Abstract
Covalently modifying a protein has proven to be a powerful mechanism of functional regulation. N-epsilon acetylation of lysine residues was initially discovered on histones and has been studied extensively in the context of chromatin and DNA metabolism, such as transcription, replication and repair. However, recent research shows that acetylation is more widespread than initially thought and that it regulates various nuclear as well as cytoplasmic and mitochondrial processes. In this review, we present the multitude of non-histone proteins targeted by lysine acetyltransferases of the large and conserved MYST family, and known functional consequences of this acetylation. Substrates of MYST enzymes include factors involved in transcription, heterochromatin formation and cell cycle, DNA repair proteins, gluconeogenesis enzymes and finally subunits of MYST protein complexes themselves. Discovering novel substrates of MYST proteins is pivotal for the understanding of the diverse functions of these essential acetyltransferases in nuclear processes, signaling, stress response and metabolism.
Keywords: MYST, Acetyltransferases, Histones, Lysines, TIP60, MOF, NuA4, HBO1
Introduction
Acetylation of the epsilon-amino group of a lysine residue of a protein is increasingly proving to be an important post-translational modification for regulating cellular phenomena. This reaction is catalyzed by specialized enzymes called lysine acetyltransferases (KAT) and is counteracted by the enzymatic activity of lysine deacetylases (KDACs) [1]. It has to be noted here that this modification is distinct from the N-alpha acetylation of the amino-terminus of proteins that occurs during translation.
The entire set of acetylated proteins in a cell is referred to as “acetylome” [2]. Over the past 5 years, several laboratories have embarked on studying the acetylome of various organisms and tissues [3–7]. A recent study identified 3,600 acetylation sites on 1,750 proteins in an analysis of three human cell lines [3]. Adding an acetyl group on an amino side chain neutralizes a positive charge, changes the overall size of the amino acid, and alters the local hydrophobicity. These changes in the properties of the substrate peptide/protein can have a significant impact on its conformation and therefore function, e.g., its enzymatic activity. Acetylation of a lysine residue also generates docking sites for binding by other proteins. A number of proteins contain acetylation-recognising modules, e.g., the bromodomain, that bind specifically to acetylated lysines [8]. Finally, an acetylated lysine can interplay with other modifications: competition with modifications on the same residue or crosstalk with modification on neighboring residues [9]. Lysine acetylation was first discovered in histones [10] and has been extensively studied in terms of its function in transcription, DNA replication and DNA repair. However, recent studies provide increasing evidence that, apart from this established role in DNA metabolism, acetylation regulates diverse cellular pathways inside and outside the nucleus [3, 5–7]. A list of processes where acetylated proteins are involved in is shown in Table 1 (see also Figs. 1 and 2.)
Table 1.
| Histones | Folding proteins |
| Regulators of transcription | Intracellular signaling pathways transducers |
| Regulators of chromatin structure | Carbohydrate metabolism enzymes |
| DNA replication factors | Fatty acid metabolism enzymes |
| DNA repair factors | Nucleotide biosynthesis enzymes |
| Splicing factors | Amino acid biosynthesis enzymes |
| Pre-mRNA processing | TCA cycle enzymes |
| mRNA stability proteins | Mitochondrial proteins |
| Translation proteins | Cytoskeleton: structural proteins, cell motility proteins |
| Cell cycle factors | Nuclear lamins |
| Circadian rhythm proteins | Longevity enzymes |
| Ubiquitylation/proteosome/stability proteins | Viral proteins |
| Chaperones |
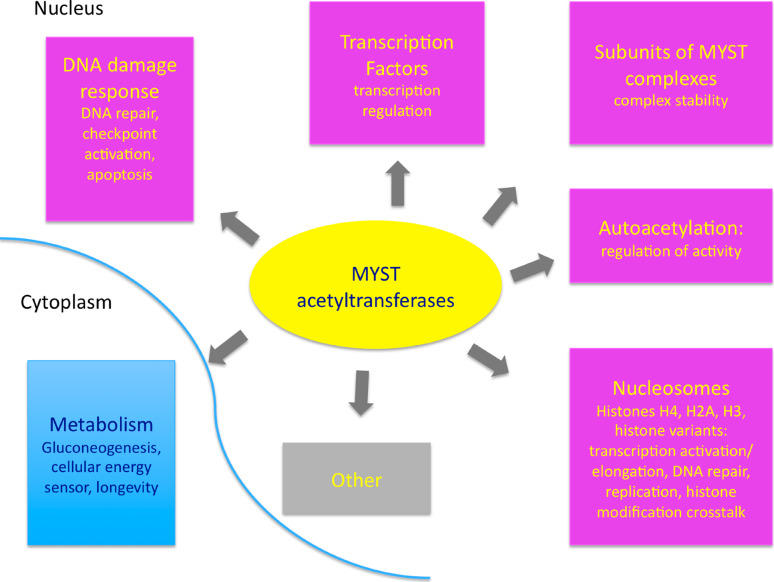
Fig. 1.
The substrates of MYST acetyltransferases can be classified in distinct classes. The majority of the MYST acetyltransferases substrates are nuclear, although cytoplasmic substrates have been reported recently. The best studied MYST substrate class is histones, namely histones H4, H3, H2A and H2A variants. Histone acetylation by MYST proteins has impacts on transcription, DNA repair, DNA replication and other nuclear processes via histone modification crosstalks. Other typical substrates of MYST enzymes are subunits of MYST multiprotein complexes, including MYST proteins themselves. Aceytlation of MYST complex subunits mostly regulates complex stability and possibly target specificity. Another class of MYST substrates are transcription factors whose protein stability and transcription activity can be modified by MYST-dependent acetylation. Proteins involved in DNA damage response can also be acetylated by MYST proteins and this modification can increase kinase activity and checkpoint activation or the choice between cell cycle arrest and apoptosis. Finally, the only known cytoplasmic substrate of MYST enzymes so far is involved in glucose metabolism and acetylation of this substrate by a MYST enzyme regulates their involvement in lifespan elongation. See text for more information
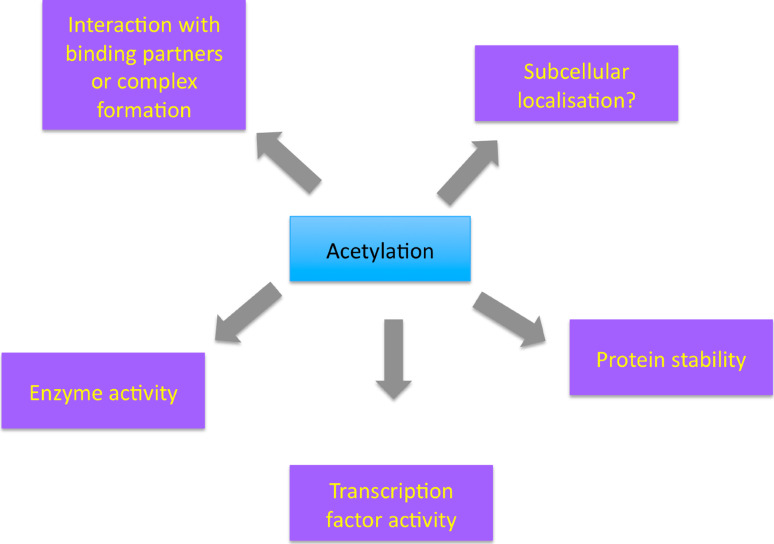
Fig. 2.
