Abstract
The cellular prion glycoprotein (PrPC) is ubiquitously expressed but its physiologic functions remain enigmatic, particularly in the immune system. Here, we demonstrate in vitro and in vivo that PrPC is involved in T lymphocytes response to oxidative stress. By monitoring the intracellular level of reduced glutathione, we show that PrP−/− thymocytes display a higher susceptibility to H2O2 exposure than PrP+/+ cells. Furthermore, we find that in mice fed with a restricted diet, a regimen known to increase the intracellular level of ROS, PrP−/− thymocytes are more sensitive to oxidative stress. PrPC function appears to be specific for oxidative stress, since no significant differences are observed between PrP−/− and PrP+/+ mice exposed to other kinds of stress. We also show a marked evolution of the redox status of T cells throughout differentiation in the thymus. Taken together, our results clearly ascribe to PrPC a protective function in thymocytes against oxidative stress.
Keywords: Cellular prion protein, T lymphocytes, Oxidative stress, Redox balance, Thymus
Introduction
PrPC is a highly conserved GPI-anchored glycoprotein [1, 2] whose true physiological function is still debated [3, 4]. Mice lacking PrPC are viable and display only minor abnormalities [5, 6]. It has been proposed that, due to its localization at the cell membrane, PrPC could play a role in ligand uptake, cell adhesion or signal transduction [7–11]. Moreover, several groups have shown the ability of this protein to bind copper with high affinity [12–14], ascribing to PrPC a role in copper homeostasis. Finally, there is increasing evidence that, in the brain, PrPC participates in the resistance against oxidative stress [15–18]. It has been shown that neurons from PrP−/− mice have an increased susceptibility to superoxide anion and hydrogen peroxide (H2O2) toxicity [19, 20].
Most studies about PrPC are focused on the brain. However, the high expression of PrPC is not restricted to the central nervous system [21, 22] but is also observed in many non-neuronal tissues including muscles, heart, kidney, hematopoietic cells and cells of the immune system [23, 24]. In the hematopoietic compartment, the level of PrPC expression is highly variable from one cell type to another and even within a given lineage, depending on the maturation steps [25, 26]. For example, it was observed that PrPC is differentially expressed on developing thymocytes, suggesting a role of this protein in the regulation of T lymphocyte differentiation [25, 27, 28].
T cell differentiation in the thymus is a precisely orchestrated process characterized by successive steps which can be followed by the expression of the cell surface molecules CD4, CD8, CD25 and CD44 [29–31]. αβT lymphocyte differentiation begins as CD4−CD8−, double-negative (DN) cells. This population is divided into four cell subpopulations based on the regulated expression of CD25 and CD44: CD44+CD25− (DN1), CD44+CD25+ (DN2), CD44−CD25+ (DN3) and CD44−CD25− (DN4). Then, DN4 thymocytes progress to CD4+CD8+ double-positive (DP) cells and express low levels of αβTCR. A fraction of the DP cells is positively selected to become either CD4+ or CD8+ single-positive (SP) cells, which finally exit the thymus and migrate to the periphery. This differentiation is highly influenced by microenvironment conditions. It is well known that the redox system and antioxidant enzymes have an effect on both cell signalization [32, 33] and apoptosis mechanisms which occur during T cell differentiation in the thymus [33, 34]. In vivo, it appears that antioxidant conditions disrupt immature thymocyte development. In thymic organ cultures, antioxidants selectively affect αβ+ thymocyte differentiation via their effects on NF-κB [35] which plays a crucial role in lymphocyte maturation [36]. Similarly, transgenic mice over-expressing Cu/Zn superoxide dismutase exhibit various immunological abnormalities, including an early thymic involution [37, 38].
We previously reported that over-expression of PrPC in mice resulted in strong alterations at different stages of T cell differentiation in the thymus. These effects could be partially reversed in vivo by copper supplementation in the drinking water, which led us to propose that the over-expression of PrPC, by chelating copper, creates an antioxidant context that is not supportive for αβ T cell development [28]. In the present study, we investigated the function of the PrPC protein in the thymic redox homeostasis. We first compared the response of PrP−/− and WT thymocytes in culture to an oxidative stress using H2O2 exposure. Then, to analyze the effects of oxidative stress in vivo, mice were submitted to a restricted feeding schedule, known to increase the intracellular level of reactive oxygen species (ROS) [39]. Finally, to assign more precisely to PrPC a role in the defense against oxidative stress, we carried out two other types of stress: mice received dexamethasone by IP injection or were submitted to gamma-irradiation. Altogether, our results demonstrate that thymocytes from PrP−/− mice are much more affected than their WT counterparts by both in vitro and in vivo oxidative stress. We therefore propose that PrPC plays an important role in the protection of thymocytes from oxidative stress, extending to the thymus the role of PrPC in the redox homeostasis previously described in the brain.
Materials and methods
Mice
C57BL/6 mice were purchased from Charles River Laboratories (l’Arbresle, France). PrP−/− mice [40] on a mixed C57BL/6/129 background were obtained from the Centre de Distribution, Typage et Archivage animal facility (CNRS, Orléans, France). Both strains were maintained under specific pathogen-free conditions in the animal facility of the Commissariat à l’Energie Atomique-Grenoble (A38 185 01), in accordance with institutional guidelines.
Cell culture and oxidative stress
Thymocytes were isolated from 4- to 6-week-old C57BL/6 or PrP−/− mice and cultured in RPMI 1640 medium supplemented with 10% FCS, 1 mM sodium pyruvate, 1% non essential amino acids, 50 μM β-mercaptoethanol, 1% penicillin (100 U/ml), 1% streptomycin (100 μg/ml) (all from InVitrogen Life Technologies, Cergy Pontoise, France). Cells (3 × 106/ml) were cultured in 24-wells plates (1 ml/well) at 37°C in humidified 5% CO2 in air. Oxidative stress was induced by addition to the cells of 200 μM H2O2 and incubation for the indicated period of time. Cell viability was determined at the end of the treatment by acridin orange/ethidium bromide staining under UV light.
Flow cytometry analysis
For the analysis of cell surface protein expression, single-cell suspensions of thymocytes (1 × 106) were stained for 30 min at 4°C with conjugated antibodies in PBS containing 3% fetal calf serum and 0.16% sodium azide. The antibodies used for staining (anti-CD4, anti-CD8, anti-CD25 and anti-CD44) were all from BD Pharmingen (le Pont de Claix, France). Various combinations of these antibodies labeled with FITC (excitation 488 nm, emission 525 nm), PE (excitation 488 nm, emission 575 nm), APC (excitation 633 nm, emission 670 nm) and PE-Cy5 (excitation 488 nm, emission 760 nm) were used. Data acquisition and analysis were performed with a FacsCalibur flow cytometer equipped with CellQuest software (Becton Dickinson).
