Abstract
Since being introduced globally as aspirin in 1899, acetylsalicylic acid has been widely used as an analgesic, anti-inflammation, anti-pyretic, and anti-thrombotic drug for years. Aspirin had been reported to down-regulate surface expression of CD40, CD80, CD86, and MHCII in myeloid dendritic cells (DC), which played essential roles in regulating the immune system. We hypothesized that the down-regulation of these surface membrane proteins is partly due to the ability of aspirin in regulating trafficking/sorting of endocytosed surface membrane proteins. By using an established epidermoid carcinoma cell line (A-431), which overexpresses the epidermal growth factor receptor (EGFR) and transferrin receptor (TfnR), we show that aspirin (1) reduces cell surface expression of EGFR and (2) accumulates endocytosed-EGFR and -TfnR in the early/sorting endosome (ESE). Further elucidation of the mechanism suggests that aspirin enhances recruitment of SNX3 and SNX5 to membranes and consistently, both SNX3 and SNX5 play essential roles in the aspirin-mediated accumulation of endocytosed-TfnR at the ESE. This study sheds light on how aspirin may down-regulate surface expression of EGFR by inhibiting/delaying the exit of endocytosed-EGFR from the ESE and recycling of endocytosed-EGFR back to the cell surface.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-011-0887-z) contains supplementary material, which is available to authorized users.
Keywords: Sorting nexins, Aspirin, Endocytosis, Epidermal growth factor receptor, Transferrin receptor, Receptor trafficking
Introduction
Aberrant signaling through epidermal growth factor (EGF) family of receptors is implicated in cancers of the breast, lung, colon, stomach, pancreas, ovary, brain, prostate, and kidney [1]. Upon EGF stimulation, epidermal growth factor receptor (EGFR) is immediately internalized via endocytosis to the early endosomes (EE). From the EE, a small pool of endocytosed-EGFR rapidly recycles to the cell surface, and the rest advances to the early/sorting endosomes (ESE). From the ESE, endocytosed-EGFR is either trafficked to the recycling endosomes (RE) for transportation back to the cell surface or to the late endosomes (LE)/lysosomes for degradation [2]. All of these endocytic organelles form a dynamic network of subcellular compartments, which actively control the timing, amplitude, and specificity of EGFR signaling [3].
Transferrin receptor 1 (CD71, TfnR), another growth-associated receptor, is a type II transmembrane protein involved in the cellular transport of iron-loaded transferrin (Tfn) [4]. Similar to EGFR, Tfn–TfnR complex is also internalized into cells via the clathrin-coated pit. Upon endosomal acidification after endocytosis, iron will dissociate from Tfn–TfnR complex and is then transported into the cytoplasm via natural resistance-associated macrophage protein 2 (NRAMP2), which is mostly found in the recycling endosomes [5].
Drugs that are found to target the EGFR and TfnR-trafficking pathways could potentially be adapted to reduce surface expression of EGFR/TfnR and hence also reduce growth-stimulatory signals arising from binding of its ligand. Since being introduced globally as aspirin in 1899, acetylsalicylic acid has been widely used as an analgesic, anti-inflammation, anti-pyretic, and anti-thrombotic drug for years. Aspirin has been reported to down-regulate cell surface expression of membrane proteins essential for T cell activation, such as CD80, CD86, and MHC class II in dendritic cells [6]. Aspirin has also been shown to regulate the expression of cysteinyl leukotriene receptor (CysLT1) on inflammatory cells [7] as well as scavenger receptor class B type I (SR-BI) in macrophages [8]. We hypothesized that the down-regulation of these surface membrane proteins is partly due to the ability of aspirin in regulating trafficking/sorting of endocytosed surface membrane proteins. In this study, we elucidated the influence of aspirin on the trafficking of EGFR and TfnR in the human epidermoid carcinoma A-431 cells [9].
Materials and methods
Materials
A-431 human epidermoid carcinoma cells (CRL-1555), HeLa (CCL-2), MCF-7 (HTB-22), and hybridoma OKT9 were obtained from ATCC, and cultured in DMEM supplemented with fetal bovine serum, l-glutamine, amino acids, HEPES buffer, and antibiotics (Gibco) at 37°C with 5% CO2. Aspirin and salicylic acid (Sigma) were prepared in plain DMEM in stock concentration of 50 mM with a shelf life of 1–7 days. Antibody against EGFR (clone F4) was purchased from Sigma, Singapore. Antibodies specific for SNX3, SNX5, caspase-3, and c-Myc were purchased from Santa Cruz Biotechnology, California. Antibodies specific for phosphorylated and non-phosphorylated forms of p38 MAPK, JNK, and p44/42 (ERK) were purchased from Cell Signalling. MAPK inhibitors were reconstituted in DMSO at a concentration of 10 mM, and the MEK1/2 inhibitor, U0126 (Cell Signalling), p38 MAPK inhibitor, SB203580 (Calbiochem), JNK inhibitor, SP600125 (Calbiochem) were used.
Flow cytometry
The assay was performed as previously described [10]. Briefly, cells treated with aspirin were dislodged from the plate using 2 mM EDTA in the presence of aspirin. The cells were then washed with PBS and stained with mouse anti-EGFR (Clone LA1, Sigma) and anti-mouse FITC (Jackson Immuno Laboratories) diluted in staining buffer (0.5% BSA, 0.05% sodium azide, 2 mM EDTA in 1× PBS, pH 7.2). Cells were fixed with 2% paraformaldehyde on ice for 30 min and samples were analyzed with Cytomics FC 500 Series Flow Cytometry Systems (Beckman Coulter).
Western-blot analysis
Western-blot analysis was performed as previously described [11]. Briefly, cells were pelleted and supernatant was removed, followed by the addition of lysis buffer (2× protease inhibitor, 1% Triton-X 100 in PBS) to the cell pellet. Cells were lysed on ice for 1 h and samples were tapped at 15-min intervals. The samples were then subjected to centrifugation at 13,000 rpm for 10 min and cell lysates were collected thereafter. Protein concentration of the lysates was quantified using Bradford solution and procedures were carried out according to the manufacturer’s protocol.
Proteins were boiled in sample buffer (stock at 5×: 250 mM Tris·Cl pH 6.8, 10% sodium dodecyl sulphate (SDS), 50% glycerol, dH2O, 5% β-mercaptoethanol) and resolved by SDS-PAGE (Bio-Rad) in running buffer (0.1% SDS, 3.02 g/l of Tris, 18.8 g/l of glycine in dH2O). Thereafter, proteins in gel were electroblotted to a nitrocellulose membrane (Hybond) in transfer buffer (20% methanol, 3.02 g/l of Tris, 18.8 g/l in dH2O). After completion of transfer, the nitrocellulose membrane was incubated overnight at 4°C with blocking buffer (PBS containing 5% skim milk and 0.1% Tween 20). Primary and secondary antibody incubations were carried out in blocking buffer. The membrane was washed with PBS containing 0.1% Tween 20 and then analyzed using the Supersignal Chemiluminescent kit (Pierce) according to the manufacturer’s recommendation. The resulting blot was then scanned and quantified using ImageJ software.
Western-blot kinetics analysis
Cells were incubated on ice with anti-EGFR (Clone 29.1) or anti-TfnR (OKT9) [12] for 1 h, and cells were replated in complete media with DMEM or aspirin, incubated at 37°C in the CO2 incubator for varying time-points, detached from the plate using EDTA and then lysed. The lysate was then analyzed by Western blot and detected using HRP-conjugated secondary antibody. The amount of endocytosed-EGFR (eEGFR) or endocytosed-TfnR (eTfnR) is depicted by the heavy chain of the antibody.
Indirect immunofluorescence (steady states)
Antibodies against VAMP3 (Synaptic Systems, Germany), EEA-1 (BD Biosciences, Singapore), LAMP-1 (eBioscience, Singapore) were used. Briefly, cells were grown on coverslips at least 12 h before use to ensure that cells adhered to the coverslips. Cells were fixed either using methanol or 4% paraformaldehyde. For methanol fixation, cells were fixed with cold methanol for 4 min, which was stored at −20°C. Thereafter, cells were washed four times with PBS to remove the methanol. As for paraformaldehyde fixation, cells were fixed using 4% paraformaldehyde for 30 min at room temperature. Cells were washed four times with PBS, followed by incubation with 0.1% saponin (Sigma) for 30 min. Cells were incubated with primary antibodies in staining buffer (1 mM CaCl2, 1 mM MgCl2, 5% goat serum, 5% FBS, 2% BSA in PBS) for 1 h at room temperature, followed by washing with PBS and saponin for methanol and paraformaldehyde fixation, respectively, for three times. Cells were then incubated with fluorescein-conjugated secondary antibody for 1 h followed by washing four times with PBS or saponin. Finally, coverslips were mounted on slides in mounting media (Vectashield) and were subjected to fluorescence (Olympus BX60) or confocal microscopy (Olympus FV500 confocal microscope or Leica TCS SP5).
Indirect immunofluorescence (kinetics)
Cells grown on coverslips were washed once with PBS, and then probed with primary antibodies against TfnR (OKT9) or EGFR (clone 29.1) on ice for 1 h, prior to treatment. After treatment, cells were fixed with cold methanol for 4 min and washed. Fluorescein-conjugated secondary antibodies was then added, incubated in the dark for 1 h, washed and mounted using mounting medium with DAPI (Vector Laboratories, Burlingame, CA, USA). Conventional fluorescent images were taken using Olympus BX-60 digital microscope with ImagePro Plus software. Confocal images were taken using either Olympus FV500/FV1000 microscope with FluoView version 2.0, Leica TCS SP5 or Zeiss LSM 510 Meta (upright stage). X–Z/Y–Z projection and stereo three-dimensional rendering were performed using Volocity visualization software from Improvision.
