Abstract
It has recently been demonstrated that not only mature B lymphocytes, but also non-lymphoid cells, including cancer cells and neurons, express IgG. In the eye, an important immune privileged site, the presence of IgG has been ascribed to IgG entering the eye through breaches of the blood–ocular barrier. Here we demonstrate that the eye itself can produce IgG intrinsically. Applying immunohistochemistry, in situ hybridization, and RT-PCR, several intraocular structures were found to express proteins and mRNA transcripts of IgG heavy chains, light chains, V(D)J rearrangements, and enzymes required for V(D)J recombination. IgG receptors were also detected in the intraocular epithelium and endothelium. The extensive distribution of IgG and its receptors in intraocular structures indicates that locally produced IgG could play a significant role in maintaining the ocular microenvironment and protection of the eyes, and it might also be involved in the pathogenesis of age-related macular degeneration and some inflammatory diseases.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0572-7) contains supplementary material, which is available to authorized users.
Keywords: Immunoglobulin G, IgG receptors, Age-related macular degeneration, Fuchs heterochromic cyclitis, Eye, Human, Murine
Introduction
A number of sites in the human body, including the brain, the reproductive organs (testis and ovary), and the eye are extremely important for normal functions, and even minor injury of such organs might lead to serious consequences that should be prevented. These organs have therefore been named “immune privileged sites”, where immune responses are regulated locally [1]. As inflammatory responses are toned down, non-MHC matched tissue grafts can survive in such a location without being rejected. In the eye, several mechanisms appear to contribute to ocular immune privilege, including the lack of lymphatic drainage, blood–ocular barrier, immune suppressive molecules in the ocular microenvironment, restricted expression of MHC molecules, expression of Fas ligands, and tumor necrosis factor-related apoptosis-inducing ligand in ocular structures and induction of suppressor immunity by regulatory T cells [1–3]. The blood–ocular barrier is constituted by the blood–retinal and blood–aqueous barriers. These barriers are composed of complex tight junctions between endothelial cells that prevent leukocytes including lymphocytes and large molecules from crossing the blood–ocular barrier under normal physiological conditions. Despite these barriers, immunoglobulins have, however, been identified in several ocular tissues. Allansmith et al. [4] detected IgG, IgA, IgE, and IgD in various ocular structures, including the cornea, choroid, conjunctiva, ciliary process, and ocular muscles using immunofluorescence and single radial immunodiffusion techniques. IgG was also demonstrated in the aqueous humor (AH) and vitreous body of the human eye under normal and pathological conditions [5–9]. It was speculated that IgG could have been transcytosed across the blood–ocular barrier by neonatal Fc receptors (FcRn), the expression of which was demonstrated in various intraocular structures, including those constituting the blood–ocular barrier [10]. IgG might also reach these intraocular structures through breaches in the blood–ocular barrier. Alternatively, the presence of IgG in the eye could be a result of local IgG production. Murray et al. [8] found increased IgG: albumin relative concentration ratios in patients with Fuchs heterochromic cyclitis and senile cataract, which were attributed to local synthesis. They suggested that B lymphocytes crossing the blood–ocular barrier were the cells responsible for IgG production. Until recently, it was widely accepted that mature B lymphocytes and plasma cells were the only cell types capable of Ig production. However, in the last few years, other cell types, including neoplastic cells and neurons of the brain have also been found to synthesize IgG [11–13].
However, the functions of IgG produced by non-lymphocytes have not been well elucidated. In cancers, tumor-derived IgG is thought to promote the growth and survival of cancer cells [13]. In the brain, IgG might act as a self-protecting factor via a mechanism of enhancing microglial endocytosis and release of TNF-α or by neutralizing complement factors [14, 15]. In the eye, a prolongation of the brain, TNF-α and the activation of the complement cascade play a key role in pathogenesis of age-related macular degeneration (AMD), the leading cause of blindness in people over 50 in the Western world [16–18]. As in the brain, IgG may also function as a self-protecting factor and be involved in the development of AMD.
Here, we show that IgG in the eye could be the result of local production by intraocular cellular elements with immunohistochemistry (IHC), in situ hybridization (ISH), and RT-PCR on eye tissues of humans and mice. Widespread expression/production of IgG was detected in the corneal epithelium and endothelium, ciliary epithelium, and retinal ganglion cells of both the human and the murine eyes. Furthermore, FcRn was detectable in corneal epithelium and endothelium, ciliary epithelium, and retinal vascular endothelium. Fc gamma receptors (FcγRs) were detected in the pigment epithelium of the iris and processes and stroma of the ciliary body only.
Materials and methods
Tissue samples
Eight human eye globes were obtained from the Eye Bank of Weifang Medical University (Weifang, PR China). These eye globes had been prepared within 12 h from postmortem donors (five males and three females, mean age 22.6 years, range 4–46 years) after their death of traffic accidents, and they all had no history of ocular disease. Normal ocular condition was confirmed by examination of the donor’s blood sample and evaluation with slit-lamp, specular microscope, and ophthalmofundoscope. Integrity of the eye globe sample was also ensured by gross anatomical and microscopic examinations. Surgically excised tonsil tissues were used as positive controls (Weifang Medical University).
Ten ICR, ten C57, ten Balb/C, ten severe combined immune deficient (SCID), and ten µ chain mutated (µMT) mice were purchased at the age of 6–7 weeks from the Experimental Animal Center of Peking University Health Science Center. Use of mice was approved by the Animal Care and Use Committee of PUHSC.
Immunohistochemistry
Human eye globes were punctured with an 18-gauge needle to make the internal components accessible to the fixation. Under anesthesia with 2% nembutal (45 mg/kg), mouse eye globes were isolated, and punctured with a 30-gauge needle. The dissected human and murine eye globes were fixed in 4% paraformaldehyde and embedded in paraffin. Lenses were eliminated before embedding. IHC was performed on consecutive paraffin-embedded tissue sections (4 µm thick) as previously described [19].
For IHC staining of human eyes, tissue sections were incubated with primary monoclonal mouse anti-human antibodies to IgG heavy chain (Igγ, 1:1,500, Sigma, St. Louis, MO, USA), Ig kappa light chain (Igκ, 1:500, Abcam, Cambridge, MA, USA), Ig lambda chain (Igλ, 1:100, Zymed, San Francisco, CA, USA), CD16 (FcγRIII), CD32 (FcγRII), and CD64 (FcγRI) (1:50; Santa Cruz, Santa Cruz, CA, USA) and polyclonal goat anti-human antibody to FcRn (1:50; Santa Cruz). Horseradish peroxidase-conjugated anti-mouse/rabbit IgG (PV9000 immunohistochemistry Kit, Zymed Laboratories) or anti-goat IgG (PV9003 Immunohistochemistry Kit, Zymed Laboratories) was added as the secondary antibody. Human tonsil tissues were used as positive controls. 3-amino-9-ethyl-carbazole (AEC) was used for visualization of signals. PBS substituted for the primary antibody was employed as a negative control.
