Abstract
Oxidative stress and low-grade inflammation are the hallmarks of the aging process and are even more enhanced in many age-related degenerative diseases. Mitochondrial dysfunction and oxidative stress can provoke and potentiate inflammatory responses, but the mechanism has remained elusive. Recent studies indicate that oxidative stress can induce the assembly of multiprotein inflammatory complexes called the inflammasomes. Nod-like receptor protein 3 (NLRP3) is the major immune sensor for cellular stress signals, e.g., reactive oxygen species, ceramides, and cathepsin B. NLRP3 activation triggers the caspase-1-mediated maturation of the precursors of IL-1β and IL-18 cytokines. During aging, the autophagic clearance of mitochondria declines and dysfunctional mitochondria provoke chronic oxidative stress, which disturbs the cellular redox balance. Moreover, increased NF-κB signaling observed during aging could potentiate the expression of NLRP3 and cytokine proforms enhancing the priming of NLRP3 inflammasomes. Recent studies have demonstrated that NLRP3 activation is associated with several age-related diseases, e.g., the metabolic syndrome. We will review here the emerging field of inflammasomes in the appearance of the proinflammatory phenotype during the aging process and in age-related diseases.
Keywords: Ageing, Autophagy, Inflammasome, Inflammation, NF-κB, NLRP3
Introduction
For centuries, scientists have postulated a number of aging theories some of which have also received substantial support from modern molecular biology. One hypothesis is the rate-of-living theory, first proposed by Max Rubner in 1908 and recently reviewed by Hulbert et al. [1]. This theory emphasizes the role of metabolic rate in the aging process, suggesting that increased energy metabolism can enhance the aging process. The free radical theory of aging, presented by Denham Harman in 1956, maintains that oxygen-derived free radicals, byproducts of metabolic reactions, can attack cellular molecules and provoke oxidative damage [2]. This mechanism fits rather well with the rate-of-living theory since mitochondria are the major source of cellular ROS production. Oxidative respiration in the mitochondrial electron transport chain, particularly in dysfunctional mitochondria, can generate a considerable amount of ROS and in that way impair the housekeeping functions within the cell. There is an extensive literature supporting this concept [3, 4], although many critical studies have been published [5]. Recently, Jones [6] presented the thiol redox hypothesis speculating that the appearance of oxidative stress is dependent on disturbances in the cellular thiol redox status. Oxidative stress is associated with the pathogenesis of several age-related diseases, e.g., metabolic syndrome and age-related macular degeneration [7, 8].
Activation of the innate immune system and the presence of low-grade inflammation is a common hallmark of the aging process [9–12]. Franceschi et al. [9] termed this chronic pro-inflammatory state as inflammaging. Innate immunity is a crucial host defence system which can recognize both invading pathogens and endogenous, stress-related signals in order to prevent host injuries [13, 14]. TLR- and NLR-mediated pathways are the two major pattern recognition receptor systems. In particular, NLRs are danger-sensing receptors which, if they encounter an insult, they stimulate the assembly of inflammatory protein complexes, called the inflammasomes [13, 14]. Consequently, inflammasomes trigger the maturation and secretion of IL-1β and IL-18 cytokines. A variety of cellular stress-related danger signals activating inflammasomes has been identified [13–16]. Several studies have indicated that oxidative stress and disturbances in thiol redox balance may activate inflammasomes [17–19]. Moreover, release of cathepsin B from ruptured lysosomes can trigger inflammasomal activation [20, 21]. The mechanism of cathepsin B-provoked activation of NLRP3 still needs to be characterized. However, it is known that the viral-induced rupture of lysosomes triggers cathepsin B-dependent mitochondrial membrane destabilization and increased ROS production [22]. Recent studies have emphasized the role of mitochondria in the control of innate immunity and, in particular, the function of autophagy in the maintenance of mitochondrial integrity and thus prevention of ROS production and inflammasome activation [23, 24]. Since many inflammasome activators are common factors in the aging process itself, and even more frequent in many age-related diseases, we will review this emerging field of inflammasomes in the aging process and associated diseases.
Inflammasomes: danger sensing inflammatory platforms
Inflammasomes are intracellular multiprotein complexes which can be activated by a large set of danger signals evoked by the presence of pathogens and cellular stress, e.g., oxidative stress, potassium efflux, and lipid accumulation [13, 14, 25]. The inflammasomal platform was discovered in the laboratory of Jürg Tschopp in 2002 [26], and, currently, inflammasomes are being intensively investigated for their role in inflammatory and metabolic diseases [15, 16, 27, 28]. The recognition component of the inflammasome is the receptor protein which subsequently recruits an inflammatory caspase, i.e., human caspase-1, -4, -5, and -12 to the complex via the CARD or PYD domains. There exist 22 human NLR proteins which can bind inflammatory caspases, normally caspase-1, either directly or via the ASC adaptor, an apoptosis-associated speck-like protein containing CARD and PYD domains. The NLR type of inflammasomes can be subdivided based on their structures and assembly model into the NLRA, NLRB, NLRC (containing, e.g., NOD1, NLRC4, NLRC5, and NLRX), and NLRP (e.g., NLRP1 and NLRP3) subfamilies [16]. The stimulation of NLRP triggers the assembly of NLRP, ASC, and caspase-1 components which subsequently oligomerize into penta- or heptameric structures. This active inflammasome cleaves the precursor forms of IL-1β and IL-18 into mature cytokines [16, 26, 28]. The secretion of these cytokines can subsequently expand the inflammatory response since these molecules signal through their specific cellular receptors [29]. Moreover, caspase-1 can also stimulate the secretion of a set of intracellular molecules, called the caspase-1 secretome, many of which are associated with inflammatory responses [30]. Lamkanfi et al. [31] demonstrated that the secretion of HMGB1, a pro-inflammatory protein, was a caspase-1-dependent process which required the assembly of NLRP3 inflammasome. In addition, cytosolic flagellin stimulated the expression of inducible NOS, which was a caspase-1-dependent response, but involved NLRC4 and NAIP5 inflammasomes in macrophages [32]. Both of these processes were caspase-1-dependent, but independent of IL-1β and IL-18 secretion. These studies indicate that inflammasomes can promote inflammation via different effector mechanisms.
A plethora of bacterial peptides and viral RNA and DNA can activate cellular inflammasomes. AIM2- and RIG-1-types of receptors recognize cytosolic RNA and DNA and trigger inflammasomes [25, 33]. Pathogenic ligands have been reviewed in detail elsewhere [33, 34]. The inflammatory responses induced by infections and tissue injury have been understood for years, but endogenous, stress-related inflammation mechanisms have remained elusive. Medzhitov [35] termed this inflammatory mode “para-inflammation”. Para-inflammation can induce adaptation to noxious stimuli, but if the stress is overwhelming or sustained, it can recruit macrophages and initiate a low-grade chronic inflammation [35]. Stress-induced, chronic inflammation aggravates the condition, and this can provoke the pathology of age-related diseases. Currently, it is known that both the TLRs and NLRs can be activated by cellular stress and damage-associated stimuli [24, 28, 36]. The stress-related activation of inflammasomes is mostly characterized by NLRP3 inflammasomes (also known as NALP3 and cryopyrin), but NLRP1 can also be activated by DAMPs.
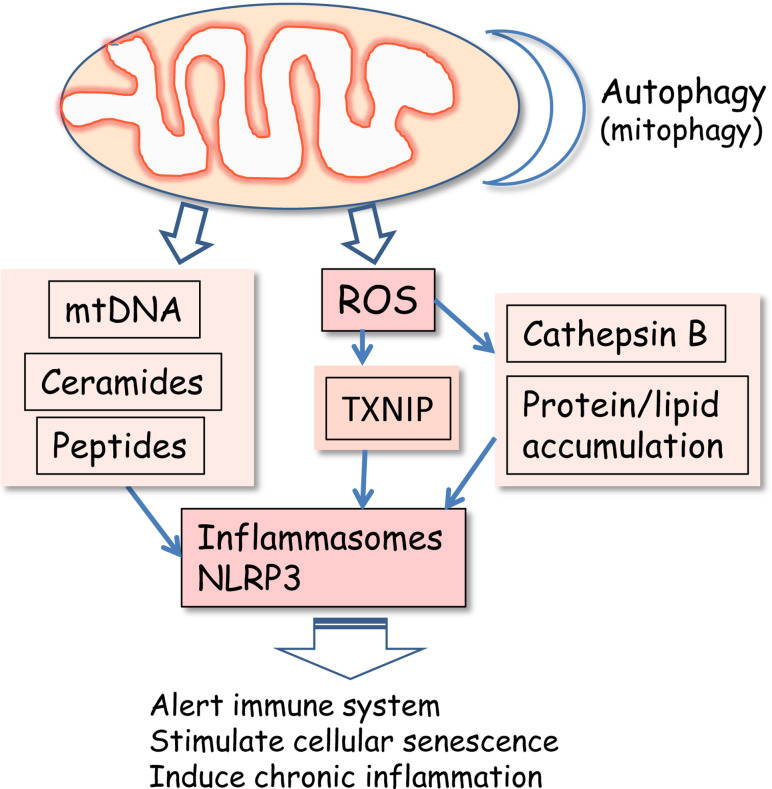
Petrilli et al. [37] were the first investigators who observed that NLRP3 inflammasomes could be activated by the efflux of potassium in human monocytes. For instance, extracellular ATP, released from damaged cells, can induce potassium efflux by activating the ATP-gated P2X7 receptors. Potassium efflux can also activate the NLRP1 but not NLRC4 inflammasomes [37]. Later studies have indicated that many other activators of NLRP3 can also reduce the intracellular potassium concentration and thus activate inflammasomes [38, 39]. However, potassium efflux is linked to increased intracellular calcium concentration. It is well known that excessive cytosolic calcium level can be balanced by its intake to mitochondria which stimulates mitochondrial ROS production [4, 40]. The exact mechanism of inflammasome activation induced by potassium efflux is not known, but it seems that it is associated with a ROS-dependent activation mechanism (see below). Uptake of certain crystal particles such as asbestos, silica, monosodium urate, and calcium phosphate crystals can also induce changes in ionic concentrations [16, 25, 28]. During aging, lipofuscin accumulates in lysosomes of post-mitotic cells. These insoluble aggregates may physically cause lysosomal damage. Lysosomes are iron-rich organelles and thus sensitive to ROS-mediated oxidation of proteins and lipids [41], which can also induce lysosomal membrane rupture. The membrane damage can lead to the release of cathepsin B, which is known to stimulate inflammasomes. For instance, cholesterol crystals activate NLRP3 inflammasomes via cathepsin B release in human macrophages [21] (Fig. 1).
