Abstract
Spider dragline silk is an outstanding material made up of unique proteins—spidroins. Analysis of the amino acid sequences of full-length spidroins reveals a tripartite composition: an N-terminal non-repetitive domain, a highly repetitive central part composed of approximately 100 polyalanine/glycine rich co-segments and a C-terminal non-repetitive domain. Recent molecular data on the terminal domains suggest that these have different functions. The composite nature of spidroins allows for recombinant production of individual and combined regions. Miniaturized spidroins designed by linking the terminal domains with a limited number of repetitive segments recapitulate the properties of native spidroins to a surprisingly large extent, provided that they are produced and isolated in a manner that retains water solubility until fibre formation is triggered. Biocompatibility studies in cell culture or in vivo of native and recombinant spider silk indicate that they are surprisingly well tolerated, suggesting that recombinant spider silk has potential for biomedical applications.
Keywords: Spidroin, Protein structure, Recombinant protein production, Biomaterial, Protein self-assembly, Biocompatibility
Introduction
Since ancient times spider silk has fascinated man because of the elegant way in which it combines strength and elasticity, and it has also been ascribed abilities to stop bleeding and promote wound healing [1]. Native dragline spider silk has a higher tensile strength and is stiffer, more extendible and less immunogenic than the commonly used native silkworm silk [2, 3], implying a wider range of applications. While the outstanding mechanical properties of spider silk have been well documented, the suggested utility of spider silk, in particular artificial mimics thereof, still needs to be tested in a rational manner. Spiders are territorial and produce low amounts of silk; therefore, they cannot not be employed for large-scale silk production. This is in direct contrast to the case of the mulberry silk worm Bombyx mori (B. mori), which is used for the industrial scale production of silk-based textiles and other materials. The practical obstacles to producing spider silk on an industrial scale have been tackled in several ways. One approach is to use Bombyx silk and try to make it functionally similar to spider silk. This requires the removal of the immunogenic coat-protein sericin if matrices suitable for biomedical applications are to be obtained. As such, Bombyx silk is often dissolved in denaturing solvents, followed by reassembly procedures, to regenerate suitable matrices. Another approach is to dissolve and regenerate silk from non-mulberry silk worms, such as Antheraea sp. [4, 5]. Antharaea silk is composed of proteins more similar in sequence to spider silk proteins, spidroins, than to Bombyx silk proteins, and can therefore be assumed to be functionally closer to spider silk. However, although silkworm silk can generate interesting materials from several aspects, this source can not result in truly spider-like silks as the constituent proteins differ. Another alternative is the recombinant overexpression of spidroins or of designed proteins with sequences inspired by the overall properties of spidroins. Often the purification processes in these cases include solubilization steps following precipitation or lyophilization, before the fibres can be generated in similar manners as for dissolved silks. All of these approaches have been subject to recent reviews [2, 6–10].
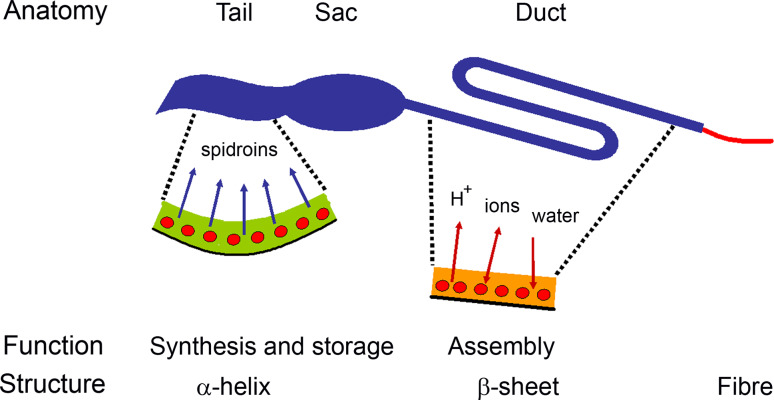
Yet another approach to produce artificial spider silk has recently been introduced [11]. This method uses recombinant production in Escherichia coli, but it differs from previously suggested methods by avoiding the resolubilization steps through the introduction of measures that assure solubility in aqueous buffers throughout the production and purification steps, until silk fiber formation is induced. This method mimics the spider’s own way of producing silk, namely, they produce an aqueous solution (dope) that is stored in the glandular sac until insoluble silk fibres are formed in the distal parts of the duct [12, 13] (Fig. 1).
Fig. 1.
The spider’s silk production system. A schematic spider silk-producing gland and duct. The main secondary structure adopted by the spidroins in the different locations as well as proposed changes in pH, salt compositions and water contents along the gland and duct are indicated
In this review, we will discuss the sequence motifs and structural and functional properties of spidroin domains and how they confer special challenges for recombinant production. We will also address how recombinant spidroins can generate novel functional materials for biomedical applications.
Spiders and spider silks
All spiders produce at least one type of silk, and some, like orb-weaving spiders, can have up to seven different types of silk glands [14] (Table 1), each producing a silk with a specific purpose and unique mechanical properties. Most studies have focussed on the dragline silk, a strong and extendible fibre used for the construction of the framework of the webs and as a lifeline, with a toughness never attained in synthetic or other natural fibres [3]. This silk is mainly composed of proteins, but there are reports of the presence of lipids and glycoproteins in the fibre, although their functions remain to be determined [15–17]. Dragline silk is produced in paired major ampullate glands, each which is connected to an S-shaped secretory duct that leads to the spinnerets on the ventral surface of the abdomen [18] (Fig. 1). The spidroins are produced and exocytosed into the lumen by a single-layered epithelium [19]. The spidroins can be stored in the glandular lumen as a dope until they are converted into a solid fibre in the end of the secretory duct [12, 13]. The silk production machinery of the spider is impressive from several points of view: (1) the spidroin genes are very large, repetitive and rich in guanine and cytosine, which puts high demands on the replication and transcription of these sequences [20–22]; (2) the glandular epithelium may multiply the entire genome without subsequent mitosis [23], and gene multiplicity and/or processing of the transcripts have been suggested [24, 25]; (3) to meet the extreme need of certain amino acids during translation, the epithelial cells of the glands have unusually large tRNA-pools for alanine and glycine [26]; (4) the spidroins are very prone to assemble, yet the spider manages to keep them in soluble helical and coil conformations [27] in the glandular lumen at very high concentrations (30–50%, w/v) [12, 27]; (5) the fibre-forming process is well controlled, enabling almost instantaneous formation of the fibre in a defined segment of the duct, thus avoiding a fatal spread of the assembly process to the dope in the gland [13]. Upon self-assembly, the spidroin’s secondary structure converts into mainly β-sheet [28]. This process probably involves shear forces, a drop in pH and changes in the ion composition along the duct [12, 29, 30] (Fig. 1).
Table 1.
Characteristics of spider silks and spidroins
| Gland | Function | Spidroin | Architecture | Length (residues)a | Amino acid motifs | Ensemble repeat length (residues) |
|---|---|---|---|---|---|---|
| Major ampullate | Web frame, life line | MaSp1 | NT-REP-CT | 3,130 | An, GX and GGXb | ~30 |
| MaSp2 | NT-REP-CT | 3,780 | An, GGX, GPX, QQ, GSGb | ~30 | ||
| Minor ampullate | Web reinforce-ment, capture silk | MiSp1 | U-REP-CT | nd | GGX, (GA)n, An, spacer (137 residues)c | ~280, Spacer[(GGX)n(GA)yAz]10; similar to MiSp1 |
| MiSp2 | U-REP-CT | nd | GGX, (GA)n, An, spacer (137 residues)c | |||
| Flagelliform | Capture spiral | Flag | NT-REP-CT | ~5,000 | GPGG(X)n, GGX, spacer (~28 residues)d | ~440 |
| Aciniform | Wrapping silk, inner egg case | AcSp1 | U-REP-CT | nd | Sn, TGPSGe | 200 |
| AcSp1 | Sn, GPSm | ~350 | ||||
| AcSp1-like | GGXf | ~187 | ||||
| Tubuliform/cylindriform | Outer egg case | TuSp1 | NT-REP-CT | 2,983 | Short An, Sn, Tn, (SX)gn | 171–182 |
| TuSp1 | Short An, Sn, Tn, (SX)hn | 180, [A0B1(A1B1)nA2], A1 = 139, B1 = 41 | ||||
| TuSp1 | Short An, Sn,Tn, (SX)i,kn | 184 | ||||
| TuSp1 | Short An, Sn, Tn, (SX)l,ln | 161, [B0(A1B1)nA2], B0 = 48, A1 = 110, B1 = 61, A2 = 98 | ||||
| TuSp1 | Short An, Sn, Tn, (SX)mn | ~200 | ||||
| TuSp1 | Short An, Sn, Tn, (SX)mn | ~300 | ||||
| TuSp1 | Short An, Sn, Tn, (SX)nn | 174 | ||||
| TuSp2 | NT-REP-CT | 3,218 | Short An, Sn, Tn, (SX)hn | 180, [A0B1(A1B1)nA2], A1 = 139, B1 = 41 | ||
| ECP-1 | Z-REP | 932 | (GA)n, short Ajn in parts of sequence | naq | ||
| ECP-2 | Z-REP | 825 | (GA)n, short Akn in parts of sequence | naq | ||
| Aggregate | Sticky coating | SCP-1 | na | 36 | nao | na |
| SCP-2 | na | 19 | nao | na | ||
| Pyriform | Attachment disk, joining fibre | PySp1 | U-REP-CT | nd | AAARAQAQAEARAKAE (A1), AAARAQAQAE (A2) and a 78 residue iteration (A3)p | [(A1A2)nA3]n |
na Not applicable, nd not determined, X any amino acid residue, U unknown N-terminal sequence, Z the ECP N-terminal region is approx.150 amino acid residues long, non-repetitive and contains 16 Cys but is not homologous to other spidroin N-terminal domains
Sources/references for amino acid motifs and ensemble repeat length (residues): b [22] (Latrodectus hesperus), c [116] (Nephila clavipes), d [46] (Nephila clavipes, Nephila madagascariensis), e [117] (Argiope trifasciata), f [118] (Latrodectus hesperus), g [119] (Argipoe aurantatia, Nephila clavipes, Araneus gemmoides), h [45] (Argiope bruennichi), I [120] (Latrodectus hesperus), j [121] (Latrodectus hesperus), k [122] ( Latrodectus hesperus), l [123] (Nephila clavata), m [35] (Deinopis spinosa, Uloborus diversus), n [124] (Nephilengys cruentata), o [125] (Latrodectus hesperus), p [126] (Latrodectus hesperus), q ECP-1 and -2 are considered to be atypical since they are much shorter than other known full-length spidroins (approximately 800–1,000 amino acid residues) and lack higher ensemble repeat units
a Length in amino acid residues. Full-length gene sequences are available for MaSp1 and MaSp2 from Latrodectus Hesperus, Nephila flagelliform silk and for the cDNAs of CySp1 and CySp2 from Argiope bruennichi
Spidroin and spider silk architecture
Spidroin gene and protein nomenclature follows a convention where the first two letters indicate the gland where it is expressed, followed by Sp for spidroin and a number referring to the different paralogues (e.g. MaSp1 for Major ampullate spidroin 1). Notable exceptions include the spidroins corresponding to the MaSp1 and MaSp2 of Araneus diadematus, which are referred to as Araneus diadematus fibroins 4 (ADF-4) and ADF-3, respectively [31]. Spidroin sequences contain N-terminal signal peptides that direct the protein to the secretory pathway [32] and which are subsequently removed in the endplasmic reticulum. Mature spidroins in general have a tripartite composition of a non-repetitive N-terminal domain (approx. 130 amino acid residues in the folded part [33]), an extensive region made up by silk-specific repeat units and a non repetitive folded C-terminal domain of approx. 110 residues [34]. The repetitive regions of different spidroins are composed of typical amino acid sequence motifs and ensemble repeat lengths (Table 1). It should, however, be noted that not all spidroins conform to these motifs and repeats [24, 25, 31, 35].
