Abstract
A hallmark of living systems is the management and the storage of information through genetic and epigenetic mechanisms. Although the notion of epigenetics was originally given to any regulation beyond DNA sequence, it has often been restricted to chromatin modifications, supposed to behave as cis-markers, specifying the sets of genes to be expressed or repressed. This definition does not take into account the initial view of epigenetics, based on nonlinear interaction networks whose “attractors” can remain stable without need for any chromatin mark. In addition, most chromatin modifications are the steady state resultants of highly dynamic modification and de-modification activities and, as such, seem poorly appropriate to work as long-term memory keepers. Instead, the basic support of epigenetic memory could remain the attractors, to which chromatin modifications belong as do many other components. The influence of chromatin modifications in memory is highly questionable when envisioned as static structural marks, but can be recovered under the dynamic circuitry perspective, thanks to their self-templating properties. Beside their standard repressive or permissive functions, chromatin modifications can also influence transcription in multiple ways such as: (1) by randomizing or inversely stabilizing gene expression, (2) by mediating cooperativity between pioneer and secondary transcription factors, and (3) in the hysteresis and the ultrasensitivity of gene expression switches, allowing the cells to take unambiguous transcriptional decisions.
Keywords: Epigenetics, Chromatin modifications, Attractors, Gene regulatory networks
Introduction
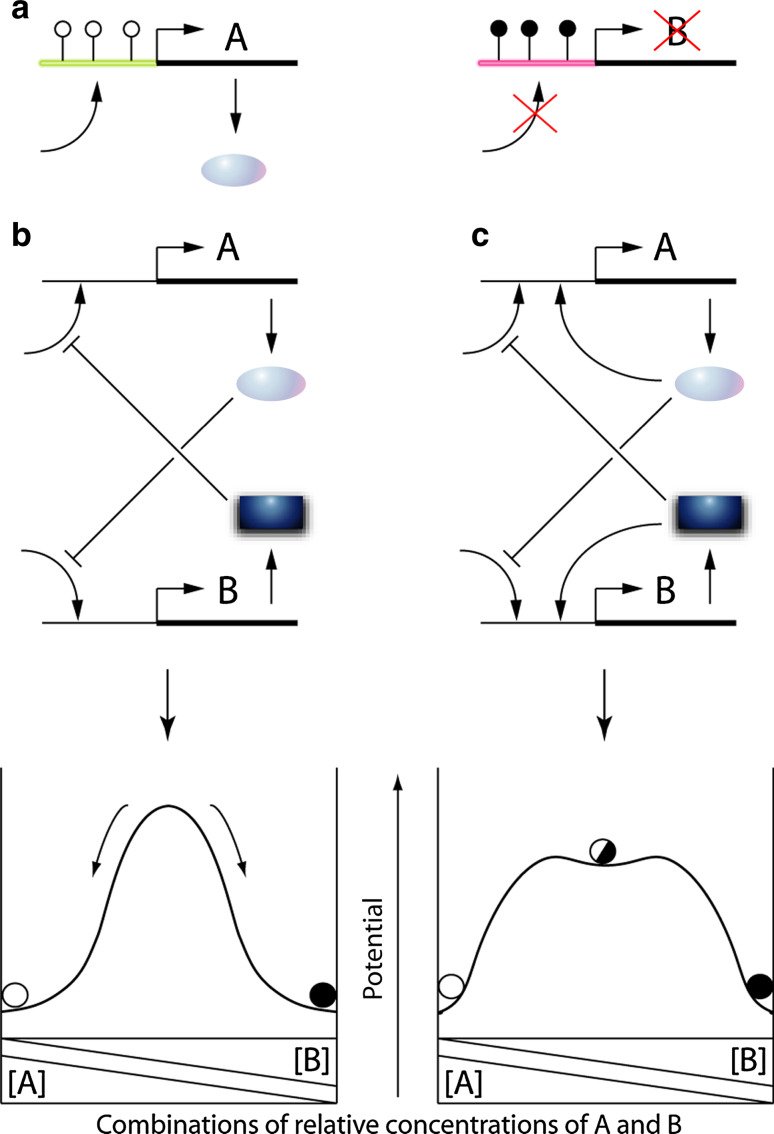
Beyond the fundamental genetic memory inscribed in DNA sequences and which allows the organisms to retain their identity, other memory mechanisms exist, called epigenetic. By memory in this context, we understand the persistence of cellular behaviors after disappearance of their initial cause. The epigenetic maintenance of gene expression memory were first established in bacteria. The induced or uninduced state of the E. coli lactose operon (lac) [1], or the capacity or incapacity of B subtilis to incorporate exogenous DNA [2], are perpetuated to their descendants without any change in DNA sequence (and, as explained in this paper, without need for gene marking). Memory effects are even more obvious in metazoans. Although most cells from a given organism have the same genome, they retain the cell-type-specific pattern of gene expression selected during embryonic differentiation and transmit it to their lineage. Furthermore, cells with both the same genome and the same differentiation state can recall certain specific treatments or exposures experienced in the past, which can influence their responses to future stimulations. The success of nuclear transfer experiments shows that most somatic cells retain the integrity of the zygotic genome but can forget their epigenetic status, which is thus erasable. Epigenetics attracted considerable attention during the past decades but, intriguingly, from different perspectives depending on the different research areas. To simplify the situation, for theoretical and modeling biologists, stable gene expression profiles essentially result from gene network configurations called attractors, whereas for most classical “eukaryotist” biologists, they rely on structural chromatin marks dictating the ability of genes to be expressed or not, depending on the cell type (Fig. 1a). This latter view neglects the attractor hypothesis and underestimates the dynamic nature of chromatin modifications. By inverting the hierarchy, chromatin marks could preferably be considered as actors of dynamical networks, which contribute to shaping high dimensional attractors. The aim of the present report is not to minimize the importance of chromatin modifications but to restore their place in biological causation and, more importantly, to suggest that we would benefit from integrating attractors into our mode of thinking and our research strategies on chromatin.
Fig. 1.
Chromatin marking versus attractor views of gene expression memory. a Persistent chromatin marking of the cis-regulatory regions of the A- and B-encoding genes, differentially designate them as expressed and repressed, respectively. b From the attractor perspective, the same result as in (a) can be obtained with a simple mutual inhibition circuit, starting from an initial condition in which A is slightly predominant. This configuration generates two attractors in which only A or B is expressed (bottom panel). c Variant scheme in which, in addition to the previous one, each gene is stimulated by its own product. The bottom panels represent sections of the corresponding 3D landscape in a plane [A]+[B] = constant. In these landscapes, inactivating transiently one gene product is sufficient to impose the migration of the system from one attractor to the other one
Static versus dynamic epigenetic memory
The two challenging conceptions of epigenetic memories can be summarized as follows.
Structural reminders
For a large fraction of searchers, epigenetics corresponds to chromatin modifications, including the structural, often covalent, chemical modifications of DNA and/or histones and the replacement of classical histones by variant isoforms [3]. These modifications are supposed to lock genes in expressible or repressed states [4–7], and also to stabilize the intensity of gene expression regardless of its level [8], which is also a sort of memory. The transcriptional instructions of chromatin marks can be read directly by transcription machineries or by specific proteins domains such as bromodomains [9]. Alternatively, the effects of chromatin marks can be indirectly mediated by higher-order chromatin structure [7], preventing transactivators from accessing the regulatory sequences of repressed genes, said to be closed. Sub-nuclear gene positioning [10, 11], or the three-dimensional genome organization [12], are also structural determinants of gene expression. Occasionally, during reprogramming, these modifications can be actively erased and reset [5]. To symbolize the principle of chromatin marking, Fig. 1a represents two genes, encoding A and B products and marked as to be expressed and repressed, respectively. These marks are supposed to be responsible for the maintenance of many cellular activities, including the cell-type-specific differentiation states established during development [5], as well as the immune memory allowing lifelong immunity against previously encountered infectious agents [13]. Certain chromatin modifications are indeed unambigously associated to the gene expression status. For example, hypermethylation of histone 3 lysine 9 (H3K9me3), linked to DNA methylation [14], reflects gene repression and chromatin condensation and can be self-perpetuated through mitosis. Conversely, hypermethylation of lysine 4 of this histone (H3K4me2-3) is a mark of active genes, correlated with H3K9 acetylation. The complex picture of chromatin modifications in relation to gene expression is reviewed in [15, 16].
Attractors
In spite of the widespread definition of epigenetics as chromatin modifications, the term epigenetic was originally used in a different context, long before the discovery of chromatin structures, according to the pioneer ideas of Waddington [17], who is recognized as the founder of the famous concept of “epigenetic landscape” [18]. In this landscape, attractors are physical entities resulting from the balance of gene interactions previously studied by Delbrück [19] and corresponding to the gene switching circuits drawn by Monod and Jacob [20]. For a given collection of genes, some of which regulate other ones, directly or indirectly, the combination of their expression levels cannot take any value but spontaneously adjust such that the strengths of interactions collectively compensate each others. The sets of compatible values are called attractors [21, 22]. The formation of attractors in gene regulatory network (GRN) does not require specific covalent gene marking, whereas most experimental studies aimed at understanding eukaryotic cell behaviors focus attention on gene structure. Though they are often envisioned as equilibria resulting from webs of interactions, attractors are precisely not equilibria but steady states in dissipative systems necessarily open, with continuous inputs of matter and free energy and associated to continuous heat dissipation. The attractors can be mathematically modeled using different tools [23–27]. Among them, sets of nonlinear differential equations are realistic enough as they avoid the simplificatory hypothesis of binary gene expression (on or off only). But, unfortunately, they are not solvable explicitly and suffer from the lack of knowledge of many interaction parameters in the cell. In the differential equations, the degradation of mRNA and proteins is satisfactorily described as a first order decay (like radioactive disintegration), but transcriptional input functions are much more difficult to capture. They are so far described using approximative and somewhat arbitrary Hill functions to satisfy sigmoidicity requirements, but which poorly reflect the complexity of transcription initiation mechanisms [28]. Hill coefficients are generally chosen more for convenience than for their biological relevance, because the realistic transcriptional functions are currently out of reach experimentally. As nonlinear equations can have several solutions, GRN can be multistable, which means that different stationary states can be obtained with exactly the same genes [19], if certain conditions are fulfilled (presence of positive circuits in the network and some nonlinearity in deterministic mechanisms) [29, 30]. In the landscape of Waddington, hills and ridges correspond to unstable gene expression combinations devoid of biological interest, while attractors are the possible stationary states. This representation is consistent with the gene expression changes observed in large-scale profiling studies, showing the progressive reshaping of expression patterns upon cellular reprogrammation in response to environmental changes or to biological signals. The same final profiles can be reached following treatment with different agents and starting from the same initial states, but through different ways [31]. For better visualization, these transient cellular changes are often compared to a sphere rolling by gravity down the Waddington landscape, from high elevation (low probability) to low elevation (high probability) states.
