Abstract
Over the last two decades the molecular and cellular mechanisms underlying T cell activation, expansion, differentiation, and memory formation have been intensively investigated. These studies revealed that the generation of memory T cells is critically impacted by a number of factors, including the magnitude of the inflammatory response and cytokine production, the type of dendritic cell [DC] that presents the pathogen derived antigen, their maturation status, and the concomitant provision of costimulation. Nevertheless, the primary stimulus leading to T cell activation is generated through the T cell receptor [TCR] following its engagement with a peptide MHC ligand [pMHC]. The purpose of this review is to highlight classical and recent findings on how antigen recognition, the degree of TCR stimulation, and intracellular signal transduction pathways impact the formation of effector and memory T cells.
Keywords: T cell differentiation, Effector and memory T cells, T cell signaling, Strength of the TCR signal
Introduction
Prior to an infection, naïve mice contain very low numbers of T cells specific to any foreign antigen. Though there is some variation, the frequency of such cells within the total CD8 or CD4 population has been estimated to be about 1 in 100,000 cells and this seems to hold true for both murine and human T cells [1–6]. After a viral or bacterial infection those rare antigen-specific T cells become activated and follow very typical response patterns. These consist of an expansion phase which lasts about 7–8 days in mice [7, 8] and about 14 days in human [9]. In this phase, antigen-specific T cells massively expand, and a single naïve T cell can undergo more than 15 consecutive divisions, and over time, one cell can generate more than 50,000 descendants [2]. Concomitant with their expansion, T cells differentiate into effector cells. The expansion phase is followed by a contraction phase during which the majority of antigen-specific T cells undergo apoptotic cell death, although a fraction of the antigen-specific T cells move on and differentiate into memory T cells.
How long does a T cell need to be stimulated by antigen?
In secondary lymphoid organs, naïve or memory T cells screen the pMHC complexes that are presented by DC. In the absence of infection, DC present only self-peptide MHC complexes, and the TCRs on the vast majority of T cells will very weakly interact with these complexes. Live cell in vivo imaging studies revealed that in this situation the DC T cell contacts are very brief, and the T cells are highly motile and quickly move from one DC to another [10, 11]. In contrast, when a DC presents a foreign-peptide MHC complex, then those T cells expressing a TCR which has a sufficient affinity for foreign pMHC (see below for details) become selectively less motile and interact for longer periods of time with the DC [11] compared to other T cells which lack specificity for the foreign pMHC. During these longer periods of interaction, the T cells are thought to be instructed to undergo proliferation and differentiation.
An important question has been how long a T cell needs to interact with a foreign antigen-presenting DC and, more precisely, how long it needs to be stimulated by the antigen in order undergo differentiation into effector and memory T cells. In vitro experiments, where cells were first exposed for a defined amount of time to antigen and then separated from the antigenic stimulus, showed that approximately 2 h of antigen exposure is sufficient to activate T cells and that the cells subsequently divide and differentiate in the absence of antigen. This observation led to the concept that a short duration of antigen exposure leads to the activation of a differentiation program that even in the absence of further antigen exposure controls T cell differentiation [12, 13]. Using the same in vitro T cell activation system, but combined with a subsequent in vivo transfer of the cells, showed that programmed T cell expansion can also occur in vivo. However, in this setup, the cells needed to be stimulated for 4–20 h and thus somewhat longer compared to continuous in vitro conditions [14].
Nevertheless, the question remained how far the programmed expansion concept is applicable to T cell differentiation occurring entirely in vivo and during a real infection where antigen presentation can persist for long periods of time [15, 16]. In vivo imaging studies using peptide-loaded DC and transgenic T cells indicate that T cell DC interactions last about 12–24 h. Afterwards, T cells dissociate from the APC and begin to proliferate [10, 17]. This behavior is well in line with the concept of programmed T cell differentiation. However, in order to formally prove that T cell programming occurs in vivo, it required a system where antigen presentation can be terminated at any given time. An approach for controlling antigen presentation in vivo is to induce T cell activation through antigen-loaded dendritic cells that transgenically express a high affinity diphtheria toxin receptor. Since mice normally lack this receptor, diphtheria toxin injection can be used to selectively deplete antigen-presenting DC. Using this system, it was interestingly observed that longer periods of presentation do not alter the differentiation of T cells but impact the numbers of effector and memory T cells that arise after the stimulation [18]. While these observations go along with the idea of programmed expansion, they also reveal that the clonal burst size is not a programmed event and that it is dependent on further antigen recognition during the T cell expansion phase. Similar observations were subsequently made in a number of systems: ablating DC at later time points during an influenza infection [19], injecting Listeria monocytogenes-infected mice at day 3 with an antibody that blocks pMHC recognition by T cells [20], and experimental reduction of virus burden at defined time points post-infection [21]. Moreover, when naïve T cells are activated in vitro and then transferred into acutely infected hosts that either present a cognate antigen for these T cells or not, then T cells show much stronger expansion in the presence of antigen [22]. Taken together, shortening antigen presentation seems to have little to no impact on the functional differentiation of effector and memory T cells, suggesting that the sole differentiation of T cells is indeed driven by a differentiation program initiated at very early time points during an infection. Nevertheless, optimum T cell expansion seems to require persistence of antigen through the T cell expansion phase.
What remains ill defined is which types of antigen-presenting cell (APC) promote or impact the clonal burst size of the T cell population. Is this a function of the same type of DC that initiates T cell priming or does it involve other types of DC or even non-professional APC? Investigating T cell migration kinetics in the spleen in response to bacterial infection revealed that, after an initial wave of proliferation, some T cells can again be found clustered with DC [20]; this DC–T cell re-association might boost the T cell responses. T cell expansion can also be boosted upon peripheral antigen exposure, and here tissue-resident DC and other types of APC might be involved. In the lungs, it has been shown that resident DC enhance T cell effector function [23], but this could be due to enhanced T cell expansion [24].
