Abstract
Organisms with bilateral symmetry elaborate patterns of neuronal projections connecting both sides of the central nervous system at all levels of the neuraxis. During development, these so-called commissural projections navigate across the midline to innervate their contralateral targets. Commissural axon pathfinding has been extensively studied over the past years and turns out to be a highly complex process, implicating modulation of axon responsiveness to the various guidance cues that instruct axon trajectories towards, within and away from the midline. Understanding the molecular mechanisms allowing these switches of response to take place at the appropriate time and place is a major challenge for current research. Recent work characterized several instructive processes controlling the spatial and temporal fine-tuning of the guidance molecular machinery. These findings illustrate the molecular strategies by which commissural axons modulate their sensitivity to guidance cues during midline crossing and show that regulation at both transcriptional and post-transcriptional levels are crucial for commissural axon guidance.
Keywords: Central nervous system, Molecular strategies, Commissural axon pathfinding, Midline crossing
Introduction
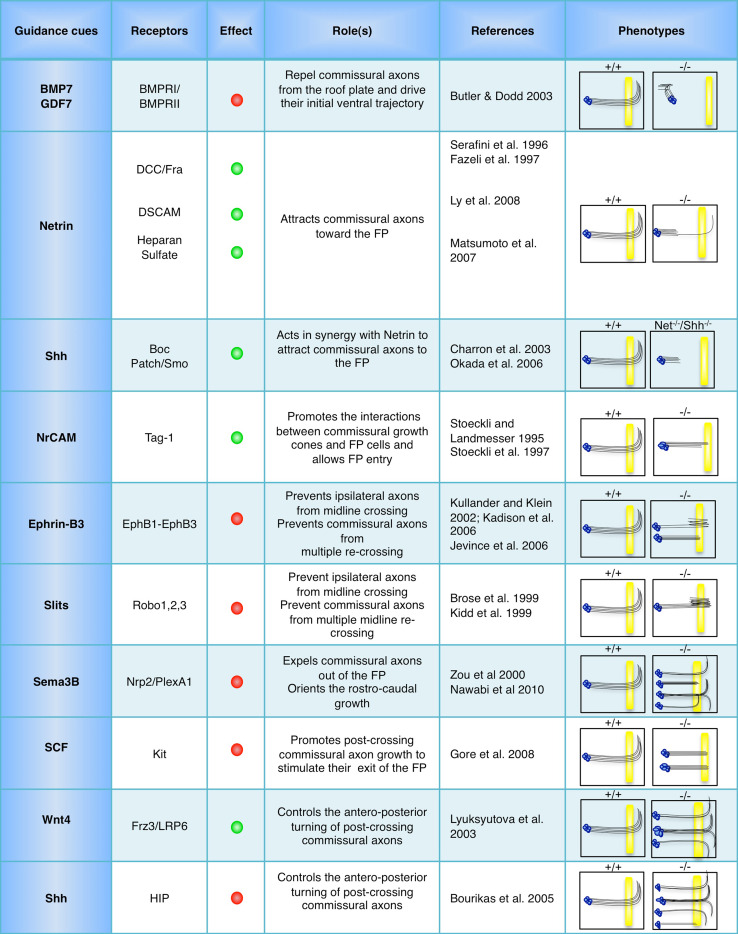
Pathfinding is highly challenging for neuronal projections that navigate very long distances in the developing body to reach their synaptic partners. This problem is solved through the subdivision of pathways into shorter sections by groups of specialized cells forming intermediate targets (also referred to as choice points). Intermediate targets provide positional information, which guide the axons from one step to the next. Nevertheless, a puzzling aspect of such stepwise navigation is that axons must be first instructed to enter and later exit the intermediate target. Major insights into the navigation at intermediate targets came with studies of commissural projections. In organisms with bilateral symmetry, multiple commissural axon tracts navigate through the midline to establish reciprocal connections between the two sides of the central nervous system. In vertebrates, a group of ventral glial cells forming the floor plate (FP), segregates two types of axonal projections: the non-crossing projections that will form ipsilateral circuits and the crossing projections that form commissural circuits (contralateral). Similarly, in the ventral cord of the fly, midline neuronal and glial cells organize ipsilateral and contralateral projections. Over the past decade, extensive work in mouse and fly animal models has highlighted the series of guidance decisions that ipsilateral and commissural axons take to navigate their respective paths. These studies revealed that ipsilateral and commissural axons respond differently to the same set of midline-derived guidance cues. Ipsilateral axons are prevented from crossing the midline by repellents to which they respond from the onset. In contrast, a temporal switch in the sensitivity of commissural axons enables them to acquire sensitivity to these repellents only once they have crossed the midline. Commissural axons are first attracted to the ventral midline by FP-derived chemo-attractants (essentially Netrins and Shh), and permissive cell-contact cues enable their entry to the FP. While navigating the FP, commissural axons lose their sensitivity to these attractants and acquire responsiveness to various FP and midline repellents such as Slit, Ephrin and Semaphorin family members [1–4]. The midline repellents then expel commissural axons from the FP (Fig. 1; Table 1; [5–23]). These findings established a crucial requirement for switches to control responsiveness to midline guidance cues during commissural axon navigation.
Fig. 1.
Schematic drawing of the spinal commissural projections and the series of guidance decisions controlling their trajectory before and after midline crossing. Commissural axons are initially repelled by BMP repellents emanating from the RP towards the ventral spinal cord. They are attracted to the FP by combinations of Netrin and Shh activity. Interactions between commissural growth cones and FP glial cells mediated by cell adhesion molecules (IgSFCAMs) regulate FP entry. After crossing, commissural axons lose their sensitivity to Netrin and acquire responsiveness to FP/midline repellents, including Slits, Ephrins, and Semaphorins. This novel sensitivity expels commissural axons away from the FP. A growth-promoting property of the FP SCF also contributes to stimulate commissural axons’ exit. Finally, responsiveness to antero-posterior gradients of repulsive and attractive cues such as Shh and Wnt4 specify commissural axon rostrocaudal turning
Table 1.
Summary of the signaling regulating commissural axon guidance
Red dots indicate repulsive effect, green dots indicate attractive effect
Guidance signals are integrated by receptors in the axon terminal, namely the growth cone, a highly motile structure composed of microtubule and actin cytoskeletal structures. Growth cones perceive gradients of guidance cues and turn either towards the source (attractive response) or turn away from it (repulsive behavior). Activation of the guidance receptors is the first step of a series of signaling cascades that leads to the remodeling of actin, microtubules, and adherent contacts needed to trigger the turning response. The level of expression of cell surface receptors will thus be the first instrumental parameter for setting the sensitivity of the growth cone to guidance cues. The targeting of guidance receptors at the appropriate level and time at the growth cone membrane is an incredibly complex challenge for the neurons, in particular when one considers that in some cases great distances separate the growth cone from the cell body. Mechanisms controlling the compartmentalized trafficking of membrane proteins have been extensively investigated in non-neuronal cells that share the characteristic asymmetric shape of neurons, particularly epithelial cells. Some of these mechanisms function in neurons to selectively deliver membrane proteins to the growth cones [24]. Pioneer and recent studies have shown that, as the growth cone progresses towards crossing, receptors and intracellular effectors undergo dynamic changes that switch growth cone responsiveness to guidance cues. Initially, two major modulations at the receptor level were characterized. First, in the fly, commissural axons are maintained unresponsive to Slit repellents until crossing via proteasomal degradation of the slit receptor Robo. This process is temporally regulated. Robo is active at the pre-crossing stage and suppressed after crossing, allowing an accumulation of the receptor at the growth cone surface and subsequent increase of response to Slits [22, 25–28]. In the vertebrate FP, mechanisms controlling the responsiveness to the Slits differ because the drosophila commissureless (comm) gene, which encodes an essential mediator of the process targeting Robo to the proteasome, has not been conserved.
A second major switch of growth cone responsiveness, underlying the silencing of Netrin-mediated attraction after midline crossing, has been identified in vertebrates. This switch has been attributed to the formation of a complex between the Netrin receptor Deleted in Colorectal Carcinoma (DCC) and the Slit receptor Roudabound (Robo) in commissural growth cones upon exposure to Slit [29]. More recently, a third mechanism of receptor regulation was identified in vertebrates, which controls the responsiveness of commissural axons to a Semaphorin midline repellent, Sema3B. Before crossing, commissural axons are maintained unresponsive to Sema3B through processing of the Sema3B signaling co-receptor, Plexin-A1. Midline-derived cues lead to suppression of this processing upon crossing, allowing Plexin-A1 accumulation in commissural growth cones and sensitization to Sema3B [16].
Diverse types of regulations of growth cone responsiveness to guidance cues have been described, which have been discussed in several recent reviews [3, 24, 30]. Among the current key questions are those which relate to the nature of the molecular pathways controlling the modulations of receptor distributions and interaction profiles. Here, we review some of the mechanisms which were found to control expression, subcellular distribution and turnover of receptors in the context of commissural axon guidance and midline crossing.
