Abstract
Invertebrate circulating hemocytes are key players in the innate immune defense and their continuous renewal from hematopoietic tissues is tightly regulated in crustaceans by astakine, a new family of cytokines sharing a prokineticin (PROK) domain. In vertebrates, brain PROKs function as transmitters of circadian rhythms and we present evidence that hemocyte release from hematopoietic tissues in crayfish is under circadian regulation, a direct result of rhythmic expression of astakine. We demonstrate that the observed variation in astakine expression has an impact on innate immunity assessed as susceptibility to a pathogenic Pseudomonas species. These findings enlighten the importance of comparing immune responses at fixed times not to neglect circadian regulation of innate immunity. Moreover, our results entail an evolutionary conserved function for prokineticins as mediators of circadian rhythm, and for the first time show a role for this domain in circadian regulation of hematopoiesis that may have implications also in vertebrates.
Keywords: Astakine, Circadian rhythm, Cytokine, Hematopoiesis, Innate immunity, Prokineticin
Introduction
Daily, circadian rhythms influence essentially all living organisms and affect many physiological processes from sleep and nutrition to immunity. This ability to respond to environmental daily rhythms has been conserved along evolution and is found among species from bacteria to mammals [1]. The circadian clock mechanism is regulated by a highly conserved set of genes encoding regulatory proteins. In mammals there is increasing evidence that stem cell activities are modulated by circadian regulatory proteins, and circadian oscillations in proliferative activity and recruitment of hematopoietic progenitor cells have been demonstrated [1, 2]. Circadian rhythmic variation of the number of blood cells may have implication in immune defense, and accordingly Lee and Edery [3] have shown circadian regulation of the innate immune response of Drosophila melanogaster. However, how this circadian rhythm is induced is unknown. Invertebrate innate immunity is a growing field of investigations, partly due to its importance for applied aquaculture of crustacean animals. During the recent decade, numerous studies about pathogen induced gene expression in crustaceans have been published, mainly in hemocytes as they are important immune cells. It is possible that neglecting natural circadian variation in hemocyte number and hemocyte synthesis has overlooked important information about immunity.
Blood cells in crustaceans (hemocytes) play an important role in the immune response and thus the regulation of hemocyte homeostasis (hematopoiesis) is essential for survival of the animal. New hemocytes are synthesized and partly differentiated in a hematopoietic tissue (HPT) situated on the dorsal part of the crayfish stomach, and then the final differentiation from stem cells into functional hemocytes expressing prophenoloxidase (proPO, the final component of an important innate immune reaction), is not completed until the hemocytes are released into the circulation [4]. A Runt family transcription factor (PlRunt) was detected in crayfish semigranular and granular hemocytes [4], and this PlRunt is needed for the differentiation of these hemocyte types. A similar function is shown for its homologue lozenge in the development of crystal cells in Drosophila [5]. Semigranular and granular hemocytes are developed through two different lineages in the HPT, and we have identified two proteins that can be used as indicators for these lineages. Cells of the granular hemocyte lineage express a superoxide dismutase (SOD) while the semigranular hemocytes are identified by the expression of a specific Kazal-type proteinase inhibitor [6]. Astakine 1, a homologue to vertebrate prokineticins, was first identified in the freshwater crayfish Pacifastacus leniusculus as a cytokine, necessary for new hemocyte synthesis and release in vivo [7]. Recently, we could show that astakine 1 alone can induce proliferation, differentiation and release of cells of the semigranular lineage, while astakine 2 is involved in granular cell maturation [unpublished]. The underlying mechanism for astakine 1 as a hematopoietic growth factor is now starting to become understood. The protein was originally purified from crayfish plasma for its ability to induce proliferation and differentiation in cultured hematopoietic stem cells (HPT cells) [7]. The expression of PlRunt as well as prophenoloxidase was induced in cultured HPT cells by the addition of astakine 1, showing the role of astakine in hemocyte differentiation. Furthermore, by in vivo gene silencing of astakine 1, hemocyte release from the hematopoietic tissue was blocked [7]. Recently, astakine 1 was shown to induce a decrease in extracellular transglutaminase activity enabling degradation of the extracellular matrix in the hematopoietic tissue and thereby inducing migration of hemocyte precursor cells [8]. With the finding of a second type of astakine in crayfish, invertebrate astakines can fall into two groups: astakine 1 and astakine 2, and astakines are now detected in several arthropod species. The main difference between invertebrate astakines and their vertebrate prokineticin homologues is that all invertebrate astakines lack an AVIT motif in their N-terminal, which is present in all members of the vertebrate prokineticin (PROK) family [9]. The AVIT motif is critical for the interaction of these peptides with their receptors, and hence we have identified a different receptor for astakine 1 on the HPT cells [10]. Prokineticins (PROKs) belong to a family of small secreted proteins of about 80 amino acids originally isolated from the venom of the black mamba, Dendroaspis polylepis in 1980 as the nontoxic MIT1 peptide [11], and later the Bv8 peptide from skin secretions of the frog Bombina variegata [12]. During recent years several different biological activities associated with vertebrate PROKs have been discovered and they influence processes related to development of the nervous system as well as to immunity [13–17]. Vertebrate PROKs are usually highly expressed in inflammatory tissues and have numerous characters in common with cytokines [14], and for example the rodent PROK, Bv8 induce migration of macrophages [17]. Although there are structural differences between astakines and vertebrate PROKs, and their binding properties differ, many functions for these small cysteine rich peptides are conserved throughout evolution.
Recent genetic as well as physiological studies have revealed a function for prokineticins as transmitters of circadian rhythms of the suprachiasmatic nucleus (SCN) in the brain of mammals [18, 19]. Although vertebrate PROKs have been assigned a role as SCN output molecule, nothing is known about their role in regulating rhythmic oscillations in hematopoiesis. Here, we provide the first evidences for an ancient role of astakine in circadian regulation of hematopoietic stem cell activity.
Materials and methods
Animals
Fresh water crayfish, Pacifastacus leniusculus, were purchased from Nils Fors, Torsång at Lake Vättern, Sweden. Healthy intermolt male crayfish were maintained in aerated tap water at 10°C, and animals of the same size were used for hemocyte and HPT sampling.
