Abstract
Myocardial infarction (MI) is a leading cause of hospitalization worldwide. A recently developed strategy to improve the management of MI is based on the use of growth factors which are able to enhance the intrinsic capacity of the heart to repair itself or regenerate after damage. Among others, hepatocyte growth factor (HGF) has been proposed as a modulator of cardiac repair of damage due to the pleiotropic effects elicited by Met receptor stimulation. In this review we describe the mechanistic basis for autocrine and paracrine protection of HGF in the injured heart. We also analyse the role of HGF/Met in stem cell maintenance and in stem cell therapies for MI. Finally, we summarize the most significant results on the use of HGF in experimental models of heart injury and discuss the potential of the molecule for treating ischaemic heart disease in humans.
Keywords: Hepatocyte growth factor, Met tyrosine-kinase receptor, Myocardial infarction, Cardiac progenitor cells, Stem cell therapy
Introduction
Ischaemic heart disease is the most common cause of death in most western countries and a major cause of hospitalization [1]. Myocardial infarction (MI) most frequently results from atherosclerosis of the coronary arteries and subsequent shortage of blood supply to the heart. The resumption of blood flow in ischaemic tissue, so-called reperfusion, has proved an effective life-saving therapy; therefore, coronary angioplastic interventions or thrombolytic treatments are currently recommended for the immediate-early treatment of acute MI.
The physiopathological response leading to the repair of the damaged tissue occurs through four independent processes:
Degeneration due to cell death for apoptosis or necrosis.
Inflammation with influx of inflammatory cells into the damaged tissue.
Fibrosis and eventual scar instauration.
Regeneration through formation of new functional tissue.
Effective repair of the damage seems to depend on the efficiency of recruitment and activation of the inflammatory cells at the site of injury [2]. However, the inflammatory response primarily triggered by reperfusion may also lead to myocardial injury. The inflammatory response is concomitant with the onset of a fibrotic process that leads to the replacement of functional heart muscle with collagen-based connective tissue. Surviving cardiomyocytes primarily react with hypertrophy, rather than proliferation, due to their limited mitotic capacity. However, the generally accepted, but never proven, dogma of the heart as a terminally differentiated organ has been challenged by the identification of cardiac progenitor cells (CPCs) in the adult heart [3–6]. CPCs are self-renewing, show unlimited replication and can generate the main cardiac cell lineages (cardiomyocytes, smooth muscle and endothelial cells).
A recently developed strategy to improve the current therapeutic arsenal against MI is based on the use of growth factors to strengthen the heart’s intrinsic capacity to react to acute damage. Stem cell transplantation has been proposed as an alternative or complementary strategy to early treatment approaches. It aims to create in the cardiac parenchyma an optimal environment for stimulating damaged myocardium self-repair. Indeed, recent studies have suggested that the beneficial effects of stem cell-based treatment of MI are mostly due to the production of trophic/proangiogenic factors [7]. Among other molecules, hepatocyte growth factor (HGF) has been proposed as a modulator of cardiac repair. This review includes the history of HGF in cardiac disease [8], unravels the mechanistic basis for HGF autocrine and paracrine protection, and explores the potential of the clinical use of HGF, with a discussion of the pros and cons in cardiac disease to date.
The HGF/Met system
HGF is synthesized as a single-chain inactive precursor (pro-HGF) and is converted by proteolysis to a two-chain, active heterodimer, composed of a 69-kDa α-chain and a 34-kDa β-chain [9]. The multiple biological functions of HGF are mediated by its specific tyrosine-kinase receptor, which is encoded by the Met protooncogene [10]. Like its ligand, the high-affinity Met receptor is a disulphide-linked heterodimer, which results from cleavage of a precursor. The mature form consists of an extracellular α-chain and a longer β-chain (for a more specific review on Met structure, signalling and targets see reference [11]). HGF was originally associated with liver regeneration, then emerged as a pleiotropic factor acting on a broad variety of cell types. HGF functions include induction of cell proliferation, motility, morphogenesis and differentiation into tubule-like structures [12]. HGF and the Met receptor are also involved in cardiomyogenesis [13].
Cardioprotective role of HGF
The expression of HGF and its secretion into the blood circulation are promoted during the early phase of MI [14]. HGF and the Met receptor are expressed at relatively low levels in normal cardiomyocytes, but their concentration increases significantly in the ischaemic myocardium and they are detectable for at least 14 days after permanent coronary artery occlusion in rats [15]. Upregulation of the Met receptor usually occurs through transcriptional activation by other molecules produced by the reactive stroma, including inflammatory cytokines and proangiogenic factors [16, 17], and HGF itself [18] or by HIF-1α induction [19]. The production of HGF and the Met receptor is also enhanced in the heart of rats subjected to myocardial ischaemia and reperfusion [20], with a slight but significant increase also in circulating plasma. An increase in protein levels might be interpreted as a mere marker of myocardial damage or as a prognostic factor in acute coronary syndromes [21]. However, a conspicuous body of evidence suggests the far broader importance of the HGF/Met system in cardiac tissue repair. First, HGF promotes enhanced survival of cardiomyocytes in vitro under ischaemic conditions [15, 22]. These effects have also been shown following administration of recombinant HGF or HGF gene transfer, which are able to reduce the infarct area and to improve cardiac function by suppressing apoptosis of cardiomyocytes [22–26]. Conversely, neutralizing endogenous HGF activity increases infarct size and cardiomyocyte death [22].
Apoptosis of cardiomyocytes is one of the main mechanisms responsible for MI and reperfusion injury [27]. HGF is a powerful survival factor for cardiac cells under oxidative stress via activation of the MEK/MAPK pathway [28], and is responsible for attenuation of cell apoptosis by activation of the PI3K/Akt pathway [29]. HGF is also able to protect against doxorubicin-induced cardiomyopathy [30, 31] through HGF-mediated ERK activation, upregulation of Met and GATA4. Met receptor activation by a specific monoclonal antibody efficiently protects cardiac muscle cells from apoptosis [32]. Liu [33] reported that HGF triggers phosphorylation and inactivation of proapoptotic Bad via the PI3K/Akt pathway and simultaneously upregulates antiapoptotic Bcl-xL. Thus, at least two additive pathways are responsible for the antiapoptotic action of HGF: (1) PI3K-dependent inactivation of Bad, and (2) upregulation of Bcl-xL, both in vitro and in vivo in cardiomyocytes [22]. The stimulation of E-twenty six (Ets) transcription factors [34] and GATA4 phosphorylation [35] are responsible for the HGF-mediated rapid induction of Bcl-xL expression.
The cardioprotective and antiapoptotic effects of HGF on cardiomyocytes confirm the importance of growth factors for cell survival in a hostile environment.
HGF-triggered biological responses for paracrine protection
Met and angiogenesis
HGF improves myocardial blood supply by stimulating angiogenesis, increasing the density of capillaries and also by direct stimulation of the endothelial cells [36, 37]. Ding et al. [38] reported that Met expression is increased shortly after switching to angiogenic growth conditions and remains high during the first steps of angiogenesis, including cell migration and proliferation. This is consistent with the evidence of Met expression in the infarcted area, where it coexists with CD31, CD34 and VWF-positive cells, suggesting that its upregulation in the injured area is essential to the promotion of angiogenesis [29]. HGF may therefore play an important role in the regeneration of endothelial cells and neovascularization during MI, thereby contributing to the protection of cardiomyocytes from ischaemic injury [24]. HGF is a potent angiogenic growth factor in mouse, rat and rabbit ischaemia models [39–42]. HGF has a stronger mitogenic effect than vascular endothelial growth factor (VEGF) and β-FGF in human aortic endothelial cells in vitro, as well as in a rabbit hind limb ischaemia model in vivo [43, 44].
Moreover, HGF can indirectly stimulate angiogenesis by inducing the release of other endothelial cell mitogens from non-endothelial cell populations [45–48]. Genes involved in angiogenesis have been found in the pathway-specific transcriptional targets of Met [49]. Among these, VEGF seems to be the most powerful in the promotion of therapeutic angiogenesis in MI [47]. However, the clinical utility of VEGF has been questioned because of the occurrence of some considerable side effects, such as an increased vascular permeability leading to oedema, leucocyte adhesion and increased expression of adhesion molecules promoting excessive inflammation. The combination of VEGF with other growth factors, such as angiopoietin-1 (Ang1), could allow alternative strategies to limit swelling and inflammation [50]. In this respect, it is interesting to note that HGF is able to promote angiogenesis without increasing vascular permeability or inflammation [51]. Moreover, the combination of VEGF and HGF has an additive effect on proliferation and migration of the endothelial cells and neovascularization in vivo [47]. In addition to angiogenesis, the formation of collateral vessels from existing arteries (arteriogenesis) plays an important role in attenuating ischaemic cardiomyopathy [52]. The angiopoietin/Tie2 ligand receptor system has been implicated in the maturation of blood vessels and arteriogenesis. The formation of mature vessels is regulated by communication between endothelial cells and smooth muscle cells (SMC), which are recruited by Ang1 to support endothelial expansion. This function could be mediated by HGF, which is induced by Ang1 and promotes the recruitment of SMC, enhancing the stabilization of the newly formed blood vessels [53].
Met and fibrosis
HGF has been shown to prevent fibrotic response in models of both acute ischaemia [24, 54] and dilated cardiomyopathy in the hamster [55, 56]. Many cytokines are known to regulate the fibrogenic process after tissue injury and transforming growth factor-beta1 (TGF-β1) in particular is believed to play a pivotal role. A large body of evidence suggests that TGF-β1 and angiotensin II (ANG II) play a crucial role in promoting an unfavourable myocardial remodelling [57–61]. ANG II increases expression of TGF-β1 in cardiac fibroblasts [57, 59] and TGF-β1 enhances fibroblast production of extracellular matrix and strengthens cellular adhesion [57, 59, 60]. TGF-β1 also induces hypertrophy and apoptotic cell death in cardiomyocytes [58] and mediates the hypertrophic cardiomyocyte growth induced by ANG II [62]. The antifibrotic effects of HGF are mediated, at least in part, by the inhibition of TGF-β1 secretion in myofibroflasts [55, 63, 64].
