Abstract
Toll-like receptors (TLR) are pattern-recognition receptors that recognize a broad variety of structurally conserved molecules derived from microbes. The recognition of TLR ligands functions as a primary sensor of the innate immune system, leading to subsequent indirect activation of the adaptive immunity as well as none-immune cells. However, TLR are also expressed by several T cell subsets, and the respective ligands can directly modulate their effector functions. The present review summarizes the recent findings of γδ T cell modulation by TLR ligands. TLR1/2/6, 3, and 5 ligands can act directly in combination with T cell receptor (TCR) stimulation to enhance cytokine/chemokine production of freshly isolated human γδ T cells. In contrast to human γδ T cells, murine and bovine γδ T cells can directly respond to TLR2 ligands with increased proliferation and cytokine production in a TCR-independent manner. Indirect stimulatory effects on IFN-γ production of human and murine γδ T cells via TLR-ligand activated dendritic cells have been described for TLR2, 3, 4, 7, and 9 ligands. In addition, TLR3 and 7 ligands indirectly increase tumor cell lysis by human γδ T cells, whereas ligation of TLR8 abolishes the suppressive activity of human tumor-infiltrating Vδ1 γδ T cells on αβ T cells and dendritic cells. Taken together, these data suggest that TLR-mediated signals received by γδ T cells enhance the initiation of adaptive immune responses during bacterial and viral infection directly or indirectly. Moreover, TLR ligands enhance cytotoxic tumor responses of γδ T cells and regulate the suppressive capacity of γδ T cells.
Keywords: γδ T cells, TLR1-10, Cytokines, Chemokines
Introduction
γδ T cells are a small subset of unconventional CD3+ T cells that display characteristics of the innate and the adaptive immune system. In contrast to αβ T cells, human γδ T cells recognize non-proteinaceous antigens without requirement of antigen processing and independently of classical MHC molecules [1, 2]. Vδ2- and Vδ1-expressing γδ T cells are presented only in small numbers in peripheral blood of humans, whereas Vδ1-expressing γδ T cells are relatively abundant in intestine, skin epithelia, and uterus of humans. Under pathological conditions, γδ T cells can quickly expand and infiltrate into lymphoid compartments and other tissues [1, 3, 4]. Human Vδ2 γδ T cells recognize phosphorylated intermediates of the non-mevalonate pathway of bacterial isoprenoid biosynthesis pathway, whereas Vδ1 γδ T cells recognize stress-inducible MHC class I chain-related antigens (MIC) A/B or self-lipid presented antigens by CD1c [5–7]. Both γδ T cell subsets rapidly release IFN-γ, TNF-α, MIP-1α/β (CCL3/4), RANTES (CCL5), and granzymes after TCR stimulation, thereby activating other cells of the immune system [4]. There are alternative nomenclatures for murine Vγ/Vδ genes in this review. We follow the nomenclature of Heilig and Tonegawa [8].
In mice, distinct differences in the T cell receptor (TCR) repertoire of γδ T cells correlate with their anatomical location. Murine Vγ3Vδ1- or Vγ5Vδ1-expressing dendritic epidermal T cells (DETC) recognize stressed, transformed, or damaged keratinocytes, whereas Vγ6Vδ1-expressing subset seeding in the uterus, the tongue, and the peritoneal cavity have a protective role against infections. Vγ1 and Vγ2 γδ T cells colonize the lymphoid system and Vγ4 γδ T cells additionally the lung, thereby playing a role in immune surveillance against inflammation and infection. Vγ7-expressing intraepithelial T cells (IEL) are important in the control of epithelial homeostasis [1, 9, 10].
Pattern-recognition receptors (PRR) such as Toll-like receptors (TLR) are widely expressed by various cells of the immune system, on none-immune cells such as epithelial cells, endothelial cells, keratinocytes, as well as on different tumor cells [11]. TLR sense diverse pathogen-associated molecular patterns (PAMP), e.g., lipid-containing ligands, proteinaceous ligands, and nucleic acid ligands [12]. Besides their direct effects on cells of the innate immunity such as dendritic cells (DC) and NK cells, TLR have also been shown to co-stimulate TCR-activated human T cells [13–15]. In γδ T cells, TLR1/TLR2, TLR2/TLR6, TLR3, and TLR5 ligands induce an enhanced production of cytokines and chemokines and an up-regulation of activation markers [16, 17].
In this review, we provide insights into the modulation of TLR on γδ T cells and their interactions with DC or tumor cells. This forms the basis for new perspectives of the immunotherapeutic manipulation of γδ T cell responses.
TLR: history, structure, and signaling
Toll was first discovered in Drosophila melanogaster involved in dorsoventral embryonic development and antifungal immune responses [18, 19]. The first human homolog of the Drosophila Toll protein was cloned in 1997 by Medzhitov et al. and was named Toll-like receptor due to the similarity to Toll protein of Drosophila [20]. So far, 12 mammalian TLR (human: TLR1-10, murine: TLR1-9 and TLR 11-13) have been discovered [11, 12, 21, 22]. TLR belong to the TLR/IL-1 receptor (TIR) superfamily and to the type I transmembrane glycoprotein receptor family. They contain an ectodomain composed of 19–25 leucine-rich repeats for recognition of ligands and an intracytoplasmic TIR domain that is conserved among all TLR [23]. Moreover, the TIR domain is required for downstream signaling by recruiting different combinations of five TIR-domain-containing adaptor molecules: Myeloid differentiation factor 88 (MyD88), MyD88-adaptor-like (MAL)/TIR-associated protein (TRIAP), TIR-domain-containing adaptor inducing interferon (IFN) β (TRIF or TICAM1), TRIF-related adaptor molecule (TRAM or TICAM2), and negative adaptor sterile α- and armadillo-motif-containing protein (SARM). MyD88 is utilized by all kinds of TLR, except for TLR3. MyD88 activates transcription factor NFκB and interferon regulatory transcription factors (IRFs)-1, -5, and -7, whereas MAL and TRAM act as bridging adaptors. MAL recruits MyD88 to TLR2 and 4, and TRAM recruits TRIF to TLR4 resulting in the activation of NFκB and IRF-3. TLR3 recruits TRIF, resulting in the induction of a signaling cascade, which activate IRF-3 and -7, and NFκB. SARM inhibits TRIF-mediated transcription factor activation [24, 25]. Most TLR including TLR1, 2, 4, 5, 6, 10, and 11 (only in mice) are expressed at the cell surface, whereas TLR 3, 7, 8, and 9 are located on membranes of intracellular compartments such as endolysosomes [12, 26]. TLR3, TLR7, and TLR9 are localized in the membrane of endoplasmic reticulum (ER) in unstimulated DC. After TLR ligand stimulation, trafficking of TLR7 and 9 from the ER to endolysosomes is regulated by an ER-localizing protein UNC93B1. TLR3 also interacts with UNC93B1, but a role of UNC93B1 in TLR3 trafficking has not been described [27]. A further protein named PRAT4A is also required for trafficking of TLR9, but not for trafficking of TLR3 [28].
TLR ligands, TLR agonists, and antagonists
TLR recognize PAMP of bacteria, viruses, parasites, and fungi either alone or as heterodimers formed with other TLR or non-TLR receptors [12, 21]. TLR agonists including natural TLR ligands or synthetic analogues of natural TLR ligands offer a therapeutic potential for treatment of cancer, infectious diseases, and type I allergy or as vaccine adjuvant [29, 30]. TLR antagonists bind to TLR but inhibit the biological activity of the respective TLR [e.g., TLR4 antagonist, lipopolysaccharide (LPS) from R. sphaeroides or TLR9 antagonist, oligonucleotide (ODN)-TTAGGG]. Moreover, neutralizing antibodies, TLR-related short hairpin (sh) RNAs and inhibitors of the TLR signaling cascade can also function as TLR antagonists [29, 31, 32].
TLR expression in T cells
TLR expression was initially detected in innate immune cells and epithelial cells where they mediate immune responses upon infection. In recent years, it has been observed that TLR expression is not restricted to the innate immune system. TLR were detected in B cells as well as in T cells and in their subpopulations [14, 15, 33]. Recent studies (as described below) identified TLR expression in γδ T cells possibly playing an important role in early immune responses of γδ T cells against different pathogens.
Co-stimulation by TLR ligands is influenced by the activation status of γδ T cells
Due to their small number in the peripheral blood, human γδ T cells are often expanded for several days, and thereby γδ T cell lines are established, before functional studies were performed [34–36]. However, the response to TLR ligand co-stimulation of expanded γδ T cell lines differs from the response of freshly isolated γδ T cells. The co-stimulation of IFN-γ production in TCR/TLR3 ligand stimulated freshly isolated γδ T cells compared to TCR stimulation alone is much higher than in similar experiments with γδ T cell lines [17, 37]. An explanation for this discrepancy is given by the enhanced IFN-γ production of γδ T cell lines after TCR stimulation compared to the production of TCR-stimulated freshly isolated γδ T cells. Therefore, an additional stimulus via TLR-ligand stimulation is more effective in freshly isolated γδ T cells than in γδ T cell lines. Moreover, the supplementation of IL-2 to the cultured γδ T cell lines might also play an important role in the extent of IFN-γ production as described in more detail below. In literature, γδ T cells are often termed “peripheral” or “circulating” γδ T cells independently of their activation status in vitro [34, 35]. To easily distinguish in this review between these different activation situations, human γδ T cells isolated from PBMC will be termed as follows:
-
(i)
“freshly isolated γδ T cells” are ex vivo isolated γδ T cells (“resting cells”), which were either positively isolated from PBMC by magnetic sorting or negatively by magnetic sorting followed by flow cytometry sorting. In general, these cells are highly purified (>98%). The initial stimulation of these cells resulted in “short-term activated γδ T cells” [16, 17, 37–42].