Measurement of intracellular glutathione
Monochlorobimane (mCB) is a thiol-reactive cell permeable reagent able to bind to the intracellular reduced glutathione (GSH) [41]. The resulting adduct is fluorescent. mCB was added to the cells as described [42]. Briefly, cells were washed twice with PBS and stained with 50 μM mCB (Molecular Probes, Interchim, Montluçon, France) in PBS for 5 min at 37°C. After a further incubation for 5 min on ice, the cellular fluorescence was analyzed by flow cytometry under UV excitation, using a Moflo cytometer (Dako, Trappes, France). In these conditions, the level of fluorescence intensity is directly correlated to the intracellular level of reduced glutathione. The numbers provided in Table 1 represent the mean fluorescence intensity (±SD) of mCB staining in the different thymocyte populations. Because identical results were obtained in WT and PrP−/− mice, results from these two groups of animals were grouped for statistical significance analysis (Student’s t test, SPSS Software ver 15.0)
Table 1.
Reduced glutathione level in the different thymic subpopulations
| DN1–DN2 | DN3 | DN4 | DP | CD4 | CD8 | |
|---|---|---|---|---|---|---|
| C57BL/6 ± SD | 3,112 ± 80 | 1,968 ± 41 | 2,053 ± 108 | 629 ± 23 | 725 ± 57 | 883 ± 53 |
| PrP−/− ± SD | 3,125 ± 73 | 2,077 ± 151 | 2,150 ± 125 | 662 ± 42 | 760 ± 85 | 1,003 ± 118 |
The results are expressed as mean fluorescence units ± SD of monochlorobimane staining intensity (n = 3)
The thymic subpopulations were defined as described in Fig. 1
Measurement of glutathione reductase levels in thymocytes
Assay of glutathione reductase (GRase) activity was adapted from Carlberg and Mannervik [43]. The principle is based on the measurement at 340 nm of NADPH consumption catalyzed by GRase during the reduction of GSSH. Results were expressed as mean international units per gram of protein ± SD of at least three experiments. Statistical significance was determined using Student’s t test.
Copper dietary supplementation
Supplemented C57BL/6 or PrP−/− mice were given water containing 250 mg/L CuSO4 and 50 mg/L sucrose, whereas the control group received only sucrose. The diet was maintained for 5 weeks and then the mice were sacrificed for analysis of the thymocytes.
Restricted feeding experiment
Mice were kept under a restricted diet consisting in the free access to food and water for only 2 h per day as described in [39]. After 7 days, the diet was stopped and mice had again unlimited access to food and water. Throughout this experiment, 3–4 mice were tested every day for their ability to respond to the stress generated by the diet. For this purpose, mice were sacrificed and their thymi recovered. Total numbers of thymocytes were measured and cells were incubated with anti-CD4 and anti-CD8 antibodies for flow cytometry analysis
Dexamethasone-mediated apoptosis
Mice were subjected to two injections of dexamethasone (2 mg/kg; i.p.) separated by 24 h. They were killed 24 h after the last injection and thymocytes were analyzed by FACS as described above.
Gamma irradiation
Mice were irradiated (6 Gy) in the “Anémone/Bio” irradiator (60Co, 2 Gy/min) in the “Arc/Nucléart” facility at the CEA-Grenoble. Mice were sacrificed 24 h post irradiation.
Results
Measurement of the intracellular redox steady state of T lymphocyte precursors
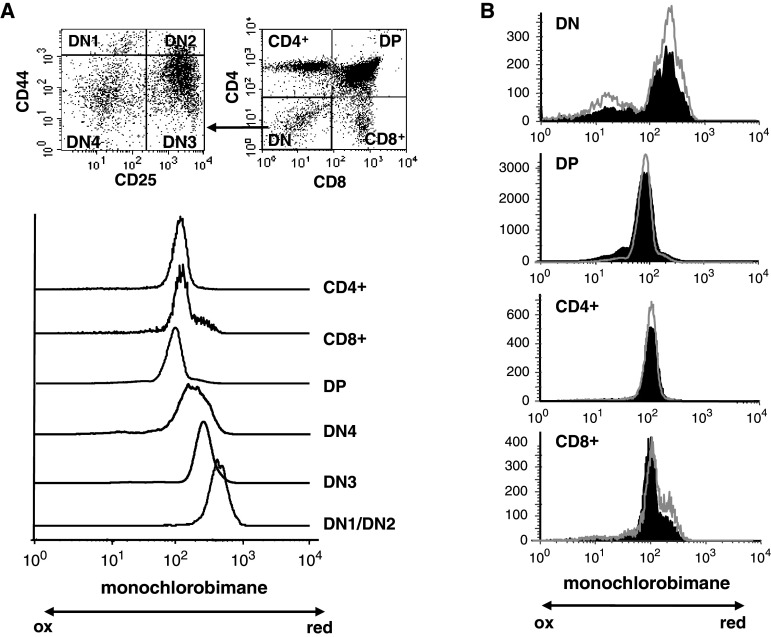
Glutathione is implicated in the protection of cells from reactive oxygen species such as free radicals and peroxides: reduced glutathione (GSH) acts as a scavenger of ROS and is regenerated through transient formation of the oxidized form (GSSG). In order to evaluate the redox state of thymocyte subpopulations, C57BL/6 and PrP−/− thymocytes were labeled with anti-CD4, anti-CD8, anti-CD44, and anti-CD25 antibodies and the content in GSH of the different subpopulations was measured by the mCB assay, as described in “Materials and methods”. We previously showed that T cell differentiation is normal in PrP−/− mice [28]. Overall, identical results were obtained by the mCB assay for both types of mice (Fig. 1; Table 1). As shown in Fig. 1a for WT mice, the most immature thymocytes (DN1/DN2 cells) contained the highest level of GSH. These cells were uniformly mCBbright. The intensity of the staining then decreased as the cells progressed until the DN3 stages (P < 0.001 when data from both types of mice are pooled) and DN4 (P < 0.05 with DN3). For both types of mice (Fig. 1a, b) there was a further reduction in the intracellular content in GSH when the developing thymocytes reached their DP stage (P < 0.001 between DN4 and DP), whereas a slight increase is noted during the final maturation of thymocytes to the SP stage (P = 0.003 between DP and SP CD4+ and P < 0.001 between DP and SP CD8+). Interestingly, while the staining was homogeneous for the DN1/DN2, DN3, DP and SP CD4+ populations, the distribution of mCB+ DN4 cells was broader and likely corresponded to an intermediate between the staining level of their DN3 precursors and their DP progeny. This dichotomy could reflect the transitional nature of the DN4 population, as these cells cycle rapidly while they exit the DN compartment to become DP. Similarly, the staining of the SP CD8+ cells was biphasic: the vast majority of the cells contained low DP-like levels of GSH while a minor population exhibited a mCB staining intensity similar to that found in DN3. This last population could represent immature SP thymocytes, an intermediate population of cycling cells on their way to become DP. Altogether, these results show that there is a marked evolution of the redox status during normal thymocyte development in mice. The same level of mCB staining is obtained for a given thymocyte population in different mice. (Fig. 1b; Table 1). Interestingly, this pattern of intracellular GSH was also found in mutant mice where T cell development is impaired because a lack of pre-TCR expression, pTα−/− mice and CD3εΔ5/Δ5 mice (data not shown), underscoring the regulated control of the redox state during thymocyte differentiation.
