Abstract
Mitochondrial dysfunction and oxidative stress occur in Parkinson’s disease (PD), but little is known about the molecular mechanisms controlling these events. Peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α) is a transcriptional coactivator that is a master regulator of oxidative stress and mitochondrial metabolism. We show here that transgenic mice overexpressing PGC-1α in dopaminergic neurons are resistant against cell degeneration induced by the neurotoxin MPTP. The increase in neuronal viability was accompanied by elevated levels of mitochondrial antioxidants SOD2 and Trx2 in the substantia nigra of transgenic mice. PGC-1α overexpression also protected against MPTP-induced striatal loss of dopamine, and mitochondria from PGC-1α transgenic mice showed an increased respiratory control ratio compared with wild-type animals. To modulate PGC-1α, we employed the small molecular compound, resveratrol (RSV) that protected dopaminergic neurons against the MPTP-induced cell degeneration almost to the same extent as after PGC-1α overexpression. As studied in vitro, RSV activated PGC-1α in dopaminergic SN4741 cells via the deacetylase SIRT1, and enhanced PGC-1α gene transcription with increases in SOD2 and Trx2. Taken together, the results reveal an important function of PGC-1α in dopaminergic neurons to combat oxidative stress and increase neuronal viability. RSV and other compounds acting via SIRT1/PGC-1α may prove useful as neuroprotective agents in PD and possibly in other neurological disorders.
Keywords: PGC-1α, RSV, SIRT1, MPTP, Dopaminergic neurons, Parkinson’s disease
Introduction
Accumulating evidence indicates that multiple factors, including genetic and environmental ones, contribute to dopaminergic neuron degeneration in PD [1–3]. Particularly, alterations in mitochondrial functions [4, 5] with an increased production of reactive oxygen species (ROS) have been associated with degeneration of midbrain dopaminergic neurons [6]. The underlying mechanisms causing neuron degeneration in PD may be related to changes in cell signaling cascades but these are not fully understood. PGC-1α is a transcriptional coactivator that is a master regulator of cell metabolism, mitochondrial biogenesis, oxidative stress, and gene expression [7, 8]. Recently, using gene-deleted mice, it was shown that a lack of PGC-1α increases the sensitivity of brain neurons against oxidative stress and excitotoxic injuries such as kainic acid [7]. Particularly, it was also shown that PGC-1α knockout mice are hypersensitive to the neurotoxin, 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP) [7] that causes degeneration of dopaminergic neurons in mice and in non-human primates. This data, together with others, underscores the importance of PGC-1α as an endogenous protective factor against cell injuries both in brain [7] as well as in other tissues such as heart [9].
In this work, we have produced transgenic mice overexpressing PGC-1α in brain neurons including dopaminergic neurons in the substantia nigra (SN), and studied cell degeneration in the SN after treatment with MPTP. In these studies, we also employed the small molecular compound resveratrol (3,4,5-trihydroxystilbene, RSV) that is known to post-transcriptionally activate PGC-1α via the histone deacetylase Sir2 (silent information regulator 2) orthologue Sirtuin-1 (SIRT1) [10–13]. In keeping with previous data using this compound [14–16], we observed that RSV significantly protected dopaminergic neurons in vivo against MPTP and the effect was similar to that observed after PGC-1α overexpression. To substantiate results obtained in vivo, we studied PGC-1α and RSV effects in mouse dopaminergic SN4741 cells in culture. The results indicate that it may be possible to modulate both the activity and levels of PGC-1α in dopaminergic neurons to increase neuronal viability and combat oxidative stress that is implicated in PD.
Materials and methods
Generation of PGC-1α transgenic mice
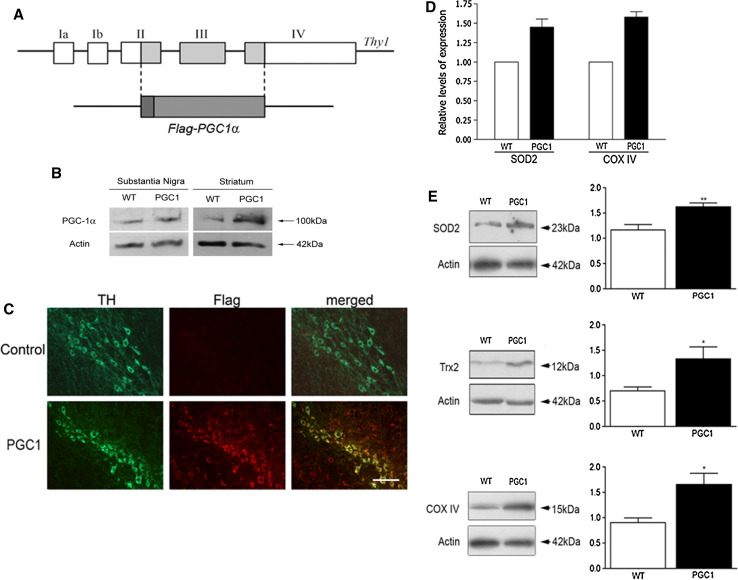
The PGC-1α cDNA with a Flag-tag was cloned into the Thy 1.2 expression cassette that drives transgene expression in brain neurons [17, 18]. Transgenic mice were generated using standard techniques at the Uppsala Transgenic Facility, at Uppsala University, Sweden. The genetic background of the mice was CBA × C57BL/6 and they were backcrossed to C57BL/6 mice for several generations to produce stable PGC-1α transgenic mouse lines, and controls were from the same breeding. The levels of PGC-1α in brain tissue including substantia nigra and striatum were studied by immunoblotting and using a polyclonal anti-PGC-1α antibody raised in rabbit against the amino acids 221–234 in the mouse sequence. Immunoblots were done as described below. To analyze the transgenic expression of PGC1 in dopaminergic neurons, immunostaining in substantia nigra was done using anti-Flag antibodies (1:300; Cell Signaling) in combination with anti-TH antibodies and employing appropriate fluorescent-labeled secondary antibodies. The mice showed expression of the PGC-1α transgene in dopaminergic neurons (Fig. 1c) Apart from midbrain, the transgene was expressed in other brain regions (data not shown), and a thorough characterization of gene expression in the PGC-1α transgenic mice will be reported later.
Fig. 1.
Analyses of transgenic mice with overexpression of PGC-1α in brain and in dopaminergic neurons. a PGC-1α cDNA containing a Flag-tag was inserted into the Thy1 expression cassette and the minigene was used to produce PGC-1α transgenic (PGC1) mice. Exons are numbered I–IV. b Immunoblots of substantia nigra and striatum from wild-type (wt) and PGC1 mice using anti-PGC-1α antibodies as described in “Methods”. c Immunostaining of sections from substantia nigra using anti-TH and anti-Flag antibodies. Upper panels control, wt mice; lower panels PGC1 mice. Note co-expression of Flag-PGC-1α with TH in the PGC1 mice but not in controls in the merged picture. Scale bar 50 μm. d Quantitative-PCR was done as described in “Methods” using RNA from substantia nigra of wild-type and PGC1 mice. Note increased expression of SOD2 and COX IV. A typical experiment is shown and was repeated with similar results. e Immunoblots were done as described in “Methods” using tissue from substantia nigra of wild-type and PGC1 mice. Protein levels of SOD2, Trx2 and COX IV were all increased in the PGC1 mice compared to wt. Left panels show typical immunoblots and right panels are quantification of the data. Values are means ± SD, n = 6 per group. *p < 0.05 and **p < 0.01 for PGC1 mice versus wild-type mice
MPTP and RSV treatments
All experiments were approved by the local ethical committee and performed in accordance with the European Communities Council Directive (86/609/EEC). Mice received three intraperitoneal (i.p.) injections of 14 mg/kg MPTP (Fluka Biochemica) within a time period of 3 h followed by a fourth injection using 7 mg/kg MPTP (see treatment schedule in Fig. 5a). This protocol induced a lesion of TH-positive neurons in the substantia nigra pars compacta (SNc) with a low degree of mortality of animals. Twenty mg/kg RSV (R5010 Sigma) was dissolved in 50% DMSO/70% ethanol and diluted in saline until to a final volume of 0.2 ml per mice and given i.p. Control mice received vehicle 50% (DMSO/70% ethanol diluted in saline) only. All mice were killed 7 days after the MPTP treatment using deep anesthesia and their brains were collected for immunohistochemistry or Western-blot analysis.
