Abstract
Allergy and anaphylaxis are inflammatory disorders caused by immune reactions mainly induced by immunoglobulin-E that signal through the high-affinity FcεRI receptor to release the inflammatory mediators from innate immune cells. The FcεRI/mast cell axis is potently involved in triggering various intracellular signaling molecules to induce calcium release from the internal stores, induction of transcription factors such as NF-kB, secretion of various cytokines as well as lipid mediators, and degranulation, resulting in the induction of allergy and anaphylaxis. In this review, we discuss various cellular and molecular mechanisms triggered through FcεRI/mast cell axis in allergy and anaphylaxis with a special emphasis on the functional genomics paradigm.
Keywords: Allergy, Anaphylaxis, FcεRI, IgE, Cytokines, Transcription, Factors, Functional, Genomics
Introduction
The term allergy was first used in 1906 by Dr. Von Pirquet, an Austrian physician, to define an Immunoglobulin E (IgE)-mediated or type I hypersensitivity reaction triggered by allergens, which are antigens that bring out an allergic reaction [1]. In 1902, Richet and Portier [2] found that a dog tolerated the administration of sea anemone toxin initially; however, it died within few minutes of administering the second dose many weeks later. They coined the term anaphylaxis, which in Greek, ana means against; phylax means guard or protection. Richet was later awarded the Nobel Prize in Medicine and Physiology in 1913 for his work in anaphylaxis. At present, anaphylaxis refers to a life-threatening IgE-mediated allergic condition characterized by multiple organ involvement and quick onset. Drugs, insect stings, latex, and foods are the common allergens that trigger anaphylactic reactions. Very rarely, death due to anaphylaxis typically results from acute respiratory or heart failure.
Mast cells play a major effector role in the initiation as well as the propagation of various inflammatory disorders such as asthma, psoriasis, arthritis, etc. [3] and are the central effector cells in the mediation of allergy and anaphylaxis [4]. IgE-dependent or IgE-independent mast cell mediated response leads to the activation, synthesis and release of inflammatory mediators in allergy, and anaphylaxis [5–8]. In the past decade, scientists have been applying the microarray technology to explore the fundamental genetic causes of inflammatory conditions [9–13]. Such transcriptomic profiling immensely helps in our critical understanding of the global effects driven by the physiological “passive sensitization” or “active stimulation” of human mast cells to design novel therapeutic strategies to ameliorate and effectively manage both allergy and anaphylaxis.
Immunoglobulins and IgE
Defense against foreign bodies and microorganisms requires the interaction between innate immunity and acquired immunity. Generally, innate and acquired responses are regulated by humoral and cell-mediated responses. The humoral responses are mediated by immunoglobulins or antibodies, which are secreted by B lymphocytes. The immunoglobulins contain a variable Fab portion in the light chains and a constant Fc portion in the heavy chains. The Fab region interacts with a specific antigen and the Fc region binds to effector molecules such as Fc receptors and complement proteins. The ability of immunoglobulins to distinguish between a variety of antigens is due to the result of somatic recombination events, which result in the alteration of their variable regions. Based on the constant Fc region, immunoglobulins are subdivided into five major classes, which includes: IgG, IgM, IgA, IgE, and IgD. The effector molecules, which distinguish these immunoglobulins, are primarily Fc receptors, transport receptors and complement [14]. Every major immunoglobulin class has its own specific Fc receptor (FcR): FcαR binds IgA, FcεR binds IgE, FcγR binds IgG, FcδR binds IgD, and FcμR binds IgM [15–18].
IgE receptors and nomenclature
FcεRI-mediated reactions are the most prevalent form of immune-related diseases among humans. FcεRs play core function in the development of allergic inflammation. There are two types of IgE receptors: the high-affinity receptor (FcεRI) and the low-affinity IgE receptor (FcεRII; CD23) [19]. FcεRI is expressed on mast cells and basophils and on antigen-presenting cells whereas, FcεRII, a Ca-dependent lectin, is expressed on the surface of B cells, as well as other hematopoietic cells including Langerhans cells, macrophages, monocytes, eosinophils, and platelets. CD23, existing as CD23a and CD23b, has been associated with facilitating antigen presentation; it also negatively regulates IgE synthesis; and transports IgE-antigen complexes across epithelium [20]. Both IgE and IL-4 are shown to upregulate expression of CD23 [21].
Unbound FcεRI on the mast cell surface has a half-life of 24 h in vitro. On the other hand, FcεRI bound to IgE seem to be expressed throughout the life of the cell [22]. Aggregation of FcεRI by multivalent antigen results in several downstream intracellular signaling events linked with mast cell or basophil activation [23, 24]. When an allergen interacts with IgE that attached to mast cells or basophils by the α chain of the high-affinity IgE receptor, it will result in the activation of mast cells and release of both stored, as well as newly synthesized mediators (FcεRIa) [25]. Release of these mediators results in local vasodilation, smooth muscle contraction, increased vascular permeability, and the initiation of inflammation. Such hypersensitivity reactions result in clinical manifestations including allergic rhinitis, atopic dermatitis, and anaphylaxis. In antigen-presenting cells (APCs), these receptors facilitate the IgE-mediated trapping and introduction of allergen to T cells [26, 27]. Eosinophils also have FcεRIa, but apart from activation and degranulation, it may aid in regulating local levels of IgE [28].
FcεRI: structure
FcεRI has a K d of over 10−10 M binding affinity with monomeric IgE, which is the strongest when compared to the rest of the other Fc receptors for their ligands [29]. The classical form of FcεRI is tetrameric (αβγ2): constitutively expressed on mast cells and basophils in humans, while the trimeric form (αγ2) is present on the APCs such as monocytes, dendritic cells and Langerhans cells. The classical form consists of an IgE-binding α chain, a β chain, and a homodimeric γ chain. The β chain in FcεRI enhances receptor maturation [30] and the γ-homodimer enhances signal transduction [31]. Studies show that even in the absence of a β chain, the trimeric form of FcεRI possesses complete function [25, 32]. Both the β and γ chains are required for the efficient cellular expression of the FcεRI-α-chain [33]. In addition, studies have shown that αγ2 complexes were translocated to the periphery of the cell, meaning that the human γ chain by itself is sufficient to fight against endoplasmic reticulum retention signals in the α chain [33].
FcεRI effector cells: mast cells and basophils
Mast cells have been considered as the most vital effector cell type for allergic conditions including, to a lesser extent, basophils and neutrophils [34, 35]. The progenitors for mast cells migrate into the peripheral tissue and undergo differentiation to become mature mast cells in situ. They are mainly located within blood vessels and the epithelial lining. Mast cells of hematopoietic origin respond to signals of both innate and acquired immune response with immediate and delayed release of inflammatory mediators. Based on their site of location and granule contents, mast cells are divided into connective tissue mast cells and mucosal mast cells. Human connective tissue mast cells are observed in the skin and intestinal sub-mucosa, and their granules comprises tryptase and chymase. Studies have also shown that the granules of tryptase were found in the mast cells located in intestinal mucosa and alveoli [36]. Mast cells are central to the pathogenesis of type I hypersensitivity and mastocytosis. Mast cells are also entailed with self-responses to pathogens, autoimmune diseases, and fibrosis. Eicosanoids and cytokines are synthesized by mast cells and contribute to inflammation. Mast cells can be specifically stained with basic dyes such as toluidine blue [37].
Basophils also express high levels of FcεRI. They are derived from CD34+ hematopoietic progenitor cells and play a vital role in the host defense against parasitic infections, as well as mediating type I hypersensitivity reactions. Basophils are present in blood as mature forms. However, they can be recruited to the site of inflammation where antigens are located. Cytoplasmic granules in the basophils comprise pro-inflammatory mediators. Because basophils share structural and functional similarities with mast cells, they trigger comparable effector responses as observed in mast cells upon aggregation of FcεRI. Apart from basophils and mast cells, FcεRI is also found to be expressed in low levels on eosinophils, monocytes, platelets, dendritic cells, and Langerhans cells [25].
Mast cells: morphology
Mast cells are up to 20 μm in diameter, are ovoid or irregularly elongated in shape [38]. Mast cells contain ample metachromatic cytoplasmic granules and they can be stained because of large sulfated proteoglycans in the granules. Tryptase staining detects all mast cell types and is the principal method for detecting tissue mast cells. The granule contents are crystalline under the electron microscope, but turn formless upon activation and before degranulation. Mast cells express several receptors including IL-3R, IL-4R, IL-5R, IL-9R, IL-10R, GM-CSFR, IFN-γR, C3a and C5a receptors, CCR3, CCR5, CXCR2, CXCR4, nerve growth factor receptor, Toll-like receptors (TLRs), and ST2 [39, 40].
