Abstract
Several mechanisms have been proposed to explain the E-cadherin dysfunction in cancer, including genetic and epigenetic alterations. Nevertheless, a significant number of human carcinomas have been seen that show E-cadherin dysfunction that cannot be explained at the genetic/epigenetic level. A substantial body of evidence has appeared recently that supports the view that other mechanisms operating at the post-translational level may also affect E-cadherin function. The present review addresses molecular aspects related to E-cadherin N-glycosylation and evidence is presented showing that the modification of N-linked glycans on E-cadherin can affect the adhesive function of this adhesion molecule. The role of glycosyltransferases involved in the remodeling of N-glycans on E-cadherin, including N-acetylglucosaminyltransferase III (GnT-III), N-acetylglucosaminyltransferase V (GnT-V), and the α1,6 fucosyltransferase (FUT8) enzyme, is also discussed. Finally, this review discusses an alternative functional regulatory mechanism for E-cadherin operating at the post-translational level, N-glycosylation, that may underlie the E-cadherin dysfunction in some carcinomas.
Keywords: E-cadherin, N-glycosylation, Glycosyltransferases, Cell adhesion, Cancer
Introduction
Cadherins comprise a large family of transmembrane glycoproteins that mediate specific cell–cell adhesion in a calcium-dependent manner, functioning as key molecules in the morphogenesis of a variety of organs [1]. E-cadherin, a type-I cadherin, is generally considered to be the prototype of all cadherins. The mature E-cadherin molecule, with an approximate molecular mass of 120 kDa, comprises a single transmembrane domain, a cytoplasmic domain (C-terminal) of about 150 amino acids, and an ectodomain of about 550 amino acids comprised of five repeated domains (EC1 to EC5) (Figs. 1, 2).
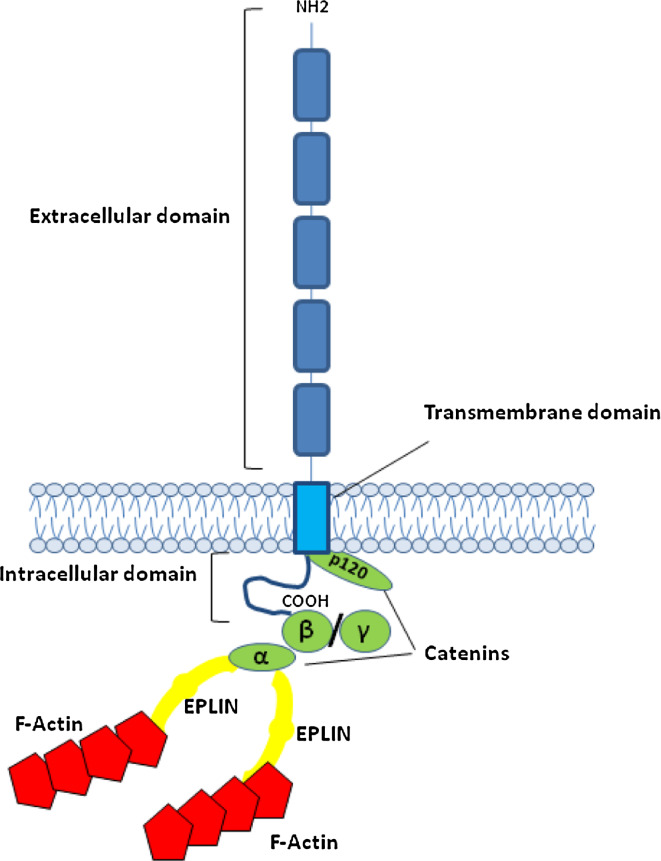
Fig. 1.
Schematic representation of the E-cadherin–catenin complex. The E-cadherin–catenin complex is proposed to interact with F-actin via α-catenin association with actin-binding proteins such as EPLIN [5]. β-Catenin and γ-catenin bind to E-cadherin in a mutually exclusive manner
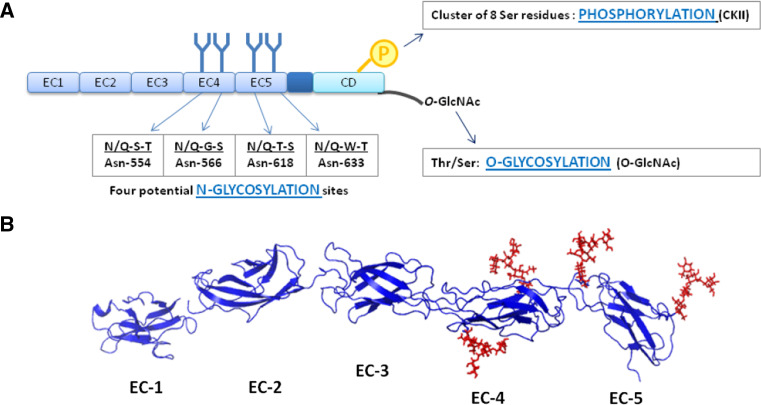
Fig. 2.
E-cadherin post-translational modifications. a The extracellular domain (EC) of human E-cadherin contains four potential N-glycosylation sites, which are located in EC4 and EC5. The phosphorylation of E-cadherin by casein kinase II (CKII) can occur in a short stretch of 30 aa in the cytoplasmic domain (CD), which contains a cluster of 8 Ser residues. Cytoplasmic O-glycosylation (O-GlcNAc addition in Thr/Ser residues) has been reported to regulate E-cadherin. These mechanims can modulate E-cadherin mediated cell–cell adhesion at a post-translational level. b Three-dimensional structure of the extracellular domain (EC1–EC5) of E-cadherin. The crystal structure of human EC1 was used in this representation and EC2–EC5 were modeled based on the crystal structure of C-cadherin. Four N-glycans were modeled with GlyProt (http://www.glycosciences.de/glyprot/), as shown in red
Crystal structures and mutagenesis studies support a model in which the first extracellular domain (EC1) is involved in binding to an opposing cadherin [2]. However, recent evidence suggests that extracellular domains in addition to EC1 regulate the cadherin function [3]. The binding between the extracellular domains of cadherin is weak, but strong cell–cell adhesion occurs during the lateral clustering of cadherins, and through interactions between the E-cadherin cytoplasmic domain and catenins (Fig. 1) [4]. β-Catenin and plakoglobin (γ-catenin) interact directly with a core region of 30 amino acids within the C-terminus of the cadherin cytoplasmic domain. The N-terminal portions of both β-catenin and plakoglobin interact with α-catenin, which links the cadherin to the cytoskeleton (Fig. 1). In fact, α-catenin has been shown to associate with actin-binding proteins such as vinculin and EPLIN [5]. Another catenin, p120-catenin, interacts with the highly conserved juxtamembrane domain of cadherins, preventing the entrance of E-cadherin into degradative endocytic membrane pathways. This cadherin-catenin complex (Fig. 1) is required for normal cell–cell adhesion.
The broad-range of their effects on physiological tissue organization makes cadherins important molecules during tumorigenesis, their dysfunction or inactivation contributing to the aberrant morphogenic events observed in cancer. Indeed, it is now clear that the classical cadherin dysfunction is a major contributor to cancer development and progression. Such dysfunction can occur through several molecular mechanisms including: mutations of the E-cadherin gene CDH1 [6]; epigenetic silencing through promoter hypermethylation [7], or transcriptional silencing through a variety of transcriptional repressors that target the CDH1 promoter [8], and by microRNAs, which have been shown to indirectly regulate the expression of E-cadherin [9].