Recently, Mizuno et al. [65] demonstrated that HGF promotes the apoptosis of myofibroblasts, resulting in a real inhibition of TGF-β1 expression in the fibrotic lung. In addition, HGF has been reported to abolish TGF-β induction [63, 65] and also intercept TGF-β-initiated Smad signalling [66, 67] in interstitial fibroblasts. HGF is also able to attenuate the translocation and accumulation of activated Smad 2/3 in the nucleus, dependent on Erk-1/2 phosphorylation [66], and induces the expression of decorin, which can bind and sequester active TGF-β [67]. Finally, HGF inhibits profibrotic signalling by downregulation of TGF-β itself and diverts Smad 2/3 signalling even in the myocardium [68].
In the case of doxorubicin-induced cardiomyopathy, HGF appears to diminish fibrosis via a different mechanism [31], which might be related to its proangiogenic function. HGF increases production of NO in different cell types, including endothelial cells [69]. Inhibition of NO production leads to the upregulation of angiotensin-converting enzyme (ACE) [70] and accelerates fibrosis [71] while inhibition of ANG II with an ACE inhibitor or with ANG II type I receptor attenuates the fibrotic changes in the heart of cardiomyopathic hamsters [63]. Of note, the antifibrogenic action resulting from the inhibition of ANG II is associated with high levels of HGF, which stimulates the degradation of extracellular matrix and inhibits collagen synthesis through stimulation of MMP1 and inhibition of TGF-β. HGF probably stimulates MMP-1 through the activation of Ets, essential transcription factors for angiogenesis and vasculogenesis. In fact, some members of the Ets family play important roles in regulating the transcription of genes, including MMP-1, stromelysin 1 and uPA [72–74], in response to multiple developmental and mitogenic signals [75, 76].
Furthermore, it has been reported that ANG II and TGF-β are strong negative regulators of local HGF production [77, 78]. This phenomenon raises the interesting hypothesis that disruption of the autocrine/paracrine local HGF system by TGF-β and ANG II may result in the abnormal accumulation of extracellular matrix.
Met and immunomodulation
Acute inflammation is initiated by cells that are already present in all tissues, mainly resident macrophages and mastocytes. Their activation leads to the early release of inflammatory mediators responsible for the clinical signs of inflammation. Vasodilation and increased vascular permeability permit the migration and extravasation of leucocytes, mainly neutrophils, out of the bloodstream into the tissue. Neutrophils migrate to the site of injury along a chemotactic gradient created by the local cells. HGF may increase the acute inflammatory response, leading to an enhanced recruitment of endothelial cells, and limit it subsequently. Monocytes are activated by HGF into macrophages [79], which are mainly involved in the second wave of the inflammatory process, when the number of neutrophils decreases, and the endothelial cells and the fibroblasts proliferate. The fibroblasts, as well as the cardiac mast cells, participate in the harmful fibrotic process.
In the monocytes, HGF upregulates IL-10 [80], a cytokine that plays an important role in limiting the inflammatory response [81], indicating that HGF may also play an immunomodulatory role. Accumulating evidence shows that IL-10 has several protective features against atherosclerotic disease. IL-10 knockout mice show enhanced formation of atherosclerotic vascular lesions [82]. Indeed, IL-10 inhibits the adhesion of monocytes to endothelial cells by downregulating the adhesion molecules CD18 and CD62-L on immune competent cells [83]. Higher IL-10 plasma levels have been found in patients with stable coronary artery disease, compared with those with unstable coronary syndromes [84]. This finding suggests that IL-10 may serve as a plaque-stabilizing cytokine and may play a protective role against atherosclerotic disease by downregulating the inflammatory process. Interestingly, the administration of HGF upregulates the serum levels of IL-10 in patients with coronary heart disease [85]. Moreover, serum IL-8 concentrations are downregulated after the administration of Ad-HGF to patients with coronary heart disease [85]. IL-8 is a proinflammatory cytokine with chemoattractant and mitogenic effects on vascular SMC [86], monocytes [87] and neutrophils, directing them to the site of tissue injury [88–90]. Thus, HGF therapy may exert protective effects against atherosclerotic disease by decreasing the inflammation through a reduction in serum IL-8 levels, as well as by increasing concentrations of IL-10. These and other [91] results suggest that increasing the local HGF concentrations may be a potential therapy for atherosclerotic lesions.
Recent studies indicate that HGF reduces cardiomyocyte apoptosis and has immunosuppressive effects also in a model of autoimmune myocarditis [92]. This protective effect appears to be due to the suppression of T cell response through the reduction of IFNγ and the increased production of IL-4 and IL-10. Interestingly, HGF promotes the differentiation of tolerogenic dendritic cells, which favour the expansion of IL-10-producing regulatory T cells, thus maintaining T cells in a low state of activation [80]. This potent immunomodulatory mechanism of action of HGF has been recently proven in an animal model of autoimmune encephalitis [93].
IFNγ plays a central role in acute rejection [94, 95]. In a study by Yamaura et al. [96], the administration of HGF resulted in a significant reduction in IFNγ expression in cardiac allografts, suggesting that the prolongation of graft survival recipients following treatment with HGF may be mainly attributable to the HGF-induced modulation of IFNγ. These results suggest that the cardioprotective and immunomodulatory potency of HGF in cardiac allografts might be at least in part due to T cell-mediated immunosuppression induced by modulation of TGF-β and IL-10 levels.
HGF and stem cell therapies
Met and resident cardiac progenitor cells
Until a few years ago, the myocardium was considered a tissue devoid of regenerative capacity. Over the last decade, however, it has been shown that the heart contains a pool of CPCs, which are still able to proliferate. A number of studies [4–6, 97–100] independently described a cardiac stem/progenitor cell population that may regenerate myocardium after injury. On the basis of specific surface markers and different isolation approaches, several types of CPCs have been described [101]. The heterogeneity of these stem cells may reflect their different origins: some stem cells reside in the myocardium from fetal life [98], and some others probably have an extracardiac origin being derived from bone marrow [102] and colonize the myocardium in the postnatal period [4, 100].
CPCs seem to possess the properties required to achieve cardiac regeneration. That is, they are autologous, can be expanded ex vivo and can differentiate into the three main cardiac populations: endothelial cells, vascular SMC and cardiomyocytes [103, 104]. When injected into infarcted murine hearts, CPCs regenerated a functionally integrated myocardium [4, 6, 100]. CPCs express receptors for many growth factors, including Met [105]. When stimulated, they respond with mobilization, expansion and differentiation into cardiomyocytes and vascular cells. Moreover, emerging evidence suggests that resident CPCs also have a paracrine function [106, 107], enhancing the survival of cardiomyocytes under hypoxic conditions as well as inducing formation of new endothelium [108]. VEGF and HGF are the soluble factors causing these effects. These findings suggest the existence of an autocrine and paracrine crosstalk in the cardiac microenvironment.
Therapeutic approaches aiming to reduce fibrosis and enhance survival and proliferation of resident cardiac stem cells might be the best option to improve the intrinsic regenerative potential of the myocardium. A valid treatment could be represented by a cocktail of growth factors, including HGF, which activate and mobilize in situ the CPCs to regenerate cardiomyocytes and vascular cells [105, 109, 110].
As a result of the elusive nature of endogenous CPCs, the attention of the scientific community is shifting towards the biology of cardiac progenitors in the embryonic and fetal heart. Interestingly, both Met and HGF are transiently expressed in the developing myocardium [13, 111–113]. Two well-defined populations of cardiac progenitors have been characterized so far. These are the first heart field and second heart field cells, which give rise to either the majority of the left ventricle and parts of the atria or the right ventricle plus parts of the atria and the proximal part of the great arteries, respectively [114]. A third lineage of progenitors has been identified in epicardial cells [115–117]. During embryonic development of the heart, the proepicardium differentiates into a migrating mesothelium, known as the epicardium. Epicardial cells in this compartment undergo the process of epithelial to mesenchymal transformation, giving origin to epicardial progenitor cells, that contribute to the cardiomyocyte lineage during the formation of the working myocardium [118, 119] and to coronary vasculogenesis [120]. Interestingly, these cells retain the ability to produce mesenchyme in response to specific growth factors and to generate SMC [121]. Thymosin-β4 has been shown to promote vessel formation and collateral growth not only during development but also in the adult epicardium [122]. Several factors initiating and/or controlling the epithelial to mesenchymal transformation in the developing myocardium have been identified, including HGF [112]. At present, no data are available on the possible expression of Met on epicardial cells, but this topic should be addressed in view of the considerable therapeutic potential of such information.
Recently, a stem cell population expressing c-kit, which has been discovered within the adult myocardium and is capable of giving origin both to vascular and cardiac cells [4], has been found in the epicardial region of embryonic [123] and fetal hearts [124]. Interestingly, the embryonic properties of the epicardium are recapitulated in the adult heart following MI and the pericardial fluid seems to be involved in the process. The increase in HGF levels in the pericardial fluid of ischaemic patients [125] suggests a role for HGF in the reactivation of a developmental programme.
Met and embryonic stem cells
Pluripotency distinguishes embryonic stem cells (ESCs) from adult stem cells: ESCs can generate all cell types in the body, while adult stem cells are multipotent and can produce a limited number of cell lineages. However, the actual amount of functional cardiac cells in the heart of a recipient after transplantation of ESCs is severely impaired by the low rate of differentiation into the cardiac lineage. HGF might improve ESC differentiation, since it significantly increases the number of beating embryoid bodies of differentiating ESCs and upregulates the expression of the heart-specific transcription factors Nkx2.5 and GATA4, and of markers of differentiated cardiomyocytes by activating the PI3K/Akt pathway [126]. Nevertheless, the clinical use of ESCs is still largely limited by the risk of inducing a teratoma and by ethical concerns. Thus, the attention of clinical science has moved to innovative therapies based on the use of adult stem and progenitor cells.
HGF in stem cell-mediated cardiac repair
The rationale for stem-cell therapies is based on their ability to secrete paracrine factors. A growing body of evidence strongly suggests that these molecules mediate a number of cardioprotective mechanisms including cell survival, neovascularization and favourable matrix remodelling. Paracrine factors are generally released from endogenous damaged cells in the heart. Thus, growth factors and cytokines secreted by exogenously transplanted cells may be advantageous by regulating endogenous processes which would be otherwise insufficient. Among these factors, HGF is released by several types of stem cells, including multipotent bone-marrow-derived cells [127], circulating mononuclear cells [128], endothelial progenitor cells [129, 130], mesenchymal stem cells [131, 132], tissue-resident c-kit+/MDR1+/Sca1+ cells [105] and adipose stem cells [133, 134]. Interestingly, HGF is pivotal for promoting the survival of adipose-derived stem cells, and for the proliferation and migration of mature and progenitor endothelial cells in vitro. Furthermore, HGF enhances reperfusion in mouse hind-limb ischaemia [133]. Regardless of the population of stem/progenitor cells involved, the release of HGF, together with a mixture of other factors, results in a therapeutic neovascularization triggered by ischaemia [135, 136]. Moreover, strategies aimed at improving the survival of stem cells themselves lead to secretion of HGF [137] and increased survival of the cardiomyocytes [138, 139].
