Abstract
B cells express immunoglobulins on their surface where they serve as antigen receptors. When secreted as antibodies, the same molecules are key elements of the humoral immune response against pathogens such as viruses. Although most antibodies are restricted to binding a specific antigen, some are polyreactive and have the ability to bind to several different ligands, usually with low affinity. Highly polyreactive antibodies are removed from the repertoire during B-cell development by physiologic tolerance mechanisms including deletion and receptor editing. However, a low level of antibody polyreactivity is tolerated and can confer additional binding properties to pathogen-specific antibodies. For example, high-affinity human antibodies to HIV are frequently polyreactive. Here we review the evidence suggesting that in the case of some pathogens like HIV, polyreactivity may confer a selective advantage to pathogen-specific antibodies.
Keywords: Polyreactivity, Antibodies, B cells, Tolerance, Viruses, HIV
Introduction
Antibodies are essential to the humoral immune response against pathogens and constitute one of the first lines of defense against re-infection. The idea that antibodies are highly specific was suggested by the “lock and key” model proposed by Emil Fisher and strongly reinforced by pioneering observations on haptens made by Karl Landsteiner [1]. However, we now know that many antibodies and T-cell antigen receptors are also polyspecific or polyreactive as defined by the ability to bind to several different ligands [2–6]. Polyreactive B cells, especially those with low levels of reactivity, are part of the physiologic repertoire and produce “natural” antibodies [7–10], which are important contributors to the innate immune responses against pathogens. In addition, recent work has shown that class-switched high-affinity antibodies that react “specifically” with infectious agents such as human immunodeficiency virus (HIV) can also be polyreactive. In this review, we discuss these recent discoveries focusing primarily on the adaptive antibody response to HIV and other viruses.
Signatures of polyreactive antibodies
David Talmage first advanced the concept of antigen receptor “multiplicity” in 1959, but this was only proven 10 years later by Herman Eisen and his colleagues who first demonstrated antibody polyspecificity in a series of experiments testing the reactivity of anti-hapten myeloma proteins [11, 12]. They showed that a myeloma protein reactive to the 2,4-dinitrophenyl (DNP) group could bind both to structurally similar (e.g., menadione) and unrelated (e.g., caffeine) molecules [12]. This “degenerate” type of binding was later documented for conventional monoclonal antibodies, for example, anti-DNA, anti-HIV-1 p24 protein and anti-DNP antibodies [13–16].
Polyreactivity is a conserved feature of antibodies among species [17] and can be found in different immunoglobulin (Ig) isotypes (IgM, IgG and IgA) [18, 19]. The affinity of polyreactive antibodies for their different ligands is generally low (K d ranging from 10−3 to 10−7 M) compared to the high affinity of monoreactive antibodies for their specific ligand (K d = 10−7–10−11 M) [2, 19, 20]. It is generally assumed that the Ig-heavy chain variable region (IgVH) accounts for most polyreactive binding [21]. Indeed, a number of molecular features of IgVH have been associated with polyreactivity, including long and hydrophobic IgH complementary determining region 3 (CDR3), but none of these are predictive [21–23]. Although the precise molecular mechanism by which polyreactive antibodies bind to multiple ligands is not known, it has been proposed that the antigen-binding site of such antibodies is more flexible than monoreactive antibodies [24–28]. Consistent with this idea, antibody molecules can adopt distinct conformations in equilibrium, which allow recognition of various antigens using different interacting residues [29, 30]. An alternative, but not necessarily mutually exclusive possibility, is that antibodies undergo conformational reconfiguration upon antigen binding, allowing antigen accommodation into the binding pocket (“induced fit” mechanism) [31]. Irrespective of the mechanism, the binding of multiple antigens by a single polyreactive antibody molecule implies that the antibody is capable of several distinct physical interactions [32, 33]. These interactions confer “promiscuous,” but usually low-affinity binding, but do not exclude high-affinity interactions as described for monoclonal antibodies specific to HIV [34] and human epidermal growth factor receptor 2 [35].
Tolerance checkpoints eliminate most self- and poly-reactive B-cell clones
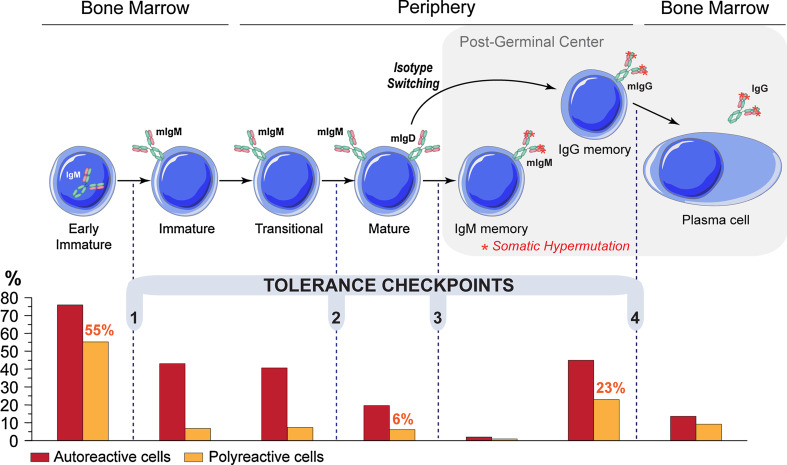
Polyreactivity and self-reactivity can arise as a result of random rearrangement of Ig genes during B-cell development [36] or by somatic mutation during the germinal center reaction [37]. Indeed, the majority of nascent B cells in the bone marrow (early immature B cells) of healthy humans express self-reactive and polyreactive BCRs (75 and 55%, respectively) [23] (Fig. 1). However, the vast majority of these autoreactive and polyreactive B cells are counterselected at two major B-cell tolerance checkpoints (Fig. 1) by clonal deletion, anergy or receptor editing [38]. As a result, only a small number of mature naïve B cells are self-reactive or polyreactive (20 and 6%, respectively) [23], and their average level of polyreactivity (as measured by ELISA) is far lower than that of their bone marrow progenitors. Polyreactivity is further removed from the B-cell repertoire during the transition to the IgM+ “memory” B-cell stage (2 and 1%, respectively) [39] (Fig. 1).
Fig. 1.
Tolerance checkpoints in human B-cell development. Tolerance checkpoints between developmental stages of B-cell lymphopoiesis; a central tolerance checkpoint in the bone marrow (1) followed by three tolerance checkpoints in periphery (2–4) ensure the removal of most autoreactive and polyreactive B cells [23, 39, 63, 154]. Bar graph shows the frequency of autoreactive (red bar) and polyreactive (orange bar) clones determined by testing monoclonal antibodies from single B cells at the different B-cell stages [23, 39, 63, 154]. The frequency of polyreactivity in the early immature, mature naïve and IgG memory B cells is indicated above the bars. mIg, membrane immunoglobulin
In contrast, tolerance checkpoints are defective in patients with autoimmune diseases i.e., systemic lupus erythematotus (SLE) [40, 41], rheumatoid arthritis [42, 43] and type 1 diabetes [44], leading to the accumulation of autoreactive and polyreactive mature naïve B cells in the periphery. The mechanisms required for establishing tolerance have been investigated in patients with immunodeficiency. Tolerance checkpoints require normal B-cell receptor (BCR) signaling [44, 45], and they can be altered by mutations in toll-like receptor signaling molecules [46] and CD40/CD40L signaling [47].
In conclusion, polyreactive B cells strongly cross-reacting with self-antigens are eliminated by B-cell tolerance mechanisms. Nevertheless, low levels of antibody polyspecificity can be tolerated to constitute a reservoir of “natural” antibodies offering structural diversity that may increase the spectrum of the B-cell repertoire involved in innate immune responses against pathogens.