For MAPK experiments, live A-431 cells grown on coverslips were probed with primary antibodies against TfnR (OKT9) on ice for 30 min to label cell surface TfnR. Cells were washed twice with pre-chilled PBS and antibody-labeled cell surface TfnR was then allowed to internalize for various time-points at 37°C in the absence or presence of aspirin and/or kinase inhibitors. Cells were fixed with methanol (pre-chilled at −20°C) for 4 min in a −20°C freezer and washed four times with PBS to remove the methanol. Cells were then incubated with primary antibodies in staining buffer (1 mM CaCl2, 1 mM MgCl2, 5% goat serum, 5% FBS, 2% BSA in PBS) for 1 h at room temperature, followed by washing three times with either PBS. Subsequently, cells were incubated with fluorescein-conjugated secondary antibody (Immuno Jackson Laboratories) for 1 h followed by washing four times with PBS. Coverslips were mounted on slides in mounting media (Vectashield) and were subjected to fluorescence (Olympus BX60).
Alternatively, live A-431 cells grown on coverslips were probed with primary antibodies against TfnR (OKT9) on ice for 30 min to label cell surface TfnR, washed twice with pre-chilled PBS and antibody-labeled cell surface TfnR was then allowed to internalize for 3 h in the presence of 10 mM aspirin at 37°C. At this time point, a cover slip was subjected to methanol fixation. The rest of the cover slips were washed three times with cold PBS to remove aspirin and treated with various concentrations of aspirin and various kinase inhibitors for 1 and 3 h at 37°C. Cells were fixed with methanol (pre-chilled at −20°C) for 4 min in a −20°C freezer and washed four times with PBS to remove the methanol. Cells were then incubated with primary antibodies in staining buffer (1 mM CaCl2, 1 mM MgCl2, 5% goat serum, 5% FBS, 2% BSA in PBS) for 1 h at room temperature, followed by washing three times with either PBS. Subsequently, cells were incubated with fluorescein-conjugated secondary antibody (Immuno Jackson Laboratories) for 1 h followed by washing four times with PBS. Coverslips were mounted on slides in mounting media (Vectashield) and were subjected to fluorescence (Olympus BX60).
Cloning and DNA plasmid transfection
The plasmids extracted from E. coli containing mouse SNX3-ligated pCR4-TOPO vector (MGC: 151262, IMAGE: 40126204, Open Biosystems), mouse SNX5-ligated pCMV-SPORT6 vector (MGC: 7534, IMAGE: 3792262, Open Biosystems) and human SNX1-ligated pOTB7 vector (MGC: 8664, IMAGE: 2964409, Open Biosystems) were subjected to PCR using pfu polymerase. For the cloning of mouse SNX5, forward primer was designed with an XbaI restriction sequence and reverse primer with a SalI sequence. For the cloning of mouse SNX3 and human SNX1, forward primer was designed with an EcoRI restriction sequence and reverse primer with a XbaI sequence.
Primers sequences for cloning: SNX1 (forward 5′ggaattccatggcgtcgggtggtg3′, reverse 5′gctctagagcttaggagatggcctttg3′), SNX3 (forward 5′ggaattccatggcggagacggtag3′, reverse 5′ gctctagagcttaatggggatg3′), SNX5 (forward 5′gctctagagatggccgcggttcccg3′, reverse 5′ ggtcgacctcagttgttcttgaataagtcg3′).
The desired DNA band was excised and purified using QIAquick Gel Extraction, and subjected to XbaI and SalI digestion (mouse SNX5), and EcoRI and XbaI digestion (mouse SNX3 and human SNX1). The inserts were ligated to pDMyc plasmid with a double-myc tag at the 5′ terminal. Transformation was performed and the clones were selected using 200 μg/ml ampicillin. Sequences were confirmed via sequencing. DNA transfection was performed using Qiagen’s Effectene transfection reagent.
Gene knockdown
Double transfection was performed using Invitrogen Life Technologies’ Lipofectamine 2000. Small interfering RNA (siRNA) directed toward human SNX1 (catalog no. LQ-017518-00), SNX3 (LQ-011521-01), SNX5 (LQ-012524-00) as well as non-targeting siRNA pool (catalog no. D-001810-10), were purchased from Dharmacon (Lafayette, CO). The amount of siRNA used for transfection was optimized at 100 pmol per well of six-well plate transfection. The cells were assayed for their activity at 72 h post-transfection.
Transferrin recycling assay
Cells were either untreated (control) or treated with 5 mM of aspirin in plain DMEM for 4 h. Subsequently, 40 μg/ml Alexa Fluor 488-conjugated transferrin (Tfn) (Invitrogen, Singapore) in complete DMEM was added and incubated at 18°C for 1 h. Cells were washed once with ice-cold PBS containing 1 mg/ml unlabeled holo-Tfn (Sigma, Singapore), and twice with ice-cold low-pH buffer (150 mM NaCl, 10 mM acetic acid pH 3.5, 1 mg/ml holo-Tfn), followed by ice-cold DMEM and ice-cold PBS. One mg/ml holo-Tfn was added and equal volume was saved for zero time point. The others were transferred to 37°C and recycling media was collected at indicated time points (5, 10, 15, 30, 45, and 60 min). Cells were lysed with ice-cold lysis buffer (50 mM Tris pH 8, 1 mM EDTA pH 8, 150 mM NaCl, 1% Triton-X 100). Duplicates of recycling media and lysates were measured using the ELISA fluorescence reader (Tecan Gemini Infinite 200) at 485/535 nm. The percentage of labeled Tfn in lysate: [lysate/(media + lysate)] × 100.
Cellular fractionation
Cells were harvested by using EDTA and washed with PBS. Cells were re-suspended with homogenizing buffer (8.6% sucrose, 25 mM HEPES, 5 mM MgCl2, 2× cocktail protease inhibitor, 1 mM PMSF) and subjected to homogenization (Potter S, Sartorius, Goettingen, Germany). The resulting homogenate was centrifuged at 150,000 × g for 1.5 h (Optima MAX Bench top Ultracentrifuge, Beckmann Coulter) to obtain the cytosol (supernatant) and membranes (pellet). Equal fractions of cytosol and membrane were analyzed by SDS-PAGE followed by immunoblotting.
Electron microscopy
Live cells were probed with mouse anti-TfnR antibody for 1 h on ice with rocking at 10-min intervals. Cold PBS was used to rinse the cells and cover cells with sufficient 1/10 dilution (diluent: in DMEM medium) of goat anti-mouse IgG (whole molecule)—10 nm gold antibody (Sigma) at 0°C with rocking for 30 min. Following this, cells are incubated at 37°C for the internalization of the receptor–antibody complex in the presence or absence of aspirin. Cells were fixed with 2.5% glutaraldehyde and scraped out followed by washing with PBS. The cells were then dehydrated by going through 25, 50, 75, 95, and 100% (v/v) (two changes) ethanol followed by infiltration with LR white embedding medium for 2 days. After embedding at 50°C for 48 h, the blocks were cut into sections with thicknesses of 100 nm. Sections were contrasted with uranyl acetate, and viewed under electron microscope, EMS208 PHILIPS.
Quantification of phospholipids in A-431 cells upon treatment with aspirin
A-431 cells were grown until confluence in 10-cm dishes, and after treatment with aspirin for 4 h, phospholipids were extracted. Cells were rinsed with ice-cold PBS, solution was aspirated from culture dishes and 1 ml ice-cold methanol:12 M HCl (96:4) supplemented with 2 mM AlCl3 was added. Cells were scraped quickly from the dish. This suspension was transferred to a 2-ml microfuge tube, added with 0.8 ml ice-cold chloroform and vortexed vigorously. Cells were homogenized and added with 0.4 ml ice-cold water. After centrifuging at 10,000 × g for 2 min at 4°C, the lower phase was collected and washed with 1 ml of cold methanol containing 2 mM oxalic acid. After another centrifugation step, the lower phase was transferred to a new tube and dried with a stream of nitrogen. Isolated phospholipids were deacylated with methylamine. The reaction was carried out at 50°C for 45 min with 0.5 ml of reagent (40% methylamine:water: n-butanol:methanol 36:8:9:47). After centrifuging for 1 min at 1,000 × g, the samples were dried in a Speed-Vac. The products were then resuspended in 0.5 ml n-butanol:petroleum ether:ethyl formate (20:40:1) and mixed with an equal volume of water (twice) to remove fatty acids. The aqueous phase was then dried in a Speed-Vac and the samples stored at −80°C for analysis. Deacylated phospholipids were resuspended in 65 μl of water, centrifuged at 1,000 × g to remove particulate matter, and analyzed. To separate and detect anionic phospholipid head groups, anion-exchange HPLC with KOH gradients followed by suppressed conductivity detection was used. The HPLC system and columns employed were all from Dionex Corporation (Sunnyvale, CA). For all results presented, an ICS-3000 HPLC system was employed with an Ionpac AS-11-HC 2 × 250 mm column and an AG11-HC 2 × 50 mm guard column. An AS-3000 autosampler was used for sample injection. Sample volumes were 60 μl, and injection volumes were 10 μl. Each sample was injected twice and samples were analyzed in triplicate. Before injection, the column was equilibrated with 10 mM KOH for 5 min, followed by 1.5 mM KOH for 2 min. The elution gradient was carried out as follows: (1) 1.5 mM to 4 mM KOH from 0–7 min postinjection, (2) from 4–16 mM from 7–12 min postinjection, (3) from 16–86 mM KOH from 12–30 min postinjection, and finally (4) 10 min isocratic elution with 86 mM KOH.
