Abstract
Gap junctions consist of arrays of intercellular channels composed of integral membrane proteins called connexin in vertebrates. Gap junction channels regulate the passage of ions and biological molecules between adjacent cells and, therefore, are critically important in many biological activities, including development, differentiation, neural activity, and immune response. Mutations in connexin genes are associated with several human diseases, such as neurodegenerative disease, skin disease, deafness, and developmental abnormalities. The activity of gap junction channels is regulated by the membrane voltage, intracellular microenvironment, interaction with other proteins, and phosphorylation. Each connexin channel has its own property for conductance and molecular permeability. A number of studies have tried to reveal the molecular architecture of the channel pore that should confer the connexin-specific permeability/selectivity properties and molecular basis for the gating and regulation. In this review, we give an overview of structural studies and describe the structural and functional relationship of gap junction channels.
Keywords: Gap junction, Connexin, Electron microscopy, X-ray diffraction, Gating, Regulation, Permeability, Selectivity
The general function of gap junctions
Intercellular signaling is fundamental to the complex biological functions of multicellular organisms such as neural transmission, immune reaction, or reproductive function [1]. Physiological functions that are critically dependent on this intracellular signaling include synaptic transmission, hormone-receptor signaling, and cell adhesion. These processes are all mediated by membrane proteins such as ion channels, G-protein coupled receptors (GPCRs), or receptor tyrosine kinases. Gap junctions are, however, unique in that they mediate intercellular signals by connecting the cytoplasms of two neighboring cells. A gap junction contains clusters of tens to thousands of intercellular channels called “gap junction channels,” each of which is formed by the end-to-end docking of two hemichannels, also referred to as “connexons.” Each connexon is composed of six connexin subunits surrounding the central pore. Connexin has been predicted to have four transmembrane alpha helices and two extracellular loops, each of which has three highly conserved cysteine residues. These cysteines make disulfide bonds between the loops [2], which are essential for the formation of functional gap junction channel [3]. There are 21 connexin (Cx) isoforms in the human proteome with different physiological properties and regulation responses. Some of them are expressed in a single cell type and form heteromeric (more than two different connexins in a connexon) or heterotypic (a gap junction channel with different connexons) channels, conferring further diversity in their composition and function.
Gap junctions are expressed in a wide variety of cells, organs, and tissues, and play essential roles in a variety of biological processes. In the developing brain, gap junctions are expressed in periventricular precursor cells and mediate synchronous Ca2+ oscillations, which coordinate and regulate the proliferation of neural cells [4–6]. The neural cells migrate along with the radial glial cell [7], and the expression pattern of connexins appears to be associated with neural differentiation [8–10]. Alterations of spatiotemporal expression patterns of connexins, Cx43, Cx40, and Cx45, are also observed in the developing heart [11–13]. It is possible that the expression of different connexins in altered spatiotemporal patterns is related to the electrical and signal patterning or formation of the electrical and signal compartments. Impairments of these expression patterns caused by knockout (KO) of certain connexins have exhibited a number of malformations or malfunctions of cardiac tissue [14–21]. Mutations of Cx43 in its C-terminal domain are associated with heart malformations [22–24], both of which support the crucial involvement of connexins in cardiac development. The inner ear in mammals consists of fluid-filled organs, the cochlea and the vestibule, which are essential for sound transduction and sensing the movement of the head. The cochlea has two major spaces, the scala media and scala tympani, filled with endolymph, high K+ and low Na+, and perilymph, Na+ and low K+, respectively. Upon sound transduction, K+ current flows through the organ of Corti to the perilymph, generating the auditory signal. K+ is subsequently taken up by transporters and recycled to the endolymph through the gap junction intercellular network. Two major connexins, Cx26 and Cx30, are co-expressed and form homo- and hetero-gap junctions in the inner ear [25–27], and mutations in these proteins are known to be associated with hearing loss [28, 29], underscoring the importance of gap junctional communication in auditory function. The lens is an extensively specialized organ that is spherical and transparent for visual function. Three different connexins, Cx43, Cx46, and Cx50, contribute to the development, maturation, and maintenance of lens fiber cells [30–32]. As in the case of the brain and the heart, the expression pattern of lens connexins changes during the differentiation of the lens fiber [33–37]. Since the lens fiber cells are devoid of mitochondria, nuclei, and other cytoplasmic organelles, thus making the tissue transparent, they must exchange metabolites and ions in a specific manner. In fact, they are coupled with the surrounding epithelial cells for transporting essential molecules and removing waste through gap junctions [38, 39]. Mutations in lens connexins are associated with cataracts [40–44], possibly because of impairment in communication with surrounding cells. In addition to the above examples, gap junctions play essential roles in a wide variety of biological processes, such as the vascular, reproductive, and immune systems, as well as in the development and progression of cancer [45–54].
Structural studies of gap junction channels
The structural information of a protein molecule is quite useful and important for studying its functions. The primary method for three-dimensional structural analysis of the gap junction channel has been electron microscopy. In the 1960s, Robertson [55] first described the hexagonal array of protein molecules on the plasma membrane of Mauthner cell synapses of goldfish, and Benedetti and Emmelot [56] identified almost the same structure in isolated rat liver. In the 1970s, Zampighi and Unwin isolated two forms of channels from rat liver and later proposed a gating model, in which sliding or tilting of each subunit closes the pore, from an 18-Å-resolution map [57, 58]. In the 1990s, Yeager et al. utilized a mammalian expression system to express a C-terminally truncated Cx43 and improved the resolution of the map to 7.5 Å [59–62], where 24 helical structures in each connexon were identified. They further improved the map up to 5.7-Å resolution [63] and proposed a helical arrangement of the four helices in a connexin subunit. More recently, Oshima et al. [64] revealed a pore plug structure in the channel vestibule of recombinant Cx26_M34A mutant at a 10-Å resolution map. Other methods, including X-ray diffraction [65–67], atomic force microscopy (AFM) [68, 69], and nuclear magnetic resonance (NMR) [68, 70–73], in combination with mutational, biochemical, and some functional studies, have added valuable structural information about the gap junction channels [74–79]. Although there has been a great deal of progress in understanding the structural biology of the gap junction channels, a high resolution structure where each amino acid could be distinguished is essential for a more detailed biochemical and physiological analysis. The long-awaited high resolution structure has been recently determined at a resolution of 3.5 Å by three-dimensional X-ray crystallographic analysis [80]. The structural determination was initiated by the single isomorphous heavy atom replacement method coupled with the anomalous dispersion method. The initial phases were refined and extended to 3.5-Å resolution by non-crystallographic symmetry averaging, multi-crystal averaging, solvent flattening, and histogram matching. Amino acid sequences and disulfide bonds were uniquely assigned by anomalous dispersion signals of the native and seleno-methionine derivative crystals [81]. Although precise modeling of side chains or atomic positions is hardly achievable at 3.5-Å resolution, the structure figures out many previously unclear features and indicates possible roles of each amino acid residue.
Molecular architecture of the Cx26 gap junction channel
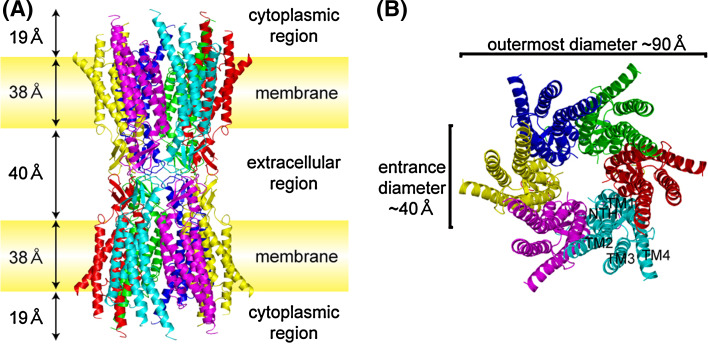
The overall structure of the Cx26 gap junction channel resembles the maps of cryo-electron microscopic (cryoEM) analysis in its shape, size, and arrangement of the transmembrane helices [62, 64]. The outer diameter of the channel at the cytoplasmic end is ~90 Å, which decreases to about 50 Å in the extracellular portion, thereby forming a structure similar to a tsuzumi, a traditional Japanese drum (Fig. 1). The inner diameter of the channel is ~40 Å at the cytoplasmic channel entrance and narrows to ~14 Å around the midpoint of the membrane region, and narrows again to ~17 Å at the extracellular boundary, where transmembrane helix 1 (TM1) is kinked followed by a 310 helix. The pore diameter widens to ~25 Å near the extracellular cavity. Although it is possible that the missing region in the cytoplasmic loop (CL) and cytoplasmic tail (CT) could form the gate, the structure is considered an open conformation, since no obstructions through the pore vestibule were identified, and the crystallization condition (calcium or magnesium free, phosphate buffer at neutral pH) generally favors the channel in its open state. The length of the channel is approximately 155 Å. The extracellular “gap,” membrane spanning region, and protruding helices into the cytoplasmic region are 40, 38, and 19 Å, respectively. This topology is roughly in agreement with the X-ray scattering density profile of the mouse liver gap junction [65], where the major connexins are Cx32 and Cx26 [82]. The extracellular surface of the connexon in the structure of Cx26 gap junction channel is rather smooth and does not have protrusions like that in the EM study [83]. The difference might be simply due to the docking interactions between apposing hemichannels or the deformation caused by urea treatment during sample preparation to split gap junctions in the EM study. In X-ray structure determination, several biochemical and biophysical data indicated that they still maintained a dodecameric gap junction channel. Thus, the X-ray structure represents more reliable configuration of the extracellular region. The channel contains substantial alpha-helical structures, as much as ~60% including the 24 transmembrane alpha helices, short helices in NT and E1. Previous circular dichroism (CD) spectroscopic study showed that rat liver gap junctions had 40–50% alpha-helical content, depending on the isolation procedure [84]. The lower estimate is probably due to the connexin composition of rat liver, where the ratio of Cx32 and Cx26 is 10:1 [82, 85], and Cx32 has a longer cytoplasmic C-terminus, considered to have a flexible structure [83, 86]. TM2 and TM3 comprise the cytoplasmic channel entrance. In contrast to the cryoEM structure of Cx43ΔCT [62], in which TM1 mostly extends into the cytoplasmic space, cryoEM and X-ray crystal structures of Cx26 [64, 80] reveal that TM2 is the helix most extending into the cytoplasmic space. Since the primary structure of the cytoplasmic region of connexin is much more variable compared to the transmembrane and extracellular regions [87], the difference might reflect the variety of the three-dimensional structures of connexins in this region.
Fig. 1.
