Abstract
Aquaporins (AQPs) are key players regulating urinary-concentrating ability. To date, eight aquaporins have been characterized and localized along the nephron, namely, AQP1 located in the proximal tubule, thin descending limb of Henle, and vasa recta; AQP2, AQP3 and AQP4 in collecting duct principal cells; AQP5 in intercalated cell type B; AQP6 in intercalated cells type A in the papilla; AQP7, AQP8 and AQP11 in the proximal tubule. AQP2, whose expression and cellular distribution is dependent on vasopressin stimulation, is involved in hereditary and acquired diseases affecting urine-concentrating mechanisms. Due to the lack of selective aquaporin inhibitors, the patho-physiological role of renal aquaporins has not yet been completely clarified, and despite extensive studies, several questions remain unanswered. Until the recent and large-scale development of genetic manipulation technology, which has led to the generation of transgenic mice models, our knowledge on renal aquaporin regulation was mainly based on in vitro studies with suitable renal cell models. Transgenic and knockout technology approaches are providing pivotal information on the role of aquaporins in health and disease. The main goal of this review is to update and summarize what we can learn from cell and animal models that will shed more light on our understanding of aquaporin-dependent renal water regulation.
Keywords: Aquaporin, Vasopressin, Nephrogenic diabetes insipidus
Introduction
Aquaporins (AQPs) are membrane proteins that mediate facilitated movement of water across the lipid bilayer in response to an osmotic gradient [141]. To date, aquaporins have been found in almost all living organisms: mammals, amphibians, invertebrates, plants, bacteria, and other microbes [38]. All aquaporins possess similar structures and consist of an approximately 30-kDa protein with six membrane-spanning domains and intracellular N- and C-termini. An NPA (Asn–Pro–Ala) box is contained in each of the highly conserved B and E loops that fold into the lipid bilayer in an ‘hourglass’ fashion, forming an aqueous pore [49, 100].
The aquaporin family can be divided into two subfamilies: aquaporins and aquaglyceroporins. The aquaporins are considered only to be permeated by water and include AQP0, AQP1, AQP2, AQP4, AQP5, AQP6 and AQP8. The aquaglyceroporins are, in addition to being permeable to water, permeable to glycerol and include AQP3, AQP7, AQP9 and AQP10. A new aquaporin subfamily has been recently identified with highly deviated NPA boxes, which are signature sequences for AQPs. With limited homology with other aquaporins, they form the “superaquaporin subfamily” that includes mammalian AQP11 and AQP12.
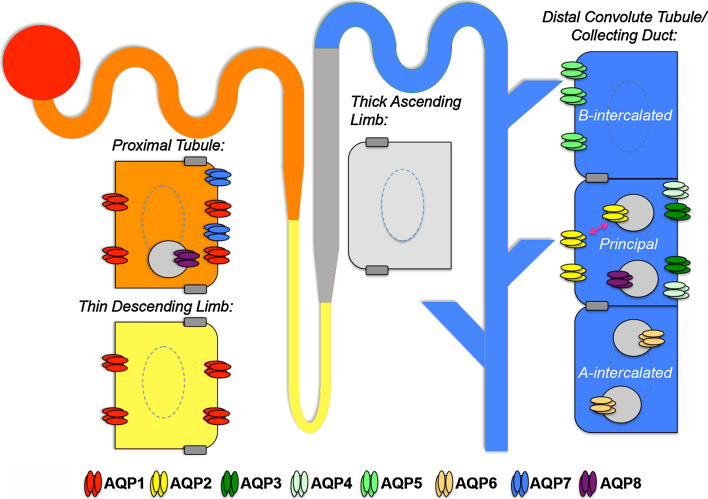
The aquaporins play different roles in body water homeostasis with respect to permeability and localization. Of the known 13 aquaporin isoforms expressed in mammals (AQP0–12), at least eight are known to be present in the kidney at distinct sites along the nephron and collecting duct: AQP1–8 (see Fig. 1 for distribution).
Fig. 1.
A single nephron showing aquaporin (AQP) distribution. AQP1 is expressed in both the apical and basolateral membrane of cells in the proximal tubule (orange) and descending thin limb (yellow), both of which are highly water permeable. AQP7 is expressed at the brush border of proximal tubule cells. AQP8 is present in intracellular vesicles in the same cells. The thick ascending limb of Henle’s loop (grey) is water-impermeable because no AQP is expressed in this tract of the nephron. AQP2 is the collecting duct (blue) water-channel that translocates from intracellular reservoir vesicles to the apical membrane in response to vasopressin in the principal cells. AQP3 and AQP4 are expressed at the basolateral membrane of the same cells. AQP6 is present in intracellular vesicles in the acid-secreting type-A intercalated cells of the collecting duct, while AQP5 is constitutively expressed at the apical membrane of the bicarbonate-secreting type-B intercalated cells
The first cloned aquaporin, AQP1, originally called CHIP-28 (channel-forming integral protein) [99], is present in both the apical and basolateral membranes of the proximal tubules and the descending thin limb of Henle’s loop and in vasa recta. AQP1 gene deletion in humans results in markedly impaired urinary concentrating ability, but only when those subjects are challenged by water deprivation [59].
AQP2 (AQP-CD) [30] is abundant in the apical membrane and in intracellular storage vesicles in the principal cells of the collecting ducts. Studies in isolated collecting ducts have shown that AQP2 is primarily expressed in the intracellular vesicles, whereas perfusion with the antidiuretic hormone arginine vasopressin (AVP) resulted in trafficking to the apical membrane in association with a dramatic increase in water permeability [91]. Upon AVP washout, AQP2 was endocytosed into the cytoplasmic vesicles with reversal of the enhanced water permeability. A multitude of studies have highlighted a critical role of AQP2 in several inherited and acquired water-balance disorders, including inherited forms of nephrogenic diabetes insipidus (NDI), acquired states of NDI, and other diseases associated with urinary concentrating defects where AQP2 expression and targeting are affected [13, 92, 108, 117]. In the same cell type where AQP2 is expressed, AQP3 (glycerol-transporting integral protein) [23] and AQP4 (mercury-insensitive water channel, MIWC) [32] are located exclusively in the basolateral membrane, thus providing channels for the final exit of water into the interstitium and ultimately into the circulation. An understanding of the physiological role of AQP3/4 has been provided by studies on knockout mice as described below.
We have recently reported compelling evidence that AQP5 is expressed in the kidney distal tubule at the apical membrane of type-B intercalated cells where it co-localizes with pendrin [102]. The possible physiological role of AQP5 in the kidney is currently under investigation. AQP6 [78, 158] is present only in renal collecting duct intercalated cells type A and under conditions of low water permeability. AQP7 [43] is expressed in the brush border of the proximal tubule [43] where it contributes to water and glycerol reabsorption [43]. AQP8 [46] has been localized to intracellular domains of proximal tubule and collecting duct cells [25]. AQP9 [45, 132] and AQP11 [31] have also been identified in kidney at low abundance by Northern blot and reverse transcription-PCR techniques. However, their functional significance within the kidney has not yet been determined [92]. AQP11-knockout mice, however, have been shown to have polycystic kidneys [86].
The high abundance of aquaporins in both kidney tubules and vasa recta suggests a major involvement in the mechanism of osmotically driven water transport from kidney tubules to bloodstream that allows urine concentration. In addition to traditional role in regulating water balance, phenotype analyses of aquaporin knockout mice have also revealed several unexpected cellular roles for aquaporins whose mechanisms are still being elucidated [145, 160]. For example, several research groups have demonstrated a role for AQP1 in tumor angiogenesis [134] and cell migration [115]. Null mice for AQP1 display an important decrease in tumor growth [115]. To explain this observation, Verkman and co-workers suggested that during cell migration, actin cleavage at the cortical membrane causes an osmotic gradient, inducing water flow. At the leading edge, water increases the local hydrostatic pressure, causing the formation of membrane protrusion [143].
Studies in cultured cells have provided a tremendous insight into the structure, function and regulation of aquaporins, leading to proposals on new roles for these proteins, such as ion transport [159].
The aim of this review is to provide insights into the physiological function of renal aquaporins. Much of this information herein is derived from studies in knockout animal models or cell culture models.
Knockout animal models
AQP1-null mice
Transgenic mouse models of aquaporin deletion and mutation (Table 1) have been very useful in elucidating the patho-physiological role of AQPs in the kidney [8, 27, 142, 143]. A fundamental feature of the proximal tubule is its ability to reabsorb most of the glomerular filtrate. At their basolateral and apical membranes, proximal tubule cells express the selective water channel AQP1 which plays a critical role in this transcellular water movement.
Table 1.
Transgenic mice models of renal aquaporins
| AQPs | Renal phenotype | References |
|---|---|---|
| AQP1−/− | Polyuria | [77, 142] |
| No sensitivity to vasopressin treatment | ||
| AQP2−/− | Polyuria | [82, 114, 121, 153, 157] |
| Papillary atrophy and hydronephrosis | ||
| AQP3−/− | Severe polyuria | [73, 75] |
| Mild responsivity under vasopressin stimulation or water deprivation | ||
| AQP4−/− | Mild polyuria | [76] |
| AQP7−/− | Mild polyuria | [120] |
| Glyceroluria | ||
| AQP8−/− | No phenotype | [155] |
| AQP11−/− | Kidney polycystic | [86] |
| AQP1−/−/AQP3−/− | Severe polyuria and hydronephrosis | [154] |
| AQP1−/−/AQP4−/− | Severe polyuria | [140] |
| AQP1−/−/AQP7−/− | Severe polyuria | [120] |
| AQP1−/−/AQP8−/− | Polyuria | [155] |
| AQP3−/−/AQP4−/− | Severe polyuria | [73, 75] |
AQP Aquaporin
A transgenic AQP1 knockout mouse has been generated with a targeted gene disruption method [77]. Genetic characterization of the offspring from heterozygous mating showed that the number of AQP1 knockout mice is below the Mendelian 1:2:1 ratio, likely indicating a lower survival rate in utero and/or in earlier development phases [142]. AQP1 knockout mice were smaller than their wild-type and heterozygous counterparts, but with normal organ morphology and physical appearance, although they showed strong dehydration and lethargy after 36 h of water deprivation [77, 142], which is in contrast to AQP1-null humans who do not show any phenotypic alteration under normal conditions [101]. AQP1 knockout mice have polyuria and are not sensitive to vasopressin treatment. Compared with their wild-type and heterozygous counterparts, their water intake was 2.5- to threefold higher [77]. Moreover, the mean arterial blood pressure was significantly lower in AQP1 knockout (88 ± 3 vs. 102 ± 4 mmHg) mice, probably due to massive water loss [142]. Transepithelial osmotic water permeability (Pf) in isolated S2 tract of proximal tubule was found to be about fivefold lower in AQP1 knockout mice than in wild-type mice (0.033 ± 0.005 vs. 0.15 ± 0.03 cm/s), highlighting the main role of AQP1 in transcellular water reabsorption in this nephron segment [116]. In addition, the proximal tubule AQP1 is highly expressed in the thin descending limb of Henle (TDLH), a nephron segment involved in urine concentration by the countercurrent multiplication mechanism. Deletion of AQP1 profoundly affects urine concentration, indicating that high TDLH water permeability is required to keep the urine concentration mechanism intact [116, 142]. Taken together, the data obtained in this AQP1 knockout model indicate that reduced urinary concentrating ability is due to an impairment in generating a hypertonic medulla.
These observations are probably relevant in humans as well as mice. In humans, AQP1 encodes the Colton blood group antigen [119]. Colton-null subjects have a reduced ability to concentrate urine when subjected to water deprivation, which is in agreement with the data obtained in AQP1 knockout mice [59].
AQP3- and AQP4-null mice
AQP3 and AQP4 are both expressed at the basolateral membrane of principal cells. AQP3 is expressed mostly in the cortical and outer/inner medullary collecting duct, whereas AQP4 is present in the inner medullary collecting duct.
