Abstract
The INTERFASCICULAR FIBERLESS/REVOLUTA (IFL1/REV) gene is essential for the normal differentiation of interfascicular fibers and secondary xylem in the inflorescence stems of Arabidopsis. It has been proposed that IFL1/REV influences auxin polar flow or the transduction of auxin signal, which is required for fiber and vascular differentiation. Assay of auxin polar transport showed that the ifl1 mutations dramatically reduced auxin polar flow along the inflorescence stems and in the hypocotyls. The null mutant allele ifl1-2 was accompanied by a significant decrease in the expression level of two putative auxin efflux carriers. The ifl1 mutants remained sensitive to auxin and an auxin transport inhibitor. The ifl1-2 mutant exhibited visible phenotypes associated with defects in auxin polar transport such as pin-like inflorescence, reduced numbers of cauline branches, reduced numbers of secondary rosette inflorescence, and dark green leaves with delayed senescence. The visible phenotypes displayed by the ifl1 mutants could be mimicked by treatment of wild-type plants with an auxin polar transport inhibitor. In addition, the auxin polar transport inhibitor altered the normal differentiation of interfascicular fibers in the inflorescence stems of wild-type Arabidopsis. Taken together, these results suggest a correlation between the reduced auxin polar transport and the alteration of cell differentiation and morphology in the ifl1 mutants.
Fiber cells, a sclerenchyma tissue, typically are long in shape and have thick secondary wall. They can be differentiated from meristematic cells such as procambium and vascular cambium or from non-meristematic parenchyma cells such as cortex and mesophyll cells. According to their origins and locations, fiber cells are classified into xylary or extraxylary fibers (Mauseth, 1988). Because of their easy identification on the basis of thick secondary walls, fibers have traditionally been used as a model for studying cell differentiation. Early physiological studies have demonstrated that auxin polar flow is essential for fiber differentiation (Aloni, 1987). However, little is known about the molecular mechanisms controlling the auxin flow paths that induce fiber differentiation.
Sachs (1972) and Aloni (1976, 1978) have pioneered the physiological studies of fiber differentiation. They have found that the signals required for the induction of fiber formation derive from young leaves and flow polarly in the basipetal direction. It has subsequently been shown that the plant hormone auxin can substitute young leaves for induction of fiber differentiation, suggesting that auxin polar flow determines fiber differentiation (Aloni, 1979). In addition to auxin, two other plant hormones, gibberellins and cytokinins, are also shown to be essential for induction of fiber differentiation (Aloni, 1979, 1982; Saks et al., 1984). In summary, these early physiological studies have clearly established that fiber cells are induced along the paths of auxin polar flow, and the induction of fiber differentiation requires three plant hormones, auxin, gibberellins, and cytokinins (Aloni, 1987).
Physiological and genetic studies have uncovered the molecular mechanisms controlling auxin polar transport (Lomax et al., 1995; Estelle, 1998; Palme and Gälweiler, 1999). It has been shown that auxin efflux carriers are located at the basal ends of cells, and are responsible for the basipetal flow of auxin in stems (Jacobs and Gilbert, 1983; Gälweiler et al., 1998). The carriers are thought to be composed of at least two separate polypeptides on which the sites for auxin efflux and N-(1-naphthyl) phthalamic acid (NPA) binding are located (Wilkinson and Morris, 1994; Dixon et al., 1996). Two genes encoding auxin efflux carriers have recently been cloned in Arabidopsis. The PIN-FORMED (PIN1) gene is shown to be highly expressed in vascular parenchyma cells. Within the cells, PIN1 is shown to be specifically localized at the basal ends, which is consistent with the expected function of an auxin efflux carrier (Gälweiler et al., 1998). Mutation of the PIN1 gene results in a pin-like inflorescence and altered vascular differentiation, indicating its important roles in the inflorescence and vascular development (Okada et al., 1991; Gälweiler et al., 1998). The alterations associated with the pin1 mutation can be mimicked by growing wild-type Arabidopsis in the presence of auxin polar transport inhibitors (Okada et al., 1991). The other auxin efflux carrier AGR/EIR1/PIN2 has been shown to be responsible for root gravitropism (Chen et al., 1998; Luschnig et al., 1998; Müller et al., 1998; Utsuno et al., 1998). In addition to these two well-characterized auxin efflux carriers, a number of genes showing high sequence homology to PIN1 have been revealed by the Arabidopsis genome sequencing project. It has been proposed that different auxin efflux carriers play roles in different aspects of plant growth and development (Estelle, 1998). However, none of these auxin polar transport carrier genes has yet been shown to be involved in fiber differentiation.
Since auxin polar transport is essential for the induction of fiber differentiation, it is reasonable to postulate that mutants with alterations in auxin polar transport may abolish normal fiber differentiation. However, none of the available Arabidopsis mutants with defects in auxin polar transport has been shown to disrupt fiber differentiation. The majority of these mutants has been found to affect vascular differentiation such as pin1 (Gälweiler et al., 1998), mp (Przemeck et al., 1996; Hardtke and Berleth, 1998), and lop1/trn1 (Carland and McHale, 1996; Cnops et al., 1996). The MONOPTEROS gene has been shown to encode a transcription factor binding to auxin responsive elements (Hardtke and Berleth, 1998). Other mutants such as pinoid and tir3 have been implicated in diverse plant morphological development (Bennett et al., 1995; Ruegger et al., 1997). The PINOID gene has been shown to encode a Ser-threonine protein kinase, which negatively regulates auxin signaling (Christensen et al., 2000). The Athb-8 gene has been found to be expressed in provascular cells in Arabidopsis (Baima et al., 1995), but it is not known whether it is involved in auxin polar transport. Although all these Arabidopsis mutants are defective in auxin polar transport in the inflorescence stems, they exhibit different tissue- or development-specific phenotypes, further supporting the hypothesis that different auxin polar transport machinery mediates various aspects of plant growth and development. It also indicates that the regulation of auxin polar flow responsible for fiber differentiation is mediated by a specific auxin polar transport mechanism.
Arabidopsis has been used as a model for studying fiber differentiation. The inflorescence stems of Arabidopsis develop xylary fibers and extraxylary fibers. The extraxylary fibers differentiate between the interfascicular regions and provide mechanical support to the stems. Typically, three to four layers of interfascicular fiber cells next to the endodermis form simultaneously, and they are easily recognized by simple histological staining. By screening ethyl methanesulfonate-mutagenized populations of Arabidopsis, we have found the ifl1 mutant with lack of normal differentiation of interfascicular fibers (Zhong et al., 1997). The ifl1 mutation has also been shown to affect the secondary xylem differentiation in the vascular bundles (Zhong and Ye, 1999). In addition to the disruption of normal differentiation of interfascicular fibers, ifl1-1 mutation causes a number of visible phenotypes such as long stems, dark green leaves with delayed senescence, and reduced numbers of cauline branches and secondary rosette inflorescence (Zhong et al., 1997). The IFL1 gene has been cloned and shown to encode a homeodomain Leu-zipper (HD-ZIP) protein (Zhong and Ye, 1999). It was found that mutations in this HD-ZIP protein (Ratcliffe et al., 2000) are responsible for the revoluta (rev) mutant phenotype with downward curling leaves (Talbert et al., 1995). The rev mutant displayed altered development of apical meristems and enlarged organs. The diverse phenotypes displayed by mutations of the HD-ZIP protein indicate that the protein might be involved in multiple processes of plant growth and development. Or, the HD-ZIP protein might affect a specific process such as auxin polar transport, which could lead to these multiple visible phenotypes.
