild-type mice: effects on life expectancy, body weight, and metal-ion levels. J Mol Med (Berl) 85:405–413 [DOI] [PubMed] [Google Scholar]
- 71.Schwarzman AL, Gregori L, Vitek MP, Lyubski S, Strittmatter WJ, Enghilde JJ, Bhasin R, Silverman J, Weisgraber KH, Coyle PK, Zagorski MG, Talafous J, Eisenberg M, Saunders AM, Roses AD, Goldgaber D (1994) Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proc Natl Acad Sci USA 91:8368–8372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sinforiani E, Citterio A, Zucchella C, Bono G, Corbetta S, Merlo P, Mauri M (2010) Impact of gender differences on the outcome of Alzheimer’s disease. Dement Geriatr Cogn Disord 30:147–154 [DOI] [PubMed] [Google Scholar]
- 73.Sultana R, Butterfield DA (2010) Role of oxidative stress in the progression of Alzheimer’s disease. J Alzheimers Dis 19:341–353 [DOI] [PubMed] [Google Scholar]
- 74.Toda T, Noda Y, Ito G, Maeda M, Shimizu T (2011) Presenilin-2 mutation causes early amyloid accumulation and memory impairment in a transgenic mouse model of Alzheimer’s disease. J Biomed Biotechnol 2011:617974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vašák M, Meloni G (2011) Chemistry and biology of mammalian metallothioneins. J Biol Inorg Chem 16:1067–1078 [DOI] [PubMed] [Google Scholar]
- 76.Vest RS, Pike CJ (2012) Gender, sex steroid hormones, and Alzheimer’s disease. Horm Behav (in press) [DOI] [PMC free article] [PubMed]
- 77.Waelput W, Broekaert D, Vandekerckhove J, Brouckaert P, Tavernier J, Libert C (2001) A mediator role for metallothionein in tumor necrosis factor-induced lethal shock. J Exp Med 194:1617–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ (2002) Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416:535–539 [DOI] [PubMed] [Google Scholar]
- 79.West AK, Hidalgo J, Eddins D, Levin ED, Aschner M (2008) Metallothionein in the central nervous system: roles in protection, regeneration and cognition. Neurotoxicology 29:489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.West AK, Leung JY, Chung RS (2011) Neuroprotection and regeneration by extracellular metallothionein via lipoprotein–receptor-related proteins. J Biol Inorg Chem 1115-1122 [DOI] [PubMed]
- 81.Widyarini S, Domanski D, Painter N, Reeve VE (2006) Estrogen receptor signaling protects against immune suppression by UV radiation exposure. Proc Natl Acad Sci USA 103:12837–12842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang X, Doser TA, Fang CX, Nunn JM, Janardhanan R, Zhu M, Sreejayan N, Quinn MT, Ren J (2006) Metallothionein prolongs survival and antagonizes senescence-associated cardiomyocyte diastolic dysfunction: role of oxidative stress. FASEB J 20:1024–1026 [DOI] [PubMed] [Google Scholar]
- 83.Zeitoun-Ghandour S, Leszczyszyn OI, Blindauer CA, Geier FM, Bundy JG, Stürzenbaum SR (2011) C. elegans metallothioneins: response to and defence against ROS toxicity. Mol Biosyst 7:2397–2406 [DOI] [PubMed] [Google Scholar]