Sampling times
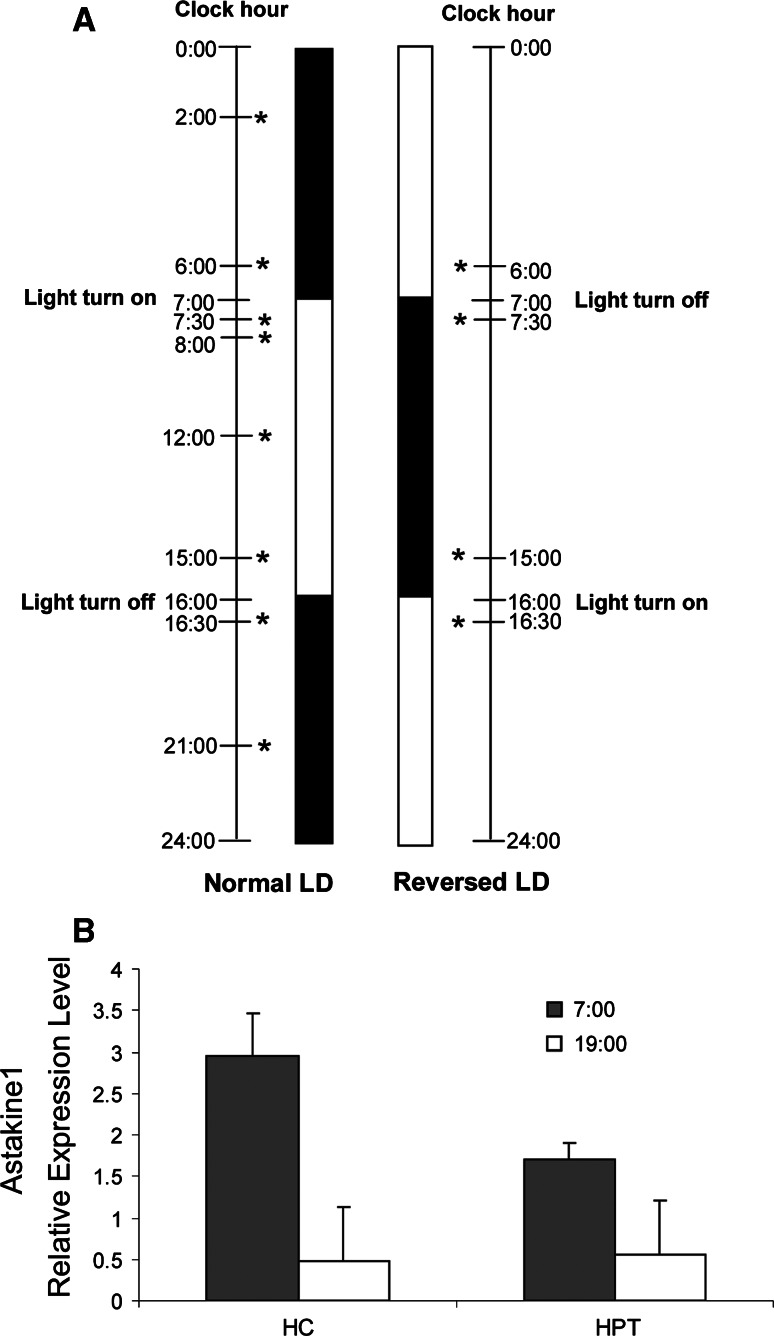
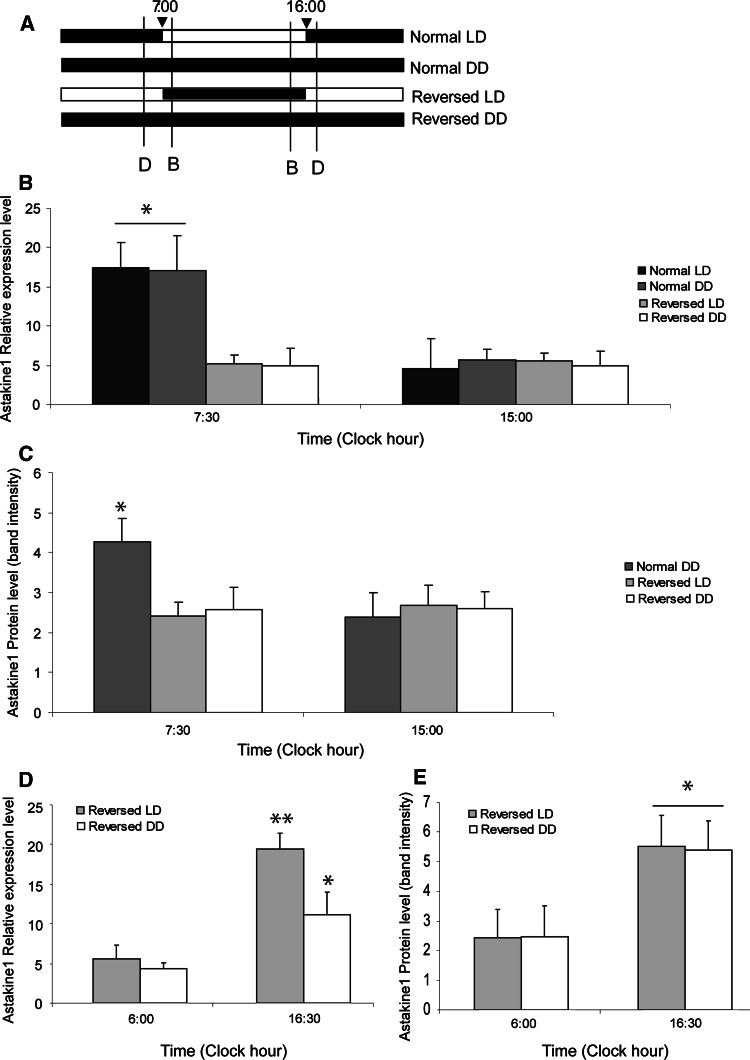
In the first experiment crayfish were held under a light/dark cycle for 12:12 (LD1) for at least 2 weeks prior to hemocyte collection or HPT preparation. This is the light/dark cycle in September. Later in November–January the cycle was changed to 9:15, which in the following text will be referred to as normal light/dark cycle (normal LD). The animals were kept for at least 2 weeks in normal LD conditions prior to hemocyte collection or HPT preparation. The light in the aquarium room was turned on and off at 7:00 and 16:00, respectively. In order to verify whether the LD rhythmic expression is due to an endogenous oscillation, the crayfish were then placed in total darkness for 3 days (normal DD) prior to sampling. Further, to confirm light as a stimuli of the rhythmic expression, the animals were subjected to reverse light conditions (reversed LD; the light cycle shifted by 12 h) for 2 weeks to adjust to a new light regime before new sampling was performed. Finally, reversed LD was followed by total darkness for 3 days (reversed DD). The different light/dark cycles and sampling times used are illustrated in Fig. 1a.