Because many of the visible phenotypes exhibited by ifl1-1 mutation are related with processes regulated by auxin, we have proposed that alterations of auxin flow or its signaling pathway might be responsible for the defect of fiber differentiation and visible phenotypes (Zhong et al., 1997; Zhong and Ye, 1999). In this study we report the analysis of auxin polar transport activity in ifl1, and we compare the ifl1 phenotypes with those of pin1 and the wild type treated with the auxin polar transport inhibitor NPA. We show that ifl1 mutations dramatically reduce auxin polar flow in the inflorescence stems and hypocotyls, and ifl1-2, which exhibits strong phenotypes, has the most dramatic reduction in auxin polar transport. We also show that the ifl1 visible phenotypes can be mimicked by treatment of the wild type with NPA. Based on these findings we propose that the ifl1 mutations cause a reduction in auxin polar transport in the interfascicular and vascular regions, which leads to alterations in the differentiation of interfascicular fiber and secondary xylem, and the overall reduction of auxin polar flow along the inflorescence stems results in most of the ifl1 visible phenotypes.
RESULTS
Auxin Polar Transport Activity in the ifl1 Mutants
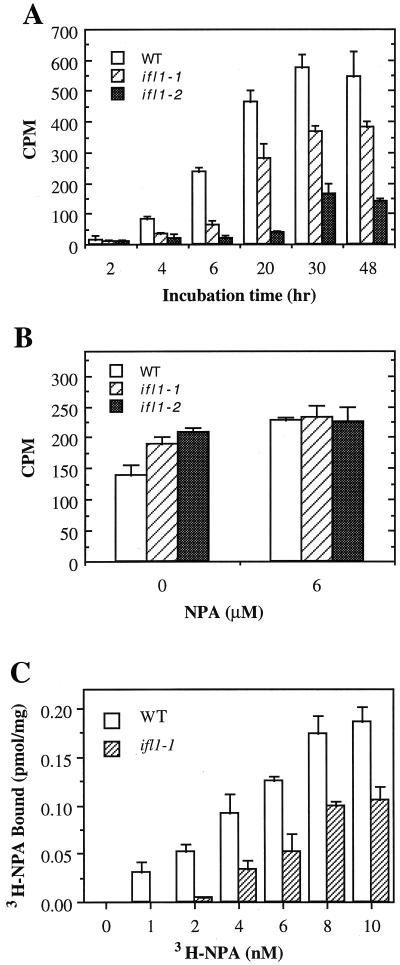
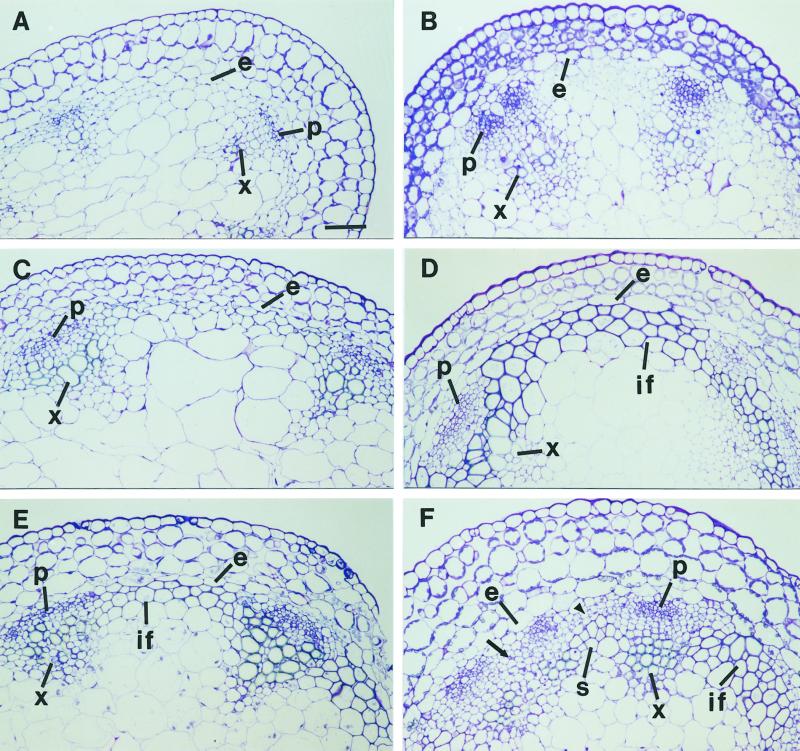
The ifl1 mutations disrupted the normal differentiation of interfascicular fibers in inflorescence stems (Fig. 1). In the wild type, four layers of interfascicular cells next to the endodermis differentiated into fiber cells (Fig. 1A). In the ifl1 mutants, the same four layers of cells next to the endodermis remained parenchymatous (Fig. 1, B and C). To investigate whether IFL1/REV might control the differentiation of interfascicular fibers by affecting auxin polar flow along the inflorescence stems we analyzed the auxin polar transport activity in ifl1 stems (Fig. 2). Top parts of the inflorescence stems that lacked interfascicular fibers were used for the assay to eliminate any possible effects of interfascicular fibers on auxin polar transport. In the wild-type stem segments, auxin was detected in the distal ends of segments after 4 h incubation, and the level of auxin in the distal ends continued to increase between 4 and 30 h of incubation period (Fig. 2A). In contrast, in ifl1-1 auxin was barely detectable in the distal ends after 6 h of incubation. Although more auxin was transported to the distal ends of the ifl1-1 segments with an increase of incubation time, the maximum auxin level in the distal ends was only approximately 65% that of the wild type. More dramatic effect on the reduction of auxin polar transport was seen in ifl1-2, which was a null mutant (Zhong and Ye, 1999). In ifl1-2, a very low level of auxin was detected in the distal ends even after 20 h of incubation. The maximum auxin level in the distal ends of the ifl1-2 segments after 30 h of incubation only reached approximately 30% that of the wild type (Fig. 2A). The auxin polar transport inhibitor NPA almost completely inhibited the auxin polar transport in the stems of the wild type and ifl1 mutants (data not shown). Little acropetal transport of auxin was detected in the stems of the wild-type (45 cpm at 48 h) and the ifl1 mutants (36 cpm at 48 h). These results demonstrate that a significant reduction in the auxin polar flow occurs in the stems of ifl1 mutants.
Figure 1.
Disruption of interfascicular fiber differentiation in the inflorescence stems of ifl1. Sections were taken from the middle portions of inflorescence stems of 6-week-old plants and were stained with toluidine blue for anatomy. A, Section from the wild type showing the presence of four layers of interfascicular fibers located next to the endodermis. B, Section from ifl1-1 showing the interfascicular cells (arrow) next to the endodermis, which were normally destined to be fibers remained parenchymatous. However, ectopic sclerification occurred in some interfascicular cells (arrowhead), which were normally not destined to be sclerified. C, Section from ifl1-2 showing lack of any sclerified cells in the interfascicular region. co, Cortex; e, endodermis; if, interfascicular fiber; pi, pith; x, xylem. Bar (A) = 5 μm (A–C).
Figure 2.
Auxin polar transport and NPA-binding activity in the wild type (WT) and ifl1. A, Time course of auxin polar transport activity in the inflorescence stems of wild type and ifl1. ifl1-1 and ifl1-2 mutants showed a dramatic reduction in the auxin polar transport activity compared with the wild type. Data are the mean values ± se of 15 plants. B, Auxin efflux in the hypocotyls of the wild type and ifl1. Auxin efflux rate in hypocotyl cells was measured by the retention of the amount of [3H] indole-3-acetic acid (IAA) in the presence or absence of NPA. The ifl1 mutants showed more retention of IAA compared with the wild type. Data are the mean values ± se of four replicates. C, 3H-NPA binding to plasma membranes prepared from the wild-type and ifl1-1 stems. Plasma membranes isolated from stems were incubated with various concentrations of 3H-NPA. Background binding activity was determined by addition of unlabeled NPA. The amount of 3H-NPA bound shown in the figure is the specific binding activity that was calculated by subtracting the background binding activity from total binding activity. ifl1-1 had a significant decrease in the NPA-binding activity compared with the wild type. Data are the mean values ± se of three assays.
