Abstract
A functional analysis of the role of glutathione in protecting plants from environmental stress was undertaken by studying Arabidopsis that had been genetically modified to have altered glutathione levels. The steady-state glutathione concentration in Arabidopsis plants was modified by expressing the cDNA for γ-glutamyl-cysteine synthetase (GSH1) in both the sense and antisense orientation. The resulting plants had glutathione levels that ranged between 3% and 200% of the level in wild-type plants. Arabidopsis plants with low glutathione levels were hypersensitive to Cd due to the limited capacity of these plants to make phytochelatins. Plants with the lowest levels of reduced glutathione (10% of wild type) were sensitive to as little as 5 μm Cd, whereas those with 50% wild-type levels required higher Cd concentrations to inhibit growth. Elevating glutathione levels did not increase metal resistance. It is interesting that the plants with low glutathione levels were also less able to accumulate anthocyanins supporting a role for glutathione S-transferases for anthocyanin formation or for the vacuolar localization and therefore accumulation of these compounds. Plants with less than 5% of wild-type glutathione levels were smaller and more sensitive to environmental stress but otherwise grew normally.
Glutathione (GSH), the tripeptide γ-glutamylcysteinyl-Gly, is the major source of non-protein thiols in most plant cells (Bergmann and Rennenberg, 1993). The chemical reactivity of the thiol group of glutathione makes it particularly suitable to serve a broad range of biochemical functions in all organisms. It has an oxidation reduction potential of −0.23 V that allows it to act as an effective electron acceptor and donor for numerous biological reactions. The nucleophilic nature of the thiol group also is important in the formation of mercaptide bonds with metals and for reacting with select electrophiles. This reactivity, along with the relative stability and high water solubility of GSH, makes it an ideal biochemical to protect plants against stress including oxidative stress, heavy metals, and certain exogenous and endogenous organic chemicals.
Electron transport reactions in plants, particularly those of the chloroplast produce reactive oxygen species including hydrogen peroxide, superoxide, and hydroxide radicals. The ascorbate/GSH cycle (Larson, 1988; Alscher, 1989; Foyer et al., 1994) is essential in removing H2O2, especially in the plastids (Foyer and Halliwell, 1976; Alscher, 1989; Noctor and Foyer, 1998; Asada, 1999). Because of the role of glutathione in ascorbate reduction, it is also essential in protecting membranes by maintaining α-tocopherol and zeaxanthin in the reduced state.
Glutathione is polymerized to form phytochelatins, (γ-Glu-Cys)2–11-Gly. This reaction is catalyzed by phytochelatin synthase (Grill et al., 1987; Clemens et al., 1999; Ha et al., 1999; Vatamaniu et al., 1999). Phytochelatins are made in the cytosol where they have a high affinity for binding with heavy metals, particularly Cd and Cu. These metal-phytochelatin complexes are then transported into the vacuole thus sequestering the metals away from sensitive enzymes (Rauser, 1990). This system provides plants with a moderate level of resistance to Cd and Cu. Arabidopsis plants that are diminished in their capacity to produce glutathione, cad2 (Howden et al., 1995a) or RML1 (Vernoux et al., 2000), or have a mutation in the gene for phytochelatin synthase, the cad1 gene (Howden et al., 1995b), make fewer phytochelatins and are hypersensitive to Cd and Cu. The enzyme phytochelatin (PC) synthase is constitutively expressed, but its activity is dependent on the presence of a heavy metal. When this enzyme is activated in the presence of Cd or Cu, this reaction becomes a major sink for glutathione.
Glutathione is also involved in the detoxification of organic compounds. Many xenobiotics as well as some metabolites like anthocyanins are reacted with GSH by a family of glutathione S-transferases (GST) and transported, possibly as GSH conjugates, into the vacuole (Marrs, 1996).
Glutathione is synthesized from standard amino acids in two steps. γ-Glutamyl-Cys (γ-EC) synthetase combines Glu and Cys in an ATP-dependent reaction to form γ-glutamyl-Cys (Hell and Bergmann, 1990). In Arabidopsis this is encoded by a single gene, GSH1 (May and Leaver, 1995). Glutathione synthetase catalyzes the ATP-dependent reaction between γ-EC and Gly to form GSH. In Arabidopsis, GSH synthetase is encoded by a single gene, GSH2 (Wang and Oliver, 1996), which through alternative mRNA splicing can produce proteins targeted to the cytosol and plastid (Skipsey et al., 1999). Chloroplastic and cytosolic isoforms of GSH reductase are also essential to reduce oxidized glutathione (GSSG) back to the reduced form, GSH.
Loss-of-function analysis is also well-suited for defining the biological roles of compounds like GSH in plants. Considering all the vital functions of glutathione, a null mutation is likely lethal. No such mutants have been isolated to date. The cad2 mutant of Arabidopsis still produces GSH at a level of 30% wild type (Howden et al., 1995a). With the exception of hypersensitivity to some toxic metals, this mutant is nearly indistinguishable from wild-type plants. Vernoux et al. (2000) have recently shown that the RML1 (ROOT MERISTEMLESS 1) mutant of Arabidopsis (Cheng et al., 1995) is due to a point mutation in the GSH1 gene. This mutant has GSH levels that are approximately 3% of wild type. The mutation precludes formation of a root meristem and is associated with a block in the G1-to-S transition in cells. Growth and fertility are both limited in this mutant. We used a transgenic approach to over-express the cDNA for γ-glutamyl-Cys synthetase in both sense and antisense orientation and produced a number of transgenic Arabidopsis lines with altered levels of GSH ranging from 3% to nearly 200% of the wild-type level. These biochemical mutants are ideal for defining biological functions of GSH in higher plants. We have demonstrated with these mutants that glutathione is essential in protecting plants from heavy metal toxicity and that glutathione levels affects the accumulation of anthocyanin. In addition, an interesting regulatory mechanism of Cys synthesis was uncovered by these mutants.