Polyreactive “natural” antibodies as a first line of defense against pathogens
B cells expressing low-affinity polyreactive surface immunoglobulins are present in the peripheral B-cell repertoire of adult individuals and represent half of cord-blood B cells [48–50]. They frequently co-express the CD5 surface molecule that is also a specific marker for mouse B-1 cells, a particular B-cell subset that was historically identified as a major source of self-reactive and polyreactive antibodies [2, 18, 51–53]. Nevertheless, other B-cell populations apart from CD5+ B-1 lymphocytes were shown to secrete “natural” antibodies cross-reacting with self-antigens; one such population is marginal zone B cells that reside in the mouse spleen [49, 50, 54–56].
Although polyreactive IgM antibodies may circulate in complex with self-antigens [57], they bind to various invading pathogens such as viruses and bacteria, thereby facilitating their elimination [58–60]. Indeed, passive transfer of normal sera to animals, subsequently infected with bacteria or viruses, can confer protection against the infectious agent [58, 59, 61, 62]. More recently, polyreactive “natural” monoclonal antibodies were shown to possess broad antibacterial activity against gram-positive and gram-negative bacteria, and the ability to neutralize the activity of LPS endotoxin [60].
Polyreactivity in antigen-experienced post-germinal center B cells
Surprisingly, B cells can re-acquire poly- and self-reactivity during the germinal center reaction. Nearly 20% of post-germinal center IgG- and IgD-expressing memory B cells are polyreactive [63, 64], as compared to 6% on the naïve B cell compartment [23] (Fig. 1). Like the “natural” IgM antibodies, polyreactive IgG antibodies may also play an important role in the immediate response to infection as suggested by the increased susceptibility to infections in the absence of IgG in patients with hyper-IgM syndrome [65].
In addition to IgG, mouse and human IgA antibodies are also polyreactive [61, 66]; 25% of intestinal IgA-expressing plasmablasts show low levels of polyreactivity with self and foreign antigens, including commensal bacteria and rotavirus [67]. Importantly, the polyreactive IgAs, like IgGs, are somatically hypermutated indicating that the reactivity arose secondarily in the germinal center [66, 67]. IgA is the most abundant Ig isotype produced in mammals, and is believed to have a major role in the protection of mucosal surfaces against toxins, bacteria, viruses and protozoa [68].
Since the germinal center is the site of somatic hypermutation, the polyreactivity observed in the human IgG and IgD memory B cells, and IgA plasmablasts, likely represents a by-product of affinity maturation. Consistent with this idea, most of the germline precursors of polyreactive IgG memory B-cell antibodies from healthy donors or SLE patients were not poly- or self-reactive [63, 69]. In addition, germline-encoded antibodies might lose their polyreactivity when somatically mutated as a result of antigen-driven affinity maturation [70]. Although the amount of polyreactive binding by IgG memory antibodies is normally low and non-pathogenic, somatic hypermutations are responsible for creating pathogenic antibodies in patients and mice with autoimmune diseases where the checkpoints that remove autoreactive B cells are altered [71–74].
Serologic polyreactivity in viral infections
Although cross-reactivity to autoantigens or polyreactivity is strongly selected against during B-cell development [75, 76], it is a common serologic feature of certain viral infections in humans, including HIV [77], Epstein–Barr virus [78–82] and hepatitis viruses [83–86]. High incidence of serologic autoreactivity was also described in patients infected with chickenpox, measles and mumps viruses [87]. Finally, mice infected with vesicular stomatitis virus, vaccinia virus and lymphocytic choriomeningitis virus also develop polyreactive autoantibody responses [88–90].
The emergence of autoantibodies in virus-infected humans and mice, as well as the role of pathogens, such as viruses, in the development of certain autoimmune diseases has been studied for over 30 years. Several mechanisms have been suggested to explain the frequent association observed between infections and autoimmunity, including molecular mimicry between self- and foreign antigens, polyclonal T- and B-cell activation (bystander activation), and viral transformation of autoreactive B cells [89, 91–95]. However, in most cases little is known about the mechanisms that facilitate the emergence of these antibodies during infection.
The relationship between antibody polyreactivity and anti-viral immune responses is best documented for HIV. Humoral self-reactivity in humans infected with HIV was first documented 25 years ago shortly after the virus was isolated [96–98]. In addition, some of the IgG monoclonal autoantibodies isolated from HIV donors with high serum autoreactivity were shown to be somatically mutated and polyreactive, cross-reacting with multiple antigens [99]. Interestingly, another lentivirus of the Retroviridae family, Visna virus, which infects sheep, also triggers the development of IgM/IgG autoantibodies similar to those found in HIV patients [100].
Polyreactivity of HIV-specific antibodies
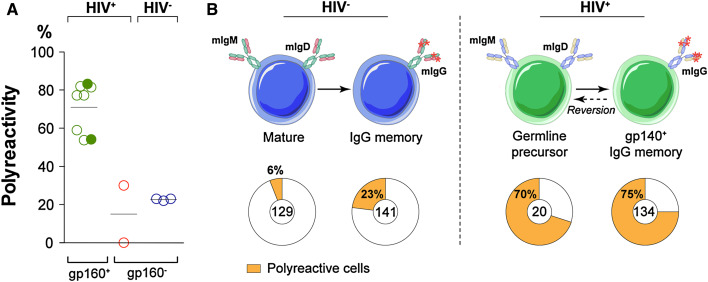
The HIV envelope protein is a trimeric glycoprotein composed of three gp120 monomers, each non-covalently bound to a transmembrane gp41 protein [101]. Two anti-HIV broad neutralizing antibodies, 2F5 and 4E10, that target a linear epitope of the membrane-proximal external region (MPER) of gp41, were found to be polyspecific and reactive against membrane phospholipids, i.e., cardiolipin [34, 102–104]. Importantly, the polyreactivity of gp160-specific IgG antibodies is a more general feature of the human memory antibody response to HIV-1. Indeed, the characterization of 134 unique anti-gp160 antibodies, isolated from IgG memory B cells in HIV clade B-infected donors, showed that the vast majority were polyreactive (75 vs. ~20% in controls), cross-reacting with both foreign and self-antigens (Fig. 2a) [105, 106]. Anti-gp41 antibodies were the most polyreactive and lipid-reactive (85–90%), as confirmed later for antibodies to the cluster II region of gp41 [107], and most recently for other human antibodies to HIV-1 gp41 [108, 109]. Although polyreactive anti-gp160 IgG antibodies isolated from clade-B infected donors have somewhat longer and more hydrophobic IgH CDR3s, the correlation was neither strong nor predictive [21–23, 106]. Similar findings were recently reported for IgG antibodies cloned from HIV clade A-infected donors (Fig. 2a) [110], strongly supporting the idea that polyreactivity is a conserved feature of HIV-specific antibodies.
Fig. 2.
Polyreactivity of anti-HIV gp160 antibodies. a Frequency of polyreactive anti-gp160 IgG memory B-cell antibodies isolated from HIV patients (HIV+ gp160+) infected with clade B (open green circles) or clade A (filled green circles) viruses compared to non gp160-binding B-cell antibodies from 2 of the clade B patients (red circles, HIV+ gp160−) [105, 106, 110], and historical control IgG memory B-cell antibodies isolated from healthy donors (HIV− gp160−) [63]. Each symbol represents a donor. b Evolution of antibody polyreactivity throughout affinity maturation (transition from mature naïve/germline precursor B cells to IgG memory B cells) for B cells in healthy donors (HIV−) and gp160-specific B cells in HIV patients (HIV+). Pie charts summarize the frequency of polyreactive (orange) and non-polyreactive (white) antibodies isolated from the B-cell compartments in HIV-infected and healthy donors indicated by the schematic diagram. The number of antibodies tested is indicated in the center of the pie chart [39, 63, 106]
Ig genes encoding high affinity antibodies to HIV-1 envelope protein are highly hypermutated [105, 110–112]. Mutation is essential for both specificity and breadth of the response as demonstrated by the finding that the germline versions of broad and potent neutralizers are generally far less active and more restricted in breadth than the mature antibody [106, 111, 113]. Surprisingly, however, the germline precursors of anti-HIV antibodies remained polyreactive (70 vs. 6% in the overall naïve repertoire) (Fig. 2b) [23, 39], indicating that polyspecific B cells may be positively selected during the anti-HIV antibody response even before the germinal center reaction [106]. This raises the fascinating question of whether polyreactive naïve B cells are able to interact with HIV and ipso facto, are preferentially recruited to mount a specific adaptive B-cell response against the virus antigens [114], as recently suggested for gp160 [109]. In support of this idea, polyspecific monoclonal antibodies/sera isolated from non-infected humans or from patients with autoimmune diseases can cross-react with viral antigens (i.e., gp120, p24) and/or bind to HIV [109, 115–118].