To exclude the possibility that the reduction in auxin polar transport in the ifl1 stems might be caused by alterations of tissues and morphology of stems, we examined the auxin transport activity in the ifl1 hypocotyls that did not show any apparent abnormality in cell organization and morphology. Because of the difficulties in handling the small size of hypocotyls, we assayed the auxin efflux rate by measuring the amount of auxin retained inside hypocotyl cells after a certain period of incubation in the solution containing auxin. It is expected that if the auxin efflux rate in the ifl1 mutants was reduced, the ifl1 hypocotyl cells should retain more auxin than the wild-type cells. The results showed that the ifl1 mutations dramatically reduced auxin efflux rate in the hypocotyl cells (Fig. 2B). The ifl1-1 and ifl1-2 mutants retained 34% and 49% more auxin compared with the wild type. The wild-type and ifl1 mutants retained the same amount of auxin in the presence of NPA (Fig. 2B), indicating that the ifl1 hypocotyls remain sensitive to NPA. This suggests that the ifl1 mutations cause a marked reduction in the auxin polar transport activity independent of alterations of cell organization and morphology.
It has been shown that NPA inhibits auxin polar transport through binding to the NPA-binding protein, which may be part of the auxin efflux carrier complex (Dixon et al., 1996). Genetic analysis has demonstrated that reduced NPA binding to plasma membranes is associated with reduced auxin polar transport activity (Ruegger et al., 1997). To investigate whether the reduced auxin polar transport activity in ifl1 stems was associated with any alterations in NPA-binding activity we analyzed the NPA-binding activity in ifl1-1 (Fig. 2C). The NPA-binding activity to the plasma membranes from wild-type and ifl1-1 stems increased linearly between 1 and 10 nm NPA. However, NPA binding to the plasma membranes from ifl1-1 remained less than 60% that of the wild type (Fig. 2C). These results indicate that the ifl1 mutation alters auxin polar transport and NPA-binding activity.
Expression of Putative Auxin Efflux Carrier Genes in ifl1
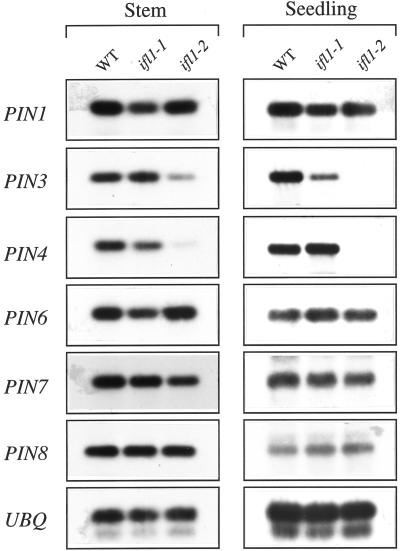
The finding that the ifl1 mutations caused reduced auxin polar transport prompted us to examine whether ifl1 mutations affect the gene expression of any auxin efflux carriers. The PIN1 and AGR/EIR1/PIN2 genes have been shown to encode auxin efflux carriers (Palme and Gälweiler, 1999). A search of the database identified five additional PIN homologs. We analyzed the expression patterns of PIN1 and its homologous genes in the stems and seedlings of the wild-type and ifl1 mutants (Fig. 3) except AGR/EIR1/PIN2, which was shown to be mainly expressed in roots (Palme and Gälweiler, 1999). The expression levels of PIN1, PIN6, PIN7, and PIN8 genes were similar between the wild-type and ifl1mutants. However, a significant decrease in the expression levels of PIN3 and PIN4 genes was seen in the ifl1-2 mutant (Fig. 3). This indicates that the dramatic reduction in auxin polar transport in the null mutant allele ifl1-2 is accompanied with a decrease in the expression of two putative auxin efflux carrier genes.
Figure 3.
Expression levels of PIN1 and other putative auxin efflux carrier genes in the wild type (WT) and ifl1 mutants. Total RNA isolated from stems or 2-week-old seedlings of the wild type and ifl1 mutants was used to analyze the expression levels of different genes by reverse transcriptase- (RT) PCR. The experiments were repeated three times and identical results were obtained. The expression level of ubiquitin (UBQ) gene was used as a control to confirm that equal amount of RNA was used for RT-PCR. The expression levels of PIN1, PIN6, PIN7, and PIN8 were similar in the stems and seedlings of the wild-type and ifl1 mutant, whereas the expression level of PIN3 and PIN4 was significantly reduced in ifl1-2 compared with the wild type.
Sensitivity of the ifl1 Mutants to Auxin and NPA
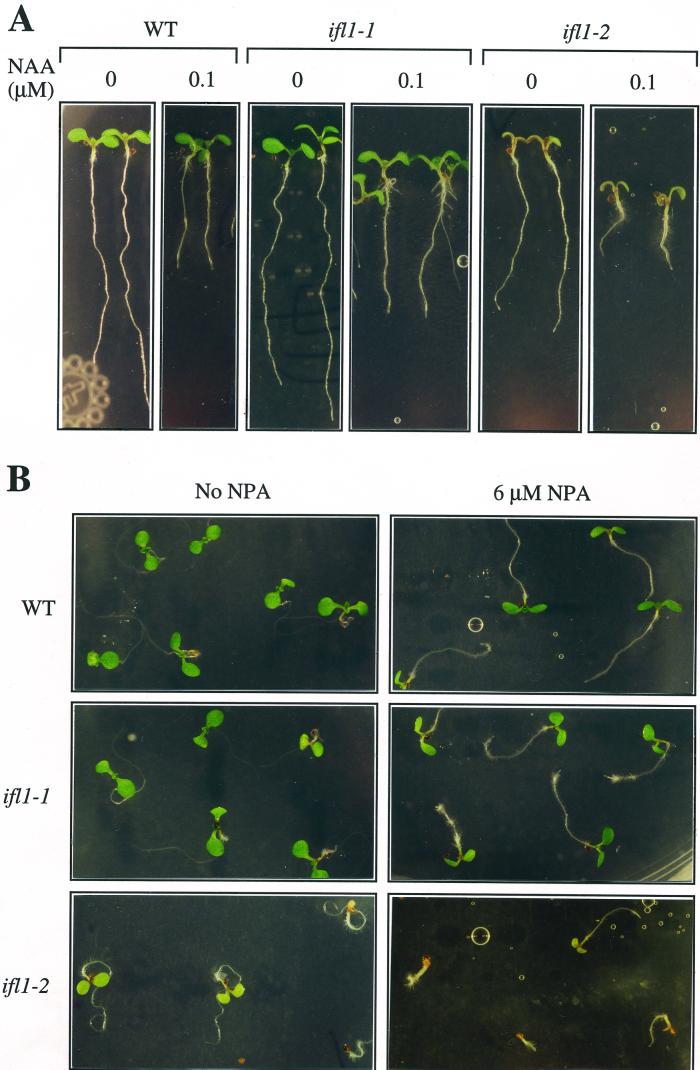
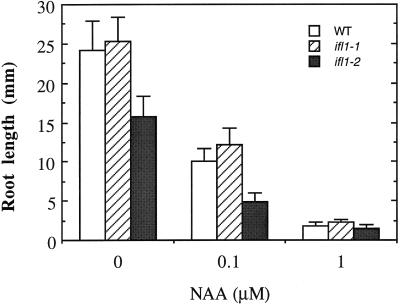
To investigate whether the ifl1 mutations altered the sensitivity to auxin, which might then cause the phenotypic changes, we assayed the ability of the ifl1 mutants in response to auxin. The results showed that the ifl1 roots remained sensitive to low levels of naphthylacetic acid (NAA; Fig. 4A). Quantitative analysis showed that NAA at 0.1 and 1 μm caused 60% and 90%, respectively, of inhibition of root growth in the wild-type and ifl1 mutants (Fig. 5). The ifl1 roots were also sensitive to NPA treatment (Fig. 4B). NPA treatment caused the roots of the wild-type and ifl1 mutants unable to grow downward into the agar medium, probably due to alterations of gravitropic response. In addition, the roots of the wild-type and ifl1 mutants grew in a relatively more straight pattern in the presence of NPA compared with the curling roots without NPA treatment (Fig. 4B). Taken together, these results suggest that the phenotypic changes caused by the ifl1 mutations are unlikely a direct result of alterations of auxin response or NPA sensitivity.
