Abstract
The synthesis of polymer nanoparticles (NPs) with controlled characteristics has become an appealing research topic lately. Nanomedicine, and especially drug delivery and imaging, are fields that require particles of a controlled size and with a tailored arrangement of functional groups. Intramolecular cross-linking or collapse of single polymer chains has emerged as an efficient alternative for the synthesis of well-defined polymer NPs. This technique allows the generation of 1.5–20 nm particles with a wide variety of chemical compositions and functionalities. This review begins by gathering synthetic strategies described in the literature and groups them into four main synthetic methods: homo-functional collapse, hetero-functional collapse, crosslinker-mediated collapse, and one-block collapse of diblock or triblock copolymers. Afterwards, the main characterization techniques and physical properties of single-chain polymer NPs (SCPNs) are exposed. Finally, several applications in nanomedicine are mentioned followed by some future perspectives.
Keywords: Polymer nanoparticles, Collapse, Single-chain polymer NPs, Intramolecular cross-linking, Drug delivery
Introduction
Currently, the synthesis of NPs is one of the key areas in any subject of research, particularly in biomedicine, as a result of the wide variety of applications they reveal. Over recent years, the development of a large number of synthetic techniques has permitted the use of many different types of materials to obtain very diverse sizes of particles, from hundreds down to just a few nanometers.
The main concern of using NPs in nanomedicine is their potential toxicity for living systems mainly derived from accumulation in different tissues and organs [1]. Drezek et al. reviewed the in vitro toxicity studies for carbon-based NPs (carbon NPs, single-wall carbon nanotubes, and multi-wall carbon nanotubes), metal-based NPs (gold NPs, gold nanorods, gold nanoshells, super-paramagnetic iron oxide NPs) and semiconductor-based NPs (cadmium selenide quantum dots, cadmium telluride quantum dots) [2]. They concluded that all of them showed low toxicity at concentrations below 10 μg/ml. However, several groups were toxic at higher concentrations in a dose- and time-dependent manner. For instance, gold nanorods visibly reduced cell viability of HeLa cells and human embryonic kidney cells. Other kinds of inorganic nanoparticles, such as those of titanium dioxide, also showed certain toxicity due to the induction of oxidative stress [3].
Dobrovolskaia et al. reviewed the immunological effect of NPs employed for biomedical applications. The study shows that NPs can be designed to induce or suppress immunological response, depending on their surface chemistry. As an example, PAMAM dendrimers did not cause human leukocytes (white blood cells) to secrete cytokines, while cationic liposomes did [4]. Ultrafine carbon black NPs can even accumulate in lung tissue after inhalation to end up in the bloodstream [5]. Moreover, neutral wax NPs are shown to be capable of entering neurons and crossing the blood–brain barrier [6]. For all these reasons, nanocarriers would need to be biocompatible, biodegradable, and specifically designed to obtain an appropriate biodistribution, so that they specifically reach a target site.
Soft matter-based nanocarriers seem particularly interesting in relation to achieving these required characteristics. The number of applications of NPs is increasing rapidly, especially in the biomedical field for drug-delivery purposes [7]. If we just consider polymer NPs, there are many examples of their use in therapies for cancer [8, 9], HIV [10–12], malaria [13] and Chagas disease [14], siRNA delivery [15], image-guided drug delivery [16], imaging agents [17], sensors and self-healing systems [18]. The applicability of those systems is related to the nature, the morphology, and the size of the nanocarriers and their structural control is essential. It has been reported that soft-matter-based delivery systems of less than 30 nm avoid accumulation in the liver and spleen, as well as unwanted immunogenic reactions [19]. Even though the preparation of polymer NPs above 20 nm in diameter is usually successful, there is a trade off with respect to tailored arrangement of functional groups and desired size. The difficulty in synthesizing polymer NPs smaller than 20 nm is rewarded with a more precise composition.
The possibility of synthesizing a NP from the collapse of a single polymer chain offers an easy procedure to obtain minuscule particles ranging between 1.5 and 20 nm in diameter. Besides, their dimensions can be tuned by controlling the molecular weight of the precursor polymer chain, as well as the quantity of intramolecular bonds generated in the collapse. On the other hand, the chemical composition and functionalities of the precursor polymer chains confer specific properties to the SCPNs. For all these reasons, SCPNs are envisaged as promising nanocarriers for applications in biomedicine.
In this review, we are going to focus on intramolecularly collapsed polymer-based NPs, their main synthetic methods, physical properties, and their actual and future use in nanomedical applications.
Synthesis and physical properties of single-chain polymer nanoparticles
As mentioned above, the general strategy for the synthesis of SCPNs consists on the collapse of a single polymer chain and the stabilization of the resulting nanoparticle by intramolecular cross-links.
The concentration of the precursor polymer chain in the reaction mixture is also a key variable in order to obtain SNCPs. At very low concentrations, intramolecular interactions dominate, whereas polymer networks predominantly form at higher concentrations due to intermolecular cross-linking. In addition, recent reports show that reaction rates are determinate not only by concentration but also by the size of the linear polymer precursors [20].
The controlled synthesis of the precursor polymer chains is a key step to produce particles with a narrow size distribution. This can be achieved synthesizing the precursors by any living free-radical polymerizations techniques.
Strategies for chain-collapse
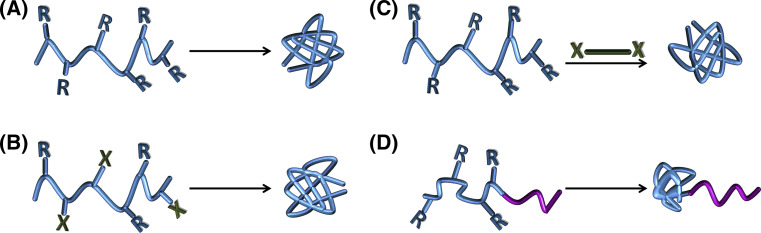
SCPNs can be classified according to the approaches used to generate the intramolecular chain-collapse. The main strategies utilized so far are illustrated in Fig. 1, and will be described in detail in the following sections. In the homofunctional chain-collapse (Fig. 1a), the copolymer chain is functionalized with reactive self-complementary “R” groups (i.e., double bonds) which are then reacted intramolecularly. The percentage of reactive groups used varies from 10–100%, and the type of cross-linking can be of covalent or non-covalent nature.