Fig. 1.
Astakine1 expression is light dependent. a Schematic diagram showing the light:dark cycles used in the experiments. The sampling times used for bleeding and dissection are indicated by asterisks. Normal LD 9 h light/15 h dark, 2 weeks adjustment, Normal DD 24 h dark 2 days after normal LD, Reversed LD 9 h dark/15 h light, 2 weeks adjustment, Reversed DD 24 h dark 2 days after Reversed LD. b Astakine 1 is differentially expressed in hemocytes (HC) and HPT at 7:00 in the morning, 30 min after the light turned on (filled square), and at 19:00 in the evening, 30 min after the light turned off (open square) (n = 6) when crayfish are held at 12 h light/12 h dark. Error bars indicate standard error of the mean (SEM)
Astakine mRNA transcription level during different LD and DD conditions
Hemocytes were collected by bleeding, and the hematopoietic tissues (HPT) were dissected as described earlier [4] at 2:00, 6:00, 7:30, 8:00, 12:00, 15:00, 16:30, and 21:00 from at least three animals at each sampling time kept at normal LD conditions. For DD, reversed LD and reversed DD conditions, samplings were made 30 min after light turn on, and 30 or 60 min before light turn off, respectively. All experiments were repeated at least twice. Total RNA (0.5 µg) was extracted from the HPT or hemocytes using GenElute™ Mammalian Total RNA Miniprep kit (Sigma) followed by RNase-Free DNase I (Ambion, Austin, TX, USA) treatment. Complementary DNA was synthesized with ThermoScript (Invitrogen) according to the instructions from the manufacturer. The expression levels of astakine 1 and astakine 2 were determined by quantitative RT-PCR. The mRNA encoding crayfish 40s ribosomal protein gene was used as an internal control. The following specific primers were used; astakine 1 (GenBank accession No. AY787656) forward, 5′-GGC TAT GGC TGG CTC TTG TAA-3′; reverse, 5′-AGG TGG CTG AAG GTC GGG TA-3′; astakine2 (GenBank accession No. EF568370) forward, 5′-ATG GTG TTA GTG TTG ATG TGT GGC-3′; reverse, 5′-GGG ACT CAG CGG AGA TTT TGC-3′; crayfish 40s ribosomal protein gene (GenBank accession No. CF542417) forward, 5′-GAC GAA TGG CAT ACA CCT GAG AGG-3′; reverse, 5′-CAG GAC TCT GCA GTT CAA GCT GAT G-3′. All cDNAs were diluted tenfold in RNase-free sterilized water and SYBR green quantitative RT-PCR amplification was performed in a Rotor-Gene 3000 (Cobett Robotics). The qPCR reactions contained 5 µl of diluted cDNA template, 1× QuantiTect® SYBR Green PCR master mix (QIAGEN) and 5 µM forward and reverse primers in a 25-μl reaction volume. For qPCR standard curve acquisition, four serial dilutions of pooled samples were prepared, and the predicted amplification efficiencies were 99% for both astakine 1 and astakine 2 primers, when amplifying the same sample with R 2 values of 0.998 and 0.994, respectively. The following amplification profile was used: 95°C for 15 min, followed by 45 cycles of 94°C for 15 s, 58°C for 30 s, and 72°C for 30 s. All qPCR reactions were performed in duplicate. Hemocytes from a least three crayfish were used for each time point. All experiments were repeated twice with similar results.
Circulating hemocyte count
Hemolymph samples (100 μl) from ten crayfish at each time point were collected and fixed with 10% formalin (200 μl) in crayfish saline (CFS; 0.2 M NaCl, 5.4 mM KCl, 10 mM CaCl2, 2 mM NaHCO3, pH 6.8). The hemocyte number was counted in a hemocytometer, and for differential hemocyte counts the proportion of granular cells was calculated.
Detection of astakine 1 protein in plasma and hemocyte lysate supernatant (HLS)
Hemolymph was collected at two time points, with highest and lowest astakine 1 mRNA transcription level, by bleeding from the abdominal hemocoel through a needle (0.8 mm) into sterile tubes with anticoagulant on ice followed by centrifugation at 800 × g for 10 min at 4°C to separate hemocytes and plasma. The hemocyte pellet was homogenized in 10 mM sodium cacodylate, 5 mM CaCl2, pH 7.0 and the homogenate was centrifuged at 25,000 × g for 20 min at 4°C and the remaining supernatant was used as HLS. Plasma proteins were precipitated with acetone. For each time point, at least three crayfish were used. The protein concentration in both HLS and plasma was determined with the Coomassie Plus-The Better Bradford Assay Kit (Thermo Scientific). The protein samples (plasma: 100 μg protein; HLS: 10 μg protein) were subjected to 12.5% SDS-PAGE and the amount of astakine was detected by Western blot using PVDF membranes. The membrane was blocked by immersion in 10% skimmed milk solution for 1 h and after washing three times in 1× TBST (10 mM Tris–HCl, pH 7.5, containing 150 mM NaCl and 0.1% Tween 20), astakine 1 was detected using an affinity purified antiastakine 1-antibody made in rabbit in TBST for 1 h as earlier described [7]. As controls for constitutive expression and as loading controls, antibodies for beta-actin (Santa Cruz, 1:2,000) and P. leniusculus beta-1,3-glucanbinding protein (1:500) [20] were used for HLS and plasma, respectively. After washing, the membrane was incubated with peroxidase-linked anti-rabbit-IgG made in donkey (GE Healthcare, 1:7,000) in 1× TBST for 1 h and finally washed with TBST for 3 × 10 min. For detection, the ECL Western blotting reagent kit (Amersham Biosciences) was used according to the manufacturer’s instructions. The intensity of the protein bands was quantified using the Quantity One software (BioRad).