Examples of simple attractors
Two-gene attractors
The circuit most often used as a model of bistability is made of two genes inhibiting each other [32]. Figure 1b represents such a reciprocal inhibition circuit between two genes otherwise expressed at a constant rate, said to be basal (without self-activation). Inhibition of an inhibition is a prototype of positive circuit, and the presence of at least one positive circuit is in theory necessary for structuring a network into multiple attractors [30]. In this scheme, the combinations of expression levels of A and B can take only two stable states, corresponding to exclusive expression of either A or B. This result is intuitively expected because, if A is slightly more expressed than B, then the gene encoding B is slightly more inhibited, and as a consequence is less able to inhibit A expression, so that A further increases (A > B => A ⋙ B). Consecutive iterations of this cycle lead to a stable situation in which only the expression of A is detectable. Hence, the same issue as previously obtained for instructive chromatin marks (A expressed and B repressed; Fig. 1a), is now obtained through a simple circuitry mechanism, without any structural gene change. In principle, the system could remain such that A and B are expressed at exactly the same level, but this state is purely theoretical since it is very fragile and nonexistent in real fluctuating cells, where any transient faint excess of one factor is explosively amplified and definitely locked. If, in addition, each gene product stimulates its own gene, in a manner that is:
(1) independent and additive with respect to the previous stimulation, and
(2) not inhibitable by the other gene product (Fig. 1c),
then a third median attractor of lower probability is predicted, in which A and B are both expressed (Fig. 1c). This scheme is the most widely used in the current literature for modeling cellular differentiation, which is conceived as a shift from the median basin into a lateral stable one [33–37]. This view of differentiation is supported by the antagonistic couples of TFs observed in progenitor cells that co-express markers of different cell lineages [38], such as the GATA/PU.1 in hematopoietic progenitors [33, 39], Pax3/Foxc2 in muscular versus vascular cells derived from somites [40], or Sox2/Oct4 in the ectodermal versus mesendodermal balance [41]. The median attractor where A and B are both expressed (Fig. 1c) corresponds to bipotent cells while the low potential attractors correspond to differentiated cells that made a choice between A and B. Based on this mechanism, development proceeds through cascades of successive binary choices that can give rise to a maximum of 2n possible cellular states after n splitting events. During embryogenesis, these choices can be:
(1) inductive and depend on the position of the cell relatively to a source of diffusing signals such as the organizers described by (once again) Waddington [42], or
(2) selective if different signals or survival factors can stabilize fractions of bipotent progenitor cells stochastically pre-committed towards A-only or B-only phenotypes. Such a selective mechanism would be much less demanding in term of number of regulators than the traditional inductive view. Stochastic jumps in the landscape can occur upon fluctuations, particularly when the genetic circuits are regulated by few molecules or of weak intrinsic robustness, or because of a lack of discernment in cellular regulations. Backward transitions, from lower elevation to upper basins of attraction, are also possible in theory [32], stochastically in case of large fluctuations, or deterministically upon forced activation or inactivation of critical gene products.
Single gene attractors
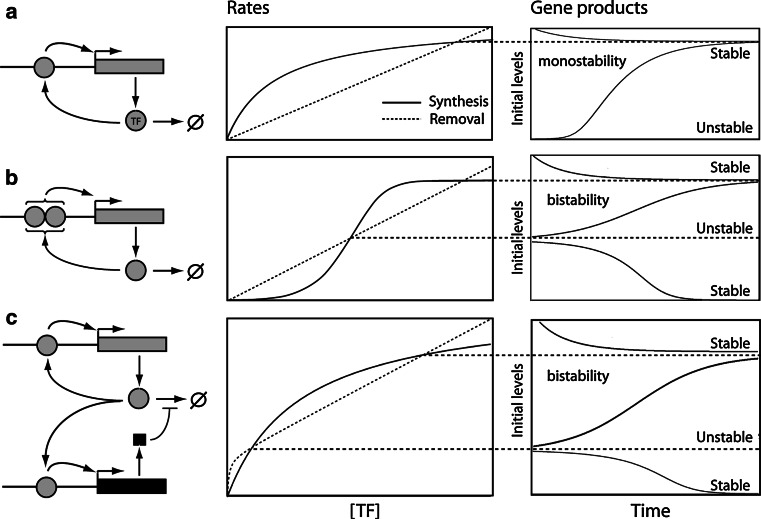
The minimal bistability-generating genetic circuit is provided by a gene whose expression is stimulated by its own product (Fig. 2). This is the archetypal memory loop which, once fired and in the absence of antagonistic influences, indefinitely locks the gene at maximal expression. This single gene scheme can generate either monostable or bistable situations with coexistence of two phenotypes in the population: either uninduced or fully induced (Fig. 2). A classical way to obtain bistability and bimodal populations is the cooperative action of the TF on its own gene promoter, for example through dimerization (Fig. 2b). In this simplistic case, the attractors are two points in a linear landscape, while for two interacting genes, the Waddington landscape is visualized as a 2D surface curved in a 3D plot whose bottom axes are the expression levels of A and B. Of course, larger systems are not representable graphically, but interestingly, the minimal circuit described above is sufficient to generate bistability and is the very common framework of many biological memory effects, as illustrated below.
Fig. 2.
Positive feedbacks with a single gene. The synthesis and degradation of the TF are two functions of its own concentration and the relative dependence on TF concentration of these two curves (left panels), determines if the system is monostable or bistable (right panels). The simple example shown is a direct positive feedback where a TF stimulates the expression of its own gene. a In the case of non-cooperative action of the TF, the amplification loop is immediately primed upon a single event, so that the system is monostable. b In the case of cooperativity illustrated in this scheme by TF dimerization, there can be a significant threshold of activation, leading to bistability. The right panels show how the bistable states are obtained when the rate of synthesis (plain line) and of removal (large dashes) equalize each other. c Bistability can also appear without sigmoidicity, as in the case of indirect cooperativity in which a target gene (black rectangle) rapidly stimulated by the TF, encodes a product stabilizing either its mRNA or the protein. In addition to these deterministic mechanisms, bistability can also be obtained stochastically for systems in which gene expression burstiness allows the crossing of amplifications thresholds (not shown)
Positive feedbacks are pivotal components of memory circuits
Feedbacks are the backbones of cybernetics in all open systems, including those of biology. Beside negative feedbacks involved in homeostasis and periodic attractors, positive feedbacks have a particular importance in point attractor formation.
State maintenance
Many examples of epigenetic state maintenance are provided by bacteria. The most famous one is the induction of the lactose operon (lac) in E. coli [1, 43]. Following treatment with a non-metabolizable inducer, the bacterial population splits into uninduced and fully-induced individual bacteria, which remain at this state over generations. This behavior is called hysteretic since, once primed, lac expression cannot be easily turned off and can survive transient periods of the absence of the inducer. A central feature of this system is a positive feedback primed by a random event [43], and clearly drawn in [1], in which the lac product permease increases the entry of the inducer in the cell, which itself stabilizes the lac repressor in a form no longer capable of binding to DNA. The random commitment of B. subtilis towards a state competent for assimilating exogenous DNA, or not, also leads to binary states [2]. Given that the master TF governing this activity, named ComK, stimulates the expression of its own gene in a constitutive manner, the competent phenotype is in theory very stable. Such memory mechanisms driven by positive feedbacks also exist in eukaryotes. For example, the behavior of aggressive cancer cells is reminiscent of the mesenchymal phenotype, acquired after the epithelial–mesenchymal transition (EMT). It is directly inherited from unicellular organisms for which migration and active division are normal activities. In metazoans, these activities remained important during development or cicatrization, but became undesirable in differentiated tissues for their associated risk of cancer. The problem is that the accidental commitment of cells into this attractor is relatively resistant to reversal, preventing metastatic cells from shifting back to the normal phenotype. One candidate feedback mechanism involved in the mesenchymal maintenance is made up of a chain of disparate components including cytokines, TFs, kinases, and micro-RNAs [44]. Other such self-sustaining circuits exist between mechanical constraints and gene expression [45]. EMT is associated with expression of genes involved in cellular migration, which goes with actin polymerization, and, in turn, actin polymerization has genetic effects favoring EMT, mediated by molecules such as MKL1 [46].
