Abstract
Cancer stem cells have been hypothesized to drive the growth and metastasis of tumors. Because they need to be targeted for cancer treatment, they have been isolated from many solid cancers. However, cancer stem cells from primary human gastric cancer tissues have not been isolated as yet. For the isolation, we used two cell surface markers: the epithelial cell adhesion molecule (EpCAM) and CD44. When analyzed by flow cytometry, the EpCAM+/CD44+ population accounts for 4.5% of tumor cells. EpCAM+/CD44+ gastric cancer cells formed tumors in immunocompromised mice; however, EpCAM−/CD44−, EpCAM+/CD44− and EpCAM−/CD44+ cells failed to do so. Xenografts of EpCAM+/CD44+ gastric cancer cells maintained a differentiated phenotype and reproduced the morphological and phenotypical heterogeneity of the original gastric tumor tissues. The tumorigenic subpopulation was serially passaged for several generations without significant phenotypic alterations. Moreover, EpCAM+/CD44+, but not EpCAM−/CD44−, EpCAM+/CD44− or EpCAM−/CD44+ cells grew exponentially in vitro as cancer spheres in serum-free medium, maintaining the tumorigenicity. Interestingly, a single cancer stem cell generated a cancer sphere that contained various differentiated cells, supporting multi-potency and self-renewal of a cancer stem cell. EpCAM+/CD44+ cells had greater resistance to anti-cancer drugs than other subpopulation cells. The above in vivo and in vitro results suggest that cancer stem cells, which are enriched in the EpCAM+/CD44+ subpopulation of gastric cancer cells, provide an ideal model system for cancer stem cell research.
Keywords: Cancer stem cells, Gastric cancer, CD44, EpCAM, Cancer sphere
Introduction
Gastric cancer is the second leading cause of cancer deaths worldwide [1, 2]. Despite advances in various diagnostic tools, including endoscopy, the 5-year relative survival rate is only 20–30% [3]. Most gastric malignancies are adenocarcinomas, which have been classified under the Lauren system into two histological types: intestinal and diffuse [4]. The intestinal type is a well-differentiated tumor characterized by cohesive neoplastic cells that form gland-like tubular structures. This type of gastric cancer usually occurs at a late age and progresses through a relatively well-defined series of pre-neoplastic histological steps. The diffuse type of gastric cancer is a poorly differentiated tumor in which individual cells infiltrate and thicken the gastric wall. Cancer cells do not form glandular structures and are not associated with intestinal metaplasia. The intestinal type is frequently accompanied by liver metastasis, whereas the diffuse type is frequently associated with peritoneal dissemination.
Two critical survival-influencing factors in gastric cancer are the depth of invasion and the presence of regional lymph node involvement [5, 6]. In other words, metastasis of cancer cells is one of the most important prognostic factors in gastric cancer, as in other epithelial cancers. Thus, studies involving the development of prognostic and therapeutic tools for gastric cancer should be focused on metastatic cancer cells.
At a recent American Association of Cancer Research (AACR) workshop, cancer stem cells have been defined as “cells within a tumor that possess the capacity for self-renewal and that can cause the heterogeneous lineages of cancer cells that constitute the tumor” [7]. Recently, a connection between metastasis and the stem cell state has been proposed [8–11]. Specifically, it was suggested that migrating cancer cells must be in a stem cell state. The ability to find macroscopic metastasis is compromised from the outset because of the limited proliferative potential if the vast majority of cells leaving a primary tumor and disseminating to distant sites lack self-renewal capability. Interestingly, induction of the epithelial-mesenchymal transition (EMT) in differentiated breast cancer cells causes the appearance of the cancer stem cell state [10]. Overexpression of Snail or Twist, important transcription factors for EMT in cancer cells, increases the self-renewal ability in vitro and tumorigenicity in vivo [10]. Taken together, it appears that cancer stem cells can drive metastasis.
Cancer stem cells have been isolated in solid tumors of a wide variety of organs using surface markers [12–17]. The existence of gastric cancer stem cells has been suggested. In gastric cancer cell lines, putative cancer stem cells have been identified using the cell surface marker, CD44 [18]. However, they did not confirm their results in primary gastric cancer tissues. Side population (SP) cells have also been isolated from gastric cancer cell lines and primary gastric cancer tissues [19, 20]. However, several studies showed evidence against an association between the SP population and cancer stem cells. SP cells exist in MKN28 gastric cancer cells [18]. However, this cell line did not produce tumor in a xenograft model. Moreover, glioma cells, which were negative for ABCG2, an ATP-binding cassette half-transporter that is associated with SP cells, produced tumors in a xenograft model [21]. Furthermore, SP and non-SP cells of colon cancer cell lines showed similar tumorigenicity in the xenograft model and equivalent multipotential differentiation potential [22]. In the present study, we were successful in enriching cancer stem cells from primary gastric cancer tissues using two cell surface markers [CD44 and the epithelial cell adhesion molecule (EpCAM), also known as epithelial specific antigen (ESA)]. EpCAM+/CD44+ cells isolated from primary cancer tissues were tumorigenic in immunocompromised mice and successfully transferred for several generations. In addition to in vivo experiments, gastric cancer stem cells generated various differentiated cells in cancer sphere culture. Because cancer stem cells can drive metastasis, strategies to target these cells will significantly advance the treatment of gastric cancer.
Materials and methods
Tumor tissues and their disaggregation
Gastric cancer tissues were obtained with informed consent from patients who underwent surgical resection at Pusan National University Hospital and Pusan National University Yangsan Hospital, and the study was approved by the institutional review boards of the respective hospital. The human gastric cancer tissues used in this study are listed in Table 1, together with clinical information related to the corresponding patients. All primary tissues were collected under protocols approved by the Pusan National University Hospital Institutional Review Board between 2007 and 2010. Tumor tissues were disaggregated as described previously, with minor modifications [23]. Solid tissues, both normal and neoplastic, collected from primary surgical specimens or mouse xenografts were mechanically and enzymatically disaggregated into single-cell suspensions and analyzed by flow cytometry. Solid tissues were minced with scissors into small (2–3 mm3) fragments and incubated for 15 min at room temperature in 100 mM phosphate buffer (pH 7.0) with 6.5 mM DTT (Sigma-Aldrich, St. Louis, MO) to remove mucus contamination. After gentle removal of the DTT solution, tissue fragments were rinsed once with phosphate solution, resuspended in serum-free RPMI medium 1640 (2 mmol/l l-glutamine, 120 µg/ml of penicillin, 100 µg/ml of streptomycin, 50 µg/ml of ceftazidime, 0.25 µg/ml of amphotericin-B, and 20 mmol/l HEPES) with 200 units/ml collagenase type III (Worthington, Lakewood, NJ) and 100 units/ml DNase I (Worthington), and incubated for 1 h at 37°C to achieve enzymatic disaggregation. The cells were then resuspended by pipetting and serially filtered by using 70-µm and 35-µm nylon meshes. The contaminating red blood cells were removed by osmotic lysis (i.e., incubation in ammonium chloride potassium phosphate hypotonic buffer for 5 min on ice).
Table 1.
