Abstract
Apoptosis is a vital component in the evolutionarily conserved host defense system. Apoptosis is the guardian of tissue integrity by removing unfit and injured cells without evoking inflammation. However, apoptosis seems to be a double-edged sword since during low-level chronic stress, such as in aging, increased resistance to apoptosis can lead to the survival of functionally deficient, post-mitotic cells with damaged housekeeping functions. Senescent cells are remarkably resistant to apoptosis, and several studies indicate that host defense mechanisms can enhance anti-apoptotic signaling, which subsequently induces a senescent, pro-inflammatory phenotype during the aging process. At the molecular level, age-related resistance to apoptosis involves (1) functional deficiency in p53 network, (2) increased activity in the NF-κB-IAP/JNK axis, and (3) changes in molecular chaperones, microRNAs, and epigenetic regulation. We will discuss the molecular basis of age-related resistance to apoptosis and emphasize that increased resistance could enhance the aging process.
Keywords: Aging, Inflammaging, NF-κB, p53, Cellular senescence, Review
Introduction
The aging process represents a progressive decline in the physiological properties of tissues and the overall fitness of the organism. The replication capacity of cells decreases and the number of cell-cycle-arrested cells increases with aging. Cells also display prominent age-related structural and functional changes, e.g., dysfunction in mitochondrial respiration, disturbances in proteasomal and autophagic degradation, and accumulation of waste material into the cytoplasm and the lysosomal compartment. Cellular senescence is the state where cells have irreversibly lost their proliferation ability, and they exhibit deficiencies in maintaining their homeostatic processes [1, 2]. The number of senescent cells increases in tissues with aging (Fig. 1). There are diverse stress conditions that damage housekeeping mechanisms during aging, e.g., oxidative, metabolic, and genotoxic stresses (Fig. 2). Age-related degeneration can be a consequence of a genetic program or it may be an entropic process [3, 4]. Ultimately, disorders in housekeeping ability jeopardize homeostasis and expose cells to both apoptotic and necrotic forms of cell death.
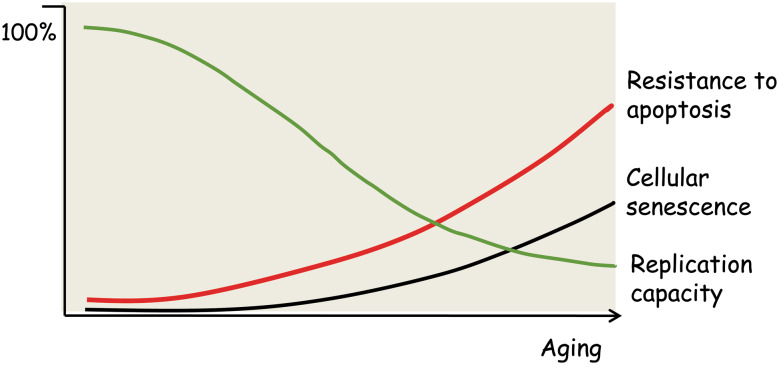
Fig. 1.
A schematic presentation of age-related changes in cellular phenotypes. Several studies indicate that cellular replication capacity declines in different tissues and immune system during aging [107–110], whereas the appearance of senescent cells increases (see text). Age-related changes are tissue specific, and the diagram depicts only the trend during aging rather than absolute changes. Alterations in cellular phenotypes are associated with an increase in the resistance to apoptosis
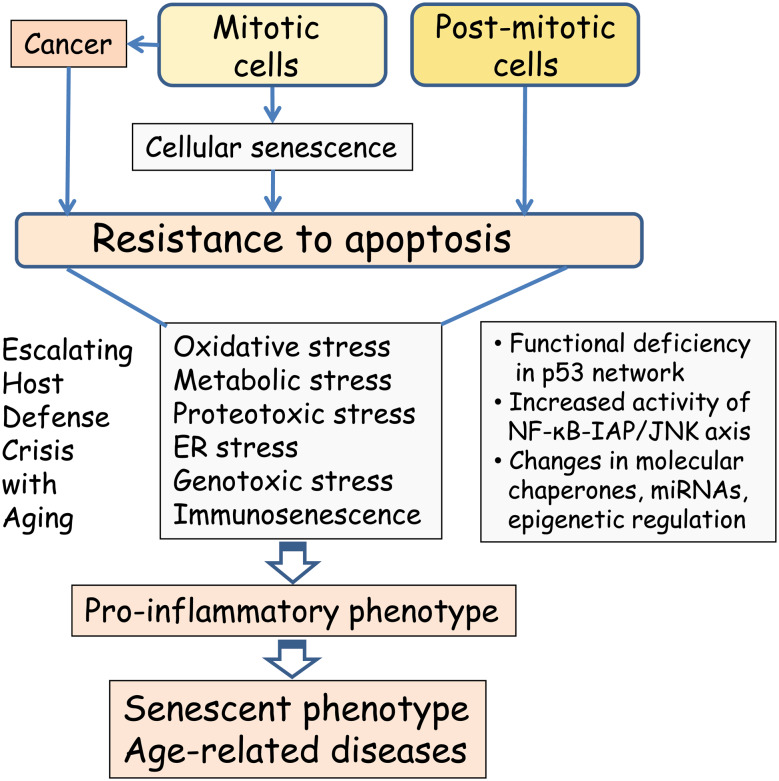
Fig. 2.
Increased resistance to apoptosis escalates the age-related host defense crisis. Persistent types of stress, e.g., oxidative, proteotoxic, and genotoxic stresses, enhance the cellular resistance to apoptosis leading to the survival of unfit, sublethally damaged cells that display a pro-inflammatory phenotype. The molecular basis of increased resistance to apoptosis involves several mechanisms, e.g., (1) functional deficiency in the p53 network, (2) increased activity in the NF-κB-IAP/JNK axis, and (3) changes in molecular chaperones, microRNAs, and epigenetic regulation. Mitotic cells can also undergo carcinogenesis, which also involves increased resistance to apoptosis but not the host defense crisis. the pro-inflammatory phenotype accelerates the aging process and aggravates age-related degenerative diseases
There are different types of cell death programs, these being triggered by the level of insult and cell type involved [5]. Apoptosis is a highly regulated suicide-type mechanism, in contrast to necrosis, which is a far less strictly controlled process. Kerr et al. [6] coined the term apoptosis, which means “falling leaves” in Greek. They characterized the morphology of apoptotic cell death and noted that apoptotic bodies can be phagocytized by neighboring cells and tissue histiocytes. In contrast to necrosis, apoptosis does not trigger inflammation and thus it saves the tissue from suffering the consequences of an inflammatory response that could aggravate the lesion. There have been many articles that have emphasized that apoptosis can proceed via multiple cascades and be balanced by pro- and antiapoptotic mechanisms [7, 8]. In addition, there is crosstalk between apoptosis and autophagy, a self-eating process [9]. All these observations indicate how versatile apoptotic cell death can be, e.g., in adapting to a variety of external and internal insults and proceeding via diverse pathways in different tissues [8, 10].