Fig. 1.
Schematic representation of the four strategies utilized for the synthesis of SCPNs: a Homo-functional chain collapse; b Hetero-bifunctional chain collapse; c Cross-linker-mediated chain collapse; d One-block collapse of diblock or triblock copolymers
The hetero-bifunctional chain collapse approach (Fig. 1b) is very similar to the previous one, but instead of using one functional group, it requires two complementary functionalities (“R” and “X”) simultaneously on the same polymer chain. In this case, the reaction also needs to be performed under diluted conditions.
The cross-linker mediated chain-collapse strategy (Fig. 1c) makes use of a cross-linker in order to synthesise the unimacromolecular NPs. The polymer chain, which is functionalized with a suitable “R” functional group, is collapsed by reacting with the two “X” end-groups of the cross-linker. Undesired intermolecular cross-linking is avoided by slowly adding one of the components to the other. The main benefits of this method are the easier synthesis of the precursor polymers and the possibility of introducing extra functionality through the cross-linking agent.
Finally, in the one-block collapse of diblock or triblock copolymers (Fig. 1d), the intramolecular collapse could be performed using any of the previous three strategies, but the outcome is different, generating the so-called molecular “tadpoles” or NP-coil copolymers. In this approach, the functional reactive groups are just in one of the blocks of the precursor copolymer chain. When the intramolecular cross-linking is forced, this block forms a globule and the rest stays as a coil.
Homo-functional chain collapse
It was around the 1980s when Martin and Eichinger initially proposed the synthesis of discrete NPs based on intramolecular coupling of single polymer chains [21, 22]. Later on, Davankov et al. published the synthesis of single-chain polystyrene (PS) NPs [23]. On this occasion, the pendant chloromethylene groups on the linear PS chains (M w = 330 kDa) were self-cross-linked via SnCl4 (catalyst). Thereby they obtained hyper-cross-linked “nanosponges” of ~17 nm in solution.
A few years later, Mecerreyes et al. carried out a very thorough study on the production of unimolecular particles from copolymers based on (1) aliphatic polyesters (caprolactone-co-ε-caprolactone or L,L-lactide), (2) poly(methyl methacrylate) (PMMA) and (3) PS [24]. Basically, all these polymers contained suspended acrylate functionalities that intramolecularly interact in ultra-diluted solutions in the presence of a radical initiator such as 2,2-azo-bis-isobutyronitrile (AIBN).
Afterwards, Jiang and Thayumanavan developed an analogous strategy based on AIBN-induced radical cross-linking of styrene groups, but on this occasion, the precursor polymer was an amine-functionalized PS [25]. As they demonstrated, these pending amino groups on the NPs could be further functionalized for different possible applications such as drug delivery.
All the approaches mentioned above show an important drawback for the synthesis of unimolecular particles on a multigram scale. The reactions need to be performed in ultra-dilute conditions (ca. 10−5 to 10−6 M) to avoid the statistically favored and competing intermolecular reactions. For this reason, in 2002, Hawker et al. developed a strategy to overcome this problem [26]. In such a method, there was a continuous addition of the linear polymer to the reaction mixture, with addition rates below cross-linking reaction rates. Maintaining these conditions the concentration of the generated NPs was constantly growing while the concentration of the reactive open-chain species was always ultra-low. For the initial approach, Hawker chose the thermal-induced coupling of benzocyclobutene (BCB) units at 250°C, and under the conditions mentioned previously, there was no substantial sign of intermolecular cross-linking.
Later on, Harth et al. developed an alternative cross-linking monomer to BCB, the vinylbenzosulfone, which turned out to be easier to synthesise [27]. This precursor was copolymerized with styrene or benzyl acrylate, and with the latter the formation of SCPNs occurred subsequent to the deprotection of the benzyl ester groups. In addition, these unimolecular NPs were soluble in physiological conditions, envisaging them as potential biomedical nanocarriers. Furthermore, the authors confirmed this hypothesis using these NPs for intracellular delivery of peptide-based therapeutics (see “Applications and future perspectives”) [19].
More recently, SCPNs synthesized from a single conducting ABA block terpolymers were studied for the encapsulation of the B block of the terpolymer, once the two A blocks were collapsed. In this specific example, B block was prepared from conducting fluorene homopolymer and fluorine/thiophene copolymer, while A blocks were synthesized from vinylbenzosulfone and PS copolymers. For certain molecular weight ABA ratios, the conducting copolymer block was site isolated into a 3D structure leading to a considerable increase in its quantum efficiency compared to the linear precursor, and hence, in its photoluminescence intensity [28].
There is another case where olefin cross-metathesis was used to obtain intramolecularly cross-linked polycarbonates by means of pending vinyl groups, once a ruthenium-based catalyst was added into a dilute polymer solution [29]. In this work, Coates et al. followed the formation of molecular NPs by atomic force microscopy (AFM), and they could visualize individual molecules at different stages of the collapse.
In the examples shown so far, the fabrication of SCPNs was accomplished using covalent bonds generated by the reaction of suitable functional groups on the polymer chain. If the intramolecular bonds used to collapse the polymer molecule are non-covalent (i.e., hydrogen bonds) the outcome is a supramolecular single-chain NP. Due to the analogy to the folding process of biomacromolecules, these types of nanocarriers generate increasing interest from a biological point of view. The development of these systems could lead to a better understanding of protein-folding processes, even though synthetic polymers are much simpler in composition than natural polymers. They could also be a good pattern to mimic some of nature’s sophisticated structures and afterwards study them in a more comprehensible scenario.