Bacterial infection
A pathogenic Pseudomonas strain, Pseudomonas libanensis/gessardii, which had originally been isolated from the hemolymph or hepatopancreas of crayfish, was cultured overnight in tryptic soy broth (TSB, Merck, Germany) at 22°C. Then, bacteria were washed three times with 0.85% NaCl by centrifugation at 900 × g for 10 min at room temperature. Cell concentrations were adjusted and verified by viable plate counts and were recorded by colony-forming units (CFU) per milliliter. Two groups of ten crayfish were injected in the base of the forth walking leg with P. libanensis/gessardii at an amount of 2.5 × 106 CFU per crayfish. One group was injected in the morning, and the other group in the afternoon. Then they were separately kept at 10–12°C, and the animals were monitored every day after inoculation and deaths were recorded. The survival analysis was performed using a Kaplan–Meier test and Logrank with the MStat 5.3 software.
Statistical analysis
All data from the analysis of relative expression levels and hemocyte counts at different sampling times were examined by one-way ANOVA followed by Duncan’s new multiple range test and Turkey test. When two treatments were compared, a t test was used. Differences were considered statistically significant at p < 0.05. Results are expressed as the mean ± SE.
Results and discussion
Astakine 1, a crustacean prokineticin, is known to induce proliferation and differentiation of hematopoietic stem cells [7] and since prokineticins in vertebrates function as mediators of circadian rhythms we decided to evaluate if there is a circadian regulation of hematopoietic stem cell proliferation and differentiation in crayfish. At first, we analyzed astakine 1 expression in hemocytes, 30 min after dawn and dusk, respectively, in crayfish adjusted to 12 h light/12 h dark cycles (LD1) for at least 2 weeks. The expression analysis revealed an apparent higher mRNA level after the dark period followed by decreasing expression during the light period (Fig. 1b). In the hematopoietic tissue (HPT), the level of astakine1 expression is lower as compared to hemocytes, but also in this tissue higher expression level was revealed in the morning (Fig. 1b).
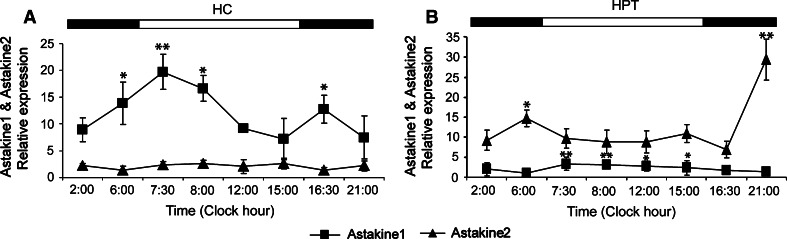
To determine in more detail a possible circadian rhythm in astakine 1 expression new animals were adjusted to 9 h light/15 h dark (normal LD) for 3 weeks and hemocyte samples were collected at six time points during the light:dark cycle as described in Fig. 1a. Since feeding has been found to influence rhythmic proliferation in neurogenic clusters in the crayfish brain [10], crayfish were not fed during the sampling period (24 h). The relative expression level of astakine 1 as analyzed by real-time PCR showed a clear rhythmicity, with highest mRNA level at the period immediately after dawn, i.e., after a long dark period (Fig. 2a, b). At the time before dusk astakine 1 showed its lowest expression in hemocytes.
Fig. 2.
Relative astakine 1 and astakine 2 mRNA expression show circadian rhythmicity. a Time course in relative expression levels of astakine 1 and astakine 2 in hemocytes (HC) at clock hour 2:00, 6:00, 7:30, 8:00, 12:00, 15:00, 16:30, and 21:00 (n = 6). b Time course in relative expression levels of astakine 1 and astakine 2 in HPT at clock hour 2:00, 6:00, 7:30, 8:00, 12:00, 15:00, 16:30, and 21:00 (n = 6). Asterisks indicate that the expression levels are significantly different (*p < 0.05, **p < 0.01). Light (open square) and dark (filled square) periods in the cycle are indicated on the top of the graph. Error bars indicate standard error of the mean (SEM)
The expression levels of astakine 2 is five to ten times lower in hemocytes as compared to astakine 1, and no significant rhythmic expression was found for hemocyte astakine 2 (Fig. 2a). In contrast, astakine 2 mRNA in HPT showed rhythmic expression with maximum level at 21:00 (Fig. 2b) in the dark phase. Moreover, the expression level of astakine 1 in hemocytes is 5–10 times higher than levels of astakine 2 (Fig. 2a) whereas the opposite relation is found in the HPT (Fig. 2b).
Photoperiodic rhythmicity has been shown to influence several processes in freshwater crayfish and these animals have served as models for studies in circadian locomotion activity [21–23]. Astacid crayfish are known to have their main locomotion activity restrained to the dark period, and usually their activity increases immediately when the light turns off, and then an activity peak is often found immediately before dawn [21, 23]. The expression of astakine 1 mRNA follows a similar pattern with very low expression before the light turns off, and then a rapid increase followed by a slightly lower expression during the night and then peak levels at dawn (Fig. 2a).