Transcriptional control of guidance receptors
Responsiveness to guidance cues requires the proper level of receptors in the growth cone. Regulation of transcription is one of the first potential ways for exerting spatio-temporal control of receptor expression. Extensive investigation of the specifics of motor neuron connectivity demonstrated that axon trajectory is one of the features defining neuronal identity, and therefore must be encoded by transcription factors. Specific combinations of transcription factors were found to control the expression of key guidance receptors in subpopulations of motoneurons, allowing their axons to make appropriate dorso-ventral choices in the target limb [31, 32]. Much less is known about the identity of transcription factors that control the crossing, or non-crossing decision, of axon subsets navigating towards the midline. Further, the extent to which transcriptional regulations impacts receptor expression levels is as yet unclear. In theory, regulation of transcription itself, as well as of processes involved in mRNA traffic, stability and mRNA modifications such as alternative splicing or editing, could impact on receptor levels.
Transcriptional control of guidance receptors in the visual system
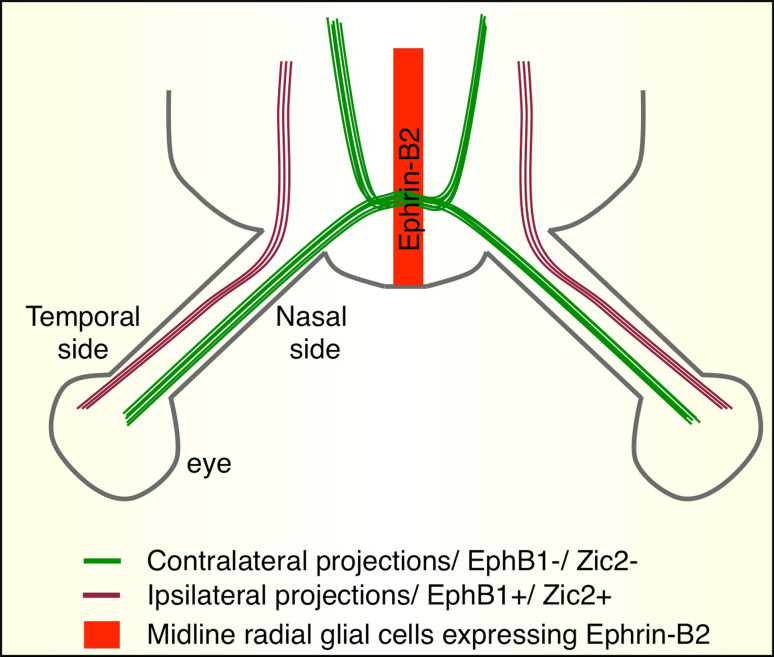
The formation of the optic chiasm has provided one of the nicest examples linking transcription factors to guidance receptor expression in commissural systems. The visual information collected by each retina must be conveyed to specific centers located on both sides of the brain, in order to allow binocular vision and spatial positioning of objects [33]. Axons from ganglion cell neurons exiting the retina are confronted by a choice: to cross or not to cross the midline. The correct answer correlates with the spatial position of the cell bodies in the retina: axons from the nasal side of the retina project to the contralateral ventral diencephalon to form the optic chiasm. Axons from the temporal side of the retina do not cross the midline, and therefore take an ipsilateral route [34, 35] (Fig. 2). At the optic chiasm, axons are exposed to a variety of guidance cues emanating from FP cells [36–38]. Ephrin signaling, especially the Ephrin-B2 ligand (expressed by FP cells) and Eph-B1 receptor (expressed by the axons), has been found to play a major role in the sorting of ipsilateral and contralateral projections [39, 40]. In EphB1-deficient mice, the number of crossing fibers is increased, suggesting that repulsion mediated by this signaling pathway may be specifically required for preventing the crossing of ipsilateral axons [40]. Similarly, inducing ectopic expression of EphB1 (but not of other Eph receptors such as EphB2) in commissural axons through targeted electroporation of the retina caused the axons to select an ipsilateral path [41]. Herrera and colleagues [42] have been interested by transcription factors from the Zic (zinc finger protein of the cerebellum) family. Zic factors (from 1 to 5) are involved in the specification of the body medial axis and in early acquisition of neuronal identity [43]. Mice lacking Zic2 develop optical nerve defects. Strikingly, these defects are very similar to those described in EphB1−/− mice. Temporal expression of Zic2 and EphB1 coincides with the navigation of projections to the ipsilateral side. These observations led two groups to investigate whether Zic2 regulates EphB1 expression [44, 45]. Zic2 was found to induce and control EphB1 expression level in the ipsilaterally projecting neuronal population [44, 45]. Furthermore, Garcia-Frigola and colleagues [44] demonstrated that ectopic Zic2 expression induced by electroporation of mouse embryos was sufficient for commissural projections to switch their trajectory from contralateral to ipsilateral. To test whether EphB1 mediates the choice specified by Zic2, the authors performed the same experiments in EphB1−/− embryos. Surprisingly, they still observed a moderate increase of ipsi-projecting axons, indicating that EphB1 may not be the only effector of Zic2-mediated pathway choice at the midline.
Fig. 2.
Midline crossing at the optic chiasm. Axons from the RGC of the nasal retina (green) are predominantly commissural and form the optic chiasm. RGC axons from the temporal retina are ipsilateral (purple). Midline glial cells express the repulsive membrane-bound EphrinB2 (red). Zic2 is a transcription factor which controls the expression of EphB1 receptor in RGCs. Commissural RGCs do not express the transcriptional factor Zic2, thus their axons lack EphB1. Consequently, they are insensitive to EphrinB2’s repulsive effect and are allowed to cross the midline. In contrast, ipsilateral RGCs express Zic2 and consequently EphB1 in their growth cones so that they are repelled by EphrinB2 and do not cross the midline
Transcriptional control of guidance receptors in spinal and ventral cord commissural projections
A recent study demonstrated that LIM transcription factors control the expression of the Robo3/Rig1 receptor in spinal commissural projections [46]. In the dorsal spinal cord, specific combinations of LIM and POU transcription factors define interneuron lineages [47–49]. Wilson and colleagues [46] concentrated on the more/most dorsal population of spinal neurons, the dl1, that lie close to the roof plate (Fig. 3; [50]). The dl1 population is derived from Math1+ progenitors cells [51, 52] and can be identified by the expression of two LIM transcription factors, Lhx2 and Lhx9 [49]. Dl1 neurons have been shown to establish predominantly commissural projections [52]. However, analysis of projection patterns in mice engineered to express reporters under the control of dll-specific promotors, such as Math1 and Barhl2, showed that the most lateral dll neurons project onto ipsilateral targets [46]. Lhx2 and Lhx9 temporal expression is dynamic and distinguishes ipsilateral and commissural pools of dll neurons. At an early stage, when postmitotic neurons migrate out of the ventricular zone, Lhx2 and Lhx9 are strongly expressed by all neurons. Later, Lhx2 but not Lhx9 disappears from the ipsilateral pool. While both of them are down-regulated in the commissural pool, Lhx2 expression remains higher than Lhx9. The authors analyzed the projection patterns of dll neurons in mice lacking Lhx2, Lhx9 or both. While no obvious commissural defects were seen in the single mutants, the dll commissural projections in the double mutants failed to cross the FP and instead fasciculated with ipsilateral tracts. Interestingly, the authors observed that these axons lack expression of a key commissural marker, Robo3/Rig1, whose genetic deletion also results in midline crossing failure. Robo3 is a divergent member of the Robo receptor family (Robo1, 2, 3) mediating the effects of the midline repellent Slits [5, 53, 54]. Using Electrophoretic Mobility Shift Assay (EMSA), Wilson and colleagues demonstrated that Robo3 expression is under the direct control of Lhx2 and Lhx9 in dl1 commissural neurons. Furthermore, the Rig1 DNA sequence contained consensus sites for both Lhx2 and Lhx9 proteins, and embryos that expressed a single allele of Lhx2/9 and Rig1, phenocopy the Rig1−/− and Lhx2−/−/Lhx9−/− embryos. This study illustrated how transcriptional activity can control specific steps of axon navigation, such as midline crossing, suggesting that vast programs might be required to specify all key steps of axon navigation.
Fig. 3.
