Abstract
Leukocyte trafficking from the bloodstream to inflamed tissues across the endothelial barrier is an essential response in innate immunity. Leukocyte adhesion, locomotion, and diapedesis induce signaling in endothelial cells and this is accompanied by a profound reorganization of the endothelial cell surfaces that is only starting to be unveiled. Here we review the current knowledge on the leukocyte-mediated alterations of endothelial membrane dynamics and their role in promoting leukocyte extravasation. The formation of protein- and lipid-mediated cell adhesion nanodomains at the endothelial apical surface, the extension of micrometric apical membrane docking structures, which are derived from microvilli and embrace adhered leukocytes, as well as the vesicle-trafficking pathways that are required for efficient leukocyte diapedesis, are discussed. The coordination between these different endothelial membrane-remodeling events probably provides the road map for transmigrating leukocytes to find exit points in the vessel wall, in a context of severe mechanical and inflammatory stress. A better understanding of how vascular endothelial cells respond to immune cell adhesion should enable new therapeutic strategies to be developed that can abrogate uncontrolled leukocyte extravasation in inflammatory diseases.
Keywords: Diapedesis, Tetraspanins, Microvilli, Docking structures, Transcellular, Caveolae, PECAM-1, ICAM-1
Introduction
Endothelial cells line the inner surface of the vascular wall, where they form a selective barrier that controls the passage of cells and small solutes between the blood and the tissue. In response to tissue injury, the endothelium from vessels surrounding the damaged area undergoes a local increase of permeability to cells and solutes that is essential for the inflammatory response [1, 2]. The increase in cell extravasation is not a passive event, but involves the active retention of blood cells, mainly leukocytes, on the endothelial surface, and the subsequent promotion by the endothelium of leukocyte extravasation toward the interstitial space [3]. Leukocyte transmigration, when pathologically altered, contributes to the development of a range of proinflammatory diseases, such as atherosclerosis, multiple sclerosis, and rheumatoid arthritis [4–6].
In the current paradigm of leukocyte transmigration, different subsets of leukocyte and endothelial receptors establish a cascade of interactions that involve initial leukocyte tethering and rolling, firm adhesion, crawling or locomotion on the luminal endothelial surface, and a final step of transendothelial migration (TEM), which is also known as diapedesis [3, 7]. Loss-of-function strategies, like blocking antibodies and genetic ablation, have enabled the identification of many surface receptors involved in the leukocyte–endothelial cell interaction [5, 8–15]. Intravital microscopy studies have also provided clarification of the chronological and hierarchical order of these interactions at each step of the cascade [16, 17]. The most important endothelial receptors involved in leukocyte transmigration through blood vessels have been extensively reviewed elsewhere and their roles often depend on the vascular bed and the leukocyte type implicated in the interaction [17–20]. In general, and from the endothelial side, initial leukocyte tethering and rolling are mediated by E- and P-selectin, and by carbohydrates exposed on the endothelial surface that are able to engage leukocyte (L)-selectin [21]. Intercellular adhesion molecule (ICAM)-1 and vascular adhesion molecule (VCAM)-1 are adhesion receptors of the immunoglobulin superfamily that are central players in the subsequent step: leukocyte firm adhesion [22]. ICAM-1 and VCAM-1 also mediate locomotion of adhered leukocytes on the endothelium and some features of diapedesis [23]. Diapedesis mostly occurs through junctions between two endothelial cells, in what is called a paracellular route. However, there exists an alternative transcellular route in which leukocytes can traverse the body of single endothelial cells [24]. A range of surface receptors localized at endothelial cell–cell junctions interacts with and facilitates extravasation of leukocytes following paracellular diapedesis. The way each of these receptors promotes local gap opening has been a matter of intense investigation in recent years [25, 26]. For instance, ICAM-1, VCAM-1, and some junctional proteins that interact with the transmigrating leukocyte have the ability to signal to and destabilize adherens junctions and, as a consequence, promote a local increase in permeability [27–29]. Some others, namely platelet endothelial cell adhesion molecule (PECAM-1), promote plasma membrane extensions at endothelial cell borders that also facilitate diapedesis. Expression levels of ICAM-1 and probably VCAM-1, also contribute to diapedesis by regulating the balance between the paracellular and transcellular routes of TEM [24, 30]. The molecular basis of this regulation will be discussed in this review.
A basal rate of leukocyte trafficking through the circulatory system is necessary for immuno-surveillance. However, leukocyte transmigration through vessels in the proximity of an inflammatory focus is increased in a spatio-temporally restricted manner. This increase is mediated by inflammatory cytokines, such as TNF-α or IL-1β, which are released by interstitial cells in response to the inflammatory upset. These stimuli upregulate the expression of E-selectin, ICAM-1, VCAM-1, and chemokines that are deposited in the luminal endothelial glycocalyx, all of which promote leukocyte capture and extravasation in the surroundings of the inflammatory focus. In addition, in response to some of these proinflammatory cytokines, junctional proteins involved in diapedesis are partially dispersed from cell–cell contacts and localize all over the luminal endothelial surface. This suggests that these proteins may play a role away from junctions in a proinflammatory context [31, 32].
Far from being passive agents exposing a plethora of receptors on their surface, endothelial cells determine the successful accomplishment of leukocyte extravasation by eliciting crucial signaling responses upon leukocyte contact [33]. In the multistep paradigm of leukocyte–endothelial interaction, the first steps (tethering, rolling, and firm adhesion) are more dependent on leukocyte behavior, whereas endothelial cell responses contribute more to modulate subsequent leukocyte locomotion and diapedesis. So, near-physiological levels of leukocyte tethering, rolling, and firm adhesion can be achieved in vitro in the absence of endothelium by providing the correct combination of laminar flow and recombinant endothelial molecules immobilized on a solid substrate [21, 34]. In contrast, endothelial adhesion receptors can be individually blocked with no apparent effect on leukocyte adhesion, but with significant consequences for the ability of leukocytes to crawl and find a passage for extravasation, indicating that the role of these endothelial receptors goes beyond their adhesive properties [30, 35]. Furthermore, altering the signaling properties of some of these receptors, for example ICAM-1, also has no effect on leukocyte adhesion but impairs posterior leukocyte transmigration [36]. In addition, once the leukocyte is engaged in crossing the endothelium, paracellular diapedesis can still be abrogated or even reversed simply by blocking the endothelial receptors involved in this step of transmigration [37]. These and several other lines of evidence indicate that leukocytes can proactively initiate the sequence of interactions that lead to the first immune reaction against tissue injury, but also that endothelial cell responses are crucial for the final outcome of this reaction, an efficient leukocyte extravasation and arrival to the focus of the inflammation.
Leukocyte interaction with the endothelial cell must be strong enough to overcome the shear stress from the blood flow and transient enough to allow leukocyte locomotion and egress from the vessel. The organization of the endothelial cell surface, the biological fence facing the vessel lumen, is thus essential to integrate signals from different sources, such as mechanical forces, cytokine signaling and cell–cell interactions. Research focused on the analysis of leukocyte–endothelial interactions at the molecular level, combining high-resolution and analytical microscopy with sophisticated in vitro settings, has been developed over recent years. These studies are finding that endothelial responses to leukocyte interaction involve a remarkable reorganization of endothelial membranes, ranging from the formation of nanodomains of adhesion receptors, based on protein–protein and protein–lipid interactions, to the extension of microvillus-like structures that engulf the leukocyte, or the formation of invaginations when leukocytes push down endothelial membranes during locomotion Importantly, this endothelial remodeling does not seem important for leukocyte adhesion itself but is essential for diapedesis. Here we provide an overview of the effect of leukocyte binding on the organization of the endothelial surface.
Submicron organization of adhesion receptors at the endothelial plasma membrane in response to leukocyte interactions
The endothelial adhesion receptors ICAM-1 and VCAM-1 are glycoproteins with an N-terminal extracellular domain containing five and seven immunoglobulin domains, respectively. They have a single transmembrane span and a short C-terminal cytoplasmic domain. ICAM-1 binding to leukocyte β2-integrins and VCAM-1 binding to β1-integrins mediate leukocyte adhesion to endothelial cells in most vascular beds [38, 39]. The structure, localization, role and dynamics of ICAM-1 and VCAM-1 are very similar, so it is conceivable that they associate and form heteroligomers or homoligomers. Using latex beads covered with anti-ICAM-1 or anti-VCAM-1 antibodies as surrogate leukocytes, van Buul and colleagues [40] have demonstrated that one receptor engagement induces association with the bead of the other. Using a different strategy, Barreiro and colleagues [41] have shown that cells overexpressing one type of integrin, and therefore engaging mainly one adhesion receptor, can indeed induce the recruitment of the other receptor to leukocyte-endothelial contacts. Interestingly, FRET studies have revealed the existence of ICAM-1 homotypic interactions but not of VCAM-1 or of ICAM-1-VCAM-1 heterotypic complexes on the endothelial surface in the absence of leukocytes. Accordingly, co-immunoprecipitation experiments of unengaged ICAM-1 and VCAM-1 have found only a negligible association between the two receptors [42]. Collectively, these data indicate that VCAM-1 and ICAM-1 follow similar dynamics upon leukocyte engagement, and become part of the same macromolecular complex in the area of contact with the leukocyte. However, in the absence of ligation, despite having a similar distribution on the endothelial surface, these two receptors are not directly associated with each other on the endothelial surface.
ICAM-1 and VCAM-1 reside in preformed tetraspanin platforms at the endothelial plasma membrane
Tetraspanins form a large group of proteins with some shared structural features: four transmembrane domains that comprise a very short intracellular segment, a first small extracellular loop or domain (SEL/SED) or EC1, and a large extracellular loop (LEL, LED) or EC2. LED contains four to eight cysteine residues, including those in a CCG motif that is conserved in all tetraspanins. LED cysteines form disulphide bonds that are necessary to maintain the structure of this domain, which is responsible for most tetraspanin interactions with other proteins [43–45]. Tetraspanins have the ability to associate with each other, forming remarkable macromolecular complexes known as tetraspanin webs [43, 46]. Cysteine residues found in close contact with the cytoplasmic leaflet of the membrane undergo palmitoylation, which is required for the formation of tetraspanin microdomains [47]. Tetraspanins interact with several other molecules, including VCAM-1 and ICAM-1 [48]. The tetraspanin web contributes to establish physical connections in large membrane domains between associate receptors that are at relatively low density within the membrane. Given the size of the contact area between the leukocyte and the endothelial cell during leukocyte adhesion, tetraspanins may form large endothelial macromolecular platforms mediated by ICAM-1 and VCAM-1 interactions with leukocyte integrins. A detailed analysis of the molecular platforms formed between tetraspanins CD9 and CD151 and VCAM-1 and ICAM-1 has been carried out using analytical microscopy techniques. By combining FRET, FRAP and FCS spectroscopy, the existence of preformed tetraspanin platforms and adhesion receptors on the endothelial surface has been suggested [41]. In contrast to the lack of association between ICAM-1 and VCAM-1, CD9 and CD51 have been linked with both receptors in immunoprecipitation experiments performed in the absence of engagement, consistent with the analytical microscopy data [41, 42]. Moreover, FRAP and FCS analyses of tetraspanins and adhesion receptors indicate that tetraspanin-receptor platforms are stabilized in the endothelial area of contact with the adhered leukocyte. Notably, a soluble recombinant protein carrying the tetraspanin LED decreased the clustering of ICAM-1 and VCAM-1 [41], and inhibited leukocyte adhesion, detachment and diapedesis. Taken together, these data indicate that ICAM-1 and VCAM-1 are pre-embedded in endothelial tetraspanin platforms, which are required for proper adhesion receptor function in leukocyte extravasation (Fig. 1).
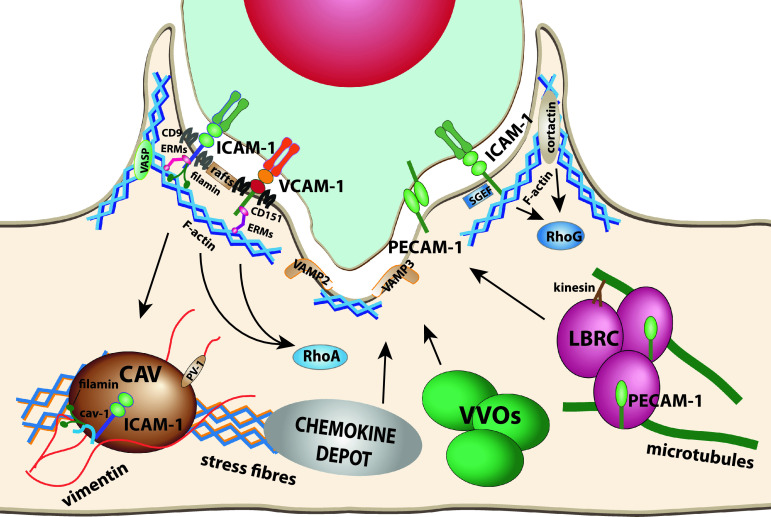
Fig. 1.