-
(ii)
“short-term γδ T cell lines” are generated out of PBMC, which were stimulated with γδ T cell-specific antigens (e.g., phosphoantigens (PAg), aminobisphosphonates (N-BP) or alkylamines) and IL-2 for 2–3 weeks. The purity of these cells was in the range of 70–95% depending on the stimulus and the frequency of IL-2 supplementation [34, 42]. Several investigators sorted these cells to enhance their purity (>98%) [36]. Then, these cells are termed “highly purified short-term γδ T cell lines”. To establish “γδ T cell lines”, allogeneic or autologous feeder cells (irradiated PBMC and/or EBV-transformed B cells), IL-2 (or other cytokines) and antigens (e.g., PAg, N-BP or PHA) were used every 2–3 weeks to expand the cells. IL-2 is given every second or third day when cells are split [17, 35, 37, 43].
-
(iii)
“γδ T cell clones” are established from freshly isolated-, short-time activated γδ T cells or short-term γδ T cell lines by limiting dilution assays or FACS cloning device. For expansion, feeder cells as mentioned under (ii) are necessary [35, 43–46].
TLR1, TLR2, and TLR6 expression
TLR2 recognizes PAMP from various pathogens including peptidoglycan (PGN), lipopeptides, glycolipids, or glycosylphosphatidylinositol-anchored structures, e.g., lipoteichoic acid (LTA), lipoarabinomannan, non-enterobacterial LPS from bacteria, zymosan from fungi, and hemagglutinin protein from measles viruses [22, 29, 47–49]. TLR 2 usually forms a heterodimer with TLR1, TLR6, TLR10, or possibly with non-TLR such as CD36 and dectin-1 [50–52]. In humans, TLR1/2 heterodimer recognizes triacylated lipopeptides such as Pam3CSK4, whereas in mice an additional TLR1-independent TLR2 activation upon activation with Pam3CSK4 has been described [53, 54]. TLR2 requires TLR6 as a co-receptor for recognition of macrophage activating lipopeptide (MALP-2) from Mycoplasma fermentans and for diacylated lipopeptide such as FSL-1, but not for Pam2CSK4 [53, 55, 56]. Rose and colleagues recently identified FSL-1 as a useful TLR2 agonist, which induced significant resistance to herpes simplex virus type II (HSV-2) infection when applied in mice or human vaginal epithelial cell cultures [57]. Moreover, Lu and colleagues described a mushroom extract named Polysaccharide Krestin (PSK) as a useful TLR2 agonist with inhibitory activity on breast cancer growth in tumor-bearing new transgenic mice, but not in TLR2-/- mice. The inhibition of tumor growth seemed to be mediated by activation of TLR2 expressing CD8+ T cells and NK cells [58]. Activation of TLR2 is also reported in several other diseases such as rheumatoid arthritis, sepsis, or diabetes. Neutralizing anti-TLR2 Ab T2.5 combined with anti-TLR4/MD-2 Ab and antibiotics protected mice against sepsis induced by E. coli or Salmonella enterica [59, 60]. An exhaustive examination of available TLR2 agonist and antagonists and the development of new compounds are under investigation [29, 30]. MAL, for example, seems to be an attractive therapeutic target for several diseases, because TLR2 requires MAL for the recruitment of MyD88 and thus for TLR2 activation [29].
TLR1, 2, and 6 expression in γδ T cells
Hedges and colleagues detected a weak TLR2 and TLR6 expression in human, short-term γδ T cell lines by Affymetrix GeneChip array [36]. Moreover, they observed TLR1-10 mRNA expression in freshly isolated, sorted bovine γδ T cells by RT-PCR. The expression of TLR1-10 mRNA in short-term γδ T cell lines (<90% purity) was reported by Deetz et al. [34]. Our own data revealed that non-activated, freshly isolated γδ T cells (>98% purity) from peripheral blood of human donors also express TLR1, 2, and 6 mRNA as measured by quantitative RT-PCR [16, 38]. Although expression levels varied among donors, and between Vδ1 and Vδ2 γδ T cell subsets, TLR1 and TLR2 expression was generally more abundant than expression of TLR6. Despite strong RNA expression, TLR1 expression on the protein level was very low on freshly isolated Vδ1 and Vδ2 γδ T cell in some donors and absent in most analyzed donors. TLR2 and TLR6 protein was slightly expressed on Vδ1 as well as on Vδ2 T cells. TCR stimulation but not TLR ligands induced an up-regulation of TLR after 20 h, also of weakly expressed TLR2 (Wesch & Peters, unpublished data). Similar results with an up-regulation of TLR2 and TLR6 after TCR stimulation were observed in freshly isolated αβ T cells [61]. In contrast, staining protocols of Deetz et al. failed to confirm TLR2 expression on the cell surface [34]. Based on our experience with different anti-TLR2 mAb and differential staining protocols, initial staining of cells with anti-TLR2 mAb and subsequent washing of the cells before staining with additional mAb (e.g., anti-TCRγδ mAb) is recommended [16]. The simultaneous staining with various mAb diluted the concentration of the applied anti-TLR2 mAb, which could result in the apparent absence of TLR2 staining [16].
Recent studies have shown that murine γδ T cells increased in the peritoneal cavity of C3H/HeN (missense mutation in the cytoplasmic domain of TLR4) and C3H/HeJ mice treated with native lipid A derived from E. coli [62]. TLR2 mRNA expression was detected in these expanded Vγ6Vδ1 γδ T cell subset (>99% after sorting), moreover, TLR2-deficient mice showed an impaired increase in γδ T cells after Lipid A injection [62]. Martin et al. [63] demonstrated that only CCR6+ IL-17-producing peritoneal γδ T cells (sorted cells) expressed TLR1, TLR2 and dectin-1 mRNA, but not TLR4 mRNA in C57BL/6 mice63. Transiently enhanced TLR2 and low TLR4 expression was observed in murine blood, spleen, and mesenteric lymph node γδ T cells 24 h after burn injury in C57BL/6 mice by flow cytometric analysis [64].
Direct co-stimulatory effects of TLR1, 2, and 6 ligands on γδ T cells
The results by Deetz et al. with short-term γδ T cell lines as well as our own results with freshly isolated γδ T cells clearly demonstrated that a cross-talk of TCR- and TLR2 signaling is necessary to enhance effector function of human peripheral blood γδ T cells [16, 34, 38]. Deetz et al. described a co-stimulatory effect of triacylated lipopeptide Pam3CysSK4 on IFN-γ production and degranulation (determined by lysosome-associated membrane CD107a protein expression on the cell surface) of activated γδ T cells. Moreover, an impaired IFN-γ production and proliferation of short-term activated Vδ2 γδ T cells mediated by the inhibition of TNF-α signaling could be partially reverted by Pam3CysSK4. In this context, the usage of TLR2 agonists during anti-TNF-α therapy could be supportive to ensure IFN-γ production of γδ T cells, which, e.g., support maturation of DC [34, 65].
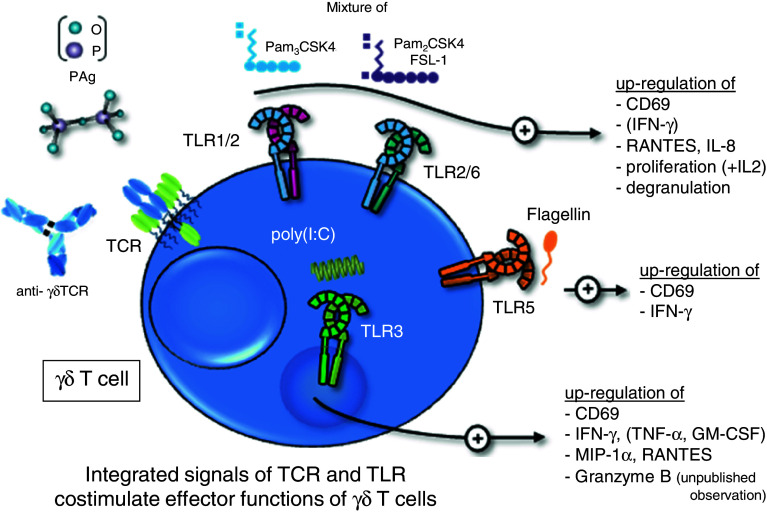
The co-stimulatory effects of Pam3CSK4 could be partially confirmed with freshly isolated γδ T cells depending on the presence of TLR1, which forms a heterodimer with TLR2 and is necessary for the recognition of triacylated lipopeptides [16, 66]. We observed a donor-dependent variability of TLR1 expression on the surface of freshly isolated γδ T cells, similarly to results with αβ T cells [16, 61]. Moreover, diacylated lipopeptides such as Pam2CSK4 and FSL-1 induced a co-stimulatory effect in freshly isolated γδ T cells in the presence of TCR stimulation. To compensate for the donor-specific individual variability in the expression of TLR1, we used a mixture of TLR2 ligands (Pam3CSK4, Pam2CSK4, and FSL-1) to investigate the co-stimulatory effects on freshly isolated γδ T cells. An enhanced production of RANTES and IL-8 involved in the recruitment of inflammatory cells was observed in freshly isolated Vδ1 as well as in Vδ2 γδ T cells. IFN-γ production was only detected in freshly isolated Vδ2 T cells (Fig. 1). The differential capacity of both γδ T cells subsets to produce IFN-γ could be explained by their distinct roles in immunity. Vδ1 γδ T cells are rare in the peripheral blood, whereas pro-inflammatory cytokine-producing Vδ2 γδ T cells involved in immune responses against bacteria are the main γδ T cell population in the peripheral blood. Additionally, Hedges and coworkers observed a slight enhancement of MIP-1α and MIP-1β mRNA in human activated γδ T cells after stimulation with TLR2 ligand PGN alone [36]. The weak response could be explained by the absence of TCR-cross linking. These data suggest that TLR-mediated signals received by γδ T cells might enhance the initiation of adaptive immune responses during bacterial infections independently of APC.
Fig. 1.
