Abstract
The classical view that endogenous antigens are processed by the proteasome and loaded on MHC class I molecules in the endoplasmic reticulum, while exogenous antigens taken up by endocytosis or phagocytosis are degraded and loaded on MHC class II in lysosome-derived organelles, has evolved along with the improvement of our understanding of the cell biology of antigen-presenting cells. In recent years, evidence for alternative presentation pathways has emerged. Exogenous antigens can be processed by the proteasome and loaded on MHC class I through a pathway called cross-presentation. Moreover, endogenous antigens can be targeted to lytic organelles for presentation on MHC class II through autophagy, a highly conserved cellular process of self-eating. Recent evidence indicates that the vacuolar degradation of endogenous antigens is also beneficial for presentation on MHC class I molecules. This review focuses on how various forms of autophagy participate to presentation of these antigens on MHC class I.
Keywords: Autophagy, MHC, Antigen presentation, Antigen processing, Lytic vacuoles
Introduction
The immune control by T lymphocytes depends on the ability of antigen-presenting cells (APC) to display peptide-MHC complexes at their surface. MHC molecules are divided into two classes that differ, among other features, by the structure of the groove where antigenic peptides are bound. The antigen-binding groove of MHC class I molecules is closed at each end, and hence binds peptides of a very defined length (8–10 amino acids) [1]. The proteolytic system that can produce these defined peptides is the proteasome, a multimeric protein complex mainly found in the cytoplasm (reviewed by Sijts and Kloetzel in this issue). Antigenic peptides generated from endogenously expressed proteins by the action of the proteasome are transported into the endoplasmic reticulum (ER) lumen by the MHC locus-encoded peptide transporter associated with antigen processing (TAP), where they can undergo additional trimming and bind nascent MHC class I molecules [2]. Peptide-MHC class I complexes reach the cell surface through the secretory pathway to stimulate CD8+ T lymphocytes. In contrast, the antigen-binding groove of MHC class II molecules is open at both ends, allowing peptides of a more variable length (13–25 amino acids) to be loaded [3]. Those peptides are efficiently generated by several proteases located in the vacuolar pathway. Hence, the requirement of a protease system with the right cleavage pattern, as well as the apparent specific distribution of the MHC class I and class II loading machinery to the biosynthetic and endo/phagocytic systems, respectively, was long thought to cause a strict segregation of the endogenous and exogenous processing/presentation pathways.
Nevertheless, it has been known for some years that the segregation of the two pathways is not perfect. Exogenous antigens that have been internalized into an endo/phagocytic compartment can efficiently be presented on MHC class I molecules by a process called cross-presentation. This process is of special significance in professional APCs, to integrate pathogenic cues into an effective cellular immune response. Because cross-presentation is much more efficient for particulate antigens (protein aggregates, bacteria, inert particles coated with proteins, viruses) than for soluble antigens [4], the majority of studies on the cross-presentation mechanisms have focused on antigens internalized into phagosomes. Different working models for the presentation of exogenous antigens on MHC class I molecules have been proposed. In a first model, antigens taken up by phagocytosis are transported across the phagosome membrane into the cytosol, by a yet unidentified transporter, where they can be further processed by the proteasome and enter the classical processing pathway for MHC class I presentation (phagosome to cytosol model) [5]. In a second model, the loading of peptides on MHC class I molecules occurs in the phagosome itself. This model requires two transmembrane transport steps of the phagocytosed antigens (phagosome to cytosol to phagosome model) [6, 7]. The first step transports peptides in the cytoplasm where the proteasome further processes the antigens. The molecules responsible for this transfer step have not been formally identified, although both sec61 and Derlin-1 have been highlighted as potential candidates [8]. The second step enables the re-entry of the processed antigens in the phagosome lumen through TAP [6, 7, 9]. Recently, it was shown that antigenic peptides that bind to MHC class I molecules can also be generated by proteases in the lumen of vacuolar organelles, making the detour to the proteasome in the cytosol unnecessary. These peptides are directly loaded onto MHC class I complexes in the vacuole (“vacuolar pathway”) [10]. In the case of phagocytosis, it was shown that the presence of a high concentration of ovalbumin in the phagosome lumen leads to the cross-presentation of the ovalbumin-derived SIINFEKL peptide in a TAP-independent manner [11], highlighting the functional properties of this compartment. While the ability of the endo/phagocytic pathway to process exogenous antigens for MHC class I presentation has been recognized for several years, it has only very recently been shown that endogenous antigens can also enter this antigen presentation pathway by autophagy [12]. In this review, we will discuss the role of autophagy in MHC class I presentation in comparison with other more characterized mechanisms of vacuolar antigen processing and presentation.