Proteins involved in endothelial apical membrane reorganization upon leukocyte interaction. ICAM-1, VCAM-1, and PECAM-1 are the three best characterized receptors that modulate the endothelial cell surfaces in contact with leukocytes. ICAM-1 and VCAM-1 are embedded into tetraspanin webs. Both receptors and E-selectin can be transiently recruited to membrane rafts upon engagement. ICAM-1 and VCAM-1 are connected to subcortical actin through different protein connectors such as ERM proteins or filamins. These receptors form signaling platforms in apical microvillar reorganization called docking structures. ICAM-1 and VCAM-1 signal to different Rho GTPases and cortactin to tune actin dynamics in the surrounding of endothelial cell-leukocyte contact areas and facilitate diapedesis. When apical ICAM-1 is engaged in caveola-rich cellular areas, can be internalized into basolateral caveolae. It has been proposed that PECAM-1 is translocated from the LBR compartment to endothelial contact areas. On the ventral side of the leukocyte, endothelial SNARE machinery participates in membrane remodeling in response to leukocytes emitting pseudopods/podosomes that scan for gateways to traverse the endothelial monolayer. VVOs participate in the formation of endothelial invaginations surrounding podosomes. Chemokine depots are intracellular compartments, still uncharacterized, which translocate chemokines to endothelial surface upon leukocyte adhesion. Actin stress fibers determine the localization of caveolae and chemokine depots. The vimentin network is associated with caveolae through PV-1. Microtubules and kinesins regulate LBRC dynamics
Coalescence of endothelial adhesion receptors into membrane rafts upon leukocyte adhesion
Membrane lipids and proteins do not diffuse freely in the bilayer, but are laterally constrained by mechanisms such as hydrophobic compatibility, membrane protein–protein interactions, or interactions between membrane proteins and the submembrane cytoskeleton. The intrinsic heterogeneity of the plasma membrane has been explained by the differential miscibility of lipids that spontaneously form microdomains of distinctive membrane order or compaction, which in turn are able to compartmentalize subsets of membrane proteins. The term “membrane rafts” designates the most popular model of membrane microdomains, which proposes the existence of liquid-ordered nano-scale assemblies of cholesterol and sphingolipids that exclude most transmembrane proteins, and preferentially include those with lipid modifications, such as glycosylphosphatidylinositol or palmitoylation. The size (10–200 nm) and dynamics of membrane rafts depend on the physiological context. Signaling events can induce the coalescence of transient and small membrane clusters into larger and more stable platforms and vice versa [49]. Crude biochemical approaches based on the different solubility properties of membrane proteins in non-ionic detergents [50] have given way to novel biophysical and microscopy techniques to investigate membrane rafts. These novel approaches are yielding evidence that confirms the partition of certain proteins into small membrane platforms regulated by cholesterol content [49]. However, the true nature of membrane rafts remains elusive, due mainly to the difficulties of clearly visualizing these domains within the cell, and this has generated skepticism and controversy [51].
Compact membrane domains of cholesterol and sphingolipids are less sensitive to detergent extraction than are fluid, non-raft membranes, so the former can be biochemically isolated as detergent-resistant membranes (DRMs). As already mentioned, this has been criticized as a strategy to characterize membrane rafts [51], but it is a simple method that provides qualitative evidence of changes occurring in the lipid context of particular proteins. On the other hand, receptor engagement with antibodies has proved to be a powerful strategy for investigating leukocyte-endothelial interactions because it enables signaling events from one cell type or the other to be identified. These can subsequently be validated by more physiological experimental approaches. Antibody-mediated engagement of E-selectin induces receptor confinement into DRMs in endothelial cells [52]. Leukocyte- or antibody-induced clustering also increases E-selectin colocalization with caveolin-1 [53], the scaffolding protein of caveolae [54–57], a vesicular compartment quite abundant in endothelial cells [58]. Caveolin-1 binds cholesterol, and caveolae are considered as specialized membrane raft domains [57]. Furthermore, endothelial raft disruption with cholesterol-sequestering reagents decreases neutrophil rolling on E-selectin and reduce the ability of E-selectin to transduce signals [59]. These findings suggest that cholesterol-rich domains may regulate leukocyte rolling by modulating signaling mediated by, at least, E-selectin. However, experimental strategies that can specifically disrupt E-selectin microdomains, or the identification of the protein signals and scaffolds responsible for the partition of E-selectin into ordered membranes, are necessary to clarify the real role of ordered membranes in these first steps in leukocyte transmigration.
Similarly, in response to leukocyte interaction or antibody crosslinking, ICAM-1 is partially confined within DRMs [52, 60]. Time-lapse analysis of ICAM-1 trafficking reveals unusual dynamics for this receptor. Following antibody clustering, ICAM-1 is translocated from actin-rich microvilli to actin stress fibers and is then partially segregated to the cell poles. Engaged ICAM-1 accumulates in areas close to, but distinct from the cell–cell junctions, where several actin bundles converge [60]. Interestingly, these areas are also enriched in caveolin-1 (Fig. 2a, b). A more detailed analysis by electron microscopy and total internal reflection fluorescence (TIRF) microscopy, which detects fluorescence at the very basal region of the cell and in close contact with the substrate, has revealed that a fraction of ICAM-1 is internalized into caveolar-like structures and is transcytosed to the abluminal endothelial membrane [60]. Thus, upon ligation, ICAM-1 is not only recruited to DRMs but is also partially segregated in a vesicular compartment enriched in membrane rafts. VCAM-1 dynamics has not been studied in such detail, but the available data suggest it is comparable to that of ICAM-1. Crosslinked VCAM-1 also segregates into membrane rafts, then aligns with stress fibers and is subsequently is partially localized in caveolin-1-rich structures [60]. Cholesterol-sequestering reagents reduce E-selectin and ICAM-1 clustering [52, 53]. However, since disruption of cholesterol-rich membrane domains inhibits the previous step of leukocyte rolling, the role of raft domains in leukocyte adhesion and locomotion has been difficult to assess using this strategy. More artificial approaches, such as analyzing apical detachment of anti-ICAM-1-bearing beads in response to the shear stress, show that cholesterol extraction increases leukocyte de-adhesion, which is also dependent on actin polymerization [61]. The role of caveolae-like domains in diapedesis has been investigated in more detail. Some groups have found that leukocytes follow a route of transcellular diapedesis preferentially in areas enriched in caveolin-1 and other caveolar components [60, 62, 63]. This suggests that clustering of at least ICAM-1 into raft-like microdomains may help guide the leukocyte towards points of extravasation. Membrane remodeling during diapedesis is reviewed in more detail below.
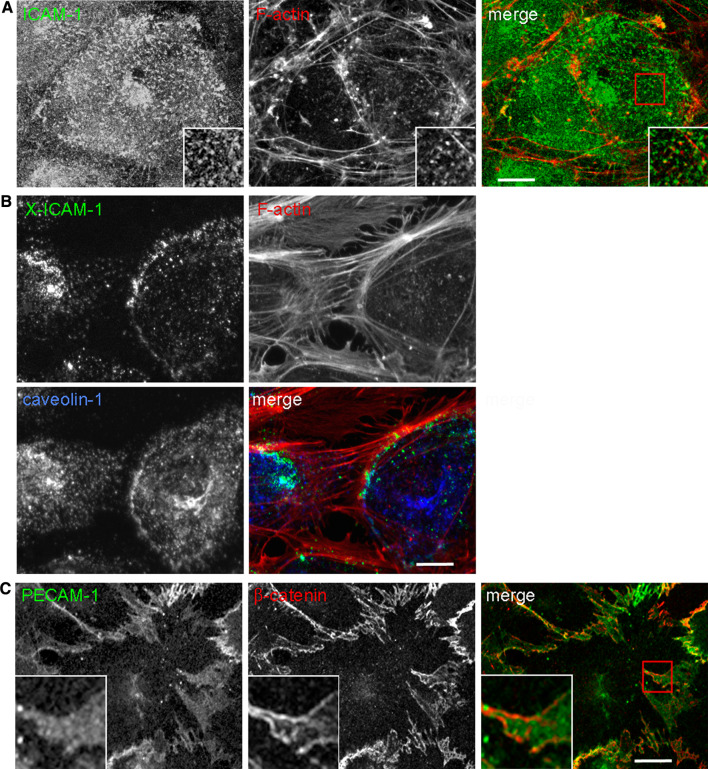
Fig. 2.
Endothelial membrane domains involved in adhesion receptor dynamics. a Microvilli. Confocal images of HUVECs stained for ICAM-1 and F-actin (phalloidin) showing the accumulation of this receptor in apical microvilli, involved in the formation of docking structures in response to leukocyte adhesion b Caveolae. Upon antibody-mediated ICAM-1 clustering (X-ICAM-1), actin stress fibers are increased, ICAM-1 aligns with actin filaments and partially colocalizes with caveolin-1. Leukocytes transmigrate transcellularly through areas rich in caveolin-1. c Lateral border recycling (LBR) compartment. PECAM-1 is diffusely localized at cell borders, labeled with anti-β-catenin antibody, in an internal compartment that is translocated to the cell surface upon leukocyte interaction. The LBR compartment is required for both paracellular and transcellular diapedesis. Bar 10 μm
Relationship between tetraspanin domains and rafts at the endothelial surface
Several lines of evidence have shown that most of the interactions between tetraspanins and other molecules are not dependent on cholesterol. However, tetraspanins can be partially recruited into DRMs isolated in isopycnic sucrose gradients using mild lysis conditions that preserve tetraspanin–tetraspanin interactions. Tetraspanin domains are though only partially sensitive to cholesterol depletion compared to membrane domains rich in prototypical raft-proteins such as GPI-linked proteins [47, 64]. Detailed measurement of the diffusion coefficient of the tetraspanin CD9 by single-molecule fluorescence microscopy indicated that cholesterol extraction affects its membrane dynamics [47]. This effect was attributed to a general alteration in membrane fluidity caused by cholesterol extraction, which also affected the dynamics of raft and non-raft protein markers. Notwithstanding a possible role of raft-lipids in the organization of tetraspanin webs [65], these webs form domains that largely depend on protein–protein interactions, with features that make them clearly distinguishable from membrane rafts [43]. Overall, distinct but complementary roles for tetraspanin and raft domains can be envisaged from a consideration of all the published evidence regarding endothelial ICAM-1 and VCAM-1 function and clustering. Data strongly support the confinement of ICAM-1 and VCAM-1 in tetraspanin nanodomains, but not in cholesterol-rich DRMs, in the absence of receptor engagement [52, 60, 66]. However, size measurements of ICAM-1 and VCAM-1 nanoclusters indicate that they coalesce and are more stable in the leukocyte contact area [41]. This coalescence parallels ICAM-1 recruitment into ordered domains, so it could well be mediated by cholesterol-enriched rafts [60]. In fact, examples of coalescence between tetraspanin and membrane rafts can be found in different cellular contexts, such as cell infection by HIV, Plasmodium sporozoite invasion, or during engagement of the B-cell receptor or the MHC-II complex [67–70]. Thus, future investigation of the relationship between the tetraspanin web, which constitutively accommodates transmembrane receptors, and membrane rafts, which can merge platforms based on protein–protein interactions, may complete the picture of how endothelial adhesion receptors are organized in the contact area between leukocyte and endothelium.
Ups and downs of the endothelial response to leukocyte interaction
Endothelial ICAM-1 and VCAM-1 can be organized into submicron membrane platforms in response to leukocyte interaction. However, leukocytes also induce supramolecular remodeling of the endothelial membrane, with the contribution of subcortical cytoskeleton, which is essential for achieving successful extravasation (Fig. 1).