Collectively, these genetic or epigenetic alterations of E-cadherin lead to alterations in the expression of this protein that often result in tissue disorders, cellular de-differentiation and increased invasiveness of tumor cells, which ultimately contributes to malignancy. However, there are several different types of invasive carcinomas in which the E-cadherin dysfunction has been observed but none of the aforementioned genetic or epigenetic mechanisms could be detected in the tumor cells. This discrepancy remains controversial, and thus other mechanisms precluding the proper function of E-cadherin may occur in cancer cells. Various lines of evidence support the conclusion that such a mechanism may also operate at the post-translational level in E-cadherin, constituting an alternative mechanism for disturbing or inhibiting the normal E-cadherin function under pathological conditions.
In this review, we will comprehensively discuss a mechanism for post-translational modification of E-cadherin, N-glycosylation, and its contribution to the biology/functionality of this adhesion molecule under homeostatic conditions and in a tumor context.
E-cadherin post-translational modifications
The significance of the post-translational modifications of proteins is gaining importance and acceptance, and, since more than 50% of all eukaryotic proteins are glycosylated, it is impossible to understand many protein functions without considering the contribution of protein post-translational modifications.
E-cadherin can be post-translationally modified through phosphorylation, O-glycosylation, and N-glycosylation (Fig. 2). These post-translational modifications have been reported to have an effect on E-cadherin functionality. E-cadherin can be phosphorylated at a cluster of eight serine residues that are located in the cytoplasmic domain of the protein, and thus phosphorylation can modulate the affinity between β-catenin and E-cadherin, ultimately modifying the strength of cell–cell adhesion [10]. Moreover, cytoplasmic O-glycosylation (O-GlcNAc) of newly synthesized E-cadherin was reported to block its cell surface transport, resulting in reduced intercellular adhesion. Zhu and co-authors proposed that it is possible that the addition of O-GlcNAc structures to E-cadherin directly prevents the binding of p120-catenin. These authors also reported that the induction of apoptosis resulted in the accumulation of E-cadherin with O-GlcNAc structures, and blocked the cell surface transport. Therefore, the authors concluded that the cytoplasmic O-GlcNAc glycosylation of E-cadherin constitutes a mechanism for regulating cell surface transport and, thus, for down-regulate adhesion in some but not all apoptosis pathways [11].
E-cadherin has four possible sites available for N-glycosylation (Fig. 2), and indeed its N-linked carbohydrate chains represent the most prominent post-translational modification of this glycoprotein. N-Glycosylation is characterized by the addition of oligosaccharide structures to the asparagine (Asn) residues of nascent proteins in a consensus sequence Asn-X-Ser/Thr, where X is any amino acid other than proline [12]. The sites of N-glycosylation of E-cadherin are localized in its extracellular domain (Fig. 2), and most of these sites are conserved among species. Both human and canine E-cadherin have four potential N-glycosylation sites, three of which are conserved in the two species. Two of the putative N-glycosylation sites of human E-cadherin are located in extracellular domain 4 (EC4), the other two sites being in EC5 (Fig. 2). On the other hand, canine E-cadherin has one potential N-glycosylation site in EC4 and two in EC5 shared with the humans. An additional site in EC5 has also been reported for canine E-cadherin from a mammary carcinoma cell line. Available evidence suggests that this N-glycosylation site, which is shared with the mouse but not with the human E-cadherin, is occupied by a β1,6 GlcNAc branched N-glycan structure [13].
Overall, the N-linked oligosaccharide chains and phosphorylation modifications represent important mechanisms affecting E-cadherin that are capable of modifying the physiological role of this molecule under homeostatic and pathological conditions.
Role of N-glycosylation of E-cadherin in its biological functions
Glycosylation is of utmost importance for the physiology of eukaryotic cells. In fact, N-glycosylation is essential for multicellular life, as shown by the fact that its complete absence has been demonstrated to be embryonically lethal [14]. Furthermore, genetic defects that affect protein glycosylation are the basis of at least 30 currently known human diseases [15]. Evidence indicates that sugar chains on glycoproteins are involved in the regulation of a myriad of relevant cellular biological functions [16]. Elucidation of the structures and functions of the oligosaccharide chains of glycoproteins is a major goal in the post-genomic era.
Several studies have shown that alterations in N-glycosylation can modulate the biological functions of E-cadherin. N-Glycans at Asn 633 are essential for E-cadherin folding, trafficking, and for its proper expression [17]. Furthermore, N-glycans have been reported to influence the stability of adherens-junction (AJ) by affecting their molecular organization [18]. The modification of E-cadherin with complex N-glycans has been shown to be associated with the formation of dynamic but weak AJ, whereas diminished N-glycosylation of E-cadherin has been reported to promote the establishment of stable AJ [19]. Consistent with this, an inverse relationship between the extent of branched N-glycans and establishment of intercellular adhesion has been reported [20].
Recent studies also demonstrated, in a canine mammary tumor cell line model, that during the acquisition of the malignant phenotype, E-cadherin undergoes extensive modification of its N-glycans, which is characterized by an increase in β1,6 branched N-glycan structures, an increase in sialylation and the presence of few high mannose structures, when compared to E-cadherin from a non-malignant cell line model. These observations support the roles that E-cadherin N-glycosylation plays in the modulation of E-cadherin-mediated cell–cell adhesion, and in the process of tumor development and progression [13].
Glycosyltransferases GnT-III, GnT-V, and FUT8 controlling crucial steps in N-glycosylation of E-cadherin
Contrary to DNA and proteins, glycosylation is a non-template-driven process, with no proofreading machinery. It is a process that involves the coordinated action of an endogenous portfolio of hundreds of specific glycosyltransferases that catalyze the addition of saccharide structures to different positions of the extending glycan, in a step-wise manner. Regarding N-glycosylation, the resulting structures are generally classified into three principal categories: (1) high mannose, in which only mannose residues are attached to the core; (2) complex, in which “antennae” are attached to the core via the action of N-acetylglucosaminyltransferases; and (3) hybrids type, in which only mannose residues are attached to the Manα1–6 arm of the core and one or two antennae to the Manα1–3 arm.