Memory of past signals
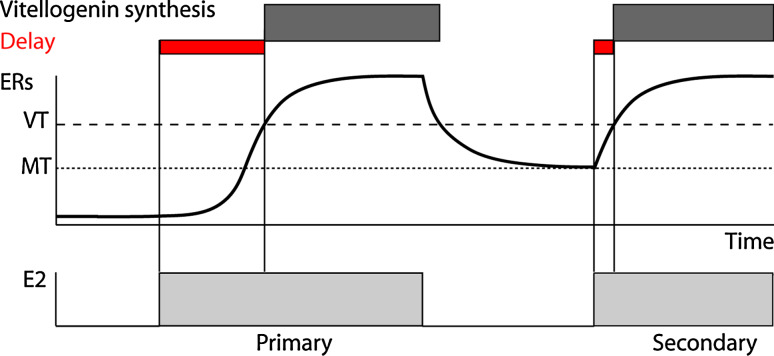
Epigenetic memory has also been involved in the delayed effects of past, sometimes neonatal or fetal exposures to hormones or xeno-hormones, which have major outcomes in toxicology [47–49]. Genetic circuits can keep alive the memory of hormones long after their removal. For example, it has long been evidenced that the production of the egg yolk in response to the sex hormone estradiol is much stronger when the animals have already been stimulated by this hormone in the past. This famous phenomenon, known as the “vitellogenesis memory effect”, is observed in all egg-laying vertebrates. Even when not expressed, the vitellogenin gene, a marker of vitellogenesis, always remains competent for expression in liver, since it can be artificially turned on in male animals by administration of estrogens. Given that the chromatin modifications of the vitellogenin gene seem not durable enough to explain the vitellogenesis memory effect [50], we proposed a novel mechanism based on active circuits primed by the first exposure to estrogens and then maintained by endogenous factors after hormone withdrawal, without postulating persistent chromatin modifications [51]. The principle of this memory is schematized in Fig. 3. The delay of response of the vitellogenin gene to hormone (E2) administration is strongly shortened during secondary administrations because the TF mediating this effect, a variant isoform of the estrogen receptor (ERs), is more abundant in the cell. This phenomenon results from: (1) the fact that the ER gene expression is itself sensitive to E2 through a direct positive feedback mediated by a consensus ER-responsive element present in its promoter, and (2) once the ER amount in the cell exceeds a memory threshold (MT; lower dashed line which is equivalent to the unstable dashed line in Fig. 2b), it can no longer fall down to its ground level owing to maintenance of a positive feedback at low level in the absence of hormone (this basal activity of ERs is not observed for normal ER isoforms). This basal activity is, however, unable to alone prime the feedback in virgin animals, thanks to a bistability threshold. Finally, the vitellogenin gene is both strictly E2-dependent and requires a minimal amount of ER (VT, upper dashed line). These features are the consequence of a strongly cooperative action of ER on the non-consensus ER-responsive elements of the vitellogenin gene (Hill coefficient over 4) and allow that vitellogenin expression is always shut off in absence in E2. A singular property of this mechanism is that: (1) it can be indefinitely perpetuated in absence of the hormone, contrary to the lac-induced state, which progressively disappears after inducer withdrawal and permease degradation, and (2) it is maintained in the absence of phenotypic change contrary to the competent state of B. subtilis, mediated by the signal-independent activity of ComK.
Fig. 3.
Dynamic model of the vitellogenesis effect. The key actor of the system is the short estrogen receptor α (ERs) which stimulates its own expression. The initial priming of this feedback is necessarily triggered by estrogen administration (E2), but it is then sustained at low level (memory threshold MT) even in absence of E2 thanks to the basal hormone-independent activity of ERs. By this way, the delay between E2 stimulation and vitellogenin expression, which requires a minimum level of ERs (VT threshold), is strongly shortened in the secondary E2 administration
Comparative properties of structural and dynamic memories
Passive versus active maintenance
While the conception of chromatin marks as static structures prevailed before discovering their dynamic nature, attractors have always been modeled as steady state phenomena underlain by continuous molecular exchanges. Structural signals can be compared to dead computer memories since, once recorded and burned, information is maintained in disks, hard-disks, or USB keys, as long as is allowed by the longevity of the material. Conversely, dynamic circuits would disappear in absence of cellular refuelling with matter and energy, in the same manner that, in computers, unsaved active files vanish in cases of electricity black-out. Energy-dependent memory seems at first glance not safe enough, but in fact could be, somewhat counter-intuitively, a safer mode of lifelong perpetuation of acquired information, given that living systems are never temporarily switched off, contrary to computing systems.
Transfer of epigenetic information across cellular generations
Both modes of epigenetic memory can survive cell division. On the one hand, attractors are preserved during mitosis thanks to the amounts of pre-synthesized molecules that can re-prime the circuits immediately after anaphasis. On the other hand, mechanisms exist to write out chromatin marks during DNA replication. The maintenance of the CG DNA methylation profile after replication can be simply achieved by the semi-conservative DNA replication and the mode of action of DNMT1 and its cofactors, recruited together with histone methylase by PCNA near the fork [52]. Chromatin proteins modifications are also duplicated by specialized machineries acting at the replication fork [6, 14, 53, 54]. In this respect, the inheritance of chromatin modifications is of the same nature as other phenomena of structural inheritance mediated by the “guided assembly” of multi-macromolecular structures with “self-templating” properties [55]. The mitotic inheritance of certain marks has, however, been shown to progressively disappear in the absence of restimulation, as for stress-induced ATF-2-dependent modifications [56].
Transfer across organism generations
The question is clearly different depending on whether the organisms correspond to single cells or to metazoans with sexual reproduction. Attractors have long been characterized in bacteria for their capacity to propagate over generations, but the question is much more complex for multicellular organisms. However, it has received great interest since it could have fundamental importance in evolution, by providing a possible basis for the Lamarkian principle of the heredity of acquired characters. Chromatin modifications could in principle be very appropriate for carrying some epigenetic information to the offspring [57]. But most chromatin marks, including DNA methylation, are supposed to be reset between the generations. An exception to this rule is, however, known. The genes subject to parental imprinting in placental mammals retain their gamete-type-specific (sperm cell or ovule) methylation pattern in the early embryo, enabling it to distinguish between the paternal and the maternal gene copies to select one of them as an exclusive transcription template. It would be crucial to determine if other genes can evade methylation erasing, if these genes are differentially modified in germ cells, as supported by certain observations [58], and if biases in germ cell chromatin modifications are robustly correlated to the living conditions, rather than randomly distributed.
A role in the assimilation of environmental conditions?
The possibility that experience- or environmentally-induced methylation can survive gametogenesis can be invoked to explain puzzling observations of transgenerational memory effects [59, 60]. This domain is somewhat related to the idea of genetic assimilation of (once again) Waddington [61], but it raises a lot of open questions. To contribute to Lamarkian evolution, chromatin modifications should influence the probability of DNA mutation. This seems to be the case for CG DNA methylation, which favors C to T mutations, with evolutionary consequences [62]. Another critical point is to understand how germ cells, which remain so far the only admitted vectors of genetic information, could assimilate the acquired characters of body tissues. If it is conceivable that certain treatments can directly affect germ cells, such as nutrient deprivation or endocrine disruptors [63], it is more difficult to understand how the repeated friction of the ventral skin of the ostrich, supposed by Waddington to have generated callosities [18], can epigenetically concern germ cells, unless postulating some intercellular transfer of information, for example ensured by RNA, such as in C. elegans [64]. This possibility cannot be ruled out considering the growing evidence of extracellular RNA. Finally, other mechanisms can be involved in biased genetic evolution, including non-random meiotic recombination or gene network-based differential sensitivity to mutations, with which epigenetic marks can also interfere. There are definitely many more questions than answers in this domain [57].
Persistence of chromatin marking in given cell lineages
Attractors are shaped by the topology of active circuits and can persist as long as the cell is refuelled with matter and free energy, a basic condition of life. Following the recent discovery of histone-demodification enzymes, chromatin marks now appear as the fragile resultants of the continuous antagonistic actions of modifiers and de-modifiers, which offers the possibility that linear changes in the ratio of modifying over de-modifying enzymes can cause brutal changes in the intensity of the covalent modifications [65]. DNA methylation is also subject to active and rapid removal [66]. These behaviors are in line with dynamic attractors but less consistent with their previously assumed locking action on cell fates, established during the embryonic stage and maintained throughout the life [67], but more interestingly, and in a completely different perspective, dynamic exchanges of chromatin modifications can also have a role in stabilizing gene expression, as will be explained later.
Coexistence of structural and epigenetic mechanisms in the classical memory of neurobiology
Genetic and epigenetic phenomena also interfere in neurobiology [68], where the concept of memory was of course first defined from our capacity to remember past experiences. The mechanisms underlying this classical memory remain far from fully understood, but it is intriguing to notice that, as for cellular memory, dynamic and structural mechanisms coexist in the brain. It is also worth noting that chromatin modifications coincide with the transient process of memory acquisition in the hippocampus [69]. A learned neuronal system clearly acquired structural modifications, including dendritic spines [70] and the stabilization or destabilization of synapses, but the phenomenon of memorization can itself involve neurone circuits with feedbacks [71, 72].
Why the attractor concept cannot be neglected in biology
Let us first examine, in practice, the consequences of the attractor principles for scientific exploration in biology.
Cellular behaviors emerge from collective molecule interactions
Attractors complicate or hinder the traditional strategies of identification of candidate genes responsible for specific biological activities. Attractor-based causation is by essence collective, in sharp contrast with the linear chains of consecutive events classically drawn by biologists [27]. Even if certain nodes in a network can occasionally acquire master roles when they are the sites of entry of signals, if they are epistatic or hubs integrating many inputs, the evolution towards steady states is always determined collectively. This revision of causation principles nullifies certain traditional experiments. If transcriptome profiling is performed using mRNA extracted following perturbation, say, for example, 2 h after hormone addition, the gene variations revealed by microarrays can correspond to transient behaviors [31] with poor predictive significance. Transcriptome analyses, performed to compare gene expression profiles following natural changes or experimental treatments, can appear disconcerting if we are looking for linear causation chains, because of the extensive transcriptome changes generally observed. Such massive reconfigurations of gene expression patterns are also obtained in experiments targeting a single gene, by genomic inactivation or siRNA. These generalized changes, which are puzzling from the traditional perspective, are expected from the attractor view. The automatic self-reorganization of a network after removing a single node can give rise to a viable variant landscape, thanks to the robustness of landscapes that is likely to have been a central criterion of evolutionary selection. This can provide a possible mechanism by which somatic mutations can be buffered and explain why no defect is observed in many gene-inactivation experiments (knock-out, KO). This result is deceiving if KOs are naïvely expected to give clear answers about the function of the targeted genes. For example, how to explain that when the celebrated actors of cell cycle (cyclins and CDK) are KO in mice, everything remains OK during the early embryonic development [73]. Even more problematically, when phenotypic defects arise in KO experiments, they can in fact reflect only the aberrant attractors generated by node elimination, leading to erroneous conclusions about its role. Diffuse causality in attractors also explains why the forced overexpression of any molecule, either mutant or wild-type, can disrupt of lot of cellular activities. It would not be acceptable to focus only on certain of these modifications to draw falsely simple stories. Traditional approaches of molecular genetics remain useful, since experimental perturbations, in conjunction with “omic” analyses, could in principle allow the reconstruction mathematically of the architectures of the underlying networks [74–76], for example, thanks to in silico tools called GRN inference algorithms, developed to uncover in a bottom-up manner the topology of a GRN [77].