RESULTS
Creating Transgenic Arabidopsis Lines with Altered GSH Levels
GSH synthesis requires both γ-EC synthetase and GSH synthetase. Therefore, GSH levels can be manipulated by altering the levels of these enzymes. Since γ-EC synthetase is thought to be the rate-limiting enzyme for GSH synthesis (Arisi et al., 1997), the single copy gene GSH1 encoding γ-EC synthetase was targeted. We used a transgenic approach to alter GSH level by over-expressing the cDNA for γ-EC synthetase in Arabidopsis. Figure 1A illustrates the genetic constructs used for plant transformation. A number of transgenic lines were produced for both sense and antisense constructs. One antisense line (R10) and two sense lines (16 and 21), which gave large consistent changes in the glutathione levels, were studied in detail. The molecular analysis of the T3 generation of one antisense line (R10-2) and two sense lines (16-A and 21-1) are shown in Figure 1B. Southern-blot analysis of the 11 or 12 individual plants from each line indicate that all three lines are homozygous for a single copy of the transgenic construct (in the 16-A line the inserted gene cannot be distinguished from the endogenous GSH1 gene).
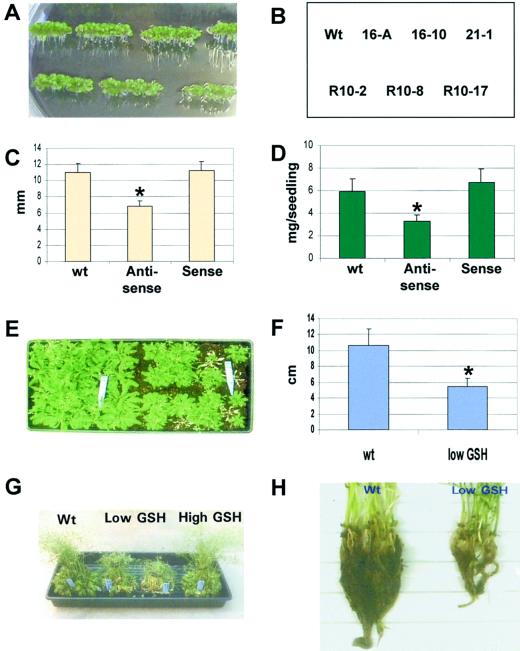
Figure 1.
Generation of transgenic Arabidopsis lines with altered GSH levels. A, The plant transforming binary vector constructs for both sense and antisense expression of GSH1. The cDNA for GSH1 was inserted into the binary vector pCB200 (Xiang and Oliver, 1999) in both orientations under the control of the 35S promoter of cauliflower mosaic virus as illustrated. The bar gene was used as the selectable marker for in-soil transformant selection and the Ω sequence of tobacco mosaic virus to enhance the translation of the transgene. B, Genomic DNA-blot analysis of three non-segregating lines (T3 generation) using GSH1 cDNA probe as indicated in A. R10-2 was an antisense line, whereas 16-A and 21-1 were lines for sense constructs. Total DNA was digested with SstI at a unique site within the T-DNA region and the presence of a single copy insert confirmed (in 16-A the band of the insert comigrates with that of the endogenous GSH1 gene). The results match the herbicide resistance phenotype of these plants. C, RNA-blot analysis of homozygous lines (T3) for both sense (16-A) and antisense (R10-2) transgene expression. Sense-specific probe (antisense GSH1) was used to estimate the GHS1 transcript levels. The mRNA was isolated from soil grown plants. D, Western-blot analysis of representative transgenic lines using antibodies raised against γ-EC synthetase of Arabidopsis. Wild-type, sense line 16-A (+), and antisense line R10-2 (−) plants were grown in liquid culture. Total protein extracts were separated by SDS-PAGE and electrotransferred to nitrocellulose before the γ-EC synthetase protein was detected using the specific polyclonal antiserum.
The transcript levels of GSH1 in these lines were shown in Figure 1C. The mRNA for γ-EC synthetase is 2.0 kb and present in the soil-grown plants at moderate levels. In the five plants of antisense line R10-2 the mRNA level is less than 10% of the wild-type level. In the four sense plants of line 16 the amount of γ-EC synthetase mRNA is increase substantially (10- to 100-fold) under control of the 35S promoter. These mRNA blots were done on leaves taken from soil grown plants. In the wild-type as well as the transgenic plants a substantial amount of the mRNA for γ-EC synthetase consistently appears to be degraded. It is unlikely that this is non-specific RNA degradation because of the quality of the mRNA on ethidium bromide stained gels. We do not observe this putative breakdown product of the γ-EC synthetase mRNA isolated from plants grown in liquid culture (Xiang and Oliver, 1998) and suggest that this might represent rapid turnover of this specific mRNA in soil-grown plants.
The protein levels for γ-EC synthetase were shown in Figure 1D for two representative transgenic lines and wild type. In this western blot, the γ-EC synthetase protein is detected as a 60-kD band in wild-type Arabidopsis plants grown in liquid culture. We were unable to detect the protein in the antisense line R10-2 and estimate that it is less than 10% of the wild-type protein level. The concentration of protein in the sense line, 16-A, is elevated to levels that are at least 25 times the amount in wild-type tissue.
The molecular characterization of these transgenic lines show that we have successfully manipulated the expression of the target gene, GSH1. The single copy sense and antisense inserts have created plants with γ-EC synthetase levels that range from a small fraction of to many times wild-type levels. The ultimate proof of the success of these transgenic modifications is to demonstrate that these plants have biochemical phenotypes with altered GSH levels. HPLC analysis of wild-type and transgenic plants demonstrated that the GSH levels were significantly modified by the sense and antisense expression of the γ-EC synthetase cDNA. Table I shows the profile of thiols in these representative lines and compares them with wild-type concentrations. The five antisense lines shown have GSH levels that range from 2.5% to 49% of wild-type concentrations. The γ-EC levels in these plants are also lower than in the wild type, although the differences were not always significant. In lines R10, R8, and R11, the concentration of Cys, which occurs before the blocked step, are increase up to 2.6-fold over wild type. The two sense lines shown have GSH levels that are 155% and 180% of wild type. Thus, we have created transgenic Arabidopsis plants with altered GSH levels ranging from approximately 3% to nearly 200% of wild type. These values are for small leaves in young soil-grown plants. As the plants age, GSH levels in the antisense plants tended to be increased (data not shown), and plants grown in liquid culture tended to have higher GSH levels.
Table I.
