Abstract
BAR domain superfamily proteins have emerged as central regulators of dynamic membrane remodeling, thereby playing important roles in a wide variety of cellular processes, such as organelle biogenesis, cell division, cell migration, secretion, and endocytosis. Here, we review the mechanistic and structural basis for the membrane curvature-sensing and deforming properties of BAR domain superfamily proteins. Moreover, we summarize the present state of knowledge with respect to their regulation by autoinhibitory mechanisms or posttranslational modifications, and their interactions with other proteins, in particular with GTPases, and with membrane lipids. We postulate that BAR superfamily proteins act as membrane-deforming scaffolds that spatiotemporally orchestrate membrane remodeling.
Keywords: BAR domain, Membrane remodeling, Membrane deformation, Curvature-sensing, Endocytosis, Synaptic vesicle recycling, Protrusion formation, Neuromorphogenesis, GTPases, Autoinhibition
Structure of Bin/amphiphysin/Rvs (BAR) domains
Eukaryotic cells are characterized by a diverse array of membraneous structures including vesicles, tubules, and pleiomorphic vacuoles that enable cellular processes such as organelle biogenesis, cell division, cell signaling and migration, secretion, and endocytosis. In many cases, dynamic membrane remodeling is accomplished by the reversible assembly of membrane-sculpting or deforming proteins [1], most notably by members of the BAR domain superfamily. Members of this protein superfamily are involved in membrane remodeling in various cellular pathways ranging from endocytic vesicle and T-tubule formation to cell migration and neuromorphogenesis [2–7]. These proteins contain BAR/N-BAR, EFC/F-BAR and IMD/I-BAR domains (Fig. 1).
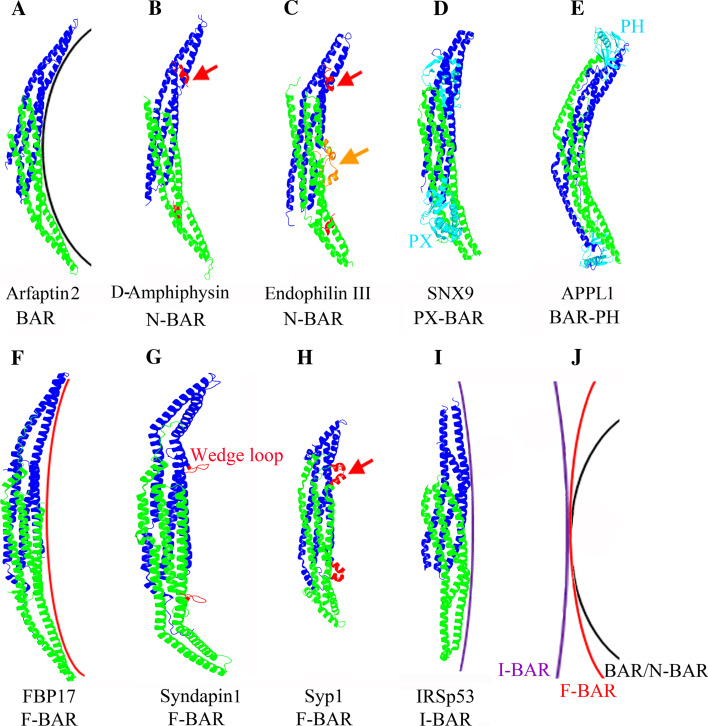
Fig. 1.
Architecture of BAR domains and their role in membrane deformation. a–e Crystal structures of BAR/N-BAR domains: the arfaptin BAR domain (1I49) (a), the Drosophila melanogaster amphiphysin BAR domain (D-Amph) (1URU) (b), the endophilin III BAR domain (2Z0V) (c), the PX-BAR domain from SNX 9 (3DYT) (d), and the BAR-PH domain from APPL1 (2Q12) (e). f–h Structural insights into F-BAR domain architecture: the F-BAR domains of FBP17 (2EFL) (f), syndapin 1 (2X3X) (g), and yeast Syp1 (3G9G) (h). i Crystal structure of the IRSp53 I-BAR domain (1WDZ). j Membrane deformation by BAR/N-BAR, F-BAR, and I-BAR domains. BAR and F-BAR domains induce positive membrane curvature, whereas I-BAR domains generate negative membrane curvature. Amphipathic α-helices are indicated by red arrows in (b), (c) and (h). The additional amphipathic α-helix within the endophilin BAR domain is indicated by an orange arrow in (c). Crystal structures were generated with Pymol
BAR/N-BAR domain
The BAR domain was originally identified as an evolutionary conserved region shared by the yeast proteins Rvs161, Rvs167 and the metazoan amphiphysins (the splice variants of which are also called Bin1) [8–11]. The crystal structure of the N-terminal domain of Arfaptin first elucidated how these domains might be able to associate with curved membrane domains [12]. BAR domains are dimerized via α-helical coiled-coils, and the dimerization module forms a positively charged surface that associates with the negatively charged inner surface of cellular membranes, mostly through interaction with negatively charged phospholipids [12–14]. BAR domain superfamily proteins deform membranes to a geometry that corresponds to the structures of the membrane-binding surface of the protein (a crescent- or banana-shaped dimer) (Fig. 1a–e, j). In vitro, BAR domains are able to bind to negatively charged phospholipids and to induce tubulation of liposomes [15]. Recent structural studies have revealed that BAR domains are frequently found in conjunction with a second membrane binding sequence such as an amphipathic α-helix (termed N-BAR domain), a PH domain (pleckstrin homology domain), or a PX domain (Phox homology domain) (Fig. 1b–e). Amphipathic α-helices are found in the endophilin and amphiphysin subfamilies, which are involved in endocytosis and the activity-dependent recycling of synaptic vesicle membranes [16–21]. Such amphipathic helices are often unstructured until they insert in an asymmetric fashion into one leaflet of the membrane. They are predicted to sit flat on the membrane surface with their hydrophobic residues dipping into the hydrophobic phase of the membrane [13, 14, 22, 23]. Mutational inactivation of this hydrophobic face will eliminate liposome binding activity and BAR domain-induced membrane tubulation in vitro [20, 21]. Biophysical and computational studies indicate that such amphipathic helices in conjunction with the concave nature of the BAR domain fold are important determinants of the ability of BAR domain proteins to sense and induce membrane curvature [13, 17, 18]. Hence, the N-BAR module serves a dual role in regulating membrane curvature: via their N-terminal amphipathic helices N-BAR domain proteins sense curved membrane domains by probing the surface for lipid packing defects; such curved microdomains then become stabilized by the lateral association of banana-shaped BAR domain assemblies [16, 17, 24, 25]. A variation of this theme is found in endophilin N-BAR, which contains an additional helix inserted within the BAR domain conserved among endophilin subfamily proteins [20, 21, 26]. This additional amphipathic helix is located in the center of the banana-shaped BAR domain and inserts into the lipid bilayer in an asymmetric fashion, similar to the N-terminal amphipathic helices found in amphiphysins [18, 20, 21, 26].
BAR domains on their own indiscriminately bind to negatively charged phospholipids. However, the presence of a neighboring PH or PX domain can confer lipid specificity, as PH or PX domains associate with phosphoinositides, including PtdIns(3)P, PtdIns(4,5)P2 or PtdIns(3,4,5)P3 [27–29], and can thus assist targeting of BAR domain-containing proteins to distinct membrane (sub)compartments. For example, the BAR-PX domain of Sorting Nexin 1 (SNX1), a critical component of the retromer complex [30, 31], has been shown to target SNX1 to a highly curved PtdIns(3)P-containing tubular microdomain on early endosomes [31], a function required for retrograde sorting of a variety of cargo ranging from mannose 6-phosphate receptors to Wnt signaling components [32, 33].
