Abstract
CD8+ T lymphocytes screen the surface of all cells in the body to detect pathogen infection or oncogenic transformation. They recognize peptides derived from cellular proteins displayed at the plasma membrane by major histocompatibility complex (MHC) class I molecules. Peptides are mostly by-products of cytosolic proteolytic enzymes. Peptidic ligands of MHC class I molecules are also generated in the secretory and vesicular pathways. Features of protein substrates, of proteases and of available MHC class I molecules for loading peptides in these compartments shape a singular collection of ligands that also contain different, longer, and lower affinity peptides than ligands produced in the cytosol. Especially in individuals who lack the transporters associated with antigen processing, TAP, and in infected and tumor cells where TAP is blocked, which thus have no supply of peptides derived from the cytosol, MHC class I ligands generated in the secretory and vesicular pathways contribute to shaping the CD8+ T lymphocyte response.
Keywords: TAP transporter, Antigenic peptide, Secretory pathway, MHC class I molecule, CD8+ T lymphocyte
MHC class I ligands generated in secretory and vesicular compartments
Major histocompatibility complex (MHC) class I molecules continuously sample the proteome and present peptides at the cell surface. In this way, cytotoxic CD8+ T lymphocytes can screen the external membrane of the cell and sense the intracellular contents. Target cell elimination by CD8+ T lymphocytes contributes to pathogen clearance and to tumor immunosurveillance while sometimes causing immunopathology. A good fraction of the peptides presented by MHC class I molecules result from proteolysis in the cytosol. These peptides are transported into the endoplasmic reticulum (ER) by TAP transporters, where they meet nascent MHC class I molecules [1–3].
Complex viruses such as herpes and poxviruses encode a variety of proteins that attenuate the cellular immune response. This results in a balance that favors both host survival and virus dissemination to new hosts. Specifically, these viruses target the MHC class I antigen processing pathway [4, 5]. As a consequence, CD8+ T cell responses are attenuated, which results in less virus control and in less severe immunopathology. TAP appears to be a significant step for virus control, as many viral functions target TAP molecules [6]. Some tumor cells also lose TAP expression [7]. This limits the supply of cytosolic peptides to the presentation pathway. Very soon after the discovery of TAP, it was recognized that some ligands can also be generated in TAP-deficient cells [8, 9]. In addition, it was noticed that TAP-independent pathways of antigen presentation appear to suffice to control viral infections in vivo in persons genetically deficient in TAP. Indeed, TAP−/− human beings are not abnormally susceptible to viral infections [10] and have virus-specific CD8+ T lymphocytes [11]. In a mouse model, TAP-deficient CD8+ T lymphocytes induced by TAP-independent MHC class I ligands expressed by a virus can mediate effective elimination of epitope-bearing cells [12]. Recognition by cytotoxic CD8+ T cells of ligands generated in the secretory pathway has been found to be relevant for induction of diabetes in animal models [13]. Some of these ligands derived from self proteins are not abundantly presented in the presence of a large pool of higher affinity cytosolic peptides. Because of this, in tumors that have lost TAP expression they behave as neoantigens and can contribute to tumor control [14]. A similar phenomenon may underlie clearance of cells infected with viruses that target TAP, as well as virus control in human TAP patients.
MHC class I ligands generated in the secretory and vesicular compartments have been studied by a variety of approaches. When cytosolic supply is impaired by inhibiting the proteasome, plasma membrane expression of MHC class I molecules is reduced in an allotype-specific way [15, 16]. However, using this approach, peptides generated by supplementary cytosolic proteases can also be loaded onto MHC class I molecules [2]. Therefore, in an attempt to limit the analysis to secretory and vesicular ligands, cells and animals deficient in TAP have been used. Plasma membrane expression of most MHC class I allotypes is also reduced to some extent in the absence of TAP. This is probably caused by two deficits: first, there is a marked reduction in supply from cytosolic peptides; second, as the number of complexes that exit the ER is reduced, any peptide exchange at later quality control sites [16–18] is severely impaired, limiting the chance of binding of peptides generated in the secretory and vesicular pathways. Epitopes presented by MHC class I molecules in TAP-deficient cells comprise at least (1) peptides generated in the secretory and vesicular pathways and (2) peptides that manage to find their way from the cytosol into the ER in a TAP-independent way (Fig. 1). As discussed below, the second group of ligands appears to be unexpectedly abundant.
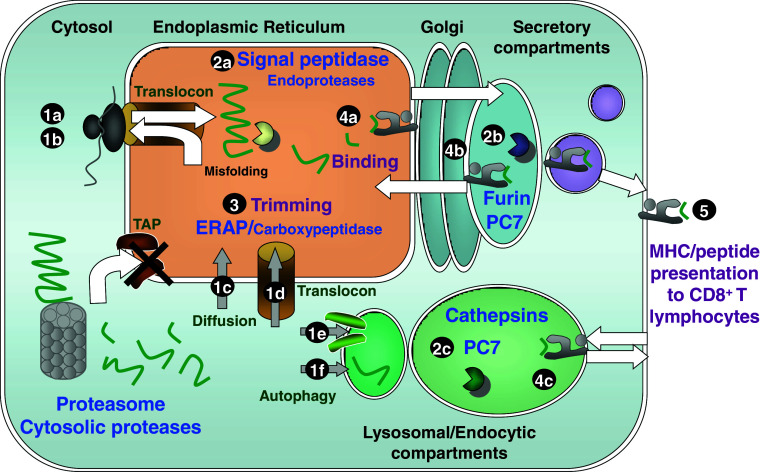
Fig. 1.
Generation of MHC class I ligands in the secretory and vesicular compartments. Peptides generated by proteasomes and other cytosolic proteases are transported to the ER via TAP. In cells deficient in TAP, membrane (1a) and secretory proteins (1b) are translocated into the ER because of their signal or transmembrane sequences. Several putative mechanisms might allow cytosolic proteins and peptides to gain access to secretory and vesicular compartments: diffusion through membranes (1c); transport through an altered ER translocon or an unidentified ER transporter (1d); transport through an unidentified endolysosomal transporter (1e); and autophagy (1f). Endoproteolytic antigen processing in the ER relies on signal peptidase and other undefined endoproteases (2a). Two proprotein convertases have been shown to process antigens in the absence of TAP: furin, located in the trans-Golgi network (2b), and PC7, located in trans-Golgi network and endocytic vesicles (2b, 2c). Cathepsins have been involved in the generation of MHC class I ligands in endolysosomal compartments (2c). Most of the products generated by these endoproteases require trimming by aminopeptidases like ERAP and by a putative carboxypeptidase in the secretory pathway (3). The canonical binding site for MHC class I ligands is the ER (4a). Alternatively peptides might be exchanged at quality control sites in the Golgi (4b) or at endosomes containing MHC class I molecules that undergo constitutive recycling (4c). Finally, MHC class I ligands generated in the secretory and vesicular routes are presented to CD8+ T lymphocytes (5). White arrows indicate forward and retrograde transport of antigens across membranes, and grey arrows indicate putative transport. Potential proteases are in lower case
Features of parental proteins and of ligands produced
Initial peptides identified in cells lacking the TAP transporters were derived from signal sequences and were biochemically isolated from HLA-A2, an allotype that preferentially selects rather hydrophobic peptides [8, 9]. Later studies concentrated on functional assays using specific CD8+ T lymphocyte lines to study antigen presentation of selected epitopes in TAP-deficient living cells. These studies greatly contribute to identifying proteases and shed light on the mechanisms involved. Recently, the sensitivity of large-scale mass spectrometry analyses has greatly improved and has allowed the study of large collections of natural peptide ligands presented by MHC class I molecules in the absence of TAP. A summary of the features of ligands and epitopes, which are detailed below, is found in Box 1.