Apoptosis is a vital component of the evolutionarily conserved host defense system of organisms. When subjected to pathological attack, apoptosis is a guardian of tissues, since it can cleanse of unfit and injured cells without evoking inflammation [5, 9]. However, apoptosis seems to be a double-edged sword since several defense mechanisms increase resistance towards apoptosis by enhancing anti-apoptotic signaling and thus prevent the apoptotic death of sublethally injured cells (see below). Increased resistance is an important safety mechanism in acute stress since after the insult, the cell may still recover and revert to homeostasis. However, in persistent stress, such as in aging, increased apoptotic resistance can lead to the survival of unfit cells that are not able to maintain proper housekeeping functions. This increase in apoptotic resistance may be relevant if one considers the context of tissue integrity during aging, but it takes place at the cost of housekeeping potential and leads to a senescent phenotype in post-mitotic cells. Escalation of the host defense crisis proceeds via the activation of low-level, chronic inflammation in aging tissues [11–13]. We will discuss the molecular mechanisms that increase the resistance to apoptosis during the aging process and hypothesize that this “age-related resistance to apoptosis” can enhance the aging process.
Apoptosis: vital force in morphogenesis and cancer
Apoptosis and embryonic development
In 1965, Lockshin and Williams [14] coined the term programmed cell death (PCD) to point out that this kind of cell death, nowadays usually called apoptosis, is a programmed process and a part of normal embryogenesis. Studies have demonstrated that PCD has an important role in morphogenesis, e.g., un shaping the central nervous system and limb development [15]. Many genes that were later identified to be important players in the mammalian apoptotic process were first described in a genetic screen for developmental defects in Caenorhabditis elegans [16–18]. For instance, the genes of ced (cell death abnormal) and ces (cell-death specification) families have conserved homologues in Drosophila melanogaster and mammals [18]. In particular, studies using model organisms have revealed the inducer mechanisms of apoptosis, e.g., reaper gene activation in D. melanogaster [19, 20] and egl-1 (egg-laying defective) in C. elegans [18]. In mammals, BH3-only proteins, such as BAD (Bcl-xL/Bcl-2 associated death promoter) and BID (BH3-interacting domain death agonist), are proapoptotic triggers [21] (see below). Currently, it is considered that PCD is an integral part of animal development, and it is often called physiological apoptosis [18, 21].
Apoptosis and cancer
Cancer is a good example of the crucial role of apoptosis in its battle to maintain tissue stability in the face of a severe pathological assault. p53 is a major tumor suppressor protein that can recognize DNA damage and subsequently arrest the cell cycle and trigger the DNA repair process. If the damage cannot be repaired, p53 induces apoptosis in order to eliminate injured cells and thus prevent possible neoplastic transformation [22]. Moreover, p53 can also combat tumor progression by triggering cellular senescence of transformed cells [23–25]. There are several other tumor suppressor proteins, e.g., the p16INK4 family, that can arrest the cell cycle, induce senescence, and in that way prevent tumor progression [26]. Interestingly, increased resistance to apoptotic cell death is a hallmark of rapidly proliferating cancer cells inhibiting intrinsic and extrinsic pro-apoptotic pathways [25, 27]. Targeted inhibition of anti-apoptotic defense will provide novel cancer therapies in the future [28]. BCL-2, survivin, and XIAP are typical target proteins. Currently, antisense oligonucleotides, e.g., oblimersen for BCL-2 mRNAs and AEG35156 for XIAP mRNA, are in phase II and III clinical studies [28]. Another approach is to design small-molecule inhibitors or mimetics for antiapoptotic proteins. ABT-737 and Obatoclax are promising BH3 domain mimetics that bind to the BCL-2 hydrophobic cleft and can kill cancer cells [29]. There is also drug development for inhibitor of apoptosis (IAP) proteins [30]. In fact, cancer is only one example of several diseases where the ability to perform apoptosis-mediated cleansing of defective cells is impaired, which aggravates the disease process [31]. Apoptosis is involved in many age-related disorders, e.g., in cardiovascular diseases and neurodegeneration [31–33].
In conclusion, apoptosis plays important roles in developmental processes and in adult tissue, where it results in removal of infected, transformed, and damaged cells. However, cancer cells can hijack the anti-apoptotic capacity and escape the pro-apoptotic control mechanisms and jeopardize tissue integrity.
Resistance to apoptosis: a hallmark of cellular senescence
By definition, cellular senescence is an irreversible cell cycle arrest, but it also involves significant changes in gene expression, cellular morphology, and function [1, 23, 24]. However, it has been difficult to identify senescent cells since there are no specific biomarkers available. SA-β-gal (senescence-associated β-galactosidase), a lysosomal hydrolytic enzyme, is a generally accepted marker although it is not an exclusive marker of senescence. Another good marker is p16INK4a, an inhibitor of cyclin-dependent kinases [24, 26]. The protein level of p16INK4a is augmented in senescent cells, but its expression also clearly increases during organismal aging, probably reflecting cell cycle exit of cells in the tissues of old animals. It seems that p16INK4a could be an effector protein of senescence, in contrast to SA-β-gal, which reflects age-related biochemical responses. For instance, p16INK4a protein can regulate the senescence-related p53 network, especially during oncogene-induced senescence. A third, chromatin-level marker of senescence is SAHF (senescence-associated heterochromatin foci), which is a chromosomal condensed heterochromatin locus, organized by histone chaperones from heterochromatin-forming proteins [34]. Most likely, SAHF can repress the expression of genes promoting cellular proliferation and thus induce irreversible replication arrest.
In addition to growth arrest, increased resistance to apoptosis is a significant functional hallmark of senescent cells. In 1995, Eugenia Wang [35] demonstrated that replicatively senescent human fibroblasts were resistant to apoptotic insults. The protein level of Bcl-2 was significantly higher in late-passage fibroblasts compared to their young counterparts, and apoptotic treatment did not affect its expression level. This original observation has been replicated since in a variety of experimental models [36, 37]. Moreover, fibroblasts of progeroid Werner syndrome patients exhibit attenuation of p53-induced apoptosis [38]. Interestingly, expression of wild-type WRN gene, mutated in Werner patients, rescued the p53-mediated apoptotic capacity. Since WRN protein binds to p53 [38], this is evidence that p53 is involved in the regulation of cellular senescence (see below). Repeated, subtoxic UVB exposure is a well-known inducer of cell cycle arrest and can cause the appearance of apoptosis resistance. Using this photoaging model, Chen et al. [39] demonstrated that p53-mediated gene expression is involved in the appearance of apoptosis resistance in human skin fibroblasts. Recently, Rochette and Brash [37] reported that the appearance of resistance to UVB-induced apoptosis clearly precedes the progressive development of replicative senescence in human fibroblasts. They observed that UVB exposure could induce a prominent increase in the expression of Bcl-xL in old cells, but not in their young counterparts. In contrast, the levels of the pro-apoptotic Bax were clearly increased in young cells and triggered apoptosis.
There are clear cell type–dependent differences in the apoptotic resistance induced by growth arrest and cellular senescence in cultured cells. Senescent keratinocytes are resistant to UV-induced apoptosis [40], as are skin fibroblasts (see above). In fact, many studies have observed that senescent endothelial cells are more vulnerable to apoptosis than their younger cohorts if examined under in vitro cell culture conditions [41]. However, endothelial cells in cultured arteries have been reported to display an increased resistance to apoptosis in long-lived rodents [42]. One explanation could be that senescent endothelial cells in culture undergo anoikis, not apoptosis. Anoikis is a specific apoptotic process triggered by the loss of extracellular matrix interactions [43]. The mechanisms conferring resistance to anoikis are quite different from those inhibiting apoptosis.
