Abstract
Long-term potentiation (LTP) defines persistent increases in neurotransmission strength at synapses that are triggered by specific patterns of neuronal activity. LTP, the most widely accepted molecular model for learning, is best characterised at glutamatergic synapses on dendritic spines. In this context, LTP involves increases in dendritic spine size and the insertion of glutamate receptors into the post-synaptic spine membrane, which together boost post-synaptic responsiveness to neurotransmitters. In dendrites, the material required for LTP is sourced from an organelle termed the endosomal-recycling compartment (ERC), which is localised to the base of dendritic spines. When LTP is induced, material derived from the recycling compartment, which contains α-amino-3-hydroxy-5-methyl-4-isoxazole propionate-type glutamate receptors (AMPARs), is mobilised into dendritic spines feeding the increased need for receptors and membrane at the spine neck and head. In this review, we discuss the importance of endosomal-recycling and the role of key proteins which control these processes in the context of LTP.
Keywords: AMPAR, Endosomal-recycling, FIP2, LTP, Myosin V, NMDAR
Background
The ability of biological organisms to acquire and retain new knowledge, skills and behaviours is commonly referred to as learning. Memories can be retained for minutes, days, weeks, months or years depending on the strength and persistence of the memory-forming stimuli involved. The exact mechanisms by which this information is retained remains somewhat mysterious. However, the widely accepted view is that synapses are major sites of information retention in the central nervous system and that changes in synaptic strength between neurons, known as synaptic plasticity, can account for the formation of new memories [1]. A significant amount of work has been undertaken to determine how neuronal cells respond to memory-inducing stimuli and execute the cellular changes that are necessary for the formation and retention of these stimuli. This review focuses on the intracellular membrane trafficking events that have been implicated in long-term potentiation (LTP) with a particular emphasis on the functions of N-methyl-d-aspartate-type glutamate receptors (NMDARs) and α-amino-3-hydroxy-5-methyl-4-isoxazole (AMPARs) and the role of endosomal-recycling in these processes.
Synaptic plasticity and long-term potentiation
The most extensively studied forms of synaptic plasticity are LTP and long-term depression (LTD). Whilst several forms of LTP/LTD exist, we focus here almost exclusively on a post-synaptic form of LTP that is dependent on the activation of NMDARs for its induction, and on the trafficking of AMPARs for its expression.
Neuronal dendrites are branched projections which protrude from the cell body of neurons and act to receive and conduct the electro-chemical stimulation from the presynapse of other neurons. Each dendrite has multiple dendritic spines which are micron-sized membranous protrusions that are major sites of excitatory synapse formation in many types of neurons. NMDARs and AMPARs are glutamate-gated cation channels that localise to the plasma membrane of neuronal dendrites and dendritic spines and mediate a large proportion of excitatory neurotransmission in the brain [2]. Whilst both receptor types are permeable to Na+ and K+, it is the permeability of NMDARs to Ca2+ that is of particular importance during LTP. This is due to the ability of Ca2+ to couple electrical-to-biochemical signalling through protein binding and conformational alteration of protein structure [2]. LTP-inducing stimuli release the Mg2+-mediated voltage-dependent block of the NMDAR, which allows these channels to open and results in an influx of cations, including Ca2+, into the cell [3]. Elevated Ca2+ levels within dendrites and dendritic spines play a major role in LTP-induction [3, 4]. From an intracellular signalling perspective, the influx of Ca2+ is considered to trigger a number of events that ultimately lead to the delivery of AMPARs and membrane, from intracellular stores, to the dendritic spine-limiting membrane with a consequential increase in synaptic strength.
Whilst the activation of NMDARs is thought to trigger cellular changes that ultimately result in the expression of LTP, it is the trafficking of AMPARs that is believed to play the major role in the increased sensitivity at the post-synapse that is associated with the formation and retention of new memories [5]. AMPARs are heterotetrameric proteins composed of combinations of four subunits: GluR1, GluR2, GluR3 and GluR4 [2]. Functional AMPARs in the hippocampus are primarily GluR2/GluR1 or GluR2/GluR3 heterotetramers [6]. The various AMPAR subunits undergo both constitutive cycling to and from the spine membrane (primarily mediated by the GluR2-subunit) and also regulated or activity-dependent trafficking leading to the expression of LTP (primarily mediated by the GluR1-subunit) [7, 8]. The trafficking of these subunits will be discussed in further detail later.
Whilst a change in glutamate receptor-mediated neurotransmission is a defining feature of LTP/LTD, a second important and closely related factor is structural plasticity of synapses. Structural plasticity has predominantly been studied in the context of dendritic spines, where the enlargement and retraction of single spines has recently been observed in association with LTP and LTD, respectively [9–12]. The dynamic actin network in spines is likely to drive plasticity-associated structural changes, but the insertion/removal of membrane is also required to modulate spine size and volume. Membrane trafficking is thus critical to changes in both spine morphology and the glutamate receptor-targeting that are associated with LTP/LTD.
The endosomal system
Endocytosis and exocytosis are important cellular processes which allow cells to internalise and sort material from the extracellular environment, and to export intracellular material to the plasma membrane or extracellular space [13]. Mammalian cells are highly compartmentalised and require a complex system of endosomal membranous compartments and pathways to facilitate intracellular transport. The Rab family of small GTPases are key regulators of membrane trafficking between intracellular compartments [14]. Rab proteins function as molecular switches and, when active, are anchored at the cytoplasmic face of membranous organelles where they direct membrane trafficking events, such as vesicle formation, motility, docking and fusion [14]. For example, Rab5 directs the initial internalisation of receptors from the plasma membrane and their delivery to peripheral early/sorting endosomes. From here, internalised material can be returned directly to the cell surface via the Rab4-dependent ‘fast’ recycling pathway, or recycled indirectly through the Rab11-mediated ‘slow’ pathway to the plasma membrane via the endosomal-recycling compartment (ERC). Alternatively, material can be delivered to late endosomes and subsequently to lysosomes for degradation, via the Rab7-mediated pathway [14]. Rab11 subfamily members (Rab11a, Rab11b and Rab11c/Rab25) are the principal Rabs currently known to be involved in regulating the trafficking of receptors back to the cell surface via the ERC [15–17].
