Abstract
The adult brain most probably reaches its highest degree of plasticity with the lifelong generation and integration of new neurons in the hippocampus and olfactory system. Neural precursor cells (NPCs) residing both in the subgranular zone of the dentate gyrus and in the subventricular zone of the lateral ventricles continuously generate neurons that populate the dentate gyrus and the olfactory bulb, respectively. The regulation of NPC proliferation in the adult brain has been widely investigated in the past few years. Yet, the intrinsic cell cycle machinery underlying NPC proliferation remains largely unexplored. In this review, we discuss the cell cycle components that are involved in the regulation of NPC proliferation in both neurogenic areas of the adult brain.
Keywords: Adult neurogenesis, Cell cycle, Proliferation, Cdks, Cyclins, Dentate gyrus, Subventricular zone
Introduction
Several decades of substantial advances in the field of neurosciences have highlighted the outstanding plasticity of the adult mammalian brain. One intriguing facet of brain plasticity is its ability to generate new neurons in two discrete areas [1]. Indeed, neural precursor cells (NPCs) of both the dentate gyrus (DG) of the hippocampus and the subventricular zone (SVZ) retain the ability to proliferate and undergo neuronal differentiation throughout adulthood [2]. Consequently, understanding the mechanisms of adult neurogenesis will contribute to the development of novel stem cell-based strategies to replace neuronal loss following neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease or stroke [3, 4].
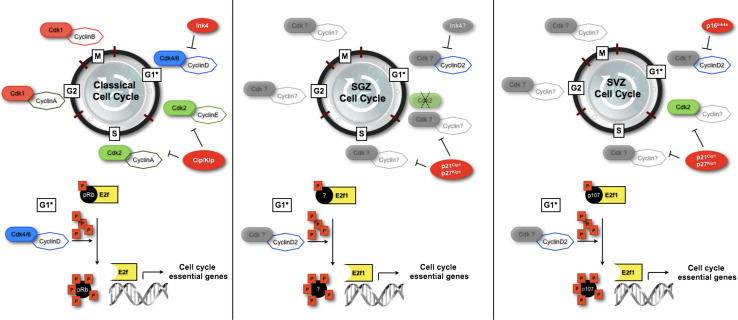
In mammals, the cell cycle is driven by the concerted action of cyclin-dependent kinases (Cdks) and their activating partners, the cyclins (Fig. 1) [5]. During the G1 phase, Cdk4/6-cyclin D complexes progressively phosphorylate the retinoblastoma protein (pRb), causing E2f transcription factors to promote the transcription of the genes required for cell cycle progression, including cyclin E [6, 7]. Cdk2-cyclin E complexes further phosphorylate pRb, leading to its complete inactivation and to a wave of transcriptional activity essential for DNA replication phase (i.e., S phase) entry [6, 7]. Cdk2 then partners with cyclin A to drive the cell through S phase, and at the end of this cell cycle phase, cyclin A associates with Cdk1 (Fig. 1). The resulting complexes facilitate the completion of the G2 phase. Finally, Cdk1-cyclin B complexes contribute to G2-M transition [8], and direct the structural and regulatory events during mitosis. Importantly, Cdk activity is negatively regulated by the members of the Ink4 and Cip/Kip family, the so-called Cdk-inhibitors (CKIs) (Fig. 1) [9]. Altogether, these elements orchestrate the progression through the different phases of the cell cycle that ultimately leads to the mitotic segregation of the two daughter cells [10, 11].
Fig. 1.
Cell cycle regulation in the adult SGZ and SVZ compared to the classical model of the cell cycle. The dashed line on Cdk2 in the SGZ cell cycle indicates its dispensability to the process. Variations of cell cycle and cell cycle phase durations may represent a key aspect of the regulation of NPC proliferation in the adult brain, as it does during embryonic brain development
A number of studies over the past few years have challenged the importance of the canonical cell cycle pathway to organismal development. Notably, the use of mutant mouse models has revealed that a considerable number of cell cycle regulators are not essential for the survival of the organism [12–14]. Indeed, the requirement for particular cell cycle regulators is often cell-type specific, and functional redundancy occurs frequently within families of cell cycle regulators. Altogether, these data highlight the need for a more integrative view of the requirement for specific cell cycle regulatory molecules in defined tissues [15], including the adult brain.
Although several studies have highlighted the importance of intrinsic cell cycle components in regulating NPC proliferation during development [16–24], their putative role in the regulation of NPC proliferation in the adult neurogenic niches has received only minor consideration. However, we believe that an improved knowledge of the intrinsic cell cycle regulation of adult NPCs will be necessary to develop new cell-based regenerative therapies. In this context, we summarise the research that has already been done on the cell cycle constituents as key regulators of adult neurogenesis.
Cdks
Five Cdks directly control the progression through the mammalian cell cycle: Cdk1, Cdk2, Cdk3, Cdk4 and Cdk6, Cdk2/3/4/6 being considered as “interphase Cdks” [25]. However, although Cdk3 is expressed in human cells [26], most laboratory mouse strains lack Cdk3 owing to a naturally occurring mutation [27]. Beyond these cell cycle-related Cdks, an atypical Cdk, Cdk5, has been characterised. Unlike Cdk1 and interphase Cdks, Cdk5 is regulated by its own activators p35 and p39 rather than by cyclins [28–30], plays no apparent role in cell cycle regulation and is predominantly expressed in postmitotic neurons [31].
The case of Cdk1 is special as mice lacking Cdk1 fail to reach the morula stage (i.e., E2.5) [32], precluding any study of its requirement for the proliferation of a specific cell type at a given developmental time point or during adulthood using Cdk1 knockout mice. This issue will be discussed below (see “Conclusions and future directions”). The remaining cell cycle-related Cdks were studied in various tissues using mutant mouse models. Surprisingly, several studies reported that knockout mice mutant for a single interphase Cdk (i.e., Cdk2, Cdk4, Cdk6) were viable and developed normally until adulthood [12, 14, 33, 34].
In addition, even if combined deletion of Cdk4/6 [12] or Cdk2/4/6 [32] causes late embryonic lethality because of a major failure in haematopoiesis, most organogenesis and tissue development appear unaffected [12, 32]. From these observations emerged the idea that Cdks are endowed with interspersed compensatory functions and that specific Cdks are required in defined proliferative niches.
Cdk2
Albeit Cdk2 was thought to be essential to promote G1/S transition [26], knockout mice for this protein are viable and develop normally with a minor body weight reduction [14, 35]. Unexpectedly, Cdk2 is only essential for meiosis, and mice lacking Cdk2 are sterile because of defects in germ cell production [14]. In the adult brain, Cdk2 has been shown to be dispensable for DG neurogenesis [36] (Table 1). Indeed, Cdk2 deletion neither impairs proliferation of adult hippocampal NPCs in basal conditions nor following seizures [36]. In addition, while Cdk2 has been involved in the regulation of neuronal survival in vitro [37–39], no defect in newborn neurons survival/apoptosis was found in the DG of Cdk2-deficient mice [36]. Accordingly, Cdk2 might be absent in DG cells or functional redundancies among Cdks likely occur in this neurogenic niche. In contrast, in vivo and vitro gain- and loss-of-function approaches established a requirement for Cdk2 in cell proliferation and self-renewal capacities of adult SVZ-derived precursors [40]. Interestingly, no defects were observed in Cdk2-deficient SVZ precursors during early postnatal life thanks to a transient functional compensation by Cdk4 [40]. Therefore, in the postnatal/adult brain, the Cdk2 requirement is highly age and cell type dependent.