Autophagy
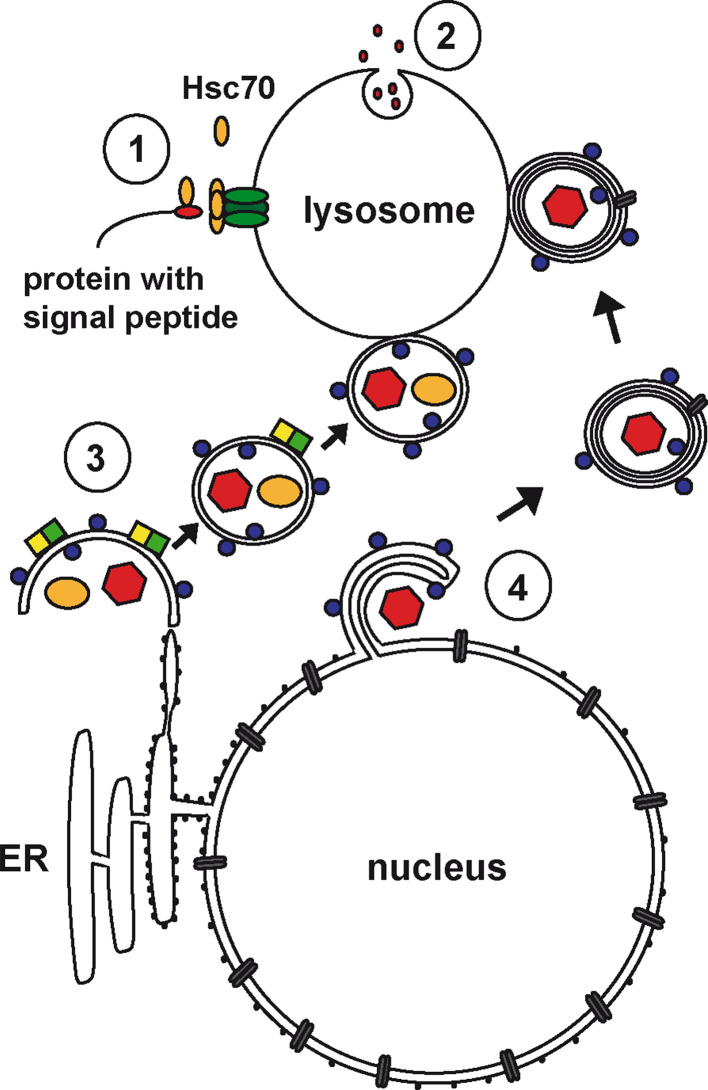
Autophagy is a fundamental, well-conserved, catabolic pathway that allows cells to recycle nutrients in starvation or stress conditions. This process is essential for cell homeostasis, survival, and differentiation. The term “autophagy” was coined almost 50 years ago by Christian de Duve to describe a mechanism leading to the sequestration of part of the cytoplasm and different organelles into double-membrane vacuoles that were observed by electron microscopy in mammalian tissues [13]. Our knowledge of the molecular processes involved in autophagy has dramatically expanded within the last decade through use of yeast genetics. In yeast, autophagosome formation can be easily followed by light microscopy, and analysis of yeast mutants has led to the identification of 32 autophagy-related proteins (atgs), many of which are conserved in higher organisms [14]. Processes involving the sequestration of components from the cytosol and their delivery to lysosomes for degradation are referred to as autophagy. At least three different forms can be distinguished in eukaryotic cells: chaperone-mediated autophagy (CMA), macroautophagy, and microautophagy (Fig. 1). The latter involves the budding of part of the lysosome membrane, enabling the uptake of nearby cytosolic elements and their degradation in the lysosome lumen [15, 16]. CMA does not require the formation of a specialized endovacuolar compartment, but rather involves a transport machinery located on the lysosomal membrane itself [17–19]. CMA specifically targets proteins containing a KFERQ motif. Hsc70 (also called hsc73) and Lamp-2a are involved in the recognition of these proteins and their transfer across the lysosomal membrane into the lumen for degradation (Fig. 1).
Fig. 1.
Mechanisms of autophagy in eukaryotic cells. In chaperone-mediated autophagy (CMA), cytoplasmic proteins containing a specific signaling motif (red) are recognized by hsc70 that subsequently associates with LAMP-2a (green) on the lysosomal membrane, allowing the translocation of the target protein into the lysosomal lumen (1). Alternatively, small portions of the cytoplasm can be taken up into lysosomes by invagination of the lysosomal membrane (microautophagy, 2). Intracellular pathogens (red hexagons), organelles and cytosolic protein aggregates (orange circles) can also be taken up by macroautophagy/xenophagy (3). A cup-shaped isolation membrane that can be in close association with the rough endoplasmic reticulum forms around the cargo. Membrane expansion and formation of the closed autophagosome requires two ubiquitin-like protein conjugation systems (blue circles: LC3, yellow and green squares: atg5/atg12). The cup closes into a vesicle bordered by two membrane layers that can subsequently fuse with lysosomes where its content and membrane components are degraded. Another autophagy pathway recently described in HSV1-infected macrophages uses the inner and outer leaflets of the nuclear envelope as a source of autophagosomal membrane (4). Protrusions originating from the nuclear membrane form a cup around virus particles or cytoplasmic components and finally close in on themselves, bud off the nucleus and fuse with lysosomes. Like the classical double-membrane autophagosomes, the 4-membrane bound vesicles generated by this process contain the autophagy marker LC3
The most widely studied form of autophagy is macroautophagy (hereafter referred to as autophagy; Fig. 1). In this process, part of the cytoplasm, protein aggregates, or even organelles like mitochondria or lysosomes, are surrounded by an expanding membrane, the phagophore, which closes in around them to form a double-membrane vesicle, the autophagosome [20]. This new compartment eventually fuses with lysosomes to facilitate the degradation of its content. The initial formation of the phagophore requires class III phosphatidylinositol 3-kinases and the autophagic protein beclin-1, together with membrane components that are recruited by a poorly characterized mechanism that might use lipid droplets, the endoplasmic reticulum, the Golgi apparatus, secretory vesicles, mitochondria, or plasma membrane as a source of lipids [21–27]. Two ubiquitin-like protein conjugation systems are involved in phagophore expansion and autophagosome formation. One of them involves the processing and tethering of the protein LC3 (atg8) to the inner and outer leaflet of the isolation membrane [21, 28, 29]. The processing of this protein is often used as a marker to measure autophagic flux. The other protein conjugation system, which associates mostly with the outer leaflet of the isolation membrane, requires the protein atg5 [21]. The lipid bilayer of the autophagosome is thin, and resembles that of the endoplasmic reticulum, cis-Golgi, mitochondria, or nuclear envelope [27]. Recent electron tomography studies have shown that the phagophore can form in close association with the rough endoplasmic reticulum [23, 24]. However, as the phagophore takes up portions of the cytoplasm and part of some organelles as cargo molecules, the presence of organelle markers on autophagosomes as an indicator of membrane origin has to be interpreted with caution.