The dragline silk is composed mainly of two similar proteins, MaSp1 and MaSp2 [21], which are large (approximately 3,500 amino acid residues long [22]) and can be encoded by several gene loci [24, 25]. In Nephila clavipes, MaSp1 is found uniformly in the fibre core, whereas MaSp2 is missing in the periphery and forms clusters in certain core areas [36]. The ratio of MaSp1 and MaSp2 in the fibre differ between spider species and probably also between individuals of one species—and perhaps even in dragline silk from one individual spider spun at different occasions [37].
In the repetitive region, tandem repeat units of a glycine-rich repeat followed by an alanine block (An, n = 6–14) appear consecutively about 100 times. Alanine and glycine residues are by far the most abundant, making up >60% of the entire sequence [22]. Certain motifs, such as An, GGX and GPGXX, have been assigned specific secondary structures in the fibre (β-sheet, 31-helix and β-spiral, respectively) that can be correlated to the mechanical characteristics [38, 39]. The alanine blocks form β-sheets that can stack to form crystalline structures in the fibre [28], linking different protein molecules together. The level of crystallinity in the fibre depends on the proportion of alanine blocks in the spidroin and can be correlated to the strength of the fibre [40, 41], although additional factors are likely to be also important [3]. The structures in the more amorphous glycine-rich regions will, on the other hand, contribute to extensibility and flexibility [39]. Thus, the nature of the repetitive region seems to determine the mechanical properties of the fibre [3, 39]. In line with this supposition, Euprosthenops australis has one of the strongest dragline silks examined (tensile strength = 1.5 GPa) and also the longest polyalanine blocks [24, 40, 41]. However, a recent molecular dynamics study of polyalanine nanocrystals suggest that the best overall mechanical performance is obtained with crystals containing about five to ten residues [42]. The reasons for these apparent discrepancies between experimental and theoretical studies are not obvious. The now available recombinant spidroins (see below) should make it possible to study the correlation between polyalanine length and mechanical properties in further detail.
Recombinant spidroin production
Several prokaryotic and eukaryotic hosts are used for recombinant protein production, each with its own pros and cons in terms of cost, ease of use, expression levels and contaminations. Many eukaryotic proteins require post-translational modifications (e.g. glycosylation and phosphorylation) in order to obtain a correct fold and biological activity [43]. Systems for most of these modifications are present only in eukaryotic cells, and the extent, pattern and types of modifications differ between host systems. Phosphorylation of tyrosine and serine residues in spidroins have been reported, but the impact on the processing and/or physical properties of dragline silk remains undetermined [44]. There are several reports on successful recombinant production of partial spidroins in prokaryotes (Table 2). Consequently, the use of eukaryotic production systems may not be crucial. However, several other properties of spidroins complicate recombinant expression. The long, repetitive and guanine/cytosine-rich gene sequences complicate sequencing, cloning work and expression, and it was only recently that all parts of the spidroins were cloned and the first full-length genes sequenced [22, 45, 46]. Genetic instability and undesirable mRNA secondary structures have led to problems, such as truncations, rearrangements and translation pauses [47, 48]. Further on, depletion of tRNA pools, low solubility of the product and suspected proteolysis have been major obstacles [48–50]. Several strategies have been used to overcome these problems, such as codon optimization [48], enriched media [51] and the use of a variety of host systems (Table 2).
Table 2.
Survey of recombinantly expressed spidroin fragments
| Host | Origin | Protein description | Terminal domains | Functionalizations | Protein size (kDa) | Biocompatibility studies | References |
|---|---|---|---|---|---|---|---|
| Prokaryotes | |||||||
| Escherichia coli | Synthetic silk like protein | Synthetic repeats | No | 76–89 | No | [127] | |
| Nephila clavipes | Repeats from MaSp1, MaSp2 | No | 11–41 | No | [49] | ||
| Not specified | Repeats from MaSp2 | No | 31–112 | No | [79] | ||
| Nephila clavipes | Repeats from MaSp1, MaSp2 | No | 65–163 | No | [48] | ||
| Nephila clavipes | Gly-rich repeats | No | 9–20 | No | [52] | ||
| Nephila clavipes | Natural C-terminal part | CT | 43 | No | [47] | ||
| Nephila clavipes | Repeats from MaSp1 | No | Met, phosphorylation site for cAPK | 25 | No | [50, 54, 65, 89] | |
| Nephila clavipes | Gly-rich repeats from flagelliform | No | 25 | No | [55] | ||
| Nephila clavipes | Repeats from MaSp1, MaSp2 | ±CT | 43–56 | No | [53] | ||
| Araneus diadematus | Repeats from ADF3, ADF4 | ±CT | 34–60 | No | [78] | ||
| Nephila clavipes | Repeats from MaSp1, MaSp2 | ±CT | 20–56 | No | [64] | ||
| Nephila clavipes | Repeats from MaSp1 | No | Not specified | No | [56] | ||
| Silk-like protein | Gly-rich repeats and Ala-blocks | No | 18–36 | No | [128] | ||
| Nephila edulis | Natural C-terminal parts, MaSp1, MaSp2 | ±CT | 10 | No | [129] | ||
| Araneus diadematus | Repeats from ADF3, ADF4 | ±CT | 48–106 | No | [51, 58, 60, 63, 81 –85, 99, 105] | ||
| Nephila clavipes | Repeats from MaSp1 | No | R5 | 45 | No | [66] | |
| Nephila clavipes | Repeats from MaSp1 | No | CRGD | 48 | hMSC | [59] | |
| Nephila clavipes | Repeats from MaSp1 | No | CDMP1 | 49–60 | No | [61] | |
| Euprosthenops australis | Natural C-terminal part from MaSp1 | ±CT, ± NT | Cys | 10–24 | HEK 293 cells, human primary fibroblasts, in vivo (rats) | [11, 32, 33, 90–93] | |
| Nephila clavipes | No | [96] | |||||
| Nephila clavipes | Repeats from MaSp1, blended with regenerated bombyx | No | CRGD | 48 | 3T3 cells | [114] | |
| Arigiope aurantia | Repeats from MaSp1 | No | 63–71 | No | [9, 62] | ||
| Nephila antipodiana | Repeats and terminal domains from TuSp1 | ±CT, ± NT | 12–15 | No | [88] | ||
| Nephila clavipes | Repeats from flagelliform | ±CT | 14–94 | No | [130] | ||
| Salmonella typhimurium | Araneus diadematus | Repeats from ADF1, ADF2, ADF3 | CT | 30–56 | No | [131] | |
| Eukaryotes | |||||||
| Pichia pastoris | Nephila clavipes | Repeats from MaSp1 | No | 65 | No | [67] | |
| Silk-like protein | Synthetic silk-like repeat | No | 28–32 | No | [68] | ||
| Nephila clavipes | Repeats from MaSp1 | No | 94–113 | No | [86] | ||
| Nephila clavipes | Repeats from MaSp1 | No | 94 | 3T3 cells | [87] | ||
| Tobacco (Nicotiana tobaccum) potato (solanum tuberosum) | Nephila clavipes | Repeats from MaSp1 | No | 100xELP | 13–100 | human chondrocytes, CHO-K1 | [80, 100] |
| Tobacco (Nicotiana tabacum) | Nephila clavipes | Repeats from MaSp1, MaSp2 | CT | 60 | No | [69] | |
| Nephila clavipes | Repeats from MaSp2 | CT | ±ELP-tag | 65 | No | [132] | |
| Arabidopsis | Nephila clavipes | Repeats from MaSp1, MaSp2 | No | 64 | No | [97] | |
| Mammalian cells (MAC-T, BHK) | Araneus diadematus, Nephila clavipes | Natural C-terminal part from ADF3, MaSp1, MaSp2 | CT | 60–140 | No | [70] | |
| Mammalian cells (COS1) | Euprosthenops sp | Repeats from MaSp1 | No | 22 | No | [133] | |
| Insect cells (Spodoptera fruiperda) | Araneus diadematus | Natural C-terminal part from ADF3, ADF4 | CT | 35–56 | No | [73] | |
| Araneus diadematus | Natural C-terminal part from ADF3, ADF4 | ±CT | 50–60 | No | [74, 134, 135] | ||
| Araneus ventricosus | Natural C-terminal part from Av Flag | CT | 28 | No | [136] | ||
| Ovary derived cell lines (Bombyx mori) | Nephila clavipes | Repeats from flagelliform | No | 37 | No | [137] | |
| Nephila clavata | Repeats from MaSp1 | No | GFP | 70 | No | 75] | |
| Transgenic animals | |||||||
| Mammary glands of mice | Nephila clavipes | Repeats from MaSp1 | 31–66 | No | [71] | ||
| Salvary glands of Bombyx mori | Nephila clavata | Repeats from MaSp1 | No | 70 | No | [76] | |
CT C-terminal domain, NT N-terminal domain, Met methionine, cAPK cyclic AMP-dependent protein kinase, R5 peptide derived from the silaffin protein of Cylindrotheca fusiformis, CRGD Cys-Arg-Gly-Asp peptide, CDMP1 carboxyl terminal domain of dentin matrix protein 1, Cys cysteine, ELP elastin-like peptide, GFP green fluorescent protein, hMSC human mesenchymal stem cells, HEK human embryonic kidney, 3T3 mouse embryonic fibroblast line, CHO-K1 Chinese hamster ovary cell line; MaSp Major ampullate spidroin, ADF Araneus diadematus fibroin
Different approaches for recombinant spidroin production have been used depending on the aim in question, ranging from biochemical characterization to the large-scale production of biomaterials. The most widely used host is the Gram-negative enterobacterium E. coli (Table 2), which offers a well-controlled, cost-efficient system suitable for large-scale production. Several successful laboratory-scale E. coli fermentation processes have been reported [47, 52–62], although instable DNA fragments [47–49], low yields [47, 49], accumulation in inclusion bodies [63] and generally low protein solubility [52, 54, 59, 64–66] have been reported. The methylotrophic yeast Pichia pastoris has been used with the purpose to minimize truncations due to translation stops [67] and to allow extracellular secretion, which has been shown to occur for an amphiphilic silk-like protein [68]. Expression in various plants (tobacco, potato, Arabidopsis) has been tried as an alternative approach aimed at efficient and cheap production suitable for scale-up. In order to optimize yields, expression strategies with retention in the endoplasmic reticulum have been used, but large-scale trials have so far only resulted in fairly low yields of spidroins [69]. Various mammalian cell systems have also been employed in an attempt to achieve the expression of high-molecular-weight spidroins, the most promising of which is a 60-kDa ADF-3 protein expressed and secreted from two cell lines (from bovine mammary epithelial alveolar cells and baby hamster kidney cells) grown in a hollow fibre reactor [70]. Transgenic production in mammary glands and secretion into milk has been tried in mice [71] and goats [72], but the fairly low yields [7] do not justify the long development times and high production cost. Insects cells have proven to be important tools for the study of assembly properties of spidroin fragments in the cytoplasm [73, 74], but these appear to be less suitable for large-scale production. Motivated by structural similarities between spider and silkworm silk, the expression of spidroins in cell lines and larvae of B. mori have been tried, with solubility as the primary limitation [75]. Expression of spidroin fragments in the silk gland epithelium of transgenic B. mori resulted in silk with improved mechanical properties [76], although the relative amount of recombinant spidroin in the final silk blend was low [77].
