Abstract
Neutrophils and macrophages are phagocytic cells that cooperate during inflammation and tissue repair. Neutrophils undergo apoptosis and are engulfed by macrophages. Engulfment modulates macrophage activation and microbicidal activity. Infection by Leishmania takes place in the context of tissue repair. This article discusses cellular and molecular mechanisms involved in the intimate cooperation of neutrophils and macrophages in Leishmania infection.
Keywords: Neutrophil, Macrophage, Leishmania, Apoptosis, Phagocytosis, Cytokines
Introduction
Leishmaniasis is a widespread disease that ranges in severity from cutaneous lesions to systemic infection. Leishmania species alternate between promastigote forms in the insect and intracellular amastigote forms in the mammalian host. Infection with Leishmania is coincident with tissue damage induced by the sand fly bite or by the laboratory needle [1]. Tissue repair is a conserved host response to injury. Repair involves recruitment of neutrophils and monocyte/macrophages, followed by apoptosis of neutrophils and engulfment of apoptotic neutrophils by macrophages [2]. Therefore, Leishmania infection co-evolved to take advantage of interactions between neutrophils and macrophages. Recent studies indicate that neutrophils mediate either host-protective or disease-promoting roles in infection. Furthermore, susceptibility to infection correlates with genetic differences involving neutrophils. Here, we discuss recent findings that help in clarifying the role of interactions between neutrophils and macrophages in Leishmania infection. Unless noted, the studies discussed here refer to infection with Leishmania major. At the end of the text, studies with other Leishmania species are presented.
Macrophage activation
Macrophage responses to microbial and immunological stimuli lead to discrete, stereotyped phenotypes [3, 4]. Classically activated, or M1, macrophages are microbicidal, while alternatively activated (M2a) and regulatory (M2b) macrophages are permissive to parasites [3, 4]. The initial contact of Leishmania with macrophages is relatively silent. In the absence of added cytokines, infection by L. mexicana results in very low transcriptional activity of macrophages, almost indistinguishable from uninfected cells [5]. Analysis of transcription suggests induction of a mixed phenotype, which is skewed towards either regulatory or alternatively activated macrophages [5, 6].
Fully differentiated macrophages develop in the context of inflammation and contact with cytokines. Classically activated macrophages display leishmanicidal activity and are required for control of Leishmania infection [4]. Alternatively activated macrophages are induced by the Th2 cytokine IL-4, and play an important role in tissue repair [3]. In addition, activation of the transcription factor PPAR-γ is important for tissue repair [7], and expression of PPAR-γ is required for induction of alternatively activated macrophages [8]. Mice lacking alternatively activated macrophages due to deficient expression of either the IL-4 receptor or PPAR-γ are less susceptible to L. major infection [8, 9]. In agreement, macrophages induced by PPAR-γ agonists are more permissive to replication of L. major [10]. In addition, a proteophosphoglycan deposited into the skin by infected sand flies induces arginase activity and alternative macrophage activation to enhance Leishmania survival [11].
Host IgG plays a disease-promoting role in infection by Leishmania [12]. Ligation of Fcγ receptors by IgG immune complexes induces IL-10 production, and a regulatory or M2b phenotype in macrophages, which is permissive for Leishmania replication [4, 12, 13]. Together, these results indicate that L. major takes advantage of alternatively activated and regulatory macrophages to replicate in the host.
Infection in the context of tissue repair
Infection by L. major takes place amid tissue repair induced by the insect bite. Tissue repair is a conserved multistep response to injury characterized by an initial influx of neutrophils, followed by monocyte/macrophages and fibroblasts [14]. Repair cannot be completed until inflammation resolves. Chronic ulcers are associated with persisting infiltrates of neutrophils [14], as in the case of L. major infection [15]. Skin ulceration correlates with keratinocyte apoptosis mediated by FasL and TRAIL [16]. Neutrophils could play a role by disrupting the basement membrane and allowing diffusion of soluble FasL and TRAIL [16]. In addition, genetic studies identify a correlation between tissue repair and the ability to clear L. major infection. Congenic mice carrying the susceptible BALB/c genetic background and the resistant loci lmr-1, -2 and -3, derived from resistant C57Bl/6 (B6) mice, clear infection by L. major [17]. Congenic mice increase the expression of genes involved in tissue repair and express faster tissue repair responses [17]. However, the lmr loci do not influence T cell cytokine responses or parasitic loads in lymph nodes, suggesting that the local response to infection is regulated by genes distinct from adaptive immunity [17].
Neutrophils as intermediate host cells
Sand fly bites create a hemorrhagic pool in the host skin for feeding. Therefore, the earliest Leishmania interactions with the host take place in whole blood [18]. Parasites react with natural antibodies and are opsonized by complement, binding to erythrocytes through CR1 [18]. Within minutes, parasites are transferred from erythrocytes to leukocytes, and are internalized by both monocytes and neutrophils [18].
