Serotonin (5-HT) is a monoamine neurotransmitter that is classically recognized for its functions in the central nervous system, where it plays important roles in regulating mood, body temperature, sleep, sexuality, appetite, and metabolism.1 Although some 5-HT is synthesized in serotonergic neurons in the central nervous system, the vast majority of 5-HT (approximately 95%) is localized to the intestine.1 In the intestine, 5-HT is synthesized in serotonergic neurons in the enteric nervous system as well as in the enterochromaffin (EC) cells of the gastrointestinal mucosa.1,2 However, EC cells secrete the vast majority of 5-HT in the gut.1
Two enzymes, tryptophan hydroxylase 1 (TPH1), in EC cells, and TPH2, in central and enteric neurons, catalyze 5-HT biosynthesis.1 Because there are no extracellular enzymes that catabolize 5-HT, uptake into cells is required for 5-HT inactivation. 5-HT is also charged at a physiologic pH, and thus, does not traverse cell membranes well. A plasmalemmal 5-HT transporter (SERT) is responsible primarily for mediating transmembrane transport of 5-HT into mucosal enterocytes3 (Figure 1).1,4
Figure 1.
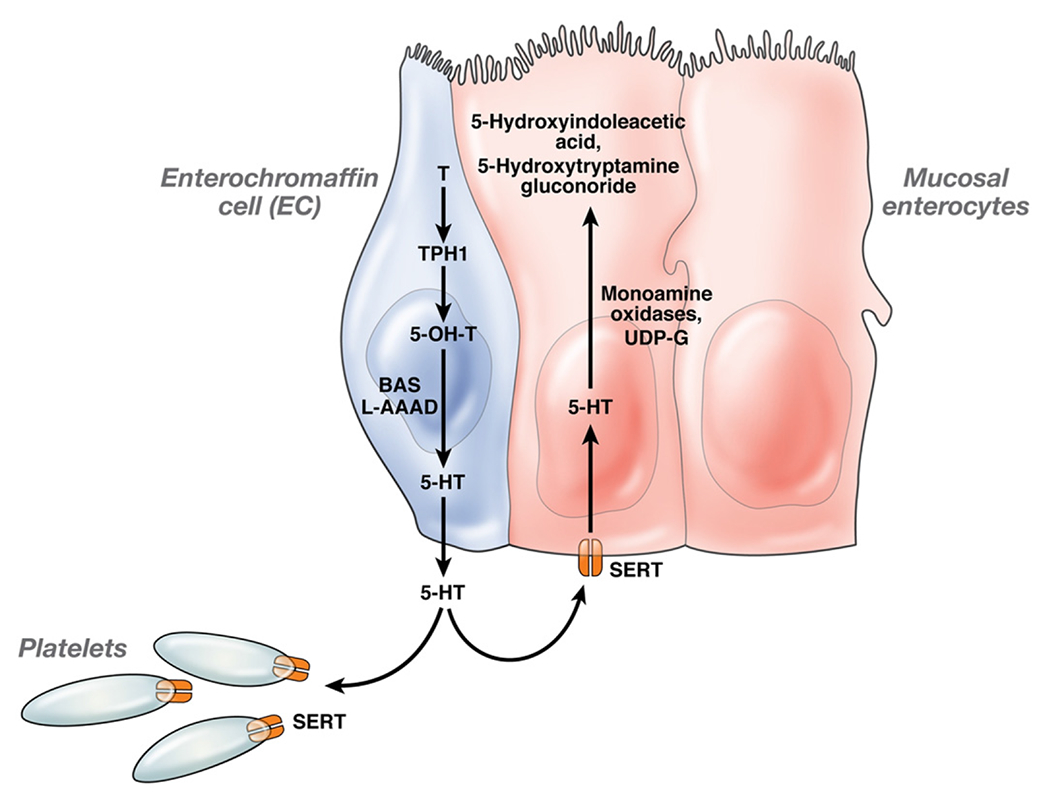
In the enterochromaffin (EC) cell, tryptophan (T) is converted to 5-hydroxytryptophan (5-OH-T) by 5-HT’s rate limiting biosynthetic enzyme TPH1. 5-OH-T is then converted to 5-HT with the assistance of L-aromatic acid decarboxylase (L-AAAD) and biogenic amine synthesis. Once secreted from the EC cell, some 5-HT is taken up by the 5-HT reuptake transporters, located mainly in mucosal enterocytes. The remainder goes into the blood where it is captured and concentrated by the platelets which release it upon stimulation. Once in the enterocyte, 5-HT is metabolized by monamine oxidases and UDP-glucuronosyltransferase to its inactive metabolites, 5-hydroxyindoleacetic acid and 5-hydroxytryptamine glucuronide. These inactive metabolites are ultimately excreted by the kidneys. In conditions where excessive 5-HT is produced, and the 5-HT reuptake transporters and platelets cannot hold more 5-HT, excess 5-HT flows into the gut lumen and blood in its active form.