Effects of acetylation on the non-histone substrates of MYST acetyltransferases. Acetylation of a non-histone substrate by a MYST enzyme can have an impact on its stability and degradation rate. Acetylation can also regulate enzymatic or transcription factor activity. Substrate modification modulates the interaction between proteins, e.g., subunits of the same complex, or affects localization and recruitment to functional sites in the cell
The vast majority of known KATs target histones, with very few exceptions, e.g., Eco1 [11]. KATs can be predominantly cytoplasmic or predominantly nuclear. In general, cytoplasmic KATs acetylate free newly synthesized histones while nuclear KATs preferentially target nucleosomal histones (assembled into chromatin). A multitude of KATs has been discovered and characterized: the list of KATs includes enzymes that have various roles, such as transcription factors, transcription coactivators and transcription elongators. By acting in vivo as parts of multisubunit complexes, KATs can optimize their efficiency and versatility: the subunits of KAT complexes can stimulate overall enzymatic activity, direct substrate specificity and allow recruitment to specific loci. Also, the KAT complex subunits sometimes facilitate the interaction between the complex and its substrate via specialized protein domains they may contain (reviewed in [12, 13]).
General information on KATs can be found in the several reviews that have been published in the recent years that analyze and summarize their functions (e.g., [13, 14]). This review will focus on one of the three major families of lysine acetyltransferases, called MYST (MOZ, Ybf2/Sas3, Sas2, TIP60). The MYST proteins have a well-established role on histone acetylation; however, their substrate spectrum is broader and new non-histone proteins regulated by MYST are continuously being discovered. Here, we will summarize what is currently known about non-chromatin substrates of MYST acetyltransferases.
MYST acetyltransferases
MYST acetyltransferases are defined by a distinct conserved histone acetyltransferase domain (reviewed in [15–19]). The MYST domain contains a C2HC zinc finger and an acetyl-CoA binding site homologous to the canonical AcCoA binding domain of another KAT family, the GNAT superfamily of acetyltransferases. Individual members of the MYST family contain additional structural features, such as chromodomains, PHD and zinc fingers. Table 2 lists the MYST proteins in the most commonly used model organisms and some of their functional features. MYST proteins are found throughout the phylogenetic tree and have a diverse variety of functions affecting the majority of cellular processes, ranging from gene regulation to DNA repair, cell cycle to stem cell homeostasis and development (reviewed in [15, 19–21]. Most of these processes are mediated via MYST-dependent regulation of chromatin; however, there are certain cell pathways in which the role of MYST is independent of histone acetylation (e.g., [22]). Previous reviews listed above provide a complete picture of the different MYST enzymes, their structural characteristics, their associated proteins and their different functions and pathological dysfunctions through acetylation of chromatin during gene regulation, DNA replication, repair and recombination, cell division/differentiation and development. This review is primarily focused on recently discovered non-chromatin substrates of MYST enzymes and their implication in regulating diverse cellular processes, expanding the already multifunctional aspect of this highly conserved and essential family of acetyltransferases.
Table 2.
MYST acetyltransferases in major model organisms
| MYST proteins | KAT5 | KAT6 | KAT7 | KAT8 |
|---|---|---|---|---|
| S. cerevisiae | Esa1 | Sas3 | Sas2 | |
| S. pombe | Mst1 | Mst2 | ||
| C. elegans | mys-1 | mys-3 mys-4 | mys-2 | |
| D. melanogaster | dTIP60 | ENOK CG1894 | CHM | MOF |
| M. musculus | TIP60 | KAT6A/MOZ/MYST3 KAT6B/Qkf/MORF/MYST4 | HBO1/MYST2 | MOF/MYST1 |
| H. sapiens | TIP60/PLIP | KAT6A/MOZ/MYST3 KAT6B/MORF/MYST4 | HBO1/MYST2 | MOF/MYST1 |
| Associated domains | Chromodomain | PHD fingers | Zn finger | Chromodomain |
| Histone specificity | H4/H2A | H3 | H4 > H3 | H4K16 |
| Roles | Gene regulation, DNA damage repair/response, development/stem cell renewal, essential for cell viability | Gene regulation, development/stem cell renewal | Gene regulation, DNA replication, chromatin boundary, development | Gene regulation, DNA damage response, dosage compensation, chromatin boundary, early development |
Non-chromatin substrates of MYST proteins
MOZ (monocytic leukemia zinc finger protein) and HBO1 (histone acetyltransferase binding to ORC1)
MOZ/MYST3/KAT6A is an acetyltransferase that targets H3, is often mutated in human leukaemia, and is essential for the maintenance of hematopoietic stem cells and normal development [20, 23–26]. MOZ is very closely related to MORF/MYST4/KAT6B, Mm Qkf and Dm ENOK. Functionally, the related protein in yeast is Sas3/KAT6, a MYST protein involved in transcription activation and elongation and DNA replication by acetylating H3 at lysines K14 and K23 [27, 28].
MOZ was found to acetylate the AML1 transcription factor in vitro [29]. MOZ is a transcriptional coactivator of AML1, a transcription factor encoded by a gene frequently translocated in many types of leukaemia. As yet, the effect of this modification has not been studied, but it is logical to presume that this acetylation is involved in AML1 coactivation.
Hs HBO1/MYST2 and Dm Chameau (KAT7) are MYST proteins involved in transcription and DNA replication via acetylation of H4 at lysines K5, K8 and K12 and H3 at lysine 14 [30–34]. HBO1 targets its associated factor JADE1 as well as MCM replicative helicase proteins in vitro (Saksouk et al., unpublished data), and these are indeed found acetylated at physiological levels in vivo [3, 5]. Moreover, in vitro experiments also suggest that the prereplicative complex components Mcm2, Cdc6, Geminin and Orc2 can be acetylated by HBO1 [35]. Taken together, these findings support a role of HBO1 in the regulation of the prereplicative complex assembly by acetylation of the chromatin at origins as well as the prereplicative complex itself [32, 35]. Acetylation of the MCM helicase could also be important for HBO1 function during replication fork progression [34].
MOF (males absent on the first)
Global histone H4 K16 acetylation in most organisms is due to the KAT8/Sas2/MOF MYST protein [13, 36–39]. In yeast, Sas2 has a well-documented role at the boundaries between euchromatin and heterochromatin such as telomeres, rDNA loci and mating-type loci, where Sas2 antagonizes the spreading of SIR proteins and regulates gene silencing [38, 39]. The best-characterized role of Dm MOF is that in sex chromosome dosage compensation, while Hs MOF/MYST1 has been implicated in transcription and DNA repair [36, 40–43].
Dosage compensation is the mechanism by which the levels of X-linked gene expression are equalized between the two sexes. In Drosophila, dosage compensation is achieved by two-fold hypertranscription of the male chromosome by dosage compensation complex (DCC). The DCC complex consists of five protein subunits, MSL1, MSL2, MSL3, MLE and MOF as well as two RNA molecules, roX1 and roX2 and, as mentioned above, is responsible for long-range acetylation of H4 at lysine K16 along the X chromosome [43].