Cutaneous infection spreads away from the hemorrhagic pool, involving phagocytes in the areas adjacent to the wound. Early studies investigated the cell types recruited to the site of L. major infection in both susceptible (BALB/c) and resistant (B6) mice [15]. Initially, both strains show similar inflammatory responses. However, neutrophils become prominent and persist in susceptible hosts, but are cleared from the sites of resistant hosts [15]. In both strains, parasites can be found inside neutrophils in the first days of infection, but L. major only replicates in immature macrophages [15]. Following complement opsonization, L. major survives inside neutrophils for more than 1 day, although the majority of parasites are killed [19]. Evasion from neutrophil microbicidal activity is an active process. Both L. major and L. donovani are ingested by neutrophils, and azurophilic granules fuse with parasite-containing phagosomes without promoting parasite killing [20]. Tertiary and specific granules, which are involved in vacuole acidification and generation of reactive oxygen species, fail to fuse with vesicles harboring parasites [20].
Studies of dynamic intravital microscopy investigated early steps of L. major infection induced by sand fly bites in the skin of resistant B6 mice [21]. After 1 day of infection, parasites localize mainly inside neutrophils. Later, neutrophils are cleared, and parasites become localized to monocytes/macrophages [21]. Although suggestive, these studies do not demonstrate transfer of parasites from neutrophils to macrophages. The time elapsed between observations allows parasite replication in macrophages. However, the study identifies viable parasites being released from apoptotic neutrophils in the vicinity of macrophages [21]. Therefore, it is possible that parasites hide in neutrophils until host cell apoptosis forces the parasite to infect a macrophage. Depletion of neutrophils reduces local incidence of infection, suggesting that neutrophils play a disease-promoting role [21]. Uptake by neutrophils could rescue parasites from death. Alternatively, passage through neutrophils could render the parasite more infective, for example, by exposing phosphatidylserine on the surface [22]. Finally, macrophages engaged in the clearance of senescent neutrophils could become permissive for intracellular parasite growth [23].
Engulfment of neutrophils regulates infection of macrophages
Neutrophils play important roles in the innate immune response. Neutrophils are short-lived cells that expose phosphatidylserine and undergo spontaneous apoptosis following transmigration of blood vessels [2]. Onset of apoptosis induces phagocytic clearance of neutrophils that is important for resolution of inflammation and tissue repair [2]. Apoptosis and engulfment of apoptotic cells play immunoregulatory and pathogenic roles in parasitic infections [24]. Engulfment of apoptotic cells induces a biochemical cascade that deactivates macrophages through TGF-β, and drives replication of an intracellular parasite through ornithine decarboxylase activity and production of polyamines [25]. Polyamine synthesis from l-ornithine is essential for intramacrophagic growth of L. major [26]. The outcome of L. major infection in macrophages engulfing neutrophils was investigated [27]. BALB/c neutrophils induce production of TGF-β, but not TNF-α in macrophages, and increase growth of L. major in a manner dependent on PGE2 and TGF-β [27]. Depletion of neutrophils reduces infection in lymph nodes of BALB/c mice, indicating a disease-promoting role [27]. An alternative to this scenario is the “Trojan horse” hypothesis, where infected neutrophils are engulfed and transfer a viable parasite to an uninfected macrophage [28]. Dynamic intravital microscopy did not observe phagocytosis of infected neutrophils [21], but this hypothesis remains a valid possibility.
Proinflammatory clearance of neutrophils
Clearance of neutrophils can be proinflammatory in the presence of additional innate immune stimuli, such as TLR ligands and heat shock proteins [29, 30]. Notably, B6, but not BALB/c, neutrophils induce production of TNF-α, but not TGF-β, in infected macrophages, and reduce parasite load in a manner dependent on TNF-α [27]. Depletion of neutrophils increases infection in lymph nodes of B6 mice, indicating a host-protective role. Proinflammatory effects of B6 neutrophils are mediated by soluble factors, and neutralization of neutrophil elastase (NE) with an inhibitory peptide abrogates the activity [27]. In agreement, injection of the NE inhibitor increases infection in lymph nodes [27]. Following neutralization of NE or TNF-α, B6 neutrophils exacerbate parasite growth [27]. The latter results suggest that NE triggers a proinflammatory response, which in turn prevents the antiinflammatory effects of apoptotic cells.
Neutrophils release granule proteins while migrating to inflammatory sites [31]. Granule proteins activate macrophages to release cytokines, and the granule protein NE activates macrophages through multiple receptors [31]. Purified NE induces TNF-α production and promotes parasite killing by macrophages [32]. B6 neutrophils release two- to threefold more NE than BALB/c neutrophils. In addition, mutant pallid B6 neutrophils, which fail to release NE, do not induce killing of L. major [32]. Elastase triggers proinflammatory responses through TLR4 [33–35]. In agreement, killing of L. major mediated by NE or neutrophils requires TLR4 expression in macrophages. Furthermore, injection of NE reduces infection in vivo in a manner dependent on TLR4 expression [32]. These results suggest that the proinflammatory and leishmanicidal activities of B6 neutrophils are due to efficient amounts of NE, and perhaps other granule proteins released into the medium. Interestingly, L. major expresses inhibitor of serine peptidase-2, a virulence factor that prevents TLR4 activation by NE, and promotes parasite survival in macrophages [36, 37].