Figure 4.
Effects of NAA and NPA on the root growth of the wild type (WT) and ifl1 mutants. A, Display of the seedlings of the wild type and ifl1 mutants grown in agar medium containing no NAA or 1 μm NAA. NAA reduced root growth of wild-type and ifl1 mutants. B, Display of the seedlings of the wild-type and ifl1 mutants grown in agar medium containing no NPA or 6 μm NPA. NPA inhibited the downward growth and curling of roots of the wild-type and ifl1 mutants.
Figure 5.
Inhibition of root growth of the wild-type (WT) and ifl1 mutants by NAA. Seeds were germinated on the agar medium containing 0, 0.1, or 1 μm NAA. Root length was measured 8 d after seedling growth. Each datum is the mean values ± se of 20 roots.
Visible Phenotypes Associated with the Reduced Auxin Polar Transport in ifl1
It has been shown that a reduction in auxin polar transport in the inflorescence stems of pin1 results in a pin phenotype, i.e. abortion of floral structures and attenuation of inflorescence meristem growth (Okada et al., 1991). Because the ifl1 mutations caused a reduction in auxin polar transport in stems, we examined whether it showed phenotypes similar to those of pin1. The ifl1-1 allele has previously been shown to have pleiotropic phenotypes, including long stems, dark green leaves with delayed senescence, and reduced numbers of cauline leaves and branches (Zhong et al., 1997). When the ifl1-1 plants were grown in the greenhouse, their inflorescence stems could grow up to approximately 80 cm in height. In contrast, inflorescence stems of the wild type arrested growth when they reached approximately 40 cm (Fig. 6A). Like ifl1-1, the ifl1-2 plants also exhibited dark green leaves with delayed senescence and reduced numbers of cauline leaves and branches (Fig. 6B). However, unlike the prolonged growth of ifl1-1, the ifl1-2 allele showed pin-like inflorescence (Fig. 6, B–D). It was typical that the ifl1-2 inflorescence meristems arrested their growth when stems reached approximately 10 cm in height. Compared with the wild type in which the inflorescence bore normal flowers (Fig. 6E), the ifl1-2 inflorescence was almost devoid of normal flowers (Fig. 6, B–D). It appeared that the floral meristems were developed and produced bulges (Fig. 6D) or thread-like structures (Fig. 6C). The inflorescence occasionally bore a few normal flowers or flowers lacking stamen and/or carpels. All these phenotypes exhibited by ifl1-2, including pin-like inflorescence, dark green leaves with delayed senescence, and reduced cauline leaves and branches (Fig. 6, B–D), resemble those found in the pin1 mutant (Fig. 6F; Okada et al., 1991), which was caused by the mutation in an auxin efflux carrier gene (Gälweiler et al., 1998). This suggests that like pin1 mutant, the visible phenotypes in ifl1 are associated with the reduced auxin polar transport.
Figure 6.
Morphological phenotypes displayed by ifl1-1 and ifl1-2. A, Prolonged inflorescence growth displayed by ifl1-1 (left and right) compared with the wild type (middle). B, Ifl1-2 showing pin-like inflorescence (arrows), dark green leaves, and reduced numbers of cauline branches. C, Close-up of ifl1-2 pin-like inflorescence showing lack of normal flowers and presence of thread-like structures. D, Close-up of ifl1-2 inflorescence apex showing lack of normal flower buds and presence of bulge-like structures. E, Wild-type inflorescence bearing normal flowers. F, pin1 mutant showing pin-like inflorescence (arrow), dark green leaves, and reduced numbers of cauline branches and secondary rosette inflorescence. These visible phenotypes resemble those seen in ifl1-2 (B and C).
We further examined the effect of ifl1 mutations on lateral root formation during seedling growth because a reduction in auxin polar transport in stems has been shown to lead to a delay of lateral root initiation (Lomax et al., 1995). The ifl1 seeds germinated and produced primary roots as normally as the wild type. However, it was evident that the ifl1-2 mutant with a more severe reduction in auxin polar transport showed a delay of 2 to 3 d in the formation of lateral roots compared with the wild type (data not shown).
NPA-Treated Wild-Type Plants Mimic the Visible Phenotypes Exhibited by ifl1
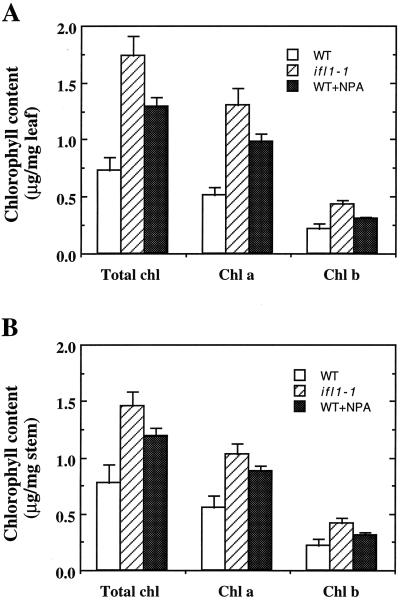
To confirm that the visible phenotypes exhibited by ifl1 were caused by reduced auxin polar transport, we tried to reduce the auxin polar transport in the wild type by treatment with NPA and then examined whether they displayed any visible phenotypes similar to ifl1. Wild-type plants grown under high concentrations of NPA showed stunted growth and their inflorescence did not bore normal flowers (data not shown; Okada et al., 1991), a phenotype similar to that of the ifl1-2 allele that had a severe reduction in auxin polar transport (Fig. 2A). When wild-type plants grew under a low concentration of NPA, their main inflorescence showed more vigorous growth than those without NPA treatment (Fig. 7A), a phenotype resembling that of the ifl1-1 allele, which had a lesser reduction in auxin polar transport (Fig. 2A). Furthermore, wild-type plants treated with NPA exhibited reduced number of secondary rosette inflorescence, reduced number of cauline branches and leaves, and greener stems and leaves with delayed senescence (Fig. 7C) compared with the wild type without NPA treatment (Fig. 7B). All these visible phenotypes were similar to those exhibited by ifl1-1 (Fig. 7D). In addition, quantitative assay of chlorophyll contents in leaves and stems showed that chlorophyll levels in leaves and stems of the wild type treated with NPA were similar to those in ifl1-1, which was approximately 60% more than those in wild type without NPA treatment (Fig. 8, A and B). Taken together, these results indicate that the reduction in auxin polar flow along the inflorescence stems of ifl1 is most likely the direct cause for the pleiotropic visible phenotypes.
Figure 7.
The morphological phenotypes of ifl1 can be mimicked by growing wild-type plants in the presence of NPA. Plants were grown on solid Murashige and Skoog medium without or with the addition of 12 μm NPA for 7 weeks and were observed for their phenotypes. A, Morphology of wild type (left), wild type grown in the presence of NPA (middle), and ifl1-1. It was evident that ifl1-1 and NPA-treated wild type showed greener leaves and fewer cauline branches and secondary rosette inflorescence compared with the wild type. B, Close-up of the rosette of the wild type showing yellow leaves, cauline branch (arrowhead), and secondary rosette inflorescence (arrow). C, Close-up of the rosette of the NPA-treated wild type showing dark green and curling leaves and a lack of cauline branches and secondary rosette inflorescence. D, Close-up of the rosette of ifl1-1 showing dark green and curling leaves and a lack of cauline branches and secondary rosette inflorescence.