Interestingly, CD4 T cells appear to significantly differ from CD8 T cells when it comes to their activation and differentiation requirements. It has been shown that longer periods of antigen presentation are needed to activate CD4 T cells and that shortening antigen presentation interferes with T cell differentiation and T cell trafficking to peripheral tissues [25, 26]. Moreover, while adoptive transfer of TCR transgenic CD8 T cells increases the precursor frequency, this does not prevent the generation of memory T cells during an infection [27]. On the other hand, the same procedure performed with CD4 transgenic T cells strongly impacts T cell differentiation and prevented the formation of memory [28]. Similar results were obtained when injecting low doses of antibodies that block pMHC and TCR interaction [29]. In addition, the generation of CD8 effector T cells seems inevitably linked to the subsequent emergence of memory T cells. In contrast, with CD4 T cells, it has been observed that the generation of functional effector T cells during an infection sometimes does not lead to the generation of T cell memory [25, 26]. These observations highlight clear differences between CD4 and CD8 T cells in terms of programmed effector and memory T cell differentiation.
Impact of the strength (quality) of the stimulus:
Flexibility of T cells to respond to different qualities of pMHC
T cells are equipped with an antigen receptor and a signaling apparatus that show an astonishing flexibility and precision when recognizing pMHC ligands. The ability of T cells to respond to pMHC that differ greatly in their strength of interaction with the TCR is best illustrated by the processes that take place during positive and negative selection in the thymus. Insight into thymic selection processes can be obtained by performing so called fetal thymic organ cultures (see Fetal thymic organ culture (FTOC)). Here, thymi harvested from TCR transgenic mice are cultured in the presence of different peptide ligands which are examined for their impact on T cell development. As explained in Fetal thymic organ culture (FTOC) and Altered peptide ligands (APL) these cultures are normally performed either with the natural “agonist” peptide corresponding to the transgenic T cells or with so called altered peptide ligands (APL) which are variants of the original peptide but which provide a lower level of stimulation to the transgenic T cells (see Altered peptide ligands (APL)). Using this method, it turned out that ligands which bind to the OT-1 TCR with a physical strength (see Defining the TCR binding and stimulatory potency of peptide MHC complexes) of 20–60 μM (R4, E1, or G4 APL) support positive selection while tenfold higher affinities (i.e. wild-type N4 ligand) have been shown to lead to negative selection in the thymus [30]. While a tenfold difference may not sound all that much at the first glance, the biological magnitude of these differences is enormous, i.e. a tenfold different affinity roughly corresponds to a 1,000-fold higher peptide concentration in functional avidity assays (see Defining the TCR binding and stimulatory potency of peptide MHC complexes). Thus, T cell selection in the thymus strongly underlines the ability of T cells to efficiently discriminate differences in the affinity of interaction between pMHC and the TCR and respond accordingly. More recent studies have defined the TCR affinity where negative selection is initiated [31]. In the presence of CD8 binding, TCRs at the negative selection threshold bind their pMHC antigens with a K D ~6 μM and have a half-life of ~2 s. The mechanism for the initiation of negative selection has been proposed to involve a TCR/co-receptor zipper [32].
In contrast to thymic selection, peripheral T cell activation was thought to require stronger TCR stimulation and high affinity pMHC and TCR interaction. This view was mainly supported by the observation that following an infection the bulk population of effector or memory T cells responds with high functional avidity (see Defining the TCR binding and stimulatory potency of peptide MHC complexes) to their antigen [33]. The assumption that strong signals are required for peripheral T cell activation was challenged by observations that, even in the absence of foreign antigen, meaning in the absence of pMHC that strongly bind to the TCR, T cells slowly proliferate and over time can obtain memory like phenotypes. This phenomenon has been termed homeostatic proliferation (HP) and plays a role in peripheral maintenance of T cells [34]. HP is thought to happen at all times, but it is most prominent when cells are in a lymphopenic host. The evidence that weak TCR pMCH interactions are the driving force behind HP was obtained in mice that present only a single pMHC that contains the very weak OT-1 APL R4. This monospecific pMHC situation was achieved by using TAP-deficient mice which fail to present peptides derived from intracellular proteins. R4 presentation in these mice was selectively restored upon using a construct that enables R4 translocation into the endoplasmic reticulum independently of TAP. Despite the weak TCR binding properties of R4 to the OT-1 TCR, this epitope nonetheless restored the ability to induce homeostatic OT-1 T cell proliferation [35]. As R4 also positively selects OT-1 in FTOC systems [30], it was furthermore concluded that both processes are driven by similar pMHC affinities.
Interestingly, T cells that underwent HP showed typical phenotypic features of memory T cells such as expressing high levels of CD44 or being able to more rapidly secrete IFN-γ or granzymes and perforin in response to TCR stimulation. In line with this, it was subsequently demonstrated that T cells, which underwent strong HP, show similar ability to protect mice from lethal pathogen challenges as conventional memory T cells [36]. This observation lead to the introduction of the term HP memory T cell. Interestingly, one study even found that, among the rare antigen-specific T cells found in the naïve T cell population, a fraction of these antigen-specific T cells show an HP memory phenotype [37]. Taken together, all these data indicate that even the weakest TCR ligand can in principle support memory T cell differentiation. Moreover, it also shows that peripheral T cells likely retain the ability to respond to those ligands by which they were positively selected in the thymus.
Background information
- Fetal thymic organ culture (FTOC)
This is an elegant system for studying T cell differentiation in the thymus [38]. Such cultures are performed with thymi harvested from day 15 embryos. These can be thymi taken from normal mice, but they are often obtained from TCR transgenic mice. For studying the ability of different APL (see Altered peptide ligands (APL)) to induce positive and negative selection very often OT-1 transgenic and TAP- or b2m-deficient donor mice are used. Any of the two deficiencies ensures that the thymus will not present endogenous peptides to the OT-1 and therefore T cell development is blocked at the double positive stage prior to positive selection. Upon adding soluble synthetic peptides (and β2m to β2m-deficient thymi), one can create a thymus that only presents a defined synthetic peptide. These cultures are used to determine whether a specific peptide causes positive or negative selection [48].
- Altered peptide ligands (APL)
Studies on how differences in the strength of pMHC and TCR interaction impact T cell responses are inevitably linked to TCR transgenic T cells and so-called altered peptide ligands. APL are ligands that differ by at least one amino acid from the original ligand against which a transgenic T cell was raised, and these substitutions impact the binding affinity of the corresponding pMHC to the TCR of the transgenic T cell. In cases of OT-1 and their natural H-2 Kb restricted ligand, SIINFEKL, one can for instance replace the amino acids at position 1 (S against E) or at position 4 (N against R or G) and thereby create ligands that only very weakly bind to the TCR of OT-1 T cells.