While AQP4 is a selective water-channel, AQP3 is not only permeable to water but functions as a glycerol transporter; therefore, it is considered to be a member of the so-called aquaglyceroporins [24, 47, 156]. AQP3 knockout mice have been generated by targeted gene deletion by Verkman’s group [75]. Genotyping characterization of offspring from AQP3 heterozygous mating indicates a normal 1:2:1 Mendelian distribution. AQP3-null mice are similar to wild-type mice in terms of growth, weight, behaviour and organ morphology [75], but they manifest profound polyuria (threefold reduction in the osmotic water permeability of the basolateral membrane compared to the wild-type) with evident signs of urinary obstruction and dilated kidney. In contrast, AQP4-null mice have mild polyuria [76]. A comparison analysis among AQP1, AQP3 and AQP4 transgenic knockout mice highlights a substantial difference in these knockout mice regarding impairment in their urinary concentrating ability [140, 154]. In AQP3 knockout mice, water intake and output is about tenfold higher than in wild-type or heterozygous mice, whereas in AQP1-null mice it is only 2.5–3.0-folds higher than in wild-type animals. In addition, urinary osmolality is significantly lower in AQP3-null mice than in wild-type or AQP1 [77] or AQP4 knockout mice [76]. In contrast to AQP1 knockout mice, the urinary concentrating ability in mice lacking AQP3 partially but significantly increases after 36 h of water deprivation or desmopressin acetate (DDAVP) treatment. These differences reflect the differential role and localization of renal aquaporins along the nephron. In fact, deletion of AQP1 severely reduces urinary concentrating ability by affecting the countercurrent multiplication mechanism, whereas in AQP3 knockout mice, in which the countercurrent multiplication mechanism is not affected, DDAVP or water deprivation results in a partial ability to concentrate urine, likely due to the expression of functional AQP4.
Alternatively, AQP4 knockout mice have very low water permeability in the inner medulla; however, this causes only a modest reduction in the kidney urinary concentrating ability. The explanation for this effect likely relates to the greater water reabsorption occurring in the connecting tubule (CNT) and cortical collecting duct where AQP3 is expressed, whereas in the mouse AQP4 is expressed predominantly in the medullary collecting ducts.
In agreement with these observations, AQP3/AQP4 double knockout mice show more severe polyuria than AQP3-knockout mice, although they still have residual water reabsorption ability [76]. Genetic deletion of AQP3 and/or AQP4 might activate different compensative mechanisms, such as modulation of urea and/or sodium reabsorption, also regulated by vasopressin to counteract water loss. Indeed, although a significant down-regulation of AQP2 expression has been detected in AQP3-null mice, it cannot be excluded that AQP2 itself relocalizes at the basolateral membrane to compensate for AQP3 and AQP4 loss, as already shown by Fenton et al. [27] in rat kidney tubules.
AQP5-null mice
AQP5 is a water-channel with a unique tissue distribution [110]. Immunocytochemical studies from several laboratories have shown AQP5 expression in the apical membranes of serous acinar cells in salivary and lacrimal glands, type I alveolar epithelial cells, cochlea and surface corneal epithelial cells [37, 48, 83, 93]. We have recently demonstrated that AQP5 is also expressed in type-B intercalated cells in the kidney CNT/collecting duct. At this site, AQP5 co-localizes with the apical transporter pendrin [102]. Ma et al. [74] generated AQP5-null mice by targeted gene disruption and observed that these mice produced low volumes of hypertonic viscous saliva, thereby providing direct evidence for a role of AQP5 in near-isosmolar fluid secretion in salivary glands. In a second study, the same authors measured lung fluid transport in AQP5-null mice and came to the conclusion that, in alveolar type-I cells, AQP5 does not appear to facilitate hydrostatically driven lung edema or active alveolar fluid absorption [73]. They described the knockout mice as ‘grossly normal’ with no kidney phenotype [74]. Further studies are required to uncover an unexpected role for AQP5 in the kidney.
AQP7-null mice
AQP7 is an aquaglyceroporin expressed in the apical membrane of epithelial cells in segment S3 of the proximal tubule [43, 90] which transports both water and glycerol [44]. AQP7 knockout mice do not display a urinary-concentrating defect [120]. Consistent with this finding, results from stop-flow light scattering studies on brush-border membrane vesicles from renal outer medulla indicate that osmotic water permeability is slightly reduced in mice lacking AQP7 (18 ± 0.4 × 10−3 cm/s) compared with wild-type animals (20 ± 0.3 × 10−3 cm/s). The lack of severe water loss in AQP7-deficient mice is in agreement with the presence of AQP1 representing the major water channel in segment S3 of the proximal tubule [140]. Interestingly, a comparative study found that urinary concentrating ability is more defective in double AQP1/AQP7 knockout mice than in AQP1 knockout mice, likely suggesting a small but significant contribution of AQP7 to proximal tubule water reabsorption [120]. Quite interestingly, despite the normal level of serum glycerol, AQP7-null mice have a severe loss of glycerol in the urine (1.7 ± 0.34 mg/ml) compared with wild-type animals (0.005 ± 0.002 mg/ml), confirming that AQP7 represents a major pathway for glycerol reabsorption in the proximal tubule. The massive loss of glycerol in urine is, however, difficult to explain given the relatively limited expression of AQP7 in segment S3 of the proximal tubule. In addition, it is surprising that such consistent glyceroluria is not associated with a parallel decrease in serum glycerol. It is possible that systemic compensative mechanisms, leading to glycerol release into the plasma, may be deregulated in AQP7 knockout mice.
AQP8-null mice
Aquaporin-8 (AQP8) is a water-transporting protein which has been proposed to be a potentially important water transporter in the gastrointestinal tract. However, AQP8 mRNA has also been detected in mouse and rat kidney, specifically in the cortex and in the outer medulla. Immunocytochemistry studies have shown that AQP8 localizes intracellularly in the proximal tubule and in collecting duct [25].
The possible involvement of AQP8 in renal function has been evaluated in AQP8-deficient mice. Specifically, urinary-concentrating ability has been examined in AQP8-null mice before and after water deprivation. In AQP8-null mice, urine osmolalities did not differ in control versus AQP8-null mice at baseline or after 36-h water deprivation [155]. In addition, the observation that urine osmolality was not significantly different in AQP1-null mice versus mice lacking both AQP8 and AQP1 indicates that AQP8 is not physiologically relevant for renal function.
AQP11-null mice
AQP11 shares homology with other members of the AQP family, although it has atypical NPA boxes [42]. Its function is still largely unknown. In the kidney, AQP11 is expressed intracellularly in the proximal tubules [86]. Mice deficient in AQP11 have been generated by gene targeting in embryonic stem cells. The transgenic mice obtained are born according to the Mendelian ratio, indicating a normal survival rate in utero; however, they start dying within 2 weeks due to severe renal failure [86]. Accurate analysis of the renal phenotype showed that their kidneys are large, anemic and polycystic. Histological studies revealed that cysts are absent in the medulla but abundant in the cortex where AQP11 is highly expressed [86]. To date, the closest human disease showing similarity with the renal phenotype observed in AQP11-deficient mice are polycystic kidney diseases (PKD), although in this severe disease, cysts are widespread throughout the kidney, whereas in AQP11 knockout mice they are confined specifically to the cortex. Nevertheless, these transgenic mice might be considered a new animal model providing an alternative pathway for studying cystogenesis in PKD. In this respect, it has recently been demonstrated that similar signals are activated in AQP11-null mice and PKD animals during cyst formation. In both mice models, results from microarray studies indicated that the genes regulating cell proliferation, reorganization of the extracellular matrix (ECM), immune system and apoptosis function atypically, leading to severe endoplasmic reticulum (ER) stress [86, 97]. On these bases, AQP11 may be involved in controlling the ER environment in which the folding of proteins occurs that causes these severe renal disorders.
AQP2 mouse models in NDI
The AQP2 water channel is functionally involved in several disorders associated with altered water balance due to excessive water reabsorption or severe polyuria. Among these, we will focus here on NDI, a genetic disease caused by the kidney’s inability to respond to vasopressin, resulting in severe water loss. Congenital NDI can be classified into X-linked and autosomal NDI. The X-linked form is due to mutations in the AVP receptor-2 (AVPR2) gene encoding the vasopressin V2 receptor, which belongs to the family of G protein-coupled receptors [4, 6, 112, 113].
Autosomal NDI
For about 5–10% of patients, congenital NDI is an autosomal disease caused by mutations in the gene encoding AQP2 (AQP2 gene mutations website http://www.medicine.mcgill.ca/nephros/aqp2.html) [21]. To date, 40 mutations have been reported, of which 32 are involved in recessive NDI. Studies in vitro using different cell models have revealed that missense mutations are located in the core region of AQP2, thus affecting its normal folding. The resulting mutated proteins accumulate in the ER and then target to proteasomes for degradation without being translocated to the apical plasma membrane [18, 54, 88, 95, 137]. In contrast, mutations causing dominant NDI are localized in the AQP2 C-terminus that contains important sorting signals. The resulting AQP2 mutants can bind wild-type AQP2 monomers, thereby generating heterotetramers that are subsequently missorted and do not reach the apical plasma membrane sufficiently to ensure normal water reabsorption [51, 55, 65]. It is still unclear whether the effects of mutant AQP2 described in cell models are similar to those causing NDI in vivo. This is particularly true considering the molecular mechanism leading to dominant NDI, where mutant AQP2 and wild-type AQP2 expression are determined by each singular allele. The recent generation of transgenic mice clearly improved the state of the art regarding the role of AQP2 in controlling water homeostasis in health and disease. In one study, AQP2-deficient mice failed to thrive and died by the age of 2 weeks, although they had appeared similar in weight to wild-type mice at birth [114]. In contrast, in the same study, transgenic mice expressing AQP2 exclusively in the CNT and deficient in the collecting duct (AQP2-CD-KO) were viable and reached adulthood even though they were significantly smaller than control mice [114]. These data indicate that AQP2 expression in the collecting duct is fundamental to the rescue of the lethal phenotype observed in total AQP2 knockout mice and that it cannot be compensated for by other mechanisms. AQP2-CD-KO mice showed a tenfold increase in urine output compared with control siblings, and water restriction caused only a slight decrease in urine output and no change in urine osmolality, revealing an absence of compensatory mechanisms.
These results indicate that AQR2 plays a fundamental role in the collecting duct system in the regulation of osmotic equilibration, although the role of AQP2 in the CNT is still not completely clear.
Similarly to mice totally lacking AQP2, the knock-in mouse model carrying the recessive NDI T126M mutation in homozygosis failed to grow, showed a severely defective urine-concentrating ability and died by day 6 if no additional fluid was administered [153]. Histological analysis revealed that its kidneys presented papillary atrophy and hydronephrosis [153]. More recently, the same authors generated a conditional knock-in adult mouse model by breeding heterozygous T126M AQP2 knock-in mice with conditional AQP2 knockout mice [157]. This mouse model has been useful for evaluating chemical strategies to rescue mutated AQP2. In recessive NDI, AQP2 missense mutants are blocked in the endoplasmic reticulum, although some mutants retain a residual function as water channels [87]. As for other proteins, missense mutations affect AQP2 folding and, consequently, proteins are trapped in the ER. In vitro studies in Madin–Darby canine kidney (MDCK) cells have shown that the chemical chaperone glycerol restores the physiological membrane localization of several AQP2 mutants, including AQP2-T126M [124]. In the inducible knock-in adult mouse model of NDI, the selective inhibitor of HSP90, 17-allylamino-17-demethoxygeldanamycin (17-AAG) caused partial rescue of defective AQP2-T126M by increasing the cell surface expression of AQP2 mutants and ameliorating urinary-concentrating ability. Of note, on the long term, 17-AAG does not alter either the gene expression of AQP2 or urine osmolality in wild-type mice, nor in total knockout AQP2 animals, indicating the highly specific effect of the drug in rescuing this specific AQP2 (T126M) mutant [157]. The results of this study underscore the importance of animal models as powerful tools for testing the efficacy of small molecules as putative therapeutic correctors of defective AQP2.