In addition to this well-characterized autophagy pathway, two other pathways have recently been proposed, that probably use a slightly different set of autophagy-related proteins, and can recruit different membranes for vacuole formation. The first pathway, Golgi-related autophagy, requires beclin-1 for initiation, but is independent of atg5 [30]. Phagophores form in close association with the trans-Golgi network and late endosomes, and probably fuse with the latter in a Rab9-dependent manner. Strikingly, the resulting autophagosomes are not decorated by LC3. Recently, we described a second alternative autophagy pathway that uses the nuclear envelope as a source of membrane [12]. Nuclear membrane-derived autophagy is observed in Herpes Simplex Virus 1 (HSV1) infected macrophages, and involves the coiling of both the inner and outer nuclear envelope around portions of the cytosol. Consequently, the autophagosomes formed by this pathway are delimited by four membranes rather than two and these are decorated by LC3 (Fig. 1). This type of autophagosome can trap viral particles. Two-membrane bound phagophores in the cytoplasm can also engulf and destroy intracellular pathogens such as viruses in a process called xenophagy [31]. Recent studies have demonstrated a contribution of autophagy to antigen presentation [12, 32–34]. Therefore, autophagy is not only important for nutrient turnover but also plays a role in innate and adaptive immunity.
Autophagy in MHC class II presentation
MHC class II molecules are constitutively expressed by specialized cells of the immune system (APCs) such as dendritic cells, macrophages, B lymphocytes, and certain activated epithelial cells. While peptides presented at the cell surface in complex with MHC class II molecules are mainly derived from the vacuolar processing of exogenous antigens internalized by endocytosis or phagocytosis, a subset of MHC class II peptides are generated from endogenous sources such as viral or cellular proteins [35, 36]. Among the latter ones, vacuolar proteins that naturally intersect the MHC class II pathway are the main endogenous source of peptides for loading on MHC class II molecules. However, endogenous proteins that are topologically isolated from the MHC class II pathway, such as cytosolic or nuclear proteins, can also be presented on MHC class II molecules, suggesting that these antigens are translocated into the MHC class II loading compartments [37–39]. Translocation of whole proteins or peptides generated by the proteasome into a MHC class II loading compartment is independent of transmembrane transporters such as TAP, but instead requires autophagy [40, 41]. This process has been reviewed in detail elsewhere [32–34]. Although the majority of the studies demonstrating the contribution of autophagy in class II presentation have focused on macroautophagy, a recent study has established that CMA can also lead to MHC class II presentation of cytosolic antigens [42].
Vacuolar pathways for MHC class I presentation
The vast majority of peptides presented on MHC class I molecules seem to be generated by the proteasome in the cytosol and transported into the ER by TAP [2]. The discovery of efficient MHC class I presentation of endogenous proteins in TAP-deficient cells has suggested that additional pathways must exist to generate and/or transport antigens to an alternative site of MHC class I loading [43–46] (Fig. 2). The ability of proteases other than the proteasome to generate MHC class I binding peptides from an endogenous protein has been addressed using an ER-targeted secretory protein artificially fused to a reporter epitope suitable for MHC class I binding [47] (for details, see the review by del Val et al. in this issue). The epitope is cleaved out of the secretory protein in the exocytic pathway by the trans-Golgi resident protease furin and is efficiently loaded onto MHC class I molecules for presentation at the cell surface. The ability of furin to generate another MHC class I epitope from a chimeric protein has also been confirmed by others [48].
Fig. 2.
Pathways of antigen presentation on MHC class I. In the classical pathway (left), endogenous antigens in the cytosol are cleaved by the proteasome (1). Resulting peptides are transported into the ER via TAP, and loaded onto MHC class I molecules that are transported to the cell surface via the secretory pathway. In an alternative vacuolar pathway (right), antigens could be degraded by proteases in a lytic vacuole either after chaperone-mediated autophagy (CMA, 2) or macroautophagy/xenophagy (3). Peptides generated in the lytic vacuole might then return to the cytosol by a transporter like sec61. These antigens could then enter the classical pathway after a potential further trimming by the proteasome (4). Alternatively, peptides could stay in the endovacuolar pathway and be loaded onto MHC class I molecules (5). Newly loaded MHC class I complexes could be transported directly back to the cell surface by recycling vesicles. This vacuolar pathway of antigen presentation might be of particular importance in TAP-deficient cells or cells infected with viruses that can interfere with TAP or MHC class I processing and transport. Peptides generated by the proteasome can also be transported to neighboring cells via gap junctions. Last but not least, peptide cleavage can also occur by the protease furin in the Golgi
Vacuoles of the endo/phagocytic pathway contain high amounts of different proteases with distinct cleavage patterns that can degrade antigens into peptides with the appropriate length for MHC class I binding [10, 48]. This vacuolar processing has been particularly studied in systems involving cross-presentation of exogenous antigens on MHC class I molecules. More than 15 years ago, it has been demonstrated that phagocytosed Escherichia coli antigens could still be presented on MHC class I molecules even when the classical MHC class I processing was inhibited [49]. More recently, Kenneth Rock and collaborators showed that cross-presentation of phagocytosed ovalbumin was mainly TAP-independent, and the antigenic peptide could in part be generated directly in the phagosome by the cystein protease cathepsin S [10]. Similarly to exogenous antigens, endogenous proteins can undergo a vacuolar processing for efficient presentation on MHC class I molecules. Reporter epitopes fused to the MHC class I heavy chain N-terminus are transported to the cell surface, internalized in endosomal compartments where aspartic cathepsins cleave out the reporter epitope suitable for MHC class I binding [48].