Schematic drawing illustrating the patterns of transcription factors specifying the dorsal spinal cord lineages. The dorsal spinal domain comprises two interneuron classes: class A (from dl1 to dl3) whose differentiation depends on signals derived from the roof plate, and class B (from dl4 to dl6 and the dlLA and dlLB interneurons) specified by RP independent signals. dlLA et dlLB classes are generated after the other dorsal interneuron lineage and are specified by distinct bHLH transcription factors. Dl1 progenitors interneuons express Math1. Ngn1 is specific for dl2 progenitors interneurons. Mash1 is expressed by progenitors cells from dl3 to dl5 populations. Specific HD factors define each neuronal population
Trancriptional regulation of guidance receptors in drosophila
Transcriptional regulations are also crucial for the formation of commissural axons in the drosophila ventral cord. Early studies demonstrated that the Commissureless (Comm) protein controls ipsilateral and contralateral choices, because expression of Comm in ipsilaterally projecting neurons was found to be sufficient to induce contralateral projections [54, 55]. Comm is normally expressed transiently in commissural neurons at the pre-crossing stage, and down-regulates the cell surface sorting of the Robo receptor. Thus, it prevents an early response to Slit, thereby allowing midline crossing [22, 25–28]. After midline crossing, Comm is itself down-regulated, resulting in Robo cell surface expression and sensitization of commissural axons to Slit (Fig. 4). Recently, Yang and colleagues [56] uncovered a new contribution of the drosophila ortholog of the Netrin receptor DCC, Frazzled (Fra), in the regulation of commissural expression. In flies lacking Fra, very little Comm mRNA is detected in commissural neurons, whereas this is not the case in other cell types. Flies expressing a dominant negative form of Fra develop a Comm-less phenotype. Re-expression of Fra in fra mutants not only rescues the crossing phenotype but also restores comm mRNA expression. Moreover, driving fra expression in a population of ipsilateral neurons is sufficient to induce ectopic commissural expression. Thus, Fra is necessary and sufficient to induce comm mRNA expression (Fig. 4). Interestingly, the authors demonstrate that this function of Fra is independent of Netrin. Liu et al. [57] have provided additional insights into the mechanisms underlying Fra expression in drosophila commissural neurons. The authors investigated the role of Midline (Mid), a member of the family of T-box transcription factors, that plays a key role in segmentation and generation of lineage diversity during neurogenesis [58–62]. The authors observed that loss of Mid induced strong defects in axonal topography but cell fates were not altered. In 65% of cases, the longitudinal tract was interrupted in Mid mutants and the posterior commissure was thinner than in wild-type flies (71%). Flies heterozygous for the mid allele showed no defects in commissural projections, but double mid-fra and mid-slit trans-heterozygotes exhibited strong alterations of midline crossing. These phenotypes were correlated with reductions of both mRNA and protein for Fra, Robo and Slit in commissural neurons. In contrast, Slit expression in ventral midline glia cells was not affected by the loss of Mid. Mid re-expression restored Fra and Robo expression and chromatin immunoprecipitation (ChIP) with anti-Mid antibody revealed that Mid binds with upstream regions of fra, slit and robo genes. Thus, Mid exerts a direct transcriptional control on fra, slit and robo gene activation.
Fig. 4.
Regulation of midline crossing in the drosophila ventral cord. At the precrossing stage, the transcription factor Midline (Mid) induces the expression of Frazzled (Fra) and Roundabound (Robo). Fra binds to the promoter region of Commissureless (Comm) and activates its transcription. Comm protein sorts Robo to the proteasome where the receptor is degraded. After midline crossing, yet unknown signaling down-regulates Comm expression. Robo accumulates at the surface of the growth cone and confers responsiveness to the midline repellent Slit. Receptor activation requires metalloprotease activity. Commissural axons are expelled from the midline and prevented from re-crossing
A gene expression code setting appropriate Robo expression profiles was also found to be crucial for other aspects of commissural axon guidance in drosophila. While Robo1 controls commissural midline crossing, Robo2 and Robo3 were shown to control the sorting of post-crossing commissural tracts into three discrete lateral tracts [63, 64]. Whether this process relied on structural differences or expression profiles was unknown as both features distinguish Robo proteins. To gain insights into this question, Spitzweck and collaborators [65] generated flies in which the expression of one robo was driven by another through introduction of the coding sequence of one robo into the loci of another (“Robo swaps”). Remarkably, analysis of the longitudinal tract positioning revealed that, although Robo proteins differ in their structure, particularly in their intracellular domain, it is their specific expression profile which accounts for the sorting of post-crossing commissural tracts. In contrast, robo1 function in midline crossing relies on additional structural specificities, as the swap of robo1 by robo2 or robo3 failed to ensure appropriate pathfinding of commissural axons across the midline [65]. This appeared to be true not only for Robo1. Analysis of the Robo swap fly lines indeed revealed the unexpected function of Robo2, in promoting midline crossing, which is unique to Robo2 and conferred by structural features of its ectodomain [65]. Exciting insights should follow from further investigation into the mechanisms controlling the robo gene expression to set this Robo code on post-crossing commissural axons.
These examples illustrate that transcriptional regulations play crucial roles in controlling the spatio-temporal distribution of key receptors involved in commissural axon guidance across the midline and even after crossing. Characterizing the transcriptional code controlling the set of receptors equipping commissural growth cones at the various steps of their navigation is certainly one of the future challenges in the field. Some other aspects remain unclear, such as the potential implication of mechanisms additional to the down-regulation of comm, which would contribute to set the spatial distribution of Robo proteins in post-crossing commissural axons.
Post transcriptional regulation of midline crossing
Regulation of guidance receptor expression by alternative splicing of mRNA
Alternative splicing of mRNA allows generating several proteins with different functional properties from a unique locus [66]. In this way, alternative splicing of neuregulin, DSCAM or neurexin mRNA largely contributes to the genesis of specific synapses [67]. Chen and colleagues [68] recently reported the implication of this mechanism during vertebrate commissural axon guidance. Robo/Slit signaling pushes axons out of the FP and prevents midline re-crossing [69]. Interestingly, two isoforms of the Robo3 receptor produced as a result of differential splicing of the 3′ end of the pre-mRNA have been identified in commissural neurons. Robo3.1 and Robo1 are expressed at the pre-crossing stage, while Robo3.2 and Robo2 are expressed after crossing [2, 68, 70]. The isoforms also differ in their functions. Over-expression of Robo3.1 by electroporation of chick neural tube produced multiple re-crossing phenotypes of commissural axons, whereas over-expression of Robo3.2 abolished the crossing. Re-expression of Robo3.1 in Robo3−/− mouse embryos restored the crossing behavior of commissural axons. This is in agreement with a model in which Robo3.1 inhibited an early response to FP Slit repellent, whereas Robo3.2 contributed to Slit-mediated FP exit. The mechanisms by which the different Robo3 isoforms play such opposing roles remain open. Whether this relies on distinct binding profiles with Slit and Robo family members is also unknown. This study highlights how regional distribution of Robos isoforms with divergent roles can modulate axon guidance. Whether such processes are used to generate functional diversity in other guidance receptors is an intriguing question.
Translational and post-translational regulation of midline crossing
Neo-synthesized receptors are sorted to the secretory pathway to be inserted in the plasma membrane. Increasing numbers of studies reported a variety of translational and post-translational regulations taking place during protein synthesis, maturation, trafficking and turn-over, which impact specific guidance decisions.
Regulation of receptor synthesis
The possibility that mechanisms regulating mRNA translation influence growth cone responsiveness to guidance cues has been suggested by several recent works. For example, Xenopus retinal growth cone responses to guidance cues were reported to require cytoplasmic polyadenylation element binding protein1 (CPEB1) [71]. CPEB binds a short sequence of the mRNA, the CPE, and nucleates a complex of factors regulating polyA elongation. By binding to certain mRNAs that are maintained in a dormant state, CPEB promotes their translation [72]. Fragile X mental retardation protein (FRMP) is an RNA binding protein, whose gene mutations are responsible for the X-linked syndrome of mental retardation. FRMP was reported to contribute to the growth cone response to Sema3A in in vitro assays [71]. A well-described role has been assigned to FRMP in the regulation of specific sets of mRNAs encoding proteins required for synaptic development and function [73]. These findings suggest that translation of a specific pool of mRNAs may be part of the mechanisms setting (and modulating) appropriate sensitivity of the growth cones to guidance cues. Recent work showed that Mushashi1 (Msi1), another RNA binding protein, controls the expression level of Robo3, a key guidance receptor for commissural systems as described above [74]. In this study, the authors reported that Msi1 regulates Robo3 expression in precerebellar neurons, which are generated dorsally in the rhombic lip in the hindbrain and migrate ventrally towards the floor plate. Among these precerebellar neurons, inferior olivary (IO) neurons are sensitive to midline-derived Slits which prevent them from midline crossing, while their axons cross the midline [75]. In contrast, the LRN/ECN neurons cross the midline to settle contralaterally. Kuwako and colleagues [74] observed that genetic loss of Msi in mice does not affect Robo3 mRNA levels, but strongly down-regulates Robo3 protein expression. Biochemical experiments suggest that Msi binds directly or indirectly to the different Robo3 transcripts, recognizing unconventional sites located in the coding sequence. Interestingly, this work highlighted differences in the mechanisms regulating Robo3 expression at different antero-posterior levels of the central nervous system. No impact of Msi loss of function was found in the pathfinding of spinal commissural neurons, in which Robo3 expression is directly controlled by the transcription factors Lhx2 and Lhx9, as described above. Interestingly, these transcription factors are not expressed by precerebellar neurons, even though in these neurons Robo3 might also be transcriptionally regulated. By using explant assays, the authors also showed that the activity of Msi1 is down-regulated by local floor plate signals, whose identities remain unknown. Thus, cue-dependent regulation of Msi expression may result in decreased downstream Robo3 levels.