Paracrine mechanisms have been shown to influence cell contractility [140]. Whether this is due to cardioprotection rather than to direct release of inotropic factors is still a matter of debate. Modulation of catecholamine levels and β-adrenoreceptor density has been proposed as the mechanism leading to enhanced contractility in a model of doxorubicin-induced heart failure [141]. The secretion of paracrine factors by the transplanted stem cells may also be responsible for a favourable remodelling of the extracellular matrix [142]. Involvement of HGF paracrine activation in cardiac remodelling, which results in preserved contractility, has been demonstrated in mesenchymal stem cells overexpressing stromal cell derived factor-1 (SDF-1), when transplanted in infarcted hearts [143].
These findings suggest the existence of a complex crosstalk between transplanted and endogenous stem cells, which may be the basis of successful cell-based therapies.
Met and homing
The regenerative potential of all nonresident stem cells, including those administered exogenously, depends on the efficacy of their recruitment at the site of injury. This process is regulated by molecular signals secreted by the damaged tissues; it is thus conceivable that a function of HGF is also as an important factor in orchestrating the process of mobilization, homing, incorporation, survival and proliferation of progenitor and stem cells, that results in myocardial repair. Several types of stem cells are able to repair the damaged myocardium by promoting angiogenesis. These include different cellular subpopulations derived from bone marrow [144]. Responding to specific signals, the stem cells migrate from their niche within the bone marrow and are released into the blood flow. Recently, it has been shown that multipotent stem cells associated with blood vessels, called mesoangioblasts [145], repair ischaemic damage as efficiently as stem cells from bone marrow [146].
Homing and engraftment are prerequisites for all cell types infused via the vascular route to provide any effect in the damaged tissues. The ability of progenitor cells to migrate, adhere to the endothelium and be retained in situ is also important for their angiogenic potential.
While the homing of leucocytes to sites of inflammation has been well studied, the mechanisms of progenitor cell homing to ischaemic areas are poorly understood. During inflammation, the recruitment of inflammatory cells requires a coordinated sequence of multistep adhesive and signalling events, including migration and invasion in the extracellular matrix, and involving matrix-degrading proteases [147]. The unique property of HGF of stimulating cell motility and expression of Met receptor in all the aforementioned stem cells [79, 131, 132, 145, 148, 149] suggests that HGF is a cytokine potentially able to attract these cells into damaged tissue, simultaneously promoting survival, angiogenesis and remodelling.
The most powerful signal for stem cell mobilization and homing is the chemokine SDF-1, which binds its specific receptor CXCR4. Under physiological conditions, various tissues, including bone marrow, constitutively express SDF-1 and a significant gradient is established between SDF-1 concentrations in the bone marrow and peripheral tissues [150]. Under different circumstances, such as hypoxia, ischaemia and inflammation, SDF-1 is upregulated and its gradient is reversed [151–153]. SDF-1 gene expression is regulated by HIF-1α. Thus, recruitment of regenerative CXCR4+ cells is mediated by hypoxic gradients via HIF-1-induced expression of SDF-1 [154]. Interestingly, hypoxia activates the transcription of the Met protooncogene [19] while Met activates HIF-1α [155] and induces CXCR4 [156].
Recently it was shown that VEGF induces the expression of perivascular SDF-1 chemokine that, in turn, is able to attract and retain in this strategic position circulating cells from the bone marrow, which act in a paracrine manner to increase in situ proliferation of activated endothelial cells [157]. Indeed, a pool of cells expressing both Met and CXCR4 exists in the bone marrow [149, 158], and these cells could respond to HGF chemotaxis and retention mediated by SDF-1, as was recently suggested for VEGF [157]. The SDF-1/HGF relationship is further interlaced since SDF-1 is also able to increase HGF release in cultured and transplanted mesenchymal stem cells [143]. This mechanism has been involved in migration and survival of different cell types, including primary myoblasts [159], satellite cells [160] and a subpopulation of bone marrow cells [149, 158].
These findings suggest an important role for HGF also in the recruitment and activation of progenitors and stem cells for regeneration.
HGF gene transfer in myocardial infarction
Due to its potential angiogenic, antiapoptotic, antifibrotic, and antiinflammatory effects, HGF gene therapy has attracted increasing attention in studies on ischaemic heart diseases (Table 1) [24–26, 161, 162].
Table 1.
Overview of preclinical and clinical studies of HGF gene therapy in MI and ischaemic disease
| Methods used in the study | Results | Model | Reference | Year | |
|---|---|---|---|---|---|
| Preclinical studies | HGF gene transfer in ischaemia | Improved angiogenesis | Rat | [40] | 2000 |
| HGF gene transfer in hind-limb ischaemia | Significantly increased blood flow | Rat | [42] | 2001 | |
| Administration of an adenoviral vector expressing HGF after ligation of coronary arteries | Preserved myocardial function and geometry; decreased left ventricular dilatation and preserved wall thickness; reduced apoptosis of interstitial cells in the infarcted tissue | Mouse | [25] | 2003 | |
| Intramyocardial injection of human HGF plasmid DNA in ischaemic cardiomyopathy | Improved cardiac function through increase in blood flow and decrease in fibrosis | Pig | [68] | 2006 | |
| Adenovirus-mediated high expression of human HGF | Increased functional arterioles and improved collateral artery growth | Pig | [163] | 2006 | |
| Bone marrow-derived mesenchymal stem cells combined with HGF transplantation in acute MI | Improved left ventricular ejection fraction, left ventricular end systolic volume and end diastolic volume; greater cardiac perfusion, growth of collateral arteries and number of vessels | Pig | [165] | 2006 | |
| Adenovirus-mediated HGF gene transfer in chronic myocardial ischaemia | Enhanced myocardial perfusion; higher density of newly formed blood vessels and number of collateral blood vessels; reduced area of myocardial ischaemia and improved left ventricular ejection fraction | Minipig | [164] | 2008 | |
| Clinical trials | Phase I clinical trials; intramuscular injection of naked HGF plasmid DNA in patients with critical limb ischaemia | No severe complications or adverse effects in any patient | Human | [168] | 2004 |
| Open-label, safety and tolerance trial of Ad-HGF in 18 patients suffering from coronary heart disease; direct intramyocardial administration of the Ad-HGF | No evidence of systemic or heart-related adverse effects; Ad-HGF was well tolerated and improved myocardial perfusion with a dose–effect relationship | Human | [166] | 2008 | |
| Phase I clinical trial; use of an adenovirus gene-transfer vector to deliver the human HGF gene to individuals with clinically significant coronary artery disease by direct intracoronary injection | HGF gene transfer is safe and feasible | Human | [167] | 2009 |
Transfer of the HGF gene in patients with MI results in a significant preservation of myocardial function and geometry, with decreased left ventricular remodelling [25]. The administration of an adenoviral vector expressing HGF leads to a reduced apoptosis of interstitial cells such as endothelial cells and myofibroblasts in the infarcted tissue in mice [25]. In vivo experiments in pigs have also demonstrated that intramyocardial injection of human HGF plasmid DNA results in a significant improvement in cardiac function through an increase in blood flow and a decrease in fibrosis in a model of ischaemic cardiomyoapthy [68]. HGF gene transfer has been shown to be effective in improving angiogenesis in several ischaemic models [40, 42]. The adenovirus-mediated expression of human HGF increases the number of functional arterioles and improves the growth of collateral artery [163].
Preclinical evaluation has shown that Ad-HGF is effective in both acute and chronic myocardial ischaemia models and no apparent toxicity and mutational effects were observed in rats [24, 40, 42], minipigs [164] or pigs [68, 163, 165]. An open-label, safety and tolerance trial of Ad-HGF in 18 patients suffering from coronary heart disease [166] showed no evidence of systemic or cardiac-related adverse events after intramyocardial administration of the Ad-HGF. These preliminary clinical data indicate that direct intramyocardial administration of Ad-HGF is well tolerated and could improve myocardial perfusion with a dose–effect relationship, encouraging larger and randomized efficacy trials. A phase I clinical trial demonstrated that it is safe to use an adenovirus gene-transfer vector to deliver the human HGF gene to individuals with clinically significant coronary artery disease by direct intracoronary injection [167]. In an another phase I clinical trial, Morishita et al. [168] evaluated the safety and curative effect of HGF plasmid DNA in patients with critical limb ischaemia. Intramuscular injection of naked HGF plasmid did not cause any severe complications or adverse effects, indicating that HGF gene transfer is feasible and safe.
The clinical application of Ad-HGF is currently approved by the State Food and Drug Administration (SFDA) of China (no. 2005L01181). However, a word of caution concerning the clinical use of HGF seems appropriate considering its unexpected possible role in atherogenesis [169].
The pros and cons of HGF in cardiac disease to date
Despite impressive data on the beneficial effects of HGF/Met in cardiac disease, the meaning of elevated levels of circulating HGF associated with an increased cardiovascular mortality in human congestive heart failure is at least questionable [170, 171]. At first glance, these results may appear to contradict a great deal of experimental data which instead suggest a cardioprotective effect of HGF. These results are very similar to those previously reported for natriuretic peptides. They are known to play a beneficial effect in patients with congestive heart failure, but elevated concentrations are strong predictors of an adverse outcome [172]. The higher angiogenic factor levels in patients with systolic dysfunction may represent a compensatory cardioprotection attempt and perhaps indicate an endogenous protective system that could be improved in view of important clinical benefits in patients with LV dysfunction. Furthermore, alongside the recognized beneficial effects on atherosclerosis, recent studies have produced controversial data suggesting that HGF may itself participate in pathological angiogenesis [173].
Finally, HGF has been identified as a potential index of the severity of hypertension [174, 175]. Increased secretion of HGF may be the physiological response counteracting endothelial dysfunction. However, further studies are needed to determine the possible role of chronic HGF stimulation in the pathogenesis of hypertension.
