Abstract
Spermatozoa acquire forward motility and fertilizing capacity during their transit through the epididymis. The maturation process involves modifications of the sperm surface by different proteins that are secreted by a series of specialized regions in the epididymal epithelium. Here we show that the rat epididymis-specific β-defensin 15 (Defb15) exhibits an androgen-dependent expression pattern, and it can bind to the acrosomal region of caput sperm. Coculture of caput spermatozoa with Defb15 antibody in vitro resulted in a significant decline in sperm motility. Moreover, the total and progressive motility of the spermatozoa dramatically decreased in rats when Defb15 was downregulated by lentivirus-mediated RNAi in vivo. Remarkably, knock down of Defb15 led to a reduction in fertility and embryonic development failure. In addition, the recombinant Defb15 showed antimicrobial activity in a dose-dependent fashion. These results suggest that Defb15 plays a dual role in both sperm maturation and pathogen defense in rat epididymis.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0478-4) contains supplementary material, which is available to authorized users.
Keywords: β-Defensin, Epididymis, Sperm maturation, Sperm motility, Male fertility, Antimicrobial activity
Introduction
Spermatozoa leaving the testes are incomplete as they can neither swim nor fertilize an egg. During their transit through the epididymis including the caput (head), corpus (body) and cauda (tail) regions, sperm undergo a series of physical, chemical and morphological alterations to acquire maturity [1–3]. The luminal fluid microenvironment is not only crucial for sperm maturation, but also protects the sperm against autoimmunity and other potentially hazardous factors such as bacteria. At present, some known and some putative host defense proteins produced and secreted by the epithelial cells of epididymides have been characterized in different mammalian species [4–12], and β-defensins are the best studied effector molecules.
The defensins are a family of cationic antimicrobial peptides that contribute to the host immune response [13]. They are classified as alpha, beta and theta defensins based on the spacing and pairing patterns of six conserved cysteine residues [14]. β-Defensins exhibit distinct antibacterial, antifungal and antiviral capacities against a wide variety of microorganisms [15–18]. Recent studies have also revealed that certain epididymal β-defensins are actively involved in sperm maturation and fertilization, physiological properties that are not related to host defense. We first reported that Spag11e/Bin1b, a rat epididymis-specific β-defensin, is not limited to the killing of bacteria but also initiates sperm motility by inducing Ca2+ uptake [19]. Moreover, another epididymal secretory β-defensin 126 (DEFB126/ESP13.2) coats the entire surface of macaque sperm and may be an important decapacitation factor that needs to be released before sperm–zona interaction and fertilization [20, 21]. On the basis of the multifunctionality of β-defensins, this family has been attracting steadily growing interest.
In follow-up studies, the complete repertoires of mammalian β-defensins have been identified using genome-wide computational searches. To date, a total of 39 human, 52 mouse and 43 rat β-defensin genes have been discovered [10], and they form four or five syntenic clusters in the respective chromosomes [10, 22]. Surprisingly, almost all rat β-defensins have been found to be preferentially expressed in the male reproductive system, particularly in the testes and different regions of the epididymis [10]. Such a unique property implies that these molecules may constitute an essential component in maintaining the normal reproductive process. Therefore, further investigations of potential roles of β-defensins in the epididymis may shed new light on our understanding of sperm maturation and reveal the therapeutic implications of sperm abnormalities.
Recently, the epididymis of Sprague-Dawley rat was microdissected into 19 intraregional segments [23]. According to the expression levels of β-defensins in each individual section, Defb15 was singled out among all family members for its higher relative abundance across the epididymis. The Defb15 gene (NM_001037520) is located on chromosome 16q12.5, and it has 75% homology with human β-defensin 6 (DEFB106, NP_001035794) and 81% homology with mouse β-defensin 15 (mDefb15, NP_631968) at the protein level (Fig. S1). Although the caput region-specific expression pattern of Defb15 mRNA has been examined by RT-PCR [10], little is known about the function of this protein. In our current study, the recombinant Defb15 protein was demonstrated to be a potent antimicrobial agent. Moreover, we prepared the sensitive and specific antibody against Defb15, and the location of Defb15 on spermatozoa and its role in maintaining sperm motility was characterized. Furthermore, by using RNAi in vivo, knock down of Defb15 gene expression in the rat epididymis provided direct evidence that Defb15 is a novel and critical molecule in the regulation of sperm motility and male fertility.
Materials and methods
Animals
Healthy Sprague-Dawley rats and New Zealand white rabbits were supplied by the Animal Center of the Chinese Academy of Sciences (Shanghai, China). Animals were housed under standard laboratory conditions. Experiments were conducted according to a protocol approved by the Institute Animal Care Committee. The protocol conformed to internationally accepted guidelines for the humane care and use of laboratory animals.
Androgen regulation and developmental change of Defb15
Castration and androgen replacement were conducted following a previously described protocol [24]. For the developmental pattern analysis, the epididymides of normal male rats were sampled at the ages of 15, 30, 45, 60, 90, 120, 270, 360 and 720 days. The epididymides of each group were excised and mixed for real-time PCR analysis (Qiagen, Valencia, CA).
Production of antibody and immunoblotting of Defb15
To raise the specific antibody to Defb15, we adopted the double-copy protein expression scheme described by Xiao et al. [25] in detail. Two Defb15 fragments corresponding to the mature Defb15 peptide of 59 amino acids (from amino acid 33 to the end) were inserted into pET-28(a) vector (Invitrogen). The recombinant protein was expressed and purified using a Novagen pET Expression Systems kit (Invitrogen). The polyclonal antisera against Defb15 were obtained and purified according to previous methods [26], and the preimmune sera were also purified as negative control following the same protocol. Western analysis, immunohistochemical staining of tissues and indirect immunofluorescence detection of proteins associated with spermatozoa were conducted following a previously described protocol [24]. Lectin peanut agglutinin (PNA) conjugated with Alexa Fluor 488 (diluted 1:500; Molecular Probes) was used to monitor the acrosomes of the spermatozoa.
Cell culture and siRNA transfection
Two potential siRNAs of 21 nucleotides (nt) specific to Defb15 mRNA (15si1 and 15si2) were selected empirically with a design tool incorporating standard parameters [27], and siRNA targeting enhanced green fluorescent protein (EGFP) was used as control for sequence specificity (Csi). The sense strands of siRNAs were: 15si1 (sequence 1), beginning at nt 124, 5′-AATGGGAGATGCACAGAATCT-3′; 15si2 (sequence 2), beginning at nt 226, 5′-AAGGGCGACGAGTTGGATGAA-3′; and Csi, beginning at nt 106, 5′-GGCGATGCCACCTACGGCAAG-3′. To examine the silencing effect of siRNAs, psiRNA vectors producing shRNAs targeting Defb15 were constructed according to the kit instructions (Invivogen), then the psiRNA constructs and a pcDNA 3.0 vector expressing Defb15 (mass ratio 1:5) were cotransfected into the mouse epididymal epithelial cells using Lipofectamine 2000 (Invitrogen). The knock-down efficiency was determined by real-time PCR and western blotting of extracts from transfected cultures 48 h after transfection.