In conclusion, as revealed in cell culture experiments, growth arrest and resistance to apoptosis are two hallmarks of senescent cells. Cellular senescence seems to be an evolutionary conserved defense against cancerous growth in multicellular organisms, but is the aging process the price?
Organismal aging: apoptosis repressed, cleansing impaired
As mentioned earlier for cultured cells, there are only a few, if any, direct indicators of cellular senescence. The expression level of p16INK4a has been a consistent senescence marker in vitro (see above), and Krishnamurthy et al. [44] demonstrated that its expression, at both mRNA and protein levels, also significantly increases in almost all rodent tissues during aging. Moreover, they noted that its expression clearly decreases during caloric restriction, a recognized longevity factor. Jeyapalan et al. [45] observed that in addition to p16INK4a, the markers of telomere damage and senescence-related heterochromatin proteins also significantly increased with aging in baboon skin fibroblasts. Melk et al. [46] demonstrated that the expressions of p16INK4a and SA-β-gal increase in rat kidney with aging. SA-β-gal staining was clearly associated with lipofuscin, a prominent aging pigment. In addition, studies on premature and accelerated aging models have revealed an increased level of senescence markers in the tissues. Hinkal et al. [47] observed that hyperactive p53+/m mice exhibited increased numbers of SA-β-gal-positive cells in all tissues studied, i.e., kidney, liver, and spleen, particularly with old age. Recent reviews have discussed in detail the role of cellular senescence in organismal aging [1, 2, 23, 48].
Increased cellular senescence is observed in several pathological conditions [1, 49, 50]. A stressful microenvironment in tissues, e.g., oxidative stress and inflammation, can trigger signaling pathways that induce the activation of cellular senescence. For instance, cellular senescence is augmented in vascular endothelium in many vascular diseases [50]. It seems that senescent endothelial cells can enhance the processes of atherosclerosis, thrombosis, and inflammation. In particular, the capacity of senescent cells to secrete inflammatory mediators may induce chronic inflammation in blood vessels. In addition, tumors often contain senescent cells, a process called oncogene-induced cellular senescence (OIS) [51, 52]. OIS is a mechanism that can restrict the tumorigenesis of benign tumors. In conclusion, it seems that the host tissues can react to pathological insults by triggering cellular senescence, which enhances the aging process (Fig. 2). This might be an important mechanism in certain age-related degenerative diseases.
It is still largely unclear whether age-related cellular senescence and quiescence of mitotic cells can elicit resistance to apoptosis in tissues, comparable to apoptotic resistance in cultured cells. The problem seems to be that apoptotic cells are not frequently encountered in tissue sections, this probably being attributable to either their fast clearance or to apoptotic resistance under normal conditions. Recently, Kavathia et al. [53] demonstrated that the intensity of global apoptosis significantly declines in humans during aging. They studied changes in serum markers of apoptosis, i.e., soluble Fas (inhibitor of apoptosis), sFasL (stimulator of apoptosis), and cytochrome c. They observed that the serum levels of total cytochrome c and sFasL decreased but the concentration of soluble Fas increased during aging. Interestingly, several studies have demonstrated that aging decreases the apoptotic response to genotoxic stress [47, 54]. Hinkal et al. [47] observed that ionizing radiation–induced apoptotic response was clearly less in the lymphoid organs of mutant p53+/m mice compared to wild-type mice. Instead, the extent of DNA damage and the levels of senescence markers, e.g., p16INK4a and p21, were significantly higher in the accelerated aging mice.
Organismal aging induces a decline in the function of the immune system, a process called immunosenescence [55]. Apoptosis is crucially involved in the age-related remodeling of the immune system, which includes thymic involution and alterations in T cells, i.e., decreased proliferation capacity and increased resistance to apoptosis [56–58]. De Martinis et al. [57] have described two different kinds of apoptotic pathways present in immune cells: (1) activation-induced cell death (AICD) and (2) damage-induced cell death (DICD). AICD eliminates superfluous clonal lymphocytes, e.g., via death receptors, whereas DICD prevents the transformation of damaged lymphocytes. Organismal aging seems to potentiate the apoptotic pathway of AICD and decreases the sensitivity to DICD [57]. A deficiency in the apoptotic capacity of the DICD pathway triggers the accumulation of dysfunctional, senescent lymphocytes. Subsequently, these lymphocytes also lose their membrane receptors and become resistant to apoptosis induced via the AICD pathway. The apoptotic resistance of senescent lymphocytes seems to be mediated by a defect in signaling of some death receptors, increased expression of anti-apoptotic proteins and inhibitory receptors, and inhibition of caspase activation [58]. The apoptotic deficiency in immunosenescent lymphocytes can also provoke autoimmune responses in the elderly. Lymphocyte senescence is probably one form of the rather common senescence-associated secretory phenotype (see below), and in that way it supports the appearance of age-related pro-inflammatory status, which accelerates the aging process and aggravates age-related degenerative diseases.
Increased resistance to apoptosis of senescent cells represents a threat to functional integrity, e.g., in the immune system, skin, and vascular endothelium. Increased tolerance of cells to molecular damage leads to the accumulation of intracellular waste products within nondividing cells, e.g., senescent cells and post-mitotic cells (Fig. 2). This activates autophagocytosis, a major cellular housekeeping mechanism that can remove damaged molecules and organelles. Recent studies have revealed that autophagy has an effector role in the induction of the senescent phenotype [59–61]. Young et al. [59] demonstrated that autophagy is clearly activated during the transition phase into cellular senescence in fibroblasts. This seems to be triggered by a negative feedback inhibition of PI3K-mTOR pathway (a well-known inhibitor of autophagy) that triggers the expression of target genes of autophagy. Zmpste24-knockout mice, an established premature aging model of Hutchinson-Gilford progeria, also exhibit increased autophagocytosis in several tissues [60]. These mice display significant metabolic changes, e.g., decreases in serum insulin and glucose concentrations, activation of LKB1-AMPK signaling, and inhibition of mTOR. The induction of autophagy in the early phase of the accelerated aging process seems to be a survival mechanism that compromises the metabolic disorders in the transition phase in order to inhibit apoptotic cell death [61]. However, many studies have demonstrated that autophagic clearance declines during the normal aging process [62, 63].