Overall structure of the human Cx26 gap junction channel in ribbon representation. a Side view of the Cx26 gap junction channel with the locations of plasma membranes and scale of each region. Each subunit is colored differently, and those associated with the crystallographic two-fold axis are in the same color. b Top view of the Cx26 gap junction channel representing the arrangement of the transmembrane helices and the N-terminal helix. The channel has a hexagonal appearance with the largest outer diameter of ~90 Å and a pore entrance of ~40 Å
Structure of the Cx26 monomer
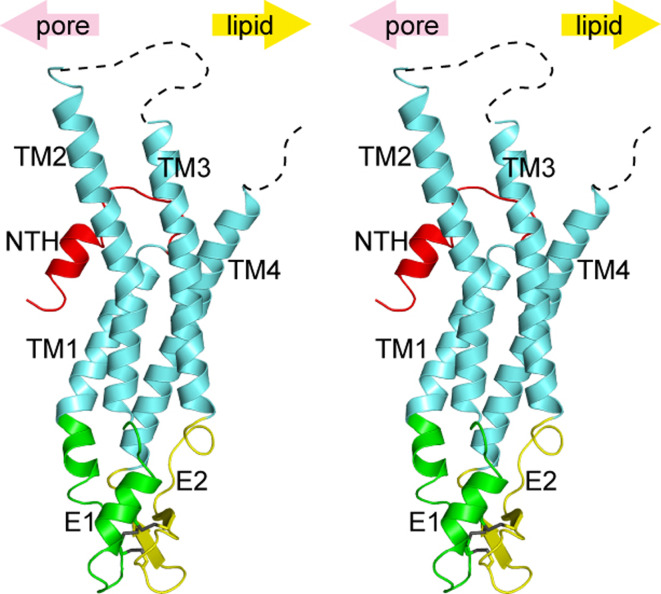
Each monomer of the Cx26 gap junction channel has four transmembrane helices (TM1-TM4), two extracellular loops (E1, E2), an N-terminal region (NT), a cytoplasmic loop (CL), and a C-terminal tail (CT; Fig. 2). The topology of connexins was proposed and tested by hydropathy plots, protease sensitivity, and site-directed antibodies to examine whether specific regions are cytoplasmic or extracellular [60, 88–97]. Although only a few connexins were subjected to the topology tests, the results were considered to be broadly applicable to all connexin family members with their conserved primary structures. The proposed topology is almost identical to that of the X-ray crystal structure. However, the atomic structure reveals a novel conformation and position of the NT. We have named this region the “N-terminal helix (NTH)” [80]. Although topologically NT has been depicted in the cytoplasmic region, it showed resistance to both proteases and antibodies [88, 89]. The structure reveals that NT is inserted into the lumen of the channel, thus accounting for the limited accessibility of proteases and antibodies.
Fig. 2.
Wall-eye stereo view of the Cx26 monomer in ribbon representation. Each region is colored differently, and the upper arrows indicate the pore side and the lipid side. Three disulfide bonds in the extracellular region are shown in stick representation. Unobserved regions in the cytoplasmic loop and the C-terminal tail are represented by dashed lines
The Cx26 monomer has the typical four-helical bundle in which any pair of adjacent helices is antiparallel. The anomalous signals from seleno-methionine derivative crystals confirm the assignment of the helices on the experimental density map [80, 81] (Fig. 2). TM1 and TM2 face the luminal side of the pore, although TM2 and the cytoplasmic half of TM1 are covered by the NTH, and they are not exposed to the lumen. TM3 and TM4 are on the perimeter of the hemichannel facing the lipid environment. There was substantial complexity and controversy surrounding the assignment of connexin alpha helices, especially on the composition of pore-exposed regions [63, 75, 76, 98]. Fleishman et al. [63] proposed an assignment of the transmembrane helices based on the EM map and theoretical models, where they proposed TM3 as the major pore helix. Biochemical studies using the substituted cysteine accessiblity method (SCAM) have been performed by several groups [75, 76, 98]. Skerrett et al. proposed TM3 as the major helix. Kronengold et al. proposed TM1 as the major helix, and Zhou et al. proposed both helices contribute to the pore. Some other studies, including domain swap chimera [99, 100] and point mutation [74, 101, 102], suggested the involvement of E1 and NT in defining the conductance properties of connexin channels. Our crystal structure is, for the most part, consistent with the SCAM result of Kronengold et al. and chimeric and mutational studies that implicated residues in NT, TM1, and E1 as pore-lining. The difference in our structure and other SCAM studies may arise as a consequence of the difference in methodology, the kind of connexins used, the state of the channels, and the form of the channels that they investigated. Baldwin et al. [103] proposed that in membrane proteins, conserved amino acid residues are likely to mediate helix-helix packing, whereas the non-conserved ones are more likely to face the lipid environment or pore lumen. The locations of the conserved and non-conserved residues among connexin family proteins are plotted on the atomic structure (Fig. 3) according to the ConSurf server [104]. Intra-molecular and inter-molecular interfaces within the hemichannel are highly conserved, whereas the pore lumen side of NTH and TM2 in the cytoplasmic region is relatively variable. The outer sides of TM3 and TM4, which are exposed to the lipid environment, are the most variable regions. The charged amino acid residues in the middle of TM3, which is unfavorable if exposed to the lipid environment, are buried within the monomer and are involved in intra-monomer interactions.
Fig. 3.
Ribbon representation of the Cx26 monomer is colored according to conservation of residues in the connexin family [104] (the gradient from white to violet indicates increasingly variable residues)
The extracellular loop E1 contains a 310 helix at the beginning and a short α-helix in its C-terminal half. E2, together with E1, contains a short antiparallel β-sheet and stretches over E1, forming the outside wall of the connexon.
Each connexin protein has three conserved cysteines in each of the extracellular loops. They are all essential for normal channel function and probably for their proper folding as well, since mutations in any of them lead to a loss of functional gap junction channels [105, 106]. (One study has reported that a cysteine-less version of Cx43 can function as the hemichannel [3].) Since a connexon docks with an apposing one from a neighboring cell in the extracellular space, it was thought that the extracellular cysteines form disulfide bridges with apposing connexon. However, it was clearly demonstrated that extracellular cysteines form disulfide bridges intramolecularly, linking the E1 and E2 of a single connexin subunit [91, 107]. Foote et al. [2] made several cysteine shift mutants of Cx32, in which the first and the third cysteines of each loop were shifted within their sequences individually or pairwise and also in some quadruple combinations. The conductance of the Cx32 gap junction channel was robustly recovered only when the first cysteine in one loop was shifted in combination with the third cysteine in the other loop. The combinations of shift indicated the pairings of disulfide bonds in which the first cysteine in each loop pairs with the third one in the other loop. The periodicity of the shifts indicated that the extracellular loops form antiparallel strands. Indeed, six conserved cysteine residues form three intramolecular disulphide bonds between E1 and E2. In X-ray crystallographic analysis, the extracellular disulfide bonds and their pairings were confirmed by collecting anomalous scattering signals from native sulfur atoms of disulfide bonds, which are much larger than that of a single sulfur atom [81]. The extracellular loops form short antiparallel beta-strand configuration, and one disulfide bond is formed between the strands, as previous studies have suggested [2]. However, the other two disulfide bonds were formed between the alpha helix and beta strand or the loop regions. In such cases, the shift of two residues would move the cysteines to the opposite side of the helix, and they would no longer form bridges. Because Cx26 and Cx32 are highly conserved members of the connexin family, it is unlikely that the discrepancy can be attributed to the difference in the connexin protein in these experiments. Rather, mutagenesis may have introduced some structural perturbation or docking of two hemichannels by external force in the Xenopus system may be possible in the biochemical study.
Structural organization of the hexameric connexon
A gap junction channel is a dodecamer, and a connexon is a hexamer of the connexin subunit with a six-fold symmetry (Fig. 1). The inter-subunit interactions within a hemichannel are mostly located in the extracellular half of transmembrane helices TM2 and TM4 and in the extracellular loops. The core of the inter-protomer interaction comprises Glu 47 (E1), Gln 48 (E1), Asn 62 (E1), Asp 66 (E1), Tyr 65 (E1), Arg 75 (TM2), and the main-chain amide of Ser 72 (E1) from one protomer, and Asp 46 (E1), Asp 50 (E1), Arg 184 (E2), Thr 186 (TM4), and Glu 187 (TM4) from the adjacent protomer (Fig. 4). In the case of multiple connexins expressed in a single cell type, there would be a variety of connexin channels. Homomeric connexons are composed of a single connexin isoform, whereas heteromeric connexons are composed of more than two different isoforms. There seem to be some rules for the formation of heteromeric connexons. The structure of the Cx26 hexamer shows that most of the intermonomer interactions are located at the extracellular side of the membrane region. These residues, as well as those of the intramonomer interactions, are conserved among connexin isoforms, suggesting that there is a conservation of monomer folding and the manner of oligomerization within the connexin family members. This idea has been validated by the similarity in cryoEM structures of two types of connexins (Cx43 and Cx26) [62, 64]. The residues of Cx26 that take part in intermonomer interactions are conserved among connexin families, making it difficult to specify the molecular determinants that specify heteromeric compatibility. The structure of the other connexins that are incompatible with Cx26 in heteromeric interactions would reveal the molecular determinants of this heteromeric compatibility. Alternatively, there might be some regulatory mechanism determining heteromeric interactions during protein synthesis, folding, oligomerization, and trafficking. Some connexins have been reported to oligomerize at the endoplasmic reticulum (ER) similarly to most membrane proteins. An exception to this rule is Cx43, which is oligomerized at the trans-Golgi network (TGN) [108–111].
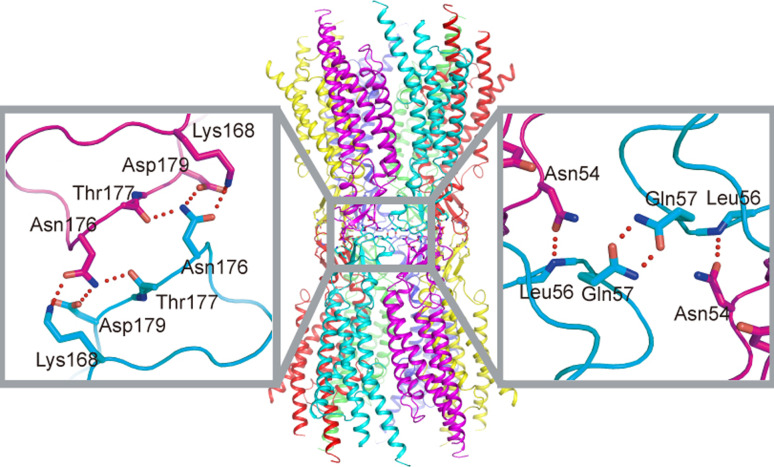
Fig. 4.