Lentiviral vector production and injection
Lentiviral particles were produced by transient cotransfection of 293T cells by pRNAi/Lenti vectors (HaiGene, Harbin, China), an encapsidation plasmid (p8.9), and a vesicular stomatitis virus (VSV) expression plasmid as previously described [28, 29]. The surgical procedures were performed according to a previously described method with modification [30]. Briefly, 1×106 transducing units of shRNA lentivirus (the viral titer was 4×107 transducing units per milliliter) was injected into the interstitial space of the caput epididymis, Defb15-shRNAs lentivirus (15si1 and 15si2) were delivered into the left epididymis and the right epididymis was treated with EGFP-shRNA lentivirus (Csi). The total RNA and protein of the caput epididymis and the spermatozoa were collected for assessment 7 days after injection.
Sperm motility analysis
We examined the sperm motility in the epididymis by computer-assisted semen analysis using sperm motility analyzer (HTM-TOXIVOS; Rat Head Toxicology, version 12.3A; Hamilton-Thorn Research, MA) [30].
Evaluation of sperm capacitation and the acrosome reaction
We applied the protocol of protein tyrosine phosphorylation and the chlortetracycline (CTC) staining assay as used in previous studies [30, 31] to assess sperm capacitation and spontaneous acrosome reaction. For the induction of acrosome reaction, spermatozoa were preincubated in the capacitation medium for 5 h, exposed to 15 μM progesterone or its solvent (DMSO; maximal concentration 1%) as control; and further incubated for 30 min. At the end of the incubations, samples were stained with CTC to assess the acrosome reaction rate.
Fertility assay
We carried out in vitro fertilization (IVF) following a standard protocol [32]. After a 48-h incubation, eggs were examined for the presence of two-cell-stage embryos as an indication of successful fertilization. The mating experiments were performed following our modified protocol [30]. Each male rat was mated with two normal females in succession 7 days after injection of the same lentivirus into the bilateral epididymides (the treated group was injected with Defb15-shRNA lentivirus; the control group with EGFP-shRNA lentivirus). The mated male rats were sampled for determining the knock-down efficiency. At 18 days the pregnant females were subjected to hysterectomy to determine the number of embryos or fetuses. After birth, the number, viability and size of the offspring in a litter were recorded.
Soluble recombinant β-defensin production and antibacterial assay
An intein-mediated expression and purification system was used to produce the recombinant β-defensins [33]. The expression plasmids were constructed by insertion of putative sequences of mature β-defensins into the pTWIN1 vector (NEB) between NcoI/XhoI sites. Protein expression was induced with 0.5 mM IPTG at 22°C for 6 h, and the optimal pH for cleavage was 5.0. Colony-forming unit (CFU) assays were employed to test the antibacterial activity as described previously [33, 34]. The elution buffer of the recombinant peptides with the same volume and pH was used as control in the antimicrobial tests.
Statistical analysis
Statistical analysis was performed using the SigmaPlot 10.0. P values were calculated using the standard t-test, and differences with a P value <0.05 were considered statistically significant.
Results
Characteristics of Defb15 mRNA expression
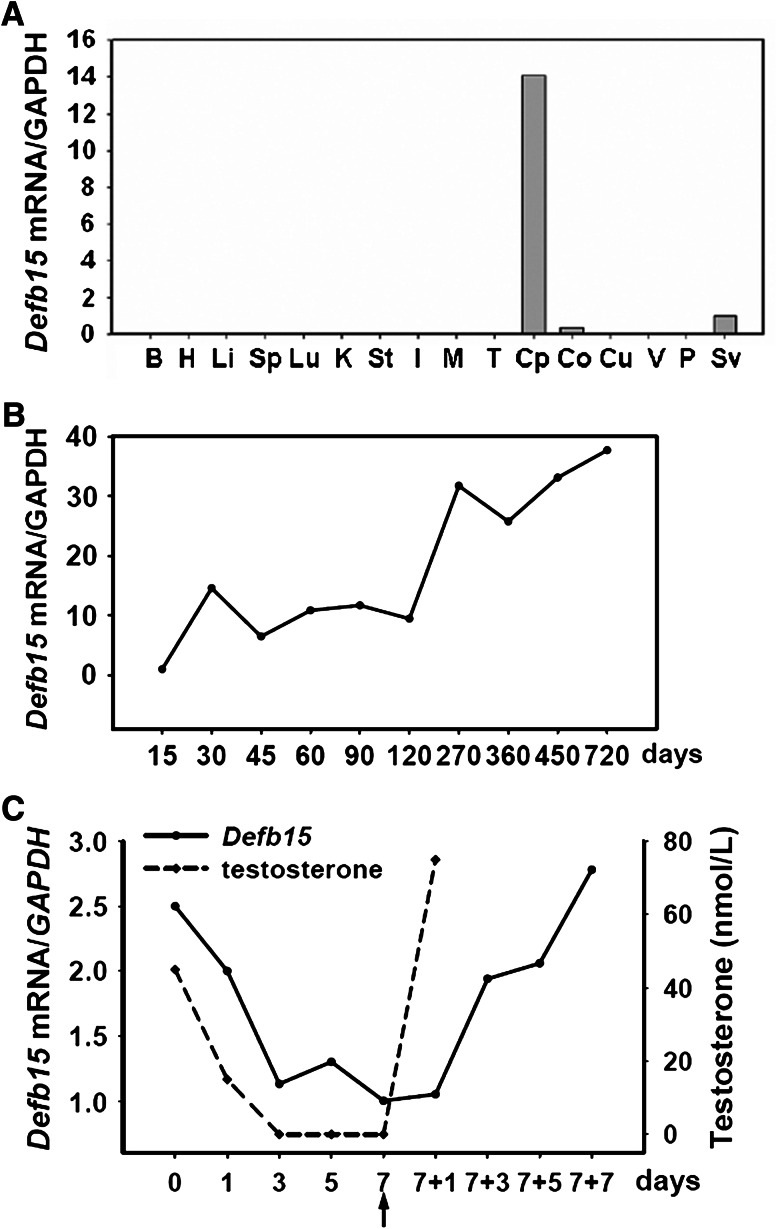
Real-time PCR analysis revealed intense expression of Defb15 mRNA in the caput epididymis (Fig. 1a), which is consistent with a previous report [10]. Monitoring Defb15 expression throughout the life-span of rats indicated that it was developmentally regulated (Fig. 1b). Its expression started at 15 days of age, reached a peak at 1 month, remained at a stable level in mature animals, and increased dramatically in aged rats. Moreover, the expression of Defb15 mRNA was upregulated by androgen in vivo as are some epididymis-specific genes reported previously [34, 35]. In castrated animals, the abundance of Defb15 mRNA in the epididymis decreased as serum testosterone levels declined rapidly. Testosterone replacement in the animals 7 days after castration resulted in a rapid increase in Defb15 mRNA levels (Fig. 1c).
Fig. 1.