In the bowel, SERT is expressed in enterocytes and in the serotonergic neurons of the enteric nervous system. If SERT is knocked out or inhibited, other transporters compensate, such as organic cation transporters 1 and 3 and the dopamine transporter (DAT) and mediate 5-HT uptake. However, these transporters have a much lower affinity for 5-HT than SERT.5 As a result, the actions of 5-HT are enhanced and prolonged when SERT is knocked out or inhibited.
Enteric mucosal 5-HT, which is produced in EC cells, and also in mast cells in rats and mice, can act as a paracrine factor or as a hormone.1,2,6 As a hormone, 5-HT is released into the blood to regulate bone density by inhibiting osteoblastic activity.7 As a paracrine factor, 5-HT targets mucosal projections of intrinsic primary afferent neurons to initiate enteric peristaltic and secretory reflexes.8,9 Because 5-HT’ has roles in increasing peristalsis and secretion, it has been thought to play a protective role against enteric infections by facilitating the rapid removal of organisms from the gut.10,11
5-HT is likely to play a role in mucosal homeostasis. There is substantial evidence that mucosal 5-HT modulates the immune response, and thus, is able potentially to influence intestinal inflammation.10 5-HT has been shown to promote lymphocyte activation and secretion of proinflammatory cytokines.12 Additionally, dendritic cells, lymphocytes, macrophages, endothelial cells, and enteric epithelial cells all express 5-HT receptors.13–16 Recent evidence from patients with inflammatory bowel disease suggests that mucosal 5-HT may play an important role in intestinal inflammation. Increased enteric 5-HT levels have been demonstrated in patients with Crohn’s disease and ulcerative colitis and this has been associated with significant increases in EC cell numbers.10,17–19 Furthermore, SERT transcription is decreased in patients with ulcerative colitis as well as patients with a recent history of diverticulitis.19,20 Both interleukin (IL)-1β and lipopolysaccharide-induced 5-HT secretion is significantly increased in EC cells derived from patients with Crohn’s disease compared with a control population.10 In patients with celiac disease, enteric 5-HT levels and duodenal EC cells are increased and a significant correlation was observed between peak postprandial 5-HT levels and postprandial dyspepsia scores, suggesting a role for 5-HT in promoting associated symptoms.21 Post-infectious irritable bowel syndrome (PI-IBS), which has been suggested by some groups to represent a “low-grade inflammatory state,” has also been associated with an increase in the peak of postprandial 5-HT release and EC cell hyperplasia.10,22 SERT polymorphisms have also been investigated in IBS. Although a meta-analysis of 7 such studies concluded that 5-HTTPLR genotypes are not risk factors for IBS, other studies have found evidence of an association when patients were stratified by the predominant clinical symptoms.23
Animal and in vitro studies further support a role for 5-HT in inflammatory states. Experimental inflammation in animals induced by TNBS or infection with either Trichinella spiralis or Citrobacter rodentium, leads to down-regulation of SERT with a concomitant increase in EC cell number and/or 5-HT release.11,24–27 Interferon-γ and tumor necrosis factor-α decrease SERT function in colonic adenocarcinoma Caco2 cells.28 In this issue of Gastroenterology, Ghia et al29 provide 1 of the first important pieces of evidence demonstrating an innovative role for mucosal 5-HT in the generation of intestinal inflammation. They are also the first to show that 5-HT promotes this inflammation through immune cell activation. Ghia et al induced dextran sulphate sodium (DSS)- or dinitrobenzene sulfonic acid (DNBS)-induced colitis in 2 mouse models in which