F-BAR domain
F-BAR proteins were formerly referred to as Pombe Cdc15 homology (PCH) proteins. Owing to primary sequence similarity, a domain with a distant relation to BAR domains was identified within them [34]. The archetypal feature of this protein family is their Fer/CIP4 homology (FCH) domain [35], which constitutes a functional unit with a neighboring coiled-coil region, together forming the F-BAR domain [34]. F-BAR domain-containing proteins often contain various combinations of SH3 domains, SH2 domains, tyrosine kinase domains, and GAP domains at their C-terminal part [33, 36]. Similar to BAR/N-BAR domains, F-BAR domains form a dimeric positively charged membrane-binding module that associates with negatively charged phospholipids. F-BAR domains usually display a more extended shape with a much shallower curvature than canonical BAR/N-BAR domains (Fig. 1f–h, j). These structural features explain why membrane tubules induced by F-BAR domains are typically of a larger diameter compared to those generated by more strongly curved BAR domain proteins [37, 38]. Similar to the N-BAR domain, an additional wedge loop or amphipathic α-helix is found in several F-BAR domains so far investigated (Fig. 1g, h). For example, X-ray crystallographic studies show that the F-BAR domain of syndapin 1 (also named as PACSIN) dimerizes into an elongated “S” shape with a wedge loop consisting of 119HKQIMGGF126 with hydrophobic residues (I122–M123) at the tip in each monomer. These residues are required for the membrane-deforming activity of syndapin [39, 40]. Replacing the hydrophobic amino acids I122 and M123 within the wedge loop by hydrophilic residues (I122T/M123Q) completely eliminated tubule formation in cells, although this mutation did not alter membrane phospholipid binding [39]. In the crystal structure of Syp 1 F-BAR, a distinctive amphipathic α-helix, similar to the amphipathic helix contained within the N-BAR domain of endophilin, protrudes from the membrane interaction face (Fig. 1h), suggesting a role in inducing and/or sensing membrane curvature. Comparison with known crystal structures of other F-BAR domains reveals differences with respect to the lateral curvature relative to the central dimerization region (Fig. 2). Specifically, this region in syndapin F-BAR domain is bent away from its central body at a ~54° angle, which generates a pronounced twisted S-shape in the dimeric molecule (Fig. 2). These differences in lateral curvature together with a distinctive amphipathic helix or wedge loop may allow F-BAR domains to interact and stabilize membranes with a broad range of curvatures.
Fig. 2.
Comparison of F-BAR domains. Monomer structures of the F-BAR domains from syndapin 1, FCHo2 (2V0O), CIP4 (2EFK), and FBP17 are shown. The degree of lateral bending was quantified by determining the angle between the long axis of the central 3-helix bundle (concave surface) and the long axis of the lateral helical region
I-BAR domain
The I-BAR domain (inverse BAR) was first identified in IRSp53 based on sequence homology as an F-actin crosslinking domain at the N-terminal region of mammalian IRSp53 and MIM (missing-in-metastasis) proteins [41]. The IRSp53 protein family comprises IRTKS (insulin receptor tyrosine kinase substrate; also known as BAIAP2L1), MIM/ABBA (missing in metastasis/actin-bundling protein with BAIAP2 homology), and FLJ22582 (BAIAP2L2) [42]. Crystal structure analysis of the I-BAR domain of IRSp53 shows strong structural similarity to the BAR domain family. One monomer consists of three α helices that dimerize into an antiparallel structure, which resembles a distinct, rather flat, cigar-shaped curvature [43]. The positively charged lipid-binding surface of I-BAR domains displays a convex geometry, thus recognizing negative membrane curvature (Fig. 1i, j) [43–45]. Like BAR/F-BAR domains, the I-BAR domains of MIM and IRSp53 can also directly bind and deform membranes into tubules in vitro [43–45]. However, I-BAR modules stabilize membrane tubules that penetrate into liposomes when bound to the membrane [45]. Strikingly, different I-BAR domains deform PI(4,5)P2-rich membranes through distinct mechanisms. The I-BAR domains of IRSp53 and IRTKS bind membranes mainly through electrostatic interactions, whereas the I-BAR domains of MIM and ABBA insert an additional amphipathic helix into the membrane bilayer, resulting in a larger tubule diameter in vitro and more efficient filopodia formation in vivo [44].
Oligomerization of BAR domains
Oligomerization around the curved membrane is essential for BAR domain function. In vivo, overexpression of BAR/F-BAR domains or BAR/F-BAR domain proteins generates membrane tubules in cells. However, different BAR domain proteins segregate within distinct areas of these tubules that correspond to their preferred curvature [15, 46]. In vitro, liposomal assays have been used to study the membrane tubulating activity of BAR domains. Previous studies have shown that BAR domain-containing proteins, such as amphiphysin, endophilin, syndapin, FBP17 and CIP4, are able to bind lipids and induce tubulation of liposomes [15, 21, 47–49]. A recent cryo-EM analysis of the CIP4 F-BAR domain illustrated how the BAR domain associates with membranes to form cylindrical tubules [49]. To form membrane tubules, F-BAR domain self-assemble into a helical oligomeric coat. Besides the tip-to-tip interactions found in the crystal structure of FBP17 F-BAR [37], the broad contacts between laterally-adjacent dimers are also required for the oligomerization of the F-BAR domain. Additionally, reorientation of the lateral interaction surface crucially triggers the oligomerization of F-BAR domain and the propagation of membrane bending. By fitting the crystal structure of the CIP4 F-BAR domain into cryo-electron microscopic (EM) reconstructions of membrane tubules, a cluster of positive residues (R/K) on the concave surface of the F-BAR module was identified that is required for membrane binding and for enabling rigid F-BAR dimers to deform the membrane [49]. This mechanism was also confirmed by a recent cryo-EM analysis of endophilin A1 N-BAR domain assemblies on liposomes [50].
BAR domain function
BAR domain superfamily proteins have been identified as central regulators of membrane remodeling, including the formation of plasma membrane protrusions and invaginations as well as formation of vesicular or tubular transport carriers (Fig. 3a). As examples of BAR domain protein function, we focus here on clathrin-mediated endocytosis and the formation of filopodial membrane protrusions.
Fig. 3.