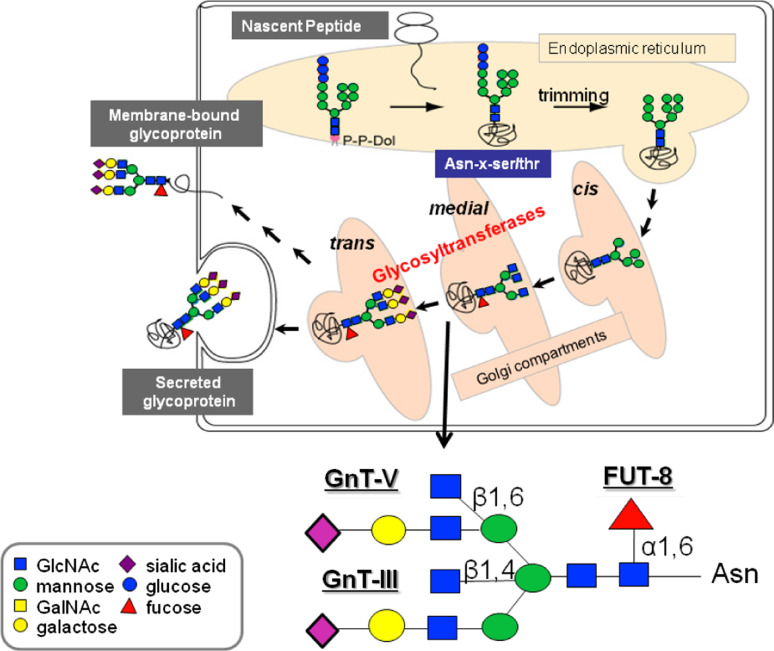
Two important glycosyltransferases involved in N-glycan biosynthesis are N-acetylglucosaminyltransferase III (GnT-III) and N-acetylglucosaminyltransferase V (GnT-V). The N-glycan products of these two enzymes are, respectively, bisecting GlcNAc structures and β1,6 GlcNAc branched structures [21, 22]. In addition, another relevant enzyme in the N-glycan biosynthetic pathway is α1,6 fucosyltransferase (FUT8) that catalyses the synthesis of an α1,6 fucose N-glycan structure [23] (Fig. 3).
Fig. 3.
Biosynthesis of N-linked glycans. Representation of a N-glycan structure with the reactions catalyzed by GnT-III, GnT-V, and FUT8
These structures produced by the above three glycosyltransferases have been associated with various biological functions of cell adhesion molecules [24–26].
GnT-III (Mgat3)
GnT-III catalyzes the addition of GlcNAc via a β1,4 linkage to the β-mannose of the mannosyl core of N-glycans (Fig. 3), and thereby alters the composition and also the conformation of the N-glycans. This bisecting GlcNAc structure, produced by GnT-III, precludes the action of GnT-V, which is no longer able to act on the biantennary oligosaccharide chains and thus prevents the formation of β1,6 branched structure.
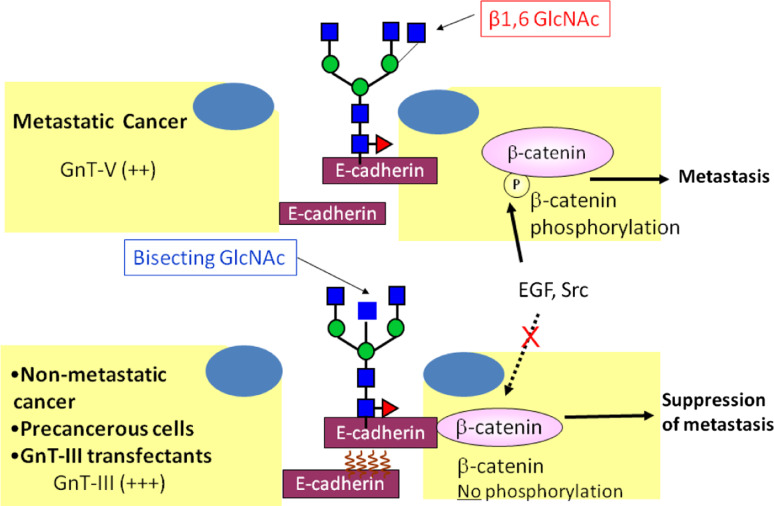
The enzymatic competition between GnT-III and GnT-V, where GnT-III has been shown to exhibit priority activity, contributes to the suppression of cancer metastasis [27]. In fact, the overexpression of GnT-III in highly metastatic B16 melanoma cells reduces β1,6 GlcNAc branching of cell-surface N-glycans and increases bisected N-glycans, which ultimately results in a significant decrease in the metastatic potential in experimental models of lung metastasis [28]. Therefore, GnT-III has been clearly shown to induce an anti-metastatic phenotype.
The general relationship between GnT-III expression and the suppression of cancer metastases has been demonstrated to be, at least partially, due to the modulation of the function of E-cadherin as a tumor suppressor protein preventing cell invasion and metastasis. In fact, previous studies demonstrated that cells expressing GnT-III exhibit enhanced cell–cell adhesion due to a delay in the turnover of E-cadherin on the cell surface [29]. Furthermore, it has also been demonstrated that the addition of bisecting GlcNAc residues to E-cadherin by GnT-III is associated with the down-regulation of tyrosine phosphorylation of β-catenin through EGFR or Src signaling. This mechanism contributes to the retention of β-catenin binding to the cytoplasmic E-cadherin domain, enhancing the homophilic interaction of E-cadherin, which leads to the suppression of cancer metastasis [30]. In addition, the suppression of metastasis induced by GnT-III has also been shown to be due to alterations of integrin functions. The overexpression of GnT-III was shown to suppress α3β1 integrin-mediated cell migration on laminin 332 [27], as well as the inhibition of α5β1 integrin-mediated cell spreading and migration. The affinity of the binding of α5β1-integrin to fibronectin was shown to be reduced upon the biosynthesis of bisecting GlcNAc on N-glycans on the α5 subunit [31]. Recently, site-4 on the integrin α5 subunit was reported to be the key site modified with bisecting GlcNAc structures catalyzed by GnT-III, which affects cell spreading and migration [32]. This comprehensive body of data supports the view that GnT-III plays roles in the suppression of tumor metastasis through at least two mechanisms: enhancement of cell–cell adhesion and down-regulation of cell-ECM adhesion.
Recent studies have shown that E-cadherin expression can also interfere with the transcription of the GnT-III gene, leading to up-regulation of GnT-III transcription [33]. In addition, Iijima et al. showed that the mRNA expression level and the activity of GnT-III were up-regulated in cells cultured under dense conditions, where E-cadherin is highly active, compared with cells cultured under sparse conditions [34]. Moreover, it was demonstrated that up-regulation of GnT-III was accompanied by the formation of bisecting GlcNAc structures on E-cadherin. These observations suggest the existence of a mutual feedback loop, and a regulatory mechanism between E-cadherin-mediated cell adhesion and GnT-III expression [33, 35]. This regulatory loop between E-cadherin and GnT-III has also been observed in some cases of human diffuse gastric carcinomas, where E-cadherin down-regulation is accompanied by reduced GnT-III functional activity [33].
Furthermore, other studies have demonstrated that the restoration of the α-catenin gene in DLD-1 cells results in a significant increase in GnT-III activity and in the production of the bisected N-glycans [36], which further supports the view that the E-cadherin–catenin complex and/or an E-cadherin-dependent signaling pathway is required for GnT-III up-regulation.
These findings suggest that this bidirectional regulatory mechanism between E-cadherin-mediated cell–cell adhesion and GnT-III expression as well as glycosyltransferase activity appear to be important for proper E-cadherin expression on the cell membrane and for the maintenance of a structured cell–cell adhesion complex, which is essential for the suppression of invasion and metastasis (Fig. 4). Interestingly, it was observed that, when GnT-III is silenced in a cell line model, there is remarkable modification of the cellular phenotype with an increase in lamellipodia and filopodia formation, and a de-localization of E-cadherin from the cell membrane to the cytoplasm [33].
Fig. 4.
