Abstract
The human intestinal mucosa is constantly exposed to commensal microbiota. Since the gut microbiota is beneficial to the host, hosts have evolved intestine-specific immune systems to co-exist with the microbiota. On the other hand, the intestinal microbiota actively regulates the host’s immune system, and recent studies have revealed that specific commensal bacterial species induce the accumulation of specific immune cell populations. For instance, segmented filamentous bacteria and Clostridium species belonging to clusters XIVa and IV induce the accumulation of Th17 cells in the small intestine and Foxp3+ regulatory T cells in the large intestine, respectively. The immune cells induced by the gut microbiota likely contribute to intestinal homeostasis and influence systemic immunity in the host.
Keywords: Gut microbiota, Clostiridium, Segmented filamentous bacteria, Regulatory T cells, Th17, Innate lymphoid cells
Introduction
The intestinal mucosa is constantly exposed to the microbiota, which is composed of more than 100 trillion commensal bacteria comprising more than 1,000 species. These bacteria are more than coincidental companions. We provide the microbiota with a secure habitat and a constant source of nutrition. In turn, the gut microbiota helps our digestion and metabolism and protects us from intestinal intrusion by foreign pathogenic microorganisms via competition for space and nutrition. Therefore, the host–microbiota relationship is mutually beneficial. To utilize the benefits, the gut mucosa has evolved a multi-component system to co-exist with the microbiota. Intestinal epithelial cells (IECs), which directly interface with the microbiota, form tight junctions and constitutively produce mucus gels and antimicrobial agents to form a physical barrier [1, 2]. IECs also constitutively produce immune regulatory cytokines. Consequently, IECs have critical roles in sequestering the microbiota in the lumen and in achieving immunological tolerance toward the microbiota. Beneath the epithelium, the intestinal mucosa is equipped with multiple lineages of cells, including innate and adoptive immune cells, that also cooperate to establish a robust symbiotic system with the microbiota [3–6].
An accumulating body of evidence suggests that the commensal microbiota actively regulates the host immune system. Using germ-free (GF) and/or gnotobiotic techniques and metagenomic approaches by massive parallel sequencing, it is becoming clear that the composition of the gut microbiota affects the structure of gut-associated lymphoid tissues (GALTs) and the activation/differentiation status of immune cells and IECs [7, 8]. Furthermore, specific commensal bacterial species induce the accumulation of specific immune cell populations [9–14]. Therefore, the alteration of the bacterial composition may result in a disruption of immune homeostasis between the host and the microbiota.
In this article, we summarize the host immune system by discussing several important cell subsets and then discuss the interactions of the microbiota with the host mucosal immune system and the consequences of these interactions (although many are still unknown). In particular, we focus on how the host immune system is adapted to co-exist with the commensal microbiota and how the commensal microbiota regulates the host immune system. A variety of cell populations and mechanisms appear to be involved in the establishment of these complicated but sophisticated relationships.
Features of the host immune system to allow co-existence with the gut microbiota
Multiple different lineages of cells in the intestines, including IECs and innate and adoptive immune cells, have their own roles to allow co-existence with commensal bacteria while protecting the host from invasion by pathogenic microbes. Recent advances have elucidated the roles of each cell population and, further, have identified several new cell populations.
Intestinal epithelial cells (IECs)
IECs are located at the interface between the host and the commensal microbiota. Accordingly, they have a critical role in sensing physical and molecular stimulation by the microbiota. IECs express multiple pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), nucleotide-binding oligomerization domain-like receptors (NLRs), and C-type lectin receptors, to sense pathogenic bacteria and commensal bacteria, both of which contain microorganism-associated molecular patterns in their structures. TLRs are expressed at low levels in normal IECs, but are upregulated in inflammatory bowel diseases (IBD) patients. Inflammatory cytokines induced by TLRs, such as interferon (IFN)-γ and tumor necrosis factor-α, can act as precipitating factors for IBD by modifying tight junction function in IECs and increasing flux across the leak pathway [15]. Therefore, a dysregulated expression and function of TLRs in IECs may be involved in IBD pathogenesis.
On the other hand, PRR signaling in IECs has been shown to contribute to the enhancement of tight junctions, epithelial cell proliferation, and antimicrobial peptide production, leading to the enhancement and maintenance of the mucosal barrier system [1]. For example, when mice kept in specific pathogen-free (SPF) conditions are treated with broad-spectrum antibiotics, they become highly susceptible to dextran sulfate sodium (DSS)-induced intestinal inflammation [16]. This is, at least in part, due to reduced constitutive TLR signaling in response to the microbiota, as oral administration of lipopolysaccharide (LPS, a TLR4 ligand) or lipoteichoic acid (a TLR2 ligand) restores resistance of antibiotic-treated mice to DSS-associated injury of the colonic epithelium [16]. Consistently, mice lacking either TLR2, TLR4, TLR9, or MyD88 are highly susceptible to DSS colitis [16]. TLR2 activation redistributes the tight junction protein zonula occludens-1 (ZO-1) in IEC cells, resulting in the enhancement of tight junctions [17, 18].
The production of antimicrobial molecules (AMMs), such as α-defensins, β-defensins, cathelicidins, C-type lectins, and lipocalin-2, is one of the pivotal roles of IECs in protecting the host from pathogenic microbiota. The expression of the C-type lectin regenerating islet-derived IIIγ (RegIIIγ) is induced in the mouse small intestine, particularly in Paneth cells, by commensal bacteria, such as Bacteroides thetaiotaomicron, whereas it is downregulated by Bifidobacterium longum [19]. This commensal-mediated induction of RegIIIγ depends on MyD88-dependent TLR activation. Indeed, IEC-specific MyD88-deficient mice have impaired production of RegIIIγ, and the transgenic expression of MyD88 specifically in Paneth cells restores the expression of RegIIIγ [20]. The antibacterial function of RegIIIγ is dependent upon binding bacterial targets through interactions with peptidoglycan, which is expressed on the surface of Gram-positive bacteria but is buried in the periplasmic space of Gram-negative bacteria [21]. Therefore, RegIIIγ has a critical role in the host defense against pathogenic Gram-positive bacteria, including vancomycin-resistant Enterococcus (VRE) [21, 22]. Furthermore, RegΙΙΙγ is critical for generating the ‘microbiota-free zone’, which is filled with mucus gels containing AMMs, including RegIIIγ, that physically separate IECs from the luminal microbiota in small intestine during steady-state conditions [23]. Therefore, TLR and RegΙΙΙγ have critical roles in maintaining intestinal homeostasis by preventing unnecessary direct contact between IECs and commensal bacteria.
It is interesting to note that IECs also produce other families of soluble-type lectins, including galectins. The production of some galectins, such as galectin-9, is up-regulated by TLRs. Galectins bind luminal β-galactoside containing glycans and modulate the mucosal and systemic immune response. Interestingly, dietary supplementation with prebiotic galacto- and fructo-oligosaccharides and Bifidobacterium breve enhances galectin-9 expression by IECs, which associates with the prevention of allergic symptoms [24].
The production of AMMs in IECs is also indirectly regulated by TLRs via cytokines, such as IL-22 [25]. Systemic injection of flagellin stimulates TLR5 on hematopoietic cells, which leads to an increase in the concentration of IL-22 in the intestine. IL-22 then stimulates IECs to produce RegIIIγ and lipocalin-2, resulting in resistance to infection by pathogens such as VRE [25]. IECs have also been shown to produce IL-17C, which is a member of the IL-17 family of cytokines. IL-17C signals through the IL-17RE and IL-17RA complex, which is expressed on IECs, in an autocrine manner, leading to the induction of AMMs, such as RegIIIβ, RegIIIγ, lipocalin-2, S100A8, and S100A9, during Citrobacter rodentium infection and DSS-induced colitis [26, 27]. IL-17C functions in synergy with IL-22 to induce these AMMs [26]. In the DSS-induced colitis model, IL-17C mRNA is induced soon after DSS administration [27], suggesting that IL-17C production is critical for the rapid production of AMMs to cope with a massive bacterial burden.
IECs have another important role as messengers that relay the signal from bacteria to the immune cells that reside underneath the IECs. For instance, IEC-conditioned intestinal dendritic cells (DCs) drive Th2 response in both human and mouse [28], suggesting that a Th2-skewed response might be required for intestinal homeostasis. IECs constitutively express thymic stromal lymphopoietin (TSLP), which prevents DCs from producing IL-12 and differentiating naïve T cells into Th1 cells [28]. Consistently, mice with the IEC-specific ablation of IκB kinase (IKK)-β exhibit decreased expression of TSLP by IECs and consequently increased production of IL-12/IL-23p40 and TNFα by intestinal DCs, which is accompanied by severe colitis characterized by increased numbers of T helper (Th) 1 and Th17 cells [29]. In addition to TSLP, IECs express IL-25 and promote the accumulation of an innate lymphocyte population in the GALT that promotes Th2 cytokine responses [30]. IECs also constitutively produce transforming growth factor-β (TGF-β) and retinoic acid, which were reported to convert human CD103− DCs into CD103+ DCs to induce de novo generation of Treg cells with gut-homing properties [31].
