Abstract
Due to the rapid emergence of resistant microbes to the currently available antibiotics, cationic antimicrobial peptides have attracted considerable interest as a possible new generation of anti-infective compounds. However, low cost development for therapeutic or industrial purposes requires, among other properties, that the peptides will be small and with simple structure. Therefore, considerable research has been devoted to optimizing peptide length combined with a simple design. This review focuses on the similarities and differences in the mode of action and target cell specificity of two families of small peptides: the naturally occurring temporins from the skin of amphibia and the engineered ultrashort lipopeptides. We will also discuss the finding that acylation of cationic peptides results in molecules with a more potent spectrum of activity and a higher resistance to proteolytic degradation. Conjugation of fatty acids to linear native peptide sequences is a powerful strategy to engineer novel successful anti-infective drugs.
Keywords: Antimicrobial peptides, Lipopeptides, LPS-neutralizing activity, Mode of action, Peptide-membrane interaction, Cell-selectivity
Introduction: antimicrobial (host-defense) peptides and lipopeptides
Host-defense cationic peptides (HDPs, also named antimicrobial peptides, AMPs) are ribosomally produced by almost all forms of life, from unicellular organisms to plants and highly evolved animal species [1]. They are essential evolutionarily conserved elements of the innate defense system of these organisms, providing them with a fast-acting weapon against microbial infections. This protection is required, in the case of vertebrates, before the adaptive immune system is activated [2–8]. Most AMPs are mobilized shortly after the attack of the pathogen and operate rapidly to neutralize a broad range of microorganisms [9]. They can be found in large quantities freely circulating or sequestered in compartments throughout the organism [10, 11]. AMPs were initially isolated more than two decades ago from various sources including insect hemolymph, the skin of frogs, and mammalian neutrophil granules [12–15].
Many antibiotic-resistant bacterial and fungal strains have emerged due to the widespread use of conventional drugs. This is a growing health concern worldwide, especially in hospitals [16, 17], and has stimulated research into alternative antibiotic agents with new mechanisms of activity. AMPs are considered among the most promising candidates for future therapeutics and have resulted in intensive research for the discovery of new types and sequences. Currently, more than 1,000 native peptides from all species of life, as well as many thousands of designed peptides have been described (see Hancock review in this MAR and database http://aps.unmc.edu/AP/main.php).
Antimicrobial lipopeptides (LiPs), on the other hand, are produced nonribosomally in bacteria and fungi during cultivation on various carbon sources. They are a class of antibiotics that are highly active against multidrug-resistant bacteria. However, some LiPs also display antifungal activity [18–29]. Most native LiPs consist of a short (six to seven amino acids) linear or cyclic peptide sequence, with either a net positive or a negative charge, to which a fatty acid moiety is covalently attached to the N-terminus. Compared to AMPs, resistance to LiPs is generally rare [20, 30].
Acylation of synthetic or natural AMPs with fatty acids has been proven to be a useful approach to improve their antimicrobial activity, as well as to endow them with antifungal properties. This has been shown with a number of LiPs consisting of cationic AMPs acylated at their N-terminus with a C8–C18 fatty acid chain length. This effect is due to changes in the overall hydrophobicity of these molecules, which affects both their oligomerization and organization in solution and in membranes and their affinity for membranes [31–40].
Searching the keywords “antimicrobial peptide” results in over 7,000 scientific publications and over 2,500 patents. Therefore, to focus this review, we will discuss only a family of short AMPs isolated from frog skin, and a de novo designed family of ultrashort LiPs (USLiPs). Due to the small size and simple composition of both these families, they are attractive and commercially feasible candidates for further development in therapeutic or industrial uses.
Does the activity of AMPs or LiPs depend on a specific structure or a defined length?
Despite differences in their conformation and length, most naturally occurring AMPs share two main features: (1) a high net positive charge under physiological conditions and (2) a potential to adopt an amphipathic structure in a hydrophobic environment (an α-helix, a β-sheet, or a combination of both [41]). These structures characterize most membrane-active peptides that can bind and permeate microbial membranes. The conformation and the length of AMPs are important biophysical parameters in determining their mode of action, which will be discussed in the next paragraphs. Generally speaking, the minimum length for a peptide in an α-helix structure required to span a membrane is ~23 amino acids or ~8 amino acids for a peptide that adopts a β-sheet structure. However, peptides that do not need to insert into the hydrophobic core of the membrane in order to exert their function can be even smaller. Nevertheless, the length of native AMPs ranges from about 8 amino acids up to more than 100 amino acids. Because most of them have an amphipathic character, many reports have focused on the role of amphipathicity in their ability to kill microorganisms.
Native LiPs on the other hand differ from AMPs by having only short chains of six to seven amino acids linked to a specific lipophilic moiety [42–44]. Both the composition of the peptide moiety and the type of the lipophilic part are sensitive to modification. In general, native LiPs belonging to this group are non-cell-selective and therefore quite toxic to mammalian cells. Despite this toxicity, daptomycin, a member of this family, which is active only toward Gram-positive bacteria, was recently approved by the FDA for the treatment of skin infections caused by Staphylococcus aureus [45]. This flagship example confirms the growing opinion that peptide-based antibiotics will be among the next generation of anti-infective therapy.
Short-length native AMPs and engineered USLiPs
Among the natural sources of AMPs, the skin of amphibia Anura is one of the richest storehouses [46–48]. These AMPs are produced by dermal glands and stored within granules that can be released on the skin surface upon stress or injury [46]. Their synthesis is induced by contact with microorganisms and is transcriptionally regulated by NF-κB/IκBα machinery [49]. Over the past 10 years, a significant number of studies have been carried out with temporins, initially isolated from the European red frog Rana temporaria [50] and subsequently from the secretions of other ranid frogs of both North American and Euroasian origin [51, 52] (see Table 1).
Table 1.
Designation and sequence of selected temporins and LiPs
aBasic residues are indicated by red letters. Underlined and bold amino acids are d-enantiomers. All the peptides are amidated at their C-terminus
Temporins are one of the largest families of AMPs (more than 100 members), and they have unique properties. (1) They are among the smallest amphipathic α-helical AMPs found in nature (10–14 amino acids, with a few longer exceptions with 16–17 amino acids). (2) Most of them have a low net positive charge at a neutral pH, ranging from +2 to +3, due to the presence of only one or two basic residues in their sequence. This is in contrast with most AMPs, which have a higher content of positive charges. (3) Some of them act efficiently and rapidly against a wide range of pathogens (including bacteria, yeasts, and protozoa of Leishmania genus) and are in general not toxic to mammalian cells. (4) Their killing kinetic is concomitant with that of membrane perturbation. (5) Some temporins have selective cytotoxic activity against neoplastic cells, such as erythroleukemic cells and cutaneous T lymphoma [53]. (6) Some have immunomodulatory activities (chemotactic to human phagocytes) [54]. (7) They retain almost all biological functions in serum. (8) Some of them have an antiendotoxin activity. (9) They show synergy with other members in their antimicrobial and antiendotoxin activity [52]. These unique properties will be outlined in the paragraphs below. Interestingly, a unique ultrashort temporin (only eight residues) has been recently isolated from the skin of the frog Phelophylax saharica. It represents the smallest naturally occurring linear AMP identified so far. It has a highly hydrophobic sequence, with the highest percentage of Phe residues (50%) of any known peptide or protein, and folds into a nonamphipathic α-helix when bound to the microbial membrane surface [55].
Among engineered small AMPs, the USLiPs represent the shortest LiPs reported so far. They are composed of two to four amino acids linked to a fatty acid (12–16 carbon chain). The net charge is positive, ranging from 1 to 4 [56, 57] (Table 1). The fatty acid tail has been shown to compensate for the hydrophobic amino acids within the peptide chain. Moreover, substituting only one out of the two to four amino acids is sufficient to create molecules with different biological functions. For example, broad spectrum compounds with activity against all strains of bacteria and fungi become selective toward Gram-positive bacteria and fungi, but not against Gram-negative bacteria [57]. USLiPs are amphiphilic molecules mimicking detergents, in which one side is hydrophilic (the peptidic side) and the other one is hydrophobic (the fatty acid side). Therefore, they can form a hydrophobic core through self-association. However, the tendency to oligomerize is offset by the resulting close proximity of the positively charged peptide moiety. For this reason, the hydrophobic moiety needs to be a long fatty acid, usually comprising at least 14 carbons [31, 56]. The finding that a single lysine attached to a palmitic acid (16 carbon atoms) is not active supports the notion that the activity of these LiPs is not dictated solely by the hydrophobic aliphatic chain, but also requires a specific amino acid sequence. Elongation of the peptide chain above four amino acids can allow the shortening of the conjugated fatty acid [31]. Regarding LiPs with a long peptide chain (more than ca. eight amino acids), oligomerization can be the result of peptide–peptide, peptide–fatty acid, and fatty acid–fatty acid interactions [58]. However, the structure and organization of the peptidic chains in solution are different for different LiPs. Most of them are found unstructured. Nevertheless, it was observed that, in solution, the peptide chains can bend and interact with the hydrophobic fatty acid part of the LiP micelles and acquire an α-helical conformation [31, 39]. Peptides that oligomerize in solution are significantly more protected from proteolytic degradation compared with peptides that do not oligomerize. Therefore, the existence of antimicrobial LiPs as aggregates may be an advantage in vivo where resistance to proteolytic degradation can influence the half-life of the peptide and its efficacy [59–62]. The self-assembly ability of antimicrobial LiPs in solution has been demonstrated with tri-amino acid LiPs of different sequences. They can form nanostructures with different morphologies, as seen by electron microscopy [57]. The different structures and the size of the assemblies determine the activity and the possibility that these LiPs will dissociate and penetrate the microorganism’s cell wall. Similarly, light microscopy analysis has shown that a new family of antimicrobial agents named oligoacyllysines tends to aggregate and form nanotubes [63–65].