Regulatory mechanism of E-cadherin-mediated cell–cell adhesion and GnT-III/GnT-V. GnT-III activity is associated with an increase in bisecting GlcNAc structures in E-cadherin, leading to a concomitant decrease in β1,6 branched structures, due to competition with GnT-V glycosyltransferase. The addition of bisecting GlcNAc residues to E-cadherin down-regulates the tyrosine phosphorylation of β-catenin and thus enhances cell–cell binding to suppress metastasis (lower figure). Conversely, in metastatic cancer cells (upper figure), the addition of β1,6 branched structures by GnT-V to E-cadherin is associated with increased tyrosine phosphorylation of β-catenin through the EGFR and Src signaling pathways, and therefore reduces E-cadherin-mediated cell–cell adhesion thereby contributing to the promotion of cancer metastasis
Altogether, these studies strongly support the biological relevance of GnT-III as to the regulation of E-cadherin functions at the post-translational level (Fig. 4).
GnT-V (Mgat5)
It is generally recognized that another change in terminal glycosylation associated with cancer comprises the size and branching of N-linked glycans [25, 37]. This increased branching is due to enhanced activity of GnT-V. This glycosyltransferase is located in the medial/trans Golgi, where it catalyzes the addition of β1,6-linked GlcNAc units to the α1,6-linked mannose of the trimannosyl core of N-linked glycans to form tri- or tetraantennary branches (Fig. 3) [22]. This branched structure can be further extended with poly-N-acetylactosamine chains that can be terminally modified with sialylated structures.
The enhanced β1,6 GlcNAc branching of N-linked structures, formed through the increased activity of GnT-V, is a common glycosylation change inducing malignancy. Alteration of the β1,6 branched N-glycans on the cell surface has been implicated in the modulation of cell–cell adhesion and migration, and has been associated with the metastatic potential of tumors. In fact, previous studies demonstrated that during the acquisition of the malignant phenotype, E-cadherin undergoes addition of β1,6 GlcNAc branches whereas E-cadherin from a benign canine mammary tumor cell line model did not contain these glycans [13]. GnT-V modification also affects other molecules that are important in the modulation of tumor adhesion, including the integrin family of adhesion molecules. Increased GnT-V expression and the consequent increased β1,6 branching on N-glycans of the β1 subunit of tumor α5β1 integrins, result in down-regulation of focal contact formation, which in turn increases tumor motility and invasiveness through the ECM [38]. Furthermore, Pierce and co-workers also reported that the GnT-V expression level modulates homotypic cell–cell adhesion depending on neural cadherin (N-cadherin). They showed that the induction of GnT-V expression causes increased levels of N-linked β1,6 branched structures on N-cadherin, resulting in a decrease in the rate of homophilic cell–cell adhesion, by promoting the phosphorylation of catenins through the EGFR and Src signaling pathways, as well as a stimulation of cadherin mediated-cell migration [39]. A recent study has demonstrated that the β1,6 branched N-glycans expressed at three sites in the EC2–3 domains regulate N-cadherin-mediated cell–cell adhesion. Inhibition or deletion of these three sites with branched N-glycans had no effect on the N-cadherin level on the cell surface or the formation of cadherin/catenin complexes, but led to increased cis-dimerization of N-cadherins, as well as increased cell–cell adhesion-mediated intracellular signaling and reduced cell migration [40]. Collectively, these results indicate that the level of β1,6 branched N-glycans, produced by the GnT-V enzyme, can modulate N-cadherin-associated homotypic cell–cell adhesion and intracellular signaling, thus playing a crucial role in the regulation of cellular motility and invasiveness.
Concerning the effects of GnT-V and its product, the β1,6 GlcNAc branched structure, on E-cadherin molecules, it has been reported that the removal of complex type N-glycans from ectodomain 4 of E-cadherin using a CHO cell line model led to an increased interaction of the E-cadherin–catenin complexes with vinculin and the actin cytoskeleton, affecting the stability of AJ [19].
In addition, previous studies have shown that GnT-III knockdown in MCF-7/AZ carcinoma cells resulted in a significant increase in β1,6 GlcNAc branched N-glycans, due to increased GnT-V enzymatic activity, concomitant with a decrease in bisecting GlcNAc structures on E-cadherin [33]. This remodeling of E-cadherin N-glycans with an increase in β1,6 branched structures had repercussions on E-cadherin cellular localization and cell morphology. These cell line models showed that there was de-localization of E-cadherin from the cell membrane with internalization to the cytoplasm, along with the formation of cellular filopodia and lamellipodia extrusions [33]. These results support the deleterious effects of β1,6 GlcNAc branched structures, by GnT-V, on E-cadherin-mediated cell–cell adhesion (Fig. 4). Furthermore, we have also observed a remarkable overexpression of these β1,6 GlcNAc branched structures in human diffuse gastric carcinoma cells that exhibited E-cadherin down-regulation [33].
In conclusion, β1,6 GlcNAc branched N-glycans play a prominent role in regulating E-cadherin-mediated adhesion and its intracellular signaling pathways, which can contribute to an increase in the migratory/invasive phenotype of cancer cells (Fig. 4).
FUT8
The introduction of an α1,6 fucose unit on the asparagine-linked N-acetylglucosamine residue of the chitobiose units of complex N-glycans was demonstrated to be catalyzed by FUT8 (Fig. 3) [23]. The action of FUT8 has been shown to be associated with oncogenesis, since the α1,6 fucosylation of α-fetoprotein is a well-known marker of hepatocellular carcinomas [41]. Taniguchi and co-workers reported that FUT8 expression is markedly enhanced in several types of cancer cell lines [42]. Furthermore, α1,6 fucosylation was reported to be essential for α3β1 integrin functions. Deletion of α1,6 fucosylation on α3β1 integrin was found to be associated with decreased α3β1 integrin-mediated cell migration and cell signaling. The reintroduction of FUT8 restored both the migration and signaling, indicating that α1,6 fucosylation is essential for α3β1 integrin functions [43].
Recent studies have also suggested that FUT8 may affect the biological functions of E-cadherin. The activity of FUT8 was involved in the appearance of a lower molecular weight population of E-cadherin and regulated its total amount [44. FUT8 transfected WiDr cells showed the accumulation of E-cadherin at cell–cell borders, and an increased cell aggregation. These observations suggest that α1,6 fucosylation regulates the processing of oligosaccharides and the turnover of E-cadherin, supporting a possible role of α1,6 fucosylation in the regulation of cell–cell adhesion in cancer [44]. In line with this study, Hu et al. proposed that α1,6-fucosylated E-cadherin is involved in the regulation of nuclear β-catenin accumulation in lung cancer cells. In FUT8 transfected cells, E-cadherin was found to be α1,6 fucosylated and there was a significant decrease in nuclear β-catenin accumulation, which may indicate an increase in binding between E-cadherin α1,6-fucosylated and β-catenin [45]. Alternatively, we could not exclude that this decrease in nuclear β-catenin accumulation is due to increased degradation of cytoplasmic β-catenin. Conversely, in FUT8 silenced cells, E-cadherin was less α1,6-fucosylated and a significant increase in nuclear β-catenin accumulation was observed [45]. However, this phenotype does not seem to be always the case. E-cadherin-mediated cell–cell adhesion was strengthened with a reduction in α1,6 fucosylation on E-cadherin after FUT8 RNAi and was weakened with elevated α1,6 fucosylation on E-cadherin by the overexpression of FUT8 in lung cancer [46].
Collectively, these studies support the possible involvement of α1,6-fucosylation of N-glycans of E-cadherin in the regulation of cell–cell adhesion and downstream signaling pathways.