Fig. 1.
Measurement of the intracellular reduced glutathione. a Thymocytes from C57BL/6 mice were labeled with anti-CD4, anti-CD8, anti-CD25 and anti-CD44 antibodies to define the different T cell subpopulations, and then incubated with monochlorobimane to evaluate their redox state, as described in “Materials and methods”. The data presented here are representative of four different experiments. b Comparison of the level of GSH between C57BL/6 (black histograms) and PrP−/− (gray line) thymocytes prepared and analyzed as in (a). The data shown here are representative of six different experiments
Thymocytes from PrP-deficient mice are more susceptible to a H2O2 stress
In order to extend our in vitro observations to an in vivo context and in order to evaluate whether PrPC expression plays a role in the modification of the cellular level of GSH in thymocytes after an oxidative stress, PrP−/− and WT cells were incubated with H2O2. Hydrogen peroxide crosses freely the membrane and induces in the cytoplasm the generation of the highly reactive hydroxyl radical OH.[44]. Thymocytes from C57BL/6 or PrP−/− mice were plated as described in “Materials and methods” and incubated for 4 h in the presence of 0.2 mM H2O2. At this concentration, which was previously used to analyze the effect of stress on antigen-presenting cells [45, 46], the cellular viability was not affected (data not shown). In C57BL/6 thymocytes, this treatment resulted in a decrease in the percentage of cells positive for mCB staining (Fig. 2a), i.e., that do contain high levels of GSH. But this decrease was much more important in the absence of PrPC expression. The PrP−/− mice used for these analyses are from a mixed C57BL/6/129 background. Experiments were repeated with mice that were backcrossed more than 10 times to C57BL/6 mice. Similar results were obtained (data not shown), showing that this effect was not due to a strain difference. Thus, in an oxidative stress situation, PrP−/− thymocytes are less able to maintain their pool of GSH, showing a difference in the capacity of these cells to respond to H2O2 exposure.
Fig. 2.
Effect of an oxidative stress on C57BL/6 and PrP-deficient thymocytes. a Control cells or cells that have been incubated with H2O2 were stained with mCB and analyzed by flow cytometry as described in “Materials and methods”. The data presented here are representative of six different experiments on PrP+/+ C57BL/6 or PrP−/− mice. b GRase activity in C57BL/6 and PrP−/− thymocytes: GRase activity was measured before (control) and after incubation of cells in the presence of H2O2 (*P < 0.05; n = 3)
In PrP−/− mice, neurons have also been shown to be over-sensitive to H2O2 exposure. It was suggested that this effect resulted from a decrease in glutathione reductase (GRase) activity [20, 47]. To determine whether the increased susceptibility to peroxide toxicity observed in PrP−/− mice was linked to an altered GRase activity, we measured it in WT and PrP−/− thymocytes, before and after incubation with H2O2 (Fig. 2b). In the absence of peroxide, GRase activity was unchanged in PrP−/− thymocytes when compared to WT. This activity was significantly increased in PrP−/− but not WT thymocytes in the presence of 0.2 mM H2O2. These results show that the increased susceptibility of PrPC-deficient thymocytes to H2O2 exposure does not result from a reduced GRase activity as this activity was on the contrary increased in this context. This increase was, however, not sufficient to prevent the decrease of the GSH level in PrP−/− thymocytes. In addition, these results show that the GRase activity is differentially regulated in neurons and thymocytes.
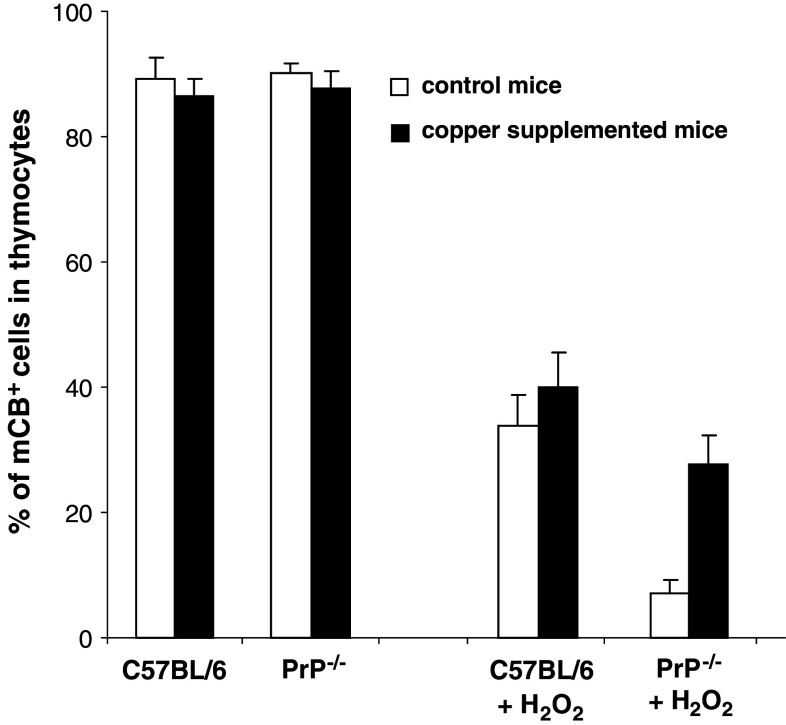
Knowing that PrPC binds copper and that this cation plays a crucial role in the regulation of the redox balance, we tried to restore, in mice lacking the PrPC protein, a correct redox intracellular environment by supplementing their drinking water with copper. The results of this copper supplementation are shown in Fig. 3. There was no difference in the content in GSH between WT and PrP−/− thymocytes when no stress was applied. Addition of copper did not change the GSH level. On the other hand, while the content of reduced glutathione after H2O2 treatment was not significantly modified in copper supplemented C57BL/6 as compared to non-treated mice, copper addition allowed the PrP−/− mice to limit the effects of the H2O2 stress: the level of reduced glutathione was significantly increased and reached nearly that of treated WT mice. This last result reinforces the hypothesis that PrPC could contribute to the preservation of the redox homeostasis.