The role of neutrophils in infection depends on the genetic profile of the host [38, 39]. The same holds true for Leishmania. B6 neutrophils induced by L. major secrete IL-12p70 and IL-10, while BALB/c neutrophils secrete IL-12p40 dimers with suppressive activity [40]. These conclusions further support the notion that B6 and BALB/c neutrophils are functionally distinct. Furthermore, after stimulation, B6 and BALB/c macrophages express proinflammatory M1 and antiinflammatory M2 phenotypes, respectively [41]. Together, the above studies [27, 32, 40] support the notion that neutrophils and macrophages play concerted roles in the inflammatory reaction [42, 43].
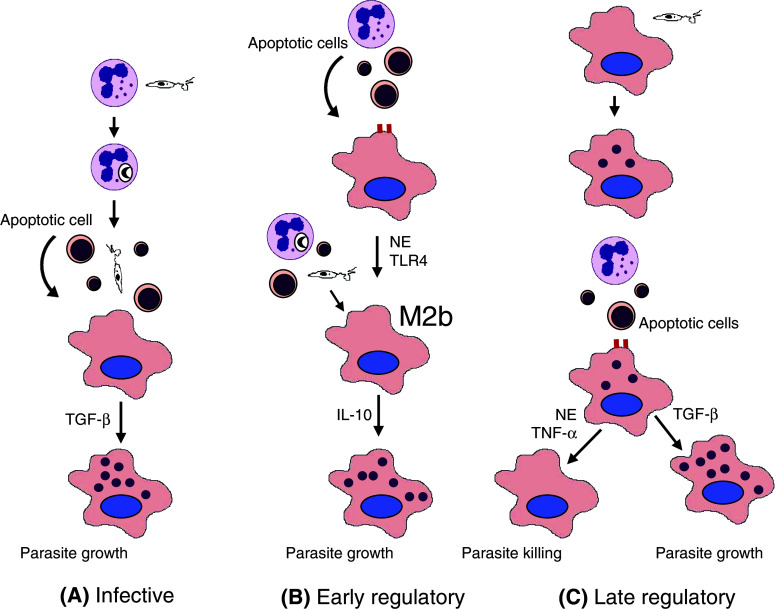
Engulfment of apoptotic cells can be either anti- or proinflammatory, depending on additional innate immune stimuli [27, 29, 30]. One important question is whether these responses imprint a particular phenotype in macrophages. Proinflammatory, but not antiinflammatory, clearance of neutrophils induces a regulatory or M2b phenotype in macrophages, characterized by low IL-12p70 and high IL-10 production following restimulation with LPS, increased expression of LIGHT/TNF superfamily 14, induction of Th2 responses, and permissive replication of L. major [44]. The ability of senescent neutrophils to induce the regulatory phenotype requires NE activity and TLR4 expression [44]. In addition, previous injection of senescent neutrophils enhances subsequent infection in vivo [44]. These results indicate that proinflammatory neutrophils induce an anti-inflammatory phenotype in macrophages that could help establishment of infection. Induction of regulatory macrophages provides an alternative explanation for the finding that depletion of neutrophils reduces infection in the sand fly bite/mouse ear model [21]. A summary of the roles proposed for engulfment of neutrophils in Leishmania infection is presented in Fig. 1.
Fig. 1.
Proposed roles of phagocytic clearance of neutrophils in Leishmania infection. a Infective role. Promastigotes infect neutrophils, delay their apoptosis and remain viable inside vacuoles [28]. Neutrophils undergo apoptosis and release viable parasites that infect macrophages [21]. In this way, parasites could be protected from extracellular killing or become more infective. In addition, uptake of the apoptotic cell bodies suppresses macrophage microbicidal activity and promotes parasite replication [21, 28]. b Early regulatory role. Neutrophil engulfment induces a regulatory or M2b phenotype in macrophages through NE and TLR4. When promastigotes are released from neutrophils, they interact with IL-10 producing M2b macrophages to increase parasite survival. [44]. c Late regulatory role. Promastigotes infect macrophages. Following engulfment of neutrophils, infected macrophages either kill parasites (through NE and TNF-α) or increase their growth (through TGF-β), depending on the genetic make up of the host [27, 32]. Replicating amastigotes are represented by small intracellular purple circles
Role of neutrophil life span
Neutrophils are potentially harmful cells that secrete toxic radicals and destructive enzymes. Therefore, their life span must be tightly regulated. Neutrophils undergo apoptosis and are engulfed by phagocytes [2]. In addition, neutrophils co-express the Fas death receptor and Fas ligand (FasL). Fas/FasL interactions accelerate [45], while inflammatory cytokines delay apoptosis of neutrophils [45, 46]. The life span of explanted BALB/c neutrophils increases by 3 h after addition of a neutralizing mAb against FasL [47]. Under these conditions, neutrophils reduce intramacrophagic growth of L. major. Furthermore, FasL-sufficient neutrophils increase parasite growth in macrophages, while FasL-deficient gld neutrophils show delayed apoptosis and promote parasite killing [47]. Extended survival of neutrophils leads to increased production of nitric oxide by macrophages, and parasite killing [47]. Finally, FasL-deficient mice are less susceptible to L. major infection [47]. Since FasL deficiency reproduces the effects of proinflammatory cytokines on neutrophil survival [45], these results highlight the complex role played by the inflammatory environment where infection takes place.