Targeting of mRNAs to dendritic spines and local protein synthesis has been demonstrated to be instrumental for synapse formation and plasticity [76–79]. In recent years, the presence of mRNAS in developing axons has raised the intriguing issue of whether local protein synthesis also participates in guidance decisions. Davis and colleagues [80] first observed that growth cones contain functional ribosomes. Campbell and Holt [81] confirmed and extended this observation, reporting that the growth cone not only contains ribosomes but also mRNAs and elongating factors. Merianda and colleagues [82] have now described that, in DRG and RGC cultured neurons, axons hold functional organelles closely related to the Golgi apparatus and the rough endoplasmic reticulum. These are comparable to the delocalized Golgi compartments found in dendrites, referred to as “Golgi outposts or satellites” [83]. This suggests that membrane-associated and secreted proteins could also be synthesized in the axons. The content of mRNAs in Xenopus embryonic and adult retinal growth cones has been analyzed recently in microarray [84]. This investigation revealed that a variety of mRNAs encoding proteins with diverse functions are trafficked to the axons and the growth cones. Interestingly, the mRNA regional distribution of Robos isoforms may exert the control, and mechanisms of compartmentalization may exist to specifically localize (or enrich) some mRNAs in the axon shaft or in the growth cone.
Up to now, functional studies essentially implicated local synthesis of cytosolic signaling molecules and cytoskeletal components in growth cone responses to guidance cues [85, 86]. The current model proposes that repulsive behavior is associated with local synthesis of signaling molecules, which destabilizes the cytoskeleton. In contrast, local synthesis of the cytoskeletal components themselves occurs in response to attractive signals. Nevertheless, local synthesis might not be required in all contexts of axon guidance, as recent reports provide evidence for local synthesis-independent responsiveness of axon growth cones to guidance cues [87]. Environmental cue-mediated stimulation of axon growth was also found to require intra-axonal protein synthesis. In this context, the authors provided evidence for the local translation of polarity components [88].
Very few examples have been found of mRNA encoding receptors being trafficked and translated locally during axon growth. However, the olfactory system has provided one of the nicest examples, with mRNAs encoding odorant receptors, which are then transported in olfactory axons and later associate with polysomes in sensory axons of the olfactory bulb [89]. These experiments were carried out in the adult, but olfactory connections are continuously renewed, strongly suggesting that immature axons contain these mRNAs. In support, the authors found that odorant receptor mRNA levels in the axons increased during periods of axon wiring, supporting a contribution of local synthesis during the formation of olfactory connections. An exciting possibility is that local synthesis of guidance receptors contributes to the switch of responsiveness of commissural axons to midline cues. A unique example supports this hypothesis [90]. In this work, the authors reported that the EphA2 transcript is locally translated in commissural growth cones upon midline crossing, and the receptor accumulates at the cell surface. The functional result of this local translation still remains unknown. Recently, an intriguing link has been made by the same group between DCC and the control of protein synthesis in neurons [91]. Starting from the observation made by immunolabeling, that DCC colocalizes with the translational machinery in the growth cone of cultured neurons, the authors characterized a Netrin-regulated “transmembrane translational regulation complex” in a cell line model. According to this model, DCC anchors components of the translational machinery, such as ribosomal proteins and translation initiation factors, at the membrane. This would allow the coupling between extracellular DCC ligands and protein synthesis at specific sites of the cell membrane where DCC is activated. In spinal cord explants, over-expression of a DCC mutant lacking the intracellular domain that interacts with ribosomal proteins prevents commissural axons from reaching the FP, suggesting a disrupted responsiveness to Netrin. It is tempting to speculate that ,since this takes place in the growth cones, such a coupling could regulate local protein synthesis according to the diverse cues encountered during axon pathfinding. Another interesting issue will be to assess whether other guidance receptors share this property.
Trafficking of guidance receptors
A variety of other molecular strategies have been retained to modulate growth cone responsiveness to guidance cues. Studies of Robo expression at the drosophila ventral cord have provided a remarkable example of the contribution of the molecular machinery controlling protein trafficking in the regulation of guidance decisions.
A genetic screen for defects of midline crossing conducted in the fly first led to the discovery of the comm gene, and the finding that Comm protein negatively regulates Robo expression at the post-transcriptional level [3, 92]. Combinations of in vitro and in vivo approaches were deployed to elucidate the mechanism by which Comm exerts its effects on Robo expression. The Comm protein is transiently expressed in commissural neurons at the pre-crossing stage, preventing Robo proteins from being trafficked to the plasma membrane by sorting them to the late endocytic pathway. As a consequence, commissural growth cones are devoid of Robo, and cannot read the Slit gradient until crossing [25, 26]. Down-regulation of comm transcription, which occurs through yet unknown mechanisms, then restores Robo expression at the surface so that commissural axons become responsive to Slit. Slit thus establishes an irreversible molecular barrier at the midline to prevent re-crossing [5, 22, 53]. Whether in vivo Robo trafficking solely depends on the comm pathway for regulation has been challenged by a report stating that flies expressing a mutant Robo deficient for comm-dependent sorting still form commissures [93].
Comm-mediated regulation of Robo appears not to apply to vertebrate commissural axon guidance since commissural orthologs have not been found in the vertebrate genome. The temporal switch of responsiveness to Slit that allows midline crossing is conserved but must be mediated by other mechanisms. Indeed, one isoform of the divergent Robo family member Robo3 exhibits functional properties resembling that of drosophila Comm, as it negatively regulates commissural axon responsiveness to Slit [70]. The dynamics of Robo protein levels from pre-crossing to crossing stages has yet to be investigated. Thus, whether regulation of Robo expression at the cell surface accounts for the temporally regulated commissural growth cone sensitivity to Slit remains an open question.
Proteolytic cleavage of guidance receptors
An additional screen for midline crossing defects in drosophila identified kuzbanian (kuz), a metalloprotease of the Adam Family as a regulator of Robo [94]. In the context of axon guidance, metalloproteases have been found in earlier work to process Eph/Ephrin family members, to interrupt ligand-receptor interactions and to allow the repulsive response [95]. Here, the authors found that genetic loss of kuz impairs commissural midline crossing. Although cleavage of Slits was known to generate fragments having specific functional properties [96], neuronal but not glial Kuz re-expression in flies could rescue the midline crossing defects. Thus, kuz is required to process Robo but not Slit. Kuz was found to mediate the cleavage of the Robo receptor, and a mutant form resistant to cleavage did not rescue the Kuz phenotype, suggesting that Robo processing by Kuz is necessary for receptor activation and initiation of transduction [94]. Thus, a temporal sequence might occur with Comm-dependent Robo degradation occurring before crossing to silence Slit responsiveness. Then, after crossing, kuz-dependent Robo processing may occur upon ligand binding to mediate Slit repulsion. An interesting question concerns how these two post-translational regulatory pathways are coordinated to set the appropriate spatio-temporal pattern of Robo expression level.
Another recent work showed that vertebrates developed some comparable cellular strategies to regulate receptor levels post-translationally during midline crossing. This study focused on Semaphorin signaling, which in addition to Slit signaling, has been shown to also contribute to commissural axon guidance at the midline [21]. The Sema3s are secreted guidance cues that play wide roles during the formation of neuronal circuits [97–102]. Sema3s activate holoreceptors composed of at least a ligand binding sub-unit, Neuropilin (Nrp; 1 or 2) [103–109] and a signaling co-receptor of one of the four Plexin-A (PlexA) family members [102, 108, 110–115]. In some contexts, recruitment of IgSuperfamily Cell Adhesion Molecules L1-CAMs by neuropilins is also required to trigger Sema3s-mediated down-regulation of adhesive contacts during the repulsive growth cone response [116]. The class 3 Semaphorin Sema3B is expressed at the ventral midline, and in explant cultures, commissural axons were found to acquire a repulsive response after midline crossing [16, 21, 117]. Mice deficient for Sema3B and for components of its receptor complex Nrp2 and Plexin-A1 all present common defects of commissural axon guidance at the midline [16, 21]. Commissural axons stall in the FP, turn prematurely before crossing or grow in an abnormal rostro-caudal direction after crossing. Using combinations of ex vivo, in vivo and biochemical approaches, Nawabi et al. [16] reported that pre-crossing commissural responsiveness to Sema3B is silenced by an endogenous proteolytic calpain1 activity. This cleaves the signaling moiety of the Sema3B receptor complex, Plexin-A1, thus preventing its cell surface expression in the growth cone. When the growth cones reach the FP, exposure to local signals suppresses this protease activity, resulting in Plexin-A1 accumulation, assembly of a functional receptor in commissural growth cones and sensitization to Sema3B [16]. By over-expressing a PlexinA1-gfpPhLuo fusion in the chick neural tube (the PhLuo is fluorescent at neutral Ph, allowing visualization of cell surface proteins), the authors were able to show that the fluorescence was very low at the pre-crossing stage and was switched on in commissural growth cones when they cross. By conditioning medium from isolated FP cultures, they found that signals released from the FP trigger Plexin-A1 accumulation and sensitize commissural growth cones to Sema3B. Using a candidate approach, the authors identified the IgSuperfamily Cell Adhesion Molecule NrCAM as an FP cue contributing to the switch of commissural responsiveness to Sema3B at the midline. Blocking calpain1 activity in culture models was sufficient to restore Plexin-A1 cell surface level and to confer sensitivity to Sema3B. In vivo, pharmacological inhibition of calpain activity impaired FP in-growth. This is consistent with a model in which suppression of Plexin-A1 processing prematurely switches on Sema3B-mediated FP repulsion. This study illustrates a mechanism of receptor processing in vertebrates which controls the temporal switch of commissural responsiveness to a midline repellent. It also implicates calpains as regulators of axon guidance decisions. Calpains are calcium-dependant proteases, which play a role in a wide variety of processes [118, 119]. Whether calpains regulate other guidance signals through receptor processing will be an interesting question to explore.