Molecular basis of increased resistance to apoptosis
The decision as to whether cells live or die by apoptosis involves the balance between pro-apoptotic and anti-apoptotic players. Intrinsic and extrinsic inducers can either enhance or repress the initiation of apoptosis. Mitochondria have a key role in the activation process but also in the repression [64]. The major components in the regulation of apoptosis include the Bcl-2 family of pro- and anti-apoptotic factors, death receptors, and caspase network. Apoptosis as a process and its regulation have been extensively reviewed recently [7, 8, 64]. However, the role of apoptosis in the aging process and in particular, the mechanisms of age-related repression, are largely unknown. Here we will discuss some mechanisms linked to age-related resistance of apoptosis and their impact on the aging process.
p53 network
Tumor suppressor protein p53 is the guardian of genomic stability, but the signaling network linked to p53 can recognize the viability of cells and choose which damaged cells will undergo apoptotic suicide. The host defense function of p53 has been thoroughly studied in the case of cancer, i.e., p53 protects tissues against tumor growth via apoptosis and cellular senescence (see above). Furthermore, mouse models of premature aging have revealed that hyperactivity of p53 may accelerate the aging process [65–67]. Increased p53 activity was induced by manipulating p53 activity directly, e.g., in p53+/m mutant mice, or indirectly via DNA damage in telomere-deficient mice, Ku80 knockout mice, and Zmpste24 null mice. However, the transgenic super-p53 mice carrying extra copies of wild-type p53 genes show no evidence of accelerated aging although they are cancer resistant [68]. Moreover, transgenic mice expressing a decreased level of Mdm2, an inhibitor of p53, displayed p53 hyperactivity linked to a normal aging process [69]. Given that the function of p53 is associated with a complex network of protein interactions, it seems that its connection to the aging process is dependent on other factors present in the network.
The p53 can regulate apoptosis in a transcription dependent and independent manner [64, 70]. The transcriptional pathway involves the induction of pro-apoptotic Bcl-2 factors, i.e., Bax, Bid, Noxa, and Puma (BH3-only proteins), and repression of anti-apoptotic Bcl-2, Bcl-xL, and survivin genes. The nontranscriptional pathway includes interactions with members of the Bcl-2 family that control MOMP (mitochondrial outer membrane permeabilization) and in that way the efflux of apoptogenic factors, e.g., cytochrome c and apoptosis-inducing factor (AIF) [7, 64, 70]. MOMP is regulated by the mitochondrial apoptosis-induced channel (MAC), which is formed by pro-apoptotic Bax and Bak proteins, but the inner membrane permeability transition pores (PTP) can also be involved in the passage of apoptogenic factors [71]. In this context, it is important to point out that a plethora of studies have indicated that mitochondria play a crucial role in the aging process [72]. Interestingly, Bax and Bak also regulate the efflux of calcium from the endoplasmic reticulum (ER) and subsequently can induce MOMP and trigger apoptosis. In fact, ER and mitochondria form a structural and functional interaction network, called mitochondria-associated membranes (MAM), which connects ER stress to mitochondrial apoptosis [73]. In MAM, sigma-1 receptors regulate calcium release from ER but also the expression of Bcl-2 protein and in that way resistance to apoptosis [74]. During aging, the efficiency of the stress recognition system in ER declines [75], which can prevent the initiation of apoptosis.
Transgenic gain-of-function models of p53 indicate that an increase in p53 activity could be the driving force of the aging process. However, Feng et al. [76] convincingly demonstrated that the functional activity of p53 declines in several murine tissues during aging. They observed that the p53-dependent apoptotic response to irradiation decreases during aging. This could be a consequence of the reduced transcriptional activity of p53 observed in several tissues with aging. Their study clearly indicates that premature aging induced by forced activation of p53 does not represent a physiological aging process. The results of Keyes et al. [77] also support this proposal since they observed that the loss of p63 protein, a p53-related protein, accelerates the aging process in mice, e.g., the expression of senescence markers SA-β-gal, p16INK4a, and PML considerably increased in adult skin after the inducible ablation of p63 in keratinocytes. The functional inefficiency of p53 could explain the reduction of mitochondrial respiration and increase in glycolytic metabolism during aging [78]. Kawauchi et al. [79] demonstrated that the deficiency in p53 activity increases glycolytic metabolism and activates IKKβ, which can stimulate the NF-κB-mediated resistance to apoptosis (see below). The reduction of p53 activity can also impair the expression of DRAM (damage-regulated autophagy modulator) [80], an inducer of autophagy, and in that way explains the paucity of autophagy during aging [63].
NF-κB-IAP/JNK axis
The NF-κB system is an ancient host defense system involved especially in the function of both adaptive and innate immunity [81]. Transcription factor NF-κB has a crucial role in the induction of resistance to apoptosis, e.g., in the carcinogenesis caused by inflammation [82]. NF-κB signaling can activate the transcription of many tumor-promoting cytokines and several anti-apoptotic survival genes, such as Bcl-xL and inhibitor of apoptosis (IAPs). The IAP family of apoptosis inhibitors includes several members, e.g., X-linked IAP (XIAP), c-IAP-1 and c-IAP-2 (cellular IAPs), and neuronal apoptosis inhibitory protein (NAIP) [83, 84]. IAPs contain BIR (baculovirus inhibitor of apoptosis protein repeat) domains, which can bind to caspases and inhibit the execution phase of apoptosis. Given that NF-κB can induce the expression of IAPs, IAPs can reciprocally stimulate the NF-κB signaling [83–85]. This positive feedback loop is one way to ensure a prompt response to acute insults. XIAP can bind via BIR domain to TAK1-binding protein 1 (TAB 1) and activate TAK1 (TGFβ -activated kinase 1). Subsequently, TAK1 activates NF-κB signaling, which further potentiates resistance to apoptosis. XIAP can also enhance NF-κB signaling via the inhibition of COMMD1, an inhibitor of NF-κB [85]. It seems that the NF-κB-IAP-axis has a crucial role in the induction of host defense, although all of the target proteins for the different IAP protein domains have not been clarified.
NF-κB signaling can repress apoptosis induced by c-Jun N-terminal kinase (JNK), an important mediator of apoptotic signals triggered by TNF-α and reactive oxygen species (ROS) [86]. NF-κB signaling can combat JNK activation by upregulating the expression of (1) Gadd45β, which inhibits MKK7, an upstream kinase of JNK and (2) manganese-dependent superoxide dismutase (Mn-SOD), which inhibits ROS-mediated JNK activation. Blocking the JNK signaling via NF-κB activation seems to be an evolutionarily conserved mechanism intended to prevent cell death in a variety of conditions of cell stress [86].
Several approaches have demonstrated that the aging process is associated with increased activation of the NF-κB system and the expression of several cytokines, e.g., IL-1β, IL-6, and IL-8 [4, 13, 87, 88]. NF-κB signaling is also one of the key factors in the appearance of the pro-inflammatory phenotype in the senescent cells [11, 12]. Chronic inflammation is also present in tissues during the aging process [13, 88, 89] as well as in several age-related diseases [90] (Fig. 2). Secreted cytokines and other inflammatory mediators can also activate NF-κB signaling and thus induce the expression of anti-apoptotic proteins, e.g., IAPs and Bcl-2, and inhibit JNK signaling, augmenting the resistance to apoptosis during aging.
Molecular chaperones
Molecular chaperones form a vital survival mechanism in cells against damaging insults. Chaperones, in particular heat shock proteins (HSPs), assist in refolding of unfolded proteins and can prevent detrimental aggregation. Moreover, HSPs can regulate different cellular functions by binding to many target proteins that support protein interactions. HSPs can be subdivided into families, including Hsp90, Hsp70, Hsp60, Hsp40, and small Hsps. Several studies have indicated that molecular chaperones increase stress resistance and cause longevity in model organisms, e.g., in Caenorhabditis elegans [91]. HSPs can regulate apoptosis and in many ways generate resistance against apoptotic cell death [92]. In particular, HSPs control the apoptotic process at the mitochondrial level targeting pro-apoptotic factors, thus preventing the release of apoptogenic factors from mitochondria. For instance, Hsp60 and Hsp70 can bind to Bax protein and block its translocation to the mitochondrial membrane [92]. Hsp27 binds to Bid and inhibits its mitochondrial redistribution. HSPs can also repress the formation of apoptosomes. Hsp70 and Hsp90 can bind to Apaf-1 and thus prevent the recruitment of procaspase-9 into the apoptosomes. All these observations indicate that HSPs are potent repressors of apoptosis. This mechanism may be important in acute cellular stress since the expression of HSPs is highly inducible by different stressors.