A role for the microbial membrane phospholipids and outer cell wall components in the function and cell specificity of AMPs and LiPs
The inner phospholipid membrane
Although the exact mode(s) of action of AMPs remains a matter of controversy, there is a consensus that the membrane of the pathogen is one of the major targets of the AMPs. This has been highlighted in various studies showing that an ample number of AMPs selectively permeate the membrane of microbial cells, causing irreversible physical damage [66–69]. Nevertheless, there is convincing evidence that membrane permeation is not the only mechanism of microbial killing. Indeed, peptides can translocate the membrane and subsequently alter the membrane septum formation, or inhibit cell-wall, nucleic-acid, protein synthesis as well as the activity of enzymes or protein folding [41, 46, 70–72]. In addition, besides a direct antimicrobial activity, AMPs from higher eukaryotes also possess anticancer activity; immunomodulatory properties (as they can activate specific immune receptors such as the TLR2/1 complex [73–75]); the ability to influence cytokine release, cell proliferation, angiogenesis, wound healing, and chemotaxis [76, 77]; and the capacity to neutralize microbial endotoxins [52, 78] (see also the review by Hancock and collaborators in this MAR).
As mentioned above, temporins and USLiPs represent two unique families of antimicrobial agents. There is a consensus that, in general, cationic AMPs and some LiPs recognize and interact with the acidic phospholipids (e.g., phosphatidylglycerol, cardiolipin) exposed on the outer leaflet of the bacterial membrane. In contrast, in mammalian cells, the outer leaflet is zwitterionic, which should reduce the binding capacity of the cationic AMPs. This can account for the preferential activity of AMPs against bacteria and partially against fungi [79, 80]. Note that temporins can also disintegrate the membrane of Leishmania parasites, causing a loss of intracellular material. The membrane of Leishmania protozoa is less anionic than that of bacteria and is devoid of phosphatidylglycerol [81]. To the best of our knowledge, temporins are among the smallest natural antiparasitic peptides reported so far that exert their activity by disrupting the parasitic membrane. Regarding LiPs, due to their high hydrophobicity some behave as non-cell-selective antimicrobials.
General models for membrane permeation
Based on the classical amphipathic α-helical or β-sheet structures of the early discovered families of AMPs, several models were proposed that could explain the mechanism of membrane permeation. These models vary from the classical “barrel stave” transmembrane pore formation mechanism [82] to a very general mechanism of membrane destabilization via the “carpet” model [83] that can involve the generation of “toroidal” pores [84, 85], channel aggregates [86], or more complex structures, depending on the length and the sequence of the peptide. An important feature of the “barrel-stave” mechanism is that it characterizes peptides that can cross the lipid bilayers regardless of the charge of the phospholipid head-group. This is because these peptides do not have a high net positive charge and therefore they are not selective to anionic membranes. Their interaction with the phospholipid bilayers is driven by hydrophobic and not electrostatic interactions. Such peptides self-associate either in solution prior to binding and insertion into the membrane, or alternatively, bind to the membrane followed by peptide oligomerization and insertion. In these oligomers, the hydrophobic surfaces of the helices interact with the fatty acid chains of the membrane phospholipids, while the hydrophilic surfaces point inward, producing a transmembrane pore. Because such AMPs interact with both zwitterionic and negatively charged membranes, they are non-cell-selective [83]. In contrast, in the “carpet model,” peptides first bind to the surface of the target membrane via electrostatic interactions and cover it in a carpet-like manner. Next, the peptides reorient themselves such that their hydrophobic face points toward the lipids, and the hydrophilic face toward the phospholipid head-groups. At very high peptide concentrations the membrane can be disintegrated in a detergent-like manner, resulting in the collapse of the membrane packing into fragments with physical dissolution of the cell wall. This mechanism does not require a specific structure, length, or specific amino acids.
In the case of LiPs, they can bind to the membrane as large oligomers followed by subsequent multimer dissociation and insertion into the phospholipid bilayers. This would be accompanied by a structural transition of LiPs and membrane perforation leading to cell death [20].
General models for cell killing
Extensive research on the mode of action of temporins has suggested the plasma membrane as their principal site of action. This has been established based on the following data: (1) a rapid induction of the collapse of membrane potential; (2) a rapid killing kinetics (within 15–20 min) concomitant with that of membrane permeation; (3) a correlation between the extent of membrane damage and the microbial killing; (4) an injured pattern of microbes (bacteria and parasites) as visualized by both transmission and scanning electron microscopy; and (5) leakage of large intracellular components (e.g., the enzyme β galactosidase) in a dose-dependent manner and with a kinetic superimposable to that of bacterial death [51, 87]. Note that the ability to strongly alter the acyl chain packing of anionic phospholipid bilayers, thereby triggering local cracks and microbial membrane damage, is maintained in the 8-mer ultrashort temporin [55].
However, the ability of several temporins to perturb the cytoplasmic membrane is not necessarily the lethal step. Some temporins can permeate the plasma membrane even at peptide concentrations markedly lower than those required to inhibit bacterial growth. This was demonstrated by using a triple-color staining method that enables simultaneous visualization of the effects of the peptide on the viability and membrane integrity of individual cells [87]. Therefore, it is reasonable to speculate that temporins can display different molecular mechanisms, depending on their concentration. Note that in contrast to other membrane-active AMPs [88], temporins do not lyse bacteria or do not induce an authophagic cell death in parasites (like indolicidin [89]), but cause the cells to have a ghost-like appearance with a deep roughening of their surface. The cell structure collapses, while membrane folding occurs with loss of intracellular material.
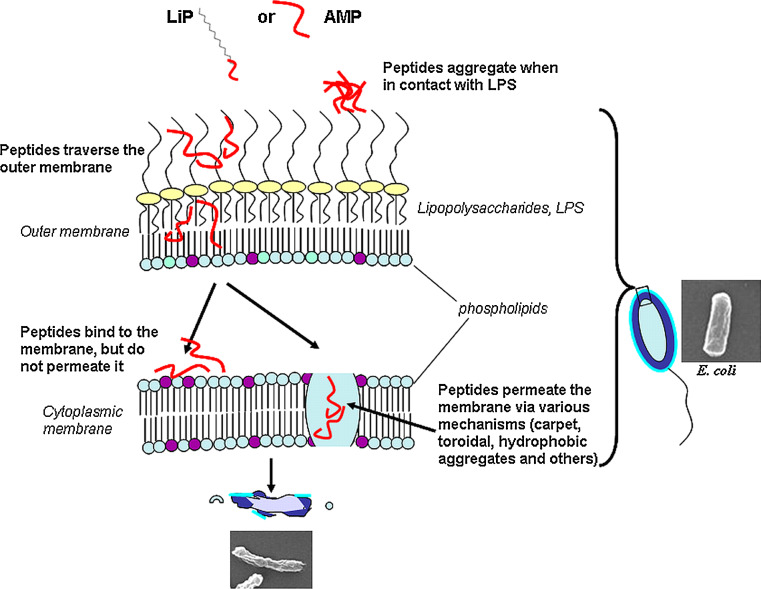
Despite differences in the composition between AMPs and USLiPs, mode of action studies have indicated a similar general mechanism. Most native LiPs act via two major mechanisms: (1) inhibiting the synthesis of cell wall compounds such as (1,3)-β-d-glucan or chitin [90–93], and (2) inducing membrane lysis [20–22, 28, 42–44, 94, 95]. However, the following data suggest that, similarly to most natural AMPs, a major target of the USLiPs is the membrane of the pathogenic microorganisms. Indeed, a direct correlation is found between the antimicrobial activity of the LiPs on different microorganisms and their ability to increase the permeability of their membranes, as well as to damage the structure of the corresponding pathogens, as shown from electron microscopy images [56]. A schematic representation of the mode of action of both short AMPs and USLiPs is shown in Fig. 1.
Fig. 1.
Schematic representation of a plausible mode of action of short native AMPs and USLiPs on Gram-negative bacteria. Both peptides can aggregate upon binding to the LPS outer layer or diffuse through it into the target cytoplasmic membrane. Then, the peptides can permeate the membrane through local breakages
The outer cell wall
Despite the fact that reaching the anionic bacterial membrane is the goal of most AMPs, the first step in the selection of target cells is governed by the electrostatic interactions between the positively charged side chains of the amino acids and the negatively charged components of the microbial cell wall, mainly lipopolysaccharide (LPS or endotoxin) in the outer membrane (OM) of Gram-negative bacteria, or lipoteichoic acid (LTA) in Gram-positive bacteria [96, 97]. Since LPS rather than LTA is the focus of a huge number of studies, it will be largely analyzed in this review. The repulsive forces of adjacent LPS molecules are neutralized by Mg2+ cations, making the OM an oriented and tightly cross-linked barrier that protects bacteria from a variety of hydrophobic molecules [98, 99], including some AMPs [100–102]. Note that for all enterobacterial and most nonenterobacterial strains, LPS is composed of three parts: (1) a hydrophobic moiety, the lipid A, consisting of fatty acid chains linked to two phosphorylated glucosamine residues; (2) an oligosaccharide core, covalently bound to the lipid A, via ketodeoxyoctonic acid; and (3) a hydrophilic O-antigenic domain, containing repeating saccharide units, which protrudes into the surrounding medium. The saccharide portion is diverse in length and composition amongst the different Gram-negative bacterial species [103, 104]. LPS covers more than 90% of the outer leaflet of the OM, whereas the inner membrane (IM, i.e., the cytoplasmic membrane) is composed of phospholipids that are distributed similarly on both sides of the bilayer. After crossing the LPS, AMPs meet this IM, which serves as an additional physical barrier to shield bacteria from antibacterial agents [105–107]. The fact that the LPS layer can protect bacteria from AMPs has been evidenced in several studies revealing that some AMPs are active against one bacterial strain but not against others, although the IMs of these bacteria have similar phospholipids composition [70]. Mode of action studies suggested that this is because of the differences in the LPS structure of the various strains [98]. Indeed, removal of the LPS from sensitive and nonsensitive bacteria ended with both bacteria being equally susceptible to the AMPs. Further support for the role of LPS in bacteria protection is manifested in studies showing lower activities for some AMPs against smooth bacteria (with a full length LPS saccharidic portion) compared to bacteria with a deep rough phenotype, characterized by the absence of LPS O-chains [108, 109].
