Abstract
Natural killer (NK) cells are innate lymphocytes involved in immunosurveillance through their cytotoxic activity and their capacity to secrete inflammatory cytokines. NK cell activation is necessary to initiate effector functions and results from a complex series of molecular and cellular events. We review here the signals that trigger NK cells and discuss recent findings showing that, besides antigen-presenting cells, T cells can play a central role in the initiation of NK cell activation in lymph nodes.
Keywords: Natural killer cells, Antigen-presenting cells, CD4+ T cells, NK receptors, IL-12, IL-2
Introduction
Natural killer (NK) cells are innate lymphocytes that circulate in the blood, lymphatics and tissues where they sample their environment to detect abnormal cells [1]. Upon stimulation, they kill tumor cells and infected cells thus contributing to tumor surveillance and pathogen clearance [2]. Natural cytotoxicity results from a coordinated series of events which include contact with target cells, adhesion, synapse formation, granule polarization and granule exocytosis [3–6]. NK cells also secrete cytokines and chemokines which regulate and orientate immune responses [7, 8]. Thus, NK cells actively interact with their environment and contribute to the fine tuning of immune responses. To exert their functions, NK cells are stimulated by different mechanisms. Schematically, NK cell activation results from a combination of soluble and contact-dependent signals. It involves cytokines, the engagement of a combination of activating and inhibitory receptors and requires cellular interactions. NK cells constitute quite a heterogeneous population of cells. They express a combination of germline-encoded NK receptors (NKRs) acquired in a stochastic and variegated manner and can display different cytokine receptor profiles [9–11]. Interestingly, some subsets were found to be associated with a specific location (i.e. decidual NK cells or mucosa-associated NK cells) [12–20]. The signals required for their activation differ with their stage of maturation, whether they have been educated, primed or previously activated (memory-like NK cells) [21].
In this review, we will examine the various components triggering NK cell activation. We will not review signaling pathways activated which are extensively described elsewhere [6, 22–25]. Rather, we will focus on signals and cellular interactions necessary to trigger NK cells in secondary lymphoid organs, at the sites of infection or within tumors. Emphasis will be put on new players, CD4+ T cells which have recently been shown to be critical for the regulation of the initiation of NK cell activation in lymph nodes [26].
Mechanisms of NK cell activation: basic principles
In contrast to T cells and B cells which predominantly use a single antigen receptor for their activation, NK cells are triggered by the combined action of cytokines and the engagement of activating and inhibitory receptors. Cytokines which have been involved in NK cell activation in vitro and in vivo include IL-12, IFN-α/β, IL-15, IL-2 and IL-18, [1, 7, 8, 27]. IL-12 is the most potent inducer of IFN-γ [28, 29] whereas type I IFNs are key regulators of NK cell cytotoxicity in the context of viral infections [30]. In addition, IL-2 and IL-15 are implicated in NK cell development, homeostasis and in activating NK cell cytotoxicity and secretion of cytokines [31]. IL-18 was also found to trigger NK cell functions in combination with IL-12 or type I IFNs [32]. Cytokines act individually or in combinations. They also often contribute to augment signaling through activating NKRs.
NK cell recognition of targets is largely dominated by the interaction of germline-encoded activating and inhibitory receptors with ligands expressed by targets [33]. The permanent expression of inhibitory NKRs specific for major histocompatibility complex (MHC) class I molecules counterbalances activating signals and confers “missing self” specificity to NK cell recognition [34–37]. Additional non-MHC ligands binding to inhibitory receptors also contribute to NK cell tolerance to self. This is the case for the mouse Clrb molecule binding to NKR-P1B/D [38, 39] and for human LLT1 binding to NKR-P1A (CD161) which provide an inhibitory signal to NK cells independent of MHC class I molecules ([40–42], and C. Germain and V.M. Braud, unpublished data). Other examples include mouse 2B4 binding CD48 [43], KLRG1 interacting with cadherins [44–47], LAIR-1 binding to collagen [48], siglecs binding to sialic acids [49] or CEACAM1 homophilic interactions [50]. NK cell tolerance to “self” implies that, in the absence of inhibitory signals, NK cells are turned on. Activation is not “spontaneous”, but it depends on the triggering of activating receptors [3]. Some activating NKRs have adapted to recognize “altered or induced” self ligands. This is the case for NKG2D which interacts with stress-induced ligands such as MICA/MICB or ULPBs in humans and Rae1, H60 or MULT-1 in mice [51, 52] or for DNAM-1 which binds to PVR or nectin-2 [53–55]. Activating KIRs may have adapted to more specifically recognize MHC class I molecules loaded with viral peptides [56, 57]. In addition, activating leukocyte immunoglobulin-like receptors (LILRs) which were shown to bind to unfolded HLA molecules may contribute to recognition of infected cells [58]. Other activating receptors interact with pathogen-encoded molecules. NK cells expressing these particular activating NKRs are increased following certain viral infections. Ly49H-expressing NK cells are expanded during MCMV infection as a result of binding to the MCMV-encoded cell surface ligand m157 [59–63]. Influenza hemagglutinin was found to interact with NKp46 receptor and this interaction is critical for the control of the infection [64, 65]. The CD94/NKG2C+ NK cell subset is increased in HCMV-seropositive individuals suggesting that they recognize a viral ligand, although this has not yet been formally demonstrated [66]. An increase of NKp30+ NK cells is also associated with chronic HCV infection [67] while an expansion of the KIR3DS1+ subset is associated with acute HIV infection [68]. In addition to this specific recognition, pathogens may also be directly recognized by NK cells through the Toll-like receptors (TLRs) they express [69–72]. Besides these mechanisms of activation, most NK cells express the FcγRIII CD16 responsible for antibody-dependent cellular cytotoxicity (ADCC).
Signals from activating NKRs are tightly regulated. This is needed to avoid uncontrolled immune response towards self. As discussed previously, the expression of inhibitory receptors to self ligands, in particular MHC class I molecules, is a major regulatory mechanism [35]. In addition, Long et al. demonstrated that cross-linking of a single activating NKR on freshly isolated “resting” NK cells was not sufficient to trigger NK cell functions. Rather, a synergy among activating receptors was needed to reach a threshold of signaling [4, 5, 73]. This threshold can also be reached when cytokine stimulation is combined with cross-linking of activating NKRs [74]. In addition, chronic exposure to activating ligands contributes to NK cell hyporesponsiveness by inducing the downregulation of the corresponding activating NKRs or signaling molecules. This was demonstrated when NK cells developed in the continuous presence of NKG2D [75–77], Ly49D [78] or Ly49H [79, 80] ligands and for mature NK cells [79]. Low level expression of NKp46, NKp44 and NKp30 has also been associated with defects in NK cell functions in human healthy donors and patients suffering from chronic myeloid leukemia [81, 82] or infected with HIV [83].