Box 1.
Features of MHC class I ligands and epitopes produced in the absence of TAP
| 1. Fit less well to binding motifs |
| 2. Some are slightly longer and have lower binding affinity |
| 3. Derived from membrane and secreted proteins |
| 4. Unexpectedly, many derived from cytosolic and nuclear proteins |
| 5. Variable numbers derived from signal sequences |
| 6. Profit from amino terminal trimming |
| 7. Unexpectedly, carboxy terminal trimming is very frequent |
| 8. Some are the exact carboxy termini of parental proteins |
Analysis of selected protein antigens and MHC class I epitopes
Defective ribosomal products, DRiPs, including errors in translation and folding and the rapidly degraded fraction of proteins, probably constitute a major source for proteasomal processing [3], although not the only one [2]. Nascent membrane proteins enter the secretory pathway en route to vesicular organelles such as the ER, Golgi apparatus, endosomes, lysosomes, plasma membrane, or on their way to the extracellular space. Cytosolic processing of these proteins by cytosolic proteasomes and auxiliary trimming enzymes poses no problem, as their DRiPs also reach the cytosol. Accordingly, many epitopes derived from these proteins are presented following the classical proteasome- and TAP-dependent pathway of antigen processing. In addition, supplementary proteases in the vesicular compartments they traverse have the chance to process these membrane proteins and provide an additional assortment of MHC class I ligands to CD8+ T lymphocytes.
Detailed analysis of individual epitopes or proteins (reviewed in [19]) has identified a number of peptides that are presented by MHC class I molecules independently of TAP. These studies not only analyze the presence of a given MHC class I ligand, but also functionally demonstrate its presentation to specific CD8+ T lymphocytes. Many studies relate to source proteins or constructs that either contain a signal sequence or are based on natural type II, III and IV membrane proteins. Thus, they are targeted for ER insertion, and it is expected that these epitopes are generated in the secretory pathway if they are presented in TAP-deficient cells. In some of these reports, the proteases involved were identified. These included the ER signal peptidase [8, 9, 20–23], the trans Golgi network protease furin [24–26], proprotein convertase 7 located in the trans Golgi network and vesicles, and in endocytic vesicles [16] and endolysosomal cathepsins [26] (Fig. 1, steps 2a, 2b, 2c). In other reports the protease could not be identified [27–35]. Frequently, some epitopes from a given protein required TAP, whereas others from the same protein were presented independently of TAP [28, 29, 31]. In some cases, the same epitope could follow both types of pathways [30]. Finally, when natural peptides have been extracted from cells involved in these functional assays, trimming at both amino and carboxy termini are found [24].
A less expected and less frequent observation was that epitopes that were presented independently of TAP previously required some type of proteolytic processing in the cytosol, for example by proteasomes [29, 31, 33]. In some instances, the puzzling observation was reported that cytosolic proteins were presented in cells lacking TAP [30, 36–38]. Clearly, this type of peptides requires a means to enter vesicular compartments in a TAP-independent way (Fig. 1, steps 1c, 1d, 1e, 1f). In one instance, a good correlation was found between hydrophobicity and TAP-independency, suggesting that peptides with a specific ability to traverse membranes have the chance to enter some type of vesicles and bind to MHC class I molecules [31, 39]. Passive diffusion, Sec61 translocon and an unidentified transporter were proposed as mechanisms [31]. It has been suggested that large amounts of hydrophobic peptides are required in the cytosol for sufficient delivery to the ER [40]. Maybe for this reason, when large-scale analyses of ligands from TAP-deficient cells have been performed, or when pathogen-derived epitopes have been defined in TAP-deficient infected cells, such a clear trend towards hydrophobicity or towards another particular biochemical feature of the ligands has not been found. This may reflect a major contribution to the pool of TAP-independent ligands of peptides actually generated in the vesicular compartments after the parental proteins have reached them. Alternatively, it may reflect the existence of a substitute peptide transporter that performs functions related to those of TAP and that has a relaxed specificity, accommodating peptides with diverse biochemical properties. TAPL at the lysosomal membrane has recently been put forward as a candidate [41].
Large-scale analyses of MHC class I ligand repertoires
Large-scale proteomic analysis of natural peptides isolated from cells offers an unbiased identification of MHC class I ligands generated in the secretory pathway of TAP-deficient cells. Ligands from three classical MHC class I molecules, Kd, HLA-A2 and HLA-B51, and from one class I-like molecule, Qa-1b, were recently analyzed [42–44]. Unexpectedly, no common picture emerges from these reports.
A first consistent observation is that TAP-independent ligands do not conform well to binding motifs deduced from large-scale analysis of cytosolic ligand repertoires.
A second finding concerns peptide length. Peptides one or two amino acids longer than canonical optimal 8mers or 9mers are frequently found. Exceptionally, several residue longer ligands are also detected. When binding is analyzed, longer peptides frequently have lower affinities. This does not mean that they are less physiologically relevant, as such weak peptides generated in the secretory pathway from insulin can trigger pancreatic β-cell destruction in diabetic patients [22].