Table 1.
Consequences of cell cycle regulatory molecule deficiency on neurogenesis in postnatal, young and old adults in vivo and in vitro
| Postnatal (P1 < P10) | Adult (P42 < P120) | Old adult (1 year < 2 years) | |||||
|---|---|---|---|---|---|---|---|
| In vitro | In vivo | In vitro | In vivo | In vitro | In vivo | ||
| Cdk2−/− | DG | N.D. | N.D. | N.D. | No change in NPC proliferation | N.D. | N.D. |
| SVZ | No change in neurosphere number | No change in NPC proliferation | Reduced neurosphere proliferation and self-renewal | Reduced NG2 + cell proliferation | N.D. | N.D. | |
| Cdk4−/− | DG | N.D. | No change in NPC proliferation | N.D. | No change in NPC proliferation | N.D. | N.D. |
| SVZ | N.D. | No change in NPC proliferation | N.D. | No change in NPC proliferation | N.D. | N.D. | |
| Cdk6−/− | DG | N.D. | Reduced NPC proliferation | N.D. | Reduced type -2b and -3 NPC proliferation | N.D. | N.D. |
| SVZ | N.D. | Reduced NPC proliferation | N.D. | Reduced type -2b and -3 NPC proliferation | N.D. | N.D. | |
| Cyclin D2−/− | DG | N.D. | No change in NPC proliferation | Some neurospheres are formed | Absent NPC proliferation | N.D. | N.D. |
| SVZ | N.D. | N.D. | N.D. | Absent NPC proliferation | N.D. | N.D. | |
| pl6Ink4a−/− | DG | N.D. | N.D. | N.D. | No change in NPC proliferation | N.D. | No change in NPC proliferation |
| SVZ | N.D. | N.D. | No change in neurosphere number nor self-renewal | No change in NPC proliferation | Increased neurosphere number and self-renewal | Increased type-B NPC proliferation | |
| p21Cip1−/− | DG | N.D. | N.D. | N.D. | Increased NPC proliferation | N.D. | N.D. |
| SVZ | No change in neurosphere number | N.D. | Increased neurosphere number and self-renewal | Increased type-B NPC proliferation | Reduced neurosphere number and self-renewal | Reduced type-B NPC proliferation | |
| p27Kipl−/− | DG | N.D. | N.D. | Increased neurosphere number | Increased NPC proliferation | N.D. | N.D. |
| SVZ | N.D. | N.D. | Increased neurosphere number | Increased type-C NPC proliferation | N.D. | N.D. | |
| pl07−/− | DG | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| SVZ | N.D. | N.D. | Increased neurosphere number and self-renewal | Increased type-B NPC proliferation | N.D. | N.D. | |
| E2 fl−/− | DG | N.D. | N.D. | N.D. | Reduced NPC proliferation | N.D. | N.D. |
| SVZ | N.D. | N.D. | N.D. | Reduced NPC proliferation | N.D. | N.D. | |
N.D. Non-determined
Cdk4/6
Since Cdk4 and Cdk6 amino acid sequences share 71% homology, it was thus suggested that these two kinases play similar activities. However, Cdk4-deficient mice exhibited pancreatic hypoplasia because of the reduced numbers of β cells [33, 34], and Cdk6-deficient mice displayed erythropenia [12]. Our laboratory recently described the expression pattern of Cdk4 and Cdk6, and showed exclusive expression in dividing NPCs of the adult SVZ and DG. Surprisingly, Cdk6 was the only one to drive NPC proliferation in vivo [41]. Specifically, the absence of Cdk6 induces a lengthening of G1 phase in neuronally committed NPCs [41]. According to the “cell cycle length hypothesis”, which predicts that G1 length influences differentiation[42], the lengthening G1 phase observed in the absence of Cdk6 may cause neuronally committed NPCs to prematurely withdraw from the cell cycle, thereby impeding their expansion capacity and thus the production of neurons in the adult brain.
Altogether, these studies demonstrate that, contrary to what occurs during embryonic development and in most adult proliferating tissues, proliferation of adult NPCs is highly dependent on the presence of specific interphase Cdks. A yet unsolved question is: why are these enzymes functionally distinct in the context of adult NPCs proliferation? A plausible explanation is that the required Cdks phosphorylate substrates that are unique to adult NPCs. It is also possible that only these Cdks can generate the necessary levels of kinase activity to drive adult NPCs division. Finally it would be interesting to determine whether or not Cdk1, the only Cdk required for embryonic development [32], is crucial for NPCs proliferation in the adult brain.
Cdk5
Cdk5 regulates multiple cellular processes of both the developing and mature CNS. Particularly, this enzyme mediates cytoskeletal changes involved in neuronal migration during embryonic development [43, 44]. Using in vitro and in vivo conditional knockout experiments, Hirota and colleagues demonstrated that Cdk5 is also a key molecule controlling migration of neuroblasts in the postnatal/adult SVZ [45]. For instance, Cdk5 deficiency impairs the chain formation, speed, directionality, and leading process extension of migrating cells in a cell-autonomous manner. Similarly, using retroviruses expressing either a dominant negative version of Cdk5 (DNCdk5) or a shRNA targeting Cdk5 mRNA, it was recently found that Cdk5 is critical for migration, dendritic extension and pathfinding of adult newborn dentate granule cells [46]. Interestingly, whereas viral expression of DNCdk5 only moderately reduced the survival of adult-born hippocampal neurons, genetic deletion of Cdk5 in adult hippocampal precursors using Nestin-CreERT2 transgenic mice resulted in dramatic reduction of neuronal survival [47], suggesting that low levels of Cdk5 activity are compatible with neuronal survival, but inadequate to promote neuritic pathfinding. Of note, ablation of Cdk5 expression only in mature dentate neurons decreases the number of neuroblasts without affecting cell proliferation, suggesting a non-cell autonomous role for Cdk5 in the survival of newborn neurons in the adult DG [47]. Overall, these studies highlighted crucial and non-redundant roles for Cdk5 during adult neurogenesis, but the molecular mechanisms underlying Cdk5 functions in that context remain to be elucidated.
D cyclins
Cyclin D family members are the regulatory subunits controlling Cdk4/6 activity [5]. Three D-type cyclins, D1, D2 and D3, have been described in mammals. Most cells express multiple D cyclins [48, 49], but studies in mutant mice revealed key roles for these proteins in specific cell types. For instance, mice lacking cyclin D1 display abnormalities in the retina and mammary glands [48–50], while mice lacking cyclin D2 have defects in the ovaries and testes [51, 52]. Finally, cyclin D3-deficient mice are less susceptible to T cell malignancies triggered by specific oncogenic pathways [53].