Currently, there are conflicting results on the role of HSPs in the aging process. Some studies have referred to decreased function of HSPs during aging [93], but in contrast, others have detected upregulation of HSP activity [94]. The controversy seems to focus on the expression levels in tissues being studied. However, one consistent observation seems to be an age-related deficiency in the induction of heat shock response via heat shock factor (HSF) [93].
Epigenetics and microRNAs
The aging process and cellular senescence have been associated with changes in epigenetic regulation, particularly in DNA methylation and chromatin modification [34, 95, 96]. Several studies have demonstrated that global DNA methylation of the genome declines with aging, whereas the promoters of certain genes can be specifically hypermethylated and genes silenced. Histones are also modified during aging, i.e., specific methylation of histones increases along with the global acetylation level. In senescent cells, there appears to be transcriptionally inactive SAHF domains [34], but also in aging tissues there seems to be significant epigenetic changes. In cancer, increased DNA methylation is linked to enhanced proliferation, but there are emerging results demonstrating that an increase in apoptosis resistance can also be caused by DNA hypermethylation of some apoptotic regulatory genes [97, 98] For instance, promoter hypermethylation can downregulate the expression of FAS and XAF1 genes. XAF1 protein is a potent inhibitor of anti-apoptotic XIAP protein and thus silencing of the XAF1 gene increases apoptosis resistance [97]. Whether age-related epigenetic changes target apoptotic pathways remains to be elucidated.
MicroRNAs (miRNAs or miRs) are small noncoding RNAs that are emerging as important gene expression regulators, e.g., in development, cancer, apoptosis, inflammation, and aging [99]. There are over 1,000 mammalian miRNAs of which about 30 are associated with the regulation of apoptosis. MiRNAs are repressors of gene expression by degrading target gene mRNAs or inhibiting their translation. MiRNAs can control both pro-apoptotic and anti-apoptotic pathways [99]. In cancer, miRNAs can induce resistance to apoptosis by downregulating pro-apoptotic miRNAs, such as the miR-15/16 and miR-34 family, or by provoking the expression of anti-apoptotic miRNAs, e.g., miR-17-92, miR-21, and miR-155 [99, 100]. Currently, there are only a few studies on changes in miRNA expression patterns in cellular senescence models and aging process. For instance, Bonifacio and Jarstfer [101] observed that miR-143 can induce senescence-associated growth arrest and it is upregulated in senescent human fibroblasts. Drummond et al. [102] demonstrated that the expression of Let-7 family members, Let-7b and Let-7e, is significantly elevated in age-related human skeletal muscle sarcopenia. Large-scale and multi-tissue studies will be required to establish whether age-related changes are associated with apoptotic miRNAs. Interestingly, some apoptosis-linked miRNAs are targets of the p53 network and under epigenetic regulation, e.g., the miR-34 family [100]. Moreover, inflammatory signals can activate a large set of miRNAs [103], some of which are targets of NF-κB signaling, such as miR-146a and miR-155, and in that way, can regulate the resistance to age-related apoptosis.
Escalation of host defense crisis: pro-inflammatory phenotype
The overwhelming resistance to apoptosis has devastating effects during aging, i.e., mitotic cells can evolve into tumorigenesis or cellular senescence, whereas post-mitotic cells can survive excessive stress, e.g., oxidative, genotoxic, and metabolic stresses, although homeostasis is compromised and sublethal damage accumulates as proposed by the so-called garbage can hypothesis. As a counterbalance, the affected cell can elicit innate immune response in order to alert neighboring cells and the organism of accumulating damage. There are receptors that can recognize the molecular patterns of self-damage, so-called damage-associated molecular patterns (DAMPs), and then the cell can send alarm molecules, e.g., cytokines and chemokines, to recruit phagocytes to support the rescue process [104]. Resistance to apoptotic cell death escalates the host defense crisis, generating a chronic inflammatory reaction (Fig. 2).
Campisi et al. [11, 12, 23] have demonstrated that senescent cells secrete pro-inflammatory proteins, such as interleukins, chemokines, growth factors, and proteases. This pro-inflammatory state of senescent cells has been called the senescence-associated secretory phenotype (SASP). SASP can be induced by several senescence inducers, e.g., DNA damage and oncogenic stress, in fibroblasts and endothelial cells but also in many other cell types [11]. Molecular regulation of the SASP is still unknown but recently Orjalo et al. [105] observed that cell surface-bound IL-1α is an important regulator of the IL-6/IL-8-driven pro-inflammatory network. NF-κB and C/EBPβ are the key transcription factors involved in this pro-inflammatory pathway.
There is a large body of evidence indicating that the aging process is associated with the appearance of a pro-inflammatory phenotype [13, 88, 89]. Franceschi et al. [89] were the first to formulate the concept of inflamm-aging, i.e., the decline in adaptive immunity with aging that generates a state of immunosenescence that can stimulate innate immunity reactions and provoke a chronic, low-level inflammatory state. Gene expression profiles from mice, rats, and human tissues have revealed that the most common age-related signature is the upregulation of inflammatory and immune response genes [88]. The levels of serum cytokines, e.g., IL-6 and TNF-α, also increase during aging [106]. Several studies have highlighted the role of NF-κB signalling in the aging process [13, 87, 88] as well as in cellular senescence [12]. Adler et al. [87] demonstrated that the NF-κB binding domain was most frequently associated with the promoters of genes upregulated during aging as well as with those that are overexpressed in Hutchinson-Guilford progeria. The NF-κB signalling pathway can be stimulated by a number of environmental and internal danger signals, e.g., oxidative stress, genotoxic stress, and tissue injuries [4, 81, 82, 90]. Interestingly, several pro-aging signals are NF-κB activators, whereas many longevity factors, e.g., SIRT1, p53, and HSPs, are NF-κB inhibitors [4]. In conclusion, the pro-inflammatory phenotype seems to be a driving force in age-related host defense crisis.
Conclusions
Apoptosis, also called programmed cell death, is a vital force in the shaping of tissues during embryogenesis. Later in life, cells can combat cancerous growth by enhancing apoptotic cell death or by triggering cellular senescence, an irreversible growth arrest in response to cellular stress and damage. There is clear evidence to indicate that senescent cells are remarkably resistant to apoptosis. Obviously, the regulation of apoptotic resistance is a crucial defense mechanism against sublethal damage induced by environmental and cellular stress situations, e.g., oxidative, proteotoxic, and genotoxic stresses. Interestingly, both cancer cells and senescent cells have adopted the same strategy, i.e., enhancement of resistance to apoptosis. The outcome may be detrimental to the organism in both cases although via different phenotypes. In post-mitotic cells, this substantial anti-apoptotic defense is a double-edged sword that increases the entropy via the accumulation of nonfunctional proteins and organelles, in particular during periods of enhanced stress. UVB-induced photoaging is a good example of this situation.