Other N-glycosylation-related enzymes
GPT (DPAGT1)
The biosynthesis of Asn-linked oligosaccharides on cellular and secreted glycoproteins has many stages along the secretory pathway, where the initial steps occur on the cytoplasmic face of the ER and the final stages on the Golgi compartment of the cell with the branching and further extension of the oligosaccharides by the Golgi glycosyltransferases [12].
The early steps in the N-glycan biosynthetic pathway are highly conserved in eukaryotes and suggest that there are important roles for the mature lipid-linked oligosaccharide for efficient N-glycosylation, protein folding and further transport from the ER [47, 48]. In fact, enzymatic defects resulting in incomplete lipid-linked oligosaccharide synthesis result in severe, multi-systemic human diseases including congenital disorders of glycosylation (CDG) type I [49].
The first gene involved in the protein N-glycosylation process is DPAGT1 which encodes the enzyme dolichol-P-dependent N-acetylglucosamine-1-phosphate-transferase (GPT) [50]. In the ER, DPAGT1 initiates the synthesis of the lipid-linked oligosaccharide precursor that will later be transferred en bloc to the Asn residues of the nascent proteins. Since it is the first gene involved in the N-glycosylation biosynthetic pathway, DPAGT1 expression has been reported to control the extent of protein N-glycosylation, being also reported to affect the N-glycosylation status of E-cadherin with an impact in its functions. Overexpression of DPAGT1 in an oral cancer cell line model was associated with modifications of E-cadherin with complex N-glycans together with alterations in the molecular organization of the adherens-junction, and a decrease in the association with γ-catenin, α-catenin and vinculin. Conversely, the partial inhibition of DPAGT1 by small interfering RNA was associated with a decrease in complex N-glycans on E-cadherin, and increased association with catenins and vinculin, which was described to promote the formation of more stable adherens-junctions and the assembly of tight-junctions in an oral carcinoma model [51]. Furthermore, DPAGT1 was also shown to regulate E-cadherin N-glycosylation in a canine cell line model (MDCK), where it was shown to have an impact on the organization and assembly of adherens-junctions and tight-junctions [52].
These studies demonstrated that DPAGT1 is an upstream regulator of protein N-glycosylation with important roles in the modulation of E-cadherin-mediated cell–cell adhesion, through control of the amount of the lipid-linked oligosaccharide precursors and thus the extent of protein N-glycosylation.
GnT-IV and α-mannosidase II
There are other glycosyltransferases involved in the N-glycosylation biosynthetic pathway that have been reported to have possible roles in cancer. Among them, N-acetylglucosaminyltransferase-IV (GnT-IV), which catalyses the formation of the β1,4 GlcNAc branch on the Manα1,3 arm of the core structure of N-glycans [53], has been reported to be significantly upregulated in human cancers such as choriocarcinomas [54], pancreatic carcinomas [55], and colorectal cancer, where GnT-IV upregulation was closely associated with the metastatic potential [56]. Regarding the specific role of this glycosyltransferase in the modulation of E-cadherin-mediated cell–cell adhesion, limited information has been reported. Although and since the activity of GnT-IV affects the antennary number of N-glycans, and consequently the terminal sialylation, it has been hypothesized that it could also play a role in the modulation of cell adhesion [57], however, this remains to be further examined.
Furthermore, processing glycosidases also play an important role in N-glycan biosynthesis by providing the correct substrates for the subsequent formation of complex and hybrid N-glycan structures. Glycosidases play crucial roles in the folding and quality control of newly synthesized glycoproteins [58]. One of the key glycosidases that is a target for the development of anti-cancer therapies is α-mannosidase II. Golgi α-mannosidase II removes the terminal α1,3- and α1,6-linked mannose from GlcNAcMan5GlcNAc2 to yield GlcNAcMan3GlcNAc2 [59, 60], which allows the further action of other glycosyltransferases, including GnT-V, involved in the branching and extension of the N-glycan structures. Inhibition of α-mannosidase II by swainsonine results in the accumulation of hybrid oligosaccharides and inhibition of the synthesis of the complex N-glycans [61]. In addition, previous in vitro and in vivo studies demonstrated that the inhibition of the synthesis of complex N-glycans by swainsonine can modulate tumor cell adhesion, invasion and progression [57, 62]. In fact, cells treated with swainsonine exhibited inhibition of the action of the GnT-V enzyme due to the unavailability of a substrate for it. This inhibition of the synthesis of β1,6GlcNAc branched N-glycan structures by swainsonine was associated with an increase in N-cadherin-mediated cell adhesion [39] as well as with stabilization of cell–cell junctions and a decrease in cell motility [20].
In summary, the interplay between glycosyltransferases and glycosidases involved in the N-glycosylation biosynthetic pathway coordinates crucial steps as to E-cadherin N-glycosylation status with a consequent impact on E-cadherin biological functions.
E-cadherin regulation in cancer: Future perspectives
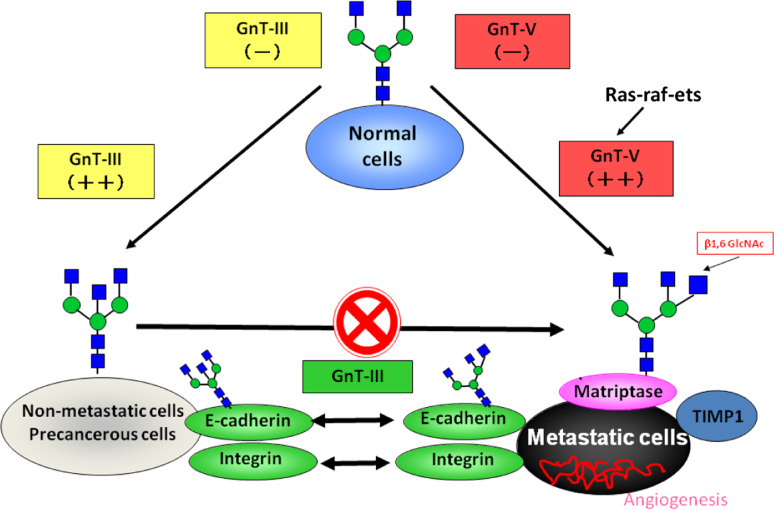
Numerous human diseases including cancer are associated with changes in glycan structures and, in some cases, it has been shown that changes in glycosylation are not consequences of altered cell physiology, but one of the causative agents of the disease [15]. The clinical relevance of glycans is currently a subject of intense interest, since the roles that glycans play in the development, regulation and progression of diseases is being unveiled. In this post-genomic era, it is crucial to identify target proteins and to determine the functions of specific oligosaccharide structures in these different proteins (Fig. 5).
Fig. 5.