In the adult brain, analysis of bromodeoxyuridine (BrdU) incorporation revealed that mice deficient for cyclin D2, but not cyclin D1, showed reduced cell proliferation in the DG and SVZ [54, 55]. Of note, the few cells that are generated within the adult DG of cyclin D2 KO mice belong to the astroglial lineage [55], suggesting that loss of cyclin D2 mostly impedes neuronally committed NPC proliferation. Moreover, an enriched environment [56–58] failed to increase neurogenesis in mice lacking cyclin D2 [55]. Altogether, these experiments revealed how essential cyclin D2 is to adult neurogenesis [55]. This is consistent with D-type cyclins being the primary cell cycle core constituents that sense growth factor stimulation to initiate cell cycle entry [59, 60].
Noteworthy, mRNA analysis suggested that cyclin D2 is the only D-type cyclin to be expressed in the adult mouse brain [55]. Using immunolabellings, another study reported that cyclin D1 is expressed in the adult DG [61]. However, the finding that adult neurogenesis is virtually absent in cyclin D2 KO mice indicates that cyclin D1 is not able to compensate for cyclin D2 during adult neurogenesis in the DG. In contrast, at early stages of life (P5), WT and cyclin D2 KO mice display strikingly similar rates of proliferation and neurogenesis [55]. Interestingly, all D-type cyclins are present at P5, and they can compensate for each other to ensure NPC proliferation and neurogenesis [55]. It has been shown that early postnatal neurogenesis is responsible for the generation of most of the granule cell neurons present in the adult DG [62, 63] and that adult neurogenesis poorly contributes to the production of granule neurons [64]. Consistently, GCL volume is only slightly reduced in the cyclin D2-deficient mice [55]. Together, these data indicate that the requirement for the different D-type cyclins varies along developmental stages, with cyclin D2 being exclusively required for adult neurogenesis. More globally, it suggests that adult neurogenesis is a specifically regulated process.
Endogenous cdk inhibitors
p16Ink4a
The four members of the Ink4 family (i.e., p16Ink4a, p15Ink4b, p18Ink4c and p19Ink4d) are specific inhibitors of Cdk4/6 activity. Among these, p16Ink4a expression increases with age in a variety of tissues [65, 66], including the brain [67]. Age is a well-described negative regulator of NPC proliferation and neurogenesis [68–70], and loss of p16Ink4a partially rescued the age-related decline in NPC proliferation in the SVZ [67]. Interestingly, in vitro and in vivo experiments showed that lack of p16Ink4a expression increased the proliferation of early/uncommitted NPCs in the SVZ (i.e., type-B stem cells) to a larger extent than lineage-determined transit-amplifying type-C cells and type-A neuroblasts [67]. This was consistent with previous findings showing that the deletion of p16Ink4a impedes embryonic neural stem but not lineage-restricted progenitor cell proliferation [71–73]. This raises the hypothesis that lineage determination modifies the cell cycle regulation of neural precursors in addition to restricting their developmental potential, causing the proliferation of lineage-determined cells to become p16Ink4a-independent [72]. In addition, neurogenesis, but not gliogenesis, in the OB was also increased in p16Ink4a-deficient old mice [67]. Altogether, these data indicate that increased expression of p16Ink4a may account for an age-related decline of type-B cell proliferation and neurogenesis in the SVZ and OB, respectively. Conversely, the absence of p16Ink4a did not detectably affect proliferation or neurogenesis in the aged DG [67], suggesting that, as exemplified by the deletion of Cdk2, the intrinsic cell cycle machinery driving NPC proliferation in the SVZ and DG is not always equivalent.
Cip/Kip family
The three members of the Cip/Kip family (p21Cip1, p27Kip1 and p57Kip2) have a broader range of Cdk inhibitory activity compared to the Ink4 inhibitors [9, 74–76]. They form stable complexes with the Cdk enzyme before cyclin binding, thereby preventing their association [9, 77, 78].
p21Cip1
Analysis of p21Cip1-deficient mice has provided new insights into the comprehension of quiescence and stem cell longevity (i.e., long-term maintenance of self-renewal ability) within the adult brain. In vitro, p21Cip1 deficiency increases the proliferation rate of SVZ-derived neurospheres from young adult (P60) mice, while the opposite situation occurs in old age (P480) [79]. However, some discrepancies were reported for in vivo analyses [79, 80], as no overall proliferation defect was reported in young adult p21Cip1 knockout mice [79, 80]. In contrast, there was a marked reduction of the number of Ki67-positive NPCs in old mice lacking p21Cip1 [79]. The reason for such a difference may be explained, as for p16Ink4a knockout mice [67], by a loss of p21Cip1 primarily affecting the proliferation of the more quiescent type-B NPCs [79], which account for a minority of actively dividing cells in the adult SVZ [81, 82]. It is possible that some rate variations of type-B precursor proliferation may not be detected when diluted within the overall cycling cell population from the young adult SVZ in vivo. However these results suggest that type-B NPCs that lack p21Cip1 expression expand themselves to a higher rate during early adult stages, but are more rapidly exhausted during ageing [79].
Altogether, these data suggest that p21Cip1 constrains SVZ NPC proliferation (i.e., the so-called relative quiescence), allowing their maintenance throughout the lifetime of the organism. This hypothesis is supported by the findings that haematopoietic stem cell quiescence is also maintained by p21Cip1 [83].
On the other hand, data regarding the importance of p21Cip1 in DG precursors are quite conflicting. For instance, although Pechnick et al. [84] observed an increase in cell proliferation in the DG in the absence of p21Cip1, another study did not report any significant modification of the mitotic activity of p21Cip-deficient DG precursors, except following ischemia. Since the genetic background and age of the animals used by these studies are similar, the observed discrepancies come probably from their different ways of evaluating cell proliferation.
p27Kip1
The absence of p27Kip1 in the adult SVZ leads to an increase in precursor proliferation [85], establishing p27Kip1 as a negative regulator of proliferation in the adult brain. Interestingly, p27Kip1 deletion induces a selective increase in transit-amplifying precursors (type-C) proliferation in the SVZ, while more quiescent precursors (type-B) are unchanged and neuroblasts (type-A cells) are decreased [85].
p27Kip1 was previously shown to induce cell cycle withdrawal in oligodendroglial precursors [86–88]. It is thus tempting to speculate that the absence of p27Kip1 prevents type-C cells from exiting the cell cycle, allowing them to perform extra rounds of divisions at the expense of lineage progression towards type-A neuroblasts [85]. This proposal correlates with findings in the haematopoietic system where p27Kip1 deletion markedly affects the proliferation of actively dividing progenitors [83, 89].