A summary of the direct co-stimulatory effects of TLR ligands on human γδ T cells. Freshly isolated human γδ T cells (Vδ1 or Vδ2 subset) are activated via their T cell receptor with anti-γδTCR mAb or phosphoantigens (PAg) together with a mixture of Pam2CSK4, Pam3CSK4, and FSL-1 (TLR1/2/6 ligands), with poly(I:C) (TLR3 ligand) or flagellin (TLR5 ligand). The co-stimulation significantly enhances the expression of activation marker CD69, cytokine/chemokine or granzyme B production, degranulation, or proliferation as indicated. Cytokines presented in brackets are exclusively produced by Vδ2 γδ T cells. In compliment for references [16, 17, 34, 37, 38, 44]
In contrast to human γδ T cells, purified neonatal bovine γδ T cells from peripheral blood expressed increased MIP-1α and RANTES levels in direct response to PGN. Moreover, Lubick et al. demonstrated the expression of CD36 on resting bovine γδ T cells [67]. CD36 has been shown to facilitate TLR2 responses against LTA in bovine γδ T cells analyzed by enhanced MIP-1α production and by blocking activity of anti-CD36 mAb [67]. CD36 expression is not analyzed for human γδ T cells, which could possibly explain the failure of LTA from S. pyrogenes or B. subtilis to enhance TCR-mediated IFN-γ production as observed by Shrestha et al. [42].
The involvement of the TCR in the recognition of TLR ligand of murine and human γδ T cells has been controversially discussed. Mokuno and coworkers described an increased proliferative of murine peritoneal Vγ6Vδ1 γδ T cells after application of lipid A from E. coli as well as from P. gingivalis in vivo and in vitro in a TCR-independent manner [62]. Studies by Mokuno and Leclercq et al. suggested that γδ T cells with invariant Vγ5Vδ1 or Vγ6Vδ1 TCR, which develop in the thymus and the epidermis of mice, rapidly respond to bacterial components via TLR2 [62, 68]. The discrimination of murine peritoneal γδ T cells with respect to their IL-17 and IFN-γ production, demonstrated that only IL-17-producing cells expressed TLR1 and 2, and could directly interact with bacterial pathogens [63]. Moreover, the addition of IL-23 induced an expansion of IL-17 production by peritoneal CCR6+ TLR1/2 expressing γδ T cells and the recruitment of neutrophils [63]. γδ T cell-deficient mice show particular defects in neutrophil-dependent inflammatory responses [69]. Moreover, adoptive transfer of γδ T cells from wild-type B6 into TLR2-deficient mice applied with Pam3CSK4 resulted in a TCR-independent direct effect against pathogen-derived molecules and an expansion of IL-17 producing γδ T cells [63]. Furthermore, an increased number of murine peripheral blood and splenic γδ T cells, which transiently up-regulated TLR2, was reported 24 h after burn injury in C57BL/6 mice [64]. Taken together, murine Vγ5Vδ1-, IL-17-producing Vγ6Vδ1-, blood and splenic γδ T cells respond to bacterial components via TLR2 in a TCR-independent manner, thereby recruiting neutrophils.
The activation and mobilization of γδ T cells in patients with systemic inflammatory response syndrome has also been observed without information of TLR expression in these γδ T cells [70].
Indirect effects of TLR2 ligands on γδ T cells response
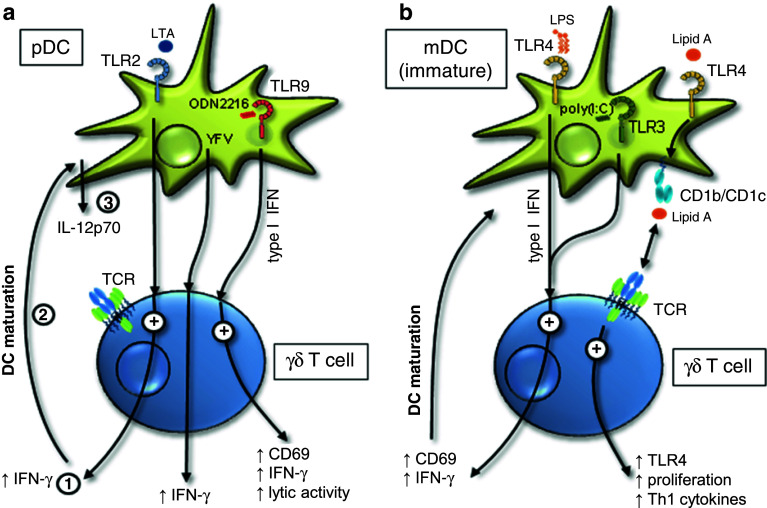
Collins et al. described an enhancement of CD25 expression on human Vδ1 γδ T cell clones derived from synovial fluid of Lyme arthritis patients or murine splenic short-term activated γδ T cell lines after stimulation with Borrelia burgdorferi in the presence of monocyte-derived immature DC. When they analyzed γδ T cell responses to B. burgdorferi in mice, they found that DC from TLR2- and MyD88-deficient mice induce only weak CD25 expression on γδ T cells after B. burgdorferi stimulation, in contrast to DC from wild-type mice. Therefore, the authors suggested that the activation of γδ T cells by B. burgdorferi is mediated indirectly via TLR stimulation on DC or monocytes. DC or monocyte-derived cytokines potentiate activation of Vδ1 γδ T cells from Lyme arthritis patients [45].
Similar to the results with Vδ1 γδ T cells, a mutually co-stimulatory effect between short-term activated Vδ2 γδ T cells and immature DC in humans has been described by Shrestha et al. [42]. TLR2 ligands such as LTA from S. aureus, S. pyrogens, and B. subtilis or mycobacteria lysates/extracts stimulated DC, thereby leading to enhanced IFN-γ production of short-term activated Vδ2 γδ T cells. Conversely, IFN-γ produced by Vδ2 γδ T cells enhanced maturation of immature DC, thereby leading to IL-12p70 production by DC (Fig. 2a). The authors suggested that IFN-γ production by short-term activated Vδ2 γδ T cells co-stimulated effective priming of Th1 CD4 T cells by DC, which is critical when the abundance of TLR2 ligand for DC maturation is limited [42]. Moreover, Shrestha suggested that an incomplete maturation of DC mediated by an intrinsically weak pathogen or by a defect TLR signaling could have particular importance for the immune response of CD4 T cells [42]. A defect in TLR2 signaling is associated with a lepromatous type but not with a tuberculoid type of leprosy and decreased IL-12 serum levels in leprosy patients [71–73]. Leprosy is associated with priming of non-protective, IL-10 secreting CD4 T cells and thereby with an uncontrolled proliferation of Mycobacteria due to the failure of TLR2 ligand mediated maturation of DC [42, 74]. Vδ2 γδ T cells were still responsive in patients, but delivered different co-stimulatory effects to DC in the absence of TLR2-signaling [42].
Fig. 2.
A summary of indirect effects of TLR ligand activated DC on γδ T cells. a TLR2 ligand LTA, TLR9 ligand CpG ODN2216, or Yellow Fever Virus (YFV) interact with plasmacytoid DC resulting in enhanced IFN-γ production of γδ T cells (1). Indirect effects of TLR2 ligands on γδ T cells trigger DC maturation (2), and IL-12p70 production of DC (3). b TLR3- and TLR4 ligands enhance the activation human γδ T cells via the stimulation of type I IFN production in myeloid DC. In addition, TLR4 ligand Lipid A presented by DC via CD1b/CD1c increases proliferation and TLR4 up-regulation in γδ T cells. In compliment for references [35, 39–42]
In both publications [42, 45], a modulation of the close interaction of γδ T cells and immature DC by TLR ligands is described, which might enhance the response to bacteria or influence the priming and cytokine production of antigen-specific αβ T cells.
TLR3 expression
TLR3 recognizes a genomic RNA purified from double-stranded RNA (dsRNA) viruses such as reoviruses, synthetic analogue of dsRNA, polyinosinic-polycytidylic acid [poly(I:C)], and small interfering (si) RNA [12, 75, 76]. Additionally, TLR3 binds to dsRNA produced during the course of replication of single-stranded RNA (ssRNA) viruses such as West Nile virus (WNV), respiratory syncytial virus (RSV) and encephalomyocarditis virus [12, 77, 78]. TLR3 triggers antiviral immune responses through the production of type I IFN. Tabeta et al. reported that TLR3−/− and TLR9CpG1/CpG1 (codominant CpG-ODN unresponsive phenotype) mice are susceptible to lethal infection with murine cytomegalovirus (MCMV) [79]. TLR3 deficiency in humans is associated with the susceptibility to HSV-1 [80]. In vivo application of the TLR3 agonist poly(I:C) is described to have a lot of side effects such as renal failure and hypersensitivity reactions, whereas Ampligen®, also known as poly[I:C(12)U] was well tolerated (e.g. after intravenous administration to HIV+ patients). Ampligen® has been produced under GMP conditions for clinical use. Ampligen® promotes DC maturation and Th1 T cell responses [81].
TLR3 expression in γδ T cells
A weak expression of TLR3 mRNA in human short-term γδ T cell lines was reported by Hedges et al. [36]. We and others identified the expression of TLR3 mRNA in freshly isolated γδ T cells from adult donors, and, additionally, demonstrated that the TLR3 mRNA expression in γδ T cells was higher than in αβ T cells [17, 38, 44]. Interestingly, TLR3 mRNA expression in γδ T cell clones derived from blood of pre-termed neonates was significantly impaired compared to γδ T cell clones from full-termed neonates or adults [44]. Moreover, we detected a prominent intracellular TLR3 expression on protein level by flow cytometry and confocal laser scanning microscopy [17]. TLR3 was not expressed on the cell surface of non-activated, freshly isolated γδ T cells, but was up-regulated on the cell surface 24 h after TCR-stimulation, and not after TLR3 ligand incubation in freshly isolated human γδ T cells [17]. The expression of TLR3 on the cell surface after TCR stimulation might provide a basis for poly(I:C) entry. TLR3 inhibition experiments with anti-TLR3 mAb suggest a functional significance of TLR3 on the cell surface [17]. Moreover, a pronounced TLR3 cell surface expression has been reported for airway epithelial cells [82, 83]. In contrast to the short-term activated γδ T cells, TLR3 was only weakly expressed on the cell surface in γδ T cell lines and clones cultured over a longer period [17].
