Abstract
Natural killer (NK) cells have originally been identified by their spontaneous cytolytic potential against tumor cells, which, however, might result from pre-activation due to prior pathogen exposure. Resting NK cells, on the contrary, require activation by bystander antigen-presenting cells to reach their full functional competence. In this review, we will summarize studies on how dendritic cells (DCs), the most potent type of antigen-presenting cell, communicate with human NK cells to activate them in secondary lymphoid organs and to integrate signals from activated NK cells at sites of inflammation for their own maturation. Furthermore, we will review aspects of the immunological synapse, which mediates this cross-talk. These studies provide the mechanistic understanding of how mature DCs can activate NK cells and survive to go on for the activation of adaptive immunity. This feature of DCs, to activate different waves of immune responses, could be harnessed for immunotherapies, including vaccinations.
Keywords: Immunological synapse, DC editing, NK cell priming, Secondary lymphoid organs, Sites of inflammation
Dendritic and natural killer cell subsets
Classically, dendritic cells (DCs) have been proposed as professional sentinels that patrol the body, searching for stress signals [1]. Indeed, DCs are found in peripheral blood and in nearly every tissue, including the entire set of lymphoid organs (thymus, lymph nodes, bone marrow, tonsils and spleen), and non-lymphoid tissues (liver, kidney, skin and gut). Therefore, it is not surprising that these antigen-presenting cells (APCs) can get in contact with a vast variety of pathogens and their products, as well as stress signals released from dying cells. After sensing disturbances in tissue homeostasis, DCs migrate to secondary lymphoid tissues to report the nature of the insult via displaying components of their original environment on major histocompatibility complex (MHC) molecules, and reflecting the conditions under which they have taken up these components, via their particular maturation pattern. In secondary lymphoid tissues, mature DCs are known to be superior to other APCs in their capacity to stimulate and propagate effective immune responses, due to antigen presentation, the expression of co-stimulatory molecules and secretion of cytokines and chemokines [2–5]. Nevertheless, it is important to note that this classical view on DCs is continually getting updated with additional DC subsets, some of which are resident in secondary lymphoid tissues and others that develop from monocytes during inflammation [6–8].
DC biology and its role in the immune system were originally dissected in mice and, until now, there has been much more knowledge of the murine DC system than of the human system. Moreover, the majority of knowledge on human DC subsets and their development derives from in vitro studies with peripheral blood DC precursors and immature DCs. However, and in agreement with the rarity and large variety of DC subsets found in mice, several DC categories can also be identified in human. According to Shortman and Naik [7], DCs can be categorized into pre-DCs, conventional DCs and inflammatory DCs. Pre-DCs include plasmacytoid DCs and monocytes and are characterized by lack of dendrites and DC function in the steady-state [9]. Conventional DCs (which display dendrites and DC functions) can be further divided into migratory DCs and lymphoid tissue-resident DCs. Migratory DCs include several subsets of blood DCs like CD1c+ (BDCA1+) DCs, CD141+ DCs and CD16+ DCs [8, 10–13], in addition to Langerhans cells and dermal DCs [14, 15]. Lymphoid tissue-resident DCs are represented by, for instance, thymic and splenic DCs. The last category of DCs consists of inflammatory DCs that are not present in the steady-state, but can differentiate from precursors upon infection or stress signals. Inflammatory DCs include Tip DCs (named after their ability to produce TNF and iNOS) [16]. However, very little is so far known about the different capacities of these DC subsets to interact with natural killer (NK) cells, an aspect of DC biology that requires further investigation.
Maturation of DCs can be achieved by sensing danger signals (for example, pathogens or their products, necrotic cells and pro-inflammatory cytokines) through recognition receptors, which are present on DCs [17–21]. Toll-like receptors (TLRs) are among the best studied receptors involved in these processes. Indeed, the expression of a unique set of TLRs renders each type of DC susceptible to particular subsets of pathogens and stress signals, and the outcome of stimulation with TLR ligands can then result in increased antigen uptake and presentation [22], expression of co-stimulatory molecules [23], secretion of cytokines [24, 25], upregulation of chemokine receptors [26] and, therefore, stimulation of distinct T cell responses (Th1, Th2, Th17 or Treg) [1, 27, 28]. Thus, DC maturation mirrors the inflammation conditions in peripheral tissues and allows the initiation of proper immune responses in secondary lymphoid tissues.
Although there are some controversies with respect to TLR expression by different DC subsets, several studies point to the expression of TLR1, TLR2, TLR3, TLR5, TLR6 and TLR8 by conventional DCs, isolated from human blood. Therefore, these DCs can sense bacteria, virus and fungi, and they produce primarily IL-12, TNF and IL-6 in response. In contrast, plasmacytoid DCs express TLR7 and TLR9, which allows them to primarily sense viruses, and they produce type I IFNs upon activation. Finally, inflammatory DCs, which have a similar repertoire of TLRs as conventional DCs, can also respond to lipopolysaccharide (LPS), due to their expression of TLR4 [24, 25, 29–33]. Thus, different DC subsets fulfill distinct and complementary functions in the innate restriction of infections and initiation of immune responses.
NK cells are among the effector cells that are activated by mature DCs. NK cells got the attention of the scientific community due to their ability to spontaneously kill tumor cells [34, 35]. In addition to their rapid responsiveness, the lack of somatic antigen receptor rearrangement placed them in the innate arm of the immune system. Human NK cells develop from common lymphoid progenitors [36] and, as in the case of DCs, they can be subcategorized into several subtypes and can have different activation states. NK cells were first phenotypically characterized by the surface presence of CD56 and lack of CD3. Furthermore, Lanier and colleagues [37] could characterize two main, functionally distinct subsets of NK cells by the density of CD56 expression. Therefore, human peripheral blood NK cells were described as consisting of a predominant (≥95%) CD56dimCD16+ subset, further characterized by high levels of perforin expression and high cytolytic activity, and a minor (≤5%) CD56brightCD16− subset, with a superior capacity to produce pro-inflammatory cytokines like IFN-γ and TNF [38–40]. Furthermore, in the steady-state, NK cells can also be found in various lymphoid and non-lymphoid tissues that contain different NK cell subsets. The reactivity of activated NK cells is guided by sets of germline-encoded activating and inhibitory receptors [41, 42]. The main activating NK cell receptors are NKG2D and the natural cytotoxicity receptors (NCRs) NKp30, NKp44 and NKp46. NK cells harboring NKG2D recognize several molecules (e.g., MICA, MICB and ULBP) that can be upregulated in cells upon infection, stress or aberrant transformation (reviewed in [43]). In contrast, the molecules recognized by the activating NCRs are still being characterized. However, studies point to a role of NKp30-mediated recognition of BAT3 in cells with DNA or endoplasmatic reticulum damage [44]. Furthermore, NKp46 and NKp44 seem to interact with hemagglutinin in viral-infected cells [45, 46], and NKp46 also participates in surveillance of cell division [47]. The inhibitory receptors (e.g., CD94/NKG2A and killer cell immunoglobulin-like receptors, KIRs) engage non-classical and classical MHC class I molecules, respectively. NK cells integrate these concurrent signaling pathways and, if activation is stronger than inhibition, they may secrete cytokines, proliferate and kill the encountered cell. While the activating NK cell receptors are homogeneously expressed on all mature NK cell subsets, the inhibitory receptors are differentially expressed on NK cell subsets [48, 49]. Therefore, different NK cell populations express a certain set of inhibitory receptors that can recognize different MHC class I alleles on target cells. Furthermore, there is an overlapping of these inhibitory receptors among the different NK cell subsets. This variegated and overlapping expression of NK cell receptors in different NK cell populations might derive from a stochastic regulation of gene expression during NK cell development and might allow the surveillance of viral-infected or tumor cells that have downregulated only a limited set of MHC class I molecules [50].
In conclusion, the complexities of human DC and NK cell populations mean that different NK cell subsets can interact with different DC populations and, depending on the place where the interaction occurs and on the maturation/activation status of the interacting cells, the outcome of this interaction may functionally differ.