In the DG, p27Kip1 is particularly expressed in the SGZ [90]. A more detailed characterisation of its expression pattern revealed its localisation in the nucleus of neuronally commited NPCs [90], which reflects its cell cycle-active form where it can effectively inhibit Cdks [91, 92]. Upon deletion of the p27Kip1 gene, the proliferation is increased in the DG [90], just as it is in the SVZ [85]. The possibility that the lack of p27Kip1 selectively enhances the proliferation of neuronally committed NPCs requires further investigation. However, it was found that the number of newly born neurons in the DG is increased in the absence of p27Kip1 [90].
Although a growing body of literature has substantiated our knowledge of the function of CKIs in adult neurogenesis, many questions remain unanswered. For instance, the reason why p16Ink4a deficiency in old animals specifically rescues the rate of neurogenesis in the SVZ-OB niche but not in the DG remains to be fully elucidated. It is well known that there is an age-related decline of neurogenesis, but this decrease is much faster in the DG as compared to the SVZ-OB system [68]. Consequently, one reasonable hypothesis is that Molofsky et al. [67] analysed DG neurogenesis in excessively old mice, when the relative paucity of dividing precursors may mask effects of p16Ink4a deletion. Hence, it will be interesting to analyse DG neurogenesis in younger p16Ink4a-deficient mice to determine if the latter may transiently increases neuronal production. In addition, it is reasonable to assume that, in the SVZ, p21Cip1 and p16Ink4a maintain precursor quiescence in the young and old animals, respectively.
Considering the Cdk-cyclin specificity of the CKIs, it appears that type-B stem cells in the old SVZ are more likely to depend on D-type cyclin activation by growth factors [59, 60] to ensure their entry into the cell cycle. This last hypothesis is strongly supported by the fact that neurogenesis in the ageing brain can be stimulated by increasing the level of FGF-2[69, 93]. On the other hand, p27Kip1 may belong to a molecular timer that defines the rate of expansion of type-C transit amplifying cells, indicating that the cell cycle machinery is clearly precursor type-specific. The expression and function of p27Kip1 in neuronally determined NPCs is likely to be the preamble of its additional role(s) in the migration and differentiation of the newly generated neurons [94, 95]. Alternatively, the functions of both p16Ink4a and p21Cip1 in type-B stem cell proliferation and p27Kip1 in type-C actively dividing NPCs might reflect the need of stem cells for an utter level of regulation of their proliferation compared to actively dividing cells. Finally one must emphasise that all studies dealing with CKI regulation of adult neurogenesis have been using germline KO mouse models. This suggests that the self-renewal phenotypes observed in these models could be at least partially non-cell autonomous and/or did not give acute information on the role of CKI in adult NPCs, specifically. Using inducible conditional gene inactivation or gene silencing is thus clearly required for deciphering the precise role of these molecules during adult neurogenesis.
Cdk-cyclin substrates (pRb/E2f)
Phosphorylation of the closely related Rb family members (i.e., pRb/p105, p107, p130) by Cdk4/6-cyclin D complexes leads to their partial inactivation and to the release of the E2f transcription factors. Among the three Rb family members, pRb function is by far the better studied and characterised [96]. However, both germline [97–99] and nervous system [100, 101] specific deletions of pRb result in embryonic/perinatal lethality, preventing any analysis of the role of pRb during adult neurogenesis. On the other hand, mice deficient in either p107 or p130 develop normally on a C57Bl/6 genetic background [102, 103]. In the adult brain, p107 is highly expressed in the SVZ, and p107-deficient mice show a twofold increase in the number of type-B stem cells compared to their WT littermates in vivo and in vitro [104]. It was later suggested that p107 regulates the neural precursor population and differentiation by the repression of Hes1, a downstream mediator of the Notch signalling pathway [105, 106]. p130 is thought to be upregulated and to maintain neurons in a differentiated state, but no specific analysis of the putative role of p130 in adult neurogenesis has been done yet.
The E2f family contains eight members most recognised for their ability to regulate cell cycle progression [107]. According to whether E2fs act positively or negatively on gene transcription, they are grouped into transcriptional activators (E2f1 to E2f3a) or suppressors (E2f3b, E2f4, E2f5, E2f7 and E2f8), E2f7 and 8 being considered as atypical E2fs [108]. As for cyclins, analysis of mutant mice revealed broad compensatory mechanisms among the E2f family members during development [109, 110]. E2f1 is the most prominently expressed in the embryonic nervous system [111, 112]. Mice with a targeted deletion of E2f1 depict testis atrophy while brain development proceeds normally [109, 110]. However, in adulthood, mice lacking E2f1 show half of the number of dividing cells within both the DG and SVZ [20]. The deficit of NPC expansion further impedes production of new neurons in the DG and OB [20]. The fact that embryonic brain development is not affected in these mutant mice [20] further suggests that cell cycle progression in adult neurogenesis is a specifically regulated process. However, the persistence of dividing cells in adult E2f1-deficient neurogenic niches indicates that other members of the E2f family are capable of some compensatory function. Supporting this is recent evidence that E2f3a or b proteins, the two products of the E2f3 gene, also sustains NPC proliferation in the adult SVZ, as mice deficient for E2f3 show a one-third reduction in NPC numbers [107].
Maintenance of adult NPCs niches: self- renewal and quiescence
A hallmark feature of adult stem cells is their capacity to self-renew over the lifespan with a very slow rate of cell division as compared to their foetal counterpart. It is widely accepted that this is linked to a quiescent state, a functionally important characteristic of adult stem cells [113]. Quiescence can be defined as a non-dividing state outside of the cell cycle (i.e., G0) where a cell can remain in stasis until activated by appropriate proliferative signals. This state is necessary in the adult brain (1) to limit the accumulation of mutations in NPCs leading to cancer and (2) to prevent the exhaustion of the NPCs pool [114]. Characterisation of the molecular regulation of NPCs-specific CDK inhibitors may provide insights into the signalling pathways underlying quiescence of adult NPCs.
Recent results showed that Bmi-1 promotes the self-renewal of NSCs by repressing the expression of p16Ink4a and p19Arf [71, 115]. However, despite ongoing Bmi-1 expression, p16Ink4a expression increases with age, potentially reducing stem cell frequency and function [67]. p53, the well-known tumour suppressor gene, is also a critical regulator of cdk inhibitors. Loss of p53 results in increased proliferation of adult NPCs by a decrease of p21cip1 expression, allowing the escape of NPCs from quiescence and promoting their entrance into the cell cycle [116, 117]. Adult NPCs lacking expression of phosphatase and tensin homolog (Pten), or forkhead box Os (FoxOs) show a similar phenotype to that of p21cip1 in terms of cell cycling and exhaustion [118–120]. Whether these effects are a consequence of the regulation of cell cycle inhibitors remains to be demonstrated.