TLR stimulation in IECs contributes to the production of IgA, which is secreted into the lumen to directly neutralize bacteria. IECs express a proliferation-inducing ligand (APRIL) upon stimulation with TLR ligands, such as lipopolysaccharide and flagellin, or with whole bacteria [32]. APRIL is functionally related to CD40L and can promote activation-induced cytidine deaminase (AID) expression in B cells. Thus, APRIL expression by IECs induces IgA class switching in B cells in a CD40L-independent manner. In addition, IECs promote IgA class switching by producing TSLP in response to TLR ligands or whole bacteria. TSLP activates DCs to produce APRIL and IL-10, which together promote switching to IgA [33].
NLR proteins, including NLRP1, NLRP3, NLRC4, and NLRP6, form cytoplasmic multiprotein complexes called inflammasomes. Upon sensing bacterial components, one of the NLRPs forms the inflammasome by associating with apoptosis-associated speck-like protein (ASC) and pro-caspase-1 in cytoplasm. Inflammasome formation triggers the activation of caspase-1, which cleaves pro-IL-1β and pro-IL-18 to generate the effector inflammatory cytokines IL-1β and IL-18. Recently, NLRP3 and NLRP6 in IECs have emerged as critical regulators of intestinal homeostasis. Several groups have shown that NLRP3−/− mice are susceptible to DSS-induced colitis [34–36]. Mice deficient in ASC or caspase-1 are similarly susceptible to experimental colitis [34–37]. NLRP6 is thought to form inflammasomes on the basis of the similarity of its molecular structure to NLRP3, although its ligands have yet to be identified. A recent report showed that deficiency in NLRP6 in mouse IECs results in a colitogenic-skewed commensal microbiota that is characterized by expanded Prevotellaceae and TM7 [38]. Indeed, the abundance of Prevotellaceae and TM7 have been implicated in human IBD [39, 40]. Mice deficient in ASC, caspase-1, or IL-18 also exhibit a significant increase in Prevotellaceae in their intestinal commensal microbiota, indicating the impact of NLRP6 and other inflammasome components on the commensal microbiota. More importantly, wild-type mice co-housed with NLRP6−/− mice acquire susceptibility to DSS-induced colitis characterized by the alteration of the microbiota toward a profile that is similar to the co-housed knockout mice, suggesting that the colitogenic microbiota of the NLRP6−/− mice is horizontally transmissible [38]. NLRP3 or NLRP6 deficiency in IECs lead to reduced IL-18 production by the IECs, and IL-18- or IL-18 receptor-deficient mice are susceptible to DSS-induced colitis [41, 42]. IEC-specific IL-18-deficient mice also display high susceptibility to DSS-induced colitis accompanied by colitogenic-skewed microbiota. However, the commensal microbiota profile in these mice is distinct from that of NLRP6-, ASC- or caspase-1-deficient mice [38], suggesting an additional NLRP6-mediated and IL-18-independent pathway of microflora regulation. Although the link between NLRP6 deficiency in IECs and the shift toward a colitogenic community is currently unknown, the studies above showed that host’s microbiota-sensing NLRPs have a critical role in maintaining the balance of commensal components.
Dendritic cells (DCs)
DCs are specialized antigen-presenting cells that have a critical role in activating T cells by presenting bacterial or food-derived antigens in both steady-state and infectious/inflammatory conditions. Although different groups differently classified intestinal lamina propria DCs according to the use of different surface markers, at least two populations (CD103+ DCs and CX3CR1+CD103− DCs) are consistent among the different classification methods. These two populations are differentiated from different precursors [43, 44], localize differently in the intestinal lamina propria, and have been reported to have different functions [45, 46]. The CX3CR1+CD103− DCs are derived from circulating Ly6Chi monocytes [44], and extend their dendrites to penetrate the IEC layer and to sample antigens from pathogenic and commensal bacteria [45, 47]. The extensions of the CX3CR1+ DCs markedly increase upon infection with pathogens, such as Salmonella, and MyD88-dependent TLR signaling by non-hematopoietic (epithelial) elements has an important role in the DC extension response [48]. Several studies show that intestinal lamina propria CX3CR1+ DCs induce Th17 cells [49, 50]. CD70+CD11c+CX3CR1+ DCs expresses ATP receptors, and can differentiate naïve T cells into Th17 cells in the presence of ATP [50]. On the other hand, intestinal CX3CR1high CD11b+ CD11c+ cells have recently been shown to inhibit CD4+ T cell proliferation in a cell contact-dependent manner and prevent T cell-dependent colitis [51]. Therefore, CX3CR1+ cells include several functionally distinct subsets.
The CD103+ DCs arise from pre-DCs [44], localize in the core of the villi and migrate to the draining mesenteric LNs [46], where they induce the gut-homing receptor CCR9 and α4β7-integrin on the responding T cells [52]. As discussed later, the CD103+ DCs may contribute to the accumulation of Treg cells induced by the gut microbiota in the steady-state colon. Indeed, CD103+ DCs express retinal dehydrogenase to synthesize retinoic acid, which in cooperation with TGF-β induces the development of Treg cells [53, 54]. In addition to CD103+ DCs, mucosal macrophages have been shown to drive the generation of Foxp3+ Treg cells within the lamina propria [55]. On the other hand, the CD103+ DCs from colitic mice exhibit impaired induction of Treg cells and instead favor the induction of IFN-γ-producing CD4+ T cells [56]. Furthermore, a recent study showed that mice with the DC-specific ablation of Notch2 exhibit a loss of CD11b+CD103+ DCs in the intestinal lamina propria and fewer Th17 cells than wild-type mice, indicating that CD103+ DCs are involved in the development of Th17 cells in the intestine [57]. These reports suggest that CD103+ DCs functionally adapt to different environmental conditions.
Interestingly, GF mice exhibit reduced CX3CR1+CD103− DCs in the colon lamina propria compared with SPF mice, although the number of CD103+ DCs remains unchanged [58]. Moreover, the number of extended dendrites on CX3CR1+CD103− DCs is greatly decreased in GF mice or antibiotic-treated mice [58]. Therefore, the presence of the gut microbiota affects the populations and functions of DCs, thereby affecting the adaptive immune system in the intestine.
IgA-producing cells
More than 80 % of the body’s effector B cells are known to reside in the intestinal mucosa. B cells develop in the bone marrow and subsequently migrate to the peripheral lymphoid organs, including GALT such as Payer’s patches (PPs) and isolated lymphoid follicles (ILFs). In these organs, the B cells mature through two processes, somatic hypermutation (SHM) and class switch recombination (CSR). The B cells acquire further diversity, and, subsequently, high-affinity immunoglobulin (Ig) mutants are selected. IgA is the dominant Ig isotype in the intestinal mucosa. Oral administration of cholera toxin induces specific IgA with high affinity; thus, this experimental system has been frequently used to study IgA responses in vivo [59]. The induction of cholera toxin-specific IgA is T cell-dependent (TD) because T cell- or MHC class II-deficient mice have a defect in the specific IgA response [60, 61]. In contrast, mice with a deficiency of T cells or MHC class II and CD40-deficient mice still have relatively abundant intestinal IgA [60], suggesting the existence of T cell-independent (TI) IgA. Upon TLR stimulation, myeloid and epithelial cells produce B cell activating factor (BAFF, also known as BLyS) and/or APRIL, which promote CSR to IgA by binding the receptors, BAFF-R and transmembrane activator, and calcium modulator and cyclophilin ligand interactor (TACI), which are expressed on B cells [32, 33, 62]. Given that IgA production via the TD pathway takes several days following antigen encounter, a faster, TI IgA response may be required to compensate for the temporal gap in the intestinal mucosa, which is constantly exposed to the microbiota. Intestinal IgA-producing plasma cells are 10- to 100-fold lower in GF mice than in SPF mice, indicating that the gut microbiota promotes the generation of IgA-producing cells [7].
IgA is secreted as a dimer or oligomer, and this dimer/oligomer binds the polymeric Ig receptor (pIgR) expressed on the basolateral surface of IECs, resulting in basolateral-to-apical transcytosis of secretory IgA (SIgA) to directly neutralize microbial toxins or pathogens [63]. PIgR-deficient mice, which lack the ability to transport IgA into the intestinal lumen, display low-grade enteropathy [64, 65], implying that IgA contributes to mucosal protection from commensal bacteria. Furthermore, the lack of activation-induced cytidine deaminase (AID), which results in a defect in CSR and thereby a lack of IgA-producing plasma cells in the intestine, leads to the excessive proliferation of anaerobic bacteria in the small intestine, particularly the segmented filamentous bacteria (SFB), accompanied by the hyperplasia of ILFs [66, 67]. The addition of IgA prevents the aberrant SFB expansion and ILF hyperplasia in the small intestine in AID-deficient mice. Importantly, mice expressing AIDG23S, which have normal levels of IgA but have a defect in SHM, have large amounts of intestinal bacteria, particularly anaerobic bacteria, that is accompanied by germinal center B cell hyperplasia in gut lymphoid tissues [68]. Therefore, TD high-affinity IgA production in GALTs is indeed required for gut homeostasis.