Another factor that can affect the ability of AMPs to insert and traverse the LPS-OM relates to their biophysical properties, specifically to their oligomeric state. When peptides’ aggregation is triggered upon binding to LPS, or it is an intrinsic property of an AMP when in solution, they tend to form bulky compounds that prevent them from crossing the OM into their target IM (Fig. 1) [107]. Once such oligomers are dissociate, the AMPs become active on Gram-negative bacteria. Three examples validate this hypothesis. Firstly, an all-l 15-amino acid peptide was compared to its d,l analog (diastereomer). Antimicrobial assays showed that the d,l peptide was more active against Gram-negative bacteria compared to the all-l counterpart (the MICs were 5 and >100 μM against E. coli, respectively). However, membrane permeation assays with spheroplasts of E. coli (lacking the cell wall) indicated that the activity of the all-l-amino acid peptide was equal to that of its diastereomer [107]. In contrast, the diastereomer was much more active (up to ten-fold) than the all-l-peptide when membrane permeation was tested on intact E. coli cells. Interestingly, biophysical studies showed that whereas the all-l-peptide formed oligomers when bound to LPS, only the diastereomer existed in a monomeric state.
The second example that supports this hypothesis involves temporins, which consist of a large number of isoforms. It has been found that isoforms that have a similar capability to permeate both zwitterionic and anionic lipid vesicles are not active towards Gram-negative bacteria and human red blood cells [110]. In another study, it was shown that whereas some isoforms oligomerized in LPS, the combination of each of them with another isoform interfered with the assembly of the peptides and high activity was observed against Gram-negative bacteria.
The final example in support of the hypothesis involves other studies of LiPs self-assembly that affected cell wall penetration and biological function of LiPs [58]. The USLiP C16-KLLL is highly potent against all microorganisms except for Gram-negative bacteria [56]. Although it strongly damages model membranes mimicking those of Gram-negative bacteria, it cannot perturb the membrane of living E. coli cells, as measured by its inability to induce an intracellular influx of the vital dye Sytox Green. A possible explanation is that the highly hydrophobic C16-KLLL organizes into aggregates that are unable to traverse the cell wall of E. coli.
Indeed, recent studies have indicated that some of the USLiPs can form fibrils similarly to amyloids [57]. However, the shape and organization of the fibrils differs for LiPs having different peptide sequences [57]. For example, LiPs composed of dodecanoic acid attached to 12-mer peptide moiety form oligomers in solution, most probably with the hydrophobic core made by the oligomerized aliphatic chains [111]. Interestingly, it has been found that temporins can also generate amyloid-type fibrils in the presence of acidic phospholipids [112, 113].
Overall, as proved for both temporins and LiPs, LPS can induce oligomerization of peptide molecules. Because of the larger size of the aggregates it is difficult for the peptides to diffuse through the LPS-leaflet into the target cytoplasmic membrane, and therefore they lose activity against Gram-negative bacteria. However, the length of the fatty acid and the amino acid composition of the peptide chain can control aggregate formation as well as the ease at which they can dissociate. Therefore, finding the correct fatty acid length and peptide sequence is important for the peptides’ ability to traverse the outer LPS barrier and hence be active against Gram-negative bacteria [56].
In the case of other microorganisms, such as parasites of Leishmania genus, the cell surface of procyclic promastigotes (the insect stage of the parasite) is surrounded by a glycocalix layer, composed mainly by the lipophosphoglycan (LPG), a highly anionic molecule encompassing phosphorylated disaccharide repeating units and bound to the membrane through a glycosylphosphoinositol anchor [114]. LPG is absent in amastigotes (the intracellular pathological form of the parasite for vertebrates) [115], and this might explain the weak activity of the majority of cationic AMPs towards this stage of the parasite. Also metacyclic promastigotes (the circulating form of Leishmania in the blood of an infected mammal for about 24 h, before being engulfed by macrophages and transformed into amastigotes) are less sensitive to the activity of AMPs. This could be related to the fact that the number of repetitive units of LPG is double on the metacyclic stage of the parasite, favoring electrostatic interactions with AMPs, and thereby limiting the peptides’ partitioning into the cytoplasmic membrane. The small size and the low net positive charge of temporins could make it easier for them to cross the parasite’s glycocalix and permeate the membrane compared with other AMPs that have a higher net positive charge and can stick easily to the negatively charged cell surface. Furthermore, unlike a few natural AMPs that exhibit antiparasitic properties, temporins preserve activity against the more resistant morphological stage of Leishmania, the amastigote [116]. This suggests that ionic interactions between the peptide and the parasite do not contribute significantly to the potency of temporins. Indeed, electrostatic interactions between these peptides and the anionic components of the parasitic cells are not critical for their mechanism of action. This argument is corroborated by (1) the marginal decrease in antiparasitic activity in the presence of the anionic heparin; (2) the invariant peptide activity under marked changes in the ionic strength of the incubation media; and (3) the strong activity against the amastigote form of the parasite in which the LPG layer is replaced by glycosylphosphoinositol of neutral or zwitterionic character [81].
Combination of AMPs in the control of cell specificity
Cell specificity can also be controlled by a combination of AMPs. As described above, the frog skin contains a wide number of isoforms of the same AMP family. Studies aimed at understanding the biological significance of the presence of closely related AMPs in a single living organism have shown a synergistic effect among several of them, suggesting that a combination of various AMPs provides the animals with maximum coverage over a broad range of possible pathogenic microbes. This has been demonstrated with dermaseptins [117] and temporins [100]. The molecular basis of this synergism was investigated by using different temporin isoforms. It was found that two isoforms, not active on Gram-negative bacteria, can synergize to overcome bacterial resistance imposed by the LPS barrier when combined each with the temporin isoform active on Gram-negative bacteria [118]. More specifically, the two inactive isoforms oligomerize when in contact with the LPS-OM. As explained before, the larger size of the peptide oligomers should hamper their translocation into the IM of these bacteria. Mechanistically, the synergistic activity between temporins on Gram-negative bacteria is related to the ability of the active peptide to prevent the self-association of the inactive isoforms. Also, spectroscopic measurements have suggested that the inactive temporin isoforms bind mostly to those portions of LPS facing the solution and not to those in proximity with the inner lipid moiety, as in the case of the active temporin, which, instead, is able to penetrate into the hydrophobic region of LPS [119]. This synergism is highly dependent on the length of the polysaccharide region of LPS and tends to disappear as the length of the carbohydrate domain becomes shorter [52, 118]. It should be noted that mechanisms underlying the synergism between AMPs in the antibacterial activity have been reported only with peptides isolated from amphibian skin. Nevertheless, they probably exist in other families of AMPs.
LPS-neutralizing activity
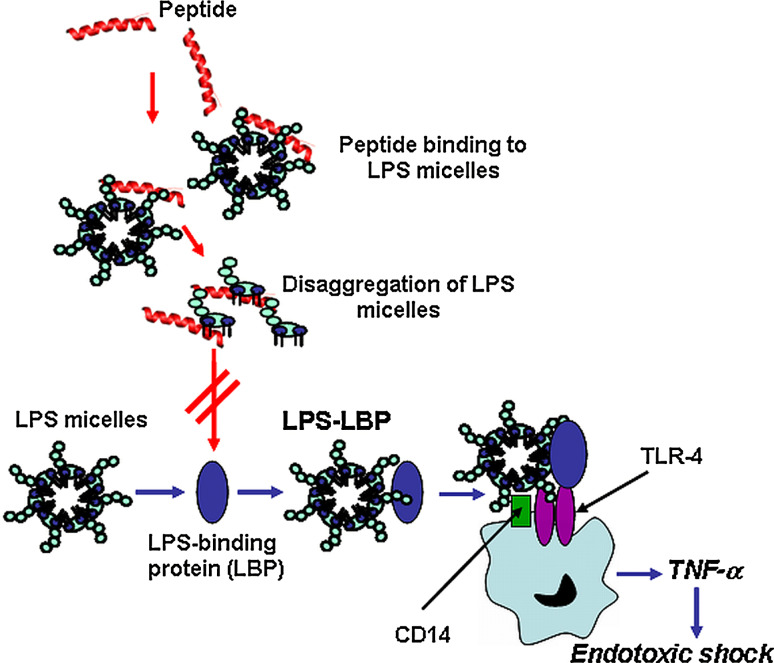
The immune system has evolved to recognize LPS as a pathogen-associated molecular pattern. Upon its recognition, LPS stimulates the innate immune cells (mainly mononuclear cells and macrophages), inducing the secretion of pro-inflammatory cytokines (e.g., tumor necrosis factor alpha, TNF-α, IL-1, IL-6) [120–122]. Antibiotic treatment is frequently associated with the release of LPS from the microbial cell wall [123, 124], giving rise to a prolonged activation of the immune system, accompanied by a massive extracellular release of cytokines. This causes a systemic inflammatory syndrome or sepsis, which, in extreme cases, leads to death [125, 126]. In contrast with conventional antibiotics, several AMPs possess dual functions: they kill bacteria and detoxify LPS. Although the exact mechanism is not yet well understood [70, 127–129], the activation mechanism of macrophages by LPS starts when LPS (through its toxic entity, lipid A) binds to the LPS-binding protein (LBP), accelerating the transfer of LPS to CD14, the primary receptor of LPS, mainly expressed on macrophages [130–132]. The LPS-CD14 complex initiates the intracellular signaling pathway by interacting with the transmembrane protein Toll-like receptor-4, which activates the NF-κB transcription factor, resulting in the production and secretion of pro-inflammatory cytokines [133–135]. In aqueous solution, LPS molecules aggregate into micelles, the biologically active form of the endotoxin. Note that disaggregation of these LPS micelles by the peptides is important to neutralize the toxic effect of LPS [100, 136] (see Fig. 2).