Direct co-stimulatory effects of TLR3 ligands on γδ T cells
In agreement with the lack of TLR3 cell surface expression on freshly isolated γδ T cells, these cells did not or only very marginally respond to TLR3 ligand poly(I:C) alone in the absence of APC [17, 40]. However, an additional signal via TCR-crosslinking (e.g. anti-TCRγδ mAb or PAg,) enhanced CD69 expression and IFN-γ, MIP-1α and RANTES production in freshly isolated, short-term activated γδ T cells [16, 17, 38] (Fig. 1). In this context, purified, short-term activated Vδ2 γδ T cells produced remarkably higher levels of IFN-γ than purified, short-term activated Vδ1 γδ T cells. The levels of MIP-1α and RANTES were comparable in both γδ T cell subsets [16]. TNF-α, GM-CSF and IL-8 were produced only by short-term activated Vδ2 γδ T cells. To rule out that positively selected γδ T cells, which are highly CD69 positive, give rise to artificial results, negatively sorted γδ T cells were also used in additional studies. In line with positively isolated γδ T cells, IFN-γ production and CD69 expression were enhanced after TCR activation with PAg and further up-regulated in the presence of poly(I:C) [37]. Increased levels of IFN-γ mRNA and IFN-γ protein were also observed in short-term activated γδ T cell lines/clones derived from human adults or term babies after TCR stimulation (anti-CD3 mAb) in combination with poly(I:C) by Gibbons et al. [44]. In comparison to freshly isolated, short-term activated γδ T cells, γδ T cell lines and clones cultured for a longer period did only slightly express TLR3 on the cell surface, which fits well with the moderate [17] or absent [35, 40] effect on IFN-γ secretion after combined poly(I:C) and TCR stimulation. The reason for this discrepancy in TLR3 cell surface expression on freshly isolated γδ T cells versus T cell lines/clones is not clear. However, the different effects of an integrated TCR/TLR3 ligand signal on freshly isolated, short-termed activated γδ T cells versus γδ T cell lines/clones could be explained by the different activation status and the IL-2 requirement of these cells, which we have previously discussed [16, 17]. Briefly, the enhanced production of IFN-γ and chemokines were measured 24 h after initial TCR/poly(I:C) stimulation in the absence or presence of low concentrations of IL-2 (25–50 IU/ml). The addition of poly(I:C) to freshly isolated γδ T cells might replace the requirement of IL-2 for enhanced IFN-γ production. Freshly isolated γδ T cells are not able to produce IL-2 [84]. The IL-2 production of γδ T cell lines/clones, or rather the addition of high concentrations of exogenous IL-2 (100–300 IU/ml), resulted in an enhanced IFN-γ production. The IL-2 production could only slightly or not at all be further increased with additional poly(I:C) stimulus, because the maximal capacity of INF-γ production has been reached. The cross-talk between TCR and TLR signaling should be investigated in more detail to reveal the difference between freshly isolated, short-term activated γδ T cells and long-term cultured γδ T cells.
In addition to the cytokines and chemokines up-regulated after co-stimulation with poly(I:C) in short-term activated γδ T cells, several interferon/virus-induced genes such as interferon-induced protein with tetratricopeptide repeats (IFIT)1, MX1 and IRF-7 as well as TLR3 and TLR7 genes were enhanced as revealed by microarray analysis and quantitative real-time RT PCR [16, 38]. These data confirmed that freshly isolated γδ T cells need an integrated signal via TCR and TLR ligand to efficiently support antiviral immunity and thus appropriate effector function against viruses. The requirement of TCR-engagement was also observed by others demonstrating a TCR-dependent expansion of Vδ2 γδ T cells in CMV-infected patients after kidney transplantation. Interestingly, TLR3 together with TLR9 seems to be involved in antiviral immunity against murine CMV infection [79]. Moreover, murine γδ T cells have also been shown to recognize HSV-1 antigen via their TCR, a pathway which protects mice from HSV-1 induced lethal encephalitis [85, 86]. The association of TLR3 deficiency with the susceptibility to HSV-1 in humans, fits well with the observation that γδ T cells from pre-term infants expressed reduced levels of TLR3, produced lower levels of IFN-γ after TCR/TLR3 ligand stimulation and were more susceptible to HSV-1 infections [44, 80, 87].
Indirect effects of TLR3 ligands on γδ T cells response
Besides the co-stimulatory effect of poly(I:C) on freshly isolated γδ T cells, other studies reported that γδ T cells were also stimulated indirectly via TLR3-mediated activation of immature myeloid DC [35, 40]. In these studies, Vδ2 γδ T cells up-regulated CD69, produced enhanced levels of IFN-γ and increased their proliferation mediated by the release of type I IFN, but not by IL-15 or IL-12p70, derived from TLR3-expressing myeloid DC (mDC). Reciprocally, activated γδ T cells promote maturation and migratory capacity of DC and thereby CD4 T cell priming [35] (Fig. 2b). Independent of direct co-stimulatory effects of poly(I:C) or indirect effects via mDC, TCR stimulation of γδ T cells is required in both situations for contribution of γδ T cells to an antiviral immune defense.
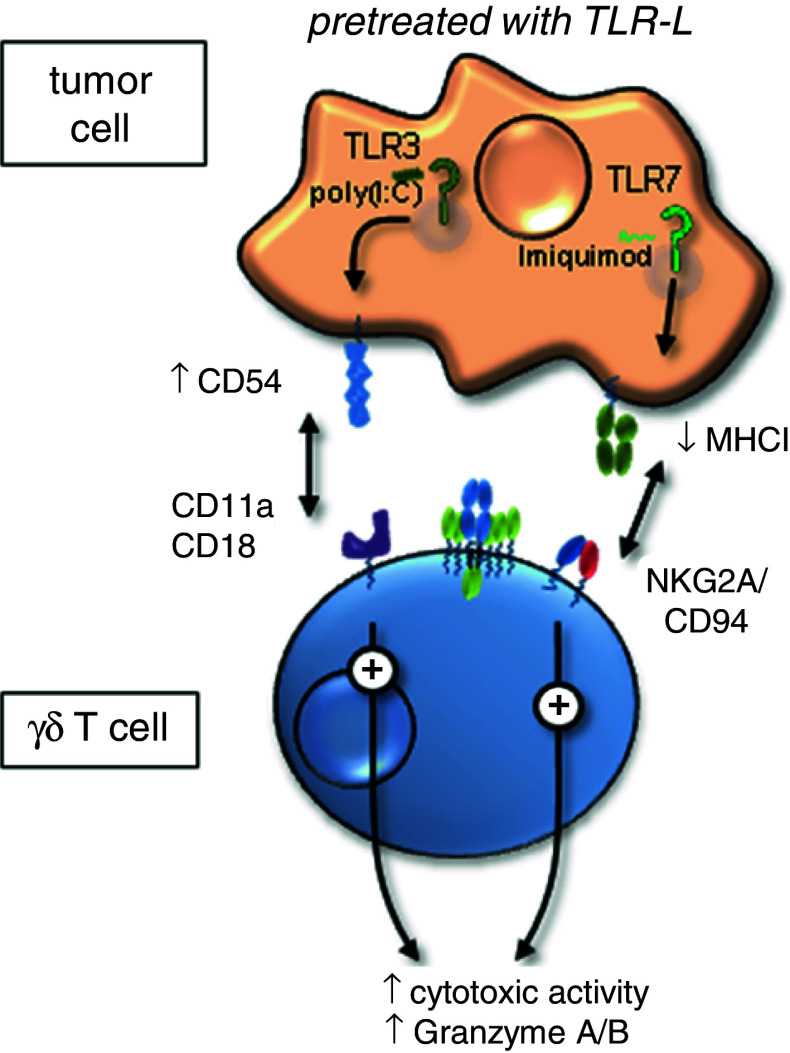
In the context of indirect poly(I:C) effects on γδ T cells, it has been shown that the time-point of administration of a TLR signal is also very important [43]. Pre-treatment of pancreatic adenocarcinomas, lung carcinomas, and squamous cell carcinomas of the head and neck with poly(I:C) for nearly 20 h was necessary to induce up-regulation of CD54 on tumor cells [43]. The interaction of CD54 and the corresponding ligand CD11a/CD18 expressed on γδ T cells is responsible for triggering cytotoxic activity of γδ T cells (Fig. 3). Thus, the TLR3 ligand pre-treatment of tumor cells enhanced the cytotoxic activity of short-term activated γδ T cells lines and γδ T cell clones, but not of freshly isolated γδ T cells. The re-stimulation of γδ T cell lines/clones via TCR further increased these TLR3 ligand mediated enhancement of cytotoxic activity.
Fig. 3.
Indirect effects of TLR ligand (TLR-L) activated tumor cells on γδ T cells. Cytotoxicity and granzyme A/B production of human γδ T cells are enhanced after pretreatment of tumor cells with poly(I:C) (TLR3 ligand) or imiquimod (TLR7 ligand). TLR3 ligand treated tumor cells up-regulate CD54. The interaction of CD54 on tumor cells with CD11a/CD18 on co-cultured γδ T cells resulted in an enhancement of cytotoxic activity by γδ T cells. TLR7 ligand induces a down-regulation of MHC class I molecules on tumor cells possibly resulting in a reduced binding affinity for inhibitory receptor NKG2A. In compliment for reference [43]
TLR4 expression
TLR4 recognizes LPS, a glycolipid component of the outer membrane of Gram-negative bacteria that can cause septic shock [88]. TLR4 forms a complex with MD-2 on the cell surface, which binds LPS. In addition, LPS-binding protein and CD14 are also involved in binding of LPS [89]. LPS consists of a hydrophobic lipid A component, a hydrophilic core oligosaccharide and an O-antigen. The best characterized ligand for the MD-2/TLR4 complex is Lipid A. TLR4 is also involved in the recognition of Streptococcus pneumoniae, RSV fusion protein and mouse mammary tumor virus (MMTV) [12]. Regarding prophylactic and therapeutic vaccine programs, 3-O-decaylated monophosphorylated Lipid A (MPLA) is a promising candidate due to its absent toxicity in humans. MPLA induces DC maturation, up-regulation of co-stimulatory molecules on DC and enhances humoral- and cell-mediated immune responses to DNA vaccination against HIV-1 [90, 91]. MPLA has already been incorporated into hepatitis B virus (HBV) vaccines and human papillomavirus (HPV) vaccine [92].