Places of NK cell activation by DCs
Although DCs and NK cells can theoretically interact in numerous places, due to their wide distribution in the body, two main locations have been the focus of most studies due to their crucial physiologic relevance during immune responses, namely secondary lymphoid organs (SLOs) and sites of inflammation. SLOs have a complex organization that allows efficient cell–cell interactions. Indeed, different hematopoietic and non-hematopoietic cells, as well as resident and migrating/homing cells, make up these structures. Resting or non-inflamed human lymph nodes harbor a significant number of lymphocytes, including NK cells [51, 52]. With a frequency of half the 10% found in peripheral blood, but 20-fold more lymphocytes being parked in SLOs than circulating through the peripheral blood at any given time point, NK cells may be 10 times more abundant in lymph nodes than in the periphery [53]. Also, in contrast to peripheral blood NK cells, lymph node NK cells mostly (around 90%) belong to the CD56bright subset [2, 52]. Indeed, circulating human CD56bright NK cells have been shown to abundantly express l-selectin, CCR7 and LFA-1, making them able to traffic and efficiently enter secondary lymphoid tissues in the steady-state [38, 51, 54–56]. Furthermore, NK cells have been found to be preferentially localized in the perifollicular T cell zones of lymph nodes [2, 57–60]. Curiously, in mice, homing of CD27bright NK cells to lymph nodes seems to be dependent on CXCR3 and not CCR7 expression [61–63]. In contrast, mouse NK cells seem to migrate into splenic white pulp due to CCR7 and CCL21 signaling [64–66]. Furthermore, some studies in mice seem to indicate that inflammation leads to increased NK cell migration into the splenic white pulp [67, 68]. Here, NK cells home to T cell zones, possibly because of their ability to migrate along fibroblastic reticular cells. Thus, NK cells traffic through secondary lymphoid tissues, in the steady-state, and may be recruited at enhanced frequencies to these tissues during inflammation.
Although in the steady-state immature DCs can slowly traffic to SLOs [69], maturation leads to the upregulation of chemokine receptors (including CCR7) on the surface of peripheral conventional DCs, rendering them more efficient in entering peripheral lymph vessels and in migrating to SLOs [3, 26, 70–72]. Furthermore, fibroblastic reticular cells in proximity to high endothelial venules (HEVs), found in the T cell area of lymph nodes and spleen, are major producers of CCL21 and CCL19, which concentrate recently homed CCR7+ mDCs around HEVs [73, 74]. In this way, DCs are able to efficiently find not only T cells, to which they can present antigen, but also resting CD56bright NK cells, enriched at these sites, in addition to NK cells that travel to the spleen upon infection [51, 56, 73]. Therefore, it has become clear that important locations of DC/NK cell interactions are SLOs and that these tissues support interactions between mDCs and naïve or resting NK cells. The mechanisms underlying these interactions and their outcomes will be reviewed in the following sections.
In addition, sites of inflammation are an important source of production of several cytokines and chemokines like CCL4, CCL5, CX3CL1 CXCL8 and CXCL10, some of them produced by subsets of dendritic cells [75]. Circulating NK cells can sense them and migrate to sites of inflammation. In contrast to lymph nodes, both subsets of NK cells seem to migrate to sites of inflammation, but there is some debate about the capacity of their retention. The type of infectious agent that triggers this inflammation might contribute to the selective enrichment of distinct NK cell subpopulations. Along these lines, the CD56bright NK cell subset was found to be enriched in sites of inflammation promoted by autoimmunity, lung cancer lesions and renal cell carcinoma [76–79]. Activated NK cells preferentially home to these sites for rapid and efficient killing of damaged cells. In inflamed tissues, activated NK cells can encounter immature DCs or DCs that are in the process of maturation and can edit their activation before they migrate to secondary lymphoid tissues. Thus, editing of DC maturation by activated NK cells probably occurs primarily at sites of inflammation.
Mechanisms of NK cell activation by DCs
NK cells are highly cytotoxic cells, ready to kill target cells, without the need of recognition of a specific antigen, but rather recognizing self-molecules, which are upregulated in response to many forms of cellular stress. These characteristics make them potent effectors against transformed and infected cells [34, 80, 81]. As exceptions from these rules, NK cells are able to initiate immune responses by direct recognition of cells infected with MCMV that express on their surface the MCMV-encoded protein m157, which is recognized by the Ly49H receptor on NK cells [82, 83], and with influenza virus, due to the ability of the NKp46 and NKp44 receptors of NK cells to recognize viral haemagglutinins on the surface of infected cells [45, 46]. However, in recent years, it has become apparent that this NK cell recognition requires pre-activation by myeloid APCs [84–89]. This pre-activation is also called NK cell priming and happens through cell contact with accessory cells and their presentation and/or secretion of cytokines. The most important accessory cells involved in NK cell priming are monocytes, macrophages and DCs. Depending on the particular conditions of immune activation, distinct subsets of these APCs will be activated and participate in NK cell stimulation. Here, we will focus on the significance of DCs as accessory cells in NK cell priming (Fig. 1).
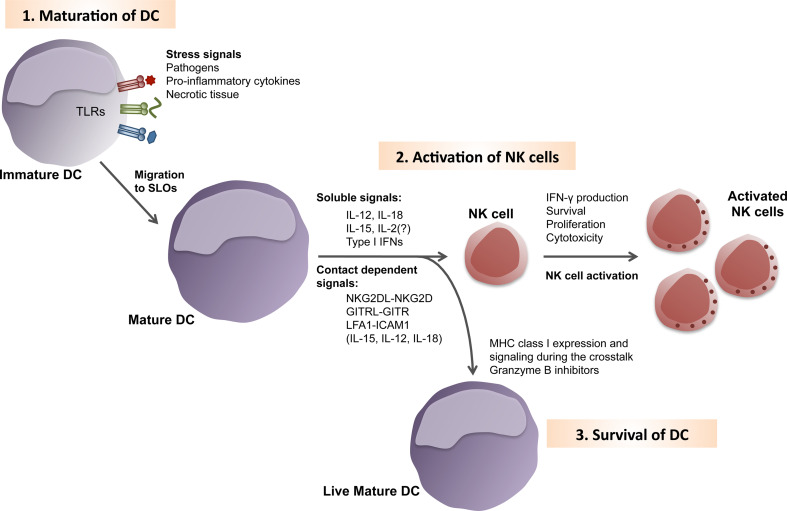
Fig. 1.
Natural killer cell activation by dendritic cells. Dendritic cells (DCs) patrol the body constantly in their immature stage. 1 Upon recognition of a stress signal, that can be pathogen-derived, necrotic cellular debris or pro-inflammatory cytokines, DCs undergo maturation. Some stress signals are detected by toll-like receptors (TLRs). During maturation, DCs up-regulate chemokine receptors, which allow them to efficiently migrate into secondary lymphoid organs (SLOs). 2 There, mature DCs can encounter autologous resting NK cells. The cross-talk between these cells differs depending on the nature of the maturation stimulus received by the DC and the particular subset of DC. Thus, NK cell activation by mature DCs can be mediated by soluble molecules to different degree. Cytokines like IL-12, IL-18 and IL-2 induce IFN-γ secretion by NK cells. Furthermore, IL-15 signaling induces NK cell proliferation and survival, while type I IFNs increases NK cell cytotoxicity. Although these cytokines can be secreted, their effect, especially when produced at limited concentrations by mature DCs, can be improved through conjugation of DCs and NK cells. Furthermore, the direct cell contact between these two cell types is also important for signals derived from receptor/ligand pairs. Along these lines, NK group 2D (NKG2D), glucocorticoid-induced TNF-receptor-related protein (GITR) and intracellular adhesion protein 1 (ICAM1) were shown to activate NK cells, after interaction with their cognate ligands (NKGD2L, GITRL and lymphocyte function-associated lymphocyte 1 (LFA1), respectively) on mature DCs. 3 Even so this cross-talk enhanced NK cell cytotoxicity, DCs survive this interaction due to the high expression of MHC class I molecules after maturation, the presence of inhibitors of granzyme B-mediated apoptosis in mature DCs, and actin-mediated stabilization of NK cell inhibitory signaling by MHC class I molecules at the immunological synapse