- Defining the TCR binding and stimulatory potency of peptide MHC complexes
-
T cells translate a molecular event, the binding of pMHC complexes to a TCR and its coreceptor, into a cellular response. In line with this, there are both bio-physical and biological parameters that can be used to describe how well the TCR or the whole cell responds to pMHC. The bio-physical parameters are TCR/pMHC affinity, on-and off-rates, and half-life times of the complex, all of which are terms commonly used to describe kinetic aspects of monomeric interactions between two molecules. These parameters can for instance be measured by surface plasmon resonance [39]. While these parameters give the most precise assessment of the ability of the TCR to bind to pMHC, it requires substantial effort and the availability of both soluble pMHC and TCR to measure them. A more practical but less precise assessment of the physical strength of pMHC and TCR interaction is to use soluble peptide MHC-tetrameric molecules and to measure binding of those molecules to the surface TCR of T cells. In analogy to the biochemical term avidity, which is normally used to describe the strength of multimeric receptor ligand interactions, these measurements are usually referred to as pMHC and TCR avidity [40, 41].
Besides the physical strength of pMHC and TCR interaction, there are a number of other factors that can influence the ability of a T cell to respond to pMHC. Moreover, T cells can differ in their ability to translate the signals received though the TCR into a cellular response [42, 43]. It is therefore also important to determine the biological activity of a given pMHC. This can be done by testing the ability of a T cell to respond to different amounts of pMHC ligands [40]. This measure is usually referred to as functional avidity (though it is also often abbreviated as avidity) and it can also be seen as a measure of antigen sensitivity of a T cell [40]. Importantly, functional avidity measurements correlate well in most cases with the physical parameters.
A broad range of pMHC TCR affinities support effector and memory differentiation during an immune response
The naïve T cell repertoire is enormously diverse and contains T cells that respond to foreign antigen with a range of high and low affinities. The population of antigen-specific T cells that forms during an infection appears to be only composed of high affinity T cells and this discrepancy raised the question what happens to the low affinity T cells during an infection?
Different observations indicate that even suboptimal levels of TCR stimulation can support effector and memory T cell differentiation. For instance, in autoimmune models where high affinity T cells have been eliminated by thymic or peripheral tolerance, lower affinity effector T cells can be detected that respond to antigen stimulation [44, 45]. Similarly, vaccines that contain so-called tumor-associated antigens usually induce lower affinity effector and memory T cells. Moreover, even T cells with mutation in the TCR signaling apparatus, which results in less potent activation (see below), can sometimes give rise to effector and memory T cells.
The most direct way to address the question of how differences in the level of TCR stimulation impact T cell responses during an infection is to use pathogens that encode APLs for TCR transgenic T cells (see Defining the TCR binding and stimulatory potency of peptide MHC complexes). Such has for instance been done using P14 TCR transgenic T cells and spontaneous mutants derived from lymphocytic choriomeningitis virus (LCMV) that contain APL for the P14 T cells. These studies showed that a broader range of pMHC and TCR affinity induces the activation of P14 cells [46], but it remained unclear why low affinity T cells cannot be detected in a polyclonal response after a pathogen infection. In a more recent study, Listeria monocytogenes strains that stably express ovalbumin containing APL for the OT-1 T cells were used [33]. Using this setup, it could be clearly demonstrated that very low potency ligands support T cell differentiation. Those even included ligands that failed to negatively select OT-1 thymocytes in FTOC systems—a notion that will be discussed in more detail below. Despite their striking affinity differences, high or very low affinity ligands induced similar initial T cell responses, i.e., in all cases, the cells went at least through 7–9 divisions and this occurred at a comparable pace with an estimated division time of 4–6 h. Only after this initial period did low and high affinity stimulated T cells begin to respond differently, in that more strongly stimulated T cells terminally accumulated at much larger numbers, proliferated longer, and began to decline in numbers later than T cells stimulated by low affinity ligands. All in all, these studies show a direct correlation between the pMHC TCR affinity and the number of divisions and extent of T cell accumulation. Most importantly, even tiny differences in the functional avidity lead to significantly different T cell numbers [33].
The different times spent in the expansion phase after high and low affinity antigen stimulation is also the reason why in a polyclonal repertoire the population of antigen-specific T cells appears to be entirely composed of high affinity T cells. High affinity T cells simply outnumber the low affinity T cells so much that we normally fail to detect them when analyzing the T cell response at the peak of expansion or at any later time point. However, when looking at polyclonal T cells at 4.5 days post-infection, low affinity T cells can be detected [33].
Rather surprisingly, major phenotypic differences between high and low affinity stimulated T cells were not observed. They all showed a typical effector signature, were CD44 high, CD62L low, expressed granzyme B, INFγ, and many also TNFα. Moreover, even very low affinity primed T cells mounted a cytotoxic response [33] and very low affinity pMHC TCR interaction support the clearance of Listeria monocytogenes (D.Z. and M.J.B., unpublished observation). Finally, no matter what type of TCR stimulation the T cell had received, they all become memory T cells and those were equally competent in mounting a secondary response. These observations led to the conclusion that very low levels of TCR stimulation are sufficient to fully differentiate T cells but fail to generate large numbers of effector and memory T cells [33].
Nonetheless, if the conclusions drawn from experiments using TCR transgenic OT-1 T cells are correct, then lower affinity effector and subsequently lower affinity memory T cells should be detectable after an infection. Indeed, using a heterologous prime/challenge setup where a Lm-N4 infection (high affinity wild-type SIINFEKL ligand) is followed by an Lm-V4 infections (low affinity APL ligand), we could detect such cells. When mice are primarily infected with Lm-N4, less than 20 % of the N4-specific T cells cross-react with the V4 epitope. When mice are first infected with Lm-N4 and later with Lm-V4, more than 50 % of the cells in the secondary infection respond to both peptides. The elevation in the numbers of N4/V4 cross-reactive T cells indicates that many of these cells are derived from memory T cells generated during the earlier Lm-N4 infection. The important detail in these experiments is that the N4/V4 cross-reactive T cells strongly react to V4 but only weakly to N4. Thus, the majority of the cross-reactive T cells are descendents of memory T cell clones that were primed by low affinity N4 stimulation [47].