In the context of DCC, MOF specifically acetylates only H4K16. However, if the DCC is not fully assembled, MOF can also have non-nucleosomal targets. MOF acetylates MSL1 in vitro [44] and MSL3 in vitro and in vivo [45]. MSL3 is acetylated in a MOF-dependent manner at lysine K116, a residue immediately C-terminal to chromodomain 1 of MSL3, a domain shown to interact with roX RNAs [46]. According to the proposed model, regulated acetylation of MSL3 causes a conformational change that results in loss of MSL3 interaction with roX2 RNA and subsequent disassembly of the complex. However, it is not clear from the literature if non-DCC-bound MOF is responsible for this modification. Rpd3 then interacts with and deacetylates MSL3, which allows re-assembly of the complex at a nearby site and spreading of DCC complex along the chromosome.
In mammals, MOF has distinct roles compared with those in Drosophila, as dosage compensation in mammals occurs by inactivating one of the copies of the X-chromosome. In mammals, too, MOF acetylates H4K16 and has a role in transcription and in DNA damage repair [36, 37, 40, 42]. In human cells, two MOF-containing complexes have been characterized: the MOF–MSL complex, which is similar to the DCC but lacks roX and MLE, and the quite distinct MOF–MSL1v1 complex, consisting of hMOF, MSL1v1, PHF20, MRCS1/2, WDR5, HCF1 and nuclear pore components [37, 41, 42, 47, 48]. Both complexes acetylate H4, but the MOF–MSL1v1 specifically acetylates p53 at lysine K120 [41, 49]. K120 lies within the DNA-binding domain of p53, and its acetylation is required for the p53-dependent transcription of preapoptotic genes such as BAX and PUMA in response to unrepairable DNA damage. In contrast, acetylation of K120 of p53 is not required for the expression of cell cycle arrest genes such as p21. TIP60 as well as hMOF targets this residue and this redundancy highlights the importance of this modification, although the preference of acetyltransferase also seems to be tissue specific [41, 49, 50]. The acetylation of p53 at K120 will be further discussed in the following section.
hMOF also interacts with ATM and DNA-PK, and it has been proposed that hMOF exerts its role in DNA damage repair via direct acetylation of ATM, although this has not yet been proven [40, 42].
Finally, another target of MOF in mammalian cells is TIP5 [51]. TIP5 is a subunit of NoRC, a potent epigenetic repressor which silences rDNA repeats via histone deacetylation/methylation and DNA methylation. MOF acetylates Mm TIP5 at lysine K633 and this is followed by deacetylation by SIRT1. It is not clear which MOF complex is responsible for this modification. The reversible acetylation of TIP5 regulates its association and dissociation with pRNA (RNA complementary to rDNA promoter) and promotes NoRC-dependent heterochromatin formation and silencing.
TIP60 (tat interacting protein 60 kD)
TIP60/KAT5 is the most studied MYST acetyltransferase. By acetylating histone H4 at lysines 5, 8, 12 and 16, and also H2A, H2A.X and H2A.Z (Dm H2Av), it regulates the transcription of genes involved in a vast variety of processes, regulates embryonic stem cell identity, and also modifies chromatin around DNA double-strand breaks (DSBs) to help repair [21, 52–57].
TIP60 has by far the most known non-histone targets. The majority of these substrates are transcription factors and almost all of them are closely related to transcriptional control. Acetylation of a non-histone substrate by TIP60 can have different consequences, but most often it affects protein stability or protein activity.
Examples of TIP60-mediated acetylation affecting protein stability are the modifications of tumor suppressor Rb and oncogene c-Myc. TIP60 acetylates the C-terminal region of Rb in whole cells, then acetylated RB is targeted for degradation by the proteosome [58]. The p14ARF tumor suppressor inhibits TIP60-dependent acetylation of Rb, thus inducing its accumulation in cells and promoting the anti-proliferative properties of Rb.
Patel et al. [59] found that the levels of acetylated c-Myc in whole cells are dependent on TIP60 and that TIP60 regulates c-Myc protein stability. However, more recent studies found that recombinant TIP60 cannot acetylate c-Myc in vitro [60]. This can be due to the fact that TIP60 needs to be part of the TIP60/NuA4 complex in order to acetylate c-Myc in vitro (as is the case for chromatin substrates [61]), or that the effect seen in whole cells is indirect, via an unknown acetylation pathway. Nevertheless, the positive role of TIP60 in c-Myc-dependent transcription is now well established in stem cells and through the TIP60-associated factor TRRAP [62–65]. On the other hand, it is difficult to draw a fully coherent picture based on the literature since TIP60 was also shown to act as a tumor suppressor in a c-Myc-driven model of lymphoma [66].
Transcription factors or enzymes whose activity is regulated by TIP60-mediated acetylation include UBF, ATM, PLAGL2, androgen receptor, Notch1 and p53. For example, UBF was reported to interact with TIP60 and to be acetylated in vitro by TIP60 [67]. Also, TIP60 seems to acetylate the DNA damage kinase ATM [68] and the PLAGL2 oncogene [69]. Gaughan et al. [70] have found that, in order for TIP60 to coactivate androgen receptor, apart from acetylating nucleosomes at androgen-responsive promoters, TIP60 directly acetylates the receptor at a KLKK site (residues 630–633) within the hinge domain.
The signaling receptor Notch1 is negatively regulated by TIP60 [71]. This regulation is presumably mediated by acetylation of four lysines on the ankyrin repeats of the receptor that results in attenuation of the transcription activity of Notch1.
The most studied non-chromatin substrate of TIP60 is the tumor suppressor p53. As mentioned in the previous section, TIP60 and MOF target p53 at lysine K120 and thus modulate the decision between cell cycle arrest and apoptosis upon p53 activation [49, 50]. It was also proposed to regulate transcription-independent apoptosis [72]. It had been known for a while that TIP60 is involved in p53 activation [73] and that it can coactivate p53-driven transcription [61, 74]. While TIP60 is important for p53-dependent expression of both cell cycle inhibitor and proapoptotic genes, acetylation of p53 by TIP60 is required only for certain proapoptotic promoters, namely PUMA and BAX [49, 50]. In agreement with these findings, cells expressing an acetylation mutant p53 have impaired apoptosis but normal cell cycle arrest in response to genotoxic stress [49, 50]. Interestingly, ChIP assays performed with wild-type (WT) or acetylation mutant p53 showed that non-acetylated p53 at K120 can still be recruited at BAX and PUMA promoters. However, it cannot fully induce transcription, as promoter-bound histone H4 acetylation and mRNA levels are low in cells expressing acetylation-mutant p53. It is not yet clear how acetylated K120 contributes to BAX and PUMA transcription, but the most probable explanation would be that it allows recruitment of specific transcriptional coactivators needed to activate these promoters [49, 50]. The emerging model is, therefore, that, in response to DNA damage, p53 is stabilized, activated and recruited with TIP60 and/or MOF at target gene promoters. In response to specific signals, e.g., the existence of unrepairable damage, TIP60/MOF then acetylate p53 at K120 thus selectively triggering apoptosis. In parallel, acetylation of K120 affects the transcription-independent apoptotic role of p53 at the mitochondria where it is required for efficient displacement of MCI-1 inhibitor from the BAK apoptotic factor [72]. K120 is a conserved residue and is found mutated in rare human tumors ([50] and references therein), highlighting the importance of this residue and its correct modification. Interestingly, loss of p53 methylation by Set7/9 correlates with loss of all p53 acetylation sites, including the ones targeted by CBP/p300, PCAF and TIP60 [75]. The lack of p53 methylation by Set7/9 prevents interaction of TIP60 with p53, explaining inhibition of acetylation. Taken together, these results support a hypothesis that there is crosstalk between Set7/9-mediated methylation of p53 and its TIP60-mediated acetylation [75].