TLR4 expression in γδ T cells
TLR4 mRNA and protein expression was not detected in highly purified, freshly isolated γδ T cells [16, 38], but was up-regulated after activation at the mRNA and protein level in human short-term γδ T cell lines [36, 39]. In accordance with human γδ T cells, murine γδ T cells did not express TLR4 on the cell surface, as analyzed with blood γδ T cells, peritoneal Vγ6Vδ1 γδ T cells, as well as Vγ3-expressing DETC or DETC lines from C57BL/6 mice [63, 64, 93]. However, TLR4/MD-2 expression was up-regulated when DETC emigrated from the epidermis during cutaneous inflammation and was slightly increased on blood γδ T cells in an experimental thermal injury model [64, 93]. Additionally, TLR4 expression was reported on murine splenic γδ T cells stimulated with anti-CD3 mAb and IL-2 [94].
In contrast to human and mice, TLR4 mRNA was detectable in purified, freshly isolated bovine γδ T cells and in non-stimulated CD8αα+ avian γδ T cells [36, 95].
Indirect effects of TLR4 ligands on γδ T cells response
Cui et al. demonstrated that “resting” human γδ T cells recognize TLR4 ligand Lipid A presented by monocyte-derived dendritic cells (moDC) in a CD1b/CD1c-restricted manner [39]. γδ T cells up-regulated TLR2 and TLR4, produced higher amounts of Th1 cytokines and proliferated much better after stimulation with Lipid A-pulsed moDC. However, only anti-TLR4 antibody could partially inhibit γδ T cell response to Lipid A. Moreover, the authors suggested that the binding of Lipid A at TLR4 on the surface of activated γδ T cells further enhanced γδ T cell effector function (Fig. 2b) which further support the elimination of Gram-negative bacteria [39]. In addition, LPS-activated myeloid DC induced a type I IFN-mediated rapid and strong IFN-γ response of human Vδ2 γδ T cells suggesting an adjuvant role of γδ T cells in the cross-talk with DC during microbial infections [35] (Fig. 2b). Moreover, they observed that Yellow Fever Virus (YFV) activated indirectly IFN-γ production of γδ T cells via plasmacytoid DC (Fig. 2a).
In contrast to freshly isolated human γδ T cells from adult donors, freshly isolated, neonatal bovine γδ T cells responded more vigorously to the TLR4 ligand. The stimulation of freshly isolated bovine γδ T cells with phenol-extracted LPS induced a rapid up-regulation of the chemokines MIP-1α and RANTES [36]. Moreover, a TCR-independent up-regulation of GM-CSF was observed in murine Vγ3 γδ T cells after LPS stimulation [36, 68].
TLR5 expression
Flagellin, a protein of the bacterial flagellae, is recognized by TLR5. Flagellin induces DC maturation and chemokine- and IL-12 production by DC, IL-12-dependent Th1 promotion and α-defensin secretion by NK cells [96, 97]. TLR5−/− mice develop spontaneous colitis, whereas high concentrations of TLR5 ligands can amplify inappropriate human T cell immune responses causing chronic inflammations such as inflammatory bowel diseases [98, 99]. In contrast, low concentrations of TLR5 ligands enhance the suppressive activity of regulatory T cells, which have a important role in maintaining gut homeostasis [98]. In this context, Gewirtz and coworkers reported that a common TLR5 polymorphism that produces dominant negative receptor is protective against Crohn's disease, but not against ulcerative colitis [100]. Flagellin derivate CBLB502 protected hematopoietic cells and cells of the gastrointestinal tract of lethally irradiated tumor-bearing mice from cell death, but did not decrease tumor radiosensitivity. Therefore, TLR5 agonists may be useful as adjuvants for cancer radiotherapy [101].
TLR5 expression in γδ T cells
Expression level of TLR5 mRNA was nearly undetectable compared to TLR1 and TLR2 expression in freshly isolated human γδ T cells [16, 36, 38]. However, Gibbons et al. mentioned in their study similar levels of TLR5 mRNA in adult and neonatal γδ T cells as demonstrated by microarray analysis [44]. In accordance with human γδ T cells, the expression of TLR5 mRNA in bovine γδ T cells is also very weak [36]. No data on TLR5 expression in mice have been reported.
Direct co-stimulatory effects of TLR5 ligands on γδ T cells
Despite the nearly absent expression of TLR5 mRNA, freshly isolated human Vδ1 γδ T cells increased IFN-γ production after stimulation with TLR5 ligand flagellin derived from Salmonella typhimurium alone (Fig. 1). IFN-γ secretion was further enhanced in the presence of TCR stimulation in these Vδ1 γδ T cells. Although the tendency towards increased IFN-γ production was observed among all tested donors, the overall level of IFN-γ production in Vδ1 γδ T cells was very low. Further experiments are necessary to examine a possible direct effect of flagellin on Vδ1 γδ T cells in more detail. In contrast to Vδ1 γδ T cells, Vδ2 γδ T cells did not further increase IFN-γ production after an combined TCR/TLR5-ligand activation compared with TCR stimulation alone [16].
TLR7/8 expression
TLR7 recognizes imidazoquinoline derivatives such as imiquimod and resiquimod (R-848), guanine analogues such as loxoribine, ssRNA derived from RNA viruses such as HIV, influenza A virus and vesicular stomatitis virus, poly(U) RNA and certain siRNAs [12, 102]. TLR7-expressing cells such as plasmacytoid DC produce large amounts of type I IFN after virus infection [103]. Viruses are internalized, recruited to the endolysosomes, and recognized by TLR7 resulting in an antiviral immune response. TLR7 also senses replicating vesicular stomatitis virus that enters the cytoplasm by autophagy [104].
Imiquimod (marketed as Aldara®) is used as first line topical therapy for malignant tumors of the skin such as basal cell carcinomas as well as for actinic keratosis and for genital condyloma. 85A is structurally related to imiquimod and is used for the treatment of melanoma [92, 105].
Phylogenetically similar to TLR7 is TLR8, which recognizes preferentially viral ssRNA from HIV and R-848, thereby inducing an antiviral immunity. Moreover, TLR8 is up-regulated in monocytes after bacterial infections [12].
TLR7/8 expression in γδ T cells
Freshly isolated human γδ T cells express TLR7 on the mRNA level in both Vδ1 and Vδ2 T cell subsets, while TLR8 mRNA was unverifiable in these cells [16, 38, 44]. At the protein level, endosomal TLR7 was observed intracellularly in Vδ1 and Vδ2 γδ T cells (unpublished observation), whereas TLR8 was only marginally detected in both subsets [16]. TLR7 mRNA expression in short-term activated γδ T cell clones derived from blood of full-termed neonates or adults was significantly improved compared to short-term activated γδ T cell clones from pre-termed neonate [44]. In addition, human tumor-infiltrating Vδ1 γδ T cells also express TLR7 and TLR8 [46].
TLR7 expression was found in purified splenic γδ T cells of mice, whereas a weak TLR8 mRNA expression was detected in purified bovine γδ T cells [36, 94].
Direct co-stimulatory effects of TLR8 ligands on γδ T cells
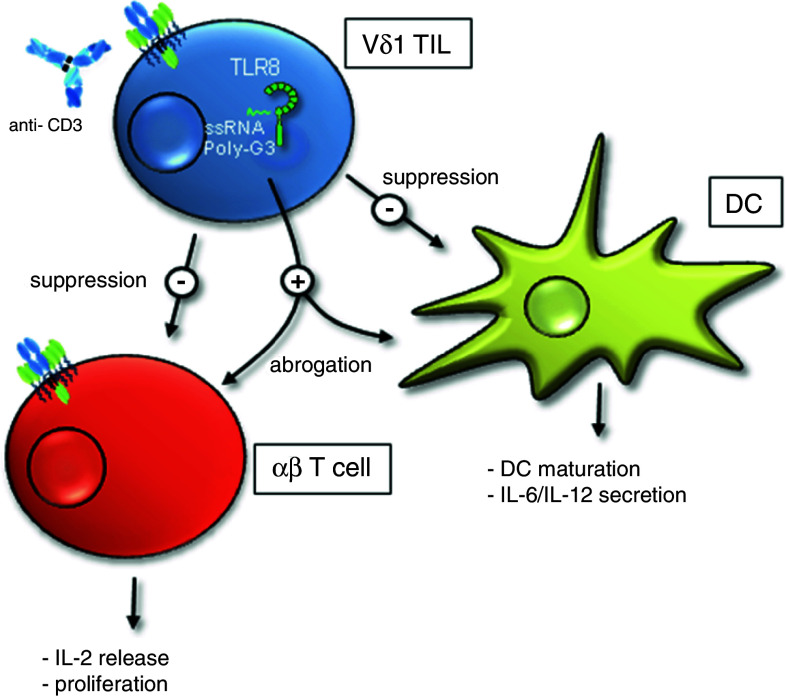
A regulatory role of TLR8 ligands for Vδ1 γδ T cells was described by Peng et al. [46]. The authors isolated transcription factor FoxP3- and CD25-negative tumor-infiltrating Vδ1 γδ T cells from breast tumor patients with tumor suppressive activity [46]. These cells posses a potent TCR-dependent, cell–cell contact-independent immunosuppressive capacity on effector functions of αβ T cells and maturation of DC. The immunosuppressive activity of these Vδ1 γδ T cells could be abolished by TLR8 ligands Poly-G3 and ssRNA40 in vitro and in vivo, but not by TLR7 ligand loxoribine or by TLR9 ligand CpG-B (Fig. 4). Knockdown experiments with siRNA demonstrated that the MyD88-dependent signaling pathway via TRAF6, IKKα, IKKβ, or p38 molecules in Vδ1 γδ T cells is required to induce a TLR8 ligand-mediated reversal of suppression [46].