In conclusion, although different mechanisms may account for the emergence of self-reactive/polyreactive antibodies in HIV-infected patients [119, 120], HIV gp160-specific antibodies frequently display polyreactivity, which may be functionally important.
Why are HIV-specific antibodies polyreactive?
In addition to the rapid mutation, several structural features of the HIV envelope protein make it a poor target for antibodies. These include: (1) carbohydrate shielding [121, 122], (2) conformation masking [123], (3) steric occlusion [124, 125] and (4) transient epitope exposure [126]. Besides these well-established “defense” mechanisms, the low density of functional HIV gp160 expressed at the viral surface may render the anti-HIV antibodies less efficient for viral neutralization by impeding bivalent binding to the virus.
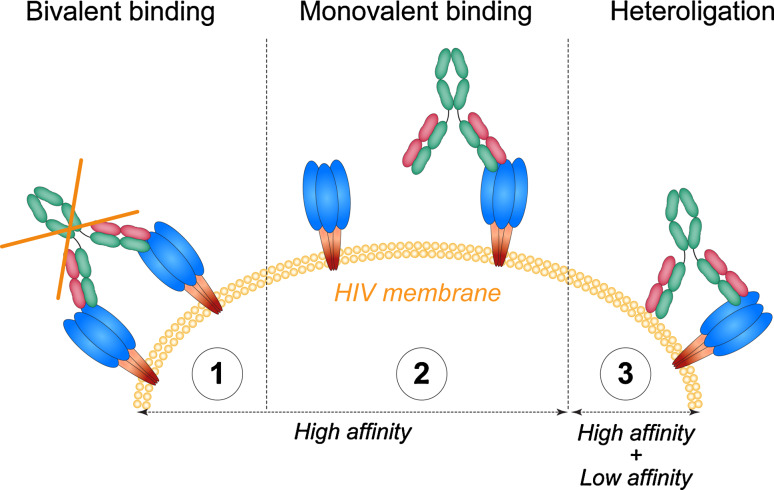
As measured by cryoelectron tomography, mature HIV viruses express only 10–15 randomly distributed viral spikes [127], which would be spaced too far apart (145 nm) for a bivalent antibody to bridge [128, 129]. Bivalent binding is an important property of antibody binding, which increases their relative binding affinity (avidity) to their targets. We have proposed that the low spike density on HIV exerts selective pressure that favors heterotypic bivalent interactions. For example, 2F5 and 4E10 bind to their epitope in the gp41 MPER, which is buried in the HIV lipid membrane, and interact simultaneously with virion lipids [130–136]. Because these polyreactive antibodies bind to lipid alone [34, 103, 132, 137, 138], it has been proposed that lipid binding by 2F5 and 4E10 increases the concentration of antibody, allowing it to “surf” the HIV virion in the vicinity of their specific epitopes on gp41 [136]. Importantly, the interaction of lipophilic residues in the 4E10 and 2F5 IgH CDR3s with HIV membrane lipids was shown to be required for their neutralizing potency [136, 139].
Heterotypic binding by anti-HIV antibodies is not limited to polyreactive lipid-binding antibodies. For example, the anti-gp120 antibody, 21c, which targets the CD4-induced co-receptor binding site of gp120 (CD4i), also binds to the CD4 molecule alone, or to both CD4i and CD4 when it is complexed with gp120 [140]. Finally, polyreactive anti-gp160 antibodies are capable of bivalent heterotypic binding or heteroligation between one high-affinity anti-HIV gp160 combining site and a second low-affinity site on currently undefined HIV-1 virus surface components [106] (Fig. 3). This unusual form of bivalent binding, where one antibody arm binds to a specific ligand and the second to a hetero-ligand, increases the antibody's apparent affinity for the virus and may in part account for the positive selection of polyreactive antibodies in the anti-HIV response in humans [106]. Heteroligation may also enhance the potency of recently described broadly neutralizing glyco-peptide-specific antibodies [141]. These antibodies bind specifically to the gp120-V3 loop and to surrounding glycans, but also have high affinity for glycans alone, which would allow for heteroligation [106, 141].
Fig. 3.
Heteroligation of polyreactive HIV gp160-specific antibodies. gp160 glycoprotein is expressed at a very low density on the HIV surface (10–15 spikes) [127], indicating that the viral spikes are spaced too far apart for a bivalent antibody to bridge [128, 129] (1). Therefore, non-polyreactive anti-gp160 antibodies likely bind to their target with only one of their two high-affinity binding sites (monovalent binding) (2). In contrast, polyreactive anti-HIV gp160 antibodies are able to bind bivalently to the virus by heteroligation between one high-affinity anti-HIV-gp160 combining site and a second low-affinity site on another molecular structure on the HIV virion (3)
Implications for vaccine development
Although not all broadly neutralizing antibodies isolated from HIV-infected humans show polyspecificity [113, 142, 143], many of them are polyreactive and cross-react with autoantigens [34, 111, 144]. Since most polyreactive B cells are removed from the repertoire early in B cell development [23, 75, 76], it has been suggested that tolerance might limit the production of broadly neutralizing HIV-1 antibodies [34, 102]. In support of this idea, knock-in B cells expressing the polyreactive IgG 2F5, a broadly neutralizing anti-HIV antibody, failed to pass central and peripheral tolerance checkpoints [145, 146]. However, the polyreactive precursors of most HIV antibodies are far less reactive than 2F5 [106, 109, 111]. In addition, chronic infection may interfere with normal selection mechanisms. Moreover, these polyreactive cells are present in low numbers in normal healthy individuals and are not pathogenic like the autoantibodies produced in the course of systemic autoimmune diseases [147, 148]. Thus, polyreactive B cells are not completely removed from the B-cell repertoire and may even be beneficial to combat the infection as discussed above. It is therefore unlikely that polyreactivity would be a significant barrier to anti-HIV vaccine development.
Conclusion and future directions
In this review, we discussed infection-associated antibody polyreactivity focusing primarily on HIV since it is the best-studied example. Since exceptionally broad anti-HIV antibodies can effectively protect animals from infection, it has been proposed that it might be possible to vaccinate against HIV by eliciting such antibodies. Since polyreactivity is linked to the specific B-cell response to HIV, it is therefore crucial to understand how such reactivity might impact vaccine development. It may be equally important to consider and study polyreactivity in antibody-based therapies against other viral infections in humans such as influenza virus [149, 150], dengue virus [151, 152] and cytomegalovirus [153].
Acknowledgments
We would like to thank Dr. Hedda Wardemann, Dr. Rushad Pavri and Caroline Eden for helpful comments on the manuscript.