Biological functions and regulation of BAR domain superfamily proteins. a BAR domain proteins function in the generation of membrane protrusions and membrane invaginations, as well as in vesicle fission. b Regulation of BAR domain proteins. Mechanisms include autoinhibition (a), phosphorylation/dephosphorylation (b), and protein–protein interactions (c). The latter may keep BAR domain proteins in an “inactive” state. c Membrane targeting of BAR domain proteins. Interactions with GTPases aid targeting of some BAR domain proteins to specific membrane sites. See text for further details
BAR domain function in clathrin-mediated endocytosis
Clathrin-mediated endocytosis is the major pathway for the uptake of nutrients and signaling molecules in higher eukaryotic cells and for the recycling or degradation of transmembrane receptors including the activity-induced reformation of synaptic vesicles at nerve terminals in neurons [51–54]. A clathrin coat is assembled on the cytoplasmic face of the plasma membrane, which progressively matures into a clathrin-coated pit (CCP). Late-stage CCPs constrict at the neck region and finally pinch off from the membrane in a reaction driven by the oligomeric GTPase dynamin. Formation of endocytic vesicles involves differentially curved membrane intermediates at each stage of the pathway, thereby necessitating tight control of membrane remodeling by BAR domain proteins including the N-BAR proteins endophilin and amphiphysin, the BAR-PX domain protein SNX9, and the F-BAR domain proteins FCHo and syndapin. Many of these factors, in addition to their BAR/F-BAR domains, also contain a Src homology 3 domain (SH3) domain that allows them to associate with other endocytic and cytoskeletal proteins, such as the GTPase dynamin (mediating vesicle fission), or N-WASP (an initiator for actin polymerization) (Fig. 3a) [6, 15, 55–59].
During clathrin-mediated endocytosis, F-BAR domain proteins typically arrive early to the site of endocytosis, and may be involved in the nucleation of CCPs [60–63]. The F-BAR-containing proteins FCHo arrive early at sites of endocytosis and specifically bind to PtdIns(4,5)P2-enriched membranes and sculpt the initial vesicle bud site by their F-BAR-encoded membrane-bending activity [61]. Recent studies suggest that another F-BAR domain protein FBP17 has a dual role in shaping and stabilizing membrane curvature via its F-BAR domain and in recruiting machinery for actin polymerization [58]. The latter may provide the force to push vesicle buds away from the plasma membrane and/or to narrow down the neck region at the stage of coat propagation. Blocking clathrin assembly prevents deep membrane invaginations even in the presence of abundant membrane-tubulating proteins in the cytosol, thereby inhibiting CCP maturation at an early stage [58].
Deep clathrin-coated membrane invaginations eventually undergo dynamin-mediated fission (Fig. 3a). Dynamin is a multidomain protein containing a GTPase domain that binds and hydrolyzes GTP, a middle domain, a PH domain for interactions with lipid membranes, a GTPase effector domain (GED) and a proline-rich domain (PRD) at the carboxyl terminal end, which facilitates dynamin recruitment by SH3 domain proteins such as SNX9, amphiphysin, endophilin, and intersectin [64–66]. Oligomeric dynamin recruited to the “neck region” of late stage CCPs hydrolyzes GTP, resulting in a conformational change within the protein that causes vesicle scission [65–68]. Dynamins function together with BAR-SH3 domain proteins, such as amphiphysin, endophilin and SNX9 in bud neck constriction and membrane fission. Consistent with this, dynamin preferentially associates with narrow tubules of a diameter similar to that generated by the N-BAR domains of amphiphysin and endophilin in vitro [47, 48, 58]. In spite of the similar morphological appearance of endophilin- and amphiphysin-coated tubules, both proteins show opposite effects with respect to the regulation of dynamin-mediated membrane fission. Amphiphysin upon co-assembly with dynamin into ring-like structures enhances the fragmentation of liposomal tubules in the presence of GTP [48], whereas endophilin inhibits dynamin-GTP-dependent vesiculation of lipid tubules [47]. Similar effects to those seen for endophilin have been reported for SNX9, which is recruited transiently to CCPs together with a burst of dynamin during late stages of vesicle formation [69]. Although the molecular mechanistic basis for the differential behavior of BAR domain proteins remains unknown, one might speculate that endophilin and SNX9 are stabilized at CCPs by the presence of the amphipathic helix inserted into the BAR domain of endophilin or the PX domain of SNX9, respectively. Hence, structural variations with respect to the interaction of BAR domain proteins with membranes may at least in part underlie their functional diversity at different stages of vesicle formation.
A common, yet poorly understood, feature of many BAR domain proteins in endocytosis is their functional and/or physical association with the machinery for actin polymerization. As shown for FBP17 and other BAR domain proteins, the concomitant presence of SH3 domains allows for recruitment of actin nucleation promoting factors including N-WASP and ARP2/3 to sites of endocytosis. Local actin polymerization may serve to push vesicle buds off from the plasma membrane, facilitate dynamin-mediated fission, and/or propel primary endocytic vesicles away from the cell surface. Clearly, further studies are needed to fully explore the underlying molecular mechanisms.
Filopodial membrane protrusions
Filopodia are often found embedded in or protruding from the lamellipodial actin network [70, 71] and play an important role in neurite outgrowth, wound healing and cell migration, and function as precursors for dendritic spines in neurons [72]. A prominent feature of I-BAR domains is their ability to induce filopodia formation (Fig. 3a). A well-studied example is the I-BAR domain protein IRSp53. This protein features an autoinhibited conformation in its “inactive” state [4]. The interaction of IRSp53 with the small GTPase Cdc42 in its GTP-bound state releases IRSp53 from autoinhibition and recruits it to the plasma membrane, where IPSp53 via its I-BAR domain induces negative membrane curvature. Induction of membrane protrusions also involves SH3 domain-mediated recruitment of the IPSp53 specific binding partners mDia (a downstream effector of the small GTPase Rho implicated in stress fiber formation and cytokinesis [73]) and Mena (a member of Ena/WASP family protein involved in actin cytoskeleton for adhesion and cell migration [74]), which synergistically promote filopodia formation [4]. Surprisingly, in addition to I-BAR domains, negative membrane curvature is also promoted by a subset of F-BAR domain-containing factors including syndapins and srGAP2. Overexpression of the F-BAR domain of syndapin in addition to the generation of positively curved membrane tubules causes the formation of membrane protrusions or filopodial extensions [39, 75]. A similar phenomenon was observed in the case of the F-BAR domain-containing protein srGAP2. Membrane protrusions formed by srGAP2 negatively regulate neuronal migration and induce neurite outgrowth and branching [76]. More recent studies have also shown that the F-BAR domain of SRGP-1 induces negative membrane curvature, thereby facilitating cell–cell adhesion during C. elegans morphogenesis [77]. The underlying mechanism for the peculiar activity of F-BAR domain to induce negative membrane curvature remains uncertain.
Filopodia formation by I-BAR domain-containing proteins depends on the inherent negative curvature of the I-BAR domain itself and on phospholipid-binding residues on the convex side of the I-BAR homodimers. Initial I-BAR-mediated membrane deformation may then be followed by actin polymerization into the space generated, i.e. by the interaction between the SH3 domain of IRSp53 and actin polymerization factors [3, 44, 45, 72]. However, work on SRGP-1 suggests that the formation of filopodia driven by its F-BAR domain occurs independently from actin polymerization [77]. In contrast, the F-BAR family protein PSTPIP 2 induces filopodia by directly associating with F-actin [78]. Hence, more than one mechanism may underlie BAR domain protein-mediated filopodia formation.
Regulation of BAR domain protein function
Since BAR domain proteins are involved in dynamic membrane remodeling by binding and bending cell membranes and by re-organizing the cytoskeleton, their function must be tightly regulated. These mechanisms of regulation include autoinhibition, phosphorylation/dephosphorylation, and interactions with other proteins (Fig. 3b).