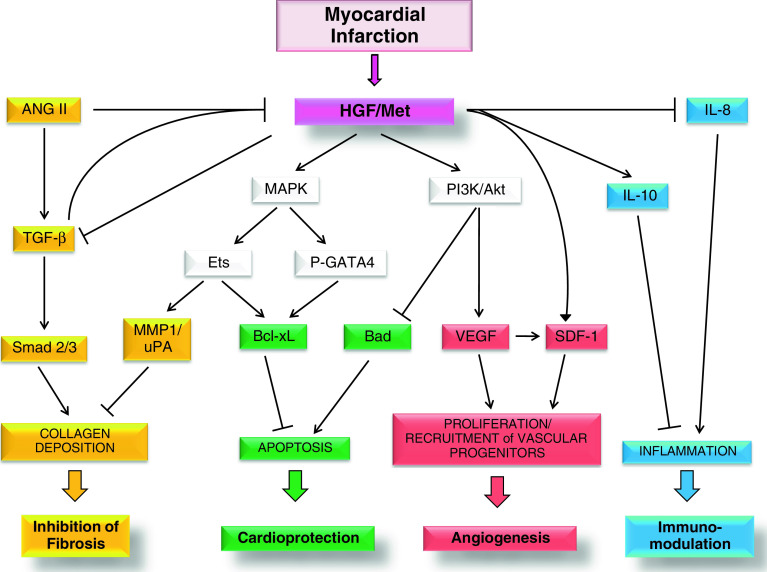
In conclusion, HGF is a cytokine that displays marked antiapoptotic activity on damaged cardiomyocytes and beneficial paracrine effects on interstitial cells, proangiogenic on endothelial cells, antifibrotic on fibroblasts and antiinflammatory on immune cells (Fig. 1). From the clinical perspective, the therapeutic efficacy of HGF in MI has only recently begun to be assessed. Moreover, new results from stem cell studies have associated HGF with cardiac muscle regeneration. These results make HGF a potential and valuable candidate molecule for regenerative medicine.
Fig. 1.
HGF and Met receptor levels increase after MI. Activation of Met receptor at the surface membrane of cardiomyocytes and interstitial cells leads to stimulation of different signalling pathways and biological responses. The local increase in secreted HGF results in inhibition of TGF-β signalling in myofibroblasts and ultimately in the inhibition of collagen deposition and fibrosis (yellow). Conversely, TGF-β and ANG II, another profibrotic factor, behave as negative regulators of HGF local production. Stimulation of Met receptor results in MAPK-mediated phosphorylation of GATA4 and Ets, which stimulate the antiapoptotic activity of Bcl-xL, while the PI3K/Akt axis inhibits Bad, a proapoptotic factor. Thus, activation of Met downstream signalling protects cardiomyocytes from programmed cell death (green). The Ets transcription factor also acts in the prevention of fibrosis by inducing extracellular matrix remodelling through MMP1 and uPA (yellow). Proliferation and recruitment of vascular progenitors are also promoted by Met activation due to induction of VEGF and SDF-1, finally enhancing angiogenesis (red). Activation of Met receptor in immune cells reduces inflammation (blue). See text for more details. ( activation,
activation,  inhibition)
inhibition)
Acknowledgments
We gratefully acknowledge Christian Leo and Amedeo Chiribiri for their critical reading of the manuscript. This work was supported by funds from the Compagnia di San Paolo and the Association Francaise contre les Myopathies (AFM). V.S. is a Fellow of Università Italo Francese.
References
- 1.Fenton D (2010) Myocardial infarction. eMedicine. http://www.emedicine.com/EMERG/topic327.htm
- 2.Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts. Curr Med Chem. 2006;13:1877–1893. doi: 10.2174/092986706777585086. [DOI] [PubMed] [Google Scholar]
- 3.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascirnbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, LeCapitaine N, Cascapera S, Beltrami AP, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 5.Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, Nakaya H, Kasanuki H, Komuro I. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 6.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gnecchi M, Zhang ZP, Ni AG, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin HK, Wyss JM, Yang RH, Schwall R. The therapeutic potential of hepatocyte growth factor for myocardial infarction and heart failure. Curr Pharm Des. 2004;10:2525–2533. doi: 10.2174/1381612043383863. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S. Molecular-cloning and expression of human hepatocyte growth-factor. Nature. 1989;342:440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 10.Park M, Dean M, Cooper CS, Schmidt M, O’Brien SJ, Blair DG, Vande Woude GF. Mechanism of met oncogene activation. Cell. 1986;45:895–904. doi: 10.1016/0092-8674(86)90564-7. [DOI] [PubMed] [Google Scholar]
- 11.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 12.Comoglio PM, Trusolino L. Invasive growth: from development to metastasis. J Clin Invest. 2002;109:857–862. doi: 10.1172/JCI15392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rappolee DA, Iyer A, Patel Y. Hepatocyte growth factor and its receptor are expressed in cardiac myocytes during early cardiogenesis. Circ Res. 1996;78:1028–1036. doi: 10.1161/01.res.78.6.1028. [DOI] [PubMed] [Google Scholar]
- 14.Matsumori A, Furukawa Y, Hashimoto T, Ono K, Shioi T, Okada M, Iwasaki A, Nishio R, Sasayama S. Increased circulating hepatocyte growth factor in the early stage of acute myocardial infarction. Biochem Biophys Res Commun. 1996;221:391–395. doi: 10.1006/bbrc.1996.0606. [DOI] [PubMed] [Google Scholar]
- 15.Ueda H, Nakamura T, Matsumoto K, Sawa Y, Matsuda H, Nakamura T. A potential cardioprotective role of hepatocyte growth factor in myocardial infarction in rats. Cardiovasc Res. 2001;51:41–50. doi: 10.1016/s0008-6363(01)00272-3. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem. 1996;119:591–600. doi: 10.1093/oxfordjournals.jbchem.a021283. [DOI] [PubMed] [Google Scholar]
- 17.Fujii K, Ishimaru F, Kozuka T, Matsuo K, Nakase K, Kataoka I, Tabayashi T, Shinagawa K, Ikeda K, Harada M, Tanimoto M. Elevation of serum hepatocyte growth factor during granulocyte colony-stimulating factor-induced peripheral blood stem cell mobilization. Br J Haematol. 2004;124:190–194. doi: 10.1046/j.1365-2141.2003.04745.x. [DOI] [PubMed] [Google Scholar]
- 18.Boccaccio C, Gaudino G, Gambarotta G, Galimi F, Comoglio PM. Hepatocyte growth-factor (Hgf) receptor expression is inducible and is part of the delayed-early response to Hgf. J Biol Chem. 1994;269:12846–12851. [PubMed] [Google Scholar]
- 19.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 20.Ono K, Matsumori A, Shioi T, Furukawa Y, Sasayama S. Enhanced expression of hepatocyte growth factor c-Met by myocardial ischemia and reperfusion in a rat model. Circulation. 1997;95:2552–2558. doi: 10.1161/01.cir.95.11.2552. [DOI] [PubMed] [Google Scholar]
- 21.Konopka A, Janas J, Piotrowski W, Stepinska J. Hepatocyte growth factor – a new marker for prognosis in acute coronary syndrome. Growth Factors. 2010;28:75–81. doi: 10.3109/08977190903403984. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Mizuno S, Matsumoto K, Sawa Y, Matsuda H, Nakamura T. Myocardial protection from ischemia/reperfusion injury by endogenous and exogenous HGF. J Clin Invest. 2000;106:1511–1519. doi: 10.1172/JCI10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funatsu T, Sawa Y, Ohtake S, Takahashi T, Matsumiya G, Matsuura N, Nakamura T, Matsuda H. Therapeutic angiogenesis in the ischemic canine heart induced by myocardial injection of naked complementary DNA plasmid encoding hepatocyte growth factor. J Thorac Cardiovasc Surg. 2002;124:1099–1105. doi: 10.1067/mtc.2002.123809. [DOI] [PubMed] [Google Scholar]
- 24.Jayasankar V, Woo YJ, Bish LT, Pirolli TJ, Chatterjee S, Berry MF, Burdick J, Gardner TJ, Sweeney HL. Gene transfer of hepatocyte growth factor attenuates postinfarction heart failure. Circulation. 2003;108(Suppl 1):II230–II236. doi: 10.1161/01.cir.0000087444.53354.66. [DOI] [PubMed] [Google Scholar]
- 25.Li YW, Takemura G, Kosai K, Yuge K, Nagano S, Esaki M, Goto K, Takahashi T, Hayakawa K, Koda M, Kawase Y, Maruyama R, Okada H, Minatoguchi S, Mizuguchi H, Fujiwara T, Fujiwara H. Postinfarction treatment with an adenoviral vector expressing hepatocyte growth factor relieves chronic left ventricular remodeling and dysfunction in mice. Circulation. 2003;107:2499–2506. doi: 10.1161/01.CIR.0000065579.19126.B8. [DOI] [PubMed] [Google Scholar]
- 26.Miyagawa S, Sawa Y, Taketani S, Kawaguchi N, Nakamura T, Matsuura N, Matsuda F. Myocardial regeneration therapy for heart failure – hepatocyte growth factor enhances the effect of cellular cardiomyoplasty. Circulation. 2002;105:2556–2561. doi: 10.1161/01.cir.0000016722.37138.f2. [DOI] [PubMed] [Google Scholar]
- 27.Scarabelli TM, Gottlieb RA. Functional and clinical repercussions of myocyte apoptosis in the multifaceted damage by ischemia/reperfusion injury: old and new concepts after 10 years of contributions. Cell Death Differ. 2004;11:S144–S152. doi: 10.1038/sj.cdd.4401544. [DOI] [PubMed] [Google Scholar]
- 28.Kitta K, Day RM, Ikeda T, Suzuki YJ. Hepatocyte growth factor protects cardiac myocytes against oxidative stress-induced apoptosis. Free Radic Biol Med. 2001;31:902–910. doi: 10.1016/s0891-5849(01)00663-3. [DOI] [PubMed] [Google Scholar]
- 29.Wang YG, Ahmad N, Wani MA, Ashraf M. Hepatocyte growth factor prevents ventricular remodeling and dysfunction in mice via Akt pathway and angiogenesis. J Mol Cell Cardiol. 2004;37:1041–1052. doi: 10.1016/j.yjmcc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Iwasaki M, Adachi Y, Nishiue T, Minamino K, Suzuki Y, Zhang YM, Nakano KJ, Koike Y, Wang JF, Mukaide H, Taketani S, Yuasa F, Tsubouchi H, Gohda E, Iwasaka T, Ikehara S. Hepatocyte growth factor delivered by ultrasound-mediated destruction of microbubbles induces proliferation of cardiomyocytes and amelioration of left ventricular contractile function in doxorubicin-induced cardiomyopathy. Stem Cells. 2005;23:1589–1597. doi: 10.1634/stemcells.2005-0049. [DOI] [PubMed] [Google Scholar]
- 31.Esaki M, Takemura G, Kosai KI, Takahashi T, Miyata S, Li LH, Goto K, Maruyama R, Okada H, Kanamori H, Ogino A, Ushikoshi H, Minatoguchi S, Fujiwara T, Fujiwara H. Treatment with an adenoviral vector encoding hepatocyte growth factor mitigates established cardiac dysfunction in doxorubicin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;294:H1048–H1057. doi: 10.1152/ajpheart.01102.2007. [DOI] [PubMed] [Google Scholar]