Fig. 1.
Mitochondrial dysfunction activates ROS production and secretion of mitochondrial DAMPs which activate NLRP3 inflammasomes and subsequently enhance the appearance of the proinflammatory phenotype during the aging process and in age-related diseases. Loss of mitochondrial integrity triggers the release of mtDNA and synthesis of ceramides and formyl peptides. Autophagic clearance of damaged mitochondria maintains mitochondrial integrity and prevents the activation of NLRP3 inflammasomes
Tattoli et al. [42] observed that NLRX1 was localized in mitochondria where it was involved in ROS generation. Moreover, NLRX1 enhanced ROS production, NF-κB signaling, and inflammatory response after TNFα exposure. However, recent studies have indicated that NLRX1 can interact with MAVS and interfere with the signaling of the RIG-1/MAVS and TRAF6/NF-κB pathways [43]. The RIG-1 pathway has a major role in the cellular viral defence system. Xia et al. [44] observed that NLRX1 negatively regulated TLR-mediated NF-κB signaling and the inflammatory response. They also revealed the mechanism upon LPS stimulation, i.e., NLRX1 was ubiquitinated and subsequently interacted with the IKKα/β complex inhibiting its activity. Knocking down of NLRX1 clearly increased NF-κB signaling and sensitized mice to LPS-induced septic shock [44]. Recently, Cui et al. [45] revealed that NLRC5 receptors inhibited the activation of IKKα and IKKβ and thus NF-κB signaling. It seems that NLRX1 and NLRC5 have an important role in the homeostatic regulation of innate immunity.
There are both structural and functional differences between NLRP1 and NLRP3 inflammasomes [16, 25, 46]. For instance, NLRP1 protein but not NLRP3 contains a CARD domain and it does not need ASC for the activation of caspase-1 [46]. These receptors also exhibit dissimilar expression profiles, e.g., NLRP1 is highly expressed in T and B lymphocytes and neurons [47, 48]. Moreover, anti-apoptotic proteins Bcl-2 and Bcl-xL can interact with NLRP1 and in that way inhibit NLRP1 activation and inflammatory responses [49]. On the other hand, the overexpression of NLRP1 can induce apoptosis, e.g., in cerebellar granule neurons [50]. Genetic variants of NLRP1 are associated with Alzheimer’s disease [51] and vitiligo-associated autoimmune diseases [52]. NLRP1 also has a crucial role in the inflammatory response initiated by spinal cord injury [53]. Potassium efflux activates both these inflammasomes [37], but it seems that ROS can activate only NLRP3 receptors. The current understanding on inflammasomes including receptor types and their activation mechanisms has been recently reviewed in detail elsewhere [15, 16, 25, 28].
Mitochondrial dysfunction stimulates inflammatory responses
Mitochondrial dysfunction is associated with the aging process as well as with the pathogenesis of several diseases, e.g., metabolic and neurodegenerative diseases [54–57]. These effects are commonly attributed to disturbances in energy metabolism and increased ROS production and to the important role of mitochondria in apoptotic cell death. Moreover, recent studies have revealed that mitochondria have a crucial role in the regulation of inflammatory responses via their ROS production [24, 57, 58]. It is well known that UCP2 and UCP3 can control immune responses and the appearance of metabolic diseases [59–61]. UCP2 proteins are expressed in most tissues whereas the presence of UCP3 is restricted to skeletal muscles and adipose tissue [62]. UCP proteins are located in the inner mitochondrial membrane where they dissipate the mitochondrial proton gradient and in that way can reduce ROS production and subsequently prevent inflammatory reactions. In ischemic brain damage, UCP2 deletion enhances cytokine production and increases neuronal damage [63]. Bai et al. [64] revealed that deletion of UCP2 stimulated NF-κB signaling under basal conditions and, in particular, after inflammatory insults. Antioxidants seem to be able to inhibit this inflammatory response which indicates that ROS production was involved. Horvath et al. [61] observed that the overexpression of UCP2 and UCP3 prevented inflammatory changes and obesity in transgenic mouse models.
Recent studies have demonstrated that a sterile cellular injury can trigger the release of DAMPs from mitochondria which can induce local and systemic inflammatory responses [65, 66]. Zhang et al. [67] demonstrated that, in tissue injury, mtDNA and formyl peptides were released from mitochondria, and, subsequently, they activated human PMNs through TLR9 and FPRL1 receptors and promoted their migration and tissue penetration. Through the same membrane receptors, mtDNA and formyl peptides stimulated inflammatory responses in local microenvironments. Moreover, at the cellular level, mitochondrial DAMPs were reported to activate inflammasomes and thus induce an inflammatory reaction [65, 66]. Interestingly, mitochondria can trigger both the assembly of apoptosomal and inflammasomal protein complexes, and thus alert the immune system about expected apoptotic cell death.
Mitochondrial origin of inflammasome activation
Recent studies have revealed that mitochondria are crucial regulators of innate immunity [24, 66, 68]. Even though these organelles have an endosymbiotic origin, mitochondria can trigger sterile inflammation by activating inflammasomes, in particular NLRP3, in cellular stress. Some inflammasomes are specialized to recognize different microbe products, i.e., flagellin activates NLRC4, bacterial muramyl peptides and lethal toxins stimulate NLRP1, and cytoplasmic DNA induces AIM2 activation [69]. For comparison, ROS is the key product of mitochondria which induces the stimulation of NLRP3 [25, 28, 57]. Recently, Bauernfeind et al. [70] demonstrated that the ROS-induced activation of NLRP3 was clearly attributable to the priming process, since ROS inhibitors blocked the priming step in NLRP3 activation. Under normal conditions, the expression levels of NLRP3 protein and pro-IL1β and pro-IL18 cytokines are low, and a clear increase in their expression level has been required prior to the efficient activation of NLRP3. Bauernfeind et al. [71] revealed that NF-κB-dependent signaling was clearly able to upregulate the expression of NLRP3 and thus permitted the activation of NLRP3 inflammasome. They also demonstrated that the priming process was only involved in the activation of NLRP3, but not in that of AIM2 or NLRC4 [71]. These studies emphasize the crucial role of NF-κB signaling in the ROS-dependent activation of NLRP3 inflammasomes.
Mitochondrial production of ROS activates inflammasomes
The mitochondrial respiratory chain, NADPH oxidases, and 5-lipoxygenase are the major cellular sources of ROS production. It is important to be aware of the origin of ROS production evoking the activation of inflammasomes if one wishes to target them through therapeutic interventions. There are many earlier observations indicating that increased ROS production could induce the expression and secretion of interleukin-1β, e.g., in PC12 cells [72] and alveolar type II epithelial cells [73]. Cruz et al. [17] revealed that ATP activated ROS production and induced IL-1β secretion via caspase-1 activation in macrophages. Anna Rubartelli [74–76] and her group have demonstrated in several experiments that the cellular redox status regulates the processing and secretion of IL-1β. Blocking of ROS production and increased expression of antioxidants can reduce IL-1β secretion. Hu et al. [77] observed that the expression of TRIM30 protein decreased NLRP3 activation by attenuating ROS production. Moreover, Tsai et al. [78] demonstrated that epigallocatechin-3-gallate, a major polyphenol in green tea, inhibited NLRP3 activation by stimulating NRF2-mediated antioxidant response and thus prevented lupus nephritis development in mice.
Several experimental approaches have demonstrated that autophagic uptake capacity can regulate mitochondrial integrity, ROS production, and subsequent NLRP3 activation [79, 80]. Recently, Zhou et al. [80] demonstrated that the inhibition of autophagy triggered the accumulation of damaged, ROS-generating mitochondria which augmented the activation of NLRP3 inflammasomes in human macrophages (Fig. 1). Earlier studies had indicated that inhibition of autophagic uptake increased the ROS production [81] and that changes in autophagy could regulate the inflammatory responses [82]. Zhou et al. [80] observed that the NLRP3 protein co-localized with ER marker in non-stimulated macrophages. After danger-signal stimulation, NLRP3 protein relocalized into the perinuclear space, and several cytochemical markers indicated that the structure was the MAM, which is the physical site of the interaction between ER and mitochondria. The MAM is known to regulate many processes, e.g., calcium homeostasis and the release of apoptogenic proteins which can subsequently assemble apoptosomes [83]. Interestingly, NLRP3 co-localized with ASC and TXNIP, which implies that the NLRP3 inflammasomes located in the MAM were active, probably being triggered by TXNIP [80]. These observations revealed an important mechanistic role for mitochondria as inducers of inflammasomes and apoptosomes, two multiprotein complexes with very different functions. Moreover, it is known that the overwhelming stimulation of macrophages with proinflammatory insults can induce the assembly of the pyroptosome complex in which ASC itself can activate caspase-1 and apoptotic cell death, a process called pyroptosis [84].