The thiol profiles of representative transgenic lines
| Transgenic Lines | Orientation of cDNA | Cys | γ-EC | GSH | GSH, % of Wild Type |
|---|---|---|---|---|---|
| Wild type | – | 47.0 ± 6.0a | 6.0 ± 1.0a | 894.0 ± 93.0 | 100.0 |
| R10 | Antisense | 122.2 ± 15.6b | 0.9 ± 0.2 | 22.4 ± 1.9a | 2.5 |
| R8 | Antisense | 61.8 ± 8.4abc | 2.0 ± 0.3a | 69.7 ± 7.3ab | 7.8 |
| R11 | Antisense | 69.3 ± 8.9abcd | 4.1 ± 0.7a | 172.5 ± 18.0abc | 19.3 |
| R4 | Antisense | 37.8 ± 4.8abcd | 3.1 ± 0.5a | 304.0 ± 31.6abcd | 34.0 |
| R6 | Antisense | 38.7 ± 5.0abcd | 4.5 ± 0.8a | 437.2 ± 45.5bcd | 48.9 |
| 16 | Sense | 55.0 ± 7.1a | 10.3 ± 1.7a | 1365.7 ± 144.2e | 155.0 |
| 21 | Sense | 51.7 ± 6.6a | 8.4 ± 1.4a | 1609.0 ± 167.4e | 180.0 |
T1 plants that survived herbicide selection were grown for 2 weeks in soil in a growth chamber at 22°C, 50 μE m−2 s−1 and 16-h light. Individual rosette leaves were harvested and the thiols determined by HPLC. The results presented are means ± se for three replicates. The concentrations reported are nanomoles per gram fresh wt. The last column shows GSH values as percent of wild-type concentration. Within a column, nos. with the same superscript letters are not significantly different.
Growth of Plants with Decreased GSH Levels
The antisense plants with low GSH levels were smaller in stature but developed at approximately the same rate as wild-type plants. On solid media, they germinated at the same time as the wild type but were noticeably smaller. The sense plants with elevated GSH levels also germinated in parallel with the wild-type plants and were slightly larger (although the difference was not statistically significant) than wild-type plants. With 1-week-old plants grown on solid media in Petri dishes (Fig. 2, A–D) the antisense plants were 55% the size (biomass) of the wild-type plants, and the sense plants were just over 100% the size of the wild type. The length of the roots of the antisense plants was approximately 60% of the wild-type value, and the over-expressing plants were 105% of wild type. There was no significant change in the root to shoot ratio as the GSH level was varied from less than wild type to greater than wild type.
Figure 2.
Reduced GSH levels result in decreased plant growth. A, Low GSH antisense (homozygous R10-2, R10-8, and R10-17 plants), high GSH sense (homozygous 16-A, 16-10, and 21-1), and wild-type plants were germinated on half-strength Murashige and Skoog salts solidified with Phytagel (3.0 g per liter) and grown under continuous light of 50 μE m−2 s−1 and at 22°C. The petri dishes were placed vertically. A plate with 5-d-old seedlings is shown. The nomenclature of the transgenic plants is that the first number indicates the primary transformant (R designated antisense constructs) and the second number or letter indicates a specific T3 line derived from that primary transformant. B, The key identifying the specific lines in A. C, Root growth of wild-type and transgenic plants. The root length of the wild-type plants, the sense lines (16-A, 16-10, and 21-1), and the antisense lines (R10-2, R10-8, and R10-17) were measured after 5 d of growth (25 plants each) and recorded along with the se. D, The same plants described in C were removed from the medium surface and the fresh weight per seedling determined. The asterisk indicates significant difference. E, Wild-type (left half of tray) and low GSH plants (R10 on right side of tray) grown for 1 month in the greenhouse. The low GSH line is still segregating. The bleached plants are segregates sensitive to Liberty herbicide. F, Rosette diameter of the wild-type and herbicide-resistant low GSH plants containing the antisense GSH1 cDNA. The wild-type line contains the control pCB200 plasmids without insert DNA. G, Photograph of 2-month-old wild-type, low GSH line R10-2, and high GSH line 16-A plants that were grown at 50 μE m−2 s−1 at 22°C before transfer to 350 μE m−2 s−1 and 26°C for 1 week. The low GSH plants are wilted under these conditions. H, Root systems of the same wild-type and low GSH plants described in G.
When the plants were grown in soil (100 μE m−2 s−1 continuous light 21°C) the antisense plants germinated at the same time as the wild type but had difficulties becoming established. The small plants were fairly fragile and easily damaged. One month after planting, both the antisense and wild-type plants bolted and began flowering on the same schedule. At this time the antisense plants were substantially smaller than wild type. At 1 month after germination the diameter of the rosette of wild-type plants was 10.5 ± 1.8 cm, whereas the diameter of the antisense plants was 5.7 ± 1.1 cm, 54% of the wild-type value (Fig. 2, E and F). This same size difference was obvious in full-grown plants. The low GSH antisense plants were smaller, but the developmental timing was indistinguishable from wild type. The decreased shoot and root mass of mature plants are shown in Figure 2, G and H. The low GSH plants are severely wilted, a phenotype we observed often when plants were placed in the greenhouse that might have been associated with decreased vigor or stress resistance.
Having engineered Arabidopsis plants with different capacities for forming glutathione and as a result different steady-state GSH levels, we then used these plants to determine the role of GSH in mitigating the effects of stress in these plants as well as its role in normal metabolic activities.
GSH Is Essential in Protecting Plant from Heavy Metal Toxicity
Howden et al. (1995a) selected for an Arabidopsis line with low γ-EC synthetase activity by screening for plants that were hypersensitive to Cd. We were interested in determining if our biochemical mutants with substantially lower GSH synthesis capacity as well as the over-expression mutants with levels twice wild type had altered sensitivity to heavy metals. Seeds from several of the different antisense and sense lines were grown on plates containing either 0 or 40 μm CdCl2 in Murashige and Skoog media solidified with Phytogel. All of the lines germinated and grew well on Cd-free medium. In the presence of 40 μm CdCl2 wild-type plants, as well as most transgenic lines containing the γ-EC synthetase sense construct, grew at rates that were very similar to the rate in the absence of Cd (Fig. 3A). The antisense lines, however, germinated in the presence of 40 μm CdCl2, but growth was substantially inhibited and the plants bleached and died within 7 d of germination (Fig. 3A). Line 20-A was a sense line with very low GSH levels, suggesting that the transgene might be cosuppressing the endogenous GSH1 gene. Like the antisense lines, it was very sensitive to Cd. This line was not studied further.