As described in the first section of this review, several DC subsets exist and they possess various means to activate NK cells. Type I IFNs (IFNα and IFNβ) primarily trigger NK cell cytotoxicity [90–92] and control IFN-γ production by these lymphocytes by regulating IL-12 production on classical DCs [93–95]. Plasmacytoid DCs seem to be a very important source of type I IFNs upon virus infections, as, for example, by MCMV and VSV [95, 96]. CpG motives of the viral genomes are recognized by TLR9, expressed in endosomes of plasmacytoid DCs, and lead to secretion of type I IFNs. Nevertheless, several in vivo studies have demonstrated that there are other sources for type I IFN production, independent of plasmacytoid DCs, and, therefore, these cells seem to represent an important, but not unique, source of this cytokine [95, 97–99]. On the other hand, recognition of MCMV by conventional DCs leads to selective secretion of IL-12 [100–102]. In this way, both subsets of DCs are activated, leading to complementary cytokine secretion and increased cytotoxicity of NK cells upon MCMV infection. Furthermore, conventional DCs-derived secretion of type I IFNs was shown to play a role during HSV1 infection [103]. In addition to type I IFN-stimulated NK cell cytotoxicity, IL-12 and IL-18 are the major promoters of IFN-γ secretion by NK cells, and both cytokines can be produced by DCs. Plasmacytoid and conventional DCs are important sources of IL-12 during MCMV infection [101, 102, 104]. Furthermore, conventional DCs activate NK cell responses to EBV via IL-12 [105]. This cytokine was shown to be particularly important for activation of CD56bright NK cells of SLOs upon encountering mature conventional DCs [2]. Furthermore, during influenza virus infection, DCs produce IL-12 and induce IFN-γ production by NK cells [90, 106]. Some responses against parasites and bacteria are also dependent on IL-12/IL-18 production by DCs, [107, 108], and probably in rare exceptions, as, for example, in response to infections with the Gram-negative bacterium Escherichia coli, Granucci and colleagues [109] found that DC-derived IL-2 secretion has a more prominent role in activating NK cells to produce IFN-γ. In addition to these cytokines that promote cytokine secretion by NK cells, IL-15 plays a crucial role in several aspects of NK cell biology. Firstly, it is important for the development of these innate lymphocytes [110–112]. Furthermore, a role of IL-15 in NK cell survival and proliferation has been documented [2, 113, 114]. Moreover, it plays an essential role during priming of protective immune responses [89, 115, 116]. Finally, a recent study showed that prolonged stimulation of NK cells with IL-15/IL-15Rα complexes leads to an impairment of NK cell activation [117], indicating a possible role in the negative-feedback mechanism that regulates NK cell activation. Of note, overstimulation of NK cells with activating-receptor ligands (e.g., Rae-1 and m157 that engage the NK cell-activating receptors NKG2D and Ly49H, respectively) or immune modulators (e.g., type I IFNs or Corynebacterium parvum) have been shown to lead to NK cell hyporesponsiveness [118–120]. DCs, especially Langerhans cells, express high levels of IL-15 and IL-15Rα, and can trans-present it to NK cells expressing IL-15Rβ and γ chains. Therefore, DCs act as accessory cells promoting NK cell survival and proliferation [113, 121]. Trans-presentation of IL-15 requires close contact between DCs and NK cells. However, IL-15 is not the only cytokine that might require conjugation of DCs and NK cells for efficient signaling. Directed secretion of DC-derived IL-12 and IL-18 towards the NK cell can be required for efficient NK cell activation, when the particular DC maturation conditions allow only limited production of these cytokines [122, 123]. Indeed, the contact-dependent transmission of signals is equally important as signaling via soluble molecules during NK cell priming by DCs [85, 86, 124]. For example, Plasmodium falciparum infection in humans confers the ability to conventional DCs to stimulate NK cells to secrete IFN-γ in a contact-dependent manner [125]. Furthermore, viruses harboring CpG motives upregulate expression of glucocorticoid-induced TNF-receptor-related protein (GITR) ligand by human plasmacytoid DCs, and the interaction of this molecule with GITR on NK cells leads to the production of IFN-γ and enhanced cytotoxicity [126]. Activation of DCs with pro-inflammatory cytokines, especially type I IFNs, promotes upregulation of NKG2D ligands leading to IFN-γ secretion and enhanced NK cell cytotoxicity [127]. NK cell activation by DCs stimulated with LPS involves TREM2 (triggering receptor expressed by myeloid cells 2), an activating immunoreceptor that signals via KARAP (killer cell activating receptor-associated protein)/DAP12 (DNAX-associated protein 12) [128]. Furthermore, adhesion molecules have been shown to be important in the DC/NK cross-talk [113, 129]. Thus, although DCs activate NK cells primarily via cytokines, cell contact is important for directed secretion during this interaction.
These studies suggest that mature DCs migrate to SLOs, where they activate NK cells in the T cell zones via directed secretion during conjugation, and these activated NK cells can then fight infections in the periphery.
The immunological synapse between DCs and NK cells
While incubation of NK cells with recombinant IL-2, IL-12 or IL-15 is sufficient to initiate cytokine production by NK cells and increase their cytotoxic activity [105, 121], NK cell activation by DCs is most efficiently performed through cell contact [113, 122]. This cell contact is mediated through immunological synapses (IS), and, thus, the study of these structures became imperative for the correct understanding of NK cell activation by DCs. Studies of IS with NK cells first focused on the interaction of NK cells and target cells that were susceptible to NK cell cytotoxicity. As the outcome of these interactions is the killing of the target cell, this IS was called cytotoxic synapse [130, 131]. The human cytotoxic NK cell IS has been extensively studied by direct visualization of cell conjugates with human primary NK cells or NK cell-like tumor cell lines. Altogether, these studies allowed the detailed description of the kinetics of formation and maturation of the cytotoxic NK cell IS. NK cell-mediated killing of allogeneic, virus-infected or tumor cells includes an initiation stage that leads to adhesion of the NK cell to the target cell (formation of the IS) and initiation of activating NK cell signaling. The following effector stage comprises reorganization of the NK cell cytoskeleton that culminates in filamentous actin polymerization at the interface between the cells, and polarization of the microtubule organizing center (MTOC) of NK cells and of cytotoxic granules towards the target cell. After lytic granule release, the termination stage begins, with the detachment of the NK cell from the apoptotic target cell. The detailed kinetics of molecule migration and signaling at the cytotoxic NK cell IS have been recently reviewed [132, 133].
During cytotoxic NK cell responses normal somatic tissue is spared to avoid immunopathology, and NK cell cytotoxicity is, therefore, tightly regulated. The expression of autologous MHC class I molecules on the surface of healthy cells, together with the absence of expression of activating ligands, inhibits initiation of cytotoxic NK cell functions [134, 135]. These inhibitory signaling pathways are also mediated through conjugates between NK cells and non-susceptible target cells. The interface of these conjugates is called the non-cytotoxic IS. The non-cytotoxic NK cell IS is characterized by the enrichment of inhibitory signaling molecules at the interface of the two cells, that inhibit NK cell–cytoskeleton rearrangements and promote NK cell detachment from the conjugated cell. In contrast to these classical classifications of NK cell IS, mature DCs activate NK cells and prevent themselves from being killed at the same time during their conjugate formation with NK cells [87]; we named this interaction a regulatory synapse [113]. While classical cytotoxic and non-cytotoxic NK cell interactions have been studied in depth, the interactions of NK cells involving regulatory synapses are just being characterized. Regulatory NK cell synapses include interactions between resting NK cells and fully matured DCs (Fig. 2). In this cross-talk, mature DCs activate NK cells to secret cytokines, to increase their cytotoxicity, to survive and to proliferate [105, 113, 121], while they escape at the same time from NK cell cytotoxicity to propagate adaptive immune responses. DC survival from NK cell cytotoxicity relies on several factors. Firstly, increased MHC class I expression after DC maturation engages inhibitory NK cell receptors, repressing NK cell cytotoxic activation. Secondly, mature DCs express protease inhibitors of the serpin family, like protease inhibitor 9 (PI9), which inhibit granzyme B-mediated apoptosis [87, 136, 137]. Furthermore, actin-dependent stabilization of MHC class I molecules at the IS between NK cells and mDCs might allow the continuous engagement of inhibitory receptors to prevent NK cell cytotoxicity (Barreira da Silva and Münz, submitted). These multiple mechanisms protect DCs from cytotoxicity, while NK cells are activated at the same time via the IS with mature DCs.
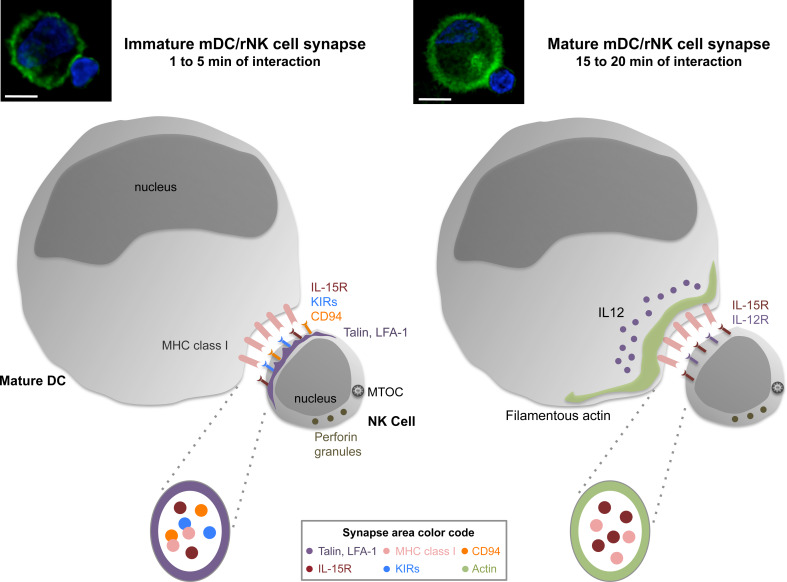
Fig. 2.