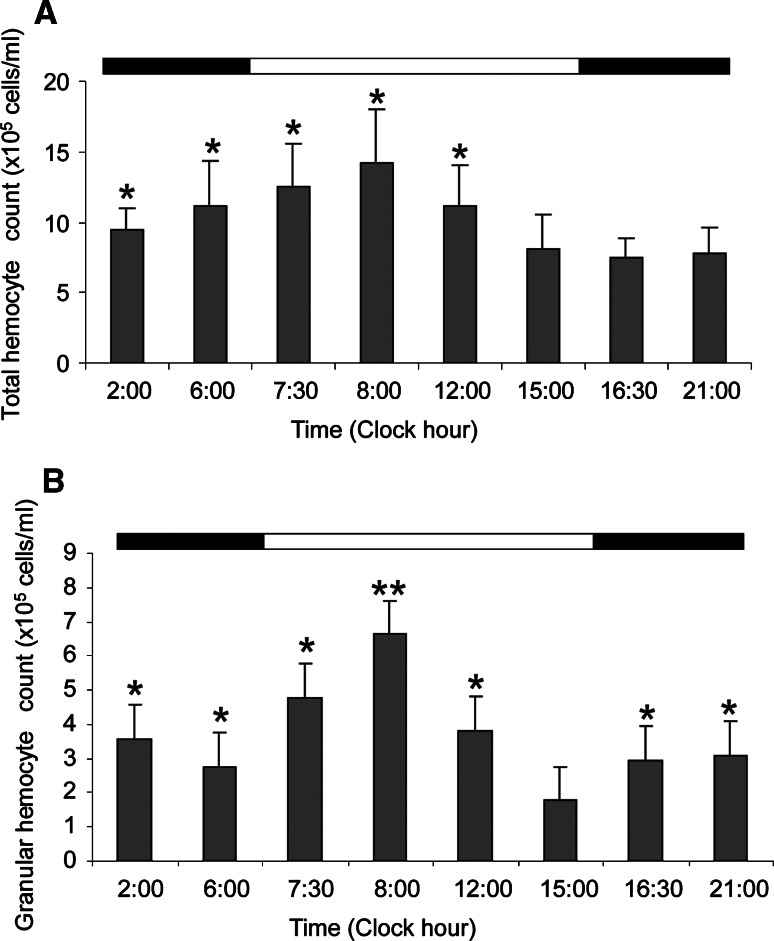
Astakine 1 expression in the HPT is lower than in hemocytes, but it also shows rhythmicity although these values also reflect the release of new hemocytes to the circulation (Fig. 2b). For example, the low astakine 1 expression at 6:00 is mirrored by high number of hemocytes found at 6:00–8:00 (Fig. 3a).
Fig. 3.
Total number of circulating hemocytes and number of granular hemocytes varies during a daily cycle. a Number of hemocytes in the hemolymph at different time points during the day (n = 10). b Number of granular hemocytes (GC) in the hemolymph at different time points (n = 4). Asterisks indicate that numbers are significantly different (*p < 0.05, **p < 0.01). The horizontal band on top of this histogram indicates illumination conditions (white:light/black:dark). Error bars indicate standard error of the mean (SEM)
Since both astakines are involved in the hematopoietic process, their oscillating expression levels would be reflected by daily oscillations of the total hemocyte count (THC) at each experimental time point. Interestingly, the numbers of hemocytes showed a pattern similar to astakine 1 mRNA levels. The numbers were slightly higher at clock hour 2:00 and continuously increased thereafter with a peak at 8:00 (Fig. 3a). Afterwards, THC decreased during the light period, and began to increase again after midnight. The granular hemocyte count (GC) showed an oscillation pattern similar to THC, with a peak at 8:00 before a significant decrease at 15:00 (Fig. 3b).
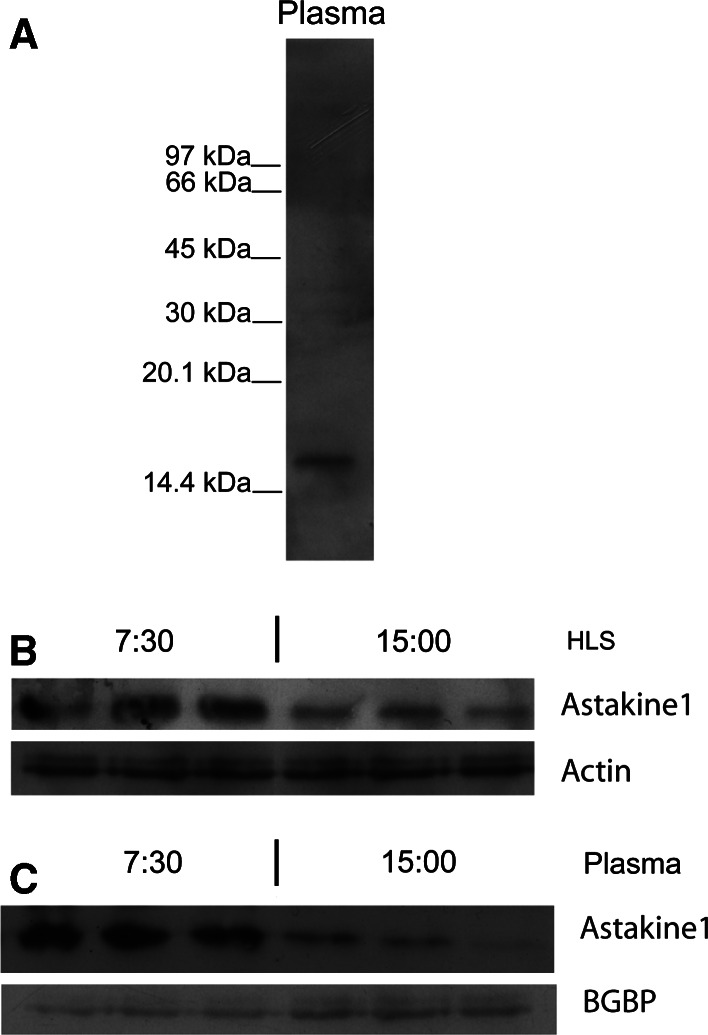
Crustacean astakine 1 is produced by hemocytes and released into the plasma. Increased plasma levels of astakine1 are found shortly after challenge of the animal with microbial polysaccharides, and before the recruitment of hemocytes from the hematopoietic tissue [7]. If the hematopoietic process is under circadian control by diurnal astakine 1 transcript levels, the protein level should have the same expression pattern like its mRNA transcription. In order to clarify if this is the case, we analyzed plasma levels by Western blot by using a specific antibody to astakine1 (Fig. 4a) [7] at the time points when astakine 1 showed the highest and the lowest transcription levels, at clock hour 7:30 and 15:00, respectively. The plasma content of astakine 1 protein at 7:30 was found to be more than double as compared to the levels at 15.00, and in some individuals hardly any astakine 1 was detected in plasma at the time before dusk. Also, in hemocyte lysates, a significant difference in astakine 1 amount was detected (Fig. 4b, c). Thus, the rhythm in protein level coincides and correlates exactly with the mRNA levels. Furthermore, astakine1 showed a significant peak in protein level at the beginning of the light period, exactly when the peak in THC was found as mentioned above (Fig. 3a).