Expression and regulation of Defb15 mRNA. a Real-time PCR analysis of Defb15 gene expression in various tissues, including the brain (B), heart (H), liver (Li), spleen (Sp), lung (Lu), kidney (K), stomach (St), intestine (I), muscle (M), testes (T), caput (Cp), corpus (Co), cauda (Cu), vas deferens (V), prostate (P), and seminal vesicle (Sv). b Relative amounts of Defb15 mRNA (real-time PCR) in rat epididymis at different developmental stages (n = 3). c Relative expression levels of Defb15 mRNA (real-time PCR) in the rat epididymis and the serum testosterone levels (expressed as nanomoles per liter) during androgen manipulation (n = 4–7). The black arrow indicates the time of androgen supplementation. GAPDH was used as internal control in a–c
Native status and localization of Defb15 protein in rat epididymis
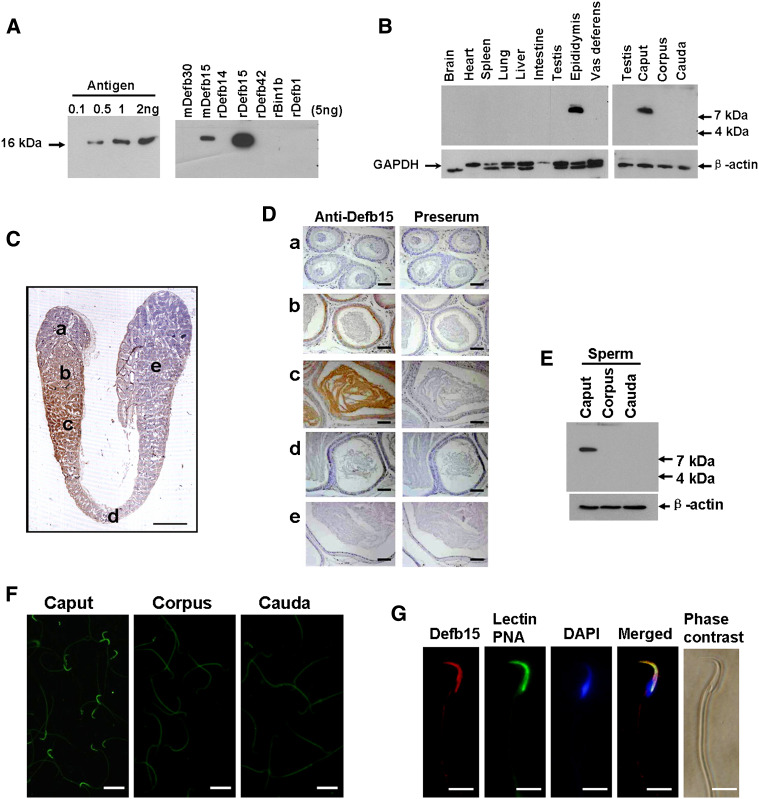
With the aid of the sensitive and specific polyclonal antibody against mature Defb15 protein (Fig. 2a), a distinct single band of approximately 8 kDa was detected in the caput but not in other portions of the epididymis or other tissues tested (Fig. 2b). Immunohistochemistry further confirmed that the positive signal was confined to the distal caput epididymis and was also present in the lumen (Fig. 2c, d). We next investigated whether Defb15 protein could interact with spermatozoa since it was subsequently secreted into the luminal fluid. Defb15 immunoreactivity was only found in the caput sperm (Fig. 2e), and indirect immunofluorescence staining further showed that the prominent signals of Defb15 sperm binding were concentrated in the acrosomal region of the caput sperm surface (Fig. 2f, g).
Fig. 2.
Localization of Defb15 protein in rat epididymis. a The sensitivity and specificity of rabbit polyclonal antibody towards Defb15 were verified by western blotting. b Protein extracts from various tissues were analyzed by western blotting analysis. GAPDH and β-actin were used to assess protein loading. c Immunohistochemical staining shows the expression pattern of Defb15 protein in the whole epididymis in a 120-day-old rat (bar 4 mm). d Magnified photographs of individual fields of c: a initial segment; b, c caput; d corpus; e cauda. Preimmune serum under the same conditions showed the background level of immunoreactivity (bars 50 μm). e Western blot analysis of Defb15 protein in total protein extracts of sperm from the caput, corpus and cauda regions of the epididymis. β-Actin was used for loading control. f Localization of Defb15 protein (FITC-labeled) on the spermatozoa by indirect immunofluorescence assays. Positive Defb15 immunoreactivity is seen at the head of sperm from the caput region, but no signal is seen on sperm from the corpus or cauda regions of the epididymis (bars 10 μm). g Refined localization of Defb15 (TRITC-labeled) on the caput sperm surface. The acrosome of the sperm was stained by Alexa Fluor 488-conjugated lectin PNA, and sperm DNA was stained with DAPI (bars 5 μm)
Defb15 protein is involved in maintaining sperm motility in vitro
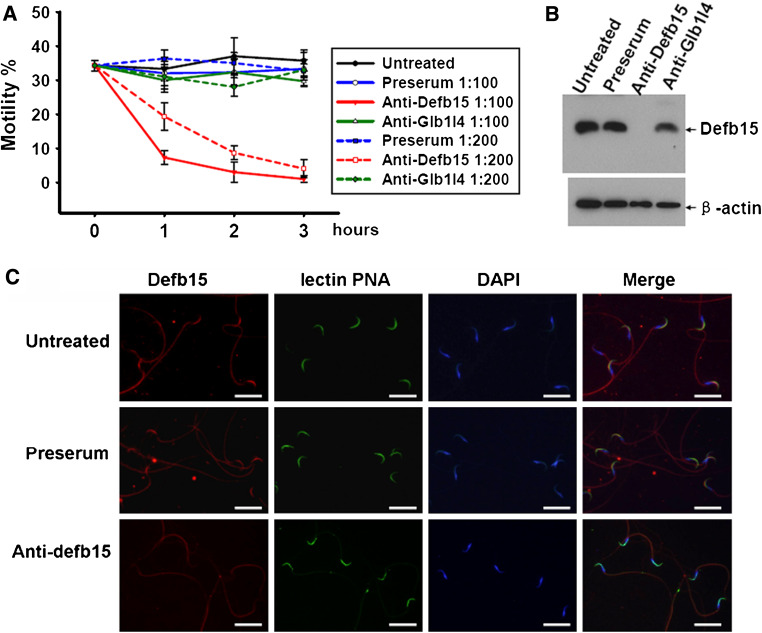
The caput region-specific localization and sperm-binding pattern of Defb15 indicate its importance in certain sperm functions during epididymal transit, since the microenvironment of the caput epididymis has been shown to be vital for sperm maturation [36]. We tested the possible role of Defb15 in sperm motility by incubating spermatozoa collected from the distal caput epididymis with the antibody in vitro. To rule out nonspecific interferences, preimmune serum and rabbit polyclonal antibody against Glb1l4 protein specifically expressed in the caput region and having no influence on sperm motility [26] were used as controls. As shown in Fig. 3a, sperm motility of samples was greatly attenuated in the presence of Defb15 antibody, whereas none of the control treatments had any effect on the spermatozoa. Moreover, we found an almost complete loss of Defb15 protein from the sperm incubated with the specific antibody for 3 h (Fig. 3b). The indirect immunofluorescence staining assay further indicated that the acrosomes of antibody-treated spermatozoa were still intact even though the signals of Defb15 had disappeared (Fig. 3c). These results suggest that the lack of Defb15 protein in the caput sperm was associated with a significant decline in sperm motility. We thus consider that Defb15 may be essential in maintaining sperm motility at the caput region of the epididymis where it binds to the sperm.
Fig. 3.