A second study also highlighted the key role of receptor processing in the sensitivity of axons to midline guidance cues. Bai and collaborators [120] conducted a genetic screen in mice exposed to a mutagenic agent to identify guidance defects of spinal nerves. They selected a mutant, which they called Columbus, that presents severe disorganized trajectories, including aberrant growth of some motor axons towards the FP and midline crossing. The mutation was found to map the gene encoding presenillin-1 (PS1), the catalytic sub-unit of γ secretase. Processing by γ secretases is known to play essential role in the function of various type 1 proteins, for example APP and Notch [121–123].
In vitro, PS-1 null motoneuron axons acquired an aberrant growth-promoting response to Netrin-1, which could be shown to be responsible for the abnormal midline directed extension of motor axons in vivo. A protease pathway was described in earlier work that first involves a metalloprotease-mediated ectodomain shedding of DCC; followed by a γ secretase-induced intracellular cleavage releasing DCC intracellular domain [124]. Bai and collaborators [120] then reported that in the wild-type context, this protease pathway actively keeps motoneurons unresponsive to Netrin-1 [120]. In commissural neurons, silencing of Netrin responsiveness is achieved through DCC-Robo receptor complex formation that is induced upon exposure to midline slit [29]. Bai et al. found that, unlike full-length DCC, the DCC stubs produced by metalloprotease cleavage do not bind Robo. The authors suggest a model in which cell-autonomous Robo-Slit signaling (motoneurons, unlike commissural neurons, express Slit) silences Netrin responsiveness. By inducing abnormal accumulation of DCC stubs which escape the silencing by Robo-Slit, inhibition of γ secretase would result in sensitivity to Netrin. Possibly in the context of commissural axon guidance, γ secretase should be active after crossing in preventing the accumulation of DCC stubs and permitting the silencing of Netrin responsiveness by Robo/Slit. Tanigushi and collaborators [124] reported that, in spinal cord extracts of PS1-deficient embryos, DCC stubs (generated by metalloprotease-mediated ectodomain shedding) are detected at much higher levels than in the wild-type embryos. Consistently, aberrant stalling of commissural axons in the FP of PS1 null embryos, reported by Bai and collaborators [120], may be the result of their continued sensitivity to Netrin. Still unclear issues relate to the exact role of this processing program of DCC in populations such as commissural neurons, which are responsive to Netrin. Altogether, these different studies illustrate the crucial role of protease activity in setting receptor expression profiles at appropriate time and space during the development of neuronal projections.
Conclusion
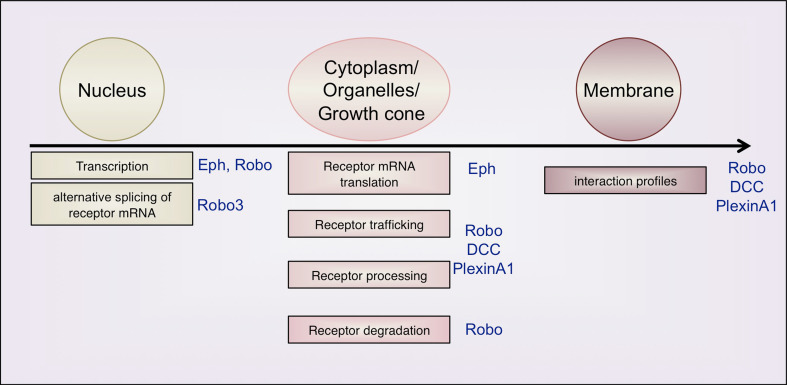
The process of midline crossing clearly exemplifies the complexity of mechanisms that allow axons projecting over long distances to navigate at intermediate targets (Fig. 5). The decision to cross the midline results from the integration of successive and intricate regulations controlling the sub-cellular distribution of guidance receptors at the growth cone surface. Studies of midline crossing in invertebrate and vertebrate models revealed key roles of mechanisms controlling protein synthesis, receptor trafficking and processing in the series of guidance decisions that commissural growth cones must take to reach the midline, cross it and move away towards the next target.
Fig. 5.
Summary of the mechanisms controlling receptor expression levels during midline crossing. In the nucleus, combinations of transcription factors set the spatio-temporal expression of guidance receptors. Structural and functional diversity of receptors can be generated by mRNA alternative splicing. Various post-translational processes control receptor function and expression in the soma and in the growth cone, such as regulated protein translation, vesicular trafficking, sorting to the plasma membrane and turn-over. At the cell membrane, regulation of the interactions between guidance receptors (e.g., modifications of the composition of receptor complexes) can also mediate switch of growth cone responses
Important processes of axon guidance are highly conserved among commissural systems, such as the switch from initial attraction towards the midline to repulsion after crossing and the conservation of Netrin and Slit signaling in mediating these growth cone responses. In contrast, the molecular strategies underlying the switch are not conserved, as species and cell-type differences have been found. Very diverse controls of guidance receptor expression in commissural neurons have been characterized, including regulation of transcription, alternative splicing and receptor processing. This raises the question of respective contribution and weight of these controls in the different guidance decisions taken by commissural axons at the midline.
What is the advantage of controlling receptor distribution through degradation or processing, rather than protein synthesis? A very simple answer is that receptor processing and degradation allow focal changes of receptor distribution. For example, regulating receptor expression specifically in a few growth cone filopodia could be achieved by spatially localized protease activities. This would allow the continued presence of unprocessed receptor forms in other subdomains of the growth cones. Receptor processing does not necessarily result in protein degradation but can ensure modulation of protein functions. This is the case in calpains, which process their target proteins to regulate their binding profiles and signal transduction properties [125]. Information is still lacking with respect to the nature of the mechanisms regulating the protease activities that limit their action in time and space, which must be closely coordinated with growth cone progression. Some equivalent outcome in terms of spatio-temporal modulation of receptor distribution could result from local translation of mRNAs asymmetrically stored. However, evidence for neo-synthesized receptors in the growth cone is still sparse. Up to now, it also remains uncertain whether the mechanisms of switch are common to all pools of commissural axons navigating together at this step of their pathfinding, or whether cell-type specificities have already been determined. Exciting insights should come from genetic manipulations of midline guidance signaling in mouse models, specifically in animals designed with traceable trajectories using fluorescent reporters of subsets of commissural axon tracts.
Acknowledgments
We acknowledge E. A. Derrington for helpful comments and manuscript reading. VC is supported by the "Fondation pour la Recherche Médicale (FRM) and the "Agence Nationale pour la Recherche" ANR.