The role of N-glycan structures in the carcinogenic process. In normal cells, the GnT-III and GnT-V enzymes are normally underexpressed. The overexpression of GnT-III is associated with increased synthesis of bisecting GlcNAc structures in some important target glycoproteins involved in cell adhesion such as E-cadherin and integrins, the modification of which by bisecting N-glycans is associated with the suppression of metastasis through enhancement of E-cadherin-mediated cell–cell adhesion and a decrease in integrin-mediated cell-extracellular matrix adhesion. Furthermore, GnT-III up-regulation precludes the availability of the substrate for the GnT-V enzyme, which is no longer able to synthesize branched structures. In a metastatic cancer situation, activation of the ras-raf-ets signaling pathway regulates the transcription of the GnT-V gene and the resulting increase in GnT-V leads to increased enzymatic production of β1,6 branched structures that modify glycoproteins involved in the carcinogenic process, including Matriptase; TIMP-1 (Tissue Inhibitor of Metalloproteinase-1, in which β1,6 branching correlates with the invasive and metastatic potential of cancer cells) as well as integrins and E-cadherin, the modification of which contributes to a decrease in cell–cell adhesion, and increase in tumor cell invasion and migration. In addition, other mechanisms also indicate that a secreted type of GnT-V may contribute to tumor angiogenesis
In fact, E-cadherin is a classical tumor suppressor gene that encodes a protein that, when dysfunctional or inactive, leads to tumor initiation and progression. In this review, we have comprehensively summarized a significant amount of data showing that, in addition to the genetic and epigenetic regulation of E-cadherin, the post-translational modification through N-glycosylation can be a mechanism that modulates and regulates E-cadherin biological functions, and in this manner influences the disease process (Fig. 5).
Since E-cadherin deregulation is a common event that occurs during tumor progression as well as a causative event in the cases of some carcinomas, such as diffuse gastric cancer and lobular breast cancer, it is essential to elucidate the in vivo molecular mechanisms underlying the N-glycan regulation of the adhesive function of E-cadherin in cancer biology. We envision that this knowledge will lead to novel therapeutic perspectives of cancer through the targeting deleterious N-glycan structures that play a role in deregulation of the functions of E-cadherin.
Acknowledgments
We thank Prof. Leonor David for the helpful suggestions regarding the manuscript. We also thank Dr. Masaki Kato of the Systems Glycobiology Research Group, RIKEN, Japan, for the technical support regarding Fig. 2b. This work was supported by grants from the Portuguese Foundation for Science and Technology (FCT), project grants [PTDC/CVT/65537/2006; PIC/IC/82716/2007; PTDC/CVT/111358/2009] and a grant in aid for Scientific Research (A) from MEXT, Japan for financial support. S.S.P. also acknowledges FCT [SFRH/BPD/63094/2009], and the Luso-American Foundation (FLAD). IPATIMUP is an Associate Laboratory of the Portuguese Ministry of Science, Technology and Higher Education, and is partially supported by FCT.
Abbreviations
- GnT-III
N-Acetylglucosaminyltransferase III
- GnT-V
N-Acetylglucosaminyltransferase V
- FUT8
α1,6 Fucosyltransferase
- GnT-IV
N-Acetylglucosaminyltransferase IV
- EC
Extracellular domain
- AJ
Adherens-junction
- ECM
Extracellular matrix
Contributor Information
Naoyuki Taniguchi, FAX: +81-6-68798414, Email: tani52@wd5.so-net.ne.jp.
Celso A. Reis, FAX: +351-2-25570799, Email: celsor@ipatimup.pt
References
- 1.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 2.Tamura K, Shan WS, Hendrickson WA, Colman DR, Shapiro L. Structure–function analysis of cell adhesion by neural (N-) cadherin. Neuron. 1998;20:1153–1163. doi: 10.1016/S0896-6273(00)80496-1. [DOI] [PubMed] [Google Scholar]
- 3.Shi QM, Maruthamuthu V, Li F, Leckband D. Allosteric cross talk between cadherin extracellular domains. Biophys J. 2010;99:95–104. doi: 10.1016/j.bpj.2010.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson WJ. Regulation of cell–cell adhesion by the cadherin–catenin complex. Biochem Soc Trans. 2008;36:149–155. doi: 10.1042/BST0360149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abe K, Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U S A. 2008;105:13–19. doi: 10.1073/pnas.0710504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berx G, Becker KF, Hofler H, van RF. Mutations of the human E-cadherin (CDH1) gene. Hum Mutat. 1998;12:226–237. doi: 10.1002/(SICI)1098-1004(1998)12:4<226::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira C, Sousa S, Pinheiro H, Karam R, Carrico R, Senz J, Kaurah P, Carvalho J, Pereira R, Gusmao L, Xiaogang W, Yokota J, Carneiro F, Huntsman D, Seruca R. Quantification of epigenetic and genetic 2nd hits in CDH1 during hereditary diffuse gastric cancer syndrome progression. Gastroenterology. 2009;136:2137–2148. doi: 10.1053/j.gastro.2009.02.065. [DOI] [PubMed] [Google Scholar]
- 8.Alves CC, Carneiro F, Hoefler H, Becker KF. Role of the epithelial-mesenchymal transition regulator Slug in primary human cancers. Front Biosci. 2009;14:3035–3050. doi: 10.2741/3433. [DOI] [PubMed] [Google Scholar]
- 9.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 10.Lickert H, Bauer A, Kemler R, Stappert J. Casein kinase II phosphorylation of E-cadherin increases E-cadherin/β-catenin interaction and strengthens cell–cell adhesion. J Biol Chem. 2000;275:5090–5095. doi: 10.1074/jbc.275.7.5090. [DOI] [PubMed] [Google Scholar]
- 11.Zhu W, Leber B, Andrews DW. Cytoplasmic O-glycosylation prevents cell surface transport of E-cadherin during apoptosis. EMBO J. 2001;20:5999–6007. doi: 10.1093/emboj/20.21.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 13.Pinho SS, Osorio H, Nita-Lazar M, Gomes J, Lopes C, Gartner F, Reis CA. Role of E-cadherin N-glycosylation profile in a mammary tumor model. Biochem Biophys Res Commun. 2009;379:1091–1096. doi: 10.1016/j.bbrc.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Ioffe E, Stanley P. Mice lacking N-acetylglucosaminyltransferase I activity die at mid-gestation, revealing an essential role for complex or hybrid N-linked carbohydrates. Proc Natl Acad Sci U S A. 1994;91:728–732. doi: 10.1073/pnas.91.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeze HH. Genetic defects in the human glycome. Nat Rev Genet. 2006;7:537–551. doi: 10.1038/nrg1894. [DOI] [PubMed] [Google Scholar]