Ups: endothelial membrane structures derived from microvilli embrace adhered leukocytes
With the advent of modern microscopy, cell surfaces have been shown to be uneven and able to emit dynamic protrusions necessary for motility and communication. Cells decorate their respective surfaces exposed to extracellular milieu with small actin-dependent projections, bearing different subsets of surface receptors and ion channels, known as microvilli (Fig. 2a) [71]. These surface organelles are important for cell communication with the extracellular space. F-actin, the cytoskeletal scaffold of the microvilli, is associated with the plasma membrane through different protein linkers that connect membrane receptors to the actin filament. In endothelial cells, ICAM-1 and VCAM-1 are enriched in microvilli and are connected to the underlying F-actin via interactions through their cytoplasmic tails with ezrin, radixin and moesin (ERM) proteins, which belong to the 4.1 superfamily of proteins. This superfamily is formed by proteins containing an N-terminal four point one, ezrin, radixin, moesin (FERM) domain that interacts with the lipids and basic amino acids in intracellular segments of membrane proteins [72]. ICAM-1 interacts preferentially with ezrin [73, 74] and VCAM-1 with moesin [66]. In their active, open conformation, ERM proteins are linked to the plasma membrane through N-terminal FERM domains and to submembrane F-actin through their C-terminal domains [75]. ERM proteins are required for microvillar elongation [74, 76–78]. Mutation of a short segment in the cytoplasmic region of ICAM-1, rich in basic amino acids, abrogates ICAM-1 interaction with ERM protein as well as the ability of this receptor to elongate microvilli [74]. ICAM-1 bearing these mutations also failed to promote leukocyte transmigration [74]. Moreover, incubation of endothelial cells with a permeable peptide blocking ICAM-1-ERM interaction did not affect leukocyte adhesion but significantly diminished leukocyte transmigration. This again supports the idea that expression of endothelial adhesion receptors is sufficient to capture and adhere leukocytes bearing active integrins, whereas ICAM-1 and probably VCAM-1 interactions with subcortical actin in microvillar structures are essential for leukocyte extravasation. The detailed molecular mechanism whereby microvilli control leukocyte transmigration is not completely understood, but probably involves substantial remodeling in response to leukocyte adhesion of the apical endothelial plasma membrane in the so-called microvillar cups or docking structures.
In 1999, Wojciak-Stothard and colleagues [79] showed that ICAM-1, VCAM-1, and E-selectin accumulated along the edges of attached monocytes, outlining fine protrusions formed by the monocyte-endothelial interface. These protrusions were positive for F-actin and morphologically reminiscent of microvilli. Barreiro and colleagues [66] subsequently reported for the first time a detailed analysis of these microvillar protrusions that surrounded adhered leukocytes, and named them docking structures. Docking structures contained F-actin, vinculin, α-actinin, VASP and phosphorylated, active ERM proteins. Analyzing various fluorescent probes suggested that the phosphoinositide PI(4,5)P2, which anchors ERM proteins to the plasma membrane, was also enriched at the tip of the protrusions [66]. Additional reports have provided substantial evidence that endothelial cup-shaped membrane projections embracing leukocytes are derived from microvilli [74, 80, 81]. Like microvilli, docking structures are sensitive to actin and microtubule depolymerization [66, 79, 80] In addition, the accumulation of VASP in these membrane projections strongly suggests that de novo actin polymerization is required for their formation, similarly to what has been found for other surface membrane extensions, such as those occurring during phagocytosis [66, 82, 83].
The investigation of the signaling pathways involved in the formation of docking structures has provided confusing information about the role of these protrusions in leukocyte extravasation. Rho family GTPases are master regulators of cytoskeletal networks. Most Rho GTPases are molecular switches that cycle between an inactive GDP-bound state and an active GTP-bound state that regulates a plethora of effectors [84]. RhoA, Rac and Cdc42 are the three most extensively studied Rho GTPases in the family, but there are many other Rho members poorly characterized [85]. In endothelial cells, RhoA controls actomyosin-mediated contractility mainly through the activation of its effector ROC kinase (ROCK) [18]. Rho and probably ROCK, activate ERM proteins, which can promote microvillar elongation [75]. Thus, the Rho-ROCK pathway could regulate the formation of docking structures, leukocyte adhesion, and transmigration. This pathway is however fundamental to endothelial architecture and function, including barrier function, so data regarding the effect of Rho or ROCK inhibition on endothelial docking structures and on leukocyte transmigration are not conclusive. For instance, treatment of endothelial cells with the toxin C3 transferase, a potent inhibitor of Rho, impairs leukocyte transmigration [79, 86, 87], but its effect on leukocyte adhesion is still unclear [86, 88]. C3 transferase has been shown to prevent [79] or not prevent [80] adhesion receptor clustering around adhered leukocytes. There is also disagreement about the role of the ROCK in leukocyte adhesion and in the formation of docking structures. Pharmacological inhibition of this kinase clearly reduced leukocyte adhesion, transmigration and formation of these microvillus-derived cups in some conditions [66], or had no effect on adhesion and formation of docking structures in others [80]. A stronger consensus has been reached concerning the effect of ROCK inhibition on leukocyte extravasation, which is clearly hampered. Taken together, these findings provide evidence of an essential role for the Rho-ROCK pathway in leukocyte transmigration in general and, probably, in the formation of docking structures in particular.
Clearer information has come from studies about the proteins in endothelial plasma membrane that are responsible for the formation of these cups. K562 cells overexpressing LFA-1 [80] or VLA-4 [66] have been used to preferentially engage ICAM-1 or VCAM-1, respectively, in endothelial cells. Both cell clones were able to promote the formation of docking structures upon adhesion to endothelial cells, which suggests that single engagement of each adhesion receptor is sufficient to generate microvillar cups. However, the blocking of the interaction of ICAM-1 with LFA-1, but not of VCAM-1 with VLA-4, reduced the formation of docking structures surrounding MCP-1-activated monocytes, suggesting a predominant role for ICAM-1 in this process [80]. Although the relative contribution of each receptor to these membrane extensions probably depends on their relative abundance, leukocyte population and type of vascular bed, further investigation of the role of adhesion receptors in microvillar cups has been mainly focused on ICAM-1. The aforementioned mutation of a domain rich in polybasic amino acids in the ICAM-1 cytoplasmic tail, which decreases ICAM-1-induced microvilli and leukocyte transmigration, also reduces the formation of endothelial docking structures upon leukocyte adhesion. This reinforces the similarities between microvilli and docking structures. ICAM-1-mediated signaling or interaction with subcortical actin thus appears to be necessary for the formation of docking complexes. Analysis of ICAM-1-mediated signaling in response to antibody engagement has revealed a major activation of a RhoA-regulated pathway [89, 90]. This, together with the fact that Rho pathway inhibition reduces the formation of the microvillar cups, further supports a role for Rho in coordinating signals originating from ICAM-1, which promote the eventual reorganization of the microvilli to embrace adhered leukocytes.
ICAM-1 can also signal to other Rho GTPases. van Buul and colleagues [81] have reported that ICAM-1 crosslinking activates RhoA, Rac1, Cdc42, and RhoG, suggesting that leukocyte adhesion orchestrates several Rho-dependent pathways to promote a profound reorganization of the endothelial apical membrane. RhoG had previously been found to be involved in the formation of the phagocytic cup [91]. Following the parallelism with the endothelial microvillus-derived cups, the role of this small GTPase has been investigated. RhoG and its guanine-nucleotide exchange factor (GEF), SH3-GEF (SGEF), were found enriched in dorsal ruffles and in membrane projections surrounding the leukocyte upon adhesion. Expression of active mutants of RhoG increased the emission of these projections, whereas RhoG or SGEF knockdown with small interfering RNA (siRNA) decreased them. It is of note that SGEF was found to be associated with the ICAM-1 cytoplasmic tail and that a tail-less ICAM-1 mutant failed to recruit RhoG to the docking structures. Future studies of the crosstalk between RhoG and other Rho GTPases will probably shed light on the signaling networks that mediate the formation of docking structures. For instance, siRNA-mediated knockdown of RhoA reduces RhoG activation upon ICAM-1 engagement, suggesting that the former GTPase is an upstream regulator of the latter [81]. VCAM-1 and ICAM-1 clustering activates Rac1 in endothelial cells [27, 81]. Analysis of the effect of Rac1 dominant negative mutants in human endothelial cells indicates that Rac1 is not required for firm adhesion of monocytes [79]. However, inhibition of Rac1 function with permeable blocking peptides or Rac1 knockdown strongly supports a role for this Rho GTPase in leukocyte extravasation [27, 92]. Notably, RhoG has the potential to regulate Rac1 activity through common GEF protein complexes [93, 94] and Rac and its effectors are in turn required for dorsal membrane dynamics such as the formation of ruffles and macropinocytosis [95–97]. Hence, a role can be envisaged for adhesion receptor-mediated Rac activation in the formation of the endothelial docking structure and in leukocyte diapedesis. Rac could contribute to RhoG or RhoA signaling pathways, as has already been observed in other cellular contexts [93, 95]. In summary, although the architecture of these docking structures seems well defined, the protein machinery recruited to these membrane protrusions and the signaling pathways emanating from these domains are still poorly defined. Nevertheless, in light of the current knowledge about the proteins that regulate microvillar cups, these structures could be also considered as hubs where supramolecular organization of protein complexes formed by adhesion receptors, tetraspanins, ERM proteins and Rho GTPases, can signal and determine the additional endothelial remodeling necessary for diapedesis. This may include microvillar elongation to ensure dynamic leukocyte retention against blood flow, but also RhoA-mediated cell contraction or Rac1-mediated weakening of cell–cell junctions.
The ability of ICAM-1 to bring together signaling machinery in the docking structure through its cytoplasmic tail seems to be of fundamental importance. Thus, the role of this receptor domain in leukocyte diapedesis may somehow denote the function of docking structures in extravasation. The reconstitution of a brain endothelioma cell line, derived from ICAM-1 and ICAM-2 double-knockout mice, with ICAM-1 mutants has elegantly demonstrated that the extracellular domain of ICAM-1 is sufficient to support T-cell adhesion, while its cytoplasmic tail is necessary for diapedesis. Truncation of this cytoplasmic domain prevented not only TEM in these cell lines but also ICAM-1-mediated RhoA activation [35]. Therefore, there are strong correlations between ICAM-1-mediated signaling, docking structures and leukocyte diapedesis, but not adhesion, which implies that the remodeling of the endothelial apical membrane helps to establish signaling platforms that facilitate the passage of leukocytes across the endothelium.
However, it is of note that docking structures have not been always observed in vitro. Luscinksas’s group has reported clustering of ICAM-1 and LFA-1 around transmigrating neutrophils under laminar flow, but not microvillus-derived structures [30, 98]. In vivo, these structures have been also difficult to detect [99]. This disagreement may be related to the different experimental settings used, which may determine different levels of integrin activation. Higher integrin activation could result in greater recruitment of endothelial counter-receptors and the subsequent stimulation of signaling pathways related to phagocytosis. This has been more extensively discussed in a previous review [100]. Further investigation into mechanisms regulating the formation of docking structures and their in vivo relevance is still required.
Downs: leukocyte-induced endothelial membrane invaginations
The scanning by adhered leukocytes for suitable areas of transmigration on the endothelial luminal membrane is defined as locomotion [23]. In this step, leukocytes search for clues to initiate diapedesis in the presence of shear stress forces from the bloodstream. Pioneering electron microscopy studies in the 1960s showed leukocytes emitting pseudopods and remodeling endothelial cells during extravasation [101–103]. Modern in vitro approaches, which combine settings reproducing leukocyte transmigration under near-physiological conditions with high-resolution fluorescent microscopy, have enabled the fine-scale analysis of leukocyte locomotion dynamics on the endothelial cells. Experiments using blocking antibodies specific for β1 and β2 integrins, VCAM-1 and ICAM-1, strongly suggest that ICAM-1 plays a predominant role in leukocyte lateral displacement on the endothelium, at least in the cases of T cells [104] and monocytes [23]. Neutrophil locomotion has been also observed in vivo in inflamed venules. Neutrophils from knockout mice of Mac-1 integrin, ligand of ICAM-1 and ICAM-2, adhered extremely well but failed to crawl on the vessel. ICAM-1, but not ICAM-2 blocking, also inhibited in vivo locomotion [105]. On the other hand, the analysis of ICAM-1 and LFA-1 distribution during leukocyte locomotion revealed that the active leukocyte integrin and endothelial ICAM-1 accumulate in the ventral area of migrating T cells. These areas of intense leukocyte–endothelial interaction were termed focal zones [106]. The formation of focal zones seems to be dependent on the association in the leukocyte of integrins with actin through talin [106]. Thus, ICAM-1 not only concentrates in membrane projections that surround leukocytes, but also accumulates beneath them, where leukocytes form integrin-dependent adhesion structures. Additional analysis of leukocyte endothelial interactions with more sophisticated in vitro approaches, including laminar shear stress and the presence of chemokines exposed on the apical surface of endothelial cells, confirmed the existence of these ventral clusters of ICAM-1 [104]. This study also shows a different distribution for VCAM-1, which surrounded preferentially the uropod pole of the adhered leukocyte, instead of accumulating in the ventral focal zone. Scanning electron microscopy also revealed the presence in crawling T cells of small and invasive filopodia that penetrate the endothelial cell. The number and size of these filopodia were increased by the shear flow and were dependent on Cdc42 activity [104]. On the other hand, visualization of ICAM-1-GFP dynamics by TIRF microscopy has shown that protrusions emitted by T cells migrating on the apical endothelial surface can reach the endothelial basal membrane [60]. In all these analyses, endothelial invaginations observed under the lymphocyte occurred during apical locomotion, before and independently of the diapedesis step.