Fig. 3.
Effect of a copper supplementation on the level of GSH. Copper was given for 5 weeks in the drinking water of supplemented mice. Thymocytes were then recovered, submitted or not to H2O2 and the level of mCB staining was determined as in Fig. 2a
Effect of a physiologic oxidative stress on mice
In order to extend our in vitro observations to an in vivo context, we exposed thymocytes in their physiological environment to an oxidative stress. To this end, mice were kept under a restricted diet: free access to food and water was allowed for only 2 h per day for 7 days. This regimen has previously been shown to increase the intracellular level of ROS [39] and to induce thymocyte apoptosis [48, 49]. We therefore compared C57BL/6 and PrP−/− mice in their ability to response to this type of stress by evaluating, each day, the thymus cellularity and the distribution of thymocyte populations. As shown in Fig. 4a, in treated C57BL/6 mice, thymic cellularity remained constant for the first 4 days of food restriction (between 216 and 197 × 106 cells). Then it fell at day 6 to reach a minimum (30 × 106 cells) 1 day after the end of the restriction period (day 8). By the second day after the return to a normal regimen (day 9), the number of thymocytes already began to increase (71 × 106 cells). In treated PrP−/− mice, thymic cellularity was found to decrease already from the first day of the food restriction period (131 vs 189 × 106 cells). This decrease was strong throughout the treatment, so that on the sixth day, PrP−/− thymus contained only 12 × 106 cells, compared to 85 × 106 cells in C57BL/6 mice. As for WT mice, this reduction in thymocytes number was prolonged after the end of the treatment: the first and the second days after the return to a normal diet, the thymus of the treated animals contained only about 2 × 106 cells. It is only at day 11, 4 days after the end of the restriction period, that the number of thymocytes began to increase, whereas this trend was already present in WT mice at day 9. Thus, the loss of cellularity began earlier, was much more pronounced and needed a longer period to be reverted in absence of PrPC expression. Altogether these results confirm that developing thymocytes are more sensitive to oxidative stress in the absence of PrP.
Fig. 4.
PrP−/− mice are more affected by a redox stress than C57BL/6 mice. a Mice were allowed a free access to food and water for 2 h per day for 7 days. After this period, they recovered a free access to food and water. The cellularity of the thymus was evaluated every day. b T cell number in each thymic subpopulation was determined by FACS analysis at day 8 after the initiation of the diet. Data are representative of n = 4 for control mice and n = 3 for the stressed mice
In parallel, we investigated the consequences of this treatment on each thymocyte subpopulation. FACS analysis revealed that thymocytes at every stage of differentiation were affected. The absolute number of cells in each of the four main compartments (DN, DP, CD4 and CD8) decreased after restricted feeding (Fig. 4b). Two days after the end of the treatment, in C57BL/6 mice, the number of cells present in the DN, DP, SP CD4 and SP CD8 compartments were only 25, 10, 30 and 48% of the numbers found in control mice, respectively. In PrP−/− mice, the consequences of the applied regimen were clearly more severe: only 4.8, 0.1, 4 and 6.5% of the cells were left in the DN, DP, SP CD4 and SP CD8 compartments in treated mice, respectively. In addition, even though DP thymocytes are the most affected cells in the thymus irrespective of PrPC expression, in the absence of PrPC these cells became a hundred times more sensitive to the oxidative stress generated by food restriction (0.11 vs 10.12% of cells remaining in PrP-deficient and WT mice, respectively). Altogether, these results reveal a higher susceptibility of PrP−/− mice to oxidative stress in vivo.
Effect of non-oxidative stress on PrP−/− mice
We next wanted to study whether the absence of PrPC had also deleterious effects on developing thymocytes in mice confronted with other types of stress. To this purpose, mice were exposed to two different types of stress. First, C57BL/6 and PrP−/− mice were injected with dexamethasone. This glucocorticoid hormone is known to induce thymocyte apoptosis [50]. C57BL/6 and PrP−/− mice received two IP injections of dexamethasone (2 mg/kg) separated by 24 h, then, 24 h after the last injection, CD4−CD8−, CD4+CD8+, SP CD4+, and SP CD8+ subpopulations were analyzed by flow cytometry. As shown in Table 2, dexamethasone induced an important reduction of the percentage of DP in C57BL/6 and PrP−/− thymocytes. This reduction was nevertheless highly comparable between both types of mice. Thus, absence of PrP expression does not influence thymocyte response to dexamethasone injection in vivo.
Table 2.
Effect of dexamethasone and γ-irradiation on the different thymic subpopulations in PrP−/− and C57BL/6 mice (n ≥ 5)
| Mice | % DN ± SD | % DP ± SD | % CD4+ ± SD | % CD8+ ± SD |
|---|---|---|---|---|
| C57BL/6 control | 3.50 ± 0.42 | 84.11 ± 1.71 | 7.67 ± 0.65 | 2.54 ± 0.49 |
| PrP−/− control | 3.36 ± 0.53 | 82.32 ± 1.76 | 8.61 ± 0.45 | 3.32 ± 0.77 |
| C57BL/6 + dexamethasone | 16.01 ± 0.94 | 6.36 ± 0.80 | 29.44 ± 0.30 | 16.43 ± 1.00 |
| PrP−/− + dexamethasone | 12.24 ± 1.05 | 8.45 ± 3.28 | 30.88 ± 2.21 | 17.88 ± 1.23 |
| C57BL/6 6 Gy | 20.39 ± 1.30 | 17.75 ± 4.69 | 43.41 ± 10.61 | 16.76 ± 3.28 |
| PrP−/− 6 Gy | 17.87 ± 4.45 | 27.58 ± 14.40 | 40.21 ± 7.29 | 14.34 ± 3.01 |
We then used gamma irradiation which is known to strongly affect thymocytes [51–53]. Both PrP−/− and C57BL/6 mice were exposed to a single dose (6 Gy) of whole body 60Co irradiation. DP cells are known to be highly radio-sensitive and, accordingly the percentage of DP was strongly reduced after irradiation, irrespective of PrPC expression (Table 2). It varied from 84.11 ± 1.71% for un-irradiated C57BL/6 mice to 17.75 ± 4.69% after irradiation and from 82.32 ± 1.76 to 27.58 ± 14.40% for PrP−/− mice. We observed compensatory increases in the other subpopulations after irradiation, but no significant differences between PrP−/− and WT mice. These results show that in vivo PrP−/− thymocytes behave like wild-type cells when exposed to no oxidative stress.