Bibliography
- 1.Black DL, Zipursky SL. To cross or not to cross: alternatively spliced forms of the Robo3 receptor regulate discrete steps in axonal midline crossing. Neuron. 2008;58(3):297–298. doi: 10.1016/j.neuron.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickson BJ, Gilestro GF. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol. 2006;22:651–675. doi: 10.1146/annurev.cellbio.21.090704.151234. [DOI] [PubMed] [Google Scholar]
- 3.Evans TA, Bashaw GJ. Axon guidance at the midline: of mice and flies. Curr Opin Neurobiol. 2010;20(1):79–85. doi: 10.1016/j.conb.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shirasaki R, Katsumata R, Murakami F. Change in chemoattractant responsiveness of developing axons at an intermediate target. Science. 1998;279(5347):105–107. doi: 10.1126/science.279.5347.105. [DOI] [PubMed] [Google Scholar]
- 5.Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96(6):795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 6.Butler SJ, Dodd J. A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron. 2003;38(3):389–401. doi: 10.1016/s0896-6273(03)00254-x. [DOI] [PubMed] [Google Scholar]
- 7.Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113(1):11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- 8.Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, Simons J, Bronson RT, Gordon JI, Tessier-Lavigne M, Weinberg RA. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386(6627):796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- 9.Gore BB, Wong KG, Tessier-Lavigne M. Stem cell factor functions as an outgrowth-promoting factor to enable axon exit from the midline intermediate target. Neuron. 2008;57(4):501–510. doi: 10.1016/j.neuron.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Jevince AR, Kadison SR, Pittman AJ, Chien CB, Kaprielian Z. Distribution of EphB receptors and ephrin-B1 in the developing vertebrate spinal cord. J Comp Neurol. 2006;497(5):734–750. doi: 10.1002/cne.21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadison SR, Makinen T, Klein R, Henkemeyer M, Kaprielian Z. EphB receptors and ephrin-B3 regulate axon guidance at the ventral midline of the embryonic mouse spinal cord. J Neurosci. 2006;26(35):8909–8914. doi: 10.1523/JNEUROSCI.1569-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3(7):475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- 13.Ly A, Nikolaev A, Suresh G, Zheng Y, Tessier-Lavigne M, Stein E. DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell. 2008;133(7):1241–1254. doi: 10.1016/j.cell.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302(5652):1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto Y, Irie F, Inatani M, Tessier-Lavigne M, Yamaguchi Y. Netrin-1/DCC signaling in commissural axon guidance requires cell-autonomous expression of heparan sulfate. J Neurosci. 2007;27(16):4342–4350. doi: 10.1523/JNEUROSCI.0700-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nawabi H, Briancon-Marjollet A, Clark C, Sanyas I, Takamatsu H, Okuno T, Kumanogoh A, Bozon M, Takeshima K, Yoshida Y, Moret F, Abouzid K, Castellani V. A midline switch of receptor processing regulates commissural axon guidance in vertebrates. Genes Dev. 2010;24(4):396–410. doi: 10.1101/gad.542510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada A, Charron F, Morin S, Shin DS, Wong K, Fabre PJ, Tessier-Lavigne M, McConnell SK. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature. 2006;444(7117):369–373. doi: 10.1038/nature05246. [DOI] [PubMed] [Google Scholar]
- 18.Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87(6):1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 19.Stoeckli ET, Landmesser LT. Axonin-1, Nr-CAM, and Ng-CAM play different roles in the in vivo guidance of chick commissural neurons. Neuron. 1995;14(6):1165–1179. doi: 10.1016/0896-6273(95)90264-3. [DOI] [PubMed] [Google Scholar]
- 20.Stoeckli ET, Sonderegger P, Pollerberg GE, Landmesser LT. Interference with axonin-1 and NrCAM interactions unmasks a floor-plate activity inhibitory for commissural axons. Neuron. 1997;18(2):209–221. doi: 10.1016/s0896-6273(00)80262-7. [DOI] [PubMed] [Google Scholar]
- 21.Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M. Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell. 2000;102(3):363–375. doi: 10.1016/s0092-8674(00)00041-6. [DOI] [PubMed] [Google Scholar]
- 22.Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96(6):785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 23.Bourikas D, Pekarik V, Baeriswyl T, Grunditz A, Sadhu R, Nardo M, Stoeckli ET. Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nat Neurosci. 2005;8(3):297–304. doi: 10.1038/nn1396. [DOI] [PubMed] [Google Scholar]
- 24.Winckler B, Mellman I. Trafficking guidance receptors. Cold Spring Harb Perspect Biol. 2010;2(7):a001826. doi: 10.1101/cshperspect.a001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keleman K, Rajagopalan S, Cleppien D, Teis D, Paiha K, Huber LA, Technau GM, Dickson BJ. Comm sorts robo to control axon guidance at the Drosophila midline. Cell. 2002;110(4):415–427. doi: 10.1016/s0092-8674(02)00901-7. [DOI] [PubMed] [Google Scholar]
- 26.Keleman K, Ribeiro C, Dickson BJ. Comm function in commissural axon guidance: cell-autonomous sorting of Robo in vivo. Nat Neurosci. 2005;8(2):156–163. doi: 10.1038/nn1388. [DOI] [PubMed] [Google Scholar]
- 27.Georgiou M, Tear G. Commissureless is required both in commissural neurones and midline cells for axon guidance across the midline. Development (Cambridge) 2002;129(12):2947–2956. doi: 10.1242/dev.129.12.2947. [DOI] [PubMed] [Google Scholar]
- 28.Georgiou M, Tear G. The N-terminal and transmembrane domains of Commissureless are necessary for its function and trafficking within neurons. Mech Dev. 2003;120(9):1009–1019. doi: 10.1016/s0925-4773(03)00179-5. [DOI] [PubMed] [Google Scholar]
- 29.Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291(5510):1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- 30.Bashaw GJ, Klein R. Signaling from axon guidance receptors. Cold Spring Harb Perspect Biol. 2010;2(5):a001941. doi: 10.1101/cshperspect.a001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kania A, Jessell TM. Topographic motor projections in the limb imposed by LIM homeodomain protein regulation of ephrin-A:EphA interactions. Neuron. 2003;38(4):581–596. doi: 10.1016/s0896-6273(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 32.Kania A, Johnson RL, Jessell TM. Coordinate roles for LIM homeobox genes in directing the dorsoventral trajectory of motor axons in the vertebrate limb. Cell. 2000;102(2):161–173. doi: 10.1016/s0092-8674(00)00022-2. [DOI] [PubMed] [Google Scholar]
- 33.Petros TJ, Rebsam A, Mason CA. Retinal axon growth at the optic chiasm: to cross or not to cross. Annu Rev Neurosci. 2008;31:295–315. doi: 10.1146/annurev.neuro.31.060407.125609. [DOI] [PubMed] [Google Scholar]
- 34.Drager UC. Birth dates of retinal ganglion cells giving rise to the crossed and uncrossed optic projections in the mouse. Proc R Soc Lond Sci. 1985;224(1234):57–77. doi: 10.1098/rspb.1985.0021. [DOI] [PubMed] [Google Scholar]
- 35.Guillery RW, Mason CA, Taylor JS. Developmental determinants at the mammalian optic chiasm. J Neurosci. 1995;15(7 Pt 1):4727–4737. doi: 10.1523/JNEUROSCI.15-07-04727.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcus RC, Mason CA. The first retinal axon growth in the mouse optic chiasm: axon patterning and the cellular environment. J Neurosci. 1995;15(10):6389–6402. doi: 10.1523/JNEUROSCI.15-10-06389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sretavan DW, Reichardt LF. Time-lapse video analysis of retinal ganglion cell axon pathfinding at the mammalian optic chiasm: growth cone guidance using intrinsic chiasm cues. Neuron. 1993;10(4):761–777. doi: 10.1016/0896-6273(93)90176-r. [DOI] [PubMed] [Google Scholar]
- 38.Wizenmann A, Thanos S, von Boxberg Y, Bonhoeffer F. Differential reaction of crossing and non-crossing rat retinal axons on cell membrane preparations from the chiasm midline: an in vitro study. Development (Cambridge) 1993;117(2):725–735. doi: 10.1242/dev.117.2.725. [DOI] [PubMed] [Google Scholar]
- 39.Nakagawa S, Brennan C, Johnson KG, Shewan D, Harris WA, Holt CE. Ephrin-B regulates the Ipsilateral routing of retinal axons at the optic chiasm. Neuron. 2000;25(3):599–610. doi: 10.1016/s0896-6273(00)81063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams SE, Mann F, Erskine L, Sakurai T, Wei S, Rossi DJ, Gale NW, Holt CE, Mason CA, Henkemeyer M. Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron. 2003;39(6):919–935. doi: 10.1016/j.neuron.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 41.Petros TJ, Shrestha BR, Mason C. Specificity and sufficiency of EphB1 in driving the ipsilateral retinal projection. J Neurosci. 2009;29(11):3463–3474. doi: 10.1523/JNEUROSCI.5655-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herrera E, Brown L, Aruga J, Rachel RA, Dolen G, Mikoshiba K, Brown S, Mason CA. Zic2 patterns binocular vision by specifying the uncrossed retinal projection. Cell. 2003;114(5):545–557. doi: 10.1016/s0092-8674(03)00684-6. [DOI] [PubMed] [Google Scholar]