Fig. 2.
Schematic representation of the LPS-neutralizing activity of HDPs. Following the interaction between LPS micelles (the biologically active form of the endotoxin) and the carrier LBP protein, the LPS–LBP complex interacts with the CD14 membrane receptor (on macrophages) and together they bind and activate the TLR4-mediated intracellular signalling pathway for the cytokine secretion (e.g., TNF-α). If peptides bind and disaggregate LPS micelles to smaller size particles, the interaction of LPB with LPS is prevented, thereby blocking the production of TNF-α and hence limiting the development of septic shock
In the case of temporins, recent studies emphasized that the combination of the isoforms that synergize in the antimicrobial activity can also display a synergistic effect in the LPS-neutralization activity, by causing the breakage of LPS aggregates into smaller size particles, to a greater extent than that induced by each temporin alone. Importantly, this synergistic effect inversely correlates with the length of the polysaccharide chain of the endotoxin molecule and only occurs with LPS having short saccharidic portions [52].
In vivo activities of temporins and USLiPs
Both temporins and USLiPs have been shown to be active in vivo. The in vivo activity of temporins was recently analyzed using the well-characterized invertebrate Caenorhabditis elegans. C. elegans is a free-living, transparent nematode, about 1 mm in length that lives in temperate soil environments. It is a differentiated roundworm with a specialized nervous system, intestine, reproductive organs, and with relevant biological processes, i.e., absorption, transport, and distribution of nutrients through various tissues and cell types. Different species of microbes such as Pseudomonas aeruginosa, Staphylococcus aureus, and Candida albicans can pass through the mouth of the animal and invade its gut, where they proliferate and kill the worm in an infection-like process. It is worthwhile noting that many virulence factors used by these microorganisms to kill C. elegans also play an important function in inducing pathogenesis in mammals. In light of these features and considering the simple structure and short life cycle (<3 days) of the nematodes as well as the ability to grow them easily in the laboratory, C. elegans represents a very suitable and convenient animal model for identifying antimicrobial compounds lacking toxicity in vivo. In addition, the C. elegans genome has been fully sequenced and annotated (http://www.wormbase.org). Even if there is a huge difference between C. elegans and mammals, it can certainly provide an advanced tool for rapid and low-cost screening of antimicrobial substances in a living organism. It has been found that almost all temporins tested reduced lethality of nematodes infected by a multidrug-resistant strain of P. aeruginosa. Interestingly, an isoform of temporins appeared to be the most active molecule in vivo, although not active in vitro. The peptide increased worm survival by approximately fourfold after 40 h of peptide treatment. Furthermore, the number of Pseudomonas colonies in the intestinal lumen of the animal dropped fivefold in comparison to untreated infected worms, within 1 day of peptide treatment [137]. The ability of this temporin to reduce Pseudomonas cells within the worm gut was also visualized by fluorescence microscopy, using nematodes infected with a strain of P. aeruginosa expressing the green-fluorescent protein (GFP). Treated animals exhibited a clear and fast decrease in fluorescence intensity along the entire intestine already within the first 90 min incubation with the peptide, due to the death of GFP-expressing Pseudomonas. This finding suggests that the mode of action used by this peptide to promote worm survival is based on its direct effect on the pathogen growth, rather than making bacteria avirulent and therefore harmless [137].
The in vivo activity of the USLiPs was analyzed in mice models for fungal infection. The USLiPs were first tested against two models: invasive aspergillosis and invasive pulmonary aspergillosis. The data indicated that the USLiPs could specifically damage fungi structures in vivo in a mouse model. Moreover, one of the USLiPs was more efficient than amphotericin B at nontoxic doses. Indeed, intratracheal treatment with this USLiP provided prolonged survival and 25% total recovery among infected immunosuppressed mice, with fungal clearance revealed by histological examination [138]. In addition, no toxic effects associated with the treatment were noticed. Observation of lung sections of USLiP-treated mice revealed phagocytosed conidia within macrophages and dendritic cells on day 4 of treatment, which could be the origin of further invasive aspergillosis once the treatment is stopped.
In another study, the skin of the mouse was infected with the pathogenic C. albicans by subcutaneous injection of yeast spores complexed to cytodex beads [139]. Note that fungi of the genus Candida are part of the normal human flora; however, C. albicans can cause disease, and it is the most frequently isolated pathogen in humans [140, 141]. It is an ubiquitous fungal organism that often colonizes the skin and the mucosal surfaces of healthy individuals giving rise to a variety of different superficial diseases. However, when the host-defense mechanisms are impaired, C. albicans can cause serious systemic infections [142, 143]. The animals were treated by administering the USLiPs subcutaneously 1 h post infection, and the treatment was repeated for the next 3 days, at a single dose, every day. Histological examination of skin samples stained with periodic acid-Schiff indicated that the USLiPs significantly reduced the number of fungal spores and hyphal forms and inhibited C. albicans skin infection [57].
Conclusions
The extensive search for alternative therapeutics has led to the discovery of cationic antibiotic peptides as potential new anti-infective compounds with new modes of action. Because of the high cost for the commercial production of long peptides, particular interest has been given to small linear peptides that can be efficiently made by chemical synthesis at competitive costs and that have reduced or no immunogenicity. Although the number of promising candidates is very high and continues to increase, we focused this review on the largest family of short naturally occurring AMPs (i.e., the temporins), as well as on the engineered USLiPs. They are different in structure and composition, yet both classes of peptides share the following features: (1) an ability to permeate both artificial and natural biological membranes and (2) an ability to drastically change the target cell morphology, thus limiting the induction of microbial resistance.
Despite their similar mechanisms of microbicidal activity, it is not an easy task to predict the target cell selectivity of these molecules, since parameters such as their particular combination, their biophysical properties, and their interactions with components on the target cell surface all play a role. However, simple modification such as acylation of very short cationic peptides has resulted in a family of compounds named USLiPs, which show an improved antimicrobial activity and a higher stability to proteolysis in solution. This attractive alternative platform provides a way to rationally design novel anti-infective agents.
Acknowledgments
This study was supported by grants from La Sapienza University of Rome and the Italian Ministero dell’Istruzione, Università e Ricerca (PRIN 2008) and partially by the German Israel Foudation to Y.S. We thank Christopher Angusch for his comments and editing the manuscript.