IL-17-producing cells, including Th17 cells and innate lymphoid cells
Th17 cells are characterized by the production of IL-17A (also referred to as IL-17), IL-17F, and IL-22. Both the small and large intestines of SPF mice harbor far more Th17 cells under steady-state conditions compared with other lymphoid or non-lymphoid organs [50]. GF mice have markedly fewer Th17 cells than SPF mice, suggesting a specific and important role of Th17 cells in intestinal mucosa immunity in the context of co-existence with commensal bacteria. In addition to Th17 cells, γδ T cells and several different innate lymphoid cell (ILC) populations are producers of high levels of IL-17 in the intestines. These cells share many characteristics with Th17 cells: the cytokines they produce, triggers for activation, and transcription factors required for their development.
Upon encountering antigens, naïve CD4 T cells are activated through the T cell receptor (TCR) and undergo differentiation into different types of effector Th cells in the presence of specific cytokines that are produced by innate cells. Th17 cells are differentiated in the presence of TGF-β and IL-6. TGF-β and IL-6 activate the signal transducer and activator of transcription 3 (STAT3), which induces the retinoic acid receptor-related orphan receptor gamma t (RORγt). RORγt is a master regulator that is essential for Th17 differentiation [69]. Other transcription factors, such as aryl hydrocarbon receptor (Ahr) and basic leucine zipper transcription factor ATF-like (BATF), also contribute to IL-17 differentiation [70, 71]. During differentiation, Th17 cells proliferate in response to IL-21, which is produced by the Th17 cells themselves [72]. IL-21 also induces the IL-23 and IL-1 receptors, which allow Th17 cells to be responsive to IL-23 and IL-1β [73]. Therefore, in the presence of highly proinflammatory cytokines (such as IL-23 and IL-1β), Th17 cells are activated to become fully inflammatory cells.
γδ T cells are constitutively activated and have the potential to produce IL-17 [74]. Mice deficient in fully functional Treg cells develop spontaneous colitis accompanied by an expansion of γδ T cells, including an IL-17-expressing population [75, 76]. The disease is abrogated in γδ T cell-deficient and antibiotic-treated mice, suggesting that the activation of γδ T cells by commensal bacteria is responsible for the colitis [76]. Therefore, Treg cells restrain the excessive activation of γδ T cells so as not to lead to damage of the host tissue. On the other hand, TCRδ−/− mice exhibit more severe colitis induced by DSS or 2,4,6-trinitrobenzene sulfonic acid (TNBS), suggesting that γδ T cells also have an important role in immunoregulation [77, 78]. Therefore, γδ T cells are likely constitutively activated by commensal bacteria to prevent the invasion of intestinal bacteria and to maintain the front-line mucosal barrier system.
Several ILCs have been identified as another important source of IL-17 in the intestines. When an agonistic antibody against CD40 is injected into recombinase-activating gene (RAG)-deficient mice, which lack lymphocytes, including Th17 cells and γδT cells, the mice develop colitis accompanied by a local elevation in IL-23 and IL-17 [79]. IL-23p40 and RAG double-deficient mice do not develop colitis upon the same treatment, suggesting that ILCs other than Th17 cells and γδT cells produce IL-17 upon IL-23 stimulation in the intestine. Following this report, several new IL-17-producing ILC populations have been identified in the lungs, skin, and intestines, i.e., the organs that are continuously exposed to the environment [80]. New populations of IL-17-producing ILCs, including CD4+ lymphoid tissue inducer (LTi) cells and Thy1+SCA1+CD3−CD4−c-KIT− cells, have been characterized. These IL-17-producing ILCs share common characteristics with differentiated Th17 cells. All the IL-17-producing ILCs require RORγt for their differentiation [69, 81–83]. Aryl hydrocarbon receptor is also expressed in γδ T cells, CD4+ LTi cells, and Thy1+SCA1+CD3−CD4−c-KIT− cells [70, 81, 84]. Many IL-17-producing ILCs constitutively express the IL-23 receptor and the IL-1β receptor, both of which allow them to produce IL-17 or IL-22 rapidly in the presence of IL-23 or IL-1β [81, 85, 86].
Thy1+SCA1+CD3−CD4−c-KIT− cells accumulate and express IL-17 in the colon lamina propria in RAG-deficient mice infected with Helicobacter hepaticus, an innate colitis model [83]. The cells markedly expand and express IL-17, IL-22, and IFN-γ during inflammation [83]. As a similar cell population to mouse Thy1+SCA1+CD3−CD4−c-KIT− cells, CD3−CD19−CD14−CD16−CD56−CD127+ cells have been reported to be increased in the inflamed ileum and colon of patients with Crohn’s disease [87]. CD3−NKp46+ cells, which are another RORγt-expressing ILC population, are present in high numbers in the intestine and express IL-22, but not IL-17. Currently, how these newly discovered IL-17- and IL-22-producing ILCs contribute to intestinal inflammation or homeostasis remains largely unknown.
Th17 cells, IL-17-producing ILCs, and their associated factors (such as IL-23, IL-17, IL-17F, IL-22, or RORγt) have been implicated in autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, uveitis, and IBD. Indeed, inflamed colon tissues in IBD patients highly express IL-23 [88], and a genome-wide study has revealed that IL23R gene is a susceptibility gene for IBD [89]. However, many studies have also shown that IL-17-producing cells have a critical role in protection rather than inflammation in the context of infection [90]. Furthermore, the transfer of IL-17A-deficient CD45RBhi CD4+ T cells has been shown to elicit a more aggressive colitis in lymphopenic mice than the transfer of wild-type cells [91], suggesting that IL-17 mediates a protective effect in intestinal inflammation. Although IL-17 has been the most intensively studied of the Th17 cytokines, the functions of other cytokines, including IL-17F and IL-22, are becoming apparent. IL-22 receptor expression is exclusively restricted to non-hematopoietic cells, particularly IECs, such that IL-22 promotes the host defense and tissue protection activities of IECs, such as the secretion of AMMs. IL-22 has protective roles in T cell transfer-induced colitis and DSS-induced colitis; transfer of IL-22-deficient naïve T cells induces more severe colitis than wild-type T cell transfer, and IL-22-deficient mice are more susceptible to DSS-induced colitis than wild-type mice [92]. During infection with the enteric pathogen C. rodentium, IL-22 protects the host by promoting the production of RegIIIβ and RegIIIγ by IECs [93]. IL-23p40-deficient mice are severely impaired in IL-22 production, leading to high susceptibility to C. rodentium infection [93]. These findings suggest that IL-22 has a more protective than pro-inflammatory role in the intestinal mucosa. In contrast, IL-17F-deficient mice exhibit milder DSS-induced colitis than wild-type mice, whereas IL-17-deficient mice have more severe colitis than wild-type mice [94], suggesting that IL-17F and IL-17 are pro-inflammatory and anti-inflammatory, respectively, during DSS-induced colitis. The individual cytokines associated with Th17 cells and IL-17-producing ILCs are probably produced at different levels and with different kinetics, which are dependent on the conditions of the mucosa, i.e., whether it is in a steady, inflammatory or bacteria-intruded state.
Regulatory T (Treg) cells
Multiple regulatory lymphoid and myeloid cell subsets are present in the intestinal mucosa. Among them, Foxp3+ Treg cells are essential for maintaining peripheral tolerance and for preventing autoimmune and inflammatory diseases. Foxp3 is a key transcription factor that is required for the development, maintenance, and function of Treg cells [95, 96]. Human IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) patients and scurfy mice, which harbor mutations in the Foxp3 gene on the X-chromosome, exhibit multi-organ inflammation via massive infiltration of immune cells [97–99]. The two subsets of Foxp3+ Treg cells differ in terms of the location of their differentiation: natural Foxp3+ Treg (nTreg) cells are differentiated in the thymus, and induced Foxp3+ Treg (iTreg) cells are differentiated in the periphery. Although a small fraction of Foxp3+ Treg cells are thought to be iTreg cells according to TCR sequence analysis [100–103], their role in physiological conditions is still unclear. The in vivo conversion of naïve cells has been observed in only a limited number of models [104–107]. Most of the models utilize foreign antigen-specific TCR-transgenic mice, such as OVA-specific TCR-transgenic mice crossed to RAG−/− mice. These mice have TCRαβ+ T cells that express only a single clonotype TCR but have no Treg cells because of the lack of an endogenous antigen. When the antigen is administered by feeding, injection, or transplantation, antigen-specific iTreg cells emerge from naïve CD4+ T cells. For example, when OVA-specific TCR transgenic RAG−/− mice are fed with OVA, Foxp3+ Treg cells appear in the mesenteric lymph node or the small intestine lamina propria [104, 107]. iTreg cells are found in lymphopenic mice, such as RAG−/− or TCRα−/− mice, after naïve T cells are transferred because the lymphopenic animals have an empty niche for Treg cells and, therefore, allow the conversion of naïve T cells into Treg cells to fill the niche [108].
