Abstract
The dynorphin/κ-opioid receptor system has been implicated in the pathogenesis and pathophysiology of several psychiatric disorders. In the present review, we present evidence indicating a key role for this system in modulating neurotransmission in brain circuits that subserve mood, motivation, and cognitive function. We overview the pharmacology, signaling, post-translational, post-transcriptional, transcriptional, epigenetic and cis regulation of the dynorphin/κ-opioid receptor system, and critically review functional neuroanatomical, neurochemical, and pharmacological evidence, suggesting that alterations in this system may contribute to affective disorders, drug addiction, and schizophrenia. We also overview the dynorphin/κ-opioid receptor system in the genetics of psychiatric disorders and discuss implications of the reviewed material for therapeutics development.
Keywords: Dynorphin, κ-Opioid receptor, Psychiatric disorder, Pharmacology, Neuroanatomy
Introduction
Psychiatric disorders constitute a global health problem. Depression, drug addiction, and schizophrenia affect ca. 120, 90, and 25 million people, respectively [1]. These numbers, however, do not accurately reflect the disease burden attributable to psychiatric disorders as one out of four families have at least one affected member. Although phenotypes differ between and within these disorders, they are characterized by alterations in cognition, emotion, motivation, and stress reactivity. Co-morbidity between disorders is high suggesting common neural substrates [2]. Consistently, imaging studies have shown altered activity in the amygdala, hippocampus, basal ganglia, and prefrontal cortex of psychiatric patients [3]; areas involved in stress responsiveness, emotional reactivity, goal-directed behavior, motivation, and executive function.
The κ-opioid receptor (KOR) and its postulated endogenous ligands, the dynorphin peptides (DYNs) [4], are enriched in the above brain regions and modulate neurotransmission therein. Increasing data suggest that dysregulation of this system may contribute to the development and maintenance of various psychiatric disorders [5], and recent years have seen an explosion of studies on this topic. In the present review, we first overview the pharmacology, signaling, post-translational, post-transcriptional, transcriptional, epigenetic, and cis regulation of the DYN/KOR system. We then review functional neuroanatomical, neurochemical and pharmacological evidence supporting a physiological role for this system in modulating neurotransmission in brain regions implicated in the pathogenesis and pathophysiology of drug addiction, affective disorders, and schizophrenia. Lastly, we overview the DYN/KOR system in the genetics of psychiatric disorders and discuss implications of the reviewed material for medications development.
DYN/KOR system—pharmacology, signaling, and post-translational regulation
DYN peptides are derived from prodynorphin (PDYN) [6]. Although DYN A1–17 (DYN A) is considered the endogenous KOR ligand [7], a number of smaller, biologically active DYN as have been identified including DYN A1–8 and DYN A1–13 [8, 9]. Other biologically active DYNs include DYN B1–13 (DYN B/rimorphin), DYN B1–29 (leumorphin), DYN A/B1–32 (big DYN) and α- and β-neoendorphin [10–14]. The affinity and efficacy of these peptides differ with DYN A being the most potent and β-neoendorphin the least [15, 16]. Importantly, DYNs bind with high affinity to other opioid and non-opioid receptors (e.g., acid-sensing ion channels and NMDA receptors), [17–19]. The CNS distribution of DYNs varies with species and brain region. In general, α-neoendorphin is the most abundant DYN and DYN A the least with the largest expression difference observed in substantia nigra pars compacta (SNc) [20–22].
Although additional of processing enzymes have been identified, DYN biosynthesis in vivo in the mouse brain appears governed primarily by the non-selective proteases cathepsin L, PC1, 2 and 3 and CPE [23–25]. Their regional expression and functional coupling them between may therefore underlie regional variations in DYN expression [26, 27]. Interestingly, cathepsin L and α-neoendorphin and DYNs A and B display vesicular co-localization in mouse primary cortical neurons [25], while PDYN is observed in vesicles in axonal and synaptic compartments in the rat brain [28]. Depolarization-induced PDYN processing and release of DYN A and B have been observed in rat primary cortical neurons. More work, however, is needed to identify which DYNs are released in vivo; clues to which may come from investigation of the roles of post-translational modifications and product inhibition in DYN biosynthesis [26, 29]. This information may help explain the relative abundance of α-neoendorphin in the brain [20–22], despite it being the least resistant to proteolytic cleavage in vitro [30].
KOR is a G-protein coupled receptor [31–33]. At least three subtypes have been suggested based on pharmacology. It seems likely, however, that these result from alternative splicing, post-translational modifications and/or protein–protein interactions [34, 35]. KOR reportedly couples to both inhibitory Gβγ, Gαi, Gαo, Gαz and Gα16, and stimulatory, Gαs, G-proteins [36]. Nanomolar ligand concentrations result in coupling of KOR to inhibitory G proteins, decreasing membrane excitability and transmitter release via stimulation of K+-channel activity (e.g., in guinea pig substantia gelatinosa slices) [37], and inhibition of Ca2+-channel and presynaptic release machinery activity (e.g., in rat primary nodose root ganglion neurons [38], and the paraventricular nucleus of the hypothalamus [39], respectively). In contrast, sub-nanomolar ligand concentrations may result in coupling of KOR to Gαs and produce opposite effects on ion channel conductance, prolonging action potential duration [40]. Not surprisingly therefore, KOR agonists produce an inverted U-shaped dose-response curve. The complexity of KOR signaling is reflected in the ability of agonists to stimulate, inhibit and/or not affect all major second messenger systems (i.e., cAMP, IP3/DAG, and Ca2+) depending on cell line/type and experimental conditions used, and in the numerous downstream effectors identified [36, 41, 42].
Post-translational regulation of KOR agonist responsiveness occurs through three distinct processes: (1) desensitization (sec–min), (2) internalization (min–h), and (3) resensitization or down-regulation (h–days) [43]. DYNs A and B, α-neoendorphin, the naturally occurring KOR agonist, salvinorin A, and several synthetic agonists (e.g., U50,488H and U69,593) induce desensitization, internalization and down-regulation of KOR in heterologous expression systems [16, 44–46]. In contrast, other synthetic agonists and antagonists (e.g., etorphine and norbinaltorphimine (BNI), respectively) have opposite effects. These paradoxical findings may be explained by chaperone-like effects of some membrane-permeant ligands as suggested by their varying ability to also promote resensitization [47, 48]. Ligands may also direct KOR signaling via differential activation of downstream effectors [49]. The notion that regulation of KOR signaling and trafficking/biosynthesis is ligand-directed has spawned interest in the development of drugs locking this receptor in favorable conformational states such that dissociation of desired and undesired behavioral responses to its activation will be maximal.
Regulation of pdyn and oprk1 in cis (gene regulation intrinsic to DNA)
No consensus gene and protein nomenclature exist across species. In accordance with the human gene nomenclature [50], we denote abbreviated gene names in italic and capital letters and abbreviated protein names in capital letters. For other species, abbreviated gene and protein names are preceded by species when appropriate and denoted in italic and lowercase letters and capital letters, respectively. The same nomenclature is applied when referring to abbreviated gene and protein names in general. We hope that these distinctions will aid interpretation of the reviewed material. pdyn has four exons in human, mouse and rat [51–53]. PDYN contains multiple transcription start sites located in exons 1 and 4 and introns 1 and 2 [54, 55]. Although in silico analysis suggests a similar transcription start site usage profile for mouse pdyn, putative human-specific alternative promoter usage has been shown/predicted for brain and testis with transcription start sites in exon 4 and intron 2, respectively. Consistently, brain- and testis-specific PDYN transcripts have been identified [54, 56, 57]. Like pdyn, the KOR gene, oprk1, has four exons in human, mouse and rat [58–60]. Mouse oprk1 reportedly has dual promoters and multiple transcription start sites located in exon 1 and intron 1 [61]. Although in silico analysis suggests a similar transcription start site usage profile for OPRK1, human-specific alternative promoter usage is predicted for brain and testis with transcription start sites in exon 2 and intron 2, respectively [55]. As transcription start sites are coupled to cis-regulatory elements (DNA sequences that influence gene expression) [62], further characterization of the promoters of PDYN and OPRK1 may provide insights into transcriptional control of these genes and their dysregulation in psychiatric disorders.
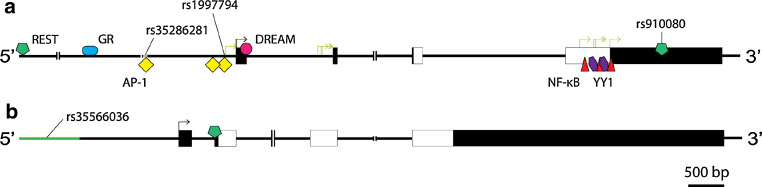
PDYN is regulated in cis by copy number variants and single nucleotide polymorphisms (SNPs) located in the promoter region of this gene (Fig. 1) [63–65]. Among these, attempts have been made to associate rs35286281 (copy number variant) (unsuccessful) and rs1997794 (SNP) (successful) with have been associated with allelic imbalance (differential expression of alleles at one or more loci) in the cortex and cerebellum [52, 64, 66, 67]. Support for a role of these variants in PDYN regulation comes from in silico analysis and in vitro binding studies suggesting that they may be targeted by the transcriptional control protein, AP-1 [68, 69]. Alternatively, the causative variant may be one in linkage disequilibrium (nonrandom association of alleles at two or more loci) with rs35286281 and rs1997794. A candidate variant is rs910080 (SNP) located in the 3′-untranslated region of PDYN [64]. Interestingly, in the one subject analyzed who was homozygous for this locus, no allelic imbalance was observed [69]. Moreover, rs910080 overlaps with a response element bound by the transcriptional control protein REST in vivo in various cell lines (Fig. 1) [70], and dominant negative REST increases endogenous PDYN expression in human neuroblastoma SH-SY5Y cells [71]. Consistent with roles of 3′-untranslated region variants in PDYN regulation, a region comprising PDYN, the neighboring gene STK-35 and regions 3′ of the two bears the signature of recent positive selection (evolutionary increase in the frequency of a genomic region) [72]. Although rs35566036 (indel) located in the promoter of OPRK1 has been shown to influence reporter gene expression in vitro (Fig. 1) [73], comparatively little is known about regulation of this gene in cis. Further assessment of the mechanisms by which rs35286281, rs1997794, rs910080, and rs35566036 affect gene expression is important as they have all been associated with psychiatric disorders (overviewed below).
Fig. 1.
To scale schematics of PDYN (a) and OPRK1 (b). Shown are functional sequence variants associated with psychiatric disorders (e.g., rs35286281) and transcriptional control proteins implicated in the regulation of these genes (positioned relative to the transcription start sites). Transcriptional control proteins for which there is in vivo evidence of binding are depicted above the sequence (e.g., REST), and those for which only in vitro evidence is available below the sequence (e.g., AP-1). ║ genomic regions omitted for clarity, boxes exons, white boxes coding regions, black arrows transcription start sites, green arrows alternative transcription start sites
Epigenetic regulation of pdyn and oprk1
Repeated cocaine administration increases pdyn expression in the striatum of rodents and non-human primates (reviewed below). Cocaine was recently shown to increase active histone marks (i.e., H4 acetylation) without affecting repressive ones (i.e., H3K9 and H3K27 methylation) on a nucleosome spanning the transcription start site in mouse pdyn [74]. Methylated CpG islands (genomic regions containing a high frequency of CpG dinucleotides) and dinucleotides have been identified in the promoters of PDYN and OPRK1 and in intron 2 of OPRK1, but the role of this epigenetic mark in the transcriptional control of these genes is unclear [75–77]. Mouse oprk1 expression is affected by trophic agents [78]. Four-day treatment of mouse P19 cells with retinoic acid results in repression of oprk1 expression via recruitment of chromatin remodeling factors (i.e., BAF155 and BRG-1) to promoter 1, acquisition of repressive histone marks (i.e., H4 deacetylation and H3K9 methylation), subsequent chromatin condensation by two adjacent nucleosomes spanning the transcription start site and parallel alterations in transcriptional control protein binding. If further differentiated with NGF, promoter 2 is activated via transcriptional control protein recruitment and acquisition of active histone marks (i.e., H3K9 demethylation and H3K4 dimethylation). It should be noted that while some predictions can be made on the basis of a ‘histone code’ [79], the presence of active or repressive chromatin marks on a promoter does not always correlate with gene expression [80]. The predictive value of a single mark or even a combination of marks on a given transcriptional outcome is therefore limited. Moreover, histone modifications and DNA methylation are not all classical epigenetic marks (i.e., heritable changes in chromatin function without alterations in the primary DNA sequence). Thus, assessment of their heritability may aid in the evaluation of PDYN and OPRK1 as susceptibility loci for psychiatric disorders.
A gene encoding a long non-coding RNA, AK090681, is transcribed from the opposite strand of PDYN [81]. Long non-coding RNAs may regulate gene expression via chromatin remodeling [82]. However, PDYN and AK090681 may be separate but overlapping transcription units as suggested by the exon locations of AK090681. Moreover, this gene appears to be actively transcribed in human embryonic stem cells while PDYN does not. Interestingly, the promoter of AK090681 contains a CpG island which methylation status may correlate with that of H3K27, suggesting that AK090681 may be involved in human embryonic stem cell differentiation [75, 76]. Moreover, rs6136489 (SNP) located in the promoter of AK090681 was recently associated with mean platelet volume (parameter affecting for example cardiovascular function) in a genome-wide meta-analysis [83]. Two transcripts lacking exons 3 and 4 have been reported for AK090681 [55, 84]. These exons enclose PDYN, and exon 3 and intron 3 of AK090681 contain response elements bound by the ‘master weaver’ CTCF and cohesin in vivo in a number of cell lines [85, 86]. CTCF may be a heritable component in epigenetic control, which regulates the interplay between DNA methylation, higher-order chromatin structure and lineage-specific gene expression [87]. Thus, the transcription units of AK090681 and PDYN may be separated by CTCF-mediated chromatin looping.
Regulation of pdyn and oprk1 in trans (gene regulation extrinsic to DNA)
Although in vitro studies have implicated numerous transcriptional control proteins in PDYN regulation (Fig. 1) [88, 89], we will limit our discussion to those for which there are evidence of binding in vivo. PDYN is targeted by the glucocorticoid receptor (GR) in human A549 cells (Fig. 1) [90]. GR binding is responsive to the synthetic GR agonist, dexamethasone, whereas PDYN expression is not. Thus, GR may either not be involved in PDYN regulation, or loss or gain of interactions in cis and/or trans are required to unmask an effect of glucocorticoid signaling on PDYN expression. Although the cognate response element(s) has not been identified, support for the latter notion comes from studies showing that treatment of rats with dexamethasone or another synthetic glucocorticoid, budesonide, alters hippocampal and spinal cord pdyn expression, respectively [91–93]. Moreover, GR binding to PDYN in A549 cells does not correlate with that of RNA polymerase II, suggesting that PDYN is not actively transcribed in these cells [90]. Although a useful indicator, binding therefore does not exclude/include involvement of a transcriptional control protein in the regulation of a given gene [94]. Moreover, whether the native chromatin state is present in immortalized cell lines/cancers has been questioned. Direct involvement of GR in PDYN regulation may be of clinical relevance given the growing body of evidence suggesting a role for the DYN/KOR system in stress (reviewed below).
The transcriptional control proteins AP-1, CREB and DREAM are implicated in a variety of physiological processes including Ca2+-signaling and synaptic plasticity [95, 96], and appear to play overlapping roles in pdyn regulation [97–100]. AP-1 and CREB may activate, repress, or not affect pdyn expression depending on interactions in cis and trans [101–104]. It should be noted, however, that with the exception of a non-canonical AP-1 site identified by Naranjo et al. [68], neither of the response elements for AP-1 and CREB identified in mouse and rat pdyn is present in PDYN. Moreover, PDYN has never been identified as a target for any AP-1 or CREB family member in vivo [105–108]. Thus, identification of the response elements in pdyn bound by AP-1 and CREB in vivo may provide valuable insights into species- and/or allele-specific regulation of this gene and its dysregulation in psychiatric disorders. Further insights into the roles of these transcriptional control proteins in PDYN regulation may also come from protein interaction studies as the CREB family member αCREM has been implicated transcriptional control of this gene via interaction with DREAM [99]. Contrary to AP-1 and CREB, the expression data on DREAM is consistent and suggest that this protein represses pdyn expression [98, 109]. Interestingly, the aversive effects of Δ-tetrahydrocannabinol, but not cocaine and morphine, are potentiated in a KOR-dependent manner in kcnip3 knockout mice, implicating this transcriptional control protein in drug-induced dysregulation of the DYN/KOR system [110].
The transcriptional control proteins AP-2, C-MYC, Ikaros, MAD and MAX are involved in cell proliferation, lineage commitment and differentiation [111–113], and have been implicated in the transcriptional control of mouse oprk1 [78]. Ikaros suppresses oprk1 expression in P19 cells, while a shift in binding of C-MYC and MAX to MAD and MAX parallels the transition from high to low constitutive expression of oprk1 transcripts initiated from promoter 1 which occurs during development. AP-2β appears to activate transcription from promoter 2, which is higher postnatally. Interestingly, AP-2β is implicated in the regulation of a number of genes involved in monoamine neurotransmission [e.g., SLC6A3 (dopamine transporter (DAT)] and TH [tyrosine hydroxylase)] and rs55733871 (copy number variant) located in intron 2 of the AP-2β gene, TFAP2B, is associated with anxiety-related personality traits [114]. Thus, gene–gene interaction studies on OPRK1 and TFAP2B are warranted. It should be noted, however, that of the cognate response elements for these transcriptional control proteins identified in mouse, only two are present in OPRK1 [60]. Moreover, OPRK1 appears not to be targeted by C-MYC in vivo [115, 116]. These findings support a role for cis-regulatory divergence (measure of the evolutionary conservation of genomic regulatory regions) in pdyn and oprk1 regulation as has been suggested for human and mouse on a genome-wide scale [117].
REST is implicated in development and disease (e.g., Huntington’s disease) [118]. Of the two response elements for REST identified in PDYN (Fig. 1) [70], binding to the one mentioned above (see Regulation of pdyn and oprk1 in cis) is not apparent in all cell lines analyzed indicating a role for REST in lineage-specific regulation of PDYN [119]. Although the cognate response element(s) has not been identified, OPRK1 may also be restrictively targeted by REST in vivo in human glioblastoma U87 cells [70, 120]. The transcriptional control protein MeCP2 has been implicated in development and disease (e.g., Rett syndrome) [121], and was recently found to target mouse oprk1 and activate its transcription in vivo in the hypothalamus perhaps via recruitment of CREB [121]. Interestingly, REST, MeCP2 and CREB are parts of a regulatory network identified using comparative genomics involving multiple microRNAs (e.g., miR-9 and miR-132/212) [123–125]. Intriguingly, like dominant negative REST, ectopic expression of miR-9 increases endogenous PDYN expression in SH-SY5Y cells [71]. Moreover, parts of this network have recently been implicated in psychiatric disorders [126–129]. Thus, aberrant network activity may underlie some instances of altered PDYN and OPRK1 expression in these disorders.
Post-transcriptional regulation of pdyn and oprk1
Multiple pdyn transcripts have been reported for human, mouse and rat [54–57, 130], and alternatively spliced pdyn mRNAs have been reported [54, 57]. Transcript multiplicity has been suggested to provide yet another level of control over PDYN and KOR expression in addition to those overviewed above [131]. In some cases, however, the translation products of these messages may have novel functions as suggested by the nuclear localization of the truncated PDYN ‘T1’ in African green monkey COS-1 cells and their sequence similarity to transcriptional control proteins [54, 132]. Multiple oprk1 transcripts have been reported for human, mouse, and rat [55, 59, 61]. Although alternative splicing has been reported for human OPRK1 [133], post-transcriptional regulation of oprk1 has been studied almost exclusively in mouse. Differential stability and translation efficacy has been reported for oprk1 transcripts in P19 cells [134, 135]. EGF stimulation results in SHP-2-mediated dephosphorylation of the growth factor receptor bound protein GRB7, recruitment of the HUR–exportin-1 complex and nuclear export of oprk1 mRNA in P19 cells and dorsal root ganglion neurons [136]. EGF and the axonal guidance cue netrin-1 also induce FAK-mediated phosphorylation of GRB7 and derepression of oprk1 mRNA translation in the cytoplasm [137–139]. Intriguingly, depolarization-induced, possibly COPB1 and HUR-mediated, axonal transport and local translation of mouse oprk1 transcripts in terminals have been demonstrated in these cell models [140–142]. The high spatial and temporal resolution analyses of mouse oprk1 epigenetic, transcriptional and post-transcriptional regulation provided by Wei Laboratories, Inc., is key to defining the physiological role of the DYN/KOR system [143], and parallel studies in other species are warranted.
Functional DYN/KOR neuroanatomy
The CNS distribution of DYN and KOR has been detailed elsewhere [144]. Therefore, this review will focus on their localization and function in brain circuitry implicated in psychiatric disorders. Moderate to high DYN and KOR expression is observed in the cortex, basal ganglia, hippocampus, amygdala, thalamus, as well as monoaminergic midbrain and brainstem structures. In some regions, clear overlap of DYN and KOR expression is not observed, suggesting that signaling therein may depend on volume transmission. Alternatively, distribution discrepancies may be explained by differences in methodology (untreated vs. colchicine-treated), species, or gender.
Dorsal and ventral striatum
The dorsal and ventral striatum, which include the nucleus accumbens (NAcc) and olfactory tubercles, are involved in movement execution/habit formation and motivation/reward, respectively. Both the dorsal and ventral striatum are primarily composed of medium-sized spiny neurons (MSNs) that receive convergent glutamatergic and dopaminergic (DAergic) inputs [145]. The ventral striatum receives dense DAergic fibers from the ventral tegmental area (VTA) and to a lesser extent from the SNc. Conversely, the dorsal striatum is primarily innervated by SNc-originating DA fibers, with little VTA innervation. KORs and DYN are highly enriched in dorsal and ventral striatal compartments (see Fig. 2) [144, 146–150]. In both, DYN is expressed in GABAergic MSNs that preferentially express D1 DA receptors [151, 152]. Electron microscopy studies have shown that striatal KORs are localized on DA varicosities in close apposition to the dopamine transporter (DAT) and, to a lesser extent, on asymmetric, presumably excitatory synapses [149, 153]. Systemic or local administration of KOR agonists into either striatal sub-region decreases basal and stimulated DA efflux [154–157]. Conversely, systemic administration of the selective KOR antagonist, norbinaltorphimine (nor-BNI) elevates NAcc dialysate DA levels [158]. Furthermore, striatal and accumbal KORs tonically inhibit basal dopamine overflow [155, 159] such that intra-striatal or intra-accumbal KOR blockade elevates DA overflow.
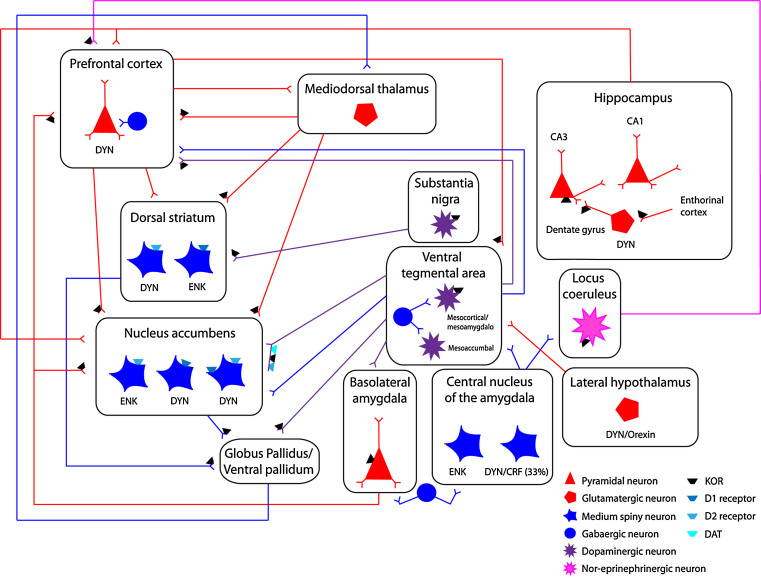
Fig. 2.
The figure depicts a simplified scheme of neuronal circuits implicated in psychiatric disorders that are modulated by DYN/KOR pathways. DYN-positive neurons are located in the hypothalamus, central nucleus of the amygdala, cortical, and striatal regions and innervate neural substrates rich in KORs. DYN-positive neurons are innervated by glutamatergic and monoaminergic fibers containing presynaptic KORs, providing a physiological substrate for DYN/KOR to modulate presynaptic monoaminergic neurotransmission. The inset provides a key of neuronal/fiber type and simplified scheme of localization of KORs and DYN-positive neurons
Striatal KORs are also found on terminals of asymmetric, presumably excitatory synapses [149, 153]. As such, KORs are positioned to affect excitatory transmission. Consistent with localization of NAcc KORs on asymmetric synapses, in vitro electrophysiological studies have shown that KOR activation inhibits presynaptic glutamate release onto MSNs in NAcc [160, 161]. This finding is in accord with in vivo and in vitro neurochemical studies [162–165] demonstrating that KOR activation decreases evoked, but not basal, glutamate overflow. More specifically, KOR activation decreases Ca2+-dependent, but not Ca2+-independent, evoked glutamate overflow. However, inhibition of glutamate release onto MSNs by the selective KOR agonist, U69,593, was not blocked by Cd2+ or the non-selective, N-Type, and P/Q-Type Ca2+ channel blockers, ω-agatoxin, or ω-conotoxin, respectively, suggesting that KOR-mediated inhibition of release is not dependent on Ca2+ channel modulation [160]. These discrepancies may be explained by the use of different rat strains (Sprague–Dawley vs. Wistar) and/or animal age (adult vs. young) between studies. The presence of KOR-immunoreactivity in astrocytic processes [149] suggests that KOR activation may modulate glutamate reuptake by glia. This is of importance since dysfunction of glutamate reuptake and glutamatergic signaling has been implicated in addiction and other psychiatric disorders [166, 167].
Individual NAcc MSNs have extensive dendritic arborization and receive synaptic inputs from thousands of neurons from limbic, cortical, and thalamic sources, rendering MSNs integrative units that compute dendritic currents funneling into the soma [145]. KOR immunoreactivity is observed in somata and proximal dendrites of MSNs [149]. Immunoreactivity in somata is typically associated with cytosolic organelle membranes such as the endoplasmic reticulum and Golgi apparatus, while dendritic KORs are associated with the plasma membrane. KORs situated on proximal dendrites may be strategically situated to shape MSN computation of diverse dendritic signals via their slow, inhibitory action on neuronal excitability and Ca2+ signaling.
As previously mentioned, medial aspects of dorsal and ventral striatum receive dense glutamatergic afferents from cortical, thalamic, and limbic sources (see Fig. 2). It is not clear, however, whether presynaptic KORs differentially modulate each of these glutamatergic NAcc and dorsal-medial striatal inputs. This is important for our understanding of NAcc and striatal function since sustained activity may result in pathway-specific inhibition by DYN/KOR systems. Additionally, since glutamate is co-released with DA from VTA inputs [168–170], activation of KORs on DA terminals may inhibit glutamate release from vesicular pools in DAergic terminals. Moreover, presynaptic NAcc DA release may be modulated by glutamatergic NAcc afferents such as the basolateral amygdala (BLA) in a manner that is independent of VTA neuronal activity [171]. Thus, NAcc DYN activation of KORs in presynaptic excitatory terminals may further inhibit NAcc DA efflux via inhibition of local glutamate release that regulates impulse-independent DA efflux.
The activity of MSNs in the NAcc and dorsal striatum is governed by glutamatergic afferents arising in thalamic nuclei, limbic sites, and cortical regions, where DA modulates these excitatory afferents [172, 173]. DA acting on DYN-positive MSN neurons produces no functional consequence in the absence of glutamatergic synaptic transmission; rather DA acting on D1 and D2 DA receptors enhances and diminishes fast excitatory transmission mediated by glutamatergic receptors (i.e., NMDA receptors), respectively [174]. Ultrastructural studies have shown that, DYN-immunoreactive axon terminals appose KOR-immunoreactive terminals, DYN-containing KOR-immunoreactive terminals, and to a lesser extent soma/dendrites [149]. Additionally, PDYN and DYN are in close proximity to D1 receptors in dendrites and axons [175]. This suggests that DYN peptides are released from terminals and possibly from dendritic sites. However, it is unclear whether dendritic DYN release occurs in striatal nuclei, as is the case in other regions [176, 177]. The release of neuropeptide transmitters from dense-core vesicles (DCVs) requires sustained neuronal activity resulting in Ca2+ accumulation away from active zones of release where DCVs are located [178]. Therefore, the possibility exists that dendritic DYN release may be triggered by back-propagating action potentials, Ca2+ influx through NMDA receptors, and/or Ca2+ spikes. PDYN synthesis and peptide release in striatal compartments is positively regulated by DA D1 and NMDA receptors [179–182]. Intra-striatal perfusion of the partial D1 receptor agonist, SKF38393, produces a concentration-dependent increase in dynorphin B dialysate levels, which correlated with a decrease in DA levels [182], presumably due to dynorphin-mediated activation of KOR that regulate DA overflow. Additionally, MK-801, a non-competitive NMDA channel blocker, attenuates elevations in PDYN synthesis induced by DA receptor agonists [182]. These findings suggest that the dynorphin/KOR system provides feedback inhibition in response to DA and glutamate transmission in the ventral and dorsal striatum [183, 184]. Thus, under normal physiological conditions dynorphins may be released from MSNs when they receive concomitant DAergic and strong glutamatergic inputs.