- 32.Pietronave S, Forte G, Locarno D, Merlin S, Zamperone A, Nicotra G, Isidoro C, Di Nardo P, Prat M. Agonist monoclonal antibodies against HGF receptor protect cardiac muscle cells from apoptosis. Am J Physiol Heart Circ Physiol. 2010;298:H1155–H1165. doi: 10.1152/ajpheart.01323.2008. [DOI] [PubMed] [Google Scholar]
- 33.Liu YH. Hepatocyte growth factor promotes renal epithelial cell survival by dual mechanisms. Am J Physiol Renal Physiol. 1999;277:F624–F633. doi: 10.1152/ajprenal.1999.277.4.F624. [DOI] [PubMed] [Google Scholar]
- 34.Cao XB, Littlejohn J, Rodarte C, Zhang LD, Martino B, Rascoe P, Hamid K, Jupiter D, Smythe WR. Up-regulation of Bcl-xl by hepatocyte growth factor in human mesothelioma cells involves ETS transcription factors. Am J Pathol. 2009;175:2207–2216. doi: 10.2353/ajpath.2009.090070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitta K, Day RM, Kim Y, Torregroza I, Evans T, Suzuki YJ. Hepatocyte growth factor induces GATA-4 phosphorylation and cell survival in cardiac muscle cells. J Biol Chem. 2003;278:4705–4712. doi: 10.1074/jbc.M211616200. [DOI] [PubMed] [Google Scholar]
- 36.Bussolino F, Direnzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM. Hepatocyte growth-factor is a potent angiogenic factor which stimulates endothelial-cell motility and growth. J Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickoloff BJ, Kinsella JL, Polverini P, Rosen EM. Scatter factor induces blood-vessel formation in vivo. Proc Natl Acad Sci U S A. 1993;90:1937–1941. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding SL, Merkulova-Rainon T, Han ZC, Tobelem G. HGF receptor up-regulation contributes to the angiogenic phenotype of human endothelial cells and promotes angiogenesis in vitro. Blood. 2003;101:4816–4822. doi: 10.1182/blood-2002-06-1731. [DOI] [PubMed] [Google Scholar]
- 39.Morishita R, Nakamura S, Hayashi S, Taniyama Y, Moriguchi A, Nagano T, Taiji M, Noguchi H, Takeshita S, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy. Hypertension. 1999;33:1379–1384. doi: 10.1161/01.hyp.33.6.1379. [DOI] [PubMed] [Google Scholar]
- 40.Aoki M, Morishita R, Taniyama Y, Kida I, Moriguchi A, Matsumoto K, Nakamura T, Kaneda Y, Higaki J, Ogihara T. Angiogenesis induced by hepatocyte growth factor in non-infarcted myocardium and infarcted myocardium: up-regulation of essential transcription factor for angiogenesis, ets. Gene Ther. 2000;7:417–427. doi: 10.1038/sj.gt.3301104. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi S, Morishita R, Nakamura S, Yamamoto K, Moriguchi A, Nagano T, Taiji M, Noguchi H, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Potential role of hepatocyte growth factor, a novel angiogenic growth factor, in peripheral arterial disease – downregulation of HGF in response to hypoxia in vascular cells. Circulation. 1999;100:301–308. doi: 10.1161/circ.100.suppl_2.Ii-301. [DOI] [PubMed] [Google Scholar]
- 42.Taniyama Y, Morishita R, Hiraoka K, Aoki M, Nakagami H, Yamasaki K, Matsumoto K, Nakamura T, Kaneda Y, Ogihara T. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat diabetic hind limb ischemia model – molecular mechanisms of delayed angiogenesis in diabetes. Circulation. 2001;104:2344–2350. doi: 10.1161/hc4401.098470. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura Y, Morishita R, Higaki J, Kida I, Aoki M, Moriguchi A, Yamada K, Hayashi S, Yo Y, Nakano H, Matsumoto K, Nakamura T, Ogihara T. Hepatocyte growth factor is a novel member of the endothelium-specific growth factors: additive stimulatory effect of hepatocyte growth factor with basic fibroblast growth factor but not with vascular endothelial growth factor. J Hypertens. 1996;14:1067–1072. doi: 10.1097/00004872-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Vale PR, Losordo DW, Milliken CE, Maysky M, Esakof DD, Symes JF, Isner JM. Left ventricular electromechanical mapping to assess efficacy of phVEGP(165) gene transfer for therapeutic angiogenesis in chronic myocardial ischemia. Circulation. 2000;102:965–974. doi: 10.1161/01.cir.102.9.965. [DOI] [PubMed] [Google Scholar]
- 45.Horiguchi N, Takayama H, Toyoda M, Otsuka T, Fukusato T, Merlino G, Takagi H, Mori M. Hepatocyte growth factor promotes hepatocarcinogenesis through c-Met autocrine activation and enhanced angiogenesis in transgenic mice treated with diethylnitrosamine. Oncogene. 2002;21:1791–1799. doi: 10.1038/sj.onc.1205248. [DOI] [PubMed] [Google Scholar]
- 46.Saucier C, Khoury H, Lai KMV, Peschard P, Dankort D, Naujokas MA, Holash J, Yancopoulos GD, Muller WJ, Pawson T, Park M. The Shc adaptor protein is critical for VEGF induction by Met/HGF and ErbB2 receptors and for early onset of tumor angiogenesis. Proc Natl Acad Sci U S A. 2004;101:2345–2350. doi: 10.1073/pnas.0308065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Belle E, Witzenbichler B, Chen DH, Silver M, Chang L, Schwall R, Isner JM. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor – the case for paracrine amplification of angiogenesis. Circulation. 1998;97:381–390. doi: 10.1161/01.cir.97.4.381. [DOI] [PubMed] [Google Scholar]
- 48.Wojta J, Kaun C, Breuss JM, Koshelnick Y, Beckmann R, Hattey E, Mildner M, Weninger W, Nakamura T, Tschachler E, Binder BR. Hepatocyte growth factor increases expression of vascular endothelial growth factor and plasminogen activator inhibitor-1 in human keratinocytes and the vascular endothelial growth factor receptor flk-1 in human endothelial cells. Lab Invest. 1999;79:427–438. [PubMed] [Google Scholar]
- 49.Kaposi-Novak P, Lee JS, Gomez-Quiroz L, Coulouarn C, Factor VM, Thorgeirsson SS. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J Clin Invest. 2006;116:1582–1595. doi: 10.1172/JCI27236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 51.Min JK, Lee YM, Kim JH, Kim YM, Kim SW, Lee SY, Gho YS, Oh GT, Kwon YG. Hepatocyte growth factor suppresses vascular endothelial growth factor-induced expression of endothelial ICAM-1 and VCAM-1 by inhibiting the nuclear factor-kappa B pathway. Circ Res. 2005;96:300–307. doi: 10.1161/01.RES.0000155330.07887.EE. [DOI] [PubMed] [Google Scholar]
- 52.Deindl E, Zaruba MM, Brunner S, Huber B, Mehl U, Assmann G, Hoefer IE, Mueller-Hoecker J, Franz WM. G-CSF administration after myocardial infarction in mice attenuates late ischemic cardiomyopathy by enhanced arteriogenesis. FASEB J. 2006;20:956–958. doi: 10.1096/fj.05-4763fje. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi H, Debusk LM, Babichev YO, Dumont DJ, Lin PC. Hepatocyte growth factor mediates angiopoietin-induced smooth muscle cell recruitment. Blood. 2006;108:1260–1266. doi: 10.1182/blood-2005-09-012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen XH, Minatoguchi S, Kosai K, Yuge K, Takahashi T, Arai M, Wang NY, Misao Y, Lu CJ, Onogi H, Kobayashi H, Yasuda S, Ezaki M, Ushikoshi H, Takemura G, Fujiwara T, Fujiwara H. In vivo hepatocyte growth factor gene transfer reduces myocardial ischemia-reperfusion injury through its multiple actions. J Card Fail. 2007;13:874–883. doi: 10.1016/j.cardfail.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 55.Nakamura T, Matsumoto K, Mizuno S, Sawa Y, Matsuda H, Nakamura T. Hepatocyte growth factor prevents tissue fibrosis, remodeling, and dysfunction in cardiomyopathic hamster hearts. Am J Physiol Heart Circ Physiol. 2005;288:H2131–H2139. doi: 10.1152/ajpheart.01239.2003. [DOI] [PubMed] [Google Scholar]
- 56.Taniyama Y, Morishita R, Aoki M, Hiraoka K, Yamasaki K, Hashiya N, Matsumoto K, Nakamura T, Kaneda Y, Ogihara T. Angiogenesis and antifibrotic action by hepatocyte growth factor in cardiomyopathy. Hypertension. 2002;40:47–53. doi: 10.1161/01.hyp.0000020755.56955.bf. [DOI] [PubMed] [Google Scholar]
- 57.Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-beta(1) in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol. 1997;29:1947–1958. doi: 10.1006/jmcc.1997.0435. [DOI] [PubMed] [Google Scholar]
- 58.Kim NN, Villarreal FJ, Printz MP, Lee AA, Dillmann WH. Trophic effects of angiotensin II on neonatal rat cardiac myocytes are mediated by cardiac fibroblasts. Am J Physiol Endocrinol Metab. 1995;32:E426–E437. doi: 10.1152/ajpendo.1995.269.3.E426. [DOI] [PubMed] [Google Scholar]
- 59.Lee AA, Dillmann WH, Mcculloch AD, Villarreal FJ. Angiotensin-II stimulates the autocrine production of transforming growth-factor-beta-1 in adult-rat cardiac fibroblasts. J Mol Cell Cardiol. 1995;27:2347–2357. doi: 10.1016/s0022-2828(95)91983-x. [DOI] [PubMed] [Google Scholar]
- 60.MacKenna D, Summerour SR, Villarreal FJ. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc Res. 2000;46:257–263. doi: 10.1016/s0008-6363(00)00030-4. [DOI] [PubMed] [Google Scholar]
- 61.Villarreal FJ, Dillmann WH. Cardiac hypertrophy-induced changes in messenger-RNA levels for TGF-beta-1, fibronectin, and collagen. Am J Physiol. 1992;262:H1861–H1866. doi: 10.1152/ajpheart.1992.262.6.H1861. [DOI] [PubMed] [Google Scholar]