FcεRI-mediated signal transduction
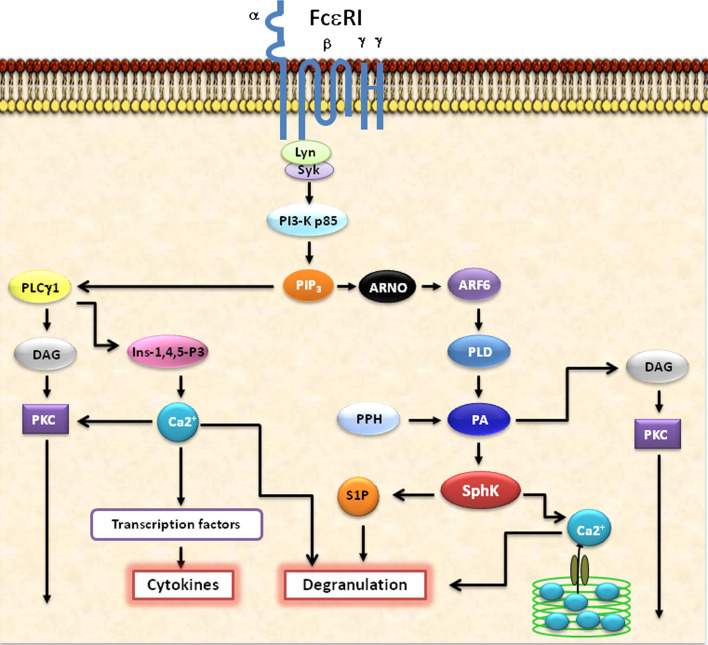
Interpreting the intracellular signaling cascade mediated by mast cell activation has major therapeutic implications for inflammatory conditions. FcεRI aggregation and mast cell activation has long been known to be an important incident in allergic conditions [41–43]. Numerous studies have focused on the intracellular signaling cascade mediated by mast cell activation and release of pro-inflammatory mediators [25, 44, 45]. Figure 1 shows the schematic representation of FcεRI-mediated intracellular signaling events. Following cross-linking of FcεRI, intracellular signal transduction is initiated by a tyrosine kinase, named Lyn, which is constitutively linked with the FcεRI receptor [46, 47]. Subsequently, Lyn phosphorylates the immunoreceptor tyrosine-based activation motif (ITAM) of the subunits of the FcεRI, leading to the activation and binding of protein tyrosine kinases (PTK) with Src homology domain 2 (SH2). One such PTK is spleen tyrosine kinase (Syk) [48]. An activated Syk subsequently leads to further tyrosine phosphorylations of several “adapter” proteins including that of linker for activation of T cells (LAT) as well as phosphorylation of phospholipase C γ (PLC-γ).
Fig. 1.
Schematic representation of FcεRI-mediated intracellular signaling events in mast cells and basophils. The allergen cross linking of FcεRI receptors initiate key cellular signaling events such as the activation of PLCγ, PKCs, SPHKs, PI3Ks, etc. An important role for these major pathways in immune cell function is predicted based on their ability to regulate the transcriptional activity of cytokine genes, calcium release from the internal stores which is essential for the degranulation and further intracellular mediation of signaling processes, and the production of lipid mediators such as prostaglandins and leukotrienes. The specific blocking of these key intracellular signaling molecules such as PLCγ, PKCs, SPHKs, S1P, PI3Ks, etc., by specific chemical inhibitors or siRNAs, or therapeutic Intrabodies would certainly be of immense use in the effective management of allergy and anaphylaxis in the near future
LAT phosphorylation leads to the activation of JNK and ERK; and the synthesis and release of cytokines and arachidonic acid (AA) metabolism result in target organs to cause the clinical syndrome of anaphylaxis [49]. On the other hand, phosphorylated PLC-γ yields diacylglycerol (DAG) and inositol-1,4,5-triphosphate (IP3) from membrane phospholipids. DAG successively activates protein kinase C (PKC), which results in the exocytosis of stored granules and cytokine release.
Furthermore, Bruton’s tyrosine kinase (Btk) is membrane targeted and activated by the binding of phosphatidylInositol-3, 4, 5-trisphosphate (PIP3) moiety to its Pleckstrin-Homology (PH) domain. Btk then phosphorylates and activates PLC-γ [50]. On the other hand, the induction of PI3 K by Syk results in the production of micromolar levels of PIP2 and PIP3. Besides, PI3 K is essential for the induction of intracellular Ca2+ release as well as mobilization across plasma membranes. Both PI3 K and PLCγ act synergistically on the common substrate PIP2 to produce PIP3 and IP3, respectively. The IP3 activates Ca2+ channels at different cellular compartments, primarily in the endoplasmic reticulum [ER], to release Ca2+ required for optimal physiological responses. As a result, the depletion of Ca2+ in the internal stores causes the influx of Ca2+ from the extracellular space through the activation of a type of store operated Ca2+ channel (SOCC) termed as Ca2+ release activated Ca2+ channel (ICRAC) [50, 51, 52].
Studies have shown that cross-linking of FcεRI in rat basophilic leukemia cells activates sphingosine kinase, which produces sphingosine-1-phosphate (S1P). S1P is a potent sphingolipid mediator, and acts as a second messenger in releasing intracellular calcium from internal stores. This process is carried through the inositol 1,4,5-triphosphate (IP3)-independent pathway [53]. Studies report that aggregation of FcεRI in human bone marrow-derived mast cells resulted in the activation of PLD1, leading to downstream activation of SPHK1 [54]. This process results in the initial release of intracellular calcium as well as degranulation of mast cell(s). Another interesting study reports that blocking SPHK activity forbids FcεRI-mediated internalization of S1P receptors and significantly reduces degranulation [55]. Also, tyrosine kinase Lyn has been shown to be associated with recruitment and activation of SPHK to FcεRI [56].
FcεRI: regulation
In 1978, Malveaux et al. [57] reported for the first time that the presence of circulating monomeric IgE could raise FcεRI cell surface expression. Many studies have been published in the 1990s showing IgE drives FcεRI expression in both human and murine mast cells, as well as basophils [58–60]. These reports have helped in understanding the mechanisms of IgE sensitization, during which FcεRI is over-expressed at cell surface, and FcεRIs bound to IgE are activated when re-exposed to allergens. FcεRI expression as well as mast cell activation upon cross-linking IgE-bound FcεRI with polyvalent antigen lead to degranulation [34, 61]. Such IgE-mediated mast cell recognition of multivalent antigen and the subsequent intracellular signaling events are well studied in various inflammatory models [62–65].
IgE-mediated FcεRI expression was also observed in monocytes [66, 67]. However, monocytes do not express FcεRIβ and are shown to express low levels of FcεRI. FcεRIβ appears to be playing a key role in the receptor expression and has also been shown to be associated with atopic diseases [68]. A single-nucleotide polymorphism (SNP) in the FcεRIβ gene has been connected to higher IgE serum levels and result in the increased FcεRI surface expression [69]. In IgE-mediated FcεRI regulation, over-expression of receptor is caused by the interaction of IgE with the receptor [70]. The total FcεRIα content increases with surface receptor up-regulation. On the other hand, the removal of IgE results in the reduction in the receptor expression, as well as FcεRIα content [71]. Though the actual mechanism still remains unclear, IgE-FcεRI binding enhances the surface expression of FcεRI [72]. Reports show that FcεRI receptor expression in mast cells are mediated by IL-4 [60, 73]. On the whole, the factors that mainly affect the expression of FcεRI are the expression of FcεRIβ and the serum levels of IgE [60, 73].
FcεRI: biological functions
FcεRI triggers IgE-mediated activation and degranulation of mast cells and basophils. Apart from mediating type I hypersensitivity reactions, FcεRI plays other biological roles, too. First of all, FcεRI and IgE are vital for the self-defense against parasitic infections [74]. In vivo studies showed that mice lacking α chain of FcεRI were also protected against parasitic infections like the normal mice. However, it is not very clear why and how the absence of functional FcεRI/mast cell axis protects these mice against parasitic infections [74]. Interestingly, very recent study has partially answered the above statement by showing that the IgG/neutrophil axis is also key for the triggering of passive and active systemic anaphylactic shock in mice [35].
The other biological role of FcεRI is their expression on APCs such as dendritic cells and monocytes [75]. FcεRI is expressed as αγ2 form on the APCs. It is known that the αγ2 structure may assist in targeting antigen-IgE-FcεRI complexes to the intracellular antigen-presenting compartment. The IgE-dependent type of antigen presentation will make sure that it will only amplify the immune responses, which were already mediated by IgE. This type of antigen presentation can mediate cytokine production from APCs and thus, helps to modulate allergic inflammation [20, 25].