Concerning these supramolecular SCPNs, the groups of Hawker and Kim [30] synthesized random PMMA copolymers having dendritic hydrogen-bonding self-complementary units. The collapse of the polymer coils was led by the formation of intramolecular H-bonds between the dendritic units, giving stable and discrete spherical SCPNs.
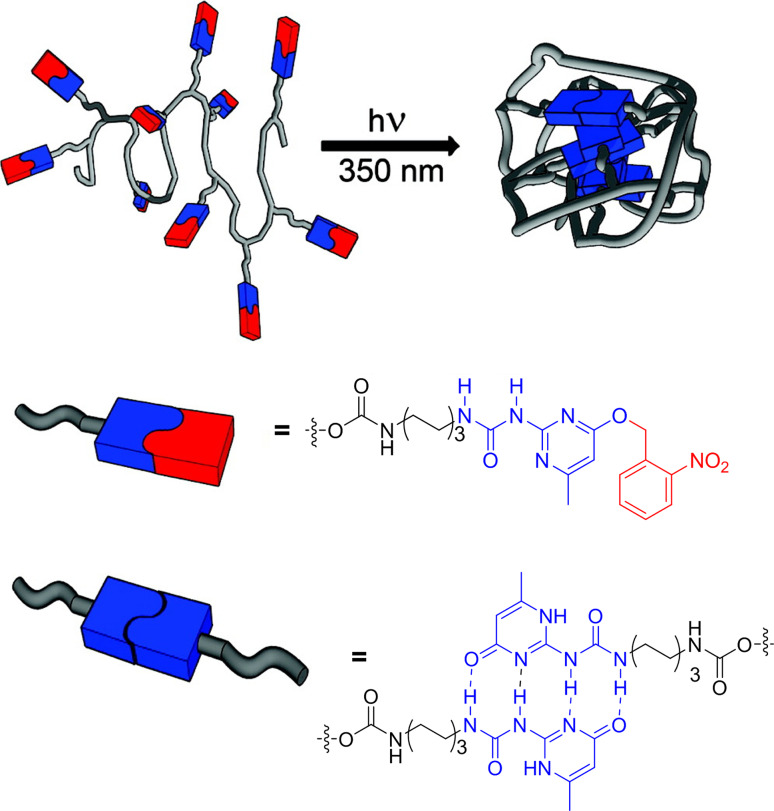
In a more recent work, Meijer et al. developed a system that could be activated by UV radiation to induce the collapse of linear precursors by supramolecular interactions [31]. For this purpose, they decorated PMMA with 2-ureidopyrimidinone (UPy) units protected with a photo-cleavable group (Scheme 1). As soon as they were irradiated with UV light, the protecting group was released and each UPy unit could very efficiently form four H-bonds, collapsing the polymer chain and obtaining the NP.
Scheme 1.
Supramolecular cross-linking of the UPy-urethane side groups after protecting group removal via UV irradiation (nitrosoaldehyde side product omitted for clarity) to induce collapse of a single polymer chain into a NP. Reproduced and adapted from [31] with permission of ACS
Hetero-bifunctional chain collapse
The fabrication of SCPNs can be carried out with two different but complementary functional side groups pending from the same polymer backbone (Fig. 1b). Loinaz et al. have utilized a “click” reaction as a strategy to produce PMMA SCPNs [32]. Such “click” reaction was an intramolecular Cu(I)-catalyzed [3 + 2] cycloaddition that takes place between azide and alkyne motifs present in the same polymer backbone at room temperature. The use of “click” chemistry [33, 34], and specifically the Cu(I)-catalyzed [3 + 2] cycloaddition of alkynes and azides [35–37], is widely used in different areas of chemistry due to its huge advantages. It is a selective and very efficient reaction with high yields, good functional group tolerance and insignificant by-products that can be performed under exceptionally mild conditions. The very mild conditions required for the synthesis of SCPNs make this method ideal for the chain-collapse of polymers that are unstable at high temperatures.
The possibility of decorating the NP after its formation with interesting biomolecules was demonstrated by Loinaz et al. In order to achieve this, the polymer chain was functionalized with an excess of azide groups, so that after the chain collapsed, an alkyne-containing molecule (propargylglycine in this case) could be attached to the surface via a second “click” reaction (Scheme 2).
Scheme 2.
SCPN formation by intramolecular “click” cycloaddition and further functionalization with a suitable biomolecule via a second “click” cycloaddition
The complexity of the synthesis of the precursor copolymers is one of the major drawbacks of this approach. It is often difficult to obtain a copolymer with two complementary reactive motifs randomly distributed over the backbone. The solution comes with the use of a suitable difunctional cross-linker, as described in the following section.
Cross-linker-mediated chain collapse
Difunctional cross-linker-mediated collapse of polymer chains is a very efficient and straightforward method for obtaining SCPNs.
Hawker’s group introduced this synthetic approach using isocyanate functionalized acrylic polymers and a diamine cross-linker under mild, room-temperature conditions to generate unimolecular NPs [38]. They obtained different sets of NPs with controlled diameters ranging from 8 to 20 nm by altering the molecular weight and the percentage of isocyanate motifs in the starting linear copolymer. The average of unreacted isocyanate groups in the final particles was always around 25% of the initially available ones, which could be utilized for further bioconjugation through primary amines.
Pomposo et al. based their NP fabrication on “click” chemistry using a difunctional cross-linker. A solution of an aliphatic dialkyne was added very slowly and with a constant rate aided with a syringe pump, to an azide containing copolymer of diverse nature [39, 40].