Structural organization of Cx26 monomer and hexamer. a Topological map of mutations associated with deafness and skin disease, adapted from [51]. b Intramolecular interactions that stabilize the monomer structure of Cx26. c Intermolecular interactions between two neighboring monomers in a connexon. Each interaction is shown in the enlarged insets
A number of mutations in connexin genes have been shown to be associated with a wide variety of inherited diseases, including deafness, skin diseases, cataracts, neuropathy, and developmental abnormalities [40–44, 112–119]. Missense mutations can abrogate protein folding or oligomerization, which are essential for the proper function of the protein. Mutations in Cx26, which is indispensable for potassium recycling in the cochlea [25–27, 120], have been associated with syndromic and nonsyndromic deafness [28, 29, 116–118, 121]. In fact, a number of residues that harbor disease-causing mutations [51] are involved in the interactions stabilizing monomer and hexamer structures (Fig. 4). These interactions are consistent with previous functional studies of mutant Cx26s. For example, W44C has been reported to accumulate in the cytoplasm, which could be attributed to the misfolding caused by the collapse of the hydrophobic core of the Cx26 monomer [122]. R184P is known as an oligomerization-deficient mutant, and this residue is involved in inter-monomer interactions [123, 124]. R75Q and R75 W, each involved in syndromic and non-syndromic deafness, respectively, are rather complicated. The structure indicates that this residue is involved in inter-monomer interactions in a connexon, which explains well the oligomerization deficiency of R75 W in detergent-solubilized form [124]. The same and other mutants of R75, however, are reported to form functional hemichannels but no functional gap junction channels in the membrane [125, 126]. R75 is located at the membrane/extracellular periphery. Interactions involving this position would contribute to appropriate folding and positioning of extracellular loops for docking as well as stabilization of inter-monomer interactions in a connexon. More detailed reviewing and examination would be necessary for some disease-associated mutants, considering the possibility of the non-straightforward effects they invoke.
Intercellular interactions in the docking of apposing connexons
There had been a presumption that gap junction channels are dissociated to hemichannnels once they are solubilized by detergents. In our experiments, however, purified connexins exist in the form of the gap junction channel, which is suggested by dynamic light scattering and size-exclusion chromatography. These results lead us to conclude that the observed structure represents a dodecameric gap junction channel that is not “re-docked” in the crystallization condition. Thus, we can discuss the inter-hemichannel interactions revealed in the crystal structure. The interactions between the two adjoining connexons of the Cx26 gap junction channel involve both E1 and E2 domains (Fig. 5). In E1, Asn 54 forms hydrogen bonds with the main-chain amide of Leu 56 in the opposite protomer, and Gln 57 forms symmetric hydrogen bonds with the same residue of the diagonally opposite protomer. These residues are highly conserved among connexins. In E2, Lys 168, Asp 179, and the main-chain carbonyl groups of Thr 177 and Asn 176 form hydrogen bonds and salt bridges with the opposite protomer. Through these interactions, the E1 and E2 domains create a tight seal in the extracellular space, isolating the channel interior from the outside environment.
Fig. 5.
Interactions between apposing connexons. Interactions of E1 and E2 are each shown in the enlarged insets
Homotypic gap junction channels are formed by a single connexin isoform. Heterotypic gap junction channels are formed by two connexons each composed of different isoforms [127, 128]. Formation of heterotypic channels provides greater variety in channel properties, including conductance, permeability, and gating, which could not be obtained with a single connexin [129]. The heterotypic gap junction channels with various combinations have been identified in lens, cardiovascular, and neural connexins [130–136]. As in the case of the heteromeric connexon, there seem to be some rules for the formation of heterotypic gap junction channels. Several groups have investigated heterotypic junction compatibility using communication deficient expression systems [129]. The extracellular loops, E1 and E2, mediate the docking of hemichannels, and thus they should determine heterotypic compatibility. Several studies have focused on identifying the structural motifs that confer specificity for heterotypic interactions by making chimeric connexins [135, 137]. In general, E2 appears to contain the determinants for heterotypic interactions, although some other regions could contribute to the specificity as well [137]. The atomic structure of the Cx26 gap junction channel provided evidence for the role of E2 in the docking of hemichannels. Since Lys168, Asn176, Thr177, and Asp179 exhibit some variation within the connexin family, we have performed homology modeling of various connexins with the crystal structure as a template and evaluated the compatibility of many combinations of connexin families (data not shown). Our preliminary result suggests that they and the corresponding residues in other connexins dictate docking compatibility.
Architecture of the channel pore
The molecular cutoff size for the gap junction channel pore is often described to be up to 1 kDa. These channels, however, are not simple and featureless tunnels for any molecules. Each homo-gap junction channel has its own conductance, permeability, and selectivity property, and hetero-gap junction channels provide further diversity, depending on the constituting connexin subunits. Conductance of a single homo-connexin channel ranges from ~20 picoSiemens (pS) to ~300 pS [138–141]. Some connexins prefer cations rather than anions [99, 142, 143], whereas others have smaller or larger molecular cutoff sizes [144–146]; yet others have a preference for permeating second messengers [147–149]. These findings imply that for certain ions, signaling molecules, or biological processes, there are specific connexin channels for which they are “fine tuned.” The most important factor for the permeability is the channel pore structure. The pore width, the electrical field within the pore, and the local electrical charges on the pore surface will affect the permeability of the ions or molecules entering and passing through the channel. These kinds of microenvironments give connexins specific permeability/selectivity properties enabling functional specialization. A large number of studies have explored the differences in permeability/selectivity of different connexin channels, including electrical conductance, pore width, and permeability of non-biological or biological molecules [142, 143, 147, 149–158].
The permeation pathway of the Cx26 gap junction channel consists of an intracellular channel entrance, the pore funnel, a negatively charged path, and an extracellular cavity (Fig. 6).
Fig. 6.
Pore architecture of the Cx26 gap junction channel. Left Cx26 gap junction channel is rendered as surface drawing and sectioned along the six-fold axis of symmetry, showing the surface potential distribution of the channel interior. Right The pore diameter is illustrated along the six-fold axis generated using the HOLE program [179]
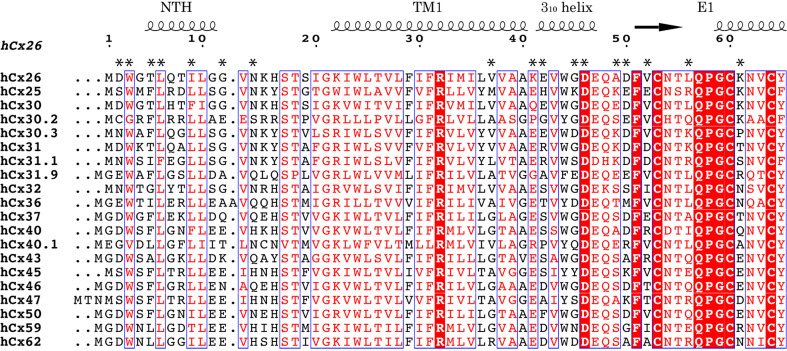
The pore funnel is formed by six NTHs located from the cytoplasmic surface to the midpoint of the plasma membrane, gradually narrowing the pore diameter. Since the bottom of the funnel is the constriction site of the pore, the identities of the surface residues of the funnel would have strong effects on both the molecular cutoff size and permeability (Figs. 6, 7). Consistent with this notion, substitutions or deletions in the NT have been reported to affect single channel conductance, molecular permeability, and charge selectivity [74, 159–163].
Fig. 7.
Sequence alignment of human connexins in the pore-lining region. Amino acid residues of Cx26 from the N-terminus to amino acid 65 are aligned with CLUSTALW [180]. Secondary structures and numbering of residues of Cx26 are represented at the top. Asterisks indicate pore-exposing residues in Cx26. Figures are created with ESPript [181] and manually modified
The negatively charged path is located at the TM1/E1 boundary, and the channel narrows again in this region. Like the residues in the NT, those in this region are exposed to the pore in a constricted site. It is, therefore, likely that these residues also contribute to channel properties. In fact, this region has been demonstrated to contain the determinants for charge selectivity in Cx46 hemichannels [99]. The pore-exposed and peripheral location, and the highly charged character of this region suggest an involvement in sensing the membrane or transjunctional voltage [164, 165]. Recent studies indicate that movement or conformational change of this region underlies the voltage-dependent “loop gating” of hemichannels [101, 166]. Lys41 and Glu42 in Cx26, which are exposed to the pore, are different from other members (Fig. 7). Cx32, which is the closest isoform of Cx26 and has negative gating polarity contrary to the positive gating polarity of Cx26, has Glu and Lys in the corresponding positions, respectively. Addition of negative charge (ES for KE) to Cx26 and positive charge (KE for ES) to Cx32 promoted faster gating kinetics and increased the gating charges, and substitution of Lys to Glu in Cx32*KE mutant reversed the gating polarity. These results suggested that the border of TM1/E1 would form a unit of voltage gating sensor with the formerly suggested one, the NT [167]. Although there is no direct interaction between Lys41 and the NT, the proximity between them (~8 Å) suggests a possibility of cooperativity between them in sensing the membrane voltage. The extracellular cavity is formed by 12 portions of E1 from each subunit, making the tight continuous inner wall of the channel in the extracellular region. Since the extracellular cavity has a wide pore diameter, the residues in this region are less likely to influence the permeability/selectivity properties.