Fig. 4.
TLR8 ligand abrogate the suppressive activity of tumor-infiltrating Vδ1 γδ T cells. Human Vδ1-expressing tumor-infiltrating lymphocytes (TIL) derived from breast tumors suppress αβ T cell effector function and DC maturation in vitro and in vivo. The suppressive activity can be reversed by TLR8 ligand. In compliment for reference [46]
Indirect effects of TLR7 ligand on γδ T cells response
Pretreatment with TLR7 ligand enhanced human γδ T cell-mediated cytotoxicity towards pancreatic adenocarcinomas, lung carcinomas, and squamous cell carcinomas of the head and neck [43]. Our results revealed a down-regulation of MHC class I molecules on tumor cells after treatment with TLR7 ligand imiquimod. The down-regulation of MHC class I molecule in the tested tumor cell lines possibly results in reduced binding affinity for inhibitory receptor NKG2A expressed on γδ T cells. NKG2A as well as activating receptor NKG2D are expressed on most human γδ T cells, thereby regulating their activation, but were not modulated by TLR7 ligand imiquimod (Fig. 3). Moreover, none of the tested cytokines, which were up-regulated after imiquimod treatment of the tumor cells influenced the γδ T cell-mediated lysis. In addition, imiquimod did not induce up-regulation of co-stimulatory molecules or cell death in the tested tumor cells [43]. The data clearly demonstrate that TLR7 agonists enhanced cytotoxicity of γδ T cell lines and clones. An intravenous administration of TLR7 agonists to cancer patients has to be carefully tested due to the unknown side-effects on other TLR7-expressing cells.
TLR9 expression
TLR9 was identified to recognize unmethylated 2′-deoxyribo (cytidine-phosphate guanosine) (CpG) DNA motifs that are frequently present in bacteria. Synthetic CpG oligodeoxynucleotides (CpG ODN) are classified according to their nucleotide sequence and their backbone modification into three classes (A-C). Class A CpG ODN directed to lysosomal compartments of plasmacytoid DC induces type I IFN production in these cells. Class B CpG ODN traffic to endosomal compartments of B cells, thereby triggering IL-12 production. Class C CpG ODN initiates both type I IFN secretion and B cell activation. All classes activate a strong Th1 response, a property that is explored for clinical trials, e.g., for treatment of Th2-mediated type I allergic disorders, as adjuvant in vaccines against HBV, HCV, or influenza or cancer therapy [12, 62, 106].
Moreover, TLR9 recognizes viral DNA from HSV-1/2 and murine CMV. Similar to TLR7, TLR9 is localized in the endolysosomes, and endosomal acidification is required for ligand binding.
TLR9 expression in γδ T cells
TLR9 mRNA expression was nearly undetectable in highly purified, freshly isolated Vδ1 and Vδ2 γδ T cells and in short-term activated γδ T cell clones [36, 38]. In purified, freshly isolated bovine γδ T cells, TLR9 mRNA expression was generally more abundant than expression of other TLR [36].
Indirect effects of TLR9 ligands on γδ T cell response
Rothenfusser et al. and Kunzmann et al. reported that TLR9 ligand CpG (ODN 1585, 2216) enhanced the activation of freshly isolated human γδ T cells via the stimulation of type I IFN production in plasmacytoid DC in vitro [40, 41]. In their studies, CpG ODN sequences interacting with plasmacytoid DC represented a strong adjuvant for short-term activated γδ T cell effector functions such as Th1-cytokine production and lytic activity [41] (Fig. 2a). Similar to the results with direct co-stimulatory effects of other TLR ligand stimulations, an additional signal via TCR on γδ T cells is required for maximal stimulation of these cells [41]. Synergistic activation of γδ T cells by phosphoantigens and adequate adjuvant induce an increased secretion of Th1-cytokine IFN-γ, which might be beneficial for potential immunotherapy of cancer or defense against viral diseases.
TLR10/11 expression
TLR10 is expressed on human B cells and plasmacytoid DC. However, TLR10 has not been detected in mice due to an incomplete TLR10 gene sequence. TLR10 forms homodimers or heterodimers with TLR1 and TLR2. The ligand for TLR10 is unknown. Similar to other TLR, TLR10 directly associates with MyD88 [50].
TLR11 is expressed in murine kidney and bladder. TLR11-deficient mice are susceptible to infection with uropathogenic bacteria, suggesting a role of TLR11 in the recognition of these bacteria. Moreover, TLR11 recognizes a profilin-like molecule derived from Toxoplasma gondii [107, 108].
TLR10 expression in γδ T cells
Deetz et al. identified the expression of TLR10 mRNA in human short-term γδ T cell lines (<90% purity) [34].
Concluding remarks
Taken together, the results with human γδ T cells demonstrate that TLR ligands on their own (except for TLR5 ligand flagellin) are not sufficient to exert a striking effect on γδ T cells. A co-stimulatory effect is induced in freshly isolated human γδ T cells after combined TCR- and TLR1/2/6, -3 or -5 ligand stimulation. However, in γδ T cell lines and clones, the addition of exogenous IL-2 (necessary for expansion of γδ T cells) might overcome the co-stimulatory effects of TLR ligands. In addition, TLR8 ligands abolish the suppressive function of tumor-infiltrating Vδ1 γδ T cells, and several other TLR ligands induce an indirect effect via DC or tumor cells on γδ T cell effector functions. These data demonstrate that TLR ligands can modulate the effector functions of γδ T cells. The combined TCR-TLR ligand stimulation of γδ T cells (directly or indirectly) could be a strategy to optimize Th1-mediated immune responses as adjuvant in vaccines against viruses or bacteria or could help to improve the therapeutic potential of cancer vaccines.
Acknowledgements
D. Wesch and D. Kabelitz gratefully acknowledge the financial support within the Priority Program 1110 (Ka 502/8-1-3) and the SFB 415 (project A15) of the Deutsche Forschungsgemeinschaft. We also thank the Werner und Klara Kreitz Stiftung for their grant support.
Conflict-of-interest disclosure
The authors declare no competing financial interests.
References
- 1.Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 2.Kabelitz D, Glatzel A, Wesch D. Antigen recognition by human γδ T lymphocytes. Int Arch Allergy Immunol. 2000;122:1–7. doi: 10.1159/000024353. [DOI] [PubMed] [Google Scholar]
- 3.Kabelitz D, Marischen L, Oberg HH, Holtmeier W, Wesch D. Epithelial defence by γδ T cells. Int Arch Allergy Immunol. 2005;137:73–81. doi: 10.1159/000085107. [DOI] [PubMed] [Google Scholar]
- 4.Wesch D, Marischen L, Kabelitz D. Regulation of cytokine production by γδ T cells. Curr Med Chem Anti-Inflamm Anti-Allergy Agents. 2005;4:153–160. doi: 10.2174/1568014053507032. [DOI] [Google Scholar]
- 5.Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, Beck E, Wiesner J, Eberl M, Jomaa H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli . FEBS Lett. 2001;509:317–322. doi: 10.1016/S0014-5793(01)03191-X. [DOI] [PubMed] [Google Scholar]
- 6.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 7.Spada FM, Grant EP, Peters PJ, Sugita M, Melian A, Leslie DS, Lee HK, Donsellar E, Hanson DA, Krensky AM, Majdic O, Porcelli SA, Morita CT, Brenner MB. Self-recognition of CD1 by γδ T cells: implications for innate immunity. J Exp Med. 2000;191:937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heilig JS, Tonegawa S. Diversity of murine γ genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836–840. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- 9.Bonneville M, O’Brien RL, Born WK. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 10.Born WK, Yin Z, Hahn YS, Sun D, O’Brien RL. Analysis of γδ T cell functions in the mouse. J Immunol. 2010;184:4055–4061. doi: 10.4049/jimmunol.0903679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang ZL. Important aspects of Toll-like receptors, ligands and their signaling pathways. Inflamm Res. 2010;59:791–808. doi: 10.1007/s00011-010-0208-2. [DOI] [PubMed] [Google Scholar]
- 12.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 13.Kabelitz D, Wesch D, Oberg HH. Regulation of regulatory T cells: role of dendritic cells and Toll-like receptors. Crit Rev Immunol. 2006;26:291–306. doi: 10.1615/critrevimmunol.v26.i4.10. [DOI] [PubMed] [Google Scholar]
- 14.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol. 2007;19:39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Kulkarni R, Behboudi S, Sharif S. Insights into the role of Toll-like receptors in modulation of T cell responses. Cell Tissue Res. 2011;343:141–152. doi: 10.1007/s00441-010-1017-1. [DOI] [PubMed] [Google Scholar]
- 16.Pietschmann K, Beetz S, Welte S, Martens I, Gruen J, Oberg HH, Wesch D, Kabelitz D. Toll-like receptor expression and function in subsets of human γδ T lymphocytes. Scand J Immunol. 2009;70:245–255. doi: 10.1111/j.1365-3083.2009.02290.x. [DOI] [PubMed] [Google Scholar]
- 17.Wesch D, Beetz S, Oberg HH, Marget M, Krengel K, Kabelitz D. Direct costimulatory effect of TLR3 ligand poly(I:C) on human γδ T lymphocytes. J Immunol. 2006;176:1348–1354. doi: 10.4049/jimmunol.176.3.1348. [DOI] [PubMed] [Google Scholar]
