Abstract
Fifty years ago, prescription of the sedative thalidomide caused a worldwide epidemic of multiple birth defects. The drug is now used in the treatment of leprosy and multiple myeloma. However, its use is limited due to its potent teratogenic activity. The mechanism by which thalidomide causes limb malformations and other developmental defects is a long-standing question. Multiple hypotheses exist to explain the molecular mechanism of thalidomide action. Among them, theories involving oxidative stress and anti-angiogenesis have been widely supported. Nevertheless, until recently, the direct target of thalidomide remained elusive. We identified a thalidomide-binding protein, cereblon (CRBN), as a primary target for thalidomide teratogenicity. Our data suggest that thalidomide initiates its teratogenic effects by binding to CRBN and inhibiting its ubiquitin ligase activity. In this review, we summarize the biology of thalidomide, focusing on the molecular mechanisms of its teratogenic effects. In addition, we discuss the questions still to be addressed.
Keywords: Thalidomide, Teratogenicity, Oxidative stress, Anti-angiogenesis, Cereblon, Fibroblast growth factor 8
Introduction
Thalidomide (α-phthalimidoglutarimide) was developed and sold by the German pharmaceutical company Grunenthal in 1957. The drug was prescribed for use as a sedative and for the treatment of morning sickness. No remarkable toxicity was observed when tested on rodents, and thalidomide was distributed in over 40 countries. However, studies by McBride and Lenz independently reported its teratogenicity in 1961 and 1962, respectively. The use of thalidomide during early pregnancy led to serious embryotoxic effects, such as limb defects (amelia and phocomelia). Over 10,000 children were affected worldwide [1–7].
Thalidomide was immediately withdrawn from the market, but studies designed to uncover the mechanism of thalidomide action have continued. Since then, several remarkable clinical effects of this drug have been discovered. In 1965, the Israeli physician, Sheskin, found thalidomide to be effective for the treatment of erythema nodosum leprosum (ENL), an inflammatory complication of leprosy that results in painful skin lesions [8]. This discovery promoted further investigation of the clinically useful effects of thalidomide. During the 1980s and early 1990s, investigators found that thalidomide was an effective treatment for certain autoimmune disorders, such as chronic graft versus host disease and rheumatoid arthritis [9–13]. In the early 1990s, it was reported that thalidomide inhibits the production of TNF-α and replication of HIV [14–16]. In 1994, thalidomide was demonstrated to have anti-angiogenic activity, suggesting that the drug has an anti-cancer effect [17]. In 1999, thalidomide was shown to be effective for the treatment of multiple myeloma, a malignant B cell lymphoma [18]. With respect to ENL and myeloma, thalidomide was approved for use by the US FDA in 1998 and 2006, respectively. Due to its serious teratogenicity, the prescription of thalidomide is strictly controlled by the System for Thalidomide Education and Prescribing Safety (STEPS) program [19]. In South America, thalidomide is widely used for the treatment of leprosy. Sadly, many children have been born with severe birth defects due to poor patient understanding of the proper use of the drug and inconsistent contraceptive administration [20, 21].
How does thalidomide induce teratogenic effects such as limb deformities? Researchers have been investigating this question for over 40 years. Elucidation of the molecular basis of thalidomide teratogenicity is important for many reasons. First, the elucidation of its mechanism of action can lead to elimination of its side effects and the development of thalidomide derivatives and related compounds that do not have teratogenic activity. Second, understanding the mechanism by which thalidomide induces limb deformities can lead to new insights into the molecular mechanisms of limb development. Previous studies have proposed many hypotheses [5, 22]. Among them, thalidomide-induced oxidative stress and anti-angiogenic action are viewed as possible and reasonable causes of thalidomide teratogenicity [17, 22–24]. However, several important questions have remained unanswered, such as the direct targets of thalidomide and how the target molecules mediate its teratogenic effects.
Recently, we identified a thalidomide-binding protein, cereblon (CRBN), using newly developed affinity purification techniques [25]. Our findings suggest that thalidomide exerts its teratogenic effects by binding to CRBN and inhibiting its activity. We concluded that CRBN is a primary target of thalidomide teratogenicity.
In this review, we summarize the biology of thalidomide, focusing on the molecular mechanism of its teratogenic effects.
Chemistry and pharmacokinetics of thalidomide
Thalidomide is a derivative of glutamic acid and contains two imide rings: glutarimide and phthalimide. The drug is poorly soluble in water and is often dissolved in dimethyl sulfoxide (DMSO) as a vehicle for experimental use. Thalidomide has two isomeric forms, S (−) and R (+). The S (−) isomer was thought to work as a teratogen and the R (+) isomer as a sedative. However, both compounds rapidly interchange under physiological conditions and it is impossible to isolate one form from the other [1, 3, 5]. Furthermore, both forms were found to be teratogenic in a rabbit model [26].
Thalidomide undergoes spontaneous non-enzymatic hydrolytic breakdown into more than a dozen products under physiological conditions [1, 3, 5]. In addition, the drug is bio-transformed by liver cytochrome P450 (CYP450) into its hydroxylated products [1, 3, 5, 27, 28]. The peak plasma concentration of thalidomide is detected at 3–6 h after administration, and the drug and its metabolic products are quickly eliminated in the urine, with a mean elimination half-life of approximately 7.3 h [3, 29, 30].
The teratogenic phenotype induced by thalidomide
When pregnant women took thalidomide for morning sickness at between 3 and 8 weeks after conception, multiple birth defects occurred [1, 4, 31]. A wide spectrum of birth defects such as malformations of the limb, ear, eye, internal organs, and central nervous system were documented [1, 4, 31]. Limb malformations were the most frequently observed defects [5, 31], as described in detail below. Ear defects were also frequent; ear malformations varied from anotia to mild malformation of the external ear. Ocular anomalies such as uveal coloboma, glaucoma, microphthalmia were also observed, and in internal organs, the most frequent defects were kidney malformations, heart defects, and structural chest defects [31]. The mortality rate for babies born with thalidomide-induced defects was very high (about 30–40%). In addition, thalidomide caused an unknown number of miscarriages. Indeed, thalidomide induced several inoperable defects such as imperforate anus and other gastrointestinal deformities, contributing significantly to early deaths [5, 7, 31–33]. Facial nerve palsy was also common, and autism and mental retardation were reported even though the incidence was low [31].
The developing limb is composed of three parts: stylopod, zeugopod, and autopod. The stylopod is the proximal part of limb and gives rise to the humerus in the forelimb and the femur in the hindlimb. The zeugopod is the mid-part that comprises the radius and ulna in the forelimb as well as the tibia and fibula in the hindlimb. The autopod is the distal part and comprises the hand or the foot, respectively. Thalidomide induces two types of limb defects: amelia and phocomelia. Amelia is complete absence of the limb, and phocomelia consists of limbs with a stylopod, a truncated or absent zeugopod, and a nearly intact autopod [4].
Thalidomide induces limb/fin deformities in humans, monkeys, rabbits, chicks, and zebrafish (Danio rerio) [4, 25]. It is noteworthy that amelia only occurs in chicks, whereas both amelia and phocomelia occur in monkeys and rabbits [4]. In zebrafish, thalidomide induces shortening of the pectoral fins along the proximodistal axis [25]. The fin is structurally different from the limb, even though the molecular pathway(s) that govern their development are shared [34, 35]. In vertebrates, fibroblast growth factor 8 (Fgf8) is essential for limb/fin development along the proximodistal axis [36, 37]. Fgf8 is expressed in the apical ectodermal ridge (AER), the distalmost end of the fin and limb, and thalidomide inhibits the expression of Fgf8 in the AER in rabbist, chicks and zebrafish [24, 25, 38]. Therefore, the basis of the teratogenic pathway of thalidomide is likely conserved from fish to mammals. However, rodents are resistant to limb deformities induced by thalidomide [1–5]. Even when administered at doses of up to 4,000 mg/kg, thalidomide did not induce limb malformation in rats [39]. However, several reports have demonstrated the effects of thalidomide (e.g., anti-angiogenic effect) in cell lines and tissues from mice and rats [5, 40, 41]. The reason for this resistance remains a mystery.