Pore funnel and implication for the Vj gating mechanism
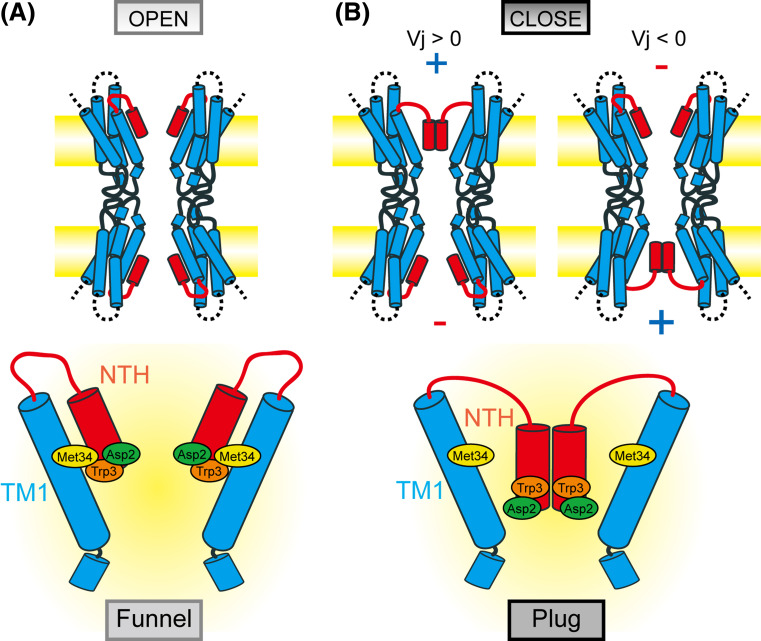
In typical topological images, the N-terminal region (NT) of connexins has been described to reside in the cytoplasmic space [60, 127]. Limited sensitivity to proteases and accessibility of antibodies suggested that NT is protected either by interaction with some other regions or by its own folding [88]. Electrophysiological studies suggested the involvement of NT in the pore lining residues and the voltage sensor [161, 163, 167–170]. NMR study revealed a short helical conformation in the NT region [71], and the EM study revealed a “pore plug,” which was formed possibly by the NT region [64, 171]. In this context, probably one of the most important and most surprising findings in the structure of the gap junction channel is the existence of the pore funnel (Fig. 8). The NT forms a short helix (NTH) and is inserted into the channel pore, and six NTHs assemble on top of the pore. This structure is termed a “pore funnel” since the appearance of the structure of six NTHs is like that of a funnel. The funnel is a narrower entrance to the channel, and the bottom is the constriction site through the channel pore. The substitution of NT residues changed the single-channel conductance or sensitivity to blockage by spermine [102, 172].
Fig. 8.
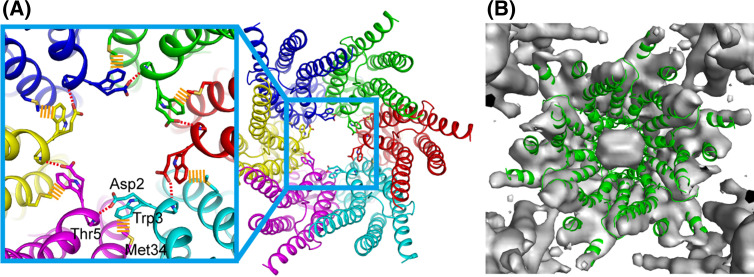
The structure of pore funnel and pore plug. a The six NTHs form pore funnel, which is stabilized by circular hydrogen bond network (red dashed lines) at the bottom of it and attached to the inner wall of the channel by hydrophobic interactions (orange dashed lines). These interactions are formed between neighboring monomers. b Superposition of the atomic model of wild-type Cx26 gap junction channel (ribbon representation: green) into the electron density map of Met34Ala mutant Cx26 (surface representation: gray)
The pore funnel is stabilized by the circular hydrogen bond network between Asp2 and the main chain of the neighboring monomer at the bottom of the funnel. Trp3, which is conserved in almost all connexins, undergoes a hydrophobic interaction with Met34 from the neighboring monomer (Fig. 8). The hydrophobic interactions draw the pore funnel onto the innermost wall of the channel, switching it to the open state. Considering the high conservation of Trp3 and the hydrophobic segment in TM1 among connexin family members, this interaction should also be conserved among most connexins. The structure of the pore funnel, along with the recent EM map of the Met34Ala mutant [64, 171] (Fig. 8), suggests the implication of the molecular consequence of the Met34Thr mutation, which is one of the most frequent deafness-associated mutations of Cx26. Substitution of the hydrophobic methionine with hydrophilic threonin would disrupt the hydrophobic interaction between Trp3, releasing the NTH from TM1. While there is no direct evidence for this, once NTHs are released from TM1, they might assemble or form some structure cooperating with the end of TM1 that physically blocks the channel at the pore vestibule and forms the “pore plug” seen in the EM map of the Met34Ala mutant.
Gap junction channels have multiple gating mechanisms, including the conventional membrane voltage-dependent gating, termed V m or V i–o gating, and the transjunctional voltage-dependent gating [164], which is specific to the gap junction channels. There are two different forms of gating mechanism in transjunctional voltage-dependence [173, 174]. One is the V j gating or fast gating, which displays fast (<10 ms) and incomplete closure to the subconductance state. Another is the loop or slow gating, which shows the slow transitions (>10 ms) from fully open to fully closed state and appears to involve the extracellular loop domains. Each connexin channel shows specific sensitivity in its gating characteristics, including its polarity [167, 168, 175]. Cx26 gap junction channels have positive V j gating polarity [167] and Cx32, the closest relative of Cx26, has negative V j gating polarity [167, 169, 170, 176]. A number of experiments using chimeras of Cx26 and Cx32 or substitution of them were performed to reveal the determinants of the voltage dependence [167, 169, 170, 176], and NT, especially the second amino acid residue, is suggested to be the voltage sensor. The pore funnel and the linker loop to TM1 are highly flexible domains, and together with the recent EM structure of Met34Ala mutant, suggest a mechanism of V j gating triggered by the movement of NT [64, 80]. In the Met34Ala mutant, the smaller side chain of Ala would be insufficient for the hydrophobic interaction with Trp3, leading to the detachment of the pore funnel from TM1 and assembly of the released NTHs at the vestibule. Since Asp2 is exposed to the pore and could sense the changes of the electric field along the pore, the application of an inside positive V j would displace Asp2 towards the cytoplasm, releasing the NTH from TM1, followed by the assembly of NTH as in the case of Met34 mutants (Fig. 9). Although this model, named the “plug gating model,” is quite different from that of the other membrane channels such as potassium channels or sodium channels, which have the S4 helix as a voltage sensor, it could account for many physiological observations. The opposite gating polarity of Cx26 and Cx32 [167, 169, 176] could be attributed to the net charge in the NT, where Cx26 has Asp2 and Cx32 has Asn2. Since up to ten amino acid residues corresponding to the end of NTH could sense the electric field [169, 176], oppositely charged residues in this region could sense and respond individually, resulting in the observed bipolar gating [169]. It is possible that the release of any one of six NTHs would break down the circular hydrogen bond network through the Asp2-Thr5, releasing them from TM1 and consequently forming the pore plug. This hypothesis can explain the reported observation that a single subunit could trigger V j gating and the bipolar gating of heteromeric hemichannels composed of subunits of different polarity [170]. Though the pore plug model seems to be a good one to explain some features of V j gating as described above, it appears that in the EM plug structure the channel pore is occluded and is unable to conduct ions. This is inconsistent with the subconductance state of V j gating. It should be noted that most of CL and CT are missing in the crystal structure, and there are some other reports that suggest the involvement of some parts of CL and CT in V j gating [177, 178], which might explain the discrepancy between subconductance state and plug structure. Undoubtedly, the high resolution structure of the whole region and in the closed state is necessary for comprehensively understanding the complex gating mechanisms of the gap junction channel.
Fig. 9.
Plug gating model for transjunctional voltage-dependent gating of the Cx26 gap junction channel. When there is no difference in membrane voltages between two neighboring cells (a), NTHs form the pore funnel and attach to TM1 by hydrophobic interactions. When there is a difference in membrane voltages between two cells (b), the positive electric field pulls up Asp2, which is exposed to the pore, in the cytoplasmic direction, releasing NTHs from TM1. Once released, NTHs will assemble on the top of the pore and form a so-called “plug” structure
Conclusion
Since its discovery in the 1960s, structural studies of the gap junction channel have been performed extensively. Determination of the recent atomic structure of the human Cx26 gap junction channel by X-ray crystallography provides an answer for long unresolved issues, such as the molecular organization, helical assignment, and pore structure. In conjunction with the previous biochemical, electrophysiological, and structural studies, the atomic structure of the N-terminal region suggests a mechanism of plug gating. The structure will be useful as a common template for any gap junction channel for functional and structural studies. There remain, however, unobserved segments in the cytoplasmic region, which are the most variable regions among connexin families contributing to the connexin-specific properties and responding to various chemical stimuli (chemical gating). Crystallographic structure of Cx26 at higher resolutions as well as those of other connexin channels will help elucidate such functional mechanisms.
Acknowledgments
This work was supported in part by grants-in-aid for scientific research (16087101, 16087206, and 21227003), the GCOE program (A-041) from the Ministry of Education, Culture, Sports, Sciences, and Technology of Japan (to T.T.).