Discussion
In a previous report, we showed that over-expression of the PrPC protein at the surface of thymocytes was at the origin of defects in the development of T cell precursors in the thymus, and that copper supplementation induced a partial restoration of T lymphocyte differentiation [28]. These results led us to propose that the over-expression of PrPC increases copper chelation and thus creates an antioxidant environment in the cells that interferes with a correct αβ T cell development. The aim of the present study was to determine whether in the absence of PrPC, thymocytes are more susceptible to oxidative stress and whether PrPC could contribute to the response of thymocytes against oxidative stress. To this purpose, we compared in vitro and in vivo the response of WT and PrP−/− thymocytes exposed to different stress. Our results show that thymocytes from mice that do not express the PrPC protein are less efficient in their capacity to respond to an oxidative stress as compared to WT mice, but behave like WT thymocytes after radiation exposure or dexamethasone treatment.
Oxidative stress is the result of the imbalance between production and destruction of ROS, leading to modification of the intracellular redox state. This imbalance can result from exposure of the cells to an excess of oxidant species or from a cellular deficit in antioxidant molecules such as glutathione [44]. Reduced glutathione is critical for the prevention of lipid and protein oxidation and the detoxification of ROS. It is essential for the regulation of the redox balance. Thymocyte development and T lymphocyte activities are markedly influenced by environment conditions. For example, exposure to ROS has been shown to impair TCR signaling [54] and T lymphocyte activation and functions [55–57]. Conversely, optimal proliferation and activation of mature lymphocytes require reducing conditions [55, 58].
Our results now show that each discrete thymocyte subpopulation is characterized by a specific level of reduced glutathione. The highest quantity of GSH is found in the more immature cells and it regularly decreases as the cells become more and more mature, to reach its lowest level in the DP/SP subpopulations. It would be interesting to determine whether this developmental regulation of the intracellular GSH is required for proper thymocyte development or is imposed onto developing thymocytes by their maturation process. This difference of redox state of the various T cell subpopulations may be used as a marker to characterize the progress in the differentiation pathway.
Glutathione is only one of the components participating in the regulation of intracellular redox balance in thymocytes. By chelating copper, the PrPC protein could also play a role in this process, but it appears dispensable in normal conditions, as similar levels of GSH are found in C57BL/6 and PrP−/− cells. However, when thymocytes are exposed to oxidative stress conditions, a marked difference between C57BL/6 and PrPC-deficient mice becomes evident: GSH is strongly diminished and/or not rapidly replenished in PrP−/− thymocytes. In PrP−/− neurons, it has been shown that this effect was the result, at least in part, of the low glutathione reductase activity [20]. Obviously, this is not the case in lymphocytes, as we found a higher GRase activity in PrP−/− thymocytes after the H2O2 stress. It seems that, after H2O2 exposure, these PrP-deficient thymocytes enhanced their GRase activity in an attempt to preserve a correct GSH pool, but this increase in GRase activity cannot maintain/restore a normal level of intracellular GSH in PrP−/− thymocytes. Thus, paradoxically, the absence of PrPC results in a lower level of GSH and a greater GRase activity in response to oxidative stress, demonstrating the importance of the PrPC protein in the regulation of the GSH pool and the redox homeostasis.
Interestingly, we can note a difference within mice that have received a copper supplementation: for WT mice, the content of GSH in thymocytes after exposure to H2O2 is not changed as compared to non-supplemented mice, whereas addition of copper allows the cells from PrP−/− mice to face the oxidative stress, having a GSH content much higher than thymocytes from mice that have not been treated with copper. This result strengthens the hypothesis that PrPC could play a role in redox homeostasis. We can thus conclude that the function of the PrPC protein is revealed under oxidation stress conditions in vitro.
Similarly, in living mice, the loss of PrPC is also correlated with an increased susceptibility to oxidative stress generated via food restriction. The decrease in total thymic cellularity is more pronounced and the effect lasts for a longer period for PrP−/− mice as compared to WT mice. In both mice, food deprivation strongly affects DP cells but this effect is much more pronounced in the case of PrP−/− mice. We propose that, due to its capacity to bind copper, PrPC participates to the fine regulation of the redox balance and consequently contributes to the regulation of thymocyte differentiation.
PrPC has been shown to be implicated in many processes where redox regulation is involved, in particular during antigen-driven interactions where PrPC appears to be mobilized at the immune synapse [59]. PrPC is associated with lipid rafts and it has been well documented that cross-linking of PrPC on mature T lymphocytes leads to PrPC capping in clusters also containing Thy-1 and molecules implicated in T cell activation, i.e., TCR/CD3, ZAP70, Fyn, Lck and LAT [60–62]. It has been recently reported that oxidative stress results in oxidation of the actin-remodeling protein cofilin [63]. Oxidation of this key integrator of T cell activation leads to impaired actin depolymerization and results in T cell hyporesponsivness under stress conditions due to incomplete immune synapse formation [63]. Antibody-mediated ligation of PrPC at the surface of T lymphocytes promotes NADPH oxidase activation and a transient accumulation of ROS which act as second messenger, through phosphorylation of MAPK-ERK1/2 [10]. In this case, ROS are no longer considered as responsible for an oxidative stress but rather as key actors of intracellular signalization [64]. The activation of ERK by NADPH oxidase products, in response to PrPC ligation, could allow the preservation of the redox status of the cell. Our results show that, both in vivo and in vitro, the response of thymocytes to oxidative stress is altered in the absence of PrPC . Taking into account published data and our present study, we propose that, in the absence of PrPC, the signaling pathway which leads to the phosphorylation of the ERK1/2 kinases is disturbed, with no direct consequences in normal conditions but leading to the disruption of the oxidant/antioxidant balance in the case of a redox stress.