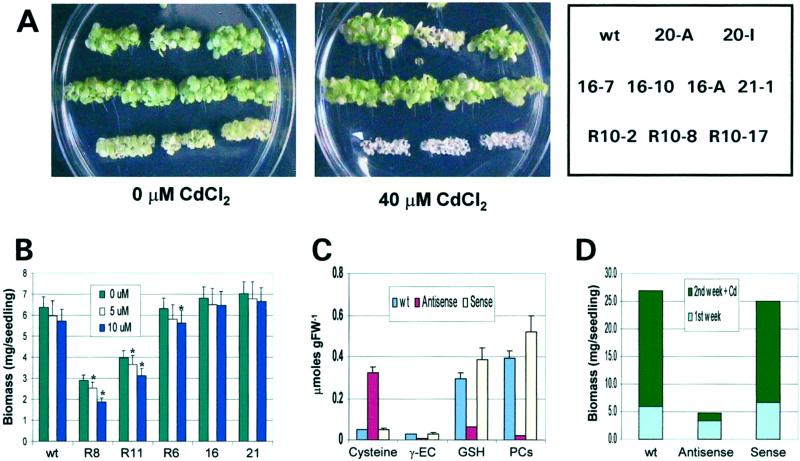
Figure 3.
Heavy metal sensitivity of the biochemical mutants with altered GSH levels. A, Heavy metal sensitivity of Arabidopsis seedlings with altered GSH levels. Wild-type and T3 Arabidopsis plants of the antisense lines with low GSH (R10-2, R10-8, R10-17) and sense lines (16-7, 16-10, 16-A, 21-1, 20-A, and 20-I) were germinated on half-strength Murashige and Skoog medium with or without 40 μm of CdCl2. The key is shown on the right. 20-A was a cosuppressed line with reduced GSH level. B, Five lots of 10 plants each were grown in Petri dishes containing the indicated concentrations of CdCl2. After 1 week the plants were harvested and the fresh weight and the GSH level determined. Those metal treatments with the asterisk had biomass accumulations that were significantly decreased by the concentration of Cd indicated. C, Thiol profile of Arabidopsis wild-type and biochemical mutants with altered GSH levels in response to CdCl2 treatment. Wild type and both sense and antisense lines as in A were germinated in liquid culture. One-week-old Arabidopsis seedlings were treated with 25 μm of CdSO4 for 16 h. Cys, γ-EC, GSH, and PCs were extracted and quantified as their monobromobimane derivatives. The values represent the average of three or more individual lines. The error bar represents the se. D, Biomass of wild-type and sense and antisense biochemical mutants. Two identical set of liquid cultures (50 seeds per flask) for lines as in C were initiated for growth measurements. One-week-old seedlings were harvested from one set of culture and fresh weight recorded. The other set of cultures were exposed to 25 μm CdCl2 for an additional week before the seedlings were harvested and their fresh weight recorded.
We focused additional attention on the R8 (10% wild-type GSH level), R11 (20% wild-type GSH), R6 (50% wild-type GSH), and 16 (150% wild-type GSH) and 21 (190% wild-type GSH) lines (Fig. 3B), which provided plants with a gradient of GSH levels. Five replicas of 10 plants each type were grown on low concentrations of CdCl2 (0, 5, and 10 μm) to determine if we could see a direct relationship between the level of GSH and the sensitivity to these levels of Cd. R8 in the absence of Cd produced 46% the biomass of wild-type plants, and its growth was significantly inhibited by 5 and 10 μm Cd. R11 had 63% as much biomass as wild type and its growth was also significantly inhibited by both 5 and 10 μm Cd. R6 with 50% wild-type GSH level had biomass accumulation that was not different from wild type and its growth was not significantly inhibited by 5 μm Cd but was by 10 μm Cd. Both of the over-expresser lines (16 and 21) had biomass accumulations that were not significantly different from wild type and, like wild type, their growth was not significantly inhibited by 5 and 10 μm Cd.
The pools of reduced thiols in mutant and wild-type plants were measured after growth in liquid culture following treatment with 25 μm CdCl2 for 16 h. This concentration was chosen because it has minimal impact on growth of wild-type tissues. Figure 3C shows that both the sense and the wild-type plants are capable of producing phytochelatins that protect these plants from the toxic effects of Cd. The antisense plants produce only very limited amounts of phytochelatins because of the decreased GSH level and are, therefore, much more metal sensitive. The resulting increased sensitivity of the antisense plants to Cd is shown in Figure 3D. Plants were grown in liquid culture without Cd for 1 week before 25 μm CdCl2 was added and the plants were allowed to continue growing for a second week. The total plant biomass at the end of the week without Cd was nearly the same for the sense plants and the wild-type plants. The antisense plants had approximately one-half the biomass of the wild-type plants. After a second week of growth in the presence of Cd, the wild-type and sense plants grew at approximately the same rate, but the growth of the antisense plants was inhibited by approximately 90% (Fig. 3D). This confirms the reduced growth rate of the antisense plants and their hypersensitivity to low concentrations of Cd due to their inability to produce phytochelatins.
Cd treatment caused activation of phytochelatin synthase and the conversion of GSH to phytochelatins (Fig. 3C). Because these plants were purposely treated with low concentrations of Cd, they produce only limited amounts of phytochelatins. Line 21-A that contains the γ-EC synthetase cDNA in the sense orientation grown in liquid culture treated with 25 μm CdCl2 has approximately 20% more GSH than wild-type plants grown under the same conditions and forms approximately 25% more phytochelatins. The antisense plants grown with 25 μm CdCl2 have GSH concentrations that are approximately 20% of wild-type values and accumulate less than 10% as much phytochelatins. Under these low Cd conditions there appears to be a direct relationship between the amount of glutathione and the amount of phytochelatins produced. The wild-type and the sense plants make enough phytochelatins to protect them from this level of Cd, whereas the antisense plants do not.