Case description and tumorigenic activity of EpCAM+/CD44+ gastric cancer cells
| Case no. | Age/sex | Lauren | T grade | N grade | Site | EpCAM+/CD44+ in tumour | Number of cells injected | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EpCAM+/CD44+ | EpCAM−/CD44− | EpCAM+/CD44− | EpCAM−/CD44+ | Unseparated | |||||||
| 1 | F/43 | Diffuse | T4a | N3a | UB, PW | 8.7% | 10,000(2/2) | 10,000(0/2) | 50,000(1/2) | ||
| Yes* | No | No | No | ||||||||
| 2 | M/78 | Diffuse | T2a | N0 | UB | 7.9% | 10,000(2/2) | 10,000(0/2) | 50,000(0/2) | ||
| 5,000(1/1) | 5,000(0/1) | 10,000(0/2) | |||||||||
| 3 | M/54 | Diffuse | T3 | N2 | Circ/LB | 6.0% | 10,000(2/2) | 10,000(0/2) | 50,000(1/1) | ||
| Yes* | No | No | No | ||||||||
| 4 | M/54 | Diffuse | T4a | N2 | Antrum | 2.4% | 10,000(1/1) | 10,000(0/1) | 10,000(1/2) | ||
| 5,000(1/2) | 5,000(0/2) | 5,000(0/2) | |||||||||
| 1,000(1/2) | 1,000(0/2) | 1,000(0/2) | |||||||||
| 500(1/2) | 500(0/2) | 500(0/4) | |||||||||
| 5 | F/48 | Diffuse | T3 | N2 | GC/LB | 1.8% | 10,000(1/1) | 10,000(0/1) | 10,000(0/2) | ||
| 5,000(1/1) | 5,000(0/1) | 5,000(0/2) | |||||||||
| 1,000(1/2) | 1,000(0/2) | 1,000(0/1) | |||||||||
| 500(0/2) | 500(0/2) | 500(0/2) | |||||||||
| 6 | M/66 | Diffuse | T3 | N3a | Antrum~LB | 14.0% | 5,000(1/1) | 5,000(0/1) | 5,000(0/1) | ||
| 1,000(1/1) | 1,000(0/1) | 1,000(0/1) | |||||||||
| 500(1/2) | 500(0/2) | 500(0/2) | |||||||||
| 7 | F/67 | Diffuse | T4a | N3a | Circ/MB | 8.6% | 5,000(1/1) | 5,000(0/1) | 5,000(0/1) | ||
| 1,000(1/1) | 1,000(0/1) | 1,000(0/1) | |||||||||
| 500(1/2) | 500(0/2) | 500(0/2) | |||||||||
| 8 | F/63 | Intestinal | T3 | N0 | Antrum | 4.7% | 1,000(1/4) | 1,000(0/4) | 1,000(0/2) | ||
| 500(1/2) | 500(0/2) | ||||||||||
| 9 | M/71 | Intestinal | T2 | N2 | Circ | 4.3% | 10,000(1/2) | 10,000(0/2) | 10,000(0/2) | 10,000(0/2) | |
| 10 | M/59 | Intestinal | T1b | N0 | LC/LB | 4.0% | 10,000(1/1) | 10,000(0/1) | 10,000(0/2) | 10,000(0/2) | |
| 11 | M/72 | Intestinal | T4a | N3a | Circ/LB | 3.5% | 10,000(1/1) | 10,000(0/1) | 10,000(0/1) | 10,000(0/1) | |
| Yes* | No | No | No | ||||||||
| 12 | M/57 | Intestinal | T3a | N3b | GC/LB | 1.5% | 5,000(1/1) | 5,000(0/1) | 5,000(0/1) | ||
| Yes* | No | No | No | ||||||||
| 13 | M/58 | Intestinal | T2 | N0 | PW/LB | 3.1% | 10,000(1/2) | 10,000(0/2) | 50,000(1/1) | ||
| 5,000(0/1) | 5,000(0/1) | 10,000(0/2) | |||||||||
| 14 | M/74 | Intestinal | T3 | N1 | AW/UB | 1.5% | 10,000(1/1) | 10,000(0/1) | 50,000(0/1) | ||
| 5,000(0/2) | 5,000(0/2) | 10,000(0/1) | |||||||||
| 15 | F/70 | Intestinal | T3 | N3a | LC/UB | 1.7% | 10,000(1/1) | 10,000(0/1) | 50,000(0/1) | ||
| 5,000(1/2) | 5,000(0/2) | 10,000(0/1) | |||||||||
| 1,000(1/2) | 1,000(0/2) | 5,000(0/1) | |||||||||
| 16 | M/60 | Intestinal | T1b | N2 | LC/LB | 4.9% | Yes* | No | No | No | |
| 17 | M/46 | Intestinal | T4a | N3a | LC/MB | 2.5% | 5,000(1/1) | 5,000(0/1) | 5,000(0/2) | ||
| 1,000(1/2) | 1,000(0/2) | 1,000(0/1) | |||||||||
| 500(0/2) | 500(0/2) | ||||||||||
| 18 | F/57 | Intestinal | T3 | N0 | LC/LB | 2.3% | 5,000(1/1) | 5,000(0/1) | 5,000(0/1) | ||
| 1,000(1/2) | 1,000(0/2) | 1,000(0/1) | |||||||||
| 500(0/2) | 500(0/2) | ||||||||||
| 19 | F/73 | Intestinal | T4a | N2 | LC,GC/LB | 1.4% | 5,000(1/1) | 5,000(0/1) | 5,000(0/1) | ||
| 1,000(0/2) | 1,000(0/2) | 1,000(0/1) | |||||||||
| 500(0/2) | 500(0/2) | ||||||||||
Freshly isolated subpopulations of gastric cancer cells generated subcutaneous tumors in mice xenografts. The success rate is shown in brackets
*In vitro sphere culture was tried. The result was presented as Yes (spheres were formed) or No
Flow cytometry and cell-sorting experiments
To minimize experimental variability and loss of cell viability, all experiments were performed on fresh tumor cell suspensions prepared shortly before flow cytometry. Antibody staining was performed in PBS supplemented with 1% bovine serum albumin. To minimize non-specific binding of antibodies, cells were incubated with 0.6% human immunoglobulins (GreenCross Corp., Seoul, South Korea) for 10 min on ice at a cell concentration of 3–5 × 105/100 µl. Cells were subsequently washed and stained with antibodies at dilutions determined by titration experiments on each xenograft line. The antibodies used in this study include anti-human ESA-APC (clone EBA-1; Becton-Dickinson, San Jose, CA) and anti-human CD44-FITC antibodies (clone G44-26; BD Biosciences, San Diego, CA). In all experiments, cells positive for the expression of non-epithelial lineage markers (Lin +) were excluded by staining with PE.Cy5-labeled antibodies using two different strategies for primary tissues and mouse xenografts. In experiments involving primary human tissues, stromal cells were excluded by simultaneous staining with anti-human CD3-PE.Cy5 (clone UCHT1: BD Biosciences), CD10-PE.Cy5 (clone HI10a: BD Biosciences) and CD45-PE.Cy5 antibodies (clone HI30; BD Biosciences). In experiments involving gastric cancer xenografts, mouse cells were excluded by simultaneous staining with anti-mouse CD45-PE.Cy5 antibody (clone 30-F11; BD Biosciences). Flow cytometry analysis was performed using a BD FACSAria cell sorter (Becton-Dickinson). The forward scatter area versus forward scatter width profiles were used to eliminate cell doublets. Dead cells were eliminated by excluding DAPI-positive cells.
Tumorigenicity experiments
Sorted cells were spun down by low-speed centrifugation (850 × g for 5 min) and resuspended in RPMI 1640 supplemented with 10% FBS, 20 mM HEPES and 2 mM l-glutamine. In all experiments, a small aliquot of cells was set aside to confirm the cell count and viability using conventional techniques (i.e., trypan blue exclusion). Once the cell count and viability were confirmed, cells were diluted to appropriate injection doses, mixed with BD Matrigel (BD Biosciences) at 1:1 ratio and injected subcutaneously in nude mice on the dorsal side of each flank. To minimize experimental variability due to individual differences in recipient mice, cell populations that were to be compared were injected on opposite flanks of the same animals. The injected mice were followed for up to 5 months and killed when tumors reached a maximum diameter of 17 mm. The Pusan National University Institutional Animal Care and Use Committee (PNUIACUC) approved the experimental procedures.
Cell cultures
Tumor samples were subjected to mechanical and enzymatic disaggregation as described above. The resulting cancer cells were cultured in Neurobasal-A medium (Gibco, Camarillo, CA) supplemented with 2 mM l-glutamine, 120 µg/ml of penicillin, 100 µg/ml of streptomycin, B27, 50 ng/ml of EGF, and 50 ng/ml of FGF-2. For differentiation, 5% FCS was added to the media instead of growth factors.
Histological evaluation
Tumor tissues were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer saline [PBS (pH 7.4)] for 4–6 h and embedded in paraffin. After cutting with a microtome, sections (4 μm thick) were deparaffinized and hydrated using xylene and graded methanol, respectively. Deparaffinized and hydrated sections were stained in a standard fashion with hematoxylin-eosin (H&E).
Cell survival assay
Cancer spheres were disaggregated, and the resulting cells were sorted into each subpopulation as described above. Sorted cells were seeded at a density of 1.5 × 104/well in 96-well plates. Next, they were treated with the indicated concentrations of various anti-cancer drugs including 5-fluorouracil (Sigma-Aldrich, St. Louis, MO), doxorubicin (Sigma-Aldrich), vinblastine (Sigma-Aldrich) and paclitaxel (Sigma-Aldrich). After 48 h, cell proliferation reagent WST-1 (Roche, Mannheim, Germany) was added at a concentration of 10 μl/well, and the cells were further incubated for 4 h at 37°C in a humidified atmosphere of 5% CO2. Cell viability was measured by absorbance at 450 nm using an enzyme-linked immunosorbent assay (ELISA) reader (TECAN, Mannedorf, Switzerland). The cell proliferation curve was plotted using the absorbance at each time point. Viability was determined in quintuplicate.
Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissue. Sections of paraffin-embedded tissue were dewaxed in xylene and rehydrated with distilled water. Sections were then incubated with 0.3% hydrogen peroxide. The slides were subsequently incubated with the following antibodies overnight at 4°C: anti-MUC5AC (Novocastra, Newcastle, UK), anti-MUC6 (Novocastra) and anti-Pan-cytokeratin antibodies (Invitrogen, Camarillo, CA). The reaction was performed using an Elite Vector Stain ABC system (Vector Laboratories, Burlingame, CA) and DAB substrate chromogen (DakoCytomation, Denmark), followed by hematoxylin counterstaining.
Transmission electron microscopy (TEM)
Cells were harvested by centrifugation, washed in PBS and then resuspended in 200 ml of PBS. Cells were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer at room temperature, and then postfixed with 1% OsO4 in the same buffer. The pellet was rinsed with distilled water, dehydrated in a graded ethanol series, and embedded in Spurr’s resin according to the recommendations of the supplier(Ladd Research Industry, Burlington, VT). Thin sections were prepared on an LKB Nova ultramicrotome (Leica, Vienna, Austria) fitted with a diamond knife and were observed in a transmission electron microscope Hitachi H-7600(Hitachi, Japan) operated by the Center for Research Facilities, Pusan National University (South Korea).