Leukocyte protrusions penetrating endothelial cells have been found to be controlled by the Src kinase and the actin regulatory protein WASP, which has led Carman and colleagues [107, 108] to propose these structures to be similar to invasive podosomes, the adhesive basal structures found in invading cancer cells. Interestingly, these authors have also found active endothelial SNARE machinery, such as VAMP2 and VAMP3, within the endothelial podoprints that surround these cognate podosomes [107], which indicates that endothelial cells provide trafficking and membrane fusion machinery to the areas where leukocytes extend their podosomes. It is of note that the treatment of endothelial cells with N-ethylmaleimide, which prevents SNARE-mediated membrane fusion [109], has a specific effect on the transcellular route of diapedesis, suggesting that proactive endothelial membrane fusion mediated by these endothelial SNARE proteins during the locomotion step is a prerequisite for the subsequent leukocyte transcellular TEM [107]. In accordance, grape-like clusters of vesicles resembling the vesiculo-vacuolar organelle (VVO) were found accumulated close to endothelial podoprints. Taken together, these findings indicate that leukocytes moving on the apical endothelial surface in search of an extravasation route induce ICAM-1-enriched endothelial invaginations that require active endothelial intracellular machinery. These invaginations have the potential to reach endothelial basolateral domains and thereby initiate transcellular diapedesis. Nonetheless, whereas most leukocytes crawling on the endothelium induce this membrane remodeling in the endothelial cell, only around 10 % of them in human vascular endothelial cells and 30 % in microvascular endothelial cells, follow transcellular diapedesis, implying that additional endothelial events may be required to facilitate the opening of a transcellular pore.
Endothelial membrane remodeling during diapedesis
Docking structures and diapedesis routes
The docking structures embracing leukocytes that adhere to the apical endothelial membrane are also observed when leukocytes undergo paracellular and transcellular diapedesis [63]. Although these membrane extensions play a role in leukocyte retention and signaling that is essential for leukocyte diapedesis, to date, no evidence has been found to suggest a function for docking structures in leukocyte guidance towards a specific route of diapedesis. Figure 3 illustrates endothelial membrane remodeling during diapedesis.
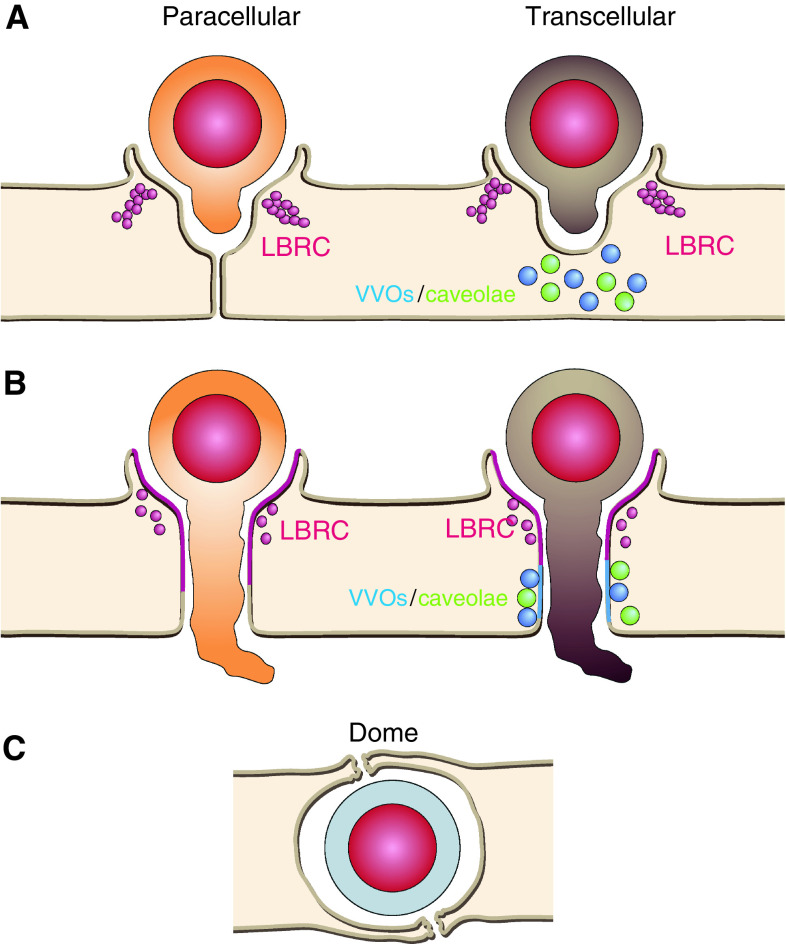
Fig. 3.
Endothelial membrane reorganization during diapedesis. Leukocytes probe the endothelial surface emitting pseudopods/podosomes (a). Once they find appropriate areas for paracellular or transcellular diapedesis (b), effective docking structures derived from microvilli must embrace apically adhered leukocytes and PECAM-1 must be relocated from the LBR compartment to leukocyte-endothelial contact areas. In paracellular diapedesis, actin-mediated cell contraction, and cell–cell junction disruption induce gap opening. In transcellular diapedesis, coordination of events described in Fig. 1 can occasionally lead to the formation of transcellular passages, in which LBR-compartment, VVOs and caveolae can potentially supply intracellular membranes. c Endothelial domes that encapsulate transmigrating leukocytes have been observed in vivo
The lateral border recycling (LBR) compartment during paracellular diapedesis
Comprehensive reviews of the endothelial signaling induced by leukocyte adhesion that control paracellular diapedesis have recently been published [24, 33, 110, 111]. Paracellular diapedesis involves disruption of cell–cell junctions, opening of intercellular gaps and reconstitution of cell–cell contacts. Adhesion receptor engagement elicits Rho- and Rac-dependent signaling pathways that induce cell contraction and junctional destabilization [27, 89, 90, 112]. This engagement also activates Src kinases and generation of reactive oxygen species (ROS) that have a direct effect on phosphorylation of junctional molecules, such as VE-cadherin [27, 28, 113–115]. In addition, endothelial cells selectively express an additional set of receptors that can be partially confined at cell borders and support paracellular diapedesis. CD99, PECAM-1 and the junctional adhesion molecules (JAMs) are the best-characterized receptors mediating diapedesis, although endothelial cell-selective adhesion molecules (ESAMs), nectins and ICAM-2 also mediate leukocyte paracellular TEM [20, 116–119]. Receptors localized at cell borders facilitate TEM by establishing new homotypic or heterotypic molecular dimers between the leukocyte and the endothelial cell, which replace interactions between two adjacent endothelial cells and thereby induce local disruption of cell–cell junctions.
With the exception of the cuboidal endothelium in high endothelial venules [120, 121], endothelial cells form relatively thin monolayers where junctions distribute in overlapping cell borders, in clear contrast with the paradigmatic straight junctional complexes found in vertical borders of columnar epithelial cells [122, 123]. One of the best characterized junctional receptors involved in diapedesis, PECAM-1, has been found in a novel intracellular compartment situated at overlapping areas of endothelial cell–cell junctions known as the LBR compartment (Fig. 2c) [124]. Electron microscopy and PECAM-1 recycling experiments suggest that this compartment is composed of concatenated vesicles that are connected to the exterior through the junction. Analysis of PECAM-1 trafficking within this compartment during monocyte paracellular diapedesis suggests that PECAM-1 is translocated from LBR vesicles towards the external contact area with the leukocyte [124]. In an attempt to characterize PECAM-1 trafficking in paracellular diapedesis, an essential role has been reported in this trafficking for the kinesin family of motor proteins, which move along the microtubular network [125]. Microtubule depolymerizing drugs or blocking antibodies against kinesins had no apparent effect on the steady-state distribution of junctional PECAM-1, but inhibited PECAM-1 trafficking during leukocyte diapedesis, blocking the passage of leukocytes through the junction. Quantitatively, the effects of these treatments were comparable to that of PECAM-1-blocking antibodies on diapedesis. JAM-A and CD99 have also been observed on the LBR, although their trafficking has yet to be investigated [126].
The protein machinery involved in the formation of LBR vesicles and the molecular clues that keep this compartment associated with cell borders have not been elucidated. Regarding the nature of the LBR compartment, endothelial cells contain unique membrane compartments involved in solute transport, such as fenestrae, caveolae and VVOs, which may potentially contribute to the LBR compartment [55, 127, 128]. Previous electron microscopy studies had suggested that PECAM-1 is concentrated in the VVOs, which are formed by numerous caveolae-like connected vesicles. However, VVOs are not restricted to perijunctional areas and are morphologically different from LBR vesicles [124, 129]. Biochemical and morphological analyses also indicate that the LBR compartment is different from caveolae [124]. The identification of the vesicle machinery and the junctional proteins involved in the genesis and regulation of the LBR compartment, and the molecular mechanisms that mediate kinesin-dependent PECAM-1 trafficking should clarify the importance of this structure in paracellular diapedesis.
The LBR compartment during transcellular diapedesis
Some junctional receptors involved in paracellular diapedesis, such as JAM-A and PECAM-1, are redistributed and localized apically, away from junctions, in response to proinflammatory cytokines such as TNF-α and INF-γ [31, 32, 130]. JAM-family proteins have the ability to establish cis and trans homophilic and heterophilic interactions between their members. In addition, JAM-A is an alternative cognate receptor for leukocyte β2-integrins that is involved in leukocyte adhesion and TEM [131–133]. JAM-C can also interact with β2-integrins [134–136], whereas JAM-B can bind β1-integrins [137], although these interactions have been less thoroughly explored. In TNF-α-stimulated HUVECs, JAM-A, CD99, and PECAM-1 accumulated within docking structures surrounding leukocytes undergoing transcellular diapedesis, away from cell–cell junctions [126]. Carman and colleagues [107] have also detected a remarkable accumulation of PECAM-1 in microvillus-derived cups embracing adhered memory T cells, although much less in the case of cups adhering monocytes [63]. Importantly, function-blocking antibodies against PECAM-1 and CD99, but not against JAM-A, can significantly diminish paracellular and transcellular diapedesis, indicating that these junctional proteins also play a role away from cell borders [107, 126]. Based on these data, Mamdouh and colleagues have proposed that the LBR compartment might supply adhesion receptors such as JAM-A, CD99 or PECAM-1 to the transcellular pores. It is of note that this unselective inhibition of both routes of diapedesis seems quite similar to the inhibition resulting from the previously enumerated strategies that target the formation of docking structures [63]. Moreover, microtubule-dependent PECAM-1 recycling from the LBR compartment was also detected in membrane extensions resembling docking structures, which surrounded leukocytes undergoing transcellular diapedesis [125, 126]. So far, the potential relationship between the LBR compartment and the docking structures in the transcellular passage has not been addressed, and may be an interesting avenue of investigation in the future.
ICAM-1 and caveolae in transcellular diapedesis
In accordance with the predominant role that ICAM-1 plays in endothelial membrane remodeling upon leukocyte adhesion and locomotion, this receptor seems also to be central for the route of transcellular extravasation in vitro. Modulation of endothelial ICAM-1 levels has an important effect on the rate of transcellular diapedesis for PMN leukocytes [30]. In endothelial cells, caveolin-1, the scaffolding protein of the caveolae, is enriched in areas where several actin stress fibers converge. Notably, in response to antibody ligation, ICAM-1 is slowly translocated to these areas along actin filaments, where the receptor is transcytosed to the endothelial basal membrane through caveolae [60]. Confocal and time-lapse microscopy reveals that engaged ICAM-1 moves to the cell periphery, whereas caveolin-1- and F-actin domains remain comparatively static (Fig. 2b). TIRF analysis of T cells crawling on the endothelial membrane reveals their capacity to step down caveolin-1 clusters that contact the basal membrane. Three research groups have found that leukocytes preferentially migrate transcellularly in areas enriched in caveolin-1 [60, 62, 63], although this has not been always observed [126]. Comparative analysis of docking structures and caveolin-1 accumulation at the transcellular pore shows that they are differently localized. Caveolae are found in a more basal plane than apical microvillar protrusions [60]. Caveolin-1 gene silencing specifically impairs the transcellular diapedesis but does not affect total or paracellular diapedesis [60, 138].
Some other caveolar components have been found in the transcellular pore, such as the protein plasmalemmal vesicle-associated protein (PV-1) [62, 139]. PV-1 is localized in stomatal diaphragms of endothelial caveolae and fenestrae [139, 140], where it is essential for the formation of these diaphragms [141]. Although the role of these structures is not well understood, stomata are thought to participate in the transcellular exchange of liquids and macromolecules [142]. PV-1-blocking antibody reduced leukocyte transmigration in vivo and in vitro, whereas adhesion was not affected. Under experimental settings that favor paracellular over transcellular TEM, anti-PV-1 antibodies had little effect on either adhesion or transmigration, suggesting that PV-1 preferentially regulates transcellular diapedesis [62]. However, further analysis of the relative contribution of PV-1 to diapedesis in cells that can follow both the transcellular and paracellular transmigration routes would definitively elucidate the true role of this protein in leukocyte egress from vessels.