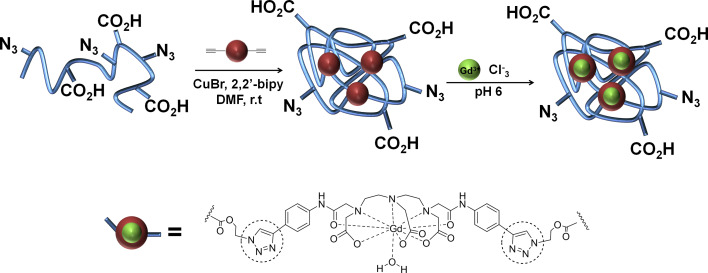
Following the same method, Odriozola et al. prepared water-soluble acrylic SCPNs utilizing a specific cross-linker based on a gadolinium(III) (Gd3+) chelating unit (diethylenetriaminepentaacetic acid, DTPA) with two terminal alkyne functionalities [41]. The incorporation of Gd3+ in the DTPA units led to paramagnetic SCPNs, which showed an increased relaxivity over their mono-gadolinium counterparts, on a per-gadolinium basis (Scheme 3). This system with multiple gadolinium centers represents the first example of potential MRI contrast agents based on SCPNs.
Scheme 3.
Synthesis and deprotection of SCPNs, and loading with Gd3+. The newly formed 1,2,3-triazole rings during the “click” cycloaddition are highlighted with dashed circles
One-block collapse of diblock or triblock copolymers
Molecular “tadpoles” or NP-coil copolymers can be obtained from a selective chain collapse of one of the blocks in an A-B type diblock copolymer (Fig. 1d). From a biological point of view, this kind of structure is especially interesting because it is known that certain natural polymers, such as peptides and proteins, can acquire diverse conformations in the same macromolecule (i.e., linear, globular) for catalysis and self-assembly purposes [42, 43]. Linear-globular architectures have successfully been used for polymer therapeutics showing a better performance than the respective natural globular protein. For instance, patients with chronic hepatitis C responded better to interferon α-2a combined with the linear synthetic polymer poly(ethylene glycol) (PEG) than to the unmodified globular protein [44].
Tao and Liu reported the preparation and isolation of diblock “tadpole” molecules in 1997 [45]. The starting copolymer was polystyrene-block-poly(2-cinnamoylethyl methacrylate), and the intramolecular collapse was forced by photo-cross-linking of the cinnamoyl units. However, the yield of the reaction happened to be very low, and purification by size-exclusion chromatography (SEC) was necessary to separate the “tadpoles” from the unwanted nanosphere by-products.
Later on, Hawker et al. used their thermal-induced method described in “Homo-functional chain collapse” to generate NP-coil structures. The linear starting diblock copolymer, poly(ethylene glycol)-block-poly[styrene-random-(4-vinylbenzocyclobutene)], was added slowly to a benzyl ether solution at 250°C for the chain-collapse [26, 46]. Based on this work, the same group prepared another similar type of NP-coil copolymers, in which the globular part was also PS-based, but the coil was a poly(n-butyl acrylate) [47].
Using the photo-cross-linking strategy of cinnamoyl-containing blocks mentioned before [45], Liu et al. synthesized molecular “tadpoles” starting from diblock copolymers of poly(tert-butyl acrylate)-block-poly(2-cinnamoyloxyethyl methacrylate) and poly(tert-butyl acrylate)-block-poly[(2-cinnamoyloxyethyl methacrylate)-random-(2-hydrocinnamoyloxyethyl methacrylate)] [48]. A solution of any of these copolymers was gradually added into a solvent under constant UV irradiation and stirring. Unlike Hawker’s BCB-based method (see “Homo-functional chain collapse”), this process was carried out at room temperature, which is a considerable advantage.
There have also been experiments designed to achieve a partial collapse of one of the blocks of an A–B–C type triblock copolymer. In a recent example, Zhu et al. synthesized a coil-nanoparticle-coil system from polystyrene-b-poly(2-vinylpyridine)-b-poly(ethylene oxide) as the triblock copolymer precursor [49]. The aim was to study programmable and hierarchical self-assembly of these amphiphilic NPs in various solvents.
Physical properties and characterization of SCPNs
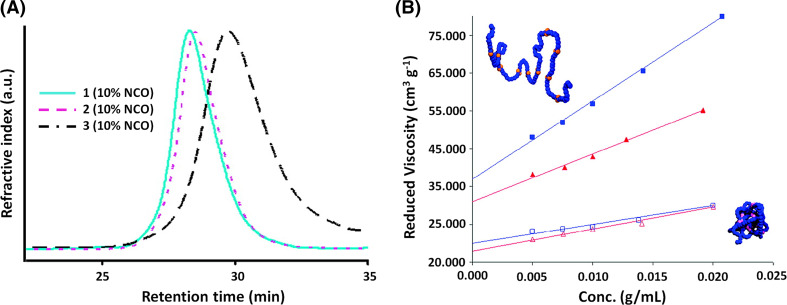
Most of the physical properties of SCPNs differ from those of the linear precursor chains, and they are usually analyzed by several established techniques. The apparent molecular weight of macromolecules can be determined by gel permeation chromatography (GPC). If intermolecular interactions are favored, polymer networks are generated and an increase of the molecular weight is observed. Although the molecular weight distribution is not altered by intramolecular cross-linking, properties related to polymer dimensions, such as intrinsic viscosity and hydrodynamic volume, are affected [38]. As GPC measurements are proportional to the hydrodynamic volume, the retention times of unimolecular polymer NPs and their linear precursors must differ to a certain extent. Consequently, NPs show a lower apparent molecular weight according to the reduction of their hydrodynamic volume, which confirms an intramolecular collapse (Fig. 2a). The behavior of intrinsic viscosity is comparable to that of hydrodynamic volume. In accordance with the similarity in nature between NPs and constant density spheres, however, analogous NPs show equivalent viscosity values even if the molecular weights differ by 50% (Fig. 2b) [38].