Modulation of NAcc DA by KOR agonists and antagonists has traditionally been ascribed to KOR-mediated inhibition and disinhibition of DA release, respectively. However, KOR-mediated alterations in dorsal and ventral striatal DA overflow may also result from modulation of DAT and DA D2 autoreceptors. KORs positively regulate DAT function in striatum and NAcc with acute KOR activation increasing DAT function [154, 185], an effect that cannot be attributed to a circuit effect since analogous results are obtained in ex vivo studies [186]. Furthermore, our recent studies in cell expression systems have not only shown that KOR regulates DAT function but that these proteins are associated [186]. An anatomical basis for the interaction of these proteins is suggested by ultrastructural studies showing that KORs are apposed to DAT in NAcc nerve terminals [150]. Moreover, KORs may also play a role in modulating presynaptic D2-like DA autoreceptors, which regulate DA release and reuptake, since repeated KOR agonist administration downregulates presynaptic D2 DA receptor function [187]. Collectively, these studies suggest the DYN/KOR system is an important modulator of presynaptic striatal DA dynamics. Modulation results from (1) direct inhibitory actions of KORs on presynaptic DA release, (2) up-regulation of transporter function, and (3) possible regulation of presynaptic D2 DA receptors that mediate DA tone. This provides evidence that KOR in dorsal and ventral striatum may be a component of a larger macromolecular complex composed of KOR, DAT, and DA D2/D3 receptors, which tightly regulate DA dynamics in the striatum. Such receptor heteromers have been proposed to regulate neurotransmission [188, 189].
VTA/substantia nigra
Midbrain monoaminergic nuclei are innervated by afferents that express PDYN and/or KORs arising from limbic and “motor” neuronal regions [190]. The VTA and substantia nigra pars compacta (SNc) are composed of DAergic and GABAergic neurons that project to striatal, pallidal, prefrontal cortical, and limbic (amygdala) structures (see Fig. 2) [145]. Dorsal and ventral striatum GABAergic MSNs which express DYN [151, 152] preferentially innervate midbrain structures including the VTA and SN [190]. This constitutes a part of the “direct pathway” of the basal ganglia. DYN pathways differentially innervate the VTA and SN depending on their origin [190]. The central nucleus of the amygdala (CeA), which contains DYN-positive striatal-like GABA neurons, sends moderate projections to the dorsal VTA, SN pars compacta, and brainstem. Additional lighter DYN inputs to the VTA and medial SN arise from the lateral and medial hypothalamus. Lateral hypothalamic neurons that express DYN also co-express orexin/hypocretin neuropeptides [191], which modulate VTA DA neuron activity [192]. Mesocortical and mesoamygdalo, but not mesolimbic, DA neurons are directly hyperpolarized by U69,593 [193, 194]. In agreement with these findings, in vivo microdialysis studies demonstrated that intra-VTA administration of KOR agonists does not decrease basal NAcc DA levels [155, 194, 195], but decreases medial PFC (mPFC) DA efflux [194]. Collectively, these studies provide evidence that whereas functional KOR expression is not present in mesoaccumbal DA soma, it is present in mesocortical DA cell bodies. At odds with these observations, U69,593 directly hyperpolarized mesolimbic and mesoamygdalo DA neurons and decreased DA-mediated inhibitory post-synaptic currents by inhibiting somatodendritic DA release in midbrain neurons from mice [196]. Factors that may account for the discrepancies include, but are not limited to, differences in species used (rat vs. mouse) and the particular neurons sampled in both studies. Administration of nor-BNI into the SNc via reverse dialysis increases local and dorsal striatal DA efflux, suggesting that KORs in the SNc tonically inhibit SNc DA neuron activity in vivo [159]. Thus, SNc KORs may differentially modulate nigrostriatal pathways as compared to mesocortical and mesolimbic DA pathways. In addition to direct action of KORs on DA neurons, KOR activation decreases VTA glutamate release [197]. Inhibition by presynaptic KORs is more robust onto cells directly hyperpolarized by the mu-opioid receptor (MOR) agonist, DAMGO, (putative GABAergic neuron), suggesting that endogenous DYN differentially regulates presynaptic glutamate release onto midbrain DA and GABAergic neurons. This may provide a physiological framework for opposing control of VTA DA neuron activity by MOR (DA neuron excitation) and KOR (DA neuron inhibition) systems that accounts for contrasting behavioral effects of these systems, although both inhibit VTA glutamate release.
Locus coeruleus
The locus coeruleus (LC) is the primary source of norepinephrine (NE) in the CNS and plays a major role in arousal, stress, and cognition [198, 199]. Dense DYN immunoreactivity is restricted to fibers interspersed within the LC (see Fig. 2) [144, 200]. Moreover, the majority of positive fibers are asymmetrical suggesting release at excitatory synapses [200]. KORs are localized on NE neuron somata and dendrites, presynaptic terminals co-expressing PDYN, and axons innervated by DYN-positive presynaptic terminals [201]. Thus, endogenous DYN may act on hetero-autoreceptors to inhibit presynaptic glutamate and/or aspartate release. Consistent with anatomical studies, in vitro electrophysiological studies have demonstrated that U50,488 and another synthetic KOR agonist, CI-977, decrease electrically stimulated excitatory post-synaptic potentials (EPSPs) in LC neurons, in a naloxone and nor-BNI-dependent manner [202, 203]. Moreover, neither agonist was effective in decreasing EPSPs elicited by pressure application of glutamic acid suggesting a presynaptic site of KOR action. Ionotophoretic application of the KOR agonist, U50,488, decreased excitation of LC neurons produced by stimuli known to excite LC neurons (sciatic nerve stimulation and opioid withdrawal) without changing tonic activity in vivo [204]. Collectively, these findings suggest that KORs may inhibit excitatory presynaptic LC inputs. Thus, DYN peptides acting on KORs may decrease synaptic transmission into the LC in response to sustained LC neuronal discharge during attentional, stressful, or arousing stimuli.
Amygdaloid structures
DYN is expressed in GABAergic neurons localized to lateral portions of the CeA. DYN-positive neurons are segregated from enkephalin-positive GABAergic neurons, which are localized to medial portions of the CeA (see Fig. 2) [144, 151]. These fibers innervate the VTA and LC. Electrolytic CeA lesions decrease DYN immunoreactivity in the LC suggesting that DYN-positive CeA neurons innervate the LC [205]. A moderate proportion of CeA DYN-containing neurons (approx. 30–40%) co-express corticotropin-releasing factor (CRF) but do not co-express enkephalin [205, 206]. Findings from ultrastructural experiments mirror the latter observations demonstrating that DYN immunoreactivity is co-localized in LC CRF-positive axon terminals [205]. This raises the possibility that CeA neurons co-release CRF and DYN in monoaminergic nuclei such as the LC and VTA. CRF can facilitate or inhibit excitatory synaptic transmission in the VTA [192]. Thus, sub-populations of DYN- and CRF-positive CeA neurons targeting VTA or LC (i.e., terminal regions rich in KORs and CRF receptors), may play a critical role in shaping synaptic activity in the VTA through concerted release of DYN and CRF. This modulation may “fine tune” DAergic neuron activity in an activity-dependent manner under normal physiological conditions.
Cortex
In situ hybridization and immunocytochemical studies have demonstrated that both cortical pyramidal and non-pyramidal cells (based on cell morphology) express PDYN and DYN peptides (see Fig. 2) [144, 207, 208]. Generally, expression is densest in layers II/III and V [144]. Dense KOR-positive neurons/fibers are observed in the same layers. Less dense staining is seen in all other layers but layer I [146, 209–211]. Given the role of layers II/III and V in intra- and extra-cortical neurotransmission, respectively, these localization studies suggest that the DYN/KOR system modulates diverse aspects of cortical information processing. With regards to cortical regions, species differences in density of DYN and KORs exist while the laminar distributions appear conserved. Although species differences are observed in relative expression of ligand and receptor between cortical regions, in situ hybridization and immunohistochemical studies have consistently shown high levels of DYN and KOR expression in the temporal and prefrontal cortex of rodents and primates relative to other cortices [147, 148] but substantial expression is also observed in other cortical regions.
KORs in the rat medial prefrontal cortex (mPFC) are localized on axonal varicosities [212]. The mPFC receives dense DA, NE, and serotonin (5-HT) varicose innervation from the VTA, LC, and dorsal raphe, respectively. KORs are also localized on presynaptic components of asymmetric synapses [212]. In vitro studies have demonstrated that KOR activation decreases [3H] DA, [3H] 5-HT, GABA, and glutamate release from mouse and rat synaptosomal preparations [213–215]. U50,488 decreases [3H] NE release from human, but not rat neo-cortical slices [216]. Using in vivo microdialysis, we have recently demonstrated that mPFC KOR activation decreases local DA overflow [217]. Additionally, mPFC KORs tonically inhibit mPFC DA overflow. The effects of intra-mPFC KOR agonist administration on local 5-HT and NE release have not been investigated in vivo. It is currently not clear if KORs similarly modulate DAT or D2 autoreceptor function in the mPFC as in the dorsal and ventral striatum since expression of both DAT and D2 autoreceptors is substantially less in cortical regions. Additionally, reuptake of DA in mPFC is not solely handled by DAT, but rather by both DAT and the NE transporter [218], suggesting that KOR modulation of DA dynamics in this region may differ from striatal regions.
Hippocampus
For a detailed overview of the functional anatomical role of the DYN/KOR system in regulating hippocampal transmission, the reader is referred to a comprehensive review by Drake et al. [176]. DYN immunoreactivity in the hippocampus is densest in the mossy fiber pathway from the dentate gyrus to CA3 with little or no expression in other hippocampal regions [219, 220]. KOR activation by endogenous or exogenous ligands decreases mossy fiber pathway synaptic transmission in guinea pigs, Long–Evans rats, DBA/2 and C57BL/6 mice, and hamster [221–224], an effect that is absent in Sprague–Dawley rats [222]. This suggests the existence of strain differences in rats. Nor-BNI bath application promotes induction of LTP of the mossy fiber pathway when sub-threshold tetanic stimulation protocols are utilized [223]. DYN inhibition of mossy fiber transmission is enhanced after tetanic stimulation of mossy fibers and this enhancement of inhibition is specific to tetanized, but not non-tetanized, mossy fiber pathway synapses in the same preparation [223]. Moreover, DYN peptides are recruited to produce heterosynaptic inhibition in the mossy fiber pathway [221, 223]. Thus, DYNs released in response to strong stimuli may decrease function of mossy fiber pathway synapses that have low levels of activity, suggesting DYN/KOR modulation of synaptic transmission appears to be synapse-selective and highly dependent on activity. DYN released from dendrites and/or local collaterals of dentate gyrus granule cells may act in a retrograde fashion and activate KORs on perforant path terminals arising from the entorhinal cortex resulting in inhibition of entorhinal cortex inputs to the dentate gyrus [224, 226]. Retrograde signaling by DYN has been reported in the hypothalamus [39], suggesting that retrograde signaling may extend to other DYN-rich regions. Collectively, these studies provide evidence that DYNs are recruited by sustained mossy fiber or perforant path activity under normal physiological conditions to control information relayed to the hippocampus from the entorhinal cortex.
Recently, the radiolabeled tracers 11C-GR103545 and MeJDTic, antagonists with high KOR affinity, were developed for positron emission topography, thus allowing in vivo assessment of KOR binding [227–229]. The results of studies using these ligands show a distribution of KORs in vivo that mirrors that of previous histological studies. These and other radiolabeled KOR ligands will enable investigation of KOR density/binding in healthy human volunteers and patients with psychiatric disorders. This will allow determination of whether there are alterations in steady-state KOR densities/binding. More importantly, these advances may eventually allow examination of KOR binding and DYN release (via a reduction in competitive radiotracer binding) during different behavioral states and in response to stimuli that exacerbate behavioral abnormalities in psychiatric disorders (i.e., stress).
Behavioral effects produced by KOR ligands in humans
In healthy humans, KOR agonists produce behavioral effects akin to those associated with schizophrenia, drug addiction, and bipolar disorder. For instance, intravenous administration of the preferential KOR agonist, (-) MR2034, produces psychotomimetic, anxiogenic, and sedative effects [229]. Psychotomimetic effects include perceptual distortion of sensory stimuli, depersonalization, speech and language impairments, and thought disorganization. Collectively, these behavioral effects may be perceived as dysphoric behavioral states. Importantly, these effects are stereo-selective and naloxone reversible, suggesting opioid receptor-mediation. Intramuscular administration of enadoline, a synthetic KOR agonist, produces effects perceived as “bad” in humans and psychotomimetic effects at higher doses [230]. Spriradoline, a synthetic KOR agonist, produces dysphoria and sedation [231–233], and psychotomimetic effects in some patients [233]. Salvia divinorum is a potent hallucinogenic herb increasingly used recreationally. The psychoactive compound in S. divinorum is salvinorin A, a selective, highly potent KOR agonist [234]. S. divinorum use in humans produces psychomimesis, sedation, speech and language impairments, and uncontrollable laughter, effects similar that of synthetic KOR ligands [235]. However, the effects are more rapid and transient as S. divinorum is usually smoked. Interestingly, a substantial proportion of S. divinorum users report that “entering another reality” (e.g., psychotomimesis) is the “best thing” about S. divinorum intoxication, while only a small proportion report unpleasant physical after-effects and “bad things” about intoxication [236]. This is in stark contrast to observations in laboratory settings where most subjects report dysphoric or unpleasant effects, suggesting that expectancy of a “trip” may determine whether the experience is deemed a positive or negative outcome. However, it should also be noted that most S. divinorum users have a high degree of cannabis and nicotine consumption [236]. Therefore, it is possible that self-reported effects of S. divinorum are influenced by interactions of several psychoactive compounds. Future research should be aimed at determining whether psychotomimetic effects produced by S. divinorum or other KOR agonists are synergistic when concomitantly consumed with cannabis. This is of particular importance since cannabinoids can have pro-psychotic and anti-psychotic effects, depending on the cannabis composition and developmental period (e.g., adolescence) of exposure [237, 238]. Recently, case reports have described recurrent psychosis-like symptoms days after S. divinorum toxicity in adolescents [239–241], which could precipitate symptoms of psychiatric disorders not under the direct pharmacological influence of S. divinorum. Recurrent psychosis is likely not frequent in S. divinorum users, but may be exhibited in subset of users with genetic predisposition or heightened risk to develop schizophrenia or other affective disorders.
Behavioral effects of KOR ligands relevant to psychiatric disorders
KOR ligands and conditioned-aversive effects
Pavlovian procedures, such as the conditioned place aversion (CPA) paradigm, have been utilized to determine the motivational effects of KOR ligands in rodents. In these procedures, drug is repeatedly paired with a conditioned stimuli (CS+; i.e., a discrete compartment) and approach (i.e., preference) or avoidance (i.e., aversion) behavior to the CS+ in the absence of drug is assessed during testing. Mucha and Herz [242] first demonstrated that systemic KOR agonist administration produces conditioned aversive effects in rodents using CPA and conditioned taste aversion paradigms. These conditioned aversive effects have been suggested to be mediated by KOR agonist-induced “dysphoria”. However, dysphoria cannot be measured in rodents. In light of the wide range of behavioral alterations KOR agonists produce, the possibility exists that psychotomimetic-like effects, anxiety-like behavior, and/or sedation may contribute to the conditioned aversive effects of KOR agonists in rodents. Microinjection of U50,488 into medial PFC, midbrain, NAcc, or lateral hypothalamus, but not to the SN or dorsal striatum, is sufficient to produce CPA [243]. This suggests that KORs in the mesolimbic DA pathway (VTA and NAcc) and limbic sites may be responsible for conditioned aversive effects produced by systemically administered KOR agonists. At odds with these findings, a recent study demonstrated that systemic, low-dose salvinorin A administration (10–40 μg/kg) produces conditioned place preference [244]. This is in contrast to the aversive effects produced by higher doses (160–3,000 μg/kg) [156, 244, 245]. Interestingly, systemic administration of a salvinorin A dose that produces CPP enhances DA overflow in the NAcc shell [244]. In contrast, higher doses decrease NAcc DA efflux [245, 246]. The conditioned rewarding effects of salvinorin A were blocked by rimonabant, a CB1 receptor antagonist as well as nor-BNI, suggesting that endogenous cannabinoid signaling may contribute to the positive conditioned effects of low-dose salvinorin A. Evidence that salvinorin A produces anxiolytic effects and decreases immobility in the forced swim test at low doses has also been obtained [247].
KOR-ligands and intracranial self-stimulation
Intracranial self-stimulation (ICSS) procedures are sensitive to manipulations that decrease motivation or decrease brain reward function [248]. Rate-frequency ICSS procedures have been widely utilized to assess the motivational and anhedonic effects of drugs. In this paradigm, stable ICSS thresholds are established and persist for days to weeks. Anhedonic behavioral states (i.e., drug withdrawal) or drugs that produce conditioned aversive effects reliably increase ICSS thresholds, suggesting that more ICSS is needed to reach “normal” brain reward levels. Systemic administration of KOR agonists increases thresholds in rats [245, 249, 250], whereas KOR antagonists are without effect, suggesting that endogenous KOR signaling does not tonically inhibit brain reward function [250, 251]. Collectively, Pavlovian and ICSS procedures demonstrate that activation of DYN/KOR systems produce negative motivational effects. However, mimicking a psychiatric behavioral phenotype does not imply that DYN/KOR dysregulation produces negative affect and anhedonia in these disorders. Future research aimed at determining whether this system contributes to negative affect/mood or anhedonia in psychiatric disease is warranted.
KOR/DA interactions and conditioned aversion
The ability of systemic KOR activation to produce CPA is dependent on intact NAcc DA signaling since DA D1 receptor antagonism and mesolimbic, but not nigrostriatal, DA pathway denervation blocks KOR agonist-induced CPA [252, 253]. NAcc KOR activation decreases extracellular DA levels by decreasing Ca2+-dependent release [163], and increasing DA uptake [153, 184]. These effects are consistent with findings that decreasing DA neuronal activity produces CPA [254, 255]. Thus, modulation of NAcc DA is a mechanism by which DYN/KORs exert negative motivational effects.
KOR-serotonin (5-HT) interactions and conditioned aversion
The conditioned aversive effects of KOR ligands may also be mediated by interactions with 5-HT systems. U50,488-induced CPA was blocked by intra-dorsal raphe nuclei (DRN) microinjections of nor-BNI, suggesting that DRN KOR activation is necessary for U50,488-mediated aversion [256]. In this study, selective expression of KORs in the DRN of oprk1 knock-out mice enabled systemic U50,488 to produce a CPA. This effect is blocked by NAcc microinjection of nor-BNI, suggesting that KOR agonists produce negative affect by modulating 5-HT signaling from the DRN to the NAcc. However, the extent to which KOR overexpression is physiologically relevant is unclear. Neurochemical studies have demonstrated that administration of U50,488 into the NAcc or DRN via-reverse microdialysis decreases terminal and somatodendritic5-HT overflow, respectively [257, 258]. As previously mentioned, KOR and D2 autoreceptors regulate monoamine function by affecting monoamine transporter function. Therefore, it is imperative to determine whether effects of KOR activation on 5-HT overflow are mediated by KOR regulation of 5-HT release and/or 5-HT transporter function. Indeed, evidence that KOR activation affects 5-HT transporter function in cells and native tissue has recently been presented [259]. Inconsistent with the notion that 5-HT neurotransmission mediates the conditioned aversive effects of KOR agonists, experimental manipulations that decrease DRN 5-HT neuron function produce reward [260]. For example, intra-DRN microinjection of GABA receptor agonists, glutamate antagonists or 5-HT1A receptor (a presynaptic 5-HT autoreceptor) agonists produce place preference and facilitate ICSS, suggesting that DRN 5-HTergic function tonically inhibits reward. Additionally, rats will readily self-administer GABA agonists and glutamate receptor antagonists into the DRN [260]. However, these manipulations decrease 5-HT neurotransmission not only in the NAcc, but, in a plethora of terminal regions (i.e., medial PFC) such that these effects may be mediated via reduced 5-HT efflux in regions other than the NAcc. Interestingly, doses of systemic salvinorin A that increase ICSS thresholds do not decrease NAcc 5-HT extracellular levels [245], suggesting that changes in 5-HT transmission may not be associated with anhedonia/negative affect produced by systemic KOR agonist administration. Future work should be aimed at determining whether the interactions between the KOR and 5-HT system are direct or indirect via 5-HT interactions with other neurotransmitter systems.
DYN/KORs, stress, and “pro-depressant-like” effects in animal models
Although acute stress is beneficial for survival by recruiting appropriate motivational and cognitive processes, chronic stress can produce long-lasting alterations in affect and mood, anhedonia, and cognitive deficits; behaviors that are relevant to depression and other psychiatric disorders. Learned helplessness and repeated forced swim stress robustly increases DYN immunoreactivity in hippocampus (CA3, dentate gyrus) and NAcc [261]. Increased hypothalamic and decreased striatal DYN A immunoreactivity after context-induced immobility (paired with electric shock) and a single forced swim exposure have also been reported [262]. Thus, KOR systems may initially recruit stress systems by stimulating the hypothalamic–pituitary adrenal (HPA) axis. CRF is a powerful mediator of behavioral stress responses and its dysregulation is implicated in depression, bipolar disorder, addiction, and schizophrenia [263]. CRF promotes DA-dependent release of DYN peptides in striatal regions [264, 265]. Moreover, enhanced phospho-KOR immunoreactivity, an index of KOR activation, is increased in the NAcc, hippocampus, BLA, bed nucleus of the stria terminalis, DRN, VTA, and ventral pallidum following central CRF administration [266]. Together, these data indicate that repeated stress and the ensuing increase in CRF enhance DYN release and KOR activation which then modulates stress reactivity. Consistent with this view, systemically administered KOR agonists increase immobility in repeated forced swim procedures whereas rodents treated with KOR antagonists and pdyn KO mice exhibit decreased immobility [251, 261, 267, 268].
KOR antagonists produce effects similar to that of traditional anti-depressants [251, 268, 269]. In the forced swim test, rodents are subjected to repeated forced swim over a 2-day period and measures of immobility (i.e., latency to immobility or time spent immobile) increase with repeated swim trials, an effect interpreted as increased “behavioral despair” that is indicative of “depressive-like” behavior [270]. Therefore, effects produced by KOR agonists and antagonists have been interpreted as “pro-depressive” and “anti-depressant”, respectively. Animal models of depression typically involve exposing subjects repeatedly to a stressful event (i.e., forced swim) from which there is no escape. Depression is characterized by persistent negative mood/affect, anhedonia, decreased motivation, and cognitive deficits. Anti-depressants widely used to treat depression target monoamine transporters. Importantly, however, therapeutic efficacy requires repeated anti-depressant treatment, suggesting that long-term alterations in neural circuitry function rather than direct pharmacological effects of antidepressants (elevating extracellular monoamine levels) mediate therapeutic efficacy. Recently, the relevance of several animal models of depression (forced swim and tail suspension tests) has been called into question due to limited face and predictive validity [271]. Indeed, in these models, antidepressant efficacy is observed after acute administration. Social defeat stress is an animal model widely used to model depression. Therapeutic efficacy with typical antidepressant is observed after chronic, but not acute, treatment [271]. Nor-BNI-treated wild-type mice exhibit decreased social defeat-induced behavior (i.e., defeat postures), suggesting that endogenous DYN release mediates stress-induced “pro-depressive-like” effects [272]. This is of interest, since in the social defeat stress model the “anti-depressant” phenotype of nor-BNI-treated mice is observed after short-term nor-BNI treatment, in contrast to the effects of typical anti-depressants. Questions, thus, remain as to whether the effects of KOR ligands observed in some animal models of depression (i.e., forced swim stress) have direct relevance to depression. Additionally, in controlled laboratory studies in humans, spiradoline did not produce depressive effects although sedation and dysphoria were reported [233]. Importantly, the aforementioned models of depression produce stress and as such may be relevant to furthering our understanding of the role of the DYN/KOR systems in stress, which is known to exacerbate behavioral abnormalities in depression and other psychiatric disorders.
Interestingly, the ability of forced swim stress and central CRF infusion to produce aversion to an odorant or compartment to which it is discretely paired is absent in wild-type mice pretreated with nor-BNI and in pdyn knock-outs [266]. This suggests that DYN activation of KOR not only contributes to stress reactivity but to the conditioned aversive effects of stress and CRF receptor activation. Stressful stimuli and CRF increases monoaminergic and glutamatergic transmission in limbic and cortical structures such as the hippocampus, mPFC, and NAcc [191]. The possibility exists that repeated stress increases monoamine and excitatory neurotransmission in these regions resulting in activity-dependent DYN release and KOR activation, and, ultimately long-lasting changes in neuronal activity in structures that regulate affect.
Neonatal stress recapitulates many facets of psychiatric disorders including anhedonia and alterations in affect and mood that persist through adulthood [273]. Neonatal maternal separation, a stressor, profoundly affects DYN peptides and phosphor-KOR immunoreactivity in regions that mediate stress responses such as the hippocampus, amygdala, hypothalamus, mPFC, and pituitary that persist into adulthood [274, 275]. Consistent with the hypothesis that neonatal stress enhances KOR signaling during adulthood, neonatal maternal separation enhances CPA produced by U50,488 during adulthood [276]. Thus, neonatal stress in rodents produces enduring alterations in DYN/KOR systems that may contribute to abnormal behavioral responses that are characteristic of a variety of psychiatric disorders and which are exacerbated by stress.
DYN/KOR alterations in human post-mortem tissue
The striatum has a “patch” and “matrix” organization, with patches richer in DYN-positive neurons than the surrounding matrix [277]. PDYN mRNA is elevated in patch compartments in post-mortem tissue of suicide subjects, an effect presumably attributed to depression [278]. As previously mentioned, similar alterations are present in animal models with a repeated stress components [279]. However, the underlying psychiatric disorder/s of the human subjects is not clear. No significant differences in either PDYN or OPRK1 mRNA levels in cingulate and dorsal lateral prefrontal cortex of patients with bipolar disorder or major depression were seen relative to controls [280]. However, PDYN expression is reduced in the amygdala of individuals suffering from depression or bipolar disorder [281]. Although these data suggest that PDYN synthesis and/or turnover is altered, the functional consequence of this decrease is not known.
CREB and the DYN/KOR system
CREB regulates pdyn expression in rodents (see trans-regulation section). Region specific alterations in CREB function is observed following stress exposure, antidepressant treatment, and in animal models of depression [282]. CREB and dominant negative CREB (mCREB) overexpression in the NAcc increases and decreases immobility in the forced swim test, respectively [269, 283]. Interestingly, the effects of CREB overexpression are ameliorated by central KOR blockade [269], suggesting that enhanced DYN mediates the effects of CREB overexpression. Importantly, nor-BNI decreased immobility in controls, NAcc CREB over-expressing, and NAcc mCREB over-expressing rats. Moreover, the effect of mCREB overexpression on forced swim is associated with decreased pdyn expression in mCREB-expressing MSNs, suggesting that decreased DYN signaling is associated with decreased immobility [283]. Intracerebroventricular and intra-accumbal nor-BNI, but not intra-dentate gyrus, treatment mimics the effects of mCREB overexpression on forced swim [283]. Moreover, elevations in ICSS thresholds produced by systemic U50,488 administration are absent in mCREB mice, suggesting that the “antidepressant” phenotype of these animals may be related to decreased KOR function [284]. However, the mechanism by which mCREB expression abolishes effects produced by KOR activation on ICSS behavior is not clear. Taken together, these studies demonstrate that enhanced NAcc DYN expression by CREB increases KOR signaling and alteration in behavior relevant to psychiatric disorders.
DYN/KOR systems and anxiety
Preclinical studies are consistent with clinical work demonstrating that KOR agonists increase anxiety [229]. Systemic KOR antagonist administration produces anxiolytic effects in elevated plus-maze, open-field, and fear-potentiated startle paradigms in rats [285], suggesting that endogenous DYN release mediates the expression of anxiety-like behavior in these behavioral paradigms. Pdyn knock-out mice exhibit decreased anxiety-like behavior in open-field, elevated plus-maze, and light/dark box paradigm. Similar effects are observed in wild-type mice pretreated with selective KOR antagonists [286]. However, inconsistent with these observations, oprk1 knock-out mice do not display altered anxiety-like behavior in these tests [287]. Consistent with the notion that DYN release occurs in response to anxiogenic environmental stimuli, systemic nor-BNI administration or pdyn ablation decreases anxiety-like behavior in procedures involving anxiety-eliciting testing conditions (brightly lit testing conditions) [288], an effect not present under normal testing conditions. Moreover, U50,488 administration produces anxiogenic effects in the elevated plus-maze and BLA KOR blockade reverses the anxiogenic effects of stress and central CRF administration. This is consistent with work demonstrating that intra-amygdala microinjections of DYN A increase anxiety-like behavior in the light–dark box test [289], and suggests that BLA DYN signaling is also a downstream mediator of the anxiogenic effects of stress and CRF. Human studies examining whether KOR antagonists decrease basal and stimulated anxiety is warranted in view of the potential implications of these findings for panic/anxiety disorders.