Finally, it was also reported that TIP60 associates with the ATM kinase to regulate the cellular response to DNA damage [68]. It is argued that TIP60-dependent acetylation of K3016 on ATM activates its kinase activity, DNA damage detection and cell cycle checkpoints [76]. Again, there are some contradictory results in the literature since down-regulation of KAT5/TIP60 in fly, yeast and human was also shown to delay repair and checkpoint release while not affecting initial DNA damage sensing (e.g., phosphorylation of H2A.X) [42, 56, 77].
Esa1 (essential Sas2-related acetyltransferase 1)
A large number of acetylated non-histone proteins have been documented in metazoans, but very few are known in yeast. It is not certain if this is circumstantial (meaning because not enough relevant research is performed to identify non-nucleosomal targets) or because the broader substrate diversity developed later in evolution. The only yeast MYST enzyme known to acetylate a protein other than histones is Esa1, the Sc orthologue of TIP60.
The Sc KAT5/Esa1 can be found in two complexes, the promoter-specific NuA4 and picoloNuA4, the latter being responsible for global histone H4 acetylation [78]. Esa1/NuA4 has a well-defined role in transcription activation and DNA repair [79–81].
Until recently, Esa1 was thought to exert the entirety of its roles in various chromatin-related processes via acetylation of histones H4 and H2A/H2A.Z. However, more and more genetic evidence showed a role for NuA4 in cellular phenomena as diverse as cell metabolism, mitochondrial processes, membrane sorting, protein trafficking, stress response and ubiquitylation [82, 83]. It therefore became plausible that not all Esa1 functions are accounted for by its role in chromatin modification.
The first evidence that NuA4 can act upon non-nucleosomal targets came from the study of Lin et al. [84] which showed that Esa1 can acetylate Yng2, an integral subunit of the NuA4 complex itself. The authors showed that Yng2 is acetylated at lysine K170 and this modification is essential for its protein stability. The authors went on to show that the acetylation/deacetylation of this residue regulates the integrity of the NuA4 complex and therefore plays a crucial role during DNA damage response [22]. According to their model, upon DNA damage, the NuA4 complex is recruited to the surroundings of the DSB, where it acetylates chromatin. Soon after this, Rpd3 is recruited and deacetylates Yng2. This results in ubiquitin-dependent degradation of Yng2 by the proteasome and the disassociation of piccolo NuA4, leaving the rest of the NuA4 subunits (e.g., Eaf1) at the break. The loss of piccolo NuA4 allows deacetylation of chromatin by Rpd3 and, presumably, processing and repair of the break. It is interesting to note that human tumor suppressor ING4, a close homolog of Yng2 found in a piccolo NuA4-like complex, is also acetylated on the same residue in human cells [3, 5, 15].
Lin and colleagues also performed a proteomic screen for new non-histone substrates of the NuA4 complex [22]. They used purified NuA4 in in vitro acetylation assays on microarrays carrying the entire yeast proteome as recombinant GST-fusions. Through this approach, they identified novel putative NuA4 targets, confirmed selected ones in vivo, and pursued the characterization of a particularly interesting one, Pck1 [22]. The NuA4 acetylation targets identified in this screen have various functions, such as chromatin dynamics, transcription regulation, cell cycle control, RNA processing, stress response and metabolism (see Table 3). Pck1, the enzyme that catalyzes the conversion of oxaloacetate into phosphoenolopyruvate at a key step of gluconeogenesis, is acetylated in vivo at lysine 514 in an Esa1-dependent manner and this modification regulates its enzymatic activity. Furthermore, the human orthologue of NuA4, the TIP60 complex, was also shown to acetylate cytoplasmic PCK1 in human cells [22]. Deacetylation of Pck1 seems to be mediated by Sir2 in yeast; however, the deacetylase involved in human cells has yet to be identified.
Table 3.
Non-histone substrates of NuA4 identified by the in vitro acetylation screen on microarray and confirmed in vivo [22]
| Cellular process | NuA4 substrate |
|---|---|
| Transcription | Nnt1 |
| Cell cycle | Cdc34, Rpt5 |
| RNA processing | Brx1, Prp19 |
| Stress response | Atg3, Atg11, Hsp104 |
| Metabolism | Gph1, Sip2, Sip5, Tap42, Pck1 |
Gluconeogenesis (GNG) is a metabolic pathway whereby non-carbohydrate carbon sources, such as glycerol or lactate, are used to generate glucose. Many steps in GNG are the opposite reactions of those in glycolysis [85]. One of the steps key to GNG is the conversion of oxaloacetate into pyruvate which, as mentioned above, is catalyzed by Pck1. Cells activate gluconeogenetic pathways when the levels of carbohydrates are low. In agreement with this, cells lacking Pck1 cannot grow well on non-fermentable carbon sources such as ethanol (EtOH) or glycerol, because they cannot complete GNG and metabolize these sources into glucose. Cells carrying a non-acetylatable arginine at residue 514 of Pck1 (pck1-K514R) also cannot grow well on EtOH or glycerol, showing that acetylation of Pck1 by NuA4 is required for GNG [22].
Additional data implicated Esa1 and Pck1 in the extension of chronological lifespan (CLS), which is the period of time that a cell can survive in stationary phase [22]. The CLS of yeast cells is longer if the yeast cells are grown under severe starvation (water) than in glucose-rich media [86]. However, cells lacking Pck1 cannot survive as long as WT cells either in glucose or in water, showing that Pck1 and gluconeogenesis are important for longer CLS. Consistent with this is that Pck1 expression is increased upon diauxic shift from glucose to poor media (3% EtOH) [22]. Strains carrying thermosensitive esa1 mutations at restrictive temperatures or the pkc1-K514R mutant show decreased CLS in comparison to WT strains. Also, strains carrying the acetyl-lysine-mimetic pck1-K514Q mutant, show an increase in CLS, to a comparative extent as a deletion of SIR2, a well-known sirtuin deacetylase that regulates CLS [22]. No genetic epistasis is seen between esa1-ts and pck1-K514R mutants, suggesting that Esa1 might have other targets in CLS control pathways. Finally, cellular uptake of EtOH from the media is quicker in strains where Pck1 and Esa1 are active and gluconeogenesis intact [22]. Altogether, these results show that acetylation by NuA4 controls gluconeogenic activity of Pck1 to convert EtOH into glucose, and this process is crucial for the longevity.
Of note is that acetylation of Pck1 by Esa1 is only promoting chronological lifespan, not replicative lifespan [22]. Replicative lifespan is defined as the number of mitotic divisions a mother yeast cell can undergo before senescence, and is in fact prolonged by Sir2 [87]. This shows that different types of lifespan are regulated by distinct, sometimes even opposite, mechanisms.