Activation of mast cells and release of mediators
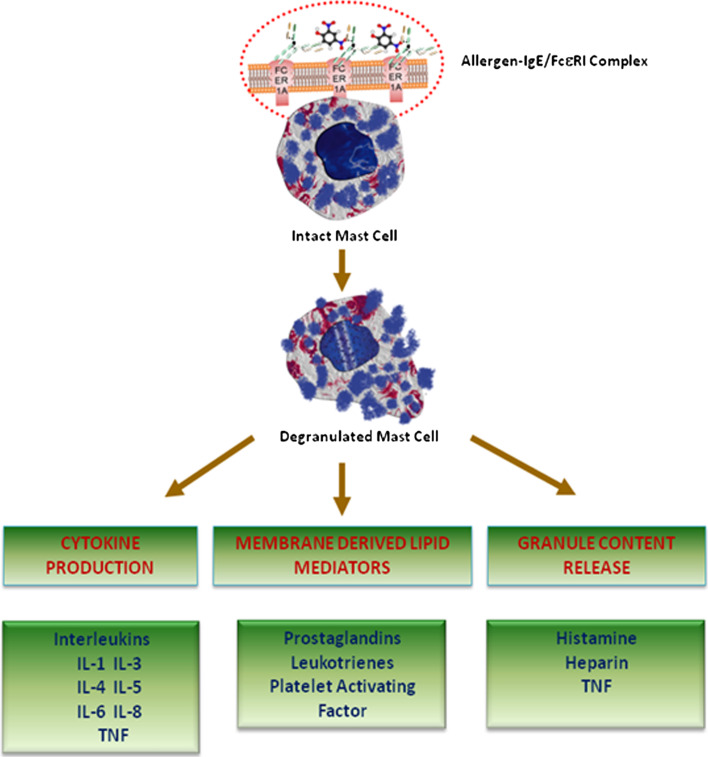
The mediators released by the mast cells can be categorized into three main groups: preformed granule content, membrane derived lipid mediators, and cytokines [76–78]. Figure 2 shows the schematic representation of major types of mediators released by the mast cells. Histamine is the most clinically evident preformed granule mediators responsible for the acute symptoms. Histamine is shown to be associated with immediate type I hypersensitivity reactions [79]. The level of histamine that is produced and stored in mast cells is roughly 1 or 2 pg/cell. Histamine receptors are further classified into H1, H2, and H3. H1 receptor stimulation results in bronchial smooth muscle contraction, increased vascular permeability, nasal mucus secretion, and increased neutrophil and eosinophil chemokinesis and chemotaxis. On the other hand, H2 receptor stimulation results in ventricular and atrial contraction, gastric acid production, airway mucus secretion, and vascular permeability as well as inhibition of basophil histamine release. Finally, H3 receptors, which are found to be in neurons and peripheral tissues, regulate the secretion and synthesis of histamine.
Fig. 2.
The key mediators of FcεRI/Mast cell axis in allergy and anaphylaxis. The schematic representation illustrates some of the key mediators released by the mast cells upon stimulation during allergy and anaphylaxis. The mediators released by the mast cells can be categorized into three main groups: preformed granule content, membrane-derived lipid mediators, and cytokines
Membrane-derived lipid mediators and cytokines are synthesized upon activation of mast cells. Prostaglandin D2 (PGD2) is the key arachidonic acid (AA) lipid metabolite released upon mast cell activation. PGD2 is synthesized from AA by the cyclooxygenase pathway and is responsible for causing bronchospasm, hypotension, and inhibition of platelet aggregation. PGD2 is roughly over 30 times more potent than histamine, especially in causing bronchoconstriction. Leukotrienes are slow-responding substances of anaphylaxis. Leukotrienes—LTB4, LTC4, LTD4, and LTE4 are synthesized from AA through the lipoxygenase pathway. Leukotrienes have been shown to be linked in increased vascular permeability, increased mucous gland production and cholinergic-independent bronchospasm. Leukotrienes have a slow onset but are 10–1,000 times added potent, compared to histamine in developing bronchoconstriction when dispensed by aerosol [80]. The other most potent compound and unstored phospholipid is platelet-activating factor (PAF). PAFs are known to cause aggregation of human platelets and release of platelet-derived vasoactive mediators. PAF has been implicated with anaphylaxis, including pulmonary edema and coronary vasoconstriction [81, 82]. Studies show that the blockade of PAF with inhibitors result in improved cardiac function, suggesting a key role for PAF in cardiac dysfunction [83]. Furthermore, TNF-α is a major cytokine that is in both stored as well as synthesized forms [84]. It over-expresses cell adhesion molecules, increases bronchial responsiveness, and also possesses antitumor effects. Other cytokines produced by mast cells include IL-1, IL-3, IL-4, IL-5, IL-6, and IL-8 [39].
Mast cells in acute and chronic allergic reactions
Mast cells play a central role in the initiation and development of atopic allergic reactions. The intracellular signaling events have been broadly studied on mast cells not only to know their physiological roles but also to find potential therapeutic targets. Type I hypersensitivity is the fundamental of acute allergic conditions [85]. It is stimulated by molecules released by mast cells when an allergen or antigen cross-react with membrane-bound IgE.
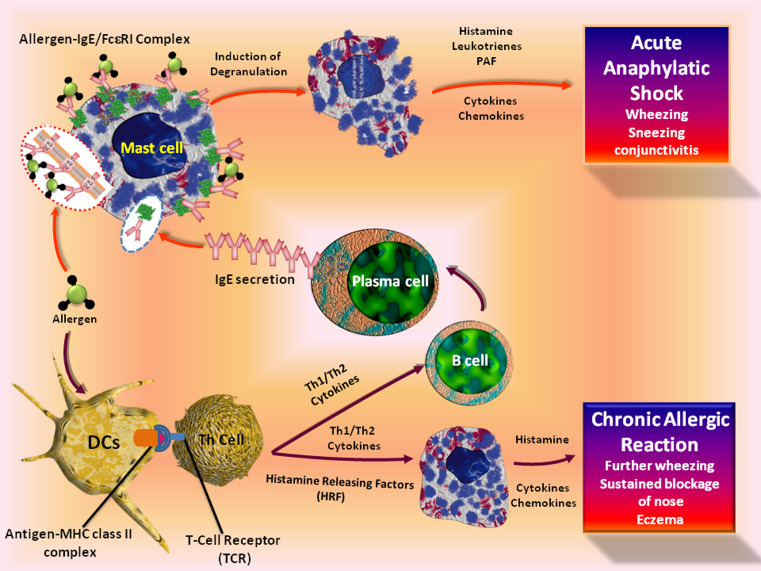
Allergic conditions broadly consist of two phases: sensitization and elicitation phase [86]. In the self, sensitization occurs upon the initial exposure to an allergen or antigen. Dendritic cells and macrophages are the first line of defense against antigens through phagocytosis and cell-mediated uptake of antigens. The antigen is degraded by the antigen-processing pathway and subsequently presented as a component of the MHC class II complexes [87]. Then the mature dendritic cells stimulate naive T cells to differentiate into either TH1- or TH2-type CD4+ T cells [88], which aids in the maturation of B-cells to IgE secreting plasma cells. FcεRI aggregation triggers the release of pre-formed mediators, production of lipid mediators and cytokines. In an atopic condition, exposure of the nose, skin, or airway to an initial dose of allergen triggers a cutaneous reaction, results runny nose, sneezing, and wheezing in minutes. In case of asthma, these mediators develop acute reactions, including mucus production and smooth muscle contraction. This depends upon the type I immediate allergic reactions and subsequently followed by a chronic allergic reaction, which peaks 6 to 9 h post-initial exposure [89]. The cellular pathways leading to acute and chronic allergic reactions are shown in Fig. 3.
Fig. 3.
The cellular pathways leading to acute and chronic allergic reactions. The binding of allergen with IgE/FcεRI complex on mast cells triggers the release of pre-formed mediators, production of lipid mediators, and cytokines leading to acute allergic conditions. Allergic conditions broadly consist of two phases: sensitization and elicitation phase. In the self, sensitization occurs upon the initial exposure to an allergen or antigen. Dendritic cells and macrophages are the first line of defense against antigens through phagocytosis and cell-mediated uptake of antigens. The antigen is subsequently degraded as a component of the MHC class II complexes. Then the mature dendritic cells stimulate naive T cells to differentiate into either TH1- or TH2-type CD4+ T cells, which aids in the maturation of B-cells to IgE-secreting plasma cells. Chronic allergic reactions, including the late-phase reaction, may rely on a combination of events and the release of mast-cell products by histamine-releasing factors from T-helper cells
A TH2 response has been observed in an atopic condition and IgE production will result in binding with FcεRI on the effector cell. This will in turn increase the efficacy during re-exposure to the same allergen [90–92]. During the late-phase reaction of the skin, neutrophils and eosinophils accumulate, followed by CD4+ T cells and basophils that infiltrate to the site of inflammation [93]. The late-phase asthmatic condition also shares a similar pattern of cellular infiltration [94–96]. However, basophils are not mainly infiltrated into the lower airways [97, 98]. Studies also show that further amplifications of chronic allergic reactions may be mediated by histamine-releasing factors [99, 100]. Cross-linking of mast-cell-bound IgE with an antibody against IgE elicits both type I and late-phase reactions [101–104]. The immediate and delayed phases of bronchial hypersensitivity are initiated by mast cells are shown in both humans, as well as in mouse models of asthma [105, 106]. It has been reported that mast cells may also be associated with regulating the early stages of autoimmunity, especially in [107–109] diseases where auto-antibodies play a vital role [110].