Our results underline the importance of the redox balance in the different steps of T cell differentiation. Moreover, by ex vivo and in vivo experiments, we have demonstrated that, in the absence of PrPC, thymocytes are less able to face an oxidative stress and to maintain their intracellular GSH level. Altogether, our results show that PrPC have a protective function against oxidative stress and in the preservation of the intracellular redox balance.
Acknowledgments
This work was supported by specific national grants from the GIS-Prion (#A108), the CEA ToxNuc program (#13-10) and European Community project Immunoprion (FP7-013144).
References
- 1.Sarnataro D, Campana V, Paladino S, Stornaiuolo M, Nitsch L, Zurzolo C. PrP(C) association with lipid rafts in the early secretory pathway stabilizes its cellular conformation. Mol Biol Cell. 2004;15:4031–4042. doi: 10.1091/mbc.E03-05-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stahl N, Borchelt DR, Prusiner SB. Differential release of cellular and scrapie prion proteins from cellular membranes by phosphatidylinositol-specific phospholipase C. Biochemistry. 1990;29:5405–5412. doi: 10.1021/bi00474a028. [DOI] [PubMed] [Google Scholar]
- 3.Aguzzi A, Baumann F, Bremer J. The prion’s elusive reason for being. Annu Rev Neurosci. 2008;31:439–477. doi: 10.1146/annurev.neuro.31.060407.125620. [DOI] [PubMed] [Google Scholar]
- 4.Hu W, Kieseier B, Frohman E, Eagar TN, Rosenberg RN, Hartung HP, Stuve O. Prion proteins: physiological functions and role in neurological disorders. J Neurol Sci. 2008;264:1–8. doi: 10.1016/j.jns.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Collinge J, Whittington MA, Sidle KC, Smith CJ, Palmer MS, Clarke AR, Jefferys JG. Prion protein is necessary for normal synaptic function. Nature. 1994;370:295–297. doi: 10.1038/370295a0. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi S, Katamine S, Nishida N, Moriuchi R, Shigematsu K, Sugimoto T, Nakatani A, Kataoka Y, Houtani T, Shirabe S, Okada H, Hasegawa S, Miyamoto T, Noda T. Loss of cerebellar Purkinje cells in aged mice homozygous for a disrupted PrP gene. Nature. 1996;380:528–531. doi: 10.1038/380528a0. [DOI] [PubMed] [Google Scholar]
- 7.Hundt C, Peyrin JM, Haik S, Gauczynski S, Leucht C, Rieger R, Riley ML, Deslys JP, Dormont D, Lasmezas C, Weiss S. Identification of interaction domains of the prion protein with its 37-kDa/67-kDa laminin receptor. EMBO J. 2001;20:5876–5886. doi: 10.1093/emboj/20.21.5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mouillet-Richard S, Ermonval M, Chebassier C, Laplanche JL, Lehmann S, Launay JM, Kellermann O. Signal transduction through prion protein. Science. 2000;289:1925–1928. doi: 10.1126/science.289.5486.1925. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt-Ulms G, Legname G, Baldwin MA, Ball HL, Bradon N, Bosque PJ, Crossin KL, Edelman GM, DeArmond SJ, Cohen FE, Prusiner SB. Binding of neural cell adhesion molecules (N-CAMs) to the cellular prion protein. J Mol Biol. 2001;314:1209–1225. doi: 10.1006/jmbi.2000.5183. [DOI] [PubMed] [Google Scholar]
- 10.Schneider B, Mutel V, Pietri M, Ermonval M, Mouillet-Richard S, Kellermann O. NADPH oxidase and extracellular regulated kinases 1/2 are targets of prion protein signaling in neuronal and nonneuronal cells. Proc Natl Acad Sci USA. 2003;100:13326–13331. doi: 10.1073/pnas.2235648100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spielhaupter C, Schatzl HM. PrPC directly interacts with proteins involved in signaling pathways. J Biol Chem. 2001;276:44604–44612. doi: 10.1074/jbc.M103289200. [DOI] [PubMed] [Google Scholar]
- 12.Brown DR, Qin K, Herms JW, Madlung A, Manson J, Strome R, Fraser PE, Kruck T, von Bohlen A, Schulz-Schaeffer W, Giese A, Westaway D, Kretzschmar H. The cellular prion protein binds copper in vivo. Nature. 1997;390:684–687. doi: 10.1038/37733. [DOI] [PubMed] [Google Scholar]
- 13.Hornshaw MP, McDermott JR, Candy JM, Lakey JH. Copper binding to the N-terminal tandem repeat region of mammalian and avian prion protein: structural studies using synthetic peptides. Biochem Biophys Res Commun. 1995;214:993–999. doi: 10.1006/bbrc.1995.2384. [DOI] [PubMed] [Google Scholar]
- 14.Stockel J, Safar J, Wallace AC, Cohen FE, Prusiner SB. Prion protein selectively binds copper(II) ions. Biochemistry. 1998;37:7185–7193. doi: 10.1021/bi972827k. [DOI] [PubMed] [Google Scholar]
- 15.Brown DR, Clive C, Haswell SJ. Antioxidant activity related to copper binding of native prion protein. J Neurochem. 2001;76:69–76. doi: 10.1046/j.1471-4159.2001.00009.x. [DOI] [PubMed] [Google Scholar]
- 16.Brown DR, Wong BS, Hafiz F, Clive C, Haswell SJ, Jones IM. Normal prion protein has an activity like that of superoxide dismutase. Biochem J. 1999;344:1–5. doi: 10.1042/0264-6021:3440001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milhavet O, Lehmann S. Oxidative stress and the prion protein in transmissible spongiform encephalopathies. Brain Res Brain Res Rev. 2002;38:328–339. doi: 10.1016/S0165-0173(01)00150-3. [DOI] [PubMed] [Google Scholar]
- 18.Wong BS, Pan T, Liu T, Li R, Gambetti P, Sy MS. Differential contribution of superoxide dismutase activity by prion protein in vivo. Biochem Biophys Res Commun. 2000;273:136–139. doi: 10.1006/bbrc.2000.2911. [DOI] [PubMed] [Google Scholar]
- 19.Brown DR, Schmidt B, Kretzschmar HA. Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature. 1996;380:345–347. doi: 10.1038/380345a0. [DOI] [PubMed] [Google Scholar]