- 18.Belvin MP, Anderson KV. A conserved signaling pathway: the Drosophila Toll-dorsal pathway. Annu Rev Cell Dev Biol. 1996;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- 19.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/S0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 20.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 21.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 23.Matsushima N, Tanaka T, Enkhbayar P, Mikami T, Taga M, Yamada K, Kuroki Y. Comparative sequence analysis of leucine-rich repeats (LRRs) within vertebrate Toll-like receptors. BMC Genomics. 2007;8:124. doi: 10.1186/1471-2164-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 25.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing Toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi K, Shibata T, Akashi-Takamura S, Kiyokawa T, Wakabayashi Y, Tanimura N, Kobayashi T, Matsumoto F, Fukui R, Kouro T, Nagai Y, Takatsu K, Saitoh S, Miyake K. A protein associated with Toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses. J Exp Med. 2007;204:2963–2976. doi: 10.1084/jem.20071132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Neill LA, Bryant CE, Doyle SL. Therapeutic targeting of Toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacol Rev. 2009;61:177–197. doi: 10.1124/pr.109.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warshakoon HJ, Hood JD, Kimbrell MR, Malladi S, Wu WY, Shukla NM, Agnihotri G, Sil D, David SA. Potential adjuvantic properties of innate immune stimuli. Hum Vaccin. 2009;5:381–394. doi: 10.4161/hv.5.6.8175. [DOI] [PubMed] [Google Scholar]
- 31.Coats SR, Pham TT, Bainbridge BW, Reife RA, Darveau RP. MD-2 mediates the ability of tetra-acylated and penta-acylated lipopolysaccharides to antagonize Escherichia coli lipopolysaccharide at the TLR4 signaling complex. J Immunol. 2005;175:4490–4498. doi: 10.4049/jimmunol.175.7.4490. [DOI] [PubMed] [Google Scholar]
- 32.Gursel I, Gursel M, Yamada H, Ishii KJ, Takeshita F, Klinman DM. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J Immunol. 2003;171:1393–1400. doi: 10.4049/jimmunol.171.3.1393. [DOI] [PubMed] [Google Scholar]
- 33.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of Toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 34.Deetz CO, Hebbeler AM, Propp NA, Cairo C, Tikhonov I, Pauza CD. Gamma interferon secretion by human Vγ2 Vδ2 T cells after stimulation with antibody against the T-cell receptor plus the Toll-like receptor 2 agonist Pam3Cys. Infect Immun. 2006;74:4505–4511. doi: 10.1128/IAI.00088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devilder MC, Allain S, Dousset C, Bonneville M, Scotet E. Early triggering of exclusive IFN-gamma responses of human Vγ9Vδ2 T cells by TLR-activated myeloid and plasmacytoid dendritic cells. J Immunol. 2009;183:3625–3633. doi: 10.4049/jimmunol.0901571. [DOI] [PubMed] [Google Scholar]
- 36.Hedges JF, Lubick KJ, Jutila MA. γδ T cells respond directly to pathogen-associated molecular patterns. J Immunol. 2005;174:6045–6053. doi: 10.4049/jimmunol.174.10.6045. [DOI] [PubMed] [Google Scholar]
- 37.Ohnesorge S, Oberg HH, Peters C, Janssen O, Kabelitz D, Wesch D. Differential poly(I:C) responses of human Vγ9Vδ2 T cells stimulated with pyrophosphates versus aminobisphosphonates. Open Immuol J. 2009;2:135–142. doi: 10.2174/1874226200902020135. [DOI] [Google Scholar]
- 38.Beetz S, Wesch D, Marischen L, Welte S, Oberg HH, Kabelitz D. Innate immune functions of human γδ T cells. Immunobiology. 2008;213:173–182. doi: 10.1016/j.imbio.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Cui Y, Kang L, Cui L, He W. Human γδ T cell recognition of lipid A is predominately presented by CD1b or CD1c on dendritic cells. Biol Direct. 2009;4:1–12. doi: 10.1186/1745-6150-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunzmann V, Kretzschmar E, Herrmann T, Wilhelm M. Polyinosinic-polycytidylic acid-mediated stimulation of human γδ T cells via CD11c dendritic cell-derived type I interferons. Immunology. 2004;112:369–377. doi: 10.1111/j.1365-2567.2004.01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rothenfusser S, Hornung V, Krug A, Towarowski A, Krieg AM, Endres S, Hartmann G. Distinct CpG oligonucleotide sequences activate human γδ T cells via interferon-alpha/-beta. Eur J Immunol. 2001;31:3525–3534. doi: 10.1002/1521-4141(200112)31:12<3525::AID-IMMU3525>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 42.Shrestha N, Ida JA, Lubinski AS, Pallin M, Kaplan G, Haslett PA. Regulation of acquired immunity by γδ T-cell/dendritic-cell interactions. Ann N Y Acad Sci. 2005;1062:79–94. doi: 10.1196/annals.1358.011. [DOI] [PubMed] [Google Scholar]
- 43.Shojaei H, Oberg HH, Juricke M, Marischen L, Kunz M, Mundhenke C, Gieseler F, Kabelitz D, Wesch D. Toll-like receptors 3 and 7 agonists enhance tumor cell lysis by human γδ T cells. Cancer Res. 2009;69:8710–8717. doi: 10.1158/0008-5472.CAN-09-1602. [DOI] [PubMed] [Google Scholar]
- 44.Gibbons DL, Haque SF, Silberzahn T, Hamilton K, Langford C, Ellis P, Carr R, Hayday AC. Neonates harbour highly active γδ T cells with selective impairments in preterm infants. Eur J Immunol. 2009;39:1794–1806. doi: 10.1002/eji.200939222. [DOI] [PubMed] [Google Scholar]
- 45.Collins C, Shi C, Russell JQ, Fortner KA, Budd RC. Activation of γδ T cells by Borrelia burgdorferi is indirect via a TLR- and caspase-dependent pathway. J Immunol. 2008;181:2392–2398. doi: 10.4049/jimmunol.181.4.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating γδ T cells suppress T and dendritic cell function via mechanisms controlled by a unique Toll-like receptor signaling pathway. Immunity. 2007;27:334–348. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 47.Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Ulmer AJ. Lipopeptide structure determines TLR2-dependent cell activation level. FEBS J. 2005;272:6354–6364. doi: 10.1111/j.1742-4658.2005.05029.x. [DOI] [PubMed] [Google Scholar]
- 48.Dziarski R, Gupta D. The peptidoglycan recognition proteins (PGRPs) Genome Biol. 2006;7:232–245. doi: 10.1186/gb-2006-7-8-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schroder NW, Morath S, Alexander C, Hamann L, Hartung T, Zahringer U, Gobel UB, Weber JR, Schumann RR. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem. 2003;278:15587–15594. doi: 10.1074/jbc.M212829200. [DOI] [PubMed] [Google Scholar]
- 50.Hasan U, Chaffois C, Gaillard C, Saulnier V, Merck E, Tancredi S, Guiet C, Briere F, Vlach J, Lebecque S, Trinchieri G, Bates EE. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J Immunol. 2005;174:2942–2950. doi: 10.4049/jimmunol.174.5.2942. [DOI] [PubMed] [Google Scholar]
- 51.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 52.Melkamu T, Squillace D, Kita H, O’Grady SM. Regulation of TLR2 expression and function in human airway epithelial cells. J Membr Biol. 2009;229:101–113. doi: 10.1007/s00232-009-9175-3. [DOI] [PubMed] [Google Scholar]
- 53.Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Akira S, Ulmer AJ. Toll-like receptor 6-independent signaling by diacylated lipopeptides. Eur J Immunol. 2005;35:282–289. doi: 10.1002/eji.200424955. [DOI] [PubMed] [Google Scholar]
- 54.Hajjar AM, O’Mahony DS, Ozinsky A, Underhill DM, Aderem A, Klebanoff SJ, Wilson CB. Cutting edge: functional interactions between Toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J Immunol. 2001;166:15–19. doi: 10.4049/jimmunol.166.1.15. [DOI] [PubMed] [Google Scholar]
- 55.Okusawa T, Fujita M, Nakamura J, Into T, Yasuda M, Yoshimura A, Hara Y, Hasebe A, Golenbock DT, Morita M, Kuroki Y, Ogawa T, Shibata K. Relationship between structures and biological activities of mycoplasmal diacylated lipopeptides and their recognition by Toll-like receptors 2 and 6. Infect Immun. 2004;72:1657–1665. doi: 10.1128/IAI.72.3.1657-1665.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeuchi O, Kawai T, Muhlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, Akira S. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13:933–940. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 57.Rose WA, McGowin CL, Pyles RB. FSL-1, a bacterial-derived Toll-like receptor 2/6 agonist, enhances resistance to experimental HSV-2 infection. Virol J. 2009;6:195. doi: 10.1186/1743-422X-6-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu H, Yang Y, Gad E, Wenner CA, Chang A, Larson ER, Dang Y, Martzen M, Standish LJ, Disis ML. Polysaccharide Krestin is a novel TLR2 agonist that mediates inhibition of tumor growth via stimulation of CD8 T cells and NK cells. Clin Cancer Res. 2011;17:67–76. doi: 10.1158/1078-0432.CCR-10-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meng YL, Liu Z, Rosen BP. As(III) and Sb(III) uptake by GlpF and efflux by ArsB in Escherichia coli . J Biol Chem. 2004;279:18334–18341. doi: 10.1074/jbc.M400037200. [DOI] [PubMed] [Google Scholar]
- 60.Spiller S, Elson G, Ferstl R, Dreher S, Mueller T, Freudenberg M, Daubeuf B, Wagner H, Kirschning CJ. TLR4-induced IFN-γ production increases TLR2 sensitivity and drives Gram-negative sepsis in mice. J Exp Med. 2008;205:1747–1754. doi: 10.1084/jem.20071990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oberg HH, Ly TT, Ussat S, Meyer T, Kabelitz D, Wesch D. Differential but direct abolishment of human regulatory T cell suppressive capacity by various TLR2 ligands. J Immunol. 2010;184:4733–4740. doi: 10.4049/jimmunol.0804279. [DOI] [PubMed] [Google Scholar]