Statistical analysis
Statistical comparisons between two groups were made using the nonparametric Mann-Whitney U test or Student’s t test. For comparison of more than three groups, one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison, was used. P values of <0.05 were considered statistically significant.
Results
Expression of surface markers in gastric cancer tissues
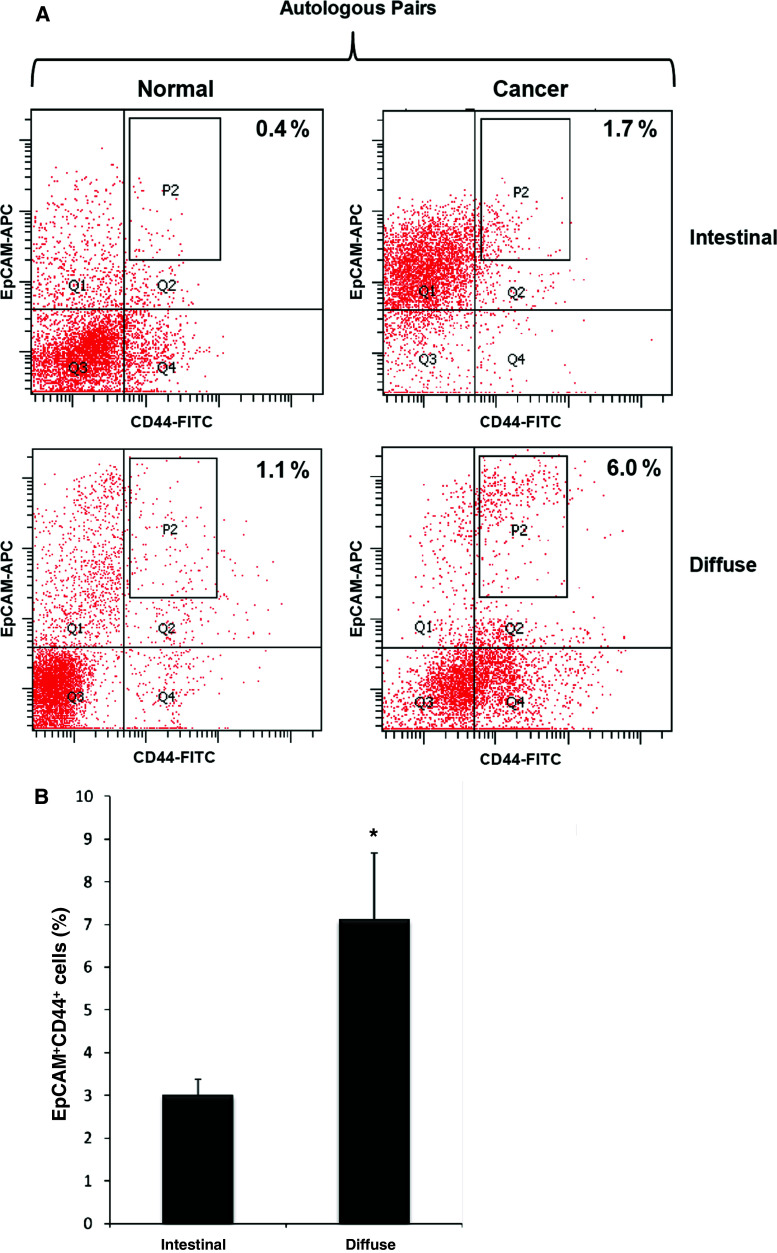
Fresh human gastric cancer specimens and corresponding normal mucosal specimens were obtained at the time of surgery from 19 patients (Table 1). To isolate cancer stem cells, the primary tissues were disaggregated into single-cell suspensions and analyzed by flow cytometry. We used two cell surface markers (CD44 and EpCAM) because these two markers have been used to isolate cancer stem cells from other cancer tissues [12, 23]. Based on the expression of CD44 and EpCAM, we were able to discriminate four main populations of epithelial cells (EpCAM+/CD44+, EpCAM−/CD44−, EpCAM+/CD44−, EpCAM−/CD44+). In normal gastric muscosa, EpCAM+/CD44+ cells were very rare compared to cancer tissues (Fig. 1). The overall frequency of EpCAM+/CD44+ was 0.5% of total live normal gastric mucosal cells. In primary gastric cancer tissues, the frequency of EpCAM+/CD44+ cells was 4.5% of total live tumor cells. Although a consistent pattern was not observed in flow cytometry plots of primary cancer tissues, more EpCAM+/CD44+ cells were observed in the diffuse type (7.1%) than in the intestinal type (3%) of gastric cancer tissues (Fig. 1).
Fig. 1.
EpCAM/CD44 expression profiles in primary gastric cancers and normal gastric mucosa. a The pattern of EpCAM/CD44 expression in primary gastric cancers and normal gastric mucosa was compared. To minimize experimental variability and contributions of genetic background, primary cancers (right panels) were compared with autologous normal mucosa (left panels) for patients 15 and 3 (Table 1), and analyzed on the same day. The upper two panels were derived from the intestinal type of gastric cancer (patient 15) and lower two panels from the diffuse type of gastric cancer (patient 3). The percentages reported in flow plots indicate the percentage of cells contained within gate P2. b Comparison of EpCAM/CD44 expression between the intestinal and the diffuse types of gastric cancers. Data are the mean ± SE of 19 patients (*P < 0.05, Student's t test)
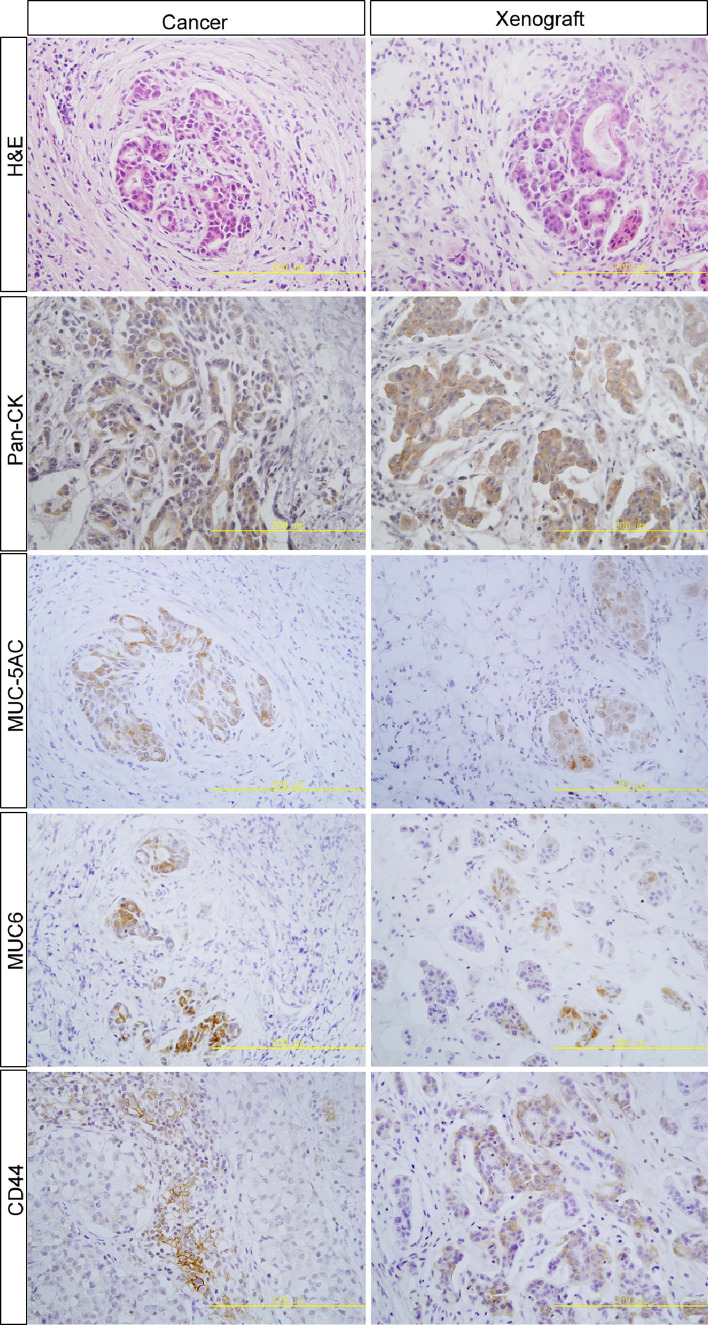
To determine the anatomic location of CD44+ cells in gastric cancer tissues, we performed immunohistochemistry in the xenograft, using gastric cancer tissues from patients and in the original patient’s tumor. CD44 expression was detected in the invasive gastric cancer cells and some immune and stromal cells (Fig. 2). However, it was hard to detect CD44 expression in normal gastric epithelial cells (data not shown).
Fig. 2.