References
- 1.Alberts B (2008) Molecular biology of the cell (various pagings). Garland Science, New York, pp 1 v
- 2.Foote CI, Zhou L, Zhu X, Nicholson BJ. The pattern of disulfide linkages in the extracellular loop regions of connexin 32 suggests a model for the docking interface of gap junctions. J Cell Biol. 1998;140:1187–1197. doi: 10.1083/jcb.140.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao X, Chen Y, Reuss L, Altenberg GA. Functional expression in Xenopus oocytes of gap-junctional hemichannels formed by a cysteine-less connexin 43. J Biol Chem. 2004;279:9689–9692. doi: 10.1074/jbc.M311438200. [DOI] [PubMed] [Google Scholar]
- 4.Bittman K, Owens DF, Kriegstein AR, LoTurco JJ. Cell coupling and uncoupling in the ventricular zone of developing neocortex. J Neurosci. 1997;17:7037–7044. doi: 10.1523/JNEUROSCI.17-18-07037.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owens DF, Kriegstein AR. Patterns of intracellular calcium fluctuation in precursor cells of the neocortical ventricular zone. J Neurosci. 1998;18:5374–5388. doi: 10.1523/JNEUROSCI.18-14-05374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- 8.Rozental R, Morales M, Mehler MF, Urban M, Kremer M, Dermietzel R, Kessler JA, Spray DC. Changes in the properties of gap junctions during neuronal differentiation of hippocampal progenitor cells. J Neurosci. 1998;18:1753–1762. doi: 10.1523/JNEUROSCI.18-05-01753.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donahue LM, Webster DR, Martinez I, Spray DC. Decreased gap-junctional communication associated with segregation of the neuronal phenotype in the RT4 cell-line family. Cell Tissue Res. 1998;292:27–35. doi: 10.1007/s004410051031. [DOI] [PubMed] [Google Scholar]
- 10.Bani-Yaghoub M, Underhill TM, Naus CC. Gap junction blockage interferes with neuronal and astroglial differentiation of mouse P19 embryonal carcinoma cells. Dev Genet. 1999;24:69–81. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<69::AID-DVG8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 11.Kaba RA, Coppen SR, Dupont E, Skepper JN, Elneil S, Haw MP, Pepper JR, Yacoub MH, Rothery S, Severs NJ. Comparison of connexin 43, 40 and 45 expression patterns in the developing human and mouse hearts. Cell Commun Adhes. 2001;8:339–343. doi: 10.3109/15419060109080750. [DOI] [PubMed] [Google Scholar]
- 12.Alcolea S, Theveniau-Ruissy M, Jarry-Guichard T, Marics I, Tzouanacou E, Chauvin JP, Briand JP, Moorman AF, Lamers WH, Gros DB. Downregulation of connexin 45 gene products during mouse heart development. Circ Res. 1999;84:1365–1379. doi: 10.1161/01.res.84.12.1365. [DOI] [PubMed] [Google Scholar]
- 13.Van Kempen MJ, Vermeulen JL, Moorman AF, Gros D, Paul DL, Lamers WH. Developmental changes of connexin40 and connexin43 mRNA distribution patterns in the rat heart. Cardiovasc Res. 1996;32:886–900. doi: 10.1016/0008-6363(96)00131-9. [DOI] [PubMed] [Google Scholar]
- 14.Kumai M, Nishii K, Nakamura K, Takeda N, Suzuki M, Shibata Y. Loss of connexin45 causes a cushion defect in early cardiogenesis. Development. 2000;127:3501–3512. doi: 10.1242/dev.127.16.3501. [DOI] [PubMed] [Google Scholar]
- 15.Kruger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K. Defective vascular development in connexin 45-deficient mice. Development. 2000;127:4179–4193. doi: 10.1242/dev.127.19.4179. [DOI] [PubMed] [Google Scholar]
- 16.Nishii K, Kumai M, Egashira K, Miwa T, Hashizume K, Miyano Y, Shibata Y. Mice lacking connexin45 conditionally in cardiac myocytes display embryonic lethality similar to that of germline knockout mice without endocardial cushion defect. Cell Commun Adhes. 2003;10:365–369. doi: 10.1080/cac.10.4-6.365.369. [DOI] [PubMed] [Google Scholar]
- 17.Simon AM, Goodenough DA, Paul DL. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr Biol. 1998;8:295–298. doi: 10.1016/S0960-9822(98)70113-7. [DOI] [PubMed] [Google Scholar]
- 18.Gu H, Smith FC, Taffet SM, Delmar M. High incidence of cardiac malformations in connexin40-deficient mice. Circ Res. 2003;93:201–206. doi: 10.1161/01.RES.0000084852.65396.70. [DOI] [PubMed] [Google Scholar]
- 19.Kirchhoff S, Kim JS, Hagendorff A, Thonnissen E, Kruger O, Lamers WH, Willecke K. Abnormal cardiac conduction and morphogenesis in connexin40 and connexin43 double-deficient mice. Circ Res. 2000;87:399–405. doi: 10.1161/01.res.87.5.399. [DOI] [PubMed] [Google Scholar]
- 20.Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- 21.Huang GY, Cooper ES, Waldo K, Kirby ML, Gilula NB, Lo CW. Gap junction-mediated cell-cell communication modulates mouse neural crest migration. J Cell Biol. 1998;143:1725–1734. doi: 10.1083/jcb.143.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Britz-Cunningham SH, Shah MM, Zuppan CW, Fletcher WH. Mutations of the Connexin43 gap-junction gene in patients with heart malformations and defects of laterality. N Engl J Med. 1995;332:1323–1329. doi: 10.1056/NEJM199505183322002. [DOI] [PubMed] [Google Scholar]
- 23.Chen P, Xie LJ, Huang GY, Zhao XQ, Chang C. Mutations of connexin43 in fetuses with congenital heart malformations. Chin Med J (Engl) 2005;118:971–976. [PubMed] [Google Scholar]
- 24.Dasgupta C, Martinez AM, Zuppan CW, Shah MM, Bailey LL, Fletcher WH. Identification of connexin43 (alpha1) gap junction gene mutations in patients with hypoplastic left heart syndrome by denaturing gradient gel electrophoresis (DGGE) Mutat Res. 2001;479:173–186. doi: 10.1016/S0027-5107(01)00160-9. [DOI] [PubMed] [Google Scholar]
- 25.Ahmad S, Chen S, Sun J, Lin X. Connexins 26 and 30 are co-assembled to form gap junctions in the cochlea of mice. Biochem Biophys Res Commun. 2003;307:362–368. doi: 10.1016/S0006-291X(03)01166-5. [DOI] [PubMed] [Google Scholar]
- 26.Kikuchi T, Kimura RS, Paul DL, Takasaka T, Adams JC. Gap junction systems in the mammalian cochlea. Brain Res Brain Res Rev. 2000;32:163–166. doi: 10.1016/S0165-0173(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 27.Forge A, Becker D, Casalotti S, Edwards J, Marziano N, Nevill G. Gap junctions in the inner ear: comparison of distribution patterns in different vertebrates and assessement of connexin composition in mammals. J Comp Neurol. 2003;467:207–231. doi: 10.1002/cne.10916. [DOI] [PubMed] [Google Scholar]
- 28.Kelsell DP, Dunlop J, Stevens HP, Lench NJ, Liang JN, Parry G, Mueller RF, Leigh IM. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature. 1997;387:80–83. doi: 10.1038/387080a0. [DOI] [PubMed] [Google Scholar]
- 29.Grifa A, Wagner CA, D’Ambrosio L, Melchionda S, Bernardi F, Lopez-Bigas N, Rabionet R, Arbones M, Monica MD, Estivill X, Zelante L, Lang F, Gasparini P. Mutations in GJB6 cause nonsyndromic autosomal dominant deafness at DFNA3 locus. Nat Genet. 1999;23:16–18. doi: 10.1038/12612. [DOI] [PubMed] [Google Scholar]
- 30.Goodenough DA. The crystalline lens. A system networked by gap junctional intercellular communication. Semin Cell Biol. 1992;3:49–58. doi: 10.1016/S1043-4682(10)80007-8. [DOI] [PubMed] [Google Scholar]
- 31.White TW, Bruzzone R. Intercellular communication in the eye: clarifying the need for connexin diversity. Brain Res Brain Res Rev. 2000;32:130–137. doi: 10.1016/S0165-0173(99)00072-7. [DOI] [PubMed] [Google Scholar]
- 32.Gerido DA, White TW. Connexin disorders of the ear, skin, and lens. Biochim Biophys Acta. 2004;1662:159–170. doi: 10.1016/j.bbamem.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 33.Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar NM, Horwitz J, Gilula NB. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–843. doi: 10.1016/S0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- 34.White TW, Bruzzone R, Goodenough DA, Paul DL. Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Mol Biol Cell. 1992;3:711–720. doi: 10.1091/mbc.3.7.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dahm R, van Marle J, Prescott AR, Quinlan RA. Gap junctions containing alpha8-connexin (MP70) in the adult mammalian lens epithelium suggests a re-evaluation of its role in the lens. Exp Eye Res. 1999;69:45–56. doi: 10.1006/exer.1999.0670. [DOI] [PubMed] [Google Scholar]
- 37.Rong P, Wang X, Niesman I, Wu Y, Benedetti LE, Dunia I, Levy E, Gong X. Disruption of Gja8 (alpha8 connexin) in mice leads to microphthalmia associated with retardation of lens growth and lens fiber maturation. Development. 2002;129:167–174. doi: 10.1242/dev.129.1.167. [DOI] [PubMed] [Google Scholar]
- 38.Donaldson P, Kistler J, Mathias RT. Molecular solutions to mammalian lens transparency. News Physiol Sci. 2001;16:118–123. doi: 10.1152/physiologyonline.2001.16.3.118. [DOI] [PubMed] [Google Scholar]
- 39.Mathias RT, Rae JL, Baldo GJ. Physiological properties of the normal lens. Physiol Rev. 1997;77:21–50. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- 40.Devi RR, Reena C, Vijayalakshmi P. Novel mutations in GJA3 associated with autosomal dominant congenital cataract in the Indian population. Mol Vis. 2005;11:846–852. [PubMed] [Google Scholar]
- 41.Devi RR, Vijayalakshmi P. Novel mutations in GJA8 associated with autosomal dominant congenital cataract and microcornea. Mol Vis. 2006;12:190–195. [PubMed] [Google Scholar]
- 42.Jiang H, Jin Y, Bu L, Zhang W, Liu J, Cui B, Kong X, Hu L. A novel mutation in GJA3 (connexin46) for autosomal dominant congenital nuclear pulverulent cataract. Mol Vis. 2003;9:579–583. [PubMed] [Google Scholar]
- 43.Shiels A, Mackay D, Ionides A, Berry V, Moore A, Bhattacharya S. A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant “zonular pulverulent” cataract, on chromosome 1q. Am J Hum Genet. 1998;62:526–532. doi: 10.1086/301762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burdon KP, Wirth MG, Mackey DA, Russell-Eggitt IM, Craig JE, Elder JE, Dickinson JL, Sale MM. A novel mutation in the connexin 46 gene causes autosomal dominant congenital cataract with incomplete penetrance. J Med Genet. 2004;41:e106. doi: 10.1136/jmg.2004.018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mesnil M, Crespin S, Avanzo JL, Zaidan-Dagli ML. Defective gap junctional intercellular communication in the carcinogenic process. Biochim Biophys Acta. 2005;1719:125–145. doi: 10.1016/j.bbamem.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Haefliger JA, Nicod P, Meda P. Contribution of connexins to the function of the vascular wall. Cardiovasc Res. 2004;62:345–356. doi: 10.1016/j.cardiores.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 47.Hill CE, Rummery N, Hickey H, Sandow SL. Heterogeneity in the distribution of vascular gap junctions and connexins: implications for function. Clin Exp Pharmacol Physiol. 2002;29:620–625. doi: 10.1046/j.1440-1681.2002.03699.x. [DOI] [PubMed] [Google Scholar]