A major aim in the recombinant production of spidroins has been to express as many continuous repetitive regions as possible (Tables 2, 3). Two strategies have been most commonly used: (1) to express parts of the native sequences (see for e.g. [47]) or (2) to generate iterated consensus repeats (see for e.g. [59, 78]). Overexpression of such recombinant spidroins has often resulted in water-insoluble products in the host and/or during downstream processing. For some applications, it is advantageous to obtain the product in an aggregated state, as the yield can be very high (up to approx. 50% of total E. coli protein content) and the aggregates can easily be isolated by centrifugation or filtration. Solubilization of aggregates is often achieved using urea, guanidine hydrochloride, LiBr, hexafluoroisopropanol or formic acid [9, 47, 48, 52, 53, 55, 57–60, 63, 64, 66, 67, 70, 78–88]. However, if the intended use of the spidroin involves biological applications, these compounds have to be removed in the final product. After removal of the solvents used for aggregate solubilization, it has generally been difficult to keep the spidoins in solution and to control assembly. Several strategies to avoid these problems have been tried, such as the introduction of methionine residues for controlled oxidation/reduction to prevent and promote assembly, respectively [50, 65, 89], kinase recognition motifs for controlled assembly via enzymatic phosphorylation [54] or solubility-enhancing fusion partners that can be released enzymatically [11]. In the latter case, spontaneous assembly into macroscopic, sterile, pyrogen-free and biocompatible silk-like fibres is possible, with the use of only physiologically compatible solvents [11, 90, 91] (Fig. 2).
Table 3.
Architecture of selected recombinant spidroin fragments
| Schematic description of constructa | Example (construct name) | Block length (residues) | Protein size (kDa) | Reference |
|---|---|---|---|---|

|
DP1B | Ala: 7; GlyX:15–20 | 65–163 | [48, 56, 67, 97] |

|
SP1 | Ala: 5; GlyX:10 | 25 | [49, 50, 54, 59] |
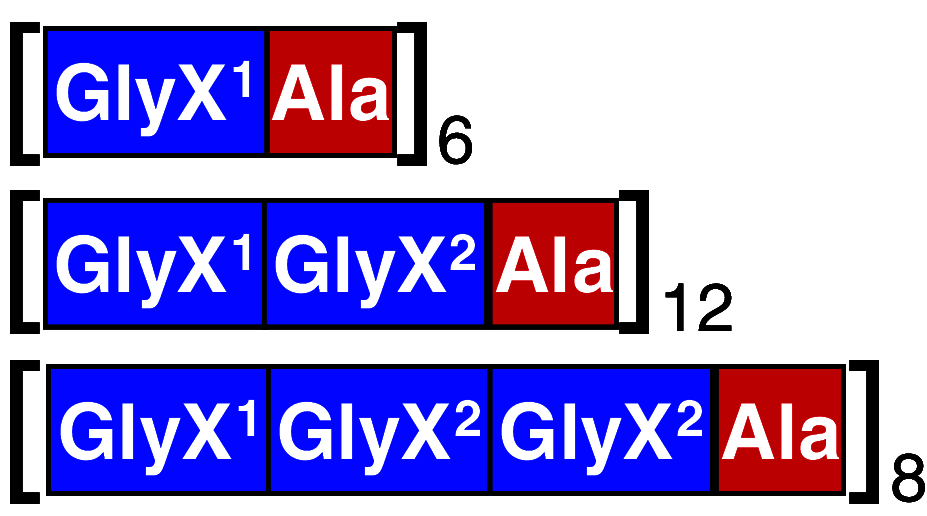
|
1E 2E 3E |
Ala: 8; GlyX: 28 | 63 | [62] |
| 71 | ||||
| 67 | ||||
|
|
1F9 | Ala: 5–7; GlyX: 20–25 | 94 | [9, 86, 87] |
|
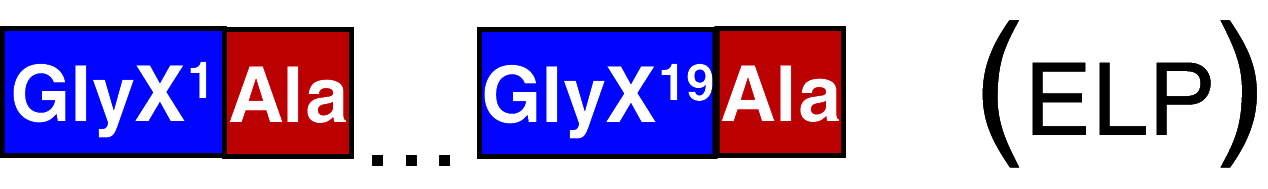
SO1 (ELP) | Ala: 6; GlyX: 20–30; 5 different GlyX | 51 | [80, 100] |
|
|
CRGD15 | Ala: 6; GlyX: 20–30 | 55 | [59, 66, 114] |
|
AQ12 (NR3); AQ24 (NR3); C16 (NR4) | Ala: 6–8; GlyX: 6–20 | 48 (60); 94 (106); 48 (58), respectively | [57, 58, 63, 78, 81, 82, 83, 84, 85, 99, 105] |

|
ADF3 | Ala: 6–8; GlyX: 6–20 | 60 | [70, 73] |

|
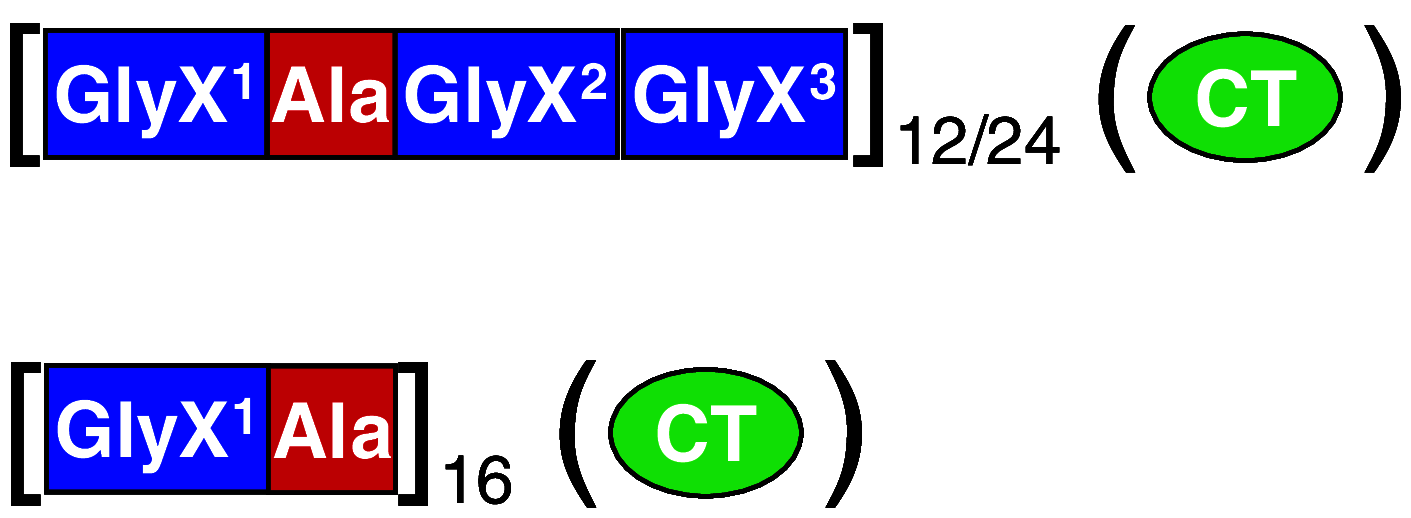
(NT)4RepCT | Ala: 12–15; GlyX: 13–19 | 12 (21) | [11, 33, 90, 91, 93] |
CT C-terminal domain, NT N-terminal domain
aThe superscript indicates the different nature of the repeat, the subscript indicates the number of bracketed repeats. For longer repeats, internal segments are indicated with dots. Domains within parenthesis are optional
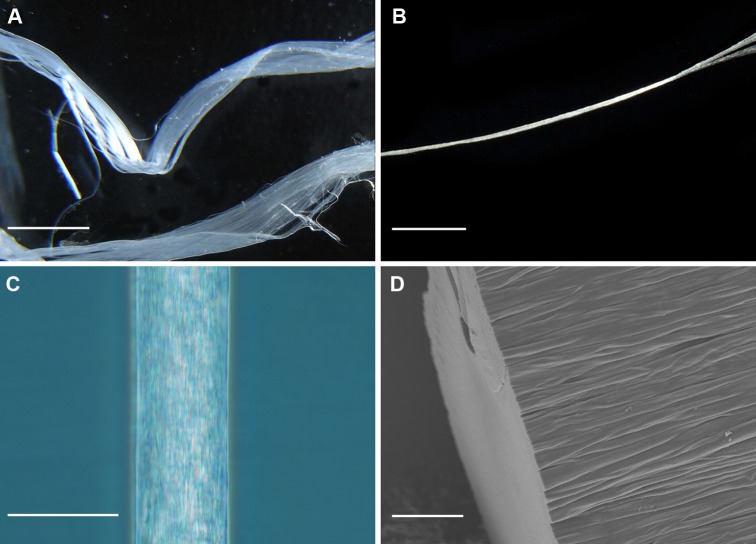
Fig. 2.
Fibres of recombinant spider silk. a Photograph of a wet fibre (scale bar 2 mm), b photograph of a dried fibre (scale bar 2 mm), c light micrograph of a dried fibre (scale bar 100 μm), d scanning electron micrograph of a dried fibre with a fracture surface obtained after rupture during tensile testing (scale bar 10 μm). All fibres were made from the 4RepCT minature spidroin (see Tables 2 and 3)
Spontaneous self-assembly into silk-like fibres (in terms of structure and stability) has so far been observed after recombinant production in the cytosol of insect cells [73] and after production and purification under non-denaturing conditions [11, 92, 93]. Solubilization of these fibres in denaturing solvents apparently destroys the ability to reassemble into silk-like fibers ([73] and unpublished observations). Huemmerich et al. [73] showed that solubilization of ADF-4 filaments in guanidinium thiocyanate followed by dialysis or dilution resulted in aggregation, but no fibrillar structures. In a similar manner, we have observed that spontaneously assembled fibres from recombinant miniature spidroin (4RepCT, see further below) dissolved in formic acid or hexafluoroisopropanol are not able to spontaneously reassemble into fibres (unpublished data). This is not surprising as not even the respinning of dissolved native spider silk results in fibres that match native silk [94, 95]. For recombinant spidroins that do not self-assemble spontaneously, different methods to convert them into fibres have been used, such as wet spinning [9, 53, 62, 70, 79, 86, 96, 97], hand drawing [82, 96], spinning though microfluidic devices [83] and electrospinning [56, 59, 66, 86, 98]. A few recombinant spidroins that generate fibres stable and long enough to allow mechanical testing have been reported [11, 70, 86, 92, 93, 96]. However, none of these fibres have replicated the extraordinary mechanical properties of the spider’s dragline silk, regardless of spidroin length. For some applications, such as scaffolds for cell culture and drug delivery, other formats than fibres are preferred. Therefore, materials have been produced by casting into films [50, 52, 53, 58–61, 65, 66, 81, 89, 99, 100], formation of microbeads [63], microspheres [57, 84], and microcapsules [101]. Three-dimensional porous scaffolds have also been produced using salt-leaching techniques [87]. Most of these techniques require post-treatment in dehydrating or salting-out solvents to increase the β-sheet contents and stability.