Autoinhibition
Multidomain proteins are frequently observed to form autoinhibitory conformations that arise from intramolecular interactions [79–82]. This is exemplified by the recently determined crystal structure of syndapin 1. Autoinhibition is achieved by the association of its F-BAR and SH3 domains, thereby blocking the membrane-tubulating activity of the F-BAR domain in living cells [39, 83]. Release from the clamped conformation is driven by association of syndapin’s SH3 domain with the PRD of dynamin 1 unlocking its potent membrane-bending activity. A similar autoinhibitory mechanism has been proposed for srGAP2 and SRGP-1 [76, 77]. A molecularly distinct autoinhibitory mechanism involving the BAR and GAP domains has recently been proposed for members of the GRAF family (GTPase regulator associated with focal adhesion kinase) and the Arf GTPase-activating protein ASAP [84, 85].
Phosphorylation/dephosphorylation
Phosphoregulation of BAR domain proteins has shown demonstrated early on for the F-BAR domain protein Cdc15, a protein with a C-terminal SH3 domain that plays important roles in a variety of cellular processes including endocytosis, cytokinesis, cell motility, and neuritogenesis [86–88]. In S. pombe, Cdc15 is an early-acting component in the formation of the contractile ring during cytokinesis. This function involves nucleation of F-actin filament assembly [88] via interactions of Cdc15 with formin and the type I myosin Myo1. Furthermore, Cdc15 stabilizes the contractile ring by associating with the C2-domain protein Fic1 and with paxillin homolog Pxl1 via its C-terminal SH3 domain [89]. Recent studies show that the biological function of Cdc15 in cytokinesis is tightly regulated by phosphorylation/dephosphorylation [90]. Multi-site phosphorylation of Cdc15 within the linker region connecting the F-BAR and the SH3 domains generates a closed inactive conformation (Fig. 3). This modification abolishes Cdc15 assembly at the division site in interphase cells and blocks complex formation with its above-mentioned binding partners. Dephosphorylation of Cdc15 releases this intramolecular block, thereby generating an open active conformation, in which the F-BAR domain engages the membrane and Cdc15 is able to interact with its binding partners [90]. A phosphoregulatory mechanism also controls the activity of Hof 1, the homolog of PSTPIP1 in mammals [91, 92], and Cdc15 in fission yeast [93], which is released from autoinhibition by Dbf2 kinase-mediated phosphorylation [94].
Regulation by protein interactions
As mentioned above, phosphorylation of Hof1 by the protein kinase Dbf2 activates its function. However, activity of Hof1 in addition is controlled by its association with other proteins. In budding yeast, Hof1 plays an essential role in the regulation of cytokinesis during which an actomyosin ring contracts to trigger cleavage furrow formation and membrane ingression [95]. Hof1 accumulates at the site of cell division once cells enter a new cell cycle [96], but its function is blocked by its direct binding to septins [94]. During cytokinesis, phosphorylation of Hof1 by Dbf2 relocates the protein from septins to the actomyosin ring.
BAR domain proteins have also been shown to be regulated by interaction with small GTPases. As association with small GTPases contributes to BAR domain targeting to membranes, these mechanism are discussed in further detail below.
Membrane targeting of BAR domain proteins
From the above, it is clear that BAR domain protein function needs to be tightly controlled. This includes the correct targeting of BAR domain proteins to organelles or organellar membrane subdomains. How is this achieved? While BAR domain proteins directly bind to membrane phospholipids, these interactions on their own are often insufficient to confer specific association with membranes. Several studies link membrane targeting of BAR domain proteins to signaling pathways involving small GTPases (Fig. 3c) [4, 6, 12, 97–99]. Small GTPases cycle between an active GTP-bound and an inactive GDP-bound state. The GTP/GDP cycle functions as a molecular switch through which effector function including membrane trafficking, actin dynamics, or cell signaling cascades are regulated [100–104]. Examples include regulation of filopodia formation by Cdc42 [4] and Rac-dependent formation of lamellipodial protrusions at the cell periphery [105]. Furthermore, subsets of small GTPases have been demonstrated to bind to and deform membranes via insertion of amphipathic helices or hydrophobic lipid moieties (i.e., myristoyl, prenyl, farnesyl, or palmitoyl groups) [106–110].
The BAR domain protein Arfaptin was originally identified as a binding partner of the Arf family of small GTPases. Recent work has shown that Arl1 associates with the BAR domain of Arfaptin in a GTP-dependent manner [97]. Overexpression of Arfaptin induces formation of Golgi-derived membrane tubules, while exogenous expression of Arl1 alone neither generates membrane tubules nor enhances Arfaptin-dependent tubulation activity. However, depletion of Arl1 abolishes the association of Arfaptin with the Golgi complex, a defect that can be cured by re-expression of exogenous Arl1. Thus, Arl1 is required for targeting Arfaptins to Golgi membranes. Given that Arl1 itself associates with membranes and generates membrane curvature, the BAR domain of Arfaptin may recognize lipid packing defects induced by Arl1-GTP, thereby facilitating the local production of highly curved membranes. Similar principles may target BAR domain proteins with GAP activity such as srGAP, GRAF, and ASAP to their correct subcellular location, although the precise molecular determinants involved may differ from those used by Arl1–Arfaptin. Indeed, biochemical analyses indicate that the early endosomal protein APPL associates with Rab5 via its BAR and PH domains, and that these interactions are critical for APPL-mediated regulation of cell proliferation [98, 99] and for its targeting to endosomal membranes [98]. Consistent with these data, overexpression of inactive GDP-bound Rab35S34N causes the redistribution of endogenous APPL endosomes to the cytoplasm [98]. Collectively, these results support the hypothesis that membrane targeting of APPL depends on the small GTPase Rab5.
Other small GTPases besides Arl1 and Rab5 have also been linked to BAR domain protein association with membranes. For example, Cdc42 has been shown to regulate membrane association of I-BAR and F-BAR domain proteins [4, 6, 111]. Cdc42 directly associates with the I-BAR domain protein IRSp53 via a partial Cdc42/Rac interactive binding region (CRIB) motif in a GTP-dependent manner. Activation of Cdc42 by expressing the DH/PH domain of FGD1, a Cdc42-specific guanine nucleotide exchange factor, dramatically increases filopodia formation, an effect blocked by an inhibitory fragment derived from IRSp53 [4]. Similarly, active Cdc42 has been observed to directly interact with the F-BAR domain proteins Toca-1 and CIP4 [6, 35, 111]. In these cases, association with Cdc42 does not involve a CRIB motif but rather occurs via a protein kinase C-related kinase homology region (HR1) domain. Disruption of complex formation between Cdc42 and Toca-1 impairs the ability of Cdc42 to facilitate N-WASP-WIP-mediated actin polymerization [6]. Activated GTP-bound Cdc42 (Q61L) significantly enhances the tubulating ability of CIP4, while the same mutant inhibits FBP17-induced membrane tubulation [112]. Cdc42 possibly also associates with other F-BAR domain proteins such as syndapin. Expression of mutant Cdc42 abrogates the ability of overexpressed syndapin to control neuromorphogenesis as evidenced by a reduced number of dendrites and branching points [2]. Whether these effects reflect a direct interaction between Cdc42 and syndapin remains to be determined. Most recently, it has been shown that the Cdc42 relative Rac1 interacts with syndapin 2 and regulates cell spreading and migration [113].