Fig. 2.
a Overlay of GPC traces for the starting copolymer 1, (Mn = 45,300), the corresponding linear control polymer 2 and NP 3 incorporating ca. 10 mol% isocyanate (NCO) cross-linking functional groups; b Plot of reduced viscosity versus concentration for control copolymers 2 (closed square 150 kDa; closed triangle 100 kDa) and their analogous cross-linked NPs 3 (open square 150 kDa; open triangle 100 kDa) in THF. Mol% NCO is 10 in this case. Reproduced and adapted from [38] with permission of ACS
The formation of SCPNs can also be confirmed by several other techniques, such as dynamic light scattering (DLS), AFM, and nuclear magnetic resonance (NMR). DLS can be utilized to measure the radius of the particles in solution, but the average sizes of some SCPNs are at the lower size limit of most of the commercial instruments. In case the SCPNs are dried on a surface, AFM is the most suitable technique to visualize and study the dimensions of the collapsed coils. One of the most detailed studies of the shape and size of this type of NPs was carried out by Berda et al. They were able to deduce the possible morphology of a single collapsed supramolecular molecule by AFM [31].
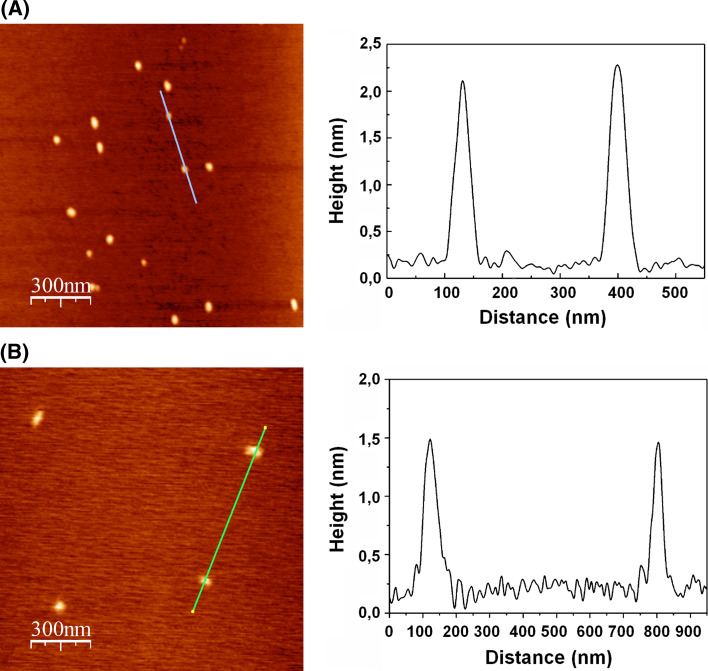
In the case of Odriozola et al., the collapse of the precursor copolymer chain was carried out through a bifunctional cross-linker, which is a Gd3+ chelating unit (see “Cross-linker-mediated chain collapse”) [41]. AFM studies clearly showed a diminution of the mean particle size of the NPs when Gd3+ was added, reducing it from ~2 nm to ~1.5 nm (Fig. 3). These NPs could probably be the smallest SCPNs so far by means of “click” chemistry.
Fig. 3.
Topographic AFM images and corresponding profiles of polyacrylic NPs showing a mean particle size of ~2 nm (a) and the same type of NPs loaded with Gd3+ with ~1.5 nm mean particle size (b). Reproduced from [41] with permission of RSC
NMR is a useful technique to confirm the formation of SCPNs due to the disappearance of several signals once the polymer chain collapses [46]. Thermal characteristics are different once the SCPNs are formed [31, 46], and the solubility of the collapsed coil may differ from the precursor polymer chain in certain solvents [30, 49].
Regarding nanomedical applications, size is an important parameter to take into account. In the case of SCPNs, size depends on the chemical nature and the molecular weight of the starting polymer chain, on top of the number of intramolecular interactions established to form the collapsed coil. The majority of SCPNs reported so far are PS-based and covalently cross-linked, and according to Hawker et al., size is basically proportional to the molecular weight and inversely proportional to the number of cross-links. To get to that conclusion, they analyzed SCPNs built on PS cross-linked by BCB chemistry [26]. Loinaz et al. identified a very similar behavior in PMMA-based copolymers [32].
Even if the collapse of a PMMA backbone precursor is performed by non-covalent cross-linking, the size change behavioral is analogous to the previously described [30, 31]. However, these supramolecular NPs are less dense and, hence, have a larger diameter than the correspondent covalent ones (around 10 nm in diameter) [50]. On the contrary, if the polymer contains bulky pending groups, the size does not diminish as the number of links increases. These have been proved by Jiang and Thayumanavan (see also “Homo-functional chain collapse”), where they study PS backbone NPs that display tert-butyloxycarbonyl (BOC) protected amino moieties [25].
Applications and future perspectives
In general, the collapse of a polymer chain due to non-covalent interactions is analogical to many folding processes in nature, so supramolecular SCPNs are envisaged as simpler patterns to mimic the behavior of biomacromolecules. For instance, these systems could allow the study of protein-folding processes in a much simpler environment than the one inside the cell, which could be useful to understand the basics of their complicated behavior in nature.
As mentioned previously, SCPNs could have a tremendous impact in the near future as drug nanocarriers due to their specific and unique characteristics for biomedical applications. With biocompatible or biodegradable chemical nature, these NPs contained perfectly defined and tailored functionalities determined by their precursor linear polymers. Some successful nanocarriers are dendrimers, and their small size (1–10 nm) seems to be their biggest advantage. That is why SCPNs (1.5–20 nm) could be challenging candidates for similar purposes.
However, their application in nanomedicine is still in its infancy. Most of the SCPNs described in this review are not soluble in aqueous media, which makes them incompatible with biomedical applications. The main issue is that synthetic routes have traditionally been developed in organic solvents for convenience (i.e., solvent evaporation, purification). Nevertheless, the expansion of medicinal chemistry has started to extrapolate the synthetic chemistry knowledge into aqueous systems. It has been just recently that a couple of publications of SCPNs in biomedicine have arisen.