For OT-1 TCR transgenic T cells, a very large number of APLs have been identified and well characterized in terms of their affinity for the OT-1 TCR and their ability to mediate positive or negative selection in FTOC systems. The border between these two categories is marked by the T4 APL which, depending on the amount of presented peptide, can either support positive or negative selection. Thus, APL with lower functional avidity than T4, i.e. Q4H7, V4, E1, or G4, induce only positive selection, and any stronger ligand, such as Q4R7, Q4, A2, or wild-type SIINFEKL, induce negative selection [48]. In contrast to their ability to stimulate positive but not negative selection in FTOC, V4, Q4H7, and T4 induced the above-described phenotypic and functionally complete differentiation of effector and memory T cells. In a Listeria infection, only the lowest affinity ligands such as E1 failed to expand OT-1 (D.Z. and M.J.B., unpublished observations). These data clearly indicate that there is a difference between the thresholds for negative selection and induction of OT-1 proliferation in the periphery. These observations are also well in line with reports showing that cells with low functional avidity escape negative selection but can be activated in the periphery and harbor the potential to cause autoimmunity [44, 45].
Number of TCR and pMHC complexes (quantity) needed for T cell activation
Following a pathogen challenge, type I and II interferons strongly upregulate MHC expression on many types of cells. While an infected cell will present virus-derived proteins fragments, many of the surface MHC are even in this situation loaded with peptides derived from self-proteins [49]. Thus, during an infection, DC will present a mixture of self- and viral peptides. An important aspect is how many copies of a distinct pathogen-derived peptide are presented by MHC molecules during an infection, and a question related to that is how many are needed to activate a naïve T cell?
While it is still very difficult and often still impossible to precisely determine the epitope density of a distinct pMHC, a few examples have been provided where numbers of specific pMHC on the cell surface could be elucidated. In 1996, a first study pointed out that even a single pMHC might be sufficient for inducing effector activation [50]. However, the conclusions in this study were drawn without direct proof that an APC presenting only a single specific pMHC can activate T cells. Such proof was provided in a later study where highly sensitive imaging techniques were used to detect low numbers of fluorescently labeled MHC bound peptides. With this approach, it was directly demonstrated that 1–3 pMHC are sufficient for triggering effector T cell functions and 10 pMHC for fully activating T cells [51–53]. That different numbers of pMHC are needed to induce different T cell functions is also supported by other circumstantial evidence. It is well known that, when T cells are exposed to titrated doses of peptides, higher concentrations of peptide are needed for inducing proliferation and cytokine secretion whereby cytotoxicity can be induced using slightly lower concentrations [54]. Despite the principal demonstration that a few pMHC can induce T cell activation in vitro, it is not known whether such low numbers are sufficient for T cell activation in vivo.
Do self-pMHC contribute to T cell activation?
It is well established that peripheral T cells weakly interact with self-pMHC complexes and that those weak engagements provide a survival signal for naïve peripheral T cells. Considering that an antigen-presenting cell even during an infection will present a large number of self-peptide MHC, an interesting question is whether these contribute to T cell activation.
In an early attempt to answer this question, RMA-S cells were used which, unlike their parental cell line RMA, are TAP-deficient and therefore largely lack surface MHC. Both types of cells can be loaded with synthetic H-2Kb binding peptidesm and the number of resulting pMHC can be measured by the 16-D1.25 antibody [54]. Interestingly, RMA-S and RMA cells that present similar numbers of pMHC can stimulate OT-1 T cells equally well and irrespective of the presence or absence of self-pMHC [55]. The same question was subsequently investigated using a very sophisticated setup. Here, recombinant MHC molecules were loaded either with a cognate peptide that efficiently stimulates TCR transgenic T cells or with a self-peptide that is weakly recognized by the same TCR. In this setup, monomeric peptide MHC molecules failed to activate the transgenic T cells while, as expected, dimeric peptide MHC molecules carrying two cognate peptides efficiently activated the T cells. Very surprisingly, even hetero-dimers composed of the cognate and the self-peptide efficiently stimulated the T cell response [56]. These studies clearly contrast with the RMA work, but a possible explanation for this discrepancy is that both RMA and RMA-S cells may present such a large number of specific pMHC that, irrespective of the presence or absence of self-pMHC, there are simply enough cognate pMHC on the surface to activate the T cells. It should also be noted that RMA-S are not completely free from self-pMHC and that they still present some pMHC loaded with peptide despite the absence of functional TAP molecules. In any case, the heterodimer observations strongly suggest that self-pMHC augment T cell activation. Other studies show a similar supporting effect of self-pMHC [57, 58], and it has been observed that, when T cells are deprived from pMHC contacts, they become less sensitive to stimulation [59].
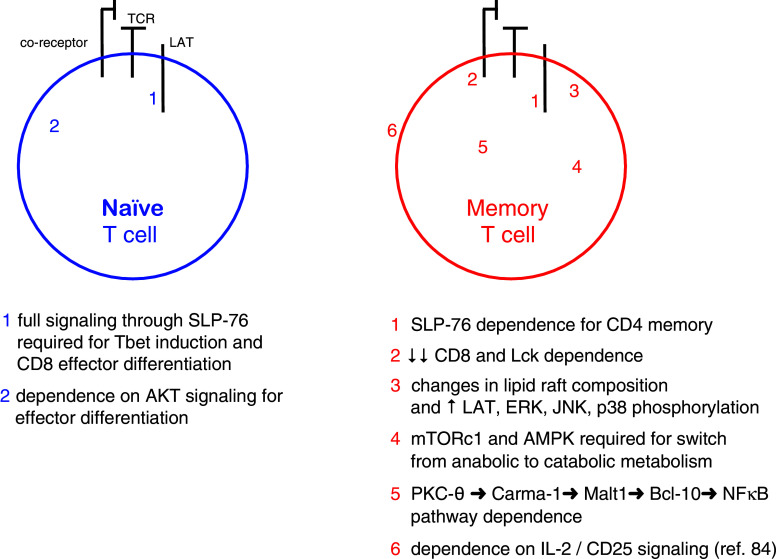
Signaling pathways in memory T cells
The difference in signaling efficiency between naïve and memory T cells could originate at multiple points along the TCR-driven signaling pathways as discussed below and shown in Fig. 1. Memory T cells are generated during or following the primary response, and there have been numerous studies which have examined the kind of TCR signals that are required for generating T cell memory. However, a second issue is to clarify whether fully developed memory T cells utilize distinct signaling pathways compared to those used by naïve T cells. Although these two problems are not always discussed separately, some of the work in this area is presented below.