the amount of intestinal 5-HT is reduced profoundly. They used Tph1+/+ mice, which lack the key biosynthetic enzyme for 5-HT in EC cells, and mice treated with the 5-HT synthesis inhibitor, parachlorylphenylalanine. Tph1−/− mice developed significantly less severe colitis following DSS or DNBS administration and parachlorylphenylalanine-treated mice developed significantly less severe DSS-induced colitis, compared with their wild-type counterparts. This was demonstrated as significantly lower disease activity, macroscopic and histologic scores, as well as diminished serum MPO and CRP levels. In Tph1−/− mice, reduced severity of intestinal inflammation was associated also with significantly less mucosal macrophage infiltration and levels of proinflammatory cytokines compared with their wild-type counterparts. To consolidate these findings, the authors restored intestinal 5-HT with subcutaneous 5-HTP, a precursor of 5-HT, in Tph1−/− mice and exposed them to DSS. Tph1−/− mice that received 5-HTP had significantly higher levels of inflammation relative to Tph1−/− mice not treated with 5-HTP. In vitro experiments demonstrated that LPS-stimulated peritoneal macrophages from Tph1+/+ mice secreted significantly greater amounts of IL-1β and IL-6 when incubated with 5-HT and this effect was inhibited by the addition of a nuclear factor (NF)-κB inhibitor. Further experiments may provide additional insights into the precise nature of 5-HT’s actions in intestinal inflammation. Stimulating intraepithelial lymphocytes with 5-HT could also potentially implicate another mechanism for 5-HT in chronic colitis. Additionally, because activation of specific signaling molecules of the NF-κB pathway may be associated with different pathophysiologic consequences pertinent to intestinal inflammation, it would be useful to identify the specific NF-κB–related signaling molecules activated by 5-HT using, for example, RNA silencing approaches. This study highlights convincingly the importance of 5-HT in the modulation of experimental colitis and provides strong evidence supporting several potential associated mechanisms, including recruitment of macrophages in intestinal inflammatory sites and an NF-κB–dependent activation of proinflammatory cytokines. These data compliment the recent study published by Bischoff et al that demonstrated that TNBS-induced colitis is increased in severity when coupled with the 5-HT–enhancing effects of the knockout of SERT.30
From the evidence presented thus far, it is apparent that 5-HT modulates intestinal inflammation (Figure 2). The precise function(s) of 5-HT in intestinal inflammation, however, are not clear. The physiologic roles of 5-HT in general have still not been elucidated completely, in part because of the multiple functions mediated by 5-HT in the intestine making it difficult to discern which of these actions are more physiologically important. The multiplicity of responses to applied 5-HT can be attributed partly to the wide number of 5-HT receptors expressed in the gut. 5-HT3, 5-HT4, and 5-HT7 receptors are expressed in various parts of the intestine and are known to serve different, and even contrasting, roles. Further complicating this picture are the multiple 5-HT receptors (5-HT1A, 5-HT1P, 5-HT2A, 2B, 5-HT3, 5-HT4, and 5-HT7) that at least potentially exert effects on GI sensorimotor function in humans.1 Because of the widespread actions of 5-HT, drugs that combat inflammation must be targeted to specific receptors. Future studies will thus likely be focused on the specific 5-HT receptor subtype(s) involved in intestinal inflammation and which aspects of the immune system they affect. Another obstacle to the development of 5-HT-related “therapies” is the limited number of suitable ligands currently available for in vivo studies.1
Figure 2.