In addition, neutrophil survival can be regulated by macrophages. Resistant B6 mice infected with L. major start clearing neutrophils after 3 days of infection, while neutrophils persist for up to 10 days in susceptible BALB/c mice [48]. B6 macrophages mediate neutrophil apoptosis through membrane bound TNF-α, and L. major infection potentiates this process [48]. BALB/c macrophages are less efficient in mediating neutrophil apoptosis, suggesting a mechanism for the different kinetics of neutrophil accumulation in the two strains.
Infection of neutrophils with L. major increases neutrophil survival by delaying spontaneous apoptosis [28, 49]. Infected neutrophils secrete increased levels of the monocyte chemokine MIP-1β, and attract monocytes in vitro [28]. Since apoptotic neutrophils release viable parasites in vivo [21], these results suggest a potential mechanism for attraction of macrophages, followed by transfer of Leishmania parasites from neutrophils to macrophages.
Neutrophil extracellular traps
A recently identified microbicidal mechanism is the release of neutrophil extracellular traps (NETs). The function of NETs is to entrap and kill microbes [50]. Following stimulation by chemokines or microbes, a proportion of neutrophils die and release NETs into the medium. NETs are composed of a backbone of chromatin and DNA, with attached antimicrobial peptides, enzymes and histones [50]. L. amazonensis induces NET formation by neutrophils, and NETs partially reduce the viability of parasites [51]. On the other hand, L. donovani evades the microbicidal effect of NETs, and leishmanial lipophosphoglycan is required for evasion [52]. The precise roles played by NETs await further investigation, including the potential role of NETs in macrophage activation.
Other Leishmania species
Infection with other pathogenic Leishmania species confirms the importance of the tissue repair response and neutrophils in leishmaniasis. Insulin-like growth factor (IGF)-I, a mediator of dermal and epidermal cell proliferation [53], increases replication of L. amazonensis and L. panamensis in vitro and in vivo [54, 55]. Apoptotic neutrophils increase the growth of L. amazonensis in human macrophages through secretion of TGF-β [56]. Necrotic neutrophils reduce parasite growth through NE and TNF-α [56]. In addition, culture with live inflammatory neutrophils increases killing of L. amazonensis in macrophages from both resistant and susceptible mouse strains [57]. Again, the mechanism involves NE and TNF-α [57]. These results suggest an important role of NE in defense against Leishmania. Neutrophils and macrophages also cooperate in resistance against L. braziliensis [58]. Neutrophils from resistant BALB/c mice induce intramacrophagic killing of L. braziliensis, which is dependent on TNF-α and reactive oxygen species [58]. Table 1 summarizes the results obtained with coculture of neutrophils and either mouse or human macrophages infected with Leishmania. It is important to bear in mind that effects of neutrophils vary according to the genetic background of the host, the proportion of apoptotic and necrotic cells in the preparations, and the activation stage of the neutrophils (resting vs activated). As shown in Table 1, secretion of TGF-β and PGE2 correlate with increased parasite replication, while NE, TNF-α and reactive oxygen species induce leishmanicidal effects (Table 1).
Table 1.
Interactions with neutrophils regulate growth of Leishmania in macrophages
| Hosta | Neutrophils | Parasite | Cell contactb | Parasite growth | Mediators involved | Ref. |
|---|---|---|---|---|---|---|
| BALB/c | APO | L. major | Yes | Increase | TGF-β, PGE2 | [27] |
| B6 | APO | L. major | No | Decrease | NE, TNF-α, ROS | [27] |
| Human | APO | L. amazonensis | N.D. | Increase | TGF-β, PGE2 | [56] |
| Human | NECRO | L. amazonensis | N.D. | Decrease | NE, TNF-α, ROS | [56] |
| Mousec | LIVE | L. amazonensis | No | Decrease | NE, TNF-α, PAF | [57] |
| BALB/c | LIVE | L. braziliensis | Yes | Decrease | TNF-α, ROS | [58] |
APO apoptotic, NECRO necrotic, LIVE live neutrophils, NE neutrophil elastase, ROS reactive oxygen species, PAF platelet-activating factor
a Mouse peritoneal inflammatory [27, 57] or resident [58] macrophages from BALB/c and B6 strains, or monocyte-derived human macrophages [56], were infected and cocultured with indicated neutrophils
b Indicates whether cell contact between neutrophils and macrophages is required for the effect
c Macrophages from both resistant (C3H/HePas) and susceptible (BALB/c) mouse strains gave similar results
Skin injury leads to rapid production of type I IFNs by plasmacytoid dendritic cells [59]. Type I IFNs promote early inflammatory responses and re-epithelization of skin wounds [59]. However, type I IFNs downregulate neutrophil responses against Leishmania. Mice deficient in type I IFN receptor show increased resistance to infection with L. amazonensis [60]. Coculture and transfer experiments indicate that neutrophils deficient in type I IFN receptor induce increased parasite killing [60]. The mechanism of regulation by type I IFN remains to be clarified.