The exact mechanism involved in ROS-induced NLRP3 activation is still unclear. Zhou et al. [18] demonstrated that oxidative stress could activate NLRP3 inflammasomes via the redox regulation of TRX/TXNIP balance (Fig. 1). They used a two-hybrid screening system to demonstrate that TXNIP could bind to the LRR domain of NLRP3 protein. The binding of TXNIP activated the NLRP3 inflammasome and increased IL-1β secretion in human macrophages. Several inflammasome inducers, such as ATP, silica, and uric acid crystals, triggered the dissociation of TXNIP from THR in a ROS-dependent manner and allowed its binding to NLRP3, and subsequently stimulated NLRP3 activation. Their experiments also revealed that the glucose-induced stimulation of IL-1β secretion was triggered by the TXNIP/NLRP3 activation in mouse beta cells. On the other hand, Masters et al. [85] could not repeat these results concerning the role of TXNIP in NLRP3 activation in bone marrow-derived macrophages. They did not perform direct binding assays between TXNIP and NLRP3. However, there are two other recent studies which have confirmed the role of TXNIP in the activation of NLRP3. Xiang et al. [19] demonstrated that ROS produced by NADPH oxidase induced the binding of TXNIP to the NLRP3, which caused inflammasome activation and IL-1β secretion from lung endothelial cells. Interestingly, they observed that HMGB1, a well-known DAMP [86], could stimulate NLRP3 via ROS production triggered by NADPH oxidase. Recently, Lunov et al. [87] demonstrated that a specific type of nanoparticles accumulated in lysosomes and induced the release of cathepsin B which stimulated ROS production from mitochondria. Subsequently, ROS triggered the dissociation of TXNIP from TRX and its binding to NLRP3 which induced caspase-1-dependent IL-1β production. They also revealed in silico that the binding of TXNIP to the NLRP3 evoked the exposure of the pyrin domain of NLRP3 to ASC which triggered inflammasomal activation. Currently, three studies have confirmed the role of TXNIP in the ROS-dependent activation of NLRP3 inflammasome.
Mitochondrial DAMPs: mtDNA, ceramides, and formyl peptides
Nakahira et al. [79] demonstrated that the block of autophagy clearly activated caspase-1 and increased the secretion of IL-1β and IL-18 in human macrophages. The inhibition of autophagic degradation impaired mitochondrial homeostasis and stimulated the generation of ROS. Subsequently, increased ROS production enhanced mitochondrial permeability and triggered the leakage of mtDNA into cytosol (Fig. 1). They observed that the release was dependent on the presence of both ROS and NLRP3. In cytoplasm, mtDNA potentiated the activation of caspase-1 in conjunction with LPS and ATP treatments. However, this response was independent of AIM2, a typical inflammasomal receptor activated as a result of viral DNA exposure. Choumar et al. [88] revealed that LPS treatment induced mtDNA depletion and mild inflammation in liver of wild-type mice whereas mitochondrial MnSOD overexpressing mice were protected. The exact mechanism how cytosolic mtDNA induces inflammasome activation is still unknown, but it seems that mtDNA and viral RNA/DNA use separate pathways. In several pathological conditions, mtDNA is known to be a potent mitochondrial DAMP and it can trigger systemic and local inflammation via TLR9 [65, 66].
Ceramides, which are composed of sphingosine and fatty acid, may be involved in the ROS-dependent regulation of TXNIP/NLRP3 balance. Vandanmagsar et al. [89] observed that the lipotoxicity-associated increase in the intracellular level of ceramides activated NLRP3 inflammasomes in macrophages and adipose tissue (Fig. 1). Ceramides and oxidative stress have been linked to each other in several pathological conditions, e.g., in aging and inflammation [90]. Yu et al. [91] demonstrated that, in stroke, mitochondrial ceramide synthase was activated and ceramides were abundantly accumulated in mitochondria where they induced mitochondrial respiratory dysfunction. Ceramides can also form channels in mitochondrial membranes [92] and thus trigger the release of mitochondrial DAMPs. Ceramides can also induce the expression of TXNIP [93, 94] and in that way activate NLRP3 inflammasomes.
N-formyl peptides are mitochondrial DAMPs which can provoke systemic inflammatory responses via formyl peptide receptors [24]. Mitochondrial N-formylated peptides activate monocytes and trigger neutrophil chemotaxis in response to tissue injuries [95]. It seems that mitochondria can also have other cell non-autonomous effects such as those involved in immune defence. Recently, Andrew Dillin and his team [96] identified so-called mitokines which could induce systemic effects on the mitochondrial pool in C. elegans. They observed that tissue-specifically induced mitochondrial unfolded protein response (UPRmt) could be translocated from the nervous system and muscle tissue into the intestine. The molecular mechanisms of this type of mitochondrial DAMP still need to be clarified. In general, UPRmt has many beneficial effects in mitochondria, e.g., it can enhance respiration and increase organismal longevity.
Potential significance of inflammasomes in the aging process and age-related diseases
Inflammasomes are sensors of intracellular sanctity when this is challenged by oxidative stress and lysosomal destabilization. The aging process involves a progressive accumulation of waste products, mostly oxidized proteins and lipids. Lipofuscin is a characteristic hallmark of the aging process in non-mitotic cells, e.g., in cardiac muscle cells [97]. There is also abundant evidence indicating that the deposition of harmful intra- and extracellular material occurs in several age-related diseases. These aggregates jeopardize cellular homeostasis and can provoke the activation of NLRP3 inflammasomes. The aging process involves a plethora of changes indicating that the pro-inflammatory phenotype could be initiated by danger-sensitive NLRP3 activation. However, direct evidence is still lacking.
Inflammasomes in age-related diseases
Recent studies indicate that inflammasomes have a crucial role in the pathogenesis of several age-related diseases, e.g., metabolic syndrome [27, 98]. Vandanmagsar et al. [89] demonstrated that, in obesity, the increased secretion of IL-1β and IL-18 cytokines was induced by NLRP3 activation in adipose tissues. The intense expression of NLRP3 protein was localized to macrophages in adipose tissues. They observed that chronic caloric restriction and physical exercise reduced the weight of animals and simultaneously clearly decreased the expression of Nlrp3, Asc, and IL-1β mRNA in both visceral and subcutaneous adipose tissues. They extended their study to type II diabetic humans who undertook a 1-year weight loss intervention. They observed that the expression levels of the same inflammasomal markers as observed in mice were also reduced in human subcutaneous adipose tissue. The decreased expression levels were coupled with lower glycemia and improved insulin resistance. Moreover, Stienstra et al. [98] observed that caspase-1 and IL-1β activities were increased in both diet-induced and genetically obese mice, and that the NLRP3-mediated caspase-1 activation directed adipocyte differentiation toward a more insulin-resistant phenotype whereas caspase-1 knockout mice were more insulin sensitive. These studies indicate that metabolic stress can trigger NLRP3 inflammasomes in adipose tissues and lead to metabolic syndrome [27].
Atherosclerosis is an inflammatory disease of the arterial vessel wall and a well-established age-related disorder [99]. Oxidative stress is a crucial factor in the pathogenesis of vascular aging and atherosclerosis [100]. Two recent studies have demonstrated that cholesterol crystals can activate NLRP3 inflammasomes in human macrophages by causing lysosomal damage and cathepsin B release [21, 101]. Duewell et al. [101] observed that microscopic cholesterol crystals were present during the very early stages of the appearance of diet-induced atherosclerotic lesions and that this coincided with the signs of inflammatory changes. After intraperitoneal injection, cholesterol crystals could induce peritonitis which did not occur in mice deficient in NLRP3, cathepsin B and L, or IL-1β [101]. Rajamäki et al. [21] demonstrated that cholesterol crystals induced leakage of lysosomal cathepsin B into the cytoplasm which subsequently triggered IL-1β secretion in human macrophages. It seems that cholesterol crystals as well as monosodium urate crystals can induce inflammation via their phagocytosis and the release of lysosomal cathepsin B, mostly in macrophages but probably also in other endocytotic cells, e.g., endothelial cells.
Halle et al. [20] revealed that fibrillary amyloid-β peptides, key players in Alzheimer’s disease, could also activate NLRP3-mediated IL-1β production in brain microglial cells. The NLRP3 activation was also dependent on the phagocytosis of aggregates and the release of cathepsin B which took place via the destabilization of lysosomes. We have recently observed that the expression of IL-18 was increased in the brains of Alzheimer’s disease patients, in particular in the frontal lobe [102]. The expression was localized in microglia, astrocytes, and also, surprisingly, in neurons. The level of IL-18 cytokine was also increased in the CSF of men with Alzheimer’s disease. These observations imply that amyloid-β oligomers or aggregates can trigger neuroinflammation via the inflammasomal activation. We have recently reviewed this topic in Alzheimer’s disease [103]. Moreover, Jha et al. [104] demonstrated that the expression of NLRP3 was greatly increased in the cuprizone-induced, mouse demyelination and neuroinflammation model. The lack of the Nlrp3 gene clearly attenuated the neuroinflammation and the amount of demyelination. A similar outcome was observed in Casp −/− and IL-18 −/− mice, but not in IL-1β −/− mice, indicating that NLRP3 inflammasomes were associated with caspase-1 activation and IL-18 secretion in mouse brain.
Necrotic cell death is involved in the aging process, although necrotic lesions are particularly present in the age-related diseases. Several studies have demonstrated that cells dying through necrosis associated with autophagic process but not via apoptosis can trigger inflammasome activation in host tissue phagocytes [82, 105–107]. In necrosis, the leakage of intracellular ATP can stimulate NLRP3 via P2X7 receptors. In addition, necrotic lesions trigger the release of DAMPs from extracellular matrix, e.g., biglycan and hyaluronan oligosaccharides, which stimulate inflammasome priming via TLRs [108]. Subsequently, the secretion of cytokines and chemokines recruit neutrophils to the sites of sterile inflammation [109].