NK cell responsiveness also depends on whether NK cells are “educated”, “licensed” or “armed” [84–87]. Many reviews have covered this topic, which will therefore not be discussed in detail here [88–92]. Briefly, NK cells are hyporesponsive when they cannot recognize self-MHC class I molecules via their inhibitory receptors. The mechanism of “hyporesponsiveness” is hotly debated and may either imply that MHC class I molecules instruct NK cells to become responsive (“licensing model”) or be due to the fact that a lack of this interaction “disarms” NK cells [85, 93]. This was originally demonstrated in developing NK cells and was later shown to be quantitative, depending on the strength of the MHC class I signal. More recent studies have also shown that this process can occur in mature NK cells which can be re-programmed [94–96]. These data suggest that a certain plasticity of NK cell responsiveness exists. In vivo, both unlicensed and licensed NK cells seem to be able to be activated, but unlicensed NK cells provided better protection to MCMV infection [97]. Whether this suggests that expression of inhibitory receptors for MHC class I molecules could be detrimental or whether other receptors such as CD94/NKG2A play a role remains to be assessed.
Specific subsets of NK cells and their location
Until recently, NK cells have been regarded as a functionally rather uniform population of cells which kill and secrete cytokines such as IFN-γ under the influence of defined stimuli. It has now become clear that NK cell diversity is broad and so are NK cell activation signals. Diversity comes partly from different development pathways [98]: NK cells originate not only from the bone marrow but also from the thymus [99]. Different stages of maturation have been identified based on the expression profile of CD11b and CD27 markers [100–102]. Signals required for their full maturation still need to be identified and may depend on the tissue-specific environment. In addition, several groups have recently demonstrated that some subsets of NK cells can become long-lived cells that mount a secondary response to specific antigens [21, 103–105]. It will be interesting to define whether this property is intrinsic to NK cells or results from a specific mode of activation or if specific NK-cell extrinsic signals generate these long-lived cells.
Diversity in NK cell subsets is often associated with distinct effector functions in specific locations. New NK cell subsets have been identified at epithelial surfaces (uterus, tonsils, intestinal mucosa and skin) [16–20, 106, 107]. Interestingly, these subsets display different transcriptional profiles and respond to specific cytokines and signals from the microflora environment [18, 20, 106–108]. Uterine NK cells seem to be specialized in the secretion of cytokines and angiogenic factors rather than cytotoxicity to allow normal placentation and successful pregnancy. Signals regulating their effector functions are still to be fully characterized but are in part regulated through inhibitory and activating NKRs [109, 110]. They likely depend on the specific local cytokine environment and on the interaction with fetal trophoblast cells. Mucosa-associated NK cells were identified in human tonsils and Peyer’s patches as NK-like cells secreting IL-22 in response to IL-23 [16]. They may correspond to human stage 3 NK cells that secrete IL-22 and require IL-1β for their maintenance and expansion [101, 111, 112]. Interestingly, they seem to display some plasticity depending on whether IL-1β, IL-7 or IL-2 are used to sustain their survival and proliferation [112]. In mice, a similar population of intestinal NK-like cells has been described with a phenotype resembling but in some aspects also distinct from lymphoid tissue inducer (LTi) cells [17–20, 113, 114]. Further investigations are needed to define the lineage relationship and physiological role of these new NK cell and NK-like cell subsets as well as the signals required to trigger their effector functions.
NK cell activation requires cellular interactions with APCs
An optimal NK cell activation results from cytokine stimulation and engagement of activating receptors, and it requires interaction with immune cells and in particular with antigen-presenting accessory cells (APCs). Initially, dendritic cells (DCs) were described to contribute to NK cell-mediated anti-tumor responses by enhancing NK cell cytotoxicity and IFN-γ production [115]. In vitro studies later confirmed that mature DCs can activate NK cell effector functions and proliferation [116–118]. DCs were also found to be required for in vivo NK cell activation upon MCMV infection [119]. Since these pioneer works, the central role of DCs and also macrophages on NK cell activation has been demonstrated in various experimental systems [120–125]. They activate NK cells via the cytokines they produce and through membrane-bound molecules. APCs seem to be crucial at several levels. First, at the initiation of NK cell activation, a first step of priming seems to be required to allow naïve NK cells to acquire NK cell functions [124, 126]. Lucas et al. [124] demonstrated that this priming occurred in the draining lymph nodes and was provided by DC trans-presenting IL-15. Membrane-bound IL-15Rα-IL-15 complexes were found to activate NK cells through direct cell–cell contact [127]. Interestingly, IL-15, together with IL-2, was found to be the most potent cytokine which induced translation of granzyme B and perforin in resting NK cells [128]. In addition, priming may involve IL-18 signaling, as the IL-18R/MyD88/IRAK4 pathway is required for ex vivo IFN-γ production by NK cells in response to IL-12 and IL-18 contributing to the NK cell response in visceral leishmaniasis [129, 130]. In addition to NK cell priming, evidence has also accumulated to show that DC/NK cross-talk is central to NK cell triggering of effector functions. Indeed, DC-derived type I IFNs mostly produced by plasmacytoid DC (pDC) stimulate NK cell cytotoxic activity but limit IFN-γ production through a control of IL-12 secretion by DCs [30, 131–133]. DC-derived IL-12 is the most potent stimulator of IFN-γ and other cytokines secreted by APCs as IL-18, IL-15 and IL-2 synergize with IL-12 to induce IFN-γ and enhance NK cell cytotoxicity [27–29, 31, 32, 134]. APCs not only produce cytokines that activate NK cells but they also physically interact with them [135–137]. This is required for several reasons. First, this interaction allows a better delivery of cytokines through the formation of immunological synapses. These synapses have been shown to promote the polarization of IL-12 or IL-18 and their delivery at the synaptic cleft [138, 139]. In addition, NK/DC interactions permit trans-presentation of IL-15 [124, 126, 127]. Interestingly, a rapid accumulation of IL-15Rα was observed at the synapse formed following NK/DC conjugate formation [140]. Lastly, direct contact of NK cells with APCs provides stimulatory and co-stimulatory signals. Expression of ligands for activating receptors such as NKG2D or NKp46 were detected on DCs, macrophages or monocytes following TLR stimulations or infections with bacteria, viruses or parasites [64, 65, 122, 141–145]. Similarly, ligands of co-stimulatory molecules have been detected on activated APCs and contribute to NK cell activation. This is the case for activation-induced C-type lectin (AICL) found expressed on activated monocytes which interacts with NKp80 on NK cells [146]; for CD48 expressed on LPS-stimulated macrophages and interacting with 2B4 [145]; for CD80 induced upon Toxoplasma gondii infection which binds CD28 on NK cells [147]; for CD40 expressed on activated macrophages interacting with CD40L [148]; and for glucocorticoid-induced TNF-receptor-related protein ligand (GITRL) expressed on virus- or CpG-activated pDCs which interacts with GITR on NK cells [149].