- 20.White AR, Collins SJ, Maher F, Jobling MF, Stewart LR, Thyer JM, Beyreuther K, Masters CL, Cappai R. Prion protein-deficient neurons reveal lower glutathione reductase activity and increased susceptibility to hydrogen peroxide toxicity. Am J Pathol. 1999;155:1723–1730. doi: 10.1016/S0002-9440(10)65487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kretzschmar HA, Prusiner SB, Stowring LE, DeArmond SJ. Scrapie prion proteins are synthesized in neurons. Am J Pathol. 1986;122:1–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Sales N, Rodolfo K, Hassig R, Faucheux B, Di Giamberardino L, Moya KL. Cellular prion protein localization in rodent and primate brain. Eur J Neurosci. 1998;10:2464–2471. doi: 10.1046/j.1460-9568.1998.00258.x. [DOI] [PubMed] [Google Scholar]
- 23.Fournier JG. Nonneuronal cellular prion protein. Int Rev Cytol. 2001;208:121–160. doi: 10.1016/S0074-7696(01)08003-2. [DOI] [PubMed] [Google Scholar]
- 24.Horiuchi M, Yamazaki N, Ikeda T, Ishiguro N, Shinagawa M. A cellular form of prion protein (PrPC) exists in many non-neuronal tissues of sheep. J Gen Virol. 1995;76:2583–2587. doi: 10.1099/0022-1317-76-10-2583. [DOI] [PubMed] [Google Scholar]
- 25.Kubosaki A, Yusa S, Nasu Y, Nishimura T, Nakamura Y, Saeki K, Matsumoto Y, Itohara S, Onodera T. Distribution of cellular isoform of prion protein in T lymphocytes and bone marrow, analyzed by wild-type and prion protein gene-deficient mice. Biochem Biophys Res Commun. 2001;282:103–107. doi: 10.1006/bbrc.2001.4538. [DOI] [PubMed] [Google Scholar]
- 26.Liu T, Li R, Wong BS, Liu D, Pan T, Petersen RB, Gambetti P, Sy MS. Normal cellular prion protein is preferentially expressed on subpopulations of murine hemopoietic cells. J Immunol. 2001;166:3733–3742. doi: 10.4049/jimmunol.166.6.3733. [DOI] [PubMed] [Google Scholar]
- 27.Isaacs JD, Jackson GS, Altmann DM. The role of the cellular prion protein in the immune system. Clin Exp Immunol. 2006;146:1–8. doi: 10.1111/j.1365-2249.2006.03194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jouvin-Marche E, Attuil-Audenis V, Aude-Garcia C, Rachidi W, Zabel M, Podevin-Dimster V, Siret C, Huber C, Martinic M, Riondel J, Villiers CL, Favier A, Naquet P, Cesbron JY, Marche PN. Overexpression of cellular prion protein induces an antioxidant environment altering T cell development in the thymus. J Immunol. 2006;176:3490–3497. doi: 10.4049/jimmunol.176.6.3490. [DOI] [PubMed] [Google Scholar]
- 29.Ceredig R, Rolink T. A positive look at double-negative thymocytes. Nat Rev Immunol. 2002;2:888–897. doi: 10.1038/nri937. [DOI] [PubMed] [Google Scholar]
- 30.Sebzda E, Mariathasan S, Ohteki T, Jones R, Bachmann MF, Ohashi PS. Selection of the T cell repertoire. Annu Rev Immunol. 1999;17:829–874. doi: 10.1146/annurev.immunol.17.1.829. [DOI] [PubMed] [Google Scholar]
- 31.Zuniga-Pflucker JC. T-cell development made simple. Nat Rev Immunol. 2004;4:67–72. doi: 10.1038/nri1257. [DOI] [PubMed] [Google Scholar]
- 32.Chaudhri G, Hunt NH, Clark IA, Ceredig R. Antioxidants inhibit proliferation and cell surface expression of receptors for interleukin-2 and transferrin in T lymphocytes stimulated with phorbol myristate acetate and ionomycin. Cell Immunol. 1988;115:204–213. doi: 10.1016/0008-8749(88)90174-8. [DOI] [PubMed] [Google Scholar]
- 33.Tome ME, Briehl MM. Thymocytes selected for resistance to hydrogen peroxide show altered antioxidant enzyme profiles and resistance to dexamethasone-induced apoptosis. Cell Death Differ. 2001;8:953–961. doi: 10.1038/sj.cdd.4400904. [DOI] [PubMed] [Google Scholar]
- 34.Freeman BD, Reaume AG, Swanson PE, Epstein CJ, Carlson EJ, Buchman TG, Karl IE, Hotchkiss RS. Role of CuZn superoxide dismutase in regulating lymphocyte apoptosis during sepsis. Crit Care Med. 2000;28:1701–1708. doi: 10.1097/00003246-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Ivanov V, Merkenschlager M, Ceredig R. Antioxidant treatment of thymic organ cultures decreases NF-kappa B and TCF1(alpha) transcription factor activities and inhibits alpha beta T cell development. J Immunol. 1993;151:4694–4704. [PubMed] [Google Scholar]
- 36.Voll RE, Jimi E, Phillips RJ, Barber DF, Rincon M, Hayday AC, Flavell RA, Ghosh S. NF-kappa B activation by the pre-T cell receptor serves as a selective survival signal in T lymphocyte development. Immunity. 2000;13:677–689. doi: 10.1016/S1074-7613(00)00067-4. [DOI] [PubMed] [Google Scholar]
- 37.Laurent J, Paly E, Marche PN, London J. Early thymic T cell development in young transgenic mice overexpressing human Cu/Zn superoxide dismutase, a model of Down syndrome. Free Radic Biol Med. 2006;40:1971–1980. doi: 10.1016/j.freeradbiomed.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 38.Peled-Kamar M, Lotem J, Okon E, Sachs L, Groner Y. Thymic abnormalities and enhanced apoptosis of thymocytes and bone marrow cells in transgenic mice overexpressing Cu/Zn-superoxide dismutase: implications for Down syndrome. EMBO J. 1995;14:4985–4993. doi: 10.1002/j.1460-2075.1995.tb00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moon EY, Han YH, Lee DS, Han YM, Yu DY. Reactive oxygen species induced by the deletion of peroxiredoxin II (PrxII) increases the number of thymocytes resulting in the enlargement of PrxII-null thymus. Eur J Immunol. 2004;34:2119–2128. doi: 10.1002/eji.200424962. [DOI] [PubMed] [Google Scholar]