A certain vanishing of the idea of gene function
The principle of the epigenetic landscape challenges the traditional attempt to classify genes into precise functional categories. Conversely, it supports the concept of biological tinkering, according to which individual gene products could be no more than tools, opportunistically adapted to many different complexes and functions [78]. This view satisfactorily explains how the apparent diversity of living organisms can be underlied by the same genes, because it results from changes in the relative amounts of gene products rather than from differences in the functions of these products; a matter of regulation rather than of structure [78, 79]. In the same manner that a screwdriver can be used to make a stair and a closet, the same genes can be involved in distantly related cellular functions. For example, Toll genes are essential during Drosophila embryogenesis to specify the dorso-ventral axis and, later, in the adult, contribute to immune defenses [80]. It is increasingly clear that many chromatin-modification machineries participate to different cellular activities including DNA repair and the control of cytoplasmic molecules. Genes previously associated with well-defined functions turn to contribute to other regulations, as NF-kB, first classified as a main regulator of inflammation, is also involved in EMT [44, 81]. Moreover, certain genes completely evade our classification attempts. This is the case of the unique gene encoding both Histone H2A and Bufforin II [82]: is it a chromatin component or an antimicrobial agent? The biological effect of a signal can also depend on the attractor of the target cell. For instance, for quiescent differentiated cells, TGFβ is a safeguard signal, preventing the cells from dividing, while conversely, if the cells are premalignant, TGFβ is a deleterious signal, aggravating the mesenchymal phenotype and leading to metastasis [83]. The same ambiguity holds for the estrogen receptor α, which is clearly involved in the initial onset of hormone-dependent breast cancers, but whose inactivation then promotes the metastatic evolution of these cancers. Such an ambivalence is also found at the level of chromatin modifications. The lysine-specific demethylase LSD1 (KDM1A), involved in cancer can demethylate histone H3 at the level of lysine 4, favoring gene repression, and at the level of lysine 9, favoring gene expression. These opposite actions are explained by the presence of LSD1 in different, mutually exclusive, protein complexes, complicating attempts at targeting LSD1 in anti-cancer strategies, and which could explain striking discrepancies in the literature, where LSD1 is presented either as a marker of cancer [84] or inversely as an anti-metastatic molecule [85]. Such contradictory conclusions are generally explained by introducing the notion of “context-specific” functions, which confusedly reflects the idea of underlying networks. Because of these pleiotropic roles of molecules, designating a particular gene as a therapeutic target is rarely successful in practice, because of the possible unanticipated and boomerang effects of the perturbations of this gene. In this respect, precise interactions [86] are more promising therapeutic target candidates than molecules by themselves.
Complexity, diversity and environment-sensitivity of genome-encoded attractors
Based on the assumption that GRNs dictate the cellular fates and that chromatin modifications dictate gene expression patterns, a tremendous status is currently attributed to these modifications. Without denying their importance, their place in the complex cellular regulatory systems can, however, be reconsidered. Before examining the specific contributions of chromatin modifications, it is first necessary to survey some features of attractors.
The complexity of biochemical networks
GRNs should be understood as very large biochemical networks made of a wide diversity of molecules, including proteins, coding and non-coding RNAs. Their different folding, localization, and post-transcriptional modification states, the organic molecules synthesized by macromolecules, as well as the inorganic molecules whose concentrations in the different cellular compartments, are also regulated by gene products. Primary transcripts are submitted to cascades of post-transcriptional and post-translational modifications, so that the functional interactions between the genes can be highly indirect. But since the machineries responsible for gene product modifications are themselves gene products, the idea that phenotypes are orchestrated by the genome can in principle be recovered. In the constellation of cellular molecules, genes are the unique components that are constant and not subject to birth and death. As the only invariant parameter of GRNs, the genome fully specifies the corpus of all possible attractors, whose number is very large but finite. Singularly, at a larger time scale, genomes result themselves from a circuitry mechanism in the biosphere system: a positive feedback based on the inverse relationship between information and entropy [87]. But more concretely, in present cells with a given genome, the precise combination of molecular actors in the cell both reflects and determines an attractor. These bidirectional relationships, which are the essence of self-organized cellular systems, also hold for chromatin modifications, which are both post-translational modifications and pre-transcriptional regulators.
GRN attractors are environment-sensitive
In the set of possible attractors inscribed in the genome, the one precisely occupied by every cell at every time point is selected by interplay between stochastic and deterministic phenomena, most of them resulting from the integration of exogenous signals. As explained in [55], phenotype depends on a web of interactions between genes, their products, and environment. Gene products are evolutionary prepared to be responsive to environmental conditions, so that the initial steps of chemical (hormones), electrical or mechanical signaling, are modifications of gene products, that can secondarily lead to a switch from the initial attractor to a new one. Pushing transiently gene product activities outside the range of values of an attractor basin, by post-translational activation or inactivation (through protein cleavage, degradation, phosphorylation, sumoylation or many other modifications), is sufficient to force the system to migrate to an adjacent basin, and ultimately to fall into a new attractor. Accordingly, the dramatic importance of the unbalanced dosage of non-mutant gene products on phenotypes has long been revealed by trisomies.
Evolutionary selected versus fortuitous attractors
The normal or “home” attractors [88], closer to the idea of “hardwired” program [89], are those resulting from evolutionary selection and robust enough to buffer strong molecular fluctuations. Certain vital stress responses are also strictly stereotyped [27]. These cellular activities correspond to profound attractor basins with acute valleys in the Waddington landscape, which can ensure the “canalization” of invariant programs [55] (once the cells have fallen into). Such attractors are likely to be predominant in development [90] and for eukaryotic cells embedded in homogeneous metazoan tissues, which have little degree of freedom. A level of flexibility in attractor selection is provided by non-deterministic quantitative and qualitative steps in the system, including, for example, bursty transcription initiation, unsettled protein folding, or stochastic post-translational modifications [91]. These loose controls offer the possibility for “unscheduled” conditions and lifestyles to force, in a retrograde manner, the cell to fall into variant secondary attractors [88] that are the mere results of nonlinear interaction networks, certain of which, called garden-of-Eden states, are devoid of precursor states [27]. These moderately stable states, not evolutionary optimized and less occupied, can lead to pathological phenotypes, if, for example, the cell sequentially jumps between many secondary attractors and climbs a rugged landscape to reach aberrant attractors close to the “archaic” attractor proposed to underly cancer [32, 92]. In this respect, pathological states like cancer can, in some cases or at early stages, be purely epigenetic, without need for genetic mutations. Mutations drive evolution by imposing the automatic reshaping of the landscape, with genesis of new fortuitous attractors, translated into new phenotypes that provide the substrates of Darwinian selection. Conversely, the occupation of certain secondary attractors in a mutation-free landscape can influence the occurrence of mutations, for example, if the expression of DNA-repair machineries is altered in this attractor. Since they are supposed to be much less stable than primary attractors [88], variant attractor are likely to not directly serve as selection substrates for metazoan evolution, but they could play this role for unicellular organisms and particularly in the clonal selection of cancer cells. This hypothesis contrasts with the more classical assumption that metastatic cells result from the accumulation of mutations, which has been contradicted by the possibility to reprogram the nuclei of malignant melanoma cells into all differentiation state-specific activities, showing that in fact most genes remained functional [93]. This example suggests that cancer can arise in a mutation-independent manner, through abnormal cell positioning in a normal landscape, while mutations can irreversibly fix cancerous phenotypes by generating abnormal landscapes.
The multifaceted contribution of chromatin modifications in eukaryotic attractors
Chromatin modifications reflect underlying attractors
In the face of the amazingly growing number of chromatin modifications recently identified, biologists hope that many of them are coordinated, to simplify the question. But from the attractor view, everything is coordinated in the cell. Chromatin modifications are the result of the relative concentrations of a wide variety of actors, including the different chromatin modifying and de-modifying enzymes, their docking and/or activating factors, the TFs responsible for their expression, and so on, which are all gene products and, as such, belong altogether to high dimensional networks. The cooperative and combinatory chromatin modifications are the emergent signatures of underlying attractors. As a matter of fact, if an aberrant DNA methylation pattern is erased using 5-aza-cytidine, then methylations are removed; but after removing 5-aza-cytidine, they reappear [94], showing that the causes remain. In this experiment, DNA methylation appears as an epiphenomenon devoid of any decisional role. This conclusion is also suggested for histone modifications, by the surprising absence of phenotypic defects caused by the vast majority of mutant histones with deregulated modifications [95]. As pointed out in [96], one cannot deny that certain chromatin modifications are associated to active genes, but this is also true for RNA polymerase. The purported activating or repressive chromatin modifications could be merely correlative rather than causal [97]. Moreover, they sometimes prove to have little influence on differentiation [98]. By contrast, the influence of TFs often prevails over pre-existing chromatin marks, as supported by the capacity of a quadroon of TFs to reprogram a somatic cell into a stem-like cell, regardless of its previous structural epigenetic status [99], or of a single TF (MyoD) to convert a fibroblast into a myoblast [100]. If TFs are strong enough, they can bypass epigenetic modifications [101]. To be further provocative in this direction, let us reveal why the sub-nuclear organization of heterochromatin domains is so important: to allow nocturnal vision. This surprising conclusion derives from a study showing that heterochromatin is confined in the nuclear center in rod receptors [102], whereas it is generally at the nuclear periphery in other cells [103]. Since to our knowledge the genetic expression of rod receptors is as accurate as that of any other cell type, this study somehow discredits the numerous studies pointing to the dramatic importance of precise subnuclear organization for gene expression. The same conclusion is also suggested by the relevant results obtained for decades in gene promoter studies through transient expression assays, in which the transfected plasmids are likely to not obey strict subnuclear localization and chromatin packaging rules.