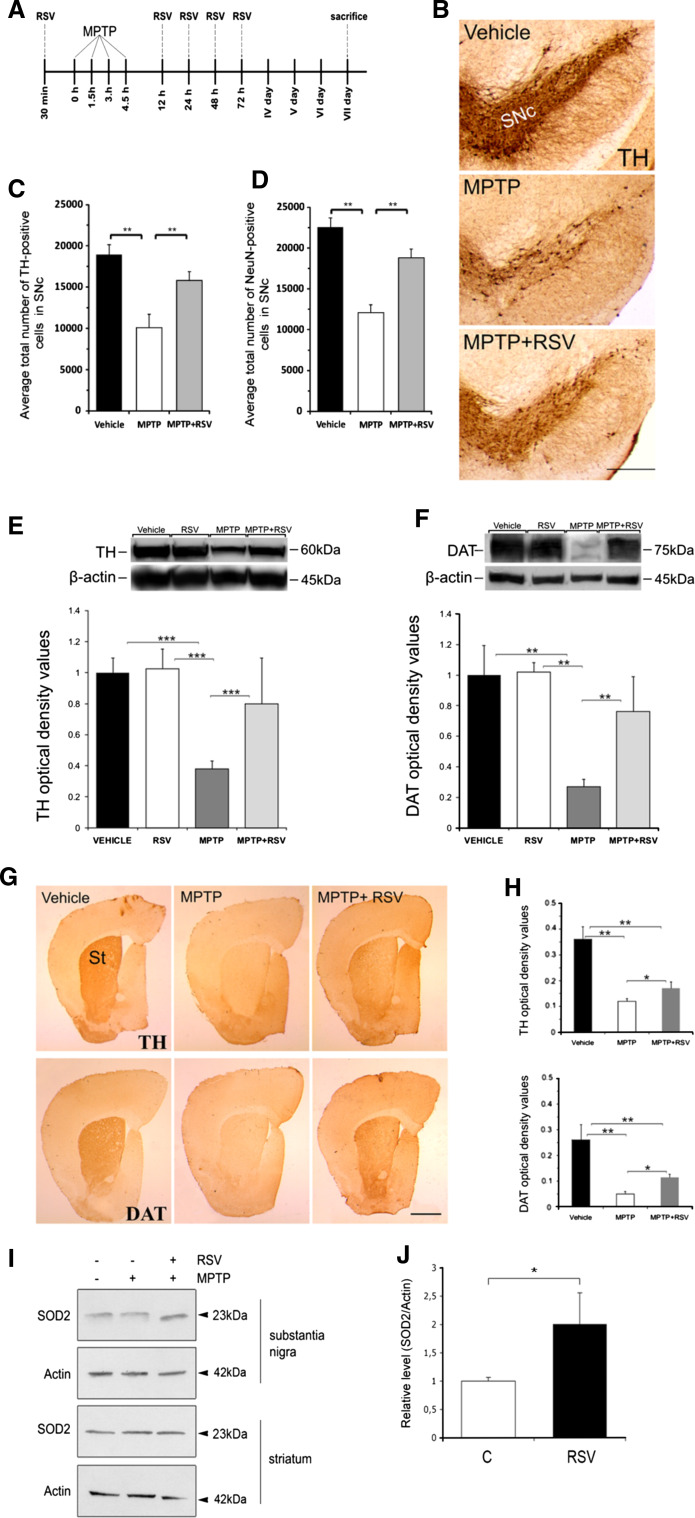
Fig. 5.
RSV protects against MPTP-induced neuronal degeneration in vivo. The neurotoxin MPTP was injected into wild-type mice as described in “Methods”. Control animals received an equal volume of vehicle. Substantia nigra and striatum were prepared and analyzed further as indicated. a Treatment schedule employing MPTP and RSV is shown with times of injections. b Immunostaining of coronal sections of SNc from control mice (vehicle) and after treatment with MPTP or MPTP + RSV. Scale bar 0.5 mm. c Histogram showing the number of TH-positive neurons in the SNc as determined by stereological analysis. Values are means ± SD, n = 5–7 per group. **p < 0.01 MPTP versus vehicle and MPTP + RSV versus MPTP. d Histogram showing the number of NeuN-positive neurons in the SNc as determined by stereological analysis. Values are means ± SD, n = 3–4 per group. **p < 0.01 MPTP versus vehicle and MPTP + RSV versus MPTP. e–f Immunoblots show declines in TH and in DAT levels induced after MPTP and their attenuation by RSV. β-actin was used as control. Values are means ± SD, n = 8–10 per group. ***p < 0.001 MPTP versus vehicle or RSV, and MPTP + RSV versus MPTP. **p < 0.01 MPTP versus vehicle or RSV, and MPTP + RSV versus MPTP. g Immunohistochemistry of TH and DAT positive neuron endings in striatum (St) was done as described in “Methods”. Note a partial preservation of dopamine fibers by RSV. h Densitometric analyses of TH and DAT-positive nerve fibers. Values are means ± SD, n = 4. TH, **p < 0.01 MPTP versus vehicle and p < 0.05 MPTP + RSV versus MPTP. DAT, **p < 0.01 MPTP versus vehicle and p < 0.05 MPTP + RSV versus MPTP. Scale bar 1 mm. i Immunoblot showing increased SOD2 levels in SN but not in striatum by RSV. j Quantification of SN data. Values are means ± SD, n = 3. *p < 0.05 for RSV versus C
Immunohistochemistry
Cell counting and measurements of fiber densities as described below were all carried out in a double-blind manner. To compare experimental groups, the sections from control and treated animals were processed at the same time and under the same conditions. Collected brains were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 3 days, immersed in a 10% sucrose solution for 1 day and in 20% sucrose for a further day and subsequently frozen in cooled isopentane and 20-μm-thick coronal sections were prepared on a cryostat (Micron, HM 500 M, Walldorf, Germany) for striatum and SN as following described. Striatum sections were prepared from A 4.70 mm to A 5.70 mm, according to the mouse atlas. For each striatum were collected 15 sections by systematic sampling every third section. Five control sections were selected to determine optical background of tyrosine hydroxylase (TH) and dopamine transporter (DAT) immunostaining for fiber density analysis. We analyzed the entire SN from bregma level A2.54 to A4. Coronal sections were made and examined for TH-positive cell count were sampled every two sections. In the SN pars compacta (SNc), cell number was determined by using TH and neuron-specific DNA-binding protein (NeuN) immunostaining. These two methods were used to explore whether the loss of cells after MPTP treatment was due to loss of antigen expression (TH) or to a loss of the cells themselves (NeuN). For TH, DAT and NeuN immunostaining floating brain sections were used. Following a washing for 5 min in PBS, the sections were preincubated for 30 min in blocking buffer consisting of 2.5% normal goat serum and 0.3% Triton X-100 and 0.3% hydrogen peroxidase, then washed two times. Sections were subsequently incubated overnight at 4°C in PBS/0.3% Triton-X-100 in the presence of the primary antibody and 1.5% blocking serum. The following antibodies and dilutions were used: mouse polyclonal anti-TH and monoclonal anti-NeuN antibodies diluted 1:1,000 and 1:300, respectively (AB152 and MAB377 Chemicon, Temecula, CA, USA); rat monoclonal anti-DAT antibodies diluted 1:200 (SC32258 Santa Cruz Biotech, CA, USA). Control sections were processed without primary antibodies in order to determine optical background of TH and DAT immunostaining.
After three 5-min washings with PBS, the sections were incubated for 1 h with a horseradish peroxidase–streptavidin complex (Vector, Burlingame, CA), diluted 1:100 in PBS. After one washing in PBS and one in Tris–HCl buffer (0.1 M pH 7.4), the peroxidase reaction was developed in the same buffer containing 0.05% 3,3-diaminobenzidine-4 HCl and 0.003% hydrogen peroxide. The sections were dehydrated and covered with Entellan.