- 62.Schultz JE, Witt SA, Glascock BJ, Nieman ML, Reiser PJ, Nix SL, Kimball TR, Doetschman T. TGF-beta 1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest. 2002;109:787–796. doi: 10.1172/JCI14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taniyama Y, Morishita R, Nakagami H, Moriguchi A, Sakonjo H, Shokei K, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Potential contribution of a novel antifibrotic factor, hepatocyte growth factor, to prevention of myocardial fibrosis by angiotensin II blockade in cardiomyopathic hamsters. Circulation. 2000;102:246–252. doi: 10.1161/01.cir.102.2.246. [DOI] [PubMed] [Google Scholar]
- 64.Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E, Fujimoto J. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226–230. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- 65.Mizuno S, Matsumoto K, Li MY, Nakamura T. HGF reduces advancing lung fibrosis in mice: a potential role for MMP-dependent myofibroblast apoptosis. FASEB J. 2005;19:580–582. doi: 10.1096/fj.04-1535fje. [DOI] [PubMed] [Google Scholar]
- 66.Yang JW, Dai CS, Liu YH. Hepatocyte growth factor suppresses renal interstitial myofibroblast activation and intercepts Smad signal transduction. Am J Pathol. 2003;163:621–632. doi: 10.1016/S0002-9440(10)63689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kobayashi E, Sasamura H, Mifune M, Shimizu-Hirota R, Kuroda M, Hayashi M, Saruta T. Hepatocyte growth factor regulates proteoglycan synthesis in interstitial fibroblasts. Kidney Int. 2003;64:1179–1188. doi: 10.1046/j.1523-1755.2003.00202.x. [DOI] [PubMed] [Google Scholar]
- 68.Azuma J, Taniyama Y, Takeya Y, Iekushi K, Aoki M, Dosaka N, Matsumoto K, Nakamura T, Ogihara T, Morishita R. Angiogenic and antifibrotic actions of hepatocyte growth factor improve cardiac dysfunction in porcine ischemic cardiomyopathy. Gene Ther. 2006;13:1206–1213. doi: 10.1038/sj.gt.3302740. [DOI] [PubMed] [Google Scholar]
- 69.Purdie KJ, Whitley GS, Johnstone AP, Cartwright JE. Hepatocyte growth factor-induced endothelial cell motility is mediated by the upregulation of inducible nitric oxide synthase expression. Cardiovasc Res. 2002;54:659–668. doi: 10.1016/s0008-6363(02)00255-9. [DOI] [PubMed] [Google Scholar]
- 70.Takemoto M, Egashira K, Tomita H, Usui M, Okamoto H, Kitabatake A, Shimokawa H, Sueishi K, Takeshita A. Chronic angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade – effects on cardiovascular remodeling in rats induced by the long-term blockade of nitric oxide synthesis. Hypertension. 1997;30:1621–1627. doi: 10.1161/01.hyp.30.6.1621. [DOI] [PubMed] [Google Scholar]
- 71.Tomita H, Egashira K, Ohara Y, Takemoto M, Koyanagi M, Katoh M, Yamamoto H, Tamaki K, Shimokawa H, Takeshita A. Early induction of transforming growth factor-beta via angiotensin II type 1 receptors contributes to cardiac fibrosis induced by long-term blockade of nitric oxide synthesis in rats. Hypertension. 1998;32:273–279. doi: 10.1161/01.hyp.32.2.273. [DOI] [PubMed] [Google Scholar]
- 72.Gum R, Lengyel E, Juarez J, Chen JH, Sato H, Seiki M, Boyd D. Stimulation of 92-kDa gelatinase B promoter activity by ras is mitogen-activated protein kinase kinase 1-independent and requires multiple transcription factor binding sites including closely spaced PEA3/ets and AP-1 sequences. J Biol Chem. 1996;271:10672–10680. doi: 10.1074/jbc.271.18.10672. [DOI] [PubMed] [Google Scholar]
- 73.Nerlov C, Rorth P, Blasi F, Johnsen M. Essential Ap-1 and Pea3 binding-elements in the human urokinase enhancer display cell type-specific activity. Oncogene. 1991;6:1583–1592. [PubMed] [Google Scholar]
- 74.Vandenbunder B, Wernert N, Queva C, Desbiens X, Stehelin D. Does the transcription factor C-Ets1 take part in the regulation of angiogenesis and tumor invasion. Folia Biol Prague. 1994;40:301–313. [PubMed] [Google Scholar]
- 75.Lewin B. Oncogenic conversion by regulatory changes in transcription factors. Cell. 1991;64:303–312. doi: 10.1016/0092-8674(91)90640-k. [DOI] [PubMed] [Google Scholar]
- 76.Iwasaka C, Tanaka K, Abe M, Sato Y. Ets-1 regulates angiogenesis by inducing the expression of urokinase-type plasminogen activator and matrix metalloproteinase-1 and the migration of vascular endothelial cells. J Cell Physiol. 1996;169:522–531. doi: 10.1002/(SICI)1097-4652(199612)169:3<522::AID-JCP12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 77.Nakano N, Moriguchi A, Morishita R, Kida I, Tomita N, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Role of angiotensin II in the regulation of a novel vascular modulator, hepatocyte growth factor (HGF), in experimental hypertensive rats. Hypertension. 1997;30:1448–1454. doi: 10.1161/01.hyp.30.6.1448. [DOI] [PubMed] [Google Scholar]
- 78.Nakano N, Morishita R, Moriguchi A, Nakamura Y, Hayashi S, Aoki M, Kida I, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Negative regulation of local hepatocyte growth factor expression by angiotensin II and transforming growth factor-beta in blood vessels – potential role of HGF in cardiovascular disease. Hypertension. 1998;32:444–451. doi: 10.1161/01.hyp.32.3.444. [DOI] [PubMed] [Google Scholar]
- 79.Galimi F, Cottone E, Vigna E, Arena N, Boccaccio C, Giordano S, Naldini L, Comoglio PM. Hepatocyte growth factor is a regulator of monocyte-macrophage function. J Immunol. 2001;166:1241–1247. doi: 10.4049/jimmunol.166.2.1241. [DOI] [PubMed] [Google Scholar]
- 80.Rutella S, Bonanno G, Procoli A, Mariotti A, de Ritis DG, Curti A, Danese S, Pessina G, Pandolfi S, Natoni F, Di Febo A, Scambia G, Manfredini R, Salati S, Ferrari S, Pierelli L, Leone G, Lemoli RM. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12(low/neg) accessory cells with dendritic-cell features. Blood. 2006;108:218–227. doi: 10.1182/blood-2005-08-3141. [DOI] [PubMed] [Google Scholar]
- 81.Yang ZQ, Zingarelli B, Szabo C. Crucial role of endogenous interleukin-10 production in myocardial ischemia/reperfusion injury. Circulation. 2000;101:1019–1026. doi: 10.1161/01.cir.101.9.1019. [DOI] [PubMed] [Google Scholar]
- 82.Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, Soubrier F, Esposito B, Duez H, Fievet C, Staels B, Duverger N, Scherman D, Tedgui A. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:E17–E24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 83.Mtairag E, Chollet-Martin S, Oudghiri M, Laquay N, Jacob MP, Michel JB, Feldman LJ. Effects of interleukin-10 on monocyte/endothelial cell adhesion and MMP-9/TIMP-1 secretion. Cardiovasc Res. 2001;49:882–890. doi: 10.1016/s0008-6363(00)00287-x. [DOI] [PubMed] [Google Scholar]
- 84.Smith DA, Irving SD, Sheldon J, Cole D, Kaski JC. Serum levels of the antiinflammatory cytokine interleukin-10 are decreased in patients with unstable angina. Circulation. 2001;104:746–749. doi: 10.1161/hc3201.094973. [DOI] [PubMed] [Google Scholar]
- 85.Yang ZJ, Xu SL, Chen B, Zhang SL, Zhang YL, Wei W, Ma DC, Wang LS, Zhu TB, Li CJ, Wang H, Cao KJ, Gao W, Huang J, Ma WZ, Wu ZZ. Hepatocyte growth factor plays a critical role in the regulation of cytokine production and induction of endothelial progenitor cell mobilization: a pilot gene therapy study in patients with coronary heart disease. Clin Exp Pharmacol Physiol. 2009;36:790–796. doi: 10.1111/j.1440-1681.2009.05151.x. [DOI] [PubMed] [Google Scholar]
- 86.Yue TL, Wang X, Sung CP, Olson B, Mckenna PJ, Gu JL, Feuerstein GZ. Interleukin-8 – a mitogen and chemoattractant for vascular smooth-muscle cells. Circ Res. 1994;75:1–7. doi: 10.1161/01.res.75.1.1. [DOI] [PubMed] [Google Scholar]
- 87.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 88.Molad Y, Haines KA, Anderson DC, Buyon JP, Cronstein BN. Immunocomplexes stimulate different signaling events to chemoattractants in the neutrophil and regulate l-selectin and beta(2)-integrin expression differently. Biochem J. 1994;299:881–887. doi: 10.1042/bj2990881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peveri P, Walz A, Dewald B, Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988;167:1547–1559. doi: 10.1084/jem.167.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schroder JM, Christophers E. Secretion of novel and homologous neutrophil-activating peptides by LPS-stimulated human-endothelial cells. J Immunol. 1989;142:244–251. [PubMed] [Google Scholar]
- 91.Watanabe K, Fukuda H, Sueda S, Funada J, Kitakaze M, Sekiya M. Metabolism of hepatocyte growth factor in the heart in patients with coronary artery disease: implication for coronary arteriosclerosis. Cardiovasc Drugs Ther. 2001;15:147–153. doi: 10.1023/a:1011174913176. [DOI] [PubMed] [Google Scholar]
- 92.Futamatsu H, Suzuki J, Mizuno S, Koga N, Adachi S, Kosuge H, Maejima Y, Hirao K, Nakamura T, Isobe M. Hepatocyte growth factor ameliorates the progression of experimental autoimmune myocarditis – a potential role for induction of T helper 2 cytokines. Circ Res. 2005;96:823–830. doi: 10.1161/01.RES.0000163016.52653.2e. [DOI] [PubMed] [Google Scholar]