Part of the machinery involved in endothelial membrane remodeling caused by apical leukocyte locomotion, such as VAMP3 [107], has been found to form transcytotic complexes with caveolin-1 in endothelial cells [143]. Stimuli like EGF can induce caveolin-1 relocalization to VAMP3-positive internal compartments, implying that the association of caveolin-1 with the SNARE machinery can be transient and regulated by extracellular stimuli [144]. Furthermore, in a series of in vitro experiments, Predescu and colleagues [145] have elegantly demonstrated the involvement of t-SNARE machinery in caveolar fusion events at the endothelial plasma membrane. On the other hand, electron microscopy of endothelial membranes in contact with pseudopods emitted by leukocytes during locomotion has revealed the accumulation of arrays of vesicles that resemble the VVO [107]. There is an unclear relationship between VVOs and caveolae. The two vesicular compartments are morphologically very similar, although VVOs form grape-like clusters of up to several hundred interconnected vesicles of heterogeneous diameter, whereas caveolar clusters are formed by the linking of a few vesicles that are more homogenous in size. Analysis of caveolin-1 distribution shows that 30–50 % of VVOs contain this signature protein of caveolae. VVO fusion events, like caveolae, are sensitive to N-ethylmaleimide (NEM) [146]. Additionally, the SNARE protein VAMP-2 has been found in VVOs and caveolae [146, 147]. However, electron microscopy studies of endothelium from caveolin-1 knockout mice revealed a comparable number of VVOs, whereas caveolar-like vesicles in contact with the apical or basolateral plasma membrane were significantly reduced in number. This indicates that caveolin-1 is not required for maintaining the morphological integrity of VVOs, but that the two compartments share molecular features. In summary, although caveolin-1 does not seem involved in the translocation of vesicle machinery found surrounding the leukocyte podosomes [107] there exists an evident molecular link between this machinery and caveolae.
Collectively, these data suggest a mechanism by which leukocytes may initiate transcellular migration. Leukocytes continuously induce transient ICAM-1 clustering, searching for sites where they can transmigrate by pushing down pseudopods or podosomes that constantly probe the endothelial surface. Leukocyte-induced endothelial invaginations recruit machinery with the potential to induce fusion of caveolae, VVOs, or both. When ICAM-1 is invaginated in areas with a high density of these intracellular compartments and F-actin, then the receptor induces vesicle fusion, resulting in the formation of a transcellular pore (Fig. 3).
Taken together, these results also indicate that the machinery involved in transcytosis of small molecules may contribute to the transcellular route of leukocyte transmigration, mediated mostly by the ICAM-1 receptor. Interestingly, Hu and colleagues have formally demonstrated that leukocyte transmigration regulates transcytosis in vitro and in vivo. PMN leukocyte adhesion through ICAM-1 or antibody-mediated ICAM-1 crosslinking induced caveolin-1-dependent albumin endocytosis and Src-mediated caveolin-1 phosphorylation in endothelial cells, which is suggestive of caveolin internalization [148].
Role of endothelial actin and vimentin networks in diapedesis
Actin stress fibers are associated with focal adhesion or cell–cell junctions in confluent endothelial cells [149]. As mentioned above, ICAM-1 and VCAM-1 clustering promotes receptor alignment with stress fibers. In addition, this clustering induces RhoA activation and stress fibers formation, which suggest that these receptors facilitate paracellular diapedesis by promoting cell contraction [27, 89]. On the other hand most transcellular pores opened by transmigrating T-cells appear in contact with stress fibers [60]. The role of these actin filaments in the formation of these passages is currently unclear. They may help to determine the cellular region that is most robust for the opening of the transcellular gap or, complementarily, they could contribute to establish a physical barrier that controls the size of the pore and preserves cell integrity. Stress fibers determine caveolae accumulation in the endothelial cell [60]. Caveolae are linked to F-actin through a direct interaction of caveolin-1 with filamin proteins [150]. Filamins coalign with caveolin-1 and stress fibers in response to activation of Rho GTPases [150]. In addition, filamin-A regulates caveolin-1 localization and dynamics [150–152]. Filamin-A, -B and caveolin-1 proteins have been found in ICAM-1 immunoprecipitates from endothelial cells [153]. The expression of filamin-B determines ICAM-1 lateral mobility, recruitment of ICAM-1 to endothelial docking structures upon leukocyte adhesion as well as leukocyte adhesion and transmigration [61, 153]. However, the particular contribution of filamins to different routes of leukocyte TEM has not yet been investigated. Cortactin, a multidomain scaffold protein involved in cortical actin assembly, also regulates ICAM-1 clustering around adhered PMN leukocytes as well as their transmigration, but had not effect on PMN cell adhesion [154, 155]. In vivo, cortactin-deficient mice display a reduced rate of neutrophil extravasation, despite vascular permeability is enhanced. This extravasation defect seems general and affects neutrophil rolling, adhesion and diapedesis [156]. These authors also observed in vitro that cortactin knockdown abrogated the formation of docking structures upon PMN leukocyte adhesion and, importantly, inhibited ICAM-1-mediated activation of RhoG. Therefore, cortactin regulates signaling pathways that are induced in response to leukocyte adhesion and are required for efficient diapedesis. Whereas filamins induce long-term stabilization of actin filaments, cortactin probably contributes to a rapid and more transient F-actin rearrangement at sites of leukocyte adhesion and transmigration through the stabilization of the Arp2/3 complex [157–159].
Finally, a complementary role has been recently proposed for actin stress fibers. Intracellular stores of chemokines that translocate to the cell surface upon leukocyte interaction have been found associated with stress fibers. Chemokines on the apical endothelial surface signal into adhered leukocytes and induce an inside-out, local activation of leukocyte integrins, thereby orientating leukocyte motility. This opens up the interesting possibility of stress fibers functioning as a railways that provide cues for leukocytes to find areas of extravasation [160].
Endothelial cells are enriched in intermediate filaments of vimentin [161, 162]. Endothelial vimentin networks mediate leukocyte transcellular TEM, probably through their association with caveolar PV-1 [163]. It is of note that vimentin is highly enriched in endothelial caveolae [164]. The relationship between endothelial F-actin, vimentin and caveolin has not been adequately addressed. However, given that the formation of a transcellular pore is likely to involve considerable mechanical stress, it is quite plausible that coordination between the different cytoskeletal scaffolds may be required.
In vivo observation of endothelial membrane reorganization and diapedesis
Technical improvement of in vivo microscopy, in combination with electron microscopy, is enabling the analysis of leukocyte-endothelial cell contact areas in animal models with a remarkable resolution. Adhesion receptor clustering or endothelial membrane remodeling in response to leukocyte interaction have been already detected in mice [165, 166]. The ratio between paracellular and transcellular diapedesis has been quantitated in venules from inflamed cremaster muscle and is similar to that found in vitro in HUVECs, but lower that in microvascular endothelial cells [60, 107, 165]. Accumulation of PECAM-1 around transcellular pores, compared to that detected in paracellular gaps opened by extravasating leukocytes, is, however, reduced in these microvessels [165]. Knock-in mice expressing VE-cadherin fused with α-catenin have highly stabilized endothelial adherens junctions and reduced capillary permeability. These mice showed a remarkable inhibition of total neutrophil extravasation and an impairment of paracellular but not transcellular diapedesis [167]. This confirms that most of leukocyte transmigration also occurs paracellularly in in vivo mouse models. The fact that levels of caveolin-1 expression in mouse venules do not correlate with increased neutrophil transmigration also supports the predominance of the paracellular transmigratory route [138].
Most of the high-resolution microscopy analysis of leukocyte transmigration has been performed in the cremaster muscle, but the molecular mechanisms regulating leukocyte diapedesis may differ depending on the endothelial bed and the inflammatory challenge. For instance, leukocyte homing to lymph nodes is not affected in the VE-cadherin-α-catenin chimeric mouse model [167], which suggests that adherens junction integrity may not be so relevant during leukocyte diapedesis through high endothelial venules. In a pioneering work on in vivo leukocyte diapedesis, Feng and colleagues [99] showed that rabbit neutrophils can emigrate from venules by a transcellular pathway in response to N-formyl-methionyl-leucyl-phenylalanine (FMLP). The endothelium of the blood–brain barrier (BBB) presents tight cell–cell junctions and low paracellular permeability [168]. In a model of experimental autoimmune encephalomyelitis, detailed electron microscopy studies suggest that mononuclear leukocytes have the ability to traverse post capillary venules of the BBB, leaving endothelial junctions intact [169]. This suggests that in vivo transcellular TEM may occur at higher rate in the BBB. The mechanisms mediating leukocyte diapedesis and endothelial membrane remodeling in these in vivo models have not been properly addressed yet. In general, detailed studies on the reorganization of endothelial adhesion receptors and the relevance of tetraspanin domains, rafts, LBR compartment dynamics, apical docking structures or endothelial membrane invaginations upon leukocyte interaction are still not available in in vivo systems.
Different endothelial membrane structures that almost completely encapsulate transmigrating leukocytes have been observed in vivo and termed “domes” [166, 170]. Domes are formed by basolateral and apical endothelial domains and may constitute the final outcome of all the different processes of membrane reorganization that are individually observed in vitro. Although little is known about the molecular players that are important for these structures, leukocyte-specific protein 1 (LSP1), an F-actin-binding protein recently shown to be expressed in the endothelium, seems essential for effective neutrophil transmigration and dome formation, supporting again the idea of a central role for subcortical actin in endothelial surface reorganization [170].
Concluding remarks
Leukocytes induce the simultaneous engagement of a plethora of endothelial receptors that orchestrate intracellular signaling pathways in order to facilitate diapedesis. The molecular complexity of diapedesis, revealed in recent years, is probably a consequence of the central and multitasking role of the endothelium in preserving the integrity of small vessels. Control of mechanical stress, plasma leakage and leukocyte transmigration all happen simultaneously in the initial inflammatory response and must be regulated by the endothelium. One important feature in this response is membrane dynamics, which has been summarized in this review that has been focused on the endothelial side. In the future, the major challenge for a deeper understanding of endothelial membrane remodeling upon leukocyte interaction will be the integration between the information on nanoscale membrane reorganization, such as the coalescence of tetraspanin-adhesion receptor domains, with that on microscale events, in which the submembrane cytoskeleton plays a complementary and scaffolding function. In this regard, both tetraspanin webs and lipid rafts have been shown to be involved in the dynamics of microvilli or in vesicle trafficking in other cellular processes [171–173], so they may well participate in the formation of docking structures or endothelial invaginations in response to leukocyte locomotion and diapedesis. It can also be noticed that the studies reviewed here have mainly been performed using sophisticated in vitro experimental techniques, whereas in vivo analyses of these endothelial responses are still rare. Thus, the in vivo validation of these in vitro results is clearly another main challenge in this exciting field. It is to be hoped that further insight into these aspects of endothelial cell–leukocyte interactions will help us to gain control of dysfunctional leukocyte extravasation in the origin of several inflammatory pathologies.
Acknowledgments
We would like to thank M.A. Alonso, E. Cernuda-Morollón and S. Gharbi for critical reading of the manuscript. We also appreciate the help of F. Belio with the design of the figures. Dr. J. Millán is supported by grants SAF2008-01936, SAF2011-22624 from Ministerio de Ciencia e Innovación, Spain, and received research support from Biogen-Idec. B. Marcos-Ramiro is supported by an FPI fellowship from Ministerio de Ciencia e Innovación. N. Reglero-Real is supported by a JAE fellowship from CSIC.