Although it was surprising to find that NuA4, a predominantly nuclear acetyltransferase with well-studied roles in the nucleus, has a cytoplasmic role, the fact that a metabolic pathway is regulated by acetylation of its enzymes was somewhat expected [88, 89]. It has to be noted here that TIP60 in human cells has been detected at the perinuclear region of the cytoplasm (reviewed in [54]). As mentioned in Table 1, studies of the acetylome show that a large part of acetylated proteins in a cell are not nuclear. Zhao et al. [7] found that every enzyme involved in glycolysis, glycogen metabolism, the TCA and urea cycles and the fatty acid metabolism can be acetylated and that changing the carbon source available results in an different pattern of acetylated metabolic pathways. The same study found that the lysine acetylation of metabolic enzymes depends on AcCoA availability. Changes in availability of AcCoA was known to affect chromatin acetylation [90, 91]. It is now clear that it also affects acetylation status of critical substrates in metabolic pathways [7, 92]. AcCoA seems to act as a “rheostat” for the acetylation of metabolic enzymes, thus regulating their activity. In parallel with these findings, a recent report showed that inducing glucose catabolism and production of AcCoA in quiescent cells specifically activates global acetylation of chromatin via stimulation of Esa1 and Gcn5 without affecting transcription [93]. These results suggest a direct metabolic regulation of KATs. Taken together, all the above findings highlight the tight connection between lysine acetylation and metabolism. Acetyltransferases seem to control metabolic pathways and vice versa.
MYST complex acetylation and autoacetylation
Proteomic studies of the acetylome in human cells indicate that proteins involved in chromatin biology are frequent targets of acetylation [3]. These include histone chaperones, subunits of ATP-dependent chromatin remodelers and histone modifiers. In fact, quite often, acetylated proteins are subunits of KAT complexes [3]. At least seven subunits of the TIP60/NuA4 complex are acetylated in vivo: TIP60 itself, TRRAP, DMAP1, ING3, EP400, EPC1/2, BRD8, MRG15, TIP49a, and EAF6. The MOZ/MORF complexes also contain a number of acetylated subunits (MOZ, MORF, ING5, BRPF1/2/3, and EAF6) and the same applies to HBO1 (HBO1, JADE2/3, ING4/5, and EAF6) and human MOF complexes (hMOF, PHF20, MSL1, TPR, MCRS1, HCF1, and WDR5) [3, 5].
This finding is not entirely surprising. It is logical that, by acetylating their associated factors in response to external signals, MYST enzymes can rapidly inflict changes on their properties (activity, stability, subunit composition, subcellular composition), thereby ensuring versatility in their function. There have been some examples of auto-acetylation of MYST complexes in the literature, such as MSL3 within Dm MOF and Yng2 within NuA4, as mentioned above [45, 84]. We now know that this is a common theme in the MYST-family of acetyltransferase complexes (Saksouk, Sapountzi, Cayrou, Cramet and Côté, unpublished).
Finally, there is increasing evidence that MYST proteins autoregulate themselves by autoacetylation. When using MYST proteins in in vitro acetylation assays using radiolabeled AcCoA, most of the enzymes show up on the radioactive gel, suggesting that MYST proteins autoacetylate in vitro. These autoacetylation events have not been extensively characterized. The catalytic action of MYST enzymes is thought to be via an acetyl-Cys enzyme intermediate [94], although it has been proposed recently that MYST enzymes might in fact function via direct nucleophillic attack of the substrate’s lysine [95]. It is therefore not clear if autoacetylation detected in radioactive assays is actually occurring on a lysine residue, although biochemical analysis of piccolo Esa1 at least suggests that autoacetylation is too stable to be an acetylated cysteine [95]. In agreement with this, Wang et al. [96] recently reported that TIP60 is indeed autoacetylated on lysines and that this is an additional mode of regulation of the acetyltransferase. The exact residue(s) targeted is not known, although proteomic analysis identified a few acetylated lysines present on TIP60 in vivo [3]. It will be interesting to find out if this type of autoregulation is widely utilized by the rest of the MYST proteins and also to understand exactly which cellular cues are inducing this.
Conclusion
Ever since the first MYST acetyltransferases were discovered, their roles in histone acetylation have been extensively studied. Apart from their established role in chromatin dynamics during transcription, DNA repair and DNA replication, there is increasing interest on their action on non-nucleosomal targets. The fact that MYST proteins have non-nucleosomal substrates proves the diversity of functions they have in the cell. The involvement of MYST proteins in so many fundamental cellular processes makes them important for the correct cell homeostasis and, by extension, important in pathological conditions. Indeed, deregulation of MYST proteins has been found to be the underlying cause of several human diseases, such as leukaemia and various other cancers [15, 97]. MYST proteins could therefore be the focus of clinical research for inhibitor/activator-based therapies. Further research into the substrate spectrum of MYST will give valuable insights into the understanding of the roles of these essential proteins, and this knowledge will certainly have therapeutic applications.
Acknowledgments
We apologize to our colleagues for work that could not be cited due to space limitation. Work in our laboratory is supported by operating grants from the Canadian Institutes of Health Research (CIHR, MOP-14308/64289). V.S. holds a CIHR fellowship and J.C. a Canada Research Chair.
References
- 1.Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, Pillus L, Reinberg D, Shi Y, Shiekhattar R, Shilatifard A, Workman J, Zhang Y. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–636. doi: 10.1016/j.cell.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 2.Smith KT, Workman JL. Introducing the acetylome. Nat Biotechnol. 2009;27:917–919. doi: 10.1038/nbt1009-917. [DOI] [PubMed] [Google Scholar]
- 3.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 4.Iwabata H, Yoshida M, Komatsu Y. Proteomic analysis of organ-specific post-translational lysine-acetylation and -methylation in mice by use of anti-acetyllysine and -methyllysine mouse monoclonal antibodies. Proteomics. 2005;5:4653–4664. doi: 10.1002/pmic.200500042. [DOI] [PubMed] [Google Scholar]
- 5.Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Yu BJ, Kim JA, Moon JH, Ryu SE, Pan JG. The diversity of lysine-acetylated proteins in Escherichia coli . J Microbiol Biotechnol. 2008;18:1529–1536. [PubMed] [Google Scholar]
- 7.Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taverna SD, Li H, Ruthenburg AJ, Allis CD, Patel DJ. How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers. Nat Struct Mol Biol. 2007;14:1025–1040. doi: 10.1038/nsmb1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latham JA, Dent SY. Cross-regulation of histone modifications. Nat Struct Mol Biol. 2007;14:1017–1024. doi: 10.1038/nsmb1307. [DOI] [PubMed] [Google Scholar]