Plants with altered levels of γ-EC synthetase have been useful in exploring other mechanisms controlling GSH and Cys biosynthesis in plants. Figure 4 shows an experiment where Arabidopsis wild-type plants, an antisense GSH1 mutant with low γ-EC synthetase, and a sense line with high γ-EC synthetase all grown in liquid culture were exposed to increasing concentrations of Cd for 24 h. The tissue was then harvested, and the levels of the major thiols determined. In this experiment the untreated wild-type plants had GSH levels of 0.19 μmol/g, whereas the low and high γ-EC synthetase plants had GSH levels of 0.03 (16%) and 0.23 (121%), respectively. Wild-type plants exposed to increasing concentrations of Cd began accumulating large amounts of phytochelatins. As glutathione was diverted to phytochelatin formation the amount of free GSH in these plants decreased. A greater flux of sulfur through the GSH pool is obvious from the substantial increase in total thiols in Cd-treated wild-type plants.
Figure 4.
Effect of increasing Cd levels on the major thiols of Arabidopsis plants. Wild-type and homozygous T3 Arabidopsis lines for both antisense low GSH (R10-2, R10-8, R10-17 combined) and sense lines (16-7, 16-10, 16-A, and 21-1 combined) with high GSH were grown in liquid culture for 1 week. Heavy metal CdCl2 at the indicated concentrations was added and incubated for 24 h. The plants were then harvested and Cys, γ-EC, GSH, and PCs were determined by HPLC as described for Figure 3B. The data presented are the mean of three flasks each containing 50 seeds. The sum of Cys, γ-EC, glutathione, and phytochelatins was represented as total thiols (Total).
In the γ-EC synthetase antisense plants we do not see this large increase in total thiols because the decreased ability to make γ-EC keeps the GSH pool low and deprives PC synthase of sufficient substrate for substantial phytochelatin formation. The antisense plants with low amounts of γ-EC synthetase protein do show that the reactions leading to Cys formation are activated by Cd treatment. In these plants the steady-state Cys concentration increases over 2-fold in response to the Cd exposure. Cys biosynthesis is probably also activated by Cd in the wild-type and sense GSH1 plants, but its accumulation is less obvious due to the increased flux through γ-EC synthetase and GSH synthetase.
At the highest Cd concentrations, γ-EC accumulates to several times the amount in untreated wild-type and high γ-EC synthetase plants. This suggests that under these conditions γ-EC synthesis is stimulated more than its conversion to GSH, or GSH synthase is more Cd sensitive. At the highest Cd level (400 μm) the increased capacity for γ-EC formation in the plants with high γ-EC synthetase protein results in γ-EC levels that are nearly twice those in wild-type plants.
One of the biggest changes in the antisense plants in the absence of Cd treatment is the substantial increase in Cys levels. Compared with the sense and wild-type plants, antisense plants have nearly five times more Cys (Fig. 4). Cys increases were also noted with the cad2 mutant (Cobbett et al., 1998). These plants have less total thiols than the wild type so the decreased GSH levels in the antisense do not result in an increased flux into the total thiol pool. The accumulation of Cys suggests that feedback inhibition by Cys and glutathione are not major determinants in Cys accumulation (Leustek et al., 2000).
GSH Levels Affect the Accumulation of Anthocyanin
The role of GSH is not just to protect plants from stress by ameliorating the effects of heavy metals and oxidative stress. Glutathione is also used for a number of metabolic functions in plants. GSH is a mandatory substrate for GST reactions. Plants with low GSH concentrations, therefore, are less able to glutathionate anthocyanins. To induce anthocyanin synthesis, Arabidopsis plants were grown at very low light (50 μE m−2 s−1 continuous light 22°C) for 1 month until the plants were just beginning to flower. These plants were then transferred to a chamber with moderate light levels (250 μE m−2 s−1 continuous light at 24°C) for 1 week. After 1 week, the amount of glutathione and anthocyanin in the leaves was determined (Fig. 5). In this experiment we used a number of antisense lines that gave glutathione levels that ranged from 20% to 45% of wild type, wild-type plants, and a sense line with nearly twice the GSH concentration of wild type (Fig. 5D). Visual observations showed that the plants with decreased GSH developed less pigmentation in the leaves (Fig. 5A). A comparison of the GSH level in these tissues (Fig. 5D) with the anthocyanin present (Fig. 5, B and C) showed a rough correlation between the amount of GSH and the resulting pigment accumulation due apparently to the large variability in anthocyanin formation between leaves in our system. When the GSH level was 20% of wild type, the resulting anthocyanin levels in the plants was very low. Maximum anthocyanin accumulated in wild-type tissue and the plants over expressing γ-EC synthetase and showing twice as much GSH did not accumulate additional pigment.
Figure 5.
Reduced anthocyanin accumulation in low GSH biochemical mutants. Low GSH antisense lines (R17-1, R17-2, R17-3, and R12-1), high GSH sense line (16-A), and wild-type plants were germinated and grown side by side under continuous light of 50 μE m−2s−1 at 22°C and well watered in a growth chamber for 5 weeks. No apparent phenotypic differences other than size were observed between low GSH plants and wild-type plants under these conditions. The plants were transferred to continuous light of 250 μE m−2 s−1 at 22°C without watering for 1 week. Anthocyanin accumulation becomes apparent in the leaves of wild-type plants (right in A) but not in low GSH antisense line R17-1 (left in A). Anthocyanin extracts of these lines are shown in B. Concentrations of anthocyanin (C) and GSH (D) were determined as described in “Materials and Methods.” The results shown are means for three to five plants.