Xenografts generated from EpCAM+/CD44+ gastric cancer cells resemble the original patient tumor (patient 6). The histology of the two tumors, as examined by H&E staining, shows similar structures. The immunohistochemical staining using various markers, including MUC5AC, MUC6, Pan-cytokeratin (Pan-CK) and CD44, showed comparable staining patterns in both the xenograft and parental tumors. In the invasive front of gastric cancer, CD44 staining was similarly observed in both tumors. Production of two gastric mucins (MUC5AC and MUC6) was also similar in both tumors. Scale bar = 200 µm
Differential tumorigenicity of gastric cancer phenotypic subpopulation
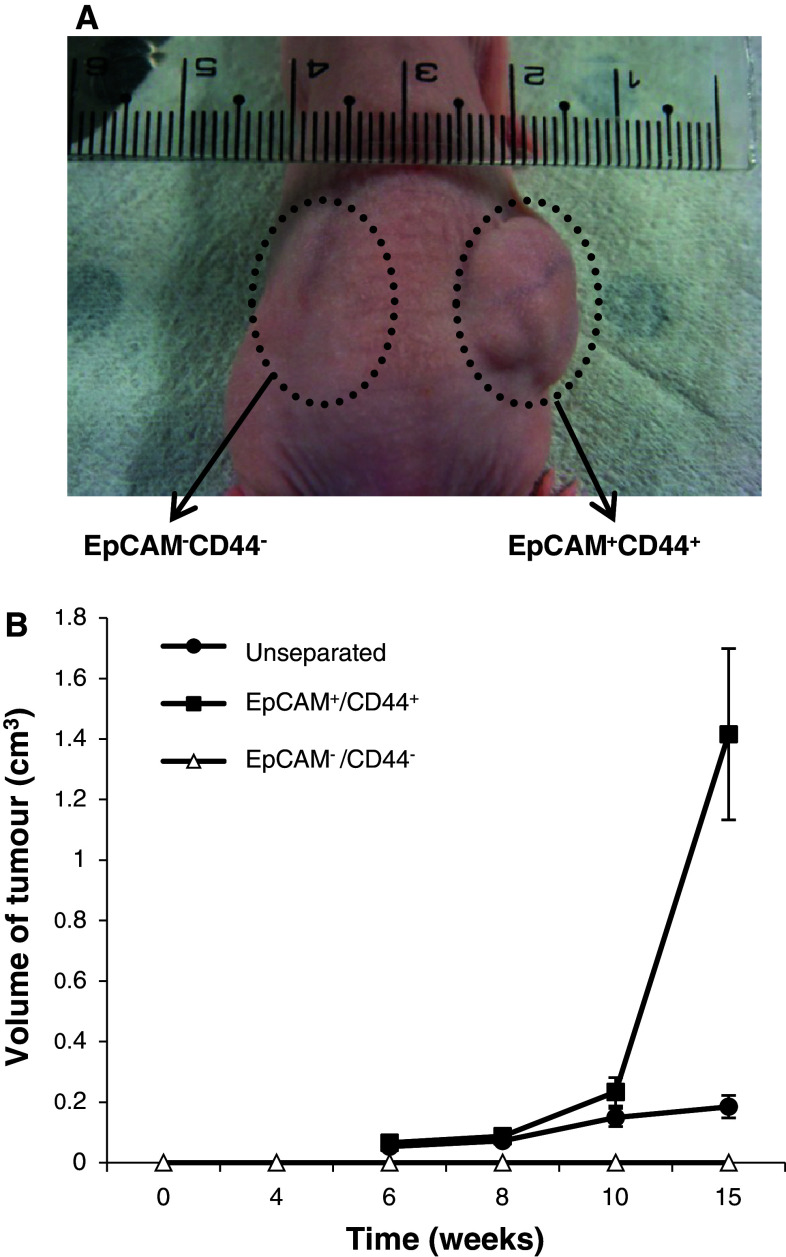
To determine whether two phenotypic subpopulations have differential tumorigenic properties, we did engraftment experiments in the immunocompromised mice. The results showed a substantial difference in tumorigenic properties. Tumors frequently arose following injection of EpCAM+/CD44+ cells, whereas EpCAM−/CD44− cells consistently failed to form tumors 20 weeks following the transplantation, indicating that EpCAM+/CD44+ cells are cancer stem cells (Fig. 3; Table 1). Moreover, EpCAM+/CD44− and EpCAM−/CD44+ cells also failed to do so 20 weeks following the transplant (data not shown). As few as 500 EpCAM+/CD44+ cells formed tumors; however, 104 EpCAM−/CD44− failed to form tumors (Table 1). In fact, 1 × 104 of the unseparated cells were required to initiate tumor formation; however, tumor formation was inefficient (1 of 10 injected hosts), suggesting that cancer stem cells were enriched with the EpCAM+/CD44+ subpopulation. Moreover, hematoxylin-eosin staining and microscopic analysis indicated that EpCAM+/CD44+-derived tumor xenografts reproduced the morphological and phenotypical heterogeneity of the primary tumor, including production of gastric mucins (Fig. 2). Staining of cytokeratin, MUC-5AC, MUC6 and CD44 showed a similar pattern between the original tumor tissue and the xenograft tumor tissue (Fig. 2). Furthermore, not all of the cells derived from the EpCAM+/CD44+ subpopulation were CD44+, despite the fact that all of the injected cells were CD44+, indicating that CD44+ cells likely generated CD44− cells. When analyzed by flow cytometry, the xenograft tumors contained both EpCAM+/CD44+ and EpCAM−/CD44− populations in proportions similar to those of the primary lesions (Fig. 4a).
Fig. 3.
The tumorigenic potential was restricted to the EpCAM+/CD44+ subpopulation. The tumorigenic potential of freshly isolated EpCAM+/CD44+, EpCAM−/CD44− and unseparated (total) gastric cancer cells was evaluated after subcutaneous injection of 104 cells in matrigel into immunocompromised mice. No tumor growth was observed on injection of EpCAM−/CD44− cells (a, circled area, patient 6). Data concerning tumor volumes are the mean ± SE of five independent experiments in duplicate (b)
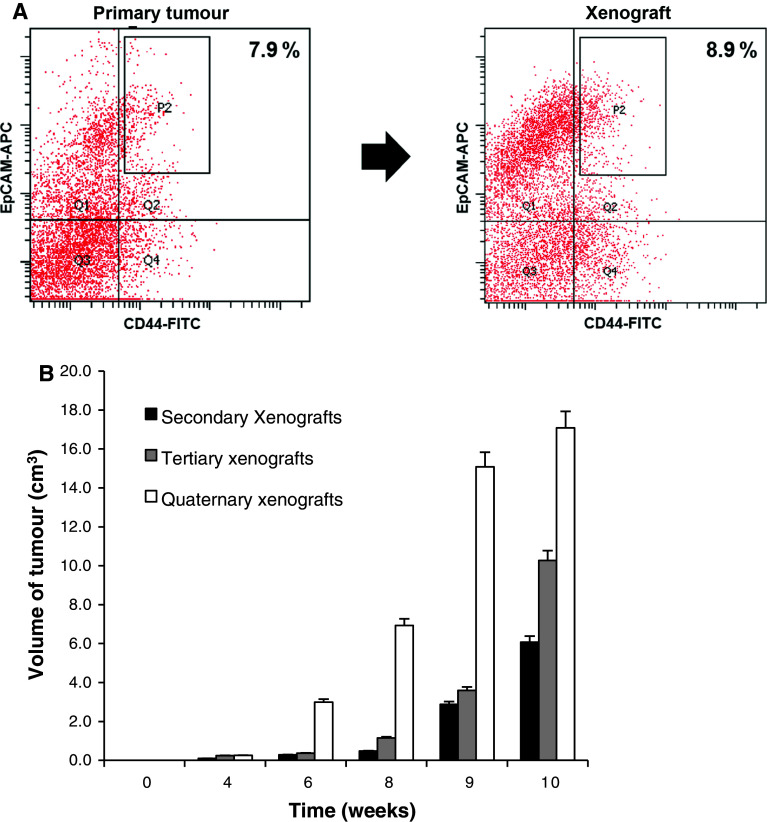
Fig. 4.
Long-term tumorigenic potential of gastric cancer EpCAM+/CD44+ cells. a Analysis of tumors grown from injection of sorted EpCAM+/CD44+ cells showed reconstitution of parental expression profiles (patient 2). Percentage reported in flow plots indicated the percentage of cells contained within gate P2. b Tumorigenic potential of gastric cancer cells derived by tumors induced by injection of 106 freshly isolated EpCAM+/CD44+ cells (secondary xenografts), gastric cancer cells derived by such secondary xenografts (tertiary xenografts) and gastric cancer cells derived by tertiary xenografts (quaternary xenografts). Data are the mean ± SE of three independent experiments in duplicate
To investigate whether EpCAM+/CD44+ gastric cancer cells display long-term tumorigenic potential, we evaluated the ability of these cells to generate tumors after serial transplantation. Tumor xenografts from the freshly isolated EpCAM+/CD44+ cells were digested to isolate EpCAM+/CD44+ and EpCAM−/CD44− cells, which in turn were transplanted. Although EpCAM−/CD44− cells were not able to form tumors into secondary mice, EpCAM+/CD44+ cells were tumorigenic. EpCAM+/CD44+ cells in secondary tumors were used as a new source of EpCAM+/CD44+ cells to generate tertiary and then quaternary tumors. During the in vivo passages, EpCAM+/CD44+ cells maintained their tumorigenicity. In addition, the aggressiveness of EpCAM+/CD44+ cells increased, as evidenced by the more rapid growth (Fig. 4b).