- 93.Benkhoucha M, Santiago-Raber ML, Schneiter G, Chofflon M, Funakoshi H, Nakamura T, Lalive PH. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25(+)Foxp3(+) regulatory T cells. Proc Natl Acad Sci U S A. 2010;107:6424–6429. doi: 10.1073/pnas.0912437107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dallman MJ, Larsen CP, Morris PJ. Cytokine gene-transcription in vascularized organ grafts – analysis using semiquantitative polymerase chain-reaction. J Exp Med. 1991;174:493–496. doi: 10.1084/jem.174.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saiura A, Mataki C, Murakami T, Umetani M, Wada Y, Kohro T, Aburatani H, Harihara Y, Hamakubo T, Yamaguchi T, Hasegawa G, Naito M, Makuuchi M, Kodama T. A comparison of gene expression in murine cardiac allografts and isografts by means DNA microarray analysis. Transplantation. 2001;72:320–329. doi: 10.1097/00007890-200107270-00027. [DOI] [PubMed] [Google Scholar]
- 96.Yamaura K, Ito K, Tsukioka K, Wada Y, Makiuchi A, Sakaguchi M, Akashima T, Fujimori M, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Suzuki J, Amano J, Isobe M. Suppression of acute and chronic rejection by hepatocyte growth factor in a murine model of cardiac transplantation – induction of tolerance and prevention of cardiac allograft vasculopathy. Circulation. 2004;110:1650–1657. doi: 10.1161/01.CIR.0000143052.45956.71. [DOI] [PubMed] [Google Scholar]
- 97.Hierlihy AM, Seale P, Lobe CG, Rudnicki MA, Megeney LA. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002;530:239–243. doi: 10.1016/s0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 98.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen YH, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JXJ, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 100.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MVG, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 101.Torella D, Ellison GM, Mendez-Ferrer S, Ibanez B, Nadal-Ginard B. Resident human cardiac stem cells: role in cardiac cellular homeostasis and potential for myocardial regeneration. Nat Clin Pract Cardiovasc Med. 2006;3:S8–S13. doi: 10.1038/ncpcardio0409. [DOI] [PubMed] [Google Scholar]
- 102.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 103.Parmacek MS, Epstein JA. Pursuing cardiac progenitors: regeneration redux. Cell. 2005;120:295–298. doi: 10.1016/j.cell.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 104.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 105.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 106.Stastna M, Abraham MR, Van Eyk JE. Cardiac stem/progenitor cells, secreted proteins, and proteomics. FEBS Lett. 2009;583:1800–1807. doi: 10.1016/j.febslet.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stastna M, Chimenti I, Marban E, Van Eyk JE. Identification and functionality of proteomes secreted by rat cardiac stem cells and neonatal cardiomyocytes. Proteomics. 2010;10:245–253. doi: 10.1002/pmic.200900515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, Marban E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:U304–U971. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rota M, Padin-Iruegas ME, Misao Y, De Angelis A, Maestroni S, Ferreira-Martins J, Fiumana E, Rastaldo R, Arcarese ML, Mitchell TS, Boni A, Bolli R, Urbanek K, Hosoda T, Anversa P, Leri A, Kajstura J. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–116. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Andermarcher E, Surani MA, Gherardi E. Co-expression of the HGF/SF and c-met genes during early mouse embryogenesis precedes reciprocal expression in adjacent tissues during organogenesis. Dev Genet. 1996;18:254–266. doi: 10.1002/(SICI)1520-6408(1996)18:3<254::AID-DVG6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 112.Song WM, Majka SM, McGuire PG. Hepatocyte growth factor expression in the developing myocardium: evidence for a role in the regulation of the mesenchymal cell phenotype and urokinase expression. Dev Dyn. 1999;214:92–100. doi: 10.1002/(SICI)1097-0177(199901)214:1<92::AID-DVDY9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 113.Romano LA, Runyan RB. Slug is an essential target of TGF beta 2 signaling in the developing chicken heart. Dev Biol. 2000;223:91–102. doi: 10.1006/dbio.2000.9750. [DOI] [PubMed] [Google Scholar]
- 114.Domian IJ, Chiravuri M, van der Meer P, Feinberg AW, Shi X, Shao Y, Wu SM, Parker KK, Chien KR. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science. 2009;326:426–429. doi: 10.1126/science.1177350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cai CL, Martin JC, Sun YF, Cui L, Wang LC, Ouyang K, Yang L, Bu L, Liang XQ, Zhang XX, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:1U4–1U104. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Muñoz-Chápuli R, Pérez-Pomares JM, Macías D, García-Garrido L, Carmona R, González-Iriarte M. The epicardium as a source of mesenchyme for the developing heart. Ital J Anat Embryol. 2001;106(2 Suppl 1):187–196. [PubMed] [Google Scholar]
- 117.Winter EM, Gittenberger-de Groot AC. Epicardium-derived cells in cardiogenesis and cardiac regeneration. Cell Mol Life Sci. 2007;64:692–703. doi: 10.1007/s00018-007-6522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Perez-Pomares JM, Carmona R, Gonzalez-Iriarte M, Atencia G, Wessels A, Munoz-Chapuli R. Origin of coronary endothelial cells from epicardial mesothelium in avian embryos. Int J Dev Biol. 2002;46:1005–1013. [PubMed] [Google Scholar]
- 119.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang DW, von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:1U5–1U109. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reese DE, Mikawa T, Bader DM. Development of the coronary vessel system. Circ Res. 2002;91:761–768. doi: 10.1161/01.res.0000038961.53759.3c. [DOI] [PubMed] [Google Scholar]
- 121.Wada AM, Smith TK, Osler ME, Reese DE, Bader DM. Epicardial/mesothelial cell line retains vasculogenic potential of embryonic epicardium. Circ Res. 2003;92:525–531. doi: 10.1161/01.RES.0000060484.11032.0B. [DOI] [PubMed] [Google Scholar]
- 122.Smart N, Risebro CA, Melville AAD, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta 4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 123.Tallini YN, Greene KS, Craven M, Spealman A, Breitbach M, Smith J, Fisher PJ, Steffey M, Hesse M, Doran RM, Woods A, Singh B, Yen A, Fleischmann BK, Kotlikoff MI. c-Kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci U S A. 2009;106:1808–1813. doi: 10.1073/pnas.0808920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Limana F, Zacheo A, Mocini D, Mangoni A, Borsellino G, Diamantini A, De Mori R, Battistini L, Vigna E, Santini M, Loiaconi V, Pompilio G, Germani A, Capogrossi MC. Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circ Res. 2007;101:1255–1265. doi: 10.1161/CIRCRESAHA.107.150755. [DOI] [PubMed] [Google Scholar]
- 125.Limana F, Bertolami C, Mangoni A, Di Carlo A, Avitabile D, Mocini D, Iannelli P, De Mori R, Marchetti C, Pozzoli O, Gentili C, Zacheo A, Germani A, Capogrossi MC. Myocardial infarction induces embryonic reprogramming of epicardial c-kit(+) cells: role of the pericardial fluid. J Mol Cell Cardiol. 2010;48:609–618. doi: 10.1016/j.yjmcc.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 126.Roggia C, Ukena C, Bohm M, Kilter H. Hepatocyte growth factor (HGF) enhances cardiac commitment of differentiating embryonic stem cells by activating P13 kinase. Exp Cell Res. 2007;313:921–930. doi: 10.1016/j.yexcr.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 127.Yoon YS, Wecker A, Heyd L, Park JS, Tkebuchava T, Kusano K, Hanley A, Scadova H, Qin GJ, Cha DH, Johnson KL, Aikawa R, Asahara T, Losordo DW. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115:326–338. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rehman J, Li JL, Orschell CM, March KL. Peripheral blood endothelial progenitor cells are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 129.Cho HJ, Lee N, Lee JY, Choi YJ, Li M, Wecker A, Jeong JO, Curry C, Qin G, Yoon YS. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007;204:3257–3269. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 131.Neuss S, Becher E, Woltje M, Tietze L, Jahnen-Dechent W. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells. 2004;22:405–414. doi: 10.1634/stemcells.22-3-405. [DOI] [PubMed] [Google Scholar]
- 132.Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cai L, Johnstone BH, Cook TG, Liang Z, Traktuev D, Cornetta K, Ingram DA, Rosen ED, Marcha KL. Suppression of hepatocyte growth factor production impairs the ability of adipose-derived stem cells to promote ischemic tissue revascularization. Stem Cells. 2007;25:3234–3243. doi: 10.1634/stemcells.2007-0388. [DOI] [PubMed] [Google Scholar]
- 134.Rehman J, Traktuev D, Li JL, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 135.Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46:7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 136.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 137.Gude N, Muraski J, Rubio M, Kajstura J, Schaefer E, Anversa P, Sussman MA. Akt promotes increased cardiomyocyte cycling and expansion of the cardiac progenitor cell population. Circ Res. 2006;99:381–388. doi: 10.1161/01.RES.0000236754.21499.1c. [DOI] [PubMed] [Google Scholar]