Role of membrane trafficking in synaptic plasticity
Two models have been presented for the increase in synaptic strength induced by LTP-stimuli: lateral movement of receptors from dendritic membrane surfaces into synapses, and delivery of material from intracellular membranous compartments to the post-synaptic membrane [18–21]. Recent data now suggest that a more accurate model for the increased AMPAR trafficking to the post-synaptic membrane may involve a combination of both these individual models [22–26]. We will first discuss the trafficking machinery proposed to be involved in the movement of receptors through the endosomal system and then examine how these two models can be linked together to ensure appropriate delivery of AMPARs to the synaptic site or the post-synaptic density (PSD).
Due to their physical size and organisation, the spatial distribution of the endosomal-system differs in neurons from that of most other cell types. Like most cell types, neuronal cells have a central transferrin-positive ERC that is localised close to the nucleus in the cell body and surrounds the minus-end of microtubules at the microtubule-organising centre (MTOC) or centrosome [27]. This tubulovesicular organelle is characterised principally by its peri-nuclear/peri-centrosomal localisation and by the presence of transferrin, the transferrin receptor, Rab11 GTPases and Rab11-effector proteins. Additionally, neuronal cells also have smaller transferrin/Rab11-positive ERCs that are positioned at the base of dendritic spines [28–30]. It is likely that these structures are also organised around the minus-end poles of microtubules given the 50/50 reversed polarity of microtubules observed in dendrites at a distance of more than 75 μm from the cell body [31]. We propose to name these compartments recycling-outposts (ROs) and will use this terminology henceforth in this review. We view these ROs in an analogous manner to that of the previously described ‘secretory-outposts’ in neuronal cells [32]. Thus, the ROs are likely to function as a local cargo reservoir and recycling-system in dendritic spines and allow for the rapid transport of material to spine heads. This specialised endosomal organisation in neurons most likely reflects their complexity and the spatial distance between the neuronal cell body and its terminal ends. The local transport from these neuronal ROs into dendritic spines is recognised as a central component in the increased trafficking of AMPARs and membrane addition to spines that are associated with LTP [19, 20, 30].
Prominent among the proteins that control trafficking through the ERC/ROs are the Rab11 GTPase subfamily and their downstream effector machinery [15, 16, 33]. Indeed, Rab11 along with its effectors, FIP2 and Myosin Vb, have been implicated in the mobilisation and insertion of AMPARs, and membrane-derived from ROs, into dendritic spines [1, 20, 34].
Endosomal-recycling and LTP
As previously outlined, dendritic spines are a major site of glutamatergic synaptic stimulation. The formation of new dendritic spines, as well as their growth in hippocampal neurons, is critical for synaptic plasticity and LTP [35, 36].
The localisation of ROs to the base of dendritic spines plays a key role in maintaining spine viability, growth and expansion [19]. Indeed, overexpression of dominant-negative mutants of Rab11a, RME-1 or syntaxin 13, which block receptor-recycling from ROs, markedly reduces the number of dendritic protrusions in hippocampal neurons [19]. On the other hand, overexpression of their wild-type counterparts increases the number of dendritic protrusions [19]. Increased spine size and number, during NMDAR-activated LTP, is also perturbed by inhibition of trafficking through the use of mutant proteins known to inhibit endosomal-recycling from the ERC/ROs [19].
During non-stimulatory conditions, ROs are primarily localised to the base of dendritic spines [28–30]. Chemical stimulation of LTP results in the dynamic mobilisation of RO-derived vesicles from the base of spines into the neck and head [19]. The movement of RO-derived material into the dendritic spine results in vesicle fusion at the post-synaptic membrane correlating with an increase in the average spine surface area facilitating spine growth and expansion [19]. Thus, the trafficking of RO-derived material from the spine base to the neck and head is a crucial component of NMDAR activation-dependent LTP [19].
Trafficking of AMPARs into dendritic spines is crucial for LTP
LTP is mediated by an increase in sensitivity of post-synaptic neurons to the neurotransmitter glutamate [34]. Increased AMPAR activity at this location mediates this increased sensitivity. Indeed, several studies have shown that prior to LTP-stimulation, AMPARs are absent from post-synaptic membranes, and at normal resting membrane potential in CA-1 pyramidal neurons, AMPARs do not generate a synaptic response upon neurotransmitter release [24, 37, 38]. However, upon LTP-stimulation during continuous NMDAR activation, AMPAR excitatory post-synaptic currents (EPSC) appear and persist [37]. This suggests that a large proportion of synapses in hippocampal neurons do not contain detectable AMPARs in the absence of LTP-stimulation, and that upon stimulation, AMPARs are mobilised towards the post-synaptic membrane. Stimulation of hippocampal neurons transiently overexpressing GFP-fused GluR1, a subunit of the AMPAR, results in the redistribution of GluR1 to dendritic spines and leads to receptor clustering in the dendritic shaft [24]. In addition, this redistribution of GluR1 is dependent on the activation of the NMDAR, suggesting that increased synaptic plasticity requires cross-talk between NMDARs and AMPARs.
Many receptors are continuously internalised from the cell surface (endocytosed) and subsequently returned (recycled) to the plasma membrane. The AMPAR undergoes continuous internalisation from and reinsertion to the post-synaptic membrane via the Rab4-directed pathway [7, 29, 39]. In hippocampal neurons, internalised AMPARs initially enter early/sorting endosomes in response to agonist stimulation (AMPA or NMDA) [29]. However, from early/sorting endosomes, they are targeted to distinct pathways depending on the persistence of agonist stimulation. AMPA-stimulation drives the transport of AMPARs towards late endosomes and thereby lysosomes for degradation [29]. On the other hand, NMDA-stimulation targets receptors towards ROs for delivery back to the post-synaptic membrane [29]. During LTP-stimulation, there is increased shuttling of AMPARs to ROs with subsequent recycling into the spine. These data illustrate that NMDAR-activation acts as a trigger to direct AMPARs back to the plasma membrane by stimulating their transport to the recycling pathway rather than the degradative pathway.