For IHC staining of murine eye tissue sections, primary goat anti-mouse polyclonal antibodies to IgG Fc fragment (1:200, Bethyl, Montgomery, TX, USA), IgG Fab fragment (1:200, Bethyl) whole molecular IgG (1:100, Jackson ImmunoResearch, West Grove, PA, USA), were used. Horseradish peroxidase-conjugated anti-goat IgG (PV9003) was added as the secondary antibody. Spleens of ICR mice served as positive controls.
RNA extraction, cDNA synthesis, and PCR
Mice were anesthetized with nembutal and perfused with PBS. Eye globes were collected via enucleation and total RNA was extracted from these eye globes following elimination of the conjunctiva and lens using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Human eye globes were dissected into cornea, ciliary body, retina and retinal pigment epithelium (RPE) under a dissection scope (SMZ-B2, Zhengzhou, China). Total RNA was isolated in each structure separately using TRIzol reagent. RQ1 Rnase-Free Dnase (Promega, Madison, WI, USA) was applied to exclude DNA contamination. Five micrograms of total RNA was reverse transcribed with oligo (dT)18 primer (Fermentas, Burlington, Ontario, Canada) using SuperScript III Reverse Transcriptase (Invitrogen) according to the manufacturers’ instructions. The reaction (RT) was performed at 55°C for 60 min.
Semi-nested RT-PCR or RT-PCR was performed as described previously [12]. In mouse ocular samples, mouse IgG heavy chain (IGHG) and Igκ mRNA transcripts were analyzed using RT-PCR or semi-nested RT-PCR (IGHG: RT-PCR, Igκ: semi-nested). In human eye samples, nested or semi-nested RT-PCR was carried out to amplify the constant region of human Igγ (IGHG1), the VDJ segment of Igγ (VDJн), Igκ, Igλ, activation-induced cytidine deaminase (AID), recombination activating gene -1 (RAG1), and recombination activating gene -2 (RAG2). In total RNA extracted from both human and mouse eye samples, CD19/20 was amplified to evaluate possible contamination by B lymphocytes and amplification of 18s rRNA or β-actin was used as an endogenous reference. Fresh mouse spleen and human tonsil tissues were used as positive controls. Details of PCR amplifying reactions are presented for each transcript in Supplementary Table 1 [11–13].
All final products were analyzed by agarose gel (2%) electrophoresis, purified with the Universal DNA purification kit (Tiangen Biotech, Beijing, China) and their identities were confirmed by DNA sequencing. Mouse Igκ PCR products were cloned into a pGEM-T vector (Tiangen, Beijing, China). Three clones were randomly chosen and subjected to sequencing by ABI 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA). The sequences of these three clones were then compared to published sequences in BLAST of the GenBank of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST). The same procedure was performed for human VDJн PCR products.
cRNA probe preparation and in situ hybridization
cRNA probes against IGHG1 and IGHG were prepared as described previously [19]. The tissue sections used for ISH were consecutive to the ones used for IHC. ISH was performed on human and mouse ocular tissue sections as described previously [11, 12]. Mouse spleen and human tonsil tissues were used as positive controls. Application of the corresponding sense probe was used as a negative control.
Results
Expression of IgG in human and murine eye tissues
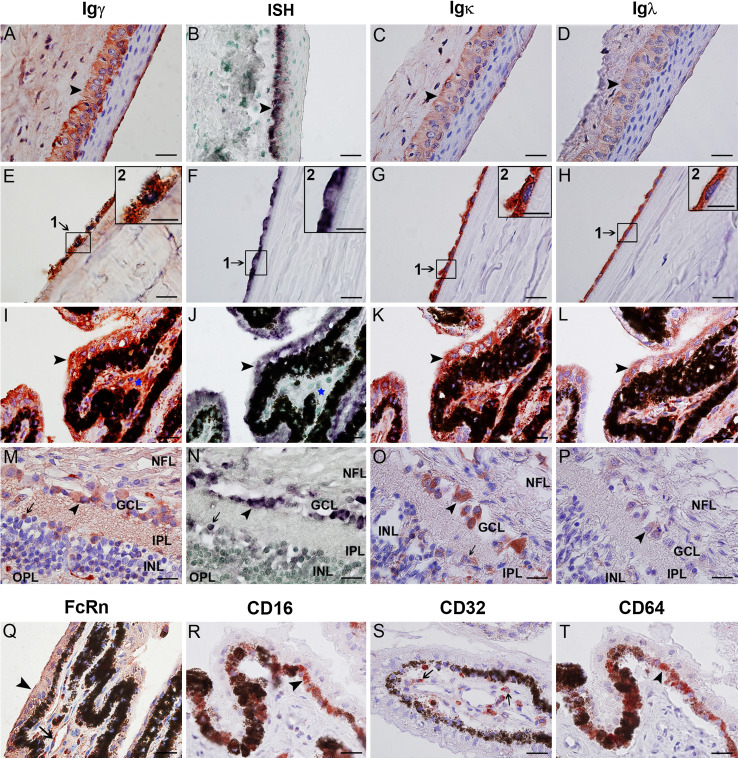
To study the distribution of IgG in the human eye, IHC with antibodies to Igγ, Igκ, and Igλ was performed on ocular tissues obtained from human autopsies. Igγ, Igκ, and Igλ were all detected in the human eye (Fig. 1). In the cornea, IgG protein was detectable in the monolayer of endothelial cells, the corneal stroma (Fig. 1e, g, h), and the basal columnar epithelial cells characterized by large oval nuclei (Fig. 1a, c, d). Consecutive sections were used to evaluate IgG expression in the iris and ciliary body. Co-expression of Igγ, Igκ, and Igλ was found in both the outer epithelial and the inner pigmented epithelial layer. However, despite identical staining procedures, the immunoreactivity of Igλ was slightly less intense than that of Igγ and Igκ (Fig. 1i, k, l). In the sclera and choroid, IgG protein was localized in the lumina of blood vessels and in the connective tissue. In the retina, Igγ immunoreactivity was found in ganglion cells, as well as in some cells of in the inner nuclear layer, the inner plexiform layer, and the nerve fiber layer (Fig. 1m, o, p). Using consecutive sections, co-expression of Igγ, Igκ, and Igλ was demonstrated in the same cells. In the tonsil tissue, Igγ, Igκ, and Igλ were localized to the cytoplasm of B lymphocytes of the germinal center. In the negative control, in which PBS was substituted for the primary antibody, no positive signal was found.
Fig. 1.