DISCUSSION
Whereas glutathione has been implicated in a number of normal metabolic functions, it is most consistently associated with protecting plants from environmental stress. It is not surprising, therefore, that Arabidopsis plants with leaf GSH levels as low as 3% of wild type grow reasonably well in low stress environments. The only consistent phenotype of the plants with low γ-EC synthetase levels, limited capacity for GSH formation, and therefore low steady-state GSH levels is that the plants were only accumulated approximately one-half of the biomass of wild-type plants. The reason for the decreased size is not obvious. Vernoux et al. (2000) have suggested a role for GSH in the cell cycle. In the RML1 mutant this results in a failure to form root apical meristems and as a result root formation is curtailed. Whereas these results suggest that the root meristem is most sensitive, it is possible that GSH plays an important role in other meristems and that when GSH levels drop to a fraction of the wild-type level, cell division within the other meristems is decreased. We were not able to document a specific inhibition of root formation in our plants. It should be noted that our plants were generated with antisense constructs using the 35S promoter and that this promoter does not express equally in all tissues. It is, as a result, possible that we are not suppressing GSH levels in meristems as much as in the rest of the plant. We have noticed that the GSH level in our plants changes with time. In those plants showing the strongest antisense phenotype, the GSH levels were lowest in young leaves (sometimes less than 5% of wild-type leaves of the same age). As the plants aged, GSH levels in mature leaves often increased to 10% to 20% of wild type. This may result from use of the 35S promoter or may indicate long term accumulation of GSH and decreased turnover in these plants.
Over expression of γ-EC synthetase caused a large increase in its steady-state mRNA and protein levels but only a modest increase in GSH concentration. This could result from a limitation in GSH synthetase activity, Cys biosynthetic capacity, feedback inhibition of GSH on γ-EC synthetase, or production of an inactive form of the enzyme. May et al. (1998) have suggested that this enzyme requires phosphorylation for full activity.
The antisense γ-EC synthetase plants with lower levels of this protein and decreased amounts of GSH were substantially more sensitive to a range of environmental stresses including the heavy metals, Cu and Cd, and photooxidative and ozone stress (data not shown). The sensitivity to Cd was most predictable. The cad2 mutant (Howden et al., 1995a; Cobbett et al., 1998) and the RML1 mutant (Vernoux et al., 2000) are also very Cd sensitive.
We were unable to document a significant increase in Cd tolerance in our GSH1 sense plants with elevated γ-EC synthetase and GSH levels despite numerous attempts. These plants did have an increased capacity to make GSH and were able to maintain GSH levels that were higher than those in untreated wild-type plants in the presence of up to 100 μm Cd (Fig. 4). This increase in GSH levels, however, did not result in increased rates of phytochelatin formation except at very low Cd levels (25 μm in Figs. 2 and 4). This suggests that under the conditions in which these plants were grown, the rate of PC formation in wild-type plants is not limited by the available GSH. Rather, phytochelatin formation is limited by either the rate of PC synthase or the capacity for further processing and/or transport of the Cd or Cd-phytochelatin complex.
In the wild-type and γ-EC synthetase sense plants, there is an increase in γ-EC level as the plants are exposed to increasing Cd concentrations. The activities of γ-EC synthetase and GSH synthetase are under multilevel controls. Transcription of both genes are induced by exposure to Cd and Cu (Schafer et al., 1998; Xiang and Oliver, 1998). The translation of at least the γ-EC synthetase mRNA is regulated by the GSH/GSSG ratio in the cell (Xiang and Oliver, submitted article). γ-EC synthetase is under feedback control by GSH and Cys, and sometimes Gly availability (Noctor et al., 1997) can limit GSH formation. Cd treatment can increase the rate of flux through this pathway by altering each of these control mechanisms. It induces GSH1 and GSH2 transcription and by inducing oxidative stress increases γ-EC synthetase mRNA translation. It lowers the GSH level and thereby lessens feedback inhibition of γ-EC synthetase. It also appears to induce formation of Cys (Fig. 4). The increase in γ-EC level following Cd exposure suggests that the reactions leading to γ-EC formation are preferentially stimulated relative to those involved in its use, presumably its conversion to GSH by GSH synthetase. These results could also happen if GSH synthetase is being inhibited by the Cd in the tissues.
Several other groups have attempted to alter GSH levels in plants by over expressing the Escherichia coli γ-EC synthetase and GSH synthetase (as opposed to the plant enzyme used in this manuscript). Elevated γ-EC synthetase, but not GSH synthetase, in transgenic poplar increased GSH levels (Noctor et al., 1996). Creissen et al. (1999) elevated GSH levels in tobacco by expressing E. coli γ-EC synthetase in chloroplasts. These plants had higher GSH levels but were also much more sensitive to oxidative stress. Nothing like this was observed in our high GSH plants. We have not identified the subcellular compartment where the GSH has accumulated within our transgenic plants. Indian mustard plants transformed with bacterial GSH1 (Zhu et al., 1999b) but not GSH2 (Zhu et al., 1999a) had a 1.5- to 2.5-fold increase in GSH, and both plants showed some increase in metal resistance.
The Cd treatment also increased the level of Cys. This is particularly obvious in the antisense plants with limited γ-EC synthetase protein. Several steps in the biosynthetic pathway for Cys are stimulated by heavy metals including APS sulfotransferase (Lee and Leustek, 1998; Heiss et al., 1999; Leustek and Saito, 1999; Leustek et al., 2000) and O-acetyl-Ser (thiol) lyase (Schafer et al., 1998; Barroso et al., 1999; Xiang and Oliver, unpublished data). In plants with decreased capacity for conversion of Cys to γ-EC Cd treatment resulted in a 2-fold increase in the Cys concentration. Under these conditions Cys becomes the major thiol in the plants.
Glutathione is required as a glutathionation of anthocyanin by a GST (e.g. the Bz2 gene of maize and the An2 gene of petunia). This formation of a glutathione-anthocyanin conjugate (or possibly a GSH-dependent reaction without conjugate formation) is an essential step in transport of the anthocyanin into the vacuole (Alfenito et al., 1998; Edwards et al., 2000). In the case of the maize Bz2 mutant, it accumulates anthocyanin in the cytosol where they give a bronze instead of purple color (Marrs et al., 1995). In petunia, the An2 deficient plants do not accumulate color in the petals. In both Bz2 and An2 mutants, the total amount of anthocyanin is decreased (Alfenito et al., 1998). In our mutants the lack of available glutathione had much the same affect as the lack of the necessary GST. Anthocyanin levels were roughly proportional to GSH levels, although plants with elevated GSH did not increase anthocyanin accumulation above wild-type levels.