The role of the class V myosins and FIP2 in LTP
Until recently, the mechanisms by which NMDAR-activation triggers the movement of RO-derived material into dendritic spines remained unclear. The mechanochemical forces necessary for such intracellular motility are typically performed by molecular motor proteins such as dyneins/kinesins and myosins. These motor proteins interact with and progressively move along the microtubule or actin cytoskeleton, respectively. F-actin is the most abundant cytoskeletal protein present in dendritic spines suggesting that an actin-based motor protein is involved in trafficking processes at these locations [40]. Indeed, it has become clear that the class V myosins play an important role in the formation, growth and maintenance of dendritic spines, an essential feature of LTP [20, 41].
The myosin V family are actin-based motor proteins involved in cell polarity and the trafficking of cellular material by interacting with microfilaments and moving towards their barbed ends [42]. Vertebrates have three class V myosins: Myosin Va (MyoVa), Myosin Vb (MyoVb) and Myosin Vc (MyoVc). MyoVa is preferentially expressed in neurons, neuroendocrine cells and melanocytes, whilst MyoVb and MyoVc are ubiquitously expressed [42]. The class V myosins are structurally similar, consisting of two heavy chains, which contain amino-terminal motor domains that have binding sites for both ATP and actin [43]. These domains are used by the protein to ‘walk’ along microfilaments [43]. The neck region contains an essential light chain and a calmodulin-binding domain which mediates conformational changes due to fluctuating intracellular Ca2+ levels [43]. Finally, class V myosins contain a tail region which is split into an extensive coiled-coil domain and a globular-tail domain that binds cargo [42–44].
MyoVb, Rab11 and FIP2 in LTP
MyoVb displays increased expression in the hippocampus and localises to dendritic spines and the ERC/ROs [20, 45]. Chemical induction of LTP increases MyoVb localisation to ROs and subsequent movement of MyoVb into dendritic spines [20]. These processes are in turn blocked using NMDAR antagonists or by removing extracellular Ca2+ [20]. This indicates that the increase in intracellular Ca2+ levels, resulting from NMDAR activation, is responsible for the association of MyoVb with ROs, and for the movement of RO-derived material into dendritic spines [20]. Short-hairpin RNA (shRNA) targeting of MyoVb results in a significant decrease in spine number and growth in chemically stimulated hippocampal neurons [46]. This effect is linked to a significant decline in recycling from ROs in dendritic spines [4, 47].
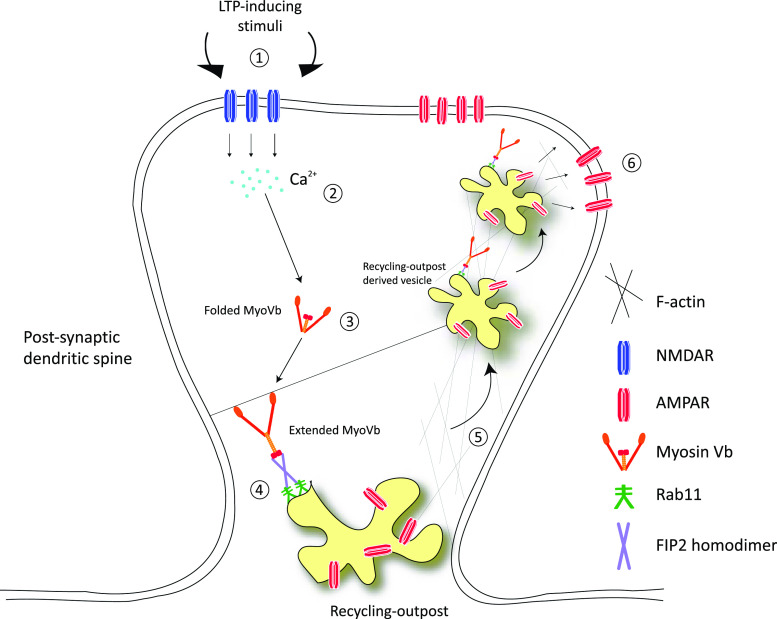
It is well established that LTP-inducing stimuli dramatically increases Ca2+ levels in dendritic spines as a result of NMDAR activation [20, 48, 49]. The class V myosins are dynamically regulated by fluxes in free intracellular Ca2+ concentrations [20, 48]. Indeed, studies on MyoVa and MyoVb have shown that, at high Ca2+ concentrations, these molecular motors adopt an extended conformation as opposed to a compacted/folded structure occurring at low Ca2+ levels (Fig. 1) [20]. The ATPase activity of MyoVb is also enhanced in response to increased concentrations of Ca2+ [20].
Fig. 1.
Model for MyoVb-dependent trafficking of recycling outpost-derived material in the post-synaptic dendritic spine during LTP-stimulation. 1 LTP-inducing stimuli at the post-synaptic dendritic spine results in the opening of NMDARs. 2 NMDAR opening results in a rapid increase in intracellular Ca2+ levels. 3 Increased intracellular Ca2+ levels stimulate an alteration of MyoVb structure, causing it to adopt its extended conformation. 4 MyoVb is recruited to the recycling-outpost (RO) where its globular-tail domain interacts with the Rab11/FIP2-complex present on the RO membrane. 5 MyoVb, tethered to RO-derived vesicles, progressively moves along actin microfilaments, and as such, these vesicles are transported to the spine neck and head. 6 The RO moves into the head and neck regions of the dendritic spine, eventually fusing with the spine membrane providing the cargo materials (membrane and AMPARs) that are required for LTP
MyoVb-dependent trafficking from ROs is mediated via interaction with Rab11 and FIP2. MyoVb binds both Rab11 and FIP2 directly and these three proteins are capable of forming a ternary complex [50, 51]. The association of the Rab11/FIP2 complex with MyoVb is believed to tether RO-derived vesicles to the actin cytoskeleton and facilitate their transport into dendritic spines (Fig. 1) [20]. Interestingly, a mutant of MyoVb that cannot interact with FIP2 shows only slight localisation to ROs and dramatically limits the motility of vesicles that emanate from these compartments [20]. On the other hand, point mutations in the MyoVb which favour the extended conformation increase co-localisation between MyoVb and ROs [20]. These data indicate that the physical association between Rab11/FIP2 and MyoVb is essential for RO-derived vesicular traffic in the post-synaptic dendritic spine during LTP stimulation.