Expression and distribution of IgG and its receptors in the normal human eye. a–h Positive immunostaining of Igγ (a, e), Igκ (c, g), and Igλ (d, h) antigens, as well as positive IGHG1 mRNA signals (b, f) are detected in the cytoplasm of the basal cells of the epithelial layer (arrow heads) and the cells of the endothelial monolayer (arrows) of the cornea. Weak positive signals are detectable in the tear film covering the outer surface of cornea. In e, f, g, h, the insert shows a higher magnification of the selected area. Scale bars, 10 µm (insert, 20µm). i–l Consecutive sections showing coexpression of Igγ (i), Igκ (k), Igλ (l) and IgG mRNA (j) in the ciliary body of the human eye. The same epithelial cell is indicated in consecutive sections by black arrow heads. Both ISH and IHC show positive staining in both layers of the ciliary epithelium, while only IHC and not ISH shows positive signals in the ciliary stroma (indicated by blue stars). Scale bars, 20 µm. m–p Consecutive sections of retina. Igγ, Igκ, Igλ, and IGHG1 mRNA are all detected in a number of ganglion cells (arrow heads) and in a few cells of the inner nuclear retinal layer (arrows). q FcRn was detected in the epithelium (arrow heads) and the microvascular endothelium (arrow) of ciliary body. r–t FcγRs expression in human eye. FcγRIII (CD16, r) and FcγRI (CD64, t) are extensively expressed in PE cells (inner layer, arrow heads), and FcγRII (CD32) is detectable in a limited number of cells in the ciliary stroma (s, arrows). Scale bars, 20 µm. NFL nerve fiber layer, GCL ganglion cell layer, IPL inner plexiform layer, INL inner nuclear layer, OPL outer plexiform layer
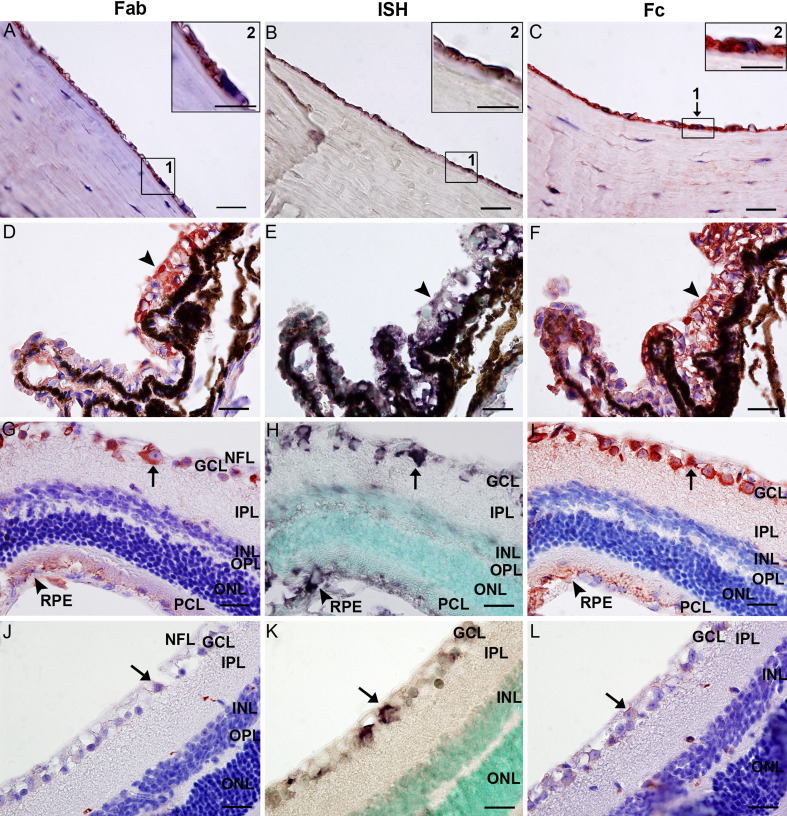
To investigate the expression of IgG in the murine eye, IHC with antibodies to IgG Fab fragment, IgG Fc fragment, and whole molecular IgG was performed on ocular tissue sections of ICR, C57 and Balb/c mice. The distribution pattern in the murine eye shared many similarities with that of the human eye. IgG Fab and Fc fragments, as well as whole molecular IgG were detected in the cornea, iris, ciliary body, retina, choroid and the sclera of eye samples from all mouse strains tested (Fig. 2 and Supplementary Fig. 1). The immunoreactivity had a cytoplasmic localization. In the cornea, IgG positive signals were clearly visible in the cytoplasm of endothelial cells, whereas epithelial cells showed no staining and cells in the substantia propria and stroma demonstrated only very weak positivity (Fig. 2a, c; Supplementary Fig. 1a, d). In the iris, positive IgG staining was found in the iris pigmented epithelium (IPE) and the stroma. In the ciliary body, IgG proteins were detected in the non-pigmented and pigmented epithelia (CPE), and the stroma (Fig. 2d, f; Supplementary Fig. 1b, e). In the retina, strong immunoreactivity was present in ganglion cells and in the retinal pigment epithelium (RPE), whereas cells in the nerve fiber layer and the photoreceptor outer segment layer showed only weak staining (Fig. 2g, i; Supplementary Fig. 1c, f). In the sclera and choroid, IgG proteins were localized to the same structure as those seen in the human eye. Despite the presence of melanin in the pigmented epithelium (including IPE, CPE, and RPE) of C57 mice (Fig. 2d, f; Supplementary Fig. 1b, e), it was relatively easy to distinguish the red IHC positive signals from the brown pigment granules. The IgG distribution in eye samples of ICR and Balb/c mice was readily visible because of the lack of melanin granules in the pigmented epithelium (Fig. 2g, l; Supplementary Fig. 1c, f).
Fig. 2.
IgG expression in the murine eye. a–c Positive IgG Fab a and IgG Fc c immunostaining and positive IGHG ISH signals b, distributed in the cytoplasm of corneal endothelial cells. Inserts show higher magnification. Scale bars, 20 µm (inserts, 20 µm). d–f Consecutive tissue sections showing IgG Fab d, IGHG mRNA e and IgG Fc f in the ciliary body of a C57 mouse. Positive IgG signals were found in the ciliary epithelial cells (black arrows). Scale bars, 20 µm. g–i Consecutive sections of the retina of an ICR mouse without melanin in its pigment epithelium. Positive IgG Fab g and Fc i immunoreactivity is detected in ganglion cells (arrows), the nerve fiber, and RPE (arrow heads). Positive IGHG mRNA signals are only detectable in ganglion cells and RPE cells. Serial sections show that IgG protein and mRNA colocalize in the cytoplasm of ganglion cells (see arrows for example). Scale bars, 20 µm. j–l Consecutive sections of the retina of a μMT mouse showing weak immunoreactivity in a few ganglion cells. Arrows indicate the same single ganglion cell in consecutive sections. Scale bars, 20 µm. NFL nerve fiber layer, GCL ganglion cell layer, IPL inner plexiform layer, INL inner nuclear layer, OPL outer plexiform layer, ONL outer nuclear layer, PCL photoreceptor cell layer, RPE retinal pigment epithelium
To exclude possible false-positive results caused by B lymphocyte contamination, both SCID and µMT mice were used. In SCID mice, which are B and T cell deficient [20], IgG protein was not detected in any intraocular structure (Supplementary Fig. 1m). In contrast, in µMT mice, which have no mature B lymphocytes due to a targeted disruption in the membrane exon of the Igµ chain gene [21], IgG proteins were expressed abundantly in the corneal endothelium and ciliary epithelium, and only weakly in retinal ganglion cells (Fig. 2j, l). The specificity of immunostaining was validated by the results in the positive and negative controls (Supplementary Fig. 1g–j).
IgG mRNA detection by ISH
Positive immune staining only confirms the presence of IgG at the protein level, but not necessarily indicates that IgG was actually produced within the eye. We therefore performed ISH to detect IgG mRNA transcripts. ISH was carried out on tissue sections consecutive to those used for IHC with antibodies to human Igγ and murine IgG Fab fragment. The human and murine probes were specific for the constant region of human IGHG1 and murine IGHG, respectively.