Fig. 4.
Astakine 1 levels in plasma and a hemocyte lysate (HLS) follow the daily rhythm in mRNA expression. a Western blot of a crude plasma sample showing specificity of the astakine 1 antibody. The molecular mass of marker proteins is indicated on the left. b Western-blot analysis of astakine1 in HLS at clock hour 7:30 and 15:00. An antibody against beta-actin was used as a control for specific down-regulation of astakine 1 and as loading control. c Western-blot analysis of astakine1 in plasma at clock hour 7:30 and 15:00. An antibody against P. leniusculus ΒGBP was used as a control for specific down-regulation of astakine 1 and as loading control
In order to determine whether the circadian rhythmic expression pattern of astakine 1 is entrained by an endogenous biological rhythm, animals that had been held in normal LD for 2 weeks were placed in total darkness for 3 days (normal DD), followed by an assessment of astakine 1 mRNA expression levels. A summary of the light:dark regimes and sampling times used in the following experiments is shown in Fig. 5a. As shown in Fig. 5b, the rhythm is endogenous, and the daily changes in astakine 1 transcript levels were observed in normal DD in a similar as in normal LD. Our first results using LD conditions of 12 h light/12 h dark (Fig. 1b) indicated that light is the external stimulus for the endogenous oscillator controlling astakine expression pattern, since after 2 weeks adjustment, the rhythm is changed according to the new external light conditions (Fig. 2a, b). To further confirm that light is responsible as stimuli of the rhythmic astakine expression, we exposed the animals to a reverse light:dark cycle (reversed LD) for 2 weeks, and then analyzed astakine 1 expression after 2 weeks adjustment. As shown in Fig. 5b, d, high astakine 1 expression levels were now phase-shifted so that high expression level was detected 30 min after the light turned on at 16:30 (Fig. 5d), although at 15:00 the expression level was low, which was maybe due to that in this reverse regime the dark period is short (9 h, Fig. 5a). Again, the endogenous nature of this rhythm was confirmed by reversed DD treatment for 2 days, showing similar expression pattern as reversed LD (Fig. 5b, d). Furthermore, the protein level of astakine 1 in plasma followed a similar pattern as the expression of astakine 1 mRNA (Fig. 5c, e).
Fig. 5.
Astakine1 mRNA expression in hemocytes and protein levels in plasma at dusk and dawn during normal LD, normal DD, reverse LD and reverse DD, respectively. a Schematic diagram showing the light:dark regimes used in the experiments. Black box indicates dark, and light box indicates light. The sampling times used in Fig. 5b and d are indicated by lines labeled B and D, respectively. b Relative expression levels of astakine 1 in hemocytes at clock hour 7:30 and 15:00 during normal LD, normal DD, reversed LD, and reversed DD, respectively (n = 3). c Western-blot analysis of astakine1 in plasma at clock hour 7:30 and 15:00, and Quantity One analysis of membranes during normal DD, reversed LD, and reversed DD, respectively (n = 3). d Relative expression levels of astakine 1 in hemocytes at clock hour 6:00 and16:30 during reversed LD and reversed DD, respectively (n = 3). e Western-blot analysis of astakine1 in plasma at clock hour 6:00 and 16:30, and Quantity One analysis of membranes during reversed LD and reversed DD, respectively (n = 3) Asterisks indicate that the expression levels are significantly different (*p < 0.05, **p < 0.01)
In mammals, there is increasing evidence that circadian regulatory proteins modulate stem cell activities, and circadian oscillations in proliferation and recruitment of hematopoietic progenitor cells have been demonstrated [1, 2]. Our results show for the first time in an invertebrate circadian control of hematopoiesis by the cytokine astakine 1. Earlier studies have revealed circadian control of cell proliferation in neuronal proliferation in the crayfish brain [21], and interestingly neuronal survival and proliferation show a similar circadian pattern as the hematopoietic process in our investigation. Our finding that diurnal expression occurs for astakine 1, a prokineticin homologue, illustrates an ancient function of these small secreted peptides as mediator of circadian rhythms across phylogenetically diverse species.