Cell proliferation assay
Cell viability was assessed by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Sigma, Singapore) as previously described [13]. Briefly, cells were seeded into 96-well plates and incubated overnight to allow cell attachment. Cells were then treated with different concentrations of aspirin in triplicates and replenished with fresh aspirin after 24 h of treatment. Assessment of cell proliferation was carried out by addition of 20 μl of 7.5 g/L MTT per well into 200 μl of media and incubated for 1–2 h at 37°C. Thereafter, 150 μl of media was removed and 100 μl solvent (40 μl conc. HCl, 600 μl Triton-X 100 in 10 ml of isopropanol) was added to dissolve the formazan blue crystals overnight. Optical density at 540 nm and background was subtracted at 690 nm. Optical density should be directly correlated with cell quantity. The optical density of each treatment group was normalized to the non-treated group to obtain the percentage of viable cells.
Statistical analysis
Student’s t test was performed for statistical analysis.
Results
Aspirin down-regulates cell surface expression of EGFR in A-431 cells
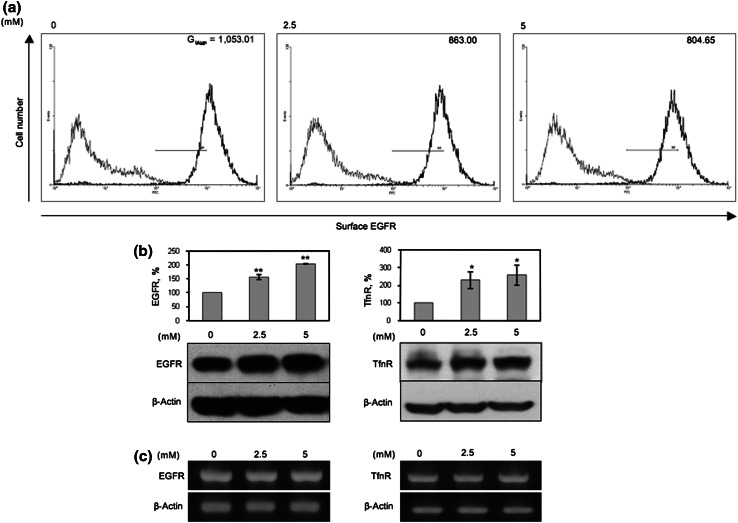
Aspirin has been reported to down-regulate cell surface expression of membrane proteins essential for T cell activation, such as CD80, CD86, and MHC class II in dendritic cells [6]. To determine whether aspirin also regulates cell surface expression of growth factor receptors, we analyzed the expression of cell surface EGFR in untreated and aspirin-treated A-431 cells by flow cytometry. As anticipated, aspirin reduced expression of cell surface EGFR in a dose-dependent manner (Fig. 1a). To determine whether the down-regulation of surface EGFR was due to a reduction in EGFR synthesis, we next examined the effect of aspirin on the expression of total EGFR and another growth factor receptor, TfnR [14] by Western-blot (WB) analysis. Intriguingly, aspirin up-regulates expression of total EGFR and TfnR in a dose-dependent manner (Fig. 1b) and this up-regulation of EGFR and TfnR expression was not due to an increase in EGFR and TfnR mRNA level upon treatment with aspirin (Fig. 1c).
Fig. 1.
Aspirin down-regulates cell surface EGFR and increases total protein expression of EGFR and TfnR. A-431 was treated with indicated concentrations of aspirin for 48 h. a Flow cytometry was performed to assess levels of cell surface EGFR. Histogram, Gmean is representative of two independent experiments. b Cell lysates were subjected to WB and total EGFR and TfnR expressions were normalized to untreated sample. Columns average of normalized values from three experiments; bars ± SD. Results with significant statistical difference from untreated sample: *p < 0.05 and **p < 0.001. c mRNA transcription was analyzed by RT-PCR
Aspirin induces accumulation of EGFR and TfnR in an intracellular aspirin-induced membrane compartment
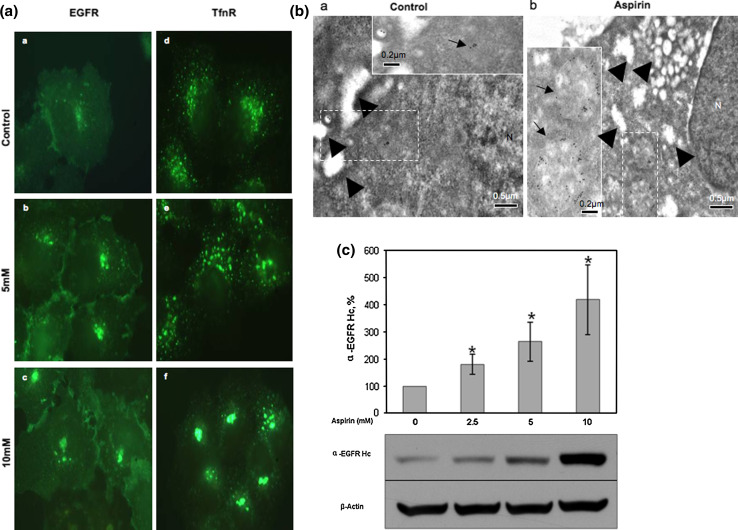
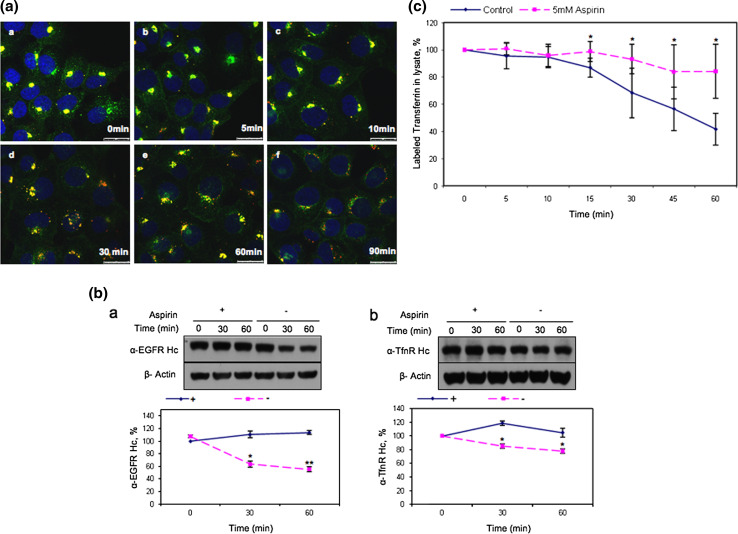
Since aspirin reduces cell surface EGFR, it is possible that aspirin accumulates the endocytosed-EGFR in an intracellular aspirin-induced membrane compartment or AMC. To determine this possibility, an immunofluorescence kinetic study was performed. A-431 cells were incubated with either EGFR- or TfnR-specific monoclonal antibody on ice to label cell surface EGFR and TfnR, washed to remove unbound antibodies, and the antibodies were endocytosed at 37°C for 2 h either in the absence or presence of various concentrations of aspirin and detected by fluorescence labeled anti-mouse secondary antibodies. As shown in Fig. 2a, aspirin treatment caused redistribution of endocytosed-EGFR and -TfnR. A major pool of the receptors was observed to accumulate at the perinuclear region in a dose-dependent manner. Furthermore, electron microscopy study confirmed that more endocytosed-TfnR (labeled by immunogold) were accumulated in larger and distinct membrane vesicles residing at the perinuclear region (arrow) in aspirin-treated cells as compared to control cells (Fig. 2b, compare panel a to panel b). Arrowheads show the presence of pinosomes in both the untreated and aspirin-treated cells (Fig. 2b, panels a and b). Generally, no endocytosed-TfnR were observed in the pinosomes. The one or two immunogold-labeled particles observed in the pinosomes could be due to the up-take of free anti-TfnR antibodies into the cells via pinocytosis (Fig. 2b, panel a, upper right corner). Taken together, our results show that endocytosed cell surface receptors accumulate in the AMC upon aspirin treatment.
Fig. 2.
Aspirin affects distribution and causes accumulation of eEGFR and eTfnR intracellularly. a Immunofluorescence (kinetics) was performed with indicated antibodies and varying concentrations of aspirin for 2 h. Image fluorescence microscope, 100×. b Cell surface TfnR in A-431 cells was labeled with anti-TfnR (OKT9) on ice for 1 h followed by staining with goat anti-mouse IgG complex (10-nm gold particles). A-431 cells were then treated in the absence or presence of 10 mM aspirin for 2 h. Thereafter, cells were subjected to electron microscopy preparation. Image from electron microscope, scale bars 0.5 μm. Insets magnification of the regions shown in the dash-lined boxes, scale bar 0.2 μm. Arrowheads less-opaque vesicles indicating macropinosomes. The majority of the macropinosomes were TfnR-negative. The one or two TfnR-positive macropinosomes seen here could be due to the uptake of unbound/free anti-TfnR antibodies together with extracellular fluid via macropinocytosis. Arrows endocytosed TfnR immunogold-conjugated antibody complex, N nucleus. c A-431 cells were incubated with anti-EGFR (clone 29.1) on ice for 1 h and then treated with varying concentrations of aspirin for 4 h. Cells were lysed and subjected to WB. EGFR expression of samples was normalized to untreated sample. Columns average of normalized values from three experiments, bars ± SD. Results with significant statistical difference from untreated sample, *p < 0.05
In view of our observation that aspirin up-regulates expression of total EGFR, we next determined whether aspirin inhibits degradation of endocytosed EGFR in the lysosomes. Cell surface EGFR of A-431 cells were labeled on ice with EGFR-specific monoclonal antibody, internalized at various time-points in the presence of aspirin, and detected for the internalized endocytosed-EGFR antibody complexes by WB. In this experiment, we assumed that one EGFR-specific monoclonal antibody molecule binds to two EGFR molecules on the cell surface and that the antibody is expected to be degraded together with the endocytosed-EGFR molecules in the lysosomes after internalization from the cell surface. Thus, the amount of intact heavy chain of the EGFR-specific monoclonal antibody detected by WB was used to measure the degradation of endocytosed-EGFR molecule in the lysosomes. As shown in Fig. 2c, aspirin reduces degradation of endocytosed-EGFR (represented by the heavy chain of the EGFR-specific monoclonal antibody) in the lysosome in A-431 cells. This is consistent with the observed accumulation of endocytosed-EGFR in the AMC.