The immunological synapse between mature dendritic cells and resting natural killer cells. Upon encounter, dendritic cells (DCs) and natural killer (NK) cells rapidly form conjugates, forming an immunological synapse (IS). The IS between mature DCs and resting NK cells has both activating and inhibitory features and can, therefore, be described as a regulatory synapse. The immature IS between DCs and NK cells (formed after 1–5 min of interaction) presents enrichment of both activating [IL-15R, talin, lymphocyte function associated molecule 1 (LFA-1)] and inhibitory [killer cell immunoglobulin like receptors (KIRs), CD94 and MHC class I] molecules at the IS (left side). The parallel signaling of these opposing interactions is possible due to the spatial separation of activating and inhibitory domains in the IS, which cluster IL-15R, MHC class I, KIRs and CD94 molecules in the central part of the IS and are surrounded by talin and LFA-1 molecules. Upon maturation of the synapse (after 15–20 min of interaction), DCs-derived filamentous actin polymerized at the interface between the cells (right side). Furthermore, IL-12 and IL-12R accumulate at the IS, while IL-15R and MHC class I polarization to the synapse is maintained. The maintenance of inhibitory signaling via MHC class I prevents polarization of the cytolytic machinery, like perforin-containing granules and the microtubule organizing center (MTOC), to the synapse. The synchronized signaling of activating and inhibitory molecules allows NK cell activation and inhibition of DC lysis by NK cells at the same time. Microscopy pictures represent conjugates between poly(I:C) matured DCs and autologous resting NK cells after 1 (left) and 20 min (right) of co-culture. Filamentous actin (green) and nuclear DNA (blue) were stained. Original magnifications ×100, scale bars 10 μm
In addition to these inhibitory interactions at the IS between DCs and NK cells, polarization of NK cell-activating cytokines to the conjugate interface can be observed. Semino and colleagues [123] investigated the interaction between immature DCs and resting NK cells. They demonstrated the importance of IL-18 accumulating at the IS for NK cell activation. In addition, the high mobility group B1 (HMGB1) protein seemed to mediate DC maturation and survival during this interaction [123, 138]. In addition, LPS-matured DCs were found to elicit IFN-γ production by resting NK cells in a cell contact-dependent fashion [122]. During this interaction, IL-12 of DCs polarized to the interface with NK cells. While these studies analyzed just a single time point after IS formation and did not optimize DC maturation for NK cell activation, we investigated the synapse maturation between NK cells and polyinosinic polycytidylic acid [poly(I:C)] matured DCs, which allows for maximal NK cell stimulation [105]. These experiments documented that the synapse between human mature DCs and autologous resting NK cells forms rapidly (within 1–5 min of coculture) and matures further after 20 min of interaction [113, 139] (Barreira da Silva and Münz, submitted). Maturation is associated with the polymerization of DC-derived filamentous actin at the IS (Barreira da Silva and Münz, submitted). Cytoskeletal stabilization of the IS was previously observed in stimulatory DC/T cell synapses [140, 141]. DC/NK cell conjugate formation also seems to depend on CX3CL1 signaling [139], as fewer conjugates are formed in the presence of blocking antibodies against CX3CL1. Upon conjugate formation, inhibitory and activating molecules are recruited in a temporally and spatially well-orchestrated manner. After 1–5 min, inhibitory KIRs and CD94 molecules on the NK cell and MHC class I molecules on the DC side, as their ligands, polarize to the DC interface with NK cells [113]. With similar kinetics, the IL-15 receptor components accumulate at the synapse. Since the interaction between KIRs and CD94 with MHC class I molecules leads to inhibitory signaling and IL-15 transpresentation is important for the activation and survival of NK cells, the synchronized polarization kinetics of all these molecules could pose problems for the parallel signaling of these inhibitory and activating pathways. The simultaneous signaling of these molecules seems to be possible because they may segregate into different domains at the center of the synapse [113]. In contrast, structural and adhesion molecules, like talin, filamentous actin and LFA-1, are located in the peripheral part of the IS [113]. In addition to these early events, maturation of the interactions between DCs and NK cells allows, after 20 min, for IL-12 polarization to the interface, which is essential for the stimulation of IFN-γ secretion by NK cells [122, 139]. Furthermore, maturation of the IS between mature DCs and resting NK cells also seems to be important for the maintenance of inhibitory signaling through KIR engagement by MHC class I molecules, and for prevention of cytotoxic granule recruitment to the interface (Barreira da Silva and Münz, submitted). Therefore, mDCs seem to stabilize their synapse with resting NK cells by actin polymerization in order to allow additional stimulatory molecules, like IL-12, to be polarized towards NK cells, but use this cytoskeletal stabilization at the same time to maintain inhibitory MHC class I molecules at the synapse to prevent being killed by NK cells.
All published studies on human DC/NK cell synapses have used monocyte-derived DCs, but the hallmarks of this interaction seem to also hold up for HLA-DR+CD11c+BDCA1+ peripheral blood DCs (Barreira da Silva and Münz, submitted). Of further note, both subsets of peripheral blood NK cells can form conjugates with mature DCs, but the CD56bright NK cell subpopulation seems to form synapses with mature DCs more readily [113, 142]. This is consistent with SLOs, as sites of CD56bright NK cell enrichment, being the primary site of interactions between mature DCs and resting NK cells.
For obvious reasons, the summarized studies on human DC interactions with NK cells have been confined to in vitro experiments. However, it remains unclear if the long lasting interactions of DCs with NK cells in vitro also occur in vivo. Initially, using an adoptive transfer strategy, Bajénoff and colleagues [58] demonstrated that NK cells can conjugate in vivo for longer time intervals with DCs (at least 25 min), both in the steady-state and upon infection with Leishmania major. In contrast, a more recent study has challenged this notion and suggested multiple short contacts of below 5 min duration for the interactions of NK cells with DCs in lymph nodes, both in the steady-state and after poly (I:C) or LPS activation [59]. Therefore, it would be interesting to discover which pattern of NK cell motility human NK cells follow in vivo, and novel mouse models with human immune compartment reconstitution might allow the addressing of this question [116, 143].
DC editing, activation and polarization by NK cells
In addition to NK cell activation by mature DCs, which might preferentially take place in secondary lymphoid tissues, activated NK cells might also influence DC maturation at sites of inflammation. This signaling from NK cells to DCs has been termed DC editing [144], and consists of killing of immature or allogeneic DCs, induction of DC maturation and modification of DC maturation for enhanced induction of Th1-polarized adaptive immune responses (Fig. 3). NK cells, as cytotoxic innate lymphocytes, can directly attack susceptible target cells. Cytotoxicity of activated NK cells is triggered if a target cell does not provide enough inhibitory signals (absence of MHC class I molecules or “missing self”) or too many activating signals (“altered self”). Due to an abundance of activating ligands, NK cells can lyse autologous myeloid cells, like microglia cells, macrophages and DCs, despite their MHC class I expression. Non-activated microglia cells were found to be susceptible to NK cell killing via recognition by the activating NKp46 and NKG2D receptors [145]. Furthermore, macrophages can also be targets of editing by NK cells. In this case, TLR-activated or infected macrophages seem to be more susceptible than non-activated macrophages and upregulate NKG2D ligands [146–148]. Moreover, the first report documenting killing of DCs by NK cells appeared in 1985 by Shah and colleagues [149]. Today, it is appreciated that immature DCs can be targets for NK cell cytotoxicity, and are more susceptible than mature DCs [150–152]. However, the ratio between DCs and NK cells seem to play an important role in whether immature DCs are targeted by NK cells. Large numbers of activated NK cells competently kill immature DCs [86, 153]. Furthermore, killing of immature DCs by NK cells is mediated by NKp30, NKp46 and the activating co-receptor DNAM-1 [87, 154, 155] in the context of insufficient inhibitory signaling due to low HLA-E expression by these APCs [156]. In contrast, upregulation of MHC class I, including non-classical HLA-E molecules, during DC maturation protects DCs against NK cell lysis [87]. After activating NK cell receptors are engaged, immature DCs are killed by NK cells through apoptosis induction, via the TNF-related apoptosis-inducing ligand (TRAIL)-death receptor 4 (DR4) pathway [153, 157]. In addition, after homing to lymph nodes, NK cells can also kill DCs by perforin-dependent mechanisms [158]. The advantages of eliminating immature DCs through NK cell-mediated cytotoxicity might be that such tolerogenic APCs can be selectively eliminated to allow only mature DCs to emigrate from sites of inflammation for efficient induction of immune responses. Moreover, DC killing by NK cells can also be harnessed clinically to remove allogeneic DCs that could elicit graft-versus-host disease during bone marrow transplantation, or allograft rejection [159, 160].