Fig. 1.
Differences between naïve and memory T cells
The difference in signaling efficiency between naïve and memory T cells could originate at multiple points along the TCR-driven signaling pathways. Using mice with inducible Lck expression, Tewari et al. [60] showed that unlike in a primary response, Lck was dispensable for the induction of a memory CD8 T cell response. The authors speculate that the Gα11-dependent, phospholipase C-β-mediated pathway may compensate for the absence of Lck expression as is the case with TCR signaling driven by bacterial super antigens. Another possibility is that memory T cells express more phosphoproteins in their lipid rafts compared to their naïve counterparts, and this provides a primed state, which compensates for the lack of Lck activity in LckOFF mice. Given the fact that much of the Lck pool is CD8-associated, it is not surprising that memory CD8 T cells are also less dependent on CD8 co-receptor function.
Using a novel approach, Au-Yeung et al. [61] engineered a mutant ZAP-70 gene, whose gene product could be inhibited by a small molecular weight inhibitor, 3-MB-PP1. By combining the genetically modified mouse strain and the inhibitor, the authors could block ZAP-70 kinase activity during various points during an immune response. In contrast to the results with Lck (see above), the activation of memory T cells requires ZAP-70 kinase activity. One caveat is that given the inhibitor’s short half-life in vivo, the inhibitor’s effect on memory T cells was assayed ex vivo. Nevertheless, if ZAP-70 but not Lck kinase activity is required to activate memory T cells, the implication is that ZAP-70 is activated by a kinase other than Lck. Whether this is Fyn or whether ZAP-70 is activated by an alternative mechanism is not yet known.
SLP-76 is an important phosphoprotein that nucleates signaling complexes required for the propagation of TCR signals [62]. Using mice, which can express mutations in the SLP-76 gene in an inducible manner, Smith-Garvin et al. [63] could show that the Y145F, and to a lesser extent the Y112F/Y118F double mutation, dampened TCR signals and that this favors the development of memory T cells at the expense of terminally differentiated effector cell. This may reflect the normal development of T cell memory, which begins following antigen clearance when T cells no longer encounter antigen-bearing APCs. T cells expressing mutant SLP-76 proteins may not be able to respond to lower levels of antigen as well as wild-type T cells and for this reason may enter the memory differentiation program at an earlier point. Given the importance of the transcription factor Tbet in driving effector T cell differentiation, attenuating SLP-76 signaling may operate by reducing Tbet expression through the SLP-76 → mTOR → Tbet pathway or alternatively a SLP-76 → ITK → Tbet pathway. Interestingly, CD8 T cell memory can be generated from T cells expressing mutant SLP-76 even when the same cells are unable to express normal amounts of inflammatory cytokines. A similar study also showed that the persistence of memory T cells was independent of SLP-76 [64].
Bushar et al. [65] examined the role of SLP-76 signaling in establishing CD4 memory T cells. In contrast to its negative role in the development of CD8 memory T cells, SLP-76 has a positive function in the development of CD4 memory T cells. SLP-76 deficiency in memory CD4 T cells inhibited expression of recall cytokines and decreased memory T cell persistence in vivo. Furthermore, SLP-76 deficiency reduced the steady state homeostasis and expansion of CD4 memory T cells despite the presence of intact IL-7 signaling. These data argue that the survival of CD4 memory T cells depends on TCR stimulation and intact SLP-76-dependent signaling pathways. Why CD4 memory T cells are more dependent on TCR and SLP-76 signaling compared to their CD8 counterparts is not yet clear.
Looking further downstream, D’Souza et al. [66] examined the effects of ERK deficiency on CD8 T cell activation, proliferation, and survival. While ERK-1 seems dispensable, the absence of ERK-2 had serious consequences for CD8 T cells. Surprisingly, ERK-2 deficiency does not affect CD8 T cell proliferation but limits clonal burst size by limiting T cell survival. This is likely mediated by decreased Bcl-2 and Bcl-XL and increased Bim expression. The observation that Bim deficiency rescues this survival defect supports this idea. As ERK phosphorylates the FoxO3 transcription factor leading to its degradation and Bim transcription is dependent on FoxO3, this is a potential mechanism of how ERK activity can inhibit Bim expression and promote CD8 T cell survival [66, 67]. A similar phenotype has been observed in PKC-θ-deficient [68] and RasGRP1-deficient [69] T cells (normal proliferation but poor survival). As PKC-θ and RasGRP1 activity each contributes to ERK activation, deficiency of either of these upstream ERK activators may similarly lead to increased Bim expression and decreased CD8 T cell survival. It should be pointed out that these studies did not directly examine memory responses, but rather survival of T cells, during the primary response. It is likely that the efficiency of T cell survival during the contraction phase of the primary response affects the number of cells, which enter the memory pool.
Arbour et al. [70] studied anti-viral responses in JNK1- and JNK2-deficient mice and observed divergent roles for these two related map kinases. Although both types of knockout mice could clear LCMV infections, antigen-specific CD8 T cells expanded poorly in JNK1-deficient animals. This was due to an increase in apoptosis of the expanding T cell population. Nevertheless, the surviving T cells expressed IFN-γ. Interestingly, memory responses to LCMV were equivalent in JNK1-deficient and wild-type mice. CD8 memory T cells may not require JNK1, and T cells surviving the primary response were selected for their JNK1 independence. In contrast, JNK2 knockout mice displayed an increased expansion of antigen-specific CD8 T cells compared to JNK2-sufficient animals. The mechanism underlying these striking differences has not yet been elucidated. Given that JNK isoforms are involved in TCR and co-stimulatory signaling pathways, it has been difficult to pin down where the JNK proteins function during the anti-viral responses. One attractive candidate for JNK1 is the co-stimulatory molecule, 4-1BB, since the 4-1BB ligand and JNK1 knockouts have similar phenotypes. The authors suggest that JNK1 may be required for transducing signals from the 4-1BB receptor.