NK cell interactions with APCs occur primarily in secondary lymphoid organs where the initiation of NK cell activation takes place. They also occur at the infection or tumor sites where NK cell effector functions participate in the control of immune responses. In draining lymph nodes, murine NK cells are detected in the paracortex in direct contact with DCs [135]. In humans, NK and DCs colocalize in T cell areas of lymph nodes [150–152]. NK cells were initially found to be slowly motile [135], but it was later established that the positive selection of NK cells using CD49b mAb decreased their motility in lymph nodes once transferred into naïve animals [135, 137]. Transfer of labeled NK cells purified by negative selection or the use of NCR1GFP/+ mice demonstrated that NK cells are in fact highly motile in lymph nodes and that they engage short and dynamic interactions with DCs or B cells [136, 137]. Such behavior may allow them to rapidly sample the local cytokine environment of DCs that contribute to their activation [136]. This finding is consistent with the observation that NK cells establish dynamic contacts with their targets [153]. Besides draining lymph nodes, NK/DC cross-talk has been reported in the spleen of animals infected with MCMV, in particular contributing to expansion of Ly49H+ NK cells [119]. NK cells were also detected in close proximity to APCs at the site of inflammation or infection. They were, for example, detected in the skin of patients infected with the yeast Malassezia [154]. Cell contact with autologous activated monocytes or macrophages was also found to be required to activate NK cells to secrete IFN-γ upon challenge with Staphylococcus aureus and Lactobacillus johnsonii [155], Influenza A or Sendai virus [142], Plasmodium falciparum [156] or Salmonella enterica [157].
A previously unappreciated role for CD4+ T cells in NK cell activation in vivo
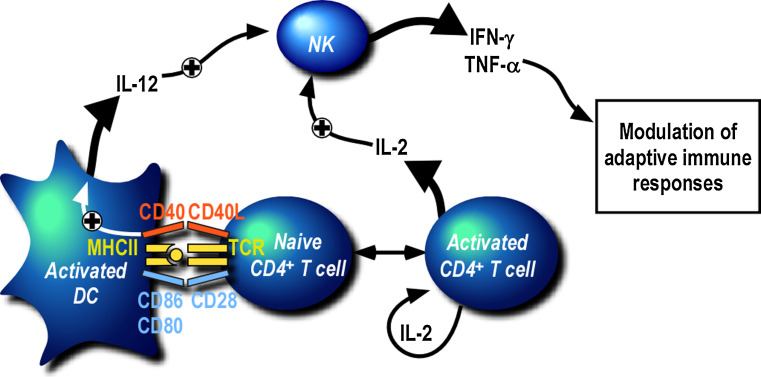
Secondary lymphoid organs are key sites where adaptive immune responses are initiated. As discussed above, they are also sites where NK cell cross-talk with DCs has been visualized [135, 136]. NK cell activation has long been considered to precede T cell activation and therefore the contribution of T cells to NK cell activation has been poorly analyzed. However, clusters of DCs, T cells and IFN-γ-secreting NK cells could be visualized very early on in the lymph nodes draining the inoculation site in Leishmania major-infected mice [135]. This suggested that a cross-talk between T cells and NK cells could occur there. How can T cells contribute to NK cell activation? Early in vitro work by Fehniger et al. [152] demonstrated that IL-2 secretion by a T cell clone could stimulate CD56bright NK cells in the presence of APCs and recombinant IL-12. In addition, He et al. [158] found that IFN-γ secretion by NK cells in PBMCs incubated with Influenza A virus was dependent on the presence of CD4+ T cells and could be abolished by neutralization of IL-2. More recently, a similar observation was made when PBMCs were challenged in vitro with P. falciparum-infected red blood cells [159]. Depletion of either CD4+ T cells, CD8+ T cells, αβTCR+ T cells or γδTCR+ T cells significantly reduced IFN-γ secretion by NK cells. In this situation, there was no requirement for NK cell contact with T cells, but T cell help was provided by the secretion of IL-2. It is clear from these in vitro studies that T cell-derived IL-2 is one mechanism that activates NK cells. This is consistent with the widely established role of IL-2 in the stimulation of NK cell proliferation and effector functions in vitro [160]. The key question remaining to answer was whether NK/T cross-talk occurs in vivo and whether T cell-derived-IL-2 also plays a central role in vivo. In humans, two populations of NK cells are distinguished based on the level of expression of CD56 [9, 161]. The CD56bright CD16− KIR− NK cell subset, which expresses the high affinity IL-2 receptor composed of IL-2R α-chain (CD25), β-chain (CD122) and γ-chain (CD132), is enriched in secondary lymphoid organs [150, 152]. The NK cell subset which is the most responsive to IL-2 therefore co-localizes with T cells, suggesting that T cell-derived IL-2 could regulate NK cell activation in vivo. Formal demonstration was provided when NK cell activation was shown to be specifically abrogated in draining lymph nodes of L. major-infected-CD4+ T cell-deficient mice [26]. Importantly, newly primed antigen-specific CD4+ T cells secreting IL-2 were required to initiate NK cell activation. But in contrast to the above in vitro studies, this work also highlighted that the role of CD4+ T cells was not simply to provide IL-2 but also to regulate DC maturation and DC secretion of IL-12. Indeed, the in vivo neutralization of both IL-2 and IL-12 was needed to significantly decrease IFN-γ secretion by NK cells in draining lymph nodes. In addition, CD4+ T cell help was found to be dependent on CD40/CD40L interaction [162, 163]. These findings indicate that the activation of antigen-specific CD4+ T cells primed by DCs presenting MHC class II/peptide complexes is a prerequisite for NK cell secretion of IFN-γ upon L. major infection. These data could be related to observations made in cancer models in which CD4+ T cell control of tumor growth was found to be dependent on a cooperation with NK cells [164] or innate immune cells expressing markers of NK cells [165]. In these situations, IFN-γ which is essential for the control of tumor growth was secreted by NK cells with the help of CD4+ T cells and a contribution of IL-2. A similar requirement for CD4+ T cells is also found following immunization with OVA where NK cell secretion of IFN-γ early on in draining lymph nodes only occurs in the presence of activated OVA-specific CD4+ T cells [26]. Based on these data, one can propose a sequential model of activation involving DC, CD4+ T cells and NK cells in lymph nodes (Fig. 1). The first event which occurs is the presentation of antigenic peptides by DC to CD4+ T cells. This step of priming triggers the activation of T cells and the secretion of IL-2. Activated CD4+ T cells upregulate CD25 to respond more efficiently to IL-2 and they also upregulate CD40L. IL-2 secreted by T cells contributes directly to NK cell activation and this is amplified as NK cells acquire the high affinity IL-2R upon activation. In addition, activated T cells contribute to the maturation of DC in a CD40/CD40L- and IL-2-dependent way resulting in the secretion of IL-12 by DCs. This cytokine synergizes with IL-2 to activate NK cells. Based on this model, one would expect that NK cells are efficiently activated by IL-2 produced by endogenous memory CD4+ T cells after a secondary challenge. Consistent with this, augmented CD4+ T cell-dependent NK cell responses were observed in PBMCs restimulated with inactivated rabies virus in vaccinated individuals [166]. But the requirement for T cell help is likely to be less critical in situations where efficient maturation of DCs can be achieved by other means and in particular through TLR stimulation. This may be the case in Listeria monocytogenes or Mycobacterium tuberculosis infections where NK cell activation has been observed in Rag −/− animals and therefore does not require CD4+ T cells [167, 168].