The effect of Defb15 antibody on sperm motility. a Changes in the sperm motility. The treatments with different concentrations of preimmune serum (Preserum), Defb15 antibody (Anti-Defb15), and Glb1l4 antibody (Anti-Glb1l4) were conducted at the time of dilution (0 h), and aliquots of spermatozoa were then taken for recording of motility. The experiment was independently replicated four times. Values are mean ± SEM. b Western blot analysis of Defb15 protein in total protein extracts of caput epididymal sperm with different treatments. β-Actin was used as the internal control. c Immunofluorescence staining of sperm from the caput epididymis treated with preimmune serum and Defb15 antibody. The acrosome of the sperm was stained by Alexa Fluor 488-conjugated lectin PNA, and sperm DNA was stained with DAPI (bars 10 μm)
Defb15 contributes to sperm motility in vivo
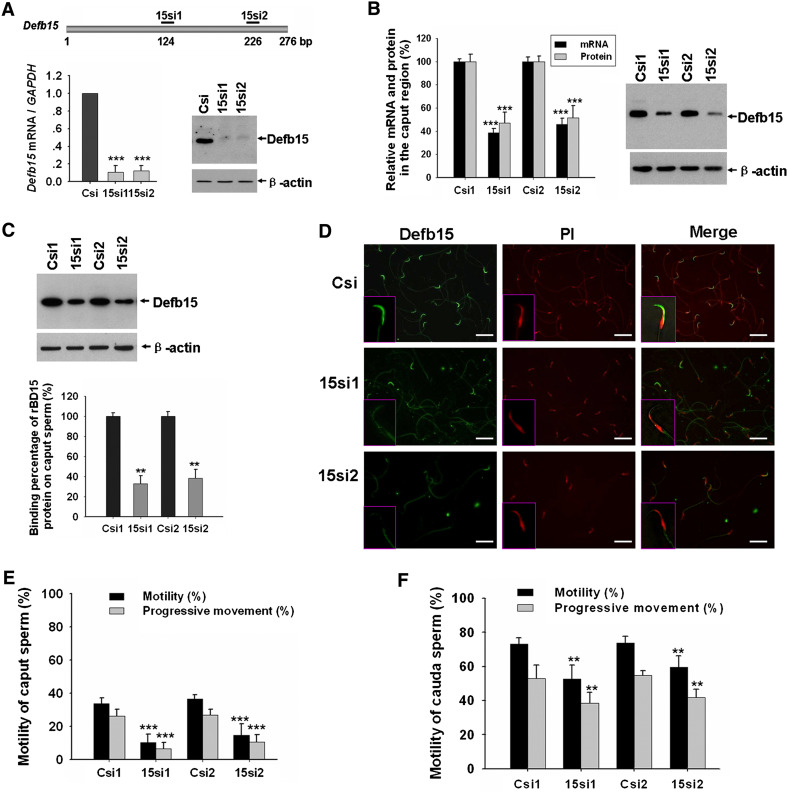
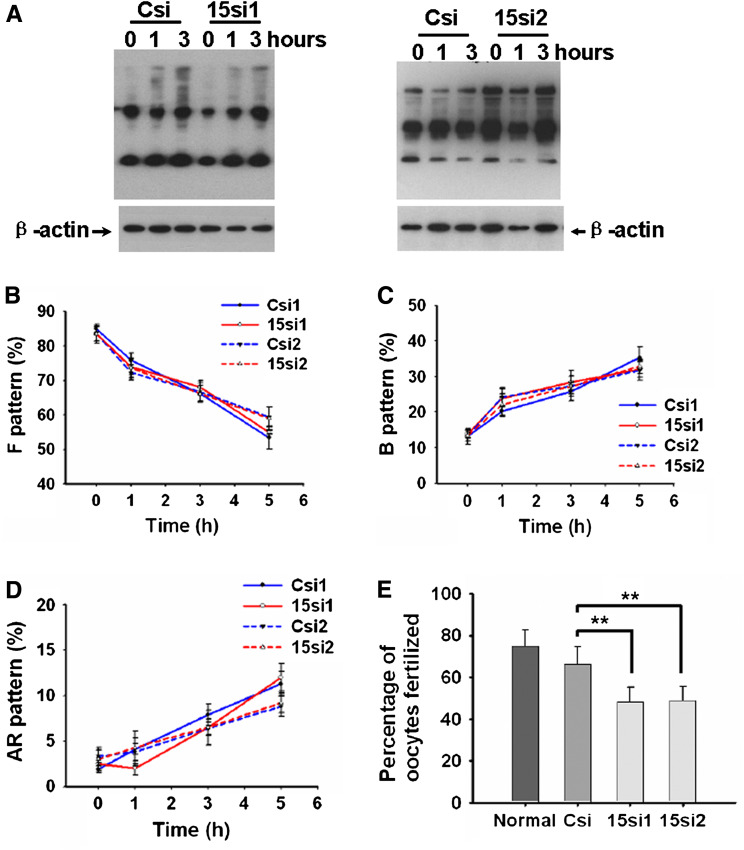
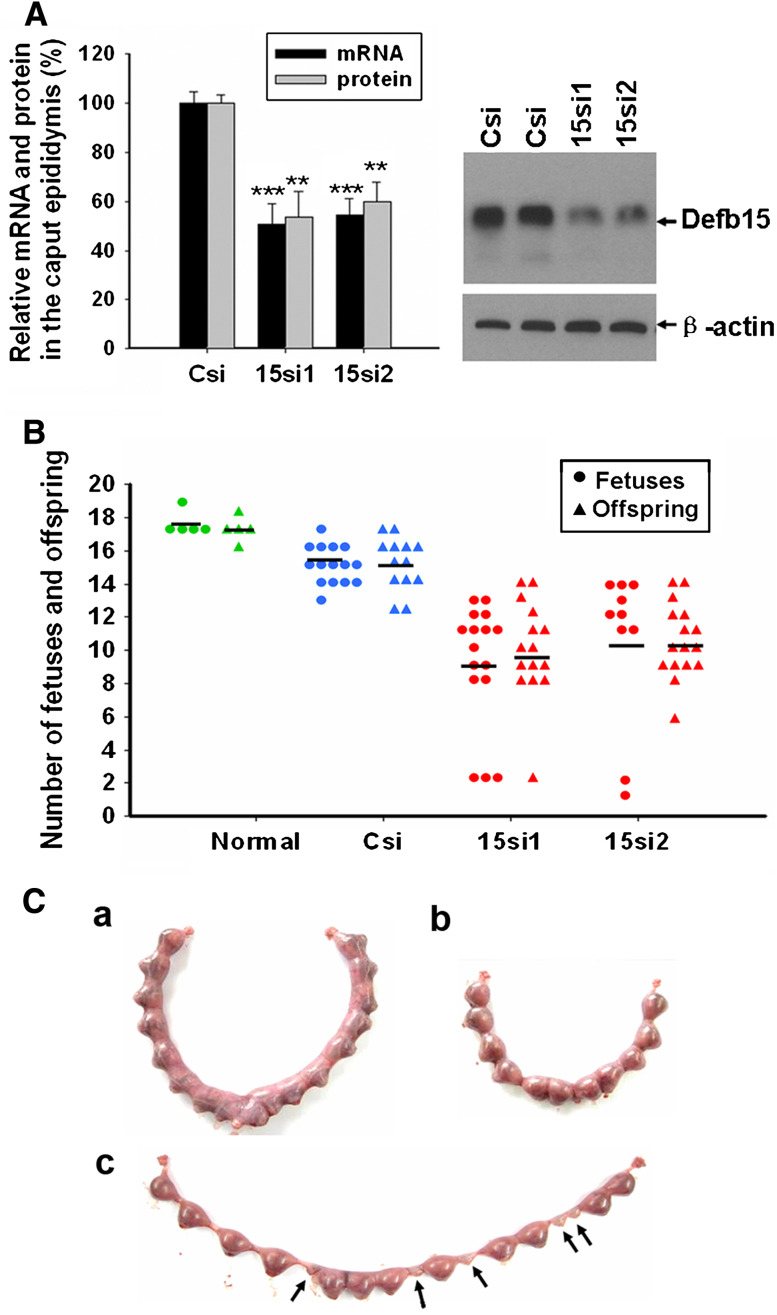
RNAi has been widely applied as a powerful technique for gene silencing in functional studies. Given the advantages over oligonucleotide siRNA, including high transduction efficiency and a stable downregulation of particular genes, we applied lentiviral vector-mediated RNAi to knock down Defb15 gene expression in rat epididymis to confirm its role in the sperm maturation process. According to the RNAi mechanism-based rules [27], two potential siRNAs (15si1 and 15si2) targeting the different site of Defb15 gene were designed and a siRNA (Csi) of EGFP was used as control for silencing specificity. Figure 4a shows that both 15si1 and 15si2 were able to inhibit Defb15 expression almost completely at the mRNA and protein levels in the mouse epididymal epithelial cell line. Lentiviral particles which generated the effective shRNAs were produced and locally injected into the caput epididymides. As shown in Fig. 4b, expression of the Defb15 gene in the caput epididymis was suppressed by about 50–60% at the mRNA and protein levels after 7 days by specific siRNAs treatment. Moreover, western blot analysis indicated a nearly 60% decrease in the Defb15 protein bound to the caput sperm in the Defb15 siRNA-treated male rats (Fig. 4c). Indirect immunofluorescence staining also confirmed the absence of Defb15 signals in partial caput spermatozoa (Fig. 4d). As expected, the reduction in Defb15 expression and sperm binding led to significant declines in total and progressive sperm motility both in caput and cauda spermatozoa (Fig. 4e, f). These results confirm that Defb15 plays a crucial role in maintaining sperm motility.
Fig. 4.
Knock down of Defb15 expression by RNAi and its effect on sperm motility. a Real-time PCR and western blot analysis show reduced expression of Defb15 mRNA and protein in vitro 48 h after siRNA treatment. Data are expressed as mean ± SEM (n = 3; ***P < 0.001 vs. control). GAPDH and β-actin were used as the internal control for RNA and protein loading. b Expression of Defb15 was significantly suppressed by 15si1 and 15si2 in the caput region of the rat epididymis at the mRNA and protein levels. Data are expressed as mean ± SEM (n = 16–18; ***P < 0.001 vs. control). GAPDH and β-actin were used as a loading controls. c Corresponding binding percentages obtained by western blot analysis. Data are expressed as mean ± SEM (n = 12; **P < 0.01 vs. control). β-Actin was used as the internal control. d Immunofluorescence staining of sperm from the caput epididymis treated with control siRNA and two specific siRNAs (bars 10 μm). e, f Total and progressive motility of spermatozoa in the caput (e) and cauda region (f) after Defb15 expression had been downregulated. Data are expressed as mean ± SEM (n = 20–24; ***P < 0.001, **P < 0.01 vs. control). In a–f: Csi (Csi1 and Csi2), EGFP siRNA control; 15si1 and 15si2, two siRNAs specifically targeting the different sites of Defb15 sequence