In the human eye, the distribution of IGHG1 mRNA showed a complex staining pattern. In the cornea, the purple signals were located in the cytoplasm of endothelial cells and basal epithelial cells (Fig. 1b, f). The corneal stroma did not show any positive signal. In the ciliary body and iris, IGHG1 mRNA was detected in the cytoplasm of cells of both epithelial layers, but not in the highly vascularized stroma (Fig. 1j). In the retina, positive signals were seen in ganglion cells, RPE, and some cells in the inner nuclear layer, the exact cell type of which cannot be ascertained, but not in the nerve fiber layer or the inner plexiform layer (Fig. 1n). Consecutive tissue sections showed co-expression of IGHG1 mRNA transcripts and Igγ proteins in the corneal endothelium, corneal epithelium, ciliary epithelium, retinal ganglion cells, RPE, and some unidentified cells in the retinal inner nuclear layer. In a number of structures that did show positive Igγ immunoreactivity, including the choroid, sclera, corneal stroma, ciliary body, and the retinal nerve fiber layer, no positive IgG mRNA signal was detectable. The negative controls validated the specificity of the ISH reaction. In the human tonsil tissue, used as a positive control, hybridization with the antisense probe yielded positive signals in the cytoplasm of B lymphocytes.
In the murine eye, IGHG mRNA was detected in the cytoplasm of cells of the following structures: corneal endothelium (Fig. 2b), iris epithelium and IPE, ciliary epithelium and CPE (Fig. 2e), as well as retinal ganglion cells and RPE (Fig. 2h). On consecutive sections, we further demonstrated co-expression of IGHG mRNA and IgG Fab protein in the same cells. However, in a number of structures that had shown positive IgG Fab IHC staining, no positive mRNA signal was detectable. These ocular structures included the choroid, sclera, substantia propria of the cornea, stroma of the ciliary body, nerve fiber layer of the retina and the outer segments of the photoreceptor layer. In eye samples of SCID mice, no positive signal was detected (Supplementary Fig. 1n), whereas in µMT mouse, Igγ mRNA transcripts were found to co-localize with IgG Fab proteins (Fig. 1j, k, l). In splenic tissue of ICR mice, which were used as positive controls, the same probes hybridized positively in the cytoplasm of B lymphocytes in the white pulp (Supplementary Fig. 1k). In the negative control, in which a sense probe was used instead of the anti-sense probe, no positive signal was detected (Supplementary Fig. 1l).
Distribution of FcRn and FcγRs in eye of human by IHC
Polyclonal goat anti-human antibody was used to detect FcRn, whereas monoclonal mouse anti-human antibodies to CD64, CD32, and CD16 were applied to detect FcγRI, FcγRII, and FcγRIII, respectively. The results are shown in Fig. 1. FcRn was detectable in the basal columnar epithelium and the endothelium of the cornea, in the two layers of the epithelium of the ciliary body (Fig. 1q), the epithelium of the iris, and the endothelial cells of the blood vessels present in the intraocular structures. FcγRI (Fig. 1t) and FcγRIII (Fig. 1r) were expressed in the cytoplasm and membrane of pigment epithelium cells. FcγRII (Fig. 1s) was only detected in some cells located in the ciliary stroma.
Detection of IgG associated transcripts
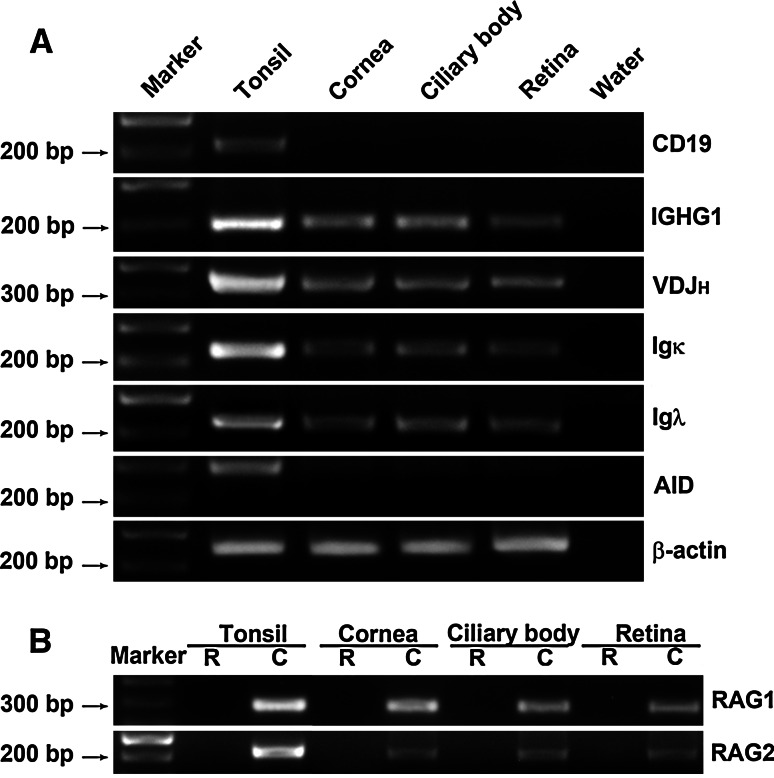
To confirm IgG expression in the individual structures of the human eye, nested or semi-nested RT-PCR was employed to identify Igγ1, Igκ, and Igλ transcripts in samples of cornea, ciliary body and retina. Igγ1, Igκ, and Igλ were positively amplified from all three intraocular structures (Fig. 3a). The expression levels in these samples appeared to be lower than that in tonsil tissue samples, which were used as positive controls. CD 19 was only amplified from the positive control but not from any of the intraocular structures, again excluding the possibility of lymphocyte contamination in the samples.
Fig. 3.