IL-1β and IL-18 secretion as marker of inflammasome activation
The activation of caspase-1 cleaves the pro-IL-1β and pro-IL-18 into mature forms, and thus the secretion levels of IL-1β and IL-18 represent the degree of inflammasomal activation. This is a widely used parameter in both cellular and in vivo experiments. However, caspase-1 is not the only IL-1β-converting enzyme which can cleave and induce the secretion of IL-1β. For instance, pro-IL-1β can be processed by the chymase enzyme in mast cells and proteinase-3 and elastase in neutrophils [110–112]. These studies indicate that there may be a cell-type-specific processing of pro-IL-1β, and that caspase-1 and inflammasomes are active mainly in monocytes and macrophages. Moreover, Harris et al. [113] demonstrated that increased autophagy reduced the secretion of IL-1β in response to inflammatory insults by sequestering the cytokine into the lysosomal degradation, whereas the inhibition of autophagy increased IL-1β secretion via the NLRP3 activation in macrophages. The inflammasome-independent IL-1β secretion has also been observed in human peripheral blood mononuclear cells [114]. The inflammasomal origin of IL-1β and IL-18 processing in non-myeloid cells needs to be clarified, although inflammasome receptors are widely expressed in different tissues [47, 48].
Bearing in mind the above limitation, several studies have been conducted using transgenic mice lacking Nlrp3, Txnip, Asc, Casp1, IL-1β, and IL-18 genes, which have verified that caspase-1-dependent inflammasome activation has a key role in IL-1β and IL-18 production and induction of inflammatory reactions, e.g., in oxidative [18, 80] and metabolic [27, 89] stresses. There is abundant evidence that increased systemic inflammation is associated with the aging process and age-related diseases and that it correlates with the mortality risk. The serum levels of TNF-α and IL-6 are clearly upregulated in elderly individuals [115, 116], although the observations on the serum levels of IL-1β and IL-18 have been conflicting. Instead, the expression levels of IL-1β and IL-18 seem to be increased with aging, e.g., in the brain tissue and vascular system [117, 118]. Recently, Niemi et al. [119] demonstrated that serum amyloid A, an acute phase protein, activated NLRP3 via the P2X7 receptors in human and mouse macrophages. They observed that NLRP3 activation was dependent on cathepsin B activity, but, interestingly, not on lysosomal destabilization. It is known that the increase in serum amyloid A level correlates with the risk for several age-related diseases.
Vascular aging evokes a clear proinflammatory shift in cytokine expression which has been linked in several studies to conditions of increased oxidative stress, in particular to mitochondrial ROS production [100, 120]. Csiszar et al. [117] demonstrated that the expressions of IL-1β, TNF-α, and IL-6 were significantly increased in the coronary arteries of the aged rat. They observed that the expression of IL-1β and IL-6 was localized in endothelium whereas TNF-α was present in smooth muscle. These age-related changes in the vascular wall can enhance the atherosclerotic process. Atherosclerosis induced by gout and hyperuricemia seems to be mediated via NLRP3 activation [121]. Yajima et al. [122] demonstrated that the expression of ASC as well as IL-1β and IL-18 were markedly increased in the lesions of wire-mediated vascular injury. The expression level of cytokines and neointimal formation were significantly lower in Asc −/− mice compared to their wild-type counterparts.
In the brain, the expression of IL-1β, IL-18, and several other cytokines are increased during aging along with the appearance of neurodegenerative changes. Gemma and Bickford [123] observed that caspase-1 activity and IL-1β expression were increased in the aged rat hippocampus. Intracerebroventricular infusion of a specific caspase-1 inhibitor decreased the caspase-1 activity and IL-1β expression and, interestingly, improved the memory functions. Gemma et al. [124] also demonstrated that the blockade of caspase-1 could increase neurogenesis in the hippocampus of aged rat. Alboni et al. [125] have recently reviewed in detail the role of IL-18 in neuroinflammation and degeneration in the central nervous system.
Several studies indicate that aging alters the function of microglial cells, e.g., they constitutively secrete TNF-α and IL-6, and their cytokine responses to insults are strikingly increased [126]. Microglial cells from old rats contain clearly reduced glutathione levels which could provoke oxidative stress and activate NLRP3. A similar shift in microglial responses has been observed in aged, transgenic Alzheimer’s mice [127]. Thiamine deficiency has been shown to trigger oxidative stress and inflammation in specific areas of brain, e.g., in thalamus [128]. The deficiency induced neuronal loss and a 43-fold increase in IL-1β expression in affected regions. In conclusion, the aging process is linked to increased inflammatory profiles in many tissues which can be triggered by inflammasomal activation.
Mitochondrial integrity, autophagy and redox balance in aging and age-related diseases
During the past decades, a strong link between mitochondrial ROS production and the aging process has been revealed [2–4]. It seems that the mitochondrial dysfunction occurring during aging is caused by impaired turnover of mitochondria via the autophagy, a process called mitophagy. Brunk and Terman [129] presented the garbage-can hypothesis of impaired autophagocytosis of damaged mitochondria during aging. Subsequently, an abundant literature has appeared demonstrating that the efficiency of autophagic uptake declines and waste material accumulates with aging [130–132]. Moreover, it has been observed that increased autophagy can extend the lifespan, and, conversely, the repression of autophagy reduces both healthspan and lifespan. Wu et al. [81] demonstrated that the inhibition of autophagy by the deletion of the Atg7 gene induced a defect in mitochondrial respiration which led to increased production of mitochondrial ROS and provoked oxidative stress. A decline in autophagic capacity has been observed in several age-related diseases, e.g., in Alzheimer’s disease [133] and type II diabetes [134]. Dysfunction of mitochondria increases the production of ROS which activates NF-κB system [135]. NF-κB signaling induces the expression of pro-IL-1β and pro-IL-18 which are required for the priming of NLRP3 inflammasomes. Under chronic conditions, NF-κB signaling can also inhibit IL-1β secretion by inducing the expression of caspase-1 inhibitors and thus form a negative feedback loop which supports the resolution of inflammation [136].
Oxidative stress regulates the TRX/TXNIP balance via different pathways, e.g., excessive ROS can oxidize TRX that induce the dissociation of TXNIP from the complex and in that way stimulate NLRP3 activation [18]. TXNIP expression can be induced by ceramides, hyperglycemia, oxidative stress, and heat shock [93, 137], all of which trigger NLRP3 activation. On the other hand, treatments that increase TRX expression can prevent oxidative stress and increase the lifespan in transgenic mice [138]. It is known that the increased expression of TRX represses inflammation whereas TXNIP is an enhancer of inflammatory diseases [137]. These studies indicate that the TRX/TXNIP balance is a crucial regulator in the aging process as well as in many age-related diseases, e.g., in Alzheimer’s disease [139] and metabolic syndrome [140].
Recently, Shi et al. [141] demonstrated that autophagocytosis regulates the clearance of NLRP3 inflammasomes and thus controls IL-1β secretion and inflammatory potential of human macrophages. The ASC component of NLRP3 inflammasomes undergoes Lys63-linked polyubiquitination and is subsequently bound by p62 adaptor protein which targets the NLRP3 complex to autophagic degradation. It seems that a decline in autophagic efficiency with aging could increase the presence of NLRP3 inflammasomes during aging.
Immunosenescence and photoaging
The age-related, progressive decline in the function of the immune system is a major hallmark of aging. This process, called immunosenescence, has recently been reviewed extensively elsewhere [10, 142–144]. Major deficiencies occur in the adaptive immunity system including thymic involution and a decline in naive CD4+ T cells concomitant with clonal expansion of CD4+ memory T cells. Interestingly, Guarda et al. [145] demonstrated that the co-culture of mouse memory CD4+ T cells with macrophages blocked the LPS-primed, ATP-dependent activation capacity of macrophage NLRP3 inflammasomes whereas other T cell subsets only slightly affected the inflammasomal activation. Memory CD4+ T cells inhibited both the NLRP1 and NLRP3 activation but they did not affect NLRC4 inflammasomes. Moreover, caspase-1 activity and secretion of IL-1β were suppressed whereas the release of other inflammatory mediators, e.g., IL-6 and TNF-α, remained intact [145]. Guarda et al. [146] also revealed that IFNβ suppressed the activation of NLRP1 and NLRP3 in mouse bone marrow-derived macrophages as well as in human primary monocytes. IFNβ inhibited NLRP3 activation in a STAT1-dependent manner. They also observed that type I IFNs increased vulnerability to C. albicans infection in mouse, probably as a result of NLRP3 inhibition. Recently, Youm et al. [147] demonstrated that the aging-associated accumulation of free cholesterol and ceramides into mouse thymus activated NLRP3 inflammasomes and clearly promoted the age-related thymic involution and decreased the number of T cell progenitors. The deletion of NLRP3 reduced thymic atrophy and improved the maintenance of different T cell subsets. This study clearly demonstrated that NLRP3 is a crucial player in age-related Immunosenescence.
The senescence of innate immunity cells, monocytes/macrophages, neutrophils, and dendritic cells, exhibits specific changes, i.e., the TLR system is repressed with aging whereas a low-grade innate immunity upregulation occurs in old tissues [10, 142–144]. There have been several observations that the functional properties of tissue macrophages change with aging. This has been mainly studied with the cells of brain, lung, and vascular wall [126, 148, 149]. Microglial cells represent the resident macrophage population within the brain. In brief, the microglial cells in old brain are converted into a “primed state” where they exhibit markers of activated microglia without secreting any inflammatory factors. After the insult, the inflammatory response of those primed microglia is more rapid and exaggerated in comparison to the microglia from young animals. Currently, there is no direct evidence whether or not inflammasomes are involved in this priming process. Nakanishi and Wu [148] have reviewed several observations indicating that autophagic dysfunction and oxidative stress induce the age-related phenotype of microglia.