The AMC is part of the EE as identified by the presence of early endosome-antigen-1 (EEA-1)
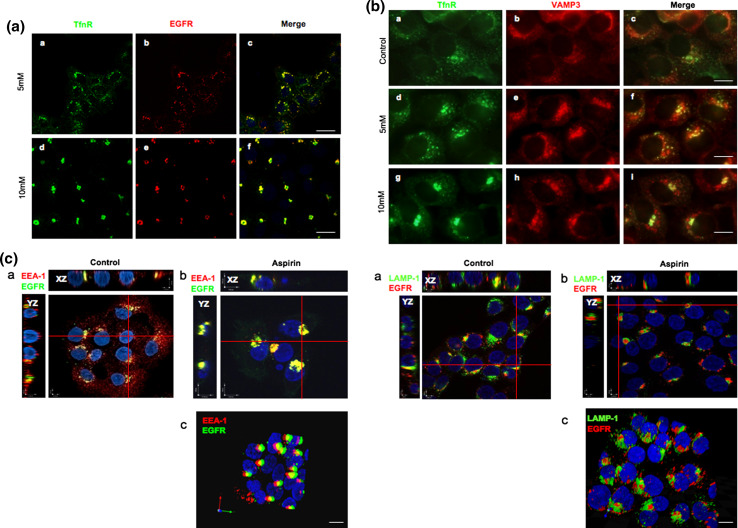
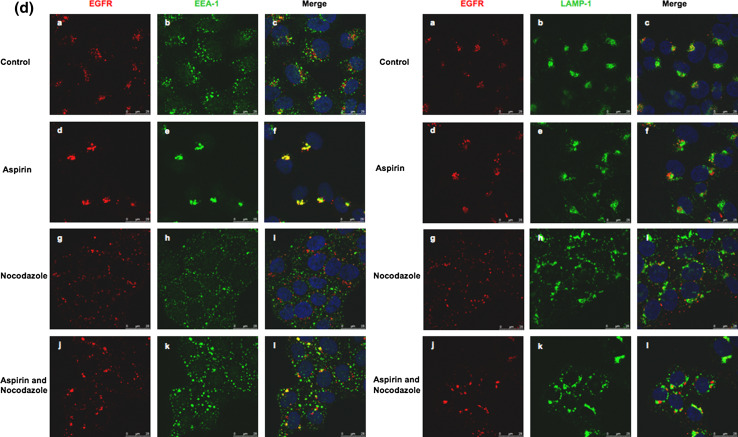
To characterize the AMC, A-431 cells were serum starved for 12 h prior to incubation with Alexa Fluor 555-conjugated EGF and anti-TfnR antibodies for 1 h on ice. Thereafter, cells were washed and further incubated with media containing varying concentrations of aspirin for 2 h at 37°C. Endocytosed-TfnR was stained with secondary antibody and analyzed by confocal immunofluorescence microscopy. Although it was shown previously that cell surface TfnR mostly traffics to the early and recycling endosomes [15], while EGFR mostly traffics to the late endosomes and lysosomes [16], we observed that both endocytosed-EGFR and endocytosed-TfnR co-localized extensively with each other upon treatment with aspirin (Fig. 3a), suggesting that aspirin affected a common trafficking pathway shared by both receptors. We next determined the effect of aspirin on the early endocytic pathway by analyzing the co-localization of VAMP3, a SNARE protein that resides in the early and recycling endosomes [17], with the endocytosed-TfnR. As shown in Fig. 3b, in the presence of aspirin, the accumulated endocytosed-TfnR partially co-localized with VAMP-3. Furthermore, in a separate experiment (Fig. 3c, XZ/YZ projection, left), endocytosed-EGFR also co-localizes with EEA-1, an early endosomal marker [18] but not LAMP-1, a late endosome/lysosomal protein [15], in the AMC upon treatment with aspirin (Fig. 3c, XZ/YZ projection, right). To further confirm the co-localization between EEA-1 and endocytosed-EGFR in the AMC, aspirin-treated A-431 cells were further incubated with nocodazole, a microtubule-depolymerizing agent that disrupts the endosomes and Golgi apparatus [19], prior to analysis by confocal immunofluorescence microscopy. Little or no co-localization between endocytosed-EGFR and EEA-1/LAMP-1 could be observed in cells treated with nocodazole alone (Fig. 3d, left and right panels g, h, and i). However, in cells treated with nocodazole and aspirin (Fig. 3d, left and right panels j, k, and l), nocodazole significantly disrupts the AMC into smaller punctate membrane structures. As anticipated, endocytosed-EGFR co-localizes well with EEA-1 (Fig. 3d, left panel l), but not LAMP-1 (Fig. 3d, right panel l) in the punctate membrane structures. Thus, our results strongly suggest that aspirin affects a common subcellular membrane compartment of EGFR and TfnR; and the AMC is part of the EE as identified by the presence of EEA-1.
Fig. 3.
AMC is part of the early endosomes. a Cells were serum starved for 12 h prior incubation with Alexa Fluor 555-conjugated EGF and anti-TfnR antibodies for 1 h on ice. Thereafter, cells were treated with varying concentrations of aspirin for 2 h. Endocytosed-TfnR was stained with secondary antibody. Image of confocal microscope, scale bars 50 μm. b Cells were treated as in a , followed by staining with polyclonal anti-VAMP-3 primary antibody and secondary antibodies. Image of fluorescence microscope, 100×. c Cells were serum starved for 12 h prior to incubation with Alexa Fluor 555-conjugated EGF for 1 h on ice. Thereafter, cells were treated with 10 mM aspirin for 2 h, stained with anti-EEA-1 (left) or anti-LAMP-1 (right) antibodies for 1 h, followed by secondary antibodies. Image of X–Z/Y–Z projection using confocal microscope (a, b). Image of stereo three-dimensional, confocal microscope (c). d Cells were serum starved for 12 h prior to incubation with Alexa Fluor 555-conjugated EGF for 1 h on ice. Thereafter, cells were treated with and without 10 mM aspirin for 1 h, followed by treatment with and without 10 μg/ml nocodazole in the presence or absence of aspirin for 1 h. Image of confocal microscope, scale bars 25 μm. The colocalizations of internalized EGF with EEA-1 and LAMP-1 in various situations are indicated by Pearson’s correlation (r). r average of values from five frames per coverslip in a representative of two independent experiments. In EEA-1 staining, control = 0.22; aspirin = 0.42; nocodazole = 0.17; aspirin and nocodazole = 0.33. In LAMP-1 staining, control = 0.31; aspirin = 0.26; nocodazole = 0.30; aspirin and nocodazole = 0.29
Aspirin mitigates exit of endocytosed-EGFR and -TfnR from the AMC
We next investigated the trafficking of endocytosed-EGFR and endocytosed-TfnR from the AMC upon aspirin removal. Surface EGFR and TfnR of A-431 cells were labeled with Alexa Fluor 555-conjugated EGF and anti-TfnR, respectively, prior to internalization in the presence of aspirin for 4 h to accumulate both endocytosed-EGFR and endocytosed-TfnR in the AMC. As shown in Fig. 4a, both endocytosed-EGFR (in red) and endocytosed-TfnR (in green) co-localizes well in the AMC in the presence of aspirin (panel a) and the magnitude of co-localization started to diminish at 30 min (panel d) after removal of aspirin. The decline in endocytosed-TfnR and endocytosed-EGFR co-localization was most apparent at 90 min after removal of aspirin (panel f). We also observed an increase in the amount of endocytosed-TfnR on the cell surface (green background) at this time point (panel f) indicating that endocytosed-TfnR from the AMC moved to the RE and recycled back to the cell surface upon removal of aspirin. To further demonstrate this point, receptor trafficking from the AMC upon aspirin removal was analyzed by WB. Cell surface EGFR of A-431 cells were labeled on ice with either EGFR- or TfnR-specific monoclonal antibody, and internalized for 4 h either in the absence or presence of aspirin. Cells were washed with cold PBS to remove aspirin, incubated at various time-points with fresh media without aspirin and detected for the internalized endocytosed-EGFR and endocytosed-TfnR antibody complexes by WB. We observed that endocytosed-EGFR was degraded more rapidly as compared to endocytosed-TfnR upon removal of aspirin (Fig. 4b, compare panel a to panel b) as depicted by the intensity of their respective antibody heavy chain bands shown in the WB. Protein bands were quantified and presented as graphs in the lower panels. Our results show that the AMC is a dynamic early endosomal compartment where both endocytosed-receptors are able to exit the AMC and continue trafficking to the late endosomes/lysosomes for degradation upon removal of aspirin.
Fig. 4.