Chromatin modifications: a link among others in biochemical circuits
As parts of attractors, chromatin modifications can be involved both in the establishment of phenotypes by GRNs and conversely the readjustment of GRNs following stochastic or deterministic conditions changes. The transfer of information into chromatin marks is believed to be mediated by TFs, but it can also be more direct. For example, the energetic status of the cell has a profound impact on chromatin modifications [104], which is expected, in turn, to influence the expression of genes involved in energetic metabolism. It is illusive in this context to identify a causal hierarchy in a cyclic circuit. For example, EMT induced by hypoxia [105] or other means upregulates the H3K9me3 demethylase JMJD2C/GASC, and, conversely, overexpression of this molecule is oncogenic [106]. The question is not to look for decisional actors in circuits but to understand how cells can fall into these circuits. In Waddington’s landscape, final cellular behaviors emerge from global molecular interactions and, reciprocally, the whole network determines the effect of any node.
Influences of chromatin modifications on GRN stochasticity
Chromatin modifications can stabilize attractors but they can also be the primers and the results of stochastic behaviors. Chromatin remodelers have been shown to affect transcription initiation steadiness [107]. By slowing the reactivity of genes to TFs, they can also widen the time windows of gene activity and inactivity, and, consequently, the burstiness of transcript production, which is a primary generator of transcriptional noise [108]. Chromatin has also long been involved in the dramatic cell-to-cell heterogeneity called variegation [109], in which the expression status of individual cells is binary at a given time, but without memory, since the clonal descendants of this cell is repartitioned into expressing or non-expressing cells. The spreading of repressive chromatin through positive feedbacks initiated on both sides of genes can explain their binary expression status [110].
In contrast, certain marks have been shown to stabilize the steady-state gene expression levels in time [8]. If cellular differentiation proceeds through differential gene closing by long-term heterochromatinization, then the number of factors necessary to regulate GRNs, and particularly repressors, is expected to decrease, with beneficial impact on global noise. Interestingly, generalized euchromatinization (gene opening) and global increase of biosynthetic activity, such as that obtained by overexpressing Myc genes, such as N-Myc [111], is associated with cancer. Hence, heterochromatin could be an essential tool developed for managing large genomes, which is not necessary in prokaryotes in which the number of repressors remains manageable.
Roles of chromatin marks in GRN memory
Although the present review is aimed at suggesting that chromatin modifications are not the main guarantors of epigenetic memory, their participation in memory phenomena cannot be ruled out. The prolonged presence of chromatin modifications can play a role in mitotic bookmarking [7]. Temporary bookmarking functions have not been extended to transgenerational batons, which remains so far purely speculative. If chromatin marks are catalyzed by constitutively expressed chromatin-modifying enzymes, they can be regulated through the docking of these enzymes to DNA, by TFs, their corepressors, and coactivators. But in this case, chromatin marks would merely behave as accessory modules of TFs and would not modify the standard description of GRNs. However, the turnovers of chromatin marks can be cancelled in case of the disappearance of certain TFs and during critical phases of the cellular physiology such as mitosis, during which global gene expression is temporarily shut off, and until the steady state attractor conditions are restored. Another possibility to achieve this goal would be to remove completely one enzymatic system (of modification or demodification) from the cell. Finally, one can also imagine that chromatin packaging triggered by the modifications in turn renders them inaccessible to the de-modifying enzymes, in a hierarchical chain of events [112]. But the existence of impenetrable forms of chromatin is questioned [113]. In fact, the more problematic feature of chromatin marks opposed to the role of chromatin modifications in epigenetic memory is their dynamic nature. Histone methylations have long been demonstrated experimentally to be transient. H3 histones are rapidly methylated in Ehrlich tumor cells, with an apparent rate of 0.21/h, and the turnover of histone-bound acetate and phosphate groups is even more rapid [114]. Moreover, the observations of these authors imply that the antagonistic methylase and demethylase activities are present simultaneously in the cells, since steady state values of mono-, di-, and tri-methylated H3 rapidly stabilize at intermediate levels. To further assess the dynamic versus static status of chromatin marks, it will be necessary to quantify the renewal cycle duration of chromatin modifications in non-dividing cells, through a pulse-chase method similar to that used to monitor nucleosome renewal [115]. The role of chromatin marks in epigenetic memory, which is highly questionable when envisioned as structural marks, can be recovered under the dynamic circuitry perspective, as discussed below.
Possible additional deterministic roles of chromatin marks
Chromatin marks can also work as would do stable TFs, by recruiting transcription machineries. For example, the general transcription factor TFIID is recruited by chromatin patches enriched in H3K4me3, the hallmark of active promoters [116]. Beside these roles in static memory during cellular and organism generations, the lagging effects of chromatin modifications could also introduce slow variables in dynamic networks. Delays in TF action could regulate the amplitude of periodic attractors generated by negative circuits and could destabilize or induce oscillations in stationary states. Finally, there are many chromatin modifications: methylation, phosphorylation, acetylation, ubiquitination, and many others [15, 117], most of them involving antagonistic enzymes, which opens the possibility of alternative roles of these modifications in the sharpness of transcriptional decisions through the so-called zero-order ultrasensitivity, initially established for the phosphorylation–dephosphorylation cycle [65] or in the hysteretic modification of chromosome regions. This latter possibility, linking chromatin modifications to dynamic circuits, is based on a feedback mechanism propagating and reinforcing local histone marks through the recruitment of the corresponding histone-modifying enzymes [118]. Such positive feedbacks have also been involved in the transfer of information between parental and newly synthesized nucleosomes during replication [119]. In yeast, a positive feedback in histone deacetylation is ensured by the reiterative recruitment of histone deacetylases [120]. Similarly, H3K9me3 can recruit Heterochromatin Protein 1 alpha (HP1α), which then recruits methyl-transferases increasing the methylation of nearby histones [121]. Accordingly, even the long-term maintenance of constitutive heterochromatin is ensured by dynamic interactions with HP1α [122]. Hence, a picture emerges in which dynamic chromatin modifications contribute to the hysteresis and the stability of cellular fates through self-templating mechanisms. In the case of cellular reprogramming, these enzymatic circuits would still remain very useful by increasing the sharpness of the switches, thereby allowing the cell to take well-defined decisions. Finally, another contribution of chromatin to attractor formation is offered by chromatin-mediated TF cooperativity, as suggested for a particular type of structural chromatin modification: the displacement of nucleosomes along DNA by ATP-dependent chromatin modelers. This phenomenon is also dynamic and could play a role in hierarchical cooperativity between TFs [123], a mechanism involved in the genesis of nonlinear relations in GRNs, necessary for creating multistability and providing the thresholds necessary for Boolean modeling. Covalent marking can also work for mediating hierarchical cooperativity between pioneer TFs that set the marks necessary to subsequent TFs.
Conclusion
Attractors and the epigenetic landscapes of Waddington are basically not biological activities but inevitable emergent physical entities applicable to any nonlinear interaction network. These general behaviors hold in different disciplines, for systems made of different components, which led von Bertalanffy to incorporate them into a “general systems theory” whose rules are transposable between distantly related fields, from quantum physics to the economy [124]. As a matter of fact, for GRNs, studies examining the outcomes of various circuitries [125] use exclusively mathematical tools. This could explain why they long remained ignored by the biologist community, when taking them into account would shed a new light on causation concepts. It is worth noting that any randomly constructed network spontaneously organizes into a given landscape with emergent properties, as long shown in Boolean simulations [126] mimicking extreme cases of nonlinear, step-like interactions. This phenomenon, coupled to Darwinian selection, typically illustrates the principle stated of self-organization out of equilibrium. According to this scenario of system evolution, the combination of interactions between the different genes from the same genome, which are mediated by cis (promoter motifs) and trans (TFs and their regulators) factors, have been evolutionary selected such that gene expression patterns converge towards discrete sets of values corresponding precisely to physiologically useful cellular phenotypes. In this way, the genuine targets of Darwinism are attractors rather than individual genes. When adapted to GRNs, the attractor view is much more elegant and integrated than the traditional causal reasoning in biology. Specifically, the attractor-based interpretation of development has the advantage to explain both: (1) the commitment of pluripotent cells and (2) the memory of lineage-committed cells. This view also enlightens previously unexplained observations, such as the fact that progenitor cells express the genes that will be active in the derived cell types [38]. This feature has an overlooked practical importance because many experiments would have been precluded in its absence. Indeed, to make engineered mouse ES cells for generating gene-inactivated mice, it is first necessary to select them by using appropriate resistance-encoding cassettes, whose expression should be driven by the promoters of differentiation genes.