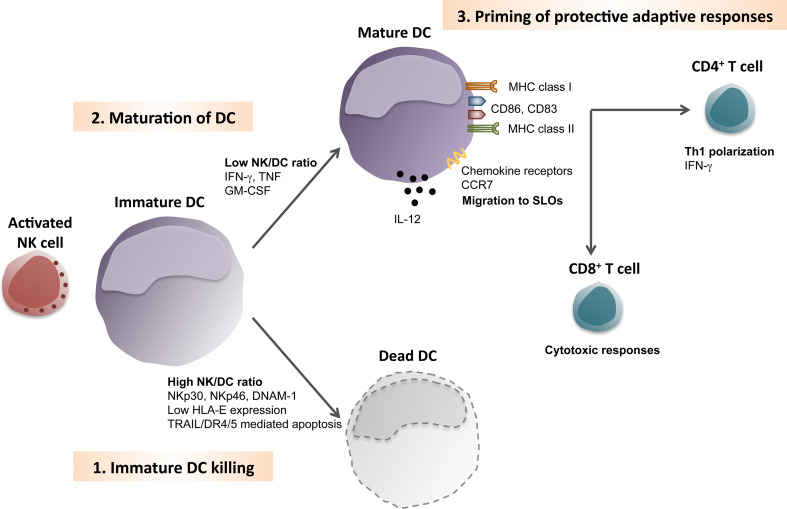
Fig. 3.
Dendritic cell editing: killing, maturation, and Th1 polarization by natural killer cells. Sites of inflammation allow activated natural killer (NK) cells to encounter immature dendritic cells (DCs). 1 At high NK cell/DC ratios, NK cells can kill immature DCs, due to the engagement of the natural cytotoxicity receptors p30 and p46 and DNAX accessory molecule 1 (NKp30, NKp46 and DNAM-1). The low levels of HLA-E expression on immature DCs are unable to inhibit this NK cell activation. It is thought that cytotoxic editing of DCs by NK cell is mediated by TRAIL/DR4/5-induced apoptosis. 2 At low NK/DC ratios, NK cells can promote maturation of DCs, via type II interferon, tumor necrosis factor and granulocyte macrophage colony-stimulating factor (IFN-γ, TNF and GM-CSF, respectively). These cytokines induce phenotypic maturation of DCs, which includes up-regulation of co-stimulatory and MHC molecules (CD83, CD86, MHC class I and MHC class II). Furthermore, in some cases, cytokine production by DCs is also induced (like IL-12 secretion). Maturation of DCs enables then to migrate into secondary lymphoid organs (SLOs), due to the upregulation of chemokine receptors, like C–C chemokine receptor 7 (CCR7). 3 Inside SLOs, DCs previously activated by NK cells were found to efficiently prime protective adaptive responses, including Th1 polarization of naïve CD4+ T cells and cytotoxic responses by CD8+ T cells
Contrary to DC killing by NK cells at high NK cell to immature DC ratios, low ratios seem to promote DC maturation and survival [86, 153]. Activated NK cells upregulate the surface expression of co-stimulatory molecules and cytokines by DCs. Both, contact-dependent mechanisms and soluble factors promote maturation of DCs by NK cells. After activation, NK cells can secrete IFN-γ and TNF and these cytokines induce differentiation of monocytes into inflammatory DCs and upregulation of co-stimulatory molecules on immature DCs [85, 86, 161, 162]. Among these, NK cells induce upregulation of CD86, CD83 and HLA-DR expression of DCs, while DC-SIGN expression was found to be downregulated [153]. Moreover, NK cells also boost plasmacytoid DC functions, like their capacity to sense virus products at suboptimal concentrations [90]. Furthermore, DC maturation by NK cells allows also for the upregulation of chemokine receptors for the migration to SLOs, the site of T cell priming. Thus, activated NK cells can mature DCs for the initiation of adaptive immune responses. However, physiologic conditions under which NK cell activation precedes DC maturation have so far been rarely reported. The notable exception being NK cell activation by tumors cells, which was found to mature DCs for the priming of protective CD8+ T cell responses [163].
In addition to DC maturation, NK cells can also assist in the polarization of Th1 responses. Especially, the CD56bright NK cell subset in SLOs is superior in IFN-γ secretion and can thereby assist Th1 priming by DCs [58, 61, 164]. The NK cell-supported initiation of Th1 immune responses allows the efficient elimination of tumor cells and several intracellular pathogens [165–167]. Indeed, Ing and Stevenson [166] showed very elegantly that Plasmodium falciparum induces activation of NK cells via mature DCs, and activated NK cells in turn augment IL-12 production by DCs to induce Th1 polarized CD4+ T cell responses. Thus, NK cells edit DC-mediated responses by killing tolerogenic immature DCs, by maturing DCs primarily via TNF, and by assisting the priming of Th1-polarized immune responses by secretion of IFN-γ.
Conclusions
Dendritic cells have now been recognized as the most efficient antigen-presenting cells for the initiation of innate and adaptive immune responses. By delicately balancing inhibitory and activating signaling, they stimulate human NK cells, preferentially those that are enriched in T cell areas of secondary lymphoid organs, and prevent themselves being killed by them at the same time. DCs preferentially migrate to these sites after maturation is set in motion through pathogen-associated molecular pattern recognition in the periphery. Activated NK cells can then, in turn, home to these peripheral sites to limit pathogen replication through cytokines and killing of infected cells, but also edit DC populations so that only mature DCs deliver signals from these sites to secondary lymphoid organs. Tolerogenic immature DCs are either matured by NK cells or destroyed at the site of infection. While the ability of NK cells to kill immature DCs is already used clinically to prevent graft-versus-host disease after HLA-mismatched bone marrow transplantation, NK cell activity is not yet consciously harnessed during vaccination by suitable adjuvant choice, which would mature DCs optimally to recruit these innate effector cells during immunization. Especially, tumor immunotherapy could benefit significantly from DC-mediated activation of NK cells.
Acknowledgments
R.B.d.S. is a GABBA PhD student and received support from the Portuguese Foundation for Science and Technology, Portugal (BD 32986/2006). Work in our laboratory is in part supported by the National Cancer Institute (R01CA108609), Cancer Research Switzerland (KFS-02652-08-2010), the Sassella Foundation (10/02) and the Swiss National Science Foundation (310030_126995) to C.M.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Ferlazzo G, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci USA. 2004;101:16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T–T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 7.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 8.Ju X, Clark G, Hart DN. Review of human DC subtypes. Methods Mol Biol. 2010;595:3–20. doi: 10.1007/978-1-60761-421-0_1. [DOI] [PubMed] [Google Scholar]
- 9.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 10.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 11.O’Doherty U, Peng M, Gezelter S, Swiggard WJ, Betjes M, Bhardwaj N, Steinman RM. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- 12.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 13.Schakel K, et al. 6-Sulfo LacNAc, a novel carbohydrate modification of PSGL-1, defines an inflammatory type of human dendritic cells. Immunity. 2002;17:289–301. doi: 10.1016/s1074-7613(02)00393-x. [DOI] [PubMed] [Google Scholar]
- 14.Romani N, Ebner S, Tripp CH, Flacher V, Koch F, Stoitzner P. Epidermal Langerhans cells–changing views on their function in vivo. Immunol Lett. 2006;106:119–125. doi: 10.1016/j.imlet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Lenz A, Heine M, Schuler G, Romani N. Human and murine dermis contain dendritic cells. Isolation by means of a novel method and phenotypical and functional characterization. J Clin Invest. 1993;92:2587–2596. doi: 10.1172/JCI116873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 17.Hartmann G, Weiner GJ, Krieg AM. CpG DNA: a potent signal for growth, activation, and maturation of human dendritic cells. Proc Natl Acad Sci USA. 1999;96:9305–9310. doi: 10.1073/pnas.96.16.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sparwasser T, Koch ES, Vabulas RM, Heeg K, Lipford GB, Ellwart JW, Wagner H. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur J Immunol. 1998;28:2045–2054. doi: 10.1002/(SICI)1521-4141(199806)28:06<2045::AID-IMMU2045>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Verdijk RM, Mutis T, Esendam B, Kamp J, Melief CJ, Brand A, Goulmy E. Polyriboinosinic polyribocytidylic acid (poly(I:C)) induces stable maturation of functionally active human dendritic cells. J Immunol. 1999;163:57–61. [PubMed] [Google Scholar]
- 20.Singh-Jasuja H, Scherer HU, Hilf N, Arnold-Schild D, Rammensee HG, Toes RE, Schild H. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur J Immunol. 2000;30:2211–2215. doi: 10.1002/1521-4141(2000)30:8<2211::AID-IMMU2211>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 23.Akira S. Mammalian toll-like receptors. Curr Opin Immunol. 2003;15:5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 24.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 28.Palliser D, Ploegh H, Boes M. Myeloid differentiation factor 88 is required for cross-priming in vivo. J Immunol. 2004;172:3415–3421. doi: 10.4049/jimmunol.172.6.3415. [DOI] [PubMed] [Google Scholar]
- 29.Ito T, et al. Interferon-alpha and interleukin-12 are induced differentially by toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002;195:1507–1512. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muzio M, et al. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 31.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–255. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 32.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 33.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 34.Herberman RB, Holden HT. Natural killer cells as antitumor effector cells. J Natl Cancer Inst. 1979;62:441–445. doi: 10.1093/jnci/62.3.441. [DOI] [PubMed] [Google Scholar]