The interaction between MyoVb and FIP2 is enhanced with increasing intracellular Ca2+ concentrations [20]. Indeed, a MyoVb mutant locked in the extended conformation (MyoVb-CCtr) strongly binds FIP2 even under low Ca2+ conditions, and also stimulates increased motility of RO-derived vesicles into dendritic spines [20]. However, this increased motility does not occur in cells expressing a mutant of MyoVb which is deficient in Rab11/FIP2-binding (MyoVb-ΔRBD). This strengthens the hypothesis that the FIP2 association with MyoVb is essential for RO-sourced trafficking into dendritic spines [20]. Indeed, NMDAR activation in response to LTP-inducing stimuli results in increased co-localisation between FIP2 and MyoVb at ROs, and this co-localisation is blocked by the expression of MyoVb-ΔRBD [20]. These data imply that the increased intracellular Ca2+ levels due to activation of NMDARs during LTP-induction directly influence the association of MyoVb with Rab11/FIP2. This is required for the trafficking of RO-derived vesicles into dendritic spines leading to their fusion with, and thus incorporation into, the spine-limiting membrane (Fig. 1).
AMPAR trafficking into post-synaptic membranes, which results in increased sensitivity to neurotransmitters, is dramatically reduced by MyoVb RNAi [20]. This finding is supported by reversal of the MyoVb knockdown phenotype with various MyoVb constructs further illustrating the importance of the formation of a MyoVb/FIP2/Rab11 ternary complex in these processes [20]. Together, this work demonstrates that both MyoVb and FIP2 are necessary to traffic AMPARs from ROs to the spine membrane (Fig. 1).
MyoVa in LTP
MyoVa is highly expressed in brain tissue and plays a role in LTP and AMPAR trafficking at the post-synaptic membrane [41, 52]. MyoVa RNAi and dominant-negative mutant studies have demonstrated that loss of MyoVa function affects NMDAR and AMPAR-mediated EPSCs [41]. The importance of MyoVa in LTP has been further demonstrated in hippocampal neurons in which loss of its function significantly decreases AMPAR-mediated EPSCs, which has been linked to the inhibition of GluR1 delivery to the post-synaptic membrane [41]. MyoVa trafficking of GluR1-positive AMPAR occurs locally from the dendritic shaft to the head and is not involved in the long-range transport of the receptor subunit along dendrites. Unlike the role proposed for MyoVb, MyoVa does not alter dendritic spine morphology [41]. Indeed, no increase in spine length or number has been observed in MyoVa loss-of-function studies which suggests that MyoVa does not play a role in spine maintenance in hippocampal neurons and that its function in LTP may be restricted to AMPAR-subunit transport into the spine head.
The role of Rab11 in the control of endosomal-recycling in epithelial cells has been well documented [15–17]. As Rab11 co-precipitates with the AMPAR, and the globular tail of MyoVa directly binds the GluR1 subunit of the AMPAR, it is likely that a link exists between Rab11 and MyoVa-dependent trafficking of AMPARs [41]. Overexpression of a dominant-negative mutant of MyoVa reduces the spine accumulation of Rab11 suggesting that MyoVa affects the trafficking from ROs into dendritic spines [41]. The active form of Rab11 (GTP-bound) enhances the association between MyoVa and GluR1 suggesting that Rab11 is an essential regulator of MyoVa-dependent trafficking of GluR1 into the dendritic spine [41]. Alternative studies suggest that MyoVa is not involved in LTP [53]. This study examined dilute lethal mice that carry a recessive functional null mutation in the MyoVa gene found that these mice have normal LTP. This study also concluded that presynaptic vesicle trafficking does not require MyoVa [53].
The seemingly contradictory findings between different groups with regard to the roles of membrane-trafficking, Rab GTPases and class V myosins in LTP are difficult to reconcile at present. Differences in experimental systems may account for some of the discrepancies. Experiments in brain slices that retain largely normal neuronal circuitry are clearly more physiological than those in dissociated neuronal cultures. Differences in methods used to induce LTP are also pertinent. It is unlikely that stimuli such as chemical induction of LTP in cultured neurons, expression of constitutively active Ca2+/calmodulin-dependent protein kinase (CaMKII) and electrophysiological induction of LTP in brain slices are completely equivalent in terms of the intracellular signalling pathways that are activated. Expression and activation of distinct complements of trafficking and accessory proteins in different experimental contexts may thus explain some of the contradictory results. In addition, studies in this area have relied heavily on experimental approaches such as the overexpression of dominant-negative constructs that have inherent limitations. Indeed, the specificity of some MyoVa dominant-negative constructs has been questioned [20]. Findings using dominant-negative approaches need to be confirmed using loss of function approaches, and in many cases this has been done. Gene knockout of the various players may ultimately be needed to tease apart their significance in vivo. Inducible knockout or inactivation approaches are preferable to avoid the possibility of compensatory changes in trafficking machinery. Such changes could account for the finding that MyoVa was not required for LTP induction in dilute-lethal mice. The strategy used by Wang et al. [20] to chemically inactivate MyoVb in an acute manner may point the way towards avoiding such confounding issues. However, knock-in of sensitised mutant versions of myosins may be preferable to transgenic overexpression of such mutants in the continued presence of the wild-type protein. The recently described ability to monitor AMPA receptor incorporation into synapses of animals that have performed a learning task would indicate that the tools to get definitive answers regarding trafficking mechanisms in vivo are now becoming available [54].