The quantification of fibers densities in striatum was performed using ImageJ software (http://rsb.info.nih.gov/ij/). The number of TH-positive DA neurons of SNc was counted using stereology procedures as described [19]. Every mounted section was numbered following the rostrocaudal level corresponding to the mouse brain atlas. The total number of cells in different groups was estimated by means of the optical fractionator, which combines the optical dissector with the fractionator-sampling scheme. Volume fraction estimations for TH-positive neurons were computed by applying the Cavalieri method based on point counts obtained during the application of the optical fractionator. An Olympus BH2 microscope (Olympus, Denmark) was interfaced with a computer (DGC systems, Stockholm, Sweden) and a color video camera (CCD-iris, Sony, Japan). The CAST-Grid software package (Olympus, Glostrup, Denmark) generated sampling frames with a known area and directed the motorized X–Y stage (Lang, Huttenberg, Germany), and a microcator (MT12, Heidenheim, Germany), which monitored the movements in the Z-axis with a resolution of 0.5 μm. Both sides of the SNc were defined using a 4× objective. After having counted the objects (∑Q−), the total number of cells in the nigral region was then estimated as: n = ∑-. fs. fa. fh, where fs is the numerical fraction of the section used and considered in the present analysis as fs = 2 since the SN sections examined were sampled every two sections, fa is the areal fraction and fh is the linear fraction of section thickness. The coefficient of error (CE) for each estimation and animal ranged from 0.03 to 0.09. The total CE of each group ranged from 0.02 to 0.06.
Preparation and immunoblotting of different brain regions
SN and striatum from brains of different experimental groups were dissected under stereomicroscopy and frozen in cooled isopentane. The SN was dissected as follows: a thick brain coronal section at SN level (A 0,500; A 1,700), according to mouse atlas was made using a brain slicer and on this section SN corresponding area, including SNc and SNr, was dissected under stereomicroscopy and used for WB analysis by pooling left and right SN. Striatum and SN tissue pieces were homogenized in cold buffer containing 50 mM Tris HCl pH 7.5, 100 mM NaCl, 1 mM DTT, 5% NP-40, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 0.1 mM phenylmethylsulfonyl fluoride, 100 μM sodium orthovanadate (Na3VO4) and 1 mM EDTA (Sigma-Aldrich, St. Louis, MO, USA). The homogenate was left on ice for 30 min and centrifuged at 10,000 × g for 15 min at 4°C to yield supernatant fractions that were stored at −80°C until use. To obtain enough material, the two SN were pooled from one animals and run together whereas individual striatum was analyzed separately. Using SDS-PAGE, 30–40 μg of protein was separated, transferred to nitrocellulose membranes (Hybond-C Extra, Amersham), blocked for 1 h in TBST and 5% skim milk, and incubated overnight at 4°C with primary antibodies, including anti-DAT, anti-TH (1:2,000), anti-SOD2 (1:5,000; Abfrontier, Seoul, Korea) and anti-COX IV (diluted 1:5,000, Abcam) antibodies. After washing, the membrane was incubated for 1 h at room temperature with peroxidase-conjugated secondary antibodies (diluted 1:5,000) and bands were visualized by enhanced chemiluminescence (Amersham ECL). β-actin (SC47778 Santa Cruz Biotech, CA, USA 1:6,000) was used as loading control. Quantification was performed using ImageJ software (http://rsb.info.nih.gov/ij/).
Quantitative RT-PCR
Total RNA was extracted from SN of wild-type and PGC1-tg mice using RNeasy tissue kit (QIAGEN) followed by cDNA synthesis. Sybergreen (Applied Biosystems) real-time quantitative PCR assays were performed on an ABI Prism 7000 Sequence Detector essentially as described [20]. Results show averages of triplicate experiments normalized to GAPDH. Primer sequences were: SOD2, forward (Fw), 5′-GACCCATTGCAAGGAACAA-3′ and reverse (Rev), 5′-GTAGTAAGCGTGCTCCCACAC-3′; COX IV, Fw, 5′-TCACTGCGCTCGTTCTGAT -3′ and Rev, 5′-CGATCGAAAGTATGAGGGATG-3′.
High-pressure liquid chromatography (HPLC)
Dopamine (DA) and 3,4-dihydroxyphenylacetic acid (DOPAC) concentrations in mouse striatal tissue were determined essentially as described before [21]. In brief, the frozen samples were homogenized in 0.5 ml of homogenization solution consisting of six parts of 0.2 M HCLO4 and one part of antioxidant solution containing oxalic acid in combination with acetic acid and l-cysteine. The homogenates were centrifuged at 20,800 × g for 35 min at 4°C. The supernatant was removed to 0.5 ml Vivaspin filter concentrators (10,000 MWCO PES; Vivascience AG, Hannover, Germany) and centrifuged at 8,600 × g at 4°C for 35 min. Filtrates containing monoamines were analyzed using high-pressure liquid chromatography with electrochemical detection.
Isolation of mitochondria and measurement of respiratory control
Mitochondria from wild-type and PGC1-tg mice were prepared essentially as described previously for rat liver mitochondria [22]. In brief, brain tissue was excised and minced into pieces in isolation medium containing 10 mM Hepes-K pH 7.4, 1 mM EGTA, and sufficient sucrose to obtain an osmolarity of 300 mOsm. The brain pieces were homogenized and centrifugated at 800 × g for 8 min, and the resulting supernatant centrifugated twice at 10,000 × g for 10 min to obtain a mitochondrial pellet that was resuspended in 300 μl of isolation medium. All steps were carried out at +4°C and mitochondria were used for measurements of membrane potential and respiration within 4 h.
Mitochondrial respiration was measured using a Clark-type electrode (Yellow Springs Instruments, USA). Mitochondria were suspended in medium containing 125 mM KCl, 10 mM Hepes-K pH 7.4, 2 mM MgCl, 1 mM Pi, 100 μM EGTA. Respiration was started by the addition of 10 mM malate plus 10 mM glutamate or 10 mM succinate. The respiratory control ratio was determined by the addition of aliquots of ADP.
Mitochondrial membrane potential was measured with the fluorescent dye tetramethylrhodamine (TMRM) using a Cary Eclipse Fluorescence Spectrophotometer (Varian, USA) set to operate with excitation at 550 nm and emission at 575 nm. The measurement medium was supplemented with 0.5 μM TMRM.
Cell cultures and immunoblotting
The SN4741 dopaminergic cell line derived from mouse SN [23] was cultured in Dulbecco’s modified Eagle’s medium/2% fetal calf serum to about 80% confluence. Cells were incubated in 96-well Costar plates and treated with different concentrations of 1-methyl-4-phenyl pyridinium (MPP+, Sigma) in the absence or presence of 5–10 μM RSV. Cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (Sigma) assay as described [24, 25]. Immunoblots were done as described [26, 27] using the following primary antibodies: SIRT1 (1:2000; Abcam, Cambridge, UK), PGC-1α (1:1000; Cell Signaling, Danvers, MA, USA), SOD1 (1:5,000; Santa Cruz Biotech), SOD2 (1:30,000; Abfrontier), Trx2 (1:1,000; Abfrontier), Catalase (1:500; Abcam), HO-1 (1:500; Stressgen, Ann Arbor, MI, USA), NRF1 (1:1,000; Molecular Probes, Invitrogen, Carlsbad, CA, USA), and XIAP (1:5,000; BD Biosciences, Franklin Lakes, NJ, USA). Appropriate peroxidase-conjugated antibodies (1:2,500, Jackson ImmunoResearch, Biofellows, Helsinki, Finland) were added for 1 h and detection was performed using SuperSignal West Pico Substrate (Pierce). Quantification was performed using ImageJ software.
Immunoprecipitation of PGC-1α
Lysates from control and RSV-treated SN4741 cells were incubated overnight at 4°C on a rotary shaker using 10 μg/ml anti-PGC-1α antibodies (Calbiochem, San Diego, CA, USA). Immune complexes were bound to Sepharose-A for 2 h at 4°C and recovered by centrifugation. Beads were washed three times and the samples resuspended in SDS-PAGE buffer and subjected to immunoblotting using either anti-PGC-1α antibodies (1:1,000; Cell Signaling) or anti-acetylated-lysine antibodies (1:1,000; Cell Signaling). The intensity of the bands reveals the degree of acetylation of PGC-1α under different conditions.
Transfection and promotor assays
SN4741 cells in six-well plates were transfected for 24 h with 0.5 μg of PGC-1α promoter constructs linked to luciferase reporter [7] using the Transfectin reagent followed by treatment with 5–10 μM RSV for 24 h. To control for transfection efficiency, 0.02 μg Renilla luciferase pRL-TK was used. Cells were harvested after 48 h using Passive Lysis Buffer, and the Renilla and the firefly luciferase activities were measured using a luminometer (Promega, Biofellows, Helsinki, Finland) [26]. Results are shown as fold increase in luciferase normalized to Renilla activity.