Multiple hypotheses for the effect of thalidomide
Studies of the molecular mechanisms of thalidomide action have been conducted for nearly half a century. More than 30 hypotheses have been proposed to explain how thalidomide causes limb defects [22, 42–44]. Many of them focus on the disruption of molecular pathways including DNA intercalation, acetylation of macromolecules, interference of glutamate metabolism and folic acid antagonism. For example, Stephens et al. [42, 44] hypothesized that thalidomide intercalates with the GC box of specific gene promoters, including insulin-like growth factor-1 (Igf1) and Fgf2, both of which are important for limb growth. There is also little or no in vitro experimental evidence to support the DNA intercalation hypothesis of Stephens et al., and it does not adequately explain the tissue specificity of the effects of thalidomide.
Oxidative stress hypothesis
In 1999, Parman et al. [23] suggested that thalidomide caused limb deformities through a mechanism involving oxidative stress. They demonstrated that thalidomide generates reactive oxygen species (ROS), oxidizes DNA and accumulates 8-hydroxy-2′-deoxyguanosine in rabbits. Moreover, they used a spin-trap reagent, α-phenyl-N-tert-butylnitrone (PBN), which has been shown to be effective in the in vitro trapping of free-radical intermediates of teratogenic anticonvulsant drugs, as well as in inhibiting the in vivo teratogenicity of an anticonvulsant phenytoin in mice [45, 46]. They demonstrated that PBN suppresses both oxidation and limb defects induced by thalidomide in rabbits. Neither oxidative stress nor limb malformation is induced by thalidomide in mice [23].
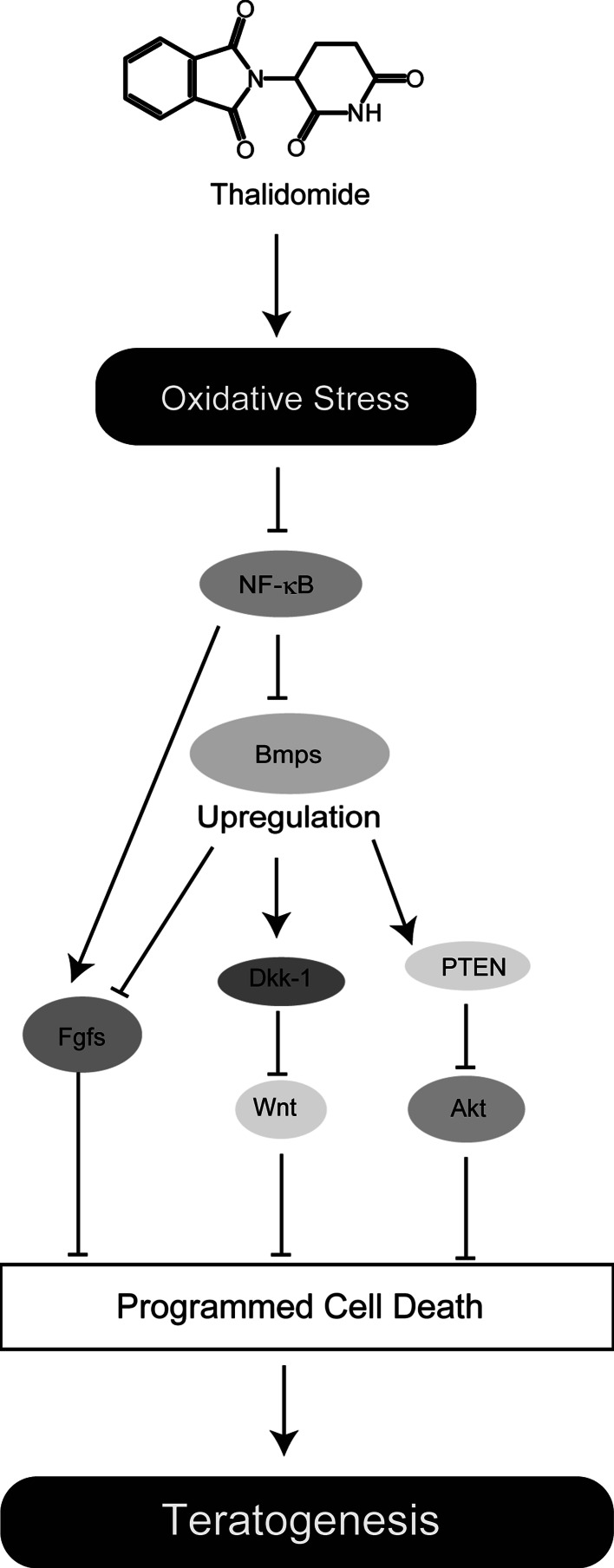
Harris et al. [38] showed that Fgf8 and Fgf10 expression is inhibited by treatment with thalidomide and that this effect is reversed by PBN pre-treatment in rabbits. Oxidative stress by thalidomide is likely to downregulate essential limb growth signaling. Fgf10 is an important regulator for limb development as well as Fgf8 and is expressed in the mesoderm beneath the AER. Fgf8 and Fgf10 form a positive feedback loop, and this Fgf signaling is important for cell proliferation and survival [47]. Fgf8 and Fgf10 have been shown to be downstream targets of Nuclear Factor-κB (NF-κB) [22, 38]. NF-κB is a redox-sensitive transcription factor whose function is affected by oxidative stress. Therefore, oxidative stress caused by thalidomide has been suggested to induce aberrant NF-κB activity, which in turn attenuates Fgf8 and Fgf10, expression, resulting in limb deformities [22].
During their study of the downstream pathways of thalidomide signaling, Knobloch et al. [48] showed that the upregulation of Bone morphogenic proteins (Bmps) and Dickkopf-1 (Dkk-1) by thalidomide is involved in its teratogenicity and is reversed by PBN. Treatment with thalidomide upregulates Bmp-4, -5,-7. Bmps belong to the transforming growth factor-β (TGF-β) family and play an essential role in multiple aspects of embryogenesis [4]. In limb development, Bmps, which are expressed in the distalmost tip of the epidermal cells, including the AER and the mesenchymal cells just beneath the AER, are important for limb patterning, and have been demonstrated to stabilize phosphatase and tensin homolog (PTEN) proteins, which block Akt survival signaling [49–52]. In addition, Bmps have been shown to inhibit Fgf signaling in mouse limb development [53]. It has been reported that NF-κB negatively inhibits Bmps expression [4, 54]. Thus, upregulation of Bmps is likely to fit with the hypothesis of Harris’s group [22]. Dkk-1 is a downstream target of Bmps and functions as an antagonist of Wnt, which regulates cell survival and proliferation [55]. Thalidomide blocks Wnt signaling and increases glycogen synthase kinase-3β (Gsk3β) activity through upregulation of Dkk-1 [48]. Stabilization of PTEN also activates Gsk3β, which promotes the programmed cell death (PCD) pathway [56].
As mentioned above, a good deal of evidence suggests that thalidomide teratogenicity is strongly linked to oxidative stress. Furthermore, downstream thalidomide signaling has been considerably clarified by these studies (Fig. 1). Oxidative stress induced by thalidomide causes PCD, possibly through blocking Fgf, Wnt, and Akt signaling. However, many questions remain. How is oxidative stress induced by thalidomide in the limb buds? And does this model explain the tissue specificity of thalidomide action? Although breakdown of thalidomide may generate free radicals [23, 57], this hypothesis does not adequately explain why defects of the limb are most frequent, if thalidomide breakdown is the only source of oxidative stress generation.
Fig. 1.
Schematic model of the oxidative stress hypothesis. Thalidomide induces ROS, and oxidative stress upregulates expression of Bmps through aberrant NF-κB activity. This alteration results in blocking Fgf (Fgf8/Fgf10), Akt, and Wnt pathways known to be important for cell survival and proliferation [4, 22, 36, 46, 47, 51]
Anti-angiogenesis hypothesis
D’Amato et al. [17] found that thalidomide inhibits Fgf2-induced angiogenesis in a rabbit cornea micropocket assay. They postulated that the inhibition of angiogenesis caused limb defects, since blood vessel formation is crucial for limb development. Since drugs with anti-angiogenic effects can often be used as anti-cancer medications, this idea was intriguing to many scientists and physicians.
Thalidomide-induced anti-angiogenesis occurs in organisms from mammals to zebrafish [17, 24, 25, 58]. Interestingly, thalidomide can inhibit angiogenesis in mouse and rat aortic ring cultures and mouse cornea models, indicating that cells and tissues from rodents are sensitive to the effects of thalidomide in vitro [40, 41].