Skin is an active immune organ housing an array of innate and adaptive immunity cells and it can respond to a variety of insults, as reviewed by Nestle et al. [150]. Keratinocytes are the immune sentinels which regulate skin pathology in conjunction with dendritic cells, macrophages, and T cells. Photoaging is a well-known pathological state of the accelerated aging process of skin induced by ultraviolet light [151]. Photoaging involves mitochondrial damage, oxidative stress, activation of NF-κB signaling, and histological characteristics of chronic skin inflammation [151, 152]. Feldmeyer et al. [153] were the first workers to demonstrate that UVB irradiation triggered the caspase-1-dependent secretion of IL-1β in human keratinocytes. The inflammatory response was mediated by NLRP3 inflammasomes. Watanabe et al. [154] revealed that isolated human keratinocytes expressed several NLRP mRNAs and, moreover, NLRP3 inflammasomes were involved in the appearance of contact hypersensitivity and eczema in skin. Cho et al. [155] observed that IL-17 and IL-22 secreted by Th17 cells can activate NLRP3 inflammasomes and stimulate IL-1β secretion in human keratinocytes. They also revealed that the IL-17- and IL-22-induced NLRP3 activation was ROS-dependent. It is observed that inflammasomal activation occurs in several skin diseases, e.g., psoriasis [156] and atopic dermatitis [157]. For instance, Dombrowski et al. [158] demonstrated that, in psoriasis, the expression of AIM2 and IL-1β were clearly increased in keratinocytes. In psoriatic lesions, increased cytoplasmic DNA activated AIM2 inflammasomes, and secretion of IL-1β was enhanced, aggravating this chronic skin disease. These observations indicate that skin will be an important tissue in the studies of inflammasomes in the aging process.
Inflammasomal concept in age-related pathology
During aging, the adaptive immunity deteriorates whereas the innate immunity is clearly activated and is more responsive to insults. The appearance of a low-grade pro-inflammatory phenotype involves, (1) serum levels of many cytokines are increased with aging [115, 116, 159], (2) the expression level of inflammatory and immune response genes is upregulated in the tissues of old rodents and humans [12, 117, 160] as well as in peripheral and intestinal leukocytes [161], and (3) NF-κB signaling is activated in several tissues [11, 162]. The NF-κB system is involved in TLR signaling but it also has a crucial role in the priming process preceding the NLRP3 activation. NF-κB signaling controls the expression of NLRP3 and in that way NF-kB signaling is required; however, it is not sufficient to trigger NLRP3 activation [71]. Currently, the molecular mechanisms of NLRP3 activation in cellular stress have not been conclusively resolved. Several studies indicate that NLRP3 activation is dependent on the increased generation of ROS, in many cases produced by mitochondria [57, 163]. Moreover, van Bruggen et al. [164] demonstrated that blocking the ROS production by Nox1-4 enzymes, another rich source of ROS generation in many cell types, did not affect NLRP3 activation. These observations imply that mitochondria may be involved but ROS do not directly activate NLRP3. The activation could be mediated indirectly by disturbances in (1) the thiol redox balance between TRX/TXNIP, (2) ceramide synthesis, (3) mitochondrial integrity leading to the leakage of mtDNA, and (4) lysosomal stability triggering release of cathepsin B (Fig. 1).
Many observations link inflammasome activation to metabolic disturbances. For instance, the promoter of TXNIP contains the transactivation site for the MondoA:Mlx complex which is a key sensor for the cellular glucose concentration [165]. Hyperglycemia is a potent activator of TXNIP-mediated caspase-1 activation and IL-1β secretion in human adipose tissue [166]. Hyperglycemia is also a potent activator of NF-κB signaling [167], and thus it can stimulate the priming process of inflammasomes and trigger the inflammatory phenotype observed in hyperglycemia. Recently, Wen et al. [168] demonstrated that saturated fatty acid palmitate, but not unsaturated oleate, activated the NLRP3 inflammasome in macrophages. They also revealed that palmitate inhibited AMP-activated kinase which is a well-known inducer of autophagy and inhibitor of inflammation [169]. Inhibition of autophagy impaired the autophagic clearance of mitochondria and in that way increased ROS production and NLRP3 activation [168]. In conclusion, there is growing evidence linking energy metabolism, autophagy, and inflammation to the regulation of healthspan and even lifespan, as proposed in the rate-of-living theory (see “Introduction”).
Acknowledgments
This study was financially supported by Grants from the Academy of Finland and the University of Eastern Finland, Kuopio, Finland. The authors thank Dr. Ewen MacDonald for checking the language of the manuscript.
Abbreviations
- AIM2
Absent in melanoma 2
- ASC
Apoptosis-associated speck-like protein containing CARD domain
- CARD
Caspase recruitment domain
- CSF
Cerebrospinal fluid
- DAMP
Damage-associated molecular pattern
- dsDNA
Double-stranded DNA
- ER
Endoplasmic reticulum
- FPR
Formyl peptide receptor
- HMGB1
High-mobility group protein B1
- IAPP
Islet amyloid polypeptide
- IFNβ
Interferon β
- IKK
Inhibitory-κB kinase
- IL
Interleukin
- LPS
Lipopolysaccharide
- LRR
Leucine-rich repeat
- MAM
Mitochondria-associated ER membrane
- MAVS
Mitochondrial antiviral signaling protein
- MnSOD
Mitochondrial manganese superoxide dismutase
- mtDNA
Mitochondrial DNA
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NF-κB
Nuclear factor-κB
- NAIP5
NLR family, apoptosis inhibitory protein
- NLR
Nucleotide-binding domain leucine-rich repeat-containing receptor family
- NLRC4
NLR family, CARD-containing 4
- NLRP3
NLR family, pyrin domain-containing 3
- NOD1
Nucleotide-binding oligomerization domain-containing protein 1
- NOS
Nitric-oxide synthase
- Nox1-4
NADPH oxidases 1–4
- NRF2
Nuclear factor E2-related factor 2
- P2X7
P2X purinoceptor 7
- PMN
Polymorphonuclear neutrophil
- PYD
Pyrin domain
- RIG-1
Retinoic acid inducible gene-1
- ROS
Reactive oxygen species
- SAMP
Senescence-accelerated prone mouse
- STAT1
Signal transducer and activator of transcription 1
- TLR
Toll-like receptors
- TNF
Tumour necrosis factor
- TRAF
TNF receptor-associated factor
- TRIM30
Tripartite-motif protein 30
- TRX
Thioredoxin
- TXNIP
Thioredoxin-interacting protein
- UCP
Uncoupling protein
- UPRmt
Mitochondrial unfolded protein response
- UVB
Ultraviolet B
References
- 1.Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev. 2007;87:1175–1213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- 2.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 3.Lenaz G. Role of mitochondria in oxidative stress and ageing. Biochim Biophys Acta. 1998;1366:53–67. doi: 10.1016/S0005-2728(98)00120-0. [DOI] [PubMed] [Google Scholar]
- 4.Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Gruber J, Schaffer S, Halliwell B. The mitochondrial free radical theory of ageing-where do we stand? Front Biosci. 2008;13:6554–6579. doi: 10.2741/3174. [DOI] [PubMed] [Google Scholar]
- 6.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feher J, Kovacs I, Artico M, Cavallotti C, Papale A, Balacco Gabrieli C. Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol Aging. 2006;27:983–993. doi: 10.1016/j.neurobiolaging.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. 2009;84:705–712. doi: 10.1016/j.lfs.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Franceschi C, Valesin S, Bonafe M, Paolisso G, Yashin AI, Monti D, De Benedictis G. The network and the remodeling theories of aging: historical background and new perspectives. Exp Gerontol. 2000;35:879–896. doi: 10.1016/S0531-5565(00)00172-8. [DOI] [PubMed] [Google Scholar]
- 10.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Age Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Activation of innate immunity system during aging: NF-κB signaling is the culprit of inflamm-aging. Ageing Res Rev. 2008;7:83–105. doi: 10.1016/j.arr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 12.de Magalhaes JP, Curado J, Church GM. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics. 2009;25:875–881. doi: 10.1093/bioinformatics/btp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunol Rev. 2009;227:95–105. doi: 10.1111/j.1600-065X.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- 14.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 15.Bauernfeind F, Ablasser A, Bartok E, Kim S, Schmid-Burgk J, Cavlar T, Hornung V. Inflammasomes: current understanding and open questions. Cell Mol Life Sci. 2011;68:765–783. doi: 10.1007/s00018-010-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kersse K, Bertrand MJM, Lamkanfi M, Vandenabeele P. NOD-like receptors and the innate immune system: coping with danger, damage and death. Cytokine Growth Factor Rev. 2011;22:257–276. doi: 10.1016/j.cytogfr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Cruz CM, Rinna A, Forman HJ, Ventura ALM, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 19.Xiang M, Shi X, Li Y, Xu J, Yin L, Xiao G, Scott MJ, Billiar TR, Wilson MA, Fan J. Hemorrhagic shock activation of NLRP3 inflammasome in lung endothelial cells. J Immunol. 2011;187:4809–4817. doi: 10.4049/jimmunol.1102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-ß. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajamäki K, Lappalainen J, Öörni K, Välimäki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGuire KA, Barlan AU, Griffin TM, Wiethoff CM. Adenovirus type 5 rupture of lysosomes leads to cathepsin B-dependent mitochondrial stress and production of reactive oxygen species. J Virol. 2011;85:10806–10813. doi: 10.1128/JVI.00675-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deretic V. Autophagy as an innate immunity paradigm: expanding the scope and repertoire of pattern recognition receptors. Curr Opinion Immunol. 2011;24:1–11. doi: 10.1016/j.coi.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunol Rev. 2011;243:136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 26.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Moll Cell. 2002;10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 27.Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 28.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr. 2006;83:447S–455S. doi: 10.1093/ajcn/83.2.447S. [DOI] [PubMed] [Google Scholar]