Abbreviations
- AMP
Antimicrobial peptide
- GFP
Green fluorescent protein
- LiPs
Lipopeptides
- LPS
Lipopolysaccharide
- LPG
Lipophosphoglycan
- LTA
Lipoteichoic acid
- IM
Inner membrane
- MIC
Minimum inhibitory concentration
- OM
Outer membrane
- TNFα
Tumor necrosis factor alpha
- USLiPs
Ultrashort lipopeptides
References
- 1.Boman HG. Antibacterial peptides: basic facts and emerging concepts. J Intern Med. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 2.Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 3.Ganz T, Lehrer RI. Antimicrobial peptides of vertebrates. Curr Opin Immunol. 1998;10:41–44. doi: 10.1016/s0952-7915(98)80029-0. [DOI] [PubMed] [Google Scholar]
- 4.Hancock REW, Brown KL, Mookherjee N. Host defence peptides from invertebrates—emerging antimicrobial strategies. Immunobiology. 2006;211:315–322. doi: 10.1016/j.imbio.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 6.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 7.Peters BM, Shirtliff ME, Jabra-Rizk MA. Antimicrobial peptides: primeval molecules or future drugs? PLoS Pathog. 2010;6:e1001067. doi: 10.1371/journal.ppat.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinstraesser L, Kraneburg U, Jacobsen F, Al-Benna S. Host defense peptides and their antimicrobial-immunomodulatory duality. Immunobiology. 2011;216:322–333. doi: 10.1016/j.imbio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 10.Lehrer RI, Ganz T. Cathelicidins: a family of endogenous antimicrobial peptides. Curr Opin Hematol. 2002;9:18–22. doi: 10.1097/00062752-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Ganz T. Defensins and other antimicrobial peptides: a historical perspective and an update. Comb Chem High Throughput Screen. 2005;8:209–217. doi: 10.2174/1386207053764594. [DOI] [PubMed] [Google Scholar]
- 12.Steiner H, Hultmark D, Engstrom A, Bennich H, Boman HG. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981;292:246–248. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- 13.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci USA. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancock REW. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect Dis. 2001;1:156–164. doi: 10.1016/S1473-3099(01)00092-5. [DOI] [PubMed] [Google Scholar]
- 15.Sahl HG, Pag U, Bonness S, Wagner S, Antcheva N, Tossi A. Mammalian defensins: structures and mechanism of antibiotic activity. J Leukoc Biol. 2005;77:466–475. doi: 10.1189/jlb.0804452. [DOI] [PubMed] [Google Scholar]
- 16.Osterholm MT. Emerging infectious diseases. A real public health crisis? Postgrad Med. 1996;100:15–16. doi: 10.3810/pgm.1996.11.105. [DOI] [PubMed] [Google Scholar]
- 17.Maviglia R, Nestorini R, Pennisi M. Role of old antibiotics in multidrug resistant bacterial infections. Curr Drug Targets. 2009;10:895–905. doi: 10.2174/138945009789108846. [DOI] [PubMed] [Google Scholar]
- 18.Denning DW. Echinocandins and pneumocandins—a new antifungal class with a novel mode of action. J Antimicrob Chemother. 1997;40:611–614. doi: 10.1093/jac/40.5.611. [DOI] [PubMed] [Google Scholar]
- 19.Denning DW. Echinocandins: a new class of antifungal. J Antimicrob Chemother. 2002;49:889–891. doi: 10.1093/jac/dkf045. [DOI] [PubMed] [Google Scholar]
- 20.Straus SK, Hancock REW. Mode of action of the new antibiotic for Gram-positive pathogens daptomycin: comparison with cationic antimicrobial peptides and lipopeptides. Biochim Biophys Acta. 2006;1758:1215–1223. doi: 10.1016/j.bbamem.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Balkovec JM. Anti-infectives: lipopeptide antifungal agents. Expert Opin Invest Drugs. 1994;3:65–82. [Google Scholar]
- 22.De Lucca AJ, Walsh TJ. Antifungal peptides: novel therapeutic compounds against emerging pathogens. Antimicrob Agents Chemother. 1999;43:1–11. doi: 10.1128/aac.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassarello C, Lazzaroni S, Bifulco G, Lo Cantore P, Iacobellis NS, Riccio R, Gomez-Paloma L, Evidente A. Tolaasins A–E, five new lipodepsipeptides produced by Pseudomonas tolaasii . J Nat Prod. 2004;67:811–816. doi: 10.1021/np0303557. [DOI] [PubMed] [Google Scholar]
- 24.Peggion C, Formaggio F, Crisma M, Epand RF, Epand RM, Toniolo C. Trichogin: a paradigm for lipopeptaibols. J Pept Sci. 2003;9:679–689. doi: 10.1002/psc.500. [DOI] [PubMed] [Google Scholar]
- 25.Tsubery H, Ofek I, Cohen S, Fridkin M. N-terminal modifications of polymyxin B nonapeptide and their effect on antibacterial activity. Peptides. 2001;22:1675–1681. doi: 10.1016/s0196-9781(01)00503-4. [DOI] [PubMed] [Google Scholar]
- 26.Fiechter A. Biosurfactants: moving towards industrial application. Trends Biotechnol. 1992;10:208–217. doi: 10.1016/0167-7799(92)90215-h. [DOI] [PubMed] [Google Scholar]
- 27.Raaijmakers JM, de Bruijn I, de Kock MJ. Cyclic lipopeptide production by plant-associated Pseudomonas spp.: diversity, activity, biosynthesis, and regulation. Mol Plant Microbe Interact. 2006;19:699–710. doi: 10.1094/MPMI-19-0699. [DOI] [PubMed] [Google Scholar]
- 28.Jeu L, Fung HB. Daptomycin: a cyclic lipopeptide antimicrobial agent. Clin Ther. 2004;26:1728–1757. doi: 10.1016/j.clinthera.2004.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauermann R, Rothenburger M, Graninger W, Joukhadar C. Daptomycin: a review 4 years after first approval. Pharmacology. 2008;81:79–91. doi: 10.1159/000109868. [DOI] [PubMed] [Google Scholar]
- 30.Moudgal V, Little T, Boikov D, Vazquez JA. Multiechinocandin- and multiazole-resistant Candida parapsilosis isolates serially obtained during therapy for prosthetic valve endocarditis. Antimicrob Agents Chemother. 2005;49:767–769. doi: 10.1128/AAC.49.2.767-769.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avrahami D, Shai Y. Conjugation of a magainin analogue with lipophilic acids controls hydrophobicity, solution assembly, and cell selectivity. Biochemistry. 2002;41:2254–2263. doi: 10.1021/bi011549t. [DOI] [PubMed] [Google Scholar]
- 32.Chicharro C, Granata C, Lozano R, Andreu D, Rivas L. N-terminal fatty acid substitution increases the leishmanicidal activity of CA(1–7)M(2–9), a cecropin-4melittin hybrid peptide. Antimicrob Agents Chemother. 2001;45:2441–2449. doi: 10.1128/AAC.45.9.2441-2449.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lockwood NA, Haseman JR, Tirrell MV, Mayo KH. Acylation of SC4 dodecapeptide increases bactericidal potency against Gram-positive bacteria, including drug-resistant strains. Biochem J. 2004;378:93–103. doi: 10.1042/BJ20031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majerle A, Kidric J, Jerala R. Enhancement of antibacterial and lipopolysaccharide binding activities of a human lactoferrin peptide fragment by the addition of acyl chain. J Antimicrob Chemother. 2003;51:1159–1165. doi: 10.1093/jac/dkg219. [DOI] [PubMed] [Google Scholar]
- 35.Mak P, Pohl J, Dubin A, Reed MS, Bowers SE, Fallon MT, Shafer WM. The increased bactericidal activity of a fatty acid-modified synthetic antimicrobial peptide of human cathepsin G correlates with its enhanced capacity to interact with model membranes. Int J Antimicrob Agents. 2003;21:13–19. doi: 10.1016/s0924-8579(02)00245-5. [DOI] [PubMed] [Google Scholar]
- 36.Radzishevsky IS, Rotem S, Zaknoon F, Gaidukov L, Dagan A, Mor A. Effects of acyl versus aminoacyl conjugation on the properties of antimicrobial peptides. Antimicrob Agents Chemother. 2005;49:2412–2420. doi: 10.1128/AAC.49.6.2412-2420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wakabayashi H, Matsumoto H, Hashimoto K, Teraguchi S, Takase M, Hayasawa H. N-Acylated and D enantiomer derivatives of a nonamer core peptide of lactoferricin B showing improved antimicrobial activity. Antimicrob Agents Chemother. 1999;43:1267–1269. doi: 10.1128/aac.43.5.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avrahami D, Shai Y. Bestowing antifungal and antibacterial activities by lipophilic acid conjugation to d, l-amino acid-containing antimicrobial peptides: a plausible mode of action. Biochemistry. 2003;42:14946–14956. doi: 10.1021/bi035142v. [DOI] [PubMed] [Google Scholar]
- 39.Avrahami D, Shai Y. A new group of antifungal and antibacterial lipopeptides derived from non-membrane active peptides conjugated to palmitic acid. J Biol Chem. 2004;279:12277–12285. doi: 10.1074/jbc.M312260200. [DOI] [PubMed] [Google Scholar]
- 40.Malina A, Shai Y. Conjugation of fatty acids with different lengths modulates the antibacterial and antifungal activity of a cationic biologically inactive peptide. Biochem J. 2005;390:695–702. doi: 10.1042/BJ20050520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mookherjee N, Hancock REW. Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell Mol Life Sci. 2007;64:922–933. doi: 10.1007/s00018-007-6475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maget-Dana R, Peypoux F. Iturins, a special class of pore-forming lipopeptides: biological and physicochemical properties. Toxicology. 1994;87:151–174. doi: 10.1016/0300-483x(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 43.Maget-Dana R, Ptak M. Interactions of surfactin with membrane models. Biophys J. 1995;68:1937–1943. doi: 10.1016/S0006-3495(95)80370-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peypoux F, Bonmatin JM, Wallach J. Recent trends in the biochemistry of surfactin. Appl Microbiol Biotechnol. 1999;51:553–563. doi: 10.1007/s002530051432. [DOI] [PubMed] [Google Scholar]