Abbreviations
- BCR
B-cell receptor
- CD4i
CD4 induced co-receptor binding site
- CDR3
Complementary determining region 3
- HIV
Human immunodeficiency virus
- Ig
Immunoglobulin
- IgH
Ig-heavy chain
- IgVH
Ig-heavy chain variable region
- MPER
Membrane-proximal external region
- SLE
Systemic lupus erythematotus
References
- 1.Landsteiner K, Pauling L, Landsteiner EK. The specificity of serological reactions. Rev. edn. Cambridge: Harvard University Press; 1945. [Google Scholar]
- 2.Casali P, Notkins AL. Probing the human B-cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Annu Rev Immunol. 1989;7:513–535. doi: 10.1146/annurev.iy.07.040189.002501. [DOI] [PubMed] [Google Scholar]
- 3.Bouvet JP, Dighiero G. From natural polyreactive autoantibodies to a la carte monoreactive antibodies to infectious agents: is it a small world after all? Infect Immun. 1998;66(1):1–4. doi: 10.1128/iai.66.1.1-4.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Notkins AL. Polyreactivity of antibody molecules. Trends Immunol. 2004;25(4):174–179. doi: 10.1016/j.it.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Zhou ZH, Tzioufas AG, Notkins AL. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J Autoimmun. 2007;29(4):219–228. doi: 10.1016/j.jaut.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisen HN, Chakraborty AK. Evolving concepts of specificity in immune reactions. Proc Natl Acad Sci USA. 2010;107(52):22373–22380. doi: 10.1073/pnas.1012051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilbert B, Dighiero G, Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982;128(6):2779–2787. [PubMed] [Google Scholar]
- 8.Dighiero G, Guilbert B, Avrameas S. Naturally occurring antibodies against nine common antigens in humans sera. II. High incidence of monoclonal Ig exhibiting antibody activity against actin and tubulin and sharing antibody specificities with natural antibodies. J Immunol. 1982;128(6):2788–2792. [PubMed] [Google Scholar]
- 9.Dighiero G, Lymberi P, Mazie JC, Rouyre S, Butler-Browne GS, Whalen RG, Avrameas S. Murine hybridomas secreting natural monoclonal antibodies reacting with self antigens. J Immunol. 1983;131(5):2267–2272. [PubMed] [Google Scholar]
- 10.Dighiero G, Guilbert B, Fermand JP, Lymberi P, Danon F, Avrameas S. Thirty-six human monoclonal immunoglobulins with antibody activity against cytoskeleton proteins, thyroglobulin, and native DNA: immunologic studies and clinical correlations. Blood. 1983;62(2):264–270. [PubMed] [Google Scholar]
- 11.Eisen HN, Michaelides MC, Underdown BJ, Schulenburg EP, Simms ES. Experimental approaches to homogenous antibody populations. Myeloma proteins with antihapten antibody activity. Fed Proc. 1970;29(1):78–84. [PubMed] [Google Scholar]
- 12.Michaelides MC, Eisen HN. The strange cross-reaction of menadione (vitamin K3) and 2, 4-dinitrophenyl ligands with a myeloma protein and some conventional antibodies. J Exp Med. 1974;140(3):687–702. doi: 10.1084/jem.140.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James LC, Tawfik DS. The specificity of cross-reactivity: promiscuous antibody binding involves specific hydrogen bonds rather than nonspecific hydrophobic stickiness. Protein Sci. 2003;12(10):2183–2193. doi: 10.1110/ps.03172703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keitel T, Kramer A, Wessner H, Scholz C, Schneider-Mergener J, Hohne W. Crystallographic analysis of anti-p24 (HIV-1) monoclonal antibody cross-reactivity and polyspecificity. Cell. 1997;91(6):811–820. doi: 10.1016/S0092-8674(00)80469-9. [DOI] [PubMed] [Google Scholar]
- 15.Kramer A, Keitel T, Winkler K, Stocklein W, Hohne W, Schneider-Mergener J. Molecular basis for the binding promiscuity of an anti-p24 (HIV-1) monoclonal antibody. Cell. 1997;91(6):799–809. doi: 10.1016/S0092-8674(00)80468-7. [DOI] [PubMed] [Google Scholar]
- 16.Shlomchik MJ, Aucoin AH, Pisetsky DS, Weigert MG. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci USA. 1987;84(24):9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchalonis JJ, Adelman MK, Robey IF, Schluter SF, Edmundson AB. Exquisite specificity and peptide epitope recognition promiscuity, properties shared by antibodies from sharks to humans. J Mol Recognit. 2001;14(2):110–121. doi: 10.1002/jmr.527. [DOI] [PubMed] [Google Scholar]
- 18.Casali P, Burastero SE, Nakamura M, Inghirami G, Notkins AL. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to Leu-1+ B-cell subset. Science. 1987;236(4797):77–81. doi: 10.1126/science.3105056. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura M, Burastero SE, Ueki Y, Larrick JW, Notkins AL, Casali P. Probing the normal and autoimmune B cell repertoire with Epstein-Barr virus. Frequency of B cells producing monoreactive high affinity autoantibodies in patients with Hashimoto’s disease and systemic lupus erythematosus. J Immunol. 1988;141(12):4165–4172. [PubMed] [Google Scholar]
- 20.Nakamura M, Burastero SE, Notkins AL, Casal P. Human monoclonal rheumatoid factor-like antibodies from CD5 (Leu-1) + B cells are polyreactive. J Immunol. 1988;140(12):4180–4186. [PubMed] [Google Scholar]
- 21.Ichiyoshi Y, Casali P. Analysis of the structural correlates for antibody polyreactivity by multiple reassortments of chimeric human immunoglobulin heavy and light chain V segments. J Exp Med. 1994;180(3):885–895. doi: 10.1084/jem.180.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguilera I, Melero J, Nunez-Roldan A, Sanchez B. Molecular structure of eight human autoreactive monoclonal antibodies. Immunology. 2001;102(3):273–280. doi: 10.1046/j.1365-2567.2001.01159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 24.Wedemayer GJ, Patten PA, Wang LH, Schultz PG, Stevens RC. Structural insights into the evolution of an antibody combining site. Science. 1997;276(5319):1665–1669. doi: 10.1126/science.276.5319.1665. [DOI] [PubMed] [Google Scholar]
- 25.Manivel V, Sahoo NC, Salunke DM, Rao KV. Maturation of an antibody response is governed by modulations in flexibility of the antigen-combining site. Immunity. 2000;13(5):611–620. doi: 10.1016/S1074-7613(00)00061-3. [DOI] [PubMed] [Google Scholar]
- 26.Jimenez R, Salazar G, Baldridge KK, Romesberg FE. Flexibility and molecular recognition in the immune system. Proc Natl Acad Sci USA. 2003;100(1):92–97. doi: 10.1073/pnas.262411399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnan L, Lomash S, Raj BP, Kaur KJ, Salunke DM. Paratope plasticity in diverse modes facilitates molecular mimicry in antibody response. J Immunol. 2007;178(12):7923–7931. doi: 10.4049/jimmunol.178.12.7923. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan L, Sahni G, Kaur KJ, Salunke DM. Role of antibody paratope conformational flexibility in the manifestation of molecular mimicry. Biophys J. 2008;94(4):1367–1376. doi: 10.1529/biophysj.107.108654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foote J, Milstein C. Conformational isomerism and the diversity of antibodies. Proc Natl Acad Sci USA. 1994;91(22):10370–10374. doi: 10.1073/pnas.91.22.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma B, Shatsky M, Wolfson HJ, Nussinov R. Multiple diverse ligands binding at a single protein site: a matter of pre-existing populations. Protein Sci. 2002;11(2):184–197. doi: 10.1110/ps.21302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhat TN, Bentley GA, Fischmann TO, Boulot G, Poljak RJ. Small rearrangements in structures of Fv and Fab fragments of antibody D1.3 on antigen binding. Nature. 1990;347(6292):483–485. doi: 10.1038/347483a0. [DOI] [PubMed] [Google Scholar]