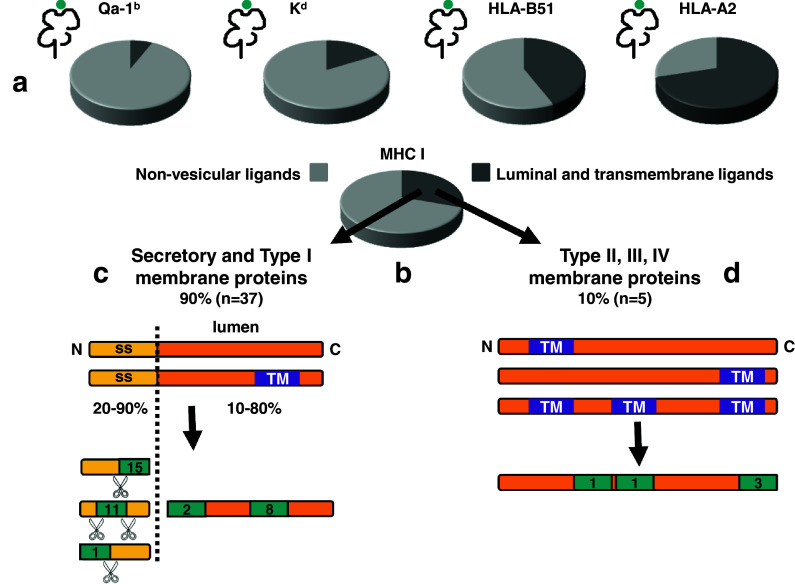
A third expected observation is that epitopes derived from luminal or transmembrane portions of parental proteins are found (Fig. 2). This source of epitopes is expected to be most easily accessible to vesicular proteases, which could process nascent or mature proteins. These ligands represent from 10 to 70% of all TAP-independent ligands for the different allotypes (10% for Qa-1b, 20% for Kd, 40% for HLA-B51, 70% for HLA-A2) (Fig. 2a). It is unclear why this fraction is so variable. It is also noteworthy that, with the exception of HLA-A2 ligands, the fraction of luminal or transmembrane ligands is not far from the estimated 25% of source proteins that cotranslationally enter the ER of mammalian cells.
Fig. 2.
Features of MHC class I ligands generated in the secretory and vesicular pathways and identified in large-scale proteomic analyses. a For the four different MHC class I allotypes indicated, the fraction of ligands derived from luminal and transmembrane regions of parental proteins is depicted in dark grey, while light grey represents the fraction of ligands derived from non-vesicular proteins (cytosolic, nuclear, mitochondrial). b Global analysis of the luminal and non-vesicular ligands shown in a. Among luminal ligands, 90% are derived from secretory and type I membrane proteins, while 10% are derived from type II, III or IV membrane proteins. c Depending on the MHC class I allotype, signal sequence-derived ligands account for 20 to 90% of the secretory and type I ligands (20% for Kd, 25% for Qa-1b, 80% for HLA-B51 and 90% for HLA-A2). Within signal peptides, signal peptidase generates the exact carboxy terminus of the ligands in almost half of the cases (all 15 of them presented by HLA-A2), whereas carboxy terminal trimming is required for the other half (presented by all four allotypes). In all cases amino terminal trimming is necessary. Ligands derived from luminal regions of the proteins other than the signal sequence also require amino terminal trimming except in two cases in which signal peptidase generates the exact amino terminus of the ligand. Dotted lines represent signal peptidase cleavage, and scissors indicate exopeptidase trimming. d The localization of ligands derived from type II, III or IV transmembrane proteins is shown. They frequently constitute the exact carboxy terminus of the parental protein. Original data from [42–44]
A fourth consistent finding is that, conversely, a variable but high fraction of ligands is unexpectedly derived from cytosolic, nuclear or mitochondrial proteins, or from the cytosolic domains of membrane proteins (Fig. 2a, b). This source of epitopes would not have access to vesicular proteases. As with wild-type cells, TAP-deficient cells are lysed to isolate MHC class I ligands, either directly or after purification of MHC class I molecules. These cells have a limited supply of peptides to the ER, and thus a larger number of peptide-receptive MHC class I molecules. During the purification procedures, soluble peptides present in any cell compartment might have the chance to artificially bind to MHC class I molecules. This has been difficult to control up till now. Efficiency of binding may be low, but with increasingly sensitive techniques, these peptides may turn up in the MHC class I ligand pool in the absence of TAP. This might be the source of peptidic ligands derived from cytosolic proteins and found in the absence of TAP. Still, these peptides might indeed gain access to vesicular compartments in the absence of TAP in living cells, maybe by using other undefined peptide transporters. Autophagy, which has recently been shown to enhance antigen processing for MHC class I presentation, may also be a potential mechanism for cytosolic substrates to gain access independently of TAP to compartments containing MHC class I molecules [45–47]. Free diffusion across membranes appears more unlikely, as most of these ligands are not hydrophobic. Interestingly, ligands isolated from cells treated with proteasome inhibitors were also abundantly derived from cytosolic or nuclear parental proteins [48]. This puzzling finding is reminiscent of peptides of cytosolic origin also found in large-scale analyses as ligands to MHC class II molecules [49] and may indicate a common pathway.
Fifth, signal sequences appear as a major source for MHC class I ligands in TAP-negative cells only in the study involving HLA-A2 and -B51 [44]. The finding for HLA-A2 follows the seminal reports on TAP-independent peptides [8, 9]. Ligands derived from signal sequences constitute more than 80% of all luminal ligands identified for these two allotypes. In contrast, only 20% of similar ligands for Kd and Qa-1b derive from this region (Fig. 2c). A low proportion was also found in cells treated with proteasome inhibitors [48]. It is unclear whether the more recent use of higher sensitivity techniques that allow detection of lower abundance ligands accounts for the abundant detection of peptides not derived from signal peptides.
The sixth observation relates to the information gained on the potential processing proteases. Signal peptidase cleaves on the carboxyl side of small and nonpolar residues, most frequently after alanine. HLA-A2 can accommodate alanine at the carboxy-terminal position of its ligands. Accordingly, signal peptidase appears to have directly produced the carboxyl termini of 75% of all signal sequence derived HLA-A2 ligands. The remaining 25% HLA-A2 ligands needed subsequent carboxy terminal trimming. For the other three MHC class I alleles, which do not share this carboxy terminal anchor motif, surprisingly all signal sequence-derived ligands had been trimmed at the carboxy terminus. On the other hand, as expected, almost all signal sequence-derived peptides showed evidence of amino-terminal trimming (Fig. 2c). On average, TAP-independent MHC class I ligands are located in the middle of the signal peptides, with HLA-A2 ligands concentrated on the carboxy terminus of the signal peptides and ligands for the remaining allotypes closer to the amino terminus.
Finally, it is noteworthy that a few peptides are found that constitute the exact carboxy-terminus of the parental proteins (Fig. 2d). This was already detected a decade ago in living cells with functional assays [34], although the endopeptidase involved has not been identified yet. In comparison, one ligand is derived from the exact amino-terminus of the parental protein, and two ligands are derived from the exact amino-terminus of the mature protein after the signal sequence has been removed (Fig. 2c).
Proteases that generate the ligands
The classical pathway of antigen processing generates peptides in the cytosol that are derived from newly synthesized proteins and from protein turnover. The cytosol is a very degradative compartment, having endoproteases that produce internal cleavages, such as the proteasome, exopeptidases that operate from either terminus and dipeptidases that deal with dipeptides [50]. If no special sequence features preclude it, the final products are amino acids. A sample of intermediate products of degradation is rescued by transport to the ER by TAP transporters [2] (Fig. 1). Proteins that reach the lysosomes are also heavily exposed to a different set of degradative proteases that operate at lower pH. In contrast to the cytosol and to lysosomes, other vesicular compartments and the secretory pathway are clearly less degradative.