The first mouse model of dominant NDI was generated by Sohara [121]. The knock-in mice were generated by homologous recombination of a part of the last exon of human AQP2 (763–772 del). Similarly to the milder phenotype described in humans, heterozygous mice exhibit an impairment in urine concentrating ability, as well as a slight but significant increase in urine osmolality after water dehydration [121]. Immunofluorescence studies of dehydrated animals revealed that AQP2 was mainly missorted to the basolateral membrane, although a weak staining of wild-type AQP2 was also detected at the apical plasma membrane, thus explaining the milder phenotype of these mice in terms of urinary-concentrating ability. Rolipram, a phosphodiesterase-4 inhibitor, was found to be able to increase urine osmolality in this knockout mouse, suggesting that phosphodiesterase inhibitors may be useful drugs for the treatment of dominant NDI. However, the patho-physiological mechanism underlying the mouse model of dominant NDI may differ from its human counterpart. In fact, rolipram administration was found not to exert any significant effect on urinary-concentrating ability in two brothers affected by congenital X-linked NDI [7], suggesting that the reduction in cAMP degradation observed in mice may not be present in humans.
Two additional mouse models of NDI have provided direct genetic evidence that specific mutations in AQP2 impair the apical accumulation of the water channel and revealed the genetic basis for a urinary-concentrating defect. McDill et al. [82] identified a mutation in the aqp2 gene responsible for a severe form of recessive NDI. Interestingly, the mutation described causes the substitution of serine 256 (S256) by a leucine, thus preventing AQP2 phosphorylation at S256 by PKA. The S256L is a spontaneous recessive mutation that causes severe hydronephrosis and obstructive nephropathy in affected mice.
No such mutation has been found as yet in humans; however, quite interestingly, the AQP2-R254L mutation found in humans also occurs at the PKA consensus site and when expressed in vitro also prevents S256 phosphorylation, thus explaining the NDI phenotype [17]. Conversely, the AQP2-P262L mutation is responsible for recessive NDI in humans, likely because the AQP2 mutant can form heterotetramers with wild-type AQP2 located at the apical plasma membrane [16]. Together, these observations suggest that the autosomal NDI phenotype might be determined by the power of the apical sorting signal of wild-type monomers compared with missorting signals in AQP2 mutants.
Another surprising finding is that described by Lloyd et al. [69], who found that although mice carrying the AQP2-F204V mutation in homozygous were affected by a severe NDI, they were viable compared with other recessive mouse models of NDI. Similarly to other missense recessive mutations, AQP2-F204V is located in the core of AQP2 and is mainly retained in the ER. Measurement of the osmotic water Pf using Xenopus oocytes demonstrated that AQP2-F204V maintains a residual function as a water channel and that a small amount of mutants escape the ER quality control and target to the plasma membrane, explaining this milder non-lethal phenotype. Therefore, these findings suggest that the severity of a recessive NDI phenotype is dependent of the degree of its misfolding, and extend to the possibility of this phenotype overcoming ER quality control.
X-linked NDI
Most patients (>90%) with congenital NDI harbor inactivating mutations in the V2 vasopressin receptor (V2R) gene [5, 6, 39, 80, 98]. This form of NDI, which is commonly referred to as X-linked NDI (XNDI), therefore almost exclusively manifests itself in males.
It has recently been reported that a generation of viable V2R mutant mice have been generated in which the V2R gene can be deleted in a conditional fashion in adults [68]. These mice excrete a large amount of hypotonic urine, show changes in renal morphology and have reduced levels of AQP2 and AQP3 [68]. The availability of such a valid animal model will help to develop a specific and effective pharmacological therapy for the treatment of NDI. A first promising result in this direction has been obtained by the same group that generated the model in which activation of the EP4 PGE2 receptor is shown to elicit a c-AMP-mediated response that could compensate for the lack of V2R in this NDI model [68]. Based on previous results obtained by our group [103, 104], we are currently investigating the potential effect of statins as a treatment for X-NDI in this mouse model.
Epithelial cell models for studying subcellular localization and function of renal aquaporins
In renal collecting duct cells, multiple types of aquaporin water channels are expressed in a polarized fashion. As discussed in preceding sections of this review, AQP1 is expressed on both apical and basolateral membranes, AQP2 is specifically targeted to the apical cell domain, whereas AQP3 and AQP4 are constitutively expressed in the basolateral plasma membrane. Polarized sorting is essential for efficient water transport. However, the molecular mechanisms involved in the polarized targeting and membrane trafficking of aquaporins remain largely unknown. Here we focus on the principal cell models used over the years to study the expression and the trafficking of renal aquaporins with particular emphasis on AQP2 as a representative example of a renal water channel fundamental for controlling osmotic water permeability.
The function of CHIP-28/AQP1 was first assessed by expressing the relative cRNA in Xenopus laevis oocytes [99]. The results showed dramatic swelling and explosion of the injected oocytes exposed to hypotonic media. Using this model, the authors were also able to calculate the Arrhenius activation energy, which was low, namely, E a < 3 kcal/mol, according to the diffusion of water through water channels.
The trafficking of AQP1 and the functional evaluation of the associated water permeability has been studied in MDCK and LLC-PK epithelial cell lines [19]. The authors of this study demonstrated that targeting of AQP1 to the apical and basolateral plasma membrane is independent of cell type and showed for the first time that water flow through a cultured epithelium can be blocked by mercurial compounds. More recently, the effect of hypertonicity on AQP1 up-regulation in the kidney has been studied using a murine renal medullary cell line, mIMCD-K2 [133].
The expression of AQP3, as well as that of AQP2, is regulated in vivo by antidiuretic hormones and water restriction [44]. In one study using MDCK cells, the authors showed that the exposure of cells to hypertonic medium induces AQP3 up-regulation at both the mRNA and protein levels [81]. MDCK cells have also been used to identify the conserved amino acid stretch YRLL as the sorting signals responsible for the basolateral targeting of AQP3 [109].
Ectopic expression of sorting signal-defective AQP4 mutants in MDCK cells has allowed the identification of the main tyrosine- and di-leucine-based sorting signals responsible for the basolateral localization of AQP4 in epithelia [79]. In the same study, the authors also identified the signal involved in AQP4 lysosomal targeting/degradation. Recent in vitro studies in MDCK cells have shown how proteins in the ECM regulate the cell surface expression of AQP4 [131]. One of the most intriguing aspects of AQP4 organization within the plasma membrane is the presence of orthogonal arrays of particles (OAPs) in all sites where AQP4 is expressed, including principal cells in the kidney, airways epithelium, gastric parietal cells, astrocytes and skeletal muscle plasmalemma [151]. CHO cells transfected with AQP4 cDNA were analyzed by freeze fracture and found to show the presence of the same OAPs as seen in collecting duct principal cells, thus indicating that AQP4 can spontaneously assemble in regular arrays [152]. More recently, a study performed by the same lab in LLC-PK cells clarified the dynamics of AQP4 assembly into OAPs [123].
A number of independent research groups have studied the polarity and regulation of AQP5 in MDCK cells. Wellner and Baum reported that AQP5 is constitutively expressed at the apical membrane of MDCK cells [146], which resembles localization in vivo. The same authors made a series of protein chimeras with other known aquaporins to identify the sorting signals responsible for AQP5 apical localization [147, 148]. Expression of green fluorescence protein-tagged AQP5 in MDCK cells provided preliminary information on the regulation of AQP5 trafficking. Kosugi-Tanaka et al. studied the role of the putative phosphorylation site at Thr-259 by mutating it to alanine and demonstrated that phosphorylation of Thr-259 is not necessary for AQP5 trafficking [63]. These authors also analyzed the contribution of the PKA-target motif at 152SRRTS of AQP5 in MDCK-II cells and came to the conclusion that AQP5 can be trafficked toward the cell membrane, irrespective of phosphorylation of the PKA target motif [56].
Permanent residence in an intracellular compartment is a unique characteristic of AQP6 that is expressed in cytoplasmic vesicles in type-A intercalated cells in the kidney, where it also co-localizes with H+-ATPase and is thought to promote urinary acid secretion [158]. Analysis of AQP6 intracellular sorting signals in transiently transfected MDCK cells led to the identification of crucial determinants for intracellular retention in the N-terminus of the protein [3]. Surprisingly, oocytes expressing rat AQP6 show a marked increase in water permeability after exposure to HgCl, a known aquaporin inhibitor [158]. Ectopic expression in oocytes also indicated that a pH of less than 5.5 activates anion conductance through AQP6 [158]. The generation of AQP6 stably transfected epithelial cells has not been successful to date.
To the best of our knowledge, the amount of published information available on AQP7 function or trafficking in cell culture models is still insignificant.
AQP8 [46] has been localized to intracellular domains of the proximal tubule and collecting duct cells [25]. This localization is different from that found in submandibular gland acinar cells [149]. The stable expression of AQP8 cDNA in MDCK cells indicates basolateral expression of the protein in cultured cells [146].
Cell models used to study AQP2 expression and trafficking
Vasopressin controls renal water excretion largely by regulating the trafficking of AQP2 in collecting duct principal cells. Vasopressin stimulates its redistribution from intracellular vesicles into the plasma membrane. This permits water to enter into the cells and exit through AQP3 and AQP4, causing variable water reabsorption from the duct lumen to the serosal side (peritubular capillaries). Our knowledge of this complex machinery has increased markedly in recent years, and several excellent reviews summarize this progress [27, 88, 89, 95, 137].
This regulating mechanism was first studied in renal-like epithelia, namely, the frog and toad urinary bladders [67]. Stimulation of these epithelia with the hormone vasopressin increases the water permeability of these bladder cells; concomitantly, aggregates of intramembrane particles appear in the luminal membranes of amphibian bladder cells after stimulation. These particles have been analyzed in various freeze-fracture studies [14, 36, 50]. Freeze-fracture electron microscopy studies, first of the amphibian urinary bladder and then of isolated renal collecting duct tubules, showed that these particles were localized in the clathrin-coated pits, leading to the suggestion that they contain vasopressin-regulated water channels [10, 11, 50]. The molecular identity of the vasopressin-sensitive water channel remained unknown for several years until the cloning of AQP2 by Fushimi et al. [30] and subsequent demonstration of its specific expression in the renal collecting duct tubules. Following identification of the mammalian AQP2 and its respective counterpart in amphibians [1], the generation of cell models contributed significantly to dissecting the complex mechanism underlining AQP2 expression and trafficking.
The expression systems can be classified into non-mammalian and mammalian systems (Table 2). The first group includes X. laevis oocytes and yeasts, while the second group comprises primary culture cells isolated from the inner medulla collecting duct tubules [80], the mouse collecting duct cell line mpkCCD [22] and different cell lines stably transfected with cDNA encoding AQP2 (Table 2).
Table 2.
Cell models for studying AQP2 regulation and trafficking
| Non mammalian | References |
|---|---|
| Xenopus laevis oocytes | [18, 30, 112] |
| Yeast | [15, 66, 118] |
| Mammalian | |
| IMCD (rat inner medulla collecting duct cell line) | [80] |
| mpkCCD (mouse cortical collecting duct cell line) | [33] |
| CD8 (rabbit cortical collecting duct cell line) | [135] |
| MDCK (Madin–Darby canine kidney epithelial cell line) | [20] |
| LLC-PK1 (Porcine renal proximal tubule cell line) | [58] |
| MCD4 (mouse cortical collecting duct cell line) | [41] |
AQP2 expression in X. laevis oocytes
Analysis of the expression and function of AQP2 has been greatly aided by studies carried out in X. laevis oocytes. The large size of the oocytes facilitates microinjection of DNA, mRNA or protein, permits manual dissection of nuclei and allows certain assays to be performed with single oocytes. Another advantage of this system is that the osmotic water permeability (Pf) of oocytes injected with the AQP2 transcript can be measured using video microscopy by analyzing the volume increase caused by an imposed osmotic gradient.
Analysis by video microscopy of the volume increase caused by an imposed osmotic gradient
Video microscopy analysis of oocytes injected with the AQP2 transcript revealed that the Pf of these cells increases 3.5-fold compared with control oocytes. The increase in Pf can be prevented by incubating the injected oocytes with 0.3 mM HgCl2, and the inhibition can be reversed by using β-mercaptoethanol, indicating that the water channel AQP2 is sensitive to mercurial compounds, just as other aquaporins [30]. This expression system has been particularly useful for the functional analysis of different AQP2 mutants that lead to NDI [112]. For example, Deen et al. found that the Pf of oocytes injected with mutant G64A cRNA did not differ from that of water-injected controls, whereas the Pf value of oocytes injected with wild-type AQP2 cRNAs was more than tenfold higher [18]. Xenopus oocytes have also been used by several groups to examine the role of vasopressin-regulated phosphorylation sites in the COOH-terminal tail of AQP2 on protein function [53, 64, 84, 85]. In particular, following the discovery of multiple phosphorylation sites in the COOH terminus of AQP2, more recent studies on this expression system have focused on determining whether phosphorylation at AQP2 S256 depends on the other phosphorylation sites. The data would seem to indicate that S264 and S269 phosphorylation depend on prior phosphorylation of S256. In contrast, AQP2 S261 phosphorylation should be independent of the phosphorylation status of S256.