- 30.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 31.Lamkanfi M, Sarkar A, Vande Walle L, Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti TD, Dixit VM. Inflammasome-dependent release of alarmin HMGB1 in endotoxemia. J Immunol. 2010;185:4385–4392. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buzzo CK, Campopiano JC, Massis LM, Lage SL, Cassado AA, Leme-Souza R, Cunha LD, Russo M, Zamboni DS, Amarante-Mendes GP, Bortoluci KR. A novel pathway for inducible nitric-oxide synthase activation through inflammasomes. J Biol Chem. 2010;285:32087–32095. doi: 10.1074/jbc.M110.124297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brodsky IE, Monack D. NLR-mediated control of inflammasome assembly in the host response against bacterial pathogens. Semin Immunol. 2009;21:199–207. doi: 10.1016/j.smim.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol. 2010;10:688–698. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 36.Bryant C, Fitzgerald KA. Molecular mechanisms involved in inflammasome activation. Trends Cell Biol. 2009;19:455–464. doi: 10.1016/j.tcb.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 38.Allam R, Darisipudi MN, Rupanagudi KV, Lichtnekert J, Tschopp J, Anders HJ. Cutting Edge: Cyclic polypeptide and aminoglycoside antibiotics trigger IL-1β secretion by activating the NLRP3 inflammasome. J Immunol. 2011;186:2714–2718. doi: 10.4049/jimmunol.1002657. [DOI] [PubMed] [Google Scholar]
- 39.Menu P, Mayor A, Zhou R, Tardivel A, Ichijo H, Mori K, Tschopp J. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis. 2012;3:e261. doi: 10.1038/cddis.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng TI, Jou MJ. Oxidative stress caused by mitochondrial calcium overload. Ann N Y Acad Sci. 2010;1201:183–188. doi: 10.1111/j.1749-6632.2010.05634.x. [DOI] [PubMed] [Google Scholar]
- 41.Kurz T, Eaton JW, Brunk UT. The role of lysosomes in iron metabolism and recycling. Int J Biochem Cell Biol. 2011;43:1686–1697. doi: 10.1016/j.biocel.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Tattoli I, Carneiro LA, Jehanno M, Magalhaes JG, Shu Y, Philpott DJ, Arnoult D, Girardin SE. NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-κB and JNK pathways by inducing reactive oxygen species production. EMBO Rep. 2008;9:293–300. doi: 10.1038/sj.embor.7401161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, Scull MA, Gris D, Roney KE, Zimmermann AG, Bowzard JB, Ranjan P, Monroe KM, Pickles R, Sambhara S, Ting JPY. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-κB signaling pathways. Immunity. 2011;34:854–865. doi: 10.1016/j.immuni.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia X, Cui J, Wang HY, Zhu L, Matsueda S, Wang Q, Yang X, Hong J, Songyang Z, Chen ZJ, Wang RF. NLRX1 negatively regulates TLR-induced NF-κB signaling by targeting TRAF6 and IKK. Immunity. 2011;34:843–853. doi: 10.1016/j.immuni.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui J, Zhu L, Xia X, Wang HY, Legras X, Hong J, Ji J, Shen P, Zheng S, Chen ZJ, Wang RF. NLRC5 negatively regulates the NF-κB and type I interferon signaling pathways. Cell. 2010;141:483–496. doi: 10.1016/j.cell.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 47.Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443–452. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- 48.Yin Y, Yan Y, Jiang X, Mai J, Chen NC, Wang H, Yang XF. Inflammasomes are differentially expressed in cardiovascular and other tissues. Int J Immunopathol Pharmacol. 2009;22:311–322. doi: 10.1177/039463200902200208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faustin B, Chen Y, Zhai D, Le Negrate G, Lartigue L, Satterthwait A, Reed JC. Mechanism of Bcl-2 and Bcl-xL inhibition of NLRP1 inflammasome: loop domain-dependent suppression of ATP binding and oligomerization. Proc Natl Acad Sci USA. 2009;106:3935–3940. doi: 10.1073/pnas.0809414106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu F, Lo CF, Ning X, Kajkowski EM, Jin M, Chiriac C, Gonzales C, Naureckiene S, Lock YW, Pong K, Zaleska MM, Jacobsen JS, Silverman S, Ozenberger BA. Expression of NALP1 in cerebellar granule neurons stimulates apoptosis. Cell Signal. 2004;16:1013–1021. doi: 10.1016/j.cellsig.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Pontillo A, Catamo E, Arosio B, Mari D, Crovella S. NALP1/NLRP1 genetic variants are associated with Alzheimer disease. Alzheimer Dis Assoc Disord. 2011 doi: 10.1097/WAD.0b013e318231a8ac. [DOI] [PubMed] [Google Scholar]
- 52.Jin Y, Mauilloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, Fain PR, Spritz RA. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356:1216–1225. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- 53.de Rivero Vaccari JP, Lotocki G, Marcillo AE, Dietrich WD, Keane RW. A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci. 2008;28:3404–3414. doi: 10.1523/JNEUROSCI.0157-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trifunovic A, Larsson NG. Mitochondrial dysfunction as a cause of ageing. J Intern Med. 2008;263:167–178. doi: 10.1111/j.1365-2796.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 55.Ren J, Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med (Berl) 2010;88:993–1001. doi: 10.1007/s00109-010-0663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coskun P, Wyrembak J, Schriner S, Chen HW, Marciniack C, Laferla F, Wallace DC. A mitochondrial etiology of Alzheimer and Parkinson disease. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbagen.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tschopp J. Mitochondria: sovereign in inflammation? Eur J Immunol. 2011;41:1196–1202. doi: 10.1002/eji.201141436. [DOI] [PubMed] [Google Scholar]
- 58.Cloonan SM, Choi AMK. Mitochondria: commanders of innate immunity and disease? Curr Opinion Immunol. 2011 doi: 10.1016/j.coi.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 59.Rousset S, Emre Y, Join-Lambert O, Hurtaud C, Ricquier D, Cassard-Doulcier AM. The uncoupling protein 2 modulates the cytokine balance in innate immunity. Cytokine. 2006;35:135–142. doi: 10.1016/j.cyto.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 60.Emre Y, Nubel T. Uncoupling protein UCP2: when mitochondrial activity meets immunity. FEBS Lett. 2010;584:1437–1442. doi: 10.1016/j.febslet.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 61.Horvath TL, Diano S, Miyamoto S, Barry S, Gatti S, Alberati D, Livak F, Lombardi A, Moreno M, Goglia F, Mor G, Hamilton J, Kachinskas D, Horwitz B, Warden CH. Uncoupling proteins-2 and 3 influence obesity and inflammation in transgenic mice. Int J Obes. 2003;27:433–442. doi: 10.1038/sj.ijo.0802257. [DOI] [PubMed] [Google Scholar]
- 62.Ricquier D. Mitochondrial uncoupling proteins. Curr Opin Drug Discov Dev. 1999;2:497–504. [PubMed] [Google Scholar]
- 63.Haines BA, Mehta SL, Pratt SM, Warden CH, Li PA. Deletion of mitochondrial uncoupling protein-2 increases ischemic brain damage after transient focal ischemia by altering gene expression patterns and enhancing inflammatory cytokines. J Cereb Blood Flow Metab. 2010;30:1825–1833. doi: 10.1038/jcbfm.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bai Y, Onuma H, Bai X, Medvedev AV, Misukonis M, Weinberg JB, Cao W, Robidoux J, Floering LM, Daniel KW, Collins S. Persistent nuclear factor-κb activation in Ucp2−/− mice leads to enhanced nitric oxide and inflammatory cytokine production. J Biol Chem. 2005;280:19062–19069. doi: 10.1074/jbc.M500566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Escames G, Lopez LC, Garcia JA, Garcia-Corzo L, Ortiz F, Acuna-Castroviejo D. Mitochondrial DNA and inflammatory diseases. Hum Genet. 2011 doi: 10.1007/s00439-011-1057-y. [DOI] [PubMed] [Google Scholar]
- 66.Krysko DV, Agostimis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32:157–164. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arnoult D, Soares F, Tattoli I, Girardin SE. Mitochondria in innate immunity. EMBO Rep. 2011;12:901–910. doi: 10.1038/embor.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Broz P, Monack DM. Molecular mechanisms of inflammasome activation during microbial infections. Immunol Rev. 2011;243:174–190. doi: 10.1111/j.1600-065X.2011.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bauernfeind FG, Bartok E, Rieger A, Franchi L, Nunez G, Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Troy CM, Stefanis L, Prochiantz A, Greene LA, Shelanski ML. The contrasting roles of ICE family proteases and interleukin-1β in apoptosis induced by trophic factor withdrawal and by copper/zinc superoxide dismutase down-regulation. Proc Natl Acad Sci USA. 1996;93:5635–5640. doi: 10.1073/pnas.93.11.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haddad JJ. Glutathione depletion is associated with augmenting a proinflammatory signal: evidence for an antioxidant/pro-oxidant mechanism regulating cytokines in the alveolar epithelium. Cytokines Cell Mol Ther. 2000;6:177–187. doi: 10.1080/mccm.6.4.177.187. [DOI] [PubMed] [Google Scholar]
- 74.Carta S, Castellani P, Delfino L, Tassi S, Vene R, Rubartelli A. DAMPs and inflammatory processes: the role of redox in the different outcomes. J Leukoc Biol. 2009;86:549–555. doi: 10.1189/jlb.1008598. [DOI] [PubMed] [Google Scholar]
- 75.Tassi S, Carta S, Vene R, Delfino L, Ciriolo MR, Rubartelli A. Pathogen-induced interleukin-1β processing and secretion is regulated by a biphasic redox response. J Immunol. 2009;183:1456–1462. doi: 10.4049/jimmunol.0900578. [DOI] [PubMed] [Google Scholar]