Wistar–Kyoto rats display enhanced stress reactivity and anxiety-like behavior relative to Sprague–Dawley rats and are considered an animal model of depression or anxiety disorders [267]. Wistar–Kyoto rats are more sensitive to the anxiolytic and stress-ameliorating effects of KOR blockade [267, 290], suggesting there is enhanced DYN/KOR signaling in this strain. Indeed, enhanced KOR- and DYN A-immunoreactivity is observed in the piriform cortex and NAcc, respectively, of Wistar–Kyoto relative to Sprague–Dawley rats [267]. Moreover, intra-piriform cortex nor-BNI administration ameliorates depressive-like behavior in Wistar–Kyoto rats. Collectively, these studies suggest that genetic differences in the DYN/KOR system may contribute to the predisposition to depressive- and anxiety-like phenotypes. As such, KOR antagonists may be useful antidepressants in discrete sub-populations with major depression and/or anxiety/panic disorders.
DYN/KOR and addiction
Drug addiction is a chronically relapsing disorder characterized by preoccupation with drug seeking and intake despite the aversive consequences that may ensue. Chronic drug use is also associated with the development of tolerance and a characteristic withdrawal syndrome. Initially, the rewarding properties of drugs of abuse drive behavior. With continued use, drug seeking/taking is driven by a balance between the positive effects produced by the drug, avoidance of the negative consequences of drug withdrawal and increased salience of drug-associated stimuli [291]. In addition, stress and conditioned stimuli associated with drug availability are capable of reinstating compulsive drug seeking and taking. The DYN/KOR system has been implicated in the development of drug addiction [183, 279, 292, 293]. Additionally, there is co-morbidity of substance use disorders and other psychiatric disorders [294]. Thus, it has been suggested that DYN/KOR dysregulation contributes to aberrant activity in brain regions that influence drug addiction and behavioral alterations in psychiatric disorders (i.e., motivational processes).
DYN/KOR system alterations in human post-mortem tissue
Since polysubstance use is common among drug addicts and drug use pattern is often unknown, it is difficult to parse the effects of individual drugs in humans. PDYN expression is enhanced in putamen “patches” but not in caudate or NAcc of cocaine users, whereas [3H]DYN binding is increased in the caudate relative to controls [277]. Enhanced DYN immunoreactivity in the caudate and ventral pallidum but no changes in the putamen and prefrontal cortex of methamphetamine and cocaine users have been reported [295, 296]. Methamphetamine users have decreased DYN immunoreactivity in the NAcc, medial pulvinar thalamic nucleus, and temporal/occipital association cortices [296]; changes not observed in cocaine users [295]. A significant correlation between recent psychostimulant use and PDYN expression in cingulate and dorsal lateral prefrontal cortices has also been reported [280]. Additionally, past, but not recent, marijuana use was associated with increased expression in these regions, suggesting that the time course of PDYN induction may vary depending on drug of abuse. Although studies to date suggest that DYN/KOR systems are altered with drug use, given limitations of post-mortem studies in humans, the only solid conclusion that can be made is that striatal PDYN expression is increased in psychostimulant users.
Cocaine and amphetamines/preclinical studies
The role of DYN/KOR systems in psychostimulant-induced drug seeking and neurochemical alterations has been extensively studied. Cocaine blocks DA, NE, and 5-HT transporters producing elevations in extracellular monoamine levels in monoaminergic nuclei (i.e., VTA) and their terminal regions (i.e., mPFC). Amphetamine, like cocaine, is a monoamine transporter substrate, but also produces reverse transport of monoamines. Both drugs produce robust elevations in extracellular monoamine levels. The ability of psychostimulants and other drugs to increase DA and monoamine levels in the NAcc and other reward-related regions (i.e., mPFC) is implicated in mediating the rewarding properties of abused drugs [297, 298].
Psychostimulant-induced changes in the DYN/KOR systems in experimental animal models
Both acute and repeated psychostimulant administration increase pdyn expression in reward-related neuronal regions in animal models, similar to what is observed in humans [151, 181]. In rodents, administration of a single or repeated injection of cocaine or amphetamine produces robust elevations in pdyn mRNA [299–301] and DYN immunoreactivity [302, 303] in the NAcc and dorsal striatum. However, cocaine-induced NAcc pdyn induction is not as robust as that resulting from amphetamine [299]. In non-human primates, high-dose cocaine self-administration acquisition and chronic high-dose cocaine, but not low-dose, self-administration increases pdyn expression in the rostral caudate and putamen [304]. In rodents, cocaine self-administration increases dorsal lateral and dorsal medial striatal, but not NAcc or limbic, pdyn expression to a similar extent in yoked and self-administering rats [305]. This suggests that pdyn elevations are due to pharmacological effects of cocaine rather than drug taking behavior per se. The effects of psychostimulants on striatal pdyn expression are dependent on D1 DA receptor signaling such that D1 receptor antagonism [306] or D1 receptor deletion [178, 302] abolish psychostimulant-induced increases in pdyn expression. Importantly, psychostimulants also elevate extracellular DYN levels in the striatum and SN [307] suggesting increased pdyn synthesis. As previously mentioned, striatal D1- and NMDA receptor interactions may play an important role in information processing [172, 173] and the regulation of DYN synthesis [181]. Thus, it is not surprising that NMDA receptor antagonism blocks the ability of psychostimulants to elevate DYN immunoreactivity in dorsal and ventral striatal compartments [181]. The PDYN increase is postulated to be a compensatory mechanism to reduce psychostimulant-induced MSN activity by activating presynaptic KORs on excitatory synapses, DAergic varicosities, and in subsets of MSNs expressing dendritic KORs [151, 308]. This hypothesis is supported by findings that nor-BNI treatment or constitutive KOR deletion enhance cocaine-evoked NAcc DA dialysate levels [153]. Importantly, nor-BNI-treated wild-type mice as well as pdyn and oprk1 knock-out mice display enhanced locomotor sensitization in response to cocaine treatment relative to controls [153, 309], suggesting that increased activity of DYN/KOR systems is a negative feedback mechanism opposing neurochemical changes produced by cocaine (i.e., elevations in DA and glutamate in the NAcc).
The influence of psychostimulants on KOR immunoreactivity and binding remains controversial. One study reported decreased KOR density in dorsal striatum after acute or repeated cocaine injections whereas decreased NAcc KOR density was only observed after repeated cocaine exposure [299]. These changes may reflect a compensatory downregulation of KORs in response to pdyn induction. However, acute and repeated amphetamine treatment decreases KOR density in NAcc, but not striatum [299], where increased pdyn induction is typically observed. Examination of [3H] bremazocine binding (in the presence of cold agonists to block other opioid receptors) 30 min after the last cocaine injection of a binge-like dosing regimen revealed increased KOR density in cingulate cortex, dorsal striatum, olfactory tubercles, and VTA. Using [3H] CI-977 and an escalating cocaine treatment regimen, however, the same group only found a significant increase in septal KOR [310]. Acute high-dose, “binge” cocaine administration decreased oprk1 mRNA in the SN [301]. However, after escalating doses of cocaine, KOR-coupling to Gi/o G-proteins increased in the VTA, as assessed by [35S] GTP binding [311]. Together, these studies suggest that changes in DYN/KOR systems are dynamic and vary according to the stage of the addiction cycle. Furthermore, it is apparent that this opioid system is recruited during normal physiological processes, but recruitment is exacerbated by psychostimulants.
Psychostimulant exposure alters the behavioral and electrophysiological effects of KOR agonists. U69,593-induced CPA is exacerbated for at least 10 days in rodents pretreated with a single cocaine injection and this effect is blocked by VTA inactivation [312], suggesting that DYN/KOR systems regulating mesocortical and/or mesolimbic neurotransmission are dysregulated following acute cocaine exposure. Indeed, the ability of DYN A and U69,593 to inhibit glutamatergic transmission in the NAcc, but not in the VTA, is disrupted during abstinence from acute amphetamine [313] or repeated cocaine injection [314]. Additionally, amphetamine-induced downregulation of KOR-mediated inhibition of glutamate transmission is reversed by concomitant amphetamine treatment with a DA D1 receptor antagonist or naltrexone [313]. These findings suggest that cocaine- and amphetamine-induced attenuation of KOR-mediated inhibition is due to D1 receptor-mediated release of endogenous DYN that down-regulates NAcc KOR function. These studies also provide evidence that psychostimulants can produce functional changes in NAcc KOR systems in the absence of changes in pdyn expression or KOR density.
Anti-psychostimulant effects of KOR agonists
Endogenous DYN/KOR systems can act as inhibitory feedback systems recruited by psychostimulants. When the “temporal order” is switched and KORs are stimulated with agonists prior to psychostimulant administration, the psychostimulant-induced behavioral, neurochemical, and molecular effects are diminished. Pretreatment with synthetic or naturally occurring KOR agonists (15–20 min prior) decreases the behavioral- and locomotor-activating effects of acute and sensitizing-regimens of systemic cocaine [156, 315–318] and amphetamine [163, 300, 319]. Acute KOR agonist administration 15–20 min prior to conditioning decreases cocaine-induced CPP in rats [320] and mice [318, 321, 322], suggesting that KOR agonists decrease the conditioned rewarding effects of psychostimulants. Prior, repeated, home cage injections of KOR agonists attenuate the subsequent development of sensitization to the conditioned reinforcing effects of cocaine [323]. This effect cannot be attributed to the aversive effects of KOR agonists or a generalized disruption of learning or memory processes since sensitization to morphine is unaltered. A recent study has shown that U69,593 doses that are ineffective in altering ICSS thresholds in drug naïve animals, block cocaine-evoked decreases in ICSS thresholds [324]. It appears likely that these actions result from the ability of KOR agonists to decrease psychostimulant-evoked increases in NAcc and dorsal striatal DA and glutamate dialysate levels [157, 163, 318, 321]. Interestingly, Thompson and colleagues [182] demonstrated that repeated cocaine administration increases NAcc DA uptake, an effect that is blocked by U69,593 treatment. Repeated co-administration of U69,593 with cocaine blocks the increased DA uptake and decreased K+-stimulated DA release in the mPFC associated with early abstinence from repeated cocaine [325]. Given these findings, the question arises as to whether KOR agonist treatment may attenuate alterations in mPFC-dependent cognitive function produced by cocaine.
Induction of the immediate early gene, fos, in neurons is an indirect marker of persistent neuronal activity and plasticity. Acute psychostimulant administration increases fos and other immediate early genes in the NAcc and prefrontal areas, and this effect is decreased by KOR agonists [151, 300]. Such findings may be of potential clinical relevance since C-FOS-positive NAcc neurons are implicated in the development of context-dependent cocaine sensitization [326]. Interestingly, oprk1 knock-out mice display decreased cocaine-induced induction of C-FOS and FOS B [153]. U69,593 pretreatment decreased cocaine-induced elevations in DA and cyclic AMP-regulated phosphoprotein (DARPP-32), a protein involved in D1 receptor signal transduction, in hippocampus, dorsal striatum, and mPFC [327]. These studies suggest that KOR agonists antagonize the actions of psychostimulants when administered shortly before psychostimulant use presumably by countering neurochemical (i.e., DA-elevating effects of psychostimulants) and molecular alterations that produce long-lasting plastic plasticity in reward-related structures. Evidence that the interaction of KOR agonists with psychostimulants depends on 5-HT signaling has also been obtained. Pretreatment with dl-p-chloroamphetamine, which depletes 5-HT stores, blocked the ability of U69,593 to decrease cocaine-stimulated locomotor activity [317], suggesting that the ability of KOR agonism to block cocaine-induced alterations in locomotor activity are partially dependent on 5-HT systems.
KORs and psychostimulant self-administration
Reports on the effects of KOR ligands on psychostimulant administration are conflicting. U50,488, spiradoline, and cyclazocine dose-dependently decrease low-dose cocaine self-administration (0.1 mg/kg) maintained under an FR1 schedule [328, 329]. This effect is stereo-selective [329] and nor-BNI reversible [328]. Importantly, responding for a natural reward (e.g., water) was only altered by high doses of U50,488 and spiradoline. Schenk et al. reported that U69,593 administration decreased self-administration of low cocaine doses (0.03–0.125 mg/kg) more robustly when cocaine doses were presented in a descending order, relative to conditions where doses were presented in a descending order [330]. Additionally, U69,593 was ineffective in attenuating self-administration of high doses of cocaine (>0.25 mg/kg). One explanation for the effect of KOR agonists on cocaine self-administration is that agonist-induced aversive or sedative effects disrupt self-administration. Indeed, chronic KOR agonists (spiradoline, bremazocine, but not cyclazocine) treatment (up to 69 injections of KOR agonists/day) decreased food-maintained responding in non-human primates [331]. However, systemic administration of KOR agonists at doses that did not alter responding for natural reinforcers was also shown to decrease cocaine self-administration [328, 331], and this may be ligand-specific [331]. Intra-VTA U50,488 microinjection does not alter responses for cocaine maintained under a FR5 schedule [332], suggesting that maintenance of cocaine self-administration is not modulated by VTA KORs. Systemic nor-BNI administration does not alter cocaine self-administration in rats under an FR1 schedule [328, 333]. However, Wee et al. using a progressive ratio schedule, recently demonstrated that a high dose of nor-BNI decreases cocaine self-administration break-points in rats with long access to cocaine, but not rats with short access. By contrast, this effect was not observed under fixed-ratio schedules [333]. This highlights the notion that the role of DYN/KORs in psychostimulant addiction is dynamic and may vary with the pattern of drug intake and duration of drug exposure.
KORs and CS+ interactions in cocaine self-administration procedures
Systemic U69,593 administration to rats produces a long-lasting decrease in cocaine-seeking behavior in a paradigm in which a cue light is associated with cocaine infusion during training and testing. However, the effect of the agonist is transient in rats that acquired cocaine responding not associated with cue light activation [334]. This work suggests that KOR agonism does not simply block the unconditioned effects of cocaine or decrease overall motivation, but rather modulates the conditioned effects of cocaine. In agreement with the latter report, recent work from our laboratory using second order schedules of cocaine self-administration has demonstrated that systemic nor-BNI increases CS+ maintained cocaine-seeking behavior but not cocaine-taking behavior maintained by both the CS+ and pharmacological effects of cocaine [335]. This suggests that endogenous KOR signaling may play a key role in modulating CS+-maintained responding. DA signaling in ventral–dorsal striatal loops is necessary for the expression of “habitual” drug seeking. Thus, one mechanism by which endogenous and exogenous KOR ligands may affect CS+ maintained behavior is through modulation of dorsal striatal DA and glutamatergic efflux. BLA function is necessary for Pavlovian conditioning. Therefore, the effects of KOR ligands may be dependent on modulation of neurotransmission in the BLA or its afferents (e.g., mPFC or NAcc). Indeed, KOR agonism decreases stimulated field excitatory potentials and synaptic plasticity in the BLA [336] providing a potential mechanisms by which endogenous and exogenous KOR agonists may decrease the conditioned effects of cocaine.
KORs and psychostimulant-primed reinstatement
Systemic administration of KOR agonists (U69,593, enadoline, and spiradoline) decreases cocaine-primed reinstatement in rodents and non-human primates [330, 337, 338]. In contrast, amphetamine-primed reinstatement in rodents is unaffected [330]. There is no evidence that KOR antagonists alter cocaine-induced self-administration or the reinstatement of CPP produced by a priming injection of cocaine [337–339]. Interestingly, KOR agonists do not alter reinstatement produced by selective DAT inhibitors [338], suggesting that KOR agonist modulation of cocaine may be mediated by interactions with other monoamine systems, including 5-HT and NE. Additionally, U69,593 is more effective at attenuating cocaine-induced reinstatement than amphetamine-induced reinstatement [335]. As previously mentioned KORs are believed to modulate monoamine transporter function. Thus subtle differences in drug action between cocaine (monoamine transporter blocker) versus amphetamine (monoamine transporter reverser) may explain why KOR agonists are more effective in attenuating cocaine-induced reinstatement. Intra-VTA microinjection of U50,488 decreases cocaine-primed cocaine reinstatement in a dose-dependent manner, and this effect was blocked by intra-VTA nor-BNI microinjection [332]. Intra-VTA U50,488 was without effect on cocaine and food responding after acquisition as well as on food-induced reinstatement of food self-administration. This suggests that effects of intra-VTA KOR administration on cocaine-primed reinstatement is specific and not due to changes in motivation or locomotor activity.
KORs and stress-induced cocaine reward and reinstatement
Stress associated with repeated forced swim or social defeat enhances cocaine-induced CPP and this effect is absent in nor-BNI-treated wild-type mice and pdyn knock-outs [268, 272]. These findings are consistent with the hypothesis that endogenous KOR systems are recruited by stressful events and enhance the motivational valence of cocaine [288]. U50,488 administered 15 min prior to conditioning sessions with cocaine or to CPP post-testing blocked whereas 60 min pretreatment potentiated cocaine CPP [322, 339], suggesting that the interaction of KOR agonists with cocaine is time-dependent. Data suggesting an involvement of DYN/KOR systems in mediating stress-induced reinstatement of cocaine-seeking behavior has also been obtained. Self-administration studies using a fixed ratio reinforcement schedule revealed an attenuation of stress-induced reinstatement of cocaine-seeking by KOR antagonists [340, 341]. Stress-induced reinstatement of cocaine CPP is also attenuated by KOR antagonism and in pdyn and oprk1 knock-out mice [342, 343]. KOR agonists also reinstate cocaine self-administration in non-human primates [344]. Interestingly, clonidine, an α2 nor-epinephrine autoreceptor and post-synaptic receptor agonist attenuated the effects of KOR agonists, suggesting that KORs may in part increase cocaine self-administration by increasing NE efflux in reward-related structures that are sensitive to stress. However, in the latter study the ability of KOR agonists to reinstate self-administration was blocked by naltrexone but not nor-BNI, suggesting a non-KOR opioid receptor-mediated effect. Analogous to reinstatement produced by a priming injection of cocaine, recent data suggest that KOR modulation of 5-HT may contribute to the stress/KOR agonists interaction. Land et al. [256] demonstrated that intra-DRN nor-BNI microinjection blocks social defeat stress-induced cocaine CPP reinstatement. This is of importance since 5-HTergic DRN neurons send efferent projections to brain regions mediating reinstatement of drug-seeking behavior such as the PFC, VTA, and amygdala [260]. Collectively, these studies suggest that endogenous KOR ligands mediate stress-induced reinstatement of self-administration and CPP and these effects are mediated by KOR regulation of monoamines.
Nicotine
Acute nicotine injection produces a dose-dependent increase in pdyn mRNA and DYN immunoreactivity in the striatum and NAcc [345]. PDYN elevations are long-lasting in striatum whereas peptide immunoreactivity is transiently increased (approx. 1 h). This is followed by a return to baseline and a rebound elevation of DYN content lasting up to 24 h. Like other drugs of abuse, systemic nicotine administration elevates extracellular NAcc DA levels [156]. Not surprisingly, D1 and NMDA receptor blockade attenuate nicotine-induced DYN elevations [345], again, highlighting the role of D1-NMDA interactions in modulating DYN synthesis in response to various drugs of abuse. Nicotine produces biphasic motivational effects across doses. Low to moderate doses produce CPP and high doses produce CPA [346, 347]. However, the neural mechanisms that mediate the aversive effects produced by acute administration of high-dose nicotine are not known. Low-dose nicotine administration does not alter the number DYN-positive neurons in the CeA or hypothalamus; nor were the number of dual-labeled DYN and C-FOS cells altered suggesting DYN neurons in these regions are not activated by low-dose nicotine administration [348]. Interestingly, doses (1–2 mg/kg; free base) that produce CPA also produce robust elevations of striatal DYN [345]. Thus, future experiments examining a role of KORs in mediating the aversive effects of high-dose nicotine are warranted. Repeated injections of low-dose nicotine decreases pdyn mRNA in the NAcc when administered three times a day [349], an effect not observed if the same nicotine dose is administered one time per day for 14 days [350]. This suggests a threshold of nicotine exposure required for long-term alterations in PDYN mRNA levels in the NAcc. Pdyn KO mice or wild-type mice treated with nor-BNI display similar nicotine-induced CPP [351, 352], suggesting that endogenous KOR signaling does not modulate the conditioned effects of nicotine. However, Galeote et al. [351] reported that acquisition of low-dose nicotine self-administration was enhanced in pdyn knock-out mice. These data may suggest that the activity of endogenous DYN/KOR systems serves an essential function in opposing the reinforcing effects of nicotine. However, as with all studies using constitutive gene deletion, compensations that arise as a consequence of gene deletion may contribute to the observed phenotype. Unpublished observations indicate systemic U50,488-induced aversion and anxiety-like behavior are enhanced in nicotine-dependent animals relative to controls as assessed by CPA and the elevated plus-maze [353]. Moreover, the ability of systemic U50,488 to decrease NAcc DA overflow is exacerbated in nicotine-dependent adult rats relative to controls. These findings suggest that chronic nicotine exposure produces changes in KOR modulation of DA neurotransmission that lead to enhanced negative affect and increased anxiogenic effects. Alterations in KOR systems produced by chronic nicotine exposure play an important role in mediating nicotine withdrawal since nor-BNI pretreatment blocks somatic signs of spontaneous withdrawal in nicotine-dependent rats. Conversely, U50,488 administration potentiates somatic withdrawal signs. In agreement with these observations, Jackson et al. [352] reported that spontaneous nicotine withdrawal-induced anxiety-like behavior in the elevated plus-maze and somatic signs of withdrawal are blocked by nor-BNI or JDTic pretreatment. Moreover, these antagonists block expression of mecamylamine-precipitated nicotine withdrawal. Collectively, these results suggest that DYN/KOR alterations produced by chronic nicotine enhance KOR signaling during nicotine withdrawal that may result in dysphoria and physical signs of withdrawal. However, it should be noted that pdyn knock-out mice display similar mecamylamine-precipitated nicotine withdrawal signs as wild-types [351].
Ethanol
In vivo microdialysis demonstrated that acute administration of ethanol produces transient elevations in extracellular DYN A 1–8 in the NAcc [354]. Prolonged elevations in DYN A 1–8 are observed in the CeA at higher doses (2.0–2.8 g/kg) [355]. Chronic ethanol treatment decreases PDYN and DYN content in the hippocampus [356, 357], and chronic ethanol consumption increases PDYN in the hypothalamus, including the paraventricular nucleus [358, 359]. Voluntary ethanol consumption also produces elevations in CeA, and mPFC [359]. NAcc pdyn mRNA expression is unchanged [349, 359] or enhanced during withdrawal from repeated ethanol injection or consumption in rodents relative to controls [360, 361]. These discrepant findings may be explained by differences in exposure (i.e., passive injection or vapor vs. voluntary consumption in home cage). Alternatively, changes in DYN/KOR systems are likely dynamic with low levels of ethanol consumption acutely engaging DYN/KOR systems reward-related structures (i.e., NAcc) and larger, prolonged consumption recruiting DYN/KOR systems involved in drug craving and stress (i.e., CeA). It is expected that these dynamic changes will also differ depending the mode of ethanol exposure. Acute ethanol exposure enhances NAcc DA release more robustly in orpk1 knock-out mice and in nor-BNI-treated wild-type mice relative to controls [362]. These findings suggest endogenous KOR systems function to decrease mesolimbic responsiveness to ethanol. Ten minutes U50,488 pretreatment prior to ethanol decreases ethanol-induced CPP using conditioning procedures under which U50,488 does not produce CPA suggesting U50,488 decreases the conditioned effects of ethanol independently of its aversive effects. Lindholm et al. [363] demonstrated that tonic inhibition of DA overflow by KORs in the NAcc is enhanced after repeated injections of moderate doses of ethanol, which may contribute to decreased motivation and negative affect after repeated ethanol exposure.
Findings regarding the effects of KOR ligands on ethanol self-administration are discrepant. Synthetic KOR agonists decrease ethanol consumption in limited-access, two-bottle choice procedures [364, 365]. Nor-BNI treatment enhances voluntary ethanol consumption in a continuous two-bottle choice paradigm. This effect occurs in rats with high, but not low, levels of ethanol consumption [366], suggesting that ethanol exposure elevates DYN tone in high drinkers. Nor-BNI pretreatment (24 h) does not alter ethanol self-administration but increases the latency for ethanol-induced NAcc DA overflow [367]. The mechanism mediating the delayed NAcc DA response is not known. However, 24 h nor-BNI pretreatment increases DA release and reuptake in mice [153], which may account for delayed mesolimbic DA responses in nor-BNI-treated rats self-administering ethanol. Overexpression of bdnf globally decreases ethanol consumption and this effect is by blocked by nor-BNI pretreatment [368], suggesting that DYN/KOR systems are a downstream effector of BDNF. Hypothalamic DYN/KOR systems have been implicated in ethanol-seeking behavior. Microinjection of U50,488 into the paraventricular nucleus of the hypothalamus decrease ethanol consumption in rats [369]. Mediodorsal hypothalamic DYN-positive neurons projecting to the paraventricular nucleus of the thalamus are activated after context-induced reinstatement of alcoholic beer self-administration and microinjection of U50,488 into this region decrease reinstatement in rats [370]. These studies suggest that hypothalamic DYN/KOR systems modulate ethanol-seeking behavior. However, it is not clear if these systems regulate ethanol consumption by modulating hypothalamic regulation of caloric intake or motivational processes associated with ethanol-seeking.
One hypothesis posits that ethanol-induced alterations in the DYN/KOR system in addiction circuitry increase negative reinforcement due to increased modulation of drug-seeking neuronal substrates by DYN/KOR systems. Systemic and intracerebroventricular administration of nor-BNI blocks alcohol self-administration after abstinence in ethanol-dependent rats exposed to chronic ethanol vapors, an effect not observed in non-dependent rats [371, 372]. However, another study failed to find any effect of nor-BNI on abstinence-induced ethanol consumption in rats with prolonged ethanol experience [373]. Interestingly, acute and chronic administration of CI-977 enhanced both basal and abstinence-induced ethanol drinking at low doses that were ineffective in modulating ethanol and water intake [373]. These studies suggest that during ethanol abstinence in dependent animals, increased DYN tone in regions mediating craving, withdrawal, and/or stress reactivity produces negative reinforcing effects and this effect is mimicked by an exogenous KOR agonist. However, it is currently not clear whether nor-BNI attenuates alcohol-seeking by diminishing the negative affective properties of withdrawal and/or decreasing stress-induced alcohol self-administration. Taken together, these studies suggest that DYN/KOR systems in different regions are recruited with different time courses after acute and repeated ethanol exposure. This time and region dependency may explain some of the discrepancies observed between studies. For instance, KOR agonists may decrease ethanol-seeking behavior by decreasing DA and glutamate transmission in the NAcc. DYN/KOR systems in stress-related systems (i.e., CeA or bed nucleus of stria terminalis) may increase withdrawal-associated dysphoria during abstinence that promotes ethanol-seeking behavior. Work examining the role of DYN/KOR systems in mediating ethanol-seeking behavior motivated by different mechanisms (i.e., positive reinforcement vs. negative reinforcement) will further our understanding of this system in ethanol addiction. However, it has been difficult to determine the role of DYN/KOR systems during different phases of ethanol-seeking behavior due to long-lasting effects of KOR antagonists and the lack of sensitive methods that permit quantification of DYN release in vivo.
The role of KOR signaling in mediating stress-induced reinstatement of drug-seeking was recently extended to include ethanol. Stress-induced enhancement of ethanol self-administration and reinstatement of CPP is absent in pdyn knock-out mice and in wild-type mice treated with nor-BNI, suggesting that stress releases DYN which mediates stress-induced potentiation of the conditioned effects of ethanol and ethanol intake [374]. Additionally, administration of U50,488 mimicked the stress-induced enhancement of ethanol CPP and oral intake. Interestingly, Matsuzawa et al. [375], reported that U50,488 and nor-BNI administration attenuated and enhanced stress-potentiated ethanol CPP in rats, respectively. Importantly, the aversive effects of U50,488 were enhanced in stress versus non-stressed rats highlighting the complexity of the interaction of stress-related DYN signaling and its role in potentiation of CPP produced by ethanol and drug of abuse.
Opioids
Like other drugs of abuse, opiate treatment has been shown to alter DYN/KOR systems in reward-related neural circuits in animal models [376]. Chronic opiate administration also increases DYN A and B immunoreactivity in the NAcc, hypothalamus, and hippocampus of rats [376–379]. Chronic morphine increases pdyn expression in the LC of mice and rats [380], which is of interest since the LC has been implicated in mediating several behavioral effects of opiates [381]. Both systemic and intra-SN morphine administration increases SN extracellular DYN B levels, while systemic, intra-SN, and intra-striatal morphine perfusion is without effect on striatal DYN B overflow [382]. Given that a large proportion of DYN inputs to the SN arise from the dorsal striatum [189], activation of MORs in the SN may disinhibit DA neurons [383, 384]. This would result in positive modulation of the direct pathway of the basal ganglia, which would enhance SN extracellular DYN levels. DYN/KOR systems have been implicated in mediating the enhanced sensitivity to the reinforcing effects of opiates and other drugs of abuse in rodents. For instance, DBA/2J mice, which have a low drug responsivity, have greater pdyn mRNA expression in the NAcc core relative to drug responsive C57BL/6J mice [385]. Interestingly, nor-BNI facilitates sub-threshold morphine CPP in DBA/2J, but not C57BL/6J, mice, suggesting that increased NAcc DYN/KOR tone in these mice decreases morphine reward in DBA/2J mice.