In spite of the success of the dynamic landscape principle, the assumption that epigenetic memory should be structurally engraved in the chromosomes, remains highly predominant. Three reasons can be proposed to explain this situation. First, chromatin marks are (misleadingly) more intuitive at first glance since, once inscribed, they are expected to be maintained passively, while attractors are necessarily active and energy-dependent. Second, the predominance of chromatin epigenetics can also result from a misinterpretation of chromatin marks as molecular flags, specifying the sets of genes to be expressed or repressed. This static view neglects the fact that the degree of chromatin modification, quantified by biochemical approaches, is the result of steady state balances between continuously active chromatin-modifying and de-modifying enzymes. Third, the highjacking of epigenetics by the chromatin community can be unintentional and due to the fact that the concept of attractor remains largely confined in the theoretical and modeling literature, even if pedagogical reviews on the subject are available, such as [32]. It is striking that animal development is envisioned either in term of chromatin marks [5] or of attractors [35], with little connection between these interpretations. Of course, the true nature of development is likely to be somewhere between these extreme conceptions. For example, the two modes of memory compared in the present study can cooperate [118] and are mixed together in a coherent mechanism fundamentally different from the principle of stable marking. The term epigenomics sometimes used, though still ambiguous, would be preferable to not pollute the more general, original meaning of epigenetics.
It will be important to more tightly link attractors and quantitative biological parameters. This rapprochement would benefit from collaborations between theorists and biologists [127], but the task will be difficult, considering the multiplicity of levels of regulation in the cell, certain ones remaining poorly explored, such as RNA editing [128]. Shifting from purely correlative to integrative approaches is a major challenge for future investigations. Together, the wide range of reciprocal influences, deterministic or non-deterministic, between chromatin modifications and cellular fates, as well as their dynamic nature, compromise the initial idea that cataloging chromatin modification patterns associated to physiological or pathological situations by high throughput array technologies and sophisticated bioinformatics can allow the unraveling of functional schemes. Exhaustively analyzing the physiological and pathological cellular states is virtually impossible. Moreover, extensive catalogues will provide useful information but will not reveal some Rosetta stone of chromatin hieroglyphs. The same illusion was common several years ago, when systematic genome sequencing was naively expected to allow the decryption of life, whereas living systems are plastic and clearly not equivalent to computers [27]. The quest for a self-consistent hidden user manual of chromatin modifications could be an impasse. Certain specialists in the field of gene expression have regretted that such descriptive research programs divert too substantial funding resources [129]. As for biochemical systems, self-organization of searchers and ideas could be more productive than managerial guidelines based on presupposals.
Whatever the difficulty of dissecting real biological attractors, it is worth noting that, contrary to chromatin marking, they are already manipulable as tools in synthetic biology approaches, for example to design memory devices [130], whereas chromatin marks are not easily programmable since they result from too complex and intricated regulations. Making simple artificial attractors is easier than modifying natural attractors.
References
- 1.Cohn M, Horibata K. Inhibition by glucose of the induced synthesis of the beta-galactoside-enzyme system of Escherichia coli. Analysis of maintenance. J Bacteriol. 1959;78:601–610. doi: 10.1128/jb.78.5.601-612.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maamar H, Raj A, Dubnau D. Noise in gene expression determines cell fate in Bacillus subtilis . Science. 2007;317:526–529. doi: 10.1126/science.1140818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y. Recent progress in the epigenetics and chromatin field. Cell Res. 2011;21:373–374. doi: 10.1038/cr.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 5.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- 6.Ng RK, Gurdon JB. Epigenetic inheritance of cell differentiation status. Cell Cycle. 2008;7:1173–1177. doi: 10.4161/cc.7.9.5791. [DOI] [PubMed] [Google Scholar]
- 7.Zaidi SK, Young DW, Montecino M, Van Wijnen AJ, Stein JL, Lian JB, Stein GS. Bookmarking the genome: maintenance of epigenetic information. J Biol Chem. 2011;286:18355–18361. doi: 10.1074/jbc.R110.197061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muramoto T, Müller I, Thomas G, Melvin A, Chubb J. Methylation of H3K4 Is required for inheritance of active transcriptional states. Curr Biol. 2010;20:397–406. doi: 10.1016/j.cub.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Yun M, Wu J, Workman JL, Li B. Readers of histone modifications. Cell Res. 2011;21:564–578. doi: 10.1038/cr.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brickner JH. Transcriptional memory: staying in the loop. Curr Biol. 2010;20:R20–R21. doi: 10.1016/j.cub.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Geyer PK, Vitalini MW, Wallrath LL. Nuclear organization: taking a position on gene expression. Curr Opin Cell Biol. 2011;23:354–359. doi: 10.1016/j.ceb.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavalli G. From linear genes to epigenetic inheritance of three-dimensional epigenomes. J Mol Biol. 2011;409:54–61. doi: 10.1016/j.jmb.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Youngblood B, Davis CW, Ahmed R. Making memories that last a lifetime: heritable functions of self-renewing memory CD8 T cells. Int Immunol. 2010;22:797–803. doi: 10.1093/intimm/dxq437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarraf SA, Stancheva I. Methyl-CpG binding protein MBD1 couples histone H3 methylation at lysine 9 by SETDB1 to DNA replication and chromatin assembly. Mol Cell. 2004;15:595–605. doi: 10.1016/j.molcel.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 15.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suganuma T, Workman JL. Signals and combinatorial functions of histone modifications. Annu Rev Biochem. 2011;80:473–499. doi: 10.1146/annurev-biochem-061809-175347. [DOI] [PubMed] [Google Scholar]
- 17.Waddington CH. The strategy of the genes. London: Allen & Unwin; 1957. [Google Scholar]
- 18.Slack JM. Conrad Hal Waddington: the last Renaissance biologist? Nat Rev Genet. 2002;3:889–895. doi: 10.1038/nrg933. [DOI] [PubMed] [Google Scholar]
- 19.Delbrück M. Unités biologiques douées de continuité génétique. Colloques Internationaux CNRS. 1949;8:33–35. [Google Scholar]
- 20.Monod J, Jacob F. General conclusions: teleonomic mechanisms in cellular metabolism, growth, and differentiation. Cold Spring Harbor Symposia on Quant Biol. 1961;26:389–401. doi: 10.1101/SQB.1961.026.01.048. [DOI] [PubMed] [Google Scholar]
- 21.Kauffman S. A proposal for using the ensemble approach to understand genetic regulatory networks. J Theor Biol. 2004;230:581–590. doi: 10.1016/j.jtbi.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Huang S. On the intrinsic inevitability of cancer: from foetal to fatal attraction. Semin Cancer Biol. 2011;21:183–199. doi: 10.1016/j.semcancer.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Thomas R. Boolean formalization of genetic control circuits. J Theor Biol. 1973;42:563–585. doi: 10.1016/0022-5193(73)90247-6. [DOI] [PubMed] [Google Scholar]
- 24.Thomas R. Laws for the dynamics of regulatory networks. Int J Dev Biol. 1998;42:479–485. [PubMed] [Google Scholar]
- 25.de Jong H. Modeling and simulation of genetic regulatory systems: a literature review. J Comput Biol. 2002;9:67–103. doi: 10.1089/10665270252833208. [DOI] [PubMed] [Google Scholar]
- 26.Karlebach G, Shamir R. Modelling and analysis of gene regulatory networks. Natl Rev Mol Cell Biol. 2008;9:770–780. doi: 10.1038/nrm2503. [DOI] [PubMed] [Google Scholar]
- 27.Bornholdt S. Boolean network models of cellular regulation: prospects and limitations. J R Soc Interface. 2008;S1:85–94. doi: 10.1098/rsif.2008.0132.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michel D. How transcription factors can adjust the gene expression floodgates. Prog Biophys Mol Biol. 2010;102:16–37. doi: 10.1016/j.pbiomolbio.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Cherry JL, Adler FR. How to make a biological switch. J Theor Biol. 2000;203:117–133. doi: 10.1006/jtbi.2000.1068. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman M, Soulé C, Thomas R. A new necessary condition on interaction graphs for multistationarity. J Theor Biol. 2007;248:675–685. doi: 10.1016/j.jtbi.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Huang S, Eichler G, Bar-Yam Y, Ingber DE. Cell fates as high-dimensional attractor states of a complex gene regulatory network. Phys Rev Lett. 2005;94:128701. doi: 10.1103/PhysRevLett.94.128701. [DOI] [PubMed] [Google Scholar]
- 32.Huang S. Reprogramming cell fates: reconciling rarity with robustness. Bioessays. 2009;31:546–560. doi: 10.1002/bies.200800189. [DOI] [PubMed] [Google Scholar]