RT-PCR amplification of IgG mRNA transcripts in the human eye. a IGHG1 (201 bp), VDJн (360–400 bp), Igκ (231 bp), and Igλ (223 bp) transcripts amplified from the cornea, ciliary body and retina of the normal human eye. AID transcripts are not detectable. No CD 19 band is identified. DEPC-treated water was used as a negative control. b RAG1 and RAG2 transcripts amplified from the normal human eye. R DNase treated RNA as template (negative control); C cDNA as template
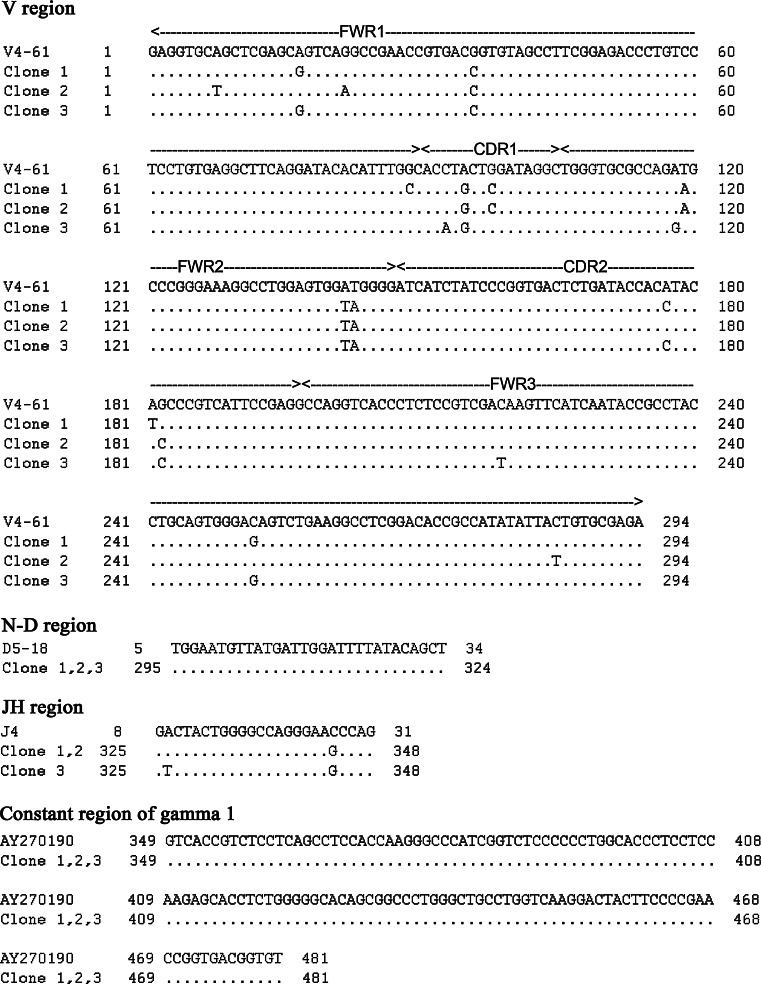
In B lymphocytes, recombination activating gene 1 and 2 (RAG 1 and RAG 2) are essential for V-(D)-J recombination, whereas activation-induced cytidine deaminase (AID) is required for class switch recombination and somatic hypermutation [22, 23]. To assess whether similar processes take place in the IgG production in the eye, we performed nested or semi-nested RT-PCR to identify mRNA transcripts of RAG1, RAG2, AID, and VDJн, which also includes a segment of about 120 bp of the constant region of IgG1. VDJн, RAG1 and RAG2 were positively detected in the same eye structures (Fig. 3a, b), indicating that V-(D)-J recombination might take place in the human eye. AID was, however, not detected (Fig. 3a). The identities of all amplified products were confirmed by DNA sequencing. Three clones of VDJH were sequenced and they showed high homology to the V4-61 germline sequence with a mutation ratio of 2.7% (Fig. 4). The constant region showed 100% homology to AY270190 further confirming the identity of the locally produced IgG. As we cloned only a limited number of recombinants, no conclusions can be drawn regarding the clonality of IgG molecules produced by the eye.
Fig. 4.
Results of sequence-blasting of IGHG1 mRNA transcripts amplified from human eye samples. The VDJн sequences of all three clones show high homology to the sequence of the V4-61 gene (mutation rate 2.7%)
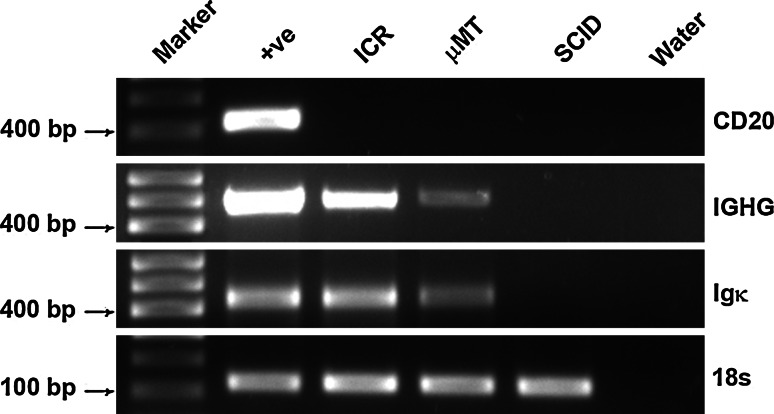
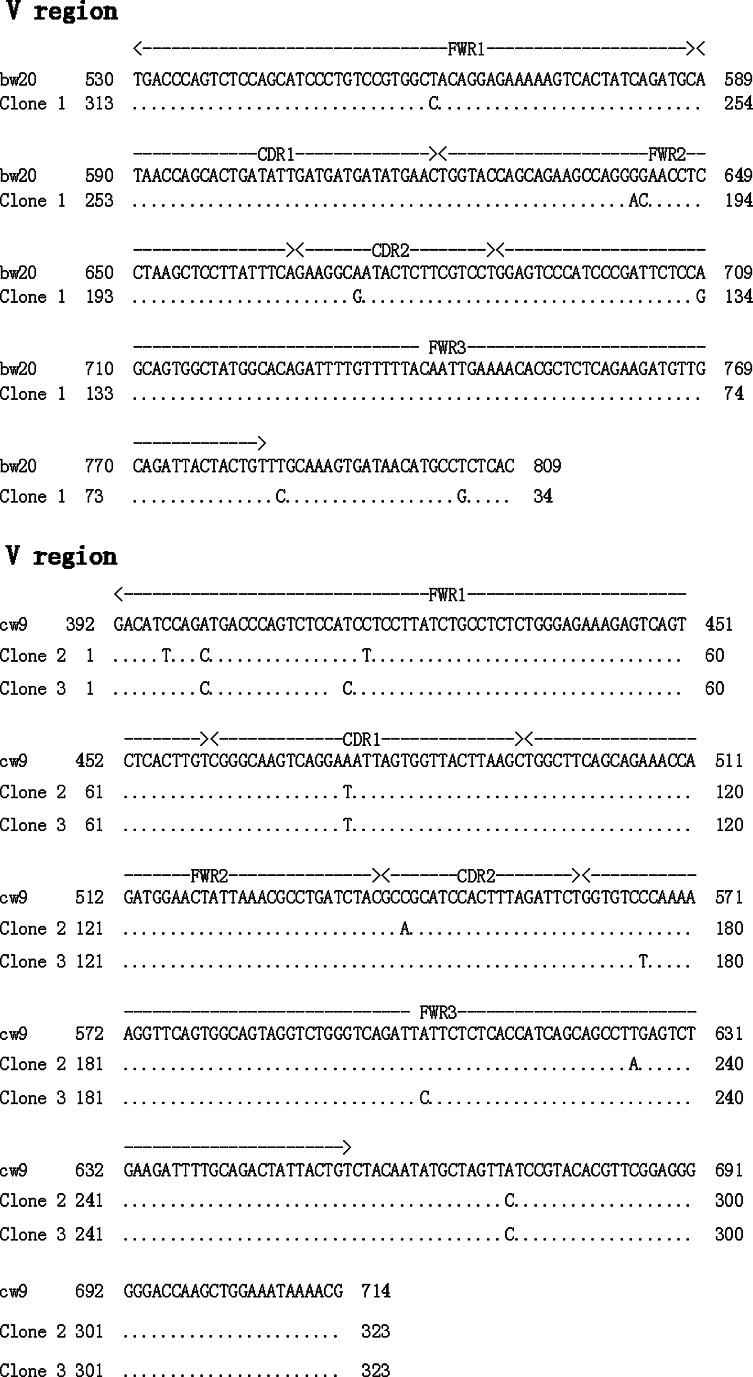
To further investigate the endogenous production of IgG in the eye, RT-PCR was performed on total RNA extracted from murine eye globes. Igγ and Igκ were both positively amplified from eye samples of ICR and µMT mice but not from those of SCID mice (Fig. 5). Transcript levels of Igγ and Igκ appeared to be expressed at lower levels in the eyes of µMT mice than in those of ICR mice. Spleen tissues of ICR mice were used as positive controls. Transcripts of CD 20, a marker for B lymphocytes, were only detected in the positive control but not in eye tissues of ICR-, µMT-, or SCID-mice, thereby firmly excluding possible contamination by lymphocytes in the tissue samples. The constant region of the Igγ PCR product was sequenced and found to be identical to that of the mouse MMU65534 Igγ germline in BLAST. Three clones of the Igκ variable region were sequenced (Fig. 6) and compared with mouse Igκ variable regions published in BLAST (http://www.ncbi.nlm.nih.gov/BLAST.cgi.). The VκJκ recombinant of 1 clone showed very high homology to bw20 germline (97.5%), with a mutation ratio of 2.5%, whereas the VκJκ recombinant of the other 2 clones showed 97.8% homology to cw9 germline with a mutation ratio of 2.2%. The constant region of Igκ showed 100% homology to AK157380.