While Xenopus oocytes have been a most valuable tool for elucidating the research aspects discussed above, this expression system does not represent a good model for studying AQP2 trafficking, probably due to the lack of several regulatory proteins required for translocating and recycling AQP2-bearing vesicles to the plasma membrane identified in epithelial cells.
AQP2 expression in yeast
Compared with X. laevis oocytes, a yeast expression system does not require intact plasma membrane expression of AQP2, which can be affected differently in different mutants. Thus wild-type and AQP2 mutants are equally expressed in vesicles, and the Pf can be calculated by using a light-scattering technique with stop-flow instrumentation. As such, the yeast system has been useful for expressing aquaporins for reconstitution studies [15, 66, 118]. Using this approach, for example, it has been shown that with respect to wild-type AQP2, L22V and P262L mutants are completely functional, which is in line with the osmotic permeability measured in oocytes [16]. In contrast, N68S, R187C and S216P mutants are only partially functional, while N123D, T125M, T126M, A147T and C181W mutants have a low water permeability [118].
AQP2 expression and sorting in epithelial cell models
Heterologous expression in epithelial cells is crucial in studies aimed at identifying the intracellular signals regulating AQP2 trafficking and sorting because the intracellular machinery regulating the expression and the functions of AQP2 is well conserved in this system. In this research field, there have been several attempts to generate both primary cultures from collecting ducts and AQP2 stably transfected cell lines since, unfortunately, the transcription of the AQP2 gene is rapidly repressed in culture conditions [29].
Primary cultured rat inner medullary collecting duct cells
The use of primary cultures to study AQP2 trafficking has the great advantage of retaining the features of the native tissue. However, cultured renal epithelial cells rapidly down-regulate the expression of AQP2 due to the internalization of the V2 receptor in culture conditions. Maric et al. [80] established a method to favor maintenance of AQP2 expression in vitro without genetic manipulation. Primary cultures of rat inner medullary collecting duct (IMCD) cells were found to retain AQP2 expression for at least 6 days when cultured in medium supplemented with dibutyryl cAMP (DBcAMP) [80]. Immunofluorescence and biochemical studies demonstrated that after stimulation with vasopressin or forskolin, AQP2-bearing vesicles relocated mainly to the basolateral rather than the apical membrane. This staining might be due to the culture conditions (600 mOsm/l) closely resembling the physiological extracellular environment osmolality for collecting duct principal cells [80]. Indeed, basolateral membrane expression of AQP2 can be seen in vivo in the distal inner medulla tubules. Consistent with this observation, it has been shown that acute vasopressin treatment can redirect AQP2 from the apical membrane to the basolateral side [138]. The primary IMCD cell model system established has the great advantage of enabling the regulation of AQP2 gene expression—as well as that of genes encoding the antidiuretic machinery at the cellular level—to be studied. IMCD cells have been useful in demonstrating that osmolality and solute composition are potent regulators of AQP2 expression in renal principal cells [122]. Moreover, the first demonstration that AQP2 translocation in response to vasopressin occurs only if protein kinase A is anchored to PKA anchoring proteins (AKAPs) was obtained in IMCD cells [60]. IMCD cells have also been used to demonstrate that vasopressin stimulation is associated with a significant decrease in the activity of Rho proteins, resulting in actin depolymerization and AQP2 targeting to the plasma membrane [61]. Further studies have revealed that activation of prostanoid receptors EP1 and EP3 counteracts vasopressin-dependent water reabsorption via the activation of Rho proteins [130].
Mouse renal collecting duct principal cells
Hasler and coworkers [33] established a very interesting mouse renal collecting duct principal cell model (mpkCCD) that endogenously expresses AQP2. This model has been particularly useful for studying vasopressin-inducible long-term AQP2 expression and regulation by hypertonicity [34, 35]. These cells have retained many fundamental features of collecting duct principal cells, including electrogenic Na+ transport stimulated by aldosterone and the expression of endogenous AQP2 when stimulated with physiological levels of vasopressin [33]. Interestingly, in a recent work, mRNA profiling in mpkCCD collecting duct cells provided the basis of cell-specific expression of AQP2 by a systems biology-based approach [111]. Moreover, mpkCCD cells have been used to identify several pathways resulting in the down-regulation of AQP2 expression [9, 12, 62, 150].
Rabbit collecting duct CD8 cells
CD8 cells were obtained from a rabbit cell line derived from the cortical collecting duct which had the characteristics of principal cells and were stably transfected with the cDNA for rat AQP2 [135]. CD8 cells were the first established AQP2 cell culture model with correct apical AQP2 sorting. Several intracellular signals and regulatory proteins involved in the translocation of AQP2 to the apical plasma membrane in the presence of vasopressin were identified in CD8 cells for the first time. In particular, it has been demonstrated in CD8 cells that a member of the Gi family, most likely Gi3, is involved in the cAMP-triggered targeting of AQP2-bearing vesicles to the apical membrane of kidney epithelial cells [136]. The crucial role of the Rho family of small GTPases as well as of annexin 2 in regulating apical AQP2 trafficking has also been demonstrated in CD8 cells [126, 128]. A detailed analysis of AQP2 biosynthesis in CD8 cells revealed that AQP2 can be phosphorylated by a kinase other than PKA during its maturation [106]. In addition, this cell line has been useful for identifying intracellular pathways counteracting cAMP-elicited AQP2 translocation in renal cells, such as bradykinin signaling [125] and extracellular calcium [107].
Madin–Darby canine kidney cell lines
AQP2-transfected Madin–Darby canine kidney cell lines (MDCK-AQP2) have been generated by several groups and represent another cell culture model in which AQP2 is sorted to the apical membrane [2, 17, 20, 84, 85, 94, 157]. However, under specific conditions, such as long-term hypertonicity, a feature of the inner medullary interstitium, AQP2 is inserted into the basolateral membrane, indicating that hypertonicity can alter the insertion of proteins from the apical membrane to the basolateral side [138]. In this respect, it is important to emphasize that correct cell polarization is crucial to ensure appropriate cellular localization of AQP2. For example, in non-polarized MDCK cells, AQP2 accumulates not only in the plasma membrane, but also in the Golgi complex [139].
Studies on MDCK cells expressing AQP2 have been crucial for gaining an understanding of the role of phosphorylation in AQP2 trafficking. A recent phospho-proteomic analysis demonstrated that AQP2 is multi-phosphorylated within the C-terminal domain at S256, S261, S264 and S269 [40]. Interestingly, the phosphorylation of these amino acids is vasopressin sensitive [28, 39, 40]. MDCK cells expressing AQP2 mutants that mimic the phosphorylated (S to D) or dephosphorylated (S to A) states, respectively, have been intensively used to clarify the importance of these post-translational modifications on AQP2 trafficking [84, 85, 127, 129]. Studies on MDCK-AQP2 also reveal that the C-tail of AQP2 is subjected to short-chain ubiquitination which controls the stability and the destiny of AQP2 [54].
Evidence for a possible critical role for the tetraspan membrane protein VIP17/MAL in the apical sorting of AQP2 has been provided in MDCK cells [52]. VIP17/MAL directly interacts with AQP2 and, in particular, the S256-phosphorylated form of AQP2 appears to interact more extensively with MAL [52]. MDCK cells expressing wild-type or mutated AQP2 have been a tremendous tool for elucidating the molecular basis of AQP2 missorting in dominant or recessive NDI (reviewed in [70]). Moreover, MDCK cells represent a good model for analysing the use of chemical chaperones as therapeutic strategies in order to correct mammalian cell processing of mutant AQP2 in NDI [124].
Porcine renal epithelial cells LLC-PK1
An epithelial cell model for studying AQP2 trafficking was generated by Katsura et al. [58] by stably transfecting porcine renal epithelial cells LLC-PK1 with cDNA encoding AQP2 (tagged with a C-terminal c-Myc epitope) in an expression vector containing a cytomegalovirus promoter. In this model, AQP2 is predominantly located on intracellular vesicles and following treatment with forskolin relocates to the basolateral plasma membrane.
Using this model, the authors demonstrated that AQP2 is internalized by clathrin-mediated endocytosis and that it can be recycled multiple times between intracellular vesicles and the plasma membrane [57]. Further investigations describe heat shock protein 70 (HSP-70) and its cognate protein Hsc-70 as new players in the endocytotic machinery controlling AQP2 internalization. Disruption of Hsc-70 activity by infecting LLC-PK1-AQP2 cells with ATPase-deficient Hsc-70 causes AQP2 accumulation at the plasma membrane, indicating a functional role of this accessory protein on AQP2 endocytosis [71]. Moreover, studies on the regulation of AQP2 trafficking by phosphorylation have been performed in LLC-PK(1) cells expressing point mutations of AQP2 S261 and S256, mimicking the phosphorylated (S to D) or dephosphorylated (S to A) states of these residues [72]. The results of this study suggest that regulated or constitutive trafficking of AQP2 is not modified by the phosphorylation state of S261. Another key result obtained in this cell line is the demonstration, through a fluorimetry assay, that vasopressin in addition to reducing AQP2 endocytosis also increases AQP2 exocytosis [96].
Mouse renal collecting duct principal cell MCD4
A useful model for studying AQP2 trafficking, i.e. MCD4 cells [41], was generated by stably transfecting mouse cortical collecting duct M1 cells [26] with human AQP2. This cell line displays an apical AQP2 targeting in response to forskolin treatment, indicating that these cells possess the intracellular machinery required for AQP2 vesicle fusion to the apical membrane in response to cAMP-elevating agent. The great advantage of this model is the species origin (mouse) that makes it possible to silence given endogenous proteins required for AQP2 trafficking. Indeed, in MCD4 cells, selective silencing of SNARE protein members provided functional evidence for their involvement in AQP2 trafficking [105]. In addition, this model has been useful for studying the association of AQP2 with membrane rafts, demonstrating that rafts may regulate both AQP2 apical sorting and endocytosis [103]. Subsequent studies have demonstrated that treatment of MCD4 with statins promote a significant accumulation of AQP2 at the apical membrane [103]. Moreover, the statin-mediated effect on AQP2 trafficking is most likely caused by statin-dependent changes in the prenylation status of key proteins regulating AQP2 trafficking in collecting duct cells, including the Rho and Rab families of proteins [104]. The interesting observation that statins also increase AQP2-mediated water reabsorption in mouse kidney in vivo [104] strongly suggests that statins may prove useful in the treatment of X-linked NDI. This hypothesis is under investigation in our laboratory using the mouse model of this disease [68].
MCD4 cells have recently proved useful for clarifying the involvement of integrin signaling on AQP2 trafficking [127, 129]. Based on the original observation that AQP2 is the only water channel containing the conserved integrin binding motif RGD, which is required for binding to integrin beta-1, we found that AQP2 relocalization to the plasma membrane is activated by peptides reproducing the interaction sequence AQP2/integrin via cAMP and Ca2+ signaling, respectively. Together, these data open up the possibility that novel custom-designed RGD-containing peptides, targeting AQP2 selectively, may be tested for ameliorating renal concentrating defects due to dysregulation of the V2R-AQP2 axis.
Conclusions and perspectives
Since the first aquaporin was identified nearly two decades ago, the generation of animal models lacking specific kidney aquaporins has resulted in clarification of the physiological role of most aquaporins involved in urinary concentrating mechanisms.