- 35.Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975;5:117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- 36.Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol. 2006;24:257–286. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- 37.Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986;136:4480–4486. [PubMed] [Google Scholar]
- 38.Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, Wu L, Butcher EC. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166:6477–6482. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 39.Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, Behrens G, Sykora KW, Schmidt RE. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol. 2001;31:3121–3127. doi: 10.1002/1521-4141(2001010)31:10<3121::aid-immu3121>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 40.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 41.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 42.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 43.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235:267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pogge von Strandmann E, et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity. 2007;27:965–974. doi: 10.1016/j.immuni.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Mandelboim O, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 46.Arnon TI, Lev M, Katz G, Chernobrov Y, Porgador A, Mandelboim O. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur J Immunol. 2001;31:2680–2689. doi: 10.1002/1521-4141(200109)31:9<2680::aid-immu2680>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 47.Nolte-‘t Hoen EN, Almeida CR, Cohen NR, Nedvetzki S, Yarwood H, Davis DM. Increased surveillance of cells in mitosis by human NK cells suggests a novel strategy for limiting tumor growth and viral replication. Blood. 2007;109:670–673. doi: 10.1182/blood-2006-07-036509. [DOI] [PubMed] [Google Scholar]
- 48.Moretta A, et al. Identification of four subsets of human CD3-CD16+ natural killer (NK) cells by the expression of clonally distributed functional surface molecules: correlation between subset assignment of NK clones and ability to mediate specific alloantigen recognition. J Exp Med. 1990;172:1589–1598. doi: 10.1084/jem.172.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valiante NM, et al. Functionally and structurally distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity. 1997;7:739–751. doi: 10.1016/s1074-7613(00)80393-3. [DOI] [PubMed] [Google Scholar]
- 50.Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 51.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 52.Ferlazzo G, Thomas D, Lin SL, Goodman K, Morandi B, Muller WA, Moretta A, Munz C. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172:1455–1462. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 53.Westermann J, Pabst R. Distribution of lymphocyte subsets and natural killer cells in the human body. Clin Investig. 1992;70:539–544. doi: 10.1007/BF00184787. [DOI] [PubMed] [Google Scholar]
- 54.Chen S, Kawashima H, Lowe JB, Lanier LL, Fukuda M. Suppression of tumor formation in lymph nodes by l-selectin-mediated natural killer cell recruitment. J Exp Med. 2005;202:1679–1689. doi: 10.1084/jem.20051473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim CH, Pelus LM, Appelbaum E, Johanson K, Anzai N, Broxmeyer HE. CCR7 ligands, SLC/6Ckine/Exodus2/TCA4 and CKbeta-11/MIP-3beta/ELC, are chemoattractants for CD56(+)CD16(−) NK cells and late stage lymphoid progenitors. Cell Immunol. 1999;193:226–235. doi: 10.1006/cimm.1999.1483. [DOI] [PubMed] [Google Scholar]
- 56.Frey M, Packianathan NB, Fehniger TA, Ross ME, Wang WC, Stewart CC, Caligiuri MA, Evans SS. Differential expression and function of l-selectin on CD56bright and CD56dim natural killer cell subsets. J Immunol. 1998;161:400–408. [PubMed] [Google Scholar]
- 57.Garrod KR, Wei SH, Parker I, Cahalan MD. Natural killer cells actively patrol peripheral lymph nodes forming stable conjugates to eliminate MHC-mismatched targets. Proc Natl Acad Sci USA. 2007;104:12081–12086. doi: 10.1073/pnas.0702867104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bajenoff M, Breart B, Huang AY, Qi H, Cazareth J, Braud VM, Germain RN, Glaichenhaus N. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J Exp Med. 2006;203:619–631. doi: 10.1084/jem.20051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beuneu H, Deguine J, Breart B, Mandelboim O, Di Santo JP, Bousso P. Dynamic behavior of NK cells during activation in lymph nodes. Blood. 2009;114:3227–3234. doi: 10.1182/blood-2009-06-228759. [DOI] [PubMed] [Google Scholar]
- 60.Walzer T, et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci USA. 2007;104:3384–3389. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 62.Watt SV, Andrews DM, Takeda K, Smyth MJ, Hayakawa Y. IFN-gamma-dependent recruitment of mature CD27(high) NK cells to lymph nodes primed by dendritic cells. J Immunol. 2008;181:5323–5330. doi: 10.4049/jimmunol.181.8.5323. [DOI] [PubMed] [Google Scholar]
- 63.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 64.Kummer JA, Kamp AM, Tadema TM, Vos W, Meijer CJ, Hack CE. Localization and identification of granzymes A and B-expressing cells in normal human lymphoid tissue and peripheral blood. Clin Exp Immunol. 1995;100:164–172. doi: 10.1111/j.1365-2249.1995.tb03619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vivier E, Munroe M, Ariniello P, Anderson P. Identification of tissue-infiltrating lymphocytes expressing PEN5, a mucin-like glycoprotein selectively expressed on natural killer cells. Am J Pathol. 1995;146:409–418. [PMC free article] [PubMed] [Google Scholar]
- 66.Timens W, Poppema S. Lymphocyte compartments in human spleen. An immunohistologic study in normal spleens and uninvolved spleens in Hodgkin’s disease. Am J Pathol. 1985;120:443–454. [PMC free article] [PubMed] [Google Scholar]
- 67.Salazar-Mather TP, Ishikawa R, Biron CA. NK cell trafficking and cytokine expression in splenic compartments after IFN induction and viral infection. J Immunol. 1996;157:3054–3064. [PubMed] [Google Scholar]
- 68.Gregoire C, et al. Intrasplenic trafficking of natural killer cells is redirected by chemokines upon inflammation. Eur J Immunol. 2008;38:2076–2084. doi: 10.1002/eji.200838550. [DOI] [PubMed] [Google Scholar]
- 69.Huang FP, MacPherson GG. Continuing education of the immune system–dendritic cells, immune regulation and tolerance. Curr Mol Med. 2001;1:457–468. doi: 10.2174/1566524013363573. [DOI] [PubMed] [Google Scholar]
- 70.Dieu MC, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 72.Sozzani S, et al. Receptor expression and responsiveness of human dendritic cells to a defined set of CC and CXC chemokines. J Immunol. 1997;159:1993–2000. [PubMed] [Google Scholar]
- 73.Bajenoff M, Granjeaud S, Guerder S. The strategy of T cell antigen-presenting cell encounter in antigen-draining lymph nodes revealed by imaging of initial T cell activation. J Exp Med. 2003;198:715–724. doi: 10.1084/jem.20030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci USA. 2000;97:12694–12699. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Megjugorac NJ, Young HA, Amrute SB, Olshalsky SL, Fitzgerald-Bocarsly P. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J Leukoc Biol. 2004;75:504–514. doi: 10.1189/jlb.0603291. [DOI] [PubMed] [Google Scholar]
- 76.Carrega P, et al. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(−) cells and display an impaired capability to kill tumor cells. Cancer. 2008;112:863–875. doi: 10.1002/cncr.23239. [DOI] [PubMed] [Google Scholar]
- 77.Schleypen JS, et al. Cytotoxic markers and frequency predict functional capacity of natural killer cells infiltrating renal cell carcinoma. Clin Cancer Res. 2006;12:718–725. doi: 10.1158/1078-0432.CCR-05-0857. [DOI] [PubMed] [Google Scholar]
- 78.Schleypen JS, Von Geldern M, Weiss EH, Kotzias N, Rohrmann K, Schendel DJ, Falk CS, Pohla H. Renal cell carcinoma-infiltrating natural killer cells express differential repertoires of activating and inhibitory receptors and are inhibited by specific HLA class I allotypes. Int J Cancer. 2003;106:905–912. doi: 10.1002/ijc.11321. [DOI] [PubMed] [Google Scholar]
- 79.Dalbeth N, Gundle R, Davies RJ, Lee YC, McMichael AJ, Callan MF. CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J Immunol. 2004;173:6418–6426. doi: 10.4049/jimmunol.173.10.6418. [DOI] [PubMed] [Google Scholar]