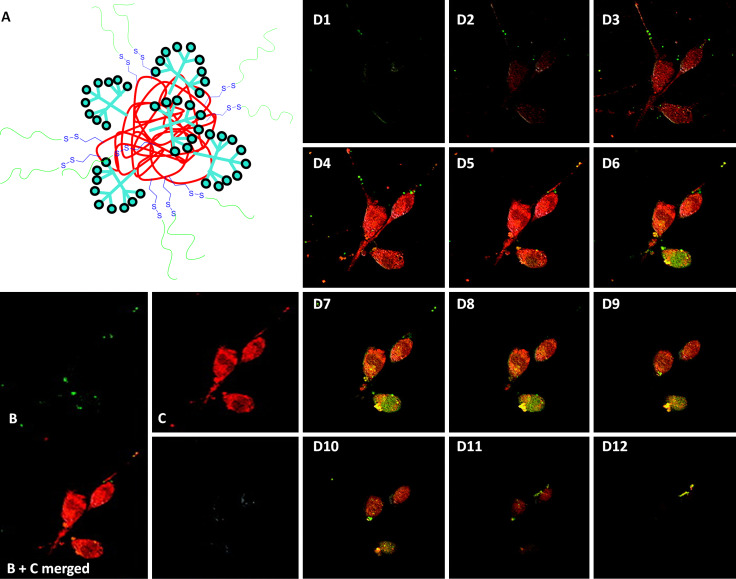
Hamilton and Harth conjugated unimolecular polymer NPs with dendritic molecular transporters to use them for intracellular delivery of peptide-based therapeutics [19]. The scaffold of the nanocarrier (Fig. 4a) contained a polymer core based on SCPN labeled with Alexa Fluor 568 (red dye) of 5–10 nm in diameter. This core was decorated with transporter dendritic units (drawn in turquoise in the figure) and functionalized with therapeutic peptides by a disulfide bond. These peptides were labeled with fluorescein to allow the detection of the nanocarrier and the cargo separately. Dendritic units seem to be critical to allow the delivery of the peptidic cargo into NIH 3T3 cells. Figure 4 shows confocal images of the uptake of these carriers into these cells. Figure 4b shows the image for fluorescein, while Fig. 4c shows the image for the fluorescence of Alexa Fluor 568. Confocal microscopy allows stack visualization of different cell layers as can be seen in Fig. 4d1–d12. Analyzing these images, the conclusion was that the cargo was released because fluorescein was accumulated in the upper layers of the cells.
Fig. 4.
Cellular uptake experiments of NP-transporter conjugate (a) into NIH 3T3 cells investigated via confocal microscopy imaged for the fluorescence of the Alexa Fluor 568 dye (c) and the fluorescein (b), with complementing z-stack (d) showing the presence of both fluorophores from the bottom (d1) to the top of the cells (d12). The cells were incubated for 30 min with a 37.2 μM NP solution in Hank’s buffered saline solution (HBSS). Reproduced and adapted from [19] with permission of ACS
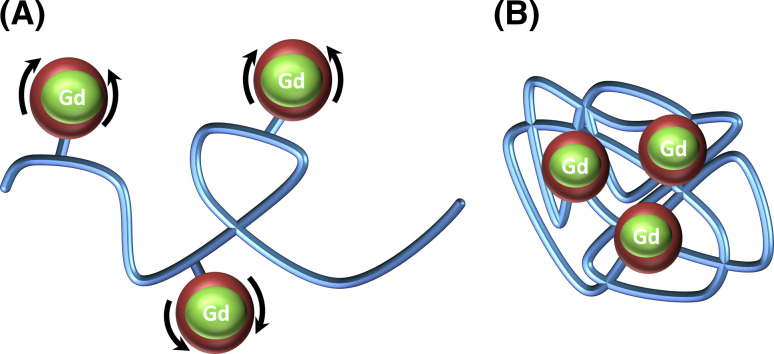
Odriozola et al. developed a new contrast agent suitable for MRI based on a new kind of paramagnetic SCPNs loaded with multiple Gd3+ ions [41]. Image contrast in MRI relies on relaxation of water protons from body tissues and fluids, and some stable paramagnetic metal ion complexes, such as Gd3+, enhance the water proton relaxivity. Commercially available agents (i.e., Magnevist® or Omniscan™) are based on low-molecular-weight Gd3+ chelates. One of the approaches to improve the relaxivity has been to graft Gd3+ onto polymeric chains, but it has been reported that internal flexibility or non-rigid attachment of the Gd3+ chelate to the macromolecule are often the limiting factors for the relaxivity of polymers containing multiple Gd3+ centers [51]. This is why Odriozola et al. chelated gadolinium ions in a DTPA-containing cross-linker to subsequently form SCPNs (Scheme 4). The originality of this approach relied on the rigidity of the system, restricting the internal rotation of the Gd-DTPA moieties and improving the relaxivity.
Scheme 4.
Schematic representation of a conventional Gd3+-grafted polymer (a) and a polymer intramolecularly cross-linked with Gd3+ chelates (b)
As we described in this review, there are high expectations placed on SCPNs in biomedicine, even though they have just started to be utilized in drug delivery and molecular imaging. Their versatility and multifunctionality are crucial for their future development into biomedical applications, as well as their compatibility with aqueous systems. Thus, new powerful SCPN nanosystems may lead to the new theranostic era.