ROS measurements
Cells were treated for 24 h with MPTP or RSV. The levels of reactive oxidative species (ROS) were measured by loading cells for 15 min with 10 μM dihydroethidium (Molecular Probes) followed by examination using fluorescence-activated cell sorter Aria (FACS; BD Biosciences) as described earlier [27, 28].
Quantitative evaluations and statistics
Quantification of fiber densities was performed using ImageJ software. Immunoblots were evaluated by one-way ANOVA with intergroup differences analyzed by Fisher’s protected least significant difference (PLSD) test, corrected by Bonferroni’s procedure for dependent samples. Statistics of cell viability were performed using Student’s t test comparing two groups, and one-way ANOVA followed by Bonferroni’s post hoc test when comparing three or more groups.
Results
PGC-1α transgenic mice are protected against MPTP-induced cell degeneration
PGC-1α is a master regulator of oxidative stress and mitochondrial metabolism. As shown in gene-deleted mice decreases in the levels of PGC-1α influences neuronal viability and responses to injury in particular parts of the brain [7]. To study whether an overexpression of PGC-1α may be neuroprotective in vivo, we generated transgenic mice expressing PGC-1α in neurons under the control of the Thy-1 promoter (Fig. 1a). Immunoblots showed that the levels of PGC-1α were increased in the nigrostriatal system in the transgenic compared with controls (Fig. 1b). Immunostaining using anti-Flag antibodies revealed that the TH-positive dopaminergic neurons expressed Flag-PGC-1α that was not observed in controls (Fig. 1c). As PGC-1α is known to influence different transcriptional programs, we studied the expression of some antioxidants and mitochondrial proteins involved in cell stress. Immunoblots of substantia nigra showed that the levels of the mitochondrial antioxidants, SOD2 and Trx2 are increased in the SN of PGC-1α transgenic mice compared with wild-type animals (Fig. 1e). Likewise, the mitochondrial enzyme COX IV was also elevated in the transgenic mice compared to controls (Fig. 1e). The increase in protein levels in the PGC-1α transgenic mice was accompanied by an enhanced gene expression, as shown here for SOD2 and COX IV using RT-PCR (Fig. 1d). This shows that overexpression of PGC-1α leads to changes in gene expression for a particular set of proteins in the SN with a potentially protective function in cell stress.
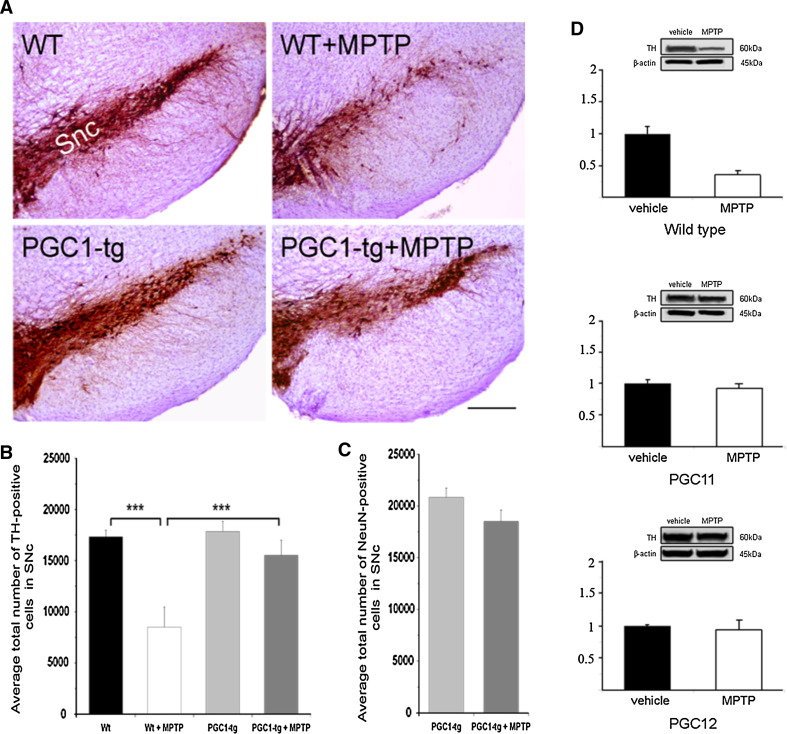
To study neuroprotection in the context of dopaminergic neurons, we treated the mice with the neurotoxin MPTP. Treatment of wild-type mice with MPTP is known to decrease the number of TH-positive neurons in the SNc [29], and this was also evident here as shown in Fig. 2a–b. However, in the PGC-1α transgenic mice, the same treatment did not significantly reduce the number of TH-positive cells, indicating a robust neuroprotection (Fig. 2a–b). Figure 2c shows that the number of NeuN-stained cells in the SNc in the PGC-1α transgenic mice did not change after MPTP. In controls, the number of NeuN-positive neurons decreased in SNc as shown in Fig 5d, and similar results using MPTP have been observed in several studies before. The preservation of NeuN-positive cells together with TH-positive neurons supports the view that PGC-1α counteracts cell degeneration in SNc after MPTP treatment. Immunoblots of TH levels in the striatum showed a decrease in wild-type but not in PGC-1α transgenic mice (Fig. 2d). In these experiments, we analyzed separately two transgenic mouse lines, PGC11 and PGC12 with similar expression of PGC-1α in the brain, and obtained the same results (Fig. 2d). This shows that the transgenic expression of PGC-1α can protect dopaminergic neurons against cell degeneration induced by MPTP.
Fig. 2.
PGC-1α protects against MPTP-induced neuronal degeneration in vivo. The neurotoxin MPTP was injected into wild-type (wt) and PGC-1α transgenic (PGC1-tg) mice as described in “Methods”. Control animals received an equal volume of vehicle. The number of TH-positive dopaminergic neurons and NeuN-positive cells in the substantia nigra pars compacta (SNc) was analyzed by using stereology as described in “Methods”. a Immunostaining of sections from SNc in vehicle and MPTP-treated wild-type and PGC1-tg mice. Scale bar 0.5 mm. b The number of TH-positive neurons in the SNc. Values are means ± SD, n = 6–12 per group. ***p < 0.001 for MPTP versus vehicle in wt animals, and for PGC1-tg + MPTP versus WT + MPTP. c The number of NeuN-positive neurons in the SNc. Values are means ± SD, n = 3–4 per group. ***p < 0.001 MPTP versus vehicle in wt animals and for PGC1-tg + MPTP versus WT + MPTP. d Immunoblots for TH. β-actin was used as control. Upper panel wild-type mice; middle and lower panels two different PGC-1α transgenic mouse lines, PGC11 and PGC12 were studied. Values are means ± SD, n = 3–6 per group. ***p < 0.001 MPTP versus vehicle in wild-type mice. There was no significant decrease in TH-positive cells in the two transgenic mouse lines
PGC-1α counteracts the decrease in striatal DA and DOPAC levels induced by MPTP
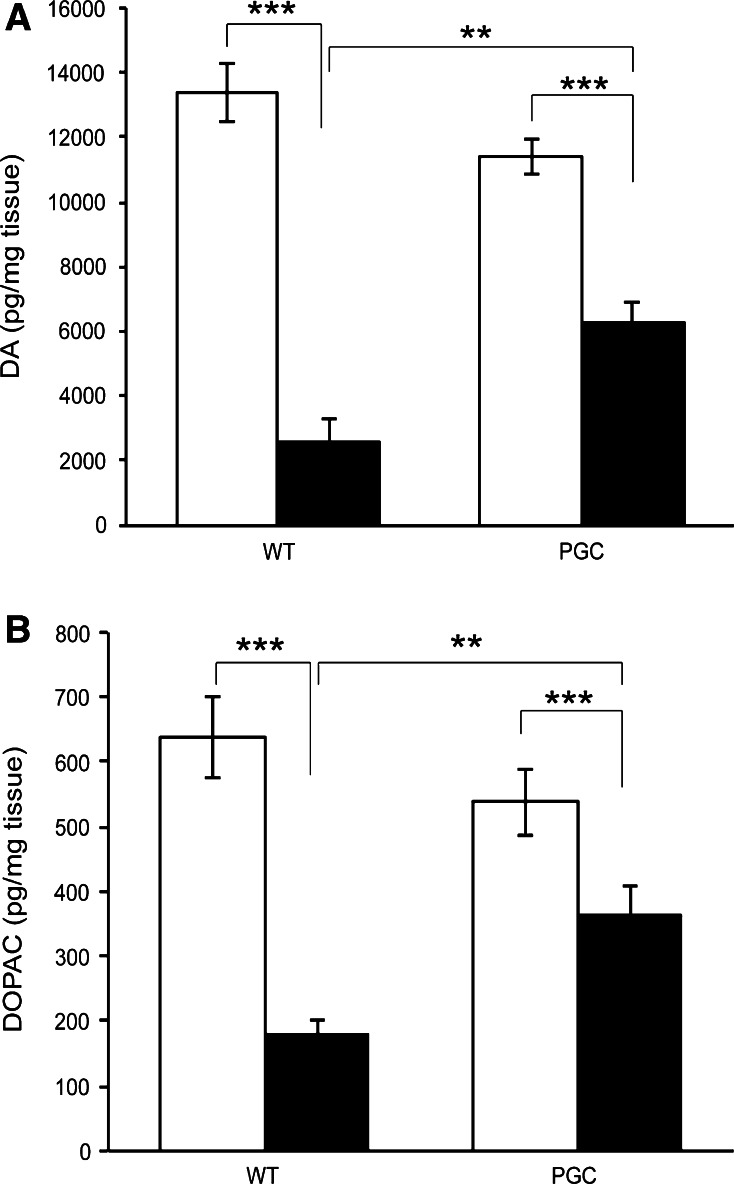
We measured the DA and DOPAC levels in wild-type and PGC-1α transgenic mice after MPTP treatment. As expected [30], MPTP decreased the striatal concentration of dopamine and DOPAC and increased the DOPAC/dopamine ratio (Fig. 3a–b). The PGC-1α transgenic mice were significantly more resistant to MPTP-induced neurotoxicity than the wild-type mice as reflected by smaller reduction of dopamine and DOPAC (Fig. 3a–b). In addition, the increase of dopamine/DOPAC ratio was lower in the PGC-1α transgenic mice as compared to controls. Taken together, this data shows that the overexpression of PGC-1α prevents changes in DA and DOPAC striatum induced by MPTP treatment showing a beneficial effect on the functional state of the nigrostriatal system in these mice.