- 45.Steenbergen JN, Alder J, Thorne GM, Tally FP. Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections. J Antimicrob Chemother. 2005;55:283–288. doi: 10.1093/jac/dkh546. [DOI] [PubMed] [Google Scholar]
- 46.Simmaco M, Mignogna G, Barra D. Antimicrobial peptides from amphibian skin: what do they tell us? Biopolymers. 1998;47:435–450. doi: 10.1002/(SICI)1097-0282(1998)47:6<435::AID-BIP3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 47.Rinaldi AC. Antimicrobial peptides from amphibian skin: an expanding scenario. Curr Opin Chem Biol. 2002;6:799–804. doi: 10.1016/s1367-5931(02)00401-5. [DOI] [PubMed] [Google Scholar]
- 48.Conlon JM, Iwamuro S, King JD. Dermal cytolytic peptides and the system of innate immunity in anurans. Ann NY Acad Sci. 2009;1163:75–82. doi: 10.1111/j.1749-6632.2008.03618.x. [DOI] [PubMed] [Google Scholar]
- 49.Miele R, Ponti D, Boman HG, Barra D, Simmaco M. Molecular cloning of a bombinin gene from Bombina orientalis: detection of NF-kappaB and NF-IL6 binding sites in its promoter. FEBS Lett. 1998;431:23–28. doi: 10.1016/s0014-5793(98)00718-2. [DOI] [PubMed] [Google Scholar]
- 50.Simmaco M, Mignogna G, Canofeni S, Miele R, Mangoni ML, Barra D. Temporins, antimicrobial peptides from the European red frog Rana temporaria . Eur J Biochem. 1996;242:788–792. doi: 10.1111/j.1432-1033.1996.0788r.x. [DOI] [PubMed] [Google Scholar]
- 51.Mangoni ML. Temporins, anti-infective peptides with expanding properties. Cell Mol Life Sci. 2006;63:1060–1069. doi: 10.1007/s00018-005-5536-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mangoni ML, Shai Y. Temporins and their synergism against Gram-negative bacteria and in lipopolysaccharide detoxification. Biochim Biophys Acta. 2009;1788:1610–1619. doi: 10.1016/j.bbamem.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 53.Rinaldi AC, Mangoni ML, Rufo A, Luzi C, Barra D, Zhao H, Kinnunen PK, Bozzi A, Di Giulio A, Simmaco M. Temporin L: antimicrobial, haemolytic and cytotoxic activities, and effects on membrane permeabilization in lipid vesicles. Biochem J. 2002;368:91–100. doi: 10.1042/BJ20020806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao H, Kinnunen PK. Modulation of the activity of secretory phospholipase A2 by antimicrobial peptides. Antimicrob Agents Chemother. 2003;47:965–971. doi: 10.1128/AAC.47.3.965-971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abbassi F, Lequin O, Piesse C, Goasdoue N, Foulon T, Nicolas P, Ladram A. Temporin-SHf, a new type of phe-rich and hydrophobic ultrashort antimicrobial peptide. J Biol Chem. 2010;285:16880–16892. doi: 10.1074/jbc.M109.097204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Makovitzki A, Avrahami D, Shai Y. Ultrashort antibacterial and antifungal lipopeptides. Proc Natl Acad Sci USA. 2006;103:15997–16002. doi: 10.1073/pnas.0606129103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Makovitzki A, Baram J, Shai Y. Antimicrobial lipopolypeptides composed of palmitoyl di- and tricationic peptides: in vitro and in vivo activities, self-assembly to nanostructures, and a plausible mode of action. Biochemistry. 2008;47:10630–10636. doi: 10.1021/bi8011675. [DOI] [PubMed] [Google Scholar]
- 58.Shai Y, Makovitzky A, Avrahami D. Host defense peptides and lipopeptides: modes of action and potential candidates for the treatment of bacterial and fungal infections. Curr Protein Pept Sci. 2006;7:479–486. doi: 10.2174/138920306779025620. [DOI] [PubMed] [Google Scholar]
- 59.Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem J. 1999;341:501–513. [PMC free article] [PubMed] [Google Scholar]
- 60.Papo N, Braunstein A, Eshhar Z, Shai Y. Suppression of human prostate tumor growth in mice by a cytolytic d-, l-amino acid peptide: membrane lysis, increased necrosis, and inhibition of prostate-specific antigen secretion. Cancer Res. 2004;64:5779–5786. doi: 10.1158/0008-5472.CAN-04-1438. [DOI] [PubMed] [Google Scholar]
- 61.Papo N, Oren Z, Pag U, Sahl HG, Shai Y. The consequence of sequence alteration of an amphipathic alpha-helical antimicrobial peptide and its diastereomers. J Biol Chem. 2002;277:33913–33921. doi: 10.1074/jbc.M204928200. [DOI] [PubMed] [Google Scholar]
- 62.Papo N, Seger D, Makovitzki A, Kalchenko V, Eshhar Z, Degani H, Shai Y. Inhibition of tumor growth and elimination of multiple metastases in human prostate and breast xenografts by systemic inoculation of a host defense-like lytic peptide. Cancer Res. 2006;66:5371–5378. doi: 10.1158/0008-5472.CAN-05-4569. [DOI] [PubMed] [Google Scholar]
- 63.Radzishevsky IS, Rotem S, Bourdetsky D, Navon-Venezia S, Carmeli Y, Mor A. Improved antimicrobial peptides based on acyl-lysine oligomers. Nat Biotechnol. 2007;25:657–659. doi: 10.1038/nbt1309. [DOI] [PubMed] [Google Scholar]
- 64.Rotem S, Radzishevsky IS, Bourdetsky D, Navon-Venezia S, Carmeli Y, Mor A. Analogous oligo-acyl-lysines with distinct antibacterial mechanisms. FASEB J. 2008;22:2652–2661. doi: 10.1096/fj.07-105015. [DOI] [PubMed] [Google Scholar]
- 65.Sarig H, Rotem S, Ziserman L, Danino D, Mor A. Impact of self-assembly properties on antibacterial activity of short acyl-lysine oligomers. Antimicrob Agents Chemother. 2008;52:4308–4314. doi: 10.1128/AAC.00656-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lehrer RI, Ganz T. Antimicrobial peptides in mammalian and insect host defence. Curr Opin Immunol. 1999;11:23–27. doi: 10.1016/s0952-7915(99)80005-3. [DOI] [PubMed] [Google Scholar]
- 67.Shai Y. From innate immunity to de novo designed antimicrobial peptides. Curr Pharm Des. 2002;8:715–725. doi: 10.2174/1381612023395367. [DOI] [PubMed] [Google Scholar]
- 68.Marcellini L, Borro M, Gentile G, Rinaldi AC, Stella L, Aimola P, Barra D, Mangoni ML. Esculentin-1b(1–18)-a membrane-active antimicrobial peptide that synergizes with antibiotics and modifies the expression level of a limited number of proteins in Escherichia coli . FEBS J. 2009;276:5647–5664. doi: 10.1111/j.1742-4658.2009.07257.x. [DOI] [PubMed] [Google Scholar]
- 69.Coccia C, Rinaldi AC, Luca V, Barra D, Bozzi A, Di Giulio A, Veerman EC, Mangoni ML. Membrane interaction and antibacterial properties of two mildly cationic peptide diastereomers, bombinins H2 and H4, isolated from Bombina skin. Eur Biophys J. 2011;40(4):577–588. doi: 10.1007/s00249-011-0681-8. [DOI] [PubMed] [Google Scholar]
- 70.Hancock REW, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8:402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 71.Patrzykat A, Friedrich CL, Zhang L, Mendoza V, Hancock REW. Sublethal concentrations of pleurocidin-derived antimicrobial peptides inhibit macromolecular synthesis in Escherichia coli . Antimicrob Agents Chemother. 2002;46:605–614. doi: 10.1128/AAC.46.03.605-614.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Subbalakshmi C, Sitaram N. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol Lett. 1998;160:91–96. doi: 10.1111/j.1574-6968.1998.tb12896.x. [DOI] [PubMed] [Google Scholar]
- 73.Chen W, Wang J, An H, Zhou J, Zhang L, Cao X. Heat shock up-regulates TLR9 expression in human B cells through activation of ERK and NF-kappaB signal pathways. Immunol Lett. 2005;98:153–159. doi: 10.1016/j.imlet.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 74.Funderburg N, Lederman MM, Feng Z, Drage MG, Jadlowsky J, Harding CV, Weinberg A, Sieg SF. Human-defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc Natl Acad Sci USA. 2007;104:18631–18635. doi: 10.1073/PNAS.0702130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu H, Arron JR. TRAF6, a molecular bridge spanning adaptive immunity, innate immunity and osteoimmunology. Bioessays. 2003;25:1096–1105. doi: 10.1002/bies.10352. [DOI] [PubMed] [Google Scholar]
- 76.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 77.Nicholls EF, Madera L, Hancock RE. Immunomodulators as adjuvants for vaccines and antimicrobial therapy. Ann NY Acad Sci. 2010;1213:46–61. doi: 10.1111/j.1749-6632.2010.05787.x. [DOI] [PubMed] [Google Scholar]
- 78.Rosenfeld Y, Sahl HG, Shai Y. Parameters involved in antimicrobial and endotoxin detoxification activities of antimicrobial peptides. Biochemistry. 2008;47:6468–6478. doi: 10.1021/bi800450f. [DOI] [PubMed] [Google Scholar]
- 79.Bechinger B, Lohner K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1529–1539. doi: 10.1016/j.bbamem.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 80.Lohner K. New strategies for novel antibiotics: peptides targeting bacterial cell membranes. Gen Physiol Biophys. 2009;28:105–116. doi: 10.4149/gpb_2009_02_105. [DOI] [PubMed] [Google Scholar]
- 81.Wassef MK, Fioretti TB, Dwyer DM. Lipid analyses of isolated surface membranes of Leishmania donovani promastigotes. Lipids. 1985;20:108–115. doi: 10.1007/BF02534216. [DOI] [PubMed] [Google Scholar]