Conclusions and future directions
The ability of the brain to undergo neurogenesis in two restricted regions has challenged our view of brain plasticity. Given that new neurons are added or replace old ones in the DG and OB, respectively [64], one can easily understand that an accurate regulation of NPC proliferation is needed to produce the correct amount of newly generated mature neurons, particularly in a perspective of cell replacement following neurodegenerative disorders [3]. In this context, cell cycle regulatory molecules represent an attractive basis of investigation. Evolution has endowed higher mammals with a wide panel of cell cycle regulatory molecules capable of redundancy in order to prevent the catastrophic consequences upon the loss of a single cell cycle component. However, recent results show that redundancy is not complete, and a careful study of knockout mouse models reveals unique functions of many of these proteins. This specificity appears at the tissue level, and even more importantly at the cell scale. Therefore, in the brain, one should determine the cell cycle machinery in proliferating cells in the DG and the SVZ, and even in each neurogenic area in different subtypes of proliferating cells, e.g., uncommitted versus neuronally determined precursors.
Besides, Cdk1 deficiency is lethal at E2.5 in mice [32], precluding any analysis of its putative roles in NPC proliferation in the adult brain using a constitutive Cdk1-mutant mouse model. This particular point highlights a significant drawback to the studies that have so far addressed the contribution of cell cycle regulatory molecules to adult neurogenesis. To our knowledge, there are currently no reports examining the function of core cell cycle components in adult neurogenesis using conditional gene manipulation. Such experiments will be necessary to fully determine the cell cycle machinery governing cell proliferation in the two neurogenic regions of the adult brain, and constitute a prerequisite for manipulating NPC proliferation and neurogenesis in the frame of stem cell-based therapies.
In conclusion, much has been uncovered in the regulation of NPC proliferation in the adult brain, but a lot remains to be determined regarding the contribution of a basic biological process that is the cell cycle to adult neurogenesis. In our view, an in depth understanding of this issue is of utter importance to foresee the use of endogenous adult NPCs as a source for regenerative therapies.
Acknowledgments
This work was supported by the Fonds Léon Frédéricq (FLF) and the Fondation Médicale Reine Elisabeth. PB was supported by the FLF and a concerted action of the French Community of Belgium (Convention no. 04/09-322). RV is supported by a postdoctoral fellowship from the Alzheimer Society of Canada. NC is a research fellow of the Fonds pour la formation à la Recherche dans l’Industrie et dans l’Agriculture (FRIA). LN and BM are respectively research associate and research director of the Belgian Fonds National de la Recherche Scientifique (FNRS).
Footnotes
P. Beukelaers and R. Vandenbosch contributed equally to this work.
References
- 1.Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009;19(6):672–682. doi: 10.1038/cr.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imayoshi I, Sakamoto M, Ohtsuka T, Kageyama R. Continuous neurogenesis in the adult brain. Dev Growth Differ. 2009;51(3):379–386. doi: 10.1111/j.1440-169X.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- 3.Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441(7097):1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 4.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 5.Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30(11):630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18(2):753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98(6):859–869. doi: 10.1016/S0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 8.Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2(1):21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- 9.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9(10):1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 10.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9(3):153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 11.Satyanarayana A, Kaldis P. Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene. 2009;28(33):2925–2939. doi: 10.1038/onc.2009.170. [DOI] [PubMed] [Google Scholar]
- 12.Malumbres M, Sotillo R, Santamaria D, Galan J, Cerezo A, Ortega S, Dubus P, Barbacid M. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell. 2004;118(4):493–504. doi: 10.1016/j.cell.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Kozar K, Ciemerych MA, Rebel VI, Shigematsu H, Zagozdzon A, Sicinska E, Geng Y, Yu Q, Bhattacharya S, Bronson RT, Akashi K, Sicinski P. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118(4):477–491. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Berthet C, Aleem E, Coppola V, Tessarollo L, Kaldis P. Cdk2 knockout mice are viable. Curr Biol. 2003;13(20):1775–1785. doi: 10.1016/j.cub.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Pagano M, Jackson PK. Wagging the dogma; tissue-specific cell cycle control in the mouse embryo. Cell. 2004;118(5):535–538. doi: 10.1016/j.cell.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai LH, Broudy V, Perlmutter RM, Kaushansky K, Roberts JM. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85(5):733–744. doi: 10.1016/S0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 17.Callaghan DA, Dong L, Callaghan SM, Hou YX, Dagnino L, Slack RS. Neural precursor cells differentiating in the absence of Rb exhibit delayed terminal mitosis and deregulated E2F 1 and 3 activity. Dev Biol. 1999;207(2):257–270. doi: 10.1006/dbio.1998.9162. [DOI] [PubMed] [Google Scholar]
- 18.Huard JM, Forster CC, Carter ML, Sicinski P, Ross ME. Cerebellar histogenesis is disturbed in mice lacking cyclin D2. Development. 1999;126(9):1927–1935. doi: 10.1242/dev.126.9.1927. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson KL, Callaghan SM, O’Hare MJ, Park DS, Slack RS. The Rb-CDK4/6 signaling pathway is critical in neural precursor cell cycle regulation. J Biol Chem. 2000;275(43):33593–33600. doi: 10.1074/jbc.M004879200. [DOI] [PubMed] [Google Scholar]
- 20.Cooper-Kuhn CM, Vroemen M, Brown J, Ye H, Thompson MA, Winkler J, Kuhn HG. Impaired adult neurogenesis in mice lacking the transcription factor E2F1. Mol Cell Neurosci. 2002;21(2):312–323. doi: 10.1006/mcne.2002.1176. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y, Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor promotes proliferation of cortical neuron precursors by regulating E2F expression. FASEB J. 2003;17(2):186–193. doi: 10.1096/fj.02-0515com. [DOI] [PubMed] [Google Scholar]