- 33.Huang S, Guo YP, May G, Enver T. Bifurcation dynamics in lineage-commitment in bipotent progenitor cells. Dev Biol. 2007;305:695–713. doi: 10.1016/j.ydbio.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 34.Foster DV, Foster JG, Huang S, Kauffman SA. A model of sequential branching in hierarchical cell fate determination. J Theor Biol. 2009;260:589–597. doi: 10.1016/j.jtbi.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Macarthur BD, Ma’ayan A, Lemischka IR. Systems biology of stem cell fate and cellular reprogramming. Natl Rev Mol Cell Biol. 2009;10:672–681. doi: 10.1038/nrm2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhattacharya S, Zhang Q, Andersen ME. A deterministic map of Waddington’s epigenetic landscape for cell fate specification. BMC Syst Biol. 2011;27:85. doi: 10.1186/1752-0509-5-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Zhang K, Xu L, Wang E. Quantifying the Waddington landscape and biological paths for development and differentiation. Proc Natl Acad Sci USA. 2011;108:8257–8262. doi: 10.1073/pnas.1017017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu M, Krause D, Greaves M, Sharkis S, Dexter M, Heyworth C, Enver T. Multilineage gene expression precedes commitment in the hemopoietic system. Genes Dev. 1997;11:774–785. doi: 10.1101/gad.11.6.774. [DOI] [PubMed] [Google Scholar]
- 39.Zhang P, Behre G, Pan J, Iwama A, Wara-Aswapati N, Radomska HS, Auron PE, Tenen DG, Sun Z. Negative cross-talk between hematopoietic regulators: GATA proteins repress PU.1. Proc Natl Acad Sci USA. 1999;96:8705–8710. doi: 10.1073/pnas.96.15.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lagha M, Brunelli S, Messina G, Cumano A, Kume T, Relaix F, Buckingham ME. Pax3:Foxc2 reciprocal repression in the somite modulates muscular versus vascular cell fate choice in multipotent progenitors. Dev Cell. 2009;17:892–899. doi: 10.1016/j.devcel.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 41.Thomson M, Liu SJ, Zou LN, Smith Z, Meissner A, Ramanathan S. Pluripotency factors in embryonic stem cells regulate differentiation into germ layers. Cell. 2011;145:875–889. doi: 10.1016/j.cell.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waddington CH (1956) Principles of embryology. Allen & Unwin, London
- 43.Novick A, Weiner M. Enzyme Induction as an all-or-none phenomenon. Proc Natl Acad Sci USA. 1957;43:553–566. doi: 10.1073/pnas.43.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang S, Ingber DE. Cell tension, matrix mechanics, and cancer development. Cancer Cell. 2005;8:175–176. doi: 10.1016/j.ccr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 46.Scharenberg MA, Chiquet-Ehrismann R, Asparuhova MB. Megakaryoblastic leukemia protein-1 (MKL1): increasing evidence for an involvement in cancer progression and metastasis. Int J Biochem Cell Biol. 2010;42:1911–1914. doi: 10.1016/j.biocel.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 47.Trichopoulos D. Hypothesis: does breast cancer originate in utero? Lancet. 1990;335:939–940. doi: 10.1016/0140-6736(90)91000-Z. [DOI] [PubMed] [Google Scholar]
- 48.Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prins GS. Estrogen imprinting: when your epigenetic memories come back to haunt you. Endocrinology. 2008;149:5919–5921. doi: 10.1210/en.2008-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burch JB, Evans MI. Chromatin structural transitions and the phenomenon of vitellogenin gene memory in chickens. Mol Cell Biol. 1986;6:1886–1893. doi: 10.1128/mcb.6.6.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicol-Benoit F, Amon A, Vaillant C, le Goff P, Dréan Y, Pakdel F, Flouriot G, Valotaire Y, Michel D. A dynamic model of transcriptional imprinting derived from the vitellogenesis memory effect. Biophys J. 2011;101:1557–1568. doi: 10.1016/j.bpj.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Estève PO, Chin HG, Smallwood A, Feehery GR, Gangisetty O, Karpf AR, Carey MF, Pradhan S. Direct interaction between DNMT1 and G9a coordinates DNA and histone methylation during replication. Genes Dev. 2006;20:3089–3103. doi: 10.1101/gad.1463706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Probst AV, Dunleavy E, Almouzni G. Epigenetic inheritance during the cell cycle. Natl Rev Mol Cell Biol. 2009;10:192–206. doi: 10.1038/nrm2640. [DOI] [PubMed] [Google Scholar]
- 54.Moazed D. Mechanisms for the inheritance of chromatin states. Cell. 2011;146:510–518. doi: 10.1016/j.cell.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jablonka E, Lamb ML. Evolution in four dimensions. Cambridge: MIT Press; 2005. [Google Scholar]
- 56.Seong K-H, Li D, Shimizu H, Nakamura R, Ishii S. Inheritance of stress-induced, ATF-2-dependent epigenetic change. Cell. 2011;145:1049–1061. doi: 10.1016/j.cell.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 57.Daxinger L, Whitelaw E. Transgenerational epigenetic inheritance: more questions than answers. Genome Res. 2010;20:1623–1628. doi: 10.1101/gr.106138.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brykczynska U, Hisano M, Erkek S, Ramos L, Oakeley EJ, Roloff TC, Beisel C, Schübeler D, Stadler MB, Peters AH. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat Struct Mol Biol. 2010;17:679–687. doi: 10.1038/nsmb.1821. [DOI] [PubMed] [Google Scholar]
- 59.Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjöström M, Golding J. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14:159–166. doi: 10.1038/sj.ejhg.5201538. [DOI] [PubMed] [Google Scholar]
- 60.Carone BR, Fauquier L, Habib N, Shea J, Hart C, Li R, Bock C, Li C, Gu H, Zamore P, Meissner A, Weng Z, Hofmann H, Friedman N, Rando O. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–1096. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waddington CH. Selection of the genetic basis for an acquired character. Nature. 1952;169:625–626. doi: 10.1038/169625b0. [DOI] [PubMed] [Google Scholar]
- 62.Galagan JE, Selker EU. RIP: the evolutionary cost of genome defense. Trends Genet. 2004;20:413–423. doi: 10.1016/j.tig.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 63.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of endocrine disruptors. Reprod Toxicol. 2011;31:337–343. doi: 10.1016/j.reprotox.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alcazar RM, Lin R, Fire AZ. Transmission dynamics of heritable silencing induced by double-stranded RNA in Caenorhabditis elegans . Genetics. 2008;180:1275–1288. doi: 10.1534/genetics.108.089433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goldbeter A, Koshland DEJ. An amplified sensitivity arising from covalent modification in biological systems. Proc Natl Acad Sci USA. 1981;78:6840–6844. doi: 10.1073/pnas.78.11.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhutani N, Burns DM, Blau HM. DNA demethylation dynamics. Cell. 2011;146:866–872. doi: 10.1016/j.cell.2011.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kundu S, Peterson CL. Role of chromatin states in transcriptional memory. Biochim Biophys Acta. 2009;1790:445–455. doi: 10.1016/j.bbagen.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Changeux JP, Mikoshiba K. Genetic and ‘epigenetic’ factors regulating synapse formation in vertebrate cerebellum and neuromuscular junction. Prog Brain Res. 1978;48:43–66. doi: 10.1016/S0079-6123(08)61015-8. [DOI] [PubMed] [Google Scholar]
- 69.Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, Paylor RE, Lubin FD. Histone methylation regulates memory formation. J Neurosci. 2010;30:3589–3599. doi: 10.1523/JNEUROSCI.3732-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hofer SB. Structural traces of past experience in the cerebral cortex. J Mol Med (Berl) 2010;88:235–239. doi: 10.1007/s00109-009-0560-2. [DOI] [PubMed] [Google Scholar]
- 71.Lisman JE, Fallon JR. What maintains memories? Science. 1999;283:339–340. doi: 10.1126/science.283.5400.339. [DOI] [PubMed] [Google Scholar]
- 72.Li WC, Soffe SR, Wolf E, Roberts A. Persistent responses to brief stimuli: feedback excitation among brainstem neurons. J Neurosci. 2006;26:4026–4035. doi: 10.1523/JNEUROSCI.4727-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 74.Goutsias J, Lee NH. Computational and experimental approaches for modeling gene regulatory networks. Curr Pharm Des. 2007;13:1415–1436. doi: 10.2174/138161207780765945. [DOI] [PubMed] [Google Scholar]
- 75.Hecker M, Lambeck S, Toepfer S, van Someren E, Guthke R. Gene regulatory network inference: data integration in dynamic models—a review. Biosystems. 2009;96:86–103. doi: 10.1016/j.biosystems.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 76.Ulitsky I, Maron-Katz A, Shavit S, Sagir D, Linhart C, Elkon R, Tanay A, Sharan R, Shiloh Y, Shamir R. Expander: from expression microarrays to networks and functions. Nat Protoc. 2010;5:303–322. doi: 10.1038/nprot.2009.230. [DOI] [PubMed] [Google Scholar]
- 77.Greenfield A, Madar A, Ostrer H, Bonneau R. DREAM4: combining genetic and dynamic information to identify biological networks and dynamical models. PLoS One. 2010;5:e13397. doi: 10.1371/journal.pone.0013397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jacob F. Evolution and tinkering. Science. 1977;196:1161–1166. doi: 10.1126/science.860134. [DOI] [PubMed] [Google Scholar]
- 79.Carroll SB. Endless forms: the evolution of gene regulation and morphological diversity. Cell. 2000;101:577–580. doi: 10.1016/S0092-8674(00)80868-5. [DOI] [PubMed] [Google Scholar]
- 80.Kambris Z, Hoffmann JA, Imler JL, Capovilla M. Tissue and stage-specific expression of the Tolls in Drosophila embryos. Gene Expr Patterns. 2002;2:311–317. doi: 10.1016/S1567-133X(02)00020-0. [DOI] [PubMed] [Google Scholar]
- 81.Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cho JH, Sung BH, Kim SC. Buforins: histone H2A-derived antimicrobial peptides from toad stomach. Biochim Biophys Acta. 2009;1788:1564–1569. doi: 10.1016/j.bbamem.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 83.Tian M, Neil JR, Schiemann WP. Transforming growth factor-beta and the hallmarks of cancer. Cell Signal. 2011;23:951–962. doi: 10.1016/j.cellsig.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lim S, Janzer A, Becker A, Zimmer A, Schüle R, Buettner R, Kirfel J. Lysine-specific demethylase 1 (LSD1) is highly expressed in ER-negative breast cancers and a biomarker predicting aggressive biology. Carcinogenesis. 2010;31:512–520. doi: 10.1093/carcin/bgp324. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y, Zhang H, Chen Y, Sun Y, Yang F, Yu W, Liang J, Sun L, Yang X, Shi L, Li R, Li Y, Zhang Y, Li Q, Yi X, Shang Y. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell. 2009;138:660–672. doi: 10.1016/j.cell.2009.05.050. [DOI] [PubMed] [Google Scholar]