Fig. 3.
PGC-1α overexpression counteracts the MPTP-induced loss of striatal dopamine and dihydroxyphenylacetic acid levels. Dopamine (DA) and dihydroxyphenylacetic acid DOPAC levels were determined by high-performance liquid chromatography as described in “Methods”. The PGC-1α transgenic mice (PGC) were significantly more resistant to MPTP-induced neurotoxicity than the wild-type mice as reflected by smaller reduction of dopamine. Values are means ± SE, n = 7–11 per group. ***p < 0.001 for MPTP versus vehicle in wild-type animals and PGC mice. **p < 0.01 for MPTP-wt animal versus MPTP-PGC1
PGC-1α affects the respiration of isolated brain mitochondria
To explore the mechanisms for neuroprotection, we isolated mitochondria from the brains of PGC-1α transgenic and wild-type mice. Measurements of mitochondrial oxygen consumption in vitro [22] showed an increased rate of respiratory control rate in mitochondria from PGC-1α transgenic mice as compared to organelles from wild-type mice (Fig. 4). The relative small difference observed (Fig. 4) may be due to the fact that mitochondria were isolated from whole brain, thus including also glial cells, which have no expression of exogenous PGC-1α driven by the Thy1 construct, being a neuron-specific promoter [17, 18]. The higher respiratory control ratio observed in brain mitochondria from PGC-1α transgenic mice indicates that the neuronal capacity for ATP production is higher in these mice compared to wild-type animals.
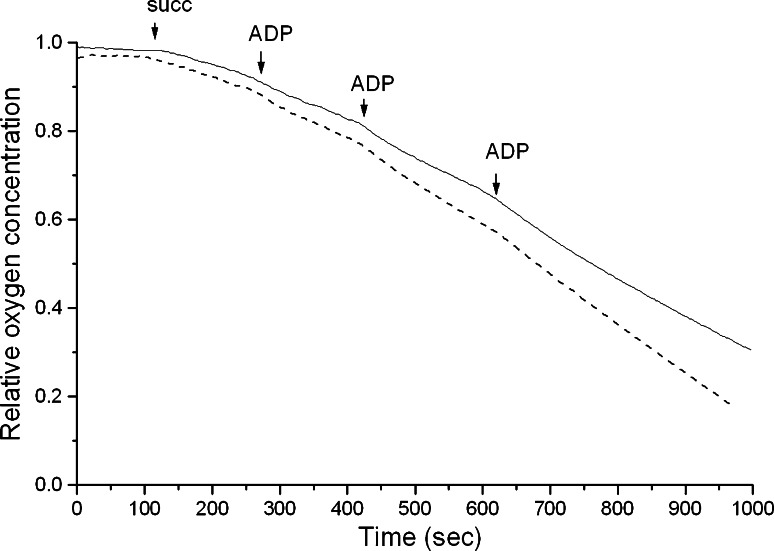
Fig. 4.
Studies of mitochondria from wild-type and PGC-1α transgenic mice. Brain mitochondria were prepared as described in “Methods”. Respiration was initiated by the addition of 10 mM succinate (succ) followed by aliquots of ADP as indicated. Whole trace, mitochondria from PGC-1α transgenic mice; broken trace, mitochondria from wild-type mice. The respiratory control ratio was 1.48 in transgenic and 1.31 in wild-type mice. One typical experiment out of three mitochondrial preparations is shown
Neuroprotective effects of RSV in dopaminergic neurons in vivo
Apart from gene expression, PGC-1α is regulated by post-transcriptional modifications such as protein deacetylation by the mammalian SIRT1 protein, which itself can be activated by RSV that is a natural product found in red grapes and wine [8, 10–13]. To study this in dopaminergic neurons, we analyzed the effects of RSV in mice treated with the neurotoxin MPTP using the treatment schedule shown in Fig. 5a. Treatment with MPTP induced a 44% reduction in the number of dopaminergic neurons in the SNc compared to vehicle-treated control mice (Fig. 5b, c). However, co-treatment with 20 mg/kg RSV restored the number of TH-positive cells to 83% of control (Fig. 5b, c). The number of NeuN-stained cells in the SNc in the three experimental groups corresponded to the data obtained with TH immunostaining showing that RSV was largely neuroprotective after the MPTP insult (Fig. 5d). There was a 63% decrease in TH protein levels in the striatum after the MPTP treatment (Fig 5e), but the administration of RSV was able to restore the TH levels to 78% of the values observed in vehicle-treated control (Fig. 5e). The levels of DAT were also reduced by MPTP treatment to about 25% of controls, and RSV also increased this value to about 75% of controls (Fig 5f). In control mice, the administration of 20 mg/kg RSV alone changed neither the TH nor the DAT protein levels (Fig. 5e, f). Immunohistochemistry combined with densitometry revealed a significant preservation of TH- and DAT-positive fibers in the striatum of MPTP-treated mice after RSV administration (Fig. 5g, h). Interestingly, the protection afforded by RSV on fiber density in striatum was less than that observed in SN on TH and DAT levels. We observed also that RSV also increased the levels of SOD in the SN (Fig. 5i, j), which is in line with the data obtained in the PGC-1α transgenic mice. Taken together, these results show that RSV has a robust neuroprotective effect in dopaminergic neurons in the SN and partially protects against dopaminergic fiber degeneration in the striatum that was induced by MPTP.