Formation of cancer spheres
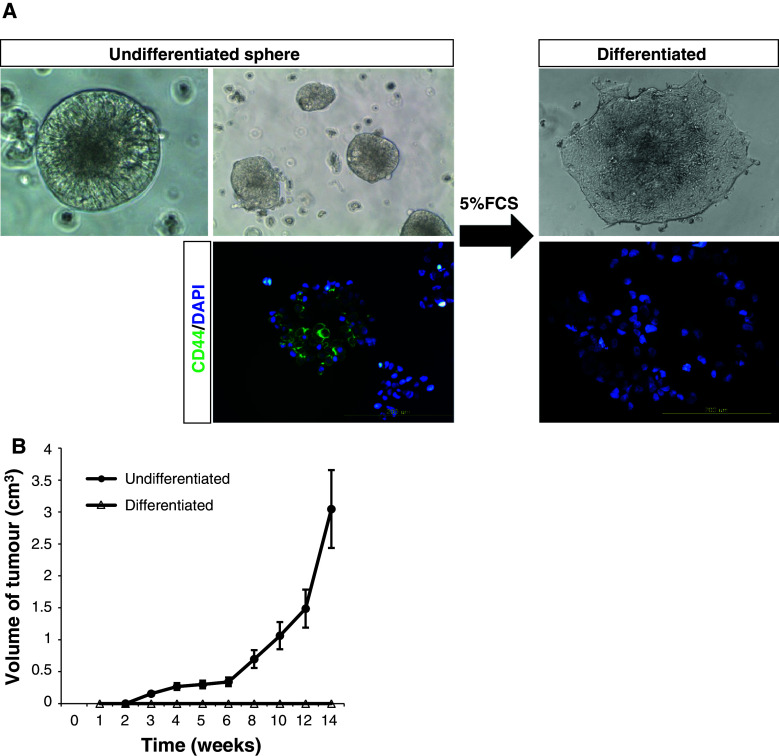
Previous reports have shown that normal and neoplastic stem cells from neural and epithelial organs can be expanded as sphere-like cellular aggregates in serum-free medium containing EGF and FGF-2 [13, 17, 24]. We tried to culture gastric cancer cells after dissociation of cancer tissues in serum-free media containing EGF and FGF-2. After we sorted EpCAM+/CD44+, EpCAM+/CD44−, EpCAM−/CD44+, and EpCAM−/CD44− subpopulations from patients’ tissues, we cultured each subpopulation to observe sphere formation. Interestingly, only the EpCAM+/CD44+ subpopulation formed cancer spheres after 4–5 weeks of culture (Table 1), although not all EpCAM+/CD44+ cells formed cancer spheres. When 5% serum was added to the media instead of EGF and FGF-2, floating cancer spheres attached to the plastic and became adherent. Following adhesion, the cells lost the expression of CD44 (Fig. 5a). To determine whether or not expanded CD44+ cells in tumor spheres maintained tumorigenic potential, we injected cancer spheres or differentiated cells subcutaneously into nude mice and monitored the formation of tumors weekly. While 104 adherent cells were not tumorigenic, cancer spheres engrafted and generated tumors (Fig. 5b).
Fig. 5.
The tumorigenic potential of EpCAM+/CD44+ gastric cancer cells is lost upon differentiation. a Microscopic analysis of gastric cancer spheres (patient 16) cultivated in differentiation condition for 7 days. CD44 expression in undifferentiated and differentiated gastric cancer cells was analyzed by immunocytochemistry. Before immunocytochemistry, undifferentiated cancer spheres were disaggregated into single cells by trypsin treatment. b Tumorigenic potential of cancer spheres and differentiated EpCAM+/CD44+ gastric cancer cells. Differentiated cells are not CD44-postive. Tumor volumes of mice injected with 104 cells are shown. Data are the mean ± SE of three independent experiments in duplicate
Differentiation of a cancer stem cell inside a cancer sphere
Next, we examined whether a single EpCAM+/CD44+ cancer stem cell can form a cancer sphere and generate differentiated cells in a cancer sphere. After sorting using FACS, we cultured a single EpCAM+/CD44+ cell in a well of a 96-well plate after limiting dilution and observed its growth. A single EpCAM+/CD44+ formed a sphere within 10 days (Fig. 6). To examine the status of differentiation of a sphere, we made a paraffin block for the sphere and labeled it with various markers including cytokeratin, MUC-5AC, MUC6, MUC2 and pepsinogen. Remarkably, we observed positive cells for various markers inside the sphere (Fig. 7a), indicating that a single cancer stem cell can differentiate into various kinds of cells. To further analyze the sphere, we observed spheres using a transmission electron microscope (TEM). TEM showed various kinds of differentiated structures including microvilli, intercellular junctions and different kinds of intracellular vesicles (Fig. 7b).
Fig. 6.
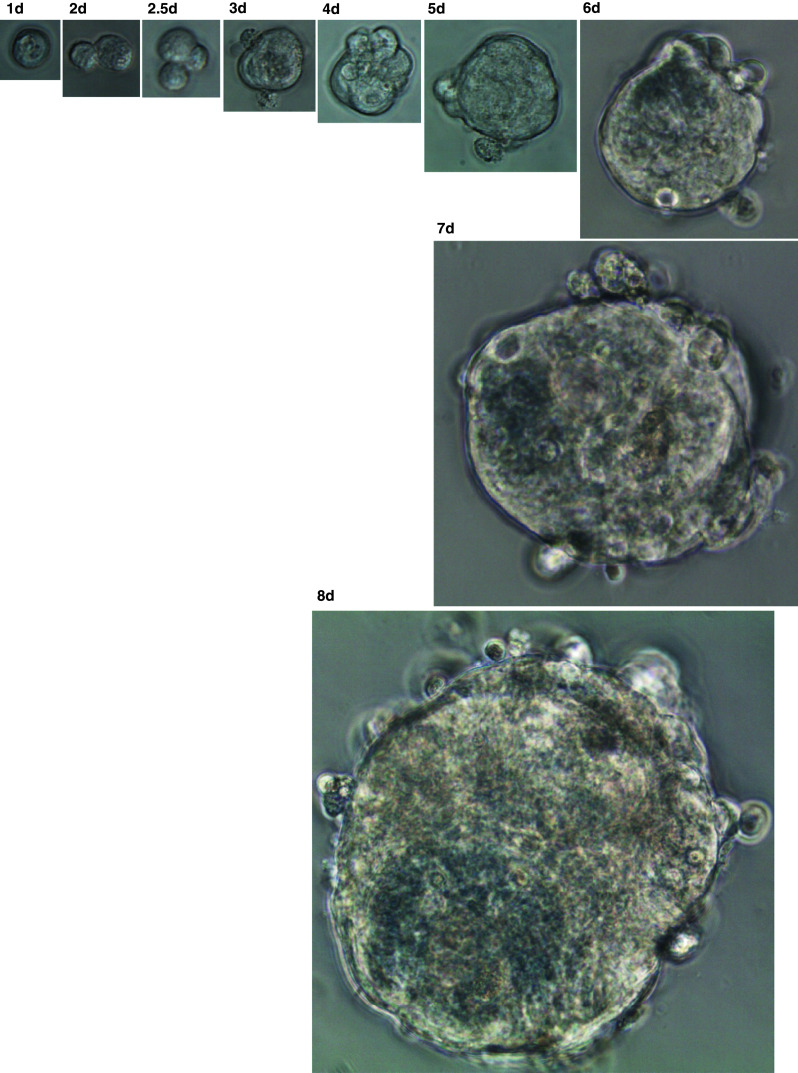
Representative example of a sphere formation originating from a single cancer stem cell. After FACS using CD44 and EpCAM antibody, a single cancer stem cell was cultured in a well of 96-well plate, and images were taken at the indicated times (patient 16). Original magnifications: days 1–3, 40× magnification; days 4–5, 20× magnification; days 6–7, 10× magnification; and day 8, 4× magnification
Fig. 7.