- 10.Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc Natl Acad Sci USA. 1964;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivanov D, Schleiffer A, Eisenhaber F, Mechtler K, Haering CH, Nasmyth K. Eco1 is a novel acetyltransferase that can acetylate proteins involved in cohesion. Curr Biol. 2002;12:323–328. doi: 10.1016/S0960-9822(02)00681-4. [DOI] [PubMed] [Google Scholar]
- 12.Carrozza MJ, Utley RT, Workman JL, Cote J. The diverse functions of histone acetyltransferase complexes. Trends Genet. 2003;19:321–329. doi: 10.1016/S0168-9525(03)00115-X. [DOI] [PubMed] [Google Scholar]
- 13.Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn’t fit all. Nat Rev Mol Cell Biol. 2007;8:284–295. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- 14.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 15.Avvakumov N, Cote J. The MYST family of histone acetyltransferases and their intimate links to cancer. Oncogene. 2007;26:5395–5407. doi: 10.1038/sj.onc.1210608. [DOI] [PubMed] [Google Scholar]
- 16.Thomas T, Voss AK. The diverse biological roles of MYST histone acetyltransferase family proteins. Cell Cycle. 2007;6:696–704. doi: 10.4161/cc.6.6.4013. [DOI] [PubMed] [Google Scholar]
- 17.Utley RT, Cote J. The MYST family of histone acetyltransferases. Curr Top Microbiol Immunol. 2003;274:203–236. doi: 10.1007/978-3-642-55747-7_8. [DOI] [PubMed] [Google Scholar]
- 18.Yang XJ. The diverse superfamily of lysine acetyltransferases and their roles in leukemia and other diseases. Nucleic Acids Res. 2004;32:959–976. doi: 10.1093/nar/gkh252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saksouk N, Avvakumov N, Cote J. (de) MYSTification and INGenuity of tumor suppressors. Cell Mol Life Sci. 2008;65:1013–1018. doi: 10.1007/s00018-008-7459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voss AK, Thomas T. MYST family histone acetyltransferases take center stage in stem cells and development. Bioessays. 2009;31:1050–1061. doi: 10.1002/bies.200900051. [DOI] [PubMed] [Google Scholar]
- 21.Squatrito M, Gorrini C, Amati B. Tip60 in DNA damage response and growth control: many tricks in one HAT. Trends Cell Biol. 2006;16:433–442. doi: 10.1016/j.tcb.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Lin YY, Lu JY, Zhang J, Walter W, Dang W, Wan J, Tao SC, Qian J, Zhao Y, Boeke JD, Berger SL, Zhu H. Protein acetylation microarray reveals that NuA4 controls key metabolic target regulating gluconeogenesis. Cell. 2009;136:1073–1084. doi: 10.1016/j.cell.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang XJ, Ullah M. MOZ and MORF, two large MYSTic HATs in normal and cancer stem cells. Oncogene. 2007;26:5408–5419. doi: 10.1038/sj.onc.1210609. [DOI] [PubMed] [Google Scholar]
- 24.Katsumoto T, Aikawa Y, Iwama A, Ueda S, Ichikawa H, Ochiya T, Kitabayashi I. MOZ is essential for maintenance of hematopoietic stem cells. Genes Dev. 2006;20:1321–1330. doi: 10.1101/gad.1393106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas T, Corcoran LM, Gugasyan R, Dixon MP, Brodnicki T, Nutt SL, Metcalf D, Voss AK. Monocytic leukemia zinc finger protein is essential for the development of long-term reconstituting hematopoietic stem cells. Genes Dev. 2006;20:1175–1186. doi: 10.1101/gad.1382606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voss AK, Collin C, Dixon MP, Thomas T. Moz and retinoic acid coordinately regulate H3K9 acetylation, Hox gene expression, and segment identity. Dev Cell. 2009;17:674–686. doi: 10.1016/j.devcel.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Taverna SD, Ilin S, Rogers RS, Tanny JC, Lavender H, Li H, Baker L, Boyle J, Blair LP, Chait BT, Patel DJ, Aitchison JD, Tackett AJ, Allis CD. Yng1 PHD finger binding to H3 trimethylated at K4 promotes NuA3 HAT activity at K14 of H3 and transcription at a subset of targeted ORFs. Mol Cell. 2006;24:785–796. doi: 10.1016/j.molcel.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howe L, Auston D, Grant P, John S, Cook RG, Workman JL, Pillus L. Histone H3 specific acetyltransferases are essential for cell cycle progression. Genes Dev. 2001;15:3144–3154. doi: 10.1101/gad.931401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitabayashi I, Aikawa Y, Nguyen LA, Yokoyama A, Ohki M. Activation of AML1-mediated transcription by MOZ and inhibition by the MOZ-CBP fusion protein. EMBO J. 2001;20:7184–7196. doi: 10.1093/emboj/20.24.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saksouk N, Avvakumov N, Champagne KS, Hung T, Doyon Y, Cayrou C, Paquet E, Ullah M, Landry AJ, Cote V, Yang XJ, Gozani O, Kutateladze TG, Cote J. HBO1 HAT complexes target chromatin throughout gene coding regions via multiple PHD finger interactions with histone H3 tail. Mol Cell. 2009;33:257–265. doi: 10.1016/j.molcel.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miotto B, Struhl K. Differential gene regulation by selective association of transcriptional coactivators and bZIP DNA-binding domains. Mol Cell Biol. 2006;26:5969–5982. doi: 10.1128/MCB.00696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miotto B, Struhl K. HBO1 histone acetylase is a coactivator of the replication licensing factor Cdt1. Genes Dev. 2008;22:2633–2638. doi: 10.1101/gad.1674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miotto B, Struhl K. HBO1 histone acetylase activity is essential for DNA replication licensing and inhibited by Geminin. Mol Cell. 2010;37:57–66. doi: 10.1016/j.molcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doyon Y, Cayrou C, Ullah M, Landry AJ, Cote V, Selleck W, Lane WS, Tan S, Yang XJ, Cote J. ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Mol Cell. 2006;21:51–64. doi: 10.1016/j.molcel.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Iizuka M, Matsui T, Takisawa H, Smith MM. Regulation of replication licensing by acetyltransferase Hbo1. Mol Cell Biol. 2006;26:1098–1108. doi: 10.1128/MCB.26.3.1098-1108.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taipale M, Rea S, Richter K, Vilar A, Lichter P, Imhof A, Akhtar A. hMOF histone acetyltransferase is required for histone H4 lysine 16 acetylation in mammalian cells. Mol Cell Biol. 2005;25:6798–6810. doi: 10.1128/MCB.25.15.6798-6810.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith ER, Cayrou C, Huang R, Lane WS, Cote J, Lucchesi JC. A human protein complex homologous to the Drosophila MSL complex is responsible for the majority of histone H4 acetylation at lysine 16. Mol Cell Biol. 2005;25:9175–9188. doi: 10.1128/MCB.25.21.9175-9188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimura A, Umehara T, Horikoshi M. Chromosomal gradient of histone acetylation established by Sas2p and Sir2p functions as a shield against gene silencing. Nat Genet. 2002;32:370–377. doi: 10.1038/ng993. [DOI] [PubMed] [Google Scholar]
- 39.Suka N, Luo K, Grunstein M. Sir2p and Sas2p opposingly regulate acetylation of yeast histone H4 lysine16 and spreading of heterochromatin. Nat Genet. 2002;32:378–383. doi: 10.1038/ng1017. [DOI] [PubMed] [Google Scholar]