KOR agonists decrease opiate self-administration maintained under limited access [328, 386], an effect associated with decreased NAcc DA levels [386]. Dorsal striatal DYN A- and B-immunoreactivity tissue content is enhanced in heroin-experienced rats when heroin access is expected but not immediately after extended heroin self-administration, an effect not observed with cocaine [387]. This suggests that DYN/KOR systems may be altered by expectancy of drug availability rather than by direct opiate exposure and these changes may result in opiate craving. However, nor-BNI does not alter heroin or morphine self-administration under limited access conditions [328, 386, 388]. Interestingly, nor-BNI enhances the increase in NAcc DA release that occurs during heroin self-administration [386], suggesting that endogenous NAcc KOR ligands may inhibit mesolimbic DA neurotransmission without altering heroin self-administration maintained under fixed ratio responding. It is of importance to determine whether endogenous DYN/KOR systems modulate withdrawal. A recent study reported that the KOR antagonist, GNTI, failed to modify withdrawal-induced choice of heroin over food in non-human primates with 21-h access to heroin [389], suggesting KORs may not regulate opiate withdrawal-induced heroin seeking. Future research aimed at determining the role of KOR systems in modulating opiate-withdrawal-induced drug-seeking and whether these processes are altered in animals with an extended history of opiate use, or stress, and in preclinical models of psychiatric disorders is needed.
Unlike other drugs of abuse, most studies have reported that KOR activation by endogenous or exogenous ligands decreases the severity of opiate withdrawal. Systemic and central administration of nor-BNI increases somatic signs of opiate withdrawal and the conditioned effects of morphine withdrawal [390]. Moreover, naloxone-precipitated morphine withdrawal decreases DA overflow in the NAcc; an effect that is more robust in nor-BNI-treated rats relative to vehicle controls [390], suggesting that mesolimbic DA responses may be associated with enhanced expression of somatic and conditioned withdrawal. Intra-NAcc microinjection of nor-BNI increases somatic signs of morphine withdrawal [103]. Interestingly, intra-LC U50,488 administration blocks morphine-withdrawal-induced excitation of LC neurons, presumably by activating presynaptic LC neurons [204]. Nor-BNI treatment can also precipitate morphine withdrawal in dependent rats [391]. Given that nor-BNI may interact with MORs for some hours after its administration, nor-BNI may precipitate withdrawal by blocking MORs. However, another study reported that nor-BNI pretreatment does not produce somatic signs of withdrawal in morphine-dependent rats [392]. Conversely, somatic signs of morphine withdrawal are decreased in oprk1 knock-out mice [393] and to a lesser extent in rats treated with JDTic, suggesting that DYN/KOR systems may contribute to somatic signs of withdrawal [394].
Therapeutic implications
Careful considerations must be made when deciding how KOR ligands should be used as therapeutic targets since the DYN/KOR system undergoes dynamic changes during various stages of the addiction cycle. Studies suggest that endogenous DYN/KOR systems are recruited in response to acute exposure to drugs of abuse and this counteracts reward/approach behaviors. As such, KOR agonists would be expected to initially decrease the rewarding effects of drugs of abuse and, perhaps, deter the development of addictive behavior. Chronic exposure and drug-seeking behavior alter the activity of the DYN/KOR system promoting negative affect and heightened vulnerability to stress during withdrawal from drugs of abuse. Thus, antagonism of KOR may ameliorate negative reinforcing behavioral states associated with drug withdrawal (e.g., nicotine or ethanol). In light of research demonstrating that KORs are powerful effectors of behavioral stress responses, KOR antagonists may be effective in ameliorating stress-induced drug-seeking behavior. Future research examining interactions between drug dependence and KOR modulation of stress responses are needed since stress systems are altered after prolonged drug exposure and contribute to drug addiction [291]. Questions remain regarding the interaction of KOR antagonists with chronic, rather than acute stress and whether antagonists would be beneficial in individuals in whom stress may be unpredictable. That is, are KOR antagonists effective if administered after the onset of stress? Importantly, conditioned stimuli associated with drug use precipitate relapse to addiction. To date, studies assessing the influence of KOR ligands on drug-seeking maintained by such stimuli are limited. Finally, increasing data indicate that with more prolonged drug experience, drug seeking that is initially goal-oriented becomes habitual [395]. Most pre-clinical studies have assessed the influence of KOR ligands during limited cocaine access, using reinforcement schedules that may not result in habitual responding. The recent description of a rodent model which allows delineation of goal-oriented versus habitual intravenous drug administration will now allow direct assessment of the role of KOR systems in these stages of addiction [396].
DYN/KOR system in schizophrenia
Schizophrenia is a disorder with strong neurodevelopmental, environmental, and polygenetic components. It is characterized by three major symptom clusters [397]: (1) positive symptoms involving auditory and to a lesser extent visual hallucinations, feelings of grandeur, delusional behavior, and thought disorder; (2) negative symptoms, which include social withdrawal, blunted affect/mood, decreased motivation, poverty of speech, and anhedonia (inability to experience pleasure); (3) cognitive deficits which include deficits in executive function, working memory, and attentional processes. Typically, schizophrenia is diagnosed during late adolescence or early adulthood in men after their first psychotic break, although negative symptoms and cognitive deficits may present prior to psychosis onset. It is believed that positive symptoms are mediated by enhanced DA transmission in the ventral striatum as they correlate with DA binding to D2 receptors [398]. Therapeutic efficacy is variable between patients and symptom cluster-dependent, with typical and atypical antipsychotics having some therapeutic value for positive symptoms [399]. However, effective therapeutics for negative symptoms or cognitive deficits are, to date, lacking since the underlying mechanisms are not understood, although they are thought to involve dysfunction of GABA and glutamate neurotransmission [400].
The DYN/KOR system has been implicated in schizophrenia in light of research demonstrating that a synthetic KOR agonist produce psychomimetic effects in humans, including hallucinations, perceptual distortions, and depersonalization [229]. Recently, converging results demonstrating that salvinorin A, a psychoactive compound in the hallucinogenic plant S. divinorum, is a potent KOR agonist have revived interest in the role of the DYN/KOR system in schizophrenia [401]. However, it should be noted that the effects of salvinorin A have not been examined in patients with schizophrenia, and salvinorin A may produce differential behavioral effects in this population.
CSF and tissue levels of DYN in schizophrenia
In the 1980s, several groups examined whether DYN peptide levels in spinal CSF were altered in schizophrenic patients. However, disparate findings were obtained. Heikkila et al. [402] reported enhanced CSF DYN A levels in unmedicated patients with schizophrenia relative to healthy and psychiatric controls using an antibody recognizing DYN A (9–17). CSF DYN A levels significantly correlated with increased psychotic rating scores on the Brief Psychiatric Ratings Scale. Increased CSF levels of DYN A have been reported in patients with schizophrenia with a poor prognosis; whereas patients with schizophrenia with a good prognosis have similar DYN A CSF levels relative to healthy controls [403]. Although correlative, these studies suggest that elevated endogenous DYN levels may contribute to the psychopathology of schizophrenia. However, Zhang et al. [404] reported lower DYN A (1–8) immunoreactivity in CSF from schizophrenia patients relative to controls with neurological disorders. The lack of healthy controls in that study makes interpretation of the findings difficult since DYN/KOR system dysregulation has been implicated in several neurological disorders. It is currently not clear to what extent spinal CSF DYN levels reflect alterations in limbic and cortical DYN activity since abnormalities in these systems has been implicated in the pathophysiology of schizophrenia. Nonetheless, these pioneering studies provided suggestive evidence that central DYN content may be altered in patients with schizophrenia. Analysis of PDYN and OPRK1 expression and KOR binding in post-mortem tissue has also yielded inconsistent results. PDYN and OPRK1 mRNA levels are unchanged in dorsal lateral prefrontal and cingulate cortex as well as in the amygdaloid nuclei of schizophrenics relative to controls [280, 281]. Fourier analysis of laminar KOR immunoreactivity in the hippocampus of patients with schizophrenia revealed that, unlike controls, the former group did not show a consistent laminar distribution of KORs [405]. It is possible that the abnormal KOR distribution observed is due to general disorganization of hippocampal circuits in schizophrenia rather than to altered KOR distribution. Moreover, sample size in this study was small.
Discriminative stimulus effects of KOR agonists
KOR agonists produce psychomimetic effects similar to ketamine and PCP. The latter drugs induce psychomimesis in healthy humans and symptoms in symptom-free patients with schizophrenia [406]. However, in non-human primates, the discriminative stimulus effects produced by salvinorin A do not generalize to those produced by ketamine or the 5-HT receptor agonist, psilocybin [407, 408]. Furthermore, Killinger et al. [409] demonstrated that the discriminative stimulus effects of salvinorin A do not generalize to LSD, another 5-HT receptor agonist. Others have reported that non-competitive NMDA receptor antagonists (ketamine, PCP, MK-801), but not competitive antagonists, generalize to the discriminative stimulus effects of U50,488, but not the KOR agonist TRK-820 [410]. Collectively, these findings suggest that psychomimetic effects produced by salvinorin A differ from those produced by classical hallucinogens. Given that PCP and ketamine produce psychomimetic effects that are qualitatively similar to sensory hallucinations and perceptual distortions in schizophrenia and KOR-agonists-induced discriminative stimulus effects may differ from PCP and ketamine, alterations in DYN/KOR function in schizophrenia appear insufficient to fully account for abnormal perception.
DYN/KORs and prepulse inhibition
Prepulse inhibition (PPI) is a sensory-gating process where a weak stimulus preceding a robust stimulus will diminish the behavior associated with the strong stimulus. PPI deficits are present in patients with schizophrenia and unaffected siblings [411]. Bortolato et al. [412] demonstrated that U50,488 disrupted PPI of the acoustic startle in rats, an effect reversed by atypical, but not typical, antipsychotic pretreatment. However, using various synthetic KOR agonists and salvinorin A, we found no effect of KOR activation on PPI [413]. Furthermore, KOR blockade did not alter basal PPI levels. Stress exacerbates or triggers symptoms in patients with schizophrenia [414]. Central CRF infusions decrease PPI, providing a framework by which stress could disrupt PPI in schizophrenics. CRF-induced PPI deficits were not altered in nor-BNI-treated rats, suggesting that endogenous DYN/KOR signaling is not necessary for basal PPI and is not a downstream mediator of CRF-induce PPI deficits. This contrasts with the downstream role of DYN/KOR in mediating stress-related anxiety and drug-seeking behavior, suggesting that CRF/KOR system interactions may be limited to certain behaviors, including affective and reward-related behaviors.
Co-morbidity of drug abuse and schizophrenia
The majority of patients with schizophrenia have co-morbid substance use disorders [397]. Given that alterations in the DYN/KOR in cortical and limbic regions implicated in schizophrenia have been reported in animal models of drug addiction and in dependent/addicted humans, it is possible that alterations that may be present in DYN/KOR systems in patients with schizophrenia stem from substance abuse. Alternatively, drugs of abuse may have differential effects on DYN synthesis and release in patients with schizophrenia or in animal models of schizophrenia relative to controls. For example, burst stimulation of the VTA results in a DA-dependent, sustained depolarization of medium-sized spiny NAcc neurons and decreased MSN firing in vivo in control rats [415]. Conversely, in neonatal ventral hippocampal lesion (NVHL) rats, a neurodevelopmental animal model of schizophrenia, VTA stimulation increases NAcc MSN activity [415]. Given that NAcc D1 DA receptor activation enhances PDYN synthesis, DYN may be synthesized in response to behaviors associated with enhanced NAcc DA release (i.e., drugs of abuse; stress) in controls, but only released in response to strong cortical or limbic drive of DYN-containing NAcc MSN neurons. In NVHL rats, drug-seeking behavior would result in exacerbated MSN activity and subsequent DYN release, resulting in enhanced DYN tone. This is consistent with elevated levels of pdyn expression in the striatum of NVHL during development [416], and during adulthood [417]. Unfortunately, to date this hypothesis has not been tested. An understanding of DYN/KOR dynamics in striatal, cortical, and limbic regions in animal models of schizophrenia will further our understanding of the role of this system in the pathophysiology of schizophrenia since it is hard to control for effects of drug abuse, stress, and antipsychotic medication in patients, all of which are associated with DYN/KOR system activation [151, 184, 279].
DYN/KOR systems in cognition
Deficits in cognitive function are observed in drug addiction, schizophrenia, and depression [418–420]. The DYN/KOR system modulates cognitive processes. This may result from control of neurotransmission by DYN/KOR systems in brain regions that mediate working memory and decision-making.
KORs and PFC-dependent processes
Systemic administration of KOR agonists decreases performance on the five-choice serial reaction time task (5-CSRTT), a task that assesses attentional processes and impulsive behavior that are dependent on appropriate PFC function [421–423]. Typically, decreased correct responses are observed after systemic KOR agonist administration, indicating impaired attentional processes. However, systemic KOR agonists also increase latency to respond and the number of trials that are omitted in the 5-CSRTT, which could reflect the influence of KOR ligands on motivation. This latter effect is a potential confound in interpreting data from cognitive tasks that require food as a motivator. Nemeth et al. [421] compared the effects of reward satiety, which decreases motivation to obtain reward, and systemic salvinorin A in the 5-CSRTT paradigm. Indeed, both reward satiety and salvinorin A decrease latency to response and trial omission indicative of decreased motivation. However, salvinorin A, but not reward satiety, decreases correct responding in the 5-CSRTT, suggesting that KOR agonism may exert detrimental effects on attentional processes independently of effects on motivation.
KOR and hippocampal-dependent cognition
Microinjections of DYN A 1–8 into CA3 of dorsal hippocampus produce a naloxone-reversible decrement of performance in tasks of spatial working memory [424]. In contrast, passive avoidance behavior is unaltered. Intra-hippocampal microinjection of DYN B or U50,488, decrease spatial working memory as assessed by the Morris water maze and this effect is reversed by nor-BNI [425, 426], suggesting that activation of CA3 hippocampal KOR modulates spatial memory. KOR blockade does not alter spatial memory [425, 426], indicating that KOR does not tonically inhibit hippocampal-dependent working memory. Consistent with pharmacological approaches, orpk1 knock-out mice do not display alterations in spatial working memory as assessed by the eight-arm radial maze and Morris water maze [427]. However, it is difficult to observe improvements in most cognitive tasks except under conditions where cognitive load is increased. Activation of the DYN/KOR system negatively modulates hippocampal synaptic plasticity. LTP has been suggested to be one type of synaptic plasticity that mediates learning and memory function [428]. As mentioned above, KOR activation decreases neurotransmission of both perforant path and mossy fiber pathways in the hippocampus [176, 223, 224]; providing a cellular basis for the detrimental effect of DYN and exogenous KOR agonists on hippocampal-dependent spatial memory.
Stress has been reported to exert both beneficial and detrimental effects on cognition. Recently, Carey et al. [429] demonstrated that the disruptive effects of forced swim stress on performance in the novel object recognition task is blocked by nor-BNI pretreatment in wild-type mice and absent in pdyn knock-out mice. Additionally, U50,488 administration decreases performance of wild-type mice in the novel object recognition task. Acute stress and CRF increase DYN synthesis and KOR phosphorylation [261, 266, 283], thereby, inhibiting glutamatergic signaling in brain regions (e.g., hippocampus, frontal and temporal cortex) that mediate cognitive function (e.g., working memory, attention, decision-making). Future research should be aimed at determining whether the modulatory role of the DYN/KOR system on cognition is altered in animals with repeated stress, a history of drug exposure, or in animal models of schizophrenia; all conditions in which DYN/KOR systems are altered.
DYN and age-related cognitive decline
Dysregulation of the DYN/KOR system is implicated in cognitive decline associated with aging. Enhanced PDYN and DYN peptide levels have been reported in the frontal cortex and hippocampus of aged rats (25–27 months of age) [430, 431]. Increased PDYN and DYN 1–8 content in the hippocampus and frontal cortex is negatively associated with performance on the Morris water maze, a spatial memory task highly dependent on proper hippocampal function [431]. Interestingly, aged rats that performed well during testing had DYN levels comparable to that of young adult controls. In partial agreement with these observations, Nguyen et al. [432] reported a small amelioration of deficits in Morris water maze performance in aged pdyn knock-out mice relative to aged wild-type controls. However, in the latter study, mice were 13–17 months of age and did not show elevated hippocampal DYN 1–8 peptide levels. Thus, the small effect size may be attributed to the use of younger animals that do not have altered DYN levels. The effects of KOR blockade on age-related cognitive effects have not been examined. However, enhanced DYN tone in the aged hippocampus may contribute to cognitive decline by decreasing hippocampal glutamatergic transmission via KOR activation or through DYN actions on non-opioid receptors.
DYN/KOR system in Alzheimer’s disease
Changes in KOR have been observed in the striatum of Alzheimer’s patients but the data are inconsistent, with decreased [433], increased [434, 435], or no difference [436] in KOR binding relative to controls. Enhanced amygdala KOR binding is present in Alzheimer’s patients [433] whereas several studies reported no differences in KOR binding in frontal and temporal cortex [433–435]. Interestingly, increased DYN A 1–8, but not DYN B, immunoreactivity is observed in frontal cortical tissue of patients with Down syndrome and Alzheimer’s disease [437]. Furthermore, DYN A 9–17 is elevated in cortex of Alzheimer’s patients relative to typical, Parkinson’s disease, and cerebrovascular disease controls, an effect not observed for PDYN and DYN B [438]. Given the existence of tonically active KORs in the mPFC that inhibit DA overflow, these findings raise the possibility that enhanced PFC DYN A tone may alter mPFC DA dynamics in Alzheimer’s disease and Down syndrome since DA signaling is critical for optimal PFC function (e.g., working memory and behavioral flexibility) [199, 439].
Beneficial effects of DYN in models of cognitive impairment
Contrary to findings that DYN/KOR activation impairs cognitive processes, a number of studies have demonstrated that DYN and synthetic KOR agonists ameliorate cognitive deficits in animal models of cognitive impairment. The detrimental effects of nicotinic acetylcholine receptor (nAChR) and muscarinic acetylcholine receptor (mAChR) ligands are reversed by DYN peptides and U50,488 in a nor-BNI-sensitive manner [440, 441]. However, it should be noted that nor-BNI was administered intracerebroventricularly 5 min prior to KOR ligand administration. Consistent with nor-BNI-sensitive effects of KOR ligands on cognitive impairment, improvement of mAChR-antagonism-induced cognitive impairment by U50,488 is blocked by antisense oligonucleotides targeting KOR [442], suggesting that KOR activation is necessary for this effect. DYN (2–13), a PDYN-derived peptide that does not interact with opioid receptors, also blocks cognitive impairments induced by mAChR ligands, suggesting that non-opioid actions of DYN peptides may contribute to this effect.
PDYN and OPRK1 in the genetics of psychiatric disorders
Heritability of psychiatric disorders is estimated to be substantial (e.g., 73–90% in schizophrenia [443]). Although the bulk of variance is yet to be explained [444, 445], multiple polymorphisms in human PDYN and OPRK1 have been associated with drug addiction or schizophrenia [446, 447]. We will limit our discussion to variants for which there is evidence of function (overviewed above). The frequency of schizophrenia is higher in individuals with three copies of rs35286281 and who are also homozygous for the C (glycine) allele of rs6280 (SNP) located in exon 2 of the DA D3 receptor gene, DRD3 [448]; mutation which confers higher DA affinity and altered signaling in vitro [449, 450]. Given the likely polygenic mode of inheritance of schizophrenia [451], and research demonstrating that the DYN/KOR system and D3 receptors regulate DA release and reuptake [185, 452, 453], this apparent gene–gene interaction suggest that concomitant dysregulation of these systems may contribute to altered DA transmission during psychosis. More specifically, altered KOR- and D3 receptor-mediated control of DA dynamics in the NAcc (where there is a high overlap between DYNs, KORs, and D3 receptors) may result in increased presynaptic control of DA efflux during development.
Although the results are divergent, rs35286281 and rs1997794 have been linked to psychiatric disorders on a number of occasions [69, 454, 455]. In some cases, however, data interpretation may have been confounded by population stratification (the presence in study samples of individuals from different population subdivisions with putatively different allele frequencies). rs910080 has been associated with alcohol dependence and episodic memory in the elderly [455, 456]. rs35566036 was associated with alcohol dependence in a family-based study [73], adding to the number of SNPs in OPRK1 associated with this disorder [455]. It should be noted that rs35566036 was not included in a linkage study on a genetically more heterogeneous sample which yielded seemingly conflicting results to those of Xuei et al. [457]. rs6985606 and rs997917 (SNPs) located in intron 2 of OPRK1, however, were associated with alcohol dependence in both studies when the variance introduced by age and gender was accounted for by Zhang et al. (p ≤ 0.05). The haplotypes (alleles on the same chromosome in linkage disequilibrium at two or more loci) associated with alcohol dependence by Zhang and colleagues were not inferred by Xuei et al. Thus, these studies are in concordance except with respect to the haplotype blocks identified, which differ at one position.
The linkage studies on PDYN and OPRK1 performed to date have been targeted to test specific hypotheses (i.e. validating previous findings) permitting a significance threshold of p < 0.05. However, genome-wide significance (i.e., p < 10−4) has not been reported for either gene; a discrepancy potentially explained by insufficient power still in genome-wide association studies (GWASes) of psychiatric disorders [444]. Power can be increased by enlarging the sample size and/or refining the phenotype. In this regard, it is interesting to note that a region on chromosome 20 comprising PDYN was recently linked to heavy alcohol consumption in a GWAS on a comparatively large sample [458], and that nominal support was found for this gene in a family-based GWAS of alcohol dependence [459]. Accurate estimates of the genotype relative risks inferred by the aforementioned SNPs in PDYN and OPRK1, however, likely awaits analyses of larger samples and/or meta-analyses.
First-generation GWASes were designed to test the common disease common variant hypothesis according to which psychiatric disorders are caused by multiple common variants (frequency of >5%) with small effect sizes (e.g., the SNPs in PDYN and OPRK1 mentioned herein) [460]. They are based on linkage disequilibrium bins (i.e., groups of highly correlated SNPs each represented by a tagging SNP) [461]. These tagging SNPs capture some 40–50% of common structural variants [sequence variants other than SNPs (e.g., rs35286281 and rs35566036)] [462]. This is a noteworthy limitation given that such variants account for an estimated 74–92% of all variant bases. Moreover, they do not capture rare variants (frequency of <1%) [463]. Thus, that only a small percentage of the genetic variance in psychiatric disorders has been explained by the GWASes performed to date is not surprising [444]. The combined results of these studies suggest that the low genotype relative risks inferred by common SNPs preclude them from being causative of the familial clustering of most psychiatric disorders [445].
Contrary to the common disease common variant hypothesis, the multiple rare variant hypothesis predicts that psychiatric disorders are the result of multiple rare, mainly non-synonymous (coding) variants with comparatively large effect sizes [464]. In addition to the apparent failure of GWASes to securely implicate specific genes in common disease [465], support for this hypothesis comes from monozygotic/dizygotic twin concordance ratios for some psychiatric disorders [e.g., drug (other than cocaine) addiction] [451]. Rare missense mutations in the coding region of PDYN were recently reported to cause the late-onset neurodegenerative disorder spinocerebellar ataxia type 23 [466]. Thus, it would be interesting to assess the prevalence of these mutations in families with multiple affected members and/or in cohorts that are at the extreme ends of psychiatric disorders. However, neither hypothesis alone is likely to explain all of the genetic variance in psychiatric disorders [460], and a complete understanding of the etiologies of psychiatric disorders in cis may await development of methods to accurately, cost-effectively and rapidly re-sequence entire genomes. Clues to the apparent shortcomings of GWASes may also come from ever more comprehensive epigenomic analyses as suggested by the identification of intergenic, often differentially methylated regions in the GWASes of disease performed to date [467].
Conclusions
DYN/KOR systems are endogenous, activity-dependent modulators of neuronal circuits that mediate affect, motivation, stress reactivity, and reward. DYN/KOR systems are recruited by various stimuli and act to shape neuronal activity, alter presynaptic neurotransmitter release, and decrease neuronal excitability. Dysregulation of neuronal activity in cortical-limbic-basal ganglia circuitry is believed to underlie behavioral abnormalities that are commonly shared by psychiatric disorders. Aberrant neuronal activity in cortical and limbic regions as well as in the basal ganglia produces long-lasting changes in DYN/KOR systems. Changes in this system may contribute to symptom clusters that are shared by various psychiatric disorders (i.e., decreased motivation and negative affect). A role for DYN/KOR in modulating drug addiction has been proposed. However, with improved research techniques and animal models of addiction it is now appreciated that the function of DYN/KOR systems in addiction is diverse and that this system may bi-directionally modulate drug-seeking behavior depending on drug history, pattern of intake, incentive salience, and stress. Future research is warranted to determine whether genetic predisposition and/or environmental factors may dictate therapeutic utility of KOR ligands. Similarly, there is compelling evidence from pre-clinical studies that the DYN/KOR system may dysregulated in affective psychiatric disorders. However, solid evidence from clinical studies is lacking. Thus, with the advent of new research tools (i.e., radiolabeled KOR ligands), work aimed at determining the physiological and pathophysiological role of DYN/KOR systems will further our understanding of this system in psychiatric disease in humans. Moreover, translational studies may provide insight to the mechanisms by which DYN/KOR contribute to brain dysfunction during the course of a disease and at what stage of the disease process, drugs that target this opioid system may have therapeutic effects.
Acknowledgments
This review was supported by the: (1) Intramural Research Program, National Institute on Drug Abuse; (2) National Institute of Mental Health (R01MH083928); (3) National Science Foundation Graduate Research Fellowship (HAT); (4) Ford Foundation Predoctoral Fellowship (HAT); (5) Meyerhoff Fellowship (HAT); and (6) Department of Clinical Neuroscience, Karolinska Institutet(RH). Special thanks to Dr. Vladimir Chefer for his thoughtful comments on the manuscript.
Conflict of interest
The authors state that they have no conflicts of interest.