In particular, from the phenotype analysis of mice deficient in specific aquaporins, it appears clear that AQP1, AQP2, AQP3 and AQP4 can facilitate transepithelial fluid absorption—albeit to differing degrees. Nevertheless, the specific contribution that each aquaporin makes in terms of fluid reabsorption in a specific nephron segment is predicted to be different based on the phenotype observed in AQP-null mice. AQP1-deficient mice are polyuric and unable to concentrate their urine in response to water deprivation or vasopressin. Observations of AQP2 conditional-knockout mice clearly demonstrate that AQP2 in the collecting duct is essential for the regulation of body water balance and that this mechanism cannot be compensated for by other mechanisms. Mice lacking AQP3 expressed on the basolateral membrane predominantly in the cortical collecting duct have severe polyuria, but they are able to generate partly concentrated urine after being deprived of water. In contrast, mice lacking AQP4 expressed mainly in the inner medullary collecting duct manifest only mild polyuria.
Nevertheless, some surprising phenotypes not related with renal water handling have been identified in AQP-null mice. For example, AQP1-null mice display a significant decrease in tumor growth, suggesting a physiological role for AQP1 in the formation of membrane protrusion [143]. Moreover, some unexpected role for renal aquaporins is predicted based on original observations. One example is AQP5, which has been recently identified in the apical membrane of beta intercalated cells which are known to play a fundamental role in acid–base control. AQP5-null mice do not apparently display a renal phenotype. The possibility that AQP5 may serve as an osmosensor for the composition of the fluid coming from the thick ascending limb is currently under investigation.
In line with all of these studies, the intense parallel work done in epithelial cell models expressing given aquaporins has contributed to clarifying the complex regulation of renal aquaporins in terms of expression and cellular localization. The functional expression of aquaporins in renal cells helped to identify several interacting proteins that mediate renal function.
However, some studies in cell models also predict unexpected cellular roles for specific aquaporins. As an example, we have recently demonstrated that AQP2 binds integrin β1 via Arg–Gly–Asp (RGD), a typical motif of cell adhesion molecules, and that this interaction regulates AQP2 trafficking in renal tissue and in AQP2-expressing MCD4 cells. This observation suggests the possibility that AQP2 ‘senses’ signals from the ECM via the integrin receptor. This interaction may have consequences on cell migration and tubular morphogenesis.
To conclude, while both cell culture models and animal models have advantages and disadvantages in terms of studying the patho-physiological role of renal aquaporins, the combinations of the findings obtained using both research strategies have greatly contributed to our current knowledge of water transport and its regulation in the mammalian kidney.
Acknowledgements
Work in the author’s laboratories is supported by the Italian Ministry of University and Research (Grant Code 2008W5AZEC_005 to GV); by Italian Telethon (Grant Code GGP04202 to GV) and by the Regional Explorative and Strategic grants from Italian Ministry of University and Research (Grant Code PE 058 to GV and CIP PS_144 to GV). We thank Anthony Green for suggesting stylistic improvements.
Footnotes
G. Tamma and G. Procino contributed equally to this work.
References
- 1.Abrami L, Simon M, Rousselet G, Berthonaud V, Buhler JM, Ripoche P. Sequence and functional expression of an amphibian water channel, FA-CHIP: a new member of the MIP family. Biochim Biophys Acta. 1994;1192:147–151. doi: 10.1016/0005-2736(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 2.Baumgarter R, Pol MH, Wetzels JF, Os CH, Deen PM. Glycosylation is not essential for vasopressindependent routing of aquaporin-2 in transfected Madin–Darbycanine kidney cells. J Am Soc Nephrol. 1998;9:1553–1559. doi: 10.1681/ASN.V991553. [DOI] [PubMed] [Google Scholar]
- 3.Beitz E, Liu K, Ikeda M, Guggino WB, Agre P, Yasui M. Determinants of AQP6 trafficking to intracellular sites versus the plasma membrane in transfected mammalian cells. Biol Cell. 2006;98(2):101–109. doi: 10.1042/BC20050025. [DOI] [PubMed] [Google Scholar]
- 4.Bichet DG. Vasopressin receptors in health and disease. Kidney Int. 1996;49:1706–1711. doi: 10.1038/ki.1996.252. [DOI] [PubMed] [Google Scholar]
- 5.Bichet DG. Nephrogenic diabetes insipidus. Am J Med. 1998;105:431–442. doi: 10.1016/s0002-9343(98)00301-5. [DOI] [PubMed] [Google Scholar]
- 6.Bichet DG. Vasopressin receptor mutations in nephrogenic diabetes insipidus. Semin Nephrol. 2008;28:245–251. doi: 10.1016/j.semnephrol.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Bichet DG, Ruel N, Arthus MF, Lonergan M. Rolipram, a phosphodiesterase inhibitor, in the treatment of two male patients with congenital nephrogenic diabetes insipidus. Nephron. 1990;56:449–450. doi: 10.1159/000186196. [DOI] [PubMed] [Google Scholar]
- 8.Boone M, Deen PM. Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption. Pflugers Arch. 2008;456:1005–1024. doi: 10.1007/s00424-008-0498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boone M, Kortenoeven ML, Robben JH, Tamma G, Deen PM. Counteracting vasopressin-mediated water reabsorption by ATP, dopamine, and phorbol esters: mechanisms of action. Am J Physiol Renal Physiol. 2011;300:F761–F771. doi: 10.1152/ajprenal.00247.2010. [DOI] [PubMed] [Google Scholar]
- 10.Bourguet J, Chevalier J, Hugon JS. Alterations in membrane-associated particle distribution during antidiuretic challenge in frog urinary bladder epithelium. Biophys J. 1976;16:627–639. doi: 10.1016/S0006-3495(76)85717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown D, Orci L. Vasopressin stimulates formation of coated pits in rat kidney collecting ducts. Nature. 1983;302:253–255. doi: 10.1038/302253a0. [DOI] [PubMed] [Google Scholar]
- 12.Bustamante M, Hasler U, Leroy V, de Seigneux S, Dimitrov M, Mordasini D, Rousselot M, Martin PY, Feraille E. Calcium-sensing receptor attenuates AVP-induced aquaporin-2 expression via a calmodulin-dependent mechanism. J Am Soc Nephrol. 2008;19:109–116. doi: 10.1681/ASN.2007010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YC, Cadnapaphornchai MA, Schrier RW. Clinical update on renal aquaporins. Biol Cell. 2005;97:357–371. doi: 10.1042/BC20040041. [DOI] [PubMed] [Google Scholar]
- 14.Chevalier J, Bourguet J, Hugon JS. Membrane associated particles: distribution in frog urinary bladder epithelium at rest and after oxytocin treatment. Cell Tissue Res. 1974;152:129–140. doi: 10.1007/BF00224690. [DOI] [PubMed] [Google Scholar]
- 15.Coury LA, Mathai JC, Prasad GV, Brodsky JL, Agre P, Zeidel ML. Reconstitution of water channel function of aquaporins 1 and 2 by expression in yeast secretory vesicles. Am J Physiol. 1998;274:F34–F42. doi: 10.1152/ajprenal.1998.274.1.F34. [DOI] [PubMed] [Google Scholar]
- 16.de Mattia F, Savelkoul PJ, Bichet DG, Kamsteeg EJ, Konings IB, Marr N, Arthus MF, Lonergan M, van Os CH, van der Sluijs P, Robertson G, Deen PM. A novel mechanism in recessive nephrogenic diabetes insipidus: wild-type aquaporin-2 rescues the apical membrane expression of intracellularly retained AQP2–P262L. Hum Mol Genet. 2004;13:3045–3056. doi: 10.1093/hmg/ddh339. [DOI] [PubMed] [Google Scholar]
- 17.de Mattia F, Savelkoul PJ, Kamsteeg EJ, Konings IB, van der Sluijs P, Mallmann R, Oksche A, Deen PM. Lack of arginine vasopressin-induced phosphorylation of aquaporin-2 mutant AQP2–R254L explains dominant nephrogenic diabetes insipidus. J Am Soc Nephrol. 2005;16:2872–2880. doi: 10.1681/ASN.2005010104. [DOI] [PubMed] [Google Scholar]
- 18.Deen PM, Croes H, van Aubel RA, Ginsel LA, van Os CH. Water channels encoded by mutant aquaporin-2 genes in nephrogenic diabetes insipidus are impaired in their cellular routing. J Clin Invest. 1995;95:2291–2296. doi: 10.1172/JCI117920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deen PM, Nielsen S, Bindels RJ, van Os CH. Apical and basolateral expression of aquaporin-1 in transfected MDCK and LLC-PK cells and functional evaluation of their transcellular osmotic water permeabilities. Pflugers Arch. 1997;433:780–787. doi: 10.1007/s004240050345. [DOI] [PubMed] [Google Scholar]
- 20.Deen PM, Rijss JP, Mulders SM, Errington RJ, van Baal J, van Os CH. Aquaporin-2 transfection of Madin–Darby canine kidney cells reconstitutes vasopressin-regulated transcellular osmotic water transport. J Am Soc Nephrol. 1997;8:1493–1501. doi: 10.1681/ASN.V8101493. [DOI] [PubMed] [Google Scholar]
- 21.Deen PM, van Aubel RA, van Lieburg AF, van Os CH. Urinary content of aquaporin 1 and 2 in nephrogenic diabetes insipidus. J Am Soc Nephrol. 1996;7:836–841. doi: 10.1681/ASN.V76836. [DOI] [PubMed] [Google Scholar]
- 22.Duong Van Huyen J, Bens M, Vandewalle A. Differential effects of aldosterone and vasopressin on chloride fluxes in transimmortalized mouse cortical collecting duct cells. J Membr Biol. 1998;164:79–90. doi: 10.1007/s002329900395. [DOI] [PubMed] [Google Scholar]
- 23.Ecelbarger CA, Terris J, Frindt G, Echevarria M, Marples D, Nielsen S, Knepper MA. Aquaporin-3 water channel localization and regulation in rat kidney. Am J Physiol. 1995;269:F663–F672. doi: 10.1152/ajprenal.1995.269.5.F663. [DOI] [PubMed] [Google Scholar]
- 24.Echevarria M, Windhager EE, Tate SS, Frindt G. Cloning and expression of AQP3, a water channel from the medullary collecting duct of rat kidney. Proc Natl Acad Sci USA. 1994;91:10997–11001. doi: 10.1073/pnas.91.23.10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elkjaer ML, Nejsum LN, Gresz V, Kwon TH, Jensen UB, Frokiaer J, Nielsen S. Immunolocalization of aquaporin-8 in rat kidney, gastrointestinal tract, testis, and airways. Am J Physiol Renal Physiol. 2001;281:F1047–F1057. doi: 10.1152/ajprenal.0158.2001. [DOI] [PubMed] [Google Scholar]
- 26.Fejes-Toth G, Naray-Fejes-Toth A. Differentiation of renal beta-intercalated cells to alpha-intercalated and principal cells in culture. Proc Natl Acad Sci USA. 1992;89:5487–5491. doi: 10.1073/pnas.89.12.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fenton RA, Knepper MA. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev. 2007;87:1083–1112. doi: 10.1152/physrev.00053.2006. [DOI] [PubMed] [Google Scholar]
- 28.Fenton RA, Moeller HB, Hoffert JD, Yu MJ, Nielsen S, Knepper MA. Acute regulation of aquaporin-2 phosphorylation at Ser-264 by vasopressin. Proc Natl Acad Sci USA. 2008;105:3134–3139. doi: 10.1073/pnas.0712338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furuno M, Uchida S, Marumo F, Sasaki S. Repressive regulation of the aquaporin-2 gene. Am J Physiol. 1996;271:F854–F860. doi: 10.1152/ajprenal.1996.271.4.F854. [DOI] [PubMed] [Google Scholar]