- 32.Sethi DK, Agarwal A, Manivel V, Rao KV, Salunke DM. Differential epitope positioning within the germline antibody paratope enhances promiscuity in the primary immune response. Immunity. 2006;24(4):429–438. doi: 10.1016/j.immuni.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Mariuzza RA. Multiple paths to multispecificity. Immunity. 2006;24(4):359–361. doi: 10.1016/j.immuni.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308(5730):1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 35.Bostrom J, Yu SF, Kan D, Appleton BA, Lee CV, Billeci K, Man W, Peale F, Ross S, Wiesmann C, Fuh G. Variants of the antibody herceptin that interact with HER2 and VEGF at the antigen binding site. Science. 2009;323(5921):1610–1614. doi: 10.1126/science.1165480. [DOI] [PubMed] [Google Scholar]
- 36.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 37.Weigert MG, Cesari IM, Yonkovich SJ, Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970;228(5276):1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- 38.Goodnow CC, Sprent J, Fazekas de St Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435(7042):590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 39.Tsuiji M, Yurasov S, Velinzon K, Thomas S, Nussenzweig MC, Wardemann H. A checkpoint for autoreactivity in human IgM+ memory B cell development. J Exp Med. 2006;203(2):393–400. doi: 10.1084/jem.20052033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yurasov S, Tiller T, Tsuiji M, Velinzon K, Pascual V, Wardemann H, Nussenzweig MC. Persistent expression of autoantibodies in SLE patients in remission. J Exp Med. 2006;203(10):2255–2261. doi: 10.1084/jem.20061446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201(5):703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp Med. 2005;201(10):1659–1667. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menard L, Samuels J, Ng YS, Meffre E. Inflammation-independent defective early B cell tolerance checkpoints in rheumatoid arthritis. Arthritis Rheum. 2011;63(5):1237–1245. doi: 10.1002/art.30164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menard L, Saadoun D, Isnardi I, Ng YS, Meyers G, Massad C, Price C, Abraham C, Motaghedi R, Buckner JH, Gregersen PK, Meffre E (2011) The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest. doi:10.1172/JCI45790 [DOI] [PMC free article] [PubMed]
- 45.Ng YS, Wardemann H, Chelnis J, Cunningham-Rundles C, Meffre E. Bruton’s tyrosine kinase is essential for human B cell tolerance. J Exp Med. 2004;200(7):927–934. doi: 10.1084/jem.20040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isnardi I, Ng YS, Srdanovic I, Motaghedi R, Rudchenko S, von Bernuth H, Zhang SY, Puel A, Jouanguy E, Picard C, Garty BZ, Camcioglu Y, Doffinger R, Kumararatne D, Davies G, Gallin JI, Haraguchi S, Day NK, Casanova JL, Meffre E. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29(5):746–757. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herve M, Xu K, Ng YS, Wardemann H, Albesiano E, Messmer BT, Chiorazzi N, Meffre E. Unmutated and mutated chronic lymphocytic leukemias derive from self-reactive B cell precursors despite expressing different antibody reactivity. J Clin Invest. 2005;115(6):1636–1643. doi: 10.1172/JCI24387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen ZJ, Wheeler CJ, Shi W, Wu AJ, Yarboro CH, Gallagher M, Notkins AL. Polyreactive antigen-binding B cells are the predominant cell type in the newborn B cell repertoire. Eur J Immunol. 1998;28(3):989–994. doi: 10.1002/(SICI)1521-4141(199803)28:03<989::AID-IMMU989>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 49.Chen ZJ, Wheeler J, Notkins AL. Antigen-binding B cells and polyreactive antibodies. Eur J Immunol. 1995;25(2):579–586. doi: 10.1002/eji.1830250241. [DOI] [PubMed] [Google Scholar]
- 50.Wang Z, Chen ZJ, Wheeler J, Shen S, Notkins AL. Characterization of murine polyreactive antigen-binding B cells: presentation of antigens to T cells. Eur J Immunol. 2001;31(4):1106–1114. doi: 10.1002/1521-4141(200104)31:4<1106::AID-IMMU1106>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 51.Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burastero SE, Casali P. Characterization of human CD5 (Leu-1, OKT1)+ B lymphocytes and the antibodies they produce. Contrib Microbiol Immunol. 1989;11:231–262. [PubMed] [Google Scholar]
- 53.Casali P, Notkins AL. CD5+ B lymphocytes, polyreactive antibodies and the human B-cell repertoire. Immunol Today. 1989;10(11):364–368. doi: 10.1016/0167-5699(89)90268-5. [DOI] [PubMed] [Google Scholar]
- 54.Martin F, Kearney JF. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity. 2000;12(1):39–49. doi: 10.1016/S1074-7613(00)80157-0. [DOI] [PubMed] [Google Scholar]
- 55.Li Y, Li H, Ni D, Weigert M. Anti-DNA B cells in MRL/lpr mice show altered differentiation and editing pattern. J Exp Med. 2002;196(12):1543–1552. doi: 10.1084/jem.20021560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou ZH, Notkins AL. Polyreactive antigen-binding B (PAB-) cells are widely distributed and the PAB population consists of both B-1+ and B-1- phenotypes. Clin Exp Immunol. 2004;137(1):88–100. doi: 10.1111/j.1365-2249.2004.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sigounas G, Kolaitis N, Monell-Torrens E, Notkins AL. Polyreactive IgM antibodies in the circulation are masked by antigen binding. J Clin Immunol. 1994;14(6):375–381. doi: 10.1007/BF01546322. [DOI] [PubMed] [Google Scholar]
- 58.Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, Zinkernagel RM. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286(5447):2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 59.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192(2):271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou ZH, Zhang Y, Hu YF, Wahl LM, Cisar JO, Notkins AL. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host Microbe. 2007;1(1):51–61. doi: 10.1016/j.chom.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quan CP, Berneman A, Pires R, Avrameas S, Bouvet JP. Natural polyreactive secretory immunoglobulin A autoantibodies as a possible barrier to infection in humans. Infect Immun. 1997;65(10):3997–4004. doi: 10.1128/iai.65.10.3997-4004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boes M, Prodeus AP, Schmidt T, Carroll MC, Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J Exp Med. 1998;188(12):2381–2386. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26(2):205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koelsch K, Zheng NY, Zhang Q, Duty A, Helms C, Mathias MD, Jared M, Smith K, Capra JD, Wilson PC. Mature B cells class switched to IgD are autoreactive in healthy individuals. J Clin Invest. 2007;117(6):1558–1565. doi: 10.1172/JCI27628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quartier P, Bustamante J, Sanal O, Plebani A, Debre M, Deville A, Litzman J, Levy J, Fermand JP, Lane P, Horneff G, Aksu G, Yalcin I, Davies G, Tezcan I, Ersoy F, Catalan N, Imai K, Fischer A, Durandy A. Clinical, immunologic and genetic analysis of 29 patients with autosomal recessive hyper-IgM syndrome due to activation-induced cytidine deaminase deficiency. Clin Immunol. 2004;110(1):22–29. doi: 10.1016/j.clim.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 66.Shimoda M, Inoue Y, Azuma N, Kanno C. Natural polyreactive immunoglobulin A antibodies produced in mouse Peyer’s patches. Immunology. 1999;97(1):9–17. doi: 10.1046/j.1365-2567.1999.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benckert J, Schmolka N, Kreschel C, Zoller MJ, Sturm A, Wiedenmann B, Wardemann H. The majority of intestinal IgA+ and IgG+ plasmablasts in the human gut are antigen-specific. J Clin Invest. 2011;121(5):1946–1955. doi: 10.1172/JCI44447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaetzel CS. Mucosal immune defense: immunoglobulin A. New York: Springer; 2007. [Google Scholar]