Mechanisms of RSV-mediated cell protection as studied in dopaminergic cells
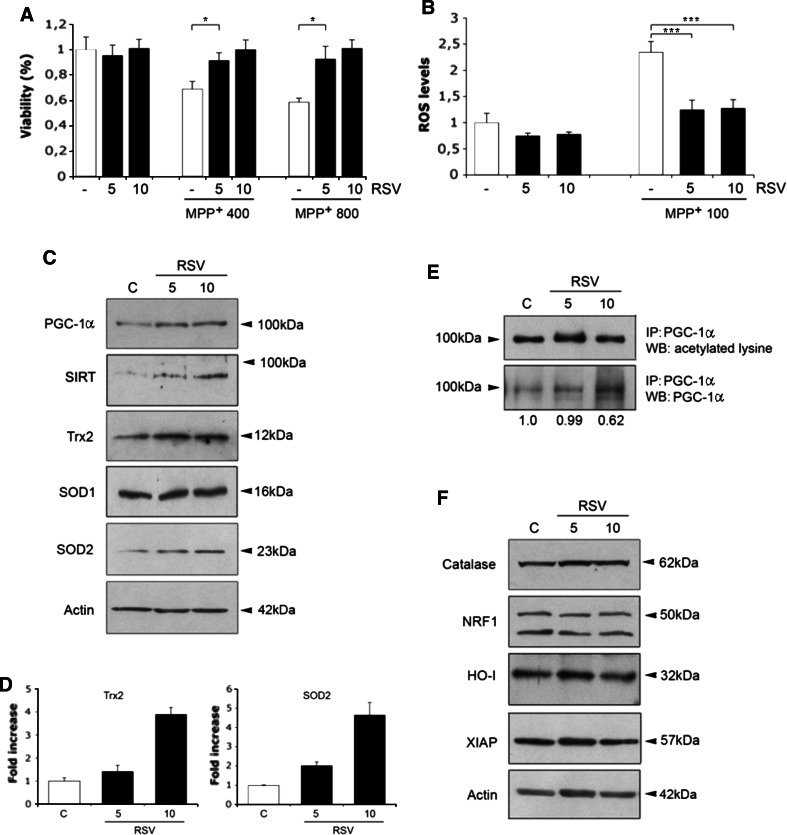
To study the mechanisms of RSV action in dopaminergic cells in more detail, we studied cultured dopaminergic SN4741 cells that were originally established from mouse SN [23]. Treatment of these cells with MPP+ produced a significant loss in cell viability that was counteracted by the addition of 5–10 μM RSV (Fig. 6a). MPP+ is known to produce ROS reactive oxidative species and oxidative stress in dopaminergic neurons. We observed that MPP+ produced an increase in ROS in the SN4741 cells, and this was counteracted by the addition of RSV (Fig. 6b). The decrease in oxidative stress by RSV may be due to increased levels of antioxidants, and we analyzed the levels of SOD2 and Trx2 using immunoblotting. Data showed that RSV elevated SOD2 and Trx2 in the SN4741 cells (Fig. 6c, d), concomitantly with increased levels of PGC-1α and SIRT1 (Fig. 6c). The increase in SOD2 by RSV was associated with an enhanced expression of SOD2-mRNA, as evaluated by RT-PCR (data not shown). In addition, the degree of acetylation of PGC-1α was reduced by about 40% (see values under Fig. 6e) in RSV-treated SN4741 cells, as shown after immunoprecipitation of the protein (Fig. 6e). Thus, RSV has a dual effect on PGC-1α in the dopaminergic cells, increasing both the activity and the levels of the protein. In contrast, SOD1, catalase, heme oxygenase, and X-chromosome linked inhibitory apoptosis protein (XIAP) were not significantly altered by RSV in the dopaminergic cells (Fig. 6c, f).
Fig. 6.
Mechanisms of RSV-mediated neuroprotection. Cell cultures and treatment of mouse SN4741 dopaminergic cells were done as described in “Methods”. a Cells were treated for 48 h with MPP+ alone or in conjunction with RSV as indicated. Cell viability was determined using the MTT assay. RSV increased cell viability comprised by 400–800 μM MPP+. Values are means ± SD, n = 4. *p < 0.05 for MPTP versus C, and for MPTP + RSV versus MPTP for both concentrations. b ROS levels were measured as described in “Methods”. RSV counteracted the increase in ROS in the SN4741 cells induced by MPP+. Values are means ± SD, n = 3. ***p < 0.001 for MPTP versus C, and for MPTP + RSV versus MPTP. c Immunoblots after 24 h treatment with RSV. Note increases in PGC-1α, SIRT1 and in SOD2 and Trx2 by RSV. β-actin was used as control. d Quantifications. SOD2 and Trx2 were increased about fourfold after 10 μM RSV. Values are means ± SD, n = 3. ***p < 0.001 for RSV versus C. Lower panel RT-PCR. SOD2 mRNA levels were increased by 10 μM RSV after 6 h. e Immunoprecipitation of PGC-1α was done as described in “Methods” followed by immunoblotting using anti-acetylated-lysine antibodies. Values below show the relative ratio of acetylated to total PGC-1α. RSV decreased acetylation of PGC-1α. A representative blot is shown and the experiments were repeated three times with similar results. f Immunoblots using specific antibodies revealed no alterations in the indicated proteins in after RSV treatment. β-actin was used as control
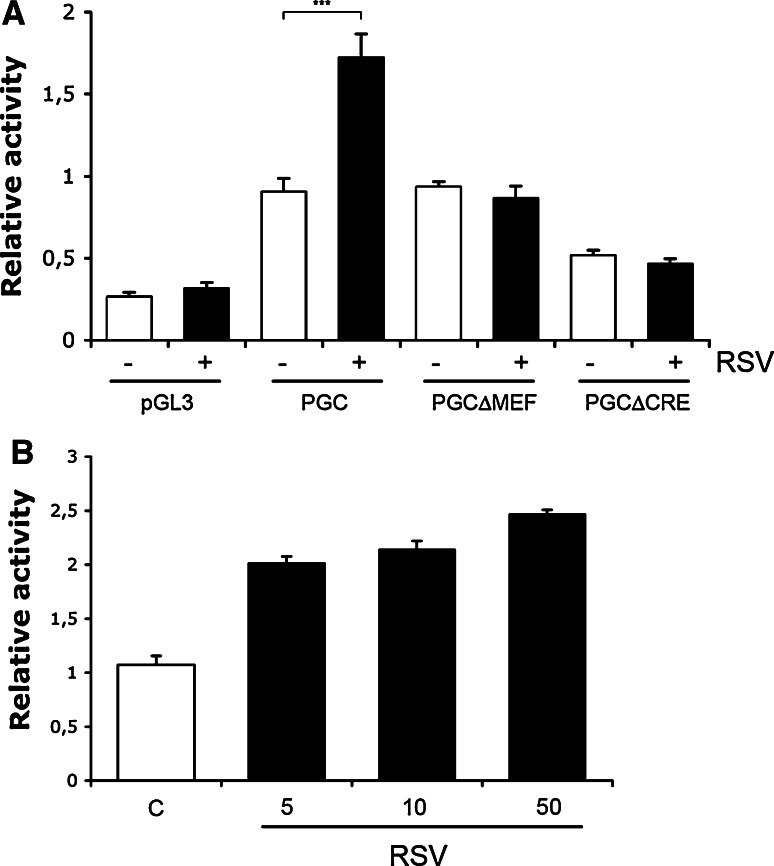
The increase in PGC-1α levels by RSV was unexpected and promoted us to study whether RSV may influence PGC-1α gene expression. To examine this, we employed PGC-1α -promoter constructs linked to a luciferase reporter gene [7]. Data showed that the gene activity of the PGC-1α was enhanced by about 80% in the SN4741 cells cultured in the presence of 10 μM RSV (Fig. 7a). Likewise, in neuronal PC6.3 cells the increase in PGC-1α-promoter activity by 10 μM RSV was about twofold (Fig. 7b). Mutation in the MAD box transcription enhancer factor (MEF) site in the PGC-1α-promoter reduced the induction in promoter activity brought about by RSV (Fig. 7a). Mutation in the cAMP response element (Cre)-binding protein (CREB) site abolished the RSV-mediated increase and reduced the overall PGC-1α-promoter activity (Fig. 7a). This shows that the activity of the PGC-1 gene in neuronal cells is enhanced by RSV and involves the binding of various transcription factors to the promoter.
Fig. 7.
RSV influences gene transcription of PGC-1α. a SN4741 cells were transfected for 24 h with PGC-1α (PGC) promoter constructs linked to a luciferase reporter gene. 10 μM RSV was added for an additional 24 h and luciferase activity was measured relative to that of Renilla as described in “Methods”. There was an about twofold increase in activity by RSV using the intact PGC-1α promoter. No increase was observed using constructs having mutations in the binding sites for MEF and CRE transcription factors. pGL3 is the basic promoter, which showed no change after RSV. Values are means ± SD, n = 3. ***p ≤ 0.001for RSV versus C for the intact promoter. b Neuronal PC6.3 cells were treated with different concentrations of RSV and the activity of the PGC-1α promoter measured as above. Values are means ± SD, n = 3. p < 0.001 for RSV versus C for all concentrations
Discussion
In the present study, we used the well-characterized promoter, Thy1 that targets expression of PGC-1α into brain neurons and allows for the study of neuron-specific changes after brain injuries [18, 31–33]. Using PGC-1α transgenic mice, we were able to demonstrate that the overexpression of PGC-1α in dopaminergic neurons partially counteracted cell degeneration induced by acute MPTP treatments. In PGC-1α transgenic the number of TH-positive neurons in the SNc declining after MPTP was significantly protected and the decreases in DA and its metabolic DOPAC observed after MPTP were significantly less in these mice compared to wild-type ones. Together, this data underscores the importance of PGC-1α signaling in neuroprotection of dopaminergic neurons, and adds to previous studies on the role of PGC-1α in models of neuronal excitotoxicity and cell stress [7, 9].