The fact that the cytosol and the endolysosomal compartment are better equipped for protein degradation correlates with the fact that these are the major sites for generation of peptides for presentation by MHC class I and class II molecules, respectively. However, for a growing number of MHC class I epitopes, the action of the proteasome is mainly destructive rather than productive as the efficiency of presentation increases in the presence of inhibitors of the proteasome [51–55]. It is probable that still many other potential epitopes are never presented because they are completely wiped out. Exopeptidases further cleave the peptides, usually to amino acids. Not surprisingly, while some precursor peptides profit from a mild exposure to them in vitro, longer incubation times usually lead to cleavage within the epitopes [56]. Peptides produced at lysosomes and presented by MHC class II molecules to CD4+ helper T lymphocytes also occasionally show this behavior [57].
In contrast, the secretory pathway is a less degradative environment. This has advantages and disadvantages. Peptides that are generated here are expected to be longer, less diverse and more stable, maybe accumulating to higher quantities. Although perhaps not many optimal epitopes are produced or correctly trimmed in these regions, these characteristics can add up and give them a good chance to bind to MHC class I molecules.
Endoproteases
Proteases in the secretory and vesicular compartments mostly belong to two categories, maturing and degradative proteases. Maturing proteases are involved in performing selective cleavages on selected substrates, often involving their maturation or activation. Signal peptidase, furin and proprotein convertases belong to this sort. They have been shown to process antigens in the absence of TAP [8, 9, 16, 20–26] (Fig. 1, steps 2a, 2b, 2c). The former enzyme recognizes and cleaves a sequence pattern common to signal peptides. The latter enzymes preferentially cleave at polybasic sites on protein substrates. This restricted cleavage specificity may limit the diversity of epitopes they can contribute to generating. This may be compensated, however, by a close to complete efficiency of cleavage at these sites, permitting the generation of close to one potential precursor peptide from each one molecule of the parental protein. In contrast, cytosolic efficiency has been measured to produce one MHC class I-peptide complex for each 500–5,000 viral translation products degraded [58, 59]. As expected, however, antigen processing by these maturing proteases liberates long peptides and thus strongly depends on trimming peptidases working processively from either end of the oligopeptides. In one instance where this has been quantitated, when furin products no longer required amino-terminal refinement, efficiency of antigen processing was increased by some 20-fold. More dramatically, when the products no longer required carboxy-terminal trimming, efficiency of antigen presentation in living cells was raised by over 1,000-fold [12].
It is not difficult to assume that the most abundant TAP-independent ligands would be those generated in the ER, as it contains trimming exopeptidases and the peptide loading complex [60, 61]. Thus, peptide products generated in the ER are optimally situated to intersect the classical antigen presentation pathway. Among the endoproteases, signal peptidase has been a frequent processing enzyme in TAP-negative cells. Signal sequences are liberated from nascent proteins by signal peptidase. In general, they have no known function and many make their way to the ER membrane or lumen. TAP-independent ligands derived from signal sequences have been mostly detected in large-scale mass spectrometric studies of peptide repertoires, but there are also reports where the implication of signal peptidase has been shown with functional assays in living cells [21, 22, 62]. Signal peptidase has an additional advantage for antigen processing among other maturing proteases, as it generates short peptides—the signal peptides—which require little subsequent trimming. A different enzyme, signal peptide peptidase, an ER endoprotease that cleaves transmembrane sections of proteins, has been shown to further process one signal peptide and favor its presentation by the classical proteasome and TAP-dependent pathway [63]. It is interesting to speculate that this activity might also contribute to trimming of some signal peptides for TAP-independent presentation.
Lysosomal proteases are powerful pH-dependent degradative enzymes, having little sequence selectivity and acting on numerous substrates. These features resemble those of the proteasome and enable them to generate most of the ligands presented by MHC class II molecules to CD4+ T lymphocytes. Accordingly, there are also examples of the involvement of cathepsins on the generation of MHC class I ligands from newly synthesized proteins [26]. It remains to be established whether this is a more general fact. Potential limiting steps in obtaining ligands derived from products of lysosomal hydrolases include availability of additional trimming enzymes, as MHC class I affinity is more sensitive than that of MHC class II to the presence of residues flanking the optimal core peptide. In addition, the balance between extensive degradation and time required to reach suitable peptide receptive MHC class I molecules could also severely limit the number of peptides presented.
Exoproteases
Aminopeptidases
Trimming at the amino terminus is the common observation for peptides that originate in the cytosol and are isolated from TAP-positive cells. There are a number of aminopeptidases in the increasingly mature compartments along the secretory pathway. The ER of human cells contains ERAP1 and ERAP2, whereas mouse cells contain the single ortholog of ERAP1 [50]. So far, ERAP1 is the only murine aminopeptidase that has been shown to be critical for the antigen processing pathway [61, 64]. Its absence in genetically deficient mice leads to the production of a greatly different repertoire of peptides [64].
Peptides generated in vesicular compartments in the absence of TAP may also have access to ERAPs. Those generated in the ER will profit most from it. Not surprisingly, a good number of TAP-independent ligands originate from signal sequences (Fig. 2c). These sequences are longer than most MHC class I ligands. Accordingly, all signal sequence derived ligands identified in large-scale proteomic analysis appear to have been trimmed at the amino terminus (Fig. 2c). Trimming of these TAP-independent ligands generated in the ER is most likely performed by ERAPs.
Peptides generated in vesicular compartments other than the ER may also have access to ERAPs. ERAPs are ER-resident luminal proteins. They have been almost exclusively detected in the ER. However, it is expected that, like all luminal proteins, they may leak from the ER and be taken back by the retrieval machinery. With limited efficiency due to the small number of molecules, they thus may have the chance to trim peptides generated in more distal cisternae of the secretory pathway.
In addition to ERAPs, aminopeptidases abound in the secretory pathway and in vesicular compartments. They enter the ER as nascent proteins and then travel to their organelle of destination or to the extracellular medium [65]. Their activity is generally not regulated so that they may trim peptides they find on their route. Further work on their possible involvement in trimming of MHC class I ligands is needed.
Carboxypeptidases
Carboxypeptidases to finely trim the carboxy terminus of peptides would also help in sampling the vesicular compartment by MHC class I molecules. Some carboxypeptidases also enter the ER as nascent proteins and, as aminopeptidases, their enzymatic activity is not strictly regulated. Most are secreted and perform their action in the extracellular medium, either as digestive enzymes or processing bioactive peptides [65]. However, contrary to what happens with aminopeptidases, no individual carboxypeptidase has been implicated in antigen processing in the vesicular pathway. Consistent with this, some secretory and vesicular MHC class I ligands correspond to the exact carboxy termini of proteins (Fig. 2d) [34, 42–44]. There is an advantage for this type of peptides as it is easy to generate them by a single amino-terminal endoproteolytic cleavage, followed by trimming optimization. This is particularly important when cleavages are expected to result from non-proteasomal supplemental proteases in vesicular compartments.