Conclusions and perspectives
It is clear that the BAR domain superfamily proteins play important roles in a wide variety of cellular processes by regulating dynamic membrane remodeling, including the formation of membrane vesicles or tubules, membrane protrusions, and invaginations as well as membrane fission. While the molecular basis for these various activities has become clear through combined structural and cell biological studies, much remains to be learnt about the physiological roles of BAR domain proteins at the organismic level. Moreover, we have only just begun to get a glimpse onto the modes and mechanisms of regulating BAR domain protein activity and function, i.e., during stimulus-induced signaling cascades. Lastly, given that the human proteome contains more than 60 members of the BAR domain protein superfamily, it is likely that many of their cell physiological functions have not been explored. All of these questions deserve attention and will need to be addressed in future studies.
Acknowledgment
We gratefully acknowledge support of our own work by the German funding agency DFG (SFB 449/TP A11; SFB 958/A07; FOR 806-HA2686/3-2).
References
- 1.McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 2.Dharmalingam E, Haeckel A, Pinyol R, Schwintzer L, Koch D, Kessels MM, Qualmann B. F-BAR proteins of the syndapin family shape the plasma membrane and are crucial for neuromorphogenesis. J Neurosci. 2009;29:13315–13327. doi: 10.1523/JNEUROSCI.3973-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim KB, Bu W, Goh WI, Koh E, Ong SH, Pawson T, Sudhaharan T, Ahmed S. The Cdc42 effector IRSp53 generates filopodia by coupling membrane protrusion with actin dynamics. J Biol Chem. 2008;283:20454–20472. doi: 10.1074/jbc.M710185200. [DOI] [PubMed] [Google Scholar]
- 4.Krugmann S, Jordens I, Gevaert K, Driessens M, Vandekerckhove J, Hall A. Cdc42 induces filopodia by promoting the formation of an IRSp53:Mena complex. Curr Biol. 2001;11:1645–1655. doi: 10.1016/s0960-9822(01)00506-1. [DOI] [PubMed] [Google Scholar]
- 5.Kamioka Y, Fukuhara S, Sawa H, Nagashima K, Masuda M, Matsuda M, Mochizuki N. A novel dynamin-associating molecule, formin-binding protein 17, induces tubular membrane invaginations and participates in endocytosis. J Biol Chem. 2004;279:40091–40099. doi: 10.1074/jbc.M404899200. [DOI] [PubMed] [Google Scholar]
- 6.Ho HY, Rohatgi R, Lebensohn AM, Le M, Li J, Gygi SP, Kirschner MW. Toca-1 mediates Cdc42-dependent actin nucleation by activating the N-WASP-WIP complex. Cell. 2004;118:203–216. doi: 10.1016/j.cell.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 7.Salazar MA, Kwiatkowski AV, Pellegrini L, Cestra G, Butler MH, Rossman KL, Serna DM, Sondek J, Gertler FB, De Camilli P. Tuba, a novel protein containing bin/amphiphysin/Rvs and Dbl homology domains, links dynamin to regulation of the actin cytoskeleton. J Biol Chem. 2003;278:49031–49043. doi: 10.1074/jbc.M308104200. [DOI] [PubMed] [Google Scholar]
- 8.Lichte B, Veh RW, Meyer HE, Kilimann MW. Amphiphysin, a novel protein associated with synaptic vesicles. EMBO J. 1992;11:2521–2530. doi: 10.1002/j.1460-2075.1992.tb05317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sivadon P, Bauer F, Aigle M, Crouzet M. Actin cytoskeleton and budding pattern are altered in the yeast rvs161 mutant: the Rvs161 protein shares common domains with the brain protein amphiphysin. Mol Gen Genet. 1995;246:485–495. doi: 10.1007/BF00290452. [DOI] [PubMed] [Google Scholar]
- 10.David C, McPherson PS, Mundigl O, de Camilli P. A role of amphiphysin in synaptic vesicle endocytosis suggested by its binding to dynamin in nerve terminals. Proc Natl Acad Sci USA. 1996;93:331–335. doi: 10.1073/pnas.93.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakamuro D, Elliott KJ, Wechsler-Reya R, Prendergast GC. BIN1 is a novel MYC-interacting protein with features of a tumour suppressor. Nat Genet. 1996;14:69–77. doi: 10.1038/ng0996-69. [DOI] [PubMed] [Google Scholar]
- 12.Tarricone C, Xiao B, Justin N, Walker PA, Rittinger K, Gamblin SJ, Smerdon SJ. The structural basis of Arfaptin-mediated cross-talk between Rac and Arf signalling pathways. Nature. 2001;411:215–219. doi: 10.1038/35075620. [DOI] [PubMed] [Google Scholar]
- 13.Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 14.Blood PD, Voth GA. Direct observation of Bin/amphiphysin/Rvs (BAR) domain-induced membrane curvature by means of molecular dynamics simulations. Proc Natl Acad Sci USA. 2006;103:15068–15072. doi: 10.1073/pnas.0603917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itoh T, Erdmann KS, Roux A, Habermann B, Werner H, De Camilli P. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev Cell. 2005;9:791–804. doi: 10.1016/j.devcel.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Drin G, Antonny B. Amphipathic helices and membrane curvature. FEBS Lett. 2010;584:1840–1847. doi: 10.1016/j.febslet.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Bhatia VK, Madsen KL, Bolinger PY, Kunding A, Hedegard P, Gether U, Stamou D. Amphipathic motifs in BAR domains are essential for membrane curvature sensing. EMBO J. 2009;28:3303–3314. doi: 10.1038/emboj.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui H, Lyman E, Voth GA. Mechanism of membrane curvature sensing by amphipathic helix containing proteins. Biophys J. 2011;100:1271–1279. doi: 10.1016/j.bpj.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jao CC, Hegde BG, Gallop JL, Hegde PB, McMahon HT, Haworth IS, Langen R. Roles of amphipathic helices and the bin/amphiphysin/rvs (BAR) domain of endophilin in membrane curvature generation. J Biol Chem. 2010;285:20164–20170. doi: 10.1074/jbc.M110.127811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masuda M, Takeda S, Sone M, Ohki T, Mori H, Kamioka Y, Mochizuki N. Endophilin BAR domain drives membrane curvature by two newly identified structure-based mechanisms. EMBO J. 2006;25:2889–2897. doi: 10.1038/sj.emboj.7601176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallop JL, Jao CC, Kent HM, Butler PJ, Evans PR, Langen R, McMahon HT. Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J. 2006;25:2898–2910. doi: 10.1038/sj.emboj.7601174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 23.Campelo F, McMahon HT, Kozlov MM. The hydrophobic insertion mechanism of membrane curvature generation by proteins. Biophys J. 2008;95:2325–2339. doi: 10.1529/biophysj.108.133173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatia VK, Hatzakis NS, Stamou D. A unifying mechanism accounts for sensing of membrane curvature by BAR domains, amphipathic helices and membrane-anchored proteins. Semin Cell Dev Biol. 2010;21:381–390. doi: 10.1016/j.semcdb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Epand RM, Shai Y, Segrest JP, Anantharamaiah GM. Mechanisms for the modulation of membrane bilayer properties by amphipathic helical peptides. Biopolymers. 1995;37:319–338. doi: 10.1002/bip.360370504. [DOI] [PubMed] [Google Scholar]