The beneficial effect observed with the PGC-1α transgenic was related to the overexpression of the PGC-1α in dopaminergic neurons in the SN. However, the detailed mechanism by which PGC-1α is neuroprotective in vivo remains to be studied, including the pattern of genes regulated PGC-1α in neurons. We observed that mitochondria isolated from PGC-1α transgenic mice had an increased respiratory control ratio compared with organelles from wild-type mice, suggesting an increased overall metabolic capacity. Whether this is due to increased turnover or dynamics of mitochondria in the PGC-1α transgenic mice, remains to be studied. Recently, it was shown that overexpression of PGC-1α in cultured neurons increased the mitochondrial capacity and protected cells against mitochondrial loss induced by mutant α-synuclein and huntingtin proteins [34]. Previous studies on PGC-1α expression in rat brain revealed a localization of PGC-1α preferentially in GABAergic neurons with a weak immunostaining of neurons in the midbrain [35]. It is reasonable to assume that the endogenous PGC-1α levels in DA neurons are too low to be protective, but that higher levels of PGC-1α can offer neuroprotection. Preliminary analyses on mice showed that other neurons than dopaminergic ones were also Flag-immunopositive in the SN of PGC-1α transgenic mice (Fig. 1c). It will be important to analyze the nature of these neurons further, and whether they may somehow contribute to the neuroprotection observed here.
Treatment with small molecular compounds appears to be an attractive means to combat nerve cell degeneration accompanying different brain diseases. We show here that treatment of control mice with RSV led to neuroprotection of dopaminergic cells against MPTP, as shown by counting the number of TH-immunopositive and NeuN-positive cells as well as by analyzing levels of TH and DAT. These results are in accordance with previous data on neuroprotection of dopaminergic neurons by RSV in different systems [14–16]. The precise mechanisms and targets for RSV in brain tissue are not fully understood at the moment. We observed for example that the protection afforded by RSV on fiber density in striatum was less than that observed in SN on TH and DAT levels, possibly reflecting a difference in the action of RSV in the cell body and in the nerve terminals of TH-positive neurons that requires further studies. One conclusion that can be drawn here is that the beneficial effect of RSV in neuroprotection was related to an increase in SOD2 in brain in vivo, probably contributing to the decrease in oxidative stress. We recently observed an increase in SOD2 and Trx2 by RSV in neuronal PC6.3 cells concomitant with a decrease in oxidative stress [36]. In line with this, we show here that 5-10 μM RSV elevates SOD2 and Trx2 in cultured dopaminergic cells. SOD2 and Trx2 regulate oxidative stress at the level of the mitochondria and these proteins are regulated in brain neurons partly by NF-κB activation that is induced by various signals including XIAP [28, 36]. In this study, we observed no change in NF-κB signaling by RSV, suggesting another mechanism of action. An obvious candidate for this is SIRT1, which is an NAD+-dependent deacetylase involved in metabolic regulations and adaptations to stress [8–10]. SIRT1 activity is linked to neuroprotection in Alzheimer’s disease [37], and the protein is altered in motor neurons in ALS models [38]. Previous studies have shown that RSV counteracts cell stress and activates PGC-1α as well as AMP kinase in neurons [13, 39]. We observed that RSV increased the levels of SIRT1 in the SN4741 cells and activated PGC-1α by reducing acetylation of the protein. Apart from this, RSV increased the levels of PGC-1α in the dopaminergic cells probably by affecting gene expression as shown using the PGC-1α gene promoter constructs. This data was unexpected, and adds to the known biological effects of RSV with a focus particularly on neuronal cells and cell protection.
Previous studies on RSV indicate that the compound is able to cross the blood–brain barrier [13]. RSV was reported to protect against brain damage induced by glutamate excitotoxicity, and after brain trauma and ischemia [13, 40, 41]. The present results obtained with RSV in dopaminergic cells suggest that RSV and other small molecular compounds acting via the SIRT1/PGC-1α system may be useful agents for neuroprotection in PD. However, the potential use of RSV in different brain disorders will require careful analyzes of the kinetics, biological actions, and pharmacological profiles of the compound in brain tissue.
Apart from RSV, other compounds related to PGC-1α action may also afford neuroprotection in the brain. PGC-1α is a transcriptional coactivator of peroxisome proliferator-activated receptor-γ (PPAR-γ) and it has been shown that the receptor agonists, such as rosiglitazone and pioglitazone, are neuroprotective in different models of PD [42–44]. These compounds activating PPAR-γ are thought to act mainly as anti-inflammatory agents in brain, but the precise mechanisms are not fully understood.
In the present work, we show that PGC-1α transgenic mice overexpressing PGC1 in dopaminergic neurons are protected against acute MPTP-induced cell degeneration in the SN and against dopamine loss in striatum. A recent meta-analysis of PD patients reported decreased levels of PGC-1α and its downstream genes in this disease identifying PGC-1α signaling as a potential target for early intervention in PD [45]. Data from the present study using the PGC-1α transgenic mice transgenic mice lends further support to this conclusion, and shows an important function of PGC-1α in dopaminergic neurons to combat oxidative stress and increase neuronal viability. Future experiments using gene profiling and proteomic analyses of the PGC-1α transgenic mice may reveal additional targets that may contribute to neuroprotection of dopaminergic neurons.
Acknowledgments
We thank M. Forsberg for help with the PGC transgenic mice, B. Spiegelmann for the PGC-1α promoter constructs, J. Son and E. Arenas for SN4741 cells, and E. Lehto for skillful technical assistance. This work was supported by the Academy of Finland, Sigrid Juselius Foundation, Finska Läkaresällskapet, Liv och Hälsa, The Finnish Parkinson Foundation, Magnus Ehrnrooth and Minerva Foundation, and Fondi di Ateneo at University of Palermo, VDL, MO and AB were supported by Ateneo of Palermo.