Collected experience from studies on the recombinant production of spidroins implies that several factors have to be taken into account to obtain silk-like materials, including which parts of the spidroins are to be expressed, structures and solubility of the recombinant protein, and assembly and spinning conditions.
Structure and function of spidroin domains
From studies at the molecular level, it is becoming increasingly clear that the three different spidroin parts are structurally and functionally distinct. The mechanical properties of the final silk fibre have traditionally been regarded as the most important functional parameter. However, it should be emphasized that the production, secretion, storage and, in particular, the controlled conversion of spidroins from soluble to insoluble states pose significant challenges. It is likely that the different domains are specialized to function during one or several—but probably not all—of these stages. The fact that the N-terminal domain is lacking in several recombinantly expressed constructs (Tables 2, 3), but that these are still able to polymerize and even form macroscopic silk-like fibres in vitro, supports this concept. Spiders manage to keep the spidroin dope at a high concentration, similar to the total protein concentration in some intracellular compartments (30–50%, w/v) without precocious aggregation; even more impressively, they manage to convert the same dope into solid fibres during the rapid transit through the extrusion duct. It is conceivable that studies of the molecular mechanisms that control spidroin solubility versus polymerization will generate insights which are of interest to the understanding of protein folding and misfolding in a general context. Given the proposed amyloid-like properties of spider silk [102], structural transitions in amyloid fibril formation may be particularly relevant.
Early studies focussed on the repetitive regions and their importance for fibre formation. For example, the absence of proline in one of the constituent proteins was suggested to be essential for fibre formation [103]. Later, an ADF-4 fragment (including the C-terminal domain) was found to form filaments in the cytosol of insect cells, although the ADF-3 counterpart stayed soluble, despite similar proline contents [78]. A significant difference in hydropathicity has been suggested as the major cause of the different solubility properties. Biochemical studies of recombinant ADF-3 and ADF-4 fragments that have been lyophilized, resolubilized in guanidinium thiocyanate and dialysed revealed that the presence of the non-repetitive C-terminal domain affects aggregation behaviour [78]. Upon expression in the cytosol of insect cells, ADF-4 fragments lacking the C-terminal domain do not self-assemble into nano fibers, as do their counterparts containing the C-terminal domain, but instead form aggregates [74]. The presence of amphipathic helices in the C-terminal domain has been proposed to be important for fibre formation [104]. In the recently determined folded structure of a C-terminal domain, the nonpolar parts of the amphipathic helices form the buried core; however, under certain experimental conditions nonpolar parts of the protein can become exposed [34]. It is possible that the C-terminal domain can adopt conformations that promote spidroin assembly (see below). Recombinant production in E. coli followed by recovery under nondenaturing conditions has generated structural and functional data of spidroin domains individually and in different combinations [11, 32–34, 92]. A construct comprising four polyalanine/glycine-rich repeats and the following C-terminal domain from MaSp1 of E. australis (4RepCT; see Tables 2, 3) and its two constituent parts have yielded information on the requirements for solubility and fibre formation [11, 92]. The repetitive part alone is unable to form fibres but aggregates into amorphous structures. Covalent linkage of the two parts (4RepCT) results in a miniature spidroin that spontaneously form metre-long macroscopic fibres under ambient conditions. Combined, these results indicate that (1) the repetitive regions mediate intermolecular contacts, (2) the C-terminal domain governs spidroin polymerization so that ordered fibres are formed rather than amorphous aggregates and (3) the N-terminal domain is not a prerequisite for in vitro fiber formation, but seems to regulate fibre formation in vivo.
The N-terminal domain is the most conserved of the spidroin domains and has evolved according to silk type [32]. Like the other spidroin domains, the N-terminal domain lacks known homologues, even in fibre-forming proteins from silkworm silk. These observations suggest that the N-terminal domain fulfils a function that is unique to spidroins and which requires structural features that can only be obtained by a folded polypeptide chain. The N-terminal domain from MaSp1 of E. australis is highly soluble and cooperatively folded [33, 92]. The recently determined high-resolution X-ray structure of recombinant N-terminal domain from the MaSp1 of E. australis [33] reveals several properties that point to specific functions. The inclusion of this N-terminal domain in miniature spidroins (NT4RepCT; see Tables 2, 3) accelerates charge-dependent self-assembly at pH values below approx. 6.4 (as observed in the spinning duct), but it delays aggregation above pH 7 (as observed in the dope). The X-ray structure analysis also revealed a homodimer of dipolar, antiparallel five-helix bundle subunits, and the overall dimeric structure and observed charge distribution are expected to be conserved through spider evolution and in all types of spidroins. These results suggest a relay-like mechanism through which the N-terminal domain regulates spidroin assembly by inhibiting precocious aggregation during storage and by accelerating and directing self-assembly as the pH is lowered along the spider’s silk extrusion duct [33]. A solution structure of a fragment of the N-terminal domain from an egg case spidroin, which after refolding forms a monomer in the presence of detergent micelles, has been reported [88]. This structure is also α-helical, but is otherwise quite different from the crystal structure of the N-terminal domain of MaSp1 of E. australis. It remains to be determined whether these differences reflect an inherent structural flexibility of the N-terminal domain, the different ways the recombinant proteins were obtained and investigated and/or other factors.
The presence of the C-terminal domain in recombinant miniature spidroins is a prerequisite for the formation of macroscopic fibres and fibrous aggregates in the test tube [92, 105]. A solution structure of the C-terminal domain from ADF-3 has recently been determined by nuclear magnetic resonance (NMR) spectroscopy [34]. This revealed a dimer of two five-helix bundles with no known structural homologues. Also, a solution structure of a monomeric C-terminal domain from an egg case spidroin, in detergent micelles, has been reported [88]. This C-terminal domain consists of a five-helix bundle but with a different topology than the ADF-3 domain. The C-terminal domain is rather well conserved at the amino acid sequence level and should consequently show a similar fold independent of origin. The reasons for the observed different folds in crystals or in solution [33, 34], on the one hand, compared to those in detergent micelles [88], on the other hand, are not clear at the present time. The ADF-3 C-terminal domain lacks surface-exposed charged residues but is quite polar due to an abundance of serines and glutamines [34]. Hagn et al. [34] further suggested that the structural state of the C-terminal domain can be regulated by chemical (in particular, the surrounding salt concentration) and mechanical stimuli and that this is a means to promote solubility versus aggregation in spidroin storage and fibre formation, respectively. The C-terminal homodimer is stabilized by a disulphide bridge. In miniature spidroins, the cysteine that forms the disulphide can be exchanged for serine without loss of ability to form fibres in the cytosol of insect cells [74] or in the test tube [93].
The N- and C-terminal spidroin domains are not related at the level of amino acid sequences, and although both fold into five-helix bundles, the topologies are different [33, 34]. There are, however, some apparent overall similarities between the N- and C-terminal domains, such as conformational flexibility and quite polar surfaces. The symmetric homodimeric nature of both domains may be important for aligning the repetitive segments [33, 34]. It has been suggested that the terminal domains form the shell of micellar structures that help to keep the spidroin repetitive segments in solution [34, 88, 106]. Micellar structures have indeed been observed in aqueous solutions of silkworm silk proteins reconstituted by polyethylene oxide treatment [106], but whether micelles exist in the spidroin dope remains to be studied. As the pH and salt composition are changed along the extrusion duct, the N- and C-terminal domains may trigger polymerization by bringing spidroins together [33, 34], but this alone does not explain what makes the many polyalanine blocks convert from helices/random coil to β-sheet. It can be speculated that once the N- and C-terminal domains have brought spidroins together in the narrowing extrusion duct, shear forces will trigger concerted conversion into β-sheet, explaining how fibre formation can occur in fractions of a second.
In summary, salient molecular determinants that currently are thought to affect spidroin solubility and conversion into fibres include: (1) the storage of a highly concentrated dope in the sac, possibly favoured by electrostatic repulsions involving the N-terminal domain and folding of polyalanine segments into α-helices; the polar nature of the serine-rich C-terminal domain and micelle formation may also contribute to high water solubility; (2) as the pH is lowered below approx. 6.4 and the salt composition is altered along the extrusion duct, the N- and C-terminal domains turn into mediators of assembly, thus bringing the spidroins together; (3) shear forces in the narrowing duct induces α-helix to β-strand conversion of polyalanine segments, which is rapidly propagated throughout the crowded spidroin population. These events eventually turn the spidroins into fibres made up of crystalline β-sheets surrounded by more flexible glycine-rich regions.
Spider silk for cell support and implantation
Even if it is not possible to use native spider silk at a larger scale, it is interesting to study its biocompatibility. The cytocompatibility of native dragline and egg-sac silk fibres has been demonstrated in a few human cell culture systems [107, 108]. Primary chondrocytes from articular cartilage were shown to attach to and survive for several weeks when grown on native dragline or egg-sac silk [108]. A study of in vitro proliferation using an endothelial cell line, however, showed that spider egg-sac silk had an inhibitory effect, although not as pronounced as that of non-mulberry silk [109]. Allmeling et al. [107] showed that in vitro human primary Schwann cells adhere quickly to and elongate along native dragline silk fibres. Later, artificial nerve constructs consisting of acellularized veins, dragline silk fibres, Schwann cells and, in some cases, extracellular matrix obtained from a tumour cell line (Matrigel; BD Biosciences, San Jose, CA) were used to replace a 2-cm deficit of the sciatic nerve in rats. These constructs promoted the regeneration of peripheral nerves with high functionality, while constructs consisting of vein and Matrigel alone resulted in nearly no myelinated nerve fibers and distinctive muscle degeneration [110]. Interestingly, successful nerve regeneration was obtained with constructs of veins and spider silk alone, which promoted the migration of autologous cells. Furthermore, no signs of inflammatory response or foreign body reaction were found, indicating the biocompatibility of the spider silk used [110]. In vivo biocompatibility of native spider silk has also been demonstrated by subcutaneous implantation of dragline silk in pigs [111] and enzyme-treated egg-sac silk in rats [112]. These results suggest that spider silk is well tolerated in vitro and in vivo.