Kersh et al. [71] studied a number of signaling components in CD8 memory T cells and observed that, despite an equivalent ability to phosphorylate CD3ζ and ZAP-70, they more efficiently phosphorylate LAT, ERK, JNK, and p38 compared to naïve and effector T cells. They linked these differences to the presence of more lipid rafts containing increased amounts of asialo-GM1 and a higher content of phosphor proteins, including LAT. As asialo-GM1 contains less negatively charged sialic acid, it is possible that this difference allows for the formation of more tightly packed clusters of lipid rafts in CD8 memory T cells. CD8 memory T cells also phosphorylated LAT more efficiently upon antigen stimulation compared to their naïve and effector counterparts. The authors suggest that these changes account for the rapid induction of TCR signaling observed in CD8 memory T cells.
NFκB signaling is important in T cell responses, and its role has been examined in the generation of T cell memory. Members of the NFκB transcription factor family are kept inactive by binding to members of the IκB family including IκBα, IκBβ, and IκBε. IKK1 and IKK2 are kinases, which phosphorylate IκB proteins leading to their ubiquitination and subsequent degradation by the 26S proteasome. This releases the NFκB allowing its translocation to the nucleus to fulfill its role as a transcription factor. Using a T cell-specific deletional approach, Schmidt-Supprian et al. found that IKK2 was not required for the survival of naïve peripheral T cells, but was essential for the generation of CD4 memory and regulatory T cells [72, 73]. A similar dependence on NFκB signaling for the generation of CD8 memory T cells was seen in mice expressing a dominant negative form of IκBα [74].
Along this line, Teixeiro et al. [75] described a mutant TCR, which supported primary CD8 T cell responses, but failed to generate a memory response. The mutation was located within the CART motif of the TCRβ chain; this is a highly conserved transmembrane sequence present within all vertebrate B and T cell receptor genes. The mutant TCR displays two obvious defects: it co-localizes poorly in the synapse and it only weakly activates NFκB signaling. Given the mutant receptor’s inability to be recruited to the synapse, it is likely that the PKC-θ → Carma-1 → Malt1 → Bcl-10 → IKK2 pathway is poorly activated, leading to inefficient and delayed NFκB activation. It is intriguing that the conserved IKK2-driven signaling pathway has relatively little impact on primary responses, but is critical for generating long-lived memory T lymphocytes.
mTOR has also been shown to have a pivotal role in the development of CD8 T cell memory. Pearce et al. [76] noticed that TRAF6-deficient T cells are unable to generate a memory response. An analysis of TRAF6 knockout T cells revealed that they were defective in activating AMP-activated kinase and were altered in mitochondrial fatty acid oxidation. Following these data, the authors treated mice harboring TRAF6-deficient T cells with metformin or rapamycin, which are known to affect cellular metabolism. Treatment with either of these inhibitors was able to restore the memory response from TRAF6-deficient T cells. Independently, Araki et al. [77] found that rapamycin increased the number of CD8 memory T cells in normal mice, which was due to its inhibition of mTOR within the mTORc1 complex. Using an RNAi knockdown approach, these authors were able to show that mTORc1 regulates the development of T cell memory. Both mTOR and AMPK regulate cell growth by controlling how the T cell produces energy. Following antigen stimulation, the T cell switches from catabolic metabolism (oxidative phosphorylation via fatty acid metabolism) to anabolic metabolism (via glycolysis) [78]. To generate memory T cells, mTOR and AMPK are involved in switching back from anabolic to catabolic metabolism. It is still not clear how changing the mode of ATP production (via catabolic metabolism) results in development of a memory phenotype. More work will likely clarify the relationship between metabolism and the establishment of T cell memory.
TCR signals have also been shown to synergize with IL-2 receptor and CD28 to activate the PI3K-dependent kinase, Akt. Although Akt has been traditionally linked with the regulation of T cell metabolism [79], recent work by Macintyre et al. [80] demonstrated normal glucose uptake and survival by Akt-inhibited T cells. Instead, Akt-mediated signals downstream from TCR and IL-2 receptor appear to control effector T cell differentiation at the expense of memory T cell generation. Microarray analysis of gene expression in Akt-inhibited T cells revealed increased expression of memory-associated genes including IL-7R, CCR7, and CD62L while effector-associated gene expression, including IFN-γ, granzyme B, and perforin, were reduced. The authors went on to show that Akt-mediated inhibition of FoxO3a-regulated gene expression is an important factor driving effector T cell differentiation. Although this work does not distinguish the independent contribution of TCR to effector T cell development, it supports a terminal differentiation model where strong/sustained signals promote full effector differentiation while weak/aborted signals promote memory T cell generation.
It is not surprising that some of the signaling pathways may be differently utilized in memory and effector T cells since these cells have different physiological roles. Memory T cells have to survive for long periods of time in the absence of cognate antigen stimulation, but must be able to quickly develop full effector function upon re-exposure to the priming antigen. The issue of how a naïve T cell develops into both effector and memory lineage T cells is a fascinating problem, which likely has parallels to gene expression programs in other differentiating systems. The elucidation of these events is ongoing, but there are clearly many unanswered questions surrounding this complex immunological problem.