Among several mechanisms for the regulation of other immune cells by Treg cells [109], IL-10 production by Treg cells has a critical role in maintaining intestinal immune tolerance. This role is demonstrated in IL-10-deficient or IL-10 receptor (IL-10R)-deficient mice, which exhibit spontaneous colitis, but not systemic autoimmune diseases [110]. The blockade of IL-10 signals skews intestinal CD4+ T cells toward the Th1 and Th17 lineages in commensal bacteria-colonized mice [111], suggesting that IL-10 restrains the basal activation of these Th cell subsets in the steady state. On the basis of the report that the genetic ablation of IL-10 specifically in Foxp3+ Treg cells elicits colitis, IL-10 produced by Treg cells has a critical role in intestinal homeostasis [112]. However, the incidence of colitis in these mice is slightly less and the onset is delayed compared with IL-10−/− mice, suggesting that IL-10 produced by other cells also contributes to the maintenance of intestinal homeostasis. Indeed, CD11b+F4/80+CD11c− macrophages in the small intestinal lamina propria are capable of producing IL-10 and involved in maintenance of mucosal homeostasis [49]. Furthermore, patients lacking a functional IL-10R develop colitis with an earlier onset and a higher penetrance compared with IPEX patients [113]. Therefore, IL-10 secretion by both Treg and non-Treg cells likely has a role in the control of intestinal immune homeostasis. Mice with a Treg-specific ablation of IL-10R or STAT3, which is a downstream signaling molecule of the IL-10R, develop spontaneous colitis with dysregulated Th17 cell responses [114, 115]. These results indicate that IL-10 signaling in Treg cells is critical for suppressing Th17 cells. IL-10R is also expressed by intestinal Th17 cells and required for the suppression of excess Th17 responses [116]. CTLA-4, which is highly expressed on intestinal Treg cells [9], is another molecule that is involved in intestinal homeostasis. On the basis of studies showing that CTLA-4 blockade abolishes Treg cell-mediated inhibition of colitis in the CD45RBhiCD4+ T cell transfer colitis model [117], and that the clinical application of an anti-CTLA-4 antibody (tremelimumab) for cancer treatment induces colitis as a side effect [118], CTLA-4 appears to be an indispensable molecule for maintaining intestinal homeostasis.
Intestinal Treg cells have suppressive activity against not only effector CD4+ T cells but also other types of cells. As mentioned above, Treg cells regulate TCRγδ T cells at steady state to maintain enteric immune tolerance [76]. Colon Foxp3+ Treg cells facilitate IgA responses to the microbiota-derived antigen flagellin, which is one of the TLR5 ligands [119]. This IgA induction could be another mechanism for the maintenance of intestinal homeostasis through Treg cells. Thus, intestinal Treg cells appear to contribute greatly to intestinal homeostasis via various mechanisms and via targeting different cell subsets.
Immune regulation by the gut microbiota
Studies using GF and gnotobiotic animals have revealed the enormous impact of commensal bacteria on a host’s systemic immune system. GF mice have smaller PPs and ILFs with fewer germinal centers, decreased IgA+ cells and lamina propria T cells, and reduced production of AMMs by IECs compared with SPF mice [21]. All these observations suggest that active immune responses occur in the intestinal mucosa in the presence of the commensal microbiota. Although the purpose of the ‘classical’ immune response is to eliminate pathogens to protect the host, the active immune response in the steady-state intestine is somehow oriented toward co-existence with the commensal microbiota. In other words, host immune cells likely function to contain the commensal bacteria to local sites and regulate local inflammation possibly elicited by the commensal bacteria.
Because more than half the intestinal microbiota is not culturable, the composition of the intestinal microbiota was until recently poorly defined. Culture-independent bacterial sequencing has now been extended to include metagenomics, which refers to studies of the structures and functions of microbial communities as well as their interactions with the habitats they occupy [120]. This analysis includes the sequencing of microbial 16S ribosomal DNA (rDNA) and the genome to determine the lineage and genes of the microbiota composing the community, and also includes characterizing the community’s expressed RNA and protein products and metabolic networks [120]. Based on this concept, the international human microbiome project has been initiated. This project has revealed that most bacteria belong to two phyla, Firmicutes and Bacteroidetes, which dominate among all the human adult commensal bacteria analyzed, and that enormous diversity exists in the types and proportions of species within the phyla [120]. The results also revealed considerable interpersonal variation [121–123].
Accumulating evidence suggests that deviations in the balance of the composition of commensal bacteria affect local and systemic diseases. Although the precise mechanisms of IBD remain unclear, the gut microbiota are thought to contribute to pathogenesis [5]. Several studies attempting to identify the causative microbiota composition for IBD have reported that the phylum Firmicutes appeared with less frequency in patients than healthy controls [122, 124–126]. Given that the samples were from patients in whom disease was established, whether the reduction in Firmicutes is a cause or a consequence of IBD is unclear.
IL-10−/− mice are often used as an IBD model, which have no colitis when they are raised under GF conditions [127]. This finding clearly demonstrates that commensal bacteria contribute to intestinal inflammation. In another IBD model, T-bet−/−RAG2−/− (TRUC) mice develop spontaneous colitis, which is horizontally and vertically transmissible to wild-type mice when they are co-housed with the TRUC mice [128], indicating that altered commensal bacteria (dysbiosis) alone have the potential to cause intestinal inflammation [128]. More recent studies have shown that mice deficient in NLRP6 have altered commensal bacteria and exhibit high sensitivity to DSS-induced colitis. The colitogenic microbiota is also transmissible to wild-type mice [38]. A 16S rDNA-based analysis revealed that TRUC mice have an increase in Klebsiella pneumoniae and Proteus mirabilis whereas NLRP6-deficient mice have an increase in Prevotellaceae and TM7 in their fecal microbiota [38, 129]. Collectively, these findings imply that the composition of the microbiota can dictate the status of local host immune system.
Induction of Th17 cells by segmented filamentous bacteria
Segmented filamentous bacteria (SFB) are yet-to-be cultured, Gram-positive, and spore-forming bacteria that are commonly considered to be nonpathogenic. SFB are host-specific members of the gut microbiota in numerous species, including mammals, birds, fishes, and insects (although whether SFB are present in the human intestine is currently unclear). Very recently, three independent groups reported the complete genome sequence of SFB indigenous to mice, and revealed that the bacteria have a highly reduced genome and are highly dependent on and utilize the host for nutrition and metabolism [130–132]. SFB colonize mainly the small intestine and exhibit a characteristic long filamentous morphology comprising multiple segments with well-defined septa. Each bacterium is likely to originate from a single, holdfast-bearing cell that tightly adheres to, and even embeds itself in, the microvilli on the IEC surface. The attachment of SFB induces morphological changes in the IECs, such as the accumulation of actin around the attachment site [133, 134]. SFB activate IECs to induce MHC class II molecules and fucosyltransferase 2 (Fut2) [135]. Fut2 fucosylates host glycoproteins on the IECs, such as asialo GM1 glycolipids. SFB are also potent stimuli of the IgA response and of the recruitment of TCRαβ+ IELs in the small intestine [136].
C57BL/6 mice from Taconic Farms have a substantial population of Th17 in the small intestine lamina propria whereas the mice from the Jackson Laboratory have only a few [12]. By comparing the components of the intestinal microbiota between the mice from the two vendors, SFB were found to be responsible for the induction of Th17 cells in the small intestine [137]. This finding was confirmed by the accumulation of Th17 cells in the small intestines of SFB-monocolonized mice [11, 137]. Furthermore, transgenic mice expressing the human antimicrobial peptide α-defensin, which exhibit the loss of SFB of the intestinal microbiota, have fewer Th17 cells in the lamina propria of the small intestine [138]. The mechanisms by which intestinal Th17 cells are induced by SFB are not at present clear. TGF-β and IL-6, which are critical for the early stages of differentiation of Th17 cells, are likely to be required for the accumulation of Th17 in the small intestine in SPF mice [12]. However, how TGF-β and IL-6 are induced by SFB colonization remains unclear. Given that SFB directly attach to IECs and induce the cytoplasmic accumulation of polymerized actin in IECs [139], SFB may have specific mechanisms for inducing TGF-β and IL-6 through direct interaction with IECs.