- 40.Bueler H, Fischer M, Lang Y, Bluethmann H, Lipp HP, DeArmond SJ, Prusiner SB, Aguet M, Weissmann C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992;356:577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- 41.Hedley DW, Chow S. Evaluation of methods for measuring cellular glutathione content using flow cytometry. Cytometry. 1994;15:349–358. doi: 10.1002/cyto.990150411. [DOI] [PubMed] [Google Scholar]
- 42.Shrieve DC, Bump EA, Rice GC. Heterogeneity of cellular glutathione among cells derived from a murine fibrosarcoma or a human renal cell carcinoma detected by flow cytometric analysis. J Biol Chem. 1988;263:14107–14114. [PubMed] [Google Scholar]
- 43.Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–490. doi: 10.1016/S0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- 44.Martinez-Cayuela M. Oxygen free radicals and human disease. Biochimie. 1995;77:147–161. doi: 10.1016/0300-9084(96)88119-3. [DOI] [PubMed] [Google Scholar]
- 45.Preynat-Seauve O, Coudurier S, Favier A, Marche PN, Villiers C. Oxidative stress impairs intracellular events involved in antigen processing and presentation to T cells. Cell Stress Chaperones. 2003;8:162–171. doi: 10.1379/1466-1268(2003)008<0162:OSIIEI>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rees AD, Donati Y, Lombardi G, Lamb J, Polla B, Lechler R. Stress-induced modulation of antigen-presenting cell function. Immunology. 1991;74:386–392. [PMC free article] [PubMed] [Google Scholar]
- 47.Bains JS, Shaw CA. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Brain Res Rev. 1997;25:335–358. doi: 10.1016/S0165-0173(97)00045-3. [DOI] [PubMed] [Google Scholar]
- 48.Malpuech-Brugere C, Nowacki W, Gueux E, Kuryszko J, Rock E, Rayssiguier Y, Mazur A. Accelerated thymus involution in magnesium-deficient rats is related to enhanced apoptosis and sensitivity to oxidative stress. Br J Nutr. 1999;81:405–411. [PubMed] [Google Scholar]
- 49.Savino W. The thymus gland is a target in malnutrition. Eur J Clin Nutr. 2002;56:S46–S49. doi: 10.1038/sj.ejcn.1601485. [DOI] [PubMed] [Google Scholar]
- 50.Sun XM, Dinsdale D, Snowden RT, Cohen GM, Skilleter DN. Characterization of apoptosis in thymocytes isolated from dexamethasone-treated rats. Biochem Pharmacol. 1992;44:2131–2137. doi: 10.1016/0006-2952(92)90339-K. [DOI] [PubMed] [Google Scholar]
- 51.Candeias SM, Durum SK, Muegge K. p53-dependent apoptosis and transcription of p21waf/cip1/sdi1 in SCID mice following gamma-irradiation. Biochimie. 1997;79:607–612. doi: 10.1016/S0300-9084(97)82010-X. [DOI] [PubMed] [Google Scholar]
- 52.Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML, Wyllie AH. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 53.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 54.Padgett DA, Glaser R. How stress influences the immune response. Trends Immunol. 2003;24:444–448. doi: 10.1016/S1471-4906(03)00173-X. [DOI] [PubMed] [Google Scholar]
- 55.Gringhuis SI, Leow A, Papendrecht-Van Der Voort EA, Remans PH, Breedveld FC, Verweij CL. Displacement of linker for activation of T cells from the plasma membrane due to redox balance alterations results in hyporesponsiveness of synovial fluid T lymphocytes in rheumatoid arthritis. J Immunol. 2000;164:2170–2179. doi: 10.4049/jimmunol.164.4.2170. [DOI] [PubMed] [Google Scholar]
- 56.Lahdenpohja N, Savinainen K, Hurme M. Pre-exposure to oxidative stress decreases the nuclear factor-kappa B-dependent transcription in T lymphocytes. J Immunol. 1998;160:1354–1358. [PubMed] [Google Scholar]
- 57.Sahaf B, Heydari K, Herzenberg LA, Herzenberg LA. Lymphocyte surface thiol levels. Proc Natl Acad Sci USA. 2003;100:4001–4005. doi: 10.1073/pnas.2628032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Angelini G, Gardella S, Ardy M, Ciriolo MR, Filomeni G, Di Trapani G, Clarke F, Sitia R, Rubartelli A. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci USA. 2002;99:1491–1496. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ballerini C, Gourdain P, Bachy V, Blanchard N, Levavasseur E, Gregoire S, Fontes P, Aucouturier P, Hivroz C, Carnaud C. Functional implication of cellular prion protein in antigen-driven interactions between T cells and dendritic cells. J Immunol. 2006;176:7254–7262. doi: 10.4049/jimmunol.176.12.7254. [DOI] [PubMed] [Google Scholar]
- 60.Mattei V, Garofalo T, Misasi R, Circella A, Manganelli V, Lucania G, Pavan A, Sorice M. Prion protein is a component of the multimolecular signaling complex involved in T cell activation. FEBS Lett. 2004;560:14–18. doi: 10.1016/S0014-5793(04)00029-8. [DOI] [PubMed] [Google Scholar]
- 61.Stuermer CA, Langhorst MF, Wiechers MF, Legler DF, Von Hanwehr SH, Guse AH, Plattner H. PrPc capping in T cells promotes its association with the lipid raft proteins reggie-1 and reggie-2 and leads to signal transduction. FASEB J. 2004;18:1731–1733. doi: 10.1096/fj.04-2150fje. [DOI] [PubMed] [Google Scholar]
- 62.Wurm S, Paar C, Sonnleitner A, Sonnleitner M, Hoglinger O, Romanin C, Wechselberger C. Co-localization of CD3 and prion protein in Jurkat lymphocytes after hypothermal stimulation. FEBS Lett. 2004;566:121–125. doi: 10.1016/j.febslet.2004.03.114. [DOI] [PubMed] [Google Scholar]
- 63.Klemke M, Wabnitz GH, Funke F, Funk B, Kirchgessner H, Samstag Y. Oxidation of cofilin mediates T cell hyporesponsiveness under oxidative stress conditions. Immunity. 2008;29:404–413. doi: 10.1016/j.immuni.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 64.Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11:173–186. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]