Figg et al. [59] suggested that metabolic breakdown by CYP450 in the liver is required for thalidomide to be active. Thalidomide exhibits its anti-angiogenic effect in the aortic ring assay only in the presence of human or rabbit liver microsomes. Interestingly, thalidomide is not activated in the presence of rat liver microsomes. In addition, thalidomide metabolites are different between multiple myeloma patients and rodents [60]; therefore, thalidomide bioactivation appears to occur in a species-specific manner.
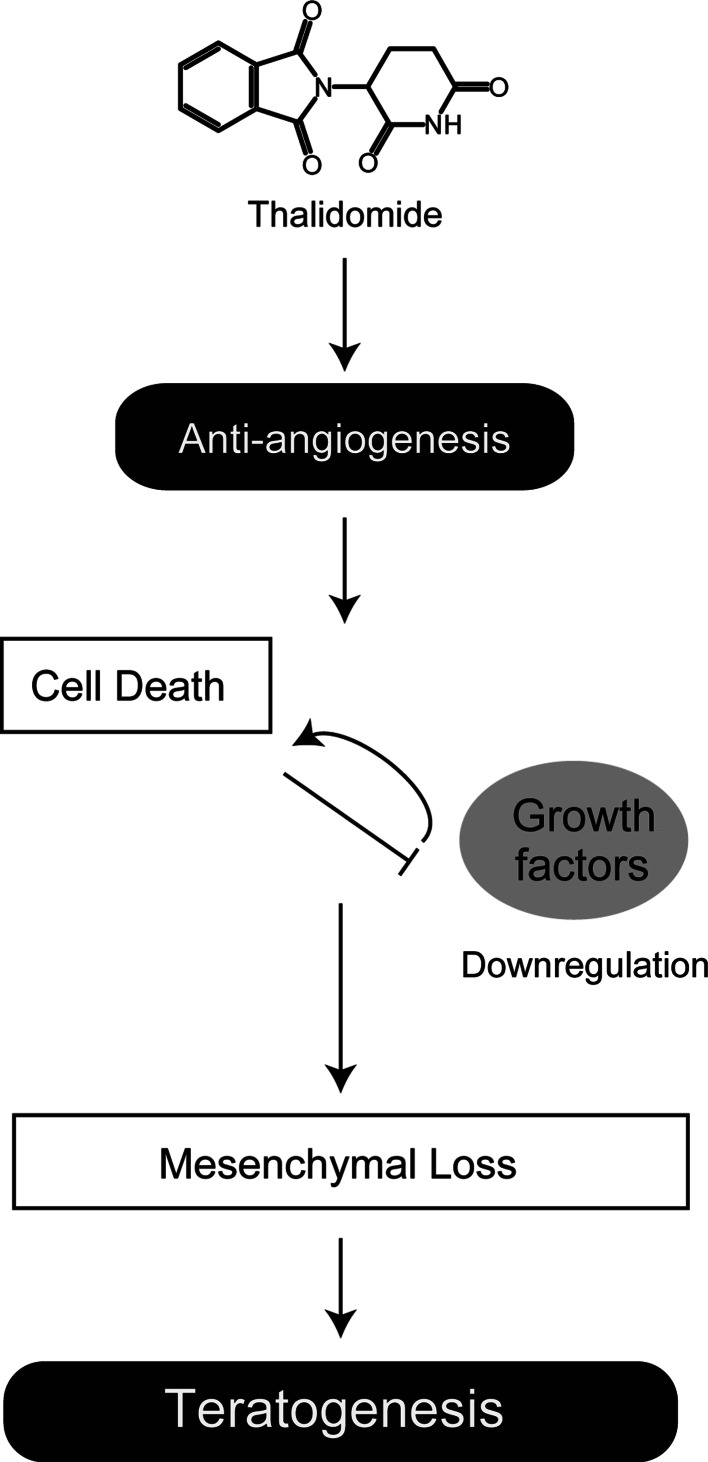
Vargesson et al. [24] recently suggested that the anti-angiogenic activity of thalidomide was a primary cause of chick limb malformations. Using CPS49, a synthetic thalidomide analog based on the anti-angiogenic activity of the parental compound [61], they demonstrated that inhibition of angiogenesis by CPS49 precedes changes in limb morphology, cell death, and inhibition of Fgf8/Fgf10 in chicks [24]. They suggested that inhibition of angiogenesis by thalidomide induces cell death and a reduction in the expression of growth factors such as Fgf8 and Fgf10, in turn resulting in mesenchymal loss in the limb bud (Fig. 2).
Fig. 2.
Schematic model of the anti-angiogenesis hypothesis. Anti-angiogenesis induced by thalidomide leads to cell death and downregulation of growth factors including Fgf8/Fgf10. Disruption of growth factor signaling pathways is also likely to be involved in cell death. The sequence of events results in mesenchymal loss and in turn limb deformities [5, 24]
These findings suggest that the anti-angiogenic effects of thalidomide are considerably linked to limb defects. However, our recent findings suggest that the inhibition of pectoral fin buds by thalidomide precedes anti-vasculogenesis in zebrafish [25]. Using fli1a:EGFP transgenic zebrafish that express EGFP in their vascular endothelial cells, we monitored vasculogenesis and fin development. The marginal blood vessel (MBV) is formed at the distal part of developing pectoral fin buds between 46 and 57 h post-fertilization (hpf), and it is only at 52–54 hpf that blood circulation initiates there [62, 63]. Consistent with the earlier studies, we showed that thalidomide inhibited MBV formation at 52 hpf and confirmed the anti-angiogenic activity of the drug. Remarkably, however, zebrafish embryos treated with thalidomide exhibited developmental defects in the fin buds at 47 hpf, when the MBV is still absent. Moreover, in 48-hpf embryos, we observed a reduction in Fgf8 expression by thalidomide treatment. Thus, morphological and transcriptional changes in pectoral fin buds precede inhibition of vasculogenesis and are therefore not secondary to the anti-angiogenic effect of thalidomide in zebrafish. Therefore, the sequence of events induced by thalidomide is different in different species. Furthermore, Lebrin et al. [64] recently suggested that thalidomide induces vessel maturation rather than inhibiting vessel sprouting in studies on heredity hemorrhagic telangiectasia (HHT) characterized by vascular malformations. In these patients, thalidomide treatment lowers nosebleed frequency in individuals with HHT and enhances blood vessel stabilization. They also showed that thalidomide treatment induces the expression of platelet-derived growth factor-b (Pdgf-b) in endothelial cells and stimulates mural cell activation, resulting in the maturation of vessels. Thus, the effects of thalidomide on vasculogenesis/angiogenesis are complicated and still poorly understood.
Identification of a direct target of thalidomide
As mentioned above, the existing hypotheses are unable to explain all aspects of thalidomide teratogenicity, although they provide important insights and have contributed to progress in studies of the mechanism of thalidomide action. Moreover, they do not answer a crucial question: what is/are the direct target(s) of thalidomide teratogenicity? Identification of thalidomide target molecule(s) is essential to elucidate the molecular mechanism of thalidomide.
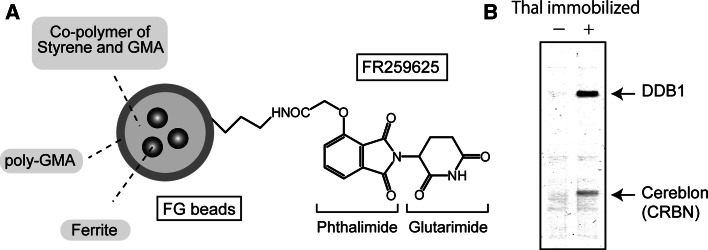
To accomplish this, we developed a new affinity purification technique. We developed ferrite-glycidyl methacrylate (FG) beads that allow single step purification of ligand target molecules [65–67] (Fig. 3a). FG beads allow for magnetic separation of target molecules from cell or tissue extracts and are suitable for use in automated high throughput screening.
Fig. 3.