Induction of Treg cells by the commensal microbiota
Foxp3+ Treg cells distribute to essentially all organs, and the frequencies of Foxp3+ cells are approximately 10 % within the CD4+ cell subset. In contrast, the frequency of Foxp3+ Treg cells in the gut lamina propria is notably higher (>30 % within CD4+ T cell) than in other organs [9]. Therefore, the intestinal microbiota likely has a critical role in the accumulation of intestinal Treg cells. Indeed, we have shown that, in the colon lamina propria, Treg cell numbers decreased in GF mice and antibiotic-treated mice [9]. However, the analyses of the number of colon Treg cells in various studies have provided discordant results. Some studies, including those from our laboratory, show a decrease in GF mice [9, 140, 141], and other studies show normal numbers of Treg cells in GF mice [111, 142–144] relative to SPF mice. These different results might be produced by different methods of isolating lymphocytes from gut lamina propria. The discrepancies could also be attributed to differences in the components of the intestinal microbiota in mice among various SPF animal facilities. Furthermore, there might be differences in the stringency of cleanliness between GF facilities. Importantly, many reports have consistently shown that the percentage and number of Helios-negative Tregs was markedly reduced in GF mice compared with SPF mice [9, 111, 143]. In SPF mice, Helios-negative and Helios-positive cells are present at approximately a 1:1 ratio within colon Treg cells [9, 111]. Helios is a putative marker for thymically derived nTreg cells [145]. Thus, Helios− Treg cells in the colon lamina propria may be iTreg cells. Although more detailed studies are required to fully characterize Helios-negative Treg cells in the intestine (discussed later), the presence of the microbiota likely affects this population of Treg cells.
The human symbiont Bacteroides fragilis is commonly isolated from human fecal samples [146, 147]. Colonization of GF mice with B. fragilis suppresses colitis by the opportunistic pathogen H. hepatics. This suppression is dependent on polysaccharide A (PSA), which is a component of B. fragilis [13]. Administration of purified PSA is sufficient to increase IL-10 production by Treg cells and to suppress IL-17 production during intestinal inflammation [142, 148]. The consumption of another human commensal species, Bifidobacterium infantis, also increases Foxp3+ Treg cells during pathogenic Salmonella typhimurium infection, leading to a reduction in disease severity and the systemic translocation of the bacteria with an attenuation of local NF-κB activation [14].
Among the microbiota that is indigenous to the murine colon, spore-forming components, particularly those of the genus Clostridium belonging to clusters XIVa and IV, are potent inducers of colon Treg cells [9]. Some species of Clostridium, such as C. perfringens (a member of Clostridium cluster I), C. difficile (a member of cluster XI), and C. tetani (a member of cluster I), produce toxins and are pathogenic, but most of the Clostridium clusters XVIa and IV maintain a commensal relationship with the host. The colonization of GF mice with a defined mixture of 46 strains of Clostridium clusters XIVa and IV, which were originally isolated from the chloroform-treated fecal material (sporulated fraction) of conventionally reared mice [149], is sufficient to induce Treg cells [9]. Importantly, the 46 strains of Clostridium spp. do not include pathogenic clostridia, such as C. difficile. Perhaps consistent with the importance of commensal Clostridium clusters XIVa and IV, colonization with altered Schaedler flora (ASF), which includes C. clostridioforme (Clostridium cluster XIVa), results in Treg cell accumulation in the colon LP [111]. Similar to mice reared in SPF conditions, Treg cells in the colon of mice colonized with Clostridium clusters XIVa and IV contain a large number of Helios− Foxp3+ cells [9]. Therefore, Clostridium clusters XIVa and IV may induce iTreg cell conversion in the colon. In addition, a large subset of the Treg cells induced by colonization by Clostridium clusters XIVa and IV expresses high levels of IL-10 and CTLA-4 [9]. Therefore, Clostridium clusters XIVa and IV may influence the qualitative properties of colon Treg cells in addition to the quantity [9].
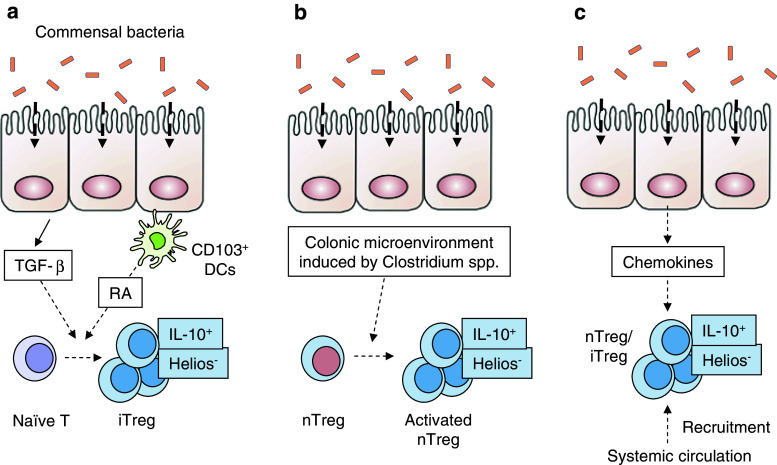
The mechanisms by which certain bacteria induce the intestinal accumulation of Treg cells are still unknown. Given that Helios is a candidate marker for nTreg cells [145], conversion from local naïve CD4 T cells may be responsible for the accumulation of Helios−Foxp3+ Treg cells in the colon (Fig. 1a). In addition, the intestinal microenvironment is rich in TGF-β and bacteria-derived antigens and, therefore, might promote iTreg cell induction (Fig. 1a). Alternatively, Helios−Foxp3+ Treg cells could be a further activated or differentiated subset of thymus-derived nTreg cells that pre-exist in the colon (Fig. 1b). The steady-state colon microenvironment might induce nTreg cells to differentiate into IL-10-producing Treg cells, resulting in the downregulation of Helios. Another possibility is that the microbiota activates the host IECs or other cells to produce chemokines to recruit Helios− Treg cells into the colon lamina propria (Fig. 1c). In support of the first scenario (the induction of iTreg cells in the colon), one recent study provided evidence that many clones of Treg cells that localize specifically in the colon react to the intestinal bacterial content in vitro [143]. Therefore, specific commensal bacteria likely induce naïve CD4+ T cells to differentiate into antigen-specific colon Treg cells that, presumably, enforce immune system tolerance toward those bacteria.
Fig. 1.
Possible mechanisms for the induction of Helios− IL-10+ Treg cells by commensal bacteria in the colon lamina propria. a Upon colonization with commensal bacteria, the colon epithelial cells produce TGF-β, which then contributes to the conversion of naive Treg cells to iTreg cells. Retinoic acid (RA) production by CD103+ DCs in the lamina propria may also contribute to the conversion. b Alternatively, the colon microenvironment that is generated in response to the commensal microbiota may contribute to the activation, differentiation, and proliferation of nTreg cells to become Helios− IL-10+ Treg cells. c Another possibility is that upon interaction with the microbiota, IECs or other intestinal cells produce chemokines that recruit Helios− IL-10+ Treg cells from other organs into the colon lamina propria
Impacts of commensal bacteria on systemic immune responses and diseases
Several models of autoimmunity have been tested in GF conditions to assess the influence of commensal bacteria on the diseases. Non-obese diabetic (NOD) mice and BioBreeding rats, which are models of type 1 diabetes (T1D), display accelerated and severe diabetes under GF conditions [150, 151]. In contrast, myelin oligodendrocyte glycoprotein (MOG)-specific TCR-transgenic mice, which spontaneously develop experimental autoimmune encephalomyelitis (EAE) in a model of multiple sclerosis, exhibit decreased severity of the disease under GF conditions [10]. The commensal bacteria-dependent activation of MOG-specific CD4+ T cells leads to an increase in MOG-specific antibody production by the draining lymph node B cells as a mechanism of altering the severity of disease by commensal bacteria [10]. For rheumatoid arthritis, different models yield different outcomes of the disease related to colonization by commensal bacteria. GF conditions ameliorate arthritis in IL-1 receptor antagonist-deficient mice and KBxN mice, both of which develop spontaneous arthritis under SPF conditions [152, 153]. In addition, GF mice have slightly less severe disease in an adjuvant-based arthritis model than SPF mice [154]. Collectively, these results imply that commensal bacteria affect autoimmune disease and that the outcome differs between the diseases.
Because SFB induce Th17 cells in the small intestine, several studies have focused on the influence of the bacteria on infections or autoimmune diseases. SFB-colonized mice are resistant to infection with C. rodentium [137]. In KBxN mice, the disease is attenuated under GF conditions, and this attenuation is accompanied by a loss in autoreactive IgG production by B cells that results from a decrease in Th17 cells [153]. Monocolonization with SFB reverts arthritis through an increase in splenic Th17 cells and IgG production [153]. Similar to the KBxN arthritis model, the severity of EAE is greatly attenuated under GF conditions [10, 155]. However, in SFB-monocolonized mice, the level of infiltration of Th17 cells in the spinal cord and the disease severity are equivalent to these measures in SPF mice [155]. These two autoimmune disease models (KBxN arthritis and EAE) have been shown to require Th17 cells for disease development, because mice with an IL-17 deficiency or subjected to IL-17-blocking treatment display attenuated disease symptoms [153, 156]. Therefore, SFB likely induce Th17 cells in the intestine, and these cells then migrate to, and accumulate in, the target organs of the autoimmune disease. NOD mice spontaneously develop a disease similar to T1D, which is a Th1-mediated disease. There is a significant difference in incidence of disease development between SFB-colonized and uncolonized NOD mice [157]. SFB-colonized NOD mice develop diabetes at a greatly reduced frequency. Interestingly, SFB colonization does not have an effect on the development of insulitis [157], suggesting that SFB colonization cannot overcome the genetic influence on the susceptibility to insulitis. However, the bacteria can modify the progress of the disease, presumably by affecting the progress of insulitis and/or the metabolism of insulin and glucose [157]. As a mechanism, the report suggests that SFB colonization directs the Th1/Th17 balance toward a Th17-skewed response and consequently suppresses the Th1 response [157]. However, how intestinal Th17 cell accumulation elicits a systemic Th17 response in the EAE, arthritis and T1D models remains unclear.