- 26.Cui H, Ayton GS, Voth GA. Membrane binding by the endophilin N-BAR domain. Biophys J. 2009;97:2746–2753. doi: 10.1016/j.bpj.2009.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang DS, Shaw G. The association of the C-terminal region of beta I sigma II spectrin to brain membranes is mediated by a PH domain, does not require membrane proteins, and coincides with a inositol-1, 4, 5 triphosphate binding site. Biochem Biophys Res Commun. 1995;217:608–615. doi: 10.1006/bbrc.1995.2818. [DOI] [PubMed] [Google Scholar]
- 28.Karathanassis D, Stahelin RV, Bravo J, Perisic O, Pacold CM, Cho W, Williams RL. Binding of the PX domain of p47(phox) to phosphatidylinositol 3, 4-bisphosphate and phosphatidic acid is masked by an intramolecular interaction. EMBO J. 2002;21:5057–5068. doi: 10.1093/emboj/cdf519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ago T, Kuribayashi F, Hiroaki H, Takeya R, Ito T, Kohda D, Sumimoto H. Phosphorylation of p47phox directs phox homology domain from SH3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation. Proc Natl Acad Sci USA. 2003;100:4474–4479. doi: 10.1073/pnas.0735712100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlton JG, Bujny MV, Peter BJ, Oorschot VM, Rutherford A, Arkell RS, Klumperman J, McMahon HT, Cullen PJ. Sorting nexin-2 is associated with tubular elements of the early endosome, but is not essential for retromer-mediated endosome-to-TGN transport. J Cell Sci. 2005;118:4527–4539. doi: 10.1242/jcs.02568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlton J, Bujny M, Peter BJ, Oorschot VM, Rutherford A, Mellor H, Klumperman J, McMahon HT, Cullen PJ. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high- curvature membranes and 3-phosphoinositides. Curr Biol. 2004;14:1791–1800. doi: 10.1016/j.cub.2004.09.077. [DOI] [PubMed] [Google Scholar]
- 32.Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, Yan D, Selva EM, Lin X. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell. 2008;14:120–131. doi: 10.1016/j.devcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Rojas R, Kametaka S, Haft CR, Bonifacino JS. Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors. Mol Cell Biol. 2007;27:1112–1124. doi: 10.1128/MCB.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh T, De Camilli P. BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochim Biophys Acta. 2006;1761:897–912. doi: 10.1016/j.bbalip.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Aspenstrom P. A Cdc42 target protein with homology to the non-kinase domain of FER has a potential role in regulating the actin cytoskeleton. Curr Biol. 1997;7:479–487. doi: 10.1016/s0960-9822(06)00219-3. [DOI] [PubMed] [Google Scholar]
- 36.Roberts-Galbraith RH, Gould KL. Setting the F-BAR: functions and regulation of the F-BAR protein family. Cell Cycle. 2010;9:4091–4097. doi: 10.4161/cc.9.20.13587. [DOI] [PubMed] [Google Scholar]
- 37.Shimada A, Niwa H, Tsujita K, Suetsugu S, Nitta K, Hanawa-Suetsugu K, Akasaka R, Nishino Y, Toyama M, Chen L, Liu ZJ, Wang BC, Yamamoto M, Terada T, Miyazawa A, Tanaka A, Sugano S, Shirouzu M, Nagayama K, Takenawa T, Yokoyama S. Curved EFC/F-BAR-domain dimers are joined end to end into a filament for membrane invagination in endocytosis. Cell. 2007;129:761–772. doi: 10.1016/j.cell.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 38.Henne WM, Kent HM, Ford MG, Hegde BG, Daumke O, Butler PJ, Mittal R, Langen R, Evans PR, McMahon HT. Structure and analysis of FCHo2 F-BAR domain: a dimerizing and membrane recruitment module that effects membrane curvature. Structure. 2007;15:839–852. doi: 10.1016/j.str.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Rao Y, Ma Q, Vahedi-Faridi A, Sundborger A, Pechstein A, Puchkov D, Luo L, Shupliakov O, Saenger W, Haucke V. Molecular basis for SH3 domain regulation of F-BAR-mediated membrane deformation. Proc Natl Acad Sci USA. 2010;107:8213–8218. doi: 10.1073/pnas.1003478107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Q, Navarro MV, Peng G, Molinelli E, Goh SL, Judson BL, Rajashankar KR, Sondermann H. Molecular mechanism of membrane constriction and tubulation mediated by the F-BAR protein Pacsin/Syndapin. Proc Natl Acad Sci USA. 2009;106:12700–12705. doi: 10.1073/pnas.0902974106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamagishi A, Masuda M, Ohki T, Onishi H, Mochizuki N. A novel actin bundling/filopodium-forming domain conserved in insulin receptor tyrosine kinase substrate p53 and missing in metastasis protein. J Biol Chem. 2004;279:14929–14936. doi: 10.1074/jbc.M309408200. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed S, Goh WI, Bu W. I-BAR domains, IRSp53 and filopodium formation. Semin Cell Dev Biol. 2010;21:350–356. doi: 10.1016/j.semcdb.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Millard TH, Bompard G, Heung MY, Dafforn TR, Scott DJ, Machesky LM, Futterer K. Structural basis of filopodia formation induced by the IRSp53/MIM homology domain of human IRSp53. EMBO J. 2005;24:240–250. doi: 10.1038/sj.emboj.7600535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saarikangas J, Zhao H, Pykalainen A, Laurinmaki P, Mattila PK, Kinnunen PK, Butcher SJ, Lappalainen P. Molecular mechanisms of membrane deformation by I-BAR domain proteins. Curr Biol. 2009;19:95–107. doi: 10.1016/j.cub.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 45.Mattila PK, Pykalainen A, Saarikangas J, Paavilainen VO, Vihinen H, Jokitalo E, Lappalainen P. Missing-in-metastasis and IRSp53 deform PI(4, 5)P2-rich membranes by an inverse BAR domain-like mechanism. J Cell Biol. 2007;176:953–964. doi: 10.1083/jcb.200609176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsujita K, Suetsugu S, Sasaki N, Furutani M, Oikawa T, Takenawa T. Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J Cell Biol. 2006;172:269–279. doi: 10.1083/jcb.200508091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farsad K, Ringstad N, Takei K, Floyd SR, Rose K, De Camilli P. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J Cell Biol. 2001;155:193–200. doi: 10.1083/jcb.200107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takei K, Slepnev VI, Haucke V, De Camilli P. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat Cell Biol. 1999;1:33–39. doi: 10.1038/9004. [DOI] [PubMed] [Google Scholar]