Abbreviations
- PMN
Polymorphonuclear
- FCS
Fluorescence correlation spectroscopy
- TIRF
Total internal reflection fluorescence
- FRET
Fluorescent resonance energy transfer
- FRAP
Fluorescence recovery after photobleaching
- TEM
Transendothelial migration
- BBB
Blood–brain barrier
- LBR
Lateral border recycling
- DRM
Detergent-resistant membranes
Footnotes
N. Reglero-Real and B. Marcos-Ramiro contributed equally to this work.
References
- 1.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7(10):803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 2.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 Suppl 1):III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 3.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 4.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 5.McMurray RW. Adhesion molecules in autoimmune disease. Semin Arthritis Rheum. 1996;25(4):215–233. doi: 10.1016/s0049-0172(96)80034-5. [DOI] [PubMed] [Google Scholar]
- 6.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 7.Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11(5):366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 8.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74(3):541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 9.Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84(4):563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- 10.Labow MA, Norton CR, Rumberger JM, Lombard-Gillooly KM, Shuster DJ, Hubbard J, Bertko R, Knaack PA, Terry RW, Harbison ML, et al. Characterization of E-selectin-deficient mice: demonstration of overlapping function of the endothelial selectins. Immunity. 1994;1(8):709–720. doi: 10.1016/1074-7613(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 11.Oppenheimer-Marks N, Davis LS, Bogue DT, Ramberg J, Lipsky PE. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J Immunol. 1991;147(9):2913–2921. [PubMed] [Google Scholar]
- 12.Jones DA, McIntire LV, Smith CW, Picker LJ. A two-step adhesion cascade for T cell/endothelial cell interactions under flow conditions. J Clin Invest. 1994;94(6):2443–2450. doi: 10.1172/JCI117612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowden RA, Ding ZM, Donnachie EM, Petersen TK, Michael LH, Ballantyne CM, Burns AR. Role of alpha4 integrin and VCAM-1 in CD18-independent neutrophil migration across mouse cardiac endothelium. Circ Res. 2002;90(5):562–569. doi: 10.1161/01.res.0000013835.53611.97. [DOI] [PubMed] [Google Scholar]
- 14.Bochner BS, Luscinskas FW, Gimbrone MA, Jr, Newman W, Sterbinsky SA, Derse-Anthony CP, Klunk D, Schleimer RP. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991;173(6):1553–1557. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nandi A, Estess P, Siegelman M. Bimolecular complex between rolling and firm adhesion receptors required for cell arrest; CD44 association with VLA-4 in T cell extravasation. Immunity. 2004;20(4):455–465. doi: 10.1016/s1074-7613(04)00077-9. [DOI] [PubMed] [Google Scholar]
- 16.Zarbock A, Ley K. New insights into leukocyte recruitment by intravital microscopy. Curr Top Microbiol Immunol. 2009;334:129–152. doi: 10.1007/978-3-540-93864-4_6. [DOI] [PubMed] [Google Scholar]
- 17.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 18.Millan J, Ridley AJ. Rho GTPases and leucocyte-induced endothelial remodelling. Biochem J. 2005;385(Pt 2):329–337. doi: 10.1042/BJ20041584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24(6):327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 20.Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7(6):467–477. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]
- 21.Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;118(26):6743–6751. doi: 10.1182/blood-2011-07-343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barreiro O, de la Fuente H, Mittelbrunn M, Sanchez-Madrid F. Functional insights on the polarized redistribution of leukocyte integrins and their ligands during leukocyte migration and immune interactions. Immunol Rev. 2007;218:147–164. doi: 10.1111/j.1600-065X.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 23.Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol. 2004;5(4):393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- 24.Wittchen ES. Endothelial signaling in paracellular and transcellular leukocyte transmigration. Front Biosci. 2009;14:2522–2545. doi: 10.2741/3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vestweber D, Broermann A, Schulte D. Control of endothelial barrier function by regulating vascular endothelial-cadherin. Curr Opin Hematol. 2010;17(3):230–236. doi: 10.1097/MOH.0b013e328338664b. [DOI] [PubMed] [Google Scholar]
- 26.Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol Rev. 2007;218:178–196. doi: 10.1111/j.1600-065X.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 27.van Wetering S, van den Berk N, van Buul JD, Mul FP, Lommerse I, Mous R, ten Klooster JP, Zwaginga JJ, Hordijk PL. VCAM-1-mediated Rac signaling controls endothelial cell–cell contacts and leukocyte transmigration. Am J Physiol Cell Physiol. 2003;285(2):C343–C352. doi: 10.1152/ajpcell.00048.2003. [DOI] [PubMed] [Google Scholar]
- 28.Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179(6):4053–4064. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- 29.Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou AP, Lampugnani MG, Gamp AC, Vestweber D, Adamson P, Dejana E, Greenwood J. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci. 2008;121(Pt 1):29–37. doi: 10.1242/jcs.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-{alpha} activated vascular endothelium under flow. Blood. 2005;106(2):584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romer LH, McLean NV, Yan HC, Daise M, Sun J, DeLisser HM. IFN-gamma and TNF-alpha induce redistribution of PECAM-1 (CD31) on human endothelial cells. J Immunol. 1995;154(12):6582–6592. [PubMed] [Google Scholar]
- 32.Martinez-Estrada OM, Manzi L, Tonetti P, Dejana E, Bazzoni G. Opposite effects of tumor necrosis factor and soluble fibronectin on junctional adhesion molecule-A in endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288(6):L1081–L1088. doi: 10.1152/ajplung.00289.2004. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Borja M, van Buul JD, Hordijk PL. The regulation of leucocyte transendothelial migration by endothelial signalling events. Cardiovasc Res. 2010;86(2):202–210. doi: 10.1093/cvr/cvq003. [DOI] [PubMed] [Google Scholar]
- 34.Shulman Z, Alon R. Real-time analysis of integrin-dependent transendothelial migration and integrin-independent interstitial motility of leukocytes. Methods Mol Biol. 2012;757:31–45. doi: 10.1007/978-1-61779-166-6_3. [DOI] [PubMed] [Google Scholar]
- 35.Lyck R, Reiss Y, Gerwin N, Greenwood J, Adamson P, Engelhardt B. T-cell interaction with ICAM-1/ICAM-2 double-deficient brain endothelium in vitro: the cytoplasmic tail of endothelial ICAM-1 is necessary for transendothelial migration of T cells. Blood. 2003;102(10):3675–3683. doi: 10.1182/blood-2003-02-0358. [DOI] [PubMed] [Google Scholar]
- 36.Greenwood J, Amos CL, Walters CE, Couraud PO, Lyck R, Engelhardt B, Adamson P. Intracellular domain of brain endothelial intercellular adhesion molecule-1 is essential for T lymphocyte-mediated signaling and migration. J Immunol. 2003;171(4):2099–2108. doi: 10.4049/jimmunol.171.4.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3(2):143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- 38.Rahman A, Fazal F. Hug tightly and say goodbye: role of endothelial ICAM-1 in leukocyte transmigration. Antioxid Redox Signal. 2009;11(4):823–839. doi: 10.1089/ars.2008.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Springer TA. Structural specializations of immunoglobulin superfamily members for adhesion to integrins and viruses. Immunol Rev. 1998;163:197–215. doi: 10.1111/j.1600-065x.1998.tb01198.x. [DOI] [PubMed] [Google Scholar]
- 40.van Buul JD, van Rijssel J, van Alphen FP, van Stalborch AM, Mul EP, Hordijk PL. ICAM-1 clustering on endothelial cells recruits VCAM-1. J Biomed Biotechnol. 2010;2010:120328. doi: 10.1155/2010/120328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barreiro O, Zamai M, Yanez-Mo M, Tejera E, Lopez-Romero P, Monk PN, Gratton E, Caiolfa VR, Sanchez-Madrid F. Endothelial adhesion receptors are recruited to adherent leukocytes by inclusion in preformed tetraspanin nanoplatforms. J Cell Biol. 2008;183(3):527–542. doi: 10.1083/jcb.200805076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards S, Lalor PF, Nash GB, Rainger GE, Adams DH. Lymphocyte traffic through sinusoidal endothelial cells is regulated by hepatocytes. Hepatology. 2005;41(3):451–459. doi: 10.1002/hep.20585. [DOI] [PubMed] [Google Scholar]
- 43.Charrin S, le Naour F, Silvie O, Milhiet PE, Boucheix C, Rubinstein E. Lateral organization of membrane proteins: tetraspanins spin their web. Biochem J. 2009;420(2):133–154. doi: 10.1042/BJ20082422. [DOI] [PubMed] [Google Scholar]
- 44.Stipp CS, Kolesnikova TV, Hemler ME. Functional domains in tetraspanin proteins. Trends Biochem Sci. 2003;28(2):106–112. doi: 10.1016/S0968-0004(02)00014-2. [DOI] [PubMed] [Google Scholar]
- 45.Kitadokoro K, Bordo D, Galli G, Petracca R, Falugi F, Abrignani S, Grandi G, Bolognesi M. CD81 extracellular domain 3D structure: insight into the tetraspanin superfamily structural motifs. EMBO J. 2001;20(1–2):12–18. doi: 10.1093/emboj/20.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang X, Claas C, Kraeft SK, Chen LB, Wang Z, Kreidberg JA, Hemler ME. Palmitoylation of tetraspanin proteins: modulation of CD151 lateral interactions, subcellular distribution, and integrin-dependent cell morphology. Mol Biol Cell. 2002;13(3):767–781. doi: 10.1091/mbc.01-05-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Espenel C, Margeat E, Dosset P, Arduise C, Le Grimellec C, Royer CA, Boucheix C, Rubinstein E, Milhiet PE. Single-molecule analysis of CD9 dynamics and partitioning reveals multiple modes of interaction in the tetraspanin web. J Cell Biol. 2008;182(4):765–776. doi: 10.1083/jcb.200803010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yanez-Mo M, Barreiro O, Gordon-Alonso M, Sala-Valdes M, Sanchez-Madrid F. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19(9):434–446. doi: 10.1016/j.tcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11(10):688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 50.Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68(3):533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 51.Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115(4):377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 52.Tilghman RW, Hoover RL. E-selectin and ICAM-1 are incorporated into detergent-insoluble membrane domains following clustering in endothelial cells. FEBS Lett. 2002;525(1–3):83–87. doi: 10.1016/s0014-5793(02)03070-3. [DOI] [PubMed] [Google Scholar]
- 53.Kiely JM, Hu Y, Garcia-Cardena G, Gimbrone MA., Jr Lipid raft localization of cell surface E-selectin is required for ligation-induced activation of phospholipase C gamma. J Immunol. 2003;171(6):3216–3224. doi: 10.4049/jimmunol.171.6.3216. [DOI] [PubMed] [Google Scholar]
- 54.Lajoie P, Goetz JG, Dennis JW, Nabi IR. Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J Cell Biol. 2009;185(3):381–385. doi: 10.1083/jcb.200811059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parton RG. Caveolae—from ultrastructure to molecular mechanisms. Nat Rev Mol Cell Biol. 2003;4(2):162–167. doi: 10.1038/nrm1017. [DOI] [PubMed] [Google Scholar]
- 56.Chidlow JH, Jr, Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res. 2010;86(2):219–225. doi: 10.1093/cvr/cvq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lajoie P, Nabi IR. Lipid rafts, caveolae, and their endocytosis. Int Rev Cell Mol Biol. 2010;282:135–163. doi: 10.1016/S1937-6448(10)82003-9. [DOI] [PubMed] [Google Scholar]
- 58.Li XA, Everson WV, Smart EJ. Caveolae, lipid rafts, and vascular disease. Trends Cardiovasc Med. 2005;15(3):92–96. doi: 10.1016/j.tcm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 59.Setiadi H, McEver RP. Clustering endothelial E-selectin in clathrin-coated pits and lipid rafts enhances leukocyte adhesion under flow. Blood. 2008;111(4):1989–1998. doi: 10.1182/blood-2007-09-113423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8(2):113–123. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- 61.van Buul JD, van Rijssel J, van Alphen FP, Hoogenboezem M, Tol S, Hoeben KA, van Marle J, Mul EP, Hordijk PL. Inside-out regulation of ICAM-1 dynamics in TNF-alpha-activated endothelium. PLoS One. 2010;5(6):e11336. doi: 10.1371/journal.pone.0011336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keuschnigg J, Henttinen T, Auvinen K, Karikoski M, Salmi M, Jalkanen S. The prototype endothelial marker PAL-E is a leukocyte trafficking molecule. Blood. 2009;114(2):478–484. doi: 10.1182/blood-2008-11-188763. [DOI] [PubMed] [Google Scholar]