- 43.Merzdorf CS. Emerging roles for zic genes in early development. Dev Dyn. 2007;236(4):922–940. doi: 10.1002/dvdy.21098. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Frigola C, Carreres MI, Vegar C, Mason C, Herrera E. Zic2 promotes axonal divergence at the optic chiasm midline by EphB1-dependent and -independent mechanisms. Development (Cambridge) 2008;135(10):1833–1841. doi: 10.1242/dev.020693. [DOI] [PubMed] [Google Scholar]
- 45.Lee R, Petros TJ, Mason CA. Zic2 regulates retinal ganglion cell axon avoidance of ephrinB2 through inducing expression of the guidance receptor EphB1. J Neurosci. 2008;28(23):5910–5919. doi: 10.1523/JNEUROSCI.0632-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson SI, Shafer B, Lee KJ, Dodd J. A molecular program for contralateral trajectory: Rig-1 control by LIM homeodomain transcription factors. Neuron. 2008;59(3):413–424. doi: 10.1016/j.neuron.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 47.Gowan K, Helms AW, Hunsaker TL, Collisson T, Ebert PJ, Odom R, Johnson JE. Crossinhibitory activities of Ngn1 and Math1 allow specification of distinct dorsal interneurons. Neuron. 2001;31(2):219–232. doi: 10.1016/s0896-6273(01)00367-1. [DOI] [PubMed] [Google Scholar]
- 48.Gross MK, Dottori M, Goulding M. Lbx1 specifies somatosensory association interneurons in the dorsal spinal cord. Neuron. 2002;34(4):535–549. doi: 10.1016/s0896-6273(02)00690-6. [DOI] [PubMed] [Google Scholar]
- 49.Lee KJ, Mendelsohn M, Jessell TM. Neuronal patterning by BMPs: a requirement for GDF7 in the generation of a discrete class of commissural interneurons in the mouse spinal cord. Genes Dev. 1998;12(21):3394–3407. doi: 10.1101/gad.12.21.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Helms AW, Johnson JE. Specification of dorsal spinal cord interneurons. Curr Opin Neurobiol. 2003;13(1):42–49. doi: 10.1016/s0959-4388(03)00010-2. [DOI] [PubMed] [Google Scholar]
- 51.Bermingham NA, Hassan BA, Wang VY, Fernandez M, Banfi S, Bellen HJ, Fritzsch B, Zoghbi HY. Proprioceptor pathway development is dependent on Math1. Neuron. 2001;30(2):411–422. doi: 10.1016/s0896-6273(01)00305-1. [DOI] [PubMed] [Google Scholar]
- 52.Helms AW, Johnson JE. Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development (Cambridge) 1998;125(5):919–928. doi: 10.1242/dev.125.5.919. [DOI] [PubMed] [Google Scholar]
- 53.Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92(2):205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- 54.Kidd T, Russell C, Goodman CS, Tear G. Dosage-sensitive and complementary functions of roundabout and commissureless control axon crossing of the CNS midline. Neuron. 1998;20(1):25–33. doi: 10.1016/s0896-6273(00)80431-6. [DOI] [PubMed] [Google Scholar]
- 55.Bonkowsky JL, Yoshikawa S, O’Keefe DD, Scully AL, Thomas JB. Axon routing across the midline controlled by the Drosophila Derailed receptor. Nature. 1999;402(6761):540–544. doi: 10.1038/990122. [DOI] [PubMed] [Google Scholar]
- 56.Yang L, Garbe DS, Bashaw GJ. A frazzled/DCC-dependent transcriptional switch regulates midline axon guidance. Science. 2009;324(5929):944–947. doi: 10.1126/science.1171320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu QX, Hiramoto M, Ueda H, Gojobori T, Hiromi Y, Hirose S. Midline governs axon pathfinding by coordinating expression of two major guidance systems. Genes Dev. 2009;23(10):1165–1170. doi: 10.1101/gad.1774209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buescher M, Svendsen PC, Tio M, Miskolczi-McCallum C, Tear G, Brook WJ, Chia W. Drosophila T box proteins break the symmetry of hedgehog-dependent activation of wingless. Curr Biol. 2004;14(19):1694–1702. doi: 10.1016/j.cub.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 59.Buescher M, Tio M, Tear G, Overton PM, Brook WJ, Chia W. Functions of the segment polarity genes midline and H15 in Drosophila melanogaster neurogenesis. Dev Biol. 2006;292(2):418–429. doi: 10.1016/j.ydbio.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 60.Gaziova I, Bhat KM. Ancestry-independent fate specification and plasticity in the developmental timing of a typical Drosophila neuronal lineage. Development (Cambridge) 2009;136(2):263–274. doi: 10.1242/dev.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leal SM, Qian L, Lacin H, Bodmer R, Skeath JB. Neuromancer1 and Neuromancer2 regulate cell fate specification in the developing embryonic CNS of Drosophila melanogaster . Dev Biol. 2009;325(1):138–150. doi: 10.1016/j.ydbio.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stennard FA, Harvey RP. T-box transcription factors and their roles in regulatory hierarchies in the developing heart. Development (Cambridge) 2005;132(22):4897–4910. doi: 10.1242/dev.02099. [DOI] [PubMed] [Google Scholar]
- 63.Rajagopalan S, Nicolas E, Vivancos V, Berger J, Dickson BJ. Crossing the midline: roles and regulation of Robo receptors. Neuron. 2000;28(3):767–777. doi: 10.1016/s0896-6273(00)00152-5. [DOI] [PubMed] [Google Scholar]
- 64.Simpson JH, Kidd T, Bland KS, Goodman CS. Short-range and long-range guidance by slit and its Robo receptors Robo and Robo2 play distinct roles in midline guidance. Neuron. 2000;28(3):753–766. doi: 10.1016/s0896-6273(00)00151-3. [DOI] [PubMed] [Google Scholar]
- 65.Spitzweck B, Brankatschk M, Dickson BJ. Distinct protein domains and expression patterns confer divergent axon guidance functions for Drosophila Robo receptors. Cell. 2010;140(3):409–420. doi: 10.1016/j.cell.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 66.Black DL. Protein diversity from alternative splicing: a challenge for bioinformatics and post-genome biology. Cell. 2000;103(3):367–370. doi: 10.1016/s0092-8674(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 67.Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17(1):43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Z, Gore BB, Long H, Ma L, Tessier-Lavigne M. Alternative splicing of the Robo3 axon guidance receptor governs the midline switch from attraction to repulsion. Neuron. 2008;58(3):325–332. doi: 10.1016/j.neuron.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 69.Mambetisaeva ET, Andrews W, Camurri L, Annan A, Sundaresan V. Robo family of proteins exhibit differential expression in mouse spinal cord and Robo-Slit interaction is required for midline crossing in vertebrate spinal cord. Dev Dyn. 2005;233(1):41–51. doi: 10.1002/dvdy.20324. [DOI] [PubMed] [Google Scholar]
- 70.Sabatier C, Plump AS, Le M, Brose K, Tamada A, Murakami F, Lee EY, Tessier-Lavigne M. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117(2):157–169. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- 71.Lin AC, Tan CL, Lin CL, Strochlic L, Huang YS, Richter JD, Holt CE. Cytoplasmic polyadenylation and cytoplasmic polyadenylation element-dependent mRNA regulation are involved in Xenopus retinal axon development. Neural Dev. 2009;4:8. doi: 10.1186/1749-8104-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Richter JD. CPEB: a life in translation. Trends Biochem Sci. 2007;32(6):279–285. doi: 10.1016/j.tibs.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 73.Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60(2):201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuwako K, Kakumoto K, Imai T, Igarashi M, Hamakubo T, Sakakibara S, Tessier-Lavigne M, Okano HJ, Okano H. Neural RNA-binding protein Musashi1 controls midline crossing of precerebellar neurons through posttranscriptional regulation of Robo3/Rig-1 expression. Neuron. 2010;67(3):407–421. doi: 10.1016/j.neuron.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Di Meglio T, Nguyen-Ba-Charvet KT, Tessier-Lavigne M, Sotelo C, Chedotal A. Molecular mechanisms controlling midline crossing by precerebellar neurons. J Neurosci. 2008;28(25):6285–6294. doi: 10.1523/JNEUROSCI.0078-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuhl D, Skehel P. Dendritic localization of mRNAs. Curr Opin Neurobiol. 1998;8(5):600–606. doi: 10.1016/s0959-4388(98)80087-1. [DOI] [PubMed] [Google Scholar]
- 77.Martin KC, Barad M, Kandel ER. Local protein synthesis and its role in synapse-specific plasticity. Curr Opin Neurobiol. 2000;10(5):587–592. doi: 10.1016/s0959-4388(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 78.Steward O. mRNA localization in neurons: a multipurpose mechanism? Neuron. 1997;18(1):9–12. doi: 10.1016/s0896-6273(01)80041-6. [DOI] [PubMed] [Google Scholar]