Neutrophils also infiltrate the dermis and serve as transient host cells in a model of visceral leishmaniasis induced by L. chagasi [61]. In addition, in visceral leishmaniasis induced by L. donovani, neutrophils play a protective role by increasing the numbers of CD4+ and CD8+ T cells secreting IFN-γ, and decreasing production of Th2 cytokines IL-4 and IL-10 [62].
Concluding remarks
Recently, the importance of neutrophils became evident in both in vitro and in vivo models of Leishmania infection. Diverse interactions between neutrophils and macrophages help to establish or to restrict infection, depending on the cytokine milieu, the activation stage of the interacting phagocytes, and on host genetic factors. In addition to effects on the innate immune responses, neutrophils interact with dendritic cells and cooperate with macrophages in adaptive immune responses [63]. As a result, neutrophils play a disease-promoting role in mice susceptible to L. major by increasing Th2 responses [64]. In addition, recruitment of neutrophils correlates with an increase of Th17 cells and immunoprotection of resistant B6 mice [65], but with aggravated infection of susceptible BALB/c mice [66].
Finally, human polymorphisms linked to neutrophil recruitment affect susceptibility to cutaneous leishmaniasis [67]. These studies suggest a role for neutrophils in human infection. Neutrophils play prominent roles as parasite vectors, immune effectors and regulators of immune responses. The intimate connection between infection and the tissue repair response opens up unexplored lines of research. Further investigation of neutrophils can lead to a better understanding of the infectious process and to better vaccines for leishmaniasis.
Acknowledgments
We thank Dr. Michelle M. Diniz (Instituto de Biofísica Carlos Chagas Filho, UFRJ) for help with the artwork. The authors receive support from Brazilian National Research Council (CNPq), Rio de Janeiro State Science Foundation (FAPERJ), and Programa Institutos Nacionais de Ciência e Tecnologia (INCT), CNPq, Brazil.
References
- 1.Peters NC, Sacks DL. The impact of vector-mediated neutrophil recruitment on cutaneous leishmaniasis. Cell Microbiol. 2009;11:1290–1296. doi: 10.1111/j.1462-5822.2009.01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 3.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 4.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S, Kim CC, Batra S, McKerrow JH, Loke P. Delineation of diverse macrophage activation programs in response to intracellular parasites and cytokines. PLoS Negl Trop Dis. 2010;4:e648. doi: 10.1371/journal.pntd.0000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez NE, Chang HK, Wilson ME. Novel program of macrophage gene expression induced by phagocytosis of Leishmania chagasi . Infect Immun. 2004;72:2111–2122. doi: 10.1128/IAI.72.4.2111-2122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michalik L, Wahli W. Involvement of PPAR nuclear receptors in tissue injury and wound repair. J Clin Invest. 2006;116:598–606. doi: 10.1172/JCI27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hölscher C, Arendse B, Schwegmann A, Myburgh E, Brombacher F. Impairment of alternative macrophage activation delays cutaneous leishmaniasis in nonhealing BALB/c mice. J Immunol. 2006;176:1115–1121. doi: 10.4049/jimmunol.176.2.1115. [DOI] [PubMed] [Google Scholar]
- 10.Gallardo-Soler A, Gómez-Nieto C, Campo ML, Marathe C, Tontonoz P, Castrillo A, Corraliza I. Arginase I induction by modified lipoproteins in macrophages: a peroxisome proliferator-activated receptor-gamma/delta-mediated effect that links lipid metabolism and immunity. Mol Endocrinol. 2008;22:1394–1402. doi: 10.1210/me.2007-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers M, Kropf P, Choi BS, Dillon R, Podinovskaia M, Bates P, Müller I. Proteophosphoglycans regurgitated by Leishmania-infected sand flies target the l-arginine metabolism of host macrophages to promote parasite survival. PLoS Pathog. 2009;5:e1000555. doi: 10.1371/journal.ppat.1000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kane MM, Mosser DM. The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol. 2001;166:1141–1147. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- 13.Miles SA, Conrad SM, Alves RG, Jeronimo SM, Mosser DM. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania . J Exp Med. 2005;201:747–754. doi: 10.1084/jem.20041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 15.Beil WJ, Meinardus-Hager G, Neugebauer DC, Sorg C. Differences in the onset of the inflammatory response to cutaneous leishmaniasis in resistant and susceptible mice. J Leukoc Biol. 1992;52:135–142. doi: 10.1002/jlb.52.2.135. [DOI] [PubMed] [Google Scholar]