Oral inoculation of SPF mice with 46 strains of Clostridium clusters XIVa and IV decreased the severity of DSS-induced colitis, and the Th2 response, including OVA-specific IgE production, upon immunization [9]. In humans, a reduction in the number of Faecalibacterium prausnitzii, which is a member of Clostridium cluster IV, was shown to be associated with a high risk of postoperative recurrence of Crohn’s disease [158]. The supernatants from F. prausnitzii cultures increase the production of IL-10 by peripheral blood mononuclear cells in vitro. In addition, the proportion of Clostridium clusters XIVa and IV in the fecal community was shown to be smaller in IBD patients than in healthy controls [122]. These reports raise the possibility that the Clostridium-dependent constitutive induction of Treg cells and their expression of IL-10 and CTLA-4 may contribute to the suppression of local and systemic autoimmunity and deleterious inflammation in humans. They further suggest that the prophylactic administration of human-gut-resident Clostridium clusters XIVa and IV could reduce susceptibility to chronic disease. However, it still remains unclear whether the defects in Treg cells actually contribute to human IBD. Interestingly, serological analysis of colitic C3H/HeJBir mice has revealed that a flagellin protein called CBir1, which contains sequences related to the flagellins of Clostridium cluster XIVa, is the dominant antigen responsible for the colitis. The adoptive transfer of a CBir1 flagellin-specific CD4+ T cell line into immunodeficient mice induced colitis in the recipients [159]. Furthermore, Crohn’s disease patients have an increased number of CD4+ T cells that are reactive to CBir1 flagellin [160]. Therefore, depending on the circumstances, clostridia-derived antigens could be causative factors for inflammatory diseases.
Concluding remarks
Considerable progress has been made in improving our understanding of intestine-residing immune cell populations and the influences of the composition of the gut microbiota on the immune system. Specific species of the gut microbiota affect the numbers and activities of intestinal immune cells. However, we are still far from achieving a comprehensive understanding of the mechanisms underlying how IECs and immune cells, including the newly discovered cells, collaborate to protect the host from pathogenic bacteria and at the same time develop tolerance to commensal bacteria. It also remains unclear whether and how the intestinal immune cells affect the systemic immune responses. In addition, it is also unclear how the host immune system maintains the diversity and balance of the microbiota. More intensive studies will be required to answer these questions.
Acknowledgments
The work was supported by the funding program for next generation world-leading researchers (NEXT program) from the Japan Society for the Promotion of Science (JSPS) to K. Honda and Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) to J. Nishio.
Abbreviations
- APRIL
A proliferation-inducing ligand
- AID
Activation-induced cytidine deaminase
- AMM
Antimicrobial molecule
- ASC
Apoptosis-associated speck-like protein
- BAFF
B cell activating factor
- CSR
Class switch recombination
- DC
Dendritic cell
- DSS
Dextran sulfate sodium
- EAE
Experimental autoimmune encephalomyelitis
- Fut2
Fucosyltransferase 2
- GF
Germ-free
- GALT
Gut-associated lymphoid tissue
- IKK
IκB kinase
- IL-10R
IL-10 receptor
- Ig
Immunoglobulin
- iTreg
Inducible Treg
- IBD
Inflammatory bowel diseases
- ILC
Innate lymphoid cell
- IECs
Intestinal epithelial cells
- ILFs
Isolated lymphoid follicles
- LPS
Lipopolysaccharide
- LTi
Lymphoid tissue inducer
- MHC
Major histocompatibility complex
- MOG
Myelin oligodendrocyte glycoprotein
- nTreg
Natural Treg
- NOD
Non-obese diabetic
- NLR
Nucleotide-binding oligomerization domain-like receptor
- PRR
Pattern recognition receptor
- PP
Payer’s patche
- pIgR
Polymeric Ig receptor
- PSA
Polysaccharide A
- RAG
Recombinase-activating gene
- RegIIIγ
Regenerating islet-derived IIIγ
- Treg
Regulatory T
- RORγt
Retinoic acid receptor-related orphan receptor gamma t
- rDNA
Ribosomal DNA
- SIgA
Secretory IgA
- SFB
Segmented filamentous bacteria
- STAT3
Signal transducer and activator of transcription 3
- SHM
Somatic hypermutation
- SPF
Specific pathogen-free
- TCR
T cell receptor
- TD
T cell-dependent
- TI
T cell-independent
- Th
T helper
- TSLP
Thymic stromal lymphopoietin
- TLR
Toll-like receptor
- TGF-β
Transforming growth factor-β
- TACI
Transmembrane activator and calcium modulator and cyclophilin ligand interactor
- T1D
Type 1 diabetes
- VRE
Vancomycin-resistant Enterococcus
References
- 1.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10(2):131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 2.Wells JM, Rossi O, Meijerink M, van Baarlen P. Epithelial crosstalk at the microbiota-mucosal interface. Proc Natl Acad Sci USA. 2010;108(Suppl 1):4607–4614. doi: 10.1073/pnas.1000092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol. 2003;3(1):63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 4.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8(6):411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 5.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 6.Rescigno M. Intestinal dendritic cells. Adv Immunol. 2010;107:109–138. doi: 10.1016/B978-0-12-381300-8.00004-6. [DOI] [PubMed] [Google Scholar]
- 7.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4(6):478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 8.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331(6015):337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479(7374):538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 11.Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, Eberl G, Snel J, Kelly D, Cerf-Bensussan N. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31(4):677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 14.O’Mahony C, Scully P, O’Mahony D, Murphy S, O’Brien F, Lyons A, Sherlock G, MacSharry J, Kiely B, Shanahan F, O’Mahony L. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog. 2008;4(8):e1000112. doi: 10.1371/journal.ppat.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 16.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127(1):224–238. doi: 10.1053/j.gastro.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132(4):1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 19.Sonnenburg JL, Chen CT, Gordon JI. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 2006;4(12):e413. doi: 10.1371/journal.pbio.0040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105(52):20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313(5790):1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, DeMatteo RP, Pamer EG. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455(7214):804–807. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334(6053):255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Kivit S, Saeland E, Kraneveld AD, van de Kant HJ, Schouten B, van Esch BC, Knol J, Sprikkelman AB, van der Aa LB, Knippels LM, Garssen J, van Kooyk Y, Willemsen LE. Galectin-9 induced by dietary synbiotics is involved in suppression of allergic symptoms in mice and humans. Allergy. 2012;67(3):343–352. doi: 10.1111/j.1398-9995.2011.02771.x. [DOI] [PubMed] [Google Scholar]
- 25.Kinnebrew MA, Ubeda C, Zenewicz LA, Smith N, Flavell RA, Pamer EG. Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J Infect Dis. 2010;201(4):534–543. doi: 10.1086/650203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song X, Zhu S, Shi P, Liu Y, Shi Y, Levin SD, Qian Y. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat Immunol. 2011;12(12):1151–1158. doi: 10.1038/ni.2155. [DOI] [PubMed] [Google Scholar]
- 27.Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, Hackney J, Kim J, Zhou M, Lai J, Modrusan Z, Sai T, Lee W, Xu M, Caplazi P, Diehl L, de Voss J, Balazs M, Gonzalez L, Jr, Singh H, Ouyang W, Pappu R. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol. 2011;12(12):1159–1166. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- 28.Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6(5):507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 29.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, Eckmann L, Karin M, Artis D. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446(7135):552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 30.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, Bhandoola A, Artis D. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464(7293):1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009;2(4):340–350. doi: 10.1038/mi.2009.13. [DOI] [PubMed] [Google Scholar]
- 32.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, Knowles DM, Rescigno M, Cerutti A. Intestinal bacteria trigger T cell-independent immunoglobulin A (2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26(6):812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, Bram RJ, Jabara H, Geha RS. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201(1):35–39. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, Herfarth HH, Jobin C, Ting JP. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207(5):1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirota SA, Ng J, Lueng A, Khajah M, Parhar K, Li Y, Lam V, Potentier MS, Ng K, Bawa M, McCafferty DM, Rioux KP, Ghosh S, Xavier RJ, Colgan SP, Tschopp J, Muruve D, MacDonald JA, Beck PL. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis. 2011;17(6):1359–1372. doi: 10.1002/ibd.21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32(3):379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N, Vallance BA, Saleh M. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32(3):367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145(5):745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucke K, Miehlke S, Jacobs E, Schuppler M. Prevalence of Bacteroides and Prevotella spp. in ulcerative colitis. J Med Microbiol. 2006;55(Pt 5):617–624. doi: 10.1099/jmm.0.46198-0. [DOI] [PubMed] [Google Scholar]
- 40.Kleessen B, Kroesen AJ, Buhr HJ, Blaut M. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand J Gastroenterol. 2002;37(9):1034–1041. doi: 10.1080/003655202320378220. [DOI] [PubMed] [Google Scholar]
- 41.Reuter BK, Pizarro TT. Commentary: the role of the IL-18 system and other members of the IL-1R/TLR superfamily in innate mucosal immunity and the pathogenesis of inflammatory bowel disease: friend or foe? Eur J Immunol. 2004;34(9):2347–2355. doi: 10.1002/eji.200425351. [DOI] [PubMed] [Google Scholar]