Rab8 in AMPAR trafficking
As outlined above, Rab11 is critical for LTP. In addition, recent work has suggested that other Rab GTPases may serve important roles in AMPAR trafficking for the expression of LTP. An initial study of Rab function at the post-synaptic terminal indicated that Rab8, but not other Rabs, significantly depressed AMPAR-mediated currents in organotypic hippocampal slice cultures [55]. Indeed, Rab8 is abundant in post-synaptic termini and accumulates in intracellular compartments in proximity to the synapse of the post-synaptic membrane [55]. Rab8 does not influence the global transport of AMPARs into spines, but it does impair the delivery of GluR2 to the surface of the spine [55]. This suggests that Rab8 may be involved in the trafficking of AMPAR subunits from an intracellular membranous compartment, within the spine, to the spine surface. Interestingly, Rab8 appears to regulate both the constitutive (activity-independent) trafficking and NMDAR/CamKII-stimulated (activity-dependent) trafficking of AMPARs which involve both the secretory-outposts and ROs [55]. Whilst Rab8 can regulate the trafficking of both GluR1 and GluR2, Rab11 appears to alter the trafficking of GluR1 alone, which suggests that Rab11 may only be involved in the activity-dependent AMPAR trafficking pathway which is most associated with NMDAR-driven LTP. Rab8 has previously been localised to both the Golgi and the ERC [56, 57]. In epithelial cells, Rab8 is thought to primarily function in the exocytic pathway to the plasma membrane, and is believed to perform this function by first directing exocytic traffic to the recycling-compartment [58]. It is likely that Rab8 functions on Golgi-outposts that are present in close proximity to ROs and participates in the shuttling of GluR2 both directly to the post-synaptic membrane and also indirectly via ROs during the constitutive trafficking of AMPARs.
Subsequent work implicates both Rab11 and Rab8 in the trafficking of the GluR1 [39]. Indeed, overexpression of dominant-negative mutants of Rab8 and Rab11 fully block activity-dependent LTP, which suggests that these Rabs may function on a common AMPAR-trafficking route to the post-synaptic membrane [39]. The increased trafficking of GluR1 is a defining feature of the activity-dependent trafficking of AMPARs that is associated with NMDAR-driven LTP [5]. Furthermore, overexpression of dominant-negative Rab8 results in increased localisation of GluR1 to dendritic spines indicating that Rab8 and Rab11 perform separate anatomical steps during activity-dependent LTP. This implies that Rab8 and Rab11 function in a sequential manner to mediate delivery of AMPARs to the post-synaptic membrane [39]. Rab8 interacts directly with the MyoVb and MyoVc motor proteins [59]. Rab8 and Rab11 both interact with an overlapping region of the globular-tail domain of MyoVb (also via FIP2 in the case of Rab11), which reinforces the hypothesis that these proteins may define different stages on a common trafficking pathway with the sequential transfer of the MyoVb protein between these Rabs [59].
The model for a co-operative ‘handover’ of AMPAR containing vesicles from Rab11 to Rab8 is reinforced by the model of Rab11/Rab8 cooperation in docking and fusion of vesicles to the base of cilia during primary ciliogenesis [60, 61]. Along with MyoVb, Rab8 function is linked to Rab11 through a common interaction with Rabin8, a guanine nucleotide exchange factor (GEF) for Rab8 which stimulates the GTP-loading of the Rab protein [61, 62]. On the other hand, Rabin8 acts as a Rab11 effector protein, and Rab11 binding to the a region flanking the GEF-domain of Rabin8 significantly increases its GEF activity on Rab8 [61]. Thus, Rab11-dependent trafficking of AMPARs to the spine may involve a ‘handover’ to Rab8 within the spine which is mediated though an interaction with Rabin8 and MyoVb. The translocation of Rabin8 into the spine could stimulate the activation of Rab8 and a possible transfer of the MyoVb from Rab11 for subsequent AMPAR-containing vesicle transport, docking and fusion.
The role of trafficking to peri-synaptic sites in LTP
AMPARs and the membrane required for increased spine volume are sourced from an intracellular ROs located at the base of dendritic spines. Two schools of thought exist on the mechanisms of receptor insertion at the synaptic site. The first argues for a direct delivery of receptors to the synaptic site from intracellular compartments [19, 30, 63, 64]. The second suggests that AMPARs are first delivered to non-synaptic sites on the membrane and then translocate via lateral diffusion through the membrane to the synaptic site/PSD [7, 26]. As discussed below, we are of the opinion that both models have value and may in fact be compatible.
As previously mentioned, functional AMPARs are composed of individual subunits (GluR1, GluR2, GluR3 and GluR4). The GluR2 subunit cycles constitutively between intracellular compartments and the synaptic site [7, 8]. The trafficking of this subunit is not influenced by NMDAR-activation and, as such, is not believed to be the primary AMPAR subunit involved in LTP [7]. On the other hand, the trafficking of GluR1 increases significantly upon NMDAR-activation, and it is thought that this subunit is the ‘driving force’ in the expression of LTP [7]. Studying one or other of the AMPAR-subunits individually in terms of LTP is fundamentally flawed, as functional AMPARs in the hippocampus require a combination of subunits to form a functional heterotetrameric receptor (although some reports suggest a minority population of GluR1 homomers exist in the hippocampus [6]). Indeed, separate temporal and spatial patterns of AMPAR-subunit trafficking exist. GluR1 is first inserted into non-synaptic regions of the neuronal surface in the first 5 min after stimulation, whilst GluR2 accumulates more rapidly at synaptic sites following stimulation [7]. This is either the result of GluR2 being inserted directly into the PSD (or in close proximity) or a more rapid diffusion from extra-synaptic sites to the PSD [7].