- 30.Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature. 1993;361:549–552. doi: 10.1038/361549a0. [DOI] [PubMed] [Google Scholar]
- 31.Gorelick DA, Praetorius J, Tsunenari T, Nielsen S, Agre P. Aquaporin-11: a channel protein lacking apparent transport function expressed in brain. BMC Biochem. 2006;7:14. doi: 10.1186/1471-2091-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasegawa H, Ma T, Skach W, Matthay MA, Verkman AS. Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J Biol Chem. 1994;269:5497–5500. [PubMed] [Google Scholar]
- 33.Hasler U, Mordasini D, Bens M, Bianchi M, Cluzeaud F, Rousselot M, Vandewalle A, Feraille E, Martin PY. Long term regulation of aquaporin-2 expression in vasopressin-responsive renal collecting duct principal cells. J Biol Chem. 2002;277:10379–10386. doi: 10.1074/jbc.M111880200. [DOI] [PubMed] [Google Scholar]
- 34.Hasler U, Nielsen S, Feraille E, Martin PY. Posttranscriptional control of aquaporin-2 abundance by vasopressin in renal collecting duct principal cells. Am J Physiol Renal Physiol. 2006;290:F177–F187. doi: 10.1152/ajprenal.00056.2005. [DOI] [PubMed] [Google Scholar]
- 35.Hasler U, Vinciguerra M, Vandewalle A, Martin PY, Feraille E. Dual effects of hypertonicity on aquaporin-2 expression in cultured renal collecting duct principal cells. J Am Soc Nephrol. 2005;16:1571–1582. doi: 10.1681/ASN.2004110930. [DOI] [PubMed] [Google Scholar]
- 36.Hays RM, Meiteles L, Fant J, Franki N, Salisbury JL. A scanning electron microscopic study of the cytoplasmic surface of the toad bladder luminal membrane. Scan Electron Microsc Part. 1982;II:789–795. [PubMed] [Google Scholar]
- 37.He X, Tse CM, Donowitz M, Alper SL, Gabriel SE, Baum BJ. Polarized distribution of key membrane transport proteins in the rat submandibular gland. Pflugers Arch. 1997;433:260–268. doi: 10.1007/s004240050276. [DOI] [PubMed] [Google Scholar]
- 38.Hoffert JD, Chou CL, Knepper MA. Aquaporin-2 in the “-omics” era. J Biol Chem. 2009;284:14683–14687. doi: 10.1074/jbc.R900006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffert JD, Nielsen J, Yu MJ, Pisitkun T, Schleicher SM, Nielsen S, Knepper MA. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am J Physiol Renal Physiol. 2007;292:F691–F700. doi: 10.1152/ajprenal.00284.2006. [DOI] [PubMed] [Google Scholar]
- 40.Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA. 2006;103:7159–7164. doi: 10.1073/pnas.0600895103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iolascon A, Aglio V, Tamma G, D’Apolito M, Addabbo F, Procino G, Simonetti MC, Montini G, Gesualdo L, Debler EW, Svelto M, Valenti G. Characterization of two novel missense mutations in the AQP2 gene causing nephrogenic diabetes insipidus. Nephron Physiol. 2007;105:p33–p41. doi: 10.1159/000098136. [DOI] [PubMed] [Google Scholar]
- 42.Ishibashi K. Aquaporin subfamily with unusual NPA boxes. Biochim Biophys Acta. 2006;1758:989–993. doi: 10.1016/j.bbamem.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 43.Ishibashi K, Imai M, Sasaki S. Cellular localization of aquaporin 7 in the rat kidney. Exp Nephrol. 2000;8:252–257. doi: 10.1159/000020676. [DOI] [PubMed] [Google Scholar]
- 44.Ishibashi K, Kuwahara M, Gu Y, Kageyama Y, Tohsaka A, Suzuki F, Marumo F, Sasaki S. Cloning and functional expression of a new water channel abundantly expressed in the testis permeable to water, glycerol, and urea. J Biol Chem. 1997;272:20782–20786. doi: 10.1074/jbc.272.33.20782. [DOI] [PubMed] [Google Scholar]
- 45.Ishibashi K, Kuwahara M, Gu Y, Tanaka Y, Marumo F, Sasaki S. Cloning and functional expression of a new aquaporin (AQP9) abundantly expressed in the peripheral leukocytes permeable to water and urea, but not to glycerol. Biochem Biophys Res Commun. 1998;244:268–274. doi: 10.1006/bbrc.1998.8252. [DOI] [PubMed] [Google Scholar]
- 46.Ishibashi K, Kuwahara M, Kageyama Y, Tohsaka A, Marumo F, Sasaki S. Cloning and functional expression of a second new aquaporin abundantly expressed in testis. Biochem Biophys Res Commun. 1997;237:714–718. doi: 10.1006/bbrc.1997.7219. [DOI] [PubMed] [Google Scholar]
- 47.Ishibashi K, Sasaki S, Fushimi K, Uchida S, Kuwahara M, Saito H, Furukawa T, Nakajima K, Yamaguchi Y, Gojobori T, et al. Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc Natl Acad Sci USA. 1994;91:6269–6273. doi: 10.1073/pnas.91.14.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishida N, Hirai SI, Mita S. Immunolocalization of aquaporin homologs in mouse lacrimal glands. Biochem Biophys Res Commun. 1997;238:891–895. doi: 10.1006/bbrc.1997.7396. [DOI] [PubMed] [Google Scholar]
- 49.Jung JS, Preston GM, Smith BL, Guggino WB, Agre P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J Biol Chem. 1994;269:14648–14654. [PubMed] [Google Scholar]
- 50.Kachadorian WA, Wade JB, DiScala VA. Vasopressin: induced structural change in toad bladder luminal membrane. Science. 1975;190:67–69. doi: 10.1126/science.809840. [DOI] [PubMed] [Google Scholar]
- 51.Kamsteeg EJ, Bichet DG, Konings IB, Nivet H, Lonergan M, Arthus MF, van Os CH, Deen PM. Reversed polarized delivery of an aquaporin-2 mutant causes dominant nephrogenic diabetes insipidus. J Cell Biol. 2003;163:1099–1109. doi: 10.1083/jcb.200309017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamsteeg EJ, Duffield AS, Konings IB, Spencer J, Pagel P, Deen PM, Caplan MJ. MAL decreases the internalization of the aquaporin-2 water channel. Proc Natl Acad Sci USA. 2007;104:16696–16701. doi: 10.1073/pnas.0708023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamsteeg EJ, Heijnen I, van Os CH, Deen PM. The subcellular localization of an aquaporin-2 tetramer depends on the stoichiometry of phosphorylated and nonphosphorylated monomers. J Cell Biol. 2000;151:919–930. doi: 10.1083/jcb.151.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kamsteeg EJ, Hendriks G, Boone M, Konings IB, Oorschot V, van der Sluijs P, Klumperman J, Deen PM. Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc Natl Acad Sci USA. 2006;103:18344–18349. doi: 10.1073/pnas.0604073103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamsteeg EJ, Wormhoudt TA, Rijss JP, van Os CH, Deen PM. An impaired routing of wild-type aquaporin-2 after tetramerization with an aquaporin-2 mutant explains dominant nephrogenic diabetes insipidus. EMBO J. 1999;18:2394–2400. doi: 10.1093/emboj/18.9.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karabasil MR, Hasegawa T, Azlina A, Purwanti N, Purevjav J, Yao C, Akamatsu T, Hosoi K. Trafficking of GFP-AQP5 chimeric proteins conferred with unphosphorylated amino acids at their PKA-target motif ((152)SRRTS) in MDCK-II cells. J Med Invest. 2009;56:55–63. doi: 10.2152/jmi.56.55. [DOI] [PubMed] [Google Scholar]
- 57.Katsura T, Ausiello DA, Brown D. Direct demonstration of aquaporin-2 water channel recycling in stably transfected LLC-PK1 epithelial cells. Am J Physiol. 1996;270:F548–F553. doi: 10.1152/ajprenal.1996.270.3.F548. [DOI] [PubMed] [Google Scholar]
- 58.Katsura T, Verbavatz JM, Farinas J, Ma T, Ausiello DA, Verkman AS, Brown D. Constitutive and regulated membrane expression of aquaporin 1 and aquaporin 2 water channels in stably transfected LLC-PK1 epithelial cells. Proc Natl Acad Sci USA. 1995;92:7212–7216. doi: 10.1073/pnas.92.16.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.King LS, Choi M, Fernandez PC, Cartron JP, Agre P. Defective urinary-concentrating ability due to a complete deficiency of aquaporin-1. N Engl J Med. 2001;345:175–179. doi: 10.1056/NEJM200107193450304. [DOI] [PubMed] [Google Scholar]
- 60.Klussmann E, Rosenthal W. Role and identification of protein kinase A anchoring proteins in vasopressin-mediated aquaporin-2 translocation. Kidney Int. 2001;60:446–449. doi: 10.1046/j.1523-1755.2001.060002446.x. [DOI] [PubMed] [Google Scholar]
- 61.Klussmann E, Tamma G, Lorenz D, Wiesner B, Maric K, Hofmann F, Aktories K, Valenti G, Rosenthal W. An inhibitory role of Rho in the vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J Biol Chem. 2001;276:20451–20457. doi: 10.1074/jbc.M010270200. [DOI] [PubMed] [Google Scholar]
- 62.Kortenoeven ML, van den Brand M, Wetzels JF, Deen PM. Hypotonicity-induced reduction of aquaporin-2 transcription in mpkCCD cells is independent of the tonicity responsive element, vasopressin, and cAMP. J Biol Chem. 2011;286:13002–13010. doi: 10.1074/jbc.M110.207878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kosugi-Tanaka C, Li X, Yao C, Akamatsu T, Kanamori N, Hosoi K. Protein kinase A-regulated membrane trafficking of a green fluorescent protein-aquaporin 5 chimera in MDCK cells. Biochim Biophys Acta. 2006;1763:337–344. doi: 10.1016/j.bbamcr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 64.Kuwahara M, Fushimi K, Terada Y, Bai L, Marumo F, Sasaki S. cAMP-dependent phosphorylation stimulates water permeability of aquaporin-collecting duct water channel protein expressed in Xenopus oocytes. J Biol Chem. 1995;270:10384–10387. doi: 10.1074/jbc.270.18.10384. [DOI] [PubMed] [Google Scholar]
- 65.Kuwahara M, Iwai K, Ooeda T, Igarashi T, Ogawa E, Katsushima Y, Shinbo I, Uchida S, Terada Y, Arthus MF, Lonergan M, Fujiwara TM, Bichet DG, Marumo F, Sasaki S. Three families with autosomal dominant nephrogenic diabetes insipidus caused by aquaporin-2 mutations in the C-terminus. Am J Hum Genet. 2001;69:738–748. doi: 10.1086/323643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuwahara M, Shinbo I, Sato K, Terada Y, Marumo F, Sasaki S. Transmembrane helix 5 is critical for the high water permeability of aquaporin. Biochemistry. 1999;38:16340–16346. doi: 10.1021/bi9916776. [DOI] [PubMed] [Google Scholar]
- 67.Leaf A. Action of neurohypophyseal hormones on the toad bladder. Gen Comp Endocrinol. 1962;2:148–160. doi: 10.1016/0016-6480(62)90035-7. [DOI] [PubMed] [Google Scholar]
- 68.Li JH, Chou CL, Li B, Gavrilova O, Eisner C, Schnermann J, Anderson SA, Deng CX, Knepper MA, Wess J. A selective EP4 PGE2 receptor agonist alleviates disease in a new mouse model of X-linked nephrogenic diabetes insipidus. J Clin Invest. 2009;119(10):3115–3126. doi: 10.1172/JCI39680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lloyd DJ, Hall FW, Tarantino LM, Gekakis N. Diabetes insipidus in mice with a mutation in aquaporin-2. PLoS Genet. 2005;1:e20. doi: 10.1371/journal.pgen.0010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loonen AJ, Knoers NV, van Os CH, Deen PM. Aquaporin 2 mutations in nephrogenic diabetes insipidus. Semin Nephrol. 2008;28:252–265. doi: 10.1016/j.semnephrol.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 71.Lu HA, Sun TX, Matsuzaki T, Yi XH, Eswara J, Bouley R, McKee M, Brown D. Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J Biol Chem. 2007;282:28721–28732. doi: 10.1074/jbc.M611101200. [DOI] [PubMed] [Google Scholar]