References
- 1.Minchin R. Sizing up targets with nanoparticles. Nat Nanotechnol. 2008;3:12–13. doi: 10.1038/nnano.2007.433. [DOI] [PubMed] [Google Scholar]
- 2.Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 3.Long TC, Saleh N, Tilton RD, Lowry GV, Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ Sci Technol. 2006;40:4346–4352. doi: 10.1021/es060589n. [DOI] [PubMed] [Google Scholar]
- 4.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 5.Shimada A, Kawamura N, Okajima M, Kaewamatawong T, Inoue H, Morita T. Translocation pathway of the intratracheally instilled ultrafine particles from the lung into the blood circulation in the mouse. Toxicol Pathol. 2006;34:949–957. doi: 10.1080/01926230601080502. [DOI] [PubMed] [Google Scholar]
- 6.Lockman PR, Koziara JM, Mumper RJ, Allen DD. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J Drug Targeting. 2004;12:635–641. doi: 10.1080/10611860400015936. [DOI] [PubMed] [Google Scholar]
- 7.Doshi N, Mitragotri S. Designer biomaterials for nanomedicine. Adv Funct Mater. 2009;19:3843–3854. doi: 10.1002/adfm.200901538. [DOI] [Google Scholar]
- 8.Bharali DJ, Khalil M, Gurbuz M, Simone TM, Mousa SA. Nanoparticles and cancer therapy: a concise review with emphasis on dendrimers. Int J Nanomedicine. 2009;4:1–7. doi: 10.2217/17435889.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaucher G, Marchessault RH, Leroux J-C. Polyester-based micelles and nanoparticles for the parenteral delivery of taxanes. J Controlled Release. 2010;143:2–12. doi: 10.1016/j.jconrel.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Gupta U, Jain NK. Non-polymeric nano-carriers in HIV/AIDS drug delivery and targeting. Adv Drug Delivery Rev. 2010;62:478–490. doi: 10.1016/j.addr.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Sharma P, Garg S. Pure drug and polymer-based nanotechnologies for the improved solubility, stability, bioavailability and targeting of anti-HIV drugs. Adv Drug Delivery Rev. 2010;62:491–502. doi: 10.1016/j.addr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Wong HL, Chattopadhyay N, Wu XY, Bendayan R. Nanotechnology applications for improved delivery of antiretroviral drugs to the brain. Adv Drug Delivery Rev. 2010;62:503–517. doi: 10.1016/j.addr.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Santos-Magalhães NS, Mosqueira VCF. Nanotechnology applied to the treatment of malaria. Adv Drug Delivery Rev. 2010;62:560–575. doi: 10.1016/j.addr.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Romero EL, Morilla MJ. Nanotechnological approaches against Chagas disease. Adv Drug Delivery Rev. 2010;62:576–588. doi: 10.1016/j.addr.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 15.Keller M. Nanomedicinal delivery approaches for therapeutic siRNA. Int J Pharm. 2009;379:210–211. doi: 10.1016/j.ijpharm.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 16.Lammers T, Subr V, Ulbrich K, Hennink WE, Storm G, Kiessling F. Polymeric nanomedicines for image-guided drug delivery and tumor-targeted combination therapy. Nano Today. 2010;5:197–212. doi: 10.1016/j.nantod.2010.05.001. [DOI] [Google Scholar]
- 17.Richard C, Chaumet-Riffaud P, Belland A, Parat A, Contino-Pepin C, Bessodes M, Scherman D, Pucci B, Mignet N. Amphiphilic perfluoroalkyl carbohydrates as new tools for liver imaging. Int J Pharm. 2009;379:301–308. doi: 10.1016/j.ijpharm.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 18.Motornov M, Roiter Y, Tokarev I, Minko S. Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems. Prog Polym Sci. 2010;35:174–211. doi: 10.1016/j.progpolymsci.2009.10.004. [DOI] [Google Scholar]
- 19.Hamilton SK, Harth E. Molecular dendritic transporter nanoparticle vectors provide efficient intracellular delivery of peptides. ACS Nano. 2009;3:402–410. doi: 10.1021/nn800679z. [DOI] [PubMed] [Google Scholar]
- 20.Li R, McCoy BJ. Inter- and intramolecular crosslinking kinetics: partitioning according to number of crosslinks. Macromol Rapid Commun. 2004;25:1059–1063. doi: 10.1002/marc.200400058. [DOI] [Google Scholar]
- 21.Martin JE, Eichinger BE. Dimensions of intramolecularly cross-linked polymers. 2. Dilute solution thermodynamic parameters and photon correlation results on the polystyrene/cyclopentane system. Macromolecules. 1983;16:1345. [Google Scholar]
- 22.Martin JE, Eichinger BE. Dimensions of intramolecularly crosslinked polymers. 1. Theory. Macromolecules. 1983;16:1345–1350. doi: 10.1021/ma00242a016. [DOI] [Google Scholar]
- 23.Davankov VA, Ilyin MM, Tsyurupa MP, Timofeeva GI, Dubrovina LV. From a dissolved polystyrene coil to an intramolecularly-hyper-cross-linked “Nanosponge”. Macromolecules. 1996;29:8398–8403. doi: 10.1021/ma951673i. [DOI] [Google Scholar]
- 24.Mecerreyes D, Lee V, Hawker CJ, Hedrick JL, Wursch A, Volksen W, Magbitang T, Huang E, Miller RD. A novel approach to functionalized nanoparticles: self-crosslinking of macromolecules in ultradilute solution. Adv Mater. 2001;13:204–208. doi: 10.1002/1521-4095(200102)13:3<204::AID-ADMA204>3.0.CO;2-9. [DOI] [Google Scholar]
- 25.Jiang J, Thayumanavan S. Synthesis and characterization of amine-functionalized polystyrene nanoparticles. Macromolecules. 2005;38:5886–5891. doi: 10.1021/ma0507286. [DOI] [Google Scholar]
- 26.Harth E, Horn BV, Lee VY, Germack DS, Gonzales CP, Miller RD, Hawker CJ. A facile approach to architecturally defined nanoparticles via intramolecular chain collapse. J Am Chem Soc. 2002;124:8653–8660. doi: 10.1021/ja026208x. [DOI] [PubMed] [Google Scholar]