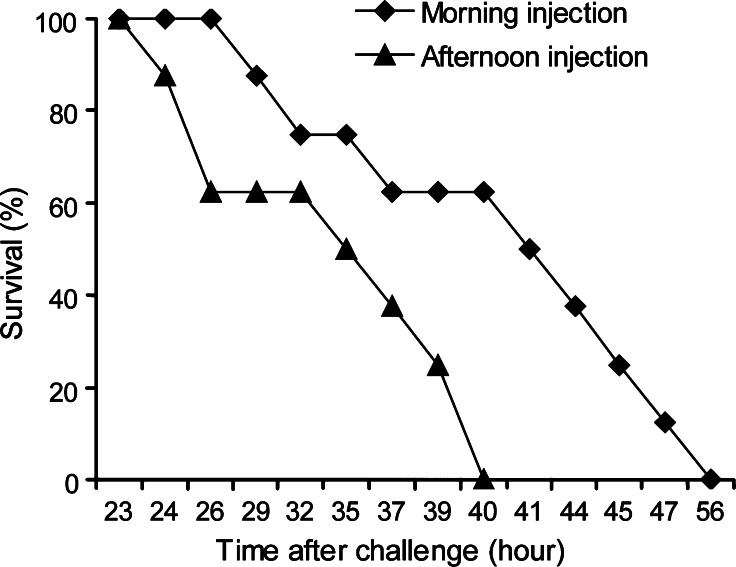
Circadian rhythmic variation of the number of blood cells may have important implication in immune defense. Invertebrate innate immunity is a growing research field of investigations, partly due to its importance for applied aquaculture of crustacean animals. During the recent decade, considerable effort has been put into gaining insight into pathogen-induced gene expression in crustaceans, mainly in hemocytes, as they have been shown to be important immune cells. It is possible that important information about immunity has been overlooked by neglecting natural circadian variation in hemocyte number and synthesis. Since astakines, the prokineticin domain containing crustacean cytokines, here are shown to exhibit circadian oscillations in their transcript levels and thereby influencing the number of circulating hemocytes, we decided to determine if this has an impact on animal health. Accordingly, we used infection of crayfish with the crayfish pathogenic P. libanensis/gessardii previously isolated from P. leniusculus [24]. In order to determine if the circadian rhythmic astakine 1 expression and hematopoiesis also modulates the innate immune system and the ability of the animals to combat a bacterial infection. Healthy crayfish adjusted to the 9-h light/15-h dark cycles for at least 2 weeks were injected before the lowest level of astakine expression at dusk (clock hour 15:00) and 30 min before dawn at the peak of expression. Cumulative mortality was recorded and as shown in Fig. 6. Significantly longer survival time was recorded for animals injected in the morning when THC and astakine 1 expression levels were high. A Kaplan–Meier test showed median survival time of 42.5 and 36 h for morning and afternoon infection, respectively, with 99% CI. Lee and Edery [3] detected circadian regulation of the defense of Drosophila melanogaster to bacterial infection. Similarly to our present results, the survival rates of the flies were clearly affected by the time of the day for infection [3]. Thus, these experiments indicate that circadian rhythms have to be considered when designing experiments to elucidate innate immune responses in invertebrate animals.
Fig. 6.
Time of infection of crayfish with P. libanensis/gessardii has an impact on survival rate. Survival rate (%) after injection of a pathogenic P. libanensis/gessardii into crayfish hemocoel in the morning (squares) or in the afternoon (triangles). A Kaplan–Meier test showed median survival time of 42.5 and 36 h for morning and afternoon infection, respectively, with 99% CI, with a p < 0.01 for survival rate after morning infection being higher than after afternoon infection, estimated by Logrank using MStat5.3
Core circadian regulatory proteins (CCRPs), such as Clock and BMAL1, have been identified and these are conserved throughout the evolution from plants and insects to mammals [1, 2]. It is now evident that CCRPs modulate stem cell proliferation and differentiation in mammals, and apart from being expressed in the central clock of the human brain (the SCN), transcripts of these genes have been detected in peripheral tissues such as the bone marrow [1]. However, the mechanism determining rhythmic variations in bone marrow activity is not clarified, although speculations about that the SCN transmit signals through the β-adrenergic system to control hematopoiesis in the bone marrow have been published [25]. Prokineticin 2 has recently been shown to be an important output molecule from mouse SCN and it is rhythmically expressed [4, 5]. Moreover, prokineticins are known to influence proliferation and differentiation of hematopoietic progenitors in mammals [26]. In the present work, we present evidence for a similar circadian expression for an invertebrate PROK homologue, and thus this molecule may be an ancient regulator of circadian stem cell activity.
Astakine 1 differs in amino acid sequence from its vertebrate homologues by lack of an AVIT motif in its N-terminal. This motif has been shown to be important for PROKs binding to their receptor proteins. So far, all invertebrate astakines lack the AVIT motif and accordingly different receptor proteins are likely. We have recently shown that astakine 1 can bind to ATP-synthase beta subunits present on the surface of a portion of HPT cells [10]. Binding of astakine 1 also affects the enzyme activity of this protein, resulting in lower ATP synthase activity. ATP is known to be a neural transmitter and relocates signals between cells in the mammalian brain. Interestingly, ATP levels in the SCN cells of rats have recently been shown to fluctuate in a circadian way, and therefore may represent a physiological output molecule [27]. Thus, our results of changes in astakine 1 protein levels in plasma urge more detailed studies of ATP levels in the crustacean hematopoietic tissue.
Conclusions
Our findings suggest that the hematopoietic process in invertebrates is under circadian control and that the ancient cytokine astakine mediates this circadian rhythm. These findings highlight the importance of examining and comparing immune responses between individuals at fixed times in order not to overlook important dynamic regulations of the innate immune system.
Moreover, our results entail an evolutionary conserved function for prokineticin domain containing proteins as mediating circadian rhythms, and for the first time show a role for this domain in circadian regulation of stem cell proliferation and differentiation that may have implications also in vertebrates.
Acknowledgments
This work was financed by the Swedish Science Research Council and Formas.