Aspirin delays the degradation of EGFR and the recycling of TfnR to the cell surface. a Cells were starved 12 h prior to incubation with Alexa Fluor 555-conjugated EGF and anti-TfnR for 1 h on ice. Thereafter, receptor–antibody complexes were allowed to be endocytosed and accumulated in AMC in the presence of 10 mM aspirin for 4 h. Upon removal of aspirin, cells were washed with PBS and further incubated in media at 37°C, fixed at the indicated time points and stained with secondary antibodies. Images of confocal microscope, scale bars 25 μm. b Cells were incubated with primary antibodies [anti-EGFR, clone 29.1.1 (a) or anti-TfnR(b)] on ice for 1 h, followed by treatment with 10 mM aspirin for 4 h. Subsequently, cells were further incubated with (+) or without (−) 10 mM aspirin and lysed at indicated time points prior to WB analysis. Receptor-specific antibody heavy chain (anti-EGFR Hc or anti-TfnR Hc) were detected with HRP-conjugated anti-mouse IgG. The amount of endocytosed receptors in each sample was normalized to zero time point. Points average of normalized values from three independent experiments; bars ±SD. Results with significant statistical difference from untreated sample: *p < 0.05 and **p < 0.001. c Transferrin recycling assay was performed. The percentage of labeled transferrin in each sample was normalized to time point zero. Points average of normalized values from five independent experiments; bars ±SD. Results with significant statistical difference from untreated sample: *p < 0.05 and **p < 0.001
Since aspirin mitigates exit of endocytosed-EGFR and endocytosed-TfnR from the AMC, it is possible that recycling of endocytosed-receptors to the cell surface is also affected by aspirin. To confirm this possibility, A-431 cells (either untreated or pre-treated with aspirin) were incubated with labeled-transferrin at 18°C for 1 h to accumulate endocytosed-TfnR with its bound labeled transferrin, in the early endosomes. Cells were washed with low pH buffer to remove surface-bound labeled-transferrin and further incubated in fresh medium containing unlabeled transferrin at 37°C. Mediums were then collected at various time-points to determine the amount of recycled labeled transferrin. As anticipated, we observed a reduction in the amount of recycled labeled transferrin in aspirin-treated A-431 cells as compared to control (Fig. 4c). Thus, aspirin reduces recycling of endocytosed-TfnR to the cell surface. Our results suggest that the AMC is an early endosomal compartment located prior to the recycling endosomes (RE) and the late endosomes/lysosomes. Hence, the AMC could possibly be the early/sorting endosomes or ESE [20] and aspirin mitigates exit of endocytosed-EGFR and -TfnR from this compartment.
The AMC is the sorting endosomes and aspirin enhances recruitment of sorting nexins (SNXs) to membranes
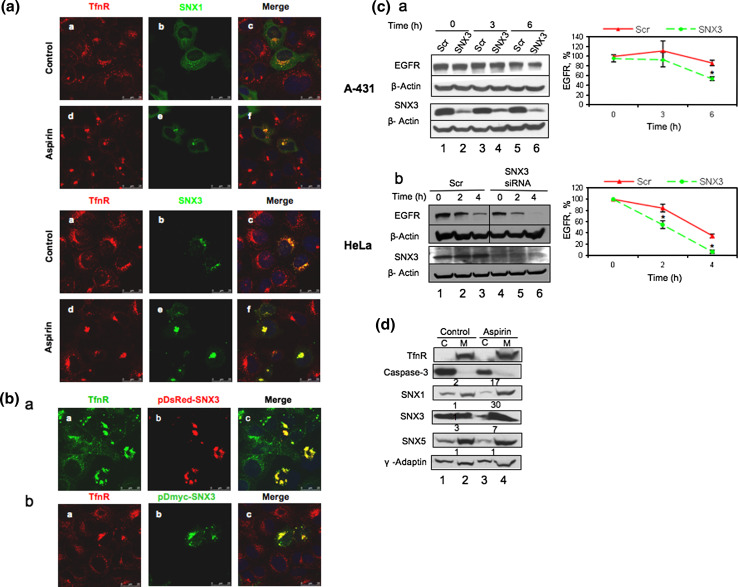
To examine the possibility that the AMC is the ESE, co-localization studies were performed for endocytosed-TfnR with several sorting nexins (SNX) proteins that had been reported previously to localize to the ESE [20]. SNX, a diverse group of trafficking proteins that have emerging roles in receptor sorting and trafficking, is unified by phospholipid-binding motif containing -phox homology (PX) domain that targets to phosphatidylinositol 3-phosphate [PI(3)P]-containing membranes and in some cases to other 3-phosphoinositides in cells that are mostly enriched in endosomal vesicles. For instance, SNX1, a well-studied member of the SNX family, recognizes the lysosomal targeting motif in the EGFR molecule and overexpression of SNX1 enhances degradation of EGFR [21]. In contrast, SNX3 [22] was shown previously to delay EGFR degradation instead in the late endosomes/lysosomes. In this study, we found that SNX1 and SNX3 co-localizes with endocytosed-TfnR in the AMC (Fig. 5a) upon aspirin treatment. Thus, taken together, our results suggest that (1) the AMC is the ESE which is located prior to the RE, and LE/lysosome and (2) aspirin inhibits the exit of endocytosed-TfnR and endocytosed-EGFR from the ESE.
Fig. 5.
AMC is the sorting endosome. a Cells transfected with Myc-SNX1 and Myc-SNX3 were subjected to immunofluorescence (kinetics). Cells were incubated with anti-TfnR mouse monoclonal antibody for 1 h on ice. Thereafter, receptor–antibody complexes were allowed to be endocytosed and accumulated in AMC in the presence of 10 mM aspirin for 2 h. Cells were fixed and stained using polyclonal anti-Myc antibodies, followed by secondary antibodies. The colocalizations of internalized TfnR with SNXs are indicated by Pearson’s correlation (r). r average of values from five frames per coverslip and a representative from two independent experiments. SNX1-overexpressed cells treated with and without aspirin, r = 0.07 and r = 0.13, respectively. SNX3-overexpressed cells treated with and without aspirin, r = 0.26 and r = 0.36 respectively. b Similar to a, cells transfected with pDsred-SNX3 (top panel) and pDmyc-SNX3 (bottom panel) were subjected to immunofluorescence (kinetics). Arrows eTfnR clumped at perinuclear region. Image of fluorescence microscope, 100×. c A-431 (a) and HeLa (b) cells transfected with either control (Scr) or SNX3-specific siRNA duplexes for 36 h, were serum starved for 12 h prior to being either untreated (control) or treated with 100 nM and 40 nM EGF, respectively, in the presence of 10 μg/ml cycloheximide. Cells were lysed at the indicated time points and subjected to WB. Total EGFR for each sample was normalized to zero time point. Points average of normalized values from three independent experiments; bars ±SD. Results with significant statistical difference from control sample, *p < 0.05. d Cells treated with varying concentrations of aspirin for 4 h were subjected to cellular fractionation. The lysate fractions were then subjected to WB using indicated antibodies. Numerical ratio of membrane to cytosol
The previous report by Xu and colleagues [22] suggested that overexpression of SNX3 in A-431 cells induced intracellular accumulation of endocytosed-EGFR in the early endosomes and delayed its degradation in the lysosomes. Similarly, our results show that overexpression of Dsred-SNX3 and Myc-SNX3 induces accumulation of endocytosed-TfnR in intracellular membrane compartments similar to the ESE/AMC (Fig. 5b). Consistent with Xu’s findings, we demonstrate that silencing of SNX3 by siRNA transfection enhances degradation of EGFR in both A-431 cells (Fig. 5c, panel a, lane 5 and lane 6) and HeLa cells (Fig. 5c, panel b, compare lane 2 to lane 5; lane 3 to lane 6). Based on these observations and the fact that aspirin mediates accumulation of endocytosed-EGFR/TfnR in the ESE/AMC and delays degradation of EGFR and TfnR, we hypothesize that aspirin induces recruitment of SNX3 (and possibly other SNXs) to membranes. As such, we performed subcellular membrane fractionation to investigate whether there is any perturbation in the recruitment of SNXs from the cytoplasm to the membrane upon aspirin treatment. The intensity of bands was quantified and membrane-to-cytoplasm (M/C) ratios was determined. As anticipated, aspirin enhances recruitment of SNX1, 3 and 5 but not γ-adaptin [23] to membrane (Fig. 5d). TfnR and caspase-3 [24] mark the membrane and cytoplasm, respectively. The magnitude of the aspirin-induced SNX3 (M/C ratio of 30) membrane recruitment is much higher compared to SNX1 (M/C ratio of 17) and SNX5 (M/C ratio of 7). Thus, we inferred that SNX3 might play a major role in regulating EGFR trafficking in the presence of aspirin.
Regulation of EGFR trafficking and degradation by aspirin is dependent on the expression of SNX3
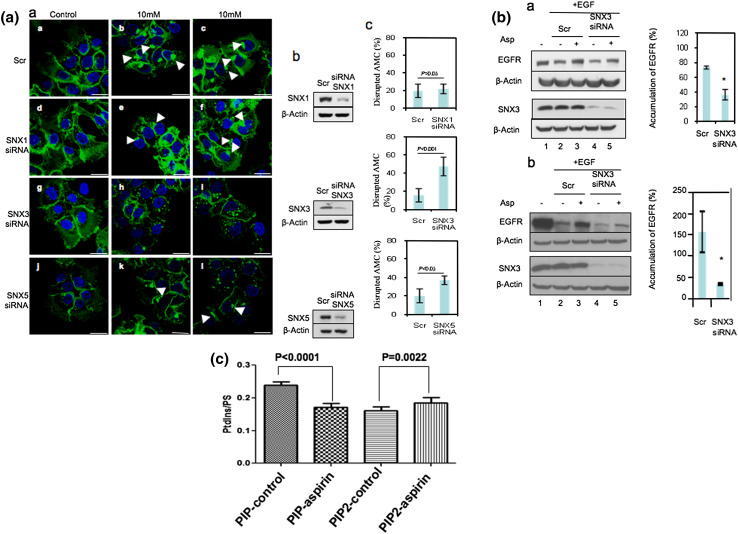
We then sought to define the role of SNX3 as well as SNX1 and SNX5 in the accumulation of endocytosed-EGFR in the ESE/AMC upon aspirin treatment by gene silencing experiments. As shown in Fig. 6a (panel a), knocking down of SNX3, SNX5, but not SNX1, abrogated the accumulation of endocytosed-EGFR in the ESE/AMC upon aspirin treatment. The gene knockdown efficiency is shown in Fig. 6a (panel b). Figure 6a (panel c) presents the percentage of cells showing inhibition of EGFR accumulation in the ESE upon aspirin treatment. In this experiment, under each condition (treatment), a total of 75 cells was randomly selected and observed for the formation of AMC.