Identification of a direct target of thalidomide. a Structure of thalidomide (thal)-immobilized FG beads. The carboxy derivative of thalidomide (FR259625) is attached covalently to the amino-group of FG beads. GMA Glycidyl methacrylate. b Thalidomide-binding proteins. CRBN and DDB1 were affinity purified from human cell extracts using thal-immobilized FG beads. No other proteins were detected. Bound proteins were visualized by silver staining. DDB1 indirectly interacts with thalidomide by binding to CRBN [25]
Using FG beads, we identified CRBN as a thalidomide-binding protein [25]. We covalently attached a carboxyl-type derivative of thalidomide to the amino-group of FG beads (Fig. 3a). We then performed affinity chromatography to purify thalidomide-binding molecules from extracts of human cell lines derived from various tissues including HeLa, 293T, HUVEC, U266, and Jurkat cells. Surprisingly, the only thalidomide-binding proteins that were identified were CRBN and damaged DNA binding protein 1 (DDB1) (Fig. 3b). CRBN directly binds to thalidomide and DDB1 indirectly interacts with thalidomide by binding to CRBN.
CRBN is a 442-amino acid protein that is conserved from plant to human. The gene that encodes CRBN was originally identified as a candidate gene of an autosomal recessive non-syndromic mild mental retardation [68]. CRBN binds to DDB1, a calcium-activated potassium channel (SLO1), and a voltage-gated chloride channel (CIC-2), but its cellular and physiological functions were largely unknown [69–71]. On the other hand, DDB1 is well known and well characterized. DDB1 was originally identified as a nucleotide excision repair protein that associates with damaged DNA binding protein 2 (DDB2) as a heterodimer [72]. However, accumulating data strongly suggest that DDB1 is a subunit of a Cul4-based E3 ubiquitin ligase complex with cullin 4 (Cul4: Cul4A or Cul4B), regulator of cullins-1 (Roc1), and a substrate receptor [69, 73–75]. The E3 ubiquitin ligase functions in the ubiquitin–proteasome protein degradation pathway and attaches polyubiquitin chains to substrate proteins for degradation via the protease complex [76]. Recent investigations show that many E3 ubiquitin ligases are important for various physiological processes such as cell cycle regulation, immune response, carcinogenesis, and development [73, 75]. In the Cul4-based E3 ubiquitin ligase complex, Roc1 and Cul4 form the catalytic core and interact with E2, an ubiquitin-conjugating enzyme. DDB1 forms a bridge between Cul4 and the substrate receptor. Substrate receptors such as DDB2 and Cockayne Syndrome A (CSA) recognize proteins targeted for polyubiquitination [77, 78].
We showed that CRBN forms an E3 ubiquitin ligase complex with DDB1, Cul4A, and Roc1. Since CRBN competes with DDB2 for DDB1-binding, CRBN is likely to function as a novel substrate receptor. Although the substrates of CRBN remain unidentified, we found that the CRBN-containing E3 complex has auto-ubiquitination activity. Moreover, we demonstrated that thalidomide inhibits auto-ubiquitination of CRBN in vitro, suggesting that thalidomide is an E3 inhibitor.
We next investigated whether CRBN is associated with thalidomide-induced defects in vivo. Our primary model animal was the zebrafish. Since zebrafish exhibit rapid growth and have transparent bodies, defects are easier to observe and analyze. The use of zebrafish leads to an advantage for genetic and chemical screening for the drugs for the treatment of human diseases [79]. Moreover, morpholino antisense oligonucleotide-mediated knockdown analysis is possible in zebrafish [80]. Zebrafish express zCrbn, which is ~70% homologous to human CRBN and binds to thalidomide. We showed that either thalidomide treatment or knockdown of zCrbn induced similar defects in zebrafish including retardation of pectoral fin and otic vesicle (ear) formation, and inhibition of Fgf8 expression in fin buds.
To unequivocally confirm that CRBN is an in vivo target of thalidomide, we constructed a CRBN mutant whose product was fully functional with the exception that it was unable to bind thalidomide. CRBNYW/AA, in which tyrosin-384 (Y) and tryptophan-386 (W) have been replaced with alanine (A), has extremely low thalidomide-binding activity. However, CRBNYW/AA can still form an E3 complex with DDB1, Cul4A and Roc1, and retains ubiquitination activity. We showed that thalidomide does not inhibit the ubiquitination activity of CRBNYW/AA. We then constructed zCrbnYW/AA (Y374A/W376A) as a counterpart of Y384A/W386A in human. Expression of zCrbnYW/AA effectively suppressed thalidomide-induced malformation in zebrafish.
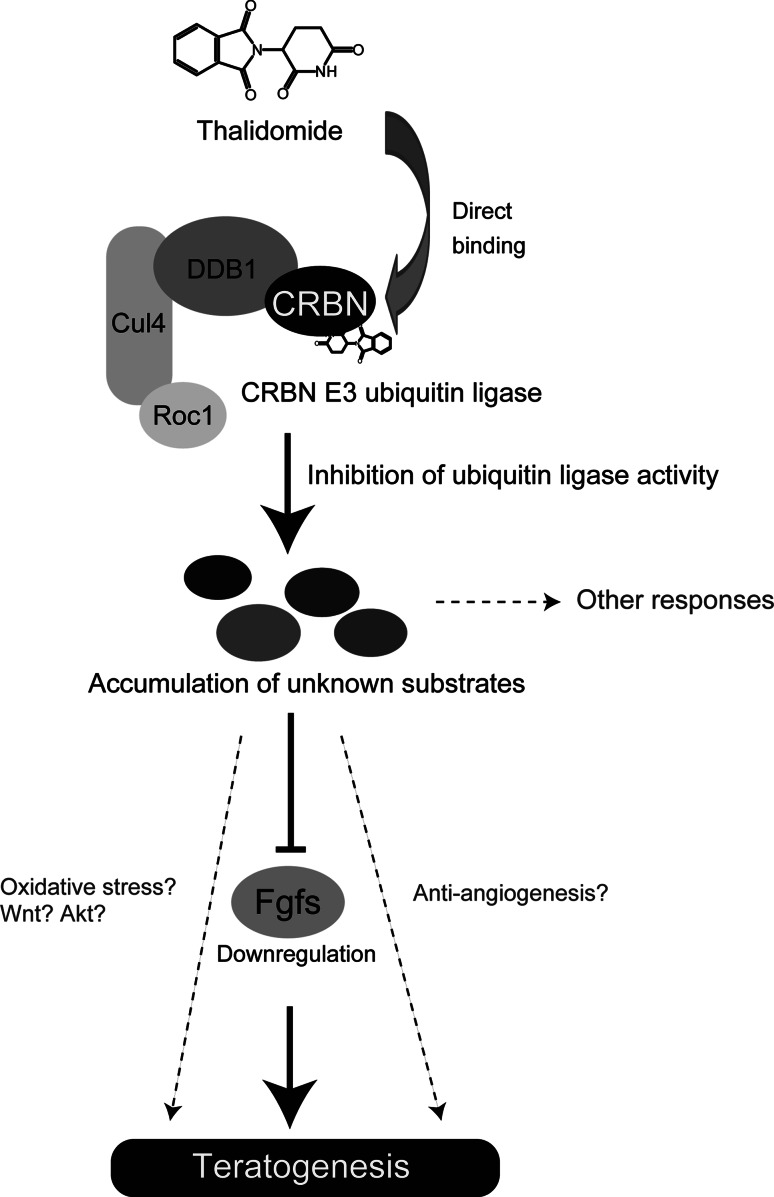
Finally, we investigated the conserved role of CRBN in zebrafish and chicks. As expected, expression of human CRBNYW/AA in chick forelimbs reduced thalidomide-induced limb deformities and prevented downregulation of Fgf8 and Fgf10. Therefore, we concluded that CRBN is a direct target of thalidomide, which binds to CRBN and inhibits its E3 ubiquitin ligase function. The subsequent aberrant accumulation of substrates results in developmental defects such as limb and ear deformities, in part through Fgf8 and Fgf10 downregulation (Fig. 4).
Fig. 4.
Schematic model of the molecular mechanisms of thalidomide teratogenicity from a CRBN-centric perspective. Thalidomide binds directly to CRBN and inhibits its E3 ubiquitin ligase activity. This inhibition results in aberrant accumulation of substrates (unknown), which in turn causes developmental defects such as limb deformities, in part through downregulation of Fgfs (Fgf8/Fgf10) [25]. We speculate that the accumulation of substrates might cause oxidative stress and induce anti-angiogenic responses, but the idea has not been experimentally proven
Our findings have identified a primary target for thalidomide activity. However, several questions still remain to be addressed and new questions are raised by the present study. How does CRBN fit into existing signaling models? Why are the effects of thalidomide tissue-specific? Why are mice and rats resistant to thalidomide? And are there other thalidomide-binding proteins?