- 40.Gupta A, Sharma GG, Young CS, Agarwal M, Smith ER, Paull TT, Lucchesi JC, Khanna KK, Ludwig T, Pandita TK. Involvement of human MOF in ATM function. Mol Cell Biol. 2005;25:5292–5305. doi: 10.1128/MCB.25.12.5292-5305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Wu L, Corsa CA, Kunkel S, Dou Y. Two mammalian MOF complexes regulate transcription activation by distinct mechanisms. Mol Cell. 2009;36:290–301. doi: 10.1016/j.molcel.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma GG, Sairie So, Gupta A, Kumar R, Cayrou C, Avvakumov N, Bhadra U, Pandita RK, Porteus MH, Chen DJ, Cote J, Pandita TK. MOF and histone H4 acetylation at lysine 16 are critical for DNA damage response and DSB repair. Mol Cell Biol. 2010;30:3582–3595. doi: 10.1128/MCB.01476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rea S, Xouri G, Akhtar A. Males absent on the first (MOF): from flies to humans. Oncogene. 2007;26:5385–5394. doi: 10.1038/sj.onc.1210607. [DOI] [PubMed] [Google Scholar]
- 44.Morales V, Straub T, Neumann MF, Mengus G, Akhtar A, Becker PB. Functional integration of the histone acetyltransferase MOF into the dosage compensation complex. EMBO J. 2004;23:2258–2268. doi: 10.1038/sj.emboj.7600235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buscaino A, Kocher T, Kind JH, Holz H, Taipale M, Wagner K, Wilm M, Akhtar A. MOF-regulated acetylation of MSL-3 in the Drosophila dosage compensation complex. Mol Cell. 2003;11:1265–1277. doi: 10.1016/S1097-2765(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 46.Akhtar A, Zink D, Becker PB. Chromodomains are protein–RNA interaction modules. Nature. 2000;407:405–409. doi: 10.1038/35030169. [DOI] [PubMed] [Google Scholar]
- 47.Cai Y, Jin J, Swanson SK, Cole MD, Choi SH, Florens L, Washburn MP, Conaway JW, Conaway RC. Subunit composition and substrate specificity of a MOF-containing histone acetyltransferase distinct from the male-specific lethal (MSL) complex. J Biol Chem. 2010;285:4268–4272. doi: 10.1074/jbc.C109.087981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mendjan S, Taipale M, Kind J, Holz H, Gebhardt P, Schelder M, Vermeulen M, Buscaino A, Duncan K, Mueller J, Wilm M, Stunnenberg HG, Saumweber H, Akhtar A. Nuclear pore components are involved in the transcriptional regulation of dosage compensation in Drosophila . Mol Cell. 2006;21:811–823. doi: 10.1016/j.molcel.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Sykes SM, Mellert HS, Holbert MA, Li K, Marmorstein R, Lane WS, McMahon SB. Acetylation of the p53 DNA-binding domain regulates apoptosis induction. Mol Cell. 2006;24:841–851. doi: 10.1016/j.molcel.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang Y, Luo J, Zhang W, Gu W. Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Mol Cell. 2006;24:827–839. doi: 10.1016/j.molcel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 51.Zhou Y, Schmitz KM, Mayer C, Yuan X, Akhtar A, Grummt I. Reversible acetylation of the chromatin remodelling complex NoRC is required for non-coding RNA-dependent silencing. Nat Cell Biol. 2009;11:1010–1016. doi: 10.1038/ncb1914. [DOI] [PubMed] [Google Scholar]
- 52.Ikura T, Tashiro S, Kakino A, Shima H, Jacob N, Amunugama R, Yoder K, Izumi S, Kuraoka I, Tanaka K, Kimura H, Ikura M, Nishikubo S, Ito T, Muto A, Miyagawa K, Takeda S, Fishel R, Igarashi K, Kamiya K. DNA damage-dependent acetylation and ubiquitination of H2AX enhances chromatin dynamics. Mol Cell Biol. 2007;27:7028–7040. doi: 10.1128/MCB.00579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murr R, Loizou JI, Yang YG, Cuenin C, Li H, Wang ZQ, Herceg Z. Histone acetylation by Trrap-Tip60 modulates loading of repair proteins and repair of DNA double-strand breaks. Nat Cell Biol. 2006;8:91–99. doi: 10.1038/ncb1343. [DOI] [PubMed] [Google Scholar]
- 54.Sapountzi V, Logan IR, Robson CN. Cellular functions of TIP60. Int J Biochem Cell Biol. 2006;38:1496–1509. doi: 10.1016/j.biocel.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Jha S, Shibata E, Dutta A. Human Rvb1/Tip49 is required for the histone acetyltransferase activity of Tip60/NuA4 and for the downregulation of phosphorylation on H2AX after DNA damage. Mol Cell Biol. 2008;28:2690–2700. doi: 10.1128/MCB.01983-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kusch T, Florens L, Macdonald WH, Swanson SK, Glaser RL, Yates JR, 3rd, Abmayr SM, Washburn MP, Workman JL. Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science. 2004;306:2084–2087. doi: 10.1126/science.1103455. [DOI] [PubMed] [Google Scholar]
- 57.Fazzio TG, Huff JT, Panning B. An RNAi screen of chromatin proteins identifies Tip60-p400 as a regulator of embryonic stem cell identity. Cell. 2008;134:162–174. doi: 10.1016/j.cell.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leduc C, Claverie P, Eymin B, Col E, Khochbin S, Brambilla E, Gazzeri S. p14ARF promotes RB accumulation through inhibition of its Tip60-dependent acetylation. Oncogene. 2006;25:4147–4154. doi: 10.1038/sj.onc.1209446. [DOI] [PubMed] [Google Scholar]
- 59.Patel JH, Du Y, Ard PG, Phillips C, Carella B, Chen CJ, Rakowski C, Chatterjee C, Lieberman PM, Lane WS, Blobel GA, McMahon SB. The c-MYC oncoprotein is a substrate of the acetyltransferases hGCN5/PCAF and TIP60. Mol Cell Biol. 2004;24:10826–10834. doi: 10.1128/MCB.24.24.10826-10834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faiola F, Liu X, Lo S, Pan S, Zhang K, Lymar E, Farina A, Martinez E. Dual regulation of c-Myc by p300 via acetylation-dependent control of Myc protein turnover and coactivation of Myc-induced transcription. Mol Cell Biol. 2005;25:10220–10234. doi: 10.1128/MCB.25.23.10220-10234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doyon Y, Selleck W, Lane WS, Tan S, Cote J. Structural and functional conservation of the NuA4 histone acetyltransferase complex from yeast to humans. Mol Cell Biol. 2004;24:1884–1896. doi: 10.1128/MCB.24.5.1884-1896.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park J, Kunjibettu S, McMahon SB, Cole MD. The ATM-related domain of TRRAP is required for histone acetyltransferase recruitment and Myc-dependent oncogenesis. Genes Dev. 2001;15:1619–1624. doi: 10.1101/gad.900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frank SR, Parisi T, Taubert S, Fernandez P, Fuchs M, Chan HM, Livingston DM, Amati B. MYC recruits the TIP60 histone acetyltransferase complex to chromatin. EMBO Rep. 2003;4:575–580. doi: 10.1038/sj.embor.embor861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martinato F, Cesaroni M, Amati B, Guccione E. Analysis of Myc-induced histone modifications on target chromatin. PLoS One. 2008;3:e3650. doi: 10.1371/journal.pone.0003650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim J, Woo AJ, Chu J, Snow JW, Fujiwara Y, Kim CG, Cantor AB, Orkin SH. A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell. 2010;143:313–324. doi: 10.1016/j.cell.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gorrini C, Squatrito M, Luise C, Syed N, Perna D, Wark L, Martinato F, Sardella D, Verrecchia A, Bennett S, Confalonieri S, Cesaroni M, Marchesi F, Gasco M, Scanziani E, Capra M, Mai S, Nuciforo P, Crook T, Lough J, Amati B. Tip60 is a haplo-insufficient tumour suppressor required for an oncogene-induced DNA damage response. Nature. 2007;448:1063–1067. doi: 10.1038/nature06055. [DOI] [PubMed] [Google Scholar]