Fig. 6.
Aspirin-induced accumulation of EGFR in the sorting endosomes is dependent on the expression of SNX3. a Cells transfected with control (Scr) or SNX-specific siRNA duplexes for 36 h were serum starved for 12 h prior to incubation with 2 μg/ml of Alexa Fluor 488-conjugated EGF on ice for 1 h. Thereafter, cells were treated with varying concentrations of aspirin for 2 h. a Image of confocal microscope, scale bars 25 μm. Arrows indicate the AMC. c Amount of AMC-negative cells upon treatment with 10 mM aspirin were counted and normalized to total number of cells. Columns average of normalized values from three independent experiments, bars ±SD. Results with significant statistical difference from Scr, *p < 0.05. b WB was performed to assess knockdown efficiency. b Serum-starved siRNA-transfected A-431 cells were treated with 100 nM of EGF and 10 μg/ml of cycloheximide, in the absence (−) or presence (+) of 5 mM aspirin for 6 h prior to WB analysis (a). A similar experiment was done in HeLa cells (b) using 40 nM EGF, 5 mM aspirin, and 2 h incubation. The change in EGFR expression in siRNA-treated samples upon treatment with aspirin was normalized to untreated sample (right). Columns average values from two independent experiments, bars ±SD. Results with significant statistical difference from Scr: *p < 0.05 and **p < 0.001. c Lipid ion chromatography analysis showed that aspirin did not elevate synthesis of total PIP lipid on membranes
To further confirm the role of SNX3 in regulating EGFR trafficking in the presence of aspirin, A-431 (panel a) and HeLa (panel b) cells transfected with either scrambled or SNX3 siRNA were either untreated or treated with aspirin for 4 and 2 h, respectively (either in the presence or absence of EGF) prior to WB using the indicated antibodies (Fig. 6b). As anticipated, EGF induces EGFR degradation (Fig. 6b, panel a and b, compare lane 1 and lane 2) whereas aspirin partially mitigates the down-regulation effect of EGF on EGFR degradation (Fig. 6b, panel a and b, compare lane 1, lane 2, and lane 3). However, the mitigation of EGFR degradation by aspirin was markedly reduced in SNX3 siRNA-treated cells as compared to scrambled siRNA-treated cells (Fig. 6b, panel a and b, compare lane 3 and lane 5). The bar charts on the right show the normalized aspirin-dependent mitigation of EGF-induced EGFR degradation (% accumulation of EGFR) in scrambled siRNA- and SNX3 siRNA-treated A-431 and HeLa cells (Fig. 6b, panel a and b, compare lane 3 to lane 5). Taken together, our results strongly suggest that the accumulation of EGFR in the ESE/AMC by aspirin is dependent on the expression of SNX3.
We next determined whether the increase in SNX3 recruitment to endosomal membranes was possibly due to the ability of aspirin in enhancing synthesis of membrane mono-phosphoinositides (PIP). Lipid ion chromatography analysis of total membranes derived from untreated and aspirin-treated A-431 cells showed that the level of total membrane PIP (normalized against total PS) was not elevated in A-431 cells upon aspirin treatment (Fig. 6c). In fact, we observed a significant drop in total PIP upon aspirin treatment. Interestingly, we did notice a slight increase in di-phosphoinositides (PIP2) in A-431 cells treated with aspirin. In view of the fact that SNX3 preferentially binds to PIP such as PI(3)P [22 and our unpublished results], the increase in PIP2 is not expected to contribute to the increase in SNX3 recruitment to membranes. Thus, the aspirin-effect on the recruitment of SNX1, SNX3, and SNX5 to membrane is not due to the aspirin-mediated enhancement of membrane PIP synthesis in A-431 cells.
The acetylating property of aspirin is not required for the aspirin-mediated intracellular accumulation of EGFR and TfnR in the ESE
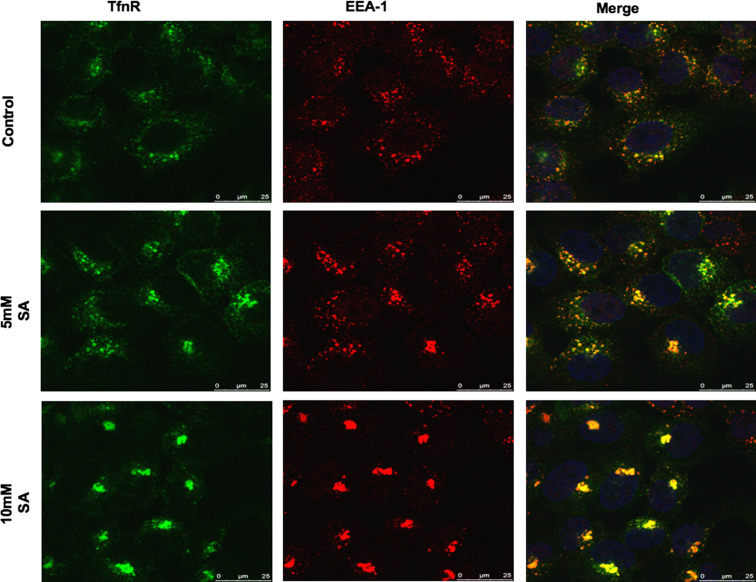
Lysine acetylation of proteins has been reported to regulate protein functions [25, 26] and a number of proteins which play important roles in regulating (either directly of indirectly) the endocytic pathway are found to be acetylated at the lysine amino acid residue [27]. To determine whether the aspirin-mediated intracellular accumulation of EGFR and TfnR observed in this study was due to the acetylating property of aspirin, A-431 cells were either untreated or treated with various concentrations (5 and 10 mM) of salicylic acid (SA) which lack the acetyl-group, fixed, stained with TfnR-specific antibodies prior to analysis by confocal microscopy. As shown in Fig. 7, like in the case of aspirin, salicylic acid accumulates endocytosed-TfnR in the ESE co-localized with EEA-1. Thus, the acetylating property of aspirin is not essential for its role in regulating receptor trafficking in A-431 cells. Since the acetylating property of aspirin is essential for modification of COX-2, and in view of the fact that SA lacks the acetyl-group (thus without acetylating property), our results suggest that aspirin regulates receptor trafficking in a COX-independent manner. Furthermore, previous studies demonstrate that aspirin can redirect the catalytic activity of COX-2 to produce aspirin-triggered 15-epi-lipoxin A4 (15-epi-LXA4) via transcellular biosynthesis [28] and the production of 15-epi-LXA4 has been shown to inhibit growth of human lung adenocarcinoma cell line [29]. However, 15-epi-LXA4 had no effect in A-431 cells (data not shown). This further suggests that the effects of aspirin observed in this study are COX-independent.
Fig. 7.
The acetylating property of aspirin is not required for the aspirin-mediated intracellular accumulation of TfnR in the ESE. Indirect immunofluorescence at kinetics was carried out for TfR in A-431 cells. Cells were incubated with anti-TfnR for 1 h on ice. Thereafter, receptor–antibody complexes were allowed to be endocytosed in the presence of indicated concentrations of salicylic acid (SA) for 2 h. Cells were fixed and then co-stained with anti-EEA-1 primary antibody, followed by secondary antibodies. Image of confocal microscope, scale bars 25 μm
Discussion
Our model (Fig. 8) suggests that aspirin enhances recruitment of SNX3/SNX5 to the ESE/AMC. Increased recruitment of SNX3/SNX5 to the ESE/AMC membrane mitigates (1) trafficking of endocytosed receptors to the LE/lysosomes for degradation and (2) recycling of endocytosed receptors to the cell surface. The increase in EGFR degradation observed in SNX3 knockdown cells as compared to control cells underscores the importance of SNX3 in negatively regulating EGFR trafficking from the ESE to the late endosomes and lysosomes for degradation (Fig. 5c, and Fig. 6b, lanes 2 and 4). In contrast, silencing of SNX1 up-regulates the expression of EGFR (data not shown), which confirms studies demonstrating SNX1 as a positive regulator of EGFR degradation [21]. The opposing effect of SNX1 and SNX3 suggest that SNX3 might be a potent “regulator” that negatively regulates SNX1-dependent EGFR trafficking from the ESE to the lysosomes for degradation. It is also worth noting that other SNXs might contribute to the aspirin-induced accumulation of EGFR in the ESE, for example, SNX5 (Fig. 6a). Silencing of SNX5 by siRNA transfection reduces (to a lesser extent) the accumulation of EGFR in the ESE/AMC by aspirin (panel j, k, and l). Thus, both SNX-3 and SNX-5 might work synergistically in the regulation of EGFR trafficking from the ESE. Since aspirin accumulates both TfnR and EGFR in the common ESE/AMC and inhibits their exit, it is highly possible that aspirin generally affect trafficking of cell surface receptor in the endocytic pathways. Ubiquitination of EGFR at its cytoplasmic domain was reported to promote sorting of EGFR to the late endosomes for degradation [30]. However, we did not examine the effect of aspirin on EGFR ubiquitination.