High-throughput functional genomics paradigm
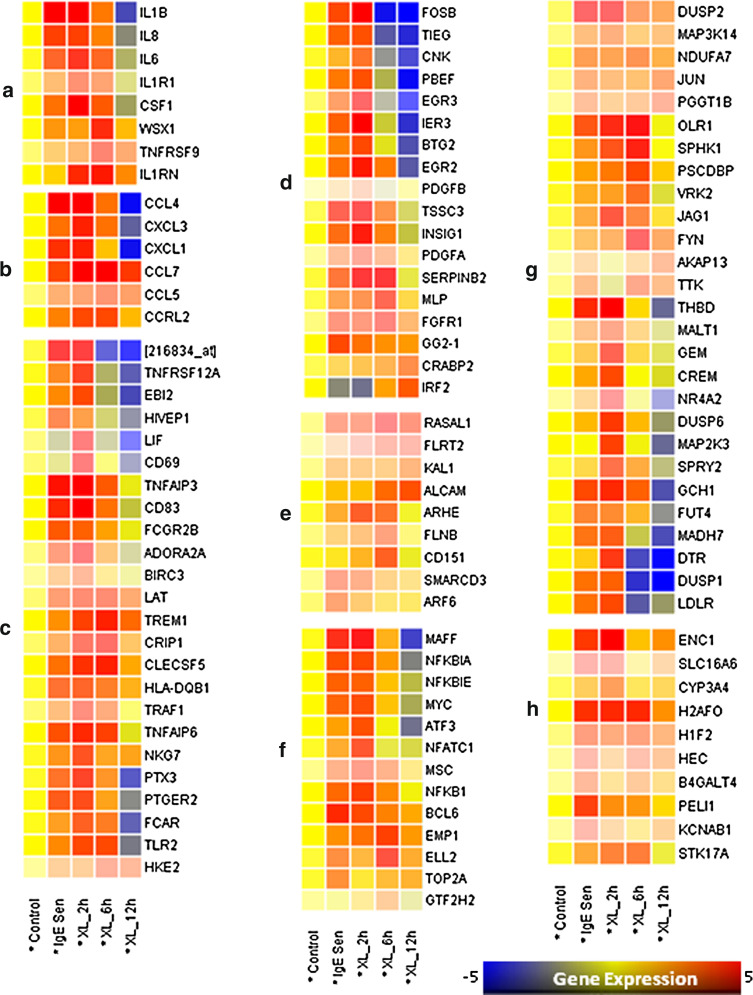
The latest developments of functional genomics tools, such as genome-wide transcriptomic analysis have revolutionized the approaches to answer complex scientific problems. Several groups have carried out low, medium, and high-throughput transcriptomic analysis of mast cell-mediated expression profiling, furnishing some novel pathogenic mechanisms of mast cell-mediated allergic responses. Table 1 shows various gene expression studies carried out on mast cells. The impact of these studies confirms that the mast cell stimulation and activation seem to be associated with number of pathologies including allergy and other immune/inflammatory conditions. A number of studies on immune/inflammatory response show the usefulness of transcriptomic analysis to understand the underlying molecular mechanisms [11, 111–114]. With transcriptomic analysis coupled with stringent statistical measures, we show the genome-wide expression patterns of differentiated human mast cells stimulated by IgE sensitization, and FcεRI aggregation at different time points. In our earlier study, we have elucidated the molecular events which the differentiated human mast cells go through upon IgE sensitization and after complete activation in a genome-wide manner [115]. Generation of a wide-variety of cytokines and chemokines upon IgE-sensitization and FcεRI aggregation on mast cells suggests that they could be the key regulators of the immune response (Fig. 4). It may also lead to the recruitment and activation of other effector cells to the site of inflammation, which may further enhance the progression of immune response [115].
Table 1.
Overview of gene expression studies performed using mast cell lines and primary mast cells
| Study | Source | Stimulation | Platform | No. of genes |
|---|---|---|---|---|
| [116] | HUCBMCs | No stimuli | RT-PCR | 32 genes |
| [117] | HUCBMCs | No stimuli | SAGE, RT-PCR | 9,000 tags- SAGE |
| [118] | Human PBMNC-MCs | IgE + Anti IgE | ELISA, RT-PCR | Cytokines and chemokines |
| [119] | HUCBMCs, eosinophils, neutrophils and PBMNC | No stimuli | ONT array | 6,000 probes |
| [120] | RBL-2H3 | IgE + DNP | ONT array | 8,799 probes |
| [121] | BM-mouse MCs | IgE + DNP | ELISA | Cytokines |
| [122] | Human lung MCs | TLR4 | QPCR, ELSIA | Cytokines |
| [123] | Human Skin MCs | IgE or PMA | ELISA, RT-PCR | Cytokines |
| [124] | HUCBMCs | IgE | ELISA | IL-8 & MCP-1 |
| [115] | HUCBMCs | IgE | ONT array | 8,763 probes |
The above evidence table depicts the authors of the study, source of mast cells used, stimulation method for activation of mast cells, platforms used for gene expression profiling, and number of genes screened.
MC mast cell, HUCBMCs human umbilical cord blood-derived mast cells, PBMNC peripheral blood mononuclear cell culture, RBL-2H3 rat basophilic leukemia cells
Fig. 4.
Gene expression pattern in human mast cells following IgE sensitization and FcεRI aggregation. The raw Affymetrix GeneChip data was downloaded from gene expression omnibus (GSE1933) and normalized with parametric test based on cross gene error model (PCGEM) and subjected to one-way ANOVA and Bonferroni-Hochberg FDR (p < 0.05) using Genespring 7.3 The differentially expressed genes by the IgE sensitization and FcεRI cross-linking for different time points (2, 6, and 12 h) were then classified and clustered based on gene ontology (GO). Analysis to decode the differentially expressed genes implicated in biological processes such as a cytokines and cytokine receptors, b chemokines and chemokine receptors, c other immunoregulatory genes, d cell proliferation and apoptosis, e adhesion and cytoskeleton remodeling, f transcription factors and regulators, g signal transduction, and h other genes [115]
FcεRI/mast cell axis: pathway analysis of differentially expressed genes
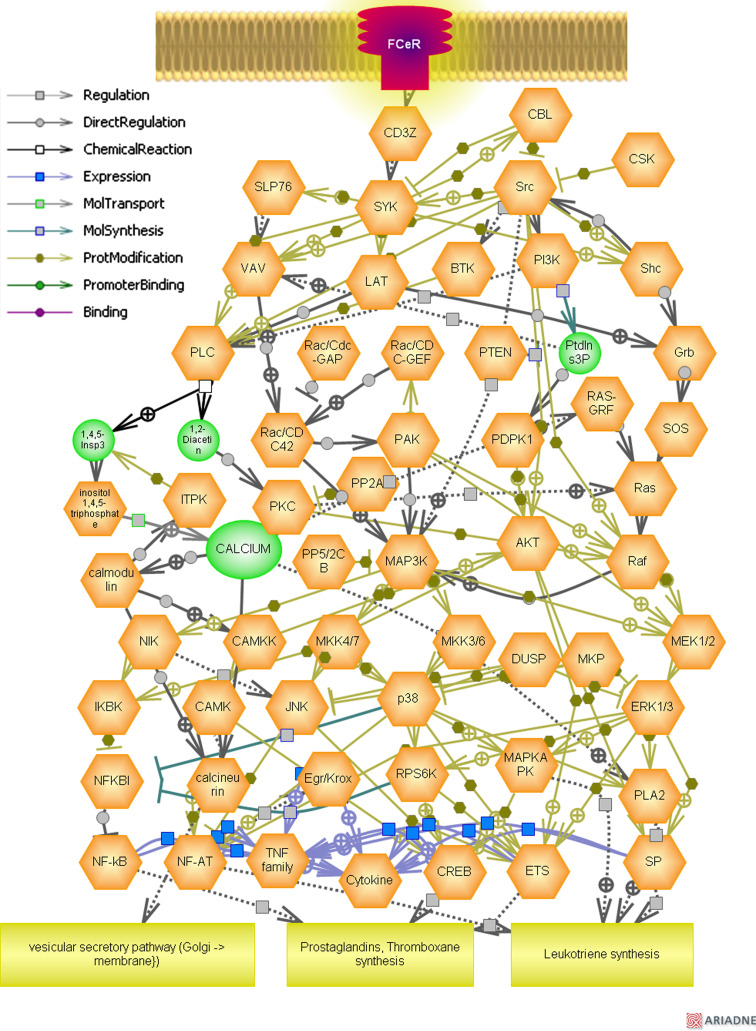
Representation of specific inflammatory and immunoregulatory pathways among the differentially expressed genes [115] was analyzed using Pathway Studio® software (Ariadne Genomics, Rockville, MD) version 6.0. The software uses information available in the current literature to identify common pathways, targets, or regulators that are associated with the altered genes to generate biological interaction networks. Microarray expression data was imported into Pathway Studio® to graphically represent all known relationships and potential interactions between the differentially expressed genes. Pathway Studio software was used to identify a possible gene network that is differentially regulated in the mast cell-mediated classical pathway. Genes were linked to each other based on the published literature (Fig. 5).
Fig. 5.
Molecular pathways triggered through FcεRI/mast cell axis. Representation of specific inflammatory and immunoregulatory pathways among the differentially expressed genes triggered through FcεRI/mast cell axis was analyzed using Pathway Studio® software (Ariadne Genomics, Rockville, MD) version 6.0. The software uses information available in the current literature to identify common pathways, targets, or regulators that are associated with the altered genes to generate biological interaction networks. Genes were linked to each other based on the published literature
Concluding remarks
Allergy and anaphylaxis comprise a wide spectrum of pathologies associated with the inappropriate activation of the immune system by environmental antigens [125]. Allergic responses to foods, insect bites, oral and injected medications, and other agents, remain huge problems, and are constantly increasing in society [125, 126]. Importantly, the FcεRI/mast cell axis is central to these immune reactions, and provides an attractive target for the inhibition of all IgE-mediated allergic diseases. Moreover, the modulation of this central axis has long been considered as a therapeutic strategy for various allergic disorders [50, 127]. Interestingly, clinical studies of allergic individuals using anti-IgE monoclonal antibody therapy have shown that the exploitation of this key axis is an effective approach to disease treatment [128, 129].