Figure 8.
Chlorophyll content in the leaves and stems of the wild type (WT) and ifl1-1. Fresh leaves and stems were extracted in ethanol and the extracts were measured for chlorophyll content spectrophotometrically. Chlorophyll content was calculated based on fresh weight of leaves or stems. A, Measurement of chlorophyll content in leaves. B, Measurement of chlorophyll content in stems. ifl1-1 and wild type grown in the presence of NPA had much higher chlorophyll level than the wild type.
ifl1 and Senescence
One of the prominent visible phenotypes of the ifl1 mutants was that their leaves remained green for a prolonged period and did not senesce as early as the wild type (Fig. 9A). To investigate whether the delay of leaf senescence was caused by the alteration in the auxin polar transport activity or by inhibition of senescence program due to the ifl1 mutations, we analyzed the senescence rate of detached leaves. It has been shown that darkness or the plant hormone abscisic acid (ABA) enhances the senescence of detached leaves (Chung et al., 1997). It was evident that leaves of the wild type and ifl1-1 senesced at the same rate under darkness or ABA treatment (Fig. 9, C and D). These results indicate that the ifl1 mutations did not cause any changes in the senescence program. Because NPA treatment causes delayed senescence, the delayed leaf senescence in the ifl1 mutants is most likely due to the alteration of auxin polar transport.
Figure 9.
Senescence of wild-type and ifl1-1 leaves. Rosette leaves were detached from plants and incubated in liquid medium for test of senescence under darkness or treatment of ABA. At left is the wild type and at right is ifl1-1. A, Natural senescence of rosette leaves of 7-week-old plants. Although wild-type rosette leaves became yellow and dying (left), leaves of ifl1-1 (right) remained dark-green and alive. B, Freshly detached leaves displaying dark green color. C, Promotion of leaf senescence by treatment of ABA. It was evident that leaves of wild type and ifl1-1 were yellowing after treatment with ABA for 3 d. Leaves without ABA treatment remained green after 3 d of incubation (data not shown). D, Promotion of leaf senescence by darkness. It was obvious that leaves of wild type and ifl1-1 were yellowing after darkness treatment for 7 d.
Effects of NPA on Interfascicular Fiber Differentiation
The above results demonstrated that the ifl1 mutations caused the reduced auxin polar flow in the inflorescence stems, thereby resulting in the pleiotropic visible phenotypes of ifl1. To investigate whether a reduction in auxin polar flow could have any effect on interfascicular fiber differentiation, we examined the formation of interfascicular fibers in the inflorescence stems of wild-type Arabidopsis plants grown in the presence of the auxin polar transport inhibitor NPA. In the 7-week-old wild-type plants without NPA treatment, interfascicular fiber cells were evident only in the basal part (Fig. 10E), but not in the top (Fig. 10A) and middle (Fig. 10C) parts of stems. Inthe wild-type plants treated with NPA, no changes in the interfascicular regions were seen in the top part of stems (Fig. 10B). However, a dramatic alteration in the interfascicular fiber differentiation was observed in the middle and lower parts of stems. In the middle part of stems from plants treated with NPA, two layers of fiber cells next to the endodermis were prominent (Fig. 10D), whereas no fiber cells had differentiated in that from plants without NPA treatment (Fig. 10C). In the lower part of stems from plants treated with NPA, fiber cells were absent in some interfascicular regions (Fig. 10F). It was also noted that some interfascicular regions had fibers next to the endodermis, whereas others developed sclerified cells a few layers away from the endodermis (Fig. 10F). This was in contrast with wild-type plants without NPA treatment in which one to two layers of fiber cells next to the endodermis were uniformly formed in all interfascicular regions of the basal stems. Although the auxin polar transport inhibitor NPA has various effects on the interfascicular fiber formation at different parts of stems, the results indicate that the normal auxin polar flow is essential for the normal differentiation of interfascicular fibers.
Figure 10.
Alteration of interfascicular fiber differentiation by treatment with the auxin polar transport inhibitor NPA. Plants were grown on the Murashige and Skoog medium with or without NPA. Inflorescence stems of 7-week-old plants were divided into three equal segments and the middle parts of stem segments were sectioned and stained with toluidine blue for anatomy. A and B, Sections from the top segments from plants grown on the medium without (A) or with (B) NPA. No secondary wall thickening was seen in the interfascicular cells. C and D, Sections from the middle segments from plants grown on the medium without (C) or with (D) NPA. Although interfascicular cells in plants treated with NPA (D) showed secondary wall thickening, those in plants without NPA treatment (C) did not. E and F, Sections from the basal segments from plants grown on the medium without (E) or with (F) NPA. Interfascicular fibers were evident in plants without NPA treatment (E), whereas some interfascicular regions in plants treated with NPA (F) were devoid of fibers (arrow and arrowhead) or had ectopic sclerification of interfascicular cells. e, Endodermis; if, interfascicular fiber; p, phloem; s, sclerenchyma; x, xylem. Bar (A) = 5 μm (A–F).
DISCUSSION
IFL1/REV encodes an HD-ZIP protein, which has been shown to be expressed in interfascicular cells and vascular bundles. Mutation of IFL1/REV not only abolishes the normal differentiation of interfascicular fibers, but also alters secondary xylem differentiation. Because auxin is known to be an inducer for differentiation of vascular bundles and fibers, it has been suggested that IFL1/REV might influence genes involved in auxin polar flow along the vascular and interfascicular regions or influence genes involved in the transduction of hormonal signals leading to vascular and interfascicular fiber differentiation (Zhong et al., 1997; Zhong and Ye, 1999). Our finding that the ifl1 mutations dramatically reduce auxin polar flow along the inflorescence stems favors the former hypothesis that IFL1/REV might influence auxin polar flow, which might in turn affect the differentiation of interfascicular fibers and secondary xylem. This also provides the first evidence that mutation of an HD-ZIP protein affects auxin polar transport.
IFL1/REV Influences Auxin Polar Flow in the Inflorescence Stems
The available evidence suggests that the reduction in auxin polar flow in the inflorescence stems of the ifl1 mutants is caused directly by the ifl1 mutations rather than indirectly by altered anatomy. First, the stems used for auxin polar transport assay were from the top parts of stems in which no fibers were present in wild type and ifl1. In addition, the interfascicular regions of ifl1 stems have the same layers of cells as in the wild type. This eliminates the possibility that the reduced auxin polar flow might be caused by the absence of fibers or lack of interfascicular cells in ifl1. Second, the ifl1 mutations reduced auxin polar transport in the hypocotyl cells in which no fibers were present. Third, simple alteration of the organization of vascular bundles and fibers may not alter auxin polar transport in stems, as demonstrated in the avb1 mutant (Zhong et al., 1999). The avb1 mutation resulted in a global alteration of vascular organizations in stems. It not only transformed the collateral vascular bundles into amphivasal bundles, but also disrupted the normal ring-like arrangement of vascular bundles in the stele. However, no alteration in the auxin polar transport activity was detected in avb1 stems compared with wild type (Zhong et al., 1999). Taken together, these lines of evidence indicate that IFL1/REV is essential for normal auxin polar flow along the inflorescence stems of Arabidopsis.