Fig. 5.
RT-PCR of murine eye samples. Both Igγ (482 bp) and Igκ (410 bp) transcripts are amplified from eye samples of ICR and µMT mice. No bands are observed in eye samples of SCID mice. Under identical conditions, the band intensity of µMT mouse appears much weaker than that of ICR mice. The band of ICR spleen sample shows the strongest intensity. In none of the eye samples CD 20 band is detected in eye samples. As a blank control, DEPC-treated water was used in place of RNA
Fig. 6.
Results of sequence-blasting of three Igκ mRNA transcripts amplified from ICR mouse eye samples. The variable region of Igκ (VκJκ) of two clones showed 97.5% homology to that of bw20. VκJκ of the other clone showed high homology to that of cw9, with a mutation ratio of 2.16%
Discussion
To our knowledge, this is the first study demonstrating local IgG synthesis by intraocular structures of human and mouse eyes. Previously, intraocular presence of IgG was thought to result from IgG entering the intraocular structures through breaches in the blood–ocular barrier or from receptor-mediated uptake. Local production of IgG by the eye itself has never been suspected or proven. Murray et al. [8] provided preliminary evidence for local synthesis of IgG1 in Fuchs heterochromic cyclitis. However, they assumed that B lymphocytes were the source of IgG1. In our study, in addition to detecting IgG in the eye, we detected expression of IgG mRNA in a number of ocular cell types including pigmented epithelial cells, corneal epithelial and endothelial cells, iris and ciliary epithelial cells, and retinal ganglion cells. It is noteworthy that in a number of sites including the corneal and ciliary stroma, the retinal nerve fiber layer, the retinal inner plexiform layer and photoreceptor outer segment layer, IgG could be detected at the protein level but not at the mRNA level. It is conceivable that following its production by corneal endothelial, corneal epithelial, ciliary epithelial, or ganglion cells, IgG passively diffuses or is actively transported into these tissues. Amplification of Igγ, Igκ, and Igλ mRNA transcripts further confirmed the ability of intra-ocular tissues to produce IgG. Sequencing of the rearranged genes of the variable regions of Igγ and Igκ was confirmed by blasting, which further supported the assumption that V(D)J recombination occurs in the eye. The amplification of RAG1 and RAG2, two enzymes constituting the RAG endonuclease essential for V(D)J recombination in B lymphocytes, provided additional evidence for such an event. Sequencing of the rearranged genes of the variable regions of Igγ and Igκ, further supported the assumption that V(D)J recombination occurs in the eye. The sequenced variable regions showed only a few point mutations. The lack of AID expression in the ocular tissues might explain this paucity of point mutations. The negative finding of IgG expression in the ocular tissues of SCID mice was due to a mutation in the gene encoding the catalytic subunit of the DNA-dependent protein kinase [20]. This enzyme is required for the repair of chromosomal breaks resulting from attempted V(D)J recombination and thus defective activity of this enzyme precludes successful completion of the V(D)J recombination process in pre B cells. As IgG was not expressed in mice deficient of this enzyme, one could speculate that V(D)J recombination is also required for the synthesis of IgG by ocular cells. Analogously, the absence of IgG expression in ocular cells of SCID mice could also be attributed to the abrogation of V(D)J recombination. In contrast to SCID mice, µMT mice did show IgG expression in ocular tissues and the distribution appeared to be similar to that in immune competent mice. Compared to SCID mice, the development of B lymphocytes in µMT mice is arrested at a later stage of B cell differentiation, i.e., the pre-B cell stage [21]. This arrest is caused by a mutation in one of the exons of the gene encoding the µ chain constant region resulting in the lack of mature B lymphocytes capable of synthesizing Igs [21]. The fact that IgG was detected in the eye of the µMT mouse indicates that intra-ocular IgG production is not dependent on the presence of mature B cells, further confirming that ocular cells themselves are capable of IgG production. In addition to the eye, we have also detected the production of IgG in other “immune privileged sites” such as the testes and placenta and have evidence to show that locally produced IgG might be involved in self-protection and “immune privileged” status of testes and immune tolerance of the placenta and the fetus. Manuscripts reporting these discoveries will be published separately.
FcRn is an important Ig receptor widely expressed in epithelium of several organs including the placenta, the intestinal tract and the breast [24, 25]. In the nervous system, it is expressed in the endothelium of brain blood vessels [26], where it is involved in the transport of IgG from the brain to the circulation across the blood–brain barrier [27]. Intravitreally injected IgG permeated the blood–retina barrier into the blood circulation in the normal retina of mice, but not in FcRn knockout mice, suggesting that FcRn is crucial for reverse transcytosis of IgG molecules [28]. In addition, FcRn protects IgG from degradation by proteinase, thus prolonging the half-life of IgG [29]. Here we demonstrated the expression of FcRn in ocular epithelial and endothelial cells in close proximity to the aqueous humor and tears and in the microvascular endothelium. It is possible that FcRn mediates the active secretion of locally produced IgG into the aqueous humor and tears, as well as the reverse transport of IgG into the circulation.
FcγRs are also essential factors in immune responses. There are three kinds of FcγRs. They are FcγRI (CD64), FcγRII (CD32) and FcγRIII (CD16), and each can be divided into several subtypes according to their gene locus [30]. Conventionally, FcγRs are expressed in most effector cells of the immune system, notably monocytes, macrophages, NK cells, mast cells, eosinophils, neutrophils and platelets. Responses triggered by IgG binding to FcγRs include phagocytosis, endocytosis, degranulation, antibody-dependent cell-mediated cytotoxicity (ADCC), transcription of cytokine genes, and release of inflammatory mediators [31–33]. In immune privilege sites, FcγRI expression has been demonstrated in microglial cells of CNS [34] and FcγRs on Schwann cells in human peripheral nerve [35]. In the present study, FcγRI and FcγRIII were found to be expressed in the PE lining the iris, ciliary body and retina. The PE is the most important protective structure in ocular physiology, whereas it is also thought to contribute to the maintenance of the immune-privileged status of the eye [36]. Given the extensive expression of IgG and FcγRs in the PE, it appears highly likely that endogenously produced IgG might play a protective role in this “immune-privileged” site. For intraocular cells (such as ciliary stromal cells) that were positive for IgG protein but negative for IgG mRNA or IgG receptors, IgG protein might be diffused into the cells from the surrounding environment via pinocytosis with help of cationic peptides [37, 38] or other unidentified mechanisms.