- 16.Tasew G, Nylén S, Lieke T, Lemu B, Meless H, Ruffin N, Wolday D, Asseffa A, Yagita H, Britton S, Akuffo H, Chiodi F, Eidsmo L. Systemic FasL and TRAIL neutralisation reduce leishmaniasis induced skin ulceration. PLoS Negl Trop Dis. 2010;4:e844. doi: 10.1371/journal.pntd.0000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakthianandeswaren A, Elso CM, Simpson K, Curtis JM, Kumar B, Speed TP, Handman E, Foote SJ. The wound repair response controls outcome to cutaneous leishmaniasis. Proc Natl Acad Sci USA. 2005;102:15551–15556. doi: 10.1073/pnas.0505630102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domínguez M, Toraño A. Immune adherence-mediated opsonophagocytosis: the mechanism of Leishmania infection. J Exp Med. 1999;189:25–35. doi: 10.1084/jem.189.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laufs H, Müller K, Fleischer J, Reiling N, Jahnke N, Jensenius JC, Solbach W, Laskay T. Intracellular survival of Leishmania major in neutrophil granulocytes after uptake in the absence of heat-labile serum factors. Infect Immun. 2002;70:826–835. doi: 10.1128/IAI.70.2.826-835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mollinedo F, Janssen H, de la Iglesia-Vicente J, Villa-Pulgarin JA, Calafat J. Selective fusion of azurophilic granules with Leishmania-containing phagosomes in human neutrophils. J Biol Chem. 2010;285:34528–34536. doi: 10.1074/jbc.M110.125302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, Lawyer P, Fay MP, Germain RN, Sacks D. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321:970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wanderley JL, Barcinski MA. Apoptosis and apoptotic mimicry: the Leishmania connection. Cell Mol Life Sci. 2010;67:1653–1659. doi: 10.1007/s00018-010-0291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribeiro-Gomes FL, Silva MT, DosReis GA. Neutrophils, apoptosis and phagocytic clearance: an innate sequence of cellular responses regulating intramacrophagic parasite infections. Parasitology. 2006;132:S61–S68. doi: 10.1017/S0031182006000862. [DOI] [PubMed] [Google Scholar]
- 24.DosReis GA, Ribeiro-Gomes FL, Guillermo LV, Lopes MF. Cross-talk between apoptosis and cytokines in the regulation of parasitic infection. Cytokine Growth Factor Rev. 2007;18:97–105. doi: 10.1016/j.cytogfr.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Freire-de-Lima CG, Nascimento DO, Soares MB, Bozza PT, Castro-Faria-Neto HC, de Mello FG, DosReis GA, Lopes MF. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- 26.Iniesta V, Gómez-Nieto LC, Corraliza I. The inhibition of arginase by N ω-hydroxy-l-arginine controls the growth of Leishmania inside macrophages. J Exp Med. 2001;193:777–784. doi: 10.1084/jem.193.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribeiro-Gomes FL, Otero AC, Gomes NA, Moniz-De-Souza MC, Cysne-Finkelstein L, Arnholdt AC, Calich VL, Coutinho SG, Lopes MF, DosReis GA. Macrophage interactions with neutrophils regulate Leishmania major infection. J Immunol. 2004;172:4454–4462. doi: 10.4049/jimmunol.172.7.4454. [DOI] [PubMed] [Google Scholar]
- 28.van Zandbergen G, Klinger M, Mueller A, Dannenberg S, Gebert A, Solbach W, Laskay T. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J Immunol. 2004;173:6521–6525. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- 29.Lucas M, Stuart LM, Savill J, Lacy-Hulbert A. Apoptotic cells and innate immune stimuli combine to regulate macrophage cytokine secretion. J Immunol. 2003;171:2610–2615. doi: 10.4049/jimmunol.171.5.2610. [DOI] [PubMed] [Google Scholar]
- 30.Zheng L, He M, Long M, Blomgran R, Stendahl O. Pathogen-induced apoptotic neutrophils express heat shock proteins and elicit activation of human macrophages. J Immunol. 2004;173:6319–6326. doi: 10.4049/jimmunol.173.10.6319. [DOI] [PubMed] [Google Scholar]
- 31.Soehnlein O, Weber C, Lindbom L. Neutrophil granule proteins tune monocytic cell function. Trends Immunol. 2009;30:538–546. doi: 10.1016/j.it.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro-Gomes FL, Moniz-de-Souza MC, Alexandre-Moreira MS, Dias WB, Lopes MF, Nunes MP, Lungarella G, DosReis GA. Neutrophils activate macrophages for intracellular killing of Leishmania major through recruitment of TLR4 by neutrophil elastase. J Immunol. 2007;179:3988–3994. doi: 10.4049/jimmunol.179.6.3988. [DOI] [PubMed] [Google Scholar]