- 138.Guo YH, He JG, Wu JL, Yang L, Dai SM, Tan XY, Liang LR. Locally overexpressing hepatocyte growth factor prevents post-ischemic heart failure by inhibition of apoptosis via calcineurin-mediated pathway and angiogenesis. Arch Med Res. 2008;39:179–188. doi: 10.1016/j.arcmed.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 139.Mirotsou M, Zhang ZY, Deb A, Zhang LN, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gnecchi M, He HM, Noiseux N, Liang OD, Zhang LM, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 141.Dhein S, Garbade J, Rouabah D, Abraham G, Ungemach FR, Schneider K, Ullmann C, Aupperle H, Gummert JF, Mohr FW. Effects of autologous bone marrow stem cell transplantation on beta-adrenoceptor density and electrical activation pattern in a rabbit model of non-ischemic heart failure. J Cardiothorac Surg. 2006;1:17. doi: 10.1186/1749-8090-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ohnishi S, Yasuda T, Kitamura S, Nagaya N. Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells. Stem Cells. 2007;25:1166–1177. doi: 10.1634/stemcells.2006-0347. [DOI] [PubMed] [Google Scholar]
- 143.Tang JM, Wang JN, Guo LY, Kong X, Yang JY, Zheng F, Zhang L, Huang YZ. Mesenchymal stem cells modified with stromal cell-derived factor 1 alpha improve cardiac remodeling via paracrine activation of hepatocyte growth factor in a rat model of myocardial infarction. Mol Cells. 2010;29:9–19. doi: 10.1007/s10059-010-0001-7. [DOI] [PubMed] [Google Scholar]
- 144.Sánchez PL, San Román JA, Villa A, Fernández ME, Fernández-Avilés F. Contemplating the bright future of stem cell therapy for cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2006;3:S138–S151. doi: 10.1038/ncpcardio0456. [DOI] [PubMed] [Google Scholar]
- 145.Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De Maria R, Boratto R, Jaffredo T, Broccoli V, Bianco P, Cossu G. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–2784. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- 146.Galli D, Innocenzi A, Staszewsky L, Zanetta L, Sampaolesi M, Bai A, Martinoli E, Carlo E, Balconi G, Fiordaliso F, Chimenti S, Cusella G, Dejana E, Cossu G, Latini R. Mesoangioblasts, vessel-associated multipotent stem cells, repair the infarcted heart by multiple cellular mechanisms – a comparison with bone marrow progenitors, fibroblasts, and endothelial cells. Arter Thromb Vasc Biol. 2005;25:692–697. doi: 10.1161/01.ATV.0000156402.52029.ce. [DOI] [PubMed] [Google Scholar]
- 147.Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4:432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- 148.Forte G, Minieri M, Cossa P, Antenucci D, Sala M, Gnocchi V, Fiaccavento R, Carotenuto F, De Vito P, Baldini PM, Prat M, Di Nardo P. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006;24:23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- 149.Kucia M, Dawn B, Hunt G, Guo YR, Wysoczynski M, Majka M, Ratajczak J, Rezzoug F, Ildstad ST, Bolli R, Ratajczak MZ. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res. 2004;95:1191–1199. doi: 10.1161/01.RES.0000150856.47324.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lataillade JJ, Domenech J, Bousse-Kerdiles MC. Stromal cell-derived factor-1 (SDF-1)/CXCR4 couple plays multiple roles on haematopoietic progenitors at the border between the old cytokine and new chemokine worlds: survival, cell cycling and trafficking. Eur Cytokine Netw. 2004;15:177–188. [PubMed] [Google Scholar]
- 151.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1 alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 152.Dentelli P, Rosso A, Balsamo A, Benedetto SC, Zeoli A, Pegoraro M, Camussi G, Pegoraro L, Brizzi MF. C-KIT, by interacting with the membrane-bound ligand, recruits endothelial progenitor cells to inflamed endothelium. Blood. 2007;109:4264–4271. doi: 10.1182/blood-2006-06-029603. [DOI] [PubMed] [Google Scholar]
- 153.Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28:299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 155.Tacchini L, Dansi P, Matteucci E, Desiderio MA. Hepatocyte growth factor signalling stimulates hypoxia inducible factor-1 (HIF-1) activity in HepG2 hepatoma cells. Carcinogenesis. 2001;22:1363–1371. doi: 10.1093/carcin/22.9.1363. [DOI] [PubMed] [Google Scholar]
- 156.Maroni P, Bendinelli P, Matteucci E, Desiderio MA. HGF induces CXCR4 and CXCL12-mediated tumor invasion through Ets1 and NF-kappa B. Carcinogenesis. 2007;28:267–279. doi: 10.1093/carcin/bgl129. [DOI] [PubMed] [Google Scholar]
- 157.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 158.Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 159.Vasyutina E, Stebler J, Brand-Saberi B, Schulz S, Raz E, Birchmeier C. CXCR4 and Gab1 cooperate to control the development of migrating muscle progenitor cells. Gene Dev. 2005;19:2187–2198. doi: 10.1101/gad.346205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ratajczak MZ, Majka M, Kucia M, Drukala J, Pietrzkowski Z, Peiper S, Janowska-Wieczorek A. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells. 2003;21:363–371. doi: 10.1634/stemcells.21-3-363. [DOI] [PubMed] [Google Scholar]
- 161.Ahmet I, Sawa Y, Yamaguchi T, Matsuda H. Gene transfer of hepatocyte growth factor improves angiogenesis and function of chronic ischemic myocardium in canine heart. Ann Thorac Surg. 2003;75:1283–1287. doi: 10.1016/s0003-4975(02)04677-5. [DOI] [PubMed] [Google Scholar]
- 162.Jayasankar V, Woo YJ, Pirolli TJ, Bish LT, Berry MF, Burdick J, Gardner TJ, Sweeney HL. Induction of angiogenesis and inhibition of apoptosis by hepatocyte growth factor effectively treats postischemic heart failure. J Card Surg. 2005;20:93–101. doi: 10.1111/j.0886-0440.2005.200373.x. [DOI] [PubMed] [Google Scholar]
- 163.Wang W, Yang ZJ, Ma DC, Wang LS, Xu SL, Zhang YR, Cao KJ, Zhang FM, Ma WZ. Induction of collateral artery growth and improvement of post-infarct heart function by hepatocyte growth factor gene transfer. Acta Pharmacol Sin. 2006;27:555–560. doi: 10.1111/j.1745-7254.2006.00306.x. [DOI] [PubMed] [Google Scholar]
- 164.Yuan B, Zhang YR, Zhao Z, Wu DL, Yuan LZ, Wu B, Wang LS, Huang J. Treatment of chronical myocardial ischemia by adenovirus-mediated hepatocyte growth factor gene transfer in minipigs. Sci China Ser C. 2008;51:537–543. doi: 10.1007/s11427-008-0073-1. [DOI] [PubMed] [Google Scholar]
- 165.Yang ZJ, Ma DC, Wang W, Xu SL, Zhang YQ, Chen B, Zhou F, Zhu TB, Wang LS, Xu ZQ, Zhang FM, Cao KJ, Ma WZ. Experimental study of bone marrow-derived mesenchymal stem cells combined with hepatocyte growth factor transplantation via noninfarct-relative artery in acute myocardial infarction. Gene Ther. 2006;13:1564–1568. doi: 10.1038/sj.gt.3302820. [DOI] [PubMed] [Google Scholar]
- 166.Yuan B, Zhao Z, Zhang YR, Wu CT, Jin WG, Zhao S, Wang W, Zhang YY, Zhu XL, Wang LS, Huang J. Short-term safety and curative effect of recombinant adenovirus carrying hepatocyte growth factor gene on ischemic cardiac disease. In Vivo. 2008;22:629–632. [PubMed] [Google Scholar]
- 167.Yang ZJ, Zhang YR, Chen B, Zhang SL, Jia EZ, Wang LS, Zhu TB, Li CJ, Wang H, Huang J, Cao KJ, Ma WZ, Wu B, Wang LS, Wu CT. Phase I clinical trial on intracoronary administration of Ad-hHGF treating severe coronary artery disease. Mol Biol Rep. 2009;36:1323–1329. doi: 10.1007/s11033-008-9315-3. [DOI] [PubMed] [Google Scholar]
- 168.Morishita R, Aoki M, Hashiya N, Makino H, Yamasaki K, Azuma J, Sawa Y, Matsuda H, Kaneda Y, Ogihara T. Safety evaluation of clinical gene therapy using hepatocyte growth factor to treat peripheral arterial disease. Hypertension. 2004;44:203–209. doi: 10.1161/01.HYP.0000136394.08900.ed. [DOI] [PubMed] [Google Scholar]
- 169.McKinnon H, Gherardi E, Reidy M, Bowyer D. Hepatocyte growth factor/scatter factor and MET are involved in arterial repair and atherogenesis. Am J Pathol. 2006;168:340–348. doi: 10.2353/ajpath.2006.050379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lamblin N, Susen S, Dagorn J, Mouquet F, Jude M, Van Belle E, Bauters C, de Groote P. Prognostic significance of circulating levels of angiogenic cytokines in patients with congestive heart failure. Am Heart J. 2005;150:137–143. doi: 10.1016/j.ahj.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 171.Ueno S, Ikeda U, Hojo Y, Arakawa H, Nonaka M, Yamamoto K, Shimada K. Serum hepatocyte growth factor levels are increased in patients with congestive heart failure. J Card Fail. 2001;7:329–334. doi: 10.1054/jcaf.2001.27686. [DOI] [PubMed] [Google Scholar]
- 172.Levin ER, Gardner DG, Samson WK. Mechanisms of disease – natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 173.Liu Y, Wilkinson FL, Kirton JP, Jeziorska M, Iizasa H, Sai Y, Nakashima E, Heagerty AM, Canfield AE, Alexander MY. Hepatocyte growth factor and c-Met expression in pericytes: implications for atherosclerotic plaque development. J Pathol. 2007;212:12–19. doi: 10.1002/path.2155. [DOI] [PubMed] [Google Scholar]
- 174.Morishita R, Moriguchi A, Higaki J, Ogihara T. Hepatocyte growth factor (HGF) as a potential index of severity of hypertension. Hypertens Res Clin Exp. 1999;22:161–167. doi: 10.1291/hypres.22.161. [DOI] [PubMed] [Google Scholar]
- 175.Nakamura S, Morishita R, Moriguchi A, Yo Y, Nakamura Y, Hayashi S, Matsumoto K, Matsumoto K, Nakamura T, Higaki J, Ogihara T. Hepatocyte growth factor as a potential index of complication in diabetes mellitus. J Hypertens. 1998;16:2019–2026. doi: 10.1097/00004872-199816121-00025. [DOI] [PubMed] [Google Scholar]