Fig. 1.
CD4+ T cell-dependent activation of NK cells in lymph nodes. Upon immunization or infection, DCs present antigen to naïve CD4+ T cells in lymph nodes draining the immunization or inoculation sites. This event results in the activation of T cells which secrete IL-2 and upregulate the high affinity IL-2 receptor and CD40L. Activated CD4+ T cells contribute to further maturation of DC and their secretion of IL-12. Both cytokines, IL-2 and IL-12, synergize to initiate NK cell activation which results in the secretion of IFN-γ and TNF-α, critical for the development of subsequent adaptive immune responses
Interestingly, iNKT cells may play a similar role. Injection of alpha-galactosyl ceramide (αGalCer) intravenously led to the activation of NK cells which was dependent on prior activation of iNKT cells and their secretion of IFN-γ [169]. Whether IL-12 also plays a role has not been examined, but activated iNKT cells have been found to contribute to DC maturation in a CD40/CD40L-dependent way [170].
Lastly, the cross-talk of NK cells with T cells may not always lead to NK cell activation but also to suppression of NK cell effector functions. Indeed, regulatory T cells have also been involved in dampening NK cell functions [171, 172].
Concluding remarks
An increasing interest to better understand the molecular and cellular requirements for NK cell activation in vivo correlates with accumulating evidence of the importance of NK cells in controlling diseases. Their role in tumor immunosurveillance in mice and humans is now well documented [2, 173]. NK cell activation is also thought to limit pathogen invasion until the adaptive immune response establishes long-lasting immune control [174]. This was elegantly illustrated recently in a model of infection with mousepox in which mice genetically resistant to mousepox lose resistance at mid-age because NK cells are no longer efficiently recruited in lymph nodes draining the primary site of infection [175]. Pursuing our efforts to decipher signals regulating NK cell activation will be useful for the development of novel NK cell-based immunotherapies as NK cell clinical relevance is emerging [173, 176–178].
References
- 1.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nature Rev Immunol. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- 3.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 4.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryceson YT, Ljunggren HG, Long EO. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood. 2009;114:2657–2666. doi: 10.1182/blood-2009-01-201632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dustin ML, Long EO. Cytotoxic immunological synapses. Immunol Rev. 2010;235:24–34. doi: 10.1111/j.0105-2896.2010.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman LA, Hunter CA. Regulatory pathways involved in the infection-induced production of IFN-gamma by NK cells. Microbes Infect. 2002;4:1531–1538. doi: 10.1016/s1286-4579(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 9.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 10.Raulet DH, Vance RE, McMahon CW. Regulation of the natural killer cell receptor repertoire. Annu Rev Immunol. 2001;19:291–330. doi: 10.1146/annurev.immunol.19.1.291. [DOI] [PubMed] [Google Scholar]
- 11.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 12.Riley JK, Yokoyama WM. NK cell tolerance and the maternal-fetal interface. Am J Reprod Immunol. 2008;59:371–387. doi: 10.1111/j.1600-0897.2008.00593.x. [DOI] [PubMed] [Google Scholar]
- 13.Trowsdale J, Moffett A. NK receptor interactions with MHC class I molecules in pregnancy. Semin Immunol. 2008;20:317–320. doi: 10.1016/j.smim.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Manaster I, Mandelboim O. The unique properties of human NK cells in the uterine mucosa. Placenta. 2008;29(Suppl A):S60–S66. doi: 10.1016/j.placenta.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Bilinski MJ, Thorne JG, Oh MJ, Leonard S, Murrant C, Tayade C, Croy BA. Uterine NK cells in murine pregnancy. Reprod Biomed Online. 2008;16:218–226. doi: 10.1016/s1472-6483(10)60577-9. [DOI] [PubMed] [Google Scholar]
- 16.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, Hardwigsen J, Anguiano E, Banchereau J, Chaussabel D, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 18.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, Diefenbach A. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 20.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leibson PJ. The regulation of lymphocyte activation by inhibitory receptors. Curr Opin Immunol. 2004;16:328–336. doi: 10.1016/j.coi.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Chini CC, Leibson PJ (2001) Signal transduction during natural killer cell activation. Curr Protoc Cytom Chapter 9, Unit 9 16 [DOI] [PubMed]
- 24.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tassi I, Klesney-Tait J, Colonna M. Dissecting natural killer cell activation pathways through analysis of genetic mutations in human and mouse. Immunol Rev. 2006;214:92–105. doi: 10.1111/j.1600-065X.2006.00463.x. [DOI] [PubMed] [Google Scholar]
- 26.Bihl F, Pecheur J, Breart B, Poupon G, Cazareth J, Julia V, Glaichenhaus N, Braud VM. Primed antigen-specific CD4+ T cells are required for NK cell activation in vivo upon Leishmania major infection. J Immunol. 2010;185:2174–2181. doi: 10.4049/jimmunol.1001486. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, Caligiuri MA, Durbin JE, Biron CA. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol. 2002;169:4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- 28.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 29.Orange JS, Biron CA. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol. 1996;156:1138–1142. [PubMed] [Google Scholar]
- 30.Colonna M, Krug A, Cella M. Interferon-producing cells: on the front line in immune responses against pathogens. Curr Opin Immunol. 2002;14:373–379. doi: 10.1016/s0952-7915(02)00349-7. [DOI] [PubMed] [Google Scholar]
- 31.Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol. 2005;86:209–239. doi: 10.1016/S0065-2776(04)86006-1. [DOI] [PubMed] [Google Scholar]
- 32.Fehniger TA, Shah MH, Turner MJ, VanDeusen JB, Whitman SP, Cooper MA, Suzuki K, Wechser M, Goodsaid F, Caligiuri MA. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–4520. [PubMed] [Google Scholar]
- 33.Joncker NT, Raulet DH. Regulation of NK cell responsiveness to achieve self-tolerance and maximal responses to diseased target cells. Immunol Rev. 2008;224:85–97. doi: 10.1111/j.1600-065X.2008.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ljunggren HG, Sturmhofel K, Wolpert E, Hammerling GJ, Karre K. Transfection of beta 2-microglobulin restores IFN-mediated protection from natural killer cell lysis in YAC-1 lymphoma variants. J Immunol. 1990;145:380–386. [PubMed] [Google Scholar]