References
- 1.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 2.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 3.Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geiger R, Duhen T, Lanzavecchia A, Sallusto F. Human naive and memory CD4+ T cell repertoires specific for naturally processed antigens analyzed using libraries of amplified T cells. J Exp Med. 2009;206:1525–1534. doi: 10.1084/jem.20090504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/S1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 8.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/S1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, Murali-Krishna K, Mahar PL, Edupuganti S, Lalor S, Germon S, Del Rio C, Mulligan MJ, Staprans SI, Altman JD, Feinberg MB, Ahmed R. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 11.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 12.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 13.Masopust D, Kaech SM, Wherry EJ, Ahmed R. The role of programming in memory T-cell development. Curr Opin Immunol. 2004;16:217–225. doi: 10.1016/j.coi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 14.van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 15.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner DL, Cauley LS, Khanna KM, Lefrancois L. Persistent antigen presentation after acute vesicular stomatitis virus infection. J Virol. 2007;81:2039–2046. doi: 10.1128/JVI.02167-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henrickson SE, Mempel TR, Mazo IB, Liu B, Artyomov MN, Zheng H, Peixoto A, Flynn MP, Senman B, Junt T, Wong HC, Chakraborty AK, von Andrian UH. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008;9:282–291. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolfi DV, Duttagupta PA, Boesteanu AC, Mueller YM, Oliai CH, Borowski AB, Katsikis PD. Dendritic cells and CD28 costimulation are required to sustain virus-specific CD8+ T cell responses during the effector phase in vivo. J Immunol. 2011;186:4599–4608. doi: 10.4049/jimmunol.1001972. [DOI] [PubMed] [Google Scholar]
- 20.Khanna KM, McNamara JT, Lefrancois L. In situ imaging of the endogenous CD8 T cell response to infection. Science. 2007;318:116–120. doi: 10.1126/science.1146291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stock AT, Mueller SN, van Lint AL, Heath WR, Carbone FR. Cutting edge: prolonged antigen presentation after herpes simplex virus-1 skin infection. J Immunol. 2004;173:2241–2244. doi: 10.4049/jimmunol.173.4.2241. [DOI] [PubMed] [Google Scholar]
- 22.Shaulov A, Murali-Krishna K. CD8 T cell expansion and memory differentiation are facilitated by simultaneous and sustained exposure to antigenic and inflammatory milieu. J Immunol. 2008;180:1131–1138. doi: 10.4049/jimmunol.180.2.1131. [DOI] [PubMed] [Google Scholar]
- 23.McGill J, Van Rooijen N, Legge KL. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med. 2008;205:1635–1646. doi: 10.1084/jem.20080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGill J, Legge KL. Cutting edge: contribution of lung-resident T cell proliferation to the overall magnitude of the antigen-specific CD8 T cell response in the lungs following murine influenza virus infection. J Immunol. 2009;183:4177–4181. doi: 10.4049/jimmunol.0901109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams MA, Bevan MJ. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- 27.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foulds KE, Shen H. Clonal competition inhibits the proliferation and differentiation of adoptively transferred TCR transgenic CD4 T cells in response to infection. Journal of immunology. 2006;176:3037–3043. doi: 10.4049/jimmunol.176.5.3037. [DOI] [PubMed] [Google Scholar]
- 29.Blair DA, Lefrancois L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc Nat Acad Sci USA. 2007;104:15045–15050. doi: 10.1073/pnas.0703767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NR. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 31.Naeher D, Daniels MA, Hausmann B, Guillaume P, Luescher I, Palmer E. A constant affinity threshold for T cell tolerance. J Exp Med. 2007;204:2553–2559. doi: 10.1084/jem.20070254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer E, Naeher D. Affinity threshold for thymic selection through a T-cell receptor-co-receptor zipper. Nat Rev Immunol. 2009;9:207–213. doi: 10.1038/nri2469. [DOI] [PubMed] [Google Scholar]
- 33.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 35.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–190. doi: 10.1016/S1074-7613(00)80093-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 37.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 39.Wu LC, Tuot DS, Lyons DS, Garcia KC, Davis MM. Two-step binding mechanism for T-cell receptor recognition of peptide MHC. Nature. 2002;418:552–556. doi: 10.1038/nature00920. [DOI] [PubMed] [Google Scholar]
- 40.Stone JD, Chervin AS, Kranz DM. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology. 2009;126:165–176. doi: 10.1111/j.1365-2567.2008.03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang XL, Altman JD. Caveats in the design of MHC class I tetramer/antigen-specific T lymphocytes dissociation assays. J Immunol Methods. 2003;280:25–35. doi: 10.1016/S0022-1759(03)00079-6. [DOI] [PubMed] [Google Scholar]
- 42.Fahmy TM, Bieler JG, Edidin M, Schneck JP. Increased TCR avidity after T cell activation: a mechanism for sensing low-density antigen. Immunity. 2001;14:135–143. [PubMed] [Google Scholar]
- 43.Slifka MK, Whitton JL. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat Immunol. 2001;2:711–717. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- 44.von Herrath MG, Dockter J, Oldstone MB. How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity. 1994;1:231–242. doi: 10.1016/1074-7613(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 45.Zehn D, Bevan MJ. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 2006;25:261–270. doi: 10.1016/j.immuni.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gronski MA, Boulter JM, Moskophidis D, Nguyen LT, Holmberg K, Elford AR, Deenick EK, Kim HO, Penninger JM, Odermatt B, Gallimore A, Gascoigne NR, Ohashi PS. TCR affinity and negative regulation limit autoimmunity. Nat Med. 2004;10:1234–1239. doi: 10.1038/nm1114. [DOI] [PubMed] [Google Scholar]
- 47.Zehn D, Turner MJ, Lefrancois L, Bevan MJ. Lack of original antigenic sin in recall CD8(+) T cell responses. Journal of immunology. 2010;184:6320–6326. doi: 10.4049/jimmunol.1000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NR, Palmer E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 49.Yewdell JW, Reits E, Neefjes J. Making sense of mass destruction: quantitating MHC class I antigen presentation. Nat Rev Immunol. 2003;3:952–961. doi: 10.1038/nri1250. [DOI] [PubMed] [Google Scholar]