- 22.Lukaszewicz A, Savatier P, Cortay V, Giroud P, Huissoud C, Berland M, Kennedy H, Dehay C. G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron. 2005;47(3):353–364. doi: 10.1016/j.neuron.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lange C, Huttner WB, Calegari F. Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell. 2009;5(3):320–331. doi: 10.1016/j.stem.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Pilaz LJ, Patti D, Marcy G, Ollier E, Pfister S, Douglas RJ, Betizeau M, Gautier E, Cortay V, Doerflinger N, Kennedy H, Dehay C. Forced G1-phase reduction alters mode of division, neuron number, and laminar phenotype in the cerebral cortex. Proc Natl Acad Sci USA. 2009;106(51):21924–21929. doi: 10.1073/pnas.0909894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malumbres M, Harlow E, Hunt T, Hunter T, Lahti JM, Manning G, Morgan DO, Tsai LH, Wolgemuth DJ. Cyclin-dependent kinases: a family portrait. Nat Cell Biol. 2009;11(11):1275–1276. doi: 10.1038/ncb1109-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262(5142):2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 27.Ye X, Zhu C, Harper JW. A premature-termination mutation in the Mus musculus cyclin-dependent kinase 3 gene. Proc Natl Acad Sci USA. 2001;98(4):1682–1686. doi: 10.1073/pnas.041596198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371(6496):419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 29.Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T, Wang JH. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371(6496):423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- 30.Tang D, Yeung J, Lee KY, Matsushita M, Matsui H, Tomizawa K, Hatase O, Wang JH. An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activator. J Biol Chem. 1995;270(45):26897–26903. doi: 10.1074/jbc.270.45.26897. [DOI] [PubMed] [Google Scholar]
- 31.Hellmich MR, Pant HC, Wada E, Battey JF. Neuronal cdc2-like kinase: a cdc2-related protein kinase with predominantly neuronal expression. Proc Natl Acad Sci USA. 1992;89(22):10867–10871. doi: 10.1073/pnas.89.22.10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santamaria D, Barriere C, Cerqueira A, Hunt S, Tardy C, Newton K, Caceres JF, Dubus P, Malumbres M, Barbacid M. Cdk1 is sufficient to drive the mammalian cell cycle. Nature. 2007;448(7155):811–815. doi: 10.1038/nature06046. [DOI] [PubMed] [Google Scholar]
- 33.Rane SG, Dubus P, Mettus RV, Galbreath EJ, Boden G, Reddy EP, Barbacid M. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat Genet. 1999;22(1):44–52. doi: 10.1038/8751. [DOI] [PubMed] [Google Scholar]
- 34.Tsutsui T, Hesabi B, Moons DS, Pandolfi PP, Hansel KS, Koff A, Kiyokawa H. Targeted disruption of CDK4 delays cell cycle entry with enhanced p27(Kip1) activity. Mol Cell Biol. 1999;19(10):7011–7019. doi: 10.1128/mcb.19.10.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortega S, Prieto I, Odajima J, Martin A, Dubus P, Sotillo R, Barbero JL, Malumbres M, Barbacid M. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet. 2003;35(1):25–31. doi: 10.1038/ng1232. [DOI] [PubMed] [Google Scholar]
- 36.Vandenbosch R, Borgs L, Beukelaers P, Foidart A, Nguyen L, Moonen G, Berthet C, Kaldis P, Gallo V, Belachew S, Malgrange B. CDK2 is dispensable for adult hippocampal neurogenesis. Cell Cycle. 2007;6(24):3065–3069. doi: 10.4161/cc.6.24.5048. [DOI] [PubMed] [Google Scholar]
- 37.Golsteyn RM. Cdk1 and Cdk2 complexes (cyclin dependent kinases) in apoptosis: a role beyond the cell cycle. Cancer Lett. 2005;217(2):129–138. doi: 10.1016/j.canlet.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Copani A, Condorelli F, Caruso A, Vancheri C, Sala A, Giuffrida Stella AM, Canonico PL, Nicoletti F, Sortino MA. Mitotic signaling by beta-amyloid causes neuronal death. FASEB J. 1999;13(15):2225–2234. [PubMed] [Google Scholar]
- 39.Rideout HJ, Wang Q, Park DS, Stefanis L. Cyclin-dependent kinase activity is required for apoptotic death but not inclusion formation in cortical neurons after proteasomal inhibition. J Neurosci. 2003;23(4):1237–1245. doi: 10.1523/JNEUROSCI.23-04-01237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jablonska B, Aguirre A, Vandenbosch R, Belachew S, Berthet C, Kaldis P, Gallo V. Cdk2 is critical for proliferation and self-renewal of neural progenitor cells in the adult subventricular zone. J Cell Biol. 2007;179(6):1231–1245. doi: 10.1083/jcb.200702031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beukelaers P, Vandenbosch R, Caron N, Nguyen L, Belachew S, Moonen G, Kiyokawa H, Barbacid M, Santamaria D, Malgrange B. Cdk6-Dependent Regulation of G(1) Length Controls Adult Neurogenesis. Stem Cells. 2011;29(4):713–724. doi: 10.1002/stem.616. [DOI] [PubMed] [Google Scholar]
- 42.Calegari F, Huttner WB. An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J Cell Sci. 2003;116(Pt 24):4947–4955. doi: 10.1242/jcs.00825. [DOI] [PubMed] [Google Scholar]
- 43.Ohshima T, Hirasawa M, Tabata H, Mutoh T, Adachi T, Suzuki H, Saruta K, Iwasato T, Itohara S, Hashimoto M, Nakajima K, Ogawa M, Kulkarni AB, Mikoshiba K. Cdk5 is required for multipolar-to-bipolar transition during radial neuronal migration and proper dendrite development of pyramidal neurons in the cerebral cortex. Development. 2007;134(12):2273–2282. doi: 10.1242/dev.02854. [DOI] [PubMed] [Google Scholar]
- 44.Ohshima T, Mikoshiba K. Reelin signaling and Cdk5 in the control of neuronal positioning. Mol Neurobiol. 2002;26(2–3):153–166. doi: 10.1385/MN:26:2-3:153. [DOI] [PubMed] [Google Scholar]
- 45.Hirota Y, Ohshima T, Kaneko N, Ikeda M, Iwasato T, Kulkarni AB, Mikoshiba K, Okano H, Sawamoto K. Cyclin-dependent kinase 5 is required for control of neuroblast migration in the postnatal subventricular zone. J Neurosci. 2007;27(47):12829–12838. doi: 10.1523/JNEUROSCI.1014-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jessberger S, Aigner S, Clemenson GD, Jr, Toni N, Lie DC, Karalay O, Overall R, Kempermann G, Gage FH. Cdk5 regulates accurate maturation of newborn granule cells in the adult hippocampus. PLoS Biol. 2008;6(11):e272. doi: 10.1371/journal.pbio.0060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lagace DC, Benavides DR, Kansy JW, Mapelli M, Greengard P, Bibb JA, Eisch AJ. Cdk5 is essential for adult hippocampal neurogenesis. Proc Natl Acad Sci USA. 2008;105(47):18567–18571. doi: 10.1073/pnas.0810137105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9(19):2364–2372. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- 49.Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, Haslam SZ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82(4):621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 50.Ma C, Papermaster D, Cepko CL. A unique pattern of photoreceptor degeneration in cyclin D1 mutant mice. Proc Natl Acad Sci USA. 1998;95(17):9938–9943. doi: 10.1073/pnas.95.17.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, Robker RL, Richards JS, McGinnis LK, Biggers JD, Eppig JJ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature. 1996;384(6608):470–474. doi: 10.1038/384470a0. [DOI] [PubMed] [Google Scholar]
- 52.Robker RL, Richards JS. Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol. 1998;12(7):924–940. doi: 10.1210/me.12.7.924. [DOI] [PubMed] [Google Scholar]