Deficient cleansing of tissues of damaged cells triggers another host defense mechanism, i.e., inflammation. Recent studies have revealed that senescent cells possess a pro-inflammatory phenotype, secreting inflammatory mediators. Inflammation is a logical cellular response to impending cell death. The cell can release inflammatory factors that then recruit phagocytes to remove the soon-to-appear apoptotic bodies or necrotic waste. An extensive literature shows that low-level, chronic inflammation is associated with the aging process. Inflammation aggravates the microenvironment in aging tissues by secreting (1) proteinases that degenerate the extracellular matrix and (2) cytokines and growth factors that can even enhance apoptotic resistance and stimulate cancerous growth. We propose that the increased resistance to apoptosis of senescent cells can aggravate the age-related host defense crisis that is enhanced by chronic inflammation.
Acknowledgments
This study was financially supported by grants from the Academy of Finland and the University of Eastern Finland, Kuopio, Finland. The authors thank Dr. Ewen MacDonald for checking the language of the manuscript.
References
- 1.Muller M. Cellular senescence: molecular mechanisms, in vivo significance, and redox considerations. Antioxid Redox Signal. 2009;11:59–98. doi: 10.1089/ars.2008.2104. [DOI] [PubMed] [Google Scholar]
- 2.Hornsby PJ. Senescence and life span. Pflugers Arch Eur J Physiol. 2010;459:291–299. doi: 10.1007/s00424-009-0723-6. [DOI] [PubMed] [Google Scholar]
- 3.Hayflick L. Entropy explains aging, genetic determinism explains longevity, and undefined terminology explains misunderstanding both. PLoS Genet. 2007;3:e220. doi: 10.1371/journal.pgen.0030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salminen A, Kaarniranta K. Genetics vs. entropy: longevity factors suppress the NF-κB-driven entropic aging process. Ageing Res Rev. 2010;9:298–314. doi: 10.1016/j.arr.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blakosglonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malomi W, Nunez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 8.Kurokawa M, Kornbluth S. Caspases and kinases in a death grip. Cell. 2009;138:838–854. doi: 10.1016/j.cell.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 10.Patel VA, Lee DJ, Feng L, Antoni A, Lieberthal W, Schwartz JH, Rauch J, Ucker DS, Levine JS. Recognition of apoptotic cells by epithelial cells. Conserved versus tissue-specific signaling responses. J Biol Chem. 2010;285:1829–1840. doi: 10.1074/jbc.M109.018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol Mech Dis. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Activation of innate immunity system during aging: NF-κB signaling is the molecular culprit of inflamm-aging. Ageing Res Rev. 2008;7:83–105. doi: 10.1016/j.arr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Lockshin RA, Williams CM. Programmed cell death-I. Cytology of degeneration in the intersegmental muscles of the Pernyi silkmoth. J Insect Physiol. 1965;11:123–133. doi: 10.1016/0022-1910(65)90099-5. [DOI] [PubMed] [Google Scholar]
- 15.Penaloza C, Lin L, Lockshin RA, Zakeri Z. Cell death in development: shaping the embryo. Histochem Cell Biol. 2006;126:149–158. doi: 10.1007/s00418-006-0214-1. [DOI] [PubMed] [Google Scholar]
- 16.Horvitz HR, Sulston JE. Isolation and genetic characterization of cell-lineage mutants of the nematode Caenorhabditis elegans . Genetics. 1980;96:435–454. doi: 10.1093/genetics/96.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Driscoll M. Cell death in C. elegans: molecular insights into mechanisms conserved between nematodes and mammals. Brain Pathol. 1996;6:411–425. doi: 10.1111/j.1750-3639.1996.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 18.Lettre G, Hengartner MO. Developmental apoptosis in C. elegans: a complex CEDnario. Nat Rev Mol Cell Biol. 2006;7:97–108. doi: 10.1038/nrm1836. [DOI] [PubMed] [Google Scholar]
- 19.White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H. Genetic control of programmed cell death in Drosophila . Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- 20.Steller H. Regulation of apoptosis in Drosophila . Cell Death Differ. 2008;15:1132–1138. doi: 10.1038/cdd.2008.50. [DOI] [PubMed] [Google Scholar]
- 21.Conradt B. Genetic control of programmed cell death during animal development. Annu Rev Genet. 2009;43:493–523. doi: 10.1146/annurev.genet.42.110807.091533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–9040. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- 23.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 25.Zuckerman V, Wolyniec K, Sionov RV, Haupt S, Haupt Y. Tumour suppression by p53: the importance of apoptosis and cellular senescence. J Pathol. 2009;219:3–15. doi: 10.1002/path.2584. [DOI] [PubMed] [Google Scholar]
- 26.Gil J, Peters G. Regulation of the INK4b-ARF-INK4a tumor suppressor locus: all for one or one for all. Nat Rev Mol Cell Biol. 2006;7:667–677. doi: 10.1038/nrm1987. [DOI] [PubMed] [Google Scholar]
- 27.Fulda S. Tumor resistance to apoptosis. Int J Cancer. 2009;124:511–515. doi: 10.1002/ijc.24064. [DOI] [PubMed] [Google Scholar]
- 28.Call JA, Eckhardt SG, Camidge DR. Targeted manipulation of apoptosis in cancer treatment. Lancet Oncol. 2009;9:1002–1011. doi: 10.1016/S1470-2045(08)70209-2. [DOI] [PubMed] [Google Scholar]
- 29.Chonghaile TN, Letai A. Mimicking the BH3 domain to kill cancer cells. Oncogene. 2009;27:S149–S157. doi: 10.1038/onc.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vucic D, Fairbrother WJ. The inhibitor of apoptosis proteins as therapeutic targets in cancer. Clin Cancer Res. 2007;13:5995–6000. doi: 10.1158/1078-0432.CCR-07-0729. [DOI] [PubMed] [Google Scholar]
- 31.Zhivotovsky B, Orrenius S. Cell death mechanisms: cross-talk and role in disease. Exp Cell Res. 2010;316:1374–1383. doi: 10.1016/j.yexcr.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 32.Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- 34.Funayama R, Ishikawa F. Cellular senescence and chromatin structure. Chromosoma. 2007;116:431–440. doi: 10.1007/s00412-007-0115-7. [DOI] [PubMed] [Google Scholar]
- 35.Wang E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res. 1995;55:2284–2292. [PubMed] [Google Scholar]
- 36.Ryu SJ, Oh YS, Park SC. Failure of stress-induced downregulation of Bcl-2 contributes to apoptosis resistance in senescent human diploid fibroblasts. Cell Death Differ. 2007;14:1020–1028. doi: 10.1038/sj.cdd.4402091. [DOI] [PubMed] [Google Scholar]
- 37.Rochette PJ, Brash DE. Progressive apoptosis resistance prior to senescence and control by the anti-apoptotic protein BCL-xL. Mech Ageing Dev. 2008;129:207–214. doi: 10.1016/j.mad.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spillare EA, Robles AI, Wang XW, Shen JC, Yu CE, Schellenberg GD, Harris CC. p53-mediated apoptosis is attenuated in Werner syndrome cells. Genes Dev. 1999;13:1355–1360. doi: 10.1101/gad.13.11.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen W, Kang J, Xia J, Li Y, Yang B, Chen B, Sun W, Song X, Xiang W, Wang X, Wang F, Wan Y, Bi Z. p53-related apoptosis resistance and tumor suppression activity in UVB-induced premature senescent human skin fibroblasts. Int J Mol Med. 2008;21:645–653. [PubMed] [Google Scholar]