- 69.Mietzner B, Tsuiji M, Scheid J, Velinzon K, Tiller T, Abraham K, Gonzalez JB, Pascual V, Stichweh D, Wardemann H, Nussenzweig MC. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci USA. 2008;105(28):9727–9732. doi: 10.1073/pnas.0803644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oppezzo P, Dumas G, Bouvet JP, Robello C, Cayota A, Pizarro JC, Dighiero G, Pritsch O. Somatic mutations can lead to a loss of superantigenic and polyreactive binding. Eur J Immunol. 2004;34(5):1423–1432. doi: 10.1002/eji.200424936. [DOI] [PubMed] [Google Scholar]
- 71.Radic MZ, Mascelli MA, Erikson J, Shan H, Shlomchik M, Weigert M. Structural patterns in anti-DNA antibodies from MRL/lpr mice. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 2):933–946. doi: 10.1101/SQB.1989.054.01.108. [DOI] [PubMed] [Google Scholar]
- 72.Radic MZ, Mackle J, Erikson J, Mol C, Anderson WF, Weigert M. Residues that mediate DNA binding of autoimmune antibodies. J Immunol. 1993;150(11):4966–4977. [PubMed] [Google Scholar]
- 73.Wellmann U, Letz M, Herrmann M, Angermuller S, Kalden JR, Winkler TH. The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci USA. 2005;102(26):9258–9263. doi: 10.1073/pnas.0500132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo W, Smith D, Aviszus K, Detanico T, Heiser RA, Wysocki LJ. Somatic hypermutation as a generator of antinuclear antibodies in a murine model of systemic autoimmunity. J Exp Med. 2010;207(10):2225–2237. doi: 10.1084/jem.20092712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol. 2008;20(6):632–638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 76.Wardemann H, Nussenzweig MC. B-cell self-tolerance in humans. Adv Immunol. 2007;95:83–110. doi: 10.1016/S0065-2776(07)95003-8. [DOI] [PubMed] [Google Scholar]
- 77.Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1(6):329–337. doi: 10.1016/S1568-9972(02)00086-1. [DOI] [PubMed] [Google Scholar]
- 78.Linder E, Kurki P, Andersson LC. Autoantibody to “intermediate filament” in infectious mononucleosis. Clin Immunol Immunopathol. 1979;14(4):411–417. doi: 10.1016/0090-1229(79)90093-X. [DOI] [PubMed] [Google Scholar]
- 79.Kataaha PK, Mortazavi-Milani SM, Russell G, Holborow EJ. Anti-intermediate filament antibodies, antikeratin antibody, and antiperinuclear factor in rheumatoid arthritis and infectious mononucleosis. Ann Rheum Dis. 1985;44(7):446–449. doi: 10.1136/ard.44.7.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Misra R, Venables PJ, Plater-Zyberk C, Watkins PF, Maini RN. Anti-cardiolipin antibodies in infectious mononucleosis react with the membrane of activated lymphocytes. Clin Exp Immunol. 1989;75(1):35–40. [PMC free article] [PubMed] [Google Scholar]
- 81.Mascia MT, Sandri G, Guerzoni C, Roncaglia R, Mantovani G, Ferri C. Detection of autoimmunity in early primary Epstein-Barr virus infection by Western blot analysis. Clin Exp Rheumatol. 2008;26(6):1034–1039. [PubMed] [Google Scholar]
- 82.Niller HH, Wolf H, Minarovits J. Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases. Autoimmunity. 2008;41(4):298–328. doi: 10.1080/08916930802024772. [DOI] [PubMed] [Google Scholar]
- 83.Lidman K, Biberfeld G, Fagraeus A, Norberg R, Torstensson R, Utter G, Carlsson L, Luca J, Lindberg U. Anti-actin specificity of human smooth muscle antibodies in chronic active hepatitis. Clin Exp Immunol. 1976;24(2):266–272. [PMC free article] [PubMed] [Google Scholar]
- 84.Kurki P, Virtanen I, Stenman S, Linder E. Characterization of human smooth muscle autoantibodies reacting with cytoplasmic intermediate filaments. Clin Immunol Immunopathol. 1978;11(4):379–387. doi: 10.1016/0090-1229(78)90165-4. [DOI] [PubMed] [Google Scholar]
- 85.Vento S, McFarlane BM, McSorley CG, Ranieri S, Giuliani-Piccari G, Dal Monte PR, Verucchi G, Williams R, Chiodo F, McFarlane IG. Liver autoreactivity in acute virus A, B and non-A, non-B hepatitis. J Clin Lab Immunol. 1988;25(1):1–7. [PubMed] [Google Scholar]
- 86.Cassani F, Cataleta M, Valentini P, Muratori P, Giostra F, Francesconi R, Muratori L, Lenzi M, Bianchi G, Zauli D, Bianchi FB. Serum autoantibodies in chronic hepatitis C: comparison with autoimmune hepatitis and impact on the disease profile. Hepatology. 1997;26(3):561–566. doi: 10.1002/hep.510260305. [DOI] [PubMed] [Google Scholar]
- 87.Toh BH, Yildiz A, Sotelo J, Osung O, Holborow EJ, Kanakoudi F, Small JV. Viral infections and IgM autoantibodies to cytoplasmic intermediate filaments. Clin Exp Immunol. 1979;37(1):76–82. [PMC free article] [PubMed] [Google Scholar]
- 88.Dales S, Fujinami RS, Oldstone MB. Infection with vaccinia favors the selection of hybridomas synthesizing autoantibodies against intermediate filaments, one of them cross-reacting with the virus hemagglutinin. J Immunol. 1983;131(3):1546–1553. [PubMed] [Google Scholar]
- 89.Hunziker L, Recher M, Macpherson AJ, Ciurea A, Freigang S, Hengartner H, Zinkernagel RM. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infections. Nat Immunol. 2003;4(4):343–349. doi: 10.1038/ni911. [DOI] [PubMed] [Google Scholar]
- 90.Ludewig B, Krebs P, Metters H, Tatzel J, Tureci O, Sahin U. Molecular characterization of virus-induced autoantibody responses. J Exp Med. 2004;200(5):637–646. doi: 10.1084/jem.20040358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Munz C, Lunemann JD, Getts MT, Miller SD. Antiviral immune responses: triggers of or triggered by autoimmunity? Nat Rev Immunol. 2009;9(4):246–258. doi: 10.1038/nri2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fujinami RS, Oldstone MB, Wroblewska Z, Frankel ME, Koprowski H. Molecular mimicry in virus infection: crossreaction of measles virus phosphoprotein or of herpes simplex virus protein with human intermediate filaments. Proc Natl Acad Sci USA. 1983;80(8):2346–2350. doi: 10.1073/pnas.80.8.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Coutelier JP, Coulie PG, Wauters P, Heremans H, van der Logt JT. In vivo polyclonal B-lymphocyte activation elicited by murine viruses. J Virol. 1990;64(11):5383–5388. doi: 10.1128/jvi.64.11.5383-5388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lunemann JD, Jelcic I, Roberts S, Lutterotti A, Tackenberg B, Martin R, Munz C. EBNA1-specific T cells from patients with multiple sclerosis cross react with myelin antigens and co-produce IFN-gamma and IL-2. J Exp Med. 2008;205(8):1763–1773. doi: 10.1084/jem.20072397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lunemann JD, Munz C. EBV in MS: guilty by association? Trends Immunol. 2009;30(6):243–248. doi: 10.1016/j.it.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 96.Hoxie JA, Fitzharris TP, Youngbar PR, Matthews DM, Rackowski JL, Radka SF. Nonrandom association of cellular antigens with HTLV-III virions. Hum Immunol. 1987;18(1):39–52. doi: 10.1016/0198-8859(87)90111-X. [DOI] [PubMed] [Google Scholar]
- 97.Kopelman RG, Zolla-Pazner S. Association of human immunodeficiency virus infection and autoimmune phenomena. Am J Med. 1988;84(1):82–88. doi: 10.1016/0002-9343(88)90012-5. [DOI] [PubMed] [Google Scholar]
- 98.Solinger AM, Adams LE, Friedman-Kien AE, Hess EV. Acquired immune deficiency syndrome (AIDS) and autoimmunity–mutually exclusive entities? J Clin Immunol. 1988;8(1):32–42. doi: 10.1007/BF00915154. [DOI] [PubMed] [Google Scholar]
- 99.Ditzel HJ, Itoh K, Burton DR. Determinants of polyreactivity in a large panel of recombinant human antibodies from HIV-1 infection. J Immunol. 1996;157(2):739–749. [PubMed] [Google Scholar]