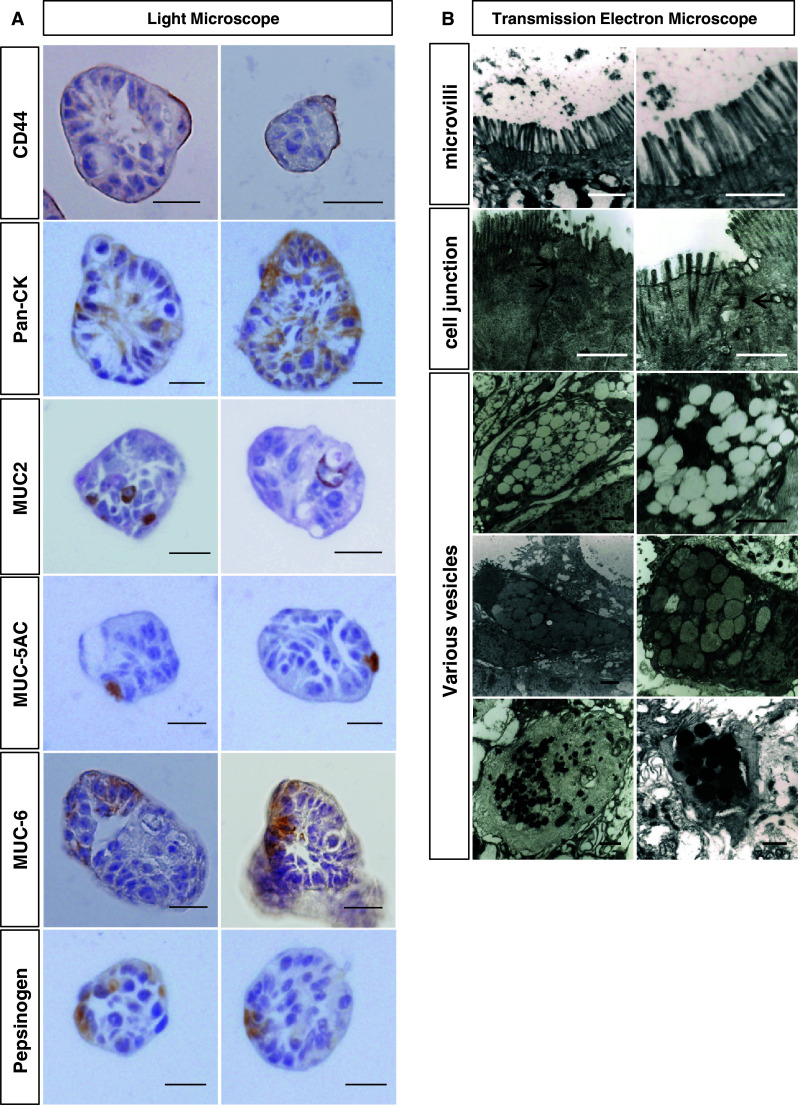
Differentiation status of cancer spheres. a Cancer spheres (patient 16) were dehydrated and processed for paraffin block. The sections were stained with the indicated markers and analyzed. Images are representative of spheres in three independent experiments. Scale bar = 20 µm. b TEM images of cancer spheres. Microvilli, interceullar junctions (arrow) and various kinds of vesicles were observed. Scale bar = 1 µm
Chemo-resistance of gastric cancer stem cells
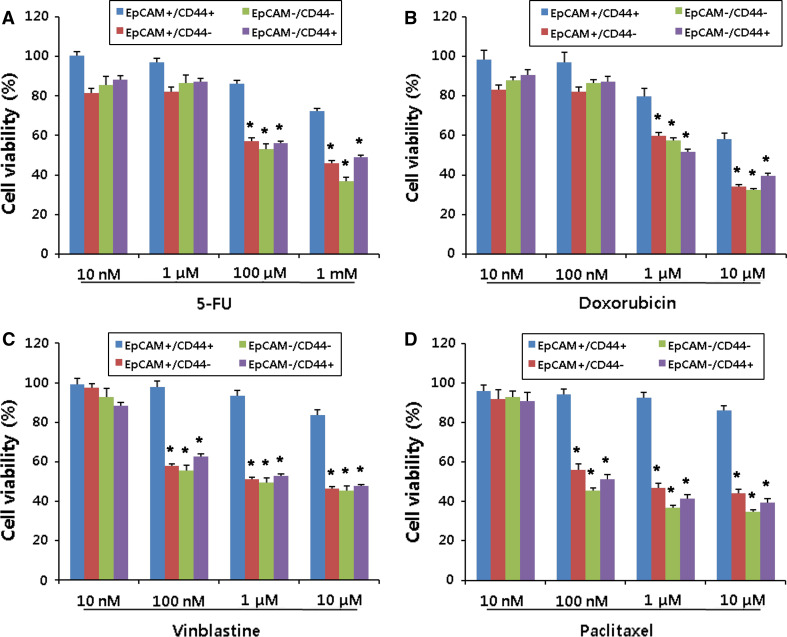
Cancer stem cells have been shown to resist chemotherapy compared to other differentiated cells [25, 26]. To examine this hypothesis, we carried out cell survival assay after sorting each subpopulation. EpCAM+/CD44+, EpCAM+/CD44−, EpCAM−/CD44+ and EpCAM−/CD44− subpopulations were treated with various concentrations of anti-cancer drugs including 5-fluorouracil, doxorubicin, vinblastine and paclitaxel (Fig. 8). The EpCAM+/CD44+ subpopulation had significantly greater resistance to all tested anti-cancer drugs than other subpopulations.
Fig. 8.
Gastric cancer stem cells resist chemotherapy. Each subpopulation EpCAM+/CD44+, EpCAM+/CD44−, EpCAM−/CD44− and EpCAM−/CD44+ was isolated from cancer spheres, and the chemo-sensitivity was assessed in each subpopulation. Cells were treated with various concentrations of anti-cancer drugs including 5-fluorouracil (5-FU, a), doxorubicin (b), vinblastine (c) and paclitaxel (d) for 48 h. Data are the mean ± SE of three independent experiments in quintuplicate [*P < 0.01, one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison]
Discussion
Lauren classification has long been applied to the pathology of gastric cancer. The diffuse type of gastric cancer showed more poorly differentiated cells than the intestinal type. Because the diffuse type has an increased propensity for intra- and trans-mural spread, it has been associated with a poorer prognosis [27]. These results are consistent with our finding that diffuse types of gastric cancer had more EpCAM+/CD44+ cells than the intestinal type (Fig. 1) because cancer stem cells are metastatic and undifferentiated. Although we could not associate the cancer stem cell population with patients’ survival rates, recent studies have shown that aberrant expression of CD44 and CD44 variants are associated with lymph node metastasis, invasion, recurrence rate and survival rate in gastric cancer [28–30]. Moreover, invasive gastric cancer cells in patients’ tissue overexpressed EpCAM and its knockdown by siRNA suppressed the in vitro and in vivo invasive abilities of gastric cancer cells [31]. In future study, the correlation of CD44 and EpCAM expression with patients’ survival rate needs to be investigated.
CD44 is a transmembrane glycoprotein involved in a variety of cellular processes including cellular adhesion [32]. Alternative splicing creates more than ten different isoforms of CD44. The expression of CD44 potentiates tumor cells to adhere to the extracellular matrix through ligands, such as hyaluronan, and facilitates the efficient formation of cell colonies. Interestingly, CD44 variants facilitate conferring a metastatic phenotype in various tumors [33–36]. CD44 has been described as a marker for cancer stem cells in a variety of cancers including colon, breast, prostate and pancreatic cancer, and leukemia [14, 23, 37–39]. Knockdown of CD44 in colon cancer stem cells prevented clonal formation and inhibited tumorigenicity in a xenograft model [40]. Collaboration between CD44 with epidermal growth factor receptor and c-MET, which are critical signaling molecules for cell migration, has been demonstrated [41]. In addition, Guo et al. [42] showed that the concentration of soluble CD44 in the serum is raised in patients with advanced gastric cancers. They also showed that the serum CD44 concentration correlated with tumor burden and metastasis of gastric cancer. Taken together, these results suggest that CD44 can be a surface marker for gastric cancer stem cells.
EpCAM is a glycosylated, 30–40-kDa type I membrane protein that is expressed in a variety of human epithelial tissues, cancers, and progenitor cells and embryonic stem cells [43–46]. Because EpCAM is expressed on essentially all human adenocarcinomas, it was used as a marker to identify disseminated cancerous cells in the circulation to predict matastasis and recurrence of the tumor [47, 48]. EpCAM is believed to mediate Ca2+-independent homotypic cell-cell adhesion [49]. However, the role of EpCAM in proliferation, migration and invasive capacity of cancer cells has been demonstrated [50]. Moreover, tumorigenic activity of EpCAM was demonstrated in a SCID mouse tumor model [51]. Taken together, these results suggest that EpCAM can be a marker for cancer stem cells. As expected, EpCAM has been used as a marker for cancer stem cells in various cancers including liver, colon, breast and pancreatic cancer [12, 16, 17, 39, 52, 53]. A high level of EpCAM in hepatocellular carcinoma was associated with expression of markers of hepatic stem cells, efficient formation of spheres and development of tumors in NOD-SCID mice [52]. Moreover, EpCAM+ hepatocellular carcinoma cells showed the abilities to self-renew and differentiate [52]. The underlying mechanism with which EpCAM regulates stemness has been suggested. In carcinoma cells, cell-to-cell contact triggers activation and proteolysis of EpCAM. The resulting intracellular domain of EpCAM travels into the nucleus and becomes part of a large complex containing the transcriptional regulators β-catenin and Lef, which are both components of the Wnt pathway, which is critical in the maintenance of normal and cancer stem cells [43]. EpCAM has also been shown to be involved in the maintenance of embryonic stem cells [46, 54, 55]. It is notable that EpCAM is overexpressed in metastatic gastric cancer cells [31].
In this study, we used two markers (CD44 and EpCAM) to isolate gastric cancer stem cells. A previous study showed that gastric cancer stem cells can be isolated from gastric cancer cell lines using CD44 alone [18]. However, in the present study, the EpCAM+/CD44− or EpCAM−/CD44+ subpopulation did not form a cancer sphere in vitro and a tumor in vivo, suggesting that both CD44 and EpCAM markers are needed for isolation of cancer stem cells directly from patients. Interestingly, the interaction between CD44 and EpCAM has been suggested before, which may support the usage of two markers. In colorectal cancer, claudin-7-associated EpCAM is recruited into tetraspanin-enriched membrane microdomains (TEM) and forms a complex with CO-029 and CD44v6 that facilitates metastasis formation [56]. Moreover, in rat pancreatic adenocarcinoma cells, interaction between CD44v4-v7 and EpCAM was demonstrated to affect cell-cell and cell-matrix adhesion and apoptosis resistance [57].