The techniques that have become available to recombinantly produce spidroins in large quantities, and to fabricate various scaffolds, provide opportunities for new biomaterial applications. To date, few in vitro studies have been carried out on the biocompatibility of recombinant spider silk, and the materials that have been studied vary considerably in terms of amino acid sequence, mode of production and format. However, the results are mainly encouraging. Agapov et al. [87] recently examined the capability of porous three-dimensional scaffolds prepared from recombinant spider silk to support mouse fibroblast ingrowth and proliferation. These scaffolds were produced by salt leaching after the recombinant 1F9 protein (Table 3) had been dissolved in formic acid containing 10% LiCl. Cells were found to attach to the scaffolds, proliferate and migrate into the deeper layers of the scaffolds within 1 week, suggesting that recombinant spider dragline silk can be used to prepare scaffolds that are stable and biocompatible and show good interconnectivity. It would be interesting to assay survival quotes and growth rates on this kind of scaffolds and to obtain more detailed information on their mechanical properties. It would also be relevant to study the ability of other cell types to grow on the scaffolds, as well as to determine their in vivo compatibility. The ability of recombinant spider silk to support cellular differentiation has also been tested in various systems; for example, human mesenchymal stem cells grown on recombinant dragline spider silk in the presence of osteogenic medium showed increased calcification compared to tissue culture plastics, indicating differentiation into osteoblast-like cells [59]. However, the authors of this study suggested that the increased calcification of spider silk films could be a result of an inflammatory response induced by lipopolysaccharide (LPS) from the expression host E. coli. LPS is an integral component of the outer membrane of Gram-negative bacteria, which is endotoxic and can activate the innate immune system even at low concentrations. After recombinant spidroin production, all traces of potentially toxic or immunogenic compounds from the host cells must be removed before usage in vivo, as well as for some in vitro applications. Affinity purification usually yields recombinantly produced proteins with over 90% purity, but a significant amount of endotoxins is retained. A production route that combines purification of a minispidroin (4RepCT; Table 3) and endotoxin removal by using a simple cell wash procedure, protein affinity purification and LPS depletion has recently been developed [91]. Applying this method, it was possible to produce fibres with in vitro pyrogenicity corresponding to less than 1 EU/mg; the fibres could be sterilized by autoclaving, and human primary fibroblasts were shown to grow on them [91] (Fig. 3). Moreover, in vivo data demonstrate the biocompatibility of recombinant spider silk, since fibres of the miniature spidroin 4RepCT subcutaneously implanted in rats are well tolerated and allow the infiltration of fibroblasts and ingrowth of capillaries [90] (Fig. 3). This is the first in vivo study to be carried out on the biocompatibility of recombinant spider silk, and the results are intriguing as they expand on the possibilities for the use of recombinant spider silk in tissue engineering and regenerative medicine. Another recent in vivo study using recombinant spider silk in a rat model of wound healing likewise shows promising results [113]. However, the proteinaceous nature of spider silk and its recombinant counterparts calls for closer immunological studies of, for example, specific antibody responses.
Fig. 3.

Cell interactions with recombinant spider silk. a Human fibroblasts grown in vitro for 3 days on 4RepCT fibres, stained for F-actin (green) and DNA (red). b Histological section stained with haematoxylin and eosin of a 4RepCT fibre bundle after 1 week of subcutaneous implantation in rat. The fibres are infiltrated by elongated cells (asterisk), likely fibroblasts and angioblasts. Arrows indicate the presence of newly formed capillaries in the implant
One obvious benefit of recombinant silk is the possibility to introduce motifs with various effects on cell growth, differentiation and migration, which can make it possible to make customized matrices. Such functionalization of spider silk has been explored to some extent; for example, Scheller et al. [100] introduced a fusion protein of an elastin motif (elastin-like polypeptide) in combination with the MaSp1-derived SO1 protein (Table 3) in order to enhance the growth of chondrocytes. This fusion protein was produced in transgenic tobacco and potato, and extraction from the leaves was achieved in a process that included heat treatment and salt precipitation. The obtained protein coated onto cell culture plates was biocompatible and supported growth of human chondrocytes in a similar manner as collagen. Chondrocyte-specific morphology was also preserved, suggesting that the spider silk-elastin protein is able to prevent dedifferentiation. However, the lack of controls using the constituent proteins separately makes it unclear whether the positive effects are a consequence of the elastin or the spider silk part, or both. Another approach is the introduction of the integrin-binding motif RGD (Arg-Gly-Asp) into sequences derived from spider silk, resulting in biocompatible materials that support cell growth [59, 114, 115]. When human mesenchymal stem cells were cultured on RGD functionalized spider dragline silk films in the presence of osteogenic medium, however, no positive effects on calcification could be observed, compared to the control without RGD [59]. Morgan et al. [114] developed RGD-containing blends of regenerated Bombyx silk and recombinant RGD-spidroin dissolved in hexafluoroisopropanol. The films were then spin-coated onto cover slips and shown to support differentiation of a murine undifferentiated osteoblastic cell line. The authors concluded that around 10% RGD-spidroin provides optimal attachment and differentiation into osteoblasts. Li et al. [115] combined RGD-containing recombinant spider silk of unspecified origin and polyvinyl alcohol polymers to prepare scaffolds supporting the growth of cells from a mouse embryonic fibroblast cell line. No comparison with non-modified silk was presented, making it difficult to estimate the effect of the introduced RGD motif. Provided that proper surface exposure of introduced motifs can be achieved, functionalized recombinant spider silk may be useful for advanced cell culture systems and implantables.
Conclusions
During evolution, spidroins have developed properties that allow the swift generation of silks with outstanding mechanical properties. Moreover, although not required for its biological functions, spider silk, as well as recombinant spider silk-like fibres, appear to be biocompatible as they support the growth of mammalian cells in vitro and can be implanted without being rejected. These features make artificial mimics of spider silk attractive alternatives for cell culture and regenerative medicine. Spidroins contain several repetitive segments capped by folded domains, all with apparently unique sequence features and specific functions. Recent developments in techniques used for the recombinant production of miniature spidroins enable the generation of sterile, pyrogen-free and biocompatible artificial spider silk with the use of physiological solvents only. This approach, in combination with an increased understanding of the structures and functions of spidroins at a molecular level, make it realistic to believe that rationally designed artificial spider silk can be used for biomedical applications in the future.
Acknowledgements
We are grateful to Prof. Wilhelm Engström for introducing spidroin research at our department. Financial support from the Swedish Research Council, Formas, Vinnova, Spiber Technologies AB, and the EU Commission is gratefully acknowledged.
Abbreviations
- ADF
Araneus diadematus fibroin
- LPS
Lipopolysaccharide
- MaSp
Major ampullate spidroin
- Spidroin
Spider silk protein
References
- 1.Bon M. A discourse upon the usefulness of the silk of spiders. Phil Trans. 1710;27:2–16. [Google Scholar]
- 2.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 3.Gosline JM, Guerette PA, Ortlepp CS, Savage KN. The mechanical design of spider silks: from fibroin sequence to mechanical function. J Exp Biol. 1999;202:3295–3303. doi: 10.1242/jeb.202.23.3295. [DOI] [PubMed] [Google Scholar]
- 4.Luan XY, Huo GH, Li MZ, Lu SZ, Zhang XG. Antheraea pernyi silk fibroin maintains the immunosupressive properties of human bone marrow mesenchymal stem cells. Cell Biol Int. 2009;33:1127–1134. doi: 10.1016/j.cellbi.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Mandal BB, Kundu SC. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials. 2009;30:2956–2965. doi: 10.1016/j.biomaterials.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Hardy JG, Scheibel TR. Silk-inspired polymers and proteins. Biochem Soc Trans. 2009;37:677–681. doi: 10.1042/BST0370677. [DOI] [PubMed] [Google Scholar]
- 7.Vendrely C, Scheibel T. Biotechnological production of spider-silk proteins enables new applications. Macromol Biosci. 2007;7:401–409. doi: 10.1002/mabi.200600255. [DOI] [PubMed] [Google Scholar]
- 8.Kluge JA, Rabotyagova O, Leisk GG, Kaplan DL. Spider silks and their applications. Trends Biotechnol. 2008;26:244–251. doi: 10.1016/j.tibtech.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Teulé F, Cooper AR, Furin WA, Bittencourt D, Rech EL, Brooks A, Lewis RV. A protocol for the production of recombinant spider silk-like proteins for artificial fiber spinning. Nat Protoc. 2009;4:324–355. doi: 10.1038/nprot.2008.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leal-Egana A, Scheibel T. Silk-based materials for biomedical applications. Biotechnol Appl Biochem. 2010;55:155–167. doi: 10.1042/BA20090229. [DOI] [PubMed] [Google Scholar]