Knock down of Defb15 expression affects the sperm–egg interaction
Capacitation represents the completion of sperm maturation that confers on mammalian sperm fertilization competence. It is widely accepted that tyrosine phosphorylation of sperm proteins is an important indicator of whether the capacitation signaling cascade has been activated [37]. There was no obvious dissimilarity in the protein tyrosine phosphorylation of caudal sperm from Defb15 siRNA-treated and control rats (Fig. 5a). This is consistent with the observation with CTC staining (Fig. 5c), another indicator for evaluating the acquisition of capacitation state. Additionally, the acrosome reaction rates were further determined by the CTC assay. There was no marked difference in the CTC staining patterns displayed by spermatozoa undergoing spontaneous and progesterone-stimulated acrosome reactions between control and treated groups (Fig. 5d; Fig. S2). Although the knock down of Defb15 might have no influence on sperm capacitation and the acrosome reactions, IVF assay showed that the fertilization rate of the cauda sperm in Defb15 siRNA-treated rats was much lower than that of the control groups (Fig. 5e).
Fig. 5.
Assessment of sperm capacitation, acrosome reaction and IVF of Defb15 knock-down male rats. The spermatozoa of the caudal epididymis from rats treated with siRNAs for 7 days were collected and incubated. Aliquots of spermatozoa were collected for protein tyrosine phosphorylation and CTC assays at different time points during incubation. a The pattern of protein tyrosine phosphorylation on caudal sperm after Defb15 expression is inhibited. β-Actin was used as loading control. b–d The changes in the sperm state of uncapacitated sperm (F pattern, b), capacitated sperm (B pattern, c), and spontaneous acrosome-reacted sperm (AR pattern, d) in the Defb15 siRNA-treated and control groups. The experiment was independently replicated three times. Values are mean ± SEM. e IVF assay of the sperm in Defb15 knock-down rats. Data are expressed as mean ± SEM (n = 6; **P < 0.01 vs. corresponding control). In a–e: Csi (Csi1 and Csi2), EGFP siRNA control; 15si1 and 15si2, two siRNAs specifically targeting the different sites of Defb15 sequence
Reduced fertility of Defb15 gene knock-down rats
Finally, we performed mating experiments to evaluate the fertility of Defb15 downregulated male rats. As shown in Fig. 6b, the numbers of live offspring and normal fetuses were considerably reduced in the litters from receptive female rats that had mated with specific siRNAs-treated male rats, compared with the numbers in the normal and control groups. Moreover, we observed some abnormal fetuses in the treated groups; Fig. 6c shows a representative example. All 17 embryos implanted in the uterus of the female rat mated with the control male rat were normal (Fig. 6c, a), whereas five fetuses appeared to be regressing in Defb15 siRNA-treated group, indicating embryonic development failure (Fig. 6c, c). These data confirm that Defb15 is essential in the sperm maturation process, and the sperm lacking Defb15 binding in the caput region seems to be the cause of fertility reduction and embryonic development failure.
Fig. 6.