The connection between CRBN and existing hypotheses
Thalidomide facilitates the formation of ROS and thereby causes oxidative DNA damage. Oxidative stress is thought to occur through the direct formation of ROS from thalidomide [23]. If so, it is clearly a CRBN-independent process. It was thought that, perhaps, damaged DNA-binding protein DDB1 may link CRBN to the induction of oxidative stress. However, our data do not support this theory. In DNA repair, DDB1 forms a complex with DDB2 that binds to damaged DNA and recognizes several substrates involved in DNA repair [77]. CRBN binds to DDB1, but not DDB2. CRBN and DDB2 interact with DDB1 in a competitive manner [25]. Hence, CRBN probably functions independently of the DDB2-mediated DNA damage response pathway. Recently, it was reported that the expression of CRBN is positively regulated by NF-E2–related factor 2 (Nrf-2) when cells are exposed to oxidative stress [81]. Nrf-2-activated ROS regulates detoxification genes, which possess an antioxidant responsive element (ARE) [82]. Overexpression of Nrf-2 upregulates CRBN, which also has an ARE [81]. If CRBN plays a specific role in the antioxidant pathway and blocks spontaneous ROS generation, CRBN is well connected to the oxidative stress hypothesis. This idea is still highly speculative, but further study of the relationship between CRBN and the antioxidant pathway might shed new light on this issue.
What about the relationship between CRBN and anti-angiogenesis? CRBN is highly expressed in head vasculature and can be purified from human umbilical vein endothelial cells using thalidomide-immobilized beads [25]. In zebrafish, thalidomide inhibits growth of the MBV in the limb at 52 hpf [25]. Even though fin deformities precede anti-angiogenesis, the presence of the MBV and blood circulation is important for fin development at later stages. Importantly, fin defects induced by thalidomide are rescued by expression of zCrbnYW/AA at 72 hpf [25]. In chicks, anti-angiogenesis has been shown to be a primary cause of thalidomide-induced defects [24]. Thalidomide-induced limb defects are suppressed by expression of a CRBN mutant deficient in thalidomide binding. It is possible that vessel defects induced by thalidomide are also at least partly rescued at this point. We thus speculate that CRBN might contribute to angiogenesis.
Although the relationship between CRBN and thalidomide teratogenicity is established, the role of CRBN in known pathways such as Fgf, Wnt, Akt and angiogenesis is just beginning to be understood. Thus far, we have only shown that CRBN is important for Fgf8/10 expression (Fig. 4). Elucidation of the role of CRBN in other signaling pathways is an important issue to be addressed.
Tissue specificity of thalidomide action
We observed strong expression of zcrbn in the pectoral fin, brain, and head vasculature in zebrafish [25]. In the pectoral fin, zcrbn is expressed in migrating and proximal mesenchyme. On the other hand, zcrbn expression is not detectable in somites in 48-hpf zebrafish embryos. The expression pattern of zcrbn is consistent with its role as a mediator of thalidomide teratogenicity. However, CRBN appears to be expressed ubiquitously in adult human and mouse tissues [83]. Therefore, CRBN is necessary but not sufficient for thalidomide teratogenicity. Other molecules, perhaps downstream targets of CRBN, are also likely to be involved. The tissue specificity of thalidomide teratogenicity may be defined in part by the expression patterns of such molecules. With regard to this, Fgf8 is particularly interesting because of its tissue-specific expression.
Species specificity of thalidomide action
Mouse/rat CRBN is ~95% homologous to human CRBN and has been shown to bind thalidomide [25]. Hence, CRBN is unlikely to direct the species specificity of thalidomide effects. Several possibilities may explain why rodents are resistant to the teratogenic effects of thalidomide.
First, differences in the pharmacokinetics of thalidomide metabolism may explain the species differences. As mentioned above, thalidomide is rapidly hydrolyzed or metabolized into over a dozen products in vitro and in vivo, and many of the breakdown products are non-teratogenic [24]. In support of this, the plasma elimination half-life of orally administered thalidomide is significantly shorter in mice (~0.5 h) than in rabbits (~2.2 h) and humans (~7.3 h) [30].
Second, it is possible that the breakdown or bioactivation of thalidomide may be required for the induction of its teratogenicity. As mentioned above, thalidomide is enzymatically or spontaneously hydrolyzed to more than a dozen breakdown products. However, evidence exists that the bioactivation of thalidomide in the liver is not critically involved in its teratogenic action, at least in zebrafish and chicks. In zebrafish, developmental defects in the ear were observed around 30 hpf, when the liver is in an early stage of development and is not yet functional. hnf4, one of the key transcription factors responsible for hepatic transcription of CYP450 genes, is not yet expressed at 30 hpf, and vascularization essential for liver function occurs only after 60 hpf [84]. In chicks, thalidomide applied directly to one of the forelimb buds causes specific defects in the thalidomide-treated limb, but not in the other limb [24, 25]. Hence, teratological outcomes can be due to the parent thalidomide. It is, however, quite difficult to demonstrate this point unequivocally in vivo. Does CRBN only bind to the parent thalidomide? Currently, we are investigating its interaction with thalidomide-related compounds. Our preliminary data showed that CRBN bound to some thalidomide analogs and derivatives. Evidently, thalidomide is not the only compound that interacts with CRBN. Therefore, it is possible that the species-dependent breakdown products that retain CRBN-binding activity contribute to developmental defects. Further study of the relationship between CRBN and thalidomide breakdown products may shed new light on this issue.
Third, thalidomide-induced formation of ROS is reported to occur in rabbits but not in mice. This may be due to the finding that the glutathione-dependent antioxidant response is stronger in mice and rats than in human [85].
Finally, interspecies differences in gestational development may result in different developmental toxic manifestations after exposure to thalidomide [86].
Other thalidomide-binding proteins
We performed affinity purification from extracts of numerous cell lines and developing zebrafish embryos. Nevertheless, the only thalidomide-binding proteins identified were CRBN and the CRBN-binding protein DDB1 [25] (Fig. 3b). CRBN may be a major target of thalidomide, but the above data do not exclude the possibility that thalidomide has other targets. The immobilization of chemical compounds on affinity beads is an important factor with respect to target purification. As previously mentioned, thalidomide is composed of phthalimide and glutarimide. CRBN was purified using thalidomide beads on which the phthalimide moiety of thalidomide is immobilized (Fig. 3a). Previously, we identified a novel target of the cancer reagent, methotrexate (MTX), using a similar technique [87]. In this study, affinity chromatography using MTX immobilized by the γ-carboxyl group of its glutamate moiety resulted in a high yield purification of a known target, dehydrofolate reductase (DHFR). On the other hand, MTX immobilized through another part of the glutamate moiety resulted in purification of deoxycytidine kinase (dCK). Changing the part of thalidomide immobilized on the beads might lead to the discovery of new targets. Currently, we are investigating other target proteins using thalidomide beads on which the glutarimide moiety is immobilized.
Conclusions
Since 1961, scientists and physicians have been investigating the way in which thalidomide causes limb malformation. However, the molecular mechanisms of thalidomide teratogenicity remain a mystery. The data accumulated thus far reveal that thalidomide induces oxidative stress, affects angiogenesis, and inhibits Fgf, Wnt, and Akt signaling. Furthermore, CRBN, a direct and primary target of thalidomide teratogenicity, has recently been identified. Thalidomide binds to CRBN and inhibits the E3 ubiquitin ligase function that is important for limb/fin and ear development. However, several questions remain to be solved, such as the mechanisms of tissue specificity and species specificity. Moreover, new questions have arisen, such as how CRBN fits into other hypotheses and models.
Finally, we discuss a possible relationship between CRBN and the therapeutic effects of thalidomide. Despite serious adverse side effects, thalidomide is now used for the treatment of multiple myeloma and leprosy. In addition, new thalidomide derivatives have been developed [2, 3]. Among them, lenalidomide (CC-5013) and pomalidomide (CC-4047) have potent pharmacological effects. Lenalidomide is effective for the treatment of myeloma, myelodisplastic syndrome (MDS), chronic lymphocytic leukemia, non-Hodgkins leukemia, and solid tumors [88–92], and has been approved for the treatment of multiple myeloma and 5q-MDS by the US FDA. Pomalidomide is used for the treatment of myeloma, myelofibrosis, sickle cell anemia, and solid tumors [93]. Due to their structural similarity to thalidomide, these compounds have teratogenic potential. Whether CRBN is involved in the pharmacological effects of thalidomide or its derivatives is unclear. If CRBN is not involved in the beneficial effects of thalidomide, non-teratogenic thalidomide derivatives could be designed. High throughput screening for thalidomide derivatives and related compounds that retain the pharmacological effects of thalidomide but lack CRBN-binding may lead to major therapeutic advances. Conversely, if CRBN is associated with the therapeutic effects of thalidomide, studies are needed to identify downstream targets and substrates. This would allow for the screening of compounds that target only the downstream signaling pathway or therapeutic substrates.