- 53.Sicinska E, Aifantis I, Le Cam L, Swat W, Borowski C, Yu Q, Ferrando AA, Levin SD, Geng Y, von Boehmer H, Sicinski P. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell. 2003;4(6):451–461. doi: 10.1016/S1535-6108(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 54.Jaholkowski P, Kiryk A, Jedynak P, Ben Abdallah NM, Knapska E, Kowalczyk A, Piechal A, Blecharz-Klin K, Figiel I, Lioudyno V, Widy-Tyszkiewicz E, Wilczynski GM, Lipp HP, Kaczmarek L, Filipkowski RK. New hippocampal neurons are not obligatory for memory formation; cyclin D2 knockout mice with no adult brain neurogenesis show learning. Learn Mem. 2009;16(7):439–451. doi: 10.1101/lm.1459709. [DOI] [PubMed] [Google Scholar]
- 55.Kowalczyk A, Filipkowski RK, Rylski M, Wilczynski GM, Konopacki FA, Jaworski J, Ciemerych MA, Sicinski P, Kaczmarek L. The critical role of cyclin D2 in adult neurogenesis. J Cell Biol. 2004;167(2):209–213. doi: 10.1083/jcb.200404181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 57.Steiner B, Zurborg S, Horster H, Fabel K, Kempermann G. Differential 24 h responsiveness of Prox1-expressing precursor cells in adult hippocampal neurogenesis to physical activity, environmental enrichment, and kainic acid-induced seizures. Neuroscience. 2008;154(2):521–529. doi: 10.1016/j.neuroscience.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 58.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1(3):191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 59.Assoian RK, Zhu X. Cell anchorage and the cytoskeleton as partners in growth factor dependent cell cycle progression. Curr Opin Cell Biol. 1997;9(1):93–98. doi: 10.1016/S0955-0674(97)80157-3. [DOI] [PubMed] [Google Scholar]
- 60.Matsushime H, Roussel MF, Ashmun RA, Sherr CJ. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65(4):701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- 61.Glickstein SB, Moore H, Slowinska B, Racchumi J, Suh M, Chuhma N, Ross ME. Selective cortical interneuron and GABA deficits in cyclin D2-null mice. Development. 2007;134(22):4083–4093. doi: 10.1242/dev.008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muramatsu R, Ikegaya Y, Matsuki N, Koyama R. Neonatally born granule cells numerically dominate adult mice dentate gyrus. Neuroscience. 2007;148(3):593–598. doi: 10.1016/j.neuroscience.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 63.Mathews EA, Morgenstern NA, Piatti VC, Zhao C, Jessberger S, Schinder AF, Gage FH. A distinctive layering pattern of mouse dentate granule cells is generated by developmental and adult neurogenesis. J Comp Neurol. 2010;518(22):4479–4490. doi: 10.1002/cne.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11(10):1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 65.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114(9):1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zindy F, Quelle DE, Roussel MF, Sherr CJ. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997;15(2):203–211. doi: 10.1038/sj.onc.1201178. [DOI] [PubMed] [Google Scholar]
- 67.Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443(7110):448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olariu A, Cleaver KM, Cameron HA. Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. J Comp Neurol. 2007;501(4):659–667. doi: 10.1002/cne.21268. [DOI] [PubMed] [Google Scholar]
- 70.Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2008;29(1):129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005;19(12):1432–1437. doi: 10.1101/gad.1299505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425(6961):962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bruggeman SW, Valk-Lingbeek ME, van der Stoop PP, Jacobs JJ, Kieboom K, Tanger E, Hulsman D, Leung C, Arsenijevic Y, Marino S, van Lohuizen M. Ink4a and Arf differentially affect cell proliferation and neural stem cell self-renewal in Bmi1-deficient mice. Genes Dev. 2005;19(12):1438–1443. doi: 10.1101/gad.1299305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee MH, Reynisdottir I, Massague J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995;9(6):639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- 75.Harper JW, Elledge SJ, Keyomarsi K, Dynlacht B, Tsai LH, Zhang P, Dobrowolski S, Bai C, Connell-Crowley L, Swindell E, et al. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995;6(4):387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78(1):59–66. doi: 10.1016/0092-8674(94)90572-X. [DOI] [PubMed] [Google Scholar]
- 77.Carnero A, Hannon GJ. The INK4 family of CDK inhibitors. Curr Top Microbiol Immunol. 1998;227:43–55. doi: 10.1007/978-3-642-71941-7_3. [DOI] [PubMed] [Google Scholar]
- 78.Vidal A, Koff A. Cell-cycle inhibitors: three families united by a common cause. Gene. 2000;247(1–2):1–15. doi: 10.1016/S0378-1119(00)00092-5. [DOI] [PubMed] [Google Scholar]
- 79.Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19(6):756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qiu J, Takagi Y, Harada J, Rodrigues N, Moskowitz MA, Scadden DT, Cheng T. Regenerative response in ischemic brain restricted by p21cip1/waf1. J Exp Med. 2004;199(7):937–945. doi: 10.1084/jem.20031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13(5):1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 82.Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22(5):1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287(5459):1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 84.Pechnick RN, Zonis S, Wawrowsky K, Pourmorady J, Chesnokova V. p21Cip1 restricts neuronal proliferation in the subgranular zone of the dentate gyrus of the hippocampus. Proc Natl Acad Sci USA. 2008;105(4):1358–1363. doi: 10.1073/pnas.0711030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Doetsch F, Verdugo JM, Caille I, Alvarez-Buylla A, Chao MV, Casaccia-Bonnefil P. Lack of the cell-cycle inhibitor p27Kip1 results in selective increase of transit-amplifying cells for adult neurogenesis. J Neurosci. 2002;22(6):2255–2264. doi: 10.1523/JNEUROSCI.22-06-02255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Durand B, Gao FB, Raff M. Accumulation of the cyclin-dependent kinase inhibitor p27/Kip1 and the timing of oligodendrocyte differentiation. EMBO J. 1997;16(2):306–317. doi: 10.1093/emboj/16.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Casaccia-Bonnefil P, Hardy RJ, Teng KK, Levine JM, Koff A, Chao MV. Loss of p27Kip1 function results in increased proliferative capacity of oligodendrocyte progenitors but unaltered timing of differentiation. Development. 1999;126(18):4027–4037. doi: 10.1242/dev.126.18.4027. [DOI] [PubMed] [Google Scholar]
- 88.Casaccia-Bonnefil P, Tikoo R, Kiyokawa H, Friedrich V, Jr, Chao MV, Koff A. Oligodendrocyte precursor differentiation is perturbed in the absence of the cyclin-dependent kinase inhibitor p27Kip1. Genes Dev. 1997;11(18):2335–2346. doi: 10.1101/gad.11.18.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng T, Rodrigues N, Dombkowski D, Stier S, Scadden DT. Stem cell repopulation efficiency but not pool size is governed by p27(kip1) Nat Med. 2000;6(11):1235–1240. doi: 10.1038/81335. [DOI] [PubMed] [Google Scholar]