- 33.Devaney JM, Greene CM, Taggart CC, Carroll TP, O’Neill SJ, McElvaney NG. Neutrophil elastase up-regulates interleukin-8 via toll-like receptor 4. FEBS Lett. 2003;544:129–132. doi: 10.1016/S0014-5793(03)00482-4. [DOI] [PubMed] [Google Scholar]
- 34.Hietaranta A, Mustonen H, Puolakkainen P, Haapiainen R, Kemppainen E. Proinflammatory effects of pancreatic elastase are mediated through TLR4 and NF-κB. Biochem Biophys Res Commun. 2004;323:192–196. doi: 10.1016/j.bbrc.2004.08.077. [DOI] [PubMed] [Google Scholar]
- 35.Johnson GB, Brunn GJ, Platt JL. Cutting edge: an endogenous pathway to systemic inflammatory response syndrome (SIRS)-like reactions through Toll-like receptor 4. J Immunol. 2004;172:20–24. doi: 10.4049/jimmunol.172.1.20. [DOI] [PubMed] [Google Scholar]
- 36.Eschenlauer SC, Faria MS, Morrison LS, Bland N, Ribeiro-Gomes FL, DosReis GA, Coombs GH, Lima AP, Mottram JC. Influence of parasite encoded inhibitors of serine peptidases in early infection of macrophages with Leishmania major . Cell Microbiol. 2009;11:106–120. doi: 10.1111/j.1462-5822.2008.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faria MS, Reis FCG, Azevedo-Pereira RL, Morrison LS, Mottram JC, Lima APCA. Leishmania inhibitor of serine peptidase 2 prevents TLR4 activation by neutrophil elastase promoting parasite survival in murine macrophages. J Immunol. 2011;186:411–422. doi: 10.4049/jimmunol.1002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pina A, Saldiva PH, Restrepo LE, Calich VL. Neutrophil role in pulmonary paracoccidioidomycosis depends on the resistance pattern of hosts. J Leukoc Biol. 2006;79:1202–1213. doi: 10.1189/jlb.0106052. [DOI] [PubMed] [Google Scholar]
- 39.Beisiegel M, Kursar M, Koch M, Loddenkemper C, Kuhlmann S, Zedler U, Stäber M, Hurwitz R, Kaufmann SH. Combination of host susceptibility and virulence of Mycobacterium tuberculosis determines dual role of nitric oxide in the protection and control of inflammation. J Infect Dis. 2009;199:1222–1232. doi: 10.1086/597421. [DOI] [PubMed] [Google Scholar]
- 40.Charmoy M, Megnekou R, Allenbach C, Zweifel C, Perez C, Monnat K, Breton M, Ronet C, Launois P, Tacchini-Cottier F. Leishmania major induces distinct neutrophil phenotypes in mice that are resistant or susceptible to infection. J Leukoc Biol. 2007;82:288–299. doi: 10.1189/jlb.0706440. [DOI] [PubMed] [Google Scholar]
- 41.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 42.Silva MT. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J Leukoc Biol. 2010;87:93–106. doi: 10.1189/jlb.0809549. [DOI] [PubMed] [Google Scholar]
- 43.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 44.Filardy AA, Pires DR, Nunes MP, Takiya CM, Freire-de-Lima CG, Ribeiro-Gomes FL, DosReis GA. Proinflammatory clearance of apoptotic neutrophils induces an IL-12lowIL-10high regulatory phenotype in macrophages. J Immunol. 2010;185:2044–2050. doi: 10.4049/jimmunol.1000017. [DOI] [PubMed] [Google Scholar]
- 45.Liles WC, Kiener PA, Ledbetter JA, Aruffo A, Klebanoff SJ. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med. 1996;184:429–440. doi: 10.1084/jem.184.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee A, Whyte MK, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol. 1993;54:283–288. [PubMed] [Google Scholar]
- 47.Ribeiro-Gomes FL, Moniz-de-Souza MC, Borges VM, Nunes MP, Mantuano-Barradas M, D’Avila H, Bozza PT, Calich VL, DosReis GA. Turnover of neutrophils mediated by Fas ligand drives Leishmania major infection. J Infect Dis. 2005;192:1127–1134. doi: 10.1086/432764. [DOI] [PubMed] [Google Scholar]
- 48.Aga E, Katschinski DM, van Zandbergen G, Laufs H, Hansen B, Müller K, Solbach W, Laskay T. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major . J Immunol. 2002;169:898–905. doi: 10.4049/jimmunol.169.2.898. [DOI] [PubMed] [Google Scholar]
- 49.Allenbach C, Zufferey C, Perez C, Launois P, Mueller C, Tacchini-Cottier F. Macrophages induce neutrophil apoptosis through membrane TNF, a process amplified by Leishmania major . J Immunol. 2006;176:6656–6664. doi: 10.4049/jimmunol.176.11.6656. [DOI] [PubMed] [Google Scholar]
- 50.Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol. 2009;30:513–521. doi: 10.1016/j.it.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Guimarães-Costa AB, Nascimento MT, Froment GS, Soares RP, Morgado FN, Conceição-Silva F, Saraiva EM. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc Natl Acad Sci USA. 2009;106:6748–6753. doi: 10.1073/pnas.0900226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gabriel C, McMaster WR, Girard D, Descoteaux A. Leishmania donovani promastigotes evade the antimicrobial activity of neutrophil extracellular traps. J Immunol. 2010;185:4319–4327. doi: 10.4049/jimmunol.1000893. [DOI] [PubMed] [Google Scholar]