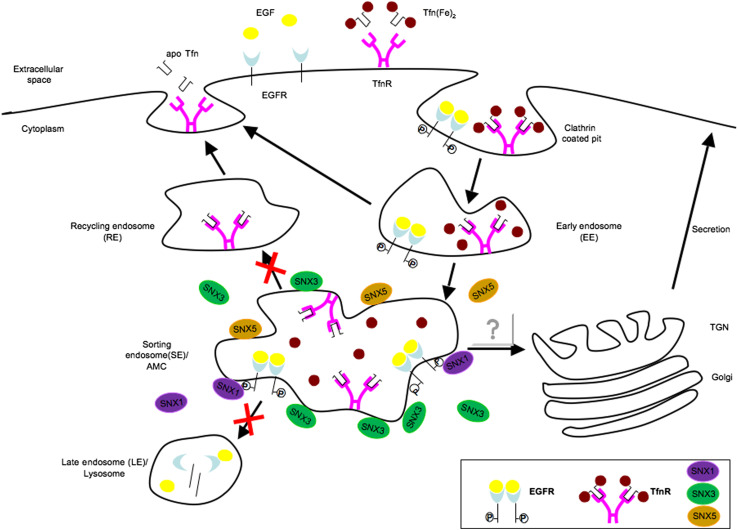
Fig. 8.
Schematic representation of the effect of aspirin on membrane-receptor trafficking. Upon binding to ligand, EGFR and TfnR are endocytosed into early endosome and then moved on to the sorting platforms. Enhanced recruitment of SNX3 and possibly SNX5 to AMC upon aspirin treatment delays the lysosomal degradation of EGFR as well as recycling of TfnR to the cell surface
The process of endocytosis and signaling is intrinsically linked and growing evidence shows the bidirectional interplay between signaling and membrane-transport networks [31]. One of the exceptionally important growth signaling pathways is the mitogen-activated protein kinases (MAPKs) pathway which includes extra-cellular signal-regulated kinase (ERK), p38 MAPK, and c-Jun NH (2)-terminal kinase (JNK). These signaling pathways regulate a variety of cellular activities including proliferation, differentiation, survival, and death [32]. Regulation of membrane trafficking machineries by signaling cascades is not uncommon [33, 34]. Recently, a study demonstrated that p38 MAPK could phosphorylates Rab5 effectors, EEA-1 and Rabenosyn5 on Thr-1392 and Ser-215, respectively, and this is essential for membrane receptor endocytosis [35]. As aspirin and indomethacin have been shown to modulate phosphorylation of p38 MAPK [36, 37], p44/42 MAPK (ERK) [38], and JNK [36, 39], it is possible that the aspirin-mediated accumulation of internalized receptors at the ESE/AMC in A-431 cells could be regulated by the MAPK signaling pathways. As shown in Supplementary Fig. 1A, aspirin explicitly increases phosphorylation of p38 MAPK, JNK and p44/42 (ERK). Interestingly, p38 MAPK inhibition could significantly abrogate the aspirin-mediated accumulation of surface-internalized TfnR (eTfnR) in the ESE/AMC as shown in Supplementary Fig. 1B. Furthermore, we also observed that the continued retention of eTfnR in the ESE/AMC by aspirin is also dependent on the activities of p38 MAPK, JNK, and ERK (Supplementary Fig. 1C). Thus, the aspirin-mediated accumulation of endocytosed cell surface receptors in the ESE is regulated by the MAPK pathway. However, it is not known how p38 MAPK, JNK, and ERK activities play a role in regulating accumulation of EGFR and TfnR in the ESE. It is possible that p38 MAPK, JNK, and ERK induce recruitment of SNX3/SNX5 to membrane by directly phosphorylating SNX3 and SNX5. However, to date, SNX3 and SNX5 have not been reported to be phosphorylated. Alternatively, p38 MAPK, JNK, and ERK might phosphorylate protein partner(s) of SNX3 and SNX5 and regulates their recruitment to membrane. Future work along this line may shed more light on this interesting question.
Aspirin has been recently reported to have anti-neoplastic properties and clinical studies have demonstrated that regular, long-term aspirin intake reduces risk of death due to cancer [40–42]. As anticipated, by using the MTT assay, we showed that aspirin inhibited A-431 cell proliferation in a dose-dependent manner (Supplementary Fig. 2). The down-regulation of surface EGFR upon aspirin treatment (Fig. 1a) due to the inhibition in the recycling pathway (Fig. 4c) might contribute partly to the mechanism underlying the anti-neoplastic activity of aspirin. Furthermore, inhibition of endosomal maturation from early to recycling and late endosomes by aspirin, as demonstrated in this study, might prevent free iron (from the Tfn–TFnR complex) from transporting into the cytoplasm, thereby causing iron deficiency and cancer cell growth arrest [43]. Further study along this line may shed some light on the link between the aspirin-induced accumulation of EGFR/TfnR in the ESE/AMC and the observed anti-neoplastic activity of aspirin.
Taken together, this study sheds light on how aspirin may down-regulate surface expression of growth factor receptors by inhibiting receptor exiting from the ESE/AMC and recycling back to the cell surface.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (TIFF 895 kb) Supplementary Fig. 1 The aspirin-mediated accumulation of endocytosed cell surface receptors in the ESE is also regulated by the MAPK pathway. (A) Cell extracts from untreated and aspirin-treated A-431 cells were analyzed by Western-blot analysis using antibodies specific for the phosphorylated forms of p38 MAPK, JNK, and ERK. Results are representative of three independent experiments. (B) Surface antibody-TfnR complexes were allowed to be internalized into cells in the absence or presence of aspirin and p38 inhibitor with concentration and duration as indicated. Image, fluorescence microscope; magnification, 100x; white arrow, the compact structure is the ESE/AMC; red arrow, reduced accumulation of eTfnR in the ESE/AMC in the presence of inhibitors and aspirin. Results are representative of at least two independent experiments. (C) Surface antibody-TfnR complexes were allowed to be internalized into A-431 cells in the presence of 10 mM aspirin for 3 h (a) and followed by treatment with 10 mM aspirin (b, j); normal complete media (f, n); aspirin and p38 inhibitor (c, k); aspirin and ERK inhibitor (d, l); aspirin and JNK inhibitor (e, m); p38 inhibitor (g, o); ERK inhibitor (h, p); and JNK inhibitor (l, q) at indicated time points. Image of fluorescence microscope; magnification, 100x; white arrow, the compact structure is the ESE/AMC; red arrow, reduced accumulation of eTfnR in the ESE/AMC in the presence of inhibitors and aspirin; yellow arrow, reduced accumulation of eTfnR in the ESE/AMC upon removal of aspirin and in the presence of inhibitors; purple arrow, reduced accumulation of eTfnR in the ESE/AMC upon removal of aspirin.
Supplementary material 2 (TIFF 61 kb) Supplementary Fig. 2 Aspirin inhibits A-431 cell proliferation. A-431 was treated with indicated concentrations of aspirin for 48 h. Cells were subjected to MTT and the number of viable cells in each sample was normalized to untreated sample. Points, average of normalized values from three independent experiments; bars, ±SD.
Acknowledgments
We would like to thank Dr. Boon Chuan Low for reading the manuscript and his valuable comments. This work was supported by research grants from the Academic Research Council-Ministry of Education, Singapore (R182-000-099-112 to S.H.W) and the Singapore National Medical Research Council (R182-000-137-213 to S.H.W).