Autophagy allows MHC class I presentation of endogenous antigens
While the studies presented above involved uptake of an external or re-uptake of an internal antigen from the cell surface, autophagy could provide a pathway to take up internal antigens and transport them directly to an endovacuolar compartment (Fig. 2). We have recently shown that autophagy contributed to MHC class I presentation of viral peptides following infection of macrophages by HSV1 [12]. Infection for 6–8 h leads to induction of autophagy, as shown by the visualization of numerous LC3-positive autophagosomes in the cytosol. While class I presentation of the immunodominant HSV1 peptide follows the classical pathway early in the infection, a vacuolar pathway that is sensitive to inhibitors of both lysosomal acidification and autophagy strongly contributes to MHC class I presentation of this viral antigen later in infection. Presentation is increased by a drug that induces autophagy. Hence, autophagy strongly contributes to MHC class I peptide generation in macrophages during the late phases of HSV1 infection. In agreement with these results, in interferon-gamma (IFN-γ)-treated tumor cells, autophagy induction or inhibition respectively enhances or decreases MHC class I antigen presentation [50].
The contribution of autophagy to MHC class I presentation has also been strongly suggested by in vivo studies. Indeed, mice infected with an attenuated form of HSV1 lacking the autophagy inhibitor ICP34.5 show an enhanced proliferation of CD8+ T cells compared to mice infected with the wild-type virus [51]. Similarly, induction of autophagy in dendritic cells infected with mycobacterium tuberculosis prior to their injection for an in vivo vaccine increases the proliferation of CD8+ T cells in injected mice [52]. Moreover, autophagic degradation of tumor antigen aggregates drastically increases MHC class I presentation of a tumor-derived peptide in implanted melanoma cells, leading to CD8+ T lymphocytes proliferation and decreased tumor growth in vivo [53]. Together, these studies suggest that autophagy enhances MHC class I antigen presentation in vivo as well as in vitro.
Besides macroautophagy, CMA has also been shown to participate in MHC class I presentation of endogenous proteins. Truncated forms of the Simian virus 40 (SV40) large T antigen that are located in the cytosol are presented on MHC class I molecules in a hsp73-mediated, but TAP-independent manner [54, 55]. These results clearly point out the ability of different forms of autophagy to contribute to MHC class I presentation.
Can both peptide generation and loading on MHC class I molecules occur in the same vacuole?
In order for MHC class I loading to occur, antigenic peptides and active MHC class I molecules must be present in the same compartment. This compartment is generally the ER where cytosolic peptides are translocated by TAP. However, TAP-independent MHC class I presentation has suggested that loading could occur in other MHC class I containing compartments. In addition to the ER and the plasma membrane, MHC class I molecules are found in vacuoles of the endocytic pathway [56]. As discussed above, peptides can efficiently be generated in endocytic and phagocytic vacuoles, suggesting that peptides could directly bind to active MHC class I molecules in the vacuole where they are generated. Indeed, epitope-carrying MHC class I-derived fusion proteins can be re-internalized from the plasma membrane and processed in recycling endosomes, where the reporter epitope is cleaved out and loaded onto recycling MHC class I molecules [48]. Recycling MHC class I molecules are also found in vesicles with characteristics similar to MHC class II-enriched lysosome-like compartments [57, 58]. As those vesicles are slightly acidic, peptides already bound to the re-internalized MHC class I molecules could be released [57]. New peptides displaying higher binding activities could then be loaded onto the now empty MHC class I molecules and transported to the cell surface for presentation [57]. The presence of the measles virus F protein in vesicles where exchange of peptides on recycling MHC class I molecules occurs strongly suggests that protein cleavage and peptide loading on MHC class I molecules take place within the same vesicle [58].
This model reveals that the different steps leading to antigen presentation on MHC class I molecules, such as protein cleavage, peptide trimming, and loading on MHC class I, can all take place in a single vacuolar compartment. However, more complex antigen trafficking between endovacuolar compartments and the cytosol are possible. At late times of macrophage infection with HSV1, both autophagolysosome formation and proteasome-activity are needed for sustained MHC class I presentation of a HSV1 glycoprotein B-derived peptide [12]. Therefore, it appears that processing of this viral antigen requires the successive action of the two degradative pathways. Antigens are first processed in autophagosomes or lysosomes, where hydrolases cleave viral proteins into large peptides. After transport into the cytoplasm, the pre-digested peptides are further processed by the proteasome, and handled by the classical MHC class I presentation pathway.
There is an interesting resemblance between these two possible pathways and the “vacuolar” and “phagosome to cytosol” models proposed for cross-presentation that have been discussed above [5]. Like phagosomes, autophagosomes could acquire the MHC class I machinery together with some potential transporters like sec61 or Derlin-1 from the ER. Indeed, this compartment might serve as a membrane donor for the biogenesis of classical or nuclear-derived autophagosomes during autophagy. Hence, antigenic peptides that are produced in autophagosomes could (1) be directly loaded on MHC class I molecules, or (2) be exported from the autophagosome to the cytosol, by a transporter like sec61 or Derlin-1, where peptides could then follow the classical MHC class I pathway. This argues for an “autophagosomal” or an “autophagosome to cytosol” pathway for endogenous antigen processing and loading on MHC class I molecules. Our data [12] rather support the second hypothesis, as MHC class I presentation is abrogated by drugs that inhibit the classical MHC class I pathway, even in the late phases of infection.
Does the quantity, structure, or subcellular localization of the antigen influence the autophagy-mediated MHC class I processing?
One question that arises is whether targeting of an endogenous antigen to a vacuole that contains proteases for antigen processing and gives access to MHC class I molecules is sufficient for a vacuolar antigen-presentation pathway, or whether other elements are also required. Autophagy contributes to the presentation of an HSV1 antigen derived from a membrane protein that is expressed at very high levels [12], which might explain why autophagy contributes so strongly to MHC class I presentation in this system. Similarly, the vacuolar pathway for cross-presentation of exogenous antigens is known to be dependent on the concentration of antigens [10], and the TAP-independent MHC class I presentation of influenza virus nucleoprotein also requires high amounts of antigen expression [44]. Hence, autophagy or other vacuolar pathways might only contribute to MHC class I presentation when antigens are present in very high amounts. However, this condition is likely to be required but not sufficient to engage autophagy in MHC class I presentation, as many studies describe the autophagy-independent MHC class I presentation of transfected antigens known to be expressed in high amounts in the cell [41].