- 80.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 81.van den Broek MF, Kagi D, Zinkernagel RM, Hengartner H. Perforin dependence of natural killer cell-mediated tumor control in vivo. Eur J Immunol. 1995;25:3514–3516. doi: 10.1002/eji.1830251246. [DOI] [PubMed] [Google Scholar]
- 82.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 83.Smith HR, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci USA. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fernandez NC, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 85.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kassim SH, Rajasagi NK, Zhao X, Chervenak R, Jennings SR. In vivo ablation of CD11c-positive dendritic cells increases susceptibility to herpes simplex virus type 1 infection and diminishes NK and T-cell responses. J Virol. 2006;80:3985–3993. doi: 10.1128/JVI.80.8.3985-3993.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gerosa F, Gobbi A, Zorzi P, Burg S, Briere F, Carra G, Trinchieri G. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–734. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 91.Nguyen KB, et al. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol. 2002;169:4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- 92.Orange JS, Biron CA. Characterization of early IL-12, IFN-alphabeta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J Immunol. 1996;156:4746–4756. [PubMed] [Google Scholar]
- 93.Dalod M, Hamilton T, Salomon R, Salazar-Mather TP, Henry SC, Hamilton JD, Biron CA. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J Exp Med. 2003;197:885–898. doi: 10.1084/jem.20021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Briere F, Trinchieri G, Biron CA. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med. 2002;195:517–528. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Asselin-Paturel C, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 97.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol. 2009;10:1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Szomolanyi-Tsuda E, Liang X, Welsh RM, Kurt-Jones EA, Finberg RW. Role for TLR2 in NK cell-mediated control of murine cytomegalovirus in vivo. J Virol. 2006;80:4286–4291. doi: 10.1128/JVI.80.9.4286-4291.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tabeta K, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krug A, et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 101.Andoniou CE, et al. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat Immunol. 2005;6:1011–1019. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- 102.Andrews DM, Andoniou CE, Scalzo AA, van Dommelen SL, Wallace ME, Smyth MJ, Degli-Esposti MA. Cross-talk between dendritic cells and natural killer cells in viral infection. Mol Immunol. 2005;42:547–555. doi: 10.1016/j.molimm.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 103.Pollara G, et al. Herpes simplex virus type-1-induced activation of myeloid dendritic cells: the roles of virus cell interaction and paracrine type I IFN secretion. J Immunol. 2004;173:4108–4119. doi: 10.4049/jimmunol.173.6.4108. [DOI] [PubMed] [Google Scholar]
- 104.Orange JS, Biron CA. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol. 1996;156:1138–1142. [PubMed] [Google Scholar]
- 105.Strowig T, Brilot F, Arrey F, Bougras G, Thomas D, Muller WA, Munz C. Tonsilar NK cells restrict B cell transformation by the Epstein-Barr virus via IFN-gamma. PLoS Pathog. 2008;4:e27. doi: 10.1371/journal.ppat.0040027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Monteiro JM, Harvey C, Trinchieri G. Role of interleukin-12 in primary influenza virus infection. J Virol. 1998;72:4825–4831. doi: 10.1128/jvi.72.6.4825-4831.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scharton-Kersten T, Afonso LC, Wysocka M, Trinchieri G, Scott P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J Immunol. 1995;154:5320–5330. [PubMed] [Google Scholar]
- 108.Nomura T, et al. Essential role of interleukin-12 (IL-12) and IL-18 for gamma interferon production induced by listeriolysin O in mouse spleen cells. Infect Immun. 2002;70:1049–1055. doi: 10.1128/IAI.70.3.1049-1055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Granucci F, Zanoni I, Pavelka N, Van Dommelen SL, Andoniou CE, Belardelli F, Degli Esposti MA, Ricciardi-Castagnoli P. A contribution of mouse dendritic cell-derived IL-2 for NK cell activation. J Exp Med. 2004;200:287–295. doi: 10.1084/jem.20040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Koka R, Burkett PR, Chien M, Chai S, Chan F, Lodolce JP, Boone DL, Ma A. Interleukin (IL)-15R[alpha]-deficient natural killer cells survive in normal but not IL-15R[alpha]-deficient mice. J Exp Med. 2003;197:977–984. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 113.Brilot F, Strowig T, Roberts SM, Arrey F, Munz C. NK cell survival mediated through the regulatory synapse with human DCs requires IL-15Ralpha. J Clin Invest. 2007;117:3316–3329. doi: 10.1172/JCI31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huntington ND, et al. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat Immunol. 2007;8:856–863. doi: 10.1038/ni1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205:1213–1225. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huntington ND, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206:25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Elpek KG, Rubinstein MP, Bellemare-Pelletier A, Goldrath AW and Turley SJ (2010). Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15R{alpha} complexes. Proc Natl Acad Sci USA (in press) [DOI] [PMC free article] [PubMed]
- 118.Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, Girardi M, Hayday AC. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 119.Saito T, Ruffman R, Welker RD, Herberman RB, Chirigos MA. Development of hyporesponsiveness of natural killer cells to augmentation of activity after multiple treatments with biological response modifiers. Cancer Immunol Immunother. 1985;19:130–135. doi: 10.1007/BF00199721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tripathy SK, Keyel PA, Yang L, Pingel JT, Cheng TP, Schneeberger A, Yokoyama WM. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J Exp Med. 2008;205:1829–1841. doi: 10.1084/jem.20072446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Münz C, Dao T, Ferlazzo G, de Cos MA, Goodman K, Young JW. Mature myeloid dendritic cell subsets have distinct roles for activation and viability of circulating human natural killer cells. Blood. 2005;105:266–273. doi: 10.1182/blood-2004-06-2492. [DOI] [PubMed] [Google Scholar]
- 122.Borg C, et al. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 2004;104:3267–3275. doi: 10.1182/blood-2004-01-0380. [DOI] [PubMed] [Google Scholar]
- 123.Semino C, Angelini G, Poggi A, Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 2005;106:609–616. doi: 10.1182/blood-2004-10-3906. [DOI] [PubMed] [Google Scholar]
- 124.Fernandez NC, Flament C, Crepineau F, Angevin E, Vivier E, Zitvogel L. Dendritic cells (DC) promote natural killer (NK) cell functions: dynamics of the human DC/NK cell cross talk. Eur Cytokine Netw. 2002;13:17–27. [PubMed] [Google Scholar]
- 125.Newman KC, Korbel DS, Hafalla JC, Riley EM. Cross-talk with myeloid accessory cells regulates human natural killer cell interferon-gamma responses to malaria. PLoS Pathog. 2006;2:e118. doi: 10.1371/journal.ppat.0020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hanabuchi S, Watanabe N, Wang YH, Ito T, Shaw J, Cao W, Qin FX, Liu YJ. Human plasmacytoid predendritic cells activate NK cells through glucocorticoid-induced tumor necrosis factor receptor-ligand (GITRL) Blood. 2006;107:3617–3623. doi: 10.1182/blood-2005-08-3419. [DOI] [PubMed] [Google Scholar]
- 127.Jinushi M, et al. Critical role of MHC class I-related chain A and B expression on IFN-alpha-stimulated dendritic cells in NK cell activation: impairment in chronic hepatitis C virus infection. J Immunol. 2003;170:1249–1256. doi: 10.4049/jimmunol.170.3.1249. [DOI] [PubMed] [Google Scholar]
- 128.Terme M, et al. IL-4 confers NK stimulatory capacity to murine dendritic cells: a signaling pathway involving KARAP/DAP12-triggering receptor expressed on myeloid cell 2 molecules. J Immunol. 2004;172:5957–5966. doi: 10.4049/jimmunol.172.10.5957. [DOI] [PubMed] [Google Scholar]
- 129.Poggi A, et al. NK cell activation by dendritic cells is dependent on LFA-1-mediated induction of calcium-calmodulin kinase II: inhibition by HIV-1 Tat C-terminal domain. J Immunol. 2002;168:95–101. doi: 10.4049/jimmunol.168.1.95. [DOI] [PubMed] [Google Scholar]
- 130.Carpen O, Virtanen I, Lehto VP, Saksela E. Polarization of NK cell cytoskeleton upon conjugation with sensitive target cells. J Immunol. 1983;131:2695–2698. [PubMed] [Google Scholar]