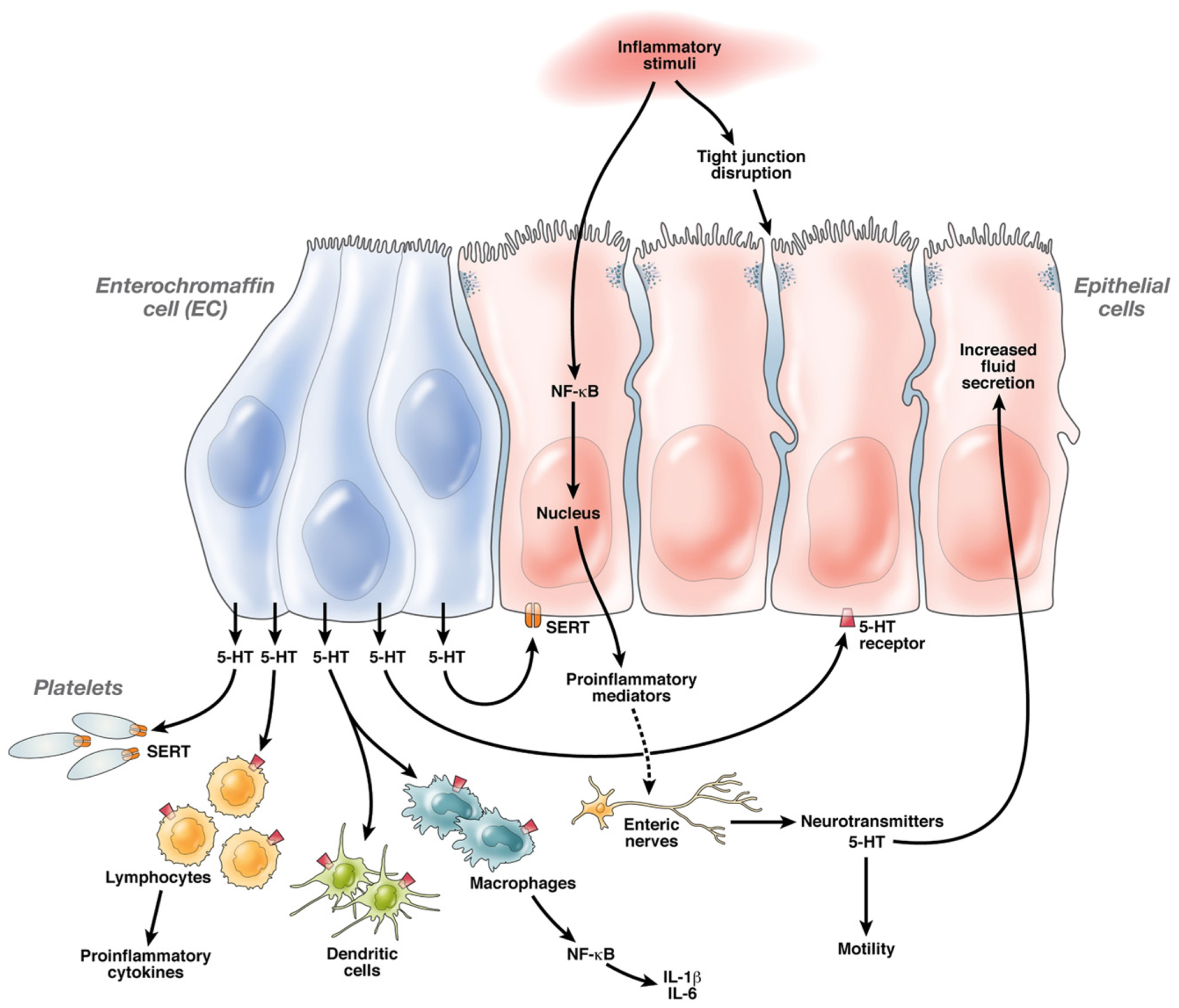
5-HT and Mechanisms of Intestinal Inflammation: Luminal inflammatory stimuli cause EC cell hyperplasia and possible activation of EC cells resulting in increased 5-HT release. While some 5-HT is taken up by SERT in epithelial cells as well as platelets in the blood, excess 5-HT circulates acts locally on epithelial cells as well as on lamina propria cells, such as macrophages, dendritic cells, lymphocytes and enteric nerves via binding on specific 5-HT receptors. This binding triggers activation of the NF-κB system and possible other proinflammatory signaling pathways resulting in transcription of proinflammatory cytokines and chemokines, such as IL-1β and IL-6. Proinflammatory mediators further amplify the intestinal inflammatory response by stimulating enteric nerves to release 5-HT and other neurotransmitters that perpetuate inflammation. 5-HT, cytokines and other neurotransmitters also act on the intestinal epithelium altering tight junctional permeability and stimulating fluid secretion, as well as on smooth muscle cells causing motility changes associated with intestinal inflammatory conditions.