- 63.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167(2):377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodriguez-Fraticelli AE, Vergarajauregui S, Eastburn DJ, Datta A, Alonso MA, Mostov K, Martin-Belmonte F. The Cdc42 GEF Intersectin 2 controls mitotic spindle orientation to form the lumen during epithelial morphogenesis. J Cell Biol. 2010;189(4):725–738. doi: 10.1083/jcb.201002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu C, Zhang YH, Thangavel M, Richardson MM, Liu L, Zhou B, Zheng Y, Ostrom RS, Zhang XA. CD82 endocytosis and cholesterol-dependent reorganization of tetraspanin webs and lipid rafts. FASEB J. 2009;23(10):3273–3288. doi: 10.1096/fj.08-123414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002;157(7):1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hogue IB, Grover JR, Soheilian F, Nagashima K, Ono A. Gag induces the coalescence of clustered lipid rafts and tetraspanin-enriched microdomains at HIV-1 assembly sites on the plasma membrane. J Virol. 2011;85(19):9749–9766. doi: 10.1128/JVI.00743-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silvie O, Charrin S, Billard M, Franetich JF, Clark KL, van Gemert GJ, Sauerwein RW, Dautry F, Boucheix C, Mazier D, Rubinstein E. Cholesterol contributes to the organization of tetraspanin-enriched microdomains and to CD81-dependent infection by malaria sporozoites. J Cell Sci. 2006;119(Pt 10):1992–2002. doi: 10.1242/jcs.02911. [DOI] [PubMed] [Google Scholar]
- 69.Zilber MT, Setterblad N, Vasselon T, Doliger C, Charron D, Mooney N, Gelin C. MHC class II/CD38/CD9: a lipid-raft-dependent signaling complex in human monocytes. Blood. 2005;106(9):3074–3081. doi: 10.1182/blood-2004-10-4094. [DOI] [PubMed] [Google Scholar]
- 70.Cherukuri A, Shoham T, Sohn HW, Levy S, Brooks S, Carter R, Pierce SK. The tetraspanin CD81 is necessary for partitioning of coligated CD19/CD21-B cell antigen receptor complexes into signaling-active lipid rafts. J Immunol. 2004;172(1):370–380. doi: 10.4049/jimmunol.172.1.370. [DOI] [PubMed] [Google Scholar]
- 71.Lange K. Fundamental role of microvilli in the main functions of differentiated cells: outline of an universal regulating and signaling system at the cell periphery. J Cell Physiol. 2011;226(4):896–927. doi: 10.1002/jcp.22302. [DOI] [PubMed] [Google Scholar]
- 72.Diakowski W, Grzybek M, Sikorski AF. Protein 4.1, a component of the erythrocyte membrane skeleton and its related homologue proteins forming the protein 4.1/FERM superfamily. Folia Histochem Cytobiol. 2006;44(4):231–248. [PubMed] [Google Scholar]
- 73.Heiska L, Alfthan K, Gronholm M, Vilja P, Vaheri A, Carpen O. Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4, 5-bisphosphate. J Biol Chem. 1998;273(34):21893–21900. doi: 10.1074/jbc.273.34.21893. [DOI] [PubMed] [Google Scholar]
- 74.Oh HM, Lee S, Na BR, Wee H, Kim SH, Choi SC, Lee KM, Jun CD. RKIKK motif in the intracellular domain is critical for spatial and dynamic organization of ICAM-1: functional implication for the leukocyte adhesion and transmigration. Mol Biol Cell. 2007;18(6):2322–2335. doi: 10.1091/mbc.E06-08-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ivetic A, Ridley AJ. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology. 2004;112(2):165–176. doi: 10.1111/j.1365-2567.2004.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11(4):276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saotome I, Curto M, McClatchey AI. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell. 2004;6(6):855–864. doi: 10.1016/j.devcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 78.Yonemura S, Tsukita S, Tsukita S. Direct involvement of ezrin/radixin/moesin (ERM)-binding membrane proteins in the organization of microvilli in collaboration with activated ERM proteins. J Cell Biol. 1999;145(7):1497–1509. doi: 10.1083/jcb.145.7.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wojciak-Stothard B, Williams L, Ridley AJ. Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering. J Cell Biol. 1999;145(6):1293–1307. doi: 10.1083/jcb.145.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carman CV, Jun CD, Salas A, Springer TA. Endothelial cells proactively form microvilli-like membrane projections upon intercellular adhesion molecule 1 engagement of leukocyte LFA-1. J Immunol. 2003;171(11):6135–6144. doi: 10.4049/jimmunol.171.11.6135. [DOI] [PubMed] [Google Scholar]
- 81.van Buul JD, Allingham MJ, Samson T, Meller J, Boulter E, Garcia-Mata R, Burridge K. RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration. J Cell Biol. 2007;178(7):1279–1293. doi: 10.1083/jcb.200612053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clancy RM, Abramson SB. Acetylcholine prevents intercellular adhesion molecule 1 (CD54)-induced focal adhesion complex assembly in endothelial cells via a nitric oxide- cGMP-dependent pathway. Arthritis Rheum. 2000;43(10):2260–2264. doi: 10.1002/1529-0131(200010)43:10<2260::AID-ANR13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 83.Booth JW, Trimble WS, Grinstein S. Membrane dynamics in phagocytosis. Semin Immunol. 2001;13(6):357–364. doi: 10.1006/smim.2001.0332. [DOI] [PubMed] [Google Scholar]
- 84.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9(9):690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 85.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16(10):522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 86.Martin-Belmonte F, Mostov K. Regulation of cell polarity during epithelial morphogenesis. Curr Opin Cell Biol. 2008;20(2):227–234. doi: 10.1016/j.ceb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 87.Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128(2):383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saito H, Minamiya Y, Saito S, Ogawa J. Endothelial Rho and Rho kinase regulate neutrophil migration via endothelial myosin light chain phosphorylation. J Leukoc Biol. 2002;72(4):829–836. [PubMed] [Google Scholar]
- 89.Thompson PW, Randi AM, Ridley AJ. Intercellular adhesion molecule (ICAM)-1, but not ICAM-2, activates RhoA and stimulates c-fos and rhoA transcription in endothelial cells. J Immunol. 2002;169(2):1007–1013. doi: 10.4049/jimmunol.169.2.1007. [DOI] [PubMed] [Google Scholar]
- 90.Etienne S, Adamson P, Greenwood J, Strosberg AD, Cazaubon S, Couraud PO. ICAM-1 signaling pathways associated with Rho activation in microvascular brain endothelial cells. J Immunol. 1998;161(10):5755–5761. [PubMed] [Google Scholar]
- 91.deBakker CD, Haney LB, Kinchen JM, Grimsley C, Lu M, Klingele D, Hsu PK, Chou BK, Cheng LC, Blangy A, Sondek J, Hengartner MO, Wu YC, Ravichandran KS. Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr Biol. 2004;14(24):2208–2216. doi: 10.1016/j.cub.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 92.Cain RJ, Vanhaesebroeck B, Ridley AJ. The PI3K p110alpha isoform regulates endothelial adherens junctions via Pyk2 and Rac1. J Cell Biol. 2010;188(6):863–876. doi: 10.1083/jcb.200907135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Katoh H, Negishi M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature. 2003;424(6947):461–464. doi: 10.1038/nature01817. [DOI] [PubMed] [Google Scholar]
- 94.Katoh H, Hiramoto K, Negishi M. Activation of Rac1 by RhoG regulates cell migration. J Cell Sci. 2006;119(Pt 1):56–65. doi: 10.1242/jcs.02720. [DOI] [PubMed] [Google Scholar]
- 95.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70(3):401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 96.Dharmawardhane S, Sanders LC, Martin SS, Daniels RH, Bokoch GM. Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J Cell Biol. 1997;138(6):1265–1278. doi: 10.1083/jcb.138.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8(1):37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 98.Shaw SK, Ma S, Kim MB, Rao RM, Hartman CU, Froio RM, Yang L, Jones T, Liu Y, Nusrat A, Parkos CA, Luscinskas FW. Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J Exp Med. 2004;200(12):1571–1580. doi: 10.1084/jem.20040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med. 1998;187(6):903–915. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Holz LE, Warren A, Le Couteur DG, Bowen DG, Bertolino P. CD8+ T cell tolerance following antigen recognition on hepatocytes. J Autoimmun. 2010;34(1):15–22. doi: 10.1016/j.jaut.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 102.Williamson JR, Grisham JW. Leucocytic emigration from inflamed capillaries. Nature. 1960;188:1203. doi: 10.1038/1881203a0. [DOI] [PubMed] [Google Scholar]
- 103.Williamson JR, Grisham JW. Electron microscopy of leukocytic margination and emigration in acute inflammation in dog pancreas. Am J Pathol. 1961;39:239–256. [PMC free article] [PubMed] [Google Scholar]
- 104.Shulman Z, Shinder V, Klein E, Grabovsky V, Yeger O, Geron E, Montresor A, Bolomini-Vittori M, Feigelson SW, Kirchhausen T, Laudanna C, Shakhar G, Alon R. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity. 2009;30(3):384–396. doi: 10.1016/j.immuni.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203(12):2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith A, Carrasco YR, Stanley P, Kieffer N, Batista FD, Hogg N. A talin-dependent LFA-1 focal zone is formed by rapidly migrating T lymphocytes. J Cell Biol. 2005;170(1):141–151. doi: 10.1083/jcb.200412032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26(6):784–797. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carman CV, Springer TA. Trans-cellular migration: cell–cell contacts get intimate. Curr Opin Cell Biol. 2008;20(5):533–540. doi: 10.1016/j.ceb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Malsam J, Kreye S, Sollner TH. Membrane fusion: SNAREs and regulation. Cell Mol Life Sci. 2008;65(18):2814–2832. doi: 10.1007/s00018-008-8352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61(1):22–32. doi: 10.1016/s1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- 111.van Buul JD, Kanters E, Hordijk PL. Endothelial signaling by Ig-like cell adhesion molecules. Arterioscler Thromb Vasc Biol. 2007;27(9):1870–1876. doi: 10.1161/ATVBAHA.107.145821. [DOI] [PubMed] [Google Scholar]
- 112.Etienne-Manneville S, Manneville JB, Adamson P, Wilbourn B, Greenwood J, Couraud PO. ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J Immunol. 2000;165(6):3375–3383. doi: 10.4049/jimmunol.165.6.3375. [DOI] [PubMed] [Google Scholar]
- 113.van Buul JD, Voermans C, van den Berg V, Anthony EC, Mul FP, van Wetering S, van der Schoot CE, Hordijk PL. Migration of human hematopoietic progenitor cells across bone marrow endothelium is regulated by vascular endothelial cadherin. J Immunol. 2002;168(2):588–596. doi: 10.4049/jimmunol.168.2.588. [DOI] [PubMed] [Google Scholar]
- 114.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121(Pt 13):2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 115.Wang Q, Pfeiffer GR, 2nd, Gaarde WA. Activation of SRC tyrosine kinases in response to ICAM-1 ligation in pulmonary microvascular endothelial cells. J Biol Chem. 2003;278(48):47731–47743. doi: 10.1074/jbc.M308466200. [DOI] [PubMed] [Google Scholar]
- 116.Bixel MG, Li H, Petri B, Khandoga AG, Khandoga A, Zarbock A, Wolburg-Buchholz K, Wolburg H, Sorokin L, Zeuschner D, Maerz S, Butz S, Krombach F, Vestweber D. CD99 and CD99L2 act at the same site as, but independently of, PECAM-1 during leukocyte diapedesis. Blood. 2010;116(7):1172–1184. doi: 10.1182/blood-2009-12-256388. [DOI] [PubMed] [Google Scholar]
- 117.Wegmann F, Petri B, Khandoga AG, Moser C, Khandoga A, Volkery S, Li H, Nasdala I, Brandau O, Fassler R, Butz S, Krombach F, Vestweber D. ESAM supports neutrophil extravasation, activation of Rho, and VEGF-induced vascular permeability. J Exp Med. 2006;203(7):1671–1677. doi: 10.1084/jem.20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reymond N, Imbert AM, Devilard E, Fabre S, Chabannon C, Xerri L, Farnarier C, Cantoni C, Bottino C, Moretta A, Dubreuil P, Lopez M. DNAM-1 and PVR regulate monocyte migration through endothelial junctions. J Exp Med. 2004;199(10):1331–1341. doi: 10.1084/jem.20032206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Woodfin A, Reichel CA, Khandoga A, Corada M, Voisin MB, Scheiermann C, Haskard DO, Dejana E, Krombach F, Nourshargh S. JAM-A mediates neutrophil transmigration in a stimulus-specific manner in vivo: evidence for sequential roles for JAM-A and PECAM-1 in neutrophil transmigration. Blood. 2007;110(6):1848–1856. doi: 10.1182/blood-2006-09-047431. [DOI] [PubMed] [Google Scholar]
- 120.Aird WC. Endothelial cell heterogeneity. Crit Care Med. 2003;31(4 Suppl):S221–S230. doi: 10.1097/01.CCM.0000057847.32590.C1. [DOI] [PubMed] [Google Scholar]
- 121.Garlanda C, Dejana E. Heterogeneity of endothelial cells. Specific markers. Arterioscler Thromb Vasc Biol. 1997;17(7):1193–1202. doi: 10.1161/01.atv.17.7.1193. [DOI] [PubMed] [Google Scholar]
- 122.Albelda SM. Endothelial and epithelial cell adhesion molecules. Am J Respir Cell Mol Biol. 1991;4(3):195–203. doi: 10.1165/ajrcmb/4.3.195. [DOI] [PubMed] [Google Scholar]