- 82.Ehrenstein G, Lecar H. Electrically gated ionic channels in lipid bilayers. Q Rev Biophys. 1977;10:1–34. doi: 10.1017/s0033583500000123. [DOI] [PubMed] [Google Scholar]
- 83.Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers. 2002;66:236–248. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 84.Ludtke SJ, He K, Heller WT, Harroun TA, Yang L, Huang HW. Membrane pores induced by magainin. Biochemistry. 1996;35:13723–13728. doi: 10.1021/bi9620621. [DOI] [PubMed] [Google Scholar]
- 85.Matsuzaki K, Murase O, Miyajima K. Kinetics of pore formation by an antimicrobial peptide, magainin 2, in phospholipid bilayers. Biochemistry. 1995;34:12553–12559. doi: 10.1021/bi00039a009. [DOI] [PubMed] [Google Scholar]
- 86.Hancock REW, Rozek A. Role of membranes in the activities of antimicrobial cationic peptides. FEMS Microbiol Lett. 2002;206:143–149. doi: 10.1111/j.1574-6968.2002.tb11000.x. [DOI] [PubMed] [Google Scholar]
- 87.Mangoni ML, Papo N, Barra D, Simmaco M, Bozzi A, Di Giulio A, Rinaldi AC. Effects of the antimicrobial peptide temporin L on cell morphology, membrane permeability and viability of Escherichia coli . Biochem J. 2004;380:859–865. doi: 10.1042/BJ20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steffen H, Rieg S, Wiedemann I, Kalbacher H, Deeg M, Sahl HG, Peschel A, Gotz F, Garbe C, Schittek B. Naturally processed dermcidin-derived peptides do not permeabilize bacterial membranes and kill microorganisms irrespective of their charge. Antimicrob Agents Chemother. 2006;50:2608–2620. doi: 10.1128/AAC.00181-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bera A, Singh S, Nagaraj R, Vaidya T. Induction of autophagic cell death in Leishmania donovani by antimicrobial peptides. Mol Biochem Parasitol. 2003;127:23–35. doi: 10.1016/s0166-6851(02)00300-6. [DOI] [PubMed] [Google Scholar]
- 90.Georgopapadakou NH, Tkacz JS. The fungal cell wall as a drug target. Trends Microbiol. 1995;3:98–104. doi: 10.1016/s0966-842x(00)88890-3. [DOI] [PubMed] [Google Scholar]
- 91.Kartsonis NA, Nielsen J, Douglas CM. Caspofungin: the first in a new class of antifungal agents. Drug Resist Updat. 2003;6:197–218. doi: 10.1016/s1368-7646(03)00064-5. [DOI] [PubMed] [Google Scholar]
- 92.Ullmann AJ. Review of the safety, tolerability, and drug interactions of the new antifungal agents caspofungin and voriconazole. Curr Med Res Opin. 2003;19:263–271. doi: 10.1185/030079903125001884. [DOI] [PubMed] [Google Scholar]
- 93.Debono M, Gordee RS. Antibiotics that inhibit fungal cell wall development. Annu Rev Microbiol. 1994;48:471–497. doi: 10.1146/annurev.mi.48.100194.002351. [DOI] [PubMed] [Google Scholar]
- 94.Evans ME, Feola DJ, Rapp RP. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann Pharmacother. 1999;33:960–967. doi: 10.1345/aph.18426. [DOI] [PubMed] [Google Scholar]
- 95.Toniolo C, Crisma M, Formaggio F, Peggion C, Epand RF, Epand RM. Lipopeptaibols, a novel family of membrane active, antimicrobial peptides. Cell Mol Life Sci. 2001;58:1179–1188. doi: 10.1007/PL00000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001;7:167–202. [PubMed] [Google Scholar]
- 97.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994;264:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- 99.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rosenfeld Y, Barra D, Simmaco M, Shai Y, Mangoni ML. A synergism between temporins toward Gram-negative bacteria overcomes resistance imposed by the lipopolysaccharide protective layer. J Biol Chem. 2006;281:28565–28574. doi: 10.1074/jbc.M606031200. [DOI] [PubMed] [Google Scholar]
- 101.Papo N, Shai Y. A molecular mechanism for lipopolysaccharide protection of Gram-negative bacteria from antimicrobial peptides. J Biol Chem. 2005;280:10378–10387. doi: 10.1074/jbc.M412865200. [DOI] [PubMed] [Google Scholar]
- 102.Sal-Man N, Oren Z, Shai Y. Preassembly of membrane-active peptides is an important factor in their selectivity toward target cells. Biochemistry. 2002;41:11921–11930. doi: 10.1021/bi0260482. [DOI] [PubMed] [Google Scholar]
- 103.Trent MS, Stead CM, Tran AX, Hankins JV. Diversity of endotoxin and its impact on pathogenesis. J Endotoxin Res. 2006;12:205–223. doi: 10.1179/096805106X118825. [DOI] [PubMed] [Google Scholar]
- 104.Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zahringer U, Seydel U, Di Padova F, Schreier M, Brade H. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 105.Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother. 1989;33:1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Friedrich CL, Moyles D, Beveridge TJ, Hancock REW. Antibacterial action of structurally diverse cationic peptides on gram-positive bacteria. Antimicrob Agents Chemother. 2000;44:2086–2092. doi: 10.1128/aac.44.8.2086-2092.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Papo N, Shai Y. Host defense peptides as new weapons in cancer treatment. Cell Mol Life Sci. 2005;62:784–790. doi: 10.1007/s00018-005-4560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Allende D, McIntosh TJ. Lipopolysaccharides in bacterial membranes act like cholesterol in eukaryotic plasma membranes in providing protection against melittin-induced bilayer lysis. Biochemistry. 2003;42:1101–1108. doi: 10.1021/bi026932s. [DOI] [PubMed] [Google Scholar]
- 109.Andra J, Koch MH, Bartels R, Brandenburg K. Biophysical characterization of endotoxin inactivation by NK-2, an antimicrobial peptide derived from mammalian NK-lysin. Antimicrob Agents Chemother. 2004;48:1593–1599. doi: 10.1128/AAC.48.5.1593-1599.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mangoni ML, Rinaldi AC, Di Giulio A, Mignogna G, Bozzi A, Barra D, Simmaco M. Structure-function relationships of temporins, small antimicrobial peptides from amphibian skin. Eur J Biochem. 2000;267:1447–1454. doi: 10.1046/j.1432-1327.2000.01143.x. [DOI] [PubMed] [Google Scholar]
- 111.Makovitzki A, Shai Y. pH-dependent antifungal lipopeptides and their plausible mode of action. Biochemistry. 2005;44:9775–9784. doi: 10.1021/bi0502386. [DOI] [PubMed] [Google Scholar]
- 112.Domanov YA, Kinnunen PK. Antimicrobial peptides temporins B and L induce formation of tubular lipid protrusions from supported phospholipid bilayers. Biophys J. 2006;91:4427–4439. doi: 10.1529/biophysj.106.091702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mahalka AK, Kinnunen PK. Binding of amphipathic alpha-helical antimicrobial peptides to lipid membranes: lessons from temporins B and L. Biochim Biophys Acta. 2009;1788:1600–1609. doi: 10.1016/j.bbamem.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 114.Turco SJ, Hull SR, Orlandi PA, Shepherd SD, Homans SW, Dwek RA, Rademacher TW. Structure of the major carbohydrate fragment of the Leishmania donovani lipophosphoglycan. Biochemistry. 1987;26:6233–6238. doi: 10.1021/bi00393a042. [DOI] [PubMed] [Google Scholar]
- 115.McConville MJ, Mullin KA, Ilgoutz SC, Teasdale RD. Secretory pathway of trypanosomatid parasites. Microbiol Mol Biol Rev. 2002;66:122–154. doi: 10.1128/MMBR.66.1.122-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mangoni ML, Saugar JM, Dellisanti M, Barra D, Simmaco M, Rivas L. Temporins, small antimicrobial peptides with leishmanicidal activity. J Biol Chem. 2005;280:984–990. doi: 10.1074/jbc.M410795200. [DOI] [PubMed] [Google Scholar]
- 117.Mor A, Hani K, Nicolas P. The vertebrate peptide antibiotics dermaseptins have overlapping structural features but target specific microorganisms. J Biol Chem. 1994;269:31635–31641. [PubMed] [Google Scholar]
- 118.Mangoni ML, Epand RF, Rosenfeld Y, Peleg A, Barra D, Epand RM, Shai Y. Lipopolysaccharide, a key molecule involved in the synergism between temporins in inhibiting bacterial growth and in endotoxin neutralization. J Biol Chem. 2008;283:22907–22917. doi: 10.1074/jbc.M800495200. [DOI] [PubMed] [Google Scholar]
- 119.Giacometti A, Cirioni O, Ghiselli R, Mocchegiani F, Orlando F, Silvestri C, Bozzi A, Di Giulio A, Luzi C, Mangoni ML, Barra D, Saba V, Scalise G, Rinaldi AC. Interaction of antimicrobial peptide temporin L with lipopolysaccharide in vitro and in experimental rat models of septic shock caused by gram-negative bacteria. Antimicrob Agents Chemother. 2006;50:2478–2486. doi: 10.1128/AAC.01553-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kubo Y, Fukuishi N, Yoshioka M, Kawasoe Y, Iriguchi S, Imajo N, Yasui Y, Matsui N, Akagi M. Bacterial components regulate the expression of Toll-like receptor 4 on human mast cells. Inflamm Res. 2007;56:70–75. doi: 10.1007/s00011-006-6064-4. [DOI] [PubMed] [Google Scholar]
- 121.Gee K, Kozlowski M, Kumar A. Tumor necrosis factor-alpha induces functionally active hyaluronan-adhesive CD44 by activating sialidase through p38 mitogen-activated protein kinase in lipopolysaccharide-stimulated human monocytic cells. J Biol Chem. 2003;278:37275–37287. doi: 10.1074/jbc.M302309200. [DOI] [PubMed] [Google Scholar]