In addition, mast cells play a major role in the pathogenesis of inflammatory diseases such as asthma, atopic dermatitis, psoriasis, and interstitial cystitis, as well as a minor role in irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), rheumatoid arthritis (RA), coronary artery disease (CAD), obesity, multiple sclerosis, and cancer [3, 4, 130]. As a result, the critical dissection of differentially expressed genes triggered through the FcεRI/mast cell axis and extensive exploration of their regulatory pathways through advancing high-throughput technologies, may allow us to selectively regulate these processes and help in the development of therapeutic modalities to potentially control and manage the exaggerated immune/inflammatory responses in allergy and anaphylaxis in the near future.
Acknowledgments
The authors would like to thank the various research groups around the globe for their exceptional contributions in the basic, translational, and clinical aspects of allergy and anaphylaxis. We really regret the omission of any of these findings or contributions on the FcεRI/Mast cell axis in this review, which is mainly due to space limitations. We extend our immense appreciation to the graphics team of Beacon Biosoft (www.beaconbiosoft.com) for the fantastic figures in our review.
Contributor Information
Manoor Prakash Hande, Phone: +65-65163664, FAX: +65-67788161, Email: phsmph@nus.edu.sg.
Peter Natesan Pushparaj, Phone: +44-141-3308133, FAX: +44-141-3304297, Email: peter.n.pushparaj@gmail.com, Email: peter.pushparaj@glasgow.ac.uk.
References
- 1.MacDonald SM, Rafnar T, Langdon J, Lichtenstein LM. Molecular identification of an IgE-dependent histamine-releasing factor. Science. 1995;269(5224):688–690. doi: 10.1126/science.7542803. [DOI] [PubMed] [Google Scholar]
- 2.Portier P, Richet C. De l’action anaphylatique de certain venins. C R Soc Biol. 1902;54:170. [Google Scholar]
- 3.Theoharides TC, Kalogeromitros D. The critical role of mast cells in allergy and inflammation. Ann NY Acad Sci. 2006;1088:78–99. doi: 10.1196/annals.1366.025. [DOI] [PubMed] [Google Scholar]
- 4.Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, Kalogeromitros D (2010) Mast cells and inflammation. Biochim Biophys Acta. doi:10.1016/j.bbadis.2010.12.014 [DOI] [PMC free article] [PubMed]
- 5.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77(4):1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 6.Munitz A, Piliponsky AM, Levi-Schaffer F. IgE-independent activation of human mast cells indicates their role in the late-phase reaction of allergic inflammation. Cell Tissue Bank. 2003;4(1):25–28. doi: 10.1023/A:1026307812980. [DOI] [PubMed] [Google Scholar]
- 7.Dudler T, Machado DC, Kolbe L, Annand RR, Rhodes N, Gelb MH, Koelsch K, Suter M, Helm BA. A link between catalytic activity, IgE-independent mast cell activation, and allergenicity of bee venom phospholipase A2. J Immunol. 1995;155(5):2605–2613. [PubMed] [Google Scholar]
- 8.Sullivan TJ, Hart DA, Streilein JW. IgE-dependent release of inflammatory mediators from hamster mast cells in vitro. Adv Exp Med Biol. 1981;134:33–41. doi: 10.1007/978-1-4757-0495-2_4. [DOI] [PubMed] [Google Scholar]
- 9.Heller RA, Schena M, Chai A, Shalon D, Bedilion T, Gilmore J, Woolley DE, Davis RW. Discovery and analysis of inflammatory disease-related genes using cDNA microarrays. Proc Natl Acad Sci USA. 1997;94(6):2150–2155. doi: 10.1073/pnas.94.6.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firoved AM, Ornatowski W, Deretic V. Microarray analysis reveals induction of lipoprotein genes in mucoid pseudomonas aeruginosa: implications for inflammation in cystic fibrosis. Infect Immun. 2004;72(9):5012–5018. doi: 10.1128/IAI.72.9.5012-5018.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spears R, Oakes R, Moore C, Bellinger LL, Hutchins B. A determination of tumor necrosis factor expression in TMJ inflammation with the use of microarray analysis. J Dent Res. 2003;82(10):807–813. doi: 10.1177/154405910308201009. [DOI] [PubMed] [Google Scholar]
- 12.Olman MA, White KE, Ware LB, Cross MT, Zhu S, Matthay MA. Microarray analysis indicates that pulmonary edema fluid from patients with acute lung injury mediates inflammation, mitogen gene expression, and fibroblast proliferation through bioactive interleukin-1. Chest. 2002;121((3 Suppl)):69S–70S. doi: 10.1378/chest.121.3_suppl.69s. [DOI] [PubMed] [Google Scholar]
- 13.Gu J, Huang F, Yu D. Analysis of inflammation related gene expression spectrum in ankylosing spondylitis patients using cDNA microarray. Zhonghua Yi Xue Za Zhi. 2001;81(17):1030–1034. [PubMed] [Google Scholar]
- 14.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 15.Unkeless JC, Scigliano E, Freedman VH. Structure and function of human and murine receptors for IgG. Annu Rev Immunol. 1988;6:251–281. doi: 10.1146/annurev.iy.06.040188.001343. [DOI] [PubMed] [Google Scholar]
- 16.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 17.Vitetta ES, Uhr JW. Immunoglobulin-receptors revisited. Science. 1975;189(4207):964–969. doi: 10.1126/science.1083069. [DOI] [PubMed] [Google Scholar]
- 18.Davie JM (1975) Immunoglobulin receptors in lymphocytes. Adv Pathobiol (1):70–79 [PubMed]
- 19.Keegan AD, Conrad DH. The receptor for the Fc region of IgE. Springer Semin Immunopathol. 1990;12(4):303–326. doi: 10.1007/BF00225321. [DOI] [PubMed] [Google Scholar]
- 20.Gould HJ, Sutton BJ, Beavil AJ, Beavil RL, McCloskey N, Coker HA, Fear D, Smurthwaite L. The biology of IGE and the basis of allergic disease. Annu Rev Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 21.Rosenwasser LJ, Meng J. Anti-CD23. Clin Rev Allergy Immunol. 2005;29(1):61–72. doi: 10.1385/CRIAI:29:1:061. [DOI] [PubMed] [Google Scholar]
- 22.MacGlashan D., Jr IgE receptor and signal transduction in mast cells and basophils. Curr Opin Immunol. 2008;20(6):717–723. doi: 10.1016/j.coi.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 2009;228(1):149–169. doi: 10.1111/j.1600-065X.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rivera J, Fierro NA, Olivera A, Suzuki R. New insights on mast cell activation via the high affinity receptor for IgE. Adv Immunol. 2008;98:85–120. doi: 10.1016/S0065-2776(08)00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 26.Stingl G, Maurer D. IgE-mediated allergen presentation via Fc epsilon RI on antigen-presenting cells. Int Arch Allergy Immunol. 1997;113(1–3):24–29. doi: 10.1159/000237499. [DOI] [PubMed] [Google Scholar]
- 27.Uehara M, Sawai T. Familial background of respiratory atopy. A factor of type I allergy to house dust mite in patients with atopic dermatitis. Arch Dermatol. 1989;125(7):939–943. doi: 10.1001/archderm.125.7.939. [DOI] [PubMed] [Google Scholar]
- 28.Smith SJ, Ying S, Meng Q, Sullivan MH, Barkans J, Kon OM, Sihra B, Larche M, Levi-Schaffer F, Kay AB. Blood eosinophils from atopic donors express messenger RNA for the alpha, beta, and gamma subunits of the high-affinity IgE receptor (Fc epsilon RI) and intracellular, but not cell surface, alpha subunit protein. J Allergy Clin Immunol. 2000;105(2 Pt 1):309–317. doi: 10.1016/s0091-6749(00)90081-2. [DOI] [PubMed] [Google Scholar]
- 29.Conrad DH, Campbell KA, Bartlett WC, Squire CM, Dierks SE. Structure and function of the low affinity IgE receptor. Adv Exp Med Biol. 1994;347:17–30. doi: 10.1007/978-1-4615-2427-4_3. [DOI] [PubMed] [Google Scholar]
- 30.Donnadieu E, Jouvin MH, Kinet JP. A second amplifier function for the allergy-associated Fc (epsilon) RI-beta subunit. Immunity. 2000;12(5):515–523. doi: 10.1016/s1074-7613(00)80203-4. [DOI] [PubMed] [Google Scholar]
- 31.Nadler MJ, Matthews SA, Turner H, Kinet JP. Signal transduction by the high-affinity immunoglobulin E receptor Fc epsilon RI: coupling form to function. Adv Immunol. 2000;76:325–355. doi: 10.1016/s0065-2776(01)76022-1. [DOI] [PubMed] [Google Scholar]
- 32.Kraft S, Bieber T. Fc epsilon ri-mediated activation of transcription factors in antigen-presenting cells. Int Arch Allergy Immunol. 2001;125(1):9–15. doi: 10.1159/000053791. [DOI] [PubMed] [Google Scholar]