- 123.Dejana E. Endothelial cell–cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5(4):261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 124.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature. 2003;421(6924):748–753. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- 125.Mamdouh Z, Kreitzer GE, Muller WA. Leukocyte transmigration requires kinesin-mediated microtubule-dependent membrane trafficking from the lateral border recycling compartment. J Exp Med. 2008;205(4):951–966. doi: 10.1084/jem.20072328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mamdouh Z, Mikhailov A, Muller WA. Transcellular migration of leukocytes is mediated by the endothelial lateral border recycling compartment. J Exp Med. 2009;206(12):2795–2808. doi: 10.1084/jem.20082745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Braet F, Riches J, Geerts W, Jahn KA, Wisse E, Frederik P. Three-dimensional organization of fenestrae labyrinths in liver sinusoidal endothelial cells. Liver Int. 2009;29(4):603–613. doi: 10.1111/j.1478-3231.2008.01836.x. [DOI] [PubMed] [Google Scholar]
- 128.Vasile E, Qu H, Dvorak HF, Dvorak AM. Caveolae and vesiculo-vacuolar organelles in bovine capillary endothelial cells cultured with VPF/VEGF on floating Matrigel-collagen gels. J Histochem Cytochem. 1999;47(2):159–167. doi: 10.1177/002215549904700205. [DOI] [PubMed] [Google Scholar]
- 129.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Ultrastructural localization of platelet endothelial cell adhesion molecule (PECAM-1, CD31) in vascular endothelium. J Histochem Cytochem. 2004;52(1):87–101. doi: 10.1177/002215540405200109. [DOI] [PubMed] [Google Scholar]
- 130.Ozaki H, Ishii K, Horiuchi H, Arai H, Kawamoto T, Okawa K, Iwamatsu A, Kita T. Cutting edge: combined treatment of TNF-alpha and IFN-gamma causes redistribution of junctional adhesion molecule in human endothelial cells. J Immunol. 1999;163(2):553–557. [PubMed] [Google Scholar]
- 131.Manes TD, Pober JS. Identification of endothelial cell junctional proteins and lymphocyte receptors involved in transendothelial migration of human effector memory CD4+ T cells. J Immunol. 2011;186(3):1763–1768. doi: 10.4049/jimmunol.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3(2):151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 133.Wojcikiewicz EP, Koenen RR, Fraemohs L, Minkiewicz J, Azad H, Weber C, Moy VT. LFA-1 binding destabilizes the JAM-A homophilic interaction during leukocyte transmigration. Biophys J. 2009;96(1):285–293. doi: 10.1529/biophysj.108.135491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Santoso S, Sachs UJ, Kroll H, Linder M, Ruf A, Preissner KT, Chavakis T. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med. 2002;196(5):679–691. doi: 10.1084/jem.20020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zen K, Babbin BA, Liu Y, Whelan JB, Nusrat A, Parkos CA. JAM-C is a component of desmosomes and a ligand for CD11b/CD18-mediated neutrophil transepithelial migration. Mol Biol Cell. 2004;15(8):3926–3937. doi: 10.1091/mbc.E04-04-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lamagna C, Meda P, Mandicourt G, Brown J, Gilbert RJ, Jones EY, Kiefer F, Ruga P, Imhof BA, Aurrand-Lions M. Dual interaction of JAM-C with JAM-B and alpha(M)beta2 integrin: function in junctional complexes and leukocyte adhesion. Mol Biol Cell. 2005;16(10):4992–5003. doi: 10.1091/mbc.E05-04-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ludwig RJ, Hardt K, Hatting M, Bistrian R, Diehl S, Radeke HH, Podda M, Schon MP, Kaufmann R, Henschler R, Pfeilschifter JM, Santoso S, Boehncke WH. Junctional adhesion molecule (JAM)-B supports lymphocyte rolling and adhesion through interaction with alpha4beta1 integrin. Immunology. 2009;128(2):196–205. doi: 10.1111/j.1365-2567.2009.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Marmon S, Hinchey J, Oh P, Cammer M, de Almeida CJ, Gunther L, Raine CS, Lisanti MP. Caveolin-1 expression determines the route of neutrophil extravasation through skin microvasculature. Am J Pathol. 2009;174(2):684–692. doi: 10.2353/ajpath.2009.080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stan RV, Ghitescu L, Jacobson BS, Palade GE. Isolation, cloning, and localization of rat PV-1, a novel endothelial caveolar protein. J Cell Biol. 1999;145(6):1189–1198. doi: 10.1083/jcb.145.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stan RV, Kubitza M, Palade GE. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc Natl Acad Sci USA. 1999;96(23):13203–13207. doi: 10.1073/pnas.96.23.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stan RV, Tkachenko E, Niesman IR. PV1 is a key structural component for the formation of the stomatal and fenestral diaphragms. Mol Biol Cell. 2004;15(8):3615–3630. doi: 10.1091/mbc.E03-08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stan RV. Endothelial stomatal and fenestral diaphragms in normal vessels and angiogenesis. J Cell Mol Med. 2007;11(4):621–643. doi: 10.1111/j.1582-4934.2007.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Predescu SA, Predescu DN, Palade GE. Endothelial transcytotic machinery involves supramolecular protein–lipid complexes. Mol Biol Cell. 2001;12(4):1019–1033. doi: 10.1091/mbc.12.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pol A, Lu A, Pons M, Peiro S, Enrich C. Epidermal growth factor-mediated caveolin recruitment to early endosomes and MAPK activation. Role of cholesterol and actin cytoskeleton. J Biol Chem. 2000;275(39):30566–30572. doi: 10.1074/jbc.M001131200. [DOI] [PubMed] [Google Scholar]
- 145.Predescu SA, Predescu DN, Shimizu K, Klein IK, Malik AB. Cholesterol-dependent syntaxin-4 and SNAP-23 clustering regulates caveolar fusion with the endothelial plasma membrane. J Biol Chem. 2005;280(44):37130–37138. doi: 10.1074/jbc.M505659200. [DOI] [PubMed] [Google Scholar]
- 146.Schnitzer JE, Allard J, Oh P. NEM inhibits transcytosis, endocytosis, and capillary permeability: implication of caveolae fusion in endothelia. Am J Physiol. 1995;268(1 Pt 2):H48–H55. doi: 10.1152/ajpheart.1995.268.1.H48. [DOI] [PubMed] [Google Scholar]
- 147.Dvorak AM, Feng D. The vesiculo-vacuolar organelle (VVO). A new endothelial cell permeability organelle. J Histochem Cytochem. 2001;49(4):419–432. doi: 10.1177/002215540104900401. [DOI] [PubMed] [Google Scholar]
- 148.Hu G, Vogel SM, Schwartz DE, Malik AB, Minshall RD. Intercellular adhesion molecule-1-dependent neutrophil adhesion to endothelial cells induces caveolae-mediated pulmonary vascular hyperpermeability. Circ Res. 2008;102(12):e120–e131. doi: 10.1161/CIRCRESAHA.107.167486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Millan J, Cain RJ, Reglero-Real N, Bigarella C, Marcos-Ramiro B, Fernandez-Martin L, Correas I, Ridley AJ. Adherens junctions connect stress fibres between adjacent endothelial cells. BMC Biol. 2010;8:11. doi: 10.1186/1741-7007-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stahlhut M, van Deurs B. Identification of filamin as a novel ligand for caveolin-1: evidence for the organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol Biol Cell. 2000;11(1):325–337. doi: 10.1091/mbc.11.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Muriel O, Echarri A, Hellriegel C, Pavon DM, Beccari L, Del Pozo MA. Phosphorylated filamin A regulates actin-linked caveolae dynamics. J Cell Sci. 2011;124(Pt 16):2763–2776. doi: 10.1242/jcs.080804. [DOI] [PubMed] [Google Scholar]
- 152.Sverdlov M, Shinin V, Place AT, Castellon M, Minshall RD. Filamin A regulates caveolae internalization and trafficking in endothelial cells. Mol Biol Cell. 2009;20(21):4531–4540. doi: 10.1091/mbc.E08-10-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kanters E, van Rijssel J, Hensbergen PJ, Hondius D, Mul FP, Deelder AM, Sonnenberg A, van Buul JD, Hordijk PL. Filamin B mediates ICAM-1-driven leukocyte transendothelial migration. J Biol Chem. 2008;283(46):31830–31839. doi: 10.1074/jbc.M804888200. [DOI] [PubMed] [Google Scholar]
- 154.Yang L, Kowalski JR, Zhan X, Thomas SM, Luscinskas FW. Endothelial cell cortactin phosphorylation by Src contributes to polymorphonuclear leukocyte transmigration in vitro. Circ Res. 2006;98(3):394–402. doi: 10.1161/01.RES.0000201958.59020.1a. [DOI] [PubMed] [Google Scholar]
- 155.Yang L, Kowalski JR, Yacono P, Bajmoczi M, Shaw SK, Froio RM, Golan DE, Thomas SM, Luscinskas FW. Endothelial cell cortactin coordinates intercellular adhesion molecule-1 clustering and actin cytoskeleton remodeling during polymorphonuclear leukocyte adhesion and transmigration. J Immunol. 2006;177(9):6440–6449. doi: 10.4049/jimmunol.177.9.6440. [DOI] [PubMed] [Google Scholar]
- 156.Schnoor M, Lai FP, Zarbock A, Klaver R, Polaschegg C, Schulte D, Weich HA, Oelkers JM, Rottner K, Vestweber D. Cortactin deficiency is associated with reduced neutrophil recruitment but increased vascular permeability in vivo. J Exp Med. 2011;208(8):1721–1735. doi: 10.1084/jem.20101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Uruno T, Liu J, Zhang P, Fan Y, Egile C, Li R, Mueller SC, Zhan X. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat Cell Biol. 2001;3(3):259–266. doi: 10.1038/35060051. [DOI] [PubMed] [Google Scholar]
- 158.Flanagan LA, Chou J, Falet H, Neujahr R, Hartwig JH, Stossel TP. Filamin A, the Arp2/3 complex, and the morphology and function of cortical actin filaments in human melanoma cells. J Cell Biol. 2001;155(4):511–517. doi: 10.1083/jcb.200105148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Viola A, Gupta N. Tether and trap: regulation of membrane-raft dynamics by actin-binding proteins. Nat Rev Immunol. 2007;7(11):889–896. doi: 10.1038/nri2193. [DOI] [PubMed] [Google Scholar]
- 160.Shulman Z, Cohen SJ, Roediger B, Kalchenko V, Jain R, Grabovsky V, Klein E, Shinder V, Stoler-Barak L, Feigelson SW, Meshel T, Nurmi SM, Goldstein I, Hartley O, Gahmberg CG, Etzioni A, Weninger W, Ben-Baruch A, Alon R. Transendothelial migration of lymphocytes mediated by intraendothelial vesicle stores rather than by extracellular chemokine depots. Nat Immunol. 2011 doi: 10.1038/ni.2173. [DOI] [PubMed] [Google Scholar]
- 161.Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, Goldman RD. Introducing intermediate filaments: from discovery to disease. J Clin Investig. 2009;119(7):1763–1771. doi: 10.1172/JCI38339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pallari HM, Eriksson JE. Intermediate filaments as signaling platforms. Sci STKE. 2006;2006(366):pe53. doi: 10.1126/stke.3662006pe53. [DOI] [PubMed] [Google Scholar]
- 163.Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8(2):156–162. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 164.Sprenger RR, Fontijn RD, van Marle J, Pannekoek H, Horrevoets AJ. Spatial segregation of transport and signalling functions between human endothelial caveolae and lipid raft proteomes. Biochem J. 2006;400(3):401–410. doi: 10.1042/BJ20060355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, Meda P, Imhof BA, Nourshargh S. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol. 2011;12(8):761–769. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Phillipson M, Kaur J, Colarusso P, Ballantyne CM, Kubes P. Endothelial domes encapsulate adherent neutrophils and minimize increases in vascular permeability in paracellular and transcellular emigration. PLoS One. 2008;3(2):e1649. doi: 10.1371/journal.pone.0001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schulte D, Kuppers V, Dartsch N, Broermann A, Li H, Zarbock A, Kamenyeva O, Kiefer F, Khandoga A, Massberg S, Vestweber D. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J. 2011;30(20):4157–4170. doi: 10.1038/emboj.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Coisne C, Engelhardt B. Tight junctions in brain barriers during central nervous system inflammation. Antioxid Redox Signal. 2011;15(5):1285–1303. doi: 10.1089/ars.2011.3929. [DOI] [PubMed] [Google Scholar]
- 169.Wolburg H, Wolburg-Buchholz K, Engelhardt B. Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol. 2005;109(2):181–190. doi: 10.1007/s00401-004-0928-x. [DOI] [PubMed] [Google Scholar]
- 170.Petri B, Kaur J, Long EM, Li H, Parsons SA, Butz S, Phillipson M, Vestweber D, Patel KD, Robbins SM, Kubes P. Endothelial LSP1 is involved in endothelial dome formation, minimizing vascular permeability changes during neutrophil transmigration in vivo. Blood. 2011;117(3):942–952. doi: 10.1182/blood-2010-02-270561. [DOI] [PubMed] [Google Scholar]
- 171.Wang HX, Kolesnikova TV, Denison C, Gygi SP, Hemler ME. The C-terminal tail of tetraspanin protein CD9 contributes to its function and molecular organization. J Cell Sci. 2011;124(Pt 16):2702–2710. doi: 10.1242/jcs.085449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bari R, Guo Q, Xia B, Zhang YH, Giesert EE, Levy S, Zheng JJ, Zhang XA. Tetraspanins regulate the protrusive activities of cell membrane. Biochem Biophys Res Commun. 2011;415(4):619–626. doi: 10.1016/j.bbrc.2011.10.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21(4):708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]