- 67.Halkidou K, Logan IR, Cook S, Neal DE, Robson CN. Putative involvement of the histone acetyltransferase Tip60 in ribosomal gene transcription. Nucleic Acids Res. 2004;32:1654–1665. doi: 10.1093/nar/gkh296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun Y, Jiang X, Chen S, Fernandes N, Price BD. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci USA. 2005;102:13182–13187. doi: 10.1073/pnas.0504211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ning J, Zheng G, Yang YC. Tip60 modulates PLAGL2-mediated transactivation by acetylation. J Cell Biochem. 2008;103:730–739. doi: 10.1002/jcb.21444. [DOI] [PubMed] [Google Scholar]
- 70.Gaughan L, Logan IR, Cook S, Neal DE, Robson CN. Tip60 and histone deacetylase 1 regulate androgen receptor activity through changes to the acetylation status of the receptor. J Biol Chem. 2002;277:25904–25913. doi: 10.1074/jbc.M203423200. [DOI] [PubMed] [Google Scholar]
- 71.Kim MY, Ann EJ, Kim JY, Mo JS, Park JH, Kim SY, Seo MS, Park HS. Tip60 histone acetyltransferase acts as a negative regulator of Notch1 signaling by means of acetylation. Mol Cell Biol. 2007;27:6506–6519. doi: 10.1128/MCB.01515-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sykes SM, Stanek TJ, Frank A, Murphy ME, McMahon SB. Acetylation of the DNA binding domain regulates transcription-independent apoptosis by p53. J Biol Chem. 2009;284:20197–20205. doi: 10.1074/jbc.M109.026096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berns K, Hijmans EM, Mullenders J, Brummelkamp TR, Velds A, Heimerikx M, Kerkhoven RM, Madiredjo M, Nijkamp W, Weigelt B, Agami R, Ge W, Cavet G, Linsley PS, Beijersbergen RL, Bernards R. A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature. 2004;428:431–437. doi: 10.1038/nature02371. [DOI] [PubMed] [Google Scholar]
- 74.Legube G, Linares LK, Tyteca S, Caron C, Scheffner M, Chevillard-Briet M, Trouche D. Role of the histone acetyl transferase Tip60 in the p53 pathway. J Biol Chem. 2004;279:44825–44833. doi: 10.1074/jbc.M407478200. [DOI] [PubMed] [Google Scholar]
- 75.Kurash JK, Lei H, Shen Q, Marston WL, Granda BW, Fan H, Wall D, Li E, Gaudet F. Methylation of p53 by Set7/9 mediates p53 acetylation and activity in vivo. Mol Cell. 2008;29:392–400. doi: 10.1016/j.molcel.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 76.Sun Y, Xu Y, Roy K, Price BD. DNA damage-induced acetylation of lysine 3016 of ATM activates ATM kinase activity. Mol Cell Biol. 2007;27:8502–8509. doi: 10.1128/MCB.01382-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Downs JA, Allard S, Jobin-Robitaille O, Javaheri A, Auger A, Bouchard N, Kron SJ, Jackson SP, Cote J. Binding of chromatin-modifying activities to phosphorylated histone H2A at DNA damage sites. Mol Cell. 2004;16:979–990. doi: 10.1016/j.molcel.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 78.Boudreault AA, Cronier D, Selleck W, Lacoste N, Utley RT, Allard S, Savard J, Lane WS, Tan S, Cote J. Yeast enhancer of polycomb defines global Esa1-dependent acetylation of chromatin. Genes Dev. 2003;17:1415–1428. doi: 10.1101/gad.1056603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu PY, Levesque N, Kobor MS. NuA4 and SWR1-C: two chromatin-modifying complexes with overlapping functions and components. Biochem Cell Biol. 2009;87:799–815. doi: 10.1139/O09-062. [DOI] [PubMed] [Google Scholar]
- 80.Doyon Y, Cote J. The highly conserved and multifunctional NuA4 HAT complex. Curr Opin Genet Dev. 2004;14:147–154. doi: 10.1016/j.gde.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 81.Altaf M, Auger A, Covic M, Cote J. Connection between histone H2A variants and chromatin remodeling complexes. Biochem Cell Biol. 2009;87:35–50. doi: 10.1139/O08-140. [DOI] [PubMed] [Google Scholar]
- 82.Hoke SM, Guzzo J, Andrews B, Brandl CJ. Systematic genetic array analysis links the Saccharomyces cerevisiae SAGA/SLIK and NuA4 component Tra1 to multiple cellular processes. BMC Genet. 2008;9:46. doi: 10.1186/1471-2156-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mitchell L, Lambert JP, Gerdes M, Al-Madhoun AS, Skerjanc IS, Figeys D, Baetz K. Functional dissection of the NuA4 histone acetyltransferase reveals its role as a genetic hub and that Eaf1 is essential for complex integrity. Mol Cell Biol. 2008;28:2244–2256. doi: 10.1128/MCB.01653-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin YY, Qi Y, Lu JY, Pan X, Yuan DS, Zhao Y, Bader JS, Boeke JD. A comprehensive synthetic genetic interaction network governing yeast histone acetylation and deacetylation. Genes Dev. 2008;22:2062–2074. doi: 10.1101/gad.1679508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scrutton MC, Utter FU. The regulation of glycolysis and gluconeogenesis in animal tissues. Annu Rev Biochem. 1968;37:249–302. doi: 10.1146/annurev.bi.37.070168.001341. [DOI] [Google Scholar]
- 86.Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, McGrew K, Longo VD. Sir2 blocks extreme life-span extension. Cell. 2005;123:655–667. doi: 10.1016/j.cell.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 87.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kouzarides T. Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 2000;19:1176–1179. doi: 10.1093/emboj/19.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Starai VJ, Celic I, Cole RN, Boeke JD, Escalante-Semerena JC. Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science. 2002;298:2390–2392. doi: 10.1126/science.1077650. [DOI] [PubMed] [Google Scholar]
- 90.Takahashi H, McCaffery JM, Irizarry RA, Boeke JD. Nucleocytosolic acetyl-coenzyme a synthetase is required for histone acetylation and global transcription. Mol Cell. 2006;23:207–217. doi: 10.1016/j.molcel.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 91.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, Li H, Xie L, Zhao W, Yao Y, Ning ZB, Zeng R, Xiong Y, Guan KL, Zhao S, Zhao GP. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science. 2010;327:1004–1007. doi: 10.1126/science.1179687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Friis RM, Wu BP, Reinke SN, Hockman DJ, Sykes BD, Schultz MC. A glycolytic burst drives glucose induction of global histone acetylation by picNuA4 and SAGA. Nucleic Acids Res. 2009;37:3969–3980. doi: 10.1093/nar/gkp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yan Y, Harper S, Speicher DW, Marmorstein R. The catalytic mechanism of the ESA1 histone acetyltransferase involves a self-acetylated intermediate. Nat Struct Biol. 2002;9:862–869. doi: 10.1038/nsb0902-638. [DOI] [PubMed] [Google Scholar]
- 95.Berndsen CE, Albaugh BN, Tan S, Denu JM. Catalytic mechanism of a MYST family histone acetyltransferase. Biochemistry. 2007;46:623–629. doi: 10.1021/bi602513x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang J, Chen J. SIRT1 regulates autoacetylation and histone acetyltransferase activity of TIP60. J Biol Chem. 2010;285:11458–11464. doi: 10.1074/jbc.M109.087585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Avvakumov N, Cote J. Functions of myst family histone acetyltransferases and their link to disease. Subcell Biochem. 2007;41:295–317. [PubMed] [Google Scholar]