- 76.Rubartelli A, Gattorno M, Netea MG, Dinarello CA. Interplay between redox status and inflammasome activation. Trends Immunol. 2011;32:559–566. doi: 10.1016/j.it.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 77.Hu Y, Mao K, Zeng Y, Chen S, Tao Z, Yang C, Sun S, Wu X, Meng G, Sun B. Tripartite-motif protein 30 negatively regulates NLRP3 inflammasome activation by modulating reactive oxygen species production. J Immunol. 2010;185:7699–7705. doi: 10.4049/jimmunol.1001099. [DOI] [PubMed] [Google Scholar]
- 78.Tsai PY, Ka SM, Chang JM, Chen HC, Shui HA, Li CY, Hua KF, Chang WL, Huang JJ, Yang SS, Chen A. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic Biol Med. 2011;51:744–754. doi: 10.1016/j.freeradbiomed.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 79.Nakahira K, Haspel JA, Rathinam VAK, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AMK. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;8:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 81.Wu JJ, Quijano C, Chen E, Liu H, Cao L, Fergusson MM, Rovira II, Gutkind S, Daniels MP, Komatsu M, Finkel T. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging. 2009;1:425–437. doi: 10.18632/aging.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fesus L, Demeny MA, Petrovski G. Autophagy shapes inflammation. Antioxid Redox Signal. 2011;14:2233–2243. doi: 10.1089/ars.2010.3485. [DOI] [PubMed] [Google Scholar]
- 83.Giorgi C, De Stefani D, Boboni A, Rizzuto R, Pinton P. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol. 2009;41:1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KHG, Mok KH, Newsholme P, Nunez G, Yodoi J, Kahn SE, Lavelle EC, O’Neill LAJ. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Castiglioni A, Canti V, Rovere-Querini P, Manfredi AA. High-mobility group box 1 (HMGB1) as a master regulator of innate immunity. Cell Tissue Res. 2011;343:189–199. doi: 10.1007/s00441-010-1033-1. [DOI] [PubMed] [Google Scholar]
- 87.Lunov O, Syrovets T, Loos C, Nienhaus GU, Mailänder V, Landfester K, Rouis M, Simmet T. Amino-functionalized polystyrene nanoparticles activate the NLRP3 inflammasome in human macrophages. ACS Nano. 2011;5:9648–9657. doi: 10.1021/nn203596e. [DOI] [PubMed] [Google Scholar]
- 88.Choumar A, Tarhuni A, Letteron P, Reyl-Desmars F, Dauhoo N, Damasse J, Vadrot N, Nahon P, Moreau R, Pessayre D, Mansouri A. Lipopolysaccharide-induced mitochondrial DNA depletion. Antioxid Redox Signal. 2011;15:2837–2854. doi: 10.1089/ars.2010.3713. [DOI] [PubMed] [Google Scholar]
- 89.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nikolova-Karakashian M, Karakashian A, Rutkute K. Role of neutral sphingomyelinases in aging and inflammation. Subcell Biochem. 2008;49:469–486. doi: 10.1007/978-1-4020-8831-5_18. [DOI] [PubMed] [Google Scholar]
- 91.Yu J, Novgorodov SA, Chudakova D, Zhu H, Bielawska A, Bielawski J, Obeid LM, Kindy MS, Gudz TI. JNK3 signaling pathway activates ceramide synthase leading to mitochondrial dysfunction. J Biol Chem. 2007;282:25940–25949. doi: 10.1074/jbc.M701812200. [DOI] [PubMed] [Google Scholar]
- 92.Siskind LJ, Kolesnick RN, Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6:118–125. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen CL, Lin CF, Chang WT, Huang WC, Teng CF, Lin YS. Ceramide induces p38 MAPK and JNK activation through a mechanism involving a thioredoxin-interacting protein-mediated pathway. Blood. 2008;111:4365–4374. doi: 10.1182/blood-2007-08-106336. [DOI] [PubMed] [Google Scholar]
- 94.Sreekumar PG, Ding Y, Ryan SJ, Kannan R, Hinton DR. Regulation of thioredoxin by ceramide in retinal pigment epithelial cells. Exp Eye Res. 2009;88:410–417. doi: 10.1016/j.exer.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raoof M, Zhang Q, Itagaki K, Hauser CJ. Mitochondrial peptides are potent immune activators that activate human neutrophils via FPR-1. J Trauma. 2010;68:1328–1332. doi: 10.1097/TA.0b013e3181dcd28d. [DOI] [PubMed] [Google Scholar]
- 96.Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sohal RS, Brunk UT. Lipofuscin as an indicator of oxidative stress and aging. Adv Exp Med Biol. 1989;266:17–26. doi: 10.1007/978-1-4899-5339-1_2. [DOI] [PubMed] [Google Scholar]
- 98.Stienstra R, Joosten LAB, Koenen T, van Tits B, van Diepen JA, van den Berg SAA, Rensen PCN, Voshol PJ, Fantuzzi G, Hijmans A, Kersten S, Muller M, van den Berg WB, van Rooijen N, Wabitsch M, Kullberg BJ, van den Meer JWM, Kanneganti T, Tack CJ, Netea MG. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stoll G, Bendszus M. Inflammation and atherosclerosis: novel insights into plaque formation and destabilization. Stroke. 2006;37:1923–1932. doi: 10.1161/01.STR.0000226901.34927.10. [DOI] [PubMed] [Google Scholar]
- 100.Ungvari Z, Kaley G, de Capo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65A:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ojala J, Alafuzoff I, Herukka SK, van Groen T, Tanila H, Pirttilä T. Expression of interleukin-18 is increased in the brains of Alzheimer’s disease patients. Neurobiol Aging. 2009;30:198–209. doi: 10.1016/j.neurobiolaging.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 103.Salminen A, Ojala J, Suuronen T, Kaarniranta K, Kauppinen A. Amyloid-β oligomers set fire to inflammasomes and induce Alzheimer’s pathology. J Cell Mol Med. 2008;12:2255–2262. doi: 10.1111/j.1582-4934.2008.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jha S, Srivastava SY, Brickey WJ, Iocca H, Toews A, Morrison JP, Chen VS, Gria D, Matsushima GK, Ting JPY. The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J Neurosci. 2010;30:15811–15820. doi: 10.1523/JNEUROSCI.4088-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, Sutterwala FS. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci USA. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li H, Ambade A, Re F. Cutting edge: necrosis activates the NLRP3 inflammasome. J Immunol. 2009;183:1528–1532. doi: 10.4049/jimmunol.0901080. [DOI] [PubMed] [Google Scholar]
- 107.Petrovski G, Ayna G, Majai G, Hodrea J, Benko S, Madi A, Fesus L. Phagocytosis of cells dying through autophagy induces inflammasome activation and IL-1β release in human macrophages. Autophagy. 2011;7:321–330. doi: 10.4161/auto.7.3.14583. [DOI] [PubMed] [Google Scholar]
- 108.Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunol Rev. 2011;243:152–162. doi: 10.1111/j.1600-065X.2011.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CCM, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 110.Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, Corr M. Caspase-1-independent activation of interleukin-1β in neutrophil-predominant inflammation. Arthritis Rheum. 2009;60:3642–3650. doi: 10.1002/art.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Joosten LAB, Netea MG, Fantuzzi G, Koenders MI, Helsen MMA, Sparrer H, Pham CT, van der Meer JWM, Dinarello CA, van den Berg WB. Inflammatory arthritis in caspase 1 gene—deficient mice. Arthritis Rheum. 2009;60:3651–3662. doi: 10.1002/art.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stehlik C. Multiple interleukin-1β-converting enzymes contribute to inflammatory arthritis. Arthritis Rheum. 2009;60:3524–3530. doi: 10.1002/art.24961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Harris J, Hartman M, Roche C, Zeng SG, O’Shea A, Sharp FA, Lambe EM, Creagh EM, Golenbock DT, Tschopp J, Kornfeld H, Fitzgerald KA, Lavelle EC. Autophagy controls IL-1β secretion by targeting pro-IL-1β for degradation. J Biol Chem. 2011;286:9587–9597. doi: 10.1074/jbc.M110.202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Crisan TO, Plantinga TS, van de Veerdonk FL, Farcas MF, Stoffels M, Kullberg BJ, van den Meer JWM, Joosten LAB, Netea MG. Inflammasome-independent modulation of cytokine response by autophagy in human cells. PLoS One. 2011;6:e18666. doi: 10.1371/journal.pone.0018666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bruunsgaard H, Andersen-Ranberg K, Hjelmborg JVB, Pedersen BK, Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med. 2003;115:278–283. doi: 10.1016/S0002-9343(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 116.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 117.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in rat coronary arteries. FASEB J. 2003;17:1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- 118.Maher FO, Martin DSD, Lynch MA. Increased IL-1β in cortex of aged rats is accompanied by downregulation of ERK and PI-3 kinase. Neurobiol Aging. 2004;25:795–806. doi: 10.1016/j.neurobiolaging.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 119.Niemi K, Teirilä L, Lappalainen J, Rajamäki K, Baumann MH, Öörni K, Wolff H, Kovanen PT, Matikainen S, Eklund KK. Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and cathepsin B-sensitive pathway. J Immunol. 2011;186:6119–6128. doi: 10.4049/jimmunol.1002843. [DOI] [PubMed] [Google Scholar]
- 120.Ungvari Z, Sonntag WE, Csiszar A. Mitochondria and aging in the vascular system. J Mol Med. 2010;88:1021–1027. doi: 10.1007/s00109-010-0667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Krishnan E. Inflammation, oxidative stress and lipids: the risk triad for atherosclerosis in gout. Rheumatology. 2010;49:1229–1238. doi: 10.1093/rheumatology/keq037. [DOI] [PubMed] [Google Scholar]
- 122.Yajima N, Takahashi M, Morimoto H, Shiba Y, Takahashi Y, Masumoto J, Ise H, Sagara J, Nakayama J, Taniguchi S, Ikeda U. Critical role of bone marrow apoptosis-associated speck-like protein, an inflammasome adaptor molecule, in neointimal formation after vascular injury in mice. Circulation. 2008;117:3079–3087. doi: 10.1161/CIRCULATIONAHA.107.746453. [DOI] [PubMed] [Google Scholar]
- 123.Gemma C, Bickford PC. Interleukin-1β and caspase-1: players in the regulation of age-related cognitive dysfunction. Rev Neurosci. 2007;18:137–148. doi: 10.1515/REVNEURO.2007.18.2.137. [DOI] [PubMed] [Google Scholar]