Footnotes
J. Mäkelä, V. Di Liberto, N. Belluardo, and D. Lindholm contributed equally to this work.
References
- 1.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 2.Gupta A, Dawson VL, Dawson TM. What causes cell death in Parkinson’s disease? Ann Neurol. 2008;64(Suppl 2):S3–S15. doi: 10.1002/ana.21573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schapira AH, Agid Y, Barone P, Jenner P, Lemke MR, Poewe W, Rascol O, Reichmann H, Tolosa E. Perspectives on recent advances in the understanding and treatment of Parkinson’s disease. Eur J Neurol. 2009;16:1090–1099. doi: 10.1111/j.1468-1331.2009.02793.x. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee R, Starkov AA, Beal MF, Thomas B. Mitochondrial dysfunction in the limelight of Parkinson’s disease pathogenesis. Biochim Biophys Acta. 2009;1792:651–663. doi: 10.1016/j.bbadis.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou C, Huang Y, Przedborski S. Oxidative stress in Parkinson’s disease: a mechanism of pathogenic and therapeutic significance. Ann N Y Acad Sci. 2008;1147:93–104. doi: 10.1196/annals.1427.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Feige JN, Auwerx J. Transcriptional targets of sirtuins in the coordination of mammalian physiology. Curr Opin Cell Biol. 2008;20:303–309. doi: 10.1016/j.ceb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Z, Xu X, Hu X, Fassett J, Zhu G, Tao Y, Li J, Huang Y, Zhang P, Zhao B, Chen Y. PGC-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid Redox Signal. 2010;13:1011–1022. doi: 10.1089/ars.2009.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pirola L, Fröjdö S. Resveratrol: one molecule, many targets. IUBMB Life. 2008;60:323–332. doi: 10.1002/iub.47. [DOI] [PubMed] [Google Scholar]
- 13.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 14.Okawara M, Katsuki H, Kurimoto E, Shibata H, Kume T, Akaike A. Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem Pharmacol. 2007;73:550–560. doi: 10.1016/j.bcp.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Chao J, Yu MS, Ho YS, Wang M, Chang RC. Dietary oxyresveratrol prevents parkinsonian mimetic 6-hydroxydopamine neurotoxicity. Free Radic Biol Med. 2008;45:1019–1026. doi: 10.1016/j.freeradbiomed.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Blanchet J, Longpré F, Bureau G, Morissette M, DiPaolo T, Bronchti G, Martinoli MG. Resveratrol, a red wine polyphenol, protects dopaminergic neurons in MPTP-treated mice. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1243–1250. doi: 10.1016/j.pnpbp.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Caroni P. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J Neurosci Methods. 1997;71:3–9. doi: 10.1016/S0165-0270(96)00121-5. [DOI] [PubMed] [Google Scholar]
- 18.Trapp T, Korhonen L, Besselmann M, Martinez R, Mercer EA, Lindholm D. Transgenic mice overexpressing XIAP in neurons show better outcome after transient cerebral ischemia. Mol Cell Neurosci. 2003;23:302–313. doi: 10.1016/S1044-7431(03)00013-7. [DOI] [PubMed] [Google Scholar]
- 19.Aguirre JA, Leo G, Cueto R, Andbjer B, Naylor A, Medhurst AD, Agnati LF, Fuxe K. The novel cyclooxygenase-2 inhibitor GW637185X protects against l-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine toxicity. Neuroreport. 2008;19:657–660. doi: 10.1097/WNR.0b013e3282fb7898. [DOI] [PubMed] [Google Scholar]
- 20.Hong C, Duit S, Jalonen P, Out R, Scheer L, Sorrentino V, Boyadjian R, Rodenburg KW, Foley E, Korhonen L, Lindholm D, Nimpf J, van Berkel TJ, Tontonoz P, Zelcer N. The E3 ubiquitin ligase IDOL induces the degradation of the low density lipoprotein receptor family members VLDLR and ApoER2. J Biol Chem. 2010;285:19720–19726. doi: 10.1074/jbc.M110.123729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Airavaara M, Mijatovic J, Vihavainen T, Piepponen TP, Saarma M, Ahtee L. In heterozygous GDNF knockout mice the response of striatal dopaminergic system to acute morphine is altered. Synapse. 2006;59:321–329. doi: 10.1002/syn.20245. [DOI] [PubMed] [Google Scholar]
- 22.Speer O, Morkunaite-Haimi S, Liobikas J, Franck M, Hensbo L, Linder MD, Kinnunen PKJ, Wallimann T, Eriksson O. Rapid suppression of mitochondrial permeability transition by methylglyoxal. Role of reversible arginine modification. J Biol Chem. 2003;278:34757–34763. doi: 10.1074/jbc.M301990200. [DOI] [PubMed] [Google Scholar]
- 23.Son JH, Chun HS, Joh TH, Cho S, Conti B, Lee JW. Neuroprotection and neuronal differentiation studies using substantia nigra dopaminergic cells derived from transgenic mouse embryos. J Neurosci. 1999;19:10–20. doi: 10.1523/JNEUROSCI.19-01-00010.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korhonen L, Belluardo N, Lindholm D. Regulation of X-chromosome-linked inhibitor of apoptosis protein in kainic acid-induced neuronal death in the rat hippocampus. Mol Cell Neurosci. 2001;17:364–372. doi: 10.1006/mcne.2000.0935. [DOI] [PubMed] [Google Scholar]
- 25.Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, Belluardo N, Lindholm D, Korhonen L. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci. 2007;27:901–908. doi: 10.1523/JNEUROSCI.4289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kairisalo M, Korhonen L, Sepp M, Pruunsild P, Kukkonen JP, Kivinen J, Timmusk T, Blomgren K, Lindholm D. NF-kappaB-dependent regulation of brain-derived neurotrophic factor in hippocampal neurons by X-linked inhibitor of apoptosis protein. Eur J Neurosci. 2009;30:958–966. doi: 10.1111/j.1460-9568.2009.06898.x. [DOI] [PubMed] [Google Scholar]
- 27.Reijonen S, Kukkonen JP, Hyrskyluoto A, Kivinen J, Kairisalo M, Takei N, Lindholm D, Korhonen L. Downregulation of NF-kappaB signaling by mutant huntingtin proteins induces oxidative stress and cell death. Cell Mol Life Sci. 2010;67:1929–1941. doi: 10.1007/s00018-010-0305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kairisalo M, Korhonen L, Blomgren K, Lindholm D. X-linked inhibitor of apoptosis protein increases mitochondrial antioxidants through NF-kappaB activation. Biochem Biophys Res Commun. 2007;364:138–144. doi: 10.1016/j.bbrc.2007.09.115. [DOI] [PubMed] [Google Scholar]
- 29.Thomas B, Beal MF. Parkinson’s disease. Hum Mol Genet. 2007;16(Spec No. 2):R183–R194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- 30.Heikkila RE, Cabbat FS, Manzino L, Duvoisin RC. Effects of 1-methyl-4-phenyl-1, 2, 5, 6-tetrahydropyridine on neostriatal dopamine in mice. Neuropharmacology. 1984;23:711–713. doi: 10.1016/0028-3908(84)90170-9. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Zhu C, Wang X, Hagberg H, Korhonen L, Sandberg M, Lindholm D, Blomgren K. X-linked inhibitor of apoptosis protein (XIAP) protects against caspase activation and tissue loss after neonatal hypoxia-ischemia. Neurobiol Dis. 2004;16:179–189. doi: 10.1016/j.nbd.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Wootz H, Hansson I, Korhonen L, Lindholm D. XIAP decreases caspase-12 cleavage and calpain activity in spinal cord of ALS transgenic mice. Exp Cell Res. 2006;312(10):1890–1898. doi: 10.1016/j.yexcr.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 33.Zhu C, Xu F, Fukuda A, Wang X, Fukuda H, Korhonen L, Hagberg H, Lannering B, Nilsson M, Eriksson PS, Northington FJ, Björk-Eriksson T, Lindholm D, Blomgren K. X-chromosome-linked inhibitor of apoptosis protein reduces oxidative stress after cerebral irradiation or hypoxia-ischemia through up-regulation of mitochondrial antioxidants. Eur J Neurosci. 2007;26:3402–3410. doi: 10.1111/j.1460-9568.2007.05948.x. [DOI] [PubMed] [Google Scholar]
- 34.Wareski P, Vaarmann A, Choubey V, Safiulina D, Liiv J, Kuum M, Kaasik A. PGC-1α and PGC-1β regulate mitochondrial density in neurons. J Biol Chem. 2009;284:21379–21385. doi: 10.1074/jbc.M109.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cowell RM, Blake KR, Russell JW. Localization of the transcriptional coactivator PGC-1alpha to GABAergic neurons during maturation of the rat brain. J Comp Neurol. 2007;502:1–18. doi: 10.1002/cne.21211. [DOI] [PubMed] [Google Scholar]
- 36.Kairisalo M, Bonomo A, Hyrskyluoto A, Mudò G, Belluardo N, Korhonen L, Lindholm D. Resveratrol reduces oxidative stress and cell death and increases mitochondrial antioxidants and XIAP in PC6.3-cells. Neurosci Lett. 2011;488:263–266. doi: 10.1016/j.neulet.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 37.Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve AA, Pasinetti GM. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- 38.Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ates O, Cayli S, Altinoz E, Gurses I, Yucel N, Sener M, Kocak A, Yologlu S. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol Cell Biochem. 2007;294:137–144. doi: 10.1007/s11010-006-9253-0. [DOI] [PubMed] [Google Scholar]
- 42.Breidert T, Callebert J, Heneka MT, Landreth G, Launay JM, Hirsch EC. Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson’s disease. J Neurochem. 2002;82:615–624. doi: 10.1046/j.1471-4159.2002.00990.x. [DOI] [PubMed] [Google Scholar]
- 43.Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB. Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J Neurochem. 2004;88:494–501. doi: 10.1046/j.1471-4159.2003.02210.x. [DOI] [PubMed] [Google Scholar]
- 44.Schintu N, Frau L, Ibba M, Caboni P, Garau A, Carboni E, Carta AR. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson’s disease. Eur J Neurosci. 2009;29:954–963. doi: 10.1111/j.1460-9568.2009.06657.x. [DOI] [PubMed] [Google Scholar]
- 45.Zheng B, et al. PGC-1α, a potential therapeutic target for early intervention in Parkinson’s disease. Science Transl Med. 2010;2(52):52ra73. doi: 10.1126/scitranslmed.3001059. [DOI] [PMC free article] [PubMed] [Google Scholar]