- 131.Carpen O, Virtanen I, Saksela E. Ultrastructure of human natural killer cells: nature of the cytolytic contacts in relation to cellular secretion. J Immunol. 1982;128:2691–2697. [PubMed] [Google Scholar]
- 132.Dustin ML, Long EO. Cytotoxic immunological synapses. Immunol Rev. 2010;235:24–34. doi: 10.1111/j.0105-2896.2010.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Masilamani M, Nguyen C, Kabat J, Borrego F, Coligan JE. CD94/NKG2A inhibits NK cell activation by disrupting the actin network at the immunological synapse. J Immunol. 2006;177:3590–3596. doi: 10.4049/jimmunol.177.6.3590. [DOI] [PubMed] [Google Scholar]
- 135.Endt J, McCann FE, Almeida CR, Urlaub D, Leung R, Pende D, Davis DM, Watzl C. Inhibitory receptor signals suppress ligation-induced recruitment of NKG2D to GM1-rich membrane domains at the human NK cell immune synapse. J Immunol. 2007;178:5606–5611. doi: 10.4049/jimmunol.178.9.5606. [DOI] [PubMed] [Google Scholar]
- 136.Medema JP, et al. Expression of the serpin serine protease inhibitor 6 protects dendritic cells from cytotoxic T lymphocyte-induced apoptosis: differential modulation by T helper type 1 and type 2 cells. J Exp Med. 2001;194:657–667. doi: 10.1084/jem.194.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hirst CE, Buzza MS, Bird CH, Warren HS, Cameron PU, Zhang M, Ashton-Rickardt PG, Bird PI. The intracellular granzyme B inhibitor, proteinase inhibitor 9, is up-regulated during accessory cell maturation and effector cell degranulation, and its overexpression enhances CTL potency. J Immunol. 2003;170:805–815. doi: 10.4049/jimmunol.170.2.805. [DOI] [PubMed] [Google Scholar]
- 138.Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, Tracey KJ, Chiorazzi N. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004;173:307–313. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 139.Pallandre JR, et al. Dendritic cell and natural killer cell cross-talk: a pivotal role of CX3CL1 in NK cytoskeleton organization and activation. Blood. 2008;112:4420–4424. doi: 10.1182/blood-2007-12-126888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Al-Alwan MM, Rowden G, Lee TD, West KA. The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J Immunol. 2001;166:1452–1456. doi: 10.4049/jimmunol.166.3.1452. [DOI] [PubMed] [Google Scholar]
- 141.Riol-Blanco L, et al. Immunological synapse formation inhibits, via NF-kappaB and FOXO1, the apoptosis of dendritic cells. Nat Immunol. 2009;10:753–760. doi: 10.1038/ni.1750. [DOI] [PubMed] [Google Scholar]
- 142.Vitale M, Della Chiesa M, Carlomagno S, Romagnani C, Thiel A, Moretta L, Moretta A. The small subset of CD56brightCD16- natural killer cells is selectively responsible for both cell proliferation and interferon-gamma production upon interaction with dendritic cells. Eur J Immunol. 2004;34:1715–1722. doi: 10.1002/eji.200425100. [DOI] [PubMed] [Google Scholar]
- 143.Strowig T, Chijioke O, Carrega P, Arrey F, Meixlsperger S, Ramer PC, Ferlazzo G and Munz C (2010). Human NK cells of mice with reconstituted human immune system components require pre-activation to acquire functional competence. Blood (in press) [DOI] [PMC free article] [PubMed]
- 144.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002;2:957–964. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 145.Lunemann A, Lunemann JD, Roberts S, Messmer B, Barreira da Silva R, Raine CS, Munz C. Human NK cells kill resting but not activated microglia via NKG2D- and NKp46-mediated recognition. J Immunol. 2008;181:6170–6177. doi: 10.4049/jimmunol.181.9.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol. 2006;177:2051–2055. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- 147.Nedvetzki S, et al. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood. 2007;109:3776–3785. doi: 10.1182/blood-2006-10-052977. [DOI] [PubMed] [Google Scholar]
- 148.Siren J, Sareneva T, Pirhonen J, Strengell M, Veckman V, Julkunen I, Matikainen S. Cytokine and contact-dependent activation of natural killer cells by influenza A or Sendai virus-infected macrophages. J Gen Virol. 2004;85:2357–2364. doi: 10.1099/vir.0.80105-0. [DOI] [PubMed] [Google Scholar]
- 149.Shah PD, Gilbertson SM, Rowley DA. Dendritic cells that have interacted with antigen are targets for natural killer cells. J Exp Med. 1985;162:625–636. doi: 10.1084/jem.162.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wilson JL, Heffler LC, Charo J, Scheynius A, Bejarano MT, Ljunggren HG. Targeting of human dendritic cells by autologous NK cells. J Immunol. 1999;163:6365–6370. [PubMed] [Google Scholar]
- 151.Carbone E, Terrazzano G, Ruggiero G, Zanzi D, Ottaiano A, Manzo C, Karre K, Zappacosta S. Recognition of autologous dendritic cells by human NK cells. Eur J Immunol. 1999;29:4022–4029. doi: 10.1002/(SICI)1521-4141(199912)29:12<4022::AID-IMMU4022>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 152.Ferlazzo G, Morandi B, D’Agostino A, Meazza R, Melioli G, Moretta A, Moretta L. The interaction between NK cells and dendritic cells in bacterial infections results in rapid induction of NK cell activation and in the lysis of uninfected dendritic cells. Eur J Immunol. 2003;33:306–313. doi: 10.1002/immu.200310004. [DOI] [PubMed] [Google Scholar]
- 153.Melki MT, Saidi H, Dufour A, Olivo-Marin JC, Gougeon ML. Escape of HIV-1-infected dendritic cells from TRAIL-mediated NK cell cytotoxicity during NK-DC cross-talk—a pivotal role of HMGB1. PLoS Pathog. 2010;6:e1000862. doi: 10.1371/journal.ppat.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pende D, et al. Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: relevance for natural killer–dendritic cell interaction. Blood. 2006;107:2030–2036. doi: 10.1182/blood-2005-07-2696. [DOI] [PubMed] [Google Scholar]
- 155.Spaggiari GM, Carosio R, Pende D, Marcenaro S, Rivera P, Zocchi MR, Moretta L, Poggi A. NK cell-mediated lysis of autologous antigen-presenting cells is triggered by the engagement of the phosphatidylinositol 3-kinase upon ligation of the natural cytotoxicity receptors NKp30 and NKp46. Eur J Immunol. 2001;31:1656–1665. doi: 10.1002/1521-4141(200106)31:6<1656::aid-immu1656>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 156.Della Chiesa M, Vitale M, Carlomagno S, Ferlazzo G, Moretta L, Moretta A. The natural killer cell-mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig-like receptors. Eur J Immunol. 2003;33:1657–1666. doi: 10.1002/eji.200323986. [DOI] [PubMed] [Google Scholar]
- 157.Hayakawa Y, Screpanti V, Yagita H, Grandien A, Ljunggren HG, Smyth MJ, Chambers BJ. NK cell TRAIL eliminates immature dendritic cells in vivo and limits dendritic cell vaccination efficacy. J Immunol. 2004;172:123–129. doi: 10.4049/jimmunol.172.1.123. [DOI] [PubMed] [Google Scholar]
- 158.Laffont S, Seillet C, Ortaldo J, Coudert JD, Guery JC. Natural killer cells recruited into lymph nodes inhibit alloreactive T-cell activation through perforin-mediated killing of donor allogeneic dendritic cells. Blood. 2008;112:661–671. doi: 10.1182/blood-2007-10-120089. [DOI] [PubMed] [Google Scholar]
- 159.Ruggeri L, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 160.Yu G, Xu X, Vu MD, Kilpatrick ED, Li XC. NK cells promote transplant tolerance by killing donor antigen-presenting cells. J Exp Med. 2006;203:1851–1858. doi: 10.1084/jem.20060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kang SJ, Liang HE, Reizis B, Locksley RM. Regulation of hierarchical clustering and activation of innate immune cells by dendritic cells. Immunity. 2008;29:819–833. doi: 10.1016/j.immuni.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vitale M, Della Chiesa M, Carlomagno S, Pende D, Arico M, Moretta L, Moretta A. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood. 2005;106:566–571. doi: 10.1182/blood-2004-10-4035. [DOI] [PubMed] [Google Scholar]
- 163.Mocikat R, et al. Natural killer cells activated by MHC class I(low) targets prime dendritic cells to induce protective CD8 T cell responses. Immunity. 2003;19:561–569. doi: 10.1016/s1074-7613(03)00264-4. [DOI] [PubMed] [Google Scholar]
- 164.Morandi B, Bougras G, Muller WA, Ferlazzo G, Munz C. NK cells of human secondary lymphoid tissues enhance T cell polarization via IFN-gamma secretion. Eur J Immunol. 2006;36:2394–2400. doi: 10.1002/eji.200636290. [DOI] [PubMed] [Google Scholar]
- 165.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 166.Ing R, Stevenson MM. Dendritic cell and NK cell reciprocal cross talk promotes gamma interferon-dependent immunity to blood-stage Plasmodium chabaudi AS infection in mice. Infect Immun. 2009;77:770–782. doi: 10.1128/IAI.00994-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Adam C, et al. DC-NK cell cross talk as a novel CD4+ T-cell-independent pathway for antitumor CTL induction. Blood. 2005;106:338–344. doi: 10.1182/blood-2004-09-3775. [DOI] [PubMed] [Google Scholar]