Most proteases recognize specific cleavage motifs for degradation of their substrate. Hence, proteasomal aminopeptidases and vacuolar proteases are likely to generate different peptides from the same protein. Still, peptides derived from ovalbumin [10] or from influenza proteins [59] could efficiently be generated by both the vacuolar and the proteasomal pathways, indicating that similar cleavage patterns can be achieved by these different degradative systems, maybe through a series of cleavage steps by different proteases in the vacuole. However, a certain protease cleavage recognition sequence might not be contained in the amino-acid sequence of the antigen to be processed, or located at a position that is not accessible to the proteases due to protein folding. In this case, the peptide repertoire produced by the vacuolar pathway would be different from the repertoire produced by the proteasome, leading to activation of different cytotoxic T lymphocytes. In the systems that we [12] and others [10, 48, 54, 55, 57, 58] have studied, MHC class I presentation levels by the vacuolar and classical pathways were assayed using monoclonal cytotoxic T lymphocytes specific for one particular peptide that is usually produced by the proteasome. Hence, the question of whether vacuolar and proteasomal antigen processing activates different sets of T lymphocytes cannot be addressed with these systems. In a different system, only two out of three antigens generated from the same protein were presented on MHC class I in a CMA-dependent manner when TAP was absent, but all three were detected in the presence of TAP [54]. This indicates that different antigen processing and presentation pathways could indeed influence the type of antigen presented, and thus indirectly the set of T lymphocytes that are recruited. As activation of different sets of T lymphocytes would ultimately result in a different repertoire of cells, antigen processing by different pathways could diversify the adaptive immune response.
Interestingly, the truncated cytosolic form but not the wild-type nuclear form of SV40 T antigen binds hsp73 in the cytosol, a step that allows processing through CMA in TAP-deficient cells [55]. While this could be explained by the presence of a binding site for hsp73 that is inaccessible on the full-length protein, it is far more likely that the localization of the truncated antigen in the cytosol enables an interaction with the cytosolic protein hsp73 and subsequent processing by CMA, whereas the wt SV40 T antigen that is located predominantly in the nucleus is simply not accessible for hsp73 binding [55]. Hence, processing for MHC class I presentation could depend on the intracellular localization of antigens, which determines the accessibility for a specific degradation pathway. This hypothesis is supported by our data showing vacuolar antigen presentation of a HSV1 protein that is present at the nuclear and the ER membrane, both of which are implicated in the biogenesis of autophagosomes [12]. Autophagy could serve as a delivery system that allows this viral antigen to access a degradative compartment and be presented on MHC class I molecules.
It is interesting to point out that the participation of autophagy in antigen processing for presentation onto MHC class II molecules also depends on the intracellular localization of antigens. Indeed, whereas only one out of three epitopes derived from the nuclear antigen EBNA1 in EBV-transformed lymphoblastoid cell lines is dependent on autophagy for MHC class II processing and presentation, autophagy largely contributes to processing of all three MHC class II epitopes when EBNA1 is expressed in the cytoplasm [60].
Is the contribution of autophagy to MHC class I presentation an anti-viral mechanism?
In the system that we studied, autophagy was induced by HSV1 infection and contributed to MHC class I presentation of a viral antigen [12]. TAP-independent MHC class I presentation has also been described for viral antigens such as the vesicular stomatitis virus nucleoprotein, Rauscher murine leukemia virus antigens, Sendai virus antigens, and influenza virus nucleoprotein [43–46, 61]. A potential contribution of autophagy or vacuolar processing was not addressed in these studies. However, MHC class I presentation of influenza virus nucleoprotein was strictly TAP-dependent after 1.5 h of infection but became TAP-independent after 3 h of infection [44], indicating that a pathway other than the classic MHC class I route contributed to antigen presentation. This is similar to our results showing a contribution of autophagy to MHC class I presentation of a viral antigen only in the late phases of HSV1 infection. Hence, the contribution of autophagy to MHC class I presentation could be particularly important during late phases of viral infections. A recent study has shown that autophagy induction increases tumor antigen processing and presentation on MHC class I molecules when cells are treated with IFN-γ [50]. As interferon is naturally produced during viral infection, exogenous addition of interferon might induce infection-specific cellular pathways. This might be a prerequisite for the contribution of autophagy to antigen presentation. This hypothesis is further supported by our data showing that a mild heat shock treatment prior to HSV1 infection enhances MHC class I presentation [12]. A heat shock mimics fever, a common symptom in viral infections, and enhances autophagy levels. The requirement for infection-induced changes in cellular pathways for the contribution of autophagy or other vacuolar pathways to MHC class I presentation could explain why MHC class I presentation is independent of autophagy in cells that are merely transformed with the herpesvirus EBV [60, 62]. Transformed cell lines profoundly differ from infected cells, potentially failing to induce specific and so far uncharacterized cellular pathways linked to viral infection, which might be necessary to enable autophagy to contribute to MHC class I presentation of endogenous antigens.