- 11.Stark M, Grip S, Rising A, Hedhammar M, Engstrom W, Hjalm G, Johansson J. Macroscopic fibers self-assembled from recombinant miniature spider silk proteins. Biomacromolecules. 2007;8:1695–1701. doi: 10.1021/bm070049y. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Knight DP, Vollrath F. Rheological characterization of nephila spidroin solution. Biomacromolecules. 2002;3:644–648. doi: 10.1021/bm0156126. [DOI] [PubMed] [Google Scholar]
- 13.Work RW. Mechanisms of major ampullate silk fiber formation by orb-web-spinning spiders. Trans Am Microsc Soc. 1977;96:170–189. [Google Scholar]
- 14.Candelas GC, Cintron J. A spider fibroin and its synthesis. J Exp Zool. 1981;216:1–6. [Google Scholar]
- 15.Schulz S (2001) Composition of the silk lipids of the spider nephila clavipes. Lipids 637-647 [DOI] [PubMed]
- 16.Sponner A, Vater W, Monajembashi S, Unger E, Grosse F, Weisshart K. Composition and hierarchical organisation of a spider silk. PLoS One. 2007;2:e998. doi: 10.1371/journal.pone.0000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Augsten K, Muhlig P, Herrmann C. Glycoproteins and skin-core structure in nephila clavipes spider silk observed by light and electron microscopy. Scanning. 2000;22:12–15. doi: 10.1002/sca.4950220103. [DOI] [PubMed] [Google Scholar]
- 18.Kovoor J (1987) Comparative structure and histochemistry of silk-producing organs in arachnids. In: Nentwig W (ed) Ecophysiology of spiders. Springer, Berlin, pp 160–186
- 19.Plazaola A, Candelas GC. Stimulation of fibroin synthesis elicits ultrastructural modifications in spider silk secretory cells. Tissue Cell. 1991;23:277–284. doi: 10.1016/0040-8166(91)90082-5. [DOI] [PubMed] [Google Scholar]
- 20.Mita K, Ichimura S, Zama M, James TC. Specific codon usage pattern and its implications on the secondary structure of silk fibroin mRNA. J Mol Biol. 1988;203:917–925. doi: 10.1016/0022-2836(88)90117-9. [DOI] [PubMed] [Google Scholar]
- 21.Hinman MB, Lewis RV. Isolation of a clone encoding a second dragline silk fibroin. Nephila clavipes dragline silk is a two-protein fiber. J Biol Chem. 1992;267:19320–19324. [PubMed] [Google Scholar]
- 22.Ayoub NA, Garb JE, Tinghitella RM, Collin MA, Hayashi CY. Blueprint for a high-performance biomaterial: full-length spider dragline silk genes. PLoS ONE. 2007;2:e514. doi: 10.1371/journal.pone.0000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregory TR, Shorthouse DP. Genome sizes of spiders. J Hered. 2003;94:285–290. doi: 10.1093/jhered/esg070. [DOI] [PubMed] [Google Scholar]
- 24.Rising A, Johansson J, Larson G, Bongcam-Rudloff E, Engstrom W, Hjalm G. Major ampullate spidroins from euprosthenops australis: multiplicity at protein, mRNA and gene levels. Insect Mol Biol. 2007;16:551–561. doi: 10.1111/j.1365-2583.2007.00749.x. [DOI] [PubMed] [Google Scholar]
- 25.Ayoub NA, Hayashi CY. Multiple recombining loci encode masp1, the primary constituent of dragline silk, in widow spiders (latrodectus: Theridiidae) Mol Biol Evol. 2008;25:277–286. doi: 10.1093/molbev/msm246. [DOI] [PubMed] [Google Scholar]
- 26.Candelas GC, Arroyo G, Carrasco C, Dompenciel R. Spider silkglands contain a tissue-specific alanine tRNA that accumulates in vitro in response to the stimulus for silk protein synthesis. Dev Biol. 1990;140:215–220. doi: 10.1016/0012-1606(90)90069-u. [DOI] [PubMed] [Google Scholar]
- 27.Hijirida DH, Do KG, Michal C, Wong S, Zax D, Jelinski LW. 13C NMR of Nephila clavipes major ampullate silk gland. Biophys J. 1996;71:3442–3447. doi: 10.1016/S0006-3495(96)79539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simmons AH, Michal CA, Jelinski LW. Molecular orientation and two-component nature of the crystalline fraction of spider dragline silk. Science. 1996;271:84–87. doi: 10.1126/science.271.5245.84. [DOI] [PubMed] [Google Scholar]
- 29.Knight DP, Vollrath F. Changes in element composition along the spinning duct in a Nephila spider. Naturwissenschaften. 2001;88:179–182. doi: 10.1007/s001140100220. [DOI] [PubMed] [Google Scholar]
- 30.Dicko C, Vollrath F, Kenney JM. Spider silk protein refolding is controlled by changing pH. Biomacromolecules. 2004;5:704–710. doi: 10.1021/bm034307c. [DOI] [PubMed] [Google Scholar]
- 31.Guerette PA, Ginzinger DG, Weber BH, Gosline JM. Silk properties determined by gland-specific expression of a spider fibroin gene family. Science. 1996;272:112–115. doi: 10.1126/science.272.5258.112. [DOI] [PubMed] [Google Scholar]
- 32.Rising A, Hjalm G, Engstrom W, Johansson J. N-terminal nonrepetitive domain common to dragline, flagelliform, and cylindriform spider silk proteins. Biomacromolecules. 2006;7:3120–3124. doi: 10.1021/bm060693x. [DOI] [PubMed] [Google Scholar]
- 33.Askarieh G, Hedhammar M, Nordling K, Saenz A, Casals C, Rising A, Johansson J, Knight SD. Self-assembly of spider silk proteins is controlled by a pH-sensitive relay. Nature. 2010;465:236–238. doi: 10.1038/nature08962. [DOI] [PubMed] [Google Scholar]
- 34.Hagn F, Eisoldt L, Hardy J, Vendrely C, Coles M, Scheibel T, Kessler H. A conserved spider silk domain acts as a molecular switch that controls fibre assembly. Nature. 2010;465:239–242. doi: 10.1038/nature08936. [DOI] [PubMed] [Google Scholar]
- 35.Garb JE, Dimauro T, Vo V, Hayashi CY. Silk genes support the single origin of orb webs. Science. 2006;312:1762. doi: 10.1126/science.1127946. [DOI] [PubMed] [Google Scholar]
- 36.Sponner A, Unger E, Grosse F, Weisshart K. Differential polymerization of the two main protein components of dragline silk during fibre spinning. Nat Mater. 2005;4:772–775. doi: 10.1038/nmat1493. [DOI] [PubMed] [Google Scholar]
- 37.Hu X, Vasanthavada K, Kohler K, McNary S, Moore AM, Vierra CA. Molecular mechanisms of spider silk. Cell Mol Life Sci. 2006;63:1986–1999. doi: 10.1007/s00018-006-6090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kummerlen J, Beek JD, Vollrath F, Meier BH. Local structure in spider dragline silk investigated by two- dimensional spin-diffusion nuclear magnetic resonance. Macromolecules. 1996;29:2920–2928. [Google Scholar]
- 39.Hayashi CY, Shipley NH, Lewis RV. Hypotheses that correlate the sequence, structure, and mechanical properties of spider silk proteins. Int J Biol Macromol. 1999;24:271–275. doi: 10.1016/s0141-8130(98)00089-0. [DOI] [PubMed] [Google Scholar]
- 40.Madsen B, Shao ZZ, Vollrath F. Variability in the mechanical properties of spider silks on three levels: Interspecific, intraspecific and intraindividual. Int J Biol Macromol. 1999;24:301–306. doi: 10.1016/s0141-8130(98)00094-4. [DOI] [PubMed] [Google Scholar]
- 41.Pouchkina-Stantcheva NN, McQueen-Mason SJ. Molecular studies of a novel dragline silk from a nursery web spider, Euprosthenops sp. (pisauridae) Comp Biochem Physiol B Biochem Mol Biol. 2004;138:371–376. doi: 10.1016/j.cbpc.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 42.Keten S, Xu Z, Ihle B, Buehler MJ. Nanoconfinement controls stiffness, strenght and mechanical toughness of β-sheet crystals in silk. Nat Mater. 2010;9:359–367. doi: 10.1038/nmat2704. [DOI] [PubMed] [Google Scholar]
- 43.Kukuruzinska MA, Lennon K. Protein N-glycosylation: molecular genetics and functional significance. Crit Rev Oral Biol Med. 1998;9:415–448. doi: 10.1177/10454411980090040301. [DOI] [PubMed] [Google Scholar]
- 44.Michal CA, Simmons AH, Chew BG, Zax DB, Jelinski LW. Presence of phosphorus in nephila clavipes dragline silk. Biophys J. 1996;70:489–493. doi: 10.1016/S0006-3495(96)79594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao AC, Zhao TF, Nakagaki K, Zhang YS, Sima YH, Miao YG, Shiomi K, Kajiura Z, Nagata Y, Takadera M, Nakagaki M. Novel molecular and mechanical properties of egg case silk from wasp spider, argiope bruennichi. Biochemistry. 2006;45:3348–3356. doi: 10.1021/bi052414g. [DOI] [PubMed] [Google Scholar]
- 46.Hayashi CY, Lewis RV. Molecular architecture and evolution of a modular spider silk protein gene. Science. 2000;287:1477–1479. doi: 10.1126/science.287.5457.1477. [DOI] [PubMed] [Google Scholar]
- 47.Arcidiacono S, Mello C, Kaplan D, Cheley S, Bayley H. Purification and characterization of recombinant spider silk expressed in escherichia coli. Appl Microbiol Biotechnol. 1998;49:31–38. doi: 10.1007/s002530051133. [DOI] [PubMed] [Google Scholar]
- 48.Fahnestock SR, Irwin SL. Synthetic spider dragline silk proteins and their production in escherichia coli. Appl Microbiol Biotechnol. 1997;47:23–32. doi: 10.1007/s002530050883. [DOI] [PubMed] [Google Scholar]
- 49.Prince J, Mcgrath K, Digirolamo C, Kaplan D. Construction, cloning, and expression of synthetic genes encoding spider dragline silk. Biochemistry. 1995;34:10879–10885. doi: 10.1021/bi00034a022. [DOI] [PubMed] [Google Scholar]
- 50.Winkler S, Szela S, Avtges P, Valluzzi R, Kirschner DA, Kaplan D. Designing recombinant spider silk proteins to control assembly. Int J Biol Macromol. 1999;24:265–270. doi: 10.1016/s0141-8130(98)00088-9. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt M, Romer L, Strehle M, Scheibel T. Conquering isoleucine auxotrophy of Escherichia coli BLR(DE3) to recombinantly produce spider silk proteins in minimal media. Biotechnol Lett. 2007;29:1741–1744. doi: 10.1007/s10529-007-9461-z. [DOI] [PubMed] [Google Scholar]
- 52.Fukushima Y. Genetically engineered syntheses of tandem repetitive polypeptides consisting of glycine-rich sequence of spider dragline silk. Biopolymers. 1998;45:269–279. doi: 10.1002/(SICI)1097-0282(19980405)45:4<269::AID-BIP1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 53.Arcidiacono S, Mello CM, Butler M, Welsh E, Soares JW, Allen A, Ziegler D, Laue T, Chase S. Aqueous processing and fiber spinning of recombinant spider silks. Macromolecules. 2002;35:1262–1266. [Google Scholar]
- 54.Winkler S, Wilson D, Kaplan DL. Controlling beta-sheet assembly in genetically engineered silk by enzymatic phosphorylation/dephosphorylation. Biochemistry. 2000;39:14002. doi: 10.1021/bi005119z. [DOI] [PubMed] [Google Scholar]
- 55.Zhou Y, Wu S, Conticello VP. Genetically directed synthesis and spectroscopic analysis of a protein polymer derived from a flagelliform silk sequence. Biomacromolecules. 2001;2:111–125. doi: 10.1021/bm005598h. [DOI] [PubMed] [Google Scholar]
- 56.Stephens JS, Fahnestock SR, Farmer RS, Kiick KL, Chase DB, Rabolt JF. Effects of electrospinning and solution casting protocols on the secondary structure of a genetically engineered dragline spider silk analogue investigated via Fourier transform Raman spectroscopy. Biomacromolecules. 2005;6:1405–1413. doi: 10.1021/bm049296h. [DOI] [PubMed] [Google Scholar]