- 72.Lu HJ, Matsuzaki T, Bouley R, Hasler U, Qin QH, Brown D. The phosphorylation state of serine 256 is dominant over that of serine 261 in the regulation of AQP2 trafficking in renal epithelial cells. Am J Physiol Renal Physiol. 2008;295:F290–F294. doi: 10.1152/ajprenal.00072.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma T, Fukuda N, Song Y, Matthay MA, Verkman AS. Lung fluid transport in aquaporin-5 knockout mice. J Clin Invest. 2000;105:93–100. doi: 10.1172/JCI8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 75.Ma T, Song Y, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc Natl Acad Sci USA. 2000;97:4386–4391. doi: 10.1073/pnas.080499597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J Clin Invest. 1997;100:957–962. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem. 1998;273:4296–4299. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]
- 78.Ma T, Yang B, Kuo WL, Verkman AS. cDNA cloning and gene structure of a novel water channel expressed exclusively in human kidney: evidence for a gene cluster of aquaporins at chromosome locus 12q13. Genomics. 1996;35:543–550. doi: 10.1006/geno.1996.0396. [DOI] [PubMed] [Google Scholar]
- 79.Madrid R, Le Maout S, Barrault MB, Janvier K, Benichou S, Merot J. Polarized trafficking and surface expression of the AQP4 water channel are coordinated by serial and regulated interactions with different clathrin-adaptor complexes. EMBO J. 2001;20:7008–7021. doi: 10.1093/emboj/20.24.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maric K, Oksche A, Rosenthal W. Aquaporin-2 expression in primary cultured rat inner medullary collecting duct cells. Am J Physiol. 1998;275:F796–F801. doi: 10.1152/ajprenal.1998.275.5.F796. [DOI] [PubMed] [Google Scholar]
- 81.Matsuzaki T, Suzuki T, Takata K. Hypertonicity-induced expression of aquaporin 3 in MDCK cells. Am J Physiol Cell Physiol. 2001;281:C55–C63. doi: 10.1152/ajpcell.2001.281.1.C55. [DOI] [PubMed] [Google Scholar]
- 82.McDill BW, Li SZ, Kovach PA, Ding L, Chen F. Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation. Proc Natl Acad Sci USA. 2006;103:6952–6957. doi: 10.1073/pnas.0602087103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mhatre AN, Steinbach S, Hribar K, Hoque AT, Lalwani AK. Identification of aquaporin 5 (AQP5) within the cochlea: cDNA cloning and in situ localization. Biochem Biophys Res Commun. 1999;264:157–162. doi: 10.1006/bbrc.1999.1323. [DOI] [PubMed] [Google Scholar]
- 84.Moeller HB, MacAulay N, Knepper MA, Fenton RA. Role of multiple phosphorylation sites in the COOH-terminal tail of aquaporin-2 for water transport: evidence against channel gating. Am J Physiol Renal Physiol. 2009;296:F649–F657. doi: 10.1152/ajprenal.90682.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moeller HB, Praetorius J, Rutzler MR, Fenton RA. Phosphorylation of aquaporin-2 regulates its endocytosis and protein–protein interactions. Proc Natl Acad Sci USA. 2009;107:424–429. doi: 10.1073/pnas.0910683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morishita Y, Matsuzaki T, Hara-chikuma M, Andoo A, Shimono M, Matsuki A, Kobayashi K, Ikeda M, Yamamoto T, Verkman A, Kusano E, Ookawara S, Takata K, Sasaki S, Ishibashi K. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol Cell Biol. 2005;25:7770–7779. doi: 10.1128/MCB.25.17.7770-7779.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mulders SM, Knoers NV, Van Lieburg AF, Monnens LA, Leumann E, Wuhl E, Schober E, Rijss JP, Van Os CH, Deen PM. New mutations in the AQP2 gene in nephrogenic diabetes insipidus resulting in functional but misrouted water channels. J Am Soc Nephrol. 1997;8:242–248. doi: 10.1681/ASN.V82242. [DOI] [PubMed] [Google Scholar]
- 88.Nedvetsky PI, Tamma G, Beulshausen S, Valenti G, Rosenthal W, Klussmann E. Regulation of aquaporin-2 trafficking. Handb Exp Pharmacol. 2009;190:133–157. doi: 10.1007/978-3-540-79885-9_6. [DOI] [PubMed] [Google Scholar]
- 89.Nejsum LN. The renal plumbing system: aquaporin water channels. Cell Mol Life Sci. 2005;62:1692–1706. doi: 10.1007/s00018-005-4549-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nejsum LN, Elkjaer M, Hager H, Frokiaer J, Kwon TH, Nielsen S. Localization of aquaporin-7 in rat and mouse kidney using RT-PCR, immunoblotting, and immunocytochemistry. Biochem Biophys Res Commun. 2000;277:164–170. doi: 10.1006/bbrc.2000.3638. [DOI] [PubMed] [Google Scholar]
- 91.Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002;82:205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 93.Nielsen S, King LS, Christensen BM, Agre P. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am J Physiol. 1997;273:C1549–C1561. doi: 10.1152/ajpcell.1997.273.5.C1549. [DOI] [PubMed] [Google Scholar]
- 94.Noda Y, Horikawa S, Kanda E, Yamashita M, Meng H, Eto K, Li Y, Kuwahara M, Hirai K, Pack C, Kinjo M, Okabe S, Sasaki S. Reciprocal interaction with G-actin and tropomyosin is essential for aquaporin-2 trafficking. J Cell Biol. 2008;182:587–601. doi: 10.1083/jcb.200709177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Noda Y, Sohara E, Ohta E, Sasaki S. Aquaporins in kidney pathophysiology. Nat Rev Nephrol. 2010;6(3):168–178. doi: 10.1038/nrneph.2009.231. [DOI] [PubMed] [Google Scholar]
- 96.Nunes P, Hasler U, McKee M, Lu HA, Bouley R, Brown D. A fluorimetry-based ssYFP secretion assay to monitor vasopressin-induced exocytosis in LLC-PK1 cells expressing aquaporin-2. Am J Physiol Cell Physiol. 2008;295:C1476–C1487. doi: 10.1152/ajpcell.00344.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Okada S, Misaka T, Tanaka Y, Matsumoto I, Ishibashi K, Sasaki S, Abe K. Aquaporin-11 knockout mice and polycystic kidney disease animals share a common mechanism of cyst formation. FASEB J. 2008;22:3672–3684. doi: 10.1096/fj.08-111872. [DOI] [PubMed] [Google Scholar]
- 98.Oksche A, Rosenthal W. The molecular basis of nephrogenic diabetes insipidus. J Mol Med. 1998;76:326–337. doi: 10.1007/s001090050224. [DOI] [PubMed] [Google Scholar]
- 99.Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 100.Preston GM, Jung JS, Guggino WB, Agre P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J Biol Chem. 1993;268:17–20. [PubMed] [Google Scholar]
- 101.Preston GM, Jung JS, Guggino WB, Agre P. Membrane topology of aquaporin CHIP. Analysis of functional epitope-scanning mutants by vectorial proteolysis. J Biol Chem. 1994;269:1668–1673. [PubMed] [Google Scholar]
- 102.Procino G, Mastrofrancesco L, Sallustio F, Costantino V, Barbieri C, Pisani F, Schena FP, Svelto M, Valenti G. AQP5 is expressed in type-B intercalated cells in the collecting duct system of the rat, mouse and human kidney. Cell Physiol Biochem. 2011a;28:683–692. doi: 10.1159/000335762. [DOI] [PubMed] [Google Scholar]
- 103.Procino G, Barbieri C, Carmosino M, Rizzo F, Valenti G, Svelto M. Lovastatin-induced cholesterol depletion affects both apical sorting and endocytosis of aquaporin-2 in renal cells. Am J Physiol Renal Physiol. 2010;298:F266–F278. doi: 10.1152/ajprenal.00359.2009. [DOI] [PubMed] [Google Scholar]
- 104.Procino G, Barbieri C, Carmosino M, Tamma G, Milano S, De Benedictis L, Mola MG, Lazo-Fernandez Y, Valenti G, Svelto M. Fluvastatin modulates renal water reabsorption in vivo through increased AQP2 availability at the apical plasma membrane of collecting duct cells. Pflugers Arch. 2011;462(5):753–766. doi: 10.1007/s00424-011-1007-5. [DOI] [PubMed] [Google Scholar]
- 105.Procino G, Barbieri C, Tamma G, De Benedictis L, Pessin JE, Svelto M, Valenti G. AQP2 exocytosis in the renal collecting duct—involvement of SNARE isoforms and the regulatory role of Munc18b. J Cell Sci. 2008;121:2097–2106. doi: 10.1242/jcs.022210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Procino G, Carmosino M, Marin O, Brunati AM, Contri A, Pinna LA, Mannucci R, Nielsen S, Kwon TH, Svelto M, Valenti G. Ser-256 phosphorylation dynamics of Aquaporin 2 during maturation from the ER to the vesicular compartment in renal cells. FASEB J. 2003;17:1886–1888. doi: 10.1096/fj.02-0870fje. [DOI] [PubMed] [Google Scholar]
- 107.Procino G, Carmosino M, Tamma G, Gouraud S, Laera A, Riccardi D, Svelto M, Valenti G. Extracellular calcium antagonizes forskolin-induced aquaporin 2 trafficking in collecting duct cells. Kidney Int. 2004;66:2245–2255. doi: 10.1111/j.1523-1755.2004.66036.x. [DOI] [PubMed] [Google Scholar]
- 108.Procino G, Mastrofrancesco L, Mira A, Tamma G, Carmosino M, Emma F, Svelto M, Valenti G. Aquaporin 2 and apical calcium-sensing receptor: new players in polyuric disorders associated with hypercalciuria. Semin Nephrol. 2008;28:297–305. doi: 10.1016/j.semnephrol.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 109.Rai T, Sasaki S, Uchida S. Polarized trafficking of the aquaporin-3 water channel is mediated by an NH2-terminal sorting signal. Am J Physiol Cell Physiol. 2006;290:C298–C304. doi: 10.1152/ajpcell.00356.2005. [DOI] [PubMed] [Google Scholar]
- 110.Raina S, Preston GM, Guggino WB, Agre P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J Biol Chem. 1995;270:1908–1912. doi: 10.1074/jbc.270.4.1908. [DOI] [PubMed] [Google Scholar]
- 111.Rinschen MM, Yu MJ, Wang G, Boja ES, Hoffert JD, Pisitkun T, Knepper MA. Quantitative phosphoproteomic analysis reveals vasopressin V2-receptor-dependent signaling pathways in renal collecting duct cells. Proc Natl Acad Sci USA. 2010;107:3882–3887. doi: 10.1073/pnas.0910646107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Robben JH, Knoers NV, Deen PM. Cell biological aspects of the vasopressin type-2 receptor and aquaporin 2 water channel in nephrogenic diabetes insipidus. Am J Physiol Renal Physiol. 2006;291:F257–F270. doi: 10.1152/ajprenal.00491.2005. [DOI] [PubMed] [Google Scholar]
- 113.Robben JH, Sze M, Knoers NV, Deen PM. Functional rescue of vasopressin V2 receptor mutants in MDCK cells by pharmacochaperones: relevance to therapy of nephrogenic diabetes insipidus. Am J Physiol Renal Physiol. 2007;292:F253–F260. doi: 10.1152/ajprenal.00247.2006. [DOI] [PubMed] [Google Scholar]
- 114.Rojek A, Fuchtbauer EM, Kwon TH, Frokiaer J, Nielsen S. Severe urinary concentrating defect in renal collecting duct-selective AQP2 conditional-knockout mice. Proc Natl Acad Sci USA. 2006;103:6037–6042. doi: 10.1073/pnas.0511324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 116.Schnermann J, Chou CL, Ma T, Traynor T, Knepper MA, Verkman AS. Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proc Natl Acad Sci USA. 1998;95:9660–9664. doi: 10.1073/pnas.95.16.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schrier RW. Vasopressin and aquaporin 2 in clinical disorders of water homeostasis. Semin Nephrol. 2008;28:289–296. doi: 10.1016/j.semnephrol.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shinbo I, Fushimi K, Kasahara M, Yamauchi K, Sasaki S, Marumo F. Functional analysis of aquaporin-2 mutants associated with nephrogenic diabetes insipidus by yeast expression. Am J Physiol. 1999;277:F734–F741. doi: 10.1152/ajprenal.1999.277.5.F734. [DOI] [PubMed] [Google Scholar]