There could be two possible mechanisms for IFL1/REV's function in auxin polar transport. One is that IFL1/REV might influence gene expression of some auxin efflux carriers. This possibility is supported by the observation that the expression level of two auxin efflux carrier genes was significantly reduced in the null mutant allele ifl1-2 (Fig. 3). The other possibility is that IFL1/REV might affect gene expression of other proteins involved in auxin polar transport. It has been proposed that auxin efflux carriers are composed of at least two separate subunits, one of which contains the site for NPA binding (Wilkinson and Morris, 1994; Dixon et al., 1996). Genetic analysis has demonstrated that a reduction in NPA-binding activity in tir3 mutant results in a decrease in auxin polar transport (Ruegger et al., 1997). Since ifl1 mutation caused the reduced NPA binding to plasma membranes, it is likely that IFL1/REV might affect the expression of NPA-binding proteins that are essential for auxin polar transport (Wilkinson and Morris, 1994; Dixon et al., 1996). Identification of the IFL1/REV target genes involved in auxin polar transport will help us to understand the mechanisms controlling auxin polar transport.
Does IFL1/REV Regulate Auxin Polar Flow in a Tissue-Specific Pattern?
One important question is whether IFL1/REV influences the global auxin polar flow or only auxin polar flow through a specific tissue. The available data suggest that IFL1/REV most likely controls auxin polar flow in some specific tissues. The main defect of the ifl1 mutations is the disruption of interfascicular fiber differentiation. This phenotype is completely different from other known Arabidopsis mutants that are defective in auxin polar transport in the stems (Bennett et al., 1995; Carland and McHale, 1996; Przemeck et al., 1996; Ruegger et al., 1997; Gälweiler et al., 1998; Hardtke and Berleth, 1998). For example, pin1 (Gälweiler et al., 1998) and mp (Przemeck et al., 1996; Hardtke and Berleth, 1998) mutants developed interfascicular fibers at the normal positions although both of them exhibited a dramatic reduction in auxin polar transport in the stems. Both mutants have been shown to have major effects on vascular differentiation. This indicates that multiple auxin polar flow paths exist in the inflorescence stems of Arabidopsis, and they are regulated by different regulatory mechanisms. Analysis of the gene expression patterns of putative auxin efflux carriers in ifl1 further suggests that IFL1/REV affects an auxin polar flow path different from that contributed by PIN1 because the ifl1 mutations did not affect the expression of PIN1 (Fig. 3). IFL1/REV appears to affect an auxin flow path that is important for the differentiation of interfascicular fibers and secondary xylem.
It was found that two putative auxin efflux carrier genes PIN3 and PIN4 had a much lower expression level in ifl1-2 compared with the wild type. This suggests that down-regulation of the expression of these two carriers might at least account for some of the reduction of auxin polar transport observed in ifl1-2. However, no significant alterations in the expression level of these two carriers were found in ifl1-1 (Fig. 3). The differential effects of the ifl1-1 and ifl1-2 mutations on the expression of these two carriers are consistent with the nature of their mutations (Zhong and Ye, 1999) and the magnitude of reduction in the auxin polar transport activity (Fig. 2A). However, how can one explain the reduction in auxin polar transport activity exhibited by ifl1-1? It is possible that an unknown auxin efflux carrier might be repressed in the ifl1 mutants, which accounts for the reduced auxin polar transport in ifl1-1. The other possibility is that components other than auxin efflux carriers, which are essential for normal auxin polar transport, might be affected by the ifl1 mutations. The exact mechanisms responsible for the alteration of auxin polar flow by the ifl1 mutations await for the characterization of the IFL1/REV target genes.
Alteration of Interfascicular Fiber and Vascular Differentiation in ifl1 Might Be Caused by Reduced Auxin Polar Flow
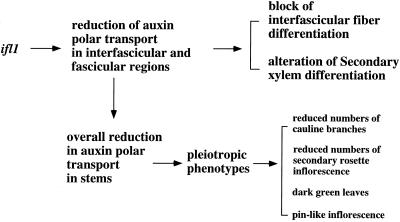
The finding that IFL1/REV influences auxin polar flow in the inflorescence stems is consistent with the phenotypes exhibited by the ifl1 mutations. The ifl1 mutations completely disrupted normal differentiation of interfascicular fibers and also reduced secondary xylem differentiation in vascular bundles (Zhong et al., 1997; Zhong and Ye, 1999). Because fiber and vascular tissues are induced along the paths of auxin polar flow, it is conceivable that a reduction in auxin polar flow in the ifl1 stems most likely accounts for the alteration of interfascicular fiber and vascular differentiation. The expression pattern of the IFL1/REV gene is also correlated with the roles of IFL1/REV in possible regulation of auxin polar flow along interfascicular and vascular regions. It is likely that IFL1/REV is essential for auxin polar flow in the interfascicular regions because the ifl1 mutations completely abolish normal differentiation of interfascicular fibers. IFL1/REV is also likely involved in auxin polar flow in vascular bundles because the ifl1 mutations reduce normal differentiation of secondary xylem (Fig. 11).
Figure 11.
A tentative explanation of the phenotypes displayed in ifl1. The finding that the ifl1 mutations dramatically reduce auxin polar transport in the inflorescence stems led us to propose that some of the observed phenotypes were likely caused by an alteration in auxin polar flow along the stems. This hypothesis was further supported by the pharmacological studies that show that most of the ifl1 phenotypes could be mimicked by the treatment of wild-type plants with NPA.
The finding that the ifl1 mutations reduce auxin polar flow along the stems can also explain the differential effects of the different ifl1 mutations on the interfascicular and fascicular activities. It has been found that some interfascicular cells other than the cells normally destined to become fiber cells are sclerified in the upper parts of ifl1-1 stems, whereas this does not happen in the lower parts of the stems (Fig. 1; Zhong et al., 1997). The ifl1-1 mutation was also shown to have much more dramatic effect on secondary xylem differentiation in the lower parts of stems than in the upper parts (Fig. 1; Zhong and Ye, 1999). Both of these phenotypes could be explained by insufficient flow of auxin from the top to the basal parts of stems in the ifl1-1 mutant.
Visible Phenotypes Exhibited by ifl1 Are Most Likely Caused by an Overall Reduction in Auxin Polar Flow
Although the ifl1 mutations cause a disruption in the differentiation of interfascicular fibers, a phenotype completely different from other mutants with defects in auxin polar transport, they do show many visible phenotypes similar to those exhibited by an auxin polar transport mutant pin1 (Okada et al., 1991; Gälweiler et al., 1998). All these visible phenotypes have been displayed by the pin1 mutant, and also can be phenocopied by reducing the auxin polar transport activity in wild type with NPA treatment. Therefore, the common visible phenotypes exhibited by ifl1 and pin1 mutants are caused by an overall reduction in auxin polar transport along the stems (Fig. 11).
It is interesting that the ifl1-1 mutant showed prolonged growth of inflorescence (Fig. 6), although it shared other visible phenotypes with ifl1-2. The only known physiological difference between ifl1-1 and ifl1-2 is that ifl1-1 causes a less reduction in auxin polar transport than does ifl1-2. Thus, it is likely that a moderate reduction in auxin polar transport might stimulate the activity of inflorescence meristems, allowing the prolonged growth. This is further supported by the NPA treatment experiment showing that plants treated with low concentration of NPA have long main inflorescence stems and other visible phenotypes similar to ifl1-1.
NPA Treatment Disrupts the Differentiation of Interfascicular Fibers in Wild-Type Inflorescence Stems
A key issue is that whether IFL1/REV influences the auxin polar flow, which in turn regulates interfasicular fiber differentiation, or the auxin polar transport and interfascicular fiber differentiation are two independent processes affected by IFL1/REV. The available evidence suggests that the former is more likely. Early physiological studies have clearly established the roles of auxin in fiber differentiation (Aloni, 1987), indicating that auxin polar transport is essential for normal induction of fiber formation. Analysis of IFL1/REV gene expression has shown its association with interfascicular cells (Zhong and Ye, 1999), suggesting that the defect in auxin polar flow resulted from the ifl1 mutations must have occurred in the interfascicular cells. This also implies that the disruption of the interfascicular fiber differentiation in ifl1 might be the result of alteration in auxin polar flow.