- 40.Chaturvedi V, Qin JZ, Stennett L, Choubey D, Nickoloff BJ. Resistance to UV-induced apoptosis in human keratinocytes during accelerated senescence is associated with functional inactivation of p53. J Cell Physiol. 2004;198:100–109. doi: 10.1002/jcp.10392. [DOI] [PubMed] [Google Scholar]
- 41.Hampel B, Malisan F, Niederegger H, Testi R, Jansen-Durr P. Differential regulation of apoptotic cell death in senescent human cells. Exp Gerontol. 2004;39:1713–1721. doi: 10.1016/j.exger.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Labinskyy N, Csiszar A, Orosz Z, Smith K, Rivera A, Buffenstein R, Ungvari Z. Comparison of endothelial function, O2−* and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol Heart Circ Physiol. 2006;291:H2698–H2704. doi: 10.1152/ajpheart.00534.2006. [DOI] [PubMed] [Google Scholar]
- 43.Chiarugi P, Giannoni E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol. 2008;76:1352–1364. doi: 10.1016/j.bcp.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 44.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink42/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev. 2007;128:36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melk A, Kittikowit W, Sandhu I, Halloran KM, Grimm P, Schmidt BM, Halloran PF. Cell senescence in rat kidney in vivo increases with growth and age despite lack of telomere shortening. Kidney Int. 2003;63:2134–2143. doi: 10.1046/j.1523-1755.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 47.Hinkal GW, Gatza CE, Parikh N, Donehower LA. Altered senescence, apoptosis, and DNA damage response in a mutant p53 model of accelerated aging. Mech Ageing Dev. 2009;130:262–271. doi: 10.1016/j.mad.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeyapalan JC, Sedivy JM. Cellular senescence and organismal aging. Mech Ageing Dev. 2008;129:467–474. doi: 10.1016/j.mad.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Evan GI, d’Adda di Fagagna F. Cellular senescence: hot or what? Curr Opin Genet Dev. 2009;19:25–31. doi: 10.1016/j.gde.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Erusalimsky JD. Vascular endothelium senescence: from mechanisms to pathophysiology. J Appl Physiol. 2009;106:326–332. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Courtois-Cox S, Jones SL, Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene. 2008;27:2801–2809. doi: 10.1038/sj.onc.1210950. [DOI] [PubMed] [Google Scholar]
- 52.Chandeck C, Mooi WJ. Oncogene-induced cellular senescence. Adv Anat Pathol. 2010;17:42–48. doi: 10.1097/PAP.0b013e3181c66f4e. [DOI] [PubMed] [Google Scholar]
- 53.Kavathia N, Jain A, Walston J, Beamer BA, Fedarko NS. Serum markers of apoptosis decrease with age and cancer stage. Aging. 2009;1:652–663. doi: 10.18632/aging.100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suh Y, Lee KA, Kim WH, Han BG, Vijg J, Park SC. Aging alters the apoptotic response to genotoxic stress. Nat Med. 2002;8:3–4. doi: 10.1038/nm0102-3. [DOI] [PubMed] [Google Scholar]
- 55.Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology. 2008;23:64–74. doi: 10.1152/physiol.00040.2007. [DOI] [PubMed] [Google Scholar]
- 56.Spaulding C, Guo W, Effros RB. Resistance to apoptosis in human CD8+ T cells that reach replicative senescence after multiple rounds of antigen-specific proliferation. Exp Gerontol. 1999;34:633–644. doi: 10.1016/S0531-5565(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 57.De Martinis M, Franceschi C, Monti D, Ginaldi L. Apoptosis remodeling in immunosenescence: implications for strategies to delay ageing. Curr Med Chem. 2007;14:1389–1397. doi: 10.2174/092986707780831122. [DOI] [PubMed] [Google Scholar]
- 58.Giovannetti A, Pierdominici M, Di Iorio A, Cianci R, Murdaca G, Puppo F, Pandolfi F, Paganelli R. Apoptosis in the homeostasis of the immune system and in human immune mediated diseases. Curr Pharm Des. 2008;14:253–268. doi: 10.2174/138161208783413310. [DOI] [PubMed] [Google Scholar]
- 59.Young AR, Narita M, Ferreira M, Kirschner K, Sadaie M, Darot JF, Tavare S, Arakawa S, Shimizu S, Watt FM, Narita M. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23:798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marino G, Ugalde AP, Salvador-Montoliu N, Varela I, Quiros PM, Cadinanos J, van der Pluijm I, Freije JM, Lopez-Otín C. Premature aging in mice activates a systemic metabolic response involving autophagy induction. Hum Mol Genet. 2008;17:2196–2211. doi: 10.1093/hmg/ddn120. [DOI] [PubMed] [Google Scholar]
- 61.Young ARJ, Narita M. Connecting autophagy to senescence in pathophysiology. Curr Opin Cell Biol. 2010;22:234–240. doi: 10.1016/j.ceb.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Rajawat YS, Hilioti Z, Bossis I. Aging: central role for autophagy and the lysosomal degradation. Ageing Res Rev. 2009;8:199–213. doi: 10.1016/j.arr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 63.Salminen A, Kaarniranta K. Regulation of the aging process by autophagy. Trends Mol Med. 2009;15:217–224. doi: 10.1016/j.molmed.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 64.Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodier F, Campisi J, Bhaumik D. Two faces of p53: aging and tumor suppression. Nucleic Acids Res. 2007;35:7475–7484. doi: 10.1093/nar/gkm744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmid G, Kramer MP, Maurer M, Wandl S, Wesierska-Gadek J. Cellular and organismal ageing: role of the p53 tumor suppressor protein in the induction of transient and terminal senescence. J Cell Biochem. 2007;101:1355–1369. doi: 10.1002/jcb.21383. [DOI] [PubMed] [Google Scholar]
- 67.Lozano G. Mouse models of p53 functions. Cold Spring Harb Perspect Biol. 2009;2:a001115. doi: 10.1101/cshperspect.a001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.García-Cao I, García-Cao M, Martín-Caballero J, Criado LM, Klatt P, Flores JM, Weill JC, Blasco MA, Serrano M. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 2002;21:6225–6235. doi: 10.1093/emboj/cdf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mendrysa SM, O’Leary KA, McElwee MK, Michalowski J, Eisenman RN, Powell DA, Perry ME. Tumor suppression and normal aging in mice with constitutively high p53 activity. Genes Dev. 2006;20:16–21. doi: 10.1101/gad.1378506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Speidel D. Transcription-independent p53 apoptosis: an alternative route to death. Trends Cell Biol. 2009;20:14–24. doi: 10.1016/j.tcb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 71.Kinnally AW, Antonsson B. A tale of two mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis. 2007;12:857–868. doi: 10.1007/s10495-007-0722-z. [DOI] [PubMed] [Google Scholar]