- 122.Mukhopadhyay S, Herre J, Brown GD, Gordon S. The potential for Toll-like receptors to collaborate with other innate immune receptors. Immunology. 2004;112:521–530. doi: 10.1111/j.1365-2567.2004.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Prins JM, Kuijper EJ, Mevissen ML, Speelman P, van Deventer SJ. Release of tumor necrosis factor alpha and interleukin 6 during antibiotic killing of Escherichia coli in whole blood: influence of antibiotic class, antibiotic concentration, and presence of septic serum. Infect Immun. 1995;63:2236–2242. doi: 10.1128/iai.63.6.2236-2242.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Byl B, Clevenbergh P, Kentos A, Jacobs F, Marchant A, Vincent JL, Thys JP. Ceftazidime and imipenem-induced endotoxin release during treatment of gram-negative infections. Eur J Clin Microbiol Infect Dis. 2001;20:804–807. doi: 10.1007/s100960100609. [DOI] [PubMed] [Google Scholar]
- 125.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 126.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 127.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock REW. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 128.Bommineni YR, Dai H, Gong YX, Soulages JL, Fernando SC, Desilva U, Prakash O, Zhang G. Fowlicidin-3 is an alpha-helical cationic host defense peptide with potent antibacterial and lipopolysaccharide-neutralizing activities. FEBS J. 2007;274:418–428. doi: 10.1111/j.1742-4658.2006.05589.x. [DOI] [PubMed] [Google Scholar]
- 129.Mookherjee N, Rehaume LM, Hancock RE. Cathelicidins and functional analogues as antisepsis molecules. Expert Opin Ther Targets. 2007;11:993–1004. doi: 10.1517/14728222.11.8.993. [DOI] [PubMed] [Google Scholar]
- 130.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 131.Schumann RR, Leong SR, Flaggs GW, Gray PW, Wright SD, Mathison JC, Tobias PS, Ulevitch RJ. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 132.Tobias PS, Ulevitch RJ. Lipopolysaccharide binding protein and CD14 in LPS dependent macrophage activation. Immunobiology. 1993;187:227–232. doi: 10.1016/S0171-2985(11)80341-4. [DOI] [PubMed] [Google Scholar]
- 133.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 134.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 135.Jiang Q, Akashi S, Miyake K, Petty HR. Lipopolysaccharide induces physical proximity between CD14 and toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa B. J Immunol. 2000;165:3541–3544. doi: 10.4049/jimmunol.165.7.3541. [DOI] [PubMed] [Google Scholar]
- 136.Rosenfeld Y, Lev N, Shai Y. Effect of the hydrophobicity to net positive charge ratio on antibacterial and anti-endotoxin activities of structurally similar antimicrobial peptides. Biochemistry. 2010;49:361–853. doi: 10.1021/bi900724x. [DOI] [PubMed] [Google Scholar]
- 137.Uccelletti D, Zanni E, Marcellini L, Palleschi C, Barra D, Mangoni ML. Anti-Pseudomonas activity of frog skin antimicrobial peptides in a Caenorhabditis elegans infection model: a plausible mode of action in vitro and in vivo. Antimicrob Agents Chemother. 2010;54:3853–3860. doi: 10.1128/AAC.00154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vallon-Eberhard A, Landsman L, Yogev N, Verrier B, Jung S. Transepithelial pathogen uptake into the small intestinal lamina propria. J Immunol. 2006;176:2465–2469. doi: 10.4049/jimmunol.176.4.2465. [DOI] [PubMed] [Google Scholar]
- 139.Lopez-Garcia B, Lee PH, Yamasaki K, Gallo RL. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol. 2005;125:108–115. doi: 10.1111/j.0022-202X.2005.23713.x. [DOI] [PubMed] [Google Scholar]
- 140.Buentke E, Scheynius A. Dendritic cells and fungi. APMIS. 2003;111:789–796. doi: 10.1034/j.1600-0463.2003.11107810.x. [DOI] [PubMed] [Google Scholar]
- 141.Van Burik JA, Magee PT. Aspects of fungal pathogenesis in humans. Annu Rev Microbiol. 2001;55:743–772. doi: 10.1146/annurev.micro.55.1.743. [DOI] [PubMed] [Google Scholar]
- 142.Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- 143.Filler SG, Sheppard DC. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog. 2006;2:e129. doi: 10.1371/journal.ppat.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lu Y, Li J, Yu H, Xu X, Liang J, Tian Y, Ma D, Lin G, Huang G, Lai R. Two families of antimicrobial peptides with multiple functions from skin of rufous-spotted torrent frog, Amolops loloensis . Peptides. 2006;27:3085–3091. doi: 10.1016/j.peptides.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 145.Ali MF, Lips KR, Knoop FC, Fritzsch B, Miller C, Conlon JM. Antimicrobial peptides and protease inhibitors in the skin secretions of the crawfish frog, Rana areolata . Biochim Biophys Acta. 2002;1601:55–63. doi: 10.1016/s1570-9639(02)00432-6. [DOI] [PubMed] [Google Scholar]
- 146.Conlon J. The temporins. In: Kastin AJ, editor. Handbook of biologically active peptides. San Diego: Elsevier; 2006. pp. 305–309. [Google Scholar]
- 147.Conlon JM, Sonnevend A, Patel M, Davidson C, Nielsen PF, Pal T, Rollins-Smith LA. Isolation of peptides of the brevinin-1 family with potent candidacidal activity from the skin secretions of the frog Rana boylii . J Pept Res. 2003;62:207–213. doi: 10.1034/j.1399-3011.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- 148.Halverson T, Basir YJ, Knoop FC, Conlon JM. Purification and characterization of antimicrobial peptides from the skin of the North American green frog Rana clamitans . Peptides. 2000;21:469–476. doi: 10.1016/s0196-9781(00)00178-9. [DOI] [PubMed] [Google Scholar]
- 149.Ali MF, Knoop FC, Vaudry H, Conlon JM. Characterization of novel antimicrobial peptides from the skins of frogs of the Rana esculenta complex. Peptides. 2003;24:955–961. doi: 10.1016/s0196-9781(03)00193-1. [DOI] [PubMed] [Google Scholar]
- 150.Conlon JM, Al-Kharrge R, Ahmed E, Raza H, Galadari S, Condamine E. Effect of aminoisobutyric acid (Aib) substitutions on the antimicrobial and cytolytic activities of the frog skin peptide, temporin-1DRa. Peptides. 2007;28:2075–2080. doi: 10.1016/j.peptides.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 151.Kim JB, Halverson T, Basir YJ, Dulka J, Knoop FC, Abel PW, Conlon JM. Purification and characterization of antimicrobial and vasorelaxant peptides from skin extracts and skin secretions of the North American pig frog Rana grylio . Regul Pept. 2000;90:53–60. doi: 10.1016/s0167-0115(00)00107-5. [DOI] [PubMed] [Google Scholar]
- 152.Zhou J, McClean S, Thompson A, Zhang Y, Shaw C, Rao P, Bjourson AJ. Purification and characterization of novel antimicrobial peptides from the skin secretion of Hylarana guentheri. Peptides. 2006;27:3077–3084. doi: 10.1016/j.peptides.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 153.Conlon JM, Coquet L, Leprince J, Jouenne T, Vaudry H, Kolodziejek J, Nowotny N, Bevier CR, Moler PE. Peptidomic analysis of skin secretions from Rana heckscheri and Rana okaloosae provides insight into phylogenetic relationships among frogs of the Aquarana species group. Regul Pept. 2007;138:87–93. doi: 10.1016/j.regpep.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 154.Goraya J, Wang Y, Li Z, O’Flaherty M, Knoop FC, Platz JE, Conlon JM. Peptides with antimicrobial activity from four different families isolated from the skins of the North American frogs Rana luteiventris, Rana berlandieri and Rana pipiens . Eur J Biochem. 2000;267:894–900. doi: 10.1046/j.1432-1327.2000.01074.x. [DOI] [PubMed] [Google Scholar]
- 155.Rollins-Smith LA, Doersam JK, Longcore JE, Taylor SK, Shamblin JC, Carey C, Zasloff M. Antimicrobial peptide defenses against pathogens associated with global amphibian declines. Dev Comp Immunol. 2002;26:63–72. doi: 10.1016/s0145-305x(01)00041-6. [DOI] [PubMed] [Google Scholar]
- 156.Kim JB, Iwamuro S, Knoop FC, Conlon JM. Antimicrobial peptides from the skin of the Japanese mountain brown frog, Rana ornativentris . J Pept Res. 2001;58:349–356. doi: 10.1034/j.1399-3011.2001.00947.x. [DOI] [PubMed] [Google Scholar]
- 157.Bevier CR, Sonnevend A, Kolodziejek J, Nowotny N, Nielsen PF, Conlon JM. Purification and characterization of antimicrobial peptides from the skin secretions of the mink frog (Rana septentrionalis) Comp Biochem Physiol C Toxicol Pharmacol. 2004;139:31–38. doi: 10.1016/j.cca.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 158.Conlon JM, Sonnevend A, Patel M, Camasamudram V, Nowotny N, Zilahi E, Iwamuro S, Nielsen PF, Pal T. A melittin-related peptide from the skin of the Japanese frog, Rana tagoi, with antimicrobial and cytolytic properties. Biochem Biophys Res Commun. 2003;306:496–500. doi: 10.1016/s0006-291x(03)00999-9. [DOI] [PubMed] [Google Scholar]
- 159.Conlon JM, Abraham B, Sonnevend A, Jouenne T, Cosette P, Leprince J, Vaudry H, Bevier CR. Purification and characterization of antimicrobial peptides from the skin secretions of the carpenter frog Rana virgatipes (Ranidae, Aquarana) Regul Pept. 2005;131:38–45. doi: 10.1016/j.regpep.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 160.Chen T, Zhou M, Rao P, Walker B, Shaw C. The Chinese bamboo leaf odorous frog (Rana (Odorrana) versabilis) and North American Rana frogs share the same families of skin antimicrobial peptides. Peptides. 2006;27:1738–1744. doi: 10.1016/j.peptides.2006.02.009. [DOI] [PubMed] [Google Scholar]