The in vitro NPA treatment experiment further supports the role of auxin polar transport in the fiber differentiation. The differentiation of interfascicular fibers in the lower parts of wild-type stems treated with NPA was significantly altered, most likely due to the insufficient flow of auxin toward the basal stems. This phenotype somewhat resembles that observed in ifl1 stems, although NPA treatment of wild type does not completely mimic the ifl1 phenotype. The different effects on fiber differentiation by NPA treatment and ifl1 mutations might reside in the nature of their actions. The ifl1 mutations might cause a dramatic reduction in auxin polar flow in the interfascicular regions so that normal fiber differentiation in this region is completely abolished. NPA is uptaken by roots and transported to shoots through xylem, where it can bind to NPA-binding proteins and affect auxin polar transport activity. However, insufficient amount of NPA might be transported to the interfascicular regions to cause a complete block of fiber differentiation. This is consistent with the phenotypes resulted from NPA treatment such as reduced number of vessel elements in the vascular bundles of the basal stems. In contrast, fiber differentiation in the upper parts of stems was actually enhanced, probably due to the availability of more auxin diverted from the vascular bundles to the interfascicular regions (Fig. 10; Mattsson et al., 1999).
However, NPA must have effectively blocked auxin polar flow toward the basal parts of stems because interfascicular fiber differentiation is significantly inhibited in the basal parts. This resembles the effects of mechanical block of auxin polar flow on fiber differentiation (Aloni, 1976). It has been shown that block of auxin flow in a specific region of coleus stems with paraffin prevents fiber differentiation in the tissues directly below the paraffin, but causes more fiber differentiation in the tissues above and lateral to the paraffin probably due to the accumulation of more auxin above the paraffin. Taken together, these results demonstrate that the normal polar flow of auxin is essential for normal differentiation of interfascicular fibers in the inflorescence stems of Arabidopsis.
In conclusion, we have shown that IFL1/REV, an HD-ZIP protein, is essential for the normal auxin polar transport and interfascicular fiber differentiation in the inflorescence stems. The finding that IFL1/REV influences auxin polar flow along the stems directly links IFL1/REV function to the early physiological studies regarding the role of auxin flow in fiber differentiation. We have recently found that overexpression of IFL1/REV leads to the promotion of secondary xylem formation in interfascicular and fascicular regions (R. Zhong and Z.-H. Ye, unpublished data), which further supports the possible role of IFL1/REV in influencing auxin polar flow. Further analysis of IFL1/REV functions will help us to understand the molecular links between auxin polar transport and fiber differentiation.
MATERIALS AND METHODS
Plant Materials
Mutants ifl1-1 (previously named ifl1) and ifl1-2 were described previously (Zhong et al., 1997; Zhong and Ye, 1999). The pin1 mutant seed was from Arabidopsis Biological Resource Center (Columbus, OH).
Histology
Arabidopsis stem segments were fixed in 2% (w/v) glutaraldehyde in phosphate-buffered saline (33 mm Na2HPO4, 1.8 mm NaH2PO4, and 140 mm NaCl, pH 7.3) solution at 4°C overnight. After fixation, segments were dehydrated in ethanol and were finally embedded in paraffin. Thin sections were prepared and stained with toluidine blue for anatomy.
Auxin Polar Transport Assay
The upper parts of inflorescence stems of 6-week-old plants were used for auxin polar transport assay as described by Okada et al. (1991). The stem segments used were at the elongating regions and they had not developed interfascicular fibers as examined with phloroglucinol HCl staining. The upper ends (relative to the apex of inflorescence) of the stem segments (2.5 cm in length) were submerged in the Murashige-Skoog medium (Murashige and Skoog, 1962) containing 1.5 μm of [3H]IAA (American Radiolabeled Chemicals, St. Louis). After incubation for various times, the opposite ends of the segments were cut and counted for the presence of radioactivity in a liquid scintillation counter. Non-polar transport of auxin in the segments was determined by addition of NPA (Chem Service, West Chester, PA) in the medium or placement of the stem segments in a reverse orientation in the medium.
Auxin efflux in hypocotyls was assayed according to Bernasconi (1996) and Garbers et al. (1996). In brief, 10 2-mm long hypocotyl segments from 10 individual seedlings were incubated in a 5 mm phosphate buffer containing 1% (w/v) Suc and 1 μm [3H]IAA in the presence or absence of 6 μm NPA. After 2 h of incubation, the segments were rinsed and then incubated for 2 h in the same buffer without IAA. After rinse, the segments were placed in the scintillation fluid and counted for the radioactivity.
NPA-Binding Assay
Inflorescence stems of 6-week-old wild-type and ifl1-1 plants were used for isolation of plasma membranes as described by Widell and Larsson (1981) and Muday et al. (1993). ifl1-2 plants were not included for the assay because they produced few seed and no enough ifl1-2 stems were available for the membrane isolation. Plasma membranes were assayed for NPA-binding activity according to Muday et al. (1993). In brief, isolated plasma membranes at the amount of 40 μg of proteins (in 200 μL of reaction mixture) were incubated with [3H]NPA (American Radiolabeled Chemicals) in a range of 1 to 10 nm. After incubation, the membranes were filtered through GFB filter (Whatman, Clifton, NJ) pretreated with 0.3% (w/v) polyethylenimine, washed, and counted for radioactivity in a liquid scintillation counter. The background binding was assayed by incubation of the reaction mixtures in the presence of 50 μm unlabeled NPA.
Gene Expression Analysis
Total RNA was isolated from the inflorescence stems of 6-week-old plants or 1-week-old seedlings. The expression level of auxin efflux carrier genes was analyzed with RT-PCR. In brief, 1 μg of total RNA was treated with DNase and used for first strand cDNA synthesis. One-tenth of the synthesized cDNA was PCR-amplified for 20 cycles with gene-specific primers corresponding to auxin efflux carrier genes. The PCR products were separated on an agarose gel and transferred onto a nylon membrane. The membrane was hybridized with digoxigenin-labeled auxin efflux carrier gene probes and the hybridized signals were detected with the chemiluminescent detection kit (Roche Molecular Biochemicals, Indianapolis) following the manufacturer's protocol.
Gene-specific primers were designed according to the non-conserved regions of putative auxin efflux carrier genes. Primers were tested to ensure that they specifically amplified the corresponding cDNAs. The information on PIN1 gene was from Gälweiler et al. (1998). A search of the GenBank database revealed five additional PIN-like genes with high amino acid sequence similarity to PIN1. These PIN-like genes were present in the following bacteria artificial chromosome clones: F15H11 (GenBank accession no. AC008148; its cDNA was named PIN3), T26J12 (AC002311; PIN7), F22K20 (AC002291; PIN6), F2I9 (AC005560; PIN4), and MQK4 (AB005242; tentatively named PIN8).
NAA and NPA Treatment of Wild-Type and ifl1 Plants
Wild-type and ifl1 plants were grown on the Murashige-Skoog medium in a growth chamber at 24°C under a 16-h light and 8-h dark photoperiod. To test the root sensitivity to NAA, plants were grown in the medium containing NAA at concentrations of 0, 0.1, 1, and 10 μm. The root length was measured after 8 d of growth. For inhibition of auxin polar transport, NPA was added in the medium at a concentration of 6, 12, 25, or 50 μm. Wild-type plants grown in the medium containing 50 μm NPA were stunted and showed pin-like inflorescence. Wild-type plants treated with 12 μm NPA grew vigorously, and were used for the comparative analysis with ifl1-1 plants.
Chlorophyll Measurement
Rosette leaves and main inflorescence stems were collected and weighed for their fresh weight. Chlorophyll was extracted with ethanol and its content was assayed based on the absorbance of the extract at 645 and 663 nm (Wintermans and De Mots, 1965).