Two additional areas in which 5-HT seems to play a role involve prenatal and adult neurogenesis. 5-HT, present in the earliest born of enteric neurons, has been demonstrated to promote neuronal development and is, therefore, in a position to shape the development of the ENS.1 Excitingly, Liu et al31 very recently demonstrated that 5-HT plays a role in adult enteric neurogenesis, which is, at least in part, dependent on the 5-HT4 receptor. These 5-HT-dependent functions may potentially be modulated with the development of receptor-specific drugs.
The most well-studied neurotransmitters in the pathogenesis of intestinal inflammation are substance P, corticotropinreleasing hormone, neurotensin, and vasoactive intestinal peptide.32 All of these transmitters bind to G-protein–coupled receptors and modulate intestinal inflammation through activation of NF-κB and other signaling pathways related to inflammation.32 From Ghia et al’s work, it is evident that 5-HT activates similar proinflammatory pathways and is likely to become a target for therapy in intestinal inflammatory disorders in the near future.
Footnotes
Conflicts of interest
The authors disclose no conflicts.
Contributor Information
KARA GROSS MARGOLIS, Department of Pediatrics, Morgan Stanley Children’s Hospital, Columbia University Medical Center, New York, New York.
CHARALABOS POTHOULAKIS, Inflammatory Bowel Disease Center, Division of Digestive Diseases, David Geffen School of Medicine—UCLA, Los Angeles, California.
References
- 1.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 2007;132:397–414. [DOI] [PubMed] [Google Scholar]
- 2.Gershon MD, Drakontides AB, Ross LL. Serotonin: Synthesis and Release from the Myenteric Plexus of the Mouse Intestine. Science 1965;149:197–199. [DOI] [PubMed] [Google Scholar]
- 3.Martel F. Recent advances on the importance of the serotonin transporter SERT in the rat intestine. Pharmacol Res 2006;54:73–76. [DOI] [PubMed] [Google Scholar]
- 4.Gerber GB, Deroo J, Mazanowska A. Metabolism of 5-hydroxytryptophan in isolated perfused intestine from normal and x-irradiated rat. Arch Int Physiol Biochim 1972;80:733–739. [DOI] [PubMed] [Google Scholar]
- 5.Chen JJ, Li Z, Pan H, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci 2001;21:6348–6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagata K, Fujimiya M, Sugiura H, et al. Intracellular localization of serotonin in mast cells of the colon in normal and colitis rats. Histochem J 2001;33:559–568. [DOI] [PubMed] [Google Scholar]
- 7.Yadav VK, Ryu JH, Suda N, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 2008;135:825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirchgessner AL, Liu MT, Gershon MD. In situ identification and visualization of neurons that mediate enteric and enteropancreatic reflexes. J Comp Neurol 1996;371:270–286. [DOI] [PubMed] [Google Scholar]
- 9.Sidhu M, Cooke HJ. Role for 5-HT and ACh in submucosal reflexes mediating colonic secretion. Am J Physiol 1995;269:G346–351. [DOI] [PubMed] [Google Scholar]
- 10.Spiller R. Serotonin, inflammation, and IBS: fitting the jigsaw together? J Pediatr Gastroenterol Nutr 2007;45 suppl 2:S115–119. [DOI] [PubMed] [Google Scholar]
- 11.Wheatcroft J, Wakelin D, Smith A, et al. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil 2005;17:863–870. [DOI] [PubMed] [Google Scholar]
- 12.Young MR, Matthews JP. Serotonin regulation of T-cell subpopulations and of macrophage accessory function. Immunology 1995;84:148–152. [PMC free article] [PubMed] [Google Scholar]