- 57.Slotta UK, Rammensee S, Gorb S, Scheibel T. An engineered spider silk protein forms microspheres. Angew Chem Int Ed Engl. 2008;47:4592–4594. doi: 10.1002/anie.200800683. [DOI] [PubMed] [Google Scholar]
- 58.Slotta U, Tammer M, Kremer F, Koelsch P, Scheibel T. Structural analysis of films cast from recombinant spider silk proteins. Supramol Chem. 2006;18:465–471. [Google Scholar]
- 59.Bini E, Foo CW, Huang J, Karageorgiou V, Kitchel B, Kaplan DL. RGD-functionalized bioengineered spider dragline silk biomaterial. Biomacromolecules. 2006;7:3139–3145. doi: 10.1021/bm0607877. [DOI] [PubMed] [Google Scholar]
- 60.Huemmerich D, Slotta U, Scheibel T. Processing and modification of films made from recombinant spider silk proteins. Appl Phys A. 2006;82:219–222. [Google Scholar]
- 61.Huang J, Wong C, George A, Kaplan DL. The effect of genetically engineered spider silk-dentin matrix protein 1 chimeric protein on hydroxyapatite nucleation. Biomaterials. 2007;28:2358–2367. doi: 10.1016/j.biomaterials.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 62.Brooks AE, Stricker SM, Joshi SB, Kamerzell TJ, Middaugh CR, Lewis RV. Properties of synthetic spider silk fibers based on argiope aurantia masp2. Biomacromolecules. 2008;9:1506–1510. doi: 10.1021/bm701124p. [DOI] [PubMed] [Google Scholar]
- 63.Liebmann B, Hummerich D, Scheibel T, Fehr M. Formulation of poorly watersoluble substances using self-assembling spider silk protein. Colloids Surf A Physicochem Eng Aspects. 2008;331:126–132. [Google Scholar]
- 64.Mello CM, Soares JW, Arcidiacono S, Butler MM. Acid extraction and purification of recombinant spider silk proteins. Biomacromolecules. 2004;5:1849–1852. doi: 10.1021/bm049815g. [DOI] [PubMed] [Google Scholar]
- 65.Szela S, Avtges P, Valluzzi R, Winkler S, Wilson D, Kirschner D, Kaplan DL. Reduction-oxidation control of beta-sheet assembly in genetically engineered silk. Biomacromolecules. 2000;1:534–542. doi: 10.1021/bm0055697. [DOI] [PubMed] [Google Scholar]
- 66.Wong Po Foo C, Patwardhan SV, Belton DJ, Kitchel B, Anastasiades D, Huang J, Naik RR, Perry CC, Kaplan DL. Novel nanocomposites from spider silk-silica fusion (chimeric) proteins. Proc Natl Acad Sci USA. 2006;103:9428–9433. doi: 10.1073/pnas.0601096103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fahnestock SR, Bedzyk LA. Production of synthetic spider dragline silk protein in pichia pastoris. Appl Microbiol Biotechnol. 1997;47:33–39. doi: 10.1007/s002530050884. [DOI] [PubMed] [Google Scholar]
- 68.Werten MW, Moers AP, Vong T, Zuilhof H, van Hest JC, de Wolf FA. Biosynthesis of an amphiphilic silk-like polymer. Biomacromolecules. 2008;9:1705–1711. doi: 10.1021/bm701111z. [DOI] [PubMed] [Google Scholar]
- 69.Menassa R, Zhu H, Karatzas CN, Lazaris A, Richman A, Brandle J. Spider dragline silk proteins in transgenic tobacco leaves: accumulation and field production. Plant Biotechnol J. 2004;2:431–438. doi: 10.1111/j.1467-7652.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- 70.Lazaris A, Arcidiacono S, Huang Y, Zhou J-F, Duguay F, Chretien N, Welsh EA, Soares JW, Karatzas CN. Spider silk fibers spun from soluble recombinant silk produced in mammalian cells. Science. 2002;259:472–476. doi: 10.1126/science.1065780. [DOI] [PubMed] [Google Scholar]
- 71.Xu HT, Fan BL, Yu SY, Huang YH, Zhao ZH, Lian ZX, Dai YP, Wang LL, Liu ZL, Fei J, Li N. Construct synthetic gene encoding artificial spider dragline silk protein and its expression in milk of transgenic mice. Anim Biotechnol. 2007;18:1–12. doi: 10.1080/10495390601091024. [DOI] [PubMed] [Google Scholar]
- 72.Williams D. Sows’ ears, silk purses and goats’ milk: new production methods and medical applications for silk. Med Device Technol. 2003;14:9–11. [PubMed] [Google Scholar]
- 73.Huemmerich D, Scheibel T, Vollrath F, Cohen S, Gat U, Ittah S. Novel assembly properties of recombinant spider dragline silk proteins. Curr Biol. 2004;14:2070–2074. doi: 10.1016/j.cub.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 74.Ittah S, Cohen S, Garty S, Cohn D, Gat U. An essential role for the C-terminal domain of a dragline spider silk protein in directing fiber formation. Biomacromolecules. 2006;7:1790–1795. doi: 10.1021/bm060120k. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y, Hu J, Miao Y, Zhao A, Zhao T, Wu D, Liang L, Miikura A, Shiomi K, Kajiura Z, Nakagaki M. Expression of EGFP-spider dragline silk fusion protein in bmn cells and larvae of silkworm showed the solubility is primary limit for dragline proteins yield. Mol Biol Rep. 2008;35:329–335. doi: 10.1007/s11033-007-9090-6. [DOI] [PubMed] [Google Scholar]
- 76.Wen H, Lan X, Zhang Y, Zhao T, Wang Y, Kajiura Z, Nakagaki M. Transgenic silkworms (Bombyx mori) produce recombinant spider dragline silk in cocoons. Mol Biol Rep. 2010;37:1815–1821. doi: 10.1007/s11033-009-9615-2. [DOI] [PubMed] [Google Scholar]
- 77.Zhu Z, Kikuchi Y, Kojima K, Tamura T, Kuwabara N, Nakamura T, Asakura T. Mechanical properties of regenerated Bombyx mori silk fibers and recombinant silk fibers produced by transgenic silkworms. J Biomater Sci Polym Ed. 2010;21:395–411. doi: 10.1163/156856209X423126. [DOI] [PubMed] [Google Scholar]
- 78.Huemmerich D, Helsen CW, Quedzuweit S, Oschmann J, Rudolph R, Scheibel T. Primary structure elements of spider dragline silks and their contribution to protein solubility. Biochemistry. 2004;43:13604–13612. doi: 10.1021/bi048983q. [DOI] [PubMed] [Google Scholar]
- 79.Lewis RV, Hinman M, Kothakota S, Fournier MJ. Expression and purification of a spider silk protein: a new strategy for producing repetitive proteins. Protein Expr Purif. 1996;7:400–406. doi: 10.1006/prep.1996.0060. [DOI] [PubMed] [Google Scholar]
- 80.Scheller J, Gührs K-H, Grosse F, Conrad U. Production of spider silk proteins in tobacco and potato. Nat Biotechnol. 2001;19:573–577. doi: 10.1038/89335. [DOI] [PubMed] [Google Scholar]
- 81.Junghans F, Morawietz U, Conrad U, Scheibel T, Heilmann A, Spohn U. Preparation and mechanical properties of layers made of recombinant spider silk proteins and silk from silk worm. Appl Phys A. 2006;82:253–260. [Google Scholar]
- 82.Exler JH, Hummerich D, Scheibel T. The amphiphilic properties of spider silks are important for spinning. Angew Chem Int Ed Engl. 2007;46:3559–3562. doi: 10.1002/anie.200604718. [DOI] [PubMed] [Google Scholar]
- 83.Rammensee S, Slotta U, Scheibel T, Bausch AR. Assembly mechanism of recombinant spider silk proteins. Proc Natl Acad Sci USA. 2008;105:6590–6595. doi: 10.1073/pnas.0709246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lammel A, Schwab M, Slotta U, Winter G, Scheibel T. Processing conditions for spider silk microsphere formation. ChemSusChem. 2008;5:413–416. doi: 10.1002/cssc.200800030. [DOI] [PubMed] [Google Scholar]
- 85.Geisler M, Pirzer T, Ackerschott C, Lud S, Garrido J, Scheibel T, Hugel T. Hydrophobic and hofmeister effects on the adhesion of spider silk proteins onto solid substrates: an AFM-based single-molecule study. Langmuir. 2008;24:1350–1355. doi: 10.1021/la702341j. [DOI] [PubMed] [Google Scholar]
- 86.Bogush VG, Sokolova OS, Davydova LI, Klinov DV, Sidoruk KV, Esipova NG, Neretina TV, Orchanskyi IA, Makeev VY, Tumanyan VG, Shaitan KV, Debabov VG, Kirpichnikov MP. A novel model system for design of biomaterials based on recombinant analogs of spider silk proteins. J Neuroimmun Pharmacol. 2009;4:17–27. doi: 10.1007/s11481-008-9129-z. [DOI] [PubMed] [Google Scholar]
- 87.Agapov II, Pustovalova OL, Moisenovich MM, Bogush VG, Sokolova OS, Sevastyanov VI, Debabov VG, Kirpichnikov MP. Three-dimensional scaffold made from recombinant spider silk protein for tissue engineering. Dokl Biochem Biophys. 2009;426:127–130. doi: 10.1134/s1607672909030016. [DOI] [PubMed] [Google Scholar]
- 88.Lin Z, Huang W, Zhang J, Fan JS, Yang D. Solution structure of eggcase silk protein and its implications for silk fiber formation. Proc Natl Acad Sci USA. 2009;106:8906–8911. doi: 10.1073/pnas.0813255106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Valluzzi R, Szela S, Avtges P, Kirschner D, Kaplan D. Methionine redox controlled crystallization of biosynthetic silk spidroin. J Phys Chem B. 1999;103:11382–11392. [Google Scholar]
- 90.Fredriksson C, Hedhammar M, Feinstein R, Nordling K, Kratz G, Johansson J, Huss F, Rising A. Tissue response to subcutaneously implanted recombinant spider silk: an in vivo study. Materials. 2009;2:1908–1922. [Google Scholar]
- 91.Hedhammar M, Bramfeldt H, Baris T, Widhe M, Askarieh G, Nordling K, von Aulock S, Johansson J. Sterilized recombinant spider silk fibers of low pyrogenicity. Biomacromolecules. 2010;11:953–959. doi: 10.1021/bm9014039. [DOI] [PubMed] [Google Scholar]
- 92.Hedhammar M, Rising A, Grip S, Martinez AS, Nordling K, Casals C, Stark M, Johansson J. Structural properties of recombinant nonrepetitive and repetitive parts of major ampullate spidroin 1 from euprosthenops australis: implications for fiber formation. Biochemistry. 2008;47:3407–3417. doi: 10.1021/bi702432y. [DOI] [PubMed] [Google Scholar]
- 93.Grip S, Johansson J, Hedhammar M. Engineered disulfides improve mechanical properties of recombinant spider silk. Protein Sci. 2009;18:1012–1022. doi: 10.1002/pro.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seidel A, Liivak O, Calve S, Adaska J, Ji G, Yang Z, Grubb D, Zax DB, Jelinski LW. Regenerated spider silk: processing, properties, and structure. Macromolecules. 2000;33:775–780. [Google Scholar]
- 95.Shao Z, Vollrath F, Yang Y, Thogersen HC. Structure and behavior of regenerated spider silk. Macromolecules. 2003;36:1157–1161. [Google Scholar]
- 96.Teulé F, Furin WA, Cooper AR, Duncan JR, Lewis RV. Modifications of spider silk sequences in an attempt to control the mechanical properties of the synthetic fibers. J Mater Sci. 2007;42:8974–8985. [Google Scholar]
- 97.Yang J, Barr LA, Fahnestock SR, Liu ZB. High yield recombinant silk-like protein production in transgenic plants through protein targeting. Transgenic Res. 2005;14:313–324. doi: 10.1007/s11248-005-0272-5. [DOI] [PubMed] [Google Scholar]
- 98.Zhou S, Peng H, Yu X, Zheng X, Cui W, Zhang Z, Li X, Wang J, Weng J, Jia W, Li F. Preparation and characterization of a novel electrospun spider silk fibroin/poly(d, l-lactide) composite fiber. J Phys Chem B. 2008;112:11209–11216. doi: 10.1021/jp800913k. [DOI] [PubMed] [Google Scholar]