The functional significance of intraocularly produced IgG warrants further investigation. In cancers, tumor-derived IgG is thought to promote the growth and survival of cancer cells. This assumption is based on the findings of Qiu et al. showing that blockade of IgG by either antisense DNA or anti-human IgG antibody increased apoptosis and inhibited growth of cancer cells in vitro and that intraperitoneal treatment with anti-IgG antibody of nude mice bearing HeLa MR tumors led to significant reduction of tumor size [13]. In the brain, a self-protecting function is also attributed to IgG. Hulse et al. [14] demonstrated that nonspecific monomeric IgG at physiological concentrations was neuroprotective in the rat brain via a mechanism that enhanced microglial endocytosis and release of TNF-α. Arumugam et al. [15] found that intravenous administration of Ig offered significant protection to neurons within ischemic regions in mice suffering from cerebral ischemia by neutralizing complement factors (C3 and C5). TNF-α and dysregulation/activation of the complement system (C3, CFH and C2, etc., especially the local complement system in the RPE-choroid complex) is thought to play a major role in the development of AMD, the leading cause of blindness in the western world [16–18]. As in the brain, IgG produced by ganglion and RPE cells of the retina could also exert a self-protecting function and stimulate growth and regeneration of such cells and may be involved in the pathobiology of AMD. In addition, IgG could also directly affect neuronal function. Binding of antigen–antibody complexes to FcγRIs expressed on dorsal root ganglia was found to lead to activation of such cells in vitro, as evidenced by the increased concentration of intra-cellular Ca2+ and the release of the neurotransmitter substance P [39].
In recent years, several immunological molecules (e.g., TNF-α, IL-6, IL-8, IL-18, and IFN-γ) that were traditionally thought to be exclusively expressed by immune cells were found to be produced by neural cells and to influence nerve cell functions [40]. Vice versa, several neurotrophic factors were found to modulate immune responses [40]. Being a classical immune mediator on the one hand, and being produced by ganglion cells on the other, IgG could be added to this list of immunological molecules mediating neuro-immune crosstalk.
Locally produced IgG could also play a role in preventing induction of immune responses by eliminating undesired antigens and invading pathogens. In a mouse model for Alzheimer’s disease, IgGs directed against amyloid β-peptide (Aβ) antigens formed immune complexes with Aβ antigens and these antigen–antibody complexes were subsequently transcytosed across the blood–brain barrier into circulation, the process of which was mediated by FcRn [41]. Intravitreally injected IgG was demonstrated to be transported across the blood–retinal barrier into the systemic circulation in a Fc-dependent manner in a rat model [27, 28]. Future studies need to investigate whether endogenous IgG also plays a role in the elimination of potentially harmful antigens from the eye.
In conclusion, our findings established that IgG can be produced locally by the pigmented epithelium, the corneal epithelium and endothelium, iris and ciliary epithelium, and retinal ganglion cells of human and mouse eyes. FcγRs possibly regulate local immune responses, whereas FcRn might mediate IgG transport in the eye. Endogenously produced IgG most likely plays a significant role in protecting the eye but further studies are needed to elucidate its exact function.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (code: 30570686), Project 111 (B07001) of the Ministry of Education, China to Dr. Jiang Gu.
Conflicts of interest
No author had any conflict of interest. Dr. Jiang Gu had full access to all the data in this study and had final responsibility for the decision of publication.
Abbreviations
- AEC
3-amino-9-ethyl-carbazole
- AH
Aqueous humor
- AID
Activation-induced cytidine deaminase
- AMD
Age-related macular degeneration
- CPE
Ciliary pigmented epithelia
- FcγRs
Fc gamma receptors
- FcRn
Neonatal Fc receptors
- Igγ
IgG Gamma chain γ
- Igκ
Ig Kappa light chain κ
- Igλ
Ig Lambda chain λ
- IGHG
Mouse IgG heavy chains
- IGHG1
Constant regions of human IgG1
- IHC
Immunohistochemistry
- IPE
Iris pigmented epithelium
- ISH
In situ hybridization
- µMT
µ chain mutated
- RAG1
Recombination activating gene -1
- RAG2
Recombination activating gene -2
- RPE
Retinal pigment epithelium
- SCID
Severe combined immune deficient
- VDJн
VDJ segment of Igγ
Footnotes
The first two institutions contributed equally to this study.
References
- 1.Ferguson TA, Griffith TS. A vision of cell death: Fas ligand and immune privilege 10 years later. Immunol Rev. 2006;213:228–238. doi: 10.1111/j.1600-065X.2006.00430.x. [DOI] [PubMed] [Google Scholar]
- 2.Taylor AW. Ocular immune privilege. Eye (Lond) 2009;23:1885–1889. doi: 10.1038/eye.2008.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee HO, Herndon JM, Barreiro R, Griffith TS, Ferguson TA. TRAIL: a mechanism of tumor surveillance in an immune privileged site. J Immunol. 2002;169:4739–4744. doi: 10.4049/jimmunol.169.9.4739. [DOI] [PubMed] [Google Scholar]
- 4.Allansmith MR, Whitney CR, McClellan BH, Newman LP. Immunoglobulins in the human eye. Location, type, and amount. Arch Ophthalmol. 1973;89:36–45. doi: 10.1001/archopht.1973.01000040038010. [DOI] [PubMed] [Google Scholar]
- 5.Waldrep JC, Schulte JR. Characterization of human IgG subclasses within intraocular compartments. Reg Immunol. 1989;2:22–32. [PubMed] [Google Scholar]
- 6.Von Sallmann L, Moore DH. Electrophoretic patterns of concentrated aqueous humor of rabbit, cattle and horse. Arch Ophthal. 1948;40:279–284. doi: 10.1001/archopht.1948.00900030285005. [DOI] [PubMed] [Google Scholar]
- 7.Sen DK, Sarin GS, Saha K. Immunoglobulins in human aqueous humour. Br J Ophthalmol. 1977;61:216–217. doi: 10.1136/bjo.61.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray PI, Hoekzema R, Luyendijk L, Konings S, Kijlstra A. Analysis of aqueous humor immunoglobulin G in uveitis by enzyme-linked immunosorbent assay, isoelectric focusing, and immunoblotting. Invest Ophthalmol Vis Sci. 1990;31:2129–2135. [PubMed] [Google Scholar]
- 9.Bloch-Michel E, Lambin P, Debbia M, Tounsi Y, Trichet C, Offret H. Local production of IgG and IgG subclasses in the aqueous humor of patients with Fuchs heterochromic cyclitis, herpetic uveitis and toxoplasmic chorioretinitis. Int Ophthalmol. 1997;21:187–194. doi: 10.1023/A:1005909331778. [DOI] [PubMed] [Google Scholar]