- 53.Edmondson SR, Thumiger SP, Werther GA, Wraight CJ. Epidermal homeostasis: the role of the growth hormone and insulin-like growth factor systems. Endocr Rev. 2003;24:737–764. doi: 10.1210/er.2002-0021. [DOI] [PubMed] [Google Scholar]
- 54.Goto H, Gomes CM, Corbett CE, Monteiro HP, Gidlund M. Insulin-like growth factor I is a growth-promoting factor for Leishmania promastigotes and amastigotes. Proc Natl Acad Sci USA. 1998;95:13211–13216. doi: 10.1073/pnas.95.22.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomes CM, Goto H, Ribeiro Da Matta VL, Laurenti MD, Gidlund M, Corbett CE. Insulin-like growth factor (IGF)-I affects parasite growth and host cell migration in experimental cutaneous leishmaniasis. Int J Exp Pathol. 2000;81:249–255. doi: 10.1046/j.1365-2613.2000.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Afonso L, Borges VM, Cruz H, Ribeiro-Gomes FL, DosReis GA, Dutra AN, Clarêncio J, de Oliveira CI, Barral A, Barral-Netto M, Brodskyn CI. Interactions with apoptotic but not with necrotic neutrophils increase parasite burden in human macrophages infected with Leishmania amazonensis . J Leukoc Biol. 2008;84:389–396. doi: 10.1189/jlb.0108018. [DOI] [PubMed] [Google Scholar]
- 57.de Souza Carmo EV, Katz S, Barbiéri CL. Neutrophils reduce the parasite burden in Leishmania (Leishmania) amazonensis infected macrophages. PLoS One. 2010;5:e13815. doi: 10.1371/journal.pone.0013815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Novais FO, Santiago RC, Báfica A, Khouri R, Afonso L, Borges VM, Brodskyn C, Barral-Netto M, Barral A, de Oliveira CI. Neutrophils and macrophages cooperate in host resistance against Leishmania braziliensis infection. J Immunol. 2009;183:8088–8098. doi: 10.4049/jimmunol.0803720. [DOI] [PubMed] [Google Scholar]
- 59.Gregorio J, Meller S, Conrad C, Di Nardo A, Homey B, Lauerma A, Arai N, Gallo RL, DiGiovanni J, Gilliet M. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921–2930. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xin L, Vargas-Inchaustegui DA, Raimer SS, Kelly BC, Hu J, Zhu L, Sun J, Soong L. Type I IFN receptor regulates neutrophil functions and innate immunity to Leishmania parasites. J Immunol. 2010;184:7047–7056. doi: 10.4049/jimmunol.0903273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thalhofer CJ, Chen Y, Sudan B, Love-Homan L, Wilson ME. Leukocytes infiltrate the skin and draining lymph nodes in response to the protozoan Leishmania infantum chagasi. Infect Immun. 2011;79:108–117. doi: 10.1128/IAI.00338-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McFarlane E, Perez C, Charmoy M, Allenbach C, Carter KC, Alexander J, Tacchini-Cottier F. Neutrophils contribute to development of a protective immune response during onset of infection with Leishmania donovani . Infect Immun. 2008;76:532–541. doi: 10.1128/IAI.01388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silva MT. Neutrophils and macrophages work in concert as inducers and effectors of adaptive immunity against extracellular and intracellular microbial pathogens. J Leukoc Biol. 2010;87:805–813. doi: 10.1189/jlb.1109767. [DOI] [PubMed] [Google Scholar]
- 64.Tacchini-Cottier F, Zweifel C, Belkaid Y, Mukankundiye C, Vasei M, Launois P, Milon G, Louis JA. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major . J Immunol. 2000;165:2628–2636. doi: 10.4049/jimmunol.165.5.2628. [DOI] [PubMed] [Google Scholar]
- 65.Wu W, Huang L, Mendez S. A live Leishmania major vaccine containing CpG motifs induces the de novo generation of Th17 cells in C57BL/6 mice. Eur J Immunol. 2010;40:2517–2527. doi: 10.1002/eji.201040484. [DOI] [PubMed] [Google Scholar]
- 66.Lopez Kostka S, Dinges S, Griewank K, Iwakura Y, Udey MC, von Stebut E. IL-17 promotes progression of cutaneous leishmaniasis in susceptible mice. J Immunol. 2009;182:3039–3046. doi: 10.4049/jimmunol.0713598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castellucci L, Jamieson SE, Miller EN, Menezes E, Oliveira J, Magalhães A, Guimarães LH, Lessa M, de Jesus AR, Carvalho EM, Blackwell JM. CXCR1 and SLC11A1 polymorphisms affect susceptibility to cutaneous leishmaniasis in Brazil: a case-control and family-based study. BMC Med Genet. 2010;11:10. doi: 10.1186/1471-2350-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]