- 13.Gershon MD. Review article: serotonin receptors and transporters—roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther 2004;20 Suppl 7:3–14. [DOI] [PubMed] [Google Scholar]
- 14.Idzko M, Panther E, Stratz C, et al. The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J Immunol 2004;172:6011–6019. [DOI] [PubMed] [Google Scholar]
- 15.McDuffie JE, Motley ED, Limbird LE, et al. 5-hydroxytryptamine stimulates phosphorylation of p44/p42 mitogen-activated protein kinase activation in bovine aortic endothelial cell cultures. J Cardiovasc Pharmacol 2000;35:398–402. [DOI] [PubMed] [Google Scholar]
- 16.Yin J, Albert RH, Tretiakova AP, et al. 5-HT(1B) receptors play a prominent role in the proliferation of T-lymphocytes. J Neuroimmunol 2006;181:68–81. [DOI] [PubMed] [Google Scholar]
- 17.El-Salhy M, Danielsson A, Stenling R, et al. Colonic endocrine cells in inflammatory bowel disease. J Intern Med 1997;242:413–419. [DOI] [PubMed] [Google Scholar]
- 18.Belai A, Boulos PB, Robson T, et al. Neurochemical coding in the small intestine of patients with Crohn’s disease. Gut 1997;40:767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 2004;126:1657–1664. [DOI] [PubMed] [Google Scholar]
- 20.Costedio MM, Coates MD, Danielson AB, et al. Serotonin signaling in diverticular disease. J Gastrointest Surg 2008;12:1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coleman NS, Foley S, Dunlop SP, et al. Abnormalities of serotonin metabolism and their relation to symptoms in untreated celiac disease. Clin Gastroenterol Hepatol 2006;4:874–881. [DOI] [PubMed] [Google Scholar]
- 22.Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol 2005;39:S184–193. [DOI] [PubMed] [Google Scholar]
- 23.Colucci R, Blandizzi C, Bellini M, et al. The genetics of the serotonin transporter and irritable bowel syndrome. Trends Mol Med 2008;14:295–304. [DOI] [PubMed] [Google Scholar]
- 24.Linden DR, Foley KF, McQuoid C, et al. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol Motil 2005;17:565–574. [DOI] [PubMed] [Google Scholar]
- 25.O’Hara JR, Ho W, Linden DR, et al. Enteroendocrine cells and 5-HT availability are altered in mucosa of guinea pigs with TNBS ileitis. Am J Physiol Gastrointest Liver Physiol 2004;287:G998–1007. [DOI] [PubMed] [Google Scholar]
- 26.O’Hara JR, Skinn AC, MacNaughton WK, et al. Consequences of Citrobacter rodentium infection on enteroendocrine cells and the enteric nervous system in the mouse colon. Cell Microbiol 2006;8:646–660. [DOI] [PubMed] [Google Scholar]
- 27.Linden DR, Chen JX, Gershon MD, et al. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol 2003;285:G207–216. [DOI] [PubMed] [Google Scholar]
- 28.Foley KF, Pantano C, Ciolino A, et al. IFN-gamma and TNF-alpha decrease serotonin transporter function and expression in Caco2 cells. Am J Physiol Gastrointest Liver Physiol 2007;292:G779–784. [DOI] [PubMed] [Google Scholar]
- 29.Ghia JE, Li N, Wang H, et al. Serotonin has a key role in the pathogenesis of experimental colitis. Gastroenterology 2009;137:1649–1660. [DOI] [PubMed] [Google Scholar]
- 30.Bischoff SC, Mailer R, Pabst O, et al. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol 2009;296:G685–695. [DOI] [PubMed] [Google Scholar]
- 31.Liu MT, Kuan YH, Wang J, et al. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci 2009;29:9683–9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gross KJ, Pothoulakis C. Role of neuropeptides in inflammatory bowel disease. Inflamm Bowel Dis 2007;13:918–932. [DOI] [PubMed] [Google Scholar]