References
- 1.WHO (2001) Mental health: new understanding, new hope. The world health report 2001
- 2.Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8:1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- 3.Henriksen G, Willoch F. Imaging of opioid receptors in the central nervous system. Brain. 2008;131:1171–1196. doi: 10.1093/brain/awm255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- 5.Shippenberg TS. The dynorphin/kappa opioid receptor system: a new target for the treatment of addiction and affective disorders? Neuropsychopharmacology. 2009;34:247. doi: 10.1038/npp.2008.165. [DOI] [PubMed] [Google Scholar]
- 6.Kakidani H, Furutani Y, Takahashi H, Noda M, Morimoto Y, Hirose T, Asai M, Inayama S, Nakanishi S, Numa S. Cloning and sequence analysis of cDNA for porcine beta-neo-endorphin/dynorphin precursor. Nature. 1982;298:245–249. doi: 10.1038/298245a0. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein A, Fischli W, Lowney LI, Hunkapiller M, Hood L. Porcine pituitary dynorphin: complete amino acid sequence of the biologically active heptadecapeptide. Proc Natl Acad Sci USA. 1981;78:7219–7223. doi: 10.1073/pnas.78.11.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein A, Tachibana S, Lowney LI, Hunkapiller M, Hood L. Dynorphin-(1-13), an extraordinarily potent opioid peptide. Proc Natl Acad Sci USA. 1979;76:6666–6670. doi: 10.1073/pnas.76.12.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minamino N, Kangawa K, Fukuda A, Matsuo H, Iagarashi M. A new opioid octapeptide related to dynorphin from porcine hypothalamus. Biochem Biophys Res Commun. 1980;95:1475–1481. doi: 10.1016/S0006-291X(80)80063-5. [DOI] [PubMed] [Google Scholar]
- 10.Kangawa K, Matsuo H. Alpha-Neo-endorphin: a “big” Leu-enkephalin with potent opiate activity from porcine hypothalami. Biochem Biophys Res Commun. 1979;86:153–160. doi: 10.1016/0006-291X(79)90394-2. [DOI] [PubMed] [Google Scholar]
- 11.Minamino N, Kangawa K, Chino N, Sakakibara S, Matsuo H. Beta-neo-endorphin, a new hypothalamic “big” Leu-enkephalin of porcine origin: its purification and the complete amino acid sequence. Biochem Biophys Res Commun. 1981;99:864–870. doi: 10.1016/0006-291X(81)91243-2. [DOI] [PubMed] [Google Scholar]
- 12.Fischli W, Goldstein A, Hunkapiller MW, Hood LE. Isolation and amino acid sequence analysis of a 4, 000-dalton dynorphin from porcine pituitary. Proc Natl Acad Sci USA. 1982;79:5435–5437. doi: 10.1073/pnas.79.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilpatrick DL, Wahlstrom A, Lahm HW, Blacher R, Udenfriend S. Rimorphin, a unique, naturally occurring [Leu]enkephalin-containing peptide found in association with dynorphin and alpha-neo-endorphin. Proc Natl Acad Sci USA. 1982;79:6480–6483. doi: 10.1073/pnas.79.21.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakao K, Suda M, Sakamoto M, Yoshimasa T, Morii N, Ikeda Y, Yanaihara C, Yanaihara N, Numa S, Imura H. Leumorphin is a novel endogenous opioid peptide derived from preproenkephalin B. Biochem Biophys Res Commun. 1983;117:695–701. doi: 10.1016/0006-291X(83)91653-4. [DOI] [PubMed] [Google Scholar]
- 15.Naqvi T, Haq W, Mathur KB. Structure-activity relationship studies of dynorphin A and related peptides. Peptides. 1998;19:1277–1292. doi: 10.1016/S0196-9781(98)00042-4. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Chen C, Liu-Chen LY. Dynorphin peptides differentially regulate the human kappa opioid receptor. Life Sci. 2007;80:1439–1448. doi: 10.1016/j.lfs.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shukla VK, Lemaire S. Non-opioid effects of dynorphins: possible role of the NMDA receptor. Trends Pharmacol Sci. 1994;15:420–424. doi: 10.1016/0165-6147(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 18.Hauser KF, Aldrich JV, Anderson KJ, Bakalkin G, Christie MJ, Hall ED, Knapp PE, Scheff SW, Singh IN, Vissel B, Woods AS, Yakovleva T, Shippenberg TS. Pathobiology of dynorphins in trauma and disease. Front Biosci. 2005;10:216–235. doi: 10.2741/1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherwood TW, Askwith CC. Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J Neurosci. 2009;29:14371–14380. doi: 10.1523/JNEUROSCI.2186-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber E, Evans CJ, Barchas JD. Predominance of the amino-terminal octapeptide fragment of dynorphin in rat brain regions. Nature. 1982;299:77–79. doi: 10.1038/299077a0. [DOI] [PubMed] [Google Scholar]
- 21.Ramsdell CD, Meador-Woodruff JH. Expression of prodynorphin-derived peptides and mRNA in guinea-pig cortex. Neuropeptides. 1993;25:131–138. doi: 10.1016/0143-4179(93)90093-P. [DOI] [PubMed] [Google Scholar]
- 22.Healy DJ, Meador-Woodruff JH. Prodynorphin-derived peptide expression in primate cortex and striatum. Neuropeptides. 1994;27:277–284. doi: 10.1016/0143-4179(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 23.Berman Y, Mzhavia N, Polonskaia A, Furuta M, Steiner DF, Pintar JE, Devi LA. Defective prodynorphin processing in mice lacking prohormone convertase PC2. J Neurochem. 2000;75:1763–1770. doi: 10.1046/j.1471-4159.2000.0751763.x. [DOI] [PubMed] [Google Scholar]
- 24.Boudarine M, Yegorov O, Sterling-Dubrovsky A, Devi LA, Berman Y. Developmental changes in opioid peptides and their receptors in Cpe(fat)/Cpe(fat) mice lacking peptide processing enzyme carboxypeptidase E. J Pharmacol Exp Ther. 2002;303:1317–1324. doi: 10.1124/jpet.102.037663. [DOI] [PubMed] [Google Scholar]
- 25.Minokadeh A, Funkelstein L, Toneff T, Hwang SR, Beinfeld M, Reinheckel T, Peters C, Zadina J, Hook V. Cathepsin L participates in dynorphin production in brain cortex, illustrated by protease gene knockout and expression. Mol Cell Neurosci. 2010;43:98–107. doi: 10.1016/j.mcn.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Day R, Lazure C, Basak A, Boudreault A, Limperis P, Dong W, Lindberg I. Prodynorphin processing by proprotein convertase 2. Cleavage at single basic residues and enhanced processing in the presence of carboxypeptidase activity. J Biol Chem. 1998;273:829–836. doi: 10.1074/jbc.273.2.829. [DOI] [PubMed] [Google Scholar]
- 27.Hook V, Funkelstein L, Lu D, Bark S, Wegrzyn J, Hwang SR. Proteases for processing proneuropeptides into peptide neurotransmitters and hormones. Annu Rev Pharmacol Toxicol. 2008;48:393–423. doi: 10.1146/annurev.pharmtox.48.113006.094812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yakovleva T, Bazov I, Cebers G, Marinova Z, Hara Y, Ahmed A, Vlaskovska M, Johansson B, Hochgeschwender U, Singh IN, Bruce-Keller AJ, Hurd YL, Kaneko T, Terenius L, Ekstrom TJ, Hauser KF, Pickel VM, Bakalkin G. Prodynorphin storage and processing in axon terminals and dendrites. FASEB J. 2006;20:2124–2126. doi: 10.1096/fj.06-6174fje. [DOI] [PubMed] [Google Scholar]
- 29.Reed B, Zhang Y, Chait BT, Kreek MJ. Dynorphin A(1–17) biotransformation in striatum of freely moving rats using microdialysis and matrix-assisted laser desorption/ionization mass spectrometry. J Neurochem. 2003;86:815–823. doi: 10.1046/j.1471-4159.2003.01859.x. [DOI] [PubMed] [Google Scholar]
- 30.Sandin J, Tan-No K, Kasakov L, Nylander I, Winter A, Silberring J, Terenius L. Differential metabolism of dynorphins in substantia nigra, striatum, and hippocampus. Peptides. 1997;18:949–956. doi: 10.1016/S0196-9781(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 31.Pert CB, Snyder SH. Opiate receptor: demonstration in nervous tissue. Science. 1973;179:1011–1014. doi: 10.1126/science.179.4077.1011. [DOI] [PubMed] [Google Scholar]
- 32.Simon EJ, Hiller JM, Edelman I. Stereospecific binding of the potent narcotic analgesic (3H) Etorphine to rat-brain homogenate. Proc Natl Acad Sci USA. 1973;70:1947–1949. doi: 10.1073/pnas.70.7.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terenius L. Stereospecific interaction between narcotic analgesics and a synaptic plasma membrane fraction of rat cerebral cortex. Acta Pharmacol Toxicol (Copenh) 1973;32:317–320. doi: 10.1111/j.1600-0773.1973.tb01477.x. [DOI] [PubMed] [Google Scholar]
- 34.Dhawan BN, Cesselin F, Raghubir R, Reisine T, Bradley PB, Portoghese PS, Hamon M. International Union of Pharmacology, XII. Classification of opioid receptors. Pharmacol Rev. 1996;48:567–592. [PubMed] [Google Scholar]
- 35.Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- 36.Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- 37.Grudt TJ, Williams JT. Kappa-opioid receptors also increase potassium conductance. Proc Natl Acad Sci USA. 1993;90:11429–11432. doi: 10.1073/pnas.90.23.11429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gross RA, Moises HC, Uhler MD, Macdonald RL. Dynorphin A and cAMP-dependent protein kinase independently regulate neuronal calcium currents. Proc Natl Acad Sci USA. 1990;87:7025–7029. doi: 10.1073/pnas.87.18.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iremonger KJ, Bains JS. Retrograde opioid signaling regulates glutamatergic transmission in the hypothalamus. J Neurosci. 2009;29:7349–7358. doi: 10.1523/JNEUROSCI.0381-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crain SM, Shen KF. Modulatory effects of Gs-coupled excitatory opioid receptor functions on opioid analgesia, tolerance, and dependence. Neurochem Res. 1996;21:1347–1351. doi: 10.1007/BF02532375. [DOI] [PubMed] [Google Scholar]
- 41.Kam AY, Chan AS, Wong YH. Kappa-opioid receptor signals through Src and focal adhesion kinase to stimulate c-Jun N-terminal kinases in transfected COS-7 cells and human monocytic THP-1 cells. J Pharmacol Exp Ther. 2004;310:301–310. doi: 10.1124/jpet.104.065078. [DOI] [PubMed] [Google Scholar]
- 42.Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem. 2006;281:18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu-Chen LY. Agonist-induced regulation and trafficking of kappa opioid receptors. Life Sci. 2004;75:511–536. doi: 10.1016/j.lfs.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 44.Blake AD, Bot G, Li S, Freeman JC, Reisine T. Differential agonist regulation of the human kappa-opioid receptor. J Neurochem. 1997;68:1846–1852. doi: 10.1046/j.1471-4159.1997.68051846.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DY, Huang P, Li JG, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of three distinct kappa ligands (Salvinorin A, TRK-820 and 3FLB) on kappa opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol Exp Ther. 2005;312:220–230. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Chen C, Wang Y, Liu-Chen LY. Ligands regulate cell surface level of the human kappa opioid receptor by activation-induced down-regulation and pharmacological chaperone-mediated enhancement: differential effects of nonpeptide and peptide agonists. J Pharmacol Exp Ther. 2006;319:765–775. doi: 10.1124/jpet.106.107987. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Liu-Chen LY. Chaperone-like effects of cell-permeant ligands on opioid receptors. Front Biosci. 2009;14:634–643. doi: 10.2741/3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li JG, Chen C, Liu-Chen LY. N-Glycosylation of the human kappa opioid receptor enhances its stability but slows its trafficking along the biosynthesis pathway. Biochemistry. 2007;46:10960–10970. doi: 10.1021/bi700443j. [DOI] [PubMed] [Google Scholar]
- 49.Bruchas MR, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl) 2010;210:137–147. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wain HM, Bruford EA, Lovering RC, Lush MJ, Wright MW, Povey S. Guidelines for human gene nomenclature. Genomics. 2002;79:464–470. doi: 10.1006/geno.2002.6748. [DOI] [PubMed] [Google Scholar]
- 51.Douglass J, McMurray CT, Garrett JE, Adelman JP, Calavetta L. Characterization of the rat prodynorphin gene. Mol Endocrinol. 1989;3:2070–2078. doi: 10.1210/mend-3-12-2070. [DOI] [PubMed] [Google Scholar]
- 52.Horikawa S, Takai T, Toyosato M, Takahashi H, Noda M, Kakidani H, Kubo T, Hirose T, Inayama S, Hayashida H, et al. Isolation and structural organization of the human preproenkephalin B gene. Nature. 1983;306:611–614. doi: 10.1038/306611a0. [DOI] [PubMed] [Google Scholar]
- 53.Sharifi N, Ament M, Brennan MB, Hochgeschwender U. Isolation and characterization of the mouse homolog of the preprodynorphin (Pdyn) gene. Neuropeptides. 1999;33:236–238. doi: 10.1054/npep.1999.0023. [DOI] [PubMed] [Google Scholar]
- 54.Nikoshkov A, Hurd YL, Yakovleva T, Bazov I, Marinova Z, Cebers G, Pasikova N, Gharibyan A, Terenius L, Bakalkin G. Prodynorphin transcripts and proteins differentially expressed and regulated in the adult human brain. FASEB J. 2005;19:1543–1545. doi: 10.1096/fj.05-3743fje. [DOI] [PubMed] [Google Scholar]
- 55.Kimura K, Wakamatsu A, Suzuki Y, Ota T, Nishikawa T, Yamashita R, Yamamoto J, Sekine M, Tsuritani K, Wakaguri H, Ishii S, Sugiyama T, Saito K, Isono Y, Irie R, Kushida N, Yoneyama T, Otsuka R, Kanda K, Yokoi T, Kondo H, Wagatsuma M, Murakawa K, Ishida S, Ishibashi T, Takahashi-Fujii A, Tanase T, Nagai K, Kikuchi H, Nakai K, Isogai T, Sugano S. Diversification of transcriptional modulation: large-scale identification and characterization of putative alternative promoters of human genes. Genome Res. 2006;16:55–65. doi: 10.1101/gr.4039406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geijer T, Telkov M, Terenius L. Characterization of human prodynorphin gene transcripts. Biochem Biophys Res Commun. 1995;215:881–888. doi: 10.1006/bbrc.1995.2546. [DOI] [PubMed] [Google Scholar]
- 57.Telkov M, Geijer T, Terenius L. Human prodynorphin gene generates several tissue-specific transcripts. Brain Res. 1998;804:284–295. doi: 10.1016/S0006-8993(98)00706-9. [DOI] [PubMed] [Google Scholar]
- 58.Liu HC, Lu S, Augustin LB, Felsheim RF, Chen HC, Loh HH, Wei LN. Cloning and promoter mapping of mouse kappa opioid receptor gene. Biochem Biophys Res Commun. 1995;209:639–647. doi: 10.1006/bbrc.1995.1547. [DOI] [PubMed] [Google Scholar]
- 59.Yakovlev AG, Krueger KE, Faden AI. Structure and expression of a rat kappa opioid receptor gene. J Biol Chem. 1995;270:6421–6424. doi: 10.1074/jbc.270.12.6421. [DOI] [PubMed] [Google Scholar]
- 60.Yuferov V, Fussell D, LaForge KS, Nielsen DA, Gordon D, Ho A, Leal SM, Ott J, Kreek MJ. Redefinition of the human kappa opioid receptor gene (OPRK1) structure and association of haplotypes with opiate addiction. Pharmacogenetics. 2004;14:793–804. doi: 10.1097/00008571-200412000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu S, Loh HH, Wei LN. Studies of dual promoters of mouse kappa-opioid receptor gene. Mol Pharmacol. 1997;52:415–420. doi: 10.1124/mol.52.3.415. [DOI] [PubMed] [Google Scholar]
- 62.Sandelin A, Carninci P, Lenhard B, Ponjavic J, Hayashizaki Y, Hume DA. Mammalian RNA polymerase II core promoters: insights from genome-wide studies. Nat Rev Genet. 2007;8:424–436. doi: 10.1038/nrg2026. [DOI] [PubMed] [Google Scholar]
- 63.Zimprich A, Kraus J, Woltje M, Mayer P, Rauch E, Hollt V. An allelic variation in the human prodynorphin gene promoter alters stimulus-induced expression. J Neurochem. 2000;74:472–477. doi: 10.1046/j.1471-4159.2000.740472.x. [DOI] [PubMed] [Google Scholar]
- 64.Babbitt CC, Silverman JS, Haygood R, Reininga JM, Rockman MV, Wray GA. Multiple functional variants in cis modulate PDYN expression. Mol Biol Evol. 2010;27:465–479. doi: 10.1093/molbev/msp276. [DOI] [PubMed] [Google Scholar]
- 65.Rouault M, Nielsen DA, Ho A, Kreek MJ, Yuferov V (2010) Cell-specific effects of variants of the 68-base pair tandem repeat on prodynorphin gene promoter activity. Addict Biol. doi:10.1111/j.1369-1600.2010.00248.x [DOI] [PMC free article] [PubMed]
- 66.Geijer T, Jonsson E, Neiman J, Gyllander A, Sedvall G, Rydberg U, Terenius L. Prodynorphin allelic distribution in Scandinavian chronic alcoholics. Alcohol Clin Exp Res. 1997;21:1333–1336. doi: 10.1111/j.1530-0277.1997.tb04456.x. [DOI] [PubMed] [Google Scholar]
- 67.Cirulli ET, Goldstein DB. In vitro assays fail to predict in vivo effects of regulatory polymorphisms. Hum Mol Genet. 2007;16:1931–1939. doi: 10.1093/hmg/ddm140. [DOI] [PubMed] [Google Scholar]
- 68.Naranjo JR, Mellstrom B, Achaval M, Sassone-Corsi P. Molecular pathways of pain: Fos/Jun-mediated activation of a noncanonical AP-1 site in the prodynorphin gene. Neuron. 1991;6:607–617. doi: 10.1016/0896-6273(91)90063-6. [DOI] [PubMed] [Google Scholar]
- 69.Yuferov V, Ji F, Nielsen DA, Levran O, Ho A, Morgello S, Shi R, Ott J, Kreek MJ. A functional haplotype implicated in vulnerability to develop cocaine dependence is associated with reduced PDYN expression in human brain. Neuropsychopharmacology. 2009;34:1185–1197. doi: 10.1038/npp.2008.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-wide mapping of in vivo protein-DNA interactions. Science. 2007;316:1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- 71.Henriksson R, Bäckman CM, Harvey BK, Bakalkin G, Shippenberg T (2010) Regulation of human prodynorphin gene (PDYN) expression by REST and miR-9 Soc Neurosci Abstr 167.5
- 72.Rockman MV, Hahn MW, Soranzo N, Zimprich F, Goldstein DB, Wray GA. Ancient and recent positive selection transformed opioid cis-regulation in humans. PLoS Biol. 2005;3:e387. doi: 10.1371/journal.pbio.0030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edenberg HJ, Wang J, Tian H, Pochareddy S, Xuei X, Wetherill L, Goate A, Hinrichs T, Kuperman S, Nurnberger JI, Jr, Schuckit M, Tischfield JA, Foroud T. A regulatory variation in OPRK1, the gene encoding the kappa-opioid receptor, is associated with alcohol dependence. Hum Mol Genet. 2008;17:1783–1789. doi: 10.1093/hmg/ddn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE, 3rd, Maze I, Sikder D, Robison AJ, LaPlant Q, Dietz DM, Russo SJ, Vialou V, Chakravarty S, Kodadek TJ, Stack A, Kabbaj M, Nestler EJ. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62:335–348. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gardiner-Garden M, Frommer M. CpG islands in vertebrate genomes. J Mol Biol. 1987;196:261–282. doi: 10.1016/0022-2836(87)90689-9. [DOI] [PubMed] [Google Scholar]
- 76.Brunner AL, Johnson DS, Kim SW, Valouev A, Reddy TE, Neff NF, Anton E, Medina C, Nguyen L, Chiao E, Oyolu CB, Schroth GP, Absher DM, Baker JC, Myers RM. Distinct DNA methylation patterns characterize differentiated human embryonic stem cells and developing human fetal liver. Genome Res. 2009;19:1044–1056. doi: 10.1101/gr.088773.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuferov V, Nielsen DA, Levran O, Randesi M, Hamon S, Ho A, Morgello S, Kreek MJ (2010) Tissue-specific DNA methylation of the human prodynorphin gene in post-mortem brain tissues and PBMCs. Pharmacogenet Genom. doi:10.1097/FPC.0b013e32833eecbc [DOI] [PMC free article] [PubMed]
- 78.Wei LN, Loh HH (2010) Transcriptional and epigenetic regulation of opioid receptor genes— present and future. Annu Rev Pharmacol Toxicol. doi:10.1146/annurev-pharmtox-010510-100605 [DOI] [PMC free article] [PubMed]
- 79.Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang MQ, Zhao K. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40:897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dulac C. Brain function and chromatin plasticity. Nature. 2010;465:728–735. doi: 10.1038/nature09231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ota T, Suzuki Y, Nishikawa T, Otsuki T, Sugiyama T, Irie R, Wakamatsu A, Hayashi K, Sato H, Nagai K, Kimura K, Makita H, Sekine M, Obayashi M, Nishi T, Shibahara T, Tanaka T, Ishii S, Yamamoto J, Saito K, Kawai Y, Isono Y, Nakamura Y, Nagahari K, Murakami K, Yasuda T, Iwayanagi T, Wagatsuma M, Shiratori A, Sudo H, Hosoiri T, Kaku Y, Kodaira H, Kondo H, Sugawara M, Takahashi M, Kanda K, Yokoi T, Furuya T, Kikkawa E, Omura Y, Abe K, Kamihara K, Katsuta N, Sato K, Tanikawa M, Yamazaki M, Ninomiya K, Ishibashi T, Yamashita H, Murakawa K, Fujimori K, Tanai H, Kimata M, Watanabe M, Hiraoka S, Chiba Y, Ishida S, Ono Y, Takiguchi S, Watanabe S, Yosida M, Hotuta T, Kusano J, Kanehori K, Takahashi-Fujii A, Hara H, Tanase TO, Nomura Y, Togiya S, Komai F, Hara R, Takeuchi K, Arita M, Imose N, Musashino K, Yuuki H, Oshima A, Sasaki N, Aotsuka S, Yoshikawa Y, Matsunawa H, Ichihara T, Shiohata N, Sano S, Moriya S, Momiyama H, Satoh N, Takami S, Terashima Y, Suzuki O, Nakagawa S, Senoh A, Mizoguchi H, Goto Y, Shimizu F, Wakebe H, Hishigaki H, Watanabe T, Sugiyama A, Takemoto M, Kawakami B, Watanabe K, Kumagai A, Itakura S, Fukuzumi Y, Fujimori Y, Komiyama M, Tashiro H, Tanigami A, Fujiwara T, Ono T, Yamada K, Fujii Y, Ozaki K, Hirao M, Ohmori Y, Kawabata A, Hikiji T, Kobatake N, Inagaki H, Ikema Y, Okamoto S, Okitani R, Kawakami T, Noguchi S, Itoh T, Shigeta K, Senba T, Matsumura K, Nakajima Y, Mizuno T, Morinaga M, Sasaki M, Togashi T, Oyama M, Hata H, Komatsu T, Mizushima-Sugano J, Satoh T, Shirai Y, Takahashi Y, Nakagawa K, Okumura K, Nagase T, Nomura N, Kikuchi H, Masuho Y, Yamashita R, Nakai K, Yada T, Ohara O, Isogai T, Sugano S. Complete sequencing and characterization of 21, 243 full-length human cDNAs. Nat Genet. 2004;36:40–45. doi: 10.1038/ng1285. [DOI] [PubMed] [Google Scholar]
- 82.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 83.Soranzo N, Spector TD, Mangino M, Kuhnel B, Rendon A, Teumer A, Willenborg C, Wright B, Chen L, Li M, Salo P, Voight BF, Burns P, Laskowski RA, Xue Y, Menzel S, Altshuler D, Bradley JR, Bumpstead S, Burnett MS, Devaney J, Doring A, Elosua R, Epstein SE, Erber W, Falchi M, Garner SF, Ghori MJ, Goodall AH, Gwilliam R, Hakonarson HH, Hall AS, Hammond N, Hengstenberg C, Illig T, Konig IR, Knouff CW, McPherson R, Melander O, Mooser V, Nauck M, Nieminen MS, O’Donnell CJ, Peltonen L, Potter SC, Prokisch H, Rader DJ, Rice CM, Roberts R, Salomaa V, Sambrook J, Schreiber S, Schunkert H, Schwartz SM, Serbanovic-Canic J, Sinisalo J, Siscovick DS, Stark K, Surakka I, Stephens J, Thompson JR, Volker U, Volzke H, Watkins NA, Wells GA, Wichmann HE, Van Heel DA, Tyler-Smith C, Thein SL, Kathiresan S, Perola M, Reilly MP, Stewart AF, Erdmann J, Samani NJ, Meisinger C, Greinacher A, Deloukas P, Ouwehand WH, Gieger C. A genome-wide meta-analysis identifies 22 loci associated with eight hematological parameters in the HaemGen consortium. Nat Genet. 2009;41:1182–1190. doi: 10.1038/ng.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim JM, Lee KH, Jeon YJ, Oh JH, Jeong SY, Song IS, Lee DS, Kim NS. Identification of genes related to Parkinson’s disease using expressed sequence tags. DNA Res. 2006;13:275–286. doi: 10.1093/dnares/dsl016. [DOI] [PubMed] [Google Scholar]
- 85.Kim TH, Abdullaev ZK, Smith AD, Ching KA, Loukinov DI, Green RD, Zhang MQ, Lobanenkov VV, Ren B. Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell. 2007;128:1231–1245. doi: 10.1016/j.cell.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmidt D, Schwalie PC, Ross-Innes CS, Hurtado A, Brown GD, Carroll JS, Flicek P, Odom DT. A CTCF-independent role for cohesin in tissue-specific transcription. Genome Res. 2010;20:578–588. doi: 10.1101/gr.100479.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phillips JE, Corces VG. CTCF: master weaver of the genome. Cell. 2009;137:1194–1211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bakalkin G, Yakovleva T, Terenius L. Prodynorphin gene expression relates to NF-kappa B factors. Brain Res Mol Brain Res. 1994;24:301–312. doi: 10.1016/0169-328X(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 89.Bakalkin G, Telkov M, Yakovleva T, Terenius L. [Leu5]enkephalin-encoding sequences are targets for a specific DNA-binding factor. Proc Natl Acad Sci USA. 1995;92:9024–9028. doi: 10.1073/pnas.92.20.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reddy TE, Pauli F, Sprouse RO, Neff NF, Newberry KM, Garabedian MJ, Myers RM. Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation. Genome Res. 2009;19:2163–2171. doi: 10.1101/gr.097022.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thai L, Lee PH, Ho J, Suh H, Hong JS. Regulation of prodynorphin gene expression in the hippocampus by glucocorticoids. Brain Res Mol Brain Res. 1992;16:150–157. doi: 10.1016/0169-328X(92)90205-P. [DOI] [PubMed] [Google Scholar]
- 92.Persson S, Schafer MK, Nohr D, Ekstrom G, Post C, Nyberg F, Weihe E. Spinal prodynorphin gene expression in collagen-induced arthritis: influence of the glucocorticosteroid budesonide. Neuroscience. 1994;63:313–326. doi: 10.1016/0306-4522(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 93.Thai L, Hong JS, Wiley RG, Gallagher M. The regulation of hippocampal dynorphin by neural/neuroendocrine pathways: models for effects of aging on an opioid peptide system. Neuroscience. 1996;70:661–671. doi: 10.1016/S0306-4522(96)83005-3. [DOI] [PubMed] [Google Scholar]
- 94.Pan Y, Tsai CJ, Ma B, Nussinov R. How do transcription factors select specific binding sites in the genome? Nat Struct Mol Biol. 2009;16:1118–1120. doi: 10.1038/nsmb1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mellstrom B, Savignac M, Gomez-Villafuertes R, Naranjo JR. Ca2+-operated transcriptional networks: molecular mechanisms and in vivo models. Physiol Rev. 2008;88:421–449. doi: 10.1152/physrev.00041.2005. [DOI] [PubMed] [Google Scholar]
- 97.Carrion AM, Mellstrom B, Luckman SM, Naranjo JR. Stimulus-specific hierarchy of enhancer elements within the rat prodynorphin promoter. J Neurochem. 1998;70:914–921. doi: 10.1046/j.1471-4159.1998.70030914.x. [DOI] [PubMed] [Google Scholar]
- 98.Carrion AM, Mellstrom B, Naranjo JR. Protein kinase A-dependent derepression of the human prodynorphin gene via differential binding to an intragenic silencer element. Mol Cell Biol. 1998;18:6921–6929. doi: 10.1128/mcb.18.12.6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ledo F, Carrion AM, Link WA, Mellstrom B, Naranjo JR. DREAM-alphaCREM interaction via leucine-charged domains derepresses downstream regulatory element-dependent transcription. Mol Cell Biol. 2000;20:9120–9126. doi: 10.1128/MCB.20.24.9120-9126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ledo F, Kremer L, Mellstrom B, Naranjo JR. Ca2+-dependent block of CREB-CBP transcription by repressor DREAM. EMBO J. 2002;21:4583–4592. doi: 10.1093/emboj/cdf440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Collins-Hicok J, Lin L, Spiro C, Laybourn PJ, Tschumper R, Rapacz B, McMurray CT. Induction of the rat prodynorphin gene through Gs-coupled receptors may involve phosphorylation-dependent derepression and activation. Mol Cell Biol. 1994;14:2837–2848. doi: 10.1128/mcb.14.5.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cole RL, Konradi C, Douglass J, Hyman SE. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron. 1995;14:813–823. doi: 10.1016/0896-6273(95)90225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ, Kumar A, Nestler EJ. An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nat Neurosci. 2006;9:205–211. doi: 10.1038/nn1636. [DOI] [PubMed] [Google Scholar]
- 104.McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- 105.Zhang X, Odom DT, Koo SH, Conkright MD, Canettieri G, Best J, Chen H, Jenner R, Herbolsheimer E, Jacobsen E, Kadam S, Ecker JR, Emerson B, Hogenesch JB, Unterman T, Young RA, Montminy M. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci USA. 2005;102:4459–4464. doi: 10.1073/pnas.0501076102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hartzell DD, Trinklein ND, Mendez J, Murphy N, Aldred SF, Wood K, Urh M. A functional analysis of the CREB signaling pathway using HaloCHIP-chip and high-throughput reporter assays. BMC Genomic. 2009;10:497. doi: 10.1186/1471-2164-10-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rozowsky J, Euskirchen G, Auerbach RK, Zhang ZD, Gibson T, Bjornson R, Carriero N, Snyder M, Gerstein MB. PeakSeq enables systematic scoring of ChIP-seq experiments relative to controls. Nat Biotechnol. 2009;27:66–75. doi: 10.1038/nbt.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ramos YF, Hestand MS, Verlaan M, Krabbendam E, Ariyurek Y, van Galen M, van Dam H, van Ommen GJ, den Dunnen JT, Zantema A, t Hoen PA. Genome-wide assessment of differential roles for p300 and CBP in transcription regulation. Nucleic Acids Res. 2010;38:5396–5408. doi: 10.1093/nar/gkq184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cheng HY, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, Kockeritz LK, Wada T, Joza NA, Crackower M, Goncalves J, Sarosi I, Woodgett JR, Oliveira-dos-Santos AJ, Ikura M, van der Kooy D, Salter MW, Penninger JM. DREAM is a critical transcriptional repressor for pain modulation. Cell. 2002;108:31–43. doi: 10.1016/S0092-8674(01)00629-8. [DOI] [PubMed] [Google Scholar]