- 16.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou F, Su J, Fu L, Yang Y, Zhang L, Wang L, Zhao H, Zhang D, Li Z, Zha X. Unglycosylation at Asn-633 made extracellular domain of E-cadherin folded incorrectly and arrested in endoplasmic reticulum, then sequentially degraded by ERAD. Glycoconj J. 2008;25:727–740. doi: 10.1007/s10719-008-9133-9. [DOI] [PubMed] [Google Scholar]
- 18.Zhao H, Liang Y, Xu Z, Wang L, Zhou F, Li Z, Jin J, Yang Y, Fang Z, Hu Y, Zhang L, Su J, Zha X. N-glycosylation affects the adhesive function of E-cadherin through modifying the composition of adherens junctions (AJs) in human breast carcinoma cell line MDA-MB-435. J Cell Biochem. 2008;104:162–175. doi: 10.1002/jcb.21608. [DOI] [PubMed] [Google Scholar]
- 19.Liwosz A, Lei T, Kukuruzinska MA. N-glycosylation affects the molecular organization and stability of E-cadherin junctions. J Biol Chem. 2006;281:23138–23149. doi: 10.1074/jbc.M512621200. [DOI] [PubMed] [Google Scholar]
- 20.Vagin O, Tokhtaeva E, Yakubov I, Shevchenko E, Sachs G. Inverse correlation between the extent of N-glycan branching and intercellular adhesion in epithelia. Contribution of the Na, K-ATPase β1 subunit. J Biol Chem. 2008;283:2192–2202. doi: 10.1074/jbc.M704713200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narasimhan S. Control of glycoprotein synthesis. UDP-GlcNAc:glycopeptide β 4-N-acetylglucosaminyltransferase III, an enzyme in hen oviduct which adds GlcNAc in β 1–4 linkage to the β-linked mannose of the trimannosyl core of N-glycosyl oligosaccharides. J Biol Chem. 1982;257:10235–10242. [PubMed] [Google Scholar]
- 22.Brockhausen I, Narasimhan S, Schachter H. The biosynthesis of highly branched N-glycans: studies on the sequential pathway and functional role of N-acetylglucosaminyltransferases I, II, III, IV, V and VI. Biochimie. 1988;70:1521–1533. doi: 10.1016/0300-9084(88)90289-1. [DOI] [PubMed] [Google Scholar]
- 23.Uozumi N, Yanagidani S, Miyoshi E, Ihara Y, Sakuma T, Gao CX, Teshima T, Fujii S, Shiba T, Taniguchi N. Purification and cDNA cloning of porcine brain GDP-L-Fuc:N-acetyl-β-D-glucosaminide α1-- > 6fucosyltransferase. J Biol Chem. 1996;271:27810–27817. doi: 10.1074/jbc.271.44.27810. [DOI] [PubMed] [Google Scholar]
- 24.Taniguchi N, Miyoshi E, Gu J, Honke K, Matsumoto A. Decoding sugar functions by identifying target glycoproteins. Curr Opin Struct Biol. 2006;16:561–566. doi: 10.1016/j.sbi.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Taniguchi N, Miyoshi E, Ko JH, Ikeda Y, Ihara Y. Implication of N-acetylglucosaminyltransferases III and V in cancer: gene regulation and signaling mechanism. Biochim Biophys Acta. 1999;1455:287–300. doi: 10.1016/s0925-4439(99)00066-6. [DOI] [PubMed] [Google Scholar]
- 26.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 27.Zhao Y, Nakagawa T, Itoh S, Inamori K, Isaji T, Kariya Y, Kondo A, Miyoshi E, Miyazaki K, Kawasaki N, Taniguchi N, Gu J. N-acetylglucosaminyltransferase III antagonizes the effect of N-acetylglucosaminyltransferase V on α3β1 integrin-mediated cell migration. J Biol Chem. 2006;281:32122–32130. doi: 10.1074/jbc.M607274200. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimura M, Nishikawa A, Ihara Y, Taniguchi S, Taniguchi N. Suppression of lung metastasis of B16 mouse melanoma by N-acetylglucosaminyltransferase III gene transfection. Proc Natl Acad Sci U S A. 1995;92:8754–8758. doi: 10.1073/pnas.92.19.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshimura M, Ihara Y, Matsuzawa Y, Taniguchi N. Aberrant glycosylation of E-cadherin enhances cell–cell binding to suppress metastasis. J Biol Chem. 1996;271:13811–13815. doi: 10.1074/jbc.271.34.20265. [DOI] [PubMed] [Google Scholar]
- 30.Kitada T, Miyoshi E, Noda K, Higashiyama S, Ihara H, Matsuura N, Hayashi N, Kawata S, Matsuzawa Y, Taniguchi N. The addition of bisecting N-acetylglucosamine residues to E-cadherin down-regulates the tyrosine phosphorylation of β-catenin. J Biol Chem. 2001;276:475–480. doi: 10.1074/jbc.M006689200. [DOI] [PubMed] [Google Scholar]
- 31.Isaji T, Gu J, Nishiuchi R, Zhao Y, Takahashi M, Miyoshi E, Honke K, Sekiguchi K, Taniguchi N. Introduction of bisecting GlcNAc into integrin α5β1 reduces ligand binding and down-regulates cell adhesion and cell migration. J Biol Chem. 2004;279:19747–19754. doi: 10.1074/jbc.M311627200. [DOI] [PubMed] [Google Scholar]
- 32.Sato Y, Isaji T, Tajiri M, Yoshida-Yamamoto S, Yoshinaka T, Somehara T, Fukuda T, Wada Y, Gu J. An N-glycosylation site on the β-propeller domain of the integrin α5 subunit plays key roles in both its function and site-specific modification by β1, 4-N-acetylglucosaminyltransferase III. J Biol Chem. 2009;284:11873–11881. doi: 10.1074/jbc.M807660200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinho SS, Reis CA, Paredes J, Magalhaes AM, Ferreira AC, Figueiredo J, Xiaogang W, Carneiro F, Gartner F, Seruca R. The role of N-acetylglucosaminyltransferase III and V in the post-transcriptional modifications of E-cadherin. Hum Mol Genet. 2009;18:2599–2608. doi: 10.1093/hmg/ddp194. [DOI] [PubMed] [Google Scholar]
- 34.Iijima J, Zhao Y, Isaji T, Kameyama A, Nakaya S, Wang X, Ihara H, Cheng X, Nakagawa T, Miyoshi E, Kondo A, Narimatsu H, Taniguchi N, Gu J. Cell–cell interaction-dependent regulation of N-acetylglucosaminyltransferase III and the bisected N-glycans in GE11 epithelial cells. Involvement of E-cadherin-mediated cell adhesion. J Biol Chem. 2006;281:13038–13046. doi: 10.1074/jbc.M601961200. [DOI] [PubMed] [Google Scholar]
- 35.Gu J, Sato Y, Kariya Y, Isaji T, Taniguchi N, Fukuda T. A mutual regulation between cell–cell adhesion and N-glycosylation: implication of the bisecting GlcNAc for biological functions. J Proteome Res. 2009;8:431–435. doi: 10.1021/pr800674g. [DOI] [PubMed] [Google Scholar]
- 36.Akama R, Sato Y, Kariya Y, Isaji T, Fukuda T, Lu L, Taniguchi N, Ozawa M, Gu J. N-acetylglucosaminyltransferase III expression is regulated by cell–cell adhesion via the E-cadherin–catenin-actin complex. Proteomics. 2008;8:3221–3228. doi: 10.1002/pmic.200800038. [DOI] [PubMed] [Google Scholar]
- 37.Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci U S A. 2002;99:10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo HB, Lee I, Kamar M, Akiyama SK, Pierce M. Aberrant N-glycosylation of β1 integrin causes reduced α5β1 integrin clustering and stimulates cell migration. Cancer Res. 2002;62:6837–6845. [PubMed] [Google Scholar]