- 49.Frost A, Perera R, Roux A, Spasov K, Destaing O, Egelman EH, De Camilli P, Unger VM. Structural basis of membrane invagination by F-BAR domains. Cell. 2008;132:807–817. doi: 10.1016/j.cell.2007.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizuno N, Jao CC, Langen R, Steven AC. Multiple modes of endophilin-mediated conversion of lipid vesicles into coated tubes: implications for synaptic endocytosis. J Biol Chem. 2010;285:23351–23358. doi: 10.1074/jbc.M110.143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jung N, Haucke V. Clathrin-mediated endocytosis at synapses. Traffic. 2007;8:1129–1136. doi: 10.1111/j.1600-0854.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- 52.Teng H, Wilkinson RS. Clathrin-mediated endocytosis near active zones in snake motor boutons. J Neurosci. 2000;20:7986–7993. doi: 10.1523/JNEUROSCI.20-21-07986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis: the physiological mechanism of vesicle retrieval at hippocampal synapses. J Physiol. 2007;585:681–686. doi: 10.1113/jphysiol.2007.139022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sato K, Ernstrom GG, Watanabe S, Weimer RM, Chen CH, Sato M, Siddiqui A, Jorgensen EM, Grant BD. Differential requirements for clathrin in receptor-mediated endocytosis and maintenance of synaptic vesicle pools. Proc Natl Acad Sci USA. 2009;106:1139–1144. doi: 10.1073/pnas.0809541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qualmann B, Kelly RB. Syndapin isoforms participate in receptor-mediated endocytosis and actin organization. J Cell Biol. 2000;148:1047–1062. doi: 10.1083/jcb.148.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dawson JC, Legg JA, Machesky LM. Bar domain proteins: a role in tubulation, scission and actin assembly in clathrin-mediated endocytosis. Trends Cell Biol. 2006;16:493–498. doi: 10.1016/j.tcb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 57.Ferguson SM, Raimondi A, Paradise S, Shen H, Mesaki K, Ferguson A, Destaing O, Ko G, Takasaki J, Cremona O, OT E, De Camilli P. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell. 2009;17:811–822. doi: 10.1016/j.devcel.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu M, Huang B, Graham M, Raimondi A, Heuser JE, Zhuang X, De Camilli P. Coupling between clathrin-dependent endocytic budding and F-BAR-dependent tubulation in a cell-free system. Nat Cell Biol. 2010;12:902–908. doi: 10.1038/ncb2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamada H, Padilla-Parra S, Park SJ, Itoh T, Chaineau M, Monaldi I, Cremona O, Benfenati F, De Camilli P, Coppey-Moisan M, Tramier M, Galli T, Takei K. Dynamic interaction of amphiphysin with N-WASP regulates actin assembly. J Biol Chem. 2009;284:34244–34256. doi: 10.1074/jbc.M109.064204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boettner DR, D’Agostino JL, Torres OT, Daugherty-Clarke K, Uygur A, Reider A, Wendland B, Lemmon SK, Goode BL. The F-BAR protein Syp1 negatively regulates WASp-Arp2/3 complex activity during endocytic patch formation. Curr Biol. 2009;19:1979–1987. doi: 10.1016/j.cub.2009.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henne WM, Boucrot E, Meinecke M, Evergren E, Vallis Y, Mittal R, McMahon HT. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science. 2010;328:1281–1284. doi: 10.1126/science.1188462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reider A, Barker SL, Mishra SK, Im YJ, Maldonado-Baez L, Hurley JH, Traub LM, Wendland B. Syp1 is a conserved endocytic adaptor that contains domains involved in cargo selection and membrane tubulation. EMBO J. 2009;28:3103–3116. doi: 10.1038/emboj.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stimpson HE, Toret CP, Cheng AT, Pauly BS, Drubin DG. Early-arriving Syp1p and Ede1p function in endocytic site placement and formation in budding yeast. Mol Biol Cell. 2009;20:4640–4651. doi: 10.1091/mbc.E09-05-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramachandran R, Surka M, Chappie JS, Fowler DM, Foss TR, Song BD, Schmid SL. The dynamin middle domain is critical for tetramerization and higher-order self-assembly. EMBO J. 2007;26:559–566. doi: 10.1038/sj.emboj.7601491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Praefcke GJ, McMahon HT. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol. 2004;5:133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- 66.Shpetner HS, Herskovits JS, Vallee RB. A binding site for SH3 domains targets dynamin to coated pits. J Biol Chem. 1996;271:13–16. doi: 10.1074/jbc.271.1.13. [DOI] [PubMed] [Google Scholar]
- 67.Hinshaw JE. Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol. 2000;16:483–519. doi: 10.1146/annurev.cellbio.16.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McFadden GI, Ralph SA. Dynamin: the endosymbiosis ring of power? Proc Natl Acad Sci USA. 2003;100:3557–3559. doi: 10.1073/pnas.0831049100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramachandran R, Schmid SL. Real-time detection reveals that effectors couple dynamin’s GTP-dependent conformational changes to the membrane. EMBO J. 2008;27:27–37. doi: 10.1038/sj.emboj.7601961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Small JV, Celis JE. Filament arrangements in negatively stained cultured cells: the organization of actin. Cytobiologie. 1978;16:308–325. [PubMed] [Google Scholar]
- 71.Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, Borisy GG. Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol. 2003;160:409–421. doi: 10.1083/jcb.200210174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- 73.Fujiwara T, Mammoto A, Kim Y, Takai Y. Rho small G-protein-dependent binding of mDia to an Src homology 3 domain-containing IRSp53/BAIAP2. Biochem Biophys Res Commun. 2000;271:626–629. doi: 10.1006/bbrc.2000.2671. [DOI] [PubMed] [Google Scholar]
- 74.Trichet L, Sykes C, Plastino J. Relaxing the actin cytoskeleton for adhesion and movement with Ena/VASP. J Cell Biol. 2008;181:19–25. doi: 10.1083/jcb.200710168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shimada A, Takano K, Shirouzu M, Hanawa-Suetsugu K, Terada T, Toyooka K, Umehara T, Yamamoto M, Yokoyama S, Suetsugu S. Mapping of the basic amino-acid residues responsible for tubulation and cellular protrusion by the EFC/F-BAR domain of pacsin2/Syndapin II. FEBS Lett. 2010;584:1111–1118. doi: 10.1016/j.febslet.2010.02.058. [DOI] [PubMed] [Google Scholar]
- 76.Guerrier S, Coutinho-Budd J, Sassa T, Gresset A, Jordan NV, Chen K, Jin WL, Frost A, Polleux F. The F-BAR domain of srGAP2 induces membrane protrusions required for neuronal migration and morphogenesis. Cell. 2009;138:990–1004. doi: 10.1016/j.cell.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zaidel-Bar R, Joyce MJ, Lynch AM, Witte K, Audhya A, Hardin J. The F-BAR domain of SRGP-1 facilitates cell–cell adhesion during C. elegans morphogenesis. J Cell Biol. 2010;191:761–769. doi: 10.1083/jcb.201005082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chitu V, Pixley FJ, Macaluso F, Larson DR, Condeelis J, Yeung YG, Stanley ER. The PCH family member MAYP/PSTPIP2 directly regulates F-actin bundling and enhances filopodia formation and motility in macrophages. Mol Biol Cell. 2005;16:2947–2959. doi: 10.1091/mbc.E04-10-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404:151–158. doi: 10.1038/35004513. [DOI] [PubMed] [Google Scholar]
- 80.Rohatgi R, Ho HY, Kirschner MW. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J Cell Biol. 2000;150:1299–1310. doi: 10.1083/jcb.150.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murayama K, Shirouzu M, Kawasaki Y, Kato-Murayama M, Hanawa-Suetsugu K, Sakamoto A, Katsura Y, Suenaga A, Toyama M, Terada T, Taiji M, Akiyama T, Yokoyama S. Crystal structure of the rac activator, Asef, reveals its autoinhibitory mechanism. J Biol Chem. 2007;282:4238–4242. doi: 10.1074/jbc.C600234200. [DOI] [PubMed] [Google Scholar]