- 119.Smith BL, Preston GM, Spring FA, Anstee DJ, Agre P. Human red cell aquaporin CHIP. J Clin Invest. 1994;94:1043–1049. doi: 10.1172/JCI117418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sohara E, Rai T, Miyazaki J, Verkman AS, Sasaki S, Uchida S. Defective water and glycerol transport in the proximal tubules of AQP7 knockout mice. Am J Physiol Renal Physiol. 2005;289:F1195–F1200. doi: 10.1152/ajprenal.00133.2005. [DOI] [PubMed] [Google Scholar]
- 121.Sohara E, Rai T, Yang SS, Uchida K, Nitta K, Horita S, Ohno M, Harada A, Sasaki S, Uchida S. Pathogenesis and treatment of autosomal-dominant nephrogenic diabetes insipidus caused by an aquaporin 2 mutation. Proc Natl Acad Sci USA. 2006;103:14217–14222. doi: 10.1073/pnas.0602331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Storm R, Klussmann E, Geelhaar A, Rosenthal W, Maric K. Osmolality and solute composition are strong regulators of AQP2 expression in renal principal cells. Am J Physiol Renal Physiol. 2003;284:F189–F198. doi: 10.1152/ajprenal.00245.2002. [DOI] [PubMed] [Google Scholar]
- 123.Tajima M, Crane JM, Verkman AS. Aquaporin-4 (AQP4) associations and array dynamics probed by photobleaching and single-molecule analysis of green fluorescent protein-AQP4 chimeras. J Biol Chem. 2010;285:8163–8170. doi: 10.1074/jbc.M109.093948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tamarappoo BK, Verkman AS. Defective aquaporin-2 trafficking in nephrogenic diabetes insipidus and correction by chemical chaperones. J Clin Invest. 1998;101:2257–2267. doi: 10.1172/JCI2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tamma G, Carmosino M, Svelto M, Valenti G. Bradykinin signaling counteracts cAMP-elicited aquaporin 2 translocation in renal cells. J Am Soc Nephrol. 2005;16:2881–2889. doi: 10.1681/ASN.2005020190. [DOI] [PubMed] [Google Scholar]
- 126.Tamma G, Klussmann E, Maric K, Aktories K, Svelto M, Rosenthal W, Valenti G. Rho inhibits cAMP-induced translocation of aquaporin-2 into the apical membrane of renal cells. Am J Physiol Renal Physiol. 2001;281:F1092–F1101. doi: 10.1152/ajprenal.0091.2001. [DOI] [PubMed] [Google Scholar]
- 127.Tamma G, Lasorsa D, Ranieri M, Mastrofrancesco L, Valenti G, Svelto M. Integrin signaling modulates AQP2 trafficking via Arg-Gly-Asp (RGD) motif. Cell Physiol Biochem. 2011;27:739–748. doi: 10.1159/000330082. [DOI] [PubMed] [Google Scholar]
- 128.Tamma G, Procino G, Mola MG, Svelto M, Valenti G. Functional involvement of Annexin-2 in cAMP induced AQP2 trafficking. Pflugers Arch. 2008;456:729–736. doi: 10.1007/s00424-008-0453-1. [DOI] [PubMed] [Google Scholar]
- 129.Tamma G, Robben JH, Trimpert C, Boone M, Deen PM. Regulation of AQP2 localization by S256 and S261 phosphorylation and ubiquitination. Am J Physiol Cell Physiol. 2011;300:C636–C646. doi: 10.1152/ajpcell.00433.2009. [DOI] [PubMed] [Google Scholar]
- 130.Tamma G, Wiesner B, Furkert J, Hahm D, Oksche A, Schaefer M, Valenti G, Rosenthal W, Klussmann E. The prostaglandin E2 analogue sulprostone antagonizes vasopressin-induced antidiuresis through activation of Rho. J Cell Sci. 2003;116:3285–3294. doi: 10.1242/jcs.00640. [DOI] [PubMed] [Google Scholar]
- 131.Tham DK, Moukhles H. Regulation of Kir4.1 and AQP4 expression and stability at the basolateral domain of epithelial MDCK cells by the extracellular matrix. Am J Physiol Renal Physiol. 2011;301:F396–F409. doi: 10.1152/ajprenal.00315.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tsukaguchi H, Shayakul C, Berger UV, Mackenzie B, Devidas S, Guggino WB, van Hoek AN, Hediger MA. Molecular characterization of a broad selectivity neutral solute channel. J Biol Chem. 1998;273:24737–24743. doi: 10.1074/jbc.273.38.24737. [DOI] [PubMed] [Google Scholar]
- 133.Umenishi F, Narikiyo T, Schrier RW. Hypertonic induction of aquaporin-1 water channel independent of transcellular osmotic gradient. Biochem Biophys Res Commun. 2004;325:595–599. doi: 10.1016/j.bbrc.2004.10.076. [DOI] [PubMed] [Google Scholar]
- 134.Vacca A, Frigeri A, Ribatti D, Nicchia GP, Nico B, Ria R, Svelto M, Dammacco F. Microvessel overexpression of aquaporin 1 parallels bone marrow angiogenesis in patients with active multiple myeloma. Br J Haematol. 2001;113:415–421. doi: 10.1046/j.1365-2141.2001.02738.x. [DOI] [PubMed] [Google Scholar]
- 135.Valenti G, Frigeri A, Ronco PM, D’Ettorre C, Svelto M. Expression and functional analysis of water channels in a stably AQP2-transfected human collecting duct cell line. J Biol Chem. 1996;271:24365–24370. doi: 10.1074/jbc.271.40.24365. [DOI] [PubMed] [Google Scholar]
- 136.Valenti G, Procino G, Liebenhoff U, Frigeri A, Benedetti PA, Ahnert-Hilger G, Nurnberg B, Svelto M, Rosenthal W. A heterotrimeric G protein of the Gi family is required for cAMP-triggered trafficking of aquaporin 2 in kidney epithelial cells. J Biol Chem. 1998;273:22627–22634. doi: 10.1074/jbc.273.35.22627. [DOI] [PubMed] [Google Scholar]
- 137.Valenti G, Procino G, Tamma G, Carmosino M, Svelto M. Minireview: aquaporin 2 trafficking. Endocrinology. 2005;146:5063–5070. doi: 10.1210/en.2005-0868. [DOI] [PubMed] [Google Scholar]
- 138.van Balkom BW, van Raak M, Breton S, Pastor-Soler N, Bouley R, van der Sluijs P, Brown D, Deen PM. Hypertonicity is involved in redirecting the aquaporin-2 water channel into the basolateral, instead of the apical, plasma membrane of renal epithelial cells. J Biol Chem. 2003;278:1101–1107. doi: 10.1074/jbc.M207339200. [DOI] [PubMed] [Google Scholar]
- 139.van Beest M, Robben JH, Savelkoul PJ, Hendriks G, Devonald MA, Konings IB, Lagendijk AK, Karet F, Deen PM. Polarisation, key to good localisation. Biochim Biophys Acta. 2006;1758:1126–1133. doi: 10.1016/j.bbamem.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 140.van Hoek AN, Ma T, Yang B, Verkman AS, Brown D. Aquaporin-4 is expressed in basolateral membranes of proximal tubule S3 segments in mouse kidney. Am J Physiol Renal Physiol. 2000;278:F310–F316. doi: 10.1152/ajprenal.2000.278.2.F310. [DOI] [PubMed] [Google Scholar]
- 141.Verkman AS. Aquaporins at a glance. J Cell Sci. 2011;124:2107–2112. doi: 10.1242/jcs.079467. [DOI] [PubMed] [Google Scholar]
- 142.Verkman AS. Lessons on renal physiology from transgenic mice lacking aquaporin water channels. J Am Soc Nephrol. 1999;10:1126–1135. doi: 10.1681/ASN.V1051126. [DOI] [PubMed] [Google Scholar]
- 143.Verkman AS. More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci. 2005;118:3225–3232. doi: 10.1242/jcs.02519. [DOI] [PubMed] [Google Scholar]
- 144.Verkman AS. Dissecting the roles of aquaporins in renal pathophysiology using transgenic mice. Semin Nephrol. 2008;28:217–226. doi: 10.1016/j.semnephrol.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Verkman AS. Knock-out models reveal new aquaporin functions. Handb Exp Pharmacol. 2009;190:359–381. doi: 10.1007/978-3-540-79885-9_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wellner RB, Baum BJ. Polarized sorting of aquaporins 5 and 8 in stable MDCK-II transfectants. Biochem Biophys Res Commun. 2001;285:1253–1258. doi: 10.1006/bbrc.2001.5287. [DOI] [PubMed] [Google Scholar]
- 147.Wellner RB, Cotrim AP, Hong S, Swaim WD, Baum BJ. Localization of AQP5/AQP8 chimeras in MDCK-II cells: exchange of the N- and C-termini. Biochem Biophys Res Commun. 2005;330:172–177. doi: 10.1016/j.bbrc.2005.02.146. [DOI] [PubMed] [Google Scholar]
- 148.Wellner RB, Hong S, Cotrim AP, Swaim WD, Baum BJ. Modifying the NH2 and COOH termini of aquaporin-5: effects on localization in polarized epithelial cells. Tissue Eng. 2005;11:1449–1458. doi: 10.1089/ten.2005.11.1449. [DOI] [PubMed] [Google Scholar]
- 149.Wellner RB, Hoque AT, Goldsmith CM, Baum BJ. Evidence that aquaporin-8 is located in the basolateral membrane of rat submandibular gland acinar cells. Pflugers Arch. 2000;441:49–56. doi: 10.1007/s004240000396. [DOI] [PubMed] [Google Scholar]
- 150.Wildman SS, Boone M, Peppiatt-Wildman CM, Contreras-Sanz A, King BF, Shirley DG, Deen PM, Unwin RJ. Nucleotides downregulate aquaporin 2 via activation of apical P2 receptors. J Am Soc Nephrol. 2009;20:1480–1490. doi: 10.1681/ASN.2008070686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wolburg H, Wolburg-Buchholz K, Fallier-Becker P, Noell S, Mack AF. Structure and functions of aquaporin-4-based orthogonal arrays of particles. Int Rev Cell Mol Biol. 2011;287:1–41. doi: 10.1016/B978-0-12-386043-9.00001-3. [DOI] [PubMed] [Google Scholar]
- 152.Yang B, Brown D, Verkman AS. The mercurial insensitive water channel (AQP-4) forms orthogonal arrays in stably transfected Chinese hamster ovary cells. J Biol Chem. 1996;271:4577–4580. [PubMed] [Google Scholar]
- 153.Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Neonatal mortality in an aquaporin-2 knock-in mouse model of recessive nephrogenic diabetes insipidus. J Biol Chem. 2001;276:2775–2779. doi: 10.1074/jbc.M008216200. [DOI] [PubMed] [Google Scholar]
- 154.Yang B, Ma T, Verkman AS. Erythrocyte water permeability and renal function in double knockout mice lacking aquaporin-1 and aquaporin-3. J Biol Chem. 2001;276:624–628. doi: 10.1074/jbc.M008664200. [DOI] [PubMed] [Google Scholar]
- 155.Yang B, Song Y, Zhao D, Verkman AS. Phenotype analysis of aquaporin-8 null mice. Am J Physiol Cell Physiol. 2005;288:C1161–C1170. doi: 10.1152/ajpcell.00564.2004. [DOI] [PubMed] [Google Scholar]
- 156.Yang B, Verkman AS. Water and glycerol permeabilities of aquaporins 1–5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J Biol Chem. 1997;272:16140–16146. doi: 10.1074/jbc.272.26.16140. [DOI] [PubMed] [Google Scholar]
- 157.Yang B, Zhao D, Verkman AS. Hsp90 inhibitor partially corrects nephrogenic diabetes insipidus in a conditional knock-in mouse model of aquaporin-2 mutation. FASEB J. 2009;23:503–512. doi: 10.1096/fj.08-118422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yasui M, Kwon TH, Knepper MA, Nielsen S, Agre P. Aquaporin-6: an intracellular vesicle water channel protein in renal epithelia. Proc Natl Acad Sci USA. 1999;96:5808–5813. doi: 10.1073/pnas.96.10.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yool AJ, Weinstein AM. New roles for old holes: ion channel function in aquaporin-1. News Physiol Sci. 2002;17:68–72. doi: 10.1152/nips.01372.2001. [DOI] [PubMed] [Google Scholar]
- 160.Zhang H, Verkman AS. Aquaporin-1 tunes pain perception by interaction with Na(v)1.8 Na+ channels in dorsal root ganglion neurons. J Biol Chem. 2010;285:5896–5906. doi: 10.1074/jbc.M109.090233. [DOI] [PMC free article] [PubMed] [Google Scholar]