- 42.Takagi H, Kanai T, Okazawa A, Kishi Y, Sato T, Takaishi H, Inoue N, Ogata H, Iwao Y, Hoshino K, Takeda K, Akira S, Watanabe M, Ishii H, Hibi T. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand J Gastroenterol. 2003;38(8):837–844. doi: 10.1080/00365520310004047. [DOI] [PubMed] [Google Scholar]
- 43.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, Stanley ER, Nussenzweig M, Lira SA, Randolph GJ, Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31(3):513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, Fehling HJ, Hardt WD, Shakhar G, Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31(3):502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 45.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307(5707):254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 46.Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206(13):3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rescigno M, Valzasina B, Bonasio R, Urbano M, Ricciardi-Castagnoli P. Dendritic cells, loaded with recombinant bacteria expressing tumor antigens, induce a protective tumor-specific response. Clin Cancer Res. 2001;7(3 Suppl):865s–870s. [PubMed] [Google Scholar]
- 48.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203(13):2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8(10):1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 50.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455(7214):808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 51.Kayama H, Ueda Y, Sawa Y, Jeon SG, Ma JS, Okumura R, Kubo A, Ishii M, Okazaki T, Murakami M, Yamamoto M, Yagita H, Takeda K. Intestinal CX3C chemokine receptor 1high (CX3CR1high) myeloid cells prevent T-cell-dependent colitis. Proc Natl Acad Sci USA. 2012;109(13):5010–5015. doi: 10.1073/pnas.1114931109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Forster R, Pabst O. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205(11):2483–2490. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204(8):1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204(8):1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, Sparwasser T, Forster R, Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34(2):237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 56.Laffont S, Siddiqui KR, Powrie F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur J Immunol. 2010;40(7):1877–1883. doi: 10.1002/eji.200939957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewis KL, Caton ML, Bogunovic M, Greter M, Grajkowska LT, Ng D, Klinakis A, Charo IF, Jung S, Gommerman JL, Ivanov II, Liu K, Merad M, Reizis B. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity. 2011;35(5):780–791. doi: 10.1016/j.immuni.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Niess JH, Adler G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J Immunol. 2010;184(4):2026–2037. doi: 10.4049/jimmunol.0901936. [DOI] [PubMed] [Google Scholar]
- 59.Macpherson AJ, Geuking MB, Slack E, Hapfelmeier S, McCoy KD. The habitat, double life, citizenship, and forgetfulness of IgA. Immunol Rev. 2012;245(1):132–146. doi: 10.1111/j.1600-065X.2011.01072.x. [DOI] [PubMed] [Google Scholar]
- 60.Bergqvist P, Gardby E, Stensson A, Bemark M, Lycke NY. Gut IgA class switch recombination in the absence of CD40 does not occur in the lamina propria and is independent of germinal centers. J Immunol. 2006;177(11):7772–7783. doi: 10.4049/jimmunol.177.11.7772. [DOI] [PubMed] [Google Scholar]
- 61.Snider DP, Liang H, Switzer I, Underdown BJ. IgA production in MHC class II-deficient mice is primarily a function of B-1a cells. Int Immunol. 1999;11(2):191–198. doi: 10.1093/intimm/11.2.191. [DOI] [PubMed] [Google Scholar]
- 62.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3(9):822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cerutti A, Chen K, Chorny A. Immunoglobulin responses at the mucosal interface. Annu Rev Immunol. 2011;29:273–293. doi: 10.1146/annurev-immunol-031210-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johansen FE, Pekna M, Norderhaug IN, Haneberg B, Hietala MA, Krajci P, Betsholtz C, Brandtzaeg P. Absence of epithelial immunoglobulin a transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J Exp Med. 1999;190(7):915–922. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimada S, Kawaguchi-Miyashita M, Kushiro A, Sato T, Nanno M, Sako T, Matsuoka Y, Sudo K, Tagawa Y, Iwakura Y, Ohwaki M. Generation of polymeric immunoglobulin receptor-deficient mouse with marked reduction of secretory IgA. J Immunol. 1999;163(10):5367–5373. [PubMed] [Google Scholar]
- 66.Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298(5597):1424–1427. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, Fagarasan S. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci USA. 2004;101(7):1981–1986. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei M, Shinkura R, Doi Y, Maruya M, Fagarasan S, Honjo T. Mice carrying a knock-in mutation of Aicda resulting in a defect in somatic hypermutation have impaired gut homeostasis and compromised mucosal defense. Nat Immunol. 2011;12(3):264–270. doi: 10.1038/ni.1991. [DOI] [PubMed] [Google Scholar]
- 69.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 70.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 71.Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, Hatton RD, Stormo GD, Weaver CT, Russell JH, Murphy TL, Murphy KM. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460(7253):405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8(9):967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 73.Awasthi A, Kuchroo VK. Th17 cells: from precursors to players in inflammation and infection. Int Immunol. 2009;21(5):489–498. doi: 10.1093/intimm/dxp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malinarich FH, Grabski E, Worbs T, Chennupati V, Haas JD, Schmitz S, Candia E, Quera R, Malissen B, Forster R, Hermoso M, Prinz I. Constant TCR triggering suggests that the TCR expressed on intestinal intraepithelial gammadelta T cells is functional in vivo. Eur J Immunol. 2010;40(12):3378–3388. doi: 10.1002/eji.201040727. [DOI] [PubMed] [Google Scholar]
- 75.Nanno M, Kanari Y, Naito T, Inoue N, Hisamatsu T, Chinen H, Sugimoto K, Shimomura Y, Yamagishi H, Shiohara T, Ueha S, Matsushima K, Suematsu M, Mizoguchi A, Hibi T, Bhan AK, Ishikawa H. Exacerbating role of gammadelta T cells in chronic colitis of T-cell receptor alpha mutant mice. Gastroenterology. 2008;134(2):481–490. doi: 10.1053/j.gastro.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 76.Park SG, Mathur R, Long M, Hosh N, Hao L, Hayden MS, Ghosh S. T regulatory cells maintain intestinal homeostasis by suppressing gammadelta T cells. Immunity. 2010;33(5):791–803. doi: 10.1016/j.immuni.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci USA. 2002;99(22):14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Inagaki-Ohara K, Chinen T, Matsuzaki G, Sasaki A, Sakamoto Y, Hiromatsu K, Nakamura-Uchiyama F, Nawa Y, Yoshimura A. Mucosal T cells bearing TCRgammadelta play a protective role in intestinal inflammation. J Immunol. 2004;173(2):1390–1398. doi: 10.4049/jimmunol.173.2.1390. [DOI] [PubMed] [Google Scholar]
- 79.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, Powrie F. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25(2):309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 80.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10(7):479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 81.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31(2):321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 82.Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, Flach M, Bengsch B, Thimme R, Holscher C, Honig M, Pannicke U, Schwarz K, Ware CF, Finke D, Diefenbach A. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33(5):736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464(7293):1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457(7230):722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O’Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206(1):35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reynders A, Yessaad N, Vu Manh TP, Dalod M, Fenis A, Aubry C, Nikitas G, Escaliere B, Renauld JC, Dussurget O, Cossart P, Lecuit M, Vivier E, Tomasello E. Identity, regulation and in vivo function of gut NKp46+ RORgammat+ and NKp46+ RORgammat-lymphoid cells. EMBO J. 2011;30(14):2934–2947. doi: 10.1038/emboj.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Geremia A, Arancibia-Carcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, Travis SP, Powrie F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208(6):1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203(11):2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314(5804):1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Connor W, Jr, Zenewicz LA, Flavell RA. The dual nature of T(H)17 cells: shifting the focus to function. Nat Immunol. 2010;11(6):471–476. doi: 10.1038/ni.1882. [DOI] [PubMed] [Google Scholar]
- 91.O’Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10(6):603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29(6):947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14(3):282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 94.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, Dong C. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205(5):1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 96.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 97.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27(1):20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 98.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27(1):68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 99.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27(1):18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 100.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21(2):267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 101.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7(4):401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 102.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+ CD4+ CD25+ T cells. Immunity. 2006;25(2):249–259. doi: 10.1016/j.immuni.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 103.Wong J, Obst R, Correia-Neves M, Losyev G, Mathis D, Benoist C. Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J Immunol. 2007;178(11):7032–7041. doi: 10.4049/jimmunol.178.11.7032. [DOI] [PubMed] [Google Scholar]