Several recent studies have now shown that GluR1 is first delivered to an extra-synaptic site and moves to the synaptic site via lateral diffusion during the induction of LTP [7, 26, 65, 66]. Indeed, recent and comprehensive electrophysiology studies, utilising theta-burst pairing in CA-1 hippocampal slices have also demonstrated a temporal separation between LTP-inducing stimuli and the full expression of LTP [26]. What is unclear regarding AMPAR trafficking is whether the GluR2/GluR1 or GluR2/GluR3 subunits composing functional heterotetramers are endocytosed/recycled/delivered to the membrane as functional AMPARs or whether individual subunits are transported and combine at the PSD to form the functional AMPAR. The different modes of transport for GFP-GluR1 and GFP-GluR2 would seem to suggest the latter model. In this case, the distinct subunits could combine at the synapse to form a functional AMPAR facilitating the increased sensitivity associated with LTP.
As outlined previously, LTP-stimuli result in NMDAR-activation and an influx of calcium which ultimately results in the association of a MyoVb/Rab11/FIP2 complex that drives the movement of vesicles from recycling-endosomes towards the synaptic site. Interestingly, intracellular Ca2+ levels has also been shown to regulate lateral diffusion of AMPAR subunits [18]. Indeed, it is known that increased intracellular Ca2+ reduces lateral diffusion of GluR2 that can result in decreased lateral movement of GluR2 from the synaptic site [18]. This reduction in GluR2 removal from the PSD coupled with increased trafficking of GluR1 to the synapse during activity-dependent LTP could result in increased numbers of both receptor types at the synapse. In this model, the increased intracellular Ca2+ concentrations associated with NMDAR activation serves two distinct purposes: to increase trafficking of GluR1 to the synapse, and also to decrease lateral diffusion and subsequent recycling of GluR2 from the synaptic site. The latter function of Ca2+ has been linked to the regulation of the GluR2 and N-ethylmaleimide Sensitive Fusion protein (NSF)/Protein Interacting with C-Kinase-1 (PICK1). At intracellular calcium levels of approximately 15 μm/L, an interaction between GluR2 and PICK1 is favoured, and there is consequent lateral diffusion of GluR2 from the PSD [65]. However, the interaction between GluR2 is biphasic and, at higher levels of intracellular Ca2+ (such as those associated with the induction of LTP), the GluR2:PICK1 interaction is disrupted by NSF, resulting in inhibition of diffusion of GluR2 away from the synaptic site [67].
Removal and endocytosis of AMPAR from the synaptic site
The trafficking of AMPARs to the peri-synapse and PSD is critical for the expression of LTP. However, maintaining functional AMPARs at the PSD, by slowing their rate of endocytosis, may be of significant importance for the expression of LTP. Indeed, the supply of AMPARs available to enter synapses is dependent on their rates of exocytosis and endocytosis. The removal of AMPARs from the synapse is not believed to occur by clathrin-mediated endocytosis at the PSD but rather by lateral diffusion away from the synaptic site [18, 21]. Dendritic spines contain specialised areas flanking, but spatially distinct from, the PSD which are characterised by the enduring presence of clathrin [66]. These areas are termed endocytic zones (EZs), or ‘endocytic hotspots’, and act to trap laterally-diffusing AMPARs for endocytosis [68]. This results in their recycling back to the plasma membrane via endocytic compartments such as ROs or their shuttling to late endosomes and subsequently to lysosomes for degradation. Indeed, it has recently been demonstrated that the displacement of these EZs from the region flanking the PSD, utilising a dynamin-3 mutant, results in a significant reduction in synaptic AMPARs [25]. This is due to the blockade of the mobile pool of AMPARs that is constitutively recycling to and from the PSD via lateral diffusion and endocytosis [25]. Thus, blocking normal endocytosis reduces the number of AMPARs that are available for exocytosis back to the PSD from the intracellular recycling compartments.
Future directions
As we have outlined above, a complex repertoire of proteins have already been implicated in endosomal trafficking during the induction of LTP. However, given the complexity of the endosomal-recycling system, it is likely that the full extent of the protein machinery involved in these processes has yet to be elucidated. Indeed, initial studies have revealed that another small GTPase, Rab10, may also play a functional role in the trafficking of AMPARs [69]. Rab10 is known to regulate the trafficking of GLR-1, one of two AMPAR-type subunits found in Caenorhabditis elegans [69]. Interestingly, Rab10 has now also been identified as an interacting partner for all three isoforms of MyoV [70]. To our knowledge, there has yet to be an in-depth study on the role of Rab10 in LTP in mammalian cells. However, it seems likely that this early work will translate into interesting findings in the future.
It is now clear that the class V myosins play a crucial role in receptor trafficking in dendritic spines. Over the years, the number of interacting proteins for these actin-binding motors has been steadily increasing. Future proteomics screens with MyoV proteins may reveal further protein trafficking machinery that could play a crucial role in the further understanding of the transport processes involved in LTP expression in dendritic spines. Similarly, whilst a role has been proposed for the Rab GTPases outlined above, the possibility that many other Rabs and indeed their effector proteins are involved cannot be ruled out. For example, FIP2, a protein which plays a crucial role in the mediation of Rab11 function during LTP expression, has recently been shown to interact with a second endosomal Rab GTPase, Rab14 [71]. This Rab has a similar subcellular distribution to Rab11; however, little is known about it with respect to its function or indeed its interaction with FIP proteins. A possible role for Rab14/FIP2 in receptor trafficking at dendritic spines should not be overlooked.
Conclusion
The cellular and molecular mechanisms which underlie the formation and retention of memories in the brain are one of the great mysteries of modern neuroscience. We now understand that the increase in synaptic strength between two neurons is mediated, at least in part, through increased dendritic spine size and increased AMPAR numbers at the spine membrane. Here, we have discussed how NMDAR-activation leads to increased trafficking from ROs into dendritic spines and how these processes contribute to the delivery of the cargo required for the expression of LTP in neuronal cells. It is clear that endosomal-recycling plays a crucial part in the generation of LTP; however, clarification of the cellular and molecular details of these processes is still in its infancy and much further investigation is required if we are to fully understand the complete role of membrane trafficking processes in memory formation.