- 110.Cheng HY, Laviolette SR, van der Kooy D, Penninger JM. DREAM ablation selectively alters THC place aversion and analgesia but leaves intact the motivational and analgesic effects of morphine. Eur J Neurosci. 2004;19:3033–3041. doi: 10.1111/j.0953-816X.2004.03435.x. [DOI] [PubMed] [Google Scholar]
- 111.Sakamuro D, Prendergast GC. New Myc-interacting proteins: a second Myc network emerges. Oncogene. 1999;18:2942–2954. doi: 10.1038/sj.onc.1202725. [DOI] [PubMed] [Google Scholar]
- 112.Grandori C, Cowley SM, James LP, Eisenman RN. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol. 2000;16:653–699. doi: 10.1146/annurev.cellbio.16.1.653. [DOI] [PubMed] [Google Scholar]
- 113.Georgopoulos K. Haematopoietic cell-fate decisions, chromatin regulation and ikaros. Nat Rev Immunol. 2002;2:162–174. doi: 10.1038/nri747. [DOI] [PubMed] [Google Scholar]
- 114.Damberg M, Garpenstrand H, Hallman J, Oreland L. Genetic mechanisms of behavior–don’t forget about the transcription factors. Mol Psychiatry. 2001;6:503–510. doi: 10.1038/sj.mp.4000935. [DOI] [PubMed] [Google Scholar]
- 115.Fernandez PC, Frank SR, Wang L, Schroeder M, Liu S, Greene J, Cocito A, Amati B. Genomic targets of the human c-Myc protein. Genes Dev. 2003;17:1115–1129. doi: 10.1101/gad.1067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zeller KI, Zhao X, Lee CW, Chiu KP, Yao F, Yustein JT, Ooi HS, Orlov YL, Shahab A, Yong HC, Fu Y, Weng Z, Kuznetsov VA, Sung WK, Ruan Y, Dang CV, Wei CL. Global mapping of c-Myc binding sites and target gene networks in human B cells. Proc Natl Acad Sci USA. 2006;103:17834–17839. doi: 10.1073/pnas.0604129103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Odom DT, Dowell RD, Jacobsen ES, Gordon W, Danford TW, MacIsaac KD, Rolfe PA, Conboy CM, Gifford DK, Fraenkel E. Tissue-specific transcriptional regulation has diverged significantly between human and mouse. Nat Genet. 2007;39:730–732. doi: 10.1038/ng2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ooi L, Wood IC. Chromatin crosstalk in development and disease: lessons from REST. Nat Rev Genet. 2007;8:544–554. doi: 10.1038/nrg2100. [DOI] [PubMed] [Google Scholar]
- 119.Bruce AW, Lopez-Contreras AJ, Flicek P, Down TA, Dhami P, Dillon SC, Koch CM, Langford CF, Dunham I, Andrews RM, Vetrie D. Functional diversity for REST (NRSF) is defined by in vivo binding affinity hierarchies at the DNA sequence level. Genome Res. 2009;19:994–1005. doi: 10.1101/gr.089086.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE, Kuehn MS, Taylor CM, Neph S, Koch CM, Asthana S, Malhotra A, Adzhubei I, Greenbaum JA, Andrews RM, Flicek P, Boyle PJ, Cao H, Carter NP, Clelland GK, Davis S, Day N, Dhami P, Dillon SC, Dorschner MO, Fiegler H, Giresi PG, Goldy J, Hawrylycz M, Haydock A, Humbert R, James KD, Johnson BE, Johnson EM, Frum TT, Rosenzweig ER, Karnani N, Lee K, Lefebvre GC, Navas PA, Neri F, Parker SC, Sabo PJ, Sandstrom R, Shafer A, Vetrie D, Weaver M, Wilcox S, Yu M, Collins FS, Dekker J, Lieb JD, Tullius TD, Crawford GE, Sunyaev S, Noble WS, Dunham I, Denoeud F, Reymond A, Kapranov P, Rozowsky J, Zheng D, Castelo R, Frankish A, Harrow J, Ghosh S, Sandelin A, Hofacker IL, Baertsch R, Keefe D, Dike S, Cheng J, Hirsch HA, Sekinger EA, Lagarde J, Abril JF, Shahab A, Flamm C, Fried C, Hackermuller J, Hertel J, Lindemeyer M, Missal K, Tanzer A, Washietl S, Korbel J, Emanuelsson O, Pedersen JS, Holroyd N, Taylor R, Swarbreck D, Matthews N, Dickson MC, Thomas DJ, Weirauch MT, Gilbert J, Drenkow J, Bell I, Zhao X, Srinivasan KG, Sung WK, Ooi HS, Chiu KP, Foissac S, Alioto T, Brent M, Pachter L, Tress ML, Valencia A, Choo SW, Choo CY, Ucla C, Manzano C, Wyss C, Cheung E, Clark TG, Brown JB, Ganesh M, Patel S, Tammana H, Chrast J, Henrichsen CN, Kai C, Kawai J, Nagalakshmi U, Wu J, Lian Z, Lian J, Newburger P, Zhang X, Bickel P, Mattick JS, Carninci P, Hayashizaki Y, Weissman S, Hubbard T, Myers RM, Rogers J, Stadler PF, Lowe TM, Wei CL, Ruan Y, Struhl K, Gerstein M, Antonarakis SE, Fu Y, Green ED, Karaoz U, Siepel A, Taylor J, Liefer LA, Wetterstrand KA, Good PJ, Feingold EA, Guyer MS, Cooper GM, Asimenos G, Dewey CN, Hou M, Nikolaev S, Montoya-Burgos JI, Loytynoja A, Whelan S, Pardi F, Massingham T, Huang H, Zhang NR, Holmes I, Mullikin JC, Ureta-Vidal A, Paten B, Seringhaus M, Church D, Rosenbloom K, Kent WJ, Stone EA, Batzoglou S, Goldman N, Hardison RC, Haussler D, Miller W, Sidow A, Trinklein ND, Zhang ZD, Barrera L, Stuart R, King DC, Ameur A, Enroth S, Bieda MC, Kim J, Bhinge AA, Jiang N, Liu J, Yao F, Vega VB, Lee CW, Ng P, Yang A, Moqtaderi Z, Zhu Z, Xu X, Squazzo S, Oberley MJ, Inman D, Singer MA, Richmond TA, Munn KJ, Rada-Iglesias A, Wallerman O, Komorowski J, Fowler JC, Couttet P, Bruce AW, Dovey OM, Ellis PD, Langford CF, Nix DA, Euskirchen G, Hartman S, Urban AE, Kraus P, Van Calcar S, Heintzman N, Kim TH, Wang K, Qu C, Hon G, Luna R, Glass CK, Rosenfeld MG, Aldred SF, Cooper SJ, Halees A, Lin JM, Shulha HP, Xu M, Haidar JN, Yu Y, Iyer VR, Green RD, Wadelius C, Farnham PJ, Ren B, Harte RA, Hinrichs AS, Trumbower H, Clawson H, Hillman-Jackson J, Zweig AS, Smith K, Thakkapallayil A, Barber G, Kuhn RM, Karolchik D, Armengol L, Bird CP, de Bakker PI, Kern AD, Lopez-Bigas N, Martin JD, Stranger BE, Woodroffe A, Davydov E, Dimas A, Eyras E, Hallgrimsdottir IB, Huppert J, Cuff J, Gnerre S, Jaffe DB, Lander ES, Koriabine M, Nefedov M, Osoegawa K, Yoshinaga Y, Zhu B, Jong PJ. Zody MC, Abecasis GR, Estivill X, Bouffard GG, Guan X, Hansen NF, Idol JR, Maduro VV, Maskeri B, McDowell JC, Park M, Thomas PJ, Young AC, Blakesley RW, Muzny DM, Sodergren E, Wheeler DA, Worley KC, Jiang H, Weinstock GM, Gibbs RA, Graves T, Fulton R, Mardis ER, Wilson RK, Clamp M. Nature. 2007;447:799–816. [Google Scholar]
- 121.Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Conaco C, Otto S, Han JJ, Mandel G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc Natl Acad Sci USA. 2006;103:2422–2427. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mortazavi A, Leeper Thompson EC, Garcia ST, Myers RM, Wold B. Comparative genomics modeling of the NRSF/REST repressor network: from single conserved sites to genome-wide repertoire. Genome Res. 2006;16:1208–1221. doi: 10.1101/gr.4997306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu J, Xie X. Comparative sequence analysis reveals an intricate network among REST, CREB and miRNA in mediating neuronal gene expression. Genome Biol. 2006;7:R85. doi: 10.1186/gb-2006-7-9-r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qiang M, Rani CS, Ticku MK. Neuron-restrictive silencer factor regulates the N-methyl-d-aspartate receptor 2B subunit gene in basal and ethanol-induced gene expression in fetal cortical neurons. Mol Pharmacol. 2005;67:2115–2125. doi: 10.1124/mol.104.010751. [DOI] [PubMed] [Google Scholar]
- 127.Pietrzykowski AZ, Friesen RM, Martin GE, Puig SI, Nowak CL, Wynne PM, Siegelmann HT, Treistman SN. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59:274–287. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hollander JA, Im HI, Amelio AL, Kocerha J, Bali P, Lu Q, Willoughby D, Wahlestedt C, Conkright MD, Kenny PJ. Striatal microRNA controls cocaine intake through CREB signalling. Nature. 2010;466:197–202. doi: 10.1038/nature09202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Im HI, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci. 2010;13:1120–1127. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Day R, Schafer MK, Collard MW, Watson SJ, Akil H. Atypical prodynorphin gene expression in corticosteroid-producing cells of the rat adrenal gland. Proc Natl Acad Sci USA. 1991;88:1320–1324. doi: 10.1073/pnas.88.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Law PY, Loh HH, Wei LN (2004) Insights into the receptor transcription and signaling: implications in opioid tolerance and dependence. Neuropharmacology 47 (Suppl 1):300–311. doi:10.1016/j.neuropharm.2004.07.013 [DOI] [PubMed]
- 132.Bakalkin G, Ponomariev D, Sarkisyan RA, Terenius L. Sequence similarity between opioid peptide precursors and DNA-binding proteins. FEBS Lett. 1991;282:175–177. doi: 10.1016/0014-5793(91)80471-E. [DOI] [PubMed] [Google Scholar]
- 133.Pruitt KD, Tatusova T, Maglott DR. NCBI Reference Sequence (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2005;33:D501–D504. doi: 10.1093/nar/gki025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wei LN, Hu X, Bi J, Loh H. Post-transcriptional regulation of mouse kappa-opioid receptor expression. Mol Pharmacol. 2000;57:401–408. [PubMed] [Google Scholar]
- 135.Hu X, Bi J, Loh HH, Wei LN. Regulation of mouse kappa opioid receptor gene expression by different 3′-untranslated regions and the effect of retinoic acid. Mol Pharmacol. 2002;62:881–887. doi: 10.1124/mol.62.4.881. [DOI] [PubMed] [Google Scholar]
- 136.Tsai NP, Lin YL, Tsui YC, Wei LN. Dual action of epidermal growth factor: extracellular signal-stimulated nuclear-cytoplasmic export and coordinated translation of selected messenger RNA. J Cell Biol. 2010;188:325–333. doi: 10.1083/jcb.200910083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tsai NP, Bi J, Loh HH, Wei LN. Netrin-1 signaling regulates de novo protein synthesis of kappa opioid receptor by facilitating polysomal partition of its mRNA. J Neurosci. 2006;26:9743–9749. doi: 10.1523/JNEUROSCI.3014-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tsai NP, Bi J, Wei LN. The adaptor Grb7 links netrin-1 signaling to regulation of mRNA translation. EMBO J. 2007;26:1522–1531. doi: 10.1038/sj.emboj.7601598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tsai NP, Tsui YC, Pintar JE, Loh HH, Wei LN. Kappa opioid receptor contributes to EGF-stimulated neurite extension in development. Proc Natl Acad Sci USA. 2010;107:3216–3221. doi: 10.1073/pnas.0912367107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bi J, Hu X, Loh HH, Wei LN. Mouse kappa-opioid receptor mRNA differential transport in neurons. Mol Pharmacol. 2003;64:594–599. doi: 10.1124/mol.64.3.594. [DOI] [PubMed] [Google Scholar]
- 141.Bi J, Tsai NP, Lin YP, Loh HH, Wei LN. Axonal mRNA transport and localized translational regulation of kappa-opioid receptor in primary neurons of dorsal root ganglia. Proc Natl Acad Sci USA. 2006;103:19919–19924. doi: 10.1073/pnas.0607394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bi J, Tsai NP, Lu HY, Loh HH, Wei LN. Copb1-facilitated axonal transport and translation of kappa opioid-receptor mRNA. Proc Natl Acad Sci USA. 2007;104:13810–13815. doi: 10.1073/pnas.0703805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wei LN (2010) The RNA superhighway: axonal RNA trafficking of kappa opioid receptor mRNA for neurite growth. Integr Biol (Camb). doi:10.1039/c0ib00107d [DOI] [PubMed]
- 144.Fallon JH, Leslie FM. Distribution of dynorphin and enkephalin peptides in the rat brain. J Comp Neurol. 1986;249:293–336. doi: 10.1002/cne.902490302. [DOI] [PubMed] [Google Scholar]
- 145.Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mansour A, Fox CA, Meng F, Akil H, Watson SJ. Kappa 1 receptor mRNA distribution in the rat CNS: comparison to kappa receptor binding and prodynorphin mRNA. Mol Cell Neurosci. 1994;5:124–144. doi: 10.1006/mcne.1994.1015. [DOI] [PubMed] [Google Scholar]
- 147.Meng F, Xie GX, Thompson RC, Mansour A, Goldstein A, Watson SJ, Akil H. Cloning and pharmacological characterization of a rat kappa opioid receptor. Proc Natl Acad Sci USA. 1993;90:9954–9958. doi: 10.1073/pnas.90.21.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xie GX, Meng F, Mansour A, Thompson RC, Hoversten MT, Goldstein A, Watson SJ, Akil H. Primary structure and functional expression of a guinea pig kappa opioid (dynorphin) receptor. Proc Natl Acad Sci USA. 1994;91:3779–3783. doi: 10.1073/pnas.91.9.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Svingos AL, Colago EE, Pickel VM. Cellular sites for dynorphin activation of kappa-opioid receptors in the rat nucleus accumbens shell. J Neurosci. 1999;19:1804–1813. doi: 10.1523/JNEUROSCI.19-05-01804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Svingos AL, Chavkin C, Colago EE, Pickel VM. Major coexpression of kappa-opioid receptors and the dopamine transporter in nucleus accumbens axonal profiles. Synapse. 2001;42:185–192. doi: 10.1002/syn.10005. [DOI] [PubMed] [Google Scholar]
- 151.Ma J, Ye N, Lange N, Cohen BM. Dynorphinergic GABA neurons are a target of both typical and atypical antipsychotic drugs in the nucleus accumbens shell, central amygdaloid nucleus and thalamic central medial nucleus. Neuroscience. 2003;121:991–998. doi: 10.1016/S0306-4522(03)00397-X. [DOI] [PubMed] [Google Scholar]
- 152.Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- 153.Meshul CK, McGinty JF. Kappa opioid receptor immunoreactivity in the nucleus accumbens and caudate-putamen is primarily associated with synaptic vesicles in axons. Neuroscience. 2000;96:91–99. doi: 10.1016/S0306-4522(99)90481-5. [DOI] [PubMed] [Google Scholar]
- 154.Chefer VI, Czyzyk T, Bolan EA, Moron J, Pintar JE, Shippenberg TS. Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine. J Neurosci. 2005;25:5029–5037. doi: 10.1523/JNEUROSCI.0854-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharmacology (Berl) 2005;179:551–558. doi: 10.1007/s00213-004-2087-0. [DOI] [PubMed] [Google Scholar]
- 158.Maisonneuve IM, Archer S, Glick SD. U50, 488, a kappa opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci Lett. 1994;181:57–60. doi: 10.1016/0304-3940(94)90559-2. [DOI] [PubMed] [Google Scholar]
- 159.You ZB, Herrera-Marschitz M, Terenius L. Modulation of neurotransmitter release in the basal ganglia of the rat brain by dynorphin peptides. J Pharmacol Exp Ther. 1999;290:1307–1315. [PubMed] [Google Scholar]
- 160.Hjelmstad GO, Fields HL. Kappa opioid receptor activation in the nucleus accumbens inhibits glutamate and GABA release through different mechanisms. J Neurophysiol. 2003;89:2389–2395. doi: 10.1152/jn.01115.2002. [DOI] [PubMed] [Google Scholar]
- 161.Hjelmstad GO, Fields HL. Kappa opioid receptor inhibition of glutamatergic transmission in the nucleus accumbens shell. J Neurophysiol. 2001;85:1153–1158. doi: 10.1152/jn.2001.85.3.1153. [DOI] [PubMed] [Google Scholar]
- 162.Rawls SM, McGinty JF. Kappa receptor activation attenuates L-trans-pyrrolidine-2, 4-dicarboxylic acid-evoked glutamate levels in the striatum. J Neurochem. 1998;70:626–634. doi: 10.1046/j.1471-4159.1998.70020626.x. [DOI] [PubMed] [Google Scholar]
- 163.Rawls SM, McGinty JF, Terrian DM. Presynaptic kappa-opioid and muscarinic receptors inhibit the calcium-dependent component of evoked glutamate release from striatal synaptosomes. J Neurochem. 1999;73:1058–1065. doi: 10.1046/j.1471-4159.1999.0731058.x. [DOI] [PubMed] [Google Scholar]
- 164.Gray AM, Rawls SM, Shippenberg TS, McGinty JF. The kappa-opioid agonist, U-69593, decreases acute amphetamine-evoked behaviors and calcium-dependent dialysate levels of dopamine and glutamate in the ventral striatum. J Neurochem. 1999;73:1066–1074. doi: 10.1046/j.1471-4159.1999.0731066.x. [DOI] [PubMed] [Google Scholar]
- 165.Hill MP, Brotchie JM. Control of glutamate release by calcium channels and kappa-opioid receptors in rodent and primate striatum. Br J Pharmacol. 1999;127:275–283. doi: 10.1038/sj.bjp.0702523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 167.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- 168.Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Floresco SB, Yang CR, Phillips AG, Blaha CD. Basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anaesthetized rat. Eur J Neurosci. 1998;10:1241–1251. doi: 10.1046/j.1460-9568.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- 172.Mogenson GJ, Yang CR, Yim CY. Influence of dopamine on limbic inputs to the nucleus accumbens. Ann N Y Acad Sci. 1988;537:86–100. doi: 10.1111/j.1749-6632.1988.tb42098.x. [DOI] [PubMed] [Google Scholar]
- 173.O’Donnell P. Dopamine gating of forebrain neural ensembles. Eur J Neurosci. 2003;17:429–435. doi: 10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- 174.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 175.Hara Y, Yakovleva T, Bakalkin G, Pickel VM. Dopamine D1 receptors have subcellular distributions conducive to interactions with prodynorphin in the rat nucleus accumbens shell. Synapse. 2006;60:1–19. doi: 10.1002/syn.20273. [DOI] [PubMed] [Google Scholar]
- 176.Drake CT, Chavkin C, Milner TA. Opioid systems in the dentate gyrus. Prog Brain Res. 2007;163:245–263. doi: 10.1016/S0079-6123(07)63015-5. [DOI] [PubMed] [Google Scholar]
- 177.Brown CH, Scott V, Ludwig M, Leng G, Bourque CW. Somatodendritic dynorphin release: orchestrating activity patterns of vasopressin neurons. Biochem Soc Trans. 2007;35:1236–1242. doi: 10.1042/BST0351166. [DOI] [PubMed] [Google Scholar]
- 178.Tallent MK. Presynaptic inhibition of glutamate release by neuropeptides: use-dependent synaptic modification. Results Probl Cell Differ. 2008;44:177–200. doi: 10.1007/400_2007_037. [DOI] [PubMed] [Google Scholar]
- 179.Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Zhang J, Xu M. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.You ZB, Herrera-Marschitz M, Nylander I, Goiny M, O’Connor WT, Ungerstedt U, Terenius L. The striatonigral dynorphin pathway of the rat studied with in vivo microdialysis-II. Effects of dopamine D1 and D2 receptor agonists. Neuroscience. 1994;63:427–434. doi: 10.1016/0306-4522(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 181.Drago J, Gerfen CR, Westphal H, Steiner H. D1 dopamine receptor-deficient mouse: cocaine-induced regulation of immediate-early gene and substance P expression in the striatum. Neuroscience. 1996;74:813–823. doi: 10.1016/0306-4522(96)00145-5. [DOI] [PubMed] [Google Scholar]
- 182.Wang JQ, McGinty JF. Glutamate-dopamine interactions mediate the effects of psychostimulant drugs. Addict Biol. 1999;4:141–150. doi: 10.1080/13556219971641. [DOI] [PubMed] [Google Scholar]
- 183.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 184.Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Thompson AC, Zapata A, Justice JB, Jr, Vaughan RA, Sharpe LG, Shippenberg TS. Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci. 2000;20:9333–9340. doi: 10.1523/JNEUROSCI.20-24-09333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shippenberg TS, Chefer VI SR. K-opioid receptor agonists up-regulate dopamine transporter (DAT) function and produce aversive effects via an ERK-dependent mechanism. Soc Neurosci Abstr. 2009;618:22. [Google Scholar]
- 187.Acri JB, Thompson AC, Shippenberg T. Modulation of pre- and postsynaptic dopamine D2 receptor function by the selective kappa-opioid receptor agonist U69593. Synapse. 2001;39:343–350. doi: 10.1002/1098-2396(20010315)39:4<343::AID-SYN1018>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 188.Ferre S, Ciruela F, Woods AS, Lluis C, Franco R. Functional relevance of neurotransmitter receptor heteromers in the central nervous system. Trends Neurosci. 2007;30:440–446. doi: 10.1016/j.tins.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 189.Fuxe K, Marcellino D, Leo G, Agnati LF. Molecular integration via allosteric interactions in receptor heteromers. A working hypothesis. Curr Opin Pharmacol. 2010;10:14–22. doi: 10.1016/j.coph.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 190.Fallon JH, Leslie FM, Cone RI. Dynorphin-containing pathways in the substantia nigra and ventral tegmentum: a double labeling study using combined immunofluorescence and retrograde tracing. Neuropeptides. 1985;5:457–460. doi: 10.1016/0143-4179(85)90053-8. [DOI] [PubMed] [Google Scholar]
- 191.Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE (2001) Orexin (hypocretin) neurons contain dynorphin. J Neurosci. pii: 21:RC168. 20015644 [DOI] [PMC free article] [PubMed]
- 192.Borgland SL, Ungless MA, Bonci A. Convergent actions of orexin/hypocretin and CRF on dopamine neurons: emerging players in addiction. Brain Res. 2010;1314:139–144. doi: 10.1016/j.brainres.2009.10.068. [DOI] [PubMed] [Google Scholar]
- 193.Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J Neurosci. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci USA. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Devine DP, Leone P, Pocock D, Wise RA. Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J Pharmacol Exp Ther. 1993;266:1236–1246. [PubMed] [Google Scholar]
- 196.Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci. 2006;26:2788–2797. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Both kappa and mu opioid agonists inhibit glutamatergic input to ventral tegmental area neurons. J Neurophysiol. 2005;93:3086–3093. doi: 10.1152/jn.00855.2004. [DOI] [PubMed] [Google Scholar]
- 198.Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- 199.Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Reyes BA, Johnson AD, Glaser JD, Commons KG, Van Bockstaele EJ. Dynorphin-containing axons directly innervate noradrenergic neurons in the rat nucleus locus coeruleus. Neuroscience. 2007;145:1077–1086. doi: 10.1016/j.neuroscience.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Reyes BA, Chavkin C, van Bockstaele EJ. Subcellular targeting of kappa-opioid receptors in the rat nucleus locus coeruleus. J Comp Neurol. 2009;512:419–431. doi: 10.1002/cne.21880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pinnock RD. A highly selective kappa-opioid receptor agonist, CI-977, reduces excitatory synaptic potentials in the rat locus coeruleus in vitro. Neuroscience. 1992;47:87–94. doi: 10.1016/0306-4522(92)90123-J. [DOI] [PubMed] [Google Scholar]
- 203.McFadzean I, Lacey MG, Hill RG, Henderson G. Kappa opioid receptor activation depresses excitatory synaptic input to rat locus coeruleus neurons in vitro. Neuroscience. 1987;20:231–239. doi: 10.1016/0306-4522(87)90015-7. [DOI] [PubMed] [Google Scholar]
- 204.Kreibich A, Reyes BA, Curtis AL, Ecke L, Chavkin C, Van Bockstaele EJ, Valentino RJ. Presynaptic inhibition of diverse afferents to the locus ceruleus by kappa-opiate receptors: a novel mechanism for regulating the central norepinephrine system. J Neurosci. 2008;28:6516–6525. doi: 10.1523/JNEUROSCI.0390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Reyes BA, Drolet G, Van Bockstaele EJ. Dynorphin and stress-related peptides in rat locus coeruleus: contribution of amygdalar efferents. J Comp Neurol. 2008;508:663–675. doi: 10.1002/cne.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Marchant NJ, Densmore VS, Osborne PB. Coexpression of prodynorphin and corticotrophin-releasing hormone in the rat central amygdala: evidence of two distinct endogenous opioid systems in the lateral division. J Comp Neurol. 2007;504:702–715. doi: 10.1002/cne.21464. [DOI] [PubMed] [Google Scholar]
- 207.Khachaturian H, Lewis ME, Haber SN, Houghten RA, Akil H, Watson SJ. Prodynorphin peptide immunocytochemistry in rhesus monkey brain. Peptides. 1985;6(Suppl 2):155–166. doi: 10.1016/0196-9781(85)90149-4. [DOI] [PubMed] [Google Scholar]
- 208.Vincent SR, Hokfelt T, Christensson I, Terenius L. Dynorphin-immunoreactive neurons in the central nervous system of the rat. Neurosci Lett. 1982;33:185–190. doi: 10.1016/0304-3940(82)90249-X. [DOI] [PubMed] [Google Scholar]
- 209.Mansour A, Burke S, Pavlic RJ, Akil H, Watson SJ. Immunohistochemical localization of the cloned kappa 1 receptor in the rat CNS and pituitary. Neuroscience. 1996;71:671–690. doi: 10.1016/0306-4522(95)00464-5. [DOI] [PubMed] [Google Scholar]
- 210.George SR, Zastawny RL, Briones-Urbina R, Cheng R, Nguyen T, Heiber M, Kouvelas A, Chan AS, O’Dowd BF. Distinct distributions of mu, delta and kappa opioid receptor mRNA in rat brain. Biochem Biophys Res Commun. 1994;205:1438–1444. doi: 10.1006/bbrc.1994.2826. [DOI] [PubMed] [Google Scholar]
- 211.Peckys D, Landwehrmeyer GB. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience. 1999;88:1093–1135. doi: 10.1016/S0306-4522(98)00251-6. [DOI] [PubMed] [Google Scholar]
- 212.Svingos AL, Colago EE. Kappa-opioid and NMDA glutamate receptors are differentially targeted within rat medial prefrontal cortex. Brain Res. 2002;946:262–271. doi: 10.1016/S0006-8993(02)02894-9. [DOI] [PubMed] [Google Scholar]
- 213.Grilli M, Neri E, Zappettini S, Massa F, Bisio A, Romussi G, Marchi M, Pittaluga A. Salvinorin A exerts opposite presynaptic controls on neurotransmitter exocytosis from mouse brain nerve terminals. Neuropharmacology. 2009;57:523–530. doi: 10.1016/j.neuropharm.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 214.Heijna MH, Padt M, Hogenboom F, Portoghese PS, Mulder AH, Schoffelmeer AN. Opioid receptor-mediated inhibition of dopamine and acetylcholine release from slices of rat nucleus accumbens, olfactory tubercle and frontal cortex. Eur J Pharmacol. 1990;181:267–278. doi: 10.1016/0014-2999(90)90088-N. [DOI] [PubMed] [Google Scholar]
- 215.Sbrenna S, Marti M, Morari M, Calo G, Guerrini R, Beani L, Bianchi C. L-glutamate and gamma-aminobutyric acid efflux from rat cerebrocortical synaptosomes: modulation by kappa- and mu- but not delta- and opioid receptor like-1 receptors. J Pharmacol Exp Ther. 1999;291:1365–1371. [PubMed] [Google Scholar]
- 216.Berger B, Rothmaier AK, Wedekind F, Zentner J, Feuerstein TJ, Jackisch R. Presynaptic opioid receptors on noradrenergic and serotonergic neurons in the human as compared to the rat neocortex. Br J Pharmacol. 2006;148:795–806. doi: 10.1038/sj.bjp.0706782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tejeda HA, Schultz K, Chefer V, Shippenberg T (2010) Modulation of mesocortical dopamine transmission by mu- and kappa-opioid receptors. Soc Neurosci Abstr 368.24
- 218.Carboni E, Silvagni A. Dopamine reuptake by norepinephrine neurons: exception or rule? Crit Rev Neurobiol. 2004;16:121–128. doi: 10.1615/CritRevNeurobiol.v16.i12.130. [DOI] [PubMed] [Google Scholar]
- 219.Chavkin C, Shoemaker WJ, McGinty JF, Bayon A, Bloom FE. Characterization of the prodynorphin and proenkephalin neuropeptide systems in rat hippocampus. J Neurosci. 1985;5:808–816. doi: 10.1523/JNEUROSCI.05-03-00808.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.McGinty JF, Henriksen SJ, Goldstein A, Terenius L, Bloom FE. Dynorphin is contained within hippocampal mossy fibers: immunochemical alterations after kainic acid administration and colchicine-induced neurotoxicity. Proc Natl Acad Sci USA. 1983;80:589–593. doi: 10.1073/pnas.80.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Castillo PE, Salin PA, Weisskopf MG, Nicoll RA. Characterizing the site and mode of action of dynorphin at hippocampal mossy fiber synapses in the guinea pig. J Neurosci. 1996;16:5942–5950. doi: 10.1523/JNEUROSCI.16-19-05942.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Salin PA, Weisskopf MG, Nicoll RA. A comparison of the role of dynorphin in the hippocampal mossy fiber pathway in guinea pig and rat. J Neurosci. 1995;15:6939–6945. doi: 10.1523/JNEUROSCI.15-10-06939.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Weisskopf MG, Zalutsky RA, Nicoll RA. The opioid peptide dynorphin mediates heterosynaptic depression of hippocampal mossy fibre synapses and modulates long-term potentiation. Nature. 1993;365:188. doi: 10.1038/365188a0. [DOI] [PubMed] [Google Scholar]