- 48.Simon AM, Goodenough DA. Diverse functions of vertebrate gap junctions. Trends Cell Biol. 1998;8:477–483. doi: 10.1016/S0962-8924(98)01372-5. [DOI] [PubMed] [Google Scholar]
- 49.Levin M. Gap junctional communication in morphogenesis. Prog Biophys Mol Biol. 2007;94:186–206. doi: 10.1016/j.pbiomolbio.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Handel A, Yates A, Pilyugin SS, Antia R. Gap junction-mediated antigen transport in immune responses. Trends Immunol. 2007;28:463–466. doi: 10.1016/j.it.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–543. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sohl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nat Rev Neurosci. 2005;6:191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- 53.Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–232. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 54.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 55.Robertson JD. The occurrence of a subunit pattern in the unit membranes of club endings in mauthner cell synapses in goldfish brains. J Cell Biol. 1963;19:201–221. doi: 10.1083/jcb.19.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benedetti EL, Emmelot P. Electron microscopic observations on negatively stained plasma membranes isolated from rat liver. J Cell Biol. 1965;26:299–305. doi: 10.1083/jcb.26.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zampighi G, Unwin PN. Two forms of isolated gap junctions. J Mol Biol. 1979;135:451–464. doi: 10.1016/0022-2836(79)90446-7. [DOI] [PubMed] [Google Scholar]
- 58.Unwin PN, Zampighi G. Structure of the junction between communicating cells. Nature. 1980;283:545–549. doi: 10.1038/283545a0. [DOI] [PubMed] [Google Scholar]
- 59.Yeager M. Electron microscopic image analysis of cardiac gap junction membrane crystals. Microsc Res Tech. 1995;31:452–466. doi: 10.1002/jemt.1070310514. [DOI] [PubMed] [Google Scholar]
- 60.Yeager M. Structure of cardiac gap junction intercellular channels. J Struct Biol. 1998;121:231–245. doi: 10.1006/jsbi.1998.3972. [DOI] [PubMed] [Google Scholar]
- 61.Unger VM, Kumar NM, Gilula NB, Yeager M. Expression, two-dimensional crystallization, and electron cryo-crystallography of recombinant gap junction membrane channels. J Struct Biol. 1999;128:98–105. doi: 10.1006/jsbi.1999.4184. [DOI] [PubMed] [Google Scholar]
- 62.Unger VM, Kumar NM, Gilula NB, Yeager M. Three-dimensional structure of a recombinant gap junction membrane channel. Science. 1999;283:1176–1180. doi: 10.1126/science.283.5405.1176. [DOI] [PubMed] [Google Scholar]
- 63.Fleishman SJ, Unger VM, Yeager M, Ben-Tal N. A Calpha model for the transmembrane alpha helices of gap junction intercellular channels. Mol Cell. 2004;15:879–888. doi: 10.1016/j.molcel.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 64.Oshima A, Tani K, Hiroaki Y, Fujiyoshi Y, Sosinsky GE. Three-dimensional structure of a human connexin26 gap junction channel reveals a plug in the vestibule. Proc Natl Acad Sci USA. 2007;104:10034–10039. doi: 10.1073/pnas.0703704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Makowski L, Caspar DL, Phillips WC, Goodenough DA. Gap junction structures. II. Analysis of the x-ray diffraction data. J Cell Biol. 1977;74:629–645. doi: 10.1083/jcb.74.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tibbitts TT, Caspar DL, Phillips WC, Goodenough DA. Diffraction diagnosis of protein folding in gap junction connexons. Biophys J. 1990;57:1025–1036. doi: 10.1016/S0006-3495(90)82621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Unwin PN, Ennis PD. Calcium-mediated changes in gap junction structure: evidence from the low angle X-ray pattern. J Cell Biol. 1983;97:1459–1466. doi: 10.1083/jcb.97.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu J, Bippes CA, Hand GM, Muller DJ, Sosinsky GE. Aminosulfonate modulated pH-induced conformational changes in connexin26 hemichannels. J Biol Chem. 2007;282:8895–8904. doi: 10.1074/jbc.M609317200. [DOI] [PubMed] [Google Scholar]
- 69.Muller DJ, Hand GM, Engel A, Sosinsky GE. Conformational changes in surface structures of isolated connexin 26 gap junctions. EMBO J. 2002;21:3598–3607. doi: 10.1093/emboj/cdf365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sorgen PL, Duffy HS, Sahoo P, Coombs W, Delmar M, Spray DC. Structural changes in the carboxyl terminus of the gap junction protein connexin43 indicates signaling between binding domains for c-Src and zonula occludens-1. J Biol Chem. 2004;279:54695–54701. doi: 10.1074/jbc.M409552200. [DOI] [PubMed] [Google Scholar]
- 71.Purnick PE, Benjamin DC, Verselis VK, Bargiello TA, Dowd TL. Structure of the amino terminus of a gap junction protein. Arch Biochem Biophys. 2000;381:181–190. doi: 10.1006/abbi.2000.1989. [DOI] [PubMed] [Google Scholar]
- 72.Duffy HS, Sorgen PL, Girvin ME, O’Donnell P, Coombs W, Taffet SM, Delmar M, Spray DC. pH-dependent intramolecular binding and structure involving Cx43 cytoplasmic domains. J Biol Chem. 2002;277:36706–36714. doi: 10.1074/jbc.M207016200. [DOI] [PubMed] [Google Scholar]
- 73.Sorgen PL, Duffy HS, Spray DC, Delmar M. pH-dependent dimerization of the carboxyl terminal domain of Cx43. Biophys J. 2004;87:574–581. doi: 10.1529/biophysj.103.039230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oh S, Verselis VK, Bargiello TA. Charges dispersed over the permeation pathway determine the charge selectivity and conductance of a Cx32 chimeric hemichannel. J Physiol. 2008;586:2445–2461. doi: 10.1113/jphysiol.2008.150805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kronengold J, Trexler EB, Bukauskas FF, Bargiello TA, Verselis VK. Single-channel SCAM identifies pore-lining residues in the first extracellular loop and first transmembrane domains of Cx46 hemichannels. J Gen Physiol. 2003;122:389–405. doi: 10.1085/jgp.200308861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skerrett IM, Aronowitz J, Shin JH, Cymes G, Kasperek E, Cao FL, Nicholson BJ. Identification of amino acid residues lining the pore of a gap junction channel. J Cell Biol. 2002;159:349–360. doi: 10.1083/jcb.200207060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suchyna TM, Xu LX, Gao F, Fourtner CR, Nicholson BJ. Identification of a proline residue as a transduction element involved in voltage gating of gap junctions. Nature. 1993;365:847–849. doi: 10.1038/365847a0. [DOI] [PubMed] [Google Scholar]
- 78.Hirst-Jensen BJ, Sahoo P, Kieken F, Delmar M, Sorgen PL. Characterization of the pH-dependent interaction between the gap junction protein connexin43 carboxyl terminus and cytoplasmic loop domains. J Biol Chem. 2007;282:5801–5813. doi: 10.1074/jbc.M605233200. [DOI] [PubMed] [Google Scholar]
- 79.Morley GE, Ek-Vitorin JF, Taffet SM, Delmar M. Structure of connexin43 and its regulation by pHi. J Cardiovasc Electrophysiol. 1997;8:939–951. doi: 10.1111/j.1540-8167.1997.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 80.Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature. 2009;458:597–602. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- 81.Suga M, Maeda S, Nakagawa S, Yamashita E, Tsukihara T. A description of the structural determination procedures of a gap junction channel at 3.5 A resolution. Acta Crystallogr D Biol Crystallogr. 2009;65:758–766. doi: 10.1107/S0907444909014711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sosinsky G. Mixing of connexins in gap junction membrane channels. Proc Natl Acad Sci USA. 1995;92:9210–9214. doi: 10.1073/pnas.92.20.9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perkins G, Goodenough D, Sosinsky G. Three-dimensional structure of the gap junction connexon. Biophys J. 1997;72:533–544. doi: 10.1016/S0006-3495(97)78693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cascio M, Gogol E, Wallace BA. The secondary structure of gap junctions. Influence of isolation methods and proteolysis. J Biol Chem. 1990;265:2358–2364. [PubMed] [Google Scholar]
- 85.Kuraoka A, Iida H, Hatae T, Shibata Y, Itoh M, Kurita T. Localization of gap junction proteins, connexins 32 and 26, in rat and guinea pig liver as revealed by quick-freeze, deep-etch immunoelectron microscopy. J Histochem Cytochem. 1993;41:971–980. doi: 10.1177/41.7.8390496. [DOI] [PubMed] [Google Scholar]
- 86.Sosinsky GE. Image analysis of gap junction structures. Electron Microsc Rev. 1992;5:59–76. doi: 10.1016/0892-0354(92)90005-B. [DOI] [PubMed] [Google Scholar]
- 87.White TW, Paul DL, Goodenough DA, Bruzzone R. Functional analysis of selective interactions among rodent connexins. Mol Biol Cell. 1995;6:459–470. doi: 10.1091/mbc.6.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hertzberg EL, Disher RM, Tiller AA, Zhou Y, Cook RG. Topology of the Mr 27, 000 liver gap junction protein. Cytoplasmic localization of amino- and carboxyl termini and a hydrophilic domain which is protease-hypersensitive. J Biol Chem. 1988;263:19105–19111. [PubMed] [Google Scholar]
- 89.Zimmer DB, Green CR, Evans WH, Gilula NB. Topological analysis of the major protein in isolated intact rat liver gap junctions and gap junction-derived single membrane structures. J Biol Chem. 1987;262:7751–7763. [PubMed] [Google Scholar]
- 90.Morley GE, Taffet SM, Delmar M. Intramolecular interactions mediate pH regulation of connexin43 channels. Biophys J. 1996;70:1294–1302. doi: 10.1016/S0006-3495(96)79686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rahman S, Evans WH. Topography of connexin32 in rat liver gap junctions. Evidence for an intramolecular disulphide linkage connecting the two extracellular peptide loops. J Cell Sci. 1991;100(Pt 3):567–578. doi: 10.1242/jcs.100.3.567. [DOI] [PubMed] [Google Scholar]
- 92.Zhang JT, Nicholson BJ. The topological structure of connexin 26 and its distribution compared to connexin 32 in hepatic gap junctions. J Membr Biol. 1994;139:15–29. doi: 10.1007/BF00232671. [DOI] [PubMed] [Google Scholar]
- 93.Yeager M, Gilula NB. Membrane topology and quaternary structure of cardiac gap junction ion channels. J Mol Biol. 1992;223:929–948. doi: 10.1016/0022-2836(92)90253-G. [DOI] [PubMed] [Google Scholar]