- 35.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 36.Ljunggren HG, Karre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985;162:1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 38.Iizuka K, Naidenko OV, Plougastel BF, Fremont DH, Yokoyama WM. Genetically linked C-type lectin-related ligands for the NKRP1 family of natural killer cell receptors. Nat Immunol. 2003;4:801–807. doi: 10.1038/ni954. [DOI] [PubMed] [Google Scholar]
- 39.Carlyle JR, Jamieson AM, Gasser S, Clingan CS, Arase H, Raulet DH. Missing self-recognition of Ocil/Clr-b by inhibitory NKR-P1 natural killer cell receptors. Proc Natl Acad Sci USA. 2004;101:3527–3532. doi: 10.1073/pnas.0308304101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aldemir H, Prod’homme V, Dumaurier MJ, Retiere C, Poupon G, Cazareth J, Bihl F, Braud VM. Cutting edge: lectin-like transcript 1 is a ligand for the CD161 receptor. J Immunol. 2005;175:7791–7795. doi: 10.4049/jimmunol.175.12.7791. [DOI] [PubMed] [Google Scholar]
- 41.Rosen DB, Cao W, Avery DT, Tangye SG, Liu YJ, Houchins JP, Lanier LL. Functional consequences of interactions between human NKR-P1A and its ligand LLT1 expressed on activated dendritic cells and B cells. J Immunol. 2008;180:6508–6517. doi: 10.4049/jimmunol.180.10.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosen DB, Bettadapura J, Alsharifi M, Mathew PA, Warren HS, Lanier LL. Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J Immunol. 2005;175:7796–7799. doi: 10.4049/jimmunol.175.12.7796. [DOI] [PubMed] [Google Scholar]
- 43.Kumar V, McNerney ME. A new self: MHC-class-I-independent natural-killer-cell self-tolerance. Nat Rev Immunol. 2005;5:363–374. doi: 10.1038/nri1603. [DOI] [PubMed] [Google Scholar]
- 44.Corral L, Hanke T, Vance RE, Cado D, Raulet DH. NK cell expression of the killer cell lectin-like receptor G1 (KLRG1), the mouse homolog of MAFA, is modulated by MHC class I molecules. Eur J Immunol. 2000;30:920–930. doi: 10.1002/1521-4141(200003)30:3<920::AID-IMMU920>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Hofmann M, Wang Q, Teng L, Chlewicki LK, Pircher H, Mariuzza RA. Structure of natural killer cell receptor KLRG1 bound to E-cadherin reveals basis for MHC-independent missing self recognition. Immunity. 2009;31:35–46. doi: 10.1016/j.immuni.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito M, Maruyama T, Saito N, Koganei S, Yamamoto K, Matsumoto N. Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. J Exp Med. 2006;203:289–295. doi: 10.1084/jem.20051986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grundemann C, Bauer M, Schweier O, von Oppen N, Lassing U, Saudan P, Becker KF, Karp K, Hanke T, Bachmann MF, et al. Cutting edge: identification of E-cadherin as a ligand for the murine killer cell lectin-like receptor G1. J Immunol. 2006;176:1311–1315. doi: 10.4049/jimmunol.176.3.1311. [DOI] [PubMed] [Google Scholar]
- 48.Meyaard L, de Vries AR, de Ruiter T, Lanier LL, Phillips JH, Clevers H. The epithelial cellular adhesion molecule (Ep-CAM) is a ligand for the leukocyte-associated immunoglobulin-like receptor (LAIR) J Exp Med. 2001;194:107–112. doi: 10.1084/jem.194.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Avril T, Floyd H, Lopez F, Vivier E, Crocker PR. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related Siglecs expressed on human monocytes and NK cells. J Immunol. 2004;173:6841–6849. doi: 10.4049/jimmunol.173.11.6841. [DOI] [PubMed] [Google Scholar]
- 50.Markel G, Lieberman N, Katz G, Arnon TI, Lotem M, Drize O, Blumberg RS, Bar-Haim E, Mader R, Eisenbach L, et al. CD66a interactions between human melanoma and NK cells: a novel class I MHC-independent inhibitory mechanism of cytotoxicity. J Immunol. 2002;168:2803–2810. doi: 10.4049/jimmunol.168.6.2803. [DOI] [PubMed] [Google Scholar]
- 51.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 52.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235:267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198:557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pende D, Castriconi R, Romagnani P, Spaggiari GM, Marcenaro S, Dondero A, Lazzeri E, Lasagni L, Martini S, Rivera P, et al. Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: relevance for natural killer-dendritic cell interaction. Blood. 2006;107:2030–2036. doi: 10.1182/blood-2005-07-2696. [DOI] [PubMed] [Google Scholar]
- 55.Tahara-Hanaoka S, Shibuya K, Onoda Y, Zhang H, Yamazaki S, Miyamoto A, Honda S, Lanier LL, Shibuya A. Functional characterization of DNAM-1 (CD226) interaction with its ligands PVR (CD155) and nectin-2 (PRR-2/CD112) Int Immunol. 2004;16:533–538. doi: 10.1093/intimm/dxh059. [DOI] [PubMed] [Google Scholar]
- 56.Hansasuta P, Dong T, Thananchai H, Weekes M, Willberg C, Aldemir H, Rowland-Jones S, Braud VM. Recognition of HLA-A3 and HLA-A11 by KIR3DL2 is peptide-specific. Eur J Immunol. 2004;34:1673–1679. doi: 10.1002/eji.200425089. [DOI] [PubMed] [Google Scholar]
- 57.Stewart CA, Laugier-Anfossi F, Vely F, Saulquin X, Riedmuller J, Tisserant A, Gauthier L, Romagne F, Ferracci G, Arosa FA, et al. Recognition of peptide-MHC class I complexes by activating killer immunoglobulin-like receptors. Proc Natl Acad Sci USA. 2005;102:13224–13229. doi: 10.1073/pnas.0503594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allen RL, Raine T, Haude A, Trowsdale J, Wilson MJ. Leukocyte receptor complex-encoded immunomodulatory receptors show differing specificity for alternative HLA-B27 structures. J Immunol. 2001;167:5543–5547. doi: 10.4049/jimmunol.167.10.5543. [DOI] [PubMed] [Google Scholar]
- 59.Brown MG, Dokun AO, Heusel JW, Smith HR, Beckman DL, Blattenberger EA, Dubbelde CE, Stone LR, Scalzo AA, Yokoyama WM. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292:934–937. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- 60.Daniels KA, Devora G, Lai WC, O’Donnell CL, Bennett M, Welsh RM. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J Exp Med. 2001;194:29–44. doi: 10.1084/jem.194.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee SH, Girard S, Macina D, Busa M, Zafer A, Belouchi A, Gros P, Vidal SM. Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nat Genet. 2001;28:42–45. doi: 10.1038/ng0501-42. [DOI] [PubMed] [Google Scholar]