- 10.Kim H, Fariss RN, Zhang C, Robinson SB, Thill M, Csaky KG. Mapping of the neonatal Fc receptor in the rodent eye. Invest Ophthalmol Vis Sci. 2008;49:2025–2029. doi: 10.1167/iovs.07-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J, Sun X, Mao Y, Zhu X, Zhang P, Zhang L, Du J, Qiu X. Expression of immunoglobulin gene with classical V-(D)-J rearrangement in mouse brain neurons. Int J Biochem Cell Biol. 2008;40:1604–1615. doi: 10.1016/j.biocel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Gu J. Immunoglobulin G expression in carcinomas and cancer cell lines. FASEB J. 2007;21:2931–2938. doi: 10.1096/fj.07-8073com. [DOI] [PubMed] [Google Scholar]
- 13.Qiu X, Zhu X, Zhang L, Mao Y, Zhang J, Hao P, Li G, Lv P, Li Z, Sun X, Wu L, Zheng J, Deng Y, Hou C, Tang P, Zhang S, Zhang Y. Human epithelial cancers secrete immunoglobulin g with unidentified specificity to promote growth and survival of tumor cells. Cancer Res. 2003;63:6488–6495. [PubMed] [Google Scholar]
- 14.Hulse RE, Swenson WG, Kunkler PE, White DM, Kraig RP. Monomeric IgG is neuroprotective via enhancing microglial recycling endocytosis and TNF-alpha. J Neurosci. 2008;28:12199–12211. doi: 10.1523/JNEUROSCI.3856-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arumugam TV, Tang SC, Lathia JD, Cheng A, Mughal MR, Chigurupati S, Magnus T, Chan SL, Jo DG, Ouyang X, Fairlie DP, Granger DN, Vortmeyer A, Basta M, Mattson MP. Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death. Proc Natl Acad Sci USA. 2007;104:14104–14109. doi: 10.1073/pnas.0700506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campa C, Costagliola C, Incorvaia C, Sheridan C, Semeraro F, De Nadai K, Sebastiani A, Parmeggiani F (2010) Inflammatory mediators and angiogenic factors in choroidal neovascularization: pathogenetic interactions and therapeutic implications. Mediators Inflamm (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- 17.Clark SJ, Bishop PN, Day AJ. Complement factor H and age-related macular degeneration: the role of glycosaminoglycan recognition in disease pathology. Biochem Soc Trans. 2010;38:1342–1348. doi: 10.1042/BST0381342. [DOI] [PubMed] [Google Scholar]
- 18.Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, Hauser MA, Rickman CB, Bok D, Hageman GS, Johnson LV. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29:95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu N, Zhang J, Guo Y, Yang C, Gu J. Cystic fibrosis transmembrane conductance regulator expression in human spinal and sympathetic ganglia. Lab Invest. 2009;89:636–644. doi: 10.1038/labinvest.2009.28. [DOI] [PubMed] [Google Scholar]
- 20.Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 21.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 22.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/S0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 23.McBlane JF, van Gent DC, Ramsden DA, Romeo C, Cuomo CA, Gellert M, Oettinger MA. Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell. 1995;83:387–395. doi: 10.1016/0092-8674(95)90116-7. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Mohanty S, Ganesan LP, Hua K, Jarjoura D, Hayton WL, Robinson JM, Anderson CL. FcRn in the yolk sac endoderm of mouse is required for IgG transport to fetus. J Immunol. 2009;182:2583–2589. doi: 10.4049/jimmunol.0803247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 26.Schlachetzki F, Zhu C, Pardridge WM. Expression of the neonatal Fc receptor (FcRn) at the blood–brain barrier. J Neurochem. 2002;81:203–206. doi: 10.1046/j.1471-4159.2002.00840.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Pardridge WM. Mediated efflux of IgG molecules from brain to blood across the blood–brain barrier. J Neuroimmunol. 2001;114:168–172. doi: 10.1016/S0165-5728(01)00242-9. [DOI] [PubMed] [Google Scholar]
- 28.Kim H, Robinson SB, Csaky KG. FcRn receptor-mediated pharmacokinetics of therapeutic IgG in the eye. Mol Vis. 2009;15:2803–2812. [PMC free article] [PubMed] [Google Scholar]
- 29.Ghetie V, Ward ES. Multiple roles for the major histocompatibility complex class I-related receptor FcRn. Annu Rev Immunol. 2000;18:739–766. doi: 10.1146/annurev.immunol.18.1.739. [DOI] [PubMed] [Google Scholar]
- 30.Ravetch JV. Fc receptors: rubor redux. Cell. 1994;78:553–560. doi: 10.1016/0092-8674(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 31.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 32.Young JD, Ko SS, Cohn ZA. The increase in intracellular free calcium associated with IgG gamma 2b/gamma 1 Fc receptor-ligand interactions: role in phagocytosis. Proc Natl Acad Sci USA. 1984;81:5430–5434. doi: 10.1073/pnas.81.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson CL, Shen L, Eicher DM, Wewers MD, Gill JK. Phagocytosis mediated by three distinct Fc gamma receptor classes on human leukocytes. J Exp Med. 1990;171:1333–1345. doi: 10.1084/jem.171.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Titus JA, Perez P, Kaubisch A, Garrido MA, Segal DM. Human K/natural killer cells targeted with hetero-cross-linked antibodies specifically lyse tumor cells in vitro and prevent tumor growth in vivo. J Immunol. 1987;139:3153–3158. [PubMed] [Google Scholar]
- 35.Kennedy PG, Lisak RP, Raff MC. Cell type-specific markers for human glial and neuronal cells in culture. Lab Invest. 1980;43:342–351. [PubMed] [Google Scholar]
- 36.Sugita S. Role of ocular pigment epithelial cells in immune privilege. Arch Immunol Ther Exp (Warsz) 2009;57:263–268. doi: 10.1007/s00005-009-0030-0. [DOI] [PubMed] [Google Scholar]
- 37.Chugh A, Eudes F, Shim YS. Cell-penetrating peptides: nanocarrier for macromolecule delivery in living cells. IUBMB Life. 2010;62:183–193. doi: 10.1002/iub.297. [DOI] [PubMed] [Google Scholar]
- 38.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 39.Andoh T, Kuraishi Y. Direct action of immunoglobulin G on primary sensory neurons through Fc gamma receptor I. FASEB J. 2004;18:182–184. doi: 10.1096/fj.02-1169fje. [DOI] [PubMed] [Google Scholar]
- 40.Kerschensteiner M, Meinl E, Hohlfeld R. Neuro-immune crosstalk in CNS diseases. Neuroscience. 2009;158:1122–1132. doi: 10.1016/j.neuroscience.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Deane R, Sagare A, Hamm K, Parisi M, LaRue B, Guo H, Wu Z, Holtzman DM, Zlokovic BV. IgG-assisted age-dependent clearance of Alzheimer’s amyloid beta peptide by the blood–brain barrier neonatal Fc receptor. J Neurosci. 2005;25:11495–11503. doi: 10.1523/JNEUROSCI.3697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.