- 94.Yancey SB, John SA, Lal R, Austin BJ, Revel JP. The 43-kD polypeptide of heart gap junctions: immunolocalization, topology, and functional domains. J Cell Biol. 1989;108:2241–2254. doi: 10.1083/jcb.108.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beyer EC, Kistler J, Paul DL, Goodenough DA. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989;108:595–605. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goodenough DA, Paul DL, Jesaitis L. Topological distribution of two connexin32 antigenic sites in intact and split rodent hepatocyte gap junctions. J Cell Biol. 1988;107:1817–1824. doi: 10.1083/jcb.107.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Milks LC, Kumar NM, Houghten R, Unwin N, Gilula NB. Topology of the 32-kd liver gap junction protein determined by site-directed antibody localizations. EMBO J. 1988;7:2967–2975. doi: 10.1002/j.1460-2075.1988.tb03159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou XW, Pfahnl A, Werner R, Hudder A, Llanes A, Luebke A, Dahl G. Identification of a pore lining segment in gap junction hemichannels. Biophys J. 1997;72:1946–1953. doi: 10.1016/S0006-3495(97)78840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Trexler EB, Bukauskas FF, Kronengold J, Bargiello TA, Verselis VK. The first extracellular loop domain is a major determinant of charge selectivity in connexin46 channels. Biophys J. 2000;79:3036–3051. doi: 10.1016/S0006-3495(00)76539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu X, Ma M, Dahl G. Conductance of connexin hemichannels segregates with the first transmembrane segment. Biophys J. 2006;90:140–150. doi: 10.1529/biophysj.105.066373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tang Q, Dowd TL, Verselis VK, Bargiello TA. Conformational changes in a pore-forming region underlie voltage-dependent “loop gating” of an unapposed connexin hemichannel. J Gen Physiol. 2009;133:555–570. doi: 10.1085/jgp.200910207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Musa H, Fenn E, Crye M, Gemel J, Beyer EC, Veenstra RD. Amino terminal glutamate residues confer spermine sensitivity and affect voltage gating and channel conductance of rat connexin40 gap junctions. J Physiol. 2004;557:863–878. doi: 10.1113/jphysiol.2003.059386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baldwin JM. The probable arrangement of the helices in G protein-coupled receptors. EMBO J. 1993;12:1693–1703. doi: 10.1002/j.1460-2075.1993.tb05814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Landau M, Mayrose I, Rosenberg Y, Glaser F, Martz E, Pupko T, Ben-Tal N. ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res. 2005;33:W299–W302. doi: 10.1093/nar/gki370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dahl G, Werner R, Levine E, Rabadan-Diehl C. Mutational analysis of gap junction formation. Biophys J. 1992;62:172–180. doi: 10.1016/S0006-3495(92)81803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dahl G, Levine E, Rabadan-Diehl C, Werner R. Cell/cell channel formation involves disulfide exchange. Eur J Biochem. 1991;197:141–144. doi: 10.1111/j.1432-1033.1991.tb15892.x. [DOI] [PubMed] [Google Scholar]
- 107.John SA, Revel JP. Connexon integrity is maintained by non-covalent bonds: intramolecular disulfide bonds link the extracellular domains in rat connexin-43. Biochem Biophys Res Commun. 1991;178:1312–1318. doi: 10.1016/0006-291X(91)91037-D. [DOI] [PubMed] [Google Scholar]
- 108.Vanslyke JK, Naus CC, Musil LS. Conformational maturation and post-ER multisubunit assembly of gap junction proteins. Mol Biol Cell. 2009;20:2451–2463. doi: 10.1091/mbc.E09-01-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993;74:1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- 110.Das Sarma J, Wang F, Koval M. Targeted gap junction protein constructs reveal connexin-specific differences in oligomerization. J Biol Chem. 2002;277:20911–20918. doi: 10.1074/jbc.M111498200. [DOI] [PubMed] [Google Scholar]
- 111.Maza J, Das Sarma J, Koval M. Defining a minimal motif required to prevent connexin oligomerization in the endoplasmic reticulum. J Biol Chem. 2005;280:21115–21121. doi: 10.1074/jbc.M412612200. [DOI] [PubMed] [Google Scholar]
- 112.Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C, Hannibal MC, Jabs EW. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet. 2003;72:408–418. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mackay D, Ionides A, Kibar Z, Rouleau G, Berry V, Moore A, Shiels A, Bhattacharya S. Connexin46 mutations in autosomal dominant congenital cataract. Am J Hum Genet. 1999;64:1357–1364. doi: 10.1086/302383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Macari F, Landau M, Cousin P, Mevorah B, Brenner S, Panizzon R, Schorderet DF, Hohl D, Huber M. Mutation in the gene for connexin 30.3 in a family with erythrokeratodermia variabilis. Am J Hum Genet. 2000;67:1296–1301. doi: 10.1016/s0002-9297(07)62957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Richard G, Smith LE, Bailey RA, Itin P, Hohl D, Epstein EH, Jr, DiGiovanna JJ, Compton JG, Bale SJ. Mutations in the human connexin gene GJB3 cause erythrokeratodermia variabilis. Nat Genet. 1998;20:366–369. doi: 10.1038/3840. [DOI] [PubMed] [Google Scholar]
- 116.Maestrini E, Korge BP, Ocana-Sierra J, Calzolari E, Cambiaghi S, Scudder PM, Hovnanian A, Monaco AP, Munro CS. A missense mutation in connexin26, D66H, causes mutilating keratoderma with sensorineural deafness (Vohwinkel’s syndrome) in three unrelated families. Hum Mol Genet. 1999;8:1237–1243. doi: 10.1093/hmg/8.7.1237. [DOI] [PubMed] [Google Scholar]
- 117.Richard G, Rouan F, Willoughby CE, Brown N, Chung P, Ryynanen M, Jabs EW, Bale SJ, DiGiovanna JJ, Uitto J, Russell L. Missense mutations in GJB2 encoding connexin-26 cause the ectodermal dysplasia keratitis-ichthyosis-deafness syndrome. Am J Hum Genet. 2002;70:1341–1348. doi: 10.1086/339986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bruzzone R, Gomes D, Denoyelle E, Duval N, Perea J, Veronesi V, Weil D, Petit C, Gabellec MM, D’Andrea P, White TW. Functional analysis of a dominant mutation of human connexin26 associated with nonsyndromic deafness. Cell Commun Adhes. 2001;8:425–431. doi: 10.3109/15419060109080765. [DOI] [PubMed] [Google Scholar]
- 119.Paznekas WA, Karczeski B, Vermeer S, Lowry RB, Delatycki M, Laurence F, Koivisto PA, Van Maldergem L, Boyadjiev SA, Bodurtha JN, Jabs EW. GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum Mutat. 2009;30:724–733. doi: 10.1002/humu.20958. [DOI] [PubMed] [Google Scholar]
- 120.Zhao HB, Kikuchi T, Ngezahayo A, White TW. Gap junctions and cochlear homeostasis. J Membr Biol. 2006;209:177–186. doi: 10.1007/s00232-005-0832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zelante L, Gasparini P, Estivill X, Melchionda S, D’Agruma L, Govea N, Mila M, Monica MD, Lutfi J, Shohat M, Mansfield E, Delgrosso K, Rappaport E, Surrey S, Fortina P. Connexin26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum Mol Genet. 1997;6:1605–1609. doi: 10.1093/hmg/6.9.1605. [DOI] [PubMed] [Google Scholar]
- 122.Martin PE, Coleman SL, Casalotti SO, Forge A, Evans WH. Properties of connexin26 gap junctional proteins derived from mutations associated with non-syndromal heriditary deafness. Hum Mol Genet. 1999;8:2369–2376. doi: 10.1093/hmg/8.13.2369. [DOI] [PubMed] [Google Scholar]
- 123.Thonnissen E, Rabionet R, Arbones ML, Estivill X, Willecke K, Ott T. Human connexin26 (GJB2) deafness mutations affect the function of gap junction channels at different levels of protein expression. Hum Genet. 2002;111:190–197. doi: 10.1007/s00439-002-0750-2. [DOI] [PubMed] [Google Scholar]
- 124.Oshima A, Doi T, Mitsuoka K, Maeda S, Fujiyoshi Y. Roles of Met-34, Cys-64, and Arg-75 in the assembly of human connexin 26. Implication for key amino acid residues for channel formation and function. J Biol Chem. 2003;278:1807–1816. doi: 10.1074/jbc.M207713200. [DOI] [PubMed] [Google Scholar]
- 125.Chen Y, Deng Y, Bao X, Reuss L, Altenberg GA. Mechanism of the defect in gap-junctional communication by expression of a connexin 26 mutant associated with dominant deafness. FASEB J. 2005;19:1516–1518. doi: 10.1096/fj.04-3491fje. [DOI] [PubMed] [Google Scholar]
- 126.Deng Y, Chen Y, Reuss L, Altenberg GA. Mutations of connexin 26 at position 75 and dominant deafness: essential role of arginine for the generation of functional gap-junctional channels. Hear Res. 2006;220:87–94. doi: 10.1016/j.heares.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 127.Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/S0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- 128.Koval M. Pathways and control of connexin oligomerization. Trends Cell Biol. 2006;16:159–166. doi: 10.1016/j.tcb.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cottrell GT, Burt JM. Functional consequences of heterogeneous gap junction channel formation and its influence in health and disease. Biochim Biophys Acta. 2005;1711:126–141. doi: 10.1016/j.bbamem.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 130.Palacios-Prado N, Bukauskas FF (2009) Heterotypic gap junction channels as voltage-sensitive valves for intercellular signaling. Proc Natl Acad Sci USA [DOI] [PMC free article] [PubMed]
- 131.Elenes S, Martinez AD, Delmar M, Beyer EC, Moreno AP. Heterotypic docking of Cx43 and Cx45 connexons blocks fast voltage gating of Cx43. Biophys J. 2001;81:1406–1418. doi: 10.1016/S0006-3495(01)75796-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Valiunas V, Weingart R, Brink PR. Formation of heterotypic gap junction channels by connexins 40 and 43. Circ Res. 2000;86:E42–E49. doi: 10.1161/01.res.86.2.e42. [DOI] [PubMed] [Google Scholar]
- 133.Cottrell GT, Burt JM. Heterotypic gap junction channel formation between heteromeric and homomeric Cx40 and Cx43 connexons. Am J Physiol Cell Physiol. 2001;281:C1559–C1567. doi: 10.1152/ajpcell.2001.281.5.C1559. [DOI] [PubMed] [Google Scholar]