In the cytosol, most carboxy termini are produced by the endoproteolytic activity of the proteasome [66], and no role for carboxypeptidases becomes evident in the classical TAP-dependent antigen-processing pathway [67]. This has discouraged the search for such enzymes (however, see [50] for a discussion of the recently discovered role of thimet oligopeptidase as cytosolic carboxypeptidase). In contrast, however, carboxy terminal trimming does contribute to the generation of secretory MHC class I ligands. Most evidence indirectly comes from identification of natural peptides. For a few natural pathogen-derived peptides, it has been shown that trimming at both peptide ends has taken place and that the exact optimal peptide has been generated [24]. Signal sequence-derived peptides isolated from TAP-deficient cells constitute a second piece of evidence. All signal sequence-derived ligands for all studied MHC class I molecules but one appear to have been trimmed at the carboxy terminus [42–44] (Fig. 2c, where they are included in the set of 12 peptides). The exception is HLA-A2, as it can accommodate the carboxy terminal alanine directly provided by signal peptidase cleavage (the set of 15 peptides in Fig. 2c). Assuming that TAP-independent epitopes derive more easily from signal peptides that are released by signal peptidase into the ER lumen or membrane and that are not exposed to cytosolic enzymes, this strongly implies a very frequent optimization of secretory ligands also at the carboxy end. It remains to be established whether this carboxy terminal trimming also contributes to optimization of the peptide supply arriving from the cytosol in cells expressing TAP.
Finally, it is not excluded that generation of the exact carboxy terminus may involve endopeptidases rather than carboxypeptidases. This would parallel the proteasome endoproteolytic activity that generates the correct carboxy termini of most peptides in the cytosol.
Location of peptide loading to MHC class I molecules
The ER is endowed with sophisticated machinery for optimizing ligand length and quality, and for facilitating peptide loading onto nascent MHC class I molecules. This includes aminopeptidases as well as folding chaperones and editing molecules in the peptide loading complex [60, 61]. Peptides generated in the ER, for example by signal peptidase, can directly benefit from this machinery. However, there is indirect evidence for peptide generation in other more distal compartments of the secretory pathway (Fig. 1, steps 2b, 2c). Subcellular localization of the processing proteases provides some clues. Cathepsins are active in endolysosomal compartments [26], while furin and protein PC7 turn fully active in the trans Golgi network and primarily reside in this organelle, in post trans Golgi network vesicles or in the endocytic system [16, 24–26], but none of them can efficiently cleave substrates in the ER. One possibility is that peptides are generated where the enzymes are active and then travel back to the ER in sufficient amounts for binding. They may use retrograde vesicular transport anywhere from the cell surface to the ER along the secretory pathway [68].
Still, peptide stability provides an additional clue suggesting the possibility of an additional peptide-loading location. As the overwhelming majority of natural peptides that have been studied, and as initially observed by Rammensee [69], the CMV 9pp89 natural peptide can only be isolated from cells that express the presenting molecule Ld [70]. This is explained by assuming that peptides are degraded in the strongly degradative environment of the cytosol if not rescued to the ER by TAP, and even so, if they do not bind in the ER they will eventually end up back in the cytosol after retrotranslocation through the Sec61 translocon [71]. However, this strong dependence on the presenting molecule is true only if the cells express TAP. When this CMV 9pp89 epitope is liberated by the trans Golgi network protease furin in a TAP-independent way, the natural peptide can be isolated from cells that have or do not have the appropriate MHC class I molecule [24]. Importantly, similar amounts are found regardless of MHC presence. This suggests that most of the peptide is not in a highly degradative compartment. Further, it suggests that most of the peptide is not in the ER, as it would be retrotranslocated to the cytosol. It follows that MHC class I molecules in TAP-deficient cells can bind this ligand even if it may not get back to the ER.
When peptidic ligands are generated and perhaps bind to MHC class I molecules in secretory compartments other than the ER, they may bind to MHC class I molecules that have suboptimally bound peptides and may exchange them at a second quality control check point in the Golgi [17, 18, 72], where peptide loading can occur closer to where these TAP-independent peptides are generated.
Finally, peptides generated further away from the ER in endolysosomal compartments may benefit from MHC class I molecules that constitutively recycle from the plasma membrane to early endosomes and back, and that upon entering gradually acidic environments may exchange their ligands [73]. As for other alternative locations for peptide loading, conclusive evidence is still lacking.
It is clear that binding can take place regardless of whether there is a supply of optimally loaded complexes coming from cytosolic processing, as TAP-deficient cells that do not have this supply of optimal ligands do present ligands generated in the vesicular compartments. Still, although conclusive evidence is lacking, it is likely that the availability of suboptimally loaded MHC class I molecules increases the efficiency of presentation of vesicular and secretory MHC class I ligands.
In summary, as the origin of protein substrates and the accessible proteases, the availability and special properties of MHC class I molecules for loading peptides contribute to shaping a singular collection of ligands in secretory and vesicular compartments.
Perspectives
Peptides derived from cellular, abnormal or pathogen proteins can be processed in the vesicular and secretory pathways and result in recognition by CD8+ T lymphocytes. These ligands are often longer and have less affinity to MHC class I molecules than those fully generated in the cytosol. Yet, they can contribute to virus clearance, tumor control and induce autoimmunity. A good number of peptides derive from proteins that mature along the secretory pathway and that are thus exposed to supplemental proteases. However, peptides that have a cytosolic origin and yet are presented independently of TAP are a recurrent finding. They even constitute the majority of TAP-independent MHC class I ligands in large-scale proteomic approaches. There is some uncertainty about whether a significant fraction of these cytosolic peptides may result from post-lysate binding. Yet, TAP-independent presentation of cytosolic proteins is also detected by cell biology approaches assaying presentation to CD8+ T lymphocytes of individual epitopes. The fact that the latter examples are less frequent might lie in their unexpectancy. The generation mechanism is difficult to envisage, and future work is needed to clarify it. In addition, there is a need to undertake systematic studies in living cells of antigen presentation to CD8+ T cell lines of large collections of proteins from complex pathogens, without selecting for membrane proteins, to assess the frequency and relevance of TAP-independent antigen presentation of cytosolic proteins and peptides, as well as from regular membrane proteins. Further, the knowledge gained about the biochemical features of the MHC class I ligands suggests the existence of more players in the secretory and vesicular pathway of antigen presentation, notably peptidases that generate the correct carboxy terminus of the ligands, aminopeptidases located in distal regions of the secretory route and endolysosomes, and novel transport mechanisms from the cytosol. Advances in this field should contribute to understanding the clinical presentation of TAP-deficient patients and help design new strategies for control of virus infections and tumors that interfere with TAP function.