- 124.Gemma C, Bachstetter AD, Cole MJ, Fister M, Hudson C, Bickford PC. Blockade of caspase-1 increases neurogenesis in the aged hippocampus. Eur J Neurosci. 2007;26:2795–2803. doi: 10.1111/j.1460-9568.2007.05875.x. [DOI] [PubMed] [Google Scholar]
- 125.Alboni S, Cervia D, Sugama S, Conti B. Interleukin 18 in CNS. J Neuroinflamm. 2010;7:9. doi: 10.1186/1742-2094-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Njie EG, Boelen E, Stassen FR, Steinbusch HWM, Borchelt, Streit WJ. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging. 2011;33:195.e1–195.e12. doi: 10.1016/j.neurobiolaging.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective β-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Karuppagounder SS, Shi Q, Xu H, Gibson GE. Changes in inflammatory processes associated with selective vulnerability following mild impairment of oxidative metabolism. Neurobiol Dis. 2007;26:353–362. doi: 10.1016/j.nbd.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brunk UT, Terman A. The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem. 2002;269:1996–2002. doi: 10.1046/j.1432-1033.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- 130.Salminen A, Kaarniranta K. Regulation of the aging process by autophagy. Trends Mol Med. 2009;15:217–224. doi: 10.1016/j.molmed.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 131.Petrovski G, Das DK. Does autophagy take a front seat in lifespan extension? J Cell Mol Med. 2010;14:2543–2551. doi: 10.1111/j.1582-4934.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weber TA, Reichert AS. Impaired quality control of mitochondria: aging from a new perspective. Exp Gerontol. 2010;45:503–511. doi: 10.1016/j.exger.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 133.Cardoso SM, Pereira CF, Moreira PI, Arduino DM, Esteves AR, Oliveira CR. Mitochondrial control of autophagic lysosomal pathway in Alzheimer’s disease. Exp Neurol. 2010;223:294–298. doi: 10.1016/j.expneurol.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 134.Las G, Shirihai OS. The role of autophagy in β-cell lipotoxicity and type 2 diabetes. Diabetes Obes Metab. 2010;12(Suppl.2):15–19. doi: 10.1111/j.1463-1326.2010.01268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gloire G, Legrand-Poels S, piette J. NF-κB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 136.Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, Göktuna SI, Neuenhahn M, Fierer J, Paxian S, Van Rooijen N, Xu Y, O’Cain T, Jaffee BB, Busch DH, Duyster J, Schmid RM, Eckmann L, Karin M. NF-κB is a negative regulator of IL-1β secretion revealed by genetic and pharmacological inhibition of IKKβ. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Watanabe R, Nakamura H, Masutani H, Yodoi J. Anti-oxidative, anti-cancer and anti-inflammatory actions by thioredoxin 1 and thioredoxin-binding protein-2. Pharmacol Ther. 2010;127:261–270. doi: 10.1016/j.pharmthera.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 138.Yoshida T, Nakamura H, Masutani H, Yodoi J. The involvement of thioredoxin and thioredoxin binding protein-2 on cellular proliferation and aging process. Ann N Y Acad Sci. 2005;1055:1–12. doi: 10.1196/annals.1323.002. [DOI] [PubMed] [Google Scholar]
- 139.Lovell MA, Xie C, Gabbita P, Markesbery WR. Decreased thioredoxin and increased thioredoxin reductase levels in Alzheimer’s disease brain. Free Rad Biol Med. 2000;28:418–427. doi: 10.1016/S0891-5849(99)00258-0. [DOI] [PubMed] [Google Scholar]
- 140.Kaimul AM, Nakamura H, Masutani H, Yodoi J. Thioredoxin and thioredoxin-binding protein-2 in cancer and metabolic syndrome. Free Rad Biol Med. 2007;43:861–868. doi: 10.1016/j.freeradbiomed.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 141.Shi CS, Shenderov K, Huang NN, Kabat J, Abu-Asab M, Fitzgerald KA, Sher A, Kehrl JH. Activation of autophagy by inflammatory signals limits !L-1β production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology. 2008;23:64–74. doi: 10.1152/physiol.00040.2007. [DOI] [PubMed] [Google Scholar]
- 143.Kovacs EJ, Palmer JL, Fortin CF, Fulop T, Jr, Goldstein DR, Linton PJ. Aging and innate immunity in the mouse: impact of intrinsic and extrinsic factors. Trends Immunol. 2009;30:319–324. doi: 10.1016/j.it.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transplant Int. 2009;22:1041–1050. doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- 145.Guarda G, Dostert C, Staehli F, Cabalzar K, Castillo R, Tardivel A, Schneider P, Tschopp J. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature. 2009;460:269–273. doi: 10.1038/nature08100. [DOI] [PubMed] [Google Scholar]
- 146.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Förster I, Farlik M, Decker T, Du Pasquier RA, Romero P, Tschopp J. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 147.Youm YH, Kanneganti TD, Vandanmagsar B, Zhu X, Ravussin A, Adijiang A, Owen JS, Thomas MJ, Francis J, Parks JS, Dixit VD. The NLRP3 inflammasome promotes age-related thymic demise and immunosenescence. Cell Rep. 2012;1:56–68. doi: 10.1016/j.celrep.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nakanishi H, Wu Z. Microglia-aging: roles of microglial lysosome- and mitochondria-derived reactive oxygen species in brain aging. Behav Brain Res. 2009;201:1–7. doi: 10.1016/j.bbr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 149.Sharma G, Hanania NA, Shim YM. The aging immune system and its relationship to the development of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:573–580. doi: 10.1513/pats.200904-022RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yaar M, Gilchrest BA. Photoaging: mechanism, prevention and therapy. Br J Dermatol. 2007;157:874–887. doi: 10.1111/j.1365-2133.2007.08108.x. [DOI] [PubMed] [Google Scholar]
- 152.Bosset S, Bonnet-Duquennoy M, Barre P, Chalon A, Kurfurst R, Bonte F, Schnebert S, Le Varlet B, Nicolas JF. Photoageing shows histological features of chronic skin inflammation without clinical and molecular abnormalities. Br J Dermatol. 2003;149:826–835. doi: 10.1046/j.1365-2133.2003.05456.x. [DOI] [PubMed] [Google Scholar]
- 153.Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, Beer HD. The inflammasome mediates UVB-induced activation and secretion of interleukin-1β by keratinocytes. Curr Biol. 2007;17:1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 154.Watanabe H, Gaide O, Petrilli V, Martinon F, Contassot E, Roques S, Kummer JA, Tschopp J, French LE. Activation of the IL-1β-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol. 2007;127:1956–1963. doi: 10.1038/sj.jid.5700819. [DOI] [PubMed] [Google Scholar]
- 155.Cho KA, Suh JW, Lee KH, Kang JL, Woo SY. IL-17 and IL-22 enhance skin inflammation by stimulating the secretion of IL-1β by keratinocytes via the ROS-NLRP3-caspase-1 pathway. Int Immunol. 2011 doi: 10.1093/intimm/dxr110. [DOI] [PubMed] [Google Scholar]
- 156.Salskov-Iversen ML, Johansen C, Kragballe K, Iversen L. Caspase-5 expression is upregulated in lesional psoriatic skin. J Invest Dermatol. 2011;131:670–676. doi: 10.1038/jid.2010.370. [DOI] [PubMed] [Google Scholar]
- 157.Dai X, Sayama K, Tohyama M, Shirakata Y, Hanakawa Y, Tokumaru S, Yang L, Hirakawa S, Hashimoto K. Mite allergen is a danger signal for the skin via activation of inflammasome in keratinocytes. J Allergy Clin Immunol. 2011;127:806–14. doi: 10.1016/j.jaci.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 158.Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Göss C, Anz D, Simanski M, Gläser R, Harder J, Hornung V, Gallo RL, Ruzicka T, Besch R, Schauber J. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med. 2011;3:82ra38. doi: 10.1126/scitranslmed.3002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2010;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Swindell WR. Genes and gene expression modules associated with caloric restriction and aging in the laboratory mouse. BMC Genomics. 2009;10:585. doi: 10.1186/1471-2164-10-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rosenstiel P, Derer S, Till A, Eberstein H, Bewig B, Nikolaus S, Nebel A, Schreiber C. Systematic expression profiling of innate immune genes defines a complex pattern of immunosenescence in peripheral and intestinal leukocytes. Genes Immun. 2008;9:103–114. doi: 10.1038/sj.gene.6364454. [DOI] [PubMed] [Google Scholar]
- 162.Adler AS, Sinha S, Kawahara TL, Zhang JY, Segal E, Chang HY. Motif module map reveals enforcement of aging by continual NF-κB activity. Genes Dev. 2007;21:3244–3257. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tschopp J, Schroeder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 164.van Bruggen R, Köker MY, Jansen M, van Houdt M, Roos D, Kuijpers TW, van den Berg TK. Human NLRP3 inflammasome activation is No1–4 independent. Blood. 2010;115:5398–5400. doi: 10.1182/blood-2009-10-250803. [DOI] [PubMed] [Google Scholar]
- 165.Stoltzman CA, Peterson CW, Breen KT, Muoio DM, Billin AN, Ayer DE. Glucose sensing by MondoA:Mlx complexes: a role for hexokinases and direct regulation of thioredoxin-interacting protein expression. Proc Natl Acad Sci USA. 2008;105:6912–6917. doi: 10.1073/pnas.0712199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Koenen TB, Stienstra R, van Tits LJ, de Graaf J, Stalenhoef AF, Joosten LA, Tack CJ, Netea MG. Hyperglycemia activates caspase-1 and TXNIP-mediated IL-1β transcription in human adipose tissue. Diabetes. 2011;60:517–524. doi: 10.2337/db10-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Salminen A, Kaarniranta K. Glycolysis links p53 function with NF-κB signaling: impact on cancer and aging process. J Cell Physiol. 2010;224:1–6. doi: 10.1002/jcp.22119. [DOI] [PubMed] [Google Scholar]
- 168.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MTH, Brickey WJ, Ting JPY. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med (Berl) 2011;89:667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]