Acknowledgments
We thank Drs. Yuki Yamaguchi, Toshihiko Ogura and Takayuki Suzuki for aiding us in our research. Our research was supported by the Global COE (Center of Excellence) Program from the Japan Ministry of Education, Culture, Sports, Science, and Technology; and by a grant for Research and Development Projects in Cooperation with Academic Institutions from the New Energy and Technology Development Organization; and by Special Coordination Funds for Promoting Science and Technology from the Japan Science and Technology Agency (JST).
References
- 1.Franks ME, Macpherson GR, Figg WD. Thalidomide. Lancet. 2004;363:1802–1811. doi: 10.1016/S0140-6736(04)16308-3. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4:314–322. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 3.Melchert M, List A. The thalidomide saga. Int J Biochem Cell Biol. 2007;39:1489–1499. doi: 10.1016/j.biocel.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Knobloch J, Ruther U. Shedding light on an old mystery: thalidomide suppresses survival pathways to induce limb defects. Cell Cycle. 2008;7:1121–1127. doi: 10.4161/cc.7.9.5793. [DOI] [PubMed] [Google Scholar]
- 5.Vargesson N. Thalidomide-induced limb defects: resolving a 50-year-old puzzle. Bioessays. 2009;31:1327–1336. doi: 10.1002/bies.200900103. [DOI] [PubMed] [Google Scholar]
- 6.McBride WG. Thalidomide embryopathy. Teratology. 1977;16:79–82. doi: 10.1002/tera.1420160113. [DOI] [PubMed] [Google Scholar]
- 7.Lenz W. A short history of thalidomide embryopathy. Teratology. 1988;38:203–215. doi: 10.1002/tera.1420380303. [DOI] [PubMed] [Google Scholar]
- 8.Sheskin J. Thalidomide in the treatment of lepra reactions. Clin Pharmacol Ther. 1965;6:303–306. doi: 10.1002/cpt196563303. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez-Rodriguez O. Thalidomide. A promising new treatment for rheumatoid arthritis. Arthritis Rheum. 1984;27:1118–1121. doi: 10.1002/art.1780271006. [DOI] [PubMed] [Google Scholar]
- 10.Hamza MH. Treatment of Behcet’s disease with thalidomide. Clin Rheumatol. 1986;5:365–371. doi: 10.1007/BF02054255. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy DM, Kanfer EJ, Barrett AJ. Thalidomide for the therapy of graft-versus-host disease following allogeneic bone marrow transplantation. Biomed Pharmacother. 1989;43:693–697. doi: 10.1016/0753-3322(89)90089-9. [DOI] [PubMed] [Google Scholar]
- 12.Vogelsang GB, Farmer ER, Hess AD, Altamonte V, Beschorner WE, Jabs DA, Corio RL, Levin LS, Colvin OM, Wingard JR, Santos GW. Thalidomide for the treatment of chronic graft-versus-host disease. N Engl J Med. 1992;326:1055–1058. doi: 10.1056/NEJM199204163261604. [DOI] [PubMed] [Google Scholar]
- 13.Atra E, Sato EI. Treatment of the cutaneous lesions of systemic lupus erythematosus with thalidomide. Clin Exp Rheumatol. 1993;11:487–493. [PubMed] [Google Scholar]
- 14.Makonkawkeyoon S, Limson-Pobre RN, Moreira AL, Schauf V, Kaplan G. Thalidomide inhibits the replication of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1993;90:5974–5978. doi: 10.1073/pnas.90.13.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampaio EP, Sarno EN, Galilly R, Cohn ZA, Kaplan G. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med. 1991;173:699–703. doi: 10.1084/jem.173.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreira AL, Sampaio EP, Zmuidzinas A, Frindt P, Smith KA, Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med. 1993;177:1675–1680. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, Munshi N, Anaissie E, Wilson C, Dhodapkar M, Zeddis J, Barlogie B. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 19.Zeldis JB, Williams BA, Thomas SD, Elsayed ME. S.T.E.P.S.: a comprehensive program for controlling and monitoring access to thalidomide. Clin Ther. 1999;21:319–330. doi: 10.1016/S0149-2918(00)88289-2. [DOI] [PubMed] [Google Scholar]
- 20.Castilla EE, Ashton-Prolla P, Barreda-Mejia E, Brunoni D, Cavalcanti DP, Correa-Neto J, Delgadillo JL, Dutra MG, Felix T, Giraldo A, Juarez N, Lopez-Camelo JS, Nazer J, Orioli IM, Paz JE, Pessoto MA, Pina-Neto JM, Quadrelli R, Rittler M, Rueda S, Saltos M, Sanchez O, Schuler L. Thalidomide, a current teratogen in South America. Teratology. 1996;54:273–277. doi: 10.1002/(SICI)1096-9926(199612)54:6<273::AID-TERA1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Schuler-Faccini L, Soares RC, de Sousa AC, Maximino C, Luna E, Schwartz IV, Waldman C, Castilla EE. New cases of thalidomide embryopathy in Brazil. Birth Defects Res A Clin Mol Teratol. 2007;79:671–672. doi: 10.1002/bdra.20384. [DOI] [PubMed] [Google Scholar]
- 22.Hansen JM, Harris C. A novel hypothesis for thalidomide-induced limb teratogenesis: redox misregulation of the NF-kappaB pathway. Antioxid Redox Signal. 2004;6:1–14. doi: 10.1089/152308604771978291. [DOI] [PubMed] [Google Scholar]
- 23.Parman T, Wiley MJ, Wells PG. Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat Med. 1999;5:582–585. doi: 10.1038/8466. [DOI] [PubMed] [Google Scholar]
- 24.Therapontos C, Erskine L, Gardner ER, Figg WD, Vargesson N. Thalidomide induces limb defects by preventing angiogenic outgrowth during early limb formation. Proc Natl Acad Sci USA. 2009;106:8573–8578. doi: 10.1073/pnas.0901505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y, Handa H. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 26.Eriksson T, Bjorkman S, Roth B, Hoglund P. Intravenous formulations of the enantiomers of thalidomide: pharmacokinetic and initial pharmacodynamic characterization in man. J Pharm Pharmacol. 2000;52:807–817. doi: 10.1211/0022357001774660. [DOI] [PubMed] [Google Scholar]
- 27.Braun AG, Harding FA, Weinreb SL. Teratogen metabolism: thalidomide activation is mediated by cytochrome P-450. Toxicol Appl Pharmacol. 1986;82:175–179. doi: 10.1016/0041-008X(86)90449-7. [DOI] [PubMed] [Google Scholar]
- 28.Ando Y, Fuse E, Figg WD. Thalidomide metabolism by the CYP2C subfamily. Clin Cancer Res. 2002;8:1964–1973. [PubMed] [Google Scholar]
- 29.Chen TL, Vogelsang GB, Petty BG, Brundrett RB, Noe DA, Santos GW, Colvin OM. Plasma pharmacokinetics and urinary excretion of thalidomide after oral dosing in healthy male volunteers. Drug Metab Dispos. 1989;17:402–405. [PubMed] [Google Scholar]
- 30.Chung F, Lu J, Palmer BD, Kestell P, Browett P, Baguley BC, Tingle M, Ching LM. Thalidomide pharmacokinetics and metabolite formation in mice, rabbits, and multiple myeloma patients. Clin Cancer Res. 2004;10:5949–5956. doi: 10.1158/1078-0432.CCR-04-0421. [DOI] [PubMed] [Google Scholar]
- 31.Miller MT, Stromland K. Teratogen update: thalidomide: a review, with a focus on ocular findings and new potential uses. Teratology. 1999;60:306–321. doi: 10.1002/(SICI)1096-9926(199911)60:5<306::AID-TERA11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 32.Mellin GW, Katzenstein M. The saga of thalidomide. Neuropathy to embryopathy, with case reports of congenital anomalies. N Engl J Med. 1962;267:1184–1192. doi: 10.1056/NEJM196212062672305. [DOI] [PubMed] [Google Scholar]