- 224.Wagner JJ, Terman GW, Chavkin C. Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature. 1993;363:451–454. doi: 10.1038/363451a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Drake CT, Terman GW, Simmons ML, Milner TA, Kunkel DD, Schwartzkroin PA, Chavkin C. Dynorphin opioids present in dentate granule cells may function as retrograde inhibitory neurotransmitters. J Neurosci. 1994;14:3736–3750. doi: 10.1523/JNEUROSCI.14-06-03736.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Schoultz BW, Hjornevik T, Willoch F, Marton J, Noda A, Murakami Y, Miyoshi S, Nishimura S, Arstad E, Drzezga A, Matsunari I, Henriksen G. Evaluation of the kappa-opioid receptor-selective tracer [(11)C]GR103545 in awake rhesus macaques. Eur J Nucl Med Mol Imaging. 2010;37:1174–1180. doi: 10.1007/s00259-010-1384-6. [DOI] [PubMed] [Google Scholar]
- 227.Talbot PS, Narendran R, Butelman ER, Huang Y, Ngo K, Slifstein M, Martinez D, Laruelle M, Hwang DR. 11C-GR103545, a radiotracer for imaging kappa-opioid receptors in vivo with PET: synthesis and evaluation in baboons. J Nucl Med. 2005;46:484–494. [PubMed] [Google Scholar]
- 228.Poisnel G, Oueslati F, Dhilly M, Delamare J, Perrio C, Debruyne D, Barre L. [11C]-MeJDTic: a novel radioligand for kappa-opioid receptor positron emission tomography imaging. Nucl Med Biol. 2008;35:561–569. doi: 10.1016/j.nucmedbio.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 229.Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- 230.Walsh SL, Strain EC, Abreu ME, Bigelow GE. Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans. Psychopharmacology (Berl) 2001;157:151–162. doi: 10.1007/s002130100788. [DOI] [PubMed] [Google Scholar]
- 231.Ur E, Wright DM, Bouloux PM, Grossman A. The effects of spiradoline (U-62066E), a kappa-opioid receptor agonist, on neuroendocrine function in man. Br J Pharmacol. 1997;120:781–784. doi: 10.1038/sj.bjp.0700971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rimoy GH, Wright DM, Bhaskar NK, Rubin PC. The cardiovascular and central nervous system effects in the human of U-62066E. A selective opioid receptor agonist. Eur J Clin Pharmacol. 1994;46:203–207. doi: 10.1007/BF00192549. [DOI] [PubMed] [Google Scholar]
- 233.Chappell PB, Leckman JF, Scahill LD, Hardin MT, Anderson G, Cohen DJ. Neuroendocrine and behavioral effects of the selective kappa agonist spiradoline in Tourette’s syndrome: a pilot study. Psychiatry Res. 1993;47:267–280. doi: 10.1016/0165-1781(93)90084-T. [DOI] [PubMed] [Google Scholar]
- 234.Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci USA. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lange JE, Daniel J, Homer K, Reed MB, Clapp JD. Salvia divinorum: effects and use among YouTube users. Drug Alcohol Depend. 2010;108:138–140. doi: 10.1016/j.drugalcdep.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 236.Gonzalez D, Riba J, Bouso JC, Gomez-Jarabo G, Barbanoj MJ. Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug Alcohol Depend. 2006;85:157–162. doi: 10.1016/j.drugalcdep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 237.Malone DT, Hill MN, Rubino T. Adolescent cannabis use and psychosis: epidemiology and neurodevelopmental models. Br J Pharmacol. 2010;160:511–522. doi: 10.1111/j.1476-5381.2010.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Fernandez-Espejo E, Viveros MP, Nunez L, Ellenbroek BA, Rodriguez de Fonseca F. Role of cannabis and endocannabinoids in the genesis of schizophrenia. Psychopharmacology (Berl) 2009;206:531–549. doi: 10.1007/s00213-009-1612-6. [DOI] [PubMed] [Google Scholar]
- 239.Przekop P, Lee T. Persistent psychosis associated with Salvia divinorum use. Am J Psychiatry. 2009;166:832. doi: 10.1176/appi.ajp.2009.08121759. [DOI] [PubMed] [Google Scholar]
- 240.Paulzen M, Grunder G. Toxic psychosis after intake of the hallucinogen salvinorin A. J Clin Psychiatry. 2008;69:1501–1502. doi: 10.4088/JCP.v69n0919c. [DOI] [PubMed] [Google Scholar]
- 241.Singh S. Adolescent salvia substance abuse. Addiction. 2007;102:823–824. doi: 10.1111/j.1360-0443.2007.01810.x. [DOI] [PubMed] [Google Scholar]
- 242.Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 1985;86:274–280. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- 243.Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- 244.Braida D, Limonta V, Capurro V, Fadda P, Rubino T, Mascia P, Zani A, Gori E, Fratta W, Parolaro D, Sala M. Involvement of kappa-opioid and endocannabinoid system on Salvinorin A-induced reward. Biol Psychiatry. 2008;63:286–292. doi: 10.1016/j.biopsych.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 245.Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- 246.Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl) 2010;210:241–252. doi: 10.1007/s00213-010-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Braida D, Capurro V, Zani A, Rubino T, Vigano D, Parolaro D, Sala M. Potential anxiolytic- and antidepressant-like effects of salvinorin A, the main active ingredient of Salvia divinorum, in rodents. Br J Pharmacol. 2009;157:844–853. doi: 10.1111/j.1476-5381.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- 249.Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC. Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 2010;210:149–159. doi: 10.1007/s00213-009-1770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- 251.Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- 252.Shippenberg TS, Herz A. Place preference conditioning reveals the involvement of D1-dopamine receptors in the motivational properties of mu- and kappa-opioid agonists. Brain Res. 1987;436:169–172. doi: 10.1016/0006-8993(87)91571-X. [DOI] [PubMed] [Google Scholar]
- 253.Shippenberg TS, Bals-Kubik R, Herz A. Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J Pharmacol Exp Ther. 1993;265:53–59. [PubMed] [Google Scholar]
- 254.Acquas E, Carboni E, Leone P, Di Chiara G. SCH 23390 blocks drug-conditioned place-preference and place-aversion: anhedonia (lack of reward) or apathy (lack of motivation) after dopamine-receptor blockade? Psychopharmacology (Berl) 1989;99:151–155. doi: 10.1007/BF00442800. [DOI] [PubMed] [Google Scholar]
- 255.Liu ZH, Shin R, Ikemoto S. Dual role of medial A10 dopamine neurons in affective encoding. Neuropsychopharmacology. 2008;33:3010–3020. doi: 10.1038/npp.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci USA. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tao R, Auerbach SB. mu-Opioids disinhibit and kappa-opioids inhibit serotonin efflux in the dorsal raphe nucleus. Brain Res. 2005;1049:70–79. doi: 10.1016/j.brainres.2005.04.076. [DOI] [PubMed] [Google Scholar]
- 258.Tao R, Auerbach SB. Opioid receptor subtypes differentially modulate serotonin efflux in the rat central nervous system. J Pharmacol Exp Ther. 2002;303:549–556. doi: 10.1124/jpet.102.037861. [DOI] [PubMed] [Google Scholar]
- 259.Shippenberg T, Jaligam V, Oz M, Mannangatti P, Jayanthi L, Ramamoorthy S (2010) K-opioid receptor agonists regulate serotonin transporter function, phosphorylation and cell surface expression. Soc Neurosci Abstr 741.16/D34
- 260.Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci Biobehav Rev. 2010;35:129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- 262.Nabeshima T, Katoh A, Wada M, Kameyama T. Stress-induced changes in brain Met-enkephalin, Leu-enkephalin and dynorphin concentrations. Life Sci. 1992;51:211–217. doi: 10.1016/0024-3205(92)90077-3. [DOI] [PubMed] [Google Scholar]
- 263.Goel N, Bale TL. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J Neuroendocrinol. 2009;21:415–420. doi: 10.1111/j.1365-2826.2009.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sirinathsinghji DJ, Nikolarakis KE, Reimer S, Herz A. Nigrostriatal dopamine mediates the stimulatory effects of corticotropin-releasing factor on methionine-enkephalin and dynorphin release from the rat neostriatum. Brain Res. 1990;526:173–176. doi: 10.1016/0006-8993(90)90268-G. [DOI] [PubMed] [Google Scholar]
- 265.Sirinathsinghji DJ, Nikolarakis KE, Herz A. Corticotropin-releasing factor stimulates the release of methionine-enkephalin and dynorphin from the neostriatum and globus pallidus of the rat: in vitro and in vivo studies. Brain Res. 1989;490:276–291. doi: 10.1016/0006-8993(89)90245-X. [DOI] [PubMed] [Google Scholar]
- 266.Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I. Antidepressant-like effects of kappa-opioid receptor antagonists in Wistar Kyoto rats. Neuropsychopharmacology. 2010;35:752–763. doi: 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 271.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neurosci Biobehav Rev. 2003;27:45–55. doi: 10.1016/S0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 274.Ploj K, Roman E, Nylander I. Long-term effects of short and long periods of maternal separation on brain opioid peptide levels in male Wistar rats. Neuropeptides. 2003;37:149–156. doi: 10.1016/S0143-4179(03)00043-X. [DOI] [PubMed] [Google Scholar]
- 275.Gustafsson L, Oreland S, Hoffmann P, Nylander I. The impact of postnatal environment on opioid peptides in young and adult male Wistar rats. Neuropeptides. 2008;42:177–191. doi: 10.1016/j.npep.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 276.Michaels CC, Holtzman SG. Early postnatal stress alters place conditioning to both mu- and kappa-opioid agonists. J Pharmacol Exp Ther. 2008;325:313–318. doi: 10.1124/jpet.107.129908. [DOI] [PubMed] [Google Scholar]
- 277.Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- 278.Hurd YL, Herman MM, Hyde TM, Bigelow LB, Weinberger DR, Kleinman JE. Prodynorphin mRNA expression is increased in the patch vs matrix compartment of the caudate nucleus in suicide subjects. Mol Psychiatry. 1997;2:495–500. doi: 10.1038/sj.mp.4000319. [DOI] [PubMed] [Google Scholar]
- 279.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Peckys D, Hurd YL. Prodynorphin and kappa opioid receptor mRNA expression in the cingulate and prefrontal cortices of subjects diagnosed with schizophrenia or affective disorders. Brain Res Bull. 2001;55:619–624. doi: 10.1016/S0361-9230(01)00525-1. [DOI] [PubMed] [Google Scholar]
- 281.Hurd YL. Subjects with major depression or bipolar disorder show reduction of prodynorphin mRNA expression in discrete nuclei of the amygdaloid complex. Mol Psychiatry. 2002;7:75–81. doi: 10.1038/sj.mp.4000930. [DOI] [PubMed] [Google Scholar]
- 282.Blendy JA. The role of CREB in depression and antidepressant treatment. Biol Psychiatry. 2006;59:1144–1150. doi: 10.1016/j.biopsych.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 283.Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, Chen J, Neve R, Nestler EJ, Duman RS. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Dinieri JA, Nemeth CL, Parsegian A, Carle T, Gurevich VV, Gurevich E, Neve RL, Nestler EJ, Carlezon WA., Jr Altered sensitivity to rewarding and aversive drugs in mice with inducible disruption of cAMP response element-binding protein function within the nucleus accumbens. J Neurosci. 2009;29:1855–1859. doi: 10.1523/JNEUROSCI.5104-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- 286.Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H, Schwarzer C. Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology. 2009;34:775–785. doi: 10.1038/npp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- 288.Bruchas MR, Land BB, Lemos JC, Chavkin C. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS One. 2009;4:e8528. doi: 10.1371/journal.pone.0008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K, Yamazaki M, Suzuki T. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006;31:739–750. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- 290.Carr GV, Lucki I. Comparison of the kappa-opioid receptor antagonist DIPPA in tests of anxiety-like behavior between Wistar Kyoto and Sprague–Dawley rats. Psychopharmacology (Berl) 2010;210:295–302. doi: 10.1007/s00213-010-1832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Gregg L, Barrowclough C, Haddock G. Reasons for increased substance use in psychosis. Clin Psychol Rev. 2007;27:494–510. doi: 10.1016/j.cpr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 295.Frankel PS, Alburges ME, Bush L, Hanson GR, Kish SJ. Striatal and ventral pallidum dynorphin concentrations are markedly increased in human chronic cocaine users. Neuropharmacology. 2008;55:41–46. doi: 10.1016/j.neuropharm.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Frankel PS, Alburges ME, Bush L, Hanson GR, Kish SJ. Brain levels of neuropeptides in human chronic methamphetamine users. Neuropharmacology. 2007;53:447–454. doi: 10.1016/j.neuropharm.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 299.Turchan J, Przewlocka B, Lason W, Przewlocki R. Effects of repeated psychostimulant administration on the prodynorphin system activity and kappa opioid receptor density in the rat brain. Neuroscience. 1998;85:1051–1059. doi: 10.1016/S0306-4522(97)00639-8. [DOI] [PubMed] [Google Scholar]
- 300.Tzaferis JA, McGinty JF. Kappa opioid receptor stimulation decreases amphetamine-induced behavior and neuropeptide mRNA expression in the striatum. Brain Res Mol Brain Res. 2001;93:27–35. doi: 10.1016/S0169-328X(01)00178-4. [DOI] [PubMed] [Google Scholar]
- 301.Spangler R, Zhou Y, Maggos CE, Schlussman SD, Ho A, Kreek MJ. Prodynorphin, proenkephalin and kappa opioid receptor mRNA responses to acute “binge” cocaine. Brain Res Mol Brain Res. 1997;44:139–142. doi: 10.1016/S0169-328X(96)00249-5. [DOI] [PubMed] [Google Scholar]
- 302.Moratalla R, Xu M, Tonegawa S, Graybiel AM. Cellular responses to psychomotor stimulant and neuroleptic drugs are abnormal in mice lacking the D1 dopamine receptor. Proc Natl Acad Sci USA. 1996;93:14928–14933. doi: 10.1073/pnas.93.25.14928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Hanson GR, Singh N, Merchant K, Johnson M, Gibb JW. The role of NMDA receptor systems in neuropeptide responses to stimulants of abuse. Drug Alcohol Depend. 1995;37:107–110. doi: 10.1016/0376-8716(94)01065-S. [DOI] [PubMed] [Google Scholar]
- 304.Fagergren P, Smith HR, Daunais JB, Nader MA, Porrino LJ, Hurd YL. Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. Eur J Neurosci. 2003;17:2212–2218. doi: 10.1046/j.1460-9568.2003.02636.x. [DOI] [PubMed] [Google Scholar]
- 305.Ziolkowska B, Stefanski R, Mierzejewski P, Zapart G, Kostowski W, Przewlocki R. Contingency does not contribute to the effects of cocaine self-administration on prodynorphin and proenkephalin gene expression in the rat forebrain. Brain Res. 2006;1069:1–9. doi: 10.1016/j.brainres.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 306.Daunais JB, McGinty JF. The effects of D1 or D2 dopamine receptor blockade on zif/268 and preprodynorphin gene expression in rat forebrain following a short-term cocaine binge. Brain Res Mol Brain Res. 1996;35:237–248. doi: 10.1016/0169-328X(95)00226-I. [DOI] [PubMed] [Google Scholar]
- 307.Bustamante D, You ZB, Castel MN, Johansson S, Goiny M, Terenius L, Hokfelt T, Herrera-Marschitz M. Effect of single and repeated methamphetamine treatment on neurotransmitter release in substantia nigra and neostriatum of the rat. J Neurochem. 2002;83:645–654. doi: 10.1046/j.1471-4159.2002.01171.x. [DOI] [PubMed] [Google Scholar]
- 308.Carlezon WA, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Bailey A, Yoo JH, Racz I, Zimmer A, Kitchen I. Preprodynorphin mediates locomotion and D2 dopamine and mu-opioid receptor changes induced by chronic ‘binge’ cocaine administration. J Neurochem. 2007;102:1817–1830. doi: 10.1111/j.1471-4159.2007.04661.x. [DOI] [PubMed] [Google Scholar]
- 310.Bailey A, Gianotti R, Ho A, Kreek MJ. Downregulation of kappa-opioid receptors in basolateral amygdala and septum of rats withdrawn for 14 days from an escalating dose “binge” cocaine administration paradigm. Synapse. 2007;61:820–826. doi: 10.1002/syn.20436. [DOI] [PubMed] [Google Scholar]
- 311.Piras AP, Zhou Y, Schlussman SD, Ho A, Kreek MJ. Acute withdrawal from chronic escalating-dose binge cocaine administration alters kappa opioid receptor stimulation of [35S] guanosine 5′-O-[gamma-thio]triphosphate acid binding in the rat ventral tegmental area. Neuroscience. 2010;169:751–757. doi: 10.1016/j.neuroscience.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Kim JA, Pollak KA, Hjelmstad GO, Fields HL. A single cocaine exposure enhances both opioid reward and aversion through a ventral tegmental area-dependent mechanism. Proc Natl Acad Sci USA. 2004;101:5664–5669. doi: 10.1073/pnas.0401373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Xia YF, He L, Whistler JL, Hjelmstad GO. Acute amphetamine exposure selectively desensitizes kappa-opioid receptors in the nucleus accumbens. Neuropsychopharmacology. 2008;33:892–900. doi: 10.1038/sj.npp.1301463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Mu P, Neumann PA, Panksepp J, Schluter OM, Dong Y (2010) Exposure to cocaine alters dynorphin-mediated regulation of excitatory synaptic transmission in nucleus accumbens neurons. Biol Psychiatry. doi:10.1016/j.biopsych.2010.09.014 [DOI] [PMC free article] [PubMed]
- 315.Chartoff EH, Potter D, Damez-Werno D, Cohen BM, Carlezon WA., Jr Exposure to the selective kappa-opioid receptor agonist salvinorin A modulates the behavioral and molecular effects of cocaine in rats. Neuropsychopharmacology. 2008;33:2676–2687. doi: 10.1038/sj.npp.1301659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Heidbreder CA, Goldberg SR, Shippenberg TS. The kappa-opioid receptor agonist U-69593 attenuates cocaine-induced behavioral sensitization in the rat. Brain Res. 1993;616:335–338. doi: 10.1016/0006-8993(93)90228-F. [DOI] [PubMed] [Google Scholar]
- 317.Zakharova E, Collins SL, Aberg M, Kumar A, Fernandez JB, Izenwasser S. Depletion of serotonin decreases the effects of the kappa-opioid receptor agonist U-69593 on cocaine-stimulated activity. Eur J Pharmacol. 2008;586:123–129. doi: 10.1016/j.ejphar.2008.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the kappa opioid agonist R-84760 on cocaine-induced increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6 J mice. Psychopharmacology (Berl) 2004;173:146–152. doi: 10.1007/s00213-003-1716-3. [DOI] [PubMed] [Google Scholar]
- 319.Vanderschuren LJ, Schoffelmeer AN, Wardeh G, De Vries TJ. Dissociable effects of the kappa-opioid receptor agonists bremazocine, U69593, and U50488H on locomotor activity and long-term behavioral sensitization induced by amphetamine and cocaine. Psychopharmacology (Berl) 2000;150:35–44. doi: 10.1007/s002130000424. [DOI] [PubMed] [Google Scholar]
- 320.Suzuki T, Shiozaki Y, Masukawa Y, Misawa M, Nagase H. The role of mu- and kappa-opioid receptors in cocaine-induced conditioned place preference. Jpn J Pharmacol. 1992;58:435–442. doi: 10.1254/jjp.58.435. [DOI] [PubMed] [Google Scholar]
- 321.Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the endogenous kappa opioid agonist dynorphin A(1–17) on cocaine-evoked increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6 J mice. Psychopharmacology (Berl) 2004;172:422–429. doi: 10.1007/s00213-003-1688-3. [DOI] [PubMed] [Google Scholar]
- 322.McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50, 488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Shippenberg TS, LeFevour A, Thompson AC. Sensitization to the conditioned rewarding effects of morphine and cocaine: differential effects of the kappa-opioid receptor agonist U69593. Eur J Pharmacol. 1998;345:27–34. doi: 10.1016/S0014-2999(97)01614-2. [DOI] [PubMed] [Google Scholar]
- 324.Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr The kappa-opioid agonist U69, 593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry. 2008;64:982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Chefer VI, Moron JA, Hope B, Rea W, Shippenberg TS. Kappa-opioid receptor activation prevents alterations in mesocortical dopamine neurotransmission that occur during abstinence from cocaine. Neuroscience. 2000;101:619–627. doi: 10.1016/S0306-4522(00)00417-6. [DOI] [PubMed] [Google Scholar]
- 326.Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, Mitchell TB, Farquhar D, Ghosh SC, Mattson BJ, Hope BT. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci. 2009;12:1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.D’Addario C, Di Benedetto M, Candeletti S, Romualdi P. The kappa-opioid receptor agonist U-69593 prevents cocaine-induced phosphorylation of DARPP-32 at Thr(34) in the rat brain. Brain Res Bull. 2007;73:34–39. doi: 10.1016/j.brainresbull.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 328.Glick SD, Maisonneuve IM, Raucci J, Archer S. Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res. 1995;681:147–152. doi: 10.1016/0006-8993(95)00306-B. [DOI] [PubMed] [Google Scholar]
- 329.Glick SD, Visker KE, Maisonneuve IM. Effects of cyclazocine on cocaine self-administration in rats. Eur J Pharmacol. 1998;357:9–14. doi: 10.1016/S0014-2999(98)00548-2. [DOI] [PubMed] [Google Scholar]
- 330.Schenk S, Partridge B, Shippenberg TS. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology (Berl) 1999;144:339–346. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- 331.Mello NK, Negus SS. Effects of kappa opioid agonists on cocaine- and food-maintained responding by rhesus monkeys. J Pharmacol Exp Ther. 1998;286:812–824. [PubMed] [Google Scholar]
- 332.Sun W, Xue Y, Huang Z, Steketee JD. Regulation of cocaine-reinstated drug-seeking behavior by kappa-opioid receptors in the ventral tegmental area of rats. Psychopharmacology (Berl) 2010;210:179–188. doi: 10.1007/s00213-010-1812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology (Berl) 2009;205:565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Schenk S, Partridge B, Shippenberg TS. Effects of the kappa-opioid receptor agonist, U69593, on the development of sensitization and on the maintenance of cocaine self-administration. Neuropsychopharmacology. 2001;24:441–450. doi: 10.1016/S0893-133X(00)00190-1. [DOI] [PubMed] [Google Scholar]
- 335.Zapata A (2010) Kappa opioid receptors and cocaine seeking habits. International Narcotics Research Conference
- 336.Huge V, Rammes G, Beyer A, Zieglgansberger W, Azad SC. Activation of kappa opioid receptors decreases synaptic transmission and inhibits long-term potentiation in the basolateral amygdala of the mouse. Eur J Pain. 2009;13:124–129. doi: 10.1016/j.ejpain.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 337.Ruedi-Bettschen D, Rowlett JK, Spealman RD, Platt DM. Attenuation of cocaine-induced reinstatement of drug seeking in squirrel monkeys: kappa opioid and serotonergic mechanisms. Psychopharmacology (Berl) 2010;210:169–177. doi: 10.1007/s00213-009-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Schenk S, Partridge B, Shippenberg TS. Reinstatement of extinguished drug-taking behavior in rats: effect of the kappa-opioid receptor agonist, U69593. Psychopharmacology (Berl) 2000;151:85–90. doi: 10.1007/s002130000476. [DOI] [PubMed] [Google Scholar]
- 339.Schindler AG, Li S, Chavkin C. Behavioral stress may increase the rewarding valence of cocaine-associated cues through a dynorphin/kappa-opioid receptor-mediated mechanism without affecting associative learning or memory retrieval mechanisms. Neuropsychopharmacology. 2010;35:1932–1942. doi: 10.1038/npp.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- 341.Beardsley PM, Pollard GT, Howard JL, Carroll FI. Effectiveness of analogs of the kappa opioid receptor antagonist (3R)-7-hydroxy-N-((1S)-1-{[(3R, 4R)-4-(3-hydroxyphenyl)-3, 4-dimethyl-1-pipe ridinyl]methyl}-2-methylpropyl)-1, 2, 3, 4-tetrahydro-3-isoquinolinecarboxami de (JDTic) to reduce U50, 488-induced diuresis and stress-induced cocaine reinstatement in rats. Psychopharmacology (Berl) 2010;210:189–198. doi: 10.1007/s00213-010-1846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berl) 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Carey AN, Borozny K, Aldrich JV, McLaughlin JP. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur J Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Valdez GR, Platt DM, Rowlett JK, Ruedi-Bettschen D, Spealman RD. Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J Pharmacol Exp Ther. 2007;323:525–533. doi: 10.1124/jpet.107.125484. [DOI] [PubMed] [Google Scholar]
- 345.Isola R, Zhang H, Tejwani GA, Neff NH, Hadjiconstantinou M. Acute nicotine changes dynorphin and prodynorphin mRNA in the striatum. Psychopharmacology (Berl) 2009;201:507–516. doi: 10.1007/s00213-008-1315-4. [DOI] [PubMed] [Google Scholar]
- 346.Torres OV, Tejeda HA, Natividad LA, O’Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav. 2008;90:658–663. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Fudala PJ, Teoh KW, Iwamoto ET. Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol Biochem Behav. 1985;22:237–241. doi: 10.1016/0091-3057(85)90384-3. [DOI] [PubMed] [Google Scholar]
- 348.Loughlin SE, Islas MI, Cheng MY, Lee AG, Villegier AS, Leslie FM. Nicotine modulation of stress-related peptide neurons. J Comp Neurol. 2006;497:575–588. doi: 10.1002/cne.20999. [DOI] [PubMed] [Google Scholar]
- 349.Mathieu-Kia AM, Besson MJ. Repeated administration of cocaine, nicotine and ethanol: effects on preprodynorphin, preprotachykinin A and preproenkephalin mRNA expression in the dorsal and the ventral striatum of the rat. Brain Res Mol Brain Res. 1998;54:141–151. doi: 10.1016/S0169-328X(97)00338-0. [DOI] [PubMed] [Google Scholar]
- 350.Mathieu AM, Caboche J, Besson MJ. Distribution of preproenkephalin, preprotachykinin A, and preprodynorphin mRNAs in the rat nucleus accumbens: effect of repeated administration of nicotine. Synapse. 1996;23:94–106. doi: 10.1002/(SICI)1098-2396(199606)23:2<94::AID-SYN5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 351.Galeote L, Berrendero F, Bura SA, Zimmer A, Maldonado R. Prodynorphin gene disruption increases the sensitivity to nicotine self-administration in mice. Int J Neuropsychopharmacol. 2009;12:615–625. doi: 10.1017/S1461145708009450. [DOI] [PubMed] [Google Scholar]
- 352.Jackson KJ, Carroll FI, Negus SS, Damaj MI. Effect of the selective kappa-opioid receptor antagonist JDTic on nicotine antinociception, reward, and withdrawal in the mouse. Psychopharmacology (Berl) 2010;210:285–294. doi: 10.1007/s00213-010-1803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Tejeda HA, Natividad LA, Torres OV, Castaneda EC, O’Dell LE. The behavioral and neurochemical effects produced by kappa-opioid receptor stimulation are diminished in nicotine-dependent adolescent versus adult rats. Soc Neurosci Abstr. 2008;360:14. [Google Scholar]
- 354.Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of dynorphin A(1–8) release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res. 2006;30:982–990. doi: 10.1111/j.1530-0277.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- 355.Lam MP, Marinelli PW, Bai L, Gianoulakis C. Effects of acute ethanol on opioid peptide release in the central amygdala: an in vivo microdialysis study. Psychopharmacology (Berl) 2008;201:261–271. doi: 10.1007/s00213-008-1267-8. [DOI] [PubMed] [Google Scholar]
- 356.Przewlocka B, Lason W, Przewlocki R. Repeated ethanol administration decreases prodynorphin biosynthesis in the rat hippocampus. Neurosci Lett. 1992;134:195–198. doi: 10.1016/0304-3940(92)90515-9. [DOI] [PubMed] [Google Scholar]
- 357.Seizinger BR, Bovermann K, Maysinger D, Hollt V, Herz A. Differential effects of acute and chronic ethanol treatment on particular opioid peptide systems in discrete regions of rat brain and pituitary. Pharmacol Biochem Behav. 1983;18(Suppl 1):361–369. doi: 10.1016/0091-3057(83)90200-9. [DOI] [PubMed] [Google Scholar]