- 62.Smith HR, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci USA. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 64.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, Davis DM, Strominger JL, Yewdell JW, Porgador A. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 65.Gazit R, Gruda R, Elboim M, Arnon TI, Katz G, Achdout H, Hanna J, Qimron U, Landau G, Greenbaum E, et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7:517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 66.Guma M, Budt M, Saez A, Brckalo T, Hengel H, Angulo A, Lopez-Botet M. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107:3624–3631. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- 67.De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P, Congia S, Mingari MC, Moretta L. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37:445–455. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 68.Alter G, Rihn S, Walter K, Nolting A, Martin M, Rosenberg ES, Miller JS, Carrington M, Altfeld M. HLA class I subtype-dependent expansion of KIR3DS1+ and KIR3DL1+ NK cells during acute human immunodeficiency virus type 1 infection. J Virol. 2009;83:6798–6805. doi: 10.1128/JVI.00256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175:1636–1642. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt KN, Leung B, Kwong M, Zarember KA, Satyal S, Navas TA, Wang F, Godowski PJ. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J Immunol. 2004;172:138–143. doi: 10.4049/jimmunol.172.1.138. [DOI] [PubMed] [Google Scholar]
- 71.Sivori S, Falco M, Della Chiesa M, Carlomagno S, Vitale M, Moretta L, Moretta A. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci USA. 2004;101:10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martinez J, Huang X, Yang Y. Direct TLR2 signaling is critical for NK cell activation and function in response to vaccinia viral infection. PLoS Pathog. 2010;6:e1000811. doi: 10.1371/journal.ppat.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ortaldo JR, Winkler-Pickett R, Wigginton J, Horner M, Bere EW, Mason AT, Bhat N, Cherry J, Sanford M, Hodge DL, et al. Regulation of ITAM-positive receptors: role of IL-12 and IL-18. Blood. 2006;107:1468–1475. doi: 10.1182/blood-2005-04-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, Girardi M, Hayday AC. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 76.Ogasawara K, Hamerman JA, Hsin H, Chikuma S, Bour-Jordan H, Chen T, Pertel T, Carnaud C, Bluestone JA, Lanier LL. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 2003;18:41–51. doi: 10.1016/s1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 77.Wiemann K, Mittrucker HW, Feger U, Welte SA, Yokoyama WM, Spies T, Rammensee HG, Steinle A. Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. J Immunol. 2005;175:720–729. doi: 10.4049/jimmunol.175.2.720. [DOI] [PubMed] [Google Scholar]
- 78.George TC, Ortaldo JR, Lemieux S, Kumar V, Bennett M. Tolerance and alloreactivity of the Ly49D subset of murine NK cells. J Immunol. 1999;163:1859–1867. [PubMed] [Google Scholar]
- 79.Tripathy SK, Keyel PA, Yang L, Pingel JT, Cheng TP, Schneeberger A, Yokoyama WM. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J Exp Med. 2008;205:1829–1841. doi: 10.1084/jem.20072446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun JC, Lanier LL. Tolerance of NK cells encountering their viral ligand during development. J Exp Med. 2008;205:1819–1828. doi: 10.1084/jem.20072448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, Gastaut JA, Pende D, Olive D, Moretta A. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99:3661–3667. doi: 10.1182/blood.v99.10.3661. [DOI] [PubMed] [Google Scholar]
- 82.Sivori S, Pende D, Bottino C, Marcenaro E, Pessino A, Biassoni R, Moretta L, Moretta A. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur J Immunol. 1999;29:1656–1666. doi: 10.1002/(SICI)1521-4141(199905)29:05<1656::AID-IMMU1656>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 83.De Maria A, Fogli M, Costa P, Murdaca G, Puppo F, Mavilio D, Moretta A, Moretta L. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) Eur J Immunol. 2003;33:2410–2418. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 84.Ohlen C, Kling G, Hoglund P, Hansson M, Scangos G, Bieberich C, Jay G, Karre K. Prevention of allogeneic bone marrow graft rejection by H-2 transgene in donor mice. Science. 1989;246:666–668. doi: 10.1126/science.2814488. [DOI] [PubMed] [Google Scholar]
- 85.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 86.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6:520–531. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 87.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 88.Hoglund P, Brodin P. Current perspectives of natural killer cell education by MHC class I molecules. Nat Rev Immunol. 2010;10:724–734. doi: 10.1038/nri2835. [DOI] [PubMed] [Google Scholar]
- 89.Held W. Tolerance and reactivity of NK cells: two sides of the same coin? Eur J Immunol. 2008;38:2930–2933. doi: 10.1002/eji.200838755. [DOI] [PubMed] [Google Scholar]
- 90.Orr MT, Lanier LL. Natural killer cell education and tolerance. Cell. 2010;142:847–856. doi: 10.1016/j.cell.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brodin P, Hoglund P. Beyond licensing and disarming: a quantitative view on NK-cell education. Eur J Immunol. 2008;38:2934–2937. doi: 10.1002/eji.200838760. [DOI] [PubMed] [Google Scholar]
- 92.Brodin P, Karre K, Hoglund P. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol. 2009;30:143–149. doi: 10.1016/j.it.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 93.Gasser S, Raulet DH. Activation and self-tolerance of natural killer cells. Immunol Rev. 2006;214:130–142. doi: 10.1111/j.1600-065X.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 94.Elliott JM, Wahle JA, Yokoyama WM. MHC class I-deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I-sufficient environment. J Exp Med. 2010;207:2073–2079. doi: 10.1084/jem.20100986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johansson MH, Bieberich C, Jay G, Karre K, Hoglund P. Natural killer cell tolerance in mice with mosaic expression of major histocompatibility complex class I transgene. J Exp Med. 1997;186:353–364. doi: 10.1084/jem.186.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Joncker NT, Shifrin N, Delebecque F, Raulet DH. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J Exp Med. 2010;207:2065–2072. doi: 10.1084/jem.20100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Orr MT, Murphy WJ, Lanier LL. ‘Unlicensed’ natural killer cells dominate the response to cytomegalovirus infection. Nat Immunol. 2010;11:321–327. doi: 10.1038/ni.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huntington ND, Vosshenrich CA, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol. 2007;7:703–714. doi: 10.1038/nri2154. [DOI] [PubMed] [Google Scholar]
- 99.Vosshenrich CA, Garcia-Ojeda ME, Samson-Villeger SI, Pasqualetto V, Enault L, Richard-Le Goff O, Corcuff E, Guy-Grand D, Rocha B, Cumano A, et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol. 2006;7:1217–1224. doi: 10.1038/ni1395. [DOI] [PubMed] [Google Scholar]