- 62.Mokuno Y, Matsuguchi T, Takano M, Nishimura H, Washizu J, Ogawa T, Takeuchi O, Akira S, Nimura Y, Yoshikai Y. Expression of Toll-like receptor 2 on γδ T cells bearing invariant V γ 6/V δ 1 induced by Escherichia coli infection in mice. J Immunol. 2000;165:931–940. doi: 10.4049/jimmunol.165.2.931. [DOI] [PubMed] [Google Scholar]
- 63.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 64.Schwacha MG, Daniel T. Up-regulation of cell surface Toll-like receptors on circulating γδ T-cells following burn injury. Cytokine. 2008;44:328–334. doi: 10.1016/j.cyto.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li H, Luo K, Pauza CD. TNF-α is a positive regulatory factor for human Vγ2 Vδ2 T cells. J Immunol. 2008;181:7131–7137. doi: 10.4049/jimmunol.181.10.7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169:10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- 67.Lubick K, Jutila MA. LTA recognition by bovine γδ T cells involves CD36. J Leukoc Biol. 2006;79:1268–1270. doi: 10.1189/jlb.1005616. [DOI] [PubMed] [Google Scholar]
- 68.Leclercq G, Plum J. Stimulation of TCR Vγ3 cells by Gram-negative bacteria. J Immunol. 1995;154:5313–5319. [PubMed] [Google Scholar]
- 69.Toth B, Alexander M, Daniel T, Chaudry IH, Hubbard WJ, Schwacha MG. The role of γδ T cells in the regulation of neutrophil-mediated tissue damage after thermal injury. J Leukoc Biol. 2004;76:545–552. doi: 10.1189/jlb.0404219. [DOI] [PubMed] [Google Scholar]
- 70.Matsushima A, Ogura H, Fujita K, Koh T, Tanaka H, Sumi Y, Yoshiya K, Hosotsubo H, Kuwagata Y, Shimazu T, Sugimoto H. Early activation of γδ T lymphocytes in patients with severe systemic inflammatory response syndrome. Shock. 2004;22:11–15. doi: 10.1097/01.shk.0000129203.84330.b3. [DOI] [PubMed] [Google Scholar]
- 71.Bochud PY, Hawn TR, Aderem A. Cutting edge: a Toll-like receptor 2 polymorphism that is associated with lepromatous leprosy is unable to mediate mycobacterial signaling. J Immunol. 2003;170:3451–3454. doi: 10.4049/jimmunol.170.7.3451. [DOI] [PubMed] [Google Scholar]
- 72.Kang TJ, Chae GT. Detection of Toll-like receptor 2 (TLR2) mutation in the lepromatous leprosy patients. FEMS Immunol Med Microbiol. 2001;31:53–58. doi: 10.1111/j.1574-695X.2001.tb01586.x. [DOI] [PubMed] [Google Scholar]
- 73.Kang TJ, Lee SB, Chae GT. A polymorphism in the Toll-like receptor 2 is associated with IL-12 production from monocyte in lepromatous leprosy. Cytokine. 2002;20:56–62. doi: 10.1006/cyto.2002.1982. [DOI] [PubMed] [Google Scholar]
- 74.Krutzik SR, Ochoa MT, Sieling PA, Uematsu S, Ng YW, Legaspi A, Liu PT, Cole ST, Godowski PJ, Maeda Y, Sarno EN, Norgard MV, Brennan PJ, Akira S, Rea TH, Modlin RL. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat Med. 2003;9:525–532. doi: 10.1038/nm864. [DOI] [PubMed] [Google Scholar]
- 75.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 76.Kariko K, Bhuyan P, Capodici J, Weissman D. Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signaling through Toll-like receptor 3. J Immunol. 2004;172:6545–6549. doi: 10.4049/jimmunol.172.11.6545. [DOI] [PubMed] [Google Scholar]
- 77.Bell JK, Askins J, Hall PR, Davies DR, Segal DM. The dsRNA binding site of human Toll-like receptor 3. Proc Natl Acad Sci USA. 2006;103:8792–8797. doi: 10.1073/pnas.0603245103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 79.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von BH, Ku CL, Casrouge A, Zhang XX, Barreiro L, Leonard J, Hamilton C, Lebon P, Heron B, Vallee L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova JL (2007) TLR3 deficiency in patients with herpes simplex encephalitis. Science 317:1522–1527 [DOI] [PubMed]
- 81.Jasani B, Navabi H, Adams M. Ampligen: a potential Toll-like 3 receptor adjuvant for immunotherapy of cancer. Vaccine. 2009;27:3401–3404. doi: 10.1016/j.vaccine.2009.01.071. [DOI] [PubMed] [Google Scholar]
- 82.Groskreutz DJ, Monick MM, Powers LS, Yarovinsky TO, Look DC, Hunninghake GW. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J Immunol. 2006;176:1733–1740. doi: 10.4049/jimmunol.176.3.1733. [DOI] [PubMed] [Google Scholar]
- 83.Hewson CA, Jardine A, Edwards MR, Laza-Stanca V, Johnston SL. Toll-like receptor 3 is induced by and mediates antiviral activity against rhinovirus infection of human bronchial epithelial cells. J Virol. 2005;79:12273–12279. doi: 10.1128/JVI.79.19.12273-12279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wesch D, Marx S, Kabelitz D. Comparative analysis of α β and γδ T cell activation by Mycobacterium tuberculosis and isopentenyl pyrophosphate. Eur J Immunol. 1997;27:952–956. doi: 10.1002/eji.1830270422. [DOI] [PubMed] [Google Scholar]
- 85.Sciammas R, Kodukula P, Tang Q, Hendricks RL, Bluestone JA. T cell receptor-γ/δ cells protect mice from herpes simplex virus type 1-induced lethal encephalitis. J Exp Med. 1997;185:1969–1975. doi: 10.1084/jem.185.11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sciammas R, Bluestone JA. HSV-1 glycoprotein I-reactive TCR γδ cells directly recognize the peptide backbone in a conformationally dependent manner. J Immunol. 1998;161:5187–5192. [PubMed] [Google Scholar]
- 87.O’Riordan DP, Golden WC, Aucott SW. Herpes simplex virus infections in preterm infants. Pediatrics. 2006;118:e1612–e1620. doi: 10.1542/peds.2005-1228. [DOI] [PubMed] [Google Scholar]
- 88.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 89.Akashi-Takamura S, Miyake K. TLR accessory molecules. Curr Opin Immunol. 2008;20:420–425. doi: 10.1016/j.coi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 90.Ismaili J, Rennesson J, Aksoy E, Vekemans J, Vincart B, Amraoui Z, Van LF, Goldman M, Dubois PM. Monophosphoryl lipid A activates both human dendritic cells and T cells. J Immunol. 2002;168:926–932. doi: 10.4049/jimmunol.168.2.926. [DOI] [PubMed] [Google Scholar]
- 91.Sasaki S, Hamajima K, Fukushima J, Ihata A, Ishii N, Gorai I, Hirahara F, Mohri H, Okuda K. Comparison of intranasal and intramuscular immunization against human immunodeficiency virus type 1 with a DNA-monophosphoryl lipid A adjuvant vaccine. Infect Immun. 1998;66:823–826. doi: 10.1128/iai.66.2.823-826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Makkouk A, Abdelnoor AM. The potential use of Toll-like receptor (TLR) agonists and antagonists as prophylactic and/or therapeutic agents. Immunopharmacol Immunotoxicol. 2009;31:331–338. doi: 10.1080/08923970902802926. [DOI] [PubMed] [Google Scholar]
- 93.Shimura H, Nitahara A, Ito A, Tomiyama K, Ito M, Kawai K. Up-regulation of cell surface Toll-like receptor 4-MD2 expression on dendritic epidermal T cells after the emigration from epidermis during cutaneous inflammation. J Dermatol Sci. 2005;37:101–110. doi: 10.1016/j.jdermsci.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 94.Fang H, Welte T, Zheng X, Chang GJ, Holbrook MR, Soong L, Wang T. γδ T cells promote the maturation of dendritic cells during West Nile virus infection. FEMS Immunol Med Microbiol. 2010;59:71–80. doi: 10.1111/j.1574-695X.2010.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pieper J, Methner U, Berndt A. Heterogeneity of avian γδ T cells. Vet Immunol Immunopathol. 2008;124:241–252. doi: 10.1016/j.vetimm.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 96.Chalifour A, Jeannin P, Gauchat JF, Blaecke A, Malissard M, N’Guyen T, Thieblemont N, Delneste Y. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood. 2004;104:1778–1783. doi: 10.1182/blood-2003-08-2820. [DOI] [PubMed] [Google Scholar]
- 97.Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003;170:5165–5175. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- 98.Crellin NK, Garcia RV, Hadisfar O, Allan SE, Steiner TS, Levings MK. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+ CD25+ T regulatory cells. J Immunol. 2005;175:8051–8059. doi: 10.4049/jimmunol.175.12.8051. [DOI] [PubMed] [Google Scholar]
- 99.Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, Neish AS, Uematsu S, Akira S, Williams IR, Gewirtz AT. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909–3921. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gewirtz AT, Vijay-Kumar M, Brant SR, Duerr RH, Nicolae DL, Cho JH. Dominant-negative TLR5 polymorphism reduces adaptive immune response to flagellin and negatively associates with Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1157–G1163. doi: 10.1152/ajpgi.00544.2005. [DOI] [PubMed] [Google Scholar]
- 101.Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, Didonato JA, Feinstein E, Gudkov AV. An agonist of Toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dockrell DH, Kinghorn GR. Imiquimod and resiquimod as novel immunomodulators. J Antimicrob Chemother. 2001;48:751–755. doi: 10.1093/jac/48.6.751. [DOI] [PubMed] [Google Scholar]
- 103.Rajagopal D, Paturel C, Morel Y, Uematsu S, Akira S, Diebold SS. Plasmacytoid dendritic cell-derived type I interferon is crucial for the adjuvant activity of Toll-like receptor 7 agonists. Blood. 2010;115:1949–1957. doi: 10.1182/blood-2009-08-238543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 105.Schon MP, Schon M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190–199. doi: 10.1038/sj.onc.1210913. [DOI] [PubMed] [Google Scholar]
- 106.Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 107.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 108.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A Toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]