Fertility of Defb15 knock-down male rats. a Relative expression levels of Defb15 including mRNA and protein in the caput region of the epididymis from all successfully mated rats after RNAi treatment. Data are expressed as the mean ± SEM (n = 12–16; ***P < 0.001, **P < 0.01 vs. corresponding control). Csi, EGFP siRNA control; 15si1 and 15si2, two siRNAs specifically targeting the different sites of Defb15 sequence. b Numbers of normal fetuses (circles) and live offspring (triangles) from normal female rats mated with normal, control (Csi) and knock-down (15si1, 15si2) male rats; horizontal lines indicate the averages. c Examples of implantation in the control and Defb15 siRNA-treated groups. There are 17 and 11 normal embryos in the control (a) and treated (b) groups, respectively. Abnormal embryos (c, arrows) appeared in the uterus of a female rat mated with a Defb15 siRNA-treated male rat
Antimicrobial activity of recombinant Defb15 protein
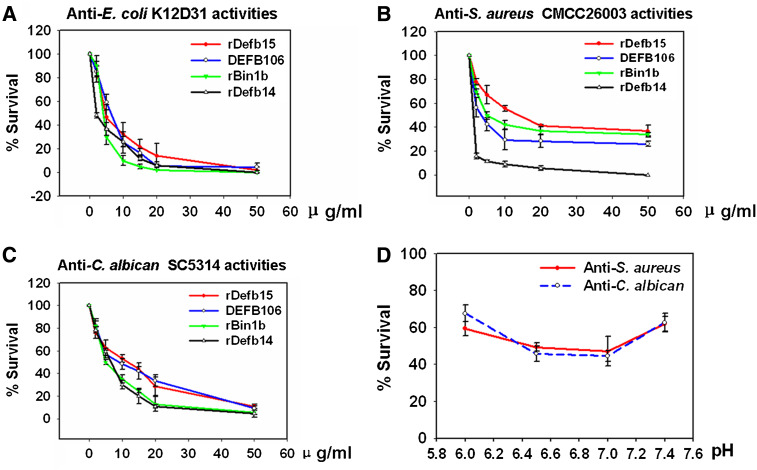
The antimicrobial properties of β-defensins have been well documented and reviewed [16]. To determine whether Defb15 also possesses microbicidal activity, we prepared some recombinant β-defensin peptides, including Defb15, Defb14 (NP_001032593.1), Bin1b [32] and DEFB106, using an intein-mediated expression and purification system (Fig. S3A). Circular dichroism spectra indicated that they all exhibited certain secondary structures (Fig. S3B). Figure 7a–c shows the antimicrobial activities of these recombinant proteins against three pathogens. By comparison, Defb15 tended to be more active against Escherichia coli and Candida albicans than against Staphylococcus aureus; whereas DEFB106, the human orthologue of Defb15, exhibited more powerful resistance to S. aureus.
Fig. 7.
Antimicrobial activities of recombinant β-defensins. a–c Dose effects of recombinant β-defensins on antimicrobial activities. E. coli K12D31 (a), S. aureus CMCC26003 (b) and C. albicans sc5314 (c) were incubated in 10 mM phosphate buffer (pH 7.4) in the presence of recombinant peptides for 2 h (E. coli and S. aureus at 37°C; C. albicans at 30°C). Incubation with the same volume of elution buffer was used as control in the antimicrobial tests. d Antimicrobial activities of Defb15 at various pH values (pH 6.0, 6.5, 7.0 and 7.5). Incubation with the elution buffer of recombinant Defb15 with the same volume and pH value was used as control. The number of CFU was determined by colony counts after serial dilutions. The experiments were independently replicated four times. Values are mean ± SEM
Discussion
Although the role of the epididymis in sperm maturation has been firmly established for nearly four decades [1, 3], the molecular basis for this process remains largely unknown. Our studies identified Defb15 as a new molecule directly linked to sperm maturation. Defb15 is a rat caput region-specific peptide with a homologue in humans (DEFB106), and it can bind in a secreted form to the caput sperm acrosome. In Defb15 gene knock-down male rats, the reduced binding of Defb15 protein to caput sperm led to a considerable attenuation of sperm motility. Our previous study has demonstrated that Bin1b, another caput region-specific β-defensin, is crucial in inducing sperm motility [19]. Taking into account the potential interaction among β-defensin members, we questioned whether the loss of sperm motility was entirely due to the effect of Defb15. When Defb15 was notably downregulated, there were no significant changes in the mRNA level of 11 β-defensins expressed predominantly in the caput epididymis [10] and the sperm-binding pattern of Bin1b protein (Fig. S4). Thus we excluded the involvement of other subfamily members in maintaining the sperm motility.
Moreover, our results showed that Defb15 binds to the sperm exclusively in the caput region. Despite the absence of Defb15 protein, the total and progressive motility of caudal sperm in Defb15 siRNA-treated male rats was also decreased (Fig. 4f), but it seemed less significant than in caput sperm (P < 0.01 in caudal sperm, P < 0.001 in caput sperm). Based on the previous research, the essential elements of sperm maturation may be “setting a series of triggers” for a sequence of cellular changes, and also “setting a safety” for each trigger to prevent inappropriate occurrence of the event [36]. Accordingly, we developed the following hypothesis that a series of molecules are involved in the achievement of sperm motility in the epididymis, and they form a long distance relay. It might be conceivable that some other proteins secreted by the epithelia of the corpus and cauda regions modulate the spermatozoa in succession to gain complete motility. As a result, the motility defect in Defb15 siRNA-treated rats could be partially rescued when poorly motile spermatozoa in the caput travel through the corpus and cauda epididymis. Further studies are needed to elucidate the molecular mechanisms by which Defb15 maintains sperm motility. Examination of target proteins of Defb15 in the sperm and related signaling pathways may contribute to addressing this issue.
Capacitation is a unique feature of mammalian sperm that occurs in the female tract and is essential for fertilization. During capacitation, spermatozoa acquire hyperactive motility and the ability to undergo a regulated acrosome reaction. Despite the fact that Defb15 has no effect on sperm capacitation and acrosome reaction (Fig. 5), results of IVF and mating experiments indicated a reduced fertilization rate and fertility in Defb15 gene knock-down rats. This supports the importance of Defb15 in maintaining normal fertilization. Since sperm motility facilitates the passage of sperm through the zona pellucida, it can be thought of as an indispensable attribute of the sperm in achieving a successful pregnancy. The decrease in progressive motility in caudal sperm may interfere with their ability to fertilize oocytes and appears to be the cause of the fertility reduction. Remarkably, embryonic development failure was observed in Defb15 siRNA-treated groups (Fig. 6c). We speculated that knock down of Defb15 might cause some changes in the essential attributes of spermatozoa besides the forward motility, and these changes to some extent influence fertility and developmental potential of embryos. It has been reported that the degree of DNA damage is clearly correlated with the impairment of embryo development and severe DNA damage causes male infertility [38, 39]. We tested whether knock down of Defb15 induced an inflammatory reaction in the epididymis and sperm DNA damage. As revealed in Fig. S5, downregulation of Defb15 expression in vivo did not cause changes in the expression of several inflammatory cytokines (e.g. TNF-α, IL-1β, IL-6 and IFN-γ) or the extent of spermatozoal DNA damage. We are trying to investigate other changes in sperm quality in Defb15 knock-down rats which might account for reduced fertility and abnormal fetuses.
Other questions arise from our data related to the effect of Defb15 on sperm motility. In spite of the reduced proportion of motile sperm, no statistically significant changes in other sperm motion variables were observed in Defb15 siRNA-treated rats (data not shown). This suggests that the velocity and movement pattern of motile sperm are not affected when Defb15 is downregulated. Moreover, successful knock-down of about 50% in Defb15 gene expression in vivo led to distinct sperm-binding patterns of Defb15 protein (Fig. 4d). Some spermatozoa were not attached to Defb15 protein, while others were still bound to Defb15. These differences may be associated with various phenotypes in the subsequent studies. However, it is difficult to separate these varied types of sperm to investigate their individual movement pattern and their roles in fertilization. Hence, our future work will concentrate on the generation of Defb15 conditional knock-out mice to reveal the phenotype associated with those spermatozoa with no Defb15 binding.