Acknowledgments
We are grateful to Dr. Daniel López (Instituto de Salud Carlos III) for helpful discussions. Work in the laboratory is supported by grants from the Spanish Ministerio de Ciencia e Innovación and Redes Temáticas de Investigación Cooperativa del Instituto de Salud Carlos III. S.I. has a Sara Borrell contract, and S.L. has a Formación de Personal Investigador contract.
References
- 1.Del Val M, López D. Multiple proteases process viral antigens for presentation by MHC class I molecules to CD8+ T lymphocytes. Mol Immunol. 2002;39(3–4):235–247. doi: 10.1016/S0161-5890(02)00104-9. [DOI] [PubMed] [Google Scholar]
- 2.Rock KL, Farfan-Arribas DJ, Shen L. Proteases in MHC class I presentation and cross-presentation. J Immunol. 2010;184(1):9–15. doi: 10.4049/jimmunol.0903399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yewdell JW. Plumbing the sources of endogenous MHC class I peptide ligands. Curr Opin Immunol. 2007;19(1):79–86. doi: 10.1016/j.coi.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Yewdell JW, Hill AB. Viral interference with antigen presentation. Nat Immunol. 2002;3(11):1019–1025. doi: 10.1038/ni1102-1019. [DOI] [PubMed] [Google Scholar]
- 5.Hansen TH, Bouvier M. MHC class I antigen presentation: learning from viral evasion strategies. Nat Rev Immunol. 2009;9(7):503–513. doi: 10.1038/nri2575. [DOI] [PubMed] [Google Scholar]
- 6.Momburg F, Hengel H. Corking the bottleneck: the transporter associated with antigen processing as a target for immune subversion by viruses. Curr Top Microbiol Immunol. 2002;269:57–74. doi: 10.1007/978-3-642-59421-2_4. [DOI] [PubMed] [Google Scholar]
- 7.Pettit SJ, Seymour K, O’Flaherty E, Kirby JA. Immune selection in neoplasia: towards a microevolutionary model of cancer development. Br J Cancer. 2000;82(12):1900–1906. doi: 10.1054/bjoc.2000.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei ML, Cresswell P. HLA-A2 molecules in an antigen-processing mutant cell contain signal sequence-derived peptides. Nature. 1992;356(6368):443–446. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- 9.Henderson RA, Michel H, Sakaguchi K, et al. HLA-A2.1-associated peptides from a mutant cell line: a second pathway of antigen presentation. Science. 1992;255(5049):1264–1266. doi: 10.1126/science.1546329. [DOI] [PubMed] [Google Scholar]
- 10.Gadola SD, Moins-Teisserenc HT, Trowsdale J, et al. TAP deficiency syndrome. Clin Exp Immunol. 2000;121(2):173–178. doi: 10.1046/j.1365-2249.2000.01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Salle H, Saulquin X, Mansour I, et al. Asymptomatic deficiency in the peptide transporter associated to antigen processing (TAP) Clin Exp Immunol. 2002;128(3):525–531. doi: 10.1046/j.1365-2249.2002.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medina F, Ramos M, Iborra S, et al. Furin-processed antigens targeted to the secretory route elicit functional TAP1−/− CD8+ T lymphocytes in vivo. J Immunol. 2009;183(7):4639–4647. doi: 10.4049/jimmunol.0901356. [DOI] [PubMed] [Google Scholar]
- 13.Brosi H, Reiser M, Rajasalu T, et al. Processing in the endoplasmic reticulum generates an epitope on the insulin A chain that stimulates diabetogenic CD8 T cell responses. J Immunol. 2009;183(11):7187–7195. doi: 10.4049/jimmunol.0901573. [DOI] [PubMed] [Google Scholar]
- 14.van Hall T, Wolpert EZ, van Veelen P, et al. Selective cytotoxic T-lymphocyte targeting of tumor immune escape variants. Nat Med. 2006;12(4):417–424. doi: 10.1038/nm1381. [DOI] [PubMed] [Google Scholar]
- 15.Benham AM, Gromme M, Neefjes J. Allelic differences in the relationship between proteasome activity and MHC class I peptide loading. J Immunol. 1998;161(1):83–89. [PubMed] [Google Scholar]
- 16.Leonhardt RM, Fiegl D, Rufer E, et al. Post-endoplasmic reticulum rescue of unstable MHC class I requires proprotein convertase PC7. J Immunol. 2010;184(6):2985–2998. doi: 10.4049/jimmunol.0900308. [DOI] [PubMed] [Google Scholar]
- 17.Howe C, Garstka M, Al Balushi M, et al. Calreticulin-dependent recycling in the early secretory pathway mediates optimal peptide loading of MHC class I molecules. EMBO J. 2009;28(23):3730–3744. doi: 10.1038/emboj.2009.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garstka M, Borchert B, Al Balushi M, et al. Peptide-receptive major histocompatibility complex class I molecules cycle between endoplasmic reticulum and cis-Golgi in wild-type lymphocytes. J Biol Chem. 2007;282(42):30680–30690. doi: 10.1074/jbc.M701721200. [DOI] [PubMed] [Google Scholar]
- 19.Johnstone C, Del Val M. Traffic of proteins and peptides across membranes for immunosurveillance by CD8+ T lymphocytes: a topological challenge. Traffic. 2007;8(11):1486–1494. doi: 10.1111/j.1600-0854.2007.00635.x. [DOI] [PubMed] [Google Scholar]
- 20.Wölfel C, Drexler I, Van Pel A, et al. Transporter (TAP)- and proteasome-independent presentation of a melanoma-associated tyrosinase epitope. Int J Cancer. 2000;88(3):432–438. doi: 10.1002/1097-0215(20001101)88:3<432::AID-IJC16>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 21.El Hage F, Stroobant V, Vergnon I, et al. Preprocalcitonin signal peptide generates a cytotoxic T lymphocyte-defined tumor epitope processed by a proteasome-independent pathway. Proc Natl Acad Sci USA. 2008;105(29):10119–10124. doi: 10.1073/pnas.0802753105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skowera A, Ellis RJ, Varela-Calvino R, et al. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J Clin Invest. 2008;118(10):3390–3402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell MJ, Abbott RJ, Croft NP, et al. An HLA-A2-restricted T-cell epitope mapped to the BNLF2a immune evasion protein of Epstein-Barr virus that inhibits TAP. J Virol. 2009;83(6):2783–2788. doi: 10.1128/JVI.01724-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gil-Torregrosa BC, Castaño AR, Del Val M. Major histocompatibility complex class I viral antigen processing in the secretory pathway defined by the trans -Golgi network protease furin. J Exp Med. 1998;188(6):1105–1116. doi: 10.1084/jem.188.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gil-Torregrosa BC, Castaño AR, López D, Del Val M. Generation of MHC class I peptide antigens by protein processing in the secretory route by furin. Traffic. 2000;1(8):641–651. doi: 10.1034/j.1600-0854.2000.010808.x. [DOI] [PubMed] [Google Scholar]