- 27.Croce TA, Hamilton SK, Chen ML, Muchalski H, Harth E. Alternative o-quinodimethane cross-linking precursors for intramolecular chain collapse nanoparticles. Macromolecules. 2007;40:6028–6031. doi: 10.1021/ma071111m. [DOI] [Google Scholar]
- 28.Adkins CT, Muchalski H, Harth E. Nanoparticles with individual site-isolated semiconducting polymers from intramolecular chain collapse processes. Macromolecules. 2009;42:5786–5792. doi: 10.1021/ma9007913. [DOI] [Google Scholar]
- 29.Cherian AE, Sun FC, Sheiko SS, Coates GW. Formation of nanoparticles by intramolecular cross-linking: following the reaction progress of single polymer chains by atomic force microscopy. J Am Chem Soc. 2007;129:11350–11351. doi: 10.1021/ja074301l. [DOI] [PubMed] [Google Scholar]
- 30.Seo M, Beck BJ, Paulusse JMJ, Hawker CJ, Kim SY. Polymeric nanoparticles via noncovalent cross-linking of linear chains. Macromolecules. 2008;41:6413–6418. doi: 10.1021/ma8009678. [DOI] [Google Scholar]
- 31.Berda EB, Foster EJ, Meijer EW. Toward controlling folding in synthetic polymers: fabricating and characterizing supramolecular single-chain nanoparticles. Macromolecules. 2010;43:1430–1437. doi: 10.1021/ma902393h. [DOI] [Google Scholar]
- 32.Ruiz de Luzuriaga A, Ormategui N, Grande HJ, Odriozola I, Pomposo JA, Loinaz I. Intramolecular click cycloaddition: an efficient room-temperature route towards bioconjugable polymeric nanoparticles. Macromol Rapid Commun. 2008;29:1156–1160. doi: 10.1002/marc.200700877. [DOI] [Google Scholar]
- 33.Iha RK, Wooley KL, Nyström AM, Burke DJ, Kade MJ, Hawker CJ. Applications of orthogonal “Click” chemistries in the synthesis of functional soft materials. Chem Rev. 2009;109:5620–5686. doi: 10.1021/cr900138t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed Engl. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 35.Meldal M, Tornøe CW. Cu-catalyzed azide-alkyne cycloaddition. Chem Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 36.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “Ligation” of azides and terminal alkynes. Angew Chem Int Ed Engl. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 37.Tornøe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 38.Beck JB, Killops KL, Kang T, Sivanandan K, Bayles A, Mackay ME, Wooley KL, Hawker CJ. Facile preparation of nanoparticles by intramolecular cross-linking of isocyanate functionalized copolymers. Macromolecules. 2009;42:5629–5635. doi: 10.1021/ma900899v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oria L, Aguado R, Pomposo JA, Colmenero J. A versatile “Click” chemistry precursor of functional polystyrene nanoparticles. Adv Mater. 2010;22:3038–3041. doi: 10.1002/adma.201000243. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz de Luzuriaga A, Perez-Baena I, Montes S, Loinaz I, Odriozola I, García I, Pomposo JA. New route to polymeric nanoparticles by click chemistry using bifunctional crosslinkers. Macromol Symp. 2010;296:303–310. doi: 10.1002/masy.201051042. [DOI] [Google Scholar]
- 41.Perez-Baena I, Loinaz I, Padro D, García I, Grande HJ, Odriozola I. Single-chain polyacrylic nanoparticles with multiple Gd(III) centres as potential MRI contrast agents. J Mater Chem. 2010;20:6916–6922. doi: 10.1039/c0jm01025a. [DOI] [Google Scholar]
- 42.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 43.Krejchi MT, Atkins EDT, Waddon AJ, Fournier MJ, Mason TL, Tirrell DA. Chemical sequence control of beta-sheet assembly in macromolecular crystals of periodic polypeptides. Science. 1994;265:1427–1432. doi: 10.1126/science.8073284. [DOI] [PubMed] [Google Scholar]
- 44.Reddy K, Wright TL, Pockros PJ, Shiffman M, Everson G, Reindollar R, Fired MW, Purdum PP, Jensen D, Smith C, Lee WM, Boyer TD, Lin A, Pedder S, DePamphilis J. Efficacy and safety of pegylated (40-kD) interferon α-2a compared with interferon α-2a in noncirrhotic patients with chronic hepatitis C. Hepatology. 2001;33:433–438. doi: 10.1053/jhep.2001.21747. [DOI] [PubMed] [Google Scholar]
- 45.Tao J, Liu G. Polystyrene-block-poly(2-cinnamoylethyl methacrylate) tadpole molecules. Macromolecules. 1997;30:2408–2411. doi: 10.1021/ma961422p. [DOI] [Google Scholar]
- 46.Kim Y, Pyun J, Frachet JMJ, Hawker CJ, Frank CW. The dramatic effect of architecture on the self-assembly of block copolymers at interfaces. Langmuir. 2005;21:10444–10458. doi: 10.1021/la047122f. [DOI] [PubMed] [Google Scholar]
- 47.Pyun J, Tang C, Kowalewski T, Frachet JMJ, Hawker CJ. Synthesis and direct visualization of block copolymers composed of different macromolecular architectures. Macromolecules. 2005;38:2674–2685. doi: 10.1021/ma047375f. [DOI] [Google Scholar]
- 48.Njikang G, Liu G, Curda SA. Tadpoles from the intramolecular photo-cross-linking of diblock copolymers. Macromolecules. 2008;41:5697–5702. doi: 10.1021/ma800642r. [DOI] [Google Scholar]
- 49.Cheng L, Hou G, Miao J, Chen D, Jiang M, Zhu L. Efficient synthesis of unimolecular polymeric Janus nanoparticles and their unique self-assembly behavior in a common solvent. Macromolecules. 2008;41:8159–8166. doi: 10.1021/ma800461z. [DOI] [Google Scholar]
- 50.Foster EJ, Berda EB, Meijer EW. Metastable supramolecular polymer nanoparticles via intramolecular collapse of single polymer chains. J Am Chem Soc. 2009;131:6964–6966. doi: 10.1021/ja901687d. [DOI] [PubMed] [Google Scholar]
- 51.Villaraza AJL, Bumb A, Brechbiel MW. Macromolecules, dendrimers, and nanomaterials in magnetic resonance imaging: the interplay between size, function, and pharmacokinetics. Chem Rev. 2010;110:2921–2959. doi: 10.1021/cr900232t. [DOI] [PMC free article] [PubMed] [Google Scholar]