At present, overwhelming evidence is accumulating that a number of epididymal β-defensins function as important effectors by providing a rapid and broad response to invading microorganisms [5–7, 11, 16, 33, 34]. Based on our results, an additional function of Defb15 can be envisioned in sperm protection since the recombinant Defb15 peptide possessed distinct and dose-dependent activity against E. coli, S. aureus and C. albicans. It is well known that the pH value and salt concentration of epididymal luminal fluid are lower than in the physiological environment [40, 41], and many studies have shown that most β-defensins lose their activity at higher sodium concentrations [6, 15, 33]. To explore whether β-defensin activity is also correlated with the degree of acidity, we examined the capacity of Defb15 to inhibit S. aureus and C. albicans at various pH values. In accordance with the localization in distal caput region, Defb15 exerted a more powerful effect at pH 6.5 and 7.0 (Fig. 7f). We are now trying to express and characterize more β-defensins of rat epididymal origin. Antimicrobial activity analyses of these peptides under simulated in vivo conditions will reveal their roles in innate immunity. There may also exist synergistic or additive effects among β-defensins clustered in the epididymis [42]. Different combinations of these agents may provide further evidence of their collaboration in microbial killing. A strategy to mimic or enhance their activities may be developed to address the increasing need for new therapeutic agents for sexually transmitted diseases.
Collectively, within the epididymis, Defb15 not only acts as a component of the immune system, but is clearly involved in the sperm maturation process by maintaining the motility of caput sperm. Determination of the molecular events by which relatively immotile quiescent sperm become incapable of fertilization may aid in the development of treatments for motility-related clinical infertility and the development of improved contraceptives.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Aihua Liu for tissue section preparation and technical assistance in examining vaginal smears of female rats, and Drs. Yuchan Zhou and Wuzi Dong for advice concerning RNAi. This work was supported by grants from the National Basic Science Research and Development Project of China (2006CB504002 and 2006CB944002), the Chinese Academy of Sciences Knowledge Innovation Program (KSCX1-YW-R-54, KSCX2-YW-R-54), and the National Natural Science Foundation Key Project of China (30930053).
References
- 1.Bedford JM. Fertile life of rabbit spermatozoa in rat uterus. Nature. 1967;213:1097–1099. doi: 10.1038/2131097a0. [DOI] [PubMed] [Google Scholar]
- 2.Jones RC. To store or mature spermatozoa? The primary role of the epididymis. Int J Androl. 1999;22:57–67. doi: 10.1046/j.1365-2605.1999.00151.x. [DOI] [PubMed] [Google Scholar]
- 3.Orgebin-Crist MC. Sperm maturation in rabbit epididymis. Nature. 1967;216:816–818. doi: 10.1038/216816a0. [DOI] [PubMed] [Google Scholar]
- 4.Li P, Chan HC, He B, So SC, Chung YW, Shang Q, Zhang YD, Zhang YL. An antimicrobial peptide gene found in the male reproductive system of rats. Science. 2001;291:1783–1785. doi: 10.1126/science.1056545. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi Y, Nagase T, Makita R, Fukuhara S, Tomita T, Tominaga T, Kurihara H, Ouchi Y. Identification of multiple novel epididymis-specific beta-defensin isoforms in humans and mice. J Immunol. 2002;169:2516–2523. doi: 10.4049/jimmunol.169.5.2516. [DOI] [PubMed] [Google Scholar]
- 6.Yenugu S, Hamil KG, Birse CE, Ruben SM, French FS, Hall SH. Antibacterial properties of the sperm-binding proteins and peptides of human epididymis 2 (HE2) family; salt sensitivity, structural dependence and their interaction with outer and cytoplasmic membranes of Escherichia coli. Biochem J. 2003;372:473–483. doi: 10.1042/BJ20030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yenugu S, Hamil KG, Radhakrishnan Y, French FS, Hall SH. The androgen-regulated epididymal sperm-binding protein, human beta-defensin 118 (DEFB118) (formerly ESC42), is an antimicrobial beta-defensin. Endocrinology. 2004;145:3165–3173. doi: 10.1210/en.2003-1698. [DOI] [PubMed] [Google Scholar]
- 8.Com E, Bourgeon F, Evrard B, Ganz T, Colleu D, Jegou B, Pineau C. Expression of antimicrobial defensins in the male reproductive tract of rats, mice, and humans. Biol Reprod. 2003;68:95–104. doi: 10.1095/biolreprod.102.005389. [DOI] [PubMed] [Google Scholar]
- 9.Avellar MC, Honda L, Hamil KG, Yenugu S, Grossman G, Petrusz P, French FS, Hall SH. Differential expression and antibacterial activity of epididymis protein 2 isoforms in the male reproductive tract of human and rhesus monkey (Macaca mulatta) Biol Reprod. 2004;71:1453–1460. doi: 10.1095/biolreprod.104.031740. [DOI] [PubMed] [Google Scholar]
- 10.Patil AA, Cai Y, Sang Y, Blecha F, Zhang G. Cross-species analysis of the mammalian beta-defensin gene family: presence of syntenic gene clusters and preferential expression in the male reproductive tract. Physiol Genomics. 2005;23:5–17. doi: 10.1152/physiolgenomics.00104.2005. [DOI] [PubMed] [Google Scholar]
- 11.Lin YQ, Li JY, Wang HY, Liu J, Zhang CL, Wang WT, Liu J, Li N, Jin SH. Cloning and identification of a novel sperm binding protein, HEL-75, with antibacterial activity and expressed in the human epididymis. Hum Reprod. 2008;23:2086–2094. doi: 10.1093/humrep/den084. [DOI] [PubMed] [Google Scholar]
- 12.O’Rand MG, Widgren EE, Sivashanmugam P, Richardson RT, Hall SH, French FS, VandeVoort CA, Ramachandra SG, Ramesh V, Jagannadha Rao A. Reversible immunocontraception in male monkeys immunized with eppin. Science. 2004;306:1189–1190. doi: 10.1126/science.1099743. [DOI] [PubMed] [Google Scholar]
- 13.Ganz T, Selsted ME, Lehrer RI. Defensins. Eur J Haematol. 1990;44:1–8. doi: 10.1111/j.1600-0609.1990.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 14.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 15.Kluver E, Adermann K, Schulz A. Synthesis and structure-activity relationship of beta-defensins, multi-functional peptides of the immune system. J Pept Sci. 2006;12:243–257. doi: 10.1002/psc.749. [DOI] [PubMed] [Google Scholar]