- 100.Harkiss GD, Veitch D, Dickson L, Watt NJ. Autoimmune reactivity in sheep induced by the visna retrovirus. J Autoimmun. 1993;6(1):63–75. doi: 10.1006/jaut.1993.1006. [DOI] [PubMed] [Google Scholar]
- 101.Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, Sodroski JG. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393(6686):705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 102.Haynes BF, Moody MA, Verkoczy L, Kelsoe G, Alam SM. Antibody polyspecificity and neutralization of HIV-1: a hypothesis. Hum Antibodies. 2005;14(3–4):59–67. [PMC free article] [PubMed] [Google Scholar]
- 103.Alam SM, McAdams M, Boren D, Rak M, Scearce RM, Gao F, Camacho ZT, Gewirth D, Kelsoe G, Chen P, Haynes BF. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J Immunol. 2007;178(7):4424–4435. doi: 10.4049/jimmunol.178.7.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Matyas GR, Beck Z, Karasavvas N, Alving CR. Lipid binding properties of 4E10, 2F5, and WR304 monoclonal antibodies that neutralize HIV-1. Biochim Biophys Acta. 2009;1788(3):660–665. doi: 10.1016/j.bbamem.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 105.Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, Phogat A, Chakrabarti B, Li Y, Connors M, Pereyra F, Walker BD, Wardemann H, Ho D, Wyatt RT, Mascola JR, Ravetch JV, Nussenzweig MC. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458(7238):636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 106.Mouquet H, Scheid JF, Zoller MJ, Krogsgaard M, Ott RG, Shukair S, Artyomov MN, Pietzsch J, Connors M, Pereyra F, Walker BD, Ho DD, Wilson PC, Seaman MS, Eisen HN, Chakraborty AK, Hope TJ, Ravetch JV, Wardemann H, Nussenzweig MC. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467(7315):591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dennison SM, Anasti K, Scearce RM, Sutherland L, Parks R, Xia SM, Liao HX, Gorny MK, Zolla-Pazner S, Haynes BF, Alam SM. Nonneutralizing HIV-1 gp41 envelope cluster II human monoclonal antibodies show polyreactivity for binding to phospholipids and protein autoantigens. J Virol. 2011;85(3):1340–1347. doi: 10.1128/JVI.01680-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morris L, Chen X, Alam M, Tomaras G, Zhang R, Marshall DJ, Chen B, Parks R, Foulger A, Jaeger F, Donathan M, Bilska M, Gray ES, Abdool Karim SS, Kepler TB, Whitesides J, Montefiori D, Moody MA, Liao HX, Haynes BF (2011) Isolation of a Human anti-HIV gp41 membrane proximal region neutralizing antibody by antigen-specific single B cell sorting. PLoS One 6 (9):e23532. doi:10.1371/journal.pone.0023532 [DOI] [PMC free article] [PubMed]
- 109.Liao HX, Chen X, Munshaw S, Zhang R, Marshall DJ, Vandergrift N, Whitesides JF, Lu X, Yu JS, Hwang KK, Gao F, Markowitz M, Heath SL, Bar KJ, Goepfert PA, Montefiori DC, Shaw GC, Alam SM, Margolis DM, Denny TN, Boyd SD, Marshal E, Egholm M, Simen BB, Hanczaruk B, Fire AZ, Voss G, Kelsoe G, Tomaras GD, Moody MA, Kepler TB, Haynes BF (2011) Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. J Exp Med. doi:10.1084/jem.20110363 [DOI] [PMC free article] [PubMed]
- 110.Mouquet H, Klein F, Scheid JF, Warncke M, Pietzsch J, Oliveira TY, Velinzon K, Seaman MS, Nussenzweig MC (2011) Memory B cell antibodies to HIV-1 gp140 cloned from individuals infected with clade A and B viruses. PLoS One 6 (9):e24078. doi:10.1371/journal.pone.0024078 [DOI] [PMC free article] [PubMed]
- 111.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Olivera TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton D, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC (2011) Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. doi:10.1126/science.1207227 [DOI] [PMC free article] [PubMed]
- 112.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O’Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329(5993):856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, Kwon YD, Scheid JF, Shi W, Xu L, Yang Y, Zhu J, Nussenzweig MC, Sodroski J, Shapiro L, Nabel GJ, Mascola JR, Kwong PD. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329(5993):811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ishigatsubo Y, Steinberg AD, Krieg A, Klinman DM. Increased utilization of polyreactive B cells during periods of generalized immune activation. Autoimmunity. 1995;22(2):113–119. doi: 10.3109/08916939508995307. [DOI] [PubMed] [Google Scholar]
- 115.Llorente M, Sanchez-Palomino S, Manes S, Lucas P, Kremer L, De Alboran IM, Toran JL, Alcami J, Del Real G, Martinez AC. Natural human antibodies retrieved by phage display libraries from healthy donors: polyreactivity and recognition of human immunodeficiency virus type 1gp120 epitopes. Scand J Immunol. 1999;50(3):270–279. doi: 10.1046/j.1365-3083.1999.00516.x. [DOI] [PubMed] [Google Scholar]
- 116.Karle S, Planque S, Nishiyama Y, Taguchi H, Zhou YX, Salas M, Lake D, Thiagarajan P, Arnett F, Hanson CV, Paul S. Cross-clade HIV-1 neutralization by an antibody fragment from a lupus phage display library. AIDS. 2004;18(2):329–331. doi: 10.1097/00002030-200401230-00026. [DOI] [PubMed] [Google Scholar]
- 117.Mancini N, Perotti M, Carletti S, Canducci F, Sampaolo M, Clementi M, Burioni R. Cloning and molecular characterization of a human recombinant IgG Fab binding to the Tat protein of human immunodeficiency virus type 1 (HIV-1) derived from the repertoire of a seronegative patient. Mol Immunol. 2006;43(9):1363–1369. doi: 10.1016/j.molimm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 118.Scherl M, Posch U, Obermoser G, Ammann C, Sepp N, Ulmer H, Dierich MP, Stoiber H, Falkensammer B. Targeting human immunodeficiency virus type 1 with antibodies derived from patients with connective tissue disease. Lupus. 2006;15(12):865–872. doi: 10.1177/0961203306071405. [DOI] [PubMed] [Google Scholar]
- 119.Russo S, Lopalco L. Is autoimmunity a component of natural immunity to HIV? Curr HIV Res. 2006;4(2):177–190. doi: 10.2174/157016206776055011. [DOI] [PubMed] [Google Scholar]
- 120.Levy JA. HIV and the pathogenesis of AIDS. 3. Washington, Oxford: ASM, Blackwell [distributor]; 2007. [Google Scholar]
- 121.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. Antibody neutralization and escape by HIV-1. Nature. 2003;422(6929):307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 122.Binley JM, Ban YE, Crooks ET, Eggink D, Osawa K, Schief WR, Sanders RW. Role of complex carbohydrates in human immunodeficiency virus type 1 infection and resistance to antibody neutralization. J Virol. 2010;84(11):5637–5655. doi: 10.1128/JVI.00105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, Steenbeke TD, Venturi M, Chaiken I, Fung M, Katinger H, Parren PW, Robinson J, Van Ryk D, Wang L, Burton DR, Freire E, Wyatt R, Sodroski J, Hendrickson WA, Arthos J. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420(6916):678–682. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 124.Labrijn AF, Poignard P, Raja A, Zwick MB, Delgado K, Franti M, Binley J, Vivona V, Grundner C, Huang CC, Venturi M, Petropoulos CJ, Wrin T, Dimitrov DS, Robinson J, Kwong PD, Wyatt RT, Sodroski J, Burton DR. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J Virol. 2003;77(19):10557–10565. doi: 10.1128/JVI.77.19.10557-10565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schief WR, Ban YE, Stamatatos L. Challenges for structure-based HIV vaccine design. Curr Opin HIV AIDS. 2009;4(5):431–440. doi: 10.1097/COH.0b013e32832e6184. [DOI] [PubMed] [Google Scholar]
- 126.Frey G, Peng H, Rits-Volloch S, Morelli M, Cheng Y, Chen B. A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc Natl Acad Sci USA. 2008;105(10):3739–3744. doi: 10.1073/pnas.0800255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhu P, Liu J, Bess J, Jr, Chertova E, Lifson JD, Grise H, Ofek GA, Taylor KA, Roux KH. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature. 2006;441(7095):847–852. doi: 10.1038/nature04817. [DOI] [PubMed] [Google Scholar]