- 33.Spouge D, Baird PA. Imperforate anus in 700,000 consecutive liveborn infants. Am J Med Genet Suppl. 1986;2:151–161. doi: 10.1002/ajmg.1320250619. [DOI] [PubMed] [Google Scholar]
- 34.Davis MC, Dahn RD, Shubin NH. An autopodial-like pattern of Hox expression in the fins of a basal actinopterygian fish. Nature. 2007;447:473–476. doi: 10.1038/nature05838. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka M, Munsterberg A, Anderson WG, Prescott AR, Hazon N, Tickle C. Fin development in a cartilaginous fish and the origin of vertebrate limbs. Nature. 2002;416:527–531. doi: 10.1038/416527a. [DOI] [PubMed] [Google Scholar]
- 36.Moon AM, Capecchi MR. Fgf8 is required for outgrowth and patterning of the limbs. Nat Genet. 2000;26:455–459. doi: 10.1038/82601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewandoski M, Sun X, Martin GR. Fgf8 signalling from the AER is essential for normal limb development. Nat Genet. 2000;26:460–463. doi: 10.1038/82609. [DOI] [PubMed] [Google Scholar]
- 38.Hansen JM, Gong SG, Philbert M, Harris C. Misregulation of gene expression in the redox-sensitive NF-kappab-dependent limb outgrowth pathway by thalidomide. Dev Dyn. 2002;225:186–194. doi: 10.1002/dvdy.10150. [DOI] [PubMed] [Google Scholar]
- 39.Brent RL. Drug testing in animals for teratogenic effects. Thalidomide in the pregnant rat. J Pediatr. 1964;64:762–770. doi: 10.1016/S0022-3476(64)80626-0. [DOI] [PubMed] [Google Scholar]
- 40.Kenyon BM, Browne F, D’Amato RJ. Effects of thalidomide and related metabolites in a mouse corneal model of neovascularization. Exp Eye Res. 1997;64:971–978. doi: 10.1006/exer.1997.0292. [DOI] [PubMed] [Google Scholar]
- 41.Fratta ID, Sigg EB, Maiorana K. Teratogenic effects of thalidomide in rabbits, rats, hamsters, and mice. Toxicol Appl Pharmacol. 1965;7:268–286. doi: 10.1016/0041-008X(65)90095-5. [DOI] [PubMed] [Google Scholar]
- 42.Stephens TD. Proposed mechanisms of action in thalidomide embryopathy. Teratology. 1988;38:229–239. doi: 10.1002/tera.1420380307. [DOI] [PubMed] [Google Scholar]
- 43.Stephens TD, Fillmore BJ. Hypothesis: thalidomide embryopathy-proposed mechanism of action. Teratology. 2000;61:189–195. doi: 10.1002/(SICI)1096-9926(200003)61:3<189::AID-TERA6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 44.Stephens TD, Bunde CJ, Fillmore BJ. Mechanism of action in thalidomide teratogenesis. Biochem Pharmacol. 2000;59:1489–1499. doi: 10.1016/S0006-2952(99)00388-3. [DOI] [PubMed] [Google Scholar]
- 45.Parman T, Chen G, Wells PG. Free radical intermediates of phenytoin and related teratogens. Prostaglandin H synthase-catalyzed bioactivation, electron paramagnetic resonance spectrometry, and photochemical product analysis. J Biol Chem. 1998;273:25079–25088. doi: 10.1074/jbc.273.39.25079. [DOI] [PubMed] [Google Scholar]
- 46.Wells PG, Winn LM. Biochemical toxicology of chemical teratogenesis. Crit Rev Biochem Mol Biol. 1996;31:1–40. doi: 10.3109/10409239609110574. [DOI] [PubMed] [Google Scholar]
- 47.Ohuchi H, Nakagawa T, Yamamoto A, Araga A, Ohata T, Ishimaru Y, Yoshioka H, Kuwana T, Nohno T, Yamasaki M, Itoh N, Noji S. The mesenchymal factor, FGF10, initiates and maintains the outgrowth of the chick limb bud through interaction with FGF8, an apical ectodermal factor. Development. 1997;124:2235–2244. doi: 10.1242/dev.124.11.2235. [DOI] [PubMed] [Google Scholar]
- 48.Knobloch J, Shaughnessy JD, Jr, Ruther U. Thalidomide induces limb deformities by perturbing the Bmp/Dkk1/Wnt signaling pathway. FASEB J. 2007;21:1410–1421. doi: 10.1096/fj.06-7603com. [DOI] [PubMed] [Google Scholar]
- 49.Knobloch J, Schmitz I, Gotz K, Schulze-Osthoff K, Ruther U. Thalidomide induces limb anomalies by PTEN stabilization, Akt suppression, and stimulation of caspase-dependent cell death. Mol Cell Biol. 2008;28:529–538. doi: 10.1128/MCB.00533-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pizette S, Niswander L. BMPs negatively regulate structure and function of the limb apical ectodermal ridge. Development. 1999;126:883–894. doi: 10.1242/dev.126.5.883. [DOI] [PubMed] [Google Scholar]
- 51.Scherz PJ, Harfe BD, McMahon AP, Tabin CJ. The limb bud Shh-Fgf feedback loop is terminated by expansion of former ZPA cells. Science. 2004;305:396–399. doi: 10.1126/science.1096966. [DOI] [PubMed] [Google Scholar]
- 52.Leslie NR, Downes CP. PTEN function: how normal cells control it and tumour cells lose it. Biochem J. 2004;382:1–11. doi: 10.1042/BJ20040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pajni-Underwood S, Wilson CP, Elder C, Mishina Y, Lewandoski M. BMP signals control limb bud interdigital programmed cell death by regulating FGF signaling. Development. 2007;134:2359–2368. doi: 10.1242/dev.001677. [DOI] [PubMed] [Google Scholar]
- 54.Andela VB, Sheu TJ, Puzas EJ, Schwarz EM, O’Keefe RJ, Rosier RN. Malignant reversion of a human osteosarcoma cell line, Saos-2, by inhibition of NFkappaB. Biochem Biophys Res Commun. 2002;297:237–241. doi: 10.1016/S0006-291X(02)02141-1. [DOI] [PubMed] [Google Scholar]
- 55.Grotewold L, Ruther U. The Wnt antagonist Dickkopf-1 is regulated by Bmp signaling and c-Jun and modulates programmed cell death. EMBO J. 2002;21:966–975. doi: 10.1093/emboj/21.5.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sauer H, Gunther J, Hescheler J, Wartenberg M. Thalidomide inhibits angiogenesis in embryoid bodies by the generation of hydroxyl radicals. Am J Pathol. 2000;156:151–158. doi: 10.1016/S0002-9440(10)64714-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yabu T, Tomimoto H, Taguchi Y, Yamaoka S, Igarashi Y, Okazaki T. Thalidomide-induced antiangiogenic action is mediated by ceramide through depletion of VEGF receptors, and is antagonized by sphingosine-1-phosphate. Blood. 2005;106:125–134. doi: 10.1182/blood-2004-09-3679. [DOI] [PubMed] [Google Scholar]
- 59.Bauer KS, Dixon SC, Figg WD. Inhibition of angiogenesis by thalidomide requires metabolic activation, which is species-dependent. Biochem Pharmacol. 1998;55:1827–1834. doi: 10.1016/S0006-2952(98)00046-X. [DOI] [PubMed] [Google Scholar]
- 60.Lu J, Palmer BD, Kestell P, Browett P, Baguley BC, Muller G, Ching LM. Thalidomide metabolites in mice and patients with multiple myeloma. Clin Cancer Res. 2003;9:1680–1688. [PubMed] [Google Scholar]
- 61.Ng SS, Gutschow M, Weiss M, Hauschildt S, Teubert U, Hecker TK, Luzzio FA, Kruger EA, Eger K, Figg WD. Antiangiogenic activity of N-substituted and tetrafluorinated thalidomide analogues. Cancer Res. 2003;63:3189–3194. [PubMed] [Google Scholar]
- 62.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 63.Grandel H, Schulte-Merker S. The development of the paired fins in the zebrafish (Danio rerio) Mech Dev. 1998;79:99–120. doi: 10.1016/S0925-4773(98)00176-2. [DOI] [PubMed] [Google Scholar]