- 82.Nezami AG, Poy F, Eck MJ. Structure of the autoinhibitory switch in formin mDia1. Structure. 2006;14:257–263. doi: 10.1016/j.str.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 83.Rao Y, Ruckert C, Saenger W, Haucke V (2011) The early steps of endocytosis: from cargo selection to membrane deformation. Eur J Cell Biol (in press) [DOI] [PubMed]
- 84.Eberth A, Lundmark R, Gremer L, Dvorsky R, Koessmeier KT, McMahon HT, Ahmadian MR. A BAR domain-mediated autoinhibitory mechanism for RhoGAPs of the GRAF family. Biochem J. 2009;417:371–377. doi: 10.1042/BJ20081535. [DOI] [PubMed] [Google Scholar]
- 85.Jian X, Brown P, Schuck P, Gruschus JM, Balbo A, Hinshaw JE, Randazzo PA. Autoinhibition of Arf GTPase-activating protein activity by the BAR domain in ASAP1. J Biol Chem. 2009;284:1652–1663. doi: 10.1074/jbc.M804218200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu JQ, Pollard TD. Counting cytokinesis proteins globally and locally in fission yeast. Science. 2005;310:310–314. doi: 10.1126/science.1113230. [DOI] [PubMed] [Google Scholar]
- 87.Wu JQ, Kuhn JR, Kovar DR, Pollard TD. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev Cell. 2003;5:723–734. doi: 10.1016/s1534-5807(03)00324-1. [DOI] [PubMed] [Google Scholar]
- 88.Carnahan RH, Gould KL. The PCH family protein, Cdc15p, recruits two F-actin nucleation pathways to coordinate cytokinetic actin ring formation in Schizosaccharomyces pombe. J Cell Biol. 2003;162:851–862. doi: 10.1083/jcb.200305012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roberts-Galbraith RH, Chen JS, Wang J, Gould KL. The SH3 domains of two PCH family members cooperate in assembly of the Schizosaccharomyces pombe contractile ring. J Cell Biol. 2009;184:113–127. doi: 10.1083/jcb.200806044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roberts-Galbraith RH, Ohi MD, Ballif BA, Chen JS, McLeod I, McDonald WH, Gygi SP, Yates JR, 3rd, Gould KL. Dephosphorylation of F-BAR protein Cdc15 modulates its conformation and stimulates its scaffolding activity at the cell division site. Mol Cell. 2010;39:86–99. doi: 10.1016/j.molcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu Y, Dowbenko D, Lasky LA. PSTPIP 2, a second tyrosine phosphorylated, cytoskeletal-associated protein that binds a PEST-type protein-tyrosine phosphatase. J Biol Chem. 1998;273:30487–30496. doi: 10.1074/jbc.273.46.30487. [DOI] [PubMed] [Google Scholar]
- 92.Spencer S, Dowbenko D, Cheng J, Li W, Brush J, Utzig S, Simanis V, Lasky LA. PSTPIP: a tyrosine phosphorylated cleavage furrow-associated protein that is a substrate for a PEST tyrosine phosphatase. J Cell Biol. 1997;138:845–860. doi: 10.1083/jcb.138.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fankhauser C, Reymond A, Cerutti L, Utzig S, Hofmann K, Simanis V. The S. pombe cdc15 gene is a key element in the reorganization of F-actin at mitosis. Cell. 1995;82:435–444. doi: 10.1016/0092-8674(95)90432-8. [DOI] [PubMed] [Google Scholar]
- 94.Meitinger F, Boehm ME, Hofmann A, Hub B, Zentgraf H, Lehmann WD, Pereira G. Phosphorylation-dependent regulation of the F-BAR protein Hof1 during cytokinesis. Genes Dev. 2011;25:875–888. doi: 10.1101/gad.622411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bi E. Cytokinesis in budding yeast: the relationship between actomyosin ring function and septum formation. Cell Struct Funct. 2001;26:529–537. doi: 10.1247/csf.26.529. [DOI] [PubMed] [Google Scholar]
- 96.Lippincott J, Li R. Dual function of Cyk2, a cdc15/PSTPIP family protein, in regulating actomyosin ring dynamics and septin distribution. J Cell Biol. 1998;143:1947–1960. doi: 10.1083/jcb.143.7.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Man Z, Kondo Y, Koga H, Umino H, Nakayama K, Shin HW. Arfaptins are localized to the trans-Golgi by interaction with Arl1, but not Arfs. J Biol Chem. 2011;286:11569–11578. doi: 10.1074/jbc.M110.201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG, Zerial M. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- 99.Zhu G, Chen J, Liu J, Brunzelle JS, Huang B, Wakeham N, Terzyan S, Li X, Rao Z, Li G, Zhang XC. Structure of the APPL1 BAR-PH domain and characterization of its interaction with Rab5. EMBO J. 2007;26:3484–3493. doi: 10.1038/sj.emboj.7601771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 101.Sit ST, Manser E. Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci. 2011;124:679–683. doi: 10.1242/jcs.064964. [DOI] [PubMed] [Google Scholar]
- 102.Aspenstrom P. The Rho GTPases have multiple effects on the actin cytoskeleton. Exp Cell Res. 1999;246:20–25. doi: 10.1006/excr.1998.4300. [DOI] [PubMed] [Google Scholar]
- 103.Hall A, Nobes CD. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos Trans R Soc Lond B Biol Sci. 2000;355:965–970. doi: 10.1098/rstb.2000.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Myers KR, Casanova JE. Regulation of actin cytoskeleton dynamics by Arf-family GTPases. Trends Cell Biol. 2008;18:184–192. doi: 10.1016/j.tcb.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 106.Huang M, Weissman JT, Beraud-Dufour S, Luan P, Wang C, Chen W, Aridor M, Wilson IA, Balch WE. Crystal structure of Sar1-GDP at 1.7 A resolution and the role of the NH2 terminus in ER export. J Cell Biol. 2001;155:937–948. doi: 10.1083/jcb.200106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rao Y, Bian C, Yuan C, Li Y, Chen L, Ye X, Huang Z, Huang M. An open conformation of switch I revealed by Sar1-GDP crystal structure at low Mg2+ Biochem Biophys Res Commun. 2006;348:908–915. doi: 10.1016/j.bbrc.2006.07.148. [DOI] [PubMed] [Google Scholar]
- 108.Antonny B, Beraud-Dufour S, Chardin P, Chabre M. N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry. 1997;36:4675–4684. doi: 10.1021/bi962252b. [DOI] [PubMed] [Google Scholar]
- 109.Goldberg J. Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell. 1998;95:237–248. doi: 10.1016/s0092-8674(00)81754-7. [DOI] [PubMed] [Google Scholar]
- 110.Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 111.Tian L, Nelson DL, Stewart DM. Cdc42-interacting protein 4 mediates binding of the Wiskott-Aldrich syndrome protein to microtubules. J Biol Chem. 2000;275:7854–7861. doi: 10.1074/jbc.275.11.7854. [DOI] [PubMed] [Google Scholar]
- 112.Toguchi M, Richnau N, Ruusala A, Aspenstrom P. Members of the CIP4 family of proteins participate in the regulation of platelet-derived growth factor receptor-beta-dependent actin reorganization and migration. Biol Cell. 2010;102:215–230. doi: 10.1042/BC20090033. [DOI] [PubMed] [Google Scholar]
- 113.de Kreuk BJ, Nethe M, Fernandez-Borja M, Anthony EC, Hensbergen PJ, Deelder AM, Plomann M, Hordijk PL (2011) The F-BAR domain protein PACSIN2 associates with Rac1 and regulates cell spreading and migration. J Cell Sci (in press) [DOI] [PubMed]