- 26.Tiwari N, Garbi N, Reinheckel T, et al. A transporter associated with antigen-processing independent vacuolar pathway for the MHC class I-mediated presentation of endogenous transmembrane proteins. J Immunol. 2007;178(12):7932–7942. doi: 10.4049/jimmunol.178.12.7932. [DOI] [PubMed] [Google Scholar]
- 27.Elliott T, Willis A, Cerundolo V, Townsend A. Processing of major histocompatibility class I-restricted antigens in the endoplasmic reticulum. J Exp Med. 1995;181(4):1481–1491. doi: 10.1084/jem.181.4.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammond SA, Bollinger RC, Tobery TW, Siliciano RF. Transporter-independent processing of HIV-1 envelope protein for recognition by CD8+ T cells. Nature. 1993;364(6433):158–161. doi: 10.1038/364158a0. [DOI] [PubMed] [Google Scholar]
- 29.Hammond SA, Johnson RP, Kalams SA, et al. An epitope-selective, transporter associated with antigen presentation (TAP)-1/2-independent pathway and a more general TAP-1/2-dependent antigen-processing pathway allow recognition of the HIV-1 envelope glycoprotein by CD8+ CTL. J Immunol. 1995;154(11):6140–6156. [PubMed] [Google Scholar]
- 30.Johnstone C, Guil S, García-Barreno B, et al. Relevance of viral context and diversity of antigen processing routes for respiratory syncytial virus cytotoxic T-lymphocyte epitopes. J Gen Virol. 2008;89(9):2194–2203. doi: 10.1099/vir.0.2008/002485-0. [DOI] [PubMed] [Google Scholar]
- 31.Lautscham G, Mayrhofer S, Taylor G, et al. Processing of a multiple membrane spanning Epstein-Barr virus protein for CD8+ T cell recognition reveals a proteasome-dependent, transporter associated with antigen processing-independent pathway. J Exp Med. 2001;194(8):1053–1068. doi: 10.1084/jem.194.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorente E, Infantes S, Barnea E, et al. TAP-independent human histocompatibility complex-Cw1 antigen processing of an HIV envelope protein conserved peptide. AIDS. 2011;25(2):265–269. doi: 10.1097/QAD.0b013e328340fe3c. [DOI] [PubMed] [Google Scholar]
- 33.Snyder HL, Bacik I, Bennink JR, et al. Two novel routes of transporter associated with antigen processing (TAP)-independent major histocompatibility complex class I antigen processing. J Exp Med. 1997;186(7):1087–1098. doi: 10.1084/jem.186.7.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snyder HL, Bacik I, Yewdell JW, et al. Promiscuous liberation of MHC-class I-binding peptides from the C termini of membrane and soluble proteins in the secretory pathway. Eur J Immunol. 1998;28(4):1339–1346. doi: 10.1002/(SICI)1521-4141(199804)28:04<1339::AID-IMMU1339>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 35.Wood P, Elliott T. Glycan-regulated antigen processing of a protein in the endoplasmic reticulum can uncover cryptic cytotoxic T cell epitopes. J Exp Med. 1998;188(4):773–778. doi: 10.1084/jem.188.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gromme M, Uytdehaag FGCM, Janssen H, et al. Recycling MHC class I molecules and endosomal peptide loading. Proc Natl Acad Sci USA. 1999;96(18):10326–10331. doi: 10.1073/pnas.96.18.10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neumeister C, Nanan R, Cornu TI, et al. Measles virus and canine distemper virus target proteins into a TAP-independent MHC class I-restricted antigen-processing pathway. J Gen Virol. 2001;82(2):441–447. doi: 10.1099/0022-1317-82-2-441. [DOI] [PubMed] [Google Scholar]
- 38.Schirmbeck R, Reimann J. Peptide transporter-independent, stress protein-mediated endosomal processing of endogenous protein antigens for major histocompatibility complex class I presentation. Eur J Immunol. 1994;24(7):1478–1486. doi: 10.1002/eji.1830240704. [DOI] [PubMed] [Google Scholar]
- 39.Lautscham G, Rickinson A, Blake N. TAP-independent antigen presentation on MHC class I molecules: lessons from Epstein-Barr virus. Microbes Infect. 2003;5(4):291–299. doi: 10.1016/S1286-4579(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 40.Ratnikov BI, Rozanov DV, Postnova TI, et al. An alternative processing of integrin alpha(v) subunit in tumor cells by membrane type-1 matrix metalloproteinase. J Biol Chem. 2002;277(9):7377–7385. doi: 10.1074/jbc.M109580200. [DOI] [PubMed] [Google Scholar]
- 41.Demirel O, Bangert I, Tampe R, Abele R. Tuning the cellular trafficking of the lysosomal peptide transporter TAPL by its N-terminal domain. Traffic. 2010;11(3):383–393. doi: 10.1111/j.1600-0854.2009.01021.x. [DOI] [PubMed] [Google Scholar]
- 42.Suri A, Walters JJ, Levisetti MG, et al. Identification of naturally processed peptides bound to the class I MHC molecule H-2Kd of normal and TAP-deficient cells. Eur J Immunol. 2006;36(3):544–557. doi: 10.1002/eji.200526235. [DOI] [PubMed] [Google Scholar]
- 43.Oliveira CC, van Veelen PA, Querido B, et al. The nonpolymorphic MHC Qa-1b mediates CD8+ T cell surveillance of antigen-processing defects. J Exp Med. 2009;207(1):207–221. doi: 10.1084/jem.20091429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinzierl AO, Rudolf D, Hillen N, et al. Features of TAP-independent MHC class I ligands revealed by quantitative mass spectrometry. Eur J Immunol. 2008;38(6):1503–1510. doi: 10.1002/eji.200838136. [DOI] [PubMed] [Google Scholar]
- 45.English L, Chemali M, Duron J, et al. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat Immunol. 2009;10(5):480–487. doi: 10.1038/ni.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munz C. Antigen processing via autophagy-not only for MHC class II presentation anymore? Curr Opin. Immunol. 2010;22:89–93. doi: 10.1016/j.coi.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chemali M, Radtke K, Desjardins M, English L (2011) Alternative pathways for MHC class I presentation: a new function for autophagy. Cell Mol Life Sci. doi:10.1007/s00018-011-0660-3 [DOI] [PMC free article] [PubMed]
- 48.Luckey CJ, Marto JA, Partridge M, et al. Differences in the expression of human class I MHC alleles and their associated peptides in the presence of proteasome inhibitors. J Immunol. 2001;167(3):1212–1221. doi: 10.4049/jimmunol.167.3.1212. [DOI] [PubMed] [Google Scholar]