- 99.Metwalli E, Slotta U, Darko C, Roth S, Scheibel T, Papadakis C. Structural changes of thin films from recombinant spider silk proteins upon post treatment. Appl Phys A. 2007;89:655–661. [Google Scholar]
- 100.Scheller J, Henggeler D, Viviani A, Conrad U. Purification of spider silk-elastin from transgenic plants and application for human chondrocyte proliferation. Transgenic Res. 2004;13:51–57. doi: 10.1023/b:trag.0000017175.78809.7a. [DOI] [PubMed] [Google Scholar]
- 101.Hermanson KD, Harasim MB, Scheibel T, Bausch AR. Permeability of silk microcapsules made by the interfacial adsorption of protein. Phys Chem Chem Phys. 2007;9:6442–6446. doi: 10.1039/b709808a. [DOI] [PubMed] [Google Scholar]
- 102.Kenney JM, Knight D, Wise MJ, Vollrath F. Amyloidogenic nature of spider silk. Eur J Biochem. 2002;269:4159–4163. doi: 10.1046/j.1432-1033.2002.03112.x. [DOI] [PubMed] [Google Scholar]
- 103.Thiel BL, Guess KB, Viney C. Non-periodic lattice crystals in the hierarchical microstructure of spider (major ampullate) silk. Biopolymers. 1997;41:703–719. doi: 10.1002/(SICI)1097-0282(199706)41:7<703::AID-BIP1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 104.Sponner A, Unger E, Grosse F, Weisshart K. Conserved C-termini of spidroins are secreted by the major ampullate glands and retained in the silk thread. Biomacromolecules. 2004;5:840–845. doi: 10.1021/bm034378b. [DOI] [PubMed] [Google Scholar]
- 105.Eisoldt L, Hardy JG, Heim M, Scheibel TR. The role of salt and shear on the storage and assembly of spider silk proteins. J Struct Biol. 2010;170:413–419. doi: 10.1016/j.jsb.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 106.Jin HJ, Kaplan DL. Mechanism of silk processing in insects and spiders. Nature. 2003;424:1057–1061. doi: 10.1038/nature01809. [DOI] [PubMed] [Google Scholar]
- 107.Allmeling C, Jokuszies A, Reimers K, Kall S, Vogt PM. Use of spider silk fibres as an innovative material in a biocompatible artificial nerve conduit. J Cell Mol Med. 2006;10:770–777. doi: 10.1111/j.1582-4934.2006.tb00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gellynck K, Verdonk PC, Van Nimmen E, Almqvist KF, Gheysens T, Schoukens G, Van Langenhove L, Kiekens P, Mertens J, Verbruggen G. Silkworm and spider silk scaffolds for chondrocyte support. J Mater Sci Mater Med. 2008;19:3399–3409. doi: 10.1007/s10856-008-3474-6. [DOI] [PubMed] [Google Scholar]
- 109.Hakimi O, Gheysens T, Vollrath F, Grahn MF, Knight DP, Vadgama P. Modulation of cell growth on exposure to silkworm and spider silk fibers. J Biomed Mater Res A. 2010;92:1366–1372. doi: 10.1002/jbm.a.32462. [DOI] [PubMed] [Google Scholar]
- 110.Allmeling C, Jokuszies A, Reimers K, Kall S, Choi CY, Brandes G, Kasper C, Scheper T, Guggenheim M, Vogt PM. Spider silk fibres in artificial nerve constructs promote peripheral nerve regeneration. Cell Prolif. 2008;41:408–420. doi: 10.1111/j.1365-2184.2008.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vollrath F, Barth P, Basedow A, Engstrom W, List H. Local tolerance to spider silks and protein polymers in vivo. In Vivo. 2002;16:229–234. [PubMed] [Google Scholar]
- 112.Gellynck K, Verdonk P, Forsyth R, Almqvist KF, Van Nimmen E, Gheysens T, Mertens J, Van Langenhove L, Kiekens P, Verbruggen G. Biocompatibility and biodegradability of spider egg sac silk. J Mater Sci Mater Med. 2008;19:2963–2970. doi: 10.1007/s10856-007-3330-0. [DOI] [PubMed] [Google Scholar]
- 113.Baoyong L, Jian Z, Denglong C, Min L (2010) Evaluation of a new type of wound dressing made from recombinant spider silk protein using rat models. Burns. doi:10.1016/j.burns.2009.12.001 [DOI] [PubMed]
- 114.Morgan AW, Roskov KE, Lin-Gibson S, Kaplan DL, Becker ML, Simon CG., Jr Characterization and optimization of rgd-containing silk blends to support osteoblastic differentiation. Biomaterials. 2008;29:2556–2563. doi: 10.1016/j.biomaterials.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 115.Wang H, Wei M, Zue Z, Li M. Cytocompatibility study of Arg-Gly-Asp-recombinant spider silk protein/poly vinyl alcohol scaffold (in Chinese) Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23:747–750. [PubMed] [Google Scholar]
- 116.Colgin MA, Lewis RV. Spider minor ampullate silk proteins contain new repetitive sequences and highly conserved non-silk-like “Spacer regions”. Protein Sci. 1998;7:667–672. doi: 10.1002/pro.5560070315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hayashi CY, Blackledge TA, Lewis RV. Molecular and mechanical characterization of aciniform silk: uniformity of iterated sequence modules in a novel member of the spider silk fibroin gene family. Mol Biol Evol. 2004;21:1950–1959. doi: 10.1093/molbev/msh204. [DOI] [PubMed] [Google Scholar]
- 118.Vasanthavada K, Hu X, Falick AM, La Mattina C, Moore AM, Jones PR, Yee R, Reza R, Tuton T, Vierra C. Aciniform spidroin, a constituent of egg case sacs and wrapping silk fibers from the black widow spider Latrodectus hesperus . J Biol Chem. 2007;282:35088–35097. doi: 10.1074/jbc.M705791200. [DOI] [PubMed] [Google Scholar]
- 119.Tian M, Lewis RV. Molecular characterization and evolutionary study of spider tubuliform (eggcase) silk protein. Biochemistry. 2005;44:8006–8012. doi: 10.1021/bi050366u. [DOI] [PubMed] [Google Scholar]
- 120.Hu X, Lawrence B, Kohler K, Falick AM, Moore AM, McMullen E, Jones PR, Vierra C. Araneoid egg case silk: a fibroin with novel ensemble repeat units from the black widow spider, Latrodectus hesperus . Biochemistry. 2005;44:10020–10027. doi: 10.1021/bi050494i. [DOI] [PubMed] [Google Scholar]
- 121.Hu X, Kohler K, Falick AM, Moore AM, Jones PR, Sparkman OD, Vierra C. Egg case protein-1. A new class of silk proteins with fibroin-like properties from the spider Latrodectus hesperus . J Biol Chem. 2005;280:21220–21230. doi: 10.1074/jbc.M412316200. [DOI] [PubMed] [Google Scholar]
- 122.Hu X, Kohler K, Falick AM, Moore AM, Jones PR, Vierra C. Spider egg case core fibers: trimeric complexes assembled from TuSP1, ECP-1, and ECP-2. Biochemistry. 2006;45:3506–3516. doi: 10.1021/bi052105q. [DOI] [PubMed] [Google Scholar]
- 123.Zhao A, Zhao T, Sima Y, Zhang Y, Nakagaki K, Miao Y, Shiomi K, Kajiura Z, Nagata Y, Nakagaki M. Unique molecular architecture of egg case silk protein in a spider, Nephila clavata . J Biochem (Tokyo) 2005;138:593–604. doi: 10.1093/jb/mvi155. [DOI] [PubMed] [Google Scholar]
- 124.Bittencourt D, Souto BM, Verza NC, Vinecky F, Dittmar K, Silva PI, Jr, Andrade AC, da Silva FR, Lewis RV, Rech EL. Spidroins from the brazilian spider Nephilengys cruentata (araneae: Nephilidae) Comp Biochem Physiol B Biochem Mol Biol. 2007;147:597–606. doi: 10.1016/j.cbpb.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 125.Hu X, Yuan J, Wang X, Vasanthavada K, Falick AM, Jones PR, La Mattina C, Vierra CA. Analysis of aqueous glue coating proteins on the silk fibers of the cob weaver, Latrodectus hesperus . Biochemistry. 2007;46:3294–3303. doi: 10.1021/bi602507e. [DOI] [PubMed] [Google Scholar]
- 126.Blasingame E, Tuton-Blasingame T, Larkin L, Falick AM, Zhao L, Fong J, Vaidyanathan V, Visperas A, Geurts P, Hu X, La Mattina C, Vierra C. Pyriform spidroin 1, a novel member of the silk gene family that anchors dragline silk fibers in attachment discs of the black widow spider, Latrodectus hesperus . J Biol Chem. 2009;284:29097–29108. doi: 10.1074/jbc.M109.021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cappello J, Crissman J, Dorman M, Mikolajczak M, Textor G, Marquet M, Ferrari F. Genetic engineering of structural protein polymers. Biotechnol Prog. 1990;6:198–202. doi: 10.1021/bp00003a006. [DOI] [PubMed] [Google Scholar]
- 128.Yang M, Asakura T. Design, expression and solid-state NMR characterization of silk-like materials constructed from sequences of spider silk, Samia cynthia ricini and Bombyx mori silk fibroins. J Biochem. 2005;137:721–729. doi: 10.1093/jb/mvi090. [DOI] [PubMed] [Google Scholar]
- 129.Sponner A, Vater W, Rommerskirch W, Vollrath F, Unger E, Grosse F, Weisshart K. The conserved C-termini contribute to the properties of spider silk fibroins. Biochem Biophys Res Commun. 2005;338:897–902. doi: 10.1016/j.bbrc.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 130.Heim M, Ackerschott CB, Scheibel T. Characterization of recombinantly produced spider flagelliform silk domains. J Struct Biol. 2010;170(2):420–425. doi: 10.1016/j.jsb.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 131.Widmaier DM, Tullman-Ercek D, Mirsky EA, Hill R, Govindarajan S, Minshull J, Voigt CA. Engineering the salmonella type III secretion system to export spider silk monomers. Mol Syst Biol. 2009;5:309. doi: 10.1038/msb.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Patel J, Zhu H, Menassa R, Gyenis L, Richman A, Brandle J. Elastin-like polypeptide fusions enhance the accumulation of recombinant proteins in tobacco leaves. Transgenic Res. 2007;16:239–249. doi: 10.1007/s11248-006-9026-2. [DOI] [PubMed] [Google Scholar]
- 133.Grip S, Rising A, Nummervoll H, Storkenfeldt E, McQueen-Mason SJ, Pouchkina-Stantcheva N, Vollrath F, Engström W, Fernandez-Arias A. Transient expression of a major ampullate spidroin 1 gene fragment from Euprosthenops sp. in mammalian cells. Cancer Genomics Proteomics. 2006;3:83–88. [PubMed] [Google Scholar]
- 134.Ittah S, Michaeli A, Goldblum A, Gat U. A model for the structure of the C-terminal domain of dragline spider silk and the role of its conserved cysteine. Biomacromolecules. 2007;8:2768–2773. doi: 10.1021/bm7004559. [DOI] [PubMed] [Google Scholar]
- 135.Ittah S, Barak N, Gat U. A proposed model for dragline spider silk self-assembly: insights from the effect of the repetitive domain size on fiber properties. Biopolymers. 2009;93:458–468. doi: 10.1002/bip.21362. [DOI] [PubMed] [Google Scholar]
- 136.Lee KS, Kim BY, Je YH, Woo SD, Sohn HD, Jin BR. Molecular cloning and expression of the c-terminus of spider flagelliform silk protein from araneus ventricosus. J Biosci. 2007;32:705–712. doi: 10.1007/s12038-007-0070-8. [DOI] [PubMed] [Google Scholar]
- 137.Miao Y, Zhang Y, Nakagaki K, Zhao T, Zhao A, Meng Y, Nakagaki M, Park EY, Maenaka K. Expression of spider flagelliform silk protein in bombyx mori cell line by a novel Bac-to-Bac/BmNPV baculovirus expression system. Appl Microbiol Biotechnol. 2006;71:192–199. doi: 10.1007/s00253-005-0127-2. [DOI] [PubMed] [Google Scholar]