- 90.Qiu J, Takagi Y, Harada J, Topalkara K, Wang Y, Sims JR, Zheng G, Huang P, Ling Y, Scadden DT, Moskowitz MA, Cheng T. p27Kip1 constrains proliferation of neural progenitor cells in adult brain under homeostatic and ischemic conditions. Stem Cells. 2009;27(4):920–927. doi: 10.1002/stem.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, Arteaga CL. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8(10):1145–1152. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- 92.Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, Lee JH, Ciarallo S, Catzavelos C, Beniston R, Franssen E, Slingerland JM. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8(10):1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 93.Mudo G, Belluardo N, Mauro A, Fuxe K. Acute intermittent nicotine treatment induces fibroblast growth factor-2 in the subventricular zone of the adult rat brain and enhances neuronal precursor cell proliferation. Neuroscience. 2007;145(2):470–483. doi: 10.1016/j.neuroscience.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 94.Nguyen L, Besson A, Heng JI, Schuurmans C, Teboul L, Parras C, Philpott A, Roberts JM, Guillemot F. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 2006;20(11):1511–1524. doi: 10.1101/gad.377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li X, Tang X, Jablonska B, Aguirre A, Gallo V, Luskin MB. p27(KIP1) regulates neurogenesis in the rostral migratory stream and olfactory bulb of the postnatal mouse. J Neurosci. 2009;29(9):2902–2914. doi: 10.1523/JNEUROSCI.4051-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer. 2008;8(9):671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clarke AR, Maandag ER, van Roon M, van der Lugt NM, van der Valk M, Hooper ML, Berns A, te Riele H. Requirement for a functional Rb-1 gene in murine development. Nature. 1992;359(6393):328–330. doi: 10.1038/359328a0. [DOI] [PubMed] [Google Scholar]
- 98.Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Effects of an Rb mutation in the mouse. Nature. 1992;359(6393):295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 99.Lee EY, Chang CY, Hu N, Wang YC, Lai CC, Herrup K, Lee WH, Bradley A. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992;359(6393):288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- 100.Ferguson KL, Vanderluit JL, Hebert JM, McIntosh WC, Tibbo E, MacLaurin JG, Park DS, Wallace VA, Vooijs M, McConnell SK, Slack RS. Telencephalon-specific Rb knockouts reveal enhanced neurogenesis, survival and abnormal cortical development. EMBO J. 2002;21(13):3337–3346. doi: 10.1093/emboj/cdf338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.MacPherson D, Sage J, Crowley D, Trumpp A, Bronson RT, Jacks T. Conditional mutation of Rb causes cell cycle defects without apoptosis in the central nervous system. Mol Cell Biol. 2003;23(3):1044–1053. doi: 10.1128/MCB.23.3.1044-1053.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cobrinik D, Lee MH, Hannon G, Mulligan G, Bronson RT, Dyson N, Harlow E, Beach D, Weinberg RA, Jacks T. Shared role of the pRB-related p130 and p107 proteins in limb development. Genes Dev. 1996;10(13):1633–1644. doi: 10.1101/gad.10.13.1633. [DOI] [PubMed] [Google Scholar]
- 103.Lee MH, Williams BO, Mulligan G, Mukai S, Bronson RT, Dyson N, Harlow E, Jacks T. Targeted disruption of p107: functional overlap between p107 and Rb. Genes Dev. 1996;10(13):1621–1632. doi: 10.1101/gad.10.13.1621. [DOI] [PubMed] [Google Scholar]
- 104.Vanderluit JL, Ferguson KL, Nikoletopoulou V, Parker M, Ruzhynsky V, Alexson T, McNamara SM, Park DS, Rudnicki M, Slack RS. p107 regulates neural precursor cells in the mammalian brain. J Cell Biol. 2004;166(6):853–863. doi: 10.1083/jcb.200403156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16(7):846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vanderluit JL, Wylie CA, McClellan KA, Ghanem N, Fortin A, Callaghan S, MacLaurin JG, Park DS, Slack RS. The Retinoblastoma family member p107 regulates the rate of progenitor commitment to a neuronal fate. J Cell Biol. 2007;178(1):129–139. doi: 10.1083/jcb.200703176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McClellan KA, Slack RS. Specific in vivo roles for E2Fs in differentiation and development. Cell Cycle. 2007;6(23):2917–2927. doi: 10.4161/cc.6.23.4997. [DOI] [PubMed] [Google Scholar]
- 108.Lammens T, Li J, Leone G, De Veylder L. Atypical E2Fs: new players in the E2F transcription factor family. Trends Cell Biol. 2009;19(3):111–118. doi: 10.1016/j.tcb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Field SJ, Tsai FY, Kuo F, Zubiaga AM, Kaelin WG, Jr, Livingston DM, Orkin SH, Greenberg ME. E2F–1 functions in mice to promote apoptosis and suppress proliferation. Cell. 1996;85(4):549–561. doi: 10.1016/S0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- 110.Yamasaki L, Jacks T, Bronson R, Goillot E, Harlow E, Dyson NJ. Tumor induction and tissue atrophy in mice lacking E2F–1. Cell. 1996;85(4):537–548. doi: 10.1016/S0092-8674(00)81254-4. [DOI] [PubMed] [Google Scholar]
- 111.Tevosian SG, Paulson KE, Bronson R, Yee AS. Expression of the E2F–1/DP-1 transcription factor in murine development. Cell Growth Differ. 1996;7(1):43–52. [PubMed] [Google Scholar]
- 112.Dagnino L, Fry CJ, Bartley SM, Farnham P, Gallie BL, Phillips RA. Expression patterns of the E2F family of transcription factors during mouse nervous system development. Mech Dev. 1997;66(1–2):13–25. doi: 10.1016/S0925-4773(97)00083-X. [DOI] [PubMed] [Google Scholar]
- 113.Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9(2):115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- 114.Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, San Emeterio J, Hortiguela R, Marques-Torrejon MA, Nakashima K, Colak D, Gotz M, Farinas I, Gage FH. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell. 2010;7(1):78–89. doi: 10.1016/j.stem.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 115.He S, Iwashita T, Buchstaller J, Molofsky AV, Thomas D, Morrison SJ. Bmi-1 over-expression in neural stem/progenitor cells increases proliferation and neurogenesis in culture but has little effect on these functions in vivo. Dev Biol. 2009;328(2):257–272. doi: 10.1016/j.ydbio.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meletis K, Wirta V, Hede SM, Nister M, Lundeberg J, Frisen J. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133(2):363–369. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- 117.Medrano S, Scrable H. Maintaining appearances–the role of p53 in adult neurogenesis. Biochem Biophys Res Commun. 2005;331(3):828–833. doi: 10.1016/j.bbrc.2005.03.194. [DOI] [PubMed] [Google Scholar]
- 118.Gregorian C, Nakashima J, Le Belle J, Ohab J, Kim R, Liu A, Smith KB, Groszer M, Garcia AD, Sofroniew MV, Carmichael ST, Kornblum HI, Liu X, Wu H. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J Neurosci. 2009;29(6):1874–1886. doi: 10.1523/JNEUROSCI.3095-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paik JH, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, Chae SS, Zheng H, Ying H, Mahoney J, Hiller D, Jiang S, Protopopov A, Wong WH, Chin L, Ligon KL, DePinho RA. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5(5):540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, Villeda SA, Thekkat PU, Guillerey C, Denko NC, Palmer TD, Butte AJ, Brunet A. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5(5):527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]