- 100.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–5496. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 101.Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, Caligiuri MA. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203:1033–1043. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 103.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 104.Cooper MA, Yokoyama WM. Memory-like responses of natural killer cells. Immunol Rev. 2010;235:297–305. doi: 10.1111/j.0105-2896.2010.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 107.Vivier E, Spits H, Cupedo T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat Rev Immunol. 2009;9:229–234. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- 108.Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hiby SE, Walker JJ, O’Shaughnessy KM, Redman CW, Carrington M, Trowsdale J, Moffett A. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, et al. Decidual NK cells regulate key developmental processes at the human fetal–maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 111.Hughes T, Becknell B, Freud AG, McClory S, Briercheck E, Yu J, Mao C, Giovenzana C, Nuovo G, Wei L, et al. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;32:803–814. doi: 10.1016/j.immuni.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc Natl Acad Sci USA. 2010;107:10961–10966. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H. Human NKp44+ IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med. 2010;207:281–290. doi: 10.1084/jem.20091509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, Flach M, Bengsch B, Thimme R, Holscher C, et al. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33:736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 116.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 120.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5:112–124. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- 121.Kassim SH, Rajasagi NK, Zhao X, Chervenak R, Jennings SR. In vivo ablation of CD11c-positive dendritic cells increases susceptibility to herpes simplex virus type 1 infection and diminishes NK and T-cell responses. J Virol. 2006;80:3985–3993. doi: 10.1128/JVI.80.8.3985-3993.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Newman KC, Riley EM. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat Rev Immunol. 2007;7:279–291. doi: 10.1038/nri2057. [DOI] [PubMed] [Google Scholar]
- 123.Schleicher U, Liese J, Knippertz I, Kurzmann C, Hesse A, Heit A, Fischer JA, Weiss S, Kalinke U, Kunz S, et al. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J Exp Med. 2007;204:893–906. doi: 10.1084/jem.20061293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kang SJ, Liang HE, Reizis B, Locksley RM. Regulation of hierarchical clustering and activation of innate immune cells by dendritic cells. Immunity. 2008;29:819–833. doi: 10.1016/j.immuni.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Koka R, Burkett P, Chien M, Chai S, Boone DL, Ma A. Cutting edge: murine dendritic cells require IL-15R alpha to prime NK cells. J Immunol. 2004;173:3594–3598. doi: 10.4049/jimmunol.173.6.3594. [DOI] [PubMed] [Google Scholar]
- 127.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205:1213–1225. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fehniger TA, Cai SF, Cao X, Bredemeyer AJ, Presti RM, French AR, Ley TJ. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 129.Chaix J, Tessmer MS, Hoebe K, Fuseri N, Ryffel B, Dalod M, Alexopoulou L, Beutler B, Brossay L, Vivier E, et al. Cutting edge: priming of NK cells by IL-18. J Immunol. 2008;181:1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Haeberlein S, Sebald H, Bogdan C, Schleicher U. IL-18, but not IL-15, contributes to the IL-12-dependent induction of NK-cell effector functions by Leishmania infantum in vivo. Eur J Immunol. 2010;40:1708–1717. doi: 10.1002/eji.200939988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gerosa F, Gobbi A, Zorzi P, Burg S, Briere F, Carra G, Trinchieri G. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–734. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 132.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Briere F, Trinchieri G, Biron CA. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med. 2002;195:517–528. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Granucci F, Zanoni I, Pavelka N, Van Dommelen SL, Andoniou CE, Belardelli F, Degli Esposti MA, Ricciardi-Castagnoli P. A contribution of mouse dendritic cell-derived IL-2 for NK cell activation. J Exp Med. 2004;200:287–295. doi: 10.1084/jem.20040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bajenoff M, Breart B, Huang AY, Qi H, Cazareth J, Braud VM, Germain RN, Glaichenhaus N. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J Exp Med. 2006;203:619–631. doi: 10.1084/jem.20051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Beuneu H, Deguine J, Breart B, Mandelboim O, Di Santo JP, Bousso P. Dynamic behavior of NK cells during activation in lymph nodes. Blood. 2009;114:3227–3234. doi: 10.1182/blood-2009-06-228759. [DOI] [PubMed] [Google Scholar]
- 137.Garrod KR, Wei SH, Parker I, Cahalan MD. Natural killer cells actively patrol peripheral lymph nodes forming stable conjugates to eliminate MHC-mismatched targets. Proc Natl Acad Sci USA. 2007;104:12081–12086. doi: 10.1073/pnas.0702867104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Borg C, Jalil A, Laderach D, Maruyama K, Wakasugi H, Charrier S, Ryffel B, Cambi A, Figdor C, Vainchenker W, et al. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 2004;104:3267–3275. doi: 10.1182/blood-2004-01-0380. [DOI] [PubMed] [Google Scholar]
- 139.Semino C, Angelini G, Poggi A, Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 2005;106:609–616. doi: 10.1182/blood-2004-10-3906. [DOI] [PubMed] [Google Scholar]
- 140.Brilot F, Strowig T, Roberts SM, Arrey F, Munz C. NK cell survival mediated through the regulatory synapse with human DCs requires IL-15Ralpha. J Clin Invest. 2007;117:3316–3329. doi: 10.1172/JCI31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hamerman JA, Ogasawara K, Lanier LL. Cutting edge: Toll-like receptor signaling in macrophages induces ligands for the NKG2D receptor. J Immunol. 2004;172:2001–2005. doi: 10.4049/jimmunol.172.4.2001. [DOI] [PubMed] [Google Scholar]
- 142.Siren J, Sareneva T, Pirhonen J, Strengell M, Veckman V, Julkunen I, Matikainen S. Cytokine and contact-dependent activation of natural killer cells by influenza A or Sendai virus-infected macrophages. J General Virol. 2004;85:2357–2364. doi: 10.1099/vir.0.80105-0. [DOI] [PubMed] [Google Scholar]
- 143.Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, Suzuki T, Miyagi T, Hayashi N. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. J Immunol. 2003;171:5423–5429. doi: 10.4049/jimmunol.171.10.5423. [DOI] [PubMed] [Google Scholar]