- 33.Miller L, Blank U, Metzger H, Kinet JP. Expression of high-affinity binding of human immunoglobulin E by transfected cells. Science. 1989;244(4902):334–337. doi: 10.1126/science.2523561. [DOI] [PubMed] [Google Scholar]
- 34.Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol. 2002;2(10):773–786. doi: 10.1038/nri914. [DOI] [PubMed] [Google Scholar]
- 35.Jonsson F, Mancardi DA, Kita Y, Karasuyama H, Iannascoli B, Van Rooijen N, Shimizu T, Daeron M, Bruhns P. Mouse and human neutrophils induce anaphylaxis. J Clin Invest. 2011;121(4):1484–1496. doi: 10.1172/JCI45232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds DS, Serafin WE, Faller DV, Wall DA, Abbas AK, Dvorak AM, Austen KF, Stevens RL. Immortalization of murine connective tissue-type mast cells at multiple stages of their differentiation by coculture of splenocytes with fibroblasts that produce Kirsten sarcoma virus. J Biol Chem. 1988;263(25):12783–12791. [PubMed] [Google Scholar]
- 37.Xu D, Jiang HR, Li Y, Pushparaj PN, Kurowska-Stolarska M, Leung BP, Mu R, Tay HK, McKenzie AN, McInnes IB, Melendez AJ, Liew FY. IL-33 exacerbates autoantibody-induced arthritis. J Immunol. 2010;184(5):2620–2626. doi: 10.4049/jimmunol.0902685. [DOI] [PubMed] [Google Scholar]
- 38.Eady RA. The mast cells: distribution and morphology. Clin Exp Dermatol. 1976;1(4):313–321. doi: 10.1111/j.1365-2230.1976.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 39.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112(4):946–956. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10(2):103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 41.Kopec A, Panaszek B, Fal AM. Intracellular signaling pathways in IgE-dependent mast cell activation. Arch Immunol Ther Exp (Warsz) 2006;54(6):393–401. doi: 10.1007/s00005-006-0049-4. [DOI] [PubMed] [Google Scholar]
- 42.Kalesnikoff J, Huber M, Lam V, Damen JE, Zhang J, Siraganian RP, Krystal G. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 2001;14(6):801–811. doi: 10.1016/s1074-7613(01)00159-5. [DOI] [PubMed] [Google Scholar]
- 43.Baumruker T, Prieschl EE. The role of sphingosine kinase in the signaling initiated at the high-affinity receptor for IgE (FcepsilonRI) in mast cells. Int Arch Allergy Immunol. 2000;122(2):85–90. doi: 10.1159/000024363. [DOI] [PubMed] [Google Scholar]
- 44.Beaven MA, Metzger H. Signal transduction by Fc receptors: the Fc epsilon RI case. Immunol Today. 1993;14(5):222–226. doi: 10.1016/0167-5699(93)90167-j. [DOI] [PubMed] [Google Scholar]
- 45.Barnes PJ, Chung KF, Page CP. Inflammatory mediators of asthma: an update. Pharmacol Rev. 1998;50(4):515–596. [PubMed] [Google Scholar]
- 46.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6(3):218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 47.Kambayashi T, Koretzky GA. Proximal signaling events in Fc epsilon RI-mediated mast cell activation. J Allergy Clin Immunol. 2007;119(3):544–552. doi: 10.1016/j.jaci.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 48.Kitaura J, Eto K, Kinoshita T, Kawakami Y, Leitges M, Lowell CA, Kawakami T. Regulation of highly cytokinergic IgE-induced mast cell adhesion by Src, Syk, Tec, and protein kinase C family kinases. J Immunol. 2005;174(8):4495–4504. doi: 10.4049/jimmunol.174.8.4495. [DOI] [PubMed] [Google Scholar]
- 49.Rivera J. Molecular adapters in Fc(epsilon)RI signaling and the allergic response. Curr Opin Immunol. 2002;14(6):688–693. doi: 10.1016/s0952-7915(02)00396-5. [DOI] [PubMed] [Google Scholar]
- 50.Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999;402(6760 Suppl):B24–B30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- 51.Goldstein B, Faeder JR, Hlavacek WS, Blinov ML, Redondo A, Wofsy C. Modeling the early signaling events mediated by FcepsilonRI. Mol Immunol. 2002;38(16–18):1213–1219. doi: 10.1016/s0161-5890(02)00066-4. [DOI] [PubMed] [Google Scholar]
- 52.Craig AW, Zirngibl R, Williams K, Cole LA, Greer PA. Mice devoid of fer protein-tyrosine kinase activity are viable and fertile but display reduced cortactin phosphorylation. Mol Cell Biol. 2001;21(2):603–613. doi: 10.1128/MCB.21.2.603-613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi OH, Kim JH, Kinet JP. Calcium mobilization via sphingosine kinase in signalling by the Fc epsilon RI antigen receptor. Nature. 1996;380(6575):634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- 54.Aarthi JJ, Darendeliler MA, Pushparaj PN. Dissecting the role of S1P/S1PR axis in health and disease. J Dent Res. 2011;90(7):841–854. doi: 10.1177/0022034510389178. [DOI] [PubMed] [Google Scholar]
- 55.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199(7):959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Urtz N, Olivera A, Bofill-Cardona E, Csonga R, Billich A, Mechtcheriakova D, Bornancin F, Woisetschlager M, Rivera J, Baumruker T. Early activation of sphingosine kinase in mast cells and recruitment to FcepsilonRI are mediated by its interaction with Lyn kinase. Mol Cell Biol. 2004;24(19):8765–8777. doi: 10.1128/MCB.24.19.8765-8777.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malveaux FJ, Conroy MC, Adkinson NF, Lichtenstein LM. IgE receptors on human basophils. Relationship to serum IgE concentration. J Clin Invest. 1978;62(1):176–181. doi: 10.1172/JCI109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsu C, MacGlashan D., Jr IgE antibody up-regulates high affinity IgE binding on murine bone marrow-derived mast cells. Immunol Lett. 1996;52(2–3):129–134. doi: 10.1016/0165-2478(96)02599-0. [DOI] [PubMed] [Google Scholar]
- 59.Lantz CS, Yamaguchi M, Oettgen HC, Katona IM, Miyajima I, Kinet JP, Galli SJ. IgE regulates mouse basophil Fc epsilon RI expression in vivo. J Immunol. 1997;158(6):2517–2521. [PubMed] [Google Scholar]
- 60.Yamaguchi M, Sayama K, Yano K, Lantz CS, Noben-Trauth N, Ra C, Costa JJ, Galli SJ. IgE enhances Fc epsilon receptor I expression and IgE-dependent release of histamine and lipid mediators from human umbilical cord blood-derived mast cells: synergistic effect of IL-4 and IgE on human mast cell Fc epsilon receptor I expression and mediator release. J Immunol. 1999;162(9):5455–5465. [PubMed] [Google Scholar]
- 61.Woolhiser MR, Okayama Y, Gilfillan AM, Metcalfe DD. IgG-dependent activation of human mast cells following up-regulation of FcgammaRI by IFN-gamma. Eur J Immunol. 2001;31(11):3298–3307. doi: 10.1002/1521-4141(200111)31:11<3298::aid-immu3298>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 62.Gurish MF, Bryce PJ, Tao H, Kisselgof AB, Thornton EM, Miller HR, Friend DS, Oettgen HC. IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis . J Immunol. 2004;172(2):1139–1145. doi: 10.4049/jimmunol.172.2.1139. [DOI] [PubMed] [Google Scholar]
- 63.Oettgen HC, Geha RS. IgE regulation and roles in asthma pathogenesis. J Allergy Clin Immunol. 2001;107(3):429–440. doi: 10.1067/mai.2001.113759. [DOI] [PubMed] [Google Scholar]
- 64.Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370(6488):367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 65.Pushparaj PN, Tay HK, H’Ng SC, Pitman N, Xu D, McKenzie A, Liew FY, Melendez AJ. The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci USA. 2009;106(24):9773–9778. doi: 10.1073/pnas.0901206106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Maurer D, Fiebiger E, Reininger B, Wolff-Winiski B, Jouvin MH, Kilgus O, Kinet JP, Stingl G. Expression of functional high affinity immunoglobulin E receptors (Fc epsilon RI) on monocytes of atopic individuals. J Exp Med. 1994;179(2):745–750. doi: 10.1084/jem.179.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reischl IG, Dubois GR, Peiritsch S, Brown KS, Wheat L, Woisetschlager M, Mudde GC. Regulation of Fc epsilonRI expression on human monocytic cells by ligand and IL-4. Clin Exp Allergy. 2000;30(7):1033–1040. doi: 10.1046/j.1365-2222.2000.00859.x. [DOI] [PubMed] [Google Scholar]
- 68.Cookson WO, Sharp PA, Faux JA, Hopkin JM. Linkage between immunoglobulin E responses underlying asthma and rhinitis and chromosome 11q. Lancet. 1989;1(8650):1292–1295. doi: 10.1016/s0140-6736(89)92687-1. [DOI] [PubMed] [Google Scholar]