- 358.Chang GQ, Karatayev O, Ahsan R, Avena NM, Lee C, Lewis MJ, Hoebel BG, Leibowitz SF. Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: relationship with circulating triglycerides. Alcohol Clin Exp Res. 2007;31:249–259. doi: 10.1111/j.1530-0277.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- 359.Chang GQ, Barson JR, Karatayev O, Chang SY, Chen YW, Leibowitz SF. Effect of chronic ethanol on enkephalin in the hypothalamus and extra-hypothalamic areas. Alcohol Clin Exp Res. 2010;34:761–770. doi: 10.1111/j.1530-0277.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Przewlocka B, Turchan J, Lason W, Przewlocki R. Ethanol withdrawal enhances the prodynorphin system activity in the rat nucleus accumbens. Neurosci Lett. 1997;238:13–16. doi: 10.1016/S0304-3940(97)00829-X. [DOI] [PubMed] [Google Scholar]
- 361.Lindholm S, Ploj K, Franck J, Nylander I. Repeated ethanol administration induces short- and long-term changes in enkephalin and dynorphin tissue concentrations in rat brain. Alcohol. 2000;22:165–171. doi: 10.1016/S0741-8329(00)00118-X. [DOI] [PubMed] [Google Scholar]
- 362.Zapata A, Shippenberg TS. Endogenous kappa opioid receptor systems modulate the responsiveness of mesoaccumbal dopamine neurons to ethanol. Alcohol Clin Exp Res. 2006;30:592–597. doi: 10.1111/j.1530-0277.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 363.Lindholm S, Rosin A, Dahlin I, Georgieva J, Franck J. Ethanol alters the effect of kappa receptor ligands on dopamine release in the nucleus accumbens. Physiol Behav. 2007;92:167–171. doi: 10.1016/j.physbeh.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 364.Nestby P, Schoffelmeer AN, Homberg JR, Wardeh G, De Vries TJ, Mulder AH, Vanderschuren LJ. Bremazocine reduces unrestricted free-choice ethanol self-administration in rats without affecting sucrose preference. Psychopharmacology (Berl) 1999;142:309–317. doi: 10.1007/s002130050894. [DOI] [PubMed] [Google Scholar]
- 365.Lindholm S, Werme M, Brene S, Franck J. The selective kappa-opioid receptor agonist U50, 488H attenuates voluntary ethanol intake in the rat. Behav Brain Res. 2001;120:137–146. doi: 10.1016/S0166-4328(00)00368-5. [DOI] [PubMed] [Google Scholar]
- 366.Mitchell JM, Liang MT, Fields HL. A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats. Psychopharmacology (Berl) 2005;182:384–392. doi: 10.1007/s00213-005-0067-7. [DOI] [PubMed] [Google Scholar]
- 367.Doyon WM, Howard EC, Shippenberg TS, Gonzales RA. Kappa-opioid receptor modulation of accumbal dopamine concentration during operant ethanol self-administration. Neuropharmacology. 2006;51:487–496. doi: 10.1016/j.neuropharm.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Logrip ML, Janak PH, Ron D. Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. FASEB J. 2008;22:2393–2404. doi: 10.1096/fj.07-099135. [DOI] [PubMed] [Google Scholar]
- 369.Barson JR, Carr AJ, Soun JE, Sobhani NC, Rada P, Leibowitz SF, Hoebel BG. Opioids in the hypothalamic paraventricular nucleus stimulate ethanol intake. Alcohol Clin Exp Res. 2010;34:214–222. doi: 10.1111/j.1530-0277.2009.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Marchant NJ, Furlong TM, McNally GP. Medial dorsal hypothalamus mediates the inhibition of reward seeking after extinction. J Neurosci. 2010;30:14102–14115. doi: 10.1523/JNEUROSCI.4079-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Walker BM, Zorrilla EP, Koob GF (2010) Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol. doi:10.1111/j.1369-1600.2010.00226.x [DOI] [PMC free article] [PubMed]
- 373.Holter SM, Henniger MS, Lipkowski AW, Spanagel R. Kappa-opioid receptors and relapse-like drinking in long-term ethanol-experienced rats. Psychopharmacology (Berl) 2000;153:93–102. doi: 10.1007/s002130000601. [DOI] [PubMed] [Google Scholar]
- 374.Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berl) 2010;210:199–209. doi: 10.1007/s00213-010-1844-5. [DOI] [PubMed] [Google Scholar]
- 375.Matsuzawa S, Suzuki T, Misawa M, Nagase H. Different roles of mu-, delta- and kappa-opioid receptors in ethanol-associated place preference in rats exposed to conditioned fear stress. Eur J Pharmacol. 1999;368:9–16. doi: 10.1016/S0014-2999(99)00008-4. [DOI] [PubMed] [Google Scholar]
- 376.Nylander I, Vlaskovska M, Terenius L. The effects of morphine treatment and morphine withdrawal on the dynorphin and enkephalin systems in Sprague–Dawley rats. Psychopharmacology (Berl) 1995;118:391–400. doi: 10.1007/BF02245939. [DOI] [PubMed] [Google Scholar]
- 377.Nylander I, Stenfors C, Tan-No K, Mathe AA, Terenius L. A comparison between microwave irradiation and decapitation: basal levels of dynorphin and enkephalin and the effect of chronic morphine treatment on dynorphin peptides. Neuropeptides. 1997;31:357–365. doi: 10.1016/S0143-4179(97)90072-X. [DOI] [PubMed] [Google Scholar]
- 378.Rattan AK, Koo KL, Tejwani GA, Bhargava HN. The effect of morphine tolerance dependence and abstinence on immunoreactive dynorphin (1–13) levels in discrete brain regions, spinal cord, pituitary gland and peripheral tissues of the rat. Brain Res. 1992;584:207–212. doi: 10.1016/0006-8993(92)90896-H. [DOI] [PubMed] [Google Scholar]
- 379.Wan XW, Li WH, Huang M, You ZD, Tan YX, Lu CL, Gong ZH. Levels of immunoreactive dynorphin A1–13 during development of morphine dependence in rats. Zhongguo Yao Li Xue Bao. 1998;19:560–563. [PubMed] [Google Scholar]
- 380.McClung CA, Nestler EJ, Zachariou V. Regulation of gene expression by chronic morphine and morphine withdrawal in the locus ceruleus and ventral tegmental area. J Neurosci. 2005;25:6005–6015. doi: 10.1523/JNEUROSCI.0062-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Van Bockstaele EJ, Reyes BA, Valentino RJ. The locus coeruleus: a key nucleus where stress and opioids intersect to mediate vulnerability to opiate abuse. Brain Res. 2010;1314:162–174. doi: 10.1016/j.brainres.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.You ZB, Herrera-Marschitz M, Nylander I, Goiny M, Kehr J, Ungerstedt U, Terenius L. Effect of morphine on dynorphin B and GABA release in the basal ganglia of rats. Brain Res. 1996;710:241–248. doi: 10.1016/0006-8993(95)01402-0. [DOI] [PubMed] [Google Scholar]
- 383.Chefer VI, Denoroy L, Zapata A, Shippenberg TS. Mu opioid receptor modulation of somatodendritic dopamine overflow: GABAergic and glutamatergic mechanisms. Eur J Neurosci. 2009;30:272–278. doi: 10.1111/j.1460-9568.2009.06827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Gieryk A, Ziolkowska B, Solecki W, Kubik J, Przewlocki R. Forebrain PENK and PDYN gene expression levels in three inbred strains of mice and their relationship to genotype-dependent morphine reward sensitivity. Psychopharmacology (Berl) 2010;208:291–300. doi: 10.1007/s00213-009-1730-1. [DOI] [PubMed] [Google Scholar]
- 386.Xi ZX, Fuller SA, Stein EA. Dopamine release in the nucleus accumbens during heroin self-administration is modulated by kappa opioid receptors: an in vivo fast-cyclic voltammetry study. J Pharmacol Exp Ther. 1998;284:151–161. [PubMed] [Google Scholar]
- 387.Cappendijk SL, Hurd YL, Nylander I, van Ree JM, Terenius L. A heroin-, but not a cocaine-expecting, self-administration state preferentially alters endogenous brain peptides. Eur J Pharmacol. 1999;365:175–182. doi: 10.1016/S0014-2999(98)00874-7. [DOI] [PubMed] [Google Scholar]
- 388.Negus SS, Henriksen SJ, Mattox A, Pasternak GW, Portoghese PS, Takemori AE, Weinger MB, Koob GF. Effect of antagonists selective for mu, delta and kappa opioid receptors on the reinforcing effects of heroin in rats. J Pharmacol Exp Ther. 1993;265:1245–1252. [PubMed] [Google Scholar]
- 389.Negus SS, Rice KC. Mechanisms of withdrawal-associated increases in heroin self-administration: pharmacologic modulation of heroin vs food choice in heroin-dependent rhesus monkeys. Neuropsychopharmacology. 2009;34:899–911. doi: 10.1038/npp.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Spanagel R, Almeida OF, Bartl C, Shippenberg TS. Endogenous kappa-opioid systems in opiate withdrawal: role in aversion and accompanying changes in mesolimbic dopamine release. Psychopharmacology (Berl) 1994;115:121–127. doi: 10.1007/BF02244761. [DOI] [PubMed] [Google Scholar]
- 391.Maldonado R, Negus S, Koob GF. Precipitation of morphine withdrawal syndrome in rats by administration of mu-, delta- and kappa-selective opioid antagonists. Neuropharmacology. 1992;31:1231–1241. doi: 10.1016/0028-3908(92)90051-P. [DOI] [PubMed] [Google Scholar]
- 392.Le Guen S, Gestreau C, Besson JM. Morphine withdrawal precipitated by specific mu, delta or kappa opioid receptor antagonists: a c-Fos protein study in the rat central nervous system. Eur J Neurosci. 2003;17:2425–2437. doi: 10.1046/j.1460-9568.2003.02678.x. [DOI] [PubMed] [Google Scholar]
- 393.Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le Meur M, Roques BP, Maldonado R, Kieffer BL. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50, 488H and attenuates morphine withdrawal. EMBO J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Carroll FI, Harris LS, Aceto MD. Effects of JDTic, a selective kappa-opioid receptor antagonist, on the development and expression of physical dependence on morphine using a rat continuous-infusion model. Eur J Pharmacol. 2005;524:89–94. doi: 10.1016/j.ejphar.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 395.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review, neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci. 2010;30:15457–15463. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Tamminga CA, Holcomb HH. Phenotype of schizophrenia: a review and formulation. Mol Psychiatry. 2005;10:27–39. doi: 10.1038/sj.mp.4001563. [DOI] [PubMed] [Google Scholar]
- 398.Guillin O, Abi-Dargham A, Laruelle M. Neurobiology of dopamine in schizophrenia. Int Rev Neurobiol. 2007;78:1–39. doi: 10.1016/S0074-7742(06)78001-1. [DOI] [PubMed] [Google Scholar]
- 399.Agid O, Kapur S, Remington G. Emerging drugs for schizophrenia. Expert Opin Emerg Drugs. 2008;13:479–495. doi: 10.1517/14728214.13.3.479. [DOI] [PubMed] [Google Scholar]
- 400.Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Sheffler DJ, Roth BL. Salvinorin A: the “magic mint” hallucinogen finds a molecular target in the kappa opioid receptor. Trends Pharmacol Sci. 2003;24:107–109. doi: 10.1016/S0165-6147(03)00027-0. [DOI] [PubMed] [Google Scholar]
- 402.Heikkila L, Rimon R, Terenius L. Dynorphin A and substance P in the cerebrospinal fluid of schizophrenic patients. Psychiatry Res. 1990;34:229–236. doi: 10.1016/0165-1781(90)90001-L. [DOI] [PubMed] [Google Scholar]
- 403.Lindstrom LH. Clinical and biological markers for outcome in schizophrenia: a review of a longitudinal follow-up study in Uppsala schizophrenia research project. Neuropsychopharmacology. 1996;14:23S–26S. doi: 10.1016/0893-133X(95)00201-N. [DOI] [PubMed] [Google Scholar]
- 404.Zhang AZ, Zhou GZ, Xi GF, Gu NF, Xia ZY, Yao JL, Chang JK, Webber R, Potkin S. Lower CSF level of dynorphin(1–8) immunoreactivity in schizophrenic patients. Neuropeptides. 1985;5:553–556. doi: 10.1016/0143-4179(85)90077-0. [DOI] [PubMed] [Google Scholar]
- 405.Royston MC, Slater P, Simpson MD, Deakin JF. Analysis of laminar distribution of kappa opiate receptor in human cortex: comparison between schizophrenia and normal. J Neurosci Methods. 1991;36:145–153. doi: 10.1016/0165-0270(91)90040-7. [DOI] [PubMed] [Google Scholar]
- 406.Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–884. doi: 10.1016/S0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 407.Butelman ER, Harris TJ, Kreek MJ. The plant-derived hallucinogen, salvinorin A, produces kappa-opioid agonist-like discriminative effects in rhesus monkeys. Psychopharmacology (Berl) 2004;172:220–224. doi: 10.1007/s00213-003-1638-0. [DOI] [PubMed] [Google Scholar]
- 408.Butelman ER, Rus S, Prisinzano TE, Kreek MJ. The discriminative effects of the kappa-opioid hallucinogen salvinorin A in nonhuman primates: dissociation from classic hallucinogen effects. Psychopharmacology (Berl) 2010;210:253–262. doi: 10.1007/s00213-009-1771-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Killinger BA, Peet MM, Baker LE. Salvinorin A fails to substitute for the discriminative stimulus effects of LSD or ketamine in Sprague–Dawley rats. Pharmacol Biochem Behav. 2010;96:260–265. doi: 10.1016/j.pbb.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 410.Mori T, Nomura M, Yoshizawa K, Nagase H, Sawaguchi T, Narita M, Suzuki T. Generalization of NMDA-receptor antagonists to the discriminative stimulus effects of kappa-opioid receptor agonists U-50, 488H, but not TRK-820 in rats. J Pharmacol Sci. 2006;100:157–161. doi: 10.1254/jphs.SCJ05006X. [DOI] [PubMed] [Google Scholar]
- 411.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 412.Bortolato M, Aru GN, Frau R, Orru M, Fa M, Manunta M, Puddu M, Mereu G, Gessa GL. Kappa opioid receptor activation disrupts prepulse inhibition of the acoustic startle in rats. Biol Psychiatry. 2005;57:1550–1558. doi: 10.1016/j.biopsych.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 413.Tejeda HA, Chefer VI, Zapata A, Shippenberg TS. The effects of kappa-opioid receptor ligands on prepulse inhibition and CRF-induced prepulse inhibition deficits in the rat. Psychopharmacology (Berl) 2010;210:231–240. doi: 10.1007/s00213-010-1799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Lodge DJ, Grace AA (2010) Developmental pathology, dopamine, stress and schizophrenia. Int J Dev Neurosci. doi:10.1016/j.ijdevneu.2010.08.002 [DOI] [PMC free article] [PubMed]
- 415.Goto Y, O’Donnell P. Delayed mesolimbic system alteration in a developmental animal model of schizophrenia. J Neurosci. 2002;22:9070–9077. doi: 10.1523/JNEUROSCI.22-20-09070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.El-Rawas R, Saade NE, Thiriet N, Atweh S, Jaber M, Al-Amin HA. Developmental changes in the mRNA expression of neuropeptides and dopamine and glutamate receptors in neonates and adult rats after ventral hippocampal lesion. Schizophr Res. 2009;113:298–307. doi: 10.1016/j.schres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 417.Lipska BK, Lerman DN, Khaing ZZ, Weinberger DR. The neonatal ventral hippocampal lesion model of schizophrenia: effects on dopamine and GABA mRNA markers in the rat midbrain. Eur J Neurosci. 2003;18:3097–3104. doi: 10.1111/j.1460-9568.2003.03047.x. [DOI] [PubMed] [Google Scholar]
- 418.Blume AW, Marlatt GA. The role of executive cognitive functions in changing substance use: what we know and what we need to know. Ann Behav Med. 2009;37:117–125. doi: 10.1007/s12160-009-9093-8. [DOI] [PubMed] [Google Scholar]
- 419.Hill SK, Harris MS, Herbener ES, Pavuluri M, Sweeney JA. Neurocognitive allied phenotypes for schizophrenia and bipolar disorder. Schizophr Bull. 2008;34:743–759. doi: 10.1093/schbul/sbn027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Royall DR. Frontal systems impairment in major depression. Semin Clin Neuropsychiatry. 1999;4:13–23. doi: 10.1053/SCNP00400013. [DOI] [PubMed] [Google Scholar]
- 421.Nemeth CL, Paine TA, Rittiner JE, Beguin C, Carroll FI, Roth BL, Cohen BM, Carlezon WA., Jr Role of kappa-opioid receptors in the effects of salvinorin A and ketamine on attention in rats. Psychopharmacology (Berl) 2010;210:263–274. doi: 10.1007/s00213-010-1834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA., Jr Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague–Dawley rats. Biol Psychiatry. 2007;62:687–693. doi: 10.1016/j.biopsych.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 423.Shannon HE, Eberle EL, Mitch CH, McKinzie DL, Statnick MA. Effects of kappa opioid receptor agonists on attention as assessed by a 5-choice serial reaction time task in rats. Neuropharmacology. 2007;53:930–941. doi: 10.1016/j.neuropharm.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 424.McDaniel KL, Mundy WR, Tilson HA. Microinjection of dynorphin into the hippocampus impairs spatial learning in rats. Pharmacol Biochem Behav. 1990;35:429–435. doi: 10.1016/0091-3057(90)90180-P. [DOI] [PubMed] [Google Scholar]
- 425.Sandin J, Nylander I, Georgieva J, Schott PA, Ogren SO, Terenius L. Hippocampal dynorphin B injections impair spatial learning in rats: a kappa-opioid receptor-mediated effect. Neuroscience. 1998;85:375–382. doi: 10.1016/S0306-4522(97)00605-2. [DOI] [PubMed] [Google Scholar]
- 426.Daumas S, Betourne A, Halley H, Wolfer DP, Lipp HP, Lassalle JM, Frances B. Transient activation of the CA3 Kappa opioid system in the dorsal hippocampus modulates complex memory processing in mice. Neurobiol Learn Mem. 2007;88:94–103. doi: 10.1016/j.nlm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 427.Jamot L, Matthes HW, Simonin F, Kieffer BL, Roder JC. Differential involvement of the mu and kappa opioid receptors in spatial learning. Genes Brain Behav. 2003;2:80–92. doi: 10.1034/j.1601-183X.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 428.Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- 429.Carey AN, Lyons AM, Shay CF, Dunton O, McLaughlin JP. Endogenous kappa opioid activation mediates stress-induced deficits in learning and memory. J Neurosci. 2009;29:4293–4300. doi: 10.1523/JNEUROSCI.6146-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Kotz CM, Weldon D, Billington CJ, Levine AS. Age-related changes in brain proDynorphin gene expression in the rat. Neurobiol Aging. 2004;25:1343–1347. doi: 10.1016/j.neurobiolaging.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 431.Jiang HK, Owyang VV, Hong JS, Gallagher M. Elevated dynorphin in the hippocampal formation of aged rats: relation to cognitive impairment on a spatial learning task. Proc Natl Acad Sci USA. 1989;86:2948–2951. doi: 10.1073/pnas.86.8.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Nguyen XV, Masse J, Kumar A, Vijitruth R, Kulik C, Liu M, Choi DY, Foster TC, Usynin I, Bakalkin G, Bing G. Prodynorphin knockout mice demonstrate diminished age-associated impairment in spatial water maze performance. Behav Brain Res. 2005;161:254–262. doi: 10.1016/j.bbr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 433.Barg J, Belcheva M, Rowinski J, Ho A, Burke WJ, Chung HD, Schmidt CA, Coscia CJ. Opioid receptor density changes in Alzheimer amygdala and putamen. Brain Res. 1993;632:209–215. doi: 10.1016/0006-8993(93)91155-L. [DOI] [PubMed] [Google Scholar]
- 434.Hiller JM, Itzhak Y, Simon EJ. Selective changes in mu, delta and kappa opioid receptor binding in certain limbic regions of the brain in Alzheimer’s disease patients. Brain Res. 1987;406:17–23. doi: 10.1016/0006-8993(87)90764-5. [DOI] [PubMed] [Google Scholar]
- 435.Mathieu-Kia AM, Fan LQ, Kreek MJ, Simon EJ, Hiller JM. Mu-, delta- and kappa-opioid receptor populations are differentially altered in distinct areas of postmortem brains of Alzheimer’s disease patients. Brain Res. 2001;893:121–134. doi: 10.1016/S0006-8993(00)03302-3. [DOI] [PubMed] [Google Scholar]
- 436.Ikeda M, Mackay KB, Dewar D, McCulloch J. Differential alterations in adenosine A1 and kappa 1 opioid receptors in the striatum in Alzheimer’s disease. Brain Res. 1993;616:211–217. doi: 10.1016/0006-8993(93)90211-5. [DOI] [PubMed] [Google Scholar]
- 437.Risser D, You ZB, Cairns N, Herrera-Marschitz M, Seidl R, Schneider C, Terenius L, Lubec G. Endogenous opioids in frontal cortex of patients with Down syndrome. Neurosci Lett. 1996;203:111–114. doi: 10.1016/0304-3940(95)12275-3. [DOI] [PubMed] [Google Scholar]
- 438.Yakovleva T, Marinova Z, Kuzmin A, Seidah NG, Haroutunian V, Terenius L, Bakalkin G. Dysregulation of dynorphins in Alzheimer disease. Neurobiol Aging. 2007;28:1700–1708. doi: 10.1016/j.neurobiolaging.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 439.Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 2006;188:567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- 440.Hiramatsu M, Murasawa H, Nabeshima T, Kameyama T. Effects of U-50, 488H on scopolamine-, mecamylamine- and dizocilpine-induced learning and memory impairment in rats. J Pharmacol Exp Ther. 1998;284:858–867. [PubMed] [Google Scholar]
- 441.Hiramatsu M, Kameyama T. Roles of kappa-opioid receptor agonists in learning and memory impairment in animal models. Methods Find Exp Clin Pharmacol. 1998;20:595–599. doi: 10.1358/mf.1998.20.7.485724. [DOI] [PubMed] [Google Scholar]
- 442.Hiramatsu M, Hoshino T. Involvement of kappa-opioid receptors and sigma receptors in memory function demonstrated using an antisense strategy. Brain Res. 2004;1030:247–255. doi: 10.1016/j.brainres.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 443.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 444.McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, Ioannidis JP, Hirschhorn JN. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet. 2008;9:356–369. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- 445.Frazer KA, Murray SS, Schork NJ, Topol EJ. Human genetic variation and its contribution to complex traits. Nat Rev Genet. 2009;10:241–251. doi: 10.1038/nrg2554. [DOI] [PubMed] [Google Scholar]
- 446.Chen AC, LaForge KS, Ho A, McHugh PF, Kellogg S, Bell K, Schluger RP, Leal SM, Kreek MJ. Potentially functional polymorphism in the promoter region of prodynorphin gene may be associated with protection against cocaine dependence or abuse. Am J Med Genet. 2002;114:429–435. doi: 10.1002/ajmg.10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Zhang CS, Tan Z, Lu L, Wu SN, He Y, Gu NF, Feng GY, He L. Polymorphism of Prodynorphin promoter is associated with schizophrenia in Chinese population. Acta Pharmacol Sin. 2004;25:1022–1026. [PubMed] [Google Scholar]
- 448.Ventriglia M, Bocchio Chiavetto L, Bonvicini C, Tura GB, Bignotti S, Racagni G, Gennarelli M. Allelic variation in the human prodynorphin gene promoter and schizophrenia. Neuropsychobiology. 2002;46:17–21. doi: 10.1159/000063571. [DOI] [PubMed] [Google Scholar]
- 449.Lundström K, Trupin MP. Proposed schizophrenia-related gene polymorphism: expression of the Ser9Gly mutant human dopamine D3 receptor with the Semliki Forest virus system. Biochem Biophys Res Commun. 1996;225:1068–1072. doi: 10.1006/bbrc.1996.1296. [DOI] [PubMed] [Google Scholar]
- 450.Hellstrand M, Danielsen EA, Steen VM, Ekman A, Eriksson E, Nilsson CL. The ser9gly SNP in the dopamine D3 receptor causes a shift from cAMP related to PGE2 related signal transduction mechanisms in transfected CHO cells. J Med Genet. 2004;41:867–871. doi: 10.1136/jmg.2004.020941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Goldman D, Oroszi G, Ducci F. The genetics of addiction: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 452.Chefer VI, Zapata A, Shippenberg TS, Bungay PM. Quantitative no-net-flux microdialysis permits detection of increases and decreases in dopamine uptake in mouse nucleus accumbens. J Neurosci Methods. 2006;155:187–193. doi: 10.1016/j.jneumeth.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 453.Zapata A, Kivell B, Han Y, Javitch JA, Bolan EA, Kuraguntla D, Jaligam V, Oz M, Jayanthi LD, Samuvel DJ, Ramamoorthy S, Shippenberg TS. Regulation of dopamine transporter function and cell surface expression by D3 dopamine receptors. J Biol Chem. 2007;282:35842–35854. doi: 10.1074/jbc.M611758200. [DOI] [PubMed] [Google Scholar]
- 454.Nikoshkov A, Drakenberg K, Wang X, Horvath MC, Keller E, Hurd YL. Opioid neuropeptide genotypes in relation to heroin abuse: dopamine tone contributes to reversed mesolimbic proenkephalin expression. Proc Natl Acad Sci USA. 2008;105:786–791. doi: 10.1073/pnas.0710902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Xuei X, Dick D, Flury-Wetherill L, Tian HJ, Agrawal A, Bierut L, Goate A, Bucholz K, Schuckit M, Nurnberger J, Jr, Tischfield J, Kuperman S, Porjesz B, Begleiter H, Foroud T, Edenberg HJ. Association of the kappa-opioid system with alcohol dependence. Mol Psychiatry. 2006;11:1016–1024. doi: 10.1038/sj.mp.4001882. [DOI] [PubMed] [Google Scholar]
- 456.Kolsch H, Wagner M, Bilkei-Gorzo A, Toliat MR, Pentzek M, Fuchs A, Kaduszkiewicz H, van den Bussche H, Riedel-Heller SG, Angermeyer MC, Weyerer S, Werle J, Bickel H, Mosch E, Wiese B, Daerr M, Jessen F, Maier W, Dichgans M. Gene polymorphisms in prodynorphin (PDYN) are associated with episodic memory in the elderly. J Neural Transm. 2009;116:897–903. doi: 10.1007/s00702-009-0238-5. [DOI] [PubMed] [Google Scholar]
- 457.Zhang H, Kranzler HR, Yang BZ, Luo X, Gelernter J. The OPRD1 and OPRK1 loci in alcohol or drug dependence: OPRD1 variation modulates substance dependence risk. Mol Psychiatry. 2008;13:531–543. doi: 10.1038/sj.mp.4002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Hansell NK, Agrawal A, Whitfield JB, Morley KI, Gordon SD, Lind PA, Pergadia ML, Montgomery GW, Madden PA, Todd RD, Heath AC, Martin NG. Can we identify genes for alcohol consumption in samples ascertained for heterogeneous purposes? Alcohol Clin Exp Res. 2009;33:729–739. doi: 10.1111/j.1530-0277.2008.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Edenberg HJ, Koller DL, Xuei X, Wetherill L, McClintick JN, Almasy L, Bierut LJ, Bucholz KK, Goate A, Aliev F, Dick D, Hesselbrock V, Hinrichs A, Kramer J, Kuperman S, Nurnberger JI, Jr, Rice JP, Schuckit MA, Taylor R, Todd Webb B, Tischfield JA, Porjesz B, Foroud T. Genome-wide association study of alcohol dependence implicates a region on chromosome 11. Alcohol Clin Exp Res. 2010;34:840–852. doi: 10.1111/j.1530-0277.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Sullivan PF, Gejman PV. Response to Mitchell and Porteus. Mol Psychiatry. 2010;15:450–452. doi: 10.1038/mp.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.LaFramboise T. Single nucleotide polymorphism arrays: a decade of biological, computational and technological advances. Nucleic Acids Res. 2009;37:4181–4193. doi: 10.1093/nar/gkp552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.McCarroll SA, Kuruvilla FG, Korn JM, Cawley S, Nemesh J, Wysoker A, Shapero MH, de Bakker PI, Maller JB, Kirby A, Elliott AL, Parkin M, Hubbell E, Webster T, Mei R, Veitch J, Collins PJ, Handsaker R, Lincoln S, Nizzari M, Blume J, Jones KW, Rava R, Daly MJ, Gabriel SB, Altshuler D. Integrated detection and population-genetic analysis of SNPs and copy number variation. Nat Genet. 2008;40:1166–1174. doi: 10.1038/ng.238. [DOI] [PubMed] [Google Scholar]
- 463.Zeggini E, Rayner W, Morris AP, Hattersley AT, Walker M, Hitman GA, Deloukas P, Cardon LR, McCarthy MI. An evaluation of HapMap sample size and tagging SNP performance in large-scale empirical and simulated data sets. Nat Genet. 2005;37:1320–1322. doi: 10.1038/ng1670. [DOI] [PubMed] [Google Scholar]
- 464.Mitchell KJ, Porteous DJ. GWAS for psychiatric disease: is the framework built on a solid foundation? Mol Psychiatry. 2009;14:740–741. doi: 10.1038/mp.2009.17. [DOI] [PubMed] [Google Scholar]
- 465.Cirulli ET, Goldstein DB. Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat Rev Genet. 2010;11:415–525. doi: 10.1038/nrg2779. [DOI] [PubMed] [Google Scholar]
- 466.Bakalkin G, Watanabe H, Jezierska J, Depoorter C, Verschuuren-Bemelmans C, Bazov I, Artemenko KA, Yakovleva T, Dooijes D, Van de Warrenburg BP, Zubarev RA, Kremer B, Knapp PE, Hauser KF, Wijmenga C, Nyberg F, Sinke RJ, Verbeek DS. Prodynorphin mutations cause the neurodegenerative disorder spinocerebellar ataxia type 23. Am J Hum Genet. 2010;87:593–603. doi: 10.1016/j.ajhg.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Feinberg AP. Epigenomics reveals a functional genome anatomy and a new approach to common disease. Nat Biotechnol. 2010;28:1049–1052. doi: 10.1038/nbt1010-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]