- 72.Lanza IR, Nair KS. Mitochondrial function as a determinant of life span. Pflugers Arch Eur J Physiol. 2010;459:277–289. doi: 10.1007/s00424-009-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giorgi C, De Stefani D, Bononi A, Rizzuto R, Pinton P. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol. 2009;41:1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meunier J, Hayashi T. Sigma-1 receptors regulate Bcl-2 expression by reactive oxygen species-dependent transcriptional regulation of nuclear factor λB. J Pharmacol Exp Ther. 2010;332:388–397. doi: 10.1124/jpet.109.160960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salminen A, Kaarniranta K. ER stress and hormetic regulation of the aging process. Ageing Res Rev. 2010;9:211–217. doi: 10.1016/j.arr.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 76.Feng Z, Hu W, Teresky AK, Hernando E, Cordon-Cardo C, Levine AJ. Declining p53 function in the aging process: a possible mechanism for the increased tumor incidence in older populations. Proc Natl Acad Sci USA. 2007;104:16633–16638. doi: 10.1073/pnas.0708043104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keyes WM, Wu Y, Vogel H, Guo X, Lowe SW, Mills AA. p63 deficiency activates a program of cellular senescence and leads to accelerated aging. Genes Dev. 2005;19:1986–1999. doi: 10.1101/gad.342305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salminen A, Kaarniranta K. Glycolysis links p53 function with NF-κB signaling: impact on cancer and aging process. J Cell Physiol. 2010;224:1–6. doi: 10.1002/jcp.22119. [DOI] [PubMed] [Google Scholar]
- 79.Kawauchi K, Araki K, Tobiume K, Tanaka N. p53 regulates glucose metabolism through an IKK-NF-κB pathway and inhibits cell transformation. Nat Cell Biol. 2008;10:611–618. doi: 10.1038/ncb1724. [DOI] [PubMed] [Google Scholar]
- 80.Crighton D, Wilkinson S, O’Prey J, Syed N, Smith P, Harrison PR, Gasco M, Garrone O, Crook T, Ryan KM. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121–134. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 81.Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 82.Karin M. NF-κB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dubrez-Daloz L, Dupoux A, Cartier J. IAPs. More than just inhibitors of apoptosis. Cell Cycle. 2008;7:1036–1046. doi: 10.4161/cc.7.8.5783. [DOI] [PubMed] [Google Scholar]
- 84.Varfolomeev E, Vucic D. (Un)expected roles of c-IAPs in apoptotic and NF-κB signaling pathways. Cell Cycle. 2008;7:1511–1521. doi: 10.4161/cc.7.11.5959. [DOI] [PubMed] [Google Scholar]
- 85.Galban S, Duckett CS. XIAP as a ubiquitin ligase in cellular signaling. Cell Death Differ. 2010;17:54–60. doi: 10.1038/cdd.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Papa S, Bubici C, Zazzeroni F, Pham CG, Kuntzen C, Knabb JR, Dean K, Franzoso G. The NF-κB-mediated control of the JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Differ. 2006;13:712–729. doi: 10.1038/sj.cdd.4401865. [DOI] [PubMed] [Google Scholar]
- 87.Adler AS, Sinha S, Kawahara TL, Zhang JY, Segal E, Chang HY. Motif module map reveals enforcement of aging by continual NF-κB activity. Genes Dev. 2007;21:3244–3257. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Magalhaes JP, Curado J, Church GM. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics. 2009;25:875–881. doi: 10.1093/bioinformatics/btp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Franceschi C, Valensin S, Bonafè M, Paolisso G, Yashin AI, Monti D, De Benedictis G. The network and the remodeling theories of aging: historical background and new perspectives. Exp Gerontol. 2000;35:879–896. doi: 10.1016/S0531-5565(00)00172-8. [DOI] [PubMed] [Google Scholar]
- 90.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-κB: its role in health and disease. J Mol Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 91.Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15:657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C. Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med. 2008;12:743–761. doi: 10.1111/j.1582-4934.2008.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Calderwood SK, Murshid A, Prince T. The shock of aging: molecular chaperones and the heat shock response in longevity and aging—a mini-review. Gerontology. 2009;55:550–558. doi: 10.1159/000225957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tower J. Hsps and aging. Trends Endocrinol Metab. 2008;20:216–222. doi: 10.1016/j.tem.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gravina S, Vijg J. Epigenetic factors in aging and longevity. Pflugers Arch Eur J Physiol. 2010;459:247–258. doi: 10.1007/s00424-009-0730-7. [DOI] [PubMed] [Google Scholar]
- 96.Munoz-Najar UM, Sedivy JM (2010) Epigenetic control of aging. Antioxid Redox Signal. doi:10.1089/ars.2010.3250 [DOI] [PMC free article] [PubMed]
- 97.Murphy TM, Perry AS, Lawler M. The emergence of DNA methylation as a key modulator of aberrant cell death in prostate cancer. Endocr Relat Cancer. 2008;15:11–25. doi: 10.1677/ERC-07-0208. [DOI] [PubMed] [Google Scholar]
- 98.Jones CL, Wain EM, Chu CC, Tosi I, Foster R, McKenzie RC, Whittaker SJ, Mitchell TJ. Downregulation of Fas gene expression in Sezary syndrome is associated with promoter hypermethylation. J Invest Dermatol. 2010;130:1116–1125. doi: 10.1038/jid.2009.301. [DOI] [PubMed] [Google Scholar]
- 99.Subramanian S, Steer CJ. MicroRNAs as gatekeepers of apoptosis. J Cell Physiol. 2010;223:289–298. doi: 10.1002/jcp.22066. [DOI] [PubMed] [Google Scholar]
- 100.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 101.Bonifacio LN, Jarstfer MB. MiRNA profile associated with replicative senescence, extended cell culture, and ectopic telomerase expression in human foreskin fibroblasts. PLoS One. 2010;5:e12519. doi: 10.1371/journal.pone.0012519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Drummond MJ, McCarthy JJ, Sinha M, Spratt HM, Volpi E, Esser KA, Rasmussen BB (2010) Aging and microRNAs expression in human skeletal muscle: a microarray and bioinformatics analysis. Physiol Genomics. doi:10.1152/physiolgenomics.00148.2010 [DOI] [PMC free article] [PubMed]
- 103.Schetter AJ, Heegaard NHH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leuk Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 105.Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J. Cell surface-bound IL-1α is an upstream regulator of senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci USA. 2009;106:17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 107.Schneider EL, Sternberg H, Tice RR, Senula GC, Kram D, Smith JR, Bynum G. Cellular replication and aging. Mech Ageing Dev. 1979;9:313–324. doi: 10.1016/0047-6374(79)90108-8. [DOI] [PubMed] [Google Scholar]
- 108.Murasko DM, Goonewardene IM. T-cell function in aging: mechanisms of decline. Annu Rev Gerontol Geriatr. 1990;10:71–96. doi: 10.1007/978-3-662-38445-9_5. [DOI] [PubMed] [Google Scholar]
- 109.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Timchenko NA. Aging and liver regeneration. Trends Endocrinol Metab. 2009;20:171–176. doi: 10.1016/j.tem.2009.01.005. [DOI] [PubMed] [Google Scholar]