- 69.Nishiyama C, Akizawa Y, Nishiyama M, Tokura T, Kawada H, Mitsuishi K, Hasegawa M, Ito T, Nakano N, Okamoto A, Takagi A, Yagita H, Okumura K, Ogawa H. Polymorphisms in the Fc epsilon RI beta promoter region affecting transcription activity: a possible promoter-dependent mechanism for association between Fc epsilon RI beta and atopy. J Immunol. 2004;173(10):6458–6464. doi: 10.4049/jimmunol.173.10.6458. [DOI] [PubMed] [Google Scholar]
- 70.MacGlashan D, Lichtenstein LM, McKenzie-White J, Chichester K, Henry AJ, Sutton BJ, Gould HJ. Upregulation of FcepsilonRI on human basophils by IgE antibody is mediated by interaction of IgE with FcepsilonRI. J Allergy Clin Immunol. 1999;104(2 Pt 1):492–498. doi: 10.1016/s0091-6749(99)70399-4. [DOI] [PubMed] [Google Scholar]
- 71.MacGlashan D, Jr, McKenzie-White J, Chichester K, Bochner BS, Davis FM, Schroeder JT, Lichtenstein LM. In vitro regulation of Fc epsilon RIalpha expression on human basophils by IgE antibody. Blood. 1998;91(5):1633–1643. [PubMed] [Google Scholar]
- 72.Borkowski TA, Jouvin MH, Lin SY, Kinet JP. Minimal requirements for IgE-mediated regulation of surface Fc epsilon RI. J Immunol. 2001;167(3):1290–1296. doi: 10.4049/jimmunol.167.3.1290. [DOI] [PubMed] [Google Scholar]
- 73.Toru H, Ra C, Nonoyama S, Suzuki K, Yata J, Nakahata T. Induction of the high-affinity IgE receptor (Fc epsilon RI) on human mast cells by IL-4. Int Immunol. 1996;8(9):1367–1373. doi: 10.1093/intimm/8.9.1367. [DOI] [PubMed] [Google Scholar]
- 74.Capron A, Dombrowicz D, Capron M. Regulation of the immune response in experimental and human schistosomiasis: the limits of an attractive paradigm. Microbes Infect. 1999;1(7):485–490. doi: 10.1016/s1286-4579(99)80086-0. [DOI] [PubMed] [Google Scholar]
- 75.Simon MR, Cooper KD, Norris RB, Blok B, King CL. Antigen presenting cell-independent cytokine and spontaneous in vitro IgE production in patients with atopic dermatitis: increased interferon-gamma production and lack of effects of in vivo low-dose interferon-gamma treatment. J Allergy Clin Immunol. 1995;96(1):84–91. doi: 10.1016/s0091-6749(95)70036-6. [DOI] [PubMed] [Google Scholar]
- 76.Fouilloux I, Duplan MB, Baroukh B, Cherruau M, Saffar JL, Lesclous P. Mast cell activation and degranulation occur early during induction of periosteal bone resorption. Bone. 2006;38(1):59–66. doi: 10.1016/j.bone.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 77.Gurish MF, Ghildyal N, Arm J, Austen KF, Avraham S, Reynolds D, Stevens RL. Cytokine mRNA are preferentially increased relative to secretory granule protein mRNA in mouse bone marrow-derived mast cells that have undergone IgE-mediated activation and degranulation. J Immunol. 1991;146(5):1527–1533. [PubMed] [Google Scholar]
- 78.Morrison DC, Roser JF, Cochrane CG, Henson PM. The initiation of mast cell degranulation: activation at the cell membrane. J Immunol. 1975;114(3):966–970. [PubMed] [Google Scholar]
- 79.Kaliner M, Shelhamer JH, Ottesen EA. Effects of infused histamine: correlation of plasma histamine levels and symptoms. J Allergy Clin Immunol. 1982;69(3):283–289. doi: 10.1016/s0091-6749(82)80005-5. [DOI] [PubMed] [Google Scholar]
- 80.Morel DR, Skoskiewicz M, Robinson DR, Bloch KJ, Hoaglin DC, Zapol WM. Leukotrienes, thromboxane A2, and prostaglandins during systemic anaphylaxis in sheep. Am J Physiol. 1991;261(3 Pt 2):H782–792. doi: 10.1152/ajpheart.1991.261.3.H782. [DOI] [PubMed] [Google Scholar]
- 81.Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, Simons FE, Simons KJ, Cass D, Yeung J. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358(1):28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 82.Pretolani M, Lellouch-Tubiana A, Lefort J, Bachelet M, Vargaftig BB. PAF-acether and experimental anaphylaxis as a model for asthma. Int Arch Allergy Appl Immunol. 1989;88(1–2):149–153. doi: 10.1159/000234770. [DOI] [PubMed] [Google Scholar]
- 83.Felix SB, Baumann G, Hashemi T, Niemczyk M, Ahmad Z, Berdel WE. Characterization of cardiovascular events mediated by platelet activating factor during systemic anaphylaxis. J Cardiovasc Pharmacol. 1990;15(6):987–997. [PubMed] [Google Scholar]
- 84.Gordon JR, Galli SJ. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J Exp Med. 1991;174(1):103–107. doi: 10.1084/jem.174.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Comaish JS. The role of the mast cells and basophils in hypersensitivity. Br J Dermatol. 1965;77:92–97. doi: 10.1111/j.1365-2133.1965.tb14605.x. [DOI] [PubMed] [Google Scholar]
- 86.von Bubnoff D, Novak N, Kraft S, Bieber T. The central role of FcepsilonRI in allergy. Clin Exp Dermatol. 2003;28(2):184–187. doi: 10.1046/j.1365-2230.2003.01209.x. [DOI] [PubMed] [Google Scholar]
- 87.Maurer D, Ebner C, Reininger B, Petzelbauer P, Fiebiger E, Stingl G. Mechanisms of Fc epsilon RI-IgE-facilitated allergen presentation by dendritic cells. Adv Exp Med Biol. 1997;417:175–178. [PubMed] [Google Scholar]
- 88.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20(12):561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 89.Karras JG, McGraw K, McKay RA, Cooper SR, Lerner D, Lu T, Walker C, Dean NM, Monia BP. Inhibition of antigen-induced eosinophilia and late phase airway hyperresponsiveness by an IL-5 antisense oligonucleotide in mouse models of asthma. J Immunol. 2000;164(10):5409–5415. doi: 10.4049/jimmunol.164.10.5409. [DOI] [PubMed] [Google Scholar]
- 90.Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med. 2001;344(1):30–37. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 91.Berin MC, Mayer L. Immunophysiology of experimental food allergy. Mucosal Immunol. 2009;2(1):24–32. doi: 10.1038/mi.2008.72. [DOI] [PubMed] [Google Scholar]
- 92.Muller UR. New developments in the diagnosis and treatment of hymenoptera venom allergy. Int Arch Allergy Immunol. 2001;124(4):447–453. doi: 10.1159/000053779. [DOI] [PubMed] [Google Scholar]
- 93.Ying S, Robinson DS, Meng Q, Barata LT, McEuen AR, Buckley MG, Walls AF, Askenase PW, Kay AB. C-C chemokines in allergen-induced late-phase cutaneous responses in atopic subjects: association of eotaxin with early 6-hour eosinophils, and of eotaxin-2 and monocyte chemoattractant protein-4 with the later 24-hour tissue eosinophilia, and relationship to basophils and other C-C chemokines (monocyte chemoattractant protein-3 and RANTES) J Immunol. 1999;163(7):3976–3984. [PubMed] [Google Scholar]
- 94.Robinson DS, Hamid Q, Jacobson M, Ying S, Kay AB, Durham SR. Evidence for Th2-type T helper cell control of allergic disease in vivo. Springer Semin Immunopathol. 1993;15(1):17–27. doi: 10.1007/BF00204623. [DOI] [PubMed] [Google Scholar]
- 95.Chang-Yeung M, Lam S, Kennedy SM, Frew AJ. Persistent asthma after repeated exposure to high concentrations of gases in pulpmills. Am J Respir Crit Care Med. 1994;149(6):1676–1680. doi: 10.1164/ajrccm.149.6.8004329. [DOI] [PubMed] [Google Scholar]
- 96.Arguelles M, Blanco I. Inflammatory bronchial polyps associated with asthma. Arch Intern Med. 1983;143(3):570–571. doi: 10.1001/archinte.1983.00350030184034. [DOI] [PubMed] [Google Scholar]
- 97.Macfarlane AJ, Kon OM, Smith SJ, Zeibecoglou K, Khan LN, Barata LT, McEuen AR, Buckley MG, Walls AF, Meng Q, Humbert M, Barnes NC, Robinson DS, Ying S, Kay AB. Basophils, eosinophils, and mast cells in atopic and nonatopic asthma and in late-phase allergic reactions in the lung and skin. J Allergy Clin Immunol. 2000;105(1 Pt 1):99–107. doi: 10.1016/s0091-6749(00)90184-2. [DOI] [PubMed] [Google Scholar]
- 98.Elmgreen J, Stahl Skov P, Permin H, Binder V, Heugh Wandall J, Norn S. Type I allergy to normal cellular constituents in chronic inflammatory bowel disease? Results from basophil histamine release test compared with total IgE and antinuclear antibodies. Allergy. 1984;39(1):23–28. doi: 10.1111/j.1398-9995.1984.tb01929.x. [DOI] [PubMed] [Google Scholar]
- 99.Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6(2):151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- 100.Holt PG, Macaubas C, Stumbles PA, Sly PD. The role of allergy in the development of asthma. Nature. 1999;402((6760 Suppl)):B12–B17. doi: 10.1038/35037009. [DOI] [PubMed] [Google Scholar]