- 50.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/S1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 51.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5:524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 52.Huppa JB, Davis MM. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol. 2003;3:973–983. doi: 10.1038/nri1245. [DOI] [PubMed] [Google Scholar]
- 53.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 54.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/S1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 55.Sporri R. Self peptide/MHC class I complexes have a negligible effect on the response of some CD8+ T cells to foreign antigen. Euro J Immunol. 2002;32:3161–70. doi: 10.1002/1521-4141(200211)32:11<3161::AID-IMMU3161>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 56.Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 57.Wulfing C, Sumen C, Sjaastad MD, Wu LC, Dustin ML, Davis MM. Costimulation and endogenous MHC ligands contribute to T cell recognition. Nat Immunol. 2002;3:42–47. doi: 10.1038/ni741. [DOI] [PubMed] [Google Scholar]
- 58.Lo WL, Felix NJ, Walters JJ, Rohrs H, Gross ML, Allen PM. An endogenous peptide positively selects and augments the activation and survival of peripheral CD4+ T cells. Nat Immunol. 2009;10:1155–1161. doi: 10.1038/ni.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 60.Tewari K, Walent J, Svaren J, Zamoyska R, Suresh M. Differential requirement for Lck during primary and memory CD8+ T cell responses. Proc Natl Acad Sci USA. 2006;103:16388–16393. doi: 10.1073/pnas.0602565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Au-Yeung BB, Levin SE, Zhang C, Hsu LY, Cheng DA, Killeen N, Shokat KM, Weiss A. A genetically selective inhibitor demonstrates a function for the kinase Zap70 in regulatory T cells independent of its catalytic activity. Nat Immunol. 2010;11:1085–1092. doi: 10.1038/ni.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jordan MS, Singer AL, Koretzky GA. Adaptors as central mediators of signal transduction in immune cells. Nat Immunol. 2003;4:110–116. doi: 10.1038/ni0203-110. [DOI] [PubMed] [Google Scholar]
- 63.Smith-Garvin JE, Burns JC, Gohil M, Zou T, Kim JS, Maltzman JS, Wherry EJ, Koretzky GA, Jordan MS. T-cell receptor signals direct the composition and function of the memory CD8+ T-cell pool. Blood. 2010;116:5548–5559. doi: 10.1182/blood-2010-06-292748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wiehagen KR, Corbo E, Schmidt M, Shin H, Wherry EJ, Maltzman JS. Loss of tonic T-cell receptor signals alters the generation but not the persistence of CD8+ memory T cells. Blood. 2010;116:5560–5570. doi: 10.1182/blood-2010-06-292458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bushar ND, Corbo E, Schmidt M, Maltzman JS, Farber DL. Ablation of SLP-76 signaling after T cell priming generates memory CD4 T cells impaired in steady-state and cytokine-driven homeostasis. Proc Natl Acad Sci USA. 2010;107:827–831. doi: 10.1073/pnas.0908126107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.D’Souza WN, Chang CF, Fischer AM, Li M, Hedrick SM. The Erk2 MAPK regulates CD8 T cell proliferation and survival. J Immunol. 2008;181:7617–7629. doi: 10.4049/jimmunol.181.11.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hedrick SM. The cunning little vixen: foxo and the cycle of life and death. Nat Immunol. 2009;10:1057–1063. doi: 10.1038/ni.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barouch-Bentov R, Lemmens EE, Hu J, Janssen EM, Droin NM, Song J, Schoenberger SP, Altman A. Protein kinase C-theta is an early survival factor required for differentiation of effector CD8+ T cells. J Immunol. 2005;175:5126–5134. doi: 10.4049/jimmunol.175.8.5126. [DOI] [PubMed] [Google Scholar]
- 69.Priatel JJ, Teh SJ, Dower NA, Stone JC, Teh HS. RasGRP1 transduces low-grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity. 2002;17:617–627. doi: 10.1016/S1074-7613(02)00451-X. [DOI] [PubMed] [Google Scholar]
- 70.Arbour N, Naniche D, Homann D, Davis RJ, Flavell RA, Oldstone MB. c-Jun NH(2)-terminal kinase (JNK)1 and JNK2 signaling pathways have divergent roles in CD8(+) T cell-mediated antiviral immunity. J . J Exp Med. 2002;195:801–810. doi: 10.1084/jem.20011481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kersh EN, Kaech SM, Onami TM, Moran M, Wherry EJ, Miceli MC, Ahmed R. TCR signal transduction in antigen-specific memory CD8 T cells. J Immunol. 2003;170:5455–5463. doi: 10.4049/jimmunol.170.11.5455. [DOI] [PubMed] [Google Scholar]
- 72.Schmidt-Supprian M, Tian J, Ji H, Terhorst C, Bhan AK, Grant EP, Pasparakis M, Casola S, Coyle AJ, Rajewsky K. I kappa B kinase 2 deficiency in T cells leads to defects in priming, B cell help, germinal center reactions, and homeostatic expansion. J Immunol. 2004;173:1612–1619. doi: 10.4049/jimmunol.173.3.1612. [DOI] [PubMed] [Google Scholar]
- 73.Schmidt-Supprian M, Courtois G, Tian J, Coyle AJ, Israel A, Rajewsky K, Pasparakis M. Mature T cells depend on signaling through the IKK complex. Immunity. 2003;19:377–389. doi: 10.1016/S1074-7613(03)00237-1. [DOI] [PubMed] [Google Scholar]
- 74.Hettmann T, Opferman JT, Leiden JM, Ashton-Rickardt PG. A critical role for NF-kappaB transcription factors in the development of CD8+ memory-phenotype T cells. Immunol Lett. 2003;85:297–300. doi: 10.1016/S0165-2478(02)00260-2. [DOI] [PubMed] [Google Scholar]
- 75.Teixeiro E, Daniels MA, Hamilton SE, Schrum AG, Bragado R, Jameson SC, Palmer E. Different T cell receptor signals determine CD8+ memory versus effector development. Science. 2009;323:502–505. doi: 10.1126/science.1163612. [DOI] [PubMed] [Google Scholar]
- 76.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pearce EL. Metabolism in T cell activation and differentiation. Curr Opin Immunol. 2010;22:314–320. doi: 10.1016/j.coi.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Juntilla MM, Koretzky GA. Critical roles of the PI3K/Akt signaling pathway in T cell development. Immunol Lett. 2008;116:104–110. doi: 10.1016/j.imlet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Macintyre AN, Finlay D, Preston G, Sinclair LV, Waugh CM, Tamas P, Feijoo C, Okkenhaug K, Cantrell DA. Protein Kinase B Controls Transcriptional Programs that Direct Cytotoxic T Cell Fate but Is Dispensable for T Cell Metabolism. Immunity. 2011;34:224–236. doi: 10.1016/j.immuni.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]