Acknowledgments
The authors are grateful to Sara Hanscom for useful discussion regarding the manuscript. This work was supported by a Science Foundation Ireland Investigator Grant (05/IN.3/B859) and a Science Foundation Ireland Research Frontiers Grant (08-RFP-NSC1499) to M. McCaffrey.
Abbreviations
- AMPAR
α-Amino-3-hydroxy-5-methyl-4-isoxazole propionate-type glutamate receptor
- EZs
Endocytic zones
- ERC
Endosomal-recycling compartment
- EPSC
Excitatory post-synaptic current
- GEF
Guanine nucleotide exchange factor
- LTP
Long-term potentiation
- LTD
Long-term depression
- MTOC
Microtubule-organising centre
- MyoV
Myosin V
- NSF
N-Ethylmaleimide sensitive fusion protein
- NMDAR
N-Methyl-d-aspartate-type glutamate receptor
- PSD
Post-synaptic density
- PICK1
Protein interacting with C-Kinase-1
- FIP
Rab11-family interacting protein
- RO
Recycling-outpost
- shRNA
Short-hairpin RNA
References
- 1.Malinow R. AMPA receptor trafficking and long-term potentiation. Philos Trans R Soc Lond B. 2003;358:707–714. doi: 10.1098/rstb.2002.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 3.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 4.Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983;305:719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- 5.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 6.Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- 8.Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/S0092-8674(01)00321-X. [DOI] [PubMed] [Google Scholar]
- 9.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 11.Nagerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 14.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 15.Schlierf B, Fey GH, Hauber J, Hocke GM, Rosorius O. Rab11b is essential for recycling of transferrin to the plasma membrane. Exp Cell Res. 2000;259:257–265. doi: 10.1006/excr.2000.4947. [DOI] [PubMed] [Google Scholar]
- 16.Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horgan CP, Hanscom SR, Jolly RS, Futter CE, McCaffrey MW. Rab11-FIP3 links the Rab11 GTPase and cytoplasmic dynein to mediate transport to the endosomal-recycling compartment. J Cell Sci. 2010;123:181–191. doi: 10.1242/jcs.052670. [DOI] [PubMed] [Google Scholar]
- 18.Borgdorff AJ, Choquet D. Regulation of AMPA receptor lateral movements. Nature. 2002;417:649–653. doi: 10.1038/nature00780. [DOI] [PubMed] [Google Scholar]
- 19.Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Edwards JG, Riley N, Provance DW, Jr, Karcher R, Li XD, Davison I, Ikebe M, Mercer JA, Kauer JA, Ehlers MD. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008;135:535–548. doi: 10.1016/j.cell.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tardin C, Cognet L, Bats C, Lounis B, Choquet D. Direct imaging of lateral movements of AMPA receptors inside synapses. EMBO J. 2003;22:4656–4665. doi: 10.1093/emboj/cdg463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 24.Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 25.Petrini EM, Lu J, Cognet L, Lounis B, Ehlers MD, Choquet D. Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation. Neuron. 2009;63:92–105. doi: 10.1016/j.neuron.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Wang XB, Frerking M, Zhou Q. Delivery of AMPA receptors to perisynaptic sites precedes the full expression of long-term potentiation. Proc Natl Acad Sci USA. 2008;105:11388–11393. doi: 10.1073/pnas.0802978105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemar A, Olivo JC, Williamson E, Saffrich R, Dotti CG. Dendroaxonal transcytosis of transferrin in cultured hippocampal and sympathetic neurons. J Neurosci. 1997;17:9026–9034. doi: 10.1523/JNEUROSCI.17-23-09026.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooney JR, Hurlburt JL, Selig DK, Harris KM, Fiala JC. Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. J Neurosci. 2002;22:2215–2224. doi: 10.1523/JNEUROSCI.22-06-02215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/S0896-6273(00)00129-X. [DOI] [PubMed] [Google Scholar]
- 30.Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- 31.Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci USA. 1988;85:8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanus C, Ehlers MD. Secretory outposts for the local processing of membrane cargo in neuronal dendrites. Traffic. 2008;9:1437–1445. doi: 10.1111/j.1600-0854.2008.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horgan CP, McCaffrey MW. The dynamic Rab11-FIPs. Biochem Soc Trans. 2009;37:1032–1036. doi: 10.1042/BST0371032. [DOI] [PubMed] [Google Scholar]
- 34.Kauer JA, Malenka RC, Nicoll RA. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron. 1988;1:911–917. doi: 10.1016/0896-6273(88)90148-1. [DOI] [PubMed] [Google Scholar]
- 35.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 36.Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- 37.Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 38.Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 39.Brown TC, Correia SS, Petrok CN, Esteban JA. Functional compartmentalization of endosomal trafficking for the synaptic delivery of AMPA receptors during long-term potentiation. J Neurosci. 2007;27:13311–13315. doi: 10.1523/JNEUROSCI.4258-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Correia SS, Bassani S, Brown TC, Lise MF, Backos DS, El-Husseini A, Passafaro M, Esteban JA. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat Neurosci. 2008;11:457–466. doi: 10.1038/nn2063. [DOI] [PubMed] [Google Scholar]
- 42.Desnos C, Huet S, Darchen F. ‘Should I stay or should I go?’: myosin V function in organelle trafficking. Biol Cell. 2007;99:411–423. doi: 10.1042/BC20070021. [DOI] [PubMed] [Google Scholar]
- 43.Reck-Peterson SL, Provance DW, Jr, Mooseker MS, Mercer JA. Class V myosins. Biochim Biophys Acta. 2000;1496:36–51. doi: 10.1016/S0167-4889(00)00007-0. [DOI] [PubMed] [Google Scholar]