- 49.Muntasell A, Carrascal M, Serradell L, et al. HLA-DR4 molecules in neuroendocrine epithelial cells associate to a heterogeneous repertoire of cytoplasmic and surface self peptides. J Immunol. 2002;169(9):5052–5060. doi: 10.4049/jimmunol.169.9.5052. [DOI] [PubMed] [Google Scholar]
- 50.van Endert P (2011) Post-proteasomal and proteasome-independent generation of MHC class I ligands. Cell Mol Life Sci. doi:10.1007/s00018-011-0662-1 [DOI] [PMC free article] [PubMed]
- 51.Vinitsky A, Anton LC, Snyder HL, et al. The generation of MHC class I associated peptides is only partially inhibited by proteasome inhibitors, Involvement of nonproteasomal cytosolic proteases in antigen processing. J Immunol. 1997;159(2):554–564. [PubMed] [Google Scholar]
- 52.Anton LC, Snyder HL, Bennink JR, et al. Dissociation of proteasomal degradation of biosynthesized viral proteins from generation of MHC class I-associated antigenic peptides. J Immunol. 1998;160(10):4859–4868. [PubMed] [Google Scholar]
- 53.Fu TM, Mylin LM, Schell TD, et al. An endoplasmic reticulum-targeting signal sequence enhances the immunogenicity of an immunorecessive simian virus 40 large T antigen cytotoxic T lymphocyte epitope. J Virol. 1998;72(2):1469–1481. doi: 10.1128/jvi.72.2.1469-1481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valmori D, Gileadi U, Servis C, et al. Modulation of proteasomal activity required for the generation of a cytotoxic T lymphocyte-defined peptide derived from the tumor antigen MAGE-3. J Exp Med. 1999;189(6):895–905. doi: 10.1084/jem.189.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guil S, Rodríguez-Castro M, Aguilar F, et al. Need for tripeptidyl-peptidase II in major histocompatibility complex class I viral antigen processing when proteasomes are detrimental. J Biol Chem. 2006;281(52):39925–39934. doi: 10.1074/jbc.M608522200. [DOI] [PubMed] [Google Scholar]
- 56.Stoltze L, Schirle M, Schwarz G, et al. Two new proteases in the MHC class I processing pathway. Nat Immunol. 2000;1(5):413–418. doi: 10.1038/80852. [DOI] [PubMed] [Google Scholar]
- 57.Watts C, Moss CX, Mazzeo D, et al. Creation versus destruction of T cell epitopes in the class II MHC pathway. Ann NY Acad Sci. 2003;987:9–14. doi: 10.1111/j.1749-6632.2003.tb06028.x. [DOI] [PubMed] [Google Scholar]
- 58.Montoya M, Del Val M. Intracellular rate-limiting steps in MHC class I antigen processing. J Immunol. 1999;163(4):1914–1922. [PubMed] [Google Scholar]
- 59.Princiotta MF, Finzi D, Qian SB, et al. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity. 2003;18(3):343–354. doi: 10.1016/S1074-7613(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 60.Chapman DC, Williams DB. ER quality control in the biogenesis of MHC class I molecules. Semin Cell Dev Biol. 2010;21:512–519. doi: 10.1016/j.semcdb.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 61.Hammer GE, Kanaseki T, Shastri N. The final touches make perfect the peptide-MHC class I repertoire. Immunity. 2007;26(4):397–406. doi: 10.1016/j.immuni.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Dorfel D, Appel S, Grunebach F, et al. Processing and presentation of HLA class I and II epitopes by dendritic cells after transfection with in vitro-transcribed MUC1 RNA. Blood. 2005;105(8):3199–3205. doi: 10.1182/blood-2004-09-3556. [DOI] [PubMed] [Google Scholar]
- 63.Bland FA, Lemberg MK, McMichael AJ, et al. Requirement of the proteasome for the trimming of signal peptide-derived epitopes presented by the nonclassical major histocompatibility complex class I molecule HLA-E. J Biol Chem. 2003;278(36):33747–33752. doi: 10.1074/jbc.M305593200. [DOI] [PubMed] [Google Scholar]
- 64.Hammer GE, Gonzalez F, Champsaur M, et al. The aminopeptidase ERAAP shapes the peptide repertoire displayed by major histocompatibility complex class I molecules. Nat Immunol. 2006;7(1):103–112. doi: 10.1038/ni1286. [DOI] [PubMed] [Google Scholar]
- 65.Barrett AJ, Rawlings ND, Woessner JF. Handbook of Proteolytic Enzymes. London: Academic Press; 2004. [Google Scholar]
- 66.Craiu A, Akoplan T, Goldberg A, Rock KL. Two distinct proteolytic processes in the generation of a major histocompatibility complex class I-presented peptide. Proc Natl Acad Sci USA. 1997;94(20):10850–10855. doi: 10.1073/pnas.94.20.10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Powis SJ, Deverson EV, Coadwell WJ, et al. Effect of polymorphism of an MHC-linked transporter on the peptides assembled in a class I molecule. Nature. 1992;357:211–215. doi: 10.1038/357211a0. [DOI] [PubMed] [Google Scholar]
- 68.Ackerman AL, Kyritsis C, Tampé R, Cresswell P. Access of soluble antigens to the endoplasmic reticulum can explain cross-presentation by dendritic cells. Nat Immunol. 2005;6(1):107–113. doi: 10.1038/ni1147. [DOI] [PubMed] [Google Scholar]
- 69.Falk K, Rotzschke O, Rammensee HG. Cellular peptide composition governed by major histocompatibility complex class I molecules. Nature. 1990;348:248–251. doi: 10.1038/348248a0. [DOI] [PubMed] [Google Scholar]
- 70.Del Val M, Schlicht HJ, Ruppert T, et al. Efficient processing of an antigenic sequence for presentation by MHC class I molecules depends on its neighboring residues in the protein. Cell. 1991;66:1145–1153. doi: 10.1016/0092-8674(91)90037-Y. [DOI] [PubMed] [Google Scholar]
- 71.Koopmann JO, Albring J, Hüter E, et al. Export of antigenic peptides from the endoplasmic reticulum intersects with retrograde protein translocation through the Sec61p channel. Immunity. 2000;13(1):117–127. doi: 10.1016/S1074-7613(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 72.Ghanem E, Fritzsche S, Al-Balushi M, et al. The transporter associated with antigen processing (TAP) is active in a post-ER compartment. J Cell Sci. 2010;123(Pt 24):4271–4279. doi: 10.1242/jcs.060632. [DOI] [PubMed] [Google Scholar]
- 73.Donaldson JG, Williams DB. Intracellular assembly and trafficking of MHC class I molecules. Traffic. 2009;10(12):1745–1752. doi: 10.1111/j.1600-0854.2009.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]