Abbreviations
- HLS
Hemocyte lysate supernatant
- HPT
Hematopoietic tissue
- PROK
Prokineticin
- SCN
Suprachiasmatic nucleus
References
- 1.Gimble JM, Floyd ZE, Bunnell BA. The 4th dimension and adult stem cells: can timing be everything? J Cell Biochem. 2009;107:569–578. doi: 10.1002/jcb.22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Méndez-Ferrer S, Chow A, Merad M, Frenette PS. Circadian rhythms influence hematopoietic stem cells. Curr Opin Hematol. 2009;16:235–242. doi: 10.1097/MOH.0b013e32832bd0f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JE, Edery I. Circadian regulation in the ability of Drosophila to combat pathogenic infections. Curr Biol. 2009;18:195–199. doi: 10.1016/j.cub.2007.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Söderhäll I, Bangyeekhun E, Mayo S, Söderhäll K. Hemocyte production and maturation in an invertebrate animal; proliferation and gene expression in hematopoietic stem cells of Pacifastacus leniusculus Dev. Comp Immunol. 2003;27:661–672. doi: 10.1016/S0145-305X(03)00039-9. [DOI] [PubMed] [Google Scholar]
- 5.Lebetsky T, Chang T, Hartenstein V, Banerjee U. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science. 2000;288:146–149. doi: 10.1126/science.288.5463.146. [DOI] [PubMed] [Google Scholar]
- 6.Wu C, Kim YA, Liu HP, Söderhäll I, Söderhäll K. Hemocyte lineage marker proteins in a crustacean, the freshwater crayfish Pacifastacus leniusculus . Proteomics. 2008;8:4226–4235. doi: 10.1002/pmic.200800177. [DOI] [PubMed] [Google Scholar]
- 7.Söderhäll I, Kim YA, Jiravanichpaisal P, Lee SY, Söderhäll K. An ancient role for a prokineticin domain in invertebrate hematopoiesis. J Immunol. 2005;174:6153–6160. doi: 10.4049/jimmunol.174.10.6153. [DOI] [PubMed] [Google Scholar]
- 8.Lin X, Söderhäll K, Söderhäll I. Transglutaminase activity in the hematopoietic tissue of a crustacean, Pacifastacus leniusculus, important in hemocyte homeostasis? BMC Immunol. 2008;9:58. doi: 10.1186/1471-2172-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaser A, Winklmayr M, Lepperdinger G, Kreil G. The AVIT protein family. Secreted cysteine-rich vertebrate proteins with diverse functions. EMBO Rep. 2003;4:469–473. doi: 10.1038/sj.embor.embor830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin X, Kim YA, Lee BL, Söderhäll K, Söderhäll I. Isolation and properties of a receptor for the invertebrate cytokine astakine, involved in hematopoiesis. Exp Cell Res. 2009;315:1171–1180. doi: 10.1016/j.yexcr.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Boisbouvier J, Albrand JP, Blackledge M, Jaquinod M, Schweitz H, Lazdunski M, Marion D. A structural homologue of colipase in black mamba venom revealed by NMR floating disulphide bridge analysis. J Mol Biol. 1998;283:205–219. doi: 10.1006/jmbi.1998.2057. [DOI] [PubMed] [Google Scholar]
- 12.Mollay C, Wechselberger C, Mignogna G, Negri L, Melchiorri P, Barra D, Kreil G. Bv8, a small protein from frog skin and its homolog from snake venom induce hyperalgesia in rats. Eur J Pharmacol. 1999;374:189–196. doi: 10.1016/S0014-2999(99)00229-0. [DOI] [PubMed] [Google Scholar]
- 13.Negri L, Lattanzi R, Giannini E, Canestrelli M, Nicotra A, Melchiorri P. Bv8/Prokineticins and their receptors: a new pronociceptive system. Int Rev Neurol. 2009;85:145–157. doi: 10.1016/S0074-7742(09)85011-3. [DOI] [PubMed] [Google Scholar]
- 14.Monnier J, Samson M. Cytokine properties of prokineticins. FEBS J. 2008;275:4014–4020. doi: 10.1111/j.1742-4658.2008.06559.x. [DOI] [PubMed] [Google Scholar]
- 15.Dorsch M, Qui Y, Soler D, Frank N, Duong T, Goodearl A, O′Neil S, Lora J, Fraser CC. PK1/EG-VEGF induces monocyte differentiation and activation. J Leukoc Biol. 2005;78:426–434. doi: 10.1189/jlb.0205061. [DOI] [PubMed] [Google Scholar]
- 16.Monnier J, Quillien V, Piquet-Pellore C, Leberre C, Preisser L, Gascan H, Samson M. Prokineticin 1 induces CCL4, CXCL1 and CXCL8 in human monocytes but not in macrophages and dendritic cells. Eur Cytokine Netw. 2008;19:166–175. doi: 10.1684/ecn.2008.0138. [DOI] [PubMed] [Google Scholar]
- 17.Martucci C, Franchi S, Giannini E, Tian H, Melchiorri P, Negri L, Sacerdote P. Bv8, the amphibian homologue of the mammalian prokineticins induces a proinflammatory phenotype of mouse macrophages. Br J Pharmacol. 2006;147:225–234. doi: 10.1038/sj.bjp.0706467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou OY, Cheng MY. Prokineticin 2 and circadian clock output. FEBS J. 2005;272:5703–5709. doi: 10.1111/j.1742-4658.2005.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C, Truong KK, Zhou QY. Efferent projections of prokineticin 2 expressing neurons in the mouse suprachiasmatic nucleus. PLoS ONE. 2009;4:e7151. doi: 10.1371/journal.pone.0007151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerenius L, Liang Z, Duvic B, Keyser P, Hellman U, Palva ET, Iwanaga S, Söderhäll K. Structure and biological activity of a 1, 3-beta-d-glucan-binding protein in crustacean blood. J Biol Chem. 1994;25:29462–29467. [PubMed] [Google Scholar]
- 21.Goergen EM, Bagay LA, Rehm K, Benton JL, Beltz BS. Circadian control of neurogenesis. Int J Neurobiol. 2002;53:90–95. doi: 10.1002/neu.10095. [DOI] [PubMed] [Google Scholar]
- 22.Castanon-Cervantes O, Battelle B, Fanjul-Moles ML. Rhythmic changes in serotonin content of the brain and eyestalk of crayfish during development. J Exp Biol. 1999;202:2823–2830. doi: 10.1242/jeb.202.20.2823. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan JM, Genco MC, Marlow ED, Benton JL, Beltz BS, Sandeman DC. Brain photoreceptor pathways contributing to circadian rhythmicity in crayfish. Chronobiol Int. 2009;26:1136–1168. doi: 10.3109/07420520903217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiravanichpaisal P, Roos S, Edsman L, Liu H, Söderhäll K. A highly virulent pathogen, Aeromonas hydrophila, from the freshwater crayfish Pacifastacus leniusculus . J Invertebr Pathol. 2009;101:56–66. doi: 10.1016/j.jip.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoitic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 26.Le Couter J, Zlot C, Tejada M, Peale F, Ferrara N. Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization. Proc Natl Acad Sci USA. 2004;101:16813–16818. doi: 10.1073/pnas.0407697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Womac AD, Burkeen JF, Neuendorff N, Earnest DJ, Zoran MJ. Circadian rhythms of extracellular ATP accumulation in suprachiasmatic nucleus cells and cultured astrocytes. Eur J Neurosci. 2009;30:869–876. doi: 10.1111/j.1460-9568.2009.06874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]