Leaf Senescence Assay
Leaves from rosettes of 5-week-old plants were detached and incubated in incubation medium [3 mm 2-(N-morpholino) ethanesulfonic acid, pH 5.8]. The leaves were kept under light or total darkness and were observed for discoloration. For ABA treatment, leaves were incubated in the incubation medium containing 50 μm ABA, and kept under light (Chung et al., 1997).
ACKNOWLEDGMENTS
We thank Gloria Muday for providing information on the source of NPA, the Arabidopsis Biological Resource Center for providing pin1 seed, and the reviewers and editor for comments.
Footnotes
This work was supported by the Cooperative State Research, Education, and Extension Service, U.S. Department of Agriculture.
LITERATURE CITED
- Aloni R. Polarity of induction and pattern of primary phloem fiber differentiation in Coleus. Am J Bot. 1976;63:877–889. [Google Scholar]
- Aloni R. Source of induction and sites of primary phloem fiber differentiation in Coleus blumei. Ann Bot. 1978;42:1261–1269. [Google Scholar]
- Aloni R. Role of auxin and gibberellin in differentiation of primary phloem fibers. Plant Physiol. 1979;63:609–614. doi: 10.1104/pp.63.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R. Role of cytokinin in differentiation of secondary xylem fibers. Plant Physiol. 1982;70:1631–1633. doi: 10.1104/pp.70.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R. Differentiation of vascular tissues. Annu Rev Plant Physiol. 1987;38:179–204. [Google Scholar]
- Baima S, Nobili F, Sessa G, Lucchetti S, Ruberti I, Morelli G. The expression of the Athb-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana. Development. 1995;12:4171–4182. doi: 10.1242/dev.121.12.4171. [DOI] [PubMed] [Google Scholar]
- Bennett SRM, Alvarez J, Bossinger G, Smyth D. Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J. 1995;8:505–520. [Google Scholar]
- Bernasconi P. Effect of synthetic and natural protein tyrosine kinase inhibitors on auxin efflux in zucchini (Cucurbita pepo) hypocotyls. Physiol Planta. 1996;96:205–210. [Google Scholar]
- Carland FM, McHale NA. LOP1: a gene involved in auxin transport and vascular patterning in Arabidopsis. Development. 1996;122:1811–1819. doi: 10.1242/dev.122.6.1811. [DOI] [PubMed] [Google Scholar]
- Chen R, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson PH. The Arabidopsis thaliana AGRAVITROPIC1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc Natl Acad Sci USA. 1998;95:15112–15117. doi: 10.1073/pnas.95.25.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SK, Dagenais N, Chory J, Weigel D. Regulation of auxin response by the protein kinase PINOID. Cell. 2000;100:469–478. doi: 10.1016/s0092-8674(00)80682-0. [DOI] [PubMed] [Google Scholar]
- Chung B-C, Lee SY, Oh SA, Rhew TH, Nam HG, Lee C-H. The promoter activity of sen1, a senescence-associated gene of Arabidopsis, is repressed by sugars. J Plant Physiol. 1997;151:339–345. [Google Scholar]
- Cnops G, den Boer B, Gerats A, Van Montagu M, Van Lijsebettens M. Chromosome landing at the Arabidopsis TORNADO1 locus using an AFLP-based strategy. Mol Gen Genet. 1996;253:32–41. doi: 10.1007/s004380050293. [DOI] [PubMed] [Google Scholar]
- Dixon MW, Jacobson JA, Cady CT, Muday GK. Cytoplasmic orientation of the naphthylphthalamic acid-binding protein in zucchini plasma membrane vesicles. Plant Physiol. 1996;112:421–432. doi: 10.1104/pp.112.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estelle M. Polar auxin transport: new support for an old model. Plant Cell. 1998;10:1775–1778. doi: 10.1105/tpc.10.11.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- Garbers C, DeLong A, Deruére J, Bernasconi P, Söll D. A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO J. 1996;15:2115–2124. [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998;17:1405–1410. doi: 10.1093/emboj/17.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M, Gilbert SF. Basal localization of the presumptive auxin transport carrier in pea stem cells. Science. 1983;220:1297–1300. doi: 10.1126/science.220.4603.1297. [DOI] [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery PH. Auxin transport. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 509–530. [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson J, Sung ZR, Berleth T. Responses of plant vascular systems to auxin transport inhibition. Development. 1999;126:2979–2991. doi: 10.1242/dev.126.13.2979. [DOI] [PubMed] [Google Scholar]
- Mauseth JD. Plant Anatomy. Menlo Park, CA: The Benjamin/Cummings Publishing Company; 1988. [Google Scholar]
- Muday GK, Brunn SA, Haworth P, Subramanian M. Evidence for a single naphthylphthalamic acid binding site on the zucchini plasma membrane. Plant Physiol. 1993;103:449–456. doi: 10.1104/pp.103.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A, Guan C, Gälweiler L, Tänzler P, Huijser P, Marchant A, Pary G, Bennett M, Wisman E, Palme K. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998;17:6903–6911. doi: 10.1093/emboj/17.23.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 1991;3:677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme K, Gälweiler L. PIN-pointing the molecular basis of auxin transport. Curr Opin Plant Biol. 1999;2:375–381. doi: 10.1016/s1369-5266(99)00008-4. [DOI] [PubMed] [Google Scholar]
- Przemeck GKH, Mattsson J, Hardtke CS, Sung ZR, Berleth T. Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta. 1996;200:229–237. doi: 10.1007/BF00208313. [DOI] [PubMed] [Google Scholar]
- Ratcliffe OJ, Riechmann JL, Zhang JZ. INTERFASCICULAR FIBERLESS1 is the same gene as REVOLUTA. Plant Cell. 2000;12:315–317. doi: 10.1105/tpc.12.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M. Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell. 1997;9:745–757. doi: 10.1105/tpc.9.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs T. The induction of fiber differentiation in peas. Ann Bot. 1972;36:189–197. [Google Scholar]
- Saks Y, Feigenbaum P, Aloni R. Regulatory effect of cytokinin on secondary xylem fiber formation in an in vivo system. Plant Physiol. 1984;76:638–642. doi: 10.1104/pp.76.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert PB, Adler HT, Parks DW, Comai L. The REVOLUTA gene is necessary for apical meristem development and for limiting cell divisions in the leaves and stems of Arabidopsis thaliana. Development. 1995;121:2723–2735. doi: 10.1242/dev.121.9.2723. [DOI] [PubMed] [Google Scholar]
- Utsuno K, Shikanai T, Yamada Y, Hashimoto T. AGR, an Agravitropic locus of Arabidopsis thaliana, encodes a novel membrane protein family member. Plant Cell Physiol. 1998;39:1111–1118. doi: 10.1093/oxfordjournals.pcp.a029310. [DOI] [PubMed] [Google Scholar]
- Widell S, Larsson C. Separation of presumptive plasma membranes from mitochondria by partition in an aqueous polymer two-phase system. Physiol Plant. 1981;51:368–374. doi: 10.1104/pp.70.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Morris DA. Targeting of auxin carriers to the plasma membrane: effects of monensin on transmembrane auxin transport in Cucurbita pepo L. tissue. Planta. 1994;193:194–202. [Google Scholar]
- Wintermans JFGM, De Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965;109:448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Zhong R, Taylor JJ, Ye Z-H. Disruption of interfascicular fiber differentiation in an Arabidopsis mutant. Plant Cell. 1997;9:2159–2170. doi: 10.1105/tpc.9.12.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Taylor JJ, Ye Z-H. Transformation of the collateral vascular bundles into amphivasal vascular bundles in an Arabidopsis mutant. Plant Physiol. 1999;120:53–64. doi: 10.1104/pp.120.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Ye Z-H. IFL1, a gene regulating interfascicular fiber differentiation in Arabidopsis, encodes a homeodomain-leucine zipper protein. Plant Cell. 1999;11:2139–2152. doi: 10.1105/tpc.11.11.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]