Abbreviations
- EGFR
Epidermal growth factor receptor
- TfnR
Transferrin receptor
- EE
Early endosome
- ESE
Early/Sorting endosomes
- RE
Recycling endosome
- LE
Late endosome
- SNX
Sorting nexin
- AMC
Aspirin-induced membrane compartment
- NRAMP2
Natural resistance-associated macrophage protein 2
- CysLT1
Cysteinyl leukotriene receptor
- SR-BI
Scavenger receptor class B type I
References
- 1.Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci. 2008;65:1566–1584. doi: 10.1007/s00018-008-7440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2009;315:683–696. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 3.Sadowski L, Pilecka I, Miaczynska M. Signaling from endosomes: location makes a difference. Exp Cell Res. 2009;315:1601–1609. doi: 10.1016/j.yexcr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence CM, Ray S, Babyonyshev M, Galluser R, Borhani DW, Harrison SC. Crystal structure of the ectodomain of human transferrin receptor. Science. 1999;286:779–782. doi: 10.1126/science.286.5440.779. [DOI] [PubMed] [Google Scholar]
- 5.Gruenheid S, Canonne-Hergaux F, Gauthier S, Hackam DJ, Grinstein S, Gros P. The iron transport protein NRAMP2 is an integral membrane glycoprotein that colocalizes with transferrin in recycling endosomes. J Exp Med. 1999;189:831–841. doi: 10.1084/jem.189.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matasic R, Dietz AB, Vuk-Pavlovic S. Cyclooxygenase-independent inhibition of dendritic cell maturation by aspirin. Immunology. 2000;101:53–60. doi: 10.1046/j.1365-2567.2000.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sousa AR, Parikh A, Scadding G, Corrigan CJ, Lee TH. Leukotriene-receptor expression on nasal mucosal inflammatory cells in aspirin-sensitive rhinosinusitis. N Engl J Med. 2002;347:1493–1499. doi: 10.1056/NEJMoa013508. [DOI] [PubMed] [Google Scholar]
- 8.Tancevski I, Wehinger A, Schgoer W, Eller P, Cuzzocrea S, Foeger B, Patsch JR, Ritsch A. Aspirin regulates expression and function of scavenger receptor-BI in macrophages: studies in primary human macrophages and in mice. FASEB J. 2006;20:1328–1335. doi: 10.1096/fj.05-5368com. [DOI] [PubMed] [Google Scholar]
- 9.Karagiannis TC, Lobachevsky PN, Leung BK, White JM, Martin RF. Receptor-mediated DNA-targeted photoimmunotherapy. Cancer Res. 2006;66:10548–10552. doi: 10.1158/0008-5472.CAN-06-1853. [DOI] [PubMed] [Google Scholar]
- 10.Huang D, Cai DT, Chua RY, Kemeny DM, Wong SH. Nitric-oxide synthase 2 interacts with CD74 and inhibits its cleavage by caspase during dendritic cell development. J Biol Chem. 2008;283:1713–1722. doi: 10.1074/jbc.M705998200. [DOI] [PubMed] [Google Scholar]
- 11.Ho YH, Cai DT, Huang D, Wang CC, Wong SH. Caspases regulate VAMP-8 expression and phagocytosis in dendritic cells. Biochem Biophys Res Commun. 2009;387:371–375. doi: 10.1016/j.bbrc.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 12.Goding JW, Burns GF. Monoclonal antibody OKT-9 recognizes the receptor for transferrin on human acute lymphocytic leukemia cells. J Immunol. 1981;127:1256–1258. [PubMed] [Google Scholar]
- 13.Pannecouque C, Daelemans D, De Clercq E. Tetrazolium-based colorimetric assay for the detection of HIV replication inhibitors: revisited 20 years later. Nat Protoc. 2008;3:427–434. doi: 10.1038/nprot.2007.517. [DOI] [PubMed] [Google Scholar]
- 14.Ponka P, Lok CN. The transferrin receptor: role in health and disease. Int J Biochem Cell Biol. 1999;31:1111–1137. doi: 10.1016/S1357-2725(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 15.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 16.Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 17.Fields IC, Shteyn E, Pypaert M, Proux-Gillardeaux V, Kang RS, Galli T, Folsch H. v-SNARE cellubrevin is required for basolateral sorting of AP-1B-dependent cargo in polarized epithelial cells. J Cell Biol. 2007;177:477–488. doi: 10.1083/jcb.200610047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patki V, Virbasius J, Lane WS, Toh BH, Shpetner HS, Corvera S. Identification of an early endosomal protein regulated by phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1997;94:7326–7330. doi: 10.1073/pnas.94.14.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong SH, Xu Y, Zhang T, Hong W. Syntaxin 7, a novel syntaxin member associated with the early endosomal compartment. J Biol Chem. 1998;273:275–380. doi: 10.1074/jbc.273.1.375. [DOI] [PubMed] [Google Scholar]
- 20.Worby CA, Dixon JE. Sorting out the cellular functions of sorting nexins. Nat Rev Mol Cell Biol. 2002;3:919–931. doi: 10.1038/nrm974. [DOI] [PubMed] [Google Scholar]
- 21.Kurten RC, Cadena DL, Gill GN. Enhanced degradation of EGF receptors by a sorting nexin, SNX1. Science. 1996;272:1008–1010. doi: 10.1126/science.272.5264.1008. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y, Hortsman H, Seet L, Wong SH, Hong W. SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns(3)P. Nat Cell Biol. 2001;3:658–666. doi: 10.1038/35083051. [DOI] [PubMed] [Google Scholar]
- 23.Takatsu H, Sakurai M, Shin HW, Murakami K, Nakayama K. Identification and characterization of novel clathrin adaptor-related proteins. J Biol Chem. 1998;273:24693–24700. doi: 10.1074/jbc.273.38.24693. [DOI] [PubMed] [Google Scholar]
- 24.Feng Y, Hu J, Xie D, Qin J, Zhong Y, Li X, Xiao W, Wu J, Tao D, Zhang M, Zhu Y, Song Y, Reed E, Li QQ, Gong J. Subcellular localization of caspase-3 activation correlates with changes in apoptotic morphology in MOLT-4 leukemia cells exposed to X-ray irradiation. Int J Oncol. 2005;27:699–704. [PubMed] [Google Scholar]
- 25.Kalgutkar AS, Crews BC, Rowlinson SW, Garner C, Seibert K, Marnett LJ. Aspirin-like molecules that covalently inactivate cyclooxygenase-2. Science. 1998;280:1268–1270. doi: 10.1126/science.280.5367.1268. [DOI] [PubMed] [Google Scholar]
- 26.Liyasova MS, Schopfer LM, Lockridge O. Reaction of human albumin with aspirin in vitro: mass spectrometric identification of acetylated lysines 199, 402, 519, and 545. Biochem Pharmacol. 2010;79:784–791. doi: 10.1016/j.bcp.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 28.Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 29.Claria J, Lee MH, Serhan CN. Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)-neutrophil interactions and are potent inhibitors of cell proliferation. Mol Med. 1996;2:583–596. [PMC free article] [PubMed] [Google Scholar]
- 30.Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Ann Rev Cell Dev Biol. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorkin A, Von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 32.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Ahn S, Kim J, Lucaveche CL, Reedy MC, Luttrell LM, Lefkowitz RJ, Daaka Y. Src-dependent tyrosine phosphorylation regulates dynamin self-assembly and ligand-induced endocytosis of the epidermal growth factor receptor. J Biol Chem. 2002;277:26642–26651. doi: 10.1074/jbc.M201499200. [DOI] [PubMed] [Google Scholar]
- 34.Confalonieri S, Salcini AE, Puri C, Tacchetti C, Di Fiore PP. Tyrosine phosphorylation of Eps15 is required for ligand-regulated, but not constitutive, endocytosis. J Cell Biol. 2000;150:905–912. doi: 10.1083/jcb.150.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mace G, Miaczynska M, Zerial M, Nebreda AR. Phosphorylation of EEA1 by p38 MAP kinase regulates mu opioid receptor endocytosis. EMBO J. 2005;24:3235–3246. doi: 10.1038/sj.emboj.7600799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oshima T, Miwa H, Joh T. Aspirin induces gastric epithelial barrier dysfunction by activating p38 MAPK via claudin-7. Am J Physiol Cell Physiol. 2008;295:C800–C806. doi: 10.1152/ajpcell.00157.2008. [DOI] [PubMed] [Google Scholar]
- 37.Ou YC, Yang CR, Cheng CL, Raung SL, Hung YY, Chen CJ. Indomethacin induces apoptosis in 786-O renal cell carcinoma cells by activating mitogen-activated protein kinases and AKT. Eur J Pharmacol. 2007;563:49–60. doi: 10.1016/j.ejphar.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 38.Becker JC, Muller-Tidow C, Stolte M, Fujimori T, Tidow N, Ilea AM, Brandts C, Tickenbrock L, Serve H, Berdel WE, Domschke W, Pohle T. Acetylsalicylic acid enhances antiproliferative effects of the EGFR inhibitor gefitinib in the absence of activating mutations in gastric cancer. Int J Oncol. 2006;29:615–623. doi: 10.3892/ijo.29.3.615. [DOI] [PubMed] [Google Scholar]
- 39.Ordan O, Rotem R, Jaspers I, Flescher E. Stress-responsive JNK mitogen-activated protein kinase mediates aspirin-induced suppression of B16 melanoma cellular proliferation. Br J Pharmacol. 2003;138:1156–1162. doi: 10.1038/sj.bjp.0705163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jana NR. NSAIDs and apoptosis. Cell Mol Life Sci. 2008;65:1295–1301. doi: 10.1007/s00018-008-7511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elwood PC, Gallagher AM, Duthie GG, Mur LA, Morgan G. Aspirin, salicylates, and cancer. Lancet. 2009;373:1301–1309. doi: 10.1016/S0140-6736(09)60243-9. [DOI] [PubMed] [Google Scholar]
- 42.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 43.Whitnall M, Howard J, Ponka P, Richardson DR. A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc Natl Acad Sci USA. 2006;103:14901–14906. doi: 10.1073/pnas.0604979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (TIFF 895 kb) Supplementary Fig. 1 The aspirin-mediated accumulation of endocytosed cell surface receptors in the ESE is also regulated by the MAPK pathway. (A) Cell extracts from untreated and aspirin-treated A-431 cells were analyzed by Western-blot analysis using antibodies specific for the phosphorylated forms of p38 MAPK, JNK, and ERK. Results are representative of three independent experiments. (B) Surface antibody-TfnR complexes were allowed to be internalized into cells in the absence or presence of aspirin and p38 inhibitor with concentration and duration as indicated. Image, fluorescence microscope; magnification, 100x; white arrow, the compact structure is the ESE/AMC; red arrow, reduced accumulation of eTfnR in the ESE/AMC in the presence of inhibitors and aspirin. Results are representative of at least two independent experiments. (C) Surface antibody-TfnR complexes were allowed to be internalized into A-431 cells in the presence of 10 mM aspirin for 3 h (a) and followed by treatment with 10 mM aspirin (b, j); normal complete media (f, n); aspirin and p38 inhibitor (c, k); aspirin and ERK inhibitor (d, l); aspirin and JNK inhibitor (e, m); p38 inhibitor (g, o); ERK inhibitor (h, p); and JNK inhibitor (l, q) at indicated time points. Image of fluorescence microscope; magnification, 100x; white arrow, the compact structure is the ESE/AMC; red arrow, reduced accumulation of eTfnR in the ESE/AMC in the presence of inhibitors and aspirin; yellow arrow, reduced accumulation of eTfnR in the ESE/AMC upon removal of aspirin and in the presence of inhibitors; purple arrow, reduced accumulation of eTfnR in the ESE/AMC upon removal of aspirin.
Supplementary material 2 (TIFF 61 kb) Supplementary Fig. 2 Aspirin inhibits A-431 cell proliferation. A-431 was treated with indicated concentrations of aspirin for 48 h. Cells were subjected to MTT and the number of viable cells in each sample was normalized to untreated sample. Points, average of normalized values from three independent experiments; bars, ±SD.