- 144.Eissmann P, Evans JH, Mehrabi M, Rose EL, Nedvetzki S, Davis DM. Multiple mechanisms downstream of TLR-4 stimulation allow expression of NKG2D ligands to facilitate macrophage/NK cell crosstalk. J Immunol. 2010;184:6901–6909. doi: 10.4049/jimmunol.0903985. [DOI] [PubMed] [Google Scholar]
- 145.Nedvetzki S, Sowinski S, Eagle RA, Harris J, Vely F, Pende D, Trowsdale J, Vivier E, Gordon S, Davis DM. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood. 2007;109:3776–3785. doi: 10.1182/blood-2006-10-052977. [DOI] [PubMed] [Google Scholar]
- 146.Welte S, Kuttruff S, Waldhauer I, Steinle A. Mutual activation of natural killer cells and monocytes mediated by NKp80-AICL interaction. Nat Immunol. 2006;7:1334–1342. doi: 10.1038/ni1402. [DOI] [PubMed] [Google Scholar]
- 147.Hunter CA, Ellis-Neyer L, Gabriel KE, Kennedy MK, Grabstein KH, Linsley PS, Remington JS. The role of the CD28/B7 interaction in the regulation of NK cell responses during infection with Toxoplasma gondii . J Immunol. 1997;158:2285–2293. [PubMed] [Google Scholar]
- 148.Atochina O, Harn D. LNFPIII/LeX-stimulated macrophages activate natural killer cells via CD40-CD40L interaction. Clin Diagn Lab Immunol. 2005;12:1041–1049. doi: 10.1128/CDLI.12.9.1041-1049.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hanabuchi S, Watanabe N, Wang YH, Ito T, Shaw J, Cao W, Qin FX, Liu YJ. Human plasmacytoid predendritic cells activate NK cells through glucocorticoid-induced tumor necrosis factor receptor-ligand (GITRL) Blood. 2006;107:3617–3623. doi: 10.1182/blood-2005-08-3419. [DOI] [PubMed] [Google Scholar]
- 150.Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, Bougras G, Muller WA, Moretta L, Munz C. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci USA. 2004;101:16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ferlazzo G, Munz C. NK cell compartments and their activation by dendritic cells. J Immunol. 2004;172:1333–1339. doi: 10.4049/jimmunol.172.3.1333. [DOI] [PubMed] [Google Scholar]
- 152.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 153.Deguine J, Breart B, Lemaitre F, Di Santo JP, Bousso P. Intravital imaging reveals distinct dynamics for natural killer and CD8(+) T cells during tumor regression. Immunity. 2010;33:632–644. doi: 10.1016/j.immuni.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 154.Buentke E, Heffler LC, Wilson JL, Wallin RP, Lofman C, Chambers BJ, Ljunggren HG, Scheynius A. Natural killer and dendritic cell contact in lesional atopic dermatitis skin—Malassezia-influenced cell interaction. J Invest Dermatol. 2002;119:850–857. doi: 10.1046/j.1523-1747.2002.00132.x. [DOI] [PubMed] [Google Scholar]
- 155.Haller D, Serrant P, Granato D, Schiffrin EJ, Blum S. Activation of human NK cells by staphylococci and lactobacilli requires cell contact-dependent costimulation by autologous monocytes. Clin Diagn Lab Immunol. 2002;9:649–657. doi: 10.1128/CDLI.9.3.649-657.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Newman KC, Korbel DS, Hafalla JC, Riley EM. Cross-talk with myeloid accessory cells regulates human natural killer cell interferon–gamma responses to malaria. PLoS Pathog. 2006;2:e118. doi: 10.1371/journal.ppat.0020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lapaque N, Walzer T, Meresse S, Vivier E, Trowsdale J. Interactions between human NK cells and macrophages in response to Salmonella infection. J Immunol. 2009;182:4339–4348. doi: 10.4049/jimmunol.0803329. [DOI] [PubMed] [Google Scholar]
- 158.He XS, Draghi M, Mahmood K, Holmes TH, Kemble GW, Dekker CL, Arvin AM, Parham P, Greenberg HB. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J Clin Invest. 2004;114:1812–1819. doi: 10.1172/JCI22797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Horowitz A, Newman KC, Evans JH, Korbel DS, Davis DM, Riley EM. Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J Immunol. 2010;184:6043–6052. doi: 10.4049/jimmunol.1000106. [DOI] [PubMed] [Google Scholar]
- 160.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 161.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 162.Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol. 2009;21:265–272. doi: 10.1016/j.smim.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 164.Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, Matzinger P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109:5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li Z, Pradera F, Kammertoens T, Li B, Liu S, Qin Z. Cross-talk between T cells and innate immune cells is crucial for IFN-gamma-dependent tumor rejection. J Immunol. 2007;179:1568–1576. doi: 10.4049/jimmunol.179.3.1568. [DOI] [PubMed] [Google Scholar]
- 166.Horowitz A, Behrens RH, Okell L, Fooks AR, Riley EM. NK cells as effectors of acquired immune responses: effector CD4(+) T cell-dependent activation of NK cells following vaccination. J Immunol. 2010;185:2808–2818. doi: 10.4049/jimmunol.1000844. [DOI] [PubMed] [Google Scholar]
- 167.Feng CG, Kaviratne M, Rothfuchs AG, Cheever A, Hieny S, Young HA, Wynn TA, Sher A. NK cell-derived IFN-gamma differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis . J Immunol. 2006;177:7086–7093. doi: 10.4049/jimmunol.177.10.7086. [DOI] [PubMed] [Google Scholar]
- 168.Thale C, Kiderlen AF. Sources of interferon-gamma (IFN-gamma) in early immune response to Listeria monocytogenes . Immunobiology. 2005;210:673–683. doi: 10.1016/j.imbio.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 169.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 170.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, et al. CD4+ CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ghiringhelli F, Menard C, Martin F, Zitvogel L. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol Rev. 2006;214:229–238. doi: 10.1111/j.1600-065X.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 173.Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol. 2008;9:486–494. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- 174.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fang M, Roscoe F, Sigal LJ. Age-dependent susceptibility to a viral disease due to decreased natural killer cell numbers and trafficking. J Exp Med. 2010;207:2369–2381. doi: 10.1084/jem.20100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 177.Chan CJ, Andrews DM, Smyth MJ. Can NK cells be a therapeutic target in human cancer? Eur J Immunol. 2008;38:2964–2968. doi: 10.1002/eji.200838764. [DOI] [PubMed] [Google Scholar]
- 178.Burke S, Lakshmikanth T, Colucci F, Carbone E. New views on natural killer cell-based immunotherapy for melanoma treatment. Trends Immunol. 2010;31:339–345. doi: 10.1016/j.it.2010.06.003. [DOI] [PubMed] [Google Scholar]