Besides being induced by infection-induced changes in the cell, a contribution of autophagy to MHC class I presentation could be induced as a consequence of the impairment of cellular functions by viral proteins. Numerous viruses have evolved mechanisms to inhibit the classical MHC class I presentation pathway, hence preventing the infected cells to alert the immune system. The proteins EBNA1 of EBV and LANA1 from Kaposi’s sarcoma-associated herpesvirus can inhibit their own proteasomal degradation, leading to decreased viral peptide presentation on MHC class I molecules [63–66]. Several herpesviruses and adenovirus can interfere with peptide loading on MHC class I through inhibition of either TAP or tapasin by a specific viral protein [67–70]. Other viral proteins can retain MHC class I molecules in the ER [67]. The HIV protein Nef, and the mouse cytomegalovirus protein gp48 can interfere with transport of MHC class I complexes to the cell surface by redirecting them to a different cellular compartment [71–73]. Therefore, another antigen-presentation pathway might counterbalance the impairment of the classical MHC class I pathway caused by the viral infection to sustain the immune response.
Autophagy enables cross-presentation of exogenous peptides on MHC class I
In addition to its function in the presentation of endogenous antigens by MHC class I molecules, autophagy is also involved in the cross-presentation of phagocytosed antigens such as dying cells (so-called donor cells) on MHC class I. Stimulation of autophagy in infected [74] or tumoral [75] donor cells leads to an enhancement of cross-presentation in dendritic cells that take up these donor cells both in vitro and in vivo. Influenza infected cells dying by programmed cell death type II (referred to as autophagic cell death) are more efficiently cross-presented by dendritic cells to stimulate CD8+ T lymphocytes than their counterpart dying by apoptosis [74]. Moreover, purified autophagic vesicles can be used as antigen carriers for cross-presentation of exogenous antigens by dendritic cells [75]. Thus autophagy not only contributes to the processing of antigens into peptides and their presentation on MHC class I in stressed or dying cells, but might also provide a transport vessel to pass these antigens on to dendritic cells and promote an optimal immune response.
Conclusions
A healthy cell gives continuous cues to its neighborhood via MHC class I molecules present at the cell surface, conveying cues about its identity and its general state. To this end, endogenous proteins are continuously processed by the proteasome, allowing the generation of peptides for MHC class I binding. The proteasome can therefore be considered as the internal sensor of the cell.
Autophagy traps cellular components and delivers them for degradation into lysosomes, allowing maintenance of cellular homeostasis. However, recent studies have shown that delivery of endogenous antigens to a degradative compartment by autophagy also contributes to optimal CD8+ T cell activation [12, 51, 52]. This mechanism seems to be induced during viral infection, suggesting the existence of one or several key virus-linked factors that enable autophagy to contribute to MHC class I presentation. It is not yet known if these factors are viral proteins or rather cellular proteins that could be induced either by a specific viral protein or by the general context of a viral infection.
Presentation of endogenous antigens on MHC class I molecules via autophagy seems to require more time and more antigen than the classical pathway involving the proteasome in the cytosol [44]. Hence, the biological relevance of this process might be limited to times of “cellular emergency”. As shown by our study, processing viral antigens by autophagy is necessary for maintaining viral antigen MHC class I presentation in infected macrophages in the late phases of HSV1 infection [12]. This suggests that the cell uses this pathway as a “spare wheel” when the classical pathway is impaired, even if this spare process is less efficient than the classical pathway. The contribution of autophagy to antigen processing and presentation on MHC class I molecules underlines the interconnection between the different pathways of antigen presentation. In times of need, the cell can take advantage of a pre-existing machinery and put it to a different use to keep up an effective immune defense against pathogens.
Footnotes
M. Chemali and K. Radtke contributed equally to this work.
References
- 1.Falk K, Rotzschke O, Stevanovic S, et al. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 2.York IA, Rock KL. Antigen processing and presentation by the class I major histocompatibility complex. Annu Rev Immunol. 1996;14:369–396. doi: 10.1146/annurev.immunol.14.1.369. [DOI] [PubMed] [Google Scholar]
- 3.Brown JH, Jardetzky TS, Gorga JC, et al. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993;364(6432):33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- 4.Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock KL. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci USA. 1993;90(11):4942–4946. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267(5195):243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 6.Houde M, Bertholet S, Gagnon E, et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425(6956):402–406. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 7.Ackerman AL, Kyritsis C, Tampe R, Cresswell P. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc Natl Acad Sci USA. 2003;100(22):12889–12894. doi: 10.1073/pnas.1735556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackerman AL, Giodini A, Cresswell P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity. 2006;25(4):607–617. doi: 10.1016/j.immuni.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Guermonprez P, Saveanu L, Kleijmeer M, et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425(6956):397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 10.Shen L, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity. 2004;21(2):155–165. doi: 10.1016/j.immuni.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Bertholet S, Goldszmid R, Morrot A, et al. Leishmania antigens are presented to CD8 + T cells by a transporter associated with antigen processing-independent pathway in vitro and in vivo. J Immunol. 2006;177(6):3525–3533. doi: 10.4049/jimmunol.177.6.3525. [DOI] [PubMed] [Google Scholar]
- 12.English L, Chemali M, Duron J et al. (2009) Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat Immunol [DOI] [PMC free article] [PubMed]