- 128.Klein JS, Bjorkman PJ (2010) Few and far between: how HIV may be evading antibody avidity. PLoS Pathog 6 (5):e1000908. doi:10.1371/journal.ppat.1000908 [DOI] [PMC free article] [PubMed]
- 129.Klein JS, Gnanapragasam PN, Galimidi RP, Foglesong CP, West AP, Jr, Bjorkman PJ. Examination of the contributions of size and avidity to the neutralization mechanisms of the anti-HIV antibodies b12 and 4E10. Proc Natl Acad Sci USA. 2009;106(18):7385–7390. doi: 10.1073/pnas.0811427106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ofek G, Tang M, Sambor A, Katinger H, Mascola JR, Wyatt R, Kwong PD. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J Virol. 2004;78(19):10724–10737. doi: 10.1128/JVI.78.19.10724-10737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cardoso RM, Zwick MB, Stanfield RL, Kunert R, Binley JM, Katinger H, Burton DR, Wilson IA. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity. 2005;22(2):163–173. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 132.Sanchez-Martinez S, Lorizate M, Katinger H, Kunert R, Nieva JL. Membrane association and epitope recognition by HIV-1 neutralizing anti-gp41 2F5 and 4E10 antibodies. AIDS Res Hum Retroviruses. 2006;22(10):998–1006. doi: 10.1089/aid.2006.22.998. [DOI] [PubMed] [Google Scholar]
- 133.Sun ZY, Oh KJ, Kim M, Yu J, Brusic V, Song L, Qiao Z, Wang JH, Wagner G, Reinherz EL. HIV-1 broadly neutralizing antibody extracts its epitope from a kinked gp41 ectodomain region on the viral membrane. Immunity. 2008;28(1):52–63. doi: 10.1016/j.immuni.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 134.Huarte N, Lorizate M, Kunert R, Nieva JL. Lipid modulation of membrane-bound epitope recognition and blocking by HIV-1 neutralizing antibodies. FEBS Lett. 2008;582(27):3798–3804. doi: 10.1016/j.febslet.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 135.Huarte N, Lorizate M, Maeso R, Kunert R, Arranz R, Valpuesta JM, Nieva JL. The broadly neutralizing anti-human immunodeficiency virus type 1 4E10 monoclonal antibody is better adapted to membrane-bound epitope recognition and blocking than 2F5. J Virol. 2008;82(18):8986–8996. doi: 10.1128/JVI.00846-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alam SM, Morelli M, Dennison SM, Liao HX, Zhang R, Xia SM, Rits-Volloch S, Sun L, Harrison SC, Haynes BF, Chen B. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc Natl Acad Sci USA. 2009;106(48):20234–20239. doi: 10.1073/pnas.0908713106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brown BK, Karasavvas N, Beck Z, Matyas GR, Birx DL, Polonis VR, Alving CR. Monoclonal antibodies to phosphatidylinositol phosphate neutralize human immunodeficiency virus type 1: role of phosphate-binding subsites. J Virol. 2007;81(4):2087–2091. doi: 10.1128/JVI.02011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Veiga AS, Pattenden LK, Fletcher JM, Castanho MA, Aguilar MI. Interactions of HIV-1 antibodies 2F5 and 4E10 with a gp41 epitope prebound to host and viral membrane model systems. Chem biochem. 2009;10(6):1032–1044. doi: 10.1002/cbic.200800609. [DOI] [PubMed] [Google Scholar]
- 139.Scherer EM, Leaman DP, Zwick MB, McMichael AJ, Burton DR. Aromatic residues at the edge of the antibody combining site facilitate viral glycoprotein recognition through membrane interactions. Proc Natl Acad Sci USA. 2010;107(4):1529–1534. doi: 10.1073/pnas.0909680107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Diskin R, Marcovecchio PM, Bjorkman PJ. Structure of a clade C HIV-1 gp120 bound to CD4 and CD4-induced antibody reveals anti-CD4 polyreactivity. Nat Struct Mol Biol. 2010;17(5):608–613. doi: 10.1038/nsmb.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, Stanfield RL, Julien JP, Ramos A, Crispin M, Depetris R, Katpally U, Marozsan A, Cupo A, Maloveste S, Liu Y, McBride R, Ito Y, Sanders RW, Ogohara C, Paulson JC, Feizi T, Scanlan CN, Wong CH, Moore JP, Olson WC, Ward AB, Poignard P, Schief WR, Burton DR, Wilson IA (2011) A Potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science. doi:10.1126/science.1213256 [DOI] [PMC free article] [PubMed]
- 142.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Principal Investigators PG, Koff WC, Wilson IA, Burton DR, Poignard P. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477(7365):466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326(5950):285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, Marshall DJ, Crump JA, Kapiga SH, Sam NE, Sinangil F, Pancera M, Yongping Y, Zhang B, Zhu J, Kwong PD, O’Dell S, Mascola JR, Wu L, Nabel GJ, Phogat S, Seaman MS, Whitesides JF, Moody MA, Kelsoe G, Yang X, Sodroski J, Shaw GM, Montefiori DC, Kepler TB, Tomaras GD, Alam SM, Liao HX, Haynes BF. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol. 2011;85(19):9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Verkoczy L, Diaz M, Holl TM, Ouyang YB, Bouton-Verville H, Alam SM, Liao HX, Kelsoe G, Haynes BF. Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance. Proc Natl Acad Sci USA. 2010;107(1):181–186. doi: 10.1073/pnas.0912914107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Verkoczy L, Chen Y, Bouton-Verville H, Zhang J, Diaz M, Hutchinson J, Ouyang YB, Alam SM, Holl TM, Hwang KK, Kelsoe G, Haynes BF. Rescue of HIV-1 broad neutralizing antibody-expressing B cells in 2F5 VH x VL Knockin mice reveals multiple tolerance controls. J Immunol. 2011;187(7):3785–3797. doi: 10.4049/jimmunol.1101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Scherer EM, Zwick MB, Teyton L, Burton DR. Difficulties in eliciting broadly neutralizing anti-HIV antibodies are not explained by cardiolipin autoreactivity. AIDS. 2007;21(16):2131–2139. doi: 10.1097/QAD.0b013e3282a4a632. [DOI] [PubMed] [Google Scholar]
- 148.Singh H, Henry KA, Wu SS, Chruscinski A, Utz PJ, Scott JK. Reactivity profiles of broadly neutralizing anti-HIV-1 antibodies are distinct from those of pathogenic autoantibodies. AIDS. 2011;25(10):1247–1257. doi: 10.1097/QAD.0b013e32834785cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453(7195):667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, Mehta A, Razavi B, Del Rio C, Zheng NY, Lee JH, Huang M, Ali Z, Kaur K, Andrews S, Amara RR, Wang Y, Das SR, O’Donnell CD, Yewdell JW, Subbarao K, Marasco WA, Mulligan MJ, Compans R, Ahmed R, Wilson PC. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208(1):181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, Chawansuntati K, Malasit P, Mongkolsapaya J, Screaton G. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328(5979):745–748. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, Sukupolvi-Petty S, Navarro-Sanchez E, Young PR, de Silva AM, Rey FA, Varani L, Whitehead SS, Diamond MS, Harris E, Lanzavecchia A, Sallusto F. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe. 2010;8(3):271–283. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Macagno A, Bernasconi NL, Vanzetta F, Dander E, Sarasini A, Revello MG, Gerna G, Sallusto F, Lanzavecchia A. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128–131A complex. J Virol. 2010;84(2):1005–1013. doi: 10.1128/JVI.01809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Scheid JF, Mouquet H, Kofer J, Yurasov S, Nussenzweig MC, Wardemann H (2011) Differential regulation of self-reactivity discriminates between IgG+ human circulating memory B cells and bone marrow plasma cells. Proc Natl Acad Sci USA. doi:10.1073/pnas.1113395108 [DOI] [PMC free article] [PubMed]