- 39.Guo HB, Lee I, Kamar M, Pierce M. N-acetylglucosaminyltransferase V expression levels regulate cadherin-associated homotypic cell–cell adhesion and intracellular signaling pathways. J Biol Chem. 2003;278:52412–52424. doi: 10.1074/jbc.M308837200. [DOI] [PubMed] [Google Scholar]
- 40.Guo HB, Johnson H, Randolph M, Pierce M. Regulation of homotypic cell–cell adhesion by branched N-glycosylation of N-cadherin extracellular EC2 and EC3 domains. J Biol Chem. 2009;284:34986–34997. doi: 10.1074/jbc.M109.060806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taketa K, Endo Y, Sekiya C, Tanikawa K, Koji T, Taga H, Satomura S, Matsuura S, Kawai T, Hirai H. A collaborative study for the evaluation of lectin-reactive α-fetoproteins in early detection of hepatocellular carcinoma. Cancer Res. 1993;53:5419–5423. [PubMed] [Google Scholar]
- 42.Miyoshi E, Uozumi N, Noda K, Hayashi N, Hori M, Taniguchi N. Expression of α1–6 fucosyltransferase in rat tissues and human cancer cell lines. Int J Cancer. 1997;72:1117–1121. doi: 10.1002/(SICI)1097-0215(19970917)72:6<1117::AID-IJC29>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, Itoh S, Wang X, Isaji T, Miyoshi E, Kariya Y, Miyazaki K, Kawasaki N, Taniguchi N, Gu J. Deletion of core fucosylation on α3β1 integrin down-regulates its functions. J Biol Chem. 2006;281:38343–38350. doi: 10.1074/jbc.M608764200. [DOI] [PubMed] [Google Scholar]
- 44.Osumi D, Takahashi M, Miyoshi E, Yokoe S, Lee SH, Noda K, Nakamori S, Gu J, Ikeda Y, Kuroki Y, Sengoku K, Ishikawa M, Taniguchi N. Core fucosylation of E-cadherin enhances cell–cell adhesion in human colon carcinoma WiDr cells. Cancer Sci. 2009;100:888–895. doi: 10.1111/j.1349-7006.2009.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu P, Shi B, Geng F, Zhang C, Wu W, Wu XZ. E-cadherin core fucosylation regulates nuclear β-catenin accumulation in lung cancer cells. Glycoconj J. 2008;25:843–850. doi: 10.1007/s10719-008-9144-6. [DOI] [PubMed] [Google Scholar]
- 46.Geng F, Shi BZ, Yuan YF, Wu XZ. The expression of core fucosylated E-cadherin in cancer cells and lung cancer patients: prognostic implications. Cell Res. 2004;14:423–433. doi: 10.1038/sj.cr.7290243. [DOI] [PubMed] [Google Scholar]
- 47.Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 48.Mendelsohn RD, Helmerhorst EJ, Cipollo JF, Kukuruzinska MA. A hypomorphic allele of the first N-glycosylation gene, ALG7, causes mitochondrial defects in yeast. Biochim Biophys Acta. 2005;1723:33–44. doi: 10.1016/j.bbagen.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 49.Wu X, Rush JS, Karaoglu D, Krasnewich D, Lubinsky MS, Waechter CJ, Gilmore R, Freeze HH. Deficiency of UDP-GlcNAc:dolichol phosphate N-acetylglucosamine-1 phosphate transferase (DPAGT1) causes a novel congenital disorder of glycosylation type ij. Hum Mutat. 2003;22:144–150. doi: 10.1002/humu.10239. [DOI] [PubMed] [Google Scholar]
- 50.Kukuruzinska MA, Apekin V, Lamkin MS, Hiltz A, Rodriguez A, Lin CC, Paz MA, Oppenheim FG. Antisense RNA to the first N-glycosylation gene, ALG7, inhibits protein N-glycosylation and secretion by Xenopus oocytes. Biochem Biophys Res Commun. 1994;198:1248–1254. doi: 10.1006/bbrc.1994.1176. [DOI] [PubMed] [Google Scholar]
- 51.Nita-Lazar M, Noonan V, Rebustini I, Walker J, Menko AS, Kukuruzinska MA. Overexpression of DPAGT1 leads to aberrant N-glycosylation of E-cadherin and cellular discohesion in oral cancer. Cancer Res. 2009;69:5673–5680. doi: 10.1158/0008-5472.CAN-08-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nita-Lazar M, Rebustini I, Walker J, Kukuruzinska MA. Hypoglycosylated E-cadherin promotes the assembly of tight junctions through the recruitment of PP2A to adherens junctions. Exp Cell Res. 2010;316:1871–1884. doi: 10.1016/j.yexcr.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oguri S, Minowa MT, Ihara Y, Taniguchi N, Ikenaga H, Takeuchi M. Purification and characterization of UDP-N-acetylglucosamine: α1, 3-D-mannoside β1, 4-N-acetylglucosaminyltransferase (N-acetylglucosaminyltransferase-IV) from bovine small intestine. J Biol Chem. 1997;272:22721–22727. doi: 10.1074/jbc.272.36.22721. [DOI] [PubMed] [Google Scholar]
- 54.Takamatsu S, Oguri S, Minowa MT, Yoshida A, Nakamura K, Takeuchi M, Kobata A. Unusually high expression of N-acetylglucosaminyltransferase-IVa in human choriocarcinoma cell lines: a possible enzymatic basis of the formation of abnormal biantennary sugar chain. Cancer Res. 1999;59:3949–3953. [PubMed] [Google Scholar]
- 55.Ide Y, Miyoshi E, Nakagawa T, Gu J, Tanemura M, Nishida T, Ito T, Yamamoto H, Kozutsumi Y, Taniguchi N. Aberrant expression of N-acetylglucosaminyltransferase-IVa and IVb (GnT-IVa and b) in pancreatic cancer. Biochem Biophys Res Commun. 2006;341:478–482. doi: 10.1016/j.bbrc.2005.12.208. [DOI] [PubMed] [Google Scholar]
- 56.D’Arrigo A, Belluco C, Ambrosi A, Digito M, Esposito G, Bertola A, Fabris M, Nofrate V, Mammano E, Leon A, Nitti D, Lise M. Metastatic transcriptional pattern revealed by gene expression profiling in primary colorectal carcinoma. Int J Cancer. 2005;115:256–262. doi: 10.1002/ijc.20883. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Zhao JH, Zhang XY, Guo HB, Liu F, Chen HL. Relations of the type and branch of surface N-glycans to cell adhesion, migration and integrin expressions. Mol Cell Biochem. 2004;260:137–146. doi: 10.1023/B:MCBI.0000026065.84798.62. [DOI] [PubMed] [Google Scholar]
- 58.Herscovics A. Importance of glycosidases in mammalian glycoprotein biosynthesis. Biochim Biophys Acta. 1999;1473:96–107. doi: 10.1016/s0304-4165(99)00171-3. [DOI] [PubMed] [Google Scholar]
- 59.Moremen KW. Golgi α-mannosidase II deficiency in vertebrate systems: implications for asparagine-linked oligosaccharide processing in mammals. Biochim Biophys Acta. 2002;1573:225–235. doi: 10.1016/s0304-4165(02)00388-4. [DOI] [PubMed] [Google Scholar]
- 60.van den Elsen JM, Kuntz DA, Rose DR. Structure of Golgi alpha-mannosidase II: a target for inhibition of growth and metastasis of cancer cells. EMBO J. 2001;20:3008–3017. doi: 10.1093/emboj/20.12.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tulsiani DR, Harris TM, Touster O. Swainsonine inhibits the biosynthesis of complex glycoproteins by inhibition of Golgi mannosidase II. J Biol Chem. 1982;257:7936–7939. [PubMed] [Google Scholar]
- 62.Goss PE, Reid CL, Bailey D, Dennis JW. Phase IB clinical trial of the oligosaccharide processing inhibitor swainsonine in patients with advanced malignancies. Clin Cancer Res. 1997;3:1077–1086. [PubMed] [Google Scholar]