- 86.Colas P. Combinatorial protein reagents to manipulate protein function. Curr Opin Chem Biol. 2000;4:54–59. doi: 10.1016/S1367-5931(99)00051-4. [DOI] [PubMed] [Google Scholar]
- 87.Michel D. Basic statistical recipes for the emergence of biochemical discernment. Prog Biophys Mol Biol. 2011;106:498–516. doi: 10.1016/j.pbiomolbio.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 88.Baverstock K. Radiation-induced genomic instability: a paradigm-breaking phenomenon and its relevance to environmentally induced cancer. Mutat Res. 2000;454:89–109. doi: 10.1016/S0027-5107(00)00100-7. [DOI] [PubMed] [Google Scholar]
- 89.Baverstock K. A comparison of two cell regulatory models entailing high dimensional attractors representing phenotype. Prog Biophys Mol Biol. 2011;106:443–449. doi: 10.1016/j.pbiomolbio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 90.Arnone MI, Davidson EH. The hardwiring of development: organization and function of genomic regulatory systems. Development. 1997;124:1851–1864. doi: 10.1242/dev.124.10.1851. [DOI] [PubMed] [Google Scholar]
- 91.Baverstock K, Rönkkö M. Epigenetic regulation of the mammalian cell. PLoS One. 2008;3:e2290. doi: 10.1371/journal.pone.0002290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang S, Ingber DE. A non-genetic basis for cancer progression and metastasis: self-organizing attractors in cell regulatory networks. Breast Dis. 2006;26:27–54. doi: 10.3233/bd-2007-26104. [DOI] [PubMed] [Google Scholar]
- 93.Hochedlinger K, Blelloch R, Brennan C, Yamada Y, Kim M, Chin L, Jaenisch R. Reprogramming of a melanoma genome by nuclear transplantation. Genes Dev. 2004;18:1875–1885. doi: 10.1101/gad.1213504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McGarvey KM, Fahrner J, Greene E, Martens J, Jenuwein T, Baylin S. Silenced tumor suppressor genes reactivated by DNA demethylation do not return to a fully euchromatic chromatin state. Cancer Res. 2006;66:3541–3549. doi: 10.1158/0008-5472.CAN-05-2481. [DOI] [PubMed] [Google Scholar]
- 95.Hyland EM, Cosgrove MS, Molina H, Wang D, Pandey A, Robert J, Cottee RJ, Boeke JD. Insights into the Role of Histone H3 and Histone H4 Core Modifiable Residues in Saccharomyces cerevisiae . Mol Cell Biol. 2005;25:10060–10070. doi: 10.1128/MCB.25.22.10060-10070.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ptashne M. On the use of the word ‘epigenetic’. Curr Biol. 2007;17:R233–R236. doi: 10.1016/j.cub.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 97.Henikoff S, Shilatifard A. Histone modification: cause or cog? Trends Genet. 2011;27:389–396. doi: 10.1016/j.tig.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 98.Wu W, Cheng Y, Keller CA, Ernst J, Kumar SA, Mishra T, Morrissey C, Dorman CM, Chen KB, Drautz D, Giardine B, Shibata Y, Song L, Pimkin M, Crawford GE, Furey TS, Kellis M, Miller W, Taylor J, Schuster SC, Zhang Y, Chiaromonte F, Blobel GA, Weiss MJ, Hardison RC. Dynamics of the epigenetic landscape during erythroid differentiation after GATA1 restoration. Genome Res. 2011 doi: 10.1101/gr.125088.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 100.Tapscott SJ, Davis RL, Thayer MJ, Cheng PF, Weintraub H, Lassar AB. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988;242:405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- 101.Koutroubas G, Merika M, Thanos D. Bypassing the requirements for epigenetic modifications in gene transcription by increasing enhancer strength. Mol Cell Biol. 2008;28:926–938. doi: 10.1128/MCB.01344-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Solovei I, Kreysing M, Lanctôt C, Kösem S, Peichl L, Cremer T, Guck J, Joffe B. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell. 2009;137:356–368. doi: 10.1016/j.cell.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 103.Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W, van Steensel B. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453:948–951. doi: 10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- 104.Wallace DC, Fan W. Energetics, epigenetics, mitochondrial genetics. Mitochondrion. 2009;10:12–31. doi: 10.1016/j.mito.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pollard PJ, Loenarz C, Mole DR, McDonough MA, Gleadle JM, Schofield CJ, Ratcliffe PJ. Regulation of Jumonji-domain-containing histone demethylases by hypoxia-inducible factor (HIF)-1alpha. Biochem J. 2008;416:387–394. doi: 10.1042/BJ20081238. [DOI] [PubMed] [Google Scholar]
- 106.Liu G, Bollig-Fischer A, Kreike B, van de Vijver MJ, Abrams J, Ethier SP, Yang ZQ. Genomic amplification and oncogenic properties of the GASC1 histone demethylase gene in breast cancer. Oncogene. 2009;28:4491–4500. doi: 10.1038/onc.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Raser JM, O’Shea EK. Control of stochasticity in eukaryotic gene expression. Science. 2004;304:1811–1814. doi: 10.1126/science.1098641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pirone JR, Elston TC. Fluctuations in transcription factor binding can explain the graded and binary responses observed in inducible gene expression. J Theor Biol. 2004;226:111–121. doi: 10.1016/j.jtbi.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 109.Fodor BD, Shukeir N, Reuter G, Jenuwein T. Mammalian Su(var) genes in chromatin control. Annu Rev Cell Dev Biol. 2010;26:471–501. doi: 10.1146/annurev.cellbio.042308.113225. [DOI] [PubMed] [Google Scholar]
- 110.Becskei A, Scherrer S, Kelemen JZ, Murray AE. Modeling of chromosomal epigenetic silencing processes. Transcription. 2011;2:173–178. doi: 10.4161/trns.2.4.16344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cotterman R, Jin VX, Krig SR, Lemen JM, Wey A, Farnham PJ, Knoepfler PS. N-Myc regulates a widespread euchromatic program in the human genome partially independent of its role as a classical transcription factor. Cancer Res. 2008;68:9654–9662. doi: 10.1158/0008-5472.CAN-08-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reik W. Wolf Reik: inheritance beyond DNA. Interview by Nicole Lebrasseur. J Cell Biol. 2010;188:302–303. doi: 10.1083/jcb.1883pi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sha K, Gu SG, Pantalena-Filho LC, Goh A, Fleenor J, Blanchard D, Krishna C, Fire A. Distributed probing of chromatin structure in vivo reveals pervasive chromatin accessibility for expressed and non-expressed genes during tissue differentiation in C. elegans . BMC genomics. 2010;11:465. doi: 10.1186/1471-2164-11-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thomas G, Lange HW, Hempel K. Kinetics of histone methylation in vivo and its relation to the cell cycle in Ehrlich ascites tumor cells. Eur J Biochem. 1975;51:609–615. doi: 10.1111/j.1432-1033.1975.tb03963.x. [DOI] [PubMed] [Google Scholar]
- 115.Deal RB, Henikoff S. Catching a glimpse of nucleosome dynamics. Cell Cycle. 2010;9:3389–3390. doi: 10.4161/cc.9.17.13091. [DOI] [PubMed] [Google Scholar]
- 116.Vermeulen M, Mulder KW, Denissov S, Pijnappel WW, van Schaik FM, Varier RA, Baltissen MP, Stunnenberg HG, Mann M, Timmers HT. Selective anchoring of TFIID to nucleosomes by trimethylation of histone H3 lysine 4. Cell. 2007;131:58–69. doi: 10.1016/j.cell.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 117.Tan M, Luo H, Lee S, Jin F, Yang JS, Montellier E, Buchou T, Cheng Z, Rousseaux S, Rajagopal N, Lu Z, Ye Z, Zhu Q, Wysocka J, Ye Y, Khochbin S, Ren B, Zhao Y. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011;146:1016–1028. doi: 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dodd IB, Micheelsen MA, Sneppen K, Thon G. Theoretical analysis of epigenetic cell memory by nucleosome modification. Cell. 2007;129:813–822. doi: 10.1016/j.cell.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 119.Annunziato AT. Split decision: what happens to nucleosomes during DNA replication? J Biol Chem. 2005;280:12065–12068. doi: 10.1074/jbc.R400039200. [DOI] [PubMed] [Google Scholar]
- 120.Rusché LN, Kirchmaier AL, Rine J. Ordered nucleation and spreading of silenced chromatin in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:2207–2222. doi: 10.1091/mbc.E02-03-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lachner M, O’Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- 122.Cheutin T, McNairn AJ, Jenuwein T, Gilbert DM, Singh PB, Misteli T. Maintenance of stable heterochromatin domains by dynamic HP1 binding. Science. 2003;299:721–725. doi: 10.1126/science.1078572. [DOI] [PubMed] [Google Scholar]
- 123.Michel D. Hierarchical cooperativity mediated by chromatin remodeling; the model of the MMTV transcription regulation. J Theor Biol. 2011;287:74–81. doi: 10.1016/j.jtbi.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 124.von Bertalanffy L. An outline of general system theory. Br J Philos Sci. 1950;1:134–165. doi: 10.1093/bjps/I.2.134. [DOI] [Google Scholar]
- 125.Shoval O, Alon U. SnapShot: network motifs. Cell. 2010;143:326-e1. doi: 10.1016/j.cell.2010.09.050. [DOI] [PubMed] [Google Scholar]
- 126.Kauffman SA. Metabolic stability and epigenesis in randomly constructed genetic nets. J Theor Biol. 1969;22:437–467. doi: 10.1016/0022-5193(69)90015-0. [DOI] [PubMed] [Google Scholar]
- 127.Huang S. Systems biology of stem cells: three useful perspectives to help overcome the paradigm of linear pathways. Philos Trans R Soc Lond B. 2011;366:2247–2259. doi: 10.1098/rstb.2011.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hayden EC. Evidence of altered RNA stirs debate. Nature. 2011;473:432. doi: 10.1038/473432a. [DOI] [PubMed] [Google Scholar]
- 129.Madhani HD, Francis NJ, Kingston RE, Kornberg RD, Moazed D, Narlikar GJ, Panning B, Struhl K. Epigenomics: a roadmap, but to where? Science. 2008;322:43–44. doi: 10.1126/science.322.5898.43b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burrill DR, Silver PA. Making cellular memories. Cell. 2010;140:13–18. doi: 10.1016/j.cell.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]