- 13.De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 14.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dean RT. Lysosomes and membrane recycling. A hypothesis. Biochem J. 1977;168(3):603–605. doi: 10.1042/bj1680603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortimore GE, Hutson NJ, Surmacz CA. Quantitative correlation between proteolysis and macro- and microautophagy in mouse hepatocytes during starvation and refeeding. Proc Natl Acad Sci USA. 1983;80(8):2179–2183. doi: 10.1073/pnas.80.8.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuervo AM, Dice JF, Knecht E. A population of rat liver lysosomes responsible for the selective uptake and degradation of cytosolic proteins. J Biol Chem. 1997;272(9):5606–5615. doi: 10.1074/jbc.272.9.5606. [DOI] [PubMed] [Google Scholar]
- 18.Chiang HL, Terlecky SR, Plant CP, Dice JF. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989;246(4928):382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- 19.Cuervo AM, Dice JF. Unique properties of lamp2a compared to other lamp2 isoforms. J Cell Sci. 2000;113(Pt 24):4441–4450. doi: 10.1242/jcs.113.24.4441. [DOI] [PubMed] [Google Scholar]
- 20.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9(10):1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z, Klionsky DJ. An overview of the molecular mechanism of autophagy. Curr Top Microbiol Immunol. 2009;335:1–32. doi: 10.1007/978-3-642-00302-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hailey DW, Rambold AS, Satpute-Krishnan P, et al. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;141(4):656–667. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi-Nishino M, Fujita N, Noda T, et al. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol. 2009;11(12):1433–1437. doi: 10.1038/ncb1991. [DOI] [PubMed] [Google Scholar]
- 24.Yla-Anttila P, Vihinen H, Jokitalo E, Eskelinen EL. 3D tomography reveals connections between the phagophore and endoplasmic reticulum. Autophagy. 2009;5(8):1180–1185. doi: 10.4161/auto.5.8.10274. [DOI] [PubMed] [Google Scholar]
- 25.Axe EL, Walker SA, Manifava M, et al. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182(4):685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravikumar B, Moreau K, Jahreiss L et al. (2010) Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol [DOI] [PMC free article] [PubMed]
- 27.Juhasz G, Neufeld TP. Autophagy: a forty-year search for a missing membrane source. PLoS Biol. 2006;4(2):e36. doi: 10.1371/journal.pbio.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2004;36(12):2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie Z, Nair U, Klionsky DJ. Atg8 controls phagophore expansion during autophagosome formation. Mol Biol Cell. 2008;19(8):3290–3298. doi: 10.1091/mbc.E07-12-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishida Y, Arakawa S, Fujitani K, et al. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461(7264):654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- 31.Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell. 2005;120(2):159–162. doi: 10.1016/j.cell.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Schmid D, Münz C. Innate and adaptive immunity through autophagy. Immunity. 2007;27(1):11–21. doi: 10.1016/j.immuni.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7(10):767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crotzer VL, Blum JS. Autophagy and its role in MHC-mediated antigen presentation. J Immunol. 2009;182(6):3335–3341. doi: 10.4049/jimmunol.0803458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobson S, Sekaly RP, Jacobson CL, et al. HLA class II-restricted presentation of cytoplasmic measles virus antigens to cytotoxic T cells. J Virol. 1989;63(4):1756–1762. doi: 10.1128/jvi.63.4.1756-1762.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudensky A, Preston-Hurlburt P, Hong SC, et al. Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991;353(6345):622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- 37.Chicz RM, Urban RG, Gorga JC, et al. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178(1):27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dengjel J, Schoor O, Fischer R, et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci USA. 2005;102(22):7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dongre AR, Kovats S, deRoos P, et al. In vivo MHC class II presentation of cytosolic proteins revealed by rapid automated tandem mass spectrometry and functional analyses. Eur J Immunol. 2001;31(5):1485–1494. doi: 10.1002/1521-4141(200105)31:5<1485::AID-IMMU1485>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 40.Nimmerjahn F, Milosevic S, Behrends U, et al. Major histocompatibility complex class II-restricted presentation of a cytosolic antigen by autophagy. Eur J Immunol. 2003;33(5):1250–1259. doi: 10.1002/eji.200323730. [DOI] [PubMed] [Google Scholar]
- 41.Dorfel D, Appel S, Grunebach F, et al. Processing and presentation of HLA class I and II epitopes by dendritic cells after transfection with in vitro-transcribed MUC1 RNA. Blood. 2005;105(8):3199–3205. doi: 10.1182/blood-2004-09-3556. [DOI] [PubMed] [Google Scholar]
- 42.Zhou D, Li P, Lin Y, et al. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity. 2005;22(5):571–581. doi: 10.1016/j.immuni.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Townsend A, Ohlen C, Bastin J, et al. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- 44.Esquivel F, Yewdell J, Bennink J. RMA/S cells present endogenously synthesized cytosolic proteins to class I-restricted cytotoxic T lymphocytes. J Exp Med. 1992;175(1):163–168. doi: 10.1084/jem.175.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hosken NA, Bevan MJ. An endogenous antigenic peptide bypasses the class I antigen presentation defect in RMA-S. J Exp Med. 1992;175(3):719–729. doi: 10.1084/jem.175.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sijts AJ, De Bruijn ML, Nieland JD, et al. Cytotoxic T lymphocytes against the antigen-processing-defective RMA-S tumor cell line. Eur J Immunol. 1992;22(6):1639–1642. doi: 10.1002/eji.1830220644. [DOI] [PubMed] [Google Scholar]
- 47.Gil-Torregrosa BC, Raul Castano A, Del Val M. Major histocompatibility complex class I viral antigen processing in the secretory pathway defined by the trans-Golgi network protease furin. J Exp Med. 1998;188(6):1105–1116. doi: 10.1084/jem.188.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tiwari N, Garbi N, Reinheckel T, et al. A transporter associated with antigen-processing independent vacuolar pathway for the MHC class I-mediated presentation of endogenous transmembrane proteins. J Immunol. 2007;178(12):7932–7942. doi: 10.4049/jimmunol.178.12.7932. [DOI] [PubMed] [Google Scholar]