MATERIALS AND METHODS
Plant Growth, Liquid Culture, and Stress Treatments
Arabidopsis (ecotype Columbia) wild-type and mutant plants were grown in a growth chamber (Percival, Boone, IA) with 12-h-light photoperiod and 22°C constant temperature or as otherwise specified. Growth of Arabidopsis plants in liquid culture and stress treatments were essentially performed as described (Xiang and Oliver, 1998). To measure root growth, seeds were germinated on half-strength Murashige and Skoog medium solidified with Phytagel (3 g per liter). The heavy metal sensitivity of Arabidopsis seedlings was examined on the same medium containing the specified concentrations of CdCl2.
DNA Manipulation and Generation of Transgenic Arabidopsis Plants
All DNA manipulations were performed as described (Ausubel et al., 1987). The GSH1 over-expression constructs were made by inserting GSH1 cDNA coding region in sense and antisense orientations in the binary vector pCB200 that was modified from plasmid pGPTV-BAR (Becker et al., 1992) by replacing the promoterless uidA with the cauliflower mosaic virus 35S promoter-driven expression cassette as described (Xiang et al., 1999). These binary vector constructs were introduced into Agrobacterium tumefaciens for Arabidopsis plant transformation as described (Xiang et al., 1999).
DNA and RNA Gel-Blot Analyses
Genomic DNA-blot analysis was performed as described (Xiang et al., 1997). Total RNA extraction and RNA-blot analysis was performed essentially as described (Xiang and Oliver, 1998).
Antibody Production and Protein Gel-Blot Analysis
To raise antibody against Arabidopsis γ-EC synthetase in rabbit, the cDNA for γ-EC synthetase was inserted in pET24a (Novagen, Madison, WI) and over expressed in E. coli strain BL21(DE3). The over-expressed protein was purified by preparative SDS-PAGE and used for antibody production in rabbits. Protein gel-blot analyses were performed as described (Falk et al., 1998).
HPLC Quantitation of Major Thiols
Cys, γ-EC, GSH, and PCs were separated and quantified by HPLC following monobromobimane derivatization of the plant extracts as described (Xiang and Oliver, 1998).
Anthocyanin Quantification
Anthocyanin extraction and spectrophotometric quantification were performed as described (Noh and Spalding, 1998). The amount of anthocyanin is presented as the values of A535 − 2(A650) per gram fresh weight.
Footnotes
This work was supported by the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (grant no. 99–35100–7545) and is a publication of the Iowa Agricultural Experiment Station.
LITERATURE CITED
- Alfenito MR, Souer E, Goodman CD, Buell R, Moi J, Koes R, Walbot V. Functional complementation of anthocyanin sequestration in the vacuole by widely divergent glutathione S-transferases. Plant Cell. 1998;10:1135–1149. doi: 10.1105/tpc.10.7.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alscher RG. Biosynthesis and antioxidant function of glutathione in plants. Physiol Plant. 1989;77:457–464. [Google Scholar]
- Arisi ACM, Noctor G, Foyer CH, Jouanin L. Modulation of the thiol contents in poplars (Populus tremula × P. alba) over-expressing enzymes involved in glutathione synthesis. Planta. 1997;202:357–369. doi: 10.1007/s004250050202. [DOI] [PubMed] [Google Scholar]
- Asada K. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Noore DD, Seidman JG, Smith JA, Struhl K. Current Protocol in Molecular Biology. Brooklyn, NY: Greene Publishing Associates; 1987. [Google Scholar]
- Barroso C, Romero LC, Cejudo FJ, Vega JM, Gotor C. Salt-specific regulation of the cytosolic O-acetylserine(thiol) lyase gene from Arabidopsis thalianais dependent on abscisic acid. Plant Mol Biol. 1999;40:729–736. doi: 10.1023/a:1006285016296. [DOI] [PubMed] [Google Scholar]
- Becker D, Kemper E, Schell J, Masterson R. New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol Biol. 1992;20:1195–1197. doi: 10.1007/BF00028908. [DOI] [PubMed] [Google Scholar]
- Bergmann L, Rennenberg H. Glutathione metabolism in plants. In: De Kok LJ, editor. Sulfur Nutrition and Assimilation in Higher Plants. The Hague, The Netherlands: SPB Academic Publishing; 1993. pp. 109–123. [Google Scholar]
- Cheng JC, Seeley K, Sung ZR. RML1 and RML2, Arabidopsisgenes required for cell proliferation at the root tip. Plant Physiol. 1995;107:365–376. doi: 10.1104/pp.107.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Kim EJ, Neumann D, Schroeder JI. Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J. 1999;15:3325–3333. doi: 10.1093/emboj/18.12.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett CS, May MJ, Howden R, Rolls B. The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thalianais deficient in γ-glutamylcysteine synthetase. Plant J. 1998;16:73–78. doi: 10.1046/j.1365-313x.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- Creissen G, Firmin J, Fryer M, Kula B, Leyland N, Reynolds H, Pastori G, Wellburn R, Baker N, Wellburn A. Elevated glutathione biosynthetic capacity in the chloroplasts of transgenic tobacco plants paradoxically causes increased oxidative stress. Plant Cell. 1999;11:1277–1291. doi: 10.1105/tpc.11.7.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R, Dixon DP, Walbot V. Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci. 2000;5:193–198. doi: 10.1016/s1360-1385(00)01601-0. [DOI] [PubMed] [Google Scholar]
- Falk KL, Behal RH, Xiang C, Oliver DJ. Metabolic bypass of the tricarboxylic acid cycle during lipid mobilization in germinating oilseeds. Plant Physiol. 1998;117:473–481. doi: 10.1104/pp.117.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Descourvieres P, Kunert KJ. Protection against oxygen radicals: an important defense mechanism studied in transgenic plants. Plant Cell Environ. 1994;17:507–523. [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Grill E, Winnacker EL, Zenk MH. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc Natl Acad Sci USA. 1987;84:439–443. doi: 10.1073/pnas.84.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha SB, Smith AP, Howden R, Dietrich WM, Bugg S, O'Connell MJ, Goldsbrough PB, Cobbett CS. Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell. 1999;11:1153–1164. doi: 10.1105/tpc.11.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss S, Schafer HJ, Haag-Kerwer A, Rausch T. Cloning sulfur assimilation genes of Brassica junceaL.: cadmium differentially affects the expression of a putative low-affinity sulfate transporter and isoforms of ATP sulfurylase and APS reductase. Plant Mol Biol. 1999;39:847–857. doi: 10.1023/a:1006169717355. [DOI] [PubMed] [Google Scholar]