- 104.Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. CD25-T cells generate CD25+ Foxp3+ regulatory T cells by peripheral expansion. J Immunol. 2004;173(12):7259–7268. doi: 10.4049/jimmunol.173.12.7259. [DOI] [PubMed] [Google Scholar]
- 105.Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172(10):6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 106.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6(12):1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 107.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115(7):1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nishio J, Feuerer M, Wong J, Mathis D, Benoist C. Anti-CD3 therapy permits regulatory T cells to surmount T cell receptor-specified peripheral niche constraints. J Exp Med. 2010;207(9):1879–1889. doi: 10.1084/jem.20100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kullberg MC, Ward JM, Gorelick PL, Caspar P, Hieny S, Cheever A, Jankovic D, Sher A. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66(11):5157–5166. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34(5):794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 112.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28(4):546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 113.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, Hatscher N, Pfeifer D, Sykora KW, Sauer M, Kreipe H, Lacher M, Nustede R, Woellner C, Baumann U, Salzer U, Koletzko S, Shah N, Segal AW, Sauerbrey A, Buderus S, Snapper SB, Grimbacher B, Klein C. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361(21):2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a stat3-dependent manner. Science. 2009;326(5955):986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Bruning JC, Muller W, Rudensky AY. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34(4):566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huber S, Gagliani N, Esplugues E, O’Connor W, Jr, Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY, Roncarolo MG, Battaglia M, Flavell RA. Th17 cells express interleukin-10 receptor and are controlled by Foxp3 and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34(4):554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192(2):295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Langer LF, Clay TM, Morse MA. Update on anti-CTLA-4 antibodies in clinical trials. Expert Opin Biol Ther. 2007;7(8):1245–1256. doi: 10.1517/14712598.7.8.1245. [DOI] [PubMed] [Google Scholar]
- 119.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci USA. 2009;106(46):19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peterson DA, Frank DN, Pace NR, Gordon JI. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe. 2008;3(6):417–427. doi: 10.1016/j.chom.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104(34):13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 124.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, Roca J, Dore J. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55(2):205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gophna U, Sommerfeld K, Gophna S, Doolittle WF, Veldhuyzen van Zanten SJ. Differences between tissue-associated intestinal microfloras of patients with Crohn’s disease and ulcerative colitis. J Clin Microbiol. 2006;44(11):4136–4141. doi: 10.1128/JCM.01004-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, Dore J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15(8):1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 127.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66(11):5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131(1):33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, Gordon JI, Onderdonk AB, Glimcher LH. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8(3):292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Prakash T, Oshima K, Morita H, Fukuda S, Imaoka A, Kumar N, Sharma VK, Kim SW, Takahashi M, Saitou N, Taylor TD, Ohno H, Umesaki Y, Hattori M. Complete genome sequences of rat and mouse segmented filamentous bacteria, a potent inducer of th17 cell differentiation. Cell Host Microbe. 2011;10(3):273–284. doi: 10.1016/j.chom.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 131.Sczesnak A, Segata N, Qin X, Gevers D, Petrosino JF, Huttenhower C, Littman DR, Ivanov II. The genome of th17 cell-inducing segmented filamentous bacteria reveals extensive auxotrophy and adaptations to the intestinal environment. Cell Host Microbe. 2011;10(3):260–272. doi: 10.1016/j.chom.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kuwahara T, Ogura Y, Oshima K, Kurokawa K, Ooka T, Hirakawa H, Itoh T, Nakayama-Imaohji H, Ichimura M, Itoh K, Ishifune C, Maekawa Y, Yasutomo K, Hattori M, Hayashi T. The lifestyle of the segmented filamentous bacterium: a non-culturable gut-associated immunostimulating microbe inferred by whole-genome sequencing. DNA Res. 2011;18(4):291–303. doi: 10.1093/dnares/dsr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Caselli M, Holton J, Boldrini P, Vaira D, Calo G. Morphology of segmented filamentous bacteria and their patterns of contact with the follicle-associated epithelium of the mouse terminal ileum: implications for the relationship with the immune system. Gut Microbes. 2010;1(6):367–372. doi: 10.4161/gmic.1.6.14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yamauchi KE, Snel J. Transmission electron microscopic demonstration of phagocytosis and intracellular processing of segmented filamentous bacteria by intestinal epithelial cells of the chick ileum. Infect Immun. 2000;68(11):6496–6504. doi: 10.1128/iai.68.11.6496-6504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Umesaki Y, Okada Y, Matsumoto S, Imaoka A, Setoyama H. Segmented filamentous bacteria are indigenous intestinal bacteria that activate intraepithelial lymphocytes and induce MHC class II molecules and fucosyl asialo GM1 glycolipids on the small intestinal epithelial cells in the ex-germ-free mouse. Microbiol Immunol. 1995;39(8):555–562. doi: 10.1111/j.1348-0421.1995.tb02242.x. [DOI] [PubMed] [Google Scholar]
- 136.Umesaki Y, Setoyama H, Matsumoto S, Imaoka A, Itoh K. Differential roles of segmented filamentous bacteria and clostridia in development of the intestinal immune system. Infect Immun. 1999;67(7):3504–3511. doi: 10.1128/iai.67.7.3504-3511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, Stoel M, Zhou Y, Sodergren E, Weinstock GM, Bevins CL, Williams CB, Bos NA. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2009;11(1):76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jepson MA, Clark MA, Simmons NL, Hirst BH. Actin accumulation at sites of attachment of indigenous apathogenic segmented filamentous bacteria to mouse ileal epithelial cells. Infect Immun. 1993;61(9):4001–4004. doi: 10.1128/iai.61.9.4001-4004.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ostman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. Eur J Immunol. 2006;36(9):2336–2346. doi: 10.1002/eji.200535244. [DOI] [PubMed] [Google Scholar]
- 141.Strauch UG, Obermeier F, Grunwald N, Gurster S, Dunger N, Schultz M, Griese DP, Mahler M, Scholmerich J, Rath HC. Influence of intestinal bacteria on induction of regulatory T cells: lessons from a transfer model of colitis. Gut. 2005;54(11):1546–1552. doi: 10.1136/gut.2004.059451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107(27):12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478(7368):250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chinen T, Volchkov PY, Chervonsky AV, Rudensky AY. A critical role for regulatory T cell-mediated control of inflammation in the absence of commensal microbiota. J Exp Med. 2010;207(11):2323–2330. doi: 10.1084/jem.20101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184(7):3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gorbach SL, Bartlett JG. Anaerobic infections (second of three parts) N Engl J Med. 1974;290(22):1237–1245. doi: 10.1056/NEJM197405302902207. [DOI] [PubMed] [Google Scholar]
- 147.Thadepalli H, Gorbach SL, Broido PW, Norsen J, Nyhus L. Abdominal trauma, anaerobes, and antibiotics. Surg Gynecol Obstet. 1973;137(2):270–276. [PubMed] [Google Scholar]
- 148.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332(6032):974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Itoh K, Mitsuoka T. Characterization of clostridia isolated from faeces of limited flora mice and their effect on caecal size when associated with germ-free mice. Lab Anim. 1985;19(2):111–118. doi: 10.1258/002367785780942589. [DOI] [PubMed] [Google Scholar]
- 150.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature. 2008;455(7216):1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rossini AA, Williams RM, Mordes JP, Appel MC, Like AA. Spontaneous diabetes in the gnotobiotic BB/W rat. Diabetes. 1979;28(11):1031–1032. doi: 10.2337/diab.28.11.1031. [DOI] [PubMed] [Google Scholar]
- 152.Abdollahi-Roodsaz S, Joosten LA, Koenders MI, Devesa I, Roelofs MF, Radstake TR, Heuvelmans-Jacobs M, Akira S, Nicklin MJ, Ribeiro-Dias F, van den Berg WB. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Invest. 2008;118(1):205–216. doi: 10.1172/JCI32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bjork J, Kleinau S, Midtvedt T, Klareskog L, Smedegard G. Role of the bowel flora for development of immunity to hsp 65 and arthritis in three experimental models. Scand J Immunol. 1994;40(6):648–652. doi: 10.1111/j.1365-3083.1994.tb03518.x. [DOI] [PubMed] [Google Scholar]
- 155.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177(1):566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 157.Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, Mathis D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci USA. 2011;108(28):11548–11553. doi: 10.1073/pnas.1108924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottiere HM, Dore J, Marteau P, Seksik P, Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105(43):16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113(9):1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shen C, Landers CJ, Derkowski C, Elson CO, Targan SR. Enhanced CBir1-specific innate and adaptive immune responses in Crohn’s disease. Inflamm Bowel Dis. 2008;14(12):1641–1651. doi: 10.1002/ibd.20645. [DOI] [PMC free article] [PubMed] [Google Scholar]