- 101.Dolovich J, Hargreave FE, Chalmers R, Shier KJ, Gauldie J, Bienenstock J. Late cutaneous allergic responses in isolated IgE-dependent reactions. J Allergy Clin Immunol. 1973;52(1):38–46. doi: 10.1016/0091-6749(73)90119-x. [DOI] [PubMed] [Google Scholar]
- 102.Milgrom H. Attainments in atop: special aspects of allergy and IgE. Adv Pediatr. 2002;49:273–297. [PubMed] [Google Scholar]
- 103.Gospos A, Dreikhausen U, Dartsch DC, Szamel M, Hockertz S. Development of an allergy test model: activation of human mast cells with potentially allergenic substances. Toxicology. 2001;166(1–2):91–96. doi: 10.1016/s0300-483x(01)00444-9. [DOI] [PubMed] [Google Scholar]
- 104.Halteren AG, Cammen MJ, Biewenga J, Savelkoul HF, Kraal G. IgE and mast cell response on intestinal allergen exposure: a murine model to study the onset of food allergy. J Allergy Clin Immunol. 1997;99(1 Pt 1):94–99. doi: 10.1016/s0091-6749(97)70305-1. [DOI] [PubMed] [Google Scholar]
- 105.Toru H, Pawankar R, Ra C, Yata J, Nakahata T. Human mast cells produce IL-13 by high-affinity IgE receptor cross-linking: enhanced IL-13 production by IL-4-primed human mast cells. J Allergy Clin Immunol. 1998;102(3):491–502. doi: 10.1016/s0091-6749(98)70140-x. [DOI] [PubMed] [Google Scholar]
- 106.Haselden BM, Kay AB, Larche M. Immunoglobulin E-independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J Exp Med. 1999;189(12):1885–1894. doi: 10.1084/jem.189.12.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Herz U, Botchkarev VA, Paus R, Renz H. Increased airway responsiveness, allergy-type-I skin responses and systemic anaphylaxis in a humanized-severe combined immuno-deficiency mouse model. Clin Exp Allergy. 2004;34(3):478–487. doi: 10.1111/j.1365-2222.2004.01887.x. [DOI] [PubMed] [Google Scholar]
- 108.Berger P, N’Guyen C, Buckley M, Scotto-Gomez E, Marthan R, Tunon-de-Lara JM. Passive sensitization of human airways induces mast cell degranulation and release of tryptase. Allergy. 2002;57(7):592–599. doi: 10.1034/j.1398-9995.2002.203545.x. [DOI] [PubMed] [Google Scholar]
- 109.Fahy JV, Fleming HE, Wong HH, Liu JT, Su JQ, Reimann J, Fick RB, Jr, Boushey HA. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med. 1997;155(6):1828–1834. doi: 10.1164/ajrccm.155.6.9196082. [DOI] [PubMed] [Google Scholar]
- 110.Benoist C, Mathis D. Mast cells in autoimmune disease. Nature. 2002;420(6917):875–878. doi: 10.1038/nature01324. [DOI] [PubMed] [Google Scholar]
- 111.Pachiappan A, Thwin MM, Manikandan J, Gopalakrishnakone P. Glial inflammation and neurodegeneration induced by candoxin, a novel neurotoxin from Bungarus candidus venom: global gene expression analysis using microarray. Toxicon. 2005;46(8):883–899. doi: 10.1016/j.toxicon.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 112.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol. 2005;77(3):388–399. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- 113.Rundle CH, Wang H, Yu H, Chadwick RB, Davis EI, Wergedal JE, Lau KH, Mohan S, Ryaby JT, Baylink DJ. Microarray analysis of gene expression during the inflammation and endochondral bone formation stages of rat femur fracture repair. Bone. 2006;38(4):521–529. doi: 10.1016/j.bone.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 114.Shiue HS, Lee YS, Tsai CN, Hsueh YM, Sheu JR, Chang HH. DNA microarray analysis of the effect on inflammation in patients treated with acupuncture for allergic rhinitis. J Altern Complement Med. 2008;14(6):689–698. doi: 10.1089/acm.2007.0669. [DOI] [PubMed] [Google Scholar]
- 115.Jayapal M, Tay HK, Reghunathan R, Zhi L, Chow KK, Rauff M, Melendez AJ. Genome-wide gene expression profiling of human mast cells stimulated by IgE or FcepsilonRI-aggregation reveals a complex network of genes involved in inflammatory responses. BMC Genomics. 2006;7:210. doi: 10.1186/1471-2164-7-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saito H, Nakajima T, Matsumoto K. Human mast cell transcriptome project. Int Arch Allergy Immunol. 2001;125(1):1–8. doi: 10.1159/000053790. [DOI] [PubMed] [Google Scholar]
- 117.Kuramasu A, Kubota Y, Matsumoto K, Nakajima T, Sun XM, Watanabe T, Saito H, Ohtsu H. Identification of novel mast cell genes by serial analysis of gene expression in cord blood-derived mast cells. FEBS Lett. 2001;498(1):37–41. doi: 10.1016/s0014-5793(01)02477-2. [DOI] [PubMed] [Google Scholar]
- 118.Wakahara S, Fujii Y, Nakao T, Tsuritani K, Hara T, Saito H, Ra C. Gene expression profiles for Fc epsilon RI, cytokines and chemokines upon Fc epsilon RI activation in human cultured mast cells derived from peripheral blood. Cytokine. 2001;16(4):143–152. doi: 10.1006/cyto.2001.0958. [DOI] [PubMed] [Google Scholar]
- 119.Nakajima T, Matsumoto K, Suto H, Tanaka K, Ebisawa M, Tomita H, Yuki K, Katsunuma T, Akasawa A, Hashida R, Sugita Y, Ogawa H, Ra C, Saito H. Gene expression screening of human mast cells and eosinophils using high-density oligonucleotide probe arrays: abundant expression of major basic protein in mast cells. Blood. 2001;98(4):1127–1134. doi: 10.1182/blood.v98.4.1127. [DOI] [PubMed] [Google Scholar]
- 120.Nakamura R, Ishida S, Ozawa S, Saito Y, Okunuki H, Teshima R, Sawada J. Gene expression profiling of Ca2+ -ATPase inhibitor DTBHQ and antigen-stimulated RBL-2H3 mast cells. Inflamm Res. 2002;51(12):611–618. doi: 10.1007/pl00012436. [DOI] [PubMed] [Google Scholar]
- 121.Nakajima T, Inagaki N, Tanaka H, Tanaka A, Yoshikawa M, Tamari M, Hasegawa K, Matsumoto K, Tachimoto H, Ebisawa M, Tsujimoto G, Matsuda H, Nagai H, Saito H. Marked increase in CC chemokine gene expression in both human and mouse mast cell transcriptomes following Fcepsilon receptor I cross-linking: an interspecies comparison. Blood. 2002;100(12):3861–3868. doi: 10.1182/blood-2002-02-0602. [DOI] [PubMed] [Google Scholar]
- 122.Okumura S, Kashiwakura J, Tomita H, Matsumoto K, Nakajima T, Saito H, Okayama Y. Identification of specific gene expression profiles in human mast cells mediated by Toll-like receptor 4 and FcepsilonRI. Blood. 2003;102(7):2547–2554. doi: 10.1182/blood-2002-12-3929. [DOI] [PubMed] [Google Scholar]
- 123.Babina M, Guhl S, Starke A, Kirchhof L, Zuberbier T, Henz BM. Comparative cytokine profile of human skin mast cells from two compartments–strong resemblance with monocytes at baseline but induction of IL-5 by IL-4 priming. J Leukoc Biol. 2004;75(2):244–252. doi: 10.1189/jlb.0403157. [DOI] [PubMed] [Google Scholar]
- 124.Matsuda K, Piliponsky AM, Iikura M, Nakae S, Wang EW, Dutta SM, Kawakami T, Tsai M, Galli SJ. Monomeric IgE enhances human mast cell chemokine production: IL-4 augments and dexamethasone suppresses the response. J Allergy Clin Immunol. 2005;116(6):1357–1363. doi: 10.1016/j.jaci.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 125.Simons FE. World Allergy Organization survey on global availability of essentials for the assessment and management of anaphylaxis by allergy-immunology specialists in health care settings. Ann Allergy Asthma Immunol. 2010;104(5):405–412. doi: 10.1016/j.anai.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 126.Demain JG, Minaei AA, Tracy JM. Anaphylaxis and insect allergy. Curr Opin Allergy Clin Immunol. 2010;10(4):318–322. doi: 10.1097/ACI.0b013e32833a6c72. [DOI] [PubMed] [Google Scholar]
- 127.Garman SC, Sechi S, Kinet JP, Jardetzky TS. The analysis of the human high affinity IgE receptor Fc epsilon Ri alpha from multiple crystal forms. J Mol Biol. 2001;311(5):1049–1062. doi: 10.1006/jmbi.2001.4929. [DOI] [PubMed] [Google Scholar]
- 128.Leavy O Therapeutic antibodies: past, present and future. Nat Rev Immunol 10 (5):297 [DOI] [PubMed]
- 129.Chan AC, Carter PJ Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol 10 (5):301–316 [DOI] [PubMed]
- 130.Bhattacharjee RN, Banerjee B, Akira S, Hande MP. Telomere-mediated chromosomal instability triggers TLR4 induced inflammation and death in mice. PLoS One. 2010;5(7):e11873. doi: 10.1371/journal.pone.0011873. [DOI] [PMC free article] [PubMed] [Google Scholar]