- Hell R, Bergmann L. γ-Glutamylcysteine synthetase in higher plants: catalytic properties and subcellular localization. Planta. 1990;180:603–612. doi: 10.1007/BF02411460. [DOI] [PubMed] [Google Scholar]
- Howden R, Andersen CR, Goldsbrough PB, Cobbett CS. A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiol. 1995a;107:1067–1073. doi: 10.1104/pp.107.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R, Goldsbrough PB, Andersen CR, Cobbett CS. Cadmium-sensitive, cad1 mutants of Arabidopsis thalianaare phytochelatin deficient. Plant Physiol. 1995b;107:1059–1066. doi: 10.1104/pp.107.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson RA. The antioxidants of higher plants. Phytochemistry. 1988;27:969–978. [Google Scholar]
- Lee S, Leustek T. APS kinase from Arabidopsis thaliana: genomic organization, expression, and kinetic analysis of the recombinant enzyme. Biochem Biophys Res Commun. 1998;247:171–175. doi: 10.1006/bbrc.1998.8751. [DOI] [PubMed] [Google Scholar]
- Leustek T, Martin MN, Bick J-A, Davies JP. Pathways and regulation of sulfur metabolism revealed through molecular and genetic studies. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:141–165. doi: 10.1146/annurev.arplant.51.1.141. [DOI] [PubMed] [Google Scholar]
- Leustek T, Saito K. Sulfate transport and assimilation in plants. Plant Physiol. 1999;120:637–644. doi: 10.1104/pp.120.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs KA. The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:127–158. doi: 10.1146/annurev.arplant.47.1.127. [DOI] [PubMed] [Google Scholar]
- May MJ, Leaver CJ. Arabidopsis thaliana γ-glutamylcysteine synthetase is structurally unrelated to mammalian, yeast, and Escherichia colihomologues. Proc Natl Acad Sci USA. 1995;91:10059–10063. doi: 10.1073/pnas.91.21.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, Vernoux T, Sanchez-Fernandez R, Van Montagu M, Inze D. Evidence for posttranscriptional activation of γ-glutamylcysteine synthetase during plant stress responses. Proc Natl Acad Sci USA. 1998;95:12049–12054. doi: 10.1073/pnas.95.20.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Arisi ACM, Jouanin L, Valadier MH, Roux Y, Foyer CH. The role of glycine in determining the rate of glutathione synthesis in poplar: possible implications for glutathione production during stress. Physiol Plant. 1997;100:255–263. [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Noctor G, Strohm M, Jouanin L, Kunert KJ, Foyer CH, Rennenberg H. Synthesis of glutathione in leaves of transgenic poplar overexpressing γ-glutamylcysteine synthetase. Plant Physiol. 1996;112:1071–1078. doi: 10.1104/pp.112.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh B, Spalding EP. Anion channels and the stimulation of anthocyanin accumulation by blue light in Arabidopsisseedlings. Plant Physiol. 1998;116:503–509. doi: 10.1104/pp.116.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauser WE. Phytochelatins. Annu Rev Biochem. 1990;59:61–86. doi: 10.1146/annurev.bi.59.070190.000425. [DOI] [PubMed] [Google Scholar]
- Schafer HJ, Haag-Kerwer A, Rausch T. cDNA cloning and expression analysis of genes encoding GSH synthesis in roots of the heavy-metal accumulator Brassica junceaL.: evidence for Cd-induction of a putative mitochondrial γ-glutamylcysteine synthetase isoform. Plant Mol Biol. 1998;37:87–97. doi: 10.1023/a:1005929022061. [DOI] [PubMed] [Google Scholar]
- Skipsey M, Andrews CJ, Townson J, Jepson I, Edwards R. Isolation of cDNA ( AJ243813) and genomic clones ( AJ243812) of glutathione synthetase containing plastidic targeting sequences from Arabidopsis thaliana. (PGR 99-137) Plant Physiol. 1999;121:312. [Google Scholar]
- Vatamaniu OK, Mari S, Lu YP, Rea PA. AtPCS1, a phytochelatin synthase from Arabidopsis: isolation and in vitro reconstitution. Proc Natl Acad Sci USA. 1999;96:7110–7115. doi: 10.1073/pnas.96.12.7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, Reichheld JP, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inze D. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione: dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell. 2000;12:97–110. doi: 10.1105/tpc.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Oliver DJ. Cloning of the cDNA and genomic clones for glutathione synthetase from Arabidopsis thaliana and complementation of a gsh2mutant in fission yeast. Plant Mol Biol. 1996;31:1093–1104. doi: 10.1007/BF00040827. [DOI] [PubMed] [Google Scholar]
- Xiang C, Han P, Lutziger I, Wang K, Oliver DJ. A mini binary vector series for plant transformation. Plant Mol Biol. 1999;40:711–717. doi: 10.1023/a:1006201910593. [DOI] [PubMed] [Google Scholar]
- Xiang C, Miao Z, Lam E. DNA binding properties, genomic organization and expression pattern of TGA6, a new member of the TGA family of bZIP transcription factors in Arabidopsis thaliana. Plant Mol Biol. 1997;34:403–415. doi: 10.1023/a:1005873500238. [DOI] [PubMed] [Google Scholar]
- Xiang C, Oliver DJ. Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell. 1998;10:1539–1550. doi: 10.1105/tpc.10.9.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YL, Pilon-Smit EAH, Jouanin L, Terry N. Overexpression of glutathione synthetase in Indian mustard enhances cadmium accumulation and tolerance. Plant Physiol. 1999a;119:73–79. doi: 10.1104/pp.119.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YL, Pilon-Smit EAH, Tarum AS, Weber SU, Jouanin L, Terry N. Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing γ-glutamylcysteine synthetase. Plant Physiol. 1999b;121:1169–1177. doi: 10.1104/pp.121.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]