- 79.Steward O, Levy WB. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci. 1982;2(3):284–291. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davis L, Dou P, De Wit M, Kater SB. Protein synthesis within neuronal growth cones. J Neurosci. 1992;12(12):4867–4877. doi: 10.1523/JNEUROSCI.12-12-04867.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32(6):1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- 82.Merianda TT, Lin AC, Lam JS, Vuppalanchi D, Willis DE, Karin N, Holt CE, Twiss JL. A functional equivalent of endoplasmic reticulum and Golgi in axons for secretion of locally synthesized proteins. Mol Cell Neurosci. 2009;40(2):128–142. doi: 10.1016/j.mcn.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Horton AC, Ehlers MD. Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J Neurosci. 2003;23(15):6188–6199. doi: 10.1523/JNEUROSCI.23-15-06188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zivraj KH, Tung YC, Piper M, Gumy L, Fawcett JW, Yeo GS, Holt CE. Subcellular profiling reveals distinct and developmentally regulated repertoire of growth cone mRNAs. J Neurosci. 2010;30(46):15464–15478. doi: 10.1523/JNEUROSCI.1800-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin AC, Holt CE. Local translation and directional steering in axons. EMBO J. 2007;26(16):3729–3736. doi: 10.1038/sj.emboj.7601808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin AC, Holt CE. Function and regulation of local axonal translation. Curr Opin Neurobiol. 2008;18(1):60–68. doi: 10.1016/j.conb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roche FK, Marsick BM, Letourneau PC. Protein synthesis in distal axons is not required for growth cone responses to guidance cues. J Neurosci. 2009;29(3):638–652. doi: 10.1523/JNEUROSCI.3845-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hengst U, Deglincerti A, Kim HJ, Jeon NL, Jaffrey SR. Axonal elongation triggered by stimulus-induced local translation of a polarity complex protein. Nat Cell Biol. 2009;11(8):1024–1030. doi: 10.1038/ncb1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dubacq C, Jamet S, Trembleau A. Evidence for developmentally regulated local translation of odorant receptor mRNAs in the axons of olfactory sensory neurons. J Neurosci. 2009;29(33):10184–10190. doi: 10.1523/JNEUROSCI.2443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brittis PA, Lu Q, Flanagan JG. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell. 2002;110(2):223–235. doi: 10.1016/s0092-8674(02)00813-9. [DOI] [PubMed] [Google Scholar]
- 91.Tcherkezian J, Brittis PA, Thomas F, Roux PP, Flanagan JG. Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation. Cell. 2010;141(4):632–644. doi: 10.1016/j.cell.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Evans TA, Bashaw GJ. Functional diversity of Robo receptor immunoglobulin domains promotes distinct axon guidance decisions. Curr Biol. 2010;20(6):567–572. doi: 10.1016/j.cub.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gilestro GF. Redundant mechanisms for regulation of midline crossing in Drosophila. PloS one. 2008;3(11):e3798. doi: 10.1371/journal.pone.0003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coleman HA, Labrador JP, Chance RK, Bashaw GJ. The Adam family metalloprotease Kuzbanian regulates the cleavage of the roundabout receptor to control axon repulsion at the midline. Development (Cambridge) 2010;137(14):2417–2426. doi: 10.1242/dev.047993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hattori M, Osterfield M, Flanagan JG. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289(5483):1360–1365. doi: 10.1126/science.289.5483.1360. [DOI] [PubMed] [Google Scholar]
- 96.Chedotal A. Slits and their receptors. Adv Exp Med Biol. 2007;621:65–80. doi: 10.1007/978-0-387-76715-4_5. [DOI] [PubMed] [Google Scholar]
- 97.Bagnard D, Lohrum M, Uziel D, Puschel AW, Bolz J. Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development (Cambridge) 1998;125(24):5043–5053. doi: 10.1242/dev.125.24.5043. [DOI] [PubMed] [Google Scholar]
- 98.Falk J, Bechara A, Fiore R, Nawabi H, Zhou H, Hoyo-Becerra C, Bozon M, Rougon G, Grumet M, Puschel AW, Sanes JR, Castellani V. Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron. 2005;48(1):63–75. doi: 10.1016/j.neuron.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 99.Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75(7):1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 100.Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75(2):217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- 101.Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- 102.Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005;6(10):789–800. doi: 10.1038/nrm1740. [DOI] [PubMed] [Google Scholar]
- 103.Fujisawa H, Ohtsuki T, Takagi S, Tsuji T. An aberrant retinal pathway and visual centers in Xenopus tadpoles share a common cell surface molecule, A5 antigen. Dev Biol. 1989;135(2):231–240. doi: 10.1016/0012-1606(89)90175-9. [DOI] [PubMed] [Google Scholar]
- 104.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90(4):739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 105.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90(4):753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 106.Satoda M, Takagi S, Ohta K, Hirata T, Fujisawa H. Differential expression of two cell surface proteins, neuropilin and plexin, in Xenopus olfactory axon subclasses. J Neurosci. 1995;15(1 Pt 2):942–955. doi: 10.1523/JNEUROSCI.15-01-00942.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takagi S, Kasuya Y, Shimizu M, Matsuura T, Tsuboi M, Kawakami A, Fujisawa H. Expression of a cell adhesion molecule, neuropilin, in the developing chick nervous system. Dev Biol. 1995;170(1):207–222. doi: 10.1006/dbio.1995.1208. [DOI] [PubMed] [Google Scholar]
- 108.Fujisawa H. Discovery of semaphorin receptors, neuropilin and plexin, and their functions in neural development. J Neurobiol. 2004;59(1):24–33. doi: 10.1002/neu.10337. [DOI] [PubMed] [Google Scholar]
- 109.Schwarz Q, Ruhrberg C. Neuropilin, you gotta let me know: should I stay or should I go? Cell Adh Migr. 2010;4(1):61–66. doi: 10.4161/cam.4.1.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kameyama T, Murakami Y, Suto F, Kawakami A, Takagi S, Hirata T, Fujisawa H. Identification of plexin family molecules in mice. Biochem Biophys Res Commun. 1996;226(2):396–402. doi: 10.1006/bbrc.1996.1367. [DOI] [PubMed] [Google Scholar]
- 111.Ohta K, Takagi S, Asou H, Fujisawa H. Involvement of neuronal cell surface molecule B2 in the formation of retinal plexiform layers. Neuron. 1992;9(1):151–161. doi: 10.1016/0896-6273(92)90230-b. [DOI] [PubMed] [Google Scholar]
- 112.Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99(1):59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- 113.Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, Tessier-Lavigne M, Comoglio PM. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99(1):71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- 114.Rohm B, Ottemeyer A, Lohrum M, Puschel AW. Plexin/neuropilin complexes mediate repulsion by the axonal guidance signal semaphorin 3A. Mech Dev. 2000;93(1–2):95–104. doi: 10.1016/s0925-4773(00)00269-0. [DOI] [PubMed] [Google Scholar]
- 115.Negishi M, Oinuma I, Katoh H. Plexins: axon guidance and signal transduction. Cell Mol Life Sci. 2005;62(12):1363–1371. doi: 10.1007/s00018-005-5018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bechara A, Nawabi H, Moret F, Yaron A, Weaver E, Bozon M, Abouzid K, Guan JL, Tessier-Lavigne M, Lemmon V, Castellani V. FAK-MAPK-dependent adhesion disassembly downstream of L1 contributes to semaphorin3A-induced collapse. EMBO J. 2008;27(11):1549–1562. doi: 10.1038/emboj.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Parra LM, Zou Y. Sonic hedgehog induces response of commissural axons to Semaphorin repulsion during midline crossing. Nat Neurosci. 2010;13(1):29–35. doi: 10.1038/nn.2457. [DOI] [PubMed] [Google Scholar]
- 118.Carragher NO, Frame MC. Calpain: a role in cell transformation and migration. Int J Biochem Cell Biol. 2002;34(12):1539–1543. doi: 10.1016/s1357-2725(02)00069-9. [DOI] [PubMed] [Google Scholar]
- 119.Wu HY, Lynch DR. Calpain and synaptic function. Mol Neurobiol. 2006;33(3):215–236. doi: 10.1385/MN:33:3:215. [DOI] [PubMed] [Google Scholar]
- 120.Bai G, Chivatakarn O, Bonanomi D, Lettieri K, Franco L, Xia C, Stein E, Ma L, Lewcock JW, Pfaff SL. Presenilin-dependent receptor processing is required for axon guidance. Cell. 2011;144(1):106–118. doi: 10.1016/j.cell.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chow VW, Mattson MP, Wong PC, Gleichmann M. An overview of APP processing enzymes and products. Neuromolecular Med. 2010;12(1):1–12. doi: 10.1007/s12017-009-8104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guardia-Laguarta C, Pera M, Lleo A. gamma-Secretase as a therapeutic target in Alzheimer’s disease. Curr Drug Targets. 2010;11(4):506–517. doi: 10.2174/138945010790980349. [DOI] [PubMed] [Google Scholar]
- 123.Jorissen E, De Strooper B. Gamma-secretase and the intramembrane proteolysis of Notch. Curr Top Dev Biol. 2010;92:201–230. doi: 10.1016/S0070-2153(10)92006-1. [DOI] [PubMed] [Google Scholar]
- 124.Taniguchi Y, Kim SH, Sisodia SS. Presenilin-dependent “gamma-secretase” processing of deleted in colorectal cancer (DCC) J Biol Chem. 2003;278(33):30425–30428. doi: 10.1074/jbc.C300239200. [DOI] [PubMed] [Google Scholar]
- 125.Sorimachi H, Hata S, Ono Y. Expanding members and roles of the calpain superfamily and their genetically modified animals. Exp Anim. 2010;59(5):549–566. doi: 10.1538/expanim.59.549. [DOI] [PubMed] [Google Scholar]