- 49.Pfeifer JD, Wick MJ, Roberts RL, et al. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361(6410):359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 50.Li B, Lei Z, Lichty BD et al. (2009) Autophagy facilitates major histocompatibility complex class I expression induced by IFN-gamma in B16 melanoma cells. Cancer Immunol Immunother [DOI] [PMC free article] [PubMed]
- 51.Broberg EK, Peltoniemi J, Nygardas M, et al. Spread and replication of and immune response to gamma134.5-negative herpes simplex virus type 1 vectors in BALB/c mice. J Virol. 2004;78(23):13139–13152. doi: 10.1128/JVI.78.23.13139-13152.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jagannath C, Lindsey DR, Dhandayuthapani S, et al. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15(3):267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 53.Fu X, Tao L, Zhang X (2010) A short polypeptide from HSV-2 ICP10 gene can induce antigen aggregation and autophagosomal degradation for enhanced immune presentation. Hum Gene Ther [DOI] [PMC free article] [PubMed]
- 54.Schirmbeck R, Bohm W, Reimann J. Stress protein (hsp73)-mediated, TAP-independent processing of endogenous, truncated SV40 large T antigen for Db-restricted peptide presentation. Eur J Immunol. 1997;27(8):2016–2023. doi: 10.1002/eji.1830270828. [DOI] [PubMed] [Google Scholar]
- 55.Schirmbeck R, Reimann J. Peptide transporter-independent, stress protein-mediated endosomal processing of endogenous protein antigens for major histocompatibility complex class I presentation. Eur J Immunol. 1994;24(7):1478–1486. doi: 10.1002/eji.1830240704. [DOI] [PubMed] [Google Scholar]
- 56.Mahmutefendic H, Blagojevic G, Kucic N, Lucin P. Constitutive internalization of murine MHC class I molecules. J Cell Physiol. 2007;210(2):445–455. doi: 10.1002/jcp.20877. [DOI] [PubMed] [Google Scholar]
- 57.Gromme M, Uytdehaag FG, Janssen H, et al. Recycling MHC class I molecules and endosomal peptide loading. Proc Natl Acad Sci USA. 1999;96(18):10326–10331. doi: 10.1073/pnas.96.18.10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kleijmeer MJ, Escola JM, UytdeHaag FG, et al. Antigen loading of MHC class I molecules in the endocytic tract. Traffic. 2001;2(2):124–137. doi: 10.1034/j.1600-0854.2001.020207.x. [DOI] [PubMed] [Google Scholar]
- 59.Schirmbeck R, Wild J, Reimann J. Similar as well as distinct MHC class I-binding peptides are generated by exogenous and endogenous processing of hepatitis B virus surface antigen. Eur J Immunol. 1998;28(12):4149–4161. doi: 10.1002/(SICI)1521-4141(199812)28:12<4149::AID-IMMU4149>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 60.Leung CS, Haigh TA, Mackay LK, et al. Nuclear location of an endogenously expressed antigen, EBNA1, restricts access to macroautophagy and the range of CD4 epitope display. Proc Natl Acad Sci USA. 2010;107(5):2165–2170. doi: 10.1073/pnas.0909448107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou X, Glas R, Momburg F, et al. TAP2-defective RMA-S cells present Sendai virus antigen to cytotoxic T lymphocytes. Eur J Immunol. 1993;23(8):1796–1801. doi: 10.1002/eji.1830230810. [DOI] [PubMed] [Google Scholar]
- 62.Paludan C, Schmid D, Landthaler M, et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307(5709):593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 63.Levitskaya J, Coram M, Levitsky V, et al. Inhibition of antigen processing by the internal repeat region of the Epstein–Barr virus nuclear antigen-1. Nature. 1995;375(6533):685–688. doi: 10.1038/375685a0. [DOI] [PubMed] [Google Scholar]
- 64.Dantuma NP, Heessen S, Lindsten K, et al. Inhibition of proteasomal degradation by the gly-Ala repeat of Epstein–Barr virus is influenced by the length of the repeat and the strength of the degradation signal. Proc Natl Acad Sci USA. 2000;97(15):8381–8385. doi: 10.1073/pnas.140217397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaldumbide A, Ossevoort M, Wiertz EJ, Hoeben RC. In cis inhibition of antigen processing by the latency-associated nuclear antigen I of Kaposi sarcoma herpes virus. Mol Immunol. 2007;44(6):1352–1360. doi: 10.1016/j.molimm.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 66.Kwun HJ, da Silva SR, Shah IM, et al. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mimics Epstein-Barr virus EBNA1 immune evasion through central repeat domain effects on protein processing. J Virol. 2007;81(15):8225–8235. doi: 10.1128/JVI.00411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hansen TH, Bouvier M. MHC class I antigen presentation: learning from viral evasion strategies. Nat Rev Immunol. 2009;9(7):503–513. doi: 10.1038/nri2575. [DOI] [PubMed] [Google Scholar]
- 68.Donaldson JG, Williams DB. Intracellular assembly and trafficking of MHC class I molecules. Traffic. 2009;10(12):1745–1752. doi: 10.1111/j.1600-0854.2009.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Loch S, Tampe R. Viral evasion of the MHC class I antigen-processing machinery. Pflugers Arch. 2005;451(3):409–417. doi: 10.1007/s00424-005-1420-8. [DOI] [PubMed] [Google Scholar]
- 70.Griffin BD, Verweij MC, Wiertz EJ. Herpesviruses and immunity: the art of evasion. Vet Microbiol. 2010;143(1):89–100. doi: 10.1016/j.vetmic.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 71.Schaefer MR, Wonderlich ER, Roeth JF, et al. HIV-1 Nef targets MHC-I and CD4 for degradation via a final common beta-COP-dependent pathway in T cells. PLoS Pathog. 2008;4(8):e1000131. doi: 10.1371/journal.ppat.1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roeth JF, Williams M, Kasper MR, et al. HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail. J Cell Biol. 2004;167(5):903–913. doi: 10.1083/jcb.200407031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reusch U, Muranyi W, Lucin P, et al. A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J. 1999;18(4):1081–1091. doi: 10.1093/emboj/18.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uhl M, Kepp O, Jusforgues-Saklani H et al. (2009) Autophagy within the antigen donor cell facilitates efficient antigen cross-priming of virus-specific CD8(+) T cells. Cell Death Differ [DOI] [PubMed]
- 75.Li Y, Wang LX, Yang G, et al. Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res. 2008;68(17):6889–6895. doi: 10.1158/0008-5472.CAN-08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]