- 44.Sellers JR, Veigel C. Walking with myosin V. Curr Opin Cell Biol. 2006;18:68–73. doi: 10.1016/j.ceb.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 45.Zhao LP, Koslovsky JS, Reinhard J, Bahler M, Witt AE, Provance DW, Jr, Mercer JA. Cloning and characterization of myr 6, an unconventional myosin of the dilute/myosin-V family. Proc Natl Acad Sci USA. 1996;93:10826–10831. doi: 10.1073/pnas.93.20.10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang F, Thirumurugan K, Stafford WF, Hammer JA, 3rd, Knight PJ, Sellers JR. Regulated conformation of myosin V. J Biol Chem. 2004;279:2333–2336. doi: 10.1074/jbc.C300488200. [DOI] [PubMed] [Google Scholar]
- 47.Malenka RC, Kauer JA, Zucker RS, Nicoll RA. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988;242:81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- 48.Li XD, Mabuchi K, Ikebe R, Ikebe M. Ca2+-induced activation of ATPase activity of myosin Va is accompanied with a large conformational change. Biochem Biophys Res Commun. 2004;315:538–545. doi: 10.1016/j.bbrc.2004.01.084. [DOI] [PubMed] [Google Scholar]
- 49.Nascimento AA, Cheney RE, Tauhata SB, Larson RE, Mooseker MS. Enzymatic characterization and functional domain mapping of brain myosin-V. J Biol Chem. 1996;271:17561–17569. doi: 10.1074/jbc.271.29.17561. [DOI] [PubMed] [Google Scholar]
- 50.Hales CM, Vaerman JP, Goldenring JR. Rab11 family interacting protein 2 associates with Myosin Vb and regulates plasma membrane recycling. J Biol Chem. 2002;277:50415–50421. doi: 10.1074/jbc.M209270200. [DOI] [PubMed] [Google Scholar]
- 51.Jagoe WN, Lindsay AJ, Read RJ, McCoy AJ, McCaffrey MW, Khan AR. Crystal structure of rab11 in complex with rab11 family interacting protein 2. Structure. 2006;14:1273–1283. doi: 10.1016/j.str.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 52.Mercer JA, Seperack PK, Strobel MC, Copeland NG, Jenkins NA. Novel myosin heavy chain encoded by murine dilute coat colour locus. Nature. 1991;349:709–713. doi: 10.1038/349709a0. [DOI] [PubMed] [Google Scholar]
- 53.Schnell E, Nicoll RA. Hippocampal synaptic transmission and plasticity are preserved in myosin Va mutant mice. J Neurophysiol. 2001;85:1498–1501. doi: 10.1152/jn.2001.85.4.1498. [DOI] [PubMed] [Google Scholar]
- 54.Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319:1104–1107. doi: 10.1126/science.1149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerges NZ, Backos DS, Esteban JA. Local control of AMPA receptor trafficking at the postsynaptic terminal by a small GTPase of the Rab family. J Biol Chem. 2004;279:43870–43878. doi: 10.1074/jbc.M404982200. [DOI] [PubMed] [Google Scholar]
- 56.Huber LA, Pimplikar S, Parton RG, Virta H, Zerial M, Simons K. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J Cell Biol. 1993;123:35–45. doi: 10.1083/jcb.123.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hattula K, Furuhjelm J, Tikkanen J, Tanhuanpaa K, Laakkonen P, Peranen J. Characterization of the Rab8-specific membrane traffic route linked to protrusion formation. J Cell Sci. 2006;119:4866–4877. doi: 10.1242/jcs.03275. [DOI] [PubMed] [Google Scholar]
- 58.Henry L, Sheff DR. Rab8 regulates basolateral secretory, but not recycling, traffic at the recycling endosome. Mol Biol Cell. 2008;19:2059–2068. doi: 10.1091/mbc.E07-09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roland JT, Kenworthy AK, Peranen J, Caplan S, Goldenring JR. Myosin Vb interacts with Rab8a on a tubular network containing EHD1 and EHD3. Mol Biol Cell. 2007;18:2828–2837. doi: 10.1091/mbc.E07-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 61.Knodler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, Guo W. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci USA. 2010;107:6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hattula K, Furuhjelm J, Arffman A, Peranen J. A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol Biol Cell. 2002;13:3268–3280. doi: 10.1091/mbc.E02-03-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gerges NZ, Backos DS, Rupasinghe CN, Spaller MR, Esteban JA. Dual role of the exocyst in AMPA receptor targeting and insertion into the postsynaptic membrane. EMBO J. 2006;25:1623–1634. doi: 10.1038/sj.emboj.7601065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanley JG, Henley JM. PICK1 is a calcium-sensor for NMDA-induced AMPA receptor trafficking. EMBO J. 2005;24:3266–3278. doi: 10.1038/sj.emboj.7600801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Racz B, Blanpied TA, Ehlers MD, Weinberg RJ. Lateral organization of endocytic machinery in dendritic spines. Nat Neurosci. 2004;7:917–918. doi: 10.1038/nn1303. [DOI] [PubMed] [Google Scholar]
- 67.Hanley JG. Molecular mechanisms for regulation of AMPAR trafficking by PICK1. Biochem Soc Trans. 2006;34:931–935. doi: 10.1042/BST0340931. [DOI] [PubMed] [Google Scholar]
- 68.Blanpied TA, Scott DB, Ehlers MD. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 2002;36:435–449. doi: 10.1016/S0896-6273(02)00979-0. [DOI] [PubMed] [Google Scholar]
- 69.Glodowski DR, Chen CC, Schaefer H, Grant BD, Rongo C. RAB-10 regulates glutamate receptor recycling in a cholesterol-dependent endocytosis pathway. Mol Biol Cell. 2007;18:4387–4396. doi: 10.1091/mbc.E07-05-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roland JT, Lapierre LA, Goldenring JR. Alternative splicing in class V myosins determines association with Rab10. J Biol Chem. 2009;284:1213–1223. doi: 10.1074/jbc.M805957200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kelly EE, Horgan CP, Adams C, Patzer TM, Ni Shuilleabhain DM, Norman JC, McCaffrey MW. Class I Rab11-family interacting proteins are binding targets for the Rab14 GTPase. Biol Cell. 2009;102:51–62. doi: 10.1042/BC20090068. [DOI] [PubMed] [Google Scholar]