- 134.Hopperstad MG, Srinivas M, Spray DC. Properties of gap junction channels formed by Cx46 alone and in combination with Cx50. Biophys J. 2000;79:1954–1966. doi: 10.1016/S0006-3495(00)76444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.White TW, Bruzzone R, Wolfram S, Paul DL, Goodenough DA. Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: the second extracellular domain is a determinant of compatibility between connexins. J Cell Biol. 1994;125:879–892. doi: 10.1083/jcb.125.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Altevogt BM, Paul DL. Four classes of intercellular channels between glial cells in the CNS. J Neurosci. 2004;24:4313–4323. doi: 10.1523/JNEUROSCI.3303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Haubrich S, Schwarz HJ, Bukauskas F, Lichtenberg-Frate H, Traub O, Weingart R, Willecke K. Incompatibility of connexin 40 and 43 Hemichannels in gap junctions between mammalian cells is determined by intracellular domains. Mol Biol Cell. 1996;7:1995–2006. doi: 10.1091/mbc.7.12.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Srinivas M, Rozental R, Kojima T, Dermietzel R, Mehler M, Condorelli DF, Kessler JA, Spray DC. Functional properties of channels formed by the neuronal gap junction protein connexin36. J Neurosci. 1999;19:9848–9855. doi: 10.1523/JNEUROSCI.19-22-09848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Teubner B, Degen J, Sohl G, Guldenagel M, Bukauskas FF, Trexler EB, Verselis VK, De Zeeuw CI, Lee CG, Kozak CA, Petrasch-Parwez E, Dermietzel R, Willecke K. Functional expression of the murine connexin 36 gene coding for a neuron-specific gap junctional protein. J Membr Biol. 2000;176:249–262. doi: 10.1007/s002320001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Veenstra RD, Wang HZ, Beyer EC, Ramanan SV, Brink PR. Connexin37 forms high conductance gap junction channels with subconductance state activity and selective dye and ionic permeabilities. Biophys J. 1994;66:1915–1928. doi: 10.1016/S0006-3495(94)80985-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kumari SS, Varadaraj K, Valiunas V, Ramanan SV, Christensen EA, Beyer EC, Brink PR. Functional expression and biophysical properties of polymorphic variants of the human gap junction protein connexin37. Biochem Biophys Res Commun. 2000;274:216–224. doi: 10.1006/bbrc.2000.3054. [DOI] [PubMed] [Google Scholar]
- 142.Veenstra RD. Size and selectivity of gap junction channels formed from different connexins. J Bioenerg Biomembr. 1996;28:327–337. doi: 10.1007/BF02110109. [DOI] [PubMed] [Google Scholar]
- 143.Suchyna TM, Nitsche JM, Chilton M, Harris AL, Veenstra RD, Nicholson BJ. Different ionic selectivities for connexins 26 and 32 produce rectifying gap junction channels. Biophys J. 1999;77:2968–2987. doi: 10.1016/S0006-3495(99)77129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gong XQ, Nicholson BJ. Size selectivity between gap junction channels composed of different connexins. Cell Commun Adhes. 2001;8:187–192. doi: 10.3109/15419060109080721. [DOI] [PubMed] [Google Scholar]
- 145.Qu Y, Dahl G. Accessibility of cx46 hemichannels for uncharged molecules and its modulation by voltage. Biophys J. 2004;86:1502–1509. doi: 10.1016/S0006-3495(04)74218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ma M, Dahl G. Cosegregation of permeability and single-channel conductance in chimeric connexins. Biophys J. 2006;90:151–163. doi: 10.1529/biophysj.105.066381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Goldberg GS, Lampe PD, Nicholson BJ. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol. 1999;1:457–459. doi: 10.1038/15693. [DOI] [PubMed] [Google Scholar]
- 148.Ayad WA, Locke D, Koreen IV, Harris AL. Heteromeric, but not homomeric, connexin channels are selectively permeable to inositol phosphates. J Biol Chem. 2006;281:16727–16739. doi: 10.1074/jbc.M600136200. [DOI] [PubMed] [Google Scholar]
- 149.Bevans CG, Kordel M, Rhee SK, Harris AL. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem. 1998;273:2808–2816. doi: 10.1074/jbc.273.5.2808. [DOI] [PubMed] [Google Scholar]
- 150.Niessen H, Harz H, Bedner P, Kramer K, Willecke K. Selective permeability of different connexin channels to the second messenger inositol 1, 4, 5-trisphosphate. J Cell Sci. 2000;113(Pt 8):1365–1372. doi: 10.1242/jcs.113.8.1365. [DOI] [PubMed] [Google Scholar]
- 151.Bedner P, Niessen H, Odermatt B, Kretz M, Willecke K, Harz H. Selective permeability of different connexin channels to the second messenger cyclic AMP. J Biol Chem. 2006;281:6673–6681. doi: 10.1074/jbc.M511235200. [DOI] [PubMed] [Google Scholar]
- 152.Veenstra RD, Wang HZ, Beblo DA, Chilton MG, Harris AL, Beyer EC, Brink PR. Selectivity of connexin-specific gap junctions does not correlate with channel conductance. Circ Res. 1995;77:1156–1165. doi: 10.1161/01.res.77.6.1156. [DOI] [PubMed] [Google Scholar]
- 153.Nicholson BJ, Weber PA, Cao F, Chang H, Lampe P, Goldberg G. The molecular basis of selective permeability of connexins is complex and includes both size and charge. Braz J Med Biol Res. 2000;33:369–378. doi: 10.1590/S0100-879X2000000400002. [DOI] [PubMed] [Google Scholar]
- 154.Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol. 2007;94:120–143. doi: 10.1016/j.pbiomolbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Veenstra RD, Wang HZ, Beyer EC, Brink PR. Selective dye and ionic permeability of gap junction channels formed by connexin45. Circ Res. 1994;75:483–490. doi: 10.1161/01.res.75.3.483. [DOI] [PubMed] [Google Scholar]
- 156.Weber PA, Chang HC, Spaeth KE, Nitsche JM, Nicholson BJ. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys J. 2004;87:958–973. doi: 10.1529/biophysj.103.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Goldberg GS, Valiunas V, Brink PR. Selective permeability of gap junction channels. Biochim Biophys Acta. 2004;1662:96–101. doi: 10.1016/j.bbamem.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 158.Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 159.Essenfelder GM, Bruzzone R, Lamartine J, Charollais A, Blanchet-Bardon C, Barbe MT, Meda P, Waksman G. Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity. Hum Mol Genet. 2004;13:1703–1714. doi: 10.1093/hmg/ddh191. [DOI] [PubMed] [Google Scholar]
- 160.Shibayama J, Paznekas W, Seki A, Taffet S, Jabs EW, Delmar M, Musa H. Functional characterization of connexin43 mutations found in patients with oculodentodigital dysplasia. Circ Res. 2005;96:e83–e91. doi: 10.1161/01.RES.0000168369.79972.d2. [DOI] [PubMed] [Google Scholar]
- 161.Gemel J, Lin X, Veenstra RD, Beyer EC. N-terminal residues in Cx43 and Cx40 determine physiological properties of gap junction channels, but do not influence heteromeric assembly with each other or with Cx26. J Cell Sci. 2006;119:2258–2268. doi: 10.1242/jcs.02953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kyle JW, Minogue PJ, Thomas BC, Domowicz DA, Berthoud VM, Hanck DA, Beyer EC. An intact connexin N-terminus is required for function but not gap junction formation. J Cell Sci. 2008;121:2744–2750. doi: 10.1242/jcs.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dong L, Liu X, Li H, Vertel BM, Ebihara L. Role of the N-terminus in permeability of chicken connexin45.6 gap junctional channels. J Physiol. 2006;576:787–799. doi: 10.1113/jphysiol.2006.113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bukauskas FF, Verselis VK. Gap junction channel gating. Biochim Biophys Acta. 2004;1662:42–60. doi: 10.1016/j.bbamem.2004.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rackauskas M, Kreuzberg MM, Pranevicius M, Willecke K, Verselis VK, Bukauskas FF. Gating properties of heterotypic gap junction channels formed of connexins 40, 43, and 45. Biophys J. 2007;92:1952–1965. doi: 10.1529/biophysj.106.099358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Verselis VK, Trelles MP, Rubinos C, Bargiello TA, Srinivas M. Loop gating of connexin hemichannels involves movement of pore-lining residues in the first extracellular loop domain. J Biol Chem. 2009;284:4484–4493. doi: 10.1074/jbc.M807430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Verselis VK, Ginter CS, Bargiello TA. Opposite voltage gating polarities of two closely related connexins. Nature. 1994;368:348–351. doi: 10.1038/368348a0. [DOI] [PubMed] [Google Scholar]
- 168.Tong JJ, Liu X, Dong L, Ebihara L. Exchange of gating properties between rat cx46 and chicken cx45.6. Biophys J. 2004;87:2397–2406. doi: 10.1529/biophysj.104.039594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Oh S, Rivkin S, Tang Q, Verselis VK, Bargiello TA. Determinants of gating polarity of a connexin 32 hemichannel. Biophys J. 2004;87:912–928. doi: 10.1529/biophysj.103.038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Oh S, Abrams CK, Verselis VK, Bargiello TA. Stoichiometry of transjunctional voltage-gating polarity reversal by a negative charge substitution in the amino terminus of a connexin32 chimera. J Gen Physiol. 2000;116:13–31. doi: 10.1085/jgp.116.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Oshima A, Tani K, Hiroaki Y, Fujiyoshi Y, Sosinsky GE. Projection structure of a N-terminal deletion mutant of connexin 26 channel with decreased central pore density. Cell Commun Adhes. 2008;15:85–93. doi: 10.1080/15419060802013588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tong JJ, Ebihara L. Structural determinants for the differences in voltage gating of chicken Cx56 and Cx45.6 gap-junctional hemichannels. Biophys J. 2006;91:2142–2154. doi: 10.1529/biophysj.106.082859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bukauskas FF, Peracchia C. Two distinct gating mechanisms in gap junction channels: CO2-sensitive and voltage-sensitive. Biophys J. 1997;72:2137–2142. doi: 10.1016/S0006-3495(97)78856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bukauskas FF, Weingart R. Voltage-dependent gating of single gap junction channels in an insect cell line. Biophys J. 1994;67:613–625. doi: 10.1016/S0006-3495(94)80521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bukauskas FF, Elfgang C, Willecke K, Weingart R. Heterotypic gap junction channels (connexin26-connexin32) violate the paradigm of unitary conductance. Pflugers Arch. 1995;429:870–872. doi: 10.1007/BF00374812. [DOI] [PubMed] [Google Scholar]
- 176.Purnick PE, Oh S, Abrams CK, Verselis VK, Bargiello TA. Reversal of the gating polarity of gap junctions by negative charge substitutions in the N-terminus of connexin 32. Biophys J. 2000;79:2403–2415. doi: 10.1016/S0006-3495(00)76485-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bukauskas FF, Bukauskiene A, Bennett MV, Verselis VK. Gating properties of gap junction channels assembled from connexin43 and connexin43 fused with green fluorescent protein. Biophys J. 2001;81:137–152. doi: 10.1016/S0006-3495(01)75687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shibayama J, Gutierrez C, Gonzalez D, Kieken F, Seki A, Carrion JR, Sorgen PL, Taffet SM, Barrio LC, Delmar M. Effect of charge substitutions at residue his-142 on voltage gating of connexin43 channels. Biophys J. 2006;91:4054–4063. doi: 10.1529/biophysj.106.085787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Smart OS, Goodfellow JM, Wallace BA. The pore dimensions of gramicidin A. Biophys J. 1993;65:2455–2460. doi: 10.1016/S0006-3495(93)81293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gouet P, Courcelle E, Stuart DI, Metoz F. ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics. 1999;15:305–308. doi: 10.1093/bioinformatics/15.4.305. [DOI] [PubMed] [Google Scholar]