- 64.Lebrin F, Srun S, Raymond K, Martin S, van den Brink S, Freitas C, Breant C, Mathivet T, Larrivee B, Thomas JL, Arthur HM, Westermann CJ, Disch F, Mager JJ, Snijder RJ, Eichmann A, Mummery CL. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med. 2010;16:420–428. doi: 10.1038/nm.2131. [DOI] [PubMed] [Google Scholar]
- 65.Sakamoto S, Kabe Y, Hatakeyama M, Yamaguchi Y, Handa H. Development and application of high-performance affinity beads: toward chemical biology and drug discovery. Chem Rec. 2009;9:66–85. doi: 10.1002/tcr.20170. [DOI] [PubMed] [Google Scholar]
- 66.Shimizu N, Sugimoto K, Tang J, Nishi T, Sato I, Hiramoto M, Aizawa S, Hatakeyama M, Ohba R, Hatori H, Yoshikawa T, Suzuki F, Oomori A, Tanaka H, Kawaguchi H, Watanabe H, Handa H. High-performance affinity beads for identifying drug receptors. Nat Biotechnol. 2000;18:877–881. doi: 10.1038/78496. [DOI] [PubMed] [Google Scholar]
- 67.Nishio K, Masaike Y, Ikeda M, Narimatsu H, Gokon N, Tsubouchi S, Hatakeyama M, Sakamoto S, Hanyu N, Sandhu A, Kawaguchi H, Abe M, Handa H. Development of novel magnetic nano-carriers for high-performance affinity purification. Colloids Surf B Biointerfaces. 2008;64:162–169. doi: 10.1016/j.colsurfb.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 68.Higgins JJ, Pucilowska J, Lombardi RQ, Rooney JP. A mutation in a novel ATP-dependent Lon protease gene in a kindred with mild mental retardation. Neurology. 2004;63:1927–1931. doi: 10.1212/01.wnl.0000146196.01316.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Angers S, Li T, Yi X, MacCoss MJ, Moon RT, Zheng N. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature. 2006;443:590–593. doi: 10.1038/nature05175. [DOI] [PubMed] [Google Scholar]
- 70.Jo S, Lee KH, Song S, Jung YK, Park CS. Identification and functional characterization of cereblon as a binding protein for large-conductance calcium-activated potassium channel in rat brain. J Neurochem. 2005;94:1212–1224. doi: 10.1111/j.1471-4159.2005.03344.x. [DOI] [PubMed] [Google Scholar]
- 71.Hohberger B, Enz R. Cereblon is expressed in the retina and binds to voltage-gated chloride channels. FEBS Lett. 2009;583:633–637. doi: 10.1016/j.febslet.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 72.Wittschieben BO, Wood RD. DDB complexities. DNA Repair (Amst) 2003;2:1065–1069. doi: 10.1016/S1568-7864(03)00113-7. [DOI] [PubMed] [Google Scholar]
- 73.Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 74.Groisman R, Polanowska J, Kuraoka I, Sawada J, Saijo M, Drapkin R, Kisselev AF, Tanaka K, Nakatani Y. The ubiquitin ligase activity in the DDB2 and CSA complexes is differentially regulated by the COP9 signalosome in response to DNA damage. Cell. 2003;113:357–367. doi: 10.1016/S0092-8674(03)00316-7. [DOI] [PubMed] [Google Scholar]
- 75.Lee J, Zhou P. DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Mol Cell. 2007;26:775–780. doi: 10.1016/j.molcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 76.Pickart CM. Back to the future with ubiquitin. Cell. 2004;116:181–190. doi: 10.1016/S0092-8674(03)01074-2. [DOI] [PubMed] [Google Scholar]
- 77.Sugasawa K, Okuda Y, Saijo M, Nishi R, Matsuda N, Chu G, Mori T, Iwai S, Tanaka K, Hanaoka F. UV-induced ubiquitylation of XPC protein mediated by UV-DDB–ubiquitin ligase complex. Cell. 2005;121:387–400. doi: 10.1016/j.cell.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 78.Groisman R, Kuraoka I, Chevallier O, Gaye N, Magnaldo T, Tanaka K, Kisselev AF, Harel-Bellan A, Nakatani Y. CSA-dependent degradation of CSB by the ubiquitin–proteasome pathway establishes a link between complementation factors of the Cockayne syndrome. Genes Dev. 2006;20:1429–1434. doi: 10.1101/gad.378206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- 80.Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 81.Lee KJ, Lee KM, Jo S, Kang KW, Park CS. Induction of cereblon by NF-E2-related factor 2 in neuroblastoma cells exposed to hypoxia-reoxygenation. Biochem Biophys Res Commun. 2010;399:711–715. doi: 10.1016/j.bbrc.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 82.Kang KW, Lee SJ, Kim SG. Molecular mechanism of nrf2 activation by oxidative stress. Antioxid Redox Signal. 2005;7:1664–1673. doi: 10.1089/ars.2005.7.1664. [DOI] [PubMed] [Google Scholar]
- 83.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Field HA, Dong PD, Beis D, Stainier DY. Formation of the digestive system in zebrafish. II. Pancreas morphogenesis. Dev Biol. 2003;261:197–208. doi: 10.1016/S0012-1606(03)00308-7. [DOI] [PubMed] [Google Scholar]
- 85.Knobloch J, Reimann K, Klotz LO, Ruther U. Thalidomide resistance is based on the capacity of the glutathione-dependent antioxidant defense. Mol Pharm. 2008;5:1138–1144. doi: 10.1021/mp8001232. [DOI] [PubMed] [Google Scholar]
- 86.Janer G, Verhoef A, Gilsing HD, Piersma AH. Use of the rat postimplantation embryo culture to assess the embryotoxic potency within a chemical category and to identify toxic metabolites. Toxicol In Vitro. 2008;22:1797–1805. doi: 10.1016/j.tiv.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 87.Uga H, Kuramori C, Ohta A, Tsuboi Y, Tanaka H, Hatakeyama M, Yamaguchi Y, Takahashi T, Kizaki M, Handa H. A new mechanism of methotrexate action revealed by target screening with affinity beads. Mol Pharmacol. 2006;70:1832–1839. doi: 10.1124/mol.106.025866. [DOI] [PubMed] [Google Scholar]
- 88.List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, Rimsza L, Heaton R, Knight R, Zeldis JB. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352:549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 89.Andritsos LA, Johnson AJ, Lozanski G, Blum W, Kefauver C, Awan F, Smith LL, Lapalombella R, May SE, Raymond CA, Wang DS, Knight RD, Ruppert AS, Lehman A, Jarjoura D, Chen CS, Byrd JC. Higher doses of lenalidomide are associated with unacceptable toxicity including life-threatening tumor flare in patients with chronic lymphocytic leukemia. J Clin Oncol. 2008;26:2519–2525. doi: 10.1200/JCO.2007.13.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chanan-Khan A, Miller KC, Musial L, Lawrence D, Padmanabhan S, Takeshita K, Porter CW, Goodrich DW, Bernstein ZP, Wallace P, Spaner D, Mohr A, Byrne C, Hernandez-Ilizaliturri F, Chrystal C, Starostik P, Czuczman MS. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006;24:5343–5349. doi: 10.1200/JCO.2005.05.0401. [DOI] [PubMed] [Google Scholar]
- 91.Chanan-Khan AA, Cheson BD. Lenalidomide for the treatment of B-cell malignancies. J Clin Oncol. 2008;26:1544–1552. doi: 10.1200/JCO.2007.14.5367. [DOI] [PubMed] [Google Scholar]
- 92.Xu Y, Li J, Ferguson GD, Mercurio F, Khambatta G, Morrison L, Lopez-Girona A, Corral LG, Webb DR, Bennett BL, Xie W. Immunomodulatory drugs reorganize cytoskeleton by modulating Rho GTPases. Blood. 2009;114:338–345. doi: 10.1182/blood-2009-02-200543. [DOI] [PubMed] [Google Scholar]
- 93.Aragon-Ching JB, Li H, Gardner ER, Figg WD. Thalidomide analogues as anticancer drugs. Recent Pat Anticancer Drug Discov. 2007;2:167–174. doi: 10.2174/157489207780832478. [DOI] [PMC free article] [PubMed] [Google Scholar]