- 16.Pazgier M, Hoover DM, Yang D, Lu W, Lubkowski J. Human beta-defensins. Cell Mol Life Sci. 2006;63:1294–1313. doi: 10.1007/s00018-005-5540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 18.Klotman ME, Chang TL. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6:447–456. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- 19.Zhou CX, Zhang YL, Xiao L, Zheng M, Leung KM, Chan MY, Lo PS, Tsang LL, Wong HY, Ho LS, Chung YW, Chan HC. An epididymis-specific beta-defensin is important for the initiation of sperm maturation. Nat Cell Biol. 2004;6:458–464. doi: 10.1038/ncb1127. [DOI] [PubMed] [Google Scholar]
- 20.Yudin AI, Tollner TL, Li MW, Treece CA, Overstreet JW, Cherr GN. ESP13.2, a member of the beta-defensin family, is a macaque sperm surface-coating protein involved in the capacitation process. Biol Reprod. 2003;69:1118–1128. doi: 10.1095/biolreprod.103.016105. [DOI] [PubMed] [Google Scholar]
- 21.Tollner TL, Yudin AI, Treece CA, Overstreet JW, Cherr GN. Macaque sperm release ESP13.2 and PSP94 during capacitation: the absence of ESP13.2 is linked to sperm–zona recognition and binding. Mol Reprod Dev. 2004;69:325–337. doi: 10.1002/mrd.20132. [DOI] [PubMed] [Google Scholar]
- 22.Schutte BC, Mitros JP, Bartlett JA, Walters JD, Jia HP, Welsh MJ, Casavant TL, McCray PB., Jr Discovery of five conserved beta-defensin gene clusters using a computational search strategy. Proc Natl Acad Sci U S A. 2002;99:2129–2133. doi: 10.1073/pnas.042692699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jelinsky SA, Turner TT, Bang HJ, Finger JN, Solarz MK, Wilson E, Brown EL, Kopf GS, Johnston DS. The rat epididymal transcriptome: comparison of segmental gene expression in the rat and mouse epididymides. Biol Reprod. 2007;76:561–570. doi: 10.1095/biolreprod.106.057323. [DOI] [PubMed] [Google Scholar]
- 24.Zhu CF, Liu Q, Zhang L, Yuan HX, Zhen W, Zhang JS, Chen ZJ, Hall SH, French FS, Zhang YL. RNase9, an androgen-dependent member of the RNase A family, is specifically expressed in the rat epididymis. Biol Reprod. 2007;76:63–73. doi: 10.1095/biolreprod.106.054635. [DOI] [PubMed] [Google Scholar]
- 25.Xiao LQ, Liu AH, Zhang YL. An effective method for raising antisera against beta-defensins: double-copy protein expression of mBin1b in E. coli. Acta Biochim Biophys Sin (Shanghai) 2004;36:571–576. doi: 10.1093/abbs/36.8.571. [DOI] [PubMed] [Google Scholar]
- 26.Zhen W, Li P, He B, Guo J, Zhang YL. The novel epididymis-specific beta-galactosidase-like gene Glb1l4 is essential in epididymal development and sperm maturation in rats. Biol Reprod. 2009;80:696–706. doi: 10.1095/biolreprod.108.071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birmingham A, Anderson E, Sullivan K, Reynolds A, Boese Q, Leake D, Karpilow J, Khvorova A. A protocol for designing siRNAs with high functionality and specificity. Nat Protoc. 2007;2:2068–2078. doi: 10.1038/nprot.2007.278. [DOI] [PubMed] [Google Scholar]
- 28.Zennou V, Serguera C, Sarkis C, Colin P, Perret E, Mallet J, Charneau P. The HIV-1 DNA flap stimulates HIV vector-mediated cell transduction in the brain. Nat Biotechnol. 2001;19:446–450. doi: 10.1038/88115. [DOI] [PubMed] [Google Scholar]
- 29.Santamaria J, Khalfallah O, Sauty C, Brunet I, Sibieude M, Mallet J, Berrard S, Lecomte MJ. Silencing of choline acetyltransferase expression by lentivirus-mediated RNA interference in cultured cells and in the adult rodent brain. J Neurosci Res. 2009;87:532–544. doi: 10.1002/jnr.21866. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Zheng M, Shi Q, Zhang L, Zhen W, Chen W, Zhang Y. An epididymis-specific secretory protein HongrES1 critically regulates sperm capacitation and male fertility. PLoS One. 2008;3:e4106. doi: 10.1371/journal.pone.0004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oberlander G, Yeung CH, Cooper TG. Influence of oral administration of ornidazole on capacitation and the activity of some glycolytic enzymes of rat spermatozoa. J Reprod Fertil. 1996;106:231–239. doi: 10.1530/jrf.0.1060231. [DOI] [PubMed] [Google Scholar]
- 32.Berger T, Miller MG, Horner CM. In vitro fertilization after in vivo treatment of rats with three reproductive toxicants. Reprod Toxicol. 2000;14:45–53. doi: 10.1016/S0890-6238(99)00062-3. [DOI] [PubMed] [Google Scholar]
- 33.Diao H, Guo C, Lin D, Zhang Y. Intein-mediated expression is an effective approach in the study of beta-defensins. Biochem Biophys Res Commun. 2007;357:840–846. doi: 10.1016/j.bbrc.2007.03.149. [DOI] [PubMed] [Google Scholar]
- 34.Yenugu S, Chintalgattu V, Wingard CJ, Radhakrishnan Y, French FS, Hall SH. Identification, cloning and functional characterization of novel beta-defensins in the rat (Rattus norvegicus) Reprod Biol Endocrinol. 2006;4:7. doi: 10.1186/1477-7827-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Q, Hamil KG, Sivashanmugam P, Grossman G, Soundararajan R, Rao AJ, Richardson RT, Zhang YL, O’Rand MG, Petrusz P, French FS, Hall SH. Primate epididymis-specific proteins: characterization of ESC42, a novel protein containing a trefoil-like motif in monkey and human. Endocrinology. 2001;142:4529–4539. doi: 10.1210/en.142.10.4529. [DOI] [PubMed] [Google Scholar]
- 36.Amann RP, Hammerstedt RH, Veeramachaneni DN. The epididymis and sperm maturation: a perspective. Reprod Fertil Dev. 1993;5:361–381. doi: 10.1071/RD9930361. [DOI] [PubMed] [Google Scholar]
- 37.Roberts KP, Wamstad JA, Ensrud KM, Hamilton DW. Inhibition of capacitation-associated tyrosine phosphorylation signaling in rat sperm by epididymal protein Crisp-1. Biol Reprod. 2003;69:572–581. doi: 10.1095/biolreprod.102.013771. [DOI] [PubMed] [Google Scholar]
- 38.Seli E, Gardner DK, Schoolcraft WB, Moffatt O, Sakkas D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil Steril. 2004;82:378–383. doi: 10.1016/j.fertnstert.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 39.Andrabi SM. Mammalian sperm chromatin structure and assessment of DNA fragmentation. J Assist Reprod Genet. 2007;24:561–569. doi: 10.1007/s10815-007-9177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levine N, Kelly H. Measurement of pH in the rat epididymis in vivo. J Reprod Fertil. 1978;52:333–335. doi: 10.1530/jrf.0.0520333. [DOI] [PubMed] [Google Scholar]
- 41.Turner TT, Hartmann PK, Howards SS. In vivo sodium, potassium, and sperm concentrations in the rat epididymis. Fertil Steril. 1977;28:191–194. doi: 10.1016/s0015-0282(16)42382-4. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Niyonsaba F, Ushio H, Okuda D, Nagaoka I, Ikeda S, Okumura K, Ogawa H. Synergistic effect of antibacterial agents human beta-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J Dermatol Sci. 2005;40:123–132. doi: 10.1016/j.jdermsci.2005.03.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.