The standard experimental method for isolation of cancer stem cells is to test the tumorigenicity of cancer cells that were separated from other cancer cells by various surface markers in immune-compromised mice [7]. However, this in vivo xenotransplantation test takes a long time. For one test, we needed to spend 5 months. A simple and easy in vitro experimental test is sphere formation assay. Various cancer stem cells from colon, brain and breast cancer, etc., formed spheres [13, 17, 58]. However, the status of cancer spheres has never been characterized.
In this study, we showed that a single gastric cancer stem cell can form a cancer sphere that contains various kinds of differentiated cells. In addition to the appearance of various differentiation markers (Fig. 7a), microvilli, intercellular junction and intracellular vesicles were confirmed by electron microscope (Fig. 7b). Because we can observe differentiation of cancer stem cells into various cells in vitro, we can apply this model for studying molecular mechanisms for the maintenance of stemness, differentiation and interaction with other kinds of cells of cancer stem cells. In this regard, we think cancer spheres isolated directly from patients are better than those from cell lines. Moreover, differentiation of cancer spheres by serum abolished the tumorigenicity of the cancer stem cells. So, we can use this model for development of therapeutic reagents.
Cancer stem cells have been associated with chemo-resistance [25, 26], which is critical for their therapeutic implications. The underlying mechanism for the chemo-resistance of cancer stem cells has been explained by several pathways including a high level of ATP-binding cassette (ABC) transporters, DNA repair capacity, detoxifying enzymes and anti-apoptotic factors [59]. It is notable that EpCAM and CD44 expressions have been associated with drug resistance by their anti-apoptotic action [60–64]. Claudin-7-EpCAM complex in tetraspanin-enriched membrane microdomains (TEM) induced extracellular signal-regulated kinase-1/2 phosphorylation, upregulation of anti-apoptotic proteins and drug resistance [65]. CD44 knockout mice showed increased apoptosis in colonic epithelium [60]. Moreover, activation of CD44 by binding with osteopontin increased apoptosis resistance via the phosphatidylinositol 3-kinase (PI3 K)/AKT pathway [64]. Furthermore, genetic variants in the CD44 gene were significantly associated with decreased cellular response to various cytotoxic chemotherapeutics [66]. In this study we showed EpCAM+/CD44+ gastric cancer stem cells are more resistant to anti-cancer drugs than other subpopulation cells. In future study, the underlying mechanism for this chemo-resistance needs to be explored.
A xenotransplantation model for the isolation of cancer stem cells has a critical problem because immunodeficient mice may not provide an ideal local microenvironment for the growth of human cancer cells [18, 67]. A recent study showed that when lymphomas and leukemias of mouse origin are transplanted into histocompatible mice, a very high frequency of the tumor cells can seed tumor growth [68]. Moreover, it appears that the severity of immunodeficiency of the recipient mice may affect the outcome of xenograft transplantation studies. The use of more highly immunocompromised NOD/SCID interleukin-2 receptor gamma chain null mice increased the detection of tumorigenic melanoma cells by several orders of magnitude [69]. Taken together, calculation of the percentage of cancer stem cells in cancer tissues may not be meaningful because the microenvironment for human cancer cells is not perfect in mice. However, we can conclude that gastric cancer stem cells are enriched in EpCAM+/CD44+ cells.
In this study, we successfully isolated gastric cancer stem cells from patients using two EpCAM and CD44 surface markers. In addition to in vivo experiments, gastric cancer stem cells generated various differentiated cells in cancer sphere culture. Therefore, these cancer spheres isolated directly from patients can be used for studying maintenance of stemness, differentiation and interaction with other cells of cancer stem cells.
Acknowledgments
This work was supported by the medical research centre program of the Ministry of Education, Science and Technology/Korea Science and Engineering Foundation (2011-0006190), the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (no. 2009-0076704) and a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family Affairs, Republic of Korea (0920050).
Conflict of interest
There are no conflicts of interest.
References
- 1.Han ME, Lee YS, Baek SY, Kim BS, Kim JB, Oh SO. Hedgehog signaling regulates the survival of gastric cancer cells by regulating the expression of bcl-2. Int J Mol Sci. 2009;10(7):3033–3043. doi: 10.3390/ijms10073033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuasa Y. Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat Rev Cancer. 2003;3(8):592–600. doi: 10.1038/nrc1141. [DOI] [PubMed] [Google Scholar]
- 3.Correia M, Machado JC, Ristimaki A. Basic aspects of gastric cancer. Helicobacter. 2009;14(Suppl 1):36–40. doi: 10.1111/j.1523-5378.2009.00696.x. [DOI] [PubMed] [Google Scholar]
- 4.Vogiatzi P, Vindigni C, Roviello F, Renieri A, Giordano A. Deciphering the underlying genetic and epigenetic events leading to gastric carcinogenesis. J Cell Physiol. 2007;211(2):287–295. doi: 10.1002/jcp.20982. [DOI] [PubMed] [Google Scholar]
- 5.Wu CW, Hsieh MC, Lo SS, Lui WY, P’Eng FK. Results of curative gastrectomy for carcinoma of the distal third of the stomach. J Am Coll Surg. 1996;183(3):201–207. [PubMed] [Google Scholar]
- 6.Wu CW, Hsieh MC, Lo SS, Tsay SH, Li AF, Lui WY, P’Eng FK. Prognostic indicators for survival after curative resection for patients with carcinoma of the stomach. Dig Dis Sci. 1997;42(6):1265–1269. doi: 10.1023/A:1018814426278. [DOI] [PubMed] [Google Scholar]
- 7.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells–perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 8.Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, Kirchner T. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells tissues organs. 2005;179(1–2):56–65. doi: 10.1159/000084509. [DOI] [PubMed] [Google Scholar]
- 9.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells-an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5(9):744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 10.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell stem cell. 2007;1(3):313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 14.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 15.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65(20):9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 17.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 18.Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem cells. 2009;27(5):1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishii T, Yashiro M, Shinto O, Sawada T, Ohira M, Hirakawa K. Cancer stem cell-like SP cells have a high adhesion ability to the peritoneum in gastric carcinoma. Cancer Sci. 2009;100(8):1397–1402. doi: 10.1111/j.1349-7006.2009.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuda K, Saikawa Y, Ohashi M, Kumagai K, Kitajima M, Okano H, Matsuzaki Y, Kitagawa Y. Tumor initiating potential of side population cells in human gastric cancer. Int J Oncol. 2009;34(5):1201–1207. [PubMed] [Google Scholar]
- 21.Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2 + and ABCG2 − cancer cells are similarly tumorigenic. Cancer Res. 2005;65(14):6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 22.Burkert J, Otto WR, Wright NA. Side populations of gastrointestinal cancers are not enriched in stem cells. J Pathol. 2008;214(5):564–573. doi: 10.1002/path.2307. [DOI] [PubMed] [Google Scholar]
- 23.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104(24):10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vescovi AL, Parati EA, Gritti A, Poulin P, Ferrario M, Wanke E, Frolichsthal-Schoeller P, Cova L, Arcellana-Panlilio M, Colombo A, Galli R. Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation. Exp Neurol. 1999;156(1):71–83. doi: 10.1006/exnr.1998.6998. [DOI] [PubMed] [Google Scholar]
- 25.Haraguchi N, Ishii H, Mimori K, Tanaka F, Ohkuma M, Kim HM, Akita H, Takiuchi D, Hatano H, Nagano H, Barnard GF, Doki Y, Mori M. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Invest. 2010;120(9):3326–3339. doi: 10.1172/JCI42550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 27.Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12(19):2979–2990. doi: 10.3748/wjg.v12.i19.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu YJ, Yan PS, Li J, Jia JF. Expression and significance of CD44 s, CD44v6, and nm23 mRNA in human cancer. World J Gastroenterol. 2005;11(42):6601–6606. doi: 10.3748/wjg.v11.i42.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoo CH, Noh SH, Kim H, Lee HY, Min JS. Prognostic significance of CD44 and nm23 expression in patients with stage II and stage IIIA gastric carcinoma. J Surg Oncol. 1999;71(1):22–28. doi: 10.1002/(SICI)1096-9098(199905)71:1<22::AID-JSO5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 30.Winder T, Ning Y, Yang D, Zhang W, Power DG, Bohanes P, Gerger A, Wilson PM, Lurje G, Tang LH, Shah M, Lenz HJ (2010) Germline polymorphisms in genes involved in the CD44 signaling pathway are associated with clinical outcome in localized gastric adenocarcinoma (GA). Int J Cancer [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 31.Du W, Ji H, Cao S, Wang L, Bai F, Liu J, Fan D. EpCAM: a potential antimetastatic target for gastric cancer. Dig Dis Sci. 2010;55(8):2165–2171. doi: 10.1007/s10620-009-1033-8. [DOI] [PubMed] [Google Scholar]
- 32.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nature Rev. 2003;4(1):33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 33.Barbour AP, Reeder JA, Walsh MD, Fawcett J, Antalis TM, Gotley DC. Expression of the CD44v2–10 isoform confers a metastatic phenotype: importance of the heparan sulfate attachment site CD44v3. Cancer Res. 2003;63(4):887–892. [PubMed] [Google Scholar]
- 34.Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65(1):13–24. doi: 10.1016/0092-8674(91)90403-L. [DOI] [PubMed] [Google Scholar]
- 35.Heider KH, Dammrich J, Skroch-Angel P, Muller-Hermelink HK, Vollmers HP, Herrlich P, Ponta H. Differential expression of CD44 splice variants in intestinal- and diffuse-type human gastric carcinomas and normal gastric mucosa. Cancer Res. 1993;53(18):4197–4203. [PubMed] [Google Scholar]
- 36.Klingbeil P, Marhaba R, Jung T, Kirmse R, Ludwig T, Zoller M. CD44 variant isoforms promote metastasis formation by a tumor cell-matrix cross-talk that supports adhesion and apoptosis resistance. Mol Cancer Res. 2009;7(2):168–179. doi: 10.1158/1541-7786.MCR-08-0207. [DOI] [PubMed] [Google Scholar]
- 37.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 38.Ponti D, Zaffaroni N, Capelli C, Daidone MG. Breast cancer stem cells: an overview. Eur J Cancer. 2006;42(9):1219–1224. doi: 10.1016/j.ejca.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 39.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 40.Du L, Wang H, He L, Zhang J, Ni B, Wang X, Jin H, Cahuzac N, Mehrpour M, Lu Y, Chen Q. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 2008;14(21):6751–6760. doi: 10.1158/1078-0432.CCR-08-1034. [DOI] [PubMed] [Google Scholar]
- 41.Matzke A, Sargsyan V, Holtmann B, Aramuni G, Asan E, Sendtner M, Pace G, Howells N, Zhang W, Ponta H, Orian-Rousseau V. Haploinsufficiency of c-Met in cd44-/- mice identifies a collaboration of CD44 and c-Met in vivo. Mol Cell Biol. 2007;27(24):8797–8806. doi: 10.1128/MCB.01355-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo YJ, Liu G, Wang X, Jin D, Wu M, Ma J, Sy MS. Potential use of soluble CD44 in serum as indicator of tumor burden and metastasis in patients with gastric or colon cancer. Cancer Res. 1994;54(2):422–426. [PubMed] [Google Scholar]
- 43.Munz M, Baeuerle PA, Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009;69(14):5627–5629. doi: 10.1158/0008-5472.CAN-09-0654. [DOI] [PubMed] [Google Scholar]
- 44.Baeuerle PA, Gires O. EpCAM (CD326) finding its role in cancer. Br J Cancer. 2007;96(3):417–423. doi: 10.1038/sj.bjc.6603494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trzpis M, McLaughlin PM, de Leij LM, Harmsen MC. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol. 2007;171(2):386–395. doi: 10.2353/ajpath.2007.070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez B, Denzel S, Mack B, Conrad M, Gires O. EpCAM is involved in maintenance of the murine embryonic stem cell phenotype. Stem cells. 2009;27(8):1782–1791. doi: 10.1002/stem.97. [DOI] [PubMed] [Google Scholar]
- 47.Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G, Pierga JY, Marth C, Oruzio D, Wiedswang G, Solomayer EF, Kundt G, Strobl B, Fehm T, Wong GY, Bliss J, Vincent-Salomon A, Pantel K. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353(8):793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 48.Pantel K, Woelfle U. Detection and molecular characterisation of disseminated tumour cells: implications for anti-cancer therapy. Biochim Biophys Acta. 2005;1756(1):53–64. doi: 10.1016/j.bbcan.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Balzar M, Winter MJ, de Boer CJ, Litvinov SV. The biology of the 17–1A antigen (Ep-CAM) J Mol Med. 1999;77(10):699–712. doi: 10.1007/s001099900038. [DOI] [PubMed] [Google Scholar]
- 50.Osta WA, Chen Y, Mikhitarian K, Mitas M, Salem M, Hannun YA, Cole DJ, Gillanders WE. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 2004;64(16):5818–5824. doi: 10.1158/0008-5472.CAN-04-0754. [DOI] [PubMed] [Google Scholar]
- 51.Maetzel D, Denzel S, Mack B, Canis M, Went P, Benk M, Kieu C, Papior P, Baeuerle PA, Munz M, Gires O. Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol. 2009;11(2):162–171. doi: 10.1038/ncb1824. [DOI] [PubMed] [Google Scholar]
- 52.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E, Reid LM, Minato H, Honda M, Kaneko S, Tang ZY, Wang XW. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136(3):1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kimura O, Takahashi T, Ishii N, Inoue Y, Ueno Y, Kogure T, Fukushima K, Shiina M, Yamagiwa Y, Kondo Y, Inoue J, Kakazu E, Iwasaki T, Kawagishi N, Shimosegawa T, Sugamura K. Characterization of the epithelial cell adhesion molecule (EpCAM) + cell population in hepatocellular carcinoma cell lines. Cancer sci. 2010;101(10):2145–2155. doi: 10.1111/j.1349-7006.2010.01661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Hofler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994;54(14):3845–3852. [PubMed] [Google Scholar]
- 55.Yokota J, Yamamoto T, Miyajima N, Toyoshima K, Nomura N, Sakamoto H, Yoshida T, Terada M, Sugimura T. Genetic alterations of the c-erbB-2 oncogene occur frequently in tubular adenocarcinoma of the stomach and are often accompanied by amplification of the v-erbA homologue. Oncogene. 1988;2(3):283–287. [PubMed] [Google Scholar]
- 56.Kuhn S, Koch M, Nubel T, Ladwein M, Antolovic D, Klingbeil P, Hildebrand D, Moldenhauer G, Langbein L, Franke WW, Weitz J, Zoller M. A complex of EpCAM, claudin-7, CD44 variant isoforms, and tetraspanins promotes colorectal cancer progression. Mol Cancer Res. 2007;5(6):553–567. doi: 10.1158/1541-7786.MCR-06-0384. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt DS, Klingbeil P, Schnolzer M, Zoller M. CD44 variant isoforms associate with tetraspanins and EpCAM. Exp Cell Res. 2004;297(2):329–347. doi: 10.1016/j.yexcr.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 58.Grimshaw MJ, Cooper L, Papazisis K, Coleman JA, Bohnenkamp HR, Chiapero-Stanke L, Taylor-Papadimitriou J, Burchell JM. Mammosphere culture of metastatic breast cancer cells enriches for tumorigenic breast cancer cells. Breast Cancer Res. 2008;10(3):R52. doi: 10.1186/bcr2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scopelliti A, Cammareri P, Catalano V, Saladino V, Todaro M, Stassi G. Therapeutic implications of cancer initiating cells. Expert Opin Biol Ther. 2009;9(8):1005–1016. doi: 10.1517/14712590903066687. [DOI] [PubMed] [Google Scholar]
- 60.Lakshman M, Subramaniam V, Wong S, Jothy S. CD44 promotes resistance to apoptosis in murine colonic epithelium. J Cell Physiol. 2005;203(3):583–588. doi: 10.1002/jcp.20260. [DOI] [PubMed] [Google Scholar]
- 61.Ohashi R, Takahashi F, Cui R, Yoshioka M, Gu T, Sasaki S, Tominaga S, Nishio K, Tanabe KK, Takahashi K. Interaction between CD44 and hyaluronate induces chemoresistance in non-small cell lung cancer cell. Cancer Lett. 2007;252(2):225–234. doi: 10.1016/j.canlet.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 62.Yaqin M, Runhua L, Fuxi Z. Analyses of Bcl-2, Survivin, and CD44v6 expressions and human papillomavirus infection in cervical carcinomas. Scand J Infect Dis. 2007;39(5):441–448. doi: 10.1080/00365540601105772. [DOI] [PubMed] [Google Scholar]
- 63.Cordo Russo RI, Garcia MG, Alaniz L, Blanco G, Alvarez E, Hajos SE. Hyaluronan oligosaccharides sensitize lymphoma resistant cell lines to vincristine by modulating P-glycoprotein activity and PI3 K/Akt pathway. Int J Cancer. 2008;122(5):1012–1018. doi: 10.1002/ijc.23122. [DOI] [PubMed] [Google Scholar]
- 64.Lin YH, Yang-Yen HF. The osteopontin-CD44 survival signal involves activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. J Biol Chem. 2001;276(49):46024–46030. doi: 10.1074/jbc.M105132200. [DOI] [PubMed] [Google Scholar]
- 65.Nubel T, Preobraschenski J, Tuncay H, Weiss T, Kuhn S, Ladwein M, Langbein L, Zoller M. Claudin-7 regulates EpCAM-mediated functions in tumor progression. Mol Cancer Res. 2009;7(3):285–299. doi: 10.1158/1541-7786.MCR-08-0200. [DOI] [PubMed] [Google Scholar]
- 66.Vazquez A, Grochola LF, Bond EE, Levine AJ, Taubert H, Muller TH, Wurl P, Bond GL. Chemosensitivity profiles identify polymorphisms in the p53 network genes 14-3-3tau and CD44 that affect sarcoma incidence and survival. Cancer Res. 2010;70(1):172–180. doi: 10.1158/0008-5472.CAN-09-2218. [DOI] [PubMed] [Google Scholar]
- 67.Marx J. Molecular biology. Cancer’s perpetual source? Science. 2007;317(5841):1029–1031. doi: 10.1126/science.317.5841.1029. [DOI] [PubMed] [Google Scholar]
- 68.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317(5836):337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 69.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456(7222):593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]